Exploring the potential of the nano-köhler theory to describe the growth of atmospheric molecular clusters by organic vapors
|
|
|
- Alisha Louisa Goodwin
- 5 years ago
- Views:
Transcription
1 Atmos. Chem. Phys. Discuss., Exploring the potential of the nano-köhler theory to describe the growth of atmospheric molecular clusters by organic vapors Jenni Kontkanen 1,2, Tinja Olenius 1, Markku Kulmala 2,3,4, and Ilona Riipinen 1 1 Department of Environmental Science and Analytical Chemistry (ACES) and Bolin Centre for Climate Research, Stockholm University, Stockholm, Sweden 2 Institute for Atmospheric and Earth System Research / Physics, Faculty of Science, University of Helsinki, Helsinki, Finland 3 Aerosol and Haze Laboratory, Beijing Advanced Innovation Center for Soft Matter Science and Engineering, Beijing University of Chemical Technology, Beijing, P.R. China 4 Joint International Research Laboratory of Atmospheric and Earth System Sciences, School of Atmospheric Sciences, Nanjing University, Nanjing, P.R. China Correspondence to: Jenni Kontkanen (jenni.kontkanen@helsinki.fi) 1 Abstract. New particle formation involving sulfuric acid, bases and oxidized organic compounds is an important source of atmospheric aerosol particles. One of the mechanisms suggested to depict this process is the nano-köhler theory, which describes the activation of inorganic molecular clusters to growth by a soluble organic vapor. In this work we studied the capability of the nano-köhler theory to describe the growth of atmospheric molecular clusters by simulating the dynamics of a cluster population in the presence of a sulfuric acid base mixture and an organic compound. We observed nano-köhler type activation in our simulations when the saturation ratio of the organic vapor and the ratio between organic and inorganic vapor concentrations were in a suitable range. However, the nano-köhler theory was unable to predict the exact size at which the activation occurred in the simulations. In some conditions apparent cluster growth rate (GR) started to increase close to the activation size determined from the simulations. Nevertheless, because the behavior of GR is also affected by other dynamic processes, GR alone cannot be used to deduce the cluster growth mechanism Introduction Atmospheric new particle formation (NPF) is a significant source of global cloud condensation nuclei (CCN) (Kerminen et al., 12; Spracklen et al., 08), and thus it affects the magnitude of indirect aerosol forcing (Kazil et al., ; Makkonen et al., 12). NPF has also been observed to contribute to particulate pollution events in urban environments, such as Chinese megacities (Guo et al., 14). Therefore, accurate understanding of NPF, including the formation of the initial nanometersized molecular clusters and their further growth to larger particles, is necessary both to decrease uncertainties in climate projections, and to tackle urban air quality problems. 1
2 Atmos. Chem. Phys. Discuss., According to current knowledge, sulfuric acid is a major driver of the first steps of NPF in most environments (Kuang et al., 08; Paasonen et al., ; Kulmala et al., 13). Base compounds, such as ammonia and amines, are also important due to their ability to cluster efficiently with sulfuric acid and stabilize the initial nanoparticles (Almeida et al., 13; Jen et al., 14, 16; Kirkby et al., 11). Low-volatile organic vapors have long been considered as responsible for growing the freshlyformed particles to larger sizes (e.g. Kuang et al., 12; Riipinen et al., 12; Yli-Juuti et al., 11). Furthermore, recent experimental results show that highly oxidized organic species, formed by oxidation of monoterpenes, can participate in NPF already at nanometer sizes (Schobesberger et al., 13; Kirkby et al., 16). However, understanding of the physics and chemistry of NPF involving organic compounds is still very limited, largely because the identities and properties of the organic species are poorly known (Donahue et al., 13; Elm et al., 17). In particular, there is a lack of a robust physical description of the formation of the initial nanoparticles in the presence of sulfuric acid, bases and organic compounds. Such a description is needed for correct interpretation of the experimental data on NPF, but also for implementing the process accurately in largerscale atmospheric transport models. NPF is often described as a two-step process (Kulmala et al., 00). In the first step, a stable cluster that is more likely to grow than to evaporate is formed by nucleation, and in the second step the cluster grows to larger sizes by condensation. The latter step is often referred to as activation of the clusters. Accordingly, particle formation involving sulfuric acid, bases, and lowvolatile organic compounds can be considered to proceed via the formation of a stable sulfuric acid-base cluster, followed by its growth by condensation of organic vapor (Kulmala, 04). However, if the participation of organic vapor in NPF occurs simply by condensation, the organic vapor needs to have an extremely high saturation ratio to be able to contribute to the growth at nanometer sizes (Kulmala et al., 04). This is due to the large surface-to-volume ratio of the clusters, which makes attachment of molecules thermodynamically unfavorable. This is qualitatively depicted by the Kelvin effect, in which the saturation ratio over the surface of a spherical particle increases with decreasing particle size. Several mechanisms have been proposed to describe how nanometer-sized clusters can overcome this thermodynamic barrier. These mechanisms include heterogeneous nucleation of organic vapors (Wang et al., 13), heterogeneous reactions between clusters and organic vapors (Wang et al., ; Zhang and Wexler, 02), adsorption of organics on cluster surface (Wang and Wexler, 13) and a nano- Köhler-type mechanism (Kulmala et al., 04). In this study, we focus on the nano-köhler mechanism and its capability to describe the growth of atmospheric clusters. The nano-köhler theory was first proposed by Kulmala et al. (04). It describes the activation of nanometer-sized inorganic clusters to growth by condensation of organic vapor, which is soluble in the inorganic compound and water. Thus, the nano- Köhler theory is analogous to the Köhler theory describing the activation of CCN to cloud droplets. The fact that the nano- Köhler theory is based on the macroscopic properties of the condensing vapors makes it appealing to use for describing the initial particle formation. The nano-köhler mechanism has been applied in aerosol dynamics models (Anttila et al., 04; Korhonen et al., 04), used to describe activation of particles inside condensation particle counters (Giechaskiel et al., 11; Kulmala et al., 07), and suggested to explain observed behavior of sub- nm particle populations in field and laboratory measurements (Kuang et al., 12; Kulmala et al., 13; Tröstl et al., 16). In a recent laboratory study Tröstl et al. (16) 2
3 Atmos. Chem. Phys. Discuss., examined particle growth rates (GR) in the presence of monoterpene oxidation products and observed accelerating growth at sizes below nm. Using a volatility distribution growth model, where the condensational growth of a single representative particle is described by the nano-köhler mechanism, the authors suggest that the particle growth at sizes below ~2 nm is governed by organic vapors with extremely low volatility. At larger sizes, where the Kelvin effect is reduced, more abundant vapors with slightly higher volatilities can start to contribute to the growth, resulting in a higher GR. Although the nano-köhler theory has been applied in aerosol dynamics and single-particle growth models, these modelling approaches provide only limited knowledge of the capability of the theory to describe the very first steps of NPF. This is because these models describe condensation macroscopically, which means that all particles of a given size grow or shrink at the same rate. However, the formation and growth of the smallest sizes is affected by stochastic collision and evaporation processes, which causes widening of the nanoparticle size distribution and enables the crossing of thermodynamic barriers by homogeneous nucleation (Wang et al., 13). Furthermore, single-particle modeling approaches assume that particles grow only by vapor monomer collisions, while both experimental and modeling studies indicate that cluster-cluster collisions may significantly contribute to particle growth in some conditions (Kontkanen et al., 16; Lehtipalo et al., 16). Modeling the initial NPF accurately requires using a cluster kinetics model, which simulates the time-development of cluster concentrations explicitly considering stochastic effects and including all collision and evaporation processes between vapor molecules and clusters (Olenius and Riipinen, 17). In this study, our aim is to investigate the potential of the nano-köhler theory to describe the growth of atmospheric molecular clusters by organic vapors. For this we use a molecular-resolution model, which allows us to explicitly simulate the timeevolution of a cluster population involving organic and inorganic species. First, we discuss similarities and differences between the nano-köhler theory and the traditional Köhler theory and compare their assumptions to real atmospheric molecular systems. Then, we apply cluster kinetics simulations to study in what kind of conditions nano-köhler type behavior can be observed. Specifically, we investigate the effects of vapor properties, such as volatility and vapor concentrations, on the dynamics of the cluster population. We also compare the results on cluster activation obtained from the simulations to the predictions of the nano-köhler theory, and assess to what extent this simplified theory is able to capture the cluster growth mechanisms. 2 Theory 30 The nano-köhler theory describes the activation of inorganic clusters to growth by spontaneous condensation of organic vapor, which is soluble in the inorganic compound and water (Kulmala et al., 04). In the original nano-köhler theory, presented by Kulmala et al. (04), a system involving sulfuric acid ammonia clusters, an organic compound, and water is studied. However, the theory can also be applied to other seed compositions and to systems containing only inorganic clusters and an organic vapor without water, which is the case in this study. 3
4 Atmos. Chem. Phys. Discuss., The nano-köhler theory is based on assuming a thermodynamic equilibrium between the clusters and the condensing organic vapor. In an analogous way to the traditional Köhler theory, the equilibrium saturation ratio of organic compound (Sorg,eq) is obtained from S org,eq = x org a org exp ( 4σ orgm org k B Tρ org d p ), (1) where x org is the molar fraction of the organic compound in the particle phase, a org is the activity coefficient, m org is the molecule mass, ρ org is the liquid-phase density, σ org is the surface tension, and d p is the cluster diameter. k B is the Boltzmann constant and T is temperature. A similar equation can be written for the equilibrium saturation ratio of water if it is included in the studied system (Kulmala et al., 04). From Eq. (1) it is possible to solve the equilibrium saturation ratio of organic compound for different cluster sizes d p in the presence of a seed cluster with diameter d p,s. When the resulting equilibrium saturation ratios are plotted as a function of the cluster size, a curve similar to traditional Köhler curves is obtained. The curve exhibits a peak resulting from the combination of Kelvin and Raoult effects, caused by the curvature of the cluster surface and solubility of the compounds (Vehkamäki and Riipinen, 12). When the saturation ratio of the organic compound is lower than the peak value, the seed clusters trapped behind the peak maintain their equilibrium size but they cannot grow further. When the saturation ratio increases beyond the peak value, the clusters start to grow spontaneously by condensation of organic vapor (see Fig. 1). Thus, the ascending part of the curve corresponds to the stable equilibrium state and the descending part to the unstable equilibrium. Note that this behavior is different than in heterogeneous nucleation, where the seed is insoluble in the nucleating vapor, and the saturation ratio needed for the cluster activation decreases as a function of cluster size without a maximum (Winkler et al., 08). Although the thermodynamic equilibrium is described in an analogous way in the nano-köhler theory and in the traditional Köhler theory, there are differences between these two theories, especially related to the systems that they are describing (Anttila et al., 04; Kulmala, 04). The comparison between the theories is presented in Table 1. Furthermore, the real systems differ significantly from the simplified system assumed in the nano-köhler theory (see Table 1 and Fig. 1). In real systems there is no specific non-evaporating seed or condensing vapor but there is a distribution of inorganic and organic vapor molecules and clusters which all can collide and evaporate. Furthermore, there does not necessarily exist an energy barrier that needs to be overcome, but the growth of clusters may be entirely governed by kinetic collisions. If an energy barrier exists, clusters may overcome the barrier even before the critical saturation ratio is reached as a result of stochastic collisions, i.e. nucleation, and thus the system is not in thermodynamic equilibrium all the time (Vehkamäki and Riipinen, 12). In addition, in real systems organic and inorganic vapors are not lost solely due to scavenging by pre-existing particle population but also due to collisions with clusters. Furthermore, vapor concentrations and external losses may vary over time, which can affect the dynamics of the cluster population. 4
5 Atmos. Chem. Phys. Discuss., Figure 1: Schematic figure illustrating how the growth of atmospheric molecular clusters is described by the nano-köhler theory, and how the growth occurs in real molecular systems affected by various dynamic processes. Table 1: Comparison between the Köhler theory, the nano-köhler theory and real systems of atmospheric molecular clusters. Köhler theory Nano-Köhler theory Real molecular systems Describes the activation of CCN (dp>0 nm) to cloud droplets by spontaneous condensation of water vapor. Describes the activation of inorganic clusters (dp = ~1 3 nm) for growth by spontaneous condensation of organic vapor. A distribution of clusters of varying sizes and compositions including inorganic and organic compounds can exist simultaneously at all times. The condensing vapor is water vapor with typical atmospheric concentrations of ~ 17 cm -3. The seed consists of a mixture of inorganic/organic compounds and is watersoluble. The seed compounds do not evaporate. Thermodynamic equilibrium between water and the seed particle is assumed. The growing cloud droplets scavenge the available water vapor thereby limiting the activation process. The condensing vapor is a water-soluble organic compound with concentrations likely ranging from ~ to 8 cm -3 (Jokinen et al., 17). The seed consists of sulfuric acid and bases and is soluble in the condensing organic compound. The seed compounds do not evaporate but condense irreversibly on the cluster. Thermodynamic equilibrium between the organic compound, the seed cluster and water is assumed. The energy barrier width in the nano-köhler is very narrow with respect to the number of molecules compared to the Köhler theory, and thus addition of only few molecules may result in overcoming the barrier. The loss rate of organic vapor is determined mainly by larger background aerosol particles, and not the growing clusters. Vapor concentrations are not constant but may vary over time. There is no seed in the same sense as in the theory. Both inorganic and organic compounds can condense and evaporate and may contribute to the growth. Clusters can nucleate over barriers and they may not be in thermodynamic equilibrium before activation to growth. The cluster population is affected by losses due to background particles, and cluster selfcoagulation may also be important. The magnitude of external losses may vary over time.
6 Atmos. Chem. Phys. Discuss., 3 Methods 3.1 Cluster kinetics model 1 We used a molecular-resolution cluster kinetics model to simulate the time-development of atmospheric cluster concentrations. Two model compounds, one corresponding to an inorganic compound and one corresponding to an organic compound, were used in the simulations (see the next section for the properties of the model compounds). The cluster population was simulated starting from vapor monomers up to clusters with mass diameter of nm, including all possible cluster compositions. The largest simulated clusters correspond to the size of a pure inorganic cluster composed of 0 molecules. In the model, the discrete General Dynamics Equation (GDE; Friedlander, 1977) is numerically solved for each cluster composition i including all processes where a cluster can be formed or lost: dc i = 1 β dt 2 j,(i j)c j C i j + Ɣ (i+j) i,j C i+j β i,j C i C j 1 Ɣ j<i j j 2 j<i i j,(i j)c i + Q i L i C i. (2) Here C i is the concentration of cluster i and β i,j is the collision rate coefficient between cluster i and cluster j. Ɣ (i+j) i,j is the evaporation rate coefficient of cluster (i+j) to clusters i and j, which we considered only for evaporation of vapor monomers. Q i is the source rate, here included only for vapor molecules. L i is the loss rate coefficient corresponding to the external sink for vapors and clusters. The set of GDEs was generated and solved with the ACDC (Atmospheric Cluster Dynamics Code) program (Olenius and Riipinen, 17). The collision rate coefficients β i,j were calculated assuming hard-sphere collisions. The evaporation rate coefficients of vapor monomers were calculated according to the Kelvin formula: Ɣ (i+j) i,j = β i,j p sat,i k B T x i exp ( 4σm i k B Tρ i d pj ) (3) Here β i,j is the collision rate coefficient between vapor compound i and cluster j. p sat,i is the saturation vapor pressure of the 2 compound, x i is the molar fraction of the compound in cluster j, σ is the cluster surface tension, ρ i is the liquid phase density of i, and d p,j is the cluster diameter. k B is the Boltzmann constant and T is the temperature. Calculating evaporation rates from Eq. (3) is consistent with calculating equilibrium saturation ratios from Eq. (1) when assuming that the activity coefficient a = 1. In most simulations the external loss coefficient L i was set to correspond to typical losses in a chamber experiment, including a size-dependent wall loss (L wall ) and a size-independent dilution loss (L dil ) according to Kürten et al. (1): L i = L wall + L dil = A wall d p,i + L dil (4) 30 Here the empirical constant A wall is nm s -1 and the loss rate due to dilution L dil is s -1 (Kürten et al., 1). In one simulation set, external losses corresponding to a sink caused by a background particle population in the planetary boundary layer were used. In this case, the loss coefficient was obtained from (Lehtinen et al., 07): L i = L ref ( d b p ) d p,ref () 6
7 Atmos. Chem. Phys. Discuss., Here L ref is the loss coefficient for a reference cluster, which in our case is the inorganic vapor monomer, and d p,ref is the diameter of the reference cluster. We set the reference loss coefficient to -3 s -1 and the exponent b to 1.6, which represent typical values in a boreal forest (Lehtinen et al., 07). 3.2 Studied compounds We simulated binary mixtures of inorganic and organic compounds relevant for atmospheric particle formation. The inorganic compound corresponds to a quasi-unary sulfuric acid base mixture, and the organic compound represents atmospheric oxidized organic species (see Table 2). All model compounds were assumed to consist of spherical molecules. In most simulations the inorganic compound was set to have the mass of a sulfuric acid dimethylamine cluster (SA DMA) and p sat = 0. Additional simulations were performed using the mass of ammonium bisulfate (SA NH 3) and p sat = -9 Pa. The properties of these model substances are consistent with observations on a 1:1 molar ratio in which dimethylamine and ammonia cluster with sulfuric acid, dimethylamine forming seemingly non-evaporating clusters (Jen et al., 14; Kürten et al., 14). In most simulations, the organic compound (ORG) was set to have a mass of 300 amu and its p sat was varied between -4 and -12 Pa. Of these saturation vapor pressures, two were selected for a more detailed study: p sat = -8 Pa, representing a lowvolatile organic compound (LVOC), and p sat = -11 Pa, representing an extremely low-volatile organic compound (ELVOC). In addition, simulations were performed using a compound with p sat = -8 Pa and a mass of 00 amu, corresponding to LVOC with a larger mass (LVOC large). The saturation vapor pressures of the LVOC and ELVOC species are consistent with the volatility basis set classification of organic compounds with low and extremely low volatilities (Donahue et al., 12). They also may represent typical saturation vapor pressures of alpha-pinene oxidation products (Tröstl et al., 16) but as different methods give very different estimates for saturation vapor pressures, the exact values are uncertain (Kurtén et al., 16). In addition to the saturation vapor pressures and molecular masses, the assumed liquid phase densities, the particle surface tension and the activity coefficients can have significant effects on the results. In earlier studies investigating the nano-köhler theory, these properties have been obtained by fitting a simple thermodynamic model to measured data on particle properties (Anttila et al., 04; Kulmala, 04). However, because of large uncertainties related to estimating these properties for nanometer-sized clusters, we chose to use a simple approach, and set all particles to have a density of 0 kg m -3 and a surface tension of N m -1. Activity coefficients were set to 1, corresponding to an ideal solution. Furthermore, we do not include water in the model system although it is incorporated in the original nano-köhler theory. Including water would lower the saturation vapor pressure of the organic compound through the solute effect (Raoult s law). We address the effect of the organic compound volatility by performing simulations with a large range of organic saturation vapor pressures. 7
8 Atmos. Chem. Phys. Discuss., Table 2: Model compounds, their molecular mass (m), saturation vapor pressure (psat) and the corresponding number concentration (Csat) and mass concentration (Cmass,sat) at T = 278 K. Note that LVOC and ELVOC are two cases of ORG selected for more detailed studies. Model substance m (amu) psat (Pa) Csat (cm -3 ) Cmass,sat (µg m -3 ) SA DMA SA NH ORG LVOC ELVOC LVOClarge Simulation sets 1 All performed simulation sets are described in Table 3. In simulation sets 1 4, the concentrations of the inorganic vapor (C SA) and the organic vapor (C ORG) were set to constant values and the simulations were run until the steady state was reached. In addition, in order to mimic a laboratory set-up, simulations with constant vapor source rates, resulting in the same steady-state vapor concentrations, were performed to study the cluster growth rates (GRs). By default, the inorganic and organic species were SA DMA and LVOC or ELVOC, unless otherwise stated. Temperature was set to 278 K in all the simulations. The sets address the effect of the volatility p sat,org and concentration C ORG of the organic vapor (set 1), the effect of relatively high sulfuric acid concentration C SA (set 2), the effect of the properties of the inorganic vapor (set 3), and the effect of the mass of the organic vapor (set 4). The simulation set addresses the effect of time-dependent vapor concentration profiles corresponding to atmospheric conditions. Two different simulations were performed: 1) a simulation where inorganic and organic vapor source rates exhibit maxima, 2) a simulation where the inorganic vapor source rate exhibits a maximum but the organic vapor source rate is constant. These simulations were run for 12 hours. 8
9 Atmos. Chem. Phys. Discuss., Table 3: Description of the simulation sets. The variables are varied at intervals of one order of magnitude. Note that the presented vapor concentrations are steady-state values for sets 1 4 and the maximum values for set. Simulation set Compounds Vapor concentrations (cm -3 ) Conditions 1 SA DMA CSA = 6 ORG, psat = CORG = 4 constant CSA and CORG, chamber experiment losses (Eq. 4) 2 3 SA DMA CSA = 7 constant CSA and CORG, LVOC or ELVOC CORG = 7 chamber experiment losses (Eq. 4) SA NH3 CSA = 6 constant CSA and CORG, LVOC CORG = 6 9 chamber experiment losses (Eq. 4) 4 SA DMA CSA = 6 LVOClarge CORG = 6 9 constant CSA and CORG, chamber experiment losses (Eq. 4) SA DMA CSA, max = 6 varying CSA and CORG, LVOC CORG, max = 6, 7 background particle losses (Eq. ) 3.4 Analysis of simulated data From the simulated cluster concentrations, we determined the contributions of different vapor monomers and clusters to the th growth over selected threshold sizes d p between 1 and 3 nm. The net cluster flux J dp th past each threshold size was determined by considering all the collisions and evaporations between different clusters or vapor molecules that lead to crossing of the threshold as J dp th = 1 2 (β i,jc i C j γ i+j i,j C i+j ), (6) where d p,i and d p,j < d th p, and d p,i+j d th p. The contribution of an individual molecule or cluster to this flux was determined so that for each collision-evaporation process between species i and j leading to the crossing of the threshold, the resulting flux was attributed to the species with the smaller number of molecules. The reasoning behind this is that when, for example, a vapor monomer i collides with cluster j composed of several molecules, the monomer is causing the cluster to grow in size, 1 and thus the flux is assigned to be due to i. In addition, we determined the apparent cluster growth rates (GR) using the experimental approach that is often applied for measured particle size distribution data (Lehtipalo et al., 14). In order to treat the simulation data similarly to measured particle concentrations, we divided the clusters into linearly-spaced size bins of a width of 0.1 nm based on their mass diameter. Vapor monomers were, however, omitted from the size bins to ensure that they do not dominate the smallest bins. Then, we determined the appearance time t app for each size bin as the time at which the concentration of the bin reaches 0% of the total 9
10 Atmos. Chem. Phys. Discuss., concentration increase in the bin (Lehtipalo et al., 14). Finally, we determined GR for each bin k with the mean diameter d p,k by numerically differentiating the (t app, d p)-data: GR k = d p,k+1 d p,k t app,k+1 t app,k (7) 3. Nano-Köhler calculations To compare the predictions of the nano-köhler theory to the results of the cluster kinetics simulations, we calculated the equilibrium saturation ratio of the organic vapor from Eq. (1) and determined the activation diameter and the corresponding saturation ratio for different seed cluster sizes. In these calculations, we used the same properties of the compounds as in the cluster population simulations (see Sect. 3.2), and varied the seed cluster size between 0.9 and 2.9 nm. 4. Results and discussion The cluster kinetics simulations indicate that nano-köhler-type activation occurs in specific conditions, determined by vapor concentrations and the organic vapor saturation ratio. In this section, we first go through two example cases: one case where nano-köhler-type activation is observed, and another case without activation. Then we discuss more generally the conditions where activation occurs, and compare the activation sizes determined from the simulations to those based on the nano-köhler theory. We also study the connection between the activation sizes and the behavior of apparent cluster GRs. Finally, we discuss 1 the sensitivity of the results to vapor properties and the time-evolution of the vapor concentrations. 4.1 Effects of organic vapor volatility and vapor concentrations on the growth mechanism Simulations with LVOC Figures 2 4 show the results for the simulations where the model compounds were SA-DMA and LVOC, C SA = 6 cm -3 and C ORG = 6 9 cm -3. Figure 2 presents the steady-state cluster distributions, illustrating how the relative contributions of SA and LVOC to the growth of the cluster population depend on their concentrations. When both C ORG and C SA are 6 cm -3, the growth proceeds mainly by additions of SA molecules, while at C ORG = 7 cm -3, both SA and LVOC participate in the growth. At C ORG = 8 cm -3 or higher, the growth is clearly dominated by LVOC. A more detailed picture of the roles of SA and LVOC in the cluster growth is obtained by studying the particle fluxes past selected threshold sizes caused by different vapor monomers or clusters. Figure 3 shows the steady-state flux fractions for five 2 threshold sizes between 1 and 3 nm in different simulations. The flux fractions are presented for SA monomer, SA dimer, pure SA clusters (trimers or larger), LVOC monomer, LVOC dimer, pure LVOC clusters (trimers or larger), and for the clusters containing both SA and LVOC (dimers or larger).
11 Atmos. Chem. Phys. Discuss., When C ORG = 6 cm -3, SA monomer dominates the flux at all sizes, and the rest of the flux is mainly caused by SA dimer and SA clusters. The contribution of LVOC monomer to the flux is minor at all sizes. When C ORG = 7 cm -3, the fractions of the flux due to SA and LVOC exhibit a distinct size-dependency. The contribution of SA monomer to the flux is highest at 1. nm and lowest at 3 nm. LVOC has a significant contribution to the flux at 1 nm, a minor contribution at 1. nm, and a larger, increasing contribution at 2 nm and above. This behavior resembles the nano-köhler type activation where the critical size, corresponding to the peak of the Köhler curve, is around 2 nm; at sizes smaller than that, the growth by organic vapor is minor due to the Kelvin barrier, while at larger sizes, organic vapor can start to spontaneously condense on the clusters. When interpreting the cluster flux results it should be kept in mind, though, only the collisions between vapor monomers and the smallest clusters can contribute to the flux past 1 nm due to their small size. When C ORG = 8 cm -3, LVOC monomer dominates the flux at 1 nm as well as at 2 nm and above. However, at 1. nm, most of the flux is caused by LVOC clusters, and the net flux of LVOC monomer is negative due to its high evaporation flux. Thus, this can also be considered as nano-köhler type situation where the critical size is around 2 nm, and at sizes just below that the growth can occur only by self-coagulation, and not by condensation of vapor monomers. It should be noted, though, that large uncertainties are related to calculating the evaporation rate of LVOC monomers from Kelvin formula (Eq. 3). Therefore, LVOC dimers and other small clusters may become unrealistically stable compared to monomers, and their contribution to the growth may be overestimated. Evaporation rates assessed by more sophisticated methods, namely quantum-mechanics-based approaches, also exhibit very high quantitative uncertainties (see e.g. Elm et al., 17 and references therein). When C ORG = 9 cm -3, LVOC monomer contributes to the flux at all sizes. The fluxes due to LVOC dimer and LVOC clusters are also significant at sizes above 1 nm. Thus, this simulation corresponds to the situation where the Kelvin barrier does not exist at any size, due to high vapor concentration. We also studied GRs determined for different size bins based on their appearance times (Fig. 4). When C ORG = 6 cm -3, GR varies between 0.2 and 0.7 nm/h, being highest at the smallest sizes. When C ORG = 7 cm -3, GR is between 0.3 and 0.9 nm/h, increasing with size in the bins above ~2.6 nm. When C ORG = 8 cm -3, GR varies between 0. and 12 nm/h, and it increases with size above ~1.6 nm. When C ORG = 9 cm -3 (not shown) GR fluctuates strongly, and it obtains either extremely high (~400 nm/h) or negative values. This is due to very rapid appearance of different sized clusters in this simulation, which makes deducing GR from appearance times ambiguous. GR also fluctuates in other simulations at the smallest sizes. This results from the fact the bins at the smallest sizes contain only a few clusters, whereas the bins at the largest sizes contain hundreds of different clusters. In previous experimental studies increase of GR as a function of size has been attributed to the activation of clusters by nano-köhler mechanism (Kulmala et al., 13; Tröstl et al., 16). We study the connection between the sizedependency of GR and nano-köhler activation in Sect
12 Atmos. Chem. Phys. Discuss., Figure 2: Steady-state cluster distributions in simulations with SA DMA and LVOC at CSA = 6 cm -3 and CORG = 6 9 cm -3. The numbers of SA molecules and organic molecules in different clusters are shown on x- and y-axis, and the color indicates the concentration of a cluster. Figure 3: Contribution of different vapor monomers and clusters to the net flux past certain threshold sizes in simulations with SA DMA and LVOC at CSA = 6 cm -3 and CORG = 6 9 cm -3. The values are at steady state. 12
13 Atmos. Chem. Phys. Discuss., Figure 4: Growth rates (GRs) determined for different size bins in simulations with SA DMA and LVOC. The colors indicate vapor concentrations in each simulation Simulations with ELVOC 1 Figures 7 present the results of the simulations where the model compounds were SA DMA and ELVOC and C SA = 6 cm -3 and C ORG = 6 9 cm -3. Thus, the only difference compared to the simulations discussed in the previous section is the lower volatility (p sat) of the organic compound. The steady-state cluster distributions show that the organic compound contributes to the growth of the cluster population clearly more in the simulations with ELVOC than with LVOC (Fig. ). When C SA and C ORG are 6 cm -3, SA and ELVOC contribute to the growth approximately equally. When C ORG = 7 cm -3 or higher, the growth is already dominated by ELVOC. Consistently with the cluster distributions, the fractions of the cluster flux caused by the organic compound are clearly higher in the simulations with SA DMA and ELVOC than in the simulations with LVOC (Fig. 6). When C ORG = 6 cm -3, SA monomer has only a rather small contribution to the fluxes at all sizes. ELVOC monomer dominates the flux at 1 nm and contributes to the fluxes significantly also at larger sizes. The clusters containing both SA and ELVOC molecules also contribute to the fluxes above 1 nm. When C ORG = 7 cm -3, SA monomer has only a negligible contribution to the fluxes at all sizes. ELVOC monomer dominates the flux at 1 nm. At larger sizes, the fluxes are mainly caused by ELVOC monomer, ELVOC dimer, larger ELVOC clusters and clusters containing both SA and ELVOC molecules. In the simulations where C ORG = 8 cm -3 or 9 cm -3, the fluxes at all sizes are due to ELVOC monomer, ELVOC dimer and ELVOC clusters. ELVOC monomer dominates the growth below 2 nm and ELVOC clusters at the larger sizes. Overall, the difference between the cluster flux results in the simulations with ELVOC and LVOC is not only the larger contribution of the organic compound to the fluxes in the simulations with ELVOC, but also the fact that in simulations with 13
14 Atmos. Chem. Phys. Discuss., ELVOC, no nano-köhler type behavior is observed. In these simulations the organic vapor can contribute to the growth at all sizes without an apparent barrier that should be overcome at small sizes. The behavior of GR is also different in simulations with ELVOC compared to simulations with LVOC (Fig. 7). When C ORG = 6 cm -3, GR varies between 0.7 and 2.2 nm/h and reaches its highest values in the smallest size bins. When C ORG = 7 cm -3, GR mostly varies between 6. and 9. nm/h, but in the smallest size bins reaches values as high as 180 nm/h. When C ORG = 8 cm -3, GR varies between 60 and 1 nm/h, without a clear size-dependency. Thus, in the simulations with ELVOC, GR does not increase with the increasing size in the same way as in the simulations with LVOC. This indicates that increasing GR observed in simulations with LVOC may be linked to the nano-köhler type activation. Figure : Steady-state cluster distributions in simulations with SA DMA and ELVOC at CSA = 6 cm -3 and CORG = 6 9 cm -3. The numbers of SA molecules and organic molecules in different clusters are shown on x- and y-axis, and the color indicates the concentration of a cluster. 14
15 Atmos. Chem. Phys. Discuss., Figure 6: Contribution of different vapor monomers and clusters to the net flux past certain threshold sizes in simulations with SA DMA and ELVOC at CSA = 6 cm -3 and CORG = 6 9 cm -3. The values are at steady state. Figure 7: Growth rates (GRs) determined for different size bins in simulations with SA DMA and ELVOC. The colors indicate vapor concentrations in each simulation. 1
16 Atmos. Chem. Phys. Discuss., Overall picture CORG/CSA 4 3 Organic dominates 2 Activation 1 0 Transition -1 SA dominates S ORG 1 2 Figure 8: Growth mechanisms with different organic vapor saturation ratios (SORG) and the ratios of organic vapor concentration (CORG) to sulfuric acid concentration (CSA). In the red area the growth is dominated by sulfuric acid, and in the green area by the organic vapor. In the yellow and gray areas neither of them clearly dominates: in the yellow area the nano-köhler type activation, involving an increasing contribution of the organic vapor with increasing particle size, is observed, and in the gray area there is a transition between these compounds without an activation. In the simulations performed with different concentrations of inorganic and organic vapors, and with a variety of organic saturation vapor pressures, different growth mechanisms can be recognized. In some simulations sulfuric acid dominates the growth of the cluster population at all sizes, whereas in other simulations the organic compound dominates. In some simulations we observe a nano-köhler type activation, where the organic vapor starts to condense on clusters and dominate the growth after a certain size is reached. In other simulations, there is also a transition between the dominance of inorganic and organic vapor but no specific size at which the dominating compound changes. The main variables determining which of the growth mechanism prevails in a simulation are the saturation ratio of the organic compound (S ORG), and the ratio between the concentrations of organic and inorganic vapors (C ORG/C SA). Figure 8 illustrates the dominating growth mechanism at different values of these variables. The nano-köhler activation is observed only in rather specific conditions: when S ORG = ~4 40 and C ORG/C SA = ~ 000. When S ORG and C ORG/C SA are lower than these values, sulfuric acid dominates the growth at all sizes, while at larger values the organic vapor dominates. When C ORG/C SA = 1 and S ORG is ~40 or larger, a transition without a clear activation is observed. It should be noted, though, that the activation may also occur at lower S ORG and C ORG/C SA values at sizes above 3 nm, which are beyond the simulated size range. In addition, it is important to keep in mind that the conditions for different growth mechanisms depend on vapor properties and environmental conditions and thus they cannot be generalized to arbitrary compounds and conditions. In simulations with SA DMA and LVOC discussed in Sect (see Fig. 3), three of the different growth mechanism can be observed: the dominance of sulfuric acid (at C SA = 6 cm -3, C ORG = 6 cm -3 ), the dominance of organic compound (at C SA = 6 cm -3, C ORG = 9 cm -3 ), and activation (at C SA = 6 cm -3, C ORG = 7 8 cm -3 ). Similar results are also obtained in a different simulation set with the same S ORG and C ORG/C SA; this is illustrated in Figure A1 which shows the results of the 16
17 Atmos. Chem. Phys. Discuss., simulations where p sat of organic compound is -7 Pa ( times higher than that of LVOC), C SA is 7 cm -3, and C ORG is varied between 7 and cm -3. In the simulations with SA DMA and ELVOC discussed in Sect (see Fig. 6), the nano-köhler type behavior cannot be seen. At C ORG = 6 cm -3, the growth occurs both by ELVOC and SA DMA without a clear size-dependency, and thus this simulation represents the transition case. With higher C ORG, ELVOC dominates the growth at all sizes. 4.2 Comparison between activation size in simulations and in the nano-köhler theory 1 To study the cluster activation size from the simulations, we determined the contribution of different compounds to cluster flux with a higher size resolution in four simulations where nano-köhler activation is observed (Fig. A2). We determined the size at which at least 0% of the flux is due to organic vapor monomer, which we define as the activation size d sim (Table 4). To compare these results to the predictions of the nano-köhler theory, we calculated the equilibrium saturation ratio of LVOC for different seed cluster sizes from Eq. (1). The resulting Köhler curves are illustrated in Fig. 9 and the critical diameters from the theory (d theory) corresponding to the simulated conditions are shown in Table 4. In addition, to study the connection between accelerating growth and nano-köhler activation, we determined the sizes at which the appearance time GR starts to significantly increase with size in each of these simulations (d GR). This was done by finding the size at which the relative increase of GR between two adjacent size bins is more than %. Note, though, that d GR could not be determined for the simulation with C ORG = 9 cm -3, due to strong fluctuation of GR. Table 4: The activation size (dsim) determined from four simulations, the corresponding critical size from the nano-köhler theory (dtheory), and the size at which GR starts to increase with size in each simulation (dgr). The activation size is here defined as the size when 0% of the cluster flux is due to organic vapor monomer. The concentrations of sulfuric acid (CSA) and organic vapor (CORG), their ratio, organic saturation vapor pressure (psat,org) and organic saturation ratio (SORG) are shown for each case. CSA (cm -3 ) CORG (cm -3 ) CORG/CSA psat,org (Pa) SORG dsim (nm) dtheory (nm) dgr (nm)
18 Atmos. Chem. Phys. Discuss., Figure 9: Köhler curves showing the equilibrium particle size at different saturation ratios for LVOC. The line color shows the diameter of the seed particle (ds). The maxima of the curves correspond to the critical sizes. 1 In the simulations where S ORG = 38, d sim and d theory are rather close to each other: the difference between them is nm depending on vapor concentrations. However, when S ORG = 3.8, d sim is clearly lower (>1 nm) than d theory. This discrepancy is likely related to the fact that the theory does not account for the stochastic collisions between inorganic and organic vapor molecules and clusters, which can result in the growth of the clusters by organic vapor already before the theoretical critical size (see also Sect. 2). Thus, although nano-köhler type behavior occurs in the simulated systems, the theory cannot be used to predict the exact size at which the organic vapor starts to dominate the cluster growth. On the other hand, the difference between d sim and d GR is only nm in different simulations. This suggests that, at least in some conditions, the increase in particle GR may indicate that the organic vapor starts to condense on the clusters around that size. However, as the behavior of GR is also affected by the variation of vapor concentrations and other conditions, this does not necessarily apply in real atmospheric systems (see Sect ). It should finally be noted that it is not entirely obvious how d sim should be defined. In some simulations there is a distinct size after which most of the flux is due to organic vapor monomer, but in some simulations the change is more gradual. In addition, in many simulations organic dimer or larger organic clusters contribute to the growth significantly at sizes where the net flux of the organic vapor monomer is negative. Thus, in these cases the growth is governed by the organic compound already before the activation size, which we determine only based on the contribution of the organic monomer. 18
19 Atmos. Chem. Phys. Discuss., Sensitivity of the results to vapor properties and time-dependency of vapor concentrations Effect of inorganic vapor properties and organic compound mass 1 To study the effect of inorganic vapor properties on the growth of the cluster population, we performed additional simulations where the organic compound was LVOC and the inorganic compound was SA NH 3, which has a non-zero saturation vapor pressure ( -9 Pa) and a slightly smaller mass than SA DMA (see Table 2). In these simulations the contribution of inorganic compound to the cluster fluxes, especially that of vapor monomer, is smaller than in the simulations with SA DMA, due to higher evaporation flux (Fig. A3). The GRs determined for different size bins are generally slightly lower in the simulations with SA NH 3 than with SA DMA (Fig. A4). However, when C SA = 6 cm -3 and C ORG = 6 cm -3, GR at sizes above 2. nm is higher in the simulation with SA NH 3 than with SA-DMA. In this case the GRs determined based on the appearance times seem not to represent the magnitude of collision-evaporation fluxes which are larger in the simulation with SA DMA. We also performed simulations with SA DMA and an organic compound with the same saturation vapor pressure as LVOC ( -8 Pa) but two times larger mass (LVOC large). In the simulations where C SA = 6 cm -3, and C ORG = 7 9 cm -3, the organic compound was found to contribute to the growth clearly less than in the simulations with LVOC (Fig. A). The main reason for the difference is that the evaporation rate is higher for the compound with a higher mass, as the molecular mass is located inside the exponent in the Kelvin formula (Eq. 3). In addition, because the diameter of LVOC large monomer is higher than 1 nm, it cannot contribute to the flux at 1 nm by default. Consistently with the lower contribution of the organic compound to the growth, the apparent cluster GRs are also lower in the simulations with LVOC large than with LVOC (Fig A6). Overall, these results demonstrate that the properties of both inorganic and organic compounds, including saturation vapor pressure but also other properties, can significantly affect the contribution of different compounds to the cluster growth. Therefore, our simulation results regarding, for example, the conditions for different growth mechanisms (Sect ) cannot be generalized for arbitrary compounds Effect of time-dependency of vapor concentrations 2 30 To study the effect of time-dependent vapor concentrations, two additional simulations were performed using SA DMA and LVOC as model compounds. In the first simulation the behavior of both vapor source rates was set to resemble the diurnal cycle of atmospheric sulfuric acid concentration (Petäjä et al., 09). In the second simulation the source rate of SA DMA had a similar behavior but the source rate of LVOC was set constant. The resulting vapor concentrations and the GRs determined for different size bins are shown in Fig.. The time-evolution of the net flux caused by different vapor monomers and clusters past different sizes in these simulations is shown in Fig. A7. Based on the flux results, in both of these simulations the organic vapor starts to significantly contribute to cluster growth around 2 nm, which corresponds to nano-köhler type activation. In the simulation where both C SA and C ORG have maxima, the activation can also be seen as an increase in GR at sizes above 2 nm. However, in the simulation with constant C ORG, GR increases with size almost linearly, without any signs 19
20 Atmos. Chem. Phys. Discuss., of activation. This shows that the size-dependency of GR is affected by dynamic variations in the system, and therefore GR alone cannot be used to deduce the cluster growth mechanism. Figure : The concentrations of SA DMA and LVOC monomers in the simulations where vapor source rates are time-dependent (upper panel), and growth rates (GRs) determined for different size bins in these simulation (lower panel). In the lower panel, the colors show the average of the appearance times of the adjacent size bins used for determining GRs. Conclusions 1 Recent experimental results indicate that organic vapors can participate in the growth of nanometer-sized atmospheric molecular clusters. One of the mechanisms proposed to depict this process is the nano-köhler theory, which describes the activation of inorganic clusters to growth by a soluble organic vapor. However, it is unclear how well the simple theory is able to describe the dynamics of real molecular systems with a distribution of clusters of varying size and composition and timedependent environmental conditions. In this work we studied the capability of the nano-köhler theory to describe the growth of atmospheric clusters by simulating the dynamics of a cluster population in the presence of a sulfuric acid base mixture and an oxidized organic compound. We found that nano-köhler type behavior occurs when the saturation ratio S ORG of the organic vapor and the ratio C ORG/C SA between organic and inorganic vapor concentrations are in the correct range, namely C ORG/C SA = ~ 000 and S ORG = ~4
21 Atmos. Chem. Phys. Discuss., When S ORG and C ORG/C SA are lower than these values, sulfuric acid dominates the growth at the studied sizes, whereas with the larger values the organic compound dominates. The conditions in which we observe nano-köhler type behavior can also occur in the atmosphere: in boreal forests C SA is typically ~ 6 cm -3 during NPF events (Petäjä et al., 09), and C ORG is likely between ~ and 8 cm -3 (Jokinen et al., 17), which would result in S ORG = ~ with p sat,org = -8 Pa and S ORG = ~ with p sat,org = -11. Although nano-köhler type activation occurs in our simulations, the nano-köhler theory is too simple to predict the exact size at which the organic vapor starts to significantly condense on the clusters. This observation demonstrates that the growth of sub-3 nm clusters can be described accurately only if the whole cluster distribution with a variety of different cluster composition and sizes is taken into account. Especially when vapor saturation ratios are high, cluster cluster collisions contribute substantially to the growth in addition to vapor monomers. Finally, we also observed that in the simulations with nano-köhler type behavior, apparent cluster GRs start to increase close to the size at which the organic vapor starts to dominate the growth. However, such behavior of GR may also be due to other dynamic processes, such as varying vapor concentrations, and therefore one should not make conclusions about the cluster growth mechanism solely based on GR. To accurately determine the initial growth mechanisms and the participating compounds would ideally require (1) quantitative measurements of the composition of sub-3 nm clusters, and (2) improved understanding of cluster thermodynamics, including composition and size-dependent evaporation rates. Combined with cluster population modeling, such assessments will be able to provide a more comprehensive understanding of the clustering processes. Code and data availability Model simulations were performed using Atmospheric Cluster Dynamics Code (ACDC). The code and the simulated data are available upon request from the authors. 21
22 Atmos. Chem. Phys. Discuss., Appendix Figure A1: Contribution of different vapor monomers and clusters to the net flux past certain threshold sizes in simulations with SA DMA and an organic compound (ORG) with psat = -7 Pa at CSA = 7 cm -3 and CORG = 7 cm -3. The values are at steady state. 22
23 Atmos. Chem. Phys. Discuss., Figure A2: Contribution of different vapor monomers and clusters to the net flux past different threshold sizes in simulations with SA DMA and an organic compound (ORG). The vapor concentrations and the saturation ratio of organic compound (SORG) are shown above the figures for each simulation. The values are at steady state. Figure A3: Contribution of different vapor monomers and clusters to the net flux past different threshold sizes in simulations with SA NH3 and LVOC at CSA = 6 cm -3 and CORG = 6 9 cm -3. The values are at steady state. 23
Growth of atmospheric clusters involving cluster cluster collisions: comparison of different growth rate methods
 doi:10.5194/acp-16-5545-2016 Author(s) 2016. CC Attribution 3.0 License. Growth of atmospheric clusters involving cluster cluster collisions: comparison of different growth rate methods Jenni Kontkanen
doi:10.5194/acp-16-5545-2016 Author(s) 2016. CC Attribution 3.0 License. Growth of atmospheric clusters involving cluster cluster collisions: comparison of different growth rate methods Jenni Kontkanen
Aerosol Dynamics. Antti Lauri NetFAM Summer School Zelenogorsk, 9 July 2008
 Aerosol Dynamics Antti Lauri NetFAM Summer School Zelenogorsk, 9 July 2008 Department of Physics, Division of Atmospheric Sciences and Geophysics, University of Helsinki Aerosol Dynamics: What? A way to
Aerosol Dynamics Antti Lauri NetFAM Summer School Zelenogorsk, 9 July 2008 Department of Physics, Division of Atmospheric Sciences and Geophysics, University of Helsinki Aerosol Dynamics: What? A way to
Supplement of Total sulfate vs. sulfuric acid monomer concenterations in nucleation studies
 Supplement of Atmos. Chem. Phys., 15, 3429 3443, 2015 http://www.atmos-chem-phys.net/15/3429/2015/ doi:10.5194/acp-15-3429-2015-supplement Author(s) 2015. CC Attribution 3.0 License. Supplement of Total
Supplement of Atmos. Chem. Phys., 15, 3429 3443, 2015 http://www.atmos-chem-phys.net/15/3429/2015/ doi:10.5194/acp-15-3429-2015-supplement Author(s) 2015. CC Attribution 3.0 License. Supplement of Total
Step 1. Step 2. g l = g v. dg = 0 We have shown that over a plane surface of water. g v g l = ρ v R v T ln e/e sat. this can be rewritten
 The basic question is what makes the existence of a droplet thermodynamically preferable to the existence only of water vapor. We have already derived an expression for the saturation vapor pressure over
The basic question is what makes the existence of a droplet thermodynamically preferable to the existence only of water vapor. We have already derived an expression for the saturation vapor pressure over
Overview of the Particle Size Magnifier (PSM)
 Overview of the Particle Size Magnifier (PSM) Joonas Vanhanen CTO, Airmodus Ltd. joonas.vanhanen@airmodus.com Airmodus Ltd. It s the small things that count Founded in 2010 A spin-off from the University
Overview of the Particle Size Magnifier (PSM) Joonas Vanhanen CTO, Airmodus Ltd. joonas.vanhanen@airmodus.com Airmodus Ltd. It s the small things that count Founded in 2010 A spin-off from the University
Slides partly by Antti Lauri and Hannele Korhonen. Liquid or solid particles suspended in a carrier gas Described by their
 Atmospheric Aerosols Slides partly by Antti Lauri and Hannele Korhonen Aerosol particles Liquid or solid particles suspended in a carrier gas Described by their Size Concentration - Number - Surface -
Atmospheric Aerosols Slides partly by Antti Lauri and Hannele Korhonen Aerosol particles Liquid or solid particles suspended in a carrier gas Described by their Size Concentration - Number - Surface -
APPLICATION OF KOHLER THEORY: MODELING CLOUD CONDENSATION NUCLEI ACTIVITY
 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 APPLICATION OF KOHLER THEORY: MODELING CLOUD CONDENSATION NUCLEI ACTIVITY Gavin Cornwell, Katherine Nadler, Alex Nguyen, and Steven Schill Department of
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 APPLICATION OF KOHLER THEORY: MODELING CLOUD CONDENSATION NUCLEI ACTIVITY Gavin Cornwell, Katherine Nadler, Alex Nguyen, and Steven Schill Department of
Köhler Curve. Covers Reading Material in Chapter 10.3 Atmospheric Sciences 5200 Physical Meteorology III: Cloud Physics
 Köhler Curve Covers Reading Material in Chapter 10.3 Atmospheric Sciences 5200 Physical Meteorology III: Cloud Physics Review of Kelvin Effect Gibbs Energy for formation of a drop G = G &'()*+, G ).'+
Köhler Curve Covers Reading Material in Chapter 10.3 Atmospheric Sciences 5200 Physical Meteorology III: Cloud Physics Review of Kelvin Effect Gibbs Energy for formation of a drop G = G &'()*+, G ).'+
Modelling the formation of organic particles in the atmosphere
 Atmos. Chem. Phys., 4, 1071 1083, 2004 SRef-ID: 1680-7324/acp/2004-4-1071 Atmospheric Chemistry and Physics Modelling the formation of organic particles in the atmosphere T. Anttila 1,2, V.-M. Kerminen
Atmos. Chem. Phys., 4, 1071 1083, 2004 SRef-ID: 1680-7324/acp/2004-4-1071 Atmospheric Chemistry and Physics Modelling the formation of organic particles in the atmosphere T. Anttila 1,2, V.-M. Kerminen
Clouds associated with cold and warm fronts. Whiteman (2000)
 Clouds associated with cold and warm fronts Whiteman (2000) Dalton s law of partial pressures! The total pressure exerted by a mixture of gases equals the sum of the partial pressure of the gases! Partial
Clouds associated with cold and warm fronts Whiteman (2000) Dalton s law of partial pressures! The total pressure exerted by a mixture of gases equals the sum of the partial pressure of the gases! Partial
ON THE GROWTH OF ATMOSPHERIC NANOPARTICLES BY ORGANIC VAPORS
 UNIVERSITY OF HELSINKI REPORT SERIES IN PHYSICS HU-P-D208 ON THE GROWTH OF ATMOSPHERIC NANOPARTICLES BY ORGANIC VAPORS TAINA YLI-JUUTI Division of Atmospheric Sciences Department of Physics Faculty of
UNIVERSITY OF HELSINKI REPORT SERIES IN PHYSICS HU-P-D208 ON THE GROWTH OF ATMOSPHERIC NANOPARTICLES BY ORGANIC VAPORS TAINA YLI-JUUTI Division of Atmospheric Sciences Department of Physics Faculty of
Chapter 9 Generation of (Nano)Particles by Growth
 Chapter 9 Generation of (Nano)Particles by Growth 9.1 Nucleation (1) Supersaturation Thermodynamics assumes a phase change takes place when there reaches Saturation of vapor in a gas, Saturation of solute
Chapter 9 Generation of (Nano)Particles by Growth 9.1 Nucleation (1) Supersaturation Thermodynamics assumes a phase change takes place when there reaches Saturation of vapor in a gas, Saturation of solute
Hydration of Atmospherically Relevant Molecular Clusters: Computational Chemistry and Classical Thermodynamics
 pubs.acs.org/jpca Hydration of Atmospherically Relevant Molecular Clusters: Computational Chemistry and Classical Thermodynamics Henning Henschel,, * Juan C. Acosta Navarro, Taina Yli-Juuti, Oona Kupiainen-Maäẗta,
pubs.acs.org/jpca Hydration of Atmospherically Relevant Molecular Clusters: Computational Chemistry and Classical Thermodynamics Henning Henschel,, * Juan C. Acosta Navarro, Taina Yli-Juuti, Oona Kupiainen-Maäẗta,
Aerosol Basics: Definitions, size distributions, structure
 Aerosol Basics: Definitions, size distributions, structure Antti Lauri NetFAM Summer School Zelenogorsk, 9 July 2008 Department of Physics, Division of Atmospheric Sciences and Geophysics, University of
Aerosol Basics: Definitions, size distributions, structure Antti Lauri NetFAM Summer School Zelenogorsk, 9 July 2008 Department of Physics, Division of Atmospheric Sciences and Geophysics, University of
Parameterization of the nitric acid effect on CCN activation
 Atmos. Chem. Phys., 5, 879 885, 25 SRef-ID: 168-7324/acp/25-5-879 European Geosciences Union Atmospheric Chemistry and Physics Parameterization of the nitric acid effect on CCN activation S. Romakkaniemi,
Atmos. Chem. Phys., 5, 879 885, 25 SRef-ID: 168-7324/acp/25-5-879 European Geosciences Union Atmospheric Chemistry and Physics Parameterization of the nitric acid effect on CCN activation S. Romakkaniemi,
Updated H 2 SO 4 -H 2 O binary homogeneous nucleation look-up tables
 Click Here for Full Article JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 113,, doi:10.1029/2008jd010527, 2008 Updated H 2 SO 4 -H 2 O binary homogeneous nucleation look-up tables Fangqun Yu 1 Received 2 June
Click Here for Full Article JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 113,, doi:10.1029/2008jd010527, 2008 Updated H 2 SO 4 -H 2 O binary homogeneous nucleation look-up tables Fangqun Yu 1 Received 2 June
Forma&on and contribu&on of highly oxygenated organic molecules (HOM) to the growth of new par&cles over the boreal forest
 Forma&on and contribu&on of highly oxygenated organic molecules (HOM) to the growth of new par&cles over the boreal forest Pontus Roldin Nuclear Physics Lund University, Lund Sweden 2017-11-23 Model &
Forma&on and contribu&on of highly oxygenated organic molecules (HOM) to the growth of new par&cles over the boreal forest Pontus Roldin Nuclear Physics Lund University, Lund Sweden 2017-11-23 Model &
Chemistry of stabilized Criegee intermediates in the CLOUD chamber
 Chemistry of stabilized Criegee intermediates in the CLOUD chamber Nina Sarnela, Mikko Sipilä, Tuija Jokinen, Heikki Junninen, and CLOUD Collaboration Citation: AIP Conference Proceedings 1527, 381 (2013);
Chemistry of stabilized Criegee intermediates in the CLOUD chamber Nina Sarnela, Mikko Sipilä, Tuija Jokinen, Heikki Junninen, and CLOUD Collaboration Citation: AIP Conference Proceedings 1527, 381 (2013);
Quasi-unary homogeneous nucleation of H 2 SO 4 -H 2 O
 THE JOURNAL OF CHEMICAL PHYSICS 122, 074501 2005 Quasi-unary homogeneous nucleation of H 2 SO 4 -H 2 O Fangqun Yu a Atmospheric Sciences Research Center, State University of New York at Albany, 251 Fuller
THE JOURNAL OF CHEMICAL PHYSICS 122, 074501 2005 Quasi-unary homogeneous nucleation of H 2 SO 4 -H 2 O Fangqun Yu a Atmospheric Sciences Research Center, State University of New York at Albany, 251 Fuller
Modeling of cloud microphysics: from simple concepts to sophisticated parameterizations. Part I: warm-rain microphysics
 Modeling of cloud microphysics: from simple concepts to sophisticated parameterizations. Part I: warm-rain microphysics Wojciech Grabowski National Center for Atmospheric Research, Boulder, Colorado parameterization
Modeling of cloud microphysics: from simple concepts to sophisticated parameterizations. Part I: warm-rain microphysics Wojciech Grabowski National Center for Atmospheric Research, Boulder, Colorado parameterization
Gas Particle Partitioning to OA
 Gas Particle Partitioning to OA Advanced Atmospheric chemistry CHEM 5152 Prof. J.L. Jimenez Last updated: Spring 2017 1 Gas Phase vs Aerosol Compounds For monofunctional compounds, alkanes beyond C 20
Gas Particle Partitioning to OA Advanced Atmospheric chemistry CHEM 5152 Prof. J.L. Jimenez Last updated: Spring 2017 1 Gas Phase vs Aerosol Compounds For monofunctional compounds, alkanes beyond C 20
On the roles of sulphuric acid and low-volatility organic vapours in the initial steps of atmospheric new particle formation
 Atmos. Chem. Phys., 10, 11223 11242, 2010 doi:10.5194/acp-10-11223-2010 Author(s) 2010. CC Attribution 3.0 License. Atmospheric Chemistry and Physics On the roles of sulphuric acid and low-volatility organic
Atmos. Chem. Phys., 10, 11223 11242, 2010 doi:10.5194/acp-10-11223-2010 Author(s) 2010. CC Attribution 3.0 License. Atmospheric Chemistry and Physics On the roles of sulphuric acid and low-volatility organic
Charging state of the atmospheric nucleation mode: Implications for separating neutral and ion-induced nucleation
 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 112,, doi:10.1029/2007jd008649, 2007 Charging state of the atmospheric nucleation mode: Implications for separating neutral and ion-induced nucleation Veli-Matti Kerminen,
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 112,, doi:10.1029/2007jd008649, 2007 Charging state of the atmospheric nucleation mode: Implications for separating neutral and ion-induced nucleation Veli-Matti Kerminen,
Study of the Mechanism of Nucleation in the Polluted Atmospheric Boundary Layer
 Study of the Mechanism of Nucleation in the Polluted Atmospheric Boundary Layer A DISSERTATION SUBMITTED TO THE FACULTY OF UNIVERSITY OF MINNESOTA BY Modi Chen IN PARTIAL FULFILLMENT OF THE REQUIREMENTS
Study of the Mechanism of Nucleation in the Polluted Atmospheric Boundary Layer A DISSERTATION SUBMITTED TO THE FACULTY OF UNIVERSITY OF MINNESOTA BY Modi Chen IN PARTIAL FULFILLMENT OF THE REQUIREMENTS
Chemical Potential. Combining the First and Second Laws for a closed system, Considering (extensive properties)
 Chemical Potential Combining the First and Second Laws for a closed system, Considering (extensive properties) du = TdS pdv Hence For an open system, that is, one that can gain or lose mass, U will also
Chemical Potential Combining the First and Second Laws for a closed system, Considering (extensive properties) du = TdS pdv Hence For an open system, that is, one that can gain or lose mass, U will also
Comprehensive analysis of particle growth rates from nucleation mode to cloud condensation nuclei in Boreal forest
 Atmos. Chem. Phys. Discuss., https://doi.org/.194/acp-18-169 Comprehensive analysis of particle growth rates from nucleation mode to cloud condensation nuclei in Boreal forest Pauli Paasonen 1, Maija Peltola
Atmos. Chem. Phys. Discuss., https://doi.org/.194/acp-18-169 Comprehensive analysis of particle growth rates from nucleation mode to cloud condensation nuclei in Boreal forest Pauli Paasonen 1, Maija Peltola
Atmospheric sulphuric acid and aerosol formation: implications from atmospheric measurements for nucleation and early growth mechanisms
 Atmospheric sulphuric acid and aerosol formation: implications from atmospheric measurements for nucleation and early growth mechanisms S.-L. Sihto, M. Kulmala, V.-M. Kerminen, M. Dal Maso, T. Petäjä,
Atmospheric sulphuric acid and aerosol formation: implications from atmospheric measurements for nucleation and early growth mechanisms S.-L. Sihto, M. Kulmala, V.-M. Kerminen, M. Dal Maso, T. Petäjä,
Université Lille 1, Villeneuve d Ascq, France Accepted author version posted online: 13 Mar 2014.
 This article was downloaded by: [213.135.234.6] On: 07 September 2015, At: 04:18 Publisher: Taylor & Francis Informa Ltd Registered in England and Wales Registered Number: 1072954 Registered office: 5
This article was downloaded by: [213.135.234.6] On: 07 September 2015, At: 04:18 Publisher: Taylor & Francis Informa Ltd Registered in England and Wales Registered Number: 1072954 Registered office: 5
Effect of ammonium bisulphate formation on atmospheric water-sulphuric acid-ammonia nucleation
 BOREAL ENVIRONMENT RESEARCH 10: 511 523 ISSN 1239-6095 Helsinki 14 December 2005 2005 Effect of ammonium bisulphate formation on atmospheric water-sulphuric acid-ammonia nucleation Tatu Anttila 1)2), Hanna
BOREAL ENVIRONMENT RESEARCH 10: 511 523 ISSN 1239-6095 Helsinki 14 December 2005 2005 Effect of ammonium bisulphate formation on atmospheric water-sulphuric acid-ammonia nucleation Tatu Anttila 1)2), Hanna
Charged and total particle formation and growth rates during EUCAARI 2007 campaign in Hyytiälä
 Atmos. Chem. Phys., 9, 4077 4089, 2009 Author(s) 2009. This work is distributed under the Creative Commons Attribution 3.0 License. Atmospheric Chemistry and Physics Charged and total particle formation
Atmos. Chem. Phys., 9, 4077 4089, 2009 Author(s) 2009. This work is distributed under the Creative Commons Attribution 3.0 License. Atmospheric Chemistry and Physics Charged and total particle formation
The size-dependent charge fraction of sub-3-nm particles as a key diagnostic of competitive nucleation mechanisms under atmospheric conditions
 The size-dependent charge fraction of sub-3-nm particles as a key diagnostic of competitive nucleation mechanisms under atmospheric conditions Fangqun Yu 1 and Richard Turco 2 1 Atmospheric Sciences Research
The size-dependent charge fraction of sub-3-nm particles as a key diagnostic of competitive nucleation mechanisms under atmospheric conditions Fangqun Yu 1 and Richard Turco 2 1 Atmospheric Sciences Research
A cloud microphysics model including trace gas condensation and sulfate chemistry
 BOREAL ENVIRONMENT RESEARCH 8: 43 424 ISSN 239-695 Helsinki December 23 23 A cloud microphysics model including trace gas condensation and sulfate chemistry Harri Kokkola ), Sami Romakkaniemi ), Markku
BOREAL ENVIRONMENT RESEARCH 8: 43 424 ISSN 239-695 Helsinki December 23 23 A cloud microphysics model including trace gas condensation and sulfate chemistry Harri Kokkola ), Sami Romakkaniemi ), Markku
Chapter 17. Fundamentals of Atmospheric Modeling
 Overhead Slides for Chapter 17 of Fundamentals of Atmospheric Modeling by Mark Z. Jacobson Department of Civil & Environmental Engineering Stanford University Stanford, CA 94305-4020 August 21, 1998 Mass
Overhead Slides for Chapter 17 of Fundamentals of Atmospheric Modeling by Mark Z. Jacobson Department of Civil & Environmental Engineering Stanford University Stanford, CA 94305-4020 August 21, 1998 Mass
Part I.
 Part I bblee@unimp . Introduction to Mass Transfer and Diffusion 2. Molecular Diffusion in Gasses 3. Molecular Diffusion in Liquids Part I 4. Molecular Diffusion in Biological Solutions and Gels 5. Molecular
Part I bblee@unimp . Introduction to Mass Transfer and Diffusion 2. Molecular Diffusion in Gasses 3. Molecular Diffusion in Liquids Part I 4. Molecular Diffusion in Biological Solutions and Gels 5. Molecular
Organic aerosol formation via sulphate cluster activation
 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 109,, doi:10.1029/2003jd003961, 2004 Organic aerosol formation via sulphate cluster activation Markku Kulmala, 1 Veli-Matti Kerminen, 2 Tatu Anttila, 1,2 Ari Laaksonen,
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 109,, doi:10.1029/2003jd003961, 2004 Organic aerosol formation via sulphate cluster activation Markku Kulmala, 1 Veli-Matti Kerminen, 2 Tatu Anttila, 1,2 Ari Laaksonen,
ANALYSES ON THE FORMATION OF ATMOSPHERIC PARTICLES AND STABILIZED SULPHURIC ACID CLUSTERS PAULI PAASONEN
 REPORT SERIES IN AEROSOL SCIENCE N:o 132 (2012) ANALYSES ON THE FORMATION OF ATMOSPHERIC PARTICLES AND STABILIZED SULPHURIC ACID CLUSTERS PAULI PAASONEN Division of Atmospheric Sciences Department of Physics
REPORT SERIES IN AEROSOL SCIENCE N:o 132 (2012) ANALYSES ON THE FORMATION OF ATMOSPHERIC PARTICLES AND STABILIZED SULPHURIC ACID CLUSTERS PAULI PAASONEN Division of Atmospheric Sciences Department of Physics
Precipitation Processes METR σ is the surface tension, ρ l is the water density, R v is the Gas constant for water vapor, T is the air
 Precipitation Processes METR 2011 Introduction In order to grow things on earth, they need water. The way that the earth naturally irrigates is through snowfall and rainfall. Therefore, it is important
Precipitation Processes METR 2011 Introduction In order to grow things on earth, they need water. The way that the earth naturally irrigates is through snowfall and rainfall. Therefore, it is important
PROBLEM 14.6 ( )( ) (b) Applying a species balance to a control volume about the hydrogen, dt 6 dt 6RAT dt 6RT dt
 PROBLEM 14.6 KNOWN: Pressure and temperature of hydrogen stored in a spherical steel tank of prescribed diameter and thickness. FIND: (a) Initial rate of hydrogen mass loss from the tank, (b) Initial rate
PROBLEM 14.6 KNOWN: Pressure and temperature of hydrogen stored in a spherical steel tank of prescribed diameter and thickness. FIND: (a) Initial rate of hydrogen mass loss from the tank, (b) Initial rate
The Sensitivity of Global Nucleation, CCN and Climate to SO2 and Criegee-Intermediate Chemistry
 The Sensitivity of Global Nucleation, CCN and Climate to SO2 and Criegee-Intermediate Chemistry Jeff Pierce, Mat Evans, Cat Scott, Steve D'Andrea, Delphine Farmer, Erik Swietlicki and Dom Spracklen Pierce,
The Sensitivity of Global Nucleation, CCN and Climate to SO2 and Criegee-Intermediate Chemistry Jeff Pierce, Mat Evans, Cat Scott, Steve D'Andrea, Delphine Farmer, Erik Swietlicki and Dom Spracklen Pierce,
AT 620 Notes. These notes were prepared by Prof. Steven A. Rutledge. (and adapted slightly for the Fall 2009 course, and again slightly for this year)
 AT 620 Notes These notes were prepared by Prof. Steven A. Rutledge (and adapted slightly for the Fall 2009 course, and again slightly for this year) You may access Prof. Cotton s notes, password cloud9
AT 620 Notes These notes were prepared by Prof. Steven A. Rutledge (and adapted slightly for the Fall 2009 course, and again slightly for this year) You may access Prof. Cotton s notes, password cloud9
Generating cloud drops from CCN. Wallace & Hobbs (1977)
 Generating cloud drops from CCN Wallace & Hobbs (1977) Cloud Drops and Equilibrium Considera3ons: review We discussed how to compute the equilibrium vapor pressure over a pure water drop, or a solu3on
Generating cloud drops from CCN Wallace & Hobbs (1977) Cloud Drops and Equilibrium Considera3ons: review We discussed how to compute the equilibrium vapor pressure over a pure water drop, or a solu3on
Lecture 7: quick review
 Lecture 7: quick review We discussed the meaning of the critical r* and ΔG*: since it s unstable, if we can form a drop with r > r*, the system will keep going falling down the energy curve, trying to
Lecture 7: quick review We discussed the meaning of the critical r* and ΔG*: since it s unstable, if we can form a drop with r > r*, the system will keep going falling down the energy curve, trying to
( $0/(TL4AE(T-.%?1$(A07*2"2-(.N(L-+30.,.?&E(
 !"##$%&'(()$%*+,-()%./"+*.0(10(23-(4-#.2-(5$%10-( 62#.783-%-'((9,."/(:";,.B(9,$%C- D E(FB(GB(H$%0-% D E(IB(@17-,- J E(4B(GB(5$",/10 K E(>B(L$00-% K E($0/(5B(G12+3& M
!"##$%&'(()$%*+,-()%./"+*.0(10(23-(4-#.2-(5$%10-( 62#.783-%-'((9,."/(:";,.B(9,$%C- D E(FB(GB(H$%0-% D E(IB(@17-,- J E(4B(GB(5$",/10 K E(>B(L$00-% K E($0/(5B(G12+3& M
Estimation of Saturated Vapor Pressure from Nucleation Data
 CHINESE JOURNAL OF CHEMICAL PHYSICS VOLUME 19, NUMBER 5 OCTOBER 27, 2006 Estimation of Saturated Vapor Pressure from Nucleation Data Mei-li Wang a, Ji-ming Yang a, Xi-sheng Luo b a. Department of Modern
CHINESE JOURNAL OF CHEMICAL PHYSICS VOLUME 19, NUMBER 5 OCTOBER 27, 2006 Estimation of Saturated Vapor Pressure from Nucleation Data Mei-li Wang a, Ji-ming Yang a, Xi-sheng Luo b a. Department of Modern
Equilibration timescale of atmospheric secondary organic aerosol partitioning
 GEOPHYSICAL RESEARCH LETTERS, VOL. 39,, doi:10.1029/2012gl054008, 2012 Equilibration timescale of atmospheric secondary organic aerosol partitioning Manabu Shiraiwa 1 and John H. Seinfeld 1 Received 25
GEOPHYSICAL RESEARCH LETTERS, VOL. 39,, doi:10.1029/2012gl054008, 2012 Equilibration timescale of atmospheric secondary organic aerosol partitioning Manabu Shiraiwa 1 and John H. Seinfeld 1 Received 25
STUDY OF AIR IONS AND NANOMETER PARTICLES IN THE ATMOSPHERE DURING NUCLEATION BURST EVENTS
 STUDY OF AIR IONS AND NANOMETER PARTICLES IN THE ATMOSPHERE DURING NUCLEATION BURST EVENTS U. HÕRRAK 1,2, H. TAMMET 1, P.P. AALTO 2, L. LAAKSO 2 and M. KULMALA 2 1 Institute of Environmental Physics, University
STUDY OF AIR IONS AND NANOMETER PARTICLES IN THE ATMOSPHERE DURING NUCLEATION BURST EVENTS U. HÕRRAK 1,2, H. TAMMET 1, P.P. AALTO 2, L. LAAKSO 2 and M. KULMALA 2 1 Institute of Environmental Physics, University
Pure Substance Properties and Equation of State
 Pure Substance Properties and Equation of State Pure Substance Content Pure Substance A substance that has a fixed chemical composition throughout is called a pure substance. Water, nitrogen, helium, and
Pure Substance Properties and Equation of State Pure Substance Content Pure Substance A substance that has a fixed chemical composition throughout is called a pure substance. Water, nitrogen, helium, and
Effects of Galactic Cosmic Rays on the Atmosphere and Climate. Jón Egill Kristjánsson, Univ. Oslo
 Effects of Galactic Cosmic Rays on the Atmosphere and Climate Jón Egill Kristjánsson, Univ. Oslo Overview of talk Hypotheses for Coupling between Galactic Cosmic Rays and Climate Observational studies
Effects of Galactic Cosmic Rays on the Atmosphere and Climate Jón Egill Kristjánsson, Univ. Oslo Overview of talk Hypotheses for Coupling between Galactic Cosmic Rays and Climate Observational studies
1.3 Molecular Level Presentation
 1.3.1 Introduction A molecule is the smallest chemical unit of a substance that is capable of stable, independent existence. Not all substances are composed of molecules. Some substances are composed of
1.3.1 Introduction A molecule is the smallest chemical unit of a substance that is capable of stable, independent existence. Not all substances are composed of molecules. Some substances are composed of
Worksheet 1.1. Chapter 1: Quantitative chemistry glossary
 Worksheet 1.1 Chapter 1: Quantitative chemistry glossary Amount The number of moles of a substance present in a sample. Aqueous solution A solution with water as the solvent. Atmosphere The unit atmosphere
Worksheet 1.1 Chapter 1: Quantitative chemistry glossary Amount The number of moles of a substance present in a sample. Aqueous solution A solution with water as the solvent. Atmosphere The unit atmosphere
Precipitation. Size! Shape! Size distribution! Agglomeration!
 Precipitation Size! Shape! Size distribution! Agglomeration! Precipitation Four major questions: 1. Why do molecules/ions precipitate? 2. What determines the size? 3. What determines the size distribution?
Precipitation Size! Shape! Size distribution! Agglomeration! Precipitation Four major questions: 1. Why do molecules/ions precipitate? 2. What determines the size? 3. What determines the size distribution?
ABSTRACT INTRODUCTION
 MODELING OF ALUMINUM NANOPARTICLE FORMATION R. Schefflan D. Kalyon S. Kovenklioglu Stevens Institute of Technology Highly Filled Materials Institute Castle Point Station Hoboken, N.J. 73 ABSTRACT Picatinny
MODELING OF ALUMINUM NANOPARTICLE FORMATION R. Schefflan D. Kalyon S. Kovenklioglu Stevens Institute of Technology Highly Filled Materials Institute Castle Point Station Hoboken, N.J. 73 ABSTRACT Picatinny
Comparative study on methodology in molecular dynamics simulation of nucleation
 THE JOURNAL OF CHEMICAL PHYSICS 126, 224517 2007 Comparative study on methodology in molecular dynamics simulation of nucleation Jan Julin, Ismo Napari, and Hanna Vehkamäki Department of Physical Sciences,
THE JOURNAL OF CHEMICAL PHYSICS 126, 224517 2007 Comparative study on methodology in molecular dynamics simulation of nucleation Jan Julin, Ismo Napari, and Hanna Vehkamäki Department of Physical Sciences,
CHAPTER 8. AEROSOLS 8.1 SOURCES AND SINKS OF AEROSOLS
 1 CHAPTER 8 AEROSOLS Aerosols in the atmosphere have several important environmental effects They are a respiratory health hazard at the high concentrations found in urban environments They scatter and
1 CHAPTER 8 AEROSOLS Aerosols in the atmosphere have several important environmental effects They are a respiratory health hazard at the high concentrations found in urban environments They scatter and
Simulating aerosol nucleation bursts in a coniferous forest
 Boreal Environment Research 12: 421 430 issn 1239-6095 Helsinki 25 June 2007 2007 Simulating aerosol nucleation bursts in a coniferous forest Hannes Tammet 1) and Markku Kulmala 2) 1) Institute of Environmental
Boreal Environment Research 12: 421 430 issn 1239-6095 Helsinki 25 June 2007 2007 Simulating aerosol nucleation bursts in a coniferous forest Hannes Tammet 1) and Markku Kulmala 2) 1) Institute of Environmental
Using COSMOTherm to evaluate properties of atmospheric autoxidation products
 Using COSMOTherm to evaluate properties of atmospheric autoxidation products 7 th March 2018 Theo Kurtén Department of Chemistry University of Helsinki, Finland 1 Co-workers and organizations COSMOtherm
Using COSMOTherm to evaluate properties of atmospheric autoxidation products 7 th March 2018 Theo Kurtén Department of Chemistry University of Helsinki, Finland 1 Co-workers and organizations COSMOtherm
9 Condensation. Learning Goals. After studying this chapter, students should be able to:
 9 Condensation Learning Goals After studying this chapter, students should be able to: 1. explain the microphysical processes that operate in clouds to influence the formation and growth of cloud droplets
9 Condensation Learning Goals After studying this chapter, students should be able to: 1. explain the microphysical processes that operate in clouds to influence the formation and growth of cloud droplets
ATOC 3500/CHEM 3152 Week 9, March 8, 2016
 ATOC 3500/CHEM 3152 Week 9, March 8, 2016 Hand back Midterm Exams (average = 84) Interaction of atmospheric constituents with light Haze and Visibility Aerosol formation processes (more detail) Haze and
ATOC 3500/CHEM 3152 Week 9, March 8, 2016 Hand back Midterm Exams (average = 84) Interaction of atmospheric constituents with light Haze and Visibility Aerosol formation processes (more detail) Haze and
CCN activation experiments with adipic acid: effect of particle phase and adipic acid coatings on soluble and insoluble particles
 Atmos. Chem. Phys., 8, 3735 3748, 2008 Author(s) 2008. This work is distributed under the Creative Commons Attribution 3.0 License. Atmospheric Chemistry and Physics CCN activation experiments with adipic
Atmos. Chem. Phys., 8, 3735 3748, 2008 Author(s) 2008. This work is distributed under the Creative Commons Attribution 3.0 License. Atmospheric Chemistry and Physics CCN activation experiments with adipic
Chemistry Review Unit 5 Physical Behavior of Matter
 Chemistry Review Phases of Matter, Changes of Phase, Substances, Mixtures, Solutions, Effect of Solute on Solution, Energy, Kinetics of Solids, Liquids and Gases Matter, Phases and Gas Laws 1. Matter is
Chemistry Review Phases of Matter, Changes of Phase, Substances, Mixtures, Solutions, Effect of Solute on Solution, Energy, Kinetics of Solids, Liquids and Gases Matter, Phases and Gas Laws 1. Matter is
Implementation of a discrete nodal model to probe the effect of size-dependent surface tension on nanoparticle formation and growth
 Aerosol Science 37 (2006) 1388 1399 www.elsevier.com/locate/jaerosci Implementation of a discrete nodal model to probe the effect of size-dependent surface tension on nanoparticle formation and growth
Aerosol Science 37 (2006) 1388 1399 www.elsevier.com/locate/jaerosci Implementation of a discrete nodal model to probe the effect of size-dependent surface tension on nanoparticle formation and growth
Lee Mauldin Airmodus University of Helsinki University of Colorado
 Nitrate Ion Chemical Ionization Lee Mauldin Airmodus University of Helsinki University of Colorado Co axial Nitrate Ion Chemical Ionization Used extensively for OH, H 2 SO 4, and MSA measurements Ground
Nitrate Ion Chemical Ionization Lee Mauldin Airmodus University of Helsinki University of Colorado Co axial Nitrate Ion Chemical Ionization Used extensively for OH, H 2 SO 4, and MSA measurements Ground
Precipitation Formation, and RADAR Equation by Dario B. Giaiotti and Fulvio Stel (1)
 PhD Environmental Fluid Mechanics Physics of the Atmosphere University of Trieste International Center for Theoretical Physics Precipitation Formation, and RADAR Equation by Dario B. Giaiotti and Fulvio
PhD Environmental Fluid Mechanics Physics of the Atmosphere University of Trieste International Center for Theoretical Physics Precipitation Formation, and RADAR Equation by Dario B. Giaiotti and Fulvio
Aerosols-Cloud-Precipitation Interactions in the Climate System. Meinrat O. Andreae Max Planck Institute for Chemistry Mainz, Germany
 Aerosols-Cloud-Precipitation Interactions in the Climate System Meinrat O. Andreae Max Planck Institute for Chemistry Mainz, Germany The global annual mean Earth s energy budget for 2000-2004 (W m 2 ).
Aerosols-Cloud-Precipitation Interactions in the Climate System Meinrat O. Andreae Max Planck Institute for Chemistry Mainz, Germany The global annual mean Earth s energy budget for 2000-2004 (W m 2 ).
Multicomponent aerosol dynamics model UHMA: model development and validation
 Atmos. Chem. Phys., 4, 757 771, 2004 SRef-ID: 1680-7324/acp/2004-4-757 Atmospheric Chemistry and Physics Multicomponent aerosol dynamics model UHMA: model development and validation H. Korhonen, K. E.
Atmos. Chem. Phys., 4, 757 771, 2004 SRef-ID: 1680-7324/acp/2004-4-757 Atmospheric Chemistry and Physics Multicomponent aerosol dynamics model UHMA: model development and validation H. Korhonen, K. E.
Response: Changes in the manuscript: Response
 We would like to thank the referee for providing valuable comments on our manuscript and we have carefully addressed the referee s comments point-by-point as follows (referee s comments in black and our
We would like to thank the referee for providing valuable comments on our manuscript and we have carefully addressed the referee s comments point-by-point as follows (referee s comments in black and our
Role of molecular size in cloud droplet activation
 GEOPHYSICAL RESEARCH LETTERS, VOL. 36, L22801, doi:10.1029/2009gl040131, 2009 Role of molecular size in cloud droplet activation M. D. Petters, 1,2 S. M. Kreidenweis, 1 A. J. Prenni, 1 R. C. Sullivan,
GEOPHYSICAL RESEARCH LETTERS, VOL. 36, L22801, doi:10.1029/2009gl040131, 2009 Role of molecular size in cloud droplet activation M. D. Petters, 1,2 S. M. Kreidenweis, 1 A. J. Prenni, 1 R. C. Sullivan,
where the terms are substituted according to Table 3 for the various conserved quantities.
 SUPPLEMENTARY MATERIAL A. Equations solved for flow field calculation Below are the equations used in the FORTRAN 77 computational fluid dynamics code used for determining the flow and other scalar fields
SUPPLEMENTARY MATERIAL A. Equations solved for flow field calculation Below are the equations used in the FORTRAN 77 computational fluid dynamics code used for determining the flow and other scalar fields
The charging of neutral dimethylamine and dimethylamine sulfuric acid clusters using protonated acetone
 doi:10.5194/amt-8-2577-2015 Author(s) 2015. CC Attribution 3.0 License. The charging of neutral dimethylamine and dimethylamine sulfuric acid clusters using protonated acetone K. Ruusuvuori 1, P. Hietala
doi:10.5194/amt-8-2577-2015 Author(s) 2015. CC Attribution 3.0 License. The charging of neutral dimethylamine and dimethylamine sulfuric acid clusters using protonated acetone K. Ruusuvuori 1, P. Hietala
MOLECULAR DYNAMICS SIMULATION OF HETEROGENEOUS NUCLEATION OF LIQUID DROPLET ON SOLID SURFACE
 MOLECULAR DYNAMICS SIMULATION OF HETEROGENEOUS NUCLEATION OF LIQUID DROPLET ON SOLID SURFACE Tatsuto Kimura* and Shigeo Maruyama** *Department of Mechanical Engineering, The University of Tokyo 7-- Hongo,
MOLECULAR DYNAMICS SIMULATION OF HETEROGENEOUS NUCLEATION OF LIQUID DROPLET ON SOLID SURFACE Tatsuto Kimura* and Shigeo Maruyama** *Department of Mechanical Engineering, The University of Tokyo 7-- Hongo,
Interactive comment on Volatility and hygroscopicity of aging secondary organic aerosol in a smog chamber by T. Tritscher et al.
 Atmos. Chem. Phys. Discuss., 11, C2397 C2406, 2011 www.atmos-chem-phys-discuss.net/11/c2397/2011/ Author(s) 2011. This work is distributed under the Creative Commons Attribute 3.0 License. Atmospheric
Atmos. Chem. Phys. Discuss., 11, C2397 C2406, 2011 www.atmos-chem-phys-discuss.net/11/c2397/2011/ Author(s) 2011. This work is distributed under the Creative Commons Attribute 3.0 License. Atmospheric
Implications of Sulfate Aerosols on Clouds, Precipitation and Hydrological Cycle
 Implications of Sulfate Aerosols on Clouds, Precipitation and Hydrological Cycle Source: Sulfate aerosols are produced by chemical reactions in the atmosphere from gaseous precursors (with the exception
Implications of Sulfate Aerosols on Clouds, Precipitation and Hydrological Cycle Source: Sulfate aerosols are produced by chemical reactions in the atmosphere from gaseous precursors (with the exception
1. Droplet Growth by Condensation
 1. Droplet Growth by Condensation It was shown before that a critical size r and saturation ratio S must be exceeded for a small solution droplet to become a cloud droplet. Before the droplet reaches the
1. Droplet Growth by Condensation It was shown before that a critical size r and saturation ratio S must be exceeded for a small solution droplet to become a cloud droplet. Before the droplet reaches the
Ammonium Bisulfate/Water Equilibrium and Metastability Phase Diagrams
 J. Phys. Chem. A 1997, 101, 4191-4195 4191 Ammonium Bisulfate/Water Equilibrium and Metastability Phase Diagrams Dan G. Imre,*, Jun Xu, I. N. Tang, and R. McGraw EnVironmental Chemistry DiVision, Department
J. Phys. Chem. A 1997, 101, 4191-4195 4191 Ammonium Bisulfate/Water Equilibrium and Metastability Phase Diagrams Dan G. Imre,*, Jun Xu, I. N. Tang, and R. McGraw EnVironmental Chemistry DiVision, Department
Rogers and Yau Chapter 10: Drop breakup, snow, precip rate, and bulk models
 Rogers and Yau Chapter 10: Drop breakup, snow, precip rate, and bulk models One explanation for the negative exponential (M-P) distribution of raindrops is drop breakup. Drop size is limited because increased
Rogers and Yau Chapter 10: Drop breakup, snow, precip rate, and bulk models One explanation for the negative exponential (M-P) distribution of raindrops is drop breakup. Drop size is limited because increased
PRECIPITATION PROCESSES
 PRECIPITATION PROCESSES Loknath Adhikari This summary deals with the mechanisms of warm rain processes and tries to summarize the factors affecting the rapid growth of hydrometeors in clouds from (sub)
PRECIPITATION PROCESSES Loknath Adhikari This summary deals with the mechanisms of warm rain processes and tries to summarize the factors affecting the rapid growth of hydrometeors in clouds from (sub)
ATMOSPHERIC THERMODYNAMICS
 ATMOSPHERIC THERMODYNAMICS 1. Introduction 1.1 The field of thermodynamics Classical thermodynamics deals with energy and the transformations of the nature of energy. To a certain extent, it classifies
ATMOSPHERIC THERMODYNAMICS 1. Introduction 1.1 The field of thermodynamics Classical thermodynamics deals with energy and the transformations of the nature of energy. To a certain extent, it classifies
Impact of new particle formation on the concentrations of aerosols and cloud condensation nuclei around Beijing
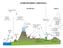 ! JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 116, D19208, doi:10.1029/2011jd016025, 2011!! Impact of new particle formation on the concentrations of aerosols and cloud condensation nuclei around Beijing H.
! JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 116, D19208, doi:10.1029/2011jd016025, 2011!! Impact of new particle formation on the concentrations of aerosols and cloud condensation nuclei around Beijing H.
2σ e s (r,t) = e s (T)exp( rr v ρ l T ) = exp( ) 2σ R v ρ l Tln(e/e s (T)) e s (f H2 O,r,T) = f H2 O
 Formulas/Constants, Physics/Oceanography 4510/5510 B Atmospheric Physics II N A = 6.02 10 23 molecules/mole (Avogadro s number) 1 mb = 100 Pa 1 Pa = 1 N/m 2 Γ d = 9.8 o C/km (dry adiabatic lapse rate)
Formulas/Constants, Physics/Oceanography 4510/5510 B Atmospheric Physics II N A = 6.02 10 23 molecules/mole (Avogadro s number) 1 mb = 100 Pa 1 Pa = 1 N/m 2 Γ d = 9.8 o C/km (dry adiabatic lapse rate)
Chemical Reaction between Solids x 2 = Kt
 2-2.1.2 Chemical Reaction between Solids The simplest system involves the reaction between two solid phases, A and B, to produce a solid solution C, A and B are commonly elements for metallic systems,
2-2.1.2 Chemical Reaction between Solids The simplest system involves the reaction between two solid phases, A and B, to produce a solid solution C, A and B are commonly elements for metallic systems,
P = x i. P i. = y i. Aerosol and Aqueous Chemistry. Raoult s Law. Raoult s Law vs. Henry s Law. or C i. = HC i. = k H
 The Great Smog Aerosol and Aqueous Chemistry Equilibrium Partitioning Oxidation and Oxidants Other Surface-driven Fogs in London were a common occurrence, but the events that began on the 5th of December
The Great Smog Aerosol and Aqueous Chemistry Equilibrium Partitioning Oxidation and Oxidants Other Surface-driven Fogs in London were a common occurrence, but the events that began on the 5th of December
The Clausius-Clapeyron and the Kelvin Equations
 PhD Environmental Fluid Mechanics Physics of the Atmosphere University of Trieste International Center for Theoretical Physics The Clausius-Clapeyron and the Kelvin Equations by Dario B. Giaiotti and Fulvio
PhD Environmental Fluid Mechanics Physics of the Atmosphere University of Trieste International Center for Theoretical Physics The Clausius-Clapeyron and the Kelvin Equations by Dario B. Giaiotti and Fulvio
Urban background aerosols: Negative correlations of particle modes and fragmentation mechanism
 Click Here for Full Article GEOPHYSICAL RESEARCH LETTERS, VOL. 34, L11811, doi:10.1029/2006gl029109, 2007 Urban background aerosols: Negative correlations of particle modes and fragmentation mechanism
Click Here for Full Article GEOPHYSICAL RESEARCH LETTERS, VOL. 34, L11811, doi:10.1029/2006gl029109, 2007 Urban background aerosols: Negative correlations of particle modes and fragmentation mechanism
Helsinki, Finland. 1 Aerodyne Research Inc., Billerica, MA, USA. 2 Chemistry Department, Boston College, Chestnut Hill, MA, USA
 Supplementary material to article: Relationship between aerosol oxidation level and hygroscopic properties of laboratory generated secondary organic aerosol (SOA) particles (paper #0GL0 to Geophysical
Supplementary material to article: Relationship between aerosol oxidation level and hygroscopic properties of laboratory generated secondary organic aerosol (SOA) particles (paper #0GL0 to Geophysical
The Concept of Equilibrium
 Chemical Equilibrium The Concept of Equilibrium Sometimes you can visually observe a certain chemical reaction. A reaction may produce a gas or a color change and you can follow the progress of the reaction
Chemical Equilibrium The Concept of Equilibrium Sometimes you can visually observe a certain chemical reaction. A reaction may produce a gas or a color change and you can follow the progress of the reaction
Effect of aging on cloud nucleating properties of atmospheric aerosols
 Effect of aging on cloud nucleating properties of atmospheric aerosols Yan Ma Nanjing University of Information Science & Technology The 1st Regional GEOS-Chem Asia Meeting 2015 年 5 月 8 日 Indirect effect
Effect of aging on cloud nucleating properties of atmospheric aerosols Yan Ma Nanjing University of Information Science & Technology The 1st Regional GEOS-Chem Asia Meeting 2015 年 5 月 8 日 Indirect effect
Enduring Understandings & Essential Knowledge for AP Chemistry
 Enduring Understandings & Essential Knowledge for AP Chemistry Big Idea 1: The chemical elements are fundamental building materials of matter, and all matter can be understood in terms of arrangements
Enduring Understandings & Essential Knowledge for AP Chemistry Big Idea 1: The chemical elements are fundamental building materials of matter, and all matter can be understood in terms of arrangements
Cloud Brightening and Climate Change
 Cloud Brightening and Climate Change 89 Hannele Korhonen and Antti-Ilari Partanen Contents Definitions... 778 Aerosols and Cloud Albedo... 778 Cloud Brightening with Sea-Salt Aerosol... 779 Climate Effects
Cloud Brightening and Climate Change 89 Hannele Korhonen and Antti-Ilari Partanen Contents Definitions... 778 Aerosols and Cloud Albedo... 778 Cloud Brightening with Sea-Salt Aerosol... 779 Climate Effects
Chapter 11. Freedom of Motion. Comparisons of the States of Matter. Liquids, Solids, and Intermolecular Forces
 Liquids, Solids, and Intermolecular Forces Chapter 11 Comparisons of the States of Matter The solid and liquid states have a much higher density than the gas state The solid and liquid states have similar
Liquids, Solids, and Intermolecular Forces Chapter 11 Comparisons of the States of Matter The solid and liquid states have a much higher density than the gas state The solid and liquid states have similar
Lecture Presentation. Chapter 12. Solutions. Sherril Soman, Grand Valley State University Pearson Education, Inc.
 Lecture Presentation Chapter 12 Solutions Sherril Soman, Grand Valley State University Thirsty Seawater Drinking seawater can cause dehydration. Seawater Is a homogeneous mixture of salts with water Contains
Lecture Presentation Chapter 12 Solutions Sherril Soman, Grand Valley State University Thirsty Seawater Drinking seawater can cause dehydration. Seawater Is a homogeneous mixture of salts with water Contains
Solar eclipse demonstrating the importance of photochemistry in new particle formation
 https://helda.helsinki.fi Solar eclipse demonstrating the importance of photochemistry in new particle formation Jokinen, Tuija 2017-04-04 Jokinen, T, Kontkanen, J, Lehtipalo, K, Manninen, H E, Aalto,
https://helda.helsinki.fi Solar eclipse demonstrating the importance of photochemistry in new particle formation Jokinen, Tuija 2017-04-04 Jokinen, T, Kontkanen, J, Lehtipalo, K, Manninen, H E, Aalto,
Assignment 70 LE CHATELIER'S PRINCIPLE AND EQUILIBRIUM CONCENTRATIONS
 BACKGROUND Assignment 70 LE CHATELIER'S PRINCIPLE AND EQUILIBRIUM CONCENTRATIONS The theoretical yield calculations of prior assignments are made on the assumption that the reaction goes to completion
BACKGROUND Assignment 70 LE CHATELIER'S PRINCIPLE AND EQUILIBRIUM CONCENTRATIONS The theoretical yield calculations of prior assignments are made on the assumption that the reaction goes to completion
A Simple Numerical Algorithm and Software for Solution of Nucleation, Surface Growth, and Coagulation Problems
 Aerosol Science and Technology, 37:892 898, 2003 Copyright c American Association for Aerosol Research ISSN: 0278-6826 print / 1521-7388 online DOI: 10.1080/02786820390225826 A Simple Numerical Algorithm
Aerosol Science and Technology, 37:892 898, 2003 Copyright c American Association for Aerosol Research ISSN: 0278-6826 print / 1521-7388 online DOI: 10.1080/02786820390225826 A Simple Numerical Algorithm
A MOLECULAR DYNAMICS SIMULATION OF A BUBBLE NUCLEATION ON SOLID SURFACE
 A MOLECULAR DYNAMICS SIMULATION OF A BUBBLE NUCLEATION ON SOLID SURFACE Shigeo Maruyama and Tatsuto Kimura Department of Mechanical Engineering The University of Tokyo 7-- Hongo, Bunkyo-ku, Tokyo -866,
A MOLECULAR DYNAMICS SIMULATION OF A BUBBLE NUCLEATION ON SOLID SURFACE Shigeo Maruyama and Tatsuto Kimura Department of Mechanical Engineering The University of Tokyo 7-- Hongo, Bunkyo-ku, Tokyo -866,
Chapter 8: Reaction Rates and Equilibrium
 Chapter 8: Reaction Rates and Equilibrium ACTIVATION ENERGY In some reaction mixtures, the average total energy of the molecules is too low at the prevailing temperature for a reaction to take place at
Chapter 8: Reaction Rates and Equilibrium ACTIVATION ENERGY In some reaction mixtures, the average total energy of the molecules is too low at the prevailing temperature for a reaction to take place at
LECTURE 5: PHASE EQUILIBRIA
 LECTURE 5: PHASE EQUILIBRIA PHASE EQUILIBRIA Phase equilibrium describes the way phases (such as solid, liquid and/or gas) co-exist at some temperatures and pressure, but interchange at others. 1 ENERGETIC
LECTURE 5: PHASE EQUILIBRIA PHASE EQUILIBRIA Phase equilibrium describes the way phases (such as solid, liquid and/or gas) co-exist at some temperatures and pressure, but interchange at others. 1 ENERGETIC
Sample Questions Chem 22 Student Chapters Page 1 of 5 Spring 2016
 Sample Questions Chem 22 Student Chapters 13-18 Page 1 of 5 1. The vapor pressure of a liquid is the pressure, at equilibrium, of the a) solid above its liquid. b) liquid above its solid. c) gas above
Sample Questions Chem 22 Student Chapters 13-18 Page 1 of 5 1. The vapor pressure of a liquid is the pressure, at equilibrium, of the a) solid above its liquid. b) liquid above its solid. c) gas above
Chapter 5 Conclusions and Future Studies
 177 Chapter 5 Conclusions and Future Studies 178 Conclusions and Future Studies The results of the studies presented in this thesis confirm that ambient and laboratory-generated aerosols exhibit complex
177 Chapter 5 Conclusions and Future Studies 178 Conclusions and Future Studies The results of the studies presented in this thesis confirm that ambient and laboratory-generated aerosols exhibit complex
Validation of a new flow-reactor for the study of secondary organic aerosol (SOA) formation
 Validation of a new flow-reactor for the study of secondary organic aerosol (SOA) formation M. Duncianu*(1,2), V. Riffault (1,2), A. Tomas (1,2), P. Coddeville (1,2) (1) Université Lille Nord de France,
Validation of a new flow-reactor for the study of secondary organic aerosol (SOA) formation M. Duncianu*(1,2), V. Riffault (1,2), A. Tomas (1,2), P. Coddeville (1,2) (1) Université Lille Nord de France,
ROBUSTNESS ANALYSIS OF ATMOSPHERIC AEROSOL REMOVAL BY PRECIPITATION
 ROBUSTNESS ANALYSIS OF ATMOSPHERIC AEROSOL REMOVAL BY PRECIPITATION C. Andronache 1 and V. T. J. Phillips 2 1 Boston College, Chestnut Hill, MA 02467, USA 2 University of Hawaii, Honolulu, HI 96822, USA
ROBUSTNESS ANALYSIS OF ATMOSPHERIC AEROSOL REMOVAL BY PRECIPITATION C. Andronache 1 and V. T. J. Phillips 2 1 Boston College, Chestnut Hill, MA 02467, USA 2 University of Hawaii, Honolulu, HI 96822, USA
