Synthesis of Pyrazine Alcaloids from Botryllus leachi. Diazines 43
|
|
|
- Moris Holland
- 5 years ago
- Views:
Transcription
1 Synthesis of Pyrazine Alcaloids from Botryllus leachi. Diazines 43 Frédéric Buron, Nelly Plé, Alain Turck,* and Guy Queguiner IRCOF, Laboratoire de Chimie Organique Fine et Hétérocyclique, UMR 6014, INSA, B.P. 08, Mont St. Aignan Cedex, France Received December 13, 2004 Regioselective metalation of pyrazines and cross-coupling reactions provides an easy access to botryllazines A and B and to an isomer of botryllazine A with good yields from chloropyrazine. Introduction Ascidians, which are small marine animals, are present in all the seas and are the source of naturally occurring alkaloids. In 1999, Duran et al. 1 isolated two pyrazine alkaloids from Botryllus leachi: botryllazines A and B (Scheme 1). These compounds exhibited cytotoxicity against human tumor cells. This antineoplasmic activity prompted us to perform a synthesis of these two compounds. In the course of this work, a synthesis of botryllazine B was described by S. Mahboobi 2 starting from 4-methoxyacetophenone by building of the pyrazine ring; the overall yield was 11%. Our syntheses are based on the regioselective functionalization of the pyrazine ring by metalation and cross-coupling reactions starting from commercial chloropyrazine 3. SCHEME 1 SCHEME 2 Results and Discussion Synthesis of Botryllazine B. In our laboratory, F. Toudic 3 performed the synthesis of 2-fluoro-6-tributylstannylpyrazine by a non-ortho-directed metalation of fluoropyrazine. The same experimental procedure was used with chloropyrazine 3 and afforded 2-chloro-6- tributylstannylpyrazine 4 with an 89% yield (Scheme 2). (1) (a) Duran, R.; Zubia, E.; Ortega, M. J.; Naranjo, S.; Salva, J. Tetrahedron 1999, 55, (b) Zubia, F.; Ortega, M. J.; Salva, J. Ciencias Marinas 2003, 29, 251. (2) Mahboobi, S.; Sellmer, A.; Burgemeister, T.; Lyssenko, A.; Shollmeyer, D. Monatsch. Chem. 2004, 135, 333. (3) Toudic, F.; Heynderickx, A.; Plé, N.; Turck, A.; Queguiner, G. Tetrahedron 2003, 59, J. Org. Chem. 2005, 70, It was demonstrated that the key intermediate was 2-chloro-3-tributylstannylpyrazine, which was again metalated and isomerized quickly to 2-chloro-3-lithio-6- tributylstannylpyrazine in the presence of an excess of LTMP affording 4 after hydrolysis /jo CCC: $ American Chemical Society Published on Web 02/25/2005
2 Pyrazine Alcaloids from Botryllus leachi SCHEME 3 SCHEME 4 SCHEME 5 TABLE 1. Metalation of 8 Followed by Reaction with Anisaldehyde a A Stille cross coupling reaction was performed with 4 and 4-methoxybenzoyl chloride 5. We first obtained a mixture of products 6 and 7 with a 64% yield (process A); product 7 resulted from the homocoupling of 4 (Scheme 3). To solve this problem, the order of introduction of the reactants was modified (process B). p-methoxy-benzoyl chloride 5 was mixed first with the palladium catalyst, and then the pyrazine 4 was introduced. Using this procedure allowed the formation of 7 to be avoided, and product 6 was obtained with a 70% yield. This product was cross-coupled again with 4-methoxyphenyl boronic acid following the Suzuki procedure to give compound 8 with good yield (Scheme 4). The last step was the cleavage of the methoxy group; the usual reagents (HI, HBr, BBr 3 ) were tested without success: a mixture of uncleaved, monocleaved, and dicleaved products was obtained. The method of Royer 4 with pyridinium hydrochloride afforded botryllazine B with excellent yield (Scheme 5). In summary, the synthesis of botryllazine B was performed in four steps from 2-chloropyrazine with an overall yield of 51%. Synthesis of Botryllazine A. The easy access to compound 8 prompted us to choose it as the starting material for synthesis of botryllazine A. The ketone was protected as dioxolane; then, the product was metalated in order to obtain a metalation ortho to the dioxolane (Scheme 6, Table 1). A metalation with dioxolane as an ortho directing group was recently reported with derivatives of acetophenone. 5 entry base equiv time temp yield ratio 10/11 1 LTMP h -75 C 2 LTMP h -75 C 70% 70/30 3 LTMP h -100 C 4 LTMP 3.1 in situ trapping -75 C 64% 70/30 5 LDA h -75 C a In the case of entries 1, 3, and 5, only the starting material was recovered. A 3-fold excess of LTMP was necessary to achieve the metalation. The need for an excess of LTMP is due to the chelating properties of the methoxy groups. 6 The metalation was not regioselective, and the major isomer was not the useful one. It was then attempted to slow the metalation rate ortho to the p-methoxyphenyl group by replacement of the hydrogen by a deuterium atom using the isotopic effect. This procedure was recently used by Y. Fort 7 et al. The deuterium atom was regioselectively introduced ortho to the chlorine atom of 12 by metalation followed by reaction with EtOD (Scheme 7). Product 13 was cross-coupled as before to afford the deuterated compound 14. The metalation of compound 14 gave a result very close to the metalation of the undeuterated compound 9, a 75/25 mixture of the two isomers 10 and 11. It must be noted that during the metalation process, the deuterium atom was replaced by a hydrogen and that compound 11 was actually recovered without the deuterium atom present in 14. This result indicated clearly that the metalation reaction was under thermodynamic control and that the main factor was the relative stability of the lithio derivatives. A PM3/Li 8 calculation of the formation energies of the two lithio derivatives gave the following values (Scheme 8). Formation energies of the two isomers are in agreement with the experimental results. Another solution for solving the regioselectivity problem would be the blocking of the 3 position with a (4) (a) Royer, R.; Buisson, J.; Demerseman, P.; Chentin, A. Bull. Soc. Chim. Fr. 1970, 10, (b) Royer, R.; Demerseman, P. Bull. Soc. Chim. Fr. 1968, 6, (5) Lukács, G.; Porcs-Makkay, M.; Simig, G. Tetrahedron Lett. 2003, 44, (6) Turck, A.; Trohay, D.; Mojovic, L.; Plé, N.; Queguiner, G., J. Organomet. Chem. 1991, 412, 301. (7) Gros, P.; Choppin, S.; Fort, Y. J. Org. Chem. 2003, 68, (8) Anders, E.; Koch, R.; Freunscht, P. J. Comput. Chem. 1993, 14, J. Org. Chem, Vol. 70, No. 7,
3 Buron et al. SCHEME 6 SCHEME 7 SCHEME 8 removable group. In this approach, a trimethylsilyl group was introduced at position 3 by a regioselective metalation of 12 followed by reaction with trimethylchlorosilane, affording compound 15. The metalation of 15 did not afford the expected product, and the desilylated compound 12 was recovered. We supposed that the desilylation reaction could be favored by the environment of the chlorine atom; we therefore performed the cross-coupling reaction with p-methoxyphenyl boronic acid, affording compound 16, and then the metalation was performed (Scheme 9). As before, the metalation afforded the desilylated product 9 along with starting material. Taking into account the failure of the blocking of position 3 by deuterium or trimethylsilyl, another route was tested. This route was based on a nucleophilic substitution of the 2-chlorine atom in order to introduce the second p-methoxy-benzoyl group on the diazine ring (Scheme 10). Products 20 and 21 were obtained in three steps from commercial 2,6-dichloropyrazine 17 with good yields. The nucleophilic substitution of the 6-chlorine atom was tested with the lithiated dithiane 9 22, the lithiated nitrile 10 23, and the p-methoxybenzaldehyde in the presence of imidazolium salts (Miyashita reaction) 11 (Scheme 11). Despite numerous experiments, the substitution failed: the starting material was recovered along with a large amount of tar. Although all the strategies attempting to access botryllazine A have failed, it was nevertheless possible to access to an isomer of this compound starting from the disubstituted pyrazine 12 (Scheme 12). The metalation of 12 was completely regioselective ortho to the chlorine atom with good yield (82%), and (9) Corey, E.; Seebach, D. Angew. Chem., Int. Ed. Engl. 1965, 4, (10) Yamanaka, H.; Ohba, S. Heterocycles 1990, 31, 895. (11) (a) Miyashita, A.; Obae, K.; Suzuki, Y.; Oishi, E.; Iwamoto, K.; Higashino, T. Heterocycles 1997, 45, (b) Miyashita, A.; Suzuki, Y.; Ohta, K.; Iwamoto, K.; Higashino, T. Heterocycles 1998, 47, 407. (c) Miyashita, A.; Matsuda, H.; Suzuki, Y.; Iwamoto, K.-i.; Higashino, T. Chem. Pharm. Bull. 1994, 42, J. Org. Chem., Vol. 70, No. 7, 2005
4 Pyrazine Alcaloids from Botryllus leachi SCHEME 9 SCHEME 10 SCHEME 11 subsequent oxidation of 24 afforded 25 (91% yield). A Suzuki cross coupling afforded 26 (87%); the deprotection of the keto group was performed with hydrochloric acid in methanol (88%), and the final cleavage of the three methoxy groups was performed with pyridinium hydrochloride (90%). Compound 28, an isomer of botryllazine A, was prepared in five steps from 12 with an overall yield of 51.5% or in eight steps from commercial chloropyrazine with a 31% yield. Another route to botryllazine A was then tested. This route presented no regioselectivity problem (Scheme 13). Two main syntheses of 2,3-dichloropyrazine 29 were described in the literature: either by chlorination of chloropyrazine 3 12 or 2-chloro-pyrazine N-oxide 13 or by reaction of 2,3-dihydroxypyrazine 14 with phosphorus oxychloride. The use of the metalation reaction of 3 followed by the reaction with C 2 Cl 6 was a new route and gave 29 with an excellent yield (90%). This was followed by two successive metalation reactions followed by reaction with p-methoxybenzaldehyde: the first with 1 equiv of LTMP affording 30 with good yield (81%), and the second with 3.1 equiv of LTMP afforded the tetrasubstituted pyrazine 31. The crude product was oxidized by manganese dioxide, and the diketone 32 was obtained with a good yield from 30 (71%). A Suzuki cross-coupling reaction with the p-methoxyphenyl boronic acid gave 33 with a 70% yield. The reduction of the last chlorine atom was (12) Okafor, C. O. J. Heterocycl. Chem. 1981, 18, 405. (13) Sato, N.; Fujii, M. J. Heterocycl. Chem. 1994, 31, (14) Palamidessi, G.; Bonanomi, M. Edizione Scientifica 1966, 21, 799. J. Org. Chem, Vol. 70, No. 7,
5 Buron et al. SCHEME 12 SCHEME 13 performed with a mixture of formic acid and triethylamine catalyzed by palladium leading to 34 in 75% yield. The cleavage of the methoxy groups was performed with pyridinium hydrochloride 4 at 215 C. Botryllazine A was obtained in seven steps from commercial chloropyrazine with an overall yield of 24.5% J. Org. Chem., Vol. 70, No. 7, 2005
6 Pyrazine Alcaloids from Botryllus leachi In conclusion, we have prepared two botryllazines A and B and an isomer of A in a few steps and with good overall yields from commercial chloropyrazine. These syntheses, which allow preparation of large quantities of these compounds, will help to advance the evaluation of their potential as drugs. This is currently under investigation in a laboratory of Artois University. 15 Supporting Information Available: Experimental procedures and product characterization for new compounds and selected 1 H and 13 C NMR spectra. This material is available free of charge via the Internet at JO (15) LBHE EA 2465, Faculté des Sciences de Lens, Pr P. Martin, SP18, Lens, France. J. Org. Chem, Vol. 70, No. 7,
Trimethylsulfonium and trimethylsulfoxonium as versatile epoxidation reagents. A comparative study
 Trimethylsulfonium and trimethylsulfoxonium as versatile epoxidation reagents. A comparative study Christelle Bermand, Alain Comel, and Gilbert Kirsch * Laboratoire de Chimie Organique, Groupe de Synthèse
Trimethylsulfonium and trimethylsulfoxonium as versatile epoxidation reagents. A comparative study Christelle Bermand, Alain Comel, and Gilbert Kirsch * Laboratoire de Chimie Organique, Groupe de Synthèse
C H Activated Trifluoromethylation
 Literature report C H Activated Trifluoromethylation Reporter:Yan Fang Superior:Prof. Yong Huang Jun. 17 th 2013 Contents Background Trifluoromethylation of sp-hybridized C-H Bonds Trifluoromethylation
Literature report C H Activated Trifluoromethylation Reporter:Yan Fang Superior:Prof. Yong Huang Jun. 17 th 2013 Contents Background Trifluoromethylation of sp-hybridized C-H Bonds Trifluoromethylation
Tautomerism and Keto Enol Equilibrium
 Tautomerism and Keto Enol Equilibrium Enols & enolates are important nucleophiles in organic & biochemistry. Keto-Enol Equilibrium: Tautomerisation can be catalyzed by either acids or bases. Relative stability
Tautomerism and Keto Enol Equilibrium Enols & enolates are important nucleophiles in organic & biochemistry. Keto-Enol Equilibrium: Tautomerisation can be catalyzed by either acids or bases. Relative stability
Total Synthesis of Verruculogen and Fumitremorgin A Enabled by Ligand-Controlled C H Borylation
 Enabled by Ligand-Controlled C H Borylation Yu Feng, Dane Holte, Jochen Zoller, Shigenobu Umemiya, Leah R. Simke, and Phil S. Baran J. Am. Chem. Soc. 2015, 137, 10160 10163. I. Introduction II. Retrosynthetic
Enabled by Ligand-Controlled C H Borylation Yu Feng, Dane Holte, Jochen Zoller, Shigenobu Umemiya, Leah R. Simke, and Phil S. Baran J. Am. Chem. Soc. 2015, 137, 10160 10163. I. Introduction II. Retrosynthetic
Title. Author(s)Ishiyama, Tatsuo; Oohashi, Zengo; Ahiko, Taka-aki; M. CitationChemistry Letters, 8: Issue Date Doc URL.
 Title Nucleophilic Borylation of Benzyl Halides with Bis(p Author(s)Ishiyama, Tatsuo; Oohashi, Zengo; Ahiko, Taka-aki; M CitationChemistry Letters, 8: 7-781 Issue Date 2002-08-05 Doc URL http://hdl.handle.net/2115/56196
Title Nucleophilic Borylation of Benzyl Halides with Bis(p Author(s)Ishiyama, Tatsuo; Oohashi, Zengo; Ahiko, Taka-aki; M CitationChemistry Letters, 8: 7-781 Issue Date 2002-08-05 Doc URL http://hdl.handle.net/2115/56196
A convenient synthesis of cyclopenta[b]pyridin-2,5-dione as a non- glycosidic cardiotonic agent
![A convenient synthesis of cyclopenta[b]pyridin-2,5-dione as a non- glycosidic cardiotonic agent A convenient synthesis of cyclopenta[b]pyridin-2,5-dione as a non- glycosidic cardiotonic agent](/thumbs/93/112723957.jpg) Issue in Honor of Prof. Guy Quéguiner ARKIVC 2008 (vii) 92-100 A convenient synthesis of cyclopenta[b]pyridin-2,5-dione as a non- glycosidic cardiotonic agent icolas Robert, Cécile Verrier, Christophe
Issue in Honor of Prof. Guy Quéguiner ARKIVC 2008 (vii) 92-100 A convenient synthesis of cyclopenta[b]pyridin-2,5-dione as a non- glycosidic cardiotonic agent icolas Robert, Cécile Verrier, Christophe
CHEM 345 Problem Set 07 Key
 CHEM 345 Problem Set 07 Key 1.) Fill in the appropriate reaction arrow. The starting material is on the left, the product is on the right. Use. Simple Ring Size. 5 and 6 are favored. 3 is not. That s it.
CHEM 345 Problem Set 07 Key 1.) Fill in the appropriate reaction arrow. The starting material is on the left, the product is on the right. Use. Simple Ring Size. 5 and 6 are favored. 3 is not. That s it.
Converting Cycloalkanones into N- Heterocycles: Formal Synthesis of ( )-Gephyrotoxin 287C. Claude Spino et al., J. Org. Chem. 2013, 78,
 Converting Cycloalkanones into N- Heterocycles: Formal Synthesis of ( )-Gephyrotoxin 287C Claude Spino et al., J. Org. Chem. 2013, 78, 12532 12544. Poison frog alkaloids Isolated from poison frog skin.
Converting Cycloalkanones into N- Heterocycles: Formal Synthesis of ( )-Gephyrotoxin 287C Claude Spino et al., J. Org. Chem. 2013, 78, 12532 12544. Poison frog alkaloids Isolated from poison frog skin.
Catellani Reaction (Pd-Catalyzed Sequential Reaction) Todd Luo
 Catellani Reaction (Pd-Catalyzed Sequential Reaction) Todd Luo 2014.1.6 1 Content Introduction Progress of Catellani Reaction o-alkylation and Applications o-arylation and Applications Conclusion and Outlook
Catellani Reaction (Pd-Catalyzed Sequential Reaction) Todd Luo 2014.1.6 1 Content Introduction Progress of Catellani Reaction o-alkylation and Applications o-arylation and Applications Conclusion and Outlook
Regioselective lithiations of 2-(3,5-dichlorophenyl)-2-(4- fluorophenyl)-1,3-dioxolane
 Issue in Honor of Prof Henk C. van der Plas ARKIVC 2009 (vi) 167-173 Regioselective lithiations of 2-(3,5-dichlorophenyl)-2-(4- fluorophenyl)-1,3-dioxolane Márta Porcs-Makkay* and Gyula Simig Chemical
Issue in Honor of Prof Henk C. van der Plas ARKIVC 2009 (vi) 167-173 Regioselective lithiations of 2-(3,5-dichlorophenyl)-2-(4- fluorophenyl)-1,3-dioxolane Márta Porcs-Makkay* and Gyula Simig Chemical
Titanacyclopropanes as versatile intermediates for carbon carbon bond formation in reactions with unsaturated compounds*
 Pure Appl. Chem., Vol. 72, No. 9, pp. 1715 1719, 2000. 2000 IUPAC Titanacyclopropanes as versatile intermediates for carbon carbon bond formation in reactions with unsaturated compounds* O. G. Kulinkovich
Pure Appl. Chem., Vol. 72, No. 9, pp. 1715 1719, 2000. 2000 IUPAC Titanacyclopropanes as versatile intermediates for carbon carbon bond formation in reactions with unsaturated compounds* O. G. Kulinkovich
I. Introduction. II. Retrosynthetic Analysis. Andrew Baggett. Liu lab
 Total Synthesis of Limonin Shuji Yamashita,* Akito Naruko, Yuki Nakazawa, Le Zhao, Yujiro Hayashi, Masahiro Hirama Tohoku University, Department of Chemistry, Aramaki-aza aoba, Aoba-ku, Sendai 980-8578
Total Synthesis of Limonin Shuji Yamashita,* Akito Naruko, Yuki Nakazawa, Le Zhao, Yujiro Hayashi, Masahiro Hirama Tohoku University, Department of Chemistry, Aramaki-aza aoba, Aoba-ku, Sendai 980-8578
Glyoxylic acid derivatives in asymmetric synthesis*
 Pure Appl. Chem., Vol. 72, No. 9, pp. 1589 1596, 2000. 2000 IUPAC Glyoxylic acid derivatives in asymmetric synthesis* Janusz Jurczak 1,2 and Tomasz Bauer 1 1 Department of Chemistry, University of Warsaw,
Pure Appl. Chem., Vol. 72, No. 9, pp. 1589 1596, 2000. 2000 IUPAC Glyoxylic acid derivatives in asymmetric synthesis* Janusz Jurczak 1,2 and Tomasz Bauer 1 1 Department of Chemistry, University of Warsaw,
KOT 222 Organic Chemistry II
 KOT 222 Organic Chemistry II Course Objectives: 1) To introduce the chemistry of alcohols and ethers. 2) To study the chemistry of functional groups. 3) To learn the chemistry of aromatic compounds and
KOT 222 Organic Chemistry II Course Objectives: 1) To introduce the chemistry of alcohols and ethers. 2) To study the chemistry of functional groups. 3) To learn the chemistry of aromatic compounds and
Copper-catalyzed cleavage of benzyl ethers with diacetoxyiodobenzene and p-toluenesulfonamide
 General Papers ARKIVC 2008 (xii) 103-108 Copper-catalyzed cleavage of benzyl ethers with diacetoxyiodobenzene and p-toluenesulfonamide Ling He a,b, Qin Wang a, Guo-Chuan Zhou b, Lei Guo b, and Xiao-Qi
General Papers ARKIVC 2008 (xii) 103-108 Copper-catalyzed cleavage of benzyl ethers with diacetoxyiodobenzene and p-toluenesulfonamide Ling He a,b, Qin Wang a, Guo-Chuan Zhou b, Lei Guo b, and Xiao-Qi
Suggested solutions for Chapter 41
 s for Chapter 41 41 PBLEM 1 Explain how this synthesis of amino acids, starting with natural proline, works. Explain the stereoselectivity of each step after the first. C 2 C 2 3 CF 3 C 2 2 Pd 2 C 2 +
s for Chapter 41 41 PBLEM 1 Explain how this synthesis of amino acids, starting with natural proline, works. Explain the stereoselectivity of each step after the first. C 2 C 2 3 CF 3 C 2 2 Pd 2 C 2 +
Synthesis of 3-halo-2,5-disubstituted furans via CuX mediated cyclization-halogenation reactions
 University of Wollongong Research Online Faculty of Science - Papers (Archive) Faculty of Science, Medicine and Health 2011 Synthesis of 3-halo-2,5-disubstituted furans via CuX mediated cyclization-halogenation
University of Wollongong Research Online Faculty of Science - Papers (Archive) Faculty of Science, Medicine and Health 2011 Synthesis of 3-halo-2,5-disubstituted furans via CuX mediated cyclization-halogenation
Supporting Information
 Supporting Information Ruthenium-Catalyzed Oxidative Annulation and Hydroarylation of Chromene-3- carboxamides with Alkynes via Double C-H Functionalization R. N. Prasad Tulichala, Mallepalli Shankar and
Supporting Information Ruthenium-Catalyzed Oxidative Annulation and Hydroarylation of Chromene-3- carboxamides with Alkynes via Double C-H Functionalization R. N. Prasad Tulichala, Mallepalli Shankar and
A. Review of Acidity and pk a Common way to examine acidity is to use the Bronsted-Lowry acid-base equation:
 1 Chapter 22: Reactions of Enols and Enolates I. Alpha Substitution verview: A. Review of Acidity and pk a Common way to examine acidity is to use the Bronsted-Lowry acid-base equation: Recall that the
1 Chapter 22: Reactions of Enols and Enolates I. Alpha Substitution verview: A. Review of Acidity and pk a Common way to examine acidity is to use the Bronsted-Lowry acid-base equation: Recall that the
Chromium Arene Complexes
 Go through Reviews Chem. Reviews Chem. Soc. Reviews Book by Prof. A. J. Elias Chromium Arene Complexes Complexation of Cr(CO) 3 with ARENES Chromium arene complexes Metal complexation is appealing in organic
Go through Reviews Chem. Reviews Chem. Soc. Reviews Book by Prof. A. J. Elias Chromium Arene Complexes Complexation of Cr(CO) 3 with ARENES Chromium arene complexes Metal complexation is appealing in organic
Chapter 16. Aldehydes and Ketones I. Nucleophilic Addition to the Carbonyl Group. Physical Properties of Aldehydes and Ketones. Synthesis of Aldehydes
 Nomenclature of Aldehydes and Ketones Chapter 16 Aldehydes and Ketones I. Aldehydes replace the -e of the parent alkane with -al The functional group needs no number Nucleophilic Addition to the Carbonyl
Nomenclature of Aldehydes and Ketones Chapter 16 Aldehydes and Ketones I. Aldehydes replace the -e of the parent alkane with -al The functional group needs no number Nucleophilic Addition to the Carbonyl
Organic Chemistry. M. R. Naimi-Jamal. Faculty of Chemistry Iran University of Science & Technology
 Organic Chemistry M. R. Naimi-Jamal Faculty of Chemistry Iran University of Science & Technology Chapter 5-2. Chemistry of Benzene: Electrophilic Aromatic Substitution Based on McMurry s Organic Chemistry,
Organic Chemistry M. R. Naimi-Jamal Faculty of Chemistry Iran University of Science & Technology Chapter 5-2. Chemistry of Benzene: Electrophilic Aromatic Substitution Based on McMurry s Organic Chemistry,
Advanced Organic Chemistry
 D. A. Evans, G. Lalic Question of the day: Chemistry 530A TBS Me 2 C Me toluene, 130 C 70% TBS C 2 Me H H Advanced rganic Chemistry Me Lecture 16 Cycloaddition Reactions Diels _ Alder Reaction Photochemical
D. A. Evans, G. Lalic Question of the day: Chemistry 530A TBS Me 2 C Me toluene, 130 C 70% TBS C 2 Me H H Advanced rganic Chemistry Me Lecture 16 Cycloaddition Reactions Diels _ Alder Reaction Photochemical
Chapter 17: Alcohols and Phenols. Based on McMurry s Organic Chemistry, 7 th edition
 Chapter 17: Alcohols and Phenols Based on McMurry s Organic Chemistry, 7 th edition Alcohols and Phenols Alcohols contain an OH group connected to a a saturated C (sp 3 ) They are important solvents and
Chapter 17: Alcohols and Phenols Based on McMurry s Organic Chemistry, 7 th edition Alcohols and Phenols Alcohols contain an OH group connected to a a saturated C (sp 3 ) They are important solvents and
Working with Hazardous Chemicals
 A Publication of Reliable Methods for the Preparation of Organic Compounds Working with Hazardous Chemicals The procedures in Organic Syntheses are intended for use only by persons with proper training
A Publication of Reliable Methods for the Preparation of Organic Compounds Working with Hazardous Chemicals The procedures in Organic Syntheses are intended for use only by persons with proper training
Electron ionization (EI) mass spectra of some 3,4-disubstituted-1,2,4- oxadiazin-5-ones and -thiones and 3,5-disubstituted 1,2,4-oxadiazin-6- ones
 Electron ionization (EI) mass spectra of some 3,4-disubstituted-1,2,4- oxadiazin-5-ones and -thiones and 3,5-disubstituted 1,2,4-oxadiazin-6- ones Kalevi Pihlaja. a* Sanna Heinonen, a Olli Martiskainen,
Electron ionization (EI) mass spectra of some 3,4-disubstituted-1,2,4- oxadiazin-5-ones and -thiones and 3,5-disubstituted 1,2,4-oxadiazin-6- ones Kalevi Pihlaja. a* Sanna Heinonen, a Olli Martiskainen,
STEREOELECTRONIC EFFECTS (S.E.) IN ORGANIC CHEMISTRY
 STEREOELECTRONIC EFFECTS (S.E.) IN ORGANIC CHEMISTRY Pierre Deslongchamps (version du 16 février 2010) Cf. pour le livre: http://pages.usherbrooke.ca/pdeslongchamps/cv.htm 1 SECTION 8 Stereoelectronic
STEREOELECTRONIC EFFECTS (S.E.) IN ORGANIC CHEMISTRY Pierre Deslongchamps (version du 16 février 2010) Cf. pour le livre: http://pages.usherbrooke.ca/pdeslongchamps/cv.htm 1 SECTION 8 Stereoelectronic
Chemistry of Benzene: Electrophilic Aromatic Substitution
 Chemistry of Benzene: Electrophilic Aromatic Substitution Why this Chapter? Continuation of coverage of aromatic compounds in preceding chapter focus shift to understanding reactions Examine relationship
Chemistry of Benzene: Electrophilic Aromatic Substitution Why this Chapter? Continuation of coverage of aromatic compounds in preceding chapter focus shift to understanding reactions Examine relationship
2/26/18. Practice Questions. Practice Questions B F. How many steps are there in this reaction?
 Practice Questions Practice Questions D B F C E A G How many steps are there in this reaction? 1 Practice Questions D B F C E A G What is the highest-energy transitions state? Practice Questions D B F
Practice Questions Practice Questions D B F C E A G How many steps are there in this reaction? 1 Practice Questions D B F C E A G What is the highest-energy transitions state? Practice Questions D B F
Sametz: CHEM 322 Spring 2012 Organic Chemistry Final
 Name: (Print your name clearly!) Sametz: CHEM 322 Spring 2012 rganic Chemistry Final All answers should be written CLEARLY in the space provided. (If it s not clear, it s wrong). FR QUESTINS 9-10: read
Name: (Print your name clearly!) Sametz: CHEM 322 Spring 2012 rganic Chemistry Final All answers should be written CLEARLY in the space provided. (If it s not clear, it s wrong). FR QUESTINS 9-10: read
II. Special Topics IIA. Enolate Chemistry & the Aldol Reaction
 P. Wipf - Chem 2320 1 3/20/2006 II. Special Topics IIA. Enolate Chemistry & the Aldol Reaction Boger Notes: p. 147-206 (Chapter VIII) Carey/Sundberg: B p. 57-95 (Chapter B 2.1) Problem of the Day: Wang,
P. Wipf - Chem 2320 1 3/20/2006 II. Special Topics IIA. Enolate Chemistry & the Aldol Reaction Boger Notes: p. 147-206 (Chapter VIII) Carey/Sundberg: B p. 57-95 (Chapter B 2.1) Problem of the Day: Wang,
CHAPTER 23 HW: ENOLS + ENOLATES
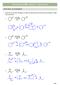 CAPTER 23 W: ENLS + ENLATES KET-ENL TAUTMERSM 1. Draw the curved arrow mechanism to show the interconversion of the keto and enol form in either trace acid or base. trace - 2 trace 3 + 2 + E1 2 c. trace
CAPTER 23 W: ENLS + ENLATES KET-ENL TAUTMERSM 1. Draw the curved arrow mechanism to show the interconversion of the keto and enol form in either trace acid or base. trace - 2 trace 3 + 2 + E1 2 c. trace
Ch 16 Electrophilic Aromatic Substitution
 Ch 16 Electrophilic Aromatic Substitution Mechanism - Aromatic rings typically undergo substitution, where an H is replaced with an electrophile (E+). - The rings do not typically undergo addition across
Ch 16 Electrophilic Aromatic Substitution Mechanism - Aromatic rings typically undergo substitution, where an H is replaced with an electrophile (E+). - The rings do not typically undergo addition across
FIVE MEMBERED AROMATIC HETEROCYCLES
 FIVE MEMBERED AROMATIC HETEROCYCLES 63 Electrophilic aromatic substitution reaction of five membered aromatic heterocycles X = O, S or NH a-substitution b-substitution The Substitution is regioselective
FIVE MEMBERED AROMATIC HETEROCYCLES 63 Electrophilic aromatic substitution reaction of five membered aromatic heterocycles X = O, S or NH a-substitution b-substitution The Substitution is regioselective
Direct Catalytic Cross-Coupling of Organolithium
 Literature report Direct Catalytic Cross-Coupling of Organolithium Compounds Reporter: Zhang-Pei Chen Checker: Mu-Wang Chen Date: 02/07/2013 Feringa, B.L.et al. Feringa, B. L. et al. Nature Chem. 2013,
Literature report Direct Catalytic Cross-Coupling of Organolithium Compounds Reporter: Zhang-Pei Chen Checker: Mu-Wang Chen Date: 02/07/2013 Feringa, B.L.et al. Feringa, B. L. et al. Nature Chem. 2013,
16. Chemistry of Benzene: Electrophilic Aromatic Substitution. Based on McMurry s Organic Chemistry, 7 th edition
 16. Chemistry of Benzene: Electrophilic Aromatic Substitution Based on McMurry s Organic Chemistry, 7 th edition Substitution Reactions of Benzene and Its Derivatives Benzene is aromatic: a cyclic conjugated
16. Chemistry of Benzene: Electrophilic Aromatic Substitution Based on McMurry s Organic Chemistry, 7 th edition Substitution Reactions of Benzene and Its Derivatives Benzene is aromatic: a cyclic conjugated
Triazabicyclodecene: an Effective Isotope. Exchange Catalyst in CDCl 3
 Triazabicyclodecene: an Effective Isotope Exchange Catalyst in CDCl 3 Supporting Information Cyrille Sabot, Kanduluru Ananda Kumar, Cyril Antheaume, Charles Mioskowski*, Laboratoire de Synthèse Bio-rganique,
Triazabicyclodecene: an Effective Isotope Exchange Catalyst in CDCl 3 Supporting Information Cyrille Sabot, Kanduluru Ananda Kumar, Cyril Antheaume, Charles Mioskowski*, Laboratoire de Synthèse Bio-rganique,
Aromatic Compounds II
 2302272 Org Chem II Part I Lecture 2 Aromatic Compounds II Instructor: Dr. Tanatorn Khotavivattana E-mail: tanatorn.k@chula.ac.th Recommended Textbook: Chapter 17 in Organic Chemistry, 8 th Edition, L.
2302272 Org Chem II Part I Lecture 2 Aromatic Compounds II Instructor: Dr. Tanatorn Khotavivattana E-mail: tanatorn.k@chula.ac.th Recommended Textbook: Chapter 17 in Organic Chemistry, 8 th Edition, L.
Alpha Substitution and Condensations of Enols and Enolate Ions. Alpha Substitution
 Alpha Substitution and ondensations of Enols and Enolate Ions hap 23 W: 27, 28, 30, 31, 37, 39, 42-44, 47, 51, 54-56 Alpha Substitution Replacement of a hydrogen on the carbon adjacent to the carbonyl,
Alpha Substitution and ondensations of Enols and Enolate Ions hap 23 W: 27, 28, 30, 31, 37, 39, 42-44, 47, 51, 54-56 Alpha Substitution Replacement of a hydrogen on the carbon adjacent to the carbonyl,
Chapter 16 Chemistry of Benzene: Electrophilic Aromatic Substitution
 John E. McMurry www.cengage.com/chemistry/mcmurry Chapter 16 Chemistry of Benzene: Electrophilic Aromatic Substitution Paul D. Adams University of Arkansas Substitution Reactions of Benzene and Its Derivatives
John E. McMurry www.cengage.com/chemistry/mcmurry Chapter 16 Chemistry of Benzene: Electrophilic Aromatic Substitution Paul D. Adams University of Arkansas Substitution Reactions of Benzene and Its Derivatives
Kinetic Isotope Effects
 1 Experiment 31 Kinetic Isotope Effects Isotopic substitution is a useful technique for the probing of reaction mechanisms. The change of an isotope may affect the reaction rate in a number of ways, providing
1 Experiment 31 Kinetic Isotope Effects Isotopic substitution is a useful technique for the probing of reaction mechanisms. The change of an isotope may affect the reaction rate in a number of ways, providing
Concise, Asymmetric, Stereocontrolled Total Synthesis of Stephacidins A, B and Notoamide B
 Concise, Asymmetric, Stereocontrolled Total Synthesis of Stephacidins A, B and otoamide B (+)-avrainvillamine (+)-notoamide B 20 51 21 55 (-)-stephacidin B In 2002, Bristol-Myers Squibb reported the biologically
Concise, Asymmetric, Stereocontrolled Total Synthesis of Stephacidins A, B and otoamide B (+)-avrainvillamine (+)-notoamide B 20 51 21 55 (-)-stephacidin B In 2002, Bristol-Myers Squibb reported the biologically
Professor Guy Quéguiner
 Professor Guy Quéguiner A Tribute Dedicated to Professor Guy Quéguiner on the occasion of his 70 th birthday and to honor his retirement from the head of the Institut de Recherche en Chimie Organique Fine
Professor Guy Quéguiner A Tribute Dedicated to Professor Guy Quéguiner on the occasion of his 70 th birthday and to honor his retirement from the head of the Institut de Recherche en Chimie Organique Fine
SUPPORTING INFORMATION
 Eur. J. Org. Chem. 2004 WILEY-VCH Verlag GmbH & Co. KGaA, 69451 Weinheim, 2004 ISSN 1434 193X SUPPORTING INFORMATION Title: The (Schiff base)vanadium(v) Complex Catalyzed Oxidation of Bromide A New Method
Eur. J. Org. Chem. 2004 WILEY-VCH Verlag GmbH & Co. KGaA, 69451 Weinheim, 2004 ISSN 1434 193X SUPPORTING INFORMATION Title: The (Schiff base)vanadium(v) Complex Catalyzed Oxidation of Bromide A New Method
Chapter 9 Aldehydes and Ketones
 Chapter 9 Aldehydes and Ketones 9.1 Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al The aldehyde functional group is always carbon
Chapter 9 Aldehydes and Ketones 9.1 Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al The aldehyde functional group is always carbon
Title of His Retirement) methyl] propenam. Author(s) Tanaka, Kazuhiko; Ushio, Hideki; Ho.
![Title of His Retirement) methyl] propenam. Author(s) Tanaka, Kazuhiko; Ushio, Hideki; Ho. Title of His Retirement) methyl] propenam. Author(s) Tanaka, Kazuhiko; Ushio, Hideki; Ho.](/thumbs/92/107930132.jpg) A New Synthetic Route to α-methylen Title Dianion of N-Phenyl-2-[ (phenylsulf Issue Dedicated to Professor Shinza of His Retirement) methyl] propenam Author(s) Tanaka, Kazuhiko; Ushio, Hideki; Ho Citation
A New Synthetic Route to α-methylen Title Dianion of N-Phenyl-2-[ (phenylsulf Issue Dedicated to Professor Shinza of His Retirement) methyl] propenam Author(s) Tanaka, Kazuhiko; Ushio, Hideki; Ho Citation
Electron-transfer Rearrangements of Dibenzodiazocine Derivatives: Effective Electron Traps for Organometallic Reactions
 The John Eisch Organometallic Symposium Binghamton University, NY Electron-transfer Rearrangements of Dibenzodiazocine Derivatives: Effective Electron Traps for Organometallic Reactions Renuka Manchanayakage
The John Eisch Organometallic Symposium Binghamton University, NY Electron-transfer Rearrangements of Dibenzodiazocine Derivatives: Effective Electron Traps for Organometallic Reactions Renuka Manchanayakage
Chapter 15. Reactions of Aromatic Compounds. Electrophilic Aromatic Substitution on Arenes. The first step is the slow, rate-determining step
 Electrophilic Aromatic Substitution on Arenes Chapter 15 Reactions of Aromatic Compounds The characteristic reaction of aromatic rings is substitution initiated by an electrophile halogenation nitration
Electrophilic Aromatic Substitution on Arenes Chapter 15 Reactions of Aromatic Compounds The characteristic reaction of aromatic rings is substitution initiated by an electrophile halogenation nitration
Synthetic Organic Chemistry Final Exam Resit (6KM33) Thursday, 26th June, 2014, 9:00 12:00 (3 h)
 Synthetic rganic Chemistry Final Exam Resit (6KM33) Thursday, 26th June, 2014, 9:00 12:00 (3 h) This is an open-book written exam. The course textbooks (Warren & Wyatt, 2 nd Ed.) with personal notes and
Synthetic rganic Chemistry Final Exam Resit (6KM33) Thursday, 26th June, 2014, 9:00 12:00 (3 h) This is an open-book written exam. The course textbooks (Warren & Wyatt, 2 nd Ed.) with personal notes and
Title. Author(s)Ishiyama, Tatsuo; Itoh, Yoshiya; Kitano, Takahiro; M. CitationTetrahedron Letters, 38(19): Issue Date
 Title Synthesis of arylboronates via the palladium(0)-cata triflates Author(s)Ishiyama, Tatsuo; Itoh, Yoshiya; Kitano, Takahiro; M CitationTetrahedron Letters, 38(19): 3447-3450 Issue Date 1997-05-12 Doc
Title Synthesis of arylboronates via the palladium(0)-cata triflates Author(s)Ishiyama, Tatsuo; Itoh, Yoshiya; Kitano, Takahiro; M CitationTetrahedron Letters, 38(19): 3447-3450 Issue Date 1997-05-12 Doc
REACTION AND SYNTHESIS REVIEW
 REACTION AND SYNTHESIS REVIEW A STUDENT SHOULD BE ABLE TO PREDICT PRODUCTS, IDENTIFY REACTANTS, GIVE REACTION CONDITIONS, PROPOSE SYNTHESES, AND PROPOSE MECHANISMS (AS LISTED BELOW). REVIEW THE MECHANISM
REACTION AND SYNTHESIS REVIEW A STUDENT SHOULD BE ABLE TO PREDICT PRODUCTS, IDENTIFY REACTANTS, GIVE REACTION CONDITIONS, PROPOSE SYNTHESES, AND PROPOSE MECHANISMS (AS LISTED BELOW). REVIEW THE MECHANISM
Module9. Nuclear Magnetic Resonance Spectroscopy Nuclear Magnetic Resonance (NMR) spectroscopy - Chemical shift - Integration of signal area
 1 CHEMISTRY 263 HOME WORK Lecture Topics: Module7. Hydrogenation of Alkenes The Function of the Catalyst - Syn and anti- addition Hydrogenation of Alkynes - Syn- addition of hydrogen: Synthesis of cis-alkenes
1 CHEMISTRY 263 HOME WORK Lecture Topics: Module7. Hydrogenation of Alkenes The Function of the Catalyst - Syn and anti- addition Hydrogenation of Alkynes - Syn- addition of hydrogen: Synthesis of cis-alkenes
CHEM 2410 Principles of Organic Chemistry I Summer Instructor: Paul Bracher. Quiz # 4
 CHEM 2410 Principles of Organic Chemistry I Summer 2016 Quiz # 4 Solutions Key Page 1 of 6 CHEM 2410 Principles of Organic Chemistry I Summer 2016 Instructor: Paul Bracher Quiz # 4 Due: Sunday, June 19
CHEM 2410 Principles of Organic Chemistry I Summer 2016 Quiz # 4 Solutions Key Page 1 of 6 CHEM 2410 Principles of Organic Chemistry I Summer 2016 Instructor: Paul Bracher Quiz # 4 Due: Sunday, June 19
1/4/2011. Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds
 Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds The Acidity of the Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons to carbonyls are unusually acidic
Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds The Acidity of the Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons to carbonyls are unusually acidic
I. Introduction. Peng Zhao. Liu lab
 Asymmetric Total Synthesis of Mycoleptodiscin A Shupeng Zhou, Hao Chen, Yijie Luo, Wenhao Zhang and Ang Li Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai
Asymmetric Total Synthesis of Mycoleptodiscin A Shupeng Zhou, Hao Chen, Yijie Luo, Wenhao Zhang and Ang Li Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai
Efficient route to 3-methoxymethylquinoline A precursor of 5- methoxymethylquinolinic acid
 Issue in onor of Prof. Charles ees AKIVC 2002 (vi) 257-263 Efficient route to 3-methoxymethylquinoline A precursor of 5- methoxymethylquinolinic acid Joel. Calvin, Gregory F. illstrom, Julie olland, Paul
Issue in onor of Prof. Charles ees AKIVC 2002 (vi) 257-263 Efficient route to 3-methoxymethylquinoline A precursor of 5- methoxymethylquinolinic acid Joel. Calvin, Gregory F. illstrom, Julie olland, Paul
Andrew Yeung CHEM636
 Andrew Yeung CHEM636 Scope verview olyketones and their synthesis Timeline of development Catalyst selection alladium vs. nickel Ligands Mechanism Initiation & termination ropagation roperties Low T g
Andrew Yeung CHEM636 Scope verview olyketones and their synthesis Timeline of development Catalyst selection alladium vs. nickel Ligands Mechanism Initiation & termination ropagation roperties Low T g
CHEMISTRY 332 FALL 08 EXAM II October 22-23, 2008
 First Three Letters of Last Name NAME Network ID CHEMISTRY 332 FALL 08 EXAM II October 22-23, 2008 The following materials are permissible during the exam: molecular model kits, course notes (printed,
First Three Letters of Last Name NAME Network ID CHEMISTRY 332 FALL 08 EXAM II October 22-23, 2008 The following materials are permissible during the exam: molecular model kits, course notes (printed,
Oxidative couplings of two nucleophiles
 Oxidative Couplings of Hydrocarbons Oxidative couplings of two nucleophiles Oxidants involved: O 2 H 2 O 2 high h valent metals(copper salts) halides(iodine(Ⅲ) oxidants) Lei, A. W. Chem. Rev., 2011, 111,
Oxidative Couplings of Hydrocarbons Oxidative couplings of two nucleophiles Oxidants involved: O 2 H 2 O 2 high h valent metals(copper salts) halides(iodine(Ⅲ) oxidants) Lei, A. W. Chem. Rev., 2011, 111,
General Papers ARKIVOC 2006 (xii)
 Synthesis of some 1-aryl-3,5-disubstituted-pyrazoles by -arylation of 3,5-disubstituted-pyrazoles with 4-fluoro and 2-fluoronitrobenzene under microwave irradiation and classical heating Ibrahim Bouabdallah,
Synthesis of some 1-aryl-3,5-disubstituted-pyrazoles by -arylation of 3,5-disubstituted-pyrazoles with 4-fluoro and 2-fluoronitrobenzene under microwave irradiation and classical heating Ibrahim Bouabdallah,
C C H 2. O p-tsoh (catalytic) H 3 O +! O
 ame Key 15 W06-Exam o. Page I. ( points) Pheromones are those chemicals that play a role in communication between individual animals or insects. Structure 1 is the pheromone of the Japanese peach moth
ame Key 15 W06-Exam o. Page I. ( points) Pheromones are those chemicals that play a role in communication between individual animals or insects. Structure 1 is the pheromone of the Japanese peach moth
Thesis of the PhD dissertation. Forintos Henrietta. Supervisor: Prof. Keglevich György Head of the Departement
 Thesis of the hd dissertation REACTIN F ETERCYCLIC TRIALKYLENYLSINE XIDES AND DIMETYL ACETYLENEDICARBXYLATE Forintos enrietta Supervisor: rof. Keglevich György ead of the Departement Research Group of
Thesis of the hd dissertation REACTIN F ETERCYCLIC TRIALKYLENYLSINE XIDES AND DIMETYL ACETYLENEDICARBXYLATE Forintos enrietta Supervisor: rof. Keglevich György ead of the Departement Research Group of
Supporting Information
 Supporting Information Incorporation of a Sugar Unit into a C C N Pincer Pd Complex Using Click Chemistry and Its Dynamic Behavior in Solution and Catalytic Ability toward the Suzuki Miyaura Coupling in
Supporting Information Incorporation of a Sugar Unit into a C C N Pincer Pd Complex Using Click Chemistry and Its Dynamic Behavior in Solution and Catalytic Ability toward the Suzuki Miyaura Coupling in
Palladium-catalyzed cross-addition of triisopropylsilylacetylene to unactivated alkynes*
 Pure Appl. Chem., Vol. 80, No. 5, pp. 1161 1166, 2008. doi:10.1351/pac200880051161 2008 IUPAC Palladium-catalyzed cross-addition of triisopropylsilylacetylene to unactivated alkynes* Naofumi Tsukada, Satoshi
Pure Appl. Chem., Vol. 80, No. 5, pp. 1161 1166, 2008. doi:10.1351/pac200880051161 2008 IUPAC Palladium-catalyzed cross-addition of triisopropylsilylacetylene to unactivated alkynes* Naofumi Tsukada, Satoshi
Chapter 3 Alkenes and Alkynes. Excluded sections 3.15&3.16
 Chapter 3 Alkenes and Alkynes Excluded sections 3.15&3.16 3.1 Definition and Classification Alkene: a hydrocarbon that contains one or more carboncarbon double bonds. ethylene is the simplest alkene. Alkyne:
Chapter 3 Alkenes and Alkynes Excluded sections 3.15&3.16 3.1 Definition and Classification Alkene: a hydrocarbon that contains one or more carboncarbon double bonds. ethylene is the simplest alkene. Alkyne:
CHEM Chapter 23. Carbonyl Condensation Reactions (quiz) W25
 CHEM 2425. Chapter 23. Carbonyl Condensation Reactions (quiz) W25 Student: 1. Which of the following statements about Aldol reactions with either aldehydes or ketones is true? Equilibrium favors the starting
CHEM 2425. Chapter 23. Carbonyl Condensation Reactions (quiz) W25 Student: 1. Which of the following statements about Aldol reactions with either aldehydes or ketones is true? Equilibrium favors the starting
Organic Chemistry. Second Edition. Chapter 19 Aromatic Substitution Reactions. David Klein. Klein, Organic Chemistry 2e
 Organic Chemistry Second Edition David Klein Chapter 19 Aromatic Substitution Reactions Copyright 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2e 19.1 Introduction to Electrophilic
Organic Chemistry Second Edition David Klein Chapter 19 Aromatic Substitution Reactions Copyright 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2e 19.1 Introduction to Electrophilic
Anomalous metathesis reactions of 3,4-disubstituted indoles
 Anomalous metathesis reactions of 3,4-disubstituted indoles Leticia Pérez-Serrano, Gema Domínguez, and Javier Pérez-Castells* Departamento de Química, Facultad de Ciencias Experimentales y de la Salud.
Anomalous metathesis reactions of 3,4-disubstituted indoles Leticia Pérez-Serrano, Gema Domínguez, and Javier Pérez-Castells* Departamento de Química, Facultad de Ciencias Experimentales y de la Salud.
Department of Chemistry, University of Saskatchewan Saskatoon SK S7N 4C9, Canada. Wipf Group. Tyler E. Benedum Current Literature February 26, 2005
 Ward, D.E; Jheengut, V.; Akinnusi, O.T. Enantioselective Direct Intermolecular Aldol Reactions with Enantiotopic Group Selectivity and Dynamic Kinetic Resolution, Organic Letters 2005, ASAP. Department
Ward, D.E; Jheengut, V.; Akinnusi, O.T. Enantioselective Direct Intermolecular Aldol Reactions with Enantiotopic Group Selectivity and Dynamic Kinetic Resolution, Organic Letters 2005, ASAP. Department
Microwave-promoted synthesis in water
 Microwave-promoted synthesis in water icholas E. Leadbeater nicholas.leadbeater@uconn.edu Outline of what we do Synthesis / Methodology / ew techniques Pure organic synthesis eg. Baylis-Hillman eaction
Microwave-promoted synthesis in water icholas E. Leadbeater nicholas.leadbeater@uconn.edu Outline of what we do Synthesis / Methodology / ew techniques Pure organic synthesis eg. Baylis-Hillman eaction
Nickel-Catalyzed Three-Component [3+2+2] Cocyclization of Ethyl Cyclopropylideneacetate and Alkynes
![Nickel-Catalyzed Three-Component [3+2+2] Cocyclization of Ethyl Cyclopropylideneacetate and Alkynes Nickel-Catalyzed Three-Component [3+2+2] Cocyclization of Ethyl Cyclopropylideneacetate and Alkynes](/thumbs/77/74720708.jpg) Nickel-Catalyzed Three-Component [3+2+2] Cocyclization of Ethyl Cyclopropylideneacetate and Alkynes Selective Synthesis of Multisubstituted Cycloheptadienes 1 2 Cat. Ni 0 1 2 Komagawa, S.; Saito, S. Angew.
Nickel-Catalyzed Three-Component [3+2+2] Cocyclization of Ethyl Cyclopropylideneacetate and Alkynes Selective Synthesis of Multisubstituted Cycloheptadienes 1 2 Cat. Ni 0 1 2 Komagawa, S.; Saito, S. Angew.
Chapter 16 Aldehydes and Ketones I. Nucleophilic Addition to the Carbonyl Group
 Chapter 16 Aldehydes and Ketones I. Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
Chapter 16 Aldehydes and Ketones I. Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
CHEM 343 Principles of Organic Chemistry II Summer Instructor: Paul J. Bracher. Quiz # 3. Monday, July 21 st, :30 a.m.
 CHEM 343 Principles of Organic Chemistry II Summer 2014 Quiz # 3 Solutions Key Page 1 of 9 CHEM 343 Principles of Organic Chemistry II Summer 2014 Instructor: Paul J. Bracher Quiz # 3 Monday, July 21 st,
CHEM 343 Principles of Organic Chemistry II Summer 2014 Quiz # 3 Solutions Key Page 1 of 9 CHEM 343 Principles of Organic Chemistry II Summer 2014 Instructor: Paul J. Bracher Quiz # 3 Monday, July 21 st,
RESEARCH REPOSITORY.
 RESEARCH REPSITRY This is the author s final version of the work, as accepted for publication following peer review but without the publisher s layout or pagination. The definitive version is available
RESEARCH REPSITRY This is the author s final version of the work, as accepted for publication following peer review but without the publisher s layout or pagination. The definitive version is available
Chemoselective deprotonative lithiation of azobenzenes. Reactions and mechanisms
 Chemoselective deprotonative lithiation of azobenzenes. Reactions and mechanisms Supporting Information Thi Thanh Thuy Nguyen, Anne Boussonnière, Estelle Banaszak, Anne-Sophie Castanet, Kim Phi Phung Nguyen,
Chemoselective deprotonative lithiation of azobenzenes. Reactions and mechanisms Supporting Information Thi Thanh Thuy Nguyen, Anne Boussonnière, Estelle Banaszak, Anne-Sophie Castanet, Kim Phi Phung Nguyen,
SECTION 3. Antiperiplanar Hypothesis. and Reactions at Unsaturated Systems
 SECTION 3 Antiperiplanar Hypothesis and Reactions at Unsaturated Systems (2016) 1 Reaction on Sp 2 Type Unsaturated System In cyclic ketone, both processes lead to a chair but we will see that C-H and
SECTION 3 Antiperiplanar Hypothesis and Reactions at Unsaturated Systems (2016) 1 Reaction on Sp 2 Type Unsaturated System In cyclic ketone, both processes lead to a chair but we will see that C-H and
Unexpected dimerization reaction of 5-methyl-6,7-methylendioxy-1- tetralone in the presence of TMSCl-ethylene glycol
 Issue in Honor of Prof. Joan Bosch ARKIVC 2007 (iv) 82-87 Unexpected dimerization reaction of 5-methyl-6,7-methylendioxy-- tetralone in the presence of TMSCl-ethylene glycol Gema Esteban and Joaquín Plumet
Issue in Honor of Prof. Joan Bosch ARKIVC 2007 (iv) 82-87 Unexpected dimerization reaction of 5-methyl-6,7-methylendioxy-- tetralone in the presence of TMSCl-ethylene glycol Gema Esteban and Joaquín Plumet
Chapter 16 Aldehydes and Ketones I Nucleophilic Addition to the Carbonyl Group
 Chapter 16 Aldehydes and Ketones I Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
Chapter 16 Aldehydes and Ketones I Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
ONE POT SYNTHESIS OF NITRILES FROM ALDEHYDES AND HYDROXYLAMINE HYDROCHLORIDE USING SODIUM SULPHATE IN DMF UNDER REFLUX CONDITION
 WORLD JOURNAL OF PHARMACY AND PHARMACEUTICAL SCIENCES Dinanath et al. Volume 3, Issue 1, 592-596. Research Article ISSN 2278 4357 ONE POT SYNTHESIS OF NITRILES FROM ALDEHYDES AND HYDROXYLAMINE HYDROCHLORIDE
WORLD JOURNAL OF PHARMACY AND PHARMACEUTICAL SCIENCES Dinanath et al. Volume 3, Issue 1, 592-596. Research Article ISSN 2278 4357 ONE POT SYNTHESIS OF NITRILES FROM ALDEHYDES AND HYDROXYLAMINE HYDROCHLORIDE
Reversible Additions to carbonyls: Weak Nucleophiles Relative Reactivity of carbonyls: Hydration of Ketones and Aldehydes
 Reversible Additions to carbonyls: Weak Nucleophiles Weak nucleophiles, such as water, alcohols, and amines, require acid or base catalysis to undergo addition to carbonyl compounds Relative Reactivity
Reversible Additions to carbonyls: Weak Nucleophiles Weak nucleophiles, such as water, alcohols, and amines, require acid or base catalysis to undergo addition to carbonyl compounds Relative Reactivity
Technical Note. Introduction
 Technical Note Characterization of Eleven 2,5-Dimethoxy-N-(2-methoxybenzyl)- phenethylamine (NBOMe) Derivatives and Differentiation from their 3- and 4- Methoxybenzyl Analogues - Part II Patrick A. Hays*,
Technical Note Characterization of Eleven 2,5-Dimethoxy-N-(2-methoxybenzyl)- phenethylamine (NBOMe) Derivatives and Differentiation from their 3- and 4- Methoxybenzyl Analogues - Part II Patrick A. Hays*,
Aldehydes and Ketones
 9 Aldehydes and Ketones hapter Summary The carbonyl group, =, is present in both aldehydes (=) and ketones ( 2 =). The IUPA ending for naming aldehydes is -al, and numbering begins with the carbonyl carbon.
9 Aldehydes and Ketones hapter Summary The carbonyl group, =, is present in both aldehydes (=) and ketones ( 2 =). The IUPA ending for naming aldehydes is -al, and numbering begins with the carbonyl carbon.
C H activation of aliphatic amines without unnecessary mask M2 Takaya Togo
 C H activation of aliphatic amines without unnecessary mask 2017.11.25 M2 Takaya Togo 1 Outline 1.Introduction 2.Free amines as DG Discovery of new activation mode Mechanistic studies Application of the
C H activation of aliphatic amines without unnecessary mask 2017.11.25 M2 Takaya Togo 1 Outline 1.Introduction 2.Free amines as DG Discovery of new activation mode Mechanistic studies Application of the
Boron containing 1,1-Dimetallicalkane Reagents
 Boron containing 1,1-Dimetallicalkane Reagents Topic Talk Xun Liu July 25, 2013 Outline 1. 1,1-Borio-lithioalkane reagents 2. 1,1-Boronalkane reagents 3. 1,1-Borio-zincioalkane Reagents and 1,1-Borio-cuprioalkane
Boron containing 1,1-Dimetallicalkane Reagents Topic Talk Xun Liu July 25, 2013 Outline 1. 1,1-Borio-lithioalkane reagents 2. 1,1-Boronalkane reagents 3. 1,1-Borio-zincioalkane Reagents and 1,1-Borio-cuprioalkane
Chapter 17 Reactions of Aromatic Compounds
 rganic Chemistry, 6 th Edition L. G. Wade, Jr. Chapter 17 Reactions of Aromatic Compounds Jo Blackburn Richland College, Dallas, TX Dallas County Community College District 2006, Prentice all Electrophilic
rganic Chemistry, 6 th Edition L. G. Wade, Jr. Chapter 17 Reactions of Aromatic Compounds Jo Blackburn Richland College, Dallas, TX Dallas County Community College District 2006, Prentice all Electrophilic
ELECTRONIC SUPPLEMENTARY INFORMATION
 Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 2017 ELECTRONIC SUPPLEMENTARY INFORMATION Non- symmetric, potentially redox non- innocent
Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 2017 ELECTRONIC SUPPLEMENTARY INFORMATION Non- symmetric, potentially redox non- innocent
Química Orgânica I. Ciências Farmacêuticas Bioquímica Química AFB QO I 2007/08 1
 Química rgânica I Ciências Farmacêuticas Bioquímica Química AFB Q I 2007/08 1 alcohols Adaptado de rganic Chemistry, 6th Edition; Wade rganic Chemistry, 6 th Edition; McMurry AFB Q I 2007/08 2 Typical
Química rgânica I Ciências Farmacêuticas Bioquímica Química AFB Q I 2007/08 1 alcohols Adaptado de rganic Chemistry, 6th Edition; Wade rganic Chemistry, 6 th Edition; McMurry AFB Q I 2007/08 2 Typical
Use of the non-aldol aldol process in the synthesis of the C1 C11 fragment of the tedanolides: use of lactol ethers in place of tetrahydrofurans
 Pergamon Tetrahedron Letters 41 (2000) 9719 9723 TETRAHEDRON LETTERS Use of the non-aldol aldol process in the synthesis of the C1 C11 fragment of the tedanolides: use of lactol ethers in place of tetrahydrofurans
Pergamon Tetrahedron Letters 41 (2000) 9719 9723 TETRAHEDRON LETTERS Use of the non-aldol aldol process in the synthesis of the C1 C11 fragment of the tedanolides: use of lactol ethers in place of tetrahydrofurans
Chem 263 Nov 14, e.g.: Fill the reagents to finish the reactions (only inorganic reagents)
 hem 263 ov 14, 2013 More examples: e.g.: Fill the reagents to finish the reactions (only inorganic reagents) Br 2 hv Br a 2 r 4 S 2 or swern oxidation Mg Li 0 0 MgBr Li e.g. : Fill the reagents (any reagents
hem 263 ov 14, 2013 More examples: e.g.: Fill the reagents to finish the reactions (only inorganic reagents) Br 2 hv Br a 2 r 4 S 2 or swern oxidation Mg Li 0 0 MgBr Li e.g. : Fill the reagents (any reagents
MECHANISM OF RIBOSYLATION OF ADENINE
 OC8 Ribosylation of Adenine 177 MECHANISM OF RIBOSYLATION OF ADENINE Jerzy BORYSKI* and Grzegorz FRAMSKI Institute of Bioorganic Chemistry, Polish Academy of Sciences, Noskowskiego 12/14, PL-61704, Poznań,
OC8 Ribosylation of Adenine 177 MECHANISM OF RIBOSYLATION OF ADENINE Jerzy BORYSKI* and Grzegorz FRAMSKI Institute of Bioorganic Chemistry, Polish Academy of Sciences, Noskowskiego 12/14, PL-61704, Poznań,
Enantioselective Borylations. David Kornfilt Denmark Group Meeting Sept. 14 th 2010
 Enantioselective Borylations David Kornfilt Denmark Group Meeting Sept. 14 th 2010 30.000-foot View Enantioenriched Organoboranes What to do with them Crudden C. M. et. al., Eur. J. Org. Chem. 2003, 46
Enantioselective Borylations David Kornfilt Denmark Group Meeting Sept. 14 th 2010 30.000-foot View Enantioenriched Organoboranes What to do with them Crudden C. M. et. al., Eur. J. Org. Chem. 2003, 46
Chem 263 Oct. 10, The strongest donating group determines where new substituents are introduced.
 Chem 263 ct. 10, 2013 The strongest donating group determines where new substituents are introduced. N 2 N 3 2 S 4 + N 3 N 2 2 S 4 N 2 N 2 + 2 N N 2 N 3 2 S 4 N 2 2 N N 2 2,4,6-trinitrophenol picric acid
Chem 263 ct. 10, 2013 The strongest donating group determines where new substituents are introduced. N 2 N 3 2 S 4 + N 3 N 2 2 S 4 N 2 N 2 + 2 N N 2 N 3 2 S 4 N 2 2 N N 2 2,4,6-trinitrophenol picric acid
Phosphine-Catalyzed Formation of Carbon-Sulfur Bonds: Catalytic Asymmetric Synthesis of gamma-thioesters
 Phosphine-Catalyzed Formation of Carbon-Sulfur Bonds: Catalytic Asymmetric Synthesis of gamma-thioesters The MIT Faculty has made this article openly available. Please share how this access benefits you.
Phosphine-Catalyzed Formation of Carbon-Sulfur Bonds: Catalytic Asymmetric Synthesis of gamma-thioesters The MIT Faculty has made this article openly available. Please share how this access benefits you.
Accepted Manuscript. One-step double reduction of aryl nitro and carbonyl groups using hydrazine. Elena Diez-Cecilia, Brendan Kelly, Isabel Rozas
 Accepted Manuscript One-step double reduction of aryl nitro and carbonyl groups using hydrazine Elena Diez-Cecilia, Brendan Kelly, Isabel Rozas PII: S0040-4039(11)01655-8 DOI: 10.1016/j.tetlet.2011.09.112
Accepted Manuscript One-step double reduction of aryl nitro and carbonyl groups using hydrazine Elena Diez-Cecilia, Brendan Kelly, Isabel Rozas PII: S0040-4039(11)01655-8 DOI: 10.1016/j.tetlet.2011.09.112
Iron Catalysed Coupling Reactions
 LONG LITERATURE REPORT Iron Catalysed Coupling Reactions Mingyu Liu 2017. 8. 31 1 Fe [Ar]3d 6 4s 2 The fourth most common element in the Earth s crust Relatively less understanding and manipulation of
LONG LITERATURE REPORT Iron Catalysed Coupling Reactions Mingyu Liu 2017. 8. 31 1 Fe [Ar]3d 6 4s 2 The fourth most common element in the Earth s crust Relatively less understanding and manipulation of
16. Chemistry of Benzene: Electrophilic Aromatic Substitution جانشینی الکتروندوستی آروماتیک شیمی آلی 2
 16. Chemistry of Benzene: Electrophilic Aromatic Substitution جانشینی الکتروندوستی آروماتیک شیمی آلی 2 Dr M. Mehrdad University of Guilan, Department of Chemistry, Rasht, Iran m-mehrdad@guilan.ac.ir Based
16. Chemistry of Benzene: Electrophilic Aromatic Substitution جانشینی الکتروندوستی آروماتیک شیمی آلی 2 Dr M. Mehrdad University of Guilan, Department of Chemistry, Rasht, Iran m-mehrdad@guilan.ac.ir Based
Spring Term 2012 Dr. Williams (309 Zurn, ex 2386)
 Chemistry 242 Organic Chemistry II Spring Term 2012 Dr. Williams (309 Zurn, ex 2386) Web Page: http://math.mercyhurst.edu/~jwilliams/ jwilliams@mercyhurst.edu (or just visit Department web site and look
Chemistry 242 Organic Chemistry II Spring Term 2012 Dr. Williams (309 Zurn, ex 2386) Web Page: http://math.mercyhurst.edu/~jwilliams/ jwilliams@mercyhurst.edu (or just visit Department web site and look
Chem 251 Fall Learning Objectives
 Learning Objectives Chapter 8 (last semester) 1. Write an electron-pushing mechanism for an SN2 reaction between an alkyl halide and a nucleophile. 2. Describe the rate law and relative rate of reaction
Learning Objectives Chapter 8 (last semester) 1. Write an electron-pushing mechanism for an SN2 reaction between an alkyl halide and a nucleophile. 2. Describe the rate law and relative rate of reaction
Metal-assisted amination with oxime derivatives*
 Pure Appl. Chem., Vol. 74, No. 1, pp. 143 149, 2002. 2002 IUPAC Metal-assisted amination with oxime derivatives* Koichi Narasaka Department of Chemistry, Graduate School of Science, The University of Tokyo,
Pure Appl. Chem., Vol. 74, No. 1, pp. 143 149, 2002. 2002 IUPAC Metal-assisted amination with oxime derivatives* Koichi Narasaka Department of Chemistry, Graduate School of Science, The University of Tokyo,
Chem 112A: Final Exam
 Chem 112A: Final Exam December 15th, 2010 Please provide all answers in the spaces provided. You are not allowed to use a calculator for this exam, but you may use molecular model kits. nly cyclohexane
Chem 112A: Final Exam December 15th, 2010 Please provide all answers in the spaces provided. You are not allowed to use a calculator for this exam, but you may use molecular model kits. nly cyclohexane
