Year 09 Science Learning Cycle 3 Overview
|
|
|
- Beverley Little
- 5 years ago
- Views:
Transcription
1 Year 09 Science Learning Cycle 3 Overview Learning Cycle Overview: Biology Hypothesis 1 Hypothesis 2 Hypothesis 3 Hypothesis 4 Hypothesis 5 Hypothesis 6 Hypothesis 7 Hypothesis 8 Hypothesis 9 Hypothesis 10 What are the basic processes that power living things? Diffusion Osmosis REQUIRED PRACTICAL Osmosis Active transport Plant tissues Plant organ system Photosynthetic reaction Rate of Photosynthesis Uses of glucose from photosynthesis REQUIRED PRACTICAL Photosynthesis Week 1-2 Chemistry Hypothesis 11 Hypothesis 12 Hypothesis 13 Hypothesis 14 Hypothesis 15 Hypothesis 16 Hypothesis 17 Hypothesis 18 How do different chemical bonds affect a substance? Properties of ionic compounds Properties of small molecules Polymers Giant covalent structures Properties of metals and alloys Metals as conductors Diamond Graphite Graphene and fullerenes Week 3-4
2 Physics How is electrical energy transferred? Hypothesis Standard circuit diagram symbols Hypothesis Electrical charge and current Direct and alternating potential difference Hypothesis Current, resistance and potential difference Week 4-5 Hypothesis Resistors Hypothesis Series and parallel circuits Hypothesis 24 REQUIRED PRACTICAL I-V Characteristics Hypothesis 25 REQUIRED PRACTICAL Resistance *Lessons in grey are higher tier only **Lessons notated RP contain a required practical N.B. For required practicals, see Science/Planning Resources/Required Practicals Combined Science for more details.
3 Biology: What are the basic processes that power living things? Intentions for learning from AQA GCSE Specification pages Lesson 1: Diffusion goes against the concentration gradient Keywords: Diffusion, concentration, membrane Students should develop an understanding that: Diffusion is the movement of particles Recall that substances move in and out of cells Describe how substances diffuse in and out of cells Explain what factors impact the rate of diffusion Teacher marked Lesson 2: Osmosis keeps plants alive Keywords: Osmosis, partially permeable membrane, concentration Osmosis is the movement of water from a high to low concentration across a partially permeable membrane Recall the definition of osmosis Describe the impact osmosis has on cell shape Explain the impact a solvent has on osmosis - Lesson 3: (RP) Osmosis can create giant potatoes Keywords: Osmosis, concentration, molar Solutions of different concentrations will cause potatoes to change in shape Recall the independent and dependent variables HIIII Predict the outcomes of the experiment Present and analyse results using graphs Lesson 4: Cells need mitochondria for active transport to take place Keywords: Active transport, energy, mitochondria Active transport is the movement of substances from a low to high concentration, this requires energy. Recall the definition of active transport Describe where active transport takes place Evaluate the difference between diffusion, osmosis and active transport Lesson 5: Plants have as many organs have humans Keywords: epidermis, mesophyll, palisade, stomata, xylem, phloem The different structures of the leaf allow the leaf to carry out its function Recall the parts of the leaf Describe the functions of the leaf Explain how the structures of the leaf allow the leaf to carry out its function TEACHER MARKED Lesson 6: Plant organs make organ systems Keywords: transpiration, translocation How the structures of the root hair cells, xylem and phloem are adapted to their functions Recall the organs of a plant Describe translocation and transpiration Explain how temperature, humidity, air movement and Lesson 7: Photosynthesis is how humans create their energy Keywords: Photosynthesis, chloroplast, endothermic Students should develop an understanding that: Plants use light to create glucose via photosynthesis Recall the photosynthesis equation Describe where each aspect of the photosynthesis equation comes from Explain why photosynthesis is an endothermic reaction - Lesson 8: Photosynthesis always takes place at the same rate Keywords: Photosynthesis, rate, factor The rate of photosynthesis changes depending on temperature, light intensity, carbon dioxide concentration, and the amount of chlorophyll. Recall what a limiting factor is Describe the factors which can limit the rate of photosynthesis Lesson 9: Glucose helps keep cell walls strong Keywords: Glucose, cellulose, starch, amino acids Glucose produced from photosynthesis can be stored as starch, oil, cellulose and for amino acids Recall the products of photosynthesis Describe how glucose is used in plants Explain what else is needed by the plant Lesson 10: (RP) Light can speed up photosynthesis Keywords: Light intensity, photosynthesis, rate By increasing the light intensity the rate of photosynthesis will increase. Recall what factors can impact the rate of photosynthesis Predict what happens to the rate of photosynthesis as the light intensity changes
4 light intensity impact the rate of transpiration Explain how the limiting factors impact the rate of photosynthesis Accurately carry out the practical and record results Chemistry: How do different chemical bonds affect a substance? Intentions for learning from AQA GCSE Specification : Pages Lesson 1: Ionic compounds can conduct electricity Keywords: solid, lattice, giant, boiling point, conduct Ionic compounds form giant ionic lattice structures and hence have specific physical properties Recall how an ionic bond forms Describe the structure of ionic bonds using scientific vocabulary Explain how this structure impacts the physical properties of covalent compound Lesson 2: Small covalent molecules have strong forces Keywords: intermolecular forces Simple molecules have weak intermolecular forces between the molecules that give them specific properties Recall how a covalent bonds forms Describe the structure of small covalent molecules Explain why small covalent molecules are gases Evaluate if covalent substances have strong or weak forces Lesson 3: All covalent compounds are gases Keywords: polymer, monomer Polymers are chains of covalent compounds Recall what a polymer is made from Recognise polymers from diagrams Describe the structure of a polymer Predict the properties of polymers based on their structure Lesson 4: Giant covalent compounds have more forces Keywords: giant, boiling point, silica Covalent compounds can form giant structures Recall what is meant by a giant covalent structure Describe a giant covalent structure Predict the properties of giant covalent structures based Lesson 5: Diamonds are fragile Keywords: strong, covalent, carbon, Lesson 6: Graphite is similar to diamond Keywords: fullerenes, graphite, slide, graphene Lesson 7: All metals are hard Keywords: delocalised, ions, slide, alloy Lesson 8: Metals conduct because of their ions Keywords: delocalised, electrons, current Diamond is made of carbon bonded to four other carbons Recall what diamond is made of Describe how diamond is structured in terms of bonding Predict the properties of diamond based on their structure Graphite, graphene and fullerenes can be used as lubricants and conductors due to their structures Recall the structure of graphite Describe the properties of graphite Explain the properties of graphite Explain the uses of graphene Evaluate the uses of fullerenes Alloys are metals with different sized ions dispersed within the layers of ions so they can t slide over each other Describe the properties of metals Explain the properties of metals in terms of ions and electrons Explain why alloys have different properties compared to pure metals Metals conduct electricity due to their electrons being able to move Recall what an electric current Describe how metals can conduct electricity Explain why covalent and ionic compounds can not conduct electricity
5 Physics: How can we measure something we can t see? Intentions for learning from AQA GCSE Specification : Pages Lesson 1: Photos of circuits are better than hand drawn diagrams Keywords: components, diagram, symbols Circuits can be drawn using scientific standard diagrams These diagrams ensure a continuity between professionals involved with electronics Recall circuit symbols. Construct circuit diagrams using standard symbols. Ask questions such as: Why are circuit symbols used? TEACHER MARKED DISTANCE/DISPLACEMENT QUESTION Lesson 2: There is one model for electricity that is better than the rest Keywords: potential difference, current, models Electric charge is carried around the circuit and we can measure this flow practically Define potential difference. State the name of the particle that carries the electrical charge round a circuit. Define an electric current. Describe and explain why an electric current will flow in a circuit. Describe different models of electricity Evaluate the benefits and drawbacks of each model. Calculate the charge flow, current or time when given the other two values. State the units used for each quantity. PEER MARKED Q=Ixt QUESTIONS Lesson 3: Current and voltage are directly proportional Keywords: current, resistance, potential difference Current, potential difference and voltage can be linked with an equation. Resistance is something we try to limit in many of our electrical devices Draw a circuit that can be used to measure the current in a component. Describe how the current varies in a series circuit. Explain why the current at each point in a series circuit must be the same in terms of electrons not being lost from the wire. Define resistance. Describe and explain how increasing the resistance in a circuit will affect the current through the circuit. Use the equation to calculate the potential difference (voltage), current or resistance when given the other two values. How does the type of metal used for a wire affect its resistance? Why do expensive scart leads have gold plating on them? What factors affect the resistance of a given length of wire? Draw a circuit that can be used to find the resistance of an electrical component using a voltmeter and an ammeter. SELF MARKED V=IR QUESTIONS Lesson 4: Ohm s law only applies to some materials Keywords: Ohm s law, temperature, conductor Resistors obey Ohm s law and can be predictable up to a point Define what is meant by an ohmic conductor. What components are ohmic conductors? Describe the conditions for which Ohm s law is valid. Explain why Ohm s law is not valid when the temperature of the conductor increases in terms of collisions.
6 Lesson 5: Christmas lights are connected in parallel to one another Keywords: series, parallel Series and parallel circuits have different properties. Components also behave differently in the two types of circuits Describe the differences between series and parallel circuits. Draw circuit diagrams for components connected in series and in parallel. Describe how ammeters and voltmeters are connected into a circuit Why does adding additional lamps in series, make them all dimmer? Explain why the current through each component in a series circuit is the same. Why does adding more lamps in series cause the current to decrease? Describe how the potential difference of the power supply is shared between the components and that the share of the potential difference a component receives depends on the resistance of that component. TEACHER MARKED SERIES/PARALLEL QUESTION Lesson 6: REQUIRED PRACTICAL I-V Characteristics Keywords: graph, filament, diode, thermistor Draw the I-V graph for an ohmic conductor, filament lamp, diode, LDR, thermistor Why do the current-potential difference graphs for diodes and filament lamps look different to that of an ohmic conductor? Calculate the resistance of an LDR or a thermistor given the range of resistances for that component and the conditions that it is placed in. Describe and explain real world applications of thermistors and LDRs including thermostats and switching on lights. Lesson 7: REQUIRED PRACTICAL Resistance Keywords: parallel, series Calculate the resistance or two components in a circuit using Use the concept of equivalent resistance. Apply knowledge of series circuits to real world applications. Students should be able to explain the design and use of d.c. series circuits for measurement and testing purposes. State that the potential difference across each component in a parallel circuit is the same. Describe how the currents in different parts of a parallel circuit change and give the reasons for this change. Describe the effect on the resistance of adding resistors in parallel. State that adding resistors in parallel will make the total resistance less than the lowest value resistor. Describe the differences between series and parallel circuits in terms of current and potential difference. Students are not required to calculate the total resistance of resistors placed in parallel. What causes resistance? Research what resistance is and why some materials have no resistance (superconductors). Explain why adding resistors in series to a circuit, increases the resistance of that circuit in terms of number of collisions. Explain why adding resistors in parallel decreases the resistance of a circuit in terms of increased number of pathways.
Year 11 Science Learning Cycle 3 Overview
 Year 11 Science Learning Cycle 3 Overview Learning Cycle Overview: Biology Hypothesis 1 Hypothesis 2 Hypothesis 3 Hypothesis 4 Hypothesis 5 Hypothesis 6 Hypothesis 7 Hypothesis 8 Hypothesis 9 How does
Year 11 Science Learning Cycle 3 Overview Learning Cycle Overview: Biology Hypothesis 1 Hypothesis 2 Hypothesis 3 Hypothesis 4 Hypothesis 5 Hypothesis 6 Hypothesis 7 Hypothesis 8 Hypothesis 9 How does
Lymm High School- KS3 Life after levels - Science Y9
 Biology BRONZE SILVER GOLD PLATINUM D and below= GCSE 1,2,3 C= GCSE 4 C/B= GCSE 5,6 A/A*= GCSE 7,8,9 Explain that cells are very Demonstrate an small and a microscope is understanding of the scale needed
Biology BRONZE SILVER GOLD PLATINUM D and below= GCSE 1,2,3 C= GCSE 4 C/B= GCSE 5,6 A/A*= GCSE 7,8,9 Explain that cells are very Demonstrate an small and a microscope is understanding of the scale needed
Unit B: Cells and Systems
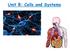 Unit B: Cells and Systems Topic 4: Fluid Movement in Cells The Cell Membrane A cell membrane allows some to enter or leave the cell, while stopping other substances. It is a selectively membrane. (A permeable
Unit B: Cells and Systems Topic 4: Fluid Movement in Cells The Cell Membrane A cell membrane allows some to enter or leave the cell, while stopping other substances. It is a selectively membrane. (A permeable
Bio Factsheet. Transport in Plants. Number 342
 Number 342 Transport in Plants This Factsheet: Explains why plants need a transport system Describes what plants transport Describes the tissues which carry out transport Outlines the position of the xylem
Number 342 Transport in Plants This Factsheet: Explains why plants need a transport system Describes what plants transport Describes the tissues which carry out transport Outlines the position of the xylem
C2 Quick Revision Questions. C2 for AQA GCSE examination 2018 onwards
 C2 Quick Revision Questions Question 1... of 50 What are the 3 main types of chemical bond? Answer 1... of 50 Ionic, Covalent & Metallic. Question 2... of 50 What force bonds atoms in an ionic bond? Answer
C2 Quick Revision Questions Question 1... of 50 What are the 3 main types of chemical bond? Answer 1... of 50 Ionic, Covalent & Metallic. Question 2... of 50 What force bonds atoms in an ionic bond? Answer
Atomic Structure and Periodic Table. HL quizzes
 Atomic Structure and Periodic Table HL quizzes Quiz 1 Ionic Bonding 1. Atoms will bond to attain a f o s of e (2 marks) 2. When metal atoms bond they always electrons to form ions ( ions). 3. When non-metal
Atomic Structure and Periodic Table HL quizzes Quiz 1 Ionic Bonding 1. Atoms will bond to attain a f o s of e (2 marks) 2. When metal atoms bond they always electrons to form ions ( ions). 3. When non-metal
LEARNING OUTCOMES CCEA GCSE BIOLOGY: UNIT 2.1: Osmosis and Plant transport
 NAME 0 LEARNING OUTCOMES CCEA GCSE BIOLOGY: 2.1.1-2.1.9 UNIT 2.1: Osmosis and Plant transport LEARNING OUTCOMES PUPIL SELF-EVALUATION Pupils should be able to: Good Average Requires Attention 1 Carry out
NAME 0 LEARNING OUTCOMES CCEA GCSE BIOLOGY: 2.1.1-2.1.9 UNIT 2.1: Osmosis and Plant transport LEARNING OUTCOMES PUPIL SELF-EVALUATION Pupils should be able to: Good Average Requires Attention 1 Carry out
2018 Version. Photosynthesis Junior Science
 2018 Version Photosynthesis Junior Science 1 Plants fill the role of Producers in a community Plants are special because they have leaves and are able to produce their own food by the process of photosynthesis
2018 Version Photosynthesis Junior Science 1 Plants fill the role of Producers in a community Plants are special because they have leaves and are able to produce their own food by the process of photosynthesis
Science Home Learning Task. Year 9. GCSE Cell structure and transport
 Science Home Learning Task Year 9 GCSE Cell structure and transport Name Tutor Group Teacher Given out: Monday 23 April Hand in: Monday 30 April Parent/Carer Comment Staff Comment GCSE level Target Investigating
Science Home Learning Task Year 9 GCSE Cell structure and transport Name Tutor Group Teacher Given out: Monday 23 April Hand in: Monday 30 April Parent/Carer Comment Staff Comment GCSE level Target Investigating
YEAR 7. St Edmund Arrowsmith CCFL Science Department Curriculum Map New AQA Course Started November 2016
 YEAR 7 St Edmund Arrowsmith CCFL Science Department Curriculum Map 201-2017 New AQA Course Started November 2016 05/09/2016 12/09/2016 19/09/2016 26/09/2016 03/10/2016 10/10/2016 17/10/2016 24/10/2016
YEAR 7 St Edmund Arrowsmith CCFL Science Department Curriculum Map 201-2017 New AQA Course Started November 2016 05/09/2016 12/09/2016 19/09/2016 26/09/2016 03/10/2016 10/10/2016 17/10/2016 24/10/2016
Plant Biology. 2. Explain why energy is lost between each trophic level (triple only).
 1. Calculate the % of energy lost between each level. A-B = B-C = C-D = 80%+ Describe quantitatively the proportion of energy transferred between trophic levels and use this to calculate efficiency (triple
1. Calculate the % of energy lost between each level. A-B = B-C = C-D = 80%+ Describe quantitatively the proportion of energy transferred between trophic levels and use this to calculate efficiency (triple
Part 4- Chemistry Paper 1 Bonding Knowledge Questions
 Part 4- Chemistry Paper 1 Bonding Knowledge Questions How bonding and structure are related to the properties of substances A simple model of the atom, symbols, relative atomic mass, electronic charge
Part 4- Chemistry Paper 1 Bonding Knowledge Questions How bonding and structure are related to the properties of substances A simple model of the atom, symbols, relative atomic mass, electronic charge
Additional Science. Important exam information and revision booklet
 Additional Science Important exam information and revision booklet CONTENTS PAGE Page Introduction 3 Section One When are my exams? What will they test 4 Useful places to help you to revise Additional
Additional Science Important exam information and revision booklet CONTENTS PAGE Page Introduction 3 Section One When are my exams? What will they test 4 Useful places to help you to revise Additional
B2 Quick Revision Questions. B2 for AQA GCSE examination 2018 onwards
 B2 Quick Revision Questions Question 1 Which raw materials are used in photosynthesis and what are the products of the reaction? Answer 1 Carbon dioxide Water Glucose Oxygen Question 2 What type of reaction
B2 Quick Revision Questions Question 1 Which raw materials are used in photosynthesis and what are the products of the reaction? Answer 1 Carbon dioxide Water Glucose Oxygen Question 2 What type of reaction
Which one of the following graphs correctly shows the relationship between potential difference (V) and current (I) for a filament lamp?
 Questions Q1. Select one answer from A to D and put a cross in the box ( ) Which one of the following graphs correctly shows the relationship between potential difference (V) and current (I) for a filament
Questions Q1. Select one answer from A to D and put a cross in the box ( ) Which one of the following graphs correctly shows the relationship between potential difference (V) and current (I) for a filament
Year 10 Science Learning Cycle 3 Overview
 Year 10 Science Learning Cycle 3 Overview Learning Cycle Overview: Biology Hypothesis 1 Hypothesis 2 Hypothesis 3 Hypothesis 4 Hypothesis 5 Hypothesis 6 Hypothesis 7 Hypothesis 8 Hypothesis 9 How does
Year 10 Science Learning Cycle 3 Overview Learning Cycle Overview: Biology Hypothesis 1 Hypothesis 2 Hypothesis 3 Hypothesis 4 Hypothesis 5 Hypothesis 6 Hypothesis 7 Hypothesis 8 Hypothesis 9 How does
AQA Chemistry GCSE. Topic 2 - Bonding, Structure and the Properties of Matter. Flashcards.
 AQA Chemistry GCSE Topic 2 - Bonding, Structure and the Properties of Matter Flashcards What is ionic bonding? What is ionic bonding? Ionic bonding is the electrostatic attraction between positive and
AQA Chemistry GCSE Topic 2 - Bonding, Structure and the Properties of Matter Flashcards What is ionic bonding? What is ionic bonding? Ionic bonding is the electrostatic attraction between positive and
Chemistry (separate) for November PPE
 1.1 Elements and 1.2 Atoms, formulae and Chapter 1 Atomic Structure and Periodic Table Identify symbols of elements from the periodic table Recognise the properties of elements and. Identify the elements
1.1 Elements and 1.2 Atoms, formulae and Chapter 1 Atomic Structure and Periodic Table Identify symbols of elements from the periodic table Recognise the properties of elements and. Identify the elements
Chemistry Paper 1 Revision Knowledge Questions. Topic 1- Atomic Structure and the periodic Table. Topic 2- Bonding, Structure and Properties of Matter
 Chemistry Paper 1 Revision Knowledge Questions Topic 1- Atomic Structure and the periodic Table Topic 2- Bonding, Structure and Properties of Matter Why am I doing this? To answer exam questions you need
Chemistry Paper 1 Revision Knowledge Questions Topic 1- Atomic Structure and the periodic Table Topic 2- Bonding, Structure and Properties of Matter Why am I doing this? To answer exam questions you need
4.2 Bonding, structure, and the properties of matter
 4.2 Bonding, structure, and the properties of matter Chemists use theories of structure and bonding to explain the physical and chemical properties of materials. Analysis of structures shows that atoms
4.2 Bonding, structure, and the properties of matter Chemists use theories of structure and bonding to explain the physical and chemical properties of materials. Analysis of structures shows that atoms
Revision checklist SP10. SP10 Electricity and Circuits. SP10a Electric circuits. SP10b Current and potential difference
 Electricity and Circuits a Electric circuits Describe the basic structure of an atom (positions, relative masses and relative charges of protons, neutrons and electrons). Recognise the circuit symbols
Electricity and Circuits a Electric circuits Describe the basic structure of an atom (positions, relative masses and relative charges of protons, neutrons and electrons). Recognise the circuit symbols
Unit 2: Structure and Bonding
 Elements vs Compounds Elements are substances made of one kind of atom. There are around 100 elements, which are listed in the Periodic Table. Elements may chemically combine (bond) together in fixed proportions
Elements vs Compounds Elements are substances made of one kind of atom. There are around 100 elements, which are listed in the Periodic Table. Elements may chemically combine (bond) together in fixed proportions
4.2.1 Chemical bonds, ionic, covalent and metallic
 4.2 Bonding, structure, and the properties of matter Chemists use theories of structure and bonding to explain the physical and chemical properties of materials. Analysis of structures shows that atoms
4.2 Bonding, structure, and the properties of matter Chemists use theories of structure and bonding to explain the physical and chemical properties of materials. Analysis of structures shows that atoms
Unit 1 Plants - Extra Worksheets
 Unit 1 Plants - Extra Worksheets 1 Complete the sentences using the words in the box. Plants can make their own using light from the. They also need carbon gas and. 2 Label the parts of the diagram to
Unit 1 Plants - Extra Worksheets 1 Complete the sentences using the words in the box. Plants can make their own using light from the. They also need carbon gas and. 2 Label the parts of the diagram to
Biology 2 Chapter 21 Review
 Biology 2 Chapter 21 Review Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Which of the following is not a tissue system of vascular plants? a. vascular
Biology 2 Chapter 21 Review Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Which of the following is not a tissue system of vascular plants? a. vascular
PHYSICS FORM 5 ELECTRICAL QUANTITES
 QUANTITY SYMBOL UNIT SYMBOL Current I Amperes A Voltage (P.D.) V Volts V Resistance R Ohm Ω Charge (electric) Q Coulomb C Power P Watt W Energy E Joule J Time T seconds s Quantity of a Charge, Q Q = It
QUANTITY SYMBOL UNIT SYMBOL Current I Amperes A Voltage (P.D.) V Volts V Resistance R Ohm Ω Charge (electric) Q Coulomb C Power P Watt W Energy E Joule J Time T seconds s Quantity of a Charge, Q Q = It
Insulators Non-metals are very good insulators; their electrons are very tightly bonded and cannot move.
 SESSION 11: ELECTRIC CIRCUITS Key Concepts Resistance and Ohm s laws Ohmic and non-ohmic conductors Series and parallel connection Energy in an electric circuit X-planation 1. CONDUCTORS AND INSULATORS
SESSION 11: ELECTRIC CIRCUITS Key Concepts Resistance and Ohm s laws Ohmic and non-ohmic conductors Series and parallel connection Energy in an electric circuit X-planation 1. CONDUCTORS AND INSULATORS
Part 6- Chemistry Paper 1 Bonding Application Questions Triple Science
 Part 6- Chemistry Paper 1 Bonding Application Questions Triple Science How bonding and structure are related to the properties of substances A simple model of the atom, symbols, relative atomic mass, electronic
Part 6- Chemistry Paper 1 Bonding Application Questions Triple Science How bonding and structure are related to the properties of substances A simple model of the atom, symbols, relative atomic mass, electronic
Cecil Jones Academy Science Fundamentals Map
 Fundamentals: Year 9 Biology Knowledge Unit 1 Skills B1 Cell Structure and Transport Unit 2 Use the terms 'eukaryotic' and 'prokaryotic' to describe types of cells Describe the features of bacterial (prokaryotic)
Fundamentals: Year 9 Biology Knowledge Unit 1 Skills B1 Cell Structure and Transport Unit 2 Use the terms 'eukaryotic' and 'prokaryotic' to describe types of cells Describe the features of bacterial (prokaryotic)
Science Year 10 Unit 1 Biology
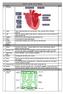 Week 1: 1. The Heart Science Year 10 Unit 1 Biology RAG 2. Artery Takes oxygenated blood away from the heart. Thick, muscular walls to withstand pressure. 3. Vein Takes deoxygenated blood towards the heart.
Week 1: 1. The Heart Science Year 10 Unit 1 Biology RAG 2. Artery Takes oxygenated blood away from the heart. Thick, muscular walls to withstand pressure. 3. Vein Takes deoxygenated blood towards the heart.
Transportation in Plants
 Transportation in Plants Bell Ringer - 5 Min Why do you need transportation in living organisms? Explain your answer with a suitable example. Water movement through plants How does water move through a
Transportation in Plants Bell Ringer - 5 Min Why do you need transportation in living organisms? Explain your answer with a suitable example. Water movement through plants How does water move through a
Level 789 Pathway: Combined Science Double Award
 Level 789 Pathway: Combined Science Double Award Yr Combined Science Targets: Pathway 8 11 Biology a) Explain in detail the role of the hormone ADH on the nephron b) Explain in detail the process by which
Level 789 Pathway: Combined Science Double Award Yr Combined Science Targets: Pathway 8 11 Biology a) Explain in detail the role of the hormone ADH on the nephron b) Explain in detail the process by which
Electron Theory of Charge. Electricity. 1. Matter is made of atoms. Refers to the generation of or the possession of electric charge.
 Electricity Refers to the generation of or the possession of electric charge. There are two kinds of electricity: 1. Static Electricity the electric charges are "still" or static 2. Current Electricity
Electricity Refers to the generation of or the possession of electric charge. There are two kinds of electricity: 1. Static Electricity the electric charges are "still" or static 2. Current Electricity
CROSS SECTION OF A LEAF INTRODUCTION
 CROSS SECTION OF A LEAF INTRODUCTION The leaf is an organ in a plant consisting of many different tissues. The primary function of a leaf is to make (synthesize) food through a chemical reaction called.
CROSS SECTION OF A LEAF INTRODUCTION The leaf is an organ in a plant consisting of many different tissues. The primary function of a leaf is to make (synthesize) food through a chemical reaction called.
Organs and leaf structure
 Organs and leaf structure Different types of tissues are arranged together to form organs. Structure: 2 parts (Petiole and Leaf Blade) Thin flat blade, large surface area Leaves contain all 3 types of
Organs and leaf structure Different types of tissues are arranged together to form organs. Structure: 2 parts (Petiole and Leaf Blade) Thin flat blade, large surface area Leaves contain all 3 types of
Calculate the total resistance of this combination. (3)
 1 The circuit shows a combination of three resistors. 22 Ω 47 Ω 620 Ω Calculate the total resistance of this combination. Total resistance = (Total for Question = 3 marks) 2 (a) Sketch a graph to show
1 The circuit shows a combination of three resistors. 22 Ω 47 Ω 620 Ω Calculate the total resistance of this combination. Total resistance = (Total for Question = 3 marks) 2 (a) Sketch a graph to show
Year 10 Chemistry TRIPLE Learning Cycle 4 Overview Can a knowledge of atomic structure allow us to predict how elements will react with eachother?
 Learning Cycle Overview: Year 10 Chemistry TRIPLE Learning Cycle 4 Overview Can a knowledge of atomic structure allow us to predict how elements will react with eachother? Commented [T1]: Good overarching
Learning Cycle Overview: Year 10 Chemistry TRIPLE Learning Cycle 4 Overview Can a knowledge of atomic structure allow us to predict how elements will react with eachother? Commented [T1]: Good overarching
Year 11 Science Learning Cycle 3 Overview
 Year 11 Science Learning Cycle 3 Overview Learning Cycle Overview: Biology Hypothesis 1 Hypothesis 2 Hypothesis 3 Hypothesis 4 Hypothesis 5 Hypothesis 6 Hypothesis 7 Hypothesis 8 Hypothesis 9 How does
Year 11 Science Learning Cycle 3 Overview Learning Cycle Overview: Biology Hypothesis 1 Hypothesis 2 Hypothesis 3 Hypothesis 4 Hypothesis 5 Hypothesis 6 Hypothesis 7 Hypothesis 8 Hypothesis 9 How does
Tissues and organs PART 2
 Tissues and organs PART 2 The structure and function of the mesophytic leaf (a plant organ) The mesopyhtic leaf (lives in a moderately moist environment) contains 7 layers of tissue: 1. Upper epidermis
Tissues and organs PART 2 The structure and function of the mesophytic leaf (a plant organ) The mesopyhtic leaf (lives in a moderately moist environment) contains 7 layers of tissue: 1. Upper epidermis
Lesson Plan: Electric Circuits (~130 minutes) Concepts
 Lesson Plan: Electric Circuits (~130 minutes) Concepts 1. Electricity is the flow of electric charge (electrons). 2. Electric Charge is a property of subatomic particles. 3. Current is the movement of
Lesson Plan: Electric Circuits (~130 minutes) Concepts 1. Electricity is the flow of electric charge (electrons). 2. Electric Charge is a property of subatomic particles. 3. Current is the movement of
Queen s Park High School Key Stage 3 Assessment Science - Earth IA6 target.
 Year 7 Enquiry Skills Queen s Park High School Key Stage 3 Assessment Science - Earth IA6 target. EARTH STRUCTURE AND UNIVERSE Draw simple graphs outlining information about planets Construct models of
Year 7 Enquiry Skills Queen s Park High School Key Stage 3 Assessment Science - Earth IA6 target. EARTH STRUCTURE AND UNIVERSE Draw simple graphs outlining information about planets Construct models of
Standard circuit diagram symbols Content Key opportunities for skills development
 4.2 Electricity Electric charge is a fundamental property of matter everywhere. Understanding the difference in the microstructure of conductors, semiconductors and insulators makes it possible to design
4.2 Electricity Electric charge is a fundamental property of matter everywhere. Understanding the difference in the microstructure of conductors, semiconductors and insulators makes it possible to design
AQA GCSE (9-1) Chemistry Three Year Scheme of Work
 This 3-Year Scheme of Work offers a flexible approach for KS4. It is based on three science lessons per fortnight (assuming a two week timetable of two lessons one week and one lesson in the other). Lessons
This 3-Year Scheme of Work offers a flexible approach for KS4. It is based on three science lessons per fortnight (assuming a two week timetable of two lessons one week and one lesson in the other). Lessons
Plants. Anatomy, Physiology & Photosynthesis
 Plants Anatomy, Physiology & Photosynthesis Plant anatomy Aerial portion absorb light energy gas exchange of O 2, CO 2 & H 2 O stomata (holes) Structural support Terrestrial portion anchorage H 2 O absorption
Plants Anatomy, Physiology & Photosynthesis Plant anatomy Aerial portion absorb light energy gas exchange of O 2, CO 2 & H 2 O stomata (holes) Structural support Terrestrial portion anchorage H 2 O absorption
Review Chemistry Paper 1
 Atomic Structure Topic Define an atom and element. Use scientific conventions to identify chemical symbols Identify elements by chemical symbols Define compound Use chemical formulae to show different
Atomic Structure Topic Define an atom and element. Use scientific conventions to identify chemical symbols Identify elements by chemical symbols Define compound Use chemical formulae to show different
BRAINSTORM ACTIVITY What do we depend on plants for?
 SBI3U1 BRAINSTORM ACTIVITY What do we depend on plants for? STOP! THINK! PAIR! SHARE! With your partner, brainstorm 5 significant uses of plants. Write them down. Now share your ideas with the rest of
SBI3U1 BRAINSTORM ACTIVITY What do we depend on plants for? STOP! THINK! PAIR! SHARE! With your partner, brainstorm 5 significant uses of plants. Write them down. Now share your ideas with the rest of
in angiosperms 10/29/08 Roots take up water via roots Large surface area is needed Roots branch and have root hairs Cortex structure also helps uptake
 in angiosperms A. Root System Roots take up water via roots Large surface area is needed Roots branch and have root hairs Cortex structure also helps uptake 1 B. Minerals Nitrogen (NO 3-,NH 4+ ) Potassium
in angiosperms A. Root System Roots take up water via roots Large surface area is needed Roots branch and have root hairs Cortex structure also helps uptake 1 B. Minerals Nitrogen (NO 3-,NH 4+ ) Potassium
The child becomes electrically charged when he goes down the slide
 P4 Revision Questions Q. The figure below shows a slide in a children s playground. (a) A child of mass 8 kilograms goes down the slide. The vertical distance from the top to the bottom of the slide is
P4 Revision Questions Q. The figure below shows a slide in a children s playground. (a) A child of mass 8 kilograms goes down the slide. The vertical distance from the top to the bottom of the slide is
Ohm s Law Book page Syllabus 2.10
 Ohm s Law Book page 85 87 Syllabus 2.10 What s wrong with this circuit diagram? Task 2 Sketch a simple series circuit containing a cell and a bulb. On your circuit diagram, show an ammeter and voltmeter
Ohm s Law Book page 85 87 Syllabus 2.10 What s wrong with this circuit diagram? Task 2 Sketch a simple series circuit containing a cell and a bulb. On your circuit diagram, show an ammeter and voltmeter
UNIT 3: Electric charge.
 UNIT 3: Electric charge Recommended Prior Knowledge Students should be aware of the two types of charge, charging by friction and by induction. They should be able to distinguish between conductors and
UNIT 3: Electric charge Recommended Prior Knowledge Students should be aware of the two types of charge, charging by friction and by induction. They should be able to distinguish between conductors and
C2 REVISION CHAPTER 1 STRUCTURES & BONDING
 C2 REVISION CHAPTER 1 STRUCTURES & BONDING Draw the symbol for sodium include its mass number and atomic number (what do they tell us) Complete the table Relative Charge Relative Mass Use pictures and
C2 REVISION CHAPTER 1 STRUCTURES & BONDING Draw the symbol for sodium include its mass number and atomic number (what do they tell us) Complete the table Relative Charge Relative Mass Use pictures and
Jeddah Knowledge International School
 Jeddah Knowledge International School Biology Revision Pack Answer key 2016-2017 Quarter 3 Grade 9 Name: Section: ANSWER KEY- SCIENCE GRADE 9, QUARTER 3 1 Mark Scheme Multiple Choice Part A 1. Which gas
Jeddah Knowledge International School Biology Revision Pack Answer key 2016-2017 Quarter 3 Grade 9 Name: Section: ANSWER KEY- SCIENCE GRADE 9, QUARTER 3 1 Mark Scheme Multiple Choice Part A 1. Which gas
WJEC England GCSE Chemistry. Topic 5: Bonding, structure and properties. Notes. (Content in bold is for Higher Tier only)
 WJEC England GCSE Chemistry Topic 5: Bonding, structure and properties Notes (Content in bold is for Higher Tier only) Chemical bonds Compounds - substances in which 2 or more elements are chemically combined.
WJEC England GCSE Chemistry Topic 5: Bonding, structure and properties Notes (Content in bold is for Higher Tier only) Chemical bonds Compounds - substances in which 2 or more elements are chemically combined.
Same theme covered in Combined but extra content Extra parts atomic symbols (first 20, Group 1 and Group 7)
 Co-teaching document new ELC Science 5960 and Foundation Level GCSE Combined Science: Trilogy (8464) Chemistry: Component 3 Elements, mixtures and compounds ELC Outcomes Summary of content covered in ELC
Co-teaching document new ELC Science 5960 and Foundation Level GCSE Combined Science: Trilogy (8464) Chemistry: Component 3 Elements, mixtures and compounds ELC Outcomes Summary of content covered in ELC
Name Date Time to Complete
 Name Date Time to Complete h m Partner Course/ Section / Grade Complex Circuits In this laboratory you will connect electric lamps together in a variety of circuits. The purpose of these exercises is to
Name Date Time to Complete h m Partner Course/ Section / Grade Complex Circuits In this laboratory you will connect electric lamps together in a variety of circuits. The purpose of these exercises is to
Transport in Plant (IGCSE Biology Syllabus )
 Transport in Plant (IGCSE Biology Syllabus 2016-2018) Plants have transport systems to move food, water and minerals around. These systems use continuous tubes called xylem and phloem: - Xylem vessels
Transport in Plant (IGCSE Biology Syllabus 2016-2018) Plants have transport systems to move food, water and minerals around. These systems use continuous tubes called xylem and phloem: - Xylem vessels
4.2.1 Current, potential difference and resistance
 4.2 Electricity Electric charge is a fundamental property of matter everywhere. Understanding the difference in the microstructure of conductors, semiconductors and insulators makes it possible to design
4.2 Electricity Electric charge is a fundamental property of matter everywhere. Understanding the difference in the microstructure of conductors, semiconductors and insulators makes it possible to design
Review. Multiple Choice Identify the letter of the choice that best completes the statement or answers the question.
 Review Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. 1. When more devices are added to a series circuit, the total circuit resistance: a.
Review Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. 1. When more devices are added to a series circuit, the total circuit resistance: a.
AQA Chemistry (Combined Science) Specification Checklists. Name: Teacher:
 AQA Chemistry (Combined Science) Specification Checklists Name: Teacher: Paper 1-4.1 Atomic structure and the periodic table 4.1.1 A simple model of the atom, symbols, relative atomic mass, electronic
AQA Chemistry (Combined Science) Specification Checklists Name: Teacher: Paper 1-4.1 Atomic structure and the periodic table 4.1.1 A simple model of the atom, symbols, relative atomic mass, electronic
Transport of substances in plants
 Transport of substances in plants We have already looked at why many organisms need transport systems with special reference to surface area and volume. The larger the volume : surface area ratio, the
Transport of substances in plants We have already looked at why many organisms need transport systems with special reference to surface area and volume. The larger the volume : surface area ratio, the
They keep the voltage the same and use this circuit to measure the current. Variable resistor. Reading on ammeter in amps
 1 Ksenia and Eva investigate five different variable resistors. They set each variable resistor to the maximum resistance. They keep the voltage the same and use this circuit to measure the current. A
1 Ksenia and Eva investigate five different variable resistors. They set each variable resistor to the maximum resistance. They keep the voltage the same and use this circuit to measure the current. A
DAY 1 Leaf Structure
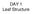 DAY 1 Leaf Structure Design a Leaf!! What would be the best structure for a leaf to carry out its major function PHOTOSYNTHESIS!!!??? Place the following in order from the top of the leaf to the bottom.
DAY 1 Leaf Structure Design a Leaf!! What would be the best structure for a leaf to carry out its major function PHOTOSYNTHESIS!!!??? Place the following in order from the top of the leaf to the bottom.
Water and Food Transportation
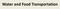 Water and Food Transportation Sugars in a Plant Sugar Form Location in Plant Organ Function of Sugar form Glucose Leaf Energy (made in photosynthesis summer, used in cellular respiration for growth-spring)
Water and Food Transportation Sugars in a Plant Sugar Form Location in Plant Organ Function of Sugar form Glucose Leaf Energy (made in photosynthesis summer, used in cellular respiration for growth-spring)
Photosynthesis. Water is one of the raw materials needed for photosynthesis When water is in short supply the rate of photosynthesis is limited
 Photosynthesis Water is one of the raw materials needed for photosynthesis When water is in short supply the rate of photosynthesis is limited Support Water is needed to ensure plant cells remain turgid
Photosynthesis Water is one of the raw materials needed for photosynthesis When water is in short supply the rate of photosynthesis is limited Support Water is needed to ensure plant cells remain turgid
4.2.1 Current, potential difference and resistance Standard circuit diagram symbols. Content. Key opportunities for skills development WS 1.
 4.2 Electricity Electric charge is a fundamental property of matter everywhere. Understanding the difference in the microstructure of conductors, semiconductors and insulators makes it possible to design
4.2 Electricity Electric charge is a fundamental property of matter everywhere. Understanding the difference in the microstructure of conductors, semiconductors and insulators makes it possible to design
Animal Cell Organelles. Plant Cell. Organelle. Cell Wall. Chloroplasts. Vacuole
 Cell Biology Higher Electron vs Light Microscope Light use light and lenses to magnify specimen Electron use a beam of electrons to form an image Electron higher magnification and higher resolution Electron
Cell Biology Higher Electron vs Light Microscope Light use light and lenses to magnify specimen Electron use a beam of electrons to form an image Electron higher magnification and higher resolution Electron
NATIONAL 5 PHYSICS ELECTRICITY
 NATIONAL 5 PHYSICS ELECTRICITY ELECTRICAL CHARGE CARRIERS AND CURRENT Electrical Charge Electrical charge exists in two distinct types positive charge and negative charge. It is also possible for an object
NATIONAL 5 PHYSICS ELECTRICITY ELECTRICAL CHARGE CARRIERS AND CURRENT Electrical Charge Electrical charge exists in two distinct types positive charge and negative charge. It is also possible for an object
OCR A GCSE Chemistry. Topic 2: Elements, compounds and mixtures. Properties of materials. Notes.
 OCR A GCSE Chemistry Topic 2: Elements, compounds and mixtures Properties of materials Notes C2.3a recall that carbon can form four covalent bonds C2.3b explain that the vast array of natural and synthetic
OCR A GCSE Chemistry Topic 2: Elements, compounds and mixtures Properties of materials Notes C2.3a recall that carbon can form four covalent bonds C2.3b explain that the vast array of natural and synthetic
 Question 1: What are the factors affecting the rate of diffusion? Diffusion is the passive movement of substances from a region of higher concentration to a region of lower concentration. Diffusion of
Question 1: What are the factors affecting the rate of diffusion? Diffusion is the passive movement of substances from a region of higher concentration to a region of lower concentration. Diffusion of
AQA GCSE (9-1) Combined Chemistry Three Year Scheme of Work
 Year 9 Term 1 1/2 1.1 Elements and compounds Year 9 Term 1 1/2 1.2 Atoms, formulae and equations Chapter 1: Atomic structure and the periodic table Identify symbols of elements from the periodic table
Year 9 Term 1 1/2 1.1 Elements and compounds Year 9 Term 1 1/2 1.2 Atoms, formulae and equations Chapter 1: Atomic structure and the periodic table Identify symbols of elements from the periodic table
Electrical Circuits Question Paper 8
 Electrical Circuits Question Paper 8 Level IGCSE Subject Physics Exam Board CIE Topic Electricity and Magnetism Sub-Topic Electrical Circuits Paper Type lternative to Practical Booklet Question Paper 8
Electrical Circuits Question Paper 8 Level IGCSE Subject Physics Exam Board CIE Topic Electricity and Magnetism Sub-Topic Electrical Circuits Paper Type lternative to Practical Booklet Question Paper 8
6 Plant Nutrition. Question Paper. Save My Exams! The Home of Revision For more awesome GCSE and A level resources, visit us at
 For more awesome GSE and level resources, visit us at www.savemyexams.co.uk/ Plant Nutrition Question Paper Level Subject Exam oard Unit ooklet IGSE iology ambridge International Examinations 6 Plant Nutrition
For more awesome GSE and level resources, visit us at www.savemyexams.co.uk/ Plant Nutrition Question Paper Level Subject Exam oard Unit ooklet IGSE iology ambridge International Examinations 6 Plant Nutrition
DAY 1 Photosynthesis. - Chemical reaction - Compared to respiration
 DAY 1 Photosynthesis - Chemical reaction - Compared to respiration Photosynthesis Photosynthesis Song Brainpop Photosynthesis The Sun is the ultimate source of mostly all energy on Earth! Autotrophs: are
DAY 1 Photosynthesis - Chemical reaction - Compared to respiration Photosynthesis Photosynthesis Song Brainpop Photosynthesis The Sun is the ultimate source of mostly all energy on Earth! Autotrophs: are
Our Interdependent World
 Planet Earth Biodiversity and Interdependence Our Interdependent World Plants, Carbon Dioxide and Oxygen Plants and food Food webs Food Pyramids In this lesson you...... will learn more about the processes
Planet Earth Biodiversity and Interdependence Our Interdependent World Plants, Carbon Dioxide and Oxygen Plants and food Food webs Food Pyramids In this lesson you...... will learn more about the processes
Foundation Cell Biology
 Foundation Cell Biology Electron vs Light Microscope Light use light and lenses to magnify specimen Electron use a beam of electrons to form an image Electron higher magnification and higher resolution
Foundation Cell Biology Electron vs Light Microscope Light use light and lenses to magnify specimen Electron use a beam of electrons to form an image Electron higher magnification and higher resolution
IGCSE Double Award Extended Coordinated Science
 IGCSE Double Award Extended Coordinated Science Biology 4.2 - Plant Nutrition Photosynthesis You need to know the definition of photosynthesis as: the fundamental process by which plants manufacture carbohydrates
IGCSE Double Award Extended Coordinated Science Biology 4.2 - Plant Nutrition Photosynthesis You need to know the definition of photosynthesis as: the fundamental process by which plants manufacture carbohydrates
Combined Science: Trilogy
 Co-teaching GCSE Chemistry and GCSE Combined Science: Trilogy This high level co-teaching guide will help you plan your route through the course. You ll be able to see what common themes and topics span
Co-teaching GCSE Chemistry and GCSE Combined Science: Trilogy This high level co-teaching guide will help you plan your route through the course. You ll be able to see what common themes and topics span
Chemistry Key Concepts - Atomic structure
 Chemistry Key Concepts - Atomic structure What is an isotope? What is the atomic number? Why do atoms have no overall charge? How are atoms of different elements different? How does the size of an atom
Chemistry Key Concepts - Atomic structure What is an isotope? What is the atomic number? Why do atoms have no overall charge? How are atoms of different elements different? How does the size of an atom
C1 REVISION 5.1 Atomic Structure
 C1 REVISION 5.1 Atomic Structure Draw the symbol for sodium include its mass number and atomic number (what do they tell us) Complete the table Relative Charge Relative Mass Balance the following equation:
C1 REVISION 5.1 Atomic Structure Draw the symbol for sodium include its mass number and atomic number (what do they tell us) Complete the table Relative Charge Relative Mass Balance the following equation:
1. Transpiration may be defined as the loss of water vapour by diffusion from a plant to its environment.
 1. Transpiration may be defined as the loss of water vapour by diffusion from a plant to its environment. The diagram below shows apparatus that can be used to estimate transpiration rates of a leafy shoot.
1. Transpiration may be defined as the loss of water vapour by diffusion from a plant to its environment. The diagram below shows apparatus that can be used to estimate transpiration rates of a leafy shoot.
Year 9 AQA GCSE Physics Revision Booklet
 Year 9 AQA GCSE Physics Revision Booklet Atomic Structure and Radioactivity Models of the atom know: Plum pudding model of the atom and Rutherford and Marsden s alpha experiments, being able to explain
Year 9 AQA GCSE Physics Revision Booklet Atomic Structure and Radioactivity Models of the atom know: Plum pudding model of the atom and Rutherford and Marsden s alpha experiments, being able to explain
SAM Teachers Guide Electricity
 SAM Teachers Guide Electricity Overview Students explore the role of electron voltage and density on electric current. They compare the movement of electrons in a conductor and an insulator. They derive
SAM Teachers Guide Electricity Overview Students explore the role of electron voltage and density on electric current. They compare the movement of electrons in a conductor and an insulator. They derive
GraspIT AQA GCSE Bonding, structure & the properties of matter
 A. Changes of State States of matter 1. Explain why different substances have different melting points. (2) strength of attractive forces between particles varies in different substances, [1] stronger
A. Changes of State States of matter 1. Explain why different substances have different melting points. (2) strength of attractive forces between particles varies in different substances, [1] stronger
1.1 Characteristics common to organisms
 Biology Form 3 Page 5 Ms. R. Buttigieg 1.1 Characteristics common to organisms see GCSE Biology pg. 292 Biology is the study of living things. We call living things organisms. Plants and animals are ALL
Biology Form 3 Page 5 Ms. R. Buttigieg 1.1 Characteristics common to organisms see GCSE Biology pg. 292 Biology is the study of living things. We call living things organisms. Plants and animals are ALL
15 min. Video reflection handout. Student s notes. Completing the Photosynthesis and Respiration Venn diagram. 20 min
 1 Friday, June 12 Objective Domain: Cells and Heredity Students differentiate how organisms from different kingdoms obtain, transform, and transport, energy and/or material. Students understand the relationships
1 Friday, June 12 Objective Domain: Cells and Heredity Students differentiate how organisms from different kingdoms obtain, transform, and transport, energy and/or material. Students understand the relationships
AQA Chemistry Checklist
 Topic 1. Atomic structure Video: Atoms, elements, compounds, mixtures Use the names and symbols of the first 20 elements in the periodic table, the elements in Groups 1 and 7, and other elements in this
Topic 1. Atomic structure Video: Atoms, elements, compounds, mixtures Use the names and symbols of the first 20 elements in the periodic table, the elements in Groups 1 and 7, and other elements in this
Theme 5: Electricity in the Home
 Theme 5: Electricity in the Home Static Electricity WHAT IS STATIC ELECTRICITY? Everything we see is made up of tiny little parts called atoms. So what are atoms made of? In the middle of each atom is
Theme 5: Electricity in the Home Static Electricity WHAT IS STATIC ELECTRICITY? Everything we see is made up of tiny little parts called atoms. So what are atoms made of? In the middle of each atom is
National 5 Physics Solutions to Electricity & Energy exam questions
 National 5 Physics Solutions to Electricity & Energy exam questions 1. (a) [number and unit must be correct] (b) (i) [number and unit must be correct] (ii) [number and unit must be correct] [or calculate
National 5 Physics Solutions to Electricity & Energy exam questions 1. (a) [number and unit must be correct] (b) (i) [number and unit must be correct] (ii) [number and unit must be correct] [or calculate
GCSE CHEMISTRY REVISION LIST
 GCSE CHEMISTRY REVISION LIST OCR Gateway Chemistry (J248) from 2016 Topic C1: Particles C1.1 Describe the main features of the particle model in terms of states of matter and change of state Explain, in
GCSE CHEMISTRY REVISION LIST OCR Gateway Chemistry (J248) from 2016 Topic C1: Particles C1.1 Describe the main features of the particle model in terms of states of matter and change of state Explain, in
Transport, Storage and Gas Exchange in Flowering Plants
 Sixth Year Biology Transport, Storage and Gas Exchange in Flowering Plants Miss Rochford In this topic: Uptake and transport of: Water and minerals Carbon dioxide Gas exchange Transport of photosynthesis
Sixth Year Biology Transport, Storage and Gas Exchange in Flowering Plants Miss Rochford In this topic: Uptake and transport of: Water and minerals Carbon dioxide Gas exchange Transport of photosynthesis
5. ELECTRIC CURRENTS
 5. ELECTRIC CURRENTS TOPIC OUTLINE Section Recommended Time Giancoli Section 5.1 Potential Difference, Current, Resistance 5.2 Electric Circuits 3h 19.1, 19.2 6.2 Electric Field and Force 6.3 Magnetic
5. ELECTRIC CURRENTS TOPIC OUTLINE Section Recommended Time Giancoli Section 5.1 Potential Difference, Current, Resistance 5.2 Electric Circuits 3h 19.1, 19.2 6.2 Electric Field and Force 6.3 Magnetic
LABORATORY 4 ELECTRIC CIRCUITS I. Objectives
 LABORATORY 4 ELECTRIC CIRCUITS I Objectives to be able to discuss potential difference and current in a circuit in terms of electric field, work per unit charge and motion of charges to understand that
LABORATORY 4 ELECTRIC CIRCUITS I Objectives to be able to discuss potential difference and current in a circuit in terms of electric field, work per unit charge and motion of charges to understand that
Structure and Bonding
 Structure and Bonding Foundation revision questions Name: Class: Date: Time: 66 minutes Marks: 65 marks Comments: Page of 25 The diagram represents a carbon atom. (a) Use words from the box to answer the
Structure and Bonding Foundation revision questions Name: Class: Date: Time: 66 minutes Marks: 65 marks Comments: Page of 25 The diagram represents a carbon atom. (a) Use words from the box to answer the
Bio Ch 6 Photosynthesis Notes
 Bio Ch 6 Photosynthesis Notes I. Photosynthesis Basics A. What is photosynthesis? 1. Photosynthesis is a chemical reaction in which light energy is converted to chemical energy in glucose. 2. It is the
Bio Ch 6 Photosynthesis Notes I. Photosynthesis Basics A. What is photosynthesis? 1. Photosynthesis is a chemical reaction in which light energy is converted to chemical energy in glucose. 2. It is the
PHOTOSYNTHESIS GR 11 LIFE SCIENCES
 PHOTOSYNTHESIS GR 11 LIFE SCIENCES Definition: Photosynthesis is the process where the energy of the sunlight is used by green plants (and some animals) to bond molecules together to form carbohydrates
PHOTOSYNTHESIS GR 11 LIFE SCIENCES Definition: Photosynthesis is the process where the energy of the sunlight is used by green plants (and some animals) to bond molecules together to form carbohydrates
1. Cell Theory Organelle containing the genetic information of the cell.
 GLOSSARY MATCHING GAME The words and definitions are all mixed up. Cut out each word and definition and glue the correct matches into your workbook. Word Definition 1. Cell Theory Organelle containing
GLOSSARY MATCHING GAME The words and definitions are all mixed up. Cut out each word and definition and glue the correct matches into your workbook. Word Definition 1. Cell Theory Organelle containing
Biology Keystone (PA Core) Quiz The Chemical Basis for Life - (BIO.A ) Water Properties, (BIO.A ) Carbon, (BIO.A.2.2.
 Biology Keystone (PA Core) Quiz The Chemical Basis for Life - (BIO.A.2.1.1 ) Water Properties, (BIO.A.2.2.1 ) Carbon, (BIO.A.2.2.2 ) Macromolecules Student Name: Teacher Name: Jared George 1) The first
Biology Keystone (PA Core) Quiz The Chemical Basis for Life - (BIO.A.2.1.1 ) Water Properties, (BIO.A.2.2.1 ) Carbon, (BIO.A.2.2.2 ) Macromolecules Student Name: Teacher Name: Jared George 1) The first
Yr. 9 Electricity WorkBook
 Yr. 9 Electricity WorkBook On completion of this booklet students should be able to: Recall the structure of a neutral atom: three particles, their charges, their location; Nucleus (Proton positive, Neutron-
Yr. 9 Electricity WorkBook On completion of this booklet students should be able to: Recall the structure of a neutral atom: three particles, their charges, their location; Nucleus (Proton positive, Neutron-
To achieve Step 1 in Science students must master the following skills and competencies.
 SCIENCE Step 1 To achieve Step 1 in Science students must master the following skills and competencies. Biology Identify the major organs of the body. Use a microscope to observe cells. Identify the reproductive
SCIENCE Step 1 To achieve Step 1 in Science students must master the following skills and competencies. Biology Identify the major organs of the body. Use a microscope to observe cells. Identify the reproductive
Photosynthesis. 1. What raw materials are used by producers for photosynthesis?
 Photosynthesis Recall that producers are found at the base of every food chain and are the foundation of ecosystems. This is due to their ability to capture light energy to produce their own food in the
Photosynthesis Recall that producers are found at the base of every food chain and are the foundation of ecosystems. This is due to their ability to capture light energy to produce their own food in the
