Tansley review. Enrico Scarpella 1,2 and Annemarie H. Meijer 3
|
|
|
- Madlyn Perry
- 5 years ago
- Views:
Transcription
1 Review Blackwell Publishing, Ltd. Pattern formation in the vascular system of monocot and dicot plant species Author for correspondence: Enrico Scarpella Tel: / Fax: / Received: 8 April 2004 Accepted: 2 July 2004 Enrico Scarpella 1,2 and Annemarie H. Meijer 3 1 Department of Botany, University of Toronto, 25 Willcocks Street, Toronto ON, Canada M5S 3B2, 2 Department of Biological Sciences, University of Alberta, CW405 Biological Sciences Building, Edmonton AB, Canada T6G 2E9, 3 Insitute of Biology, Leiden University, Clusius Laboratory, Wassenaarseweg 64, 2333 AL Leiden, The Netherlands Contents Summary 209 I. Introduction 209 II. The plant vascular system 210 III. Ontogeny of the vascular tissues 210 IV. Procambium development 210 V. The organisation of the vascular tissues 212 VI. The regulation of longitudinal vascular pattern formation 214 VII. The regulation of radial vascular pattern formation 220 VIII. Genetic screens for vascular development mutants 231 IX. Genes involved in vascular development identified through reverse genetics approaches 235 X. Conclusions and perspectives 235 Note added at the revision stage 236 Acknowledgements 236 References 236 Summary Plant vascular tissues are organised in continuous strands, the longitudinal and radial patterns of which are intimately linked to the signals that direct plant architecture as a whole. Therefore, understanding the mechanisms underlying vascular tissue patterning is expected to shed light on patterning events beyond those that organise the vascular system, and thus represents a central issue in plant developmental biology. A number of recent advances, reviewed here, are leading to a more precise definition of the signals that control the formation of vascular tissues and their integration into a larger organismal context. New Phytologist (2004) 164: New Phytologist (2004) doi: /j x I. Introduction The colonisation of land by plants occurred more than 400 million years ago and represents one of the most important events in the history of the biological world. To accomplish the transition from aquatic to terrestrial life successfully, several structural and functional changes had to occur in plants. For example, adaptation was necessary for protection against water loss, for absorption of nutrients and water from the soil, for efficient transport of assimilates throughout the 209
2 210 Review plant body, and for mechanical support. A fundamental role in this process of adaptation has been played by the evolutionary development of vascular tissues, which, by solving the problem of long-distance transport of water and nutrients and by providing rigidity to the plant body, allowed the gradual colonisation of land by the first vascular plants. Primitive vascular plants had vascular tissues organised in a very simple fashion, but with the evolution of novel structures, such as the leaf, and the colonisation of various terrestrial habitats by different plant species, vascular tissues diversified to a variety of organisations. Previous excellent reviews have discussed anatomical, physiological, cell biological, and developmental aspects of vascular patterning and differentiation, mostly in dicot plant species (Northcote, 1995; Fukuda, 1996, 1997, 2000, 2004; McCann, 1997; Nelson & Dengler, 1997; Sjölund, 1997; Berleth et al., 2000a,b; Roberts & McCann, 2000; Sachs, 2000; Aloni, 2001; Dengler & Kang, 2001; Dengler, 2001; Kuriyama & Fukuda, 2001; Turner & Sieburth, 2002; van Bel et al., 2002; Ye, 2002). The present review will first provide a brief description of the structure of the plant vascular system and will deal with its ontogeny and levels of organisation in both monocot and dicot species. Subsequently, it will discuss the regulation of vascular tissue patterning and its relationship to the signals that direct the organisation of the whole plant organism. Finally, it will summarise recent genetic approaches to identify genes involved in different aspects of vascular development in monocots and dicots. II. The plant vascular system The vascular system of contemporary seed plants is composed of a coherent and continuous network of strands called vascular bundles (Esau, 1965a). These structures extend through each organ and throughout the entire plant, functionally connecting every part of the shoot with the root system. Vascular bundles typically represent the organisation of two vascular tissues, the phloem and the xylem. The phloem is the route for dissolved carbohydrates, which are transported from tissues that are net producers of photoassimilates to tissues that are net users. Additionally, the phloem provides the path for the translocation of peptides, proteins, and mrnas involved in plant growth and development and in defence against pathogens (Citovsky & Zambyski, 2000; Oparka & Santa Cruz, 2000; Ruiz- Medrano et al., 2001; van Bel et al., 2002). The xylem is the main conduit for water and mineral nutrients that travel from the root to the sites of evapo-transpiration in the shoot system. Furthermore, the xylem transports hormones such as abscisic acid and cytokinin (Haberer & Kieber, 2002; Hartung et al., 2002). Both phloem and xylem typically comprise a number of specialised vascular cell types, including conducting elements (tracheary elements in the xylem and sieve elements in the phloem), parenchyma, and sclerenchyma cells. III. Ontogeny of the vascular tissues In spite of the large structural and functional diversification displayed by the different cell types of the phloem and xylem, all these highly specialised vascular cell types differentiate, during the course of normal development, from a single primary meristematic tissue: the procambium, or provascular tissue (Esau, 1965a). In plants that undergo radial thickening (gymnosperms and dicots), secondary vascular tissues are formed from a lateral meristem called (vascular) cambium, the initials of which originate in part from the procambium within the vascular bundles, and in part from the parenchyma between the vascular bundles. Recently, a number of studies have advanced our understanding of the environmental and hormonal signals and the genetic cues that regulate the formation and the activity of the cambium. Because these subjects do not strictly fall into the realm of vascular pattern formation, the reader is referred to more specific, excellent reviews (e.g. Lachaud et al., 1999; Savidge et al., 2000; Han, 2001; Savidge, 2001; Bhalerao et al., 2003; Helariutta & Bhalerao, 2003). Monocot species usually lack secondary growth from a cambium, but may develop substantial stems (e.g. palms) by a thickening growth resulting from division and enlargement of parenchyma cells of the ground tissue (Esau, 1965b). Secondary growth by means of a special kind of cambium does occur in certain other monocots (e.g. Agave, Aloe, and Yucca). However, in these woody monocots, the cambium arises from the parenchyma outside the primary vascular bundles and produces new parenchyma and secondary vascular bundles. Finally, vascular elements can differentiate from parenchyma cells, in order to regenerate a vascular connection interrupted as a consequence of wounding or grafting in many dicot species, or to generate de novo a connection between the existing vasculature and a developing adventitious organ in both dicots and monocots (Sachs, 1981). IV. Procambium development There is a vast body of literature that describes the anatomy of vascular tissue development (reviewed in Nelson & Dengler, 1997). These classical investigations have provided a strictly structural definition of procambium. According to this definition, procambium becomes recognisable during organ development as continuous strands of cytoplasmically dense and narrow cells oriented with their major axes parallel to that of the strand itself (Fig. 1a). Procambial cells and their initials (preprocambial cells) preferentially divide parallel to the axis of the developing vascular strand. Because the axis of the strand itself lies almost invariably parallel to the direction of local organ growth (pre)procambial cells also divide parallel to the direction of growth. This orientation of the division plane represents a characteristic of procambial cells, in that normally the plane of cell division in the surrounding tissues is perpendicular to the direction of growth (Smith, 2001). New Phytologist (2004) 164: New Phytologist (2004)
3 Review 211 Fig. 1 Procambium development. (a) Detail of a longitudinal section through a mature rice embryo showing a mature procambial strand. Procambial cells are elongated and have a dense cytoplasm. Parenchyma cells at the left side of the procambial strand are also elongated, but they are more vacuolated. Like procambial cells, cells of the epidermis are cytoplasmically dense, but they are isodiametric. Parenchyma cells between the epidermis and the procambial strand are isodiametric and have a vacuolated cytoplasm. (b) Detail of a transverse section through the shoot apical region of a mature carrot plant showing a ring of provascular tissue / residual meristem. (c) Detail of a developing Arabidopsis leaf showing the onset of GUS reporter gene expression in the enhancer-trap ET1335 (Sundaresan et al., 1995) marking procambial cells (outlined in red, as opposed to the surrounding cells of the ground meristem, which are outlined in yellow). (d) Detail of a developing Arabidopsis leaf showing the onset of Athb8-GUS reporter gene expression (Baima et al., 1995) marking preprocambial cells (outlined in red, as opposed to the surrounding cells of the ground meristem, which are outlined in yellow). e, epidermis; p, pith; pc, procambium; pv, provascular tissue / residual meristem. Photograph in (b) reproduced with permission from Xia & Steeves (1999). Photographs in (c) and (d) reproduced with permission from Scarpella et al. (2004). In general, preprocambial cells are polygonal and isodiametric and do not differ from the surrounding cells. However, in the axis immediately below the shoot apical meristem of some dicots and gymnosperms, it has been possible to distinguish a faintly delimited ring of meristematic tissue from which procambial cells will later emerge (Esau, 1965a) (Fig. 1b). There are two different interpretations of this meristematic tissue, which are reflected in the terminology used to describe it. In one interpretation, these prodesmogen, provascular, or prestelar cells are considered to be the first stage of vascular differentiation occurring independently of the leaves, but in relation to the activity of the shoot apical meristem. In some species, the presence of carboxylesterase activity in the provascular tissue, but not in the cells of the shoot apical meristem that are continuous with this tissue, and/or the developmental fate of the provascular tissue in the absence of leaf primordia seem to support this view (Wardlaw, 1950; McArthur & Steeves, 1972; Xia & Steeves, 1999, 2000). However, similar studies performed in other species seem to support the alternative interpretation, which is that the tissue in question does not represent the first step in the differentiation towards vascular tissues, but rather is a continuation of the shoot apical meristem (Helm, 1932; Ball, 1952; Young, 1954; Gahan & Bellani, 1984; Müller, 1995). Therefore, the authors refer to this tissue as meristem ring, residual meristem, or restmeristem, which they consider anatomically recognisable only because of the precocious vacuolation and expansion of the cells of the surrounding tissues. According to this interpretation, this tissue may or may not become procambium depending on its relation to the developing leaf primordia. More recently, the use of the term provascular tissue, historically linked to vascular development stages in the stem, has been extended to refer to preprocambial cells in all plant organs. However, different opinions exist as to which cells should be regarded as provascular in the same organs at comparable developmental stages (Clay & Nelson, 2002; Holding & Springer, 2002). Furthermore, as mentioned above, certain authors consider provascular tissue as a synonym of procambium (e.g. Esau, 1965a). Therefore, in order to avoid any confusion, throughout this review, only the term procambial will be used to describe anatomically identifiable precursors of vascular cells, and the stages prior to procambial differentiation will be regarded as preprocambial. The arrangement of vascular strands along the main axis of the plant is largely maintained through the elongation and elaboration of existing strand patterns, rather than being newly generated. By contrast, vascular patterns in the leaf are created de novo during the development of each leaf primordium. Therefore, the study of vascular development in the leaf represents an attractive and convenient system to study the dynamics underlying vascular pattern formation. However, at the same time, understanding the mechanistic basis of vascular tissue patterning is particularly challenging in leaves. In fact, the exact position in the developing leaf primordium at which procambial strands will be formed is largely unpredictable. Because of this variability inherent in leaf vascular pattern formation, its study requires the analysis of extremely large numbers of individual samples, a task that is incompatible with the necessary, but time-consuming and labourintensive, histological or optical tissue sectioning. Thus, the identification of genes specifically expressed at early stages of vascular development (Baima et al., 1995; Hiwatashi & Fukuda, 2000; Scarpella et al., 2000; Clay & Nelson, 2002; Kang & Dengler, 2002, 2004; Ohashi-Ito et al., 2002; Groover et al., 2003; Ohashi-Ito & Fukuda, 2003; Scarpella et al., 2004), and the isolation of numerous lines displaying early vascular reporter gene expression from different enhancer- and gene-trap lines New Phytologist (2004) New Phytologist (2004) 164:
4 212 Review (Sundaresan et al., 1995; Malamy & Benfey, 1997; Clay & Nelson, 2002; Holding & Springer, 2002; Scarpella et al., 2004) have generated invaluable tools to uncover the anatomically unidentifiable, early stages of procambium formation. An Arabidopsis (Arabidopsis thaliana) enhancer-trap line in which the onset of reporter gene expression reproducibly marks procambial cell identity acquisition has recently been identified and characterised, allowing the study of procambium formation in large populations of samples (Scarpella et al., 2004) (Fig. 1c). An additional source for the isolation of genes specifically expressed in the procambium is potentially represented by the in vitro system for xylogenesis developed in the dicot species Zinnia elegans (Fukuda, 1996, 1997, 2000; McCann, 1997; Roberts & McCann, 2000; Kuriyama & Fukuda, 2001; Fukuda, 2004). In this cell culture system, mesophyll cells from young leaves are induced to transdifferentiate into xylem tracheary elements in the presence of a suitable hormone combination, and a large number of genes selectively associated with different steps of tracheary element formation have been isolated and characterised by using this system. Expression studies have demonstrated the colinearity between the different steps of the transdifferentiation of mesophyll cells into tracheary elements that takes place in this in vitro system and the process of xylem differentiation from procambial cells that occurs in planta, simultaneously leading to the discovery of early markers of procambium development in Zinnia (Demura & Fukuda, 1994; Ohashi-Ito et al., 2002; Ohashi-Ito & Fukuda, 2003). Unfortunately, promoter-reporter gene constructs for most of these genes are not available yet, and thus these potentially useful markers cannot be directly tested in other plant species. However, available evidence suggests that at least some of the genes specifically expressed in the early steps of mesophyll cell transdifferentiation could be employed in numerous species as markers of different stages of procambium development (Igarashi et al., 1998; Demura et al., 2002; Milioni et al., 2002; Ohashi-Ito et al., 2002; Ohashi-Ito & Fukuda, 2003). A further difficulty intrinsic to the study of the dynamics underlying vascular pattern formation in leaves is that, in these organs, preprocambial cells are recruited from the apparently homogenous subepidermal ground meristem tissue, which will later give rise to both procambium and mesophyll. Therefore, in the leaf (unlike in the stem) preprocambial cells do not differ in any respect from the surrounding cells with regard to cell shape and extent of vacuolation. Because of this lack of anatomical distinction, preprocambial cells can only be distinguished from the surrounding ground meristem on the basis of differential gene expression (Scarpella et al., 2004). The onset of expression of the Arabidopsis thaliana homeobox gene8 (Athb8; Baima et al., 1995), which encodes a member of the homeodomain-leucine zipper (HD-Zip) III family of transcription factors (Sessa et al., 1994), marks the earliest stages of preprocambial development (Kang & Dengler, 2004; Scarpella et al., 2004) (Fig. 1d). The dynamics of Athb8 expression suggest that in Arabidopsis leaves, preprocambial strands develop in continuity with the pre-existing vasculature and extend progressively away form their point of origin. Once the Athb8 preprocambial domain has reached its maximal extension, procambial cells differentiate nearly simultaneously along the length of the entire preprocambial strand (Scarpella et al., 2004). V. The organisation of the vascular tissues Two levels of organisation can be distinguished within the vascular system (Esau, 1965b; Dengler & Kang, 2001): the longitudinal pattern, which is the array of vascular bundles within a certain organ, and the radial (or transverse, or transectional) pattern, which is the spatial arrangement of the phloem and xylem, and of their different cell types, within each vascular bundle. Both levels of organisation are organspecific. In fact, developing vascular tissues must maintain their functionality while being integrated into the context of expanding organs with radically different morphology and anatomy, such as leaves, stems, roots, and flowers. This eventually gives rise to the typical organ-specific position and internal organisation of vascular bundles, and the reproducible differentiation of nonvascular cell types in fixed spatial relationships to vascular bundles (Fig. 2). The existence of organ-specific vascular patterns suggests that genetic instructions likely constrain the variety of possible organisations in order to integrate vascular and nonvascular tissue patterns functionally into the context of a determined organ. Furthermore, species-specific cues are also likely to be involved in the coherent organisation of the vascular tissues into a specific organ context, as shown, for example, by the successful use of species-specific leaf vascular patterns as taxonomic diagnostic features (e.g. Klucking, 1995). Dicot and monocot leaves, in fact, display highly divergent vascular patterns (Nelson & Dengler, 1997). Most dicot leaves show a reticulate pattern, in which veins of different size orders form a highly branched network, whereas most monocot leaves show a typical striate venation pattern, in which major longitudinal veins lie parallel along the proximo-distal axis of Fig. 2 Organ-specific vascular tissue organisation in the monocot rice and the dicot Arabidopsis. (a) Transverse section through a mature rice root. Vascular tissues are organised in a central vascular cylinder surrounded by the endodermis and embedded in the cortex. (b) Transverse section through a mature rice stem internode. Vascular tissues are organised in small and large bundles, which are arranged in two concentric circles. The small vascular bundles of the outer circle are located between two rings of sclerenchyma and embedded in a photosynthetic cortex. The large vascular bundles of the inner circle are embedded in a parenchymatous pith. (c) Detail of a transverse section through a mature rice leaf. Vascular tissues are organised in large and small longitudinal bundles and transverse commissural bundles, which are not visible in this New Phytologist (2004) 164: New Phytologist (2004)
5 Review 213 section (see also Fig. 3). Longitudinal bundles are surrounded by a parenchymatous bundle sheath and embedded in the mesophyll. (d) Detail of the vascular cylinder in (a). The xylem forms a solid central core with ridgelike projections extending towards the periphery of the vascular cylinder. The phloem is organised in separate strands alternating with the xylem ridges. The number of xylem ridges varies in different species. Typically, monocot roots have more than four, and rice roots have six (Scarpella et al., 2003). (e) Detail of the internode wall in (b). Vascular tissues are organised in collateral bundles, where the phloem pole at the outer side of the bundle is juxtaposed with the xylem pole at the inner side of the bundle. (f) Detail of a large vascular bundle in (c). Vascular tissues are organised in collateral bundles, where the phloem pole at the abaxial (lower) side of the bundle is juxtaposed with the xylem pole at the adaxial (upper) side of the bundle. (g) Transverse section through a mature Arabidopsis root. Vascular tissues are organised in a central vascular cylinder surrounded by the endodermis and embedded in the cortex. (h) Transverse section through a mature Arabidopsis stem internode. Vascular tissues are organised in bundles that are arranged in a circle and connected by interfascicular fibres. The vascular bundles and the interfascicular fibres form a hollow cylinder that surrounds a central parenchymatous pith and is embedded in a photosynthetic cortex. (i) Detail of a transverse section through a mature Arabidopsis leaf. Vascular tissues are organised in bundles of different sizes that are surrounded by a parenchymatous bundle sheath and embedded in the mesophyll (see also Fig. 3). (j) Detail of the vascular cylinder in (g). The xylem forms a solid central core with ridgelike projections extending towards the periphery of the vascular cylinder. The phloem is organised in separate strands alternating with the xylem ridges. The number of xylem ridges varies in different species. Typically, dicot roots have up to four, and Arabidopsis roots have two (Dolan et al., 1993). (k) Detail of a transverse section through a mature Arabidopsis stem internode. Vascular tissues are organised in collateral bundles, where the phloem pole at the outer side of the bundle is juxtaposed with the xylem pole at the inner side of the bundle. (l) Detail of a vascular bundle in (i). Vascular tissues are organised in collateral bundles, where the phloem pole at the abaxial (lower) side of the bundle is juxtaposed with the xylem pole at the adaxial (upper) side of the bundle. 1, Primary vein (midvein); 2, secondary vein; 3, tertiary vein; b, bundle sheath; c, cortex; en, endodermis; if, interfascicular fibres; lv, large vascular bundle; m, mesophyll; p, phloem; pi, pith; s, sclerenchyma; sv, small vascular bundle; v, vascular cylinder; vb, vascular bundle; x, xylem. Slides in g,h,j,k courtesy of N. Dengler. Slides in i,l courtesy of J. Kang. New Phytologist (2004) New Phytologist (2004) 164:
6 214 Review VI. The regulation of longitudinal vascular pattern formation Fig. 3 Species-specific vascular tissue organisation in the leaf. (a) Whole-mount clearing of a mature Arabidopsis leaf viewed with dark-field illumination and showing a reticulate vascular pattern. The midvein, or primary vein, of the Arabidopsis leaf is a single vein that contains more vascular cells than the lower orders of veins because of a cell division activity that persists even after all other veins have completely differentiated (Donnelly et al., 1999; Kang & Dengler, 2002). (b) Detail of a whole-mount clearing of a mature rice leaf viewed with dark-field illumination and showing a striate vascular pattern. The midvein of the rice leaf is a complex structure consisting of one large and seven small veins (Scarpella et al., 2003). The midvein is usually separated from the first large vein by two or three small veins. Large veins are separated by an average of five small veins, except at the margins of the leaf, where one or two small veins separate the two most external large veins. 1, Primary vein (midvein); 2, secondary vein; 3, tertiary vein; 4, quaternary vein; cv, commissural vein; lv, large vein; mv, midvein; sv, small vein. the leaf and are connected transversally by minor commissural veins (Fig. 3). Although the organ- and species-specific vascular tissue organisations are highly reproducible, the final result is not a static and completely predictable picture. In fact, vascular patterns are simultaneously both consistent in their integration into the local tissue context and, with the possible exception of the midvein in the leaf, unpredictable in the precise course and arrangement of the vascular strands. Furthermore, the vascular system, at least in dicot species, retains a high level of plasticity and flexibility, in that fully expanded leaves and emerging adventitious organs can develop additional connections with the existing vasculature, and older parts of the root, stem, and leaf can still form new vascular bridges to circumvent a wound (Sachs, 1981 and references therein; Sachs, 1989). The apparent contradiction lying in the simultaneous existence of a high level of reproducibility and variability in the organisation of the vascular tissues suggests the involvement of directional signals that, in combination with the selforganising capacities of vascular tissues, generate continuous and perfectly aligned strands within variable and unpredictable vascular networks. The vascular system therefore represents a reproducible, yet flexible, entity where the overall pattern and arrangement of cell types is functional, but not completely identical from individual to individual. 1. Polar auxin transport and vascular development At present, the molecular mechanisms underlying the different aspects of vascular tissue pattern formation are not known. However, various plant hormones have been reported to promote vascular differentiation in different species and experimental conditions (e.g. Aloni, 1987; Fukuda, 1997). Nevertheless, the role of the phytohormone auxin is unique. In fact, auxin not only triggers vascular differentiation per se, but also induces the transdifferentiation of a slender strip of parenchyma cells into a continuous vascular strand that will extend towards the basal pole of the plant in wounded stems and roots of various dicot species (Sachs, 1981) (Fig. 4a). However, a source of auxin alone does not seem to induce procambium formation in stems where all leaf primordia are excised (Young, 1954; McArthur & Steeves, 1972), although it can promote differentiation of procambium into xylem (Jost, 1939; Wangermann, 1967). Furthermore, vascular differentiation in response to auxin application does not readily occur in all species. For example, monocots are known for their recalcitrance (Aloni & Plotkin, 1985; our unpublished observations). These findings suggest that further factors acting in concert with auxin are probably required. However, the capacity of a simple signal to trigger a complex and oriented cellular response suggests that the signalling mechanism recruits polar cues already present in the organism. These directional signals integrating cell polarity and aligned differentiation could be provided by the polar auxin transport (PAT) that normally occurs in plants (Goldsmith, 1977; Lomax et al., 1995; Muday & DeLong, 2001; Friml & Palme, 2002; Muday & Murphy, 2002). Auxin is synthesised predominantly in young apical regions, such as leaf primordia and floral buds (Sheldrake, 1973; Goodwin, 1978; Ljung et al., 2001). In the stem, auxin moves unidirectionally through the vascular tissues from the apex to the base (Goldsmith, 1977; Lomax et al., 1995) (Fig. 4b). In the root, two distinct polarities of PAT exist. Auxin moves acropetally through the central vascular cylinder and, after being redistributed at the root tip, proceeds basipetally towards the elongation zone through the cells of the epidermis and/or outer cortical layers of the root (Goldsmith, 1977; Lomax et al., 1995) (Fig. 4b). At the cellular level, auxin is thought to be translocated through the action of specific membrane-localised influx and efflux carriers. Auxin enters plant cells both by diffusion and through the facilitating action of an auxin influx carrier (Goldsmith, 1977; Lomax et al., 1995) that is thought to be encoded by AUX1 (Marchant et al., 1999) and possibly by related genes (Parry et al., 2001). Auxin cannot diffuse out of plant cells, and can thus exit only through an efflux carrier apparatus that requires the activity of at least two polypeptides (Morris, 2000; Muday & DeLong, 2001; Muday & Murphy, 2002) (Fig. 4b). The first New Phytologist (2004) 164: New Phytologist (2004)
7 Review 215 Fig. 4 Polar auxin transport and vascular development. (a) Local application of auxin (black blob) induces the transdifferentiation of parenchymatic cells to form vascular strands (black wavy lines) in hypocotyl segments (Sachs, 1981). The vascular differentiation response is characterised by specific properties. First, the response is local, as the newly formed vascular strands extend from the precise site of auxin application. Second, the response is polar, as auxin induces vascular strands only towards the morphologically basal pole of the organ. Third, the response is continuous, as the responding cells differentiate in continuity with one another, eventually connecting the auxin source with pre-existing vasculature (grey). Fourth, the differentiation zone is restricted in the radial dimension, as differentiation occurs only within a narrow strip of cells. Fifth, the patterned vascular differentiation response requires the use of polarly transported auxins, and is obstructed in the presence of auxin transport inhibitors. (b) Left: routes of polar auxin transport in the plant. In the shoot, auxin is transported mainly through the vascular tissues in a single direction, from the shoot apex to the base. The directionality of polar auxin transport within leaf vascular networks is not clear, and therefore has not been represented. In the root, movement of auxin from the shoot is from the base of the root towards the root apex through cells of the central vascular cylinder. Lower right: additionally, in the root, auxin is transported from the root tip towards the base through cells of the cortex and/or epidermis. Upper right: hypothetical cellular mechanism for polar auxin transport (redrawn from Muday & DeLong, 2001). Basal localisation of the putative auxin efflux carrier PIN1 protein (black oval) depends on vesicle transport, and polar auxin transport requires an intact actin cytoskeleton. Cortical actin filaments (black lines) might thus serve as tracks for vesicle delivery cycling of the PIN1 protein between the plasma membrane and undefined endosomal membrane structures (dotted) (Geldner et al., 2001, 2003). A high-affinity NPAbinding protein (white circle), known to interact with actin, may connect the transport vesicles (grey) with the actin tracks. of them is an integral membrane transporter thought to be encoded by members of the PIN FORMED (PIN) gene family (Palme & Gälweiler, 1999). The second component of the auxin efflux carrier apparatus performs a regulatory function, and represents a high-affinity binding site for PAT inhibitors (PATIs), such as 1-N-naphthylphthalamic acid (NPA) (Rubery, 1990). Several studies indicate that this NPAbinding protein (NBP) is a peripheral membrane protein associated with the cytosolic face of the plasma membrane, and that this protein interacts with the actin cytoskeleton (Morris, 2000; Muday, 2000). Interestingly, both the AUX1 and PIN proteins show an asymmetric localisation in the plasma membrane that is consistent with a role in controlling the polarity of auxin movement (Gälweiler et al., 1998; Müller et al., 1998; Swarup et al., 2001; Friml et al., 2002, 2003; Benková et al., 2003). Although the molecular details remain hypothetical, PAT itself is experimentally well-documented, and its characteristics could account for the geometrical properties of vascular strand formation. A number of different hypotheses have been postulated to explain the link between auxin physiology and the different aspects of vascular development (Mitchison, 1980; Sachs, 1981; Meinhardt, 1982; Nelson & Dengler, 1997; Aloni, 2001). However, ultimate experimental evidence has been difficult to obtain. With the currently available genetic and experimental tools, the only possibility to test the hypothesis that PAT and auxin signalling have a role in vascular development is by manipulating auxin flow and response. This has been performed in a variety of ways, and the results are consistent with a specific role of PAT and auxin signalling in vascular development, as detailed in the following paragraphs. The application of several classes of PATIs to Arabidopsis seedlings has been used in two studies as a means to investigate the development of the vascular system under conditions of reduced PAT (Mattsson et al., 1999; Sieburth, 1999). Both studies report similar vascular tissue responses in several organs. Vascular cells were less aligned with each other, and generally more vascular tissues were formed. These findings seem to support a role for PAT in the concerted regulation of oriented cell differentiation and the restriction of vascular differentiation to narrow zones. Chemical interference with PAT also significantly affected leaf venation pattern (Mattsson et al., 1999; Sieburth, 1999). Increasing inhibition of PAT progressively restricted vascular differentiation towards the margins of the leaf, suggesting that this region harbours major auxin sources critical for vein formation. This pattern shift was already observed at low concentrations of PATIs that did not significantly alter leaf morphology. Furthermore, different orders of veins in the leaf became unresponsive to PAT inhibition at the time of their emergence as procambial strands, New Phytologist (2004) New Phytologist (2004) 164:
8 216 Review suggesting that these cells express auxin efflux carriers that are insensitive to the applied inhibitors. Consistent with this hypothesis, increased levels of expression of the Oryza sativa homeobox1 (Oshox1) HD-Zip II gene, a positive regulator of procambial development in rice (Oryza sativa), reduced the sensitivity of the PAT machinery towards inhibition (Scarpella et al., 2000, 2002). An approach similar to that undertaken in Arabidopsis has also been employed to study the possible, and historically more controversial, relationship between PAT and vascular development in two monocot species (Tsiantis et al., 1999a; Scarpella et al., 2002). In both maize (Zea mays) and rice, PATI application resulted in thickening of the vascular bundles of the leaf, suggesting a role for PAT in constraining the regions of vascular differentiation. Furthermore, rice leaves formed under conditions of PAT inhibition displayed reduced distance between longitudinal veins and increased distance between transverse veins, providing experimental evidence for a role of PAT in vascular patterning in a monocot species (Scarpella et al., 2002). In addition to the results obtained through the experimental manipulation of PAT with chemical inhibitors, the molecular features of the PIN1, or AtPIN1, gene in Arabidopsis support a role for PAT in vascular development. The PIN1 gene encodes a putative auxin efflux carrier protein that is localised to the basal membrane of procambial and xylem parenchyma cells (Gälweiler et al., 1998; Steinmann et al., 1999) (Table 1). Mutations at the PIN1 locus result in reduced PAT in the stem, pin-shaped inflorescence morphology (a feature also observed upon chemical inhibition of PAT), and aberrant vascular patterning (Okada et al., 1991; Gälweiler et al., 1998; Mattsson et al., 1999). Excess vascular tissue formation is observed at sites of leaf insertions in mutant stems and at the margins of mutant leaves. All these abnormalities are similar to those found in Arabidopsis plants treated with PATIs (Mattsson et al., 1999; Sieburth, 1999). However, vascular defects in pin1 mutants resemble those evoked by treating wild-type plants with low concentrations of PATIs. This suggests that multiple redundantly acting genes are involved in PAT. Inhibition of PAT with high concentrations of PATIs probably mimics the appearance of a mutant simultaneously defective in all these genes. Such a mutant would be expected to have additional severe embryonic defects, since application of PATIs at early embryo stages results in the loss of embryonic axis formation and the consequent generation of ball-shaped embryos (Hadfi et al., 1998; Friml et al., 2003). However, these defects were not observed in the studies mentioned above, since PATIs were applied at germination. The phenotype of the gnom/emb30 ( gn/emb30) mutant of Arabidopsis matches that predicted for a mutant strongly impaired in PAT. Mutant embryos and seedlings have no detectable apical-basal polarity, and the vascular system is limited to a centrally located series of vascular cells that are entirely disconnected and randomly oriented (Mayer et al., 1993) (Table 1). The GN/EMB30 gene encodes a guanine exchange factor required for vesicle transport-mediated polar localisation of PIN1 and possibly other auxin efflux membrane proteins (Steinmann et al., 1999). Interestingly, seedlings simultaneously carrying mutations in multiple PIN genes display phenotypes reminiscent of gn/emb30 mutants, supporting a redundant role of these genes in PAT (Friml et al., 2003). Furthermore, weaker gn/emb30 alleles exhibit leaf vascular pattern defects similar to those induced by postembryonic PAT inhibition (Koizumi et al., 2000; Geldner et al., 2004). Therefore, all available data are consistent with the view that both organismal apical-basal polarity and positioning of procambial strands depend on PAT. The observation that changes in the expression of genes encoding proposed regulators of vascular development display alterations in PAT capacity (Carland & McHale, 1996; Przemeck et al., 1996; Tsiantis et al., 1999a; Scarpella et al., 2000; Scanlon et al., 2002) has been tentatively interpreted as independent evidence confirming the role of PAT in vascular tissue development. However, in these studies, the vascular tissue organisation was altered to different extents at the developmental stages at which the organs were analysed for their PAT properties. This made it difficult to eventually resolve the causal relationship between the vascular phenotypes and the associated PAT alterations. However, attempts to assess PAT capacity at developmental stages at which the vascular defects could not yet be anatomically identified, or in organs that showed wild-type anatomy, seem to support the view that alterations in PAT capacity represent the direct cause of aberrant vascular phenotypes (Zhong & Ye, 2001; Scarpella et al., 2002). In summary, vascular strand formation is strictly dependent on apical-basal organismal polarity and PAT. This correlation suggests that apical-basal axis establishment and vascular strand formation have a common origin in the orientation and distribution of PAT. However, while obviously necessary, PAT is probably not sufficient to ensure the precision of aligned vascular differentiation, which is likely to be further promoted by other, unidentified mechanisms. 2. Auxin response and vascular development Although PAT seems to account for the geometric properties of the vascular differentiation response, proper auxin response should nevertheless be essential for the relay of auxin signals in this process. In leaves, vascular differentiation indeed occurs at sites of maximum auxin response (Mattsson et al., 2003; Scarpella et al., 2003) (Fig. 5a,b), and proper positioning of these auxin response maxima requires PAT (Mattsson et al., 2003). Furthermore, vascular defects have been reported for auxin response mutants. For example, the auxin-resistant1 (axr1) mutant of Arabidopsis displays smaller vascular bundles in the stem (Lincoln et al., 1990). However, severe auxin insensitivity might have more dramatic effects resulting in embryo or seedling lethality, and therefore many mutants New Phytologist (2004) 164: New Phytologist (2004)
9 New Phytologist (2004) New Phytologist (2004) 164: Table 1 Genes involved in longitudinal vascular pattern formation and organismal apical-basal polarity establishment Gene Organism Mutant phenotype Vascular anatomy (At)PIN1 Arabidopsis Increased and discontinuous vascularisation in the stem of the homozygous mutant. Increased and marginalised vascularisation in leaves AXR6/AtCUL1 Arabidopsis Reduced, discontinuous, and centralised vascularisation in cotyledons of the homozygous mutant BDL/IAA12 Arabidopsis Reduced and discontinuous vascularisation in cotyledons and leaves of the homozygous mutant EMB30/GN/VAN7 Arabidopsis Disconnected, randomly oriented vascular cells in homozygous mutant seedlings MP/ARF5 Arabidopsis Reduced, misaligned, and discontinuous vascularisation in the stem of the homozygous mutant. Reduced, misaligned, discontinuous, and centralised vascularisation in cotyledons and leaves. Discontinuous vascularisation in adventitious roots RAL1 Rice Reduced, discontinuous and misaligned vascularisation in scutella and leaves of the homozygous mutant. Altered timing of vascular development in leaves. Reduced vascularisation in stem and adventitious roots Auxin physiology Reduced auxin transport Reduced auxin sensitivity (dominant trait) Reduced auxin sensitivity (dominant trait) Overexpressor phenotype* Vascular anatomy Auxin physiology Molecular identity Unknown Unknown Putative auxin efflux carrier Unknown Unknown Member of the cullin/cdc53 family of proteins Unknown Unknown Member of the Aux/ IAA family of shortlived nuclear proteins Unknown Unknown Unknown Guanine exchange factor Reduced auxin sensitivity and transport Reduced auxin sensitivity Normal at the seedling stage Unknown *Unless otherwise indicated, overexpression is achieved through expression driven by the Cauliflower Mosaic Virus 35S promoter. Transcription factor of the ARF family References Okada et al. (1991); Gälweiler et al. (1998); Mattsson et al. (1999) Hobbie et al. (2000); Hellmann et al. (2003) Hamann et al. (1999), (2002) Mayer et al. (1993); Shevell et al. (1994); Busch et al. (1996); Steinmann et al. (1999); Koizumi et al. (2000) Berleth & Jürgens (1993); Przemeck et al. (1996); Hardtke & Berleth (1998); Mattsson et al. (2003) Unknown Unknown Unknown Scarpella et al. (2003) Review 217
10 218 Review Fig. 5 Auxin response and vascular development. (a,b) Spatial patterns of auxin response in leaves of Arabidopsis (a) (Mattsson et al., 2003) and rice (b) (Scarpella et al., 2003), visualised through β-glucuronidase reporter gene expression driven by the DR5 synthetic auxin-inducible promoter (Ulmasov et al., 1997b). (a) Whole-mount preparation of a developing Arabidopsis first leaf. (b) Transverse section through the shoot apex of a rice seedling. (c) Hypothetical model of auxin regulation of gene expression in vascular development (for excellent, more comprehensive reviews on auxin regulation of gene expression, the reader is referred to Dharmasiri & Estelle, 2002; Hellmann & Estelle, 2002; Kepinski & Leyser, 2002; Leyser, 2002). In the presence of low cellular levels of auxin, MP/ARF5 (green) activity is repressed through the interaction with Aux/IAA proteins, such as BDL/IAA12 (red). In the presence of high cellular concentrations of auxin, the levels of Aux/IAA proteins are depleted by proteolysis through the 26S proteosome. Auxin-mediated Aux/IAA protein degradation is regulated through the E3 ubiquitin-ligase function of SCF TIR1 (light blue), which is composed of four subunits (RBX1, AXR6/CUL1, ASK1/2, and TIR1). Auxin promotes the interaction between the F-box substrate recognition subunit TIR1 and the targeted Aux/IAA protein (Dharmasiri et al., 2003; Tian et al., 2003). Upon auxin-mediated Aux/IAA proteolysis, MP/ARF5 can activate transcription of targets, which include genes expressed at early stages of vascular development, such as Athb8, and early auxin-inducible genes, such as Aux/IAAs (Mattsson et al., 2003). Therefore, auxin acts antagonistically by simultaneously promoting Aux/IAA protein degradation and Aux/IAA gene transcription, thus making Aux/IAA and ARF proteins central components of a potential regulatory feedback loop. This mechanism allows the rapid restoration of Aux/IAA protein abundance when auxin levels diminish, therefore providing a means to quickly and efficiently respond to auxin-mediated endogenous inputs or external signals. Photographs in (a) and (b) reproduced with permission from Mattsson et al. (2003) and Scarpella et al. (2003), respectively. 4, 5, 6, Fourth, fifth, and sixth leaf primordium, respectively; E1 (orange), ubiquitin-activating enzyme, E2 (yellow), ubiquitin-conjugating enzyme, white, ubiquitin. in this class might still be unidentified. This is suggested by the fact that mutations in the MONOPTEROS (MP) and AUXIN-RESISTANT6 (AXR6) genes of Arabidopsis lead to seedling lethality associated with a complex phenotype characterised by impaired auxin response, severely reduced vascular system, defective embryo axis formation, and consequent failure to produce an embryonic root (Berleth & Jürgens, 1993; Przemeck et al., 1996; Hobbie et al., 2000; Mattsson et al., 2003). Seedling lethality in mp mutants can be bypassed by generating adventitious roots in tissue culture, enabling studies of New Phytologist (2004) 164: New Phytologist (2004)
11 Review 219 postembryonic stages (Berleth & Jürgens, 1993). Throughout mutant development, vascular tissues are incompletely differentiated (Przemeck et al., 1996) (Table 1). In leaves, the vascular system is reduced to its most central part: the midvein and a few secondary veins. Mutant stems show reduced PAT, but, because of the vascular defects, it is not clear whether these alterations in auxin flow represent the cause or the consequence of the vascular defects. Homozygous axr6 mutant seedlings display defects very similar to those of mp mutants, but putative postembryonic functions of the AXR6 gene have not been studied because of the seedling lethal phenotype of the mutant (Hobbie et al., 2000) (Table 1). However, one important difference from mp, whose defects are recessive, is that the auxin sensitivity defects caused by the axr6 mutation are dominant. Heterozygous axr6 mutants are bushy, form fewer lateral roots than normal, and show auxin-resistant root elongation and gravitropism. Since vascular patterns have not been investigated in heterozygous axr6 mutants, it remains to be established whether the function of the mutated AXR6 gene also acts in a dominant fashion in vascular development. However, it is important to realise that inferring the wild-type function of a gene from a dominant phenotype can be difficult because the change caused by the mutation may give rise to novel functions not normally present in wild-type plants. Mutation of the BODENLOS (BDL) gene of Arabidopsis results in a phenotype similar to those of the mp and axr6 mutants, but the defects in bdl are weaker and do not result in seedling lethality (Hamann et al., 1999) (Table 1). Development of the vascular system is reduced, and a hypocotyl of variable length ends in a basal peg rather than in a primary root meristem. Homozygous bdl mutant seedlings display reduced auxin responses, such as reduced hypocotyl swelling and callus formation. Similar to the axr6 mutation, the auxin sensitivity defects in the bdl mutant are dominant (Hamann et al., 2002). A recessive mutation in the RADICLELESS1 (RAL1) gene of rice is characterised by defects very similar to those displayed by the above Arabidopsis mutants (Scarpella et al., 2003) (Table 1). Mutant ral1 embryos fail to form a primary root, and the embryonic procambial system is reduced to an interrupted and prematurely aborted primary strand with misaligned procambial cells. Like bdl, ral1 seedlings spontaneously produce adventitious roots, allowing the study of the postembryonic function of the RAL1 gene. In ral1 leaves, longitudinal and commissural (transverse) veins display altered spacing, and the commissural veins show interruptions in their continuity and display abnormal branching. All these vascular patterning aberrations originate from defects in the procambium, the formation of which is delayed in ral1 leaf primordia. Finally, the ral1 mutant displays a defective response to auxin and an enhanced sensitivity to cytokinin. The similarities between the phenotypes of the mp, axr6, and bdl mutants of Arabidopsis and the ral1 mutant of rice suggest that they have related primary defects in the molecular machinery underlying the alignment of cell differentiation with the axis of auxin flow. Strong support for this interpretation comes from the fact that MP is a member of the auxin response factor (ARF) family of transcriptional regulators (Hardtke & Berleth, 1998). The MP DNA-binding domain can bind to auxin response elements, which are short conserved sequences essential for the rapid auxin regulation of certain classes of auxin inducible genes (Ulmasov et al., 1997a). ARF proteins have two conserved C-terminal domains in common with the related class of short-lived, nuclear-localised Aux/IAA proteins (Abel et al., 1994). Unlike ARF genes, Aux/IAA genes are rapidly induced by auxin, and their abundance seems to reflect the strength of the auxin response. The conserved C-terminal domains have been shown to mediate homo- and heterodimerisation within each class and between members of the ARF and Aux/IAA classes (Kim et al., 1997). It has been suggested that the specificity of the auxin response could be conferred by nuclear complex combinations of ARF and Aux/IAA proteins, some of which could specifically promote the differentiation of vascular cells or other cells aligned with the axis of PAT (Berleth et al., 2000a). Consistent with this interpretation is the recently determined molecular identity of the BDL and AXR6 genes. The BDL gene encodes a member of the Aux/IAA family, IAA12, which is capable of interacting with the MP protein in yeast (Hamann et al., 2002). The bdl mutation is similar to mutations reported for other Aux/IAA genes which have been shown to result in the stabilisation of the mutant protein. Interpreting such a phenotype is somewhat problematic because the stabilised IAA12 protein in bdl mutant plants might be present at sufficiently high concentrations to interact with ARF proteins that it would not normally contact. If this is true, then the phenotype of the bdl mutant may reflect the expression patterns of the mutated gene, rather than its real function. Recent genetic evidence, however, suggests that, although the BDL protein does not display any preferential interaction with MP/ ARF5 in transient expression systems, a highly specific antagonistic interaction of both proteins in a variety of developmental contexts occurs in planta (Hardtke et al., 2004). The AXR6 gene encodes AtCUL1 (Shen et al., 2002; Hellmann et al., 2003), a member of the cullin/cdc53 family of proteins, and is therefore one of the different subunits of the SCF (for SKP1, Cullin/CDC53, F-box protein) ubiquitin ligase (Gray et al., 1999). The SCF TIR1 ubiquitin ligase complex, which contains the TRANSPORT INHIBITOR RESISTANT1 (TIR1) F-box protein and the Arabidopsis SKP1-like protein, has been implicated in auxin-dependent degradation of Aux/IAA proteins (Gray et al., 1999, 2001). Taken together, these data suggest a simplistic, but experimentally testable model for the auxin-mediated regulation of vascular pattern formation (Fig. 5c). In the absence of an auxin signal, Aux/IAA proteins such as BDL/IAA12 would New Phytologist (2004) New Phytologist (2004) 164:
12 220 Review act as inhibitors of MP transcriptional activator function. In the presence of auxin, Aux/IAA proteins would be targeted for SCF-mediated degradation, releasing MP from the inhibitory interaction. MP would then be free to activate the transcription of genes involved in vascular development, as well as that of Aux/IAA genes. The activation of Aux/IAA transcription by MP, with consequent inhibition of MP function, would be necessary to limit the duration of the auxin response, thus ensuring its prompt modulation upon changes in auxin signals. This model has found further experimental support in the recent discovery that enhanced levels of MP, in the absence of recognisable phenotypes, result in increased transcript abundance of genes expressed early in vascular development, such as the HD-Zip genes Athb8 and Athb20, as well as in the enhanced auxin inducibility of these and some Aux/ IAA genes (Mattsson et al., 2003). VII. The regulation of radial vascular pattern formation Primary morphogenesis of the leaf temporally coincides with the appearance of the procambial strands that will give rise to its major veins (Nelson & Dengler, 1997; Dengler & Kang, 2001). Numerous mutants have been identified from screens for aberrant leaf shape in many species, but vascular pattern has been characterised only for a few of those (Tables 2 and 3). The most conspicuous vascular pattern defects observed in these leaf shape mutants are abnormalities in the number and placement of the major veins (i.e. those that are formed early at the morphogenetic stage of leaf development) (Tables 2 and 3). Similarly, enhanced and ectopic expression or antisense silencing of various genes results in leaf aberrations that are accompanied by vascular development effects (Tables 2 and 3). The most striking leaf shape mutations that also alter vascular pattern are those in genes thought to be responsible for the maintenance of leaf dorsoventral (or adaxial-abaxial) polarity (Table 2). The formation of flat lateral organs involves the specification of adaxial and abaxial cell identities. Leaf anatomy and gene expression studies suggest that incipient leaf primordia are initially uniform and become polarized in the adaxial-abaxial dimension as soon as the primordium emerges from the flanks of the shoot apical meristem (Hudson & Waites, 1998; Bowman, 2000). A growing number of regulatory genes have been implicated in adaxial-abaxial patterning (Sessions & Yanofsky, 1999; Bowman et al., 2002). The adaxial-abaxial polarity of a leaf is reflected in the polar organisation of leaf veins, with xylem located towards the adaxial side of the leaf and phloem facing the abaxial side, referred to as collateral bundle organisation (Fig. 6). An integrated regulation of leaf and vascular strand polarity is suggested by the phenotypes resulting from mutations in various genes. Both adaxialised and abaxialised leaves do not form blades and thus develop as cylindrical organs with a single central vascular strand. A polarity shift of the surrounding organ is reflected in a corresponding shift towards amphivasal (i.e. a xylem ring surrounds a central phloem cylinder) or amphicribral (i.e. the phloem is organised as a sheath around a xylem cylinder) organisation (Fig. 6). Examples of leaves that develop as cylindrical organs with correspondingly altered internal vein organisation have been observed in Arabidopsis argonaute1 (ago1) mutant plants heterozygous for zwille/pinhead (zll/ pnh) (Lynn et al., 1999), in the phantastica (phan) single mutant and the phan handlebar (hb) double mutant of Anthirrinum (Waites & Hudson, 1995; Waites et al., 1998; Waites & Hudson, 2001), in the phabulosa ( phb) (McConnell & Barton, 1998; McConnell et al., 2001) and the amphivasal bundle1 (avb1) dominant mutants of Arabidopsis (Zhong et al., 1999), and in the Rough sheath1 (Rs1) dominant mutant of maize (Becraft & Freeling, 1994). In phb, avb1, and Rs1, the vascular strands are amphivasal, whereas in phan, phan hb, and the ago1 mutant in the zll/pnh heterozygous background, the single vein is amphicribral. Furthermore, ectopic overexpression of genes involved in leaf abaxial polarity acquisition, such as the KANADI (KAN) genes (Eshed et al., 2001; Kerstetter et al., 2001) and the FILAMENTOUS FLOWER/ YABBY1 gene (Sawa et al., 1999), results in abaxially radialized leaves that do not develop any vasculature. Vascular bundles are also missing from most leaves of severely adaxialised phb plants (McConnell & Barton, 1998). As both extreme phenotypes are incompatible with vascular tissue formation, it is possible that intermediate positional values or adaxialabaxial polarity per se may be essential for vascular tissue formation. Adaxial-abaxial polarity may be derived from ancestral central-peripheral polarity in cylindrical organs (Tasaka, 2001), and this seems to be reflected in the organization of vascular bundles in stems and in the central-peripheral organization of hypocotyls, which in cases of extreme adaxial-abaxial shifts may be devoid of any vascular tissues (Kerstetter et al., 2001). In further support of this view, both leaves and stems of the avb1 mutant display amphivasal bundles (Zhong et al., 1999). A dominant mutation in the REVOLUTA/INTERFASCICU- LAR FIBERLESS1 (REV/IFL1) gene, which encodes a member of the same family of HD-Zip III transcription factors as PHB/Athb14 and PHAVOLUTA (PHV)/Athb9 (Zhong & Ye, 1999; Ratcliffe et al., 2000; McConnell et al., 2001; Otsuga et al., 2001), results in amphivasal bundles in the stem (Emery et al., 2003). Similarly, stems of kan1 kan2 kan3 triple mutants also show amphivasal bundles (Emery et al., 2003). Conversely, rev phb phv triple loss-of-function mutants, which fail to develop postembryonic organs, display amphicribral vascular bundles in their radialised cotyledons (Emery et al., 2003). While radialised vascular bundles in leaves of the phb dominant mutant could be viewed as a consequence of the radialisation of the leaves, the altered vascular patterning in the stems of rev dominant mutants and kan1 kan2 kan3 plants seems to suggest a direct role for KANADI and HD-Zip III genes in vascular patterning. New Phytologist (2004) 164: New Phytologist (2004)
13 New Phytologist (2004) New Phytologist (2004) 164: Table 2 Genes involved in radial vascular pattern formation and organismal adaxial-abaxial polarity establishment Gene AGO1 and ZLL/PNH Organism Arabidopsis Mutant phenotype Over/misexpressor phenotype* Vascular anatomy Auxin physiology Vascular anatomy Auxin physiology Molecular identity References Amphicribral vascular bundles in the cylindrical leaves of the ago1 homozygous mutant in the zll/pnh heterozygous background AVB1 Arabidopsis Amphivasal vascular bundles in stem and leaves of homozygous and heterozygous mutants KAN1, 2 and 3 Arabidopsis Amphivasal bundles in the stem of the triple mutant PHB/Athb14 and PHV/Athb9 Arabidopsis Amphivasal vascular bundles in funnel-shaped leaves of the phb heterozygous dominant mutant. Absence of phloem or of both phloem and xylem in cylindrical leaves of the phb heterozygous dominant mutant. Amphicribral bundles in the cylindrical cotyledons of the phab phav rev triple loss-of-function mutant PHAN and HB Anthirrinum In the phan homozygous mutant, cylindrical leaves, which develop at the upper part of the stem, contain amphicribral vascular bundles. In the phan hb double mutant, all leaves are cylindrical and contain amphicribral vascular bundles REV/IFL1 Arabidopsis Absence of interfascicular fibres and reduced secondary xylem in the stem of the homozygous recessive mutant. Amphivasal bundles in the stem of the heterozygous and homozygous dominant mutant RS1 Maize Disordered vascular anastomosis at the convoluted and sheath-like blade sheath boundary in heterozygous and homozygous mutants. Increased and ectopic xylem production. Occasional amphivasal vascular bundles in the leaf Unknown Unknown Unknown Piwi/PAZ-domain proteins Normal auxin transport Unknown Unknown Unknown Transcription factor of the HD-Zip class Absence of vasculature in the cylindrical leaves of 35S-KAN1, AS1pro- KAN1 and AS1pro-KAN2 Unknown Transcripton factors of the GARP class Unknown Unknown Unknown PHB/Athb14, PHV/ Athb9, and REV/IFL1 are transcription factors of the HD-Zip III family Uknown Unknown Unknown PHAN is a transcription factor of the MYB class. The molecular identity of HB is unknown Normal auxin sensitivity. Reduced auxin transport Unknown Unknown Transcription factor of the HD-Zip III family Unknown Unknown Unknown Transcription factor of the KNOX class *Unless otherwise indicated, overexpression is achieved through expression driven by the Cauliflower Mosaic Virus 35S promoter. Lynn et al. (1999); Cerutti et al. (2000) Zhong et al. (1999); Ye (2002) Eshed et al. (2001); Kerstetter et al. (2001); Emery et al. (2003) McConnell & Barton (1998); McConnell et al. (2001); Emery et al. (2003) Waites & Hudson (1995); Waites et al. (1998); Waites & Hudson (2001) Zhong et al. (1997); Zhong & Ye (1999), (2001); Ratcliffe et al. (2000); Otsuga et al. (2001); Emery et al. (2003) Becraft & Freeling (1994); Schneeberger et al. (1995) Review 221
14 New Phytologist (2004) 164: New Phytologist (2004) Table 3 Genes involved in vascular development but with no clear effects on organismal polarity establishment Mutant phenotype Over/misexpressor phenotype* Gene Organism Vascular anatomy Auxin physiology Vascular anatomy Auxin physiology Molecular identity References AGO1 Arabidopsis Reduced number or absence of higher-order veins in the narrow leaves of the homozygous mutant. Absence of vascularisation in the cylindrical leaves AMP1/COP2/HPT/PT Arabidopsis Simplified venation resembling that of cotyledons in the cotyledon-shaped leaves of the homozygous mutant API7 Arabidopsis Primary vein often splits and rejoins in the pointed leaves with slightly incised margins of the homozygous mutant APL Arabidopsis Absence of phloem in the homozygous mutant. Formation of ectopic xylem at positions where normally phloem differentiates AS1 Arabidopsis Reduced vascularisation in the small cotyledons and in the small and lobed leaves of the homozygous mutant. Secondary veins join the primary vein more proximally than in wild type AS2 Arabidopsis Reduced size or absence of recognisable primary vein in the asymmetric and lobed leaves of the homozygous mutant. Secondary veins run parallel and separately from the primary vein. Reduced number of higher-order veins. Discontinuous vascularisation. Delayed xylem differentiation Unknown Unknown Unknown Piwi/PAZ-domain protein Unknown Unknown Unknown Glutamate carboxypeptidase Bohmert et al. (1998); Cerutti et al. (2000) Jürgens et al. (1991); Chaudhury et al. (1993); Hou et al. (1993); Conway & Poethig (1997); Telfer et al. (1997); Mordhorst et al. (1998); Helliwell et al. (2001) Unknown Unknown Unknown Unknown Berna et al. (1999); Candela et al. (2001) Unknown Absence of xylem in the root of WOLpro-APL Unknown MYB-related transcription factor Unknown Unknown Unknown Transcription factor of the MYB class Unknown Amphivasal vascular bundles in the cylindrical leaves Bonke et al. (2003) Byrne et al. (2000); Sun et al. (2002) Unknown LOB-domain protein Semiarti et al. (2001); Iwakawa et al. (2002 ); Shuai et al. (2002); Lin et al. (2003) 222 Review
15 New Phytologist (2004) New Phytologist (2004) 164: Table 3 Continued Mutant phenotype Athb8 Arabidopsis Normal Unknown Increased and precocious vascularisation in root, stem, and leaves BOP1 Arabidopsis Multiple primary veins associated with ectopic outgrowths in the petiole of the homozygous mutant COV1 Arabidopsis Increased vascularisation in the stem of the homozygous mutant CPD/DWF3/CBB3 Arabidopsis Increased phloem to xylem ratio in the vascular bundles of the stem of the homozygous mutant CVP1/SMT2 Arabidopsis Reduced, misaligned, and discontinuous vascularisation in cotyledons of the homozygous mutant. Increased vascularisation in the stem CVP2 Arabidopsis Reduced and discontinuous vascularisation in cotyledons and leaves of the homozygous mutant DC2.15 Carrot Decreased phloem to xylem ratio in the veins of the twisted leaves of 35S-DC2.15 antisense. Twisting of one of the vascular bundles of the midvein DL Rice Absence of recognisable midrib in the narrow and ribbon-like leaves of the homozygous mutant DWF7/STE1/BUL1 Arabidopsis Delayed procambium initiation in the small leaves of the homozygous mutant. Increased phloem to xylem ratio in the irregularly spaced vascular bundles of the stem Unknown Transcription factor of the HD-Zip III family Baima et al. (1995; 2001) Unknown Unknown Unknown Unknown Ha et al. (2003) Normal auxin sensitivity Unknown Unknown Putative integral membrane protein Parker et al. (2003) Unknown Unknown Unknown Cytochrome P450 Szeckeres et al. (1996); Choe et al. (1999) Normal auxin content, sensitivity and transport Unknown Unknown Sterol Methyltransferase2 Carland et al. (1999; 2002) Normal auxin content, sensitivity and transport Unknown Unknown Unknown Carland et al. (1999) Unknown Unknown Unknown Putative cell wallplasmamembrane Holk et al. (2002) linker protein Unknown Over/misexpressor phenotype* Gene Organism Vascular anatomy Auxin physiology Vascular anatomy Auxin physiology Molecular identity References Multiple midribs in the leaves of ACT1pro-DL Unknown Transcription factor of the YABBY class Unknown Unknown Unknown 7 sterol C-5 desaturase Iwata & Omura (1971); Nagasawa et al. (2003); Yamaguchi et al. (2004) Gashotte et al. (1995; 1996); Choe et al. (1999); Catterou et al. (2001 ) Review 223
16 New Phytologist (2004) 164: New Phytologist (2004) Table 3 Continued Mutant phenotype ELI1 Arabidopsis Discontinuous and misaligned xylem strands in the root of the homozygous mutant. Increased xylem in the stem FIL/YAB1 and YAB3 Arabidopsis Reduced number of higherorder veins and discontinuous vascularisation in the petalshaped and occasionally bifurcated leaves of the fil/ yab1 yab3 double mutant FK/HYD2/ELL1 Arabidopsis Increased phloem to xylem ratio in the supernumerary/ ectopic vascular bundles of the hypocotyl of the homozygous mutant. Reduced and discontinuous vascularisation in the small and abnormally shaped cotyledons and leaves FKD1 and 2 Arabidopsis Secondary veins fail to connect distally to preexisting vasculature in cotyledons and leaves of single and double homozygous mutants. Increased number of freely ending veins. Discontinuous vascularisation. Delayed vascular development in fkd1 FZY Petunia Absence of secondary veins in the wide, curled-up leaves of the homozygous mutant HVE Arabidopsis Reduced vascularisation in cotyledons and leaves of the homozygous mutant HYD1 Arabidopsis Reduced and discontinuous vascularisation in the small and irregularly shaped cotyledons and leaves of the homozygous mutant. Reduced phloem to xylem ratio in the supernumerary/ectopic vascular bundles of the hypocotyl Unknown Unknown Unknown Unknown Caño-Delgado et al. (2000) Unknown Increased auxin sensitivity Reduced auxin response in fkd1 Normal auxin content Normal auxin sensitivity Increased auxin sensitivity Over/misexpressor phenotype* Gene Organism Vascular anatomy Auxin physiology Vascular anatomy Auxin physiology Molecular identity References Absence of vasculature in cylindrical leaves Unknown Transcription factors of the YABBY class Sawa et al. (1999); Siegfried et al. (1999) Unknown Unknown Sterol C-14 reductase Mayer et al. (1991); Jang et al. (2000); Schrick et al. (2000); Souter et al. (2002) Unknown Unknown Unknown Steynen & Schultz (2003) Unknown Increased auxin content Flavin monooxygenase-like protein Tobeña-Santamaria et al. (2002) Unknown Unknown Unknown Candela et al. (1999; 2001) Unknown Unknown Sterol 8-7 isomerase Topping et al. (1997); Souter et al. (2002) 224 Review
17 New Phytologist (2004) New Phytologist (2004) 164: Table 3 Continued Mutant phenotype KN1 Maize Increased distance between longitudinal veins and vein zig-zagging in the wide, occasionally twisted and convoluted leaves of the homozygous and heterozygous mutants KNAT1/BP Arabidopsis Premature and enhanced xylem and sclerenchyma differentiation in the stem of the homozygous mutant Unknown Unknown Unknown Transcription factor of the KNOX class Unknown Over/misexpressor phenotype* Gene Organism Vascular anatomy Auxin physiology Vascular anatomy Auxin physiology Molecular identity References Decreased size of the primary vein in the small leaves of PETEpro- KNAT1 lettuce plants. Increased size and number of veins at the leaf margins. Delayed xylem and sclerenchyma differentiation, and ectopic lignification of cortical and epidermal cells in stems of 35S- KNAT1 Arabidopsis plants LEM7 Arabidopsis Secondary veins do not join the primary vein and run parallel in the narrow and asymmetric leaves of the homozygous mutant. Reduced number of higherorder veins LEP Arabidopsis Unknown Unknown Increased xylem in the midvein of the leaves, which display blade growth on both sides of the petiole Unknown Transcription factor of the KNOX class Freeling and Hake (1985); Vollbrecht et al. (1991); Kerstetter et al. (1994) Lincoln et al. (1994); Frugis et al. (2001); Douglas et al. (2002); Mele et al. (2003) Unknown Unknown Unknown Unknown Meisel et al. (1996) Unknown Transcription factor of the AP2/EREBP class van der Graaff et al. (2000; 2002) Review 225
18 New Phytologist (2004) 164: New Phytologist (2004) Table 3 Continued Mutant phenotype LOP1/TRN1 Arabidopsis Bifurcated and twisted primary vein and reduced vascularisation in the narrow, asymmetric, and irregularly shaped leaves with twisted petiole of the homozygous mutant LSN1 Maize Occasional absence of recognisable midvein in the narrow and wavy leaves with curved and rolled margins of the homozygous mutant. Reduced distance between longitudinal veins. Altered reciprocal positioning of xylem and phloem in the vascular bundles of the leaves ISR and SR2 Maize Reduced distance between longitudinal veins at the margins of the narrow leaves of the isr sr2 double mutant MBL Millet Absence of recognisable midvein in the narrow and ribbon-like leaves of the homozygous mutant. Reduced number of large veins. Increased number of small veins between large veins MRL1 and 2 Millet Absence of recognisable midvein in the narrow and ribbon-like leaves of the single homozygous mutants. Reduced number of longitudinal veins Reduced auxin transport Over/misexpressor phenotype* Gene Organism Vascular anatomy Auxin physiology Vascular anatomy Auxin physiology Molecular identity References Oshox1 Rice Unknown Unknown Precocious vascular differentiation in stem and root Unknown Unknown Unknown Carland & McHale (1996); Cnops et al. (2000) Unknown Unknown Unknown Unknown Landoni et al. (2000) Unknown Unknown Unknown ISR is a chloroplast protein similar to bacterial phosphatases. The molecular identity of SR2 is unknown Park et al. (2000) Unknown Unknown Unknown Unknown Fladung et al. (1991); Fladung (1994) Unknown Unknown Unknown Unknown Rao et al. (1988; 1989) Increased auxin sensitivity and transport Transcription factor of the HD-Zip II family Scarpella et al. (2000; 2002) 226 Review
19 New Phytologist (2004) New Phytologist (2004) 164: Table 3 Continued Mutant phenotype OsKn2 and 3 Rice Unknown Unknown Reduced number and increased size of longitudinal veins in the narrow, irregularly shaped, and convoluted leaves. Irregular cellular arrangment in the xylem OsPNH Rice Reduced number of longitudinal veins in the narrow, asymmetric, fused, and twisted leaves of ACT1pro-OsPNH antisense. Absence of vascularisation in the small cylindrical leaves OV1 Barley Absence of recognisable midvein in the narrow and ribbon-like leaves of the homozygous mutant PLS Arabidopsis Reduced vascularisation in the leaves of the homozygous mutant RON1 Arabidopsis Increased number of freely ending veins near the margins of the broad and rounded leaves of the homozygous mutant RS2 Maize Multiple midveins and/or increased size of midvein in the narrow and twisted leaves of the homozygous mutant. Increased size and number of longitudinal veins and increased distance between them in the wide leaves Normal auxin transport Transcription factors of the KNOX class Unknown Unknown Unknown Piwi/PAZ-domain protein Postma-Haarsma et al. (2002) Nishimura et al. (2002) Unknown Unknown Unknown Unknown Seip & Tsuchiya (1979) Reduced auxin sensitivity Increased leaf vascularisation Normal auxin sensitivity Short polypeptide with no significant homology with known proteins Casson et al. (2002) Unknown Unknown Unknown Unknown Berna et al. (1999); Candela et al. (2001) Reduced auxin transport Over/misexpressor phenotype* Gene Organism Vascular anatomy Auxin physiology Vascular anatomy Auxin physiology Molecular identity References Unknown Unknown Transcription factor of the MYB class Schneeberger et al. (1998); Tsiantis et al. (1999a,b); Timmermans et al. (1999) Review 227
20 New Phytologist (2004) 164: New Phytologist (2004) Table 3 Continued Mutant phenotype SEM1 Maize Reduced number and size of transverse veins in the leaf sheath of the homozygous mutant. Reticulated vascularisation in sheath marginal outgrowths developing at the boundary with the blade SFC Arabidopsis Reduced and discontinuous vascularisation in cotyledons and leaves of the homozygous mutant SMT1/CPH/ORC Arabidopsis Reduced and discontinuous vascularisation in the small and lobed cotyledons of the homozygous mutant TAN1 Maize The position of the veins relative to each other and the surrounding tissues is highly irregular/disorganised in the leaves of the homozygous mutant VAN1 4 and VAN6 Arabidopsis Reduced and discontinuous vascularisation in cotyledons and leaves of the homozygous mutants. Altered reciprocal positioning of xylem and phloem in the hypocotyl of van1, 2, 4 and 6 VAN5 Arabidopsis Increased size of the veins in the cotyledons and in the narrow and irregularly shaped leaves of the homozygous mutant. Reduced number or absence of higher-order veins. Discontinuous and misaligned vascularisation Reduced auxin transport Increased auxin sensitivity Reduced auxin transport Over/misexpressor phenotype* Gene Organism Vascular anatomy Auxin physiology Vascular anatomy Auxin physiology Molecular identity References VAS Arabidopsis Normal Unknown Increased phloem in hypocotyls, stems, and leaf petioles Unknown Unknown Unknown Scanlon et al. (2002) Unknown Unknown Unknown Deyholos et al. (2000) Unknown Unknown Sterol Methyltransferase1 Unknown Unknown Unknown Microtubule binding protein Scheres et al. (1996); Diener et al. (2000); Schrick et al. (2002); Willemsen et al. (2003) Smith et al. (1996); Smith (2001) Unknown Unknown Unknown Unknown Koizumi et al. (2000) Unknown Unknown Unknown Unknown Koizumi et al. (2000) Unknown Weak similarity to non-specific lipid transfer proteins van der Graaff et al. (2002) 228 Review
21 New Phytologist (2004) New Phytologist (2004) 164: Table 3 Continued Mutant phenotype VCS Arabidopsis Slightly reduced vascularisation in the pointy leaves of the homozygous mutant grown at lower temperatures. Highly reduced vascularisation in the narrow, asymmetric, epinastic, and chlorotic leaves at higher temperatures VEP1/AWI31 Arabidopsis Reduced and discontinuous vascularisation in the leaves of VEP1/AWI31 antisense. Reduced vascularisation in cotyledons, stem, and root VH1 Arabidopsis Reduced phloem transport at early stages of leaf development in the homozygous mutant WI1 Maize Delayed or absent metaxylem element differentiation in the stem of the homozygous mutant WOL/CRE1/AHK4 Arabidopsis Absence of phloem in the root and basal part of the hypocotyl of the homozygous mutant XTC1 and 2 Arabidopsis Simplified venation resembling that of cotyledons in the cotyledon-shaped leaves of the homozygous mutants Over/misexpressor phenotype* Gene Organism Vascular anatomy Auxin physiology Vascular anatomy Auxin physiology Molecular identity References Normal Unknown Unknown WD-domain protein Deyholos et al. (2003) Unknown Unknown Unknown Polypeptide similar to animal proteins involved in apoptosis (death-domain proteins) Unknown Reduced vascularisation in juvenile leaves Jun et al. (2002); Yang et al. (1997) Unknown LRR receptor kinase Clay & Nelson (2002) Unknown Unknown Unknown Unknown Postlethwait & Nelson (1957) Unknown Unknown Unknown Cytokinin receptor two-component histidine kinase Scheres et al. (1995); Mähönen et al. (2000); Inoue et al. (2001); Ueguchi et al. (2001a,b) Unknown Unknown Unknown Unknown Conway & Poethig (1997) *Unless otherwise indicated, overexpression is achieved through expression driven by the Cauliflower Mosaic Virus 35S promoter. Review 229
22 230 Review Fig. 6 Radial vascular tissue patterns in vascular bundles of the shoot. (a) Transverse section through a mature Ranunculus stem showing a collateral vascular bundle organisation. The phloem is located towards the abaxial side in flat organs, and towards the outside in cylindrical organs. The xylem is located towards the adaxial side in flat organs, and towards the inside in cylindrical organs. (b) Transverse section through a mature Polypodium rhizome showing an amphicribral vascular bundle organisation. The phloem surrounds the xylem. (c) Transverse section through a mature Cordyline (Acorus calamus) rhizome showing an amphivasal vascular bundle organisation. The xylem encloses the phloem. (d) Transverse section through a mature Cucurbita stem showing a bicollateral vascular bundle organisation. The phloem is located towards both the abaxial and the adaxial sides in flat organs, and towards both the inside and the outside in cylindrical organs. The xylem is located between the phloem poles. p, phloem; x, xylem. Slides courtesy of N. Dengler. Recently, micrornas (mirnas) were identified in Arabidopsis that are complementary to a specific region of the PHB, PHV, and REV/IFL1 transcripts where the dominant mutations in these genes occur (Reinhart et al., 2002; Rhoades et al., 2002; Emery et al., 2003). This discovery suggests that the phenotypes induced by these HD-Zip III gene mutations may be due to the disruption of mirna regulation (Reinhart et al., 2002; Tang et al., 2003). Accordingly, a modified REV/IFL1 sequence with reduced complementarity to these mirnas, but with no change in amino acid sequence, when introduced into wild-type plants, gave rise to phenotypes similar to those induced by the dominant rev mutation (Emery et al., 2003). The AGO1 and ZLL/PNH genes, which encode members of a family of key components in RNAmediated gene-silencing pathways that use short interfering RNAs or mirnas to select their targets in a sequence-dependent manner (Cerutti et al., 2000; Carrington & Ambros, 2003), are expressed in a pattern that overlaps with that of PHB, PHV, and REV/IFL1 (Moussian et al., 1998; Lynn et al., 1999; Zhong & Ye, 1999; Otsuga et al., 2001; Emery et al., 2003). Recent evidence suggests that the mirna-dependent regulation of at least some of these HD-Zip genes may be mediated by AGO1, and possibly by ZLL/PNH (Kidner & Martienssen, 2004). The maize gene ROLLED LEAF1 is the homolog of the Arabidopsis REV/IFL1 gene, and the semidominant Rld1-O mutation results in adaxialisation or partial reversal of adaxial-abaxial polarity in the leaf (Nelson et al., 2002; Juarez et al., 2004). However, it remains to be established New Phytologist (2004) 164: New Phytologist (2004)
 Question 1: State the location and function of different types of meristem. Meristems are specialised regions of plant growth. The meristems mark the regions where active cell division and rapid division
Question 1: State the location and function of different types of meristem. Meristems are specialised regions of plant growth. The meristems mark the regions where active cell division and rapid division
Class XI Chapter 6 Anatomy of Flowering Plants Biology
 Class XI Chapter 6 Anatomy of Flowering Plants Biology Question 1: State the location and function of different types of meristem. Meristems are specialised regions of plant growth. The meristems mark
Class XI Chapter 6 Anatomy of Flowering Plants Biology Question 1: State the location and function of different types of meristem. Meristems are specialised regions of plant growth. The meristems mark
Outline. Leaf Development. Leaf Structure - Morphology. Leaf Structure - Morphology
 Outline 1. Leaf Structure: Morphology & Anatomy 2. Leaf Development A. Anatomy B. Sector analysis C. Leaf Development Leaf Structure - Morphology Leaf Structure - Morphology 1 Leaf Structure - Morphology
Outline 1. Leaf Structure: Morphology & Anatomy 2. Leaf Development A. Anatomy B. Sector analysis C. Leaf Development Leaf Structure - Morphology Leaf Structure - Morphology 1 Leaf Structure - Morphology
Plant Structure and Organization - 1
 Plant Structure and Organization - 1 In our first unit of Biology 203 we will focus on the structure and function of the higher plants, in particular the angiosperms, or flowering plants. We will look
Plant Structure and Organization - 1 In our first unit of Biology 203 we will focus on the structure and function of the higher plants, in particular the angiosperms, or flowering plants. We will look
Actions of auxin. Hormones: communicating with chemicals History: Discovery of a growth substance (hormone- auxin)
 Hormones: communicating with chemicals History- discovery of plant hormone. Auxin Concepts of hormones Auxin levels are regulated by synthesis/degradation, transport, compartmentation, conjugation. Polar
Hormones: communicating with chemicals History- discovery of plant hormone. Auxin Concepts of hormones Auxin levels are regulated by synthesis/degradation, transport, compartmentation, conjugation. Polar
Plants. Tissues, Organs, and Systems
 Plants Tissues, Organs, and Systems Meristematic cells Specialized cells that are responsible for producing specialized cells, they produce three types of tissue in the body of a plant. Meristematic Cells
Plants Tissues, Organs, and Systems Meristematic cells Specialized cells that are responsible for producing specialized cells, they produce three types of tissue in the body of a plant. Meristematic Cells
Lab Exercise 4: Primary Growth and Tissues in Stems
 Lab Exercise 4: Primary Growth and Tissues in Stems Tissues of the plant body can be classified in a variety of ways: functionally (based on the tissue function, e.g. vascular tissue ), morphologically
Lab Exercise 4: Primary Growth and Tissues in Stems Tissues of the plant body can be classified in a variety of ways: functionally (based on the tissue function, e.g. vascular tissue ), morphologically
Plant Structure. Lab Exercise 24. Objectives. Introduction
 Lab Exercise Plant Structure Objectives - Be able to identify plant organs and give their functions. - Learn distinguishing characteristics between monocot and dicot plants. - Understand the anatomy of
Lab Exercise Plant Structure Objectives - Be able to identify plant organs and give their functions. - Learn distinguishing characteristics between monocot and dicot plants. - Understand the anatomy of
NOTES: CH 35 - Plant Structure & Growth
 NOTES: CH 35 - Plant Structure & Growth In their evolutionary journey, plants adapted to the problems of a terrestrial existence as they moved from water to land ANGIOSPERMS (flowering plants) -most diverse
NOTES: CH 35 - Plant Structure & Growth In their evolutionary journey, plants adapted to the problems of a terrestrial existence as they moved from water to land ANGIOSPERMS (flowering plants) -most diverse
The Shoot System: Primary Stem Structure - 1
 The Shoot System: Primary Stem Structure - 1 Shoot System The shoot system comprises the leaves and stems of plants. Leaves are located at nodes on the stem; the distance along the stem between nodes is
The Shoot System: Primary Stem Structure - 1 Shoot System The shoot system comprises the leaves and stems of plants. Leaves are located at nodes on the stem; the distance along the stem between nodes is
Chapter 6. Biology of Flowering Plants. Anatomy Seedlings, Meristems, Stems, and Roots
 BOT 3015L (Outlaw/Sherdan/Aghoram); Page 1 of 6 Chapter 6 Biology of Flowering Plants Anatomy Seedlings, Meristems, Stems, and Roots Objectives Seedling germination and anatomy. Understand meristem structure
BOT 3015L (Outlaw/Sherdan/Aghoram); Page 1 of 6 Chapter 6 Biology of Flowering Plants Anatomy Seedlings, Meristems, Stems, and Roots Objectives Seedling germination and anatomy. Understand meristem structure
23-. Shoot and root development depend on ratio of IAA/CK
 Balance of Hormones regulate growth and development Environmental factors regulate hormone levels light- e.g. phototropism gravity- e.g. gravitropism temperature Mode of action of each hormone 1. Signal
Balance of Hormones regulate growth and development Environmental factors regulate hormone levels light- e.g. phototropism gravity- e.g. gravitropism temperature Mode of action of each hormone 1. Signal
Anatomy of Flowering Plants. K C Meena PGT Biology
 Anatomy of Flowering Plants K C Meena PGT Biology Tissues A group of similar cells performing same function. Types of plant tissues - Meristematic tissues and permanent tissues. Meristematic tissues Have
Anatomy of Flowering Plants K C Meena PGT Biology Tissues A group of similar cells performing same function. Types of plant tissues - Meristematic tissues and permanent tissues. Meristematic tissues Have
Plant Tissues and Organs. Topic 13 Plant Science Subtopics , ,
 Plant Tissues and Organs Topic 13 Plant Science Subtopics 13.1.2, 13.1.3, 13.1.4 Objectives: List and describe the major plant organs their structure and function List and describe the major types of plant
Plant Tissues and Organs Topic 13 Plant Science Subtopics 13.1.2, 13.1.3, 13.1.4 Objectives: List and describe the major plant organs their structure and function List and describe the major types of plant
SESSION 6: SUPPORT AND TRANSPORT SYSTEMS IN PLANTS PART 1
 SESSION 6: SUPPORT AND TRANSPORT SYSTEMS IN PLANTS PART 1 KEY CONCEPTS In this session we will focus on summarising what you need to know about: - Anatomy of dicotyledonous plants Root and stem: distribution
SESSION 6: SUPPORT AND TRANSPORT SYSTEMS IN PLANTS PART 1 KEY CONCEPTS In this session we will focus on summarising what you need to know about: - Anatomy of dicotyledonous plants Root and stem: distribution
Topic 2: Plant Structure & Growth Ch. 35 Angiosperms are the most complex plants. They are composed of cells, tissues, organs and organ systems.
 Topic 2: Plant Structure & Growth Ch. 35 Angiosperms are the most complex plants. They are composed of cells, tissues, organs and organ systems. Fig. 35.8 Plant Cells pp.798-802 Types of plant cells Include:
Topic 2: Plant Structure & Growth Ch. 35 Angiosperms are the most complex plants. They are composed of cells, tissues, organs and organ systems. Fig. 35.8 Plant Cells pp.798-802 Types of plant cells Include:
The plant body has a hierarchy of organs, tissues, and cells. Plants, like multicellular animals:
 Chapter 28 The plant body has a hierarchy of organs, tissues, and cells Plants, like multicellular animals: o Have organs composed of different tissues, which are in turn composed of cells 3 basic organs:
Chapter 28 The plant body has a hierarchy of organs, tissues, and cells Plants, like multicellular animals: o Have organs composed of different tissues, which are in turn composed of cells 3 basic organs:
Plant Structure. Objectives At the end of this sub section students should be able to:
 Name: 3.2 Organisation and the Vascular Structures 3.2.1 Flowering plant structure and root structure Objectives At the end of this sub section students should be able to: 1. Label a diagram of the external
Name: 3.2 Organisation and the Vascular Structures 3.2.1 Flowering plant structure and root structure Objectives At the end of this sub section students should be able to: 1. Label a diagram of the external
The three principal organs of seed plants are roots, stems, and leaves.
 23 1 Specialized Tissues in Plants Seed Plant Structure The three principal organs of seed plants are roots, stems, and leaves. 1 of 34 23 1 Specialized Tissues in Plants Seed Plant Structure Roots: absorb
23 1 Specialized Tissues in Plants Seed Plant Structure The three principal organs of seed plants are roots, stems, and leaves. 1 of 34 23 1 Specialized Tissues in Plants Seed Plant Structure Roots: absorb
Plants. Plant Form and Function. Tissue Systems 6/4/2012. Chapter 17. Herbaceous (nonwoody) Woody. Flowering plants can be divided into two groups:
 Monocots Dicots 6/4/2012 Plants Plant Form and Function Chapter 17 Herbaceous (nonwoody) In temperate climates, aerial parts die back Woody In temperate climates, aerial parts persist The Plant Body Functions
Monocots Dicots 6/4/2012 Plants Plant Form and Function Chapter 17 Herbaceous (nonwoody) In temperate climates, aerial parts die back Woody In temperate climates, aerial parts persist The Plant Body Functions
Plant Structure, Growth, and Development
 Plant Structure, Growth, and Development Plant hierarchy: Cells Tissue: group of similar cells with similar function: Dermal, Ground, Vascular Organs: multiple kinds of tissue, very diverse function Organ
Plant Structure, Growth, and Development Plant hierarchy: Cells Tissue: group of similar cells with similar function: Dermal, Ground, Vascular Organs: multiple kinds of tissue, very diverse function Organ
Useful Propagation Terms. Propagation The application of specific biological principles and concepts in the multiplication of plants.
 Useful Propagation Terms Propagation The application of specific biological principles and concepts in the multiplication of plants. Adventitious Typically describes new organs such as roots that develop
Useful Propagation Terms Propagation The application of specific biological principles and concepts in the multiplication of plants. Adventitious Typically describes new organs such as roots that develop
Topic 14. The Root System. II. Anatomy of an Actively Growing Root Tip
 Topic 14. The Root System Introduction. This is the first of two lab topics that focus on the three plant organs (root, stem, leaf). In these labs we want you to recognize how tissues are organized in
Topic 14. The Root System Introduction. This is the first of two lab topics that focus on the three plant organs (root, stem, leaf). In these labs we want you to recognize how tissues are organized in
The RADICLELESS1 gene is required for vascular pattern formation in rice
 Development 130, 645-658 2003 The Company of Biologists Ltd doi:10.1242/dev.00243 645 The RADICLELESS1 gene is required for vascular pattern formation in rice Enrico Scarpella*, Saskia Rueb and Annemarie
Development 130, 645-658 2003 The Company of Biologists Ltd doi:10.1242/dev.00243 645 The RADICLELESS1 gene is required for vascular pattern formation in rice Enrico Scarpella*, Saskia Rueb and Annemarie
Chapter 29: Plant Tissues
 Chapter 29: Plant Tissues Shoots and Roots Shoots (Leaves and Stem) Produce food by photosynthesis Carry out reproductive functions Roots Anchor the plant Penetrate the soil and absorb water and dissolved
Chapter 29: Plant Tissues Shoots and Roots Shoots (Leaves and Stem) Produce food by photosynthesis Carry out reproductive functions Roots Anchor the plant Penetrate the soil and absorb water and dissolved
IX. PRIMARY STEM STRUCTURE AND DEVELOPMENT Bot 404 Fall 2004
 IX. PRIMARY STEM STRUCTURE AND DEVELOPMENT Bot 404 Fall 2004 A. Shoot apex -plants have an open system of growth, therefore the ability (at least potentially) to continue growth because there is a meristem
IX. PRIMARY STEM STRUCTURE AND DEVELOPMENT Bot 404 Fall 2004 A. Shoot apex -plants have an open system of growth, therefore the ability (at least potentially) to continue growth because there is a meristem
Primary Plant Body: Embryogenesis and the Seedling
 BIOL 221 Concepts of Botany Primary Plant Body: Embryogenesis and the Seedling (Photo Atlas: Figures 1.29, 9.147, 9.148, 9.149, 9.150, 9.1, 9.2) A. Introduction Plants are composed of fewer cell types,
BIOL 221 Concepts of Botany Primary Plant Body: Embryogenesis and the Seedling (Photo Atlas: Figures 1.29, 9.147, 9.148, 9.149, 9.150, 9.1, 9.2) A. Introduction Plants are composed of fewer cell types,
The mode of development in animals and plants is different
 The mode of development in animals and plants is different Outcome of animal embryogenesis is a mini edition of the adult Outcome of plant embryogenesis is a simple structure with -root apical meristem
The mode of development in animals and plants is different Outcome of animal embryogenesis is a mini edition of the adult Outcome of plant embryogenesis is a simple structure with -root apical meristem
Arabidopsis. The FORKED genes are essential for distal vein meeting in. Research article. Summary. Introduction
 Research article 4695 The FORKED genes are essential for distal vein meeting in Arabidopsis Quintin J. Steynen and Elizabeth A. Schultz Department of Biological Sciences, University of Lethbridge, Lethbridge,
Research article 4695 The FORKED genes are essential for distal vein meeting in Arabidopsis Quintin J. Steynen and Elizabeth A. Schultz Department of Biological Sciences, University of Lethbridge, Lethbridge,
Bring Your Text to Lab!!!
 Bring Your Text to Lab!!! Vascular Plant Anatomy: Flowering Plants Objectives: 1. To observe what the basic structure of vascular plants is, and how and where this form originates. 2. To begin to understand
Bring Your Text to Lab!!! Vascular Plant Anatomy: Flowering Plants Objectives: 1. To observe what the basic structure of vascular plants is, and how and where this form originates. 2. To begin to understand
Auxin Signaling in Arabidopsis Leaf Vascular Development 1
 Auxin Signaling in Arabidopsis Leaf Vascular Development 1 Jim Mattsson 2, Wenzislava Ckurshumova, and Thomas Berleth* Department of Botany, University of Toronto, 25 Willcocks Street, Toronto, Canada
Auxin Signaling in Arabidopsis Leaf Vascular Development 1 Jim Mattsson 2, Wenzislava Ckurshumova, and Thomas Berleth* Department of Botany, University of Toronto, 25 Willcocks Street, Toronto, Canada
Forms strands that conduct water, minerals, and organic compounds. Much of the inside of nonwoody parts of plants. Includes roots, stems, and leaves
 Biology II Vascular plants have 3 tissue systems: Dermal Protective outer layer of plant Vascular Forms strands that conduct water, minerals, and organic compounds Ground Much of the inside of nonwoody
Biology II Vascular plants have 3 tissue systems: Dermal Protective outer layer of plant Vascular Forms strands that conduct water, minerals, and organic compounds Ground Much of the inside of nonwoody
UNIT 6 - STRUCTURES OF FLOWERING PLANTS & THEIR FUNCTIONS
 6.1 Plant Tissues A tissue is a group of cells with common function, structures or both. In plants we can find 2 types of tissues: Meristem Permanent tissues Meristem is found in regions with continuous
6.1 Plant Tissues A tissue is a group of cells with common function, structures or both. In plants we can find 2 types of tissues: Meristem Permanent tissues Meristem is found in regions with continuous
STEMS Anytime you use something made of wood, you re using something made from the stem of a plant. Stems are linear structures with attached leaves
 STEMS OUTLINE External Form of a Woody Twig Stem Origin and Development Stem Tissue Patterns Herbaceous Dicotyledonous Stems Woody Dicotyledonous Stems Monocotyledonous Stems Specialized Stems Wood and
STEMS OUTLINE External Form of a Woody Twig Stem Origin and Development Stem Tissue Patterns Herbaceous Dicotyledonous Stems Woody Dicotyledonous Stems Monocotyledonous Stems Specialized Stems Wood and
Major Plant Hormones 1.Auxins 2.Cytokinins 3.Gibberelins 4.Ethylene 5.Abscisic acid
 Plant Hormones Lecture 9: Control Systems in Plants What is a Plant Hormone? Compound produced by one part of an organism that is translocated to other parts where it triggers a response in target cells
Plant Hormones Lecture 9: Control Systems in Plants What is a Plant Hormone? Compound produced by one part of an organism that is translocated to other parts where it triggers a response in target cells
BIOL 305L Laboratory One
 Please print Full name clearly: BIOL 305L Laboratory One General plant anatomy a great place to start! Introduction Botany is the science of plant life. Traditionally, the science included the study of
Please print Full name clearly: BIOL 305L Laboratory One General plant anatomy a great place to start! Introduction Botany is the science of plant life. Traditionally, the science included the study of
Plant Anatomy: roots, stems and leaves
 Plant Anatomy: roots, stems and leaves The plant body has a hierarchy of organs, tissues and cells Plants, like animals, have organs composed of different tissues, which are composed of cells. Tissue is
Plant Anatomy: roots, stems and leaves The plant body has a hierarchy of organs, tissues and cells Plants, like animals, have organs composed of different tissues, which are composed of cells. Tissue is
Visit For All NCERT solutions, CBSE sample papers, Question papers, Notes for Class 6 to 12. Chapter-6 ANATOMY OF FLOWERING PLANTS
 Chapter-6 ANATOMY OF FLOWERING PLANTS POINTS TO REMEMBER Anatomy : Anatomy is the study of internal structure of organisms. Plant anatomy includes organisation and structure of tissues. Tissue : A group
Chapter-6 ANATOMY OF FLOWERING PLANTS POINTS TO REMEMBER Anatomy : Anatomy is the study of internal structure of organisms. Plant anatomy includes organisation and structure of tissues. Tissue : A group
Genetic Regulation of Vascular Tissue Patterning in Arabidopsis
 The Plant Cell, Vol. 11, 2123 2137, November 1999, www.plantcell.org 1999 American Society of Plant Physiologists Genetic Regulation of Vascular Tissue Patterning in Arabidopsis Francine M. Carland, a,1
The Plant Cell, Vol. 11, 2123 2137, November 1999, www.plantcell.org 1999 American Society of Plant Physiologists Genetic Regulation of Vascular Tissue Patterning in Arabidopsis Francine M. Carland, a,1
13.2 The Vascular Plant Body (textbook p )
 13.2 The Vascular Plant Body (textbook p544 550) Learning Goal: Label and explain the anatomy of the Vascular Plant and it's Tissue Types Plants are classified into two main groups: and. Vascular plants
13.2 The Vascular Plant Body (textbook p544 550) Learning Goal: Label and explain the anatomy of the Vascular Plant and it's Tissue Types Plants are classified into two main groups: and. Vascular plants
Plant Anatomy and Tissue Structures
 Plant Anatomy and Tissue Structures The Two Major Plant Systems Reproductive shoot (flower) Terminal bud Node Internode Angiosperm plants have threse major organs: Roots Stems Leaves & Flowers Terminal
Plant Anatomy and Tissue Structures The Two Major Plant Systems Reproductive shoot (flower) Terminal bud Node Internode Angiosperm plants have threse major organs: Roots Stems Leaves & Flowers Terminal
Plant Anatomy. By Umanga Chapagain
 Plant Anatomy By Umanga Chapagain PLANT ANATOMY The science of the structure of the organized plant body learned by dissection is called Plant Anatomy. In general, Plant Anatomy refers to study of internal
Plant Anatomy By Umanga Chapagain PLANT ANATOMY The science of the structure of the organized plant body learned by dissection is called Plant Anatomy. In general, Plant Anatomy refers to study of internal
Stems and Transport in Vascular Plants. Herbaceous Stems. Herbaceous Dicot Stem 3/12/2012. Chapter 34. Basic Tissues in Herbaceous Stems.
 Bud scale Terminal bud Stems and Transport in Plants One year's growth Terminal bud scale scars Axillary bud Leaf scar Node Internode Node Chapter 34 Lenticels Terminal bud scale scars Bundle scars A Woody
Bud scale Terminal bud Stems and Transport in Plants One year's growth Terminal bud scale scars Axillary bud Leaf scar Node Internode Node Chapter 34 Lenticels Terminal bud scale scars Bundle scars A Woody
Chapter 35~ Plant Structure and Growth
 Chapter 35~ Plant Structure and Growth Plant Organization Plant morphology is based on plant s evolutionary history Need to draw in nutrients from the ground and the air Plant Organs Root system = roots
Chapter 35~ Plant Structure and Growth Plant Organization Plant morphology is based on plant s evolutionary history Need to draw in nutrients from the ground and the air Plant Organs Root system = roots
ENDODERMIS & POLARITY
 https://en.wikipedia.org/wiki/casparian_strip ENDODERMIS & POLARITY Niloufar Pirayesh 13.01.2016 PCDU SEMINAR 2 What is Endodermis? It helps with Regulate the movement of water ions and hormones. (in and
https://en.wikipedia.org/wiki/casparian_strip ENDODERMIS & POLARITY Niloufar Pirayesh 13.01.2016 PCDU SEMINAR 2 What is Endodermis? It helps with Regulate the movement of water ions and hormones. (in and
Topic 15. The Shoot System
 Topic 15. The Shoot System Introduction. This is the second of two lab topics that focus on the three plant organs (root, stem, leaf). In these labs we want you to recognize how tissues are organized in
Topic 15. The Shoot System Introduction. This is the second of two lab topics that focus on the three plant organs (root, stem, leaf). In these labs we want you to recognize how tissues are organized in
PLANT STRUCTURE AND FUNCTION Read pages Re-read and then complete the questions below.
 PLANT STRUCTURE AND FUNCTION Read pages 600-602. Re-read and then complete the questions below. 1. PLANT TISSUES - plant tissues are made up of 3 basic cell types: Parenchyma, Collenchyma or Sclerenchyma
PLANT STRUCTURE AND FUNCTION Read pages 600-602. Re-read and then complete the questions below. 1. PLANT TISSUES - plant tissues are made up of 3 basic cell types: Parenchyma, Collenchyma or Sclerenchyma
Plant morphogenesis: long-distance coordination and local patterning Thomas Berleth* and Tsvi Sachs
 57 Plant morphogenesis: long-distance coordination and local patterning Thomas Berleth* and Tsvi Sachs The overall morphology of a plant is largely determined by developmental decisions taken within or
57 Plant morphogenesis: long-distance coordination and local patterning Thomas Berleth* and Tsvi Sachs The overall morphology of a plant is largely determined by developmental decisions taken within or
Plant Anatomy: roots, stems and leaves
 Plant Anatomy: roots, stems and leaves The plant body has a hierarchy of organs, tissues and cells Plants, like animals, have organs composed of different tissues, which are composed of cells. Tissue is
Plant Anatomy: roots, stems and leaves The plant body has a hierarchy of organs, tissues and cells Plants, like animals, have organs composed of different tissues, which are composed of cells. Tissue is
ARE YOU familiar with the sayings Get to
 Root Anatomy ARE YOU familiar with the sayings Get to the root of the problem or the root of all evil? Both these sayings suggest that the root is an essential part of something. With plants, the essential
Root Anatomy ARE YOU familiar with the sayings Get to the root of the problem or the root of all evil? Both these sayings suggest that the root is an essential part of something. With plants, the essential
Biology Slide 1 of 36
 Biology 1 of 36 2 of 36 Types of Roots Types of Roots What are the two main types of roots? 3 of 36 Types of Roots The two main types of roots are: taproots, which are found mainly in dicots, and fibrous
Biology 1 of 36 2 of 36 Types of Roots Types of Roots What are the two main types of roots? 3 of 36 Types of Roots The two main types of roots are: taproots, which are found mainly in dicots, and fibrous
Honors Biology I Ch 29 Plant Structure & Function
 3 Basic types of plant cells Honors Biology I Ch 29 Plant Structure & Function 1) Parenchyma cells- loosely packed or cells with a and thin, Involved in metabolic functions 2) Collenchyma cells- thicker
3 Basic types of plant cells Honors Biology I Ch 29 Plant Structure & Function 1) Parenchyma cells- loosely packed or cells with a and thin, Involved in metabolic functions 2) Collenchyma cells- thicker
ROOTS. Syllabus Theme A Plant Structure and Function. Root systems. Primary Growth of Roots. Taproot system. Fibrous root system.
 Syllabus Theme A lant Structure and Function A2: Structure and function of the basic plant organs ampbell & Reece hap. 35 Selected page numbers ROOTS Functions Anchors the vascular plant Absorbs minerals
Syllabus Theme A lant Structure and Function A2: Structure and function of the basic plant organs ampbell & Reece hap. 35 Selected page numbers ROOTS Functions Anchors the vascular plant Absorbs minerals
2/25/2013. o Plants take up water and minerals from below ground o Plants take up CO2 and light from above ground THREE BASIC PLANT ORGANS ROOTS
 o Plants take up water and minerals from below ground o Plants take up CO2 and light from above ground THREE BASIC PLANT ORGANS o Roots o Stems o Leaves ROOTS o Anchor plant o Absorb water and minerals
o Plants take up water and minerals from below ground o Plants take up CO2 and light from above ground THREE BASIC PLANT ORGANS o Roots o Stems o Leaves ROOTS o Anchor plant o Absorb water and minerals
23 2 Roots Slide 2 of 36
 2 of 36 Types of Roots Types of Roots What are the two main types of roots? 3 of 36 Types of Roots The two main types of roots are: taproots, which are found mainly in dicots, and fibrous roots, which
2 of 36 Types of Roots Types of Roots What are the two main types of roots? 3 of 36 Types of Roots The two main types of roots are: taproots, which are found mainly in dicots, and fibrous roots, which
today finish up cell division Continue intro to plant anatomy main plant organs basic anatomy: monocots versus dicots How to tell the organs apart
 Download as an RTF file Download as a PDF file Biology 20 Fall 2001 Lecture #4 Jan 18, 2001 What did we get from last lecture? Plant anatomy introduction Tissue Types in plants Four basic tissue: meristem,
Download as an RTF file Download as a PDF file Biology 20 Fall 2001 Lecture #4 Jan 18, 2001 What did we get from last lecture? Plant anatomy introduction Tissue Types in plants Four basic tissue: meristem,
CONTROL OF PLANT GROWTH AND DEVELOPMENT BI-2232 RIZKITA R E
 CONTROL OF PLANT GROWTH AND DEVELOPMENT BI-2232 RIZKITA R E The development of a plant the series of progressive changes that take place throughout its life is regulated in complex ways. Factors take part
CONTROL OF PLANT GROWTH AND DEVELOPMENT BI-2232 RIZKITA R E The development of a plant the series of progressive changes that take place throughout its life is regulated in complex ways. Factors take part
Plant Structure and Growth
 Plant Structure and Growth A. Flowering Plant Parts: The flowering plants or are the most diverse group of plants. They are divided into 2 classes and. Examples of monocots: Examples of dicots: The morphology
Plant Structure and Growth A. Flowering Plant Parts: The flowering plants or are the most diverse group of plants. They are divided into 2 classes and. Examples of monocots: Examples of dicots: The morphology
Molecular Genetics of. Plant Development STEPHEN H. HOWELL CAMBRIDGE UNIVERSITY PRESS
 Molecular Genetics of Plant Development STEPHEN H. HOWELL CAMBRIDGE UNIVERSITY PRESS Contents Preface A Word on Genetic Nomenclature page xiii xvii 1 Approaches to the Study of Plant Development 1 Pattern
Molecular Genetics of Plant Development STEPHEN H. HOWELL CAMBRIDGE UNIVERSITY PRESS Contents Preface A Word on Genetic Nomenclature page xiii xvii 1 Approaches to the Study of Plant Development 1 Pattern
Types of Plants. Unit 6 Review 5/2/2011. Plants. A. pine B. moss C. corn plant D. bean plant E. liverwort
 Unit 6 Review Plants Initial questions are worth 1 point each. Each question will be followed by an explanation All questions will be asked a second time at the very end, each of those questions will be
Unit 6 Review Plants Initial questions are worth 1 point each. Each question will be followed by an explanation All questions will be asked a second time at the very end, each of those questions will be
Plant Anatomy Lab 7 - Stems II
 Plant Anatomy Lab 7 - Stems II This exercise continues the previous lab in studying primary growth in the stem. We will be looking at stems from a number of different plant species, and emphasize (1) the
Plant Anatomy Lab 7 - Stems II This exercise continues the previous lab in studying primary growth in the stem. We will be looking at stems from a number of different plant species, and emphasize (1) the
Plant Structure and Function
 Plant Structure and Function A Meridian Biology AP Study Guide by John Ho and Tim Qi Plant Terms Growth: Growth Types Type Location Description Primary Primary Vertical growth (up-down), dominant direction
Plant Structure and Function A Meridian Biology AP Study Guide by John Ho and Tim Qi Plant Terms Growth: Growth Types Type Location Description Primary Primary Vertical growth (up-down), dominant direction
Roots and Soil Chapter 5
 Roots and Soil Chapter 5 Plant Organs Plant organs are groups of several types of tissues that together perform a particular function. Vegetative organs roots, stems, leaves make and use food, absorb water
Roots and Soil Chapter 5 Plant Organs Plant organs are groups of several types of tissues that together perform a particular function. Vegetative organs roots, stems, leaves make and use food, absorb water
Downloaded from
 POINTS TO REMEMBER : 6. Anatomy of Flowering Plants Study of internal structure of plant is called anatomy. In plants cells are the basic unit. Cells organized into tissues and tissues organized into organs.
POINTS TO REMEMBER : 6. Anatomy of Flowering Plants Study of internal structure of plant is called anatomy. In plants cells are the basic unit. Cells organized into tissues and tissues organized into organs.
Plant Structure and Function (Ch. 23)
 Plant Structure and Function (Ch. 23) Basic plant anatomy 1 root root tip root hairs Roots Roots anchor plant in soil, absorb minerals & water, & store food fibrous roots (1) mat of thin roots that spread
Plant Structure and Function (Ch. 23) Basic plant anatomy 1 root root tip root hairs Roots Roots anchor plant in soil, absorb minerals & water, & store food fibrous roots (1) mat of thin roots that spread
Lecture 4 Root Put line under your answer! There is only one correct answer in the multiple choice questions
 Lecture 4 Root Put line under your answer! There is only one correct answer in the multiple choice questions 1. The perception of gravity by a root is thought to take place in a) root hairs b) the region
Lecture 4 Root Put line under your answer! There is only one correct answer in the multiple choice questions 1. The perception of gravity by a root is thought to take place in a) root hairs b) the region
Auxin transport-feedback models of patterning in plantspce_1997
 1258..1271 Plant, Cell and Environment (2009) 32, 1258 1271 doi: 10.1111/j.1365-3040.2009.01997.x Auxin transport-feedback models of patterning in plantspce_1997 RICHARD S. SMITH & EMMANUELLE M. BAYER
1258..1271 Plant, Cell and Environment (2009) 32, 1258 1271 doi: 10.1111/j.1365-3040.2009.01997.x Auxin transport-feedback models of patterning in plantspce_1997 RICHARD S. SMITH & EMMANUELLE M. BAYER
Chapter 23 Notes Roots Stems Leaves
 Chapter 23 Notes Roots Stems Leaves I. Specialized tissue in plants - effective way to ensure the plant s survival A. Seed plant structure 1. Roots - a. Absorbs water and dissolves nutrients b. anchors
Chapter 23 Notes Roots Stems Leaves I. Specialized tissue in plants - effective way to ensure the plant s survival A. Seed plant structure 1. Roots - a. Absorbs water and dissolves nutrients b. anchors
Big Advantage!:Vegetative reproduction is a faster way to reproduce compared to sexual reproduction if the environment is favorable.
 DAY 5 OF CHAPTER 25 NOTES http://www.toto.com/misha/mavica/folliage2.jpg Asexual reproduction in plants is also known as vegetative reproduction. Methods of vegetative reproduction include plant structures
DAY 5 OF CHAPTER 25 NOTES http://www.toto.com/misha/mavica/folliage2.jpg Asexual reproduction in plants is also known as vegetative reproduction. Methods of vegetative reproduction include plant structures
PLS 622: Plant Physiology I, Wednesday, September 27, Vegetative development: Vascular tissue differentiation:
 PLS 622: Plant Physiology I, Wednesday, September 27, 2006. Vegetative development: Vascular tissue differentiation: Just prior to the completion of seed germination, the provascular tissue in the embryo
PLS 622: Plant Physiology I, Wednesday, September 27, 2006. Vegetative development: Vascular tissue differentiation: Just prior to the completion of seed germination, the provascular tissue in the embryo
Division Ave. High School AP Biology
 Monocots & dicots Angiosperm are divide into 2 classes dicots (eudicot) 2 cotyledons (seed leaves) leaves with network of veins woody plants, trees, shrubs, beans monocots 1 cotyledon leaves with parallel
Monocots & dicots Angiosperm are divide into 2 classes dicots (eudicot) 2 cotyledons (seed leaves) leaves with network of veins woody plants, trees, shrubs, beans monocots 1 cotyledon leaves with parallel
Chapter #35~ Plant Structure and Growth
 Chapter #35~ Plant Structure and Growth What part of a plant is represented by each of these: Carrot Celery Red Pepper Tomato Lettuce Garbanzo Bean Angiosperm structure Three basic organs: Roots (root
Chapter #35~ Plant Structure and Growth What part of a plant is represented by each of these: Carrot Celery Red Pepper Tomato Lettuce Garbanzo Bean Angiosperm structure Three basic organs: Roots (root
Page 1. Gross Anatomy of a typical plant (Angiosperm = Flowering Plant): Gross Anatomy of a typical plant (Angiosperm = Flowering Plant):
 Chapter 43: Plant Form and Function Gross Anatomy of a typical plant (Angiosperm = Flowering Plant): Root System Anchor plant Absorb water / nutrients Store surplus sugars Transport materials from / to
Chapter 43: Plant Form and Function Gross Anatomy of a typical plant (Angiosperm = Flowering Plant): Root System Anchor plant Absorb water / nutrients Store surplus sugars Transport materials from / to
Regulation of Vascular Development
 J Plant Growth Regul (2001) 20:1 13 DOI: 10.1007/s003440010008 2001 Springer-Verlag Thematic Articles Regulation of Vascular Development Nancy G. Dengler* Department of Botany, University of Toronto, 25
J Plant Growth Regul (2001) 20:1 13 DOI: 10.1007/s003440010008 2001 Springer-Verlag Thematic Articles Regulation of Vascular Development Nancy G. Dengler* Department of Botany, University of Toronto, 25
Plant Structure and Function Extension
 Plant Structure and Function Extension NGSSS: SC.912.L.14.7 Relate the structure of each of the major plant organs and tissues to physiological processes. (AA) Part 1A: Leaves The leaf of a plant serves
Plant Structure and Function Extension NGSSS: SC.912.L.14.7 Relate the structure of each of the major plant organs and tissues to physiological processes. (AA) Part 1A: Leaves The leaf of a plant serves
(A) Buds (B) Lateral meristem (C) Apical meristem (D) Stem (E) Trichomes
 AP Biology - Problem Drill 17: Plant Structure Question No. 1 of 10 1. What are hair-like outgrowths that protect and absorb nutrients? Question #01 (A) Buds (B) Lateral meristem (C) Apical meristem (D)
AP Biology - Problem Drill 17: Plant Structure Question No. 1 of 10 1. What are hair-like outgrowths that protect and absorb nutrients? Question #01 (A) Buds (B) Lateral meristem (C) Apical meristem (D)
Ginkgo leaf. Ginkgo is dioecious, separate sexes: male and female plants are separate. Monoecious plants have both male and female parts.
 Ginkgo leaf Figure 22-30 Ginkgo tree. Ginkgo is dioecious, separate sexes: male and female plants are separate. Monoecious plants have both male and female parts. The vein pattern is dichotomous: Divided
Ginkgo leaf Figure 22-30 Ginkgo tree. Ginkgo is dioecious, separate sexes: male and female plants are separate. Monoecious plants have both male and female parts. The vein pattern is dichotomous: Divided
Plant Organs. Roots & Stems
 Plant Organs Roots & Stems I. Roots A. F(x)s = grow underground 1. Absorb water & nutrients from soil 2. Anchor plant in the soil 3. Make hormones important for growth & development I. Roots B. Structure
Plant Organs Roots & Stems I. Roots A. F(x)s = grow underground 1. Absorb water & nutrients from soil 2. Anchor plant in the soil 3. Make hormones important for growth & development I. Roots B. Structure
(Photo Atlas: Figures 9.147, 9.148, 9.150, 9.1, 9.2, )
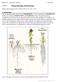 BIOL 221 Concepts of Botany Fall 2007 Topic 07: Primary Plant Body: The Root System (Photo Atlas: Figures 9.147, 9.148, 9.150, 9.1, 9.2, 9.5 9.23) A. Introduction The root has the primary functions of
BIOL 221 Concepts of Botany Fall 2007 Topic 07: Primary Plant Body: The Root System (Photo Atlas: Figures 9.147, 9.148, 9.150, 9.1, 9.2, 9.5 9.23) A. Introduction The root has the primary functions of
Plant Anatomy AP Biology
 Plant Anatomy 2006-2007 Basic plant anatomy 1 root root tip root hairs Roots 1 Roots anchor plant in soil, absorb minerals & water, & store food fibrous roots (1) mat of thin roots that spread out monocots
Plant Anatomy 2006-2007 Basic plant anatomy 1 root root tip root hairs Roots 1 Roots anchor plant in soil, absorb minerals & water, & store food fibrous roots (1) mat of thin roots that spread out monocots
CONTROL SYSTEMS IN PLANTS
 AP BIOLOGY PLANTS FORM & FUNCTION ACTIVITY #5 NAME DATE HOUR CONTROL SYSTEMS IN PLANTS HORMONES MECHANISM FOR HORMONE ACTION Plant Form and Function Activity #5 page 1 CONTROL OF CELL ELONGATION Plant
AP BIOLOGY PLANTS FORM & FUNCTION ACTIVITY #5 NAME DATE HOUR CONTROL SYSTEMS IN PLANTS HORMONES MECHANISM FOR HORMONE ACTION Plant Form and Function Activity #5 page 1 CONTROL OF CELL ELONGATION Plant
Anatomy of Plants Student Notes
 Directions: Fill in the blanks. Anatomy of Plants Student Notes Plant Cell Biology Segment 1. Plants Plants are organisms are incapable of movement produce food through 2. Animals Animals are multicellular
Directions: Fill in the blanks. Anatomy of Plants Student Notes Plant Cell Biology Segment 1. Plants Plants are organisms are incapable of movement produce food through 2. Animals Animals are multicellular
Formation of polarity convergences underlying shoot outgrowths
 RESEARCH ARTICLE Formation of polarity convergences underlying shoot outgrowths Katie Abley, Susanna Sauret-Güeto, Athanasius FM Marée, Enrico Coen* John Innes Centre, Norwich Research Park, Norwich, United
RESEARCH ARTICLE Formation of polarity convergences underlying shoot outgrowths Katie Abley, Susanna Sauret-Güeto, Athanasius FM Marée, Enrico Coen* John Innes Centre, Norwich Research Park, Norwich, United
Leaf and Internode. Introduction. Parts of the Monocot and Dicot Leaf. Introductory article
 Andrew Hudson, University of Edinburgh, Edinburgh, UK Christopher Jeffree, University of Edinburgh, Edinburgh, UK Leaves of different species show wide variation in morphology and anatomy, usually associated
Andrew Hudson, University of Edinburgh, Edinburgh, UK Christopher Jeffree, University of Edinburgh, Edinburgh, UK Leaves of different species show wide variation in morphology and anatomy, usually associated
Chapter 29. Table of Contents. Section 1 Plant Cells and Tissues. Section 2 Roots. Section 3 Stems. Section 4 Leaves. Plant Structure and Function
 Plant Structure and Function Table of Contents Section 1 Plant Cells and Tissues Section 2 Roots Section 3 Stems Section 4 Leaves Section 1 Plant Cells and Tissues Objectives Describe the three basic types
Plant Structure and Function Table of Contents Section 1 Plant Cells and Tissues Section 2 Roots Section 3 Stems Section 4 Leaves Section 1 Plant Cells and Tissues Objectives Describe the three basic types
PLANTS FORM AND FUNCTION PLANT MORPHOLOGY PART I: BASIC MORPHOLOGY. Plant Form & Function Activity #1 page 1
 AP BIOLOGY PLANTS FORM AND FUNCTION ACTIVITY #1 NAME DATE HOUR PLANT MORPHOLOGY PART I: BASIC MORPHOLOGY Plant Form & Function Activity #1 page 1 PART II: ROOTS 1. Examine the examples of the two root
AP BIOLOGY PLANTS FORM AND FUNCTION ACTIVITY #1 NAME DATE HOUR PLANT MORPHOLOGY PART I: BASIC MORPHOLOGY Plant Form & Function Activity #1 page 1 PART II: ROOTS 1. Examine the examples of the two root
Apical dominance models can generate basipetal patterns of bud activation
 Apical dominance models can generate basipetal patterns of bud activation Przemyslaw Prusinkiewicz 1, Richard S. Smith 1 and Ottoline Leyser 2 1 Department of Computer Science, University of Calgary 2
Apical dominance models can generate basipetal patterns of bud activation Przemyslaw Prusinkiewicz 1, Richard S. Smith 1 and Ottoline Leyser 2 1 Department of Computer Science, University of Calgary 2
Today: Plant Structure Exam II is on F March 31
 Next few lectures are on plant form and function Today: Plant Structure Exam II is on F March 31 Outline Plant structure I. Plant Cells structure & different types II. Types of meristems Apical meristems:
Next few lectures are on plant form and function Today: Plant Structure Exam II is on F March 31 Outline Plant structure I. Plant Cells structure & different types II. Types of meristems Apical meristems:
Plant Development. Chapter 31 Part 1
 Plant Development Chapter 31 Part 1 Impacts, Issues Foolish Seedlings, Gorgeous Grapes Gibberellin and other plant hormones control the growth and development of plants environmental cues influence hormone
Plant Development Chapter 31 Part 1 Impacts, Issues Foolish Seedlings, Gorgeous Grapes Gibberellin and other plant hormones control the growth and development of plants environmental cues influence hormone
Introduction to Botany. Lecture 25
 Introduction to Botany. Lecture 25 Alexey Shipunov Minot State University November 2, 2015 Shipunov (MSU) Introduction to Botany. Lecture 25 November 2, 2015 1 / 33 Outline 1 Questions and answers 2 Stem
Introduction to Botany. Lecture 25 Alexey Shipunov Minot State University November 2, 2015 Shipunov (MSU) Introduction to Botany. Lecture 25 November 2, 2015 1 / 33 Outline 1 Questions and answers 2 Stem
Level 2 Plant Growth and Development Part I Toby Day MSU Extension Horticulture Associate Specialist
 Level 2 Plant Growth and Development Part I Toby Day MSU Extension Horticulture Associate Specialist Pages 24-38 Montana Master Gardener Handbook Plant Growth and Development Whole Plant Organs Tissues
Level 2 Plant Growth and Development Part I Toby Day MSU Extension Horticulture Associate Specialist Pages 24-38 Montana Master Gardener Handbook Plant Growth and Development Whole Plant Organs Tissues
Plant Structure And Growth
 Plant Structure And Growth The Plant Body is Composed of Cells and Tissues Tissue systems (Like Organs) made up of tissues Made up of cells Plant Tissue Systems Ground Tissue System Ø photosynthesis Ø
Plant Structure And Growth The Plant Body is Composed of Cells and Tissues Tissue systems (Like Organs) made up of tissues Made up of cells Plant Tissue Systems Ground Tissue System Ø photosynthesis Ø
TARGET STUDY MATERIAL
 TARGET STUDY MATERIAL Plus-1 Botany VOL I TARGET EDUCATIONAL INSTITUTION Target Educational institution is the one and only Entrance coaching and CBSE 10 th coaching centre at Mukkam with advanced technologies
TARGET STUDY MATERIAL Plus-1 Botany VOL I TARGET EDUCATIONAL INSTITUTION Target Educational institution is the one and only Entrance coaching and CBSE 10 th coaching centre at Mukkam with advanced technologies
PLANT STRUCTURE: PARTS (ORGANS) Roots Leaves Stems
 PLANT STRUCTURE: PARTS (ORGANS) Roots Leaves Stems ROOTS El Hiquieron. Strangulating Plant Ficusjimenezii The trees you see growing on the wall are the Higueron. The Higueronsare plants that can grow in
PLANT STRUCTURE: PARTS (ORGANS) Roots Leaves Stems ROOTS El Hiquieron. Strangulating Plant Ficusjimenezii The trees you see growing on the wall are the Higueron. The Higueronsare plants that can grow in
Plant Stimuli pp Topic 3: Plant Behaviour Ch. 39. Plant Behavioural Responses. Plant Hormones. Plant Hormones pp
 Topic 3: Plant Behaviour Ch. 39 Plants exist in environments that are constantly changing. Like animals, plants must be able to detect and react to stimuli in the environment. Unlike animals, plants can
Topic 3: Plant Behaviour Ch. 39 Plants exist in environments that are constantly changing. Like animals, plants must be able to detect and react to stimuli in the environment. Unlike animals, plants can
Cytokinin. Fig Cytokinin needed for growth of shoot apical meristem. F Cytokinin stimulates chloroplast development in the dark
 Cytokinin Abundant in young, dividing cells Shoot apical meristem Root apical meristem Synthesized in root tip, developing embryos, young leaves, fruits Transported passively via xylem into shoots from
Cytokinin Abundant in young, dividing cells Shoot apical meristem Root apical meristem Synthesized in root tip, developing embryos, young leaves, fruits Transported passively via xylem into shoots from
can affect division, elongation, & differentiation of cells to another region of plant where they have an effect
 Note that the following is a rudimentary outline of the class lecture; it does not contain everything discussed in class. Plant Hormones Plant Hormones compounds regulators growth or can affect division,
Note that the following is a rudimentary outline of the class lecture; it does not contain everything discussed in class. Plant Hormones Plant Hormones compounds regulators growth or can affect division,
Reproduction, Seeds and Propagation
 Reproduction, Seeds and Propagation Diploid (2n) somatic cell Two diploid (2n) somatic cells Telophase Anaphase Metaphase Prophase I One pair of homologous chromosomes (homologues) II Homologues condense
Reproduction, Seeds and Propagation Diploid (2n) somatic cell Two diploid (2n) somatic cells Telophase Anaphase Metaphase Prophase I One pair of homologous chromosomes (homologues) II Homologues condense
CBSE Quick Revision Notes (Class-11 Biology) CHAPTER-15 PLANT GROWTH AND DEVELOPMENT
 CBSE Quick Revision Notes (Class-11 Biology) CHAPTER-15 PLANT GROWTH AND DEVELOPMENT Root, stem leaves, flower, fruits and seeds arise in orderly manner in plants. The sequence of growth is as follows-
CBSE Quick Revision Notes (Class-11 Biology) CHAPTER-15 PLANT GROWTH AND DEVELOPMENT Root, stem leaves, flower, fruits and seeds arise in orderly manner in plants. The sequence of growth is as follows-
Embryo Development. Embryo Development. Embryo Development. Embryo Development (Cont.) Vegetative Plant Development
 Vegetative Plant Development Chapter 37 Embryo Development Begins once the egg cell is fertilized -The growing pollen tube enters angiosperm embryo sac and releases two sperm cells -One sperm fertilizes
Vegetative Plant Development Chapter 37 Embryo Development Begins once the egg cell is fertilized -The growing pollen tube enters angiosperm embryo sac and releases two sperm cells -One sperm fertilizes
