Structure of the Vertical and Horizontal System Neurons of the Lobula Plate in Drosophila
|
|
|
- Tyler McLaughlin
- 6 years ago
- Views:
Transcription
1 THE JOURNAL OF COMPARATIVE NEUROLOGY 454: (2002) Structure of the Vertical and Horizontal System Neurons of the Lobula Plate in Drosophila ETHAN K. SCOTT, 1 * THOMAS RAABE, 2 AND LIQUN LUO 1,3 1 Department of Biological Sciences, Stanford University, Stanford, California Department of Genetics and Neurobiology, University of Wuerzburg, Biozentrum, Am Hubland, and Institute of Medical Radiation and Cell Research, Wuerzburg, Germany 3 Neurosciences Program, Stanford University, Stanford, California ABSTRACT The lobula plate in the optic lobe of the fly brain is a high-order processing center for visual information. Within the lobula plate lie a small number of giant neurons that are responsible for the detection of wide field visual motion. Although the structure and motion sensitivity of these cells have been extensively described in large flies, the system has not been described systematically in Drosophila. Here, we use the mosaic analysis with a repressible cell marker (MARCM) system to analyze a subset of these cells, the horizontal and vertical systems. Our results suggest that the Drosophila horizontal system is similar to those described in larger flies, with three neurons fanning their dendrites over the lobula plate. We found that there are six neurons in the Drosophila vertical system, a figure that compares with 9 11 neurons in large flies. Even so, the Drosophila vertical system closely resembles the systems of larger flies, with each neuron in Drosophila having an approximate counterpart in large flies. This anatomical similarity implies that the inputs to the vertical system are similarly organized in these various fly species, and that it is likely that the Drosophila neurons respond to motions similar to those sensed by their specific structural counterparts in large flies. Additionally, the similar appearance of vertical system cells in multiple cell clones demonstrates that they share a common developmental lineage. Access to these cells in Drosophila should allow for the use of genetic tools in future studies of horizontal and vertical system function. J. Comp. Neurol. 454: , Wiley-Liss, Inc. Indexing terms: dendrites; optic flow; optic lobe In order to navigate through their complex natural habitat, many flying insects rely heavily on vision. Particularly important for flight control is information on the movement of the fly s body itself. When a fly changes orientation or moves in some direction, its entire visual field shifts in a manner specific to the maneuver being performed (Koenderink and van Doorn, 1987). For example, as an insect engages in straight level flight, objects in its surroundings flow from the front to the back of its visual field. In dipteran insects, the integration of such inputs is carried out largely by giant neurons of the lobula plate in the lobula complex (Dvorak et al., 1975). These neurons have been characterized in great anatomical and physiological detail in various large flies. Initial anatomical analyses of the lobula plate in the housefly (Musca domestica) revealed two systems of giant neurons, which were termed the horizontal system (HS) and vertical system (VS) (Pierantoni, 1976; Strausfeld, 1976a). The horizontal system comprises three giant neurons, HS north (HSN), HS equatorial (HSE), and HS south (HSS), whose overlapping dendrites fan out over the dorsal, middle, and ventral parts of the lobula plate, respectively. The Grant sponsor: the National Institutes of Health; Grant numbers: 2 TR32 HD and R01-NS *Correspondence to: Ethan K. Scott, Department of Biological Sciences, Stanford University, Stanford, California escott@stanfordalumni.org Received 3 April 2002; Revised 8 August 2002; Accepted 23 August 2002 DOI /cne Published online the week of November 11, 2002 in Wiley InterScience ( WILEY-LISS, INC.
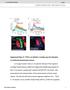
2 ANATOMY OF THE DROSOPHILA VERTICAL SYSTEM vertical system in the housefly was shown to have nine cells, each of which has a dendritic arborization covering a band stretching out along the dorsal-ventral axis of the lobula plate. The axons of these cells extend to the posterior slope in the periesophageal region, where they synapse onto the ocellar fibers and a branch of a huge fiber of the cervical connective (Strausfeld, 1976a; Heisenberg et al., 1978). Detailed descriptions in the blowflies Calliphora erythrocephala (Hausen et al., 1980; Hengstenberg et al., 1982) and Phaenicia sericata (Eckart and Bishop, 1978) indicate that the vertical systems in these species have 9 11 cells with structures similar to their counterparts in Musca. The fly visual system is organized as a retinotopic map, whereby the visual field is represented spatially as input into a given layer of the optic lobe (Strausfeld, 1976b). This map persists through the first and second optic neuropils, the lamina and medulla, and through the input to the lobula complex, comprising the lobula and the lobula plate. However, output of the vertical system and horizontal system from this neuropil is not organized into a retinotopic map (Strausfeld, 1976b). These facts imply that the conversion from the input of spatially organized visual information to output relating to specific wide-field stimuli takes place in part in these cells. In support of this idea, studies have shown that activity in discrete anteriorposterior layers of the lobula plate correspond not to regions of the visual field, but to directions of optic flow in the visual field as a whole. Specifically, four layers are active in response to front to back (most anterior layer), back to front (middle anterior), upward (middle posterior), and downward (most posterior) motions (Buchner and Buchner, 1984; Buchner et al., 1984). This schematic organization of the input to the lobula plate agrees well with experiments that have characterized the function of the vertical system in vivo. In numerous experiments on blowflies, VS neurons have been shown to depolarize in response to wide-field motion in their preferred direction. In its simplest form, this stimulus can be a downward moving pattern of horizontal stripes (Hengstenberg, 1977; Soohoo and Bishop, 1980; Hengstenberg, 1982; Single and Borst, 1998). Certain VS neurons have also shown responses to horizontal shifts in the visual field (Eckert and Bishop, 1978; Eckert, 1982). These observations and others led to models in which certain neurons in the lobula and lobula plate act as detectors of wide-field motions in the visual field (Egelhaaf et al., 1988; Borst and Egelhaaf, 1989; Egelhaaf and Borst, 1993). In accordance with these models, a more extensive analysis has indicated that VS neurons respond most robustly to wide-field shifts that would accompany flight motions such as changes in pitch or roll (Krapp et al., 1998). It is therefore postulated that the vertical system functions in sensing self-motion during flight. Although the horizontal and vertical systems have been characterized extensively in the housefly and blowfly, structural information on these cells in Drosophila is somewhat sparse. In terms of cellular composition, the Drosophila horizontal system appears to be similar to those in large flies, with three cells extending their fanshaped dendrites through the lobula plate (Heisenberg et al., 1978; Fischbach and Dittrich, 1989). The vertical system is simpler than those of the housefly and blowfly, with only five to seven cells (Heisenberg et al., 1978). Golgi staining in Drosophila has shown the VS neurons to be 471 generally similar to those described in larger flies (Fischbach and Dittrich, 1989; Buschbeck and Strausfeld, 1997), but the system as a whole has yet to be described in detail. In the current study, we report the general structures of the HS and VS neurons in the lobula plate in Drosophila and provide a detailed analysis of the dendritic organization of individual VS neurons. MATERIALS AND METHODS Fly culture, histology, and microscopy Drosophila melanogaster were grown on standard media at 25 C. During clonal analysis, larvae hatched over a 2-hour interval were moved to plastic vials containing approximately 10 ml of food. Larvae were kept at a concentration of 80 per vial. Mitotic recombination was induced via heat shock (40 minutes in a 37 C water bath, 30 minutes at room temperature, 40 minutes in 37 C water bath) at 2 and 3 days after hatching. Adult female flies between 2 and 5 days after eclosure were dissected, fixed, and stained as described (Lee and Luo, 1999). Briefly, dissected brains were fixed in 4% paraformaldehyde in 100 mm phosphate buffer (ph 7.2) for 30 minutes at room temperature and then washed 6 10 minutes in phosphate buffer (ph 7.2) with 0.1% Triton X-100 (PBT). They were then incubated in PBT with 5% normal goat serum for 30 minutes and stained with 100 diluted rat antimcd8- chain (Caltag Laboratories. Burlingame, CA) overnight at 4 C. They were washed 6 10 minutes in PBT and then incubated for 2 hours with 500 diluted goat anti-rat CY3-conjugated antibody (Jackson ImmunoResearch, West Grove, PA) in PBT. A final minute washes in PBT preceded mounting using Slow- Fade (Molecular Probes, Eugene, OR). A Bio-Rad (Hercules, CA) MRC 1024 laser scanning confocal microscope and the Laser Sharp image collection program were used. Images were processed using Adobe Photoshop. Three-dimensional traces of the dendrites were produced from confocal stacks using MicroBright- Field Neurolucida software. Briefly, dendritic branches were traced such that turning points, branch points, and endpoints were specified in X, Y, and Z positions within the confocal stack. These points were then combined to provide the overall shape of the dendritic arborizations. Traces were then rotated using Rotator 3.5 software. Fly strains and the MARCM system The Gal4-3A line was isolated in an enhancer-trap screen for lines that show specific expression patterns in the adult brain (T.R., unpublished data). P[Gal4] lines were generated as described (Brand and Perrimon, 1993) and crossed to the UAS (Upstream Activating Sequence) -tau reporter (Ito et al., 1997b). The progeny were analyzed by mass histology (Heisenberg and Bohl, 1979) and immunohistochemistry with an anti-tau antibody. Once the Gal4-3A driver was isolated, its nonclonal expression pattern was characterized in a UAS-mCD8-GFP / ; GAL4-3A / female. The MARCM system requires that the GAL80 gene, a repressor of GAL4-mediated expression from a UAS, be eliminated from a cell or clone of cells, thus allowing for the expression of a UAS-driven marker gene (Lee and Luo, 1999). Elimination of GAL80 is allowed by the inclusion of Flp recombinase target (FRT) proximal to GAL80 on the
3 472 E.K. SCOTT ET AL. Fig. 1. Expression pattern of Gal4-3A. A: Posterior view of the entire brain. The cell bodies (arrowhead) and dendrites (arrows) of the VS neurons can be seen. Other cells and their neurites, which have not been characterized, are visible, particularly in the protocerebrum. The neurons of the horizontal system are too faint to be seen in a nonclonal analysis. They are only visualized when MARCM is performed. The box shows the area magnified in B. B: Close-up of the optic lobe showing the position of the vertical system dendrites (arrows) and cell bodies (arrowhead). Scale bar 100 m ina;50 m in B. chromosome being used for recombination and on the homologous chromosome. The flp gene is included and driven by a heat shock promoter, thus allowing for heat shockinduced mitotic recombination at the FRT sites. The resulting elimination of GAL80 in this cell or clone of cells allows the driver (Gal4-3A), to cause expression of the membrane localized marker, mcd8-gfp (Lee and Luo, 1999), which is later used to visualize the cell or cells. The limited expression pattern of Gal4-3A prevents the marking of a large number of background clones. This results in a system that allows for the positive marking of a cell or clone of cells in a dark background. Such positive staining is critical for the analysis of fine cellular structures such as dendrites. Inverse PCR showed the Gal4-3A driver to be located at 66A on 3L. Accordingly, the following female genotypes were created for the analysis using MARCM: tubp- GAL80, hs-flp, FRT 19A / yw, FRT 19A ; UAS-mCD8-GFP / ; GAL4-3A / for recombination on X, hs-flp, UASmCD8-GFP / ; tubp-gal80, FRT 40A / FRT 40A ; GAL4-3A / for recombination on 2L, hs-flp / ; FRT G13, tubp-gal80 / FRT G13, UAS-mCD8-GFP ; GAL4-3A / for recombination on 2R, and hs-flp, UAS-mCD8-GFP / ; tubp-gal80, FRT 2A / GAL4-3A, FRT 2A for recombination on 3L. RESULTS The Gal4-3A driver The lobula complex is in the most medial portion of the optic lobe and comprises the anterior lobula and the posterior lobula plate. A previous analysis of the Drosophila lobula plate has revealed three systems of giant neurons: the vertical system with five to seven cells, the horizontal system with three cells, and the two M-cells (Heisenberg et al., 1978). To describe the fine structural details of these cells, we were interested in visualizing them using the MARCM system, which depends on a tissue-specific Gal4 driver (Lee and Luo, 1999). The Gal4-3A driver shows expression in a limited number of cell types throughout the brain (Fig. 1A). Within the optic lobe, the cells of the vertical system are notable among those expressing Gal4-3A, but a generally high background of marked cells indicates that Gal4-3A is expressed in cells other than the VS neurons (Fig. 1). Giant neurons of the Drosophila lobula plate The MARCM system in Drosophila allows for the marking of individual cells or groups of clonally related cells in an otherwise unlabeled background (Lee and Luo, 1999). We have used this system in conjunction the Gal4-3A driver to label the HS and VS neurons of the lobula plate. Using MARCM, we can label the cells of each of these systems in great structural detail. Although the HS and VS neurons are the most prominent among the clones that we create using Gal4-3A, a few other cell types appear in the optic lobes and elsewhere in the brain (data not shown). Because mitotic recombination can lead to Gal4-UAS activity either in a dividing neuroblast or in a ganglion mother cell, clones with different numbers of cells form (Fig. 2A). When a neuroblast loses the Gal80 repressor as a result of recombination early in development, all the progeny of this cell have the potential to be marked (Lee and Luo, 1999). This can lead to a neuroblast clone in which the rest of the of this lineage is labeled. Interestingly, we find that the cells of the vertical system frequently appear in the same neuroblast clone, indicating that they share a common lineage. Such neuroblast clones in the vertical system allow for a characterization of the system as a whole (Fig. 2B). As in larger flies, the dendrites of the vertical system cover the lobula plate broadly in the dorsal-ventral and
4 ANATOMY OF THE DROSOPHILA VERTICAL SYSTEM Fig. 2. Clone formation using MARCM. A: Schematic diagram showing a typical CNS neuroblast division pattern in Drosophila. NB, neuroblast; G, ganglion mother cell; N, neuron. Shaded circles represent those that will be marked as members of the clone lacking GAL80. Mitotic recombination caused by the expression of Flipase (FLP) in a neuroblast can result in an NB clone (i), or a two-cell clone (ii), depending on whether the daughter gaining Gal4-UAS activity is the neuroblast (i) or the ganglion mother cell (ii). Recombination in a ganglion mother cell results in a single cell clone (iii). (Adapted from Lee et al., 1999). B: Posterior view of the entire brain. Neuroblast clones containing most or all of both vertical systems can be seen with their dendrites (small arrows) covering the lobula plates dorsoventrally and mediolaterally. The anterior-posterior axis will be described in later figures. The axons extend to the center of the protocerebrum and divide into dorsal (arrows) and ventral (arrowhead) branches. The dotted line outlines the brain. Scale bar 100 m. medial-lateral axes (Fig. 2B). The anterior-posterior structure of these dendrites will be addressed as we describe these cells individually (see Figs. 4 9). The axons of these cells travel medially and terminate near the esophagus (Fig. 2B). The horizontal system comprises three individually identifiable cells extending their dendrites over the dorsal (HSN, Fig. 3A), central (HSE, Fig. 3B), and ventral (HSS, Fig. 3C) lobula plate.through extensive bifurcation, the dendrites of each of these cells covers a wide area of the lobula plate, resulting in significant overlap of the 473 dendritic fields of the HS cells. As judged from confocal images, these dendritic fields are quite flat in the anteriorposterior axis. Their axons extend medially and ventrally within the brain in a manner similar to that described in large flies (Strausfeld, 1976a). In all these respects, the HS neurons appear to be similar to those described in Drosophila using Golgi staining (Fischbach and Dittrich, 1989). In addition to the cells of the horizontal system and vertical system, we occasionally see other giant neurons in the lobula plate (data not shown), possibly representing the previously described M-cells (Heisenberg et al., 1978). Individual neurons in the vertical system To characterize the complex and overlapping dendrites of the VS neurons, we have imaged and traced single cell clones and observed their dendritic structures in three dimensions. As in other flies vertical systems, the most complex cells extend their dendrites into the most lateral area of the lobula plate. The outermost cell, VS1, is characterized by a main dendritic shaft that produces one or a few dorsally projecting branches before sweeping ventrally (Fig. 4A). As the main shaft extends ventrally, it continues to produce smaller branches that combine to form a narrow band covering the most lateral part of the lobula plate. Like its counterparts in larger flies, this cell is relatively flat but extends anteriorly in the dorsal aspect of its dendritic tree (Fig. 4D). The VS2 cell extends its dendrites into a region of the lobula plate that is medial to, but overlapping that of the VS1 cell (see below). Its overall dendritic structure, with a major dendritic shaft sweeping from dorsal to ventral, is similar to that of VS1, but there are a few recognizable differences (Fig. 5). The VS2 cell is less complex, with fewer dendritic branches than VS1. Additionally, it invariably has a single dorsally projecting dendrite, which often extends away from the main dendritic shaft before branching further (Fig. 5A). By comparison, VS1 has dorsal dendrites that branch almost immediately upon leaving the main dendritic shaft (Fig. 4A). Finally, VS2 is extremely flat in the anterior-posterior axis, with all of its dendritic structures among the posterior dendrites of the vertical system (Fig. 5D; also see below). The central two cells of the vertical system are similar in structure to one another. Compared with VS1 and VS2, VS3 and VS4 are less defined by a dominant dendritic shaft that sweeps from dorsal to ventral (Figs. 6A, 7A). Although VS3 and VS4 cells have a ventrally sweeping dendrite, they also have major dendrites projecting and branching dorsally. This makes these dendritic trees more nearly symmetrical along their dorsal-ventral axis than the other members of the vertical system. Also unlike most other VS neurons, VS3 and VS4 elaborate their dorsal dendrites at different anterior-posterior levels of the dorsal lobula plate (Figs. 6C,D, 7C,D). There are two criteria that allow VS3 and VS4 to be distinguished. First, the dorsal dendrites of VS3 continue to slant laterally as they extend dorsally (Fig. 6A), whereas these dendrites in VS4 curve so that they extend directly dorsally and even slightly medially (Fig. 7A). Second, the dorsal dendrites of VS3 have a major component that extends anteriorly (Fig. 6D), whereas anterior projections are few and simple in VS4 cells (Fig. 7D). Using these criteria, it is generally possible to distinguish between single VS3 and single VS4
5 474 E.K. SCOTT ET AL. Fig. 3. The HS neurons. A: A single cell clone of an HSN cell. Its dendrites can be seen covering the dorsal aspect of the lobula plate (arrows), and its axon extends medially and ventrally into the protocerebrum (arrowhead). B: An HSE cell. The dendrites are in the center of the lobula plate dorsoventrally. C: An HSS cell. The dendrites are in the ventral part of the lobula plate. Dotted lines show the edges of the optic lobe. D, dorsal; V, ventral; M, medial; L, lateral. Scale bars 25 m. Fig. 4. The VS1 neuron. A: Posterior view of the dendrites of a VS1 neuron. The dendritic tree is dominated by a main dendritic shaft, which sweeps from dorsal to ventral. Branches depart in an approximately radial fashion from this main shaft to cover the characteristic field of VS1. B: A three-dimensional tracing of the dendrites from A, viewed from the same perspective. C: The same tracing, rotated 45. D: The same tracing, rotated 90 to show anterior extension by the dorsal dendrites (arrowhead). For details on the structure of this cell, see the text. A, anterior; P, posterior. Scale bar 25 m and applies to all panels. cells, but the structures are sufficiently similar that this distinction sometimes cannot be made. The final two members of the vertical system are notable for branching predominantly in the dorsal lobula plate (Figs. 8, 9). Both VS5 and VS6 have ventral dendrites with relatively little complexity. They can be distinguished based on two characteristics. First, VS5 projects at least two major dendritic branches dorsally, one from the initial dorsal extension, and one from a major branch that initially grows into the central lobula plate before contributing a dorsally extending branch (Fig. 8A). In contrast, VS6 has a single major dorsal branch that is an extension of the original dorsal arborization (Fig. 9A). Additionally, VS5 sends its dorsal dendrites dramatically anteriorly in the lobula plate, generally 20 m or more out of the plane formed by the ventral dendrites (as judged from confocal stacks; data not shown). VS6 has anteriorly projecting dorsal dendrites, but they tend to extend approximately 10 m out of this plane.
6 ANATOMY OF THE DROSOPHILA VERTICAL SYSTEM 475 Fig. 5. The VS2 neuron. A: Posterior view of the dendrites of a VS2 neuron. The overall shape of the dendritic field is similar to that seen for VS1, but the dendrites are less complex. B: A three-dimensional tracing of the dendrites from A, viewed from the same perspective. C: The same tracing, rotated 45. D: The same tracing, rotated 90, shows the dendritic structure to be flat in the anterior-posterior axis. For details on the structure of this cell, see the text. Scale bar 25 m and applies to all panels. Stereotypy in the VS1 neuron One important property of any cellular system used for the genetic study of dendrites is stereotypy in the dendritic structure from animal to animal. In order to characterize the level of stereotypy among vertical system dendrites, we have analyzed one example, the VS1 neuron, in detail for 12 different animals. Examples can be seen in Figure 10. All VS1 dendrites observed are dominated by a major dendritic shaft that sweeps from the dorsal to the ventral part of the dendritic field. In most cases, this shaft gives rise to smaller branches but is itself unbranched (Fig. 10A C). Occasionally, there is a bifurcation of this major shaft in the ventral aspect of the field (Fig. 10D). The other major variability found among VS1 dendrites is the manner in which the dorsal part of the field is innervated. In some cases, a single major branch accounts for all the dorsal dendrites (Fig. 10A,B), whereas other cells have multiple dorsal dendrites (Fig. 10C,D). Despite these two variations in the details of VS1 dendrites, there are important ways in which VS1 dendrites are invariant. The overall three-dimensional dendritic field is almost identical from one animal to another regardless of the specific branching characteristics. This field involves dorsal dendrites that extend anteriorly in the lobula plate and ventral dendrites that remain in the posterior (Fig. 4D; data not shown for dendrites in Fig. 10). Also, the total complexity of the dendritic trees and total length of the dendrites in a given VS1 cell are highly consistent, regardless of the manner in which the dendrite branches (unpublished observations). These observations indicate that, although there are some differences in the structural details of these dendrites from animal to animal, there are important invariant features that should allow members of the vertical system to be used in studies of dendrite development. Our experience with all the members of the vertical system leads us to believe that the level of stereotypy is similar among other cells in the vertical system (data not shown). Relative position of the vertical system cells Although looking at these cells individually is necessary for characterizing their structures in detail, we need to consider them as a group in order to characterize the overall dendritic structure of the vertical system. This can be done by analyzing neuroblast clones (Fig. 11). As mentioned previously, the VS neurons as a group send their dendrites over a large medial-lateral and dorsal-ventral field. In the ventral part of the lobula plate, the dendrites of individual cells form distinct but overlapping bands of innervation (Fig. 11A,B). In the dorsal area, the fields of these cells dendrites overlap more extensively and are difficult to distinguish without information about their anterior-posterior position. Looking at neuroblast clones from various angles reveals that the ventral dendrites of the system as a whole are extremely flat, with little anterior-posterior depth (Fig. 11C). The dorsal dendrites, as we have already shown for individual cells, spread over a much greater anterior-posterior range (Fig. 11C E). An interesting detail of this factor is that anteriorly extending
7 476 E.K. SCOTT ET AL. Fig. 6. The VS3 neuron. A: Posterior view of the dendrites of the VS3 neuron. The dendrites are almost symmetrical dorsoventrally and are not as heavily defined by a single main dendritic shaft as those of VS1 and VS2. Note that the major dorsal dendrite continues to extend slightly laterally as it extends dorsally (arrowhead). B: Three-dimensional tracings of the dendrites from A, viewed from the same perspective. C: The same tracing, rotated 45. D: The same tracing, rotated 90, showing complex anterior extensions (arrowhead). For details on the structure of this cell, see the text. Scale bar 25 m and applies to all panels. dendrites tend to remain unbranched until they reach a certain anterior-posterior depth, at which point they elaborate in a nearly two-dimensional manner (Fig. 11C E). Among the cells extending dendrites anteriorly, VS5 appears to innervate most anteriorly, whereas VS1 and VS3 extend dendrites less dramatically in the anterior direction. The VS2, VS4, and VS6 neurons appear to branch mostly or completely posteriorly. This may mean that the cells receive specific types of visual information based on the anterior-posterior depth that their dendrites reach (see Discussion). DISCUSSION Lineage of the VS neurons Using the MARCM system, we have individually labeled the giant neurons of the horizontal system and vertical system in the Drosophila lobula plate. All show complex dendritic arborizations through wide areas of the lobula plate and resemble their counterparts in large flies in all major respects. In addition to labeling individual cells, the MARCM system can be used to study the lineage and birth order of groups of cells. By varying induction times for mitotic recombination and by looking at the content of partial neuroblast clones, we have previously been able to infer the birth orders for distinct groups of mushroom body neurons and for antennal lobe projection neurons (Lee et al., 1999; Jefferis et al., 2001). These experiments and others have allowed for the cellular characterization of development in various neural tissues. For example, the approximately 2,400 cells of the Drosophila mushroom bodies have been shown to be derived from four neuroblasts, which divide throughout development in the manner diagrammed in Figure 2A (Ito and Hotta, 1992). Three morphologically distinct neuron types are among those born from these neuroblasts, and the different types are born during consistent windows during development (Lee et al., 1999). This provides an example of a neural tissue in which numerous and diverse cells are born from a small number of neuroblasts, each of which makes an indistinguishable contribution (Ito et al., 1997a). The projection neurons of the Drosophila olfactory system provide a contrasting program, in which a large number of cells with various different structures are derived from three neuroblasts, each of which makes a unique contribution (Jefferis et al., 2001). These two programs have in common the fact that different morphologically distinct neurons are born in an invariant order and during specific and consistent times of development. Experiments along these lines were uninformative with regard to the order in which the VS neurons are born. Among single cell clones, we found no correlation between the time of recombination (via heatshock from 0 to 7 days after larval hatching) and the member of the vertical system that was labeled. Indeed, only heatshocks at 2 or 3 days after larval hatching produced single cell clones consistently, and both times provided single cell clones rep-
8 ANATOMY OF THE DROSOPHILA VERTICAL SYSTEM 477 Fig. 7. The VS4 neuron. A: Posterior view of the dendrites of the VS4 neuron. Overall, the structure is similar to that described for VS3 (see text and Fig. 6). Unlike VS3, the dorsal dendrite curves medially as it extends dorsally (arrowhead). B: Three-dimensional tracings of the dendrites from A, viewed from the same perspective. C: The same tracings, rotated 45. D: The same tracings, rotated 90. Compared with VS3, this dendritic tree shows few and simple anterior extensions among the dorsal dendrites (arrows). For details on the structure of this cell, see text. Scale bar 25 m and applies to all panels. resenting a full range of VS neuron types. This would not be unexpected if the neurons were all born in rapid succession, because there is some heterogeneity in the speed at which different animals develop. We also seldom saw neuroblast clones with fewer than six cells. This, too, is to be expected if the neurons are all born rapidly. With only a narrow window of time between the first and last division of the neuroblast, it would be difficult to create a partial vertical system neuroblast clone. Such a short window is quite possible if only three neuroblast divisions are required for the creation of the vertical system. Combining these results, it is possible that there is no consistent birth order, but it seems likely that the VS neurons are all born in too short a temporal window for them to be distinguished using these methods. More generally, however, we can use neuroblast clones to infer the lineage of the horizontal and vertical systems. The fact that we see neuroblast clones containing all the VS neurons (Figs. 2, 10) demonstrates that they are related in lineage. The lack of HS cells in vertical system neuroblast clones and the lack of multiple HS cells in the same clone argue that there is no clear lineage shared by the VS and HS neurons, or among the HS neurons. These negative results are, however, less conclusive than the strong positive evidence in favor of lineage within the vertical system. Therefore, as in the mushroom bodies and projection neurons, it seems that the different VS neurons are derived from the same neuroblast, but it is unclear that the cells are born at a precise time in development, or even that there is a particular order in which they are born. Stereotypy of the VS dendrites As integrators of information from broad regions of the visual field, VS neurons have dendrites spread over large areas of the lobula plate. Given the retinotopic map present in input to the lobula plate and the separation of motion information into different anterior-posterior layers, it seems likely that the three-dimensional structure of these dendrites is critical to their function. Experiments on dark-reared flies have shown that the function of these cells is not dependent on experience (Karmeiere et al., 2001). This indicates that the inputs to the lobula plate are largely hard-wired and that the dendrites of the VS neurons probably form normally in visually deprived flies. Alternatively, it is possible that the function of these cells might be maintained even if their architecture were slightly perturbed. This possibility was supported by a computer model indicating that variability in the input or specific connections made would probably be tolerated and that the neurons would maintain normal function (Douglass and Strausfeld, 2000a, b). In an effort to see how consistent the structure of the dendrites is in wild-type animals, we have looked at 12 VS1 neurons from different animals in detail and compared their structures (see Fig. 10 for examples). By observing natural levels of variation, we hope to gauge how strictly the development of these dendrites is regulated. We have found that there is variation in certain branching characteristics but that the overall size and threedimensional shape of the dendritic field are highly stereotyped. We have also observed that the structure is not
9 478 E.K. SCOTT ET AL. Fig. 8. The VS5 neuron. A: Posterior view of the dendrites of a VS5 neuron. The structure is characterized by two separate large branches, which extend dorsally (arrowheads). B: Three-dimensional tracing of the dendrites from A, viewed from the same perspective. C: The same tracing, rotated 45. D: The same tracing, rotated 90, showing a relatively dramatic anterior extension in the dorsal dendrites (arrowhead). For details on the structure of this cell, see the text. Scale bar 25 m and applies to all panels. changed in any obvious manner in dark-reared flies (unpublished data). This indicates that the region covered by the dendrites of the VS1 cell is consistent but does not answer the question as to whether or not this consistent structure is necessary for the normal function of the cell, nor does it tell whether the input to the structurally normal VS1 cell is perturbed in visually deprived flies. Similarity to large flies Our analysis of the Drosophila horizontal system has shown that there are three HS neurons that send their dendrites into distinct but overlapping fields in the lobula plate. In these respects, our results are consistent with past studies in Drosophila (Fischbach and Dittrich, 1989) and indicate that the horizontal system in Drosophila is identical schematically to those described for larger flies (Pierantoni, 1976; Strausfeld, 1976a). A detailed structural analysis of the Drosophila vertical system allows us to make comparisons between it and the systems of large flies. The most noticeable difference between Drosophila and large flies is the reduced number of VS neurons. Whereas blowflies and houseflies have 9 11 vertical system cells, we found 6 in Drosophila. Because the marking of cells with the MARCM system depends on the expression of a Gal4 driver, it is possible that there are additional members of the Drosophila vertical system that lack expression by Gal4-3A. Such cells would not be stained in these experiments. There are a few arguments against this possibility. First, the VS neurons that we have described all share a common lineage, as shown by their common appearance in neuroblast clones. They also have in common strong Gal4-3A expression (Fig. 1B). It seems unlikely that one or a few additional cells sharing the structural and functional characteristics of VS1-6 would appear in the vertical system without sharing lineage and gene expression with the others. Second, our total of six VS neurons is in accord with a previous estimate of five to seven (Heisenberg et al., 1978), and we have described all the vertical system members identified in this previous study. Since the estimate of five to seven was reached via silver staining, it was not dependent on the expression of Gal4-3A or any other driver. Finally, the cellular structures that we have described here provide a structural counterpart for the vertical systems of larger flies, albeit with fewer cells (see below). There are few or no VS neurons in large flies that lack close structural relatives among VS1 6. This provides support for the idea that six Drosophila VS neurons would be sufficient to carry out the same function as the blowfly and housefly vertical systems. Although the Drosophila vertical system has fewer cells, the individual neurons bear a striking resemblance to those in the housefly and blowfly. The blowfly, for instance, has 11 VS neurons with characteristic dendritic structures that have been described in detail (Eckert and Bishop, 1978; Hengstenberg et al., 1982; Krapp et al., 1998). VS1 in the blowfly and VS1 in Drosophila closely resemble each other, with a ventrally sweeping dendritic shaft that remains at the posterior edge of the lobula plate and an elaborate dorsally projecting dendrite that extends anteriorly. In the blowfly, VS2 6 show a mix of dendritic structures that either sweep ventrally as VS1 does or are
10 ANATOMY OF THE DROSOPHILA VERTICAL SYSTEM 479 Fig. 9. The VS6 neuron. A: Posterior view of the dendrites of a VS6 neuron. Like VS5, VS6 has its dendrites predominantly projecting dorsally. Unlike for VS5, these dorsal dendrites derive from a single major dorsal branch (arrowhead). B: Three-dimensional tracing of the dendrites from A, viewed from the same perspective. C: The same tracing, rotated 45. D: The same tracing, rotated 90, shows anterior extension of the dorsal dendrites, but not as dramatically as in VS5 (arrowhead). For details on the structure of this cell, see text. Scale bar 25 m and applies to all panels. Fig. 10. Stereotypy in the VS1 dendrites. All panels show examples of wild-type VS1 dendrites. A,B: Dendritic trees in which single large branches (arrows) account for all of the dorsal dendrites. C,D: Dendrites with multiple dorsal branches filling this same space. A C: Single, unbranched dendritic shafts that extend to the ventral tip of the dendritic tree. D: A cell with a branched dendritic shaft (arrowhead). Scale bar 25 m.
11 480 E.K. SCOTT ET AL. Fig. 11. The three-dimensional structure of a vertical system neuroblast clone A: Posterior view of the dendrites of the vertical system. All six cells are visible. B: Tracing of the dendrites from A, with each cell s dendrites in a different color. The color for each individual cell is indicated. Due to the difficulty of determining the origin of certain dendrites, some have been left out of the traces. Most notably, the dorsally projecting dendrite of VS2 is absent from the tracing, and major aspects of VS3 and VS4 have been left out. C: The same tracing from B, rotated 90 to show anterior-posterior depth. D: Closer view of the boxed area in C showing the anterior-posterior details of the dorsal dendrites. E: Dorsal view of the tracing from B. Scale bar 50 m and applies to all panels except for D. approximately symmetrical along the dorsal-ventral axis. For the most part, the dendrites of these neurons are on the posterior face of the lobula plate, but some have a few anteriorly extending dendrites in the dorsal lobula plate. These cells in the blowfly correspond structurally to VS2 4 in Drosophila. The remainder of the blowfly VS neurons have dendrites similar to VS5 and VS6 in Drosophila. In both flies, the ventral dendrites of these neurons show few if any major branches, and the complex dorsal dendrites extend anteriorly. A recent study in the blowfly linked members of the vertical system with the specific optic flow events to which they responded optimally (Krapp et al., 1998). The conclusion was that different members of the vertical system are most responsive to optic flow events that would accompany different rotational body movements during flight. As a group, the vertical system neurons combine to be sensitive to rotations around various body axes, ranging from pure pitch changes to pure roll. Generally, it was found that the three-dimensional structures of given cells dendrites correspond to the part of the visual field sensed and the type of motion to which the cell is optimally responsive (Krapp et al., 1998). Of four discrete layers of input into the Drosophila lobula plate, the anterior two contain horizontal flow information whereas the posterior two layers contain vertical flow information (Buchner and Buchner, 1984). The similarities in dendritic structure between blowflies and Drosophila and the nature of the input into the lobula plate support the idea that the Drosophila VS neurons may respond to pitch and roll stimuli similar to those sensed by their counterparts in large flies. Of course, the current evidence is indirect, and functional studies similar to those done in blowfly (Krapp et al., 1998) would be necessary to confirm these functions for the dendritic structures described here for Drosophila. The clear positive staining of these cells in a dark background leads us to believe that the vertical system will serve as a system in which Drosophila genetics can be employed to study the function and importance of these cells for flight control or other visually dependent behaviors. For instance, Gal4-3A could be used in conjunction with a UAS transgene that interferes with changes in membrane potential (reviewed by White et al., 2001) or a UAS-driven apoptotic gene such as hid to kill developing VS neurons (Grether et al., 1995). However, more vertical system-specific GAL4 drivers may be necessary for these experiments to be most effective. In addition to its potential usefulness in studies of visual behavior, the VS neurons could be used as a model for studying dendrites in general. Through use of the MARCM system, loss of function alleles in genes of interest could be made homozygous in individual VS neurons, and the effects on their complex yet stereotyped dendritic structures could be studied. ACKNOWLEDGMENTS We thank Martin Heisenberg for his support in isolating the Gal4-3A line and for comments on the manuscript and John Reuter providing technical assistance. We also
12 ANATOMY OF THE DROSOPHILA VERTICAL SYSTEM thank Takahiro Chihara, Bryan Gore, and Greg Jefferis for their comments on the article. LITERATURE CITED Borst A, Egelhaaf M Principles of visual motion detection. Trends Neurosci 12: Brand AH, Perrimon N Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118: Buchner E, Buchner S Neuroanatomical mapping of visually induced nervous activity in insects by 3 H-deoxyglucose. In: Ali MA, editor. Photoreceptions and vision in invertebrates. New York: Plenum Press. p Buchner E, Buchner S, Bulthoff I Deoxyglucose mapping of nervous activity induced in Drosophila brain by visual movement. J Comp Physiol 155: Buschbeck EK, Strausfeld NJ The relevance of neural architecture to visual performance: phylogenetic conservation and variation in Dipteran visual systems. J Comp Neurol 383: Douglass JK, Strausfeld NJ. 2000a. Optic flow representation in the optic lobes of Diptera: modeling the role of T5 directional tuning properties. J Comp Physiol A 186: Douglass JK, Strausfeld NJ. 2000b. Optic flow representation in the optic lobes of Diptera: modeling innervation matrices onto collators and their evolutionary implications. J Comp Physiol A 186: Dvorak DR, Bishop LG, Eckert HE On the identification of movement detectors in the fly optic lobe. J Comp Physiol 100:5 23. Eckert HE The vertical-horizontal neurone (VH) in the lobula plate of the blowfly, Phaenicia. J Comp Physiol 149: Eckert HE, Bishop LG Anatomical and physiological properties of the vertical cells in the third optic ganglion of Phaenicia sericata (Diptera, Calliphoridae). J Comp Physiol 126: Egelhaaf M, Borst A A look into the cockpit of the fly: visual orientation, algorithms, and identified neurons. J Neurosci 13: Egelhaaf M, Hausen K, Reichardt W, Wehrhahn C Visual course control in flies relies on neuronal computation of object and background motion. Trends Neurosci 11: Fischbach KF, Dittrich APM The optic lobe of Drosophila melanogaster. I. A Golgi analysis of wild-type structures. Cell Tissue Res 258: Grether ME, Abrams JM, Agapite J, White K, Steller H The head involution defective gene of Drosophila melanogaster functions in programmed cell death. Genes Dev 9: Hausen K, Wolburg-Buchholz W, Ribi WA The synaptic organization of visual interneurons in the lobula complex of flies. A light and electron microscopical study using silver-intensified cobaltimpregnations. Cell Tissue Res 208: Heisenberg M, Bohl K Isolation of anatomical brain mutants of Drosophila by histological means. Z Naturf 34: Heisenberg M, Wonneberger R, Wolf R optomotor-blind H31 a Drosophila mutant of the lobula plate giant neurons. J Comp Physiol 124: Hengstenberg R Spike responses of non-spiking visual interneurone. Nature 270: Hengstenberg R Common visual response properties of giant vertical cells in the lobula plate of the blowfly Calliphora. J Comp Physiol 149: Hengstenberg R, Hausen K, Hengstenberg B The number and structure of the giant vertical cells (VS) in the lobula plate on the blowfly Calliphora erythrocephala. J Comp Physiol 149: Ito K, Hotta Y Proliferation pattern of postembryonic neuroblasts in the brain of Drosophila melanogaster. Dev Biol 149: Ito K, Awano W, Suzuki K, Hiromi Y, Yamamoto D. 1997a. The Drosophila mushroom body is a quadruple structure of clonal units each of which contains a virtually identical set of neurons and glial cells. Development 124: Ito K, Sass H, Urban J, Hofbauer A, Schneuwly S. 1997b. Gal4-responsive UAS-tau as a tool for studying the anatomy and development of the Drosophila central nervous system. Cell Tissue Res 290:1 10. Jefferis GS, Marin EC, Stocker RF, Luo L Target neuron prespecification in the olfactory map of Drosophila. Nature 414: Karmeier K, Tabor R, Egelhaaf M, Krapp HG Early visual experience and the receptive-field organization of optic flow processing interneurons in the fly motion pathway. Vis Neurosci 18:1 8. Koenderink JJ, van Doorn AJ Facts on optic flow. Biol Cybernetics 56: Krapp HG, Hengstenberg B, Hengstenberg R Dendritic structure and receptive-field organization of optic flow processing interneurons in the fly. J Neurophysiol 79: Lee T, Luo L Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron 22: Lee T, Lee A, Luo L Development of the Drosophila mushroom bodies: sequential generation of three distinct types of neurons from a neuroblast. Development 126: Pierantoni R A look into the cock-pit of the fly. The architecture of the lobular plate. Cell Tissue Res 171: Single S, Borst A Dendritic integration and its role in computing image velocity. Science 281: Soohoo SL, Bishop LG Intensity and motion responses of giant vertical neurons of the fly eye. J Neurobiol 11: Strausfeld NJ. 1976a. Atlas of an insect brain. Berlin: Springer-Verlag. Strausfeld NJ. 1976b. Mosaic organizations, layers, and visual pathways in the insect brain. In: Zettler F, Weiler R, editors. Neural principles in vision. Berlin: Springer-Verlag. p White B, Osterwalder T, Keshishian H Molecular genetic approaches to the targeted suppression of neuronal activity. Curr Biol 11:R1041 R1053.
Development of the Drosophila mushroom bodies: sequential generation of three distinct types of neurons from a neuroblast
 Development 126, 4065-4076 (1999) Printed in Great Britain The Company of Biologists Limited 1999 DEV8625 4065 Development of the Drosophila mushroom bodies: sequential generation of three distinct types
Development 126, 4065-4076 (1999) Printed in Great Britain The Company of Biologists Limited 1999 DEV8625 4065 Development of the Drosophila mushroom bodies: sequential generation of three distinct types
Baz, Par-6 and apkc are not required for axon or dendrite specification in Drosophila
 Baz, Par-6 and apkc are not required for axon or dendrite specification in Drosophila Melissa M. Rolls and Chris Q. Doe, Inst. Neurosci and Inst. Mol. Biol., HHMI, Univ. Oregon, Eugene, Oregon 97403 Correspondence
Baz, Par-6 and apkc are not required for axon or dendrite specification in Drosophila Melissa M. Rolls and Chris Q. Doe, Inst. Neurosci and Inst. Mol. Biol., HHMI, Univ. Oregon, Eugene, Oregon 97403 Correspondence
Follow this and additional works at: Part of the Medical Sciences Commons
 Bucknell University Bucknell Digital Commons Master s Theses Student Theses 2010 The overexpression of homeotic complex gene Ultrabithorax in the post-embryonic neuronal lineages of the ventral nervous
Bucknell University Bucknell Digital Commons Master s Theses Student Theses 2010 The overexpression of homeotic complex gene Ultrabithorax in the post-embryonic neuronal lineages of the ventral nervous
NN-A29932: Supplementary Data 1 Diversity and Wiring Variability of Olfactory Local Interneurons in the Drosophila Antennal Lobe
 NN-A29932: Supplementary Data 1 Diversity and Wiring Variability of Olfactory Local Interneurons in the Drosophila Antennal Lobe Ya-Hui Chou, Maria L. Spletter, Emre Yaksi, Jonathan C. S. Leong, Rachel
NN-A29932: Supplementary Data 1 Diversity and Wiring Variability of Olfactory Local Interneurons in the Drosophila Antennal Lobe Ya-Hui Chou, Maria L. Spletter, Emre Yaksi, Jonathan C. S. Leong, Rachel
The Number and Structure of Giant Vertical Cells (VS) in the Lobula Plate of the Blowfly Calliphora erythrocephala
 J Comp Physiol (1982) 149:163-177 Journal of Comparative Physiology A Springer-Verlag 1982 The Number and Structure of Giant Vertical Cells (VS) in the Lobula Plate of the Blowfly Calliphora erythrocephala
J Comp Physiol (1982) 149:163-177 Journal of Comparative Physiology A Springer-Verlag 1982 The Number and Structure of Giant Vertical Cells (VS) in the Lobula Plate of the Blowfly Calliphora erythrocephala
Neural Action Fields for Optic Flow Based Navigation: A Simulation Study of the Fly Lobula Plate Network
 Neural Action Fields for Optic Flow Based Navigation: A Simulation Study of the Fly Lobula Plate Network Alexander Borst*, Franz Weber Department of Systems and Computational Neurobiology, Max-Planck-Institute
Neural Action Fields for Optic Flow Based Navigation: A Simulation Study of the Fly Lobula Plate Network Alexander Borst*, Franz Weber Department of Systems and Computational Neurobiology, Max-Planck-Institute
Contributions of the 12 Neuron Classes in the Fly Lamina to Motion Vision
 Article Contributions of the 1 Neuron Classes in the Fly mina to Motion Vision John C. Tuthill, 1,,3 Aljoscha Nern, 1, Stephen L. Holtz, 1 Gerald M. Rubin, 1 and Michael B. Reiser 1, * 1 HHMI/Janelia Farm
Article Contributions of the 1 Neuron Classes in the Fly mina to Motion Vision John C. Tuthill, 1,,3 Aljoscha Nern, 1, Stephen L. Holtz, 1 Gerald M. Rubin, 1 and Michael B. Reiser 1, * 1 HHMI/Janelia Farm
Nature Biotechnology: doi: /nbt Supplementary Figure 1. Overexpression of YFP::GPR-1 in the germline.
 Supplementary Figure 1 Overexpression of YFP::GPR-1 in the germline. The pie-1 promoter and 3 utr were used to express yfp::gpr-1 in the germline. Expression levels from the yfp::gpr-1(cai 1.0)-expressing
Supplementary Figure 1 Overexpression of YFP::GPR-1 in the germline. The pie-1 promoter and 3 utr were used to express yfp::gpr-1 in the germline. Expression levels from the yfp::gpr-1(cai 1.0)-expressing
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/6/301/ra98/dc1 Supplementary Materials for Regulation of Epithelial Morphogenesis by the G Protein Coupled Receptor Mist and Its Ligand Fog Alyssa J. Manning,
www.sciencesignaling.org/cgi/content/full/6/301/ra98/dc1 Supplementary Materials for Regulation of Epithelial Morphogenesis by the G Protein Coupled Receptor Mist and Its Ligand Fog Alyssa J. Manning,
Clonal analysis of Drosophila antennal lobe neurons: diverse neuronal architectures in the lateral neuroblast lineage
 Access the Development most First recent posted version epress online at http://dev.biologists.org/lookup/doi/10.1242/dev.024380 on online 24 July publication 2008 as 10.1242/dev.024380 date 23 July 2008
Access the Development most First recent posted version epress online at http://dev.biologists.org/lookup/doi/10.1242/dev.024380 on online 24 July publication 2008 as 10.1242/dev.024380 date 23 July 2008
Analysis of non-olfactory sensory pathways that are necessary for the olfactory associative learning of the Drosophila mushroom body
 15 12601 B 2009 2011 21300117 Analysis of non-olfactory sensory pathways that are necessary for the olfactory associative learning of the Drosophila mushroom body ITO, Kei 00311192 The mushroom body is
15 12601 B 2009 2011 21300117 Analysis of non-olfactory sensory pathways that are necessary for the olfactory associative learning of the Drosophila mushroom body ITO, Kei 00311192 The mushroom body is
Fig. S1. Expression pattern of moody-gal4 in third instar. Maximum projection illustrating a dissected moody-gal4>ngfp L3 larva stained for Repo
 Fig. S1. Expression pattern of moody-gal4 in third instar. Maximum projection illustrating a dissected moody-gal4>ngfp L3 larva stained for Repo (magenta), Fas2 (blue) and GFP (green) in overview (A) and
Fig. S1. Expression pattern of moody-gal4 in third instar. Maximum projection illustrating a dissected moody-gal4>ngfp L3 larva stained for Repo (magenta), Fas2 (blue) and GFP (green) in overview (A) and
10/2/2015. Chapter 4. Determination and Differentiation. Neuroanatomical Diversity
 Chapter 4 Determination and Differentiation Neuroanatomical Diversity 1 Neurochemical diversity: another important aspect of neuronal fate Neurotransmitters and their receptors Excitatory Glutamate Acetylcholine
Chapter 4 Determination and Differentiation Neuroanatomical Diversity 1 Neurochemical diversity: another important aspect of neuronal fate Neurotransmitters and their receptors Excitatory Glutamate Acetylcholine
Current Opinion in Neurobiology 2002, 12: /02/$ see front matter 2002 Elsevier Science Ltd. All rights reserved.
 80 Development of neuronal connectivity in Drosophila antennal lobes and mushroom bodies Gregory SXE Jefferis*, Elizabeth C Marin, Ryan J Watts and Liqun Luo* Recent advances in the study of the connectivity
80 Development of neuronal connectivity in Drosophila antennal lobes and mushroom bodies Gregory SXE Jefferis*, Elizabeth C Marin, Ryan J Watts and Liqun Luo* Recent advances in the study of the connectivity
Supplementary Materials for
 www.sciencemag.org/cgi/content/full/science.1244624/dc1 Supplementary Materials for Cytoneme-Mediated Contact-Dependent Transport of the Drosophila Decapentaplegic Signaling Protein Sougata Roy, Hai Huang,
www.sciencemag.org/cgi/content/full/science.1244624/dc1 Supplementary Materials for Cytoneme-Mediated Contact-Dependent Transport of the Drosophila Decapentaplegic Signaling Protein Sougata Roy, Hai Huang,
Midterm 1. Average score: 74.4 Median score: 77
 Midterm 1 Average score: 74.4 Median score: 77 NAME: TA (circle one) Jody Westbrook or Jessica Piel Section (circle one) Tue Wed Thur MCB 141 First Midterm Feb. 21, 2008 Only answer 4 of these 5 problems.
Midterm 1 Average score: 74.4 Median score: 77 NAME: TA (circle one) Jody Westbrook or Jessica Piel Section (circle one) Tue Wed Thur MCB 141 First Midterm Feb. 21, 2008 Only answer 4 of these 5 problems.
Reading: Chapter 5, pp ; Reference chapter D, pp Problem set F
 Mosaic Analysis Reading: Chapter 5, pp140-141; Reference chapter D, pp820-823 Problem set F Twin spots in Drosophila Although segregation and recombination in mitosis do not occur at the same frequency
Mosaic Analysis Reading: Chapter 5, pp140-141; Reference chapter D, pp820-823 Problem set F Twin spots in Drosophila Although segregation and recombination in mitosis do not occur at the same frequency
Neural development its all connected
 Neural development its all connected How do you build a complex nervous system? How do you build a complex nervous system? 1. Learn how tissue is instructed to become nervous system. Neural induction 2.
Neural development its all connected How do you build a complex nervous system? How do you build a complex nervous system? 1. Learn how tissue is instructed to become nervous system. Neural induction 2.
Linear Combinations of Optic Flow Vectors for Estimating Self-Motion a Real-World Test of a Neural Model
 Linear Combinations of Optic Flow Vectors for Estimating Self-Motion a Real-World Test of a Neural Model Matthias O. Franz MPI für biologische Kybernetik Spemannstr. 38 D-72076 Tübingen, Germany mof@tuebingen.mpg.de
Linear Combinations of Optic Flow Vectors for Estimating Self-Motion a Real-World Test of a Neural Model Matthias O. Franz MPI für biologische Kybernetik Spemannstr. 38 D-72076 Tübingen, Germany mof@tuebingen.mpg.de
Building the brain (1): Evolutionary insights
 Building the brain (1): Evolutionary insights Historical considerations! Initial insight into the general role of the brain in human behaviour was already attained in antiquity and formulated by Hippocrates
Building the brain (1): Evolutionary insights Historical considerations! Initial insight into the general role of the brain in human behaviour was already attained in antiquity and formulated by Hippocrates
Chapter 11. Development: Differentiation and Determination
 KAP Biology Dept Kenyon College Differential gene expression and development Mechanisms of cellular determination Induction Pattern formation Chapter 11. Development: Differentiation and Determination
KAP Biology Dept Kenyon College Differential gene expression and development Mechanisms of cellular determination Induction Pattern formation Chapter 11. Development: Differentiation and Determination
Chapter 4 Evaluating a potential interaction between deltex and git in Drosophila: genetic interaction, gene overexpression and cell biology assays.
 Evaluating a potential interaction between deltex and git in Drosophila: genetic interaction, gene overexpression and cell biology assays. The data described in chapter 3 presented evidence that endogenous
Evaluating a potential interaction between deltex and git in Drosophila: genetic interaction, gene overexpression and cell biology assays. The data described in chapter 3 presented evidence that endogenous
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature10923 Supplementary Figure 1 Ten-a and Ten-m antibody and cell type specificities. a c, Representative single confocal sections of a Drosophila NMJ stained with antibodies to Ten-a (red),
doi:10.1038/nature10923 Supplementary Figure 1 Ten-a and Ten-m antibody and cell type specificities. a c, Representative single confocal sections of a Drosophila NMJ stained with antibodies to Ten-a (red),
18.4 Embryonic development involves cell division, cell differentiation, and morphogenesis
 18.4 Embryonic development involves cell division, cell differentiation, and morphogenesis An organism arises from a fertilized egg cell as the result of three interrelated processes: cell division, cell
18.4 Embryonic development involves cell division, cell differentiation, and morphogenesis An organism arises from a fertilized egg cell as the result of three interrelated processes: cell division, cell
Modulation of central pattern generator output by peripheral sensory cells in Drosophila larvae. BioNB4910 Cornell University.
 Modulation of central pattern generator output by peripheral sensory cells in Drosophila larvae BioNB4910 Cornell University Goals 1) Observe the behavioral effects of remotely activating different populations
Modulation of central pattern generator output by peripheral sensory cells in Drosophila larvae BioNB4910 Cornell University Goals 1) Observe the behavioral effects of remotely activating different populations
SUPPLEMENTARY INFORMATION
 Supplemental Figure S1. WISL is not elicited by courtship song and is dependent on chordotonal mechanosensory neurons. (a) Average locomotor velocity vs. time plots for wild-type CS flies exposed to recordings
Supplemental Figure S1. WISL is not elicited by courtship song and is dependent on chordotonal mechanosensory neurons. (a) Average locomotor velocity vs. time plots for wild-type CS flies exposed to recordings
System Identification of Optic-Flow Processing Neurons in the Fly: Single Cell and Small Circuit Models. Franz Weber
 System Identification of Optic-Flow Processing Neurons in the Fly: Single Cell and Small Circuit Models Franz Weber München 211 System Identification of Optic-Flow Processing Neurons in the Fly: Single
System Identification of Optic-Flow Processing Neurons in the Fly: Single Cell and Small Circuit Models Franz Weber München 211 System Identification of Optic-Flow Processing Neurons in the Fly: Single
Nature Neuroscience: doi: /nn.2662
 Supplementary Figure 1 Atlastin phylogeny and homology. (a) Maximum likelihood phylogenetic tree based on 18 Atlastin-1 sequences using the program Quicktree. Numbers at internal nodes correspond to bootstrap
Supplementary Figure 1 Atlastin phylogeny and homology. (a) Maximum likelihood phylogenetic tree based on 18 Atlastin-1 sequences using the program Quicktree. Numbers at internal nodes correspond to bootstrap
Sensory/ Motor Systems March 10, 2008
 Sensory/ Motor Systems March 10, 2008 Greg Suh greg.suh@med.nyu.edu Title: Chemical Senses- periphery Richard Axel and Linda Buck Win Nobel Prize for Olfactory System Research 2 3 After Leslie Vosshal
Sensory/ Motor Systems March 10, 2008 Greg Suh greg.suh@med.nyu.edu Title: Chemical Senses- periphery Richard Axel and Linda Buck Win Nobel Prize for Olfactory System Research 2 3 After Leslie Vosshal
Diverse Functions of N-Cadherin in Dendritic and Axonal Terminal Arborization of Olfactory Projection Neurons
 Neuron, Vol. 42, 63 75, April 8, 2004, Copyright 2004 by Cell Press Diverse Functions of N-Cadherin in Dendritic and Axonal Terminal Arborization of Olfactory Projection Neurons Haitao Zhu and Liqun Luo*
Neuron, Vol. 42, 63 75, April 8, 2004, Copyright 2004 by Cell Press Diverse Functions of N-Cadherin in Dendritic and Axonal Terminal Arborization of Olfactory Projection Neurons Haitao Zhu and Liqun Luo*
Report. Walking Modulates Speed Sensitivity in Drosophila Motion Vision
 Current Biology 2, 147 1475, August 24, 21 ª21 Elsevier Ltd All rights reserved DOI 1.116/j.cub.21.6.72 Walking Modulates Speed Sensitivity in Drosophila Motion Vision Report M. Eugenia Chiappe, 1 Johannes
Current Biology 2, 147 1475, August 24, 21 ª21 Elsevier Ltd All rights reserved DOI 1.116/j.cub.21.6.72 Walking Modulates Speed Sensitivity in Drosophila Motion Vision Report M. Eugenia Chiappe, 1 Johannes
Developmental genetics: finding the genes that regulate development
 Developmental Biology BY1101 P. Murphy Lecture 9 Developmental genetics: finding the genes that regulate development Introduction The application of genetic analysis and DNA technology to the study of
Developmental Biology BY1101 P. Murphy Lecture 9 Developmental genetics: finding the genes that regulate development Introduction The application of genetic analysis and DNA technology to the study of
purpose of this Chapter is to highlight some problems that will likely provide new
 119 Chapter 6 Future Directions Besides our contributions discussed in previous chapters to the problem of developmental pattern formation, this work has also brought new questions that remain unanswered.
119 Chapter 6 Future Directions Besides our contributions discussed in previous chapters to the problem of developmental pattern formation, this work has also brought new questions that remain unanswered.
Characterization of the apoptotic functions of the HID hmolog isolated from Megaselia scalaris
 University of Tennessee, Knoxville Trace: Tennessee Research and Creative Exchange University of Tennessee Honors Thesis Projects University of Tennessee Honors Program 5-2013 Characterization of the apoptotic
University of Tennessee, Knoxville Trace: Tennessee Research and Creative Exchange University of Tennessee Honors Thesis Projects University of Tennessee Honors Program 5-2013 Characterization of the apoptotic
Introduction Principles of Signaling and Organization p. 3 Signaling in Simple Neuronal Circuits p. 4 Organization of the Retina p.
 Introduction Principles of Signaling and Organization p. 3 Signaling in Simple Neuronal Circuits p. 4 Organization of the Retina p. 5 Signaling in Nerve Cells p. 9 Cellular and Molecular Biology of Neurons
Introduction Principles of Signaling and Organization p. 3 Signaling in Simple Neuronal Circuits p. 4 Organization of the Retina p. 5 Signaling in Nerve Cells p. 9 Cellular and Molecular Biology of Neurons
Segment boundary formation in Drosophila embryos
 Segment boundary formation in Drosophila embryos Development 130, August 2003 Camilla W. Larsen, Elizabeth Hirst, Cyrille Alexandre and Jean Paul Vincent 1. Introduction: - Segment boundary formation:
Segment boundary formation in Drosophila embryos Development 130, August 2003 Camilla W. Larsen, Elizabeth Hirst, Cyrille Alexandre and Jean Paul Vincent 1. Introduction: - Segment boundary formation:
Transient and steady-state response properties of movement detectors
 116 J. Opt. Soc. Am. A/Vol. 6, No. 1/January 1989 M. Egelhaaf and A. Borst Transient and steady-state response properties of movement detectors Martin Egelhaaf and Alexander Borst Max-Planck-nstitut fr
116 J. Opt. Soc. Am. A/Vol. 6, No. 1/January 1989 M. Egelhaaf and A. Borst Transient and steady-state response properties of movement detectors Martin Egelhaaf and Alexander Borst Max-Planck-nstitut fr
1. Draw, label and describe the structure of DNA and RNA including bonding mechanisms.
 Practicing Biology BIG IDEA 3.A 1. Draw, label and describe the structure of DNA and RNA including bonding mechanisms. 2. Using at least 2 well-known experiments, describe which features of DNA and RNA
Practicing Biology BIG IDEA 3.A 1. Draw, label and describe the structure of DNA and RNA including bonding mechanisms. 2. Using at least 2 well-known experiments, describe which features of DNA and RNA
Mr.checker Wikipedia Commons. Louise Docker from sydney, Australia, Wikimedia Commons. Angelique Paulk
 Mr.checker Wikipedia Commons Louise Docker from sydney, Australia, Wikimedia Commons Angelique Paulk Objectives How does learning and memory operate at the behavioral level? How can you train an insect?
Mr.checker Wikipedia Commons Louise Docker from sydney, Australia, Wikimedia Commons Angelique Paulk Objectives How does learning and memory operate at the behavioral level? How can you train an insect?
Lecture 6: Non-Cortical Visual Pathways MCP 9.013/7.68, 03
 Lecture 6: Non-Cortical Visual Pathways MCP 9.013/7.68, 03 Roger W. Sperry The problem of central nervous reorganization after nerve regeneration and muscle transposition. R.W. Sperry. Quart. Rev. Biol.
Lecture 6: Non-Cortical Visual Pathways MCP 9.013/7.68, 03 Roger W. Sperry The problem of central nervous reorganization after nerve regeneration and muscle transposition. R.W. Sperry. Quart. Rev. Biol.
MCB 141 Midterm I Feb. 19, 2009
 Write your name and student ID# on EVERY PAGE of your exam MCB 141 Midterm I Feb. 19, 2009 Circle the name of your TA Jessica Lyons Alberto Stolfi Question #1 Question #2 Question #3 Question #4 TOTAL
Write your name and student ID# on EVERY PAGE of your exam MCB 141 Midterm I Feb. 19, 2009 Circle the name of your TA Jessica Lyons Alberto Stolfi Question #1 Question #2 Question #3 Question #4 TOTAL
Axis determination in flies. Sem 9.3.B.5 Animal Science
 Axis determination in flies Sem 9.3.B.5 Animal Science All embryos are in lateral view (anterior to the left). Endoderm, midgut; mesoderm; central nervous system; foregut, hindgut and pole cells in yellow.
Axis determination in flies Sem 9.3.B.5 Animal Science All embryos are in lateral view (anterior to the left). Endoderm, midgut; mesoderm; central nervous system; foregut, hindgut and pole cells in yellow.
Figure 1. Automated 4D detection and tracking of endosomes and polarity markers.
 A t = 0 z t = 90 B t = 0 z t = 90 C t = 0-90 t = 0-180 t = 0-300 Figure 1. Automated 4D detection and tracking of endosomes and polarity markers. A: Three different z planes of a dividing SOP shown at
A t = 0 z t = 90 B t = 0 z t = 90 C t = 0-90 t = 0-180 t = 0-300 Figure 1. Automated 4D detection and tracking of endosomes and polarity markers. A: Three different z planes of a dividing SOP shown at
Neural Diversity in the Drosophila Olfactory Circuitry: A Dissertation
 University of Massachusetts Medical School escholarship@umms GSBS Dissertations and Theses Graduate School of Biomedical Sciences 2007-07-31 Neural Diversity in the Drosophila Olfactory Circuitry: A Dissertation
University of Massachusetts Medical School escholarship@umms GSBS Dissertations and Theses Graduate School of Biomedical Sciences 2007-07-31 Neural Diversity in the Drosophila Olfactory Circuitry: A Dissertation
Announcements: Test4: Wednesday on: week4 material CH5 CH6 & NIA CAPE Evaluations please do them for me!! ask questions...discuss listen learn.
 Announcements: Test4: Wednesday on: week4 material CH5 CH6 & NIA CAPE Evaluations please do them for me!! ask questions...discuss listen learn. The Chemical Senses: Olfaction Mary ET Boyle, Ph.D. Department
Announcements: Test4: Wednesday on: week4 material CH5 CH6 & NIA CAPE Evaluations please do them for me!! ask questions...discuss listen learn. The Chemical Senses: Olfaction Mary ET Boyle, Ph.D. Department
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature11589 Supplementary Figure 1 Ciona intestinalis and Petromyzon marinus neural crest expression domain comparison. Cartoon shows dorsal views of Ciona mid gastrula (left) and Petromyzon
doi:10.1038/nature11589 Supplementary Figure 1 Ciona intestinalis and Petromyzon marinus neural crest expression domain comparison. Cartoon shows dorsal views of Ciona mid gastrula (left) and Petromyzon
AFRL-RW-EG-TR Optic Glomeruli: Biological Circuits that Compute Target Identity. November Nicholas J. Strausfeld
 Nicholas J. Strausfeld AFRL-RW-EG-TR-2013-116 Optic Glomeruli: Biological Circuits that Compute Target Identity Department of Neuroscience and Center for Insect Science University of Arizona Tucson, AZ
Nicholas J. Strausfeld AFRL-RW-EG-TR-2013-116 Optic Glomeruli: Biological Circuits that Compute Target Identity Department of Neuroscience and Center for Insect Science University of Arizona Tucson, AZ
A mosaic genetic screen for genes necessary for Drosophila mushroom body neuronal morphogenesis
 Development 130, 1203-1213 2003 The Company of Biologists Ltd doi:10.1242/dev.00319 1203 A mosaic genetic screen for genes necessary for Drosophila mushroom body neuronal morphogenesis John E. Reuter 1,
Development 130, 1203-1213 2003 The Company of Biologists Ltd doi:10.1242/dev.00319 1203 A mosaic genetic screen for genes necessary for Drosophila mushroom body neuronal morphogenesis John E. Reuter 1,
SUPPLEMENTARY INFORMATION
 med!1,2 Wild-type (N2) end!3 elt!2 5 1 15 Time (minutes) 5 1 15 Time (minutes) med!1,2 end!3 5 1 15 Time (minutes) elt!2 5 1 15 Time (minutes) Supplementary Figure 1: Number of med-1,2, end-3, end-1 and
med!1,2 Wild-type (N2) end!3 elt!2 5 1 15 Time (minutes) 5 1 15 Time (minutes) med!1,2 end!3 5 1 15 Time (minutes) elt!2 5 1 15 Time (minutes) Supplementary Figure 1: Number of med-1,2, end-3, end-1 and
Solutions to Problem Set 4
 Question 1 Solutions to 7.014 Problem Set 4 Because you have not read much scientific literature, you decide to study the genetics of garden peas. You have two pure breeding pea strains. One that is tall
Question 1 Solutions to 7.014 Problem Set 4 Because you have not read much scientific literature, you decide to study the genetics of garden peas. You have two pure breeding pea strains. One that is tall
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb3267 Supplementary Figure 1 A group of genes required for formation or orientation of annular F-actin bundles and aecm ridges: RNAi phenotypes and their validation by standard mutations.
DOI: 10.1038/ncb3267 Supplementary Figure 1 A group of genes required for formation or orientation of annular F-actin bundles and aecm ridges: RNAi phenotypes and their validation by standard mutations.
Distinct memory traces for two visual features in the Drosophila brain
 Distinct memory traces for two visual features in the Drosophila brain Supplementary Information Gang Liu 1,2, Holger Seiler 3, Ai Wen 1, Troy Zars 3,4, Kei Ito 5, Reinhard Wolf 3, Martin Heisenberg 3,
Distinct memory traces for two visual features in the Drosophila brain Supplementary Information Gang Liu 1,2, Holger Seiler 3, Ai Wen 1, Troy Zars 3,4, Kei Ito 5, Reinhard Wolf 3, Martin Heisenberg 3,
Supplementary Figure 1
 Supplementary Figure 1 Single-cell RNA sequencing reveals unique sets of neuropeptides, transcription factors and receptors in specific types of sympathetic neurons (a) Dissection of paravertebral SGs
Supplementary Figure 1 Single-cell RNA sequencing reveals unique sets of neuropeptides, transcription factors and receptors in specific types of sympathetic neurons (a) Dissection of paravertebral SGs
Visual Motion Analysis by a Neural Network
 Visual Motion Analysis by a Neural Network Kansai University Takatsuki, Osaka 569 1095, Japan E-mail: fukushima@m.ieice.org (Submitted on December 12, 2006) Abstract In the visual systems of mammals, visual
Visual Motion Analysis by a Neural Network Kansai University Takatsuki, Osaka 569 1095, Japan E-mail: fukushima@m.ieice.org (Submitted on December 12, 2006) Abstract In the visual systems of mammals, visual
Positional Cues in the Drosophila Nerve Cord: Semaphorins Pattern the Dorso-Ventral Axis
 Positional Cues in the Drosophila Nerve Cord: Semaphorins Pattern the Dorso-Ventral Axis Marta Zlatic 1,2,3 *, Feng Li 1, Maura Strigini 4, Wesley Grueber 2, Michael Bate 1 * 1 Department of Zoology, University
Positional Cues in the Drosophila Nerve Cord: Semaphorins Pattern the Dorso-Ventral Axis Marta Zlatic 1,2,3 *, Feng Li 1, Maura Strigini 4, Wesley Grueber 2, Michael Bate 1 * 1 Department of Zoology, University
Supporting Information
 Supporting Information Cao et al. 10.1073/pnas.1306220110 Gram - bacteria Hemolymph Cytoplasm PGRP-LC TAK1 signalosome Imd dfadd Dredd Dnr1 Ikk signalosome P Relish Nucleus AMP and effector genes Fig.
Supporting Information Cao et al. 10.1073/pnas.1306220110 Gram - bacteria Hemolymph Cytoplasm PGRP-LC TAK1 signalosome Imd dfadd Dredd Dnr1 Ikk signalosome P Relish Nucleus AMP and effector genes Fig.
Respective roles of the DRL receptor and its ligand WNT5 in Drosophila mushroom body development
 RESEARCH ARTICLE 3089 Development 134, 3089-3097 (2007) doi:10.1242/dev.02876 Respective roles of the DRL receptor and its ligand WNT5 in Drosophila mushroom body development Nicola Grillenzoni*, Adrien
RESEARCH ARTICLE 3089 Development 134, 3089-3097 (2007) doi:10.1242/dev.02876 Respective roles of the DRL receptor and its ligand WNT5 in Drosophila mushroom body development Nicola Grillenzoni*, Adrien
Construction of Anatomically Correct Models of Mouse Brain Networks 1
 Construction of Anatomically Correct Models of Mouse Brain Networks 1 B. H. McCormick a, W. Koh a Y. Choe a L. C. Abbott b J. Keyser a D. Mayerich a Z. Melek a P. Doddapaneni a a Department of Computer
Construction of Anatomically Correct Models of Mouse Brain Networks 1 B. H. McCormick a, W. Koh a Y. Choe a L. C. Abbott b J. Keyser a D. Mayerich a Z. Melek a P. Doddapaneni a a Department of Computer
MCDB 4777/5777 Molecular Neurobiology Lecture 29 Neural Development- In the beginning
 MCDB 4777/5777 Molecular Neurobiology Lecture 29 Neural Development- In the beginning Learning Goals for Lecture 29 4.1 Describe the contributions of early developmental events in the embryo to the formation
MCDB 4777/5777 Molecular Neurobiology Lecture 29 Neural Development- In the beginning Learning Goals for Lecture 29 4.1 Describe the contributions of early developmental events in the embryo to the formation
The functional organization of the visual cortex in primates
 The functional organization of the visual cortex in primates Dominated by LGN M-cell input Drosal stream for motion perception & spatial localization V5 LIP/7a V2 V4 IT Ventral stream for object recognition
The functional organization of the visual cortex in primates Dominated by LGN M-cell input Drosal stream for motion perception & spatial localization V5 LIP/7a V2 V4 IT Ventral stream for object recognition
9/4/2015 INDUCTION CHAPTER 1. Neurons are similar across phyla Thus, many different model systems are used in developmental neurobiology. Fig 1.
 INDUCTION CHAPTER 1 Neurons are similar across phyla Thus, many different model systems are used in developmental neurobiology Fig 1.1 1 EVOLUTION OF METAZOAN BRAINS GASTRULATION MAKING THE 3 RD GERM LAYER
INDUCTION CHAPTER 1 Neurons are similar across phyla Thus, many different model systems are used in developmental neurobiology Fig 1.1 1 EVOLUTION OF METAZOAN BRAINS GASTRULATION MAKING THE 3 RD GERM LAYER
Drosophila melanogaster- Morphogen Gradient
 NPTEL Biotechnology - Systems Biology Drosophila melanogaster- Morphogen Gradient Dr. M. Vijayalakshmi School of Chemical and Biotechnology SASTRA University Joint Initiative of IITs and IISc Funded by
NPTEL Biotechnology - Systems Biology Drosophila melanogaster- Morphogen Gradient Dr. M. Vijayalakshmi School of Chemical and Biotechnology SASTRA University Joint Initiative of IITs and IISc Funded by
Essential Roles of Drosophila RhoA in the Regulation of Neuroblast Proliferation and Dendritic but Not Axonal Morphogenesis
 Neuron, Vol. 25, 307 316, February, 2000, Copyright 2000 by Cell Press Essential Roles of Drosophila RhoA in the Regulation of Neuroblast Proliferation and Dendritic but Not Axonal Morphogenesis Tzumin
Neuron, Vol. 25, 307 316, February, 2000, Copyright 2000 by Cell Press Essential Roles of Drosophila RhoA in the Regulation of Neuroblast Proliferation and Dendritic but Not Axonal Morphogenesis Tzumin
SHORT COMMUNICATION MULTIMODALITY OF OCELLAR INTERNEURONES OF THE AMERICAN COCKROACH BY TAKAHIRO OHYAMA AND YOSHIHIRO TOH
 J. exp. Biol. 125, 405-409 (1986) 405 Printed in Great Britain The Company of Biologists Limited 1986 SHORT COMMUNICATION MULTIMODALITY OF OCELLAR INTERNEURONES OF THE AMERICAN COCKROACH BY TAKAHIRO OHYAMA
J. exp. Biol. 125, 405-409 (1986) 405 Printed in Great Britain The Company of Biologists Limited 1986 SHORT COMMUNICATION MULTIMODALITY OF OCELLAR INTERNEURONES OF THE AMERICAN COCKROACH BY TAKAHIRO OHYAMA
Spatiotemporal Response Properties of Optic-Flow Processing Neurons
 Article Spatiotemporal Response Properties of Optic-Flow Processing Neurons Franz Weber, 1,3, * Christian K. Machens, 2 and Alexander Borst 1 1 Department of Systems and Computational Neurobiology, Max-Planck-Institute
Article Spatiotemporal Response Properties of Optic-Flow Processing Neurons Franz Weber, 1,3, * Christian K. Machens, 2 and Alexander Borst 1 1 Department of Systems and Computational Neurobiology, Max-Planck-Institute
Wide-Field Motion Integration in Fly VS Cells: Insights from an Inverse Approach
 Wide-Field Motion Integration in Fly VS Cells: Insights from an Inverse Approach Benjamin Torben-Nielsen*, Klaus M. Stiefel* Theoretical and Experimental Neurobiology Unit, Okinawa Institute of Science
Wide-Field Motion Integration in Fly VS Cells: Insights from an Inverse Approach Benjamin Torben-Nielsen*, Klaus M. Stiefel* Theoretical and Experimental Neurobiology Unit, Okinawa Institute of Science
Neuronal cell death in grasshopper embryos: variable patterns in different species, clutches, and clones
 J. Embryol. exp. Morph. 78, 169-182 (1983) Printed in Great Britain The Company of Biologists Limited 1983 Neuronal cell death in grasshopper embryos: variable patterns in different species, clutches,
J. Embryol. exp. Morph. 78, 169-182 (1983) Printed in Great Britain The Company of Biologists Limited 1983 Neuronal cell death in grasshopper embryos: variable patterns in different species, clutches,
Modeling retinal high and low contrast sensitivity lters. T. Lourens. Abstract
 Modeling retinal high and low contrast sensitivity lters T. Lourens Department of Computer Science University of Groningen P.O. Box 800, 9700 AV Groningen, The Netherlands E-mail: tino@cs.rug.nl Abstract
Modeling retinal high and low contrast sensitivity lters T. Lourens Department of Computer Science University of Groningen P.O. Box 800, 9700 AV Groningen, The Netherlands E-mail: tino@cs.rug.nl Abstract
Lobula Plate Tangential Cells in Drosophila melanogaster ; Response Properties, Synaptic Organization & Input Channels
 Lobula Plate Tangential Cells in Drosophila melanogaster ; Response Properties, Synaptic Organization & Input Channels Maximilian Albert Jösch Krotki München 2009 2 Lobula Plate Tangential Cells in Drosophila
Lobula Plate Tangential Cells in Drosophila melanogaster ; Response Properties, Synaptic Organization & Input Channels Maximilian Albert Jösch Krotki München 2009 2 Lobula Plate Tangential Cells in Drosophila
Why Flies? stages of embryogenesis. The Fly in History
 The Fly in History 1859 Darwin 1866 Mendel c. 1890 Driesch, Roux (experimental embryology) 1900 rediscovery of Mendel (birth of genetics) 1910 first mutant (white) (Morgan) 1913 first genetic map (Sturtevant
The Fly in History 1859 Darwin 1866 Mendel c. 1890 Driesch, Roux (experimental embryology) 1900 rediscovery of Mendel (birth of genetics) 1910 first mutant (white) (Morgan) 1913 first genetic map (Sturtevant
AP Biology Essential Knowledge Cards BIG IDEA 1
 AP Biology Essential Knowledge Cards BIG IDEA 1 Essential knowledge 1.A.1: Natural selection is a major mechanism of evolution. Essential knowledge 1.A.4: Biological evolution is supported by scientific
AP Biology Essential Knowledge Cards BIG IDEA 1 Essential knowledge 1.A.1: Natural selection is a major mechanism of evolution. Essential knowledge 1.A.4: Biological evolution is supported by scientific
Development of Drosophila
 Development of Drosophila Hand-out CBT Chapter 2 Wolpert, 5 th edition March 2018 Introduction 6. Introduction Drosophila melanogaster, the fruit fly, is found in all warm countries. In cooler regions,
Development of Drosophila Hand-out CBT Chapter 2 Wolpert, 5 th edition March 2018 Introduction 6. Introduction Drosophila melanogaster, the fruit fly, is found in all warm countries. In cooler regions,
Nature Neuroscience: doi: /nn Supplementary Figure 1. Expression of GCaMP6s in LGN and their axons in V1.
 Supplementary Figure 1 Expression of GCaMP6s in LGN and their axons in V1. (a, b) Coronal section of LGN and V1 expressing GCaMP6s. a Coronal slice (bregma -2.3 mm) including thalamus confirmed that GCaMP6s
Supplementary Figure 1 Expression of GCaMP6s in LGN and their axons in V1. (a, b) Coronal section of LGN and V1 expressing GCaMP6s. a Coronal slice (bregma -2.3 mm) including thalamus confirmed that GCaMP6s
The foraging locus: behavioral tests for normal muscle movement in rover and sitter Drosophila melanogaster larvae
 Genetica 85: 205-209, 1992. 0 1992 Kluwer Acadernic Publishers. Primed in the Nerhrrlands. The foraging locus: behavioral tests for normal muscle movement in rover and sitter Drosophila melanogaster larvae
Genetica 85: 205-209, 1992. 0 1992 Kluwer Acadernic Publishers. Primed in the Nerhrrlands. The foraging locus: behavioral tests for normal muscle movement in rover and sitter Drosophila melanogaster larvae
Selection for late pupariation affects diapause incidence and duration in the flesh fly, Sarcophaga bullata
 Selection for late pupariation affects diapause incidence and duration in the flesh fly, Sarcophaga bullata By: Vincent C. Henrich and David L. Denlinger Henrich, V.C., and D.L. Denlinger (1982) Selection
Selection for late pupariation affects diapause incidence and duration in the flesh fly, Sarcophaga bullata By: Vincent C. Henrich and David L. Denlinger Henrich, V.C., and D.L. Denlinger (1982) Selection
Neural basis of a visuo-motor transformation in the fly
 Neural basis of a visuo-motor transformation in the fly Stephen James Huston Churchill College Department of Zoology University of Cambridge A dissertation submitted in partial fulfillment of the requirements
Neural basis of a visuo-motor transformation in the fly Stephen James Huston Churchill College Department of Zoology University of Cambridge A dissertation submitted in partial fulfillment of the requirements
Global Layout Optimization of Olfactory Cortex and of Amygdala of Rat
 UMIACS-TR-2007-14 CS-TR-4861 Global Layout Optimization of Olfactory Cortex and of Amygdala of Rat Raul Rodriguez-Esteban and Christopher Cherniak June 2005 Committee for Philosophy and the Sciences, Department
UMIACS-TR-2007-14 CS-TR-4861 Global Layout Optimization of Olfactory Cortex and of Amygdala of Rat Raul Rodriguez-Esteban and Christopher Cherniak June 2005 Committee for Philosophy and the Sciences, Department
Comprehensive Maps of Drosophila Higher Olfactory Centers: Spatially Segregated Fruit and Pheromone Representation
 Comprehensive Maps of Drosophila Higher Olfactory Centers: Spatially Segregated Fruit and Pheromone Representation Gregory S.X.E. Jefferis, 1,5,7, * Christopher J. Potter, 1,4,7 Alexander M. Chan, 1 Elizabeth
Comprehensive Maps of Drosophila Higher Olfactory Centers: Spatially Segregated Fruit and Pheromone Representation Gregory S.X.E. Jefferis, 1,5,7, * Christopher J. Potter, 1,4,7 Alexander M. Chan, 1 Elizabeth
Physiology 2 nd year. Neuroscience Optional Lecture
 Academic year 2018/2019 Physiology 2 nd year Semester 1 Curricula Nervous system physiology Blood physiology Acid-base equilibrium Bibliography: Boron & Boulpaep Medical Physiology, 3 rd edition Physiology
Academic year 2018/2019 Physiology 2 nd year Semester 1 Curricula Nervous system physiology Blood physiology Acid-base equilibrium Bibliography: Boron & Boulpaep Medical Physiology, 3 rd edition Physiology
INTERSEGMENTAL TO INTRASEGMENTAL CONVERSION BY GANGLIONIC FUSION IN LATERAL GIANT INTERNEURONES OF CRAYFISH
 J. exp. Biol. 107, 515-519 (1983) 5 \ 5 Printed in Great Britain The Company of Biologists Limited 1983 INTERSEGMENTAL TO INTRASEGMENTAL CONVERSION BY GANGLIONIC FUSION IN LATERAL GIANT INTERNEURONES OF
J. exp. Biol. 107, 515-519 (1983) 5 \ 5 Printed in Great Britain The Company of Biologists Limited 1983 INTERSEGMENTAL TO INTRASEGMENTAL CONVERSION BY GANGLIONIC FUSION IN LATERAL GIANT INTERNEURONES OF
Big Idea 1: The process of evolution drives the diversity and unity of life.
 Big Idea 1: The process of evolution drives the diversity and unity of life. understanding 1.A: Change in the genetic makeup of a population over time is evolution. 1.A.1: Natural selection is a major
Big Idea 1: The process of evolution drives the diversity and unity of life. understanding 1.A: Change in the genetic makeup of a population over time is evolution. 1.A.1: Natural selection is a major
AP Curriculum Framework with Learning Objectives
 Big Ideas Big Idea 1: The process of evolution drives the diversity and unity of life. AP Curriculum Framework with Learning Objectives Understanding 1.A: Change in the genetic makeup of a population over
Big Ideas Big Idea 1: The process of evolution drives the diversity and unity of life. AP Curriculum Framework with Learning Objectives Understanding 1.A: Change in the genetic makeup of a population over
Principles of Experimental Embryology
 Biology 4361 Developmental Biology Principles of Experimental Embryology September 19, 2006 Major Research Questions How do forces outside the embryo affect its development? (Environmental Developmental
Biology 4361 Developmental Biology Principles of Experimental Embryology September 19, 2006 Major Research Questions How do forces outside the embryo affect its development? (Environmental Developmental
Optic glomeruli and their inputs in Drosophila share an organizational ground pattern with the antennal lobes
 Optic glomeruli and their inputs in Drosophila share an organizational ground pattern with the antennal lobes Article (Published Version) Mu, Laiyong, Ito, Kei, Bacon, Jonathan P and Strausfeld, Nicholas
Optic glomeruli and their inputs in Drosophila share an organizational ground pattern with the antennal lobes Article (Published Version) Mu, Laiyong, Ito, Kei, Bacon, Jonathan P and Strausfeld, Nicholas
Neural encoding of motion visual cues in horizontally sensitive neurons of Drosophila
 Neural encoding of motion visual cues in horizontally sensitive neurons of Drosophila André Marques andre.f.r.marques@tecnico.ulisboa.pt Instituto Superior Técnico, Lisboa, Portugal September 216 Abstract
Neural encoding of motion visual cues in horizontally sensitive neurons of Drosophila André Marques andre.f.r.marques@tecnico.ulisboa.pt Instituto Superior Técnico, Lisboa, Portugal September 216 Abstract
Cell division takes place next to the RPE. Neuroblastic cells have the capacity to differentiate into any of the cell types found in the mature retina
 RPE is a monolayer of hexagonal shaped neural epithelial cells that have the same embryological origin as the neural retina. They mature before the neural retina and play a key role in metabolic support
RPE is a monolayer of hexagonal shaped neural epithelial cells that have the same embryological origin as the neural retina. They mature before the neural retina and play a key role in metabolic support
Supporting Online Material for
 www.sciencemag.org/cgi/content/full//576/65/dc Supporting Online Material for Evolution of a Polyphenism by Genetic Accommodation Yuichiro Suzuki* and H. Frederik Nijhout *To whom correspondence should
www.sciencemag.org/cgi/content/full//576/65/dc Supporting Online Material for Evolution of a Polyphenism by Genetic Accommodation Yuichiro Suzuki* and H. Frederik Nijhout *To whom correspondence should
Developmental Biology
 Developmental Biology 320 (2008) 30 38 Contents lists available at ScienceDirect Developmental Biology journal homepage: www. el sevier. com/ developmental bi ology Branch architecture of the fly larval
Developmental Biology 320 (2008) 30 38 Contents lists available at ScienceDirect Developmental Biology journal homepage: www. el sevier. com/ developmental bi ology Branch architecture of the fly larval
Enduring understanding 1.A: Change in the genetic makeup of a population over time is evolution.
 The AP Biology course is designed to enable you to develop advanced inquiry and reasoning skills, such as designing a plan for collecting data, analyzing data, applying mathematical routines, and connecting
The AP Biology course is designed to enable you to develop advanced inquiry and reasoning skills, such as designing a plan for collecting data, analyzing data, applying mathematical routines, and connecting
Genetic assimilation can occur in the absence of selection for the assimilating phenotype, suggesting a role for the canalization heuristic
 doi: 10.1111/j.1420-9101.2004.00739.x Genetic assimilation can occur in the absence of selection for the assimilating phenotype, suggesting a role for the canalization heuristic J. MASEL Department of
doi: 10.1111/j.1420-9101.2004.00739.x Genetic assimilation can occur in the absence of selection for the assimilating phenotype, suggesting a role for the canalization heuristic J. MASEL Department of
Linking Cell Fate, Trajectory Choice, and Target Selection: Genetic Analysis of Sema-2b in Olfactory Axon Targeting
 Article Linking Cell Fate, Trajectory Choice, and Target Selection: Genetic Analysis of Sema-2b in Olfactory Axon Targeting William J. Joo, 1,2 Lora B. Sweeney, 1,2,4 Liang Liang, 1,3 and Liqun Luo 1,2,
Article Linking Cell Fate, Trajectory Choice, and Target Selection: Genetic Analysis of Sema-2b in Olfactory Axon Targeting William J. Joo, 1,2 Lora B. Sweeney, 1,2,4 Liang Liang, 1,3 and Liqun Luo 1,2,
NEURONS, SENSE ORGANS, AND NERVOUS SYSTEMS CHAPTER 34
 NEURONS, SENSE ORGANS, AND NERVOUS SYSTEMS CHAPTER 34 KEY CONCEPTS 34.1 Nervous Systems Are Composed of Neurons and Glial Cells 34.2 Neurons Generate Electric Signals by Controlling Ion Distributions 34.3
NEURONS, SENSE ORGANS, AND NERVOUS SYSTEMS CHAPTER 34 KEY CONCEPTS 34.1 Nervous Systems Are Composed of Neurons and Glial Cells 34.2 Neurons Generate Electric Signals by Controlling Ion Distributions 34.3
Genetic Lab 3. Drosophila Fly
 Genetic Lab 3 Drosophila Fly An Introduction to fruit or vinegar fly Drosophila Melanogaster Is a small (about 3mm long), common fly found near unripe and rotted fruit, so that it called fruit or vinegar
Genetic Lab 3 Drosophila Fly An Introduction to fruit or vinegar fly Drosophila Melanogaster Is a small (about 3mm long), common fly found near unripe and rotted fruit, so that it called fruit or vinegar
MCN. Complex Genetic Interactions among Four Receptor Tyrosine Phosphatases Regulate Axon Guidance in Drosophila
 MCN Molecular and Cellular Neuroscience 17, 274 291 (2001) doi:10.1006/mcne.2000.0939, available online at http://www.idealibrary.com on Complex Genetic Interactions among Four Receptor Tyrosine Phosphatases
MCN Molecular and Cellular Neuroscience 17, 274 291 (2001) doi:10.1006/mcne.2000.0939, available online at http://www.idealibrary.com on Complex Genetic Interactions among Four Receptor Tyrosine Phosphatases
Natural Variation, Development, and Adaptive Phenotypes in C. elegans
 Natural Variation, Development, and Adaptive Phenotypes in C. elegans Bradly Alicea http://publish.illinois.edu/bradly-alicea Fly/Worm Club, UIUC, April 22, 2015 with input from Nathan Schroeder, Rebecca
Natural Variation, Development, and Adaptive Phenotypes in C. elegans Bradly Alicea http://publish.illinois.edu/bradly-alicea Fly/Worm Club, UIUC, April 22, 2015 with input from Nathan Schroeder, Rebecca
Figure S1. Programmed cell death in the AB lineage occurs in temporally distinct
 SUPPLEMENTAL FIGURE LEGENDS Figure S1. Programmed cell death in the AB lineage occurs in temporally distinct waves. (A) A representative sub-lineage (ABala) of the C. elegans lineage tree (adapted from
SUPPLEMENTAL FIGURE LEGENDS Figure S1. Programmed cell death in the AB lineage occurs in temporally distinct waves. (A) A representative sub-lineage (ABala) of the C. elegans lineage tree (adapted from
CHAPTER 9 BODY ORGANIZATION. Copyright 2007 by Mosby, Inc., an affiliate of Elsevier Inc. 1
 CHAPTER 9 BODY ORGANIZATION Copyright 2007 by Mosby, Inc., an affiliate of Elsevier Inc. 1 Anatomy and Physiology Four basic properties of life: Reception The ability of the organism to control its actions
CHAPTER 9 BODY ORGANIZATION Copyright 2007 by Mosby, Inc., an affiliate of Elsevier Inc. 1 Anatomy and Physiology Four basic properties of life: Reception The ability of the organism to control its actions
Vision as a Compensatory Mechanism for Disturbance Rejection in Upwind Flight
 Vision as a Compensatory Mechanism for Disturbance Rejection in Upwind Flight Michael. Reiser, J. Sean Humbert, Mary J. Dunlop, Domitilla Del Vecchio, Richard M. Murray, and Michael H. Dickinson Abstract
Vision as a Compensatory Mechanism for Disturbance Rejection in Upwind Flight Michael. Reiser, J. Sean Humbert, Mary J. Dunlop, Domitilla Del Vecchio, Richard M. Murray, and Michael H. Dickinson Abstract
The student might demonstrate the ability to achieve this standard by: Making a chart comparing the similar functions of plant and animal cells.
 1: Life, Cell Biology: All living organisms are composed of cells, from just one to many trillions, whose details are usually visible only through a microscope. s 1.a) Cells function similarly in all living
1: Life, Cell Biology: All living organisms are composed of cells, from just one to many trillions, whose details are usually visible only through a microscope. s 1.a) Cells function similarly in all living
80% of all excitatory synapses - at the dendritic spines.
 Dendritic Modelling Dendrites (from Greek dendron, tree ) are the branched projections of a neuron that act to conduct the electrical stimulation received from other cells to and from the cell body, or
Dendritic Modelling Dendrites (from Greek dendron, tree ) are the branched projections of a neuron that act to conduct the electrical stimulation received from other cells to and from the cell body, or
