On Formulas for Equivalent Potential Temperature
|
|
|
- Leo McDonald
- 6 years ago
- Views:
Transcription
1 SEPTEMBER 2009 N O T E S A N D C O R R E S P O N D E N C E 3137 On Formulas for Equivalent Potential Temperature ROBERT DAVIES-JONES NOAA/National Severe Storms aboratory, Norman, Oklahoma (Manuscript received 28 August 2008, in final form 16 April 2009 ABSTRACT Several new formulas for pseudoadiabatic equivalent potential temperature (EPT are devised and compared to previous ones. The maximum errors of all the formulas are determined from calculations on a dense grid of points in the region of a thermodynamic diagram defined by wet-bulb potential temperature #328C (EPT # 400 K and pressure between 100 and 1050 mb. One of the new formulas has an accuracy of K in the specified region. Finding the imitation first law of thermodynamics that they satisfy approximately reveals how the formulas work. 1. Introduction Corresponding author address: Dr. Robert Davies-Jones, NOAA/ National Severe Storms aboratory, National Weather Center, 120 David. Boren Blvd., Norman, OK bob.davies-jones@noaa.gov There are several formulas for equivalent potential temperature (EPT in the literature (Bolton 1980; Bryan Rossby (1932 devised the first such formula by dropping an intractable term from the equation governing pseudoadiabatic water-saturation processes. This term is part of the entropy of water vapor. Its omission is equivalent to setting the specific heat of water to zero. Owing to the missing entropy, the Rossby formula underestimates the EPT by several degrees in very warm and moist environments. By using an optimized constant latent heat instead of the actual one, which is a linear function of temperature, Bryan (2008 obtained a simple formula that is accurate to about half a degree. His formula is associated with a simple version of the first law of thermodynamics, which is useful in some theoretical studies. Except at very cold temperatures where latent heat release is small, Bryan s constant latent heat is larger than the actual one. The upward adjustment in latent heat compensates to a large extent for the missing term. Bolton s (1980 modification of the Betts and Dugan (1973 formula is similar to Bryan s formula, and is also associated with a constant latent heat (Davies-Jones The most accurate formula to date is from Bolton (1980. It computes EPT to accuracy, relative to numerical solutions of the governing equation, better than 0.02 K. Note that accuracies quoted in the literature are based on a limited number of calculations (e.g., Bolton s Table 3 and Bryan s Table 1. Bolton s formula has three adjustable parameters and is hard to interpret physically. In this paper, the gap between Bryan s formula with one adjustable parameter and Bolton s very accurate formula with three is bridged by constructing formulas that have two degrees of freedom and intermediate accuracies. These new formulas are obtained by optimally adjusting the line in a graph of latent heat versus temperature instead of constraining it to have zero slope. Also, understanding of how the formulas work is gained by working backward from the formulas to an imitation first law of thermodynamics that they satisfy. This procedure identifies the adjustments needed to compensate for setting the specific heat of water to zero. Bolton s formula is not amenable to this approach so a very similar, but more manageable formula with the same accuracy is devised. ast, maximum errors of each formula are determined more systematically than previously by calculating EPT at 1209 points in a grid covering a wide area of a thermodynamic diagram. 2. EPT formulas As pointed out by Davies-Jones (2008, almost all of the formulas for the EPT of an air parcel have the following form: DOI: /2009MWR2774.1
2 3138 M O N T H Y W E A T H E R R E V I E W VOUME 137 * 5Qexp * # *(T 0 1 C r(1 1 k T 2 r, (2.1 where 0 * (.0, 1 * ($0, and k 2 ($0 are constants, * is the estimate of, is the temperature at the lifting condensation level (C, and r is the mixing ratio. All symbols and a few useful relationships are listed in the appendix. The quantity Q is either the potential temperature u of moist air, which is conserved in unsaturated isentropic motion, or the potential temperature of dry air at the C, u D. These formulas apply to all levels because, u D, r, and e can be replaced by, u D, r s (, p, and e s (, respectively, above the C. Associated with (2.1 is the approximate equation for pseudoadiabatic ascent: d ln * 5 d lnq1d * *(T 0 1 C r(1 1 k T 2 r 5 0, (2.2 fit to data match the data more accurately. The third degree of freedom, k 2, is needed for a very accurate fit. Using this approach Betts and Dugan (1973, Simpson (1978, Bolton (1980, and Bryan (2008 have devised formulas that are similar to (2.4, but that match the exact pseudoadiabats far better. Since the equations in Bolton s paper are referenced many times, we will preface his equations numbers with a B. Similarly, Bryan s (2008 equations are prefaced with Br. We do not consider Simpson s formula [(B33 after Bolton s modification] further because it is less accurate than (B38 and (B39, has a more cumbersome form, assumes that u D is conserved during adiabatic ascent to the C, and is not easily associated with an imitation first law of thermodynamics. Bolton simplified Betts and Dugan s (1973 formula to 5 u exp *! 0 r, * T Jkg 1, * 1 5 0, (2.5 and the adjusted entropy: s p * [ ln * 5 lnq1 * *(T 0 1 C r(1 1 k T 2 r. (2.3 The first and least accurate formula for EPT is the one devised by Rossby: u9 E exp ( r #, (2.4 where 9 denotes the Rossby EPT and is the actual latent heat. It underestimates the actual EPT by up to 58 in extremely humid air masses because it uses the actual latent heat [ ( 2 C and ignores the term involving the specific heat of water in the entropy formulation. However, it sufficed for its original purpose, the identification of air masses. Because the omitted entropy term c w r s d ln is positive, 9 is always an underestimate of. The Rossby formula has no parameter that can be adjusted to compensate for the missing entropy. A further approximation consists of replacing u D by u (Iribarne and Godson 1973, p When k 2 5 0, comparison of (2.1 and (2.4 shows that we can regard * [ 0 * 2 1 *( 2 C as a surrogate for. We can obtain more accurate formulas for EPT by adjusting the two latent heat coefficients. Generally, formulas with more degrees of freedom (the number of independent parameters that are varied to obtain a best which is equivalent to (B35. This has one degree of freedom, *, 0 an optimum constant value of latent heat. Bryan s (2008 formula (Br13 similarly has one degree of freedom. It has the following form:! H R y r/c *r pd exp, (2.6 with * 5 0 * Jkg 21. Here, the relative humidity H has been introduced to avoid having to compute, and u D is used instead of u. Bolton s two new formulas, (B38 and (B39, are the most accurate to date. They have the form (2.1 with k 2 6¼ 0 so they both contain a r 2 term. The difference between them is the use of u D in (B39 and u in the less precise (B38. Although (B38 ostensibly has three degrees of freedom, it in fact has only two because * is proportional to (i.e., 0 * and 1 * are not varied independently. Equation (B39 does have three degrees of freedom, and this seems to be the number required for an estimate with maximum error (relative to the results of numerical integration of the differential equation governing pseudoadiabatic ascent to within 0.03 K. The actual error is around 0.2 K, owing mainly to the specific heat at constant pressure for dry air, assumed to be a constant, varying slightly with temperature and pressure. The corresponding error in pseudo-wet-bulb temperature is less than 0.2 K (Davies-Jones The third-order term, 2k 2 1 *( 2 Cr 2, in (2.1 is much smaller in magnitude than the term k 2 0 *r 2. When this term is neglected, (2.1 simplifies to
3 SEPTEMBER 2009 N O T E S A N D C O R R E S P O N D E N C E 3139 ( * 5Qexp [* *(T 0 1 C]r 1 K 2 r 2, (2.7 d 5 R y d lne s, (3.2 T 2 K where K 2 ( k 2 0 * is a constant. A formula of this form that is as accurate as (B39 is developed later. Note that all the formulas with k are included in this form. 3. The formulas imitation first law of thermodynamics In many cloud models, simplified versions of the first law of thermodynamics are used (Wilhelmson Only some of these are exact differentials for pseudoadiabatic processes and so have exact corresponding analytical formulas for EPT. On the other hand, the approximation to the entropy form of the first law (Holton 1992, p. 51 that corresponds to an analytical approximate formula for EPT can always be found by differentiating the formula as in (2.1 and (2.2. For use in theoretical studies, Bryan (2008 found an EPT formula that is associated with a simplified version of the first law. Here, we work backward from the entropy formulation to find an imitation first law that the formulas satisfy with the purpose of understanding how the formulas work. As is customary, we neglect the ice phase, assume that the phases of water are in equilibrium (so that there is no supersaturation and that the volume of any liquid water in the parcel is negligible, and consider the two extreme cases of adiabatic ascent of air that is saturated with respect to a plane surface of water (Bryan In the first extreme case, the liquid water remains in the parcel and the total water mixing ratio, Q [ r 1 r w, is constant. This is a reversible process (Saunders 1957 and the liquid water content is said to be adiabatic. The second case is the pseudoadiabatic process where condensate falls out of the parcel immediately after it forms. Normally, the liquid water content in clouds lies between the two extremes (Wilhelmson 1977 although occasionally it may exceed the adiabatic value. For the reversible process, the differential equation governing entropy has the exact integral: s r 5 lnu D 1 rc w ln R y r lnh 1 ( r/ 1 r w c w ln (3.1 [Emanuel 1994, Eq. (4.5.9]. After multiplying the differential form of (3.1 by and using (B23, the ideal gas laws for dry air and vapor, and the Clausius Clapeyron equation: we find that ds r 5 dh a d dp 5 0, (3.3 where h [ ( 1 Qc w 1 r is the specific moist (reversible enthalpy (Emanuel 1994, p By applying Kirchoff s equation: we obtain the first law d d 5 c py c w, (3.4 ds r 5 ( 1 rc py 1 r w c w d a d dp 1 dr50. (3.5 In the pseudoadiabatic process, the liquid water is removed immediately from the parcel (r w 5 0 so total water is no longer conserved. The entropy, EPT and first law are now s p 5 lnu D 1 c w ð TK Y(t dt 1 ( Y( t5t 0 R y r lnh, (3.6 ( H R y r/c Y(T pd exp K (T c K 1 c w pd Y( ð # TK 3 Y(t dt, (3.7 T 0 and ds p 5 ( 1 rc py d a d dp 1 ( dr 5 0, (3.8 where Y( [ r/ and T 0 is a very cold temperature where the integrand is effectively zero. The integral term in (3.6 is part of the entropy of water vapor. It is not zero because, in the pseudoadiabatic process, water condenses and then is removed instantly, along with its entropy, as the parcel is rising and cooling, not while it is maintained at a constant temperature (Bolton The exact formula for is (3.7, but it is not useful because it contains an integral. Since the integral is not an exact differential, the integration has to be performed numerically along a pseudoadiabat. This is tantamount to generating pseudoadiabats by stepwise numerical integration so nothing has been gained. Useful formulas
4 3140 M O N T H Y W E A T H E R R E V I E W VOUME 137 for s p and that depend only on the initial and final states of the parcel have either ignored this term or replaced it implicitly by a compensating term that is an exact differential. Alternatively, we can set c w 5 0 and explicitly adjust some other coefficients to minimize the resulting error. This is the approach adopted here. In fact, the formulas that have the simplified form in (2.7 may all be obtained by setting c w 5 0, which eliminates the computationally expensive integral term, and adjusting (and K 2 if nonzero to minimize error. In other words, c w / c w * 5 0, 0 / 0 * and 1 / 1 * where the superscript asterisk denotes that the coefficients have been adjusted. This gives us the estimate # u* E H R y r/c *(T pd exp K r 1 K 2 r 2. (3.9a The EPT estimate, *, should be conserved below the C. We can ensure this by integrating the entropy equation just for the pseudoadiabatic part of the ascent, and evaluating the constant of integration at the C. This yields u* E exp # *( r s ( 1 K 2 r 2 s. (3.9b We now find that an imitation first law that corresponds to (3.9. In turns out that in the temperature range 2478, 2 C # 328C, 1, */, 1.06 for the formulas with Q5u D, so to a quite good approximation we can use (3.2 with * instead of. From (3.9a and the modified (3.2, we obtain 0 5 ds p * 5 a d dp 1 d(*r dh* 1 K 2 d(r 2 /. (3.10 Since r 2 varies much more rapidly than at high mixing ratios where the last term is important, we can approximate (3.10 by where ds p * 5 dh* a d dp 5 0, (3.11 h* 5 1 *( r 1 K 2 r 2 (3.12 is an enthalpy that exists in this simple form because c w * 5 0. Expanding (3.12 gives us the following first law : ds p * 5 ( 1 *r d 1 [ 0 * 1 *( C] dr 1 K 2 d(r 2 a d dp 5 0. (3.13 If we assume Kirchoff s Eq. (3.4 for the adjusted parameters, then we can define an adjusted specific heat of water vapor, c py * 52* 1 and (3.13 becomes ds p * 5 ( 1 rc py * d a d dp 1 *( dr 1 K 2 d(r (3.14 Bryan (2008 set 1 * 5 0 (and K Then, (3.6, (3.7, and (3.9 reduce to (Br11, (Br13, and (Br10, respectively, and the enthalpy becomes h*5 1 0 *r. We can see how the formulas work by comparing (3.8 and (3.14. The adjustment of the specific heat of water to zero and Kirchoff s equation makes the specific heat of water vapor negative (or zero if 1 * 5 0. Thus, the specific heat of moist air is reduced artificially. From a first-law viewpoint, an artificial increase in latent heat, (* 2 dr, is required to compensate for the decrease in specific heat. The small r 2 term is extra compensation needed for very accurate calculations of very warm EPTs. From an entropy viewpoint, the entropy increase associated with the increase in latent heat is needed to compensate the entropy of water vapor for the part that is missing. Which of the forms (3.9a or (3.9b is preferable? Equation (3.9a has the advantage that it does not require computation of temperature at the C. But, unlike (3.9b, it does not conserve below the C (Bryan Thus, a disadvantage of (3.9a is the introduction of additional error below the C as shown below. Since r is constant below the C, the integrand in (3.7 has an antiderivative and so (3.7 becomes ( H R y r/c r (T pd exp K 1 c T w ln T K, K T 1 (3.15 where T 1 is an arbitrary constant of integration. Thus, by conservation of and (3.15, u D and u D are related by ( u D H R y r/c r (T pd exp K ( 1 c w ln T K. (3.16 Substituting for u D in (3.9b gives us where ( u* E H R y r/c r *(T pd exp K 1 K 2 r f ( 1 f (, (3.17
5 SEPTEMBER 2009 N O T E S A N D C O R R E S P O N D E N C E 3141 f (t [ *(t (t c t w lnt 1 K 2 r t. (3.18 So (3.9b implies (3.17, not (3.9a. Although we can apply a correction factor to (3.9a [to obtain (3.17], this involves, so we might as well use (3.9b in the first place. Using Q5u instead of u D is less expensive computationally, but also leads to more approximation. Bolton states For most purposes (B38 is probably to be preferred, since it makes use of u. As a basis for very accurate work, however, (B39 with u D given by (B24 is suggested. However, the author prefers using formulas that use u D instead of u for the following reasons. First note that from (B4, (B5, (B7, (B24, and the ideal gas laws for moist and dry air, u D, and its value at the C, u D, are related to u by u 0.28r u D 5 u 1 1 r # kd, «u 0.28r u D 5 u 1 1 r # kd. «(3.19 Thus, the extra work involved in computing u D or u D is relatively minor, and simply replacing u D with u in the exact Eq. (3.7 introduces error by tacitly setting the above quantities in square brackets to 1. Since u, u D,the adjustment to latent heat has to be larger when Q5u. 4. Approach for devising new formulas The approach is to minimize the difference between the true specific pseudoentropy and the one associated with a given formula. From (3.6 and (3.9a this difference for a saturated parcel is ( ð # c TK s p * s p 5 Y(, *( 1 K 2 r s ( w Y(t, u Y(, E dt, (4.1 T 0 for Q5u D. [When Q5u, the term ln(u/u D is added to the right side.] Here, Y(, [ r s (, /.To compensate for the omission of the c w term in (3.9b, we seek the optimum *, defined as the one that minimizes the maximum error, js p * 2 s p j max, in a specified region of a thermodynamic diagram. At cold temperatures and hence small mixing ratios, js p * 2 s p j is small because Y is small. We need to make this difference small at large r s. For large mixing ratios, js p * 2 s p j is small only if *( 1 K 2 r s (, ( ð c TK w Y(t, u Y(, E dt, (4.2 T 0 where * 5 at the cold temperature T 0. For estimation of * 1 K 2 r s, the quantity, [( 1 ð c TK w Y(t, u Y(, E dt T 0 5 Y( ln u D (4.3 [from (3.7] was evaluated, using results from the numerical integration procedure described below. We may regard as the data that * 1 K 2 r s should fit at large mixing ratios, and thus satisfy the approximation in (4.2. The procedure (Bolton 1980 finds the true EPT of a parcel as follows. From (3.6, the differential equation defining a pseudoadiabat is 0 5 d ln 5 d lnu x 1 c w ( x d, (4.4 where lnu x 5 lnu D 1 x and x [ r s /. The saturation vapor pressure is computed from (B10, the dewpoint temperature is calculated from the inverse of (B10, and the temperature at the C is given by (B15. The quantities u x and x along a pseudoadiabat are found by numerically integrating (4.4 from the C where u x [ 9, using the (second order modified Euler method here and small temperatures steps. To reduce round-off error slightly, the prognostic variable in (B31 is changed here to ln(u x /u x0 where u x0 is the value of u x at p 5 p 0. At each step, x is recovered from u x by solving (B32 via Newton s method. The pressure at each step is given by p 5 e s (1 1 «/ x. The EPT of the pseudoadiabat is the value of u x obtained by integrating to a very cold temperature T 0 (in K where the value of u x no longer changes. 5. Optimization procedure First, consider formulas with two degrees of freedom, 0 * and 1 * (K We seek the values of 0 * and 1 * that minimize the maximum absolute error jdsj max in the region of a thermodynamic diagram defined by
6 3142 M O N T H Y W E A T H E R R E V I E W VOUME 137 FIG. 1. Contours of jd j max in ( 0 *, 1 * space for Q5u D. The grid spacings are D 0 * Jkg 21 and D 1 * 5 10 J kg 21 K 21. The contour levels and the minimum value in the grid are shown at the top. The letters R, D, and the large O are centered on the points ( 0, 1 5 ( , 2370 used in (2.4, ( 0 *, 1 * 5 ( , 0 used in (6.2, and ( 0 *, 1 * 5 ( , 900 used in (6.4, respectively (units are SI. 100 # p # 1050 mb and u w # 328C ( # 400 K. The procedure used to find the optimal values of the parameters 0 * and 1 * in a formula consists of the following steps. 1 Choose the set of grid points {2208, 2188,...,328C} 3 {100, 125,..., 1050 mb} in (u w, p space. 2 From the initial condition 5 u w, p 5 p 0, integrate along pseudoadiabats as described in section 3 to determine the true value of the EPT,, that corresponds to each WBPT, u w. 3 The integration procedure yields pressure on a regular grid in (u w, space. Obtain the values of temperature at the grid points in (u w, p space by interpolation. 4 Select initial values for 0 * and 1 *. 5 Use the formula to compute the estimated EPT, *, of a saturated parcel at each point. 6 At each point, calculate the difference d [ * 2 between the EPT computed from the formula and the true EPT. Save d (u w, p. 7 Find the maximum absolute difference jd j max. 8 If the formula has two degrees of freedom, set up a mesh system in the 2D ( 0 *, 1 * space centered on the initial value with uniform spacing D 0 * and D 1 * directions. Repeat steps 4 7 at each grid point and contour the jd j max field. This is done for (2.7 with K 2 5 0inFigs.1and2fora grid in the domain defined by 0 * 2 [ , ]J kg 21 and 1 * 2 [0, 2500] J kg 21 K 21. At this high resolution it is evident that there is only one minimum in the domain when either Q5u D (Fig. 1 or Q5u (Fig Record the minimum value and locate the grid point where it occurs. At this point center a new grid with the spacing D 0 * and D 1 * reduced by
7 SEPTEMBER 2009 N O T E S A N D C O R R E S P O N D E N C E 3143 FIG. 2. As in Fig. 1, but for Q5u. The letters A and the large O locate the points ( 0 *, 1 * 5 ( , 0 used in (6.1 and ( 0 *, 1 * 5 ( , 1109 used in (6.3, respectively. factors of 30 and 10, respectively, and find the minimum value and its location in this grid. 10 The optimum values of the parameters have now been determined. The accuracy E of the formula is the minimum value of jd j max in ( 0 *, 1 * space. The parameters have to be found quite precisely because the error is sensitive to it [as found also by Bryan (2008]. 11 Data are also evaluated at points {u w 5 348,368,388, 408C} 3 {p 5 100, 125,..., 1050 mb} in a grid extension for plotting in some of the figures. These data are not used in optimization. 12 Steps 8 10 are similar for a formula with only one parameter 0 *, except the search for the minimum is one dimensional (along the line 1 * 5 0. For the formula with three parameters, steps 8 10 are performed for different values of the third parameter, K 2, until the minimum in the 3D parameter space is found. The method is quite general. It can be easily adapted to work with values of the physical constants and formulas for saturation vapor pressure and condensation temperature that differ from those that Bolton used. 6. Optimized formulas We first consider the class of formulas with * The optimized version of (B35 is 5 u exp Ar, A [ */c T 0 pd K, (6.1 where the optimum value of A is only slightly different from Bolton s value of 2675 K. Using u D instead of u provides us with a new more accurate formula:! *r exp 0, (6.2
8 3144 M O N T H Y W E A T H E R R E V I E W VOUME 137 TABE 1. The formulas for and their errors. Here E is the maximum error in for saturated parcels in the region of a thermodynamic diagram defined by 2208C # u w # 328C and 100 mb # p # 1050 mb. The formulas are listed in order of increasing accuracy. The numbers in parentheses in the fourth column are the maximum errors for u w # 408C. Formula Equation Values of constants E (K Degrees of freedom (2.4 exp 0 1 (T # C Jkg 21, r J kg 21 K (11.1 0! (Br13 H k d r/«exp * 0 r * Jkg ( T! (6.1 *r 5 u exp 0 * Jkg (1.32 1! (6.2 *r exp 0 * Jkg ( # (6.3 5 u exp * 0 *(T 1 C * r Jkg 21, 0.18 ( * J kg 21 K 21 (6.4 (B38** (B39 (6.5 exp * # 0 *(T 1 C r # 5 u exp *r (1 1 k T 2 r # exp *r (1 1 k T 2 r exp ( # * 1 K 2 rr ** (B38 has only two degrees of freedom because * * Jkg 21, 0.11 ( * J kg 21 K 21 0 * Jkg 21, 1 * J kg 21 K 21, k * Jkg 21, 1 * J kg 21 K 21, k * Jkg 21, 1 * J kg 21 K 21, K Jkg ( ( ( where 0 * Jkg 21 is the optimum value, which is close to the value Jkg 21 obtained by Bryan (2008 despite the different optimization criteria. Optimizing (2.7 with K and two degrees of freedom, 0 * and 1 *, results in the new formulas: and 5 u exp * # *(T 0 1 C r, 8 < * Jkg 1 : * J kg 1 K 1 exp * # 0 *(T 1 C r, ( * Jkg 1. * J kg 1 K 1 (6.3 (6.4 In addition, a new formula with three degrees of freedom is found: ( exp [* 0 *(T 1 C 1 K 2 r]r, (6.5 where 0 * Jkg 21, 1 * J kg 21 K 21, and K Jkg Accuracy of the formulas The previous and new formulas are ranked in terms of the accuracy E in Table 1 and their maximum errors as functions of u w are shown in Figs The accuracies of the formulas in the region 100 # p # 1050 mb and u w # 328C are determined first of all by the number of degrees of freedom and then, with one exception, by the choice for Q as anticipated in section 3. The Rossby equation (2.4, which has no degrees of freedom, shows the maximum error (up to 5 K in the above region that
9 SEPTEMBER 2009 N O T E S A N D C O R R E S P O N D E N C E 3145 FIG. 3. Maximum absolute error j * 2 j as a function of u w for the Rossby formula, (Ro or (2.4, which has no degrees of freedom, and for the Eqs. (6.1, (6.2, and (Br13 that have one degree of freedom. The ordinate scale is logarithmic. arises from neglecting the c w term and utilizing the actual latent heat. The Rossby formula has a maximum error with respect to pressure (with u w held fixed that varies almost exponentially with u w (Fig. 3. Of the formulas with a constant adjusted latent heat (one degree of freedom, (6.2 has the least maximum error, 0.38 K (Fig. 3 because it uses u D. The modified Betts Dugan equation (6.1, which contains u, is accurate to 0.49 K. Even though it has the same form as (6.2 for a saturated parcel, Bryan s formula (Br13 with a maximum error of 0.57 K is slightly less accurate than (6.1. This is probably due to it being optimized by minimizing the mean square error (instead of the maximum error for a limited number of cases. Because it uses u, (6.3 with a maximum error of 0.18 K is the least accurate of the formulas with two degrees of freedom (Fig. 4. Even though it also uses u and (6.4 uses u D, (B38 is slightly the more accurate with a maximum error of 0.09 K compared to 0.11 K. The better accuracy of (B38 is probably because of its use of k 2 [see (2.1] as the second adjustable parameter and because of the fixed parameter 1 * being determined by * } instead of being set to zero. In the category with three degrees of freedom, (6.5 is accurate to 0.15 K and (B39 to K (Fig. 5. However, (B39 is more accurate for u w, 248C so there is little to choose between the two formulas. Surprisingly, (B38 is used most frequently (Emanuel 1994; Bryan 2008 even though it is less accurate than (B39. FIG. 4. As in Fig. 3, but for (B38, (6.3, and (6.4, which have two degrees of freedom. The maximum errors are not restrained by optimization beyond u w 5 328C and grow rapidly (Figs For u w # 408C, the widely used Eq. (B38 has a maximum error of 0.94 K (Table 1. In contrast, (6.5 and (B39 contain the error to 0.1 K (Fig. 5. The quantity defined by (4.3 is the adjusted latent heat according to the data. Figure 6 is a scattergram of FIG. 5. As in Fig. 3, but for (6.5 and (B39, which have three degrees of freedom, and the ordinate scale is now linear.
10 3146 M O N T H Y W E A T H E R R E V I E W VOUME 137 FIG. 6. Scatter diagram for [ ln( /u D /Y vs temperature T (8C for data at all the points in the grid for which r s $ (small dots and at the points in the grid extension (circled dots. The lines identified by, (6.2, (6.4, and (6.5 and the dashed line [near the (6.5 line] are the actual latent heat of vaporization according to (B2, the constant adjusted latent heat used in (6.2, and the adjusted latent heats used in (6.4, (6.5, and (B39, respectively. The data for u w. 328C is not used in the determination of the adjusted latent heats (and K 2. versus T for all the points in the grid for which r s $ ower values of r s were excluded because / 0/0 as r s / 0. The data do not lie precisely on a straight line, indicating that, although is mainly a linear function of, it is slightly dependent on r. The straight lines are the actual latent heat, T used in (2.4, the constant latent heat, * 5 0 *, used in (6.2, and the adjusted latent heats, * 5 0 * 2 1 *T, used in (6.4, (6.5, and (B39. The constant latent heat used in (6.2 is equal to the computed adjusted latent heats within a few degrees of 208C. The line for (6.4 fits the data well. The (6.5 and (B39 lines are not a good fit at cold temperatures (and small mixing ratios, but this is not crucial because * is multiplied by r in the formulas. These lines underestimate the observed adjusted latent heats at warm temperatures to make room for functions of r, which are not represented in Fig. 6. Figure 7 is a scattergram for /*(B39 versus r s to isolate the (1 1 k 2 r factor in (B39 according to the data. The points spread out at small mixing ratio as the numerator and denominator both tend to zero. Bolton s line r s fits the data nicely, thus verifying (B39. Similarly a scattergram for 2*(6.5 versus r s (Fig. 8 isolates the K 2 r term in (6.5 according to the data. The line K 2 r s, K Jkg 21, obtained by optimization, is also a good fit to the data. FIG. 7. Scatter diagram for /*(B39 vs r s for the same data points as in Fig. 6, where *(B39 is the adjusted latent heat in (B39. Also shown is the line /*(B k 2 r s, k , which is Bolton s fit to the data. For formulas with 1 * 5 k 2 5 K 2 5 0, it is apparent from (2.7 that the adjusted latent heat for a formula that uses u must be larger than the corresponding formula that uses u D by an amount that is approximately equal to ( /r ln(u D /u. Over the grid this quantity ranges from to Jkg 21 with a mean around FIG. 8. Scatter diagram for 2*(6.5 vs r s for the same data points as in Fig. 6, where *(6.5 is the adjusted latent heat in (6.5. Also shown is the line [ 2*(6.5] 5 K 2 r s, K Jkg 21, used in (6.5.
11 SEPTEMBER 2009 N O T E S A N D C O R R E S P O N D E N C E Jkg 21. From Table 1 we see that this agrees with the difference ( Jkg 21 between 0 * for (6.1 and (6.2. Finally, we stress that all the accuracies given above are relative to the results from numerical integration of (B25. Owing to not being strictly constant, there is an uncertainty of up to 0.2 K, which has not been taken into account (Bolton Summary The accuracies of several formulas for equivalent potential temperature have been assessed by computing their maximum error throughout the region of a thermodynamic diagram defined by 100 # p # 1050 mb and u w # 328C ( # 400 K. The more accurate formulas contain u D rather than u, and,noth. Some new formulas have been devised. These have two or three degrees of freedom and minimize the above maximum error. With one degree of freedom, a constant latent heat, it is possible to fit the data to 0.4 K. This error is reduced to 0.1 K [in (6.4] by utilizing an adjusted latent heat that depends linearly with temperature. The residual error is not a function of temperature. It must therefore be a function of r since and r alone (or and r s if the parcel is saturated determine. To achieve greater accuracy than 0.1 K, a formula must contain this extra dependence on r. Bolton s (B38 and (B39 do so by containing an r 2 term. Equation (B39 is the more accurate because it uses u D and has an extra degree of freedom. Finding the imitation first law of thermodynamics that the formulas satisfy approximately reveals how the formulas work. Adjusting the adjusted specific heat of water to zero to eliminate the integral term in the entropy formulation and Kirchoff s equation makes the specific heat of water vapor negative (or zero if the formula uses a constant latent heat. Thus, the specific heat of moist air is reduced artificially. An artificial increase in latent heat, (* 2 dr, is required to compensate for the decrease in specific heat. The highly accurate Eqs. (B38 and (B39 are not easily reconciled with an imitation first law. Therefore, a formula, (6.5, is devised herein that has a slightly simpler formula than (B39, has comparable accuracy, and can be related to an imitation first law if a small r 2 term is added to the imitation enthalpy. This small boost in enthalpy is additional compensation that is needed at high mixing ratios. Acknowledgments. This work was supported in part by NSF Grant ATM I thank Dr. George Bryan and two anonymous reviewers for their thoughtprovoking comments. APPENDIX ist of Symbols A K, the constant in (B35. C K (absolute zero 5 0K C. E: Maximum absolute error in for u w # 328C, 100 # p # 1050 mb. H: Relative humidity [ e/e s. K 2 : A constant in (2.7 and ( Jkg 21 in (6.5. ( : atent heat of vaporization, ( 2 C Jkg J kg 21 K 21 ( c w 2 c py, Kirchoff s equation. * Adjusted latent heat, 5* 0 2 *(T 1 K 2 C. Q: Total water mixing ratio (g g 21. R d : Gas constant for dry air J kg 21 K 21. R m : Gas constant for moist air 5 R d (1 1 r/«/(1 1 r R d ( r. R y : Gas constant for water vapor J kg 21 K 21 (5R d /«. T, : Temperature (8C and K, respectively. : Temperature at the C (K. T 0 : A very cold temperature (K. Y [ r/ (K 21. : Specific heat at constant pressure of dry air J kg 21 K 21 ( R d. c pm : Specific heat at constant pressure of moist air 5 ( 1 rc py /(1 1 r 5 ( r. c py : Specific heat at constant pressure of water vapor J kg 21 K 21 ( 4R y. c py * : Adjusted value of c py 52*. 1 c w, c w *: Specific heat of water, J kg 21 K 21 and adjusted value (50. e: Vapor pressure (mb, e 5 e s (T D, T D 5 dewpoint temperature (K. e s ( : Saturation vapor pressure (mb exp[17.67( 2 C/( 2 C ]. de s /d 5 e s /R y T 2 K (Clausius Clapeyron equation. h, h*: Specific enthalpy, adjusted specific enthalpy (J kg 21. k 2 : A nondimensional constant equal to 0.81 in (B38 and in (B39. p: Pressure (mb. p 0 : mb. r: Mixing ratio (g g 21, 5«e/(p 2 e. r s : Saturation mixing ratio (g g 21 5 «e s /(p 2 e s. r w : iquid water mixing ratio (g g 21. s p, s p *: Actual and adjusted specific pseudoadiabatic entropy. s r : Total (reversible specific entropy of moist air [ s d 1 rs y 1 r w s w. D*, 0 D*: 1 Grid spacings in (*, 0 * 1 space. : Defined in (4.3. Q: Either u or u D.
12 3148 M O N T H Y W E A T H E R R E V I E W VOUME 137 a d : Specific volume of dry air (m 3. x [ r s /. «5 R d /R y k d 5 R d / ( 2/7. k m 5 R m /c pm 5 k d ( r. u: Potential temperature of moist air (K 5 (p 0 /p k m. u D : Potential temperature of dry air (K 5 [p 0 / (p e] k d. u D : Value of u D at the C (K., 9 : EPT and Rossby EPT (K. *: Estimate of by a formula. u w : Wet-bulb potential temperature (WBPT (8C. u x [ u D exp(x. jd j max : Maximum error in as a function of 0 * and 1 *. REFERENCES Betts, A. K., and F. J. Dugan, 1973: Empirical formula for saturation pseudo-adiabats and saturation equivalent potential temperature. J. Appl. Meteor., 12, Bolton, D., 1980: The computation of equivalent potential temperature. Mon. Wea. Rev., 108, Bryan, G. H., 2008: On the computation of pseudoadiabatic entropy and equivalent potential temperature. Mon. Wea. Rev., 136, Davies-Jones, R., 2008: An efficient and accurate method for computing the wet-bulb temperature along pseudoadiabats. Mon. Wea. Rev., 136, Emanuel, K. A., 1994: Atmospheric Convection. Oxford University Press, 580 pp. Holton, J. R., 1992: An Introduction to Dynamic Meteorology. 3rd ed. Academic Press, 511 pp. Iribarne, J. V., and W.. Godson, 1973: Atmospheric Thermodynamics. Reidel, 222 pp. Rossby, C. G., 1932: Thermodynamics applied to air mass analysis. MIT Meteorology Papers 1, No. 3, 48 pp. Saunders, P. M., 1957: The thermodynamics of saturated air: A contribution to the classical theory. Quart. J. Roy. Meteor. Soc., 83, Simpson, R. H., 1978: On the computation of equivalent potential temperature. Mon. Wea. Rev., 106, Wilhelmson, R. B., 1977: On the thermodynamic equation for deep convection. Mon. Wea. Rev., 105,
1. Water Vapor in Air
 1. Water Vapor in Air Water appears in all three phases in the earth s atmosphere - solid, liquid and vapor - and it is one of the most important components, not only because it is essential to life, but
1. Water Vapor in Air Water appears in all three phases in the earth s atmosphere - solid, liquid and vapor - and it is one of the most important components, not only because it is essential to life, but
Lecture Ch. 6. Condensed (Liquid) Water. Cloud in a Jar Demonstration. How does saturation occur? Saturation of Moist Air. Saturation of Moist Air
 Lecture Ch. 6 Saturation of moist air Relationship between humidity and dewpoint Clausius-Clapeyron equation Dewpoint Temperature Depression Isobaric cooling Moist adiabatic ascent of air Equivalent temperature
Lecture Ch. 6 Saturation of moist air Relationship between humidity and dewpoint Clausius-Clapeyron equation Dewpoint Temperature Depression Isobaric cooling Moist adiabatic ascent of air Equivalent temperature
Thermodynamics Review [?] Entropy & thermodynamic potentials Hydrostatic equilibrium & buoyancy Stability [dry & moist adiabatic]
![Thermodynamics Review [?] Entropy & thermodynamic potentials Hydrostatic equilibrium & buoyancy Stability [dry & moist adiabatic] Thermodynamics Review [?] Entropy & thermodynamic potentials Hydrostatic equilibrium & buoyancy Stability [dry & moist adiabatic]](/thumbs/81/83694823.jpg) Thermodynamics Review [?] Entropy & thermodynamic potentials Hydrostatic equilibrium & buoyancy Stability [dry & moist adiabatic] Entropy 1. (Thermodynamics) a thermodynamic quantity that changes in a
Thermodynamics Review [?] Entropy & thermodynamic potentials Hydrostatic equilibrium & buoyancy Stability [dry & moist adiabatic] Entropy 1. (Thermodynamics) a thermodynamic quantity that changes in a
Quasi-equilibrium transitions
 Quasi-equilibrium transitions We have defined a two important equilibrium conditions. he first is one in which there is no heating, or the system is adiabatic, and dh/ =0, where h is the total enthalpy
Quasi-equilibrium transitions We have defined a two important equilibrium conditions. he first is one in which there is no heating, or the system is adiabatic, and dh/ =0, where h is the total enthalpy
Parcel Model. Meteorology September 3, 2008
 Parcel Model Meteorology 5210 September 3, 2008 1 Governing Equations for Precipitating Convection For precipitating convection, we have the following set of equations for potential temperature, θ, mixing
Parcel Model Meteorology 5210 September 3, 2008 1 Governing Equations for Precipitating Convection For precipitating convection, we have the following set of equations for potential temperature, θ, mixing
Radiative equilibrium Some thermodynamics review Radiative-convective equilibrium. Goal: Develop a 1D description of the [tropical] atmosphere
![Radiative equilibrium Some thermodynamics review Radiative-convective equilibrium. Goal: Develop a 1D description of the [tropical] atmosphere Radiative equilibrium Some thermodynamics review Radiative-convective equilibrium. Goal: Develop a 1D description of the [tropical] atmosphere](/thumbs/72/66535634.jpg) Radiative equilibrium Some thermodynamics review Radiative-convective equilibrium Goal: Develop a 1D description of the [tropical] atmosphere Vertical temperature profile Total atmospheric mass: ~5.15x10
Radiative equilibrium Some thermodynamics review Radiative-convective equilibrium Goal: Develop a 1D description of the [tropical] atmosphere Vertical temperature profile Total atmospheric mass: ~5.15x10
Chapter 4 Water Vapor
 Chapter 4 Water Vapor Chapter overview: Phases of water Vapor pressure at saturation Moisture variables o Mixing ratio, specific humidity, relative humidity, dew point temperature o Absolute vs. relative
Chapter 4 Water Vapor Chapter overview: Phases of water Vapor pressure at saturation Moisture variables o Mixing ratio, specific humidity, relative humidity, dew point temperature o Absolute vs. relative
Chapter 5. On-line resource
 Chapter 5 The water-air heterogeneous system On-line resource on-line analytical system that portrays the thermodynamic properties of water vapor and many other gases http://webbook.nist.gov/chemistry/fluid/
Chapter 5 The water-air heterogeneous system On-line resource on-line analytical system that portrays the thermodynamic properties of water vapor and many other gases http://webbook.nist.gov/chemistry/fluid/
Exam 1 (Chaps. 1-6 of the notes)
 10/12/06 ATS 541 - Atmospheric Thermodynamics and Cloud Physics 1 Exam 1 (Chaps. 1-6 of the notes) ATS 541 students: Answer all questions ATS 441 students: You may delete problem 3 or problem 5 1. [10
10/12/06 ATS 541 - Atmospheric Thermodynamics and Cloud Physics 1 Exam 1 (Chaps. 1-6 of the notes) ATS 541 students: Answer all questions ATS 441 students: You may delete problem 3 or problem 5 1. [10
Measuring State Parameters of the Atmosphere
 Measuring State Parameters of the Atmosphere Some Applications of Atmospheric Thermodynamics Earth Observing Laboratory, NCAR IDEAS-4 Tutorial Introduction Goals of This Presentation Present two complementary
Measuring State Parameters of the Atmosphere Some Applications of Atmospheric Thermodynamics Earth Observing Laboratory, NCAR IDEAS-4 Tutorial Introduction Goals of This Presentation Present two complementary
Parcel Model. Atmospheric Sciences September 30, 2012
 Parcel Model Atmospheric Sciences 6150 September 30, 2012 1 Governing Equations for Precipitating Convection For precipitating convection, we have the following set of equations for potential temperature,
Parcel Model Atmospheric Sciences 6150 September 30, 2012 1 Governing Equations for Precipitating Convection For precipitating convection, we have the following set of equations for potential temperature,
1. Heterogeneous Systems and Chemical Equilibrium
 1. Heterogeneous Systems and Chemical Equilibrium The preceding section involved only single phase systems. For it to be in thermodynamic equilibrium, a homogeneous system must be in thermal equilibrium
1. Heterogeneous Systems and Chemical Equilibrium The preceding section involved only single phase systems. For it to be in thermodynamic equilibrium, a homogeneous system must be in thermal equilibrium
Project 3 Convection and Atmospheric Thermodynamics
 12.818 Project 3 Convection and Atmospheric Thermodynamics Lodovica Illari 1 Background The Earth is bathed in radiation from the Sun whose intensity peaks in the visible. In order to maintain energy balance
12.818 Project 3 Convection and Atmospheric Thermodynamics Lodovica Illari 1 Background The Earth is bathed in radiation from the Sun whose intensity peaks in the visible. In order to maintain energy balance
Synoptic Meteorology I: Skew-T Diagrams and Thermodynamic Properties
 Synoptic Meteorology I: Skew-T Diagrams and Thermodynamic Properties For Further Reading Most information contained within these lecture notes is drawn from Chapters 1, 2, 4, and 6 of The Use of the Skew
Synoptic Meteorology I: Skew-T Diagrams and Thermodynamic Properties For Further Reading Most information contained within these lecture notes is drawn from Chapters 1, 2, 4, and 6 of The Use of the Skew
ATMO 551a Moist Adiabat Fall Change in internal energy: ΔU
 Enthalpy and the Moist Adiabat We have described the dry adiabat where an air parcel is lifted rapidly causing the air parcel to expand as the environmental pressure decreases and the air parcel does work
Enthalpy and the Moist Adiabat We have described the dry adiabat where an air parcel is lifted rapidly causing the air parcel to expand as the environmental pressure decreases and the air parcel does work
ATMO 551a Fall 08. Equivalent Potential Temperature
 Equivalent Potential emperature he equivalent potential temperature, θ e, is the potential temperature that would result if all of the water in the air parcel were condensed and rained out by raising the
Equivalent Potential emperature he equivalent potential temperature, θ e, is the potential temperature that would result if all of the water in the air parcel were condensed and rained out by raising the
Introduction. Lecture 6: Water in Atmosphere. How Much Heat Is Brought Upward By Water Vapor?
 Lecture 6: Water in Atmosphere Introduction Over 70% of the planet is covered by water Water is unique in that it can simultaneously exist in all three states (solid, liquid, gas) at the same temperature
Lecture 6: Water in Atmosphere Introduction Over 70% of the planet is covered by water Water is unique in that it can simultaneously exist in all three states (solid, liquid, gas) at the same temperature
MEA 716 Exercise, BMJ CP Scheme With acknowledgements to B. Rozumalski, M. Baldwin, and J. Kain Optional Review Assignment, distributed Th 2/18/2016
 MEA 716 Exercise, BMJ CP Scheme With acknowledgements to B. Rozumalski, M. Baldwin, and J. Kain Optional Review Assignment, distributed Th 2/18/2016 We have reviewed the reasons why NWP models need to
MEA 716 Exercise, BMJ CP Scheme With acknowledgements to B. Rozumalski, M. Baldwin, and J. Kain Optional Review Assignment, distributed Th 2/18/2016 We have reviewed the reasons why NWP models need to
GEF2200 atmospheric physics 2018
 GEF2200 atmospheric physics 208 Solutions: thermodynamics 3 Oppgaver hentet fra boka Wallace and Hobbs (2006) er merket WH06 WH06 3.8r Unsaturated air is lifted (adiabatically): The first pair of quantities
GEF2200 atmospheric physics 208 Solutions: thermodynamics 3 Oppgaver hentet fra boka Wallace and Hobbs (2006) er merket WH06 WH06 3.8r Unsaturated air is lifted (adiabatically): The first pair of quantities
Diagnosing the Intercept Parameter for Exponential Raindrop Size Distribution Based on Video Disdrometer Observations: Model Development
 Diagnosing the Intercept Parameter for Exponential Raindrop Size Distribution Based on Video Disdrometer Observations: Model Development Guifu Zhang 1, Ming Xue 1,2, Qing Cao 1 and Daniel Dawson 1,2 1
Diagnosing the Intercept Parameter for Exponential Raindrop Size Distribution Based on Video Disdrometer Observations: Model Development Guifu Zhang 1, Ming Xue 1,2, Qing Cao 1 and Daniel Dawson 1,2 1
arxiv: v1 [physics.ao-ph] 12 Oct 2015
![arxiv: v1 [physics.ao-ph] 12 Oct 2015 arxiv: v1 [physics.ao-ph] 12 Oct 2015](/thumbs/77/74746419.jpg) arxiv:1510.03239v1 [physics.ao-ph] 12 Oct 2015 Parameterization of Atmospheric Convection (COST-ES0905 book) in R. S. Plant and J.-I. Yano, editors. Vol. 2: Current issues and new theories. Chapter 22:
arxiv:1510.03239v1 [physics.ao-ph] 12 Oct 2015 Parameterization of Atmospheric Convection (COST-ES0905 book) in R. S. Plant and J.-I. Yano, editors. Vol. 2: Current issues and new theories. Chapter 22:
Theory. Humidity h of an air-vapor mixture is defined as the mass ratio of water vapor and dry air,
 Theory Background In a cooling tower with open water circulation, heat is removed from water because of the material and heat exchange between the water and the ambient air. The cooling tower is a special
Theory Background In a cooling tower with open water circulation, heat is removed from water because of the material and heat exchange between the water and the ambient air. The cooling tower is a special
9D.3 THE INFLUENCE OF VERTICAL WIND SHEAR ON DEEP CONVECTION IN THE TROPICS
 9D.3 THE INFLUENCE OF VERTICAL WIND SHEAR ON DEEP CONVECTION IN THE TROPICS Ulrike Wissmeier, Robert Goler University of Munich, Germany 1 Introduction One does not associate severe storms with the tropics
9D.3 THE INFLUENCE OF VERTICAL WIND SHEAR ON DEEP CONVECTION IN THE TROPICS Ulrike Wissmeier, Robert Goler University of Munich, Germany 1 Introduction One does not associate severe storms with the tropics
Sec Water vapour variables each has its own usefulness 2/11 The ideal gas law inter-relates vapour pressure (e) & absolute humidity ( ρv) 1 e
 Ch7. Water vapour: the most variable gas & most important GHG Absolute humidity Specific humidity ρv ρv = q q= mass of water vapour volume of sample EAS270_Ch7_WaterVapour_A.odp JDW, EAS Ualberta, last
Ch7. Water vapour: the most variable gas & most important GHG Absolute humidity Specific humidity ρv ρv = q q= mass of water vapour volume of sample EAS270_Ch7_WaterVapour_A.odp JDW, EAS Ualberta, last
df dz = dp dt Essentially, this is just a statement of the first law in one of the forms we derived earlier (expressed here in W m 3 ) dq p dt dp
 A problem with using entropy as a variable is that it is not a particularly intuitive concept. The mechanics of using entropy for evaluating system evolution is well developed, but it sometimes feels a
A problem with using entropy as a variable is that it is not a particularly intuitive concept. The mechanics of using entropy for evaluating system evolution is well developed, but it sometimes feels a
PALM - Cloud Physics. Contents. PALM group. last update: Monday 21 st September, 2015
 PALM - Cloud Physics PALM group Institute of Meteorology and Climatology, Leibniz Universität Hannover last update: Monday 21 st September, 2015 PALM group PALM Seminar 1 / 16 Contents Motivation Approach
PALM - Cloud Physics PALM group Institute of Meteorology and Climatology, Leibniz Universität Hannover last update: Monday 21 st September, 2015 PALM group PALM Seminar 1 / 16 Contents Motivation Approach
Name 28-MAY-08. FA RP 1 Mr. Chase. 1. Which weather-station model shows an air pressure of millibars?
 FA RP 1 Mr. Chase Name 28-MAY-08 1. Which weather-station model shows an air pressure of 993.4 millibars? 2. Which station model shows the correct form for indicating a northwest wind at 25 knots and an
FA RP 1 Mr. Chase Name 28-MAY-08 1. Which weather-station model shows an air pressure of 993.4 millibars? 2. Which station model shows the correct form for indicating a northwest wind at 25 knots and an
Clouds and atmospheric convection
 Clouds and atmospheric convection Caroline Muller CNRS/Laboratoire de Météorologie Dynamique (LMD) Département de Géosciences ENS M2 P7/ IPGP 1 What are clouds? Clouds and atmospheric convection 3 What
Clouds and atmospheric convection Caroline Muller CNRS/Laboratoire de Météorologie Dynamique (LMD) Département de Géosciences ENS M2 P7/ IPGP 1 What are clouds? Clouds and atmospheric convection 3 What
MT3230 Supplemental Data 4.2 Spring, 2018 Dr. Sam Miller COMPUTING THE LATENT HEAT OF VAPORIZATION OF WATER AS A FUNCTION OF TEMPERATURE.
 MT3230 Supplemental Data 4.2 Spring, 2018 Dr. Sam Miller COMPUTING THE LATENT HEAT OF VAPORIZATION OF WATER AS A FUNCTION OF TEMPERATURE Abstract The latent heat of vaporization parameterizes the amount
MT3230 Supplemental Data 4.2 Spring, 2018 Dr. Sam Miller COMPUTING THE LATENT HEAT OF VAPORIZATION OF WATER AS A FUNCTION OF TEMPERATURE Abstract The latent heat of vaporization parameterizes the amount
Mixing and Turbulence
 Mixing and Turbulence November 3, 2012 This section introduces some elementary concepts associated with mixing and turbulence in the environment. 1 Conserved Variables Studies of mixing of different airmasses
Mixing and Turbulence November 3, 2012 This section introduces some elementary concepts associated with mixing and turbulence in the environment. 1 Conserved Variables Studies of mixing of different airmasses
2σ e s (r,t) = e s (T)exp( rr v ρ l T ) = exp( ) 2σ R v ρ l Tln(e/e s (T)) e s (f H2 O,r,T) = f H2 O
 Formulas/Constants, Physics/Oceanography 4510/5510 B Atmospheric Physics II N A = 6.02 10 23 molecules/mole (Avogadro s number) 1 mb = 100 Pa 1 Pa = 1 N/m 2 Γ d = 9.8 o C/km (dry adiabatic lapse rate)
Formulas/Constants, Physics/Oceanography 4510/5510 B Atmospheric Physics II N A = 6.02 10 23 molecules/mole (Avogadro s number) 1 mb = 100 Pa 1 Pa = 1 N/m 2 Γ d = 9.8 o C/km (dry adiabatic lapse rate)
Outline. Aim. Gas law. Pressure. Scale height Mixing Column density. Temperature Lapse rate Stability. Condensation Humidity.
 Institute of Applied Physics University of Bern Outline A planetary atmosphere consists of different gases hold to the planet by gravity The laws of thermodynamics hold structure as vertical coordinate
Institute of Applied Physics University of Bern Outline A planetary atmosphere consists of different gases hold to the planet by gravity The laws of thermodynamics hold structure as vertical coordinate
Lecture 07 February 10, 2010 Water in the Atmosphere: Part 1
 Lecture 07 February 10, 2010 Water in the Atmosphere: Part 1 About Water on the Earth: The Hydrological Cycle Review 3-states of water, phase change and Latent Heat Indices of Water Vapor Content in the
Lecture 07 February 10, 2010 Water in the Atmosphere: Part 1 About Water on the Earth: The Hydrological Cycle Review 3-states of water, phase change and Latent Heat Indices of Water Vapor Content in the
Lecture 7. Science A-30 February 21, 2008 Air may be forced to move up or down in the atmosphere by mechanical forces (wind blowing over an obstacle,
 Lecture 7. Science A-30 February 21, 2008 Air may be forced to move up or down in the atmosphere by mechanical forces (wind blowing over an obstacle, like a mountain) or by buoyancy forces. Air that is
Lecture 7. Science A-30 February 21, 2008 Air may be forced to move up or down in the atmosphere by mechanical forces (wind blowing over an obstacle, like a mountain) or by buoyancy forces. Air that is
Buoyancy and Coriolis forces
 Chapter 2 Buoyancy and Coriolis forces In this chapter we address several topics that we need to understand before starting on our study of geophysical uid dynamics. 2.1 Hydrostatic approximation Consider
Chapter 2 Buoyancy and Coriolis forces In this chapter we address several topics that we need to understand before starting on our study of geophysical uid dynamics. 2.1 Hydrostatic approximation Consider
Simplified Microphysics. condensation evaporation. evaporation
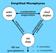 Simplified Microphysics water vapor condensation evaporation cloud droplets evaporation condensation collection rain drops fall out (precipitation) = 0 (reversible) = (irreversible) Simplified Microphysics
Simplified Microphysics water vapor condensation evaporation cloud droplets evaporation condensation collection rain drops fall out (precipitation) = 0 (reversible) = (irreversible) Simplified Microphysics
P sat = A exp [B( 1/ /T)] B= 5308K. A=6.11 mbar=vapor press. 0C.
![P sat = A exp [B( 1/ /T)] B= 5308K. A=6.11 mbar=vapor press. 0C. P sat = A exp [B( 1/ /T)] B= 5308K. A=6.11 mbar=vapor press. 0C.](/thumbs/79/79184286.jpg) Lecture 5. Water and water vapor in the atmosphere 14 Feb 2008 Review of buoyancy, with an unusual demonstration of Archimedes principle. Water is a polar molecule that forms hydrogen bonds. Consequently
Lecture 5. Water and water vapor in the atmosphere 14 Feb 2008 Review of buoyancy, with an unusual demonstration of Archimedes principle. Water is a polar molecule that forms hydrogen bonds. Consequently
Chapter 5 - Atmospheric Moisture
 Chapter 5 - Atmospheric Moisture Understanding Weather and Climate Aguado and Burt Water Water Vapor - water in a gaseous form, not droplets. Water can also achieve solid and liquid phases on Earth Temperature
Chapter 5 - Atmospheric Moisture Understanding Weather and Climate Aguado and Burt Water Water Vapor - water in a gaseous form, not droplets. Water can also achieve solid and liquid phases on Earth Temperature
2.1 Effects of a cumulus ensemble upon the large scale temperature and moisture fields by induced subsidence and detrainment
 Atmospheric Sciences 6150 Cloud System Modeling 2.1 Effects of a cumulus ensemble upon the large scale temperature and moisture fields by induced subsidence and detrainment Arakawa (1969, 1972), W. Gray
Atmospheric Sciences 6150 Cloud System Modeling 2.1 Effects of a cumulus ensemble upon the large scale temperature and moisture fields by induced subsidence and detrainment Arakawa (1969, 1972), W. Gray
Naraine Persaud, Entry Code ME-11 1
 Naraine Persaud, Entry Code ME-11 1 Persaud, N. 2005. Adiabatic cooling. In: Water Encyclopedia Volume 4: Oceanography; Meteorology; Physics and Chemistry; Water Law; and Water History, Art, and Culture.
Naraine Persaud, Entry Code ME-11 1 Persaud, N. 2005. Adiabatic cooling. In: Water Encyclopedia Volume 4: Oceanography; Meteorology; Physics and Chemistry; Water Law; and Water History, Art, and Culture.
Supporting Information for The origin of water-vapor rings in tropical oceanic cold pools
 GEOPHYSICAL RESEARCH LETTERS Supporting Information for The origin of water-vapor rings in tropical oceanic cold pools Wolfgang Langhans 1 and David M. Romps 1,2 Contents of this file 1. Texts S1 to S2
GEOPHYSICAL RESEARCH LETTERS Supporting Information for The origin of water-vapor rings in tropical oceanic cold pools Wolfgang Langhans 1 and David M. Romps 1,2 Contents of this file 1. Texts S1 to S2
5/6/ :41 PM. Chapter 6. Using Entropy. Dr. Mohammad Abuhaiba, PE
 Chapter 6 Using Entropy 1 2 Chapter Objective Means are introduced for analyzing systems from the 2 nd law perspective as they undergo processes that are not necessarily cycles. Objective: introduce entropy
Chapter 6 Using Entropy 1 2 Chapter Objective Means are introduced for analyzing systems from the 2 nd law perspective as they undergo processes that are not necessarily cycles. Objective: introduce entropy
Final Examination. Part A Answer ONLY TWELVE QUESTIONS in Part A. (Each question is 3 points)
 ATS 210 Spring Term 2001 NAME: Final Examination This is a 2 hour, closed-book examination. Calculators may be used. All answers should be written on the examination paper. Use the final sheet for any
ATS 210 Spring Term 2001 NAME: Final Examination This is a 2 hour, closed-book examination. Calculators may be used. All answers should be written on the examination paper. Use the final sheet for any
Clouds associated with cold and warm fronts. Whiteman (2000)
 Clouds associated with cold and warm fronts Whiteman (2000) Dalton s law of partial pressures! The total pressure exerted by a mixture of gases equals the sum of the partial pressure of the gases! Partial
Clouds associated with cold and warm fronts Whiteman (2000) Dalton s law of partial pressures! The total pressure exerted by a mixture of gases equals the sum of the partial pressure of the gases! Partial
7. The weather instrument below can be used to determine relative humidity.
 1. What is the dewpoint temperature when the dry-bulb temperature is 12 C and the wet-bulb temperature is 7 C? A) 1 C B) -2 C C) -5 C D) 4 C 2. A parcel of air has a dry-bulb temperature reading of 16
1. What is the dewpoint temperature when the dry-bulb temperature is 12 C and the wet-bulb temperature is 7 C? A) 1 C B) -2 C C) -5 C D) 4 C 2. A parcel of air has a dry-bulb temperature reading of 16
Clausius Clapeyron Equation
 Course - BSc. Applied Physical Science (Computer Science) Year & Semester - Ist, IInd Subject - Physics Paper No. - 6 Paper Title - Thermal Physics Lecture No. 18 Clausius Clapeyron Equation Hello friends,
Course - BSc. Applied Physical Science (Computer Science) Year & Semester - Ist, IInd Subject - Physics Paper No. - 6 Paper Title - Thermal Physics Lecture No. 18 Clausius Clapeyron Equation Hello friends,
( ) = 1005 J kg 1 K 1 ;
 Problem Set 3 1. A parcel of water is added to the ocean surface that is denser (heavier) than any of the waters in the ocean. Suppose the parcel sinks to the ocean bottom; estimate the change in temperature
Problem Set 3 1. A parcel of water is added to the ocean surface that is denser (heavier) than any of the waters in the ocean. Suppose the parcel sinks to the ocean bottom; estimate the change in temperature
Numerical Example An air parcel with mass of 1 kg rises adiabatically from sea level to an altitude of 3 km. What is its temperature change?
 Numerical Example An air parcel with mass of 1 kg rises adiabatically from sea level to an altitude of 3 km. What is its temperature change? From the 1 st law, T = -g/c p z + Q/m air /c p Here, Q = 0,
Numerical Example An air parcel with mass of 1 kg rises adiabatically from sea level to an altitude of 3 km. What is its temperature change? From the 1 st law, T = -g/c p z + Q/m air /c p Here, Q = 0,
Lecture 10: Climate Sensitivity and Feedback
 Lecture 10: Climate Sensitivity and Feedback Human Activities Climate Sensitivity Climate Feedback 1 Climate Sensitivity and Feedback (from Earth s Climate: Past and Future) 2 Definition and Mathematic
Lecture 10: Climate Sensitivity and Feedback Human Activities Climate Sensitivity Climate Feedback 1 Climate Sensitivity and Feedback (from Earth s Climate: Past and Future) 2 Definition and Mathematic
3. As warm, moist air moves into a region, barometric pressure readings in the region will generally 1. decrease 2. increase 3.
 Teacher: Mr. Prizzi Castle Learning Review 1 1. Which process most directly results in cloud formation? 1. condensation 3. precipitation 2. transpiration 4. radiation 2. An air mass originating over north
Teacher: Mr. Prizzi Castle Learning Review 1 1. Which process most directly results in cloud formation? 1. condensation 3. precipitation 2. transpiration 4. radiation 2. An air mass originating over north
First Law of Thermodynamics
 First Law of Thermodynamics September 11, 2013 The first law of thermodynamics is the conservation of energy applied to thermal systems. Here, we develop the principles of thermodynamics for a discrete
First Law of Thermodynamics September 11, 2013 The first law of thermodynamics is the conservation of energy applied to thermal systems. Here, we develop the principles of thermodynamics for a discrete
Atmospheric Dynamics: lecture 3
 Atmospheric Dynamics: lecture 3 Moist convection Dew point temperature/lapse rate/lcl Equivalent potential temperature Conditional and potential instability Thermodynamic diagram CAPE Introduction to Python
Atmospheric Dynamics: lecture 3 Moist convection Dew point temperature/lapse rate/lcl Equivalent potential temperature Conditional and potential instability Thermodynamic diagram CAPE Introduction to Python
Today s Lecture: Atmosphere finish primitive equations, mostly thermodynamics
 Today s Lecture: Atmosphere finish primitive equations, mostly thermodynamics Reference Peixoto and Oort, Sec. 3.1, 3.2, 3.4, 3.5 (but skip the discussion of oceans until next week); Ch. 10 Thermodynamic
Today s Lecture: Atmosphere finish primitive equations, mostly thermodynamics Reference Peixoto and Oort, Sec. 3.1, 3.2, 3.4, 3.5 (but skip the discussion of oceans until next week); Ch. 10 Thermodynamic
Atmospheric Dynamics: lecture 2
 Atmospheric Dynamics: lecture 2 Topics Some aspects of advection and the Coriolis-effect (1.7) Composition of the atmosphere (figure 1.6) Equation of state (1.8&1.9) Water vapour in the atmosphere (1.10)
Atmospheric Dynamics: lecture 2 Topics Some aspects of advection and the Coriolis-effect (1.7) Composition of the atmosphere (figure 1.6) Equation of state (1.8&1.9) Water vapour in the atmosphere (1.10)
Review of Mathematics
 Review for Exam #1 Review of Mathematics 2 Weighted Mean A certain property of material 1 is P 1 and that of material 2 is P 2 If x 1 amount (weight or volume) of material 1 is mixed with x 2 amount of
Review for Exam #1 Review of Mathematics 2 Weighted Mean A certain property of material 1 is P 1 and that of material 2 is P 2 If x 1 amount (weight or volume) of material 1 is mixed with x 2 amount of
USING PROGRAM HURRICANE. Download all the files, including the (empty) output subdirectory into a new folder on your machine.
 USING PROGRAM HURRICANE Download all the files, including the (empty) output subdirectory into a new folder on your machine. QuickStart: First compile hurr.f. Modify the first column of the input file
USING PROGRAM HURRICANE Download all the files, including the (empty) output subdirectory into a new folder on your machine. QuickStart: First compile hurr.f. Modify the first column of the input file
Vapor Pressure of Liquids Equilibria and Thermodynamics
 Chemistry 1B-Foothill College Vapor Pressure of Liquids Equilibria and Thermodynamics In this exercise, you will investigate the relationship between the vapor pressure of a liquid and the thermodynamic
Chemistry 1B-Foothill College Vapor Pressure of Liquids Equilibria and Thermodynamics In this exercise, you will investigate the relationship between the vapor pressure of a liquid and the thermodynamic
GPS RO Retrieval Improvements in Ice Clouds
 Joint COSMIC Tenth Data Users Workshop and IROWG-6 Meeting GPS RO Retrieval Improvements in Ice Clouds Xiaolei Zou Earth System Science Interdisciplinary Center (ESSIC) University of Maryland, USA September
Joint COSMIC Tenth Data Users Workshop and IROWG-6 Meeting GPS RO Retrieval Improvements in Ice Clouds Xiaolei Zou Earth System Science Interdisciplinary Center (ESSIC) University of Maryland, USA September
Monteverdi Metr 201 Quiz #4 100 pts.
 DEPARTMENT OF GEOSCIENCES Name San Francisco State University April 27, 2012 Monteverdi Metr 201 Quiz #4 100 pts. A. Definitions. (5 points each for a total of 25 points in this section). (a) Convective
DEPARTMENT OF GEOSCIENCES Name San Francisco State University April 27, 2012 Monteverdi Metr 201 Quiz #4 100 pts. A. Definitions. (5 points each for a total of 25 points in this section). (a) Convective
Orographic Precipitation II: Effects of Phase Change on Orographic Flow. Richard Rotunno. National Center for Atmospheric Research, USA
 Orographic Precipitation II: Effects of Phase Change on Orographic Flow Richard Rotunno National Center for Atmospheric Research, USA Condensation D Dt vs w( x, ) vs U Large-Scale Flow 0 H L Dynamics w
Orographic Precipitation II: Effects of Phase Change on Orographic Flow Richard Rotunno National Center for Atmospheric Research, USA Condensation D Dt vs w( x, ) vs U Large-Scale Flow 0 H L Dynamics w
Water Vapor and the Dynamics of Climate Changes
 Water Vapor and the Dynamics of Climate Changes Tapio Schneider California Institute of Technology (based on Rev. Geophys. article with Xavier Levine and Paul O Gorman) Water vapor dynamics in warming
Water Vapor and the Dynamics of Climate Changes Tapio Schneider California Institute of Technology (based on Rev. Geophys. article with Xavier Levine and Paul O Gorman) Water vapor dynamics in warming
Atmospheric Composition הרכב האטמוספירה
 Atmospheric Composition הרכב האטמוספירה N 2 O 2 Trace Gases Water Vapor (H 2 O) Argon (Ar) Carbon Dioxide (CO 2 ) Neon (Ne) Helium (He) Methane (CH 4 ) Nitrous Oxide (N 2 O) Ozone (O 3 ) Nitrogen and oxygen
Atmospheric Composition הרכב האטמוספירה N 2 O 2 Trace Gases Water Vapor (H 2 O) Argon (Ar) Carbon Dioxide (CO 2 ) Neon (Ne) Helium (He) Methane (CH 4 ) Nitrous Oxide (N 2 O) Ozone (O 3 ) Nitrogen and oxygen
Clouds and turbulent moist convection
 Clouds and turbulent moist convection Lecture 2: Cloud formation and Physics Caroline Muller Les Houches summer school Lectures Outline : Cloud fundamentals - global distribution, types, visualization
Clouds and turbulent moist convection Lecture 2: Cloud formation and Physics Caroline Muller Les Houches summer school Lectures Outline : Cloud fundamentals - global distribution, types, visualization
THERMODYNAMICS NOTES. These notes give a brief overview of engineering thermodynamics. They are based on the thermodynamics text by Black & Hartley.
 THERMODYNAMICS NOTES These notes give a brief overview of engineering thermodynamics. They are based on the thermodynamics text by Black & Hartley. Topics covered include: concepts; properties; conservation
THERMODYNAMICS NOTES These notes give a brief overview of engineering thermodynamics. They are based on the thermodynamics text by Black & Hartley. Topics covered include: concepts; properties; conservation
Name: First three letters of last name
 Name: First three letters of last name Chemistry 342 Third Exam April 22, 2005 2:00 PM in C6 Lecture Center Write all work you want graded in the spaces provided. Both the logical solution to the problem
Name: First three letters of last name Chemistry 342 Third Exam April 22, 2005 2:00 PM in C6 Lecture Center Write all work you want graded in the spaces provided. Both the logical solution to the problem
where p oo is a reference level constant pressure (often 10 5 Pa). Since θ is conserved for adiabatic motions, a prognostic temperature equation is:
 1 Appendix C Useful Equations Purposes: Provide foundation equations and sketch some derivations. These equations are used as starting places for discussions in various parts of the book. C.1. Thermodynamic
1 Appendix C Useful Equations Purposes: Provide foundation equations and sketch some derivations. These equations are used as starting places for discussions in various parts of the book. C.1. Thermodynamic
A Numerical Study of Methods for Moist Atmospheric Flows: Compressible Equations
 NOVEMBER 2014 D U A R T E E T A L. 4269 A Numerical Study of Methods for Moist Atmospheric Flows: Compressible Equations MAX DUARTE ANN S. ALMGREN KAUSHIK BALAKRISHNAN AND JOHN B. BELL Center for Computational
NOVEMBER 2014 D U A R T E E T A L. 4269 A Numerical Study of Methods for Moist Atmospheric Flows: Compressible Equations MAX DUARTE ANN S. ALMGREN KAUSHIK BALAKRISHNAN AND JOHN B. BELL Center for Computational
Governing Equations and Scaling in the Tropics
 Governing Equations and Scaling in the Tropics M 1 ( ) e R ε er Tropical v Midlatitude Meteorology Why is the general circulation and synoptic weather systems in the tropics different to the those in the
Governing Equations and Scaling in the Tropics M 1 ( ) e R ε er Tropical v Midlatitude Meteorology Why is the general circulation and synoptic weather systems in the tropics different to the those in the
Moist Convection. Chapter 6
 Moist Convection Chapter 6 1 2 Trade Cumuli Afternoon cumulus over land 3 Cumuls congestus Convectively-driven weather systems Deep convection plays an important role in the dynamics of tropical weather
Moist Convection Chapter 6 1 2 Trade Cumuli Afternoon cumulus over land 3 Cumuls congestus Convectively-driven weather systems Deep convection plays an important role in the dynamics of tropical weather
Chapter 7 PHASE EQUILIBRIUM IN A ONE-COMPONENT SYSTEM
 Chapter 7 PHASE EQUILIBRIUM IN A ONE-COMPONENT SYSTEM 7.1 INTRODUCTION The intensive thermodynamic properties of a system are temperature, pressure, and the chemical potentials of the various species occurring
Chapter 7 PHASE EQUILIBRIUM IN A ONE-COMPONENT SYSTEM 7.1 INTRODUCTION The intensive thermodynamic properties of a system are temperature, pressure, and the chemical potentials of the various species occurring
12. Heat of melting and evaporation of water
 VS 12. Heat of melting and evaporation of water 12.1 Introduction The change of the physical state of a substance in general requires the absorption or release of heat. In this case, one speaks of a first
VS 12. Heat of melting and evaporation of water 12.1 Introduction The change of the physical state of a substance in general requires the absorption or release of heat. In this case, one speaks of a first
ln P s T and P s T where R 22,105, D A 27, E B 97.
 ASAE D271.2 DEC94 Psychrometric Data Reviewed by ASAE s Structures and Environment Division and the Food Engineering Division Standards Committees; approved by the Electric Power and Processing Division
ASAE D271.2 DEC94 Psychrometric Data Reviewed by ASAE s Structures and Environment Division and the Food Engineering Division Standards Committees; approved by the Electric Power and Processing Division
Weather, Atmosphere and Meteorology
 S c i e n c e s Weather, Atmosphere and Meteorology Key words: Atmosphere, Ozone, Water vapor, solar radiation, Condensation, Evaporation, Humidity, Dew-Point Temperature, Cirrus Clouds, Stratus Clouds,
S c i e n c e s Weather, Atmosphere and Meteorology Key words: Atmosphere, Ozone, Water vapor, solar radiation, Condensation, Evaporation, Humidity, Dew-Point Temperature, Cirrus Clouds, Stratus Clouds,
4 WATER VAPOR. Contents 4.1. VAPOR PRESSURE AT SATURATION
 Copyright 2017 by Roland Stull. Practical Meteorology: An Algebra-based Survey of Atmospheric Science. v1.02b 4 WATER VAPOR Contents 4.1. Vapor Pressure at Saturation 87 HIGHER MATH Clausius-Clapeyron
Copyright 2017 by Roland Stull. Practical Meteorology: An Algebra-based Survey of Atmospheric Science. v1.02b 4 WATER VAPOR Contents 4.1. Vapor Pressure at Saturation 87 HIGHER MATH Clausius-Clapeyron
Applied Thermodynamics for Marine Systems Prof. P. K. Das Department of Mechanical Engineering Indian Institute of Technology, Kharagpur
 Applied Thermodynamics for Marine Systems Prof. P. K. Das Department of Mechanical Engineering Indian Institute of Technology, Kharagpur Lecture - 21 Psychometric Processes Good afternoon, yesterday we
Applied Thermodynamics for Marine Systems Prof. P. K. Das Department of Mechanical Engineering Indian Institute of Technology, Kharagpur Lecture - 21 Psychometric Processes Good afternoon, yesterday we
The Effect of Sea Spray on Tropical Cyclone Intensity
 The Effect of Sea Spray on Tropical Cyclone Intensity Jeffrey S. Gall, Young Kwon, and William Frank The Pennsylvania State University University Park, Pennsylvania 16802 1. Introduction Under high-wind
The Effect of Sea Spray on Tropical Cyclone Intensity Jeffrey S. Gall, Young Kwon, and William Frank The Pennsylvania State University University Park, Pennsylvania 16802 1. Introduction Under high-wind
General Concepts of Atmospheric Thermodynamic Atmospheric Thermodynamic Theory
 General Concepts of Atmospheric Thermodynamic Atmospheric Thermodynamic Theory The theory of thermodynamics is one of the important cornerstones of classical physics. It has applications not only in physics,
General Concepts of Atmospheric Thermodynamic Atmospheric Thermodynamic Theory The theory of thermodynamics is one of the important cornerstones of classical physics. It has applications not only in physics,
Accurate computation of moist available potential energy with the Munkres algorithm
 Quarterly Journal of the Royal Meteorological Society Q. J. R. Meteorol. Soc. 143: 288 292, January 2017 A DOI:10.1002/qj.2921 Accurate computation of moist available potential energy with the Munkres
Quarterly Journal of the Royal Meteorological Society Q. J. R. Meteorol. Soc. 143: 288 292, January 2017 A DOI:10.1002/qj.2921 Accurate computation of moist available potential energy with the Munkres
A Large-Eddy Simulation Study of Moist Convection Initiation over Heterogeneous Surface Fluxes
 A Large-Eddy Simulation Study of Moist Convection Initiation over Heterogeneous Surface Fluxes Song-Lak Kang Atmospheric Science Group, Texas Tech Univ. & George H. Bryan MMM, NCAR 20 th Symposium on Boundary
A Large-Eddy Simulation Study of Moist Convection Initiation over Heterogeneous Surface Fluxes Song-Lak Kang Atmospheric Science Group, Texas Tech Univ. & George H. Bryan MMM, NCAR 20 th Symposium on Boundary
METEO 431: Atmospheric Thermodynamics
 METEO 431: Atmospheric Thermodynamics Final Exam (100 points) INSTRUCTIONS: Please write as legibly as you can and make sure your thoughts are organized. I am looking for your understanding of the key
METEO 431: Atmospheric Thermodynamics Final Exam (100 points) INSTRUCTIONS: Please write as legibly as you can and make sure your thoughts are organized. I am looking for your understanding of the key
Experiments with Idealized Supercell Simulations
 Latent Heat Nudging in almo: Experiments with Idealized Supercell Simulations Daniel Leuenberger and Andrea Rossa MeteoSwiss Talk Outline Introduction Supercell Storms Characteristics Reference simulation
Latent Heat Nudging in almo: Experiments with Idealized Supercell Simulations Daniel Leuenberger and Andrea Rossa MeteoSwiss Talk Outline Introduction Supercell Storms Characteristics Reference simulation
A 3 Vapor pressure of volatile liquids
 Versuchsanleitungen zum Praktikum Physikalische Chemie für Anfänger 1 A 3 Vapor pressure of volatile liquids Purpose The purpose of this experiment is to determine the vaporization enthalpy, entropy and
Versuchsanleitungen zum Praktikum Physikalische Chemie für Anfänger 1 A 3 Vapor pressure of volatile liquids Purpose The purpose of this experiment is to determine the vaporization enthalpy, entropy and
Solutions to Comprehensive Final Examination Given on Thursday, 13 December 2001
 Name & Signature Dr. Droegemeier Student ID Meteorology 1004 Introduction to Meteorology Fall, 2001 Solutions to Comprehensive Final Examination Given on Thursday, 13 December 2001 BEFORE YOU BEGIN!! Please
Name & Signature Dr. Droegemeier Student ID Meteorology 1004 Introduction to Meteorology Fall, 2001 Solutions to Comprehensive Final Examination Given on Thursday, 13 December 2001 BEFORE YOU BEGIN!! Please
Lecture 9: Climate Sensitivity and Feedback Mechanisms
 Lecture 9: Climate Sensitivity and Feedback Mechanisms Basic radiative feedbacks (Plank, Water Vapor, Lapse-Rate Feedbacks) Ice albedo & Vegetation-Climate feedback Cloud feedback Biogeochemical feedbacks
Lecture 9: Climate Sensitivity and Feedback Mechanisms Basic radiative feedbacks (Plank, Water Vapor, Lapse-Rate Feedbacks) Ice albedo & Vegetation-Climate feedback Cloud feedback Biogeochemical feedbacks
2 Equations of Motion
 2 Equations of Motion system. In this section, we will derive the six full equations of motion in a non-rotating, Cartesian coordinate 2.1 Six equations of motion (non-rotating, Cartesian coordinates)
2 Equations of Motion system. In this section, we will derive the six full equations of motion in a non-rotating, Cartesian coordinate 2.1 Six equations of motion (non-rotating, Cartesian coordinates)
Entropy and the Second Law of Thermodynamics
 Entropy and the Second Law of Thermodynamics Reading Problems 7-1 7-3 7-88, 7-131, 7-135 7-6 7-10 8-24, 8-44, 8-46, 8-60, 8-73, 8-99, 8-128, 8-132, 8-1 8-10, 8-13 8-135, 8-148, 8-152, 8-166, 8-168, 8-189
Entropy and the Second Law of Thermodynamics Reading Problems 7-1 7-3 7-88, 7-131, 7-135 7-6 7-10 8-24, 8-44, 8-46, 8-60, 8-73, 8-99, 8-128, 8-132, 8-1 8-10, 8-13 8-135, 8-148, 8-152, 8-166, 8-168, 8-189
GEF2200 Atmosfærefysikk 2012
 GEF2200 Atmosfærefysikk 2012 Løsningsforslag til oppgavesett 4 WH06 3.46 (WH 2.49) The air parcel has the properties p = 1000hPa, T = 15 C and T d = 4 C. b Lifting the air parcel to p 2 = 900hPa, T 2 we
GEF2200 Atmosfærefysikk 2012 Løsningsforslag til oppgavesett 4 WH06 3.46 (WH 2.49) The air parcel has the properties p = 1000hPa, T = 15 C and T d = 4 C. b Lifting the air parcel to p 2 = 900hPa, T 2 we
Experiment Flow Analysis
 MASSACHUSETTS INSTITUTE OF TECHNOLOGY Department of Physics Physics 8.1X Fall Term 22 Handed out: November 26 Due: Dec 6 at 4 pm Experiment Flow Analysis Problem 1: Here is a summary of the measurements,
MASSACHUSETTS INSTITUTE OF TECHNOLOGY Department of Physics Physics 8.1X Fall Term 22 Handed out: November 26 Due: Dec 6 at 4 pm Experiment Flow Analysis Problem 1: Here is a summary of the measurements,
Surface Tension Kelvin Effect
 Lecture Ch. 5a Surface tension (Kelvin effect) Hygroscopic growth (subsaturated humidity) Saturation Chemical potential (Raoult effect) (partly from 4.5.1) Curry and Webster, Ch. 5 (skip 5.6, 5.7); also
Lecture Ch. 5a Surface tension (Kelvin effect) Hygroscopic growth (subsaturated humidity) Saturation Chemical potential (Raoult effect) (partly from 4.5.1) Curry and Webster, Ch. 5 (skip 5.6, 5.7); also
Equation for Global Warming
 Equation for Global Warming Derivation and Application Contents 1. Amazing carbon dioxide How can a small change in carbon dioxide (CO 2 ) content make a critical difference to the actual global surface
Equation for Global Warming Derivation and Application Contents 1. Amazing carbon dioxide How can a small change in carbon dioxide (CO 2 ) content make a critical difference to the actual global surface
ATMO/OPTI 656b Spring 08. Physical Properties of the Atmosphere
 Physical Properties of the Atmosphere Thin as a piece of paper The atmosphere is a very thin layer above the solid Earth and its oceans. This is true of the atmospheres of all of the terrestrial planets.
Physical Properties of the Atmosphere Thin as a piece of paper The atmosphere is a very thin layer above the solid Earth and its oceans. This is true of the atmospheres of all of the terrestrial planets.
ATMOSPHERIC THERMODYNAMICS
 ATMOSPHERIC THERMODYNAMICS 1. Introduction 1.1 The field of thermodynamics Classical thermodynamics deals with energy and the transformations of the nature of energy. To a certain extent, it classifies
ATMOSPHERIC THERMODYNAMICS 1. Introduction 1.1 The field of thermodynamics Classical thermodynamics deals with energy and the transformations of the nature of energy. To a certain extent, it classifies
Page 1. Name: 1) The graph below shows air temperature for an area near the Earth's surface during a 12-hour period.
 Name: 1) The graph below shows air temperature for an area near the Earth's surface during a 12-hour period. Which graph best illustrates the probable change in air pressure during the same time period?
Name: 1) The graph below shows air temperature for an area near the Earth's surface during a 12-hour period. Which graph best illustrates the probable change in air pressure during the same time period?
Thermodynamics of Atmospheres and Oceans
 Thermodynamics of Atmospheres and Oceans Judith A. Curry and Peter J. Webster PROGRAM IN ATMOSPHERIC AND OCEANIC SCIENCES DEPARTMENT OF AEROSPACE ENGINEERING UNIVERSITY OF COLORADO BOULDER, COLORADO USA
Thermodynamics of Atmospheres and Oceans Judith A. Curry and Peter J. Webster PROGRAM IN ATMOSPHERIC AND OCEANIC SCIENCES DEPARTMENT OF AEROSPACE ENGINEERING UNIVERSITY OF COLORADO BOULDER, COLORADO USA
1 in = ft (round answer to nearest integer)
 Hydrology LAB 1: DRAINAGE-BASIN PROPERTIES AND SIMPLE METEOROLOGIC CALCULATIONS OBJECTIVES: a. to develop your ability to extract basic drainage-basin data from topographic maps b. to learn how to make
Hydrology LAB 1: DRAINAGE-BASIN PROPERTIES AND SIMPLE METEOROLOGIC CALCULATIONS OBJECTIVES: a. to develop your ability to extract basic drainage-basin data from topographic maps b. to learn how to make
Chapter 6. Using Entropy
 Chapter 6 Using Entropy Learning Outcomes Demonstrate understanding of key concepts related to entropy and the second law... including entropy transfer, entropy production, and the increase in entropy
Chapter 6 Using Entropy Learning Outcomes Demonstrate understanding of key concepts related to entropy and the second law... including entropy transfer, entropy production, and the increase in entropy
Köhler Curve. Covers Reading Material in Chapter 10.3 Atmospheric Sciences 5200 Physical Meteorology III: Cloud Physics
 Köhler Curve Covers Reading Material in Chapter 10.3 Atmospheric Sciences 5200 Physical Meteorology III: Cloud Physics Review of Kelvin Effect Gibbs Energy for formation of a drop G = G &'()*+, G ).'+
Köhler Curve Covers Reading Material in Chapter 10.3 Atmospheric Sciences 5200 Physical Meteorology III: Cloud Physics Review of Kelvin Effect Gibbs Energy for formation of a drop G = G &'()*+, G ).'+
Reconstruction of Thermodynamic Cycles in a High-Resolution Simulation of a Hurricane
 OCTOBER 2017 P A U L U I S A N D Z H A N G 3367 Reconstruction of Thermodynamic Cycles in a High-Resolution Simulation of a Hurricane OLIVIER M. PAULUIS Courant Institute of Mathematical Sciences, New
OCTOBER 2017 P A U L U I S A N D Z H A N G 3367 Reconstruction of Thermodynamic Cycles in a High-Resolution Simulation of a Hurricane OLIVIER M. PAULUIS Courant Institute of Mathematical Sciences, New
p = ρrt p = ρr d = T( q v ) dp dz = ρg
 Chapter 1: Properties of the Atmosphere What are the major chemical components of the atmosphere? Atmospheric Layers and their major characteristics: Troposphere, Stratosphere Mesosphere, Thermosphere
Chapter 1: Properties of the Atmosphere What are the major chemical components of the atmosphere? Atmospheric Layers and their major characteristics: Troposphere, Stratosphere Mesosphere, Thermosphere
Kelvin Effect. Covers Reading Material in Chapter 10.3 Atmospheric Sciences 5200 Physical Meteorology III: Cloud Physics
 Kelvin Effect Covers Reading Material in Chapter 10.3 Atmospheric Sciences 5200 Physical Meteorology III: Cloud Physics Vapor Pressure (e) e < e # e = e # Vapor Pressure e > e # Relative humidity RH =
Kelvin Effect Covers Reading Material in Chapter 10.3 Atmospheric Sciences 5200 Physical Meteorology III: Cloud Physics Vapor Pressure (e) e < e # e = e # Vapor Pressure e > e # Relative humidity RH =
