Principles of Food and Bioprocess Engineering (FS 231) Solutions to Example Problems on Freezing
|
|
|
- Darlene Newton
- 6 years ago
- Views:
Transcription
1 Principles o Food and Bioprocess Engineering (FS 21) Solutions to Example Problems on Freezing 1. In order to determine the a to be used in the above equation, e recall that Plank s equation is valid or 1-D reezing only. Thus, the sides o the container that are 80 cm and 90 cm long are considered to be ininitely long, and hence a = 5 cm = 0.05 m For a slab, P' = 1/2, R' = 1/8 2 = 1,100 kg/m, usion (product) = 0.8 (.5 kj/kg) = kj/kg, h = 140 W/m K k = 1.6 W/m K (make sure that the properties used are or the rozen product and not liquid) T F= -1.5 C, T a = -0 C Thus, t = 850 s (= 64 min) 2. We use the olloing equation (Plank s equation) to determine the reezing time: Here, = 1,000 kg/m, k = 1.5 W/m K, usion (product) = 0.75 (.5) = kj/kg The product can be considered to be an ininite cylinder o radius cm (a = 6 cm = 0.06 m) since the height (= 65 cm) is large (greater than ten times) in comparison ith the diameter. Thus, P' = 1/4, R' = 1/16. Using our knoledge on unsteady state heat transer, e kno that as long as N Bi > 40 (here, N Bi = 75), e can assume that the resistance to heat transer at the surace is negligible (i.e., h = ). Also, T F = 271 K, T = -5 C = 28 K Substituting these values, e get: t = 1,17 s. T = 27 K, T' = 271 K, usion (solvent) = 6,00 J/mol, R g = 8.14 J/mol K Thus, X = 0.98 = (500/18)/[(500/18) + (m s/40)] Thus, m = g s 4. We irst determine the initial reezing temperature o the solution as ollos: In the above equation, Here, m = 1,500 g, M = 18 g, m s = 60 g, M s = 45. Thus, e get: X = 0.984
2 Substituting X = 0.984, T = 27 K, R g = 8.14 J/mol K, usion (solvent) = 6,00 J/mol, yields: T' = K. This is the initial reezing point (= T F) We then use the olloing equation (Plank s equation) to determine the reezing time: We assume that the product is an ininite cylinder and hence, P' = 1/4, R' = 1/16 2 a = 0.05 m, h = 120 W/m K, k = 0.6 W/m K, = 1,000 kg/m, T a = 2 K usion (product) = (1500/1560) (.5 x 10 ) = 20.7 kj/kg This yields: t = 045 s (= 51 min) 5. We use the olloing equation: usion (solvent) = 6,00 J/mol, R g = 8.14 J/mol K, T = 27 K, T' = 271 K This yields, X = 0.98 Considering a 100 g solution, e have m = 90 g, m = 10 g. Also, M = 18 g. s Substituting these values in:, yields: M = 98 g s 6. The irst method involves the use o the Clausius-Clapeyron equation given belo: ith Here, m = 600 g, M = 18 g, m s = 400 g, M s = 0 g Thus, e get: X = 0.71 Substituting, usion (solvent) = 6,00 J/mol, R g = 8.14 J/mol K, T = 27 K, X = 0.71, e get: T = K Thus, depression in reezing point = = K = 1.2 C The second method involves the use o the olloing equation: Here, m = number o moles o solute per kg o solvent = (400/0)*(1000/600) = 22.2 Also, k = 1.86 C/mol Thus, T = (1.86)(22.2) = 41. C There is a dierence in the ansers obtained by the to methods because the second method is not valid in this case since e do not have a dilute solution. 7. We use the olloing equation (Plank s equation) to determine the reezing time:
3 Here, = 950 kg/m, k = 0.5 W/m K, usion = 0.8 (.5) = kj/kg The product can be considered to be an ininite slab o thickness 5 cm (= a) since the other to dimensions are large in comparison to this dimension. Thus, P' = 1/2, R' = 1/8. Since the Biot number is 100, e can assume that the resistance to heat transer at the surace is negligible (i.e., h = ). Also, T F= 271 K, T a = -40 C = 2 K Substituting these values, e get: t = 4169 s 8. a. In order to determine the reezing point o the solution, e need to use the Calusius- Clapeyron equation given belo: (1) ith, (2) In equation 2, m = 2 kg, M = 18 g, m s = 1 kg, M s = 110 g Thus, X = In equation 1, usion (solvent) = 6,00 J/mol, R = 8.14 J/mol K, T = 27 K Substituting, e get: T' = 265 K (= - 8 C) b. I NaCl (molecular eight = 58.5 g) as used instead o CaCl 2, X ould be loer than and hence T' ould be loer than -8 C. 9. We begin by using Plank s equation (given belo): In this problem, e do not truly have an ininite cylinder (since height 10*diameter). Hoever, it is close enough that e can use Plank s equation and e ill be able to obtain an estimate o the reezing time. Here, P' = 1/4, R' = 1/16 2 a = 0.1 m, h = 500 W/m K, k = 0.5 W/m K, = 950 kg/m usion (product) = (0.9) (.5 kj/kg) = kj/kg = 00,150 J/kg R g = 8.14 J/mol K T = - 5 C a Thus, e can solve or the reezing time i e kno the initial reezing point, T F. The initial reezing point can be determined using the Clausius-Clapeyron equation (given belo): (1)
4 = 6,00 J/mol, R = 8.14 J/mol K usion (solvent) g and (2) Since the product has 10% solids, the remaining 90% is ater. In computing, X, e only need the ratio o mass o ater to mass o solids and do not actually need the exact amount o solids and ater. I e consider the total product eight to be 100 g, then: m = 90 g, m s = 10 g No, M = 18 g, M s = 50 g Substituting these values in equation 2, e get: X = 0.96 Substituting this in equation 1, e get: T' = K = -4.1 C (= T F) Substituting this in Plank s equation, e get: t = 11,996 s (= ~200 min = ~. hrs) 10. Consider a 100 g product. Initially, the product has 90 g ater and 10 g solids. Ater 40 % o this ater has rozen, the product has 54 g ater (40 % o 90 g = 6 g; 6 g o ater is no rozen, thereby leaving 54 g o ater) and 10 g o solids The mole raction o ater in solution is given by: We then use the olloing equation (Clausius-Clapeyron equation): Here, usion (solvent) = 6,00 J/mol, R g = 8.14 J/mol K Solving, e get: T' = K = C 11. We begin by using Plank s equation (given belo): In this problem, e have an ininite cylinder since the height is at least 10 times the diameter. Hence, the value o a in this problem is 0.08 m. Thus, P' = 1/4, R' = 1/16 2 h = 250 W/m K, k = 0.6 W/m K, = 850 kg/m usion = (0.75) (.5 kj/kg) = kj/kg = 250,125 J/kg R g = 8.14 J/mol K T = - 0 C = 24 K a Thus, e can solve or the reezing time i e kno the initial reezing point, T F. The initial reezing point (T ) can be determined using the Clausius-Clapeyron equation (given F
5 belo): (1) = 6,00 J/mol, R = 8.14 J/mol K usion g ith (2) Since the product has 25% solids, the remaining 75% is ater. In equation 2, m = 0.75, M = 18 g, m s = 0.25, M s = 175 g (Note that e do not need the actual values o the mass o solids & ater, but only the ratios hence e use m = 0.75 and m s = 0.25) Thus, X = 0.97 In equation 1, usion = 600 J/mol, R = 8.14 J/mol K, T = 27 K Substituting, e get: T' = K = T F Substituting this in Plank s equation, e get: t = 5,901 s (= ~1.64 h) 12. We begin ith the Clausius-Clapeyron equation: Here, T = 27 K, T = 268 K, usion (solvent) = 600 J/mol, R g = 8.14 J/mol K Solving, e get: X = No, Here, m = 2000, M = 18, M s = 60 Solving, e get: m =.6 g s We then use the Plank s equation (belo) to determine the reezing time: Here, = 975 kg/m k = 0.45 W/m K usion (product) = m.c. o product ( usion (ater)) = [200/( )]{.5 x 10 }J/kg = x 10 J/kg T F = -5 C = 268 K T a = -40 C = 2 K h = 200 W/m 2 K
6 Since to o the dimensions o the tray are more than 10 times the third dimension, e can approximate the tray to be an ininite slab. For an ininite slab, a = thickness = 0.04 m, P' = ½, R' = 1/8 Substituting these values, e get: t = 41 s
Principles of Food and Bioprocess Engineering (FS 231) Exam 2 Part A -- Closed Book (50 points)
 Principles of Food and Bioprocess Engineering (FS 231) Exam 2 Part A -- Closed Book (50 points) 1. Are the following statements true or false? (20 points) a. Thermal conductivity of a substance is a measure
Principles of Food and Bioprocess Engineering (FS 231) Exam 2 Part A -- Closed Book (50 points) 1. Are the following statements true or false? (20 points) a. Thermal conductivity of a substance is a measure
ENGINEERING OF NUCLEAR REACTORS. Tuesday, October 9 th, 2014, 1:00 2:30 p.m.
 .31 ENGINEERING OF NUCLEAR REACTORS Tuesday, October 9 th, 014, 1:00 :30 p.m. OEN BOOK QUIZ 1 (solutions) roblem 1 (50%) Loss o condensate pump transient in a LWR condenser i) Consider the seaater in the
.31 ENGINEERING OF NUCLEAR REACTORS Tuesday, October 9 th, 014, 1:00 :30 p.m. OEN BOOK QUIZ 1 (solutions) roblem 1 (50%) Loss o condensate pump transient in a LWR condenser i) Consider the seaater in the
1. Nusselt number and Biot number are computed in a similar manner (=hd/k). What are the differences between them? When and why are each of them used?
 1. Nusselt number and Biot number are computed in a similar manner (=hd/k). What are the differences between them? When and why are each of them used?. During unsteady state heat transfer, can the temperature
1. Nusselt number and Biot number are computed in a similar manner (=hd/k). What are the differences between them? When and why are each of them used?. During unsteady state heat transfer, can the temperature
Principles of Food and Bioprocess Engineering (FS 231) Problems on Heat Transfer
 Principles of Food and Bioprocess Engineering (FS 1) Problems on Heat Transfer 1. What is the thermal conductivity of a material 8 cm thick if the temperature at one end of the product is 0 C and the temperature
Principles of Food and Bioprocess Engineering (FS 1) Problems on Heat Transfer 1. What is the thermal conductivity of a material 8 cm thick if the temperature at one end of the product is 0 C and the temperature
Principles of Food and Bioprocess Engineering (FS 231) Solutions to Example Problems on Evaporation
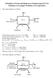 Princiles of Food and Biorocess Engineering (FS 231) Solutions to Examle Problems on Eaoration 1. The system diagram is as follows: Solids balance equation: 0.1 (m f) = 0.5 (1,000) Thus, m f = 5,000 kg/hr
Princiles of Food and Biorocess Engineering (FS 231) Solutions to Examle Problems on Eaoration 1. The system diagram is as follows: Solids balance equation: 0.1 (m f) = 0.5 (1,000) Thus, m f = 5,000 kg/hr
Chemistry 163B Colligative Properties Challenged Penpersonship Notes
 Chemistry 163 Colligative Properties Challenged Penpersonship Notes 1 colligative properties o solutions colligative One entry ound. Main Entry: col li ga tive Pronunciation: kä-lə- gā-tiv, kə- li-gə-tiv
Chemistry 163 Colligative Properties Challenged Penpersonship Notes 1 colligative properties o solutions colligative One entry ound. Main Entry: col li ga tive Pronunciation: kä-lə- gā-tiv, kə- li-gə-tiv
Introduction To Resonant. Circuits. Resonance in series & parallel RLC circuits
 Introduction To esonant Circuits esonance in series & parallel C circuits Basic Electrical Engineering (EE-0) esonance In Electric Circuits Any passive electric circuit ill resonate if it has an inductor
Introduction To esonant Circuits esonance in series & parallel C circuits Basic Electrical Engineering (EE-0) esonance In Electric Circuits Any passive electric circuit ill resonate if it has an inductor
CHEM 1451 Lab Week 09 Colligative Properties Postlab Report. Coach s Name
 Instructions To complete the table entry titled Measured, copy your measurement that you made in the previous week. If you ran the experiment again to obtain a different measurement, then check the box
Instructions To complete the table entry titled Measured, copy your measurement that you made in the previous week. If you ran the experiment again to obtain a different measurement, then check the box
Why do Golf Balls have Dimples on Their Surfaces?
 Name: Partner(s): 1101 Section: Desk # Date: Why do Golf Balls have Dimples on Their Surfaces? Purpose: To study the drag force on objects ith different surfaces, ith the help of a ind tunnel. Overvie
Name: Partner(s): 1101 Section: Desk # Date: Why do Golf Balls have Dimples on Their Surfaces? Purpose: To study the drag force on objects ith different surfaces, ith the help of a ind tunnel. Overvie
Principles of Food and Bioprocess Engineering (FS 231) Solutions to Example Problems on Psychrometrics
 Principles of Food and Bioprocess Engineering (FS 21) Solutions to Example Problems on Psychrometrics 1. We begin by identifying the conditions of the two streams on the psychrometric chart as follows.
Principles of Food and Bioprocess Engineering (FS 21) Solutions to Example Problems on Psychrometrics 1. We begin by identifying the conditions of the two streams on the psychrometric chart as follows.
CENG 5210 Advanced Separation Processes. Reverse osmosis
 Reverse osmosis CENG 510 Advanced Separation Processes In osmosis, solvent transports from a dilute solute or salt solution to a concentrated solute or salt solution across a semipermeable membrane hich
Reverse osmosis CENG 510 Advanced Separation Processes In osmosis, solvent transports from a dilute solute or salt solution to a concentrated solute or salt solution across a semipermeable membrane hich
Chapter 12 Intermolecular Forces of Attraction
 Chapter 12 Intermolecular Forces of Attraction Intermolecular Forces Attractive or Repulsive Forces between molecules. Molecule - - - - - - Molecule Intramolecular Forces bonding forces within the molecule.
Chapter 12 Intermolecular Forces of Attraction Intermolecular Forces Attractive or Repulsive Forces between molecules. Molecule - - - - - - Molecule Intramolecular Forces bonding forces within the molecule.
Chapter 4: Transient Heat Conduction. Dr Ali Jawarneh Department of Mechanical Engineering Hashemite University
 Chapter 4: Transient Heat Conduction Dr Ali Jawarneh Department of Mechanical Engineering Hashemite University Objectives When you finish studying this chapter, you should be able to: Assess when the spatial
Chapter 4: Transient Heat Conduction Dr Ali Jawarneh Department of Mechanical Engineering Hashemite University Objectives When you finish studying this chapter, you should be able to: Assess when the spatial
= = mol J K K mol N m. N/m N/m ( )( ) is the universal gas constant.
 THE THERMAL BEHAVIOR OF MATTER 7 EXERCISES Section 7. Gases 7. INTERPRET This problem involves the ideal-gas law, which we can use to ind the volume o mol o Martian atmosphere given its temperature and
THE THERMAL BEHAVIOR OF MATTER 7 EXERCISES Section 7. Gases 7. INTERPRET This problem involves the ideal-gas law, which we can use to ind the volume o mol o Martian atmosphere given its temperature and
Unit 7. Solution Concentrations and Colligative Properties
 Unit 7 Solution Concentrations and Colligative Properties Molarity Most widely used concentration unit [HCl] means concentration of HCl in mol/l Notice volume is total volume of solution Molarity (M)=
Unit 7 Solution Concentrations and Colligative Properties Molarity Most widely used concentration unit [HCl] means concentration of HCl in mol/l Notice volume is total volume of solution Molarity (M)=
Heat Transfer: A Practical Approach - Yunus A Cengel Assignment 11 Fall 2003 Tuesday, November 18, 2003 Chapter 11, Problem 49
 Heat Transer: A Practical Approach - Yunus A Cengel Assignment Fall 00 Tuesday, November 8, 00 Chapter, Problem 9 The variation o the spectral transmissivity o a 0.6- cm-thick glass window is as given
Heat Transer: A Practical Approach - Yunus A Cengel Assignment Fall 00 Tuesday, November 8, 00 Chapter, Problem 9 The variation o the spectral transmissivity o a 0.6- cm-thick glass window is as given
Heat. Heat is energy transferred between a system and its surroundings because of a temperature difference between them.
 What is heat? Heat Heat Heat is energy transferred between a system and its surroundings because of a temperature difference between them. Specific heat The specific heat of a material is the amount of
What is heat? Heat Heat Heat is energy transferred between a system and its surroundings because of a temperature difference between them. Specific heat The specific heat of a material is the amount of
Examination Heat Transfer
 Examination Heat Transfer code: 4B680 date: June 13, 2008 time: 14.00-17.00 Note: There are 4 questions in total. The first one consists of independent subquestions. If possible and necessary, guide numbers
Examination Heat Transfer code: 4B680 date: June 13, 2008 time: 14.00-17.00 Note: There are 4 questions in total. The first one consists of independent subquestions. If possible and necessary, guide numbers
Principles of Food and Bioprocess Engineering (FS 231) Solutions to Example Problems on Mass Balance
 Principles of Food and Bioprocess Engineering (FS 231) Solutions to Example Problems on Mass Balance 1. The mass balance equation for the system is: 2 + 3 = m This yields, m = 5 kg 2. The mass balance
Principles of Food and Bioprocess Engineering (FS 231) Solutions to Example Problems on Mass Balance 1. The mass balance equation for the system is: 2 + 3 = m This yields, m = 5 kg 2. The mass balance
University of Rome Tor Vergata
 University of Rome Tor Vergata Faculty of Engineering Department of Industrial Engineering THERMODYNAMIC AND HEAT TRANSFER HEAT TRANSFER dr. G. Bovesecchi gianluigi.bovesecchi@gmail.com 06-7259-727 (7249)
University of Rome Tor Vergata Faculty of Engineering Department of Industrial Engineering THERMODYNAMIC AND HEAT TRANSFER HEAT TRANSFER dr. G. Bovesecchi gianluigi.bovesecchi@gmail.com 06-7259-727 (7249)
Problem Set #10 Assigned November 8, 2013 Due Friday, November 15, 2013 Please show all work for credit. To Hand in
 Problem Set #10 Assigned November 8, 013 Due Friday, November 15, 013 Please show all work or credit To Hand in 1. 1 . A least squares it o ln P versus 1/T gives the result 3. Hvaporization = 5.8 kj mol
Problem Set #10 Assigned November 8, 013 Due Friday, November 15, 013 Please show all work or credit To Hand in 1. 1 . A least squares it o ln P versus 1/T gives the result 3. Hvaporization = 5.8 kj mol
Week 14/Tu: Lecture Units 33 & 34
 Week 14/Tu: Lecture Units 33 & 34 Exam 3 Unit 33: Colligative Properties -- Vapor pressure of solutions -- Freezing, boiling of solutions -- Osmotic pressure Unit 34: Introduction to Equilibria -- Rate
Week 14/Tu: Lecture Units 33 & 34 Exam 3 Unit 33: Colligative Properties -- Vapor pressure of solutions -- Freezing, boiling of solutions -- Osmotic pressure Unit 34: Introduction to Equilibria -- Rate
UNIT 2 SOLUTION. Q. 1. The vapour pressure of deliquescent substance is less or more than that of water vapours in air?
 UNIT 2 SOLUTION 1 MARK QUESTIONS Q. 1. The vapour pressure of deliquescent substance is less or more than that of water vapours in air? Ans. Less than that of water vapours in air. Q. 2. If is the degree
UNIT 2 SOLUTION 1 MARK QUESTIONS Q. 1. The vapour pressure of deliquescent substance is less or more than that of water vapours in air? Ans. Less than that of water vapours in air. Q. 2. If is the degree
Freezing Loads and Freezing Time Calculation
 6 Freezing Loads and Freezing Time Calculation Gauri S. Mittal University of Guelph, Guelph, Ontario, Canada CONTENTS I. Introduction... 17 II. Freezing Load...... 18 A. Calculation.... 18 B. Freezing
6 Freezing Loads and Freezing Time Calculation Gauri S. Mittal University of Guelph, Guelph, Ontario, Canada CONTENTS I. Introduction... 17 II. Freezing Load...... 18 A. Calculation.... 18 B. Freezing
SOLUTIONS. Chapter Test B. A. Matching. Column A. Column B. Name Date Class. 418 Core Teaching Resources
 16 SOLUTIONS Chapter Test B A. Matching Match each term in Column B to the correct description in Column A. Write the letter of the correct term on the line. Column A Column B 1. the number of moles of
16 SOLUTIONS Chapter Test B A. Matching Match each term in Column B to the correct description in Column A. Write the letter of the correct term on the line. Column A Column B 1. the number of moles of
1. What is a solution? and think
 1. What is a solution? and think Solutions Properties of Solutions Solutions: 1. Have no visible parts (particles are small) 2. They are homogeneous evenly distributed particles 3. The particles do not
1. What is a solution? and think Solutions Properties of Solutions Solutions: 1. Have no visible parts (particles are small) 2. They are homogeneous evenly distributed particles 3. The particles do not
SOLUTION CONCENTRATIONS
 SOLUTION CONCENTRATIONS The amount of solute in a solution (concentration) is an important property of the solution. A dilute solution contains small quantities of solute relative to the solvent, while
SOLUTION CONCENTRATIONS The amount of solute in a solution (concentration) is an important property of the solution. A dilute solution contains small quantities of solute relative to the solvent, while
Examination Heat Transfer
 Examination Heat Transfer code: 4B680 date: 17 january 2006 time: 14.00-17.00 hours NOTE: There are 4 questions in total. The first one consists of independent sub-questions. If necessary, guide numbers
Examination Heat Transfer code: 4B680 date: 17 january 2006 time: 14.00-17.00 hours NOTE: There are 4 questions in total. The first one consists of independent sub-questions. If necessary, guide numbers
If there is convective heat transfer from outer surface to fluid maintained at T W.
 Heat Transfer 1. What are the different modes of heat transfer? Explain with examples. 2. State Fourier s Law of heat conduction? Write some of their applications. 3. State the effect of variation of temperature
Heat Transfer 1. What are the different modes of heat transfer? Explain with examples. 2. State Fourier s Law of heat conduction? Write some of their applications. 3. State the effect of variation of temperature
Time-Dependent Conduction :
 Time-Dependent Conduction : The Lumped Capacitance Method Chapter Five Sections 5.1 thru 5.3 Transient Conduction A heat transfer process for which the temperature varies with time, as well as location
Time-Dependent Conduction : The Lumped Capacitance Method Chapter Five Sections 5.1 thru 5.3 Transient Conduction A heat transfer process for which the temperature varies with time, as well as location
QUESTION ANSWER. . e. Fourier number:
 QUESTION 1. (0 pts) The Lumped Capacitance Method (a) List and describe the implications of the two major assumptions of the lumped capacitance method. (6 pts) (b) Define the Biot number by equations and
QUESTION 1. (0 pts) The Lumped Capacitance Method (a) List and describe the implications of the two major assumptions of the lumped capacitance method. (6 pts) (b) Define the Biot number by equations and
concentration of solute (molality) Freezing point depression constant (for SOLVENT)
 74 FREEZING POINT DEPRESSION concentration of solute (molality) Freezing point depression constant (for SOLVENT) Freezing point depression: The amount the freezing temperature is LOWERED by the solute.
74 FREEZING POINT DEPRESSION concentration of solute (molality) Freezing point depression constant (for SOLVENT) Freezing point depression: The amount the freezing temperature is LOWERED by the solute.
75 A solution of 2.500g of unknown dissolved in g of benzene has a freezing point of C. What is the molecular weight of the unknown?
 75 A solution of 2.500g of unknown dissolved in 100.0 g of benzene has a freezing point of 4.880 C. What is the molecular weight of the unknown? Solving for Cm (molality) will allow us to calculate how
75 A solution of 2.500g of unknown dissolved in 100.0 g of benzene has a freezing point of 4.880 C. What is the molecular weight of the unknown? Solving for Cm (molality) will allow us to calculate how
Physical Properties of Solutions
 Physical Properties of Solutions Chapter 12 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 12.1- Types of solutions A solution is a homogenous mixture of 2 or
Physical Properties of Solutions Chapter 12 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 12.1- Types of solutions A solution is a homogenous mixture of 2 or
70 Example: If a solution is m citric acid, what is the molar concentration (M) of the solution? The density of the solution is 1.
 70 Example: If a solution is 0.688 m citric acid, what is the molar concentration (M) of the solution? The density of the solution is 1.049 g/ml molality definition molarity definition To solve the problem,
70 Example: If a solution is 0.688 m citric acid, what is the molar concentration (M) of the solution? The density of the solution is 1.049 g/ml molality definition molarity definition To solve the problem,
Physical Chemistry I for Biochemists Chem340. Lecture 26 (3/14/11)
 Physical Cheistry I or Biocheists Che340 Lecture 26 (3/14/11) Yoshitaka Ishii Ch 7.2, 7.4-5, & 7.10 Announceent Exa 2 this Friday. Please be well prepared! HW average 80-85. You will probably have one
Physical Cheistry I or Biocheists Che340 Lecture 26 (3/14/11) Yoshitaka Ishii Ch 7.2, 7.4-5, & 7.10 Announceent Exa 2 this Friday. Please be well prepared! HW average 80-85. You will probably have one
Snow Parameter Caused Uncertainty of Predicted Snow Metamorphism Processes
 Sno Parameter Caused Uncertainty of Predicted Sno Metamorphism Processes Report on the Research Performed during the REU Program at the University of Alaska Fairbanks, Geophysical Institute, 903 Koyukuk
Sno Parameter Caused Uncertainty of Predicted Sno Metamorphism Processes Report on the Research Performed during the REU Program at the University of Alaska Fairbanks, Geophysical Institute, 903 Koyukuk
22.06 ENGINEERING OF NUCLEAR SYSTEMS OPEN BOOK FINAL EXAM 3 HOURS
 22.6 ENGINEERING OF NUCLEAR SYSTEMS OPEN BOOK FINAL EXAM 3 HOURS Short Questions (1% each) a) The specific power in a UO 2 pellet of a certain LWR is q"'=2 W/cm 3. The fuel 235 U enrichment is 4 % by weight.
22.6 ENGINEERING OF NUCLEAR SYSTEMS OPEN BOOK FINAL EXAM 3 HOURS Short Questions (1% each) a) The specific power in a UO 2 pellet of a certain LWR is q"'=2 W/cm 3. The fuel 235 U enrichment is 4 % by weight.
COLLIGATIVE PROPERTIES OF SOLUTIONS
 NAME: UNIT #9: MOLARITY DILUTIONS SOLUBILITY CURVES COLLIGATIVE PROPERTIES OF SOLUTIONS 1. MOLARITY a) Molarity is a measurement of the concentration of a solution in Chemistry. b) When making solutions,
NAME: UNIT #9: MOLARITY DILUTIONS SOLUBILITY CURVES COLLIGATIVE PROPERTIES OF SOLUTIONS 1. MOLARITY a) Molarity is a measurement of the concentration of a solution in Chemistry. b) When making solutions,
PHY 335 Data Analysis for Physicists
 PHY 335 Data Analysis or Physicists Instructor: Kok Wai Ng Oice CP 171 Telephone 7 1782 e mail: kng@uky.edu Oice hour: Thursday 11:00 12:00 a.m. or by appointment Time: Tuesday and Thursday 9:30 10:45
PHY 335 Data Analysis or Physicists Instructor: Kok Wai Ng Oice CP 171 Telephone 7 1782 e mail: kng@uky.edu Oice hour: Thursday 11:00 12:00 a.m. or by appointment Time: Tuesday and Thursday 9:30 10:45
- Applications: In chemistry, this effect is often used to determine the molecular weight of an unknown molecule.
 73 FREEZING POINT DEPRESSION concentration of solute (molality) Freezing point depression constant (for SOLVENT) Freezing point depression: The amount the freezing temperature is LOWERED by the solute.
73 FREEZING POINT DEPRESSION concentration of solute (molality) Freezing point depression constant (for SOLVENT) Freezing point depression: The amount the freezing temperature is LOWERED by the solute.
Dual Program Level 1 Physics Course
 Dual Program Level 1 Physics Course Assignment 15 Due: 11/Feb/2012 14:00 Assume that water has a constant specific heat capacity of 4190 J/kg K at all temperatures between its melting point and boiling
Dual Program Level 1 Physics Course Assignment 15 Due: 11/Feb/2012 14:00 Assume that water has a constant specific heat capacity of 4190 J/kg K at all temperatures between its melting point and boiling
64 previous solution
 64 previous solution mole fraction (definition) 1 - Convert 29.6 grams sodium sulfate to moles. We already did this to find molality, so we can re-use the number. 2 - This is the total moles of both sodium
64 previous solution mole fraction (definition) 1 - Convert 29.6 grams sodium sulfate to moles. We already did this to find molality, so we can re-use the number. 2 - This is the total moles of both sodium
1. A solution that is 9% by mass glucose contains 9 g of glucose in every g of solution.
 Solutions molarity (Homework) For answers, send email to: admin@tutor-homework.com. Include file name: Chemistry_Worksheet_0144 Price: $3 (c) 2012 www.tutor-homework.com: Tutoring, homework help, help
Solutions molarity (Homework) For answers, send email to: admin@tutor-homework.com. Include file name: Chemistry_Worksheet_0144 Price: $3 (c) 2012 www.tutor-homework.com: Tutoring, homework help, help
EFFECTS OF VISCOUS DISSIPATION ON FREE CONVECTION BOUNDARY LAYER FLOW TOWARDS A HORIZONTAL CIRCULAR CYLINDER
 EFFECTS OF VISCOUS DISSIPATION ON FREE CONVECTION BOUNDARY LAYER FLOW TOWARDS A HORIZONTAL CIRCULAR CYLINDER Muhammad Khairul Anuar Mohamed 1, Norhaizah Md Sari 1, Abdul Rahman Mohd Kasim 1, Nor Aida Zuraimi
EFFECTS OF VISCOUS DISSIPATION ON FREE CONVECTION BOUNDARY LAYER FLOW TOWARDS A HORIZONTAL CIRCULAR CYLINDER Muhammad Khairul Anuar Mohamed 1, Norhaizah Md Sari 1, Abdul Rahman Mohd Kasim 1, Nor Aida Zuraimi
Freezing point depression - The freezing temperature of a SOLUTION gets lower as the CONCENTRATION of a solution increases.
 73 COLLIGATIVE PROPERTIES - properties unique to solutions. - depend only on the CONCENTRATION of a solution and not the IDENTITY of the solute** **ionic solutes: Remember that they dissociate into MULTIPLE
73 COLLIGATIVE PROPERTIES - properties unique to solutions. - depend only on the CONCENTRATION of a solution and not the IDENTITY of the solute** **ionic solutes: Remember that they dissociate into MULTIPLE
The temperature of a body, in general, varies with time as well
 cen58933_ch04.qd 9/10/2002 9:12 AM Page 209 TRANSIENT HEAT CONDUCTION CHAPTER 4 The temperature of a body, in general, varies with time as well as position. In rectangular coordinates, this variation is
cen58933_ch04.qd 9/10/2002 9:12 AM Page 209 TRANSIENT HEAT CONDUCTION CHAPTER 4 The temperature of a body, in general, varies with time as well as position. In rectangular coordinates, this variation is
Principles of Food and Bioprocess Engineering (FS 231) Example Problems on Units and Dimensions
 Principles of Food and Bioprocess Engineering (FS 231) Example Problems on Units and Dimensions 1. Determine the dimensions of the following quantities starting from their units: a. Work b. Specific heat
Principles of Food and Bioprocess Engineering (FS 231) Example Problems on Units and Dimensions 1. Determine the dimensions of the following quantities starting from their units: a. Work b. Specific heat
 CHAPTER 9 SOLUTIONS SHORT QUESTIONS WITH ANSWER Q.1 Binary solution can be homogenous or heterogeneous explain? The solutions which contain two components only are called as binary solution. If binary
CHAPTER 9 SOLUTIONS SHORT QUESTIONS WITH ANSWER Q.1 Binary solution can be homogenous or heterogeneous explain? The solutions which contain two components only are called as binary solution. If binary
Physics 111. Lecture 35 (Walker: ) Latent Heat Internal Energy First Law of Thermodynamics. Latent Heats. Latent Heat
 Physics 111 Lecture 35 (Walker: 17.4-5) Latent Heat Internal Energy First Law of Thermodynamics Latent Heats The heat required to convert from one phase to another is called the latent heat. The latent
Physics 111 Lecture 35 (Walker: 17.4-5) Latent Heat Internal Energy First Law of Thermodynamics Latent Heats The heat required to convert from one phase to another is called the latent heat. The latent
Physics 111. Lecture 39 (Walker: 17.6, 18.2) Latent Heat Internal Energy First Law of Thermodynamics May 8, Latent Heats
 Physics 111 Lecture 39 (Walker: 17.6, 18.2) Latent Heat Internal Energy First Law of Thermodynamics May 8, 2009 Lecture 39 1/26 Latent Heats The heat required to convert from one phase to another is called
Physics 111 Lecture 39 (Walker: 17.6, 18.2) Latent Heat Internal Energy First Law of Thermodynamics May 8, 2009 Lecture 39 1/26 Latent Heats The heat required to convert from one phase to another is called
Chapter 4: Properties of Pure Substances. Pure Substance. Phases of a Pure Substance. Phase-Change Processes of Pure Substances
 Chapter 4: roperties o ure Substances ure Substance A substance that has a ixed chemical composition throughout is called a pure substance such as water, air, and nitrogen A pure substance does not hae
Chapter 4: roperties o ure Substances ure Substance A substance that has a ixed chemical composition throughout is called a pure substance such as water, air, and nitrogen A pure substance does not hae
IE312 1 HW#2 Solution_Fall06
 IE3. Section 3.3: Problem -3 Problem P.68 Max z 4x +x s.t. 8x +x 6 ---- C 5x +x ---- C xx 0 ---- C3 X 8 Feasible Region 6 C 4 C CASE The LP has alternative or multiple optimal solutions: To extreme points
IE3. Section 3.3: Problem -3 Problem P.68 Max z 4x +x s.t. 8x +x 6 ---- C 5x +x ---- C xx 0 ---- C3 X 8 Feasible Region 6 C 4 C CASE The LP has alternative or multiple optimal solutions: To extreme points
LIQUID SOLUTION EXERCISE # 2
 LIQUID OLUION EXERCIE # 4. m d of solute. 4.. ().97 6. he concentration of solution (ppm) t. of solute 6 t.of solvent 5 6 5 ppm 6. m k.69.5 C.69.5 C solvent.59 C 99.75 C.59 C.789 C. i k f [NaCl Na + +
LIQUID OLUION EXERCIE # 4. m d of solute. 4.. ().97 6. he concentration of solution (ppm) t. of solute 6 t.of solvent 5 6 5 ppm 6. m k.69.5 C.69.5 C solvent.59 C 99.75 C.59 C.789 C. i k f [NaCl Na + +
CHEM1109 Answers to Problem Sheet Isotonic solutions have the same osmotic pressure. The osmotic pressure, Π, is given by:
 CHEM1109 Answers to Problem Sheet 5 1. Isotonic solutions have the same osmotic pressure. The osmotic pressure, Π, is given by: Π = MRT where M is the molarity of the solution. Hence, M = Π 5 (8.3 10 atm)
CHEM1109 Answers to Problem Sheet 5 1. Isotonic solutions have the same osmotic pressure. The osmotic pressure, Π, is given by: Π = MRT where M is the molarity of the solution. Hence, M = Π 5 (8.3 10 atm)
Dual porosity DRM formulation for flow and transport through fractured porous media
 Boundary Eleents XXVII 407 Dual porosity DRM orulation or lo and transport through ractured porous edia T. aardzioska & V. Popov Wessex Institute o Technology, UK Abstract The ain objective o this ork
Boundary Eleents XXVII 407 Dual porosity DRM orulation or lo and transport through ractured porous edia T. aardzioska & V. Popov Wessex Institute o Technology, UK Abstract The ain objective o this ork
Chemistry. TOPIC : Solution and colligative properties
 TOPIC : Solution and colligative properties Date : Marks : 20 mks Time : ½ hr. If 5.85 g of NaCl (molecular weight 58.5) is dissolved in water and the solution is made up to 0.5 litre, the molarity of
TOPIC : Solution and colligative properties Date : Marks : 20 mks Time : ½ hr. If 5.85 g of NaCl (molecular weight 58.5) is dissolved in water and the solution is made up to 0.5 litre, the molarity of
Julkaisu 112. Freezing Point Depressions of Dilute Solutions of Alkali Metal Chlorides and Bromides. Jaakko I. Partanen [ ] γ ) ln( m d(ln ± I = ±)
![Julkaisu 112. Freezing Point Depressions of Dilute Solutions of Alkali Metal Chlorides and Bromides. Jaakko I. Partanen [ ] γ ) ln( m d(ln ± I = ±) Julkaisu 112. Freezing Point Depressions of Dilute Solutions of Alkali Metal Chlorides and Bromides. Jaakko I. Partanen [ ] γ ) ln( m d(ln ± I = ±)](/thumbs/89/98776372.jpg) Julkaisu 112 Freezing Point Depressions o Dilute Solutions o Alkali Metal Chlorides and Bromides Jaakko I. Partanen = = + + + + ) / ( ) ( 2 ) ( 2 us 1 1 H I m RM I m M R [ ] ) / ( ) ( 2 / ) ) ln ( ( us
Julkaisu 112 Freezing Point Depressions o Dilute Solutions o Alkali Metal Chlorides and Bromides Jaakko I. Partanen = = + + + + ) / ( ) ( 2 ) ( 2 us 1 1 H I m RM I m M R [ ] ) / ( ) ( 2 / ) ) ln ( ( us
1 R-value = 1 h ft2 F. = m2 K btu. W 1 kw = tons of refrigeration. solar = 1370 W/m2 solar temperature
 Quick Reference for Heat Transfer Analysis compiled by Jason Valentine and Greg Walker Please contact greg.alker@vanderbilt.edu ith corrections and suggestions copyleft 28: You may copy, distribute, and
Quick Reference for Heat Transfer Analysis compiled by Jason Valentine and Greg Walker Please contact greg.alker@vanderbilt.edu ith corrections and suggestions copyleft 28: You may copy, distribute, and
FORMULA SHEET (tear off)
 FORMULA SHEET (tear off) N A = 6.022 x 10 23 C = ( 5 / 9 ) ( F - 32) F = ( 9 / 5 )( C) + 32 1 amu = 1.661 x 10-27 kg C = K - 273.15 K = C + 273.15 1 atm = 760 torr = 760 mm Hg 1 atm = 1.013 bar pv = nrt
FORMULA SHEET (tear off) N A = 6.022 x 10 23 C = ( 5 / 9 ) ( F - 32) F = ( 9 / 5 )( C) + 32 1 amu = 1.661 x 10-27 kg C = K - 273.15 K = C + 273.15 1 atm = 760 torr = 760 mm Hg 1 atm = 1.013 bar pv = nrt
Graduate Preliminary Examination, Thursday, January 6, Part I 2
 Graduate Preliminary Examination, Thursday, January 6, 2011 - Part I 2 Section A. Mechanics 1. ( Lasso) Picture from: The Lasso: a rational guide... c 1995 Carey D. Bunks A lasso is a rope of linear mass
Graduate Preliminary Examination, Thursday, January 6, 2011 - Part I 2 Section A. Mechanics 1. ( Lasso) Picture from: The Lasso: a rational guide... c 1995 Carey D. Bunks A lasso is a rope of linear mass
ANSWERS CIRCLE CORRECT SECTION
 CHEMISTRY 162 - EXAM I June 08, 2009 Name: SIGN: RU ID Number Choose the one best answer for each question and write the letter preceding it in the appropriate space on this answer sheet. Only the answer
CHEMISTRY 162 - EXAM I June 08, 2009 Name: SIGN: RU ID Number Choose the one best answer for each question and write the letter preceding it in the appropriate space on this answer sheet. Only the answer
Phase Change (State Change): A change in physical form but not the chemical identity of a substance.
 CHM 123 Chapter 11 11.1-11.2 Phase change, evaporation, vapor pressure, and boiling point Phase Change (State Change): A change in physical form but not the chemical identity of a substance. Heat (Enthalpy)
CHM 123 Chapter 11 11.1-11.2 Phase change, evaporation, vapor pressure, and boiling point Phase Change (State Change): A change in physical form but not the chemical identity of a substance. Heat (Enthalpy)
Soluble: A solute that dissolves in a specific solvent. Insoluble: A solute that will not dissolve in a specific solvent. "Like Dissolves Like"
 Solutions Homogeneous Mixtures Solutions: Mixtures that contain two or more substances called the solute and the solvent where the solute dissolves in the solvent so the solute and solvent are not distinguishable
Solutions Homogeneous Mixtures Solutions: Mixtures that contain two or more substances called the solute and the solvent where the solute dissolves in the solvent so the solute and solvent are not distinguishable
Ch 7 Practice Problems
 Ch 7 Practice Problems 1. For the equilibrium that exists in an aqueous solution of nitrous acid (HNO 2, a eak acid), the equilibrium constant expression is [H ] [NO 2 ] = [HNO ] 2 [H ][N][O] [HNO 2] =
Ch 7 Practice Problems 1. For the equilibrium that exists in an aqueous solution of nitrous acid (HNO 2, a eak acid), the equilibrium constant expression is [H ] [NO 2 ] = [HNO ] 2 [H ][N][O] [HNO 2] =
Properties of Solutions. Chapter 13
 Properties of Solutions Chapter 13 Sodium acetate crystals rapidly form when a seed crystal is added to a supersaturated solution of sodium acetate. Saturated solution: contains the maximum amount of a
Properties of Solutions Chapter 13 Sodium acetate crystals rapidly form when a seed crystal is added to a supersaturated solution of sodium acetate. Saturated solution: contains the maximum amount of a
(a) How much work is done by the gas? (b) Assuming the gas behaves as an ideal gas, what is the final temperature? V γ+1 2 V γ+1 ) pdv = K 1 γ + 1
 P340: hermodynamics and Statistical Physics, Exam#, Solution. (0 point) When gasoline explodes in an automobile cylinder, the temperature is about 2000 K, the pressure is is 8.0 0 5 Pa, and the volume
P340: hermodynamics and Statistical Physics, Exam#, Solution. (0 point) When gasoline explodes in an automobile cylinder, the temperature is about 2000 K, the pressure is is 8.0 0 5 Pa, and the volume
How Cold is Freezing?
 Details Completion About one period Permission: Download, Share, and Remix How Cold is Freezing? Overview How can the ocean be colder than 0 C, the temperature at which water freezes? As it turns out,
Details Completion About one period Permission: Download, Share, and Remix How Cold is Freezing? Overview How can the ocean be colder than 0 C, the temperature at which water freezes? As it turns out,
BOUNDARY LAYER ANALYSIS ALONG A STRETCHING WEDGE SURFACE WITH MAGNETIC FIELD IN A NANOFLUID
 Proceedings o the International Conerence on Mechanical Engineering and Reneable Energy 7 (ICMERE7) 8 December, 7, Chittagong, Bangladesh ICMERE7-PI- BOUNDARY LAYER ANALYSIS ALONG A STRETCHING WEDGE SURFACE
Proceedings o the International Conerence on Mechanical Engineering and Reneable Energy 7 (ICMERE7) 8 December, 7, Chittagong, Bangladesh ICMERE7-PI- BOUNDARY LAYER ANALYSIS ALONG A STRETCHING WEDGE SURFACE
2015 American Journal of Engineering Research (AJER)
 American Journal o Engineering Research (AJER) 2015 American Journal o Engineering Research (AJER) e-issn: 2320-0847 p-issn : 2320-0936 Volume-4, Issue-7, pp-33-40.ajer.org Research Paper Open Access The
American Journal o Engineering Research (AJER) 2015 American Journal o Engineering Research (AJER) e-issn: 2320-0847 p-issn : 2320-0936 Volume-4, Issue-7, pp-33-40.ajer.org Research Paper Open Access The
oz ounce (mass) = L = cm 3
 Memorize relationships shown in each box! NOTE: Exact quantities are specified as exact. Also, consider 1 as exact. mass (M) Common unit abbreviations (singular) 1 kg = 2.20462 lb m = 35.27392 oz L liter
Memorize relationships shown in each box! NOTE: Exact quantities are specified as exact. Also, consider 1 as exact. mass (M) Common unit abbreviations (singular) 1 kg = 2.20462 lb m = 35.27392 oz L liter
ENERGY ANALYSIS: CLOSED SYSTEM
 ENERGY ANALYSIS: CLOSED SYSTEM A closed system can exchange energy with its surroundings through heat and work transer. In other words, work and heat are the orms that energy can be transerred across the
ENERGY ANALYSIS: CLOSED SYSTEM A closed system can exchange energy with its surroundings through heat and work transer. In other words, work and heat are the orms that energy can be transerred across the
Introduction to Heat and Mass Transfer. Week 9
 Introduction to Heat and Mass Transfer Week 9 補充! Multidimensional Effects Transient problems with heat transfer in two or three dimensions can be considered using the solutions obtained for one dimensional
Introduction to Heat and Mass Transfer Week 9 補充! Multidimensional Effects Transient problems with heat transfer in two or three dimensions can be considered using the solutions obtained for one dimensional
INTERMOLECULAR FORCES AND COLLIGATIVE PROPERTIES HOMEWORK ANSWERS
 INTERMOLECULAR FORCES AND COLLIGATIVE PROPERTIES OMEWORK ANSWERS 1. 12.2 a)-c) intermolecular d) intramolecular 2. 12.5 a), b) intermolecular; c), d) intramolecular. 12.15 No. At 1.1 atm, water boils at
INTERMOLECULAR FORCES AND COLLIGATIVE PROPERTIES OMEWORK ANSWERS 1. 12.2 a)-c) intermolecular d) intramolecular 2. 12.5 a), b) intermolecular; c), d) intramolecular. 12.15 No. At 1.1 atm, water boils at
11/4/2017. General Chemistry CHEM 101 (3+1+0) Dr. Mohamed El-Newehy. Chapter 4 Physical Properties of Solutions
 General Chemistry CHEM 11 (3+1+) Dr. Mohamed El-Newehy http://fac.ksu.edu.sa/melnewehy Chapter 4 Physical Properties of Solutions 1 Types of Solutions A solution is a homogenous mixture of 2 or more substances.
General Chemistry CHEM 11 (3+1+) Dr. Mohamed El-Newehy http://fac.ksu.edu.sa/melnewehy Chapter 4 Physical Properties of Solutions 1 Types of Solutions A solution is a homogenous mixture of 2 or more substances.
CP Chapter 15/16 Solutions What Are Solutions?
 CP Chapter 15/16 Solutions What Are Solutions? What is a solution? A solution is uniform that may contain solids, liquids, or gases. Known as a mixture Solution = + o Solvent The substance in abundance
CP Chapter 15/16 Solutions What Are Solutions? What is a solution? A solution is uniform that may contain solids, liquids, or gases. Known as a mixture Solution = + o Solvent The substance in abundance
A) sublimation. B) liquefaction. C) evaporation. D) condensation. E) freezing. 11. Below is a phase diagram for a substance.
 PX0411-1112 1. Which of the following statements concerning liquids is incorrect? A) The volume of a liquid changes very little with pressure. B) Liquids are relatively incompressible. C) Liquid molecules
PX0411-1112 1. Which of the following statements concerning liquids is incorrect? A) The volume of a liquid changes very little with pressure. B) Liquids are relatively incompressible. C) Liquid molecules
CH 302 Spring 2008 Worksheet 4 Answer Key Practice Exam 1
 CH 302 Spring 2008 Worksheet 4 Answer Key Practice Exam 1 1. Predict the signs of ΔH and ΔS for the sublimation of CO 2. a. ΔH > 0, ΔS > 0 b. ΔH > 0, ΔS < 0 c. ΔH < 0, ΔS > 0 d. ΔH < 0, ΔS < 0 Answer:
CH 302 Spring 2008 Worksheet 4 Answer Key Practice Exam 1 1. Predict the signs of ΔH and ΔS for the sublimation of CO 2. a. ΔH > 0, ΔS > 0 b. ΔH > 0, ΔS < 0 c. ΔH < 0, ΔS > 0 d. ΔH < 0, ΔS < 0 Answer:
Chemistry 2000 Lecture 12: Temperature dependence of the equilibrium constant
 Chemistry 2000 Lecture 12: Temperature dependence of the equilibrium constant Marc R. Roussel February 12, 2019 Marc R. Roussel Temperature dependence of equilibrium February 12, 2019 1 / 15 Temperature
Chemistry 2000 Lecture 12: Temperature dependence of the equilibrium constant Marc R. Roussel February 12, 2019 Marc R. Roussel Temperature dependence of equilibrium February 12, 2019 1 / 15 Temperature
6. (6) Show all the steps of how to convert 50.0 F into its equivalent on the Kelvin scale.
 General Physics I Quiz 8 - Ch. 13 - Temperature & Kinetic Theory July 30, 2009 Name: Make your work clear to the grader. Show formulas used. Give correct units and significant figures. Partial credit is
General Physics I Quiz 8 - Ch. 13 - Temperature & Kinetic Theory July 30, 2009 Name: Make your work clear to the grader. Show formulas used. Give correct units and significant figures. Partial credit is
Use the Equations given in your notes to solve the Colligative Property Questions. Freezing Boiling Point ( C)
 Colligative Properties of Solvents 8.HW Colligative Properties.doc Use the Equations given in your notes to solve the Colligative Property Questions. ΔT b m K b, ΔT f m Solvent Formula Freezing Point (
Colligative Properties of Solvents 8.HW Colligative Properties.doc Use the Equations given in your notes to solve the Colligative Property Questions. ΔT b m K b, ΔT f m Solvent Formula Freezing Point (
Useful Information Provided on Exam 1. Sections Covered on Exam , 10.2, 10.8,
 Chem 101B Exam 1 Study Questions Name: Chapters 10(partial), 11 & 12(partial) Review Tuesday 2/7/2017 Due on Exam Thursday 2/9/2017 (Exam 1 date) This is a homework assignment. Please show your work for
Chem 101B Exam 1 Study Questions Name: Chapters 10(partial), 11 & 12(partial) Review Tuesday 2/7/2017 Due on Exam Thursday 2/9/2017 (Exam 1 date) This is a homework assignment. Please show your work for
Born-Haber Cycle: ΔH hydration
 Born-Haber Cycle: ΔH hydration ΔH solution,nacl = ΔH hydration,nacl(aq) U NaCl ΔH hydration,nacl(aq) = ΔH hydration,na + (g) + ΔH hydration,cl (g) Enthalpies of Hydration 1 Sample Exercise 11.3 Use the
Born-Haber Cycle: ΔH hydration ΔH solution,nacl = ΔH hydration,nacl(aq) U NaCl ΔH hydration,nacl(aq) = ΔH hydration,na + (g) + ΔH hydration,cl (g) Enthalpies of Hydration 1 Sample Exercise 11.3 Use the
Critical Conditions for Water-based Suppression of Plastic Pool Fires. H. Li 1, A. S. Rangwala 1 and J.L. Torero 2
 Paper # 070FR-0069 Topic: Fire 8 th U. S. National Combustion Meeting Organized by the Western States Section of the Combustion Institute and hosted by the University of Utah May 19-22, 2013 Critical Conditions
Paper # 070FR-0069 Topic: Fire 8 th U. S. National Combustion Meeting Organized by the Western States Section of the Combustion Institute and hosted by the University of Utah May 19-22, 2013 Critical Conditions
Thermodynamics. Thermodynamics is the study of the collective properties of a system containing many bodies (typically of order 10 23!
 Thermodynamics Thermodynamics is the study of the collective properties of a system containing many bodies (typically of order 10 23!) Chapter18 Thermodynamics Thermodynamics is the study of the thermal
Thermodynamics Thermodynamics is the study of the collective properties of a system containing many bodies (typically of order 10 23!) Chapter18 Thermodynamics Thermodynamics is the study of the thermal
Version 001 HW 15 Thermodynamics C&J sizemore (21301jtsizemore) 1
 Version 001 HW 15 Thermodynamics C&J sizemore 21301jtsizemore 1 This print-out should have 38 questions. Multiple-choice questions may continue on the next column or page find all choices before answering.
Version 001 HW 15 Thermodynamics C&J sizemore 21301jtsizemore 1 This print-out should have 38 questions. Multiple-choice questions may continue on the next column or page find all choices before answering.
Analysis of Non-Thermal Equilibrium in Porous Media
 Analysis o Non-Thermal Equilibrium in Porous Media A. Nouri-Borujerdi, M. Nazari 1 School o Mechanical Engineering, Shari University o Technology P.O Box 11365-9567, Tehran, Iran E-mail: anouri@shari.edu
Analysis o Non-Thermal Equilibrium in Porous Media A. Nouri-Borujerdi, M. Nazari 1 School o Mechanical Engineering, Shari University o Technology P.O Box 11365-9567, Tehran, Iran E-mail: anouri@shari.edu
Thermophysical properties of foods and unsteady heat transfer
 Thermophysical properties of foods and unsteady heat transfer Questions 1. Nominate the main goals of the foods heating and nominate the basic operations having these goals 2. Define thermal conductivity,
Thermophysical properties of foods and unsteady heat transfer Questions 1. Nominate the main goals of the foods heating and nominate the basic operations having these goals 2. Define thermal conductivity,
EXTENDED SURFACES / FINS
 EXTENDED SURFACES / FINS Convection: Heat transer etween a solid surace and a moving luid is governed y the Newton s cooling law: q = ha(t s -T ). Thereore, to increase the convective heat transer, one
EXTENDED SURFACES / FINS Convection: Heat transer etween a solid surace and a moving luid is governed y the Newton s cooling law: q = ha(t s -T ). Thereore, to increase the convective heat transer, one
AP CHEMISTRY NOTES 15-1 INTERMOLECULAR FORCES
 AP CHEMISTRY NOTES 15-1 INTERMOLECULAR FORCES INTERMOLECULAR FORCES In addition to the covalent bonds that exist between atoms in a molecule (H2O for instance), there are also weak attractions between
AP CHEMISTRY NOTES 15-1 INTERMOLECULAR FORCES INTERMOLECULAR FORCES In addition to the covalent bonds that exist between atoms in a molecule (H2O for instance), there are also weak attractions between
CHEM 121b Exam 1 Spring 1999
 Name SSN CHEM 121b Exam 1 Spring 1999 This exam consists of 15 multiple choice questions (each worth 2 points), and 5 written problems (points noted below for each). There are a total of 100 possible points.
Name SSN CHEM 121b Exam 1 Spring 1999 This exam consists of 15 multiple choice questions (each worth 2 points), and 5 written problems (points noted below for each). There are a total of 100 possible points.
CHEM N-7 November 2005
 CHEM1909 2005-N-7 November 2005 Calcium chloride (3.42 g) is completely dissolved in 200 ml of water at 25.00 ºC in a coffee cup calorimeter. The temperature of the water after dissolution is 27.95 ºC.
CHEM1909 2005-N-7 November 2005 Calcium chloride (3.42 g) is completely dissolved in 200 ml of water at 25.00 ºC in a coffee cup calorimeter. The temperature of the water after dissolution is 27.95 ºC.
Thermal behaviour of latent thermal energy storage unit during charging and discharging processes: effects of HTF inlet velocity
 Revue des Energies Renouvelables Vol. 0 N (017) 85-93 Thermal behaviour o latent thermal energy storage unit during charging and discharging processes: eects o HTF inlet velocity F. Benmoussa 1 *, A. Benzaoui
Revue des Energies Renouvelables Vol. 0 N (017) 85-93 Thermal behaviour o latent thermal energy storage unit during charging and discharging processes: eects o HTF inlet velocity F. Benmoussa 1 *, A. Benzaoui
Phase Diagrams: Conditions for Equilibrium (CfE)
 Phase Equilibrium: Conditions for Equilibrium (CfE) Phase Diagrams: Conditions for Equilibrium (CfE) Write down the conditions for equilibrium for: a pure single phase system, a pure multi-phase system,
Phase Equilibrium: Conditions for Equilibrium (CfE) Phase Diagrams: Conditions for Equilibrium (CfE) Write down the conditions for equilibrium for: a pure single phase system, a pure multi-phase system,
FIND: (a) Sketch temperature distribution, T(x,t), (b) Sketch the heat flux at the outer surface, q L,t as a function of time.
 PROBLEM 5.1 NOWN: Electrical heater attached to backside of plate while front surface is exposed to convection process (T,h); initially plate is at a uniform temperature of the ambient air and suddenly
PROBLEM 5.1 NOWN: Electrical heater attached to backside of plate while front surface is exposed to convection process (T,h); initially plate is at a uniform temperature of the ambient air and suddenly
THERMODYNAMICS, FLUID AND PLANT PROCESSES. The tutorials are drawn from other subjects so the solutions are identified by the appropriate tutorial.
 THERMODYNAMICS, FLUID AND PLANT PROCESSES The tutorials are drawn from other subjects so the solutions are identified by the appropriate tutorial. THERMODYNAMICS TUTORIAL 1 LIQUIDS VAPOURS - GASES SAE
THERMODYNAMICS, FLUID AND PLANT PROCESSES The tutorials are drawn from other subjects so the solutions are identified by the appropriate tutorial. THERMODYNAMICS TUTORIAL 1 LIQUIDS VAPOURS - GASES SAE
Chemistry 6A F2007. Dr. J.A. Mack. Freezing Point Depression: 11/16/07. t f = nk f M
 Chemistry 6A F2007 Dr. J.A. Mack 11/16/07 11/14/07 Dr. Mack. CSUS 1 Freezing Point Depression: Similarly: The Freezing point of a solution is always lower than the freezing point of the pure solvent of
Chemistry 6A F2007 Dr. J.A. Mack 11/16/07 11/14/07 Dr. Mack. CSUS 1 Freezing Point Depression: Similarly: The Freezing point of a solution is always lower than the freezing point of the pure solvent of
Unit Conversions, Important Constants and Relationships
 NOTE: Exact quantities are specified as exact. Consider 1 as exact! mass (M) 1 kg = 2.20462 lb m = 35.27392 oz 1 lb m = 16 oz (exact)= 453.593 g length (L) 1 m = 10 10 (exact) angstroms (Å) = 100 cm =
NOTE: Exact quantities are specified as exact. Consider 1 as exact! mass (M) 1 kg = 2.20462 lb m = 35.27392 oz 1 lb m = 16 oz (exact)= 453.593 g length (L) 1 m = 10 10 (exact) angstroms (Å) = 100 cm =
10) On a solubility curve, the points on the curve indicate a solution. 11) Values on the graph a curve represent unsaturated solutions.
 Unit 11 Solutions- Funsheets Part A: Solubility Curves- Answer the following questions using the solubility curve below. Include units! 1) What mass of each solute will dissolve in 100mL of water at the
Unit 11 Solutions- Funsheets Part A: Solubility Curves- Answer the following questions using the solubility curve below. Include units! 1) What mass of each solute will dissolve in 100mL of water at the
 CHEMISTRY 110 EXAM 3 Nov. 11, 2013 ORM A!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!" 1. The cylinder shown below is filled with enough N 2 gas at 25 o C to reach a
CHEMISTRY 110 EXAM 3 Nov. 11, 2013 ORM A!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!" 1. The cylinder shown below is filled with enough N 2 gas at 25 o C to reach a
