(b) The heat transfer can be determined from an energy balance on the system
|
|
|
- Cameron Lynch
- 5 years ago
- Views:
Transcription
1 8-5 Heat is transferred to a iston-cylinder device wit a set of stos. e work done, te eat transfer, te exergy destroyed, and te second-law efficiency are to be deterined. Assutions e device is stationary and kinetic and otential energy canges are zero. ere is no friction between te iston and te cylinder. Analysis (a) e roerties of te refrigerant at te initial and final states are (ables A- troug A-) ka.65 /kg v C 8. kj/kg u s.6 kj/kg.k 8 ka.756 /kg v C.96 kj/kg u s.8 kj/kg.k e boundary work is deterined to be W ( ) (. kg)(8 ka)( ) b, out v v /kg.57 kj (b) e eat transfer can be deterined fro an energy balance on te syste in ( u u) + Wb, out (. kg)(.96 8.)kJ/kg +.57 kj 9.8 kj (c) e exergy difference between te inlet and exit states is X u [ u ( s s ) + ( v v )] (. kg).6 kj [(.96 8.)kJ/kg (98 K)(.8.6)kg.K + ( ka)( ) /kg] e useful work outut for te rocess is W u, out Wb,out ( v v).57 kj (. kg)( ka)( ) /kg. kj e exergy destroyed is te difference between te exergy difference and te useful work outut X X.6..7 kj dest W u,out (d) e second-law efficiency for tis rocess is Wu,out η II X. kj.6 kj.78 -a. kg ka C
2 8-9 Stea exands in a turbine, wic is not insulated. e reversible ower, te exergy destroyed, te second-law efficiency, and te ossible increase in te turbine ower if te turbine is well insulated are to be deterined. Assutions Steady oerating conditions exist. otential energy cange is negligible. Analysis (a) e roerties of te stea at te inlet and exit of te turbine are (ables A- troug A-6) a 8.7 kj/kg 55 C s kj/kg.k ka 9. kj/kg x.95 s kj/kg.k e entaly at te dead state is 5 C.8 kj/kg x e ass flow rate of stea ay be deterined fro an energy balance on te turbine V V & + & + + & out + a (6 /s) kj/kg ( /s) & 8.7 kj/kg + 9. kj/kg & + /s + 5 kw + 5 kw &.69 kg/s e reversible ower ay be deterined fro V -V W & rev & ( s s ) + (6 /s) (.69) (8.7 9.) (98)( ) + 7kW ( /s) kj/kg /s kj/kg /s (b) e exergy destroyed in te turbine is X & dest rev a kW (c) e second-law efficiency is a 5 kw η II.7 rev 7 kw (d) e energy of te stea at te turbine inlet in te given dead state is & & ( ) (.69 kg/s)( )kJ/kg 995 kw e fraction of energy at te turbine inlet tat is converted to ower is a 5 kw f.79 & 995 kw Assuing tat te sae fraction of eat loss fro te turbine could ave been converted to work, te ossible increase in te ower if te turbine is to be well-insulated becoes W & f & (.79)(5 kw). kw increase out Stea a 55 C, 6 /s urbine ka /s x.95
3 8-6 Argon gas is exanded adiabatically in an exansion valve. e exergy of argon at te inlet, te exergy destruction, and te second-law efficiency are to be deterined. Assutions Steady oerating conditions exist. Kinetic and otential energy canges are zero. Argon is an ideal gas wit constant secific eats. roerties e roerties of argon gas are.8 kj/kg.k, c.5 kj/kg.ºc (able A-). Analysis (a) e exergy of te argon at te inlet is x c ( ( s s ) ) c ln ln (.5 kj/kg.k)( 5) C (98 K) (.5 kj/kg.k)ln.7 kj/kg 7 K 98 K (.8 kj/kg.k)ln (b) oting tat te teerature reains constant in a trottling rocess of an ideal gas, te exergy destruction is deterined fro x dest s ( s gen s ) ln (98 K) (.8 kj/kg.k)ln.7 kj/kg (c) e second-law efficiency is x η II x x dest (.7.7)kJ/kg.6.7 kj/kg Argon.5 a C 5 ka 5 ka 5 ka 5 ka ka
4 -9 A geoteral eat u is considered. e degrees of subcooling done on te refrigerant in te condenser, te ass flow rate of te refrigerant, te eating load, te of te eat u, te iniu ower inut are to be deterined. Assutions Steady oerating conditions exist. Kinetic and otential energy canges are negligible. Analysis (a) Fro te refrigerant-a tables. (ables A- troug A-) x C. & 57. ka. kj/kg in ( out H & ( ) (.96 kg/s)(8..) kj/kg.7 kw & H.7 kw.68 in.656 kw (d) e reversible of te cycle is rev.9 L / H (5 + 7) /(5 + 7) e corresonding iniu ower inut is &.7 kw H in,in.8 kw.9 rev H Condenser Exansion 57. ka 6.59 kj/kg valve Coressor x (sat. va.) s.9 kj/kg ka 8. kj/kg s s Evaorator C sat. va. Fro te stea tables (able A-) x. w 5 C 9. kj/kg C Water w C 67.5 kj/kg 5 C e saturation teerature at te condenser ressure of ka and te actual teerature at te condenser outlet are ka 5. C H. a ka 8.59 C (fro EES). kj/kg en, te degrees of subcooling is subcool sat C s (b) e rate of eat absorbed fro te L geoteral water in te evaorator is & L & w ( w w ) (.65 kg/s)( )kJ/kg.78 kw is eat is absorbed by te refrigerant in te evaorator & L.78 kw &.96 kg/s (6.59.)kJ/kg (c) e ower inut to te coressor, te eating load and te are & ) + & (.96 kg/s)( )kJ/kg.656 kw. a s s W in s. W in
5 -66 A gas refrigeration cycle wit eliu as te working fluid is considered. e iniu teerature in te cycle, te, and te ass flow rate of te eliu are to be deterined. Assutions Steady oerating conditions exist. Heliu is an ideal gas wit constant secific eats. Kinetic and otential energy canges are negligible. roerties e roerties of eliu are c 5.96 kj/kgk and k.667 (able A-). Analysis (a) Fro te isentroic relations, s ( k ) / k H.667 /.667 s ( 6K)( ) 8.K 5 C ( k ) / k.667 / C s ( K) 8.K and s efrig s η η ( s ) (.8)( 8.) s s. K ηc s s + s.5 K (b) e of tis gas refrigeration cycle is deterined fro q w net,in w w ( ) ( ) ( ) ( ) L co,in 6. (.5 6) (.) q L turb,out in ( )/ η 6 + ( 8. 6) /(.8).56 (c) e ass flow rate of eliu is deterined fro & refrig & refrig & refrig 8 kj/s &.9 kg/s q c 5.96 kj/kg K 6. K L ( ) ( )( ) C
6 -8E A two-evaorator coression refrigeration cycle wit refrigerant-a as te working fluid is considered. e cooling load of bot evaorators er unit of flow troug te coressor and te of te syste are to be deterined. Assutions Steady oerating conditions exist. Kinetic and otential energy canges are negligible. Coressor Exansion Exansion valve valve Evaorator 5 Exansion valve 7 Evaorator Analysis Fro te refrigerant tables (ables A-E, A-E, and A-E), 6 6 sia 6 sia 8.59 Btu/lb sat. liquid 6 5 F sat. vaor 8.59 Btu/lb 5 Condenser F (trottling) 7. Btu/lb F F Btu/lb sat. vaor For a unit ass flowing troug te coressor, te fraction of ass flowing troug Evaorator II is denoted by x and tat troug Evaorator I is y (y -x). Fro te cooling loads secification, & L,e va & L,e va x( 5 ) y( 7 6 ) were x y Cobining tese results and solving for y gives y.698 ( 7 6 ) + ( 5 ) ( ) + ( ) en, x y Alying an energy balance to te oint in te syste were te two evaorator streas are recobined gives x5 + y7 (.6)(7.) + (.698)(98.68) x 5 + y7.8 Btu/lb en, 6 sia 6 F H -9.5 F 5 L 7 W in s
7 9.5 F.8 Btu/lb s 6 sia s sia s. Btu/lb.8 Btu/lb e cooling load of bot evaorators er unit ass troug te coressor is q L x( 5 ) + y( 7 6 ) (.6)( ) Btu/lb + (.698)( ) Btu/lb Btu/lb e work inut to te coressor is w in (..8) Btu/lb 6.96 Btu/lb e of tis refrigeration syste is deterined fro its definition, q L w in Btu/lb 6.96 Btu/lb.6 - e ass fractions of te constituents of a gas ixture are given. e ole fractions of te gas and gas constant are to be deterined. roerties e olar asses of, and are 6. and. kg/kol, resectively (able A-) Analysis For convenience, consider kg of te ixture. en te nuber of oles of eac coonent and te total nuber of oles are 75 kg 5 kg kol.568 kol + en te ole fraction of eac coonent becoes y y.688 kol.89 or 89.% 5.56 kol.568 kol.8 or.8% 5.56 kol 75 kg.688 kol 6 kg/kol 5 kg.568 kol kg/kol 5.56 kol e olar ass and te gas constant of te ixture are deterined fro teir definitions, and u kg 9. kg/ kol 5.56 kol 8. kj/kol K.7 kj/kg K 9. kg/kol ass 75% 5% - e asses, teeratures, and ressures of two gases contained in two tanks connected to eac oter are given. e valve connecting te tanks is oened and te final teerature is easured. e volue of eac tank and te final ressure are to be deterined.
8 Assutions Under secified conditions bot and can be treated as ideal gases, and te ixture as an ideal gas ixture roerties e olar asses of and are 8. and. kg/kol, resectively. e gas constants of and are.968 and.598 ka /kgk, resectively (able A-). Analysis e volues of te tanks are V V ( kg)(.968 ka /kg K)(98 K).95 ka ( kg)(.598 ka /kg K)(98 K).65 5 ka kg 5 C ka kg 5 C 5 ka Also, us, V total V + V.95 kg 8 kg/kol +.65 kg.975 kol kg/kol.57 kol kol kol u V (.95 kol)(8. ka kol /kol K)(98 K). ka
9 -7 Heat is transferred to a gas ixture contained in a iston cylinder device. e initial state and te final teerature are given. e eat transfer is to be deterined for te ideal gas and non-ideal gas cases. roerties e olar asses of H and are., and 8. kg/kol. (able A-). Analysis Fro te energy balance relation, E E E in in W out b,out in U H H + H ( ) H + ( ) H H since W b and U cobine into H for quasi-equilibriu constant ressure rocesses H H 6kg kol H kg / kol kg.75 kol 8 kg / kol (a) Assuing ideal gas beavior, te inlet and exit entalies of H and are deterined fro te ideal gas tables to be K,55. kj / K 5,669. kj / kol :,68 kj / kol, 5,8 kj / K us, ideal ( 5,669.,55.) +.75 ( 5,8, 68) 7 kj (b) Using Aagat's law and te generalized entaly dearture cart, te entaly cange of eac gas is deterined to be 6,.85,H cr,h. Z 5 H :.86,H,H cr,h. Z, 6.6,H cr,h. us H can be treated as an ideal gas during tis rocess. :, erefore,,,, cr, cr,,, ( ) ( ) 6 6. cr, ( ) ( Z Z ) + ( ) H u cr H,ideal Z.7 Z..7 5,669.,55.,.8kJ/kol ideal (Fig. A-9) (Fig. A-9) (8.ka /kol K)(6.K)(..7) + (5,8,68)kJ/kol,79.5kJ/kol in kol,.8 kj/kol +.75 kol,79.5 kj/kol Substituting, ( )( ) ( )( ) 75 kj 6 kg H kg 5 a 6 K
10 - e asses of coonents of a gas ixture are given. is ixture is exanded in an adiabatic, steady-flow turbine of secified isentroic efficiency. e second law efficiency and te exergy destruction during tis exansion rocess are to be deterined. Assutions All gases will be odeled as ideal gases wit constant secific eats. roerties e olar asses of,, and He are.,., and. kg/kol, resectively (able A-). e constant-ressure secific eats of tese gases at roo teerature are.98,.86, and 5.96 kj/kg K, resectively (able A-a). Analysis e total ass of te ixture is + + He kg ka 7 C e ole nubers of eac coonent are.kg.5 kol kg/kol kg.7 kol kg/kol He.5 kg He.5 kol He kg/kol e ole nuber of te ixture is + + He e aarent olecular weigt of te ixture is.6 kg.6 kg/kol.586 kol e aarent gas constant of te ixture is u 8. kj/kol K.786 kj/kg K.6 kg/kol e ass fractions are f f f He He.kg.65.6 kg kg.6 kg.65.5 kg.5.6 kg e constant-ressure secific eat of te ixture is deterined fro c f c, + f c, + f Hec kj/kg K en te constant-volue secific eat is c v, He c kj/kg K e secific eat ratio is c.9 k.55 c v.5 e teerature at te end of te exansion for te isentroic rocess is.586 kol,, He ixture ka
11 ( k ) / k.55/.55 ka s (6 K) 65 K ka Using te definition of turbine isentroic efficiency, te actual outlet teerature is ηturb ( s ) (6 K) (.9)(6 65) e entroy cange of te gas ixture is 99 K 99 s c ln ln (.9) ln (.786) ln.658 kj/kg K 6 s e actual work roduced is wout c ( ) (.9 kj/kg K)(6 99) K 665 kj/kg e reversible work outut is w rev, out ( s s ) 665 kj/kg (98 K)(.658 kj/kg K) 7 kj/kg e second-law efficiency and te exergy destruction are ten η x II dest w w out rev,out w w kj/kg rev,out out
3 Thermodynamics and Statistical mechanics
 Therodynaics and Statistical echanics. Syste and environent The syste is soe ortion of atter that we searate using real walls or only in our ine, fro the other art of the universe. Everything outside the
Therodynaics and Statistical echanics. Syste and environent The syste is soe ortion of atter that we searate using real walls or only in our ine, fro the other art of the universe. Everything outside the
Chapter 9 Practical cycles
 Prof.. undararajan Chater 9 Practical cycles 9. Introduction In Chaters 7 and 8, it was shown that a reversible engine based on the Carnot cycle (two reversible isothermal heat transfers and two reversible
Prof.. undararajan Chater 9 Practical cycles 9. Introduction In Chaters 7 and 8, it was shown that a reversible engine based on the Carnot cycle (two reversible isothermal heat transfers and two reversible
CHAPTER 2 THERMODYNAMICS
 CHAPER 2 HERMODYNAMICS 2.1 INRODUCION herodynaics is the study of the behavior of systes of atter under the action of external fields such as teerature and ressure. It is used in articular to describe
CHAPER 2 HERMODYNAMICS 2.1 INRODUCION herodynaics is the study of the behavior of systes of atter under the action of external fields such as teerature and ressure. It is used in articular to describe
I. Concepts and Definitions. I. Concepts and Definitions
 F. Properties of a syste (we use the to calculate changes in energy) 1. A property is a characteristic of a syste that can be given a nuerical value without considering the history of the syste. Exaples
F. Properties of a syste (we use the to calculate changes in energy) 1. A property is a characteristic of a syste that can be given a nuerical value without considering the history of the syste. Exaples
Kelvin Planck Statement of the Second Law. Clausius Statement of the Second Law
 Kelv Planck Statement of te Second aw It is imossible to construct an enge wic, oeratg a cycle, will roduce no oter effect tan te extraction of eat from a sgle reservoir and te erformance of an equivalent
Kelv Planck Statement of te Second aw It is imossible to construct an enge wic, oeratg a cycle, will roduce no oter effect tan te extraction of eat from a sgle reservoir and te erformance of an equivalent
1. (2.5.1) So, the number of moles, n, contained in a sample of any substance is equal N n, (2.5.2)
 Lecture.5. Ideal gas law We have already discussed general rinciles of classical therodynaics. Classical therodynaics is a acroscoic science which describes hysical systes by eans of acroscoic variables,
Lecture.5. Ideal gas law We have already discussed general rinciles of classical therodynaics. Classical therodynaics is a acroscoic science which describes hysical systes by eans of acroscoic variables,
Efficiencies. Damian Vogt Course MJ2429. Nomenclature. Symbol Denotation Unit c Flow speed m/s c p. pressure c v. Specific heat at constant J/kgK
 Turbomachinery Lecture Notes 1 7-9-1 Efficiencies Damian Vogt Course MJ49 Nomenclature Subscrits Symbol Denotation Unit c Flow seed m/s c Secific heat at constant J/kgK ressure c v Secific heat at constant
Turbomachinery Lecture Notes 1 7-9-1 Efficiencies Damian Vogt Course MJ49 Nomenclature Subscrits Symbol Denotation Unit c Flow seed m/s c Secific heat at constant J/kgK ressure c v Secific heat at constant
General Physical Chemistry I
 General Physical Cheistry I Lecture 12 Aleksey Kocherzhenko Aril 2, 2015" Last tie " Gibbs free energy" In order to analyze the sontaneity of cheical reactions, we need to calculate the entroy changes
General Physical Cheistry I Lecture 12 Aleksey Kocherzhenko Aril 2, 2015" Last tie " Gibbs free energy" In order to analyze the sontaneity of cheical reactions, we need to calculate the entroy changes
First Name Last Name CIRCLE YOUR LECTURE BELOW: Div. 1 10:30 am Div. 2 2:30 pm Div. 3 4:30 pm Prof. Gore Prof. Udupa Prof. Chen
 CIRCLE YOUR LECURE BELOW: Div. 1 10:30 am Div. :30 m Div. 3 4:30 m Prof. Gore Prof. Udua Prof. Chen EXAM # 3 INSRUCIONS 1. his is a closed book examination. All needed roerty tables are rovided.. Do not
CIRCLE YOUR LECURE BELOW: Div. 1 10:30 am Div. :30 m Div. 3 4:30 m Prof. Gore Prof. Udua Prof. Chen EXAM # 3 INSRUCIONS 1. his is a closed book examination. All needed roerty tables are rovided.. Do not
Chapter 4 Practice Problems
 Chater 4 ractice roblems (4.) (a) the number of microstates is N (b) 3 articles total 3! {, } 3 number microstates of secific arrangement (macrostate)!*! robability = (# microstates of secific arrangement)/(total
Chater 4 ractice roblems (4.) (a) the number of microstates is N (b) 3 articles total 3! {, } 3 number microstates of secific arrangement (macrostate)!*! robability = (# microstates of secific arrangement)/(total
Exergy and the Dead State
 EXERGY The energy content of the universe is constant, just as its mass content is. Yet at times of crisis we are bombarded with speeches and articles on how to conserve energy. As engineers, we know that
EXERGY The energy content of the universe is constant, just as its mass content is. Yet at times of crisis we are bombarded with speeches and articles on how to conserve energy. As engineers, we know that
Lecture 38: Vapor-compression refrigeration systems
 ME 200 Termodynamics I Lecture 38: Vapor-compression refrigeration systems Yong Li Sangai Jiao Tong University Institute of Refrigeration and Cryogenics 800 Dong Cuan Road Sangai, 200240, P. R. Cina Email
ME 200 Termodynamics I Lecture 38: Vapor-compression refrigeration systems Yong Li Sangai Jiao Tong University Institute of Refrigeration and Cryogenics 800 Dong Cuan Road Sangai, 200240, P. R. Cina Email
Section A 01. (12 M) (s 2 s 3 ) = 313 s 2 = s 1, h 3 = h 4 (s 1 s 3 ) = kj/kgk. = kj/kgk. 313 (s 3 s 4f ) = ln
 0. (a) Sol: Section A A refrigerator macine uses R- as te working fluid. Te temperature of R- in te evaporator coil is 5C, and te gas leaves te compressor as dry saturated at a temperature of 40C. Te mean
0. (a) Sol: Section A A refrigerator macine uses R- as te working fluid. Te temperature of R- in te evaporator coil is 5C, and te gas leaves te compressor as dry saturated at a temperature of 40C. Te mean
ESE (Prelims) - Offline Test Series MECHANICAL ENGINEERING SUBJECT: FLUID MECHANICS AND TURBO MACHINERY SOLUTIONS
 ES I: 0 ESE- 09 (Prelis) - Offline est Series est- 0. Ans: (a) MECANICAL ENGINEERING SUBJEC: FLUI MECANICS AN URBO MACINERY SOLUIONS Fro Newton s Law of viscosity: du dy = du for a ie dr d r = u ax dr
ES I: 0 ESE- 09 (Prelis) - Offline est Series est- 0. Ans: (a) MECANICAL ENGINEERING SUBJEC: FLUI MECANICS AN URBO MACINERY SOLUIONS Fro Newton s Law of viscosity: du dy = du for a ie dr d r = u ax dr
NEWCOMEN ATMOSPHERIC ENGINE
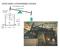 NECMEN AMSPERIC ENGINE atmoseric ressure Ford Museum, 760 strokes/m F 7 stroke 5 sia steam 8 50 F water NECMEN AMSPERIC ENGINE atmoseric ressure Ford Museum, 760 strokes/m 5 sia steam 8 F 50 F water 7
NECMEN AMSPERIC ENGINE atmoseric ressure Ford Museum, 760 strokes/m F 7 stroke 5 sia steam 8 50 F water NECMEN AMSPERIC ENGINE atmoseric ressure Ford Museum, 760 strokes/m 5 sia steam 8 F 50 F water 7
Engineering Services Examination - UPSC MECHANICAL ENGINEERING. Topic-wise Conventional Papers I & II (Last 30 Years Questions)
 Engineering Services Examination - UPSC MECHANICAL ENGINEERING Toic-wise Conventional Paers I & II (Last 0 Years Questions) Dedicated to Mechanical Engineers and Engineering Services asirants. 04 by Engineers
Engineering Services Examination - UPSC MECHANICAL ENGINEERING Toic-wise Conventional Paers I & II (Last 0 Years Questions) Dedicated to Mechanical Engineers and Engineering Services asirants. 04 by Engineers
Theory of turbomachinery. Chapter 1
 Theory of turbomachinery Chater Introduction: Basic Princiles Take your choice of those that can best aid your action. (Shakeseare, Coriolanus) Introduction Definition Turbomachinery describes machines
Theory of turbomachinery Chater Introduction: Basic Princiles Take your choice of those that can best aid your action. (Shakeseare, Coriolanus) Introduction Definition Turbomachinery describes machines
Chapter 1 Fundamentals
 Chater Fundamentals. Overview of Thermodynamics Industrial Revolution brought in large scale automation of many tedious tasks which were earlier being erformed through manual or animal labour. Inventors
Chater Fundamentals. Overview of Thermodynamics Industrial Revolution brought in large scale automation of many tedious tasks which were earlier being erformed through manual or animal labour. Inventors
Unit code: H/ QCF level: 5 Credit value: 15 OUTCOME 1 - THERMODYNAMIC SYSTEMS TUTORIAL 2
 Unit 43: Plant and Process Princiles Unit code: H/60 44 QCF level: 5 Credit value: 5 OUCOME - HERMODYNAMIC SYSEMS UORIAL Understand thermodynamic systems as alied to lant engineering rocesses hermodynamic
Unit 43: Plant and Process Princiles Unit code: H/60 44 QCF level: 5 Credit value: 5 OUCOME - HERMODYNAMIC SYSEMS UORIAL Understand thermodynamic systems as alied to lant engineering rocesses hermodynamic
Given: Hot fluid oil, Cold fluid - water (T 1, T 2 ) (t 1, t 2 ) Water
 . In a counter flow double pipe eat excanger, oil is cooled fro 85 to 55 by water entering at 5. Te ass flow rate of oil is 9,800 kg/ and specific eat f oil is 000 J/kg K. Te ass flow rate of water is
. In a counter flow double pipe eat excanger, oil is cooled fro 85 to 55 by water entering at 5. Te ass flow rate of oil is 9,800 kg/ and specific eat f oil is 000 J/kg K. Te ass flow rate of water is
Today lecture. 1. Entropy change in an isolated system 2. Exergy
 Today lecture 1. Entropy change in an isolated system. Exergy - What is exergy? - Reversible Work & Irreversibility - Second-Law Efficiency - Exergy change of a system For a fixed mass For a flow stream
Today lecture 1. Entropy change in an isolated system. Exergy - What is exergy? - Reversible Work & Irreversibility - Second-Law Efficiency - Exergy change of a system For a fixed mass For a flow stream
4. How a psychrometer works
 1 4. How a sychroeter works Identical theroeters Dry bulb (war Gauze around the wet bulb ool water Wet bulb (cool Saturated air Huidity to be easured T dry at Water being condensated, waring the gauze
1 4. How a sychroeter works Identical theroeters Dry bulb (war Gauze around the wet bulb ool water Wet bulb (cool Saturated air Huidity to be easured T dry at Water being condensated, waring the gauze
Compressor 1. Evaporator. Condenser. Expansion valve. CHE 323, October 8, Chemical Engineering Thermodynamics. Tutorial problem 5.
 CHE 33, October 8, 014. Cemical Engineering Termodynamics. Tutorial problem 5. In a simple compression refrigeration process, an adiabatic reversible compressor is used to compress propane, used as a refrigerant.
CHE 33, October 8, 014. Cemical Engineering Termodynamics. Tutorial problem 5. In a simple compression refrigeration process, an adiabatic reversible compressor is used to compress propane, used as a refrigerant.
02. Equilibrium Thermodynamics II: Engines
 University of Rhode Island DigitalCommons@URI Equilibrium Statistical Physics Physics Course Materials 205 02. Equilibrium Thermodynamics II: Engines Gerhard Müller University of Rhode Island, gmuller@uri.edu
University of Rhode Island DigitalCommons@URI Equilibrium Statistical Physics Physics Course Materials 205 02. Equilibrium Thermodynamics II: Engines Gerhard Müller University of Rhode Island, gmuller@uri.edu
Molecular Speeds. Real Gasses. Ideal Gas Law. Reasonable. Why the breakdown? P-V Diagram. Using moles. Using molecules
 Kinetic Theory of Gases Connect icroscopic properties (kinetic energy and oentu) of olecules to acroscopic state properties of a gas (teperature and pressure). P v v 3 3 3 But K v and P kt K v kt Teperature
Kinetic Theory of Gases Connect icroscopic properties (kinetic energy and oentu) of olecules to acroscopic state properties of a gas (teperature and pressure). P v v 3 3 3 But K v and P kt K v kt Teperature
Basic Thermodynamic Relations
 Basic herodynaic Relations Isolated syste: this is a syste that does not exchange energy with the surrounding edia. First Postulate (equilibriu theore) : Isolated syste always reaches the equilibriu state
Basic herodynaic Relations Isolated syste: this is a syste that does not exchange energy with the surrounding edia. First Postulate (equilibriu theore) : Isolated syste always reaches the equilibriu state
Actual exergy intake to perform the same task
 CHAPER : PRINCIPLES OF ENERGY CONSERVAION INRODUCION Energy conservation rinciles are based on thermodynamics If we look into the simle and most direct statement of the first law of thermodynamics, we
CHAPER : PRINCIPLES OF ENERGY CONSERVAION INRODUCION Energy conservation rinciles are based on thermodynamics If we look into the simle and most direct statement of the first law of thermodynamics, we
Chapters 19 & 20 Heat and the First Law of Thermodynamics
 Capters 19 & 20 Heat and te First Law of Termodynamics Te Zerot Law of Termodynamics Te First Law of Termodynamics Termal Processes Te Second Law of Termodynamics Heat Engines and te Carnot Cycle Refrigerators,
Capters 19 & 20 Heat and te First Law of Termodynamics Te Zerot Law of Termodynamics Te First Law of Termodynamics Termal Processes Te Second Law of Termodynamics Heat Engines and te Carnot Cycle Refrigerators,
PHYS 1101 Practice problem set 5, Chapter 18: 4, 9, 15, 23, 27, 32, 40, 43, 55, 56, 59 1 = = = Nk T Nk T Nk T B 1 B 2 B 1
 PHYS 0 Practice roble set, Chater 8: 4, 9,,, 7,, 40, 4,, 6, 9 8.4. Sole: (a he ean free ath of a olecule in a gas at teerature, olue V, and ressure is λ 00 n. We also know that λ λ V 4 π ( N V r Although,
PHYS 0 Practice roble set, Chater 8: 4, 9,,, 7,, 40, 4,, 6, 9 8.4. Sole: (a he ean free ath of a olecule in a gas at teerature, olue V, and ressure is λ 00 n. We also know that λ λ V 4 π ( N V r Although,
MAE 110A. Homework 6: Solutions 11/9/2017
 MAE 110A Hoework 6: Solutions 11/9/2017 H6.1: Two kg of H2O contained in a piston-cylinder assebly, initially at 1.0 bar and 140 C undergoes an internally ersible, isotheral copression to 25 bar. Given
MAE 110A Hoework 6: Solutions 11/9/2017 H6.1: Two kg of H2O contained in a piston-cylinder assebly, initially at 1.0 bar and 140 C undergoes an internally ersible, isotheral copression to 25 bar. Given
Find: a) Mass of the air, in kg, b) final temperature of the air, in K, and c) amount of entropy produced, in kj/k.
 PROBLEM 6.25 Three m 3 of air in a rigid, insulated container fitted with a paddle wheel is initially at 295 K, 200 kpa. The air receives 1546 kj of work from the paddle wheel. Assuming the ideal gas model,
PROBLEM 6.25 Three m 3 of air in a rigid, insulated container fitted with a paddle wheel is initially at 295 K, 200 kpa. The air receives 1546 kj of work from the paddle wheel. Assuming the ideal gas model,
NUCLEAR THERMAL-HYDRAULIC FUNDAMENTALS
 NUCLEAR THERMAL-HYDRAULIC FUNDAMENTALS Dr. J. Micael Doster Departent of Nuclear Engineering Nort Carolina State University Raleig, NC Copyrigted POER CYCLES Te analysis of Terodynaic Cycles is based alost
NUCLEAR THERMAL-HYDRAULIC FUNDAMENTALS Dr. J. Micael Doster Departent of Nuclear Engineering Nort Carolina State University Raleig, NC Copyrigted POER CYCLES Te analysis of Terodynaic Cycles is based alost
Notes on the function gsw_enthalpy_first_derivatives_ct_exact(sa,ct,p)
 Notes on gsw_entaly_first_derivatives_c_exact 1 Notes on te function gsw_entaly_first_derivatives_c_exact(c) is function gsw_entaly_first_derivatives_c_exact(c) evaluates two of te first order artial derivatives
Notes on gsw_entaly_first_derivatives_c_exact 1 Notes on te function gsw_entaly_first_derivatives_c_exact(c) is function gsw_entaly_first_derivatives_c_exact(c) evaluates two of te first order artial derivatives
6-5. H 2 O 200 kpa 200 C Q. Entropy Changes of Pure Substances
 Canges f ure Substances 6-0C Yes, because an ternally reversible, adiabatic prcess vlves n irreversibilities r eat transfer. 6- e radiatr f a steam eatg system is itially filled wit supereated steam. e
Canges f ure Substances 6-0C Yes, because an ternally reversible, adiabatic prcess vlves n irreversibilities r eat transfer. 6- e radiatr f a steam eatg system is itially filled wit supereated steam. e
Main components of the above cycle are: 1) Boiler (steam generator) heat exchanger 2) Turbine generates work 3) Condenser heat exchanger 4) Pump
 Introducton to Terodynacs, Lecture -5 Pro. G. Cccarell (0 Applcaton o Control olue Energy Analyss Most terodynac devces consst o a seres o coponents operatng n a cycle, e.g., stea power plant Man coponents
Introducton to Terodynacs, Lecture -5 Pro. G. Cccarell (0 Applcaton o Control olue Energy Analyss Most terodynac devces consst o a seres o coponents operatng n a cycle, e.g., stea power plant Man coponents
CHAPTER 2 ENERGY INTERACTION (HEAT AND WORK)
 CHATER ENERGY INTERACTION (HEAT AND WORK) Energy can cross the boundary of a closed system in two ways: Heat and Work. WORK The work is done by a force as it acts upon a body moving in direction of force.
CHATER ENERGY INTERACTION (HEAT AND WORK) Energy can cross the boundary of a closed system in two ways: Heat and Work. WORK The work is done by a force as it acts upon a body moving in direction of force.
CHEM 305 Solutions for assignment #2
 CHEM 05 Solutions for assignent #. (a) Starting fro C C show that C C Substitute the result into the original expression for C C : C C (b) Using the result fro (a), evaluate C C for an ideal gas. a. Both
CHEM 05 Solutions for assignent #. (a) Starting fro C C show that C C Substitute the result into the original expression for C C : C C (b) Using the result fro (a), evaluate C C for an ideal gas. a. Both
The First Law of Thermodynamics. By: Yidnekachew Messele
 The First Law of Thermodynamics By: Yidnekachew Messele It is the law that relates the various forms of energies for system of different types. It is simply the expression of the conservation of energy
The First Law of Thermodynamics By: Yidnekachew Messele It is the law that relates the various forms of energies for system of different types. It is simply the expression of the conservation of energy
= T. (kj/k) (kj/k) 0 (kj/k) int rev. Chapter 6 SUMMARY
 Capter 6 SUMMARY e second la of termodynamics leads to te definition of a ne property called entropy ic is a quantitative measure of microscopic disorder for a system. e definition of entropy is based
Capter 6 SUMMARY e second la of termodynamics leads to te definition of a ne property called entropy ic is a quantitative measure of microscopic disorder for a system. e definition of entropy is based
Chapter 7 Energy Principle
 Chater 7: Energy Princile By Dr Ali Jawarneh Hashemite University Outline In this chater we will: Derive and analyse the Energy equation. Analyse the flow and shaft work. Derive the equation for steady
Chater 7: Energy Princile By Dr Ali Jawarneh Hashemite University Outline In this chater we will: Derive and analyse the Energy equation. Analyse the flow and shaft work. Derive the equation for steady
ME 354 THERMODYNAMICS 2 MIDTERM EXAMINATION. Instructor: R. Culham. Name: Student ID Number: Instructions
 ME 354 THERMODYNAMICS 2 MIDTERM EXAMINATION February 14, 2011 5:30 pm - 7:30 pm Instructor: R. Culham Name: Student ID Number: Instructions 1. This is a 2 hour, closed-book examination. 2. Answer all questions
ME 354 THERMODYNAMICS 2 MIDTERM EXAMINATION February 14, 2011 5:30 pm - 7:30 pm Instructor: R. Culham Name: Student ID Number: Instructions 1. This is a 2 hour, closed-book examination. 2. Answer all questions
Daniel López Gaxiola 1 Student View Jason M. Keith
 Suppleental Material for Transport Process and Separation Process Principles Chapter Principles of Moentu Transfer and Overall Balances In fuel cells, the fuel is usually in gas or liquid phase. Thus,
Suppleental Material for Transport Process and Separation Process Principles Chapter Principles of Moentu Transfer and Overall Balances In fuel cells, the fuel is usually in gas or liquid phase. Thus,
ME 300 Thermodynamics II Exam 2 November 13, :00 p.m. 9:00 p.m.
 ME 300 Therodynaics II Exa 2 Noveber 3, 202 8:00 p.. 9:00 p.. Nae: Solution Section (Circle One): Sojka Naik :30 a.. :30 p.. Instructions: This is a closed book/notes exa. You ay use a calculator. You
ME 300 Therodynaics II Exa 2 Noveber 3, 202 8:00 p.. 9:00 p.. Nae: Solution Section (Circle One): Sojka Naik :30 a.. :30 p.. Instructions: This is a closed book/notes exa. You ay use a calculator. You
The Realm of Hydrogeology
 The Real of Hydrogeology In class exercise Stagnant Flow Plot hydraulic head and ressure vs. deth for (also indicate the hydrostatic line) Stagnant flow (no flow) Steady downward flow Steady uward flow
The Real of Hydrogeology In class exercise Stagnant Flow Plot hydraulic head and ressure vs. deth for (also indicate the hydrostatic line) Stagnant flow (no flow) Steady downward flow Steady uward flow
Thermodynamic Properties are Measurements p,t,v, u,h,s - measure directly -measure by change
 Thermodynamic Proerties are Measurements,T,, u,h,s - measure directly -measure by change s Tables T T Proerty Data Cure its Tables Correlation's, Boyles Law Tables c@tc limited hand calculations Equations
Thermodynamic Proerties are Measurements,T,, u,h,s - measure directly -measure by change s Tables T T Proerty Data Cure its Tables Correlation's, Boyles Law Tables c@tc limited hand calculations Equations
Answer Key THERMODYNAMICS TEST (a) 33. (d) 17. (c) 1. (a) 25. (a) 2. (b) 10. (d) 34. (b) 26. (c) 18. (d) 11. (c) 3. (d) 35. (c) 4. (d) 19.
 HERMODYNAMICS ES Answer Key. (a) 9. (a) 7. (c) 5. (a). (d). (b) 0. (d) 8. (d) 6. (c) 4. (b). (d). (c) 9. (b) 7. (c) 5. (c) 4. (d). (a) 0. (b) 8. (b) 6. (b) 5. (b). (d). (a) 9. (a) 7. (b) 6. (a) 4. (d).
HERMODYNAMICS ES Answer Key. (a) 9. (a) 7. (c) 5. (a). (d). (b) 0. (d) 8. (d) 6. (c) 4. (b). (d). (c) 9. (b) 7. (c) 5. (c) 4. (d). (a) 0. (b) 8. (b) 6. (b) 5. (b). (d). (a) 9. (a) 7. (b) 6. (a) 4. (d).
The average velocity of water in the tube and the Reynolds number are Hot R-134a
 hater 0:, 8, 4, 47, 50, 5, 55, 7, 75, 77, 8 and 85. 0- Refrigerant-4a is cooled by water a double-ie heat exchanger. he overall heat transfer coefficient is to be determed. Assumtions he thermal resistance
hater 0:, 8, 4, 47, 50, 5, 55, 7, 75, 77, 8 and 85. 0- Refrigerant-4a is cooled by water a double-ie heat exchanger. he overall heat transfer coefficient is to be determed. Assumtions he thermal resistance
374 Exergy Analysis. sys (u u 0 ) + P 0 (v v 0 ) T 0 (s s 0 ) where. e sys = u + ν 2 /2 + gz.
 374 Exergy Analysis The value of the exergy of the system depends only on its initial and final state, which is set by the conditions of the environment The term T 0 P S is always positive, and it does
374 Exergy Analysis The value of the exergy of the system depends only on its initial and final state, which is set by the conditions of the environment The term T 0 P S is always positive, and it does
NODIA AND COMPANY. GATE SOLVED PAPER Mechanical Engineering Thermodynamics. Copyright By NODIA & COMPANY
 No art of this ublication may be reroduced or distributed in any form or any means, electronic, mechanical, hotocoying, or otherwise without the rior ermission of the author. GAE SOLVED PAPER Mechanical
No art of this ublication may be reroduced or distributed in any form or any means, electronic, mechanical, hotocoying, or otherwise without the rior ermission of the author. GAE SOLVED PAPER Mechanical
Phase transitions. Lectures in Physical Chemistry 4. Tamás Turányi Institute of Chemistry, ELTE. Phases
 Phase transitions Lectures in Physical Cheistry 4 Taás Turányi Institute of Cheistry, ELTE Phases DEF a syste is hoogeneous, if () it does not contain arts searated by acroscoic surfaces, and () all intensive
Phase transitions Lectures in Physical Cheistry 4 Taás Turányi Institute of Cheistry, ELTE Phases DEF a syste is hoogeneous, if () it does not contain arts searated by acroscoic surfaces, and () all intensive
ME Thermodynamics I
 Homework - Week 01 HW-01 (25 points) Given: 5 Schematic of the solar cell/solar panel Find: 5 Identify the system and the heat/work interactions associated with it. Show the direction of the interactions.
Homework - Week 01 HW-01 (25 points) Given: 5 Schematic of the solar cell/solar panel Find: 5 Identify the system and the heat/work interactions associated with it. Show the direction of the interactions.
SPC 407 Sheet 6 - Solution Compressible Flow Fanno Flow
 SPC 407 Sheet 6 - Solution Comressible Flow Fanno Flow 1. What is the effect of friction on flow velocity in subsonic and suersonic Fanno flow? Friction increases the flow velocity in subsonic Fanno flow,
SPC 407 Sheet 6 - Solution Comressible Flow Fanno Flow 1. What is the effect of friction on flow velocity in subsonic and suersonic Fanno flow? Friction increases the flow velocity in subsonic Fanno flow,
8-4 P 2. = 12 kw. AIR T = const. Therefore, Q &
 8-4 8-4 Air i compreed teadily by a compreor. e air temperature i mataed contant by eat rejection to te urroundg. e rate o entropy cange o air i to be determed. Aumption i i a teady-low proce ce tere i
8-4 8-4 Air i compreed teadily by a compreor. e air temperature i mataed contant by eat rejection to te urroundg. e rate o entropy cange o air i to be determed. Aumption i i a teady-low proce ce tere i
5/6/ :41 PM. Chapter 6. Using Entropy. Dr. Mohammad Abuhaiba, PE
 Chapter 6 Using Entropy 1 2 Chapter Objective Means are introduced for analyzing systems from the 2 nd law perspective as they undergo processes that are not necessarily cycles. Objective: introduce entropy
Chapter 6 Using Entropy 1 2 Chapter Objective Means are introduced for analyzing systems from the 2 nd law perspective as they undergo processes that are not necessarily cycles. Objective: introduce entropy
Humidity parameters. Saturation (equilibrium) vapor pressure Condensation balances evaporation
 uidity paraeters Saturation (equilibriu) vapor pressure Condensation balances evaporation Miing ratio & specific huidity Mass ratio of water vapor and air and water content and wet air. Dew point & frost
uidity paraeters Saturation (equilibriu) vapor pressure Condensation balances evaporation Miing ratio & specific huidity Mass ratio of water vapor and air and water content and wet air. Dew point & frost
1 = The rate at which the entropy of the high temperature reservoir changes, according to the definition of the entropy, is
 8-7 8-9 A reverible eat um wit eciied reervoir temerature i conidered. e entroy cange o two reervoir i to be calculated and it i to be determed i ti eat um atiie te creae entroy rcile. Aumtion e eat um
8-7 8-9 A reverible eat um wit eciied reervoir temerature i conidered. e entroy cange o two reervoir i to be calculated and it i to be determed i ti eat um atiie te creae entroy rcile. Aumtion e eat um
[95/95] APPROACH FOR DESIGN LIMITS ANALYSIS IN VVER. Shishkov L., Tsyganov S. Russian Research Centre Kurchatov Institute Russian Federation, Moscow
![[95/95] APPROACH FOR DESIGN LIMITS ANALYSIS IN VVER. Shishkov L., Tsyganov S. Russian Research Centre Kurchatov Institute Russian Federation, Moscow [95/95] APPROACH FOR DESIGN LIMITS ANALYSIS IN VVER. Shishkov L., Tsyganov S. Russian Research Centre Kurchatov Institute Russian Federation, Moscow](/thumbs/81/82687654.jpg) [95/95] APPROACH FOR DESIGN LIMITS ANALYSIS IN VVER Shishkov L., Tsyganov S. Russian Research Centre Kurchatov Institute Russian Federation, Moscow ABSTRACT The aer discusses a well-known condition [95%/95%],
[95/95] APPROACH FOR DESIGN LIMITS ANALYSIS IN VVER Shishkov L., Tsyganov S. Russian Research Centre Kurchatov Institute Russian Federation, Moscow ABSTRACT The aer discusses a well-known condition [95%/95%],
MAE 320 HW 7B. 1e. For an isolated system, please circle the parameter which will change with time. (a) Total energy;
 MAE 320 HW 7B his comprehensive homework is due Monday, December 5 th, 206. Each problem is worth the points indicated. Copying of the solution from another is not acceptable. Multi-choice, multi-answer
MAE 320 HW 7B his comprehensive homework is due Monday, December 5 th, 206. Each problem is worth the points indicated. Copying of the solution from another is not acceptable. Multi-choice, multi-answer
Department of Mechanical Engineering ME 322 Mechanical Engineering Thermodynamics. Ideal Gas Mixtures II. Lecture 32
 Departent of Mechanical Engineering ME 322 Mechanical Engineering Therodnaics Ideal Gas Mixtures II Lecture 32 The Gibbs Phase Rule The nuber of independent, intensive properties required to fix the state
Departent of Mechanical Engineering ME 322 Mechanical Engineering Therodnaics Ideal Gas Mixtures II Lecture 32 The Gibbs Phase Rule The nuber of independent, intensive properties required to fix the state
Lecture 13. Heat Engines. Thermodynamic processes and entropy Thermodynamic cycles Extracting work from heat
 Lecture 3 Heat Engines hermodynamic rocesses and entroy hermodynamic cycles Extracting work from heat - How do we define engine efficiency? - Carnot cycle: the best ossible efficiency Reading for this
Lecture 3 Heat Engines hermodynamic rocesses and entroy hermodynamic cycles Extracting work from heat - How do we define engine efficiency? - Carnot cycle: the best ossible efficiency Reading for this
300 kpa 77 C. (d) If we neglect kinetic energy in the calculation of energy transport by mass
 6-6- Air flows steadily a ie at a secified state. The diameter of the ie, the rate of flow energy, and the rate of energy transort by mass are to be determed. Also, the error oled the determation of energy
6-6- Air flows steadily a ie at a secified state. The diameter of the ie, the rate of flow energy, and the rate of energy transort by mass are to be determed. Also, the error oled the determation of energy
ME Thermodynamics I
 HW-22 (25 points) Given: 1 A gas power cycle with initial properties as listed on the EFD. The compressor pressure ratio is 25:1 Find: 1 Sketch all the processes on a p-h diagram and calculate the enthalpy,
HW-22 (25 points) Given: 1 A gas power cycle with initial properties as listed on the EFD. The compressor pressure ratio is 25:1 Find: 1 Sketch all the processes on a p-h diagram and calculate the enthalpy,
Chapter 7. Dr Ali Jawarneh. Department of Mechanical Engineering Hashemite University
 Chapter 7 ENTROPY Dr Ali Jawarneh Department of Mechanical Engineering Hashemite University Objectives Apply the second law of thermodynamics to processes. Define a new property called entropy to quantify
Chapter 7 ENTROPY Dr Ali Jawarneh Department of Mechanical Engineering Hashemite University Objectives Apply the second law of thermodynamics to processes. Define a new property called entropy to quantify
The Schrödinger Equation and the Scale Principle
 Te Scrödinger Equation and te Scale Princile RODOLFO A. FRINO Jul 014 Electronics Engineer Degree fro te National Universit of Mar del Plata - Argentina rodolfo_frino@aoo.co.ar Earlier tis ear (Ma) I wrote
Te Scrödinger Equation and te Scale Princile RODOLFO A. FRINO Jul 014 Electronics Engineer Degree fro te National Universit of Mar del Plata - Argentina rodolfo_frino@aoo.co.ar Earlier tis ear (Ma) I wrote
first law of ThermodyNamics
 first law of ThermodyNamics First law of thermodynamics - Principle of conservation of energy - Energy can be neither created nor destroyed Basic statement When any closed system is taken through a cycle,
first law of ThermodyNamics First law of thermodynamics - Principle of conservation of energy - Energy can be neither created nor destroyed Basic statement When any closed system is taken through a cycle,
Lecture 13 Heat Engines
 Lecture 3 Heat Engines hermodynamic rocesses and entroy hermodynamic cycles Extracting work from heat - How do we define engine efficiency? - Carnot cycle: the best ossible efficiency Reading for this
Lecture 3 Heat Engines hermodynamic rocesses and entroy hermodynamic cycles Extracting work from heat - How do we define engine efficiency? - Carnot cycle: the best ossible efficiency Reading for this
CHAPTER 7 ENTROPY. Copyright Hany A. Al-Ansary and S. I. Abdel-Khalik (2014) 1
 CHAPTER 7 ENTROPY S. I. Abdel-Khalik (2014) 1 ENTROPY The Clausius Inequality The Clausius inequality states that for for all cycles, reversible or irreversible, engines or refrigerators: For internally-reversible
CHAPTER 7 ENTROPY S. I. Abdel-Khalik (2014) 1 ENTROPY The Clausius Inequality The Clausius inequality states that for for all cycles, reversible or irreversible, engines or refrigerators: For internally-reversible
Study of the circulation theory of the cooling system in vertical evaporative cooling generator
 358 Science in China: Series E Technological Sciences 006 Vol.49 No.3 358 364 DOI: 10.1007/s11431-006-0358-1 Study of the circulation theory of the cooling system in vertical evaorative cooling generator
358 Science in China: Series E Technological Sciences 006 Vol.49 No.3 358 364 DOI: 10.1007/s11431-006-0358-1 Study of the circulation theory of the cooling system in vertical evaorative cooling generator
F = U TS G = H TS. Availability, Irreversibility S K Mondal s Chapter 5. max. actual. univ. Ns =
 Availability, Irreversibility S K Mondal s Chater 5 I = W W 0 max ( S) = Δ univ actual 7. Irreversibility rate = I = rate of energy degradation = rate of energy loss (Wlost) = 0 S gen for all rocesses
Availability, Irreversibility S K Mondal s Chater 5 I = W W 0 max ( S) = Δ univ actual 7. Irreversibility rate = I = rate of energy degradation = rate of energy loss (Wlost) = 0 S gen for all rocesses
FINITE TIME THERMODYNAMIC MODELING AND ANALYSIS FOR AN IRREVERSIBLE ATKINSON CYCLE. By Yanlin GE, Lingen CHEN, and Fengrui SUN
 FINIE IME HERMODYNAMIC MODELING AND ANALYSIS FOR AN IRREVERSIBLE AKINSON CYCLE By Yanlin GE, Lingen CHEN, and Fengrui SUN Performance of an air-standard Atkinson cycle is analyzed by using finite-time
FINIE IME HERMODYNAMIC MODELING AND ANALYSIS FOR AN IRREVERSIBLE AKINSON CYCLE By Yanlin GE, Lingen CHEN, and Fengrui SUN Performance of an air-standard Atkinson cycle is analyzed by using finite-time
MAHALAKSHMI ENGINEERING COLLEGE
 MAHALAKSHMI ENGINEERING COLLEGE TIRUCHIRAPALLI-621213. Department: Mechanical Subject Code: ME2202 U N IT - 1 Semester: III Subject Name: ENGG. THERMODYNAMICS 1. 1 kg of gas at 1.1 bar, 27 o C is compressed
MAHALAKSHMI ENGINEERING COLLEGE TIRUCHIRAPALLI-621213. Department: Mechanical Subject Code: ME2202 U N IT - 1 Semester: III Subject Name: ENGG. THERMODYNAMICS 1. 1 kg of gas at 1.1 bar, 27 o C is compressed
the first derivative with respect to time is obtained by carefully applying the chain rule ( surf init ) T Tinit
 .005 ermal Fluids Engineering I Fall`08 roblem Set 8 Solutions roblem ( ( a e -D eat equation is α t x d erfc( u du π x, 4αt te first derivative wit respect to time is obtained by carefully applying te
.005 ermal Fluids Engineering I Fall`08 roblem Set 8 Solutions roblem ( ( a e -D eat equation is α t x d erfc( u du π x, 4αt te first derivative wit respect to time is obtained by carefully applying te
5. Dimensional Analysis. 5.1 Dimensions and units
 5. Diensional Analysis In engineering the alication of fluid echanics in designs ake uch of the use of eirical results fro a lot of exerients. This data is often difficult to resent in a readable for.
5. Diensional Analysis In engineering the alication of fluid echanics in designs ake uch of the use of eirical results fro a lot of exerients. This data is often difficult to resent in a readable for.
I. (20%) Answer the following True (T) or False (F). If false, explain why for full credit.
 I. (20%) Answer the following True (T) or False (F). If false, explain why for full credit. Both the Kelvin and Fahrenheit scales are absolute temperature scales. Specific volume, v, is an intensive property,
I. (20%) Answer the following True (T) or False (F). If false, explain why for full credit. Both the Kelvin and Fahrenheit scales are absolute temperature scales. Specific volume, v, is an intensive property,
Thermodynamics. Temperature Scales Fahrenheit: t F. Thermal Expansion and Strss. Temperature and Thermal Equilibrium
 herodynaics Fro the Greek theros eaning heat and dynais eaning power is a branch of physics that studies the effects of changes in teperature, pressure, and volue on physical systes at the acroscopic scale
herodynaics Fro the Greek theros eaning heat and dynais eaning power is a branch of physics that studies the effects of changes in teperature, pressure, and volue on physical systes at the acroscopic scale
Unit B-1: List of Subjects
 ES31 Energy Transfer Fundamentals Unit B: The First Law of Thermodynamics ROAD MAP... B-1: The Concept of Energy B-: Work Interactions B-3: First Law of Thermodynamics B-4: Heat Transfer Fundamentals Unit
ES31 Energy Transfer Fundamentals Unit B: The First Law of Thermodynamics ROAD MAP... B-1: The Concept of Energy B-: Work Interactions B-3: First Law of Thermodynamics B-4: Heat Transfer Fundamentals Unit
CHAPTER 5 MASS AND ENERGY ANALYSIS OF CONTROL VOLUMES
 Thermodynamics: An Engineering Approach 8th Edition in SI Units Yunus A. Çengel, Michael A. Boles McGraw-Hill, 2015 CHAPTER 5 MASS AND ENERGY ANALYSIS OF CONTROL VOLUMES Lecture slides by Dr. Fawzi Elfghi
Thermodynamics: An Engineering Approach 8th Edition in SI Units Yunus A. Çengel, Michael A. Boles McGraw-Hill, 2015 CHAPTER 5 MASS AND ENERGY ANALYSIS OF CONTROL VOLUMES Lecture slides by Dr. Fawzi Elfghi
Name: I have observed the honor code and have neither given nor received aid on this exam.
 ME 235 FINAL EXAM, ecember 16, 2011 K. Kurabayashi and. Siegel, ME ept. Exam Rules: Open Book and one page of notes allowed. There are 4 problems. Solve each problem on a separate page. Name: I have observed
ME 235 FINAL EXAM, ecember 16, 2011 K. Kurabayashi and. Siegel, ME ept. Exam Rules: Open Book and one page of notes allowed. There are 4 problems. Solve each problem on a separate page. Name: I have observed
Thermodynamics. Temperature Scales Fahrenheit: t F. Thermal Expansion and Stress. Temperature and Thermal Equilibrium
 herodynaics Fro the Greek theros eaning heat and dynais eaning power is a branch of physics that studies the effects of changes in teperature, pressure, and volue on physical systes at the acroscopic scale
herodynaics Fro the Greek theros eaning heat and dynais eaning power is a branch of physics that studies the effects of changes in teperature, pressure, and volue on physical systes at the acroscopic scale
Chapter 5. Mass and Energy Analysis of Control Volumes
 Chapter 5 Mass and Energy Analysis of Control Volumes Conservation Principles for Control volumes The conservation of mass and the conservation of energy principles for open systems (or control volumes)
Chapter 5 Mass and Energy Analysis of Control Volumes Conservation Principles for Control volumes The conservation of mass and the conservation of energy principles for open systems (or control volumes)
Dishwasher. Heater. Homework Solutions ME Thermodynamics I Spring HW-1 (25 points)
 HW-1 (25 points) (a) Given: 1 for writing given, find, EFD, etc., Schematic of a household piping system Find: Identify system and location on the system boundary where the system interacts with the environment
HW-1 (25 points) (a) Given: 1 for writing given, find, EFD, etc., Schematic of a household piping system Find: Identify system and location on the system boundary where the system interacts with the environment
Outline. Review for Final Exam. But, First a Word About Units. The Final. Transitions Between Phases. Density and Related Summary
 Review for Final Exa May 6, 008 Review for Final Exa Larry Caretto Mechanical Engineering 390 Fluid Mechanics May 6, 008 Outline Proerties, statics and anoeters Bernoulli and continuity equation Moentu
Review for Final Exa May 6, 008 Review for Final Exa Larry Caretto Mechanical Engineering 390 Fluid Mechanics May 6, 008 Outline Proerties, statics and anoeters Bernoulli and continuity equation Moentu
ENGR Thermodynamics
 ENGR 224 - hermodynamics #1 - Diagram for a Cascade VCR Cycle (21 ts) Baratuci Final 13-Jun-11 On a full sheet of paper, construct a complete Diagram for the cascade cascade vapor-compression refrigeration
ENGR 224 - hermodynamics #1 - Diagram for a Cascade VCR Cycle (21 ts) Baratuci Final 13-Jun-11 On a full sheet of paper, construct a complete Diagram for the cascade cascade vapor-compression refrigeration
Chapter 5. Mass and Energy Analysis of Control Volumes. by Asst. Prof. Dr.Woranee Paengjuntuek and Asst. Prof. Dr.Worarattana Pattaraprakorn
 Chapter 5 Mass and Energy Analysis of Control Volumes by Asst. Prof. Dr.Woranee Paengjuntuek and Asst. Prof. Dr.Worarattana Pattaraprakorn Reference: Cengel, Yunus A. and Michael A. Boles, Thermodynamics:
Chapter 5 Mass and Energy Analysis of Control Volumes by Asst. Prof. Dr.Woranee Paengjuntuek and Asst. Prof. Dr.Worarattana Pattaraprakorn Reference: Cengel, Yunus A. and Michael A. Boles, Thermodynamics:
 CHAPTER INTRODUCTION AND BASIC PRINCIPLES. (Tutorial). Determine if the following properties of the system are intensive or extensive properties: Property Intensive Extensive Volume Density Conductivity
CHAPTER INTRODUCTION AND BASIC PRINCIPLES. (Tutorial). Determine if the following properties of the system are intensive or extensive properties: Property Intensive Extensive Volume Density Conductivity
Phys102 First Major-112 Zero Version Coordinator: Wednesday, March 07, 2012 Page: 1
 Coordinator: Wednesday, March 07, 01 Page: 1 Q1. A transverse sinusoidal wave, travelling in the positive x direction along a string, has an aplitude of 0 c. The transverse position of an eleent of the
Coordinator: Wednesday, March 07, 01 Page: 1 Q1. A transverse sinusoidal wave, travelling in the positive x direction along a string, has an aplitude of 0 c. The transverse position of an eleent of the
In the next lecture...
 16 1 In the next lecture... Solve problems from Entropy Carnot cycle Exergy Second law efficiency 2 Problem 1 A heat engine receives reversibly 420 kj/cycle of heat from a source at 327 o C and rejects
16 1 In the next lecture... Solve problems from Entropy Carnot cycle Exergy Second law efficiency 2 Problem 1 A heat engine receives reversibly 420 kj/cycle of heat from a source at 327 o C and rejects
ECE309 INTRODUCTION TO THERMODYNAMICS & HEAT TRANSFER. 20 June 2005
 ECE309 INTRODUCTION TO THERMODYNAMICS & HEAT TRANSFER 20 June 2005 Midterm Examination R. Culham & M. Bahrami This is a 90 minute, closed-book examination. You are permitted to use one 8.5 in. 11 in. crib
ECE309 INTRODUCTION TO THERMODYNAMICS & HEAT TRANSFER 20 June 2005 Midterm Examination R. Culham & M. Bahrami This is a 90 minute, closed-book examination. You are permitted to use one 8.5 in. 11 in. crib
Chapter 7. Entropy. by Asst.Prof. Dr.Woranee Paengjuntuek and Asst. Prof. Dr.Worarattana Pattaraprakorn
 Chapter 7 Entropy by Asst.Prof. Dr.Woranee Paengjuntuek and Asst. Prof. Dr.Worarattana Pattaraprakorn Reference: Cengel, Yunus A. and Michael A. Boles, Thermodynamics: An Engineering Approach, 5th ed.,
Chapter 7 Entropy by Asst.Prof. Dr.Woranee Paengjuntuek and Asst. Prof. Dr.Worarattana Pattaraprakorn Reference: Cengel, Yunus A. and Michael A. Boles, Thermodynamics: An Engineering Approach, 5th ed.,
JJMIE Jordan Journal of Mechanical and Industrial Engineering
 JJMIE Jordan Journal of Mechanical and Industrial Engineering Volume, Number, Jun. 8 ISSN 995-6665 Pages 7-75 Efficiency of Atkinson Engine at Maximum Power Density using emerature Deendent Secific Heats
JJMIE Jordan Journal of Mechanical and Industrial Engineering Volume, Number, Jun. 8 ISSN 995-6665 Pages 7-75 Efficiency of Atkinson Engine at Maximum Power Density using emerature Deendent Secific Heats
HEAT, WORK, AND THE FIRST LAW OF THERMODYNAMICS
 HET, ORK, ND THE FIRST L OF THERMODYNMIS 8 EXERISES Section 8. The First Law of Thermodynamics 5. INTERPRET e identify the system as the water in the insulated container. The roblem involves calculating
HET, ORK, ND THE FIRST L OF THERMODYNMIS 8 EXERISES Section 8. The First Law of Thermodynamics 5. INTERPRET e identify the system as the water in the insulated container. The roblem involves calculating
ME Thermodynamics I. Lecture Notes and Example Problems
 ME 227.3 Thermodynamics I Lecture Notes and Example Problems James D. Bugg September 2018 Department of Mechanical Engineering Introduction Part I: Lecture Notes This part contains handout versions of
ME 227.3 Thermodynamics I Lecture Notes and Example Problems James D. Bugg September 2018 Department of Mechanical Engineering Introduction Part I: Lecture Notes This part contains handout versions of
Then the amount of water that flows through the pipe during a differential time interval dt is (1) 4
 5-98 Review Problems 5-45 A tank oen to te atmosere is itially filled wit. e tank discarges to te atmosere troug a long ie connected to a valve. e itial discarge velocity from te tank and te time required
5-98 Review Problems 5-45 A tank oen to te atmosere is itially filled wit. e tank discarges to te atmosere troug a long ie connected to a valve. e itial discarge velocity from te tank and te time required
7. Momentum balances Partly based on Chapter 7 of the De Nevers textbook (sections ).
 Lecture Notes CHE 31 Fluid Mechanics (Fall 010) 7 Moentu balances Partly based on Chater 7 of the De Neers tetbook (sections 71-73) Introduction Net to ass and energy oentu is an iortant quantity fluid
Lecture Notes CHE 31 Fluid Mechanics (Fall 010) 7 Moentu balances Partly based on Chater 7 of the De Neers tetbook (sections 71-73) Introduction Net to ass and energy oentu is an iortant quantity fluid
Developing Transfer Functions from Heat & Material Balances
 Colorado Sool of Mine CHEN43 Stirred ank Heater Develoing ranfer untion from Heat & Material Balane Examle ranfer untion Stirred ank Heater,,, A,,,,, We will develo te tranfer funtion for a tirred tank
Colorado Sool of Mine CHEN43 Stirred ank Heater Develoing ranfer untion from Heat & Material Balane Examle ranfer untion Stirred ank Heater,,, A,,,,, We will develo te tranfer funtion for a tirred tank
Thermodynamics I Chapter 6 Entropy
 hermodynamics I hater 6 Entroy Mohsin Mohd ies Fakulti Keuruteraan Mekanikal, Uniersiti eknologi Malaysia Entroy (Motiation) he referred direction imlied by the nd Law can be better understood and quantified
hermodynamics I hater 6 Entroy Mohsin Mohd ies Fakulti Keuruteraan Mekanikal, Uniersiti eknologi Malaysia Entroy (Motiation) he referred direction imlied by the nd Law can be better understood and quantified
Content. Entropy and principle of increasing entropy. Change of entropy in an ideal gas.
 Entropy Content Entropy and principle of increasing entropy. Change of entropy in an ideal gas. Entropy Entropy can be viewed as a measure of molecular disorder, or molecular randomness. As a system becomes
Entropy Content Entropy and principle of increasing entropy. Change of entropy in an ideal gas. Entropy Entropy can be viewed as a measure of molecular disorder, or molecular randomness. As a system becomes
Chapter 20: Exercises: 3, 7, 11, 22, 28, 34 EOC: 40, 43, 46, 58
 Chater 0: Exercises:, 7,,, 8, 4 EOC: 40, 4, 46, 8 E: A gasoline engine takes in.80 0 4 and delivers 800 of work er cycle. The heat is obtained by burning gasoline with a heat of combustion of 4.60 0 4.
Chater 0: Exercises:, 7,,, 8, 4 EOC: 40, 4, 46, 8 E: A gasoline engine takes in.80 0 4 and delivers 800 of work er cycle. The heat is obtained by burning gasoline with a heat of combustion of 4.60 0 4.
THERMODYNAMICS, FLUID AND PLANT PROCESSES. The tutorials are drawn from other subjects so the solutions are identified by the appropriate tutorial.
 THERMODYNAMICS, FLUID AND PLANT PROCESSES The tutorials are drawn from other subjects so the solutions are identified by the appropriate tutorial. THERMODYNAMICS TUTORIAL 2 THERMODYNAMIC PRINCIPLES SAE
THERMODYNAMICS, FLUID AND PLANT PROCESSES The tutorials are drawn from other subjects so the solutions are identified by the appropriate tutorial. THERMODYNAMICS TUTORIAL 2 THERMODYNAMIC PRINCIPLES SAE
ECE309 THERMODYNAMICS & HEAT TRANSFER MIDTERM EXAMINATION. Instructor: R. Culham. Name: Student ID Number:
 ECE309 THERMODYNAMICS & HEAT TRANSFER MIDTERM EXAMINATION June 19, 2015 2:30 pm - 4:30 pm Instructor: R. Culham Name: Student ID Number: Instructions 1. This is a 2 hour, closed-book examination. 2. Permitted
ECE309 THERMODYNAMICS & HEAT TRANSFER MIDTERM EXAMINATION June 19, 2015 2:30 pm - 4:30 pm Instructor: R. Culham Name: Student ID Number: Instructions 1. This is a 2 hour, closed-book examination. 2. Permitted
