The binding of positronium to lithium
|
|
|
- Austin Little
- 5 years ago
- Views:
Transcription
1 J. Phys. B: At. Mol. Opt. Phys. 31 (1998) L103 L107. Printed in the UK PII: S (98) LETTER TO THE EDITOR The binding of positronium to lithium G G Ryzhikh and J Mitroy Faculty of Science, Northern Territory University, Casuarina, NT, 0909, Australia Received 29 September 1997 Abstract. The wavefunction of the lithium positride (LiPs) ground state is calculated using an ab initio approach which uses a Gaussian basis with rij 2 correlation factors. The energy of the LiPs ground state, Hartree, has a total energy more negative than the sum of the positronium ( Hartree) and lithium ( Hartree) ground states. The ground state is therefore stable against dissociation into Li + Ps with a binding energy of Hartree. The use of positrons as a tool for spectroscopy, for example, positron annihilation spectroscopy in condensed matter physics, has gained widespread acceptance over the years [1, 2]. One of the questions that is raised by the use of positrons as a spectroscopic tool is of course the possibility that chemically stable systems containing a positron, or positronium, could be formed in the various targets under investigation [1, 2]. In spite of much work (and a lot of speculation), little hard information is known about the ability of a positron or positronium to form chemically stable systems in atoms, molecules or condensed matter. For instance, a recent review identified only five atoms and molecules (this excludes Ps and Ps 2 ) as permitting positronium binding with any degree of certainty [3]. These species are H, F, Cl, Br and OH. Most of the evidence for binding is based on calculation, and only for one species, namely H, could the result be classed as rigorous. While the most recent results for the other species come from high-quality calculations [4 6], they are based on model potentials for open-shell systems and do not have the same degree of certainty as the result for hydrogen which has been the subject of numerous high-quality calculations [7 11] and a recent experiment [12]. Recently, the e + Li species has been shown to be chemically stable with a binding energy of ɛ = Hartree [13]. This result, and the fact the PsH is chemically stable, immediately suggests that lithium positride, LiPs, would also be chemically stable. However, two earlier calculations on LiPs had failed to show that positronium could be bound to neutral lithium [14, 15]. Fortunately, we were ignorant of these results when deciding whether to investigate the LiPs species for possible binding. The condition for the LiPs ground state to be chemically stable is that the total energy of the LiPs ground state be more negative than the sum ( Hartree) of the Ps ground state energy ( Hartree) and the Li ground state energy ( Hartree) [13, 16]. The decay into Li + e + does not enter into the picture since the binding energy of the Li ground state [13, 17] is only Hartree. In this letter, a large variational calculation of the LiPs system is performed using the stochastic variational method (SVM) as implemented by Varga and Suzuki [18, 19]. The SVM was initially proposed as a method suitable for solving nuclear structure problems /98/ $19.50 c 1998 IOP Publishing Ltd L103
2 L104 involving a small number of particles [20, 21]. The SVM uses a Gaussian basis which has a number of features that make it possible to generate very accurate wavefunctions for few-body systems. First, the matrix elements of the interaction Hamiltonian can be calculated analytically, or at worst, reduced to a one-dimensional integral for any number of particles. Second, the Gaussian basis contains terms with rij 2 correlation factors. In addition, that part of the wavefunction concerned with the spatial coordinates maintains its functional form after any possible permutation of the particles. This is a very useful property for studying systems containing identical particles. One of the important aspects of any variational calculation is the optimization of the nonlinear parameters. In the SVM the exponents of the Gaussian basis are optimized using stochastic techniques. In recent years, the SVM and related methods have been used to perform high-precision variational calculations in atomic, mesoatomic, hypernuclear and multiquark systems [11, 22 24]. The program of Varga and Suzuki [18] was used for the present set of calculations. An initial series of calculations on a variety of related species were performed to validate the method. Results of our calculations for Li +, Li, Li and PsH are shown in table 1 and compared with other accurate nonrelativistic calculations. To simplify comparison with other results in table 1, all results were computed with an infinite nuclear mass. Our calculation for positronium hydride, PsH, agrees with the best previous calculation to within Hartree [11]. Results for Li underestimate the best calculation [17] by less than Hartree. Table 1. Non-relativistic energies (in Hartree) of various atomic systems compared with previous accurate results. In these calculations the nuclear mass has been assumed to be infinite. The number in parentheses refers to the total dimension of the Gaussian basis. Species Present SVM Other calculations Li (300) a PsH (400) b Li (400) c Li (600) d Li + e (800) LiPs (600) a Reference [25]. b Reference [11]. c Reference [16]. d Reference [17]. The convergence of energy of the LiPs system as a function of the dimension of the Gaussian basis is shown in table 2. In contradiction with previous works [14, 15] it is found that the ground state is chemically stable. It proved relatively easy to demonstrate that the system was stable since binding was achieved with less than 200 basis functions. The largest calculation included 600 basis functions, and resulted in a total energy of E = Hartree which is equivalent to a binding energy of ɛ = Hartree. When reference is made to the binding energy of the LiPs system it should be noted that the binding energy is relative to breakup into Li and Ps. It is clear from the convergence pattern shown in table 2 that the present energy does not represent the converged limit for the LiPs total energy. This is not surprising since there are five active particles, four electrons and one positron in the LiPs system. We suspect that the present estimate of the binding energy is accurate to about 10%.
3 L105 Table 2. Convergence of the LiPs energy (in Hartree) as a function of basis size. The third column shows the binding energy (in Hartree) relative to the Li + Ps threshold at Hartree. Basis size Total energy Binding energy Energy expectation values were also computed with the present optimized wavefunctions for a finite mass. The 7 Li nucleus has a mass of M = m e and for this species we obtained E = Hartree for Li, and E = Hartree for LiPs giving a binding energy of ɛ = Hartree. Finite-mass considerations have almost no effect on whether the system will be bound or not. Relativistic effects will also not affect whether binding is possible or not since one estimate of the relativistic energy correction for neutral Li is Hartree [26]. Although the best ab initio estimate of the LiPs binding energy, Hartree, is subject to uncertainties due to incomplete convergence of the LiPs energy, and less importantly subject to relativistic and finite mass corrections, the statement that the system is chemically stable will remain valid under any possible refinements of the model. While the LiPs ground state is chemically stable, it is not stable against electron positron annihilation. The dominant decay process for electron positron annihilation is into two γ - rays. The two-photon annihilation rate Ɣ 2γ was computed using the general formula [11], namely Ɣ 2γ = πα 4 c δ /a 0 = δ s 1. (1) In the above expression, δ is the expectation value of electron positron Dirac δ-function, / δ = δ(r i r 0 ) (2) i where r 0 is the positron coordinate and the r i (i = 1, 2, 3, 4) are the electron coordinates. The value obtained for the LiPs annihilation rate, Ɣ 2γ, was s 1. The annihilation rate for PsH was also computed for validation reasons and the value we obtained, Ɣ 2γ = s 1, was consistent with the best previous estimate [11], namely Ɣ 2γ = s 1. The probability density for finding an electron or positron as a function of the distance from the nucleus is depicted in figure 1. P (r) is the electron probability density and P + (r) is the positron probability density. These functions are normalized so that and 0 0 P (r) dr = 4 (3) P + (r) dr = 1. (4) The electron density exhibits the shell structure of the atom with the probability densities for the core and valence electrons having two distinct maxima. The peak of the probability
4 L106 Figure 1. The electron and positron probability densities, P ± (r), plotted as a function of r (in units of a 0 ). The electron density is represented by the full curve while the positron density is shown by the broken curve. density for the valence electrons occurs near 3.6 a 0. The peak of the probability density for the positron occurs near 5.2 a 0. That the positron orbits at a larger distance than the electrons is expected since the electrons are attracted to the nucleus, while the positron is repelled from the nucleus. The binding of positronium to lithium immediately raises the question as to whether it is possible to bind positronium to the heavier alkali atoms such as sodium and potassium. This question cannot be directly answered by SVM calculations since a full n-body calculation on a system containing 12 electrons and one positron (for sodium) is out of the question. The complexity of the problem needs to be reduced by using the frozen-core approximation before progress can be made. The authors would like to thank Dr D M Schrader for useful correspondence and Dr K Varga for the use of the SVM program. This work was supported by a research grant from the Australian Research Council. References [1] Schrader D M and Y C Jean (eds) 1988 Positron and Positronium Chemistry (Amsterdam: Elsevier) [2] Schrader D M and Wedlich D M 1989 From Atoms to Polymers: Isoelectronic Analogies ed J F Liebman and A Greenburg (New York: Wiley) [3] Schrader D M Bound states of positrons with atoms and molecules: theory Nucl. Instrum. Methods under review [4] Schrader D M, Yoshida T and Iguchi K 1993 J. Chem. Phys [5] Schrader D M, Yoshida T and Iguchi K 1992 Phys. Rev. Lett [6] Yoshida T, Miyako G, Jiang N and Schrader D M 1996 Phys. Rev. A [7] Ore A 1951 Phys. Rev [8] Lebeda C F and Schrader D M 1969 Phys. Rev [9] Clary D C 1976 J. Phys. B: At. Mol. Phys [10] Ho Y K 1986 Phys. Rev. A [11] Frolov A M and Smith VHJr1997 Phys. Rev. A [12] Schrader D M, Jacobsen F M, Frandsen N-P and Mikkelsen U 1992 Phys. Rev. Lett [13] Ryzhikh G G and Mitroy J 1997 Phys. Rev. Lett
5 L107 [14] Harju A, Barbiellini B and Nieminen R M 1996 Phys. Rev. A [15] Saito S L 1995 Chem. Phys. Lett [16] McKenzie D K and Drake GWF1991 Phys. Rev. A 44 R6973 [17] Fischer C F 1993 J. Phys. B: At. Mol. Opt. Phys [18] Varga K and Suzuki Y 1998 Comput. Phys. Commun [19] Varga K and Suzuki Y 1995 Phys. Rev. C [20] Kukulin V I 1975 Izv. Acad. Nauk [21] Kukulin V I and Krasnopolsky V M 1977 J. Phys. G: Nucl. Phys [22] Kopylov V A, Frolov A M and Kolesnikov N N 1984 Izv. Vuzov [23] Kolesnikov N N and Tarasov V I 1982 Sov. J. Nucl. Phys [24] Barbour I M and Ponting D K 1979 Nucl. Phys [25] Pekeris C L 1958 Phys. Rev [26] Davidson E R, Hagstrom S A, Chakaravorty S J, Umar V M and Froese Fischer C 1991 Phys. Rev. A
Positron binding to atomic zinc
 J. Phys. B: At. Mol. Opt. Phys. 32 (1999) 1375 1383. Printed in the UK PII: S0953-4075(99)99402-6 Positron binding to atomic zinc J Mitroy and G Ryzhikh Atomic and Molecular Physics Laboratories, Research
J. Phys. B: At. Mol. Opt. Phys. 32 (1999) 1375 1383. Printed in the UK PII: S0953-4075(99)99402-6 Positron binding to atomic zinc J Mitroy and G Ryzhikh Atomic and Molecular Physics Laboratories, Research
Structure of the LiPs and e + Be systems. 1 Introduction. J. Mitroy
 J. At. Mol. Sci. doi: 10.4208/jams.071510.072110a Vol. 1, No. 4, pp. 275-279 November 2010 Structure of the LiPs and e + Be systems J. Mitroy ARC Centre for Anti-matter Matter Studies and School of Engineering,
J. At. Mol. Sci. doi: 10.4208/jams.071510.072110a Vol. 1, No. 4, pp. 275-279 November 2010 Structure of the LiPs and e + Be systems J. Mitroy ARC Centre for Anti-matter Matter Studies and School of Engineering,
MANY-BODY THEORY CALCULATIONS OF POSITRON BINDING TO NEGATIVE IONS
 International Science Press, Many-body ISSN: 2229-3159 Theory Calculations of Positron Binding to Negative Ions RESEARCH ARTICLE MANY-BODY THEORY CALCULATIONS OF POSITRON BINDING TO NEGATIVE IONS J.A.
International Science Press, Many-body ISSN: 2229-3159 Theory Calculations of Positron Binding to Negative Ions RESEARCH ARTICLE MANY-BODY THEORY CALCULATIONS OF POSITRON BINDING TO NEGATIVE IONS J.A.
Positron chemistry by quantum Monte Carlo. II. Ground-state of positronpolar molecule complexes
 JOURAL OF CHEMICAL PHYSICS VOLUME 109, UMBER 5 1 AUGUST 1998 Positron chemistry by quantum Monte Carlo. II. Ground-state of positronpolar molecule complexes Dario Bressanini a) Istituto di Scienze Matematiche,
JOURAL OF CHEMICAL PHYSICS VOLUME 109, UMBER 5 1 AUGUST 1998 Positron chemistry by quantum Monte Carlo. II. Ground-state of positronpolar molecule complexes Dario Bressanini a) Istituto di Scienze Matematiche,
arxiv:physics/ v1 [physics.atom-ph] 8 Oct 2006
![arxiv:physics/ v1 [physics.atom-ph] 8 Oct 2006 arxiv:physics/ v1 [physics.atom-ph] 8 Oct 2006](/thumbs/90/103719618.jpg) The existence of a 2 P o excited state for the e + Ca system M.W.J.Bromley Department of Physics, San Diego State University, San Diego CA 9282, USA arxiv:physics/060045v [physics.atom-ph] 8 Oct 2006 J.Mitroy
The existence of a 2 P o excited state for the e + Ca system M.W.J.Bromley Department of Physics, San Diego State University, San Diego CA 9282, USA arxiv:physics/060045v [physics.atom-ph] 8 Oct 2006 J.Mitroy
Charge renormalization at the large-d limit for N-electron atoms and weakly bound systems
 Charge renormalization at the large-d limit for N-electron atoms and weakly bound systems S. Kais and R. Bleil Department of Chemistry, Purdue University, West Lafayette, Indiana 47907 Received 25 January
Charge renormalization at the large-d limit for N-electron atoms and weakly bound systems S. Kais and R. Bleil Department of Chemistry, Purdue University, West Lafayette, Indiana 47907 Received 25 January
Analysis of the ultrafast dynamics of the silver trimer upon photodetachment
 J. Phys. B: At. Mol. Opt. Phys. 29 (1996) L545 L549. Printed in the UK LETTER TO THE EDITOR Analysis of the ultrafast dynamics of the silver trimer upon photodetachment H O Jeschke, M E Garcia and K H
J. Phys. B: At. Mol. Opt. Phys. 29 (1996) L545 L549. Printed in the UK LETTER TO THE EDITOR Analysis of the ultrafast dynamics of the silver trimer upon photodetachment H O Jeschke, M E Garcia and K H
Positronium molecule
 Positronium molecule e + e - e - (0γ) 2γ (4γ) (1γ) 3γ (5γ) e + PSAS 2008 University of Windsor, July 26, 2008 Andrzej Czarnecki University of Alberta Outline Positronium, its ion, and the molecule 1951
Positronium molecule e + e - e - (0γ) 2γ (4γ) (1γ) 3γ (5γ) e + PSAS 2008 University of Windsor, July 26, 2008 Andrzej Czarnecki University of Alberta Outline Positronium, its ion, and the molecule 1951
CHEM6085: Density Functional Theory
 Lecture 5 CHEM6085: Density Functional Theory Orbital-free (or pure ) DFT C.-K. Skylaris 1 Consists of three terms The electronic Hamiltonian operator Electronic kinetic energy operator Electron-Electron
Lecture 5 CHEM6085: Density Functional Theory Orbital-free (or pure ) DFT C.-K. Skylaris 1 Consists of three terms The electronic Hamiltonian operator Electronic kinetic energy operator Electron-Electron
Structure and resonances of the e + -He(1s2s 3 S e ) system
 Structure and resonances of the e + -He(1s2s 3 S e ) system Zhenzhong Ren, 1, 2 Huili Han, 1, Tingyun Shi, 1 and J. Mitroy 3 1 State Key Laboratory of Magnetic Resonance and Atomic and Molecular Physics,
Structure and resonances of the e + -He(1s2s 3 S e ) system Zhenzhong Ren, 1, 2 Huili Han, 1, Tingyun Shi, 1 and J. Mitroy 3 1 State Key Laboratory of Magnetic Resonance and Atomic and Molecular Physics,
Relativistic corrections of energy terms
 Lectures 2-3 Hydrogen atom. Relativistic corrections of energy terms: relativistic mass correction, Darwin term, and spin-orbit term. Fine structure. Lamb shift. Hyperfine structure. Energy levels of the
Lectures 2-3 Hydrogen atom. Relativistic corrections of energy terms: relativistic mass correction, Darwin term, and spin-orbit term. Fine structure. Lamb shift. Hyperfine structure. Energy levels of the
Critical Behavior of Electron Impact Ionization of Atoms
 Critical Behavior of Electron Impact Ionization of Atoms IMAD LADADWA, 1,2 SABRE KAIS 1 1 Department of Chemistry, Purdue University, West Lafayette, Indiana 47907 2 Department of Physics, University of
Critical Behavior of Electron Impact Ionization of Atoms IMAD LADADWA, 1,2 SABRE KAIS 1 1 Department of Chemistry, Purdue University, West Lafayette, Indiana 47907 2 Department of Physics, University of
Chapter 4: Structure of the Atom Science
 Chapter 4: Structure of the Atom Science 1. What are canal rays? Canal rays are positively charged radiations that can pass through perforated cathode plate. These rays consist of positively charged particles
Chapter 4: Structure of the Atom Science 1. What are canal rays? Canal rays are positively charged radiations that can pass through perforated cathode plate. These rays consist of positively charged particles
Coulomb trajectory effects on electron-impact detachment of negative ions and on mutual neutralization cross sections
 J. Phys. B: At. Mol. Opt. Phys. 29 (1996) 6175 6184. Printed in the UK Coulomb trajectory effects on electron-impact detachment of negative ions and on mutual neutralization cross sections J T Lin, T F
J. Phys. B: At. Mol. Opt. Phys. 29 (1996) 6175 6184. Printed in the UK Coulomb trajectory effects on electron-impact detachment of negative ions and on mutual neutralization cross sections J T Lin, T F
Electron Configuration in Ionic Bonding Ionic Bonds Bonding in Metals
 Electron Configuration in Ionic Bonding Ionic Bonds Bonding in Metals Valence Electrons Electrons in the highest occupied energy level of an element s atoms Examples Mg: 1s 2 2s 2 2p 6 3s 2 2 valence e
Electron Configuration in Ionic Bonding Ionic Bonds Bonding in Metals Valence Electrons Electrons in the highest occupied energy level of an element s atoms Examples Mg: 1s 2 2s 2 2p 6 3s 2 2 valence e
(e, 2e) spectroscopy of atomic clusters
 J. Phys. B: At. Mol. Opt. Phys. 30 (1997) L703 L708. Printed in the UK PII: S0953-4075(97)86235-9 LETTER TO THE EDITOR (e, 2e) spectroscopy of atomic clusters S Keller, E Engel, H Ast and R M Dreizler
J. Phys. B: At. Mol. Opt. Phys. 30 (1997) L703 L708. Printed in the UK PII: S0953-4075(97)86235-9 LETTER TO THE EDITOR (e, 2e) spectroscopy of atomic clusters S Keller, E Engel, H Ast and R M Dreizler
Chem Unit: Part I Atoms and the EM Force
 Chem Unit: Part I Atoms and the EM Force I. Electric Charge & the EM Force of Attraction and Repulsion Some of the fundamental particles that make up the STUFF in this universe have a property called electric
Chem Unit: Part I Atoms and the EM Force I. Electric Charge & the EM Force of Attraction and Repulsion Some of the fundamental particles that make up the STUFF in this universe have a property called electric
The Shell Model This activity is modified from Chemistry: A Guided Inquiry (3/e) by R.S. Moog and J.J. Farrell, Wiley, 2006.
 The Shell Model This activity is modified from Chemistry: A Guided Inquiry (3/e) by R.S. Moog and J.J. Farrell, Wiley, 2006. The first ionization energy (IE 1 ) is the minimum energy required to remove
The Shell Model This activity is modified from Chemistry: A Guided Inquiry (3/e) by R.S. Moog and J.J. Farrell, Wiley, 2006. The first ionization energy (IE 1 ) is the minimum energy required to remove
A quasifree model for the absorption effects in positron scattering by atoms
 J. Phys. B: At. Mol. Opt. Phys. 29 (1996) L127 L133. Printed in the UK LETTER TO THE EDITOR A quasifree model for the absorption effects in positron scattering by atoms David D Reid and J M Wadehra Department
J. Phys. B: At. Mol. Opt. Phys. 29 (1996) L127 L133. Printed in the UK LETTER TO THE EDITOR A quasifree model for the absorption effects in positron scattering by atoms David D Reid and J M Wadehra Department
Chapter 30 Questions 8. Quoting from section 30-3, K radioactivity was found in every case to be unaffected
 Physics 111 Fall 007 Homework Solutions Week #10 Giancoli Chapter 30 Chapter 30 Questions 8. Quoting from section 30-3, K radioactivity was found in every case to be unaffected by the strongest physical
Physics 111 Fall 007 Homework Solutions Week #10 Giancoli Chapter 30 Chapter 30 Questions 8. Quoting from section 30-3, K radioactivity was found in every case to be unaffected by the strongest physical
Forbidden Electric Dipole Transitions in the Hydrogen Molecular Ion First Estimates
 Bulg. J. Phys. 44 (2017) 76 83 Forbidden Electric Dipole Transitions in the Hydrogen Molecular Ion First Estimates P. Danev Institute for Nuclear Research and Nuclear Energy, Bulgarian Academy of Sciences,
Bulg. J. Phys. 44 (2017) 76 83 Forbidden Electric Dipole Transitions in the Hydrogen Molecular Ion First Estimates P. Danev Institute for Nuclear Research and Nuclear Energy, Bulgarian Academy of Sciences,
Total and state-selective electron capture cross sections for C 3+ + H collisions
 J. Phys. B: At. Mol. Opt. Phys. 32 (1999) 5271 5278. Printed in the UK PII: S0953-4075(99)06231-8 Total and state-selective electron capture cross sections for C 3+ + H collisions H C Tseng and C D Lin
J. Phys. B: At. Mol. Opt. Phys. 32 (1999) 5271 5278. Printed in the UK PII: S0953-4075(99)06231-8 Total and state-selective electron capture cross sections for C 3+ + H collisions H C Tseng and C D Lin
Use of intermediate coupling relationships to test measured branching fraction data
 J. Phys. B: At. Mol. Opt. Phys. 31 (1998) L769 L774. Printed in the UK PII: S0953-4075(98)95845-X LETTER TO THE EDITOR Use of intermediate coupling relationships to test measured branching fraction data
J. Phys. B: At. Mol. Opt. Phys. 31 (1998) L769 L774. Printed in the UK PII: S0953-4075(98)95845-X LETTER TO THE EDITOR Use of intermediate coupling relationships to test measured branching fraction data
U N I T T E S T P R A C T I C E
 South Pasadena AP Chemistry Name 8 Atomic Theory Period Date U N I T T E S T P R A C T I C E Part 1 Multiple Choice You should allocate 25 minutes to finish this portion of the test. No calculator should
South Pasadena AP Chemistry Name 8 Atomic Theory Period Date U N I T T E S T P R A C T I C E Part 1 Multiple Choice You should allocate 25 minutes to finish this portion of the test. No calculator should
Open problems about many-body Dirac operators
 Open problems about many-body Dirac operators Jan Dereziński Dep. Math. Meth. in Phys., Faculty of Physics, University of Warsaw, Hoża 74, 00-682 Warszawa, Poland email: Jan.Derezinski@fuw.edu.pl October
Open problems about many-body Dirac operators Jan Dereziński Dep. Math. Meth. in Phys., Faculty of Physics, University of Warsaw, Hoża 74, 00-682 Warszawa, Poland email: Jan.Derezinski@fuw.edu.pl October
Chapter 6 The Atom Study Guide
 Chapter 6 The Atom Study Guide Read pages 118-125 Look at all words in bold or colored print Look at the paragraph summaries on the sides Section 6.1 Fundamental Particles and Forces Vocabulary Definition
Chapter 6 The Atom Study Guide Read pages 118-125 Look at all words in bold or colored print Look at the paragraph summaries on the sides Section 6.1 Fundamental Particles and Forces Vocabulary Definition
ATOMIC STRUCTURE. Atomic Structure. Atomic orbitals and their energies (a) Hydrogenic radial wavefunctions
 ATOMIC STRUCTURE Atomic orbitals and their energies (a) Hydrogenic radial wavefunctions Bundet Boekfa Chem Div, Fac Lib Arts & Sci Kasetsart University Kamphaeng Saen Campus 1 2 Atomic orbitals and their
ATOMIC STRUCTURE Atomic orbitals and their energies (a) Hydrogenic radial wavefunctions Bundet Boekfa Chem Div, Fac Lib Arts & Sci Kasetsart University Kamphaeng Saen Campus 1 2 Atomic orbitals and their
ELASTIC POSITRON SCATTERING FROM ZINC AND CADMIUM IN THE RELATIVISTIC POLARIZED ORBITAL APPROXIMATION
 Vol. 84 (1993) ACTA PHYSICA POLONICA A No. 6 ELASTIC POSITRON SCATTERING FROM ZINC AND CADMIUM IN THE RELATIVISTIC POLARIZED ORBITAL APPROXIMATION RADOSLAW SZMYTKOWSKI Institute of Theoretical Physics
Vol. 84 (1993) ACTA PHYSICA POLONICA A No. 6 ELASTIC POSITRON SCATTERING FROM ZINC AND CADMIUM IN THE RELATIVISTIC POLARIZED ORBITAL APPROXIMATION RADOSLAW SZMYTKOWSKI Institute of Theoretical Physics
CHAPTER 2 MANY-ELECTRON ATOMS AND THE PERIODIC TABLE
 CHAPTER MANY-ELECTRON ATOMS AND THE PERIODIC TABLE.1 (a) is incorrect because the magnetic quantum number ml can have only whole number values. (c) is incorrect because the maximum value of the angular
CHAPTER MANY-ELECTRON ATOMS AND THE PERIODIC TABLE.1 (a) is incorrect because the magnetic quantum number ml can have only whole number values. (c) is incorrect because the maximum value of the angular
Recoil ion and electronic angular asymmetry parameters for photo double ionization of helium at 99 ev
 J. Phys. B: At. Mol. Opt. Phys. 3 (997) L649 L655. Printed in the UK PII: S953-475(97)85842-7 LETTER TO THE EDITOR Recoil ion and electronic angular asymmetry parameters for photo double ionization of
J. Phys. B: At. Mol. Opt. Phys. 3 (997) L649 L655. Printed in the UK PII: S953-475(97)85842-7 LETTER TO THE EDITOR Recoil ion and electronic angular asymmetry parameters for photo double ionization of
Calculation of the Isotope Shifts on 5S 1/2 4D 3/2,5/2 Transitions of 87,88 Sr +
 Commun. Theor. Phys. (Beijing, China) 37 (22) pp 76 7 c International Academic Publishers Vol. 37, No. 6, June 5, 22 Calculation of the Isotope Shifts on 5S /2 4D 3/2,5/2 Transitions of 87,88 Sr + LI Yong,,2
Commun. Theor. Phys. (Beijing, China) 37 (22) pp 76 7 c International Academic Publishers Vol. 37, No. 6, June 5, 22 Calculation of the Isotope Shifts on 5S /2 4D 3/2,5/2 Transitions of 87,88 Sr + LI Yong,,2
All-electron quantum Monte Carlo calculations for the noble gas atoms He to Xe
 All-electron quantum Monte Carlo calculations for the noble gas atoms He to Xe A. Ma, N. D. Drummond, M. D. Towler, and R. J. Needs Theory of Condensed Matter Group, Cavendish Laboratory, University of
All-electron quantum Monte Carlo calculations for the noble gas atoms He to Xe A. Ma, N. D. Drummond, M. D. Towler, and R. J. Needs Theory of Condensed Matter Group, Cavendish Laboratory, University of
CHEM 1305: Introductory Chemistry
 CHEM 1305: Introductory Chemistry The Periodic Table From Chapter 5 Textbook Introductory Chemistry: Concepts and Critical Thinking Seventh Edition by Charles H. Corwin Classification of Elements By 1870,
CHEM 1305: Introductory Chemistry The Periodic Table From Chapter 5 Textbook Introductory Chemistry: Concepts and Critical Thinking Seventh Edition by Charles H. Corwin Classification of Elements By 1870,
AP Biology. Why are we studying chemistry? Chapter 2. The Chemical Context of Life. The Basics. The World of Elements.
 Chapter 2. The Chemical Context of Life Why are we studying chemistry? Biology has chemistry at its foundation The Basics The World of Elements Everything is made of matter Matter is made of atoms Atoms
Chapter 2. The Chemical Context of Life Why are we studying chemistry? Biology has chemistry at its foundation The Basics The World of Elements Everything is made of matter Matter is made of atoms Atoms
COLLEGE PHYSICS. Chapter 30 ATOMIC PHYSICS
 COLLEGE PHYSICS Chapter 30 ATOMIC PHYSICS Matter Waves: The de Broglie Hypothesis The momentum of a photon is given by: The de Broglie hypothesis is that particles also have wavelengths, given by: Matter
COLLEGE PHYSICS Chapter 30 ATOMIC PHYSICS Matter Waves: The de Broglie Hypothesis The momentum of a photon is given by: The de Broglie hypothesis is that particles also have wavelengths, given by: Matter
Ab-initio Calculations for Forbidden M1/E2 Decay Rates in Ti XIX ion
 EJTP 3, No. 11 (2006) 111 122 Electronic Journal of Theoretical Physics Ab-initio Calculations for Forbidden M1/E2 Decay Rates in Ti XIX ion A. Farrag Physics Department,Faculty of Science, Cairo University,
EJTP 3, No. 11 (2006) 111 122 Electronic Journal of Theoretical Physics Ab-initio Calculations for Forbidden M1/E2 Decay Rates in Ti XIX ion A. Farrag Physics Department,Faculty of Science, Cairo University,
FACTS WHY? C. Alpha Decay Probability 1. Energetics: Q α positive for all A>140 nuclei
 C. Alpha Decay Probability 1. Energetics: Q α positive for all A>140 nuclei 2. Range of Measured Half-Lives (~10 44 ) 10 16 y > t 1/2 > 10 21 s 3. Why α? a. Proton & Neutron Emission: Q p, Q n are negative
C. Alpha Decay Probability 1. Energetics: Q α positive for all A>140 nuclei 2. Range of Measured Half-Lives (~10 44 ) 10 16 y > t 1/2 > 10 21 s 3. Why α? a. Proton & Neutron Emission: Q p, Q n are negative
Physics 107 Final Exam May 6, Your Name: 1. Questions
 Physics 107 Final Exam May 6, 1996 Your Name: 1. Questions 1. 9. 17. 5.. 10. 18. 6. 3. 11. 19. 7. 4. 1. 0. 8. 5. 13. 1. 9. 6. 14.. 30. 7. 15. 3. 8. 16. 4.. Problems 1. 4. 7. 10. 13.. 5. 8. 11. 14. 3. 6.
Physics 107 Final Exam May 6, 1996 Your Name: 1. Questions 1. 9. 17. 5.. 10. 18. 6. 3. 11. 19. 7. 4. 1. 0. 8. 5. 13. 1. 9. 6. 14.. 30. 7. 15. 3. 8. 16. 4.. Problems 1. 4. 7. 10. 13.. 5. 8. 11. 14. 3. 6.
An ab initio treatment of the X-ray emission spectra of the HCl molecule
 Home Search Collections Journals About Contact us My IOPscience An ab initio treatment of the X-ray emission spectra of the HCl molecule This article has been downloaded from IOPscience. Please scroll
Home Search Collections Journals About Contact us My IOPscience An ab initio treatment of the X-ray emission spectra of the HCl molecule This article has been downloaded from IOPscience. Please scroll
Particle Behavior of Light 1. Calculate the energy of a photon, mole of photons 2. Find binding energy of an electron (know KE) 3. What is a quanta?
 Properties of Electromagnetic Radiation 1. What is spectroscopy, a continuous spectrum, a line spectrum, differences and similarities 2. Relationship of wavelength to frequency, relationship of E to λ
Properties of Electromagnetic Radiation 1. What is spectroscopy, a continuous spectrum, a line spectrum, differences and similarities 2. Relationship of wavelength to frequency, relationship of E to λ
 TitleAnalytical Expression of the Hartre Author(s) Mukoyama, Takeshi; Yasui, Jun Citation Bulletin of the Institute for Chemi University (1992), 70(4): 385-391 Issue Date 1992-11-30 URL http://hdl.handle.net/2433/77474
TitleAnalytical Expression of the Hartre Author(s) Mukoyama, Takeshi; Yasui, Jun Citation Bulletin of the Institute for Chemi University (1992), 70(4): 385-391 Issue Date 1992-11-30 URL http://hdl.handle.net/2433/77474
Chapter 2: The Structure of the Atom and the Periodic Table
 Chapter 2: The Structure of the Atom and the Periodic Table 1. What are the three primary particles found in an atom? A) neutron, positron, and electron B) electron, neutron, and proton C) electron, proton,
Chapter 2: The Structure of the Atom and the Periodic Table 1. What are the three primary particles found in an atom? A) neutron, positron, and electron B) electron, neutron, and proton C) electron, proton,
Higher-order C n dispersion coefficients for hydrogen
 Higher-order C n dispersion coefficients for hydrogen J Mitroy* and M W J Bromley Faculty of Technology, Charles Darwin University, Darwin NT 0909, Australia Received 2 November 2004; published 11 March
Higher-order C n dispersion coefficients for hydrogen J Mitroy* and M W J Bromley Faculty of Technology, Charles Darwin University, Darwin NT 0909, Australia Received 2 November 2004; published 11 March
The Shell Model (II)
 22 ChemActivity 5 The Shell Model (II) Model 1: Valence Electrons, Inner-Shell Electrons, and Core Charge. The electrons in the outermost shell of an atom are referred to as valence electrons. Electrons
22 ChemActivity 5 The Shell Model (II) Model 1: Valence Electrons, Inner-Shell Electrons, and Core Charge. The electrons in the outermost shell of an atom are referred to as valence electrons. Electrons
Ground-state correlations in magnesium: electron momentum spectroscopy of the valence region
 J. Phys. B: At. Mol. Opt. Phys. 21 (1988) 4239-4247. Printed in the UK Ground-state correlations in magnesium: electron momentum spectroscopy of the valence region R Pascual, J Mitroyt, L Frost$ and E
J. Phys. B: At. Mol. Opt. Phys. 21 (1988) 4239-4247. Printed in the UK Ground-state correlations in magnesium: electron momentum spectroscopy of the valence region R Pascual, J Mitroyt, L Frost$ and E
DENSITY FUNCTIONAL THEORY FOR NON-THEORISTS JOHN P. PERDEW DEPARTMENTS OF PHYSICS AND CHEMISTRY TEMPLE UNIVERSITY
 DENSITY FUNCTIONAL THEORY FOR NON-THEORISTS JOHN P. PERDEW DEPARTMENTS OF PHYSICS AND CHEMISTRY TEMPLE UNIVERSITY A TUTORIAL FOR PHYSICAL SCIENTISTS WHO MAY OR MAY NOT HATE EQUATIONS AND PROOFS REFERENCES
DENSITY FUNCTIONAL THEORY FOR NON-THEORISTS JOHN P. PERDEW DEPARTMENTS OF PHYSICS AND CHEMISTRY TEMPLE UNIVERSITY A TUTORIAL FOR PHYSICAL SCIENTISTS WHO MAY OR MAY NOT HATE EQUATIONS AND PROOFS REFERENCES
PHYS3113, 3d year Statistical Mechanics Tutorial problems. Tutorial 1, Microcanonical, Canonical and Grand Canonical Distributions
 1 PHYS3113, 3d year Statistical Mechanics Tutorial problems Tutorial 1, Microcanonical, Canonical and Grand Canonical Distributions Problem 1 The macrostate probability in an ensemble of N spins 1/2 is
1 PHYS3113, 3d year Statistical Mechanics Tutorial problems Tutorial 1, Microcanonical, Canonical and Grand Canonical Distributions Problem 1 The macrostate probability in an ensemble of N spins 1/2 is
Stability conditions for hydrogen-antihydrogen-like quasimolecules
 Purdue University Purdue e-pubs Birck and NCN Publications Birck Nanotechnology Center May 8 Stability conditions for hydrogen-antihydrogen-like quasimolecules Alejandro Ferrón Universidad Nacional de
Purdue University Purdue e-pubs Birck and NCN Publications Birck Nanotechnology Center May 8 Stability conditions for hydrogen-antihydrogen-like quasimolecules Alejandro Ferrón Universidad Nacional de
Bohr-Coster diagrams for multiply ionized states of light elements in the range Z--10 to 20
 Pramhn.a-J. Phys., Vol. 29, No. 3, September 1987, pp. 253-260. Printed in India. Bohr-Coster diagrams for multiply ionized states of light elements in the range Z--10 to 20 LAKSH'MI NATARAJAN, A V TANKHIWALE
Pramhn.a-J. Phys., Vol. 29, No. 3, September 1987, pp. 253-260. Printed in India. Bohr-Coster diagrams for multiply ionized states of light elements in the range Z--10 to 20 LAKSH'MI NATARAJAN, A V TANKHIWALE
CHAPTER 2 Atoms and the Periodic Table
 CHAPTER 2 and the Periodic Table General, Organic, & Biological Chemistry Janice Gorzynski Smith CHAPTER 2: & the Periodic Table Learning Objectives:! Elemental Symbols! Metals vs Nonmetals vs Metalloids
CHAPTER 2 and the Periodic Table General, Organic, & Biological Chemistry Janice Gorzynski Smith CHAPTER 2: & the Periodic Table Learning Objectives:! Elemental Symbols! Metals vs Nonmetals vs Metalloids
The Nucleus. PHY 3101 D. Acosta
 The Nucleus PHY 30 D. Acosta Rutherford Scattering Experiments by Geiger & Marsden in 909 /5/005 PHY 30 -- D. Acosta Rutherford Model of the Atom Conclusion: the atom contains a positive nucleus < 0 fm
The Nucleus PHY 30 D. Acosta Rutherford Scattering Experiments by Geiger & Marsden in 909 /5/005 PHY 30 -- D. Acosta Rutherford Model of the Atom Conclusion: the atom contains a positive nucleus < 0 fm
Atoms with More than One Electron
 Fun with the Periodic Table Activity 6 Atoms with More than One Electron GOALS In this activity you will: View the spectra of various materials. Graphically analyze patterns in the amounts of energy required
Fun with the Periodic Table Activity 6 Atoms with More than One Electron GOALS In this activity you will: View the spectra of various materials. Graphically analyze patterns in the amounts of energy required
Phase transitions and the stability of atomic and molecular ions
 International Journal of Mass Spectrometry 182/183 (1999) 23 29 Phase transitions and the stability of atomic and molecular ions Sabre Kais a, *, Juan Pablo Neirotti a, Pablo Serra b a Department of Chemistry,
International Journal of Mass Spectrometry 182/183 (1999) 23 29 Phase transitions and the stability of atomic and molecular ions Sabre Kais a, *, Juan Pablo Neirotti a, Pablo Serra b a Department of Chemistry,
arxiv:physics/ v1 [physics.atom-ph] 4 Nov 2004
![arxiv:physics/ v1 [physics.atom-ph] 4 Nov 2004 arxiv:physics/ v1 [physics.atom-ph] 4 Nov 2004](/thumbs/88/117718694.jpg) 1 arxiv:physics/0411043v1 [physics.atom-ph] 4 Nov 2004 S tudies of Lanthanides 6s Ionization Energy G. Gaigalas, Z. Rudzikas and T. Žalandauskas, Vilnius University Research Institute of Theoretical Physics
1 arxiv:physics/0411043v1 [physics.atom-ph] 4 Nov 2004 S tudies of Lanthanides 6s Ionization Energy G. Gaigalas, Z. Rudzikas and T. Žalandauskas, Vilnius University Research Institute of Theoretical Physics
The boundaries of an atom are fuzzy, and an atom s radius can vary under different conditions.
 Atomic Radii The boundaries of an atom are fuzzy, and an atom s radius can vary under different conditions. To compare different atomic radii, they must be measured under specified conditions. Atomic radius
Atomic Radii The boundaries of an atom are fuzzy, and an atom s radius can vary under different conditions. To compare different atomic radii, they must be measured under specified conditions. Atomic radius
2.Ionization Energies
 2.Ionization Energies Ionization energy, IE, is the energy required to remove one electron from an atom or ion; an endothermic process that is, A A + + 1 e - H = +ve The energy, in kj mol -1, required
2.Ionization Energies Ionization energy, IE, is the energy required to remove one electron from an atom or ion; an endothermic process that is, A A + + 1 e - H = +ve The energy, in kj mol -1, required
Beyond the Hartree-Fock Approximation: Configuration Interaction
 Beyond the Hartree-Fock Approximation: Configuration Interaction The Hartree-Fock (HF) method uses a single determinant (single electronic configuration) description of the electronic wavefunction. For
Beyond the Hartree-Fock Approximation: Configuration Interaction The Hartree-Fock (HF) method uses a single determinant (single electronic configuration) description of the electronic wavefunction. For
Chapter 2: Atoms and the Periodic Table
 1. Which element is a nonmetal? A) K B) Co C) Br D) Al Ans: C Difficulty: Easy 2. Which element is a metal? A) Li B) Si C) Cl D) Ar E) More than one of the elements above are metals. 3. Which element is
1. Which element is a nonmetal? A) K B) Co C) Br D) Al Ans: C Difficulty: Easy 2. Which element is a metal? A) Li B) Si C) Cl D) Ar E) More than one of the elements above are metals. 3. Which element is
Dominance of short-range correlations in photoejection-induced excitation processes
 J. Phys. B: At. Mol. Opt. Phys. 30 (1997) L641 L647. Printed in the UK PII: S0953-4075(97)86594-7 LETTER TO THE EDITOR Dominance of short-range correlations in photoejection-induced excitation processes
J. Phys. B: At. Mol. Opt. Phys. 30 (1997) L641 L647. Printed in the UK PII: S0953-4075(97)86594-7 LETTER TO THE EDITOR Dominance of short-range correlations in photoejection-induced excitation processes
Atomic Structure. 1. For a hydrogen atom which electron transition requires the largest amount of energy?
 Atomic Structure 1. For a hydrogen atom which electron transition requires the largest amount of energy? A. n = 4 to n = 10 B. n = 3 to n = 2 C. n = 3 to n = 4 D. n = 1 to n = 3 E. n = 2 to n = 4 2. Which
Atomic Structure 1. For a hydrogen atom which electron transition requires the largest amount of energy? A. n = 4 to n = 10 B. n = 3 to n = 2 C. n = 3 to n = 4 D. n = 1 to n = 3 E. n = 2 to n = 4 2. Which
Chapter 6 Test. name. The Structure of Matter
 Chapter 6 Test The Structure of Matter MULTIPLE CHOICE. Write the letter of the term or phrase that best completes each statement or best answers each question on the answer sheet provided. 1. A compound
Chapter 6 Test The Structure of Matter MULTIPLE CHOICE. Write the letter of the term or phrase that best completes each statement or best answers each question on the answer sheet provided. 1. A compound
Solving the Schrödinger equation for the Sherrington Kirkpatrick model in a transverse field
 J. Phys. A: Math. Gen. 30 (1997) L41 L47. Printed in the UK PII: S0305-4470(97)79383-1 LETTER TO THE EDITOR Solving the Schrödinger equation for the Sherrington Kirkpatrick model in a transverse field
J. Phys. A: Math. Gen. 30 (1997) L41 L47. Printed in the UK PII: S0305-4470(97)79383-1 LETTER TO THE EDITOR Solving the Schrödinger equation for the Sherrington Kirkpatrick model in a transverse field
ATOMIC STRUCTURE, ELECTRONS, AND PERIODICITY
 ATOMIC STRUCTURE, ELECTRONS, AND PERIODICITY All matter is made of atoms. There are a limited number of types of atoms; these are the elements. (EU 1.A) Development of Atomic Theory Atoms are so small
ATOMIC STRUCTURE, ELECTRONS, AND PERIODICITY All matter is made of atoms. There are a limited number of types of atoms; these are the elements. (EU 1.A) Development of Atomic Theory Atoms are so small
Atomic Theory and Periodic Trends Practice AP Chemistry Questions
 AP Chemistry/1516 Atomic Theory and Periodic Trends Practice AP Chemistry Questions 1. 2007 B, question #2 Answer the following problems about gases. (b) A major line in the emission spectrum of neon corresponds
AP Chemistry/1516 Atomic Theory and Periodic Trends Practice AP Chemistry Questions 1. 2007 B, question #2 Answer the following problems about gases. (b) A major line in the emission spectrum of neon corresponds
ψ s a ˆn a s b ˆn b ψ Hint: Because the state is spherically symmetric the answer can depend only on the angle between the two directions.
 1. Quantum Mechanics (Fall 2004) Two spin-half particles are in a state with total spin zero. Let ˆn a and ˆn b be unit vectors in two arbitrary directions. Calculate the expectation value of the product
1. Quantum Mechanics (Fall 2004) Two spin-half particles are in a state with total spin zero. Let ˆn a and ˆn b be unit vectors in two arbitrary directions. Calculate the expectation value of the product
Midterm Exam I. CHEM 181: Introduction to Chemical Principles September 24, 2015 Key
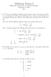 Midterm Exam I CHEM 8: Introduction to Chemical Principles September 24, 205 Key. A Li 2+ ion in an unknown, highly excited electronic state, first emits a photon at a wavelength of 4.36 µm, and following
Midterm Exam I CHEM 8: Introduction to Chemical Principles September 24, 205 Key. A Li 2+ ion in an unknown, highly excited electronic state, first emits a photon at a wavelength of 4.36 µm, and following
Highly accurate evaluation of the few-body auxiliary functions and four-body integrals
 INSTITUTE OF PHYSICSPUBLISHING JOURNAL OFPHYSICSB: ATOMIC, MOLECULAR AND OPTICAL PHYSICS J. Phys. B: At. Mol. Opt. Phys. 36 (2003) 1857 1867 PII: S0953-4075(03)58918-0 Highly accurate evaluation of the
INSTITUTE OF PHYSICSPUBLISHING JOURNAL OFPHYSICSB: ATOMIC, MOLECULAR AND OPTICAL PHYSICS J. Phys. B: At. Mol. Opt. Phys. 36 (2003) 1857 1867 PII: S0953-4075(03)58918-0 Highly accurate evaluation of the
Physics of atoms and molecules
 Physics of atoms and molecules 2nd edition B.H. Bransden and C.J. Joachain Prentice Hall An imprint of Pearson Education Harlow, England London New York Boston San Francisco Toronto Sydney Singapore Hong
Physics of atoms and molecules 2nd edition B.H. Bransden and C.J. Joachain Prentice Hall An imprint of Pearson Education Harlow, England London New York Boston San Francisco Toronto Sydney Singapore Hong
Silicon / Si If not silicon then CE = 0 / 3 1
 M.(a) Silicon / Si If not silicon then CE = 0 / 3 covalent (bonds) M3 dependent on correct M2 Strong or many of the (covalent) bonds need to be broken / needs a lot of energy to break the (covalent) bonds
M.(a) Silicon / Si If not silicon then CE = 0 / 3 covalent (bonds) M3 dependent on correct M2 Strong or many of the (covalent) bonds need to be broken / needs a lot of energy to break the (covalent) bonds
IONIC AND METALLIC BONDING
 Name IONIC AND METALLIC BONDING Chem 512 Homework rint this sheet, answer the questions and turn it in as a HARD COY A. Matching Match each description in Column B with the correct term in Column A. Write
Name IONIC AND METALLIC BONDING Chem 512 Homework rint this sheet, answer the questions and turn it in as a HARD COY A. Matching Match each description in Column B with the correct term in Column A. Write
Fully differential cross sections for transfer ionization a sensitive probe of high level correlation effects in atoms
 INSTITUTE OF PHYSICS PUBLISHING JOURNAL OF PHYSICS B: ATOMIC, MOLECULAR AND OPTICAL PHYSICS J. Phys. B: At. Mol. Opt. Phys. 37 (2004) L201 L208 PII: S0953-4075(04)75475-9 LETTER TO THE EDITOR Fully differential
INSTITUTE OF PHYSICS PUBLISHING JOURNAL OF PHYSICS B: ATOMIC, MOLECULAR AND OPTICAL PHYSICS J. Phys. B: At. Mol. Opt. Phys. 37 (2004) L201 L208 PII: S0953-4075(04)75475-9 LETTER TO THE EDITOR Fully differential
Generalized seniority scheme in light Sn isotopes. Abstract
 Generalized seniority scheme in light Sn isotopes N. Sandulescu a,b, J. Blomqvist b,t. Engeland c, M. Hjorth-Jensen d,a.holt c,r.j. Liotta b and E. Osnes c a Institute of Atomic Physics, P.O.Box MG-6,
Generalized seniority scheme in light Sn isotopes N. Sandulescu a,b, J. Blomqvist b,t. Engeland c, M. Hjorth-Jensen d,a.holt c,r.j. Liotta b and E. Osnes c a Institute of Atomic Physics, P.O.Box MG-6,
Electronic Structure and Bonding Review
 Name: Band: Date: Electronic Structure and Bonding Review 1. For electrons: a. What is the relative charge? b. What is the relative mass? c. What is the symbol? d. Where are they located in the modern
Name: Band: Date: Electronic Structure and Bonding Review 1. For electrons: a. What is the relative charge? b. What is the relative mass? c. What is the symbol? d. Where are they located in the modern
Lecture 32: The Periodic Table
 Lecture 32: The Periodic Table (source: What If by Randall Munroe) PHYS 2130: Modern Physics Prof. Ethan Neil (ethan.neil@colorado.edu) Announcements Homework #9 assigned, due next Wed. at 5:00 PM as usual.
Lecture 32: The Periodic Table (source: What If by Randall Munroe) PHYS 2130: Modern Physics Prof. Ethan Neil (ethan.neil@colorado.edu) Announcements Homework #9 assigned, due next Wed. at 5:00 PM as usual.
Chapter 9 Ionic and Covalent Bonding
 Chem 1045 Prof George W.J. Kenney, Jr General Chemistry by Ebbing and Gammon, 8th Edition Last Update: 06-April-2009 Chapter 9 Ionic and Covalent Bonding These Notes are to SUPPLIMENT the Text, They do
Chem 1045 Prof George W.J. Kenney, Jr General Chemistry by Ebbing and Gammon, 8th Edition Last Update: 06-April-2009 Chapter 9 Ionic and Covalent Bonding These Notes are to SUPPLIMENT the Text, They do
Non-sequential and sequential double ionization of NO in an intense femtosecond Ti:sapphire laser pulse
 J. Phys. B: At. Mol. Opt. Phys. 30 (1997) L245 L250. Printed in the UK PII: S0953-4075(97)80013-2 LETTER TO THE EDITOR Non-sequential and sequential double ionization of NO in an intense femtosecond Ti:sapphire
J. Phys. B: At. Mol. Opt. Phys. 30 (1997) L245 L250. Printed in the UK PII: S0953-4075(97)80013-2 LETTER TO THE EDITOR Non-sequential and sequential double ionization of NO in an intense femtosecond Ti:sapphire
MITOCW ocw f08-lec08_300k
 MITOCW ocw-5-111-f08-lec08_300k The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high quality educational resources for free.
MITOCW ocw-5-111-f08-lec08_300k The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high quality educational resources for free.
The periodic system of the elements. Predict. (rather than passively learn) Chemical Properties!
 The periodic system of the elements Study of over 100 elements daunting task! Nature has provided the periodic table Enables us to correlate an enormous amount of information Predict (rather than passively
The periodic system of the elements Study of over 100 elements daunting task! Nature has provided the periodic table Enables us to correlate an enormous amount of information Predict (rather than passively
Name(s) with Lab section in Group. How do we use the model of the electronic structure of the atom to understand periodic trends of the elements?
 Chem 1314 Sections 14 and 15 Periodicity Laboratory Fall 2004 Monday, November 22, 2004 Name(s) with Lab section in Group How do we use the model of the electronic structure of the atom to understand periodic
Chem 1314 Sections 14 and 15 Periodicity Laboratory Fall 2004 Monday, November 22, 2004 Name(s) with Lab section in Group How do we use the model of the electronic structure of the atom to understand periodic
Activity # 2. Name. Date due. Assignment on Atomic Structure
 Activity # 2 10 Name Date Date due Assignment on Atomic Structure NOTE: This assignment is based on material on the Power Point called Atomic Structure, as well as pages 167-173 in the Science Probe textbook.
Activity # 2 10 Name Date Date due Assignment on Atomic Structure NOTE: This assignment is based on material on the Power Point called Atomic Structure, as well as pages 167-173 in the Science Probe textbook.
Electronic Structure of Atoms and the Periodic table. Electron Spin Quantum # m s
 Electronic Structure of Atoms and the Periodic table Chapter 6 & 7, Part 3 October 26 th, 2004 Homework session Wednesday 3:00 5:00 Electron Spin Quantum # m s Each electron is assigned a spinning motion
Electronic Structure of Atoms and the Periodic table Chapter 6 & 7, Part 3 October 26 th, 2004 Homework session Wednesday 3:00 5:00 Electron Spin Quantum # m s Each electron is assigned a spinning motion
Instructor background for the discussion points of Section 2
 Supplementary Information for: Orbitals Some fiction and some facts Jochen Autschbach Department of Chemistry State University of New York at Buffalo Buffalo, NY 14260 3000, USA Instructor background for
Supplementary Information for: Orbitals Some fiction and some facts Jochen Autschbach Department of Chemistry State University of New York at Buffalo Buffalo, NY 14260 3000, USA Instructor background for
Learning Guide 2C Small Molecules Chem What are the two most common types of physical changes that we encounter in chemistry?
 Review Learning Guide 2C Small Molecules Chem 1010 What are the two most common types of physical changes that we encounter in chemistry? 1) 2) What are the three most common types of nuclear changes,
Review Learning Guide 2C Small Molecules Chem 1010 What are the two most common types of physical changes that we encounter in chemistry? 1) 2) What are the three most common types of nuclear changes,
Evaluate Scientific Models for Atomic Structure
 Evaluate Scientific Models for Atomic Structure Directions: Answer all parts of each question below. Make sure your answers are in complete sentences and are concise, including ONLY necessary details.
Evaluate Scientific Models for Atomic Structure Directions: Answer all parts of each question below. Make sure your answers are in complete sentences and are concise, including ONLY necessary details.
Equivalence principle for free and bound antiparticles
 Equivalence principle for free and bound antiparticles Savely G Karshenboim D.I. Mendeleev Institute for Metrology (St. Petersburg) and Max-Planck-Institut für Quantenoptik (Garching) How accurate are
Equivalence principle for free and bound antiparticles Savely G Karshenboim D.I. Mendeleev Institute for Metrology (St. Petersburg) and Max-Planck-Institut für Quantenoptik (Garching) How accurate are
Science Olympiad Regional UW-Milwaukee Chemistry test 2013
 Science Olympiad Regional UW-Milwaukee Chemistry test 2013 Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. 1. What is another name for the representative
Science Olympiad Regional UW-Milwaukee Chemistry test 2013 Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. 1. What is another name for the representative
Periodic Relationships
 Periodic Relationships 1 Tabulation of Elements Mendeleev (1869) Arranged by mass Tabulation by chem.& physical properties Predicted missing elements and properties 2 Modern Periodic Table Argon vs. potassium
Periodic Relationships 1 Tabulation of Elements Mendeleev (1869) Arranged by mass Tabulation by chem.& physical properties Predicted missing elements and properties 2 Modern Periodic Table Argon vs. potassium
A1: Atomic Structure Worksheet (Goals 1 3, Chapter 4)
 Unit 3 Assignment Packet Name: Period: A1: Atomic Structure Worksheet (Goals 1 3, Chapter 4) 1. Democritus, who lived in Greece during the 4 th century B.C., suggested that is made up of tiny particles
Unit 3 Assignment Packet Name: Period: A1: Atomic Structure Worksheet (Goals 1 3, Chapter 4) 1. Democritus, who lived in Greece during the 4 th century B.C., suggested that is made up of tiny particles
Low energy positron interactions with rare gas atoms: threshold features and benchmark cross sections
 Journal of Physics: Conference Series Low energy positron interactions with rare gas atoms: threshold features and benchmark cross sections To cite this article: James Sullivan et al 2011 J. Phys.: Conf.
Journal of Physics: Conference Series Low energy positron interactions with rare gas atoms: threshold features and benchmark cross sections To cite this article: James Sullivan et al 2011 J. Phys.: Conf.
Research Letter On the Appearance of a Cooper Minimum in the Photoionization Cross Sections of the Plasma-Embedded Li Atom
 Research Letters in Physics Volume 29, Article ID 832413, 5 pages doi:1.1155/29/832413 Research Letter On the Appearance of a Cooper Minimum in the Photoionization Cross Sections of the Plasma-Embedded
Research Letters in Physics Volume 29, Article ID 832413, 5 pages doi:1.1155/29/832413 Research Letter On the Appearance of a Cooper Minimum in the Photoionization Cross Sections of the Plasma-Embedded
Multi-electron coincidence spectroscopy: double photoionization from molecular inner-shell orbitals
 Journal of Physics: Conference Series OPEN ACCESS Multi-electron coincidence spectroscopy: double photoionization from molecular inner-shell orbitals To cite this article: Y Hikosaka et al 2014 J. Phys.:
Journal of Physics: Conference Series OPEN ACCESS Multi-electron coincidence spectroscopy: double photoionization from molecular inner-shell orbitals To cite this article: Y Hikosaka et al 2014 J. Phys.:
Unit 2 Review Please note that this does not start on question 1.
 Unit 2 Review Please note that this does not start on question 1. 21. Of the three particles; protons, neutrons, and electrons, which one(s) are responsible for most of the mass of an atom? a) the protons
Unit 2 Review Please note that this does not start on question 1. 21. Of the three particles; protons, neutrons, and electrons, which one(s) are responsible for most of the mass of an atom? a) the protons
8. Which of the following could be an isotope of chlorine? (A) 37 Cl 17 (B) 17 Cl 17 (C) 37 Cl 17 (D) 17 Cl 37.5 (E) 17 Cl 37
 Electronic Structure Worksheet 1 Given the following list of atomic and ionic species, find the appropriate match for questions 1-4. (A) Fe 2+ (B) Cl (C) K + (D) Cs (E) Hg + 1. Has the electron configuration:
Electronic Structure Worksheet 1 Given the following list of atomic and ionic species, find the appropriate match for questions 1-4. (A) Fe 2+ (B) Cl (C) K + (D) Cs (E) Hg + 1. Has the electron configuration:
Metal + water -> metal hydroxide + hydrogen Metal + acid -> metal salt + hydrogen
 Name of Formula Formula of ion Name of salt Hydrochloric Sulphuric HCl Cl - Chloride H 2 SO 4 SO 4-2 Sulphate Key words: Oxidation: loss of electrons Reduction: gain of electrons Displacement reaction:
Name of Formula Formula of ion Name of salt Hydrochloric Sulphuric HCl Cl - Chloride H 2 SO 4 SO 4-2 Sulphate Key words: Oxidation: loss of electrons Reduction: gain of electrons Displacement reaction:
CHAPTER-3 CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES OF ELEMENTS
 CHAPTER-3 CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES OF ELEMENTS Mandeleev s Periodic Law:- The properties of the elements are the periodic function of their atomic masses. Moseley, the English
CHAPTER-3 CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES OF ELEMENTS Mandeleev s Periodic Law:- The properties of the elements are the periodic function of their atomic masses. Moseley, the English
Exercises 16.3a, 16.5a, 16.13a, 16.14a, 16.21a, 16.25a.
 SPECTROSCOPY Readings in Atkins: Justification 13.1, Figure 16.1, Chapter 16: Sections 16.4 (diatomics only), 16.5 (omit a, b, d, e), 16.6, 16.9, 16.10, 16.11 (omit b), 16.14 (omit c). Exercises 16.3a,
SPECTROSCOPY Readings in Atkins: Justification 13.1, Figure 16.1, Chapter 16: Sections 16.4 (diatomics only), 16.5 (omit a, b, d, e), 16.6, 16.9, 16.10, 16.11 (omit b), 16.14 (omit c). Exercises 16.3a,
D) g. 2. In which pair do the particles have approximately the same mass?
 1. A student constructs a model for comparing the masses of subatomic particles. The student selects a small, metal sphere with a mass of gram to represent an electron. A sphere with which mass would be
1. A student constructs a model for comparing the masses of subatomic particles. The student selects a small, metal sphere with a mass of gram to represent an electron. A sphere with which mass would be
Chapter 2 Chemistry. The World of Elements. Why are we studying chemistry? Models of atoms. The Basics. Atomic structure determines behavior
 Chapter 2 Chemistry The World of Elements What? You thought you were all done with the Periodic Table? NEVER! Why are we studying chemistry? Biology has chemistry at its foundation Models of atoms Yeah,
Chapter 2 Chemistry The World of Elements What? You thought you were all done with the Periodic Table? NEVER! Why are we studying chemistry? Biology has chemistry at its foundation Models of atoms Yeah,
CHEM3023: Spins, Atoms and Molecules
 CHEM3023: Spins, Atoms and Molecules Lecture 3 The Born-Oppenheimer approximation C.-K. Skylaris Learning outcomes Separate molecular Hamiltonians to electronic and nuclear parts according to the Born-Oppenheimer
CHEM3023: Spins, Atoms and Molecules Lecture 3 The Born-Oppenheimer approximation C.-K. Skylaris Learning outcomes Separate molecular Hamiltonians to electronic and nuclear parts according to the Born-Oppenheimer
4. A hydrogen bond is formed between a hydrogen atom and a negative atom, usually a nitrogen or oxygen.
 Name Biology Summer Assignment Print, complete and bring this assignment with you on the first day of classes. Use the background information document to help you complete this assignment. Modified True/False
Name Biology Summer Assignment Print, complete and bring this assignment with you on the first day of classes. Use the background information document to help you complete this assignment. Modified True/False
