Post-Show. Chemistry. Periodic Table of the Elements. After the Show. Traveling Science Shows
|
|
|
- Harvey Patrick
- 5 years ago
- Views:
Transcription
1 Traveling Science Shows Post-Show Chemistry After the Show We recently presented a Chemistry show at your school, and thought you and your students might like to continue investigating this topic. The following activities are designed to review and extend the ideas covered in the show. To most people, solutions mean finding answers. But to chemists, solutions are things that are still all mixed up. - anonymous 5th grader Please remember to use appropriate safety measures for all activities. An adult instructor should always supervise students during experiments. Materials in chemistry experiments should never be ingested. Goggles or safety glasses are recommended for all activities, but not required unless stated. Visit us online at or contact us at tss@fi.edu. Periodic Table of the Elements
2 FIZZY, BUBBLY FUN FOR GRADES 1-4 As we learned in the Chemistry show, a chemical reaction occurs when two or more substances form new substances. You can tell when a chemical reaction takes place if it produces heat or light, a color change, or bubbles. Here, students will conduct an experiment to determine how temperature impacts a chemical reaction. EQUIPMENT 3 clear glass or plastic cups Hot, cold, and room-temperature water Seltzer tablets Stop-watch and thermometer (optional) PROCEDURE 1. Fill one cup with hot water, another cup with room-temperature water, and the third with ice water. 2. Place a seltzer tablet in each cup and watch the reaction. What signs of a chemical reaction can you observe? 3. In which temperature water did the reaction take place the fastest? The slowest? How can you tell which reaction happened the fastest and slowest? Older students could graph the time it took to complete the reaction against the temperature of the water. 4. Brainstorm other ways to make the reaction happen faster for instance, breaking the tablet into smaller pieces, or adding vinegar to the water. Now test out your hypotheses! WHY? Most seltzer tablets are a mixture of two chemicals: sodium bicarbonate (the main ingredient in baking soda) and citric acid. When mixed with water, a chemical reaction produces sodium citrate and carbon dioxide, which bubbles out of the water. In this experiment, students will discover that as temperature increases, the chemical reaction speeds up. This is because heat energy speeds up the molecules, increasing the rate at which they bump into each other and react.
3 MYSTERY MATTER FOR GRADES 3-6 Chemists often have to identify unknown materials. During the show, you saw how chemists can use a flame test to identify certain salts. In this experiment, students will observe chemical reactions in order to identify chemicals. Caution students not to taste any of the materials. EQUIPMENT Small quantities of salt, granulated sugar, baking soda, flour, and cornstarch Plastic wrap Small quantities of water, vinegar, and iodine Eye droppers Toothpicks Magnifying glasses PROCEDURE Part I 1. In your small group, collect a small sample of each white powder. Place a small spoonful of each powder in a row on top of a sheet of plastic wrap. Label the powders or note the order on a sheet of paper. Then record some observations of each powder for instance, the color, shape, and size of the particles. 2. You will conduct tests to observe what happens when each powder is exposed to a different indicator (water, vinegar, and iodine). For the water test, use the eye-dropper to add three drops of water to each sample. Mix with a toothpick. Record your observations in the data table on the next page. 3. Using new samples, Repeat step 2 with each of the other indicators (vinegar and iodine). Part 2 4. Collect samples of three un-labeled powders. Your task is to identify each powder using your observations of the powder s properties and its reaction with indicators. 5. Present your procedures and conclusions. 6. Dispose of all white powders in the trash. Do not rinse powders down the sink, as they may clog the drain. Wash your hands when finished. WHY? Various substances react in characteristic ways with other substances. An indicator is any substance that reacts in a particular way and helps us identify an unknown chemical. In this case, the white powders react differently from one another when mixed with water, iodine, or vinegar. By comparing the reaction of a mystery powder to the known reactions of similar powders, you can draw a conclusion about the mystery powder. Chemists often use indicators and known reactions to identify unknown products of chemical reactions. As a challenge, try to identify a mixture of two different powders during step 4!
4 MYSTERY MATTER Data Table White powder Observations of its properties Reaction with water Reaction with vinegar Reaction with iodine Salt Sugar Baking soda Flour Cornstarch Unknown sample #1 Unknown sample #2 Unknown sample #3 Sample #1 is. Our evidence: Sample #2 is. Our evidence: Sample #3 is. Our evidence:
5 KITCHEN CHEMISTRY FOR GRADES 5-8 Our Traveling Scientist demonstrated how acids and bases react with each other and with indicators. In this activity, students will use indicator paper to classify common household substances as acids or bases. Before conducting this experiment with students, test out the goldenrod paper; some varieties will work better than others. Students should avoid skin contact with the liquids. Never mix bleach with other chemicals. EQUIPMENT Assortment of liquid bases (such as ammonia, bleach, baking soda and water, milk of magnesia) Assortment of liquid acids (such as vinegar, lemon juice, orange juice, soda) Cotton swabs Goldenrod-colored copy paper PROCEDURE 1. Create a data table to record how each substance reacts with the paper and whether it is an acid or a base. 2. Obtain a sheet of goldenrod-colored copy paper and a sample of each household substance. 3. Select one of the substances. Predict whether it is an acid or a base. Dip a cotton swab into that substance. Then press the tip of the swab onto the goldenrod paper. 4. What reaction do you observe? Decide whether that substance is an acid or a base, and record your findings in your data table. 5. Test the rest of the substances. Were any of your results surprising? 6. After you have determined which substances are bases, soak a strip of goldenrod paper in a base. Then dip it into one of the acids. What happens? Why? WHY? Goldenrod copy paper acts as a ph indicator, meaning that it can be used to determine whether a substance is an acid or a base. Acids and bases react differently to an indicator because of differences in their chemical composition. A base will change the goldenrod paper to red; an acid will not change the color of the paper. An acid can neutralize, or cancel out, a base and return the indicator to its original color.
6 MORE INFORMATION We ve provided the following information to help refresh your memory about the topics we covered during the show, and to deepen your understanding about important chemistry topics. Chemistry: The scientific study of matter, its properties, and interactions with other matter and with energy. Matter: Any substance that has mass and volume; in other words, all the stuff around us. Matter is made up of atoms and molecules. A chemical is simply any matter with a definite, known composition. Chemicals are all around us! Element: A pure chemical substance consisting of only one type of atom. There are 118 known elements, which make up all the matter in the universe. Periodic Table: An arrangement of the elements by increasing atomic number (the number of protons in an element). The periodic table displays the elements so that one may see trends in their properties and predict how an element will react with another element. Atom: The basic unit of matter. An atom consists of a nucleus surrounded by electrons. The nucleus is the very dense region at the center of an atom. It consists of protons (which have an electrical charge of +1) and neutrons (which have no electrical charge). Electrons (which have an electrical charge of -1) move very rapidly around the nucleus. Molecule: An electrically neutral group of at least two atoms held together by strong chemical bonds. Compound: A group of two or more different elements, held together by strong chemical bonds. Solid: A state of matter with very tightly packed particles that do not have enough energy to move freely. For this reason, solids have a fixed shape and volume. Liquid: A state of matter in which particles have enough energy to move freely relative to each other. Liquids have a fixed volume, but not a fixed shape. Gas: A state of matter in which the molecules have enough energy to spread out and occupy the entire space of the container confining it. A gas has no definite shape or volume. Physical Reaction: A change where the form of matter is altered, not its chemical substance. Melting ice and evaporating water are examples of physical reactions. In the end, the water is in a different state of matter but it is still water. Chemical Reaction: A change where ingredients, or reactants, are chemically altered to form new substances, or products. An acid-base reaction and combustion are examples of chemical reactions. Combustion: A chemical reaction in which a substance combines with oxygen, producing energy in the form of heat and light. Combustion requires fuel, oxygen, and heat.
7 Substitution Reaction: A type of chemical reaction where an atom, ion, or group of atoms or ions in one reactant molecule is replaced by another atom, ion, or group from another reactant. Also called a displacement reaction, it results in two or more new products. Solution: A blend of two or more liquid substances with a uniform composition throughout. When you dissolve sugar in a glass of water, the ingredients are evenly distributed through the solution. Mixture: A combination of chemicals in which each substance retains its own chemical identity. That is, the ingredients are not chemically combined. A salad is an example of a mixture there are no chemical reactions between the tomatoes and the lettuce. Polymer: A large molecule made up of identical units which are chemically cross-linked. Plastics are petroleumbased polymers. ph Scale: A number line scale that goes from zero through fourteen. ph is a measure of the concentration of hydrogen ions in a liquid. As we move from zero to fourteen along the scale, the concentration of hydrogen ions decreases. Acid: Any substance with a ph less than seven. The lower the ph, the stronger the acid. Acids tend to taste sour, and can be corrosive. Base: Any substance with a ph greater than seven. The higher the ph, the stronger the base. Substances that are basic or alkaline taste bitter and often have a slippery feel. MORE RESOURCES Introduction to Matter: Visit for chemistry activities and resources. You ll find lesson plans, games, videos, and more! Careers in Science and Technology: Check out to meet a chemist, a geologist, a meteorologist, and more fascinating scientists. Follow Ashish through a typical day in the life of a chemist, and then find out what it takes to become a chemist yourself!
Post-Show HOT AND COLD. Gases. Liquids. Solids. After the Show. Traveling Science Shows
 Traveling Science Shows Post-Show HOT AND COLD After the Show We recently presented a Hot and Cold show at your school, and thought you and your students might like to continue investigating this topic.
Traveling Science Shows Post-Show HOT AND COLD After the Show We recently presented a Hot and Cold show at your school, and thought you and your students might like to continue investigating this topic.
Page 1 / 12. Chemistry Exam. Name: Matter Properties, Structure. Question 1 (1 point) The atomic number of an atom is. A. The mass of the atom.
 Chemistry Exam Matter Properties, Structure Name: Question 1 (1 point) The atomic number of an atom is A. The mass of the atom. B. The number of protons added to the number of neutrons in the nucleus.
Chemistry Exam Matter Properties, Structure Name: Question 1 (1 point) The atomic number of an atom is A. The mass of the atom. B. The number of protons added to the number of neutrons in the nucleus.
Chapter 6, Lesson 9: Neutralizing Acids and Bases
 Chapter 6, Lesson 9: Neutralizing Acids and Bases Key Concepts ph is a measure of the concentration of H 3 O + ions in a solution. Adding an acid increases the concentration of H 3 O + ions in the solution.
Chapter 6, Lesson 9: Neutralizing Acids and Bases Key Concepts ph is a measure of the concentration of H 3 O + ions in a solution. Adding an acid increases the concentration of H 3 O + ions in the solution.
LEVEL ZERO VOICE CATALYST (10 minutes, individual work): 1. Counting atoms
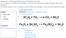 Assignment 4 Chemical Detectives at the Molecular Level LO: To explain the differences between chemical and physical changes through observable evidence. EQ: What evidence can be used to tell the difference
Assignment 4 Chemical Detectives at the Molecular Level LO: To explain the differences between chemical and physical changes through observable evidence. EQ: What evidence can be used to tell the difference
5 th GRADE PHYSICAL AND CHEMICAL TESTS
 5 th GRADE PHYSICAL AND CHEMICAL TESTS Summary: Students investigate 5 unknown white powders. They gather clues by observing the physical and chemical changes of the powders. At the end of the activity,
5 th GRADE PHYSICAL AND CHEMICAL TESTS Summary: Students investigate 5 unknown white powders. They gather clues by observing the physical and chemical changes of the powders. At the end of the activity,
STEMscopedia: PHYSICAL AND CHEMICAL PROPERTIES AND CHANGES 8P1CD
 Reflect STEMscopedia: PHYSICAL AND CHEMICAL 8P1CD What do ice cream, root beer, and carbon dioxide gas have in common? Not only do these ingredients combine to make a good treat on a hot summer day, but
Reflect STEMscopedia: PHYSICAL AND CHEMICAL 8P1CD What do ice cream, root beer, and carbon dioxide gas have in common? Not only do these ingredients combine to make a good treat on a hot summer day, but
Chapter 6, Lesson 7: Energy Changes in Chemical Reactions
 Chapter 6, Lesson 7: Energy Changes in Chemical Reactions Key Concepts If two substances react and the temperature of the mixture decreases, the reaction is endothermic. If two substances react and the
Chapter 6, Lesson 7: Energy Changes in Chemical Reactions Key Concepts If two substances react and the temperature of the mixture decreases, the reaction is endothermic. If two substances react and the
Atoms. - Proton - Neutron. - Electron
 Chemistry of Life Atoms - The basic unit of matter is called an Atom - Atoms are incredibly small, but despite its extremely small size, an atom contains subatomic particles that are even smaller - Three
Chemistry of Life Atoms - The basic unit of matter is called an Atom - Atoms are incredibly small, but despite its extremely small size, an atom contains subatomic particles that are even smaller - Three
By All INdICATIONS (2 Hours)
 By All INdICATIONS (2 Hours) Addresses NGSS Level of Difficulty: 5 Grade Range: 6-8 OVERVIEW In this activity, students create an acid-base indicator using red cabbage extract. Students then use this indicator
By All INdICATIONS (2 Hours) Addresses NGSS Level of Difficulty: 5 Grade Range: 6-8 OVERVIEW In this activity, students create an acid-base indicator using red cabbage extract. Students then use this indicator
2-1: Describing Matter. 8 th Grade Physical Sciences
 8 th Grade Physical Sciences What is Matter? Matter is anything that has mass and takes up space. Properties of Matter Matter can be described in many ways; hard, soft, heavy, light, rough, smooth,
8 th Grade Physical Sciences What is Matter? Matter is anything that has mass and takes up space. Properties of Matter Matter can be described in many ways; hard, soft, heavy, light, rough, smooth,
Physical Changes vs Chemical Changes Lab
 Physical Changes vs Chemical Changes Lab What was done? What can you observe? Is it a physical or chemical change and why? What else have you learned during the demonstration? #1) Crumpling Paper Notice
Physical Changes vs Chemical Changes Lab What was done? What can you observe? Is it a physical or chemical change and why? What else have you learned during the demonstration? #1) Crumpling Paper Notice
Physical & Chemical PROPERTIES
 Name Test Date Hour Group Chem #4 Notebook Properties & Changes LEARNING TARGETS I can give examples of physical and chemical properties. I can give examples of physical and chemical changes. I can identify
Name Test Date Hour Group Chem #4 Notebook Properties & Changes LEARNING TARGETS I can give examples of physical and chemical properties. I can give examples of physical and chemical changes. I can identify
Physical and Chemical Changes Or How Do You Know When You ve Made Something New?
 Introduction Or How Do You Know When You ve Made Something New? Remember that all matter has characteristic physical and chemical properties. Matter can also undergo physical and chemical changes. How
Introduction Or How Do You Know When You ve Made Something New? Remember that all matter has characteristic physical and chemical properties. Matter can also undergo physical and chemical changes. How
2/22/2019 NEW UNIT! Chemical Interactions. Atomic Basics #19
 NEW UNIT! Chemical Interactions Atomic Basics #19 1 Vocabulary: Matter: Anything that has mass and takes up space. Atom: the smallest particle of matter. Element: A pure substance made up of only one type
NEW UNIT! Chemical Interactions Atomic Basics #19 1 Vocabulary: Matter: Anything that has mass and takes up space. Atom: the smallest particle of matter. Element: A pure substance made up of only one type
Chemistry Lab Define Acids and Bases
 Introduction Chemistry Lab Define Acids and Bases By the 1500 s chemists recognized that certain substances shared a common property a sour taste. These substances possessed other characteristic properties
Introduction Chemistry Lab Define Acids and Bases By the 1500 s chemists recognized that certain substances shared a common property a sour taste. These substances possessed other characteristic properties
How Do Scientists Measure Acidity?
 4.5 Investigate How Do Scientists Measure Acidity? ph scale: a measure of the concentration of hydrogen ions in a substance. neutral: a solution with a ph of 7. ph 7 has an equal number of hydrogen ions
4.5 Investigate How Do Scientists Measure Acidity? ph scale: a measure of the concentration of hydrogen ions in a substance. neutral: a solution with a ph of 7. ph 7 has an equal number of hydrogen ions
Student Exploration: Chemical Changes
 Name: Date: Student Exploration: Chemical Changes Vocabulary: acid, base, catalyst, chemical change, coefficient, conservation of matter, decomposition, dissolve, double replacement, endothermic, exothermic,
Name: Date: Student Exploration: Chemical Changes Vocabulary: acid, base, catalyst, chemical change, coefficient, conservation of matter, decomposition, dissolve, double replacement, endothermic, exothermic,
Chemistry Final Study Guide KEY. 3. Define physical changes. A change in any physical property of a substance, not in the substance itself.
 Chemistry Final Study Guide KEY Unit 2: Matter & Its Properties, Lesson 1: Physical and Chemical Properties & Changes 1. Define physical properties. The characteristics of a substance that can be observed
Chemistry Final Study Guide KEY Unit 2: Matter & Its Properties, Lesson 1: Physical and Chemical Properties & Changes 1. Define physical properties. The characteristics of a substance that can be observed
Physical Science SPS 6 Review Activity
 Properties of Acids & Bases: in this table, list everything that you know about acids, bases, & their properties. List specific things and general things. (how do you identify them? What makes them, them?
Properties of Acids & Bases: in this table, list everything that you know about acids, bases, & their properties. List specific things and general things. (how do you identify them? What makes them, them?
Physical Science Lecture Notes Chapters 17, 18 & 19
 Physical Science Lecture Notes Chapters 17, 18 & 19 I. 17-1: Matter & Its Changes a. Changes in matter i. Physical Changes Alters form or appearance but doesn t change it into another substance ie. Water
Physical Science Lecture Notes Chapters 17, 18 & 19 I. 17-1: Matter & Its Changes a. Changes in matter i. Physical Changes Alters form or appearance but doesn t change it into another substance ie. Water
6.1- Chemical vs. Physical - Pre-Lab Questions
 6.1- Chemical vs. Physical - Pre-Lab Questions Name: Instructor: Date: Section/Group: 1. Using the procedures for each station provided as a guide, predict which properties you will be looking for in each
6.1- Chemical vs. Physical - Pre-Lab Questions Name: Instructor: Date: Section/Group: 1. Using the procedures for each station provided as a guide, predict which properties you will be looking for in each
Name: Section: Matter: Atoms and Properties Practice Test
 Name: Section: Matter: Atoms and Properties Practice Test Directions: For each of the questions or incomplete statements below, choose the best of the answer choices given and write your answer on the
Name: Section: Matter: Atoms and Properties Practice Test Directions: For each of the questions or incomplete statements below, choose the best of the answer choices given and write your answer on the
Lesson 2. Color change
 Lesson 2 Color change T E A C H E R G U I D E Lesson summary Students meet marine chemist Sera Tuikabe, who is studying ocean acidification in the water surrounding the Republic of the Fiji Islands. Students
Lesson 2 Color change T E A C H E R G U I D E Lesson summary Students meet marine chemist Sera Tuikabe, who is studying ocean acidification in the water surrounding the Republic of the Fiji Islands. Students
Saturday Science Lesson Plan Fall 2008
 Saturday Science Lesson Plan Fall 2008 LEARNING OBJECTIVES STANDARDS 1.1.1 Observe, describe, draw, and sort objects carefully to learn about them. 1.2.6 Describe and compare objects in terms of number,
Saturday Science Lesson Plan Fall 2008 LEARNING OBJECTIVES STANDARDS 1.1.1 Observe, describe, draw, and sort objects carefully to learn about them. 1.2.6 Describe and compare objects in terms of number,
Lesson 1: What are chemical changes?
 Lesson 1 Summary Lesson 1: What are chemical changes? Vocabulary Use with pp. 375 377 physical change a change in which the material keeps its identify chemical change a change in which one substance or
Lesson 1 Summary Lesson 1: What are chemical changes? Vocabulary Use with pp. 375 377 physical change a change in which the material keeps its identify chemical change a change in which one substance or
CHAPTER 2--LIFE'S CHEMICAL BASIS
 CHAPTER 2--LIFE'S CHEMICAL BASIS Student: 1. People are most likely to ingest large amounts of mercury by eating A. soy products. B. chicken. C. beef. D. large predatory fish. E. small herbivorous fish.
CHAPTER 2--LIFE'S CHEMICAL BASIS Student: 1. People are most likely to ingest large amounts of mercury by eating A. soy products. B. chicken. C. beef. D. large predatory fish. E. small herbivorous fish.
Chemistry Unit Test 1 Review
 Chemistry Unit Test 1 Review Name: S8P1a. Students will distinguish between atoms and molecules. 1. Which of the following particles combine to form molecules? a. Atoms b. Protons c. Electrons d. Compounds
Chemistry Unit Test 1 Review Name: S8P1a. Students will distinguish between atoms and molecules. 1. Which of the following particles combine to form molecules? a. Atoms b. Protons c. Electrons d. Compounds
Lab: Cabbages in Chemistry 3pts ec printing in COLOR / 2pts B&W
 Lab: Cabbages in Chemistry 3pts ec printing in COLOR / 2pts B&W Telltale Colors 1. In the mixing tray, place 5 drops of the chemical in 13 compartments 2. DIP test the ph paper & record the ph measurement
Lab: Cabbages in Chemistry 3pts ec printing in COLOR / 2pts B&W Telltale Colors 1. In the mixing tray, place 5 drops of the chemical in 13 compartments 2. DIP test the ph paper & record the ph measurement
Every living and nonliving things is made up of matter. MATTER: anything that has mass & takes up space. What does all matter have in common?
 the basics Every living and nonliving things is made up of matter MATTER: anything that has mass & takes up space What does all matter have in common? Smallest unit of matter ALL matter is made of particles
the basics Every living and nonliving things is made up of matter MATTER: anything that has mass & takes up space What does all matter have in common? Smallest unit of matter ALL matter is made of particles
Student Notes Acids and Bases
 Name: Class: Date: Student Notes Acids and Bases Many foods that we eat contain acids, such as lemons, oranges, apples, vinegar, grapes, and soda pop. Lemons and oranges contain citric acid, while apples
Name: Class: Date: Student Notes Acids and Bases Many foods that we eat contain acids, such as lemons, oranges, apples, vinegar, grapes, and soda pop. Lemons and oranges contain citric acid, while apples
The grade 5 English science unit, Acids and Bases, meets the academic content standards set in the Korean curriculum, which state students should:
 This area addresses ph among different characteristics of solutions. It will be interesting for students to classify a variety of solutions into acids and bases by using the characteristics of the solutions.
This area addresses ph among different characteristics of solutions. It will be interesting for students to classify a variety of solutions into acids and bases by using the characteristics of the solutions.
Science 10. Unit 2: Chemistry. Book 5: Acid -Base Chemistry & the ph Scale. Block: Name:
 Science 10 Unit 2: Chemistry Book 5: Acid -Base Chemistry & the ph Scale Name: Block: 1 Classifying Substances There are other ways you can use to classify compounds. For example, you can classify some
Science 10 Unit 2: Chemistry Book 5: Acid -Base Chemistry & the ph Scale Name: Block: 1 Classifying Substances There are other ways you can use to classify compounds. For example, you can classify some
Chemistry: classifying chemical and physical changes in various materials/substances
 Chemistry: classifying chemical and physical changes in various materials/substances Nikki Schilling, Ames, St. Paul Mn Based on original activity from Cool Chemistry Concoctions by Joe Rhatigan and Veronika
Chemistry: classifying chemical and physical changes in various materials/substances Nikki Schilling, Ames, St. Paul Mn Based on original activity from Cool Chemistry Concoctions by Joe Rhatigan and Veronika
SECTION 19.1 ACID BASE THEORIES
 Name: Period: Chapter 19 Acids, Bases, and Salts Final Exam Study Guide SECTION 19.1 ACID BASE THEORIES (pages 587 593) Define the following vocabulary terms: 1. monoprotic acids 2. diprotic acids 3. triprotic
Name: Period: Chapter 19 Acids, Bases, and Salts Final Exam Study Guide SECTION 19.1 ACID BASE THEORIES (pages 587 593) Define the following vocabulary terms: 1. monoprotic acids 2. diprotic acids 3. triprotic
Author(s): Tim Bumpus, Stephanie Sanders, Camilo Sarmiento, Daniel Urul. Standards: Next Generation Science Standards (www.nextgenscience.
 Unknown Powders Author(s): Tim Bumpus, Stephanie Sanders, Camilo Sarmiento, Daniel Urul Date Created: February, 2016 Subject: Chemistry Grade Level: Grades 3-6 Standards: Next Generation Science Standards
Unknown Powders Author(s): Tim Bumpus, Stephanie Sanders, Camilo Sarmiento, Daniel Urul Date Created: February, 2016 Subject: Chemistry Grade Level: Grades 3-6 Standards: Next Generation Science Standards
MiSP CHEMICAL REACTIONS, L3 Teacher Guide. Introduction
 MiSP CHEMICAL REACTIONS, L3 Teacher Guide Introduction This weeklong unit should be included with other chemistry content teaching and learning. It is designed to follow Intermediate Level Science Core
MiSP CHEMICAL REACTIONS, L3 Teacher Guide Introduction This weeklong unit should be included with other chemistry content teaching and learning. It is designed to follow Intermediate Level Science Core
Alka Seltzer Lab: Reaction rate - Factors that affect the rate of chemical reactions
 Alka Seltzer Lab: Reaction rate - Factors that affect the rate of chemical reactions Materials Lead Scribe Reporter/Questioner Clean-up WRITE ALL NOTES / DATA / OBSERVATIONS ON YOUR DATA SHEET The rate
Alka Seltzer Lab: Reaction rate - Factors that affect the rate of chemical reactions Materials Lead Scribe Reporter/Questioner Clean-up WRITE ALL NOTES / DATA / OBSERVATIONS ON YOUR DATA SHEET The rate
Chapter 6, Lesson 1: What is a Chemical Reaction?
 Chapter 6, Lesson 1: What is a Chemical Reaction? Key Concepts: A physical change, such as a state change or dissolving, does not create a new substance, but a chemical change does. In a chemical reaction,
Chapter 6, Lesson 1: What is a Chemical Reaction? Key Concepts: A physical change, such as a state change or dissolving, does not create a new substance, but a chemical change does. In a chemical reaction,
CO 2. Lesson 1. Production of a gas
 Lesson 1 Production of a gas T E A C H E R G U I D E CO 2 Lesson summary Students meet volcanologist Victor Helguson, who is studying the gases released by volcanoes in Iceland. Students conduct a chemical
Lesson 1 Production of a gas T E A C H E R G U I D E CO 2 Lesson summary Students meet volcanologist Victor Helguson, who is studying the gases released by volcanoes in Iceland. Students conduct a chemical
Elements, Compounds, and Mixtures. Matter: Properties and Changes
 Elements, Compounds, and Mixtures Matter: Properties and Changes Warm up Observe the pictures and use the following terms to name them: element, compound, or mixture. Explain your answer. Warm up answers
Elements, Compounds, and Mixtures Matter: Properties and Changes Warm up Observe the pictures and use the following terms to name them: element, compound, or mixture. Explain your answer. Warm up answers
Name: Date: Period: Identifying & Analyzing Powders Lab [Adapted from
 Name: Date: Period: Identifying & Analyzing Powders Lab [Adapted from http://www.csun.edu/chemteach/activities/beginninganalysis.pdf ] In this lab, you will be observing physical and chemical properties
Name: Date: Period: Identifying & Analyzing Powders Lab [Adapted from http://www.csun.edu/chemteach/activities/beginninganalysis.pdf ] In this lab, you will be observing physical and chemical properties
Mixtures, Solubility, and Acid/Base Solutions
 Mixtures, Solubility, and Acid/Base Soluts Acid and Base Soluts What do you think? Read the two statements below and decide whether you agree or disagree with them. Place an A in the Before column if you
Mixtures, Solubility, and Acid/Base Soluts Acid and Base Soluts What do you think? Read the two statements below and decide whether you agree or disagree with them. Place an A in the Before column if you
LESSON 9: Ageless Apples ESTIMATED TIME Setup: 5 10 minutes Procedure: The effects can be observed over a 24-hour time period.
 LESSON 9: Ageless Apples ESTIMATED TIME Setup: 5 10 minutes Procedure: The effects can be observed over a 24-hour time period. DESCRIPTION Place apple slices into solutions with different levels of acidity
LESSON 9: Ageless Apples ESTIMATED TIME Setup: 5 10 minutes Procedure: The effects can be observed over a 24-hour time period. DESCRIPTION Place apple slices into solutions with different levels of acidity
States of Matter in Food
 Science Unit: Lesson 13: Matter States of Matter in Food Summary: Science skills: In this lesson, students explore the concept of state change, using various food and drink, in three activities: (1) Students
Science Unit: Lesson 13: Matter States of Matter in Food Summary: Science skills: In this lesson, students explore the concept of state change, using various food and drink, in three activities: (1) Students
Classifying Substances
 Classifying Substances There are other ways you can use to classify compounds. For example, you can classify some compounds as acids or bases. You are very familiar with acids and bases because you see
Classifying Substances There are other ways you can use to classify compounds. For example, you can classify some compounds as acids or bases. You are very familiar with acids and bases because you see
8.5E: Chemical Reactions
 Reflect Have you ever seen fireworks explode in the sky? If so, you may have observed a variety of different colors and shapes in the sky and watched with amazement as the colors changed overhead. These
Reflect Have you ever seen fireworks explode in the sky? If so, you may have observed a variety of different colors and shapes in the sky and watched with amazement as the colors changed overhead. These
To measure ph s in a variety of solutions and mixtures and to account for the results obtained.
 Acid-Base Studies PURPOSE To measure ph s in a variety of solutions and mixtures and to account for the results obtained. GOALS 1 To learn to use ph paper and a ph meter to measure the ph of a given solution.
Acid-Base Studies PURPOSE To measure ph s in a variety of solutions and mixtures and to account for the results obtained. GOALS 1 To learn to use ph paper and a ph meter to measure the ph of a given solution.
Liquid X Lab. Number of Drops Before Spilling Trial 1 Trial 2 Trial 3. Write a conclusion: How do your results for Liquid X compare to water?
 Names Block Date BIG QUESTION: Liquid X Lab Station 1 Surface Tension, Cohesion, and Adhesion Water is cohesive, adhesive, and has surface tension. Does Liquid X have the same properties? 1. Use a pipette
Names Block Date BIG QUESTION: Liquid X Lab Station 1 Surface Tension, Cohesion, and Adhesion Water is cohesive, adhesive, and has surface tension. Does Liquid X have the same properties? 1. Use a pipette
Lesson 4. Temperature change
 Lesson 4 Temperature change T E A C H E R G U I D E Lesson summary Students meet scientist Jason Williams, an industrial chemist who designs the materials and processes for making solar cells. He explains
Lesson 4 Temperature change T E A C H E R G U I D E Lesson summary Students meet scientist Jason Williams, an industrial chemist who designs the materials and processes for making solar cells. He explains
Introducing Science Summary Sheet
 Introducing Science Summary Sheet Acids in the laboratory Dilute acids You will have used some dilute acids at school, such as hydrochloric acid, sulphuric acid and nitric acid. Their bottles are labelled
Introducing Science Summary Sheet Acids in the laboratory Dilute acids You will have used some dilute acids at school, such as hydrochloric acid, sulphuric acid and nitric acid. Their bottles are labelled
Exploring Acids & Bases
 Food Explorations Lab: Exploring Acids & Bases STUDENT LAB INVESTIGATIONS Name: Lab Overview In this investigation, you will use cabbage juice indicator to determine if two unknown samples are acids or
Food Explorations Lab: Exploring Acids & Bases STUDENT LAB INVESTIGATIONS Name: Lab Overview In this investigation, you will use cabbage juice indicator to determine if two unknown samples are acids or
Name: Date: Period: Identifying & Analyzing Powders Lab [Adapted from
 Name: Date: Period: Identifying & Analyzing Powders Lab [Adapted from http://www.csun.edu/chemteach/activities/beginninganalysis.pdf ] In this lab, you will be observing physical and chemical properties
Name: Date: Period: Identifying & Analyzing Powders Lab [Adapted from http://www.csun.edu/chemteach/activities/beginninganalysis.pdf ] In this lab, you will be observing physical and chemical properties
LAB TEST Physical and Chemical Changes
 NAME: DATE: STATION: LAB TEST Physical and Chemical Changes PURPOSE: To observe physical and chemical changes in matter MATERIALS: 3 medium test tubes 1 small test tube test tube rack test tube holder
NAME: DATE: STATION: LAB TEST Physical and Chemical Changes PURPOSE: To observe physical and chemical changes in matter MATERIALS: 3 medium test tubes 1 small test tube test tube rack test tube holder
Pure Substances and Mixtures
 Pure Substances and Mixtures Unit 1 Lesson 4 Mix It Up! Make two mixtures in transparent containers with lids. 1. Find two transparent containers with tight lids. In one container, mix either salt or sugar
Pure Substances and Mixtures Unit 1 Lesson 4 Mix It Up! Make two mixtures in transparent containers with lids. 1. Find two transparent containers with tight lids. In one container, mix either salt or sugar
Acids and Bases. How does ph affect biological solutions? Introduction. Prelab Preparation Review Section 2.3 on acids and bases in your textbook.
 Acids and Bases How does ph affect biological solutions? Learning Objectives To relate the ph scale to how acidic or basic a solution is. To explain how a buffer affects the ph of a solution. Process Objectives
Acids and Bases How does ph affect biological solutions? Learning Objectives To relate the ph scale to how acidic or basic a solution is. To explain how a buffer affects the ph of a solution. Process Objectives
A simple equation of what happens when you add baking soda to vinegar:
 What s the Matter? Matter is anything that takes up space and has mass. Mass is the stuff that matter is made of, or the amount of particles in a substance or object. Matter has physical and chemical properties
What s the Matter? Matter is anything that takes up space and has mass. Mass is the stuff that matter is made of, or the amount of particles in a substance or object. Matter has physical and chemical properties
Substances and Mixtures:Separating a Mixture into Its Components
 MiraCosta College Introductory Chemistry Laboratory Substances and Mixtures:Separating a Mixture into Its Components EXPERIMENTAL TASK To separate a mixture of calcium carbonate, iron and sodium chloride
MiraCosta College Introductory Chemistry Laboratory Substances and Mixtures:Separating a Mixture into Its Components EXPERIMENTAL TASK To separate a mixture of calcium carbonate, iron and sodium chloride
Experiment 8 - Chemical Changes
 Experiment 8 - Chemical Changes When a chemical change occurs, the chemicals that you start with are changed into different chemicals. We know when this happens because the new chemicals have different
Experiment 8 - Chemical Changes When a chemical change occurs, the chemicals that you start with are changed into different chemicals. We know when this happens because the new chemicals have different
Liquid X Lab. Station 1 The Penny Lab Water is cohesive, adhesive, and has surface tension. Does Liquid X have the same properties?
 Names Block Date Liquid X Lab Station 1 The Penny Lab Water is cohesive, adhesive, and has surface tension. Does Liquid X have the same properties? 1. Use a pipette to carefully place drops of each liquid
Names Block Date Liquid X Lab Station 1 The Penny Lab Water is cohesive, adhesive, and has surface tension. Does Liquid X have the same properties? 1. Use a pipette to carefully place drops of each liquid
Name. Pre-Test. Directions: Circle the T if a statement is true or F if it is false (F). 1. Matter always stays the same.
 1 Pre-est Directions: Circle the if a statement is true or if it is false (). 1. Matter always stays the same. 2. A physical change is a change in size, shape or phase of matter, without the matter changing
1 Pre-est Directions: Circle the if a statement is true or if it is false (). 1. Matter always stays the same. 2. A physical change is a change in size, shape or phase of matter, without the matter changing
Table of Contents. Performance Standards
 Table of Contents Letter to the Student............................................ 5 Letter to the Family............................................. 6 Georgia Performance Standards Correlation Chart..................
Table of Contents Letter to the Student............................................ 5 Letter to the Family............................................. 6 Georgia Performance Standards Correlation Chart..................
The Chemistry of Color Tie Die
 Ages: 6 to 12 The Chemistry of Color Tie Die Contributor: Dr. Aaron Judy Couture, Laboratory of Atomic and Solid State Physics, Cornell University Main idea: What is an acid, and what is a base? How is
Ages: 6 to 12 The Chemistry of Color Tie Die Contributor: Dr. Aaron Judy Couture, Laboratory of Atomic and Solid State Physics, Cornell University Main idea: What is an acid, and what is a base? How is
Aim: What is ph? Mr. M. Gonzalez. What is ph?
 Aim: Mr. M. Gonzalez Do now: What happens when you combine baking soda and vinegar? A Chemical Reaction!!! Answer The reaction between baking soda (NaHCO3) and vinegar (CH3COOH) is actually two reactions,
Aim: Mr. M. Gonzalez Do now: What happens when you combine baking soda and vinegar? A Chemical Reaction!!! Answer The reaction between baking soda (NaHCO3) and vinegar (CH3COOH) is actually two reactions,
Bellevue College CHEM& 121 Experiment: Stoichiometric Analysis of an Antacid 1
 Experiment: Stoichiometric Analysis of an Antacid 1 Introduction In this lab, you will use the concept of stoichiometry to solve two sequential problems. First, you will try to determine the products of
Experiment: Stoichiometric Analysis of an Antacid 1 Introduction In this lab, you will use the concept of stoichiometry to solve two sequential problems. First, you will try to determine the products of
Matter, mass, and volume are related.
 Suppose you had all of the parts needed to make an at home aquarium: a tank, water, rocks, plants, and some fish. To put the aquarium together, you would need to arrange the rocks and plants in the tank.
Suppose you had all of the parts needed to make an at home aquarium: a tank, water, rocks, plants, and some fish. To put the aquarium together, you would need to arrange the rocks and plants in the tank.
Understand what acids and alkalis are, and where they are found.
 Lesson Aims- Understand what acids and alkalis are, and where they are found. Test a range of household products with litmus indicator to see whether they are acidic or alkaline. Found in citrus fruit
Lesson Aims- Understand what acids and alkalis are, and where they are found. Test a range of household products with litmus indicator to see whether they are acidic or alkaline. Found in citrus fruit
What Do You Think? Investigate GOALS
 Activity 2 More Chemical Changes GOALS In this activity you will: Observe several typical examples of evidence that a chemical change is occurring. Make generalizations about the combinations of materials
Activity 2 More Chemical Changes GOALS In this activity you will: Observe several typical examples of evidence that a chemical change is occurring. Make generalizations about the combinations of materials
Chapter 2: Properties of Matter Student Outline 2.1 Classifying Matter A. Pure Substances
 Name: Date: Physical Science Period: Chapter 2: Properties of Matter Student Outline GA Performance Standards SPS1. Students will investigate our current understanding of the atom. SPS2. Students will
Name: Date: Physical Science Period: Chapter 2: Properties of Matter Student Outline GA Performance Standards SPS1. Students will investigate our current understanding of the atom. SPS2. Students will
Cell Biology. Water, Acids, Bases and Buffers. Water makes up 70-99% of the weight of most living organisms Water
 Cell Biology Water, Acids, Bases and Buffers WATER CHEMISTRY Water makes up 70-99% of the weight of most living organisms Water Hydrogen bonded to Oxygen by covalent bond Polar molecule (electrons find
Cell Biology Water, Acids, Bases and Buffers WATER CHEMISTRY Water makes up 70-99% of the weight of most living organisms Water Hydrogen bonded to Oxygen by covalent bond Polar molecule (electrons find
Science in the Kitchen
 Program Support Notes by: Margaret Bishop B.Ed, Dip T Produced by: VEA Pty Ltd Commissioning Editor: Sandra Frerichs B.Ed, M.Ed. Executive Producer: Simon Garner B.Ed, Dip Management Davis Film and Video
Program Support Notes by: Margaret Bishop B.Ed, Dip T Produced by: VEA Pty Ltd Commissioning Editor: Sandra Frerichs B.Ed, M.Ed. Executive Producer: Simon Garner B.Ed, Dip Management Davis Film and Video
KITCHEN CHEMISTRY Identifying acids and bases with red cabbage indicator
 KITCHEN CHEMISTRY Identifying acids and bases with red cabbage indicator By Darby Sloss and Marianne Smith Edited by Anne Starace Abstract Chemistry is an important part of our lives. Kitchen Chemistry
KITCHEN CHEMISTRY Identifying acids and bases with red cabbage indicator By Darby Sloss and Marianne Smith Edited by Anne Starace Abstract Chemistry is an important part of our lives. Kitchen Chemistry
Chemistry Teleclass Webinar!
 Welcome to the Supercharged Science Chemistry Teleclass Webinar! You can fill out this worksheet as we go along to get the most out of time together, or you can use it as a review exercise at the end of
Welcome to the Supercharged Science Chemistry Teleclass Webinar! You can fill out this worksheet as we go along to get the most out of time together, or you can use it as a review exercise at the end of
Lab #2 Biology 10 BCC Topic: Chemistry in Practice
 Lab #2 Biology 10 BCC Topic: Chemistry in Practice Chemistry is a vast field of study in and of itself. In Biology, we use chemistry as a tool to help us understand how things relate to each other on a
Lab #2 Biology 10 BCC Topic: Chemistry in Practice Chemistry is a vast field of study in and of itself. In Biology, we use chemistry as a tool to help us understand how things relate to each other on a
11. Introduction to Acids, Bases, ph, and Buffers
 11. Introduction to Acids, Bases, ph, and Buffers What you will accomplish in this experiment You ll use an acid-base indicating paper to: Determine the acidity or basicity of some common household substances
11. Introduction to Acids, Bases, ph, and Buffers What you will accomplish in this experiment You ll use an acid-base indicating paper to: Determine the acidity or basicity of some common household substances
CHEMISTRY'S RAINBOW: THE POWER OF ph
 CHEMISTRY'S RAINBOW: THE POWER OF ph MATERIALS PER GROUP: Water Citric acid (C 6 H 8 O 7 ) Sodium carbonate (Na 2 CO 3 ) Universal indicator Universal indicator color chart Nine small (3 oz.) clear plastic
CHEMISTRY'S RAINBOW: THE POWER OF ph MATERIALS PER GROUP: Water Citric acid (C 6 H 8 O 7 ) Sodium carbonate (Na 2 CO 3 ) Universal indicator Universal indicator color chart Nine small (3 oz.) clear plastic
The Water Molecule. Like all molecules, a water molecule is neutral. Water is polar. Why are water molecules polar?
 Properties of Water The Water Molecule Like all molecules, a water molecule is neutral. Water is polar Why are water molecules polar? Polarity oxygen atom 8 protons in its nucleus has a much stronger attraction
Properties of Water The Water Molecule Like all molecules, a water molecule is neutral. Water is polar Why are water molecules polar? Polarity oxygen atom 8 protons in its nucleus has a much stronger attraction
Chapter 6, Lesson 10: Carbon Dioxide Can Make a Solution Acidic
 Chapter 6, Lesson 10: Carbon Dioxide Can Make a Solution Acidic Key Concepts Carbon dioxide (CO 2 ) gas dissolved in water can cause water to become acidic. The acidity of water from dissolved CO 2 can
Chapter 6, Lesson 10: Carbon Dioxide Can Make a Solution Acidic Key Concepts Carbon dioxide (CO 2 ) gas dissolved in water can cause water to become acidic. The acidity of water from dissolved CO 2 can
Chapter 2: Chemical Basis of Life
 Chapter 2: Chemical Basis of Life Honors Biology 2011 1 Chemistry of Life Living organisms are composed of about 25 chemical elements Matter - anything that occupies space and has mass Matter is composed
Chapter 2: Chemical Basis of Life Honors Biology 2011 1 Chemistry of Life Living organisms are composed of about 25 chemical elements Matter - anything that occupies space and has mass Matter is composed
Science 5 - Sawyer Matter [Exam ID:6291]
![Science 5 - Sawyer Matter [Exam ID:6291] Science 5 - Sawyer Matter [Exam ID:6291]](/thumbs/94/121035538.jpg) Science 5 - Sawyer Matter [Exam I:6291] 1 Which of the diagrams best shows the arrangement of molecules in a solid? 2 Water, ice, and steam are alike because they are the same compound have the same shape
Science 5 - Sawyer Matter [Exam I:6291] 1 Which of the diagrams best shows the arrangement of molecules in a solid? 2 Water, ice, and steam are alike because they are the same compound have the same shape
Student Notes. Chemical Reactions LINK
 LCPS Core Experience Chemical Reactions Student Notes OBJECTIVES Students will: investigate the relationship between reactants and products. investigate an exothermic reaction. investigate an endothermic
LCPS Core Experience Chemical Reactions Student Notes OBJECTIVES Students will: investigate the relationship between reactants and products. investigate an exothermic reaction. investigate an endothermic
Characteristics of Chemical Change
 Section 2 Characteristics of Chemical Change What Do You See? Learning Outcomes In this section you will Observe several typical examples of evidence that a chemical change is occurring. Make generalizations
Section 2 Characteristics of Chemical Change What Do You See? Learning Outcomes In this section you will Observe several typical examples of evidence that a chemical change is occurring. Make generalizations
Chemical reactions are processes in which the atoms of one or more substances are rearranged to form different chemical compounds.
 Chapter 11 Chemical Reactions Notes Chemical Reactions: Chemical reactions are processes in which the atoms of one or more substances are rearranged to form different chemical compounds. How to tell if
Chapter 11 Chemical Reactions Notes Chemical Reactions: Chemical reactions are processes in which the atoms of one or more substances are rearranged to form different chemical compounds. How to tell if
Wherever chemical solutions are involved, ph matters. Some
 47 Acids, Bases, and the ph Scale R EA D I N G Wherever chemical solutions are involved, ph matters. Some important chemical reactions, such as those involved in corrosion of iron or digestion of food,
47 Acids, Bases, and the ph Scale R EA D I N G Wherever chemical solutions are involved, ph matters. Some important chemical reactions, such as those involved in corrosion of iron or digestion of food,
Chemical reactions are processes in which the atoms of one or more substances are rearranged to form different chemical compounds.
 Chapter 9 Chemical Reactions Notes Chemical Reactions: Chemical reactions are processes in which the atoms of one or more substances are rearranged to form different chemical compounds. How to tell if
Chapter 9 Chemical Reactions Notes Chemical Reactions: Chemical reactions are processes in which the atoms of one or more substances are rearranged to form different chemical compounds. How to tell if
THIRD GRADE OCEANS 1 WEEK LESSON PLANS AND ACTIVITIES
 THIRD GRADE OCEANS 1 WEEK LESSON PLANS AND ACTIVITIES WATER CYCLE OVERVIEW OF THIRD GRADE WATER WEEK 1. PRE: Comparing the different components of the water cycle. LAB: Contrasting water with hydrogen
THIRD GRADE OCEANS 1 WEEK LESSON PLANS AND ACTIVITIES WATER CYCLE OVERVIEW OF THIRD GRADE WATER WEEK 1. PRE: Comparing the different components of the water cycle. LAB: Contrasting water with hydrogen
INTRODUCTION TO LESSON CLUSTER 5
 INTRODUCTION TO LESSON CLUSTER 5 EXPLAINING DISSOLVING A. Lesson Cluster Goals and Lesson Objectives Goals Students should be able to explain dissolving of solids in liquids in terms of molecules. Lesson
INTRODUCTION TO LESSON CLUSTER 5 EXPLAINING DISSOLVING A. Lesson Cluster Goals and Lesson Objectives Goals Students should be able to explain dissolving of solids in liquids in terms of molecules. Lesson
Chemistry Part 2: We re Not Done Yet!
 Chemistry Part 2: We re Not Done Yet! WOW! Learning about atoms sure was fun! Let s continue our fascinating exploration of chemicals. What is a Chemical? Chemical: A substance having a constant composition
Chemistry Part 2: We re Not Done Yet! WOW! Learning about atoms sure was fun! Let s continue our fascinating exploration of chemicals. What is a Chemical? Chemical: A substance having a constant composition
Foundations of Chemistry
 Name Foundations of Chemistry What is matter, and how does it change? Date Before You Read Before you read the chapter, think about what you know about matter and how it changes Record three things that
Name Foundations of Chemistry What is matter, and how does it change? Date Before You Read Before you read the chapter, think about what you know about matter and how it changes Record three things that
Chapter 2 The Chemistry of Life
 Chapter 2 The Chemistry of Life The Water - polarity H 2 O is a polar A water is polar because there is an uneven distribution of electrons between the oxygen and hydrogen atoms. Water Sec. 2-2 Properties
Chapter 2 The Chemistry of Life The Water - polarity H 2 O is a polar A water is polar because there is an uneven distribution of electrons between the oxygen and hydrogen atoms. Water Sec. 2-2 Properties
States of Matter: A Solid Lesson where Liquids Can be a Gas!
 TEACHER GUIDE STATES OF MATTER 60 Minute Physical Science Lesson Science- to- Go! Program Grades: 1-3 States of Matter: A Solid Lesson where Liquids Can be a Gas! Description Your classroom will be converted
TEACHER GUIDE STATES OF MATTER 60 Minute Physical Science Lesson Science- to- Go! Program Grades: 1-3 States of Matter: A Solid Lesson where Liquids Can be a Gas! Description Your classroom will be converted
Creative Classroom Lessons
 Matter, Matter Everywhere! Solids, Liquids and Gases Unit Creative Classroom Lessons Contents: 1. Solids, Liquids and gases student folder cover 2. Introduction Activity: Mystery Box 3. Solids, Liquids
Matter, Matter Everywhere! Solids, Liquids and Gases Unit Creative Classroom Lessons Contents: 1. Solids, Liquids and gases student folder cover 2. Introduction Activity: Mystery Box 3. Solids, Liquids
Welcome to the Supercharged Science. Chemistry Course!
 Welcome to the Supercharged Science Chemistry Course! You can fill out this worksheet as we go along to get the most out of time together, or you can use it as a review exercise at the end of the class
Welcome to the Supercharged Science Chemistry Course! You can fill out this worksheet as we go along to get the most out of time together, or you can use it as a review exercise at the end of the class
6TH GRADE UNIT 2 STUDY GUIDE
 6TH GRADE UNIT 2 STUDY GUIDE 6thgradeUnit2StudyGuide:StatesofMatter I. Vocabulary Indicate how students are going to demonstrate accountability for completing the correctives. Example Signature. Matter:
6TH GRADE UNIT 2 STUDY GUIDE 6thgradeUnit2StudyGuide:StatesofMatter I. Vocabulary Indicate how students are going to demonstrate accountability for completing the correctives. Example Signature. Matter:
Matter and Its Properties
 Section 2 4A, 4B, 4C, 4D Main Ideas Atoms are the building blocks of matter. All substances have characteristic properties. Matter can be a pure substance or a mixture. 4A differentiate between physical
Section 2 4A, 4B, 4C, 4D Main Ideas Atoms are the building blocks of matter. All substances have characteristic properties. Matter can be a pure substance or a mixture. 4A differentiate between physical
CLASS COPY Structure and Properties of Matter Parts of the atom
 CLASS COPY Structure and Properties of Matter Parts of the atom An atom is made up of protons, neutrons, and electrons. Look at the model of a carbon atom from the graphite in the point of a pencil. Protons
CLASS COPY Structure and Properties of Matter Parts of the atom An atom is made up of protons, neutrons, and electrons. Look at the model of a carbon atom from the graphite in the point of a pencil. Protons
Interactions of Matter/ Chemistry Grade 8
 Interactions of Matter/ Chemistry Grade 8 Page 1 The activities in this teacher s guide were created by the Center for Inquiry- Based Learning (CIBL) to accompany the materials in the Interactions of Matter/
Interactions of Matter/ Chemistry Grade 8 Page 1 The activities in this teacher s guide were created by the Center for Inquiry- Based Learning (CIBL) to accompany the materials in the Interactions of Matter/
Chapter 15 Study Questions
 Chapter 15 Study Questions Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. 1. Which of the following describes lipids? a. used to store energy
Chapter 15 Study Questions Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. 1. Which of the following describes lipids? a. used to store energy
Name: Date: Number: Acids
 Acids The sour taste of the lemon juice tells us that it is an acid. Acids are special kinds of chemicals. They are common in everyday life. Some are helpful, others are harmful. There are some that are
Acids The sour taste of the lemon juice tells us that it is an acid. Acids are special kinds of chemicals. They are common in everyday life. Some are helpful, others are harmful. There are some that are
CHEMICAL REACTION IN A BAGGY (MODIFIED FOR ADEED)
 CHEMICAL REACTION IN A BAGGY (MODIFIED FOR ADEED) Overview: Students investigate chemical reactions using given substances. Students identify chemical reactions in their daily lives. Objectives: The student
CHEMICAL REACTION IN A BAGGY (MODIFIED FOR ADEED) Overview: Students investigate chemical reactions using given substances. Students identify chemical reactions in their daily lives. Objectives: The student
Unit 2.3: Water, Acids, and Bases
 Unit 2.3: Water, Acids, and Bases Lesson Objectives Describe the distribution of Earth s water. Identify water s structure and properties. Define acids, bases, and ph. Explain why water is essential for
Unit 2.3: Water, Acids, and Bases Lesson Objectives Describe the distribution of Earth s water. Identify water s structure and properties. Define acids, bases, and ph. Explain why water is essential for
