LESSON 9: Ageless Apples ESTIMATED TIME Setup: 5 10 minutes Procedure: The effects can be observed over a 24-hour time period.
|
|
|
- Eleanor Alyson Harris
- 5 years ago
- Views:
Transcription
1 LESSON 9: Ageless Apples ESTIMATED TIME Setup: 5 10 minutes Procedure: The effects can be observed over a 24-hour time period. DESCRIPTION Place apple slices into solutions with different levels of acidity to change the rate at which the apples turn brown. OBJECTIVE This lesson introduces acids, bases, and the ph scale as a measure of acidity. Students apply solutions with different levels of acidity to apple slices to determine what level of acidity works best to keep the apple slices from browning. This lesson can be extended to introduce proteins, amino acids, and enzymes. CONTENT TOPICS Scientific inquiry; properties of matter; chemical reactions; acids and bases; food chemistry; chemistry in the human body MATERIALS o Lemon juice o Baking soda o Water (preferably distilled) o Apples o Plastic sandwich bags with a snap or zip closure (or small bowls with lids) o Permanent markers o Measuring cups and spoons o Knife or apple slicer Always remember to use the appropriate safety equipment when conducting your experiment. Refer to the Safety First section in the Resource Guide on pages for more detailed information about safety in the classroom. Jump ahead to page 117 to view the Experimental Procedure. NATIONAL SCIENCE EDUCATION STANDARDS SUBJECT MATTER This lesson applies both Dimension 1: Scientific and Engineering Practices and Dimension 2: Crosscutting Concepts from A Framework for K 12 Science Education, established as a guide for the updated National Science Education Standards. In addition, this lesson covers the following Disciplinary Core Ideas from that framework: PS1.A: Structure and Properties of Matter PS1.B: Chemical Reactions ETS2.B: Influence of Engineering, Technology, and Science on Society and the Natural World (see Analysis & Conclusion) OBSERVATION & RESEARCH BACKGROUND Matter may be classified as either a pure substance or a mixture. A mixture is made of two or more substances that are combined physically. The different parts of a mixture have different properties. Scientists are able to separate mixtures into their original parts. A solution is a uniform mixture in which one or more substances (solutes) are dissolved in another substance (solvent). For example, salt may be dissolved in water to form a saltwater solution. The salt is the solute, and the water is the solvent. Pure substances and mixtures may experience changes, and these changes can be either physical or chemical. A physical change is any change in a substance s form that does not change its chemical makeup. The chemical formula of the substance stays the same before and after the change. Cutting an apple is an example of a physical change. A chemical change or chemical reaction is a change that takes place when the structure or composition of the materials changes. When a chemical change is complete, the resulting substance(s) is/are different from the original substance(s). The browning of an apple exposed to air over time is an example of a chemical change. You Be The LESSON Chemist 1: Activity Goofy Guides Putty page
2 LESSON 9: Ageless Apples CONNECT TO THE YOU BE THE CHEMIST CHALLENGE For additional background information, please review CEF s Challenge study materials online at Additional information on physical and chemical properties and changes can be found in the Classification of Matter section of CEF s Passport to Science Exploration: The Core of Chemistry. Additional information on acids and bases can be found in the Acids, Bases, and ph section of CEF s Passport to Science Exploration: Chemistry Connections. Additional information on food chemistry and preservation can be found in the Applications of Chemistry in Everyday Life section of CEF s Passport to Science Exploration: Chemistry Concepts in Action. To describe certain substances, chemists may use the terms acid and base. The ph scale measures how acidic or basic a substance is, and it ranges from 0 to 14. Acids generally have a ph below 7, have a sour taste (be sure to remind students that they should never taste an unknown substance to determine what it is!), and are corrosive. Common household acids include lemon juice, vinegar, soda pop, and orange juice. Bases generally have a ph above 7 and are slippery to the touch (again, remind students they should never touch an unknown substance without proper protection, like gloves). Common household bases include ammonia, baking soda, milk of magnesia, borax, and bleach. Apples (and apple juice) are acidic. They are also perishable, which means that they will eventually go bad or decay. When apple slices are exposed to oxygen, certain compounds in the apple, called enzymes, cause the exposed flesh of the apple to turn brown. The rate at which this browning process occurs can be slowed down by making those enzymes less active. The enzymes become less active at lower ph levels. Therefore, applying an acid to the surface of the apple slice slows the activity of the enzymes and keeps the apple from browning. Basic solutions, such as a baking soda solution, will not lower the ph level on the surface of the apple slices. Lemon juice is often used to keep fruit from browning because lemon juice has a very low ph level. Applying lemon juice to the surface of sliced apples increases the acidity on the surface to a ph level below 2. This low ph reduces the activity of the enzymes in the apple and slows down the browning process. FORMULAS & EQUATIONS Lemon juice gets its acidity from citric acid. The chemical formula for citric acid is C6H8O7. Ascorbic acid, commonly known as vitamin C, is also found in many fruits. The chemical formula for ascorbic acid is C6H8O6. The vitamin C and citric acid found in citrus fruits, such as lemons, oranges, and limes, help to prevent browning. Sodium bicarbonate is commonly known as baking soda. The chemical formula for sodium bicarbonate is NaHCO3. The baking soda solution used in this lesson has a higher concentration of hydroxide ions and a ph level of about 9.0. Distilled water (pure water) is neutral. It has a ph level of 7.0 and is, therefore, neither acidic nor basic. The chemical formula for pure water is H2O. Regular tap water may be slightly acidic. If you use regular tap water, you may want to discuss the difference between tap water and distilled water with your students. HYPOTHESIS uacids and bases will have different effects on the rate at which apple slices turn brown because of the differences in their ph levels. You Be The Chemist Activity Guide page 115
3 LESSON 9: Ageless Apples DIFFERENTIATION IN THE CLASSROOM LOWER GRADE LEVELS/BEGINNERS Use the experiment to discuss physical and chemical properties. Physical properties can be observed by using our senses and taking measurements. Some examples of physical properties are color, texture, shape, melting point, and density. Chemical properties can be identified by observing how a chemical reacts with other substances. Some examples of chemical properties include acidity, toxicity, and flammability. During the experiment, students can observe the different physical and chemical properties of the substances. Likewise, use the same method to go over physical and chemical changes in more detail. For example, show a picture of cake batter and then a baked cake. Ask how they know it s a chemical change. Then, show a picture of a cake cut in pieces. It s a physical change! HIGHER GRADE LEVELS/ADVANCED STUDENTS DESCRIPTION Use solutions of varying ph levels to affect the enzyme activity in an apple and change the rate at which the apple slices turn brown. OBJECTIVE This lesson introduces acids, bases, and the ph scale as a measure of acidity. Students apply different solutions to apple slices to determine what ph level works best to keep the apple slices from browning and learn how acidity relates to enzyme activity. OBSERVATION & RESEARCH To describe certain chemical compounds, chemists may use the terms acid and base. In general, a solution that contains a concentration of hydrogen ions (H + ) greater than the H + concentration in pure water is called an acid. Common household acids include lemon juice, vinegar, soda pop, and orange juice. Likewise, a solution containing an excess of hydroxide ions (OH - ) or an H + concentration less than that of pure water is called a base. Common household bases include ammonia, baking soda, milk of magnesia, borax, and bleach. Solutions containing H + concentrations equal to that of pure water are neutral. The concentrations of hydrogen ions in acids and bases are measured on the ph scale. The higher the concentration of H +, the lower the ph will be. A substance with a ph lower than 7 is considered to be acidic. The lower the concentration of H +, the higher the ph will be. A substance with a ph higher than 7 is considered to be basic. Most substances range from 0 to 14 on the ph scale, with 0 being the most acidic, 7 being neutral, and 14 being the most basic. Pure water is a neutral substance with a ph of 7. Most foods contain proteins. Proteins are complex organic compounds that are involved in almost all cell functions. Enzymes are proteins that can help to increase the rate of chemical reactions. When apples and many other fruits are cut, they may turn brown quickly. This browning occurs as a result of the exposure to oxygen in air. When a fruit is cut or bruised, its cells become damaged, allowing oxygen in the air to react with a certain type of chemical compound called a phenol. The rate of this oxidation reaction is increased by a certain enzyme, which is also present in the fruit. To slow down the rate at which a fruit turns brown, chemical compounds are used to control the activity of the enzymes. The activity of enzymes can be reduced by lowering the ph on the surface of the exposed fruit. The most common treatment is to apply ascorbic acid (vitamin C) to the fruit. The increase in the acidity decreases the rate of the chemical reaction between the phenols in the fruit and the oxygen. Therefore, the apple slice will remain crisp and white in color for a longer period of time. CONNECT TO THE YOU BE THE CHEMIST CHALLENGE For additional background information, please review CEF s Challenge study materials online at Additional information on physical and chemical changes can be found in the Classification of Matter section of CEF s Passport to Science Exploration: The Core of Chemistry. Additional information on acids and bases can be found in the Acids, Bases, and ph section of CEF s Passport to Science Exploration: Chemistry Connections. Additional information on proteins can be found in the Organic Chemistry section of CEF s Passport to Science Exploration: Chemistry Concepts in Action. You Be The Chemist Activity Guide page 116
4 EXPERIMENTATION LESSON 9: Ageless Apples As the students perform the experiment, challenge them to identify the independent, dependent, and controlled variables, as well as whether there is a control setup for the experiment. (Hint: If the level of acidity of the solutions changes, will the results change?) Review the information in the Scientific Inquiry section on pages to discuss variables. EXPERIMENTAL PROCEDURE 1. Use a marker to label three plastic bags. Label one lemon juice, the second baking soda, and the third water. 2. Pour ¼ cup of lemon juice into the bag labeled lemon juice. 3. Create a baking soda solution by mixing ¼ cup of water with one tablespoon of baking soda. Pour the baking soda solution into the plastic bag labeled baking soda. 4. Pour ¼ cup of water into the plastic bag labeled water. 5. Cut an apple or apples into evenly sliced pieces. Sharp objects like knives can be dangerous. For safety, cut the apples for the students, and then have them come to the front of the class to get their apple slices once they have prepared their bags. Although common household acids and bases are diluted, they can still pose risks. Proper safety procedures should be followed to protect your eyes, skin, clothing, and work surfaces. Never use the sense of taste in the lab. Do not allow students to eat the apples or taste the solutions. DATA COLLECTION Have students record data in their science notebooks or on the following activity sheet. For example, which substances were acids and which were bases? How long did the apple slices take to brown in each bag? You can use the table provided in the activity sheet (or a similar one of your own) for students to record their data. NOTES 6. Place two to three apple slices into each of the three bags. Seal the bags tightly. Gently shake the bag to make sure the apple slices are completely coated by the solution in the bag. 7. Take the apples out of the lemon juice bag, and place them on a table on top of the bag. Do the same for the apples in the other bags. Be sure to lay them on top of the corresponding bag, so you know which apples were coated with which solution. 8. Observe the apple slices immediately following the treatment. Then, set the apple slices aside for a few hours (or a day or two), and observe the differences in the color of the apples. You Be The Chemist Activity Guide page 117
5 LESSON 9: Ageless Apples ANALYSIS & CONCLUSION Use the questions from the activity sheet or your own questions to discuss the experimental data. Ask students to determine whether they should accept or reject their hypotheses. Review the information in the Scientific Inquiry section on pages to discuss valid and invalid hypotheses. ASSESSMENT/GOALS Upon completion of this lesson, students should be able to Apply a scientific inquiry process and perform an experiment. Define acids and bases and explain the differences between them. Identify common household acids and bases. Explain ph and the ph scale. Define and identify chemical reactions. Understand the process of oxidation as a chemical reaction (see Differentiation in the Classroom). Understand the role of enzymes in chemical reactions (see Differentiation in the Classroom). Fun Fact Our stomachs contain gastric acid, which is mainly hydrochloric acid. It has a ph varying from 1 to 3. The high level of acidity is one of the causes of heartburn. Fun Fact An antacid is taken to relieve heartburn. It is comprised of basic solutions that neutralize the stomach acids to provide relief. MODIFICATIONS/EXTENSIONS Modifications and extensions provide alternative methods for performing the lesson or similar lessons. They also introduce ways to expand on the content topics presented and think beyond those topics. Use the following examples or have a discussion to generate other ideas as a class. Consider using different household acids and bases to test their effects on the apple slices. You could bring in various substances, such as lemon juice, vinegar, cola, a baking soda solution, and milk of magnesia. Then, allow students to choose two substances. Once they observe the reactions, have them guess whether the substance is an acid or base. Instead of water, use plain apple slices as a control. (Do not put them in any substance. Just cut them and leave them out.) Then discuss the use of controls in experiments. REAL-WORLD APPLICATIONS Chemistry is important in keeping food fresh. Chemicals are added to foods to preserve their freshness, shelf life, and flavor. Challenge your students to look for a list of chemical preservatives on the labels of on their favorite foods. Also discuss the importance of certain preservation methods to their health. For example, pasteurization kills bacteria in milk so that it is safer to drink. The acidity of foods can also affect your health. Discuss the discovery of citrus fruits as a means of preventing scurvy among explorers, pirates, and other early sailors. Talk about how it is important to consume sources of vitamin C daily. COMMUNICATION Discuss the results as a class and review the activity sheet. Review the information in the Scientific Inquiry section on pages to discuss the importance of communication to scientific progress. You Be The Chemist Activity Guide page 118
6 OBSERVE & RESEARCH 1. Write down the materials you see. 2. How might these materials be used? 3. Define the following key terms. Then, provide an example of each by writing the example or drawing/pasting an image of the example. Term Definition Example (write or add image) Mixture Solution Physical change Chemical reaction Acid Base 4. Consider what will happen if apple slices are coated with the different solutions and why. uwrite your hypothesis. You Be The Chemist Activity Guides page 119
7 PERFORM YOUR EXPERIMENT 1. Use a marker to label three plastic bags. Label one lemon juice. Label the second baking soda. Label the third water. 2. Pour ¼ cup of lemon juice into the bag labeled lemon juice. 3. Create a baking soda solution. Mix ¼ cup of water with one tablespoon of baking soda. Pour the baking soda solution into the plastic bag labeled baking soda. 4. Pour ¼ cup of water into the plastic bag labeled water. 5. Get six to nine apple slices from your teacher. 6. Place two to three apple slices into each of the three bags. Seal the bags tightly. Gently shake the bag to make sure the apple slices are completely coated by the solution in the bag. 7. Take the apple slices out of the lemon juice bag. Place them on a table on top of the bag. Do the same for the apple slices in the other bags. Be sure to lay them on top of the right bag so you know which apple slices were coated with which solution. 8. Observe the apple slices immediately following the treatment. Then, set the apple slices aside. Observe them later at your teacher s direction. ANALYZE & CONCLUDE 1. Record your observations of the reactions in the table below at different time intervals. For example the first row of the table might read: lemon juice, 30 minutes, white. Coating Substance Time Passed Color of Apple Slices You Be The Chemist Activity Guide page 120
8 2. What effect did bases have on the apple slices? What effect did acids have? 3. List other household substances that you think are acids. 4. List other household substances that you think are bases. 5. Why is it useful for a chemist to know if a chemical is an acid or a base? 6. If you have an apple for lunch but don t finish it, what is the best way to keep it fresh and crisp? 7. Is your hypothesis valid? Why or why not? If not, what would be your next steps? You Be The Chemist Activity Guides page 121
9 EXPAND YOUR KNOWLEDGE ADVANCED 1. Define the following key terms. Then, provide an example of each by writing the example or drawing/pasting an image of the example. Term Definition Example (write or add image) ph scale Protein Enzyme 2. Where are proteins found in the human body? What is their role? 3. List some chemical reactions in the human body that use enzymes. You Be The Chemist Activity Guide page 122
10 ANSWER KEY Below are suggested answers. Other answers may also be acceptable. OBSERVE & RESEARCH 1. Write down the materials you see. Lemon juice, baking soda, water, apples 2. How might these materials be used? Lemon juice and baking soda may be used for cooking. Water is used for drinking, cleaning, and many other things. Apples are a healthy food to eat. The different materials may be combined so the browning of apple slices (a chemical reaction) occurs at different rates. 3. Define the following key terms. Then, provide an example of each by writing the example or drawing/pasting an image of the example. Term Definition Example (write or add image) Mixture A physical combination of two or more substances that can be physically separated. Solution A homogeneous (uniform) mixture in which one or more substances (solutes) are dissolved in another substance (solvent). Physical change Chemical reaction Acid A change that alters the form or appearance of a substance but does not change its chemical makeup or create a new substance. A change that takes place when atoms of one or more substances are rearranged, and the bonds between the atoms are broken or formed to produce new substances; also known as a chemical change. A solution that contains an excess of hydrogen ions (H + ); acids have a higher concentration of hydrogen ions than pure water. Base A solution that has an excess of hydroxide ions (OH - ); bases have a lower concentration of hydrogen ions than pure water. 4. Consider what will happen if apple slices are coated with the different solutions and why. uwrite your hypothesis. Acids and bases will have different effects on the rate at which apple slices turn brown because of the differences in their ph levels. You Be The Chemist Activity Guide page 123
11 ANSWER KEY Below are suggested answers. Other answers may also be acceptable. PERFORM YOUR EXPERIMENT 1. Use a marker to label three plastic bags. Label one lemon juice. Label the second baking soda. Label the third water. 2. Pour ¼ cup of lemon juice into the bag labeled lemon juice. 3. Create a baking soda solution. Mix ¼ cup of water with one tablespoon of baking soda. Pour the baking soda solution into the plastic bag labeled baking soda. 4. Pour ¼ cup of water into the plastic bag labeled water. 5. Get six to nine apple slices from your teacher. 6. Place two to three apple slices into each of the three bags. Seal the bags tightly. Gently shake the bag to make sure the apple slices are completely coated by the solution in the bag. 7. Take the apple slices out of the lemon juice bag. Place them on a table on top of the bag. Do the same for the apple slices in the other bags. Be sure to lay them on top of the right bag so you know which apple slices were coated with which solution. 8. Observe the apple slices immediately following the treatment. Then, set the apple slices aside. Observe them later at your teacher s direction. ANALYZE & CONCLUDE 1. Record your observations of the reactions in the table below at different time intervals. For example the first row of the table might read: lemon juice, 30 minutes, white. Coating Substance Time Passed Color of Apple Slices Lemon juice 30 minutes White Baking soda solution 30 minutes Slightly brown Water 30 minutes Slightly brown Lemon juice 1 day White or slightly brown Baking soda solution 1 day Completely brown (or dark brown) Water 1 day Completely brown (or dark brown) You Be The Chemist Activity Guide page 124
12 ANSWER KEY Below are suggested answers. Other answers may also be acceptable. 2. What effect did bases have on the apple slices? What effect did acids have? The bases did not have any effect on the browning reaction of the apple slices, so the apples still turned brown quickly. The acids slowed the rate at which the apples turned brown, so they stayed white and crisp longer. 3. List other household substances that you think are acids. Soda pop, vinegar, orange juice, milk, coffee List other household substances that you think are bases. Ammonia, bleach, milk of magnesia, antacids (such as Tums ) Why is it useful for a chemist to know if a chemical is an acid or a base? Acids and bases react differently with other substances and can be dangerous if handled incorrectly. Acids and bases should also never be stored together because they can react violently. Determining the ph of a substance is important to lab safety. 6. If you have an apple for lunch but don t finish it, what is the best way to keep it fresh and crisp? To keep the apple crisp, add some lemon juice or another edible acid to the apple. 7. Is your hypothesis valid? Why or why not? If not, what would be your next steps? Answer 1: Valid because the data support my hypothesis. Answer 2: Invalid because the data do not support my hypothesis. I would reject my hypothesis and could form a new one, such as You Be The Chemist Activity Guide page 125
13 ANSWER KEY Below are suggested answers. Other answers may also be acceptable. EXPAND YOUR KNOWLEDGE ADVANCED Have students complete this section if you used the advanced differentiation information, or challenge them to find the answers to these questions at home and discuss how these terms relate to the experiment in class the next day. 1. Define the following key terms. Then, provide an example of each by writing the example or drawing/pasting an image of the example. Term Definition Example (write or add image) ph scale A scale that is used to measure the acidity of (concentration of hydrogen ions in) a solution; the ph scale generally ranges from 0 to 14. Protein A group of complex organic compounds made up of amino acids and involved in almost all cell functions; proteins help the body to grow and repair damage. Enzyme A type of protein found in living cells that acts as catalyst by increasing the rate of a chemical reaction in living organisms. 2. Where are proteins found in the human body? What is their role? Proteins can be found in human hair, nails, organs, muscles, ligaments, and skin. They help the body to grow and to repair damage. 3. List some chemical reactions in the human body that use enzymes. Enzymes help with digestion and metabolism, as well as cellular respiration. You Be The Chemist Activity Guide page 126
LESSON 6: Dew Drops ESTIMATED TIME Setup: 5 10 minutes Procedure: minutes
 LESSON 6: Dew Drops ESTIMATED TIME Setup: 5 10 minutes Procedure: 15 20 minutes DESCRIPTION Use jars of hot and cold water to demonstrate how water changes states. OBJECTIVE This lesson demonstrates the
LESSON 6: Dew Drops ESTIMATED TIME Setup: 5 10 minutes Procedure: 15 20 minutes DESCRIPTION Use jars of hot and cold water to demonstrate how water changes states. OBJECTIVE This lesson demonstrates the
By All INdICATIONS (2 Hours)
 By All INdICATIONS (2 Hours) Addresses NGSS Level of Difficulty: 5 Grade Range: 6-8 OVERVIEW In this activity, students create an acid-base indicator using red cabbage extract. Students then use this indicator
By All INdICATIONS (2 Hours) Addresses NGSS Level of Difficulty: 5 Grade Range: 6-8 OVERVIEW In this activity, students create an acid-base indicator using red cabbage extract. Students then use this indicator
LESSON 15: Marshmallow Launcher ESTIMATED TIME Setup: minutes Procedure: minutes
 LESSON 15: Marshmallow Launcher ESTIMATED TIME Setup: 10 15 minutes Procedure: 15 20 minutes DESCRIPTION Launch marshmallows from a plastic-spoon catapult to demonstrate the differences between potential
LESSON 15: Marshmallow Launcher ESTIMATED TIME Setup: 10 15 minutes Procedure: 15 20 minutes DESCRIPTION Launch marshmallows from a plastic-spoon catapult to demonstrate the differences between potential
LESSON 26: Swimming Specks ESTIMATED TIME Setup: 5 minutes Procedure: 5 10 minutes
 LESSON 26: Swimming Specks ESTIMATED TIME Setup: 5 minutes Procedure: 5 10 minutes DESCRIPTION Add black pepper to the surface of a bowl of water to observe the properties of density and surface tension.
LESSON 26: Swimming Specks ESTIMATED TIME Setup: 5 minutes Procedure: 5 10 minutes DESCRIPTION Add black pepper to the surface of a bowl of water to observe the properties of density and surface tension.
LESSON 4: Buoyant Butter ESTIMATED TIME Setup: 5 minutes Procedure: 5 10 minutes
 LESSON 4: Buoyant Butter ESTIMATED TIME Setup: 5 minutes Procedure: 5 10 minutes DESCRIPTION Calculate the density of a stick of butter to determine if it will sink or float in water. OBJECTIVE This lesson
LESSON 4: Buoyant Butter ESTIMATED TIME Setup: 5 minutes Procedure: 5 10 minutes DESCRIPTION Calculate the density of a stick of butter to determine if it will sink or float in water. OBJECTIVE This lesson
Understand what acids and alkalis are, and where they are found.
 Lesson Aims- Understand what acids and alkalis are, and where they are found. Test a range of household products with litmus indicator to see whether they are acidic or alkaline. Found in citrus fruit
Lesson Aims- Understand what acids and alkalis are, and where they are found. Test a range of household products with litmus indicator to see whether they are acidic or alkaline. Found in citrus fruit
Post-Show. Chemistry. Periodic Table of the Elements. After the Show. Traveling Science Shows
 Traveling Science Shows Post-Show Chemistry After the Show We recently presented a Chemistry show at your school, and thought you and your students might like to continue investigating this topic. The
Traveling Science Shows Post-Show Chemistry After the Show We recently presented a Chemistry show at your school, and thought you and your students might like to continue investigating this topic. The
Chapter 6, Lesson 9: Neutralizing Acids and Bases
 Chapter 6, Lesson 9: Neutralizing Acids and Bases Key Concepts ph is a measure of the concentration of H 3 O + ions in a solution. Adding an acid increases the concentration of H 3 O + ions in the solution.
Chapter 6, Lesson 9: Neutralizing Acids and Bases Key Concepts ph is a measure of the concentration of H 3 O + ions in a solution. Adding an acid increases the concentration of H 3 O + ions in the solution.
The grade 5 English science unit, Acids and Bases, meets the academic content standards set in the Korean curriculum, which state students should:
 This area addresses ph among different characteristics of solutions. It will be interesting for students to classify a variety of solutions into acids and bases by using the characteristics of the solutions.
This area addresses ph among different characteristics of solutions. It will be interesting for students to classify a variety of solutions into acids and bases by using the characteristics of the solutions.
Mixtures, Solubility, and Acid/Base Solutions
 Mixtures, Solubility, and Acid/Base Soluts Acid and Base Soluts What do you think? Read the two statements below and decide whether you agree or disagree with them. Place an A in the Before column if you
Mixtures, Solubility, and Acid/Base Soluts Acid and Base Soluts What do you think? Read the two statements below and decide whether you agree or disagree with them. Place an A in the Before column if you
Student Notes Acids and Bases
 Name: Class: Date: Student Notes Acids and Bases Many foods that we eat contain acids, such as lemons, oranges, apples, vinegar, grapes, and soda pop. Lemons and oranges contain citric acid, while apples
Name: Class: Date: Student Notes Acids and Bases Many foods that we eat contain acids, such as lemons, oranges, apples, vinegar, grapes, and soda pop. Lemons and oranges contain citric acid, while apples
Acids and Bases. How does ph affect biological solutions? Introduction. Prelab Preparation Review Section 2.3 on acids and bases in your textbook.
 Acids and Bases How does ph affect biological solutions? Learning Objectives To relate the ph scale to how acidic or basic a solution is. To explain how a buffer affects the ph of a solution. Process Objectives
Acids and Bases How does ph affect biological solutions? Learning Objectives To relate the ph scale to how acidic or basic a solution is. To explain how a buffer affects the ph of a solution. Process Objectives
2. What type of bonding allows water to attract other water molecules? 3. What is the difference between solutions and mixtures?
 Biology Lab Name(s) Period: Date: Purpose: To investigate the properties of water, ph, and enzymes that biologically impact biological functions. Background Information: Water: Sometimes we call water
Biology Lab Name(s) Period: Date: Purpose: To investigate the properties of water, ph, and enzymes that biologically impact biological functions. Background Information: Water: Sometimes we call water
CHEMISTRY'S RAINBOW: THE POWER OF ph
 CHEMISTRY'S RAINBOW: THE POWER OF ph MATERIALS PER GROUP: Water Citric acid (C 6 H 8 O 7 ) Sodium carbonate (Na 2 CO 3 ) Universal indicator Universal indicator color chart Nine small (3 oz.) clear plastic
CHEMISTRY'S RAINBOW: THE POWER OF ph MATERIALS PER GROUP: Water Citric acid (C 6 H 8 O 7 ) Sodium carbonate (Na 2 CO 3 ) Universal indicator Universal indicator color chart Nine small (3 oz.) clear plastic
Wherever chemical solutions are involved, ph matters. Some
 47 Acids, Bases, and the ph Scale R EA D I N G Wherever chemical solutions are involved, ph matters. Some important chemical reactions, such as those involved in corrosion of iron or digestion of food,
47 Acids, Bases, and the ph Scale R EA D I N G Wherever chemical solutions are involved, ph matters. Some important chemical reactions, such as those involved in corrosion of iron or digestion of food,
Chemistry: classifying chemical and physical changes in various materials/substances
 Chemistry: classifying chemical and physical changes in various materials/substances Nikki Schilling, Ames, St. Paul Mn Based on original activity from Cool Chemistry Concoctions by Joe Rhatigan and Veronika
Chemistry: classifying chemical and physical changes in various materials/substances Nikki Schilling, Ames, St. Paul Mn Based on original activity from Cool Chemistry Concoctions by Joe Rhatigan and Veronika
SNC2D CHEMISTRY 2/24/2013. CHEMICAL REACTIONS L Acids & Bases (P ) Activity: Introduction to (2DCHEM-ASG3) Introduction to Acids & Bases
 SNC2D CHEMISTRY CHEMICAL REACTIONS L Acids & Bases (P.198-201) Activity: Introduction to (2DCHEM-ASG3) INSTRUCTIONS A. Read the activity 2DCHEM - ASG3 (Introduction to Acids & Bases). B. Follow the instructions
SNC2D CHEMISTRY CHEMICAL REACTIONS L Acids & Bases (P.198-201) Activity: Introduction to (2DCHEM-ASG3) INSTRUCTIONS A. Read the activity 2DCHEM - ASG3 (Introduction to Acids & Bases). B. Follow the instructions
Introducing Science Summary Sheet
 Introducing Science Summary Sheet Acids in the laboratory Dilute acids You will have used some dilute acids at school, such as hydrochloric acid, sulphuric acid and nitric acid. Their bottles are labelled
Introducing Science Summary Sheet Acids in the laboratory Dilute acids You will have used some dilute acids at school, such as hydrochloric acid, sulphuric acid and nitric acid. Their bottles are labelled
Science 10. Unit 2: Chemistry. Book 5: Acid -Base Chemistry & the ph Scale. Block: Name:
 Science 10 Unit 2: Chemistry Book 5: Acid -Base Chemistry & the ph Scale Name: Block: 1 Classifying Substances There are other ways you can use to classify compounds. For example, you can classify some
Science 10 Unit 2: Chemistry Book 5: Acid -Base Chemistry & the ph Scale Name: Block: 1 Classifying Substances There are other ways you can use to classify compounds. For example, you can classify some
Name: Date: Number: Acids
 Acids The sour taste of the lemon juice tells us that it is an acid. Acids are special kinds of chemicals. They are common in everyday life. Some are helpful, others are harmful. There are some that are
Acids The sour taste of the lemon juice tells us that it is an acid. Acids are special kinds of chemicals. They are common in everyday life. Some are helpful, others are harmful. There are some that are
Lab: Cabbages in Chemistry 3pts ec printing in COLOR / 2pts B&W
 Lab: Cabbages in Chemistry 3pts ec printing in COLOR / 2pts B&W Telltale Colors 1. In the mixing tray, place 5 drops of the chemical in 13 compartments 2. DIP test the ph paper & record the ph measurement
Lab: Cabbages in Chemistry 3pts ec printing in COLOR / 2pts B&W Telltale Colors 1. In the mixing tray, place 5 drops of the chemical in 13 compartments 2. DIP test the ph paper & record the ph measurement
2. What characteristic of water makes it the universal solvent? Nonpolar large molecules long-chain hydrocarbon molecules polar
 PS Chemistry Chapter 22 & 23 Review Test Date Chapter 22 Suggestions for Studying Section 1 Know that a solution is made up of a solute and solvent. Be able to provide an example of a solute and a solvent.
PS Chemistry Chapter 22 & 23 Review Test Date Chapter 22 Suggestions for Studying Section 1 Know that a solution is made up of a solute and solvent. Be able to provide an example of a solute and a solvent.
Unit 2.3: Water, Acids, and Bases
 Unit 2.3: Water, Acids, and Bases Lesson Objectives Describe the distribution of Earth s water. Identify water s structure and properties. Define acids, bases, and ph. Explain why water is essential for
Unit 2.3: Water, Acids, and Bases Lesson Objectives Describe the distribution of Earth s water. Identify water s structure and properties. Define acids, bases, and ph. Explain why water is essential for
Exploring Acids & Bases
 Food Explorations Lab: Exploring Acids & Bases STUDENT LAB INVESTIGATIONS Name: Lab Overview In this investigation, you will use cabbage juice indicator to determine if two unknown samples are acids or
Food Explorations Lab: Exploring Acids & Bases STUDENT LAB INVESTIGATIONS Name: Lab Overview In this investigation, you will use cabbage juice indicator to determine if two unknown samples are acids or
How Do Scientists Measure Acidity?
 4.5 Investigate How Do Scientists Measure Acidity? ph scale: a measure of the concentration of hydrogen ions in a substance. neutral: a solution with a ph of 7. ph 7 has an equal number of hydrogen ions
4.5 Investigate How Do Scientists Measure Acidity? ph scale: a measure of the concentration of hydrogen ions in a substance. neutral: a solution with a ph of 7. ph 7 has an equal number of hydrogen ions
Aim: What is ph? Mr. M. Gonzalez. What is ph?
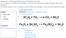
 Aim: Mr. M. Gonzalez Do now: What happens when you combine baking soda and vinegar? A Chemical Reaction!!! Answer The reaction between baking soda (NaHCO3) and vinegar (CH3COOH) is actually two reactions,
Aim: Mr. M. Gonzalez Do now: What happens when you combine baking soda and vinegar? A Chemical Reaction!!! Answer The reaction between baking soda (NaHCO3) and vinegar (CH3COOH) is actually two reactions,
Bio 105 Lab 3: Chemistry: ph and solutions
 1 Bio 105 Lab 3: Chemistry: ph and solutions Part 1. Acid and Base Chemistry A. Introduction BIO 105 Summer 2013 Name One of the most important concepts in biology is acid/base chemistry. We are familiar
1 Bio 105 Lab 3: Chemistry: ph and solutions Part 1. Acid and Base Chemistry A. Introduction BIO 105 Summer 2013 Name One of the most important concepts in biology is acid/base chemistry. We are familiar
Classifying Substances
 Classifying Substances There are other ways you can use to classify compounds. For example, you can classify some compounds as acids or bases. You are very familiar with acids and bases because you see
Classifying Substances There are other ways you can use to classify compounds. For example, you can classify some compounds as acids or bases. You are very familiar with acids and bases because you see
UC Irvine FOCUS! 5 E Lesson Plan Title: Acid/Base-pH Lab Grade Level and Course: 8 th grade Physical Science, Grades 9-12 Chemistry Materials:
 UC Irvine FOCUS! 5 E Lesson Plan Title: Acid/Base-pH Lab Grade Level and Course: 8 th grade Physical Science, Grades 9-12 Chemistry Materials: Detergent-quart, shampoo- quart Lemon-juice-quart Vinegar
UC Irvine FOCUS! 5 E Lesson Plan Title: Acid/Base-pH Lab Grade Level and Course: 8 th grade Physical Science, Grades 9-12 Chemistry Materials: Detergent-quart, shampoo- quart Lemon-juice-quart Vinegar
The Determination of ph of some Common Acids & Bases
 The Determination of ph of some Common Acids & Bases Introduction: An acid is a substance that when dissolved in water produces hydrogen ions, H +. Inorganic acids do not contain carbon but organic acids
The Determination of ph of some Common Acids & Bases Introduction: An acid is a substance that when dissolved in water produces hydrogen ions, H +. Inorganic acids do not contain carbon but organic acids
6.2 The ph Scale and Indicators
 6.2 The ph Scale and Indicators In May 2001, 49 people who attended a dance festival in Dauphin, Manitoba, became sick within a week. The evidence suggests that the source of the illness was the hotel
6.2 The ph Scale and Indicators In May 2001, 49 people who attended a dance festival in Dauphin, Manitoba, became sick within a week. The evidence suggests that the source of the illness was the hotel
CHEMICAL REACTION IN A BAGGY (MODIFIED FOR ADEED)
 CHEMICAL REACTION IN A BAGGY (MODIFIED FOR ADEED) Overview: Students investigate chemical reactions using given substances. Students identify chemical reactions in their daily lives. Objectives: The student
CHEMICAL REACTION IN A BAGGY (MODIFIED FOR ADEED) Overview: Students investigate chemical reactions using given substances. Students identify chemical reactions in their daily lives. Objectives: The student
ACIDS & BASES. Acids & Bases 1
 ACIDS & BASES Acids and bases have real-life significance. The human body functions properly only when delicate acid-base balances are maintained and crops grow best in soil with the proper ph. In addition,
ACIDS & BASES Acids and bases have real-life significance. The human body functions properly only when delicate acid-base balances are maintained and crops grow best in soil with the proper ph. In addition,
Physical & Chemical PROPERTIES
 Name Test Date Hour Group Chem #4 Notebook Properties & Changes LEARNING TARGETS I can give examples of physical and chemical properties. I can give examples of physical and chemical changes. I can identify
Name Test Date Hour Group Chem #4 Notebook Properties & Changes LEARNING TARGETS I can give examples of physical and chemical properties. I can give examples of physical and chemical changes. I can identify
MONDAY (12/12) TUESDAY (12/13) WEDNESDAY (12/14) THURSDAY (12/15) FRIDAY (12/16) Making Acid Rain (a lab) Quiz
 Homework Activities Name: Date: Period: This week, we will be using our knowledge of acids and bases and studying how acids, specifically acid rain, affect our lives and our environment. We will also end
Homework Activities Name: Date: Period: This week, we will be using our knowledge of acids and bases and studying how acids, specifically acid rain, affect our lives and our environment. We will also end
Unit 5 Lesson 2 Acids, Bases, and Salts. Copyright Houghton Mifflin Harcourt Publishing Company
 Donations Accepted What are acids and bases? Acids and bases are chemicals that increase the number of ions present in a water solution when they dissolve. Lemon juice and vinegar both contain acid. Shampoo
Donations Accepted What are acids and bases? Acids and bases are chemicals that increase the number of ions present in a water solution when they dissolve. Lemon juice and vinegar both contain acid. Shampoo
Friday, 09/09/16. Topic: Acids, Bases & Salts (I) Essential Question: By the end of today, Explain differences in acids, bases & salts
 Friday, 09/09/16 P.S.1; P.S. 2-11: Distinguish between chemical and physical properties and changes. Essential Question: How do we differentiate between acids, bases and salts? By the end of today, IWBAT
Friday, 09/09/16 P.S.1; P.S. 2-11: Distinguish between chemical and physical properties and changes. Essential Question: How do we differentiate between acids, bases and salts? By the end of today, IWBAT
EXPERIMENT. for a Weak Acid. Determination of K a
 EXPERIMENT Determination of K a for a Weak Acid Hands-On Labs, Inc. Version 42-0151-00-02 Review the safety materials and wear goggles when working with chemicals. Read the entire exercise before you begin.
EXPERIMENT Determination of K a for a Weak Acid Hands-On Labs, Inc. Version 42-0151-00-02 Review the safety materials and wear goggles when working with chemicals. Read the entire exercise before you begin.
Characteristics of Chemical Change
 Section 2 Characteristics of Chemical Change What Do You See? Learning Outcomes In this section you will Observe several typical examples of evidence that a chemical change is occurring. Make generalizations
Section 2 Characteristics of Chemical Change What Do You See? Learning Outcomes In this section you will Observe several typical examples of evidence that a chemical change is occurring. Make generalizations
What Do You Think? Investigate GOALS
 Activity 2 More Chemical Changes GOALS In this activity you will: Observe several typical examples of evidence that a chemical change is occurring. Make generalizations about the combinations of materials
Activity 2 More Chemical Changes GOALS In this activity you will: Observe several typical examples of evidence that a chemical change is occurring. Make generalizations about the combinations of materials
Name: Block: Date: Student Notes
 Name: Block: Date: LCPS Core Experience Acids and Bases Student Notes OBJECTIVES Students will: recognize some acids and bases as common and familiar household chemicals. realize that acids and bases are
Name: Block: Date: LCPS Core Experience Acids and Bases Student Notes OBJECTIVES Students will: recognize some acids and bases as common and familiar household chemicals. realize that acids and bases are
KITCHEN CHEMISTRY Identifying acids and bases with red cabbage indicator
 KITCHEN CHEMISTRY Identifying acids and bases with red cabbage indicator By Darby Sloss and Marianne Smith Edited by Anne Starace Abstract Chemistry is an important part of our lives. Kitchen Chemistry
KITCHEN CHEMISTRY Identifying acids and bases with red cabbage indicator By Darby Sloss and Marianne Smith Edited by Anne Starace Abstract Chemistry is an important part of our lives. Kitchen Chemistry
Liquid X Lab. Number of Drops Before Spilling Trial 1 Trial 2 Trial 3. Write a conclusion: How do your results for Liquid X compare to water?
 Names Block Date BIG QUESTION: Liquid X Lab Station 1 Surface Tension, Cohesion, and Adhesion Water is cohesive, adhesive, and has surface tension. Does Liquid X have the same properties? 1. Use a pipette
Names Block Date BIG QUESTION: Liquid X Lab Station 1 Surface Tension, Cohesion, and Adhesion Water is cohesive, adhesive, and has surface tension. Does Liquid X have the same properties? 1. Use a pipette
Unit 1: Chemistry in Action
 Unit 1: Chemistry in Action Intermediate 1 Chemistry Learning Outcomes Substances Elements Everything in the world is made from about 100 elements. Each element has a name and a symbol. Chemists have arranged
Unit 1: Chemistry in Action Intermediate 1 Chemistry Learning Outcomes Substances Elements Everything in the world is made from about 100 elements. Each element has a name and a symbol. Chemists have arranged
Drinking water is allowed to contain up to 1.3 parts per million of copper (by mass) and be considered safe. What does parts per million (ppm) mean?
 E One in a Million Drinking water is allowed to contain up to 1. parts per million of copper (by mass) and be considered safe. What does parts per million (ppm) mean? Living things and the environment
E One in a Million Drinking water is allowed to contain up to 1. parts per million of copper (by mass) and be considered safe. What does parts per million (ppm) mean? Living things and the environment
Lyniece McKim Biology Instructor Star Valley High Afton, WY.
 Lyniece McKim Biology Instructor Star Valley High Afton, WY lmckm@lcsd2.org TEACHER SHARE-A-THON NABT 2010 Biology: Chemistry of Life Lab: Testing Mystery Substances Inquiry activity Using indicators;
Lyniece McKim Biology Instructor Star Valley High Afton, WY lmckm@lcsd2.org TEACHER SHARE-A-THON NABT 2010 Biology: Chemistry of Life Lab: Testing Mystery Substances Inquiry activity Using indicators;
Examples of Strong Acids: Strong Acid Formula Common Source Hydrochloric Acid HCl Stomach Acid
 ACIDS AND BASES: PH AND BUFFERS PURPOSE: To determine the ph of common acids and bases using a ph meter, ph paper, and red cabbage indicator. To test the effect of adding an acid or base to a buffer solution.
ACIDS AND BASES: PH AND BUFFERS PURPOSE: To determine the ph of common acids and bases using a ph meter, ph paper, and red cabbage indicator. To test the effect of adding an acid or base to a buffer solution.
Families of Chemical Compounds. Chapter 9
 Families of Chemical Compounds Chapter 9 Groups of Compounds Compounds are grouped based on physical and chemical properties Types: Organic, Acids, Bases, and Salts Acids and Bases Examples of Acids Aspirin
Families of Chemical Compounds Chapter 9 Groups of Compounds Compounds are grouped based on physical and chemical properties Types: Organic, Acids, Bases, and Salts Acids and Bases Examples of Acids Aspirin
Mixing Acids and Bases
 Mixing Acids and Bases Acids and bases are two different kinds of substances. They each produce different kinds of charged particles, called ions, when they dissolve. An acid produces positive hydrogen
Mixing Acids and Bases Acids and bases are two different kinds of substances. They each produce different kinds of charged particles, called ions, when they dissolve. An acid produces positive hydrogen
Standards 8.5.c. I know chemical reactions usually liberate or absorbs heat.
 6 March 2013 Give an example of a physical change and a chemical change, and then describe how they are different from the other. Explain your answer in 2-3 sentences. Standards 8.5.c. I know chemical
6 March 2013 Give an example of a physical change and a chemical change, and then describe how they are different from the other. Explain your answer in 2-3 sentences. Standards 8.5.c. I know chemical
Bags of Reactions Chemistry I Acc
 Introduction: Bags of Reactions Chemistry I Acc Plop, plop, fizz, fizz, oh, what a relief it is. claims the old TV ad for a popular antacid. Just what is in the tablet that is relieving the upset stomach?
Introduction: Bags of Reactions Chemistry I Acc Plop, plop, fizz, fizz, oh, what a relief it is. claims the old TV ad for a popular antacid. Just what is in the tablet that is relieving the upset stomach?
Acids, Bases and ph. Biology Honors. Acidic, Basic or Neutral (indicate if strong or weak acid or base) Substance ph More H + More OH - Vitamin C
 Biology Honors Name Acids, Bases and ph Substance ph More H + More OH - Acidic, Basic or Neutral (indicate if strong or weak acid or base) Vitamin C Tap water Hydrochloric acid (HCl) Sodium hydroxide (NaOH)
Biology Honors Name Acids, Bases and ph Substance ph More H + More OH - Acidic, Basic or Neutral (indicate if strong or weak acid or base) Vitamin C Tap water Hydrochloric acid (HCl) Sodium hydroxide (NaOH)
Chapter 7 Properties of Matter
 Chapter 7 Properties of Matter ALABAMA 8TH Gi&n SCINC STANDARDS COVRD IN THIS CHAPTR INCLUD: Define solution in terms of solute and solvent Defining diffusion and osmosis Defining isotonic, hypertonic
Chapter 7 Properties of Matter ALABAMA 8TH Gi&n SCINC STANDARDS COVRD IN THIS CHAPTR INCLUD: Define solution in terms of solute and solvent Defining diffusion and osmosis Defining isotonic, hypertonic
1 Chemistry Notes Dr. Reeves Science Class (This was me when I had hair.)
 1 Chemistry Notes Dr. Reeves Science Class (This was me when I had hair.) Table of Contents Introduction Slide 5 Topics of Discussion Slide 6 Periodic Table Slide 10 Elements Slide 15 Chemical Formulas
1 Chemistry Notes Dr. Reeves Science Class (This was me when I had hair.) Table of Contents Introduction Slide 5 Topics of Discussion Slide 6 Periodic Table Slide 10 Elements Slide 15 Chemical Formulas
Chapter 6, Lesson 7: Energy Changes in Chemical Reactions
 Chapter 6, Lesson 7: Energy Changes in Chemical Reactions Key Concepts If two substances react and the temperature of the mixture decreases, the reaction is endothermic. If two substances react and the
Chapter 6, Lesson 7: Energy Changes in Chemical Reactions Key Concepts If two substances react and the temperature of the mixture decreases, the reaction is endothermic. If two substances react and the
Properties of Acids and Bases
 Page I - Identification and Classification Introduction Acids and bases are useful reagents in the chemistry laboratory and play an important role in biology and nature. What are acids and bases? What
Page I - Identification and Classification Introduction Acids and bases are useful reagents in the chemistry laboratory and play an important role in biology and nature. What are acids and bases? What
3/26/2011. explosion
 Chemistry Acids and Bases Year 10 Study of Chemicals and how they react Everything is made of chemicals They are made of elements. There are over 100 elements Acids are common Some are dangerous and can
Chemistry Acids and Bases Year 10 Study of Chemicals and how they react Everything is made of chemicals They are made of elements. There are over 100 elements Acids are common Some are dangerous and can
The Chemistry of Color Tie Die
 Ages: 6 to 12 The Chemistry of Color Tie Die Contributor: Dr. Aaron Judy Couture, Laboratory of Atomic and Solid State Physics, Cornell University Main idea: What is an acid, and what is a base? How is
Ages: 6 to 12 The Chemistry of Color Tie Die Contributor: Dr. Aaron Judy Couture, Laboratory of Atomic and Solid State Physics, Cornell University Main idea: What is an acid, and what is a base? How is
Physical Changes vs Chemical Changes Lab
 Physical Changes vs Chemical Changes Lab What was done? What can you observe? Is it a physical or chemical change and why? What else have you learned during the demonstration? #1) Crumpling Paper Notice
Physical Changes vs Chemical Changes Lab What was done? What can you observe? Is it a physical or chemical change and why? What else have you learned during the demonstration? #1) Crumpling Paper Notice
Compound. Math Focus. What are compounds? What is a chemical reaction? How are compounds used in everyday life?
 CHAPTER 3 2 Compounds SECTION Elements, Compounds, and Mixtures BEFORE YOU READ After you read this section, you should be able to answer these questions: What are compounds? What is a chemical reaction?
CHAPTER 3 2 Compounds SECTION Elements, Compounds, and Mixtures BEFORE YOU READ After you read this section, you should be able to answer these questions: What are compounds? What is a chemical reaction?
Liquid X Lab. Station 1 The Penny Lab Water is cohesive, adhesive, and has surface tension. Does Liquid X have the same properties?
 Names Block Date Liquid X Lab Station 1 The Penny Lab Water is cohesive, adhesive, and has surface tension. Does Liquid X have the same properties? 1. Use a pipette to carefully place drops of each liquid
Names Block Date Liquid X Lab Station 1 The Penny Lab Water is cohesive, adhesive, and has surface tension. Does Liquid X have the same properties? 1. Use a pipette to carefully place drops of each liquid
3 Chemical Properties
 CHAPTER 7 3 Chemical Properties SECTION The Properties of Matter BEFORE YOU READ After you read this section, you should be able to answer these questions: What are chemical properties of matter? What
CHAPTER 7 3 Chemical Properties SECTION The Properties of Matter BEFORE YOU READ After you read this section, you should be able to answer these questions: What are chemical properties of matter? What
I. What Are the Properties of Acids?
 Chapter 6.3 DESCRIBING ACIDS AND BASES I. What Are the Properties of Acids? I. An Acid reacts with metals, reacts wth carbonates, tastes sour, and turns blue litmus paper red. A. REACTIONS WITH METALS
Chapter 6.3 DESCRIBING ACIDS AND BASES I. What Are the Properties of Acids? I. An Acid reacts with metals, reacts wth carbonates, tastes sour, and turns blue litmus paper red. A. REACTIONS WITH METALS
Unit 13 Acids and Bases E.Q. What are the differences between acids and bases?
 Unit 13 Acids and Bases E.Q. What are the differences between acids and bases? What are Properties of Acids? They taste sour (don t try this in lab). They can conduct electricity. Can be strong or weak
Unit 13 Acids and Bases E.Q. What are the differences between acids and bases? What are Properties of Acids? They taste sour (don t try this in lab). They can conduct electricity. Can be strong or weak
Student Exploration: Chemical Changes
 Name: Date: Student Exploration: Chemical Changes Vocabulary: acid, base, catalyst, chemical change, coefficient, conservation of matter, decomposition, dissolve, double replacement, endothermic, exothermic,
Name: Date: Student Exploration: Chemical Changes Vocabulary: acid, base, catalyst, chemical change, coefficient, conservation of matter, decomposition, dissolve, double replacement, endothermic, exothermic,
Creating Science Chemical Indicators
 Creating Science Chemical Indicators Chemicals have many properties, and there are some fun ways to learn what some of them are! #CreatingScienceRedCabbageIndicator Suggested Outcomes (NOTE: This is by
Creating Science Chemical Indicators Chemicals have many properties, and there are some fun ways to learn what some of them are! #CreatingScienceRedCabbageIndicator Suggested Outcomes (NOTE: This is by
Acids, Bases and Salts
 Acids, Bases and Salts Synopsis Substances are classified into acids, bases and salts depending on their chemical behavior and properties. Acids are of sour taste and turn blue litmus to red. Bases are
Acids, Bases and Salts Synopsis Substances are classified into acids, bases and salts depending on their chemical behavior and properties. Acids are of sour taste and turn blue litmus to red. Bases are
Have a ph value less than ph 7 Turn blue litmus indicator red Can neutralise an alkali Have a sour taste (WARNING: never taste any chemicals)
 Acids and Alkalis ACIDS (acidic solutions) Acids have the following properties: Have a ph value less than ph 7 Turn blue litmus indicator red Can neutralise an alkali Have a sour taste (WARNING: never
Acids and Alkalis ACIDS (acidic solutions) Acids have the following properties: Have a ph value less than ph 7 Turn blue litmus indicator red Can neutralise an alkali Have a sour taste (WARNING: never
Name Date Class TEKS/TAKS TEST PREPARATION FOR SCIENCE. D Not Here. F It does not show which atoms compose. G It does not show the correct shape of
 CAPTER 4 TEKS/TAKS TEST PREPARATION FOR SCIENCE Practice Test A 1 A sulfur atom must gain two electrons to have a filled outermost energy level. Which statement describes an atom from an element in the
CAPTER 4 TEKS/TAKS TEST PREPARATION FOR SCIENCE Practice Test A 1 A sulfur atom must gain two electrons to have a filled outermost energy level. Which statement describes an atom from an element in the
Chemistry Lab Define Acids and Bases
 Introduction Chemistry Lab Define Acids and Bases By the 1500 s chemists recognized that certain substances shared a common property a sour taste. These substances possessed other characteristic properties
Introduction Chemistry Lab Define Acids and Bases By the 1500 s chemists recognized that certain substances shared a common property a sour taste. These substances possessed other characteristic properties
About Science Prof Online PowerPoint Resources
 About Science Prof Online PowerPoint Resources Science Prof Online (SPO) is a free science education website that provides fully-developed Virtual Science Classrooms, science-related PowerPoints, articles
About Science Prof Online PowerPoint Resources Science Prof Online (SPO) is a free science education website that provides fully-developed Virtual Science Classrooms, science-related PowerPoints, articles
Determining ph. Standards: 5.e. Students know how to determine whether a solution is acidic, basic, or neutral
 Science Stars: 8 th grade Lesson Plan Determining ph Standards: 5.e. Students know how to determine whether a solution is acidic, basic, or neutral Suggested time: 50 minutes Anticipatory Set (Engage):
Science Stars: 8 th grade Lesson Plan Determining ph Standards: 5.e. Students know how to determine whether a solution is acidic, basic, or neutral Suggested time: 50 minutes Anticipatory Set (Engage):
Author: Alan Moghaddam and Rachael Cunningham Title: phun with ph
 Author: Alan Moghaddam and Rachael Cunningham Title: phun with ph Synopsis The audience will be introduced to the main concepts of the ph scale. They will learn the definition of ph and the logarithmic
Author: Alan Moghaddam and Rachael Cunningham Title: phun with ph Synopsis The audience will be introduced to the main concepts of the ph scale. They will learn the definition of ph and the logarithmic
Chapter 6, Lesson 10: Carbon Dioxide Can Make a Solution Acidic
 Chapter 6, Lesson 10: Carbon Dioxide Can Make a Solution Acidic Key Concepts Carbon dioxide (CO 2 ) gas dissolved in water can cause water to become acidic. The acidity of water from dissolved CO 2 can
Chapter 6, Lesson 10: Carbon Dioxide Can Make a Solution Acidic Key Concepts Carbon dioxide (CO 2 ) gas dissolved in water can cause water to become acidic. The acidity of water from dissolved CO 2 can
Chapter 14. Preview Then skip to section 2. Section 1 Ionic and Covalent Compounds. Section 2 Acids and Bases. Section 3 Solutions of Acids and Bases
 Chemical Compounds Preview Then skip to section 2 Section 1 Ionic and Covalent Compounds Section 2 Acids and Bases Section 3 Solutions of Acids and Bases Section 4 Organic Compounds Concept Mapping Section
Chemical Compounds Preview Then skip to section 2 Section 1 Ionic and Covalent Compounds Section 2 Acids and Bases Section 3 Solutions of Acids and Bases Section 4 Organic Compounds Concept Mapping Section
Choose the correct answer. velocity changes in every two seconds by ms -1
 Acceleration Q I Choose the correct answer 1. If a body moves with uniform velocity its acceleration is -----zero. 2. Acceleration of a body is the rate of change of ----velocity 3. The acceleration of
Acceleration Q I Choose the correct answer 1. If a body moves with uniform velocity its acceleration is -----zero. 2. Acceleration of a body is the rate of change of ----velocity 3. The acceleration of
Copyright 2011, 2013 Gravitas Publications, Inc.
 Illustrations: Rebecca W. Keller, PhD Copyright 2011, 2013 Gravitas Publications, Inc. All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted,
Illustrations: Rebecca W. Keller, PhD Copyright 2011, 2013 Gravitas Publications, Inc. All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted,
11. Introduction to Acids, Bases, ph, and Buffers
 11. Introduction to Acids, Bases, ph, and Buffers What you will accomplish in this experiment You ll use an acid-base indicating paper to: Determine the acidity or basicity of some common household substances
11. Introduction to Acids, Bases, ph, and Buffers What you will accomplish in this experiment You ll use an acid-base indicating paper to: Determine the acidity or basicity of some common household substances
Lesson 2. Color change
 Lesson 2 Color change T E A C H E R G U I D E Lesson summary Students meet marine chemist Sera Tuikabe, who is studying ocean acidification in the water surrounding the Republic of the Fiji Islands. Students
Lesson 2 Color change T E A C H E R G U I D E Lesson summary Students meet marine chemist Sera Tuikabe, who is studying ocean acidification in the water surrounding the Republic of the Fiji Islands. Students
Modeling Conservation of Matter
 Modeling Conservation of Matter Imagine that you and two of your classmates want to make a strawberry banana smoothie. You lay out the ingredients: one banana, five strawberries and two scoops of ice cream.
Modeling Conservation of Matter Imagine that you and two of your classmates want to make a strawberry banana smoothie. You lay out the ingredients: one banana, five strawberries and two scoops of ice cream.
Reactants and Products
 Biological Chemical Reactions Chemical reactions allow living things to grow, develop, reproduce, and adapt. Real-World Reading Link When you lie down for the night, you might think that your body is completely
Biological Chemical Reactions Chemical reactions allow living things to grow, develop, reproduce, and adapt. Real-World Reading Link When you lie down for the night, you might think that your body is completely
5 ACIDS, BASES AND SALTS
 5 ACIDS, BASES AND SALTS TEXTBOOK QUESTIONS AND THEIR ANSWERS Q.1. Taste the following substances and enter the result in the following table : Substance Taste (Sour / bitter / any other) Lemon juice Orange
5 ACIDS, BASES AND SALTS TEXTBOOK QUESTIONS AND THEIR ANSWERS Q.1. Taste the following substances and enter the result in the following table : Substance Taste (Sour / bitter / any other) Lemon juice Orange
Online Resource 10. December 2016
 Designing for the Next Generation Standards: Educative Curriculum Materials and Measures of Teacher Knowledge Online Resource 10 December 2016 Toward High School Biology: Tables 1-4 provide evidence that
Designing for the Next Generation Standards: Educative Curriculum Materials and Measures of Teacher Knowledge Online Resource 10 December 2016 Toward High School Biology: Tables 1-4 provide evidence that
Bay Area Scientists in Schools Presentation Plan
 Bay Area Scientists in Schools Presentation Plan Lesson Name Presenter(s) Chemical Reactions Mercedes Taylor, Nick Settineri, Jessica Ziegler, Tyler Hurlburt, Parker Deal Grade Level 5 Standards Connection(s)
Bay Area Scientists in Schools Presentation Plan Lesson Name Presenter(s) Chemical Reactions Mercedes Taylor, Nick Settineri, Jessica Ziegler, Tyler Hurlburt, Parker Deal Grade Level 5 Standards Connection(s)
LEVEL ZERO VOICE CATALYST (10 minutes, individual work): 1. Counting atoms
 Assignment 4 Chemical Detectives at the Molecular Level LO: To explain the differences between chemical and physical changes through observable evidence. EQ: What evidence can be used to tell the difference
Assignment 4 Chemical Detectives at the Molecular Level LO: To explain the differences between chemical and physical changes through observable evidence. EQ: What evidence can be used to tell the difference
Acids, Bases, and Salts
 Acids, Bases, and Salts 1 An acid is a substance that releases hydrogen ions when it dissociates. H +1 Cl -1 Cl -1 H +1 2 An acid is a substance that releases hydrogen ions when it dissociates in water.
Acids, Bases, and Salts 1 An acid is a substance that releases hydrogen ions when it dissociates. H +1 Cl -1 Cl -1 H +1 2 An acid is a substance that releases hydrogen ions when it dissociates in water.
Topic 5 National Chemistry Summary Notes. Acids and Alkalis
 Topic 5 National Chemistry Summary Notes Acids and Alkalis Experiment Collect some samples of rain water LI 1 The ph Scale The ph scale is a continuous range of numbers from below 0 to above 14. Acids
Topic 5 National Chemistry Summary Notes Acids and Alkalis Experiment Collect some samples of rain water LI 1 The ph Scale The ph scale is a continuous range of numbers from below 0 to above 14. Acids
Page 1. Acids, Bases and Salts. Activity series of metals. HA Acids and bases H + + A - A very important class of chemicals.
 Acids, Bases and Salts Acids and bases Acid-Base Theory A very important class of chemicals. Self-Ionization of Water Properties of Acids and Bases The ph Concept Strengths of Acids and Bases Analysis
Acids, Bases and Salts Acids and bases Acid-Base Theory A very important class of chemicals. Self-Ionization of Water Properties of Acids and Bases The ph Concept Strengths of Acids and Bases Analysis
LAB TEST Physical and Chemical Changes
 NAME: DATE: STATION: LAB TEST Physical and Chemical Changes PURPOSE: To observe physical and chemical changes in matter MATERIALS: 3 medium test tubes 1 small test tube test tube rack test tube holder
NAME: DATE: STATION: LAB TEST Physical and Chemical Changes PURPOSE: To observe physical and chemical changes in matter MATERIALS: 3 medium test tubes 1 small test tube test tube rack test tube holder
Experiment 8 - Chemical Changes
 Experiment 8 - Chemical Changes When a chemical change occurs, the chemicals that you start with are changed into different chemicals. We know when this happens because the new chemicals have different
Experiment 8 - Chemical Changes When a chemical change occurs, the chemicals that you start with are changed into different chemicals. We know when this happens because the new chemicals have different
Lab: Detecting ph of Commonly Used Acids and Bases
 Lab: Detecting ph of Commonly Used Acids and Bases FOR THE TEACHER Summary In this lab, students will use their knowledge of acids and bases to determine the acidity and basicity of every day items by
Lab: Detecting ph of Commonly Used Acids and Bases FOR THE TEACHER Summary In this lab, students will use their knowledge of acids and bases to determine the acidity and basicity of every day items by
INTRODUCTION TO LESSON CLUSTER 5
 INTRODUCTION TO LESSON CLUSTER 5 EXPLAINING DISSOLVING A. Lesson Cluster Goals and Lesson Objectives Goals Students should be able to explain dissolving of solids in liquids in terms of molecules. Lesson
INTRODUCTION TO LESSON CLUSTER 5 EXPLAINING DISSOLVING A. Lesson Cluster Goals and Lesson Objectives Goals Students should be able to explain dissolving of solids in liquids in terms of molecules. Lesson
EXPERIMENT. Acid-Base Chemistry
 EXPERIMENT Acid-Base Chemistry Hands-On Labs, Inc. Version 42-0137-00-02 Review the safety materials and wear goggles when working with chemicals. Read the entire exercise before you begin. Take time to
EXPERIMENT Acid-Base Chemistry Hands-On Labs, Inc. Version 42-0137-00-02 Review the safety materials and wear goggles when working with chemicals. Read the entire exercise before you begin. Take time to
The Chemistry of Biology
 The Chemistry of Biology Life depends on chemistry. Living things are composed of chemical compounds. If order to understand biology, one must first understand the chemistry of life. I. The Nature of Matter
The Chemistry of Biology Life depends on chemistry. Living things are composed of chemical compounds. If order to understand biology, one must first understand the chemistry of life. I. The Nature of Matter
Physical Science SPS 6 Review Activity
 Properties of Acids & Bases: in this table, list everything that you know about acids, bases, & their properties. List specific things and general things. (how do you identify them? What makes them, them?
Properties of Acids & Bases: in this table, list everything that you know about acids, bases, & their properties. List specific things and general things. (how do you identify them? What makes them, them?
HarperCollinsPublishers Licensed for home use only. Not for whiteboard or general classroom use
 BIG IDEAS Acids and alkalis You are learning to: Sort common materials into acids and alkalis Explain some properties of acids and alkalis Recognise the common chemical properties of acids and alkalis
BIG IDEAS Acids and alkalis You are learning to: Sort common materials into acids and alkalis Explain some properties of acids and alkalis Recognise the common chemical properties of acids and alkalis
Chemical Reactions. Unit 4
 Chemical Reactions Unit 4 Lesson 1: Chemical Bonds Unit 4: Reactions Compounds Most substances around you are NOT elements. There are around 100 elements, but millions of different substances. Most substances
Chemical Reactions Unit 4 Lesson 1: Chemical Bonds Unit 4: Reactions Compounds Most substances around you are NOT elements. There are around 100 elements, but millions of different substances. Most substances
Science Home Learning Task. Year 8. Acids and alkalis
 Science Home Learning Task Year 8 Acids and alkalis Name Tutor Group Teacher Given out: Monday 6 November Hand in: Monday 13 November Parent/Carer Comment Staff Comment Target Investigating science Welcome
Science Home Learning Task Year 8 Acids and alkalis Name Tutor Group Teacher Given out: Monday 6 November Hand in: Monday 13 November Parent/Carer Comment Staff Comment Target Investigating science Welcome
6.1. How Compounds Form. Chemicals Everywhere. Hydrogen peroxide. Compounds
 6.1 How Compounds Form Here is a summary of what you will learn in this section: Compounds are composed of two or more elements that combine in a specific ratio. Ionic compounds form when metallic and
6.1 How Compounds Form Here is a summary of what you will learn in this section: Compounds are composed of two or more elements that combine in a specific ratio. Ionic compounds form when metallic and
Community in the Classroom Presentation Plan
 Community in the Classroom Presentation Plan Lesson Name How Can You Tell One Clear Gas From Another? Presenter(s) Michael Grass Grade Level 5th Standards Connection(s) Abstract: Many gases are clear and
Community in the Classroom Presentation Plan Lesson Name How Can You Tell One Clear Gas From Another? Presenter(s) Michael Grass Grade Level 5th Standards Connection(s) Abstract: Many gases are clear and
