Chem 350 Jasperse Ch. 6 Summary of Reaction Types, Ch. 4-6, Test 2 1. Radical Halogenation (Ch. 4)
|
|
|
- Vernon Lane
- 5 years ago
- Views:
Transcription
1 Chem 350 Jasperse Ch. 6 Summary of Reaction Types, Ch. 4-6, Test 2 1. Radical alogenation (Ch. 4) 2, hv resonance stabilized>3º>2º>1º>alkenyl 1 Recognition: X 2, hv Predicting product: Identify which carbon could give the most stable radical, and substitute a for an on that carbon. Stereochemistry: Leads to racemic, due to achiral radical intermediate. Mech: Radical. Be able to draw propagation steps. slow step - 2. S N 2 Substitution NaC 3 C 3 ready to repeat first step S N 2: 1º>2º>3º> alkenyl Any of a large variety of nuclophiles or electrophiles can work. Recognition: A. Anionic Nucleophile, and B. 1 or 2º alkyl halide (3º alkyl halides fail, will give E2 upon treatment with Anionic Nucleophile/Base. For 2º alkyl halides, S N 2 is often accompanied by variable amounts of E2.) Predicting product: Replace the halide with the anion nucleophile Stereochemistry: Leads to Inversion of Configuration Mech: Be able to draw completely. nly one concerted step! C 3 C 3 S N 2: 1º>2º>3º> alkenyl 3. E2 Reactions. NaC 3 C 3 E2: 3º>2º>1º> alkenyl Mech: C 3 C 3 Recognition: A. Anionic Nucleophile/Base, and B. 3º or 2º alkyl halide (1º alkyl halides undergo S N 2 instead. For 2º alkyl halides, E2 is often accompanied by variable amounts of S N 2.) rientation: The most substituted alkene forms (unless a bulky base is used, ch. 7) Predicting product: Remove halide and a hydrogen from the neighboring carbon that can give the most highly substituted alkene. The hydrogen on the neighboring carbon must be trans, however. Stereochemistry: Anti elimination. The hydrogen on the neighbor carbon must be trans/anti. Mech: Concerted. Uses anion. Be able to draw completely. nly one concerted step!
2 4. S N 1 Reactions. C 3 C 3 S N 1: resonance >3º>2º>1º> alkenyl 2 Recognition: A. Neutral, weak nucleophile. No anionic nucleophile/base, and B. 3º or 2º alkyl halide. (Controlled by cation stability). (1º alkyl halides undergo S N 2 instead. For 2º alkyl halides, S N 1 is often accompanied by variable amounts of E1.) Predicting product: Remove halide and replace it with the nucleophile (minus an atom!) Stereochemistry: Racemization. The achiral cation intermediate forgets any stereochem. Mech: Stepwise, 3 steps, via carbocation. Be able to draw completely. slow step C 3 C 3 C 3 5. E1 Reactions. 3º > 2º > 1º (Controlled by cation stability) C 3 E1: 3º>2º>1 Recognition: A. Neutral, weak nucleophile. No anionic nucleophile/base, and B. 3º or 2º alkyl halide. (Controlled by cation stability). (For 2º alkyl halides, E1 is often accompanied by variable amounts of S N 1.) rientation: The most substituted alkene forms Predicting the major product: Remove halide and a hydrogen from the neighboring carbon that can give the most highly substituted alkene. The hydrogen on the neighboring carbon can be cis or trans. Stereochemistry: Not an issue. The eliminating hydrogen can be cis or trans.. Mech: Stepwise, 2 steps, via carbocation. Be able to draw completely. slow step Sorting among S N 2, S N 1, E2, E1: ow do I predict? Step 1: Check nucleophile/base. If neutral, then S N 1/E1 mixture of both If anionic, then S N 2/E2. Step 2: If anionic, and in the S N 2/E2, then Check the substrate. o 1º S N 2 o 2º S N 2/E2 mixture. ften more S N 2, but not reliable o 3º E2
3 Ch. 5 Alkyl alides: Nucleophilic Substitution and Elimination 3 6.1,2 Classification, Nomenclature A. General Classification alkyl halide Connected to an sp 3 carbon vinyl halide Connected to an sp 2 alkene carbon aryl halide Connected to an sp 2 aryl carbon allylic halide Connected to an sp 3 carbon that is connected to an sp 2 carbon B. 1º, 2º, 3º Classification 3º 2º 1º C. Systematic Naming: x-aloalkane (test responsible) (Include number!) D. Common Naming: alkyl halide (not tested) Structure Formal Name Common Name 1-chloropropane Propyl chloride Cl 2-bromopentane I 2-iodopropane Isopropyl iodide Systematic Nomenclature: x-aloalkane (test responsible) Common: alkyl halide (not tested) 6.3 Uses: solvents anesthetics refrigerants pesticides reactants
4 6.4 Structure: 4 A. Polar C! X!" B. Weak Bonds, eakable Stability Bond Bond Strength Reactivity Toward eakage Most C-Cl 81 Least C- 68 least C-I 53 Most 6.5 Physical Properties boiling point: controlled by molecular weight (London force) water solubility: low, no hydrogen-bonding density: greater than water, so they sink (unlike hydrocarbons, which float) 6.6 Preparation of Alkyl alides Review: R- 2 R (under photolysis, Ch. 4) We will learn other preparations in chapters 8 and 11
5 5 6.7 Basic verview/preview of Alkyl alide Reactions: Substitution (S N 2 or S N 1) or Elimination (E2 or E1) Because R-X bonds are weak, halides are good leaving groups. A. Substitution R-X NaZ or Z R-Z NaX or X Anion or neutral 2 Variants 1. S N 2: Anionic nucleophile The R-X bond breaking is simultaneous with R-Z bond formation C 3 C 3 S N 2: 1º>2º>3º> alkenyl 2. S N 1: Neutral nucleophile The R-X bond breaks first to give a carbocation in the rate determining step; formation of the R-Z bond comes later slow step C 3 C 3 C 3 B. Elimination X C C NaZ or Z C C NaZ or Z anion or neutral 2 Variants 1. E2: Anionic base The R-X and C- bond breaking is simultaneous with C=C bond formation C 3 C 3 2. E1: Neutral base The R-X bond breaks first to give a carbocation in the rate determining step. C- bond cleavage and C=C bond formation comes later slow step
6 6.8 The S N 2 Reaction 6 General: Z "nucleophile" C X!!" "electrophile" Z C X "leaving group" Example, with test-level mechanism: Na 3 C C 3 Na X double-barbed arrows (electron pairs move) Na is a spectator More Detailed Mechanism: C C Transition-State C Notes: Simple, concerted one-step mechanism. No intermediates. The anion needs to be very reactive and thus not too stable. Normally ANINIC NUCLEPILE. Both nucleophile and electrophile are involved in the rate determining step. Rate = k[anion] 1 [R-X] 1 2 nd order rate law is why it s called S N 2: Substitution Nucleophilic 2nd order The nucleophile attacks opposite side from the leaving group. This backside attack (or opposite side attack) results in inversion of stereochemistry when a chiral, 2º R-X is involved Na Inversion of Stereochemistry at Chiral Center The transition state involves a 5-bonded, trigonal bipyramidal carbon that is more cluttered than either the original tetrahedral reactant or the final tetrahedral product Steric crowding in the transition-state makes the reaction very, very, very sensitive to steric factors o For the electrophile R-X: C 3 -X > 1º R-X > 2º R-X > 3º R-X for steric reasons o For the nucleophile it also helps to be smaller rather than larger
7 7 6.9 Generality of S N 2 Reactions -many kinds of nucleophiles, give many products R-X Na R- R-X NaR R--R Alcohols Ethers R-X Na R R R Esters R-X KI R-I R-X NaCN R-CN Iodides Nitriles R-X R R Etc. R Alkynes Notes Most nucleophiles are ANINS Various oxygen anions are good to make alcohols, ethers, or esters alogen exchange useful route to iodides (more valuable and less accessible) There are a few neutral nucleophiles (not for test): nitrogen family Predicting Products for S N 2 Reactions 1. Don t change the structure for the carbon skeleton 2. Put the nucleophile in exactly the spot where the halide began 3. Unless the halide was attached to a chiral center; in that case invert the configuration for the product If the halide was wedged, the nucleophile should be hashed If the halide was hashed, the nucleophile should be wedged 4. Don t mess with any spectator portions: whatever was attached to the nucleophilic anion at the beginning should still be attached at the end
8 8 6.10, 6.11 Structural Factors that Impact S N 2 1. Nucleophile a. Anion versus Neutral: Should be ANINIC b. Anion Stability: Less Stable should be More Reactive (Reactant Stability-Reactivity Principle) 1) -anion nucleophilicity decreases across a horizontal row (electronegativity factor) C 2 Na > NNa > Na > NaF 2) -anion nucleophilicity decreases when an anion is stabilized by resonance Na > Na 3) -anion nucleophilicity increases down a vertical column NaSe > NaS > Na c. Size: all else equal, smaller is better than bigger Na > Na 2. Electrophile Substrate: Allylic > 1º > 2º > >> 3º, alkenyl, aryl o 3º and alkenyl, aryl never do S N 2 o transition-state stability-reactivity principle o Steric clutter in the transition state explains the 1º > 2º > >> 3º pattern o Allylic benefits from a complex orbital resonance effect in the T-state o Alkenyl/aryl halides are bad for some molecular orbital reasons (backside attack doesn t work, particularly for aryl halides) Leaving Group: R-I > R- > R-Cl o reactant stability-reactivity principle o weaker bonds break faster
9 6.12 Inversion of Stereochem in S N 2 In the mechanism, the nucleophile attacks from the backside or opposite side from the leaving group inverts configuration 9 C C Transition-State C Inversion occurs mechanistically in every S N 2 reaction But inversion is chemically relevant only when a chiral carbon is involved C 3 NaC 3 NaC3 C 3 Inversion matters, since product is chiral Inversion doesn t matter, for achiral product Predicting products when chiral carbons undergo inversion: Keep the carbon skeleton fixed If leaving group is hashed, the nucleophile will end up wedged in the product If leaving group is wedged, the nucleophile will end up hashed in the product NaC 2 C 3 C 2 C 3 Na 3 C cis 3 C trans Two Standard Proofs for S N 2 mechanism: Inversion of configuration on a chiral carbon 2 nd order rate law Predicting Products for S N 2 Reactions 1. Don t change the structure for the carbon skeleton 2. Put the nucleophile in exactly the spot where the halide began 3. Unless the halide was attached to a chiral center; in that case invert the configuration for the product If the halide was wedged, the nucleophile should be hashed If the halide was hashed, the nucleophile should be wedged 4. Don t mess with any spectator portions: whatever was attached to the nucleophilic anion at the beginning should still be attached at the end
10 S N 2 Problems: For each of the following a. Identify whether or not an S N 2 reaction would take place? b. If not, why not? c. For those that could undergo S N 2 substitution, draw in the product I 2 Not, neutral 2. Na 3. Na Not, 3º 4. NaC 3 C 3 Inversion 5. KC 2 C 3 C 2 C 3 Inversion 6. KCN CN 7. C 3 Not, neutral 8. NaSC 3 SC3 9. Na Not, vinyl 10. NaC 3 3 C NaC 3 Na Not, 3º Not, aryl
11 11 More S N 2 Problems 1. Rank the reactivity toward NaC 3 (For any problem like this, try to recognize what kind of a reaction it is, so that you know what stability/reactivity issues apply). I I Issues: S N 2 with anion. Iodide > bromide. Allyl > 1º > 2º > vinyl. #4 is sterically more cluttered than #3 2. Rank Reactivity toward (For any problem like this, try to recognize what kind of a reaction it is, so that you know what stability/reactivity issues apply). NaN 2 NaC 3 Na C Issues: Stability/reactivity principles. 1. Anion vs neutral. (Why methanol is way last) 2. Electronegativity. (N vs anions) 3. Resonance (#2 vs #3) 3. What nucleophile should you use to accomplish the following transformations? SPh PhSNa C C C C Ph Ph 4. Draw the Products, Including Stereochemistry. (Stereochemistry will matter for S N 2 and S N 1 reactions anytime the haloalkane is 2º) Na 3 C KCN 3 C CN Issue: Inversion. Notice that ring goes from cis to trans. 5. Choose Reactants to make the following, from a haloalkane and some nucleophile. Issues: Electrophile needs to be 1º or 2º, but can t be 3º
12 S N 1 = Substitution Nucleophilic 1st rder = Solvolysis Dramatic difference in mechanism, rates, structure dependence, and stereochemical outcome (compared to S N 2) General: R-X Z- R-X X neutral Neutral, non-anionic nucleophiles do the substitution ften this is just the solvent ( 2, R, RC 2 are common) o For this reasons, these reactions are often called solvolysis reactions eat is often required Acid is sometimes used to accelerate S N 1 reactions Predicting Products for S N 1 Reactions 1. Don t change the structure for the carbon skeleton 2. Connect R and Z, while taking the halide off of the electrophile and off of the nucleophile 3. Unless the halide was attached to a chiral center, a racemic mixture will result 4. Maintain the integrity of the spectator attachments Examples: Cl 2 I C 3 C 3 3-Step Mechanism slow C 3 step C 3 C 3 Step 1: Carbocation Formation. TIS IS TE SLW STEP o Therefore the rate is controlled by cation stability! Step 2: Carbocation capture by neutral molecule (usually a solvent molecule) o When cation and neutral combine, a cation is produced! Step 3: Deprotonation to get neutral Notes: 1. Carbocation formation is key 2. Rate = k[r-x] First order 3. Rate does not depend on concentration of nucleophile 4. See cations, not anions. Acidic, not basic conditions. Neutral, not anionic nucleophile. 5. Charge and atoms must balance in step 2. Thus, the oxygen retains the hydrogen. 6. xygen eventually loses the, but only in step Rate can be enhanced by AgN 3. The Ag cation helps strip the halide off in step 1.
13 Structural Factors that Impact S N 1 Rates 13 Nucleophile: Should be NEUTRAL, but otherwise non-factor Electrophile 1. Substrate: Allylic > 3º > 2º > > 1º > alkenyl, aryl o Resonance is huge o alkenyl, aryl never do S N 2, 1º only with AgN 3 o product stability-reactivity principle: in the rate-determining step, the more stable the product cation, the faster it will form o In terms of 1º, 2º, 3º, S N 1 and S N 2 have exactly opposite patterns 2. Leaving Group: R-I > R- > R-Cl o reactant stability-reactivity principle: in the rate determining step, the weaker the C-X bond, the faster it will break o This pattern is the same as for S N 2 3. AgN 3 elps o Ag helps strip the halide off in step one 4. Polar Solvent elps o A polar solvent helps to stabilize the ions that form in the ratedetermining step Solvent Polarity: Most Least Solvent 2 C 3 Relative Rate S N 1 Stereo: Racemization riginal stereochemistry is forgotten at the carbocation stage, get racemic R/S mixture 3 C S optically active 2 Why? Carbocation forgets original stereo: 3 C S optically active Ex. C 3 Achiral cation 3 C C 3 S R Racemic Mixture Front Attack 2 Back Attack C C 2 C 3 3 C 3 C 3 C 3 C 2 C 3 C 2 C 3 C 3 C 3 3 C 2 2 R C C S R C 3
14 14 S N 1 Problems: For the following, which are and aren t S N 1 candidates? If not, why not? What would be the product if they are S N 1 candidates? 1. I 2 Not, 1º NaC 3 Not, S N 2, anionic 4. C 3 C 3 Racemic 3 C 5. Na Not, S N 2, anionic C 3 C 3 3 C Racemic 8. C 3 Not, vinyl Not, Aryl 11. Rank Reactivity towards (For any problem like this, try to recognize what kind of a reaction it is, so that you know what stability/reactivity issues apply). I Issues: Allylic > 3º > 2º > 1º I > > Cl Cl I I Cl
15 6.16 Comparing S N 2 vs S N 1 15 S N 1 S N 2 1 Nucleophile Neutral, weak Anionic, strong 2 Substrate 3º R-X > 2º R-X 1º R-X > 2º R-X Allylic effect Allylic elps Allylic helps 3 Leaving Group I > > Cl I > > Cl 4 Solvent Polar needed Non-factor 5 Rate Law K[RX] k[rx][anion] 6 Stereochemistry Racemization Inversion (on chiral, normally 2º R-X) 7 Ions Cationic Anionic 8 Rearrangements Problem at times Never 1. Identify as S N 1 or S N 2 or No Reaction. Draw the Product(s), if a reaction occurs. a. NaC 2 C 3 C 2 C 3 SN 2, anion, 1º b. 2 No reaction, aryl c. 2 d. C 3 SNa 3 CS e. C 3 S 3 CS SC 3 Racemic 2. Which fit S N 1, which fit S N 2? a. Faster in presence of silver nitrate? S N 1 b. Faster in water than in hexane? S N 1 c. When the moles of reactant is kept the same, but the volume of solvent is cut in half, the reaction rate increases by 2-fold? S N 1 d. By 4-fold? S N 2 e. 2-bromobutane reacts faster than 1-bromobutane? S N 1 f. 2-bromobutane reacts slower than 1-bromobutane? S N 2
16 6-17 E1 Elimination Reactions Examples: 16 2 S N 1 E1 E1 (major E1) (minor E1) C 3 C 3 S N 1 E1 (major E1) E1 (minor E1) Notes Under S N 1 conditions, some elimination product(s) form as well E1 and S N 1 normally compete, resulting in mixtures o This is not good from a synthetic perspective. Structurally Isomeric Alkenes can form o The double bond must involve the original halogenated carbon and any neighbor carbon (that had a hydrogen to begin with that can be eliminated) o Normally the alkene with fewer alkene s is formed more extensively over alkenes with more alkene s. (More C-substituted alkene is major). Neutral/acidic (the formula starts neutral, but acid is produced) 1 st order rate law r = k[rx] 1 E1 Mechanism: 2 Steps slow step Step 1: Carbocation Formation. TIS IS TE SLW STEP o Therefore the rate is controlled by cation stability! Just like S N 1! o Benefits from exactly the same factors that speed up S N 1 (3º > 2º, RI > R, polar solvent, etc..) Step 2: Deprotonation from a carbon that neighbors the cation (and the original halogenated carbon) o Draw bromide as base for simplicity o But often it s actually water or alcohol solvent that picks up the proton E1 Summary Recognition: A. Neutral, weak nucleophile. No anionic nucleophile/base, and B. 3º or 2º alkyl halide. (Controlled by cation stability). (For 2º alkyl halides, E1 is often accompanied by variable amounts of S N 1.) rientation: The most substituted alkene forms Predicting the major product: Remove halide and a hydrogen from the neighboring carbon that can give the most highly substituted alkene. The hydrogen on the neighboring carbon can be cis or trans. Stereochemistry: Not an issue. The eliminating hydrogen can be cis or trans.. Mech: Stepwise, 2 steps, via carbocation. Be able to draw completely.
17 E2 Reaction (2 nd rder, Under Anionic/Basic S N 2 type Conditions) Examples 3º R-X NaC 3 E2 E2 (major) (minor E2) C 3 No Competing S N 2 for 3º R-X 3º R-X Na major minor 2 No Competing S N 2 for 3º R-X Na 2 2º R-X S N 2 More of this than either E2 product major E2 (of the E2's) minor E2 (of the E2's) S N 2 and E2 Compete for 2º R-X Normally there is more S N 2 than E2 1º R-X Na S N 2 Na S N 2 only, no competing E2 Compete for 1º R-X Notes E2 happens with anionic nucleophiles/bases, when S N 2 is hindered Reactivity: 3º R-X > 2º R-X. o 1º R-X and vinyl or aryl halides do not undergo E2. Structurally Isomeric Alkenes can form o The double bond must involve the original halogenated carbon and any neighbor carbon (that had a hydrogen to begin with that can be eliminated) o Normally the alkene with fewer alkene s is formed more extensively over alkenes with more alkene s. (More C-substituted alkene is major). Mech C 3 C 3 anionic. Anion base gets things started. 2 nd order rate law. Rate = k[r-x] 1 [anion base] 1 It all happens in one concerted step, but there are three arrow to show all the bond making and breaking Bonds Made Bonds oken Base to hydrogen C-X bond C=C pi bond C- bond
18 E2 Summary Recognition: A. Anionic Nucleophile/Base, and B. 3º or 2º alkyl halide (1º alkyl halides undergo SN2 instead. For 2º alkyl halides, E2 is often accompanied by variable amounts of SN2.) rientation: The most substituted alkene forms (unless a bulky base is used, ch. 7) Predicting product: Remove halide and a hydrogen from the neighboring carbon that can give the most highly substituted alkene. The hydrogen on the neighboring carbon must be trans, however. Stereochemistry: Anti elimination. The hydrogen on the neighbor carbon must be trans/anti. Mech: Concerted. Uses anion. Be able to draw completely. nly one concerted step! 18 S N 1 vs E1 S N 1 C C C C C C E1 C C 2 C C 3 Both satisfy the carbocation. They just meet it s bonding need with different electrons. S N 2 vs E2 S N 2 C C C C E2 C C C C 2 Both provide an electron pair to displace the C- bond pair. They just use different electrons. Both involve the anion. It s called the nucleophile in the S N 2, the base in the E2. The S N 2 involves a crowded transition state, and thus is strongly impacted by steric factors. The E2 does not have any steric problems (and in fact alleviates them). The difference in steric profile explains why for S N 2, 1º > 2º > 3º, but that for E2, the reactivity of 3º is just fine.
19 6-18 Zaitsev s Rule: When E1 or E2 elimination can give more than 1 structurally isomeric alkene, the more highly Carbon-substituted alkene form will predominate over a less highly carbon-substituted alkene. The fewer s on the product alkene the better. o Every Alkene has four attachments. The fewer of these that are s, the better. o When pictures are drawn in which the s are not shown, the more highly substituted alkenes turn out to be the best. Why? Product Stability-Reactivity Rule. Alkenes with more C s and fewer s attached are more stable. Alkene Stability is shown below: tetra- > tri- > di- > mono- > unsubstituted o Why? Alkene carbons are somewhat electron poor due to the inferior overlap of pi bonds. (ne carbon doesn t really get as much of the other carbon s electron as is the case in a nice sigma bond). Since alkyl groups are electron donors, they stabilize electron-deficient alkene carbons. Analogous to why electron-donating alkyls give the 3º > 2º > 1º stability pattern for cations and radicals 19 C C C C C C di-subbed tetrasubbed > C C C C C C C C C > C C C C C C > C C C C C > trisubbed monosubbed C C unsubbed > > > > di-subbed tetrasubbed trisubbed monosubbed unsubbed Examples 2 S N 1 E1 E1 (major E1) (minor E1) 3º R-X Na major minor 2 No Competing S N 2 for 3º R-X Na 2 2º R-X S N 2 More of this than either E2 product major E2 (of the E2's) minor E2 (of the E2's) S N 2 and E2 Compete for 2º R-X Normally there is more S N 2 than E2
20 6-20 Stereochemistry of E2 Eliminations For E2 (not for E1) C- and C-X bonds must be in the same plane (coplanar) The halogen and the hydrogen being removed must be trans to each Why? o Due to orbital overlap requirements. o In the concerted E2 mechanism, the electrons from the hydrogen must essentially come in backside to the leaving halide just as in backside-attack S N 2 mechanism 20 D Na D major D minor "D" is deuterium isotopically labeled hydrogen Sometimes, a molecule will need to single-bond spin into an eclipsed conformation to enable it to do a trans-elimination. Can't react Na spin Can React Na Eliminations in Cyclic Compounds are ften impacted by the Trans Requirement D C 3 C 3 Na C 3 D C 3 C 3 D C 3 C 3 C 3 Na C 3 C Comparing E2 vs E1 E1 E2 1 Nucleophile/Base Neutral, weak, acidic Anionic, strong, basic 2 Substrate 3º R-X > 2º R-X 3º RX > 2º RX > 1º RX Allylic effect Allylic elps Non-factor 3 Leaving Group I > > Cl I > > Cl 4 Solvent Polar needed Non-factor 5 Rate Law K[RX] k[rx][anion] 6 Stereochemistry Non-selective Trans requirement 7 Ions Cationic Anionic 8 Rearrangements Problem at times Never 9 rientation Zaitsev s Rule: Prefer more substituted alkene Zaitsev s Rule: Prefer more Substituted alkene (assuming trans requirement permits)
21 Elimination Problems: Draw the major Elimination Product for the following Reactions. Classify as E1 or E2. (There may be accompanying S N 2 or S N 1 material, but to whatever degree elimination occurs, draw the major product.) C 3 E1 2. C 3 E1 3. C 3 Na E2 4. C 3 Na E2 5. C 3 Na E2 6. D C 3 C 3 C 3 D C 3 C 3 E1 7. D C 3 C 3 C 3 Na C 3 C 3 E2 8. D C3 C 3 Na C 3 D C 3 C 3 E2
22 22 Comparing S N 2 vs S N 1 vs E2 vs E1: appens When? ow Do I Predict Which Step 1: Check nucleophile/base. If neutral, then S N 1/E1 mixture of both If anionic, then S N 2/E2. Step 2: If anionic, and in the S N 2/E2 pool, then Check the substrate. o 1º S N 2 o 2º S N 2/E2 mixture. ften more S N 2, but not reliable o 3º E2 Notes: 1º R-X S N 2 only No E2 or S N 1/E1 (cation too lousy for S N 1/E1; S N 2 too fast for E2 to compete) 3º R-X E2 (anionic) or No S N 2 (sterics too lousy) S N 1/E1 (neutral/acidic) 2º R-X mixtures common Neutral Q1: Anion or Neutral Nucleophile/Base? S N 1/E1 Mix S N 2/E2 Q2: Is substrate 1º, 2º or 3º R-X? 1º R-X 3º R-X S N 2 nly 2º R-X S N 2/E2 Mix (normally favoring S N 2) E2 nly Note: Aryl and Vinyl alides will not undergo any of these types of reactions. If you see 2 /hv type recipe, then you re back in the chapter 4 world of radical halogenation
23 For each mixture, Classify the Type of Reaction (or no reaction ) Draw the major product. (r both a substitution and elim product..) Na SN2, anionic, 1º 2. Na E2 Anionic, 3º 3. I NaC 3 C Can't make an sp center in a ring, bond angle reasons 6. K No reaction, vinyl 7. I 2 No reaction, vinyl PhS SPh No reaction, neutral, 1º hv
24 24 Design Synthetic Plans for converting the starting materials into the target molecules. In each case, more than one chemical operation will be required. Strategy: R- R- (via bromination) Substitution product (via S N 2) or alkene (via E2) 1. 2, hv (makes 2. NaC 1. 3 C , hv (makes 2. NaC 3 (or any anionic E2 base) (no substitution side product) , hv (makes 2. NaI I , hv (makes 2. C 3 C 3 (some alkene would accompany this product) Keys: These can t be done directly, in a single operation Each sequence ends up increasing the number of functional groups in the ultimate product. The key reaction for increasing the functionality: R- R- nce you re converted the starting material to an R- you can interconvert that functional group into something else by substitution, or into an alkene by elimination
25 25 Draw the mechanism for formation of the major product in each of the following reactions. In some cases where both elimination and substitution might compete, the problem specifies whether to draw the substitution or elimination mechanism. 2 (Subst.) D NaC 3 elim. 2. Cl 2 Cl Cl 3. C 3 Na Subst. C elim 2 5.
26 26 Rank the Reactivity of the chemicals shown toward the thing in the box. Keys: Identify the type of reaction that would be involved Think about the rate-determining step and how reactant or product or transitionstate stability would influence the rate. 1. Neutral, S N 1/E , hv 3 C C 3 2. Radical alogenation Radical Stability Cl I 3. Radical alogenation Reactant Stability/Reactivity I C 3 4. Na Primary, S N Neutral resonance Na 5. (S N 1) Solvent: Pentane Na I I 6. Anionic, S N
27 27 Give the Major Product(s) for each of the following. If it s likely to give a mixture of both substitution and elimination, just draw the substitution product. Designate stereochemical outcomes when stereochemistry is relevant (2º substrates). Key: Try to recognize what type of reaction will happen first. NaC 3 C 3 2 hv SC 3 C 3 SNa show sub only I C 3 C 3 3 C NaC 3 Na show sub only 2 show sub only cis/trans mixture due to racemization type process D NaC 3 show elim only
28 28 Provide Reactants for the Following (ne of the Starting Chemicals must be an R-) 1 CN CN 2 3 C C 3 3 C C 3 (trans only) 3 B r C 3 (or any anionic E2 base) (no substitution side product formed) 4 (no elimination side product formed) 5 3 C C 3 C 3 (or any other anionic E2 base) C 3 Draw the Major Alkene Isomer, Following Elimination 6. NC Cl NaC 3 show elim only CN Cl 7. 2 show elim only
29 Cation Rearrangements (and their impact in S N 1 and E1 reactions) 1. Carbocations are very unstable, and sometimes rearrange to other carbocations that are more stable. 2. A rearrangement requires that a superior cation will result. Four cases: 2º 3º non-allylic allylic strained ring unstrained or less strained ring 1º cation 2º or 3º cation (rare, since 1º cations are hard to make and pretty rare) ydride Shifts Alkyl Shifts C 3 2º 3º C 3 2º 3º 2º 2º allylic 3. Two processes for cation rearrangement: ydride shift (an jumps over) Alkyl shift (a carbon jumps over) 4. The resulting rearranged cation must always be on a carbon directly adjacent to the original 5. Cation rearrangement does not occur if you start with a pretty good cation in the first place. Thus, most cation mechanisms that start with 2º or 3º cations don t undergo rearrangement because rearrangement does not lead to improved cation stability Why Bother? No Stability Gain, No Motive, Won t appen Examples in SN1 2 3º 2º 2º some E1 alkenes 2º minor - major - Path A 2 2 Path B 2 2 Product mixture results from competition between Path A and Path B. I via C 3 C 3 via C 3
Some Arrow-Pushing Guidelines (Section 1.14) 1. Arrows follow electron movement.
 Chem 350 Jasperse Ch. 1 Notes 1 Note: The headers and associated chapters don t actually jive with the textbook we are using this summer. But otherwise this highlights a lot of the chemistry from Organic
Chem 350 Jasperse Ch. 1 Notes 1 Note: The headers and associated chapters don t actually jive with the textbook we are using this summer. But otherwise this highlights a lot of the chemistry from Organic
Knowing how many elements of unsaturation are present helps to classify, and helps in isomer problems. Theory # H's - Actual # H's 2 (2C + 2) - H
 hem 341 Jasperse h. 6 andouts 1 h. 6 Structure and Synthesis of lkenes Review ond Strength - σ ond 83 kcal/mol = π ond 63 kcal/mol π onds are much weaker π onds are thus more breakable and more reactive
hem 341 Jasperse h. 6 andouts 1 h. 6 Structure and Synthesis of lkenes Review ond Strength - σ ond 83 kcal/mol = π ond 63 kcal/mol π onds are much weaker π onds are thus more breakable and more reactive
7. Haloalkanes (text )
 2009, Department of hemistry, The University of Western Ontario 7.1 7. aloalkanes (text 7.1 7.10) A. Structure and Nomenclature Like hydrogen, the halogens have a valence of one. Thus, a halogen atom can
2009, Department of hemistry, The University of Western Ontario 7.1 7. aloalkanes (text 7.1 7.10) A. Structure and Nomenclature Like hydrogen, the halogens have a valence of one. Thus, a halogen atom can
Organic Chemistry CHM 314 Dr. Laurie S. Starkey, Cal Poly Pomona Alkyl Halides: Substitution Reactions - Chapter 6 (Wade)
 rganic Chemistry CM 314 Dr. Laurie S. Starkey, Cal Poly Pomona Alkyl alides: Substitution Reactions - Chapter 6 (Wade) Chapter utline I. Intro to RX (6-1 - 6-7) II. Substitution Reactions A) S N 2 (6-8,
rganic Chemistry CM 314 Dr. Laurie S. Starkey, Cal Poly Pomona Alkyl alides: Substitution Reactions - Chapter 6 (Wade) Chapter utline I. Intro to RX (6-1 - 6-7) II. Substitution Reactions A) S N 2 (6-8,
Basic Organic Chemistry Course code : CHEM (Pre-requisites : CHEM 11122)
 Basic Organic Chemistry Course code : CHEM 12162 (Pre-requisites : CHEM 11122) Chapter 01 Mechanistic Aspects of S N2,S N1, E 2 & E 1 Reactions Dr. Dinesh R. Pandithavidana Office: B1 222/3 Phone: (+94)777-745-720
Basic Organic Chemistry Course code : CHEM 12162 (Pre-requisites : CHEM 11122) Chapter 01 Mechanistic Aspects of S N2,S N1, E 2 & E 1 Reactions Dr. Dinesh R. Pandithavidana Office: B1 222/3 Phone: (+94)777-745-720
CHAPTER 7. Further Reactions of Haloalkanes: Unimolecular Substitution and Pathways of Elimination
 CHAPTER 7 Further Reactions of Haloalkanes: Unimolecular Substitution and Pathways of Elimination 7-1 Solvolysis of Tertiary and Secondary Haloalkanes The rate of S N 2 reactions decrease dramatically
CHAPTER 7 Further Reactions of Haloalkanes: Unimolecular Substitution and Pathways of Elimination 7-1 Solvolysis of Tertiary and Secondary Haloalkanes The rate of S N 2 reactions decrease dramatically
C h a p t e r S e v e n : Haloalkanes: Nucleophilc Substitution and Elimination Reactions S N 2
 C h a p t e r S e v e n : Haloalkanes: Nucleophilc Substitution and Elimination Reactions S N 2 CHM 321: Summary of Important Concepts Concepts for Chapter 7: Substitution Reactions I. Nomenclature of
C h a p t e r S e v e n : Haloalkanes: Nucleophilc Substitution and Elimination Reactions S N 2 CHM 321: Summary of Important Concepts Concepts for Chapter 7: Substitution Reactions I. Nomenclature of
Classes of Halides. Chapter 6 Alkyl Halides: Nucleophilic Substitution and Elimination. Polarity and Reactivity. Classes of Alkyl Halides
 rganic hemistry, 5 th Edition L. G. Wade, Jr. hapter 6 Alkyl alides: Nucleophilic Substitution and Elimination lasses of alides Alkyl: alogen, X, is directly bonded to sp 3 carbon. Vinyl: X is bonded to
rganic hemistry, 5 th Edition L. G. Wade, Jr. hapter 6 Alkyl alides: Nucleophilic Substitution and Elimination lasses of alides Alkyl: alogen, X, is directly bonded to sp 3 carbon. Vinyl: X is bonded to
Chapter 7 Substitution Reactions 7.1 Introduction to Substitution Reactions Substitution Reactions: two reactants exchange parts to give new products
 hapter 7 Substitution eactions 7.1 Introduction to Substitution eactions Substitution eactions: two reactants exchange parts to give new products A-B + -D A-D + B- 3 2 + Br 3 2 Br + Elimination eaction:
hapter 7 Substitution eactions 7.1 Introduction to Substitution eactions Substitution eactions: two reactants exchange parts to give new products A-B + -D A-D + B- 3 2 + Br 3 2 Br + Elimination eaction:
Chapter 9. Nucleophilic Substitution and ß-Elimination
 Chapter 9 Nucleophilic Substitution and ß-Elimination Nucleophilic Substitution Nucleophile: From the Greek meaning nucleus loving. A molecule or ion that donates a pair of electrons to another atom or
Chapter 9 Nucleophilic Substitution and ß-Elimination Nucleophilic Substitution Nucleophile: From the Greek meaning nucleus loving. A molecule or ion that donates a pair of electrons to another atom or
Organic Halogen Compounds
 8 Organic alogen ompounds APTER SUMMARY 8.1 Introduction Although organic halogen compounds are rarely found in nature, they do have a variety of commercial applications including use as insecticides,
8 Organic alogen ompounds APTER SUMMARY 8.1 Introduction Although organic halogen compounds are rarely found in nature, they do have a variety of commercial applications including use as insecticides,
Learning Guide for Chapter 17 - Dienes
 Learning Guide for Chapter 17 - Dienes I. Isolated, conjugated, and cumulated dienes II. Reactions involving allylic cations or radicals III. Diels-Alder Reactions IV. Aromaticity I. Isolated, Conjugated,
Learning Guide for Chapter 17 - Dienes I. Isolated, conjugated, and cumulated dienes II. Reactions involving allylic cations or radicals III. Diels-Alder Reactions IV. Aromaticity I. Isolated, Conjugated,
COURSE OBJECTIVES / OUTCOMES / COMPETENCIES.
 COURSE OBJECTIVES / OUTCOMES / COMPETENCIES. By the end of the course, students should be able to do the following: See Test1-4 Objectives/Competencies as listed in the syllabus and on the main course
COURSE OBJECTIVES / OUTCOMES / COMPETENCIES. By the end of the course, students should be able to do the following: See Test1-4 Objectives/Competencies as listed in the syllabus and on the main course
The Electrophile. S N 2 and E2 least stable most stable least hindered most hindered. S N 1 and E1. > x > >
 The Electrophile 1 Recall that electrophile means electron- loving. When considering substitution and elimination reactions we must consider the carbon attached to the leaving group. Is it a primary, secondary,
The Electrophile 1 Recall that electrophile means electron- loving. When considering substitution and elimination reactions we must consider the carbon attached to the leaving group. Is it a primary, secondary,
Chapter 8. Substitution reactions of Alkyl Halides
 Chapter 8. Substitution reactions of Alkyl Halides There are two types of possible reaction in organic compounds in which sp 3 carbon is bonded to an electronegative atom or group (ex, halides) 1. Substitution
Chapter 8. Substitution reactions of Alkyl Halides There are two types of possible reaction in organic compounds in which sp 3 carbon is bonded to an electronegative atom or group (ex, halides) 1. Substitution
Preparation of Alkyl Halides, R-X. Reaction of alkanes with Cl 2 & Br 2 (F 2 is too reactive, I 2 is unreactive): R + X X 2.
 Preparation of Alkyl alides, R-X Reaction of alkanes with Cl 2 & Br 2 (F 2 is too reactive, I 2 is unreactive): UV R + X 2 R X or heat + X This mechanism involves a free radical chain reaction. A chain
Preparation of Alkyl alides, R-X Reaction of alkanes with Cl 2 & Br 2 (F 2 is too reactive, I 2 is unreactive): UV R + X 2 R X or heat + X This mechanism involves a free radical chain reaction. A chain
Homework problems Chapters 6 and Give the curved-arrow formalism for the following reaction: CH 3 OH + H 2 C CH +
 omework problems hapters 6 and 7 1. Give the curved-arrow formalism for the following reaction: : 3 - : 2 : 3 2-3 3 2. In each of the following sets, arrange the compounds in order of decreasing pka and
omework problems hapters 6 and 7 1. Give the curved-arrow formalism for the following reaction: : 3 - : 2 : 3 2-3 3 2. In each of the following sets, arrange the compounds in order of decreasing pka and
Essential Organic Chemistry. Chapter 9
 Essential Organic Chemistry Paula Yurkanis Bruice Chapter 9 Substitution and Elimination Reactions of Alkyl Halides 9.1 How Alkyl Halides React Substitution Reactions One group takes the place of another.
Essential Organic Chemistry Paula Yurkanis Bruice Chapter 9 Substitution and Elimination Reactions of Alkyl Halides 9.1 How Alkyl Halides React Substitution Reactions One group takes the place of another.
CONCERTED sp 2 H. HO Et
 Alkyl alides Substitution and Elimination 1 Substitutions (Quick Review) 1.1 SN2 Reactions LB nucleophile "backside attack!" NERTED reaction This is fundamentally just a Lewis acid/base reaction, the Lewis
Alkyl alides Substitution and Elimination 1 Substitutions (Quick Review) 1.1 SN2 Reactions LB nucleophile "backside attack!" NERTED reaction This is fundamentally just a Lewis acid/base reaction, the Lewis
Nuggets of Knowledge for Chapter 17 Dienes and Aromaticity Chem 2320
 Nuggets of Knowledge for Chapter 17 Dienes and Aromaticity Chem 2320 I. Isolated, cumulated, and conjugated dienes A diene is any compound with two or C=C's is a diene. Compounds containing more than two
Nuggets of Knowledge for Chapter 17 Dienes and Aromaticity Chem 2320 I. Isolated, cumulated, and conjugated dienes A diene is any compound with two or C=C's is a diene. Compounds containing more than two
11. Reactions of Alkyl Halides: Nucleophilic Substitutions and Eliminations
 11. Reactions of Alkyl Halides: Nucleophilic Substitutions and Eliminations Based on McMurry s Organic Chemistry, 6 th edition 2003 Ronald Kluger Department of Chemistry University of Toronto Alkyl Halides
11. Reactions of Alkyl Halides: Nucleophilic Substitutions and Eliminations Based on McMurry s Organic Chemistry, 6 th edition 2003 Ronald Kluger Department of Chemistry University of Toronto Alkyl Halides
Chem 341 Jasperse Ch Handouts 1
 Chem 341 Jasperse Ch. 5 + 10 Handouts 1 Ch. 5 The Study of Chemical Reactions 5.1 Four general types of chemical reactions 1. Addition reactions 2. Elimination Reactions 3. Substitution Reactions 4. Rearrangement
Chem 341 Jasperse Ch. 5 + 10 Handouts 1 Ch. 5 The Study of Chemical Reactions 5.1 Four general types of chemical reactions 1. Addition reactions 2. Elimination Reactions 3. Substitution Reactions 4. Rearrangement
Chem 341 Jasperse Ch. 9 Handouts 1
 Chem 341 Jasperse Ch. 9 andouts 1 Ch. 9 Stereochemistry Stereoisomers have the same condensed formulas and basic bonding sequence, but have different 3-dimensional shape and cannot be interconverted 9.1,2
Chem 341 Jasperse Ch. 9 andouts 1 Ch. 9 Stereochemistry Stereoisomers have the same condensed formulas and basic bonding sequence, but have different 3-dimensional shape and cannot be interconverted 9.1,2
Homework - Review of Chem 2310
 omework - Review of Chem 2310 Chapter 1 - Atoms and Molecules Name 1. What is organic chemistry? 2. Why is there an entire one year course devoted to the study of organic compounds? 3. Give 4 examples
omework - Review of Chem 2310 Chapter 1 - Atoms and Molecules Name 1. What is organic chemistry? 2. Why is there an entire one year course devoted to the study of organic compounds? 3. Give 4 examples
Chapter 5. Nucleophilic aliphatic substitution mechanism. by G.DEEPA
 Chapter 5 Nucleophilic aliphatic substitution mechanism by G.DEEPA 1 Introduction The polarity of a carbon halogen bond leads to the carbon having a partial positive charge In alkyl halides this polarity
Chapter 5 Nucleophilic aliphatic substitution mechanism by G.DEEPA 1 Introduction The polarity of a carbon halogen bond leads to the carbon having a partial positive charge In alkyl halides this polarity
Chapter 6: Organic Halogen Compounds; Substitution and Elimination Reactions
 Chapter 6: Organic Halogen Compounds; Substitution and Elimination Reactions Halogen compounds are important for several reasons. Simple alkyl and aryl halides, especially chlorides and bromides, are versatile
Chapter 6: Organic Halogen Compounds; Substitution and Elimination Reactions Halogen compounds are important for several reasons. Simple alkyl and aryl halides, especially chlorides and bromides, are versatile
BSc. II 3 rd Semester. Submitted By Dr. Sangita Nohria Associate Professor PGGCG-11 Chandigarh 1
 BSc. II 3 rd Semester Submitted By Dr. Sangita Nohria Associate Professor PGGCG-11 Chandigarh 1 Introduction to Alkyl Halides Alkyl halides are organic molecules containing a halogen atom bonded to an
BSc. II 3 rd Semester Submitted By Dr. Sangita Nohria Associate Professor PGGCG-11 Chandigarh 1 Introduction to Alkyl Halides Alkyl halides are organic molecules containing a halogen atom bonded to an
Elimination Reactions. Chapter 6 1
 Elimination Reactions Chapter 6 1 E1 Mechanism Step 1: halide ion leaves, forming a carbocation. Step 2: Base abstracts H + from adjacent carbon forming the double bond. Chapter 6 2 E1 Energy Diagram E1:
Elimination Reactions Chapter 6 1 E1 Mechanism Step 1: halide ion leaves, forming a carbocation. Step 2: Base abstracts H + from adjacent carbon forming the double bond. Chapter 6 2 E1 Energy Diagram E1:
Chapter 8: Nucleophilic Substitution 8.1: Functional Group Transformation By Nucleophilic Substitution
 hapter 8: Nucleophilic Substitution 8.1: Functional Group Transformation By Nucleophilic Substitution Nu: = l,, I Nu - Nucleophiles are Lewis bases (electron-pair donor) Nucleophiles are often negatively
hapter 8: Nucleophilic Substitution 8.1: Functional Group Transformation By Nucleophilic Substitution Nu: = l,, I Nu - Nucleophiles are Lewis bases (electron-pair donor) Nucleophiles are often negatively
Chapter 11: Nucleophilic Substitution and Elimination Walden Inversion
 hapter 11: Nucleophilic Substitution and Elimination Walden Inversion (S)-(-) Malic acid [a] D = -2.3 Ag 2, 2 Pl 5 l Ag 2, 2 ()-2-hlorosuccinic acid l (-)-2-hlorosuccinic acid Pl 5 ()-() Malic acid [a]
hapter 11: Nucleophilic Substitution and Elimination Walden Inversion (S)-(-) Malic acid [a] D = -2.3 Ag 2, 2 Pl 5 l Ag 2, 2 ()-2-hlorosuccinic acid l (-)-2-hlorosuccinic acid Pl 5 ()-() Malic acid [a]
c. Cl H Page 1 of 7 major P (E > Z and more substituted over less substituted alkene) LG must be axial are the same Cl -
 CEM 109A 1. Predict the products of the following reactions (a-c E2, d-f E1 KEY focuses only on elimination products, in most cases there will also be substitution products.) a. - LG must be axial - are
CEM 109A 1. Predict the products of the following reactions (a-c E2, d-f E1 KEY focuses only on elimination products, in most cases there will also be substitution products.) a. - LG must be axial - are
Chapter 6 Ionic Reactions-Nucleophilic Substitution and Elimination Reactions of Alkyl Halides"
 Chapter 6 Ionic Reactions-Nucleophilic Substitution and Elimination Reactions of Alkyl Halides" t Introduction" The polarity of a carbon-halogen bond leads to the carbon having a partial positive charge"
Chapter 6 Ionic Reactions-Nucleophilic Substitution and Elimination Reactions of Alkyl Halides" t Introduction" The polarity of a carbon-halogen bond leads to the carbon having a partial positive charge"
Chapter 11, Part 1: Polar substitution reactions involving alkyl halides
 hapter 11, Part 1: Polar substitution reactions involving alkyl halides Overview: The nature of alkyl halides and other groups with electrophilic sp 3 hybridized leads them to react with nucleophiles and
hapter 11, Part 1: Polar substitution reactions involving alkyl halides Overview: The nature of alkyl halides and other groups with electrophilic sp 3 hybridized leads them to react with nucleophiles and
Organic Reactions Susbstitution S N. Dr. Sapna Gupta
 Organic Reactions Susbstitution S N 2 Dr. Sapna Gupta Kinetics of Nucleophilic Reaction Rate law is order of reaction 0 order is when rate of reaction is unaffected by change in concentration of the reactants
Organic Reactions Susbstitution S N 2 Dr. Sapna Gupta Kinetics of Nucleophilic Reaction Rate law is order of reaction 0 order is when rate of reaction is unaffected by change in concentration of the reactants
Elimination Reactions Heating an alkyl halide with a strong base causes elimination of a. molecule of HX
 Elimination eactions eating an alkyl halide with a strong base causes elimination of a molecule of X 1. Potassium hydroxide dissolved in ethanol and the sodium salts of alcohols (such as sodium ethoxide)
Elimination eactions eating an alkyl halide with a strong base causes elimination of a molecule of X 1. Potassium hydroxide dissolved in ethanol and the sodium salts of alcohols (such as sodium ethoxide)
Reactions SN2 and SN1
 Reactions SN2 and SN1 Reactivity: Functional groups can be interconverted using a great variety of reagents. Millions of organic molecules have been synthesized via a series of functional-group interconversions.
Reactions SN2 and SN1 Reactivity: Functional groups can be interconverted using a great variety of reagents. Millions of organic molecules have been synthesized via a series of functional-group interconversions.
Nucleophilic Substitution and Elimination
 Nucleophilic Substitution and Elimination Alkyl halides react with a nucleophile in one of two ways. Either they eliminate an X to form an alkene, or they undergo a substitution with the nucleophile, Nu,
Nucleophilic Substitution and Elimination Alkyl halides react with a nucleophile in one of two ways. Either they eliminate an X to form an alkene, or they undergo a substitution with the nucleophile, Nu,
Chapter 8: Alkene Structure and Preparation via Elimination Reactions
 Nature of the pi bond Chapter 8: Alkene Structure and Preparation via Elimination eactions [Sections: 8.1-8.13] C C 3 C C 3 bond length bond strength 2 C C 2 a C=C double bond is stronger than a C C single
Nature of the pi bond Chapter 8: Alkene Structure and Preparation via Elimination eactions [Sections: 8.1-8.13] C C 3 C C 3 bond length bond strength 2 C C 2 a C=C double bond is stronger than a C C single
Chapter 7 Alkenes; Elimination Reactions
 hapter 7 Alkenes; Elimination Reactions Alkenes Alkenes contain a carbon-carbon double bond. They are named as derivatives of alkanes with the suffix -ane changed to -ene. The parent alkane is the longest
hapter 7 Alkenes; Elimination Reactions Alkenes Alkenes contain a carbon-carbon double bond. They are named as derivatives of alkanes with the suffix -ane changed to -ene. The parent alkane is the longest
1. What are the respective hybridizations of the atoms numbered 1 to 4 in this compound?
 CEM 331: Chapter 1/2: Structures (Atoms, Molecules, Bonding) 1. What are the respective hybridizations of the atoms numbered 1 to 4 in this compound? N C 2 C N C 2 C N 1 2 3 4 1: three sigma bonds and
CEM 331: Chapter 1/2: Structures (Atoms, Molecules, Bonding) 1. What are the respective hybridizations of the atoms numbered 1 to 4 in this compound? N C 2 C N C 2 C N 1 2 3 4 1: three sigma bonds and
Dr. Anand Gupta Mr Mahesh Kapil
 Dr. Anand Gupta Mr Mahesh Kapil 09356511518 09888711209 anandu71@yahoo.com mkapil_foru@yahoo.com Preparation of Haloalkanes From Alkanes Alkenes Alcohols Carboxylic Acids (Hundsdicker Reaction) Halide
Dr. Anand Gupta Mr Mahesh Kapil 09356511518 09888711209 anandu71@yahoo.com mkapil_foru@yahoo.com Preparation of Haloalkanes From Alkanes Alkenes Alcohols Carboxylic Acids (Hundsdicker Reaction) Halide
CHE1502. Tutorial letter 201/1/2016. General Chemistry 1B. Semester 1. Department of Chemistry CHE1502/201/1/2016
 CE1502/201/1/2016 Tutorial letter 201/1/2016 General Chemistry 1B CE1502 Semester 1 Department of Chemistry This tutorial letter contains the answers to the questions in assignment 1. FIRST SEMESTER: KEY
CE1502/201/1/2016 Tutorial letter 201/1/2016 General Chemistry 1B CE1502 Semester 1 Department of Chemistry This tutorial letter contains the answers to the questions in assignment 1. FIRST SEMESTER: KEY
Chapter 8: Alkene Structure and Preparation via Elimination Reactions
 1. Nature of the pi bond Chapter 8: Alkene Structure and Preparation via Elimination eactions [Sections: 8.1-8.13] C C bond length bond strength 3 C C 3 3 C C 3 3 C C 3 3 C 2 C C 2 3 C a C=C double bond
1. Nature of the pi bond Chapter 8: Alkene Structure and Preparation via Elimination eactions [Sections: 8.1-8.13] C C bond length bond strength 3 C C 3 3 C C 3 3 C C 3 3 C 2 C C 2 3 C a C=C double bond
ORGANIC - EGE 5E CH. 7 - NUCLEOPHILIC SUBSTITUTION AND ELIMINATION REACTIONS
 !! www.clutchprep.com CONCEPT: INTRODUCTION TO SUBSTITUTION Previously, we discussed the various ways that acids could react with bases: Recall that in these mechanisms, electrons always travel from density
!! www.clutchprep.com CONCEPT: INTRODUCTION TO SUBSTITUTION Previously, we discussed the various ways that acids could react with bases: Recall that in these mechanisms, electrons always travel from density
C h a p t e r S e v e n : Substitution Reactions S N 2 O H H H O H H. Br -
 C h a p t e r S e v e n : Substitution Reactions Br Br S N 2 CM 321: Summary of Important Concepts YConcepts for Chapter 7: Substitution Reactions I. Nomenclature of alkyl halides, R X A. Common name:
C h a p t e r S e v e n : Substitution Reactions Br Br S N 2 CM 321: Summary of Important Concepts YConcepts for Chapter 7: Substitution Reactions I. Nomenclature of alkyl halides, R X A. Common name:
CHAPTER 9 HW SOLUTIONS: ALCOHOLS + ETHERS
 CAPTER 9 W SLUTNS: ALCLS + ETERS ALCL + ETER NMENCLATURE. Give the UPAC name for each compound. nclude cis/trans or R/S stereochemistry if necessary. Structure C Name -methyl--heptanol -t-butylcyclopentanol
CAPTER 9 W SLUTNS: ALCLS + ETERS ALCL + ETER NMENCLATURE. Give the UPAC name for each compound. nclude cis/trans or R/S stereochemistry if necessary. Structure C Name -methyl--heptanol -t-butylcyclopentanol
REACTIONS OF HALOALKANES - SUBSTITUTION AND ELIMINATION
 REACTIONS OF HALOALKANES - SUBSTITUTION AND ELIMINATION Haloalkanes (also known as halogenoalkanes and alkyl halides) are organic compounds where one of the hydrogens of an alkane or cycloalkane has been
REACTIONS OF HALOALKANES - SUBSTITUTION AND ELIMINATION Haloalkanes (also known as halogenoalkanes and alkyl halides) are organic compounds where one of the hydrogens of an alkane or cycloalkane has been
Organic Chemistry I Review: Highlights of Key Reactions, Mechanisms, and Principles 1 Some Arrow-Pushing Guidelines (Section 1.14)
 rganic Chemistry I Review: ighlights of Key Reactions, Mechanisms, and Principles 1 Some Arrow-Pushing Guidelines (Section 1.14) 1. Arrows follow electron movement. 2. Some rules for the appearance of
rganic Chemistry I Review: ighlights of Key Reactions, Mechanisms, and Principles 1 Some Arrow-Pushing Guidelines (Section 1.14) 1. Arrows follow electron movement. 2. Some rules for the appearance of
OChem 1 Mechanism Flashcards. Dr. Peter Norris, 2018
 OChem 1 Mechanism Flashcards Dr. Peter Norris, 2018 Mechanism Basics Chemical change involves bonds forming and breaking; a mechanism describes those changes using curved arrows to describe the electrons
OChem 1 Mechanism Flashcards Dr. Peter Norris, 2018 Mechanism Basics Chemical change involves bonds forming and breaking; a mechanism describes those changes using curved arrows to describe the electrons
OChem 1 Mechanism Flashcards. Dr. Peter Norris, 2015
 OChem 1 Mechanism Flashcards Dr. Peter Norris, 2015 Mechanism Basics Chemical change involves bonds forming and breaking; a mechanism describes those changes using curved arrows to describe the electrons
OChem 1 Mechanism Flashcards Dr. Peter Norris, 2015 Mechanism Basics Chemical change involves bonds forming and breaking; a mechanism describes those changes using curved arrows to describe the electrons
Learning Guide for Chapter 11 - Alkenes I
 Learning Guide for Chapter 11 - Alkenes I I. Introduction to alkenes - p 1 bond structure, classifying alkenes, reactivity, physical properties, occurrences and uses, spectroscopy, stabilty II. Unsaturation
Learning Guide for Chapter 11 - Alkenes I I. Introduction to alkenes - p 1 bond structure, classifying alkenes, reactivity, physical properties, occurrences and uses, spectroscopy, stabilty II. Unsaturation
Walden discovered a series of reactions that could interconvert (-)-malic acid and (+)-malic acid.
 Chapter 11: Reactions of alkyl halides: nucleophilic substitutions and eliminations Alkyl halides are polarized in the C-X bond, making carbon δ+ (electrophilic). A nucleophilecan attack this carbon, displacing
Chapter 11: Reactions of alkyl halides: nucleophilic substitutions and eliminations Alkyl halides are polarized in the C-X bond, making carbon δ+ (electrophilic). A nucleophilecan attack this carbon, displacing
The General Stabilization Effect of Conjugation (Section 15.1, 2, 3, 8, 9) Conjugated (more stable)
 Chem 0 Jasperse Ch Notes. Conjugation Ch. Conjugated Systems The General Stabilization Effect of Conjugation (Section.,,, 8, 9) Conjugated (more stable) Isolated (less stable) Notes: Cations Radicals Anions
Chem 0 Jasperse Ch Notes. Conjugation Ch. Conjugated Systems The General Stabilization Effect of Conjugation (Section.,,, 8, 9) Conjugated (more stable) Isolated (less stable) Notes: Cations Radicals Anions
Real life example 1 Let s look at this series of chloroalcohols, and how fast the chloride gets displaced by an external nucleophile.
 Class 2 Carbocations Last time we talked about neighboring group participation in substitution reactions. I want to start today by talking about a few more real life examples. Real life example 1 Let s
Class 2 Carbocations Last time we talked about neighboring group participation in substitution reactions. I want to start today by talking about a few more real life examples. Real life example 1 Let s
10. Organohalides. Based on McMurry s Organic Chemistry, 7 th edition
 10. Organohalides Based on McMurry s Organic Chemistry, 7 th edition What Is an Alkyl Halide An organic compound containing at least one carbonhalogen bond (C-X) X (F, Cl, Br, I) replaces H Can contain
10. Organohalides Based on McMurry s Organic Chemistry, 7 th edition What Is an Alkyl Halide An organic compound containing at least one carbonhalogen bond (C-X) X (F, Cl, Br, I) replaces H Can contain
3-chloro-1-propene 1-chloropropane 2-chloropropene
 ANSWERS #1. (from 50 minute exam #3, Fall 2000) 5. (6 points) For each group of 3 compounds, identify the compound that expresses the indicated property the MOST and the compound that expresses it the
ANSWERS #1. (from 50 minute exam #3, Fall 2000) 5. (6 points) For each group of 3 compounds, identify the compound that expresses the indicated property the MOST and the compound that expresses it the
Lecture Topics: I. Electrophilic Aromatic Substitution (EAS)
 Reactions of Aromatic Compounds Reading: Wade chapter 17, sections 17-1- 17-15 Study Problems: 17-44, 17-46, 17-47, 17-48, 17-51, 17-52, 17-53, 17-59, 17-61 Key Concepts and Skills: Predict and propose
Reactions of Aromatic Compounds Reading: Wade chapter 17, sections 17-1- 17-15 Study Problems: 17-44, 17-46, 17-47, 17-48, 17-51, 17-52, 17-53, 17-59, 17-61 Key Concepts and Skills: Predict and propose
(CH 3 ) 3 COH. CH 3 ONa
 1. Rank the following compounds in the trend requested. (15 points each) a. Rank by nucleophilicity. The strongest nucleophile is 1, while the weakest nucleophile is 5. C 3 PNa (C 3 ) 3 C C 3 Na C 3 C
1. Rank the following compounds in the trend requested. (15 points each) a. Rank by nucleophilicity. The strongest nucleophile is 1, while the weakest nucleophile is 5. C 3 PNa (C 3 ) 3 C C 3 Na C 3 C
75. A This is a Markovnikov addition reaction. In these reactions, the pielectrons in the alkene act as a nucleophile. The strongest electrophile will
 71. B SN2 stands for substitution nucleophilic bimolecular. This means that there is a bimolecular rate-determining step. Therefore, the reaction will follow second-order kinetics based on the collision
71. B SN2 stands for substitution nucleophilic bimolecular. This means that there is a bimolecular rate-determining step. Therefore, the reaction will follow second-order kinetics based on the collision
Substitution and Elimination reactions
 PART 3 Substitution and Elimination reactions Chapter 8. Substitution reactions of RX 9. Elimination reactions of RX 10. Substit n/elimin n of other comp ds 11. Organometallic comp ds 12. Radical reactions
PART 3 Substitution and Elimination reactions Chapter 8. Substitution reactions of RX 9. Elimination reactions of RX 10. Substit n/elimin n of other comp ds 11. Organometallic comp ds 12. Radical reactions
CHAPTER 8 HW SOLUTIONS: ELIMINATIONS REACTIONS
 APTER 8 W SLUTNS: ELMNATNS REATNS S-TRANS SMERSM 1. Use a discussion and drawing of orbitals to help explain why it is generally easy to rotate around single bonds at room temperature, while it is difficult
APTER 8 W SLUTNS: ELMNATNS REATNS S-TRANS SMERSM 1. Use a discussion and drawing of orbitals to help explain why it is generally easy to rotate around single bonds at room temperature, while it is difficult
S N 1 Displacement Reactions
 S N 1 Displacement Reactions Tertiary alkyl halides cannot undergo S N 2 reactions because of the severe steric hindrance blocking a backside approach of the nucleophile. They can, however, react via an
S N 1 Displacement Reactions Tertiary alkyl halides cannot undergo S N 2 reactions because of the severe steric hindrance blocking a backside approach of the nucleophile. They can, however, react via an
ORGANIC - CLUTCH CH. 8 - ELIMINATION REACTIONS.
 !! www.clutchprep.com CONCEPT: THE E2 MECHANISM A strong nucleophile reacts with an inaccessible leaving group to produce beta-elimination in one-step. E2 Properties (Circle One) Nucleophile = Strong /
!! www.clutchprep.com CONCEPT: THE E2 MECHANISM A strong nucleophile reacts with an inaccessible leaving group to produce beta-elimination in one-step. E2 Properties (Circle One) Nucleophile = Strong /
Classes of Alkenes. Alkenes and Alkynes. Saturated compounds (alkanes): Have the maximum number of hydrogen atoms attached to each carbon atom.
 Alkenes and Alkynes Saturated compounds (alkanes): ave the maximum number of hydrogen atoms attached to each carbon atom. Unsaturated compounds: ave fewer hydrogen atoms attached to the carbon chain than
Alkenes and Alkynes Saturated compounds (alkanes): ave the maximum number of hydrogen atoms attached to each carbon atom. Unsaturated compounds: ave fewer hydrogen atoms attached to the carbon chain than
How alkyl halides react
 Chapter 10 1 How alkyl halides react δ+ δ- RCH 2 -X X= halogen X = higher EN C = lower EN This polar carbon-halogen bond causes alkyl halide to undergo S N and elimination reaction. 2 The mechanism of
Chapter 10 1 How alkyl halides react δ+ δ- RCH 2 -X X= halogen X = higher EN C = lower EN This polar carbon-halogen bond causes alkyl halide to undergo S N and elimination reaction. 2 The mechanism of
Lecture 21 Organic Chemistry 1
 CEM 232 Organic Chemistry I at Chicago Lecture 21 Organic Chemistry 1 Professor Duncan Wardrop March 30, 2010 1 Self Test Question Ethanolysis of alkyl halide 1 gives ether 2 as one product; however, several
CEM 232 Organic Chemistry I at Chicago Lecture 21 Organic Chemistry 1 Professor Duncan Wardrop March 30, 2010 1 Self Test Question Ethanolysis of alkyl halide 1 gives ether 2 as one product; however, several
Alkyl Halides. Alkyl halides are a class of compounds where a halogen atom or atoms are bound to an sp 3 orbital of an alkyl group.
 Alkyl Halides Alkyl halides are a class of compounds where a halogen atom or atoms are bound to an sp 3 orbital of an alkyl group. CHCl 3 (Chloroform: organic solvent) CF 2 Cl 2 (Freon-12: refrigerant
Alkyl Halides Alkyl halides are a class of compounds where a halogen atom or atoms are bound to an sp 3 orbital of an alkyl group. CHCl 3 (Chloroform: organic solvent) CF 2 Cl 2 (Freon-12: refrigerant
Structure and Preparation of Alkenes: Elimination Reactions
 Structure and Preparation of Alkenes: Elimination Reactions Alkene Nomenclature First identify the longest continuous chain that includes the double bond. Replace the -ane ending of the corresponding unbranched
Structure and Preparation of Alkenes: Elimination Reactions Alkene Nomenclature First identify the longest continuous chain that includes the double bond. Replace the -ane ending of the corresponding unbranched
CHEM 261 HOME WORK Lecture Topics: MODULE 1: The Basics: Bonding and Molecular Structure Text Sections (N0 1.9, 9-11) Homework: Chapter 1:
 CHEM 261 HOME WORK Lecture Topics: MODULE 1: The Basics: Bonding and Molecular Structure Atomic Structure - Valence Electrons Chemical Bonds: The Octet Rule - Ionic bond - Covalent bond How to write Lewis
CHEM 261 HOME WORK Lecture Topics: MODULE 1: The Basics: Bonding and Molecular Structure Atomic Structure - Valence Electrons Chemical Bonds: The Octet Rule - Ionic bond - Covalent bond How to write Lewis
Chapter 7 - Alkenes and Alkynes I
 Andrew Rosen Chapter 7 - Alkenes and Alkynes I 7.1 - Introduction - The simplest member of the alkenes has the common name of ethylene while the simplest member of the alkyne family has the common name
Andrew Rosen Chapter 7 - Alkenes and Alkynes I 7.1 - Introduction - The simplest member of the alkenes has the common name of ethylene while the simplest member of the alkyne family has the common name
OChem1 Old Exams. Chemistry 3719 Practice Exams
 OChem1 Old Exams Chemistry 3719 Practice Exams Fall 2017 Chemistry 3719 Practice Exam A1 This exam is worth 100 points out of a total of 600 points for Chemistry 3719/3719L. You have 50 minutes to complete
OChem1 Old Exams Chemistry 3719 Practice Exams Fall 2017 Chemistry 3719 Practice Exam A1 This exam is worth 100 points out of a total of 600 points for Chemistry 3719/3719L. You have 50 minutes to complete
Loudon Chapter 10 Review: Alcohols & Thiols Jacquie Richardson, CU Boulder Last updated 4/26/2016
 Alcohols (R) and thiols (RS) have many reactions in common with alkyl halides, but they don t do everything exactly the same. The main difference between this and alkyl halide chemistry is that unlike
Alcohols (R) and thiols (RS) have many reactions in common with alkyl halides, but they don t do everything exactly the same. The main difference between this and alkyl halide chemistry is that unlike
CHEM Lecture 7
 CEM 494 Special Topics in Chemistry Illinois at Chicago CEM 494 - Lecture 7 Prof. Duncan Wardrop ctober 22, 2012 CEM 494 Special Topics in Chemistry Illinois at Chicago Preparation of Alkenes Elimination
CEM 494 Special Topics in Chemistry Illinois at Chicago CEM 494 - Lecture 7 Prof. Duncan Wardrop ctober 22, 2012 CEM 494 Special Topics in Chemistry Illinois at Chicago Preparation of Alkenes Elimination
Halo Alkanes and Halo Arenes
 alo Alkanes and alo Arenes Short Answer Questions: **1. Write the isomers of the compound having formula C 4 9 Br? Sol. There are five isomers of C 4 9 Br. These are: 2-bromobutane is expected to exhibit
alo Alkanes and alo Arenes Short Answer Questions: **1. Write the isomers of the compound having formula C 4 9 Br? Sol. There are five isomers of C 4 9 Br. These are: 2-bromobutane is expected to exhibit
PRACTICE PROBLEMS UNIT 8
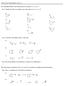 PRACTICE PROBLEMS UNIT 8 8A. Identify halides and carbocations as being 1 o, 2 o, or 3 o 8A.1 assify the following halides and carbocations as 1 o, 2 o, or 3 o. 8A.2 Circle the most stable cation in each
PRACTICE PROBLEMS UNIT 8 8A. Identify halides and carbocations as being 1 o, 2 o, or 3 o 8A.1 assify the following halides and carbocations as 1 o, 2 o, or 3 o. 8A.2 Circle the most stable cation in each
Glendale Community College Chemistry 105 Exam. 3 Lecture Notes Chapters 6 & 7
 Sevada Chamras, Ph.D. Glendale Community College Chemistry 105 Exam. 3 Lecture Notes Chapters 6 & 7 Description: Examples: 3 Major Types of Organic Halides: 1. Alkyl Halides: Chapter 6 (Part 1/2) : Alkyl
Sevada Chamras, Ph.D. Glendale Community College Chemistry 105 Exam. 3 Lecture Notes Chapters 6 & 7 Description: Examples: 3 Major Types of Organic Halides: 1. Alkyl Halides: Chapter 6 (Part 1/2) : Alkyl
Lab Activity 9: Introduction to Organic Chemical Reactivity, Lab 5 Prelab, Reflux
 Lab Activity 9: Introduction to Organic Chemical Reactivity, Lab 5 Prelab, Reflux Objectives 1. Identify structural features (pi bonds, bond polarity, lone pairs) of a compound 2. Determine whether a structural
Lab Activity 9: Introduction to Organic Chemical Reactivity, Lab 5 Prelab, Reflux Objectives 1. Identify structural features (pi bonds, bond polarity, lone pairs) of a compound 2. Determine whether a structural
Organic Chemistry Practice Problems: Solutions
 rganic Chemistry Practice Problems: Solutions 1. 2. a. B, A b. D, B c. A, D d. D, A a. Resonance b. Electronegativity of fluorine atoms F F c. Neither is very acidic, but the oxygen will help stabilise
rganic Chemistry Practice Problems: Solutions 1. 2. a. B, A b. D, B c. A, D d. D, A a. Resonance b. Electronegativity of fluorine atoms F F c. Neither is very acidic, but the oxygen will help stabilise
1. Root of name depends on longest chain of C containing the double bond; ends in "ene"
 Alkenes (β-carotene, an antioxidant pigment) n 2n (acyclic) n 2n-2 (cyclic) R R R R Key features sp 2 -hybridized carbons, 120 o bond angles σ + π bonding between = planar geometry around = "unsaturated"
Alkenes (β-carotene, an antioxidant pigment) n 2n (acyclic) n 2n-2 (cyclic) R R R R Key features sp 2 -hybridized carbons, 120 o bond angles σ + π bonding between = planar geometry around = "unsaturated"
(Neither an oxidation or reduction: Addition or loss of H +, H 2 O, HX).
 eactions of Alcohols Alcohols are versatile organic compounds since they undergo a wide variety of transformations the majority of which are either oxidation or reduction type reactions. xidation is a
eactions of Alcohols Alcohols are versatile organic compounds since they undergo a wide variety of transformations the majority of which are either oxidation or reduction type reactions. xidation is a
Chapter 8 Alkyl Halides and Elimination Reactions
 Organic Chemistry, Second Edition Janice Gorzynski Smith University of Hawai i Chapter 8 Alkyl Halides and Elimination Reactions Prepared by Rabi Ann Musah State University of New York at Albany Copyright
Organic Chemistry, Second Edition Janice Gorzynski Smith University of Hawai i Chapter 8 Alkyl Halides and Elimination Reactions Prepared by Rabi Ann Musah State University of New York at Albany Copyright
Ch. 4 Alkane Halogenation and The General Study of Chemical Reactions
 Chem 350 Jasperse Ch. 4. Chemical Reactions and Alkane alogenation 1 Ch. 4 Alkane alogenation and The General Study of Chemical Reactions Three Factors in Every Reaction: 1. Mechanism: what is the step-by-step
Chem 350 Jasperse Ch. 4. Chemical Reactions and Alkane alogenation 1 Ch. 4 Alkane alogenation and The General Study of Chemical Reactions Three Factors in Every Reaction: 1. Mechanism: what is the step-by-step
1.13 Acid-Base Reactions: Lone-Pair Donors & Acceptors
 1.13 Acid-Base Reactions: Lone-Pair Donors & Acceptors I, Cl, N 3, 3 P 4 pka 10 to 5 Super strong acids 3 + pka 1.7 RC 2 pka ~ 5 acids Ph pka ~ 10 get 2, R pka ~ 16 weaker RCC (alkynes) pka ~ 26 RN 2 pka
1.13 Acid-Base Reactions: Lone-Pair Donors & Acceptors I, Cl, N 3, 3 P 4 pka 10 to 5 Super strong acids 3 + pka 1.7 RC 2 pka ~ 5 acids Ph pka ~ 10 get 2, R pka ~ 16 weaker RCC (alkynes) pka ~ 26 RN 2 pka
Chapter 8: Chemistry of Alkynes (C n H 2n-2 )
 hapter 8: hemistry of Alkynes ( n 2n-2 ) Bonding & hybridization Both are sp-hybridized Bond angles = 180 o 1 σ + 2 π bonds Linear around lassification R R R' σ bond energy: 88 kcal/mol π bond energy:
hapter 8: hemistry of Alkynes ( n 2n-2 ) Bonding & hybridization Both are sp-hybridized Bond angles = 180 o 1 σ + 2 π bonds Linear around lassification R R R' σ bond energy: 88 kcal/mol π bond energy:
Understanding the basics. Mechanisms 5/24/11
 Understanding the basics Mechanisms Some Basic rgo I Reactions Mechanisms are the most mind-boggling part of organic chemistry. Students, generally speaking, have spent their time memorizing their way
Understanding the basics Mechanisms Some Basic rgo I Reactions Mechanisms are the most mind-boggling part of organic chemistry. Students, generally speaking, have spent their time memorizing their way
Chem 350 Jasperse Ch. 8 Notes 1
 hem 350 Jasperse h. 8 Notes 1 Summary of Alkene Reactions, h. 8. Memorize Reaction, rientation where Appropriate, Stereochemistry where Appropriate, and Mechanism where Appropriate. -all are drawn using
hem 350 Jasperse h. 8 Notes 1 Summary of Alkene Reactions, h. 8. Memorize Reaction, rientation where Appropriate, Stereochemistry where Appropriate, and Mechanism where Appropriate. -all are drawn using
MCAT Organic Chemistry Problem Drill 10: Aldehydes and Ketones
 MCAT rganic Chemistry Problem Drill 10: Aldehydes and Ketones Question No. 1 of 10 Question 1. Which of the following is not a physical property of aldehydes and ketones? Question #01 (A) Hydrogen bonding
MCAT rganic Chemistry Problem Drill 10: Aldehydes and Ketones Question No. 1 of 10 Question 1. Which of the following is not a physical property of aldehydes and ketones? Question #01 (A) Hydrogen bonding
UNIT.10 HALOALKANES AND HALOARENES
 UNIT.10 ALOALKANES AND ALOARENES ONE MARKS QUESTIONS 1. What are haloalkanes? aloalkane is a derivative obtained by replacing hydrogen atom of alkane by halogen atom. 2. What is the hybridization of the
UNIT.10 ALOALKANES AND ALOARENES ONE MARKS QUESTIONS 1. What are haloalkanes? aloalkane is a derivative obtained by replacing hydrogen atom of alkane by halogen atom. 2. What is the hybridization of the
Chapter 8 Reactions of Alkenes
 Chapter 8 Reactions of Alkenes Electrophilic Additions o Regio vs stereoselectivity Regio where do the pieces add? Markovnikov s rule hydrogen will go to the side of the double bond with most hydrogens.
Chapter 8 Reactions of Alkenes Electrophilic Additions o Regio vs stereoselectivity Regio where do the pieces add? Markovnikov s rule hydrogen will go to the side of the double bond with most hydrogens.
Chem 3719 Example Exams. Chemistry 3719 Practice Exams
 Chem 3719 Example Exams Chemistry 3719 Practice Exams Fall 2018 Chemistry 3719, Fall 2017 Exam 1 Student Name: Y Number: This exam is worth 100 points out of a total of 700 points for Chemistry 3719/3719L.
Chem 3719 Example Exams Chemistry 3719 Practice Exams Fall 2018 Chemistry 3719, Fall 2017 Exam 1 Student Name: Y Number: This exam is worth 100 points out of a total of 700 points for Chemistry 3719/3719L.
Alcohols: Contain a hydroxy group( OH) bonded to an sp 2 or sp 3 hybridized
 Lecture Notes hem 51B S. King hapter 9 Alcohols, Ethers, and Epoxides I. Introduction Alcohols, ether, and epoxides are 3 functional groups that contain σ-bonds. Alcohols: ontain a hydroxy group( ) bonded
Lecture Notes hem 51B S. King hapter 9 Alcohols, Ethers, and Epoxides I. Introduction Alcohols, ether, and epoxides are 3 functional groups that contain σ-bonds. Alcohols: ontain a hydroxy group( ) bonded
Once familiar with chiral centers, models, drawings and mental images NOW: Final representation of chiral centers: Fischer Projections
 Once familiar with chiral centers, models, drawings and mental images NOW: Final representation of chiral centers: Fischer Projections Fischer Projections are 2-dimensional representations of 3-dimensional
Once familiar with chiral centers, models, drawings and mental images NOW: Final representation of chiral centers: Fischer Projections Fischer Projections are 2-dimensional representations of 3-dimensional
PAPER No. : 5; Organic Chemistry-II MODULE No. : 13; Mixed S N 1 and S N 2 Reactions
 Subject Chemistry Paper No and Title Module No and Title Module Tag 5; Organic Chemistry-II 13; Mixed S N 1 and S N 2 Reactions CHE_P5_M13 TABLE OF CONTENTS 1. Learning Outcomes 2. Introduction 3. Nature
Subject Chemistry Paper No and Title Module No and Title Module Tag 5; Organic Chemistry-II 13; Mixed S N 1 and S N 2 Reactions CHE_P5_M13 TABLE OF CONTENTS 1. Learning Outcomes 2. Introduction 3. Nature
Chapter 8 I. Nucleophilic Substitution (in( II. Competion with Elimination. Nucleophilic Substitution
 hapter 8 I. Nucleophilic Substitution (in( depth) II. ompetion with Elimination Nucleophilic Substitution Substrate is a sp3 hybridized carbon atom (cannot be an a vinylic halide or an aryl halide except
hapter 8 I. Nucleophilic Substitution (in( depth) II. ompetion with Elimination Nucleophilic Substitution Substrate is a sp3 hybridized carbon atom (cannot be an a vinylic halide or an aryl halide except
Elimination reactions
 Chapter 9 Elimination reactions E2 and E1 reactions Competition between S N and E Elimination reactions Ch 9 #2 elimination and/or substitution 2 mechanisms ~ E2 and E1 E2: bimolecular elimination rxn
Chapter 9 Elimination reactions E2 and E1 reactions Competition between S N and E Elimination reactions Ch 9 #2 elimination and/or substitution 2 mechanisms ~ E2 and E1 E2: bimolecular elimination rxn
Lecture 20 Organic Chemistry 1
 CEM 232 rganic Chemistry I at Chicago Lecture 20 rganic Chemistry 1 Professor Duncan Wardrop March 18, 2010 1 Self-Test Question Capnellene (4) is a marine natural product that was isolated from coral
CEM 232 rganic Chemistry I at Chicago Lecture 20 rganic Chemistry 1 Professor Duncan Wardrop March 18, 2010 1 Self-Test Question Capnellene (4) is a marine natural product that was isolated from coral
Loudon Chapter 17 Review: Allylic/Benzylic Reactivity
 Chapter 17 is all about reactions that happen at the position one away from an aromatic ring, or one away from a double bond. These are called the benzylic and allylic positions respectively. Benzyl and
Chapter 17 is all about reactions that happen at the position one away from an aromatic ring, or one away from a double bond. These are called the benzylic and allylic positions respectively. Benzyl and
Chemistry 2050 Introduction to Organic Chemistry Fall Semester 2011 Dr. Rainer Glaser
 Chemistry 2050 Introduction to Organic Chemistry Fall Semester 2011 Dr. Rainer Glaser Examination #2 Practice Edition Arenes, Stereochemistry, and Organic Halogen Compounds, with Nucleophilic Substitution
Chemistry 2050 Introduction to Organic Chemistry Fall Semester 2011 Dr. Rainer Glaser Examination #2 Practice Edition Arenes, Stereochemistry, and Organic Halogen Compounds, with Nucleophilic Substitution
(b) (CH 3 ) 2 CCH 2 CH 3 D 2 O. (e) A. CH 3 CCl OSO 2 CH 3 C 6 H 5 H 3 C
 278 h a p t e r 7 Further Reactions of aloalkanes 3. arbocations are stabilized by hyperconjugation: Tertiary are the most stable, followed by secondary. Primary and methyl cations are too unstable to
278 h a p t e r 7 Further Reactions of aloalkanes 3. arbocations are stabilized by hyperconjugation: Tertiary are the most stable, followed by secondary. Primary and methyl cations are too unstable to
HALOALKANES. Structure Contain the functional group C-X where X is a halogen (F,Cl,Br or I)
 aloalkanes AS3 1 ALOALKANES Structure ontain the functional group X where X is a halogen (F,l, or I) Types aloalkanes halogen is attached to an aliphatic skeleton alkyl group aloarenes halogen is attached
aloalkanes AS3 1 ALOALKANES Structure ontain the functional group X where X is a halogen (F,l, or I) Types aloalkanes halogen is attached to an aliphatic skeleton alkyl group aloarenes halogen is attached
