You must have your answers written in PERMANENT ink if you want a regrade!!!! This means no test written in pencil or ERASABLE INK will be regraded.
|
|
|
- Domenic Skinner
- 5 years ago
- Views:
Transcription
1 NAME (Print): SIGNATURE: hemistry 320N Dr. Brent Iverson 3rd Midterm April 20, 2017 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long, but there is a reason. I would like to give you a lot of little questions, so you can find ones you can answer and show me what you know, rather than just a few questions that may be testing the one thing you forgot. I recommend you look the exam over and answer the questions you are sure of first, then go back and try to figure out the rest. Also make sure to look at the point totals on the questions as a guide to help budget your time. You must have your answers written in PERMANENT ink if you want a regrade!!!! This means no test written in pencil or ERASABLE INK will be regraded. Please note: We routinely xerox a number of exams following initial grading to guard against receiving altered answers during the regrading process. FINALLY, DUE T SME UNFRTUNATE REENT INIDENTS YU ARE NT ALLWED T INTERAT WIT YUR ELL PNE IN ANY WAY. IF YU TU YUR ELL PNE DURING TE EXAM YU WILL GET A "0" N MATTER WAT YU ARE DING WIT TE PNE. PUT IT AWAY AND LEAVE IT TERE!!!
2 Page Points Total (49) (24) (20) (23) (26) (18) (25) (19) (14) (10) (19) (19) (16) (19) (301)
3 Student onor ode As a student of The University of Texas at Austin, I shall abide by the core values of the University and uphold academic integrity. (Your signature)
4 ompound pk a ydrochloric acid -l -7 Protonated alcohol R ydronium ion arboxylic acids Ammonium ion β-dicarbonyls 3 R - 4 N R 2 R' Primary ammonium β-ketoesters β-diesters Water Alcohols Acid chlorides Aldehydes Ketones Esters R R 3 N R 2 R 2 R 2 2 R' R 2 2 R' l R 2 R' R' Terminal alkynes R 25 LDA -N(i- 3 7 ) 2 40 Terminal alkenes R 2 44 Alkanes
5 Pg 1 (49) D NT TEAR UT TIS PAGE!! We are trying something new to improve grading accuracy. You must write the answers for the questions on the next three pages on this single sheet. Question 1, page 2 (13 pts) True false questions. As appropriate, circle True or False in each space corresponding to the statements on page True False 1.2 True False 1.3 True False 1.4 True False 1.5 True False 1.6 True False 1.7 True False 1.8 True False 1.9 True False 1.10 True False 1.11 True False 1.12 True False 1.13 True False Question 2, page 3 (16 pts) Write the word that best completes the sentences laisen aldol hydroxy aldehyde Michael Question 3, page 3 (8 pts) For each molecule, write onjugated or "Not onjugated 3.1 Not onjugated 3.2 onjugated 3.3 Not onjugated Not onjugated Question 4, page 4 (4 pts) Rank by relative acidity with a 1 under the most acidic and a 4 under the least acidic onjugated onjugated onjugated 3.8 Not onjugated Question 5, page 4 (4 pts) Rank by relative acidity with a 1 under the most acidic and a 4 under the least acidic nucleophile beta (β) Question 6, page 4 (4 pts) Write the correct statement. Wait, I will give you these four points! 2.8 Michael 2.9 aldol d. all of the above (of course) Fluorescence Phosphorescence hemiluminescence reflected absorbed filled 2.16 unfilled
6 Pg 2 (13) Write your answers to these questions on the answer sheet on page 1 1. (13 pts). n page 1, circle True or False to indicate whether each of the following statements is true or false. 1.1 Kinetic control in a chemical reaction refers to situations at low temperature in which the predominant product is the one that forms the fastest. 1.2 Kinetic control in a chemical reaction refers to situations at low temperature in which the predominant product is the one that is more stable (lower in energy). 1.3 Thermodynamic control in a chemical reaction refers to situations at low temperature in which the predominant product is the one that is more stable (lower in energy). 1.4 Thermodynamic control in a chemical reaction refers to situations at high temperature in which the predominant product is the one that is more stable (lower in energy). 1.5 Thermodynamic control in a chemical reaction refers to situations at high temperature in which the predominant product is the one that the predominate product is the one that forms the fastest. 1.6 In the first step of a laisen condensation with ethyl acetate using NaEt as the base, equilibrium favors formation of the enolate. 1.7 In the first step of a laisen condensation with ethyl acetate using LDA as the base, equilibrium favors the starting ethyl acetate. 1.8 In the first step of a laisen condensation with ethyl acetate using LDA as the base, equilibrium favors the enolate. 1.9 According to molecular orbital theory, you generate as many new molecular orbitals as atomic orbitals used to create them onjugation refers to the situation in which 2p orbitals on more than two adjacent atoms in the same molecule overlap, allowing the pi electron density to delocalize into all the adjacent 2p orbitals to provide for extra stability The greater the number of pi bonds in conjugation, the smaller the energy difference between filled and unfilled orbitals, so the longer the wavelength of light that is absorbed (wavelength is inversely proportional to energy of light) The greater the number of pi bonds in conjugation, the larger the energy difference between filled and unfilled orbitals, so the shorter the wavelength of light that is absorbed (wavelength is inversely proportional to energy of light) X adds to conjugated dienes to give both 1,2 and 1,4 addition products, via a resonance stabilized allylic cation intermediate.
7 Pg 3 (24) Write your answers to these questions on the answer sheet on page 1 2. (16 pts). n page 1, fill in each blank with the one word that best completes the following sentences. A. In a (2.1) condensation reaction, two esters react to create a β-ketoester product. B. In the (2.2) reaction, two aldehyde molecules react to create initially a β- (2.3) aldehyde product, that can dehydrate in the presence of acid and heating to give a conjugated α,β-unsaturated (2.4).. In one example of the (2.5) reaction, a (2.6) such as an enolate or enamine reacts with an electrophile such as an α,β-unsaturated ketone to give a new carbon-carbon bond at the (2.7) carbon atom of the ketone. D. The Robinson annulation reaction involves a (2.8) reaction followed by an (2.9) reaction then dehydration to make a six-membered ring. E. (2.10) occurs when there are not vibrations possible (a rigid molecule) so the photon is emitted as the electron goes back to ground state. F. (2.11) (glow in the dark) happens when the excited electron has a flipped spin, and must reflip back before entering the original filled orbital while emitting a photon. G. (2.12) (firefly light, "light sticks") happens when a chemical reaction produces an excited electron in a rigid molecule that then emits a photon as it transitions back to a ground state.. When illuminated with normal white light, a substance appears to our eyes to be the combination of the wavelengths (2.13) minus the wavelengths (2.14). I. When light of the proper frequency is absorbed by a molecule, an electron in a(n) (filled/unfilled) (2.15) orbital will be excited to a(n) (filled/unfilled) (2.16) orbital. 3. (8 pts). n page 1, for the following list of molecules, write conjugated or not conjugated to indicate whether or not the given molecule contains a conjugated pi system
8 Pg 4 (23) Write your answers to these questions on the answer sheet on page 1 4. (4 pts). n page 1, rank the following from most to least acidic, with a 1 corresponding to the number of the molecule that is most acidic and 4 next the number of the molecule that is least acidic and 2 and 3 for the molecules with those relative acidities. N LDA (4 pts). n page 1, rank the following from most to least acidic, with a 1 corresponding to the number of the molecule that is most acidic and 4 next the number of the molecule that is least acidic and 2 and 3 for the molecules with those relative acidities. 6. (4 pts). n page 1, write down the correct statement. a. An average adult burns about 100 calories for every mile they run. b. After about age 25 you will need to actively take care of your body to remain healthy, and running should be part of your exercise routine. c. A very recent study, correcting for all other factors, has offered evidence that running is the best form of exercise for staying healthy, and that running for one hour adds an average of seven hours to your life. d. All of the above! F This would have been the nomenclature section. Because your class KILLED the Longhorn run with 164 runners there is none. Great job!
9 Pg 5 (24) 7. (2 pts) What is the most important question in chemistry? Where are the electrons? 8. (14 points) Suppose a relative of yours is having an MRI. In no more than four sentences, explain to them what is happening when they have the MRI scan. We wil be looking for a minumum of 7 key points here. The popular medical diagnostic technique of magnetic resonance imaging (MRI) is based on the same principles as NMR, namely the flipping (i.e. resonance) of nuclear spins of protons by radio frequency irradiation when a patient is placed in a strong magnetic field. Magnetic field gradients are used to gain imaging information, and rotation of the gradient around the center of the object gives imaging in an entire plane (i.e. slice inside patient). In an MRI image, you are looking at individual slices that when stacked make up the three-dimensional image of relative amounts of protons, especially the protons from water and fat, in the different tissues. 9. (8 points) Draw the two most important resonance contributing structures of the amide shown below. Be sure to show all lone pairs and formal charges. You do not have to draw arrows on this one. N N N
10 Pg 6 (20) 10. (20 pts) In each of the boxes over an arrow, write the minimum number of equivalents of the specified reagent required to carry out the reaction shown to completion. If only a catalytic amount is needed, write "AT". Note: You must assume the carbonyl compound starting material is initially present in an amount of 1.0 equivalent. A) 1) AT equivalents 2) mild 3 + (heat) Na B) 1) 0.5 equivalents NaEt 2) mild 3 + ) 1) 1.0 equivalents LDA 2) * racemic l D) 1) 1.0 equivalents Na E) N 1) 1.0 equivalents 3 N 3 For these next two we have provided the product, you need to draw the starting material as well as fill in the number of equivalents. F) 1) AT equivalents Na ) mild (heat) 4 6 G) 6 5 1) 1.0 equivalents NaEt ) mild racemic *
11 Pg 7 (23) (23 pts) omplete the mechanism for the following laisen condensation reaction. Be sure to show arrows to indicate movement of all electrons, write all lone pairs, all formal charges, and all the products for each step. Remember, I said all the products for each step. IF A NEW IRAL ENTER IS REATED IN AN INTERMEDIATE R PRDUT, MARK IT WIT AN ASTERISK AND LABEL TE MLEULE AS RAEMI IF APPRPRIATE. In the boxes provided, write which of the 4 mechanistic elements describes each step (make a bond, break a bond, etc.). 3 Take a proton away 3 Make a bond 3 racemic Break a bond * * racemic Take a proton away 3 3 Note you will have to write a balanced equation for this mechanism on PAGE 9
12 Pg 8 (26) 12. (26 pts) omplete the mechanism for the following Michael reaction. Be sure to show arrows to indicate movement of all electrons, write all lone pairs, all formal charges, and all the products for each step. Remember, I said all the products for each step. IF A NEW IRAL ENTER IS REATED IN AN INTERMEDIATE R PRDUT, MARK IT WIT AN ASTERISK AND LABEL TE MLEULE AS RAEMI IF APPRPRIATE. In the boxes provided, write which of the 4 mechanistic elements describes each step (make a bond, break a bond, etc.). 2 1) 2 3 Take a proton away 2 3 2) β α N2 Make a bond Enol β α N Add a proton β α N2 Tautomerization β α N2 2 3 Take a proton away β α N2 Products 2 3 Note you will have to write a balanced equation for the above mechanism on PAGE 9
13 Pg 9 (18) 13. (18 pts) Write BALANED equations for all three of the mechanisms that you drew on the last two pages. Note that because we want balanced equations you will need to specify the amount of each of the reagents you start with as well as the equivalents of each of the products made. Any reagent used in a catalytic amount should be placed in the box over the arrow (not all of the boxes over the arrows will necessarily have anything in them). Write a balanced equation for the overall process described by mechanism 1 from page equivalent equivalent 0.5 equivalent equivalent Write a balanced equation for the overall process described by mechanism 2 from page equivalent β α N2 1.0 equivalent 2 3 N2 1.0 equivalent 1.0 equivalent 1.0 equivalent
14 Pg 10 (25) 14. (3, 4,5 or 7 pts.) Write the predominant carbon containing product or products that will occur for each transformation. If there are multiple carbon containing products, WRITE ALL F TEM. If a new chiral center is created and a racemic mixture is formed, label the chiral center with an asterisk (*) and write racemic. If an E,Z mixture is created as the products, YU NEED T DRAW BT TE E AND Z PRDUT. No need for wedges and dashes. Also, do not worry about balancing these equations, but you do need to show us ALL of the major carbon-containing products of these transformations. (You should recognize this page from a recent homework) * Racemic 1.0 eq. LDA 1.0 eq. Na 1) 0.5 eq. NaEt 2) 3 (Mild acid) 0.5 eq. LDA 1) 1.0 eq. NaEt 2) 3 (Mild acid) 1) 0.5 eq. LDA 2) 3 (Mild acid) * Racemic * Racemic
15 Pg 11 (19) 14. (3, 4,5 or 7 pts.) Write the predominant carbon containing product or products that will occur for each transformation. If there are multiple carbon containing products, WRITE ALL F TEM. If a new chiral center is created and a racemic mixture is formed, label the chiral center with an asterisk (*) and write racemic. If an E,Z mixture is created as the products, YU NEED T DRAW BT TE E AND Z PRDUT. No need for wedges and dashes. Also, do not worry about balancing these equations, but you do need to show us ALL of the major carbon-containing products of these transformations. 1) Na (catalytic) 2) 3 heat Aldol followed by dehydration 1) Na (catalytic) 2) 3 heat E,Z mixture Aldol followed by dehydration 1) 1.0 eq. NaEt 2) 3) 3 mild Michael * Robinson Na (catalytic) heat Racemic Et Et Et Et Diels-Alder Et Et
16 Pg 12 (14) 14. (3, 4,5 or 7 pts.) Write the predominant carbon containing product or products that will occur for each transformation. If there are multiple carbon containing products, WRITE ALL F TEM. If a new chiral center is created and a racemic mixture is formed, label the chiral center with an asterisk (*) and write racemic. If an E,Z mixture is created as the products, YU NEED T DRAW BT TE E AND Z PRDUT. No need for wedges and dashes. Also, do not worry about balancing these equations, but you do need to show us ALL of the major carbon-containing products of these transformations. N 1) 2) 3 heat Br * Racemic N Enamine alkylation Be sure to write down all the carbon containing products. 1) 1.0 eq. NaEt 2) Br 2 3) 3 strong and heat 2 Alkylation followed by ester hydrolysis and decarboxylation
17 Pg 13 (10) 15. Using any reagents turn the starting material into the indicated product. All carbon atoms in the product must come from the starting material. Draw all molecules synthesized along the way. When in doubt, draw the molecule! Label all chiral centers with an asterisk (*) and make sure to write "Racemic" where appropriate. You will notice a theme in these problems in that you will be starting with very simple structures and making more complex products. Remember, all of the carbons of the product must come from the given starting material. (10 pts) 2 carbons 6 carbons A) 2 r 4? New - bond 1) 0.5 Eq. NaEt 2) mild 3 (laisen) Sl 2 l The key recognition element is the β-keto ester product indicating a laisen as the last step. Alternatively, the ethyl ester could have been made from a reaction of ethanol with sufuric acid directly, avoiding the acid chloride intermediate.
18 Pg 14 (19) 15. Using any reagents turn the starting material into the indicated product. All carbon atoms in the product must come from the starting material. Draw all molecules synthesized along the way. When in doubt, draw the molecule! Label all chiral centers with an asterisk (*) and make sure to write "Racemic" where appropriate. You will notice a theme in these problems in that you will be starting with very simple structures and making more complex products. Remember, all of the carbons of the product must come from the given starting material. (19 pts) B) 2 carbons? New - bonds 5 carbons 2 r 4 heat β-keto acid Et 2 S 4 (catalytic) or 1) Sl 2 2) Et 1) 0.5 eq. NaEt 2) 3 (mild acid) PBr 3 Br * racemic 2 S 4 / 2 (strong acid) * racemic β-keto ester 1.0 eq. NaEt Recognize the product as being a metyl ketone with 5 carbons. A methyl ketone is the KRE for the acetoester synthesis. The starting alcohol has 2 carbons. Therefore, assume that there are two new - bonds formed as shown and 1 carbon must be lost. The most logical way to lose a single carbon (consistent with the acetoester synthesis) is from a decarboxylation step. For this to occur, the carboxylic acid would have to be β to the carbonyl, so putting all of this information together predict the β-íketo acid intermediate shown. Recognize the β-keto acid intermediate as being derived from the corresponding β-keto ester, that in turn, is the product of a two carbon alkylation of the enolate of ethyl acetoacetate. The anion is derived from deprotanation of ethyl acetoacetate using 1.0 equivalent of NaEt and the required bromoethane can be derived from the starting ethanol by reaction with PBr 3 (or Sl 2 if chloroethane is used). Recognize that the ethyl acetoacetate is the product of a laisen condensation with the ester of acetic acid, such as ethyl acetate. Recognize that the ester could be derived by reacting acetic acid with an alcohol such as ethanol in the presence of catalytic 2 S 4. Recognize that acetic acid can be derived from the oxidation of the starting ethanol with 2 r 4. An aldol approach would be possilble here. Ethanol could be converted to acetaldehyde, that is used for an aldol. The aldol product could be oxidized to the corresponding β-keto acid. You would need to make the β- keto ester from the β-keto acid to allow for alkylation of the enolate in the next step. (The β-keto acid cannot be used as an enolate because the carboxylic acid function would deprotonate, not the β-carbon.)
19 Pg 15 (19) 15. Using any reagents turn the starting material into the indicated product. All carbon atoms in the product must come from the starting material. Draw all molecules synthesized along the way. When in doubt, draw the molecule! Label all chiral centers with an asterisk (*) and make sure to write "Racemic" where appropriate. You will notice a theme in these problems in that you will be starting with very simple structures and making more complex products. Remember, all of the carbons of the product must come from the given starting material. (19 pts) 1) B 3 2) 2 2 / Na 4 carbons )? 1) 3 2) ( 3 ) 2 S 1) NaB 4 2) 2 11 carbons New - bond New - bond * Racemic 1) 0.5 eq. NaPr 2) 3 (mild acid) 2 r 4 Sl 2 l Recognize the product as a symmetric β-keto ester, the KRE of a laisen condensation reaction. Recognize further that there is a three-carbon alkoxy group on the ester portion of the product. The starting material has 4 carbons, while the product is an 8-carbon laisen product with a three-carbon alkoxy group. Therefore propose to create a four-carbon carboxylic acid through the sequence of non-markovnikov hydroboration oxidation followed by 2 r 4 oxidation. The three-carbon piece can be made from the stating alkene by the sequence of ozonlolysis followed by reduction.
20 Pg 16 (19) 15. Using any reagents turn the starting material into the indicated product. All carbon atoms in the product must come from the starting material. Draw all molecules synthesized along the way. When in doubt, draw the molecule! Label all chiral centers with an asterisk (*) and make sure to write "Racemic" where appropriate. You will notice a theme in these problems in that you will be starting with very simple structures and making more complex products. Remember, all of the carbons of the product must come from the given starting material. (16 pts) 4 carbons 3 carbons 6 carbons? D) New = bond 1) 0.5 eq. NaEt 2) 3 (mild acid) laisen condensation P 1) catalytic Na 2) 3 (heat) Aldol 1) 1.0 eq. NaEt 2) Michael heat (- 2 ) 2 S 4 / 2 * (strong acid) * racemic racemic Recognize the product as an α,β-unsaturated ketone in a ring, the KRE of a cyclic aldol reaction followed by dehydration. This can be difficult to spot. The starting material for that reaction is the methyl ketone aldehyde that can be recognized as coming from the Michael reaction of acetoester with the Michael acceptor shown followed by decarboxylation. Acetoester can be made from the laisen reaction of the starting ethyl acetate and the required Michael acceptor can be derived from the P oxidation of the starting alcohol. Notice that this synthetic scheme uses the big three of enolate reactions, a laisen, a Michael and an aldol.
21 Pg 17 (19) 15. Using any reagents turn the starting material into the indicated product. All carbon atoms in the product must come from the starting material. Draw all molecules synthesized along the way. When in doubt, draw the molecule! Label all chiral centers with an asterisk (*) and make sure to write "Racemic" where appropriate. You will notice a theme in these problems in that you will be starting with very simple structures and making more complex products. Remember, all of the carbons of the product must come from the given starting material. This one is much harder because there is no real KRE in the product. Save it until last and be creative! The chemistry is not hard to understand once you are on the right track. 7 carbons 7 carbons (19 pts) E)? 1) 3 2) ( 3 ) 2 S heat (- 2 ) * racemic 2 S 4 / 2 (strong acid) 2 r 4 * R racemic 1) 1.0 eq. NaR l Sl 2 l 2 R R 2) 3 (mild acid) R This is a difficult problem because there are no real KREs in the product. Instead, we were counting on you recognize that there are 6 carbons in the product but 7 carbons in the starting material. Further, we hoped that you would remember that the best way to lose one carbon is decarboxylation, and the only way you know to make a ring is through enolate chemistry. Putting those two clues together should lead you to a Dieckmann. Therefore, propose a Dieckman from the corresponding 7-carbon diester. Note that because the alkoxy portion of the ester does not show up in the product, you can use any alcohol (ethanol, methanol etc.) as long as the base used in the Dieckmann matches. The diester can be derived from the starting cycloheptanone through the sequence of ozonolysis, oxidation, conversion to the diacid chloride followed by reaction with alcohol.
You must have your answers written in PERMANENT ink if you want a regrade!!!! This means no test written in pencil or ERASABLE INK will be regraded.
 AME (Print): SIGATURE: hemistry 320 Dr. Brent Iverson 3rd Midterm April 23, 2015 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long, but there
AME (Print): SIGATURE: hemistry 320 Dr. Brent Iverson 3rd Midterm April 23, 2015 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long, but there
You must have your answers written in PERMANENT ink if you want a regrade!!!! This means no test written in pencil or ERASABLE INK will be regraded.
 NAME (Print): SIGNATURE: hemistry 310N Dr. Brent Iverson 2nd Midterm March 25, 2010 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long, but
NAME (Print): SIGNATURE: hemistry 310N Dr. Brent Iverson 2nd Midterm March 25, 2010 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long, but
Please note: We routinely xerox a number of exams following initial grading to guard against receiving altered answers during the regrading process.
 NAME (Print): SIGNATURE: hemistry 320M/328M Dr. ent Iverson 2nd Midterm ctober 25, 2018 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long,
NAME (Print): SIGNATURE: hemistry 320M/328M Dr. ent Iverson 2nd Midterm ctober 25, 2018 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long,
You must have your answers written in PERMANENT ink if you want a regrade!!!! This means no test written in pencil or ERASABLE INK will be regraded.
 NAME (Print): SIGNATURE: Chemistry 320N Dr. Brent Iverson 1st Midterm Feb. 13, 2014 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long, but
NAME (Print): SIGNATURE: Chemistry 320N Dr. Brent Iverson 1st Midterm Feb. 13, 2014 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long, but
Please note: We routinely xerox a number of exams following initial grading to guard against receiving altered answers during the regrading process.
 NAME (Print): SIGNATURE: Chemistry 320N Dr. Brent Iverson 1st Midterm Feb. 18, 2016 Please print the first three letters of your LAST name in the three boxes Please Note: This test may be a bit long, but
NAME (Print): SIGNATURE: Chemistry 320N Dr. Brent Iverson 1st Midterm Feb. 18, 2016 Please print the first three letters of your LAST name in the three boxes Please Note: This test may be a bit long, but
Note: You must have your answers written in pen if you want a regrade!!!!
 AME (Print): SIGATURE: hemistry 310 Dr. Brent Iverson 2nd Midterm March 29, 2007 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long, but there
AME (Print): SIGATURE: hemistry 310 Dr. Brent Iverson 2nd Midterm March 29, 2007 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long, but there
You must have your answers written in PERMANENT ink if you want a regrade!!!! This means no test written in pencil or ERASABLE INK will be regraded.
 AME (Print): SIGATURE: Chemistry 320 Dr. Brent Iverson 3rd Midterm April 18, 2013 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long, but
AME (Print): SIGATURE: Chemistry 320 Dr. Brent Iverson 3rd Midterm April 18, 2013 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long, but
Note: You must have your answers written in pen if you want a regrade!!!!
 NAME (Print): SIGNATURE: hemistry 310N Dr. Brent Iverson 2nd Midterm March 29, 2007 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long, but
NAME (Print): SIGNATURE: hemistry 310N Dr. Brent Iverson 2nd Midterm March 29, 2007 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long, but
Please note: We routinely xerox a number of exams following initial grading to guard against receiving altered answers during the regrading process.
 NAME (Print): SIGNATURE: hemistry 30N Dr. Brent Iverson st Midterm Feb. 9, 05 Please print the first three letters of your LAST name in the three boxes Please Note: This test may be a bit long, but there
NAME (Print): SIGNATURE: hemistry 30N Dr. Brent Iverson st Midterm Feb. 9, 05 Please print the first three letters of your LAST name in the three boxes Please Note: This test may be a bit long, but there
You must have your answers written in PERMANENT ink if you want a regrade!!!! This means no test written in pencil or ERASABLE INK will be regraded.
 NAME (Print): SIGNATURE: hemistry 320M/328M Dr. ent Iverson 3rd Midterm November 15, 2012 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long,
NAME (Print): SIGNATURE: hemistry 320M/328M Dr. ent Iverson 3rd Midterm November 15, 2012 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long,
Note: You must have your answers written in pen if you want a regrade!!!!
 AME (Print): SIGATURE: hemistry 310 Dr. ent Iverson 3rd Midterm April 26, 2007 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long, but there
AME (Print): SIGATURE: hemistry 310 Dr. ent Iverson 3rd Midterm April 26, 2007 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long, but there
You must have your answers written in PERMANENT ink if you want a regrade!!!! This means no test written in pencil or ERASABLE INK will be regraded.
 NAME (Print): SIGNATURE: Chemistry 310M/318M Dr. ent Iverson 3rd Midterm November 18, 2010 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit
NAME (Print): SIGNATURE: Chemistry 310M/318M Dr. ent Iverson 3rd Midterm November 18, 2010 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit
Note: You must have your answers written in pen if you want a regrade!!!!
 AME (Print): SIGATURE: hemistry 30 Dr. Brent Iverson nd Midterm March 7, 008 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long, but there
AME (Print): SIGATURE: hemistry 30 Dr. Brent Iverson nd Midterm March 7, 008 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long, but there
You must have your answers written in PERMANENT ink if you want a regrade!!!! This means no test written in pencil or ERASABLE INK will be regraded.
 AME (Print): SIGATURE: hemistry 320 Dr. Brent Iverson 2nd Midterm March 26, 2015 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long, but there
AME (Print): SIGATURE: hemistry 320 Dr. Brent Iverson 2nd Midterm March 26, 2015 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long, but there
You must have your answers written in PERMANENT ink if you want a regrade!!!! This means no test written in pencil or ERASABLE INK will be regraded.
 AME (Print): SIGATURE: hemistry 320 Dr. Brent Iverson 3rd Midterm April 17, 2014 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long, but there
AME (Print): SIGATURE: hemistry 320 Dr. Brent Iverson 3rd Midterm April 17, 2014 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long, but there
You must have your answers written in PERMANENT ink if you want a regrade!!!! This means no test written in pencil or ERASABLE INK will be regraded.
 AME (Print): SIGATURE: Chemistry 320 Dr. Brent Iverson 2nd Midterm March 23, 2017 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long, but
AME (Print): SIGATURE: Chemistry 320 Dr. Brent Iverson 2nd Midterm March 23, 2017 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long, but
Note: You must have your answers written in pen if you want a regrade!!!!
 NAME (Print): SIGNATURE: Chemistry 310N Dr. Brent Iverson 2nd Midterm March 27, 2008 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long,
NAME (Print): SIGNATURE: Chemistry 310N Dr. Brent Iverson 2nd Midterm March 27, 2008 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long,
You must have your answers written in PERMANENT ink if you want a regrade!!!! This means no test written in pencil or ERASABLE INK will be regraded.
 AME (Print): SIGATURE: hemistry 310 Dr. Brent Iverson 2nd Midterm March 22, 2012 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long, but there
AME (Print): SIGATURE: hemistry 310 Dr. Brent Iverson 2nd Midterm March 22, 2012 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long, but there
Note: You must have your answers written in pen if you want a regrade!!!!
 NAME (Print): SIGNATURE: hemistry 310M/318M Dr. Brent Iverson 2nd Midterm November 1, 2007 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit
NAME (Print): SIGNATURE: hemistry 310M/318M Dr. Brent Iverson 2nd Midterm November 1, 2007 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit
Score: Homework Problem Set 9 Iverson CH320N Due Monday, April 17. NAME (Print): Chemistry 320N Dr. Brent Iverson 9th Homework April 10, 2017
 omework Problem Set 9 Iverson C0N Due Monday, April 7 NAME (Print): SIGNATURE: Chemistry 0N Dr. ent Iverson 9th omework April 0, 07 Please print the first three letters of your last name in the three boxes
omework Problem Set 9 Iverson C0N Due Monday, April 7 NAME (Print): SIGNATURE: Chemistry 0N Dr. ent Iverson 9th omework April 0, 07 Please print the first three letters of your last name in the three boxes
Please note: We routinely xerox a number of exams following initial grading to guard against receiving altered answers during the regrading process.
 NAME (Print): SIGNATURE: Chemistry 320N Dr. ent Iverson 1st Midterm Feb. 18, 2016 Please print the first three letters of your LAST name in the three boxes Please Note: This test may be a bit long, but
NAME (Print): SIGNATURE: Chemistry 320N Dr. ent Iverson 1st Midterm Feb. 18, 2016 Please print the first three letters of your LAST name in the three boxes Please Note: This test may be a bit long, but
Note: You must have your answers written in pen if you want a regrade!!!!
 NAME (Print): SIGNATURE: hemistry 310M/318M Dr. ent Iverson 2nd Midterm ctober 29, 2009 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long,
NAME (Print): SIGNATURE: hemistry 310M/318M Dr. ent Iverson 2nd Midterm ctober 29, 2009 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long,
Note: You must have your answers written in pen if you want a regrade!!!!
 NAME (Print): SIGNATURE: Chemistry 310N Dr. Brent Iverson 1st Midterm Feb. 21, 2008 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long, but
NAME (Print): SIGNATURE: Chemistry 310N Dr. Brent Iverson 1st Midterm Feb. 21, 2008 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long, but
Note: You must have your answers written in pen if you want a regrade!!!!
 NAME (Print): SIGNATURE: hemistry 310M/318M Dr. ent Iverson 2nd Midterm November 1, 2007 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long,
NAME (Print): SIGNATURE: hemistry 310M/318M Dr. ent Iverson 2nd Midterm November 1, 2007 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long,
You must have your answers written in PERMANENT ink if you want a regrade!!!! This means no test written in pencil or ERASABLE INK will be regraded.
 AME (Print): SIGATURE: hemistry 310M/318M Dr. ent Iverson Final exam December 8, 2010 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long,
AME (Print): SIGATURE: hemistry 310M/318M Dr. ent Iverson Final exam December 8, 2010 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long,
You must have your answers written in PERMANENT ink if you want a regrade!!!! This means no test written in pencil or ERASABLE INK will be regraded.
 AME (Print): SIGATURE: Chemistry 320 Dr. Brent Iverson Final May 7, 2014 Please print the first three letters of your last name in the three boxes Please ote: You will do your best if you are relaxed.
AME (Print): SIGATURE: Chemistry 320 Dr. Brent Iverson Final May 7, 2014 Please print the first three letters of your last name in the three boxes Please ote: You will do your best if you are relaxed.
Note: You must have your answers written in pen if you want a regrade!!!!
 AME (Print): SIGATURE: Chemistry 310 Dr. Brent Iverson Final May 11, 2006 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long, but there is
AME (Print): SIGATURE: Chemistry 310 Dr. Brent Iverson Final May 11, 2006 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long, but there is
Note: You must have your answers written in pen if you want a regrade!!!!
 AME (Print): SIGATURE: Chemistry 310 Dr. Brent Iverson 1st Midterm Feb. 22, 2007 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long, but there
AME (Print): SIGATURE: Chemistry 310 Dr. Brent Iverson 1st Midterm Feb. 22, 2007 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long, but there
You must have your answers written in PERMANENT ink if you want a regrade!!!! This means no test written in pencil or ERASABLE INK will be regraded.
 AME (Print): IGATURE: hemistry 320M/328M Dr. Brent Iverson 2nd Midterm ctober 25, 2012 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long,
AME (Print): IGATURE: hemistry 320M/328M Dr. Brent Iverson 2nd Midterm ctober 25, 2012 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long,
You must have your answers written in PERMANENT ink if you want a regrade!!!! This means no test written in pencil or ERASABLE INK will be regraded.
 AME (Print): SIGATURE: hemistry 320 Dr. Brent Iverson 1st Midterm Feb. 14, 2013 (Sorry about the Valentine's day exam!) Please print the first three letters of your last name in the three boxes Please
AME (Print): SIGATURE: hemistry 320 Dr. Brent Iverson 1st Midterm Feb. 14, 2013 (Sorry about the Valentine's day exam!) Please print the first three letters of your last name in the three boxes Please
Score: Homework Problem Set 6 Iverson CH320N Due Friday, March 10. NAME (Print): Chemistry 320N Dr. Brent Iverson 6th Homework March 3, 2017
 omework Problem Set 6 Iverson 320 Due Friday, March 10 AME (Print): SIGATURE: hemistry 320 Dr. Brent Iverson 6th omework March 3, 2017 Please print the first three letters of your last name in the three
omework Problem Set 6 Iverson 320 Due Friday, March 10 AME (Print): SIGATURE: hemistry 320 Dr. Brent Iverson 6th omework March 3, 2017 Please print the first three letters of your last name in the three
New bond. ph 4.0. Fischer esterification. New bond 2 O * New bond. New bond H 2N. New C-C bond. New C-C bond. New C-C bond. O Cl.
 Iverson C 0N KRE Table: For use in synthesis problems, count carbons in products and starting materials then identify location(s) of new s, especially C-C or C=C s. With that information, use the following
Iverson C 0N KRE Table: For use in synthesis problems, count carbons in products and starting materials then identify location(s) of new s, especially C-C or C=C s. With that information, use the following
Please note: We routinely xerox a number of exams following initial grading to guard against receiving altered answers during the regrading process.
 NAME (Print): SIGNATURE: hemistry 320M/328M Dr. ent Iverson Final December 16, 2017 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long, but
NAME (Print): SIGNATURE: hemistry 320M/328M Dr. ent Iverson Final December 16, 2017 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long, but
Note: You must have your answers written in pen if you want a regrade!!!!
 NAME (Print): SIGNATURE: Chemistry 310N Dr. Brent Iverson 1st Midterm Feb. 23, 2006 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long, but
NAME (Print): SIGNATURE: Chemistry 310N Dr. Brent Iverson 1st Midterm Feb. 23, 2006 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long, but
Score: NAME (Print): Chemistry 320N Dr. Brent Iverson 3rd Homework February 3, 2017 SIGNATURE:
 omework Problem Set 3 Iverson 30N Due Friday February 10 NAME (Print): SIGNATURE: hemistry 30N Dr. ent Iverson 3rd omework February 3, 017 Please print the first three letters of your last name in the
omework Problem Set 3 Iverson 30N Due Friday February 10 NAME (Print): SIGNATURE: hemistry 30N Dr. ent Iverson 3rd omework February 3, 017 Please print the first three letters of your last name in the
Homework Problem Set 11 Iverson CH320N Due Friday, May 5
 omework Problem Set 11 Iverson 320 Due Friday, May 5 AME (Print): SIGATURE: hemistry 320 Dr. Brent Iverson Problem Set 11 April 28, 2017 Please print the first three letters of your last name in the three
omework Problem Set 11 Iverson 320 Due Friday, May 5 AME (Print): SIGATURE: hemistry 320 Dr. Brent Iverson Problem Set 11 April 28, 2017 Please print the first three letters of your last name in the three
Alpha Substitution and Condensations of Enols and Enolate Ions. Alpha Substitution
 Alpha Substitution and ondensations of Enols and Enolate Ions hap 23 W: 27, 28, 30, 31, 37, 39, 42-44, 47, 51, 54-56 Alpha Substitution Replacement of a hydrogen on the carbon adjacent to the carbonyl,
Alpha Substitution and ondensations of Enols and Enolate Ions hap 23 W: 27, 28, 30, 31, 37, 39, 42-44, 47, 51, 54-56 Alpha Substitution Replacement of a hydrogen on the carbon adjacent to the carbonyl,
Please note: We routinely xerox a number of exams following initial grading to guard against receiving altered answers during the regrading process.
 NAME (Print): SIGNATURE: Chemistry 320M/328M Dr. ent Iverson Final December 13, 2018 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long,
NAME (Print): SIGNATURE: Chemistry 320M/328M Dr. ent Iverson Final December 13, 2018 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long,
You must have your answers written in PERMANENT ink if you want a regrade!!!! This means no test written in pencil or ERASABLE INK will be regraded.
 ME (Print): SIGTURE: hemistry 320 Dr. rent Iverson 2nd Midterm March 26, 2015 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long, but there
ME (Print): SIGTURE: hemistry 320 Dr. rent Iverson 2nd Midterm March 26, 2015 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long, but there
You must have your answers written in PERMANENT ink if you want a regrade!!!! This means no test written in pencil or ERASABLE INK will be regraded.
 AME (Print): SIGATURE: hemistry 310 Dr. ent Iverson Final Exam May 14, 2010 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long, but there
AME (Print): SIGATURE: hemistry 310 Dr. ent Iverson Final Exam May 14, 2010 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long, but there
Please note: We routinely xerox a number of exams following initial grading to guard against receiving altered answers during the regrading process.
 AME (Print): SIGATURE: hemistry 320M/328M Dr. ent Iverson Final December 13, 2018 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long, but
AME (Print): SIGATURE: hemistry 320M/328M Dr. ent Iverson Final December 13, 2018 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long, but
You must have your answers written in PERMANENT ink if you want a regrade!!!! This means no test written in pencil or ERASABLE INK will be regraded.
 AM (Print): SIGATUR: hemistry 320 Dr. ent Iverson Final May 7, 2014 Please print the first three letters of your last name in the three boxes Please ote: You will do your best if you are relaxed. Take
AM (Print): SIGATUR: hemistry 320 Dr. ent Iverson Final May 7, 2014 Please print the first three letters of your last name in the three boxes Please ote: You will do your best if you are relaxed. Take
Homework Problem Set 7 Iverson CH320M/328M Due Friday, Nov. 2
 NAME (Print): SIGNATURE: hemistry 320M/328M Dr. Brent Iverson 7th omework October 29, 2018 Please print the first three letters of your last name in the three boxes 1 Score: 2 1. For the following reactions,
NAME (Print): SIGNATURE: hemistry 320M/328M Dr. Brent Iverson 7th omework October 29, 2018 Please print the first three letters of your last name in the three boxes 1 Score: 2 1. For the following reactions,
Lecture Notes Chem 51C S. King Chapter 24 Carbonyl Condensation Reactions
 Lecture Notes Chem 51C S. King Chapter 24 Carbonyl Condensation Reactions I. Reaction of Enols & Enolates with ther Carbonyls Enols and enolates are electron rich nucleophiles that react with a number
Lecture Notes Chem 51C S. King Chapter 24 Carbonyl Condensation Reactions I. Reaction of Enols & Enolates with ther Carbonyls Enols and enolates are electron rich nucleophiles that react with a number
What is in Common for the Following Reactions, and How Do They Work?
 What is in Common for the Following Reactions, and ow Do They Work? You should eventually be able to draw the mechanism for these (and other) reactions 13 Key Intermediate 1 Br-Br Na Br 2 C 3 -I Me NaMe
What is in Common for the Following Reactions, and ow Do They Work? You should eventually be able to draw the mechanism for these (and other) reactions 13 Key Intermediate 1 Br-Br Na Br 2 C 3 -I Me NaMe
Note: You must have your answers written in pen if you want a regrade!!!!
 AME (Print): SIGATURE: Chemistry 310 Dr. Brent Iverson Final May 18, 2009 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long, but there is
AME (Print): SIGATURE: Chemistry 310 Dr. Brent Iverson Final May 18, 2009 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long, but there is
You must have your answers written in PERMANENT ink if you want a regrade!!!! This means no test written in pencil or ERASABLE INK will be regraded.
 NAME (Print): SIGNATURE: hemistry 320M/328M Dr. ent Iverson Final exam December 15, 2012 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long,
NAME (Print): SIGNATURE: hemistry 320M/328M Dr. ent Iverson Final exam December 15, 2012 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long,
Michael and Aldol CH391 December 4, 2002
 Michael and Aldol H391 December 4, 2002 RH 2 H pk a = 16-20 + H RHH + Enolate anions... So.a basic solution contains comparable amounts of the aldehyde and its enolate. Which gives rise to..the Aldol ondensation
Michael and Aldol H391 December 4, 2002 RH 2 H pk a = 16-20 + H RHH + Enolate anions... So.a basic solution contains comparable amounts of the aldehyde and its enolate. Which gives rise to..the Aldol ondensation
Enols and Enolates. A type of reaction with carbonyl compounds is an α-substitution (an electrophile adds to the α carbon of a carbonyl)
 Enols and Enolates A type of reaction with carbonyl compounds is an α-substitution (an electrophile adds to the α carbon of a carbonyl) E+ E In the preceding chapters, we primarily studied nucleophiles
Enols and Enolates A type of reaction with carbonyl compounds is an α-substitution (an electrophile adds to the α carbon of a carbonyl) E+ E In the preceding chapters, we primarily studied nucleophiles
Reactions at α-position
 Reactions at α-position In preceding chapters on carbonyl chemistry, a common reaction mechanism observed was a nucleophile reacting at the electrophilic carbonyl carbon site NUC NUC Another reaction that
Reactions at α-position In preceding chapters on carbonyl chemistry, a common reaction mechanism observed was a nucleophile reacting at the electrophilic carbonyl carbon site NUC NUC Another reaction that
You must have your answers written in PERMANENT ink if you want a regrade!!!! This means no test written in pencil or ERASABLE INK will be regraded.
 NAME (Print): SIGNATURE: hemistry 310M/318M Dr. ent Iverson Final December 15, 2009 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long, but
NAME (Print): SIGNATURE: hemistry 310M/318M Dr. ent Iverson Final December 15, 2009 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long, but
CHAPTER 19: CARBONYL COMPOUNDS III
 CHAPTER 19: CARBONYL COMPOUNDS III A hydrogen bonded to a carbon adjacent to a carbonyl carbon is sufficiently acidic to be removed by a strong base. The carbon adjacent to a carbonyl carbon is called
CHAPTER 19: CARBONYL COMPOUNDS III A hydrogen bonded to a carbon adjacent to a carbonyl carbon is sufficiently acidic to be removed by a strong base. The carbon adjacent to a carbonyl carbon is called
CHAPTER 23 HW: ENOLS + ENOLATES
 CAPTER 23 W: ENLS + ENLATES KET-ENL TAUTMERSM 1. Draw the curved arrow mechanism to show the interconversion of the keto and enol form in either trace acid or base. trace - 2 trace 3 + 2 + E1 2 c. trace
CAPTER 23 W: ENLS + ENLATES KET-ENL TAUTMERSM 1. Draw the curved arrow mechanism to show the interconversion of the keto and enol form in either trace acid or base. trace - 2 trace 3 + 2 + E1 2 c. trace
Chapter 21 Ester Enolates
 hapter 21 Ester Enolates Introduction R R' H H The preparation and reactions of β-dicarbonyl compounds, especially β-keto esters, is the main focus of this chapter. A proton on the carbon flanked by the
hapter 21 Ester Enolates Introduction R R' H H The preparation and reactions of β-dicarbonyl compounds, especially β-keto esters, is the main focus of this chapter. A proton on the carbon flanked by the
NAME (Print): Chemistry 610A/618A Dr. Brent Iverson 2nd Exam Oct. 29, 2003 SIGNATURE:
 NAME (Print): SIGNATURE: hemistry 610A/618A Dr. ent Iverson 2nd Exam ct. 29, 2003 Please Note: This test may be a bit long, but there is a reason. I would like to give you a lot of little questions, so
NAME (Print): SIGNATURE: hemistry 610A/618A Dr. ent Iverson 2nd Exam ct. 29, 2003 Please Note: This test may be a bit long, but there is a reason. I would like to give you a lot of little questions, so
ORGANIC - BRUICE 8E CH CARBONYL COMPOUNDS III: REACTIONS AT THE ALPHA-CARBON
 !! www.clutchprep.com CNCEPT: ALPHA CARBNS AND TAUTMERIZATIN We have discussed the high reactivity of the carbonyl carbon. However, carbonyls contain another highly reactive component. What is the acidity
!! www.clutchprep.com CNCEPT: ALPHA CARBNS AND TAUTMERIZATIN We have discussed the high reactivity of the carbonyl carbon. However, carbonyls contain another highly reactive component. What is the acidity
1/4/2011. Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds
 Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds The Acidity of the Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons to carbonyls are unusually acidic
Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds The Acidity of the Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons to carbonyls are unusually acidic
Note: You must have your answers written in pen if you want a regrade!!!!
 NAME (Print): SIGNATURE: hemistry 310M/318M Dr. Brent Iverson 1st Midterm ctober 4, 2007 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long,
NAME (Print): SIGNATURE: hemistry 310M/318M Dr. Brent Iverson 1st Midterm ctober 4, 2007 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long,
Lecture 24 Two Germans and an Englishman
 Lecture 24 Two Germans and an Englishman Robert Robinson 1886-1975 Nobel Laureate 1947 April 17, 2018 tto Paul Hermann Diels 1876-1954 Nobel Laureates 1950 Kurt Alder 1902-1958 Exam III Tomorrow Wed April
Lecture 24 Two Germans and an Englishman Robert Robinson 1886-1975 Nobel Laureate 1947 April 17, 2018 tto Paul Hermann Diels 1876-1954 Nobel Laureates 1950 Kurt Alder 1902-1958 Exam III Tomorrow Wed April
Aldehydes and Ketones : Aldol Reactions
 Aldehydes and Ketones : Aldol Reactions The Acidity of the a Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons a to carbonyls are unusually acidic The resulting anion is stabilized by
Aldehydes and Ketones : Aldol Reactions The Acidity of the a Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons a to carbonyls are unusually acidic The resulting anion is stabilized by
Homework Problem Set 10 Iverson CH320M/CH328M Due Monday, December 10
 omework Problem Set 0 Iverson 30M/38M ue Monday, ecember 0 NME (Print): SIGNTURE: hemistry 30M/38M r. rent Iverson 0th omework ecember 3, 08 Please print the first three letters of your last name in the
omework Problem Set 0 Iverson 30M/38M ue Monday, ecember 0 NME (Print): SIGNTURE: hemistry 30M/38M r. rent Iverson 0th omework ecember 3, 08 Please print the first three letters of your last name in the
You must have your answers written in PERMANENT ink if you want a regrade!!!! This means no test written in pencil or ERASABLE INK will be regraded.
 AME (Print): SIGATURE: hemistry 310M/318M Dr. Brent Iverson 1st Midterm September 29, 2011 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long,
AME (Print): SIGATURE: hemistry 310M/318M Dr. Brent Iverson 1st Midterm September 29, 2011 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long,
CHAPTER 24 HW: CARBONYL CONDENSATIONS
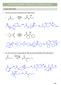 CAPTER 24 W: CARBNYL CNDENSATINS ALDL REACTIN 1. Draw the curved arrow mechanism for each aldol reaction. Na 2 product Na Et prod. 2. Give the curved arrow mechanism for this aldol reaction (and dehydration
CAPTER 24 W: CARBNYL CNDENSATINS ALDL REACTIN 1. Draw the curved arrow mechanism for each aldol reaction. Na 2 product Na Et prod. 2. Give the curved arrow mechanism for this aldol reaction (and dehydration
ORGANIC - CLUTCH CH CONDENSATION CHEMISTRY.
 !! www.clutchprep.com CNCEPT: CNDENSATIN REACTINS A condensation reaction spontaneously combines two or more molecules with the loss of a smaller molecule. Instead of just reacting with electophiles, enolates
!! www.clutchprep.com CNCEPT: CNDENSATIN REACTINS A condensation reaction spontaneously combines two or more molecules with the loss of a smaller molecule. Instead of just reacting with electophiles, enolates
CHEM 347 Organic Chemistry II (for Majors) Instructor: Paul J. Bracher. Quiz # 4. Due in Monsanto Hall 103 by: Friday, April 4 th, 2014, 7:00 p.m.
 CHEM 347 Quiz # 4 Spring 2014 Page 1 of 9 CHEM 347 Organic Chemistry II (for Majors) Instructor: Paul J. Bracher Quiz # 4 Due in Monsanto Hall 103 by: Friday, April 4 th, 2014, 7:00 p.m. Student Name (Printed)
CHEM 347 Quiz # 4 Spring 2014 Page 1 of 9 CHEM 347 Organic Chemistry II (for Majors) Instructor: Paul J. Bracher Quiz # 4 Due in Monsanto Hall 103 by: Friday, April 4 th, 2014, 7:00 p.m. Student Name (Printed)
CH310N Spring Anslyn. March 23, Exam 2
 C310N Spring 2010 Anslyn March 23, 2010 Exam 2 Please PRINT the first three letters of your last name in the boxes below. PRINT Full Name UT-EID 1) ( 8 pts ) 2) ( 5 pts ) 3) ( 5 pts ) 4) ( 22 pts ) 5)
C310N Spring 2010 Anslyn March 23, 2010 Exam 2 Please PRINT the first three letters of your last name in the boxes below. PRINT Full Name UT-EID 1) ( 8 pts ) 2) ( 5 pts ) 3) ( 5 pts ) 4) ( 22 pts ) 5)
Please note: We routinely xerox a number of exams following initial grading to guard against receiving altered answers during the regrading process.
 AME (Print): SIGATUE: hemistry 0M/8M Dr. Brent Iverson 1st Midterm September 8, 017 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long, but
AME (Print): SIGATUE: hemistry 0M/8M Dr. Brent Iverson 1st Midterm September 8, 017 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long, but
Answers to HT2, Chemistry A
 Answers to T2, hemistry 302-302A - 2004 1. There are many ways to do these problems - here is just one. For all three parts we can use diphenyl ketone (benzophenone) so let s make that first: 1. 2 Eq.
Answers to T2, hemistry 302-302A - 2004 1. There are many ways to do these problems - here is just one. For all three parts we can use diphenyl ketone (benzophenone) so let s make that first: 1. 2 Eq.
2.222 Practice Problems 2003
 2.222 Practice Problems 2003 Set #1 1. Provide the missing starting compound(s), reagent/solvent, or product to correctly complete each of the following. Most people in the class have not done this type
2.222 Practice Problems 2003 Set #1 1. Provide the missing starting compound(s), reagent/solvent, or product to correctly complete each of the following. Most people in the class have not done this type
NAME (Print): Chemistry 320M/328M Dr. Brent Iverson 1st Midterm September 27, 2018 SIGNATURE:
 AME (Print): SIGATURE: hemistry 320M/328M Dr. Brent Iverson 1st Midterm September 27, 2018 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long,
AME (Print): SIGATURE: hemistry 320M/328M Dr. Brent Iverson 1st Midterm September 27, 2018 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long,
Chapter 19. Carbonyl Compounds III Reaction at the α-carbon
 Chapter 19. Carbonyl Compounds III Reaction at the α-carbon There is a basic hydrogen (α hydrogen) on α carbon, which can be removed by a strong base. 19.1 The Acidity of α-hydrogens A hydrogen bonded
Chapter 19. Carbonyl Compounds III Reaction at the α-carbon There is a basic hydrogen (α hydrogen) on α carbon, which can be removed by a strong base. 19.1 The Acidity of α-hydrogens A hydrogen bonded
Objective 14. Develop synthesis strategies for organic synthesis.
 Objective 14. Develop synthesis strategies for organic synthesis. Skills: Draw structure ID structural features and reactive sites (alpha C, beta C, LG, etc.) ID Nu - and E + use curved arrows to show
Objective 14. Develop synthesis strategies for organic synthesis. Skills: Draw structure ID structural features and reactive sites (alpha C, beta C, LG, etc.) ID Nu - and E + use curved arrows to show
When we deprotonate we generate enolates or enols. Mechanism for deprotonation: Resonance form of the anion:
 Lecture 5 Carbonyl Chemistry III September 26, 2013 Ketone substrates form tertiary alcohol products, and aldehyde substrates form secondary alcohol products. The second step (treatment with aqueous acid)
Lecture 5 Carbonyl Chemistry III September 26, 2013 Ketone substrates form tertiary alcohol products, and aldehyde substrates form secondary alcohol products. The second step (treatment with aqueous acid)
A. Review of Acidity and pk a Common way to examine acidity is to use the Bronsted-Lowry acid-base equation:
 1 Chapter 22: Reactions of Enols and Enolates I. Alpha Substitution verview: A. Review of Acidity and pk a Common way to examine acidity is to use the Bronsted-Lowry acid-base equation: Recall that the
1 Chapter 22: Reactions of Enols and Enolates I. Alpha Substitution verview: A. Review of Acidity and pk a Common way to examine acidity is to use the Bronsted-Lowry acid-base equation: Recall that the
Note: You must have your answers written in blue or black pen if you want a regrade!!!!
 AME (Print): SIGATURE: hemistry 30M/38M Dr. Brent Iverson st Midterm Sept. 8, 005 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long, but
AME (Print): SIGATURE: hemistry 30M/38M Dr. Brent Iverson st Midterm Sept. 8, 005 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long, but
Chap 11. Carbonyl Alpha-Substitution Reactions and Condensation Reactions
 Chap 11. Carbonyl Alpha-Substitution eactions and Condensation eactions Four fundamental reactions of carbonyl compounds 1) Nucleophilic addition (aldehydes and ketones) ) Nucleophilic acyl substitution
Chap 11. Carbonyl Alpha-Substitution eactions and Condensation eactions Four fundamental reactions of carbonyl compounds 1) Nucleophilic addition (aldehydes and ketones) ) Nucleophilic acyl substitution
Lecture 23. The Aldol Condensation. an Aldol! April 12, 2018 O H O H - Chemistry 328N
 Lecture 23 The Aldol Condensation - b a an Aldol! April 12, 2018 Exam III - Wed April 18 WEL 2.122 7-9 PM Covers thru today omework ydrolysis Reactions Synthesis Get an A!!! Lithium diisopropylamide (LDA)
Lecture 23 The Aldol Condensation - b a an Aldol! April 12, 2018 Exam III - Wed April 18 WEL 2.122 7-9 PM Covers thru today omework ydrolysis Reactions Synthesis Get an A!!! Lithium diisopropylamide (LDA)
Chapter 22 Enols and Enolates
 Chapter Enols and Enolates Acidity of the α hydrogen o The position next door to a carbonyl is called the α position o When an α proton is abstracted, the resulting carbanion is resonancestabilized. This
Chapter Enols and Enolates Acidity of the α hydrogen o The position next door to a carbonyl is called the α position o When an α proton is abstracted, the resulting carbanion is resonancestabilized. This
Chapter 19. Synthesis and Reactions of b-dicarbonyl Compounds: More Chemistry of Enolate Anions. ß-dicarbonyl compounds. Why are ß-dicarbonyls useful?
 Chapter 19 Synthesis and Reactions of b-dicarbonyl Compounds: More Chemistry of Enolate Anions ß-dicarbonyl compounds Two carbonyl groups separated by a carbon Three common types ß-diketone ß-ketoester
Chapter 19 Synthesis and Reactions of b-dicarbonyl Compounds: More Chemistry of Enolate Anions ß-dicarbonyl compounds Two carbonyl groups separated by a carbon Three common types ß-diketone ß-ketoester
Homework 6 Organic Chemistry MCAT Review Summer 2012 Brent Iverson
 omework 6 rganic hemistry MAT Review Summer 2012 Brent Iverson 1 1. Fill in each blank with the word that best completes the following sentences about NMR. The two most important isotopes for organic chemistry
omework 6 rganic hemistry MAT Review Summer 2012 Brent Iverson 1 1. Fill in each blank with the word that best completes the following sentences about NMR. The two most important isotopes for organic chemistry
Lecture 23. Amines. Chemistry 328N. April 12, 2016
 Lecture 23 Amines April 12, 2016 Michael Reaction Michael reaction: conjugate addition of an enolate Arthur Michael anion to an, -unsaturated carbonyl compound!! Following are two examples in the first,
Lecture 23 Amines April 12, 2016 Michael Reaction Michael reaction: conjugate addition of an enolate Arthur Michael anion to an, -unsaturated carbonyl compound!! Following are two examples in the first,
Chapter 19. Organic Chemistry. Carbonyl Compounds III. Reactions at the a-carbon. 4 th Edition Paula Yurkanis Bruice
 Organic Chemistry 4 th Edition Paula Yurkanis Bruice Chapter 19 Carbonyl Compounds III Reactions at the a-carbon Disampaikan oleh: Dr. Sri Handayani 2013 Irene Lee Case Western Reserve University Cleveland,
Organic Chemistry 4 th Edition Paula Yurkanis Bruice Chapter 19 Carbonyl Compounds III Reactions at the a-carbon Disampaikan oleh: Dr. Sri Handayani 2013 Irene Lee Case Western Reserve University Cleveland,
CHEM Chapter 23. Carbonyl Condensation Reactions (quiz) W25
 CHEM 2425. Chapter 23. Carbonyl Condensation Reactions (quiz) W25 Student: 1. Which of the following statements about Aldol reactions with either aldehydes or ketones is true? Equilibrium favors the starting
CHEM 2425. Chapter 23. Carbonyl Condensation Reactions (quiz) W25 Student: 1. Which of the following statements about Aldol reactions with either aldehydes or ketones is true? Equilibrium favors the starting
State University of New York at Stony Brook Department of Chemistry. CHE 322, Organic Chemistry II Exam II March 17, 2004 Form 1
 State University of New York at Stony Brook Department of Chemistry CHE 22, rganic Chemistry II Exam II March 17, 2004 Form 1 Please answer all questions specifically, concisely, and readably in the spaces
State University of New York at Stony Brook Department of Chemistry CHE 22, rganic Chemistry II Exam II March 17, 2004 Form 1 Please answer all questions specifically, concisely, and readably in the spaces
Lecture 3: Aldehydes and ketones
 Lecture 3: Aldehydes and ketones I want to start by talking about the mechanism of hydroboration/ oxidation, which is a way to get alcohols from alkenes. This gives the anti-markovnikov product, primarily
Lecture 3: Aldehydes and ketones I want to start by talking about the mechanism of hydroboration/ oxidation, which is a way to get alcohols from alkenes. This gives the anti-markovnikov product, primarily
Aldol Reactions pka of a-h ~ 20
 Enolate Anions Chapter 17 Hydrogen on a carbons a to a carbonyl is unusually acidic The resulting anion is stabilized by resonance to the carbonyl Aldehydes and Ketones II Aldol Reactions pka of a-h ~
Enolate Anions Chapter 17 Hydrogen on a carbons a to a carbonyl is unusually acidic The resulting anion is stabilized by resonance to the carbonyl Aldehydes and Ketones II Aldol Reactions pka of a-h ~
CHEM 343 Principles of Organic Chemistry II Summer Instructor: Paul J. Bracher. Quiz # 3. Monday, July 21 st, :30 a.m.
 CHEM 343 Principles of Organic Chemistry II Summer 2014 Quiz # 3 Solutions Key Page 1 of 9 CHEM 343 Principles of Organic Chemistry II Summer 2014 Instructor: Paul J. Bracher Quiz # 3 Monday, July 21 st,
CHEM 343 Principles of Organic Chemistry II Summer 2014 Quiz # 3 Solutions Key Page 1 of 9 CHEM 343 Principles of Organic Chemistry II Summer 2014 Instructor: Paul J. Bracher Quiz # 3 Monday, July 21 st,
Ch 22 Carbonyl Alpha ( ) Substitution
 Ch 22 Carbonyl Alpha () Substitution The overall reaction replaces an H with an E + The acid-catalyzed reaction has an enol intermediate The base-catalyzed reaction has an enolate intermediate Keto-Enol
Ch 22 Carbonyl Alpha () Substitution The overall reaction replaces an H with an E + The acid-catalyzed reaction has an enol intermediate The base-catalyzed reaction has an enolate intermediate Keto-Enol
When H and OH add to the alkyne, an enol is formed, which rearranges to form a carbonyl (C=O) group:
 Next Up: Addition of, : The next two reactions are the Markovnikov and non-markovnikov additions of and to an alkyne But you will not see alcohols form in this reaction! When and add to the alkyne, an
Next Up: Addition of, : The next two reactions are the Markovnikov and non-markovnikov additions of and to an alkyne But you will not see alcohols form in this reaction! When and add to the alkyne, an
CH 3 CHCH 3 CH 3 CHCH 3 Isopropyl cation. Oxomium ion intermediate. intermediate (an electrophile)
 Understanding (as opposed to memorizing) mechanisms is critical to mastering organic chemistry. Although the mechanisms you encounter throughout the course may seem entirely different, they are actually
Understanding (as opposed to memorizing) mechanisms is critical to mastering organic chemistry. Although the mechanisms you encounter throughout the course may seem entirely different, they are actually
Final Exam Version 1. Chemistry 140C. Fall Good Luck! Dec 5, :30 am 2:30 pm This exam accounts for 50% of the final grade.
 Chemistry 140C Final Exam Version 1 Fall 2006 Dec 5, 2006 11:30 am 2:30 pm This exam accounts for 50% of the final grade. Mark your final answer clearly. Completely erase irrelevant information! Exams
Chemistry 140C Final Exam Version 1 Fall 2006 Dec 5, 2006 11:30 am 2:30 pm This exam accounts for 50% of the final grade. Mark your final answer clearly. Completely erase irrelevant information! Exams
Organic Chemistry, Third Edition. Chapter 24 Carbonyl condensations
 rganic Chemistry, Third Edition Chapter 24 Carbonyl condensations 1 Review: enolates LDA, -78 C TF kinetic RX R = Me, 1 alkyl R Na TF, RT RX R = Me, 1 alkyl thermodynamic R enolates = nucleophiles React
rganic Chemistry, Third Edition Chapter 24 Carbonyl condensations 1 Review: enolates LDA, -78 C TF kinetic RX R = Me, 1 alkyl R Na TF, RT RX R = Me, 1 alkyl thermodynamic R enolates = nucleophiles React
Note: You must have your answers written in pen if you want a regrade!!!!
 NAME (Print): SIGNATURE: hemistry 310M/318M Dr. Brent Iverson 1st Midterm ctober 1, 2009 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long,
NAME (Print): SIGNATURE: hemistry 310M/318M Dr. Brent Iverson 1st Midterm ctober 1, 2009 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long,
Suggested solutions for Chapter 28
 s for Chapter 28 28 PBLEM 1 ow would you make these four compounds? Give your disconnections, explain why you chose them and then give reagents for the. 2 2 Me S Exercises in basic one- group C X disconnections.
s for Chapter 28 28 PBLEM 1 ow would you make these four compounds? Give your disconnections, explain why you chose them and then give reagents for the. 2 2 Me S Exercises in basic one- group C X disconnections.
Spring Term 2012 Dr. Williams (309 Zurn, ex 2386)
 Chemistry 242 Organic Chemistry II Spring Term 2012 Dr. Williams (309 Zurn, ex 2386) Web Page: http://math.mercyhurst.edu/~jwilliams/ jwilliams@mercyhurst.edu (or just visit Department web site and look
Chemistry 242 Organic Chemistry II Spring Term 2012 Dr. Williams (309 Zurn, ex 2386) Web Page: http://math.mercyhurst.edu/~jwilliams/ jwilliams@mercyhurst.edu (or just visit Department web site and look
Exam 1 (Monday, July 6, 2015)
 Chem 231 Summer 2015 Assigned Homework Problems Last updated: Friday, July 24, 2015 Problems Assigned from Essential Organic Chemistry, 2 nd Edition, Paula Yurkanis Bruice, Prentice Hall, New York, NY,
Chem 231 Summer 2015 Assigned Homework Problems Last updated: Friday, July 24, 2015 Problems Assigned from Essential Organic Chemistry, 2 nd Edition, Paula Yurkanis Bruice, Prentice Hall, New York, NY,
Sul Ross State University Syllabus for Organic Chemistry II: CHEM 3408 (Spring 2017)
 Sul Ross State University Syllabus for Organic Chemistry II: CHEM 3408 (Spring 2017) Class: Organic Chemistry II Instructor: Dr. David J. Leaver Room: WSB 307 Office: WSB 318 Time: MWF 9:00-9:50am Office
Sul Ross State University Syllabus for Organic Chemistry II: CHEM 3408 (Spring 2017) Class: Organic Chemistry II Instructor: Dr. David J. Leaver Room: WSB 307 Office: WSB 318 Time: MWF 9:00-9:50am Office
Chem 22 Final Exam Practice
 Chem 22 Final Exam Practice Questions taken from regular tests given during the previous semesters. Only one answer is correct unless the question says otherwise. The questions are somewhat scrambled with
Chem 22 Final Exam Practice Questions taken from regular tests given during the previous semesters. Only one answer is correct unless the question says otherwise. The questions are somewhat scrambled with
Chem 342 Organic Chemistry II Final Exam 13 May 2009
 hem 342 rganic hemistry II Final Exam 13 May 2009 KEY Please read through each question carefully and answer in the spaces provided. A good strategy is to go through the test and answer all the questions
hem 342 rganic hemistry II Final Exam 13 May 2009 KEY Please read through each question carefully and answer in the spaces provided. A good strategy is to go through the test and answer all the questions
You must have your answers written in PERMANENT ink if you want a regrade!!!! This means no test written in pencil or ERASABLE INK will be regraded.
 AME (Print): SIGATURE: hemistry 0M/8M Dr. Brent Iverson st Midterm September 7, 0 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long, but
AME (Print): SIGATURE: hemistry 0M/8M Dr. Brent Iverson st Midterm September 7, 0 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long, but
