CHAPTER 16 CONJUGATE ADDITION
|
|
|
- Noah Barber
- 5 years ago
- Views:
Transcription
1 HAPTER 16 NJUGATE ADDITIN
2 onjugated Pi Bonds onjugated double bonds are separated by only one single bond. Isolated double bonds are separated by two or more single bonds.
3 arbonyl ompounds with onjugated Pi Bonds A carbon-carbon double bond can also be conjugated pi bond of a carbonyl group in an aldehyde, ketone, carboxylic acid and its derivatives. H 2 H H 3 H H H H 3 All are onjugated Pi Bonds
4 H Examples onjugated onjugated onjugated Isolated
5 1,2-Addition H 2 H H H 2 Addition occurs here examples or here H 3 H H H HH 3 l 2 H 3 H l H H HH 3 l
6 1,4-Addition = onjugate addition H 2 H H H 2 H 2 H H Addition occurs here example
7 1,2 and 1,4- Addition to onjugated Mechanism Dienes
8 ther electrophilic reagents add to conjugated dienes in similar fashion
9 Kinetic ontrol versus Thermodynamic ontrol of a hemical Reaction In addition of HBr to 1,3-butadiene the temperature of reaction greatly affects the distribution of 1,2 and 1,4 products.
10 Heating the 1,2-addition product leads to an equilibrium which favors the 1,4-addition product Because equilibrium conditions favor the 1,4-addition product it must be the most stable
11 1,2-addition product is formed faster and is the major product at low temperatures due to DG of 1,2-addition product is lower than for 1,4-addition product The reaction is said to be under kinetic control At higher temperatures when an equilibrium is established, the most stable product predominates 1,4-addition product is more stable and is the major product at high temperatures The reaction is said to be under thermodynamic control The 1,4 product is most stable because it leads to a disubstituted double bond 1,2-addition product has a less stable monosubstituted double bond
12 1,4-Addition Polymers X H 3 H 2 H H 2 isoprene catalyst 1,4-addition H 3 H 3 H 2 HH 2 H 2 HH 2 polyisoprene X Natural rubber = polyisoprene with all cis double bonds Hard polymer (gutta-percha) = trans polyisoprene
13 DIELS- ALDER REATIN
14 The Diels-Alder Reaction concerted three pairs of electrons move at one time + heat ring-forming reaction diene + dienophile cyclohexene NTE: = gain of bond order = loss of bond order
15 EXAMPLES F DIELS-ALDER REATIN H 3 H 3 H 3 H 3 H 3 H 3 Diene Dienophile A yclohexene
16 THE DIENE MUST BE ABLE T ADPT THE S-IS NFRMATIN rotate + reacts s-trans conformation s-cis conformation does not react reacts normally +
17 Some s-cis Dienes can be used in Diels Alder Reaction All are s-cis
18 MRE DIELS-ALDER REATINS
19
20 cis cis
21 If the dienophile is an alkyne a cyclohexadiene is formed. H 3 H 3 H 3
22 1- Electrophilic 1,4-Addition to α,β- Unsaturated arbonyl ompounds Weak Nu: catalyzed by H + Mechanism H 2 H R H H 2 H R + + H + H H 2 H R H H 2 H R + H 2 H 2 R u product Nu Nu reactant H H H 2 H R H 2 H R Nu Nu H 2 H R
23 Under acid condition Nu: attacks the β- carbon Examples H 2 HH H 3 H HH 3 Hl H 2 l H + H 2 H H 2 H 3 HH 2 H 3 H H + H 3 H H 3
24 2- Nucleophilic Addition to α,β unsaturated carbonyl compounds H 2 H R - H 2 H R + arbonyl is + Nu: attacks here β carbon is + ; Nu: - attacks here + H 2 H R -
25 Example H H 2 H H N HN H 2 HH 3 N Attack of N - on the carbonyl carbon Mechanism H 2 H H N H 2 HH 3 N or H 2 H 2 H 3 N - HN H H 2 HH 3 N
26 Attack of N - on the β carbon - Mechanism H 2 H H 3 - N H H 2 H H 3 enol N H 2 H 2 H 3 N H + H 2 H H 3 N - H 2 H H 3 N -
27 Which of the two addition reactions occurs? A mixture of products results but the major product depends on the nature of nucleophiles 1. Stronger bases attack 1,2 (at =). eg. LiAlH 4, NaBH 4, RMgX 2. Weaker bases attack 1,4 ( at =) e.g. N -, NH 3, RNH 2 3. uprates (R 2 uli) attack 1,4 (at =).
28 Stronger bases attack 1,2 (at =) H H 2 H 3 1) H 3 H 2 MgBr 2) H +, H 2 H 3 H HH 3 H 2 H 3 H 3 1) LiAlH 4 2) H +, H 2 1) NaBH 4 2) H +, H 2 H H 3 H HH 3 H H H 2 HH 3 H 3
29 Weaker bases attack 1,4 ( at =) H 3 H HH 3 H 2 N - H 3 HN NH 3 H 3 H H 2 H 3 NH 2 H 3 N H 3 H 2 HH 3 (H 3 ) 2 HH 3 H 3 NH 2 (H 3 ) 2 H 2 H 3 NHH 3
30 Lithium dialkylcuprates attack 1,4 at = H 3 1) (H 3 ) 2 uli 2) H +, H 2 H 3 H 3 H 3 H HH 3 1) (H 3 ) 2 uli 2) H +, H 2 H 3 H H 2 H 3 H 3
31 α,β- Unsaturated carboxylic acid A conjugated carboxylic acid behaves like conjugated aldehydes and ketones H 2 H H N - H 2 H 2 H N 2 H H 2 H NH 3 NH 4 l H 2 H NH 2 H 2 2 H
32 Examples H H +, H 2 H H H 2 H 3 NH 2 - NHH 3
Michael and Aldol CH391 December 4, 2002
 Michael and Aldol H391 December 4, 2002 RH 2 H pk a = 16-20 + H RHH + Enolate anions... So.a basic solution contains comparable amounts of the aldehyde and its enolate. Which gives rise to..the Aldol ondensation
Michael and Aldol H391 December 4, 2002 RH 2 H pk a = 16-20 + H RHH + Enolate anions... So.a basic solution contains comparable amounts of the aldehyde and its enolate. Which gives rise to..the Aldol ondensation
Conjugated Systems & Pericyclic Reactions
 onjugated Systems & Pericyclic Reactions 1 onjugated Dienes from heats of hydrogenation-relative stabilities of conjugated vs unconjugated dienes can be studied: Name 1-Butene 1-Pentene Structural Formula
onjugated Systems & Pericyclic Reactions 1 onjugated Dienes from heats of hydrogenation-relative stabilities of conjugated vs unconjugated dienes can be studied: Name 1-Butene 1-Pentene Structural Formula
Lecture Notes Chem 51B S. King I. Conjugation
 Lecture Notes Chem 51B S. King Chapter 16 Conjugation, Resonance, and Dienes I. Conjugation Conjugation occurs whenever p-orbitals can overlap on three or more adjacent atoms. Conjugated systems are more
Lecture Notes Chem 51B S. King Chapter 16 Conjugation, Resonance, and Dienes I. Conjugation Conjugation occurs whenever p-orbitals can overlap on three or more adjacent atoms. Conjugated systems are more
and Ultraviolet Spectroscopy
 Organic Chemistry, 7 th Edition L. G. Wade, Jr. Chapter 15 Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy 2010, Prentice all Conjugated Systems Conjugated double bonds are separated
Organic Chemistry, 7 th Edition L. G. Wade, Jr. Chapter 15 Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy 2010, Prentice all Conjugated Systems Conjugated double bonds are separated
Dr. Dina akhotmah-232 1
 Dr. Dina akhotmah-232 1 Chemistry of polyfunction 1. Types of carbon atom Dr. Dina akhotmah-232 2 Classification of multiple bonds of polyunsaturated compounds Dr. Dina akhotmah-232 3 Organic chemistry,
Dr. Dina akhotmah-232 1 Chemistry of polyfunction 1. Types of carbon atom Dr. Dina akhotmah-232 2 Classification of multiple bonds of polyunsaturated compounds Dr. Dina akhotmah-232 3 Organic chemistry,
11/5/ Conjugated Dienes. Conjugated Dienes. Conjugated Dienes. Heats of Hydrogenation
 8.12 Sites of unsaturation Many compounds have numerous sites of unsaturation If sites are well separated in molecule they react independently If sites are close together they may interact with one another
8.12 Sites of unsaturation Many compounds have numerous sites of unsaturation If sites are well separated in molecule they react independently If sites are close together they may interact with one another
Chapter 13 Conjugated Unsaturated Systems
 Chapter 13 Conjugated Unsaturated Systems Introduction Conjugated unsaturated systems have a p orbital on a carbon adjacent to a double bond The p orbital can come from another double or triple bond The
Chapter 13 Conjugated Unsaturated Systems Introduction Conjugated unsaturated systems have a p orbital on a carbon adjacent to a double bond The p orbital can come from another double or triple bond The
Chapter 14: Conjugated Dienes
 Chapter 14: Conjugated Dienes Coverage: 1. Conjugated vs Nonconjugated dienes and Stability 2. MO picture of 1,3-butadiene 3. Electrophilic addition to Dienes 4. Kinetic vs Thermodynamic Control 5. Diels-Alder
Chapter 14: Conjugated Dienes Coverage: 1. Conjugated vs Nonconjugated dienes and Stability 2. MO picture of 1,3-butadiene 3. Electrophilic addition to Dienes 4. Kinetic vs Thermodynamic Control 5. Diels-Alder
Nuggets of Knowledge for Chapter 17 Dienes and Aromaticity Chem 2320
 Nuggets of Knowledge for Chapter 17 Dienes and Aromaticity Chem 2320 I. Isolated, cumulated, and conjugated dienes A diene is any compound with two or C=C's is a diene. Compounds containing more than two
Nuggets of Knowledge for Chapter 17 Dienes and Aromaticity Chem 2320 I. Isolated, cumulated, and conjugated dienes A diene is any compound with two or C=C's is a diene. Compounds containing more than two
Conjugated Dienes and Ultraviolet Spectroscopy
 Conjugated Dienes and Ultraviolet Spectroscopy Key Words Conjugated Diene Resonance Structures Dienophiles Concerted Reaction Pericyclic Reaction Cycloaddition Reaction Bridged Bicyclic Compound Cyclic
Conjugated Dienes and Ultraviolet Spectroscopy Key Words Conjugated Diene Resonance Structures Dienophiles Concerted Reaction Pericyclic Reaction Cycloaddition Reaction Bridged Bicyclic Compound Cyclic
Dienes & Polyenes: An overview and two key reactions (Ch )
 Dienes & Polyenes: An overview and two key reactions (h. 14.1-14.5) Polyenes contain more than one double bond and are very common in natural products (ex: carotene). Diene chemistry applies to trienes,
Dienes & Polyenes: An overview and two key reactions (h. 14.1-14.5) Polyenes contain more than one double bond and are very common in natural products (ex: carotene). Diene chemistry applies to trienes,
Organic Chemistry II / CHEM 252 Chapter 13 Conjugated Unsaturated Systems
 Organic Chemistry II / CHEM 252 Chapter 13 Conjugated Unsaturated Systems Bela Torok Department of Chemistry University of Massachusetts Boston Boston, MA 1 Introduction - Conjugated unsaturated systems
Organic Chemistry II / CHEM 252 Chapter 13 Conjugated Unsaturated Systems Bela Torok Department of Chemistry University of Massachusetts Boston Boston, MA 1 Introduction - Conjugated unsaturated systems
Conjugated Dienes. Chapter 14 Organic Chemistry, 8 th Edition John E. McMurry
 Conjugated Dienes Chapter 14 Organic Chemistry, 8 th Edition John E. McMurry 1 Dienes Propadiene (allene) is a cumulated diene 1,3-Butadiene is a conjugated diene. 1,4-Pentadiene is an isolated diene.
Conjugated Dienes Chapter 14 Organic Chemistry, 8 th Edition John E. McMurry 1 Dienes Propadiene (allene) is a cumulated diene 1,3-Butadiene is a conjugated diene. 1,4-Pentadiene is an isolated diene.
CHAPTER 9 THEORY OF RESONANCE BY, G.DEEPA
 CHAPTER 9 THEORY OF RESONANCE BY, G.DEEPA Conjugation in Alkadienes and Allylic Systems conjugation a series of overlapping p orbitals The Allyl Group allylic position is the next to a double bond 1 allyl
CHAPTER 9 THEORY OF RESONANCE BY, G.DEEPA Conjugation in Alkadienes and Allylic Systems conjugation a series of overlapping p orbitals The Allyl Group allylic position is the next to a double bond 1 allyl
Conjugated Dienes. Chapter 14 Organic Chemistry, 8 th Edition John E. McMurry
 Conjugated Dienes Chapter 14 Organic Chemistry, 8 th Edition John E. McMurry 1 Dienes Propadiene (allene) is a cumulated diene 1,3-Butadiene is a conjugated diene. 1,4-Pentadiene is an isolated diene.
Conjugated Dienes Chapter 14 Organic Chemistry, 8 th Edition John E. McMurry 1 Dienes Propadiene (allene) is a cumulated diene 1,3-Butadiene is a conjugated diene. 1,4-Pentadiene is an isolated diene.
Conjugated Systems. With conjugated double bonds resonance structures can be drawn
 Conjugated Systems Double bonds in conjugation behave differently than isolated double bonds With conjugated double bonds resonance structures can be drawn With isolated double bonds cannot draw resonance
Conjugated Systems Double bonds in conjugation behave differently than isolated double bonds With conjugated double bonds resonance structures can be drawn With isolated double bonds cannot draw resonance
18.10 Conjugate Additions O O. O X BrCH 2 CH 2 CH 2 CCH 3 ± ± BrCH 2 CH 2 CH 2 CH 3. TsOH 1 O C O O W 3 CH 3 CCH 3 CH 3 CCH 2 CH 2 CH 2 CH 3
 80 NJUGATE ADDITINS 779 An attempt to form a Grignard reagent from 5-bromo- 2-pentanone is doomed to failure because the Grignard will react with the carbonyl group Therefore, the carbonyl group is first
80 NJUGATE ADDITINS 779 An attempt to form a Grignard reagent from 5-bromo- 2-pentanone is doomed to failure because the Grignard will react with the carbonyl group Therefore, the carbonyl group is first
Lecture 3: Aldehydes and ketones
 Lecture 3: Aldehydes and ketones I want to start by talking about the mechanism of hydroboration/ oxidation, which is a way to get alcohols from alkenes. This gives the anti-markovnikov product, primarily
Lecture 3: Aldehydes and ketones I want to start by talking about the mechanism of hydroboration/ oxidation, which is a way to get alcohols from alkenes. This gives the anti-markovnikov product, primarily
Aldehydes and Ketones : Aldol Reactions
 Aldehydes and Ketones : Aldol Reactions The Acidity of the a Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons a to carbonyls are unusually acidic The resulting anion is stabilized by
Aldehydes and Ketones : Aldol Reactions The Acidity of the a Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons a to carbonyls are unusually acidic The resulting anion is stabilized by
Conjugate Addition Reactions 2:02 PM
 1 Conjugate Addition vs Direct Addition What is Conjugate Addition? Conjugate addition refers to nucleophilic addition directed to the electrophilic carbon of the C=C (double bond) in a,bunsaturated systems.
1 Conjugate Addition vs Direct Addition What is Conjugate Addition? Conjugate addition refers to nucleophilic addition directed to the electrophilic carbon of the C=C (double bond) in a,bunsaturated systems.
Chapter 15: Conjugated Systems, Orbital Symmetry, and UV Spectroscopy
 Chapter 15: Conjugated Systems, Orbital Symmetry, and UV Spectroscopy Conjugated unsaturated systems have a p orbital on a carbon adjacent to a double bond The p orbital can come from another double (e.g.
Chapter 15: Conjugated Systems, Orbital Symmetry, and UV Spectroscopy Conjugated unsaturated systems have a p orbital on a carbon adjacent to a double bond The p orbital can come from another double (e.g.
Conjugated Systems, Orbital Symmetry and UV Spectroscopy
 Conjugated Systems, Orbital Symmetry and UV Spectroscopy Introduction There are several possible arrangements for a molecule which contains two double bonds (diene): Isolated: (two or more single bonds
Conjugated Systems, Orbital Symmetry and UV Spectroscopy Introduction There are several possible arrangements for a molecule which contains two double bonds (diene): Isolated: (two or more single bonds
CHEMISTRY Topic #3: Addition Reactions of Conjugated Dienes Spring 2017 Dr. Susan Findlay
 CEMISTRY 2600 Topic #3: Addition Reactions of Conjugated Dienes Spring 2017 Dr. Susan Findlay Different Kinds of Dienes When a molecule contains multiple π-bonds, their reactivity is dictated in part by
CEMISTRY 2600 Topic #3: Addition Reactions of Conjugated Dienes Spring 2017 Dr. Susan Findlay Different Kinds of Dienes When a molecule contains multiple π-bonds, their reactivity is dictated in part by
Alpha Substitution and Condensations of Enols and Enolate Ions. Alpha Substitution
 Alpha Substitution and ondensations of Enols and Enolate Ions hap 23 W: 27, 28, 30, 31, 37, 39, 42-44, 47, 51, 54-56 Alpha Substitution Replacement of a hydrogen on the carbon adjacent to the carbonyl,
Alpha Substitution and ondensations of Enols and Enolate Ions hap 23 W: 27, 28, 30, 31, 37, 39, 42-44, 47, 51, 54-56 Alpha Substitution Replacement of a hydrogen on the carbon adjacent to the carbonyl,
Ch 14 Conjugated Dienes and UV Spectroscopy
 Ch 14 Conjugated Dienes and UV Spectroscopy Conjugated Systems - Conjugated systems have alternating single and double bonds. For example: C=C C=C C=C and C=C C=O - This is not conjugated because the double
Ch 14 Conjugated Dienes and UV Spectroscopy Conjugated Systems - Conjugated systems have alternating single and double bonds. For example: C=C C=C C=C and C=C C=O - This is not conjugated because the double
3 - CONJUGATION. More than one double bond can be in a given compound: n=0
 3 - NJUGATIN 1. Terminology and Nomenclature (SF 13.1 13.6; SFS 13.1 13.6) A compound containing a double bond is called an alkene, olefin or maybe simply "ene". There are often other names associated
3 - NJUGATIN 1. Terminology and Nomenclature (SF 13.1 13.6; SFS 13.1 13.6) A compound containing a double bond is called an alkene, olefin or maybe simply "ene". There are often other names associated
Carbonyl Chemistry V + C O C. Chemistry /30/02
 arbonyl hemistry V Ō - + Keto-enol enol Tautomerism H 3 H 3 Ketone H H R 2 H R' H H 3 H 2 H Enol H Acid atalyzed α Halogenation R 2 R' + X 2 H + R 2 R' + HX H X X 2 can be l 2, Br 2, or I 2. Substitution
arbonyl hemistry V Ō - + Keto-enol enol Tautomerism H 3 H 3 Ketone H H R 2 H R' H H 3 H 2 H Enol H Acid atalyzed α Halogenation R 2 R' + X 2 H + R 2 R' + HX H X X 2 can be l 2, Br 2, or I 2. Substitution
A. Review of Acidity and pk a Common way to examine acidity is to use the Bronsted-Lowry acid-base equation:
 1 Chapter 22: Reactions of Enols and Enolates I. Alpha Substitution verview: A. Review of Acidity and pk a Common way to examine acidity is to use the Bronsted-Lowry acid-base equation: Recall that the
1 Chapter 22: Reactions of Enols and Enolates I. Alpha Substitution verview: A. Review of Acidity and pk a Common way to examine acidity is to use the Bronsted-Lowry acid-base equation: Recall that the
Reactions of Alkenes and Alkynes
 5 2 2 2 2 2 2 2 Reactions of Alkenes and Alkynes APTER SUMMARY Addition is the characteristic reaction of alkenes and alkynes. Since the carbons of a double or triple bond do not have the maximum number
5 2 2 2 2 2 2 2 Reactions of Alkenes and Alkynes APTER SUMMARY Addition is the characteristic reaction of alkenes and alkynes. Since the carbons of a double or triple bond do not have the maximum number
Physical Properties. Alcohols can be: CH CH 2 OH CH 2 CH 3 C OH CH 3. Secondary alcohol. Primary alcohol. Tertiary alcohol
 Chapter 10: Structure and Synthesis of Alcohols 100 Physical Properties Alcohols can be: CH 3 CH 3 CH CH 2 OH * Primary alcohol CH 3 OH CH * CH 2 CH 3 Secondary alcohol CH 3 CH 3 * C OH CH 3 Tertiary alcohol
Chapter 10: Structure and Synthesis of Alcohols 100 Physical Properties Alcohols can be: CH 3 CH 3 CH CH 2 OH * Primary alcohol CH 3 OH CH * CH 2 CH 3 Secondary alcohol CH 3 CH 3 * C OH CH 3 Tertiary alcohol
Chapter 14: Conjugated Dienes and Ultraviolet Spectroscopy Diene: molecule with two double bonds Conjugated diene: alternating double and single bonds
 Chapter 14: Conjugated Dienes and Ultraviolet Spectroscopy Diene: molecule with two double bonds Conjugated diene: alternating double and single bonds C-C single bond lkene Diene C=C double bonds Conjugate
Chapter 14: Conjugated Dienes and Ultraviolet Spectroscopy Diene: molecule with two double bonds Conjugated diene: alternating double and single bonds C-C single bond lkene Diene C=C double bonds Conjugate
THE DIELS-ALDER REACTION
 22.6 TE DIELS-ALDER REATIN 977 2 Both overlaps are bonding. ± 2 ± 2 2 M of the diene LUM of the alkene ( 2 ) (*) The [ + 2] cycloaddition is allowed by a thermal pathway. Both overlaps are bonding, so
22.6 TE DIELS-ALDER REATIN 977 2 Both overlaps are bonding. ± 2 ± 2 2 M of the diene LUM of the alkene ( 2 ) (*) The [ + 2] cycloaddition is allowed by a thermal pathway. Both overlaps are bonding, so
Chapter 20: Aldehydes and Ketones
 hem A225 Notes Page 67 I. Introduction hapter 20: Aldehydes and Ketones Aldehydes and ketones contain a carbonyl group (=) with no other heteroatoms attached. An aldehyde has at least one hydrogen attached;
hem A225 Notes Page 67 I. Introduction hapter 20: Aldehydes and Ketones Aldehydes and ketones contain a carbonyl group (=) with no other heteroatoms attached. An aldehyde has at least one hydrogen attached;
Learning Guide for Chapter 17 - Dienes
 Learning Guide for Chapter 17 - Dienes I. Isolated, conjugated, and cumulated dienes II. Reactions involving allylic cations or radicals III. Diels-Alder Reactions IV. Aromaticity I. Isolated, Conjugated,
Learning Guide for Chapter 17 - Dienes I. Isolated, conjugated, and cumulated dienes II. Reactions involving allylic cations or radicals III. Diels-Alder Reactions IV. Aromaticity I. Isolated, Conjugated,
Key ideas: In EAS, pi bond is Nu and undergoes addition.
 Objective 7. Apply addition and elimination concepts to predict electrophilic aromatic substitution reactions (EAS) of benzene and monosubstituted benzenes. Skills: Draw structure ID structural features
Objective 7. Apply addition and elimination concepts to predict electrophilic aromatic substitution reactions (EAS) of benzene and monosubstituted benzenes. Skills: Draw structure ID structural features
Conjugated Systems. Organic Compounds That Conduct Electricity
 Conjugated Systems Organic Compounds That Conduct Electricity Conjugated and Nonconjugated Dienes Compounds can have more than one double or triple bond If they are separated by only one single bond they
Conjugated Systems Organic Compounds That Conduct Electricity Conjugated and Nonconjugated Dienes Compounds can have more than one double or triple bond If they are separated by only one single bond they
Additions to the Carbonyl Groups
 Chapter 18 Additions to the Carbonyl Groups Nucleophilic substitution (S N 2andS N 1) reaction occurs at sp3 hybridized carbons with electronegative leaving groups Why? The carbon is electrophilic! Addition
Chapter 18 Additions to the Carbonyl Groups Nucleophilic substitution (S N 2andS N 1) reaction occurs at sp3 hybridized carbons with electronegative leaving groups Why? The carbon is electrophilic! Addition
Enols and Enolates. A type of reaction with carbonyl compounds is an α-substitution (an electrophile adds to the α carbon of a carbonyl)
 Enols and Enolates A type of reaction with carbonyl compounds is an α-substitution (an electrophile adds to the α carbon of a carbonyl) E+ E In the preceding chapters, we primarily studied nucleophiles
Enols and Enolates A type of reaction with carbonyl compounds is an α-substitution (an electrophile adds to the α carbon of a carbonyl) E+ E In the preceding chapters, we primarily studied nucleophiles
Chapter 15 Dienes, Resonance, and Aromaticity
 Instructor Supplemental Solutions to Problems 2010 Roberts and Company Publishers Chapter 15 Dienes, Resonance, and Aromaticity Solutions to In-Text Problems 15.2 The delocalization energy is the energy
Instructor Supplemental Solutions to Problems 2010 Roberts and Company Publishers Chapter 15 Dienes, Resonance, and Aromaticity Solutions to In-Text Problems 15.2 The delocalization energy is the energy
Lecture 24 Two Germans and an Englishman
 Lecture 24 Two Germans and an Englishman Robert Robinson 1886-1975 Nobel Laureate 1947 April 17, 2018 tto Paul Hermann Diels 1876-1954 Nobel Laureates 1950 Kurt Alder 1902-1958 Exam III Tomorrow Wed April
Lecture 24 Two Germans and an Englishman Robert Robinson 1886-1975 Nobel Laureate 1947 April 17, 2018 tto Paul Hermann Diels 1876-1954 Nobel Laureates 1950 Kurt Alder 1902-1958 Exam III Tomorrow Wed April
Carboxylic Acids O R C + H + O - Chemistry 618B
 arboxylic Acids R H R + H + - R - Nomenclature - IUPA IUPA names: drop the -e from the parent alkane and add the suffix -oic acid If the compound contains a carbon-carbon double bond, change the infix
arboxylic Acids R H R + H + - R - Nomenclature - IUPA IUPA names: drop the -e from the parent alkane and add the suffix -oic acid If the compound contains a carbon-carbon double bond, change the infix
The General Stabilization Effect of Conjugation (Section 15.1, 2, 3, 8, 9) Conjugated (more stable)
 Chem 0 Jasperse Ch Notes. Conjugation Ch. Conjugated Systems The General Stabilization Effect of Conjugation (Section.,,, 8, 9) Conjugated (more stable) Isolated (less stable) Notes: Cations Radicals Anions
Chem 0 Jasperse Ch Notes. Conjugation Ch. Conjugated Systems The General Stabilization Effect of Conjugation (Section.,,, 8, 9) Conjugated (more stable) Isolated (less stable) Notes: Cations Radicals Anions
2016 Pearson Education, Inc. Isolated and Conjugated Dienes
 2016 Pearson Education, Inc. Isolated and Conjugated Dienes 2016 Pearson Education, Inc. Reactions of Isolated Dienes 2016 Pearson Education, Inc. The Mechanism Double Bonds can have Different Reactivities
2016 Pearson Education, Inc. Isolated and Conjugated Dienes 2016 Pearson Education, Inc. Reactions of Isolated Dienes 2016 Pearson Education, Inc. The Mechanism Double Bonds can have Different Reactivities
REACTION AND SYNTHESIS REVIEW
 REACTION AND SYNTHESIS REVIEW A STUDENT SHOULD BE ABLE TO PREDICT PRODUCTS, IDENTIFY REACTANTS, GIVE REACTION CONDITIONS, PROPOSE SYNTHESES, AND PROPOSE MECHANISMS (AS LISTED BELOW). REVIEW THE MECHANISM
REACTION AND SYNTHESIS REVIEW A STUDENT SHOULD BE ABLE TO PREDICT PRODUCTS, IDENTIFY REACTANTS, GIVE REACTION CONDITIONS, PROPOSE SYNTHESES, AND PROPOSE MECHANISMS (AS LISTED BELOW). REVIEW THE MECHANISM
Chapter 13. Conjugated Unsaturated Systems. +,., - Allyl. What is a conjugated system? AllylicChlorination (High Temperature)
 What is a conjugated system? Chapter 13 Conjugated Unsaturated Systems Conjugated unsaturated systems have a p orbital on a carbon adjacent to a double bond The p orbital may be empty (a carbocation The
What is a conjugated system? Chapter 13 Conjugated Unsaturated Systems Conjugated unsaturated systems have a p orbital on a carbon adjacent to a double bond The p orbital may be empty (a carbocation The
When we deprotonate we generate enolates or enols. Mechanism for deprotonation: Resonance form of the anion:
 Lecture 5 Carbonyl Chemistry III September 26, 2013 Ketone substrates form tertiary alcohol products, and aldehyde substrates form secondary alcohol products. The second step (treatment with aqueous acid)
Lecture 5 Carbonyl Chemistry III September 26, 2013 Ketone substrates form tertiary alcohol products, and aldehyde substrates form secondary alcohol products. The second step (treatment with aqueous acid)
1/4/2011. Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds
 Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds The Acidity of the Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons to carbonyls are unusually acidic
Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds The Acidity of the Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons to carbonyls are unusually acidic
Lecture 22 Organic Chemistry 1
 CEM 232 rganic Chemistry I at Chicago Lecture 22 rganic Chemistry 1 Professor Duncan Wardrop April 1, 2010 1 Self Test Question Which starting material could not be used to prepare the tribromide below?
CEM 232 rganic Chemistry I at Chicago Lecture 22 rganic Chemistry 1 Professor Duncan Wardrop April 1, 2010 1 Self Test Question Which starting material could not be used to prepare the tribromide below?
Review and Preview. Coursepack: pp (14.6 is FYI only)
 Review and Preview We have studied many compounds with pi bonds. When a compound has more than one multiple bond in its structure, the relative positions of those multiple bonds with respect to one another
Review and Preview We have studied many compounds with pi bonds. When a compound has more than one multiple bond in its structure, the relative positions of those multiple bonds with respect to one another
2.222 Practice Problems 2003
 2.222 Practice Problems 2003 Set #1 1. Provide the missing starting compound(s), reagent/solvent, or product to correctly complete each of the following. Most people in the class have not done this type
2.222 Practice Problems 2003 Set #1 1. Provide the missing starting compound(s), reagent/solvent, or product to correctly complete each of the following. Most people in the class have not done this type
Chapter 19 Substitutions at the Carbonyl Group
 Chapter 19 Substitutions at the Carbonyl Group In Chapter 18 Additions to the Carbonyl Groups In Chapter 19 Substitutions at the Carbonyl Group O O - - O - O R Y R C+ Y R Y Nu -Ȳ R N u + Y=goodleavinggroup
Chapter 19 Substitutions at the Carbonyl Group In Chapter 18 Additions to the Carbonyl Groups In Chapter 19 Substitutions at the Carbonyl Group O O - - O - O R Y R C+ Y R Y Nu -Ȳ R N u + Y=goodleavinggroup
LECTURE #22 Thurs., Nov.15, 2007
 Provide a rxn sequence to make these as the major products Answers: 1. i Pr-Cl, AlCl 3 2. conc. fuming? H 2 S 4 3. Cl 2, FeCl 3 or AlCl 3 4. dilute H 2 S 4 note: normally aqueous workup after step 1, but
Provide a rxn sequence to make these as the major products Answers: 1. i Pr-Cl, AlCl 3 2. conc. fuming? H 2 S 4 3. Cl 2, FeCl 3 or AlCl 3 4. dilute H 2 S 4 note: normally aqueous workup after step 1, but
CYCLOADDITIONS IN ORGANIC SYNTHESIS
 CYCLOADDITIONS IN ORGANIC SYNTHESIS 1 CYCLOADDITIONS IN ORGANIC SYNTHESIS Introduction Cycloaddition describes the union of two independent π-systems through a concerted process involving a cyclic movement
CYCLOADDITIONS IN ORGANIC SYNTHESIS 1 CYCLOADDITIONS IN ORGANIC SYNTHESIS Introduction Cycloaddition describes the union of two independent π-systems through a concerted process involving a cyclic movement
Chapter 3 Alkenes and Alkynes. Excluded sections 3.15&3.16
 Chapter 3 Alkenes and Alkynes Excluded sections 3.15&3.16 3.1 Definition and Classification Alkene: a hydrocarbon that contains one or more carboncarbon double bonds. ethylene is the simplest alkene. Alkyne:
Chapter 3 Alkenes and Alkynes Excluded sections 3.15&3.16 3.1 Definition and Classification Alkene: a hydrocarbon that contains one or more carboncarbon double bonds. ethylene is the simplest alkene. Alkyne:
Exam. Name. MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 Exam Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which of the following statements is incorrect about benzene? 1) A) All of the carbon
Exam Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which of the following statements is incorrect about benzene? 1) A) All of the carbon
Chapter 11, Part 1: Polar substitution reactions involving alkyl halides
 hapter 11, Part 1: Polar substitution reactions involving alkyl halides Overview: The nature of alkyl halides and other groups with electrophilic sp 3 hybridized leads them to react with nucleophiles and
hapter 11, Part 1: Polar substitution reactions involving alkyl halides Overview: The nature of alkyl halides and other groups with electrophilic sp 3 hybridized leads them to react with nucleophiles and
1. Radical Substitution on Alkanes. 2. Radical Substitution with Alkenes. 3. Electrophilic Addition
 1. Radical Substitution on Alkanes Only Cl and Br are useful at the laboratory level. Alkane reactivity: tertiary > secondary > primary > methyl Numbers below products give their relative yield. Relative
1. Radical Substitution on Alkanes Only Cl and Br are useful at the laboratory level. Alkane reactivity: tertiary > secondary > primary > methyl Numbers below products give their relative yield. Relative
Organic Chemistry II KEY March 25, a) I only b) II only c) II & III d) III & IV e) I, II, III & IV
 rganic Chemistry II KEY March 25, 2015 Exam 2: VERSIN A 1. Which of the following compounds will give rise to an aromatic conjugate base? E a) I only b) II only c) II & III d) III & IV e) I, II, III &
rganic Chemistry II KEY March 25, 2015 Exam 2: VERSIN A 1. Which of the following compounds will give rise to an aromatic conjugate base? E a) I only b) II only c) II & III d) III & IV e) I, II, III &
Ch 19 Aldehydes and Ketones
 Ch 19 Aldehydes and Ketones Aldehydes (RCHO), with the exception of formaldehyde (H 2 CO), are compounds with both an H and an organic group attached to a carbonyl. Ketones (R 2 CO) are compounds with
Ch 19 Aldehydes and Ketones Aldehydes (RCHO), with the exception of formaldehyde (H 2 CO), are compounds with both an H and an organic group attached to a carbonyl. Ketones (R 2 CO) are compounds with
Chapter 17 Aldehydes and Ketones
 hapter 17 Aldehydes and Ketones arbonyl Groups polarized (1) Aldehydes and Ketones ' aldehydes ketones : and : are poor leaving groups (2) arboxylic Acid Derivatives l ' ' 2 carboxylic acid substituent
hapter 17 Aldehydes and Ketones arbonyl Groups polarized (1) Aldehydes and Ketones ' aldehydes ketones : and : are poor leaving groups (2) arboxylic Acid Derivatives l ' ' 2 carboxylic acid substituent
Organic Chemistry I (Chem340), Spring Final Exam
 rganic Chemistry I (Chem340), pring 2005 Final Exam This is a closed-book exam. No aid is to be given to or received from another person. Model set and calculator may be used, but cannot be shared. Please
rganic Chemistry I (Chem340), pring 2005 Final Exam This is a closed-book exam. No aid is to be given to or received from another person. Model set and calculator may be used, but cannot be shared. Please
Chem 263 Sept 22, Beta-carotene (depicted below) is the orange-red colour in carrots. β-carotene
 hem 263 Sept 22, 2009 onjugated Dienes and olour ontinued Beta-carotene (depicted below) is the orange-red colour in carrots. Xanthophylls β-carotene Xanthophylls are oxygenated carotene molecules. Zeaxanthin,
hem 263 Sept 22, 2009 onjugated Dienes and olour ontinued Beta-carotene (depicted below) is the orange-red colour in carrots. Xanthophylls β-carotene Xanthophylls are oxygenated carotene molecules. Zeaxanthin,
Organic Chemistry: CHEM2322
 Conjugated Systems Organic Chemistry: We met in Chem 2321 unsaturated bonds as either a C=C bond or C C bond. If these unsaturated bonds are well separated then they react independently however if there
Conjugated Systems Organic Chemistry: We met in Chem 2321 unsaturated bonds as either a C=C bond or C C bond. If these unsaturated bonds are well separated then they react independently however if there
CHEM Chapter 23. Carbonyl Condensation Reactions (quiz) W25
 CHEM 2425. Chapter 23. Carbonyl Condensation Reactions (quiz) W25 Student: 1. Which of the following statements about Aldol reactions with either aldehydes or ketones is true? Equilibrium favors the starting
CHEM 2425. Chapter 23. Carbonyl Condensation Reactions (quiz) W25 Student: 1. Which of the following statements about Aldol reactions with either aldehydes or ketones is true? Equilibrium favors the starting
235 Organic II. Final Exam Review REACTIONS OF CONJUGATED DIENES 1,2 VS 1,4 ADDITION REACTIONS OF CONJUGATED DIENES
 b. the ompound 7 i 1 Spectral Data: singlet, 196.5 ppm singlet, 14.1 ppm singlet, 14.4 ppm doublet, 19.1 ppm doublet, 18.5 ppm 1 MR Mass Spectrum Absorbance Intensity Infrared Spectrum 65 91 9. Structure:
b. the ompound 7 i 1 Spectral Data: singlet, 196.5 ppm singlet, 14.1 ppm singlet, 14.4 ppm doublet, 19.1 ppm doublet, 18.5 ppm 1 MR Mass Spectrum Absorbance Intensity Infrared Spectrum 65 91 9. Structure:
Chap 11. Carbonyl Alpha-Substitution Reactions and Condensation Reactions
 Chap 11. Carbonyl Alpha-Substitution eactions and Condensation eactions Four fundamental reactions of carbonyl compounds 1) Nucleophilic addition (aldehydes and ketones) ) Nucleophilic acyl substitution
Chap 11. Carbonyl Alpha-Substitution eactions and Condensation eactions Four fundamental reactions of carbonyl compounds 1) Nucleophilic addition (aldehydes and ketones) ) Nucleophilic acyl substitution
ORGANIC - CLUTCH CH CONDENSATION CHEMISTRY.
 !! www.clutchprep.com CNCEPT: CNDENSATIN REACTINS A condensation reaction spontaneously combines two or more molecules with the loss of a smaller molecule. Instead of just reacting with electophiles, enolates
!! www.clutchprep.com CNCEPT: CNDENSATIN REACTINS A condensation reaction spontaneously combines two or more molecules with the loss of a smaller molecule. Instead of just reacting with electophiles, enolates
Lecture Notes Chem 51C S. King Chapter 24 Carbonyl Condensation Reactions
 Lecture Notes Chem 51C S. King Chapter 24 Carbonyl Condensation Reactions I. Reaction of Enols & Enolates with ther Carbonyls Enols and enolates are electron rich nucleophiles that react with a number
Lecture Notes Chem 51C S. King Chapter 24 Carbonyl Condensation Reactions I. Reaction of Enols & Enolates with ther Carbonyls Enols and enolates are electron rich nucleophiles that react with a number
Background Information
 ackground nformation ntroduction to Condensation eactions Condensation reactions occur between the α-carbon of one carbonyl-containing functional group and the carbonyl carbon of a second carbonyl-containing
ackground nformation ntroduction to Condensation eactions Condensation reactions occur between the α-carbon of one carbonyl-containing functional group and the carbonyl carbon of a second carbonyl-containing
Aldehydes and Ketones: Nucleophilic Addition Reactions
 Aldehydes and Ketones: Nucleophilic Addition Reactions Why this Chapter? Much of organic chemistry involves the chemistry of carbonyl compounds Aldehydes/ketones are intermediates in synthesis of pharmaceutical
Aldehydes and Ketones: Nucleophilic Addition Reactions Why this Chapter? Much of organic chemistry involves the chemistry of carbonyl compounds Aldehydes/ketones are intermediates in synthesis of pharmaceutical
Chapter 19. Carbonyl Compounds III Reaction at the α-carbon
 Chapter 19. Carbonyl Compounds III Reaction at the α-carbon There is a basic hydrogen (α hydrogen) on α carbon, which can be removed by a strong base. 19.1 The Acidity of α-hydrogens A hydrogen bonded
Chapter 19. Carbonyl Compounds III Reaction at the α-carbon There is a basic hydrogen (α hydrogen) on α carbon, which can be removed by a strong base. 19.1 The Acidity of α-hydrogens A hydrogen bonded
Exam 1 (Monday, July 6, 2015)
 Chem 231 Summer 2015 Assigned Homework Problems Last updated: Friday, July 24, 2015 Problems Assigned from Essential Organic Chemistry, 2 nd Edition, Paula Yurkanis Bruice, Prentice Hall, New York, NY,
Chem 231 Summer 2015 Assigned Homework Problems Last updated: Friday, July 24, 2015 Problems Assigned from Essential Organic Chemistry, 2 nd Edition, Paula Yurkanis Bruice, Prentice Hall, New York, NY,
Aldehydes and Ketones 2. Based on Organic Chemistry, J. G. Smith 3rde.
 Aldehydes and Ketones 2 Based on Organic Chemistry, J. G. Smith 3rde. The Wittig Reaction Wittig reaction, named for German chemist Georg Wittig, who was awarded the Nobel Prize in Chemistry in 1979 for
Aldehydes and Ketones 2 Based on Organic Chemistry, J. G. Smith 3rde. The Wittig Reaction Wittig reaction, named for German chemist Georg Wittig, who was awarded the Nobel Prize in Chemistry in 1979 for
Chapter 8: Chemistry of Alkynes (C n H 2n-2 )
 hapter 8: hemistry of Alkynes ( n 2n-2 ) Bonding & hybridization Both are sp-hybridized Bond angles = 180 o 1 σ + 2 π bonds Linear around lassification R R R' σ bond energy: 88 kcal/mol π bond energy:
hapter 8: hemistry of Alkynes ( n 2n-2 ) Bonding & hybridization Both are sp-hybridized Bond angles = 180 o 1 σ + 2 π bonds Linear around lassification R R R' σ bond energy: 88 kcal/mol π bond energy:
CH318N Spring 2012 Final Exam. Chemistry 318N. Spring 2012 Dr. Willson. Final Exam
 318N Spring 2012 Final Exam 3 hemistry 318N Spring 2012 Dr. Willson Final Exam This afternoon you will take two tests, one in chemistry and one in integrity. I want you to get A s on both of these tests
318N Spring 2012 Final Exam 3 hemistry 318N Spring 2012 Dr. Willson Final Exam This afternoon you will take two tests, one in chemistry and one in integrity. I want you to get A s on both of these tests
There are several possible arrangements for a molecule which contains two double bonds (diene): 1. Isolated: (two or more single bonds between them)
 1 Chapter 15: Conjugation and Reactions of Dienes I. Introduction to Conjugation There are several possible arrangements for a molecule which contains two double bonds (diene): 1. Isolated: (two or more
1 Chapter 15: Conjugation and Reactions of Dienes I. Introduction to Conjugation There are several possible arrangements for a molecule which contains two double bonds (diene): 1. Isolated: (two or more
Another Equilibrium: Reaction At The α-position
 Another Equilibrium: Reaction At The α-position D 3 + D 3 C CD 3 2 C 2 the keto form the enol form chanism: + C 2 C 2 C 2 D D 2 D 2 repeat 5 times D 3 C CD 3 alogenation At The α-position Br 2, 2 C 2 Br
Another Equilibrium: Reaction At The α-position D 3 + D 3 C CD 3 2 C 2 the keto form the enol form chanism: + C 2 C 2 C 2 D D 2 D 2 repeat 5 times D 3 C CD 3 alogenation At The α-position Br 2, 2 C 2 Br
1) What is the major product of the following reaction? HBr. HBr+BrBr
 1) What is the major product of the following reaction? HBr HBr+BrBr 2) What is the major product of the following reaction? HBrperoxide BrHBrBrBr 3) What is the major product of the following reaction?
1) What is the major product of the following reaction? HBr HBr+BrBr 2) What is the major product of the following reaction? HBrperoxide BrHBrBrBr 3) What is the major product of the following reaction?
Aldol Reactions pka of a-h ~ 20
 Enolate Anions Chapter 17 Hydrogen on a carbons a to a carbonyl is unusually acidic The resulting anion is stabilized by resonance to the carbonyl Aldehydes and Ketones II Aldol Reactions pka of a-h ~
Enolate Anions Chapter 17 Hydrogen on a carbons a to a carbonyl is unusually acidic The resulting anion is stabilized by resonance to the carbonyl Aldehydes and Ketones II Aldol Reactions pka of a-h ~
Aldehydes and Ketones
 Aldehydes and Ketones Preparation of Aldehydes xidation of Primary Alcohols --- 2 P 1o alcohol ydroboration of a Terminal Alkyne, followed by Tautomerization --- 1. B 3, TF 2. 2 2, K 2 terminal alkyne
Aldehydes and Ketones Preparation of Aldehydes xidation of Primary Alcohols --- 2 P 1o alcohol ydroboration of a Terminal Alkyne, followed by Tautomerization --- 1. B 3, TF 2. 2 2, K 2 terminal alkyne
Diels-Alder Reaction
 Diels-Alder Reaction Method for synthesis of 6-membered ring ne-step, concerted reaction Termed [4+2] cycloaddition reaction where 4 and 2 electrons react. + Diels-Alder Reaction Discovered by. Diels and
Diels-Alder Reaction Method for synthesis of 6-membered ring ne-step, concerted reaction Termed [4+2] cycloaddition reaction where 4 and 2 electrons react. + Diels-Alder Reaction Discovered by. Diels and
Chapter 18 Ketones and Aldehydes. Carbonyl Compounds. Chapter 18: Aldehydes and Ketones Slide 18-2
 hapter 18 Ketones and Aldehydes arbonyl ompounds hapter 18: Aldehydes and Ketones Slide 18-2 1 arbonyl Structure arbon is sp 2 hybridized. = bond is shorter, stronger, and more polar than = bond in alkenes.
hapter 18 Ketones and Aldehydes arbonyl ompounds hapter 18: Aldehydes and Ketones Slide 18-2 1 arbonyl Structure arbon is sp 2 hybridized. = bond is shorter, stronger, and more polar than = bond in alkenes.
ELECTROPHILIC ADDITIONS OF ALKENES AS THE COUNTERPART OF ELIMINATIONS
 ELECTRPHILIC ADDITINS F ALKENES AS THE CUNTERPART F ELIMINATINS INTRDUCTIN - Chapter 8 is mostly about alkene reactions. That is, how one can transform alkenes into other functional groups. Most of these
ELECTRPHILIC ADDITINS F ALKENES AS THE CUNTERPART F ELIMINATINS INTRDUCTIN - Chapter 8 is mostly about alkene reactions. That is, how one can transform alkenes into other functional groups. Most of these
CHEM2077 HONORS ORGANIC CHEMISTRY SYLLABUS
 CHEM2077 HONORS ORGANIC CHEMISTRY SYLLABUS 1. STRUCTURE AND BONDING a] Atomic structure and bonding b] Hybridization and MO Theory c] Drawing chemical structures 2. POLAR COVALENT BONDS: ACIDS AND BASES
CHEM2077 HONORS ORGANIC CHEMISTRY SYLLABUS 1. STRUCTURE AND BONDING a] Atomic structure and bonding b] Hybridization and MO Theory c] Drawing chemical structures 2. POLAR COVALENT BONDS: ACIDS AND BASES
Organic Chemistry CHM 224
 rganic Chemistry CM 224 Final Exam Review Questions This is a compilation example final exam questions. Provide IUPAC names for each of the structures below. 2 ! Propose a structure for the compound that
rganic Chemistry CM 224 Final Exam Review Questions This is a compilation example final exam questions. Provide IUPAC names for each of the structures below. 2 ! Propose a structure for the compound that
10.12 The Diels-Alder Reaction. Synthetic method for preparing compounds containing a cyclohexene ring
 10.12 The Diels-Alder Reaction Synthetic method for preparing compounds containing a cyclohexene ring In general... + conjugated diene alkene (dienophile) cyclohexene via transition state Mechanistic features
10.12 The Diels-Alder Reaction Synthetic method for preparing compounds containing a cyclohexene ring In general... + conjugated diene alkene (dienophile) cyclohexene via transition state Mechanistic features
Dr. Mohamed El-Newehy
 By Dr. Mohamed El-Newehy Chemistry Department, College of Science, King Saud University http://fac.ksu.edu.sa/melnewehy Aldehydes and Ketones 1 Structure of Aldehydes and Ketones - Aldehydes and ketones
By Dr. Mohamed El-Newehy Chemistry Department, College of Science, King Saud University http://fac.ksu.edu.sa/melnewehy Aldehydes and Ketones 1 Structure of Aldehydes and Ketones - Aldehydes and ketones
Lecture 23. Amines. Chemistry 328N. April 12, 2016
 Lecture 23 Amines April 12, 2016 Michael Reaction Michael reaction: conjugate addition of an enolate Arthur Michael anion to an, -unsaturated carbonyl compound!! Following are two examples in the first,
Lecture 23 Amines April 12, 2016 Michael Reaction Michael reaction: conjugate addition of an enolate Arthur Michael anion to an, -unsaturated carbonyl compound!! Following are two examples in the first,
ROADMAP FOR REACTIONS Chapter 6
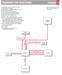 RADMAP FR REACTINS Chapter 6 (A) (B) (C) (D) (E) (F) (G) (H) (I) (J) (K) (L) (M) (N) () Regiochemistry: Markovnikov addition to a bond Stereochemistry: anti-addition Regiochemistry: non-markovnikov addition
RADMAP FR REACTINS Chapter 6 (A) (B) (C) (D) (E) (F) (G) (H) (I) (J) (K) (L) (M) (N) () Regiochemistry: Markovnikov addition to a bond Stereochemistry: anti-addition Regiochemistry: non-markovnikov addition
75. A This is a Markovnikov addition reaction. In these reactions, the pielectrons in the alkene act as a nucleophile. The strongest electrophile will
 71. B SN2 stands for substitution nucleophilic bimolecular. This means that there is a bimolecular rate-determining step. Therefore, the reaction will follow second-order kinetics based on the collision
71. B SN2 stands for substitution nucleophilic bimolecular. This means that there is a bimolecular rate-determining step. Therefore, the reaction will follow second-order kinetics based on the collision
3) The delocalized π system in benzene is formed by a cyclic overlap of 6 orbitals. A) s B) p C) sp D) sp2 E) sp3
 Chapter 8 Questions 1) Which of the following statements is incorrect about benzene? A) All of the carbon atoms are sp hybridized. B) It has delocalized electrons. C) The carbon-carbon bond lengths are
Chapter 8 Questions 1) Which of the following statements is incorrect about benzene? A) All of the carbon atoms are sp hybridized. B) It has delocalized electrons. C) The carbon-carbon bond lengths are
COURSE OBJECTIVES / OUTCOMES / COMPETENCIES.
 COURSE OBJECTIVES / OUTCOMES / COMPETENCIES. By the end of the course, students should be able to do the following: See Test1-4 Objectives/Competencies as listed in the syllabus and on the main course
COURSE OBJECTIVES / OUTCOMES / COMPETENCIES. By the end of the course, students should be able to do the following: See Test1-4 Objectives/Competencies as listed in the syllabus and on the main course
CHEMISTRY MIDTERM # 2 ANSWER KEY July 10, 2002
 EMISTRY 314-81 MIDTERM # 2 ANSWER KEY July 10, 2002 Statistics: Average: 47 pts (67%); ighest: 69 pts (99%); Lowest: 26 pts (37%) Number of students performing at or above average: 12 (46%) 1. (7 pts)
EMISTRY 314-81 MIDTERM # 2 ANSWER KEY July 10, 2002 Statistics: Average: 47 pts (67%); ighest: 69 pts (99%); Lowest: 26 pts (37%) Number of students performing at or above average: 12 (46%) 1. (7 pts)
When H and OH add to the alkyne, an enol is formed, which rearranges to form a carbonyl (C=O) group:
 Next Up: Addition of, : The next two reactions are the Markovnikov and non-markovnikov additions of and to an alkyne But you will not see alcohols form in this reaction! When and add to the alkyne, an
Next Up: Addition of, : The next two reactions are the Markovnikov and non-markovnikov additions of and to an alkyne But you will not see alcohols form in this reaction! When and add to the alkyne, an
Aldehydes and Ketones Reactions. Dr. Sapna Gupta
 Aldehydes and Ketones Reactions Dr. Sapna Gupta Reactions of Aldehydes and Ketones Nucleophilic Addition A strong nucleophile attacks the carbonyl carbon, forming an alkoxide ion that is then protonated.
Aldehydes and Ketones Reactions Dr. Sapna Gupta Reactions of Aldehydes and Ketones Nucleophilic Addition A strong nucleophile attacks the carbonyl carbon, forming an alkoxide ion that is then protonated.
CHE 275 REACTIONS OF ALKENES: ADDITION REACTIONS CHAP 8 ASSIGN
 HE 275 REATINS F ALKENES: ADDITIN REATINS HAP 8 ASSIGN 1. Which reagent or test could be used to distinguish between 2-methyl-2-pentene and 2-methylpentane? A. Br 2/l 4 B. KMn 4,. oncd. H 2S 4 D. Two of
HE 275 REATINS F ALKENES: ADDITIN REATINS HAP 8 ASSIGN 1. Which reagent or test could be used to distinguish between 2-methyl-2-pentene and 2-methylpentane? A. Br 2/l 4 B. KMn 4,. oncd. H 2S 4 D. Two of
Homework for Chapter 17 Chem 2320
 Homework for Chapter 17 Chem 2320 I. Cumulated, isolated, and conjugated dienes Name 1. Draw structures which fit the following descriptions. Use correct geometry! a conjugated diene with the formula C
Homework for Chapter 17 Chem 2320 I. Cumulated, isolated, and conjugated dienes Name 1. Draw structures which fit the following descriptions. Use correct geometry! a conjugated diene with the formula C
Solution problem 22: Non-Benzoid Aromatic Sytems
 Solution problem 22: on-enzoid Aromatic Sytems 22.1 & 22.2 Each double bond and each heteroatom (, ) with lone pairs donates 2 π- electrons as well as a negative charge. oron or a positive charge does
Solution problem 22: on-enzoid Aromatic Sytems 22.1 & 22.2 Each double bond and each heteroatom (, ) with lone pairs donates 2 π- electrons as well as a negative charge. oron or a positive charge does
ADDITION REACTIONS OF ALKYNES
 ADDITIN REATINS F ALKYNES ADDITIN REATINS F ALKYNES two pi bonds A carbon-carbon triple bond is like two carbon-carbon double bonds. Most double bond reagents will react twice. ALKYNES ARE LESS REATIVE
ADDITIN REATINS F ALKYNES ADDITIN REATINS F ALKYNES two pi bonds A carbon-carbon triple bond is like two carbon-carbon double bonds. Most double bond reagents will react twice. ALKYNES ARE LESS REATIVE
Basic Organic Chemistry
 Basic rganic hemistry ourse code: EM 12162 (Pre-requisites : EM 11122) hapter 06 hemistry of Aldehydes & Ketones Dr. Dinesh R. Pandithavidana ffice: B1 222/3 Phone: (+94)777-745-720 (Mobile) Email: dinesh@kln.ac.lk
Basic rganic hemistry ourse code: EM 12162 (Pre-requisites : EM 11122) hapter 06 hemistry of Aldehydes & Ketones Dr. Dinesh R. Pandithavidana ffice: B1 222/3 Phone: (+94)777-745-720 (Mobile) Email: dinesh@kln.ac.lk
