Patrick, An Introduction to Medicinal Chemistry 5e Chapter 7 Enzymes as drug targets. 1) The structures of isoleucine and valine are as follows.
|
|
|
- Dwayne Booth
- 6 years ago
- Views:
Transcription
1 Answers to end-of-chapter questions 1) The structures of isoleucine and valine are as follows. 2 C 2 2 C 2 3 C C 3 3 C C 3 Isoleucine Valine Isoleucine has a larger side chain than valine, and so there is less space available in that region of the active site for CX-1 than there is in the corresponding region in CX-2. Drugs can be designed that take advantage of this difference such that they fit into the active site of CX-2 but not the active site of CX-1. 2) The reaction catalysed by acetylcholinesterase on acetylcholine is the hydrolysis of the ester to produce acetic acid and choline. With neostigmine, the corresponding reaction would be the following eostigmine In fact, this does not happen and a stable complex with the enzyme is formed. Following the mechanism through, serine acts as a nucleophile and a nucleophilic substitution reaction takes place on the urethane with loss of the phenol group. owever, once this has occurred, serine is 'capped' with a stable urethane group that is resistant to hydrolysis. xford University Press, All rights reserved.
2 Patrick, An Introduction to dicinal Chemistry 5e Stage 1 Stage 2 C Ar Ar Physostigmine Stage 3 -Ar Stage 4 C Ar C Ar + 2 ydrolysis VERY SLW Stable carbamoyl intermediate Full details of this process are described in section ) A transition-state inhibitor is a drug which mimics the transition state for an enzyme-catalysed reaction. Such a drug should bind more strongly than either the substrate or the product, and be a strong inhibitor as a result. The enzyme-catalysed hydrolysis of the peptide bond between Phe and Pro should involve the enzyme using a nucleophilic group to form a bond to the carbonyl group, resulting in the intermediate shown. xford University Press, All rights reserved.
3 EZ u 2 C 2 2 EZ u C 2 The transition state for this stage should resemble the intermediate more than the starting material and so drugs mimicking the transition state should have characteristics also seen in the intermediate. The similarities in structure I to the intermediate are highlighted in colour below. extra methylene group C tetrahedral centre ote the presence of the tetrahedral centre bearing the alcohol group - also present in the intermediate. ote also that there is an extra methylene group between this tetrahedral centre and proline. This is necessary if the inhibitor is to be stable to the enzyme-catalysed reaction. Further details regarding the design of this inhibitor can be found in sections and An IC nm means that the concentration of inhibitor required to inhibit the enzyme by 50% is 6500 nanomolar. 4) It is proposed that the binding interactions between an enzyme and a substrate are optimal during the transition state of the enzyme-catalysed reaction. This is a reasonable proposition since the speed and effectiveness of a catalysed reaction is crucially dependent on how much the catalyst stablises the transition state. The more stable the transition state, the lower the activation energy. This is in turn results in a faster rate of reaction, which explains why binding interactions play an important role in an enzyme s role as a catalyst. Therefore, it is more important that enzymes form their strongest interactions with 'guest molecules' at the transition state of the reaction, rather than to the substrate at the beginning of the process, or the product at the end. Indeed, strong interactions with substrate or product are likely to be detrimental since it could result in a slow 'turnover' with substrate and product spending too much time in the active site. 5) In both cases, the enzyme-catalysed process is stalled. Both 5-azacytidine and 5- fluoro-2'-deoxycytidine can react with the enzyme to form structures I and II xford University Press, All rights reserved.
4 respectively. These are the equivalent structures to the normal intermediate III. In the normal enzyme mechanism, the final stage involves loss of a hydrogen from structure III to form a double bond within the ring, resulting in cleavage of the C-S bond linking the structure to the enzyme. This cannot occur in either structures I or II. In structure II, this mechanism would require the loss of a highly unstable F +. In structure I, one could draw a mechanism involving the lone pair of the nitrogen, but this would involve the nitrogen gaining a positive charge which is not favoured. F R S-Enz R S-Enz R S-Enz I II III 5) The following table shows the values necessary for both the Michaelis nton and Lineweaver-Burk plots in the absence of inhibitor. Substrate concentration [S] (mol dm -3 ) Initial rate (mol dm -3 s -1 ) 1/(substrate concentration) 1/[S] (mol -1 dm 3 ) 1/Initial rate (mol -1 dm 3 s) The Lineweaver-Burke plot has 1/initial rate on the y-axis and 1/substrate concentration on the x-axis. This should give a straight line having an intercept of on the y-axis and a slope of Therefore, the maximum rate of reaction = 1/intercept = mol dm -3 s -1 K M = slope x (maximum rate of reaction) = mol dm -3 The maximum rate of reaction is achieved at high substrate concentration. K M is the Michaelis constant and corresponds to the substrate concentration at which the initial rate of reaction is half of J max. A Michaelis nton plot of the maximum rate of reaction versus substrate concentration shows a curve where the line is approaching a maximum plateau, but has not yet reached it. Therefore, any attempt to define the maximum rate of reaction from this kind of plot can only be an estimate. Since we need to know the maximum rate of reaction in order to define K M, the value of K M will also be inaccurate. The Lineweaver-Burke plot gives a straight line and allows us define the maximum rate of reaction and K M more accurately. xford University Press, All rights reserved.
5 The table for the experiment carried out in presence of 4nM inhibitor is as follows Substrate concentration [S] (mol dm -3 ) Initial rate J (mol dm -3 s -1 ) 1/(substrate concentration) 1/[S] (mol -1 dm 3 ) 1/Initial rate 1/J (mol -1 dm 3 s) The Lineweaver-Burk plot in the presence of the inhibitor should give a straight line plot crossing the y-axis at the same point as the Lineweaver-Burk plot in the absence of inhibitor. As the lines cross the y-axis at the same point, this indicates competitive inhibition. The slope of the line in the presence of inhibitor is steeper than the line in the absence of inhibitor. Slope in the presence of inhibitor = Maximum rate of reaction = K M (app)=1.19 alpha = K M app/k M = 1.19/0.387 =3.075 Therefore, Ki = [I] / (alpha-1) = ) Analogue I is less active than the original quinazoline as there is no nitrogen to participate in the hydrogen bonding network involving water and two tyrosine residues. Moreover, the additional hydrogen atom would clash with the water molecule and displace it. Therefore, there are fewer binding interactions for analogue II, making it a weaker inhibitor Analogue II has a nitrile substituent which will also displace the water molecule. owever, the nitrile group can form a hydrogen bonding interaction directly to one of the tyrosine residues. This binding interaction must be stronger than the original hydrogen bonding network involving the quinazoline structure. xford University Press, All rights reserved.
6 8) The reaction catalysed by cytidine deaminase is as follows and includes an intermediate where an group has been added to form a tetrahedral centre. The group is provided by the conserved water molecule present in the active site A possible mechanism is as follows The proton provided in the mechanism is likely to be provided by an amino acid residue such as histidine. The enzyme-catalysed reaction mechanism carried out on zebularine will create the structure below which serves as a transition-state analogue and enzyme inhibitor. A transition state analogue is an effective inhibitor as it is believed that the transition state of an enzyme-catalysed reaction mechanism is bound more strongly than either the substrate or the product. Although a transition state is a highly unstable species, it is believed that it bears more of a similarity to the reaction intermediate than to the substrate or product. Therefore, a transition state analogue should contain a tetrahedral centre and relevant binding groups. In this case, the group can form crucial hydrogen-bonding interactions and is orientated in a similar manner to the normal reaction intermediate. Unlike the usual reaction intermediate, however, the transition xford University Press, All rights reserved.
7 state analogue is stable as there is no leaving group available to allow the formation of the carbonyl group. The transition state analogue formed from zebularine 3,4-Dihydrozebularine lacks the C= bond within the heterocyclic ring, which means that an enzyme-catalysed reaction is not possible with the conserved water molecule. Therefore, the structure binds without any alteration and no transition-state analogue is formed. The structure binds more weakly as there is no group present to form an additional hydrogen-bonding interaction. xford University Press, All rights reserved.
OXFORD H i g h e r E d u c a t i o n Oxford University Press, All rights reserved.
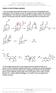 Patrick, An Introduction to dicinal hemistry 4e Answers to end-of-chapter questions 1) The mechanism below shows the release of one molecule of formaldehyde from methenamine. The mechanism can then be
Patrick, An Introduction to dicinal hemistry 4e Answers to end-of-chapter questions 1) The mechanism below shows the release of one molecule of formaldehyde from methenamine. The mechanism can then be
It is generally believed that the catalytic reactions occur in at least two steps.
 Lecture 16 MECHANISM OF ENZYME ACTION A chemical reaction such as A ----> P takes place because a certain fraction of the substrate possesses enough energy to attain an activated condition called the transition
Lecture 16 MECHANISM OF ENZYME ACTION A chemical reaction such as A ----> P takes place because a certain fraction of the substrate possesses enough energy to attain an activated condition called the transition
Lecture 12: Burst Substrates and the V vs [S] Experiment
![Lecture 12: Burst Substrates and the V vs [S] Experiment Lecture 12: Burst Substrates and the V vs [S] Experiment](/thumbs/95/124907449.jpg) Biological Chemistry Laboratory Biology 3515/Chemistry 3515 Spring 2019 Lecture 12: Burst Substrates and the V vs [S] Experiment 14 February 2019 c David P. Goldenberg University of Utah goldenberg@biology.utah.edu
Biological Chemistry Laboratory Biology 3515/Chemistry 3515 Spring 2019 Lecture 12: Burst Substrates and the V vs [S] Experiment 14 February 2019 c David P. Goldenberg University of Utah goldenberg@biology.utah.edu
Biochemistry 462a - Enzyme Kinetics Reading - Chapter 8 Practice problems - Chapter 8: (not yet assigned); Enzymes extra problems
 Biochemistry 462a - Enzyme Kinetics Reading - Chapter 8 Practice problems - Chapter 8: (not yet assigned); Enzymes extra problems Introduction Enzymes are Biological Catalysis A catalyst is a substance
Biochemistry 462a - Enzyme Kinetics Reading - Chapter 8 Practice problems - Chapter 8: (not yet assigned); Enzymes extra problems Introduction Enzymes are Biological Catalysis A catalyst is a substance
Biochemistry Enzyme kinetics
 1 Description of Module Subject Name Paper Name Module Name/Title Enzyme Kinetics Dr. Vijaya Khader Dr. MC Varadaraj 2 1. Objectives 2. Enzymes as biological catalyst 3. Enzyme Catalysis 4. Understanding
1 Description of Module Subject Name Paper Name Module Name/Title Enzyme Kinetics Dr. Vijaya Khader Dr. MC Varadaraj 2 1. Objectives 2. Enzymes as biological catalyst 3. Enzyme Catalysis 4. Understanding
Biochemistry. Lecture 8 Enzyme Kinetics
 Biochemistry Lecture 8 Enzyme Kinetics Why Enzymes? igher reaction rates Greater reaction specificity Milder reaction conditions Capacity for regulation C - - C N 2 - C N 2 - C - C Chorismate mutase -
Biochemistry Lecture 8 Enzyme Kinetics Why Enzymes? igher reaction rates Greater reaction specificity Milder reaction conditions Capacity for regulation C - - C N 2 - C N 2 - C - C Chorismate mutase -
CHAPTER 29 HW: AMINO ACIDS + PROTEINS
 CAPTER 29 W: AMI ACIDS + PRTEIS For all problems, consult the table of 20 Amino Acids provided in lecture if an amino acid structure is needed; these will be given on exams. Use natural amino acids (L)
CAPTER 29 W: AMI ACIDS + PRTEIS For all problems, consult the table of 20 Amino Acids provided in lecture if an amino acid structure is needed; these will be given on exams. Use natural amino acids (L)
A. Reaction Mechanisms and Catalysis (1) proximity effect (2) acid-base catalysts (3) electrostatic (4) functional groups (5) structural flexibility
 (P&S Ch 5; Fer Ch 2, 9; Palm Ch 10,11; Zub Ch 9) A. Reaction Mechanisms and Catalysis (1) proximity effect (2) acid-base catalysts (3) electrostatic (4) functional groups (5) structural flexibility B.
(P&S Ch 5; Fer Ch 2, 9; Palm Ch 10,11; Zub Ch 9) A. Reaction Mechanisms and Catalysis (1) proximity effect (2) acid-base catalysts (3) electrostatic (4) functional groups (5) structural flexibility B.
Patrick: An Introduction to Medicinal Chemistry 5e Chapter 03
 01) Which of the following statements is not true regarding the active site of an enzyme? a. An active site is normally on the surface of an enzyme. b. An active site is normally hydrophobic in nature.
01) Which of the following statements is not true regarding the active site of an enzyme? a. An active site is normally on the surface of an enzyme. b. An active site is normally hydrophobic in nature.
Reading for today: Chapter 16 (selections from Sections A, B and C) Friday and Monday: Chapter 17 (Diffusion)
 Lecture 29 Enzymes Reading for today: Chapter 6 (selections from Sections, B and C) Friday and Monday: Chapter 7 (Diffusion) 4/3/6 Today s Goals Michaelis-Menten mechanism for simple enzyme reactions:
Lecture 29 Enzymes Reading for today: Chapter 6 (selections from Sections, B and C) Friday and Monday: Chapter 7 (Diffusion) 4/3/6 Today s Goals Michaelis-Menten mechanism for simple enzyme reactions:
Affinity labels for studying enzyme active sites. Irreversible Enzyme Inhibition. Inhibition of serine protease with DFP
 Irreversible Enzyme Inhibition Irreversible inhibitors form stable covalent bonds with the enzyme (e.g. alkylation or acylation of an active site side chain) There are many naturally-occurring and synthetic
Irreversible Enzyme Inhibition Irreversible inhibitors form stable covalent bonds with the enzyme (e.g. alkylation or acylation of an active site side chain) There are many naturally-occurring and synthetic
2. Which of the following are nucleophiles and which are electrophiles?
 Life Sciences 1a ractice roblems 7 1. a) ow many intermediates are there in the reaction? b) ow many transition states are there? c) What is the fastest step in the reaction? d) Which is more stable, A
Life Sciences 1a ractice roblems 7 1. a) ow many intermediates are there in the reaction? b) ow many transition states are there? c) What is the fastest step in the reaction? d) Which is more stable, A
2013 W. H. Freeman and Company. 6 Enzymes
 2013 W. H. Freeman and Company 6 Enzymes CHAPTER 6 Enzymes Key topics about enzyme function: Physiological significance of enzymes Origin of catalytic power of enzymes Chemical mechanisms of catalysis
2013 W. H. Freeman and Company 6 Enzymes CHAPTER 6 Enzymes Key topics about enzyme function: Physiological significance of enzymes Origin of catalytic power of enzymes Chemical mechanisms of catalysis
C a h p a t p e t r e r 6 E z n y z m y e m s
 Chapter 6 Enzymes 4. Examples of enzymatic reactions acid-base catalysis: give and take protons covalent catalysis: a transient covalent bond is formed between the enzyme and the substrate metal ion catalysis:
Chapter 6 Enzymes 4. Examples of enzymatic reactions acid-base catalysis: give and take protons covalent catalysis: a transient covalent bond is formed between the enzyme and the substrate metal ion catalysis:
Synthesis of Nitriles a. dehydration of 1 amides using POCl 3 : b. SN2 reaction of cyanide ion on halides:
 I. Nitriles Nitriles consist of the CN functional group, and are linear with sp hybridization on C and N. Nitriles are non-basic at nitrogen, since the lone pair exists in an sp orbital (50% s character
I. Nitriles Nitriles consist of the CN functional group, and are linear with sp hybridization on C and N. Nitriles are non-basic at nitrogen, since the lone pair exists in an sp orbital (50% s character
Module No. 31: Peptide Synthesis: Definition, Methodology & applications
 PAPER 9: TECHNIQUES USED IN MOLECULAR BIOPHYSICS I Module No. 31: Peptide Synthesis: Definition, Methodology & applications Objectives: 1. Introduction 2. Synthesis of peptide 2.1. N-terminal protected
PAPER 9: TECHNIQUES USED IN MOLECULAR BIOPHYSICS I Module No. 31: Peptide Synthesis: Definition, Methodology & applications Objectives: 1. Introduction 2. Synthesis of peptide 2.1. N-terminal protected
From Friday s material
 5.111 Lecture 35 35.1 Kinetics Topic: Catalysis Chapter 13 (Section 13.14-13.15) From Friday s material Le Chatelier's Principle - when a stress is applied to a system in equilibrium, the equilibrium tends
5.111 Lecture 35 35.1 Kinetics Topic: Catalysis Chapter 13 (Section 13.14-13.15) From Friday s material Le Chatelier's Principle - when a stress is applied to a system in equilibrium, the equilibrium tends
Biochemical Kinetics: the science that studies rates of chemical reactions An example is the reaction (A P), The velocity, v, or rate, of the
 Biochemical Kinetics: the science that studies rates of chemical reactions An example is the reaction (A P), The velocity, v, or rate, of the reaction A P is the amount of P formed or the amount of A consumed
Biochemical Kinetics: the science that studies rates of chemical reactions An example is the reaction (A P), The velocity, v, or rate, of the reaction A P is the amount of P formed or the amount of A consumed
Problem Set #3 Solutions
 Problem Set #3 Solutions 1. a) C 3 (methyl group) Since carbon (E = 2.5) is slightly more electronegative than hydrogen (E = 2.2), there will be a small dipole moment pulling electron density away from
Problem Set #3 Solutions 1. a) C 3 (methyl group) Since carbon (E = 2.5) is slightly more electronegative than hydrogen (E = 2.2), there will be a small dipole moment pulling electron density away from
G. GENERAL ACID-BASE CATALYSIS
 G. GENERAL ACID-BASE CATALYSIS Towards a Better Chemical Mechanism via Catalysis There are two types of mechanisms we ll be discussing this semester. Kinetic mechanisms are concerned with rate constants
G. GENERAL ACID-BASE CATALYSIS Towards a Better Chemical Mechanism via Catalysis There are two types of mechanisms we ll be discussing this semester. Kinetic mechanisms are concerned with rate constants
Lecture # 3, 4 Selecting a Catalyst (Non-Kinetic Parameters), Review of Enzyme Kinetics, Selectivity, ph and Temperature Effects
 1.492 - Integrated Chemical Engineering (ICE Topics: Biocatalysis MIT Chemical Engineering Department Instructor: Professor Kristala Prather Fall 24 Lecture # 3, 4 Selecting a Catalyst (Non-Kinetic Parameters,
1.492 - Integrated Chemical Engineering (ICE Topics: Biocatalysis MIT Chemical Engineering Department Instructor: Professor Kristala Prather Fall 24 Lecture # 3, 4 Selecting a Catalyst (Non-Kinetic Parameters,
Chemistry Problem Set #9 Due on Thursday 11/15/18 in class.
 Chemistry 391 - Problem Set #9 Due on Thursday 11/15/18 in class. Name 1. There is a real enzyme called cocaine esterase that is produced in bacteria that live at the base of the coca plant. The enzyme
Chemistry 391 - Problem Set #9 Due on Thursday 11/15/18 in class. Name 1. There is a real enzyme called cocaine esterase that is produced in bacteria that live at the base of the coca plant. The enzyme
CHEM 251 (4 credits): Description
 CHEM 251 (4 credits): Intermediate Reactions of Nucleophiles and Electrophiles (Reactivity 2) Description: An understanding of chemical reactivity, initiated in Reactivity 1, is further developed based
CHEM 251 (4 credits): Intermediate Reactions of Nucleophiles and Electrophiles (Reactivity 2) Description: An understanding of chemical reactivity, initiated in Reactivity 1, is further developed based
Problems from Previous Class
 1 Problems from Previous lass 1. What is K m? What are the units of K m? 2. What is V max? What are the units of V max? 3. Write down the Michaelis-Menten equation. 4. What order of reaction is the reaction
1 Problems from Previous lass 1. What is K m? What are the units of K m? 2. What is V max? What are the units of V max? 3. Write down the Michaelis-Menten equation. 4. What order of reaction is the reaction
Solutions In each case, the chirality center has the R configuration
 CAPTER 25 669 Solutions 25.1. In each case, the chirality center has the R configuration. C C 2 2 C 3 C(C 3 ) 2 D-Alanine D-Valine 25.2. 2 2 S 2 d) 2 25.3. Pro,, Trp, Tyr, and is, Trp, Tyr, and is Arg,
CAPTER 25 669 Solutions 25.1. In each case, the chirality center has the R configuration. C C 2 2 C 3 C(C 3 ) 2 D-Alanine D-Valine 25.2. 2 2 S 2 d) 2 25.3. Pro,, Trp, Tyr, and is, Trp, Tyr, and is Arg,
Chapter 25: The Chemistry of Life: Organic and Biological Chemistry
 Chemistry: The Central Science Chapter 25: The Chemistry of Life: Organic and Biological Chemistry The study of carbon compounds constitutes a separate branch of chemistry known as organic chemistry The
Chemistry: The Central Science Chapter 25: The Chemistry of Life: Organic and Biological Chemistry The study of carbon compounds constitutes a separate branch of chemistry known as organic chemistry The
Enzymes Part III: Enzyme kinetics. Dr. Mamoun Ahram Summer semester,
 Enzymes Part III: Enzyme kinetics Dr. Mamoun Ahram Summer semester, 2015-2016 Kinetics Kinetics is deals with the rates of chemical reactions. Chemical kinetics is the study of the rates of chemical reactions.
Enzymes Part III: Enzyme kinetics Dr. Mamoun Ahram Summer semester, 2015-2016 Kinetics Kinetics is deals with the rates of chemical reactions. Chemical kinetics is the study of the rates of chemical reactions.
ENZYME KINETICS. Medical Biochemistry, Lecture 24
 ENZYME KINETICS Medical Biochemistry, Lecture 24 Lecture 24, Outline Michaelis-Menten kinetics Interpretations and uses of the Michaelis- Menten equation Enzyme inhibitors: types and kinetics Enzyme Kinetics
ENZYME KINETICS Medical Biochemistry, Lecture 24 Lecture 24, Outline Michaelis-Menten kinetics Interpretations and uses of the Michaelis- Menten equation Enzyme inhibitors: types and kinetics Enzyme Kinetics
BSc and MSc Degree Examinations
 Examination Candidate Number: Desk Number: BSc and MSc Degree Examinations 2018-9 Department : BIOLOGY Title of Exam: Molecular Biology and Biochemistry Part I Time Allowed: 1 hour and 30 minutes Marking
Examination Candidate Number: Desk Number: BSc and MSc Degree Examinations 2018-9 Department : BIOLOGY Title of Exam: Molecular Biology and Biochemistry Part I Time Allowed: 1 hour and 30 minutes Marking
Chem 204. Mid-Term Exam I. July 21, There are 3 sections to this exam: Answer ALL questions
 Chem 204 Mid-Term Exam I July 21, 2009 Name: Answer Key Student ID: There are 3 sections to this exam: Answer ALL questions Section I: Multiple-Choice 20 questions, 2 pts each Section II: Fill-in-the-Blank
Chem 204 Mid-Term Exam I July 21, 2009 Name: Answer Key Student ID: There are 3 sections to this exam: Answer ALL questions Section I: Multiple-Choice 20 questions, 2 pts each Section II: Fill-in-the-Blank
CHEM J-8 June Complete the following table. Make sure you give the name of the starting material where indicated. REAGENTS/ CONDITIONS
 CEM1102 2014-J-8 June 2014 Complete the following table. Make sure you give the name of the starting material where indicated. STARTIG MATERIAL REAGETS/ CDITIS STRUCTURAL FRMULA(S) F MAJR RGAIC PRDUCT(S)
CEM1102 2014-J-8 June 2014 Complete the following table. Make sure you give the name of the starting material where indicated. STARTIG MATERIAL REAGETS/ CDITIS STRUCTURAL FRMULA(S) F MAJR RGAIC PRDUCT(S)
Catalysis. Instructor: Dr. Tsung-Lin Li Genomics Research Center Academia Sinica
 Catalysis Instructor: Dr. Tsung-Lin Li Genomics Research Center Academia Sinica References: Biochemistry" by Donald Voet and Judith G. Voet Biochemistry" by Christopher K. Mathews, K. E. Van Hold and Kevin
Catalysis Instructor: Dr. Tsung-Lin Li Genomics Research Center Academia Sinica References: Biochemistry" by Donald Voet and Judith G. Voet Biochemistry" by Christopher K. Mathews, K. E. Van Hold and Kevin
Enzymes Enzyme Mechanism
 Mechanisms of Enzymes BCMB 3100 Chapters 6, 7, 8 Enzymes Enzyme Mechanism 1 Energy diagrams Binding modes of enzyme catalysis Chemical modes of enzyme catalysis Acid-Base catalysis Covalent catalysis Binding
Mechanisms of Enzymes BCMB 3100 Chapters 6, 7, 8 Enzymes Enzyme Mechanism 1 Energy diagrams Binding modes of enzyme catalysis Chemical modes of enzyme catalysis Acid-Base catalysis Covalent catalysis Binding
Lecture 16 (10/23/17) Lecture 16 (10/23/17)
 Lecture 16 (10/23/17) Reading: Ch6; 207-210 Ch6; 192-193, 195-196, 205-206 Problems: Ch6 (text); 18, 19, 20, 21, 22 Ch6 (study guide-facts); 9, 11 Ch6 (study guide-applying); 2 NEXT Reading: Ch6; 213-218
Lecture 16 (10/23/17) Reading: Ch6; 207-210 Ch6; 192-193, 195-196, 205-206 Problems: Ch6 (text); 18, 19, 20, 21, 22 Ch6 (study guide-facts); 9, 11 Ch6 (study guide-applying); 2 NEXT Reading: Ch6; 213-218
Enzymes Enzyme Mechanism
 BCMB 3100 Chapters 6, 7, 8 Enzymes Enzyme Mechanism 1 Mechanisms of Enzymes Energy diagrams Binding modes of enzyme catalysis Chemical modes of enzyme catalysis Acid-Base catalysis Covalent catalysis Binding
BCMB 3100 Chapters 6, 7, 8 Enzymes Enzyme Mechanism 1 Mechanisms of Enzymes Energy diagrams Binding modes of enzyme catalysis Chemical modes of enzyme catalysis Acid-Base catalysis Covalent catalysis Binding
CHEM April 10, Exam 3
 Name CHEM 3511 April 10, 2009 Exam 3 Name Page 1 1. (12 points) Give the name of your favorite Tech professor and in one sentence describe why you like him/her. 2. (10 points) An enzyme cleaves a chemical
Name CHEM 3511 April 10, 2009 Exam 3 Name Page 1 1. (12 points) Give the name of your favorite Tech professor and in one sentence describe why you like him/her. 2. (10 points) An enzyme cleaves a chemical
Effect of Temperature Increasing the temperature increases the energy in the system. Two effects kinetic. denaturing
 Effect of Temperature Increasing the temperature increases the energy in the system Two effects kinetic denaturing Kinetic effect Increased motion of molecules Increased collisions between enzyme/substrate
Effect of Temperature Increasing the temperature increases the energy in the system Two effects kinetic denaturing Kinetic effect Increased motion of molecules Increased collisions between enzyme/substrate
Exam 3 11/10/2014 Last Name (PRINT): First Name: Pg Topic Pts Total possible 3 Multiple. 12 choice 4 Multiple. 9 choice 5 Multiple
 Last Name (PRINT): First Name: Pg Topic Pts Total possible 3 Multiple 12 choice 4 Multiple 9 choice 5 Multiple 12 choice 6 Multiple 16 choice, start T/F 7 T/F and Fill in Blank 22 8 Binding problems 12
Last Name (PRINT): First Name: Pg Topic Pts Total possible 3 Multiple 12 choice 4 Multiple 9 choice 5 Multiple 12 choice 6 Multiple 16 choice, start T/F 7 T/F and Fill in Blank 22 8 Binding problems 12
Chapter 20 Carboxylic Acid Derivatives Nucleophilic Acyl Substitution
 Chapter 20 Carboxylic Acid Derivatives Nucleophilic Acyl Substitution Nomenclature: In carboxylic acid chlorides, anhydrides, esters and amides, the parent is the carboxylic acid. In each case be sure
Chapter 20 Carboxylic Acid Derivatives Nucleophilic Acyl Substitution Nomenclature: In carboxylic acid chlorides, anhydrides, esters and amides, the parent is the carboxylic acid. In each case be sure
Chemistry 112 Chemical Kinetics. Kinetics of Simple Enzymatic Reactions: The Case of Competitive Inhibition
 Chemistry Chemical Kinetics Kinetics of Simple Enzymatic Reactions: The Case of Competitive Inhibition Introduction: In the following, we will develop the equations describing the kinetics of a single
Chemistry Chemical Kinetics Kinetics of Simple Enzymatic Reactions: The Case of Competitive Inhibition Introduction: In the following, we will develop the equations describing the kinetics of a single
Lecture 15: Enzymes & Kinetics. Mechanisms ROLE OF THE TRANSITION STATE. H-O-H + Cl - H-O δ- H Cl δ- HO - + H-Cl. Margaret A. Daugherty.
 Lecture 15: Enzymes & Kinetics Mechanisms Margaret A. Daugherty Fall 2004 ROLE OF THE TRANSITION STATE Consider the reaction: H-O-H + Cl - H-O δ- H Cl δ- HO - + H-Cl Reactants Transition state Products
Lecture 15: Enzymes & Kinetics Mechanisms Margaret A. Daugherty Fall 2004 ROLE OF THE TRANSITION STATE Consider the reaction: H-O-H + Cl - H-O δ- H Cl δ- HO - + H-Cl Reactants Transition state Products
4 Examples of enzymes
 Catalysis 1 4 Examples of enzymes Adding water to a substrate: Serine proteases. Carbonic anhydrase. Restrictions Endonuclease. Transfer of a Phosphoryl group from ATP to a nucleotide. Nucleoside monophosphate
Catalysis 1 4 Examples of enzymes Adding water to a substrate: Serine proteases. Carbonic anhydrase. Restrictions Endonuclease. Transfer of a Phosphoryl group from ATP to a nucleotide. Nucleoside monophosphate
I5 ELECTROPHILIC SUBSTITUTIONS OF
 Section I Aromatic chemistry I5 ELECTPILIC SUBSTITUTINS F MN-SUBSTITUTED AMATIC INGS Key Notes ortho, meta and para substitution Substituent effect eaction profile Activating groups inductive o/p Deactivating
Section I Aromatic chemistry I5 ELECTPILIC SUBSTITUTINS F MN-SUBSTITUTED AMATIC INGS Key Notes ortho, meta and para substitution Substituent effect eaction profile Activating groups inductive o/p Deactivating
Chapter 8. Enzymes: basic concept and kinetics
 Chapter 8 Enzymes: basic concept and kinetics Learning objectives: mechanism of enzymatic catalysis Michaelis -Menton Model Inhibition Single Molecule of Enzymatic Reaction Enzymes: catalysis chemical
Chapter 8 Enzymes: basic concept and kinetics Learning objectives: mechanism of enzymatic catalysis Michaelis -Menton Model Inhibition Single Molecule of Enzymatic Reaction Enzymes: catalysis chemical
Amino Acids and Peptides
 Amino Acids Amino Acids and Peptides Amino acid a compound that contains both an amino group and a carboxyl group α-amino acid an amino acid in which the amino group is on the carbon adjacent to the carboxyl
Amino Acids Amino Acids and Peptides Amino acid a compound that contains both an amino group and a carboxyl group α-amino acid an amino acid in which the amino group is on the carbon adjacent to the carboxyl
Lecture 11: Enzymes: Kinetics [PDF] Reading: Berg, Tymoczko & Stryer, Chapter 8, pp
![Lecture 11: Enzymes: Kinetics [PDF] Reading: Berg, Tymoczko & Stryer, Chapter 8, pp Lecture 11: Enzymes: Kinetics [PDF] Reading: Berg, Tymoczko & Stryer, Chapter 8, pp](/thumbs/74/70848503.jpg) Lecture 11: Enzymes: Kinetics [PDF] Reading: Berg, Tymoczko & Stryer, Chapter 8, pp. 216-225 Updated on: 2/4/07 at 9:00 pm Key Concepts Kinetics is the study of reaction rates. Study of enzyme kinetics
Lecture 11: Enzymes: Kinetics [PDF] Reading: Berg, Tymoczko & Stryer, Chapter 8, pp. 216-225 Updated on: 2/4/07 at 9:00 pm Key Concepts Kinetics is the study of reaction rates. Study of enzyme kinetics
Ch 20 Carboxylic Acids and Nitriles
 Ch 20 Carboxylic Acids and Nitriles Carboxylic Acids (RCO 2 H) are compounds with an OH attached to a carbonyl. Nitriles (RC N) are compounds a carbon-nitrogen triple bond. Naming Carboxylic Acids 1. Replace
Ch 20 Carboxylic Acids and Nitriles Carboxylic Acids (RCO 2 H) are compounds with an OH attached to a carbonyl. Nitriles (RC N) are compounds a carbon-nitrogen triple bond. Naming Carboxylic Acids 1. Replace
THE CHEMISTRY OF THE CARBONYL GROUP
 TE CEMISTY F TE CABYL GUP Professor Tim Donohoe 8 lectures, T, weeks 1-4, 2007 andout A C C You will be able to download copies of the handouts from this course at http://users.ox.ac.uk/~magd1571/teaching/teaching.htm
TE CEMISTY F TE CABYL GUP Professor Tim Donohoe 8 lectures, T, weeks 1-4, 2007 andout A C C You will be able to download copies of the handouts from this course at http://users.ox.ac.uk/~magd1571/teaching/teaching.htm
A) at equilibrium B) endergonic C) endothermic D) exergonic E) exothermic.
 CHEM 2770: Elements of Biochemistry Mid Term EXAMINATION VERSION A Date: October 29, 2014 Instructor: H. Perreault Location: 172 Schultz Time: 4 or 6 pm. Duration: 1 hour Instructions Please mark the Answer
CHEM 2770: Elements of Biochemistry Mid Term EXAMINATION VERSION A Date: October 29, 2014 Instructor: H. Perreault Location: 172 Schultz Time: 4 or 6 pm. Duration: 1 hour Instructions Please mark the Answer
Lecture 27. Transition States and Enzyme Catalysis
 Lecture 27 Transition States and Enzyme Catalysis Reading for Today: Chapter 15 sections B and C Chapter 16 next two lectures 4/8/16 1 Pop Question 9 Binding data for your thesis protein (YTP), binding
Lecture 27 Transition States and Enzyme Catalysis Reading for Today: Chapter 15 sections B and C Chapter 16 next two lectures 4/8/16 1 Pop Question 9 Binding data for your thesis protein (YTP), binding
Michaelis-Menton kinetics
 Michaelis-Menton kinetics The rate of an enzyme catalyzed reaction in which substrate S is converted into products P depends on the concentration of the enzyme E even though the enzyme does not undergo
Michaelis-Menton kinetics The rate of an enzyme catalyzed reaction in which substrate S is converted into products P depends on the concentration of the enzyme E even though the enzyme does not undergo
Enzymes and Enzyme Kinetics I. Dr.Nabil Bashir
 Enzymes and Enzyme Kinetics I Dr.Nabil Bashir Enzymes and Enzyme Kinetics I: Outlines Enzymes - Basic Concepts and Kinetics Enzymes as Catalysts Enzyme rate enhancement / Enzyme specificity Enzyme cofactors
Enzymes and Enzyme Kinetics I Dr.Nabil Bashir Enzymes and Enzyme Kinetics I: Outlines Enzymes - Basic Concepts and Kinetics Enzymes as Catalysts Enzyme rate enhancement / Enzyme specificity Enzyme cofactors
It s the amino acids!
 Catalytic Mechanisms HOW do enzymes do their job? Reducing activation energy sure, but HOW does an enzyme catalysis reduce the energy barrier ΔG? Remember: The rate of a chemical reaction of substrate
Catalytic Mechanisms HOW do enzymes do their job? Reducing activation energy sure, but HOW does an enzyme catalysis reduce the energy barrier ΔG? Remember: The rate of a chemical reaction of substrate
Chem 263 March 28, 2006
 Chem 263 March 28, 2006 Properties of Carboxylic Acids Since carboxylic acids are structurally related to both ketones and aldehydes, we would expect to see some similar structural properties. The carbonyl
Chem 263 March 28, 2006 Properties of Carboxylic Acids Since carboxylic acids are structurally related to both ketones and aldehydes, we would expect to see some similar structural properties. The carbonyl
NH 2. Biochemistry I, Fall Term Sept 9, Lecture 5: Amino Acids & Peptides Assigned reading in Campbell: Chapter
 Biochemistry I, Fall Term Sept 9, 2005 Lecture 5: Amino Acids & Peptides Assigned reading in Campbell: Chapter 3.1-3.4. Key Terms: ptical Activity, Chirality Peptide bond Condensation reaction ydrolysis
Biochemistry I, Fall Term Sept 9, 2005 Lecture 5: Amino Acids & Peptides Assigned reading in Campbell: Chapter 3.1-3.4. Key Terms: ptical Activity, Chirality Peptide bond Condensation reaction ydrolysis
BA, BSc, and MSc Degree Examinations
 Examination Candidate Number: Desk Number: BA, BSc, and MSc Degree Examinations 2017-8 Department : BIOLOGY Title of Exam: Molecular Biology and Biochemistry Part I Time Allowed: 1 hour and 30 minutes
Examination Candidate Number: Desk Number: BA, BSc, and MSc Degree Examinations 2017-8 Department : BIOLOGY Title of Exam: Molecular Biology and Biochemistry Part I Time Allowed: 1 hour and 30 minutes
Amines and Heterocycles. McMurry: Chapter 24
 Amines and Heterocycles McMurry: Chapter 24 Introduction to Amines and Heterocycles Amines and heterocycles (cyclic amines) are ammonia derivatives, many of whichare found widely in livingorganisms: 2
Amines and Heterocycles McMurry: Chapter 24 Introduction to Amines and Heterocycles Amines and heterocycles (cyclic amines) are ammonia derivatives, many of whichare found widely in livingorganisms: 2
Chem Lecture 4 Enzymes Part 2
 Chem 452 - Lecture 4 Enzymes Part 2 Question of the Day: Is there some easy way to clock how many reactions one enzyme molecule is able to catalyze in an hour? Thermodynamics I think that enzymes are molecules
Chem 452 - Lecture 4 Enzymes Part 2 Question of the Day: Is there some easy way to clock how many reactions one enzyme molecule is able to catalyze in an hour? Thermodynamics I think that enzymes are molecules
BIOCHEMISTRY - CLUTCH REVIEW 2.
 !! www.clutchprep.com CONCEPT: BINDING AFFINITY Protein-ligand binding is reversible, like a chemical equilibrium [S] substrate concentration [E] enzyme concentration Ligands bind to proteins via the same
!! www.clutchprep.com CONCEPT: BINDING AFFINITY Protein-ligand binding is reversible, like a chemical equilibrium [S] substrate concentration [E] enzyme concentration Ligands bind to proteins via the same
Enzyme Inhibition and Drug Action
 Enzyme Inhibition and Drug Action Malfunction of enzyme Introduction of enzyme by microorganism Disease Inhibition of enzyme - Interesting but difficult drug strategy Inhib. of enzymes from microorganisms
Enzyme Inhibition and Drug Action Malfunction of enzyme Introduction of enzyme by microorganism Disease Inhibition of enzyme - Interesting but difficult drug strategy Inhib. of enzymes from microorganisms
Discussion Section (Day, Time): TF:
 ame: Chemistry 27 Professor Gavin MacBeath arvard University Spring 2004 Final Exam Thursday, May 28, 2004 2:15 PM - 5:15 PM Discussion Section (Day, Time): Directions: TF: 1. Do not write in red ink.
ame: Chemistry 27 Professor Gavin MacBeath arvard University Spring 2004 Final Exam Thursday, May 28, 2004 2:15 PM - 5:15 PM Discussion Section (Day, Time): Directions: TF: 1. Do not write in red ink.
Janice Gorzynski Smith University of Hawai i. Chapter 6. Modified by Dr. Juliet Hahn
 Organic Chemistry, Fifth Edition Janice Gorzynski Smith University of Hawai i Chapter 6 Modified by Dr. Juliet Hahn Copyright 2017 McGraw-Hill Education. All rights reserved. No reproduction or distribution
Organic Chemistry, Fifth Edition Janice Gorzynski Smith University of Hawai i Chapter 6 Modified by Dr. Juliet Hahn Copyright 2017 McGraw-Hill Education. All rights reserved. No reproduction or distribution
Biochemistry 3100 Sample Problems Binding proteins, Kinetics & Catalysis
 (1) Draw an approximate denaturation curve for a typical blood protein (eg myoglobin) as a function of ph. (2) Myoglobin is a simple, single subunit binding protein that has an oxygen storage function
(1) Draw an approximate denaturation curve for a typical blood protein (eg myoglobin) as a function of ph. (2) Myoglobin is a simple, single subunit binding protein that has an oxygen storage function
MITOCW watch?v=gboyppj9ok4
 MITOCW watch?v=gboyppj9ok4 The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high quality educational resources for free. To
MITOCW watch?v=gboyppj9ok4 The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high quality educational resources for free. To
Amides, Amino acids and Chirality
 R hemistry A 432 Amides, Amino Acids & hirality Amides, Amino acids and hirality aming of Amides The amide functional group consists of a carbonyl group bonded to the nitrogen of an amine. Like amines,
R hemistry A 432 Amides, Amino Acids & hirality Amides, Amino acids and hirality aming of Amides The amide functional group consists of a carbonyl group bonded to the nitrogen of an amine. Like amines,
After lectures by. disappearance of reactants or appearance of. measure a reaction rate we monitor the. Reaction Rates (reaction velocities): To
 Revised 3/21/2017 After lectures by Dr. Loren Williams (GeorgiaTech) Protein Folding: 1 st order reaction DNA annealing: 2 nd order reaction Reaction Rates (reaction velocities): To measure a reaction
Revised 3/21/2017 After lectures by Dr. Loren Williams (GeorgiaTech) Protein Folding: 1 st order reaction DNA annealing: 2 nd order reaction Reaction Rates (reaction velocities): To measure a reaction
Energy, Enzymes, and Metabolism. Energy, Enzymes, and Metabolism. A. Energy and Energy Conversions. A. Energy and Energy Conversions
 Energy, Enzymes, and Metabolism Lecture Series 6 Energy, Enzymes, and Metabolism B. ATP: Transferring Energy in Cells D. Molecular Structure Determines Enzyme Fxn Energy is the capacity to do work (cause
Energy, Enzymes, and Metabolism Lecture Series 6 Energy, Enzymes, and Metabolism B. ATP: Transferring Energy in Cells D. Molecular Structure Determines Enzyme Fxn Energy is the capacity to do work (cause
Part II => PROTEINS and ENZYMES. 2.7 Enzyme Kinetics 2.7a Chemical Kinetics 2.7b Enzyme Inhibition
 Part II => PROTEINS and ENZYMES 2.7 Enzyme Kinetics 2.7a Chemical Kinetics 2.7b Enzyme Inhibition Section 2.7a: Chemical Kinetics Synopsis 2.7a - Chemical kinetics (or reaction kinetics) is the study of
Part II => PROTEINS and ENZYMES 2.7 Enzyme Kinetics 2.7a Chemical Kinetics 2.7b Enzyme Inhibition Section 2.7a: Chemical Kinetics Synopsis 2.7a - Chemical kinetics (or reaction kinetics) is the study of
Proteins Act As Catalysts
 Proteins Act As Catalysts Properties of Enzymes Catalyst - speeds up attainment of reaction equilibrium Enzymatic reactions -10 3 to 10 17 faster than the corresponding uncatalyzed reactions Substrates
Proteins Act As Catalysts Properties of Enzymes Catalyst - speeds up attainment of reaction equilibrium Enzymatic reactions -10 3 to 10 17 faster than the corresponding uncatalyzed reactions Substrates
Chapter 6: Outline-2. Chapter 6: Outline Properties of Enzymes. Introduction. Activation Energy, E act. Activation Energy-2
 Chapter 6: Outline- Properties of Enzymes Classification of Enzymes Enzyme inetics Michaelis-Menten inetics Lineweaver-Burke Plots Enzyme Inhibition Catalysis Catalytic Mechanisms Cofactors Chapter 6:
Chapter 6: Outline- Properties of Enzymes Classification of Enzymes Enzyme inetics Michaelis-Menten inetics Lineweaver-Burke Plots Enzyme Inhibition Catalysis Catalytic Mechanisms Cofactors Chapter 6:
[Urea] (M) k (s -1 )
![[Urea] (M) k (s -1 ) [Urea] (M) k (s -1 )](/thumbs/96/126856044.jpg) BMB178 Fall 2018 Problem Set 1 Due: 10/26/2018, noon Office hour: 10/25/2018, SFL GSR218 7 9 pm Problem 1. Transition state theory (20 points): Consider a unimolecular reaction where a substrate S is converted
BMB178 Fall 2018 Problem Set 1 Due: 10/26/2018, noon Office hour: 10/25/2018, SFL GSR218 7 9 pm Problem 1. Transition state theory (20 points): Consider a unimolecular reaction where a substrate S is converted
[Urea] (M) k (s -1 )
![[Urea] (M) k (s -1 ) [Urea] (M) k (s -1 )](/thumbs/89/98097251.jpg) BMB178 Fall 2018 Problem Set 1 Due: 10/26/2018, noon Office hour: 10/25/2018, SFL GSR218 7 9 pm Problem 1. Transition state theory (20 points): Consider a unimolecular reaction where a substrate S is converted
BMB178 Fall 2018 Problem Set 1 Due: 10/26/2018, noon Office hour: 10/25/2018, SFL GSR218 7 9 pm Problem 1. Transition state theory (20 points): Consider a unimolecular reaction where a substrate S is converted
Chem 263 Notes March 2, 2006
 Chem 263 Notes March 2, 2006 Average for the midterm is 102.5 / 150 (approx. 68%). Preparation of Aldehydes and Ketones There are several methods to prepare aldehydes and ketones. We will only deal with
Chem 263 Notes March 2, 2006 Average for the midterm is 102.5 / 150 (approx. 68%). Preparation of Aldehydes and Ketones There are several methods to prepare aldehydes and ketones. We will only deal with
Chapter 19 Carboxylic Acids
 Carboxylic acids have the formula RCO2H. Nomenclature Chapter 19 Carboxylic Acids For the parent alkane, drop the terminal e and add the suffix oic acid. The parent alkane is the longest continuous chain
Carboxylic acids have the formula RCO2H. Nomenclature Chapter 19 Carboxylic Acids For the parent alkane, drop the terminal e and add the suffix oic acid. The parent alkane is the longest continuous chain
Life Sciences 1a Lecture Slides Set 10 Fall Prof. David R. Liu. Lecture Readings. Required: Lecture Notes McMurray p , O NH
 Life ciences 1a Lecture lides et 10 Fall 2006-2007 Prof. David R. Liu Lectures 17-18: The molecular basis of drug-protein binding: IV protease inhibitors 1. Drug development and its impact on IV-infected
Life ciences 1a Lecture lides et 10 Fall 2006-2007 Prof. David R. Liu Lectures 17-18: The molecular basis of drug-protein binding: IV protease inhibitors 1. Drug development and its impact on IV-infected
SAR. Structure - Activity Relationships (alkoholy, amíny, aldehydy, ketóny, estery, amidy, kyseliny, uhľovodíky) 2/28/2016
 Structure Activity elationships (SA) identifies which functional groups are important for binding and activity SA Structure - Activity elationships (alkoholy, amíny, aldehydy, ketóny, estery, amidy, kyseliny,
Structure Activity elationships (SA) identifies which functional groups are important for binding and activity SA Structure - Activity elationships (alkoholy, amíny, aldehydy, ketóny, estery, amidy, kyseliny,
Solutions. Solutions. Water Basics 10/24/ Water Properties
 0/24/206 O Water Basics Polar: part of a molecule is slightly positive, while another part is slightly negative Oxygen hogs electrons from hydrogen; results in negative charge on oxygen and positive charge
0/24/206 O Water Basics Polar: part of a molecule is slightly positive, while another part is slightly negative Oxygen hogs electrons from hydrogen; results in negative charge on oxygen and positive charge
The problem is that your product still has a-protons, and can keep on forming enolates to get more methyl groups added:
 Lecture 14 ovember 3, 2011 OK I want to continue briefly with the topic of proline catalysis that we discussed last time. In particular, the idea of using secondary amines to catalyze carbonyl chemistry
Lecture 14 ovember 3, 2011 OK I want to continue briefly with the topic of proline catalysis that we discussed last time. In particular, the idea of using secondary amines to catalyze carbonyl chemistry
Chemical Kinetics. Goal. Objectives
 4 Chemical Kinetics To understand the physical Goal factors that determine reaction rates. bjectives After this chapter, you should be able to: describe the factors that determine reaction rates. identify
4 Chemical Kinetics To understand the physical Goal factors that determine reaction rates. bjectives After this chapter, you should be able to: describe the factors that determine reaction rates. identify
B X A X. In this case the star denotes a chiral center.
 Lecture 13 Chirality III October 29, 2013 We can also access chiral molecules through the use of something called chiral auxiliaries, which basically is a chiral attachment that you add to your molecule
Lecture 13 Chirality III October 29, 2013 We can also access chiral molecules through the use of something called chiral auxiliaries, which basically is a chiral attachment that you add to your molecule
Enzyme Kinetics 2014
 V 41 Enzyme Kinetics 2014 Atkins Ch.23, Tinoco 4 th -Ch.8 Enzyme rxn example Catalysis/Mechanism: E + S k -1 ES k 1 ES E is at beginning and k 2 k -2 E + P at end of reaction Catalyst: No consumption of
V 41 Enzyme Kinetics 2014 Atkins Ch.23, Tinoco 4 th -Ch.8 Enzyme rxn example Catalysis/Mechanism: E + S k -1 ES k 1 ES E is at beginning and k 2 k -2 E + P at end of reaction Catalyst: No consumption of
Chem. 27 Section 1 Conformational Analysis Week of Feb. 6, TF: Walter E. Kowtoniuk Mallinckrodt 303 Liu Laboratory
 Chem. 27 Section 1 Conformational Analysis TF: Walter E. Kowtoniuk wekowton@fas.harvard.edu Mallinckrodt 303 Liu Laboratory ffice hours are: Monday and Wednesday 3:00-4:00pm in Mallinckrodt 303 Course
Chem. 27 Section 1 Conformational Analysis TF: Walter E. Kowtoniuk wekowton@fas.harvard.edu Mallinckrodt 303 Liu Laboratory ffice hours are: Monday and Wednesday 3:00-4:00pm in Mallinckrodt 303 Course
The Study of Chemical Reactions. Mechanism: The complete, step by step description of exactly which bonds are broken, formed, and in which order.
 The Study of Chemical Reactions Mechanism: The complete, step by step description of exactly which bonds are broken, formed, and in which order. Thermodynamics: The study of the energy changes that accompany
The Study of Chemical Reactions Mechanism: The complete, step by step description of exactly which bonds are broken, formed, and in which order. Thermodynamics: The study of the energy changes that accompany
Enzymes are macromolecules (proteins) that act as a catalyst
 Chapter 8.4 Enzymes Enzymes speed up metabolic reactions by lowering energy barriers Even though a reaction is spontaneous (exergonic) it may be incredibly slow Enzymes cause hydrolysis to occur at a faster
Chapter 8.4 Enzymes Enzymes speed up metabolic reactions by lowering energy barriers Even though a reaction is spontaneous (exergonic) it may be incredibly slow Enzymes cause hydrolysis to occur at a faster
Conformational Analysis
 Conformational Analysis C01 3 C C 3 is the most stable by 0.9 kcal/mole C02 K eq = K 1-1 * K 2 = 0.45-1 * 0.048 = 0.11 C04 The intermediate in the reaction of 2 has an unfavorable syn-pentane interaction,
Conformational Analysis C01 3 C C 3 is the most stable by 0.9 kcal/mole C02 K eq = K 1-1 * K 2 = 0.45-1 * 0.048 = 0.11 C04 The intermediate in the reaction of 2 has an unfavorable syn-pentane interaction,
Suggested solutions for Chapter 29
 s for Chapter 29 29 PRBLEM 1 or each of the following reactions (a) state what kind of substitution is suggested and (b) suggest what product might be formed if monosubstitution occured. Br 2 3 2 S 4 S
s for Chapter 29 29 PRBLEM 1 or each of the following reactions (a) state what kind of substitution is suggested and (b) suggest what product might be formed if monosubstitution occured. Br 2 3 2 S 4 S
Chapter 6 Overview. Enzymes. Catalysis most important function of proteins. Globular protein Increase rate of metabolic processes
 Chapter 6 Overview Enzymes Catalysis most important function of proteins n Enzymes protein catalysts Globular protein Increase rate of metabolic processes Enzymes kinetics info on reaction rates & measure
Chapter 6 Overview Enzymes Catalysis most important function of proteins n Enzymes protein catalysts Globular protein Increase rate of metabolic processes Enzymes kinetics info on reaction rates & measure
Biochemistry. Lecture 8
 Biochemistry Lecture 8 Why Enzymes? igher reaction rates Greater reaction specificity Milder reaction conditions Capacity for regulation C - - C N 2 - C N 2 - C - C Chorismate mutase - C - C - C Metabolites
Biochemistry Lecture 8 Why Enzymes? igher reaction rates Greater reaction specificity Milder reaction conditions Capacity for regulation C - - C N 2 - C N 2 - C - C Chorismate mutase - C - C - C Metabolites
Chapter 5 Metabolism: Energy & Enzymes
 Energy Energy is the capacity to do work Kinetic energy Energy of motion Potential energy Stored energy What do you use for energy? Where do you think the energy is stored these molecules? The BONDS! Every
Energy Energy is the capacity to do work Kinetic energy Energy of motion Potential energy Stored energy What do you use for energy? Where do you think the energy is stored these molecules? The BONDS! Every
Conformational Geometry of Peptides and Proteins:
 Conformational Geometry of Peptides and Proteins: Before discussing secondary structure, it is important to appreciate the conformational plasticity of proteins. Each residue in a polypeptide has three
Conformational Geometry of Peptides and Proteins: Before discussing secondary structure, it is important to appreciate the conformational plasticity of proteins. Each residue in a polypeptide has three
Prof. Jason D. Kahn Your Signature: Exams written in pencil or erasable ink will not be re-graded under any circumstances.
 Biochemistry 461, Section I May 6, 1997 Exam #3 Prof. Jason D. Kahn Your Printed Name: Your SS#: Your Signature: You have 80 minutes for this exam. Exams written in pencil or erasable ink will not be re-graded
Biochemistry 461, Section I May 6, 1997 Exam #3 Prof. Jason D. Kahn Your Printed Name: Your SS#: Your Signature: You have 80 minutes for this exam. Exams written in pencil or erasable ink will not be re-graded
4. The Michaelis-Menten combined rate constant Km, is defined for the following kinetic mechanism as k 1 k 2 E + S ES E + P k -1
 Fall 2000 CH 595C Exam 1 Answer Key Multiple Choice 1. One of the reasons that enzymes are such efficient catalysts is that a) the energy level of the enzyme-transition state complex is much higher than
Fall 2000 CH 595C Exam 1 Answer Key Multiple Choice 1. One of the reasons that enzymes are such efficient catalysts is that a) the energy level of the enzyme-transition state complex is much higher than
Loudon Chapter 20 & 21 Review: Carboxylic Acids & Derivatives CHEM 3331, Jacquie Richardson, Fall Page 1
 Loudon Chapter 20 & 21 eview: Carboxylic Acids & Derivatives CEM 3331, Jacquie ichardson, Fall 2010 - Page 1 These two chapters cover compounds which are all at the three bonds to more electronegative
Loudon Chapter 20 & 21 eview: Carboxylic Acids & Derivatives CEM 3331, Jacquie ichardson, Fall 2010 - Page 1 These two chapters cover compounds which are all at the three bonds to more electronegative
BCMB 3100 Chapters 6,7,8 Enzyme Basics. Six Classes (IUBMB) Kinetics Michaelis-Menten Equation Vo, Km, Vmax, Kcat Lineweaver-Burk Plot
 BCMB 3100 Chapters 6,7,8 Enzyme Basics Six Classes (IUBMB) Kinetics Enzymes are biological macromolecules that increase the rate of the reaction. Six major groups of enzymes (pgs. 94-95/98-99) Oxidoreductases:
BCMB 3100 Chapters 6,7,8 Enzyme Basics Six Classes (IUBMB) Kinetics Enzymes are biological macromolecules that increase the rate of the reaction. Six major groups of enzymes (pgs. 94-95/98-99) Oxidoreductases:
Nucleophilic Addition Reactions of Carboxylic Acid Derivatives
 Lecture 5: bjectives: Nucleophilic Addition eactions of Carboxylic Acid Derivatives By the end of this lecture you will be able to: draw the mechanism of a nucleophilic addition-elimination reaction with
Lecture 5: bjectives: Nucleophilic Addition eactions of Carboxylic Acid Derivatives By the end of this lecture you will be able to: draw the mechanism of a nucleophilic addition-elimination reaction with
Final Exam. Chem 3B, Fall 2016 Monday, Dec 12, pm. Name Answer Key. Student ID. If you are making up an incomplete, list the semester here:
 Final Exam Chem 3B, Fall 2016 Monday, Dec 12, 2016 3-6 pm ame Answer Key Student ID If you are making up an incomplete, list the semester here: You have 180 minutes to complete this exam. Please provide
Final Exam Chem 3B, Fall 2016 Monday, Dec 12, 2016 3-6 pm ame Answer Key Student ID If you are making up an incomplete, list the semester here: You have 180 minutes to complete this exam. Please provide
BCMB 3100 Chapters 6,7,8 Enzyme Basics. Six Classes (IUBMB) Kinetics Michaelis-Menten Equation Vo, Km, Vmax, Kcat Lineweaver-Burk Plot
 BCMB 3100 Chapters 6,7,8 Enzyme Basics Six Classes (IUBMB) Kinetics Michaelis-Menten Equation Vo, Km, Vmax, Kcat Lineweaver-Burk Plot Enzymes are biological macromolecules that increase the rate of the
BCMB 3100 Chapters 6,7,8 Enzyme Basics Six Classes (IUBMB) Kinetics Michaelis-Menten Equation Vo, Km, Vmax, Kcat Lineweaver-Burk Plot Enzymes are biological macromolecules that increase the rate of the
BCMB 3100 Chapters 6,7,8 Enzyme Basics. Six Classes (IUBMB) Kinetics Michaelis-Menten Equation Vo, Km, Vmax, Kcat Lineweaver-Burk Plot
 BCMB 3100 Chapters 6,7,8 Enzyme Basics Six Classes (IUBMB) Kinetics Michaelis-Menten Equation Vo, Km, Vmax, Kcat Lineweaver-Burk Plot Enzymes are biological macromolecules that increase the rate of the
BCMB 3100 Chapters 6,7,8 Enzyme Basics Six Classes (IUBMB) Kinetics Michaelis-Menten Equation Vo, Km, Vmax, Kcat Lineweaver-Burk Plot Enzymes are biological macromolecules that increase the rate of the
Reactions of Haloalkanes
 Reactions of aloalkanes N Goalby chemrevise.org aloalkanes ontain a halogen atom covalently bonded to a carbon atom General formula: R-X where X is a halogen atom (F, l,, I) and R is the carbon chain.
Reactions of aloalkanes N Goalby chemrevise.org aloalkanes ontain a halogen atom covalently bonded to a carbon atom General formula: R-X where X is a halogen atom (F, l,, I) and R is the carbon chain.
CHE1502. Tutorial letter 201/1/2016. General Chemistry 1B. Semester 1. Department of Chemistry CHE1502/201/1/2016
 CE1502/201/1/2016 Tutorial letter 201/1/2016 General Chemistry 1B CE1502 Semester 1 Department of Chemistry This tutorial letter contains the answers to the questions in assignment 1. FIRST SEMESTER: KEY
CE1502/201/1/2016 Tutorial letter 201/1/2016 General Chemistry 1B CE1502 Semester 1 Department of Chemistry This tutorial letter contains the answers to the questions in assignment 1. FIRST SEMESTER: KEY
