SAR. Structure - Activity Relationships (alkoholy, amíny, aldehydy, ketóny, estery, amidy, kyseliny, uhľovodíky) 2/28/2016
|
|
|
- Zoe Marybeth Dickerson
- 5 years ago
- Views:
Transcription
1 Structure Activity elationships (SA) identifies which functional groups are important for binding and activity SA Structure - Activity elationships (alkoholy, amíny, aldehydy, ketóny, estery, amidy, kyseliny, uhľovodíky) Method alter, remove or mask a functional group test the analogue for activity method of testing: in vitro target - trarget activity response in vitro on cells or in vivo - biological response (binding interactions with target (e.g. enzyme)) biological response (target binding pharmacokinetic properties) M-II Andrej Boháč if group is removed or modified and in vitro activity: drops or diminished => group was important for binding unaffected => group is not important onsider by analogues: 5. Structure Activity elationships (SA) modifications may disrupt binding by steric or electronic effects easiest analogues are those made directly from a lead compound 3 some analogues have to be made by a full (de novo) synthesis (e.g. replacing an aromatic ring with a heterocyclic ring) SA allows identification of important groups involved in binding SA allows identification of the pharmacophore MPIE an opioid analgesic drug DEIE antitussive drug AALGESI ATIVITY DPS 1
2 IMPTAT GUPS DETEMIED F ATIVITY MPIE 6-YMPIE ATIVITY UAFFETED MPIE AALGESI PAMAPE F PIATES 3 MPIE 3 METAZIE LEVPAL 2
3 5.1 SA on Alcohols Possible effect of analogues on binding (e.g. ether) BD Possible analogues = or BA Ether analogue 3 = or Ether analogue steric shield 3 Ether o interaction as BD o interaction as BA Ester Alkane 5.2 SA on 1 o, 2 o & 3 o Amines ( 2,, 3 ) if amine is ionised 5.2 SA on 1 o, 2 o & 3 o Amines ( 2,, 3 ) for free base -Bonding Ionic 2-2 BD = or BA -Bonding BD 2 = or 3 acts as a strong BD ote: 3 o Amines are only able to act as BA s - no hydrogen available to act as BD 3
4 5.2 SA on 1 o, 2 o & 3 o Amines ( 2,, 3 ) s of 3 o amines containing a methyl substituent s of 1 o & 2 o amines Effect on binding 2 3l Amide analogue Demethylation l 3 V-l 2 o amine 3 l 3 o amide 3 o interaction 1 o and 2 o amines are converted to 2 o and 3 o amides respectively amides cannot ionise and so ionic bonding is not possible an amide is a poor BA and so this eliminates BA interactions steric effect of acyl group is likely to hinder acting as a BD (2 o amide) PPSE A MEAISM of demetylation from nitrogen by V-l? 5.3 SA on Quaternary Ammonium Salts ( 4 ) 5.4 SA on Aldehydes and Ketones 2-3 Ionic bonding 3 d d- Induced dipole interactions s Dipole-dipole interaction BA -Bonding (= or ) s Full synthesis of 1 o -2 o amines and nitrogen to form permanent ion subsequently amides to disable ' Ketone Planar sp 2 carbon centre ab 4 or LiAl 4 ' 2 o Alcohol Tetrahedral sp 3 carbon centre 4
5 5.5 SA on Esters Effect on binding hange in stereochemistry (planar to tetrahedral) May move oxygen out of range Alcohol analogue (= or ) If still active, further reactions can be carried out on alcohol to establish importance of oxygen -bonding as BA by either oxygen s 3 a LiAl 4 ydrolysis splits molecule and may lead to a loss of activity due to loss of other functional groups - only suitable for simple esters. ydrolysis leads to a dramatic increase in polarity which may influence ability of analogue to reach target if in vivo tests are used. eduction to alcohol removes carbonyl group and can establish importance of the carbonyl oxygen, but reaction can be difficult to do if other labile functional groups are present. arboxylic acid 2 1 o Alcohol Alcohol 3 Esters are usually hydrolysed by esterases Esters are more likely to be important for pharmacokinetic reasons acting as prodrugs. 5.6 SA on Amides Prodrug Fatty Barrier Esterase BA BD Prodrug Ester masks polar groups Allows passage through fatty cell membranes Esterase (= or ) (= or ) The nitrogen of an amide cannot act as a BA - lone pair interacts with carbonyl group Tertiary amides unable to act as BD s 5
6 s ' a arboxylic acid 2 Amine ' s LiAl o Amine a / MeI 3 ' 3 o Amide ydrolysis splits molecule and may lead to loss of activity due to loss of other functional groups - only suitable for simple amides. ydrolysis leads to dramatic increase in polarity which may affect ability of analogue to reach target if in vivo tests are done eduction to amine removes carbonyl group and can establish importance of the carbonyl oxygen, but reaction may be difficult to do if other labile groups are present. -alkylation will disable BD properties of group in 2 amides. -Methylation prevents BD interaction and may introduce a steric effect that prevents also an BA interaction steric shield 3 3 o binding as BD Binding of as BA hindered 5.7 SA on arboxylic Acids as free acid as carboxylate ion BA BA BA Ionic bonding (= or ) (= or ) (= or ) (= or ) BD harged oxygen atoms are strong BA s. Group can interact by ionic and hydrogen bonding at the same time. (= or ) 6
7 5.8 SA on Aromatic ings and Alkenes Possible analogues / ' LiAl 4 ' Ester 2 Possible effects esterification prevents ionisation, BD interactions and may hinder BA by a steric effect reduction removes carbonyl oxygen as potential BA and prevents ionisation 3 2 steric shield 3 1 o Alcohol vdw hydrophobic pocket Possible analogues ' 2 / ai 2 / Pd/ ' vdw hydrophobic region o ionic bonding possible -Bonding hindered Possible effects on binding 5.10 SA of Alkyl Groups Possible interactions o fit hydrophobic pocket Buffers hydrophobic region 3 hydrophobic slot van der Waals interactions hydrophobic pocket 7
8 5.10 SA of Alkyl Groups s Easiest alkyl groups to vary are substituents on heteroatoms. Vary length and bulk of alkyl group to test space available. 3 V-l 3 Br ' ' ' ' 5.9 Miscellaneous Functional Groups in s acid chlorides - too reactive to be of used acid anhydrides - too reactive to be of used - present in anticancer drugs (alkyl. agents) -react with nucleophiles in DA Ar - commonly present (liphophilic int., fluorine F...= interactions, or halogen bond:..., bond) 2 - sometimes present but often toxic -:::- alkynes - sometimes present, but not usually important in binding interactions -S thiols - present in some drugs as important binding group to transition metals (e.g. Zn in zinc metalloproteinases MMPs) - - present in some drugs but rarely involved in binding 3 ydrolysis ' ' functional groups that may be important for electronic reasons (e.g. nitro, cyano, aryl halides) functional groups that may be important for steric reasons (e.g. alkynes) 8
OXFORD H i g h e r E d u c a t i o n Oxford University Press, All rights reserved.
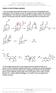 Patrick, An Introduction to dicinal hemistry 4e Answers to end-of-chapter questions 1) The mechanism below shows the release of one molecule of formaldehyde from methenamine. The mechanism can then be
Patrick, An Introduction to dicinal hemistry 4e Answers to end-of-chapter questions 1) The mechanism below shows the release of one molecule of formaldehyde from methenamine. The mechanism can then be
ORGANIC - EGE 5E CH. 2 - COVALENT BONDING AND CHEMICAL REACTIVITY
 !! www.clutchprep.com CONCEPT: HYBRID ORBITAL THEORY The Aufbau Principle states that electrons fill orbitals in order of increasing energy. If carbon has only two unfilled orbitals, why does it like to
!! www.clutchprep.com CONCEPT: HYBRID ORBITAL THEORY The Aufbau Principle states that electrons fill orbitals in order of increasing energy. If carbon has only two unfilled orbitals, why does it like to
Chapter 22: Amines. Organic derivatives of ammonia, NH 3. Nitrogen atom have a lone pair of electrons, making the amine both basic and nucleophilic
 hapter 22: Amines. rganic derivatives of ammonia, 3. itrogen atom have a lone pair of electrons, making the amine both basic and nucleophilic 22.1: Amines omenclature. (please read) sp 3 Amines are classified
hapter 22: Amines. rganic derivatives of ammonia, 3. itrogen atom have a lone pair of electrons, making the amine both basic and nucleophilic 22.1: Amines omenclature. (please read) sp 3 Amines are classified
Amines. Amines are organic compounds containing a nitrogen functionality. primary secondary tertiary quaternary
 Amines Amines are organic compounds containing a nitrogen functionality Depending upon the number of alkyl, or aryl, groups attached to nitrogen determines its classification, or order 2 primary secondary
Amines Amines are organic compounds containing a nitrogen functionality Depending upon the number of alkyl, or aryl, groups attached to nitrogen determines its classification, or order 2 primary secondary
Chapter 20 Carboxylic Acid Derivatives. Nucleophilic Acyl Substitution
 ucleophilic Acyl Substitution hapter 20 arboxylic Acid Derivatives ucleophilic Acyl Substitution Y (1) need to have Y as a u Y u u + Y (2) could not happen with aldehydes or ketones as : and : are poor
ucleophilic Acyl Substitution hapter 20 arboxylic Acid Derivatives ucleophilic Acyl Substitution Y (1) need to have Y as a u Y u u + Y (2) could not happen with aldehydes or ketones as : and : are poor
Look for absorption bands in decreasing order of importance:
 1. Match the following to their IR spectra (30 points) Look for absorption bands in decreasing order of importance: a e a 2941 1716 d f b 3333 c b 1466 1.the - absorption(s) between 3100 and 2850 cm-1.
1. Match the following to their IR spectra (30 points) Look for absorption bands in decreasing order of importance: a e a 2941 1716 d f b 3333 c b 1466 1.the - absorption(s) between 3100 and 2850 cm-1.
Synthesis of Nitriles a. dehydration of 1 amides using POCl 3 : b. SN2 reaction of cyanide ion on halides:
 I. Nitriles Nitriles consist of the CN functional group, and are linear with sp hybridization on C and N. Nitriles are non-basic at nitrogen, since the lone pair exists in an sp orbital (50% s character
I. Nitriles Nitriles consist of the CN functional group, and are linear with sp hybridization on C and N. Nitriles are non-basic at nitrogen, since the lone pair exists in an sp orbital (50% s character
MOLECULAR REPRESENTATIONS AND INFRARED SPECTROSCOPY
 MOLEULAR REPRESENTATIONS AND INFRARED SPETROSOPY A STUDENT SOULD BE ABLE TO: 1. Given a Lewis (dash or dot), condensed, bond-line, or wedge formula of a compound draw the other representations. 2. Give
MOLEULAR REPRESENTATIONS AND INFRARED SPETROSOPY A STUDENT SOULD BE ABLE TO: 1. Given a Lewis (dash or dot), condensed, bond-line, or wedge formula of a compound draw the other representations. 2. Give
Chapter 1 Reactions of Organic Compounds. Reactions Involving Hydrocarbons
 Chapter 1 Reactions of Organic Compounds Reactions Involving Hydrocarbons Reactions of Alkanes Single bonds (C-C) are strong and very hard to break, therefore these compounds are relatively unreactive
Chapter 1 Reactions of Organic Compounds Reactions Involving Hydrocarbons Reactions of Alkanes Single bonds (C-C) are strong and very hard to break, therefore these compounds are relatively unreactive
Learning Organic Chemistry
 Objective 1 Represent organic molecules with chemical formulas, expanded formulas, Lewis structures, skeletal structures. Determine shape (VSEPR), bond polarity, and molecule polarity. Identify functional
Objective 1 Represent organic molecules with chemical formulas, expanded formulas, Lewis structures, skeletal structures. Determine shape (VSEPR), bond polarity, and molecule polarity. Identify functional
Identifying Functional Groups. Why is this necessary? Alkanes. Why is this so important? What is a functional group? 2/1/16
 Identifying Functional Groups The Key to Survival Why is this so important? ver and over again, you will be asked to do reactions, the details to which you will receive in lecture and via your textbook.
Identifying Functional Groups The Key to Survival Why is this so important? ver and over again, you will be asked to do reactions, the details to which you will receive in lecture and via your textbook.
Lecture Notes Chemistry Mukund P. Sibi Lecture 36 Synthesis of Amines
 Lecture otes hemistry 42-2008 Mukund P. Sibi Synthesis of Amines Amines can be prepared from a variety of starting materials. All of these methods involve functional group transformations. The main methods
Lecture otes hemistry 42-2008 Mukund P. Sibi Synthesis of Amines Amines can be prepared from a variety of starting materials. All of these methods involve functional group transformations. The main methods
CHEMISTRY Topic #1: Functional Groups and Drawing Organic Molecules Fall 2014 Dr. Susan Findlay
 EMISTRY 2500 Topic #1: Functional Groups and Drawing rganic Molecules Fall 2014 Dr. Susan Findlay Drawing rganic Molecules (Basics) Recall the steps for drawing Lewis structures in EM 1000: 1. Determine
EMISTRY 2500 Topic #1: Functional Groups and Drawing rganic Molecules Fall 2014 Dr. Susan Findlay Drawing rganic Molecules (Basics) Recall the steps for drawing Lewis structures in EM 1000: 1. Determine
Chapter 24. Amines. Based on McMurry s Organic Chemistry, 7 th edition
 Chapter 24. Amines Based on McMurry s Organic Chemistry, 7 th edition Amines Organic Nitrogen Compounds Organic derivatives of ammonia, NH 3, Nitrogen atom with a lone pair of electrons, making amines
Chapter 24. Amines Based on McMurry s Organic Chemistry, 7 th edition Amines Organic Nitrogen Compounds Organic derivatives of ammonia, NH 3, Nitrogen atom with a lone pair of electrons, making amines
Drug Discovery. Zainab Al Kharusi Office: 33-10
 Drug Discovery Zainab Al Kharusi Office: 33-10 Objectives: To understand the processes of Drug Development To be able to develop a plan for drug discovery Reference: Patrick G L. An Introduction to Medicinal
Drug Discovery Zainab Al Kharusi Office: 33-10 Objectives: To understand the processes of Drug Development To be able to develop a plan for drug discovery Reference: Patrick G L. An Introduction to Medicinal
Exam 1 (Monday, July 6, 2015)
 Chem 231 Summer 2015 Assigned Homework Problems Last updated: Friday, July 24, 2015 Problems Assigned from Essential Organic Chemistry, 2 nd Edition, Paula Yurkanis Bruice, Prentice Hall, New York, NY,
Chem 231 Summer 2015 Assigned Homework Problems Last updated: Friday, July 24, 2015 Problems Assigned from Essential Organic Chemistry, 2 nd Edition, Paula Yurkanis Bruice, Prentice Hall, New York, NY,
Chapter 20: Carboxylic Acids
 1 Chapter 20: Carboxylic Acids I. Introduction: Carboxylic acid structure: Classification of carboxylic acids: A carboxylic acid donates protons by the heterocyclic cleavage of the O-H bond, generating
1 Chapter 20: Carboxylic Acids I. Introduction: Carboxylic acid structure: Classification of carboxylic acids: A carboxylic acid donates protons by the heterocyclic cleavage of the O-H bond, generating
Patrick: An Introduction to Medicinal Chemistry 5e Chapter 01
 Questions Patrick: An Introduction to Medicinal Chemistry 5e 01) Which of the following molecules is a phospholipid? a. i b. ii c. iii d. iv 02) Which of the following statements is false regarding the
Questions Patrick: An Introduction to Medicinal Chemistry 5e 01) Which of the following molecules is a phospholipid? a. i b. ii c. iii d. iv 02) Which of the following statements is false regarding the
R N R N R N. primary secondary tertiary
 Chapter 19 Amines omenclature o assification of amines Amines are classified as 1, 2, or 3 based on how many R groups are attached to the nitrogen R R R R R R primary secondary tertiary When there are
Chapter 19 Amines omenclature o assification of amines Amines are classified as 1, 2, or 3 based on how many R groups are attached to the nitrogen R R R R R R primary secondary tertiary When there are
Loudon Chapter 23 Review: Amines Jacquie Richardson, CU Boulder Last updated 4/22/2018
 This chapter is about the chemistry of nitrogen. We ve seen it before in several places, but now we can look at several reactions that are specific to nitrogen. Amines can be subdivided based on how many
This chapter is about the chemistry of nitrogen. We ve seen it before in several places, but now we can look at several reactions that are specific to nitrogen. Amines can be subdivided based on how many
Classes of Organic Compounds
 Unit 1 Functional Groups Depicting Structures of rganic ompounds Lewis Structures ondensed structural formulas Line angle drawings 3-dimensional structures Resonance Structures Acid-Base Reactions urved
Unit 1 Functional Groups Depicting Structures of rganic ompounds Lewis Structures ondensed structural formulas Line angle drawings 3-dimensional structures Resonance Structures Acid-Base Reactions urved
Carbon Compounds. Chemical Bonding Part 2
 Carbon Compounds Chemical Bonding Part 2 Introduction to Functional Groups: Alkanes! Alkanes Compounds that contain only carbons and hydrogens, with no double or triple bonds.! Alkyl Groups A part of a
Carbon Compounds Chemical Bonding Part 2 Introduction to Functional Groups: Alkanes! Alkanes Compounds that contain only carbons and hydrogens, with no double or triple bonds.! Alkyl Groups A part of a
Table 8.2 Detailed Table of Characteristic Infrared Absorption Frequencies
 Table 8.2 Detailed Table of Characteristic Infrared Absorption Frequencies The hydrogen stretch region (3600 2500 cm 1 ). Absorption in this region is associated with the stretching vibration of hydrogen
Table 8.2 Detailed Table of Characteristic Infrared Absorption Frequencies The hydrogen stretch region (3600 2500 cm 1 ). Absorption in this region is associated with the stretching vibration of hydrogen
Aromatic Hydrocarbons
 Aromatic Hydrocarbons Aromatic hydrocarbons contain six-membered rings of carbon atoms with alternating single and double carbon-carbon bonds. The ring is sometimes shown with a circle in the center instead
Aromatic Hydrocarbons Aromatic hydrocarbons contain six-membered rings of carbon atoms with alternating single and double carbon-carbon bonds. The ring is sometimes shown with a circle in the center instead
b.p.=100 C b.p.=65 C b.p.=-25 C µ=1.69 D µ=2.0 D µ=1.3 D
 Alcohols I eading: Wade chapter 10, sections 10-1- 10-12 Study Problems: 10-35, 10-37, 10-38, 10-39, 10-40, 10-42, 10-43 Key Concepts and Skills: Show how to convert alkenes, alkyl halides, and and carbonyl
Alcohols I eading: Wade chapter 10, sections 10-1- 10-12 Study Problems: 10-35, 10-37, 10-38, 10-39, 10-40, 10-42, 10-43 Key Concepts and Skills: Show how to convert alkenes, alkyl halides, and and carbonyl
12.1 The Nature of Organic molecules
 12.1 The Nature of Organic molecules Organic chemistry: : The chemistry of carbon compounds. Carbon is tetravalent; it always form four bonds. Prentice Hall 2003 Chapter One 2 Organic molecules have covalent
12.1 The Nature of Organic molecules Organic chemistry: : The chemistry of carbon compounds. Carbon is tetravalent; it always form four bonds. Prentice Hall 2003 Chapter One 2 Organic molecules have covalent
Chapter 2. Molecular Representations
 hapter 2. Molecular Representations 3 () 3 ( 3 ) 2 3 3 3 8 Lewis (Kekule) structure ondensed and par6ally condensed structure Skeletal (bond- line) structure Molecular formula Amoxicillin a widely prescribed
hapter 2. Molecular Representations 3 () 3 ( 3 ) 2 3 3 3 8 Lewis (Kekule) structure ondensed and par6ally condensed structure Skeletal (bond- line) structure Molecular formula Amoxicillin a widely prescribed
2016 Pearson Education, Inc. Isolated and Conjugated Dienes
 2016 Pearson Education, Inc. Isolated and Conjugated Dienes 2016 Pearson Education, Inc. Reactions of Isolated Dienes 2016 Pearson Education, Inc. The Mechanism Double Bonds can have Different Reactivities
2016 Pearson Education, Inc. Isolated and Conjugated Dienes 2016 Pearson Education, Inc. Reactions of Isolated Dienes 2016 Pearson Education, Inc. The Mechanism Double Bonds can have Different Reactivities
Nucleophilic Addition Reactions of Carboxylic Acid Derivatives
 Lecture 5: bjectives: Nucleophilic Addition eactions of Carboxylic Acid Derivatives By the end of this lecture you will be able to: draw the mechanism of a nucleophilic addition-elimination reaction with
Lecture 5: bjectives: Nucleophilic Addition eactions of Carboxylic Acid Derivatives By the end of this lecture you will be able to: draw the mechanism of a nucleophilic addition-elimination reaction with
Amines Reading Study Problems Key Concepts and Skills Lecture Topics: Amines: structure and nomenclature
 Amines Reading: Wade chapter 19, sections 19-1-19-19 Study Problems: 19-37, 19-39, 19-40, 19-41, 19-44, 19-46, 19-47, 19-48, 19-51, 19-54 Key Concepts and Skills: Explain how the basicity of amines varies
Amines Reading: Wade chapter 19, sections 19-1-19-19 Study Problems: 19-37, 19-39, 19-40, 19-41, 19-44, 19-46, 19-47, 19-48, 19-51, 19-54 Key Concepts and Skills: Explain how the basicity of amines varies
Loudon Chapter 23 Review: Amines CHEM 3331, Jacquie Richardson, Fall Page 1
 Loudon Chapter 23 eview: Amines CEM 3331, Jacquie ichardson, Fall 2010 - Page 1 This chapter is about the chemistry of nitrogen. We ve seen it before in several places, but now we can look at several reactions
Loudon Chapter 23 eview: Amines CEM 3331, Jacquie ichardson, Fall 2010 - Page 1 This chapter is about the chemistry of nitrogen. We ve seen it before in several places, but now we can look at several reactions
CHEMISTRY 263 HOME WORK
 Lecture Topics: CHEMISTRY 263 HOME WORK Module7: Hydrogenation of Alkenes Hydrogenation - syn and anti- addition - hydrogenation of alkynes - synthesis of cis-alkenes -synthesis of trans-alkenes Text sections:
Lecture Topics: CHEMISTRY 263 HOME WORK Module7: Hydrogenation of Alkenes Hydrogenation - syn and anti- addition - hydrogenation of alkynes - synthesis of cis-alkenes -synthesis of trans-alkenes Text sections:
Keynotes in Organic Chemistry
 Keynotes in Organic Chemistry Second Edition ANDREW F. PARSONS Department of Chemistry, University of York, UK Wiley Contents Preface xi 1 Structure and bonding 1 1.1 Ionic versus covalent bonds 1 1.2
Keynotes in Organic Chemistry Second Edition ANDREW F. PARSONS Department of Chemistry, University of York, UK Wiley Contents Preface xi 1 Structure and bonding 1 1.1 Ionic versus covalent bonds 1 1.2
Chapter 25: The Chemistry of Life: Organic and Biological Chemistry
 Chemistry: The Central Science Chapter 25: The Chemistry of Life: Organic and Biological Chemistry The study of carbon compounds constitutes a separate branch of chemistry known as organic chemistry The
Chemistry: The Central Science Chapter 25: The Chemistry of Life: Organic and Biological Chemistry The study of carbon compounds constitutes a separate branch of chemistry known as organic chemistry The
Organic Chemistry. Introduction to Organic Molecules and Functional Groups
 For updated version, please click on http://ocw.ump.edu.my Organic Chemistry Introduction to Organic Molecules and Functional Groups by Dr. Seema Zareen & Dr. Izan Izwan Misnon Faculty Industrial Science
For updated version, please click on http://ocw.ump.edu.my Organic Chemistry Introduction to Organic Molecules and Functional Groups by Dr. Seema Zareen & Dr. Izan Izwan Misnon Faculty Industrial Science
Module9. Nuclear Magnetic Resonance Spectroscopy Nuclear Magnetic Resonance (NMR) spectroscopy - Chemical shift - Integration of signal area
 1 CHEMISTRY 263 HOME WORK Lecture Topics: Module7. Hydrogenation of Alkenes The Function of the Catalyst - Syn and anti- addition Hydrogenation of Alkynes - Syn- addition of hydrogen: Synthesis of cis-alkenes
1 CHEMISTRY 263 HOME WORK Lecture Topics: Module7. Hydrogenation of Alkenes The Function of the Catalyst - Syn and anti- addition Hydrogenation of Alkynes - Syn- addition of hydrogen: Synthesis of cis-alkenes
CHAPTER 2: Structure and Properties of Organic Molecules
 1 HAPTER 2: Structure and Properties of Organic Molecules Atomic Orbitals A. What are atomic orbitals? Atomic orbitals are defined by special mathematical functions called wavefunctions-- (x, y, z). Wavefunction,
1 HAPTER 2: Structure and Properties of Organic Molecules Atomic Orbitals A. What are atomic orbitals? Atomic orbitals are defined by special mathematical functions called wavefunctions-- (x, y, z). Wavefunction,
General Infrared Absorption Ranges of Various Functional Groups
 General Infrared Absorption Ranges of Various Functional Groups Frequency Range Bond Type of Compound cm -1 Intensity C Alkanes 2850-2970 Strong 1340-1470 Strong C Alkenes 3010-3095 Medium 675-995 Strong
General Infrared Absorption Ranges of Various Functional Groups Frequency Range Bond Type of Compound cm -1 Intensity C Alkanes 2850-2970 Strong 1340-1470 Strong C Alkenes 3010-3095 Medium 675-995 Strong
Lecture 27 Organic Chemistry 1
 CHEM 232 rganic Chemistry I at Chicago Lecture 27 rganic Chemistry 1 Professor Duncan Wardrop April 20, 2010 1 Self Test Question Nitrosonium (not nitronium) cations can be generated by treating sodium
CHEM 232 rganic Chemistry I at Chicago Lecture 27 rganic Chemistry 1 Professor Duncan Wardrop April 20, 2010 1 Self Test Question Nitrosonium (not nitronium) cations can be generated by treating sodium
Chapter 10: Carboxylic Acids and Their Derivatives
 Chapter 10: Carboxylic Acids and Their Derivatives The back of the white willow tree (Salix alba) is a source of salicylic acid which is used to make aspirin (acetylsalicylic acid) The functional group
Chapter 10: Carboxylic Acids and Their Derivatives The back of the white willow tree (Salix alba) is a source of salicylic acid which is used to make aspirin (acetylsalicylic acid) The functional group
Chem 263 Nov 24, Properties of Carboxylic Acids
 Chem 263 ov 24, 2009 Properties of Carboxylic Acids Since carboxylic acids are structurally related to both ketones and aldehydes, we would expect to see some similar structural properties. The carbonyl
Chem 263 ov 24, 2009 Properties of Carboxylic Acids Since carboxylic acids are structurally related to both ketones and aldehydes, we would expect to see some similar structural properties. The carbonyl
UNIT 4 REVISION CHECKLIST CHEM 4 AS Chemistry
 UNIT 4 REVISION CHECKLIST CHEM 4 AS Chemistry Topic 4.1 Kinetics a) Define the terms: rate of a reaction, rate constant, order of reaction and overall order of reaction b) Deduce the orders of reaction
UNIT 4 REVISION CHECKLIST CHEM 4 AS Chemistry Topic 4.1 Kinetics a) Define the terms: rate of a reaction, rate constant, order of reaction and overall order of reaction b) Deduce the orders of reaction
Chem 1075 Chapter 19 Organic Chemistry Lecture Outline
 Chem 1075 Chapter 19 Organic Chemistry Lecture Outline Slide 2 Introduction Organic chemistry is the study of and its compounds. The major sources of carbon are the fossil fuels: petroleum, natural gas,
Chem 1075 Chapter 19 Organic Chemistry Lecture Outline Slide 2 Introduction Organic chemistry is the study of and its compounds. The major sources of carbon are the fossil fuels: petroleum, natural gas,
More information can be found in Chapter 12 in your textbook for CHEM 3750/ 3770 and on pages in your laboratory manual.
 CHEM 3780 rganic Chemistry II Infrared Spectroscopy and Mass Spectrometry Review More information can be found in Chapter 12 in your textbook for CHEM 3750/ 3770 and on pages 13-28 in your laboratory manual.
CHEM 3780 rganic Chemistry II Infrared Spectroscopy and Mass Spectrometry Review More information can be found in Chapter 12 in your textbook for CHEM 3750/ 3770 and on pages 13-28 in your laboratory manual.
Electronegativity Scale F > O > Cl, N > Br > C, H
 Organic Chem Chapter 12 Alkanes Organic chemistry is the study of carbon compounds. Carbon has several properties that are worth discussing: Tetravalent Always forms 4 bonds Can form multiple bonds (double
Organic Chem Chapter 12 Alkanes Organic chemistry is the study of carbon compounds. Carbon has several properties that are worth discussing: Tetravalent Always forms 4 bonds Can form multiple bonds (double
ALCOHOLS: Properties & Preparation
 ALLS: Properties & Preparation General formula: -, where is alkyl or substitued alkyl. Ar-: phenol - different properties. Nomenclature 1. ommon names: Name of alkyl group, followed by word alcohol. 2.
ALLS: Properties & Preparation General formula: -, where is alkyl or substitued alkyl. Ar-: phenol - different properties. Nomenclature 1. ommon names: Name of alkyl group, followed by word alcohol. 2.
TOK: The relationship between a reaction mechanism and the experimental evidence to support it could be discussed. See
 Option G: Further organic chemistry (15/22 hours) SL students study the core of these options and HL students study the whole option (the core and the extension material). TOK: The relationship between
Option G: Further organic chemistry (15/22 hours) SL students study the core of these options and HL students study the whole option (the core and the extension material). TOK: The relationship between
Alcohol Synthesis. Dr. Sapna Gupta
 Alcohol Synthesis Dr. Sapna Gupta Synthesis of Alcohols Alcohols can be synthesized from several functional groups. Nucleophilic substitution of O - on alkyl halide ydration of alkenes water in acid solution
Alcohol Synthesis Dr. Sapna Gupta Synthesis of Alcohols Alcohols can be synthesized from several functional groups. Nucleophilic substitution of O - on alkyl halide ydration of alkenes water in acid solution
Mechanism Summary for A-level AQA Chemistry
 Mechanism Summary for Alevel AQA hemistry Electrophilic Addition of Alkenes with omine Electrophilic Addition of Alkenes with sulphuric acid 3 S 2 3 3 S 2 S 2 Electrophilic Addition of Alkenes with hydrogen
Mechanism Summary for Alevel AQA hemistry Electrophilic Addition of Alkenes with omine Electrophilic Addition of Alkenes with sulphuric acid 3 S 2 3 3 S 2 S 2 Electrophilic Addition of Alkenes with hydrogen
Carbon-heteroatom single bonds basic C N C X. X= F, Cl, Br, I Alkyl Halide C O. epoxide Chapter 14 H. alcohols acidic H C S C. thiols.
 hapter 13: Alcohols and Phenols 13.1 Structure and Properties of Alcohols Alkanes arbon - arbon Multiple Bonds arbon-heteroatom single bonds basic Alkenes X X= F, l,, I Alkyl alide amines hapter 23 nitro
hapter 13: Alcohols and Phenols 13.1 Structure and Properties of Alcohols Alkanes arbon - arbon Multiple Bonds arbon-heteroatom single bonds basic Alkenes X X= F, l,, I Alkyl alide amines hapter 23 nitro
Chapter 2: An Introduction to Organic Compounds
 Chapter : An Introduction to Organic Compounds I. FUNCTIONAL GROUPS: Functional groups with similar structure/reactivity may be "grouped" together. A. Functional Groups With Carbon-Carbon Multiple Bonds.
Chapter : An Introduction to Organic Compounds I. FUNCTIONAL GROUPS: Functional groups with similar structure/reactivity may be "grouped" together. A. Functional Groups With Carbon-Carbon Multiple Bonds.
CHEM 203. Midterm Exam 1 October 31, 2008 ANSWERS. This a closed-notes, closed-book exam. You may use your set of molecular models
 CEM 203 Midterm Exam 1 ctober 31, 2008 Your name: ANSWERS This a closed-notes, closed-book exam You may use your set of molecular models This exam contains 8 pages Time: 1h 30 min 1. / 15 2. / 16 3. /
CEM 203 Midterm Exam 1 ctober 31, 2008 Your name: ANSWERS This a closed-notes, closed-book exam You may use your set of molecular models This exam contains 8 pages Time: 1h 30 min 1. / 15 2. / 16 3. /
Chem 263 March 28, 2006
 Chem 263 March 28, 2006 Properties of Carboxylic Acids Since carboxylic acids are structurally related to both ketones and aldehydes, we would expect to see some similar structural properties. The carbonyl
Chem 263 March 28, 2006 Properties of Carboxylic Acids Since carboxylic acids are structurally related to both ketones and aldehydes, we would expect to see some similar structural properties. The carbonyl
CHEM3331: Fundamentals of Organic Chemistry I Prof. Ognjen Š. Miljanić December 11, 2012
 HEM3331: Fundamentals of rganic hemistry I Final Exam Prof. gnjen Š. Miljanić December 11, 2012 Name: Last First Student ID Number: ead all directions very carefully, think about your answer, and then
HEM3331: Fundamentals of rganic hemistry I Final Exam Prof. gnjen Š. Miljanić December 11, 2012 Name: Last First Student ID Number: ead all directions very carefully, think about your answer, and then
Definition: A carboxylic acid derivative undergoes hydrolysis (bond breaking with water) to form a carboxylic acid R C N + H 2 O + H O R
 arboxylic Acid Derivatives Addition/Elimination Definition: A carboxylic acid derivative undergoes hydrolysis (bond breaking with water) to form a carboxylic acid X = l, Br addition/elimination X acid
arboxylic Acid Derivatives Addition/Elimination Definition: A carboxylic acid derivative undergoes hydrolysis (bond breaking with water) to form a carboxylic acid X = l, Br addition/elimination X acid
Organic Chemistry Review: Topic 10 & Topic 20
 Organic Structure Alkanes C C σ bond Mechanism Substitution (Incoming atom or group will displace an existing atom or group in a molecule) Examples Occurs with exposure to ultraviolet light or sunlight,
Organic Structure Alkanes C C σ bond Mechanism Substitution (Incoming atom or group will displace an existing atom or group in a molecule) Examples Occurs with exposure to ultraviolet light or sunlight,
Bio-elements. Living organisms requires only 27 of the 90 common chemical elements found in the crust of the earth, to be as its essential components.
 Bio-elements Living organisms requires only 27 of the 90 common chemical elements found in the crust of the earth, to be as its essential components. Most of the chemical components of living organisms
Bio-elements Living organisms requires only 27 of the 90 common chemical elements found in the crust of the earth, to be as its essential components. Most of the chemical components of living organisms
Alkanes, Alkenes and Alkynes
 Alkanes, Alkenes and Alkynes Hydrocarbons Hydrocarbons generally fall into 2 general groupings, aliphatic hydrocarbons and aromatic hydrocarbons. Aliphatic hydrocarbons contain chains and rings of hydrocarbons,
Alkanes, Alkenes and Alkynes Hydrocarbons Hydrocarbons generally fall into 2 general groupings, aliphatic hydrocarbons and aromatic hydrocarbons. Aliphatic hydrocarbons contain chains and rings of hydrocarbons,
Chapter 24 From Petroleum to Pharmaceuticals
 hapter 24 From Petroleum to Pharmaceuticals 24.1 Petroleum Refining and the ydrocarbons 24.2 Functional Groups and Organic Synthesis 24.3 Pesticides and Pharmaceuticals IR Tutor and Infrared Spectroscopy
hapter 24 From Petroleum to Pharmaceuticals 24.1 Petroleum Refining and the ydrocarbons 24.2 Functional Groups and Organic Synthesis 24.3 Pesticides and Pharmaceuticals IR Tutor and Infrared Spectroscopy
Alcohols. Alcohol any organic compound containing a hydroxyl (R-OH) group. Alcohols are an extremely important organic source
 Alcohols Alcohol any organic compound containing a hydroxyl (R-OH) group Uses: synthetic intermediate, cleanser, cosmetics, fuel, alcoholic beverages, etc. Alcohols are an extremely important organic source
Alcohols Alcohol any organic compound containing a hydroxyl (R-OH) group Uses: synthetic intermediate, cleanser, cosmetics, fuel, alcoholic beverages, etc. Alcohols are an extremely important organic source
Organic Chemistry Lecture 2 - Hydrocarbons, Alcohols, Substitutions
 ALKANES Water-insoluble, low density C-C single bonds Higher MW -> higher BP, higher MP Branching -> lower BP, higher MP Forms cycloalkanes which can have ring strain Cyclohexane: chair vs. boat configuration
ALKANES Water-insoluble, low density C-C single bonds Higher MW -> higher BP, higher MP Branching -> lower BP, higher MP Forms cycloalkanes which can have ring strain Cyclohexane: chair vs. boat configuration
Option G: Further organic chemistry (15/22 hours)
 Option G: Further organic chemistry (15/) TOK: The relationship between a reaction mechanism and the experimental evidence to support it could be discussed. See 16... Core material: G1 G8 are core material
Option G: Further organic chemistry (15/) TOK: The relationship between a reaction mechanism and the experimental evidence to support it could be discussed. See 16... Core material: G1 G8 are core material
DAMIETTA UNIVERSITY. Energy Diagram of One-Step Exothermic Reaction
 DAMIETTA UNIVERSITY CHEM-103: BASIC ORGANIC CHEMISTRY LECTURE 5 Dr Ali El-Agamey 1 Energy Diagram of One-Step Exothermic Reaction The vertical axis in this graph represents the potential energy. The transition
DAMIETTA UNIVERSITY CHEM-103: BASIC ORGANIC CHEMISTRY LECTURE 5 Dr Ali El-Agamey 1 Energy Diagram of One-Step Exothermic Reaction The vertical axis in this graph represents the potential energy. The transition
Chapter 12. Alcohols from Carbonyl Compounds Oxidation-Reduction & Organometallic Compounds. Structure
 Chapter 12 Alcohols from Carbonyl Compounds xidation-eduction & rganometallic Compounds Created by Professor William Tam & Dr. Phillis Chang Structure ~ 120 o ~ 120 o C ~ 120 o Carbonyl carbon: sp 2 hybridized
Chapter 12 Alcohols from Carbonyl Compounds xidation-eduction & rganometallic Compounds Created by Professor William Tam & Dr. Phillis Chang Structure ~ 120 o ~ 120 o C ~ 120 o Carbonyl carbon: sp 2 hybridized
Chem 263 Notes March 2, 2006
 Chem 263 Notes March 2, 2006 Average for the midterm is 102.5 / 150 (approx. 68%). Preparation of Aldehydes and Ketones There are several methods to prepare aldehydes and ketones. We will only deal with
Chem 263 Notes March 2, 2006 Average for the midterm is 102.5 / 150 (approx. 68%). Preparation of Aldehydes and Ketones There are several methods to prepare aldehydes and ketones. We will only deal with
Introduction. A1.1 (a) Shell number and number of subshells 1. A1.1 (b) Orbitals 2. A1.1 (c ) Orbital shapes (s, p & d) 2
 Preface Table of Contents Introduction i A1.1 (a) Shell number and number of subshells 1 A1.1 (b) Orbitals 2 A1.1 (c ) Orbital shapes (s, p & d) 2 A1.1 (d) Relative energies of s,p,d,f sub-shells 4 A 1.1
Preface Table of Contents Introduction i A1.1 (a) Shell number and number of subshells 1 A1.1 (b) Orbitals 2 A1.1 (c ) Orbital shapes (s, p & d) 2 A1.1 (d) Relative energies of s,p,d,f sub-shells 4 A 1.1
Amines. Chapter 24 Organic Chemistry, 8th Edition. John McMurry
 Amines Chapter 24 Organic Chemistry, 8th Edition John McMurry 1 Introduction Amines are stronger bases and better nucleophiles than other neutral organic compounds. 2 Nomenclature 1 Amines are named using
Amines Chapter 24 Organic Chemistry, 8th Edition John McMurry 1 Introduction Amines are stronger bases and better nucleophiles than other neutral organic compounds. 2 Nomenclature 1 Amines are named using
Aromatic Compounds II
 2302272 Org Chem II Part I Lecture 2 Aromatic Compounds II Instructor: Dr. Tanatorn Khotavivattana E-mail: tanatorn.k@chula.ac.th Recommended Textbook: Chapter 17 in Organic Chemistry, 8 th Edition, L.
2302272 Org Chem II Part I Lecture 2 Aromatic Compounds II Instructor: Dr. Tanatorn Khotavivattana E-mail: tanatorn.k@chula.ac.th Recommended Textbook: Chapter 17 in Organic Chemistry, 8 th Edition, L.
Reduction. Boron based reagents. NaBH 4 / NiCl 2. Uses: Zn(BH 4 ) 2. Preparation: Good for base sensitive groups Chelation control model.
 Uses: Ar N 2 Ar N 2 Ar N Ar N 2 eduction Boron based reagents NaB 4 / NiCl 2 2 Ar C N Ar C N 2 Preparation: Zn(B 4 ) 2 ZnCl 2 (Ether) NaB 4 Zn(B 4 ) 2 Good for base sensitive groups Chelation control model
Uses: Ar N 2 Ar N 2 Ar N Ar N 2 eduction Boron based reagents NaB 4 / NiCl 2 2 Ar C N Ar C N 2 Preparation: Zn(B 4 ) 2 ZnCl 2 (Ether) NaB 4 Zn(B 4 ) 2 Good for base sensitive groups Chelation control model
Organic Chemistry 112 A B C - Syllabus Addendum for Prospective Teachers
 Chapter Organic Chemistry 112 A B C - Syllabus Addendum for Prospective Teachers Ch 1-Structure and bonding Ch 2-Polar covalent bonds: Acids and bases McMurry, J. (2004) Organic Chemistry 6 th Edition
Chapter Organic Chemistry 112 A B C - Syllabus Addendum for Prospective Teachers Ch 1-Structure and bonding Ch 2-Polar covalent bonds: Acids and bases McMurry, J. (2004) Organic Chemistry 6 th Edition
Paper 9: ORGANIC CHEMISTRY-III (Reaction Mechanism-2) Module 16: Reduction by Metal hydrides Part-I
 Subject Chemistry Paper No and Title Module No and Title Module Tag 9: ORGANIC -III (Reaction Mechanism-2) 16: Reduction by Metal hydrides Part-I CHE_P9_M16 TABLE OF CONTENTS 1. Learning Outcomes 2. Introduction
Subject Chemistry Paper No and Title Module No and Title Module Tag 9: ORGANIC -III (Reaction Mechanism-2) 16: Reduction by Metal hydrides Part-I CHE_P9_M16 TABLE OF CONTENTS 1. Learning Outcomes 2. Introduction
Molecular Geometry: VSEPR model stand for valence-shell electron-pair repulsion and predicts the 3D shape of molecules that are formed in bonding.
 Molecular Geometry: VSEPR model stand for valence-shell electron-pair repulsion and predicts the 3D shape of molecules that are formed in bonding. Sigma and Pi Bonds: All single bonds are sigma(σ), that
Molecular Geometry: VSEPR model stand for valence-shell electron-pair repulsion and predicts the 3D shape of molecules that are formed in bonding. Sigma and Pi Bonds: All single bonds are sigma(σ), that
Q.1 Draw structures for all amines of molecular formula C 4 H 11 N. Classify them as primary, secondary or tertiary amines.
 1 AMIES Structure ontain the 2 group. lassification primary (1 ) amines secondary (2 ) amines tertiary (3 ) amines quarternary (4 ) ammonium salts + 1 2 3 4 Aliphatic Aromatic methylamine, ethylamine,
1 AMIES Structure ontain the 2 group. lassification primary (1 ) amines secondary (2 ) amines tertiary (3 ) amines quarternary (4 ) ammonium salts + 1 2 3 4 Aliphatic Aromatic methylamine, ethylamine,
Chapter 20. Amines. Nomenclature for amines. Aryl amines
 Nomenclature for amines Chapter 20 Common names are widely used, named as alkylamines Systematic (IUPAC) nomenclature replaces the -e of the corresponding parent alkane with -amine Amines Simple secondary
Nomenclature for amines Chapter 20 Common names are widely used, named as alkylamines Systematic (IUPAC) nomenclature replaces the -e of the corresponding parent alkane with -amine Amines Simple secondary
Alkyl phenyl ketones are usually named by adding the acyl group as prefix to phenone.
 Aldehydes, Ketones and Carboxylic Acids Nomenclature of aldehydes and ketones Aldehydes: Often called by their common names instead of IUPAC names. Ketones: Derived by naming two alkyl or aryl groups bonded
Aldehydes, Ketones and Carboxylic Acids Nomenclature of aldehydes and ketones Aldehydes: Often called by their common names instead of IUPAC names. Ketones: Derived by naming two alkyl or aryl groups bonded
BIOB111_CHBIO - Tutorial activity for Session 10. Conceptual multiple choice questions:
 BIOB111_CHBIO - Tutorial activity for Session 10 General Topics for Session 10 Week 5 Properties of the functional groups and examples. Amines, amides and Esters Physical properties and chemical reactions:
BIOB111_CHBIO - Tutorial activity for Session 10 General Topics for Session 10 Week 5 Properties of the functional groups and examples. Amines, amides and Esters Physical properties and chemical reactions:
Chapter 19: Amines. Introduction
 Chapter 19: Amines Chap 19 HW: (be able to name amines); 37, 39, 41, 42, 44, 46, 47, 48, 53-55, 57, 58 Introduction Organic derivatives of ammonia. Many are biologically active. Chap 19: Amines Slide 19-2
Chapter 19: Amines Chap 19 HW: (be able to name amines); 37, 39, 41, 42, 44, 46, 47, 48, 53-55, 57, 58 Introduction Organic derivatives of ammonia. Many are biologically active. Chap 19: Amines Slide 19-2
All Classes of Organic Compounds
 Amines All Classes of Organic Compounds ydrocarbons Functionalized ydrocarbons F,Cl,Br O,S, Alkanes Alkenes Alkynes Aromatics alides -O- -S- -- Alcohols Phenols Ethers Thiols Dissulfides Amines O C O C
Amines All Classes of Organic Compounds ydrocarbons Functionalized ydrocarbons F,Cl,Br O,S, Alkanes Alkenes Alkynes Aromatics alides -O- -S- -- Alcohols Phenols Ethers Thiols Dissulfides Amines O C O C
10. Amines (text )
 2009, Department of Chemistry, The University of Western Ontario 10.1 10. Amines (text 10.1 10.6) A. Structure and omenclature Amines are derivatives of ammonia (H 3 ), where one or more H atoms has been
2009, Department of Chemistry, The University of Western Ontario 10.1 10. Amines (text 10.1 10.6) A. Structure and omenclature Amines are derivatives of ammonia (H 3 ), where one or more H atoms has been
ALCOHOLS AND PHENOLS
 ALCOHOLS AND PHENOLS ALCOHOLS AND PHENOLS Alcohols contain an OH group connected to a a saturated C (sp3) They are important solvents and synthesis intermediates Phenols contain an OH group connected to
ALCOHOLS AND PHENOLS ALCOHOLS AND PHENOLS Alcohols contain an OH group connected to a a saturated C (sp3) They are important solvents and synthesis intermediates Phenols contain an OH group connected to
H H O C C O H Carboxylic Acids and Derivatives C CH 2 C. N Goalby chemrevise.org. Strength of carboxylic acids.
 19 arboxylic Acids and Derivatives Naming arboxylic acids These have the ending -oic acid but no number is necessary for the acid group as it must always be at the end of the chain. The numbering always
19 arboxylic Acids and Derivatives Naming arboxylic acids These have the ending -oic acid but no number is necessary for the acid group as it must always be at the end of the chain. The numbering always
ORGANIC CHEMISTRY. Wiley STUDY GUIDE AND SOLUTIONS MANUAL TO ACCOMPANY ROBERT G. JOHNSON JON ANTILLA ELEVENTH EDITION. University of South Florida
 STUDY GUIDE AND SOLUTIONS MANUAL TO ACCOMPANY ORGANIC CHEMISTRY ELEVENTH EDITION T. W. GRAHAM SOLOMONS University of South Florida CRAIG B. FRYHLE Pacific Lutheran University SCOTT A. SNYDER Columbia University
STUDY GUIDE AND SOLUTIONS MANUAL TO ACCOMPANY ORGANIC CHEMISTRY ELEVENTH EDITION T. W. GRAHAM SOLOMONS University of South Florida CRAIG B. FRYHLE Pacific Lutheran University SCOTT A. SNYDER Columbia University
Alkenes. Alkenes-hydrocarbons with a carbon-carbon double bond. Alkenes have the formula C n H 2n. Nomenclature
 Alkenes Alkenes-hydrocarbons with a carbon-carbon double bond. Alkenes have the formula n 2n. Nomenclature Alkenes are named in the same manner as alkanes with the following adjustments. 1. Find the longest
Alkenes Alkenes-hydrocarbons with a carbon-carbon double bond. Alkenes have the formula n 2n. Nomenclature Alkenes are named in the same manner as alkanes with the following adjustments. 1. Find the longest
KOT 222 Organic Chemistry II
 KOT 222 Organic Chemistry II Course Objectives: 1) To introduce the chemistry of alcohols and ethers. 2) To study the chemistry of functional groups. 3) To learn the chemistry of aromatic compounds and
KOT 222 Organic Chemistry II Course Objectives: 1) To introduce the chemistry of alcohols and ethers. 2) To study the chemistry of functional groups. 3) To learn the chemistry of aromatic compounds and
Organic Chemistry I Exam 3 Fall 2001 November 30, Which of the following compounds corresponds to the spectral data given below?
 . Which of the following compounds corresponds to the spectral data given below? one of these. The reaction energy diagram given below corresponds to which of the following reactions? TS TS TS Br + R RI
. Which of the following compounds corresponds to the spectral data given below? one of these. The reaction energy diagram given below corresponds to which of the following reactions? TS TS TS Br + R RI
1. LiAlH4 :.. :.. 2. H3O +
 Ch 24 Amines Description of Amines - An amine is a compound with a nitrogen atom that has single bonds to carbon and hydrogen atoms. - An uncharged nitrogen atom normally has three bonds and a lone pair.
Ch 24 Amines Description of Amines - An amine is a compound with a nitrogen atom that has single bonds to carbon and hydrogen atoms. - An uncharged nitrogen atom normally has three bonds and a lone pair.
Reducing Agents. Linda M. Sweeting 1998
 Reducing Agents Linda M. Sweeting 1998 Reduction is defined in chemistry as loss of oxygen, gain of hydrogen or gain of electrons; the gain of electrons enables you to calculate an oxidation state. Hydride
Reducing Agents Linda M. Sweeting 1998 Reduction is defined in chemistry as loss of oxygen, gain of hydrogen or gain of electrons; the gain of electrons enables you to calculate an oxidation state. Hydride
Chapters 2 & 25: Covalent bonds & Organic Chemistry
 hapters 2 & 25: ovalent bonds & Organic hemistry Read: BLB 2.6, 2.9; 25.1-25.4 (only nomenclature in Table 25.1, NOT reactions) W: BLB 2:43, 45, 69, 76, 77 BLB 25:11, 12, 25, 40a, c-f Packet Organic:1
hapters 2 & 25: ovalent bonds & Organic hemistry Read: BLB 2.6, 2.9; 25.1-25.4 (only nomenclature in Table 25.1, NOT reactions) W: BLB 2:43, 45, 69, 76, 77 BLB 25:11, 12, 25, 40a, c-f Packet Organic:1
Carboxylic Acids and Nitriles
 Carboxylic Acids and Nitriles Why this Chapter? Carboxylic acids present in many industrial processes and most biological processes They are the starting materials from which other acyl derivatives are
Carboxylic Acids and Nitriles Why this Chapter? Carboxylic acids present in many industrial processes and most biological processes They are the starting materials from which other acyl derivatives are
Alcohols, Ethers and Epoxides. Chapter Organic Chemistry, 8th Edition John McMurry
 Alcohols, Ethers and Epoxides Chapter 17-18 Organic Chemistry, 8th Edition John McMurry 1 Introduction Structure and Bonding Alcohols contain a hydroxy group (OH) bonded to an sp 3 hybridized carbon. 2
Alcohols, Ethers and Epoxides Chapter 17-18 Organic Chemistry, 8th Edition John McMurry 1 Introduction Structure and Bonding Alcohols contain a hydroxy group (OH) bonded to an sp 3 hybridized carbon. 2
2Dstructuredrawing Chem314 Beauchamp
 2Dstructuredrawing hem314 Beauchamp 3 2 3 3 2 2 3 2 2 3 2 2 (neutral) (cation) (anion) (free radical) use zig-zag drawing for sp 3 chains 1 o carbocation 1 o carbanion 1 o free radical 3 3 3 3 3 3 (cation)
2Dstructuredrawing hem314 Beauchamp 3 2 3 3 2 2 3 2 2 3 2 2 (neutral) (cation) (anion) (free radical) use zig-zag drawing for sp 3 chains 1 o carbocation 1 o carbanion 1 o free radical 3 3 3 3 3 3 (cation)
Module 4 revision guide: Compounds with C=O group
 opyright N Goalby Bancroft's School Module 4 revision guide: ompounds with = group arbonyls: Aldehydes and Ketones arbonyls are compounds with a = bond, they can be either aldehydes or ketones. 3 ethanal
opyright N Goalby Bancroft's School Module 4 revision guide: ompounds with = group arbonyls: Aldehydes and Ketones arbonyls are compounds with a = bond, they can be either aldehydes or ketones. 3 ethanal
Carboxylic acid derivatives
 arboxylic acid derivatives Acyl hlorides and Acid Anhydrides N Goalby hemrevise.org arboxylic acid derivatives carboxylic acid (ethanoic acid) 3 l Acyl hloride (ethanoyl chloride) key 3 3 Acid Anhydride
arboxylic acid derivatives Acyl hlorides and Acid Anhydrides N Goalby hemrevise.org arboxylic acid derivatives carboxylic acid (ethanoic acid) 3 l Acyl hloride (ethanoyl chloride) key 3 3 Acid Anhydride
Class XII: Chemistry Chapter 13: Amines Top concepts
 Class XII: Chemistry Chapter 13: Amines Top concepts 1. Amines are regarded as derivatives of ammonia in which one, two or all three hydrogen atoms are replaced by alkyl or aryl group 2. Classification
Class XII: Chemistry Chapter 13: Amines Top concepts 1. Amines are regarded as derivatives of ammonia in which one, two or all three hydrogen atoms are replaced by alkyl or aryl group 2. Classification
Chimica Farmaceutica
 Chimica Farmaceutica Drug Targets Why should chemicals, some of which have remarkably simple structures, have such an important effect «in such a complicated and large structure as a human being? The answer
Chimica Farmaceutica Drug Targets Why should chemicals, some of which have remarkably simple structures, have such an important effect «in such a complicated and large structure as a human being? The answer
Carboxylic Acid Derivatives and Nucleophilic Acyl Substitution Reactions. McMurray Text Chapter 21
 Carboxylic Acid Derivatives and Nucleophilic Acyl Substitution eactions McMurray Text Chapter 21 Carboxylic Acid Derivatives X Acid alide Ester ' Acid Anhydride ' N 2 Amide (1 ) Nomenclature Acid alides
Carboxylic Acid Derivatives and Nucleophilic Acyl Substitution eactions McMurray Text Chapter 21 Carboxylic Acid Derivatives X Acid alide Ester ' Acid Anhydride ' N 2 Amide (1 ) Nomenclature Acid alides
Lecture 13A 05/11/12. Amines. [Sn2; Hofmann elimination; reduction of alkyl azides, amides, nitriles, imines; reductive amination; Gabriel synthesis]
![Lecture 13A 05/11/12. Amines. [Sn2; Hofmann elimination; reduction of alkyl azides, amides, nitriles, imines; reductive amination; Gabriel synthesis] Lecture 13A 05/11/12. Amines. [Sn2; Hofmann elimination; reduction of alkyl azides, amides, nitriles, imines; reductive amination; Gabriel synthesis]](/thumbs/86/93838793.jpg) Lecture 13A 05/11/12 Amines [Sn2; ofmann elimination; reduction of alkyl azides, amides, nitriles, imines; reductive amination; Gabriel synthesis] Curtius and ofmann rearrangements Both of these, in principle,
Lecture 13A 05/11/12 Amines [Sn2; ofmann elimination; reduction of alkyl azides, amides, nitriles, imines; reductive amination; Gabriel synthesis] Curtius and ofmann rearrangements Both of these, in principle,
CHEM 203. Final Exam December 15, 2010 ANSWERS. This a closed-notes, closed-book exam. You may use your set of molecular models
 CEM 203 Final Exam December 15, 2010 Your name: ANSWERS This a closed-notes, closed-book exam You may use your set of molecular models This test contains 15 pages Time: 2h 30 min 1. / 16 2. / 15 3. / 24
CEM 203 Final Exam December 15, 2010 Your name: ANSWERS This a closed-notes, closed-book exam You may use your set of molecular models This test contains 15 pages Time: 2h 30 min 1. / 16 2. / 15 3. / 24
75. A This is a Markovnikov addition reaction. In these reactions, the pielectrons in the alkene act as a nucleophile. The strongest electrophile will
 71. B SN2 stands for substitution nucleophilic bimolecular. This means that there is a bimolecular rate-determining step. Therefore, the reaction will follow second-order kinetics based on the collision
71. B SN2 stands for substitution nucleophilic bimolecular. This means that there is a bimolecular rate-determining step. Therefore, the reaction will follow second-order kinetics based on the collision
Amines and Heterocycles. McMurry: Chapter 24
 Amines and Heterocycles McMurry: Chapter 24 Introduction to Amines and Heterocycles Amines and heterocycles (cyclic amines) are ammonia derivatives, many of whichare found widely in livingorganisms: 2
Amines and Heterocycles McMurry: Chapter 24 Introduction to Amines and Heterocycles Amines and heterocycles (cyclic amines) are ammonia derivatives, many of whichare found widely in livingorganisms: 2
