CHARACTERIZATION OF INTERCALATED SMECTITES USING XRD PROFILE ANALYSIS IN THE LOW-ANGLE REGION
|
|
|
- Silas Austin
- 6 years ago
- Views:
Transcription
1 Clays and Clay Minerals, Vol. 46, No. l, CHARACTERIZATION OF INTERCALATED SMECTITES USING XRD PROFILE ANALYSIS IN THE LOW-ANGLE REGION DANIEL JANEBA, 1 PAVLA CAPKOVA,! ZDENEK WEISS 2 AND HENK SCHENK 3 I Faculty of Mathematics and Physics, Charles University Prague, Ke Karlovu 5, Prague, Czech Republic 2 Central Analytical Laboratory, Technical University of Mining and Metallurgy, Ostrava, Czech Republic 3 Laboratory of Crystallography, University of Amsterdam, Nieuwe Achtergracht 166, 118 WV Amsterdam, The Netherlands Abstract---X-ray diffraction (XRD) characterization of natural and intercalated smectites is usually limited to the apparent d-value estimated from the peak maxima in the raw data. This can lead to the misinterpretation of the measured data. In the case of XRD, the interference function is modulated by instrumental factors (Lorentz-polarization factor, diffraction geometry) and physical factors (structure factor, surface roughness effect). These effects lead to diffraction profile distortions, depending on the diffraction angle and peak full width at half maximum (FWHM). As a result, the diffraction profiles for structures with large line broadening (FWHM > 1 ~ exhibit a significant peak shift (Ad A), especially at low angles (2 --< 1~ The present work deals with the detailed analysis of all these effects, their corrections and their consequences for the interpretation of diffraction patterns (including possible errors in determining lattice parameters or the structure model). The investigated materials were montmorillonites (MMT) intercalated with hydroxy-a1 polymers. Diffraction profile analysis revealed the corrected d-values and showed that the intercalated sample is not a mixed-layered structure. As a result a structural model of the interlayer is presented. Key Words--Montmorillonite, Profile Analysis, Smectite, XRD. INTRODUCTION The search for highly efficient sorbents and catalysts has led to the development of 2-dimensional molecular sieve-type materials based on clays. These new classes of materials, known as pillared interlayer clays (PILC), are generally prepared by intercalation of smectites with inorganic polymeric cations (Mitchell 199). The technology of PILC synthesis requires careful monitoring based on the accurate and reliable characterization of prepared material. X-ray diffraction (XRD) characterization of original and pillared montmorillonites presented in the literature is usually limited to the apparent d-values estimated from the peak maxima in the raw data (Figueras et al. 199; Hsu 1992). It will be shown that this method of analysis can lead to misinterpretations of measured data. For structures exhibiting diffraction line broadening, the low-angle region of a diffraction pattern shows certain characteristic distortions arising from instrumental factors such as Lorentz-polarization factor or the diffraction geometry and physical factors such as the structure factor or the surface roughness effect. The distortions are a continuous function of diffraction angle, regardless of the cause of the line broadening. This work analyzes these effects and discusses potential consequences for interpretation of diffraction patterns such as the incorrect determination of lattice parameters or possible incorrect conclusions concerning structural disorder or phase identification. A method to determine the d-value of basal planes in the montmorillonite structure (that is, determination of the lattice parameter c) is given. This method of profile analysis can also help to determine the phase composition of intercalated smecrite (that is, to distinguish an interstratified structure with an irregular mixing of layers of 2 different d- values corresponding to the intercalated and nonintercalated smectites from a mixture of 2 segregated phases, intercalated and nonintercalated). THEORY Turbostratic arrangements of silicate layers in certain clay minerals, notably in those of the smectite group, strongly affect the diffraction pattern. Consequently the powder diffraction pattern shows mainly basal (OOl) reflections and 2-dimensional hk diffraction bands. These hk bands are not well suited to structure analysis, and the identification and characterization of intercalated clays is mainly based on the basal reflections. The (/) line broadening may be caused by distortion of the silicate layers, by small particle size and by irregular mixing of layers of different basal spacings in the layer stacking. Moreover, the intensities are reduced by thermal motion. As a result of all these effects, only 2 or 3 basal reflections are usually observable and the information contained in the diffraction pattern is very limited. The present work deals with profile analysis of the (l) series. The integrated intensity I1 and profile intensity /(2) of basal (/) reflections are (in case of powder sample) given by: Copyright , The Clay Minerals Society 63
2 64 Janeba, Capkov& Weiss and Schenk Clays and Clay Minerals I; = S LplFl21A [1] I(2) = S LplF[2ACb [23 where S is a scale, Lp is the Lorentz polarization factor, ]b-j2/ is the structure factor squared, A is an absorption factor (representing the volume absorption, surface absorption and the absorption in the sample holder) and qb is the profile interference function. In the case of narrow diffraction lines we can assume that the angle-dependent terms (Lp, If'-12, A) do not change across the line width and therefore the line position can be taken as the position of maximum intensity. If the diffraction profiles are broadened, the angle dependence of these factors must be taken into account (Reynolds 198). The considerable change of the line profiles, and the consequent shift of the peak maxima to lower 2, have been observed after the correction of experimental diffraction diagrams for these angledependent factors (Lp, [b-12, A), These changes stimulated us to a more detailed investigation of these effects. Effects Leading To Diffraction Profile Distortions LORENTZ-POLARIZATION FACTOR. The Lorentz-polarization correction for Bragg-Brentano geometry (without a monochromator) is given by (see Klug and Alexander 1974): 1 + cos 2 2 Lp = 2 sin 2 cos [3] STRUCTURE FACTOR. The angular dependency of structure factor is given by: F(2) ~ /sin \ /4~rzisin.) = j=l njfj~----~)c~ ~ +i ~ /sin \ /4"rrzisin ) J=, njfj/---~--)sin ~- ~ [4, where h is the wavelength, nj is the number of atoms of each type in unit cell in positions zj (in,~), fj is the atomic scattering amplitudes which can be calculated using the coefficients for analytical approximation to the scattering factors: f(si~ O)~ - - = L r '/sin O\ /] 2 ] aiexp -bil-----~] l +c [5] j=l and al, be, c are the coefficients taken from lbers and Hamilton (1974). VOLUME ABSORPTION. The volume absorption for the symmetrical Bragg-Brentano geometry and thick sample (t ~ % where t is the thickness) is: 1 A = -- [6] 2Ix where p~ is the absorption coefficient. This kind of absorption is angular independent and does not contrib- ute to the angular dependent corrections for thick sample. THE LOW-ANGLE DIFFRACTION GEOMETRY. As the interplanar spacing of intercalated smectites is between 15 and 2 A, the diffraction angles for (1) and (2) peaks are very low (2 < 12~ Diffraction peaks at such low angles are affected much more by the instrumental and physical factors than the peaks at higher diffraction angles and that is why more attention should be paid to their detailed analysis. Surface Roughness Effect. There is strong absorption by the rough surface of the sample which affects especially the intensity of diffraction peaks at lower 2 angles. If the surface is modeled as a surface with cubes upon it, the radiation has to pass the cube before reaching the detector and the intensity (Berg and Wachters, personal communication 1992) is: l(a, [3) = 1(9 ~ 9~ - e(cot et + cot [3)] [7] e = Nd2[1 - exp(-lxd)] [8] where N is the number of cubes per unit area, d is the edge of the cube, c~, [3 --< 9 ~ a + [3 = 2, et is the angle between the incident beam and the surface of the sample, [3 is the angle between the diffracted beam and the surface of the sample and Ix is the absorption coefficient. The Primary Beam Intensity. At low angles the trace of the primary beam is very large, even larger than the area of the sample, and part of the primary beam irradiates the sample holder. At higher angles the trace is getting smaller and the whole primary beam irradiates only the sample. At Bragg-Brentano geometry the change of the trace of the primary beam is given by: 1/sin [9] This effect can be replaced when using the automatic divergence slit. The Absorption Of The Sample Holder. Part of the diffracted beam is absorbed by the sample holder which also affects the diffraction profile. EXPERIMENTAL X-ray powder diffraction diagrams were obtained using a Philips powder diffractometer with a vertical goniometer, using CuKet radiation and a Ni filter. A step size of.2 ~ in 2 and a measuring time of 2 s per step were used to record the diffraction pattern. The divergence slit was V6 ~ and.1 mm scatter and receiving slit were used. The radius of the goniometer was 17 cm. As the fiat sample holder is in the horizontal position during the measurement, powder can be pressed into the sample holder without any binding agent. The
3 Vol. 46, No. 1, 1998 Intercalated smectites: XRD profile analysis ////," 17.5 (I. ~ 1' '1'5 2' 2'5 3' s'5 4 FWHM (~ Figure 1. Magnitude of the peak shift Ad after the Lp correction for 3 different d-values as a function of peak width for CuKct (d = 18,~, triangles; d = 15.2 A, circles; d = 12.5,~, squares). of the peak shift is higher for peaks with larger FWHM and for peaks at lower diffraction angles. Specific examples are discussed below. The Lorentz-Polarization Factor We calculated peak position shifts caused by the Lorentz-polarization factor for different FWHMs for 3 different peaks. Diffraction profiles were simulated (Rafaja 1988) for different particle sizes and different values of microstrain, and the correction for the Lorentz-polarization factor was carried out. The peak position shifts are shown in Figure 1. It can be seen that the shift is much more significant for lower diffraction angles and higher FWHM. This is exactly the case of intercalated smectites where the (1) line corresponds to d-values between 15 and 2,~ and is broadened (see above). samples prepared in this fashion show l preferred orientation. Natural Na-MMT was intercalated in an aqueous solution in which Na + ions were replaced by Al-hydroxy complexes. The intercalated sample contained 25% Na-MMT as determined by chemical analysis. Chemical analysis showed 2.66 A1 atoms in interlayer per unit cell corresponding to either Keggin 5 + ions [AllaO4(OH)26(H2)1] 5+ (described by Johansson 1962) or to gibbsite-type double-ring sandwiches [A12(OH)54(H2)22] 6+. For structure models see Gapkovti et al. (1998). Computer simulations (CERIUS 2) gave the same d-values [d(1) = 19.6,~] for both (Capkov~ et al. 1998). We assumed that the structure of clay sheet did not change during the process of intercalation. RESULT AND DISCUSSION The above-described corrections were investigated and then applied to the measured diffractogram of intercalated montmorillonite. Each of the effects (instrumental or physical) is expressed by a mathematical function that modifies the interference function. All of the described effects represent multiplicative influences to the profile function. To obtain the profile function it is necessary to correct the measured data using the described correcting functions. This operation leads to a change in the shape of the profile and the peak positions may be shifted. The magnitude of the shift of peak positions is proportional to the FWHM of the peak and also to the d-value, and the direction of the shift depends on the behavior of the correcting function. Strictly speaking, the magnitude of the shift is given by the first derivative of the correcting function and the direction of the peak shift depends on the sign of the first derivative of the correcting function. For example, steeply decreasing Lp dependence shifts the peak position to higher 2 angles. Also, the magnitude The Structure Factor A knowledge of the structure model is necessary for the structure factor correction. There are large differences in angular dependencies of the structure factor for different types of Al-hydroxy cations. Two cations were assumed in the interlayer: the Keggin 5+ and gibbsite-like double-ring sandwich. The structure factor calculations used structural models from molecular simulations as reported by Capkov~i et al. (1998). As the angular dependence of the square of structure factor for montmorillonite intercalated with Al-hydroxy polymers is not monotonic, the diffraction peaks (1) and (2) can be shifted in opposite directions depending on the sign of the derivative of the structure factor curve: if the sign of derivative is negative, the peak position is shifted to higher angles, as in the case of Lorentz-polarization factor, whereas if the sign of the derivative of the correcting function is positive, the peak position is shifted to lower angles. The angular dependences of the square of the structure factor for Na-MMT, MMT intercalated with Keggin 5 + and MMT intercalated with gibbsite-like double-ring sandwiches are shown in Figure 2. The Low-Angle Diffraction Geometry To get reasonable results, it is necessary to take into account the specific features of the low-angle geometry, as follows. THE ABSORPTION OF THE SAMPLE HOLDER. The sample was placed in a square-shaped plastic holder (see Figure 3). Part of the diffracted radiation is absorbed by the holder so the irradiated volume of sample is not equal to the volume from which the diffracted radiation is detected. The diffracting volume for Bragg-Brentano geometry is in the shape of a prism with a triangular base and the volume is proportional to tan. THE SURFACE ROUGHNESS EFFECT, To get a correcting function describing the surface roughness effect, we
4 66 Janeba, Capkov~i, Weiss and Schenk Clays and Clay Minerals MMT intercalated wi~ Keggin ',... Na-MMT t ",, - - MK/IT in tercalated with x~ 1 te-ring sandwiches ~n ~ S a m p l e Holder 2 I t 12 Angle 2 Figure 2. The angular dependences of the structure factor for MMT (Na-MMT, MMT intercalated with Keggin 5+ cation and MMT intercalated with gibbsite-type double-ring sandwich). The concentration of A1 is 2.66 atoms per unit interlayer. assumed that we are dealing with an ideal rough surface. For such a surface, half of the surface area is covered by cubes. The absorption coefficient for AL- MMT is tx = 52.2 cm J (the value was calculated according to the chemical composition). The cube edge was assumed to be d = 5 Ixm, which is the edge of the cubic grains of our sample. Then the intensity IBB for symmetrical Bragg-Brentano geometry (from Equations [7] and [8]) is given by: IBB = 1 -- cot [1] The Profile Analysis of Powder Diffractogram of Intercalated Montmorillonite With all these corrections together, the detailed profile analysis of intercalated montmorillonite was made possible. The measured diffractogram without any corrections and the diffractogram corrected for the Lp factor are shown in Figure 4. Many authors use the raw data or the Lp-corrected diagram for lattice parameters estimation which might cause the differences in d-values published in literature. The corresponding d-values are included in Table 1. The peaks were located using the computer program Difpatan (Ku~el 199). The misfit of the d-values indicates that the more detailed profile analysis is necessary. Our aim was to obtain an interference function that is not affected by any instrumental or physical factors and which can give the most accurate information about the d-values. To obtain the interference function, the above-discussed effects were taken into account and the correcting functions were taken as indicated above: Equation [3] for Lorentzpolarization factor, Equation [4] for the square of the structure factor, Equation [9] for the primary beam intensity and Equation [1] for the surface roughness effect. The absorption in the sample holder was taken Figure 3. The absorption of sample holder for Bragg-Brentano geometry: the actual diffracting volume is a prism with a triangular base, v is the depth of penetration, is the diffraction angle. to be dependent on tan, and the volume absorption was taken to be angular independent. The correction for the square of the structure factor was done for both models of the interlayer, that is, for the Keggin 5+ cation and for the gibbsite-type doublering sandwich (Figure 5). We obtained an "impossi OO '~ 15 c c: 1 5OO ~2 2 o = 4.74 ~ a) ~ d = 1.18 ~ A angle 2 (o) 2 = 4.98 ~ f 5 d1 = A 4 / ~ 2o = 8.82 ~ 3 1 o b) angle 2 (o) Figure 4. a) Measured profile of MMT intercalated with gihbsite-type double-ring sandwich (concentration 2.66 A1 atoms per unit interlayer, Bragg-Brentano geometry); b) profile of MMT intercalated with gibbsite-type double-ring sandwich after the Loreutz-polarization factor correction.
5 Vol. 46, No. 1, 1998 Intercalated smectites: XRD profile analysis 67 Table 1. Diffraction line positions and corresponding d-values for (1) and (2) line of intercalated MMT in raw data and after corrections. Measured profile Lp-corrected profile Interference function for gibbsite-type double-ring sandwich (Na-MMT included) Interference function for gibbsite-type double-ring sandwich (Na-MMT not included) Simulated interference function for mixed structure 75% A1-MMT + 25% Na-MMT (oot) (OO2) Position Position (~ d(1) (A) (~ 2d(2) (A) ble" interference function from the correction for Keggin 5+ cation with an artificial peak which does not have any physical meaning and indicates an incorrect structural model of the interlayer. In addition 27A1 NMR spectroscopy did not indicate the tetrahedral coordination of AI typical for Keggin cations. In contrast, the peak positions for the gibbsite-type doublering sandwich correction fit perfectly with the computer simulations. Moreover, the d(1) and 2d(2) are very similar to each other (they are in the range of errors estimated as.2,~). This is in the case when 25% of Na-MMT is included in the structure factor calculation (d-values in Table 1), as the chemical analysis showed 25% of nonintercalated Na-MMT. The presence of a nonintercalated Na-MMT causes a strong asymmetry of the (1) peak as seen in Figure 4. The resulting interference function (after all corrections) for MMT intercalated with gibbsite-type doublering sandwiches is shown in Figure 6. The peak positions and profiles of the interference function were compared with the calculated peak positions and profiles of the interference function of a 2- phase AI-MMT + Na-MMT sample [d(1) = ~ 3 == angle 2 (o) Figure 5. Correction for structure factor: solid line = data corrected for MMT intercalated with gibbsite-type doublering sandwich; dashed line = data corrected for MMT intercalated with Keggin 5 + cation. The extra peak in the interference function indicates an incorrect structural model of interlayer.,~, d(1) = 12.5.~] and with peak positions and profiles simulated for a mixed structure [75% AI-MMT with d(1) = 19.5,~ and 25% Na-MMT with d(1) = 12.5,~] (using formulas from Seul and Torney 1989). The peak positions of the measured interference function match with the calculated positions for a 2- phase sample, but not with the peak positions for a mixed-layered structure (Table 1). Consequently, we can conclude that there is no evidence that this sample is a mixed structure. The interference function was simulated using the computer program Simul (Rafaja 1988) for different particle sizes and for different values of microstrain, and the simulated interference function was corrected in the opposite way using the same system of corrections (as used to correct the measured data). The aim was to get a diffraction profile similar to that which we measured. Good agreement was achieved for particle sizes 12,~ and microstrain Ad/d =.1 for A1- MMT, and for particle size 15 tk and Ad/d =.1 for the nonintercalated Na-MMT. The comparison of the calculated and measured profiles is in Figure 7. CONCLUSIONS Present results confirm that XRD profile analysis of clay minerals exhibiting diffraction line broadening 1.8 "~ o.a 1.6 f-- 2a = 4.59 o 14 /~ o=.=19.2sa 1.2? / \ ~ 1 =,r-- 2,=o3o~. A ~ ~ ; ; angle 2 (o) Figure 6. Resulting interference function for MMT intercalated with gibbsite-type double-ring sandwich, 25% of nonintercalated Na-MMT included in the structure factor.
6 68 Janeba, Capkov~i, Weiss and Schenk Clays and Clay Minerals 14O O 12 1O 8OOO " 6 4 2O AI-MMT (gibbsite ring in interlayer) D=12 A, Ad/d=.1... Na-MMT D=15 A, ~dld'=.1 angle 2 (o) 2 ~ Measured '~" I 15 / 5 ~ / ~ angle 2 (~) Figure 7. Calculated profile for a 2-phase sample (AI-MMT + Na-MMT) and measured profile (after background correction) of AI-MMT containing 25% of nonintercalated Na-MMT. should include the corrections for all the angle-dependent factors in the intensity formula. For conclusions concerning the structural characteristics the consequences of neglecting these corrections were illustrated quantitatively. Lattice spacings may differ significantly from those corresponding to the raw data or to the Lp-corrected data. For a correct interpretation of diffraction data and a reliable conclusion about the structure, correction of the diffraction pattern is necessary. This profile analysis can also detect an incorrect model as shown for the Keggin 5+ cation in the interlayer. This may occur because of different angular dependences of the square of the structure factor. The shape of the corrected profiles (the shape of the interference function) yields additional information about the phase homogenity of the sample, which is especially interesting in the case of intercalation. The asymmetry of the (1) peak indicates that both forms, the A1 intercalated MMT and the nonintercalated Na- MMT, are present in the sample. This conclusion is supported also by chemical analysis. ACKNOWLEDGMENTS This work was supported by the Grant Agency of the Czech Republic (25/94/468), the Grant Agency of the Charles University (33) and by the US-Czechoslovak Science and Technology Joint Fund grant No The authors are thankful to the crystallographers A. J. v, d. Berg and J. J. Wachters for submitting their work concerning surface roughness effect (personal communication, 1992). REFERENCES Capkov~i R Driessen RAJ, Numan M, Schenk H, Weiss Z, Klika Z Molecular simulations of the montmorillonite intercalated with aluminium complex cations. Parts I and II. Clays Clay Miner: in press. Figueras E Klapyta Z, Massiani R Mountassir Z, Tichit D, Fajula E Gueguen C, Bousquet J, Auroux A Use of competetive ion exchange for intercalation of montrnorillonite with hydroxy-aluminium species. Clays Clay Miner 38: Hsu PH Reaction of OH-AI polymers with smectites and vermiculites. Clays Clay Miner 4:3-35. lbers JA, Hamilton WC International tables for X-ray crystallography, Vol. IV--Revised and supplementary tables. Birmingham: Kynoch Pr. 366 p. Johansson G The crystal structures of [A12(OH)2(H2)8] (SO4)z.2H2 and [AI~(OHh(H2)s](SeO4)2-2HzO. Acta Chem Scand 16: Klug HE Alexander LE X-ray diffraction procedures for polycrystalline and amorphous materials. New York: Wiley Intersci Publ. 966 p. Ku~el R Difpatan [computer program], Faculty of Math and Physics, Charles Univ, Prague, Czech Republic; available from kuzel@karlov.mff.cuni.cz. Mitchell WI Pillared layered structures. London: Elsevier. 252 p. Rafaja D Simul [computer program], Faculty of Math and Physics, Charles Univ, Prague, Czech Republic; available from rafaja@karlov.mff.cuni.cz. Reynolds RC. 198: Interstratified clay minerals. In: Brindley GW, Brown G, editors. Crystal structures of clay minerals and their X-ray identification. London: Mineral Soc. Seul M, Torney DC Statistical theory of X-ray scattering from crystals of finite size with pure displacement disorder in one dimension. Acta Crystallogr A45: (Received 25 October 1996; accepted 3 April 1997; Ms. 2823)
MOLECULAR SIMULATIONS OF MONTMORILLONITE INTERCALATED WITH ALUMINUM COMPLEX CATIONS. PART II: INTERCALATION WITH AI(OH)3-FRAGMENT POLYMERS
 Clays and Clay Minerals. Vol. 46. No. 3, 240-244, 1998. MOLECULAR SIMULATIONS OF MONTMORILLONITE INTERCALATED WITH ALUMINUM COMPLEX CATIONS. PART II: INTERCALATION WITH AI(OH)3-FRAGMENT POLYMERS P. CAPK()V./~,
Clays and Clay Minerals. Vol. 46. No. 3, 240-244, 1998. MOLECULAR SIMULATIONS OF MONTMORILLONITE INTERCALATED WITH ALUMINUM COMPLEX CATIONS. PART II: INTERCALATION WITH AI(OH)3-FRAGMENT POLYMERS P. CAPK()V./~,
ANALYSIS OF LOW MASS ABSORPTION MATERIALS USING GLANCING INCIDENCE X-RAY DIFFRACTION
 173 ANALYSIS OF LOW MASS ABSORPTION MATERIALS USING GLANCING INCIDENCE X-RAY DIFFRACTION N. A. Raftery, L. K. Bekessy, and J. Bowpitt Faculty of Science, Queensland University of Technology, GPO Box 2434,
173 ANALYSIS OF LOW MASS ABSORPTION MATERIALS USING GLANCING INCIDENCE X-RAY DIFFRACTION N. A. Raftery, L. K. Bekessy, and J. Bowpitt Faculty of Science, Queensland University of Technology, GPO Box 2434,
Setting The motor that rotates the sample about an axis normal to the diffraction plane is called (or ).
 X-Ray Diffraction X-ray diffraction geometry A simple X-ray diffraction (XRD) experiment might be set up as shown below. We need a parallel X-ray source, which is usually an X-ray tube in a fixed position
X-Ray Diffraction X-ray diffraction geometry A simple X-ray diffraction (XRD) experiment might be set up as shown below. We need a parallel X-ray source, which is usually an X-ray tube in a fixed position
Basics of XRD part III
 Basics of XRD part III Dr. Peter G. Weidler Institute of Functional Interfaces IFG 1 10/31/17 KIT The Research University of the Helmholtz Association Name of Institute, Faculty, Department www.kit.edu
Basics of XRD part III Dr. Peter G. Weidler Institute of Functional Interfaces IFG 1 10/31/17 KIT The Research University of the Helmholtz Association Name of Institute, Faculty, Department www.kit.edu
The effect of isomorphous substitutions on the intensities of (OO1) reflections of mica- and chlorite-type structures.
 657 The effect of isomorphous substitutions on the intensities of (OO1) reflections of mica- and chlorite-type structures. By GEORGE BROWN', B.Sc. Pedology Department, Rothamsted Experimental Station,
657 The effect of isomorphous substitutions on the intensities of (OO1) reflections of mica- and chlorite-type structures. By GEORGE BROWN', B.Sc. Pedology Department, Rothamsted Experimental Station,
THE USE OF PIPERIDINE AS AN AID TO CLAY-MINERAL IDENTIFICATION
 THE USE OF PIPERIDINE AS AN AID TO CLAY-MINERAL IDENTIFICATION By J. M. OADES* and W. N. TOWNSEND Department of Agriculture, The University of Leeds. [Received 30th August, 1962] ABSTRACT It is suggested
THE USE OF PIPERIDINE AS AN AID TO CLAY-MINERAL IDENTIFICATION By J. M. OADES* and W. N. TOWNSEND Department of Agriculture, The University of Leeds. [Received 30th August, 1962] ABSTRACT It is suggested
X-rays. X-ray Radiography - absorption is a function of Z and density. X-ray crystallography. X-ray spectrometry
 X-rays Wilhelm K. Roentgen (1845-1923) NP in Physics 1901 X-ray Radiography - absorption is a function of Z and density X-ray crystallography X-ray spectrometry X-rays Cu K α E = 8.05 kev λ = 1.541 Å Interaction
X-rays Wilhelm K. Roentgen (1845-1923) NP in Physics 1901 X-ray Radiography - absorption is a function of Z and density X-ray crystallography X-ray spectrometry X-rays Cu K α E = 8.05 kev λ = 1.541 Å Interaction
Lecture 23 X-Ray & UV Techniques
 Lecture 23 X-Ray & UV Techniques Schroder: Chapter 11.3 1/50 Announcements Homework 6/6: Will be online on later today. Due Wednesday June 6th at 10:00am. I will return it at the final exam (14 th June).
Lecture 23 X-Ray & UV Techniques Schroder: Chapter 11.3 1/50 Announcements Homework 6/6: Will be online on later today. Due Wednesday June 6th at 10:00am. I will return it at the final exam (14 th June).
Study on Magnetic Properties of Vermiculite Intercalation compounds
 Study on Magnetic Properties of Vermiculite Intercalation compounds M. Suzuki and I.S. Suzuki Department of Physics, State University of New York at Binghamton (October, ) I. INTRODUCTION In recent years
Study on Magnetic Properties of Vermiculite Intercalation compounds M. Suzuki and I.S. Suzuki Department of Physics, State University of New York at Binghamton (October, ) I. INTRODUCTION In recent years
Structure analysis of montmorillonite intercalated with rhodamine B: modeling and experiment
 J Mol Model (2003) 9:39 46 DOI 10.1007/s00894-002-0107-8 ORIGINAL PAPER Miroslav Pospíšil Pavla Čapková Helena Weissmannová Zdeněk Klika Miroslava Trchová Marta Chmielová Zdeněk Weiss Structure analysis
J Mol Model (2003) 9:39 46 DOI 10.1007/s00894-002-0107-8 ORIGINAL PAPER Miroslav Pospíšil Pavla Čapková Helena Weissmannová Zdeněk Klika Miroslava Trchová Marta Chmielová Zdeněk Weiss Structure analysis
SOLID STATE 18. Reciprocal Space
 SOLID STATE 8 Reciprocal Space Wave vectors and the concept of K-space can simplify the explanation of several properties of the solid state. They will be introduced to provide more information on diffraction
SOLID STATE 8 Reciprocal Space Wave vectors and the concept of K-space can simplify the explanation of several properties of the solid state. They will be introduced to provide more information on diffraction
Analysis of Clays and Soils by XRD
 Analysis of Clays and Soils by XRD I. Introduction Proper sample preparation is one of the most important requirements in the analysis of powder samples by X-ray diffraction (XRD). This statement is especially
Analysis of Clays and Soils by XRD I. Introduction Proper sample preparation is one of the most important requirements in the analysis of powder samples by X-ray diffraction (XRD). This statement is especially
Crystal Structure Determination II
 Crystal Structure Determination II Dr. Falak Sher Pakistan Institute of Engineering and Applied Sciences 09/10/2010 Diffraction Intensities The integrated intensity, I (hkl) (peak area) of each powder
Crystal Structure Determination II Dr. Falak Sher Pakistan Institute of Engineering and Applied Sciences 09/10/2010 Diffraction Intensities The integrated intensity, I (hkl) (peak area) of each powder
Introduction to X-ray and neutron scattering
 UNESCO/IUPAC Postgraduate Course in Polymer Science Lecture: Introduction to X-ray and neutron scattering Zhigunov Alexander Institute of Macromolecular Chemistry ASCR, Heyrovsky sq., Prague -16 06 http://www.imc.cas.cz/unesco/index.html
UNESCO/IUPAC Postgraduate Course in Polymer Science Lecture: Introduction to X-ray and neutron scattering Zhigunov Alexander Institute of Macromolecular Chemistry ASCR, Heyrovsky sq., Prague -16 06 http://www.imc.cas.cz/unesco/index.html
CRYSTAL STRUCTURE MODELING OF A HIGHLY DISORDERED POTASSIUM BIRNESSITE
 Clays and Clay Minerals, Vol. 44, No. 6, 744-748, 1996. CRYSTAL STRUCTURE MODELING OF A HIGHLY DISORDERED POTASSIUM BIRNESSITE KERRY L. HOLLANDt AND JEFFREY R. WALKER Department of Geology and Geography,
Clays and Clay Minerals, Vol. 44, No. 6, 744-748, 1996. CRYSTAL STRUCTURE MODELING OF A HIGHLY DISORDERED POTASSIUM BIRNESSITE KERRY L. HOLLANDt AND JEFFREY R. WALKER Department of Geology and Geography,
X-ray diffraction geometry
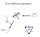 X-ray diffraction geometry Setting controls sample orientation in the diffraction plane. most important for single-crystal diffraction For any poly- (or nano-) crystalline specimen, we usually set: 1 X-ray
X-ray diffraction geometry Setting controls sample orientation in the diffraction plane. most important for single-crystal diffraction For any poly- (or nano-) crystalline specimen, we usually set: 1 X-ray
X-ray, Neutron and e-beam scattering
 X-ray, Neutron and e-beam scattering Introduction Why scattering? Diffraction basics Neutrons and x-rays Techniques Direct and reciprocal space Single crystals Powders CaFe 2 As 2 an example What is the
X-ray, Neutron and e-beam scattering Introduction Why scattering? Diffraction basics Neutrons and x-rays Techniques Direct and reciprocal space Single crystals Powders CaFe 2 As 2 an example What is the
NAME GEOL FORENSIC GEOLOGY X-RAY DIFFRACTION AND FORENSIC GEOLOGY
 NAME GEOL.2150 - FORENSIC GEOLOGY X-RAY DIFFRACTION AND FORENSIC GEOLOGY I. Introduction Minerals are crystalline solids. Individual minerals are distinguished on the basis of chemistry and the way in
NAME GEOL.2150 - FORENSIC GEOLOGY X-RAY DIFFRACTION AND FORENSIC GEOLOGY I. Introduction Minerals are crystalline solids. Individual minerals are distinguished on the basis of chemistry and the way in
An Introduction to Diffraction and Scattering. School of Chemistry The University of Sydney
 An Introduction to Diffraction and Scattering Brendan J. Kennedy School of Chemistry The University of Sydney 1) Strong forces 2) Weak forces Types of Forces 3) Electromagnetic forces 4) Gravity Types
An Introduction to Diffraction and Scattering Brendan J. Kennedy School of Chemistry The University of Sydney 1) Strong forces 2) Weak forces Types of Forces 3) Electromagnetic forces 4) Gravity Types
RIETVELD REFINEMENT WITH XRD AND ND: ANALYSIS OF METASTABLE QANDILITE-LIKE STRUCTURES
 Copyright JCPDS - International Centre for Diffraction Data 2004, Advances in X-ray Analysis, Volume 47. 261 RIETVELD REFINEMENT WITH XRD AND ND: ANALYSIS OF METASTABLE QANDILITE-LIKE STRUCTURES G. Kimmel
Copyright JCPDS - International Centre for Diffraction Data 2004, Advances in X-ray Analysis, Volume 47. 261 RIETVELD REFINEMENT WITH XRD AND ND: ANALYSIS OF METASTABLE QANDILITE-LIKE STRUCTURES G. Kimmel
Röntgenpraktikum. M. Oehzelt. (based on the diploma thesis of T. Haber [1])
![Röntgenpraktikum. M. Oehzelt. (based on the diploma thesis of T. Haber [1]) Röntgenpraktikum. M. Oehzelt. (based on the diploma thesis of T. Haber [1])](/thumbs/91/106501237.jpg) Röntgenpraktikum M. Oehzelt (based on the diploma thesis of T. Haber [1]) October 21, 2004 Contents 1 Fundamentals 2 1.1 X-Ray Radiation......................... 2 1.1.1 Bremsstrahlung......................
Röntgenpraktikum M. Oehzelt (based on the diploma thesis of T. Haber [1]) October 21, 2004 Contents 1 Fundamentals 2 1.1 X-Ray Radiation......................... 2 1.1.1 Bremsstrahlung......................
Scattering Techniques and Geometries How to choose a beamline. Christopher J. Tassone
 Scattering Techniques and Geometries How to choose a beamline Christopher J. Tassone Why Care About Geometries? How do you decide which beamline you want to use? Questions you should be asking Do I want
Scattering Techniques and Geometries How to choose a beamline Christopher J. Tassone Why Care About Geometries? How do you decide which beamline you want to use? Questions you should be asking Do I want
THE EFFECTS OF ADDING 1 M NaOH, KOH AND HCl SOLUTION TO THE FRAMEWORK STRUCTURE OF NATURAL ZEOLITE
 MATERIALS SCIENCE and TECHNOLOGY Edited by Evvy Kartini et.al. THE EFFECTS OF ADDING 1 M NaOH, KOH AND HCl SOLUTION TO THE FRAMEWORK STRUCTURE OF NATURAL ZEOLITE Supandi Suminta, Supardi and Parikin Center
MATERIALS SCIENCE and TECHNOLOGY Edited by Evvy Kartini et.al. THE EFFECTS OF ADDING 1 M NaOH, KOH AND HCl SOLUTION TO THE FRAMEWORK STRUCTURE OF NATURAL ZEOLITE Supandi Suminta, Supardi and Parikin Center
The Controlled Evolution of a Polymer Single Crystal
 Supporting Online Material The Controlled Evolution of a Polymer Single Crystal Xiaogang Liu, 1 Yi Zhang, 1 Dipak K. Goswami, 2 John S. Okasinski, 2 Khalid Salaita, 1 Peng Sun, 1 Michael J. Bedzyk, 2 Chad
Supporting Online Material The Controlled Evolution of a Polymer Single Crystal Xiaogang Liu, 1 Yi Zhang, 1 Dipak K. Goswami, 2 John S. Okasinski, 2 Khalid Salaita, 1 Peng Sun, 1 Michael J. Bedzyk, 2 Chad
CHARACTERIZATION A NICKEL HYDROXIDE-VERMICULITE COMPLEX: PREPARATION AND
 Clays and Clay Minerals, Vol. 47, No. 6, 726-731, 1999. A NICKEL HYDROXIDE-VERMICULITE COMPLEX: PREPARATION AND CHARACTERIZATION MOTOKI UEHARA, 1 ATSUSHI YAMZAKI, 2 TAKU UMEZAWA, 3 KOICHIRO TAKAHASHI,
Clays and Clay Minerals, Vol. 47, No. 6, 726-731, 1999. A NICKEL HYDROXIDE-VERMICULITE COMPLEX: PREPARATION AND CHARACTERIZATION MOTOKI UEHARA, 1 ATSUSHI YAMZAKI, 2 TAKU UMEZAWA, 3 KOICHIRO TAKAHASHI,
Crystal Structure and Electron Diffraction
 Crystal Structure and Electron Diffraction References: Kittel C.: Introduction to Solid State Physics, 8 th ed. Wiley 005 University of Michigan, PHY441-44 (Advanced Physics Laboratory Experiments, Electron
Crystal Structure and Electron Diffraction References: Kittel C.: Introduction to Solid State Physics, 8 th ed. Wiley 005 University of Michigan, PHY441-44 (Advanced Physics Laboratory Experiments, Electron
Lesson 2 Diffractometers & Phase Identification
 Lesson 2 Diffractometers & Phase Identification Nicola Döbelin RMS Foundation, Bettlach, Switzerland February 11 14, 2013, Riga, Latvia Repetition: Generation of X-rays Kα 1 Target (Cu, Mo, Fe, Co,...)
Lesson 2 Diffractometers & Phase Identification Nicola Döbelin RMS Foundation, Bettlach, Switzerland February 11 14, 2013, Riga, Latvia Repetition: Generation of X-rays Kα 1 Target (Cu, Mo, Fe, Co,...)
FORMATION OF CRYSTAL STRUCTURES DURING ACTIVATED CARBON PRODUCTION FROM TURKISH ELBISTAN LIGNITE
 FORMATION OF CRYSTAL STRUCTURES DURING ACTIVATED CARBON PRODUCTION FROM TURKISH ELBISTAN LIGNITE Billur Sakintuna 1, Sevil Çetinkaya 2, and Yuda Yürüm 1 1 Sabanci University Faculty of Engineering and
FORMATION OF CRYSTAL STRUCTURES DURING ACTIVATED CARBON PRODUCTION FROM TURKISH ELBISTAN LIGNITE Billur Sakintuna 1, Sevil Çetinkaya 2, and Yuda Yürüm 1 1 Sabanci University Faculty of Engineering and
Study of the Phase Composition of Fe 2 O 3 Nanoparticles
 WDS'9 Proceedings of Contributed Papers, Part III, 28 212, 29. ISBN 978-8-7378-13-3 MATFYZPRESS Study of the Phase Composition of Fe 2 O 3 Nanoparticles V. Valeš, J. Poltierová-Vejpravová, A. Mantlíková,
WDS'9 Proceedings of Contributed Papers, Part III, 28 212, 29. ISBN 978-8-7378-13-3 MATFYZPRESS Study of the Phase Composition of Fe 2 O 3 Nanoparticles V. Valeš, J. Poltierová-Vejpravová, A. Mantlíková,
Data Acquisition. What choices need to be made?
 1 Specimen type and preparation Radiation source Wavelength Instrument geometry Detector type Instrument setup Scan parameters 2 Specimen type and preparation Slide mount Front loading cavity Back loading
1 Specimen type and preparation Radiation source Wavelength Instrument geometry Detector type Instrument setup Scan parameters 2 Specimen type and preparation Slide mount Front loading cavity Back loading
MONTMORILLONITE AND VERMICULITE MODIFIED BY N-VINYLCAPROLACTAM AND POLY(N-VINYLCAPROLACTAM) PREPARATION AND CHARACTERIZATION
 MONTMORILLONITE AND VERMICULITE MODIFIED BY N-VINYLCAPROLACTAM AND POLY(N-VINYLCAPROLACTAM) PREPARATION AND CHARACTERIZATION Lenka PAZOURKOVÁ 1,2, Gražyna SIMHA MARTYNKOVÁ 1,2, Marianna HUNDÁKOVÁ 1,2,
MONTMORILLONITE AND VERMICULITE MODIFIED BY N-VINYLCAPROLACTAM AND POLY(N-VINYLCAPROLACTAM) PREPARATION AND CHARACTERIZATION Lenka PAZOURKOVÁ 1,2, Gražyna SIMHA MARTYNKOVÁ 1,2, Marianna HUNDÁKOVÁ 1,2,
Crystals, X-rays and Proteins
 Crystals, X-rays and Proteins Comprehensive Protein Crystallography Dennis Sherwood MA (Hons), MPhil, PhD Jon Cooper BA (Hons), PhD OXFORD UNIVERSITY PRESS Contents List of symbols xiv PART I FUNDAMENTALS
Crystals, X-rays and Proteins Comprehensive Protein Crystallography Dennis Sherwood MA (Hons), MPhil, PhD Jon Cooper BA (Hons), PhD OXFORD UNIVERSITY PRESS Contents List of symbols xiv PART I FUNDAMENTALS
Chapter 1 X-ray Absorption Fine Structure (EXAFS)
 1 Chapter 1 X-ray Absorption Fine Structure (EXAFS) 1.1 What is EXAFS? X-ray absorption fine structure (EXAFS, XAFS) is an oscillatory modulation in the X-ray absorption coefficient on the high-energy
1 Chapter 1 X-ray Absorption Fine Structure (EXAFS) 1.1 What is EXAFS? X-ray absorption fine structure (EXAFS, XAFS) is an oscillatory modulation in the X-ray absorption coefficient on the high-energy
DELAMINATION OF NATURAL VERMICULITE USING OXALIC ACID
 DELAMINATION OF NATURAL VERMICULITE USING OXALIC ACID Petra Majorová a, Jana Seidlerová a, Gražyna Simha Martynková a, Eva Gryčová a, a Centrum nanotechnologií VŠB TU Ostrava, 17.listopadu 15, 708 33,
DELAMINATION OF NATURAL VERMICULITE USING OXALIC ACID Petra Majorová a, Jana Seidlerová a, Gražyna Simha Martynková a, Eva Gryčová a, a Centrum nanotechnologií VŠB TU Ostrava, 17.listopadu 15, 708 33,
NOTE. Separation of chlorophenols using columns of hydroxyaluminium interlayered clays
 Clay Minerals (1997) 32, 143-147 NOTE Separation of chlorophenols using columns of hydroxyaluminium interlayered clays Clay minerals play an important role in the retention, transport and chemistry of
Clay Minerals (1997) 32, 143-147 NOTE Separation of chlorophenols using columns of hydroxyaluminium interlayered clays Clay minerals play an important role in the retention, transport and chemistry of
Analysis of Nanostructured Materials Ph.D. Course*
 Int. J. Engng Ed. Vol. 18, No. 5, pp. 532±538, 2002 0949-149X/91 $3.00+0.00 Printed in Great Britain. # 2002 TEMPUS Publications. Analysis of Nanostructured Materials Ph.D. Course* Z. WEISS, P. WYSLYCH,
Int. J. Engng Ed. Vol. 18, No. 5, pp. 532±538, 2002 0949-149X/91 $3.00+0.00 Printed in Great Britain. # 2002 TEMPUS Publications. Analysis of Nanostructured Materials Ph.D. Course* Z. WEISS, P. WYSLYCH,
THE IMPORTANCE OF THE SPECIMEN DISPLACEMENT CORRECTION IN RIETVELD PATTERN FITTING WITH SYMMETRIC REFLECTION-OPTICS DIFFRACTION DATA
 Copyright(c)JCPDS-International Centre for Diffraction Data 2001,Advances in X-ray Analysis,Vol.44 96 THE IMPORTANCE OF THE SPECIMEN DISPLACEMENT CORRECTION IN RIETVELD PATTERN FITTING WITH SYMMETRIC REFLECTION-OPTICS
Copyright(c)JCPDS-International Centre for Diffraction Data 2001,Advances in X-ray Analysis,Vol.44 96 THE IMPORTANCE OF THE SPECIMEN DISPLACEMENT CORRECTION IN RIETVELD PATTERN FITTING WITH SYMMETRIC REFLECTION-OPTICS
IMPROVING THE ACCURACY OF RIETVELD-DERIVED LATTICE PARAMETERS BY AN ORDER OF MAGNITUDE
 Copyright (c)jcpds-international Centre for Diffraction Data 2002, Advances in X-ray Analysis, Volume 45. 158 IMPROVING THE ACCURACY OF RIETVELD-DERIVED LATTICE PARAMETERS BY AN ORDER OF MAGNITUDE B. H.
Copyright (c)jcpds-international Centre for Diffraction Data 2002, Advances in X-ray Analysis, Volume 45. 158 IMPROVING THE ACCURACY OF RIETVELD-DERIVED LATTICE PARAMETERS BY AN ORDER OF MAGNITUDE B. H.
research papers 1. Introduction 2. Experimental E. Rossmanith, a * A Hupe, a R. Kurtz, a H. Schmidt a and H.-G. Krane b
 Journal of Applied Crystallography ISSN 0021-8898 Received 14 September 2000 Accepted 17 January 2001 Kinematical two-dimensional multiple-diffraction intensity profiles. Application to x±w scans of silicon
Journal of Applied Crystallography ISSN 0021-8898 Received 14 September 2000 Accepted 17 January 2001 Kinematical two-dimensional multiple-diffraction intensity profiles. Application to x±w scans of silicon
Scattering Lecture. February 24, 2014
 Scattering Lecture February 24, 2014 Structure Determination by Scattering Waves of radiation scattered by different objects interfere to give rise to an observable pattern! The wavelength needs to close
Scattering Lecture February 24, 2014 Structure Determination by Scattering Waves of radiation scattered by different objects interfere to give rise to an observable pattern! The wavelength needs to close
Effect of EcSS 3000 on Expansive Clays
 Effect of EcSS 3000 on Expansive Clays R. Malek Director, Particle Characterization Laboratory, Materials Research Institute, Pennsylvania State University, University Park, PA 16802. RQM@PSU.EDU (814)
Effect of EcSS 3000 on Expansive Clays R. Malek Director, Particle Characterization Laboratory, Materials Research Institute, Pennsylvania State University, University Park, PA 16802. RQM@PSU.EDU (814)
NOTE TOSUDITE CRYSTALLIZATION IN THE KAOLINIZED GRANITIC CUPOLA OF MONTEBRAS, CREUSE, FRANCE
 Clay Minerals (1986) 21, 225-230 225 NOTE TOSUDITE CRYSTALLIZATION IN THE KAOLINIZED GRANITIC CUPOLA OF MONTEBRAS, CREUSE, FRANCE Albite, muscovite granite and greisens of the Montebras cupola, Creuse,
Clay Minerals (1986) 21, 225-230 225 NOTE TOSUDITE CRYSTALLIZATION IN THE KAOLINIZED GRANITIC CUPOLA OF MONTEBRAS, CREUSE, FRANCE Albite, muscovite granite and greisens of the Montebras cupola, Creuse,
Analytical Methods for Materials
 Analytical Methods for Materials Laboratory Module # Crystal Structure Determination for Non-Cubic Crystals Suggested Reading 1. Y. Waseda, E. Matsubara, and K. Shinoda, X-ray Diffraction Crystallography,
Analytical Methods for Materials Laboratory Module # Crystal Structure Determination for Non-Cubic Crystals Suggested Reading 1. Y. Waseda, E. Matsubara, and K. Shinoda, X-ray Diffraction Crystallography,
DIFFRACTION PHYSICS THIRD REVISED EDITION JOHN M. COWLEY. Regents' Professor enzeritus Arizona State University
 DIFFRACTION PHYSICS THIRD REVISED EDITION JOHN M. COWLEY Regents' Professor enzeritus Arizona State University 1995 ELSEVIER Amsterdam Lausanne New York Oxford Shannon Tokyo CONTENTS Preface to the first
DIFFRACTION PHYSICS THIRD REVISED EDITION JOHN M. COWLEY Regents' Professor enzeritus Arizona State University 1995 ELSEVIER Amsterdam Lausanne New York Oxford Shannon Tokyo CONTENTS Preface to the first
Kirchhoff prestack depth migration in velocity models with and without rotation of the tensor of elastic moduli: Orthorhombic and triclinic anisotropy
 Kirchhoff prestack depth migration in velocity models with and without rotation of the tensor of elastic moduli: Orthorhombic and triclinic anisotropy Václav Bucha Department of Geophysics, Faculty of
Kirchhoff prestack depth migration in velocity models with and without rotation of the tensor of elastic moduli: Orthorhombic and triclinic anisotropy Václav Bucha Department of Geophysics, Faculty of
Characterization. of solid catalysts. 7. X-ray Absorption. XANES and EXAFS. Prof dr J W (Hans) Niemantsverdriet.
 www.catalysiscourse.com Characterization of solid catalysts 7. X-ray Absorption XANES and EXAFS Prof dr J W (Hans) Niemantsverdriet Schuit Institute of Catalysis J.W. Niemantsverdriet, TU/e, Eindhoven,
www.catalysiscourse.com Characterization of solid catalysts 7. X-ray Absorption XANES and EXAFS Prof dr J W (Hans) Niemantsverdriet Schuit Institute of Catalysis J.W. Niemantsverdriet, TU/e, Eindhoven,
The Reciprocal Lattice
 59-553 The Reciprocal Lattice 61 Because of the reciprocal nature of d spacings and θ from Bragg s Law, the pattern of the diffraction we observe can be related to the crystal lattice by a mathematical
59-553 The Reciprocal Lattice 61 Because of the reciprocal nature of d spacings and θ from Bragg s Law, the pattern of the diffraction we observe can be related to the crystal lattice by a mathematical
Surface Sensitivity & Surface Specificity
 Surface Sensitivity & Surface Specificity The problems of sensitivity and detection limits are common to all forms of spectroscopy. In its simplest form, the question of sensitivity boils down to whether
Surface Sensitivity & Surface Specificity The problems of sensitivity and detection limits are common to all forms of spectroscopy. In its simplest form, the question of sensitivity boils down to whether
Transmission Electron Microscopy and Diffractometry of Materials
 Brent Fultz James Howe Transmission Electron Microscopy and Diffractometry of Materials Fourth Edition ~Springer 1 1 Diffraction and the X-Ray Powder Diffractometer 1 1.1 Diffraction... 1 1.1.1 Introduction
Brent Fultz James Howe Transmission Electron Microscopy and Diffractometry of Materials Fourth Edition ~Springer 1 1 Diffraction and the X-Ray Powder Diffractometer 1 1.1 Diffraction... 1 1.1.1 Introduction
Effects of sample transparency in powder diffraction
 Effects of sample transparency in powder diffraction Derk Reefman Philips Research Laboratories, Prof. Holstlaan 4, 5656 AA Eindhoven, the Netherlands (Received 26 June 1995; accepted 15 December 1995)
Effects of sample transparency in powder diffraction Derk Reefman Philips Research Laboratories, Prof. Holstlaan 4, 5656 AA Eindhoven, the Netherlands (Received 26 June 1995; accepted 15 December 1995)
EXPERT SYSTEM FOR STRUCTURAL CHARACTERIZATION OF PHYLLOSILICATES: II. APPLICATION TO MIXED-LAYER MINERALS
 Clay Minerals (1994) 29, 39-45 EXPERT SYSTEM FOR STRUCTURAL CHARACTERIZATION OF PHYLLOSILICATES: II. APPLICATION TO MIXED-LAYER MINERALS V.A. DRITS AND A. PLAN~ON* Geological Institute, Academy of Sciences,
Clay Minerals (1994) 29, 39-45 EXPERT SYSTEM FOR STRUCTURAL CHARACTERIZATION OF PHYLLOSILICATES: II. APPLICATION TO MIXED-LAYER MINERALS V.A. DRITS AND A. PLAN~ON* Geological Institute, Academy of Sciences,
High-Resolution Neutron Diffraction Monochromators for Neutron Diffractometry
 High-Resolution Neutron Diffraction Monochromators for Neutron Diffractometry Pavol Mikula, Nuclear Physics Institute ASCR 25 68 Řež near Prague, Czech Republic NMI3-Meeting, Barcelona, 21 Motivation Backscattering
High-Resolution Neutron Diffraction Monochromators for Neutron Diffractometry Pavol Mikula, Nuclear Physics Institute ASCR 25 68 Řež near Prague, Czech Republic NMI3-Meeting, Barcelona, 21 Motivation Backscattering
Structure Analysis by Small-Angle X-Ray and Neutron Scattering
 Structure Analysis by Small-Angle X-Ray and Neutron Scattering L. A. Feigin and D. I. Svergun Institute of Crystallography Academy of Sciences of the USSR Moscow, USSR Edited by George W. Taylor Princeton
Structure Analysis by Small-Angle X-Ray and Neutron Scattering L. A. Feigin and D. I. Svergun Institute of Crystallography Academy of Sciences of the USSR Moscow, USSR Edited by George W. Taylor Princeton
WORKED EXAMPLES IN X-RAY ANALYSIS
 WORKED EXAMPLES IN X-RAY ANALYSIS PHILIPS TECHNICAL LIBRARY WORKED EXAMPLES IN X-RAY ANALYSIS R. JENKINS J.L. DE VRIES Springer Science+Business Media, LLC Springer Science+Business Media New York 1970
WORKED EXAMPLES IN X-RAY ANALYSIS PHILIPS TECHNICAL LIBRARY WORKED EXAMPLES IN X-RAY ANALYSIS R. JENKINS J.L. DE VRIES Springer Science+Business Media, LLC Springer Science+Business Media New York 1970
SAMANTHA GORHAM FRANK H. MORRELL CAMPUS ADVISOR: Prof. TREVOR A. TYSON (NJIT)
 SAMANTHA GORHAM FRANK H. MORRELL CAMPUS ADVISOR: Prof. TREVOR A. TYSON (NJIT) I WANT TO THANK PROFESSOR TREVOR A. TYSON FOR HIS HELP IN ASSISTING ME THROUGHOUT THE COURSE OF THIS PRJECT AND RESEARCH. I
SAMANTHA GORHAM FRANK H. MORRELL CAMPUS ADVISOR: Prof. TREVOR A. TYSON (NJIT) I WANT TO THANK PROFESSOR TREVOR A. TYSON FOR HIS HELP IN ASSISTING ME THROUGHOUT THE COURSE OF THIS PRJECT AND RESEARCH. I
X-ray practical: Crystallography
 X-ray practical: Crystallography Aim: To familiarise oneself with the operation of Tex-X-Ometer spectrometer and to use it to determine the lattice spacing in NaCl and LiF single crystals. Background:
X-ray practical: Crystallography Aim: To familiarise oneself with the operation of Tex-X-Ometer spectrometer and to use it to determine the lattice spacing in NaCl and LiF single crystals. Background:
Soil Colloidal Chemistry. Compiled and Edited by Dr. Syed Ismail, Marthwada Agril. University Parbhani,MS, India
 Soil Colloidal Chemistry Compiled and Edited by Dr. Syed Ismail, Marthwada Agril. University Parbhani,MS, India 1 The Colloidal Fraction Introduction What is a colloid? Why this is important in understanding
Soil Colloidal Chemistry Compiled and Edited by Dr. Syed Ismail, Marthwada Agril. University Parbhani,MS, India 1 The Colloidal Fraction Introduction What is a colloid? Why this is important in understanding
PERFORMANCE OF PP/CLAY NANOCOMPOSITES WITH EDGE FUNCTIONALIZED CLAY
 PERFORMANCE OF PP/CLAY NANOCOMPOSITES WITH EDGE FUNCTIONALIZED CLAY Sharad Kumar and K. Jayaraman Department of Chemical Engineering and Materials Science Michigan State University, East Lansing, MI 48824
PERFORMANCE OF PP/CLAY NANOCOMPOSITES WITH EDGE FUNCTIONALIZED CLAY Sharad Kumar and K. Jayaraman Department of Chemical Engineering and Materials Science Michigan State University, East Lansing, MI 48824
Structure Report for J. Reibenspies
 X-ray Diffraction Laboratory Center for Chemical Characterization and Analysis Department of Chemistry Texas A & M University Structure Report for J. Reibenspies Project Name: Sucrose Date: January 29,
X-ray Diffraction Laboratory Center for Chemical Characterization and Analysis Department of Chemistry Texas A & M University Structure Report for J. Reibenspies Project Name: Sucrose Date: January 29,
CHANGES THE STRUCTURE AND CAFFEINE ADSORPTION PROPERTY OF CALCINED MONTMORILLONITE
 Geotec., Const. Mat. & Env., ISSN: 2186-2982(Print), 2186-2990(Online), Japan CHANGES THE STRUCTURE AND CAFFEINE ADSORPTION PROPERTY OF CALCINED MONTMORILLONITE Kenichiro Yamamoto 1, Takashi Shiono 1,
Geotec., Const. Mat. & Env., ISSN: 2186-2982(Print), 2186-2990(Online), Japan CHANGES THE STRUCTURE AND CAFFEINE ADSORPTION PROPERTY OF CALCINED MONTMORILLONITE Kenichiro Yamamoto 1, Takashi Shiono 1,
High-Resolution. Transmission. Electron Microscopy
 Part 4 High-Resolution Transmission Electron Microscopy 186 Significance high-resolution transmission electron microscopy (HRTEM): resolve object details smaller than 1nm (10 9 m) image the interior of
Part 4 High-Resolution Transmission Electron Microscopy 186 Significance high-resolution transmission electron microscopy (HRTEM): resolve object details smaller than 1nm (10 9 m) image the interior of
X-ray Diffraction. Interaction of Waves Reciprocal Lattice and Diffraction X-ray Scattering by Atoms The Integrated Intensity
 X-ray Diraction Interaction o Waves Reciprocal Lattice and Diraction X-ray Scattering by Atoms The Integrated Intensity Basic Principles o Interaction o Waves Periodic waves characteristic: Frequency :
X-ray Diraction Interaction o Waves Reciprocal Lattice and Diraction X-ray Scattering by Atoms The Integrated Intensity Basic Principles o Interaction o Waves Periodic waves characteristic: Frequency :
J. Med. Chem., 1996, 39(14), , DOI: /jm960098l
 J. Med. Chem., 1996, 39(14), 2835-2843, DOI:10.1021/jm960098l Terms & Conditions Electronic Supporting Information files are available without a subscription to ACS Web Editions. The American Chemical
J. Med. Chem., 1996, 39(14), 2835-2843, DOI:10.1021/jm960098l Terms & Conditions Electronic Supporting Information files are available without a subscription to ACS Web Editions. The American Chemical
ZZZZZZZZZZYZZZZZZZZZZZZZz6
 USOO5898752A United States Patent (19) 11 Patent Number: Van Der Wal (45) Date of Patent: Apr. 27, 1999 54) X-RAY ANALYSIS APPARATUS PROVIDED Primary Examiner-Craig E. Church WITH A DOUBLE COLLIMATOR MASK
USOO5898752A United States Patent (19) 11 Patent Number: Van Der Wal (45) Date of Patent: Apr. 27, 1999 54) X-RAY ANALYSIS APPARATUS PROVIDED Primary Examiner-Craig E. Church WITH A DOUBLE COLLIMATOR MASK
Data processing and reduction
 Data processing and reduction Leopoldo Suescun International School on Fundamental Crystallography 2014 May 1st, 2014 Reciprocal lattice c* b* b * dh' k' l' 1 dh' k' l' * dhkl 1 dhkl a a* 0 d hkl c bc
Data processing and reduction Leopoldo Suescun International School on Fundamental Crystallography 2014 May 1st, 2014 Reciprocal lattice c* b* b * dh' k' l' 1 dh' k' l' * dhkl 1 dhkl a a* 0 d hkl c bc
X-ray absorption at the L 2,3 edge of an anisotropic single crystal: Cadmium 0001
 PHYSICAL REVIEW B VOLUME 54, NUMBER 4 5 JULY 996-II X-ray absorption at the L 2,3 edge of an anisotropic single crystal: Cadmium 000 P. Le Fèvre Laboratoire pour l Utilisation du Rayonnement Electromagnetique
PHYSICAL REVIEW B VOLUME 54, NUMBER 4 5 JULY 996-II X-ray absorption at the L 2,3 edge of an anisotropic single crystal: Cadmium 000 P. Le Fèvre Laboratoire pour l Utilisation du Rayonnement Electromagnetique
The ideal fiber pattern exhibits 4-quadrant symmetry. In the ideal pattern the fiber axis is called the meridian, the perpendicular direction is
 Fiber diffraction is a method used to determine the structural information of a molecule by using scattering data from X-rays. Rosalind Franklin used this technique in discovering structural information
Fiber diffraction is a method used to determine the structural information of a molecule by using scattering data from X-rays. Rosalind Franklin used this technique in discovering structural information
J. PETROVIČ. Institute of Inorganic Chemistry, Slovak Academy of Sciences, Bratislava 9. Received March 13, 1969
 Isomorphous Substitution of Aluminium for Silicon in Tobermoritic Structure. II. The Mixtures Prepared from Different Starting Materials and from Gels Containing Aluminium Ion J. PETROVIČ Institute of
Isomorphous Substitution of Aluminium for Silicon in Tobermoritic Structure. II. The Mixtures Prepared from Different Starting Materials and from Gels Containing Aluminium Ion J. PETROVIČ Institute of
Chapter 2. X-ray X. Diffraction and Reciprocal Lattice. Scattering from Lattices
 Chapter. X-ray X Diffraction and Reciprocal Lattice Diffraction of waves by crystals Reciprocal Lattice Diffraction of X-rays Powder diffraction Single crystal X-ray diffraction Scattering from Lattices
Chapter. X-ray X Diffraction and Reciprocal Lattice Diffraction of waves by crystals Reciprocal Lattice Diffraction of X-rays Powder diffraction Single crystal X-ray diffraction Scattering from Lattices
General theory of diffraction
 General theory of diffraction X-rays scatter off the charge density (r), neutrons scatter off the spin density. Coherent scattering (diffraction) creates the Fourier transform of (r) from real to reciprocal
General theory of diffraction X-rays scatter off the charge density (r), neutrons scatter off the spin density. Coherent scattering (diffraction) creates the Fourier transform of (r) from real to reciprocal
PHOTOCATALYTIC DEGRADATION STUDIES OF POLYANILINE BASED ZnO-Al 2 O 3 NANOCOMPOSITE
 PHOTOCATALYTIC DEGRADATION STUDIES OF POLYANILINE BASED ZnO-Al 2 O 3 NANOCOMPOSITE Baiju V 1, Dedhila Devadathan 2, Biju R 3, Raveendran R 4 Nanoscience Research Laboratory, Department of Physics, Sree
PHOTOCATALYTIC DEGRADATION STUDIES OF POLYANILINE BASED ZnO-Al 2 O 3 NANOCOMPOSITE Baiju V 1, Dedhila Devadathan 2, Biju R 3, Raveendran R 4 Nanoscience Research Laboratory, Department of Physics, Sree
ANALYSIS OF NANOSTRUCTURED MATERIALS - Ph.D. STUDENTS COURSE
 ANALYSIS OF NANOSTRUCTURED MATERIALS - Ph.D. STUDENTS COURSE Z. Weiss 1, P. Wyslych 2, P. Èapková 3, M. Køístková 4, D. Havlová 5 Abstract The Ph.D. student course includes four areas oriented on analytical
ANALYSIS OF NANOSTRUCTURED MATERIALS - Ph.D. STUDENTS COURSE Z. Weiss 1, P. Wyslych 2, P. Èapková 3, M. Køístková 4, D. Havlová 5 Abstract The Ph.D. student course includes four areas oriented on analytical
3R Phase of MoS 2 and WS 2 Outperforms Corresponding 2H Phase for Hydrogen Evolution
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2017 3R Phase of MoS 2 and WS 2 Outperforms Corresponding 2H Phase for Hydrogen Evolution Rou Jun Toh,
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2017 3R Phase of MoS 2 and WS 2 Outperforms Corresponding 2H Phase for Hydrogen Evolution Rou Jun Toh,
The University of Hong Kong Department of Physics
 The University of Hong Kong Department of Physics Physics Laboratory PHYS3551 Introductory Solid State Physics Experiment No. 3551-2: Electron and Optical Diffraction Name: University No: This experiment
The University of Hong Kong Department of Physics Physics Laboratory PHYS3551 Introductory Solid State Physics Experiment No. 3551-2: Electron and Optical Diffraction Name: University No: This experiment
X-ray diffraction is a non-invasive method for determining many types of
 Chapter X-ray Diffraction.1 Introduction X-ray diffraction is a non-invasive method for determining many types of structural features in both crystalline and amorphous materials. In the case of single
Chapter X-ray Diffraction.1 Introduction X-ray diffraction is a non-invasive method for determining many types of structural features in both crystalline and amorphous materials. In the case of single
Morphological Characterization by Powder X-Ray Diffraction for the Proposed System xagi-(1-x)nh4i
 Morphological Characterization by Powder X-Ray Diffraction for the Proposed System xagi-(1-x)nh4i E.J. Cañate-Gonzalez 1#, W. Fong-Silva 2#, C.A. Severiche-Sierra 3&, Y.A. Marrugo-Ligardo 4&, J. Jaimes-Morales
Morphological Characterization by Powder X-Ray Diffraction for the Proposed System xagi-(1-x)nh4i E.J. Cañate-Gonzalez 1#, W. Fong-Silva 2#, C.A. Severiche-Sierra 3&, Y.A. Marrugo-Ligardo 4&, J. Jaimes-Morales
Rajesh Prasad Department of Applied Mechanics Indian Institute of Technology New Delhi
 TEQIP WORKSHOP ON HIGH RESOLUTION X-RAY AND ELECTRON DIFFRACTION, FEB 01, 2016, IIT-K. Introduction to x-ray diffraction Peak Positions and Intensities Rajesh Prasad Department of Applied Mechanics Indian
TEQIP WORKSHOP ON HIGH RESOLUTION X-RAY AND ELECTRON DIFFRACTION, FEB 01, 2016, IIT-K. Introduction to x-ray diffraction Peak Positions and Intensities Rajesh Prasad Department of Applied Mechanics Indian
Chemistry Instrumental Analysis Lecture 19 Chapter 12. Chem 4631
 Chemistry 4631 Instrumental Analysis Lecture 19 Chapter 12 There are three major techniques used for elemental analysis: Optical spectrometry Mass spectrometry X-ray spectrometry X-ray Techniques include:
Chemistry 4631 Instrumental Analysis Lecture 19 Chapter 12 There are three major techniques used for elemental analysis: Optical spectrometry Mass spectrometry X-ray spectrometry X-ray Techniques include:
Which of the following can be used to calculate the resistive force acting on the brick? D (Total for Question = 1 mark)
 1 A brick of mass 5.0 kg falls through water with an acceleration of 0.90 m s 2. Which of the following can be used to calculate the resistive force acting on the brick? A 5.0 (0.90 9.81) B 5.0 (0.90 +
1 A brick of mass 5.0 kg falls through water with an acceleration of 0.90 m s 2. Which of the following can be used to calculate the resistive force acting on the brick? A 5.0 (0.90 9.81) B 5.0 (0.90 +
XRD Intensity Calculations -Example FCC Cu (centric)
 Ihkl F hkl F hkl hkl f f Cu Cu ( e (1 e XRD Intensity Calculations -Example FCC Cu (centric) Consider Copper which is F 3 with a=3.615å; atoms in positions [0,0,0] m m [½,½,0][½,0,½][0,½,½] and l=1.54å
Ihkl F hkl F hkl hkl f f Cu Cu ( e (1 e XRD Intensity Calculations -Example FCC Cu (centric) Consider Copper which is F 3 with a=3.615å; atoms in positions [0,0,0] m m [½,½,0][½,0,½][0,½,½] and l=1.54å
X-ray Absorption Spectroscopy. Kishan K. Sinha Department of Physics and Astronomy University of Nebraska-Lincoln
 X-ray Absorption Spectroscopy Kishan K. Sinha Department of Physics and Astronomy University of Nebraska-Lincoln Interaction of X-rays with matter Incident X-ray beam Fluorescent X-rays (XRF) Scattered
X-ray Absorption Spectroscopy Kishan K. Sinha Department of Physics and Astronomy University of Nebraska-Lincoln Interaction of X-rays with matter Incident X-ray beam Fluorescent X-rays (XRF) Scattered
Clay interactions at high temperature by molecular dynamics, thermodynamic modelling and laboratory experiments and analysis
 VTT TECHNICAL RESEARCH CENTRE OF FINLAND LTD Clay interactions at high temperature by molecular dynamics, thermodynamic modelling and laboratory experiments and analysis IGD-TP 7th Exchange Forum, Cordoba,
VTT TECHNICAL RESEARCH CENTRE OF FINLAND LTD Clay interactions at high temperature by molecular dynamics, thermodynamic modelling and laboratory experiments and analysis IGD-TP 7th Exchange Forum, Cordoba,
UNIVERSITY OF SURREY DEPARTMENT OF PHYSICS. Level 1: Experiment 2F THE ABSORPTION, DIFFRACTION AND EMISSION OF X- RAY RADIATION
 UNIVERSITY OF SURREY DEPARTMENT OF PHYSICS Level 1: Experiment 2F THE ABSORPTION, DIFFRACTION AND EMISSION OF X- RAY RADIATION 1 AIMS 1.1 Physics These experiments are intended to give some experience
UNIVERSITY OF SURREY DEPARTMENT OF PHYSICS Level 1: Experiment 2F THE ABSORPTION, DIFFRACTION AND EMISSION OF X- RAY RADIATION 1 AIMS 1.1 Physics These experiments are intended to give some experience
A. BEN HAJ AMARA. L.P.M., Facultd des Sciences de Bizerte, 7021 Zarzouna, B&erte, Tunisia
 Clay Minerals (1997) 32, 46347 X-ray diffraction, analysis of infrared and TGA/DTG hydrated nacrite A. BEN HAJ AMARA L.P.M., Facultd des Sciences de Bizerte, 721 Zarzouna, B&erte, Tunisia (Received 25
Clay Minerals (1997) 32, 46347 X-ray diffraction, analysis of infrared and TGA/DTG hydrated nacrite A. BEN HAJ AMARA L.P.M., Facultd des Sciences de Bizerte, 721 Zarzouna, B&erte, Tunisia (Received 25
Diffractometer. Geometry Optics Detectors
 Diffractometer Geometry Optics Detectors Diffractometers Debye Scherrer Camera V.K. Pecharsky and P.Y. Zavalij Fundamentals of Powder Diffraction and Structural Characterization of Materials. Diffractometers
Diffractometer Geometry Optics Detectors Diffractometers Debye Scherrer Camera V.K. Pecharsky and P.Y. Zavalij Fundamentals of Powder Diffraction and Structural Characterization of Materials. Diffractometers
Copyright SOIL STRUCTURE and CLAY MINERALS
 SOIL STRUCTURE and CLAY MINERALS Soil Structure Structure of a soil may be defined as the mode of arrangement of soil grains relative to each other and the forces acting between them to hold them in their
SOIL STRUCTURE and CLAY MINERALS Soil Structure Structure of a soil may be defined as the mode of arrangement of soil grains relative to each other and the forces acting between them to hold them in their
Fourier Syntheses, Analyses, and Transforms
 Fourier Syntheses, Analyses, and Transforms http://homepages.utoledo.edu/clind/ The electron density The electron density in a crystal can be described as a periodic function - same contents in each unit
Fourier Syntheses, Analyses, and Transforms http://homepages.utoledo.edu/clind/ The electron density The electron density in a crystal can be described as a periodic function - same contents in each unit
SUPPLEMENTARY INFORMATION
 Anisotropic phase segregation and migration of Pt in nanocrystals en route to nanoframe catalysts Zhiqiang Niu 1, Nigel Becknell 1, Yi Yu 1,2, Dohyung Kim 3, Chen Chen 1,4, Nikolay Kornienko 1, Gabor A.
Anisotropic phase segregation and migration of Pt in nanocrystals en route to nanoframe catalysts Zhiqiang Niu 1, Nigel Becknell 1, Yi Yu 1,2, Dohyung Kim 3, Chen Chen 1,4, Nikolay Kornienko 1, Gabor A.
Electronic Supplementary Information (ESI) Green synthesis of shape-defined anatase TiO 2 nanocrystals wholly exposed with {001} and {100} facets
 Electronic Supplementary Information (ESI) Green synthesis of shape-defined anatase TiO 2 nanocrystals wholly exposed with {001} and {100} facets Lan Wang, a Ling Zang, b Jincai Zhao c and Chuanyi Wang*
Electronic Supplementary Information (ESI) Green synthesis of shape-defined anatase TiO 2 nanocrystals wholly exposed with {001} and {100} facets Lan Wang, a Ling Zang, b Jincai Zhao c and Chuanyi Wang*
Laser Optics-II. ME 677: Laser Material Processing Instructor: Ramesh Singh 1
 Laser Optics-II 1 Outline Absorption Modes Irradiance Reflectivity/Absorption Absorption coefficient will vary with the same effects as the reflectivity For opaque materials: reflectivity = 1 - absorptivity
Laser Optics-II 1 Outline Absorption Modes Irradiance Reflectivity/Absorption Absorption coefficient will vary with the same effects as the reflectivity For opaque materials: reflectivity = 1 - absorptivity
X-ray Diffraction from Materials
 X-ray Diffraction from Materials 008 Spring Semester Lecturer; Yang Mo Koo Monday and Wednesday 4:45~6:00 8. X-ray Diffractometers Diffractometer: a measuring instrument for analyzing the structure of
X-ray Diffraction from Materials 008 Spring Semester Lecturer; Yang Mo Koo Monday and Wednesday 4:45~6:00 8. X-ray Diffractometers Diffractometer: a measuring instrument for analyzing the structure of
NEW CORRECTION PROCEDURE FOR X-RAY SPECTROSCOPIC FLUORESCENCE DATA: SIMULATIONS AND EXPERIMENT
 Copyright JCPDS - International Centre for Diffraction Data 2005, Advances in X-ray Analysis, Volume 48. 266 NEW CORRECTION PROCEDURE FOR X-RAY SPECTROSCOPIC FLUORESCENCE DATA: SIMULATIONS AND EXPERIMENT
Copyright JCPDS - International Centre for Diffraction Data 2005, Advances in X-ray Analysis, Volume 48. 266 NEW CORRECTION PROCEDURE FOR X-RAY SPECTROSCOPIC FLUORESCENCE DATA: SIMULATIONS AND EXPERIMENT
Lecture 6. Physical Properties. Solid Phase. Particle Composition
 Lecture 6 Physical Properties Solid Phase Particle Composition 1 Questions What are tetrahedrons and octahedrons? How do silica tetrahedra bonds affect mineral weathering? Difference between primary and
Lecture 6 Physical Properties Solid Phase Particle Composition 1 Questions What are tetrahedrons and octahedrons? How do silica tetrahedra bonds affect mineral weathering? Difference between primary and
Protein crystallography. Garry Taylor
 Protein crystallography Garry Taylor X-ray Crystallography - the Basics Grow crystals Collect X-ray data Determine phases Calculate ρ-map Interpret map Refine coordinates Do the biology. Nitrogen at -180
Protein crystallography Garry Taylor X-ray Crystallography - the Basics Grow crystals Collect X-ray data Determine phases Calculate ρ-map Interpret map Refine coordinates Do the biology. Nitrogen at -180
Name :. Roll No. :... Invigilator s Signature :.. CS/B. Tech (New)/SEM-1/PH-101/ PHYSICS-I
 Name :. Roll No. :..... Invigilator s Signature :.. CS/B. Tech (New)/SEM-1/PH-101/2011-12 2011 PHYSICS-I Time Allotted : 3 Hours Full Marks : 70 The figures in the margin indicate full marks. Candidates
Name :. Roll No. :..... Invigilator s Signature :.. CS/B. Tech (New)/SEM-1/PH-101/2011-12 2011 PHYSICS-I Time Allotted : 3 Hours Full Marks : 70 The figures in the margin indicate full marks. Candidates
X Rays & Crystals. Characterizing Mineral Chemistry & Structure. J.D. Price
 X Rays & Crystals Characterizing Mineral Chemistry & Structure J.D. Price Light - electromagnetic spectrum Wave behavior vs. particle behavior If atoms are on the 10-10 m scale, we need to use sufficiently
X Rays & Crystals Characterizing Mineral Chemistry & Structure J.D. Price Light - electromagnetic spectrum Wave behavior vs. particle behavior If atoms are on the 10-10 m scale, we need to use sufficiently
THE USE OF PEARSON VII DISTRIBUTION FUNCTIONS IN X-RA Y DIFFR ACTION RESIDUAL STRESS MEASUREMENT
 THE USE OF PEARSON VII DISTRIBUTION FUNCTIONS IN X-RA Y DIFFR ACTION RESIDUAL STRESS MEASUREMENT Paul S. Prevéy Lambda Research ABSTRACT The fitting of a parabola by least squares regression to the upper
THE USE OF PEARSON VII DISTRIBUTION FUNCTIONS IN X-RA Y DIFFR ACTION RESIDUAL STRESS MEASUREMENT Paul S. Prevéy Lambda Research ABSTRACT The fitting of a parabola by least squares regression to the upper
LAB 01 X-RAY EMISSION & ABSORPTION
 LAB 0 X-RAY EMISSION & ABSORPTION REPORT BY: TEAM MEMBER NAME: Ashley Tsai LAB SECTION No. 05 GROUP 2 EXPERIMENT DATE: Feb., 204 SUBMISSION DATE: Feb. 8, 204 Page of 3 ABSTRACT The goal of this experiment
LAB 0 X-RAY EMISSION & ABSORPTION REPORT BY: TEAM MEMBER NAME: Ashley Tsai LAB SECTION No. 05 GROUP 2 EXPERIMENT DATE: Feb., 204 SUBMISSION DATE: Feb. 8, 204 Page of 3 ABSTRACT The goal of this experiment
Structure of organo-clays--an X-ray diffraction and thermogravimetric analysis study
 Structure of organo-clays--an X-ray diffraction and thermogravimetric analysis study Yunfei Xi, Zhe Ding, Ray L. Frost Inorganic Materials Research Group, School of Physical and Chemical Sciences, Queensland
Structure of organo-clays--an X-ray diffraction and thermogravimetric analysis study Yunfei Xi, Zhe Ding, Ray L. Frost Inorganic Materials Research Group, School of Physical and Chemical Sciences, Queensland
CRYSTAL STRUCTURE OF TETRAMETHYLAMMONIUM-EXCHANGED VERMICULITE
 Clays and Clay Minerals, Vol. 45, No. 6, 859-866, 1997. CRYSTAL STRUCTURE OF TETRAMETHYLAMMONIUM-EXCHANGED VERMICULITE A. VAHEDI-FARIDI AND STEPHEN GUGGENHEIM Department of Geological Sciences, University
Clays and Clay Minerals, Vol. 45, No. 6, 859-866, 1997. CRYSTAL STRUCTURE OF TETRAMETHYLAMMONIUM-EXCHANGED VERMICULITE A. VAHEDI-FARIDI AND STEPHEN GUGGENHEIM Department of Geological Sciences, University
