Lecture 23 X-Ray & UV Techniques
|
|
|
- Jonas Hunter
- 5 years ago
- Views:
Transcription
1 Lecture 23 X-Ray & UV Techniques Schroder: Chapter /50 Announcements Homework 6/6: Will be online on later today. Due Wednesday June 6th at 10:00am. I will return it at the final exam (14 th June). I will make the solutions available on June 6 th. Office Hours Next week office hours will be Today 13:00-14:00. 2/50 1
2 Announcements Final Exam The final is scheduled for Thursday June 14 th at 14:00. Strand Agriculture Hall 113. The exam will be 90 minutes long. More information will be provided next week. The next lecture with content will be today. Wednesday June 6 th will be a review lecture. I will go through some examples. There will be no lecture on Friday June 8 th. It will be treated as an opportunity to prepare for the exam. 3/50 Lecture 23 X-Ray Fluorescence. X-Ray Photoelectron Spectroscopy. Ultraviolet Photoelectron Spectroscopy. X-Ray Diffraction Spectroscopy. 4/50 2
3 X-Ray Fluorescence 5/50 X-Rays X-rays are just photons (like light). Defined in the range of λ = 10 pm to 10 nm In terms of energy this is: ~100 ev to 100 kev. A lot of materials are transparent to X-rays. Hence it is a good bulkprobe, since X-rays can travel a long way in materials. 6/50 3
4 X-Ray Fluorescence X-ray fluorescence (XRF) involves x-ray-induced ionization of inner core electrons and detection of secondary x-rays emitted as a consequence of relaxation from an excited state. X-rays hν L hν Also X-rays The x-ray energy and intensity establish the atomic identity and concentration, respectively. Atomic identification is readily accomplished, since x- ray energies are well known and x-ray lines are relatively few in number. K 7/50 X-Ray Fluorescence XRF is a bulk technique. In Si for example the penetration depth is ~ 1 10 μm. XRF is a nondestructive elemental analysis technique and is applicable to solids, liquids, and thin films. It has a sensitivity of ~0.01% or ~5 X cm -3 and an analysis area of ~1 cm 2. 8/50 4
5 Total Reflection XRF Total Reflection XRF X-ray fluorescence (TXRF) is a surface-sensitive version of XRF. We use of a grazing incidence angle of θ < 0.1. incident x-ray beam θ detector secondary x-ray beam substrate reflected x-ray beam This results in a theoretical beam penetration distance of ~2-3 nm. This may be slightly higher due to finite roughness, wafer warpage or beam divergence. 9/50 Total Reflection XRF TXRF has a detection sensitivity of ~ cm -2 for metallic impurities in silicon, which can be enhanced by about an order of magnitude by HF vapor-etching of the surface native oxide, which is allowed to dry and then analyzed by TXRF. 10/50 5
6 Applications of XRF In reality XRF is ideally suited to rapidly survey samples, for more detailed analysis later. It is non-destructive and can be used in air. Can be used for thickness measurements if standards are used. 11/50 X-Ray Photoelectron Spectroscopy 12/50 6
7 XPS X-Ray Photoelectron Spectroscopy (XPS) is sometimes also known as Electron Spectroscopy for Chemical Analysis (ESCA). We use it to evaluate elemental composition and properties of chemical bonds. As usual we fire radiation (X-Rays) at the sample and measure what comes off. In this case we measure electrons coming emitted (hence the term photoelectron). Typically energies are ~1,250 1,500 ev. We are probing core levels. 13/50 XPS Normally the X-Ray beam is large, but we can use focused beam to provide spatial information. 14/50 7
8 XPS The photon comes in and kicks off a core (e.g. L level) electron. E vacuum hν The electron then will be detected with a metal spectrometer. L K 15/50 Binding Energy Emitted electrons are energy analyzed and then plotted in terms of the binding energy E B : Where: known h = Planck Constant. E B = hν KE eφ sp measured ν = Frequency of incoming photon. KE = Kinetic energy of emitted electron. e = Fundamental unit of charge. calibrated φ sp = Work function of detector (in V). 16/50 8
9 XPS Spectrum Below is what a typical XPS spectrum looks like 17/50 Bonding The primary reason why XPS is of interest (rather than the many other elemental analysis techniques) is that it can provide information on bonding and local environment. The figure to the right shows what happens as Pb bonds with O. Schroder p /50 9
10 XPS is a Surface Technique X-rays are photons and observe the Beer Lambert law. For inelastic scattering only: Where: I x = I 0 exp x l cos θ I x = X-Ray intensity at distance x into sample. I 0 = X-Ray intensity at sample surface. l = Inelastic mean free path. θ = Angle to surface normal (0 is normal to surface). 19/50 XPS is a Surface Technique At 1000 ev, λ = 1.6 nm. For example if θ = 0 the intensity received as a function of depth could look like this: 95% of X-Ray absorption takes place within first 5nm. 95% of electrons will be emitted from first 5nm. This is a surface technique. 20/50 10
11 XPS vs AES XPS depth profiling is analogous to Auger electron spectroscopy (AES) depth profiling. However, in conventional XPS the x-ray excitation area is quite large (~mm s). Modern XPS systems offer a small-spot option (~1-100 μm), either by restricting the size of the x-ray source or modifying the detector design to accept photoelectrons from a restricted portion of the sample surface. 21/50 XPS vs AES XPS, rather than AES, is better suited to the surface assessment of delicate materials such as polymers, since x-rays induce less surface damage than electrons. In angle-resolved XPS, the surface sensitivity may be modified by varying the take-off angle. I x = I 0 exp x l cos θ XPS elemental sensitivity is ~1%. 22/50 11
12 XPS vs AES Quantitative XPS is accomplished using relative sensitivity factors and standards and has an accuracy of ~10%. The two most common XPS x-ray sources are the Kα lines of Al and Mg at ev and ev, respectively. One disadvantage of these sources are their relatively large linewidths of 0.85 ev and 0.7 ev for Al and Mg, respectively. Also, the lineshapes of these x-ray sources are asymmetric. 23/50 XPS vs AES A broad and asymmetric line results in poorer resolution. Resolution improves with the use of an x-ray monochromator to reduce the linewidth to ~0.1 ev. However, this drastically decreases the x-ray intensity and the signal-to-noise ratio. 24/50 12
13 Ultraviolet Photoelectron Spectroscopy 25/50 UPS Ultraviolet photoelectron spectroscopy (UPS) is another type of photoemission. UPS is basically the same technique as XPS, but employing ultraviolet radiation rather than X-rays. UPS is usually accomplished using a helium lamp emitting at either 21.2 ev (He I radiation) or 40.8 ev (He II radiation). He linewidths are very narrow, ~1 mev, so that resolution is determined by the analyzer. 26/50 13
14 UPS The process is the same as XPS, but we are now looking at much lower energies shallower states (including valence band states). E vacuum VB hν VB /50 UPS Data This is an example of real data. We are looking for the energy after which no electrons are emitted top of valence band. This can be tricky and subjective. Secondary electron edge Note VBE here is relative to Fermi Energy. Intensity a.u. Intensity a.u. He I Source Energy SEE ev VBE EF Evac Binding Energy w.r.t. E Fermi ev VBE Valence band edge Work Function VBE 0.82 ev EFermi Evac 4.69 ev Binding Energy w.r.t. E Fermi ev 28/50 14
15 UPS Data We can combine our knowledge of the valence band energy with other techniques. E.g. using Tauc analysis of optical absorption data can give us band gap. Together this allows us to produce a basic band structure of our material. This information is critically important for device design. 29/50 ARPES Up to now the UPS measurements that we have discussed have implicitly been angle-integrated, since most photoelectron energy analyzers accept electrons over a wide range of take-off angles. More detailed information is available from angleresolved photoemission spectroscopy (ARPES) measurements, in which you can map out the valence band structure in k-space. 30/50 15
16 ARPES Data This information can be used to reconstruct, experimentally, the density of states in the semiconductor. This is something that traditionally is calculated and direct access is very difficult. B.B.Y. Hsu, Adv. Mater. 27 (2015) /50 ARPES Data By measuring the emission as a function of angle we can evaluate the entire band structure (in k-space). T. Cuk, PRL. 93 (2004) This allows us to evaluate the effective mass of carriers in this material. 32/50 16
17 X-Ray Diffraction 33/50 XRD The final technique we will talk about is X-ray diffraction (XRD). We primarily use XRD for crystal structure determination and chemical identification. It is a routine technique for many materials (not just electronic materials) because it is reasonably quick and unambiguous. 34/50 17
18 XRD Consider a monochromatic beam of x-rays incident upon a crystal surface at an angle θ. incident θ θ reflected d Under certain circumstances this incident beam is specularly reflected from the surface. Constructive interference occurs for certain angles θ, depending on the separation of crystal planes d. 35/50 XRD The incident and reflected beams are described by their wave vectors k i and k r, respectively, where: k = 2π λ λ is wavelength of the incident X-rays. The incident x-ray beam penetrates the lattice and scatters from atoms in the lattice. If the crystal is represented as a series of parallel atomic planes (as indicated on the previous page) the incident beam is partially reflected at each plane. 36/50 18
19 Bragg s Law If the reflected rays from each plane to the detector have a difference in path length equal to an integral number of x-ray wavelengths, these rays constructively interfere, resulting in specular reflection. This requirement leads to Bragg s Law: 2d sin θ = nλ Where: n N. 37/50 Bragg s Law Note two important ramifications of Bragg s Law. First, the interplanar spacing may be found from the angle and the wavelength. Second, diffraction (i.e., scattering and constructive interference) can occur only if λ < 2d. An alternative way to express Bragg s Law involves an Ewald construction in reciprocal space. Reciprocal space is the Fourier transform of real space. 38/50 19
20 Ewald Construction An alternative way to express Bragg s Law involves an Ewald construction in reciprocal space. Reciprocal space is the Fourier transform of real space. reciprocal space k r k i Ewald sphere G hkl 39/50 Ewald Construction The Ewald construction is accomplished as follows: (i) Draw k i in reciprocal space. (ii) Use k i to generate an Ewald sphere. (iii) Draw k r as a vector from the center of the Ewald sphere to another point located on the sphere surface. (iv) Draw a vector from k i to k r, and identify this vector as the reciprocal lattice vector G hkl, where (hkl) specifies the diffraction plane Miller indices. 40/50 20
21 Ewald Construction The utility of the Ewald construction is that the diffraction condition equivalent to Bragg s law is k r k i = G hkl This equation is particularly interesting when it is recognized that: d hkl = 2π G hkl d hkl is the interplanar distance. 41/50 Bragg Reflection The geometry of Bragg reflection is summarized as follows. real space incident θ k i k r θ θ reflected G hkl d hkl = 2π d hkl G hkl 42/50 21
22 Powder XRD There are several ways to accomplish XRD, such as the powder method, shown below. focussing circle incident x-ray beam θ sample detector scattered x-ray beam 2θ rotate sample & detector relative to x-ray source in a θτ2θ manner intensity (counts) d hkl λ 2sin θ θ 43/50 Powder Database Bragg s Law specifies XRD peak locations. We can use this information to fingerprint compounds and structures. Tens of thousands of XRD powder diffraction files are available for crystal identification from the Joint Committee on Powder Diffraction Standards (JCPDS). The best known database is maintained by Cambridge University: 44/50 22
23 Real Data Typically one would calculate an XRD pattern from a known structure then compare it with experimental data. This can be used to confirm that you have the material / phase / orientation that you think you do. 45/50 Real Data Often when plotted on a linear scale a small number of peaks dominate, and obscure a lot of information. Experimental XRD specialists often will require XRD data plotted on a logarithmic scale to make convincing statements on structure. Red = experimental asterisks = unidentified peaks Blue = calculated 46/50 23
24 Scherrer Formula The width of an XRD peak also provides interesting information. The maximum grain size of a polycrystalline thin film can be estimated from the Scherrer formula: Where: d grain = 0.9λ Δ cos θ d grain = maximum grain size. Δ = full-width-at-half-maximum (in radians) of the XRD peak. 47/50 Rocking Curve For an epitaxial film, the degree of crystalline perfection is estimated from a rocking curve assessment in which the intensity of a diffraction peak is monitored as a function of a small rotation along an axis perpendicular to the sample surface. sample normal ~0.1 ω sample sample rocking 48/50 24
25 Rocking Curve sample normal ~0.1 ω sample sample rocking The sharpness of this rocking curve is a measure of crystalline perfection. A perfectly flat surface at T=0K would in theory have a delta function as its rocking curve. 49/50 Next Time Course Summary. Review Lecture for Final Exam. Exam structure / regulations. Example questions. 50/50 25
Lecture 22 Ion Beam Techniques
 Lecture 22 Ion Beam Techniques Schroder: Chapter 11.3 1/44 Announcements Homework 6/6: Will be online on later today. Due Wednesday June 6th at 10:00am. I will return it at the final exam (14 th June).
Lecture 22 Ion Beam Techniques Schroder: Chapter 11.3 1/44 Announcements Homework 6/6: Will be online on later today. Due Wednesday June 6th at 10:00am. I will return it at the final exam (14 th June).
Surface Sensitivity & Surface Specificity
 Surface Sensitivity & Surface Specificity The problems of sensitivity and detection limits are common to all forms of spectroscopy. In its simplest form, the question of sensitivity boils down to whether
Surface Sensitivity & Surface Specificity The problems of sensitivity and detection limits are common to all forms of spectroscopy. In its simplest form, the question of sensitivity boils down to whether
Electron Spectroscopy
 Electron Spectroscopy Photoelectron spectroscopy is based upon a single photon in/electron out process. The energy of a photon is given by the Einstein relation : E = h ν where h - Planck constant ( 6.62
Electron Spectroscopy Photoelectron spectroscopy is based upon a single photon in/electron out process. The energy of a photon is given by the Einstein relation : E = h ν where h - Planck constant ( 6.62
MS482 Materials Characterization ( 재료분석 ) Lecture Note 4: XRF
 2016 Fall Semester MS482 Materials Characterization ( 재료분석 ) Lecture Note 4: XRF Byungha Shin Dept. of MSE, KAIST 1 Course Information Syllabus 1. Overview of various characterization techniques (1 lecture)
2016 Fall Semester MS482 Materials Characterization ( 재료분석 ) Lecture Note 4: XRF Byungha Shin Dept. of MSE, KAIST 1 Course Information Syllabus 1. Overview of various characterization techniques (1 lecture)
Energy Spectroscopy. Excitation by means of a probe
 Energy Spectroscopy Excitation by means of a probe Energy spectral analysis of the in coming particles -> XAS or Energy spectral analysis of the out coming particles Different probes are possible: Auger
Energy Spectroscopy Excitation by means of a probe Energy spectral analysis of the in coming particles -> XAS or Energy spectral analysis of the out coming particles Different probes are possible: Auger
Methods of surface analysis
 Methods of surface analysis Nanomaterials characterisation I RNDr. Věra Vodičková, PhD. Surface of solid matter: last monoatomic layer + absorbed monolayer physical properties are effected (crystal lattice
Methods of surface analysis Nanomaterials characterisation I RNDr. Věra Vodičková, PhD. Surface of solid matter: last monoatomic layer + absorbed monolayer physical properties are effected (crystal lattice
Lecture 5. X-ray Photoemission Spectroscopy (XPS)
 Lecture 5 X-ray Photoemission Spectroscopy (XPS) 5. Photoemission Spectroscopy (XPS) 5. Principles 5.2 Interpretation 5.3 Instrumentation 5.4 XPS vs UV Photoelectron Spectroscopy (UPS) 5.5 Auger Electron
Lecture 5 X-ray Photoemission Spectroscopy (XPS) 5. Photoemission Spectroscopy (XPS) 5. Principles 5.2 Interpretation 5.3 Instrumentation 5.4 XPS vs UV Photoelectron Spectroscopy (UPS) 5.5 Auger Electron
Birck Nanotechnology Center XPS: X-ray Photoelectron Spectroscopy ESCA: Electron Spectrometer for Chemical Analysis
 Birck Nanotechnology Center XPS: X-ray Photoelectron Spectroscopy ESCA: Electron Spectrometer for Chemical Analysis Dmitry Zemlyanov Birck Nanotechnology Center, Purdue University Outline Introduction
Birck Nanotechnology Center XPS: X-ray Photoelectron Spectroscopy ESCA: Electron Spectrometer for Chemical Analysis Dmitry Zemlyanov Birck Nanotechnology Center, Purdue University Outline Introduction
Chemistry Instrumental Analysis Lecture 19 Chapter 12. Chem 4631
 Chemistry 4631 Instrumental Analysis Lecture 19 Chapter 12 There are three major techniques used for elemental analysis: Optical spectrometry Mass spectrometry X-ray spectrometry X-ray Techniques include:
Chemistry 4631 Instrumental Analysis Lecture 19 Chapter 12 There are three major techniques used for elemental analysis: Optical spectrometry Mass spectrometry X-ray spectrometry X-ray Techniques include:
An Introduction to Diffraction and Scattering. School of Chemistry The University of Sydney
 An Introduction to Diffraction and Scattering Brendan J. Kennedy School of Chemistry The University of Sydney 1) Strong forces 2) Weak forces Types of Forces 3) Electromagnetic forces 4) Gravity Types
An Introduction to Diffraction and Scattering Brendan J. Kennedy School of Chemistry The University of Sydney 1) Strong forces 2) Weak forces Types of Forces 3) Electromagnetic forces 4) Gravity Types
Photoelectron Spectroscopy. Xiaozhe Zhang 10/03/2014
 Photoelectron Spectroscopy Xiaozhe Zhang 10/03/2014 A conception last time remain Secondary electrons are electrons generated as ionization products. They are called 'secondary' because they are generated
Photoelectron Spectroscopy Xiaozhe Zhang 10/03/2014 A conception last time remain Secondary electrons are electrons generated as ionization products. They are called 'secondary' because they are generated
X-Ray Photoelectron Spectroscopy (XPS)
 X-Ray Photoelectron Spectroscopy (XPS) Louis Scudiero http://www.wsu.edu/~scudiero; 5-2669 Electron Spectroscopy for Chemical Analysis (ESCA) The basic principle of the photoelectric effect was enunciated
X-Ray Photoelectron Spectroscopy (XPS) Louis Scudiero http://www.wsu.edu/~scudiero; 5-2669 Electron Spectroscopy for Chemical Analysis (ESCA) The basic principle of the photoelectric effect was enunciated
MS482 Materials Characterization ( 재료분석 ) Lecture Note 2: UPS
 2016 Fall Semester MS482 Materials Characterization ( 재료분석 ) Lecture Note 2: UPS Byungha Shin Dept. of MSE, KAIST 1 Course Information Syllabus 1. Overview of various characterization techniques (1 lecture)
2016 Fall Semester MS482 Materials Characterization ( 재료분석 ) Lecture Note 2: UPS Byungha Shin Dept. of MSE, KAIST 1 Course Information Syllabus 1. Overview of various characterization techniques (1 lecture)
Lecture 20 Optical Characterization 2
 Lecture 20 Optical Characterization 2 Schroder: Chapters 2, 7, 10 1/68 Announcements Homework 5/6: Is online now. Due Wednesday May 30th at 10:00am. I will return it the following Wednesday (6 th June).
Lecture 20 Optical Characterization 2 Schroder: Chapters 2, 7, 10 1/68 Announcements Homework 5/6: Is online now. Due Wednesday May 30th at 10:00am. I will return it the following Wednesday (6 th June).
Film Characterization Tutorial G.J. Mankey, 01/23/04. Center for Materials for Information Technology an NSF Materials Science and Engineering Center
 Film Characterization Tutorial G.J. Mankey, 01/23/04 Theory vs. Experiment A theory is something nobody believes, except the person who made it. An experiment is something everybody believes, except the
Film Characterization Tutorial G.J. Mankey, 01/23/04 Theory vs. Experiment A theory is something nobody believes, except the person who made it. An experiment is something everybody believes, except the
X- ray Photoelectron Spectroscopy and its application in phase- switching device study
 X- ray Photoelectron Spectroscopy and its application in phase- switching device study Xinyuan Wang A53073806 I. Background X- ray photoelectron spectroscopy is of great importance in modern chemical and
X- ray Photoelectron Spectroscopy and its application in phase- switching device study Xinyuan Wang A53073806 I. Background X- ray photoelectron spectroscopy is of great importance in modern chemical and
Energy Spectroscopy. Ex.: Fe/MgO
 Energy Spectroscopy Spectroscopy gives access to the electronic properties (and thus chemistry, magnetism,..) of the investigated system with thickness dependence Ex.: Fe/MgO Fe O Mg Control of the oxidation
Energy Spectroscopy Spectroscopy gives access to the electronic properties (and thus chemistry, magnetism,..) of the investigated system with thickness dependence Ex.: Fe/MgO Fe O Mg Control of the oxidation
Photoelectron spectroscopy Instrumentation. Nanomaterials characterization 2
 Photoelectron spectroscopy Instrumentation Nanomaterials characterization 2 RNDr. Věra V Vodičkov ková,, PhD. Photoelectron Spectroscopy general scheme Impact of X-ray emitted from source to the sample
Photoelectron spectroscopy Instrumentation Nanomaterials characterization 2 RNDr. Věra V Vodičkov ková,, PhD. Photoelectron Spectroscopy general scheme Impact of X-ray emitted from source to the sample
Setting The motor that rotates the sample about an axis normal to the diffraction plane is called (or ).
 X-Ray Diffraction X-ray diffraction geometry A simple X-ray diffraction (XRD) experiment might be set up as shown below. We need a parallel X-ray source, which is usually an X-ray tube in a fixed position
X-Ray Diffraction X-ray diffraction geometry A simple X-ray diffraction (XRD) experiment might be set up as shown below. We need a parallel X-ray source, which is usually an X-ray tube in a fixed position
Silver Thin Film Characterization
 Silver Thin Film Characterization.1 Introduction Thin films of Ag layered structures, typically less than a micron in thickness, are tailored to achieve desired functional properties. Typical characterization
Silver Thin Film Characterization.1 Introduction Thin films of Ag layered structures, typically less than a micron in thickness, are tailored to achieve desired functional properties. Typical characterization
MS482 Materials Characterization ( 재료분석 ) Lecture Note 5: RBS
 2016 Fall Semester MS482 Materials Characterization ( 재료분석 ) Lecture Note 5: RBS Byungha Shin Dept. of MSE, KAIST 1 Course Information Syllabus 1. Overview of various characterization techniques (1 lecture)
2016 Fall Semester MS482 Materials Characterization ( 재료분석 ) Lecture Note 5: RBS Byungha Shin Dept. of MSE, KAIST 1 Course Information Syllabus 1. Overview of various characterization techniques (1 lecture)
The design of an integrated XPS/Raman spectroscopy instrument for co-incident analysis
 The design of an integrated XPS/Raman spectroscopy instrument for co-incident analysis Tim Nunney The world leader in serving science 2 XPS Surface Analysis XPS +... UV Photoelectron Spectroscopy UPS He(I)
The design of an integrated XPS/Raman spectroscopy instrument for co-incident analysis Tim Nunney The world leader in serving science 2 XPS Surface Analysis XPS +... UV Photoelectron Spectroscopy UPS He(I)
Introduction to X-ray Photoelectron Spectroscopy (XPS) XPS which makes use of the photoelectric effect, was developed in the mid-1960
 Introduction to X-ray Photoelectron Spectroscopy (XPS) X-ray Photoelectron Spectroscopy (XPS), also known as Electron Spectroscopy for Chemical Analysis (ESCA) is a widely used technique to investigate
Introduction to X-ray Photoelectron Spectroscopy (XPS) X-ray Photoelectron Spectroscopy (XPS), also known as Electron Spectroscopy for Chemical Analysis (ESCA) is a widely used technique to investigate
X-ray Photoelectron Spectroscopy (XPS)
 X-ray Photoelectron Spectroscopy (XPS) As part of the course Characterization of Catalysts and Surfaces Prof. Dr. Markus Ammann Paul Scherrer Institut markus.ammann@psi.ch Resource for further reading:
X-ray Photoelectron Spectroscopy (XPS) As part of the course Characterization of Catalysts and Surfaces Prof. Dr. Markus Ammann Paul Scherrer Institut markus.ammann@psi.ch Resource for further reading:
MS482 Materials Characterization ( 재료분석 ) Lecture Note 5: RBS. Byungha Shin Dept. of MSE, KAIST
 2015 Fall Semester MS482 Materials Characterization ( 재료분석 ) Lecture Note 5: RBS Byungha Shin Dept. of MSE, KAIST 1 Course Information Syllabus 1. Overview of various characterization techniques (1 lecture)
2015 Fall Semester MS482 Materials Characterization ( 재료분석 ) Lecture Note 5: RBS Byungha Shin Dept. of MSE, KAIST 1 Course Information Syllabus 1. Overview of various characterization techniques (1 lecture)
X-Ray Photoelectron Spectroscopy (XPS) Prof. Paul K. Chu
 X-Ray Photoelectron Spectroscopy (XPS) Prof. Paul K. Chu X-ray Photoelectron Spectroscopy Introduction Qualitative analysis Quantitative analysis Charging compensation Small area analysis and XPS imaging
X-Ray Photoelectron Spectroscopy (XPS) Prof. Paul K. Chu X-ray Photoelectron Spectroscopy Introduction Qualitative analysis Quantitative analysis Charging compensation Small area analysis and XPS imaging
CHARACTERIZATION of NANOMATERIALS KHP
 CHARACTERIZATION of NANOMATERIALS Overview of the most common nanocharacterization techniques MAIN CHARACTERIZATION TECHNIQUES: 1.Transmission Electron Microscope (TEM) 2. Scanning Electron Microscope
CHARACTERIZATION of NANOMATERIALS Overview of the most common nanocharacterization techniques MAIN CHARACTERIZATION TECHNIQUES: 1.Transmission Electron Microscope (TEM) 2. Scanning Electron Microscope
The photoelectric effect
 The photoelectric effect E K hν-e B E F hν E B A photoemission experiment Lifetime broadening ΔE.Δτ~ħ ΔE~ħ/Δτ + Experimental resolution Hüfner, Photoelectron Spectroscopy (Springer) A photoemission experiment
The photoelectric effect E K hν-e B E F hν E B A photoemission experiment Lifetime broadening ΔE.Δτ~ħ ΔE~ħ/Δτ + Experimental resolution Hüfner, Photoelectron Spectroscopy (Springer) A photoemission experiment
Chemical Analysis in TEM: XEDS, EELS and EFTEM. HRTEM PhD course Lecture 5
 Chemical Analysis in TEM: XEDS, EELS and EFTEM HRTEM PhD course Lecture 5 1 Part IV Subject Chapter Prio x-ray spectrometry 32 1 Spectra and mapping 33 2 Qualitative XEDS 34 1 Quantitative XEDS 35.1-35.4
Chemical Analysis in TEM: XEDS, EELS and EFTEM HRTEM PhD course Lecture 5 1 Part IV Subject Chapter Prio x-ray spectrometry 32 1 Spectra and mapping 33 2 Qualitative XEDS 34 1 Quantitative XEDS 35.1-35.4
print first name print last name print student id grade
 print first name print last name print student id grade Experiment 2 X-ray fluorescence X-ray fluorescence (XRF) and X-ray diffraction (XRD) may be used to determine the constituent elements and the crystalline
print first name print last name print student id grade Experiment 2 X-ray fluorescence X-ray fluorescence (XRF) and X-ray diffraction (XRD) may be used to determine the constituent elements and the crystalline
Good Diffraction Practice Webinar Series
 Good Diffraction Practice Webinar Series High Resolution X-ray Diffractometry (1) Mar 24, 2011 www.bruker-webinars.com Welcome Heiko Ress Global Marketing Manager Bruker AXS Inc. Madison, Wisconsin, USA
Good Diffraction Practice Webinar Series High Resolution X-ray Diffractometry (1) Mar 24, 2011 www.bruker-webinars.com Welcome Heiko Ress Global Marketing Manager Bruker AXS Inc. Madison, Wisconsin, USA
Name: (a) What core levels are responsible for the three photoelectron peaks in Fig. 1?
 Physics 243A--Surface Physics of Materials: Spectroscopy Final Examination December 16, 2014 (3 problems, 100 points total, open book, open notes and handouts) Name: [1] (50 points), including Figures
Physics 243A--Surface Physics of Materials: Spectroscopy Final Examination December 16, 2014 (3 problems, 100 points total, open book, open notes and handouts) Name: [1] (50 points), including Figures
X-Ray Photoelectron Spectroscopy (XPS)
 X-Ray Photoelectron Spectroscopy (XPS) Louis Scudiero http://www.wsu.edu/~scudiero; 5-2669 Fulmer 261A Electron Spectroscopy for Chemical Analysis (ESCA) The basic principle of the photoelectric effect
X-Ray Photoelectron Spectroscopy (XPS) Louis Scudiero http://www.wsu.edu/~scudiero; 5-2669 Fulmer 261A Electron Spectroscopy for Chemical Analysis (ESCA) The basic principle of the photoelectric effect
Röntgenpraktikum. M. Oehzelt. (based on the diploma thesis of T. Haber [1])
![Röntgenpraktikum. M. Oehzelt. (based on the diploma thesis of T. Haber [1]) Röntgenpraktikum. M. Oehzelt. (based on the diploma thesis of T. Haber [1])](/thumbs/91/106501237.jpg) Röntgenpraktikum M. Oehzelt (based on the diploma thesis of T. Haber [1]) October 21, 2004 Contents 1 Fundamentals 2 1.1 X-Ray Radiation......................... 2 1.1.1 Bremsstrahlung......................
Röntgenpraktikum M. Oehzelt (based on the diploma thesis of T. Haber [1]) October 21, 2004 Contents 1 Fundamentals 2 1.1 X-Ray Radiation......................... 2 1.1.1 Bremsstrahlung......................
V 11: Electron Diffraction
 Martin-Luther-University Halle-Wittenberg Institute of Physics Advanced Practical Lab Course V 11: Electron Diffraction An electron beam conditioned by an electron optical system is diffracted by a polycrystalline,
Martin-Luther-University Halle-Wittenberg Institute of Physics Advanced Practical Lab Course V 11: Electron Diffraction An electron beam conditioned by an electron optical system is diffracted by a polycrystalline,
Inelastic soft x-ray scattering, fluorescence and elastic radiation
 Inelastic soft x-ray scattering, fluorescence and elastic radiation What happens to the emission (or fluorescence) when the energy of the exciting photons changes? The emission spectra (can) change. One
Inelastic soft x-ray scattering, fluorescence and elastic radiation What happens to the emission (or fluorescence) when the energy of the exciting photons changes? The emission spectra (can) change. One
X Rays & Crystals. Characterizing Mineral Chemistry & Structure. J.D. Price
 X Rays & Crystals Characterizing Mineral Chemistry & Structure J.D. Price Light - electromagnetic spectrum Wave behavior vs. particle behavior If atoms are on the 10-10 m scale, we need to use sufficiently
X Rays & Crystals Characterizing Mineral Chemistry & Structure J.D. Price Light - electromagnetic spectrum Wave behavior vs. particle behavior If atoms are on the 10-10 m scale, we need to use sufficiently
X-ray diffraction geometry
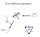 X-ray diffraction geometry Setting controls sample orientation in the diffraction plane. most important for single-crystal diffraction For any poly- (or nano-) crystalline specimen, we usually set: 1 X-ray
X-ray diffraction geometry Setting controls sample orientation in the diffraction plane. most important for single-crystal diffraction For any poly- (or nano-) crystalline specimen, we usually set: 1 X-ray
Chemistry 311: Instrumentation Analysis Topic 2: Atomic Spectroscopy. Chemistry 311: Instrumentation Analysis Topic 2: Atomic Spectroscopy
 Topic 2b: X-ray Fluorescence Spectrometry Text: Chapter 12 Rouessac (1 week) 4.0 X-ray Fluorescence Download, read and understand EPA method 6010C ICP-OES Winter 2009 Page 1 Atomic X-ray Spectrometry Fundamental
Topic 2b: X-ray Fluorescence Spectrometry Text: Chapter 12 Rouessac (1 week) 4.0 X-ray Fluorescence Download, read and understand EPA method 6010C ICP-OES Winter 2009 Page 1 Atomic X-ray Spectrometry Fundamental
Electron Microprobe Analysis and Scanning Electron Microscopy
 Electron Microprobe Analysis and Scanning Electron Microscopy Electron microprobe analysis (EMPA) Analytical technique in which a beam of electrons is focused on a sample surface, producing X-rays from
Electron Microprobe Analysis and Scanning Electron Microscopy Electron microprobe analysis (EMPA) Analytical technique in which a beam of electrons is focused on a sample surface, producing X-rays from
Interaction X-rays - Matter
 Interaction X-rays - Matter Pair production hν > M ev Photoelectric absorption hν MATTER hν Transmission X-rays hν' < hν Scattering hν Decay processes hν f Compton Thomson Fluorescence Auger electrons
Interaction X-rays - Matter Pair production hν > M ev Photoelectric absorption hν MATTER hν Transmission X-rays hν' < hν Scattering hν Decay processes hν f Compton Thomson Fluorescence Auger electrons
Spectroscopy on Mars!
 Spectroscopy on Mars! Pathfinder Spirit and Opportunity Real World Friday H2A The Mars Pathfinder: Geological Elemental Analysis On December 4th, 1996, the Mars Pathfinder was launched from earth to begin
Spectroscopy on Mars! Pathfinder Spirit and Opportunity Real World Friday H2A The Mars Pathfinder: Geological Elemental Analysis On December 4th, 1996, the Mars Pathfinder was launched from earth to begin
Structure analysis: Electron diffraction LEED TEM RHEED
 Structure analysis: Electron diffraction LEED: Low Energy Electron Diffraction SPA-LEED: Spot Profile Analysis Low Energy Electron diffraction RHEED: Reflection High Energy Electron Diffraction TEM: Transmission
Structure analysis: Electron diffraction LEED: Low Energy Electron Diffraction SPA-LEED: Spot Profile Analysis Low Energy Electron diffraction RHEED: Reflection High Energy Electron Diffraction TEM: Transmission
Fundamentals of Nanoscale Film Analysis
 Fundamentals of Nanoscale Film Analysis Terry L. Alford Arizona State University Tempe, AZ, USA Leonard C. Feldman Vanderbilt University Nashville, TN, USA James W. Mayer Arizona State University Tempe,
Fundamentals of Nanoscale Film Analysis Terry L. Alford Arizona State University Tempe, AZ, USA Leonard C. Feldman Vanderbilt University Nashville, TN, USA James W. Mayer Arizona State University Tempe,
QUESTIONS AND ANSWERS
 QUESTIONS AND ANSWERS (1) For a ground - state neutral atom with 13 protons, describe (a) Which element this is (b) The quantum numbers, n, and l of the inner two core electrons (c) The stationary state
QUESTIONS AND ANSWERS (1) For a ground - state neutral atom with 13 protons, describe (a) Which element this is (b) The quantum numbers, n, and l of the inner two core electrons (c) The stationary state
X-ray diffraction and Crystal Structure Solutions from Thin Films
 X-ray diffraction and Crystal Structure Solutions from Thin Films Ingo Salzmann Humboldt-Universität zu Berlin Institut für Physik Overview Experimental technique X-ray diffraction The principal phenomenon
X-ray diffraction and Crystal Structure Solutions from Thin Films Ingo Salzmann Humboldt-Universität zu Berlin Institut für Physik Overview Experimental technique X-ray diffraction The principal phenomenon
EE 527 MICROFABRICATION. Lecture 5 Tai-Chang Chen University of Washington
 EE 527 MICROFABRICATION Lecture 5 Tai-Chang Chen University of Washington MICROSCOPY AND VISUALIZATION Electron microscope, transmission electron microscope Resolution: atomic imaging Use: lattice spacing.
EE 527 MICROFABRICATION Lecture 5 Tai-Chang Chen University of Washington MICROSCOPY AND VISUALIZATION Electron microscope, transmission electron microscope Resolution: atomic imaging Use: lattice spacing.
X-Ray Photoelectron Spectroscopy (XPS) Auger Electron Spectroscopy (AES)
 X-Ray Photoelectron Spectroscopy (XPS) Auger Electron Spectroscopy (AES) XPS X-ray photoelectron spectroscopy (XPS) is one of the most used techniques to chemically characterize the surface. Also known
X-Ray Photoelectron Spectroscopy (XPS) Auger Electron Spectroscopy (AES) XPS X-ray photoelectron spectroscopy (XPS) is one of the most used techniques to chemically characterize the surface. Also known
Studying Metal to Insulator Transitions in Solids using Synchrotron Radiation-based Spectroscopies.
 PY482 Lecture. February 28 th, 2013 Studying Metal to Insulator Transitions in Solids using Synchrotron Radiation-based Spectroscopies. Kevin E. Smith Department of Physics Department of Chemistry Division
PY482 Lecture. February 28 th, 2013 Studying Metal to Insulator Transitions in Solids using Synchrotron Radiation-based Spectroscopies. Kevin E. Smith Department of Physics Department of Chemistry Division
Nearly Free Electron Gas model - II
 Nearly Free Electron Gas model - II Contents 1 Lattice scattering 1 1.1 Bloch waves............................ 2 1.2 Band gap formation........................ 3 1.3 Electron group velocity and effective
Nearly Free Electron Gas model - II Contents 1 Lattice scattering 1 1.1 Bloch waves............................ 2 1.2 Band gap formation........................ 3 1.3 Electron group velocity and effective
IV. Surface analysis for chemical state, chemical composition
 IV. Surface analysis for chemical state, chemical composition Probe beam Detect XPS Photon (X-ray) Photoelectron(core level electron) UPS Photon (UV) Photoelectron(valence level electron) AES electron
IV. Surface analysis for chemical state, chemical composition Probe beam Detect XPS Photon (X-ray) Photoelectron(core level electron) UPS Photon (UV) Photoelectron(valence level electron) AES electron
Because light behaves like a wave, we can describe it in one of two ways by its wavelength or by its frequency.
 Light We can use different terms to describe light: Color Wavelength Frequency Light is composed of electromagnetic waves that travel through some medium. The properties of the medium determine how light
Light We can use different terms to describe light: Color Wavelength Frequency Light is composed of electromagnetic waves that travel through some medium. The properties of the medium determine how light
PHOTOELECTRON SPECTROSCOPY (PES)
 PHOTOELECTRON SPECTROSCOPY (PES) NTRODUCTON Law of Photoelectric effect Albert Einstein, Nobel Prize 1921 Kaiser-Wilhelm-nstitut (now Max-Planck- nstitut) für Physik Berlin, Germany High-resolution electron
PHOTOELECTRON SPECTROSCOPY (PES) NTRODUCTON Law of Photoelectric effect Albert Einstein, Nobel Prize 1921 Kaiser-Wilhelm-nstitut (now Max-Planck- nstitut) für Physik Berlin, Germany High-resolution electron
Core Level Spectroscopies
 Core Level Spectroscopies Spectroscopies involving core levels are element-sensitive, and that makes them very useful for understanding chemical bonding, as well as for the study of complex materials.
Core Level Spectroscopies Spectroscopies involving core levels are element-sensitive, and that makes them very useful for understanding chemical bonding, as well as for the study of complex materials.
Welcome back to PHY 3305
 Welcome back to PHY 3305 Today s Lecture: Double Slit Experiment Matter Waves Louis-Victor-Pierre-Raymond, 7th duc de Broglie 1892-1987 AnNouncements Reading Assignment for Thursday, Sept 28th: Chapter
Welcome back to PHY 3305 Today s Lecture: Double Slit Experiment Matter Waves Louis-Victor-Pierre-Raymond, 7th duc de Broglie 1892-1987 AnNouncements Reading Assignment for Thursday, Sept 28th: Chapter
Advanced Lab Course. X-Ray Photoelectron Spectroscopy 1 INTRODUCTION 1 2 BASICS 1 3 EXPERIMENT Qualitative analysis Chemical Shifts 7
 Advanced Lab Course X-Ray Photoelectron Spectroscopy M210 As of: 2015-04-01 Aim: Chemical analysis of surfaces. Content 1 INTRODUCTION 1 2 BASICS 1 3 EXPERIMENT 3 3.1 Qualitative analysis 6 3.2 Chemical
Advanced Lab Course X-Ray Photoelectron Spectroscopy M210 As of: 2015-04-01 Aim: Chemical analysis of surfaces. Content 1 INTRODUCTION 1 2 BASICS 1 3 EXPERIMENT 3 3.1 Qualitative analysis 6 3.2 Chemical
Secondary Ion Mass Spectrometry (SIMS)
 CHEM53200: Lecture 10 Secondary Ion Mass Spectrometry (SIMS) Major reference: Surface Analysis Edited by J. C. Vickerman (1997). 1 Primary particles may be: Secondary particles can be e s, neutral species
CHEM53200: Lecture 10 Secondary Ion Mass Spectrometry (SIMS) Major reference: Surface Analysis Edited by J. C. Vickerman (1997). 1 Primary particles may be: Secondary particles can be e s, neutral species
Lecture 5: Characterization methods
 Lecture 5: Characterization methods X-Ray techniques Single crystal X-Ray Diffration (XRD) Powder XRD Thin film X-Ray Reflection (XRR) Microscopic methods Optical microscopy Electron microscopies (SEM,
Lecture 5: Characterization methods X-Ray techniques Single crystal X-Ray Diffration (XRD) Powder XRD Thin film X-Ray Reflection (XRR) Microscopic methods Optical microscopy Electron microscopies (SEM,
General theory of diffraction
 General theory of diffraction X-rays scatter off the charge density (r), neutrons scatter off the spin density. Coherent scattering (diffraction) creates the Fourier transform of (r) from real to reciprocal
General theory of diffraction X-rays scatter off the charge density (r), neutrons scatter off the spin density. Coherent scattering (diffraction) creates the Fourier transform of (r) from real to reciprocal
X-rays. X-ray Radiography - absorption is a function of Z and density. X-ray crystallography. X-ray spectrometry
 X-rays Wilhelm K. Roentgen (1845-1923) NP in Physics 1901 X-ray Radiography - absorption is a function of Z and density X-ray crystallography X-ray spectrometry X-rays Cu K α E = 8.05 kev λ = 1.541 Å Interaction
X-rays Wilhelm K. Roentgen (1845-1923) NP in Physics 1901 X-ray Radiography - absorption is a function of Z and density X-ray crystallography X-ray spectrometry X-rays Cu K α E = 8.05 kev λ = 1.541 Å Interaction
5.8 Auger Electron Spectroscopy (AES)
 5.8 Auger Electron Spectroscopy (AES) 5.8.1 The Auger Process X-ray and high energy electron bombardment of atom can create core hole Core hole will eventually decay via either (i) photon emission (x-ray
5.8 Auger Electron Spectroscopy (AES) 5.8.1 The Auger Process X-ray and high energy electron bombardment of atom can create core hole Core hole will eventually decay via either (i) photon emission (x-ray
Auger Electron Spectroscopy (AES)
 1. Introduction Auger Electron Spectroscopy (AES) Silvia Natividad, Gabriel Gonzalez and Arena Holguin Auger Electron Spectroscopy (Auger spectroscopy or AES) was developed in the late 1960's, deriving
1. Introduction Auger Electron Spectroscopy (AES) Silvia Natividad, Gabriel Gonzalez and Arena Holguin Auger Electron Spectroscopy (Auger spectroscopy or AES) was developed in the late 1960's, deriving
Chapter 4 Scintillation Detectors
 Med Phys 4RA3, 4RB3/6R03 Radioisotopes and Radiation Methodology 4-1 4.1. Basic principle of the scintillator Chapter 4 Scintillation Detectors Scintillator Light sensor Ionizing radiation Light (visible,
Med Phys 4RA3, 4RB3/6R03 Radioisotopes and Radiation Methodology 4-1 4.1. Basic principle of the scintillator Chapter 4 Scintillation Detectors Scintillator Light sensor Ionizing radiation Light (visible,
SOLID STATE 18. Reciprocal Space
 SOLID STATE 8 Reciprocal Space Wave vectors and the concept of K-space can simplify the explanation of several properties of the solid state. They will be introduced to provide more information on diffraction
SOLID STATE 8 Reciprocal Space Wave vectors and the concept of K-space can simplify the explanation of several properties of the solid state. They will be introduced to provide more information on diffraction
Electron Microscopy I
 Characterization of Catalysts and Surfaces Characterization Techniques in Heterogeneous Catalysis Electron Microscopy I Introduction Properties of electrons Electron-matter interactions and their applications
Characterization of Catalysts and Surfaces Characterization Techniques in Heterogeneous Catalysis Electron Microscopy I Introduction Properties of electrons Electron-matter interactions and their applications
Appearance Potential Spectroscopy
 Appearance Potential Spectroscopy Submitted by Sajanlal P. R CY06D009 Sreeprasad T. S CY06D008 Dept. of Chemistry IIT MADRAS February 2006 1 Contents Page number 1. Introduction 3 2. Theory of APS 3 3.
Appearance Potential Spectroscopy Submitted by Sajanlal P. R CY06D009 Sreeprasad T. S CY06D008 Dept. of Chemistry IIT MADRAS February 2006 1 Contents Page number 1. Introduction 3 2. Theory of APS 3 3.
Reference literature. (See: CHEM 2470 notes, Module 8 Textbook 6th ed., Chapters )
 September 17, 2018 Reference literature (See: CHEM 2470 notes, Module 8 Textbook 6th ed., Chapters 13-14 ) Reference.: https://slideplayer.com/slide/8354408/ Spectroscopy Usual Wavelength Type of Quantum
September 17, 2018 Reference literature (See: CHEM 2470 notes, Module 8 Textbook 6th ed., Chapters 13-14 ) Reference.: https://slideplayer.com/slide/8354408/ Spectroscopy Usual Wavelength Type of Quantum
Interaction of particles with matter - 2. Silvia Masciocchi, GSI and University of Heidelberg SS2017, Heidelberg May 3, 2017
 Interaction of particles with matter - 2 Silvia Masciocchi, GSI and University of Heidelberg SS2017, Heidelberg May 3, 2017 Energy loss by ionization (by heavy particles) Interaction of electrons with
Interaction of particles with matter - 2 Silvia Masciocchi, GSI and University of Heidelberg SS2017, Heidelberg May 3, 2017 Energy loss by ionization (by heavy particles) Interaction of electrons with
Skoog Chapter 6 Introduction to Spectrometric Methods
 Skoog Chapter 6 Introduction to Spectrometric Methods General Properties of Electromagnetic Radiation (EM) Wave Properties of EM Quantum Mechanical Properties of EM Quantitative Aspects of Spectrochemical
Skoog Chapter 6 Introduction to Spectrometric Methods General Properties of Electromagnetic Radiation (EM) Wave Properties of EM Quantum Mechanical Properties of EM Quantitative Aspects of Spectrochemical
Solid State Physics Lecture 3 Diffraction and the Reciprocal Lattice (Kittel Ch. 2)
 Solid State Physics 460 - Lecture 3 Diffraction and the Reciprocal Lattice (Kittel Ch. 2) Diffraction (Bragg Scattering) from a powder of crystallites - real example of image at right from http://www.uni-wuerzburg.de/mineralogie/crystal/teaching/pow.html
Solid State Physics 460 - Lecture 3 Diffraction and the Reciprocal Lattice (Kittel Ch. 2) Diffraction (Bragg Scattering) from a powder of crystallites - real example of image at right from http://www.uni-wuerzburg.de/mineralogie/crystal/teaching/pow.html
The Fundamentals of Spectroscopy: Theory BUILDING BETTER SCIENCE AGILENT AND YOU
 The Fundamentals of Spectroscopy: Theory BUILDING BETTER SCIENCE AGILENT AND YOU 1 Agilent is committed to the educational community and is willing to provide access to company-owned material. This slide
The Fundamentals of Spectroscopy: Theory BUILDING BETTER SCIENCE AGILENT AND YOU 1 Agilent is committed to the educational community and is willing to provide access to company-owned material. This slide
5) Surface photoelectron spectroscopy. For MChem, Spring, Dr. Qiao Chen (room 3R506) University of Sussex.
 For MChem, Spring, 2009 5) Surface photoelectron spectroscopy Dr. Qiao Chen (room 3R506) http://www.sussex.ac.uk/users/qc25/ University of Sussex Today s topics 1. Element analysis with XPS Binding energy,
For MChem, Spring, 2009 5) Surface photoelectron spectroscopy Dr. Qiao Chen (room 3R506) http://www.sussex.ac.uk/users/qc25/ University of Sussex Today s topics 1. Element analysis with XPS Binding energy,
ECE Semiconductor Device and Material Characterization
 ECE 4813 Semiconductor Device and Material Characterization Dr. Alan Doolittle School of Electrical and Computer Engineering Georgia Institute of Technology As with all of these lecture slides, I am indebted
ECE 4813 Semiconductor Device and Material Characterization Dr. Alan Doolittle School of Electrical and Computer Engineering Georgia Institute of Technology As with all of these lecture slides, I am indebted
Lecture 7 Chemical/Electronic Structure of Glass
 Lecture 7 Chemical/Electronic Structure of Glass Syllabus Topic 6. Electronic spectroscopy studies of glass structure Fundamentals and Applications of X-ray Photoelectron Spectroscopy (XPS) a.k.a. Electron
Lecture 7 Chemical/Electronic Structure of Glass Syllabus Topic 6. Electronic spectroscopy studies of glass structure Fundamentals and Applications of X-ray Photoelectron Spectroscopy (XPS) a.k.a. Electron
Last Lecture. Overview and Introduction. 1. Basic optics and spectroscopy. 2. Lasers. 3. Ultrafast lasers and nonlinear optics
 Last Lecture Overview and Introduction 1. Basic optics and spectroscopy. Lasers 3. Ultrafast lasers and nonlinear optics 4. Time-resolved spectroscopy techniques Jigang Wang, Feb, 009 Today 1. Spectroscopy
Last Lecture Overview and Introduction 1. Basic optics and spectroscopy. Lasers 3. Ultrafast lasers and nonlinear optics 4. Time-resolved spectroscopy techniques Jigang Wang, Feb, 009 Today 1. Spectroscopy
Nanoelectronics 09. Atsufumi Hirohata Department of Electronics. Quick Review over the Last Lecture
 Nanoelectronics 09 Atsufumi Hirohata Department of Electronics 13:00 Monday, 12/February/2018 (P/T 006) Quick Review over the Last Lecture ( Field effect transistor (FET) ): ( Drain ) current increases
Nanoelectronics 09 Atsufumi Hirohata Department of Electronics 13:00 Monday, 12/February/2018 (P/T 006) Quick Review over the Last Lecture ( Field effect transistor (FET) ): ( Drain ) current increases
Reflection = EM strikes a boundary between two media differing in η and bounces back
 Reflection = EM strikes a boundary between two media differing in η and bounces back Incident ray θ 1 θ 2 Reflected ray Medium 1 (air) η = 1.00 Medium 2 (glass) η = 1.50 Specular reflection = situation
Reflection = EM strikes a boundary between two media differing in η and bounces back Incident ray θ 1 θ 2 Reflected ray Medium 1 (air) η = 1.00 Medium 2 (glass) η = 1.50 Specular reflection = situation
X-ray Absorption Spectroscopy
 X-ray Absorption Spectroscopy Nikki Truss November 26, 2012 Abstract In these experiments, some aspects of x-ray absorption spectroscopy were investigated. The x-ray spectrum of molybdenum was recorded
X-ray Absorption Spectroscopy Nikki Truss November 26, 2012 Abstract In these experiments, some aspects of x-ray absorption spectroscopy were investigated. The x-ray spectrum of molybdenum was recorded
The Use of Synchrotron Radiation in Modern Research
 The Use of Synchrotron Radiation in Modern Research Physics Chemistry Structural Biology Materials Science Geochemical and Environmental Science Atoms, molecules, liquids, solids. Electronic and geometric
The Use of Synchrotron Radiation in Modern Research Physics Chemistry Structural Biology Materials Science Geochemical and Environmental Science Atoms, molecules, liquids, solids. Electronic and geometric
Review of Optical Properties of Materials
 Review of Optical Properties of Materials Review of optics Absorption in semiconductors: qualitative discussion Derivation of Optical Absorption Coefficient in Direct Semiconductors Photons When dealing
Review of Optical Properties of Materials Review of optics Absorption in semiconductors: qualitative discussion Derivation of Optical Absorption Coefficient in Direct Semiconductors Photons When dealing
Electron Spettroscopies
 Electron Spettroscopies Spettroscopy allows to characterize a material from the point of view of: chemical composition, electronic states and magnetism, electronic, roto-vibrational and magnetic excitations.
Electron Spettroscopies Spettroscopy allows to characterize a material from the point of view of: chemical composition, electronic states and magnetism, electronic, roto-vibrational and magnetic excitations.
CHEM*3440. X-Ray Energies. Bremsstrahlung Radiation. X-ray Line Spectra. Chemical Instrumentation. X-Ray Spectroscopy. Topic 13
 X-Ray Energies very short wavelength radiation 0.1Å to 10 nm (100 Å) CHEM*3440 Chemical Instrumentation Topic 13 X-Ray Spectroscopy Visible - Ultraviolet (UV) - Vacuum UV (VUV) - Extreme UV (XUV) - Soft
X-Ray Energies very short wavelength radiation 0.1Å to 10 nm (100 Å) CHEM*3440 Chemical Instrumentation Topic 13 X-Ray Spectroscopy Visible - Ultraviolet (UV) - Vacuum UV (VUV) - Extreme UV (XUV) - Soft
4. Other diffraction techniques
 4. Other diffraction techniques 4.1 Reflection High Energy Electron Diffraction (RHEED) Setup: - Grazing-incidence high energy electron beam (3-5 kev: MEED,
4. Other diffraction techniques 4.1 Reflection High Energy Electron Diffraction (RHEED) Setup: - Grazing-incidence high energy electron beam (3-5 kev: MEED,
Chemistry Instrumental Analysis Lecture 8. Chem 4631
 Chemistry 4631 Instrumental Analysis Lecture 8 UV to IR Components of Optical Basic components of spectroscopic instruments: stable source of radiant energy transparent container to hold sample device
Chemistry 4631 Instrumental Analysis Lecture 8 UV to IR Components of Optical Basic components of spectroscopic instruments: stable source of radiant energy transparent container to hold sample device
MSE 321 Structural Characterization
 Auger Spectroscopy Auger Electron Spectroscopy (AES) Scanning Auger Microscopy (SAM) Incident Electron Ejected Electron Auger Electron Initial State Intermediate State Final State Physical Electronics
Auger Spectroscopy Auger Electron Spectroscopy (AES) Scanning Auger Microscopy (SAM) Incident Electron Ejected Electron Auger Electron Initial State Intermediate State Final State Physical Electronics
Auger Electron Spectroscopy
 Auger Electron Spectroscopy Auger Electron Spectroscopy is an analytical technique that provides compositional information on the top few monolayers of material. Detect all elements above He Detection
Auger Electron Spectroscopy Auger Electron Spectroscopy is an analytical technique that provides compositional information on the top few monolayers of material. Detect all elements above He Detection
X-ray, Neutron and e-beam scattering
 X-ray, Neutron and e-beam scattering Introduction Why scattering? Diffraction basics Neutrons and x-rays Techniques Direct and reciprocal space Single crystals Powders CaFe 2 As 2 an example What is the
X-ray, Neutron and e-beam scattering Introduction Why scattering? Diffraction basics Neutrons and x-rays Techniques Direct and reciprocal space Single crystals Powders CaFe 2 As 2 an example What is the
Data collection Strategy. Apurva Mehta
 Data collection Strategy Apurva Mehta Outline Before.. Resolution, Aberrations and detectors During.. What is the scientific question? How will probing the structure help? Is there an alternative method?
Data collection Strategy Apurva Mehta Outline Before.. Resolution, Aberrations and detectors During.. What is the scientific question? How will probing the structure help? Is there an alternative method?
de Broglie Waves h p de Broglie argued Light exhibits both wave and particle properties
 de Broglie argued de Broglie Waves Light exhibits both wave and particle properties Wave interference, diffraction Particle photoelectric effect, Compton effect Then matter (particles) should exhibit both
de Broglie argued de Broglie Waves Light exhibits both wave and particle properties Wave interference, diffraction Particle photoelectric effect, Compton effect Then matter (particles) should exhibit both
X-Ray Photoelectron Spectroscopy (XPS)-2
 X-Ray Photoelectron Spectroscopy (XPS)-2 Louis Scudiero http://www.wsu.edu/~scudiero; 5-2669 Fulmer 261A Electron Spectroscopy for Chemical Analysis (ESCA) The 3 step model: 1.Optical excitation 2.Transport
X-Ray Photoelectron Spectroscopy (XPS)-2 Louis Scudiero http://www.wsu.edu/~scudiero; 5-2669 Fulmer 261A Electron Spectroscopy for Chemical Analysis (ESCA) The 3 step model: 1.Optical excitation 2.Transport
Interaction of Ionizing Radiation with Matter
 Type of radiation charged particles photonen neutronen Uncharged particles Charged particles electrons (β - ) He 2+ (α), H + (p) D + (d) Recoil nuclides Fission fragments Interaction of ionizing radiation
Type of radiation charged particles photonen neutronen Uncharged particles Charged particles electrons (β - ) He 2+ (α), H + (p) D + (d) Recoil nuclides Fission fragments Interaction of ionizing radiation
XPS & Scanning Auger Principles & Examples
 XPS & Scanning Auger Principles & Examples Shared Research Facilities Lunch Talk Contact info: dhu Pujari & Han Zuilhof Lab of rganic Chemistry Wageningen University E-mail: dharam.pujari@wur.nl Han.Zuilhof@wur.nl
XPS & Scanning Auger Principles & Examples Shared Research Facilities Lunch Talk Contact info: dhu Pujari & Han Zuilhof Lab of rganic Chemistry Wageningen University E-mail: dharam.pujari@wur.nl Han.Zuilhof@wur.nl
An introduction to X- ray photoelectron spectroscopy
 An introduction to X- ray photoelectron spectroscopy X-ray photoelectron spectroscopy belongs to a broad class of spectroscopic techniques, collectively called, electron spectroscopy. In general terms,
An introduction to X- ray photoelectron spectroscopy X-ray photoelectron spectroscopy belongs to a broad class of spectroscopic techniques, collectively called, electron spectroscopy. In general terms,
Graphene films on silicon carbide (SiC) wafers supplied by Nitride Crystals, Inc.
 9702 Gayton Road, Suite 320, Richmond, VA 23238, USA Phone: +1 (804) 709-6696 info@nitride-crystals.com www.nitride-crystals.com Graphene films on silicon carbide (SiC) wafers supplied by Nitride Crystals,
9702 Gayton Road, Suite 320, Richmond, VA 23238, USA Phone: +1 (804) 709-6696 info@nitride-crystals.com www.nitride-crystals.com Graphene films on silicon carbide (SiC) wafers supplied by Nitride Crystals,
Contrasted strengths and weakness of EDS, WDS and AES for determining the composition of samples
 Contrasted strengths and weakness of EDS, WDS and AES for determining the composition of samples Ana-Marija Nedić Course 590B 12/07/2018 Iowa State University Contrasted strengths and weakness of EDS,
Contrasted strengths and weakness of EDS, WDS and AES for determining the composition of samples Ana-Marija Nedić Course 590B 12/07/2018 Iowa State University Contrasted strengths and weakness of EDS,
3.17 Strukturanalyse mit Röntgenstrahlen nach Debye- Scherrer
 3.17 Strukturanalyse mit Röntgenstrahlen nach Debye- Scherrer Ausarbeitung (engl.) Fortgeschrittenenpraktikum an der TU Darmstadt Versuch durchgeführt von: Mussie Beian, Jan Schupp, Florian Wetzel Versuchsdatum:
3.17 Strukturanalyse mit Röntgenstrahlen nach Debye- Scherrer Ausarbeitung (engl.) Fortgeschrittenenpraktikum an der TU Darmstadt Versuch durchgeführt von: Mussie Beian, Jan Schupp, Florian Wetzel Versuchsdatum:
Ultraviolet Photoelectron Spectroscopy (UPS)
 Ultraviolet Photoelectron Spectroscopy (UPS) Louis Scudiero http://www.wsu.edu/~scudiero www.wsu.edu/~scudiero; ; 5-26695 scudiero@wsu.edu Photoemission from Valence Bands Photoelectron spectroscopy is
Ultraviolet Photoelectron Spectroscopy (UPS) Louis Scudiero http://www.wsu.edu/~scudiero www.wsu.edu/~scudiero; ; 5-26695 scudiero@wsu.edu Photoemission from Valence Bands Photoelectron spectroscopy is
EEE4106Z Radiation Interactions & Detection
 EEE4106Z Radiation Interactions & Detection 2. Radiation Detection Dr. Steve Peterson 5.14 RW James Department of Physics University of Cape Town steve.peterson@uct.ac.za May 06, 2015 EEE4106Z :: Radiation
EEE4106Z Radiation Interactions & Detection 2. Radiation Detection Dr. Steve Peterson 5.14 RW James Department of Physics University of Cape Town steve.peterson@uct.ac.za May 06, 2015 EEE4106Z :: Radiation
Physics with Neutrons I, WS 2015/2016. Lecture 11, MLZ is a cooperation between:
 Physics with Neutrons I, WS 2015/2016 Lecture 11, 11.1.2016 MLZ is a cooperation between: Organization Exam (after winter term) Registration: via TUM-Online between 16.11.2015 15.1.2015 Email: sebastian.muehlbauer@frm2.tum.de
Physics with Neutrons I, WS 2015/2016 Lecture 11, 11.1.2016 MLZ is a cooperation between: Organization Exam (after winter term) Registration: via TUM-Online between 16.11.2015 15.1.2015 Email: sebastian.muehlbauer@frm2.tum.de
Structural characterization. Part 1
 Structural characterization Part 1 Experimental methods X-ray diffraction Electron diffraction Neutron diffraction Light diffraction EXAFS-Extended X- ray absorption fine structure XANES-X-ray absorption
Structural characterization Part 1 Experimental methods X-ray diffraction Electron diffraction Neutron diffraction Light diffraction EXAFS-Extended X- ray absorption fine structure XANES-X-ray absorption
