Ultraviolet Spectroscopy. CH- 521 Course on Interpreta2ve Molecular Spectroscopy; Course Instructor: Krishna P. Kaliappan
|
|
|
- Merry Carr
- 6 years ago
- Views:
Transcription
1 Ultraviolet Spectroscopy CH- 521 Course on Interpreta2ve Molecular Spectroscopy; Course Instructor: Krishna P. Kaliappan
2 Ultraviolet Spectroscopy UV light can be absorbed by molecules to excite higher energy (most loosely bound) electrons from lower energy states to higher states. Such transitions can be studied extensively to understand the binding energy of the corresponding electrons undergoing transition. Since π-electrons are most loosely bound in an organic molecule, UV spectroscopy yields a lot of information about the degree of unsaturation in a molecule. When the wavelength of the transition exceeds the UV range, based on the same principle, even the colours of molecules can be explained on the basis of absorption of visible light. β-carotene λ max = 452 nm 1,3-butadiene λ max = 217 nm CH- 521 Course on Interpreta2ve Molecular Spectroscopy; Course Instructor: Krishna P. Kaliappan
3 Electromagnetic Spectrum Energy is proportional to frequency Frequency is inversely proportional wavelength CH- 521 Course on Interpreta2ve Molecular Spectroscopy; Course Instructor: Krishna P. Kaliappan 3
4 The Visible Spectrum CH- 521 Course on Interpreta2ve Molecular Spectroscopy; Course Instructor: Krishna P. Kaliappan 4
5 Some Natural rganic Pigments H H H CH 3 C 2 H H N Z Alizarin H H Kermesic Acid H Z N H Z=H, Indigo Z=Br Punicin/Tyrian Purple H CH 3 CH 3 Crocetin H CH 3 CH 3 β-carotene (from carrots) CH- 521 Course on Interpreta2ve Molecular Spectroscopy; Course Instructor: Krishna P. Kaliappan 5
6 Instrumentation Double Beam UV Spectrometer: CH- 521 Course on Interpreta2ve Molecular Spectroscopy; Course Instructor: Krishna P. Kaliappan 6
7 Double Beam UV Spectrometer Light Source: Combined sources used to cover a range of nm Deuterium lamp for UV range Tungsten/halogen for visible range Diffraction Grating & The Slit: Diffraction grating splits lights to its component colors like a prism Slit allows to pass only a narrow range of wavelengths to the rotating disk Rotating Disks: Rotating disks are made of different number of segments CH- 521 Course on Interpreta2ve Molecular Spectroscopy; Course Instructor: Krishna P. Kaliappan
8 Rotating Disks: Double Beam UV Spectrometer If light hits the mirrored section, it bounces back to a mirror. The reflected light meets the transparent section of the second disk and passes through it to the detector. If light hits the transparent section, it will pass through and bounced by a mirror onto a second rotating disk. Light meets the mirrored section of the second disk and bounces onto the detector. CH- 521 Course on Interpreta2ve Molecular Spectroscopy; Course Instructor: Krishna P. Kaliappan
9 Double Beam UV Spectrometer Sample & Reference Cells: Small rectangular glass/quartz containers. Designed in such a manner that light has to travel 1 cm through the contents. Detector & Computer: Detector converts light to current. The greater is the intensity of light, the higher is the current. An absorbance (A) could be written as- Intensity of light passing through reference cell = I o Intensity of light passing through sample = I CH- 521 Course on Interpreta2ve Molecular Spectroscopy; Course Instructor: Krishna P. Kaliappan
10 Principle Behind UV Spectrometers Lambert-Beer s Law: Molar absorptivity = Absorbance of 1 mol dm -3 solution if cell length = 1 cm. The intensity of an absorption band in UV is expressed as the molar absorptivity at maximum absorption ε max. CH- 521 Course on Interpreta2ve Molecular Spectroscopy; Course Instructor: Krishna P. Kaliappan 10
11 Principle Behind UV Spectrometers Relative Energies of Various rbitals: σ (Anti-bonding) π (Anti-bonding) These energy gaps are different in different compounds. Energy n (Non-bonding) π (Bonding) The difference in energy between two orbitals- σ (Bonding) When light passes through a compound, some of its energy promotes an electron from one of the bonding or non-bonding orbitals to one of the anti-bonding orbitals. The frequency (or wavelength) of absorption depends on the energy gaps between those two energy levels. CH- 521 Course on Interpreta2ve Molecular Spectroscopy; Course Instructor: Krishna P. Kaliappan 11
12 Electronic Transitions Energy σ (Anti-bonding) π (Anti-bonding) n (Non-bonding) π (Bonding) σ (Bonding) The higher is the energy gap, the lower is the wavelength of the light absorbed. Bigger jumps requires more energy, so absorb light with a shorter wavelength. Not all electronic transitions are allowed. Certain restrictions should be considered for electronic transitions, called selection rules Energy σ (Anti-bonding) π (Anti-bonding) n (Non-bonding) π (Bonding) σ (Bonding) (1) The spin quantum number of an electron should not change during the electronic transition. (2) The transition between two orbitals should be symmetry allowed. Any transition that violates these rules are called forbidden transition. Most common forbidden transition is n è π*. CH- 521 Course on Interpreta2ve Molecular Spectroscopy; Course Instructor: Krishna P. Kaliappan 12
13 Electronic Transitions Important Electronic Transitions: From π (bonding) orbital to π* (anti-bonding) orbital (π è π*). From n (non-bonding) orbital to π* (anti-bonding) orbital (n è π*). From n (non-bonding) orbital to σ* (anti-bonding) orbital (n è σ*). In Alkanes: σ è σ* or n è σ* Usually weak absorptions Absorption Characteristics of n è π* Compound λ max ε max Solvent Methanol Hexane 1-Hexanethiol 224 (s) 126 Cyclohexane Trimethylamine Hexane N-methylpiperidine Ether Diethyl ether Gas phase Methyl chloride Hexane Methyl iodide Hexane CH- 521 Course on Interpreta2ve Molecular Spectroscopy; Course Instructor: Krishna P. Kaliappan 13
14 In Alkenes: Electronic Transitions In unconjugated alkenes π è π* transition takes place around nm. Absorption Data for Conjugated Alkenes (π è π*) Compound λ max εmax Solvent 1,3-Butadiene ,000 Hexane 2,3-Dimethyl-1,3-butadiene ,400 Cyclohexane 1,3,5-Hexatriene ,000 Isooctane , ,000 1,3-Cyclohexadiene 256 8,000 Hexane 1,3-Cyclopentadiene 239 3,400 Hexane In Carbonyls: π è π* around 190 nm (ε = 900) & n è π* around 280 nm (ε = 15). Since n è π* transition is a symmetry forbidden transition, intensity of this transition is much lower than other allowed transitions. CH- 521 Course on Interpreta2ve Molecular Spectroscopy; Course Instructor: Krishna P. Kaliappan 14
15 ther Examples Chromophore Example Excitation λ max ε max Solvent C=C Ethene π è π* 165 nm 15,000 hexane C C 1-Hexyne π è π* 173 nm 10,000 hexane C= Ethanal n è π* π è π* 290 nm 180 nm 15 10,000 hexane hexane N= Nitromethane n è π* π è π* 275 nm 200 nm 17 5,000 ethanol ethanol C-X X=Br X=I Methyl bromide Methyl Iodide n è σ* n è σ* 205 nm 255 nm hexane hexane CH- 521 Course on Interpreta2ve Molecular Spectroscopy; Course Instructor: Krishna P. Kaliappan 15
16 Conventions & Terminologies Chromophore: A covalently unsaturated group responsible for electronic absorption (e.g., C=C, C=, esters, amides, -N 2 etc.). Auxochrome: A saturated group with non-bonded electrons which, when attached to a chromohore, alters both the wavelength and the intensity of the absorption (e.g., -H, -NH 2, -NR 2 -SH etc.) Bathochromic Shift: The shift of absorption to a longer wavelength (also known as red shift ). Hypsochromic Shift: The shift of absorption to a shorter wavelength (also known as blue shift ). Hyperchromic Effect: An increase in absorption intensity. Hypochromic Effect: A decrease in absorption intensity. CH- 521 Course on Interpreta2ve Molecular Spectroscopy; Course Instructor: Krishna P. Kaliappan 16
17 Solvents & Solutions Solvents should not absorb UV-radiation within same range as the substance. Acetonitrile 190 nm n-hexane 201 nm Chloroform 240 nm Methanol 205 nm Cyclohexane 195 nm Isooctane 195 nm 1,4-Dioxane 215 nm Water 190 nm 95% Ethanol 205 nm Trimethyl phosphate 210 nm A strong absorbing solvent allows very little amount of light to pass through the sample. Non-polar solvents do not form H- bond with solute, so fine structure is often observed. Polar solvents form solute-solvent complexes through H-bonding, hence, fine structure may disappear. UV spectra of phenol in ethanol and isooctane. CH- 521 Course on Interpreta2ve Molecular Spectroscopy; Course Instructor: Krishna P. Kaliappan 17
18 Solvents & Solutions Solvent Effects: The position and intensity of an absorption band may shift if the spectrum was recorded in different solvents. Conjugated dienes and aromatic hydrocarbons experience very less solvent effect. α,β-unsaturated carbonyls show two different shifts in bands for changing solvents from non-polar to a polar protic one. π π s π è π* band moves to longer wavelength, n è π* band moves to Energy n shorter wavelength. π n s π* orbitals get stabilized (due to more polarity) by solvation than π π s orbitals. n orbitals get stabilized π π > π s π s mainly by H-bonding. n π < n s π s CH- 521 Course on Interpreta2ve Molecular Spectroscopy; Course Instructor: Krishna P. Kaliappan 18
19 Effect of Conjugation Ψ 4 * Ψ 2 * Ψ 4 * Ψ 2 * E Ψ 2 * Ψ 3 * Ψ 2 * E ΔE 1 ΔE 2 Ψ 3 * Ψ 2 Ψ 2 Ψ 1 Ψ 1 Ψ 1 Ψ 1 Ψ 1 Ψ 1 CH- 521 Course on Interpreta2ve Molecular Spectroscopy; Course Instructor: Krishna P. Kaliappan 19
20 Effect of Conjugation Energy Ethylene Butadiene Hexatriene Molecule Ethylene 165 1,3-butadiene 217 1,3,5-hexatriene 258 β-carotene 470 λ max (nm) CH 3 CH 3 CH 3 H 3 C H 3 C CH 3 CH 3 CH 3 CH 3 CH 3 CH- 521 Course on Interpreta2ve Molecular Spectroscopy; Course Instructor: Krishna P. Kaliappan 20
21 Effect of Conjugation Effect of s-cis & s-trans Conformers: λ max π è π* (Ψ 2 è Ψ 3 *): s-trans 230 nm s-cis 271 nm Probably repulsion between terminal lobes of Ψ 2 increases energy of HM (Ψ 2 ) in s-cis form. Hence, less energy (ie. Higher wavelength) is required for Ψ 2 è Ψ 3 * transition. H 3 C H 3 C CH 3 H3 C Substitution may force a molecule to take s-cis form, therefore, absorbs energy from a longer wavelength (shows a red shift) than usual s-trans conformer. CH- 521 Course on Interpreta2ve Molecular Spectroscopy; Course Instructor: Krishna P. Kaliappan 21
22 Effect of Conjugation In cyclic system a double bond is forced to stay in s-cis (Cisoid) form, therefore, shows a red shift with a drop in intensity. Homoannular & Heteroannular Dienes: λ max = 234 nm ε max = λ max = 273 nm ε max = CH- 521 Course on Interpreta2ve Molecular Spectroscopy; Course Instructor: Krishna P. Kaliappan 22
23 Woodward Fieser Rules for Diene Woodward (1941) predicted λ max values only for the lowest energy transition (π è π*) from HM to LUM. Base values: Base value for an unsubstituted, conjugated, acyclic or heteroannular diene 214 nm Base value for an unsubstituted, conjugated, homoannular diene 253 nm Increments for: Each extra double bonds in conjugation Exocyclic double bond (effect is two fold if the bond is exocyclic to two rings) Substituent effect: A. -CR or CAr + 0 nm B. Simple alkyl substituents or ring residue + 5 nm C. Halogen (-Cl, -Br) + 5 nm D. R (R=Alkyl) + 6 nm E. SR (R=Alkyl) + 30 nm F. NR 2 (R=Alkyl) + 60 nm + 30 nm + 5 nm CH- 521 Course on Interpreta2ve Molecular Spectroscopy; Course Instructor: Krishna P. Kaliappan 23
24 Woodward Fieser Rules for Diene Transoid (base): 214 nm 3 ring residues : exocyclic C=C: nm bserved: 235 nm Et Transoid (base): 214 nm 3 ring residues: exocyclic C=C: + 5 -R: nm bserved: 241 nm Transoid (base): 214 nm 3 Ring residues: Alkyl substituent: Exocyclic C=C: nm CH- 521 Course on Interpreta2ve Molecular Spectroscopy; Course Instructor: Krishna P. Kaliappan 24
25 Woodward Fieser Rules for Diene Cisoid (base): 253 nm 3 ring residues: exocyclic C=C: nm bserved: 275 nm Cisoid (base): 253 nm 3 Ring residues: Exocyclic C=C: + 5 Double-bond Extending Conjugation: nm bserved: 304 nm Base value: 214 nm 2 Ring residue +10 Exocyclic C=C: nm bserved: 230 nm Base value: 214 nm 2 Ring residue: +10 Exocyclic C=C: + 5. Total 229 nm bserved: 236 nm CH- 521 Course on Interpreta2ve Molecular Spectroscopy; Course Instructor: Krishna P. Kaliappan 25
26 Woodward Fieser Rules for Diene R Transoid (base): 214 nm 5 ring residues: DEC: exocyclic C=C nm bserved: 283 nm HC Cisoid (base): 253 nm 3 ring residues: Alkyl subs: +5 1 exocyclic C=C nm bserved: 275 nm Ac Cisoid (base): 253 nm 5 ring residues: DEC: exocyclic C=C nm bserved: 355 nm CH- 521 Course on Interpreta2ve Molecular Spectroscopy; Course Instructor: Krishna P. Kaliappan 26
27 Woodward Fieser Rules for Diene Base value: 214 nm 3 Alkyl grs: nm bserved: 232 nm Base value: 214 nm 4 Alkyl grs: nm bserved: 235 nm Base value: 253 nm 4 Ring residues: Exocyclic C=C: nm bserved: 282 nm Base value: 253 nm 5 Ring residues: Exocyclic C=C: +15 DEC: +30. Total 323 nm bserved: 325 nm CH- 521 Course on Interpreta2ve Molecular Spectroscopy; Course Instructor: Krishna P. Kaliappan
28 Woodward-Fieser Rule for Enones Base values: Rules of Enone & Dienone Absorption Acyclic α,β-unsaturated ketones 215 nm 6-membered cyclic α,β-unsaturated ketones 215 nm 5-membered cyclic α,β-unsaturated ketones 202 nm α,β-unsaturated aldehydes 210 nm α,β-unsaturated carboxylic acid & esters 195 nm Increments for: Double bond extending conjugation (DEC): +30 Exocyclic double bond: + 5 Homodiene component: +39 CH- 521 Course on Interpreta2ve Molecular Spectroscopy; Course Instructor: Krishna P. Kaliappan 28
29 Woodward-Fieser Rule for Enones Increments for: Alkyl group/ring residue: α +10 β +12 γ & higher +18 Polar groups: -H: α +35 β +30 δ +50 -Ac: α,β,γ + 6 -Me: α +35 β +30 γ +17 δ +31 -SAlk: β +85 -Cl: α +15 β +12 -Br: α +25 β +30 -NR 2 : β +95 CH- 521 Course on Interpreta2ve Molecular Spectroscopy; Course Instructor: Krishna P. Kaliappan 29
30 Woodward-Fieser Rule for Enones 1β' 2 CH 3 β β Base value: 215 nm Δ 4,5 system (base): 215 nm α substituent: β substituents: +24 β substituent: exocyclic C=C: nm 244 nm bserved: 232 nm bserved: 245 nm β H H β α α β Base value: 202 nm β substituent: +12 Base value: 215 nm α-h: β substituents: nm α-h: +35. bserved: 247 nm 274 nm bserved: 270 nm CH- 521 Course on Interpreta2ve Molecular Spectroscopy; Course Instructor: Krishna P. Kaliappan 30
31 Woodward-Fieser Rule for Enones α β γ CH3 CCH 3 δ Base value: 215 nm 1 DEC: +30 Homocyclic diene: +39 δ ring residue: nm bserved: 300 nm Base value: 202 nm Exocyclic C=C: β-ring residues: nm bserved: 226 nm R Br Base value: 202 nm 1 α-br: β-ring residue: +24 Exocyclic C=C: nm bserved: 251 nm Base value: 215 nm 1 DEC: +30 β-ring residue: +12 δ ring residue: Exocyclic C=C: nm bserved: 280 nm CH- 521 Course on Interpreta2ve Molecular Spectroscopy; Course Instructor: Krishna P. Kaliappan 31
32 Woodward-Fieser Rule for Enones Base value: 215 nm α alkyl substituent: +10 β alkyl substituent: nm Base value: 215 nm α alkyl: +10 β alkyl: nm Base value: 215 nm 1 DEC: +30 Exocyclic C=C: + 5 β-alkyl substituent: +12 γ-alkyl substituent: +18 δ-alkyl substituent: nm Base value: 215 nm 1 α-alkyl: β-alkyl: Exocyclic C=C: nm CH- 521 Course on Interpreta2ve Molecular Spectroscopy; Course Instructor: Krishna P. Kaliappan 32
33 Aromatic Compounds Parent chromophore: Ar = C 6 H 5 Ar-C-R Ar-CH Ar-CH or Ar-CR 246 nm 250 nm 230 nm Increment for each substituent on Ar: Alkyl or ring residue o, m + 3 nm p + 10 nm H, CH 3, Alk o, m + 7 nm p + 25 nm NH 2 o, m + 13 nm p + 58 nm NHCCH 3 o,m + 20 nm p + 45 nm NHMe p + 73 nm NMe 2 o, m + 20 nm p + 85 nm Cl o, m + 0 nm p + 10 nm Br o, m + 2 nm p + 15 nm CH- 521 Course on Interpreta2ve Molecular Spectroscopy; Course Instructor: Krishna P. Kaliappan 33
34 Aromatic Compounds Me EtH Calc λ max = 246 (parent chromophore) + 3 (o-ring residue) + 25 (p-me) = 274 nm EtH bs λ max = 276 nm Cl H C 2 Et EtH Calc λ max = (o-ring residue) + 7 (o-h) = 256 nm EtH bs λ max = 257 nm Me Me +25 EtH Calc λ max = = 281 nm EtH bs λ max = 278 nm CH- 521 Course on Interpreta2ve Molecular Spectroscopy; Course Instructor: Krishna P. Kaliappan 34
35 Biphenyls: Violations of Woodward Rules CH 3 CH 3 H CH CH 3 C 3 3 H 3 C CH 3 H 3 C CH nm 237 nm CH nm Not planar 45 0 C angle Substitutions cause loss of co-planarity of orbitals. Loss of overlap, blue shift with reduced intensity. CH- 521 Course on Interpreta2ve Molecular Spectroscopy; Course Instructor: Krishna P. Kaliappan 35
36 (1) In alkenes, trans isomers exhibit absorption at longer wavelength and high intensity. (2) Woodward rules are applicable only if there is no strain around chromophore. Steric Inhibition of Resonance Ring strain Cross conjugation Steric inhibition of resonance (3) If calculated and experimental values of λ max do not match, one can say that the molecule must have some strain. (4) Thus UV can be used indirectly to determine if the molecule has any strain. CH- 521 Course on Interpreta2ve Molecular Spectroscopy; Course Instructor: Krishna P. Kaliappan 36
37 Fieser-Kuhn rules for Conjugated Polyenes λ max = M + n( n) R endo 10R exo ε max = (1.74 X 10 4 )n Where n = number of conjugated double bonds M = number of alkyl or alkyl like substituents on the conjugated system R endo = number of rings with endocyclic double bonds in the conjugated system R exo = number of rings with exocyclic double bonds CH- 521 Course on Interpreta2ve Molecular Spectroscopy; Course Instructor: Krishna P. Kaliappan 37
38 Lycopene H 3 C CH 3 CH 3 CH 3 CH 3 CH 3 CH 3 CH 3 CH 3 CH 3 calc λ max = (8) + 11[ (11)] = 476 nm obs λ max = 474 nm (hexane) cal ε max = 1.74 X 10 4 (11) = 19.1 X 10 4 obs ε max = 18.6 X 10 4 (hexane) CH 3 CH 3 CH 3 β-carotene H 3 C H 3 C CH 3 CH 3 calc λ max = nm obs λ max = 452 nm (hexane) CH 3 CH 3 CH 3 cal ε max = 19.1 X 10 4 obs ε max = 15.2 X 10 4 CH- 521 Course on Interpreta2ve Molecular Spectroscopy; Course Instructor: Krishna P. Kaliappan 38
39 Home Work Predict λ max values of the following compounds using Woodward- Fieser rules: Me Me Me Me R Me Me CH 3 Me Me CH 3 Ac Is it possible to use UV spectroscopy as a tool to predict the course of this reaction? hν CH- 521 Course on Interpreta2ve Molecular Spectroscopy; Course Instructor: Krishna P. Kaliappan 39
40 Home Work Match three steroid structures shown below to the following values hexane for λ max : Compound A, 275 nm; B, 304 nm, and C, 356 nm. C 9 H 19 C 9 H 19 C 8 H 17 H 3 C H 3 C H Partial hydrogenation of the triene shown below results in two compounds, D and E, both of molecular formula C 10 H 14. Compound D hexane shows a λ max = 235 nm and E, 275 nm. Assign the structures. CH- 521 Course on Interpreta2ve Molecular Spectroscopy; Course Instructor: Krishna P. Kaliappan 40
ULTRAVIOLET SPECTROSCOPY or ELECTRONIC SPECTROSCOPY
 ULTRAVILET SPECTRSCPY or ELECTRNIC SPECTRSCPY S. SANKARARAMAN Department of Chemistry Indian Institute of Technology Madras Chennai 600036, INDIA Sanka@iitm.ac.in Absorption of electromagnetic radiation
ULTRAVILET SPECTRSCPY or ELECTRNIC SPECTRSCPY S. SANKARARAMAN Department of Chemistry Indian Institute of Technology Madras Chennai 600036, INDIA Sanka@iitm.ac.in Absorption of electromagnetic radiation
UV-Vis Spectroscopy. Chem 744 Spring Gregory R. Cook, NDSU Thursday, February 14, 13
 UV-Vis Spectroscopy Chem 744 Spring 2013 UV-Vis Spectroscopy Every organic molecule absorbs UV-visible light Energy of electronic transitions saturated functionality not in region that is easily accessible
UV-Vis Spectroscopy Chem 744 Spring 2013 UV-Vis Spectroscopy Every organic molecule absorbs UV-visible light Energy of electronic transitions saturated functionality not in region that is easily accessible
UV Spectroscopy: Empirical Approach to Molecular Structures. Dr. Mishu Singh Department of Chemistry M. P.Govt P. G.
 UV Spectroscopy: Empirical Approach to Molecular Structures Dr. Mishu Singh Department of Chemistry M. P.Govt P. G.College, Hardoi WHAT IS SPECTROSCOPY? Atoms and molecules interact with electromagnetic
UV Spectroscopy: Empirical Approach to Molecular Structures Dr. Mishu Singh Department of Chemistry M. P.Govt P. G.College, Hardoi WHAT IS SPECTROSCOPY? Atoms and molecules interact with electromagnetic
Electronic Excitation by UV/Vis Spectroscopy :
 Electronic Excitation by UV/Vis Spectroscopy : X-ray: core electron excitation UV: valance electronic excitation IR: molecular vibrations Radio waves: Nuclear spin states (in a magnetic field) The wavelength
Electronic Excitation by UV/Vis Spectroscopy : X-ray: core electron excitation UV: valance electronic excitation IR: molecular vibrations Radio waves: Nuclear spin states (in a magnetic field) The wavelength
Molecular Spectroscopy. H 2 O e -
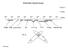 Molecular Spectroscopy ν (cm -1 ) λ (cm) 10 6 10 8 10 10 10 12 10 14 10 16 10 18 10 20 10 22 ν (Hz) NMR ESR microwave IR UV/Vis VUV X-Ray Gamma Ray H 2 e - UV/Vis Spectroscopy absorption technique X hν
Molecular Spectroscopy ν (cm -1 ) λ (cm) 10 6 10 8 10 10 10 12 10 14 10 16 10 18 10 20 10 22 ν (Hz) NMR ESR microwave IR UV/Vis VUV X-Ray Gamma Ray H 2 e - UV/Vis Spectroscopy absorption technique X hν
Terms used in UV / Visible Spectroscopy
 Terms used in UV / Visible Spectroscopy Chromophore The part of a molecule responsible for imparting color, are called as chromospheres. OR The functional groups containing multiple bonds capable of absorbing
Terms used in UV / Visible Spectroscopy Chromophore The part of a molecule responsible for imparting color, are called as chromospheres. OR The functional groups containing multiple bonds capable of absorbing
Electronic Excitation by UV/Vis Spectroscopy :
 Electronic Excitation by UV/Vis Spectroscopy : X-ray: core electron excitation UV: valance electronic excitation IR: molecular vibrations Radio waves: Nuclear spin states (in a magnetic field) The wavelength
Electronic Excitation by UV/Vis Spectroscopy : X-ray: core electron excitation UV: valance electronic excitation IR: molecular vibrations Radio waves: Nuclear spin states (in a magnetic field) The wavelength
Molecular Spectroscopy
 Molecular Spectroscopy Types of transitions: 1) Electronic (UV-Vis-Near IR) 2) Vibrational (IR) 3) Rotational (microwave) Electronic Absorption Spectra π π* Gary L. Miessler and Donald A. Tarr, Inorganic
Molecular Spectroscopy Types of transitions: 1) Electronic (UV-Vis-Near IR) 2) Vibrational (IR) 3) Rotational (microwave) Electronic Absorption Spectra π π* Gary L. Miessler and Donald A. Tarr, Inorganic
Spectroscopy may be defined as the study of interaction between electromagnetic radiations and matter.
 Spectroscopy may be defined as the study of interaction between electromagnetic radiations and matter. Spectroscopy has a wide range of applications. It is heavily used in astronomy and remote sensing.
Spectroscopy may be defined as the study of interaction between electromagnetic radiations and matter. Spectroscopy has a wide range of applications. It is heavily used in astronomy and remote sensing.
Spektroskopi Molekul Organik
 Spektroskopi Molekul rganik Chapter 7: UV & electronic transitions Usable ranges & observations Selection rules Band Structure Instrumentation & Spectra Beer-Lambert Law Application of UV-spec 1 Dosen:
Spektroskopi Molekul rganik Chapter 7: UV & electronic transitions Usable ranges & observations Selection rules Band Structure Instrumentation & Spectra Beer-Lambert Law Application of UV-spec 1 Dosen:
Lecture 09 MO theory. (Refer Slide Time: 00:33)
 (Refer Slide Time: 00:33) Atomic and Molecular Absorption Spectrometry for Pollution Monitoring Dr. J R Mudakavi Department of Chemical Engineering Indian Institute of Science, Bangalore Lecture 09 MO
(Refer Slide Time: 00:33) Atomic and Molecular Absorption Spectrometry for Pollution Monitoring Dr. J R Mudakavi Department of Chemical Engineering Indian Institute of Science, Bangalore Lecture 09 MO
Terms used in UV / Visible Spectroscopy
 Terms used in UV / Visible Spectroscopy Chromophore The part of a molecule responsible for imparting color, are called as chromospheres. OR The functional groups containing multiple bonds capable of absorbing
Terms used in UV / Visible Spectroscopy Chromophore The part of a molecule responsible for imparting color, are called as chromospheres. OR The functional groups containing multiple bonds capable of absorbing
Advanced Analytical Chemistry
 84.514 Advanced Analytical Chemistry Part III Molecular Spectroscopy (continued) Website http://faculty.uml.edu/david_ryan/84.514 http://www.cem.msu.edu/~reusch/virtualtext/ Spectrpy/UV-Vis/spectrum.htm
84.514 Advanced Analytical Chemistry Part III Molecular Spectroscopy (continued) Website http://faculty.uml.edu/david_ryan/84.514 http://www.cem.msu.edu/~reusch/virtualtext/ Spectrpy/UV-Vis/spectrum.htm
9/28/10. Visible and Ultraviolet Molecular Spectroscopy - (S-H-C Chapters 13-14) Valence Electronic Structure. n σ* transitions
 Visible and Ultraviolet Molecular Spectroscopy - (S-H-C Chapters 13-14) Electromagnetic Spectrum - Molecular transitions Widely used in chemistry. Perhaps the most widely used in Biological Chemistry.
Visible and Ultraviolet Molecular Spectroscopy - (S-H-C Chapters 13-14) Electromagnetic Spectrum - Molecular transitions Widely used in chemistry. Perhaps the most widely used in Biological Chemistry.
Conjugated Dienes and Ultraviolet Spectroscopy
 Conjugated Dienes and Ultraviolet Spectroscopy Key Words Conjugated Diene Resonance Structures Dienophiles Concerted Reaction Pericyclic Reaction Cycloaddition Reaction Bridged Bicyclic Compound Cyclic
Conjugated Dienes and Ultraviolet Spectroscopy Key Words Conjugated Diene Resonance Structures Dienophiles Concerted Reaction Pericyclic Reaction Cycloaddition Reaction Bridged Bicyclic Compound Cyclic
UV / Visible Spectroscopy. Click icon to add picture
 UV / Visible Spectroscopy Click icon to add picture Spectroscopy It is the branch of science that deals with the study of interaction of matter with light. OR It is the branch of science that deals with
UV / Visible Spectroscopy Click icon to add picture Spectroscopy It is the branch of science that deals with the study of interaction of matter with light. OR It is the branch of science that deals with
Ferdowsi University of Mashhad
 Spectroscopy in Inorganic Chemistry 2 Diatomic molecule C v and D h HCN H-H 3 contribution orbital electron Σ 0 σ 1 Π 1 π 1 Δ 2 δ 1 Φ 3 δ 1 Σ + Σ - 4 Linear molecule NO 2s+1 2 Π A 1 =Σ + 0 A 2 =Σ - 0 E
Spectroscopy in Inorganic Chemistry 2 Diatomic molecule C v and D h HCN H-H 3 contribution orbital electron Σ 0 σ 1 Π 1 π 1 Δ 2 δ 1 Φ 3 δ 1 Σ + Σ - 4 Linear molecule NO 2s+1 2 Π A 1 =Σ + 0 A 2 =Σ - 0 E
Spectroscopy. Page 1 of 8 L.Pillay (2012)
 Spectroscopy Electromagnetic radiation is widely used in analytical chemistry. The identification and quantification of samples using electromagnetic radiation (light) is called spectroscopy. Light has
Spectroscopy Electromagnetic radiation is widely used in analytical chemistry. The identification and quantification of samples using electromagnetic radiation (light) is called spectroscopy. Light has
Spectroscopy in Inorganic Chemistry. Electronic Absorption Spectroscopy
 Spectroscopy in Inorganic Chemistry Diatomic molecule C v and D h NO H-H 2 contribution orbital Σ 0 σ Π 1 π Δ 2 δ Φ 3 δ 3 Linear molecule NO 2s+1 2 Π A 1 =Σ + 0 A 2 =Σ - 0 E 1 =Π 1 E 2 =Δ 2 E 3 =Φ 3 4
Spectroscopy in Inorganic Chemistry Diatomic molecule C v and D h NO H-H 2 contribution orbital Σ 0 σ Π 1 π Δ 2 δ Φ 3 δ 3 Linear molecule NO 2s+1 2 Π A 1 =Σ + 0 A 2 =Σ - 0 E 1 =Π 1 E 2 =Δ 2 E 3 =Φ 3 4
Chapter 13 Conjugated Unsaturated Systems
 Chapter 13 Conjugated Unsaturated Systems Introduction Conjugated unsaturated systems have a p orbital on a carbon adjacent to a double bond The p orbital can come from another double or triple bond The
Chapter 13 Conjugated Unsaturated Systems Introduction Conjugated unsaturated systems have a p orbital on a carbon adjacent to a double bond The p orbital can come from another double or triple bond The
Instrumental Chemical Analysis
 L6 page 1 Instrumental Chemical Analysis Ultraviolet and visible spectroscopy Dr. Ahmad Najjar Philadelphia University Faculty of Pharmacy Department of Pharmaceutical Sciences 2 nd semester, 2016/2017
L6 page 1 Instrumental Chemical Analysis Ultraviolet and visible spectroscopy Dr. Ahmad Najjar Philadelphia University Faculty of Pharmacy Department of Pharmaceutical Sciences 2 nd semester, 2016/2017
Instrumental Chemical Analysis
 L6 page 1 Instrumental Chemical Analysis Ultraviolet and visible spectroscopy Dr. Ahmad Najjar Philadelphia University Faculty of Pharmacy Department of Pharmaceutical Sciences 2 nd semester, 2016/2017
L6 page 1 Instrumental Chemical Analysis Ultraviolet and visible spectroscopy Dr. Ahmad Najjar Philadelphia University Faculty of Pharmacy Department of Pharmaceutical Sciences 2 nd semester, 2016/2017
Chapter 13. Conjugated Unsaturated Systems. +,., - Allyl. What is a conjugated system? AllylicChlorination (High Temperature)
 What is a conjugated system? Chapter 13 Conjugated Unsaturated Systems Conjugated unsaturated systems have a p orbital on a carbon adjacent to a double bond The p orbital may be empty (a carbocation The
What is a conjugated system? Chapter 13 Conjugated Unsaturated Systems Conjugated unsaturated systems have a p orbital on a carbon adjacent to a double bond The p orbital may be empty (a carbocation The
and Ultraviolet Spectroscopy
 Organic Chemistry, 7 th Edition L. G. Wade, Jr. Chapter 15 Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy 2010, Prentice all Conjugated Systems Conjugated double bonds are separated
Organic Chemistry, 7 th Edition L. G. Wade, Jr. Chapter 15 Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy 2010, Prentice all Conjugated Systems Conjugated double bonds are separated
CHEM 261 Notes Nov 22, 2017 REVIEW:
 155 CEM 261 Notes Nov 22, 2017 REVIEW: Recall how we can show the energy levels of the atomic orbitals of C. If the C is sp 2 hybridized, two of the 2p orbitals combine with the 2s orbital to form two
155 CEM 261 Notes Nov 22, 2017 REVIEW: Recall how we can show the energy levels of the atomic orbitals of C. If the C is sp 2 hybridized, two of the 2p orbitals combine with the 2s orbital to form two
UV Visible Spectroscopy
 UV Visible Spectroscopy It involves the measurement of absorption of light in the UV region(10-200(far UV)-200-400nm(near UV) and visible region(400-800nm)by the compound under investigation. It is also
UV Visible Spectroscopy It involves the measurement of absorption of light in the UV region(10-200(far UV)-200-400nm(near UV) and visible region(400-800nm)by the compound under investigation. It is also
Química Orgânica I. Ciências Farmacêuticas Bioquímica Química. Análise estrutural AFB QO I 2007/08 1 AFB QO I 2007/08 2
 Química Orgânica I Ciências Farmacêuticas Bioquímica Química AFB QO I 2007/08 1 Análise estrutural AFB QO I 2007/08 2 1 Adaptado de: Organic Chemistry, 6th Edition; L. G. Wade, Jr. Organic Chemistry, William
Química Orgânica I Ciências Farmacêuticas Bioquímica Química AFB QO I 2007/08 1 Análise estrutural AFB QO I 2007/08 2 1 Adaptado de: Organic Chemistry, 6th Edition; L. G. Wade, Jr. Organic Chemistry, William
Conjugated Systems, Orbital Symmetry and UV Spectroscopy
 Conjugated Systems, Orbital Symmetry and UV Spectroscopy Introduction There are several possible arrangements for a molecule which contains two double bonds (diene): Isolated: (two or more single bonds
Conjugated Systems, Orbital Symmetry and UV Spectroscopy Introduction There are several possible arrangements for a molecule which contains two double bonds (diene): Isolated: (two or more single bonds
Most organic molecules and functional groups are transparent in the portions of the electromagnetic ULTRAVIOLET SPECTROSCOPY
 14782_07_h7_p381-417.pp2.qxd 2/2/08 1:22 AM Page 381 A P T E R 7 ULTRAVILET SPETRSPY Most organic molecules and functional groups are transparent in the portions of the electromagnetic spectrum that we
14782_07_h7_p381-417.pp2.qxd 2/2/08 1:22 AM Page 381 A P T E R 7 ULTRAVILET SPETRSPY Most organic molecules and functional groups are transparent in the portions of the electromagnetic spectrum that we
More information can be found in Chapter 12 in your textbook for CHEM 3750/ 3770 and on pages in your laboratory manual.
 CHEM 3780 rganic Chemistry II Infrared Spectroscopy and Mass Spectrometry Review More information can be found in Chapter 12 in your textbook for CHEM 3750/ 3770 and on pages 13-28 in your laboratory manual.
CHEM 3780 rganic Chemistry II Infrared Spectroscopy and Mass Spectrometry Review More information can be found in Chapter 12 in your textbook for CHEM 3750/ 3770 and on pages 13-28 in your laboratory manual.
Chemistry 304B, Spring 1999 Lecture 5 1. UV Spectroscopy:
 Chemistry 304B, Spring 1999 Lecture 5 1 Ultraviolet spectroscopy; UV Spectroscopy: Infrared spectroscopy; Nuclear magnetic resonance spectroscopy General basis of spectroscopy: Shine light at a collection
Chemistry 304B, Spring 1999 Lecture 5 1 Ultraviolet spectroscopy; UV Spectroscopy: Infrared spectroscopy; Nuclear magnetic resonance spectroscopy General basis of spectroscopy: Shine light at a collection
17.1 Classes of Dienes
 17.1 Classes of Dienes There are three categories for dienes: Cumulated: pi bonds are adjacent. Conjugated: pi bonds are separated by exactly ONE single bond. Isolated: pi bonds are separated by any distance
17.1 Classes of Dienes There are three categories for dienes: Cumulated: pi bonds are adjacent. Conjugated: pi bonds are separated by exactly ONE single bond. Isolated: pi bonds are separated by any distance
Ultraviolet and Visible Spectroscopy
 BSC 3 rd YEAR SUBJECT CHEMISTRY SESSION 2016-2017 ORGANIC PORTION(B) UNIT I Ultraviolet and Visible Spectroscopy ELECTROMAGNETIC RADIATIONS Visible light is a form of energy which can be described by two
BSC 3 rd YEAR SUBJECT CHEMISTRY SESSION 2016-2017 ORGANIC PORTION(B) UNIT I Ultraviolet and Visible Spectroscopy ELECTROMAGNETIC RADIATIONS Visible light is a form of energy which can be described by two
Table 8.2 Detailed Table of Characteristic Infrared Absorption Frequencies
 Table 8.2 Detailed Table of Characteristic Infrared Absorption Frequencies The hydrogen stretch region (3600 2500 cm 1 ). Absorption in this region is associated with the stretching vibration of hydrogen
Table 8.2 Detailed Table of Characteristic Infrared Absorption Frequencies The hydrogen stretch region (3600 2500 cm 1 ). Absorption in this region is associated with the stretching vibration of hydrogen
E35 SPECTROSCOPIC TECHNIQUES IN ORGANIC CHEMISTRY
 E35 SPECTRSCPIC TECNIQUES IN RGANIC CEMISTRY Introductory Comments. These notes are designed to introduce you to the basic spectroscopic techniques which are used for the determination of the structure
E35 SPECTRSCPIC TECNIQUES IN RGANIC CEMISTRY Introductory Comments. These notes are designed to introduce you to the basic spectroscopic techniques which are used for the determination of the structure
17.1 Classes of Dienes
 W 2/1 Due: HW14, spec03 Due: n/a M 2/6 Lecture HW14 grading Lect17a Conjugated π systems Lecture quiz Lect17b Lab Lab02 Qual Analysis II (cont) 7-1 17.1 Classes of Dienes There are three categories for
W 2/1 Due: HW14, spec03 Due: n/a M 2/6 Lecture HW14 grading Lect17a Conjugated π systems Lecture quiz Lect17b Lab Lab02 Qual Analysis II (cont) 7-1 17.1 Classes of Dienes There are three categories for
Structure Determination. How to determine what compound that you have? One way to determine compound is to get an elemental analysis
 Structure Determination How to determine what compound that you have? ne way to determine compound is to get an elemental analysis -basically burn the compound to determine %C, %H, %, etc. from these percentages
Structure Determination How to determine what compound that you have? ne way to determine compound is to get an elemental analysis -basically burn the compound to determine %C, %H, %, etc. from these percentages
Chapter 14: Conjugated Dienes and Ultraviolet Spectroscopy Diene: molecule with two double bonds Conjugated diene: alternating double and single bonds
 Chapter 14: Conjugated Dienes and Ultraviolet Spectroscopy Diene: molecule with two double bonds Conjugated diene: alternating double and single bonds C-C single bond lkene Diene C=C double bonds Conjugate
Chapter 14: Conjugated Dienes and Ultraviolet Spectroscopy Diene: molecule with two double bonds Conjugated diene: alternating double and single bonds C-C single bond lkene Diene C=C double bonds Conjugate
Conjugated Dienes. Chapter 14 Organic Chemistry, 8 th Edition John E. McMurry
 Conjugated Dienes Chapter 14 Organic Chemistry, 8 th Edition John E. McMurry 1 Dienes Propadiene (allene) is a cumulated diene 1,3-Butadiene is a conjugated diene. 1,4-Pentadiene is an isolated diene.
Conjugated Dienes Chapter 14 Organic Chemistry, 8 th Edition John E. McMurry 1 Dienes Propadiene (allene) is a cumulated diene 1,3-Butadiene is a conjugated diene. 1,4-Pentadiene is an isolated diene.
09/05/40 MOLECULAR ABSORPTION METHODS
 MOLECULAR ABSORPTION METHODS Absorption spectroscopy refers to spectroscopic techniques that measure the absorption of radiation, as a function of wavelength ( absorption spectrum ), due to its interaction
MOLECULAR ABSORPTION METHODS Absorption spectroscopy refers to spectroscopic techniques that measure the absorption of radiation, as a function of wavelength ( absorption spectrum ), due to its interaction
General Infrared Absorption Ranges of Various Functional Groups
 General Infrared Absorption Ranges of Various Functional Groups Frequency Range Bond Type of Compound cm -1 Intensity C Alkanes 2850-2970 Strong 1340-1470 Strong C Alkenes 3010-3095 Medium 675-995 Strong
General Infrared Absorption Ranges of Various Functional Groups Frequency Range Bond Type of Compound cm -1 Intensity C Alkanes 2850-2970 Strong 1340-1470 Strong C Alkenes 3010-3095 Medium 675-995 Strong
Introduction. The analysis of the outcome of a reaction requires that we know the full structure of the products as well as the reactants
 Introduction The analysis of the outcome of a reaction requires that we know the full structure of the products as well as the reactants Spectroscopy and the Electromagnetic Spectrum Unlike mass spectrometry,
Introduction The analysis of the outcome of a reaction requires that we know the full structure of the products as well as the reactants Spectroscopy and the Electromagnetic Spectrum Unlike mass spectrometry,
CHAPTER 9 THEORY OF RESONANCE BY, G.DEEPA
 CHAPTER 9 THEORY OF RESONANCE BY, G.DEEPA Conjugation in Alkadienes and Allylic Systems conjugation a series of overlapping p orbitals The Allyl Group allylic position is the next to a double bond 1 allyl
CHAPTER 9 THEORY OF RESONANCE BY, G.DEEPA Conjugation in Alkadienes and Allylic Systems conjugation a series of overlapping p orbitals The Allyl Group allylic position is the next to a double bond 1 allyl
two slits and 5 slits
 Electronic Spectroscopy 2015January19 1 1. UV-vis spectrometer 1.1. Grating spectrometer 1.2. Single slit: 1.2.1. I diffracted intensity at relative to un-diffracted beam 1.2.2. I - intensity of light
Electronic Spectroscopy 2015January19 1 1. UV-vis spectrometer 1.1. Grating spectrometer 1.2. Single slit: 1.2.1. I diffracted intensity at relative to un-diffracted beam 1.2.2. I - intensity of light
Chem 1075 Chapter 19 Organic Chemistry Lecture Outline
 Chem 1075 Chapter 19 Organic Chemistry Lecture Outline Slide 2 Introduction Organic chemistry is the study of and its compounds. The major sources of carbon are the fossil fuels: petroleum, natural gas,
Chem 1075 Chapter 19 Organic Chemistry Lecture Outline Slide 2 Introduction Organic chemistry is the study of and its compounds. The major sources of carbon are the fossil fuels: petroleum, natural gas,
Alkanes 3/27/17. Hydrocarbons: Compounds made of hydrogen and carbon only. Aliphatic (means fat ) - Open chain Aromatic - ring. Alkane Alkene Alkyne
 Alkanes EQ 1. How will I define Hydrocarbons? 2. Compare and contrast the 3 types of hydrocarbons (Alkanes, alkenes, alkynes). Hydrocarbons: Compounds made of hydrogen and carbon only. Aliphatic (means
Alkanes EQ 1. How will I define Hydrocarbons? 2. Compare and contrast the 3 types of hydrocarbons (Alkanes, alkenes, alkynes). Hydrocarbons: Compounds made of hydrogen and carbon only. Aliphatic (means
ORGANIC SPECTROSCOPY NOTES
 - 1 - ORGANIC SPECTROSCOPY NOTES Basics of Spectroscopy UV/vis, IR and NMR are all types of Absorption Spectroscopy, where EM radiation corresponding to exactly the energy of specific excitations in molecules
- 1 - ORGANIC SPECTROSCOPY NOTES Basics of Spectroscopy UV/vis, IR and NMR are all types of Absorption Spectroscopy, where EM radiation corresponding to exactly the energy of specific excitations in molecules
Chapter 15 Dienes, Resonance, and Aromaticity
 Instructor Supplemental Solutions to Problems 2010 Roberts and Company Publishers Chapter 15 Dienes, Resonance, and Aromaticity Solutions to In-Text Problems 15.2 The delocalization energy is the energy
Instructor Supplemental Solutions to Problems 2010 Roberts and Company Publishers Chapter 15 Dienes, Resonance, and Aromaticity Solutions to In-Text Problems 15.2 The delocalization energy is the energy
1. Which compound would you expect to have the lowest boiling point? A) NH 2 B) NH 2
 MULTIPLE CICE QUESTINS Topic: Intermolecular forces 1. Which compound would you expect to have the lowest boiling point? A) N 2 B) N 2 C) N D) E) N Ans: : N 2 D Topic: Molecular geometry, dipole moment
MULTIPLE CICE QUESTINS Topic: Intermolecular forces 1. Which compound would you expect to have the lowest boiling point? A) N 2 B) N 2 C) N D) E) N Ans: : N 2 D Topic: Molecular geometry, dipole moment
Chapter 12 Mass Spectrometry and Infrared Spectroscopy
 Organic Chemistry, 6 th Edition L. G. Wade, Jr. Chapter 12 Mass Spectrometry and Infrared Spectroscopy Jo Blackburn Richland College, Dallas, TX Dallas County Community College District 2006, Prentice
Organic Chemistry, 6 th Edition L. G. Wade, Jr. Chapter 12 Mass Spectrometry and Infrared Spectroscopy Jo Blackburn Richland College, Dallas, TX Dallas County Community College District 2006, Prentice
Chapter 25: The Chemistry of Life: Organic and Biological Chemistry
 Chemistry: The Central Science Chapter 25: The Chemistry of Life: Organic and Biological Chemistry The study of carbon compounds constitutes a separate branch of chemistry known as organic chemistry The
Chemistry: The Central Science Chapter 25: The Chemistry of Life: Organic and Biological Chemistry The study of carbon compounds constitutes a separate branch of chemistry known as organic chemistry The
Chapter 4 Ultraviolet and visible spectroscopy Molecular Spectrophotometry
 Chapter 4 Ultraviolet and visible spectroscopy Molecular Spectrophotometry Properties of light Electromagnetic radiation and electromagnetic spectrum Absorption of light Beer s law Limitation of Beer s
Chapter 4 Ultraviolet and visible spectroscopy Molecular Spectrophotometry Properties of light Electromagnetic radiation and electromagnetic spectrum Absorption of light Beer s law Limitation of Beer s
Infra-red Spectroscopy
 Molecular vibrations are associated with the absorption of energy (infrared activity) by the molecule as sets of atoms (molecular moieties) vibrate about the mean center of their chemical bonds. Infra-red
Molecular vibrations are associated with the absorption of energy (infrared activity) by the molecule as sets of atoms (molecular moieties) vibrate about the mean center of their chemical bonds. Infra-red
Organic Chemistry: CHEM2322
 Conjugated Systems Organic Chemistry: We met in Chem 2321 unsaturated bonds as either a C=C bond or C C bond. If these unsaturated bonds are well separated then they react independently however if there
Conjugated Systems Organic Chemistry: We met in Chem 2321 unsaturated bonds as either a C=C bond or C C bond. If these unsaturated bonds are well separated then they react independently however if there
Conjugated Systems. With conjugated double bonds resonance structures can be drawn
 Conjugated Systems Double bonds in conjugation behave differently than isolated double bonds With conjugated double bonds resonance structures can be drawn With isolated double bonds cannot draw resonance
Conjugated Systems Double bonds in conjugation behave differently than isolated double bonds With conjugated double bonds resonance structures can be drawn With isolated double bonds cannot draw resonance
Increasing energy. ( 10 4 cm -1 ) ( 10 2 cm -1 )
 The branch of science which deals with the interaction of electromagnetic radiation with matter is called spectroscopy The energy absorbed or emitted in each transition corresponds to a definite frequency
The branch of science which deals with the interaction of electromagnetic radiation with matter is called spectroscopy The energy absorbed or emitted in each transition corresponds to a definite frequency
Infrared Spectroscopy
 Infrared Spectroscopy Introduction Spectroscopy is an analytical technique which helps determine structure. It destroys little or no sample. The amount of light absorbed by the sample is measured as wavelength
Infrared Spectroscopy Introduction Spectroscopy is an analytical technique which helps determine structure. It destroys little or no sample. The amount of light absorbed by the sample is measured as wavelength
Conjugated Dienes. Chapter 14 Organic Chemistry, 8 th Edition John E. McMurry
 Conjugated Dienes Chapter 14 Organic Chemistry, 8 th Edition John E. McMurry 1 Dienes Propadiene (allene) is a cumulated diene 1,3-Butadiene is a conjugated diene. 1,4-Pentadiene is an isolated diene.
Conjugated Dienes Chapter 14 Organic Chemistry, 8 th Edition John E. McMurry 1 Dienes Propadiene (allene) is a cumulated diene 1,3-Butadiene is a conjugated diene. 1,4-Pentadiene is an isolated diene.
FAMILIES of ORGANIC COMPOUNDS
 1 SCH4U October 2016 Organic Chemistry Chemistry of compounds that contain carbon (except: CO, CO 2, HCN, CO 3 - ) Carbon is covalently bonded to another carbon, hydrogen and possibly to oxygen, a halogen
1 SCH4U October 2016 Organic Chemistry Chemistry of compounds that contain carbon (except: CO, CO 2, HCN, CO 3 - ) Carbon is covalently bonded to another carbon, hydrogen and possibly to oxygen, a halogen
Chem 263 Notes Sept. 26, 2013
 Chem 263 Notes Sept. 26, 2013 Example of Predicting Stereochemistry The following example uses 2Z, 4E-hexadiene (diene) and cyclopentene (dienophile) to produce an endo product. As shown before with the
Chem 263 Notes Sept. 26, 2013 Example of Predicting Stereochemistry The following example uses 2Z, 4E-hexadiene (diene) and cyclopentene (dienophile) to produce an endo product. As shown before with the
Infrared Spectroscopy: Identification of Unknown Substances
 Infrared Spectroscopy: Identification of Unknown Substances Suppose a white powder is one of the four following molecules. How can they be differentiated? H N N H H H H Na H H H H H A technique that is
Infrared Spectroscopy: Identification of Unknown Substances Suppose a white powder is one of the four following molecules. How can they be differentiated? H N N H H H H Na H H H H H A technique that is
Ch 14 Conjugated Dienes and UV Spectroscopy
 Ch 14 Conjugated Dienes and UV Spectroscopy Conjugated Systems - Conjugated systems have alternating single and double bonds. For example: C=C C=C C=C and C=C C=O - This is not conjugated because the double
Ch 14 Conjugated Dienes and UV Spectroscopy Conjugated Systems - Conjugated systems have alternating single and double bonds. For example: C=C C=C C=C and C=C C=O - This is not conjugated because the double
Ethers. Chapter 14: Ethers, Epoxides, & Sulfides. General Formula: Types: a) Symmetrical: Examples: b) Unsymmetrical: Examples: Physical Properties:
 Chamras Chemistry 106 Lecture Notes Examination 1 Materials Chapter 14: Ethers, Epoxides, & Sulfides Ethers General Formula: Types: a) Symmetrical: Examples: b) Unsymmetrical: Examples: Physical Properties:
Chamras Chemistry 106 Lecture Notes Examination 1 Materials Chapter 14: Ethers, Epoxides, & Sulfides Ethers General Formula: Types: a) Symmetrical: Examples: b) Unsymmetrical: Examples: Physical Properties:
12. Structure Determination: Mass Spectrometry and Infrared Spectroscopy
 12. Structure Determination: Mass Spectrometry and Infrared Spectroscopy Determining the Structure of an Organic Compound The analysis of the outcome of a reaction requires that we know the full structure
12. Structure Determination: Mass Spectrometry and Infrared Spectroscopy Determining the Structure of an Organic Compound The analysis of the outcome of a reaction requires that we know the full structure
PAPER No.12 :Organic Spectroscopy MODULE No.29: Combined problem on UV, IR, 1 H NMR, 13 C NMR and Mass - Part I
 Subject Chemistry Paper No and Title Module No and Title Module Tag 12: rganic Spectroscopy 29: Combined problem on UV, IR, 1 H NMR, 13 C NMR and Mass - Part I CHE_P12_M29 TABLE F CNTENTS 1. Learning utcomes
Subject Chemistry Paper No and Title Module No and Title Module Tag 12: rganic Spectroscopy 29: Combined problem on UV, IR, 1 H NMR, 13 C NMR and Mass - Part I CHE_P12_M29 TABLE F CNTENTS 1. Learning utcomes
Answers to Problems. Chapter 2 2. (J' ~ 5. (a) n ~ Chapter 3. n* > n ~ n* n* < n ~ n* < n ~ (J'* > n ~ er* > n ~
 Answers to Problems Chapter 1 3. Gamma rays > X-rays > UV > Visible > IR > Radio waves 6. (a) (i) 4 cm- 1 (ii) 3587.7 cm- 1 (iii) 3355.7 cm- 1 (b) 7.495 x 1 8 MHz to 3.748 x 1 8 MHz 7. (a) 7.15 kcal/mole
Answers to Problems Chapter 1 3. Gamma rays > X-rays > UV > Visible > IR > Radio waves 6. (a) (i) 4 cm- 1 (ii) 3587.7 cm- 1 (iii) 3355.7 cm- 1 (b) 7.495 x 1 8 MHz to 3.748 x 1 8 MHz 7. (a) 7.15 kcal/mole
Experiment 3. Condensation Reactions of Ketones and Aldehydes: The Aldol Condensation Reaction.
 Experiment 3. Condensation Reactions of Ketones and Aldehydes: The Aldol Condensation Reaction. References: Brown & Foote, Chapters 16, 19, 23 INTRODUCTION: This experiment continues the saga of carbon-carbon
Experiment 3. Condensation Reactions of Ketones and Aldehydes: The Aldol Condensation Reaction. References: Brown & Foote, Chapters 16, 19, 23 INTRODUCTION: This experiment continues the saga of carbon-carbon
Chapter 9. Organic Chemistry: The Infinite Variety of Carbon Compounds. Organic Chemistry
 Chapter 9 Organic Chemistry: The Infinite Variety of Carbon Compounds Organic Chemistry Organic chemistry is defined as the chemistry of carbon compounds. Of tens of millions of known chemical compounds,
Chapter 9 Organic Chemistry: The Infinite Variety of Carbon Compounds Organic Chemistry Organic chemistry is defined as the chemistry of carbon compounds. Of tens of millions of known chemical compounds,
7a. Structure Elucidation: IR and 13 C-NMR Spectroscopies (text , , 12.10)
 2009, Department of Chemistry, The University of Western Ontario 7a.1 7a. Structure Elucidation: IR and 13 C-NMR Spectroscopies (text 11.1 11.5, 12.1 12.5, 12.10) A. Electromagnetic Radiation Energy is
2009, Department of Chemistry, The University of Western Ontario 7a.1 7a. Structure Elucidation: IR and 13 C-NMR Spectroscopies (text 11.1 11.5, 12.1 12.5, 12.10) A. Electromagnetic Radiation Energy is
Can you differentiate A from B using 1 H NMR in each pair?
 Can you differentiate A from B using 1 H NMR in each pair? To be NMR active any nucleus must have a spin quantum number, different from zero (I 0) As in 1 H, the spin quantum number (I) of 13 C is 1/2
Can you differentiate A from B using 1 H NMR in each pair? To be NMR active any nucleus must have a spin quantum number, different from zero (I 0) As in 1 H, the spin quantum number (I) of 13 C is 1/2
MOLECULAR ABSORPTION METHODS
 MOLECULAR ABSORPTION METHODS Absorption spectroscopy refers to spectroscopic techniques that measure the absorption of radiation, as a function of wavelength ( absorption spectrum ), due to its interaction
MOLECULAR ABSORPTION METHODS Absorption spectroscopy refers to spectroscopic techniques that measure the absorption of radiation, as a function of wavelength ( absorption spectrum ), due to its interaction
Advanced Pharmaceutical Analysis
 Lecture 2 Advanced Pharmaceutical Analysis IR spectroscopy Dr. Baraa Ramzi Infrared Spectroscopy It is a powerful tool for identifying pure organic and inorganic compounds. Every molecular compound has
Lecture 2 Advanced Pharmaceutical Analysis IR spectroscopy Dr. Baraa Ramzi Infrared Spectroscopy It is a powerful tool for identifying pure organic and inorganic compounds. Every molecular compound has
CONJUGATED PI SYSTEMS AND PERICYCLIC REACTIONS
 CNJUGATED PI SYSTEMS AND PERICYCLIC REACTINS A STUDENT SULD BE ABLE T: 1. Recognize and give examples of conjugated, isolated, and cumulated dienes, and of allylic intermediates. Also, name given the structure,
CNJUGATED PI SYSTEMS AND PERICYCLIC REACTINS A STUDENT SULD BE ABLE T: 1. Recognize and give examples of conjugated, isolated, and cumulated dienes, and of allylic intermediates. Also, name given the structure,
Detailed Course Content
 Detailed Course Content Chapter 1: Carbon Compounds and Chemical Bonds The Structural Theory of Organic Chemistry 4 Chemical Bonds: The Octet Rule 6 Lewis Structures 8 Formal Charge 11 Resonance 14 Quantum
Detailed Course Content Chapter 1: Carbon Compounds and Chemical Bonds The Structural Theory of Organic Chemistry 4 Chemical Bonds: The Octet Rule 6 Lewis Structures 8 Formal Charge 11 Resonance 14 Quantum
4.3A: Electronic transitions
 Ashley Robison My Preferences Site Tools Popular pages MindTouch User Guide FAQ Sign Out If you like us, please share us on social media. The latest UCD Hyperlibrary newsletter is now complete, check it
Ashley Robison My Preferences Site Tools Popular pages MindTouch User Guide FAQ Sign Out If you like us, please share us on social media. The latest UCD Hyperlibrary newsletter is now complete, check it
EXPT. 7 CHARACTERISATION OF FUNCTIONAL GROUPS USING IR SPECTROSCOPY
 EXPT. 7 CHARACTERISATION OF FUNCTIONAL GROUPS USING IR SPECTROSCOPY Structure 7.1 Introduction Objectives 7.2 Principle 7.3 Requirements 7.4 Strategy for the Interpretation of IR Spectra 7.5 Practice Problems
EXPT. 7 CHARACTERISATION OF FUNCTIONAL GROUPS USING IR SPECTROSCOPY Structure 7.1 Introduction Objectives 7.2 Principle 7.3 Requirements 7.4 Strategy for the Interpretation of IR Spectra 7.5 Practice Problems
AP Chemistry Chapter 22 - Organic and Biological Molecules
 AP Chemistry Chapter - Organic and Biological Molecules.1 Alkanes: Saturated Hydrocarbons A. Straight-chain Hydrocarbons 1. Straight-chain alkanes have the formula C n H n+. Carbons are sp hybridized The
AP Chemistry Chapter - Organic and Biological Molecules.1 Alkanes: Saturated Hydrocarbons A. Straight-chain Hydrocarbons 1. Straight-chain alkanes have the formula C n H n+. Carbons are sp hybridized The
Radiant energy is proportional to its frequency (cycles/s = Hz) as a wave (Amplitude is its height) Different types are classified by frequency or
 CHEM 241 UNIT 5: PART B INFRA-RED RED SPECTROSCOPY 1 Spectroscopy of the Electromagnetic Spectrum Radiant energy is proportional to its frequency (cycles/s = Hz) as a wave (Amplitude is its height) Different
CHEM 241 UNIT 5: PART B INFRA-RED RED SPECTROSCOPY 1 Spectroscopy of the Electromagnetic Spectrum Radiant energy is proportional to its frequency (cycles/s = Hz) as a wave (Amplitude is its height) Different
Learning Guide for Chapter 3 - Infrared Spectroscopy
 Learning Guide for hapter 3 - Infrared Spectroscopy I. Introduction to spectroscopy - p 1 II. Molecular vibrations - p 3 III. Identifying functional groups - p 6 IV. Interpreting an IR spectrum - p 12
Learning Guide for hapter 3 - Infrared Spectroscopy I. Introduction to spectroscopy - p 1 II. Molecular vibrations - p 3 III. Identifying functional groups - p 6 IV. Interpreting an IR spectrum - p 12
CH 3. mirror plane. CH c d
 CAPTER 20 Practice Exercises 20.1 The index of hydrogen deficiency is two. The structural possibilities include two double bonds, a double do 20.3 (a) As this is an alkane, it contains only C and and has
CAPTER 20 Practice Exercises 20.1 The index of hydrogen deficiency is two. The structural possibilities include two double bonds, a double do 20.3 (a) As this is an alkane, it contains only C and and has
Introduc)on to Func)onal Groups in Organic Molecules
 Introduc)on to Func)onal Groups in rganic Molecules CH 3 H 3 C N C C N C C N N CH CH 3 Caffeine Func)onal Group Func%onal group - collec)on of atoms at a site that have a characteris)c behavior in all
Introduc)on to Func)onal Groups in rganic Molecules CH 3 H 3 C N C C N C C N N CH CH 3 Caffeine Func)onal Group Func%onal group - collec)on of atoms at a site that have a characteris)c behavior in all
Paper: 12, Organic Spectroscopy Module: 5, Applications of UV spectroscopy
 Subject Chemistry Paper No and Title Module No and Title Module Tag Paper 12: Organic Spectroscopy Applications of UV-visible Spectroscopy CHE_P12_M5 TABLE OF CONTENTS 1. Learning Outcomes 2. Introduction
Subject Chemistry Paper No and Title Module No and Title Module Tag Paper 12: Organic Spectroscopy Applications of UV-visible Spectroscopy CHE_P12_M5 TABLE OF CONTENTS 1. Learning Outcomes 2. Introduction
1. How do you account for the formation of ethane during chlorination of methane?
 1. How do you account for the formation of ethane during chlorination of methane? The formation of ethane is due to the side reaction in termination step by the combination of two CH 3 free radicals. 2.
1. How do you account for the formation of ethane during chlorination of methane? The formation of ethane is due to the side reaction in termination step by the combination of two CH 3 free radicals. 2.
Paper 12: Organic Spectroscopy
 Subject Chemistry Paper No and Title Module No and Title Module Tag Paper 12: Organic Spectroscopy 31: Combined problem on UV, IR, 1 H NMR, 13 C NMR and Mass - Part III CHE_P12_M31 TABLE OF CONTENTS 1.
Subject Chemistry Paper No and Title Module No and Title Module Tag Paper 12: Organic Spectroscopy 31: Combined problem on UV, IR, 1 H NMR, 13 C NMR and Mass - Part III CHE_P12_M31 TABLE OF CONTENTS 1.
Chapter 2: An Introduction to Organic Compounds
 Chapter : An Introduction to Organic Compounds I. FUNCTIONAL GROUPS: Functional groups with similar structure/reactivity may be "grouped" together. A. Functional Groups With Carbon-Carbon Multiple Bonds.
Chapter : An Introduction to Organic Compounds I. FUNCTIONAL GROUPS: Functional groups with similar structure/reactivity may be "grouped" together. A. Functional Groups With Carbon-Carbon Multiple Bonds.
The International Union of Pure and Applied Chemistry has developed a system of rules for naming organic molecules.
 HYDRCARBNS AND THEIR DERIVATIVES The field of organic chemistry includes the study of hydrocarbons (compounds composed of carbon and hydrogen atoms covalently bonded together) and their derivatives (variations
HYDRCARBNS AND THEIR DERIVATIVES The field of organic chemistry includes the study of hydrocarbons (compounds composed of carbon and hydrogen atoms covalently bonded together) and their derivatives (variations
1.1. IR is part of electromagnetic spectrum between visible and microwave
 CH2SWK 44/6416 IR Spectroscopy 2013Feb5 1 1. Theory and properties 1.1. IR is part of electromagnetic spectrum between visible and microwave 1.2. 4000 to 400 cm -1 (wave numbers) most interesting to organic
CH2SWK 44/6416 IR Spectroscopy 2013Feb5 1 1. Theory and properties 1.1. IR is part of electromagnetic spectrum between visible and microwave 1.2. 4000 to 400 cm -1 (wave numbers) most interesting to organic
Application of IR Raman Spectroscopy
 Application of IR Raman Spectroscopy 3 IR regions Structure and Functional Group Absorption IR Reflection IR Photoacoustic IR IR Emission Micro 10-1 Mid-IR Mid-IR absorption Samples Placed in cell (salt)
Application of IR Raman Spectroscopy 3 IR regions Structure and Functional Group Absorption IR Reflection IR Photoacoustic IR IR Emission Micro 10-1 Mid-IR Mid-IR absorption Samples Placed in cell (salt)
Organic Chemistry. FAMILIES of ORGANIC COMPOUNDS
 1 SCH4U September 2017 Organic Chemistry Is the chemistry of compounds that contain carbon (except: CO, CO 2, HCN, CO 3 2- ) Carbon is covalently bonded to another carbon, hydrogen and possibly to oxygen,
1 SCH4U September 2017 Organic Chemistry Is the chemistry of compounds that contain carbon (except: CO, CO 2, HCN, CO 3 2- ) Carbon is covalently bonded to another carbon, hydrogen and possibly to oxygen,
California State Polytechnic University, Pomona
 alifornia State Polytechnic University, Pomona 2-1 Dr. Laurie S. Starkey, rganic hemistry M 314, Wade hapter 2: Structure and Physical Properties of rganic Molecules hapter utline 1) rbitals and Bonding
alifornia State Polytechnic University, Pomona 2-1 Dr. Laurie S. Starkey, rganic hemistry M 314, Wade hapter 2: Structure and Physical Properties of rganic Molecules hapter utline 1) rbitals and Bonding
Chapter 1 Reactions of Organic Compounds. Reactions Involving Hydrocarbons
 Chapter 1 Reactions of Organic Compounds Reactions Involving Hydrocarbons Reactions of Alkanes Single bonds (C-C) are strong and very hard to break, therefore these compounds are relatively unreactive
Chapter 1 Reactions of Organic Compounds Reactions Involving Hydrocarbons Reactions of Alkanes Single bonds (C-C) are strong and very hard to break, therefore these compounds are relatively unreactive
Infrared Spectroscopy An Instrumental Method for Detecting Functional Groups
 Infrared Spectroscopy An Instrumental Method for Detecting Functional Groups 1 The Electromagnetic Spectrum Infrared Spectroscopy I. Physics Review Frequency, υ (nu), is the number of wave cycles that
Infrared Spectroscopy An Instrumental Method for Detecting Functional Groups 1 The Electromagnetic Spectrum Infrared Spectroscopy I. Physics Review Frequency, υ (nu), is the number of wave cycles that
Lesmahagow High School CfE Advanced Higher Chemistry. Unit 2 Organic Chemistry and Instrumental Analysis. Molecular Orbitals and Structure
 Lesmahagow High School CfE Advanced Higher Chemistry Unit 2 Organic Chemistry and Instrumental Analysis Molecular Orbitals and Structure 1 Molecular Orbitals Orbitals can be used to explain the bonding
Lesmahagow High School CfE Advanced Higher Chemistry Unit 2 Organic Chemistry and Instrumental Analysis Molecular Orbitals and Structure 1 Molecular Orbitals Orbitals can be used to explain the bonding
13.24: Mass Spectrometry: molecular weight of the sample
 hapter 13: Spectroscopy Methods of structure determination Nuclear Magnetic Resonances (NMR) Spectroscopy (Sections 13.3-13.19) Infrared (IR) Spectroscopy (Sections 13.20-13.22) Ultraviolet-visible (UV-Vis)
hapter 13: Spectroscopy Methods of structure determination Nuclear Magnetic Resonances (NMR) Spectroscopy (Sections 13.3-13.19) Infrared (IR) Spectroscopy (Sections 13.20-13.22) Ultraviolet-visible (UV-Vis)
OAT Organic Chemistry - Problem Drill 19: NMR Spectroscopy and Mass Spectrometry
 OAT Organic Chemistry - Problem Drill 19: NMR Spectroscopy and Mass Spectrometry Question No. 1 of 10 Question 1. Which statement concerning NMR spectroscopy is incorrect? Question #01 (A) Only nuclei
OAT Organic Chemistry - Problem Drill 19: NMR Spectroscopy and Mass Spectrometry Question No. 1 of 10 Question 1. Which statement concerning NMR spectroscopy is incorrect? Question #01 (A) Only nuclei
Lecture 13 Organic Chemistry 1
 EM 232 rganic hemistry I at hicago Lecture 13 rganic hemistry 1 Professor Duncan Wardrop February 23, 2010 1 EM 232 rganic hemistry I at hicago Spectroscopy & Spectrometry hapter 13 2 EM 232 rganic hemistry
EM 232 rganic hemistry I at hicago Lecture 13 rganic hemistry 1 Professor Duncan Wardrop February 23, 2010 1 EM 232 rganic hemistry I at hicago Spectroscopy & Spectrometry hapter 13 2 EM 232 rganic hemistry
SPECTROSCOPY MEASURES THE INTERACTION BETWEEN LIGHT AND MATTER
 SPECTROSCOPY MEASURES THE INTERACTION BETWEEN LIGHT AND MATTER c = c: speed of light 3.00 x 10 8 m/s (lamda): wavelength (m) (nu): frequency (Hz) Increasing E (J) Increasing (Hz) E = h h - Planck s constant
SPECTROSCOPY MEASURES THE INTERACTION BETWEEN LIGHT AND MATTER c = c: speed of light 3.00 x 10 8 m/s (lamda): wavelength (m) (nu): frequency (Hz) Increasing E (J) Increasing (Hz) E = h h - Planck s constant
Infrared Spectroscopy
 x-rays ultraviolet (UV) visible Infrared (I) microwaves radiowaves near I middle I far I λ (cm) 8 x 10-5 2.5 x 10-4 2.5 x 10-3 2.5 x 10-2 µ 0.8 2.5 25 250 ν (cm -1 ) 13,000 4,000 400 40 ν (cm -1 1 ) =
x-rays ultraviolet (UV) visible Infrared (I) microwaves radiowaves near I middle I far I λ (cm) 8 x 10-5 2.5 x 10-4 2.5 x 10-3 2.5 x 10-2 µ 0.8 2.5 25 250 ν (cm -1 ) 13,000 4,000 400 40 ν (cm -1 1 ) =
Ultraviolet Spectroscopy
 This work by IJARBEST is licensed under a Creative Commons Attribution 4.0 International License. Available at https://www.ijarbest.com Ultraviolet Spectroscopy 1 D. Farvez Basha, 2 C. Santhiya, 2 K. Tharani
This work by IJARBEST is licensed under a Creative Commons Attribution 4.0 International License. Available at https://www.ijarbest.com Ultraviolet Spectroscopy 1 D. Farvez Basha, 2 C. Santhiya, 2 K. Tharani
Chapter 15: Conjugated Systems, Orbital Symmetry, and UV Spectroscopy
 Chapter 15: Conjugated Systems, Orbital Symmetry, and UV Spectroscopy Conjugated unsaturated systems have a p orbital on a carbon adjacent to a double bond The p orbital can come from another double (e.g.
Chapter 15: Conjugated Systems, Orbital Symmetry, and UV Spectroscopy Conjugated unsaturated systems have a p orbital on a carbon adjacent to a double bond The p orbital can come from another double (e.g.
