Kinetic Limitations on Cloud Droplet Formation and Impact on Cloud Albedo
|
|
|
- Joshua Osborne
- 6 years ago
- Views:
Transcription
1 1 Appeared in Tellus B, 53, , Kinetic Limitations on Cloud Droplet Formation and Impact on Cloud Albedo Athanasios Nenes Department of Chemical Engineering,Mail Code , California Institute of Technology, Pasadena, California, 91125, USA Steven Ghan Pacific Northwest National Laboratory, Richland, Washington, 99352, USA Hayder Abdul-Razzak Texas A&M University-Kingsville, Kingsville, Texas, 78363, USA Patrick Y.Chuang NCAR, Boulder, Colorado, 80307, USA John H. Seinfeld Department of Chemical Engineering,Mail Code , California Institute of Technology, Pasadena, California, 91125, USA Abstract. Under certain conditions mass transfer limitations on the growth of cloud condensation nuclei (CCN) may have a significant impact on the number of droplets that can form in a cloud. The assumption that particles remain in equilibrium until activated may therefore not always be appropriate for aerosol populations existing in the atmosphere. This work identifies three mechanisms that lead to kinetic limitations, the effect of which on activated cloud droplet number and cloud albedo is assessed using a one-dimensional cloud parcel model with detailed microphysics for a variety of aerosol size distributions and updraft velocities. In assessing the effect of kinetic limitations, we have assumed as cloud droplets not only those that are strictly activated (as dictated by classical Köhler theory), but also unactivated drops large enough to have an impact on cloud optical properties. Aerosol number concentration is found to be the key parameter that controls the significance of kinetic effects. Simulations indicate that the equilibrium assumption leads to an overprediction of droplet number by less than 10% for marine aerosol; this overprediction can exceed 40% for urban type aerosol. Overall, the effect of kinetic limitations on cloud albedo can be considered important when equilibrium activation theory consistently overpredicts droplet number by more than 10%. The maximum change in cloud albedo as a result of kinetic limitations is less than for cases such as marine aerosol; however albedo differences can exceed 0.1 under more polluted conditions. Kinetic limitations are thus not expected to be climatically significant on a global scale, but can regionally have a large impact on cloud albedo.
2 1. Introduction Much of the uncertainty associated with quantifying the indirect climatic effect of aerosols originates in the complex relationship between aerosols and cloud droplets. Approximate analytical expressions that predict those aerosol particles that activate to form cloud droplets have been proposed for implementation in general circulation models (GCMs) (Ghan et al. [1993]; Liu and Wang [1996]; Abdul-Razzak et al. [1998]). These parameterizations generally rely on the assumption that particles are at equilibrium with the ambient (supersaturated) water vapor concentration until activated as cloud condensation nuclei (CCN). The number of droplets formed in a cloud can therefore be estimated from the number of CCN active at the maximum supersaturation in the cloud updraft. The problem of droplet nucleation parameterization is then reduced to the problem of determining the maximum supersaturation in the cloud parcel. The assumption that all particles respond instantaneously to any changes in supersaturation leads to a problem: the amount of water absorbed by the largest aerosol particles when they activate can be larger than the amount of water vapor available in the cloud parcel. This problem, known for a long time, is not serious when predicting the number of droplets, because although not activated, these particles have an equilibrium saturation ratio very close to unity, as activated particles do. As a consequence, their growth can be parameterized as if they were activated. In addition, the number of large particles that give rise to this problem are usually negligible compared to the concentration of smaller particles of the distribution, so the errors in cloud droplet number overall are expected to be small. The assumption of equilibrium, however, can lead to a discrepancy in droplet number as a result of mass transfer limitations. Chuang et al. [1997] have shown that under certain circumstances growth kinetics may retard the growth of CCN sufficiently to limit the number of activated droplets formed. By comparing the time scale for particle growth at equilibrium with that for actual condensational growth, Chuang et al. [1997] conclude that particles with critical supersaturation less than a threshold value do not have time to grow larger than their critical size, and thus do not activate. This suggests that equilibrium models that diagnose droplet formation from maximum supersaturation may systematically overestimate the number of activated droplets formed. Such a systematic bias could have implications for estimates of indirect aerosol radiative forcing of climate. It is difficult, however, to draw firm conclusions simply from a comparison of timescales because growth kinetics depend on the full time history of supersaturation and particle growth. Furthermore, the timescales controlling the growth of the droplets change drastically as the populations grows. A more conclusive method of evaluating the importance of growth kinetics is obtained by explicitly simulating the kinetic growth process and then comparing the simulated number of droplets formed with that predicted by equilibrium theory for the same CCN concentration and maximum supersaturation. Furthermore, it is incorrect to consider cloud drops as only those that are strictly activated (as defined by Köhler theory); the presence of large unactivated droplets cannot be neglected. This issue becomes even more important when slightly soluble substances are present in the aerosol (Laaksonen et al. [1998]). The condensational growth of an aerosol population prescribes that particles with very large dry diameters, although not activated, lead to roughly the same size of activated droplets and thus belong to the cloud droplet population. With this in mind, one can define as CCN all particles that produce droplets that are larger than the smallest particle that is strictly activated. This cloud droplet definition differs slightly from that given by Pandis et al. [1984]; we do not consider as droplets all the particles that exceed their critical diameter or have a critical supersaturation lower than ambient supersaturation, but only those comparable in size to those that are strictly activated. Based on the previous discussion, the inertial mechanism of Chuang et al. [1997] is believed not to contribute to any bias in the predicted droplet number when the equilibrium assumption is invoked. However, there are other kinetic limitation mechanisms that produce particles that are much smaller than activated drops; in this sense, these mechanisms act to decrease the number of cloud droplets from that predicted strictly on the basis of equilibrium activation. This study will focus mainly on these mechanisms. In the sections that follow, the mechanisms that lead to kinetic limitations in cloud droplet formation are presented. The models used for evaluating kinetic effects are then described, together with the relevant criteria used for assessing the potential climatic importance of these effects. Finally, simulations are presented, for a variety of updraft conditions and aerosol types, from which conclusions can be derived regarding the effect of kinetic limitations on cloud droplet number concentration and albedo. 2
3 3 Figure 1. Illustration of the kinetic limitation mechanisms. 2. Kinetic Limitation Mechanisms There are three kinetic limitation processes that can inhibit the formation of cloud droplets. These mechanisms will be explained with the help of Figure 1, which illustrates typical cloud parcel and droplet equilibrium supersaturation profiles (S and S eq, respectively) as a function of cloud depth, as predicted by adiabatic parcel theory. Each aerosol equilibrium curve corresponds to particles containing a different amount of solute. The equilibrium curves vary with respect to cloud depth as a consequence of droplets changing size as they traverse through the cloud column. According to equilibrium activation theory, any particle with a critical supersaturation, S c, less than the maximum supersaturation, S max, encountered in the parcel will activate. However, the time which particles are exposed to a supersaturation level is a crucial parameter; that time must be sufficiently long to allow the particle to reach its critical diameter. The droplet can be considered activated only when the wet diameter exceeds its critical value. Furthermore, to ensure constant growth of the droplet, the ambient supersaturation should be high enough for activated droplets to continuously grow throughout the duration of cloud formation. The yellow curve of Figure 1 represents an aerosol particle that activates and remains so throughout the entire time of cloud formation. The critical supersaturation of the particle, S c3, is less than S max ; the time needed for activation is also less than the time during which S S c3. When this particle activates, its equilibrium curve will be at a maximum (with S eq = S c ); subsequently, the particle is activated and S eq drops. As can be seen, the particle S eq is always less than the parcel supersaturation, so the driving force for growth, S S eq, is always positive. This guarantees that the particle will remain activated throughout the cloud. The same cannot be said for all the aerosol types depicted in Figure 1. The first mechanism that limits the formation of activated droplets is the inertial mechanism described by Chuang et al. [1997]. This mechanism is illustrated in Figure 1 for the particles with a critical supersaturation S c4 (blue curve). These particles have a large dry diameter and a very low critical supersaturation. The timescale of cloud formation is not sufficient for these particles to reach their critical diameter. Nonetheless, the driving force for growth is always positive, and these particles continuously grow, attaining a wet diameter similar (and actually larger) to those of the activated droplets. Thus, even though these particles do not activate, they cannot be distinguished from activated droplets, and so should be treated as such. The red curve corresponds to a particle with a relatively high critical supersaturation, S c1. The time during which S > S c1 is not sufficient for activation. As a result, the particle initially grows, but subsequently evaporates to become an interstitial aerosol particle. This kinetic effect is the second of the three mechanisms identified, and is termed the evaporation mechanism. Although this is an inertial mechanism (in the sense that small particles do not respond fast enough to changes in ambient supersaturation), a different name is assigned because the particles affected behave much differently than those subject to the inertial mechanism of Chuang et al. [1997]. Finally, some particles can initially activate but become interstitial aerosol through the third mechanism, the so-called deactivation mechanism. This is illustrated in Figure 1 for particles of critical supersaturation S c2 (green curve). These particles are exposed to a supersaturation that exceeds their critical values sufficiently long to activate and do so initially. However, after a while, the parcel supersaturation drops below the droplet S eq. When this happens, the growth driving force, S S eq, becomes negative, and these droplets begins evaporating. The rate of evaporation can be quite fast, and the droplet may deactivate and become part of the interstitial aerosol, thus decreasing the number of cloud droplets. This mechanism is not a result of mass
4 4 transfer kinetic limitation, but rather a dynamic effect arising from the limited available water vapor; water transfers from activated drops to other sizes that can still grow. The deactivation and evaporation mechanisms render the affected aerosol much smaller than the activated droplet size, leading to considerably smaller contribution to cloud optical properties. On the other hand, whereas the inertial mechanism prevents droplets from activating, it does not produce droplets that are differentiated from other activated droplets as the other two mechanisms do. When using the equilibrium assumption, it is expected that the inertial mechanism does not lead to any bias in predicted droplet number. The same cannot be said for the evaporation and deactivation mechanisms, which tend to decrease the number of cloud droplets that are formed. Therefore using the equilibrium assumption in the presence of these two mechanisms will tend to overestimate the droplet number. In summary, if kinetic limitations affect mainly larger particles (through the inertial mechanism), the equilibrium assumption should not induce a large error in predicted droplet number. However, if kinetic effects apply mainly to the smaller particles of a distribution, not only can the equilibrium assumption yield large error in predicted droplet number, but the droplet number will be sensitive to fluctuations in parcel supersaturation. Any factor that can influence supersaturation history (such as mode radius, number concentration, and updraft velocity) will, in turn, affect all the relevant time scales and thus the extent and type of kinetic limitations. 3. Measures of Kinetic Limitations Assessing the effect of kinetic limitations on cloud droplet formation requires first the calculation of two quantities, N eq and N kn, the number concentrations of droplets based on equilibrium and kinetic approaches, respectively. N eq is equal to the concentration of particles with critical supersaturation, S c, less than or equal to the maximum supersaturation, S max, achieved in the ascending air parcel (as calculated by the parcel model). N eq is based on the assumption that the particles that can activate do so instantaneously, and is the upper limit to the number of droplets that can be formed. N kn is the actual droplet concentration, and is equal to the number of particles that are larger than the activated particle with the smallest dry diameter (i.e. that has a wet radius larger than its critical value). This number contains droplets that have a critical supersaturation less than the parcel maximum supersaturation but which are not larger than their critical size. Critical parameters are calculated from classical Köhler theory (e.g., Seinfeld and Pandis [1998]). The importance of kinetic growth limitations on droplet formation will be measured in terms of both the activated droplet number and the cloud albedo. Based on the variation of N kn and N eq with cloud depth, z, one can define the total droplet ratio at any height z, α (z), α (z) = N kn (z)/n eq (z) (1) which expresses the ratio of actual droplet number to the maximum droplets attainable at a certain distance above cloud base. The droplet ratio includes both strictly activated and unactivated droplets. It is also useful, when assessing kinetic effects, to examine the portion of the droplet population that is strictly activated. For this purpose, the unactivation ratio, φ (z), defined as the fraction of droplets that are not activated, is used: φ (z) = N unact (z)/n kn (z) (2) where N unact (z) is the number of unactivated droplets in the distribution. For example, a φ (z) = 0.2 means that 20% of the droplets are smaller than their activation diameter and thus are not strictly activated. Profiles of α (z) and φ (z) can provide insight regarding the kinetic limitation mechanisms present. Figure 2 presents a qualitative sketch of the three types of α (z) profiles seen in the simulations. Each type represents a case where different kinetic limitation mechanisms are active. The type 1 profile is observed when the inertial mechanism is the only type of kinetic limitation active. In this case, φ (z) initially attains large values that decreases further up in the cloud column (not shown), and α (z) approaches unity with increasing z. If the evaporation mechanism is active, α (z) initially increases and approaches an asymptotic value less than unity; the fraction that never activates are the small particles that evaporate. This is the type 2 profile of Figure 2. Finally, if the deactivation mechanism is present, then the droplet ratio would initially increase, reach a maximum, and then begin to decrease as particles evaporate and deactivate. It is difficult to determine a priori when a discrepancy in cloud droplet number is important. Placed in the context of the effect on albedo, this issue becomes more straightforward; the discrepancy in droplet
5 hensive models (Considine and Curry [1998]), so this variation in droplet number with cloud height must be considered when calculating optical properties. Finally, existing theoretical aerosol-cloud parameterizations in GCMs are based on adiabatic cloud parcel model equations, and so estimating cloud albedo sensitivity to kinetic effects is appropriately carried out using adiabatic parcel model calculations. In order to assess the differences arising from the kinetic and thermodynamic assumptions, a droplet growth model has been incorporated within the framework of an adiabatic parcel model Cloud Parcel Model 5 Figure 2. Illustration of the three types of droplet ratio profiles seen in the simulations. number can be considered significant when the albedo is biased by an amount comparable to the change induced by anthropogenic effects. Furthermore, GCMs currently implement a cloud drop number calculation for determining cloud albedo, so it is directly relevant to examine the potential error from the equilibrium activation assumption. In calculating cloud albedo, the cloud liquid water content and effective radius of the droplet distribution are used, with the assumption that the droplet distribution is narrow. Furthermore, the effect of interstitial aerosol on the liquid water content and optical properties are neglected. 4. Cloud Parcel and Albedo Models A cloud parcel model is the simplest tool that can be used to simulate the evolution of droplet distributions throughout a non-precipitating cloud column. These models predict a number concentration profile that starts from zero at cloud base and reaches an asymptotic value further up. In reality, droplet number and size are also affected by turbulent mixing and downdrafts, which cannot be correctly accounted for in a single parcel model. Near cloud base, where kinetic effects are strongest, droplets from upper levels tend to dry out and do not participate in the droplet distribution. Therefore, one would still expect a droplet number concentration that is zero at cloud base and quickly reaches an asymptotic value. Such distributions have been measured and predicted by more compre- The adiabatic cloud parcel model is based upon the parcel model described by Pruppacher and Klett [1997] and Seinfeld and Pandis [1998]. Conservation of heat and moisture for a rising air parcel can be expressed as dt dt = gv c p dw v dt L c p dw v dt = dw c dt (3) (4) where T is the temperature of the air, V is the updraft velocity, and w v and w c are the mixing ratios of water vapor, and liquid water in the parcel, respectively. In (4), the condensation rate for a population of water droplets consisting of N i droplets of radius r i, i = 1...n can be expressed as dw c dt = 4πρ w ρ a n N i ri 2 i=1 dr i dt where the particle growth rate is determined from, with G given by (5) dr dt = G r (S S eq) (6) 1 G = ρ wrt p v D v M + Lρ w (7) w k a T( LM w 1) RT The supersaturation S is given by w v /w v 1. By integration of the supersaturation balance equation, ds dt = 1 w v [ dwv dt (S + 1) ( w v T dt dt w v p a ρ a gv )] (8)
6 6 and the equilibrium supersaturation S eq is given by Köhler theory, S eq = exp 2M wσ w RT ρ w r i 3n s M w 4πρ w (r 3 i r3 dry,i ) 1 (9) Here we have used the hydrostatic relation to relate changes in atmospheric pressure to vertical velocity in (8). D v in (9) is the diffusivity of water vapor in air, modified for non-continuum effects, D v = 1 + Dv a cr D v 2πM w RT (10) where a c = 1.0 is the condensation coefficient. k a in (7) is the thermal conductivity of air modified for noncontinuum effects, k a = k a 1 + k a 2πM a a T rρc p RT (11) where a T = 0.96 is the thermal accommodation coefficient, and n s in (9) is the number of moles of solute per particle, n s = 4ρ pενπd 3 i 3M s (12) where d i is the dry particle diameter, and ρ p = [(1 ε) /ρ u + ε/ρ s ] 1 (13) is the mean particle density. Surface tension σ w is expressed (in J m 2 ) as a function of the parcel temperature T (Seinfeld and Pandis [1998]), σ w = (T 273). Other symbols are defined in the Appendix. Equations (3)-(8) constitute a closed system of ordinary differential equations that are solved numerically using the LSODE solver of Hindmarsh [1983] Cloud Albedo Cloud albedo, R c, is calculated based on the twostream approximation of a non-absorbing, horizontally homogeneous cloud (Lacis and Hansen [1974]), R c = τ τ where τ is the cloud optical depth, (14) τ = H 0 3ρ a w L (z) dz (15) 2ρ w r eff (z) where w L (z) is the liquid water mixing ratio profile along the cloud column, calculated from the parcel model simulations (after transforming the Lagrangian solution into Eulerian form by setting w L (z) = w L (t) at t = z/v ).ρ w is the water density, ρ a is the air density and r eff (z) is the cloud droplet distribution effective radius, r eff = r 3 n(r)dr 0 r 2 n(r)dr 0 (16) where n(r) is the droplet size distribution. These expressions yield values for cloud albedo that are of reasonable accuracy for relatively thick clouds composed of narrow distributions of large droplets (Hatzianastassiou et al. [1997]). Assuming that the interstitial aerosol has a negligible amount of liquid water, and that the droplet population is effectively monodisperse, the expression for r eff (z) is given by r eff (z) = 3w L (z) 4πN i (z) ρ w (17) where N i (z) can be either N kn (z) or N eq (z). In the first case, the albedo calculated will be the kinetic albedo, while the second will be the thermodynamic albedo. The thermodynamic albedo tends to be higher than the kinetic albedo. This is because for the same amount of cloud liquid water, the number of droplets predicted is larger in the thermodynamic case, hence the effective radius according to (17) would be smaller than in the kinetic case. The quantity used for assessing the importance of kinetic effects, in terms of cloud albedo, is the difference between thermodynamic and kinetic albedo. Because the thermodynamic albedo is larger than the kinetic (as explained before), this difference will be positive. Furthermore, since this difference depends on the cloud depth, we select the cloud depth for which this difference is maximum. 5. Simulation Parameters The importance of kinetic limitations depends on cloud thickness, updraft velocity, and aerosol character-
7 7 istics. To explore the dependence on these parameters, we shall consider a range of values spanning observations of boundary layer clouds. This study does not examine all possible phenomena that influence aerosol activation behavior, such as changes in surface tension, and the presence of slightly soluble substances. For example, a decrease in surface tension (from surfactant species in the aerosol) should enhance kinetic effects, because a) the critical radius for activation becomes larger, so particles need more time to activate, and b) a larger fraction of the aerosol population can activate, so more particles compete for cloud water. Solution nonidealities are not considered; they do not have a significant impact since droplets dilute considerably under supersaturated conditions (Young and Warren [1992]). Finally, the effect of uncertainty in the accommodation coefficient is not examined Key Parameters Cloud thickness influences the transit time of air parcels rising through a cloud, and hence the time available for particle growth. Boundary layer clouds are typically m thick, with most in the range m (Nichols [1984]; Duynkerke et al. [1995]; Frisch et al. [1995a]; White et al. [1995]). To explore the dependence of kinetic effects on cloud thickness, we consider values ranging from 10 to 1000 m. Updraft velocity influences both the transit time and the maximum supersaturation in a cloud updraft. The maximum supersaturation achieved is lower in weaker updrafts, so only particles with relatively low critical supersaturations can be activated. Observed updraft velocities in boundary layer clouds vary widely, but values derived from measured vertical velocity variance are typically cm s 1 (Nichols [1984]; Duynkerke et al. [1995]; Frisch et al. [1995b]). We explore the dependence on updraft by considering updraft velocities of 10, 30, 100, and 300 cm s Aerosol characteristics The dependence of kinetic effects on aerosol number concentration and mean radius will be explored by considering a variety of aerosol size distributions. We consider idealized size distributions in which the number concentration and mean radius are prescribed in order to clearly sort out the physics involved. We then consider size distributions that more closely approximate ambient distributions. Size distributions are of the single or multiple lognormal form, nm [ ] dn (r) d ln r = N i exp ln2 (r/r g,i ) 2π ln σi 2 ln 2 σ i i=1 (18) where N i is the aerosol number concentration, r g,i the number mode radius, σ i is the geometric standard deviation for mode i, and n m is the number of modes in the distribution. For single modes we consider a single value for σ = 2, but a wide range in number concentration ( cm 3 ) and mode radius ( µm). The range in mode radius and number concentration is appropriate for accumulation mode particles, which comprise most CCN. For multiple modes we have selected four of Whitby [1978] trimodal representations, namely the marine, clean continental, average background, and urban aerosol. The parameters of these four distributions are listed in Table 1. The size distributions refer to dry size, while the chemical composition of the aerosol is assumed pure ammonium sulfate. In all kinetic simulations, particles are assumed initially to be in equilibrium with a relative humidity of 98%. For the idealized size distributions, we consider 200 size bins spaced equally in log radius. Using a size range between about D p,g/ 10σ and 10σD p,g covers total particle number to within 10 7 %. The simulations exhibit little sensitivity with respect to initial relative humidity concentrations and denser discretization schemes. 6. Effect of Kinetic Limitations on Cloud Droplet Number We first explore the parametric dependence of kinetic limitations using the single log-normal size distributions. We then consider various multimode lognormal distributions that resemble ambient aerosol Single log-normal size distributions From the simulations we calculate the α (z) and φ (z) profiles. The results of these simulations are summarized in Table 2, which gives the characteristics of these ratios for all the mode radii, number concentrations, and updrafts examined. As expected, the droplet ratio (for constant mode radius and number concentration) in most cases approaches an asymptotic value for large cloud depths. Kinetic effects become more apparent as updraft velocity decreases and aerosol concentrations increase. For a mode radius of 0.03 µm, the droplet number is reduced by 40% for an updraft velocity of 10 cm s 1 and an aerosol number concentration of 3000
8 8 Table 1. Aerosol distribution parameters (r g,i in µm, N i in cm 3 ) [Whitby, 1978] Aerosol type Nuclei Mode Accumulation Mode Coarse Mode r g,1 σ 1 N 1 r g,2 σ 2 N 2 r g,3 σ 3 N 3 Marine Clean Continental Average Background Urban cm 3. In this particular example, the unactivation ratio φ (z) is close to zero. This means that most (more than 95%) of the cloud drops are activated. In this particular case, the droplet ratio profile is type 3, so both evaporation and deactivation mechanisms are present. The dependence of kinetic effects on N i is further pronounced as mode radius increases. For example, the droplet ratio for an aerosol number concentration of 3000 cm 3 and a mode radius of 0.1 µm can be close to zero for a large portion of the cloud, because the time required for growth is very large for weak updrafts (this is also seen in φ (z), which ranges between 1.0 and 0.083). In this particular case, the α (z) profile is type 2, so the evaporation mechanism is present. The fact that α (z) is maximum at 700 m above cloud base indicates that kinetic effects are very strong. This is not surprising, given the mode radius and concentration of particles. As updraft velocity increases, supersaturation, being the driving force for particle growth, also increases and activates particles lower in the cloud. The dependence on cloud thickness is also stronger for a mode radius of 0.1 µm than for 0.03 µm because a) maximum supersaturation is achieved further up from cloud base, and, b) the larger particles respond more slowly to variations in supersaturation. The deactivation mechanism is responsible for decreasing the droplet ratio for low updrafts and high number concentrations (> 300 cm 3 ). Another striking feature, as evidenced by φ (z), is that the inertial mechanism is negligible for particles of smaller modal diameter; in the larger size distributions, roughly half of the particles are not activated during the first m of the cloud. Despite the difference in modal size, the fraction of small particles that fail to activate is roughly the same at large cloud depths. The simulations indicate that a large mode radius tends to accentuate kinetic effects. By examining α (z) and φ (z) as a function of mode radius for a number concentration of 100 cm 3, we can see that evaporation and deactivation effects are negligible for all mode radii; most droplet ratio profiles are of type 1. Inertial effects (as shown by the value of φ (z) at 20 m) become increasingly important as mode radius increases. On the other hand, α (z) never drops below This is an important point: for low concentration of particles, kinetic effects that influence droplet number are negligible regardless of particle diameter. The inertial mechanism is the only type of limitation experienced by these particles. Kinetic effects are more prominent when the number concentration increases to 1000 cm 3. When the concentration of particles is this high, α (z) profile types indicate that the deactivation and evaporation mechanisms are much more prominent than for a particle concentration of 100 cm 3. This suggests that these kinetic limitation mechanisms are present primarily at high number concentrations Trimodal log-normal size distributions From the previous section it is clear that kinetic limitations on droplet formation are important for high number concentrations, and large mean particle size can further enhance these effects. Although one can imagine combinations of number concentration and mean particle size that would yield significant kinetic limitations, such combinations may not be realistic. High particle concentrations are normally associated with small particle sizes, and vice versa. An assessment of the importance of kinetic limitations on ambient droplet formation should therefore consider size distributions that represent ambient aerosol. In doing so, we examine the trimodal log-normal fits to measured size distributions for a variety of aerosol types in Table 1. Although the number concentration for the nuclei mode can be quite large, the particles are so small that the supersaturation necessary for activating them
9 9 Table 2. Characteristics of α(z) and φ(z) profiles for single lognormal aerosol distribution runs. α max is the maximum value of α(z), encountered at z αmax above cloud base. r g,i (µm) N i (cm 3 ) V (m s 1 ) Profile type α max z αmax (m) α (1000m) φ (20m) φ (1000m)
10 10 R atio R atio Figure 3 Background C lo u d D epth (m ) M arin e C lo u d D epth (m ) R atio R atio U p d ra ft V e lo city (m s -1 ) Clean Continental C lo ud D epth (m ) U rb a n C lo ud D ep th (m ) Figure 3. Droplet ratio as a function of updraft velocity and cloud thickness for the background, clean continental, marine, and urban distributions of Table 1. Solid lines represent the droplet ratio α (z), while dashed lines represent the unactivation ratio φ (z). is never encountered within a cloud. Furthermore, the mean particle size of the coarse mode is large enough for significant kinetic effects, but the number concentrations are usually too small to have a significant impact on droplet number. Thus, the distribution characteristics of the accumulation mode is expected to determine the kinetic effects of the aerosol types in Table 1. Figure 3 shows α (z) and φ (z) as a function of updraft velocity and cloud thickness for the aerosol types shown in Table 1. Simulations reveal that kinetic effects for the marine aerosol are negligible regardless of updraft velocity and cloud thickness. This is not surprising, given that the number concentration of the accumulation mode, 60 cm 3, is too low for kinetic effects to be important. The accumulation mode number concentration for the clean continental aerosol is much higher, 800 cm 3, so kinetic effects become evident at weak updraft velocities: α (z) is always below 0.85 for V =10 cm s 1, and 0.95 for V =30 cm s 1. Results for the clean continental aerosol are comparable to those illustrated in Table 2 for a number mode radius of 0.03 µm and a number concentration of 1000 cm 3, but with kinetic effects being somewhat weaker because of the lower number concentration. The ratio φ (z), however, is low and the droplet ratio approaches an asymptotic value, so the aerosol is subject to the evaporation mechanism, as some of the particles fail to activate in the initial stages of cloud formation. Kinetic limitations on droplet formation are quite important for the average background aerosol, which has an accumulation mode number concentration of 2300 cm 3. The droplet ratio is a little over 0.8 for a 30 cm s 1 updraft, which is consistent with the single lognormal size distribution with the same number concentration. Although there are strong inertial effects in the coarse mode, its contribution to the total droplet number concentration is rather small. Because of this, φ (z) is low, less than 0.1 for cloud depths larger than 20 m, indicating that most of the droplets are strictly activated. For weak updrafts, because of the high concentration of accumulation mode particles, deactivation plays a significant role and reduces the droplet number by 20%. Overall, the aerosol seems to be subject to both deactivation and evaporation mechanisms, the degree to which depends on the updraft velocity. With an accumulation mode number concentration of 32,000 cm 3, one might expect substantial kinetic effects for the urban aerosol, and indeed this is the case. In fact, kinetic effects are so strong, that α (z) is zero until 70 m above cloud base. The activation ratio never exceeds 0.8, even for the strongest updrafts. The latter indicates that many particles fail to activate as the supersaturation decreases following the maximum supersaturation in all updraft cases examined. Inertial effects are also significant; even for clouds of 200 m thickness, φ (z) is above 0.2. Finally, deactivation is also very strong since the decrease in α (z) after the maximum is about 20%. 7. Effect of Kinetic Limitations on Cloud Albedo For very thin clouds (on the order of 10 m), the optical depth is small, so the albedo difference itself is small. As the cloud depth increases, so does optical depth. This initially leads to an increase in the albedo
11 11 Figure 4. Maximum difference between thermodynamic and kinetic cloud albedo. The single mode lognormal distribution aerosol is used with (a) number mode radius 0.03 µm, (b) number mode radius 0.1 µm, (c) number concentration of 100 cm 3, and (d) number concentration of 1000 cm 3. difference; after a certain point, the optical depth is so large, that the albedo difference begins to decrease. The point of maximum difference between thermodynamic and kinetic albedo is used for evaluating kinetic effects. A climatically important kinetic effect would be considered for a difference between kinetic and thermodynamic albedo larger than about 0.005; this albedo change, if relevant globally, could yield an uncertainty in radiative forcing comparable to that estimated for anthropogenic indirect aerosol radiative forcing (Facchini et al. [1999]). It should also be noted that the maximum albedo difference in the simulations (both single mode and trimodal distributions) was encountered in the first 300 m of the cloud. As expected, increasing the kinetic effects in droplet number tends to shift the locus of the maximum higher up in the cloud Single log-normal size distributions Since the difference between thermodynamic and kinetic cloud albedo depends on the difference in cloud droplet number, it is expected that the largest difference would be seen for high number concentrations. The discrepancy is also expected to be enhanced if the distribution has a large modal radius. This can be seen in the two top panels of Figure 4, which shows the maximum albedo difference, as a function of number concentration, for two modal radii. For a number mode radius of 0.03 µm (top left panel), there is large sensitivity of kinetic effects on albedo to number concentration. For low concentrations (100 cm 3 ), kinetic limitations are not strong enough to yield a significant difference in cloud albedo; this difference becomes very large however, close to 0.05, when the concentration is around 3000 cm 3. For a number mode radius of 0.1 µm (top right panel), these effects are enhanced considerably and the albedo difference approaches 0.7 for the lowest updraft and highest number concentrations. The cases where albedo difference is below seem to be the same for both mode radius cases, when the number concentration is below 300 cm 3. The above statements are further supported by the two bottom panels of Figure 4. These show the maximum albedo difference as a function of mode radius, for two number concentrations. Regardless of modal radius, when the concentration is 100 cm 3 (bottom left panel) the albedo difference is insignificant. However, when the concentration becomes 1000 cm 3, kinetic effects are always significant. When the albedo difference is around 0.1, the effect of mode radius seems to be small. By comparing Figure 4 with Table 2, we observe a consistent trend; the maximum albedo difference tends to exceed the value whenever the droplet ratio consistently (that is, for all cloud depths) is below 0.9. This is an important point, since it provides a quantitative measure for when the bias in droplet number becomes important Trimodal log-normal size distributions Figure 5 shows the maximum difference in cloud albedo between the thermodynamic and kinetic activation models, for the four log-normal distributions of Table 1. Albedo differences are shown for both the total number concentration in Table 1 and twice these values. The urban aerosol distribution exhibits the largest difference in albedo, which is expected, given that it is the distribution with the largest kinetic limitation effects in droplet number. Of the other aerosol classes, the largest sensitivity is experienced by the clean continental aerosol, where a doubling in concentration leads to a fivefold increase in albedo difference (at the highest updraft velocities). As in section 7.1, kinetic limitations become important for cloud albedo whenever α (z) is
12 12 Figure 5. Maximum difference between thermodynamic and kinetic cloud albedo. The aerosol distributions in Table 1 are used with (a) given number concentrations, and (d) doubled number concentrations. consistently below Summary and Conclusions There are three mechanisms that lead to kinetic limitations on cloud droplet formation. The first, identified by Chuang et al. [1997], and termed the inertial mechanism, arises when particles with critical supersaturation less than a threshold value do not have time to grow larger than their critical size and thus do not activate. In the second deactivation mechanism, activated droplets evaporate to form interstitial aerosol when the parcel supersaturation drops below the droplet equilibrium saturation ratio. In the third mechanism, the evaporation mechanism, particles that could potentially activate (because their critical supersaturation is low enough) evaporate to form interstitial aerosol because the time they are exposed to high supersaturation is not sufficient to reach their critical radius. Large particles, which are subject to the inertial mechanism, are also large enough to be considered cloud droplets even when they are not strictly activated. Particles, however, that are subject to the deactivation and evaporation mechanisms become much smaller than activated drops and hence should not be considered cloud droplets. Thus, if deactivation and evaporation significantly reduce the droplet number concentration, these processes should be accounted for in parameterizations of the activation process. The inertial mechanism, although a type of kinetic limitation, does not generate a discrepancy in cloud droplet number. Most of the discrepancy is a result of the smaller particles that fail to activate, through the two other mechanisms identified. This also implies that for cases which kinetic effects are significant, the droplet number is quite sensitive to fluctuations in supersaturation. In terms of number of cloud droplets, the conditions most likely to yield considerable kinetic effects are those of high aerosol number concentrations. Weak updrafts and large mode radius tend to enhance kinetic effects. We have also investigated a variety of aerosol types to determine those subject to important kinetic effects under a variety of cloud formation conditions. We have found that urban aerosol is strongly affected by kinetic growth limitations, but relatively pristine marine aerosols are not. We have found that for most aerosols the percentage errors from neglecting kinetic effects are larger for weak updrafts than for strong updrafts, even though the residence time of air parcels in clouds is shorter for strong updrafts. However, absolute errors in droplet number are often largest for moderate updrafts because aerosol activation is inefficient for weak updrafts, and kinetic limitations are smaller for strong updrafts. The conditions most likely to yield considerable differences in cloud albedo are similar to those for droplet number, i.e., weak updrafts, large mode radius, and high aerosol concentrations. Simulations indicate that the maximum albedo difference is less than for marine aerosol (where kinetic limitations in cloud droplet number are also not significant). Albedo differences can, however, exceed 0.1 for urban aerosol. By comparing the droplet ratio and albedo difference plots, it can be seen that kinetic effects become important whenever the droplet ratio is consistently below 0.9. Of the three kinetic limitation mechanisms, the evaporation and deactivation mechanisms can influence droplet number concentrations. However, the deactivation mechanism does not affect aerosol throughout the
13 13 cloud and always appears together with the evaporation mechanism. So, it is believed that conditions that are conducive towards the appearance of the evaporation mechanism can lead to substantial kinetic effects. As a consequence, kinetic limitations are not expected overall to be climatically significant, but can have a noticeable local impact. Existing parameterizations of aerosol activation in GCMs do not account for kinetic limitations. Because closed-form solutions of the activation process are not available, parameterizations can be fit to kinetic simulations. Future parameterizations should therefore be matched to kinetic simulations that extend beyond the point of maximum supersaturation, classify activated particles on the basis of their size rather than their critical supersaturation, and also account for fluctuations in cloud supersaturation. Acknowledgments. This work was supported at PNNL by the NASA Earth Science Enterprise under contract NAS and by the U.S. Department of Energy Atmospheric Radiation Measurement Program, which is part of the DOE Biological and Environmental Research Program. PNNL is operated for the DOE by Battelle Memorial Institute under contract DE-AC06-76RLO This work as supported at the California Institute of Technology by the Office of Naval Research. Notation a c a T c p r dry D v D v condensation coefficient thermal accommodation coefficient specific heat of dry air at constant pressure particle dry radius water vapor diffusivity in air water vapor diffusivity in air, corrected for non-continuum effects g acceleration of gravity G particle growth parameter, as given by (7) H cloud thickness k a thermal conductivity of air k a thermal conductivity of air, corrected for non-continuum effects L latent heat of condensation of water M a molecular weight of dry air M s molecular weight of the solute M w molecular weight of water n (r) number size distribution n m number of modes in idealized log-normal distributions n s number of moles of solute per particle N aerosol number concentration N eq number concentration of particles with S c smaller than or equal to S max number concentration of particles with dry radius N kn larger than that of the smallest activated particle N unact number concentration of large unactivated particles (with dry radius larger than that of the smallest activated particle, but with a wet radius less than the critical size) p a air pressure p v saturation vapor pressure of water r particle wet radius r g,i number mode radius of log-normal size distribution r eff size distribution effective radius R universal gas constant R c cloud albedo S supersaturation S c critical supersaturation for activating according to Köhler theory S eq supersaturation in equilibrium with particle size S max maximum supersaturation in a cloud parcel t time T temperature of air parcel V parcel updraft velocity w v water vapor mixing ratio in air parcel w c cloud liquid water mixing ratio wv p v/p a saturation mixing ratio of water z cloud depth as measured from cloud base (or where S=0%) α droplet ratio N kn /N eq ε mass fraction of soluble material in the dry particle ν number of ions the solute dissociates into in solution ρ a density of air ρ w density of water ρ p mean density of particle ρ s density of soluble component of particle ρ u density of insoluble component of particle σ geometric standard deviation of the aerosol size distribution σ w water surface tension τ optical depth φ unactivation ratio N unact /N kn References Abdul-Razzak, H., S. Ghan, and C. Rivera-Carpio, A parameterization of aerosol activation. part i: Single aerosol type, J. Geophys. Res., 103, , Chuang, P., R. Charlson, and J. Seinfeld, Kinetic limitation on droplet formation in clouds, Nature, 390, , Considine, G., and J. Curry, Effects of entrainment and droplet sedimentation on the microphysical structure of stratus and stratocumulus clouds, Q.J.R.Meteorol.Soc., 124, , Duynkerke, P., H. Zhang, and P. Jonker, Microphysical and turbulent structure of nocturnal stratocumulus as observed during astex, J. Aerosol Sci., 124, , Facchini, M., M. Mircea, S. Fuzzi, and R. Charlson, Cloud
Parameterization of cloud droplet formation in global climate models
 1 Parameterization of cloud droplet formation in global climate models ATHANASIOS NENES 1 and JOHN H. SEINFELD Department of Chemical Engineering, Mail Code 210-41, California Institute of Technology,
1 Parameterization of cloud droplet formation in global climate models ATHANASIOS NENES 1 and JOHN H. SEINFELD Department of Chemical Engineering, Mail Code 210-41, California Institute of Technology,
Black carbon radiative heating effects on cloud microphysics and implications for the aerosol indirect effect: 2. Cloud microphysics
 Black carbon radiative heating effects on cloud microphysics and implications for the aerosol indirect effect: 2. Cloud microphysics Athanasios Nenes, William C. Conant and John H. Seinfeld Departments
Black carbon radiative heating effects on cloud microphysics and implications for the aerosol indirect effect: 2. Cloud microphysics Athanasios Nenes, William C. Conant and John H. Seinfeld Departments
Parameterization of the nitric acid effect on CCN activation
 Atmos. Chem. Phys., 5, 879 885, 25 SRef-ID: 168-7324/acp/25-5-879 European Geosciences Union Atmospheric Chemistry and Physics Parameterization of the nitric acid effect on CCN activation S. Romakkaniemi,
Atmos. Chem. Phys., 5, 879 885, 25 SRef-ID: 168-7324/acp/25-5-879 European Geosciences Union Atmospheric Chemistry and Physics Parameterization of the nitric acid effect on CCN activation S. Romakkaniemi,
Droplet Nucleation: Physically-Based Parameterizations and Comparative
 Droplet Nucleation: Physically-Based Parameterizations and Comparative Evaluation 1 1 1 1 1 1 1 1 0 1 0 1 Steven J. Ghan 1, Hayder Abdul-Razzak, Athanasios Nenes, Yi Ming, Xiaohong Liu 1, Mikhail Ovchinnikov
Droplet Nucleation: Physically-Based Parameterizations and Comparative Evaluation 1 1 1 1 1 1 1 1 0 1 0 1 Steven J. Ghan 1, Hayder Abdul-Razzak, Athanasios Nenes, Yi Ming, Xiaohong Liu 1, Mikhail Ovchinnikov
Black carbon radiative heating effects on cloud microphysics and implications for the aerosol indirect effect 2. Cloud microphysics
 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 107, NO. D21, 4605, doi:10.1029/2002jd002101, 2002 Black carbon radiative heating effects on cloud microphysics and implications for the aerosol indirect effect 2.
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 107, NO. D21, 4605, doi:10.1029/2002jd002101, 2002 Black carbon radiative heating effects on cloud microphysics and implications for the aerosol indirect effect 2.
Sensitivity of cloud condensation nuclei activation processes to kinetic parameters
 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 111,, doi:10.1029/2005jd006529, 2006 Sensitivity of cloud condensation nuclei activation processes to kinetic parameters P. Y. Chuang 1 Received 25 July 2005; revised
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 111,, doi:10.1029/2005jd006529, 2006 Sensitivity of cloud condensation nuclei activation processes to kinetic parameters P. Y. Chuang 1 Received 25 July 2005; revised
Generating cloud drops from CCN. Wallace & Hobbs (1977)
 Generating cloud drops from CCN Wallace & Hobbs (1977) Cloud Drops and Equilibrium Considera3ons: review We discussed how to compute the equilibrium vapor pressure over a pure water drop, or a solu3on
Generating cloud drops from CCN Wallace & Hobbs (1977) Cloud Drops and Equilibrium Considera3ons: review We discussed how to compute the equilibrium vapor pressure over a pure water drop, or a solu3on
Chemical and Dynamical Effects on Cloud Droplet Number: Implications for Estimates of the Aerosol Indirect Effect
 Chemical and Dynamical Effects on Cloud Droplet Number: Implications for Estimates of the Aerosol Indirect Effect SARA LANCE 1, A. NENES 1,2*, T. RISSMAN 3 1 School of Earth and Atmospheric Sciences and
Chemical and Dynamical Effects on Cloud Droplet Number: Implications for Estimates of the Aerosol Indirect Effect SARA LANCE 1, A. NENES 1,2*, T. RISSMAN 3 1 School of Earth and Atmospheric Sciences and
Step 1. Step 2. g l = g v. dg = 0 We have shown that over a plane surface of water. g v g l = ρ v R v T ln e/e sat. this can be rewritten
 The basic question is what makes the existence of a droplet thermodynamically preferable to the existence only of water vapor. We have already derived an expression for the saturation vapor pressure over
The basic question is what makes the existence of a droplet thermodynamically preferable to the existence only of water vapor. We have already derived an expression for the saturation vapor pressure over
Effect of Turbulent Enhancemnt of Collision-coalescence on Warm Rain Formation in Maritime Shallow Convection
 Effect of Turbulent Enhancemnt of Collision-coalescence on Warm Rain Formation in Maritime Shallow Convection A. A. WYSZOGRODZKI 1 W. W. GRABOWSKI 1,, L.-P. WANG 2, AND O. AYALA 2 1 NATIONAL CENTER FOR
Effect of Turbulent Enhancemnt of Collision-coalescence on Warm Rain Formation in Maritime Shallow Convection A. A. WYSZOGRODZKI 1 W. W. GRABOWSKI 1,, L.-P. WANG 2, AND O. AYALA 2 1 NATIONAL CENTER FOR
Importance of vertical velocity variations in the cloud droplet nucleation process of marine stratus clouds
 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 110,, doi:10.1029/2004jd004922, 2005 Importance of vertical velocity variations in the cloud droplet nucleation process of marine stratus clouds Yiran Peng, 1,2 Ulrike
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 110,, doi:10.1029/2004jd004922, 2005 Importance of vertical velocity variations in the cloud droplet nucleation process of marine stratus clouds Yiran Peng, 1,2 Ulrike
Modeling of cloud microphysics: from simple concepts to sophisticated parameterizations. Part I: warm-rain microphysics
 Modeling of cloud microphysics: from simple concepts to sophisticated parameterizations. Part I: warm-rain microphysics Wojciech Grabowski National Center for Atmospheric Research, Boulder, Colorado parameterization
Modeling of cloud microphysics: from simple concepts to sophisticated parameterizations. Part I: warm-rain microphysics Wojciech Grabowski National Center for Atmospheric Research, Boulder, Colorado parameterization
APPLICATION OF KOHLER THEORY: MODELING CLOUD CONDENSATION NUCLEI ACTIVITY
 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 APPLICATION OF KOHLER THEORY: MODELING CLOUD CONDENSATION NUCLEI ACTIVITY Gavin Cornwell, Katherine Nadler, Alex Nguyen, and Steven Schill Department of
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 APPLICATION OF KOHLER THEORY: MODELING CLOUD CONDENSATION NUCLEI ACTIVITY Gavin Cornwell, Katherine Nadler, Alex Nguyen, and Steven Schill Department of
Evaluation of a new Cloud Droplet Activation Parameterization with In Situ Data from CRYSTAL-FACE and CSTRIPE
 Evaluation of a new Cloud Droplet Activation Parameterization with In Situ Data from CRYSTAL-FACE and CSTRIPE Nicholas Meskhidze 1, Athanasios Nenes 1,2*, William C. Conant 3, John H. Seinfeld 3,4 1 School
Evaluation of a new Cloud Droplet Activation Parameterization with In Situ Data from CRYSTAL-FACE and CSTRIPE Nicholas Meskhidze 1, Athanasios Nenes 1,2*, William C. Conant 3, John H. Seinfeld 3,4 1 School
Warm Rain Precipitation Processes
 Warm Rain Precipitation Processes Cloud and Precipitation Systems November 16, 2005 Jonathan Wolfe 1. Introduction Warm and cold precipitation formation processes are fundamentally different in a variety
Warm Rain Precipitation Processes Cloud and Precipitation Systems November 16, 2005 Jonathan Wolfe 1. Introduction Warm and cold precipitation formation processes are fundamentally different in a variety
How Important Is the Spectral Ripening Effect in Stratiform Boundary Layer Clouds? Studies Using Simple Trajectory Analysis
 2681 How Important Is the Spectral Ripening Effect in Stratiform Boundary Layer Clouds? Studies Using Simple Trajectory Analysis R. WOOD Met Office, Bracknell, Berkshire, United Kingdom S. IRONS AND P.
2681 How Important Is the Spectral Ripening Effect in Stratiform Boundary Layer Clouds? Studies Using Simple Trajectory Analysis R. WOOD Met Office, Bracknell, Berkshire, United Kingdom S. IRONS AND P.
Precipitation Processes
 Precipitation Processes Dave Rahn Precipitation formation processes may be classified into two categories. These are cold and warm processes, where cold processes can only occur below 0 C and warm processes
Precipitation Processes Dave Rahn Precipitation formation processes may be classified into two categories. These are cold and warm processes, where cold processes can only occur below 0 C and warm processes
Clouds associated with cold and warm fronts. Whiteman (2000)
 Clouds associated with cold and warm fronts Whiteman (2000) Dalton s law of partial pressures! The total pressure exerted by a mixture of gases equals the sum of the partial pressure of the gases! Partial
Clouds associated with cold and warm fronts Whiteman (2000) Dalton s law of partial pressures! The total pressure exerted by a mixture of gases equals the sum of the partial pressure of the gases! Partial
1. Droplet Growth by Condensation
 1. Droplet Growth by Condensation It was shown before that a critical size r and saturation ratio S must be exceeded for a small solution droplet to become a cloud droplet. Before the droplet reaches the
1. Droplet Growth by Condensation It was shown before that a critical size r and saturation ratio S must be exceeded for a small solution droplet to become a cloud droplet. Before the droplet reaches the
A cloud microphysics model including trace gas condensation and sulfate chemistry
 BOREAL ENVIRONMENT RESEARCH 8: 43 424 ISSN 239-695 Helsinki December 23 23 A cloud microphysics model including trace gas condensation and sulfate chemistry Harri Kokkola ), Sami Romakkaniemi ), Markku
BOREAL ENVIRONMENT RESEARCH 8: 43 424 ISSN 239-695 Helsinki December 23 23 A cloud microphysics model including trace gas condensation and sulfate chemistry Harri Kokkola ), Sami Romakkaniemi ), Markku
CHAPTER 8. AEROSOLS 8.1 SOURCES AND SINKS OF AEROSOLS
 1 CHAPTER 8 AEROSOLS Aerosols in the atmosphere have several important environmental effects They are a respiratory health hazard at the high concentrations found in urban environments They scatter and
1 CHAPTER 8 AEROSOLS Aerosols in the atmosphere have several important environmental effects They are a respiratory health hazard at the high concentrations found in urban environments They scatter and
Modelling Cloud Droplet Formation
 Modelling Cloud Droplet Formation Rosalind West, August 2008 Transfer of status report Supervised by Dr. Philip Stier, Dr. Don Grainger and Dr. Andy Jones 1 2 Contents Contents 1 Introduction.........................................
Modelling Cloud Droplet Formation Rosalind West, August 2008 Transfer of status report Supervised by Dr. Philip Stier, Dr. Don Grainger and Dr. Andy Jones 1 2 Contents Contents 1 Introduction.........................................
Use of In Situ Data to Test a Raman Lidar Based Cloud Condensation Nuclei Remote Sensing Method
 FEBRUARY 2004 NOTES AND CORRESPONDENCE 387 Use of In Situ Data to Test a Raman Lidar Based Cloud Condensation Nuclei Remote Sensing Method STEVEN J. GHAN Pacific Northwest National Laboratory, Richland,
FEBRUARY 2004 NOTES AND CORRESPONDENCE 387 Use of In Situ Data to Test a Raman Lidar Based Cloud Condensation Nuclei Remote Sensing Method STEVEN J. GHAN Pacific Northwest National Laboratory, Richland,
Exam 2: Cloud Physics April 16, 2008 Physical Meteorology Questions 1-10 are worth 5 points each. Questions are worth 10 points each.
 Exam : Cloud Physics April, 8 Physical Meteorology 344 Name Questions - are worth 5 points each. Questions -5 are worth points each.. Rank the concentrations of the following from lowest () to highest
Exam : Cloud Physics April, 8 Physical Meteorology 344 Name Questions - are worth 5 points each. Questions -5 are worth points each.. Rank the concentrations of the following from lowest () to highest
Problem Session (afternoon, 9/19/11)
 Problem Session (afternoon, 9/19/11) CCN growth; supersaturation in a updraft (finish up last few slides from Lecture 12) Atmospheric Aerosols (2 page notes + spreadsheet) Computing CCN spectra (spreadsheet)
Problem Session (afternoon, 9/19/11) CCN growth; supersaturation in a updraft (finish up last few slides from Lecture 12) Atmospheric Aerosols (2 page notes + spreadsheet) Computing CCN spectra (spreadsheet)
Introduction. Effect of aerosols on precipitation: - challenging problem - no agreement between the results (quantitative and qualitative)
 Introduction Atmospheric aerosols affect the cloud mycrophysical structure & formation (observations, numerical studies) An increase of the aerosol particles: - increases CCN concentrations - decreases
Introduction Atmospheric aerosols affect the cloud mycrophysical structure & formation (observations, numerical studies) An increase of the aerosol particles: - increases CCN concentrations - decreases
Updated H 2 SO 4 -H 2 O binary homogeneous nucleation look-up tables
 Click Here for Full Article JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 113,, doi:10.1029/2008jd010527, 2008 Updated H 2 SO 4 -H 2 O binary homogeneous nucleation look-up tables Fangqun Yu 1 Received 2 June
Click Here for Full Article JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 113,, doi:10.1029/2008jd010527, 2008 Updated H 2 SO 4 -H 2 O binary homogeneous nucleation look-up tables Fangqun Yu 1 Received 2 June
Importance of Composition and Hygroscopicity of BC Particles to the Effect of BC Mitigation on Cloud Properties: Application to California Conditions
 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 Importance of Composition and Hygroscopicity of BC Particles to the Effect of BC Mitigation on Cloud Properties:
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 Importance of Composition and Hygroscopicity of BC Particles to the Effect of BC Mitigation on Cloud Properties:
Implications of Sulfate Aerosols on Clouds, Precipitation and Hydrological Cycle
 Implications of Sulfate Aerosols on Clouds, Precipitation and Hydrological Cycle Source: Sulfate aerosols are produced by chemical reactions in the atmosphere from gaseous precursors (with the exception
Implications of Sulfate Aerosols on Clouds, Precipitation and Hydrological Cycle Source: Sulfate aerosols are produced by chemical reactions in the atmosphere from gaseous precursors (with the exception
PRECIPITATION PROCESSES
 PRECIPITATION PROCESSES Loknath Adhikari This summary deals with the mechanisms of warm rain processes and tries to summarize the factors affecting the rapid growth of hydrometeors in clouds from (sub)
PRECIPITATION PROCESSES Loknath Adhikari This summary deals with the mechanisms of warm rain processes and tries to summarize the factors affecting the rapid growth of hydrometeors in clouds from (sub)
Precipitation Formation, and RADAR Equation by Dario B. Giaiotti and Fulvio Stel (1)
 PhD Environmental Fluid Mechanics Physics of the Atmosphere University of Trieste International Center for Theoretical Physics Precipitation Formation, and RADAR Equation by Dario B. Giaiotti and Fulvio
PhD Environmental Fluid Mechanics Physics of the Atmosphere University of Trieste International Center for Theoretical Physics Precipitation Formation, and RADAR Equation by Dario B. Giaiotti and Fulvio
9 Condensation. Learning Goals. After studying this chapter, students should be able to:
 9 Condensation Learning Goals After studying this chapter, students should be able to: 1. explain the microphysical processes that operate in clouds to influence the formation and growth of cloud droplets
9 Condensation Learning Goals After studying this chapter, students should be able to: 1. explain the microphysical processes that operate in clouds to influence the formation and growth of cloud droplets
Aerosol Dynamics. Antti Lauri NetFAM Summer School Zelenogorsk, 9 July 2008
 Aerosol Dynamics Antti Lauri NetFAM Summer School Zelenogorsk, 9 July 2008 Department of Physics, Division of Atmospheric Sciences and Geophysics, University of Helsinki Aerosol Dynamics: What? A way to
Aerosol Dynamics Antti Lauri NetFAM Summer School Zelenogorsk, 9 July 2008 Department of Physics, Division of Atmospheric Sciences and Geophysics, University of Helsinki Aerosol Dynamics: What? A way to
Köhler Curve. Covers Reading Material in Chapter 10.3 Atmospheric Sciences 5200 Physical Meteorology III: Cloud Physics
 Köhler Curve Covers Reading Material in Chapter 10.3 Atmospheric Sciences 5200 Physical Meteorology III: Cloud Physics Review of Kelvin Effect Gibbs Energy for formation of a drop G = G &'()*+, G ).'+
Köhler Curve Covers Reading Material in Chapter 10.3 Atmospheric Sciences 5200 Physical Meteorology III: Cloud Physics Review of Kelvin Effect Gibbs Energy for formation of a drop G = G &'()*+, G ).'+
An investigation into the aerosol dispersion effect through the activation process in marine stratus clouds
 1 An investigation into the aerosol dispersion effect through the activation process in marine stratus clouds Yiran Peng, 1, Ulrike Lohmann, 2 Richard Leaitch 3 and Markku Kulmala 4 Short title: 1 Max
1 An investigation into the aerosol dispersion effect through the activation process in marine stratus clouds Yiran Peng, 1, Ulrike Lohmann, 2 Richard Leaitch 3 and Markku Kulmala 4 Short title: 1 Max
Aerosol Effects on Water and Ice Clouds
 Aerosol Effects on Water and Ice Clouds Ulrike Lohmann Department of Physics and Atmospheric Science, Dalhousie University, Halifax, N. S., Canada Contributions from Johann Feichter, Johannes Hendricks,
Aerosol Effects on Water and Ice Clouds Ulrike Lohmann Department of Physics and Atmospheric Science, Dalhousie University, Halifax, N. S., Canada Contributions from Johann Feichter, Johannes Hendricks,
Multi-Scale Modeling of Turbulence and Microphysics in Clouds. Steven K. Krueger University of Utah
 Multi-Scale Modeling of Turbulence and Microphysics in Clouds Steven K. Krueger University of Utah 10,000 km Scales of Atmospheric Motion 1000 km 100 km 10 km 1 km 100 m 10 m 1 m 100 mm 10 mm 1 mm Planetary
Multi-Scale Modeling of Turbulence and Microphysics in Clouds Steven K. Krueger University of Utah 10,000 km Scales of Atmospheric Motion 1000 km 100 km 10 km 1 km 100 m 10 m 1 m 100 mm 10 mm 1 mm Planetary
Melting of ice particles:
 Melting of ice particles: When ice particles fall below 0 C they begin to melt, but the process takes some time since heat transfer needs to occur (heat from ambient environment has to supply the latent
Melting of ice particles: When ice particles fall below 0 C they begin to melt, but the process takes some time since heat transfer needs to occur (heat from ambient environment has to supply the latent
Aerosol Basics: Definitions, size distributions, structure
 Aerosol Basics: Definitions, size distributions, structure Antti Lauri NetFAM Summer School Zelenogorsk, 9 July 2008 Department of Physics, Division of Atmospheric Sciences and Geophysics, University of
Aerosol Basics: Definitions, size distributions, structure Antti Lauri NetFAM Summer School Zelenogorsk, 9 July 2008 Department of Physics, Division of Atmospheric Sciences and Geophysics, University of
Numerical simulation of marine stratocumulus clouds Andreas Chlond
 Numerical simulation of marine stratocumulus clouds Andreas Chlond Marine stratus and stratocumulus cloud (MSC), which usually forms from 500 to 1000 m above the ocean surface and is a few hundred meters
Numerical simulation of marine stratocumulus clouds Andreas Chlond Marine stratus and stratocumulus cloud (MSC), which usually forms from 500 to 1000 m above the ocean surface and is a few hundred meters
Lecture 7: quick review
 Lecture 7: quick review We discussed the meaning of the critical r* and ΔG*: since it s unstable, if we can form a drop with r > r*, the system will keep going falling down the energy curve, trying to
Lecture 7: quick review We discussed the meaning of the critical r* and ΔG*: since it s unstable, if we can form a drop with r > r*, the system will keep going falling down the energy curve, trying to
An investigation into the aerosol dispersion effect through the activation process in marine stratus clouds
 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 112,, doi:10.1029/2006jd007401, 2007 An investigation into the aerosol dispersion effect through the activation process in marine stratus clouds Yiran Peng, 1 Ulrike
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 112,, doi:10.1029/2006jd007401, 2007 An investigation into the aerosol dispersion effect through the activation process in marine stratus clouds Yiran Peng, 1 Ulrike
Investigation of Droplet Size Distributions and Drizzle Formation Using A New Trajectory Ensemble Model. Part II: Lucky Parcels
 VOLUME 66 J O U R N A L O F T H E A T M O S P H E R I C S C I E N C E S APRIL 2009 Investigation of Droplet Size Distributions and Drizzle Formation Using A New Trajectory Ensemble Model. Part II: Lucky
VOLUME 66 J O U R N A L O F T H E A T M O S P H E R I C S C I E N C E S APRIL 2009 Investigation of Droplet Size Distributions and Drizzle Formation Using A New Trajectory Ensemble Model. Part II: Lucky
Rain rate. If the drop size distribu0on is n(d), and fall speeds v(d), net ver0cal flux of drops (m - 2 s - 1 )
 Rain rate If the drop size distribu0on is n(d), and fall speeds v(d), net ver0cal flux of drops (m - 2 s - 1 ) Φ = 0 (w v(d))n(d)dd The threshold diameter has v(d th ) = w. Smaller drops move up, larger
Rain rate If the drop size distribu0on is n(d), and fall speeds v(d), net ver0cal flux of drops (m - 2 s - 1 ) Φ = 0 (w v(d))n(d)dd The threshold diameter has v(d th ) = w. Smaller drops move up, larger
Radiative influences on ice crystal and droplet growth within mixed-phase stratus clouds
 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 113,, doi:10.1029/2007jd009262, 2008 Radiative influences on ice crystal and droplet growth within mixed-phase stratus clouds Z. J. Lebo, 1,2 N. C. Johnson, 1 and
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 113,, doi:10.1029/2007jd009262, 2008 Radiative influences on ice crystal and droplet growth within mixed-phase stratus clouds Z. J. Lebo, 1,2 N. C. Johnson, 1 and
Thermodynamics of Atmospheres and Oceans
 Thermodynamics of Atmospheres and Oceans Judith A. Curry and Peter J. Webster PROGRAM IN ATMOSPHERIC AND OCEANIC SCIENCES DEPARTMENT OF AEROSPACE ENGINEERING UNIVERSITY OF COLORADO BOULDER, COLORADO USA
Thermodynamics of Atmospheres and Oceans Judith A. Curry and Peter J. Webster PROGRAM IN ATMOSPHERIC AND OCEANIC SCIENCES DEPARTMENT OF AEROSPACE ENGINEERING UNIVERSITY OF COLORADO BOULDER, COLORADO USA
Chapter 7 Precipitation Processes
 Chapter 7 Precipitation Processes Chapter overview: Supersaturation and water availability Nucleation of liquid droplets and ice crystals Liquid droplet and ice growth by diffusion Collision and collection
Chapter 7 Precipitation Processes Chapter overview: Supersaturation and water availability Nucleation of liquid droplets and ice crystals Liquid droplet and ice growth by diffusion Collision and collection
Progress on Application of Modal Aerosol Dynamics to CAM
 Progress on Application of Modal Aerosol Dynamics to CAM Xiaohong Liu, Steve Ghan, Richard Easter, Rahul Zaveri, Yun Qian (Pacific Northwest National Laboratory) Jean-Francois Lamarque, Peter Hess, Natalie
Progress on Application of Modal Aerosol Dynamics to CAM Xiaohong Liu, Steve Ghan, Richard Easter, Rahul Zaveri, Yun Qian (Pacific Northwest National Laboratory) Jean-Francois Lamarque, Peter Hess, Natalie
2σ e s (r,t) = e s (T)exp( rr v ρ l T ) = exp( ) 2σ R v ρ l Tln(e/e s (T)) e s (f H2 O,r,T) = f H2 O
 Formulas/Constants, Physics/Oceanography 4510/5510 B Atmospheric Physics II N A = 6.02 10 23 molecules/mole (Avogadro s number) 1 mb = 100 Pa 1 Pa = 1 N/m 2 Γ d = 9.8 o C/km (dry adiabatic lapse rate)
Formulas/Constants, Physics/Oceanography 4510/5510 B Atmospheric Physics II N A = 6.02 10 23 molecules/mole (Avogadro s number) 1 mb = 100 Pa 1 Pa = 1 N/m 2 Γ d = 9.8 o C/km (dry adiabatic lapse rate)
Cloud Droplet Growth by Condensation and Aggregation EPM Stratocumulus and Arctic Stratocumulus
 Cloud Droplet Growth by Condensation and Aggregation EPM Stratocumulus and Arctic Stratocumulus US Department of Energy, ARM http://www.arm.gov/science/highlights/rntm3/view Typical EPMS Characteristics
Cloud Droplet Growth by Condensation and Aggregation EPM Stratocumulus and Arctic Stratocumulus US Department of Energy, ARM http://www.arm.gov/science/highlights/rntm3/view Typical EPMS Characteristics
Slides partly by Antti Lauri and Hannele Korhonen. Liquid or solid particles suspended in a carrier gas Described by their
 Atmospheric Aerosols Slides partly by Antti Lauri and Hannele Korhonen Aerosol particles Liquid or solid particles suspended in a carrier gas Described by their Size Concentration - Number - Surface -
Atmospheric Aerosols Slides partly by Antti Lauri and Hannele Korhonen Aerosol particles Liquid or solid particles suspended in a carrier gas Described by their Size Concentration - Number - Surface -
UNRESOLVED ISSUES. 1. Spectral broadening through different growth histories 2. Entrainment and mixing 3. In-cloud activation
 URESOLVED ISSUES. Spectral broadening through different growth histories 2. Entrainment and mixing. In-cloud activation /4 dr dt ξ ( S ) r, ξ F D + F K 2 dr dt 2ξ ( S ) For a given thermodynamic conditions
URESOLVED ISSUES. Spectral broadening through different growth histories 2. Entrainment and mixing. In-cloud activation /4 dr dt ξ ( S ) r, ξ F D + F K 2 dr dt 2ξ ( S ) For a given thermodynamic conditions
Physical Processes & Issues
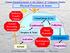 Physical Processes & Issues Radiative Transfer Climate VIS IR Cloud Drops & Ice Aerosol Processing Air quality Condensation Droplets & Xtals Cloud Dynamics Collection Aerosol Activation Hydrological Cycle
Physical Processes & Issues Radiative Transfer Climate VIS IR Cloud Drops & Ice Aerosol Processing Air quality Condensation Droplets & Xtals Cloud Dynamics Collection Aerosol Activation Hydrological Cycle
Humidity impact on the aerosol effect in warm cumulus clouds
 GEOPHYSICAL RESEARCH LETTERS, VOL. 35, L17804, doi:10.1029/2008gl034178, 2008 Humidity impact on the aerosol effect in warm cumulus clouds O. Altaratz, 1 I. Koren, 1 and T. Reisin 2 Received 31 March 2008;
GEOPHYSICAL RESEARCH LETTERS, VOL. 35, L17804, doi:10.1029/2008gl034178, 2008 Humidity impact on the aerosol effect in warm cumulus clouds O. Altaratz, 1 I. Koren, 1 and T. Reisin 2 Received 31 March 2008;
Theoretical analysis of mixing in liquid clouds Part IV: DSD evolution and mixing diagrams
 https://doi.org/10.5194/acp-18-3659-2018 Author(s) 2018. This work is distributed under the Creative Commons Attribution 3.0 License. Theoretical analysis of mixing in liquid clouds Part IV: DSD evolution
https://doi.org/10.5194/acp-18-3659-2018 Author(s) 2018. This work is distributed under the Creative Commons Attribution 3.0 License. Theoretical analysis of mixing in liquid clouds Part IV: DSD evolution
Impact of biomass burning on cloud properties in the Amazon Basin
 Preprint Copy 1 Impact of biomass burning on cloud properties in the Amazon Basin G. C. Roberts Max Planck Institute for Chemistry; California Institute of Technology A. Nenes and J. H. Seinfeld California
Preprint Copy 1 Impact of biomass burning on cloud properties in the Amazon Basin G. C. Roberts Max Planck Institute for Chemistry; California Institute of Technology A. Nenes and J. H. Seinfeld California
1. Water Vapor in Air
 1. Water Vapor in Air Water appears in all three phases in the earth s atmosphere - solid, liquid and vapor - and it is one of the most important components, not only because it is essential to life, but
1. Water Vapor in Air Water appears in all three phases in the earth s atmosphere - solid, liquid and vapor - and it is one of the most important components, not only because it is essential to life, but
An Annual Cycle of Arctic Cloud Microphysics
 An Annual Cycle of Arctic Cloud Microphysics M. D. Shupe Science and Technology Corporation National Oceanic and Atmospheric Administration Environmental Technology Laboratory Boulder, Colorado T. Uttal
An Annual Cycle of Arctic Cloud Microphysics M. D. Shupe Science and Technology Corporation National Oceanic and Atmospheric Administration Environmental Technology Laboratory Boulder, Colorado T. Uttal
How important is the spectral ripening effect in stratiform boundary layer clouds? Studies using simple trajectory analysis ROBERT WOOD 1 Meteorologic
 How important is the spectral ripening effect in stratiform boundary layer clouds? Studies using simple trajectory analysis ROBERT WOOD 1 Meteorological Research Flight, Met. Office, Farnborough, UK. SARAH
How important is the spectral ripening effect in stratiform boundary layer clouds? Studies using simple trajectory analysis ROBERT WOOD 1 Meteorological Research Flight, Met. Office, Farnborough, UK. SARAH
P1.61 Impact of the mass-accomodation coefficient on cirrus
 P1.61 Impact of the mass-accomodation coefficient on cirrus Robert W. Carver and Jerry Y. Harrington Department of Meteorology, Pennsylvania State University, University Park, PA 1. Introduction Recent
P1.61 Impact of the mass-accomodation coefficient on cirrus Robert W. Carver and Jerry Y. Harrington Department of Meteorology, Pennsylvania State University, University Park, PA 1. Introduction Recent
Sungsu Park, Chris Bretherton, and Phil Rasch
 Improvements in CAM5 : Moist Turbulence, Shallow Convection, and Cloud Macrophysics AMWG Meeting Feb. 10. 2010 Sungsu Park, Chris Bretherton, and Phil Rasch CGD.NCAR University of Washington, Seattle,
Improvements in CAM5 : Moist Turbulence, Shallow Convection, and Cloud Macrophysics AMWG Meeting Feb. 10. 2010 Sungsu Park, Chris Bretherton, and Phil Rasch CGD.NCAR University of Washington, Seattle,
Black carbon radiative heating effects on cloud microphysics and implications for the aerosol indirect effect 1. Extended Köhler theory
 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 107, NO. D21, 4604, doi:10.1029/2002jd002094, 2002 Black carbon radiative heating effects on cloud microphysics and implications for the aerosol indirect effect 1.
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 107, NO. D21, 4604, doi:10.1029/2002jd002094, 2002 Black carbon radiative heating effects on cloud microphysics and implications for the aerosol indirect effect 1.
2 DESCRIPTION OF THE LES MODEL
 SENSITIVITY OF THE MARINE STRATOCUMULUS DIURNAL CYCLE TO THE AEROSOL LOADING I. Sandu 1, J.L. Brenguier 1, O. Geoffroy 1, O. Thouron 1, V. Masson 1 1 GAME/CNRM, METEO-FRANCE - CNRS, FRANCE 1 INTRODUCTION
SENSITIVITY OF THE MARINE STRATOCUMULUS DIURNAL CYCLE TO THE AEROSOL LOADING I. Sandu 1, J.L. Brenguier 1, O. Geoffroy 1, O. Thouron 1, V. Masson 1 1 GAME/CNRM, METEO-FRANCE - CNRS, FRANCE 1 INTRODUCTION
P1.17 SENSITIVITY OF THE RETRIEVAL OF STRATOCUMULUS CLOUD LIQUID WATER AND PRECIPITATION FLUX TO DOPPLER RADAR PARAMETERS
 P1.17 SENSITIVITY OF THE RETRIEVAL OF STRATOCUMULUS CLOUD LIQUID WATER AND PRECIPITATION FLUX TO DOPPLER RADAR PARAMETERS Yefim L. Kogan*, Zena N. Kogan, and David B. Mechem Cooperative Institute for Mesoscale
P1.17 SENSITIVITY OF THE RETRIEVAL OF STRATOCUMULUS CLOUD LIQUID WATER AND PRECIPITATION FLUX TO DOPPLER RADAR PARAMETERS Yefim L. Kogan*, Zena N. Kogan, and David B. Mechem Cooperative Institute for Mesoscale
References: Cloud Formation. ESCI Cloud Physics and Precipitation Processes Lesson 1 - Cloud Types and Properties Dr.
 ESCI 34 - Cloud Physics and Precipitation Processes Lesson 1 - Cloud Types and Properties Dr. DeCaria References: Glossary of Meteorology, 2nd ed., American Meteorological Society A Short Course in Cloud
ESCI 34 - Cloud Physics and Precipitation Processes Lesson 1 - Cloud Types and Properties Dr. DeCaria References: Glossary of Meteorology, 2nd ed., American Meteorological Society A Short Course in Cloud
Impact of biomass burning on cloud properties in the Amazon Basin
 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 108, NO. D2, 4062, doi:10.1029/2001jd000985, 2003 Impact of biomass burning on cloud properties in the Amazon Basin G. C. Roberts, 1 A. Nenes, 2 J. H. Seinfeld, 3
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 108, NO. D2, 4062, doi:10.1029/2001jd000985, 2003 Impact of biomass burning on cloud properties in the Amazon Basin G. C. Roberts, 1 A. Nenes, 2 J. H. Seinfeld, 3
Parcel Model. Atmospheric Sciences September 30, 2012
 Parcel Model Atmospheric Sciences 6150 September 30, 2012 1 Governing Equations for Precipitating Convection For precipitating convection, we have the following set of equations for potential temperature,
Parcel Model Atmospheric Sciences 6150 September 30, 2012 1 Governing Equations for Precipitating Convection For precipitating convection, we have the following set of equations for potential temperature,
Warm Cloud Processes. Some definitions. Two ways to make big drops: Effects of cloud condensation nuclei
 Warm Cloud Processes Dr. Christopher M. Godfrey University of North Carolina at Asheville Warm clouds lie completely below the 0 isotherm 0 o C Some definitions Liquid water content (LWC) Amount of liquid
Warm Cloud Processes Dr. Christopher M. Godfrey University of North Carolina at Asheville Warm clouds lie completely below the 0 isotherm 0 o C Some definitions Liquid water content (LWC) Amount of liquid
Precipitation Processes METR σ is the surface tension, ρ l is the water density, R v is the Gas constant for water vapor, T is the air
 Precipitation Processes METR 2011 Introduction In order to grow things on earth, they need water. The way that the earth naturally irrigates is through snowfall and rainfall. Therefore, it is important
Precipitation Processes METR 2011 Introduction In order to grow things on earth, they need water. The way that the earth naturally irrigates is through snowfall and rainfall. Therefore, it is important
A Novel Approach for Simulating Droplet Microphysics in Turbulent Clouds
 A Novel Approach for Simulating Droplet Microphysics in Turbulent Clouds Steven K. Krueger 1 and Alan R. Kerstein 2 1. University of Utah 2. Sandia National Laboratories Multiphase Turbulent Flows in the
A Novel Approach for Simulating Droplet Microphysics in Turbulent Clouds Steven K. Krueger 1 and Alan R. Kerstein 2 1. University of Utah 2. Sandia National Laboratories Multiphase Turbulent Flows in the
1. INTRODUCTION. investigating the differences in actual cloud microphysics.
 MICROPHYSICAL PROPERTIES OF DEVELOPING VERSUS NON-DEVELOPING CLOUD CLUSTERS DURING TROPICAL CYCLOGENESIS 4B.5 Nathan D. Johnson,* William C. Conant, and Elizabeth A. Ritchie Department of Atmospheric Sciences,
MICROPHYSICAL PROPERTIES OF DEVELOPING VERSUS NON-DEVELOPING CLOUD CLUSTERS DURING TROPICAL CYCLOGENESIS 4B.5 Nathan D. Johnson,* William C. Conant, and Elizabeth A. Ritchie Department of Atmospheric Sciences,
Cloud Condensation Nuclei Hygroscopic Parameter Kappa
 Cloud Condensation Nuclei Hygroscopic Parameter Kappa Covers Reading Material in Chapter 17.5 Atmospheric Sciences 5200 Physical Meteorology III: Cloud Physics Cloud Condensation Nuclei (CCN) Form a cloud
Cloud Condensation Nuclei Hygroscopic Parameter Kappa Covers Reading Material in Chapter 17.5 Atmospheric Sciences 5200 Physical Meteorology III: Cloud Physics Cloud Condensation Nuclei (CCN) Form a cloud
Influence of Organic-Containing Aerosols on Marine Boundary Layer Processes
 DISTRIBUTION STATEMENT A. Approved for public release; distribution is unlimited. Influence of Organic-Containing Aerosols on Marine Boundary Layer Processes John H. Seinfeld California Institute of Technology,
DISTRIBUTION STATEMENT A. Approved for public release; distribution is unlimited. Influence of Organic-Containing Aerosols on Marine Boundary Layer Processes John H. Seinfeld California Institute of Technology,
5. General Circulation Models
 5. General Circulation Models I. 3-D Climate Models (General Circulation Models) To include the full three-dimensional aspect of climate, including the calculation of the dynamical transports, requires
5. General Circulation Models I. 3-D Climate Models (General Circulation Models) To include the full three-dimensional aspect of climate, including the calculation of the dynamical transports, requires
Satellite measurements of CCN and cloud properties at the cloudy boundary layer: The Holy Grail is it achievable?
 Satellite measurements of CCN and cloud properties at the cloudy boundary layer: The Holy Grail is it achievable? C 1 Daniel Rosenfeld, The Hebrew University of Jerusalem E -85 D C -89-93 E -85 D C -93-89
Satellite measurements of CCN and cloud properties at the cloudy boundary layer: The Holy Grail is it achievable? C 1 Daniel Rosenfeld, The Hebrew University of Jerusalem E -85 D C -89-93 E -85 D C -93-89
Lecture Ch. 6. Condensed (Liquid) Water. Cloud in a Jar Demonstration. How does saturation occur? Saturation of Moist Air. Saturation of Moist Air
 Lecture Ch. 6 Saturation of moist air Relationship between humidity and dewpoint Clausius-Clapeyron equation Dewpoint Temperature Depression Isobaric cooling Moist adiabatic ascent of air Equivalent temperature
Lecture Ch. 6 Saturation of moist air Relationship between humidity and dewpoint Clausius-Clapeyron equation Dewpoint Temperature Depression Isobaric cooling Moist adiabatic ascent of air Equivalent temperature
PALM - Cloud Physics. Contents. PALM group. last update: Monday 21 st September, 2015
 PALM - Cloud Physics PALM group Institute of Meteorology and Climatology, Leibniz Universität Hannover last update: Monday 21 st September, 2015 PALM group PALM Seminar 1 / 16 Contents Motivation Approach
PALM - Cloud Physics PALM group Institute of Meteorology and Climatology, Leibniz Universität Hannover last update: Monday 21 st September, 2015 PALM group PALM Seminar 1 / 16 Contents Motivation Approach
Outline. Aim. Gas law. Pressure. Scale height Mixing Column density. Temperature Lapse rate Stability. Condensation Humidity.
 Institute of Applied Physics University of Bern Outline A planetary atmosphere consists of different gases hold to the planet by gravity The laws of thermodynamics hold structure as vertical coordinate
Institute of Applied Physics University of Bern Outline A planetary atmosphere consists of different gases hold to the planet by gravity The laws of thermodynamics hold structure as vertical coordinate
Parcel Model. Meteorology September 3, 2008
 Parcel Model Meteorology 5210 September 3, 2008 1 Governing Equations for Precipitating Convection For precipitating convection, we have the following set of equations for potential temperature, θ, mixing
Parcel Model Meteorology 5210 September 3, 2008 1 Governing Equations for Precipitating Convection For precipitating convection, we have the following set of equations for potential temperature, θ, mixing
Prediction of cirrus clouds in GCMs
 Prediction of cirrus clouds in GCMs Bernd Kärcher, Ulrike Burkhardt, Klaus Gierens, and Johannes Hendricks DLR Institut für Physik der Atmosphäre Oberpfaffenhofen, 82234 Wessling, Germany bernd.kaercher@dlr.de
Prediction of cirrus clouds in GCMs Bernd Kärcher, Ulrike Burkhardt, Klaus Gierens, and Johannes Hendricks DLR Institut für Physik der Atmosphäre Oberpfaffenhofen, 82234 Wessling, Germany bernd.kaercher@dlr.de
저작권법에따른이용자의권리는위의내용에의하여영향을받지않습니다.
 저작자표시 - 비영리 - 변경금지 2.0 대한민국 이용자는아래의조건을따르는경우에한하여자유롭게 이저작물을복제, 배포, 전송, 전시, 공연및방송할수있습니다. 다음과같은조건을따라야합니다 : 저작자표시. 귀하는원저작자를표시하여야합니다. 비영리. 귀하는이저작물을영리목적으로이용할수없습니다. 변경금지. 귀하는이저작물을개작, 변형또는가공할수없습니다. 귀하는, 이저작물의재이용이나배포의경우,
저작자표시 - 비영리 - 변경금지 2.0 대한민국 이용자는아래의조건을따르는경우에한하여자유롭게 이저작물을복제, 배포, 전송, 전시, 공연및방송할수있습니다. 다음과같은조건을따라야합니다 : 저작자표시. 귀하는원저작자를표시하여야합니다. 비영리. 귀하는이저작물을영리목적으로이용할수없습니다. 변경금지. 귀하는이저작물을개작, 변형또는가공할수없습니다. 귀하는, 이저작물의재이용이나배포의경우,
Rogers and Yau Chapter 10: Drop breakup, snow, precip rate, and bulk models
 Rogers and Yau Chapter 10: Drop breakup, snow, precip rate, and bulk models One explanation for the negative exponential (M-P) distribution of raindrops is drop breakup. Drop size is limited because increased
Rogers and Yau Chapter 10: Drop breakup, snow, precip rate, and bulk models One explanation for the negative exponential (M-P) distribution of raindrops is drop breakup. Drop size is limited because increased
Aerosols and climate. Rob Wood, Atmospheric Sciences
 Aerosols and climate Rob Wood, Atmospheric Sciences What are aerosols? Solid or liquid particles suspended in air Sizes range from a few nm to a few thousand nm Huge range of masses Where do aerosols come
Aerosols and climate Rob Wood, Atmospheric Sciences What are aerosols? Solid or liquid particles suspended in air Sizes range from a few nm to a few thousand nm Huge range of masses Where do aerosols come
Mid High Latitude Cirrus Precipitation Processes. Jon Sauer, Dan Crocker, Yanice Benitez
 Mid High Latitude Cirrus Precipitation Processes Jon Sauer, Dan Crocker, Yanice Benitez Department of Chemistry and Biochemistry, University of California, San Diego, CA 92093, USA *To whom correspondence
Mid High Latitude Cirrus Precipitation Processes Jon Sauer, Dan Crocker, Yanice Benitez Department of Chemistry and Biochemistry, University of California, San Diego, CA 92093, USA *To whom correspondence
Characteristic updrafts for computing distribution averaged cloud droplet number and stratocumulus cloud properties
 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 115,, doi:1.129/29jd13233, 21 Characteristic updrafts for computing distribution averaged cloud droplet number and stratocumulus cloud properties R. Morales 1 and
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 115,, doi:1.129/29jd13233, 21 Characteristic updrafts for computing distribution averaged cloud droplet number and stratocumulus cloud properties R. Morales 1 and
Revisiting Twomey s approximation for peak supersaturation
 Atmos. Chem. Phys., 15, 383 3814, 15 www.atmos-chem-phys.net/15/383/15/ doi:1.5194/acp-15-383-15 Authors) 15. CC Attribution 3. License. Revisiting Twomey s approximation for peak supersaturation B. J.
Atmos. Chem. Phys., 15, 383 3814, 15 www.atmos-chem-phys.net/15/383/15/ doi:1.5194/acp-15-383-15 Authors) 15. CC Attribution 3. License. Revisiting Twomey s approximation for peak supersaturation B. J.
An Intercomparison of Single-Column Model Simulations of Summertime Midlatitude Continental Convection
 An Intercomparison of Single-Column Model Simulations of Summertime Midlatitude Continental Convection S. J. Ghan Pacific Northwest National Laboratory Richland, Washington D. A. Randall, K.-M. Xu, and
An Intercomparison of Single-Column Model Simulations of Summertime Midlatitude Continental Convection S. J. Ghan Pacific Northwest National Laboratory Richland, Washington D. A. Randall, K.-M. Xu, and
Parameterization of cloud droplet size distributions: Comparison with parcel models and observations
 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 114, D1125, doi:1.129/28jd11387, 29 Parameterization of cloud droplet size distributions: Comparison with parcel models and observations W. C. Hsieh, 1 A. Nenes, 1,2
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 114, D1125, doi:1.129/28jd11387, 29 Parameterization of cloud droplet size distributions: Comparison with parcel models and observations W. C. Hsieh, 1 A. Nenes, 1,2
Can a change of single scattering albedo in Amami-Oshima in a low pressure condition be explained by GCM simulations?
 Can a change of single scattering albedo in Amami-Oshima in a low pressure condition be explained by GCM simulations? Daisuke Goto 1, Toshihiko Takemura 2, Nick Schutgens 1, Haruo Tsuruta 1, and Teruyuki
Can a change of single scattering albedo in Amami-Oshima in a low pressure condition be explained by GCM simulations? Daisuke Goto 1, Toshihiko Takemura 2, Nick Schutgens 1, Haruo Tsuruta 1, and Teruyuki
Aerosols AP sizes AP types Sources Sinks Amount and lifetime Aerosol radiative effects. Aerosols. Trude Storelvmo Aerosols 1 / 21
 Aerosols Trude Storelvmo Aerosols 1 / 21 Aerosols: Definition Definition of an aerosol: disperse system with air as carrier gas and a solid or liquid or a mixture of both as disperse phases. Aerosol particles
Aerosols Trude Storelvmo Aerosols 1 / 21 Aerosols: Definition Definition of an aerosol: disperse system with air as carrier gas and a solid or liquid or a mixture of both as disperse phases. Aerosol particles
ENVR 116 Introduction to Aerosol Science December 17, 2001 FINAL EXAMINATION
 ENVR 116 Introduction to Aerosol Science December 17, 2001 FINAL EXAMINATION Please answer all questions on the attached sheets Answer Question No. 1 and 4 of the remaining 6 questions. No extra credit
ENVR 116 Introduction to Aerosol Science December 17, 2001 FINAL EXAMINATION Please answer all questions on the attached sheets Answer Question No. 1 and 4 of the remaining 6 questions. No extra credit
On-line Aerosols in the Oslo Version of CAM3: Some shortcomings. Seland,
 On-line Aerosols in the Oslo Version of CAM3: Some shortcomings Trond Iversen,, Alf Kirkevåg, Øyvind Seland, Jon Egill Kristjansson, Trude Storelvmo,, Jens Debernard Norwegian Meteorological Institute
On-line Aerosols in the Oslo Version of CAM3: Some shortcomings Trond Iversen,, Alf Kirkevåg, Øyvind Seland, Jon Egill Kristjansson, Trude Storelvmo,, Jens Debernard Norwegian Meteorological Institute
Clouds, Haze, and Climate Change
 Clouds, Haze, and Climate Change Jim Coakley College of Oceanic and Atmospheric Sciences Earth s Energy Budget and Global Temperature Incident Sunlight 340 Wm -2 Reflected Sunlight 100 Wm -2 Emitted Terrestrial
Clouds, Haze, and Climate Change Jim Coakley College of Oceanic and Atmospheric Sciences Earth s Energy Budget and Global Temperature Incident Sunlight 340 Wm -2 Reflected Sunlight 100 Wm -2 Emitted Terrestrial
A FROZEN DROP PRECIPITATION MECHANISM OVER AN OPEN OCEAN AND ITS EFFECT ON RAIN, CLOUD PATTERN, AND HEATING
 A FROZEN DROP PRECIPITATION MECHANISM OVER AN OPEN OCEAN AND ITS EFFECT ON RAIN, CLOUD PATTERN, AND HEATING 13.6 Tsutomu Takahashi* University of Hawaii, Honolulu, Hawaii Kazunori Shimura JFE Techno-Research
A FROZEN DROP PRECIPITATION MECHANISM OVER AN OPEN OCEAN AND ITS EFFECT ON RAIN, CLOUD PATTERN, AND HEATING 13.6 Tsutomu Takahashi* University of Hawaii, Honolulu, Hawaii Kazunori Shimura JFE Techno-Research
Solutions to questions from chapter 8 in GEF Cloud Physics
 Solutions to questions from chapter 8 in GEF4310 - Cloud Physics i.h.h.karset@geo.uio.no Problem 1 a) What is expressed by the equation below? Answer: The left side is the time rate of change of the mass
Solutions to questions from chapter 8 in GEF4310 - Cloud Physics i.h.h.karset@geo.uio.no Problem 1 a) What is expressed by the equation below? Answer: The left side is the time rate of change of the mass
The Clausius-Clapeyron and the Kelvin Equations
 PhD Environmental Fluid Mechanics Physics of the Atmosphere University of Trieste International Center for Theoretical Physics The Clausius-Clapeyron and the Kelvin Equations by Dario B. Giaiotti and Fulvio
PhD Environmental Fluid Mechanics Physics of the Atmosphere University of Trieste International Center for Theoretical Physics The Clausius-Clapeyron and the Kelvin Equations by Dario B. Giaiotti and Fulvio
Parameterization of cirrus cloud formation in large-scale models: Homogeneous nucleation
 Click Here for Full Article JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 113,, doi:10.1029/2007jd009355, 2008 Parameterization of cirrus cloud formation in large-scale models: Homogeneous nucleation Donifan Barahona
Click Here for Full Article JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 113,, doi:10.1029/2007jd009355, 2008 Parameterization of cirrus cloud formation in large-scale models: Homogeneous nucleation Donifan Barahona
Parameterization of cloud droplet activation using a simplified treatment of the aerosol number size distribution
 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 113,, doi:10.1029/2007jd009485, 2008 Parameterization of cloud droplet activation using a simplified treatment of the aerosol number size distribution Niku Kivekäs,
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 113,, doi:10.1029/2007jd009485, 2008 Parameterization of cloud droplet activation using a simplified treatment of the aerosol number size distribution Niku Kivekäs,
P = x i. P i. = y i. Aerosol and Aqueous Chemistry. Raoult s Law. Raoult s Law vs. Henry s Law. or C i. = HC i. = k H
 The Great Smog Aerosol and Aqueous Chemistry Equilibrium Partitioning Oxidation and Oxidants Other Surface-driven Fogs in London were a common occurrence, but the events that began on the 5th of December
The Great Smog Aerosol and Aqueous Chemistry Equilibrium Partitioning Oxidation and Oxidants Other Surface-driven Fogs in London were a common occurrence, but the events that began on the 5th of December
Clouds and turbulent moist convection
 Clouds and turbulent moist convection Lecture 2: Cloud formation and Physics Caroline Muller Les Houches summer school Lectures Outline : Cloud fundamentals - global distribution, types, visualization
Clouds and turbulent moist convection Lecture 2: Cloud formation and Physics Caroline Muller Les Houches summer school Lectures Outline : Cloud fundamentals - global distribution, types, visualization
