The Clausius-Clapeyron and the Kelvin Equations
|
|
|
- Natalie Washington
- 5 years ago
- Views:
Transcription
1 PhD Environmental Fluid Mechanics Physics of the Atmosphere University of Trieste International Center for Theoretical Physics The Clausius-Clapeyron and the Kelvin Equations by Dario B. Giaiotti and Fulvio Stel (1) Regional Meteorological Observatory, via Oberdan, 18/A I Visco (UD) - ITALY Abstract This lecture deals with the phase changes. In particular, starting from heuristic considerations, a quantity called Gibbs free energy is defined. This quantity is conserved during isothermal and isobaric transformations and is used to determine how the equilibrium pressure between two different phases changes with temperature. This law is called the Clausius-Clapeyron equation and is one of the fundamental laws of cloud microphysics. The Clausius-Clapeyron equation for the three different phase changes is represented in the P-T diagram. The properties of these relationships are briefly described as well as some examples of their applications. Some preliminary considerations are done concerning the role of Clausius-Clapeyron equation on the precipitation formation. The Clausius-Clapeyron equation does not describe the most general possible relationship between the equilibrium pressure and temperature because it does not take into account the geometry of the system. A generalized form of the Gibbs free energy that takes into account even the energy needed to create a surface is developed. With this generalized form the dependency of equilibrium pressure in a spherical geometry is developed. This relationship is called Kelvin's law. The role of Kelvin's law in the occurrence of supersaturation, then in the formation of cloud droplets is discussed. The Gibbs free energy Water vapour can be considered as a perfect gas (i.e., described by the ideal gas law e= ρrt where e is its partial pressure, R its constant and ρ its density) almost for all the real situations apart when condensation occurs. When condensation takes place, e.g., during an isothermal compression, what is observed is that even the vapour pressure remains constant up to the moment in which al the vapour is condensed. This fact cannot be explained nor by the ideal gas law, neither by the Van der Waals equation of state, moreover the pressure value at which condensation begins is, for pure water, function of temperature only. This value is called saturation vapour pressure and it should be extremely interesting to know in advance its value for a given temperature to realize how far we are, in terms of amount of vapour pressure from saturation. To know how saturation vapour pressure changes as a function of temperature we cannot, obviously, use nor the ideal gas law, neither the van der Waals but we have to base our thoughts on more fundamental laws, in particular on the first law of thermodynamic. dq dt = du dv e dt dt We apply the first law to the idealized transformation above mentioned, that is the isothermal compression at saturation on a system composed by liquid water and water vapour. If we integrate the first law between the times t 1 and t 2 we obtain
2 t2 dq t1 dt dt= t2 du t1 dt dt t2 dv t1 e dt dt. Because in the above transformation (constant temperature) during the condensation e remains constant, defining as l the amount of heat released (absorbed) when a unitary mass of vapour is condensed (evaporated), we can write l Δ M =U t 2 U t 1 e V t 2 V t 1 dividing by the total amount of water vapour ΔM condensed and introducing the specific variables for internal energy and volume (i.e., u and α) we have l=u t 2 u t 1 e α t 2 α t 1. At this point it is useful to introduce the specific entropy s for unit mass defined as ds dt = 1 T dq dt Because in the above transformation temperature T is kept constant, integrating the specific temperature between the same times t 1 and t 2 we obtain t2 ds t1 dt dt= t2 1 t1 T that is s t 2 s t 1 = t1 T or equivalently dq dt dt t2 1 T s t 2 s t 1 = t1 dq dt dt= 1 T l t2 dq dt dt=l Equating the two specific latent heats we obtain the relationship T s t 2 s t 1 =u t 2 u t 1 e α t 2 α t 1 that rearranged becomes u t 1 e α t 1 T s t 1 =u t 2 e α t 2 T s t 2 which states that we can define a quantity (usually denoted by the g letter) which is constant during the phase transitions (in isobaric and isothermal transformations). This quantity is called Gibbs free energy (i.e., g = u + pα - Ts) and it is essentially the specific enthalpy (i.e., h = u + pα) diminished by the product of temperature for specific entropy (i.e., Ts). Thanks to its constance during phase transitions, specific Gibbs internal energy can be used to describe how the saturation vapour pressure changes with temperature, in fact the specific Gibbs free energy is constant for the two phases (vapour and liquid), then we can write dg l = dg v
3 equation that, using the definition of Gibbs free energy, becomes d u eα Ts l = d u eα Ts v expanding the derivatives we obtain du l de l α e dα l l l s T ds l l = du v de v α e dα v v v s T ds v v or equivalently du l e dα l l de l α s T ds l l l = du v e dα v v de v α s T ds v v v then applying the following form of the first law of thermodynamics dq dt du dv = e dt and the following definition of entropy ds dt = 1 T dq dt both based on the fact that the only quantity we varies is temperature, the above equation becomes dq l de l α s dq l l l = dq v de v α s dq v v v then de l α l s l = de v α v s v. Since the vapour and liquid pressure are the same, i.e., the saturation vapour pressure e v =e l e s, we obtain de s = s v s l α v α l This equation solves formally our problem, because it gives a defined form for the variation of saturation vapour pressure with temperature, moreover it gives us useful hints for the physical interpretation of the saturation vapour pressure derivative. In fact we can say that the rate of change of saturation vapour pressure with temperature is the entropy rate of change for the given change in specific volume in the transition between the two phases. In other words we can say that saturation vapour pressure changes (increases) with temperature because specific entropy increases with temperature more in vapour than in liquid phase. Apart from its interesting physical interpretation the above equation is still in a poorly useful form. A better form can be easily obtained remembering the result we obtained to define the Gibbs internal energy, i.e., the difference in the specific entropies of liquid and solid water during the phase transition is the specific latent heat of
4 vaporization (condensation) divided by the temperature at which the transition takes place. With this in mind we obtain de s = 1 T l α v α l which is the usually adopted functional dependence of saturation vapour pressure from absolute temperature, universally known as Clausius-Clapeyron equation. This equation mutatis mutandis can be used to describe all the change of phase from vapour to liquid, from vapour to solid and from liquid to solid. In the latter cases latent heats of sublimation and solidification (i.e., sublimation minus condensation) have to be used instead of latent heat of condensation. This equation can be integrated in principle but practically this can be done only assuming a functional dependency from temperature of latent heats and specific volumes. Some considerations, however, can be done even without the integration. In particular usually, being latent heats positive defined, the saturation pressure increases or decreases with temperature according to the interplay between the specific volumes of the two phases. In other words, usually saturation pressure for vapour and liquid phases increases with temperature because vapour density is lower than liquid density. The same happens for many liquid and solid phases, apart from water because its density is lower for ice than for liquid As told before, the water Clausius-Clapeyron equation for the usual atmospheric conditions can be quite easily integrated with a few simple assumptions. The simplest possible situation is that of vapour and liquid phases. We start assuming that latent heat of condensation is constant with temperature and that liquid water specific volume (inverse of density) can be neglected when compared with vapour specific volume. Water latent heat of condensation changes by about 6% from its l=2501 J / g at 0 C. We can go a step further just using one more assumption, that is the ideal gas law for the water vapour to describe the functional dependence of α v from temperature, i.e., eα v = RT. With these assumptions the Clausius-Clapeyron equation becomes de s 1 T l α v 1 T l e s RT = l e s RT 2 which can be easily integrated giving an exponential dependence of e s from temperature. A greater accuracy (i.e., ability of reproducing reality) can be obtained introducing into the above equation the dependence of latent heat from temperature. This can be easily done using the first law of thermodynamics and in particular a result we obtained in defining the Gibbs free energy to determine the latent heat of condensation, that is l=u t 2 u t 1 e α t 2 α t 1 where the index t 1 is referring to the liquid phase while t 2 to the vapour one (in this way l is positive). This equation can be simplified remembering that α 2 α 1 and confusing again water vapour with an ideal gas, then l=u t 2 u t 1 eα t 1 =u t 2 u t 1 RT Differentiating the above equation with respect to temperature, we obtain dl = d u t 2 d u t 1 d RT remembering that u(t2) and u(t1) are the internal energies of liquid water and water vapour, by
5 definition their derivatives represent the specific heats of water vapour at constant volume c vv (remember that we still consider vapour as an ideal gas) and the specific heat of water c w. Then we obtain dl =c vv c w R or, remembering that R=c pv c vv dl =c pv c w which states us that if you do not want to consider constant with temperature the latent heat of condensation, you can at least consider constant with temperature its derivative. Then, fixing a reference status l 0,T 0, we can obtain the functional dependence of latent heat from temperature l= c pv c w T T 0 l 0 that can be used into the Clausius-Clapeyron equation to obtain a preciser dependency of saturation vapour pressure from temperature. Apart from this specific use, the above equation tells us that Δl=c pv ΔT c w ΔT =ΔQ pv ΔQ w which means that when temperature changes the variations in latent heat of condensation is equal to the difference in the amount of heat absorbed (released) by two unitary masses of vapour and liquid water at constant pressure. This is not surprising, since that difference in heats has to be released (absorbed) when water vapour is made to condense (evaporate) keeping constant temperature (remember that condensation is a constant pressure process). Before to conclude the discussion on the Clausius-Clapeyron equation it is just worth to spend a few more words on the liquid and solid transitions of phase. For them the equation assumes the form de s = 1 T l α l α i where l in this case is the latent heat of solidification and e s in this case is the pressure exerted by liquid over solid phase when they are in equilibrium (i.e., nor increasing of liquid part neither solid one for a closed system). It is interesting to apply this equation to water because, since T and l are positive defined, the sign of the derivative (i.e., the slope of e s in the PT diagram) is that of (α_l - α_i). For water this is negative since ice density is lower than liquid one. With this in mind we can produce the PT diagram for the three phases (liquid, solid and vapour) of water.
6 Pressure (hpa) Temperature (C) From the above picture it is quite clear that equilibrium pressure over liquid is slightly higher over liquid than over ice. From the molecular point of view this means that water molecules escape easily from water than from ice, then reaching farther (i.e, for higher pressures) the equilibrium with the vapour. Concerning the equilibrium pressure between liquid and solid, as described above, it decreases with increasing temperature. According to our naïve interpretation, we can imagine that for a fixed negative temperature you have to compress a lot ice to reduce its density -then reducing the intramolecular distances- making a little bit easy their escape from the ice phase in favour of the liquid one. The slope of equilibrium pressure between solid and liquid phase in the P-T diagram is pretty steep (almost vertical) of the order of de s/~ 10 5 hpa K 1, inverting it we can see that freezing level decreases by nearly ΔT ~ 0.01 K when we increase pressure by one atmosphere (1013 hpa). This means that you can find liquid water even below the classical freezing level just increasing (a lot) pressure. However the sensitivity of melting point from pressure is so low that only in systems characterized by high pressures can take advantage from this mechanism. The classical example is represented by glaciers, whose base can host a thin liquid layer even for negative Celsius temperatures. Of course, dealing with glaciers composed by other substances, e.g. C0 2, as can happen in other planets, this effect might not take place when the solid phase of the specific substance has a higher density than the liquid one. All the three equilibrium pressures for all the substances intersect on a point (called triple point) that for water coincides with the zero Celsius (i.e., K). At the triple point all the three phases can coexist. The triple point is the key quantity when dealing with changes of phase, in fact as shown in the previous lectures, even if at standard atmospheric pressures both water and carbon dioxide are vapours, their behaviour is quite different. We have to take care of water vapour for the change of phase, while we can almost forget carbon dioxide. The reason why this happens is that the triple point for carbon dioxide (~216.5 K, -56 ºC) is lower than for water. Is the distance from the triple e_liq e_ice p_ice Figure. Water equilibrium pressures of liquid over solid (solid blue line), of vapour over liquid (dashed red line) and of vapour over solid (solid green).
7 point that makes impossible the phase transition of the carbon dioxide, otherwise we should take care even of its latent heat of evaporation (lv ~ 574 J/kg) and of solidification (ls ~ 184 J/kg) to understand and forecast the atmospheric behaviour. Table. Saturation vapour pressure over liquid water, ice and the corresponding latent heats. Reproduced from the Smithsonian Meteorological Tables (List, 1951). T C e_s (Pa) e _i (Pa) L_v->l (J/kg) L_v->s (J/kg) L_l->s(J/kg) The dependence of saturation vapour pressure from geometry Obtaining the Clausius-Clapeyron equation and the dependence of equilibrium pressure from temperature we use the free energy of Gibbs. This free energy is conserved during the bulk transition between vapour and liquid as demonstrated above. However, in some circumstances (very often observed in nature), dealing with bulk phases is not enough, in fact geometry may play a major role. We can tackle the problem just with some heuristic considerations, in fact if we want to split a water droplet in two parts we have to spend energy. This expenditure of energy is needed to break the links between water molecules to produce two distinct parts. But we can consider this energy expenditure as due to the formation of a greater surface enveloping water. Even if this can seem counter-intuitive, it is particularly convenient under the analytical point of view. Returning back to our initial problem, defining as g v and g w the Gibbs free energies for unit mass of water vapour and liquid water respectively, assuming that the energy required to produce a unit surface of liquid phase is σ, the conservation of total energy requires d dt M v g v M w g w σ A =0 where A is the surface of liquid phase and Mw and Mv are respectively the masses of liquid and vapour phases. Mass conservation during phase transition requires that
8 d dt M v = d dt M w then expanding the above derivative we obtain g w g v dm w σ d dt A dt=0 Assuming a spherical geometry we can express the water mass as a function of water density ρ and of the droplet radius r. Moreover we can rewrite even the droplet surface obtaining g w g v ρ w dr dr π 3r σ 4π 2r dt dt =0 where we assumed constant both the surface energy for unit surface and the density. Simplifying we obtain g g 2σ w v rρ w dr dt =0 Since energy has to be conserved independently from the radius variation with time, we can conclude that g v =g w 2σ rρ w This equation means that not all the bulk Gibbs free energy of vapour transfers into the liquid phase (per unit mass): an amount of it is spent in producing the surface. If the droplet radius diverge (infinite curvature, e.g., a plane droplet), we return back to what we used at the beginning of this lecture. To obtain the equilibrium pressure dependence from the geometry (radius in this case), we will now use the above generalize version of the Gibbs free energy conservation. In particular we will differentiate with respect to the radius r. dg v dr = dg w dr d dr 2σ rρ w To expand this derivative we have to remember that Gibbs free energy depend only from pressure p and temperature T (which we consider not dependent from radius), then g v p dp dr = g w dp p dr 2 r 2 σ ρ w from the functional dependency of specific Gibbs free energy we have g v p = 1 ρ v and g w p = 1 ρ w Then with simple algebra we obtain dp 2σ ρ = dr r v 2 ρ w ρ v
9 Remembering that at ordinary atmospheric conditions water vapour can be considered an ideal gas, we can write vapour density as a function of pressure and temperature, that is ρ v = p R v T With relatively simple algebra we obtain the following differential equation ρ w R v T p p dp 2σ = dr r 2 which can be solved integrating it from a generic radius r up to an infinite radius, giving ln p p = 2 σ r ρ w R v T ρ v p p ρ w p Since water density is higher than vapour density we can delete the last term on the right member obtaining the so called Kelvin law for the equilibrium pressure: p= p e 2σ r ρ w R v T The interpretation of p is quite straightforward, remembering that the infinite radius corresponds to a flat surface. In other words it is the bulk equilibrium pressure we obtained with the Clausius Clapeyron equation. Using the values normally observed in atmospheric conditions for the above Kelvin's law (Rv = 461 J/kg K ; σ = N m and ρ w = 10 3 kg/m 3 ) we obtain r p= p e where r is measured in microns. From the above law it is clear that differences in the saturation (equilibrium) vapour pressure over water is really a weak function of radius. Nevertheless this dependence is extremely important foe the development of cloud droplets and then the formation of precipitations. In particular it is clear that the saturation vapour pressure required to admit the formation of droplets of the order of a few hundredths of micron is extremely high, more than twice the maximum observed in nature. This means that condensation of water vapour is extremely difficult. Very small droplets can form easily under the statistical point of view, but they are stable only for very high pressures. On the contrary the pressure required for the equilibrium of large droplets (a few microns) is not so high, but they are statistically disadvantaged. This is the reason why condensation of pure water vapour (homogeneous condensation or nucleation) is quite rare in nature and the formation of clouds has to take place for different reasons, in particular we need what is called heterogeneous condensation which requires the presence of condensation nuclei. The same is true for the solid phase formation.
10 Figure. The ratio between equilibrium pressure for water over a spherical and flat surface as a function of the sphere radius p/p 2.2 p/p_inf r (μm)
Kelvin Effect. Covers Reading Material in Chapter 10.3 Atmospheric Sciences 5200 Physical Meteorology III: Cloud Physics
 Kelvin Effect Covers Reading Material in Chapter 10.3 Atmospheric Sciences 5200 Physical Meteorology III: Cloud Physics Vapor Pressure (e) e < e # e = e # Vapor Pressure e > e # Relative humidity RH =
Kelvin Effect Covers Reading Material in Chapter 10.3 Atmospheric Sciences 5200 Physical Meteorology III: Cloud Physics Vapor Pressure (e) e < e # e = e # Vapor Pressure e > e # Relative humidity RH =
Köhler Curve. Covers Reading Material in Chapter 10.3 Atmospheric Sciences 5200 Physical Meteorology III: Cloud Physics
 Köhler Curve Covers Reading Material in Chapter 10.3 Atmospheric Sciences 5200 Physical Meteorology III: Cloud Physics Review of Kelvin Effect Gibbs Energy for formation of a drop G = G &'()*+, G ).'+
Köhler Curve Covers Reading Material in Chapter 10.3 Atmospheric Sciences 5200 Physical Meteorology III: Cloud Physics Review of Kelvin Effect Gibbs Energy for formation of a drop G = G &'()*+, G ).'+
Basic Thermodynamics Prof. S. K. Som Department of Mechanical Engineering Indian Institute of Technology, Kharagpur. Lecture No 16
 Basic Thermodynamics Prof. S. K. Som Department of Mechanical Engineering Indian Institute of Technology, Kharagpur Lecture No 16 Properties of Pure Substances-I Good afternoon. In the last class, we were
Basic Thermodynamics Prof. S. K. Som Department of Mechanical Engineering Indian Institute of Technology, Kharagpur Lecture No 16 Properties of Pure Substances-I Good afternoon. In the last class, we were
1. Heterogeneous Systems and Chemical Equilibrium
 1. Heterogeneous Systems and Chemical Equilibrium The preceding section involved only single phase systems. For it to be in thermodynamic equilibrium, a homogeneous system must be in thermal equilibrium
1. Heterogeneous Systems and Chemical Equilibrium The preceding section involved only single phase systems. For it to be in thermodynamic equilibrium, a homogeneous system must be in thermal equilibrium
Precipitation Formation, and RADAR Equation by Dario B. Giaiotti and Fulvio Stel (1)
 PhD Environmental Fluid Mechanics Physics of the Atmosphere University of Trieste International Center for Theoretical Physics Precipitation Formation, and RADAR Equation by Dario B. Giaiotti and Fulvio
PhD Environmental Fluid Mechanics Physics of the Atmosphere University of Trieste International Center for Theoretical Physics Precipitation Formation, and RADAR Equation by Dario B. Giaiotti and Fulvio
ln( P vap(s) / torr) = T / K ln( P vap(l) / torr) = T / K
 Chem 4501 Introduction to Thermodynamics, 3 Credits Kinetics, and Statistical Mechanics Fall Semester 2017 Homework Problem Set Number 9 Solutions 1. McQuarrie and Simon, 9-4. Paraphrase: Given expressions
Chem 4501 Introduction to Thermodynamics, 3 Credits Kinetics, and Statistical Mechanics Fall Semester 2017 Homework Problem Set Number 9 Solutions 1. McQuarrie and Simon, 9-4. Paraphrase: Given expressions
Chapter 5. On-line resource
 Chapter 5 The water-air heterogeneous system On-line resource on-line analytical system that portrays the thermodynamic properties of water vapor and many other gases http://webbook.nist.gov/chemistry/fluid/
Chapter 5 The water-air heterogeneous system On-line resource on-line analytical system that portrays the thermodynamic properties of water vapor and many other gases http://webbook.nist.gov/chemistry/fluid/
Chemical Potential. Combining the First and Second Laws for a closed system, Considering (extensive properties)
 Chemical Potential Combining the First and Second Laws for a closed system, Considering (extensive properties) du = TdS pdv Hence For an open system, that is, one that can gain or lose mass, U will also
Chemical Potential Combining the First and Second Laws for a closed system, Considering (extensive properties) du = TdS pdv Hence For an open system, that is, one that can gain or lose mass, U will also
Phase Diagrams. NC State University
 Chemistry 433 Lecture 18 Phase Diagrams NC State University Definition of a phase diagram A phase diagram is a representation of the states of matter, solid, liquid, or gas as a function of temperature
Chemistry 433 Lecture 18 Phase Diagrams NC State University Definition of a phase diagram A phase diagram is a representation of the states of matter, solid, liquid, or gas as a function of temperature
Physical transformations of pure substances Boiling, freezing, and the conversion of graphite to diamond examples of phase transitions changes of
 Physical transformations of pure substances Boiling, freezing, and the conversion of graphite to diamond examples of phase transitions changes of phase without change of chemical composition. In this chapter
Physical transformations of pure substances Boiling, freezing, and the conversion of graphite to diamond examples of phase transitions changes of phase without change of chemical composition. In this chapter
Physics Nov Phase Transitions
 Physics 301 11-Nov-1999 15-1 Phase Transitions Phase transitions occur throughout physics. We are all familiar with melting ice and boiling water. But other kinds of phase transitions occur as well. Some
Physics 301 11-Nov-1999 15-1 Phase Transitions Phase transitions occur throughout physics. We are all familiar with melting ice and boiling water. But other kinds of phase transitions occur as well. Some
5.60 Thermodynamics & Kinetics Spring 2008
 MIT OpenCourseWare http://ocw.mit.edu 5.60 Thermodynamics & Kinetics Spring 2008 For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms. 5.60 Spring 2008 Lecture
MIT OpenCourseWare http://ocw.mit.edu 5.60 Thermodynamics & Kinetics Spring 2008 For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms. 5.60 Spring 2008 Lecture
Basic Thermodynamics Prof. S. K. Som Department of Mechanical Engineering Indian Institute of Technology, Kharagpur
 Basic Thermodynamics Prof. S. K. Som Department of Mechanical Engineering Indian Institute of Technology, Kharagpur Lecture - 15 Joule-Kelvin Expansion; Properties of Pure Substances Good morning. Last
Basic Thermodynamics Prof. S. K. Som Department of Mechanical Engineering Indian Institute of Technology, Kharagpur Lecture - 15 Joule-Kelvin Expansion; Properties of Pure Substances Good morning. Last
Phase Diagrams. Department of Mechanical Engineering Indian Institute of Technology Kanpur Kanpur India
 Phase Diagrams 1 Increasing the temperature isobarically T-v diagram of constant-pressure phase-change processes of a pure substance at various pressures numerical values are for water. 2 Temperature -
Phase Diagrams 1 Increasing the temperature isobarically T-v diagram of constant-pressure phase-change processes of a pure substance at various pressures numerical values are for water. 2 Temperature -
Chapter 6. Phase transitions. 6.1 Concept of phase
 Chapter 6 hase transitions 6.1 Concept of phase hases are states of matter characterized by distinct macroscopic properties. ypical phases we will discuss in this chapter are liquid, solid and gas. Other
Chapter 6 hase transitions 6.1 Concept of phase hases are states of matter characterized by distinct macroscopic properties. ypical phases we will discuss in this chapter are liquid, solid and gas. Other
The Gibbs Phase Rule F = 2 + C - P
 The Gibbs Phase Rule The phase rule allows one to determine the number of degrees of freedom (F) or variance of a chemical system. This is useful for interpreting phase diagrams. F = 2 + C - P Where F
The Gibbs Phase Rule The phase rule allows one to determine the number of degrees of freedom (F) or variance of a chemical system. This is useful for interpreting phase diagrams. F = 2 + C - P Where F
The Second Law of Thermodynamics (Chapter 4)
 The Second Law of Thermodynamics (Chapter 4) First Law: Energy of universe is constant: ΔE system = - ΔE surroundings Second Law: New variable, S, entropy. Changes in S, ΔS, tell us which processes made
The Second Law of Thermodynamics (Chapter 4) First Law: Energy of universe is constant: ΔE system = - ΔE surroundings Second Law: New variable, S, entropy. Changes in S, ΔS, tell us which processes made
Clausius Clapeyron Equation
 Course - BSc. Applied Physical Science (Computer Science) Year & Semester - Ist, IInd Subject - Physics Paper No. - 6 Paper Title - Thermal Physics Lecture No. 18 Clausius Clapeyron Equation Hello friends,
Course - BSc. Applied Physical Science (Computer Science) Year & Semester - Ist, IInd Subject - Physics Paper No. - 6 Paper Title - Thermal Physics Lecture No. 18 Clausius Clapeyron Equation Hello friends,
Thermodynamic condition for equilibrium between two phases a and b is G a = G b, so that during an equilibrium phase change, G ab = G a G b = 0.
 CHAPTER 5 LECTURE NOTES Phases and Solutions Phase diagrams for two one component systems, CO 2 and H 2 O, are shown below. The main items to note are the following: The lines represent equilibria between
CHAPTER 5 LECTURE NOTES Phases and Solutions Phase diagrams for two one component systems, CO 2 and H 2 O, are shown below. The main items to note are the following: The lines represent equilibria between
Phase Transitions. µ a (P c (T ), T ) µ b (P c (T ), T ), (3) µ a (P, T c (P )) µ b (P, T c (P )). (4)
 Phase Transitions A homogeneous equilibrium state of matter is the most natural one, given the fact that the interparticle interactions are translationally invariant. Nevertheless there is no contradiction
Phase Transitions A homogeneous equilibrium state of matter is the most natural one, given the fact that the interparticle interactions are translationally invariant. Nevertheless there is no contradiction
 Physical Chemistry Physical chemistry is the branch of chemistry that establishes and develops the principles of Chemistry in terms of the underlying concepts of Physics Physical Chemistry Main book: Atkins
Physical Chemistry Physical chemistry is the branch of chemistry that establishes and develops the principles of Chemistry in terms of the underlying concepts of Physics Physical Chemistry Main book: Atkins
Atmospheric Thermodynamics
 Atmospheric Thermodynamics R. Wordsworth February 12, 2015 1 Objectives Derive hydrostatic equation Derive dry and moist adiabats Understand how the theory relates to observed properties of real atmospheres
Atmospheric Thermodynamics R. Wordsworth February 12, 2015 1 Objectives Derive hydrostatic equation Derive dry and moist adiabats Understand how the theory relates to observed properties of real atmospheres
Pure Substance. Properties of Pure Substances & Equations of State. Vapour-Liquid-Solid Phase Equilibrium
 Pure Substance Properties of Pure Substances & Equations of State Dr. d. Zahurul Haq Professor Department of echanical Engineering Bangladesh University of Engineering & Technology (BUET) Dhaka-1000, Bangladesh
Pure Substance Properties of Pure Substances & Equations of State Dr. d. Zahurul Haq Professor Department of echanical Engineering Bangladesh University of Engineering & Technology (BUET) Dhaka-1000, Bangladesh
CHEMICAL ENGINEERING THERMODYNAMICS. Andrew S. Rosen
 CHEMICAL ENGINEERING THERMODYNAMICS Andrew S. Rosen SYMBOL DICTIONARY 1 TABLE OF CONTENTS Symbol Dictionary... 3 1. Measured Thermodynamic Properties and Other Basic Concepts... 5 1.1 Preliminary Concepts
CHEMICAL ENGINEERING THERMODYNAMICS Andrew S. Rosen SYMBOL DICTIONARY 1 TABLE OF CONTENTS Symbol Dictionary... 3 1. Measured Thermodynamic Properties and Other Basic Concepts... 5 1.1 Preliminary Concepts
MS212 Thermodynamics of Materials ( 소재열역학의이해 ) Lecture Note: Chapter 7
 2017 Spring Semester MS212 Thermodynamics of Materials ( 소재열역학의이해 ) Lecture Note: Chapter 7 Byungha Shin ( 신병하 ) Dept. of MSE, KAIST Largely based on lecture notes of Prof. Hyuck-Mo Lee and Prof. WooChul
2017 Spring Semester MS212 Thermodynamics of Materials ( 소재열역학의이해 ) Lecture Note: Chapter 7 Byungha Shin ( 신병하 ) Dept. of MSE, KAIST Largely based on lecture notes of Prof. Hyuck-Mo Lee and Prof. WooChul
Step 1. Step 2. g l = g v. dg = 0 We have shown that over a plane surface of water. g v g l = ρ v R v T ln e/e sat. this can be rewritten
 The basic question is what makes the existence of a droplet thermodynamically preferable to the existence only of water vapor. We have already derived an expression for the saturation vapor pressure over
The basic question is what makes the existence of a droplet thermodynamically preferable to the existence only of water vapor. We have already derived an expression for the saturation vapor pressure over
Lecture Phase transformations. Fys2160,
 Lecture 12 01.10.2018 Phase transformations Fys2160, 2018 1 A phase transformation Discontinuous change in the properties of substance when the environent is changed infinitesimaly. Change between phases
Lecture 12 01.10.2018 Phase transformations Fys2160, 2018 1 A phase transformation Discontinuous change in the properties of substance when the environent is changed infinitesimaly. Change between phases
Thermodynamics I. Properties of Pure Substances
 Thermodynamics I Properties of Pure Substances Dr.-Eng. Zayed Al-Hamamre 1 Content Pure substance Phases of a pure substance Phase-change processes of pure substances o Compressed liquid, Saturated liquid,
Thermodynamics I Properties of Pure Substances Dr.-Eng. Zayed Al-Hamamre 1 Content Pure substance Phases of a pure substance Phase-change processes of pure substances o Compressed liquid, Saturated liquid,
Physics 5D PRACTICE FINAL EXAM Fall 2013
 Print your name: Physics 5D PRACTICE FINAL EXAM Fall 2013 Real Exam is Wednesday December 11 Thimann Lecture 3 4:00-7:00 pm Closed book exam two 8.5x11 sheets of notes ok Note: Avogadro s number N A =
Print your name: Physics 5D PRACTICE FINAL EXAM Fall 2013 Real Exam is Wednesday December 11 Thimann Lecture 3 4:00-7:00 pm Closed book exam two 8.5x11 sheets of notes ok Note: Avogadro s number N A =
At this point, we've developed the tools and basic concepts necessary to apply
 18 Lecture 0 At this point, we've developed the tools and basic concepts necessary to apply thermodynamics to a number of different systems, with the ultimate goal of describing chemically reacting systems.
18 Lecture 0 At this point, we've developed the tools and basic concepts necessary to apply thermodynamics to a number of different systems, with the ultimate goal of describing chemically reacting systems.
MT3230 Supplemental Data 4.2 Spring, 2018 Dr. Sam Miller COMPUTING THE LATENT HEAT OF VAPORIZATION OF WATER AS A FUNCTION OF TEMPERATURE.
 MT3230 Supplemental Data 4.2 Spring, 2018 Dr. Sam Miller COMPUTING THE LATENT HEAT OF VAPORIZATION OF WATER AS A FUNCTION OF TEMPERATURE Abstract The latent heat of vaporization parameterizes the amount
MT3230 Supplemental Data 4.2 Spring, 2018 Dr. Sam Miller COMPUTING THE LATENT HEAT OF VAPORIZATION OF WATER AS A FUNCTION OF TEMPERATURE Abstract The latent heat of vaporization parameterizes the amount
Application of Thermodynamics in Phase Diagrams. Today s Topics
 Lecture 23 Application of Thermodynamics in Phase Diagrams The Clausius Clapeyron Equation A. K. M. B. Rashid Professor, Department of MME BUET, Dhaka Today s Topics The Clapeyron equation Integration
Lecture 23 Application of Thermodynamics in Phase Diagrams The Clausius Clapeyron Equation A. K. M. B. Rashid Professor, Department of MME BUET, Dhaka Today s Topics The Clapeyron equation Integration
2σ e s (r,t) = e s (T)exp( rr v ρ l T ) = exp( ) 2σ R v ρ l Tln(e/e s (T)) e s (f H2 O,r,T) = f H2 O
 Formulas/Constants, Physics/Oceanography 4510/5510 B Atmospheric Physics II N A = 6.02 10 23 molecules/mole (Avogadro s number) 1 mb = 100 Pa 1 Pa = 1 N/m 2 Γ d = 9.8 o C/km (dry adiabatic lapse rate)
Formulas/Constants, Physics/Oceanography 4510/5510 B Atmospheric Physics II N A = 6.02 10 23 molecules/mole (Avogadro s number) 1 mb = 100 Pa 1 Pa = 1 N/m 2 Γ d = 9.8 o C/km (dry adiabatic lapse rate)
Chapter 3 PROPERTIES OF PURE SUBSTANCES SUMMARY
 Chapter 3 PROPERTIES OF PURE SUBSTANCES SUMMARY PURE SUBSTANCE Pure substance: A substance that has a fixed chemical composition throughout. Compressed liquid (sub-cooled liquid): A substance that it is
Chapter 3 PROPERTIES OF PURE SUBSTANCES SUMMARY PURE SUBSTANCE Pure substance: A substance that has a fixed chemical composition throughout. Compressed liquid (sub-cooled liquid): A substance that it is
For an incompressible β and k = 0, Equations (6.28) and (6.29) become:
 Internal Energy and Entropy as Functions of T and V These are general equations relating the internal energy and entropy of homogeneous fluids of constant composition to temperature and volume. Equation
Internal Energy and Entropy as Functions of T and V These are general equations relating the internal energy and entropy of homogeneous fluids of constant composition to temperature and volume. Equation
Chapter 12 Intermolecular Forces of Attraction
 Chapter 12 Intermolecular Forces of Attraction Intermolecular Forces Attractive or Repulsive Forces between molecules. Molecule - - - - - - Molecule Intramolecular Forces bonding forces within the molecule.
Chapter 12 Intermolecular Forces of Attraction Intermolecular Forces Attractive or Repulsive Forces between molecules. Molecule - - - - - - Molecule Intramolecular Forces bonding forces within the molecule.
Lecture Notes 1: Physical Equilibria Vapor Pressure
 Lecture Notes 1: Physical Equilibria Vapor Pressure Our first exploration of equilibria will examine physical equilibria (no chemical changes) in which the only changes occurring are matter changes phases.
Lecture Notes 1: Physical Equilibria Vapor Pressure Our first exploration of equilibria will examine physical equilibria (no chemical changes) in which the only changes occurring are matter changes phases.
THERMODYNAMICS NOTES. These notes give a brief overview of engineering thermodynamics. They are based on the thermodynamics text by Black & Hartley.
 THERMODYNAMICS NOTES These notes give a brief overview of engineering thermodynamics. They are based on the thermodynamics text by Black & Hartley. Topics covered include: concepts; properties; conservation
THERMODYNAMICS NOTES These notes give a brief overview of engineering thermodynamics. They are based on the thermodynamics text by Black & Hartley. Topics covered include: concepts; properties; conservation
Pure Substance. Properties of Pure Substances & Equations of State. Vapour-Liquid-Solid Phase Equilibrium
 Pure Substance Properties of Pure Substances & Equations of State Dr. d. Zahurul Haq Professor Department of echanical Engineering Bangladesh University of Engineering & Technology (BUET) Dhaka-1000, Bangladesh
Pure Substance Properties of Pure Substances & Equations of State Dr. d. Zahurul Haq Professor Department of echanical Engineering Bangladesh University of Engineering & Technology (BUET) Dhaka-1000, Bangladesh
Chapter 8 Phase Diagram, Relative Stability of Solid, Liquid, and Gas
 Chapter 8 Phase Diagram, Relative Stability of Solid, Liquid, and Gas Three states of matter: solid, liquid, gas (plasma) At low T: Solid is most stable. At high T: liquid or gas is most stable. Ex: Most
Chapter 8 Phase Diagram, Relative Stability of Solid, Liquid, and Gas Three states of matter: solid, liquid, gas (plasma) At low T: Solid is most stable. At high T: liquid or gas is most stable. Ex: Most
Physics and Thermodynamics of Water and Ice. Ottmar Möhler
 Forschungszentrum Karlsruhe in der Helmholtz-Gemeinschaft Physics and Thermodynamics of Water and Ice Ottmar Möhler Institute for Meteorology and Climate Research (IMK-AAF) ESF workshop on Microbiological
Forschungszentrum Karlsruhe in der Helmholtz-Gemeinschaft Physics and Thermodynamics of Water and Ice Ottmar Möhler Institute for Meteorology and Climate Research (IMK-AAF) ESF workshop on Microbiological
Thermodynamics (Classical) for Biological Systems Prof. G. K. Suraishkumar Department of Biotechnology Indian Institute of Technology Madras
 Thermodynamics (Classical) for Biological Systems Prof. G. K. Suraishkumar Department of Biotechnology Indian Institute of Technology Madras Module No. # 01 Lecture No. # 01 Introduction and Review Welcome
Thermodynamics (Classical) for Biological Systems Prof. G. K. Suraishkumar Department of Biotechnology Indian Institute of Technology Madras Module No. # 01 Lecture No. # 01 Introduction and Review Welcome
Supporting Information: On Localized Vapor Pressure Gradients Governing Condensation and Frost Phenomena
 Supporting Information: On Localized Vapor Pressure Gradients Governing Condensation and Frost Phenomena Saurabh Nath and Jonathan B. Boreyko Department of Biomedical Engineering and Mechanics, Virginia
Supporting Information: On Localized Vapor Pressure Gradients Governing Condensation and Frost Phenomena Saurabh Nath and Jonathan B. Boreyko Department of Biomedical Engineering and Mechanics, Virginia
1. Second Law of Thermodynamics
 1. Second Law of hermodynamics he first law describes how the state of a system changes in response to work it performs and heat absorbed. he second law deals with direction of thermodynamic processes
1. Second Law of hermodynamics he first law describes how the state of a system changes in response to work it performs and heat absorbed. he second law deals with direction of thermodynamic processes
Classical Thermodynamics. Dr. Massimo Mella School of Chemistry Cardiff University
 Classical Thermodynamics Dr. Massimo Mella School of Chemistry Cardiff University E-mail:MellaM@cardiff.ac.uk The background The field of Thermodynamics emerged as a consequence of the necessity to understand
Classical Thermodynamics Dr. Massimo Mella School of Chemistry Cardiff University E-mail:MellaM@cardiff.ac.uk The background The field of Thermodynamics emerged as a consequence of the necessity to understand
Chapter 2 Carnot Principle
 Chapter 2 Carnot Principle 2.1 Temperature 2.1.1 Isothermal Process When two bodies are placed in thermal contact, the hotter body gives off heat to the colder body. As long as the temperatures are different,
Chapter 2 Carnot Principle 2.1 Temperature 2.1.1 Isothermal Process When two bodies are placed in thermal contact, the hotter body gives off heat to the colder body. As long as the temperatures are different,
Chapter 3 PROPERTIES OF PURE SUBSTANCES
 Thermodynamics: An Engineering Approach Seventh Edition Yunus A. Cengel, Michael A. Boles McGraw-Hill, 2011 Chapter 3 PROPERTIES OF PURE SUBSTANCES Copyright The McGraw-Hill Companies, Inc. Permission
Thermodynamics: An Engineering Approach Seventh Edition Yunus A. Cengel, Michael A. Boles McGraw-Hill, 2011 Chapter 3 PROPERTIES OF PURE SUBSTANCES Copyright The McGraw-Hill Companies, Inc. Permission
Thermodynamic Properties
 Thermodynamic Properties (TP) Thermodynamic Properties Define and articulate some of the critical language and concepts of Thermodynamics Distinguish between the universe, system, surroundings, and boundary
Thermodynamic Properties (TP) Thermodynamic Properties Define and articulate some of the critical language and concepts of Thermodynamics Distinguish between the universe, system, surroundings, and boundary
PROPERTIES OF PURE SUBSTANCES. Chapter 3. Mehmet Kanoglu. Thermodynamics: An Engineering Approach, 6 th Edition. Yunus A. Cengel, Michael A.
 Thermodynamics: An Engineering Approach, 6 th Edition Yunus A. Cengel, Michael A. Boles McGraw-Hill, 2008 Chapter 3 PROPERTIES OF PURE SUBSTANCES Mehmet Kanoglu Copyright The McGraw-Hill Companies, Inc.
Thermodynamics: An Engineering Approach, 6 th Edition Yunus A. Cengel, Michael A. Boles McGraw-Hill, 2008 Chapter 3 PROPERTIES OF PURE SUBSTANCES Mehmet Kanoglu Copyright The McGraw-Hill Companies, Inc.
NENG 301 Week 8 Unary Heterogeneous Systems (DeHoff, Chap. 7, Chap )
 NENG 301 Week 8 Unary Heterogeneous Systems (DeHoff, Chap. 7, Chap. 5.3-5.4) Learning objectives for Chapter 7 At the end of this chapter you will be able to: Understand the general features of a unary
NENG 301 Week 8 Unary Heterogeneous Systems (DeHoff, Chap. 7, Chap. 5.3-5.4) Learning objectives for Chapter 7 At the end of this chapter you will be able to: Understand the general features of a unary
Chapter 3 PROPERTIES OF PURE SUBSTANCES. Thermodynamics: An Engineering Approach, 6 th Edition Yunus A. Cengel, Michael A. Boles McGraw-Hill, 2008
 Chapter 3 PROPERTIES OF PURE SUBSTANCES Thermodynamics: An Engineering Approach, 6 th Edition Yunus A. Cengel, Michael A. Boles McGraw-Hill, 2008 Objectives Introduce the concept of a pure substance. Discuss
Chapter 3 PROPERTIES OF PURE SUBSTANCES Thermodynamics: An Engineering Approach, 6 th Edition Yunus A. Cengel, Michael A. Boles McGraw-Hill, 2008 Objectives Introduce the concept of a pure substance. Discuss
MME 2010 METALLURGICAL THERMODYNAMICS II. Fundamentals of Thermodynamics for Systems of Constant Composition
 MME 2010 METALLURGICAL THERMODYNAMICS II Fundamentals of Thermodynamics for Systems of Constant Composition Thermodynamics addresses two types of problems: 1- Computation of energy difference between two
MME 2010 METALLURGICAL THERMODYNAMICS II Fundamentals of Thermodynamics for Systems of Constant Composition Thermodynamics addresses two types of problems: 1- Computation of energy difference between two
(Heat capacity c is also called specific heat) this means that the heat capacity number c for water is 1 calorie/gram-k.
 Lecture 23: Ideal Gas Law and The First Law of Thermodynamics 1 (REVIEW) Chapter 17: Heat Transfer Origin of the calorie unit A few hundred years ago when people were investigating heat and temperature
Lecture 23: Ideal Gas Law and The First Law of Thermodynamics 1 (REVIEW) Chapter 17: Heat Transfer Origin of the calorie unit A few hundred years ago when people were investigating heat and temperature
df dz = dp dt Essentially, this is just a statement of the first law in one of the forms we derived earlier (expressed here in W m 3 ) dq p dt dp
 A problem with using entropy as a variable is that it is not a particularly intuitive concept. The mechanics of using entropy for evaluating system evolution is well developed, but it sometimes feels a
A problem with using entropy as a variable is that it is not a particularly intuitive concept. The mechanics of using entropy for evaluating system evolution is well developed, but it sometimes feels a
Phase Equilibrium: Preliminaries
 Phase Equilibrium: Preliminaries Phase diagrams for two one component systems, CO 2 and H 2 O, are shown below. The main items to note are the following: The lines represent equilibria between two phases.
Phase Equilibrium: Preliminaries Phase diagrams for two one component systems, CO 2 and H 2 O, are shown below. The main items to note are the following: The lines represent equilibria between two phases.
12. Heat of melting and evaporation of water
 VS 12. Heat of melting and evaporation of water 12.1 Introduction The change of the physical state of a substance in general requires the absorption or release of heat. In this case, one speaks of a first
VS 12. Heat of melting and evaporation of water 12.1 Introduction The change of the physical state of a substance in general requires the absorption or release of heat. In this case, one speaks of a first
Chapter 3 PROPERTIES OF PURE SUBSTANCES
 Thermodynamics: An Engineering Approach Seventh Edition in SI Units Yunus A. Cengel, Michael A. Boles McGraw-Hill, 2011 Chapter 3 PROPERTIES OF PURE SUBSTANCES Copyright The McGraw-Hill Companies, Inc.
Thermodynamics: An Engineering Approach Seventh Edition in SI Units Yunus A. Cengel, Michael A. Boles McGraw-Hill, 2011 Chapter 3 PROPERTIES OF PURE SUBSTANCES Copyright The McGraw-Hill Companies, Inc.
Unit 7 (B) Solid state Physics
 Unit 7 (B) Solid state Physics hermal Properties of solids: Zeroth law of hermodynamics: If two bodies A and B are each separated in thermal equilibrium with the third body C, then A and B are also in
Unit 7 (B) Solid state Physics hermal Properties of solids: Zeroth law of hermodynamics: If two bodies A and B are each separated in thermal equilibrium with the third body C, then A and B are also in
Thermodynamics of solids 5. Unary systems. Kwangheon Park Kyung Hee University Department of Nuclear Engineering
 Thermodynamics of solids 5. Unary systems Kwangheon ark Kyung Hee University Department of Nuclear Engineering 5.1. Unary heterogeneous system definition Unary system: one component system. Unary heterogeneous
Thermodynamics of solids 5. Unary systems Kwangheon ark Kyung Hee University Department of Nuclear Engineering 5.1. Unary heterogeneous system definition Unary system: one component system. Unary heterogeneous
CHEM-UA 652: Thermodynamics and Kinetics
 1 CHEM-UA 652: Thermodynamics and Kinetics Notes for Lecture 13 I. PHASE DIAGRAMS The different phases of substances are characterized by different ranges of thermodynamic variables in which these phasesarethestablephases.
1 CHEM-UA 652: Thermodynamics and Kinetics Notes for Lecture 13 I. PHASE DIAGRAMS The different phases of substances are characterized by different ranges of thermodynamic variables in which these phasesarethestablephases.
MAHALAKSHMI ENGINEERING COLLEGE
 MAHALAKSHMI ENGINEERING COLLEGE TIRUCHIRAPALLI 621 213. Department: Mechanical Subject Code: ME2202 Semester: III Subject Name: ENGG. THERMODYNAMICS UNIT-I Basic Concept and First Law 1. What do you understand
MAHALAKSHMI ENGINEERING COLLEGE TIRUCHIRAPALLI 621 213. Department: Mechanical Subject Code: ME2202 Semester: III Subject Name: ENGG. THERMODYNAMICS UNIT-I Basic Concept and First Law 1. What do you understand
ATMO 551a Moist Adiabat Fall Change in internal energy: ΔU
 Enthalpy and the Moist Adiabat We have described the dry adiabat where an air parcel is lifted rapidly causing the air parcel to expand as the environmental pressure decreases and the air parcel does work
Enthalpy and the Moist Adiabat We have described the dry adiabat where an air parcel is lifted rapidly causing the air parcel to expand as the environmental pressure decreases and the air parcel does work
Chapter 3 PROPERTIES OF PURE SUBSTANCES
 Chapter 3 PROPERTIES OF PURE SUBSTANCES PURE SUBSTANCE Pure substance: A substance that has a fixed chemical composition throughout. Air is a mixture of several gases, but it is considered to be a pure
Chapter 3 PROPERTIES OF PURE SUBSTANCES PURE SUBSTANCE Pure substance: A substance that has a fixed chemical composition throughout. Air is a mixture of several gases, but it is considered to be a pure
Exam 2: Cloud Physics April 16, 2008 Physical Meteorology Questions 1-10 are worth 5 points each. Questions are worth 10 points each.
 Exam : Cloud Physics April, 8 Physical Meteorology 344 Name Questions - are worth 5 points each. Questions -5 are worth points each.. Rank the concentrations of the following from lowest () to highest
Exam : Cloud Physics April, 8 Physical Meteorology 344 Name Questions - are worth 5 points each. Questions -5 are worth points each.. Rank the concentrations of the following from lowest () to highest
Liquids and Solids. Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display.
 Liquids and Solids Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 1 Gases, Liquids and Solids Gases are compressible fluids. They have no proper volume and proper
Liquids and Solids Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 1 Gases, Liquids and Solids Gases are compressible fluids. They have no proper volume and proper
Lecture Ch. 6. Condensed (Liquid) Water. Cloud in a Jar Demonstration. How does saturation occur? Saturation of Moist Air. Saturation of Moist Air
 Lecture Ch. 6 Saturation of moist air Relationship between humidity and dewpoint Clausius-Clapeyron equation Dewpoint Temperature Depression Isobaric cooling Moist adiabatic ascent of air Equivalent temperature
Lecture Ch. 6 Saturation of moist air Relationship between humidity and dewpoint Clausius-Clapeyron equation Dewpoint Temperature Depression Isobaric cooling Moist adiabatic ascent of air Equivalent temperature
Physics 360 Review 3
 Physics 360 Review 3 The test will be similar to the second test in that calculators will not be allowed and that the Unit #2 material will be divided into three different parts. There will be one problem
Physics 360 Review 3 The test will be similar to the second test in that calculators will not be allowed and that the Unit #2 material will be divided into three different parts. There will be one problem
International Academy Invitational Tournament Keep the Heat Test Team Name. Team Number. Predicted Water Temp C
 International Academy Invitational Tournament Keep the Heat Test 2-4-2012 Team Name Team Number Predicted Water Temp C Circle the all of the correct answer to the below questions. One or more of the answers
International Academy Invitational Tournament Keep the Heat Test 2-4-2012 Team Name Team Number Predicted Water Temp C Circle the all of the correct answer to the below questions. One or more of the answers
Generating cloud drops from CCN. Wallace & Hobbs (1977)
 Generating cloud drops from CCN Wallace & Hobbs (1977) Cloud Drops and Equilibrium Considera3ons: review We discussed how to compute the equilibrium vapor pressure over a pure water drop, or a solu3on
Generating cloud drops from CCN Wallace & Hobbs (1977) Cloud Drops and Equilibrium Considera3ons: review We discussed how to compute the equilibrium vapor pressure over a pure water drop, or a solu3on
Basic Thermodynamics Module 1
 Basic Thermodynamics Module 1 Lecture 9: Thermodynamic Properties of Fluids Thermodynamic Properties of fluids Most useful properties: Properties like pressure, volume and temperature which can be measured
Basic Thermodynamics Module 1 Lecture 9: Thermodynamic Properties of Fluids Thermodynamic Properties of fluids Most useful properties: Properties like pressure, volume and temperature which can be measured
1. Second Law of Thermodynamics
 1. Second Law of hermodynamics he first law describes how the state of a system changes in response to work it performs and heat absorbed. However, the first law cannot explain certain facts about thermal
1. Second Law of hermodynamics he first law describes how the state of a system changes in response to work it performs and heat absorbed. However, the first law cannot explain certain facts about thermal
Melting of ice particles:
 Melting of ice particles: When ice particles fall below 0 C they begin to melt, but the process takes some time since heat transfer needs to occur (heat from ambient environment has to supply the latent
Melting of ice particles: When ice particles fall below 0 C they begin to melt, but the process takes some time since heat transfer needs to occur (heat from ambient environment has to supply the latent
Lecture Presentation. Chapter 11. Liquids and Intermolecular Forces Pearson Education, Inc.
 Lecture Presentation Chapter 11 Liquids and States of Matter The fundamental difference between states of matter is the strength of the intermolecular forces of attraction. Stronger forces bring molecules
Lecture Presentation Chapter 11 Liquids and States of Matter The fundamental difference between states of matter is the strength of the intermolecular forces of attraction. Stronger forces bring molecules
UNIVERSITY OF SOUTHAMPTON
 UNIVERSIY OF SOUHAMPON PHYS1013W1 SEMESER 2 EXAMINAION 2013-2014 Energy and Matter Duration: 120 MINS (2 hours) his paper contains 9 questions. Answers to Section A and Section B must be in separate answer
UNIVERSIY OF SOUHAMPON PHYS1013W1 SEMESER 2 EXAMINAION 2013-2014 Energy and Matter Duration: 120 MINS (2 hours) his paper contains 9 questions. Answers to Section A and Section B must be in separate answer
Lecture Notes 2: Physical Equilibria Phase Diagrams
 Lecture Notes 2: Physical Equilibria Phase Diagrams There are number of graphical means to help to understand the relationships between the different phases of a particular substance. The first thing we
Lecture Notes 2: Physical Equilibria Phase Diagrams There are number of graphical means to help to understand the relationships between the different phases of a particular substance. The first thing we
Properties of Entropy
 Properties of Entropy Due to its additivity, entropy is a homogeneous function of the extensive coordinates of the system: S(λU, λv, λn 1,, λn m ) = λ S (U, V, N 1,, N m ) This means we can write the entropy
Properties of Entropy Due to its additivity, entropy is a homogeneous function of the extensive coordinates of the system: S(λU, λv, λn 1,, λn m ) = λ S (U, V, N 1,, N m ) This means we can write the entropy
Surface chemistry. Liquid-gas, solid-gas and solid-liquid surfaces.
 Surface chemistry. Liquid-gas, solid-gas and solid-liquid surfaces. Levente Novák & István Bányai, University of Debrecen Dept of Colloid and Environmental Chemistry http://kolloid.unideb.hu/~kolloid/
Surface chemistry. Liquid-gas, solid-gas and solid-liquid surfaces. Levente Novák & István Bányai, University of Debrecen Dept of Colloid and Environmental Chemistry http://kolloid.unideb.hu/~kolloid/
Lecture Presentation. Chapter 11. Liquids and Intermolecular Forces. John D. Bookstaver St. Charles Community College Cottleville, MO
 Lecture Presentation Chapter 11 Liquids and Intermolecular Forces John D. Bookstaver St. Charles Community College Cottleville, MO Properties of Gases, Liquids, and Solids State Volume Shape of State Density
Lecture Presentation Chapter 11 Liquids and Intermolecular Forces John D. Bookstaver St. Charles Community College Cottleville, MO Properties of Gases, Liquids, and Solids State Volume Shape of State Density
Simplified Microphysics. condensation evaporation. evaporation
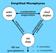 Simplified Microphysics water vapor condensation evaporation cloud droplets evaporation condensation collection rain drops fall out (precipitation) = 0 (reversible) = (irreversible) Simplified Microphysics
Simplified Microphysics water vapor condensation evaporation cloud droplets evaporation condensation collection rain drops fall out (precipitation) = 0 (reversible) = (irreversible) Simplified Microphysics
Lecture 20. Phase Transitions. Phase diagrams. Latent heats. Phase-transition fun. Reading for this Lecture: Elements Ch 13.
 Lecture 20 Phase ransitions Phase diagrams Latent heats Phase-transition fun Reading for this Lecture: Elements Ch 13 Lecture 20, p 1 Solid-gas equilibrium: vapor pressure Consider solid-gas equilibrium
Lecture 20 Phase ransitions Phase diagrams Latent heats Phase-transition fun Reading for this Lecture: Elements Ch 13 Lecture 20, p 1 Solid-gas equilibrium: vapor pressure Consider solid-gas equilibrium
Thermodynamics of phase transitions
 Thermodynamics of phase transitions Katarzyna Sznajd-Weron Department of Theoretical of Physics Wroc law University of Science and Technology, Poland March 12, 2017 Katarzyna Sznajd-Weron (WUST) Thermodynamics
Thermodynamics of phase transitions Katarzyna Sznajd-Weron Department of Theoretical of Physics Wroc law University of Science and Technology, Poland March 12, 2017 Katarzyna Sznajd-Weron (WUST) Thermodynamics
Paper-II Chapter- TS-equation, Maxwell s equation. z = z(x, y) dz = dx + dz = Mdx + Ndy. dy Now. = 2 z
 aper-ii Chapter- S-equation, Maxwell s equation Let heorem: Condition o exact dierential: Where M Hence, z x dz dx and N Q. Derive Maxwell s equations z x z zx, z dx + dz Mdx + Nd z d Now 2 z x M N x x
aper-ii Chapter- S-equation, Maxwell s equation Let heorem: Condition o exact dierential: Where M Hence, z x dz dx and N Q. Derive Maxwell s equations z x z zx, z dx + dz Mdx + Nd z d Now 2 z x M N x x
Chapter 7 PHASE EQUILIBRIUM IN A ONE-COMPONENT SYSTEM
 Chapter 7 PHASE EQUILIBRIUM IN A ONE-COMPONENT SYSTEM 7.1 INTRODUCTION The intensive thermodynamic properties of a system are temperature, pressure, and the chemical potentials of the various species occurring
Chapter 7 PHASE EQUILIBRIUM IN A ONE-COMPONENT SYSTEM 7.1 INTRODUCTION The intensive thermodynamic properties of a system are temperature, pressure, and the chemical potentials of the various species occurring
6 Physical transformations of pure substances
 6 Physical transformations of pure substances E6.b E6.2b E6.3b E6.4b Solutions to exercises Discussion questions Refer to Fig. 6.8. The white lines represent the regions of superheating and supercooling.
6 Physical transformations of pure substances E6.b E6.2b E6.3b E6.4b Solutions to exercises Discussion questions Refer to Fig. 6.8. The white lines represent the regions of superheating and supercooling.
Phases of matter and phase diagrams
 Phases of matter and phase diagrams Transition to Supercritical CO2 Water Ice Vapor Pressure and Boiling Point Liquids boil when the external pressure equals the vapor pressure. Temperature of boiling
Phases of matter and phase diagrams Transition to Supercritical CO2 Water Ice Vapor Pressure and Boiling Point Liquids boil when the external pressure equals the vapor pressure. Temperature of boiling
Chemistry: The Central Science
 Chemistry: The Central Science Fourteenth Edition Chapter 11 Liquids and Intermolecular Forces Intermolecular Forces The attractions between molecules are not nearly as strong as the intramolecular attractions
Chemistry: The Central Science Fourteenth Edition Chapter 11 Liquids and Intermolecular Forces Intermolecular Forces The attractions between molecules are not nearly as strong as the intramolecular attractions
Ch. 9 Liquids and Solids
 Intermolecular Forces I. A note about gases, liquids and gases. A. Gases: very disordered, particles move fast and are far apart. B. Liquid: disordered, particles are close together but can still move.
Intermolecular Forces I. A note about gases, liquids and gases. A. Gases: very disordered, particles move fast and are far apart. B. Liquid: disordered, particles are close together but can still move.
PHASE CHANGE. Freezing Sublimation
 Melting Graphic Organizer Deposition PHASE CHANGE Freezing Sublimation Boiling Evaporation Condensation PHASE CHANGE Phase change happens as the temperature changes. All matter can move from one state
Melting Graphic Organizer Deposition PHASE CHANGE Freezing Sublimation Boiling Evaporation Condensation PHASE CHANGE Phase change happens as the temperature changes. All matter can move from one state
Thermodynamics 1 Lecture Note 2
 Thermodynamics 1 Lecture Note 2 March 20, 2015 Kwang Kim Yonsei University kbkim@yonsei.ac.kr 39 8 7 34 53 Y O N Se I 88.91 16.00 14.01 78.96 126.9 Physical Chemistry Chemistry is the study of Matter and
Thermodynamics 1 Lecture Note 2 March 20, 2015 Kwang Kim Yonsei University kbkim@yonsei.ac.kr 39 8 7 34 53 Y O N Se I 88.91 16.00 14.01 78.96 126.9 Physical Chemistry Chemistry is the study of Matter and
So far in talking about thermodynamics, we ve mostly limited ourselves to
 251 Lecture 33 So far in talking about thermodynamics, we ve mostly limited ourselves to discussions of thermochemistry, a quantification of the heat absorbed or given off as the result of a chemical reaction.
251 Lecture 33 So far in talking about thermodynamics, we ve mostly limited ourselves to discussions of thermochemistry, a quantification of the heat absorbed or given off as the result of a chemical reaction.
Phase Equilibria in a One-Component System I
 5.60 spring 2005 Lecture #17 page 1 Phase Equilibria in a One-Component System I Goal: Understand the general phenomenology of phase transitions and phase coexistence conditions for a single component
5.60 spring 2005 Lecture #17 page 1 Phase Equilibria in a One-Component System I Goal: Understand the general phenomenology of phase transitions and phase coexistence conditions for a single component
4) It is a state function because enthalpy(h), entropy(s) and temperature (T) are state functions.
 Chemical Thermodynamics S.Y.BSc. Concept of Gibb s free energy and Helmholtz free energy a) Gibb s free energy: 1) It was introduced by J.Willard Gibb s to account for the work of expansion due to volume
Chemical Thermodynamics S.Y.BSc. Concept of Gibb s free energy and Helmholtz free energy a) Gibb s free energy: 1) It was introduced by J.Willard Gibb s to account for the work of expansion due to volume
Lecture 4: The First Law of Thermodynamics
 Lecture 4: The First Law of Thermodynamics Latent Heat Last lecture, we saw that adding heat to an object does not always change the temperature of the object In some cases, the heat causes a phase change
Lecture 4: The First Law of Thermodynamics Latent Heat Last lecture, we saw that adding heat to an object does not always change the temperature of the object In some cases, the heat causes a phase change
1. Water Vapor in Air
 1. Water Vapor in Air Water appears in all three phases in the earth s atmosphere - solid, liquid and vapor - and it is one of the most important components, not only because it is essential to life, but
1. Water Vapor in Air Water appears in all three phases in the earth s atmosphere - solid, liquid and vapor - and it is one of the most important components, not only because it is essential to life, but
States of matter Part 2
 Physical Pharmacy Lecture 2 States of matter Part 2 Assistant Lecturer in Pharmaceutics Overview The Liquid State General properties Liquefaction of gases Vapor pressure of liquids Boiling point The Solid
Physical Pharmacy Lecture 2 States of matter Part 2 Assistant Lecturer in Pharmaceutics Overview The Liquid State General properties Liquefaction of gases Vapor pressure of liquids Boiling point The Solid
More Thermodynamics. Specific Specific Heats of a Gas Equipartition of Energy Reversible and Irreversible Processes
 More Thermodynamics Specific Specific Heats of a Gas Equipartition of Energy Reversible and Irreversible Processes Carnot Cycle Efficiency of Engines Entropy More Thermodynamics 1 Specific Heat of Gases
More Thermodynamics Specific Specific Heats of a Gas Equipartition of Energy Reversible and Irreversible Processes Carnot Cycle Efficiency of Engines Entropy More Thermodynamics 1 Specific Heat of Gases
UNIT 15: THERMODYNAMICS
 UNIT 15: THERMODYNAMICS ENTHALPY, DH ENTROPY, DS GIBBS FREE ENERGY, DG ENTHALPY, DH Energy Changes in Reactions Heat is the transfer of thermal energy between two bodies that are at different temperatures.
UNIT 15: THERMODYNAMICS ENTHALPY, DH ENTROPY, DS GIBBS FREE ENERGY, DG ENTHALPY, DH Energy Changes in Reactions Heat is the transfer of thermal energy between two bodies that are at different temperatures.
Outline. Aim. Gas law. Pressure. Scale height Mixing Column density. Temperature Lapse rate Stability. Condensation Humidity.
 Institute of Applied Physics University of Bern Outline A planetary atmosphere consists of different gases hold to the planet by gravity The laws of thermodynamics hold structure as vertical coordinate
Institute of Applied Physics University of Bern Outline A planetary atmosphere consists of different gases hold to the planet by gravity The laws of thermodynamics hold structure as vertical coordinate
Introduction. Statistical physics: microscopic foundation of thermodynamics degrees of freedom 2 3 state variables!
 Introduction Thermodynamics: phenomenological description of equilibrium bulk properties of matter in terms of only a few state variables and thermodynamical laws. Statistical physics: microscopic foundation
Introduction Thermodynamics: phenomenological description of equilibrium bulk properties of matter in terms of only a few state variables and thermodynamical laws. Statistical physics: microscopic foundation
Today s Lecture: Atmosphere finish primitive equations, mostly thermodynamics
 Today s Lecture: Atmosphere finish primitive equations, mostly thermodynamics Reference Peixoto and Oort, Sec. 3.1, 3.2, 3.4, 3.5 (but skip the discussion of oceans until next week); Ch. 10 Thermodynamic
Today s Lecture: Atmosphere finish primitive equations, mostly thermodynamics Reference Peixoto and Oort, Sec. 3.1, 3.2, 3.4, 3.5 (but skip the discussion of oceans until next week); Ch. 10 Thermodynamic
