Sensitivity of cloud condensation nuclei activation processes to kinetic parameters
|
|
|
- Piers Bradley
- 6 years ago
- Views:
Transcription
1 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 111,, doi: /2005jd006529, 2006 Sensitivity of cloud condensation nuclei activation processes to kinetic parameters P. Y. Chuang 1 Received 25 July 2005; revised 5 November 2005; accepted 4 January 2006; published 5 May [1] The activation of cloud condensation nuclei to form cloud drops can be a kinetically limited process under some circumstances. Here the sensitivity of particle activation to parameters that are related to kinetic processes, in particular condensational growth and ambient supersaturation, is examined. Specifically, the parameters studied are the thermal and mass accommodation coefficients, the rate of solute dissolution for a particle, and the updraft velocity. Results are based on the equation for condensational growth and simulations using adiabatic parcel models. We conclude that activation is not very sensitive to the thermal accommodation coefficient but is sensitive to the mass accommodation coefficient below some critical value of 0.1 to Dissolution rates (defined for 100 nm dry particles) in the range of 10 min to 1 day can significantly affect activation. Sensitivity to updraft velocity uncertainties can be significant and are likely to be more important for polluted rather than clean conditions. Citation: Chuang, P. Y. (2006), Sensitivity of cloud condensation nuclei activation processes to kinetic parameters, J. Geophys. Res., 111,, doi: /2005jd Introduction [2] Clouds are an important component of the atmosphere, representing a key uncertainty in our understanding of both weather and climate. The activation of cloud condensation nuclei (CCN) to form cloud droplets is one of the first steps in cloud formation, and therefore is one of the processes that determines the microphysical properties and evolution of a cloud. CCN activation is not always well described by equilibrium theory [Chuang et al., 1997; Nenes et al., 2001]. Model calculations have shown that changes in droplet growth rate can potentially lead to significant changes in cloud properties, in particular cloud effective radius and albedo [Feingold and Chuang, 2002]. [3] Here, the sensitivity of activation-related processes to a variety of kinetic parameters, i.e., parameters that are related to droplet growth in a cloud updraft, is studied. Specifically, we focus on: (1) mass and thermal accommodation coefficients, which are relevant to the rate of condensational growth; (2) the timescale of dissolution of solutes, which leads to a time dependence in the equilibrium size of a droplet; and (3) the updraft velocity, an important parameter for determining cloud drop number concentration and cloud supersaturation, both of which are coupled to the total sink of water vapor by droplets growing by condensation. [4] We define sensitivity of some process P(x) to an independent variable x as: Sensitivity ¼ d ~P d~x ¼ dp=p ð 0Þ x0 dx=x ð 0 Þ ¼ dðln PÞ dðln xþ ¼ x 0 dp x0 P 0 dx 1 Department of Earth Sciences, University of California, Santa Cruz, Santa Cruz, California, USA. Copyright 2006 by the American Geophysical Union /06/2005JD006529$09.00 ð1þ where ~P and ~x are dimensionless, referenced to values P 0 and x 0. According to this definition, a sensitivity of 1 means that a 10% change (or uncertainty) in x leads to a 10% change (or uncertainty) in P(x). A sensitivity of 0.2 means that a 10% change in x leads to only a 2% change in P(x), whereas a sensitivity of 5 means that a 10% change in x leads to a 50% change in P(x). 2. Sensitivity of the Condensational Growth Rate [5] We first examine the sensitivity of condensational growth to two parameters, the mass (a c ) and thermal (a T ) accommodation coefficients. Condensational growth rate is given by the equation [Fukuta and Walter, 1970]: a da dt r w RT 1 p sat ðt 1 ÞD þ Lr w v Mw S 1 S eq ð2þ LM w ka T1 RT 1 1 where some of the multiplicative terms have been removed for clarity. See Appendix A for all nomenclature. The numerator represents the driving force for condensation, i.e., the difference between the partial pressure of water far from the droplet (S 1 ) and that at equilibrium with the droplet surface (S eq ). We will examine sensitivity of activation to maximum S 1 in section 4. The first term in the denominator represents growth due to diffusion of water vapor and the second term in the denominator represents the change in mass transfer due to latent heat release from condensation, with both terms corrected for noncontinuum effects. We rewrite the growth equation as: a da dt K S 1 S eq ð3þ 1of7
2 Figure 1. Sensitivity of condensational growth rate to the thermal accommodation coefficient for droplets of different diameters as shown. Both axes are dimensionless. where we will now call K the mass transfer coefficient which then has the form r K ¼ w RT 1 p sat ðt 1 ÞD v M þ Lr w LM 1 w w ka T 1 ð4þ 1 RT 1 The modified mass diffusivity is in turn given by [Fukuta and Walter, 1970]: D v D v * ¼ 1=2 ð5þ a aþd þ Dv aa c 2pM w RT a does not become significant relative to the other (diffusion) term except for these small values of a T. Physically, the interpretation is that the latent heat released does not substantially change the surface temperature of the drop. The lack of sensitivity of growth rates to a T has been understood in the community for a long time; they are presented here partly to show how small a T must be to be significant, but primarily for completeness and to serve as a contrast with the results for a c. The results in Figure 1 are weakly sensitive to the choice of T and a c used during this calculation, but the main conclusions remain the same. [7] Figure 2 shows the sensitivity of K to a c. Here, we see that depending on droplet size, the sensitivity asymptotes at a value of 1 given sufficiently small a c, with values on the order of 10 1 to 10 3 depending on droplet size. In contrast with a T, however, laboratory measurements of a c show that these values can be as low as 10 5 in the presence of organic films on the surface of the droplets [Rubel and Gentry, 1984; Seaver et al., 1992], and evidence that such particles may exist in the atmosphere has been presented [Chuang, 2003]. See Barnes [1986] and Chuang [2003] for more comprehensive reviews of this subject. Note that not all studies seeking to observe such effects yielded positive results [e.g., Cruz and Pandis, 1998]. These results show that the uncertainty in droplet growth rate is equal to the uncertainty in a c (i.e., sensitivity = 1) if a c is below 10 1 to 10 3, depending on droplet size. To what extent such values of a c exist for real CCN is not known, but these results show that it is important that future work addresses this issue. 3. Sensitivity to Dissolution Rate [8] The activation of cloud droplets for simple salts can be described by classic Köhler theory [e.g., Pruppacher and Klett, 1997]: where now the mass accommodation coefficient, a c, is explicitly written. Similarly, the modified thermal diffusivity is given by [Fukuta and Walter, 1970]: k a k a * ¼ 1=2 ð6þ a aþd þ ka aa T r a c pa 2pM a RT 1 ln S ¼ 4M ws w RTr w D p 6n sm w pr w D 3 p ¼ A D p B D 3 p ð7þ where the thermal accommodation coefficient, a T, is explicitly written. [6] We show here the calculated sensitivities of the mass transfer coefficient K to the two accommodation coefficients a T and a c based on equations (2) to (6). Figure 1 shows the results for a T. The sensitivity is a function of droplet size a, but in all cases it is clear that the condensational growth of droplets 0.3 mm in radius and above exhibit very little sensitivity to a T down to a T 10 2, and only grow above 0.1 for a T values 10 3 and smaller. It is typically argued that a T is close to unity [Rogers and Yau, 1989], an assertion that has some experimental support [Shaw and Lamb, 1999]. These results show that it is not important to know accurately the value of a T in order to predict accurately the condensational growth rate because the sensitivity of K to a T is very small in this range of a T. The explanation of this result is that the heat transfer-related term in equation (2) (i.e., the second term in the denominator) Figure 2. Sensitivity of condensational growth rate to the mass accommodation coefficient for droplets of different diameters as shown. Both axes are dimensionless. 2of7
3 From this equation, the critical supersaturation S c of a particle can be shown to be: ln S c ¼ 1=2 4A3 ð8þ 27B Normally, it is assumed that the number of moles in solution, n s, is a constant, which implies that B is constant. In this section, we explore what happens if dissolution is not instantaneous, but instead is associated with some finite timescale. [9] It has been hypothesized that the dissolution of some solutes can require long timescales despite the fact that complete dissolution is the thermodynamically favorable (i.e., equilibrium) state [Hegg et al., 2001; Shantz et al., 2003]. If the solute in a growing droplet is unable to dissolve sufficiently rapidly to maintain a saturated solution (i.e., is kinetically limited from maintaining equilibrium), then this decreases the amount of solute present n s, which increases the particle critical supersaturation S c and can therefore prevent the particle from activating even if an equilibrium calculation says it would. Here, we explore the parameter space for dissolution rates in order to study what range of dissolution timescales can cause appreciable changes in activation. We note that because this is an exploratory study, we seek an order-of-magnitude result for the range of dissolution timescales that are significant for this problem. Also, it is typically believed that a larger fraction of particles are present in their deliquesced state (either stable or metastable) rather than their crystallized state. Dissolution timescales are not relevant to the activation of such particles because they have no solid phase. [10] We first define dissolution timescale in a very simple manner: t sol is the time required for a dry 100 nm solute particle to fully dissolve. We also assume that the rate limiting step for dissolution is a solid-phase surface process, which implies that the rate of dissolution is proportional to surface area and independent of the solute concentration in solution. Given these assumptions, the rate of dissolution of a solid solute particle can be expressed as: dv sol dt ¼ ka sol Assuming a spherical solute inclusion, it is easy to show that equation (9) can be expressed as: dd sol dt ¼ k ð9þ ð10þ where k has absorbed more factors but remains a constant. Therefore equation (10) shows that the decrease in particle diameter due to dissolution proceeds at a constant rate, and thus the rate constant for dissolution, k, is inversely proportional to t sol, which we have defined as the time for a dry 100 nm particle to dissolve. We will express dissolution rate using t sol since it is more physically intuitive. [11] To study the effect of dissolution rate, we use results from an adiabatic 1-D cloud parcel model (model adapted from Feingold and Heymsfield [1992]). Two different cases Figure 3. Time profile of relative humidity for two conditions, clean and polluted, used for calculating relevant dissolution timescales t sol. For times less than 400 s, the RH increases linearly with time, with initial condition of RH = 90% at t =0s. (which we will term clean and polluted ) are used to calculate the relative humidity as a function of time in a rising air parcel, as shown in Figure 3. The clean case has fewer particles and therefore a higher maximum supersaturation. We then consider whether or not a single particle in this environment will activate as a function of the particle dry size (expressed as particle equilibrium critical supersaturation, S c ), and t sol for that particle. We assume that the model solute has similar properties (molecular mass, density) to NaCl, but the results here do not depend significantly on the solute properties chosen. [12] A simple model was used to determine whether or not a particular particle activated. Particles are initially assumed to be at equilibrium at a RH of 90%. Using the desired time profile of RH (either clean or polluted), the particle solute content n s in the next time step is determined by the dissolution rate t sol, with the constraint that it cannot exceed the equilibrium n s at that RH. The calculation proceeds until (1) activation is achieved, defined as the ambient supersaturation S exceeding the particle supersaturation, or (2) the simulated cloud updraft exceeds 270 s after peak supersaturation. In principle, (1) is not a perfect criterion for activation. It is possible that a particle that satisfies (1) at some moment could fail that same criterion a short period of time later, thus de-activating. This could occur if a particle activates according to (1) when ambient S is decreasing. Once activated, the particle grows very quickly by condensation, and this dilution reduces the particle equilibrium S. However, if the ambient S decreases even more quickly, this would lead to net evaporation and eventually de-activation (such behavior was observed in simulations by Nenes et al. [2001]). For realistic adiabatic 3of7
4 velocity, aerosol number and size distribution), and many more calculations could be performed to better map out the parameter space. However, the purpose here is to get some order-of-magnitude estimate of the kinetic dissolution timescales that may be relevant to the activation of real aerosol under cloud conditions, and perhaps guide future experiments. These results suggest that the minimum t sol that is relevant is on the order of 10 min, while values of 1 day would lead to significant changes in activation behavior over a range of particle size. Intuitively, these values do not seem out of the realm of possibility, but whether or not atmospheric aerosol contains solutes that exhibit such timescales remains unknown. These results serve as a useful guide for future experiments. Figure 4. Grid showing those combinations of dissolution timescale t sol and particle equilibrium critical supersaturation (S c ) that are kinetically inhibited from activating. Results are for clean aerosol conditions (see Figure 3). situations, however, this seems unlikely, though updrafts with significant entrainment to dilute ambient S might see such an effect. Also, the criterion chosen in (2) is somewhat arbitrary, but will not significantly affect the model predictions. It should also be noted that these simulations represent a single particle in this updraft environment, surrounded by other particles that do not exhibit dissolution kinetics. If, instead, all particles exhibited some finite t sol, the total water uptake would decrease at any given time, and therefore S max for that updraft would increase, rather than being a constant as assumed here. [13] Figure 4 shows the results for the clean case, which exhibits a maximum ambient supersaturation, S max, of 0.39%. The shortest t sol where any inhibition of activation is observed is s(1 hr), and this occurs for particles whose S c is 0.33%, which is very close to S max and therefore require only a small decrease in solute concentration to be unable to activate. The discrepancy between S max of 0.39% and S c of 0.33% is due to the limited resolution of the calculations. The range of particles that are inhibited from activating because of dissolution kinetics increases as t sol increases, with particles in the range of S c from 0.18% to 0.39% inhibited for the largest t sol considered here, 10 5 s (1 day). This represents a range of dry diameters from 60 to 90 nm. [14] Figure 5 shows the results for the polluted case, which has S max = 0.19%. In general, the lower supersaturation environment leads to a broader range of particles that are kinetically inhibited from activating. The lowest value of t sol that prevents activation is 10 3 s(15 min). At the largest t sol of 10 5 s, particles with S c between 0.09% and 0.19% do not activate, corresponding to particles in the diameter size range of 180 to 280 nm. [15] Clearly, these results depend on the choice of parameters used for these specific calculations (e.g., updraft 4. Sensitivity to Uncertainties in Updraft Velocity (w) [16] Cloud droplet closure experiments compare measured cloud droplet number (CDN) concentrations with that predicted from measured aerosol size distribution and chemical composition along with measured cloud updraft velocity (w). The adiabatic CDN cannot be predicted using an equilibrium model since it depends on the balance of two time-dependent processes: the time evolution of supersaturation, which depends primarily on w and temperature; and the rate of water vapor uptake by condensation onto existing particles. [17] Accurately measuring w from aircraft is a nontrivial exercise. A typical estimated w uncertainty is 0.1 m/s [Khelif et al., 1999; Kalogiros and Wang, 2002]. In this section, we study the sensitivity of S max and CDN to the uncertainty in measured w using an adiabatic parcel model (we refer the reader to Lance et al. [2004] and Rissman et Figure 5. Grid showing those combinations of dissolution timescale t sol and particle equilibrium critical supersaturation (S c ) that are kinetically inhibited from activating. Results are for polluted aerosol conditions (see Figure 3). 4of7
5 Figure 6. Aerosol size distributions [Whitby, 1978] used for calculating sensitivities to updraft velocity (results shown in Figures 7 and 8). al. [2004] for alternate approaches to this topic). The simulations are performed using three different aerosol size distributions, which are the model marine, clean continental, and urban distributions proposed by Whitby [1978]. Each aerosol is assumed to be composed only of ammonium sulfate. The aerosol size distributions are plotted in Figure 6. A range of w values between 0.1 to 10 m/s are modeled. Activation of CCN to form cloud droplets is determined kinetically, but under these conditions, any deviation from the predicted equilibrium CDN is very small. [18] Cloud parcel model predictions of S max are calculated for a given updraft velocity and aerosol size distribution, as shown in Figure 7. The slope of the curve of log S max versus log w is a measure of the sensitivity of S max on w (see equation (1)). Figure 7 shows that such a curve fits the model results very well (R 2 > 0.99) for all three aerosol cases. The modeled sensitivity of S max to w is 0.56, 0.59, and 0.69 for the marine, continental and urban aerosol over the range of w studied. These values are fairly consistent with each other despite the large differences in the aerosol size distributions, suggesting that the sensitivity of S max to w is not a strong function of aerosol type. A careful study in order to understand exactly why these cases differ is not presented here because, again, the goal is to estimate the sensitivities, and therefore deviations on the order of 10 to 20% are not significant. [19] These sensitivity results suggest that the uncertainty in predicting S max can be very significant under weak updraft conditions, such as stratiform clouds, where mean w can be in the range of 0.1 to 0.3 m/s. The uncertainty in measured w of 0.1 m/s would then represent a w uncertainty of 100% to 33%, which translates into uncertainties in S max of 60% to 20%, assuming a sensitivity of 0.6. Understanding the microphysical properties of stratiform clouds is climatically important because their albedo is sensitive to the presence of anthropogenic aerosol [Charlson et al., 1992]. For more strongly convective clouds, where w is 1 m/s or larger, these results predict that the uncertainty in measured Figure 7. Maximum supersaturation as a function of updraft velocity for different aerosol size distributions. The slope of the curve represents the sensitivity of S max to w and is given in parentheses for each aerosol type. w will lead to uncertainties in S max of less than 6% in regions that are well represented by a single updraft. [20] The sensitivity of CDN to the uncertainty in w is also studied. The results are presented as the fraction of particles that form cloud drops (rather than absolute cloud drop concentrations), as shown in Figure 8. The average sensitivity of cloud drop fraction for the marine, continental and Figure 8. Fraction of particles that activate to form cloud drops as a function of updraft velocity for different aerosol size distributions. The slope of the curve represents the sensitivity of the cloud drop fraction to w and is given in parentheses for each aerosol type. 5of7
6 urban aerosol cases are 0.39, 0.34 and 1.0 based on the best fit curves in Figure 8. The much larger sensitivity for the urban case is a striking result. The likely explanation for this is that S max is much smaller in the urban case (Figure 7), and this means that the minimum particle size for activation is much larger (since the aerosol is assumed to be internally mixed). For the urban case, the slope of the aerosol size distribution is fairly steep in the size range of 0.05 to 0.5 um. As w increases, S max increases, and therefore the minimum size for activation decreases, but because of this steep slope, the number of particles which can activate increases very quickly, leading to a large change in CDN, and therefore in cloud drop fraction. For the other two aerosol cases, the values of S max are larger, which means that the minimum size is smaller, and the slope of the aerosol size distribution at these sizes is generally flat or sloping in the opposite direction, leading to a much smaller sensitivity. For the continental case, the fitted curve does not seem to be the best representation of the model results, as there is a clear concavity to the results, i.e., sensitivity is higher for low w than high w. The reason this occurs is likely the same explanation as for the increased sensitivity in the urban case discussed above. [21] Given these variations, the general conclusion is that CDN is reasonably sensitive to w (sensitivity 0.3 to 0.4) for the cleaner aerosol cases, with increased sensitivity (1) for the polluted case. These results are consistent with Feingold [2003], who showed that drop effective radius exhibits stronger sensitivity to w for higher aerosol extinctions. The exact value of the sensitivity appears to depend quite strongly on the shape of the aerosol size distribution, which is in contrast to the sensitivity results for S max, which appear to be fairly insensitive to the aerosol size distribution. 5. Summary [22] A number of processes related to cloud droplet activation that involve time dependence are studied. The focus is on estimating a reasonable value for the sensitivity of these processes to parameters that are not well characterized for real atmospheric aerosols. Because many of the parameters are not well constrained, a much more detailed analysis of the sensitivity is not attempted here. The main conclusions of this work are: [23] 1. Droplet growth rate is not very sensitive to the thermal accommodation coefficient for the range of values that are believed to be realistic. Improved constraints of a T are therefore not of any significant value for understanding droplet activation. [24] 2. Droplet growth rate is sensitive (sensitivity 1) to the mass accommodation coefficient below some critical value whose range is from 10 1 to Whether these values are exhibited for atmospheric aerosols remains an open question. [25] 3. If solute dissolution exhibits timescales on the order of 10 min to 1 day, then this can significantly affect whether or not a nondeliquesced particle composed of this solute will activate. [26] 4. The sensitivity of maximum parcel supersaturation to uncertainties in measured updraft velocity w are 0.6 to 0.7, and appears not to be strongly dependent on the aerosol selected. [27] 5. The sensitivity of the fraction of particles that activate to w is in the range of 0.3 to 1, depending on the aerosol size distribution chosen. Notation A Köhler equation term A sol surface area of solute a droplet radius B Köhler equation term CDN cloud droplet number c pa heat capacity of air at constant pressure D p particle diameter D sol solid solute particle diameter D v water vapor diffusivity k dissolution rate constant K mass transfer coefficient for condensational growth k a thermal diffusivity L latent heat of evaporation M w molecular weight of water n s moles of solute P some process that depends on independent variable x p sat saturation water vapor pressure r particle radius R ideal gas law constant S supersaturation S c critical supersaturation for a particle S max maximum supersaturation achieved in a rising adiabatic air parcel T temperature t time t sol time constant for dissolution V sol volume of solute w updraft velocity x some independent variable D flux correction length scale a c mass accommodation coefficient a T thermal accommodation coefficient r a density of air r w density of liquid water s w surface tension of water Subscripts 1 infinity, a distance far away from the droplet a air eq at equilibrium with the droplet surface sat at saturation sol solute v vapor w water Superscripts * modified diffusivities denotes a normalized quantity [28] Acknowledgments. This work was partly funded by NASA under grant NNG04GE16G. The author also thanks G. Feingold (NOAA ETL) and A. Nenes (Georgia Tech) for their generous contributions of model output and useful discussion, B. Ervens (Colorado State Univ./ NOAA), and one anonymous reviewer for useful comments. References Barnes, G. T. (1986), The effects of monolayers on the evaporation of liquids, Adv. Colloid Interface Sci., 25, of7
7 Charlson, R. J., S. E. Schwartz, J. M. Hales, R. D. Cess, J. A. Coakley, J. E. Hansen, and D. J. Hofmann (1992), Climate forcing by anthropogenic aerosols, Science, 255, Chuang, P. Y. (2003), Measurement of the timescale of hygroscopic growth for atmospheric aerosols, J. Geophys. Res., 108(D9), 4282, doi: / 2002JD Chuang, P. Y., R. J. Charlson, and J. H. Seinfeld (1997), Kinetic limitations on droplet formation in clouds, Nature, 390, Cruz, C. N., and S. N. Pandis (1998), The effect of organic coatings on the cloud condensation nuclei activation of inorganic atmospheric aerosol, J. Geophys. Res., 103, 13,111 13,123. Feingold, G. (2003), Modeling of the first indirect effect: Analysis of measurement requirements, Geophys. Res. Lett., 30(19), 1997, doi: / 2003GL Feingold, G., and P. Y. Chuang (2002), Analysis of the influence of filmforming compounds on droplet growth: Implications for cloud microphysical processes and climate, J. Atmos. Sci., 59, Feingold, G., and A. J. Heymsfield (1992), Parameterizations of condensational growth of droplets for use in general circulation models, J. Atmos. Sci., 49, Fukuta, N., and L. A. Walter (1970), Kinetics of hydrometeor growth from a vapor-spherical model, J. Atmos. Sci., 27, Hegg, D. A., S. Gao, W. Hoppel, G. Frick, P. Caffrey, W. R. Leaitch, N. Shantz, J. Ambrusko, and T. Albrechcinski (2001), Laboratory studies of the efficiency of selected organic aerosols as CCN, Atmos. Res., 58, Kalogiros, J. A., and Q. Wang (2002), Calibration of a radome-differential GPS system on a Twin Otter research aircraft for turbulence measurements, J. Atmos. Oceanic Technol., 19, Khelif, D., S. P. Burns, and C. A. Friehe (1999), Improved wind measurements on research aircraft, J. Atmos. Oceanic Technol., 16, Lance, S., A. Nenes, and T. A. Rissman (2004), Chemical and dynamical effects on cloud droplet number: Implications for estimates of the aerosol indirect effect, J. Geophys. Res., 109, D22208, doi: / 2004JD Nenes, A., S. Ghan, H. Abdul-Razzak, P. Y. Chuang, and J. H. Seinfeld (2001), Kinetic limitations on cloud droplet formation and impact on cloud albedo, Tellus, Ser. B, 53, Pruppacher, H. R., and J. D. Klett (1997), Microphysics of Clouds and Precipitation, D. Reidel, Norwell, Mass. Rissman, T. A., A. Nenes, and J. H. Seinfeld (2004), Chemical amplification (or dampening) of the Twomey effect: Conditions derived from droplet activation theory, J. Atmos. Sci., 61, Rogers, R. R., and M. K. Yau (1989), A Short Course in Cloud Physics, 3rd ed., Butterworth-Heinemann, Woburn, Mass. Rubel, G. O., and J. W. Gentry (1984), Measurement of the kinetics of solution droplets in the presence of adsorbed monolayers: Determination of water accommodation coefficients, J. Phys. Chem., 88, Seaver, M., J. R. Peele, T. J. Manuccia, G. O. Rubel, and G. Ritchie (1992), Evaporation kinetics of ventilated waterdrops coated with octadecanol monolayers, J. Phys. Chem., 96, Shantz, N. C., W. R. Leaitch, and P. F. Caffrey (2003), Effect of organics of low solubility on the growth rate of cloud droplets, J. Geophys. Res., 108(D5), 4168, doi: /2002jd Shaw, R. A., and D. Lamb (1999), Experimental determination of the thermal accommodation and condensation coefficients of water, J. Chem. Phys., 111, 10,659 10,663. Whitby, K. T. (1978), Physical characteristics of sulfur aerosols, Atmos. Environ., 12, P. Y. Chuang, Department of Earth Sciences, University of California, Santa Cruz, 1156 High Street, Santa Cruz, CA 95064, USA. (pchuang@ es.ucsc.edu) 7of7
Parameterization of the nitric acid effect on CCN activation
 Atmos. Chem. Phys., 5, 879 885, 25 SRef-ID: 168-7324/acp/25-5-879 European Geosciences Union Atmospheric Chemistry and Physics Parameterization of the nitric acid effect on CCN activation S. Romakkaniemi,
Atmos. Chem. Phys., 5, 879 885, 25 SRef-ID: 168-7324/acp/25-5-879 European Geosciences Union Atmospheric Chemistry and Physics Parameterization of the nitric acid effect on CCN activation S. Romakkaniemi,
Updated H 2 SO 4 -H 2 O binary homogeneous nucleation look-up tables
 Click Here for Full Article JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 113,, doi:10.1029/2008jd010527, 2008 Updated H 2 SO 4 -H 2 O binary homogeneous nucleation look-up tables Fangqun Yu 1 Received 2 June
Click Here for Full Article JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 113,, doi:10.1029/2008jd010527, 2008 Updated H 2 SO 4 -H 2 O binary homogeneous nucleation look-up tables Fangqun Yu 1 Received 2 June
Chemical and Dynamical Effects on Cloud Droplet Number: Implications for Estimates of the Aerosol Indirect Effect
 Chemical and Dynamical Effects on Cloud Droplet Number: Implications for Estimates of the Aerosol Indirect Effect SARA LANCE 1, A. NENES 1,2*, T. RISSMAN 3 1 School of Earth and Atmospheric Sciences and
Chemical and Dynamical Effects on Cloud Droplet Number: Implications for Estimates of the Aerosol Indirect Effect SARA LANCE 1, A. NENES 1,2*, T. RISSMAN 3 1 School of Earth and Atmospheric Sciences and
Kinetic Limitations on Cloud Droplet Formation and Impact on Cloud Albedo
 1 Appeared in Tellus B, 53, 133 149, 2001. Kinetic Limitations on Cloud Droplet Formation and Impact on Cloud Albedo Athanasios Nenes Department of Chemical Engineering,Mail Code 210-41, California Institute
1 Appeared in Tellus B, 53, 133 149, 2001. Kinetic Limitations on Cloud Droplet Formation and Impact on Cloud Albedo Athanasios Nenes Department of Chemical Engineering,Mail Code 210-41, California Institute
Black carbon radiative heating effects on cloud microphysics and implications for the aerosol indirect effect 2. Cloud microphysics
 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 107, NO. D21, 4605, doi:10.1029/2002jd002101, 2002 Black carbon radiative heating effects on cloud microphysics and implications for the aerosol indirect effect 2.
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 107, NO. D21, 4605, doi:10.1029/2002jd002101, 2002 Black carbon radiative heating effects on cloud microphysics and implications for the aerosol indirect effect 2.
Generating cloud drops from CCN. Wallace & Hobbs (1977)
 Generating cloud drops from CCN Wallace & Hobbs (1977) Cloud Drops and Equilibrium Considera3ons: review We discussed how to compute the equilibrium vapor pressure over a pure water drop, or a solu3on
Generating cloud drops from CCN Wallace & Hobbs (1977) Cloud Drops and Equilibrium Considera3ons: review We discussed how to compute the equilibrium vapor pressure over a pure water drop, or a solu3on
Parameterization of cloud droplet formation in global climate models
 1 Parameterization of cloud droplet formation in global climate models ATHANASIOS NENES 1 and JOHN H. SEINFELD Department of Chemical Engineering, Mail Code 210-41, California Institute of Technology,
1 Parameterization of cloud droplet formation in global climate models ATHANASIOS NENES 1 and JOHN H. SEINFELD Department of Chemical Engineering, Mail Code 210-41, California Institute of Technology,
An investigation into the aerosol dispersion effect through the activation process in marine stratus clouds
 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 112,, doi:10.1029/2006jd007401, 2007 An investigation into the aerosol dispersion effect through the activation process in marine stratus clouds Yiran Peng, 1 Ulrike
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 112,, doi:10.1029/2006jd007401, 2007 An investigation into the aerosol dispersion effect through the activation process in marine stratus clouds Yiran Peng, 1 Ulrike
Importance of vertical velocity variations in the cloud droplet nucleation process of marine stratus clouds
 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 110,, doi:10.1029/2004jd004922, 2005 Importance of vertical velocity variations in the cloud droplet nucleation process of marine stratus clouds Yiran Peng, 1,2 Ulrike
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 110,, doi:10.1029/2004jd004922, 2005 Importance of vertical velocity variations in the cloud droplet nucleation process of marine stratus clouds Yiran Peng, 1,2 Ulrike
Black carbon radiative heating effects on cloud microphysics and implications for the aerosol indirect effect: 2. Cloud microphysics
 Black carbon radiative heating effects on cloud microphysics and implications for the aerosol indirect effect: 2. Cloud microphysics Athanasios Nenes, William C. Conant and John H. Seinfeld Departments
Black carbon radiative heating effects on cloud microphysics and implications for the aerosol indirect effect: 2. Cloud microphysics Athanasios Nenes, William C. Conant and John H. Seinfeld Departments
Influence of Organic-Containing Aerosols on Marine Boundary Layer Processes
 DISTRIBUTION STATEMENT A. Approved for public release; distribution is unlimited. Influence of Organic-Containing Aerosols on Marine Boundary Layer Processes John H. Seinfeld California Institute of Technology,
DISTRIBUTION STATEMENT A. Approved for public release; distribution is unlimited. Influence of Organic-Containing Aerosols on Marine Boundary Layer Processes John H. Seinfeld California Institute of Technology,
Evaluation of a new Cloud Droplet Activation Parameterization with In Situ Data from CRYSTAL-FACE and CSTRIPE
 Evaluation of a new Cloud Droplet Activation Parameterization with In Situ Data from CRYSTAL-FACE and CSTRIPE Nicholas Meskhidze 1, Athanasios Nenes 1,2*, William C. Conant 3, John H. Seinfeld 3,4 1 School
Evaluation of a new Cloud Droplet Activation Parameterization with In Situ Data from CRYSTAL-FACE and CSTRIPE Nicholas Meskhidze 1, Athanasios Nenes 1,2*, William C. Conant 3, John H. Seinfeld 3,4 1 School
An investigation into the aerosol dispersion effect through the activation process in marine stratus clouds
 1 An investigation into the aerosol dispersion effect through the activation process in marine stratus clouds Yiran Peng, 1, Ulrike Lohmann, 2 Richard Leaitch 3 and Markku Kulmala 4 Short title: 1 Max
1 An investigation into the aerosol dispersion effect through the activation process in marine stratus clouds Yiran Peng, 1, Ulrike Lohmann, 2 Richard Leaitch 3 and Markku Kulmala 4 Short title: 1 Max
Droplet Nucleation: Physically-Based Parameterizations and Comparative
 Droplet Nucleation: Physically-Based Parameterizations and Comparative Evaluation 1 1 1 1 1 1 1 1 0 1 0 1 Steven J. Ghan 1, Hayder Abdul-Razzak, Athanasios Nenes, Yi Ming, Xiaohong Liu 1, Mikhail Ovchinnikov
Droplet Nucleation: Physically-Based Parameterizations and Comparative Evaluation 1 1 1 1 1 1 1 1 0 1 0 1 Steven J. Ghan 1, Hayder Abdul-Razzak, Athanasios Nenes, Yi Ming, Xiaohong Liu 1, Mikhail Ovchinnikov
Black carbon radiative heating effects on cloud microphysics and implications for the aerosol indirect effect 1. Extended Köhler theory
 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 107, NO. D21, 4604, doi:10.1029/2002jd002094, 2002 Black carbon radiative heating effects on cloud microphysics and implications for the aerosol indirect effect 1.
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 107, NO. D21, 4604, doi:10.1029/2002jd002094, 2002 Black carbon radiative heating effects on cloud microphysics and implications for the aerosol indirect effect 1.
Hygroscopic growth of ammonium sulfate/dicarboxylic acids
 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 108, NO. D20, 4638, doi:10.1029/2003jd003775, 2003 Hygroscopic growth of ammonium sulfate/dicarboxylic acids Matthew E. Wise, Jason D. Surratt, Daniel B. Curtis, John
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 108, NO. D20, 4638, doi:10.1029/2003jd003775, 2003 Hygroscopic growth of ammonium sulfate/dicarboxylic acids Matthew E. Wise, Jason D. Surratt, Daniel B. Curtis, John
Warm Rain Precipitation Processes
 Warm Rain Precipitation Processes Cloud and Precipitation Systems November 16, 2005 Jonathan Wolfe 1. Introduction Warm and cold precipitation formation processes are fundamentally different in a variety
Warm Rain Precipitation Processes Cloud and Precipitation Systems November 16, 2005 Jonathan Wolfe 1. Introduction Warm and cold precipitation formation processes are fundamentally different in a variety
Cloud Condensation Nuclei Hygroscopic Parameter Kappa
 Cloud Condensation Nuclei Hygroscopic Parameter Kappa Covers Reading Material in Chapter 17.5 Atmospheric Sciences 5200 Physical Meteorology III: Cloud Physics Cloud Condensation Nuclei (CCN) Form a cloud
Cloud Condensation Nuclei Hygroscopic Parameter Kappa Covers Reading Material in Chapter 17.5 Atmospheric Sciences 5200 Physical Meteorology III: Cloud Physics Cloud Condensation Nuclei (CCN) Form a cloud
A cloud microphysics model including trace gas condensation and sulfate chemistry
 BOREAL ENVIRONMENT RESEARCH 8: 43 424 ISSN 239-695 Helsinki December 23 23 A cloud microphysics model including trace gas condensation and sulfate chemistry Harri Kokkola ), Sami Romakkaniemi ), Markku
BOREAL ENVIRONMENT RESEARCH 8: 43 424 ISSN 239-695 Helsinki December 23 23 A cloud microphysics model including trace gas condensation and sulfate chemistry Harri Kokkola ), Sami Romakkaniemi ), Markku
Modeling of cloud microphysics: from simple concepts to sophisticated parameterizations. Part I: warm-rain microphysics
 Modeling of cloud microphysics: from simple concepts to sophisticated parameterizations. Part I: warm-rain microphysics Wojciech Grabowski National Center for Atmospheric Research, Boulder, Colorado parameterization
Modeling of cloud microphysics: from simple concepts to sophisticated parameterizations. Part I: warm-rain microphysics Wojciech Grabowski National Center for Atmospheric Research, Boulder, Colorado parameterization
Humidity impact on the aerosol effect in warm cumulus clouds
 GEOPHYSICAL RESEARCH LETTERS, VOL. 35, L17804, doi:10.1029/2008gl034178, 2008 Humidity impact on the aerosol effect in warm cumulus clouds O. Altaratz, 1 I. Koren, 1 and T. Reisin 2 Received 31 March 2008;
GEOPHYSICAL RESEARCH LETTERS, VOL. 35, L17804, doi:10.1029/2008gl034178, 2008 Humidity impact on the aerosol effect in warm cumulus clouds O. Altaratz, 1 I. Koren, 1 and T. Reisin 2 Received 31 March 2008;
Step 1. Step 2. g l = g v. dg = 0 We have shown that over a plane surface of water. g v g l = ρ v R v T ln e/e sat. this can be rewritten
 The basic question is what makes the existence of a droplet thermodynamically preferable to the existence only of water vapor. We have already derived an expression for the saturation vapor pressure over
The basic question is what makes the existence of a droplet thermodynamically preferable to the existence only of water vapor. We have already derived an expression for the saturation vapor pressure over
Aerosol Effects on Water and Ice Clouds
 Aerosol Effects on Water and Ice Clouds Ulrike Lohmann Department of Physics and Atmospheric Science, Dalhousie University, Halifax, N. S., Canada Contributions from Johann Feichter, Johannes Hendricks,
Aerosol Effects on Water and Ice Clouds Ulrike Lohmann Department of Physics and Atmospheric Science, Dalhousie University, Halifax, N. S., Canada Contributions from Johann Feichter, Johannes Hendricks,
APPLICATION OF KOHLER THEORY: MODELING CLOUD CONDENSATION NUCLEI ACTIVITY
 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 APPLICATION OF KOHLER THEORY: MODELING CLOUD CONDENSATION NUCLEI ACTIVITY Gavin Cornwell, Katherine Nadler, Alex Nguyen, and Steven Schill Department of
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 APPLICATION OF KOHLER THEORY: MODELING CLOUD CONDENSATION NUCLEI ACTIVITY Gavin Cornwell, Katherine Nadler, Alex Nguyen, and Steven Schill Department of
Consistent estimates from satellites and models for the first aerosol indirect forcing
 GEOPHYSICAL RESEARCH LETTERS, VOL. 39,, doi:10.1029/2012gl051870, 2012 Consistent estimates from satellites and models for the first aerosol indirect forcing Joyce E. Penner, 1 Cheng Zhou, 1 and Li Xu
GEOPHYSICAL RESEARCH LETTERS, VOL. 39,, doi:10.1029/2012gl051870, 2012 Consistent estimates from satellites and models for the first aerosol indirect forcing Joyce E. Penner, 1 Cheng Zhou, 1 and Li Xu
The impact of microphysical parameters, ice nucleation mode, and habit growth on the ice/liquid partitioning in mixed phase Arctic clouds
 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 116,, doi:10.1029/2011jd015729, 2011 The impact of microphysical parameters, ice nucleation mode, and habit growth on the ice/liquid partitioning in mixed phase Arctic
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 116,, doi:10.1029/2011jd015729, 2011 The impact of microphysical parameters, ice nucleation mode, and habit growth on the ice/liquid partitioning in mixed phase Arctic
Clouds associated with cold and warm fronts. Whiteman (2000)
 Clouds associated with cold and warm fronts Whiteman (2000) Dalton s law of partial pressures! The total pressure exerted by a mixture of gases equals the sum of the partial pressure of the gases! Partial
Clouds associated with cold and warm fronts Whiteman (2000) Dalton s law of partial pressures! The total pressure exerted by a mixture of gases equals the sum of the partial pressure of the gases! Partial
CHAPTER 8. AEROSOLS 8.1 SOURCES AND SINKS OF AEROSOLS
 1 CHAPTER 8 AEROSOLS Aerosols in the atmosphere have several important environmental effects They are a respiratory health hazard at the high concentrations found in urban environments They scatter and
1 CHAPTER 8 AEROSOLS Aerosols in the atmosphere have several important environmental effects They are a respiratory health hazard at the high concentrations found in urban environments They scatter and
Exam 2: Cloud Physics April 16, 2008 Physical Meteorology Questions 1-10 are worth 5 points each. Questions are worth 10 points each.
 Exam : Cloud Physics April, 8 Physical Meteorology 344 Name Questions - are worth 5 points each. Questions -5 are worth points each.. Rank the concentrations of the following from lowest () to highest
Exam : Cloud Physics April, 8 Physical Meteorology 344 Name Questions - are worth 5 points each. Questions -5 are worth points each.. Rank the concentrations of the following from lowest () to highest
Surface Tension Kelvin Effect
 Lecture Ch. 5a Surface tension (Kelvin effect) Hygroscopic growth (subsaturated humidity) Saturation Chemical potential (Raoult effect) (partly from 4.5.1) Curry and Webster, Ch. 5 (skip 5.6, 5.7); also
Lecture Ch. 5a Surface tension (Kelvin effect) Hygroscopic growth (subsaturated humidity) Saturation Chemical potential (Raoult effect) (partly from 4.5.1) Curry and Webster, Ch. 5 (skip 5.6, 5.7); also
Aerosol--cloud drop concentration closure in warm cumulus
 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 109,, doi:10.1029/2003jd004324, 2004 Aerosol--cloud drop concentration closure in warm cumulus W. C. Conant, 1 T. M. VanReken, 2 T. A. Rissman, 2 V. Varutbangkul,
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 109,, doi:10.1029/2003jd004324, 2004 Aerosol--cloud drop concentration closure in warm cumulus W. C. Conant, 1 T. M. VanReken, 2 T. A. Rissman, 2 V. Varutbangkul,
An adsorption model of insoluble particle activation: Application to black carbon
 Click Here for Full Article JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 112,, doi:10.1029/2007jd008549, 2007 An adsorption model of insoluble particle activation: Application to black carbon B. F. Henson 1 Received
Click Here for Full Article JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 112,, doi:10.1029/2007jd008549, 2007 An adsorption model of insoluble particle activation: Application to black carbon B. F. Henson 1 Received
Investigating the simulation of cloud microphysical processes in numerical models using a one-dimensional dynamical framework
 ATMOSPHERIC SCIENCE LETTERS Atmos. Sci. Let. 10: 207 21 (2009) Published online in Wiley InterScience (www.interscience.wiley.com).239 Investigating the simulation of cloud microphysical processes in numerical
ATMOSPHERIC SCIENCE LETTERS Atmos. Sci. Let. 10: 207 21 (2009) Published online in Wiley InterScience (www.interscience.wiley.com).239 Investigating the simulation of cloud microphysical processes in numerical
Lecture 7: quick review
 Lecture 7: quick review We discussed the meaning of the critical r* and ΔG*: since it s unstable, if we can form a drop with r > r*, the system will keep going falling down the energy curve, trying to
Lecture 7: quick review We discussed the meaning of the critical r* and ΔG*: since it s unstable, if we can form a drop with r > r*, the system will keep going falling down the energy curve, trying to
Aerosol Basics: Definitions, size distributions, structure
 Aerosol Basics: Definitions, size distributions, structure Antti Lauri NetFAM Summer School Zelenogorsk, 9 July 2008 Department of Physics, Division of Atmospheric Sciences and Geophysics, University of
Aerosol Basics: Definitions, size distributions, structure Antti Lauri NetFAM Summer School Zelenogorsk, 9 July 2008 Department of Physics, Division of Atmospheric Sciences and Geophysics, University of
Implications of Sulfate Aerosols on Clouds, Precipitation and Hydrological Cycle
 Implications of Sulfate Aerosols on Clouds, Precipitation and Hydrological Cycle Source: Sulfate aerosols are produced by chemical reactions in the atmosphere from gaseous precursors (with the exception
Implications of Sulfate Aerosols on Clouds, Precipitation and Hydrological Cycle Source: Sulfate aerosols are produced by chemical reactions in the atmosphere from gaseous precursors (with the exception
Use of In Situ Data to Test a Raman Lidar Based Cloud Condensation Nuclei Remote Sensing Method
 FEBRUARY 2004 NOTES AND CORRESPONDENCE 387 Use of In Situ Data to Test a Raman Lidar Based Cloud Condensation Nuclei Remote Sensing Method STEVEN J. GHAN Pacific Northwest National Laboratory, Richland,
FEBRUARY 2004 NOTES AND CORRESPONDENCE 387 Use of In Situ Data to Test a Raman Lidar Based Cloud Condensation Nuclei Remote Sensing Method STEVEN J. GHAN Pacific Northwest National Laboratory, Richland,
Commentary on cloud modelling and the mass accommodation coefficient of water
 Commentary on cloud modelling and the mass accommodation coefficient of water A. Laaksonen, T. Vesala, M. Kulmala, P. M. Winkler, P. E. Wagner To cite this version: A. Laaksonen, T. Vesala, M. Kulmala,
Commentary on cloud modelling and the mass accommodation coefficient of water A. Laaksonen, T. Vesala, M. Kulmala, P. M. Winkler, P. E. Wagner To cite this version: A. Laaksonen, T. Vesala, M. Kulmala,
Ammonium Bisulfate/Water Equilibrium and Metastability Phase Diagrams
 J. Phys. Chem. A 1997, 101, 4191-4195 4191 Ammonium Bisulfate/Water Equilibrium and Metastability Phase Diagrams Dan G. Imre,*, Jun Xu, I. N. Tang, and R. McGraw EnVironmental Chemistry DiVision, Department
J. Phys. Chem. A 1997, 101, 4191-4195 4191 Ammonium Bisulfate/Water Equilibrium and Metastability Phase Diagrams Dan G. Imre,*, Jun Xu, I. N. Tang, and R. McGraw EnVironmental Chemistry DiVision, Department
9 Condensation. Learning Goals. After studying this chapter, students should be able to:
 9 Condensation Learning Goals After studying this chapter, students should be able to: 1. explain the microphysical processes that operate in clouds to influence the formation and growth of cloud droplets
9 Condensation Learning Goals After studying this chapter, students should be able to: 1. explain the microphysical processes that operate in clouds to influence the formation and growth of cloud droplets
Chasing Aerosol Particles Down to Nano Sizes
 Chasing Aerosol Particles Down to Nano Sizes ERC Seminar 13 June 2013 Armin Sorooshian Chemical & Environmental Engineering Atmospheric Sciences University of Arizona Outline of Talk 1. What are aerosol
Chasing Aerosol Particles Down to Nano Sizes ERC Seminar 13 June 2013 Armin Sorooshian Chemical & Environmental Engineering Atmospheric Sciences University of Arizona Outline of Talk 1. What are aerosol
Aerosol-cloud drop concentration closure in warm cumulus
 Aerosol-cloud drop concentration closure in warm cumulus W.C. Conant 1, T.M. VanReken 1, T.A. Rissman 1, V. Varutbangkul 1, H.H. Jonsson 2, A. Nenes 3, J.L. Jimenez 4, A.E. Delia 4, R. Bahreini 1, G.C.
Aerosol-cloud drop concentration closure in warm cumulus W.C. Conant 1, T.M. VanReken 1, T.A. Rissman 1, V. Varutbangkul 1, H.H. Jonsson 2, A. Nenes 3, J.L. Jimenez 4, A.E. Delia 4, R. Bahreini 1, G.C.
Supplement of Multiphase oxidation of SO 2 by NO 2 on CaCO 3 particles
 Supplement of Atmos. Chem. Phys., 18, 2481 2493, 2018 https://doi.org/10.5194/acp-18-2481-2018-supplement Author(s) 2018. This work is distributed under the Creative Commons Attribution 4.0 License. Supplement
Supplement of Atmos. Chem. Phys., 18, 2481 2493, 2018 https://doi.org/10.5194/acp-18-2481-2018-supplement Author(s) 2018. This work is distributed under the Creative Commons Attribution 4.0 License. Supplement
THE RELATIONSHIP BETWEEN CLOUD DROPLET AND AEROSOL NUMBER CONCENTRATIONS FOR CLIMATE MODELS
 INTERNATIONAL JOURNAL OF CLIMATOLOGY, VOL. 16, 94 1-946 (1 996) 551.521.1 l(4) THE RELATIONSHIP BETWEEN CLOUD DROPLET AND AEROSOL NUMBER CONCENTRATIONS FOR CLIMATE MODELS 1. GULTEPE and G. A. ISAAC Cloud
INTERNATIONAL JOURNAL OF CLIMATOLOGY, VOL. 16, 94 1-946 (1 996) 551.521.1 l(4) THE RELATIONSHIP BETWEEN CLOUD DROPLET AND AEROSOL NUMBER CONCENTRATIONS FOR CLIMATE MODELS 1. GULTEPE and G. A. ISAAC Cloud
Aerosol-Cloud-Radiation Interactions in Atmospheric Forecast Models
 Aerosol-Cloud-Radiation Interactions in Atmospheric Forecast Models John H. Seinfeld, Principal Investigator California Institute of Technology 1200 E. California Blvd., M/C 210-41 Pasadena, CA 91125 (626)
Aerosol-Cloud-Radiation Interactions in Atmospheric Forecast Models John H. Seinfeld, Principal Investigator California Institute of Technology 1200 E. California Blvd., M/C 210-41 Pasadena, CA 91125 (626)
Aerosols influence on the interplay between condensation, evaporation and rain in warm cumulus cloud
 Atmos. Chem. Phys., 8, 15 24, 2008 Author(s) 2008. This work is licensed under a Creative Commons License. Atmospheric Chemistry and Physics Aerosols influence on the interplay between condensation, evaporation
Atmos. Chem. Phys., 8, 15 24, 2008 Author(s) 2008. This work is licensed under a Creative Commons License. Atmospheric Chemistry and Physics Aerosols influence on the interplay between condensation, evaporation
Response: Changes in the manuscript: Response
 We would like to thank the referee for providing valuable comments on our manuscript and we have carefully addressed the referee s comments point-by-point as follows (referee s comments in black and our
We would like to thank the referee for providing valuable comments on our manuscript and we have carefully addressed the referee s comments point-by-point as follows (referee s comments in black and our
Aerosol Dynamics. Antti Lauri NetFAM Summer School Zelenogorsk, 9 July 2008
 Aerosol Dynamics Antti Lauri NetFAM Summer School Zelenogorsk, 9 July 2008 Department of Physics, Division of Atmospheric Sciences and Geophysics, University of Helsinki Aerosol Dynamics: What? A way to
Aerosol Dynamics Antti Lauri NetFAM Summer School Zelenogorsk, 9 July 2008 Department of Physics, Division of Atmospheric Sciences and Geophysics, University of Helsinki Aerosol Dynamics: What? A way to
Influence of Organic-Containing Aerosols on Marine Boundary Layer Processes
 DISTRIBUTION STATEMENT A. Approved for public release; distribution is unlimited. Influence of Organic-Containing Aerosols on Marine Boundary Layer Processes John H. Seinfeld California Institute of Technology
DISTRIBUTION STATEMENT A. Approved for public release; distribution is unlimited. Influence of Organic-Containing Aerosols on Marine Boundary Layer Processes John H. Seinfeld California Institute of Technology
Radiative influences on ice crystal and droplet growth within mixed-phase stratus clouds
 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 113,, doi:10.1029/2007jd009262, 2008 Radiative influences on ice crystal and droplet growth within mixed-phase stratus clouds Z. J. Lebo, 1,2 N. C. Johnson, 1 and
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 113,, doi:10.1029/2007jd009262, 2008 Radiative influences on ice crystal and droplet growth within mixed-phase stratus clouds Z. J. Lebo, 1,2 N. C. Johnson, 1 and
Precipitation Processes
 Precipitation Processes Dave Rahn Precipitation formation processes may be classified into two categories. These are cold and warm processes, where cold processes can only occur below 0 C and warm processes
Precipitation Processes Dave Rahn Precipitation formation processes may be classified into two categories. These are cold and warm processes, where cold processes can only occur below 0 C and warm processes
Modification of aerosol mass and size distribution due to aqueous-phase SO 2 oxidation in clouds: Comparisons of several models
 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 108, NO. D7, 4213, doi:10.1029/2002jd002697, 2003 Modification of aerosol mass and size distribution due to aqueous-phase SO 2 oxidation in clouds: Comparisons of
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 108, NO. D7, 4213, doi:10.1029/2002jd002697, 2003 Modification of aerosol mass and size distribution due to aqueous-phase SO 2 oxidation in clouds: Comparisons of
Parameterization of cloud droplet size distributions: Comparison with parcel models and observations
 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 114, D1125, doi:1.129/28jd11387, 29 Parameterization of cloud droplet size distributions: Comparison with parcel models and observations W. C. Hsieh, 1 A. Nenes, 1,2
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 114, D1125, doi:1.129/28jd11387, 29 Parameterization of cloud droplet size distributions: Comparison with parcel models and observations W. C. Hsieh, 1 A. Nenes, 1,2
The Importance of Adsorption for CCN Activity and Hygroscopic Properties of Mineral Dust Aerosol
 1 2 The Importance of Adsorption for CCN Activity and Hygroscopic Properties of Mineral Dust Aerosol 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 Prashant Kumar 1, Athanasios Nenes 1,2, and
1 2 The Importance of Adsorption for CCN Activity and Hygroscopic Properties of Mineral Dust Aerosol 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 Prashant Kumar 1, Athanasios Nenes 1,2, and
Physio-chemical and Optical Characterization of Anthropogenic and Natural Aerosol: Implications for Assessing Global Effects
 Physio-chemical and Optical Characterization of Anthropogenic and Natural Aerosol: Implications for Assessing Global Effects GLOBE Pollution Southern Japan TRACE-P, 2001 Dust Antony Clarke, University
Physio-chemical and Optical Characterization of Anthropogenic and Natural Aerosol: Implications for Assessing Global Effects GLOBE Pollution Southern Japan TRACE-P, 2001 Dust Antony Clarke, University
2σ e s (r,t) = e s (T)exp( rr v ρ l T ) = exp( ) 2σ R v ρ l Tln(e/e s (T)) e s (f H2 O,r,T) = f H2 O
 Formulas/Constants, Physics/Oceanography 4510/5510 B Atmospheric Physics II N A = 6.02 10 23 molecules/mole (Avogadro s number) 1 mb = 100 Pa 1 Pa = 1 N/m 2 Γ d = 9.8 o C/km (dry adiabatic lapse rate)
Formulas/Constants, Physics/Oceanography 4510/5510 B Atmospheric Physics II N A = 6.02 10 23 molecules/mole (Avogadro s number) 1 mb = 100 Pa 1 Pa = 1 N/m 2 Γ d = 9.8 o C/km (dry adiabatic lapse rate)
Clouds, Haze, and Climate Change
 Clouds, Haze, and Climate Change Jim Coakley College of Oceanic and Atmospheric Sciences Earth s Energy Budget and Global Temperature Incident Sunlight 340 Wm -2 Reflected Sunlight 100 Wm -2 Emitted Terrestrial
Clouds, Haze, and Climate Change Jim Coakley College of Oceanic and Atmospheric Sciences Earth s Energy Budget and Global Temperature Incident Sunlight 340 Wm -2 Reflected Sunlight 100 Wm -2 Emitted Terrestrial
Warm Cloud Processes. Some definitions. Two ways to make big drops: Effects of cloud condensation nuclei
 Warm Cloud Processes Dr. Christopher M. Godfrey University of North Carolina at Asheville Warm clouds lie completely below the 0 isotherm 0 o C Some definitions Liquid water content (LWC) Amount of liquid
Warm Cloud Processes Dr. Christopher M. Godfrey University of North Carolina at Asheville Warm clouds lie completely below the 0 isotherm 0 o C Some definitions Liquid water content (LWC) Amount of liquid
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 109, D20207, doi: /2004jd004666, 2004
 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 109,, doi:10.1029/2004jd004666, 2004 Effects of film-forming compounds on the growth of giant cloud condensation nuclei: Implications for cloud microphysics and the
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 109,, doi:10.1029/2004jd004666, 2004 Effects of film-forming compounds on the growth of giant cloud condensation nuclei: Implications for cloud microphysics and the
Mixing and Turbulence
 Mixing and Turbulence November 3, 2012 This section introduces some elementary concepts associated with mixing and turbulence in the environment. 1 Conserved Variables Studies of mixing of different airmasses
Mixing and Turbulence November 3, 2012 This section introduces some elementary concepts associated with mixing and turbulence in the environment. 1 Conserved Variables Studies of mixing of different airmasses
Chapter 7 Precipitation Processes
 Chapter 7 Precipitation Processes Chapter overview: Supersaturation and water availability Nucleation of liquid droplets and ice crystals Liquid droplet and ice growth by diffusion Collision and collection
Chapter 7 Precipitation Processes Chapter overview: Supersaturation and water availability Nucleation of liquid droplets and ice crystals Liquid droplet and ice growth by diffusion Collision and collection
Phase Transformations of the Ternary System (NH 4 ) 2 SO 4 - H 2 SO 4 -H 2 O and the Implications for Cirrus Cloud Formation
 Phase Transformations of the Ternary System (NH 4 ) 2 SO 4 - H 2 SO 4 -H 2 O and the Implications for Cirrus Cloud Formation Scot T. Martin Department of Environmental Sciences and Engineering, The University
Phase Transformations of the Ternary System (NH 4 ) 2 SO 4 - H 2 SO 4 -H 2 O and the Implications for Cirrus Cloud Formation Scot T. Martin Department of Environmental Sciences and Engineering, The University
Köhler Curve. Covers Reading Material in Chapter 10.3 Atmospheric Sciences 5200 Physical Meteorology III: Cloud Physics
 Köhler Curve Covers Reading Material in Chapter 10.3 Atmospheric Sciences 5200 Physical Meteorology III: Cloud Physics Review of Kelvin Effect Gibbs Energy for formation of a drop G = G &'()*+, G ).'+
Köhler Curve Covers Reading Material in Chapter 10.3 Atmospheric Sciences 5200 Physical Meteorology III: Cloud Physics Review of Kelvin Effect Gibbs Energy for formation of a drop G = G &'()*+, G ).'+
Physical Processes & Issues
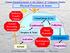 Physical Processes & Issues Radiative Transfer Climate VIS IR Cloud Drops & Ice Aerosol Processing Air quality Condensation Droplets & Xtals Cloud Dynamics Collection Aerosol Activation Hydrological Cycle
Physical Processes & Issues Radiative Transfer Climate VIS IR Cloud Drops & Ice Aerosol Processing Air quality Condensation Droplets & Xtals Cloud Dynamics Collection Aerosol Activation Hydrological Cycle
Parameterization of cloud droplet activation using a simplified treatment of the aerosol number size distribution
 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 113,, doi:10.1029/2007jd009485, 2008 Parameterization of cloud droplet activation using a simplified treatment of the aerosol number size distribution Niku Kivekäs,
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 113,, doi:10.1029/2007jd009485, 2008 Parameterization of cloud droplet activation using a simplified treatment of the aerosol number size distribution Niku Kivekäs,
Surfactant properties of atmospheric and model humic-like substances (HULIS)
 Click Here for Full Article GEOPHYSICAL RESEARCH LETTERS, VOL. 34, L16807, doi:10.1029/2007gl029576, 2007 Surfactant properties of atmospheric and model humic-like substances (HULIS) Ilya Taraniuk, 1 Ellen
Click Here for Full Article GEOPHYSICAL RESEARCH LETTERS, VOL. 34, L16807, doi:10.1029/2007gl029576, 2007 Surfactant properties of atmospheric and model humic-like substances (HULIS) Ilya Taraniuk, 1 Ellen
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 112, D10S30, doi: /2006jd007272, 2007
 Click Here for Full Article JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 112,, doi:10.1029/2006jd007272, 2007 Aerosol cloud drop concentration closure for clouds sampled during the International Consortium for
Click Here for Full Article JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 112,, doi:10.1029/2006jd007272, 2007 Aerosol cloud drop concentration closure for clouds sampled during the International Consortium for
Introduction. Effect of aerosols on precipitation: - challenging problem - no agreement between the results (quantitative and qualitative)
 Introduction Atmospheric aerosols affect the cloud mycrophysical structure & formation (observations, numerical studies) An increase of the aerosol particles: - increases CCN concentrations - decreases
Introduction Atmospheric aerosols affect the cloud mycrophysical structure & formation (observations, numerical studies) An increase of the aerosol particles: - increases CCN concentrations - decreases
Urban background aerosols: Negative correlations of particle modes and fragmentation mechanism
 Click Here for Full Article GEOPHYSICAL RESEARCH LETTERS, VOL. 34, L11811, doi:10.1029/2006gl029109, 2007 Urban background aerosols: Negative correlations of particle modes and fragmentation mechanism
Click Here for Full Article GEOPHYSICAL RESEARCH LETTERS, VOL. 34, L11811, doi:10.1029/2006gl029109, 2007 Urban background aerosols: Negative correlations of particle modes and fragmentation mechanism
Role of molecular size in cloud droplet activation
 GEOPHYSICAL RESEARCH LETTERS, VOL. 36, L22801, doi:10.1029/2009gl040131, 2009 Role of molecular size in cloud droplet activation M. D. Petters, 1,2 S. M. Kreidenweis, 1 A. J. Prenni, 1 R. C. Sullivan,
GEOPHYSICAL RESEARCH LETTERS, VOL. 36, L22801, doi:10.1029/2009gl040131, 2009 Role of molecular size in cloud droplet activation M. D. Petters, 1,2 S. M. Kreidenweis, 1 A. J. Prenni, 1 R. C. Sullivan,
Satellite-based estimate of global aerosol-cloud radiative forcing by marine warm clouds
 SUPPLEMENTARY INFORMATION DOI: 10.1038/NGEO2214 Satellite-based estimate of global aerosol-cloud radiative forcing by marine warm clouds Y.-C. Chen, M. W. Christensen, G. L. Stephens, and J. H. Seinfeld
SUPPLEMENTARY INFORMATION DOI: 10.1038/NGEO2214 Satellite-based estimate of global aerosol-cloud radiative forcing by marine warm clouds Y.-C. Chen, M. W. Christensen, G. L. Stephens, and J. H. Seinfeld
The role of surfactants in Köhler theory reconsidered
 Atmos. Chem. Phys., 4, 2107 2117, 2004 SRef-ID: 1680-7324/acp/2004-4-2107 Atmospheric Chemistry and Physics The role of surfactants in Köhler theory reconsidered R. Sorjamaa 1, B. Svenningsson 2, T. Raatikainen
Atmos. Chem. Phys., 4, 2107 2117, 2004 SRef-ID: 1680-7324/acp/2004-4-2107 Atmospheric Chemistry and Physics The role of surfactants in Köhler theory reconsidered R. Sorjamaa 1, B. Svenningsson 2, T. Raatikainen
Modelling Cloud Droplet Formation
 Modelling Cloud Droplet Formation Rosalind West, August 2008 Transfer of status report Supervised by Dr. Philip Stier, Dr. Don Grainger and Dr. Andy Jones 1 2 Contents Contents 1 Introduction.........................................
Modelling Cloud Droplet Formation Rosalind West, August 2008 Transfer of status report Supervised by Dr. Philip Stier, Dr. Don Grainger and Dr. Andy Jones 1 2 Contents Contents 1 Introduction.........................................
P = x i. P i. = y i. Aerosol and Aqueous Chemistry. Raoult s Law. Raoult s Law vs. Henry s Law. or C i. = HC i. = k H
 The Great Smog Aerosol and Aqueous Chemistry Equilibrium Partitioning Oxidation and Oxidants Other Surface-driven Fogs in London were a common occurrence, but the events that began on the 5th of December
The Great Smog Aerosol and Aqueous Chemistry Equilibrium Partitioning Oxidation and Oxidants Other Surface-driven Fogs in London were a common occurrence, but the events that began on the 5th of December
How Important Is the Spectral Ripening Effect in Stratiform Boundary Layer Clouds? Studies Using Simple Trajectory Analysis
 2681 How Important Is the Spectral Ripening Effect in Stratiform Boundary Layer Clouds? Studies Using Simple Trajectory Analysis R. WOOD Met Office, Bracknell, Berkshire, United Kingdom S. IRONS AND P.
2681 How Important Is the Spectral Ripening Effect in Stratiform Boundary Layer Clouds? Studies Using Simple Trajectory Analysis R. WOOD Met Office, Bracknell, Berkshire, United Kingdom S. IRONS AND P.
A REVIEW OF OUR UNDERSTANDING OF THE AEROSOL CLOUD INTERACTION FROM THE PERSPECTIVE OF A BIN RESOLVED CLOUD SCALE MODELLING
 JP3.4 A REVIEW OF OUR UNDERSTANDING OF THE AEROSOL CLOUD INTERACTION FROM THE PERSPECTIVE OF A BIN RESOLVED CLOUD SCALE MODELLING Andrea I. Flossmann and W. Wobrock Clermont University, Aubière, France
JP3.4 A REVIEW OF OUR UNDERSTANDING OF THE AEROSOL CLOUD INTERACTION FROM THE PERSPECTIVE OF A BIN RESOLVED CLOUD SCALE MODELLING Andrea I. Flossmann and W. Wobrock Clermont University, Aubière, France
Cloud Droplet Growth by Condensation and Aggregation EPM Stratocumulus and Arctic Stratocumulus
 Cloud Droplet Growth by Condensation and Aggregation EPM Stratocumulus and Arctic Stratocumulus US Department of Energy, ARM http://www.arm.gov/science/highlights/rntm3/view Typical EPMS Characteristics
Cloud Droplet Growth by Condensation and Aggregation EPM Stratocumulus and Arctic Stratocumulus US Department of Energy, ARM http://www.arm.gov/science/highlights/rntm3/view Typical EPMS Characteristics
Title of file for HTML: Supplementary Information Description: Supplementary Figures, Supplementary Tables and Supplementary References
 Title of file for HTML: Supplementary Information Description: Supplementary Figures, Supplementary Tables and Supplementary References Title of file for HTML: Peer Review File Description: g e (RH) (bulk)
Title of file for HTML: Supplementary Information Description: Supplementary Figures, Supplementary Tables and Supplementary References Title of file for HTML: Peer Review File Description: g e (RH) (bulk)
MT3230 Supplemental Data 4.2 Spring, 2018 Dr. Sam Miller COMPUTING THE LATENT HEAT OF VAPORIZATION OF WATER AS A FUNCTION OF TEMPERATURE.
 MT3230 Supplemental Data 4.2 Spring, 2018 Dr. Sam Miller COMPUTING THE LATENT HEAT OF VAPORIZATION OF WATER AS A FUNCTION OF TEMPERATURE Abstract The latent heat of vaporization parameterizes the amount
MT3230 Supplemental Data 4.2 Spring, 2018 Dr. Sam Miller COMPUTING THE LATENT HEAT OF VAPORIZATION OF WATER AS A FUNCTION OF TEMPERATURE Abstract The latent heat of vaporization parameterizes the amount
Lecture 7: The Monash Simple Climate
 Climate of the Ocean Lecture 7: The Monash Simple Climate Model Dr. Claudia Frauen Leibniz Institute for Baltic Sea Research Warnemünde (IOW) claudia.frauen@io-warnemuende.de Outline: Motivation The GREB
Climate of the Ocean Lecture 7: The Monash Simple Climate Model Dr. Claudia Frauen Leibniz Institute for Baltic Sea Research Warnemünde (IOW) claudia.frauen@io-warnemuende.de Outline: Motivation The GREB
References: Cloud Formation. ESCI Cloud Physics and Precipitation Processes Lesson 1 - Cloud Types and Properties Dr.
 ESCI 34 - Cloud Physics and Precipitation Processes Lesson 1 - Cloud Types and Properties Dr. DeCaria References: Glossary of Meteorology, 2nd ed., American Meteorological Society A Short Course in Cloud
ESCI 34 - Cloud Physics and Precipitation Processes Lesson 1 - Cloud Types and Properties Dr. DeCaria References: Glossary of Meteorology, 2nd ed., American Meteorological Society A Short Course in Cloud
The Atmospheric Chemistry and Physics of Ammonia
 The Atmospheric Chemistry and Physics of Ammonia Russell Dickerson Dept. Meteorology, The University of Maryland Presented at the National Atmospheric Deposition Program Ammonia Workshop October 23, 2003
The Atmospheric Chemistry and Physics of Ammonia Russell Dickerson Dept. Meteorology, The University of Maryland Presented at the National Atmospheric Deposition Program Ammonia Workshop October 23, 2003
Characteristic updrafts for computing distribution averaged cloud droplet number and stratocumulus cloud properties
 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 115,, doi:1.129/29jd13233, 21 Characteristic updrafts for computing distribution averaged cloud droplet number and stratocumulus cloud properties R. Morales 1 and
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 115,, doi:1.129/29jd13233, 21 Characteristic updrafts for computing distribution averaged cloud droplet number and stratocumulus cloud properties R. Morales 1 and
Water activity and activation diameters from hygroscopicity data - Part II: Application to organic species
 Water activity and activation diameters from hygroscopicity data - Part II: Application to organic species K. A. Koehler, S. M. Kreidenweis, P. J. Demott, A. J. Prenni, C. M. Carrico, B. Ervens, G. Feingold
Water activity and activation diameters from hygroscopicity data - Part II: Application to organic species K. A. Koehler, S. M. Kreidenweis, P. J. Demott, A. J. Prenni, C. M. Carrico, B. Ervens, G. Feingold
CCN activation experiments with adipic acid: effect of particle phase and adipic acid coatings on soluble and insoluble particles
 Atmos. Chem. Phys., 8, 3735 3748, 2008 Author(s) 2008. This work is distributed under the Creative Commons Attribution 3.0 License. Atmospheric Chemistry and Physics CCN activation experiments with adipic
Atmos. Chem. Phys., 8, 3735 3748, 2008 Author(s) 2008. This work is distributed under the Creative Commons Attribution 3.0 License. Atmospheric Chemistry and Physics CCN activation experiments with adipic
Melting of ice particles:
 Melting of ice particles: When ice particles fall below 0 C they begin to melt, but the process takes some time since heat transfer needs to occur (heat from ambient environment has to supply the latent
Melting of ice particles: When ice particles fall below 0 C they begin to melt, but the process takes some time since heat transfer needs to occur (heat from ambient environment has to supply the latent
Solutions to questions from chapter 8 in GEF Cloud Physics
 Solutions to questions from chapter 8 in GEF4310 - Cloud Physics i.h.h.karset@geo.uio.no Problem 1 a) What is expressed by the equation below? Answer: The left side is the time rate of change of the mass
Solutions to questions from chapter 8 in GEF4310 - Cloud Physics i.h.h.karset@geo.uio.no Problem 1 a) What is expressed by the equation below? Answer: The left side is the time rate of change of the mass
Slides partly by Antti Lauri and Hannele Korhonen. Liquid or solid particles suspended in a carrier gas Described by their
 Atmospheric Aerosols Slides partly by Antti Lauri and Hannele Korhonen Aerosol particles Liquid or solid particles suspended in a carrier gas Described by their Size Concentration - Number - Surface -
Atmospheric Aerosols Slides partly by Antti Lauri and Hannele Korhonen Aerosol particles Liquid or solid particles suspended in a carrier gas Described by their Size Concentration - Number - Surface -
Precipitation Processes METR σ is the surface tension, ρ l is the water density, R v is the Gas constant for water vapor, T is the air
 Precipitation Processes METR 2011 Introduction In order to grow things on earth, they need water. The way that the earth naturally irrigates is through snowfall and rainfall. Therefore, it is important
Precipitation Processes METR 2011 Introduction In order to grow things on earth, they need water. The way that the earth naturally irrigates is through snowfall and rainfall. Therefore, it is important
PALM - Cloud Physics. Contents. PALM group. last update: Monday 21 st September, 2015
 PALM - Cloud Physics PALM group Institute of Meteorology and Climatology, Leibniz Universität Hannover last update: Monday 21 st September, 2015 PALM group PALM Seminar 1 / 16 Contents Motivation Approach
PALM - Cloud Physics PALM group Institute of Meteorology and Climatology, Leibniz Universität Hannover last update: Monday 21 st September, 2015 PALM group PALM Seminar 1 / 16 Contents Motivation Approach
Kelvin Effect. Covers Reading Material in Chapter 10.3 Atmospheric Sciences 5200 Physical Meteorology III: Cloud Physics
 Kelvin Effect Covers Reading Material in Chapter 10.3 Atmospheric Sciences 5200 Physical Meteorology III: Cloud Physics Vapor Pressure (e) e < e # e = e # Vapor Pressure e > e # Relative humidity RH =
Kelvin Effect Covers Reading Material in Chapter 10.3 Atmospheric Sciences 5200 Physical Meteorology III: Cloud Physics Vapor Pressure (e) e < e # e = e # Vapor Pressure e > e # Relative humidity RH =
Chapter 2 Aerosol Mass Transfer
 Chapter 2 Aerosol Mass Transfer The size distribution of aerosol particles has a significant impact on their chemical and physical properties, including their optical properties and ability to act as cloud
Chapter 2 Aerosol Mass Transfer The size distribution of aerosol particles has a significant impact on their chemical and physical properties, including their optical properties and ability to act as cloud
Importance of Composition and Hygroscopicity of BC Particles to the Effect of BC Mitigation on Cloud Properties: Application to California Conditions
 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 Importance of Composition and Hygroscopicity of BC Particles to the Effect of BC Mitigation on Cloud Properties:
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 Importance of Composition and Hygroscopicity of BC Particles to the Effect of BC Mitigation on Cloud Properties:
Lecture Ch. 6. Condensed (Liquid) Water. Cloud in a Jar Demonstration. How does saturation occur? Saturation of Moist Air. Saturation of Moist Air
 Lecture Ch. 6 Saturation of moist air Relationship between humidity and dewpoint Clausius-Clapeyron equation Dewpoint Temperature Depression Isobaric cooling Moist adiabatic ascent of air Equivalent temperature
Lecture Ch. 6 Saturation of moist air Relationship between humidity and dewpoint Clausius-Clapeyron equation Dewpoint Temperature Depression Isobaric cooling Moist adiabatic ascent of air Equivalent temperature
GEOPHYSICAL RESEARCH LETTERS, VOL. 33, L21804, doi: /2006gl027150, 2006
 Click Here for Full Article GEOPHYSICAL RESEARCH LETTERS, VOL. 33, L21804, doi:10.1029/2006gl027150, 2006 Influence of aerosols on the shortwave cloud radiative forcing from North Pacific oceanic clouds:
Click Here for Full Article GEOPHYSICAL RESEARCH LETTERS, VOL. 33, L21804, doi:10.1029/2006gl027150, 2006 Influence of aerosols on the shortwave cloud radiative forcing from North Pacific oceanic clouds:
Boundary layer equilibrium [2005] over tropical oceans
![Boundary layer equilibrium [2005] over tropical oceans Boundary layer equilibrium [2005] over tropical oceans](/thumbs/96/128963638.jpg) Boundary layer equilibrium [2005] over tropical oceans Alan K. Betts [akbetts@aol.com] Based on: Betts, A.K., 1997: Trade Cumulus: Observations and Modeling. Chapter 4 (pp 99-126) in The Physics and Parameterization
Boundary layer equilibrium [2005] over tropical oceans Alan K. Betts [akbetts@aol.com] Based on: Betts, A.K., 1997: Trade Cumulus: Observations and Modeling. Chapter 4 (pp 99-126) in The Physics and Parameterization
Highly Biased Hygroscopicity Derived from Size-Resolved Cloud Condensation Nuclei Activation Ratios without Data Inversion
 ATMOSPHERIC AND OCEANIC SCIENCE LETTERS, 2014, VOL. 7, NO. 3, 254 259 Highly Biased Hygroscopicity Derived from Size-Resolved Cloud Condensation Nuclei Activation Ratios without Data Inversion DENG Zhao-Ze
ATMOSPHERIC AND OCEANIC SCIENCE LETTERS, 2014, VOL. 7, NO. 3, 254 259 Highly Biased Hygroscopicity Derived from Size-Resolved Cloud Condensation Nuclei Activation Ratios without Data Inversion DENG Zhao-Ze
Cloud processing, cloud evaporation and Angström exponent
 Atmos. Chem. Phys., 9, 71 80, 2009 Author(s) 2009. This work is distributed under the Creative Commons Attribution 3.0 License. Atmospheric Chemistry and Physics Cloud processing, cloud evaporation and
Atmos. Chem. Phys., 9, 71 80, 2009 Author(s) 2009. This work is distributed under the Creative Commons Attribution 3.0 License. Atmospheric Chemistry and Physics Cloud processing, cloud evaporation and
ROBUSTNESS ANALYSIS OF ATMOSPHERIC AEROSOL REMOVAL BY PRECIPITATION
 ROBUSTNESS ANALYSIS OF ATMOSPHERIC AEROSOL REMOVAL BY PRECIPITATION C. Andronache 1 and V. T. J. Phillips 2 1 Boston College, Chestnut Hill, MA 02467, USA 2 University of Hawaii, Honolulu, HI 96822, USA
ROBUSTNESS ANALYSIS OF ATMOSPHERIC AEROSOL REMOVAL BY PRECIPITATION C. Andronache 1 and V. T. J. Phillips 2 1 Boston College, Chestnut Hill, MA 02467, USA 2 University of Hawaii, Honolulu, HI 96822, USA
PRECIPITATION PROCESSES
 PRECIPITATION PROCESSES Loknath Adhikari This summary deals with the mechanisms of warm rain processes and tries to summarize the factors affecting the rapid growth of hydrometeors in clouds from (sub)
PRECIPITATION PROCESSES Loknath Adhikari This summary deals with the mechanisms of warm rain processes and tries to summarize the factors affecting the rapid growth of hydrometeors in clouds from (sub)
