Metathesis and Polyfuranones,Polylactams and dendrimers
|
|
|
- Lee Lee
- 6 years ago
- Views:
Transcription
1 Metathesis and Polyfuranones,Polylactams and dendrimers By obert B. Login Metathesis is now closing in on more than three decades of spectacular development and some of the problems with metathesis are being resolved. ecently patents have appeared claiming supported catalysts that are easy to remove and could be re-used even in flow reactors. ence, the problem of heavy metal contamination can be eliminated. (USP 6,921,735 and 7,632,772 for example). Economics are a chief concern but new more robust catalyst continue to be developed that are much more cost effective. I find metathesis chemistry fascinating. When one thinks about the scope of these reactions, that double or triple bonds can be readily manipulated by CM, MP, CEYM, ADMET, CM, ene-yne etc techniques. If you are unfamiliar with these acronyms, there are many good reviews, for example the recently published, lefin Metathesis theory and practice, by Karol Grela ed. Wiley, My background as the Director of Polymer Science for ISP leads me to think of acetylenic chemistry practiced by that company and how to find new and useful products based on it. I therefore began to think about 1,4-dihydroxy-2-butene an intermediate in the preparation of 1,4-butanediol, BL, and VP. Because it has a double bond it should react with metathesis catalysts like Grubbs 2 or others and undergo ing Closure Metathesis (CM). Therefore I think the following scheme would be very interesting:
2 Grubbs Cat. 2-furan-5-one Grubbs cat. Scheme 1A In this scheme, nothing is wasted, producing two moles of the expensive 2(5)-furanones and ethylene. The diester acrylate might give two furanones directly. Cl Grubbs Cat. 2X 2-furan-5-one Bull. Korean Chem. Soc. 2011, Vol. 32, o The new journal for organic synthesis, V28, Issue 2, rg. Lett. 2005,7(9), pp Scheme 1B
3 2(5)-furanone can be synthesized from the allyl alcohol acrylate ester, as shown above, by CM(rg. Lett. 2005, 7(9), pp1805. And allyl alcohol can be prepared by the hydrogenation of propargyl alcohol. Whichever of the above approaches, is easier or less expensive or both, should be considered. I have copied some of the introduction to an article; Tetrahedron 65 (2009) that illustrates the interest in this compound. Furan-2(5)-ones have attracted the attention of several organic chemists as valuable targets due to their presence as a subunit in many natural products isolated from a variety of sources like sponges, algae, animals, plants and insects. According to the literature reports this subunit is present in as many as more than 13,000 natural products. This core unit is the key structure to induce a wide range of biological activities like antimicrobial, anti-fungal,anti-inflammatory, anticancer and anti-viral etc. The Butenolide synthon is the core structural unit found in many bioactive natural products such as sarcophine1and rubrolide, which is isolated from the colonial tunicate itterela rubra, as well as in synthetic drug benfurodil hemisuccinate (Eucilat). 2(5)-furanone (aka butanolide) is an excellent Michael receptor and will react with ammonia or an ammonia surrogates to form a new monomer. Scheme 2 illustrates this reaction.
4 n n Scheme 2 The last step of this scheme is new as far as I can tell; however, several patents have issued concerning the addition of primary amines to this unsaturated lactone affording the hydroxy containing polyamides(usatuated SIDE CAI PLYAMIDE Polymers by.ollingworth and G Wang, USP 6,541,601 B1; Zhi _ uang,et. al., US 6,399,714 B1; awle I. ollingsworth, usp 6,153,724). These patents do not show how to further react the hydroxy group with the amide groups to generate pyrrolidones The polylactams resulting from this chemistry would be analogous to the nylon family with the added benefit of water solubility. If the MW is in the nylon range, I would predict a tough polymer possibly even a fiber former that could be handled as an aqueous solution. Potential uses for these polyamides would revolve around their water solubility. Temporary medical applications such as sutures, erode-able inplants, cell scaffolds, bandages, drug delivery etc. Such uses would be very valuable. Free adical Polymerization:
5 F Polymerization Yoon et al. USP 7,241,552 B2 Jul. 10, 2007 illustrates the F polymerization of the 2(5)-furanones. In this case they used angelic lactone and they claim that the lactone can polymerize in two ways: n F Polymerization ot only can the 2(5)-furanones readily polymerize they can copolymerized with a variety of F monomers. nce this polymer is available, it can be post reacted with primary amines to form hydroxy and amide pendant groups. These poly lactones can have a variety of uses but they could also further react to form pyrrolidone groups. This would be another analog to the one above(scheme 2)and the resulting polymers would have similar uses. Acrylamide derivatives: Cl Bull. Korean Chem. Soc. 2011, Vol. 32, o In much the same way as acrylic esters, -unsaturated acrylamides will also undergo CM to form 2-pyrrol-5-ones. Presumably, 2-pyrrol-5-ones will polymerize by a F mechanism? I have not found any reference to the n Grubbs cat. - -alkyl-2-pyrrol-5-one n
6 polymerization of this monomer? It is possible that the reason is that the actual structure of this monomer is the hydroxypyrrol which does not polymerize? owever, if it did polymerize, Said polymers would contain pendant pyrrolidone groups with the lactams pointing out away from the backbone. Such polypyrrolidones would have unique properties; for example, water solubility, complexation, iodophors, hair fixatives, tablet binders, excipients, solublizers of water insoluble drugs and so forth. In short, every job that PVP does might be done better by this polymer. 2-pyrrol-5-one might also copolymerize with a variety of monomers. In fact this monomer would be compatible with ATP and other living F polymerization techniques. Should the unreactivity be true then the poly(2(5)-furanone) post reaction route would afford similar polymers by post reaction with amines followed by condensation to pendant pyrrolidone. It would be very nice if 2-pyrrol-5-one's would polymerize as a plethora of interesting uses can be visualized. owever the lack of references makes me think this is not the case. I would appreciate being informed if anyone knows references that would shed light on this chemistry. Ene-Yne Metathesis: Since proparyl derivatives are available from acetylenic chemistry, the following could be considered:
7 EneYne Metathesis vinylation ADMET Metathesis Polymer Br Scheme 3 Polymer The 4-vinyl derivative will undergo the Diels-Alder reaction which as I show can lead to another set of polymers. The Diels-Alder reaction can be performed along with metathesis in-situ or as a post reaction. ther dimer types have been discussed in previous reports available on my web site (rloginconsulting.com) The ester of propargyl acid and allyl alcohol, the reverse of whats shown above, will also react by the Ene-Yne
8 mechanism. bviously other unsaturated primary amines such as allylamines can also condense with these anhydride and lactone groups and undergo ADMET polymerization. The same set of reactions are feasible with 2-pyrrol-5- ones replacing maleic anhydride and might require fewer steps. The point is that dimer type pyrrolidone vinyl derivative can be polymerized to useful polymers. Simply by anhydrous acid catalyzed condensation or by metathesis. Cl EneYne Metathesis vinylation ADMET Metathesis Polymer Br Polymer Scheme 4 bviously, one can use -substituted maleimides or - substituted 2-pyrrol-5-ones as the dieneophiles. They would broaden the conceivable structures resulting in a variety of new dimer pyrrolidone analogs. Another approach to linear pyrrolidone polymers by metathesis might be as
9 follows: 2 Cl 2 EneYne Metathesis 1 1 n Scheme 5 I would think that 5-membered heterocyclics would be favored over large macrocycles and that the Diels-Alder reaction is considered a click reaction reliably resulting in a polymer. Bis-maleimides can also be employed. ote that other isomers with the pyrrolidone carbonyls pointing in the other position after the Diels-Alder reaction can occur in all the above examples. Inaddition, the 3-methylene pyrrolidone can be polymerized as a diamine dimer;
10 x ADMET x n Pyrrolidone Dendrimer-like compounds If you are following my reports on my web page(rloginconsulting.com) then you realize that I have a strong belief in the value of the lactam as a functional group especially the pyrrolidone ring because in this lactam the orbitals allow the best overlap for charge separation. This is why PVP is water soluble and able to complex anions like the triiodide and phenolics. This is why on my blog I have tried to figure out how to use it in a wide variety of compounds where I believe it has never been used before. I do this because I'm interested in it and because it deserves to be pursued. I would hope that the ever growing list of VP manufacturers and those who polymerize it in its various forms and grades would see the value of doing more innovative research involving new uses. bviously I would love to be involved as a consultant. My first approach to a pyrrolidone dendrimer (actually hyperbranched polymers) is the following:
11 reduction allylchloride amine protection BC SCl 2 Cl Cl Propargylamine BC -BC acroylchloride BC Metathesis catalyst Dendrimer Scheme 1 Several problems can be visualized with scheme 1; the unsaturation could react by metathesis in unexpected ways to form large rings, the 3-pyrrolin-2-ones could isomerize to the 4-pyrrolin-2-one which might not enter the Diels- Alder reaction? bviously other things can go wrong-but that is the nature of research. If the Diels-Alder reactions go as predicted then a partially growing
12 dendrimer would look like: Etc. Scheme 2 bviously, the build out is not so neat with chains growing at different rates resulting in chains of various lengths. They would not be as neat as some of the well known dendrimers, but dendrimer like. Actually the dendrimers are usually prepared stepwise by an iterative approach. Another route to pyrrolidone dendrimers (actually hyperbranched polymers) is shown in scheme 3. In this case a double CEYM takes place leading to a diene which is reacted with a preformed 2-pyrrol-2-one. bviously, these reactions are possible but they could take alternate routes. owever a pyrrolidone dendrimer would be very interesting for a wide variety of uses, such as drug delivery, capture of and transport of DA, scaffolds for enzymes, catalysis by carrying metal containing structures. I think the best approach to the utility of such dendrimers is to look at Prof. Tomalia et.al new book Dendrimers, Dendrons, and Dendritic Polymers Cambridge, e shows a wealth of Dendrimer uses some of which could be of
13 immense value. CEYM CM Diels-Alder Vinylation Br Dendrimer Scheme 3 My concern is that the unsaturation from CEYM might isomerize rather than produce a diene that would undergo the Diels-Alder reaction. emember, dendrimers and dendrons that are reviewed in Tomalia's book are prepared by complicated multistep procedures. The hyper branched polymers are simpler and can also have interesting properties. The point is to stimulate
14 thinking about new unique uses for the pyrrolidone functionality. Conclusion: Metathesis can lead to numerous compounds of value especially to those companies who are involved with PVP and all its variations. I would like to help any company that sees value in my ideas. If there are mistakes in any proposed chemistry, I would appreciate your comments. Thank you, Dr. obert B. Login rloginconsulting.com
Reverse Polyvinylpyrrolidones (PVP) By: Robert B Login
 everse Polyvinylpyrrolidones (PVP) By: obert B Login Years ago, Dr. Dandreau and I received several patents for reverse PVP whose structures are as shown in the following patents (PLYMEIZABLE PYLIDYL XAZLIE
everse Polyvinylpyrrolidones (PVP) By: obert B Login Years ago, Dr. Dandreau and I received several patents for reverse PVP whose structures are as shown in the following patents (PLYMEIZABLE PYLIDYL XAZLIE
Pyrrolidone Dimers and Pendant Pyrromethane Polymers with MIP potential
 Pyrrolidone Dimers and Pendant Pyrromethane Polymers with MIP potential Proposed methods to synthesize Pyrrolidone Dimers of various structures. By obert B. Login I have been interested in the pyrrolidone
Pyrrolidone Dimers and Pendant Pyrromethane Polymers with MIP potential Proposed methods to synthesize Pyrrolidone Dimers of various structures. By obert B. Login I have been interested in the pyrrolidone
Nucleic Acid Derivatised Pyrrolidone By: Robert B. Login
 ucleic Acid Derivatised Pyrrolidone By: Robert B. Login Templated polymer synthesis is a growing technology with many recent references illustrating for example how employing ucleic acid bases attached
ucleic Acid Derivatised Pyrrolidone By: Robert B. Login Templated polymer synthesis is a growing technology with many recent references illustrating for example how employing ucleic acid bases attached
Modified Baylis-Hillman Reaction with Epoxides. Can the Baylis-Hillman reaction be modified to work with epoxides?
 Modified Baylis-Hillman Reaction with Epoxides By: Robert B. Login Can the Baylis-Hillman reaction be modified to work with epoxides? Several B-H reviews have appeared covering this reaction in very great
Modified Baylis-Hillman Reaction with Epoxides By: Robert B. Login Can the Baylis-Hillman reaction be modified to work with epoxides? Several B-H reviews have appeared covering this reaction in very great
Learning Organic Chemistry
 Objective 1 Represent organic molecules with chemical formulas, expanded formulas, Lewis structures, skeletal structures. Determine shape (VSEPR), bond polarity, and molecule polarity. Identify functional
Objective 1 Represent organic molecules with chemical formulas, expanded formulas, Lewis structures, skeletal structures. Determine shape (VSEPR), bond polarity, and molecule polarity. Identify functional
A)-Reaction of Primary Amines with Maleate half esters to prepare Polymaleimides In-Situ without Isolating the corresponding Monomers.
 In-Situ Synthesis of Maleimides leading to Polymaleimides without isolating the Monomers and subsequent reduction. By: Robert B. Login Goals: An inexpensive method of producing polymaleimides that are
In-Situ Synthesis of Maleimides leading to Polymaleimides without isolating the Monomers and subsequent reduction. By: Robert B. Login Goals: An inexpensive method of producing polymaleimides that are
Chem 1075 Chapter 19 Organic Chemistry Lecture Outline
 Chem 1075 Chapter 19 Organic Chemistry Lecture Outline Slide 2 Introduction Organic chemistry is the study of and its compounds. The major sources of carbon are the fossil fuels: petroleum, natural gas,
Chem 1075 Chapter 19 Organic Chemistry Lecture Outline Slide 2 Introduction Organic chemistry is the study of and its compounds. The major sources of carbon are the fossil fuels: petroleum, natural gas,
Name Date Class. aryl halides substitution reaction
 23.1 INTRODUCTION TO FUNCTIONAL GROUPS Section Review Objectives Explain how organic compounds are classified Identify the IUPAC rules for naming halocarbons Describe how halocarbons can be prepared Vocabulary
23.1 INTRODUCTION TO FUNCTIONAL GROUPS Section Review Objectives Explain how organic compounds are classified Identify the IUPAC rules for naming halocarbons Describe how halocarbons can be prepared Vocabulary
Olefin Metathesis ROMP. L n Ru= ROMP n RCM. dilute
 lefin Metathesis MP: ing-opening metathesis polymerization Thermodynamically favored for 3,4, 8, larger ring systems Bridging groups (bicyclic olefins) make ΔG polymerization more favorable as a result
lefin Metathesis MP: ing-opening metathesis polymerization Thermodynamically favored for 3,4, 8, larger ring systems Bridging groups (bicyclic olefins) make ΔG polymerization more favorable as a result
Chapter 25: The Chemistry of Life: Organic and Biological Chemistry
 Chemistry: The Central Science Chapter 25: The Chemistry of Life: Organic and Biological Chemistry The study of carbon compounds constitutes a separate branch of chemistry known as organic chemistry The
Chemistry: The Central Science Chapter 25: The Chemistry of Life: Organic and Biological Chemistry The study of carbon compounds constitutes a separate branch of chemistry known as organic chemistry The
Molecular Geometry: VSEPR model stand for valence-shell electron-pair repulsion and predicts the 3D shape of molecules that are formed in bonding.
 Molecular Geometry: VSEPR model stand for valence-shell electron-pair repulsion and predicts the 3D shape of molecules that are formed in bonding. Sigma and Pi Bonds: All single bonds are sigma(σ), that
Molecular Geometry: VSEPR model stand for valence-shell electron-pair repulsion and predicts the 3D shape of molecules that are formed in bonding. Sigma and Pi Bonds: All single bonds are sigma(σ), that
ummary Manipulating Radicals
 Manipulating Radicals ummary Modern catalysis research tries to address issues such as material scarcity, sustainability or process costs. One solution is to replace expensive and scarce noble metal catalysts
Manipulating Radicals ummary Modern catalysis research tries to address issues such as material scarcity, sustainability or process costs. One solution is to replace expensive and scarce noble metal catalysts
An alcohol is a compound obtained by substituting a hydoxyl group ( OH) for an H atom on a carbon atom of a hydrocarbon group.
 Derivatives of Hydrocarbons A functional group is a reactive portion of a molecule that undergoes predictable reactions. All other organic compounds can be considered as derivatives of hydrocarbons (i.e.,
Derivatives of Hydrocarbons A functional group is a reactive portion of a molecule that undergoes predictable reactions. All other organic compounds can be considered as derivatives of hydrocarbons (i.e.,
Chapter 13 Alkenes and Alkynes Based on Material Prepared by Andrea D. Leonard University of Louisiana at Lafayette
 Chapter 13 Alkenes and Alkynes Based on Material Prepared by Andrea D. Leonard University of Louisiana at Lafayette 1 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display.
Chapter 13 Alkenes and Alkynes Based on Material Prepared by Andrea D. Leonard University of Louisiana at Lafayette 1 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display.
CH318N Spring 2012 Final Exam. Chemistry 318N. Spring 2012 Dr. Willson. Final Exam
 318N Spring 2012 Final Exam 3 hemistry 318N Spring 2012 Dr. Willson Final Exam This afternoon you will take two tests, one in chemistry and one in integrity. I want you to get A s on both of these tests
318N Spring 2012 Final Exam 3 hemistry 318N Spring 2012 Dr. Willson Final Exam This afternoon you will take two tests, one in chemistry and one in integrity. I want you to get A s on both of these tests
Isobenzofuran by itself is not stable enough to be isolated, but various analogues of it are isolable.
 Diels Alder Class 2 March 8, 2011 Last time we talked a lot about highly reactive dienes, especially those developed by the group of Sam Danishefsky. ne more example of an interesting diene is isobenzofuran:
Diels Alder Class 2 March 8, 2011 Last time we talked a lot about highly reactive dienes, especially those developed by the group of Sam Danishefsky. ne more example of an interesting diene is isobenzofuran:
DAMIETTA UNIVERSITY CHEM-405: PERICYCLIC REACTIONS LECTURE
 DAMIETTA UNIVERSITY CHEM-405: PERICYCLIC REACTIONS LECTURE 8 Dr Ali El-Agamey 1 LEARNING OUTCOMES LECTURE 8 (1) The Woodward-Hoffmann rules for [1,n] sigmatropic rearrangements -[1,2] cationic shift -[1,2]
DAMIETTA UNIVERSITY CHEM-405: PERICYCLIC REACTIONS LECTURE 8 Dr Ali El-Agamey 1 LEARNING OUTCOMES LECTURE 8 (1) The Woodward-Hoffmann rules for [1,n] sigmatropic rearrangements -[1,2] cationic shift -[1,2]
Chapter 12: Unsaturated Hydrocarbons
 Chapter 12: Unsaturated Hydrocarbons UNSATURATED HYDROCARBONS contain carbon-carbon multiple bonds. Alkenes C=C double bonds Alkynes triple bonds Aromatics benzene rings 1 2 NAMING ALKENES Step 1: Name
Chapter 12: Unsaturated Hydrocarbons UNSATURATED HYDROCARBONS contain carbon-carbon multiple bonds. Alkenes C=C double bonds Alkynes triple bonds Aromatics benzene rings 1 2 NAMING ALKENES Step 1: Name
UNIT 4 REVISION CHECKLIST CHEM 4 AS Chemistry
 UNIT 4 REVISION CHECKLIST CHEM 4 AS Chemistry Topic 4.1 Kinetics a) Define the terms: rate of a reaction, rate constant, order of reaction and overall order of reaction b) Deduce the orders of reaction
UNIT 4 REVISION CHECKLIST CHEM 4 AS Chemistry Topic 4.1 Kinetics a) Define the terms: rate of a reaction, rate constant, order of reaction and overall order of reaction b) Deduce the orders of reaction
A polymer is a very large molecule that is built from monomers. A monomer is one of the repeating units that make up a polymer.
 1.8 Polymers The General Structure of Polymers A polymer is a very large molecule that is built from monomers. A monomer is one of the repeating units that make up a polymer. Many biological molecules,
1.8 Polymers The General Structure of Polymers A polymer is a very large molecule that is built from monomers. A monomer is one of the repeating units that make up a polymer. Many biological molecules,
Plastics are synthetic substances that can be moulded (often under heat and pressure) and retain the shape they are moulded into.
 5.7: Polymers Plastics are synthetic substances that can be moulded (often under heat and pressure) and retain the shape they are moulded into. Polymers are large molecules that are made by linking together
5.7: Polymers Plastics are synthetic substances that can be moulded (often under heat and pressure) and retain the shape they are moulded into. Polymers are large molecules that are made by linking together
Rhenium-Catalyzed Synthesis of Multisubstituted Aromatic Compounds via C-C Single-Bond Cleavage
 henium-catalyzed Synthesis of Multisubstituted Aromatic Compounds via C-C Single-Bond Cleavage Kuninobu, Y.; Takata,.; Kawata, A.; Takai, K. rg. Lett. ASAP Et 5 6 cat. [ebr(c) 3 (thf)] 2 5 6 Current Literature
henium-catalyzed Synthesis of Multisubstituted Aromatic Compounds via C-C Single-Bond Cleavage Kuninobu, Y.; Takata,.; Kawata, A.; Takai, K. rg. Lett. ASAP Et 5 6 cat. [ebr(c) 3 (thf)] 2 5 6 Current Literature
This reactivity makes alkenes an important class of organic compounds because they can be used to synthesize a wide variety of other compounds.
 This reactivity makes alkenes an important class of organic compounds because they can be used to synthesize a wide variety of other compounds. Mechanism for the addition of a hydrogen halide What happens
This reactivity makes alkenes an important class of organic compounds because they can be used to synthesize a wide variety of other compounds. Mechanism for the addition of a hydrogen halide What happens
Chapter 3 Alkenes and Alkynes. Excluded sections 3.15&3.16
 Chapter 3 Alkenes and Alkynes Excluded sections 3.15&3.16 3.1 Definition and Classification Alkene: a hydrocarbon that contains one or more carboncarbon double bonds. ethylene is the simplest alkene. Alkyne:
Chapter 3 Alkenes and Alkynes Excluded sections 3.15&3.16 3.1 Definition and Classification Alkene: a hydrocarbon that contains one or more carboncarbon double bonds. ethylene is the simplest alkene. Alkyne:
Electronegativity Scale F > O > Cl, N > Br > C, H
 Organic Chem Chapter 12 Alkanes Organic chemistry is the study of carbon compounds. Carbon has several properties that are worth discussing: Tetravalent Always forms 4 bonds Can form multiple bonds (double
Organic Chem Chapter 12 Alkanes Organic chemistry is the study of carbon compounds. Carbon has several properties that are worth discussing: Tetravalent Always forms 4 bonds Can form multiple bonds (double
Organic Chemistry. Alkynes
 For updated version, please click on http://ocw.ump.edu.my Organic Chemistry Alkynes by Dr. Seema Zareen & Dr. Izan Izwan Misnon Faculty Industrial Science & Technology seema@ump.edu.my & iezwan@ump.edu.my
For updated version, please click on http://ocw.ump.edu.my Organic Chemistry Alkynes by Dr. Seema Zareen & Dr. Izan Izwan Misnon Faculty Industrial Science & Technology seema@ump.edu.my & iezwan@ump.edu.my
Chapter 7 Alkenes and Alkynes I: Properties and Synthesis Elimination Reactions of Alkyl Halides"
 Chapter 7 Alkenes and Alkynes I: Properties and Synthesis Elimination Reactions of Alkyl Halides The (E)-(Z) System for Designating Alkene Diastereomers The Cahn-Ingold-Prelog convention is used to assign
Chapter 7 Alkenes and Alkynes I: Properties and Synthesis Elimination Reactions of Alkyl Halides The (E)-(Z) System for Designating Alkene Diastereomers The Cahn-Ingold-Prelog convention is used to assign
Organic Chemistry. Organic chemistry is the chemistry of compounds containing carbon.
 Organic Chemistry Organic Chemistry Organic chemistry is the chemistry of compounds containing carbon. In this chapter we will discuss the structural features of organic molecules, nomenclature, and a
Organic Chemistry Organic Chemistry Organic chemistry is the chemistry of compounds containing carbon. In this chapter we will discuss the structural features of organic molecules, nomenclature, and a
11/5/ Conjugated Dienes. Conjugated Dienes. Conjugated Dienes. Heats of Hydrogenation
 8.12 Sites of unsaturation Many compounds have numerous sites of unsaturation If sites are well separated in molecule they react independently If sites are close together they may interact with one another
8.12 Sites of unsaturation Many compounds have numerous sites of unsaturation If sites are well separated in molecule they react independently If sites are close together they may interact with one another
Dr. Dina akhotmah-232 1
 Dr. Dina akhotmah-232 1 Chemistry of polyfunction 1. Types of carbon atom Dr. Dina akhotmah-232 2 Classification of multiple bonds of polyunsaturated compounds Dr. Dina akhotmah-232 3 Organic chemistry,
Dr. Dina akhotmah-232 1 Chemistry of polyfunction 1. Types of carbon atom Dr. Dina akhotmah-232 2 Classification of multiple bonds of polyunsaturated compounds Dr. Dina akhotmah-232 3 Organic chemistry,
Glyoxylic acid derivatives in asymmetric synthesis*
 Pure Appl. Chem., Vol. 72, No. 9, pp. 1589 1596, 2000. 2000 IUPAC Glyoxylic acid derivatives in asymmetric synthesis* Janusz Jurczak 1,2 and Tomasz Bauer 1 1 Department of Chemistry, University of Warsaw,
Pure Appl. Chem., Vol. 72, No. 9, pp. 1589 1596, 2000. 2000 IUPAC Glyoxylic acid derivatives in asymmetric synthesis* Janusz Jurczak 1,2 and Tomasz Bauer 1 1 Department of Chemistry, University of Warsaw,
Chem101 General Chemistry. Lecture 11 Unsaturated Hydrocarbons
 hem101 General hemistry Lecture 11 Unsaturated ydrocarbons Unsaturated ydrocarbons ontain one or more double or triple carbon-carbon bond. University of Wisconsin-Eau laire hem101 - Lecture 11 2 Unsaturated
hem101 General hemistry Lecture 11 Unsaturated ydrocarbons Unsaturated ydrocarbons ontain one or more double or triple carbon-carbon bond. University of Wisconsin-Eau laire hem101 - Lecture 11 2 Unsaturated
1.1 Basic Polymer Chemistry. 1.2 Polymer Nomenclature. 1.3 Polymer Synthesis. 1.4 Chain Growth Polymerization. Polymer =
 1.1 Basic Polymer hemistry Polymers are the largest class of soft materials: over 100 billion pounds of polymers made in US each year lassification systems 1.2 Polymer Nomenclature Polymer = Monomer =
1.1 Basic Polymer hemistry Polymers are the largest class of soft materials: over 100 billion pounds of polymers made in US each year lassification systems 1.2 Polymer Nomenclature Polymer = Monomer =
Synthesis of Nitriles a. dehydration of 1 amides using POCl 3 : b. SN2 reaction of cyanide ion on halides:
 I. Nitriles Nitriles consist of the CN functional group, and are linear with sp hybridization on C and N. Nitriles are non-basic at nitrogen, since the lone pair exists in an sp orbital (50% s character
I. Nitriles Nitriles consist of the CN functional group, and are linear with sp hybridization on C and N. Nitriles are non-basic at nitrogen, since the lone pair exists in an sp orbital (50% s character
Chapter 12 Alkenes and Alkynes
 BR M 102 lass Notes hapter 12 Page 1 of 8 hapter 12 Alkenes and Alkynes * alkenes = double bonds * alkynes triple bonds * aromatics or arenes alternating double and single bonds such as in benzene * saturated
BR M 102 lass Notes hapter 12 Page 1 of 8 hapter 12 Alkenes and Alkynes * alkenes = double bonds * alkynes triple bonds * aromatics or arenes alternating double and single bonds such as in benzene * saturated
Introduction to Synthetic Methods in Step-Growth Polymers 1.1 INTRODUCTION Historical Perspective Some of the earliest useful polymeric
 Synthetics Polymers Introduction to Synthetic Methods in Step-Growth Polymers 1.1 INTRODUCTION 1.1.1 Historical Perspective Some of the earliest useful polymeric materials, the Bakelite resins formed from
Synthetics Polymers Introduction to Synthetic Methods in Step-Growth Polymers 1.1 INTRODUCTION 1.1.1 Historical Perspective Some of the earliest useful polymeric materials, the Bakelite resins formed from
Suggested solutions for Chapter 41
 s for Chapter 41 41 PBLEM 1 Explain how this synthesis of amino acids, starting with natural proline, works. Explain the stereoselectivity of each step after the first. C 2 C 2 3 CF 3 C 2 2 Pd 2 C 2 +
s for Chapter 41 41 PBLEM 1 Explain how this synthesis of amino acids, starting with natural proline, works. Explain the stereoselectivity of each step after the first. C 2 C 2 3 CF 3 C 2 2 Pd 2 C 2 +
Hydrocarbons and their Functional Groups
 Hydrocarbons and their Functional Groups Organic chemistry is the study of compounds in which carbon is the principal element. carbon atoms form four bonds long chains, rings, spheres, sheets, and tubes
Hydrocarbons and their Functional Groups Organic chemistry is the study of compounds in which carbon is the principal element. carbon atoms form four bonds long chains, rings, spheres, sheets, and tubes
CARBOXYLIC ACIDS and their Derivatives Nucleophilic Acyl substitution - Review the nomenclature for these compounds in your textbook
 CARBXYLIC ACIDS and their Derivatives Nucleophilic Acyl substitution - Review the nomenclature for these compounds in your textbook R Z R Z R Z - the basicity of Z determines the relative stability of
CARBXYLIC ACIDS and their Derivatives Nucleophilic Acyl substitution - Review the nomenclature for these compounds in your textbook R Z R Z R Z - the basicity of Z determines the relative stability of
Introduction to Alkenes and Alkynes
 Introduction to Alkenes and Alkynes In an alkane, all covalent bonds between carbon were σ (σ bonds are defined as bonds where the electron density is symmetric about the internuclear axis) In an alkene,
Introduction to Alkenes and Alkynes In an alkane, all covalent bonds between carbon were σ (σ bonds are defined as bonds where the electron density is symmetric about the internuclear axis) In an alkene,
Advanced Organic Chemistry
 D. A. Evans, G. Lalic Question of the day: Chemistry 530A TBS Me 2 C Me toluene, 130 C 70% TBS C 2 Me H H Advanced rganic Chemistry Me Lecture 16 Cycloaddition Reactions Diels _ Alder Reaction Photochemical
D. A. Evans, G. Lalic Question of the day: Chemistry 530A TBS Me 2 C Me toluene, 130 C 70% TBS C 2 Me H H Advanced rganic Chemistry Me Lecture 16 Cycloaddition Reactions Diels _ Alder Reaction Photochemical
QuickTime and a TIFF (Uncompressed) decompressor are needed to see this picture. Organic Chemistry. QuickTime and a are needed to see this picture.
 QuickTime and a TIFF (Uncompressed) decompressor are needed to see this picture. Organic Chemistry QuickTime and a TIFF (Uncompressed) decompressor are needed to see this picture. Organic Chemistry Has
QuickTime and a TIFF (Uncompressed) decompressor are needed to see this picture. Organic Chemistry QuickTime and a TIFF (Uncompressed) decompressor are needed to see this picture. Organic Chemistry Has
Repeated insertion. Multiple insertion leads to dimerization, oligomerization or polymerization. κ 1: mainly dimerization κ
 Repeated insertion ultiple insertion leads to dimerization, oligomerization or polymerization. k prop Et Key factor: k CT / k prop = κ κ 1: mainly dimerization κ 0.1-1.0: oligomerization (always mixtures)
Repeated insertion ultiple insertion leads to dimerization, oligomerization or polymerization. k prop Et Key factor: k CT / k prop = κ κ 1: mainly dimerization κ 0.1-1.0: oligomerization (always mixtures)
Lecture 4 Chapter 13 - Polymers. Functional Groups Condensation Rxns Free Radical Rxns
 Lecture 4 Chapter 13 - Polymers Functional Groups Condensation Rxns Free Radical Rxns Chemistry the whole year on one page Last semester Basic atomic theory Stoichiometry, balancing reactions Thermodynamics
Lecture 4 Chapter 13 - Polymers Functional Groups Condensation Rxns Free Radical Rxns Chemistry the whole year on one page Last semester Basic atomic theory Stoichiometry, balancing reactions Thermodynamics
Organic Chemistry I (Chem340), Spring Final Exam
 rganic Chemistry I (Chem340), pring 2005 Final Exam This is a closed-book exam. No aid is to be given to or received from another person. Model set and calculator may be used, but cannot be shared. Please
rganic Chemistry I (Chem340), pring 2005 Final Exam This is a closed-book exam. No aid is to be given to or received from another person. Model set and calculator may be used, but cannot be shared. Please
Homogeneous Catalysis - B. List
 omogeneous Catalysis - B. List 2.2.2 Research Area "rganocatalytic Asymmetric α-alkylation of Aldehydes" (B. List) Involved:. Vignola, A. Majeed Seayad bjective: α-alkylations of carbonyl compounds are
omogeneous Catalysis - B. List 2.2.2 Research Area "rganocatalytic Asymmetric α-alkylation of Aldehydes" (B. List) Involved:. Vignola, A. Majeed Seayad bjective: α-alkylations of carbonyl compounds are
Chapter 19: Alkenes and Alkynes
 Chapter 19: Alkenes and Alkynes The vast majority of chemical compounds that we know anything about and that we synthesize in the lab or the industrial plant are organic compounds. The simplest organic
Chapter 19: Alkenes and Alkynes The vast majority of chemical compounds that we know anything about and that we synthesize in the lab or the industrial plant are organic compounds. The simplest organic
2.222 Practice Problems 2003
 2.222 Practice Problems 2003 Set #1 1. Provide the missing starting compound(s), reagent/solvent, or product to correctly complete each of the following. Most people in the class have not done this type
2.222 Practice Problems 2003 Set #1 1. Provide the missing starting compound(s), reagent/solvent, or product to correctly complete each of the following. Most people in the class have not done this type
KOT 222 Organic Chemistry II
 KOT 222 Organic Chemistry II Course Objectives: 1) To introduce the chemistry of alcohols and ethers. 2) To study the chemistry of functional groups. 3) To learn the chemistry of aromatic compounds and
KOT 222 Organic Chemistry II Course Objectives: 1) To introduce the chemistry of alcohols and ethers. 2) To study the chemistry of functional groups. 3) To learn the chemistry of aromatic compounds and
Suggested solutions for Chapter 29
 s for Chapter 29 29 PRBLEM 1 or each of the following reactions (a) state what kind of substitution is suggested and (b) suggest what product might be formed if monosubstitution occured. Br 2 3 2 S 4 S
s for Chapter 29 29 PRBLEM 1 or each of the following reactions (a) state what kind of substitution is suggested and (b) suggest what product might be formed if monosubstitution occured. Br 2 3 2 S 4 S
3.10 Benzene : Aromatic Hydrocarbons / Arenes
 3.10 Benzene : Aromatic ydrocarbons / Arenes There are two major classes of organic chemicals aliphatic : straight or branched chain organic substances aromatic or arene: includes one or more ring of six
3.10 Benzene : Aromatic ydrocarbons / Arenes There are two major classes of organic chemicals aliphatic : straight or branched chain organic substances aromatic or arene: includes one or more ring of six
Chapter 10: Carboxylic Acids and Their Derivatives
 Chapter 10: Carboxylic Acids and Their Derivatives The back of the white willow tree (Salix alba) is a source of salicylic acid which is used to make aspirin (acetylsalicylic acid) The functional group
Chapter 10: Carboxylic Acids and Their Derivatives The back of the white willow tree (Salix alba) is a source of salicylic acid which is used to make aspirin (acetylsalicylic acid) The functional group
Chapter 15 Dienes, Resonance, and Aromaticity
 Instructor Supplemental Solutions to Problems 2010 Roberts and Company Publishers Chapter 15 Dienes, Resonance, and Aromaticity Solutions to In-Text Problems 15.2 The delocalization energy is the energy
Instructor Supplemental Solutions to Problems 2010 Roberts and Company Publishers Chapter 15 Dienes, Resonance, and Aromaticity Solutions to In-Text Problems 15.2 The delocalization energy is the energy
Chapter 15: Conjugated Systems, Orbital Symmetry, and UV Spectroscopy
 Chapter 15: Conjugated Systems, Orbital Symmetry, and UV Spectroscopy Conjugated unsaturated systems have a p orbital on a carbon adjacent to a double bond The p orbital can come from another double (e.g.
Chapter 15: Conjugated Systems, Orbital Symmetry, and UV Spectroscopy Conjugated unsaturated systems have a p orbital on a carbon adjacent to a double bond The p orbital can come from another double (e.g.
2. Amorphous or Crystalline Structurally, polymers in the solid state may be amorphous or crystalline. When polymers are cooled from the molten state
 2. Amorphous or Crystalline Structurally, polymers in the solid state may be amorphous or crystalline. When polymers are cooled from the molten state or concentrated from the solution, molecules are often
2. Amorphous or Crystalline Structurally, polymers in the solid state may be amorphous or crystalline. When polymers are cooled from the molten state or concentrated from the solution, molecules are often
Suggested solutions for Chapter 32
 s for Chapter 32 32 PBLEM 1 Explain how the stereo- and regio- chemistry of these reactions are controlled. Why is the epoxidation only moderately diastereoselective, and why does the amine attack where
s for Chapter 32 32 PBLEM 1 Explain how the stereo- and regio- chemistry of these reactions are controlled. Why is the epoxidation only moderately diastereoselective, and why does the amine attack where
Chapter 9. Organic Chemistry: The Infinite Variety of Carbon Compounds. Organic Chemistry
 Chapter 9 Organic Chemistry: The Infinite Variety of Carbon Compounds Organic Chemistry Organic chemistry is defined as the chemistry of carbon compounds. Of tens of millions of known chemical compounds,
Chapter 9 Organic Chemistry: The Infinite Variety of Carbon Compounds Organic Chemistry Organic chemistry is defined as the chemistry of carbon compounds. Of tens of millions of known chemical compounds,
CHEMISTRY 252 Exam pts. Section 703 Grand Rapids 27 July 2006
 ame PID CMISTRY 252 xam 1 100 pts. Section 70 Grand Rapids 27 July 2006 Make sure you have all 12 exam pages You will have 90 minutes to complete the 5 questions Please sign your name at the bottom of
ame PID CMISTRY 252 xam 1 100 pts. Section 70 Grand Rapids 27 July 2006 Make sure you have all 12 exam pages You will have 90 minutes to complete the 5 questions Please sign your name at the bottom of
Loudon Chapter 20 & 21 Review: Carboxylic Acids & Derivatives CHEM 3331, Jacquie Richardson, Fall Page 1
 Loudon Chapter 20 & 21 eview: Carboxylic Acids & Derivatives CEM 3331, Jacquie ichardson, Fall 2010 - Page 1 These two chapters cover compounds which are all at the three bonds to more electronegative
Loudon Chapter 20 & 21 eview: Carboxylic Acids & Derivatives CEM 3331, Jacquie ichardson, Fall 2010 - Page 1 These two chapters cover compounds which are all at the three bonds to more electronegative
Pechmann Dye Homopolymers By: Robert B. Login rloginconsulting.com
 Pechmann Dye omopolymers By: obert B. Login rloginconsulting.com rganic semiconductors compounds continues to fascinate me. Especially those related to the pyrrolidone type derivatives. It just strikes
Pechmann Dye omopolymers By: obert B. Login rloginconsulting.com rganic semiconductors compounds continues to fascinate me. Especially those related to the pyrrolidone type derivatives. It just strikes
CHAPTER 3 ALKENES, ALKYNES & CONJUGATE DIENES
 CHEM 244 PRINCIPLES OF ORGANIC CHEMISTRY I FOR CHEMICAL ENGINEERING STUDENTS, COLLEGE OF ENGINEERING PRE-REQUISITES COURSE; CHEM 101 CREDIT HOURS; 2 (2+0) Dr. Mohamed El-Newehy Chemistry Department, College
CHEM 244 PRINCIPLES OF ORGANIC CHEMISTRY I FOR CHEMICAL ENGINEERING STUDENTS, COLLEGE OF ENGINEERING PRE-REQUISITES COURSE; CHEM 101 CREDIT HOURS; 2 (2+0) Dr. Mohamed El-Newehy Chemistry Department, College
How to make pyridines: the Hantzsch pyridine synthesis
 ow to make pyridines: the antzsch pyridine synthesis 1191 Zinc in acetic acid (Chapter 24) reduces the oxime to the amine and we can start the synthesis by doing the conjugate addition and then reducing
ow to make pyridines: the antzsch pyridine synthesis 1191 Zinc in acetic acid (Chapter 24) reduces the oxime to the amine and we can start the synthesis by doing the conjugate addition and then reducing
New Heterocyclic Monomers and Polymers By Robert B Login
 ew eterocyclic Monomers and Polymers By obert B Login I find the ability to search the literature using Google very stimulating. Who would have thought that you could in a flash search all US patents,
ew eterocyclic Monomers and Polymers By obert B Login I find the ability to search the literature using Google very stimulating. Who would have thought that you could in a flash search all US patents,
Amines. Amines are organic compounds containing a nitrogen functionality. primary secondary tertiary quaternary
 Amines Amines are organic compounds containing a nitrogen functionality Depending upon the number of alkyl, or aryl, groups attached to nitrogen determines its classification, or order 2 primary secondary
Amines Amines are organic compounds containing a nitrogen functionality Depending upon the number of alkyl, or aryl, groups attached to nitrogen determines its classification, or order 2 primary secondary
Synthesis and Sustainable Chemistry
 Synthesis and Sustainable Chemistry Considering % yield and % Atom Economy: high % yield means very efficient conversion from reactants to products increasing % yield means more efficient use of starting
Synthesis and Sustainable Chemistry Considering % yield and % Atom Economy: high % yield means very efficient conversion from reactants to products increasing % yield means more efficient use of starting
Chem 145 Unsaturated hydrocarbons Alkynes
 Dr. Seham ALTERARY Chem 145 Unsaturated hydrocarbons Alkynes Chapter 4 1434-1435 2013-2014 2 st semester By the end of this chapter you should be familiar with: Definition for Alkynes. Nomenclature of
Dr. Seham ALTERARY Chem 145 Unsaturated hydrocarbons Alkynes Chapter 4 1434-1435 2013-2014 2 st semester By the end of this chapter you should be familiar with: Definition for Alkynes. Nomenclature of
Journal of Chemical and Pharmaceutical Research
 Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research ISSN No: 0975-7384 CODEN(USA): JCPRC5 J. Chem. Pharm. Res., 2011, 3(5):519-523 Indole: The molecule of diverse pharmacological
Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research ISSN No: 0975-7384 CODEN(USA): JCPRC5 J. Chem. Pharm. Res., 2011, 3(5):519-523 Indole: The molecule of diverse pharmacological
N-Heterocyclic Carbenes (NHCs)
 N-Heterocyclic Carbenes (NHCs) In contrast to Fischer and Schrock type carbenes NHCs are extremely stable, inert ligands when complexed to a metal centre. Similar to phosphine ligands they are electronically
N-Heterocyclic Carbenes (NHCs) In contrast to Fischer and Schrock type carbenes NHCs are extremely stable, inert ligands when complexed to a metal centre. Similar to phosphine ligands they are electronically
There are several possible arrangements for a molecule which contains two double bonds (diene): 1. Isolated: (two or more single bonds between them)
 1 Chapter 15: Conjugation and Reactions of Dienes I. Introduction to Conjugation There are several possible arrangements for a molecule which contains two double bonds (diene): 1. Isolated: (two or more
1 Chapter 15: Conjugation and Reactions of Dienes I. Introduction to Conjugation There are several possible arrangements for a molecule which contains two double bonds (diene): 1. Isolated: (two or more
Aromatic Compounds. A number of these compounds had a distinct odor. Hence these compounds were called aromatic
 Aromatic Compounds Early in the history of organic chemistry (late 18 th, early 19 th century) chemists discovered a class of compounds which were unusually stable A number of these compounds had a distinct
Aromatic Compounds Early in the history of organic chemistry (late 18 th, early 19 th century) chemists discovered a class of compounds which were unusually stable A number of these compounds had a distinct
- Overview of polymeriza1on catalysis
 - verview of polymeriza1on catalysis Different coordina-on polymeriza-on mechanisms: RP, RMP, (meth)acrylate polymeriza6on, olefin polymeriza6on. Different catalysts: Metal- based catalysts, organic catalysts
- verview of polymeriza1on catalysis Different coordina-on polymeriza-on mechanisms: RP, RMP, (meth)acrylate polymeriza6on, olefin polymeriza6on. Different catalysts: Metal- based catalysts, organic catalysts
Chapter 20 Carboxylic Acid Derivatives Nucleophilic Acyl Substitution
 Chapter 20 Carboxylic Acid Derivatives Nucleophilic Acyl Substitution Nomenclature: In carboxylic acid chlorides, anhydrides, esters and amides, the parent is the carboxylic acid. In each case be sure
Chapter 20 Carboxylic Acid Derivatives Nucleophilic Acyl Substitution Nomenclature: In carboxylic acid chlorides, anhydrides, esters and amides, the parent is the carboxylic acid. In each case be sure
Chapter 8: Chemistry of Alkynes (C n H 2n-2 )
 hapter 8: hemistry of Alkynes ( n 2n-2 ) Bonding & hybridization Both are sp-hybridized Bond angles = 180 o 1 σ + 2 π bonds Linear around lassification R R R' σ bond energy: 88 kcal/mol π bond energy:
hapter 8: hemistry of Alkynes ( n 2n-2 ) Bonding & hybridization Both are sp-hybridized Bond angles = 180 o 1 σ + 2 π bonds Linear around lassification R R R' σ bond energy: 88 kcal/mol π bond energy:
Hyperlearning MCAT Instructor Qualifying Exam Organic Chemistry
 Hyperlearning MCAT Instructor Qualifying Exam Organic Chemistry 30 Questions (5 pages); Time limit = 45 minutes Use of books or notes is not permitted. 1. When analyzed with a polarimeter, which of the
Hyperlearning MCAT Instructor Qualifying Exam Organic Chemistry 30 Questions (5 pages); Time limit = 45 minutes Use of books or notes is not permitted. 1. When analyzed with a polarimeter, which of the
COURSE OBJECTIVES / OUTCOMES / COMPETENCIES.
 COURSE OBJECTIVES / OUTCOMES / COMPETENCIES. By the end of the course, students should be able to do the following: See Test1-4 Objectives/Competencies as listed in the syllabus and on the main course
COURSE OBJECTIVES / OUTCOMES / COMPETENCIES. By the end of the course, students should be able to do the following: See Test1-4 Objectives/Competencies as listed in the syllabus and on the main course
CHEM 203. Final Exam December 15, 2010 ANSWERS. This a closed-notes, closed-book exam. You may use your set of molecular models
 CEM 203 Final Exam December 15, 2010 Your name: ANSWERS This a closed-notes, closed-book exam You may use your set of molecular models This test contains 15 pages Time: 2h 30 min 1. / 16 2. / 15 3. / 24
CEM 203 Final Exam December 15, 2010 Your name: ANSWERS This a closed-notes, closed-book exam You may use your set of molecular models This test contains 15 pages Time: 2h 30 min 1. / 16 2. / 15 3. / 24
Background Information
 ackground nformation ntroduction to Condensation eactions Condensation reactions occur between the α-carbon of one carbonyl-containing functional group and the carbonyl carbon of a second carbonyl-containing
ackground nformation ntroduction to Condensation eactions Condensation reactions occur between the α-carbon of one carbonyl-containing functional group and the carbonyl carbon of a second carbonyl-containing
Outline. Organic Compounds. Overview: Carbon: The Backbone of Life. I. Organic compounds II. Bonding with Carbon III. Isomers IV.
 Chapter 4 Carbon and the Molecular Diversity of Life Outline I. Organic compounds II. Bonding with Carbon III. Isomers IV. Functional Groups Organic Compounds What is organic We think of organic produce
Chapter 4 Carbon and the Molecular Diversity of Life Outline I. Organic compounds II. Bonding with Carbon III. Isomers IV. Functional Groups Organic Compounds What is organic We think of organic produce
COURSE UNIT DESCRIPTION. Dept. Organic Chemistry, Vilnius University. Type of the course unit
 Course unit title Organic Chemistry II Lecturer(s) Rimantas Vaitkus COURSE UNIT DESCRIPTION Department Dept. Organic Chemistry, Vilnius University Cycle First Type of the course unit Mode of delivery Period
Course unit title Organic Chemistry II Lecturer(s) Rimantas Vaitkus COURSE UNIT DESCRIPTION Department Dept. Organic Chemistry, Vilnius University Cycle First Type of the course unit Mode of delivery Period
Lecture Notes Chem 51C S. King Chapter 24 Carbonyl Condensation Reactions
 Lecture Notes Chem 51C S. King Chapter 24 Carbonyl Condensation Reactions I. Reaction of Enols & Enolates with ther Carbonyls Enols and enolates are electron rich nucleophiles that react with a number
Lecture Notes Chem 51C S. King Chapter 24 Carbonyl Condensation Reactions I. Reaction of Enols & Enolates with ther Carbonyls Enols and enolates are electron rich nucleophiles that react with a number
Massachusetts Institute of Technology Organic Chemistry Problem Set 1. Functional Group Transformations Study Guide
 Massachusetts Institute of Technology rganic Chemistry 5.511 Problem Set 1 September, 2007 Prof. Rick L. Danheiser Functional Group Transformations Study Guide The purpose of this three-part study guide
Massachusetts Institute of Technology rganic Chemistry 5.511 Problem Set 1 September, 2007 Prof. Rick L. Danheiser Functional Group Transformations Study Guide The purpose of this three-part study guide
Organic Synthesis III x 1hr Lectures: Michaelmas Term Weeks 5-8 Tues; Thrs at 10am Dyson Perrins lecture theatre
 Organic Synthesis III 2015 8 x 1hr Lectures: Michaelmas Term Weeks 5-8 Tues; Thrs at 10am Dyson Perrins lecture theatre Copies of this handout will be available at http://donohoe.chem.ox.ac.uk/page16/index.html
Organic Synthesis III 2015 8 x 1hr Lectures: Michaelmas Term Weeks 5-8 Tues; Thrs at 10am Dyson Perrins lecture theatre Copies of this handout will be available at http://donohoe.chem.ox.ac.uk/page16/index.html
Suggested solutions for Chapter 34
 s for Chapter 34 34 PRBLEM 1 Predict the structure of the product of this Diels- Alder reaction. C 2 +? 3 Si Can you deal with a moderately complicated Diels- Alder? The diene is electron- rich and will
s for Chapter 34 34 PRBLEM 1 Predict the structure of the product of this Diels- Alder reaction. C 2 +? 3 Si Can you deal with a moderately complicated Diels- Alder? The diene is electron- rich and will
Organic Chemistry II / CHEM 252 Chapter 13 Conjugated Unsaturated Systems
 Organic Chemistry II / CHEM 252 Chapter 13 Conjugated Unsaturated Systems Bela Torok Department of Chemistry University of Massachusetts Boston Boston, MA 1 Introduction - Conjugated unsaturated systems
Organic Chemistry II / CHEM 252 Chapter 13 Conjugated Unsaturated Systems Bela Torok Department of Chemistry University of Massachusetts Boston Boston, MA 1 Introduction - Conjugated unsaturated systems
Chem 263 Nov 24, Properties of Carboxylic Acids
 Chem 263 ov 24, 2009 Properties of Carboxylic Acids Since carboxylic acids are structurally related to both ketones and aldehydes, we would expect to see some similar structural properties. The carbonyl
Chem 263 ov 24, 2009 Properties of Carboxylic Acids Since carboxylic acids are structurally related to both ketones and aldehydes, we would expect to see some similar structural properties. The carbonyl
Chapter 13 Conjugated Unsaturated Systems
 Chapter 13 Conjugated Unsaturated Systems Introduction Conjugated unsaturated systems have a p orbital on a carbon adjacent to a double bond The p orbital can come from another double or triple bond The
Chapter 13 Conjugated Unsaturated Systems Introduction Conjugated unsaturated systems have a p orbital on a carbon adjacent to a double bond The p orbital can come from another double or triple bond The
Chapter 27 Pericyclic Reactions
 Instructor Supplemental Solutions to Problems 2010 Roberts and Company Publishers Chapter 27 Pericyclic Reactions Solutions to In-Text Problems 27.1 (b) This is a sigmatropic reaction; two electrons are
Instructor Supplemental Solutions to Problems 2010 Roberts and Company Publishers Chapter 27 Pericyclic Reactions Solutions to In-Text Problems 27.1 (b) This is a sigmatropic reaction; two electrons are
Synthesis of Resorcinylic Macrolides
 Synthesis of Resorcinylic Macrolides X H H H H Cl X= Radicicol (1) X= CH2 Cycloproparadicicol (2) Danishefsky, S. J. J. Am. Chem. Soc. 2004, 126, ASAP Danishefsky, S. J. rg. Lett. 2004, 6, 413-416 Danishefsky,
Synthesis of Resorcinylic Macrolides X H H H H Cl X= Radicicol (1) X= CH2 Cycloproparadicicol (2) Danishefsky, S. J. J. Am. Chem. Soc. 2004, 126, ASAP Danishefsky, S. J. rg. Lett. 2004, 6, 413-416 Danishefsky,
Chapter 13 Alkenes and Alkynes & Aromatic Compounds
 Chapter 13 Alkenes and Alkynes & Aromatic Compounds Chapter Outline 13.1 Alkenes and Alkynes 13.2 Nomenclature of Alkenes and Alkynes 13.3 Cis Trans Isomers 13.4 Alkenes in Food and Medicine 13.6 Reactions
Chapter 13 Alkenes and Alkynes & Aromatic Compounds Chapter Outline 13.1 Alkenes and Alkynes 13.2 Nomenclature of Alkenes and Alkynes 13.3 Cis Trans Isomers 13.4 Alkenes in Food and Medicine 13.6 Reactions
Organic Chemistry. M. R. Naimi-Jamal. Faculty of Chemistry Iran University of Science & Technology
 Organic Chemistry M. R. Naimi-Jamal Faculty of Chemistry Iran University of Science & Technology Chapter 4-3: Continue Alkynes: An Introduction to Organic Synthesis Based on: McMurry s Organic Chemistry,
Organic Chemistry M. R. Naimi-Jamal Faculty of Chemistry Iran University of Science & Technology Chapter 4-3: Continue Alkynes: An Introduction to Organic Synthesis Based on: McMurry s Organic Chemistry,
Synthesis of coumarins by ring-closing metathesis*
 Pure Appl. Chem., Vol. 75, No. 4, pp. 421 425, 2003. 2003 IUPAC Synthesis of coumarins by ring-closing metathesis* Arnab K. Chatterjee, F. Dean Toste, Steven D. Goldberg, and Robert H. Grubbs Arnold and
Pure Appl. Chem., Vol. 75, No. 4, pp. 421 425, 2003. 2003 IUPAC Synthesis of coumarins by ring-closing metathesis* Arnab K. Chatterjee, F. Dean Toste, Steven D. Goldberg, and Robert H. Grubbs Arnold and
What is in Common for the Following Reactions, and How Do They Work?
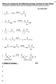 What is in Common for the Following Reactions, and ow Do They Work? You should eventually be able to draw the mechanism for these (and other) reactions 13 Key Intermediate 1 Br-Br Na Br 2 C 3 -I Me NaMe
What is in Common for the Following Reactions, and ow Do They Work? You should eventually be able to draw the mechanism for these (and other) reactions 13 Key Intermediate 1 Br-Br Na Br 2 C 3 -I Me NaMe
Aromatic Hydrocarbons
 Aromatic Hydrocarbons Aromatic hydrocarbons contain six-membered rings of carbon atoms with alternating single and double carbon-carbon bonds. The ring is sometimes shown with a circle in the center instead
Aromatic Hydrocarbons Aromatic hydrocarbons contain six-membered rings of carbon atoms with alternating single and double carbon-carbon bonds. The ring is sometimes shown with a circle in the center instead
Curriculum Vitae. Professional Experiences Department of Chemistry Indian Institute of Science Education and Research Pune , India
 Dr. B. Gnanaprakasam Curriculum Vitae Assistant Professor Department of Chemistry Indian Institute of Science Education and Research (IISER) Pune-411021, India E-mail: gnanaprakasam@iiserpune.ac.in, gnanam21@gmail.com
Dr. B. Gnanaprakasam Curriculum Vitae Assistant Professor Department of Chemistry Indian Institute of Science Education and Research (IISER) Pune-411021, India E-mail: gnanaprakasam@iiserpune.ac.in, gnanam21@gmail.com
Introduction to Organic Chemistry. Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display.
 Introduction to Organic Chemistry Copyright The McGraw-ill Companies, Inc. Permission required for reproduction or display. 1 Common Elements in Organic Compounds 2 Classification of ydrocarbons ydrocarbons
Introduction to Organic Chemistry Copyright The McGraw-ill Companies, Inc. Permission required for reproduction or display. 1 Common Elements in Organic Compounds 2 Classification of ydrocarbons ydrocarbons
Mechanistic Studies of Allylsilane Rearrangement
 chanistic Studies of Allylsilane Rearrangement Thesis: The goal of our project is to determine the operating mechanism in the transformation of α-siloxy allylsilanes to vinyl silanes. Elucidating the mechanism
chanistic Studies of Allylsilane Rearrangement Thesis: The goal of our project is to determine the operating mechanism in the transformation of α-siloxy allylsilanes to vinyl silanes. Elucidating the mechanism
Solution problem 22: Non-Benzoid Aromatic Sytems
 Solution problem 22: on-enzoid Aromatic Sytems 22.1 & 22.2 Each double bond and each heteroatom (, ) with lone pairs donates 2 π- electrons as well as a negative charge. oron or a positive charge does
Solution problem 22: on-enzoid Aromatic Sytems 22.1 & 22.2 Each double bond and each heteroatom (, ) with lone pairs donates 2 π- electrons as well as a negative charge. oron or a positive charge does
Chapter 22 Hydrocarbon Compounds
 Chapter 22 Hydrocarbon Compounds 1 ORGANIC COMPOUNDS Organic compounds are carbon compounds and there are over a million. The simplest organic compounds are hydrocarbons and they are composed of hydrogen
Chapter 22 Hydrocarbon Compounds 1 ORGANIC COMPOUNDS Organic compounds are carbon compounds and there are over a million. The simplest organic compounds are hydrocarbons and they are composed of hydrogen
CHAPTER 9 THEORY OF RESONANCE BY, G.DEEPA
 CHAPTER 9 THEORY OF RESONANCE BY, G.DEEPA Conjugation in Alkadienes and Allylic Systems conjugation a series of overlapping p orbitals The Allyl Group allylic position is the next to a double bond 1 allyl
CHAPTER 9 THEORY OF RESONANCE BY, G.DEEPA Conjugation in Alkadienes and Allylic Systems conjugation a series of overlapping p orbitals The Allyl Group allylic position is the next to a double bond 1 allyl
Reactions at α-position
 Reactions at α-position In preceding chapters on carbonyl chemistry, a common reaction mechanism observed was a nucleophile reacting at the electrophilic carbonyl carbon site NUC NUC Another reaction that
Reactions at α-position In preceding chapters on carbonyl chemistry, a common reaction mechanism observed was a nucleophile reacting at the electrophilic carbonyl carbon site NUC NUC Another reaction that
