0.5. Dip. Freq. HkHzL D HppmL
|
|
|
- Madeleine Davidson
- 5 years ago
- Views:
Transcription
1 D HppmL 3-2 Dip. Freq. HkHzL Figure 5-11: Graph of the CT function for the Ix self-magnetization, when σ()=ix for an oriented sample,, as a function of the dipolar coupling frequency in the lab frame in khz, and the chemical shift difference in the lab frame where t=3ms,and the CS average frequency in the lab frame is set as Σ =5 ppm. The scalar coupling J=. 135
2 S HppmL 3-2 Dip. Freq. HkHzL Figure 5-12: Graph of the CT function for the Ix self-magnetization for an oriented sample,, when σ()=ix, as a function of the dipolar coupling frequency in the lab frame in khz, and the chemical shift average Σ in the lab frame where t=3ms,and the CS average frequency in the lab frame is set as =5ppm; The scalar coupling J=. 136
3 time HmsL 3-2 Dip. Freq. HkHzL Figure 5-13: Graph of the CT function for the Ix self-magnetization for an oriented sample,, when σ()=ix, as a function of the dipolar coupling frequency in the lab frame in khz, and the chemical shift difference is set to =12ppm and the CS average frequency (both in the lab frame) is set as Σ =8 ppm. The scalar coupling J=. Time is in ms. 137
4 There are several characteristics in the CT from the transverse self-magnetization term that can be deduced from Eq. 5-23a. The first term, which we will call the Ix even term cos[.5bt]cos[σt] cos[.5at] contains the B-term dipolar coefficient in a cosine as frequency.5b, modulated by a cosine dependent on the average of the chemical shift frequencies. It this term which retains the original dipolar coupling frequency in the cosine. The cos[σt] contribution from the chemical shift modifies the usual DCT form of the CT coefficients The second term in Eq. 5-23a is equal to /R cos[.5at] sin[.5bt] sin [Σt]. This will be called the Ix odd term as it is a function dependent on two sine terms which mix the chemical shift frequencies into a sine function of the dipolar coupling that is not present in the evolution of coherence from H D (see Chapter 4, specifically Table 4-1a and b). The odd term in Ix has an amplitude modulated by the coefficient /R which represents the contribution of the chemical shift difference to the tilted frame. This amplitude acts to modulate the contribution of the second term in Eq. 5-23a, so that a small value of results in only small contributions of the Ix odd term. The Ix coefficient is dominated by the Ix even term for small values of, while the odd term will contribute significantly for larger values of. Essentially, the Ix odd term results from the mixing of the chemical shift interaction into the coupling Hamiltonian. Figure 5-3 graphs the dependence of /R as a function of dipolar coupling and, for when J= and J=53Hz. This represents the contribution of the second term in Eq. 5-23a over a range of dipolar coupling values important in 13 C homonuclear experiments on peptides. The range of, representing the average of the chemical shifts in ppm, was chosen to illustrate the fast rise to full contribution; greater values of follow an asymptotic rise towards the value of 1. The even and odd transfer components of the Ix Ix transfer coefficient are graphed in Figure 5-4a and 5-4b as a function of and Σ to show the even and odd dependencies on both Σ and. While acts as the contribution of the Ix odd term to the 138
5 total coherence transfer, Σ acts as a superposing frequency over the DCT frequencies usually encountered during pure dipolar coupling. The odd component in Ix also contains a sine term for the chemical shift contribution as well. As the difference in laboratory-frame chemical shifts ( ) becomes smaller, that is, as the chemical shifts become equal, the sine term goes to zero as the term /R and the total coherence transfer approaches H T Ix Ixcos[ At ]cos[ Bt ]cos[ ω cst] + o. t. (5-24a) Ix 2 2 H T ω cst] + o. t. (5-24b) Sxsin[ At ]sin[ Bt ]cos[ 2 2 which are the expected dipolar coherence transfer terms multiplied by the average of the chemical shifts in the laboratory frame; since they are identical, it will be simply the shared chemical shift ω cs modulating the dipolar coherence transfer as a multiplicative frequency. This special case only occurs for nuclei sharing the same chemical shift. Defining the dipolar coherence transfer frequency to represent two nuclei separated by 1.53 Å, the coherence transfer for the Ix odd term can be graphed as a function of both chemical shift frequencies in the laboratory frame in Figure 5-4. One can also vary the dipolar coupling and hold either or Σ constant to examine the coupling for either the Ix odd term (Figures 5-5 and 5-6) and the Ix even term (Figure 5-7) as well as the Sx coefficient (Figures 5-8 through 5-1). It can be observed that each variable changes the value of the coherence transfer, making the value of the coherence transfer a five-variable function involving, Σ, t, D, and J. The behavior of the entire coherence transfer as a function of chemical shift values for the self magnetization Ix coefficient is a sum of the Ix odd and even terms, and is therefore heavily dependent on the value of the chemical shift difference. Figure 5-11 plots the complete form of the Ix Ix coherence transfer for various values of the dipolar coupling against the chemical shift difference, setting Σ constant at an arbitrary 139
6 5ppm, J at zero, and time at an arbitrary 1ms, which allows for easy viewing of the - dependent trends in the self-magnetization. It is obvious that the value of does modulate the contribution of the two terms to the CT. Additionally, Figure 5-12 shows the same plot for the variation in Σ, which acts as an additional modulation of the usual dipolar coherence transfer functions. Figure 5-13 gives the total coherence transfer as a function of time and dipolar coupling for arbitrary values of and Σ. Compare this result to Figure 3-2a which has no chemical shift component. Even small values of the chemical shift can significantly change the behavior of the coherence transfer. The transverse case of coherence transfer is much more complicated than the longitudinal case. The coherence transfer for the longitudinal case, Equation 5-25, for the Iz magnetization coefficient: σ()=iz H t R +? + B 2R ( 2 cos[bt] )Iz + o.t. (5-25) under the total Hamiltonian results in constant terms in R and which predict that, as the chemical shift increases in comprison to the dipolar coupling frequency, longitudinal coherence transfer will disappear. When one sets the chemical shift to zero, the Iz coefficient in Eq reduces to 2 2 B + B cos[bt] 1 ( ) = (1 + cos[bt]) (5-26) 2 2B 2 and returns the proper coefficient as seen for longitudinal coherence transfer under just H D (see Table 4-1). Likewise, when the dipolar coherence transfer is zero, the coefficient reduces to 1, meaning there is no coherence transfer for σ()=iz as expected. The chemical shift acts as an additional attenuating factor for Iz. Figure 5-5 shows the dependence of the coherence transfer of Iz on the chemical shift difference in an oriented sample. Figure 5-14 and 5-15 graph the Iz and Sz coherence transfer respectively as a function of time and chemical shift difference. Figures 5-16 and 5-17 correspond to a CT as a function of dipolar coupling and time for Iz and Sz when σ()=iz. 14
7 The most apparent effect of appreciable chemical shift to the dipolar coupling in the longitudinal case is the dwindling of longitudinal coherence transfer between spins I and S. Looking, for example, at Figure 5-14, it is apparent that as grows in magnitude, the system loses its ability to transfer coherence. This also predicts that nuclei with the same chemical shift, that is, when = will have the same coherence transfer behavior as a system with no chemical shift present, as there is no Σ-dependence within the longitudinal functions. Examining Figures 5-16 and 5-17 for a single time constant of t=5ms, it is apparent that the CT is rapidly lost with an increase in as compared to the dipolar coupling constant. Again, this shows that even over various dipolar coupling constants typical of 13 C experiments, CT is significantly reduced at t=5ms when takes on the small value of 2ppm. 141
8 time HmsL 2 2 DHppmL 1 1 Figure 5-14: Graph of the CT function for the Iz self-magnetization for an oriented sample, when σ()=iz, as a function of time and the chemical shift difference in ppm. The dipolar coupling is set to correspond to an internuclear distance of 1.53 Å. The scalar coupling J=. Time is in ms. 142
9 time HmsL 2 2 DHppmL 1 1 Figure 5-15: Graph of the CT function for the Sz transferred-magnetization for an oriented sample, when σ()=iz, as a function of time and the chemical shift difference in ppm. The dipolar coupling is set to correspond to an internuclear distance of 1.53 Å. The scalar coupling J=. Time is in ms. 143
10 dip. coupling HkHzL -2 2 DHppmL 1-3 Figure 5-16: Graph of the CT function for the Iz self-magnetization for an oriented sample, when σ()=iz, as a function of dipolar coupling frequency in khz and the chemical shift difference in ppm. The constant time is set to 5ms. The scalar coupling J=. 144
11 dip. coupling HkHzL -2 2 DHppmL 1-3 Figure 5-17: Graph of the CT function for the Sz transferred coherence for an oriented sample, when σ()=iz, as a function of dipolar coupling frequency in khz and the chemical shift difference in ppm. The constant time is set to 5ms. The scalar coupling J=. 145
12 The program IS.F was written (See Appendix A) which uses numerical calculations with the Wigner transformation matrices to transform the principal-axis system frequencies as in Equation 5-16 and then integrating over all possible angles in the longitudinal and transverse magnetization functions to simulate a powder averaging effect. For longitudinal coherence transfer, simulations were done with parameters smaller in magnitude than or equal to those used for transverse magnetization modeling. Except for the very smallest anisotropies, the longitudinal equation was unable to transfer coherence; that is, the self-magnetization of spin I in state Iz remained constant over a sample time of up to 1ms. We therefore set out to model the transverse magnetization transfer exclusively. The first step was to verify that the output of the program matched the case of pure DCT. Simulations were done with zero chemical shift as well as with equal chemical shifts. In the case of Iz -> Iz simulation, the sample returned the identical result to the pure dipolar recoupling case, as did the Ix simulation, both verified with comparison against the output of the POWDER.F program, also in the Appendix.. Simulations were performed with the FORTRAN program IS.F (see Appendix A) using inputs of: Euler angles from the spin 2 CS frame and for the spin 2 CS frame to the molecular frame, chemical shift principal values σ 11, σ 22, and σ 33 for both spin 1 and spin 2, magic-angle sample spinning parameters, rotor tilt, dipolar coupling, and spin coupling. We chose an arbitrary C α -C O distance of 1.55 Å. Figs through 5-23 all model the transverse coherence transfer in an unoriented sample for various parameters. Because the simulation is dependent on 22 separate parameters, an exhaustive examination of often-similar results would result in repetition. Instead, we look at various cases in each simulation run to see trends in the behaviors of the variables within the unoriented system simulations. 146
13 Varying the angles between the chemical shift PAS frame and the molecular frame had a noticeable effect on the rapid decay of coherence in the unoriented sample simulation. Adjusting the values of σ 11 =σ 22 =σ 33 for both spin I and spin S also made (expected) differences in the coherence transfer patterns. Figure 5-18 is a comparison between the conditions of no chemical shift (solid line), a small isotropic chemical shift in both nuclei (dashed line), and a small anisotropy of 5ppm in nucleus 1 (dashed-dot line). The left shoulder of the plot, falling from 1% magnetization (normalized to 1), can be followed for each of the examples as a measure for the rate of coherence transfer from spin I to spin S. The case of non-zero chemical shift yields a powder pattern familiar from Chapter 4 (Fig 4-1a) and is used as a comparison. The other two traces, those with nonzero chemical shift, show that the addition of chemical shift either aids in the loss of coherence due to a faster transverse dephasing, or actually increases the rate of coherent transfer to the second spin. To examine which case it might be, we model the response of the second spin, Sx, whose coefficient (from Eq. 5-21) is B At Bt cos( Σ t) sin( )sin( ) (5-27) 2 2 R Figure 5-19 models the case of the Sx magnetization when the initial state σ() = Ix. Although it is understood that introduction of a chemical shift interaction reduces the transfer of transverse coherent magnetization, it was pleasant to see that the analytical solution yielded the same results. It can be seen that even for a small chemical shift (1 ppm) in the two nuclei, the addition of chemical shift has decreased both the magnitude of the resulting transverse magnetization and the speed at which it is transferred, as the shoulder obviously lags behind that for the pure coupling Hamiltonian (solid line). Adding a small anisotropy (3 ppm) to the sample does change the phase pattern of the curve, but does not appreciably impact the coherence transfer rate. Examining Eq once again, it can be seen that the greater the difference in the chemical shifts of the two nuclei, at each angle in the crystallite sampling, the smaller the 147
14 term B/R will become, reducing the coherence transfer magnitude for Sx, eventually by the reciprocal of the chemical shift difference. For Sx, the term cos(σt), sampled for each crystallite orientation, will also begin to dominate the powder pattern for greater values of Σ, resulting in a wider range of frequencies and therefore a faster decay of transverse magnetization. In comparison, as increases, the Ix self-magnetization gains the full contribution of the "odd term." If the difference in chemical shift decreases transverse coherent transfer of magnetization, then where does the coherence go? Comparing Eq to the results of Chapter 3 shows that new coherences arise as compared to the simple case of DCT free of chemical shift. The operators that appear that seem "out of the ordinary" are Iy, Sy, IxSz, all of which operators are outside the coherent transfer group discussed in Chapter 2. In fact, the cylindrical symmetry of DCT is broken by any anisotropy in the chemical shift, which manifests as the term. This symmetry breaking reduces the coherent transfer of magentization since the transfer is no longer contained in the cyclical groups shown in Table 2-1. Therefore, the effect of anisotropies in the chemical shift act to break the symmetry of cylindrical mixing present in the dipolar Hamiltonian. The chemical shift is a transverse interaction which breaks the cylindrical symmetry apparent in the spin operators of H D. As the chemical shift difference increases to a magnitude much larger than the dipolar coupling term B (Eq. 5-19f), the coherence becomes shared equally among the antiphase operators (-IySz, IxSz) and the transverse operators (Iy, Ix). The fact that these operators dominate, and longitudinal CT is impossible, means that the effects of chemical shift act as a kind of dissapative rip in the symmetry present in the dipolar Hamiltonian. Zero- and double-quantum operators cannot be generated during free evolution in the presence of chemical shift, unlike in the case of dipolar coupling where the Iz group of Table 2-5 has IxSy and IySx as members. Therefore, magnetization cannot be "stored" in unobservable operators when DCT operates in the presence of chemical shift. 148
15 Figure 5-18: Graph of the CT function for the Ix self-magnetization of an unoriented sample, when σ()=ix, as a function of time. The parameters are given below. All three simulations share the listed constants, with specific principal values given below: The Euler angles were chosen arbitrarily. Note that the chaotic behavior of the dephasing is not due to the limits of resolution. Shared: 6., 6727., 1.55,. H larmor magnet, gamma, internuc dist in angs, J in Hz 4, 3., 2 Steps in angle summation, sim. time (ms), graph points 1., 75., 5. Euler angles from cs PAS to dipolar frame for spin I 2., 145.,9 Euler angles from cs PAS to dipolar frame for spin S.1,54.7,. Zero, rotor-b angle, wr*t both in degrees from rotor to lab Solid line:.,.,. σ11, σ22, σ33 for spin I (ppm).,., σ11, σ22, σ33 for spin S (ppm) Dashed line: 15.,1.,1. σ11, σ22, σ33 for spin I (ppm) 1.,1.,1 σ11, σ22, σ33 for spin S (ppm) Dashed-dot line: 1.,1.,1. σ11, σ22, σ33 for spin I (ppm) 1.,1.,1 σ11, σ22, σ33 for spin S (ppm) 149
16 Figure 5-19: Graph of the CT function for the Sx (transferred) self-magnetization for an unoriented sample, when σ()=ix, as a function of time. The parameters are given below. Note that the chaotic behavior of the dephasing is not due to the limits of resolution. Euler angles were chosen arbitrarily. Note that the chaotic behavior of the dephasing is not due to the limits of resolution. Both simulations use the following parameters, with the differences given below. 6., 6727., 1.55,. H larmor magnet, gamma, internuc dist in angs, J in Hz 5, 1., 5 Steps in angle summation, sim. time (ms), graph points. 1., 75., 5. Euler angles from cs PAS to dipolar frame for spin I 2., 145.,9 Euler angles from cs PAS to dipolar frame for spin S., 54.7,. Zero, rotor to B angle, wr*t both in degrees from rotor to lab Solid line:.,.,. σ11, σ 22, σ33 for spin I.,.,..σ11, σ22, σ33 for spin S Dashed line: 13.,1.,1. σ11, σ 22, σ33 for spin I 13.,1.,1..σ11, σ22, σ33 for spin S Dashed-dot line: 1.,1.,1. σ11, σ 22, σ33 for spin I 1.,1.,1..σ11, σ22, σ33 for spin S 15
17 Figure 5-2: Graph of the CT function for the Ix self-magnetization for an unoriented sample, when σ()=ix, as a function of time for two sets of principle values of the chemical shift. The parameters are given below. Note that the chaotic behavior of the dephasing is not due to the limits of resolution. All lines have the following values in common: 6., 6727., 1.55,. H larmor magnet, gamma, internuc dist in angs, J in Hz. 4,.5,2 Steps in angle summation, sim. time (ms), time steps Euler angles from cs PAS to dipolar frame for spin I Euler angles from cs PAS to dipolar frame for spin S.,.,. Zero, rotor to B angle, wr*t both in degrees from rotor to lab Solid line: 1.,1.,1. σ11, σ22, σ33 for spin I (ppm) 1.,1.,1 σ11, σ22, σ33 for spin S (ppm) Dashed line: σ11, σ22, σ33 for spin I (ppm) σ11, σ22, σ33 for spin S (ppm) 151
18 * Figure 5-21: Graph of the CT function for the Ix self-magnetization for an unoriented sample, when σ()=ix, as a function of time for three sets of principal values of the chemical shift. The parameters are given below. Note that the chaotic behavior of the dephasing is not due to the limits of resolution. All lines have the following values in common: 6., 6727., 1.55,. H larmor magnet, gamma, internuc dist in angs, J in Hz. 4,.5,2 Steps in angle summation, sim. time (ms), time steps Euler angles from cs PAS to dipolar frame for spin I Euler angles from cs PAS to dipolar frame for spin S.,.,. Zero, rotor to B angle, wr*t both in degrees from rotor to lab Solid line: 1.,1.,1. σ11, σ22, σ33 for spin I (ppm) 1.,1.,1 σ11, σ22, σ33 for spin S (ppm) Dashed line: 1.,1.,5. σ11, σ22, σ33 for spin I (ppm) 1.,1.,5 σ11, σ22, σ33 for spin S (ppm) Dashed -dot line (marked with * and the fastest decay) σ11, σ22, σ33 for spin I (ppm) σ11, σ22, σ33 for spin S (ppm) 152
19 Figure 5-22: Graph of the CT function for the Ix self-magnetization for an unoriented sample, when σ()=ix, as a function of time for three sets of principal values of the chemical shift. The parameters are given below. Note that the chaotic behavior of the dephasing is not due to the limits of resolution. All lines have the following values in common: 6., 6727., 1.55,. H larmor magnet, gamma, internuc dist in angs, J in Hz. 4,.5,2 Steps in angle summation, sim. time (ms), time steps Euler angles from cs PAS to dipolar frame for spin I Euler angles from cs PAS to dipolar frame for spin S.,.,. Zero, rotor to B angle, wr*t both in degrees from rotor to lab Solid line: 1,1.,1. σ11, σ22, σ33 for spin I (ppm) 1.,1.,1 σ11, σ22, σ33 for spin S (ppm) Dashed line: 1.,1.,1. σ11, σ22, σ33 for spin I (ppm) 1.,1.,1 σ11, σ22, σ33 for spin S (ppm) Dashed -dot line σ11, σ22, σ33 for spin I (ppm) σ11, σ22, σ33 for spin S (ppm) 153
20 The anisotropy of chemical shift seems to affect the rapidity of dephasing. Figure 5-2 is an example of extreme variations in the anisotropy between two nuclei. It can be easily seen that while the pattern of evolution is affected, the added anisotropy causes a rapid loss of coherence in the Ix self-magnetization. Figure 5-21 gives an example of the change in coherence transfer when only one set of anisotropies (along σ zz ) is varied. The coherence loss in Ix becomes more rapid for greater values of σ zz. Fig 5-22 shows that the variation of the most anisotropic CS principal value does not change the rate of coherence loss in Ix, which can be seen by the decay of the shoulder to the left of the graph. This leads to the conclusion that the same isotropic chemical shift with the same angles between the chemical shift PAS and the dipolar PAS will yield the same coherence transfer rate, no matter what the individual principal axis values might be. Conclusions This work has given the simple analytical results for the coherence transfer in the presence of chemical shift. Examination of the analytical equations sheds light on the reasons for many well-known experimental facts. Dipolar coherence transfer in the presence of chemical shift is much reduced. Even in oriented samples, the rapid dephasing due to (even small) anisotropic chemical shifts can cause coherence transfer to be rapidly lost as we have seen in the simulations earlier in this chapter. The dephasing in unoriented samples with small chemical shifts is of sufficient rapidity to cause the dipolar coherence transfer to be rapidly lost to dephasing processes. The solution, as always, involves experimentally maniplulating the chemical shift. This is especially true when attempting to transfer longitudinal 154
21 magnetization, which loses coherence transfer ability for even small values of the chemical shift. 155
CHAPTER 5 DIPOLAR COHERENCE TRANSFER IN THE PRESENCE OF CHEMICAL SHIFT FOR UNORIENTED AND ORIENTED HOMONUCLEAR TWO SPIN-½ SOLID STATE SYSTEMS
 CHAPTE 5 DIPOLA COHEENCE TANSFE IN THE PESENCE OF CHEMICAL SHIFT FO UNOIENTED AND OIENTED HOMONUCLEA TWO SPIN-½ SOLID STATE SYSTEMS Introduction In this chapter, coherence transfer is examined for the
CHAPTE 5 DIPOLA COHEENCE TANSFE IN THE PESENCE OF CHEMICAL SHIFT FO UNOIENTED AND OIENTED HOMONUCLEA TWO SPIN-½ SOLID STATE SYSTEMS Introduction In this chapter, coherence transfer is examined for the
to refine, or to explore the limitations of, existing sequences. spins. In this paper, we will employ theoretical tools to explore various modes of
 study is also important for the design of better dipolar recovery pulse sequences as well to refine, or to explore the limitations of, existing sequences. Because of the nature of the dipolar interaction
study is also important for the design of better dipolar recovery pulse sequences as well to refine, or to explore the limitations of, existing sequences. Because of the nature of the dipolar interaction
An introduction to Solid State NMR and its Interactions
 An introduction to Solid State NMR and its Interactions From tensor to NMR spectra CECAM Tutorial September 9 Calculation of Solid-State NMR Parameters Using the GIPAW Method Thibault Charpentier - CEA
An introduction to Solid State NMR and its Interactions From tensor to NMR spectra CECAM Tutorial September 9 Calculation of Solid-State NMR Parameters Using the GIPAW Method Thibault Charpentier - CEA
6 NMR Interactions: Zeeman and CSA
 6 NMR Interactions: Zeeman and CSA 6.1 Zeeman Interaction Up to this point, we have mentioned a number of NMR interactions - Zeeman, quadrupolar, dipolar - but we have not looked at the nature of these
6 NMR Interactions: Zeeman and CSA 6.1 Zeeman Interaction Up to this point, we have mentioned a number of NMR interactions - Zeeman, quadrupolar, dipolar - but we have not looked at the nature of these
4 Spin-echo, Spin-echo Double Resonance (SEDOR) and Rotational-echo Double Resonance (REDOR) applied on polymer blends
 4 Spin-echo, Spin-echo ouble Resonance (SEOR and Rotational-echo ouble Resonance (REOR applied on polymer blends The next logical step after analyzing and concluding upon the results of proton transversal
4 Spin-echo, Spin-echo ouble Resonance (SEOR and Rotational-echo ouble Resonance (REOR applied on polymer blends The next logical step after analyzing and concluding upon the results of proton transversal
Principios Básicos de RMN en sólidos destinado a usuarios. Gustavo Monti. Fa.M.A.F. Universidad Nacional de Córdoba Argentina
 Principios Básicos de RMN en sólidos destinado a usuarios Gustavo Monti Fa.M.A.F. Universidad Nacional de Córdoba Argentina CONTENIDOS MODULO 2: Alta resolución en sólidos para espines 1/2 Introducción
Principios Básicos de RMN en sólidos destinado a usuarios Gustavo Monti Fa.M.A.F. Universidad Nacional de Córdoba Argentina CONTENIDOS MODULO 2: Alta resolución en sólidos para espines 1/2 Introducción
Biochemistry 530 NMR Theory and Practice
 Biochemistry 530 NMR Theory and Practice Gabriele Varani Department of Biochemistry and Department of Chemistry University of Washington 1D spectra contain structural information.. but is hard to extract:
Biochemistry 530 NMR Theory and Practice Gabriele Varani Department of Biochemistry and Department of Chemistry University of Washington 1D spectra contain structural information.. but is hard to extract:
Product Operator Formalism: A Brief Introduction
 Product Operator Formalism: A Brief Introduction Micholas D. Smith Physics Department, Drexel University, Philadelphia, PA 19104 May 14, 2010 Abstract Multiple spin systems allow for the presence of quantum
Product Operator Formalism: A Brief Introduction Micholas D. Smith Physics Department, Drexel University, Philadelphia, PA 19104 May 14, 2010 Abstract Multiple spin systems allow for the presence of quantum
Chem8028(1314) - Spin Dynamics: Spin Interactions
 Chem8028(1314) - Spin Dynamics: Spin Interactions Malcolm Levitt see also IK m106 1 Nuclear spin interactions (diamagnetic materials) 2 Chemical Shift 3 Direct dipole-dipole coupling 4 J-coupling 5 Nuclear
Chem8028(1314) - Spin Dynamics: Spin Interactions Malcolm Levitt see also IK m106 1 Nuclear spin interactions (diamagnetic materials) 2 Chemical Shift 3 Direct dipole-dipole coupling 4 J-coupling 5 Nuclear
Spin Interactions. Giuseppe Pileio 24/10/2006
 Spin Interactions Giuseppe Pileio 24/10/2006 Magnetic moment µ = " I ˆ µ = " h I(I +1) " = g# h Spin interactions overview Zeeman Interaction Zeeman interaction Interaction with the static magnetic field
Spin Interactions Giuseppe Pileio 24/10/2006 Magnetic moment µ = " I ˆ µ = " h I(I +1) " = g# h Spin interactions overview Zeeman Interaction Zeeman interaction Interaction with the static magnetic field
Author(s) Takegoshi, K; Nakamura, S; Terao, T.
 Title C-13-H-1 dipolar-driven C-13-C-13 r irradiation in nuclear magnetic res Author(s) Takegoshi, K; Nakamura, S; Terao, T Citation JOURNAL OF CHEMICAL PHYSICS (2003), 2341 Issue Date 2003-02-01 URL http://hdl.handle.net/2433/49979
Title C-13-H-1 dipolar-driven C-13-C-13 r irradiation in nuclear magnetic res Author(s) Takegoshi, K; Nakamura, S; Terao, T Citation JOURNAL OF CHEMICAL PHYSICS (2003), 2341 Issue Date 2003-02-01 URL http://hdl.handle.net/2433/49979
Sideband Patterns from Rotor-Encoded Longitudinal Magnetization in MAS Recoupling Experiments
 Journal of Magnetic Resonance 146, 140 156 (2000) doi:10.1006/jmre.2000.2119, available online at http://www.idealibrary.com on Sideband Patterns from Rotor-Encoded Longitudinal Magnetization in MAS Recoupling
Journal of Magnetic Resonance 146, 140 156 (2000) doi:10.1006/jmre.2000.2119, available online at http://www.idealibrary.com on Sideband Patterns from Rotor-Encoded Longitudinal Magnetization in MAS Recoupling
NMR Dynamics and Relaxation
 NMR Dynamics and Relaxation Günter Hempel MLU Halle, Institut für Physik, FG Festkörper-NMR 1 Introduction: Relaxation Two basic magnetic relaxation processes: Longitudinal relaxation: T 1 Relaxation Return
NMR Dynamics and Relaxation Günter Hempel MLU Halle, Institut für Physik, FG Festkörper-NMR 1 Introduction: Relaxation Two basic magnetic relaxation processes: Longitudinal relaxation: T 1 Relaxation Return
Second Order Effects, Overtone NMR, and their Application to Overtone Rotary Recoupling of 14 N- 13 C Spin Pairs under Magic-Angle-Spinning
 Second Order Effects, Overtone NMR, and their Application to Overtone Rotary Recoupling of 14 N- 13 C Spin Pairs under Magic-Angle-Spinning 1. The NMR Interactions NMR is defined by a series of interactions,
Second Order Effects, Overtone NMR, and their Application to Overtone Rotary Recoupling of 14 N- 13 C Spin Pairs under Magic-Angle-Spinning 1. The NMR Interactions NMR is defined by a series of interactions,
Solid-state NMR of spin > 1/2
 Solid-state NMR of spin > 1/2 Nuclear spins with I > 1/2 possess an electrical quadrupole moment. Anisotropic Interactions Dipolar Interaction 1 H- 1 H, 1 H- 13 C: typically 50 khz Anisotropy of the chemical
Solid-state NMR of spin > 1/2 Nuclear spins with I > 1/2 possess an electrical quadrupole moment. Anisotropic Interactions Dipolar Interaction 1 H- 1 H, 1 H- 13 C: typically 50 khz Anisotropy of the chemical
Cross Polarization 53 53
 Cross Polarization 53 Why don t we normally detect protons in the solid-state BPTI Strong couplings between protons ( >20kHz) Homogeneous interaction Not readily averaged at moderate spinning speeds Rhodopsin
Cross Polarization 53 Why don t we normally detect protons in the solid-state BPTI Strong couplings between protons ( >20kHz) Homogeneous interaction Not readily averaged at moderate spinning speeds Rhodopsin
Chem 325 NMR Intro. The Electromagnetic Spectrum. Physical properties, chemical properties, formulas Shedding real light on molecular structure:
 Physical properties, chemical properties, formulas Shedding real light on molecular structure: Wavelength Frequency ν Wavelength λ Frequency ν Velocity c = 2.998 10 8 m s -1 The Electromagnetic Spectrum
Physical properties, chemical properties, formulas Shedding real light on molecular structure: Wavelength Frequency ν Wavelength λ Frequency ν Velocity c = 2.998 10 8 m s -1 The Electromagnetic Spectrum
14. Coherence Flow Networks
 14. Coherence Flow Networks A popular approach to the description of NMR pulse sequences comes from a simple vector model 1,2 in which the motion of the spins subjected to RF pulses and chemical shifts
14. Coherence Flow Networks A popular approach to the description of NMR pulse sequences comes from a simple vector model 1,2 in which the motion of the spins subjected to RF pulses and chemical shifts
12 Understanding Solution State NMR Experiments: Product Operator Formalism
 12 Understanding Solution State NMR Experiments: Product Operator Formalism As we saw in the brief introduction in the previous chapter, solution state NMR pulse sequences consist of building blocks. Product
12 Understanding Solution State NMR Experiments: Product Operator Formalism As we saw in the brief introduction in the previous chapter, solution state NMR pulse sequences consist of building blocks. Product
Spin Relaxation and NOEs BCMB/CHEM 8190
 Spin Relaxation and NOEs BCMB/CHEM 8190 T 1, T 2 (reminder), NOE T 1 is the time constant for longitudinal relaxation - the process of re-establishing the Boltzmann distribution of the energy level populations
Spin Relaxation and NOEs BCMB/CHEM 8190 T 1, T 2 (reminder), NOE T 1 is the time constant for longitudinal relaxation - the process of re-establishing the Boltzmann distribution of the energy level populations
8 NMR Interactions: Dipolar Coupling
 8 NMR Interactions: Dipolar Coupling 8.1 Hamiltonian As discussed in the first lecture, a nucleus with spin I 1/2 has a magnetic moment, µ, associated with it given by µ = γ L. (8.1) If two different nuclear
8 NMR Interactions: Dipolar Coupling 8.1 Hamiltonian As discussed in the first lecture, a nucleus with spin I 1/2 has a magnetic moment, µ, associated with it given by µ = γ L. (8.1) If two different nuclear
Hyperfine interaction
 Hyperfine interaction The notion hyperfine interaction (hfi) comes from atomic physics, where it is used for the interaction of the electronic magnetic moment with the nuclear magnetic moment. In magnetic
Hyperfine interaction The notion hyperfine interaction (hfi) comes from atomic physics, where it is used for the interaction of the electronic magnetic moment with the nuclear magnetic moment. In magnetic
Simulations of spectra and spin relaxation
 43 Chapter 6 Simulations of spectra and spin relaxation Simulations of two-spin spectra We have simulated the noisy spectra of two-spin systems in order to characterize the sensitivity of the example resonator
43 Chapter 6 Simulations of spectra and spin relaxation Simulations of two-spin spectra We have simulated the noisy spectra of two-spin systems in order to characterize the sensitivity of the example resonator
Solid-state NMR and proteins : basic concepts (a pictorial introduction) Barth van Rossum,
 Solid-state NMR and proteins : basic concepts (a pictorial introduction) Barth van Rossum, 16.02.2009 Solid-state and solution NMR spectroscopy have many things in common Several concepts have been/will
Solid-state NMR and proteins : basic concepts (a pictorial introduction) Barth van Rossum, 16.02.2009 Solid-state and solution NMR spectroscopy have many things in common Several concepts have been/will
CONTENTS. 2 CLASSICAL DESCRIPTION 2.1 The resonance phenomenon 2.2 The vector picture for pulse EPR experiments 2.3 Relaxation and the Bloch equations
 CONTENTS Preface Acknowledgements Symbols Abbreviations 1 INTRODUCTION 1.1 Scope of pulse EPR 1.2 A short history of pulse EPR 1.3 Examples of Applications 2 CLASSICAL DESCRIPTION 2.1 The resonance phenomenon
CONTENTS Preface Acknowledgements Symbols Abbreviations 1 INTRODUCTION 1.1 Scope of pulse EPR 1.2 A short history of pulse EPR 1.3 Examples of Applications 2 CLASSICAL DESCRIPTION 2.1 The resonance phenomenon
Physics 127b: Statistical Mechanics. Landau Theory of Second Order Phase Transitions. Order Parameter
 Physics 127b: Statistical Mechanics Landau Theory of Second Order Phase Transitions Order Parameter Second order phase transitions occur when a new state of reduced symmetry develops continuously from
Physics 127b: Statistical Mechanics Landau Theory of Second Order Phase Transitions Order Parameter Second order phase transitions occur when a new state of reduced symmetry develops continuously from
Direct dipolar interaction - utilization
 Direct dipolar interaction - utilization Two main uses: I: magnetization transfer II: probing internuclear distances Direct dipolar interaction - utilization Probing internuclear distances ˆ hetero D d
Direct dipolar interaction - utilization Two main uses: I: magnetization transfer II: probing internuclear distances Direct dipolar interaction - utilization Probing internuclear distances ˆ hetero D d
Introduction to Relaxation Theory James Keeler
 EUROMAR Zürich, 24 Introduction to Relaxation Theory James Keeler University of Cambridge Department of Chemistry What is relaxation? Why might it be interesting? relaxation is the process which drives
EUROMAR Zürich, 24 Introduction to Relaxation Theory James Keeler University of Cambridge Department of Chemistry What is relaxation? Why might it be interesting? relaxation is the process which drives
Slow symmetric exchange
 Slow symmetric exchange ϕ A k k B t A B There are three things you should notice compared with the Figure on the previous slide: 1) The lines are broader, 2) the intensities are reduced and 3) the peaks
Slow symmetric exchange ϕ A k k B t A B There are three things you should notice compared with the Figure on the previous slide: 1) The lines are broader, 2) the intensities are reduced and 3) the peaks
Magnetic Resonance Imaging. Pål Erik Goa Associate Professor in Medical Imaging Dept. of Physics
 Magnetic Resonance Imaging Pål Erik Goa Associate Professor in Medical Imaging Dept. of Physics pal.e.goa@ntnu.no 1 Why MRI? X-ray/CT: Great for bone structures and high spatial resolution Not so great
Magnetic Resonance Imaging Pål Erik Goa Associate Professor in Medical Imaging Dept. of Physics pal.e.goa@ntnu.no 1 Why MRI? X-ray/CT: Great for bone structures and high spatial resolution Not so great
Spectral Broadening Mechanisms
 Spectral Broadening Mechanisms Lorentzian broadening (Homogeneous) Gaussian broadening (Inhomogeneous, Inertial) Doppler broadening (special case for gas phase) The Fourier Transform NC State University
Spectral Broadening Mechanisms Lorentzian broadening (Homogeneous) Gaussian broadening (Inhomogeneous, Inertial) Doppler broadening (special case for gas phase) The Fourier Transform NC State University
Ferdowsi University of Mashhad
 Spectroscopy in Inorganic Chemistry Nuclear Magnetic Resonance Spectroscopy spin deuterium 2 helium 3 The neutron has 2 quarks with a -e/3 charge and one quark with a +2e/3 charge resulting in a total
Spectroscopy in Inorganic Chemistry Nuclear Magnetic Resonance Spectroscopy spin deuterium 2 helium 3 The neutron has 2 quarks with a -e/3 charge and one quark with a +2e/3 charge resulting in a total
Kay Saalwächter and Hans W. Spiess b) Max-Planck-Institute for Polymer Research, Postfach 3148, D Mainz, Germany
 JOURNAL OF CHEMICAL PHYSICS VOLUME 114, NUMBER 13 1 APRIL 2001 Heteronuclear 1 H 13 C multiple-spin correlation in solid-state nuclear magnetic resonance: Combining rotational-echo double-resonance recoupling
JOURNAL OF CHEMICAL PHYSICS VOLUME 114, NUMBER 13 1 APRIL 2001 Heteronuclear 1 H 13 C multiple-spin correlation in solid-state nuclear magnetic resonance: Combining rotational-echo double-resonance recoupling
T 1, T 2, NOE (reminder)
 T 1, T 2, NOE (reminder) T 1 is the time constant for longitudinal relaxation - the process of re-establishing the Boltzmann distribution of the energy level populations of the system following perturbation
T 1, T 2, NOE (reminder) T 1 is the time constant for longitudinal relaxation - the process of re-establishing the Boltzmann distribution of the energy level populations of the system following perturbation
Classical behavior of magnetic dipole vector. P. J. Grandinetti
 Classical behavior of magnetic dipole vector Z μ Y X Z μ Y X Quantum behavior of magnetic dipole vector Random sample of spin 1/2 nuclei measure μ z μ z = + γ h/2 group μ z = γ h/2 group Quantum behavior
Classical behavior of magnetic dipole vector Z μ Y X Z μ Y X Quantum behavior of magnetic dipole vector Random sample of spin 1/2 nuclei measure μ z μ z = + γ h/2 group μ z = γ h/2 group Quantum behavior
The Physical Basis of the NMR Experiment
 The Physical Basis of the NMR Experiment 1 Interaction of Materials with Magnetic Fields F F S N S N Paramagnetism Diamagnetism 2 Microscopic View: Single Spins an electron has mass and charge in addition
The Physical Basis of the NMR Experiment 1 Interaction of Materials with Magnetic Fields F F S N S N Paramagnetism Diamagnetism 2 Microscopic View: Single Spins an electron has mass and charge in addition
Spectral Broadening Mechanisms. Broadening mechanisms. Lineshape functions. Spectral lifetime broadening
 Spectral Broadening echanisms Lorentzian broadening (Homogeneous) Gaussian broadening (Inhomogeneous, Inertial) Doppler broadening (special case for gas phase) The Fourier Transform NC State University
Spectral Broadening echanisms Lorentzian broadening (Homogeneous) Gaussian broadening (Inhomogeneous, Inertial) Doppler broadening (special case for gas phase) The Fourier Transform NC State University
Tutorial Session - Exercises. Problem 1: Dipolar Interaction in Water Moleclues
 Tutorial Session - Exercises Problem 1: Dipolar Interaction in Water Moleclues The goal of this exercise is to calculate the dipolar 1 H spectrum of the protons in an isolated water molecule which does
Tutorial Session - Exercises Problem 1: Dipolar Interaction in Water Moleclues The goal of this exercise is to calculate the dipolar 1 H spectrum of the protons in an isolated water molecule which does
Center for Sustainable Environmental Technologies, Iowa State University
 NMR Characterization of Biochars By Catherine Brewer Center for Sustainable Environmental Technologies, Iowa State University Introduction Nuclear magnetic resonance spectroscopy (NMR) uses a very strong
NMR Characterization of Biochars By Catherine Brewer Center for Sustainable Environmental Technologies, Iowa State University Introduction Nuclear magnetic resonance spectroscopy (NMR) uses a very strong
Andreas Brinkmann Physical Chemistry/Solid State NMR, NSRIM Center, University of Nijmegen, Toernooiveld 1, 6525 ED Nijmegen, The Netherlands
 JOURNAL OF CHEMICAL PHYSICS VOLUME 20, NUMBER 24 22 JUNE 2004 Second order average Hamiltonian theory of symmetry-based pulse schemes in the nuclear magnetic resonance of rotating solids: Application to
JOURNAL OF CHEMICAL PHYSICS VOLUME 20, NUMBER 24 22 JUNE 2004 Second order average Hamiltonian theory of symmetry-based pulse schemes in the nuclear magnetic resonance of rotating solids: Application to
Classical Description of NMR Parameters: The Bloch Equations
 Classical Description of NMR Parameters: The Bloch Equations Pascale Legault Département de Biochimie Université de Montréal 1 Outline 1) Classical Behavior of Magnetic Nuclei: The Bloch Equation 2) Precession
Classical Description of NMR Parameters: The Bloch Equations Pascale Legault Département de Biochimie Université de Montréal 1 Outline 1) Classical Behavior of Magnetic Nuclei: The Bloch Equation 2) Precession
Advanced Quadrupolar NMR. Sharon Ashbrook School of Chemistry, University of St Andrews
 Advanced Quadrupolar NMR Sharon Ashbrook School of Chemistry, University of St Andrews Quadrupolar nuclei: revision single crystal powder ST 500 khz ST ω 0 MAS 1 khz 5 khz second-order broadening Example:
Advanced Quadrupolar NMR Sharon Ashbrook School of Chemistry, University of St Andrews Quadrupolar nuclei: revision single crystal powder ST 500 khz ST ω 0 MAS 1 khz 5 khz second-order broadening Example:
Lecture 01. Introduction to Elementary Particle Physics
 Introduction to Elementary Particle Physics Particle Astrophysics Particle physics Fundamental constituents of nature Most basic building blocks Describe all particles and interactions Shortest length
Introduction to Elementary Particle Physics Particle Astrophysics Particle physics Fundamental constituents of nature Most basic building blocks Describe all particles and interactions Shortest length
Magic-Angle Spinning (MAS) drive bearing
 Magic-Angle Spinning (MAS) magic-angle spinning is done pneumatically spinning frequency can be stabilized within a few Hz Magic-Angle Spinning (MAS) drive bearing Magic-Angle Spinning (MAS) Maximum spinning
Magic-Angle Spinning (MAS) magic-angle spinning is done pneumatically spinning frequency can be stabilized within a few Hz Magic-Angle Spinning (MAS) drive bearing Magic-Angle Spinning (MAS) Maximum spinning
Biophysical Chemistry: NMR Spectroscopy
 Relaxation & Multidimensional Spectrocopy Vrije Universiteit Brussel 9th December 2011 Outline 1 Relaxation 2 Principles 3 Outline 1 Relaxation 2 Principles 3 Establishment of Thermal Equilibrium As previously
Relaxation & Multidimensional Spectrocopy Vrije Universiteit Brussel 9th December 2011 Outline 1 Relaxation 2 Principles 3 Outline 1 Relaxation 2 Principles 3 Establishment of Thermal Equilibrium As previously
An Introduction to Hyperfine Structure and Its G-factor
 An Introduction to Hyperfine Structure and Its G-factor Xiqiao Wang East Tennessee State University April 25, 2012 1 1. Introduction In a book chapter entitled Model Calculations of Radiation Induced Damage
An Introduction to Hyperfine Structure and Its G-factor Xiqiao Wang East Tennessee State University April 25, 2012 1 1. Introduction In a book chapter entitled Model Calculations of Radiation Induced Damage
NMR (CHEM8028) Solid-state NMR: Anisotropic interactions and how we use them. Dr Philip Williamson January 2015
 NMR (CEM808) Solid-state NMR: Anisotropic interactions and how we use them Dr Philip Williamson January 015 NMR: From Molecular to Cellular Level Cell Solid State NMR Mitochondrion Membrane Liquid NMR
NMR (CEM808) Solid-state NMR: Anisotropic interactions and how we use them Dr Philip Williamson January 015 NMR: From Molecular to Cellular Level Cell Solid State NMR Mitochondrion Membrane Liquid NMR
4 DQF-COSY, Relayed-COSY, TOCSY Gerd Gemmecker, 1999
 44 4 DQF-COSY, Relayed-COSY, TOCSY Gerd Gemmecker, 1999 Double-quantum filtered COSY The phase problem of normal COSY can be circumvented by the DQF-COSY, using the MQC term generated by the second 90
44 4 DQF-COSY, Relayed-COSY, TOCSY Gerd Gemmecker, 1999 Double-quantum filtered COSY The phase problem of normal COSY can be circumvented by the DQF-COSY, using the MQC term generated by the second 90
Truncated Dipolar Recoupling in Solid-State Nuclear Magnetic Resonance
 Truncated Dipolar Recoupling in Solid-State Nuclear Magnetic Resonance Ildefonso Marin-Montesinos a, Giulia Mollica b, Marina Carravetta a, Axel Gansmüller a, Giuseppe Pileio a, Matthias Bechmann c, Angelika
Truncated Dipolar Recoupling in Solid-State Nuclear Magnetic Resonance Ildefonso Marin-Montesinos a, Giulia Mollica b, Marina Carravetta a, Axel Gansmüller a, Giuseppe Pileio a, Matthias Bechmann c, Angelika
NMR-spectroscopy of proteins in solution. Peter Schmieder
 NMR-spectroscopy of proteins in solution Basic aspects of NMR-Spektroskopie Basic aspects of NMR-spectroscopy 3/84 Prerequisite for NMR-spectroscopy is a nuclear spin that can be thought of as a mixture
NMR-spectroscopy of proteins in solution Basic aspects of NMR-Spektroskopie Basic aspects of NMR-spectroscopy 3/84 Prerequisite for NMR-spectroscopy is a nuclear spin that can be thought of as a mixture
The NMR Inverse Imaging Problem
 The NMR Inverse Imaging Problem Nuclear Magnetic Resonance Protons and Neutrons have intrinsic angular momentum Atoms with an odd number of proton and/or odd number of neutrons have a net magnetic moment=>
The NMR Inverse Imaging Problem Nuclear Magnetic Resonance Protons and Neutrons have intrinsic angular momentum Atoms with an odd number of proton and/or odd number of neutrons have a net magnetic moment=>
Spin Dynamics Basics of Nuclear Magnetic Resonance. Malcolm H. Levitt
 Spin Dynamics Basics of Nuclear Magnetic Resonance Second edition Malcolm H. Levitt The University of Southampton, UK John Wiley &. Sons, Ltd Preface xxi Preface to the First Edition xxiii Introduction
Spin Dynamics Basics of Nuclear Magnetic Resonance Second edition Malcolm H. Levitt The University of Southampton, UK John Wiley &. Sons, Ltd Preface xxi Preface to the First Edition xxiii Introduction
Motion of a Spinning Symmetric Top
 Motion of a Spinning Symmetric Top V. Tanrıverdi tanriverdivedat@googlemail.com Abstract We firstly reviewed the symmetric top problem, then we have solved different possible motions numerically. We have
Motion of a Spinning Symmetric Top V. Tanrıverdi tanriverdivedat@googlemail.com Abstract We firstly reviewed the symmetric top problem, then we have solved different possible motions numerically. We have
II. Spontaneous symmetry breaking
 . Spontaneous symmetry breaking .1 Weinberg s chair Hamiltonian rotational invariant eigenstates of good angular momentum: M > have a density distribution that is an average over all orientations with
. Spontaneous symmetry breaking .1 Weinberg s chair Hamiltonian rotational invariant eigenstates of good angular momentum: M > have a density distribution that is an average over all orientations with
Robotics I. February 6, 2014
 Robotics I February 6, 214 Exercise 1 A pan-tilt 1 camera sensor, such as the commercial webcams in Fig. 1, is mounted on the fixed base of a robot manipulator and is used for pointing at a (point-wise)
Robotics I February 6, 214 Exercise 1 A pan-tilt 1 camera sensor, such as the commercial webcams in Fig. 1, is mounted on the fixed base of a robot manipulator and is used for pointing at a (point-wise)
Principles of Nuclear Magnetic Resonance in One and Two Dimensions
 Principles of Nuclear Magnetic Resonance in One and Two Dimensions Richard R. Ernst, Geoffrey Bodenhausen, and Alexander Wokaun Laboratorium für Physikalische Chemie Eidgenössische Technische Hochschule
Principles of Nuclear Magnetic Resonance in One and Two Dimensions Richard R. Ernst, Geoffrey Bodenhausen, and Alexander Wokaun Laboratorium für Physikalische Chemie Eidgenössische Technische Hochschule
PHYSICAL REVIEW B VOLUME 58, NUMBER 5
 PHYSICAL REVIEW B VOLUME 58, NUMBER 5 1 AUGUST 1998-I Scaling and labeling the high-resolution isotropic axis of two-dimensional multiple-quantum magic-angle-spinning spectra of half-integer quadrupole
PHYSICAL REVIEW B VOLUME 58, NUMBER 5 1 AUGUST 1998-I Scaling and labeling the high-resolution isotropic axis of two-dimensional multiple-quantum magic-angle-spinning spectra of half-integer quadrupole
HS Trigonometry Mathematics CC
 Course Description A pre-calculus course for the college bound student. The term includes a strong emphasis on circular and triangular trigonometric functions, graphs of trigonometric functions and identities
Course Description A pre-calculus course for the college bound student. The term includes a strong emphasis on circular and triangular trigonometric functions, graphs of trigonometric functions and identities
Lecture #6 Chemical Exchange
 Lecture #6 Chemical Exchange Topics Introduction Effects on longitudinal magnetization Effects on transverse magnetization Examples Handouts and Reading assignments Kowalewski, Chapter 13 Levitt, sections
Lecture #6 Chemical Exchange Topics Introduction Effects on longitudinal magnetization Effects on transverse magnetization Examples Handouts and Reading assignments Kowalewski, Chapter 13 Levitt, sections
ANALYTICAL AND NUMERICAL INVESTIGATIONS *
 Romanian Reports in Physics, Vol. 64, No. 1, P. 127 134, 2012 1 H DOUBLE QUANTUM NMR AT ULTRA FAST MAS: ANALYTICAL AND NUMERICAL INVESTIGATIONS * C. TRIPON 1, X. FILIP 1, M. ALUAS 2, C. FILIP 1 1 National
Romanian Reports in Physics, Vol. 64, No. 1, P. 127 134, 2012 1 H DOUBLE QUANTUM NMR AT ULTRA FAST MAS: ANALYTICAL AND NUMERICAL INVESTIGATIONS * C. TRIPON 1, X. FILIP 1, M. ALUAS 2, C. FILIP 1 1 National
H ψ = E ψ. Introduction to Exact Diagonalization. Andreas Läuchli, New states of quantum matter MPI für Physik komplexer Systeme - Dresden
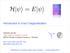 H ψ = E ψ Introduction to Exact Diagonalization Andreas Läuchli, New states of quantum matter MPI für Physik komplexer Systeme - Dresden http://www.pks.mpg.de/~aml laeuchli@comp-phys.org Simulations of
H ψ = E ψ Introduction to Exact Diagonalization Andreas Läuchli, New states of quantum matter MPI für Physik komplexer Systeme - Dresden http://www.pks.mpg.de/~aml laeuchli@comp-phys.org Simulations of
Chapter 2 Multiple Quantum NMR
 Chapter 2 Multiple Quantum NMR In the following sections, we want to elucidate the meaning of multiple quantum (MQ) coherence in the special case of dipolar coupled spin- 1 / 2 systems, and to illustrate
Chapter 2 Multiple Quantum NMR In the following sections, we want to elucidate the meaning of multiple quantum (MQ) coherence in the special case of dipolar coupled spin- 1 / 2 systems, and to illustrate
Principles of Magnetic Resonance Imaging
 Principles of Magnetic Resonance Imaging Hi Klaus Scheffler, PhD Radiological Physics University of 1 Biomedical Magnetic Resonance: 1 Introduction Magnetic Resonance Imaging Contents: Hi 1 Introduction
Principles of Magnetic Resonance Imaging Hi Klaus Scheffler, PhD Radiological Physics University of 1 Biomedical Magnetic Resonance: 1 Introduction Magnetic Resonance Imaging Contents: Hi 1 Introduction
NMR course at the FMP: NMR of organic compounds and small biomolecules - III-
 NMR course at the FMP: NMR of organic compounds and small biomolecules - III- 23.03.2009 The program 2/82 The product operator formalism (the PROF ) Basic principles Building blocks Two-dimensional NMR:
NMR course at the FMP: NMR of organic compounds and small biomolecules - III- 23.03.2009 The program 2/82 The product operator formalism (the PROF ) Basic principles Building blocks Two-dimensional NMR:
Spin mixing at level anti-crossings in the rotating frame makes high-field SABRE feasible
 Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2014 Electronic Supplementary Information for the article Spin mixing at level anti-crossings
Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2014 Electronic Supplementary Information for the article Spin mixing at level anti-crossings
Lecture #6 NMR in Hilbert Space
 Lecture #6 NMR in Hilbert Space Topics Review of spin operators Single spin in a magnetic field: longitudinal and transverse magnetiation Ensemble of spins in a magnetic field RF excitation Handouts and
Lecture #6 NMR in Hilbert Space Topics Review of spin operators Single spin in a magnetic field: longitudinal and transverse magnetiation Ensemble of spins in a magnetic field RF excitation Handouts and
free space (vacuum) permittivity [ F/m]
![free space (vacuum) permittivity [ F/m] free space (vacuum) permittivity [ F/m]](/thumbs/79/79624275.jpg) Electrostatic Fields Electrostatic fields are static (time-invariant) electric fields produced by static (stationary) charge distributions. The mathematical definition of the electrostatic field is derived
Electrostatic Fields Electrostatic fields are static (time-invariant) electric fields produced by static (stationary) charge distributions. The mathematical definition of the electrostatic field is derived
Introduction to NMR Product Operators. C. Griesinger. Max Planck Institute for Biophysical Chemistry. Am Faßberg 11. D Göttingen.
 ntroduction to NMR Product Operato C. Griesinger Max Planck nstitute for Biophysical Chemistry Am Faßberg 11 D-3777 Göttingen Germany cigr@nmr.mpibpc.mpg.de http://goenmr.de EMBO Coue Heidelberg Sept.
ntroduction to NMR Product Operato C. Griesinger Max Planck nstitute for Biophysical Chemistry Am Faßberg 11 D-3777 Göttingen Germany cigr@nmr.mpibpc.mpg.de http://goenmr.de EMBO Coue Heidelberg Sept.
2 = = 0 Thus, the number which is largest in magnitude is equal to the number which is smallest in magnitude.
 Limits at Infinity Two additional topics of interest with its are its as x ± and its where f(x) ±. Before we can properly discuss the notion of infinite its, we will need to begin with a discussion on
Limits at Infinity Two additional topics of interest with its are its as x ± and its where f(x) ±. Before we can properly discuss the notion of infinite its, we will need to begin with a discussion on
Classical Description of NMR Parameters: The Bloch Equations
 Classical Description of NMR Parameters: The Bloch Equations Pascale Legault Département de Biochimie Université de Montréal 1 Outline 1) Classical Behavior of Magnetic Nuclei: The Bloch Equation 2) Precession
Classical Description of NMR Parameters: The Bloch Equations Pascale Legault Département de Biochimie Université de Montréal 1 Outline 1) Classical Behavior of Magnetic Nuclei: The Bloch Equation 2) Precession
APPENDIX E SPIN AND POLARIZATION
 APPENDIX E SPIN AND POLARIZATION Nothing shocks me. I m a scientist. Indiana Jones You ve never seen nothing like it, no never in your life. F. Mercury Spin is a fundamental intrinsic property of elementary
APPENDIX E SPIN AND POLARIZATION Nothing shocks me. I m a scientist. Indiana Jones You ve never seen nothing like it, no never in your life. F. Mercury Spin is a fundamental intrinsic property of elementary
Quantum Analogs Chapter 3 Student Manual
 Quantum Analogs Chapter 3 Student Manual Broken Symmetry in the Spherical Resonator and Modeling a Molecule Professor Rene Matzdorf Universitaet Kassel 3. Broken symmetry in the spherical resonator and
Quantum Analogs Chapter 3 Student Manual Broken Symmetry in the Spherical Resonator and Modeling a Molecule Professor Rene Matzdorf Universitaet Kassel 3. Broken symmetry in the spherical resonator and
Lecture #9 Redfield theory of NMR relaxation
 Lecture 9 Redfield theory of NMR relaxation Topics Redfield theory recap Relaxation supermatrix Dipolar coupling revisited Scalar relaxation of the st kind Handouts and Reading assignments van de Ven,
Lecture 9 Redfield theory of NMR relaxation Topics Redfield theory recap Relaxation supermatrix Dipolar coupling revisited Scalar relaxation of the st kind Handouts and Reading assignments van de Ven,
Motion of a Spinning Symmetric Top
 Motion of a Spinning Symmetric Top V. Tanrıverdi tanriverdivedat@googlemail.com Abstract We firstly reviewed the symmetric top problem, then we have solved different possible motions numerically. We have
Motion of a Spinning Symmetric Top V. Tanrıverdi tanriverdivedat@googlemail.com Abstract We firstly reviewed the symmetric top problem, then we have solved different possible motions numerically. We have
Lecture 11 Spin, orbital, and total angular momentum Mechanics. 1 Very brief background. 2 General properties of angular momentum operators
 Lecture Spin, orbital, and total angular momentum 70.00 Mechanics Very brief background MATH-GA In 9, a famous experiment conducted by Otto Stern and Walther Gerlach, involving particles subject to a nonuniform
Lecture Spin, orbital, and total angular momentum 70.00 Mechanics Very brief background MATH-GA In 9, a famous experiment conducted by Otto Stern and Walther Gerlach, involving particles subject to a nonuniform
Long-lived spin echoes in magnetically diluted system: an NMR study of the Ge single crystals Alexander M. Panich,
 Long-lived spin echoes in magnetically diluted system: an NMR study of the Ge single crystals Alexander M. Panich, Department of Physics, Ben-Gurion University of the Negev, Beer Sheva, Israel N. A. Sergeev,
Long-lived spin echoes in magnetically diluted system: an NMR study of the Ge single crystals Alexander M. Panich, Department of Physics, Ben-Gurion University of the Negev, Beer Sheva, Israel N. A. Sergeev,
Molecules in Magnetic Fields
 Molecules in Magnetic Fields Trygve Helgaker Hylleraas Centre, Department of Chemistry, University of Oslo, Norway and Centre for Advanced Study at the Norwegian Academy of Science and Letters, Oslo, Norway
Molecules in Magnetic Fields Trygve Helgaker Hylleraas Centre, Department of Chemistry, University of Oslo, Norway and Centre for Advanced Study at the Norwegian Academy of Science and Letters, Oslo, Norway
R Function Tutorial in Module 8.2
 R Function Tutorial in Module 8.2 Introduction to Computational Science: Modeling and Simulation for the Sciences, 2 nd Edition Angela B. Shiflet and George W. Shiflet Wofford College 2014 by Princeton
R Function Tutorial in Module 8.2 Introduction to Computational Science: Modeling and Simulation for the Sciences, 2 nd Edition Angela B. Shiflet and George W. Shiflet Wofford College 2014 by Princeton
Dipolar Recoupling: Heteronuclear
 Dipolar Recoupling: Heteronuclear Christopher P. Jaroniec Ohio State University, Columbus, OH, USA 1 Introduction 1 MAS Hamiltonian 3 3 Heteronuclear Dipolar Recoupling in Spin Pairs 4 4 Heteronuclear
Dipolar Recoupling: Heteronuclear Christopher P. Jaroniec Ohio State University, Columbus, OH, USA 1 Introduction 1 MAS Hamiltonian 3 3 Heteronuclear Dipolar Recoupling in Spin Pairs 4 4 Heteronuclear
CS273: Algorithms for Structure Handout # 13 and Motion in Biology Stanford University Tuesday, 11 May 2003
 CS273: Algorithms for Structure Handout # 13 and Motion in Biology Stanford University Tuesday, 11 May 2003 Lecture #13: 11 May 2004 Topics: Protein Structure Determination Scribe: Minli Zhu We acknowledge
CS273: Algorithms for Structure Handout # 13 and Motion in Biology Stanford University Tuesday, 11 May 2003 Lecture #13: 11 May 2004 Topics: Protein Structure Determination Scribe: Minli Zhu We acknowledge
A Hands on Introduction to NMR Lecture #1 Nuclear Spin and Magnetic Resonance
 A Hands on Introduction to NMR 22.920 Lecture #1 Nuclear Spin and Magnetic Resonance Introduction - The aim of this short course is to present a physical picture of the basic principles of Nuclear Magnetic
A Hands on Introduction to NMR 22.920 Lecture #1 Nuclear Spin and Magnetic Resonance Introduction - The aim of this short course is to present a physical picture of the basic principles of Nuclear Magnetic
Arthur S. Edison Department of Biochemistry & Molecular Biology
 Theory and Applications of NMR Spectroscopy Arthur S. Edison Department of Biochemistry & Molecular Biology Summary Week 1 Notes: Introduction to the basics: Bloch equations Week 1 Homework Week 2 Notes:
Theory and Applications of NMR Spectroscopy Arthur S. Edison Department of Biochemistry & Molecular Biology Summary Week 1 Notes: Introduction to the basics: Bloch equations Week 1 Homework Week 2 Notes:
Magnets, 1D quantum system, and quantum Phase transitions
 134 Phys620.nb 10 Magnets, 1D quantum system, and quantum Phase transitions In 1D, fermions can be mapped into bosons, and vice versa. 10.1. magnetization and frustrated magnets (in any dimensions) Consider
134 Phys620.nb 10 Magnets, 1D quantum system, and quantum Phase transitions In 1D, fermions can be mapped into bosons, and vice versa. 10.1. magnetization and frustrated magnets (in any dimensions) Consider
Introduction to MRI. Spin & Magnetic Moments. Relaxation (T1, T2) Spin Echoes. 2DFT Imaging. K-space & Spatial Resolution.
 Introduction to MRI Spin & Magnetic Moments Relaxation (T1, T2) Spin Echoes 2DFT Imaging Selective excitation, phase & frequency encoding K-space & Spatial Resolution Contrast (T1, T2) Acknowledgement:
Introduction to MRI Spin & Magnetic Moments Relaxation (T1, T2) Spin Echoes 2DFT Imaging Selective excitation, phase & frequency encoding K-space & Spatial Resolution Contrast (T1, T2) Acknowledgement:
Chapter 29. Quantum Chaos
 Chapter 29 Quantum Chaos What happens to a Hamiltonian system that for classical mechanics is chaotic when we include a nonzero h? There is no problem in principle to answering this question: given a classical
Chapter 29 Quantum Chaos What happens to a Hamiltonian system that for classical mechanics is chaotic when we include a nonzero h? There is no problem in principle to answering this question: given a classical
NMR-spectroscopy. I: basics. Peter Schmieder
 NMR-spectroscopy I: basics Why spectroscopy? 2/102 Why spectroscopy It is well established that all biological relevant processes take place via interactions of molecules, either small ones (metall ions,
NMR-spectroscopy I: basics Why spectroscopy? 2/102 Why spectroscopy It is well established that all biological relevant processes take place via interactions of molecules, either small ones (metall ions,
NMR, the vector model and the relaxation
 NMR, the vector model and the relaxation Reading/Books: One and two dimensional NMR spectroscopy, VCH, Friebolin Spin Dynamics, Basics of NMR, Wiley, Levitt Molecular Quantum Mechanics, Oxford Univ. Press,
NMR, the vector model and the relaxation Reading/Books: One and two dimensional NMR spectroscopy, VCH, Friebolin Spin Dynamics, Basics of NMR, Wiley, Levitt Molecular Quantum Mechanics, Oxford Univ. Press,
conventions and notation
 Ph95a lecture notes, //0 The Bloch Equations A quick review of spin- conventions and notation The quantum state of a spin- particle is represented by a vector in a two-dimensional complex Hilbert space
Ph95a lecture notes, //0 The Bloch Equations A quick review of spin- conventions and notation The quantum state of a spin- particle is represented by a vector in a two-dimensional complex Hilbert space
In this lecture, we will go through the hyperfine structure of atoms. The coupling of nuclear and electronic total angular momentum is explained.
 Lecture : Hyperfine Structure of Spectral Lines: Page- In this lecture, we will go through the hyperfine structure of atoms. Various origins of the hyperfine structure are discussed The coupling of nuclear
Lecture : Hyperfine Structure of Spectral Lines: Page- In this lecture, we will go through the hyperfine structure of atoms. Various origins of the hyperfine structure are discussed The coupling of nuclear
Measuring Spin-Lattice Relaxation Time
 WJP, PHY381 (2009) Wabash Journal of Physics v4.0, p.1 Measuring Spin-Lattice Relaxation Time L.W. Lupinski, R. Paudel, and M.J. Madsen Department of Physics, Wabash College, Crawfordsville, IN 47933 (Dated:
WJP, PHY381 (2009) Wabash Journal of Physics v4.0, p.1 Measuring Spin-Lattice Relaxation Time L.W. Lupinski, R. Paudel, and M.J. Madsen Department of Physics, Wabash College, Crawfordsville, IN 47933 (Dated:
Introduction solution NMR
 2 NMR journey Introduction solution NMR Alexandre Bonvin Bijvoet Center for Biomolecular Research with thanks to Dr. Klaartje Houben EMBO Global Exchange course, IHEP, Beijing April 28 - May 5, 20 3 Topics
2 NMR journey Introduction solution NMR Alexandre Bonvin Bijvoet Center for Biomolecular Research with thanks to Dr. Klaartje Houben EMBO Global Exchange course, IHEP, Beijing April 28 - May 5, 20 3 Topics
3. Perturbed Angular Correlation Spectroscopy
 3. Perturbed Angular Correlation Spectroscopy Dileep Mampallil Augustine K.U.Leuven, Belgium Perturbed Angular Correlation Spectroscopy (PAC) is a gamma ray spectroscopy and can be used to investigate
3. Perturbed Angular Correlation Spectroscopy Dileep Mampallil Augustine K.U.Leuven, Belgium Perturbed Angular Correlation Spectroscopy (PAC) is a gamma ray spectroscopy and can be used to investigate
VIII Chemical Exchange
 VIII Chemical Exchange Lecture notes by Assaf Tal Chemical exchange has surprising ties with relaxation as we shall see. Understanding exchange lets us understand phenomena, some of which at first glance
VIII Chemical Exchange Lecture notes by Assaf Tal Chemical exchange has surprising ties with relaxation as we shall see. Understanding exchange lets us understand phenomena, some of which at first glance
COPYRIGHTED MATERIAL. Production of Net Magnetization. Chapter 1
 Chapter 1 Production of Net Magnetization Magnetic resonance (MR) is a measurement technique used to examine atoms and molecules. It is based on the interaction between an applied magnetic field and a
Chapter 1 Production of Net Magnetization Magnetic resonance (MR) is a measurement technique used to examine atoms and molecules. It is based on the interaction between an applied magnetic field and a
Axion Detection With NMR
 PRD 84 (2011) arxiv:1101.2691 + to appear Axion Detection With NMR Peter Graham Stanford with Dmitry Budker Micah Ledbetter Surjeet Rajendran Alex Sushkov Dark Matter Motivation two of the best candidates:
PRD 84 (2011) arxiv:1101.2691 + to appear Axion Detection With NMR Peter Graham Stanford with Dmitry Budker Micah Ledbetter Surjeet Rajendran Alex Sushkov Dark Matter Motivation two of the best candidates:
Intro to Nuclear and Particle Physics (5110)
 Intro to Nuclear and Particle Physics (5110) March 13, 009 Nuclear Shell Model continued 3/13/009 1 Atomic Physics Nuclear Physics V = V r f r L r S r Tot Spin-Orbit Interaction ( ) ( ) Spin of e magnetic
Intro to Nuclear and Particle Physics (5110) March 13, 009 Nuclear Shell Model continued 3/13/009 1 Atomic Physics Nuclear Physics V = V r f r L r S r Tot Spin-Orbit Interaction ( ) ( ) Spin of e magnetic
Symmetry-Based Double Quantum Recoupling in Solid State NMR. Marina Carravetta
 Symmetry-Based Double Quantum Recoupling in Solid State NMR Marina Carravetta N. S + NH Physical Chemistry Division Arrhenius Laboratory Stockholm University 2002 PAGINA VUOTA Abstract This thesis deals
Symmetry-Based Double Quantum Recoupling in Solid State NMR Marina Carravetta N. S + NH Physical Chemistry Division Arrhenius Laboratory Stockholm University 2002 PAGINA VUOTA Abstract This thesis deals
Electron spins in nonmagnetic semiconductors
 Electron spins in nonmagnetic semiconductors Yuichiro K. Kato Institute of Engineering Innovation, The University of Tokyo Physics of non-interacting spins Optical spin injection and detection Spin manipulation
Electron spins in nonmagnetic semiconductors Yuichiro K. Kato Institute of Engineering Innovation, The University of Tokyo Physics of non-interacting spins Optical spin injection and detection Spin manipulation
Appendix A: Matrices
 Appendix A: Matrices A matrix is a rectangular array of numbers Such arrays have rows and columns The numbers of rows and columns are referred to as the dimensions of a matrix A matrix with, say, 5 rows
Appendix A: Matrices A matrix is a rectangular array of numbers Such arrays have rows and columns The numbers of rows and columns are referred to as the dimensions of a matrix A matrix with, say, 5 rows
NMR journey. Introduction to solution NMR. Alexandre Bonvin. Topics. Why use NMR...? Bijvoet Center for Biomolecular Research
 2 NMR journey Introduction to solution NMR Alexandre Bonvin Bijvoet Center for Biomolecular Research with thanks to Dr. Klaartje Houben EMBO Global Exchange course, CCMB, Hyderabad, India November 29th
2 NMR journey Introduction to solution NMR Alexandre Bonvin Bijvoet Center for Biomolecular Research with thanks to Dr. Klaartje Houben EMBO Global Exchange course, CCMB, Hyderabad, India November 29th
