VANDERBILT STUDENT VOLUNTEERS FOR SCIENCE
|
|
|
- Felicia Reed
- 5 years ago
- Views:
Transcription
1 VANDERBILT STUDENT VOLUNTEERS FOR SCIENCE Evidence of a Chemical Reaction Fall 2018 Goal: To show students evidence of a chemical change. Fits TN standards:7ps1.2, 7PS1.3, 7PS1.4 VSVSer Lesson Outline I. Introduction Question students about the difference between physical and chemical changes. Explain what constitutes evidence of chemical reactions. II. Safety Concerns Discuss safety issues. Demonstrate how students will use the small dispensing bottles and the 24-well culture plate. III. Determining if a Chemical Change has Occurred Tell students to follow the instructions on the instruction sheet. You will still need to guide them through the procedures, making sure they understand the instructions. Discuss results with students after they finish each row. Chemical equations for Rows A, B, C are given. Row A: Chemical Reactions That Give a Precipitate (solid) Students should realize that if the solution turns cloudy, a solid (precipitate) is forming. Row B: Chemical Reactions That Involve a Color Change Formation of complex ions cause color changes Row C: Chemical Reactions That Produce a Gas Students look carefully at the bubbles (CO 2) produced in solutions IV. Analyzing Results Emphasize the chemistry of carbonates and bicarbonates. Students predict the reaction between marble and HCl V. Review LOOK AT THE VIDEO BEFORE YOU GO OUT TO YOUR CLASSROOM ( USE THE PPT AND VIDEO TO VISUALIZE THE MATERIALS USED IN EACH SECTION. 1. In the car ride, read through this quiz together as a team. Make sure each team member has read the lesson and has a fundamental understanding of the material. Lesson Quiz 1) What are some basic differences between a physical and chemical change? 2) What are signs of a chemical change? 3) What are the major safety concerns in this lesson for students? 4) What is important about the reaction between a carbonate/bicarbonate and an acid? 5) What is a precipitate? 6) Briefly describe the process students will use to identify the unknown at the end of the lesson 7) If a reaction causes the solution to go from colored to clear, does it count as a color change? 8) In all of the reactions in this lesson for which a gas is given off, what is the gas?
2 2. Use these fun facts during the lesson: Chemical changes constantly occur in living organisms. The human body is made up of proteins that help catalyze complex chemical reactions. The products of a chemical reaction have the same total mass as the reactants (law of conservation of mass). Unpacking the Kit What you will need for each section: VSVSers do this while 1 person is giving the Introduction. Note that students are put into pairs and should have their pencils ready While one team member starts the introduction, another should write the following vocabulary words on the board: physical change, chemical change, chemical reaction, formula, solution, precipitate, compound, mixture For Part III. Determining If a Chemical Change Has Occurred 34 safety goggles for students and VSVS members well culture plates with lids 16 plastic plates Instruction sheets, obs sheets 15 sets of dropper bottles of each: 1.0 M HCl hydrochloric acid 2.5 M Na 2CO 3 sodium carbonate 0.5 M NaHCO 3 sodium bicarbonate 0.1 M Cu(NO 3) 2 copper (II) nitrate 0.1 M Fe(NO 3) 3 iron (III) nitrate 0.1 M KSCN potassium thiocyanate A. Demonstration Bag A 2 oz bottle of 0.1 M CaCl 2, 2 oz bottle of 1 M Na 2CO 3, 2 10 oz clear cups 4 jars containing the products, with precipitate in bottom B. Demonstration Bag B 2 oz bottle of 0.05 M Na 2S 2O 3, 2 oz bottle of 0.01 M I 2, 2 10 oz clear cups C. Demonstration Bag C - 2 oz bottle of 1 M HCl, jar of Na 2CO 3, 1 teaspoon, and 1 10 oz clear cup For Part IV. Analyzing Results 15 small pieces of marble CaCO 3 (in a small bag) For Part V. Optional: Identifying an Unknown 2 sets of 1oz dropper bottles of each of the following unknowns: Unknown A: NaHCO 3 3, Unknown B: CaCl 2, Unknown C: HCl, Unknown D: KSCN, I. Introduction Learning Goals: Students can name the different indicators that a chemical reaction has occurred.
3 Ask students: What is the difference between a physical change and a chemical change? Be sure to include the following information in the discussion: A physical change does not change the chemical properties of a substance. o No new substance is formed during a physical change. o Only the physical properties are changed. o Examples of physical changes include changes in the size, shape, or state of matter. For example, ice, liquid water, and steam. In each of these states, water has physically changed (from solid, liquid, gas) but not chemically. A chemical change does change the chemical properties of a substance. o One or more new substances are formed in a chemical change. o A chemical change cannot be easily reversed. o Examples include: burning paper, digestion of food, bananas browning Ask students: How can you tell when a chemical change has occurred? Some answers may include: a gas given off, color change, precipitation, explosion, burning, etc. Tell students what to look for to determine if a chemical change has occurred: When solutions of two compounds are mixed, it is often possible to determine whether or not a chemical reaction has occurred through visual observation. Evidence of a chemical change might be a color change, a gas given off (it may smell), the formation of a precipitate (a new solid), or an energy (temperature, light) change. Write these observations on the board and share the following explanation with students. 1. A color change occurs when two solutions are mixed and a new color is produced. o BUT, if the color of one solution becomes a paler shade, that change is caused by dilution from the other solution and does not qualify as a color change. 2. Bubbles or fizz indicate that a gas is given off. o BUT, make sure that students understand that the bubbles given off in a soda pop drink is NOT evidence of a chemical change This is just excess gas that is released when the top is opened. Carbonated beverages contain carbon dioxide gas dissolved under pressure, and removing the top lowers the pressure and allows carbon dioxide bubbles to escape. 3. A precipitate forms when two substances react to give a new solid compound that does not dissolve in water. o A precipitate will MOST LIKELY look like a cloudy solution, fine grains in a solution, a swirl, or a fluffy solid. The solution cannot be seen through. Note: When two clear solutions are mixed and a white precipitate forms, this whitish color does not count as a color change. The change should be recorded only as the formation of a precipitate. 4. An energy change (temperature or light) can be either a physical or chemical change. A chemical energy change occurs in a glow stick when chemicals mix to produces light. A physical energy change occurs when you freeze water. Important: Scientists do not rely on just visual observations to determine if a chemical or physical change has occurred. The only real evidence is the formation of new substances with different chemical formulas from the reactants.
4 II. Safety Concerns Tell students they must put on safety goggles before mixing any solutions. Students should not directly smell any chemicals. If anyone gets any of the chemicals on their skin or in their eyes, they should flush immediately with water. Although the solutions are dilute, they could still cause eye damage, especially the 1.0 M HCl. Emphasize to students how important it is for them to follow directions. Organize students in pairs and distribute the following materials to each pair of students: 2 safety goggles 1 24-well culture plate 1 plastic plate 6 dropper bottles of solutions 2 Instruction Sheets 2 Chemical Reactions Observation Sheets VSVS volunteers should put on their safety goggles and keep them on until students are finished mixing chemicals. letters Have students look at the 24-well plate and the instructions at the top of the Chemical Reactions Lab Sheet. Show students how to find the letters A, B, C, D as well as the numbers 1-6 on the 24-well plate. (Letters are imprinted in the plastic along the right side; numbers are imprinted across the top and the bottom. These are tiny and may be difficult to see.) Show students how to match the grid on the lab sheet to the 24-well plate. Tell students to place the 24-well plate on the plastic plate. Give the following instructions to the students: 1. The names and formulas of the compounds being used in this experiment are listed at the bottom of the observation sheet. Have students look at these names and formulas while you pronounce them for the class. The labels on the dropper bottles list both the name and formula. of the compounds. Show students how to be careful when matching the formulas (some of the formulas are very similar). 2. Show students one of the bottles and demonstrate how to get drops out of the bottle. Dropper bottles are easy to use. Apply slow, gentle pressure. Do not remove the red cap from a bottle until it is to be used. Put the cap back on the bottle immediately after use. When using two solutions, put a squirt of the first solution in the correct well so that it is one-fourth full (we do not want students to spend time counting drops). Then add one squirt of the second solution. The well should now be half full. numbers 3. Tell students they will perform the reactions for one row only then stop and discuss the results with the VSVS members. Tell the partners to take turns doing the experiments as they follow the grid on the lab sheet. Both students record their observations on the lab sheet. Students can record if No
5 Reaction occurs. Otherwise, they will record color change, gas given off, or precipitate formed. 4. Tell students to follow the instructions on the instruction sheet for mixing solutions. (The instruction sheet lists the same directions as are given below.) III. Determining If a Chemical Change Has Occurred Learning Goals: Students can name the different indicators that a chemical reaction has occurred. Students can identify the specific indicators of a reaction and explain how to look for them One team member should draw a grid of the well plate on the board with all of the rows labeled. Write on this when discussing the results with the students. Note: VSVS volunteers need to monitor the students closely to be sure contamination does not occur. Ensure that students use the correct bottle. Stop and discuss results with students after each row. This is preferable to waiting until students finish all of the experiment since some will finish very quickly and then be bored waiting for others to catch up. The beginning of each reaction is given on the student observation sheet. Students and VSVSers should complete each equation on the board after the reactions in each row are completed. A. Chemical Reactions that Give Precipitates - Row A Demonstration: Show students what a precipitate looks like by doing the following demonstration. Take the demonstration bag marked ROW A. Remove the 2 oz bottles of solutions and the two 10 oz clear cups. Empty each 2 oz bottles into separate cups. Hold the two cups up so the students can see what happens, and then pour one solution into the other. A white solid (precipitate) forms. Point this out as an example of a chemical reaction in which a precipitate forms to the students. Show the students the jars with the clear liquid and precipitate in the bottom. Tell them that these are the products from the same reaction just done, but the products were allowed to stand for several hours. Shake the jar to show the students that the solution will become cloudy again.
6 Note: For each activity DO NOT record the results until the students have completed the experiments for the row since they may wait to copy the answers from the board. Tell students to use the grid on their observation sheet to perform the experiments in Row A. Make sure that they correctly identify the formulas of the compounds being used in a reaction ( listed on the observation sheet). Review and Equations: Ask students what evidence indicated that a chemical reaction occurred. o A precipitate formed in A1 and A2. Put the results on the board. A1: precipitate A2: precipitate Students can look at the equations on the observation sheet: Demo: CaCl 2 + Na 2CO 3 CaCO NaCl A1: 2 Fe(NO 3) Na 2CO 3 Fe 2(CO 3) NaNO 3 A2: Cu(NO 3) 2 + Na 2CO 3 CuCO NaNO 3 B. Chemical Reactions that Involve a Color Change - Row B Demonstration: Show students what a color change looks like by doing the following demonstration. Take the demonstration bag marked ROW B. Remove the 2 oz bottles of solutions and the two 10oz clear plastic cups. Empty each 2 oz bottles into separate cups. Hold the two clear containers up, and tell students to notice that one is a clear colorless solution and the other is a clear, brown solution. Pour the colorless solution into the brown solution, and ask students to describe what happens. The brown solution turns colorless, but it is still clear (i.e no precipitation). Explain to students that a chemical reaction has taken place because the brown solution turned colorless upon addition of the clear solution. Tell students to use the grid on the lab sheet to perform the experiments in Row B. Background for VSVS Members Only: This is an oxidation-reduction reaction in which iodine is reduced to iodide ion, and thiosulfate ion, S 2O 4 2-, is oxidized to tetrathionate ion, S 4O Review and Equations: Ask students what evidence indicated that a chemical reaction occurred. o A color change occurred. Put the results on the board. B1: color change to deep red B2: color change to pale green.
7 Students can look at the equations on their observation sheet. Demo: I Na 2S 2O 3 2 NaI + Na 2S 4O 6 B1: Fe(NO 3) 3 + KSCN Fe(SCN)(NO 3) 2 + KNO 3 intense red color B2: Cu(NO 3) 2 + KSCN Cu(SCN)(NO 3) + KNO 3 pale green color Background for VSVS Members Only: Color changes with metal ion solutions are caused by the formation of complex ions. In the present case, the SCN - (thiocyanate) anion bonds strongly to the Fe 3+ (iron) ion in solution to give an intense deep red color. The SCN - anion also bonds to Cu(II) (copper) ion. C. Chemical Reactions that Produce a Gas - Row C Note: Tell students they will have to look very closely and quickly as soon as they add the second solution to the first solution. The bubbles of gas are small and come off as soon as the solutions are mixed. Demonstration: Show students what a chemical change that produces a gas looks like by performing the demonstration first. Take the demonstration bag marked ROW C. Hold the cup up and ask students to watch very carefully what happens. Put 1 tsp of the solid (Na 2CO 3) into the cup and empty the 2oz bottle (HCl) into it. Ask students to describe what happens. A bubbling up (slight foaming) which quickly subsides indicates a gas is given off. Tell students to watch very carefully for bubbles of gas when they are doing Row C because they may be difficult to see. Tell students to use the grid on the lab sheet to perform all the experiments in Row C. Review and Equations: Ask students what evidence indicated that a chemical reaction occurred. o Bubbles/gas was given off. Put the results on the board. C1: bubbles/gas C2: bubbles/gas Students can look at the equations on the observation sheet. Demo: Na 2CO 3 + 2HCl 2NaCl + CO 2 + H 2O C1: NaHCO 3 + HCl NaCl + CO 2 (g) + H 2O C2: Na 2CO 3 + 2HCl 2NaCl + CO 2 (g) + H 2O
8 IV. Analyzing Results Learning Goals: Students can analyze compounds reactions with one another and use that data to identify an unknown compound. Carbonates: Write CO 3 on the board and tell students that formulas that include CO 3 as part of the formula are called carbonates. One form of carbonate, sodium carbonate is commonly referred to as "washing soda." It is part of many laundry detergents and dish washing detergents. Tell students to look at the ingredients of the copy of a washing soda box on their Instruction sheet. Sodium carbonate is listed as an ingredient. Bicarbonates: Write HCO 3 on the board and tell students that formulas that include HCO 3 as part of the formula are called bicarbonates. A common source of bicarbonate is baking soda, whose scientific name is sodium bicarbonate. Sodium bicarbonate is used for baking and as a deodorizer. Tell students to look at the ingredient label on baking soda and notice it says sodium bicarbonate. Reactions of Carbonates and Bicarbonates: Ask students to look at each box in row C and circle the formulas that have CO 3 or HCO 3 as part of the formula.. Write HCl on the board. o Tell students that HCl is an acid called hydrochloric acid. o Tell students to put boxes around all the HCl s in Row C Ask students what happened in row C when an acid was added to a carbonate or bicarbonate. o o A gas was given off. Ask students if they have ever made a volcano at home or at school. Ask students if they remembered what they added to make the lava. Most likely, they used vinegar and baking soda. Vinegar is an acid, and when added to baking soda (sodium bicarbonate), it bubbles as it releases a gas. Ask students what happens when you add an acid to carbonate or bicarbonate. When an acid is added to a carbonate or bicarbonate a chemical reaction occurs. This is evidenced by the fact that a gas is given off. Tell students that the gas given off in these reactions is carbon dioxide. Identifying Carbonates Using Chemical Reactions: Tell students that chalk, limestone and marble are all calcium carbonate. If the formula for calcium carbonate is CaCO 3, what might happen if an acid (HCl) is added to marble? It will bubble (give off a gas). Write the first half of the equation, CaCO 3 (s) + HCl (aq)...on the board, and ask students to hypothesize what will happen, and what the products will be. The gas will be CO2.
9 The full equation is: CaCO 3 + 2HCl CaCl 2 + CO 2 + H 2O Have VSVS members hand out a piece of marble to each pair of students. Tell students that they are going to test their hypothesis to see if the marble will bubble when HCl is added. Have students add the marble to C5 and to squirt some HCl into the well. Ask students what happened. Was their hypothesis correct? Yes, it should bubble when HCl is added. Tell students that many statues are made of limestone and marble. Ask students what they think happens to statues when acid rain falls on them. The carbonate in the statue reacts with acid liberating a gas and causing the statue to decompose. Background Information (in case students ask about acid rain): Acid rain is caused by the presence of varying amounts of sulfuric acid and nitric acid in rain drops. Fossil fuels, particularly coal and oil, contain sulfur as an impurity. When fossil fuels are burned, the sulfur combines with oxygen to form sulfur oxides that are gases released into the air. When it rains, the water reacts with these gases to form sulfuric acid. When vehicles burn gasoline or diesel fuel, nitrogen oxides are emitted into the air, and these react with water to produce nitric acid. The amount of sulfuric acid and nitric acid in acid rain depends on the location. The acid rain in Nashville is primarily caused by nitric acid from the high density of vehicles, while the acidity of rain in industrialized areas will be caused by the presence of both sulfuric and nitric acids. As a result of the Clean Air Act, industrial and power plants are emitting much lower amounts of sulfur oxides; however, the emission of nitric oxides from vehicle exhausts is still a problem. V. Optional: Identifying an Unknown Tell students they will be given a colorless solution that contains one of the following compounds dissolved in water Unknown A: NaHCO 3 Unknown B: CaCl 2 Unknown C: HCl Unknown D: KSCN These are the same colorless solutions that they have been doing the experiments with, except that CaCl 2 was used only in Demonstration A. Tell them that they will follow a plan, using the dropper bottles from the chemical reaction kit, to determine the identity of the unknown. Students can work in pairs, groups, or as a class. Distribute the 8 unknown dropper bottles throughout the class, so that pairs or groups of students have access to one unknown. Tell students to: 1. Follow the plan in Row D and add a squirt of their unknown to the 6 wells in Row D of the well plate. 2. Add a few drops from their known dropper bottles, one well at a time. 3. Write down the observation immediately after each addition. 4. Look at the results in Rows A, B and C, including the demonstration reactions, to determine what the possible reactions are.
10 Ask students: What do you think your unknown is? What is your proof for what you ve listed in (a)? The proof should be stated something like the answer response in the plans given above but specific for the unknown. For example, if the unknown was CaCl 2, the proof statement could be: A precipitate formed when Na 2CO 3 solution was added. The CaCl 2 solution is the only one of the possible unknowns that will give a precipitate with Na 2CO 3. Write balanced chemical equations for the reactions you used to determine the identity of the unknown. (Refer to equations in lesson (Section VIII)) Optional: Answer these questions after finishing the chemical reactions observation sheet. 1. Write complete, balanced equations for the following: a. One reaction in Row A that gave a precipitate. Refer to equations in lesson (Section VIII) b. One reaction in Row B that gave a color change. Refer to equations in lesson (Section VIII) c. One reaction in Row C that gave a gas. Refer to equations in lesson (Section VIII) VI. Review Questions Ask students: What is a physical change? What is a chemical change? What are the chemical changes we saw today? How do we know when a chemical change has occurred? (answer on p. 3) VII. Clean-up Have students put the dropper bottles back in the Ziploc bag. Make sure that the bottles are all upright. Leaks make for nasty clean-up tasks. Collect the Ziploc bags and the goggles. Place the lids on the 24-well plates and carefully put them in the Rubbermaid container. Place the lid on the Rubbermaid container and put it in the bottom of the box. (If you can rinse them out at the school, do so, PLEASE) Place the ziploc bags and other materials in the box. Collect all instruction sheets in sheet protectors and put them in the box. Lesson written by Dr. Melvin Joesten, Chemistry Department, Vanderbilt University Pat Tellinghuisen, Coordinator of VSVS, Vanderbilt University
11 NAME Class Activity Sheet Extension Activity 1. You will be given an unknown colorless solution that contains one of the following compounds dissolved in water: CaCl 2, KSCN, HCl, NaHCO 3. Follow Row D to determine the identity of your unknown. a. What do you think your unknown is? If there is more than one possibility, list both. b. What is your proof for what you ve listed in (a)? c. Write balanced chemical equations for the reactions you used to determine the identity of the unknown. 1. Write complete, balanced equations for the following (choose a correct equation from the list below): a. One reaction in Row A that gave a precipitate. b. One reaction in Row B that gave a color change. c. One reaction in Row C that gave a gas. Chemical Equations for Chemical Reactions in this Lesson Abbreviations: s = solid, aq = aqueous, g = gas, l = liquid CaCl 2 (aq) + Na 2CO 3 (aq) CaCO 3 (s) + 2 NaCl (aq) 2 Fe(NO 3) 3 (aq) + 3 Na 2CO 3 (aq) Fe 2(CO 3) 3 (s) + 6 NaNO 3 (aq) Cu(NO 3) 2 (aq) + Na 2CO 3 (aq) CuCO 3 (s) + 2 NaNO 3 (aq) I 2(aq) + 2 Na 2S2O 3(aq) 2 NaI(aq) + Na 2S 4O 6 (aq) Fe(NO 3) 3 (aq) + KSCN (aq) Fe(SCN)(NO 3) 2 (aq) + KNO 3 (aq) Cu(NO 3) 2 (aq) + KSCN (aq) Cu(SCN)(NO 3) (aq) + KNO 3 (aq) Na 2CO 3 (s) + 2HCl (aq) 2NaCl (aq) + CO 2 (g) + H 2O (l) NaHCO 3 (aq) + HCl (aq) NaCl (aq) + CO 2 (g) + H 2O (l) Na 2CO 3 (aq) + 2HCl (aq) 2NaCl (aq) + CO 2 (g) + H 2O (l) CaCO 3 (s) + 2HCl (aq) CaCl 2 (aq) + CO 2 (g) + H 2O (l)
12 Class Activity Sheet ANSWER SHEET Answer these questions after finishing the chemical reactions observation sheet. 2. Write complete, balanced equations for the following: a. One reaction in Row A that gave a precipitate. Refer to equations on worksheet One reaction in Row B that gave a color change. Refer to equations on worksheet One reaction in Row C that gave a gas. Refer to equations in lesson (Section VIII) You will be given an unknown colorless solution that contains one of the following compounds dissolved in water: CaCl 2, KSCN, HCl, NaHCO 3. Follow Row D to determine the identity of your unknown. What do you think your unknown is? 5. What is your proof for what you ve listed in (a)? The proof should be stated something like the answer response in the plans given above, but specific for the unknown. For example, if the unknown was CaCl 2, the proof statement could be: A precipate formed when NaHCO 3solution was added. The CaCl 2 solution is the only one of the possible unknowns that will give a precipitate with NaHCO Write balanced chemical equations for the reactions you used to determine the identity of the unknown. Refer to equations below
13 Chemical Reactions Observation Sheet Name Vocabulary Words: physical change, chemical change, chemical reaction, formula, solution, precipitate, compound, mixture In each box, use the following choices to record your observations: color change, precipitate formed, gas given off, (no reaction) Add other observations if you wish. Ex: lots of bubbles, fizz, small or large bubbles, cloudy precipitate. A1 Fe(NO 3) 3 + Na 2CO 3 A2 Cu(NO 3) 2 + Na 2CO 3 A3 A4 A5 A6 Demonstration CaCl 2 + Na 2CO 3 CaCl2 + Na2CO3 CaCO3 + 2NaCl 2 Fe(NO3)3 + 3 Na2CO3 Fe2(CO3)3 + 6 NaNO3 Cu(NO3)2 + Na2CO3 CuCO3 + 2 NaNO3 B1 Fe(NO 3) 3 +KSCN B2 Cu(NO 3) 2 +KSCN B3 B4 B5 B6 Demonstration I 2 + 2Na 2S2O 3 I2 + 2 Na2S2O3 2 NaI + Na2S4O6 Fe(NO3)3 + KSCN Fe(SCN)(NO3)2 + KNO3 Cu(NO3)2 + KSCN (aq) Cu(SCN)(NO3) + KNO3 C1 NaHCO 3 + HCl C2 Na 2CO 3 + HCl C3 C4 C5 marble solid CaCO 3 + HCl C6 Demonstration Na 2CO 3 + 2HCl Na2CO3 + 2HCl 2NaCl + CO2+ H2O NaHCO3 + HCl NaCl + CO2 + H2O Na2CO3 + 2HCl 2NaCl + CO2 + H2O CaCO3 + 2HCl CaCl2 + CO2+ H2O D1 NaHCO 3 D2 KSCN D3 Fe(NO 3) 3 D4 Cu(NO 3) 2 D5 HCl D6 HCl - hydrochloric acid Na 2CO 3 - sodium carbonate NaHCO 3 - sodium bicarbonate Cu(NO 3) 2 - copper (II) nitrate Fe(NO 3) 3 - iron (III) nitrate KSCN - potassium thiocyanate CaCl 2 - calcium chloride
14 A1 Fe(NO 3) 3 + Na 2CO 3 precipitate A2 Cu(NO 3) 2 + Na 2CO 3 precipitate Chemical Reactions Lab Sheet Answer Key A3 A4 A5 A6 Demonstration CaCl 2 + Na 2CO 3 white precipitate B1 Fe(NO 3) 3 +KSCN color changedeep red B2 Cu(NO 3) 2 +KSCN color change - pale green color B3 B4 B5 B6 Demonstration I Na 2S2O 3 color change brown to colorless C1 baking soda NaHCO 3 + HCl gas given off C2 washing soda Na 2CO 3 + HCl gas given off C3 C4 C5 marble solid CaCO 3 + HCl gas given off C6 Demonstration Na 2CO 3 + 2HCl gas given off D1 baking soda NaHCO 3 D2 potassium thiocyanate KSCN D3 iron nitrate Fe(NO 3) 3 D4 copper nitrate Cu(NO 3) 2 D5 hydrochloric acid HCl D6 HCl - hydrochloric acid Na 2CO 3 - sodium carbonate NaHCO 3 - sodium bicarbonate Cu(NO 3) 2 - copper (II) nitrate Fe(NO 3) 3 - iron (III) nitrate KSCN - potassium thiocyanate CaCl 2 - calcium chloride
15 Answer Sheet for Unknown Solutions baking soda NaHCO 3 A (Na 2CO 3) potassium thiocyanate KSCN A (Na 2CO 3) iron nitrate Fe(NO 3) 3 A (Na 2CO 3) copper nitrate Cu(NO 3) 2 A (Na 2CO 3) hydrochloric acid HCl A (Na 2CO 3) PPT PPT Gas given off baking soda NaHCO 3 B ( CaCl 2) potassium thiocyanate KSCN B ( CaCl 2) iron nitrate Fe(NO 3) 3 B ( CaCl 2) copper nitrate Cu(NO 3) 2 B ( CaCl 2) hydrochloric acid HCl B ( CaCl 2) PPT baking soda NaHCO 3 C (HCl) potassium thiocyanate KSCN C (HCl) iron nitrate Fe(NO 3) 3 C (HCl) copper nitrate Cu(NO 3) 2 C (HCl) hydrochloric acid HCl C (HCl) Gas given off baking soda NaHCO 3 D (KSCN) potassium thiocyanate KSCN D (KSCN) Color change iron nitrate Fe(NO 3) 3 D (KSCN) Color change copper nitrate Cu(NO 3) 2 D (KSCN) hydrochloric acid HCl D (KSCN) baking soda NaHCO 3 E ( NaHCO 3) potassium thiocyanate KSCN E ( NaHCO 3) Color change iron nitrate Fe(NO 3) 3 E ( NaHCO 3) Color change copper nitrate Cu(NO 3) 2 E ( NaHCO 3) hydrochloric acid HCl E ( NaHCO 3) PPT PPT Gas given off
16 Important: Instruction Sheet Evidence of a Chemical Reaction 7th Grade Always wear safety goggles when dealing with chemicals Pay close attention to which solutions you are using To use dropper bottles apply slow, gentle pressure Don t fill more than halfway full Record observations: = no reaction, PPT= precipitate, color change, gas formed Chemical Name Chemical Formula 1. Iron III Nitrate Fe(NO 3) 3 2. Copper Nitrate Cu (NO 3) 2 3. Sodium Carbonate Na 2CO 3 4. Potassium Thiocyanate KSCN 5. Sodium Bicarbonate NaHCO 3 6. Hydrochloric Acid HCl I. Setting Up A. Receive and put on safety goggles B. Materials 1. First set of solutions well plate 3. plastic plate (place well plate on plastic plate) 4. Observation Sheet III A. Row A -Precipitate C. Watch VSVS members complete Demo A D. What you need: 1. Fe(NO 3) 3 ---> squirt into well A1, ¼ full 2. Cu(NO 3) 2 ---> squirt into well A2, ¼ full 3. Na 2CO > squirt into both A1 and A2 until ½ full C. Record your observations for each well on your observation chart. D. Wait for instructions from VSVS member before moving on III. B Row B Color Change A. Watch VSVS members complete Demo B B. What you need 1. Fe(NO 3) 3 ---> squirt into well B1, ¼ full 2. Cu(NO 3) > squirt into well B2, ¼ full 3. KSCN ----> squirt into both B1 and B2 until ½ full C. Record your observations for each well on your observation chart. D. Wait for instructions from VSVS members before moving on
17 III. Row C Produce a Gas A. Watch VSVS members perform Demo C B. What you need 1. NaHCO3 ---> squirt into well C1, ¼ full 2. Na2CO3 ----> squirt into well C2, ¼ full 3. HCL ---> squirt into both C1 and C2 until ½ full C. Record your observations for each well on your observation chart D. Wait for instructions from VSVS members before moving on. IV. Analyzing the Results Carbonates and Bicarbonates A. Discuss results with the class or small groups B. Predict what will happen if marble and an acid interact? 1. Receive a piece of marble from VSVS member 2. Place marble in well C5 3. Add a few drops of HCL 4. Record observations V. Optional Identifying an Unknown A. Receive an unknown solution from VSVS member B. Record which unknown you are given on your observation sheet C. Squirt unknown into wells D1 - D6 until ¼ full 1. D1 ---> squirt NaHCO3, until ½ full a. Record Observations for each cell immediately after addition. 2. D2 ---> squirt Na2CO3, until ½ full 3. D3 --->squirt KSCN, until ½ full 4. D4 ---> squirt Fe(NO3)3, until ½ full 5. D5 ---> squirt Cu(NO3)2, until ½ full 6. D6 ---> squirt HCl, until ½ full VII. D. Determine which solution your unknown is (ask VSVS member if you need help Clean- Up A. Place red caps on all the dropper bottles B. Give goggles back to VSVS members C. Wash off the well plates if available
What Do You Think? Investigate GOALS
 Activity 2 More Chemical Changes GOALS In this activity you will: Observe several typical examples of evidence that a chemical change is occurring. Make generalizations about the combinations of materials
Activity 2 More Chemical Changes GOALS In this activity you will: Observe several typical examples of evidence that a chemical change is occurring. Make generalizations about the combinations of materials
Characteristics of Chemical Change
 Section 2 Characteristics of Chemical Change What Do You See? Learning Outcomes In this section you will Observe several typical examples of evidence that a chemical change is occurring. Make generalizations
Section 2 Characteristics of Chemical Change What Do You See? Learning Outcomes In this section you will Observe several typical examples of evidence that a chemical change is occurring. Make generalizations
More Chemical Changes
 Activity 2 More Chemical Changes Activity 2 More Chemical Changes GOALS In this activity you will: Observe several typical examples of evidence that a chemical change is occurring. Make generalizations
Activity 2 More Chemical Changes Activity 2 More Chemical Changes GOALS In this activity you will: Observe several typical examples of evidence that a chemical change is occurring. Make generalizations
CHEMICAL REACTION IN A BAGGY (MODIFIED FOR ADEED)
 CHEMICAL REACTION IN A BAGGY (MODIFIED FOR ADEED) Overview: Students investigate chemical reactions using given substances. Students identify chemical reactions in their daily lives. Objectives: The student
CHEMICAL REACTION IN A BAGGY (MODIFIED FOR ADEED) Overview: Students investigate chemical reactions using given substances. Students identify chemical reactions in their daily lives. Objectives: The student
CO 2. Lesson 1. Production of a gas
 Lesson 1 Production of a gas T E A C H E R G U I D E CO 2 Lesson summary Students meet volcanologist Victor Helguson, who is studying the gases released by volcanoes in Iceland. Students conduct a chemical
Lesson 1 Production of a gas T E A C H E R G U I D E CO 2 Lesson summary Students meet volcanologist Victor Helguson, who is studying the gases released by volcanoes in Iceland. Students conduct a chemical
Chemical Energy Conversions. Vanderbilt Student Volunteers for Science VINSE/VSVS Rural
 Chemical Energy Conversions Vanderbilt Student Volunteers for Science 2018-2019 VINSE/VSVS Rural Important!!! Please use this resource to reinforce your understanding of the lesson! Make sure you have
Chemical Energy Conversions Vanderbilt Student Volunteers for Science 2018-2019 VINSE/VSVS Rural Important!!! Please use this resource to reinforce your understanding of the lesson! Make sure you have
Acid Rain Drops Keep Falling on My Head Investigating Acid Rain
 Investigating Acid Rain Carbonic acid is produced when carbon dioxide gas dissolves in rain droplets of unpolluted air: (1) CO 2(g) + H 2 O (l) H 2 CO 3(aq) CO 2 Nitrous acid and nitric acid result from
Investigating Acid Rain Carbonic acid is produced when carbon dioxide gas dissolves in rain droplets of unpolluted air: (1) CO 2(g) + H 2 O (l) H 2 CO 3(aq) CO 2 Nitrous acid and nitric acid result from
Goal: To measure the conductivity of solids and solutions using an LED in a circuit. TN Curriculum Alignment:
 VANDERBILT STUDENT VOLUNTEERS FOR SCIENCE http://studentorgs.vanderbilt.edu/vsvs Investigating Ionic, Covalent and Metallic Bonding 2018-2019 VINSE/VSVS Rural Acknowledgement: We want to thank NASA and
VANDERBILT STUDENT VOLUNTEERS FOR SCIENCE http://studentorgs.vanderbilt.edu/vsvs Investigating Ionic, Covalent and Metallic Bonding 2018-2019 VINSE/VSVS Rural Acknowledgement: We want to thank NASA and
Relative Solubility of Transition Elements
 Microscale Relative Solubility of Transition Elements The transition elements are found in periods 4, 5, and 6 between groups 2 and 13 of the periodic table. As the atomic number increases across a row
Microscale Relative Solubility of Transition Elements The transition elements are found in periods 4, 5, and 6 between groups 2 and 13 of the periodic table. As the atomic number increases across a row
What Do You Think? Investigate GOALS
 Cool Chemistry Show Activity 4 Chemical Equations GOALS In this activity you will: Represent chemical changes using word equations and chemical equations. Distinguish between different classes of chemical
Cool Chemistry Show Activity 4 Chemical Equations GOALS In this activity you will: Represent chemical changes using word equations and chemical equations. Distinguish between different classes of chemical
7 th Grade Fall 2018 Lesson Plans. Vanderbilt Student Volunteers for Science
 7 th Grade Fall 2018 Lesson Plans Vanderbilt Student Volunteers for Science http://studentorgs.vanderbilt.edu/vsvs/ VOLUNTEER INFORMATION Team Member Contact Information Name: Phone Number: _ Name: Phone
7 th Grade Fall 2018 Lesson Plans Vanderbilt Student Volunteers for Science http://studentorgs.vanderbilt.edu/vsvs/ VOLUNTEER INFORMATION Team Member Contact Information Name: Phone Number: _ Name: Phone
Physical & Chemical PROPERTIES
 Name Test Date Hour Group Chem #4 Notebook Properties & Changes LEARNING TARGETS I can give examples of physical and chemical properties. I can give examples of physical and chemical changes. I can identify
Name Test Date Hour Group Chem #4 Notebook Properties & Changes LEARNING TARGETS I can give examples of physical and chemical properties. I can give examples of physical and chemical changes. I can identify
Chapter 6, Lesson 7: Energy Changes in Chemical Reactions
 Chapter 6, Lesson 7: Energy Changes in Chemical Reactions Key Concepts If two substances react and the temperature of the mixture decreases, the reaction is endothermic. If two substances react and the
Chapter 6, Lesson 7: Energy Changes in Chemical Reactions Key Concepts If two substances react and the temperature of the mixture decreases, the reaction is endothermic. If two substances react and the
Chemistry 141 Samuel A. Abrash Chemical Reactions Lab Lecture 9/5/2011
 Chemistry 141 Samuel A. Abrash Chemical Reactions Lab Lecture 9/5/2011 Q: Before we start discussing this week s lab, can we talk about our lab notebooks? Sure. Q: What makes a lab notebook a good notebook?
Chemistry 141 Samuel A. Abrash Chemical Reactions Lab Lecture 9/5/2011 Q: Before we start discussing this week s lab, can we talk about our lab notebooks? Sure. Q: What makes a lab notebook a good notebook?
Experiment 7: SIMULTANEOUS EQUILIBRIA
 Experiment 7: SIMULTANEOUS EQUILIBRIA Purpose: A qualitative view of chemical equilibrium is explored based on the reaction of iron(iii) ion and thiocyanate ion to form the iron(iii) thiocyanate complex
Experiment 7: SIMULTANEOUS EQUILIBRIA Purpose: A qualitative view of chemical equilibrium is explored based on the reaction of iron(iii) ion and thiocyanate ion to form the iron(iii) thiocyanate complex
Lab: Chemical Reactions
 Lab: Chemical Reactions PRE-LAB: Write the chemical equations (skeleton equations) for the following. Make sure to clearly label what type of product was produced (solid, liquid, gas, or no reaction).
Lab: Chemical Reactions PRE-LAB: Write the chemical equations (skeleton equations) for the following. Make sure to clearly label what type of product was produced (solid, liquid, gas, or no reaction).
Reaction Types and Chemical Equations
 Cool Chemistry Show Section 4 Reaction Types and Chemical Equations What Do You See? Learning Outcomes In this section you will Represent chemical changes using word equations and chemical equations. Distinguish
Cool Chemistry Show Section 4 Reaction Types and Chemical Equations What Do You See? Learning Outcomes In this section you will Represent chemical changes using word equations and chemical equations. Distinguish
General Stoichiometry Notes STOICHIOMETRY: tells relative amts of reactants & products in a chemical reaction
 General Stoichiometry Notes STOICHIOMETRY: tells relative amts of reactants & products in a chemical reaction Given an amount of a substance involved in a chemical reaction, we can figure out the amount
General Stoichiometry Notes STOICHIOMETRY: tells relative amts of reactants & products in a chemical reaction Given an amount of a substance involved in a chemical reaction, we can figure out the amount
Signs of Chemical Reactions
 Signs of Chemical Reactions Tell the difference between changes which are PHYSICAL and those which involve a CHEMICAL reaction Doing six different experiments and observing the changes which
Signs of Chemical Reactions Tell the difference between changes which are PHYSICAL and those which involve a CHEMICAL reaction Doing six different experiments and observing the changes which
Equilibrium and LeChatelier s Principle
 1 Equilibrium and LeChatelier s Principle Purpose: To examine LeChatelier s Principle by studying disturbances applied to several equilibrium systems. Introduction Many chemical reactions reach a state
1 Equilibrium and LeChatelier s Principle Purpose: To examine LeChatelier s Principle by studying disturbances applied to several equilibrium systems. Introduction Many chemical reactions reach a state
Classifying Chemical Reactions
 1 Classifying Chemical Reactions Analyzing and Predicting Products Introduction The power of chemical reactions to transform our lives is visible all around us-in our cars, even in our bodies. Chemists
1 Classifying Chemical Reactions Analyzing and Predicting Products Introduction The power of chemical reactions to transform our lives is visible all around us-in our cars, even in our bodies. Chemists
The ABCs of Chemistry
 Hands-On Science The ABCs of Chemistry Michael Margolin illustrated by Lloyd Birmingham WALCH EDUCATION Contents To the Teacher... v... vii... viii... xvi... 1... 9.... 17... 28... 38... 45.... 52... 62...
Hands-On Science The ABCs of Chemistry Michael Margolin illustrated by Lloyd Birmingham WALCH EDUCATION Contents To the Teacher... v... vii... viii... xvi... 1... 9.... 17... 28... 38... 45.... 52... 62...
MONDAY (12/12) TUESDAY (12/13) WEDNESDAY (12/14) THURSDAY (12/15) FRIDAY (12/16) Making Acid Rain (a lab) Quiz
 Homework Activities Name: Date: Period: This week, we will be using our knowledge of acids and bases and studying how acids, specifically acid rain, affect our lives and our environment. We will also end
Homework Activities Name: Date: Period: This week, we will be using our knowledge of acids and bases and studying how acids, specifically acid rain, affect our lives and our environment. We will also end
General Stoichiometry Notes STOICHIOMETRY: tells relative amts of reactants & products in a chemical reaction
 General Stoichiometry Notes STOICHIOMETRY: tells relative amts of reactants & products in a chemical reaction Given an amount of a substance involved in a chemical reaction, we can figure out the amount
General Stoichiometry Notes STOICHIOMETRY: tells relative amts of reactants & products in a chemical reaction Given an amount of a substance involved in a chemical reaction, we can figure out the amount
Modeling Conservation of Matter
 Modeling Conservation of Matter Imagine that you and two of your classmates want to make a strawberry banana smoothie. You lay out the ingredients: one banana, five strawberries and two scoops of ice cream.
Modeling Conservation of Matter Imagine that you and two of your classmates want to make a strawberry banana smoothie. You lay out the ingredients: one banana, five strawberries and two scoops of ice cream.
The grade 5 English science unit, Acids and Bases, meets the academic content standards set in the Korean curriculum, which state students should:
 This area addresses ph among different characteristics of solutions. It will be interesting for students to classify a variety of solutions into acids and bases by using the characteristics of the solutions.
This area addresses ph among different characteristics of solutions. It will be interesting for students to classify a variety of solutions into acids and bases by using the characteristics of the solutions.
Student Exploration: Chemical Changes
 Name: Date: Student Exploration: Chemical Changes Vocabulary: acid, base, catalyst, chemical change, coefficient, conservation of matter, decomposition, dissolve, double replacement, endothermic, exothermic,
Name: Date: Student Exploration: Chemical Changes Vocabulary: acid, base, catalyst, chemical change, coefficient, conservation of matter, decomposition, dissolve, double replacement, endothermic, exothermic,
Signs of Chemical Reactions
 Signs of Chemical Reactions Tell the difference between changes which are PHYSICAL and those which involve a CHEMICAL reaction Doing six different experiments and observing the changes which
Signs of Chemical Reactions Tell the difference between changes which are PHYSICAL and those which involve a CHEMICAL reaction Doing six different experiments and observing the changes which
Chemical Reactions Unit
 Name: Hour: Teacher: ROZEMA / Chemistry Chemical Reactions Unit 1 P a g e 2 P a g e 3 P a g e 4 P a g e 5 P a g e 6 P a g e Chemistry Balancing Equations Balance the following equations by inserting the
Name: Hour: Teacher: ROZEMA / Chemistry Chemical Reactions Unit 1 P a g e 2 P a g e 3 P a g e 4 P a g e 5 P a g e 6 P a g e Chemistry Balancing Equations Balance the following equations by inserting the
Chemical Reaction Lab Bagged Chemical Reactions
 Learning Target: The student experiments and determines that the rates of reaction among atoms and molecules depend on the concentration, pressure, and temperature of the reactants and the presence or
Learning Target: The student experiments and determines that the rates of reaction among atoms and molecules depend on the concentration, pressure, and temperature of the reactants and the presence or
Lesson 2. Color change
 Lesson 2 Color change T E A C H E R G U I D E Lesson summary Students meet marine chemist Sera Tuikabe, who is studying ocean acidification in the water surrounding the Republic of the Fiji Islands. Students
Lesson 2 Color change T E A C H E R G U I D E Lesson summary Students meet marine chemist Sera Tuikabe, who is studying ocean acidification in the water surrounding the Republic of the Fiji Islands. Students
A Visual Introduction to Ionic and Net Ionic Equations
 Overview This activity explores the phenomenon of chemical precipitation and asks students to construct an atomic level model of precipitation using ionic and net ionic equations. Initially, students run
Overview This activity explores the phenomenon of chemical precipitation and asks students to construct an atomic level model of precipitation using ionic and net ionic equations. Initially, students run
LEVEL ZERO VOICE CATALYST (10 minutes, individual work): 1. Counting atoms
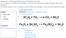 Assignment 4 Chemical Detectives at the Molecular Level LO: To explain the differences between chemical and physical changes through observable evidence. EQ: What evidence can be used to tell the difference
Assignment 4 Chemical Detectives at the Molecular Level LO: To explain the differences between chemical and physical changes through observable evidence. EQ: What evidence can be used to tell the difference
Read the lab thoroughly. Answer the pre-lab questions that appear at the end of this lab exercise.
 Experiment 10 Stoichiometry- Gravimetric Analysis Pre-lab Assignment Read the lab thoroughly. Answer the pre-lab questions that appear at the end of this lab exercise. Purpose The purpose this experiment
Experiment 10 Stoichiometry- Gravimetric Analysis Pre-lab Assignment Read the lab thoroughly. Answer the pre-lab questions that appear at the end of this lab exercise. Purpose The purpose this experiment
Chemical Reactions. Chemical Reactions Chemical reactions have a standard format when written:
 0.3.notebook A chemical property is a behaviour that occurs when substances change to create a new substance. When a new substance is created, a chemical change has occurred. New colour Evidence of chemical
0.3.notebook A chemical property is a behaviour that occurs when substances change to create a new substance. When a new substance is created, a chemical change has occurred. New colour Evidence of chemical
Chemical Names and Formulas
 Cool Chemistry Show Activity 3 Chemical Names and Formulas GOALS In this activity you will: Predict the charges of ions of some elements. Determine the formulas of ionic compounds. Write the conventional
Cool Chemistry Show Activity 3 Chemical Names and Formulas GOALS In this activity you will: Predict the charges of ions of some elements. Determine the formulas of ionic compounds. Write the conventional
Ocean Acidification in a Cup Materials
 Ocean Acidification in a Cup Ocean acidification is a problem that humans will have to deal with as we release more and more carbon dioxide into the atmosphere. This activity demonstrates how water can
Ocean Acidification in a Cup Ocean acidification is a problem that humans will have to deal with as we release more and more carbon dioxide into the atmosphere. This activity demonstrates how water can
Physical and Chemical Changes Or How Do You Know When You ve Made Something New?
 Introduction Or How Do You Know When You ve Made Something New? Remember that all matter has characteristic physical and chemical properties. Matter can also undergo physical and chemical changes. How
Introduction Or How Do You Know When You ve Made Something New? Remember that all matter has characteristic physical and chemical properties. Matter can also undergo physical and chemical changes. How
Chapter 6, Lesson 1: What is a Chemical Reaction?
 Chapter 6, Lesson 1: What is a Chemical Reaction? Key Concepts: A physical change, such as a state change or dissolving, does not create a new substance, but a chemical change does. In a chemical reaction,
Chapter 6, Lesson 1: What is a Chemical Reaction? Key Concepts: A physical change, such as a state change or dissolving, does not create a new substance, but a chemical change does. In a chemical reaction,
Examples of Strong Acids: Strong Acid Formula Common Source Hydrochloric Acid HCl Stomach Acid
 ACIDS AND BASES: PH AND BUFFERS PURPOSE: To determine the ph of common acids and bases using a ph meter, ph paper, and red cabbage indicator. To test the effect of adding an acid or base to a buffer solution.
ACIDS AND BASES: PH AND BUFFERS PURPOSE: To determine the ph of common acids and bases using a ph meter, ph paper, and red cabbage indicator. To test the effect of adding an acid or base to a buffer solution.
5.1. The Classification of Matter
 5.1 The Classification of Matter Chemistry is the study of matter. Matter is anything that has mass and volume. Mass is the amount of matter that an object has. Volume is the amount of space that an object
5.1 The Classification of Matter Chemistry is the study of matter. Matter is anything that has mass and volume. Mass is the amount of matter that an object has. Volume is the amount of space that an object
Classifying Chemical Reactions Analyzing and Predicting Products
 Classifying Chemical Reactions Analyzing and Predicting Products Background A chemical reaction is defined as any process in which one or more substances are converted into new substances with different
Classifying Chemical Reactions Analyzing and Predicting Products Background A chemical reaction is defined as any process in which one or more substances are converted into new substances with different
Stoichiometry: Baking Soda and Vinegar Reactions Student Version
 Stoichiometry: Baking Soda and Vinegar Reactions Student Version In this lab, students will examine the chemical reaction between baking soda and vinegar, and mix different amounts of these household chemicals
Stoichiometry: Baking Soda and Vinegar Reactions Student Version In this lab, students will examine the chemical reaction between baking soda and vinegar, and mix different amounts of these household chemicals
INSPIRE GK12 Lesson Plan. Compare Relate, Predict National Standards
 Lesson Title Halloween Chemistry Length of Lesson 30 minutes Created By Erin Anderson Subject Chemistry Grade Level 9-12 State Standards 7a, b, c, d, g DOK Level II DOK Application Compare Relate, Predict
Lesson Title Halloween Chemistry Length of Lesson 30 minutes Created By Erin Anderson Subject Chemistry Grade Level 9-12 State Standards 7a, b, c, d, g DOK Level II DOK Application Compare Relate, Predict
8 Grade. Vanderbilt Student Volunteers for Science. Fall 2017 Lesson Plans. Chemistry.
 th 8 Grade Fall 207 Lesson Plans Chemistry Vanderbilt Student Volunteers for Science http://studentorgs.vanderbilt.edu/vsvs/ VOLUNTEER INFORMATION Team Member Contact Information Name: Phone Number: _
th 8 Grade Fall 207 Lesson Plans Chemistry Vanderbilt Student Volunteers for Science http://studentorgs.vanderbilt.edu/vsvs/ VOLUNTEER INFORMATION Team Member Contact Information Name: Phone Number: _
Chapter 6, Lesson 10: Carbon Dioxide Can Make a Solution Acidic
 Chapter 6, Lesson 10: Carbon Dioxide Can Make a Solution Acidic Key Concepts Carbon dioxide (CO 2 ) gas dissolved in water can cause water to become acidic. The acidity of water from dissolved CO 2 can
Chapter 6, Lesson 10: Carbon Dioxide Can Make a Solution Acidic Key Concepts Carbon dioxide (CO 2 ) gas dissolved in water can cause water to become acidic. The acidity of water from dissolved CO 2 can
Classifying Chemical Reactions: Lab Directions
 Classifying Chemical Reactions: Lab Directions Please Return Background: The power of chemical reactions to transform our lives is visible all around us in our homes, in our cars, even in our bodies. Chemists
Classifying Chemical Reactions: Lab Directions Please Return Background: The power of chemical reactions to transform our lives is visible all around us in our homes, in our cars, even in our bodies. Chemists
Pre-Lab Read the entire laboratory assignment. Answer all pre-lab questions before beginning the lab.
 Name: Date: Pd: Lab Partner: Lab # 13: Types of Reactions, Predicting Products of Chemical Reactions Lab Accelerated Chemistry 1 Introduction: If you examine your bicycle after it has been left out in
Name: Date: Pd: Lab Partner: Lab # 13: Types of Reactions, Predicting Products of Chemical Reactions Lab Accelerated Chemistry 1 Introduction: If you examine your bicycle after it has been left out in
Conservation of Mass in Chemical Reactions Lab. Experiment Question: What happens to the total mass of substances when a chemical reaction occurs?
 Conservation of Mass in Chemical Reactions Lab Name: 5 th Grade PSI Science Score: / 5 Experiment Question: What happens to the total mass of substances when a chemical reaction occurs? Hypothesis Starters:
Conservation of Mass in Chemical Reactions Lab Name: 5 th Grade PSI Science Score: / 5 Experiment Question: What happens to the total mass of substances when a chemical reaction occurs? Hypothesis Starters:
Chemical reaction? Food color. Which photo shows a physical change and which shows a chemical reaction?
 Chemical reactions Chemical reaction? Food color Which photo shows a physical change and which shows a chemical reaction? Starter: chemical reactions Aim 17 May 2016 By the end of this lesson I will be
Chemical reactions Chemical reaction? Food color Which photo shows a physical change and which shows a chemical reaction? Starter: chemical reactions Aim 17 May 2016 By the end of this lesson I will be
Limiting Reactants Lab
 Name: Teacher s Name: Class: Block: Date: Partners: Limiting Reactants Lab Purpose: Through experimentation, determine the limiting reactant and the percent yield in a chemical reaction that generates
Name: Teacher s Name: Class: Block: Date: Partners: Limiting Reactants Lab Purpose: Through experimentation, determine the limiting reactant and the percent yield in a chemical reaction that generates
5 th Grade Lesson Plan: Matter and Chemical Reactions
 5 th Grade Lesson Plan: Matter and Chemical Reactions Objective: Teach students that matter is neither created nor destroyed during a chemical reaction, rather, it is transformed. Identify evidence that
5 th Grade Lesson Plan: Matter and Chemical Reactions Objective: Teach students that matter is neither created nor destroyed during a chemical reaction, rather, it is transformed. Identify evidence that
Chemistry: classifying chemical and physical changes in various materials/substances
 Chemistry: classifying chemical and physical changes in various materials/substances Nikki Schilling, Ames, St. Paul Mn Based on original activity from Cool Chemistry Concoctions by Joe Rhatigan and Veronika
Chemistry: classifying chemical and physical changes in various materials/substances Nikki Schilling, Ames, St. Paul Mn Based on original activity from Cool Chemistry Concoctions by Joe Rhatigan and Veronika
Experiment 8 - Double Displacement Reactions
 Experiment 8 - Double Displacement Reactions A double displacement reaction involves two ionic compounds that are dissolved in water. In a double displacement reaction, it appears as though the ions are
Experiment 8 - Double Displacement Reactions A double displacement reaction involves two ionic compounds that are dissolved in water. In a double displacement reaction, it appears as though the ions are
The Eight Solution Problem Exploring Reactions of Aqueous Ionic Compounds
 The Eight Solution Problem Exploring Reactions of Aqueous Ionic Compounds About this Lesson This activity allows students to mix a variety of known ionic solutions while making careful observations. After
The Eight Solution Problem Exploring Reactions of Aqueous Ionic Compounds About this Lesson This activity allows students to mix a variety of known ionic solutions while making careful observations. After
The Eight Solution Problem Exploring Reactions of Aqueous Ionic Compounds
 15 Exploring Reactions of Aqueous Ionic Compounds INTRODUCTION Your goal in this lab is to identify eight unknown solutions. You and your partner will first collect data by observing reactions between
15 Exploring Reactions of Aqueous Ionic Compounds INTRODUCTION Your goal in this lab is to identify eight unknown solutions. You and your partner will first collect data by observing reactions between
Separation and Qualitative Determination of Cations
 Separation and Qualitative Determination of Cations Introduction Much of laboratory chemistry is focused on the question of how much of a given substance is contained in a sample. Sometimes, however, the
Separation and Qualitative Determination of Cations Introduction Much of laboratory chemistry is focused on the question of how much of a given substance is contained in a sample. Sometimes, however, the
Chemical Reactions. Chemical changes are occurring around us all the time
 Chemical changes are occurring around us all the time Food cooking Fuel being burned in a car s engine Oxygen being used in the human body The starting materials are called reactants The ending materials
Chemical changes are occurring around us all the time Food cooking Fuel being burned in a car s engine Oxygen being used in the human body The starting materials are called reactants The ending materials
2/22/2019 NEW UNIT! Chemical Interactions. Atomic Basics #19
 NEW UNIT! Chemical Interactions Atomic Basics #19 1 Vocabulary: Matter: Anything that has mass and takes up space. Atom: the smallest particle of matter. Element: A pure substance made up of only one type
NEW UNIT! Chemical Interactions Atomic Basics #19 1 Vocabulary: Matter: Anything that has mass and takes up space. Atom: the smallest particle of matter. Element: A pure substance made up of only one type
Elements and Their Oxides
 Elements and Their Oxides An oxide is a. Oxides can form when an element reacts with oxygen, often in air. This reaction can be rapid with the release of a great deal of energy, as in the combustion of
Elements and Their Oxides An oxide is a. Oxides can form when an element reacts with oxygen, often in air. This reaction can be rapid with the release of a great deal of energy, as in the combustion of
Types of Chemical Reactions. Synthesis, Combustion, Decomposition and Replacement
 Types of Chemical Reactions Synthesis, Combustion, Decomposition and Replacement You can think of atoms as people getting together as couples... Analogy One person A couple Switching partners Chemical
Types of Chemical Reactions Synthesis, Combustion, Decomposition and Replacement You can think of atoms as people getting together as couples... Analogy One person A couple Switching partners Chemical
Supernatant: The liquid layer lying above the solid layer after a precipitation reaction occurs.
 Limiting Reagent Introduction The quantities of substances involved in a chemical reaction represented by a balanced equation are often referred to as stoichiometric amounts. Solution stoichiometry is
Limiting Reagent Introduction The quantities of substances involved in a chemical reaction represented by a balanced equation are often referred to as stoichiometric amounts. Solution stoichiometry is
Chemical Reactions BASICS
 Chemical Reactions BASICS There are 5 simple reactions in this chemistry class (but more are coming later in the year). They are synthesis, decomposition, single replacement, double replacement, and combustion.
Chemical Reactions BASICS There are 5 simple reactions in this chemistry class (but more are coming later in the year). They are synthesis, decomposition, single replacement, double replacement, and combustion.
Stoichiometry: Baking Soda and Vinegar Reactions Teacher Version
 Stoichiometry: Baking Soda and Vinegar Reactions Teacher Version In this lab, students will examine the chemical reaction between baking soda and vinegar, and mix different amounts of these household chemicals
Stoichiometry: Baking Soda and Vinegar Reactions Teacher Version In this lab, students will examine the chemical reaction between baking soda and vinegar, and mix different amounts of these household chemicals
Name: Date: Class: Physical Weathering. As you go from station to station, please follow all directions as they are presented to you.
 Name: Date: Class: Weathering Stations Physical Weathering As you go from station to station, please follow all directions as they are presented to you. Station 1 Frost Wedging At this station you should
Name: Date: Class: Weathering Stations Physical Weathering As you go from station to station, please follow all directions as they are presented to you. Station 1 Frost Wedging At this station you should
Section 1: What is a Chemical Reaction
 Section 1: What is a Chemical Reaction I can describe and give examples of physical and chemical changes. I can identify reactants and products. I can explain what happens to molecules in chemical reactions
Section 1: What is a Chemical Reaction I can describe and give examples of physical and chemical changes. I can identify reactants and products. I can explain what happens to molecules in chemical reactions
Experiment 7 Can You Slow It Down?
 Experiment 7 Can You Slow It Down? OUTCOMES After completing this experiment, the student should be able to: tell which factors influence the reaction rate and how they influence the rate. change the temperature
Experiment 7 Can You Slow It Down? OUTCOMES After completing this experiment, the student should be able to: tell which factors influence the reaction rate and how they influence the rate. change the temperature
Matter Lesson 2. Learning Goal 3: I can describe the differences between physical and chemical changes of matter.
 Matter Lesson 2 Learning Goal 2: I can describe the differences between intensive physical properties, extensive physical properties, and chemical properties of matter. Learning Goal 3: I can describe
Matter Lesson 2 Learning Goal 2: I can describe the differences between intensive physical properties, extensive physical properties, and chemical properties of matter. Learning Goal 3: I can describe
Lesson 1: What are chemical changes?
 Lesson 1 Summary Lesson 1: What are chemical changes? Vocabulary Use with pp. 375 377 physical change a change in which the material keeps its identify chemical change a change in which one substance or
Lesson 1 Summary Lesson 1: What are chemical changes? Vocabulary Use with pp. 375 377 physical change a change in which the material keeps its identify chemical change a change in which one substance or
Post-Show. Chemistry. Periodic Table of the Elements. After the Show. Traveling Science Shows
 Traveling Science Shows Post-Show Chemistry After the Show We recently presented a Chemistry show at your school, and thought you and your students might like to continue investigating this topic. The
Traveling Science Shows Post-Show Chemistry After the Show We recently presented a Chemistry show at your school, and thought you and your students might like to continue investigating this topic. The
Activity 2 Elements and Their Properties
 Activity 2 Elements and Their Properties Activity 2 Elements and Their Properties GOALS In this activity you will: Apply ancient definitions of elements to materials you believe are elements. Test some
Activity 2 Elements and Their Properties Activity 2 Elements and Their Properties GOALS In this activity you will: Apply ancient definitions of elements to materials you believe are elements. Test some
Stoichiometry: Baking Soda and Vinegar Reactions Student Advanced Version
 Stoichiometry: Baking Soda and Vinegar Reactions Student Advanced Version In this lab, students will examine the chemical reaction between baking soda and vinegar, and mix different amounts of these household
Stoichiometry: Baking Soda and Vinegar Reactions Student Advanced Version In this lab, students will examine the chemical reaction between baking soda and vinegar, and mix different amounts of these household
What does rate of reaction mean?
 Junior Science What does rate of reaction mean? It is not how much of a product is made, but instead how quickly a reaction takes place. The speed of a reaction is called the rate of the reaction. What
Junior Science What does rate of reaction mean? It is not how much of a product is made, but instead how quickly a reaction takes place. The speed of a reaction is called the rate of the reaction. What
MiSP CHEMICAL REACTIONS, L3 Teacher Guide. Introduction
 MiSP CHEMICAL REACTIONS, L3 Teacher Guide Introduction This weeklong unit should be included with other chemistry content teaching and learning. It is designed to follow Intermediate Level Science Core
MiSP CHEMICAL REACTIONS, L3 Teacher Guide Introduction This weeklong unit should be included with other chemistry content teaching and learning. It is designed to follow Intermediate Level Science Core
Name Period Date. Lab 1: Mass of Ice Materials: beaker, ice and balance.
 Name Period Date Testing the Law of Conservation of MASS! Introduction: Does mass change in a chemical or physical reaction? In this series of experiments you will find the answer to this question. Lab
Name Period Date Testing the Law of Conservation of MASS! Introduction: Does mass change in a chemical or physical reaction? In this series of experiments you will find the answer to this question. Lab
Section I: Synthesis reactions Synthesis reactions occur when two or more substances come together to form a single new substance.
 TYPES OF CHEMICAL REACTIONS A Laboratory Investigation Purpose: Observe the five major types of reactions. Record observations for these reactions. Complete balanced equations for these reactions. Introduction:
TYPES OF CHEMICAL REACTIONS A Laboratory Investigation Purpose: Observe the five major types of reactions. Record observations for these reactions. Complete balanced equations for these reactions. Introduction:
Name Period Date. Lab 9: Analysis of Commercial Bleach
 Name Period Date Lab 9: Analysis of Commercial Bleach Introduction Many common products are effective because they contain oxidizing agents. Some products, which contain oxidizing agents, are bleaches,
Name Period Date Lab 9: Analysis of Commercial Bleach Introduction Many common products are effective because they contain oxidizing agents. Some products, which contain oxidizing agents, are bleaches,
Topic 5 National Chemistry Summary Notes. Acids and Alkalis
 Topic 5 National Chemistry Summary Notes Acids and Alkalis Experiment Collect some samples of rain water LI 1 The ph Scale The ph scale is a continuous range of numbers from below 0 to above 14. Acids
Topic 5 National Chemistry Summary Notes Acids and Alkalis Experiment Collect some samples of rain water LI 1 The ph Scale The ph scale is a continuous range of numbers from below 0 to above 14. Acids
A student investigated three glow sticks. One was placed in water at 5 C, one in water at 40 C and one in water at 70 C.
 1 The picture shows three glowsticks. Photograph supplied by istockphoto/thinktsock Glow sticks contain several chemicals. When a glow stick is bent the chemicals mix. A chemical reaction takes place which
1 The picture shows three glowsticks. Photograph supplied by istockphoto/thinktsock Glow sticks contain several chemicals. When a glow stick is bent the chemicals mix. A chemical reaction takes place which
Shifts in Equilibrium: Le Châtelier s Principle
 6 Shifts in Equilibrium: Le Châtelier s Principle Introduction Whenever a chemical reaction occurs, the reverse reaction can also occur. As the original reactants, on the left side of the equation, react
6 Shifts in Equilibrium: Le Châtelier s Principle Introduction Whenever a chemical reaction occurs, the reverse reaction can also occur. As the original reactants, on the left side of the equation, react
TYPES OF CHEMICAL REACTIONS
 EXPERIMENT 11 (2 Weeks) Chemistry 110 Laboratory TYPES OF CHEMICAL REACTIONS PURPOSE: The purpose of this experiment is perform, balance and classify chemical reactions based on observations. Students
EXPERIMENT 11 (2 Weeks) Chemistry 110 Laboratory TYPES OF CHEMICAL REACTIONS PURPOSE: The purpose of this experiment is perform, balance and classify chemical reactions based on observations. Students
AP Chemistry Unit 2 Test (Chapters 3 and 4)
 AP Chemistry Unit 2 Test (Chapters 3 and 4) NAME: 1. A student is assigned the task of determining the mass percent of silver in an alloy of copper and silver by dissolving a sample of the alloy in excess
AP Chemistry Unit 2 Test (Chapters 3 and 4) NAME: 1. A student is assigned the task of determining the mass percent of silver in an alloy of copper and silver by dissolving a sample of the alloy in excess
Limiting Reagent. Introduction
 Limiting Reagent Introduction The concept of the limiting reagent can be demonstrated by an analogy: Jerry works in the Purchasing Department at a specialty car manufacturing plant in Detroit. The plant
Limiting Reagent Introduction The concept of the limiting reagent can be demonstrated by an analogy: Jerry works in the Purchasing Department at a specialty car manufacturing plant in Detroit. The plant
Supernatant: The liquid layer lying above the solid layer after a precipitation reaction occurs.
 Limiting Reagent Introduction The quantities of substances involved in a chemical reaction represented by a balanced equation are often referred to as stoichiometric amounts. Solution stoichiometry is
Limiting Reagent Introduction The quantities of substances involved in a chemical reaction represented by a balanced equation are often referred to as stoichiometric amounts. Solution stoichiometry is
CHM-201 General Chemistry and Laboratory I Laboratory 4. Introduction to Chemical Reactions (based in part on Small Scale Chemistry methodology as
 CHM-201 General Chemistry and Laboratory I Laboratory 4. Introduction to Chemical Reactions (based in part on Small Scale Chemistry methodology as described in Chemtrek by Stephen Thompson at Colorado
CHM-201 General Chemistry and Laboratory I Laboratory 4. Introduction to Chemical Reactions (based in part on Small Scale Chemistry methodology as described in Chemtrek by Stephen Thompson at Colorado
Figure 1. Oxygen. (g) +... (g)... SO 3. The pressure of the reacting gases was increased.
 Q1. Figure 1 represents a reaction in the production of sulfuric acid. Figure 1 Oxygen Sulfur dioxide Sulfur trioxide (a) Complete and balance the equation for the reaction.... SO 2 (g) +... (g)... SO
Q1. Figure 1 represents a reaction in the production of sulfuric acid. Figure 1 Oxygen Sulfur dioxide Sulfur trioxide (a) Complete and balance the equation for the reaction.... SO 2 (g) +... (g)... SO
Lab #14: Qualitative Analysis of Cations and Anions
 Lab #14: Qualitative Analysis of Cations and Anions Objectives: 1. To understand the rationale and the procedure behind the separation for various cations and anions. 2. To perform qualitative analysis
Lab #14: Qualitative Analysis of Cations and Anions Objectives: 1. To understand the rationale and the procedure behind the separation for various cations and anions. 2. To perform qualitative analysis
TYPES OF CHEMICAL REACTIONS SYNTHESIS (COMPOSITION), DECOMPOSITION AND REPLACEMENT (SINGLE AND DOUBLE), AND COMBUSTION
 TYPES OF CHEMICAL REACTIONS SYNTHESIS (COMPOSITION), DECOMPOSITION AND REPLACEMENT (SINGLE AND DOUBLE), AND COMBUSTION YOU CAN THINK OF ATOMS AS PEOPLE GETTING TOGETHER AS COUPLES... Analogy One person
TYPES OF CHEMICAL REACTIONS SYNTHESIS (COMPOSITION), DECOMPOSITION AND REPLACEMENT (SINGLE AND DOUBLE), AND COMBUSTION YOU CAN THINK OF ATOMS AS PEOPLE GETTING TOGETHER AS COUPLES... Analogy One person
Representing Chemical Change
 Representing Chemical Change As we have already mentioned, a number of changes can occur when elements react with one another. These changes may either be physical or chemical. One way of representing
Representing Chemical Change As we have already mentioned, a number of changes can occur when elements react with one another. These changes may either be physical or chemical. One way of representing
Laboratory 3. Development of an Equation. Objectives. Introduction
 Laboratory 3 Development of an Equation Objectives Apply laboratory procedures and make observations to investigate a chemical reaction. Based on these observations, identify the pattern of reactivity
Laboratory 3 Development of an Equation Objectives Apply laboratory procedures and make observations to investigate a chemical reaction. Based on these observations, identify the pattern of reactivity
Unit 3: Physical Science Classifying Matter in our Daily Lives
 Science 7 Unit 3: Physical Science Classifying Matter in our Daily Lives Name Period Purpose: I understand the relationship between atoms, molecules, elements, compounds, and mixtures and can also provide
Science 7 Unit 3: Physical Science Classifying Matter in our Daily Lives Name Period Purpose: I understand the relationship between atoms, molecules, elements, compounds, and mixtures and can also provide
Recognizing Chemical_Reactions By Dr. Kathleen Vandiver
 Recognizing Chemical_Reactions By Dr. Kathleen Vandiver Hello. My name is Dr. Kathy Vandiver. And we're here at the Edgerton Center at MIT, a university in the United States. And the Edgerton Center is
Recognizing Chemical_Reactions By Dr. Kathleen Vandiver Hello. My name is Dr. Kathy Vandiver. And we're here at the Edgerton Center at MIT, a university in the United States. And the Edgerton Center is
Saturday Science Lesson Plan Fall 2008
 Saturday Science Lesson Plan Fall 2008 LEARNING OBJECTIVES STANDARDS 1.1.1 Observe, describe, draw, and sort objects carefully to learn about them. 1.2.6 Describe and compare objects in terms of number,
Saturday Science Lesson Plan Fall 2008 LEARNING OBJECTIVES STANDARDS 1.1.1 Observe, describe, draw, and sort objects carefully to learn about them. 1.2.6 Describe and compare objects in terms of number,
CHEMISTRY CORE PRACTICALS
 CHEMISTRY CORE PRACTICALS Science (9-1) Combined Science / Chemistry Core Practicals www.chemistryinfo.co.uk Modified 23/03/2018 (MJB) Core Practical INDEX Paper 1 Paper 2 CP1a: Topic: 2.11 Investigate
CHEMISTRY CORE PRACTICALS Science (9-1) Combined Science / Chemistry Core Practicals www.chemistryinfo.co.uk Modified 23/03/2018 (MJB) Core Practical INDEX Paper 1 Paper 2 CP1a: Topic: 2.11 Investigate
HYSICAL AND CHEMICAL PROPERTIES AND PHYSIC AND CHEMICAL CHANGES
 Experiment 4 Name: 15 P HYSICAL AND CHEMICAL PROPERTIES AND PHYSIC AND CHEMICAL CHANGES 13 Al e In this experiment, you will also observe physical and chemical properties and physical and chemical changes.
Experiment 4 Name: 15 P HYSICAL AND CHEMICAL PROPERTIES AND PHYSIC AND CHEMICAL CHANGES 13 Al e In this experiment, you will also observe physical and chemical properties and physical and chemical changes.
Lesson 4. Temperature change
 Lesson 4 Temperature change T E A C H E R G U I D E Lesson summary Students meet scientist Jason Williams, an industrial chemist who designs the materials and processes for making solar cells. He explains
Lesson 4 Temperature change T E A C H E R G U I D E Lesson summary Students meet scientist Jason Williams, an industrial chemist who designs the materials and processes for making solar cells. He explains
Photosynthesis Lab. (adapted from: Photosynthesis.pdf )
 Photosynthesis Lab (adapted from: http://media.collegeboard.com/digitalservices/pdf/ap/bio-manual/bio_lab5- Photosynthesis.pdf ) Background and PreLab: Photosynthesis fuels ecosystems and replenishes the
Photosynthesis Lab (adapted from: http://media.collegeboard.com/digitalservices/pdf/ap/bio-manual/bio_lab5- Photosynthesis.pdf ) Background and PreLab: Photosynthesis fuels ecosystems and replenishes the
H 2 SO 4 HCN H 3 PO 4 HNO 3 HCl. Ca(OH) 2 C 3 H 5 (OH) 3 NaOH NH 4 OH. form LOTS of ions and. 2PbCO 3 Pb(OH) 2. VERY LITTLE ions
 2-15-17 WARM UP: INTRO TO ACIDS AND BASES.. Acids & Bases 14.2 14.3, p. 422-431 1) A few common Acids What do you notice about the formulas?? sulphuric acid hydrogen cyanide (prussic acid) phosphoric acid
2-15-17 WARM UP: INTRO TO ACIDS AND BASES.. Acids & Bases 14.2 14.3, p. 422-431 1) A few common Acids What do you notice about the formulas?? sulphuric acid hydrogen cyanide (prussic acid) phosphoric acid
What Do You Think? Investigate GOALS
 Activity 1 Elements and Compounds GOALS In this activity you will: Decompose water by electrolysis into the two elements from which it is composed. Test the two elements to determine their identities.
Activity 1 Elements and Compounds GOALS In this activity you will: Decompose water by electrolysis into the two elements from which it is composed. Test the two elements to determine their identities.
PDFMAILER.COM Print and send PDF files as s with any application, ad-sponsored and free of charge Activity # 14.
 Activity # 14 Name Purpose Date Date due Activities 10c and 10d - Performing More Examples of Chemical Reactions To perform a number of different chemical reactions, determine what the reactants and products
Activity # 14 Name Purpose Date Date due Activities 10c and 10d - Performing More Examples of Chemical Reactions To perform a number of different chemical reactions, determine what the reactants and products
