Ligand-Interaction Kinetics of the Pheromone- Binding Protein from the Gypsy Moth, L. dispar: Insights into the Mechanism of Binding and Release
|
|
|
- Hector George
- 5 years ago
- Views:
Transcription
1 Article Ligand-Interaction Kinetics of the Pheromone- Binding Protein from the Gypsy Moth, L. dispar: Insights into the Mechanism of Binding and Release Yongmei Gong, 1 Tamara C.S. Pace, 2 Carlos Castillo, 1 Cornelia Bohne, 2 Melanie A. O Neill, 1 and Erika Plettner 1, * 1 Department of Chemistry, Simon Fraser University, Burnaby, BC V5A 1S6, Canada 2 Department of Chemistry, University of Victoria, Victoria, BC V8W 3V6, Canada *Correspondence: plettner@sfu.ca DOI /j.chembiol SUMMARY The pheromone-binding proteins (PBPs), which exist at a high concentration in the sensillum lymph surrounding olfactory neurons, are proposed to be important in pheromone detection and discrimination in insects. Here, we present a systematic study of PBP-ligand interaction kinetics. We find that PBP2, from the gypsy moth, Lymantria dispar, associates and dissociates slowly with its biofunctional ligands, (+)- and ( )-disparlure. Tryptophan anisotropy measurements detect PBP multimers in solution as well as an increase in the multimeric state of the protein upon long exposure to ligand. We propose a kinetic model that includes monomer/multimer equilibria and a two-step binding process: (1) external binding of the pheromone assisted by the C terminus of PBP2, and (2) slow embedding of the pheromone into the internal pocket. This experimentally derived model sheds light on the potential biological function and mechanism of PBPs as ligand scavengers. INTRODUCTION Communication with species-specific signal chemicals (pheromones) plays an important role in insect reproduction. For example, in the case of moths, the female releases the pheromone, and the males detect and follow the pheromone plume upwind to mate. The feather-like antennae of the moth act as a very sensitive chemoreceptor. The antenna is covered with hollow sensory hairs that are innervated by the dendrites of sensory neurons (sensilla). The cuticular hair wall is penetrated by a system of pore tubules that are thought to act as a pathway for the signal molecules passing from the outside of the hair to the interior (Steinbrecht, 1997). The lumen of the hair is filled with a protein-rich solution, the sensillum lymph. The most abundant proteins in the lymph of pheromone-sensitive hairs are the pheromone-binding proteins (PBPs) (Vogt and Riddiford, 1981)(Figure 1). PBPs are small (15 kda), hydrophilic proteins that are specialized members of the insect odorant-binding protein (OBP) family. These proteins have been recently classified into three structural classes: long, medium, and short, differing mainly in the length of their C-terminal segment (Pesenti et al., 2008). Insect OBPs can bind odorants with some selectivity (reviewed: [Honson et al., 2005]); PBPs bind pheromone components. OBPs (including PBPs) are essential for insect olfaction (Kim et al., 1998). For example, OBP76a (LUSH) in Drosophila is required for vaccenyl acetate (cva, a pheromone) detection (Ha and Smith, 2006; Xu et al., 2005) and has been further proven recently to adopt a conformation that activates the odorant receptor itself (Laughlin et al., 2008). Although it is clear now that OBPs can actively present ligands to the receptor, there are other possible functions for OBPs. First, they can transport hydrophobic odorants across the lymph to the receptors (Krieger and Breer, 1999)(Figure 1). Second, the OBPs have been shown to be necessary for both neuronal background activity and odor-evoked activity (Benton et al., 2007; Grosse-Wilde et al., 2007; Laughlin et al., 2008; Xu et al., 2005). Third, OBPs may act as scavengers, buffering high doses of odorant and thereby preventing the neurons from saturating (Honson et al., 2003). The presence of multiple PBPs (Graham and Davies, 2002; Krieger et al., 1991; NagnanLeMeillour et al., 1996; Vogt et al., 1989) indicates that these proteins may also take part in the olfactory coding or signal filtering. Odorant receptors show different activity profiles for a set of ligands in the presence of different PBPs (Grosse-Wilde et al., 2007). PBPs bind individual pheromone components with subtle differences in the binding affinities (Du and Prestwich, 1995; Honson et al., 2003; Plettner et al., 2000). Also, PBPs have flexible binding pockets that can host a wide variety of compounds, but not all of the bound ligands seem to trigger an expedient conformational change of the protein (Kowcun et al., 2001; Lartigue et al., 2003; Lautenschlager et al., 2007; Pesenti et al., 2008). The question that arises now is: are these differences in equilibrium binding affinity functionally meaningful? OBP-ligand interactions require > 30 min to establish equilibrium (Plettner et al., 2000), whereas a moth responds to the pheromonal stimulus in milliseconds; thus, the interactions between the olfactory components (OBPs, ligands, and odorant receptors, etc.) may not be under thermodynamic control. The purpose of this work is to provide a dynamic perspective of the PBP functions. Here, we focus on a lepidopteran, the gypsy moth, Lymantria dispar. The female gypsy moths emit (+)-disparlure ((7R, 8S)-7,8- epoxy-2-methyloctadecane) as the main sex attractant pheromone component (Adler et al., 1972; Bierl et al., 1970, 1972). The antipode, ( )-disparlure, is a behavioral antagonist of upwind flight in gypsy moth males (Vite et al., 1976). The male 162 Chemistry & Biology 16, , February 27, 2009 ª2009 Elsevier Ltd All rights reserved
2 C A Figure 1. Schematic Illustrations of the Moth Olfactory System (A) A close-up view of the hairy branches of moth antenna. (B) Diagram of the olfactory sensillum ([1] olfactory receptor neuron; [2] auxillary supporting cells; [3] dendrite of an olfactory receptor neuron projecting into the hollow space of the sensillum; [4] cuticle wall of the hair; [5] cuticular pores). (C) The peripheral components of the sensillum trichodeum ([6] sensory neuron membrane protein [SNMP]; [7] olfactory receptor and coreceptor; [8] phospholipid bilayer of the neuronal membrane; [9] micelles formed by fatty acids; shaded triangles, pheromone-binding proteins (PBPs); open circles, pheromone molecules). The pheromone molecules adsorbed on the cuticle wall of the sensillum migrate along the surface into the pore canal penetrating the cuticle and diffuse through the pore tubules into the sensillum lymph. PBPs come to interact with the ligands. The pheromone molecule may diffuse by itself through the barrier to associate with the membrane protein and then activate the receptor (Benton et al., 2007). Alternatively, ligand can either activate the PBP (Laughlin et al., 2008) or be delivered by the micelles (Honson, 2006). B moth antenna has separate sensilla populations specialized on either (+)-disparlure or on ( )-disparlure, but not both (Hansen, 1984). The gypsy moth has two known PBPs: LdisPBP1 and LdisPBP2 (PBP1 and PBP2 from here on). The sexual dimorphism, ontogeny (Vogt et al., 1989), and ligand binding affinities of these PBPs (Honson et al., 2003; Inkster et al., 2005; Kowcun et al., 2001; Paduraru et al., 2008; Plettner et al., 2000) have been studied. PBP2 binds (+)-disparlure slightly more strongly than ( )-disparlure. The timescale of PBP-ligand equilibration is much slower than the timescale at which individual insect sensilla are activated after the onset of a stimulus. Thus, kinetic studies are necessary to understand the mechanism of ligand binding and the biological function of PBPs. In this paper, we report the first, to our knowledge, systematic study of the kinetics and mechanism for association and dissociation of physiologically significant pheromone compounds with PBP2. Our experimental approach relies on two techniques. First, we have applied our previous binding assay, in which bound and free ligand are separated by passage of the equilibrated mixture through a size-exclusion filter (Paduraru et al., 2008; Plettner et al., 2000). Second, we have developed a fluorescence assay, which relies on surface dansylation of the PBP and a decrease in dansyl (DNS) fluorescence upon ligand addition. Furthermore, since previous studies have suggested that PBPs multimerize (Danty et al., 1999; Honson et al., 2003; Leal, 2000; Maida et al., 1993; Plettner et al., 2000), we have also used Trp fluorescence anisotropy to examine the possible connection between PBP aggregation and ligand binding. Our results indicate a two-step interaction process between PBP2 and ligand. A fast uptake of the hydrophobic ligand from the buffer is followed by slow embedding of the ligand into the binding pocket. The first process, rapid capture of the ligand on a millisecond timescale, has also been detected in a previous study with the PBP from the silk moth (Bombyx mori), BmorPBP (Leal et al., 2005). We have detected this rapid process indirectly (see below), and we have found no selectivity between (+)- and ( )-disparlure for PBP2. The second slow-binding step was found to be selective. Therefore, we have followed the second process, in which PBP2 shows similar discrimination between (+)- and ( )-disparlure in the kinetic constants (this paper) as in the dissociation constants (Plettner et al., 2000). We propose that the rapid-binding step mainly involves the C-terminal region of the protein, and that the slow-binding step mainly involves the core of the protein. This proposal is supported by the kinetics of a truncated form of PBP2 (T-PBP2) that lacks the 17 residues from the C terminus. Without the C-terminal peptide, T-PBP2 binds (+)-disparlure 103 weaker externally and exhibits much slower kinetic behavior. We believe that the C termini of longchain PBPs play an important role in interactions with ligands. An effective conformational change could be triggered by a ligand either in the first step or the second step. In our simulation with all of the available kinetic constants, the results support that PBP does not need to associate quickly to produce sufficient PBPligand complex, i.e., properly shaped PBP (either P.L ex or P.L) (Equation 4), to activate the olfactory receptor (see Supplemental Data available online). We also present evidence for more than one population of PBP2 in solution. These populations are in equilibrium with each other and consist of high-order multimers. We propose a role for these multimers in ligand scavenging. RESULTS Ligand Binding Affinities of the Dansylated PBP2 and of the C Terminus-Truncated PBP2 The average amount of disparlure bound to dansylated PBP2 (DNS-PBP2) was compared to that bound to unlabeled PBP2. The result showed no statistically significant difference (six replicates, t test, p > 0.05, see Supplemental Data), and showed that the two proteins have very similar dissociation constants (Table 1). Therefore, the DNS modification did not affect the binding affinities of PBP2. This might be explained by the modification sites K31 and 38 being on the surface of the homologymodeled PBP2 structure. However, the C-terminally truncated PBP2, T-PBP2, exhibited a significantly reduced thermodynamic binding affinity toward (+)-disparlure (Table 1). Two observations suggest similar secondary structures for PBP2 and T-PBP2: (1) T-PBP2 reacts with antiserum raised against PBP2; and (2) both proteins have similar far-uv CD spectra (Figure S5). (Details of T-PBP2 preparation and conditions under which the K D s were measured can be found in Supplemental Data.) Chemistry & Biology 16, , February 27, 2009 ª2009 Elsevier Ltd All rights reserved 163
3 Table 1. The Comparison of the Dissociation Constants between PBP2, DNS-PBP2, and T-PBP2 with (+)-Disparlure by GC Assay [L] total (mm) [L] bound (mm) K D = [L] free [P] free / [L] bound (mm) [P] total Protein (mm) PBP ± ± ± 0.9 DNS-PBP ± ± ± 1.8 T-PBP ± ± ± 3 Means ± SE of six replicates. The correlation between the change in I dns and the concentration of ligand-bound protein is not dependent on total protein concentration; I dns is observed to decrease with the same slope for either 2 or 4 mm protein (Figure 2B, procedures are described in Supplemental Data). Similar results were obtained with (+)-disparlure, ( )-disparlure, or the racemic mixture (data not shown). Since I dns is more sensitive than I trp to ligand binding, we have used I dns to monitor the kinetics of protein-ligand association and dissociation. Optical Properties of DNS-PBP2 When excited at 295 nm, DNS-PBP2 gives two emission peaks, assigned to Trp at 327 nm and DNS at 520 nm. The intensity of each is designated as I trp and I dns respectively (Figure 2A). The I dns /I trp ratio decreased significantly (>20%) upon ligand binding, dominated by a decrease in I dns. With increasing concentration of ligand-bound protein, both I dns and I trp are observed to decrease linearly, although the change in I trp is substantially smaller (Figure 2A). For this reason, subsequent experiments were conducted with selective excitation of DNS at 340 nm. Kinetic Studies Association of DNS-PBP2 with (+)- and ( )-Disparlure We have observed a slow association of ligands to PBP2 and DNS-PBP2, in seconds, and an even slower association to T-PBP2, in minutes (Figures 2C 2E). We consistently observed in both fluorescence and gas chromatography (GC) assays a non-zero physical quantity at time 0, which suggested some kinetic behavior of the protein that was not resolved on our experimental timescale (5 s). This is clearly visible in our fluorescence assay (Figure 2C), in which 50% of the total A B C D E Figure 2. The Optical Properties of DNS-PBP2 Related to Ligand Binding and the Slow Association Kinetics of PBP2, DNS-PBP2, and T-PBP2 (A) An example to show the I trp and I dns change in tryptophan and dansyl fluorescence intensity, with increasing concentration of the protein-ligand complex for DNS-PBP2-(+)-disparlure, when the total protein concentration was kept at 2 mm. Samples were excited at 295 nm. The inset shows the spectra (black, without ligand; pale, with ligand). (B) I dns decreased linearly, corresponding to the increase in the concentration of ligand-bound protein. Samples were excited at 340 nm, for two different total protein concentrations (squares, 2 mm; diamonds, 4 mm). (C) I dns decreased with time upon ligand addition (black line), whereas the solvent for the ligand, EtOH, showed no effect (pale line, lower). The DNS fluorescence was stable with time when there was no treatment (pale line, upper). (D and E) The association of (D) 2 mm PBP2 and (E) T-PBP2 with 10 mm (+)-disparlure determined by a GC assay (see Experimental Procedures). Each point represents the average of at least three replicates, and bars indicate the SE. The slope represents V 0, the initial binding velocity used in the determination of the order in Figure 3B. T-PBP2 shows much slower kinetics. 164 Chemistry & Biology 16, , February 27, 2009 ª2009 Elsevier Ltd All rights reserved
4 A B C fluorescence quenching associated with ligand binding is static. Similar behavior is detected in our GC-based assay. The shortest feasible incubation time for GC-based assays is similar to the time resolution of the fluorescence experiments. Within this time window, we also observed by GC that 40% of the total concentration of PBP2-ligand complexes (monitored over 100 s) had already formed (Figure 2D and Figure S6). We are unable to resolve the kinetics of PBP2 with (+)-disparlure in < 5 s with the current methods. However, we have carefully validated our measurements with different methods for the slower (>5 s) kinetic behavior, and the results are consistent. In this work, we have probed the slow association by measuring the initial binding rate as a function of ligand concentration according to Equation 1: V 0 = k on ½PŠ m 0 ½LŠn 0 ; (1) where V 0 is the initial linear rate of the slow phase, k on is the association constant, P 0 is the initial protein concentration, L 0 is the initial ligand concentration, and m and n reflect the reaction order of the protein and ligand, respectively. With increasing ligand concentration in the low-concentration regime in which ligand is limiting ([L] % 2 mm, 2 mm DNS-PBP2), the initial rate increased linearly. From the slope, we have obtained the k on values for both ligands (Figure 3A). The reaction order, n for ligand, was obtained from the slope of a plot of logv 0 versus log[l] 0 (Table 2). At ligand concentrations exceeding that of protein, V 0 becomes independent of ligand concentration. This result is very important. It indicates that PBP2 becomes saturated with excess ligand and reaches its maximum association velocity, which is independent of the ligand concentration. We have observed an offline point at 2 mm ligand for both ligands, in both methods. This is consistent with our previous observation that binding affinity is related to the protein:ligand ratio (Honson et al., 2003). One explanation is that PBP acts differently at high and low ligand concentration, and that 2 mm, corresponding to 1:1 PBP:ligand ratio in our case, is a switch point for different PBP functions. In a second series of experiments, the protein concentration was varied from 1 to 8 mm, whereas the ligand concentration was in constant excess (10 mm). The initial rates thus obtained correspond to the maximum at each protein concentration. These are shown in Figure 3B derived from fluorescence and GC assays. These data may be fit to Equation 2: log V max = log k + m log P 0 ; (2) Figure 3. Binding Rates of PBP2 with (+)- or ( )-Disparlure and the Dissociation of DNS-PBP2-Ligand Complexes with Time (A C) For DNS-PBP2, rates were obtained by excitation at 340 nm and after changes in DNS fluorescence upon addition of ligand. For PBP2 and T-PBP2, rates were obtained from plots of GC-based data, such as the one in Figures 2D and 2E. Bars indicate SE for fluorescence-based data and fitting errors for GC-based data. (A) The plot of V 0 against L 0 when the protein concentration was 2 mm and the ligand concentration was varied between 0.6 and 8 mm. V max is the maximal rate of ligand binding at ligand saturation. Curves are fitted to the Michaelis-Menten equation (squares, DNS-PBP2 and (+)-disparlure; circles, DNS-PBP2 and ( )-disparlure; triangles, T-PBP2 and (+)-disparlure). (B) Based on Equation 2, the plot of logv max against logp 0 when the ligand concentration was held constant at 10 mm and protein concentration was varied between 1 and 8 mm (diamonds, PBP2; squares, where m represents the general association order of the protein, whereas the parameter k typically represents the rate constant. The meaning of this parameter for PBP2 ligand binding will be discussed below. The association orders for PBP2 from either fluorescence (0.81 ± 0.03) or GC (0.35 ± 0.02) assays are both smaller than 1 (Table 2). DNS-PBP2). The slopes give the reaction orders in protein, which are both smaller than 1 (see text). (C) The amount of the complex that is needed to be consumed to reach the final equilibrium was plotted against time (Equation 1). Fitting of the data to the first-order exponential decay presented the apparent dissociation rate constants, which are s 1 for (+)-disparlure and s 1 for ( )-disparlure. Fitting results are listed on the top right corner. Chemistry & Biology 16, , February 27, 2009 ª2009 Elsevier Ltd All rights reserved 165
5 Table 2. Summary of Ligand-Binding Kinetics and Thermodynamics for PBP2 Measurement Ligand (+)-Disparlure ( )-Disparlure k on /M -1 s 1a DNS-PBP2 (4.8 ± 0.4) (1.6 ± 0.2) T-PBP2 (0.12 ± 0.01) N.D. b k off /s 1a Flu. (4.7 ± 0.4) (5.0 ± 0.2) Radio c n 1.1 ± ± 0.3 K D /mm Flu. k off /k on Radio c K 0 D /mm d DNS-PBP2 0.9 ± ± 0.6 T-PBP2 10 ± 3 N.D. k 2 /s 1e DNS-PBP T-PBP N.D. m Flu ± 0.03 N.D. GC 0.35 ± 0.02 N.D. a V on =k on [P] m [L] n ;V off =k off [P$L]. b N.D., not determined. c Assays with radio-labeled disparlure (Honson et al., 2005; Plettner et al., 2000). d The dissociation constant of the hypothetical intermediate P$L ex from the fitting of PBP2 association data (Figure 3A) with the Michaelis-Menten equation: e 0 k 2 =K D 3 k on (Equation 4). K M = k 1 + k 2 z k 1 = k 0 D k 1 k : 2 The association curve of T-PBP2 with (+)-disparlure is very similar to that of PBP2 (Figures 2E and 3A), except that the binding is much slower (for experimental procedures, see Supplemental Data). Dissociation of DNS-PBP2-Ligand Complexes Dissociation of DNS-PBP2-ligand complexes (Figure 3C) follows an apparent first-order exponential decay (Equation 3, derived in Supplemental Data): x = Ae kappt ; (3) where x represents the amount of complex dissociated to reach the final equilibrium, and k app is the apparent dissociation rate constant, k off. DNS-PBP2 complexes with either (+)- or ( )-disparlure dissociate with similar, extremely slow, kinetics (Table 2). Our rate constants for ligand binding and release yield dissociation constants (K D =k off /k on, Table 2) that compare well to literature values of 1.8 and 3.2 mm for (+)- and ( )-disparlure, respectively (Plettner et al., 2000). The slight selectivity between these two ligands was preserved. That is, PBP2 binds (+)-disparlure more strongly than ( )-disparlure, either in equilibrated conditions or as indicated from the derived kinetic constants. These results are important, as they indicate that the processes we are following represent the rate-limiting steps for binding and dissociation. Molecular Size of PBP2-Ligand Complexes Evaluated by Tryptophan Anisotropy We have measured the Trp fluorescence anisotropy of unlabeled PBP2, and its ligand complexes, as a function of solvent viscosity in order to evaluate their hydrodynamic volumes and, correspondingly, their degree of multimerization. Double reciprocal plots of anisotropy versus viscosity are linear, as predicted by Equation S6 (Figure S8). To extract the volume, V, from these data, we also require the limiting anisotropy, r 0, and fluorescence lifetime, t, of Trp in each sample and the hydrodynamic volume of monomeric PBP. The former, r 0, was obtained from the linear fitting of the data, and t (Table 3) has been measured experimentally (Supplemental Data). The hydrodynamic volume of monomeric PBP2 is estimated to be 29 nm 3 according to Equation S7. Based on crystallographic data, the approximate dimensions of the PBP from the silkworm moth Bombyx mori (BmorPBP) (15.9 kda, 142 aa) are Å (Sandler et al., 2000). Its corresponding volume, approximating an ellipsoid, is 22.0 nm 3. Considering that PBP2 (16 kda, 145 aa) is longer by 3 amino acids than BmorPBP, the calculated 29 nm 3 volume for PBP2 monomer is reasonable. Hydrodynamic volumes of PBP2 and its complexes are evaluated according to Equation S6, yielding values ranging between 40 and 90 nm 3 depending on protein concentration and ligand incubation time (Table 3). Within experimental error (±5 nm 3 ), the average hydrodynamic volume of apo-pbp2 was independent of protein concentration between 2 and 10 mm, and the averaged volume for an overall population of PBP2 (free and ligand-bound forms) was unchanged by short incubation with ligand. A consistent increase in volume was detected after overnight incubation with ligand. In all cases, the numbers of monomers in each rotational unit (evaluated as V sample /V monomer ) are nonintegral. This is not surprising given that we are measuring the average molecular volume of an equilibrium population of PBP2 species, e.g., Table 3. Trp Anisotropy Parameters for PBP2 and PBP2-(+)-Disparlure Complexes after Different Incubation Conditions Conditions 2 mm 10 mm Without Ligand 3 min Incubation Overnight Without Ligand 3 min Incubation Overnight r t/ns a b b 4.74 V/nm Number of monomers c 1.8 ± ± ± ± ± ± 0.8 a Amplitude average lifetime. b Average of the lifetimes obtained without ligand and incubated with ligand overnight. c V sample /V monomer. 166 Chemistry & Biology 16, , February 27, 2009 ª2009 Elsevier Ltd All rights reserved
6 monomer and dimer. We further note the errors associated with these measurements and the theoretical approximations made, including modeling the rotating particle as a sphere. However, in all cases, the number of monomers is greater than unity, largely between 1 and 2 within experimental error. This consistently suggests that PBP2 does not exist as homogenous monomers in solution, either with or without ligand; instead, an equilibrium population of monomers and dimers is the simplest explanation for our data. The change of the multimerization equilibrium induced by ligand was significantly slower than the binding kinetics. Therefore, the change in multimer states during the time window of the binding kinetics was insignificant, simplifying data analysis. DISCUSSION Fluorescent PBP2 Mimics Wild-Type Protein As confirmed by the GC assay, PBP2 and DNS-PBP2 have the same dissociation constants within experimental error (Table 1); the modification does not change the binding affinities of PBP2. It is therefore likely that the binding pocket and corresponding ligand interactions are unaffected by the fluorophore, consistent with the labeling sites (K31 and/or K38) being on the surface of the protein. Furthermore, two different methods, GC and fluorescence, gave fractional association orders, m, for PBP2 with (+)-disparlure (Figure 3B), and K D values estimated from k on and k off for both ligands are close to those previously reported (Table 2). Thus, the fluorescent-labeled PBP2 is a valid mimic of wild-type protein and provides a method for measurements of native ligand binding kinetics. Kinetic Pathway for the PBP2-Ligand Interaction Previous studies have found that a decrease of the ph can induce a significant conformational change on the C termini of long-chain PBPs (Damberger et al., 2007). The newly formed seventh a helix then occupies the binding pocket and therefore is proposed to be responsible for the release of the ligand near the olfactory neuron membrane, where the ph is assumed to be significantly lower than the lymph ph. However, from a more recent study, it seems that a small local conformational change of the PBP (not caused by ph changes) is sufficient to trigger the receptor response (Laughlin et al., 2008). We did not investigate the ph effect on PBP-ligand interaction kinetics in this paper. Based on the behaviors of PBP2 under physiological ph we have found here, we propose an alternative pathway of ligand binding and releasing, without invoking a significant conformational change of the C terminus induced by a ph decrease. Based on the measured association and dissociation kinetics of DNS-PBP2 with (+)- and ( )-disparlure, we propose that PBP2 associates with the ligand first at a peripheral site, building equilibrium promptly with the rate constants k 1 and k 1 for the forward and reverse interactions, respectively (Equation 4). The PBP2-ligand complex intermediate, P$L ex, may then be transformed to the specific complex P$L, by properly orienting the ligand and docking it into the inner binding pocket. The rate constant for this step is designated as k 2. Complex P$L dissociates to the intermediate P$L ex with a very small rate constant, k 2 (Equation 4). The rate-limiting step for binding is internalization of the initially associated ligand, whereas exhalation of the bound ligand to the peripherally associated species rate limits the dissociation process (k 1»k 2,k 1»k 2 ): P + L % k 1 k 1 P$L ex % k2 P$L: (4) k 2 The proposed intermediate in the binding pathway is necessary to rationalize the saturability of the association curves observed in both fluorescence and GC-based assays (Figure 3A). Two-phase binding kinetics have been observed during earlier work on PBPs (Honson et al., 2005; Leal et al., 2005), and a similar ligand-binding pathway has been observed for human cytochrome P450 3A4 (Isin and Guengerich, 2007), for which substrate binds to a peripheral site before entering the catalytic pocket. Here, we find that the initial association proceeds at a fast rate, which cannot be resolved on the timescale we are using, suggesting that it is more easily accessed by ligand. Our recent results with a fluorescent ligand and stopped-flow measurements confirm the existence of this fast step (unpublished data). We propose that this first binding site is a hydrophobic patch on the protein surface, specifically a site near the C terminus of the PBP. This model is based on several facts. First, the C terminus is sufficiently hydrophobic to accommodate the aliphatic chain of the ligand (Figure 4A). Second, the flexibility of the C terminus provides a greater opportunity for protein-ligand collision than the less flexible core of the protein. Third, the C terminus, the N terminus, and the loop between a helices 2 and 3 comprise an opening of the binding pocket with considerable mobility (Zubkov et al., 2005). Our proposed model is also supported by two other pieces of experimental data. First, in the photoaffinity labeling of Antheraea polyphemus PBP (ApolPBP) with an analog of its pheromone, the exclusively labeled residue (Thr44) is located on the a2/a3 loop in a conformation pointing outward relative to the binding pocket (Du and Prestwich, 1995; Mohanty et al., 2004). Second and most importantly, the 403 slower kinetic behavior of T-PBP2 (this study) indicates damage to the association phase when the C terminus is missing (Table 2). We also suggest that the external binding site possibly involves Trp37, which is highly conserved in all long-chain PBPs and is in the a2-a3 loop (Figures 4A and 4B). Assays of ligand binding based on changes in Trp fluorescence are consistent with our proposed two-step binding model. Binding of bombykol, the cognate ligand for BmorPBP, was shown to quench the intrinsic Trp fluorescence of BmorPBP in milliseconds with no spectral shift (Leal et al., 2005). Also, titration of ligand into ApolPBP elicited a change of the Trp37 fluorescence intensity with no shift in emission wavelength (Bette et al., 2002). These changes in Trp fluorescence intensity with no spectral shift appear to be characteristic of a rapid interaction between a ligand and the external binding site. The process of ligand translocation from the external, peripheral site to the internal binding pocket has been the focus of the current kinetic study. Due to technical problems with adsorption of very hydrophobic ligands in the stopped-flow fluorimeter, we have put our efforts toward studying the slow step. We have observed the first step, using a more soluble fluorescent dye as a surrogate ligand (unpublished data). With DNS-PBP2 and disparlure, we could not separate the initial association from Chemistry & Biology 16, , February 27, 2009 ª2009 Elsevier Ltd All rights reserved 167
7 A B C Figure 4. Sequence Alignments and Models (A) Sequence alignments of the PBPs of the moths Antheraea polyphemus, A. pernyi, Argyrotaenia velutinana, Bombyx mori, Heliothis virescens, and Lymantria dispar. An asterisk indicates a fully conserved residue, a colon specifies strongly conserved residues, and a period indicates a weakly conserved residue. Overall, the C termini of the long-chain PBPs, starting from the second framed Trp, share high similarity and are considerably hydrophobic. (B) The threading structures of PBP2 on 20 of the NMR structures of BmorPBP (PDB ID: 1LS8) (left) (Lee et al., 2002) and ApolPBP (PDB ID: 1QWV) (right) (Mohanty et al., 2004). Trp37 and Trp129 are shown. The C terminus and the loop between a helices 2 and 3 present multiple conformations. Models were prepared with Spdb-viewer. (C) Model of the equilibrium between the PBP dimer and monomer. Addition of ligand or high protein concentration favors the formation of dimers (step a), in which two populations of PBP exist (type A and type B). Type B PBP has a more blocked ligand entrance to the inner binding site. The binding process of PBP molecules is labeled as step b. Dimeric PBP has smaller binding capacity (one ligand per two proteins) than the monomeric PBP (one ligand per protein) (ellipses, ligand; triangles, PBP). The binding capacity is shown schematically below the line. 168 Chemistry & Biology 16, , February 27, 2009 ª2009 Elsevier Ltd All rights reserved
8 the translocation process. However, the overall binding process is saturable (Figure 3A), meaning that a steady-state population of the intermediate (P$L ex ) builds up rapidly, and that the externally adsorbed ligand is then slowly internalized. The k on values obtained represent the overall rate constant for both steps. The overall process (Equation 4) is analogous to a pathway for onesite enzyme catalysis. A plot of the initial rate against ligand concentration at constant protein concentration may thus be fit to the Michealis-Menten equation to estimate the K M value. This will be close to the dissociation constant, K 0 D, of the intermediate when k 1»k 2 (Table 2). This is related to the concentration of the intermediate, Equation 5, ½P$L ex Š = ½PŠ½LŠ z ½PŠ½LŠ ; (5) K 0 D K M and, correspondingly, to the overall rate constant for ligand binding, k on, Equation 6, V on = d½p$lš = k 2 ½P$L ex Šz k 2 ½PŠ½LŠ = k on ½PŠ½LŠ: (6) dt K M The values of K 0 d for (+)- and ( )-disparlure with DNS-PBP2 are similar, (0.9 ± 0.5) mm and (1.6 ± 0.6) mm, respectively. However, the binding affinity of (+)-disparlure at the peripheral site of T-PBP2 is 103 weaker (K 0 D =10mM). This agrees well with the hypothesis that the C terminus is the major component of the peripheral binding site. The translocation rate constant k 2, as a product of K 0 D and k on, is a first-order rate constant with the unit of s 1. The k 2 values show slight difference between ligands ( s 1 for (+)-disparlure and s 1 for ( )-disparlure), suggesting that the slow second translocation step is ligand selective. This value for T-PBP2 with (+)-disparlure is smaller ( s 1 ), which means that the loss of the C terminus affected the internalization of the ligand to some extent (Table 2). The dissociation of the P$L complex is extremely slow. This is likely caused by complete enclosure of the ligand in the binding pocket, such as bombykol in BmorPBP and cva in LUSH (Laughlin et al., 2008; Sandler et al., 2000). Furthermore, our observation that T-PBP2 dissociates from ligand more easily (larger K D, Table 1) indicates that the removal of the C terminus has lowered the barrier to dissociation. Recent research has shown the importance of the subtle conformational changes of the C termini of medium-chain PBPs induced by ligand binding (Laughlin et al., 2008; Pesenti et al., 2008). This could also be the case for the C termini in the long-chain PBPs. Based on our study, we propose that the C terminus and the other components of the external binding site may act as a stepping stone that assists the entry of the ligand into the interior binding pocket, as well as a gate that may lock the ligand inside and make dissociation difficult. Monomer and Multimer Equilibrium in Solution Aggregation of OBPs in solution has been reported several times (Danty et al., 1999; Honson et al., 2003; Leal, 2000; Plettner et al., 2000) and has been observed directly in the solid state by X-ray crystallography (Kruse et al., 2003; Lartigue et al., 2003, 2004; Laughlin et al., 2008; Sandler et al., 2000). Since the oligomeric state of the proteins may affect the interactions with ligand, we need to understand the aggregation forms of the PBP under the kinetic test conditions. We propose that, in solution, the PBP monomer exists in equilibrium with at least one other population of higher-order aggregate, most likely a dimer under our experimental conditions. This proposal is supported by values for PBP2 hydrodynamic volumes, estimated by Trp fluorescence anisotropy, that are intermediate between monomeric and dimeric forms, even at a low concentration of 2 mm (Table 3). Additional evidence for protein multimerization comes from measurements of the initial association rate of PBP2 with an excess of ligand, which provides the maximum rate, V max,at that protein concentration (Figure 3B). We found that V max is proportional to [P] m,m<1(table 2), which means that V max /[P] will decrease with increasing protein concentration. Since V max represents the maximum number of ligand molecules that can be bound to the protein per second, V max /[P] represents the maximum number of bound ligand molecules per protein molecule per second in other words, the binding capacity of the PBP per second. At increased protein concentration, this binding capacity decreases. One explanation is that, at high protein concentration, the aggregated protein blocks the binding of ligand to some extent. If there are two populations of protein in the solution (Figure 4C), they may or may not have the same conformation. One population of PBP can bind ligand directly (A in steps b1 and b3), whereas another, whose binding pocket is blocked in the multimeric form, needs to dissociate first (B in step b2). The monomer-multimer equilibrium will shift toward multimer with increasing protein concentration (step a), accounting for the decreased per-second binding capacity in the higher protein concentration regime. Consequently, we suggest addition of another component to the core PBP2 kinetics scheme, namely, equilibrium between monomeric and dimer/multimeric forms of PBP2. The shift of this equilibrium induced by ligand is slow and therefore will not interfere with the PBP-ligand interaction kinetics. Steady-state kinetics do not resolve the equilibrium distribution; our measurements reflect the population-weighted average kinetic properties of all PBP2 species, of which only ligand-binding-competent type A are represented by P in Equation 4. The maximal initial velocity measured by both fluorescence and GC methods (Figure 3B) corresponds to the P A $L ex concentration at its maximum. In excess ligand, the concentration of the intermediate P A $L ex is proportional to the concentration of P A, which is linked to the initial protein concentration, P 0, and the dimer-monomer equilibrium constant. Since we have no information on the latter, we make no attempt to derive or solve an expression for the parameter k in Equation 2. Considering the high concentration of PBPs in the sensillum lymph (average of 600 mm for LdisPBPs [Honson, 2006]), it is unlikely that PBP will reach maximum velocity at odorant doses encountered in typical plumes. These doses range from 10 molecules$s 1 $ sensillum 1 (17 pm) to 10 8 molecules$s 1 $sensillum 1 (170 mm) (Supplemental Data). Multifunctional PBPs Based on previous work, C terminally activated PBP can be an activator of pheromone-sensitive neurons (Laughlin et al., 2008). Our study reveals two-step association kinetics of a ligand with PBP2 and indicates a role of the C terminus in ligand Chemistry & Biology 16, , February 27, 2009 ª2009 Elsevier Ltd All rights reserved 169
9 binding. The rapid phase is proposed to involve the uptake of the ligand by the C terminus of PBP2, whereas the slow phase is proposed to involve specific internalization of the ligand into the binding pocket. The selectivity of this slow step parallels the thermodynamic binding selectivity of PBP2 with different ligands, consistent with the observation that pheromone ligand can specifically trigger an active conformation of the PBP (Laughlin et al., 2008). However, the selectivity here is still not strong enough to count for the high specificity of the moth olfactory system. Therefore, as indicated by Laughlin et al. (2008) and Pesenti et al. (2008), the selectivity of the peripheral olfaction may rely on the differences of the C-terminal conformations of PBPs with different ligands. In our hypothesis, the insect olfactory sensing may not be under thermodynamic, but kinetic, control. The most active ligand might be the one that most efficiently triggers the C terminus conformational change. We do not know at which stage the C terminus of the PBP is activated the external binding stage or the internal binding stage. However, because the odorant receptor is sensitive enough to detect a few molecules, sufficient amount of P$L ex or P$L complexes could be formed in milliseconds, even for a slow-binding protein such as PBP2 (Figure S9). Another PBP from the same species, PBP1, binds ligand 23 faster (unpublished data). In addition to a ligand transporter and activator, PBP may also act as a scavenger. PBPs can multimerize into an asymmetric dimer. Each monomer of this asymmetrical unit presents a different conformation of the C terminus (Laughlin et al., 2008; Sandler et al., 2000). Specifically, it is the monomer B (whose C terminus is relatively more blocked) that retains the active conformation when a ligand is bound (Laughlin et al., 2008). The active conformation could very possibly be deactivated through PBP dimerization. We have observed slow multimerization of PBP2, and we hypothesize that, at high ligand concentration, this could be one mechanism used to protect the receptors from being saturated. SIGNIFICANCE Odorant-binding proteins play a significant role in odorant detection in insects. Pheromone-binding proteins, PBPs, are members of this protein family, specialized in binding pheromones. Various biological functions have been proposed for PBPs, and a lot of their static properties have been unveiled, such as the 3D structures and binding affinities with different ligands. However, little effort has been made to understand the dynamic interactions between a PBP and a ligand. To our knowledge, we have presented here the first systematic kinetic study on the interaction between one PBP, PBP2, from the gypsy moth, with its natural ligands, (+)- and ( )-disparlure. We have shown that PBP2, which belongs to the long category PBPs (with aa hydrophobic C-terminal peptide), binds hydrophobic ligands in two steps: one rapid, the other slow. We suggest that the slow one corresponds to the specific reorienting and embedding of the ligand to the internal binding pocket, whereas the rapid one is from the binding to an external site close to the C terminus. Based on recent literature (Laughlin et al., 2008), the PBP-ligand complex, with ligand bound at an internal site, is an active ligand of the odorant receptor. Our study has revealed an important role for the C terminus of long-chain PBPs: to act as a gate and as part of a path for the ligand. We also provide more evidence for the existence of PBP multimers in solution, and we provide new, to our knowledge, evidence that, over a long time (hours), the multimerization state increases in the presence of ligand. Our results support the hypothesis that, in addition to a carrier of ligand, PBP is also a scavenger. EXPERIMENTAL PROCEDURES Preparation of Dansylated PBP2 PBP2 was expressed and purified as previously described (Plettner et al., 2000) and was stored at 37 C in 20 mm Tris-HCl buffer (ph 7.4). Before the reaction, 10 ml of mm PBP2 solution was dialyzed against L 20 mm NaHCO 3 buffer (ph 10.3) overnight at 4 C, to replace the Tris. Two times excess of 53.0 mm fresh dansyl chloride (DNS-Cl) in EtOH was slowly added to the protein solution every half hour. The reaction was conducted at room temperature on a stirring plate and stopped after 1 hr by running the crude product on preparative 12% native PAGE gels directly. Fluorescent fractions were pooled together and dialyzed against 20 mm Tris-HCl buffer (ph 7.4). The dansylation was confirmed by MALDI mass spectrometry, the composition, with respect to the number of dansyl groups attached (see Supplemental Data), was evaluated by FPLC, and the attachment sites (K31 and 38) were identified by peptide mapping (Table S3). The apparent molecular weights of the protein samples were calculated according to their compositions. Extinction coefficients at 280 nm, 3 280, evaluated from known quantities of pure nondansylated and dansylated PBP2 compare well to calculated estimates, which are based on the amino acid composition of the PBP2 (Pace et al., 1995) and the absorbance of DNS group at 280 nm (3 280 = 1920 M 1 cm 1 for dansyl t-butylamine): M 1 cm 1 = #Trp #Tyr #Cys #DNS (#Trp = 2, #Tyr = 2, #Cys = 6, #DNS = P i0 a ið#dnsþ i )(Table S3). The experimentally measured values were used to evaluate protein concentration. All protein samples used for the experiments were in 20 mm Tris-HCl buffer (ph 7.4) unless otherwise indicated. Association of DNS-PBP2 with (+)- and ( )-Disparlure Ligand association with DNS-PBP2 was investigated at a constant protein concentration (2 mm) with varied ligand concentrations (0.6 8 mm), and also at a constant ligand concentration (excess, 10 mm) with varied protein concentration (1 8 mm). Each protein sample was equilibrated in the fluorescence cuvette for at least 2 min before ligand addition, and all measurements were made at 20 C. At least four replicates were performed. Controls, in which the same volume of EtOH was added to the protein as that of the ligand stock, were performed in parallel. Samples were excited at 340 nm, and the emission of DNS-PBP2 was monitored at 522 nm one point per second for at least 90 s. To validate the optical measurements, we have performed a second series of experiments, in which the protein concentration was varied and the ligand concentration was constant and in excess by using a GC assay. In this assay, 100 ml PBP2 samples of 1, 2, 4, and 8 mm were incubated with 10 mm (+)-disparlure (1 ml of 1 mm ethanol stock) for various lengths of time (5 90 s), and each point was tested in triplicate. Pheromone bound to PBP was separated from the free pheromone by gel filtration on small columns of Bio-Gel P2 (0.06 g) in a 200 ml pipette tip. The filtrate was extracted with ml hexane:ethyl acetate (1:1) mixture, and the recovered ligand was quantitated by GC. Dissociation of DNS-PBP2 with (+)- and ( )-Disparlure The DNS-PBP-ligand complexes were obtained after an overnight incubation as described above, and they were diluted to the desired concentration of 2 mm. The DNS fluorescence intensity was monitored immediately after preparation for about 3 hr at 20 C. Dissociation rate constants were obtained by fitting the data to an exponential decay. 170 Chemistry & Biology 16, , February 27, 2009 ª2009 Elsevier Ltd All rights reserved
10 Tryptophan Anisotropy Measurements Samples (20 mm Tris buffer [ph 7.4]) were prepared as described by Flecha and Levi (2003), and sample viscosity was varied with glycerol, by using the same composition for each set of samples (four replicates). Two protein concentrations (2 and 10 mm) were chosen. Experiments were conducted without ligand and with 10 mm of the most strongly bound ligand for each protein. Ligand-containing samples were measured after 3 min and again after overnight incubation. Tryptophan was excited at 280 nm, and its emission was monitored at 335 nm (20 C) by using a HORIBA Jobin Yvon SPEX spectrofluorometer (Fluorolog-3) equipped with Glan-Thompson autopolarizers (5 nm bandwidth). Reported anisotropy values, determined from the intensity of the horizontally (H) and vertically (V) polarized emission components according to Equation 7, are averages of at least three measurements: SUPPLEMENTAL DATA r = I VV G,I VH I VV + 2,G,I VH ; with G = I HV I HH : (7) Supplemental Data include Supplemental Experimental Procedures, Supplemental References, nine figures, and three tables and are available with this article online at S (09) ACKNOWLEDGMENTS We thank J. Huang for the help with peptide mapping and Q. Yang (School of Engineering Science, Simon Fraser University, Burnaby, British Columbia) for the help with mathematics in Equation 3. This work was supported by the Natural Sciences and Engineering Research Council of Canada (NSERC) for C.B., a Canada Graduate Scholarship for NSERC for T.C.S.P., and RGPIN (NSERC Operating) and STGRP (NSERC Strategic) for E.P. Received: October 31, 2008 Revised: December 25, 2008 Accepted: January 13, 2009 Published: February 26, 2009 REFERENCES Adler, V.E., Bierl, B.A., Beroza, M., and Sarmient, R. (1972). Electroantennograms and field attraction of gypsy moth Lepidoptera-Lymantriidae sex attractant disparlure and related compounds. J. Econ. Entomol. 65, Benton, R., Vannice, K.S., and Vosshall, L.B. (2007). An essential role for a CD36-related receptor in pheromone detection in Drosophila. Nature 450, Bette, S., Breer, H., and Krieger, J. (2002). Probing a pheromone binding protein of the silkmoth Antheraea polyphemus by endogenous tryptophan fluorescence. Insect Biochem. Mol. Biol. 32, Bierl, B.A., Beroza, M., and Collier, C.W. (1970). Potent sex attractant of gypsy moth: its isolation, identification, and synthesis. Science 170, Bierl, B.A., Beroza, M., and Collier, C.W. (1972). Isolation, identification, and synthesis of gypsy moth Lepidoptera-Lymantriidae sex attractant. J. Econ. Entomol. 65, 659. Damberger, F.F., Ishida, Y., Leal, W.S., and Wuethrich, K. (2007). Structural basis of ligand binding and release in insect pheromone-binding proteins: NMR structure of Antheraea polyphemus PBP1 at ph 4.5. J. Mol. Biol. 373, Danty, E., Briand, L., Michard-Vanhee, C., Perez, V., Arnold, G., Gaudemer, O., Huet, D., Huet, J.C., Ouali, C., Masson, C., and Pernollet, J.C. (1999). Cloning and expression of a queen pheromone-binding protein in the honeybee: an olfactory-specific, developmentally regulated protein. J. Neurosci. 19, Du, G.H., and Prestwich, G.D. (1995). Protein-structure encodes the ligandbinding specificity in pheromone binding-proteins. Biochemistry 34, Flecha, F.L.G., and Levi, V. (2003). Determination of the molecular size of BSA by fluorescence anisotropy. Biochem. Mol. Biol. Educ. 31, Graham, L.A., and Davies, P.L. (2002). The odorant-binding proteins of Drosophila melanogaster: annotation and characterization of a divergent gene family. Gene 292, Grosse-Wilde, E., Gohl, T., Bouche, E., Breer, H., and Krieger, J. (2007). Candidate pheromone receptors provide the basis for the response of distinct antennal neurons to pheromonal compounds. Eur. J. Neurosci. 25, Ha, T.S., and Smith, D.P. (2006). A pheromone receptor mediates 11-cis-vaccenyl acetate-induced responses in Drosophila. J. Neurosci. 26, Hansen, K. (1984). Discrimination and production of disparlure enantiomers by the gypsy-moth and the nun moth. Physiol. Entomol. 9, Honson, N. (2006). Structure and Function of Pheromone-Binding Proteins from the Gypsy Moth, Lymantria Dispar (Burnaby, Canada: Simon Fraser University). Honson, N., Johnson, M.A., Oliver, J.E., Prestwich, G.D., and Plettner, E. (2003). Structure-activity studies with pheromone-binding proteins of the gypsy moth, Lymantria dispar. Chem. Senses 28, Honson, N., Gong, Y., and Plettner, E. (2005). Structure and function of insect odorant and pheromone-binding proteins (OBPs and PBPs) and chemosensory-specific proteins (CSPs). In Chemical Ecology and Phytochemistry of Forest Ecosystems, J.T. Romeo, ed. (Oxford: Elsevier Ltd.), pp Inkster, J.A.H., Ling, I., Honson, N.S., Jacquet, L., Gries, R., and Plettner, E. (2005). Synthesis of disparlure analogues, using resolution on microcrystalline cellulose triacetate-i. Tetrahedron Asymmetry 16, Isin, E.M., and Guengerich, F.P. (2007). Multiple sequential steps involved in the binding of inhibitors to cytochrome p450 3A4. J. Biol. Chem. 282, Kim, M.S., Repp, A., and Smith, D.P. (1998). LUSH odorant-binding protein mediates chemosensory responses to alcohols in Drosophila melanogaster. Genetics 150, Kowcun, A., Honson, N., and Plettner, E. (2001). Olfaction in the gypsy moth, Lymantria dispar: effect of ph, ionic strength, and reductants on pheromone transport by pheromone-binding proteins. J. Biol. Chem. 276, Krieger, J., and Breer, H. (1999). Olfactory reception in invertebrates. Science 286, Krieger, J., Raming, K., and Breer, H. (1991). Cloning of genomic and complementary-dna encoding insect pheromone binding-proteins: evidence for microdiversity. Biochim. Biophys. Acta 1088, Kruse, S.W., Zhao, R., Smith, D.P., and Jones, D.N.M. (2003). Structure of a specific alcohol-binding site defined by the odorant binding protein LUSH from Drosophila melanogaster. Nat. Struct. Biol. 10, Lartigue, A., Gruez, A., Spinelli, S., Riviere, S., Brossut, R., Tegoni, M., and Cambillau, C. (2003). The crystal structure of a cockroach pheromone-binding protein suggests a new ligand binding and release mechanism. J. Biol. Chem. 278, Lartigue, A., Gruez, A., Briand, L., Blon, F., Bezirard, V., Walsh, M., Pernollet, J.C., Tegoni, M., and Cambillau, C. (2004). Sulfur single-wavelength anomalous diffraction crystal structure of a pheromone-binding protein from the honeybee Apis mellifera L. J. Biol. Chem. 279, Laughlin, J.D., Ha, T.S., Jones, D.N.M., and Smith, D.P. (2008). Activation of pheromone-sensitive neurons is mediated by conformational activation of pheromone-binding protein. Cell 133, Lautenschlager, C., Leal, W.S., and Clardy, J. (2007). Bombyx mori pheromone-binding protein binding nonpheromone ligands: implications for pheromone recognition. Structure 15, Leal, W.S. (2000). Duality monomer-dimer of the pheromone-binding protein from Bombyx mori. Biochem. Biophys. Res. Commun. 268, Leal, W.S., Chen, A.M., Ishida, Y., Chiang, V.P., Erickson, M.L., Morgan, T.I., and Tsuruda, J.M. (2005). Kinetics and molecular properties of pheromone binding and release. Proc. Natl. Acad. Sci. USA 102, Chemistry & Biology 16, , February 27, 2009 ª2009 Elsevier Ltd All rights reserved 171
CHEMICAL COMMUNICATION IN INSECTS, FROM PHEROMONE IDENTIFICATION TO PROTEOMICS OF OLFACTION
 CHEMICAL COMMUNICATION IN INSECTS, FROM PHEROMONE IDENTIFICATION TO PROTEOMICS OF OLFACTION Francesca R. Dani Dipartimento di Biologia; Università di Firenze CISM, Centro di Spettrometria di Massa; Università
CHEMICAL COMMUNICATION IN INSECTS, FROM PHEROMONE IDENTIFICATION TO PROTEOMICS OF OLFACTION Francesca R. Dani Dipartimento di Biologia; Università di Firenze CISM, Centro di Spettrometria di Massa; Università
CD Basis Set of Spectra that is used is that derived from comparing the spectra of globular proteins whose secondary structures are known from X-ray
 CD Basis Set of Spectra that is used is that derived from comparing the spectra of globular proteins whose secondary structures are known from X-ray crystallography An example of the use of CD Modeling
CD Basis Set of Spectra that is used is that derived from comparing the spectra of globular proteins whose secondary structures are known from X-ray crystallography An example of the use of CD Modeling
Isothermal experiments characterize time-dependent aggregation and unfolding
 1 Energy Isothermal experiments characterize time-dependent aggregation and unfolding Technical ote Introduction Kinetic measurements have, for decades, given protein scientists insight into the mechanisms
1 Energy Isothermal experiments characterize time-dependent aggregation and unfolding Technical ote Introduction Kinetic measurements have, for decades, given protein scientists insight into the mechanisms
Protein Structure Analysis and Verification. Course S Basics for Biosystems of the Cell exercise work. Maija Nevala, BIO, 67485U 16.1.
 Protein Structure Analysis and Verification Course S-114.2500 Basics for Biosystems of the Cell exercise work Maija Nevala, BIO, 67485U 16.1.2008 1. Preface When faced with an unknown protein, scientists
Protein Structure Analysis and Verification Course S-114.2500 Basics for Biosystems of the Cell exercise work Maija Nevala, BIO, 67485U 16.1.2008 1. Preface When faced with an unknown protein, scientists
it is assumed that only EH and ESH are catalytically active Michaelis-Menten equation for this model is:
 initial rates for many enzymatic reactions exhibit bell-shaped curves as a function of ph curves reflect the ionizations of certain amino acid residues that must be in a specific ionization state for enzyme
initial rates for many enzymatic reactions exhibit bell-shaped curves as a function of ph curves reflect the ionizations of certain amino acid residues that must be in a specific ionization state for enzyme
Lecture 27. Transition States and Enzyme Catalysis
 Lecture 27 Transition States and Enzyme Catalysis Reading for Today: Chapter 15 sections B and C Chapter 16 next two lectures 4/8/16 1 Pop Question 9 Binding data for your thesis protein (YTP), binding
Lecture 27 Transition States and Enzyme Catalysis Reading for Today: Chapter 15 sections B and C Chapter 16 next two lectures 4/8/16 1 Pop Question 9 Binding data for your thesis protein (YTP), binding
Substrate Specificity of Alcohol Dehydrogenase
 0 Substrate Specificity of Alcohol Dehydrogenase Roshan Roshan Chikarmane and Jonathan White Department of Chemistry University of Oregon Eugene, OR 97403 April 26, 2014 Abstract: The substrate specificity
0 Substrate Specificity of Alcohol Dehydrogenase Roshan Roshan Chikarmane and Jonathan White Department of Chemistry University of Oregon Eugene, OR 97403 April 26, 2014 Abstract: The substrate specificity
Crystal Violet as a Fluorescent Switch-On Probe for I-Motif: Label-Free DNA-Based Logic Gate
 Crystal Violet as a Fluorescent Switch-On Probe for I-Motif: Label-Free DNA-Based Logic Gate Dik-Lung Ma,* a Maria Hiu-Tung Kwan, a Daniel Shiu-Hin Chan, a Paul Lee, a Hui Yang, a Victor Pui-Yan Ma, a
Crystal Violet as a Fluorescent Switch-On Probe for I-Motif: Label-Free DNA-Based Logic Gate Dik-Lung Ma,* a Maria Hiu-Tung Kwan, a Daniel Shiu-Hin Chan, a Paul Lee, a Hui Yang, a Victor Pui-Yan Ma, a
Supplementary figure 1 Application of tmfret in LeuT. (a) To assess the feasibility of using tmfret for distance-dependent measurements in LeuT, a
 Supplementary figure 1 Application of tmfret in LeuT. (a) To assess the feasibility of using tmfret for distance-dependent measurements in LeuT, a series of tmfret-pairs comprised of single cysteine mutants
Supplementary figure 1 Application of tmfret in LeuT. (a) To assess the feasibility of using tmfret for distance-dependent measurements in LeuT, a series of tmfret-pairs comprised of single cysteine mutants
Chem 204. Mid-Term Exam I. July 21, There are 3 sections to this exam: Answer ALL questions
 Chem 204 Mid-Term Exam I July 21, 2009 Name: Answer Key Student ID: There are 3 sections to this exam: Answer ALL questions Section I: Multiple-Choice 20 questions, 2 pts each Section II: Fill-in-the-Blank
Chem 204 Mid-Term Exam I July 21, 2009 Name: Answer Key Student ID: There are 3 sections to this exam: Answer ALL questions Section I: Multiple-Choice 20 questions, 2 pts each Section II: Fill-in-the-Blank
BSc and MSc Degree Examinations
 Examination Candidate Number: Desk Number: BSc and MSc Degree Examinations 2018-9 Department : BIOLOGY Title of Exam: Molecular Biology and Biochemistry Part I Time Allowed: 1 hour and 30 minutes Marking
Examination Candidate Number: Desk Number: BSc and MSc Degree Examinations 2018-9 Department : BIOLOGY Title of Exam: Molecular Biology and Biochemistry Part I Time Allowed: 1 hour and 30 minutes Marking
Lecture 11: Enzymes: Kinetics [PDF] Reading: Berg, Tymoczko & Stryer, Chapter 8, pp
![Lecture 11: Enzymes: Kinetics [PDF] Reading: Berg, Tymoczko & Stryer, Chapter 8, pp Lecture 11: Enzymes: Kinetics [PDF] Reading: Berg, Tymoczko & Stryer, Chapter 8, pp](/thumbs/74/70848503.jpg) Lecture 11: Enzymes: Kinetics [PDF] Reading: Berg, Tymoczko & Stryer, Chapter 8, pp. 216-225 Updated on: 2/4/07 at 9:00 pm Key Concepts Kinetics is the study of reaction rates. Study of enzyme kinetics
Lecture 11: Enzymes: Kinetics [PDF] Reading: Berg, Tymoczko & Stryer, Chapter 8, pp. 216-225 Updated on: 2/4/07 at 9:00 pm Key Concepts Kinetics is the study of reaction rates. Study of enzyme kinetics
Membrane Protein Channels
 Membrane Protein Channels Potassium ions queuing up in the potassium channel Pumps: 1000 s -1 Channels: 1000000 s -1 Pumps & Channels The lipid bilayer of biological membranes is intrinsically impermeable
Membrane Protein Channels Potassium ions queuing up in the potassium channel Pumps: 1000 s -1 Channels: 1000000 s -1 Pumps & Channels The lipid bilayer of biological membranes is intrinsically impermeable
Supplementary Figure 1 Crystal contacts in COP apo structure (PDB code 3S0R)
 Supplementary Figure 1 Crystal contacts in COP apo structure (PDB code 3S0R) Shown in cyan and green are two adjacent tetramers from the crystallographic lattice of COP, forming the only unique inter-tetramer
Supplementary Figure 1 Crystal contacts in COP apo structure (PDB code 3S0R) Shown in cyan and green are two adjacent tetramers from the crystallographic lattice of COP, forming the only unique inter-tetramer
Serine-7 but not serine-5 phosphorylation primes RNA polymerase II CTD for P-TEFb recognition
 Supplementary Information to Serine-7 but not serine-5 phosphorylation primes RNA polymerase II CTD for P-TEFb recognition Nadine Czudnochowski 1,2, *, Christian A. Bösken 1, * & Matthias Geyer 1 1 Max-Planck-Institut
Supplementary Information to Serine-7 but not serine-5 phosphorylation primes RNA polymerase II CTD for P-TEFb recognition Nadine Czudnochowski 1,2, *, Christian A. Bösken 1, * & Matthias Geyer 1 1 Max-Planck-Institut
XV 74. Flouorescence-Polarization-Circular-Dichroism- Jablonski diagram Where does the energy go?
 XV 74 Flouorescence-Polarization-Circular-Dichroism- Jablonski diagram Where does the energy go? 1) Excite system through A Absorbance S 0 S n Excite from ground excited singlet S = 0 could be any of them
XV 74 Flouorescence-Polarization-Circular-Dichroism- Jablonski diagram Where does the energy go? 1) Excite system through A Absorbance S 0 S n Excite from ground excited singlet S = 0 could be any of them
Lecture # 3, 4 Selecting a Catalyst (Non-Kinetic Parameters), Review of Enzyme Kinetics, Selectivity, ph and Temperature Effects
 1.492 - Integrated Chemical Engineering (ICE Topics: Biocatalysis MIT Chemical Engineering Department Instructor: Professor Kristala Prather Fall 24 Lecture # 3, 4 Selecting a Catalyst (Non-Kinetic Parameters,
1.492 - Integrated Chemical Engineering (ICE Topics: Biocatalysis MIT Chemical Engineering Department Instructor: Professor Kristala Prather Fall 24 Lecture # 3, 4 Selecting a Catalyst (Non-Kinetic Parameters,
Interpreting and evaluating biological NMR in the literature. Worksheet 1
 Interpreting and evaluating biological NMR in the literature Worksheet 1 1D NMR spectra Application of RF pulses of specified lengths and frequencies can make certain nuclei detectable We can selectively
Interpreting and evaluating biological NMR in the literature Worksheet 1 1D NMR spectra Application of RF pulses of specified lengths and frequencies can make certain nuclei detectable We can selectively
Table S1. Overview of used PDZK1 constructs and their binding affinities to peptides. Related to figure 1.
 Table S1. Overview of used PDZK1 constructs and their binding affinities to peptides. Related to figure 1. PDZK1 constru cts Amino acids MW [kda] KD [μm] PEPT2-CT- FITC KD [μm] NHE3-CT- FITC KD [μm] PDZK1-CT-
Table S1. Overview of used PDZK1 constructs and their binding affinities to peptides. Related to figure 1. PDZK1 constru cts Amino acids MW [kda] KD [μm] PEPT2-CT- FITC KD [μm] NHE3-CT- FITC KD [μm] PDZK1-CT-
BA, BSc, and MSc Degree Examinations
 Examination Candidate Number: Desk Number: BA, BSc, and MSc Degree Examinations 2017-8 Department : BIOLOGY Title of Exam: Molecular Biology and Biochemistry Part I Time Allowed: 1 hour and 30 minutes
Examination Candidate Number: Desk Number: BA, BSc, and MSc Degree Examinations 2017-8 Department : BIOLOGY Title of Exam: Molecular Biology and Biochemistry Part I Time Allowed: 1 hour and 30 minutes
Protein separation and characterization
 Address:800 S Wineville Avenue, Ontario, CA 91761,USA Website:www.aladdin-e.com Email USA: tech@aladdin-e.com Email EU: eutech@aladdin-e.com Email Asia Pacific: cntech@aladdin-e.com Protein separation
Address:800 S Wineville Avenue, Ontario, CA 91761,USA Website:www.aladdin-e.com Email USA: tech@aladdin-e.com Email EU: eutech@aladdin-e.com Email Asia Pacific: cntech@aladdin-e.com Protein separation
I690/B680 Structural Bioinformatics Spring Protein Structure Determination by NMR Spectroscopy
 I690/B680 Structural Bioinformatics Spring 2006 Protein Structure Determination by NMR Spectroscopy Suggested Reading (1) Van Holde, Johnson, Ho. Principles of Physical Biochemistry, 2 nd Ed., Prentice
I690/B680 Structural Bioinformatics Spring 2006 Protein Structure Determination by NMR Spectroscopy Suggested Reading (1) Van Holde, Johnson, Ho. Principles of Physical Biochemistry, 2 nd Ed., Prentice
Molecular dynamics simulations of anti-aggregation effect of ibuprofen. Wenling E. Chang, Takako Takeda, E. Prabhu Raman, and Dmitri Klimov
 Biophysical Journal, Volume 98 Supporting Material Molecular dynamics simulations of anti-aggregation effect of ibuprofen Wenling E. Chang, Takako Takeda, E. Prabhu Raman, and Dmitri Klimov Supplemental
Biophysical Journal, Volume 98 Supporting Material Molecular dynamics simulations of anti-aggregation effect of ibuprofen Wenling E. Chang, Takako Takeda, E. Prabhu Raman, and Dmitri Klimov Supplemental
Supplementary Information. Overlap between folding and functional energy landscapes for. adenylate kinase conformational change
 Supplementary Information Overlap between folding and functional energy landscapes for adenylate kinase conformational change by Ulrika Olsson & Magnus Wolf-Watz Contents: 1. Supplementary Note 2. Supplementary
Supplementary Information Overlap between folding and functional energy landscapes for adenylate kinase conformational change by Ulrika Olsson & Magnus Wolf-Watz Contents: 1. Supplementary Note 2. Supplementary
Supplemental Materials and Methods
 Supplemental Materials and Methods Time-resolved FRET (trfret) to probe for changes in the Box A/A stem upon complex assembly U3 MINI was folded and the decay of Fl fluorescence was measured at 20 ºC (see
Supplemental Materials and Methods Time-resolved FRET (trfret) to probe for changes in the Box A/A stem upon complex assembly U3 MINI was folded and the decay of Fl fluorescence was measured at 20 ºC (see
Supporting online material
 Supporting online material Materials and Methods Target proteins All predicted ORFs in the E. coli genome (1) were downloaded from the Colibri data base (2) (http://genolist.pasteur.fr/colibri/). 737 proteins
Supporting online material Materials and Methods Target proteins All predicted ORFs in the E. coli genome (1) were downloaded from the Colibri data base (2) (http://genolist.pasteur.fr/colibri/). 737 proteins
SIMPLE MODEL Direct Binding Analysis
 Neurochemistry, 56:120:575 Dr. Patrick J. McIlroy Supplementary Notes SIMPLE MODEL Direct Binding Analysis The interaction of a (radio)ligand, L, with its receptor, R, to form a non-covalent complex, RL,
Neurochemistry, 56:120:575 Dr. Patrick J. McIlroy Supplementary Notes SIMPLE MODEL Direct Binding Analysis The interaction of a (radio)ligand, L, with its receptor, R, to form a non-covalent complex, RL,
Nature Structural and Molecular Biology: doi: /nsmb Supplementary Figure 1. Definition and assessment of ciap1 constructs.
 Supplementary Figure 1 Definition and assessment of ciap1 constructs. (a) ciap1 constructs used in this study are shown as primary structure schematics with domains colored as in the main text. Mutations
Supplementary Figure 1 Definition and assessment of ciap1 constructs. (a) ciap1 constructs used in this study are shown as primary structure schematics with domains colored as in the main text. Mutations
Chapter 2 The Chemistry of Life
 Chapter 2 The Chemistry of Life I. Water Liquid Naturally occurring It expands liquid to solid Covers more than 75% of our surface Most abundant in living organisms most important inorganic compound for
Chapter 2 The Chemistry of Life I. Water Liquid Naturally occurring It expands liquid to solid Covers more than 75% of our surface Most abundant in living organisms most important inorganic compound for
Supporting Information. Time-Resolved Botulinum Neurotoxin A Activity Monitored using. Peptide-Functionalized Au Nanoparticle Energy Transfer Sensors
 Electronic Supplementary Material (ESI) for Chemical Science. This journal is The Royal Society of Chemistry 2014 Supporting Information Time-Resolved Botulinum Neurotoxin A Activity Monitored using Peptide-Functionalized
Electronic Supplementary Material (ESI) for Chemical Science. This journal is The Royal Society of Chemistry 2014 Supporting Information Time-Resolved Botulinum Neurotoxin A Activity Monitored using Peptide-Functionalized
Substrate-dependent switching of the allosteric binding mechanism of a dimeric enzyme
 Supplementary Information: Substrate-dependent switching of the allosteric binding mechanism of a dimeric enzyme Lee Freiburger, 1 Teresa Miletti, 1 Siqi Zhu, 1 Oliver Baettig, Albert Berghuis, Karine
Supplementary Information: Substrate-dependent switching of the allosteric binding mechanism of a dimeric enzyme Lee Freiburger, 1 Teresa Miletti, 1 Siqi Zhu, 1 Oliver Baettig, Albert Berghuis, Karine
Scale in the biological world
 Scale in the biological world 2 A cell seen by TEM 3 4 From living cells to atoms 5 Compartmentalisation in the cell: internal membranes and the cytosol 6 The Origin of mitochondria: The endosymbion hypothesis
Scale in the biological world 2 A cell seen by TEM 3 4 From living cells to atoms 5 Compartmentalisation in the cell: internal membranes and the cytosol 6 The Origin of mitochondria: The endosymbion hypothesis
Supplementary Information for. Direct nitration and azidation of aliphatic carbons by an iron-dependent halogenase
 Supplementary Information for Direct nitration and azidation of aliphatic carbons by an iron-dependent halogenase Megan L Matthews, Wei-chen Chang, Andrew P Layne, Linde A Miles, Carsten Krebs, J Martin
Supplementary Information for Direct nitration and azidation of aliphatic carbons by an iron-dependent halogenase Megan L Matthews, Wei-chen Chang, Andrew P Layne, Linde A Miles, Carsten Krebs, J Martin
Protein Structure Prediction II Lecturer: Serafim Batzoglou Scribe: Samy Hamdouche
 Protein Structure Prediction II Lecturer: Serafim Batzoglou Scribe: Samy Hamdouche The molecular structure of a protein can be broken down hierarchically. The primary structure of a protein is simply its
Protein Structure Prediction II Lecturer: Serafim Batzoglou Scribe: Samy Hamdouche The molecular structure of a protein can be broken down hierarchically. The primary structure of a protein is simply its
Structural basis for catalytically restrictive dynamics of a high-energy enzyme state
 Supplementary Material Structural basis for catalytically restrictive dynamics of a high-energy enzyme state Michael Kovermann, Jörgen Ådén, Christin Grundström, A. Elisabeth Sauer-Eriksson, Uwe H. Sauer
Supplementary Material Structural basis for catalytically restrictive dynamics of a high-energy enzyme state Michael Kovermann, Jörgen Ådén, Christin Grundström, A. Elisabeth Sauer-Eriksson, Uwe H. Sauer
type GroEL-GroES complex. Crystals were grown in buffer D (100 mm HEPES, ph 7.5,
 Supplementary Material Supplementary Materials and Methods Structure Determination of SR1-GroES-ADP AlF x SR1-GroES-ADP AlF x was purified as described in Materials and Methods for the wild type GroEL-GroES
Supplementary Material Supplementary Materials and Methods Structure Determination of SR1-GroES-ADP AlF x SR1-GroES-ADP AlF x was purified as described in Materials and Methods for the wild type GroEL-GroES
Discussion Session prior to the Second Examination: Sunday evening April 13 6 to 8 pm. 161 Noyes Laboratory
 Discussion Session prior to the Second Examination: Sunday evening April 13 6 to 8 pm 161 Noyes Laboratory Determination of the Stokes Radius by measuring the Rotational Diffusion Coefficient: D rot D
Discussion Session prior to the Second Examination: Sunday evening April 13 6 to 8 pm 161 Noyes Laboratory Determination of the Stokes Radius by measuring the Rotational Diffusion Coefficient: D rot D
Gel Permeation Chromatography (GPC) or Size Exclusion Chromatography (SEC)
 Gel Permeation Chromatography (GPC) or Size Exclusion Chromatography (SEC) Size Exclusion Chromatography (SEC) is a non-interaction based separation mechanism in which compounds are retained for different
Gel Permeation Chromatography (GPC) or Size Exclusion Chromatography (SEC) Size Exclusion Chromatography (SEC) is a non-interaction based separation mechanism in which compounds are retained for different
Chemical kinetics and catalysis
 Chemical kinetics and catalysis Outline Classification of chemical reactions Definition of chemical kinetics Rate of chemical reaction The law of chemical raction rate Collision theory of reactions, transition
Chemical kinetics and catalysis Outline Classification of chemical reactions Definition of chemical kinetics Rate of chemical reaction The law of chemical raction rate Collision theory of reactions, transition
Membrane Proteins: 1. Integral proteins: 2. Peripheral proteins: 3. Amphitropic proteins:
 Membrane Proteins: 1. Integral proteins: proteins that insert into/span the membrane bilayer; or covalently linked to membrane lipids. (Interact with the hydrophobic part of the membrane) 2. Peripheral
Membrane Proteins: 1. Integral proteins: proteins that insert into/span the membrane bilayer; or covalently linked to membrane lipids. (Interact with the hydrophobic part of the membrane) 2. Peripheral
Supplementary materials. Crystal structure of the carboxyltransferase domain. of acetyl coenzyme A carboxylase. Department of Biological Sciences
 Supplementary materials Crystal structure of the carboxyltransferase domain of acetyl coenzyme A carboxylase Hailong Zhang, Zhiru Yang, 1 Yang Shen, 1 Liang Tong Department of Biological Sciences Columbia
Supplementary materials Crystal structure of the carboxyltransferase domain of acetyl coenzyme A carboxylase Hailong Zhang, Zhiru Yang, 1 Yang Shen, 1 Liang Tong Department of Biological Sciences Columbia
Chemical Exchange and Ligand Binding
 Chemical Exchange and Ligand Binding NMR time scale Fast exchange for binding constants Slow exchange for tight binding Single vs. multiple binding mode Calcium binding process of calcium binding proteins
Chemical Exchange and Ligand Binding NMR time scale Fast exchange for binding constants Slow exchange for tight binding Single vs. multiple binding mode Calcium binding process of calcium binding proteins
A) at equilibrium B) endergonic C) endothermic D) exergonic E) exothermic.
 CHEM 2770: Elements of Biochemistry Mid Term EXAMINATION VERSION A Date: October 29, 2014 Instructor: H. Perreault Location: 172 Schultz Time: 4 or 6 pm. Duration: 1 hour Instructions Please mark the Answer
CHEM 2770: Elements of Biochemistry Mid Term EXAMINATION VERSION A Date: October 29, 2014 Instructor: H. Perreault Location: 172 Schultz Time: 4 or 6 pm. Duration: 1 hour Instructions Please mark the Answer
Lecture 26: More on Gel Filtration Chromatography and the Trypsin Resurrection Experiment
 Biological Chemistry Laboratory Biology 3515/Chemistry 3515 Spring 2018 Lecture 26: More on Gel Filtration Chromatography and the Trypsin Resurrection Experiment 12 April 2018 c David P. Goldenberg University
Biological Chemistry Laboratory Biology 3515/Chemistry 3515 Spring 2018 Lecture 26: More on Gel Filtration Chromatography and the Trypsin Resurrection Experiment 12 April 2018 c David P. Goldenberg University
Laser. emission W FL
 W Laser Laser emission W FL Supplementary Figure 1. Comparison between fluorescence and laser emission spectra. The fluorescence has broad spectrum whereas the laser has very narrow spectrum. W FL and
W Laser Laser emission W FL Supplementary Figure 1. Comparison between fluorescence and laser emission spectra. The fluorescence has broad spectrum whereas the laser has very narrow spectrum. W FL and
NH 2. Biochemistry I, Fall Term Sept 9, Lecture 5: Amino Acids & Peptides Assigned reading in Campbell: Chapter
 Biochemistry I, Fall Term Sept 9, 2005 Lecture 5: Amino Acids & Peptides Assigned reading in Campbell: Chapter 3.1-3.4. Key Terms: ptical Activity, Chirality Peptide bond Condensation reaction ydrolysis
Biochemistry I, Fall Term Sept 9, 2005 Lecture 5: Amino Acids & Peptides Assigned reading in Campbell: Chapter 3.1-3.4. Key Terms: ptical Activity, Chirality Peptide bond Condensation reaction ydrolysis
Problems from Previous Class
 1 Problems from Previous lass 1. What is K m? What are the units of K m? 2. What is V max? What are the units of V max? 3. Write down the Michaelis-Menten equation. 4. What order of reaction is the reaction
1 Problems from Previous lass 1. What is K m? What are the units of K m? 2. What is V max? What are the units of V max? 3. Write down the Michaelis-Menten equation. 4. What order of reaction is the reaction
The ph-responsive behaviour of aqueous solutions of poly(acrylic acid) is dependent on molar mass
 Electronic Supplementary Material (ESI) for Soft Matter. This journal is The Royal Society of Chemistry 2016 The ph-responsive behaviour of aqueous solutions of poly(acrylic acid) is dependent on molar
Electronic Supplementary Material (ESI) for Soft Matter. This journal is The Royal Society of Chemistry 2016 The ph-responsive behaviour of aqueous solutions of poly(acrylic acid) is dependent on molar
4. Circular Dichroism - Spectroscopy
 4. Circular Dichroism - Spectroscopy The optical rotatory dispersion (ORD) and the circular dichroism (CD) are special variations of absorption spectroscopy in the UV and VIS region of the spectrum. The
4. Circular Dichroism - Spectroscopy The optical rotatory dispersion (ORD) and the circular dichroism (CD) are special variations of absorption spectroscopy in the UV and VIS region of the spectrum. The
Biochemistry Quiz Review 1I. 1. Of the 20 standard amino acids, only is not optically active. The reason is that its side chain.
 Biochemistry Quiz Review 1I A general note: Short answer questions are just that, short. Writing a paragraph filled with every term you can remember from class won t improve your answer just answer clearly,
Biochemistry Quiz Review 1I A general note: Short answer questions are just that, short. Writing a paragraph filled with every term you can remember from class won t improve your answer just answer clearly,
I. Proteomics by Mass Spectrometry 1. What is an internal standard and what does it accomplish analytically?
 Name I. Proteomics by Mass Spectrometry 1. What is an internal standard and what does it accomplish analytically? Internal standards are standards added intentionally to all samples, standards and blanks.
Name I. Proteomics by Mass Spectrometry 1. What is an internal standard and what does it accomplish analytically? Internal standards are standards added intentionally to all samples, standards and blanks.
Research Science Biology The study of living organisms (Study of life)
 Scientific method Why is there a hypothesis and prediction? If only prediction: then there is no way to finish the prediction and conclude whether the results support the hypothesis If surfaces are sampled
Scientific method Why is there a hypothesis and prediction? If only prediction: then there is no way to finish the prediction and conclude whether the results support the hypothesis If surfaces are sampled
SUPPLEMENTARY INFORMATION
 Ultrafast proton transport in sub-1-nm diameter carbon nanotube porins Ramya H. Tunuguntla, 1 Frances I. Allen, 2 Kyunghoon Kim, 1, Allison Belliveau, 1 Aleksandr Noy 1,3, * * Correspondence to AN: noy1@llnl.gov
Ultrafast proton transport in sub-1-nm diameter carbon nanotube porins Ramya H. Tunuguntla, 1 Frances I. Allen, 2 Kyunghoon Kim, 1, Allison Belliveau, 1 Aleksandr Noy 1,3, * * Correspondence to AN: noy1@llnl.gov
Chemical Kinetics. Topic 7
 Chemical Kinetics Topic 7 Corrosion of Titanic wrec Casón shipwrec 2Fe(s) + 3/2O 2 (g) + H 2 O --> Fe 2 O 3.H 2 O(s) 2Na(s) + 2H 2 O --> 2NaOH(aq) + H 2 (g) Two examples of the time needed for a chemical
Chemical Kinetics Topic 7 Corrosion of Titanic wrec Casón shipwrec 2Fe(s) + 3/2O 2 (g) + H 2 O --> Fe 2 O 3.H 2 O(s) 2Na(s) + 2H 2 O --> 2NaOH(aq) + H 2 (g) Two examples of the time needed for a chemical
Introduction... Theory Influence of Excitation Pulse Shape...
 1. Fluorescence Anisotropy: Theory and Applications Robert F. Steiner 1.1. 1.2. 1.3. 1.4. 1.5. 1.6. Introduction... Theory... 1.2.1. Meaning of Anisotropy... 1.2.2. Influence of Excitation Pulse Shape...
1. Fluorescence Anisotropy: Theory and Applications Robert F. Steiner 1.1. 1.2. 1.3. 1.4. 1.5. 1.6. Introduction... Theory... 1.2.1. Meaning of Anisotropy... 1.2.2. Influence of Excitation Pulse Shape...
Interaction of Gold Nanoparticle with Proteins
 Chapter 7 Interaction of Gold Nanoparticle with Proteins 7.1. Introduction The interfacing of nanoparticle with biomolecules such as protein is useful for applications ranging from nano-biotechnology (molecular
Chapter 7 Interaction of Gold Nanoparticle with Proteins 7.1. Introduction The interfacing of nanoparticle with biomolecules such as protein is useful for applications ranging from nano-biotechnology (molecular
Theoretical Models for Chemical Kinetics
 Theoretical Models for Chemical Kinetics Thus far we have calculated rate laws, rate constants, reaction orders, etc. based on observations of macroscopic properties, but what is happening at the molecular
Theoretical Models for Chemical Kinetics Thus far we have calculated rate laws, rate constants, reaction orders, etc. based on observations of macroscopic properties, but what is happening at the molecular
Supporting Information for: Kinetic Mechanisms Governing Stable Ribonucleotide Incorporation in Individual DNA Polymerase Complexes
 Supporting Information for: Kinetic Mechanisms Governing Stable Ribonucleotide Incorporation in Individual DNA Polymerase Complexes Joseph M. Dahl, Hongyun Wang, José M. Lázaro, Margarita Salas, and Kate
Supporting Information for: Kinetic Mechanisms Governing Stable Ribonucleotide Incorporation in Individual DNA Polymerase Complexes Joseph M. Dahl, Hongyun Wang, José M. Lázaro, Margarita Salas, and Kate
Comparison of the Diffusion Coefficients Obtained for Latex Film Formation Studied by FRET and Pyrene Excimer Formation
 Comparison of the Diffusion Coefficients Obtained for Latex Film Formation Studied by FRT and Pyrene xcimer Formation Remi Casier, Jean Duhamel, Mario Gauthier Institute for Polymer Research, Department
Comparison of the Diffusion Coefficients Obtained for Latex Film Formation Studied by FRT and Pyrene xcimer Formation Remi Casier, Jean Duhamel, Mario Gauthier Institute for Polymer Research, Department
ion mobility spectrometry IR spectroscopy
 Debasmita Gho 29.10.2016 Introducti on Owing to its accuracy, sensitivity, and speed, mass spectrometry (MS) coupled to fragmentation techniques is the method of choice for determining the primary structure
Debasmita Gho 29.10.2016 Introducti on Owing to its accuracy, sensitivity, and speed, mass spectrometry (MS) coupled to fragmentation techniques is the method of choice for determining the primary structure
Direct, Reliable, and Correct Oxygen Measurement
 Direct, Reliable, and Correct Oxygen Measurement February, 2015 Oxygen-dependent quenching of phosphorescence has been used extensively for the last 25 years as a reliable method for measuring oxygen.
Direct, Reliable, and Correct Oxygen Measurement February, 2015 Oxygen-dependent quenching of phosphorescence has been used extensively for the last 25 years as a reliable method for measuring oxygen.
Application of time resolved area normalized emission spectroscopy to multicomponent systems
 JOURNAL OF CHEMICAL PHYSICS VOLUME 115, NUMBER 15 15 OCTOBER 2001 Application of time resolved area normalized emission spectroscopy to multicomponent systems A. S. R. Koti and N. Periasamy a) Department
JOURNAL OF CHEMICAL PHYSICS VOLUME 115, NUMBER 15 15 OCTOBER 2001 Application of time resolved area normalized emission spectroscopy to multicomponent systems A. S. R. Koti and N. Periasamy a) Department
Hole s Human Anatomy and Physiology Eleventh Edition. Chapter 2
 Hole s Human Anatomy and Physiology Eleventh Edition Shier Butler Lewis Chapter 2 1 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. CHAPTER 2 CHEMICAL BASIS OF
Hole s Human Anatomy and Physiology Eleventh Edition Shier Butler Lewis Chapter 2 1 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. CHAPTER 2 CHEMICAL BASIS OF
Raman spectroscopy of phthalocyanines and their sulfonated derivatives
 Journal of Molecular Structure 744 747 (2005) 481 485 www.elsevier.com/locate/molstruc Raman spectroscopy of phthalocyanines and their sulfonated derivatives B. Brożek-Płuska*, I. Szymczyk, H. Abramczyk
Journal of Molecular Structure 744 747 (2005) 481 485 www.elsevier.com/locate/molstruc Raman spectroscopy of phthalocyanines and their sulfonated derivatives B. Brożek-Płuska*, I. Szymczyk, H. Abramczyk
Protein folding. Today s Outline
 Protein folding Today s Outline Review of previous sessions Thermodynamics of folding and unfolding Determinants of folding Techniques for measuring folding The folding process The folding problem: Prediction
Protein folding Today s Outline Review of previous sessions Thermodynamics of folding and unfolding Determinants of folding Techniques for measuring folding The folding process The folding problem: Prediction
Protein Dynamics. The space-filling structures of myoglobin and hemoglobin show that there are no pathways for O 2 to reach the heme iron.
 Protein Dynamics The space-filling structures of myoglobin and hemoglobin show that there are no pathways for O 2 to reach the heme iron. Below is myoglobin hydrated with 350 water molecules. Only a small
Protein Dynamics The space-filling structures of myoglobin and hemoglobin show that there are no pathways for O 2 to reach the heme iron. Below is myoglobin hydrated with 350 water molecules. Only a small
AP CHEMISTRY CHAPTER 12 KINETICS
 AP CHEMISTRY CHAPTER 12 KINETICS Thermodynamics tells us if a reaction can occur. Kinetics tells us how quickly the reaction occurs. Some reactions that are thermodynamically feasible are kinetically so
AP CHEMISTRY CHAPTER 12 KINETICS Thermodynamics tells us if a reaction can occur. Kinetics tells us how quickly the reaction occurs. Some reactions that are thermodynamically feasible are kinetically so
Supplementary Materials for
 advances.sciencemag.org/cgi/content/full/3/4/e1600663/dc1 Supplementary Materials for A dynamic hydrophobic core orchestrates allostery in protein kinases Jonggul Kim, Lalima G. Ahuja, Fa-An Chao, Youlin
advances.sciencemag.org/cgi/content/full/3/4/e1600663/dc1 Supplementary Materials for A dynamic hydrophobic core orchestrates allostery in protein kinases Jonggul Kim, Lalima G. Ahuja, Fa-An Chao, Youlin
LABORATORY OF ELEMENTARY BIOPHYSICS
 LABORATORY OF ELEMENTARY BIOPHYSICS Experimental exercises for III year of the First cycle studies Field: Applications of physics in biology and medicine Specialization: Molecular Biophysics Fluorescence
LABORATORY OF ELEMENTARY BIOPHYSICS Experimental exercises for III year of the First cycle studies Field: Applications of physics in biology and medicine Specialization: Molecular Biophysics Fluorescence
Supporting Informations for. 1,8-Naphthyridine-based molecular clips for off-on fluorescence sensing
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2015 Supporting Informations for 1,8-aphthyridine-based molecular clips for off-on fluorescence
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2015 Supporting Informations for 1,8-aphthyridine-based molecular clips for off-on fluorescence
schematic diagram; EGF binding, dimerization, phosphorylation, Grb2 binding, etc.
 Lecture 1: Noncovalent Biomolecular Interactions Bioengineering and Modeling of biological processes -e.g. tissue engineering, cancer, autoimmune disease Example: RTK signaling, e.g. EGFR Growth responses
Lecture 1: Noncovalent Biomolecular Interactions Bioengineering and Modeling of biological processes -e.g. tissue engineering, cancer, autoimmune disease Example: RTK signaling, e.g. EGFR Growth responses
Experimental Methods in Kinetics (2014)
 IIIa- 35 Experimental Methods in Kinetics (2014) Initial examples assume start R = A 0 + B 0 etc., P = 0 follow reaction forward initial rate, etc. measure [A] vs. t Chemical methods: Could mix and take
IIIa- 35 Experimental Methods in Kinetics (2014) Initial examples assume start R = A 0 + B 0 etc., P = 0 follow reaction forward initial rate, etc. measure [A] vs. t Chemical methods: Could mix and take
Biochemistry 3100 Sample Problems Binding proteins, Kinetics & Catalysis
 (1) Draw an approximate denaturation curve for a typical blood protein (eg myoglobin) as a function of ph. (2) Myoglobin is a simple, single subunit binding protein that has an oxygen storage function
(1) Draw an approximate denaturation curve for a typical blood protein (eg myoglobin) as a function of ph. (2) Myoglobin is a simple, single subunit binding protein that has an oxygen storage function
Supplementary Figure 1. Structures of substrates tested with 1. Only one enantiomer is shown.
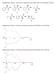 Supplementary Figure 1. Structures of substrates tested with 1. Only one enantiomer is shown. Supplementary Figure 2. CD spectra obtained using 1 and (R)-3 (blue) and (S)-3 (red) Supplementary Figure 3.
Supplementary Figure 1. Structures of substrates tested with 1. Only one enantiomer is shown. Supplementary Figure 2. CD spectra obtained using 1 and (R)-3 (blue) and (S)-3 (red) Supplementary Figure 3.
Receptor Based Drug Design (1)
 Induced Fit Model For more than 100 years, the behaviour of enzymes had been explained by the "lock-and-key" mechanism developed by pioneering German chemist Emil Fischer. Fischer thought that the chemicals
Induced Fit Model For more than 100 years, the behaviour of enzymes had been explained by the "lock-and-key" mechanism developed by pioneering German chemist Emil Fischer. Fischer thought that the chemicals
Measurement Examples. Excitation and Emission Scans. Steady State Fluorescence Anisotropy. Kinetic Measurements
 Measurement Examples A division of Edinburgh Instruments Ltd. Excitation and Emission Scans Excitation and emission spectra are standard measurements in fluorescence spectroscopy. The figure demonstrates
Measurement Examples A division of Edinburgh Instruments Ltd. Excitation and Emission Scans Excitation and emission spectra are standard measurements in fluorescence spectroscopy. The figure demonstrates
Membrane proteins Porins: FadL. Oriol Solà, Dimitri Ivancic, Daniel Folch, Marc Olivella
 Membrane proteins Porins: FadL Oriol Solà, Dimitri Ivancic, Daniel Folch, Marc Olivella INDEX 1. INTRODUCTION TO MEMBRANE PROTEINS 2. FADL: OUTER MEMBRANE TRANSPORT PROTEIN 3. MAIN FEATURES OF FADL STRUCTURE
Membrane proteins Porins: FadL Oriol Solà, Dimitri Ivancic, Daniel Folch, Marc Olivella INDEX 1. INTRODUCTION TO MEMBRANE PROTEINS 2. FADL: OUTER MEMBRANE TRANSPORT PROTEIN 3. MAIN FEATURES OF FADL STRUCTURE
Reading for today: Chapter 16 (selections from Sections A, B and C) Friday and Monday: Chapter 17 (Diffusion)
 Lecture 29 Enzymes Reading for today: Chapter 6 (selections from Sections, B and C) Friday and Monday: Chapter 7 (Diffusion) 4/3/6 Today s Goals Michaelis-Menten mechanism for simple enzyme reactions:
Lecture 29 Enzymes Reading for today: Chapter 6 (selections from Sections, B and C) Friday and Monday: Chapter 7 (Diffusion) 4/3/6 Today s Goals Michaelis-Menten mechanism for simple enzyme reactions:
Introduction The gramicidin A (ga) channel forms by head-to-head association of two monomers at their amino termini, one from each bilayer leaflet. Th
 Abstract When conductive, gramicidin monomers are linked by six hydrogen bonds. To understand the details of dissociation and how the channel transits from a state with 6H bonds to ones with 4H bonds or
Abstract When conductive, gramicidin monomers are linked by six hydrogen bonds. To understand the details of dissociation and how the channel transits from a state with 6H bonds to ones with 4H bonds or
Principles of Physical Biochemistry
 Principles of Physical Biochemistry Kensal E. van Hold e W. Curtis Johnso n P. Shing Ho Preface x i PART 1 MACROMOLECULAR STRUCTURE AND DYNAMICS 1 1 Biological Macromolecules 2 1.1 General Principles
Principles of Physical Biochemistry Kensal E. van Hold e W. Curtis Johnso n P. Shing Ho Preface x i PART 1 MACROMOLECULAR STRUCTURE AND DYNAMICS 1 1 Biological Macromolecules 2 1.1 General Principles
Fluorescence 2009 update
 XV 74 Fluorescence 2009 update Jablonski diagram Where does the energy go? Can be viewed like multistep kinetic pathway 1) Excite system through A Absorbance S 0 S n Excite from ground excited singlet
XV 74 Fluorescence 2009 update Jablonski diagram Where does the energy go? Can be viewed like multistep kinetic pathway 1) Excite system through A Absorbance S 0 S n Excite from ground excited singlet
After lectures by. disappearance of reactants or appearance of. measure a reaction rate we monitor the. Reaction Rates (reaction velocities): To
 Revised 3/21/2017 After lectures by Dr. Loren Williams (GeorgiaTech) Protein Folding: 1 st order reaction DNA annealing: 2 nd order reaction Reaction Rates (reaction velocities): To measure a reaction
Revised 3/21/2017 After lectures by Dr. Loren Williams (GeorgiaTech) Protein Folding: 1 st order reaction DNA annealing: 2 nd order reaction Reaction Rates (reaction velocities): To measure a reaction
Kinetic Isotope Effects
 1 Experiment 31 Kinetic Isotope Effects Isotopic substitution is a useful technique for the probing of reaction mechanisms. The change of an isotope may affect the reaction rate in a number of ways, providing
1 Experiment 31 Kinetic Isotope Effects Isotopic substitution is a useful technique for the probing of reaction mechanisms. The change of an isotope may affect the reaction rate in a number of ways, providing
Anatoly B. Kolomeisky. Department of Chemistry CAN WE UNDERSTAND THE COMPLEX DYNAMICS OF MOTOR PROTEINS USING SIMPLE STOCHASTIC MODELS?
 Anatoly B. Kolomeisky Department of Chemistry CAN WE UNDERSTAND THE COMPLEX DYNAMICS OF MOTOR PROTEINS USING SIMPLE STOCHASTIC MODELS? Motor Proteins Enzymes that convert the chemical energy into mechanical
Anatoly B. Kolomeisky Department of Chemistry CAN WE UNDERSTAND THE COMPLEX DYNAMICS OF MOTOR PROTEINS USING SIMPLE STOCHASTIC MODELS? Motor Proteins Enzymes that convert the chemical energy into mechanical
Copyright Mark Brandt, Ph.D A third method, cryogenic electron microscopy has seen increasing use over the past few years.
 Structure Determination and Sequence Analysis The vast majority of the experimentally determined three-dimensional protein structures have been solved by one of two methods: X-ray diffraction and Nuclear
Structure Determination and Sequence Analysis The vast majority of the experimentally determined three-dimensional protein structures have been solved by one of two methods: X-ray diffraction and Nuclear
Enzymes Part III: Enzyme kinetics. Dr. Mamoun Ahram Summer semester,
 Enzymes Part III: Enzyme kinetics Dr. Mamoun Ahram Summer semester, 2015-2016 Kinetics Kinetics is deals with the rates of chemical reactions. Chemical kinetics is the study of the rates of chemical reactions.
Enzymes Part III: Enzyme kinetics Dr. Mamoun Ahram Summer semester, 2015-2016 Kinetics Kinetics is deals with the rates of chemical reactions. Chemical kinetics is the study of the rates of chemical reactions.
Rational design of light-directed dynamic spheres
 Electronic Supplementary Information (ESI) Rational design of light-directed dynamic spheres Yumi Okui a and Mina Han* a,b a Department of Chemistry and Department of Electronic Chemistry Tokyo Institute
Electronic Supplementary Information (ESI) Rational design of light-directed dynamic spheres Yumi Okui a and Mina Han* a,b a Department of Chemistry and Department of Electronic Chemistry Tokyo Institute
Fluorescence Visual Gel-separation of Dansylated BSA-protected Gold-Nanoclusters
 Fluorescence Visual Gel-separation of Dansylated BSA-protected Gold-Nanoclusters (Electronic Supporting Information) Hong-Wei Li, a,b Kelong Ai b and Yuqing Wu a * Experimental Section All chemicals were
Fluorescence Visual Gel-separation of Dansylated BSA-protected Gold-Nanoclusters (Electronic Supporting Information) Hong-Wei Li, a,b Kelong Ai b and Yuqing Wu a * Experimental Section All chemicals were
Protein-Ligand Interactions Are Responsible for Signal Transduction
 Proteinigand Interactions Are Responsible for Signal ransduction ypes of Interactions: 1. ProteinProtein 2. ProteinDNA (RNA) 3. Proteinsmall molecule Dynamic Proteinigand Interaction Dynamic interaction
Proteinigand Interactions Are Responsible for Signal ransduction ypes of Interactions: 1. ProteinProtein 2. ProteinDNA (RNA) 3. Proteinsmall molecule Dynamic Proteinigand Interaction Dynamic interaction
Structure of the α-helix
 Structure of the α-helix Structure of the β Sheet Protein Dynamics Basics of Quenching HDX Hydrogen exchange of amide protons is catalyzed by H 2 O, OH -, and H 3 O +, but it s most dominated by base
Structure of the α-helix Structure of the β Sheet Protein Dynamics Basics of Quenching HDX Hydrogen exchange of amide protons is catalyzed by H 2 O, OH -, and H 3 O +, but it s most dominated by base
A Determination of DNA-DAPI Binding using Fluorescence Spectroscopy
 CHEM 311L Revision 1.2 A Determination of DNA-DAPI Binding using Fluorescence Spectroscopy In this Laboratory Exercise, we will determine the binding constant K f for complex formation between 4'-6-diamidino-2-phenylindole
CHEM 311L Revision 1.2 A Determination of DNA-DAPI Binding using Fluorescence Spectroscopy In this Laboratory Exercise, we will determine the binding constant K f for complex formation between 4'-6-diamidino-2-phenylindole
Supporting Information
 Supporting Information Materials and Methods: Tris-hydroxymethylaminomethane (Tris) was purchased from USB. All the other reagents used in the experiments were purchased from Sigma. All the DNA oligonucleotides
Supporting Information Materials and Methods: Tris-hydroxymethylaminomethane (Tris) was purchased from USB. All the other reagents used in the experiments were purchased from Sigma. All the DNA oligonucleotides
Secondary Structure. Bioch/BIMS 503 Lecture 2. Structure and Function of Proteins. Further Reading. Φ, Ψ angles alone determine protein structure
 Bioch/BIMS 503 Lecture 2 Structure and Function of Proteins August 28, 2008 Robert Nakamoto rkn3c@virginia.edu 2-0279 Secondary Structure Φ Ψ angles determine protein structure Φ Ψ angles are restricted
Bioch/BIMS 503 Lecture 2 Structure and Function of Proteins August 28, 2008 Robert Nakamoto rkn3c@virginia.edu 2-0279 Secondary Structure Φ Ψ angles determine protein structure Φ Ψ angles are restricted
Membranes 2: Transportation
 Membranes 2: Transportation Steven E. Massey, Ph.D. Associate Professor Bioinformatics Department of Biology University of Puerto Rico Río Piedras Office & Lab: NCN#343B Tel: 787-764-0000 ext. 7798 E-mail:
Membranes 2: Transportation Steven E. Massey, Ph.D. Associate Professor Bioinformatics Department of Biology University of Puerto Rico Río Piedras Office & Lab: NCN#343B Tel: 787-764-0000 ext. 7798 E-mail:
Certificate of Analysis
 Certificate of Analysis 10 Old Barn Road Lake Placid, NY 12946 Technical Support: T: 800 548-7853 F: 518 523-4513 email: techserv@upstate.com Sales Department: T: 800 233-3991 F: 781 890-7738 Licensing
Certificate of Analysis 10 Old Barn Road Lake Placid, NY 12946 Technical Support: T: 800 548-7853 F: 518 523-4513 email: techserv@upstate.com Sales Department: T: 800 233-3991 F: 781 890-7738 Licensing
On the status of the Michaelis-Menten equation and its implications for enzymology
 1 On the status of the Michaelis-Menten equation and its implications for enzymology Sosale Chandrasekhar 1 Department of Organic Chemistry, Indian Institute of Science, Bangalore 560 012, India 1 E-mail:
1 On the status of the Michaelis-Menten equation and its implications for enzymology Sosale Chandrasekhar 1 Department of Organic Chemistry, Indian Institute of Science, Bangalore 560 012, India 1 E-mail:
Singlet. Fluorescence Spectroscopy * LUMO
 Fluorescence Spectroscopy Light can be absorbed and re-emitted by matter luminescence (photo-luminescence). There are two types of luminescence, in this discussion: fluorescence and phosphorescence. A
Fluorescence Spectroscopy Light can be absorbed and re-emitted by matter luminescence (photo-luminescence). There are two types of luminescence, in this discussion: fluorescence and phosphorescence. A
Enzyme Kinetics Using Isothermal Calorimetry. Malin Suurkuusk TA Instruments October 2014
 Enzyme Kinetics Using Isothermal Calorimetry Malin Suurkuusk TA Instruments October 2014 ITC is a powerful tool for determining enzyme kinetics Reactions, including enzymatic reactions, produce or absorb
Enzyme Kinetics Using Isothermal Calorimetry Malin Suurkuusk TA Instruments October 2014 ITC is a powerful tool for determining enzyme kinetics Reactions, including enzymatic reactions, produce or absorb
Detection of toxic mercury ions using a ratiometric CdSe/ZnS nanocrystal sensor
 Supporting Information for Detection of toxic mercury ions using a ratiometric CdSe/ZnS nanocrystal sensor Leah E. Page, Xi Zhang, Ali M. Jawaid, and Preston T. Snee * Department of Chemistry, University
Supporting Information for Detection of toxic mercury ions using a ratiometric CdSe/ZnS nanocrystal sensor Leah E. Page, Xi Zhang, Ali M. Jawaid, and Preston T. Snee * Department of Chemistry, University
Protein Synthetic Lipid Interactions
 Acta Biophysica Romana 2006 22-24 Febbraio Università di Roma - Tor Vergata Protein Synthetic Lipid Interactions Silvia Tardioli and Adalberto Bonincontro CNISM-Dipartimento di Fisica Camillo La Mesa Dipartimento
Acta Biophysica Romana 2006 22-24 Febbraio Università di Roma - Tor Vergata Protein Synthetic Lipid Interactions Silvia Tardioli and Adalberto Bonincontro CNISM-Dipartimento di Fisica Camillo La Mesa Dipartimento
