Polarization is out of the plane
|
|
|
- Louise Collins
- 5 years ago
- Views:
Transcription
1 Physics 055 Lecture 2 Recall from last time the various crystal classes and the dierent translational and rotational symmetry operations which made this enumeration possible. You should also be familiar with the distinction between the CONVENTIONL and the PRIMITIVE unit cells. (Refer to the 2-D Centered Rectangular Bravais Lattice.) Example: Consider the 2-D close-packed structure formed by a uniform collection of marbles. a 2 a + a 2 a For this system a \hexagonal" lattice (lattice vectors ~a and ~a 2 ) is formed with a single \marble" per unit cell. Notice that the Wigner-Seitz cells form hexagons. If identical 2-D planes of marbles are placed directly on top of one another, a simple (primitive) hexagonal 3-D lattice is formed. a 3 unit cell a 2 marble center a Simple Hexagonal (one marble basis) In this case j~a j = j~a 2 j = j~a 3 j =60,==90 Note: this system is no longer close-packed. Let us dene this stacking as //. Looking back at our 2-D hexagonal planes, notice that marbles could be packed more tightly if the marbles are displaced (~a 3 + ~a 2 ) (denoted by B's) or 2(~a 3 + ~a 2 ) (denoted by C's) from one layer to the next so that the marble in the next layer reside in the hollows forms by three neighboring marble in the layer. 7
2 B B C C B B C C B B C C Notice that the B's and C's themselves form a hexagonal 2-D lattice. Now assume that the rst two layers stack to B. For the third layer, either or C translations (assume close-packing) are now possible. If the stacking is BB..., the hexagonal close packed structure (HCP) has been formed. It has a hexagonal 3-D lattice and a two marble basis j ~a j=j ~a 2 j, j ~a 3 j= q 8=3 j ~a j, =60,==90 with marble at (0,0,0) and marble 2 at ( 3 ; 3 ; 2 ) in terms of (~a ;~a 2 ;~a 3 ). If instead the stacking is BCBC..., we have a primitive rhombohedral lattice with a single atom basis j~a j = j~a 2 j = j~a 3 j and = = =60. lternately, we could form a hexagonal unit cell with a 3 marble basis. In addition, this packing is also the same as the conventional face-centered cubic structure (FCC) which has a cubic unit cell and a four atom basis! Question: How are directions in the \real" space lattice designated? For this, a common notation of [u v w] in brackets is used to select a real space vector ~R = u~a + v~a 2 + w~a 3. P Example: Cubic lattice using [3 2 ] ( 3 R ~P is perpendicular to the plane formed by the [3 2 ] lattice vector ~R =3~a + 2 ~a 2 +~a 3 Notice that we form a plane which intercepts the axes at said points. How do we describe the plane? ( u v ) Lowest Common Denominator w )x6=(236) 2 R P For cubic symmetry []? (). In general, this is NOT true. 8
3 Experimental Methods Now that we have dened a system of crystals, it is important to develop a series of experimental procedures in order to study \real" crystals. For this there are a wide variety of techniques to study surfaces and the bulk. \Real" space images! Transmission electron microscopy (TEM) Scanning tunneling microscopy (STM) Diraction Images! -ray diraction, neutron scattering We will discuss diraction techniques: Sources of x-rays - conventional, synchrotron Sources of neutrons - reactors, spellation Conventional x-ray source Filament - e s h ν Low energy electron diraction (LEED) Metal target Fe, Co, Cu, g, Mo, Cr Two types of radiation:. Bremsstrahlung - \de"acceleration of incident e's 2. Characteristic line spectra - photons from atomic transition n=2 to K n=3 to K However, the incident e's must excite s electrons to the rst allowable transition KeV K α Synchrotron Light Source Bremsstrahlung light cone E H Photon Intensity K β a electrons/positrons move in a ring Bremsstahlung λ increasing cceleration Potential limit Photon Intensity (log scale) Photon Energy (kev) 9
4 Neutrons: Reactor - Thermal neutrons from ssion events E neutron =0.025 ev = 25 mev :5 Spellation - High energy protons impinge on a uranium pile. Reactor (steady state beam) High Flux Neutron neutrons sample Reactor collimators Spellation source (pulsed beam) Proton accelerator high energy neutrons Moderator sample Uranium target low energy neutrons Scattering of x-rays: This can be solved classically by using Maxwell's equations, or quantum mechanically. In either case the calculation is involved, and will not be reproduced here. (See e.g., -Ray Diraction, zaro, Kaplow, Kato, Weiss, Wilson, Young.) The J. J. Thomson model of scattering due to acceleration of the electrons follows. The incident photon E ~ = E ~ 0 expfi(!t kz)g, can be described as a travelling wave and the electron is considered to be xed by an attached spring with spring constant K. y-z plane y E H z x-z plane -x electron k is the spring constant 0
5 ~F e = e[ E ~ + (_xh)] kx c m! x= e ~ E k~x! 0 = q k=m! x= e m ~ E! 2 0 ~x Solution: ~x = x 0 e i!t^x x 0 =- ee 0 m a =j ~ x j=!2 ee 0 m(! 2 0! 2 ) (! 2 0! 2 ) Since m nucleus >> m electron No contribution by the nucleus. The electron is accelerated in the x-direction and radiates energy. For exact details refer to any text on classical electrodynamics (e.g., Jackson, Reitz & Milford). Time and usefulness do not allow us to delve further. There are however two components to the incident radiation: Polarization is out of the plane Es φ.. x φ E s Ei y θ z Ei y x θ z Polarization is in the plane Es Ei x θ φ z (ssume that your eye /detector is in the x-z plane) Note: is the angle between the scattered beam and the direction of the accelerated electron is the angle from the ^z-axis E s = a e sin Rc 2 E s E 0 = e 2! 2 sin Rc 2 m(! 2 0! 2 ) I s I 0 = E2 s E 2 0 e 4! 4 sin 2 =+ R 2 m 2 c 4 (! 0 2! 2 ) 2
6 [Note: If!! 0 (i.e., at an absorption edge) Thompson theory breaks down.] In general,!>>! 0 and the x-ray intensity / (a \light bulb") R 2 [Incoherent (i.e., Compton scattering) is also present.] I s = e 4 R 2 m 2 c 4 sin2 I 0 s was just shown by viewing only in the x-z plane: For x-rays polarized in the x-dir + = =2 For x-rays polarized in the y-dir = /2 Thus for an unpolarized beam I = I 2 0(x z)+ I 2 0(y z) I s =! 2 e2 hsin i 2 =2+sin 2 (=2 ) mc 2 R 2 2 I s = R 2 e 2 mc 2! 2 +cos 2 2 Polarization factor The polarization factor is just one of many necessary \corrections." 2
7 Physics 055 Lecture 3 Title: Scattering Due to toms Last time we calculated the scattering of photons (using unpolarized incident radiation) from a single classically bound electron. I s =! I o R e 2 2 +cos 2 2 mc 2 2 Thus the total scattering cross section is given by: T = ZZ Is I o R 2 sindd = e mc 2! 2 =0: cm 2 : In reality atoms are a collection of electrons. These electrons are spatially separated and to rst order non-interacting. Thus, their scattering will not superimpose completely coherently. For example, assume two electrons such that one is at the origin whilst the other is located at ~r i. Scattered radiation k Incident radiation s k 0 r i p 2 p electron at origin (reference point) The incoming wave is described by ~E i = E ~ 0 exp ni(! o 0 t ~ k0 ~r) 3
8 and the outgoing wave is given by ~E f = E ~ s exp ni(! o s t ~ ks ~r) Recall " photon =h! =hjkjc; = 2 jkj ; = c Since each electron independently scatters the incoming photon eld, there is necessarily a path dierence p +p 2 between the scattered waves. This path dierence implies a phase dierence. p = ^k 0 ~r i p 2 =+^k s ~r i pp +p 2 = ^ks ^k0 ~ri phase dierence ssume elastic scattering 0 = s so path 2 = = ~ ks ~ k0 ~ri =~s~r i and ~ k s ~ k0 is the change in the \momentum" of the scattered waves (i.e., diraction vector). Thus we have a new and important quantity called the scattering vector or diraction vector. ~s ~ k d ~ k s ~ k0 To get the total scattered amplitude for a single atom, it is clearly necessary to take the superposition of scattering from all the electrons. The amplitude for the i th electron is i = f e exp i(~s ~r i ) where f e is the amplitude for an electron at the origin. Thus, the total amplitude is = where Z is the atomic number. Z i= I intensity = =f 2 e i = f e exp i(~s ~r i ) i j I=f 2 e e i~s~r j i;j i e i~s~r i e i~s(~r i ~r j ) This summation is for the superposition of point-like e's. This distribution clearly changes with time. In actuality, the time averaged distribution is required. Replacing the point-like e's with a charge distribution gives Z = f e V (r)e i~s~r dv 4
9 Thus a new quantity, f atom f e tomic scattering form factor, is realized. Notice that as ~s! 0, (towards the forward scattering direction) the phase dierences go to zero. Thus, intuitively one expects a smoothly decreasing intensity with increasing angle. Now for the scattering from an arbitrary collection of atoms all atoms T = atom = atoms k where f k = f e R k (r)e i(~s~r) d~r for each atom f k e i~s~r k ~r k position of the k th atom f k atomic form factor for the k th atom The total observed intensity is I T = T T= k;` f k f ` ei~s(~r k ~r`) Notice that at no point has the assumed periodicity of a crystalline lattice centered into this discussion! This will be done next time. However, we will go a bit further. SSUME a collection of identical atoms: I T = k=` + k6=` fk f ` ei~s(~r k ~r`) I T = k=` f k f k ei~s(~r k ~r ) k + f k f ` ei~s(~r k k6=` ~r`) ssume there are N identical atoms I T = Nf 2 + k6=` f k f ` ei~s(~r k ~r`) () The rst term has no phase or angular dependence. It represents a coherent background and is called the self-scattering term. nother venture: ssume we replace the discrete sum of T function: with an atomic distribution Z T (~s) =f (~r)e i~s~r dv V where f is the individual atom form factor and (~r) is the atomic number density. Then Z I (~s) =(~s) (~s)=jfj 2 (~r)(~r 0 ) e i~s(~r ~r0) d~rd~r 0 5
10 R r r Origin Then the second term in Eqn. (on the previous page) becomes I 0 (s) =jfj 2 Z Z (~r)(~r+ ~ R)e i~s~r d~rd(~r+ ~ R) We can dene a new quantity P ( ~ R) Z (~r) Let ~r 0 = ~r+ ~ R ~r + R ~ d~r pair distribution function Now I 0 (~s) =jfj 2 Z ( ~ R)e i~s~r d ~ R Clearly, I 0 (~s) is just the Fourier transform of the pair distribution function. IMPORTNT - This quantity is what a diraction experiment extracts From this information and insight the actual atomic positions can often be ascertained. 6
11 Physics 055 Lecture 4 Title: Coherent Scattering from a crystal. Scattering from a periodic arrangement of atoms and the construction of the reciprocal lattice. Recall the total atomic scattering amplitude Now invoke lattice periodicity (~s) = all atoms k f k e i~s~r k R = n ~a + n 2 ~a 2 + n 3 ~a 3 nd also assume (at present) there is just one atom per unit cell Thus ~r k = n ~a + n 2 ~a 2 + n 3 ~a 3 f k = f (~s) =f all atoms k e i~s(n ~a +n 2 ~a 2 +n 3 ~a 3 ) This sum is somewhat awkward to follow ifn ;n 2 ;n 3!. Let us examine the sum in the range 0 n ;n 2 ;n 3 N-. Hence there are only N 3 atoms to deal with. (~s) =f N n =0 N e i(~sa )n n 2 =0 N e i(~s~a 2 )n 2 n 3 =0 e i(~s ~a 3)n 3 Now each sum can be simplied if the following substitution is made: 0 finite crystal N- N n=0 e i(~s~a)n = 7 = n=0 e i(~s~a)n n=n e i(~s~a)n e i(~s~a)n e i(~s~a)n n=0 = e in (~s~a) n=0 n=0 e i(~s~a)n e i(~s~a)n = e in (~s~a) e i(~s~a) e i(~s~a) e i(~s~a) e in (~s~a) e i(~s~a) = 2( cos ~s ~a)
12 or (~s) = f h e in (~s~a ) ih ih e in (~s~a 2 ) e in (~s~a 3 )i e i~s~a 2 e i~s~a 2 e ~s~a 3 2( cos ~s ~a )2( cos ~s ~a 2 )2( cos ~s ~a 3 ) The intensity I = * gives I = jfj 2 ( cos N ~s ~a )( cos ~s ~a ) :::: ( cos N ~s ~a 3 )( cos ~s ~a 3 ) ( cos ~s ~a ) 2 :::: ( cos ~s ~a 3 ) 2 I = jfj 2 ( cos N ~s ~a )( cosn (~s ~a 2 )) ( cosn (~s ~a 3 )) ( cos ~s ~a )( cos ~s ~a 2 )( cos ~s ~a 3 ) or I = jf j 2 sin 2 (N (~s~a )=2)sin 2 (N~s~a 2 =2)sin 2 (N~s~a 3 ) sin 2 (~s~a =2)sin 2 (~s~a 2 =2) sin 2 (~s~a 3 =2) The behavior of the intensity is relatively straightforward. ~s ~a 2 =2n and ~s ~a 3 =2m. Thus (for one set of terms) Let N=0 and let both sin 2 N~s ~a 2 =2 sin 2 ~s ~a 2 =2 = sin 2 Nn sin 2 n = N2 (using L'Hopital's rule) or since sin(nn)! 0 expand top and bottom in Taylor's series sin x x if x! 0 (choose n =0) Thus sin 2 Nn sin 2 n (Nn)2 (n) 2 = N 2 I = jfj 2 sin2 N~sa sin 2 ~s ~a N 4 Plot: Numerator = sin 2 5(~s ~a ) and Denominator = sin 2 (~s ~a )(0~s~a 2) ~s ~a ( radians) ~s ~a ( radians) 8
13 Now for sin 2 5~s ~a 00 sin 2 ~s ~a So ~s ~a ( radians) I(0) = jfj = jfj 2 N 6 Now compare this intensity with the self scattering whichwas proportional to the number of atoms or N 3 (in this case) when N atoms! In addition, as N becomes large the intensity due to nite size eects (so called \F" peaks) become unobservable. However, these fringes can be seen in very small crystals using electron diraction. They can also be seen in surface scattering from crystals with steps. s N!, the curves become -functions with FWHM (Full widths at half maximum) of 2/N and height N 6 at the positions ~s ~a = 2h ~s ~a 2 = 2k h; k; ` are integers ~s ~a 3 = 2` 9 >= This is the Laue condition! >; This set of expressions are called the Laue diraction conditions. When ~s~a ;~s~aa 2 ;~s~a 3 are all multiples of 2, the amplitudes interfere constructively. This condition is equivalent to Bragg's law n = 2d sin. (Constructive interference a scattered waves) To review: One atom per unit with a crystal length of N- on a side 9
14 N 3 sites (~s) =f i e i~s(n ~a +n 2 ~a 2 + n 3 ~a 3 ) gives I = jfj 2 sin2 (N~s ~a )=2:::: sin 2 (~s ~a )=2::::: If we restrict our sum to ~s's such that the Laue conditions are met then ~s ~a i =2` and all of the scattering is coherent. (i =,2,3) (~s) =fn 3 If we dene a normalizing f = f 0 =N 3, then (~s) =f 0 (N independent) What if there are two atoms per cell tom is located at n ~a + n 2 ~a 2 + n 3 ~a 3 While tom B is at n ~a + n 2 ~a 2 n 3 ~a 3 + ~ where ~ is not a lattice vector. N3 sites The total (~s) =f j N 3 e i~s(n ~a +n 2 ~a 2 +n 3 ~a 3) sites + f B j e i~s(n ~a +n 2 ~a 2 +n 3 ~a 3 + ~ ) Restrict sum to those that satisfy the Laue condition. The set of ~s's which satisfy the Laue condition are designated ~ G and are called the reciprocal lattice vectors. ( G)=f ~ N 3 +f B N 3 e i~ G ~ f 0 =f=n 3 ( G)=f ~ +f 0 0 B ei~ G ~!there are two terms here, one for each atom within the unit cell ( the basis). Generalizing gives unit cell atoms ~G = j f j e i ~ G~r j and I ~G = structure factor ~r j is the relative position of atoms within the unit cell. 20
Solid State Physics Lecture 3 Diffraction and the Reciprocal Lattice (Kittel Ch. 2)
 Solid State Physics 460 - Lecture 3 Diffraction and the Reciprocal Lattice (Kittel Ch. 2) Diffraction (Bragg Scattering) from a powder of crystallites - real example of image at right from http://www.uni-wuerzburg.de/mineralogie/crystal/teaching/pow.html
Solid State Physics 460 - Lecture 3 Diffraction and the Reciprocal Lattice (Kittel Ch. 2) Diffraction (Bragg Scattering) from a powder of crystallites - real example of image at right from http://www.uni-wuerzburg.de/mineralogie/crystal/teaching/pow.html
Crystals Statics. Structural Properties. Geometry of lattices. Aug 23, 2018
 Crystals Statics. Structural Properties. Geometry of lattices Aug 23, 2018 Crystals Why (among all condensed phases - liquids, gases) look at crystals? We can take advantage of the translational symmetry,
Crystals Statics. Structural Properties. Geometry of lattices Aug 23, 2018 Crystals Why (among all condensed phases - liquids, gases) look at crystals? We can take advantage of the translational symmetry,
Solid State Physics 460- Lecture 5 Diffraction and the Reciprocal Lattice Continued (Kittel Ch. 2)
 Solid State Physics 460- Lecture 5 Diffraction and the Reciprocal Lattice Continued (Kittel Ch. 2) Ewald Construction 2θ k out k in G Physics 460 F 2006 Lect 5 1 Recall from previous lectures Definition
Solid State Physics 460- Lecture 5 Diffraction and the Reciprocal Lattice Continued (Kittel Ch. 2) Ewald Construction 2θ k out k in G Physics 460 F 2006 Lect 5 1 Recall from previous lectures Definition
Surface Sensitivity & Surface Specificity
 Surface Sensitivity & Surface Specificity The problems of sensitivity and detection limits are common to all forms of spectroscopy. In its simplest form, the question of sensitivity boils down to whether
Surface Sensitivity & Surface Specificity The problems of sensitivity and detection limits are common to all forms of spectroscopy. In its simplest form, the question of sensitivity boils down to whether
Introduction to Solid State Physics or the study of physical properties of matter in a solid phase
 Introduction to Solid State Physics or the study of physical properties of matter in a solid phase Prof. Germar Hoffmann 1. Crystal Structures 2. Reciprocal Lattice 3. Crystal Binding and Elastic Constants
Introduction to Solid State Physics or the study of physical properties of matter in a solid phase Prof. Germar Hoffmann 1. Crystal Structures 2. Reciprocal Lattice 3. Crystal Binding and Elastic Constants
An Introduction to Diffraction and Scattering. School of Chemistry The University of Sydney
 An Introduction to Diffraction and Scattering Brendan J. Kennedy School of Chemistry The University of Sydney 1) Strong forces 2) Weak forces Types of Forces 3) Electromagnetic forces 4) Gravity Types
An Introduction to Diffraction and Scattering Brendan J. Kennedy School of Chemistry The University of Sydney 1) Strong forces 2) Weak forces Types of Forces 3) Electromagnetic forces 4) Gravity Types
2. Diffraction as a means to determine crystal structure
 Page 1 of 22 2. Diffraction as a means to determine crystal structure Recall de Broglie matter waves: 2 p h E = where p = 2m λ h 1 E = ( ) 2m λ hc E = hυ = ( photons) λ ( matter wave) He atoms: [E (ev)]
Page 1 of 22 2. Diffraction as a means to determine crystal structure Recall de Broglie matter waves: 2 p h E = where p = 2m λ h 1 E = ( ) 2m λ hc E = hυ = ( photons) λ ( matter wave) He atoms: [E (ev)]
(10%) (c) What other peaks can appear in the pulse-height spectrum if the detector were not small? Give a sketch and explain briefly.
 Sample questions for Quiz 3, 22.101 (Fall 2006) Following questions were taken from quizzes given in previous years by S. Yip. They are meant to give you an idea of the kind of questions (what was expected
Sample questions for Quiz 3, 22.101 (Fall 2006) Following questions were taken from quizzes given in previous years by S. Yip. They are meant to give you an idea of the kind of questions (what was expected
Phys 412 Solid State Physics. Lecturer: Réka Albert
 Phys 412 Solid State Physics Lecturer: Réka Albert What is a solid? A material that keeps its shape Can be deformed by stress Returns to original shape if it is not strained too much Solid structure
Phys 412 Solid State Physics Lecturer: Réka Albert What is a solid? A material that keeps its shape Can be deformed by stress Returns to original shape if it is not strained too much Solid structure
X-ray Diffraction. Interaction of Waves Reciprocal Lattice and Diffraction X-ray Scattering by Atoms The Integrated Intensity
 X-ray Diraction Interaction o Waves Reciprocal Lattice and Diraction X-ray Scattering by Atoms The Integrated Intensity Basic Principles o Interaction o Waves Periodic waves characteristic: Frequency :
X-ray Diraction Interaction o Waves Reciprocal Lattice and Diraction X-ray Scattering by Atoms The Integrated Intensity Basic Principles o Interaction o Waves Periodic waves characteristic: Frequency :
Geometry of Crystal Lattice
 0 Geometry of Crystal Lattice 0.1 Translational Symmetry The crystalline state of substances is different from other states (gaseous, liquid, amorphous) in that the atoms are in an ordered and symmetrical
0 Geometry of Crystal Lattice 0.1 Translational Symmetry The crystalline state of substances is different from other states (gaseous, liquid, amorphous) in that the atoms are in an ordered and symmetrical
Interaction of particles with matter - 2. Silvia Masciocchi, GSI and University of Heidelberg SS2017, Heidelberg May 3, 2017
 Interaction of particles with matter - 2 Silvia Masciocchi, GSI and University of Heidelberg SS2017, Heidelberg May 3, 2017 Energy loss by ionization (by heavy particles) Interaction of electrons with
Interaction of particles with matter - 2 Silvia Masciocchi, GSI and University of Heidelberg SS2017, Heidelberg May 3, 2017 Energy loss by ionization (by heavy particles) Interaction of electrons with
2. Diffraction as a means to determine crystal structure
 2. Diffraction as a means to determine crystal structure Recall de Broglie matter waves: He atoms: [E (ev)] 1/2 = 0.14 / (Å) E 1Å = 0.0196 ev Neutrons: [E (ev)] 1/2 = 0.28 / (Å) E 1Å = 0.0784 ev Electrons:
2. Diffraction as a means to determine crystal structure Recall de Broglie matter waves: He atoms: [E (ev)] 1/2 = 0.14 / (Å) E 1Å = 0.0196 ev Neutrons: [E (ev)] 1/2 = 0.28 / (Å) E 1Å = 0.0784 ev Electrons:
Crystal planes. Neutrons: magnetic moment - interacts with magnetic materials or nuclei of non-magnetic materials. (in Å)
 Crystallography: neutron, electron, and X-ray scattering from periodic lattice, scattering of waves by periodic structures, Miller indices, reciprocal space, Ewald construction. Diffraction: Specular,
Crystallography: neutron, electron, and X-ray scattering from periodic lattice, scattering of waves by periodic structures, Miller indices, reciprocal space, Ewald construction. Diffraction: Specular,
Summary Chapter 2: Wave diffraction and the reciprocal lattice.
 Summary Chapter : Wave diffraction and the reciprocal lattice. In chapter we discussed crystal diffraction and introduced the reciprocal lattice. Since crystal have a translation symmetry as discussed
Summary Chapter : Wave diffraction and the reciprocal lattice. In chapter we discussed crystal diffraction and introduced the reciprocal lattice. Since crystal have a translation symmetry as discussed
ESSENTIAL QUANTUM PHYSICS PETER LANDSHOFF. University of Cambridge ALLEN METHERELL. University of Central Florida GARETH REES. University of Cambridge
 ESSENTIAL QUANTUM PHYSICS PETER LANDSHOFF University of Cambridge ALLEN METHERELL University of Central Florida GARETH REES University of Cambridge CAMBRIDGE UNIVERSITY PRESS Constants of quantum physics
ESSENTIAL QUANTUM PHYSICS PETER LANDSHOFF University of Cambridge ALLEN METHERELL University of Central Florida GARETH REES University of Cambridge CAMBRIDGE UNIVERSITY PRESS Constants of quantum physics
Roger Johnson Structure and Dynamics: X-ray Diffraction Lecture 6
 6.1. Summary In this Lecture we cover the theory of x-ray diffraction, which gives direct information about the atomic structure of crystals. In these experiments, the wavelength of the incident beam must
6.1. Summary In this Lecture we cover the theory of x-ray diffraction, which gives direct information about the atomic structure of crystals. In these experiments, the wavelength of the incident beam must
Neutron and x-ray spectroscopy
 Neutron and x-ray spectroscopy B. Keimer Max-Planck-Institute for Solid State Research outline 1. self-contained introduction neutron scattering and spectroscopy x-ray scattering and spectroscopy 2. application
Neutron and x-ray spectroscopy B. Keimer Max-Planck-Institute for Solid State Research outline 1. self-contained introduction neutron scattering and spectroscopy x-ray scattering and spectroscopy 2. application
PART 1 Introduction to Theory of Solids
 Elsevier UK Job code: MIOC Ch01-I044647 9-3-2007 3:03p.m. Page:1 Trim:165 240MM TS: Integra, India PART 1 Introduction to Theory of Solids Elsevier UK Job code: MIOC Ch01-I044647 9-3-2007 3:03p.m. Page:2
Elsevier UK Job code: MIOC Ch01-I044647 9-3-2007 3:03p.m. Page:1 Trim:165 240MM TS: Integra, India PART 1 Introduction to Theory of Solids Elsevier UK Job code: MIOC Ch01-I044647 9-3-2007 3:03p.m. Page:2
is the minimum stopping potential for which the current between the plates reduces to zero.
 Module 1 :Quantum Mechanics Chapter 2 : Introduction to Quantum ideas Introduction to Quantum ideas We will now consider some experiments and their implications, which introduce us to quantum ideas. The
Module 1 :Quantum Mechanics Chapter 2 : Introduction to Quantum ideas Introduction to Quantum ideas We will now consider some experiments and their implications, which introduce us to quantum ideas. The
Particles and Waves Particles Waves
 Particles and Waves Particles Discrete and occupy space Exist in only one location at a time Position and velocity can be determined with infinite accuracy Interact by collisions, scattering. Waves Extended,
Particles and Waves Particles Discrete and occupy space Exist in only one location at a time Position and velocity can be determined with infinite accuracy Interact by collisions, scattering. Waves Extended,
Interaction X-rays - Matter
 Interaction X-rays - Matter Pair production hν > M ev Photoelectric absorption hν MATTER hν Transmission X-rays hν' < hν Scattering hν Decay processes hν f Compton Thomson Fluorescence Auger electrons
Interaction X-rays - Matter Pair production hν > M ev Photoelectric absorption hν MATTER hν Transmission X-rays hν' < hν Scattering hν Decay processes hν f Compton Thomson Fluorescence Auger electrons
Phonons I - Crystal Vibrations (Kittel Ch. 4)
 Phonons I - Crystal Vibrations (Kittel Ch. 4) Displacements of Atoms Positions of atoms in their perfect lattice positions are given by: R 0 (n 1, n 2, n 3 ) = n 10 x + n 20 y + n 30 z For simplicity here
Phonons I - Crystal Vibrations (Kittel Ch. 4) Displacements of Atoms Positions of atoms in their perfect lattice positions are given by: R 0 (n 1, n 2, n 3 ) = n 10 x + n 20 y + n 30 z For simplicity here
Setting The motor that rotates the sample about an axis normal to the diffraction plane is called (or ).
 X-Ray Diffraction X-ray diffraction geometry A simple X-ray diffraction (XRD) experiment might be set up as shown below. We need a parallel X-ray source, which is usually an X-ray tube in a fixed position
X-Ray Diffraction X-ray diffraction geometry A simple X-ray diffraction (XRD) experiment might be set up as shown below. We need a parallel X-ray source, which is usually an X-ray tube in a fixed position
Keble College - Hilary 2012 Section VI: Condensed matter physics Tutorial 2 - Lattices and scattering
 Tomi Johnson Keble College - Hilary 2012 Section VI: Condensed matter physics Tutorial 2 - Lattices and scattering Please leave your work in the Clarendon laboratory s J pigeon hole by 5pm on Monday of
Tomi Johnson Keble College - Hilary 2012 Section VI: Condensed matter physics Tutorial 2 - Lattices and scattering Please leave your work in the Clarendon laboratory s J pigeon hole by 5pm on Monday of
4. Other diffraction techniques
 4. Other diffraction techniques 4.1 Reflection High Energy Electron Diffraction (RHEED) Setup: - Grazing-incidence high energy electron beam (3-5 kev: MEED,
4. Other diffraction techniques 4.1 Reflection High Energy Electron Diffraction (RHEED) Setup: - Grazing-incidence high energy electron beam (3-5 kev: MEED,
(Re-write, January 2011, from notes of S. C. Fain Jr., L. Sorensen, O. E. Vilches, J. Stoltenberg and D. B. Pengra, Version 1, preliminary)
 Electron Diffraction (Re-write, January 011, from notes of S. C. Fain Jr., L. Sorensen, O. E. Vilches, J. Stoltenberg and D. B. Pengra, Version 1, preliminary) References: Any introductory physics text
Electron Diffraction (Re-write, January 011, from notes of S. C. Fain Jr., L. Sorensen, O. E. Vilches, J. Stoltenberg and D. B. Pengra, Version 1, preliminary) References: Any introductory physics text
Applied Nuclear Physics (Fall 2006) Lecture 19 (11/22/06) Gamma Interactions: Compton Scattering
 .101 Applied Nuclear Physics (Fall 006) Lecture 19 (11//06) Gamma Interactions: Compton Scattering References: R. D. Evans, Atomic Nucleus (McGraw-Hill New York, 1955), Chaps 3 5.. W. E. Meyerhof, Elements
.101 Applied Nuclear Physics (Fall 006) Lecture 19 (11//06) Gamma Interactions: Compton Scattering References: R. D. Evans, Atomic Nucleus (McGraw-Hill New York, 1955), Chaps 3 5.. W. E. Meyerhof, Elements
Road map (Where are we headed?)
 Road map (Where are we headed?) oal: Fairly high level understanding of carrier transport and optical transitions in semiconductors Necessary Ingredients Crystal Structure Lattice Vibrations Free Electron
Road map (Where are we headed?) oal: Fairly high level understanding of carrier transport and optical transitions in semiconductors Necessary Ingredients Crystal Structure Lattice Vibrations Free Electron
CHAPTER 5 Wave Properties of Matter and Quantum Mechanics I
 CHAPTER 5 Wave Properties of Matter and Quantum Mechanics I 5.1 X-Ray Scattering 5.2 De Broglie Waves 5.3 Electron Scattering 5.4 Wave Motion 5.5 Waves or Particles? 5.6 Uncertainty Principle 5.7 Probability,
CHAPTER 5 Wave Properties of Matter and Quantum Mechanics I 5.1 X-Ray Scattering 5.2 De Broglie Waves 5.3 Electron Scattering 5.4 Wave Motion 5.5 Waves or Particles? 5.6 Uncertainty Principle 5.7 Probability,
Experiment 12 ELECTRON DIFFRACTION. Diffraction by Graphite 1. Diffraction by Aluminum 3. The Electron Diffraction Apparatus 5
 1-i Experiment 1 ELECTRON DIFFRACTION Introduction 1 Diffraction by Graphite 1 Diffraction by Aluminum 3 The Electron Diffraction Apparatus 5 Procedure and Analysis 6 References 7 Prelab Problems 8 Appendix
1-i Experiment 1 ELECTRON DIFFRACTION Introduction 1 Diffraction by Graphite 1 Diffraction by Aluminum 3 The Electron Diffraction Apparatus 5 Procedure and Analysis 6 References 7 Prelab Problems 8 Appendix
High-Resolution. Transmission. Electron Microscopy
 Part 4 High-Resolution Transmission Electron Microscopy 186 Significance high-resolution transmission electron microscopy (HRTEM): resolve object details smaller than 1nm (10 9 m) image the interior of
Part 4 High-Resolution Transmission Electron Microscopy 186 Significance high-resolution transmission electron microscopy (HRTEM): resolve object details smaller than 1nm (10 9 m) image the interior of
M2 TP. Low-Energy Electron Diffraction (LEED)
 M2 TP Low-Energy Electron Diffraction (LEED) Guide for report preparation I. Introduction: Elastic scattering or diffraction of electrons is the standard technique in surface science for obtaining structural
M2 TP Low-Energy Electron Diffraction (LEED) Guide for report preparation I. Introduction: Elastic scattering or diffraction of electrons is the standard technique in surface science for obtaining structural
 Name : Roll No. :.. Invigilator s Signature :.. CS/B.Tech/SEM-2/PH-201/2010 2010 ENGINEERING PHYSICS Time Allotted : 3 Hours Full Marks : 70 The figures in the margin indicate full marks. Candidates are
Name : Roll No. :.. Invigilator s Signature :.. CS/B.Tech/SEM-2/PH-201/2010 2010 ENGINEERING PHYSICS Time Allotted : 3 Hours Full Marks : 70 The figures in the margin indicate full marks. Candidates are
disordered, ordered and coherent with the substrate, and ordered but incoherent with the substrate.
 5. Nomenclature of overlayer structures Thus far, we have been discussing an ideal surface, which is in effect the structure of the topmost substrate layer. The surface (selvedge) layers of the solid however
5. Nomenclature of overlayer structures Thus far, we have been discussing an ideal surface, which is in effect the structure of the topmost substrate layer. The surface (selvedge) layers of the solid however
Crystallographic structure Physical vs Chemical bonding in solids
 Crystallographic structure Physical vs Chemical bonding in solids Inert gas and molecular crystals: Van der Waals forces (physics) Water and organic chemistry H bonds (physics) Quartz crystal SiO 2 : covalent
Crystallographic structure Physical vs Chemical bonding in solids Inert gas and molecular crystals: Van der Waals forces (physics) Water and organic chemistry H bonds (physics) Quartz crystal SiO 2 : covalent
Name :. Roll No. :... Invigilator s Signature :.. CS/B. Tech (New)/SEM-1/PH-101/ PHYSICS-I
 Name :. Roll No. :..... Invigilator s Signature :.. CS/B. Tech (New)/SEM-1/PH-101/2011-12 2011 PHYSICS-I Time Allotted : 3 Hours Full Marks : 70 The figures in the margin indicate full marks. Candidates
Name :. Roll No. :..... Invigilator s Signature :.. CS/B. Tech (New)/SEM-1/PH-101/2011-12 2011 PHYSICS-I Time Allotted : 3 Hours Full Marks : 70 The figures in the margin indicate full marks. Candidates
3.012 Structure An Introduction to X-ray Diffraction
 3.012 Structure An Introduction to X-ray Diffraction This handout summarizes some topics that are important for understanding x-ray diffraction. The following references provide a thorough explanation
3.012 Structure An Introduction to X-ray Diffraction This handout summarizes some topics that are important for understanding x-ray diffraction. The following references provide a thorough explanation
Introduction to crystallography The unitcell The resiprocal space and unitcell Braggs law Structure factor F hkl and atomic scattering factor f zθ
 Introduction to crystallography The unitcell The resiprocal space and unitcell Braggs law Structure factor F hkl and atomic scattering factor f zθ Introduction to crystallography We divide materials into
Introduction to crystallography The unitcell The resiprocal space and unitcell Braggs law Structure factor F hkl and atomic scattering factor f zθ Introduction to crystallography We divide materials into
Crystal Structure. Dr Bindu Krishnan
 Solid State Physics-1 Crystal Structure Dr Bindu Krishnan CRYSTAL LATTICE What is crystal (space) lattice? In crystallography, only the geometrical properties of the crystal are of interest, therefore
Solid State Physics-1 Crystal Structure Dr Bindu Krishnan CRYSTAL LATTICE What is crystal (space) lattice? In crystallography, only the geometrical properties of the crystal are of interest, therefore
PROBING CRYSTAL STRUCTURE
 PROBING CRYSTAL STRUCTURE Andrew Baczewski PHY 491, October 10th, 2011 OVERVIEW First - we ll briefly discuss Friday s quiz. Today, we will answer the following questions: How do we experimentally probe
PROBING CRYSTAL STRUCTURE Andrew Baczewski PHY 491, October 10th, 2011 OVERVIEW First - we ll briefly discuss Friday s quiz. Today, we will answer the following questions: How do we experimentally probe
SOLID STATE 18. Reciprocal Space
 SOLID STATE 8 Reciprocal Space Wave vectors and the concept of K-space can simplify the explanation of several properties of the solid state. They will be introduced to provide more information on diffraction
SOLID STATE 8 Reciprocal Space Wave vectors and the concept of K-space can simplify the explanation of several properties of the solid state. They will be introduced to provide more information on diffraction
U(x) Finite Well. E Re ψ(x) Classically forbidden
 Final Exam Physics 2130 Modern Physics Tuesday December 18, 2001 Point distribution: All questions are worth points 8 points. Answers should be bubbled onto the answer sheet. 1. At what common energy E
Final Exam Physics 2130 Modern Physics Tuesday December 18, 2001 Point distribution: All questions are worth points 8 points. Answers should be bubbled onto the answer sheet. 1. At what common energy E
PARTICLES AND WAVES CHAPTER 29 CONCEPTUAL QUESTIONS
 CHAPTER 29 PARTICLES AND WAVES CONCEPTUAL QUESTIONS 1. REASONING AND SOLUTION A monochromatic light source emits photons of a single frequency. According to Equation 29.2, the energy, E, of a single photon
CHAPTER 29 PARTICLES AND WAVES CONCEPTUAL QUESTIONS 1. REASONING AND SOLUTION A monochromatic light source emits photons of a single frequency. According to Equation 29.2, the energy, E, of a single photon
Today, I will present the first of two lectures on neutron interactions.
 Today, I will present the first of two lectures on neutron interactions. I first need to acknowledge that these two lectures were based on lectures presented previously in Med Phys I by Dr Howell. 1 Before
Today, I will present the first of two lectures on neutron interactions. I first need to acknowledge that these two lectures were based on lectures presented previously in Med Phys I by Dr Howell. 1 Before
Phys 460 Describing and Classifying Crystal Lattices
 Phys 460 Describing and Classifying Crystal Lattices What is a material? ^ crystalline Regular lattice of atoms Each atom has a positively charged nucleus surrounded by negative electrons Electrons are
Phys 460 Describing and Classifying Crystal Lattices What is a material? ^ crystalline Regular lattice of atoms Each atom has a positively charged nucleus surrounded by negative electrons Electrons are
X-ray, Neutron and e-beam scattering
 X-ray, Neutron and e-beam scattering Introduction Why scattering? Diffraction basics Neutrons and x-rays Techniques Direct and reciprocal space Single crystals Powders CaFe 2 As 2 an example What is the
X-ray, Neutron and e-beam scattering Introduction Why scattering? Diffraction basics Neutrons and x-rays Techniques Direct and reciprocal space Single crystals Powders CaFe 2 As 2 an example What is the
Atomic Physics. Chapter 6 X ray. Jinniu Hu 24/12/ /20/13
 Atomic Physics Chapter 6 X ray 11/20/13 24/12/2018 Jinniu Hu 1!1 6.1 The discovery of X ray X-rays were discovered in 1895 by the German physicist Wilhelm Roentgen. He found that a beam of high-speed electrons
Atomic Physics Chapter 6 X ray 11/20/13 24/12/2018 Jinniu Hu 1!1 6.1 The discovery of X ray X-rays were discovered in 1895 by the German physicist Wilhelm Roentgen. He found that a beam of high-speed electrons
Fundamentals of X-ray diffraction
 Fundamentals of X-ray diffraction Elena Willinger Lecture series: Modern Methods in Heterogeneous Catalysis Research Outline History of X-ray Sources of X-ray radiation Physics of X-ray scattering Fundamentals
Fundamentals of X-ray diffraction Elena Willinger Lecture series: Modern Methods in Heterogeneous Catalysis Research Outline History of X-ray Sources of X-ray radiation Physics of X-ray scattering Fundamentals
48 K. B. Korotchenko, Yu. L. Pivovarov, Y. Takabayashi where n is the number of quantum states, L is the normalization length (N = 1 for axial channel
 Pis'ma v ZhETF, vol. 95, iss. 8, pp. 481 { 485 c 01 April 5 Quantum Eects for Parametric X-ray Radiation during Channeling: Theory and First Experimental Observation K. B. Korotchenko 1), Yu. L. Pivovarov,
Pis'ma v ZhETF, vol. 95, iss. 8, pp. 481 { 485 c 01 April 5 Quantum Eects for Parametric X-ray Radiation during Channeling: Theory and First Experimental Observation K. B. Korotchenko 1), Yu. L. Pivovarov,
Notes on x-ray scattering - M. Le Tacon, B. Keimer (06/2015)
 Notes on x-ray scattering - M. Le Tacon, B. Keimer (06/2015) Interaction of x-ray with matter: - Photoelectric absorption - Elastic (coherent) scattering (Thomson Scattering) - Inelastic (incoherent) scattering
Notes on x-ray scattering - M. Le Tacon, B. Keimer (06/2015) Interaction of x-ray with matter: - Photoelectric absorption - Elastic (coherent) scattering (Thomson Scattering) - Inelastic (incoherent) scattering
Basic Crystallography Part 1. Theory and Practice of X-ray Crystal Structure Determination
 Basic Crystallography Part 1 Theory and Practice of X-ray Crystal Structure Determination We have a crystal How do we get there? we want a structure! The Unit Cell Concept Ralph Krätzner Unit Cell Description
Basic Crystallography Part 1 Theory and Practice of X-ray Crystal Structure Determination We have a crystal How do we get there? we want a structure! The Unit Cell Concept Ralph Krätzner Unit Cell Description
Quantum Condensed Matter Physics Lecture 5
 Quantum Condensed Matter Physics Lecture 5 detector sample X-ray source monochromator David Ritchie http://www.sp.phy.cam.ac.uk/drp2/home QCMP Lent/Easter 2019 5.1 Quantum Condensed Matter Physics 1. Classical
Quantum Condensed Matter Physics Lecture 5 detector sample X-ray source monochromator David Ritchie http://www.sp.phy.cam.ac.uk/drp2/home QCMP Lent/Easter 2019 5.1 Quantum Condensed Matter Physics 1. Classical
Chapter 2. X-ray X. Diffraction and Reciprocal Lattice. Scattering from Lattices
 Chapter. X-ray X Diffraction and Reciprocal Lattice Diffraction of waves by crystals Reciprocal Lattice Diffraction of X-rays Powder diffraction Single crystal X-ray diffraction Scattering from Lattices
Chapter. X-ray X Diffraction and Reciprocal Lattice Diffraction of waves by crystals Reciprocal Lattice Diffraction of X-rays Powder diffraction Single crystal X-ray diffraction Scattering from Lattices
Transmission Electron Microscopy
 L. Reimer H. Kohl Transmission Electron Microscopy Physics of Image Formation Fifth Edition el Springer Contents 1 Introduction... 1 1.1 Transmission Electron Microscopy... 1 1.1.1 Conventional Transmission
L. Reimer H. Kohl Transmission Electron Microscopy Physics of Image Formation Fifth Edition el Springer Contents 1 Introduction... 1 1.1 Transmission Electron Microscopy... 1 1.1.1 Conventional Transmission
Reciprocal Lattice. Let A(r) be some function featuring discrete translation symmetry:
 Reciprocal Lattice From Quantum Mechanics we know that symmetries have kinematic implications. Each symmetry predetermines its specific quantum numbers and leads to certain constraints (conservation laws/selection
Reciprocal Lattice From Quantum Mechanics we know that symmetries have kinematic implications. Each symmetry predetermines its specific quantum numbers and leads to certain constraints (conservation laws/selection
Crystal Structure SOLID STATE PHYSICS. Lecture 5. A.H. Harker. thelecture thenextlecture. Physics and Astronomy UCL
 Crystal Structure thelecture thenextlecture SOLID STATE PHYSICS Lecture 5 A.H. Harker Physics and Astronomy UCL Structure & Diffraction Crystal Diffraction (continued) 2.4 Experimental Methods Notes: examples
Crystal Structure thelecture thenextlecture SOLID STATE PHYSICS Lecture 5 A.H. Harker Physics and Astronomy UCL Structure & Diffraction Crystal Diffraction (continued) 2.4 Experimental Methods Notes: examples
Structure analysis: Electron diffraction LEED TEM RHEED
 Structure analysis: Electron diffraction LEED: Low Energy Electron Diffraction SPA-LEED: Spot Profile Analysis Low Energy Electron diffraction RHEED: Reflection High Energy Electron Diffraction TEM: Transmission
Structure analysis: Electron diffraction LEED: Low Energy Electron Diffraction SPA-LEED: Spot Profile Analysis Low Energy Electron diffraction RHEED: Reflection High Energy Electron Diffraction TEM: Transmission
Nuclear Physics. (PHY-231) Dr C. M. Cormack. Nuclear Physics This Lecture
 Nuclear Physics (PHY-31) Dr C. M. Cormack 11 Nuclear Physics This Lecture This Lecture We will discuss an important effect in nuclear spectroscopy The Mössbauer Effect and its applications in technology
Nuclear Physics (PHY-31) Dr C. M. Cormack 11 Nuclear Physics This Lecture This Lecture We will discuss an important effect in nuclear spectroscopy The Mössbauer Effect and its applications in technology
Wave properties of matter & Quantum mechanics I. Chapter 5
 Wave properties of matter & Quantum mechanics I Chapter 5 X-ray diffraction Max von Laue suggested that if x-rays were a form of electromagnetic radiation, interference effects should be observed. Crystals
Wave properties of matter & Quantum mechanics I Chapter 5 X-ray diffraction Max von Laue suggested that if x-rays were a form of electromagnetic radiation, interference effects should be observed. Crystals
Handout 7 Reciprocal Space
 Handout 7 Reciprocal Space Useful concepts for the analysis of diffraction data http://homepages.utoledo.edu/clind/ Concepts versus reality Reflection from lattice planes is just a concept that helps us
Handout 7 Reciprocal Space Useful concepts for the analysis of diffraction data http://homepages.utoledo.edu/clind/ Concepts versus reality Reflection from lattice planes is just a concept that helps us
22.54 Neutron Interactions and Applications (Spring 2004) Chapter 1 (2/3/04) Overview -- Interactions, Distributions, Cross Sections, Applications
 .54 Neutron Interactions and Applications (Spring 004) Chapter 1 (/3/04) Overview -- Interactions, Distributions, Cross Sections, Applications There are many references in the vast literature on nuclear
.54 Neutron Interactions and Applications (Spring 004) Chapter 1 (/3/04) Overview -- Interactions, Distributions, Cross Sections, Applications There are many references in the vast literature on nuclear
E. K. A. ADVANCED PHYSICS LABORATORY PHYSICS 3081, 4051
 E. K. A. ADVANCED PHYSICS LABORATORY PHYSICS 38, 45 ELECTRON DIFFRACTION Introduction This experiment, using a cathode ray tube, permits the first hand observation that electrons, which are usually though
E. K. A. ADVANCED PHYSICS LABORATORY PHYSICS 38, 45 ELECTRON DIFFRACTION Introduction This experiment, using a cathode ray tube, permits the first hand observation that electrons, which are usually though
Review of Last Class 1
 Review of Last Class 1 X-Ray diffraction of crystals: the Bragg formulation Condition of diffraction peak: 2dd sin θθ = nnλλ Review of Last Class 2 X-Ray diffraction of crystals: the Von Laue formulation
Review of Last Class 1 X-Ray diffraction of crystals: the Bragg formulation Condition of diffraction peak: 2dd sin θθ = nnλλ Review of Last Class 2 X-Ray diffraction of crystals: the Von Laue formulation
de Broglie Waves h p de Broglie argued Light exhibits both wave and particle properties
 de Broglie argued de Broglie Waves Light exhibits both wave and particle properties Wave interference, diffraction Particle photoelectric effect, Compton effect Then matter (particles) should exhibit both
de Broglie argued de Broglie Waves Light exhibits both wave and particle properties Wave interference, diffraction Particle photoelectric effect, Compton effect Then matter (particles) should exhibit both
Experimental Determination of Crystal Structure
 Experimental Determination of Crystal Structure Branislav K. Nikolić Department of Physics and Astronomy, University of Delaware, U.S.A. PHYS 624: Introduction to Solid State Physics http://www.physics.udel.edu/~bnikolic/teaching/phys624/phys624.html
Experimental Determination of Crystal Structure Branislav K. Nikolić Department of Physics and Astronomy, University of Delaware, U.S.A. PHYS 624: Introduction to Solid State Physics http://www.physics.udel.edu/~bnikolic/teaching/phys624/phys624.html
research papers Calculation of crystal truncation rod structure factors for arbitrary rational surface terminations
 Journal of Applied Crystallography ISSN 0021-8898 Received 22 April 2002 Accepted 5 August 2002 Calculation of crystal truncation rod structure factors for arbitrary rational surface terminations Thomas
Journal of Applied Crystallography ISSN 0021-8898 Received 22 April 2002 Accepted 5 August 2002 Calculation of crystal truncation rod structure factors for arbitrary rational surface terminations Thomas
DIFFRACTION PHYSICS THIRD REVISED EDITION JOHN M. COWLEY. Regents' Professor enzeritus Arizona State University
 DIFFRACTION PHYSICS THIRD REVISED EDITION JOHN M. COWLEY Regents' Professor enzeritus Arizona State University 1995 ELSEVIER Amsterdam Lausanne New York Oxford Shannon Tokyo CONTENTS Preface to the first
DIFFRACTION PHYSICS THIRD REVISED EDITION JOHN M. COWLEY Regents' Professor enzeritus Arizona State University 1995 ELSEVIER Amsterdam Lausanne New York Oxford Shannon Tokyo CONTENTS Preface to the first
Imaging Methods: Scanning Force Microscopy (SFM / AFM)
 Imaging Methods: Scanning Force Microscopy (SFM / AFM) The atomic force microscope (AFM) probes the surface of a sample with a sharp tip, a couple of microns long and often less than 100 Å in diameter.
Imaging Methods: Scanning Force Microscopy (SFM / AFM) The atomic force microscope (AFM) probes the surface of a sample with a sharp tip, a couple of microns long and often less than 100 Å in diameter.
Physics with Neutrons I, WS 2015/2016. Lecture 11, MLZ is a cooperation between:
 Physics with Neutrons I, WS 2015/2016 Lecture 11, 11.1.2016 MLZ is a cooperation between: Organization Exam (after winter term) Registration: via TUM-Online between 16.11.2015 15.1.2015 Email: sebastian.muehlbauer@frm2.tum.de
Physics with Neutrons I, WS 2015/2016 Lecture 11, 11.1.2016 MLZ is a cooperation between: Organization Exam (after winter term) Registration: via TUM-Online between 16.11.2015 15.1.2015 Email: sebastian.muehlbauer@frm2.tum.de
The Wave Nature of Matter *
 OpenStax-CNX module: m42576 1 The Wave Nature of Matter * OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 Abstract Describe the Davisson-Germer
OpenStax-CNX module: m42576 1 The Wave Nature of Matter * OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 Abstract Describe the Davisson-Germer
Energy Spectroscopy. Excitation by means of a probe
 Energy Spectroscopy Excitation by means of a probe Energy spectral analysis of the in coming particles -> XAS or Energy spectral analysis of the out coming particles Different probes are possible: Auger
Energy Spectroscopy Excitation by means of a probe Energy spectral analysis of the in coming particles -> XAS or Energy spectral analysis of the out coming particles Different probes are possible: Auger
Elastic and Inelastic Scattering in Electron Diffraction and Imaging
 Elastic and Inelastic Scattering in Electron Diffraction and Imaging Contents Introduction Symbols and definitions Part A Diffraction and imaging of elastically scattered electrons Chapter 1. Basic kinematical
Elastic and Inelastic Scattering in Electron Diffraction and Imaging Contents Introduction Symbols and definitions Part A Diffraction and imaging of elastically scattered electrons Chapter 1. Basic kinematical
VICTORIA JUNIOR COLLEGE 2014 JC2 PRELIMINARY EXAMINATION. 23/9/ h 1600h
 Name: Class: 13S VICTORIA JUNIOR COLLEGE 2014 JC2 PRELIMINARY EXAMINATION PHYSICS 9646/3 Higher 2 Paper 3 23/9/2014 1400h 1600h TUESDAY (2 Hours) This paper consists of two sections: Section A (40 marks)
Name: Class: 13S VICTORIA JUNIOR COLLEGE 2014 JC2 PRELIMINARY EXAMINATION PHYSICS 9646/3 Higher 2 Paper 3 23/9/2014 1400h 1600h TUESDAY (2 Hours) This paper consists of two sections: Section A (40 marks)
The Bohr Model of Hydrogen
 The Bohr Model of Hydrogen Suppose you wanted to identify and measure the energy high energy photons. One way to do this is to make a calorimeter. The CMS experiment s electromagnetic calorimeter is made
The Bohr Model of Hydrogen Suppose you wanted to identify and measure the energy high energy photons. One way to do this is to make a calorimeter. The CMS experiment s electromagnetic calorimeter is made
APEX CARE INSTITUTE FOR PG - TRB, SLET AND NET IN PHYSICS
 Page 1 1. Within the nucleus, the charge distribution A) Is constant, but falls to zero sharply at the nuclear radius B) Increases linearly from the centre, but falls off exponentially at the surface C)
Page 1 1. Within the nucleus, the charge distribution A) Is constant, but falls to zero sharply at the nuclear radius B) Increases linearly from the centre, but falls off exponentially at the surface C)
Transmission Electron Microscopy and Diffractometry of Materials
 Brent Fultz James Howe Transmission Electron Microscopy and Diffractometry of Materials Fourth Edition ~Springer 1 1 Diffraction and the X-Ray Powder Diffractometer 1 1.1 Diffraction... 1 1.1.1 Introduction
Brent Fultz James Howe Transmission Electron Microscopy and Diffractometry of Materials Fourth Edition ~Springer 1 1 Diffraction and the X-Ray Powder Diffractometer 1 1.1 Diffraction... 1 1.1.1 Introduction
X-ray diffraction is a non-invasive method for determining many types of
 Chapter X-ray Diffraction.1 Introduction X-ray diffraction is a non-invasive method for determining many types of structural features in both crystalline and amorphous materials. In the case of single
Chapter X-ray Diffraction.1 Introduction X-ray diffraction is a non-invasive method for determining many types of structural features in both crystalline and amorphous materials. In the case of single
WAVE PARTICLE DUALITY
 WAVE PARTICLE DUALITY Evidence for wave-particle duality Photoelectric effect Compton effect Electron diffraction Interference of matter-waves Consequence: Heisenberg uncertainty principle PHOTOELECTRIC
WAVE PARTICLE DUALITY Evidence for wave-particle duality Photoelectric effect Compton effect Electron diffraction Interference of matter-waves Consequence: Heisenberg uncertainty principle PHOTOELECTRIC
Chapter 37 Early Quantum Theory and Models of the Atom. Copyright 2009 Pearson Education, Inc.
 Chapter 37 Early Quantum Theory and Models of the Atom Planck s Quantum Hypothesis; Blackbody Radiation Photon Theory of Light and the Photoelectric Effect Energy, Mass, and Momentum of a Photon Compton
Chapter 37 Early Quantum Theory and Models of the Atom Planck s Quantum Hypothesis; Blackbody Radiation Photon Theory of Light and the Photoelectric Effect Energy, Mass, and Momentum of a Photon Compton
General theory of diffraction
 General theory of diffraction X-rays scatter off the charge density (r), neutrons scatter off the spin density. Coherent scattering (diffraction) creates the Fourier transform of (r) from real to reciprocal
General theory of diffraction X-rays scatter off the charge density (r), neutrons scatter off the spin density. Coherent scattering (diffraction) creates the Fourier transform of (r) from real to reciprocal
Physics of Radiotherapy. Lecture II: Interaction of Ionizing Radiation With Matter
 Physics of Radiotherapy Lecture II: Interaction of Ionizing Radiation With Matter Charge Particle Interaction Energetic charged particles interact with matter by electrical forces and lose kinetic energy
Physics of Radiotherapy Lecture II: Interaction of Ionizing Radiation With Matter Charge Particle Interaction Energetic charged particles interact with matter by electrical forces and lose kinetic energy
Crystals, X-rays and Proteins
 Crystals, X-rays and Proteins Comprehensive Protein Crystallography Dennis Sherwood MA (Hons), MPhil, PhD Jon Cooper BA (Hons), PhD OXFORD UNIVERSITY PRESS Contents List of symbols xiv PART I FUNDAMENTALS
Crystals, X-rays and Proteins Comprehensive Protein Crystallography Dennis Sherwood MA (Hons), MPhil, PhD Jon Cooper BA (Hons), PhD OXFORD UNIVERSITY PRESS Contents List of symbols xiv PART I FUNDAMENTALS
Chapter V: Interactions of neutrons with matter
 Chapter V: Interactions of neutrons with matter 1 Content of the chapter Introduction Interaction processes Interaction cross sections Moderation and neutrons path For more details see «Physique des Réacteurs
Chapter V: Interactions of neutrons with matter 1 Content of the chapter Introduction Interaction processes Interaction cross sections Moderation and neutrons path For more details see «Physique des Réacteurs
Outline. Chapter 6 The Basic Interactions between Photons and Charged Particles with Matter. Photon interactions. Photoelectric effect
 Chapter 6 The Basic Interactions between Photons and Charged Particles with Matter Radiation Dosimetry I Text: H.E Johns and J.R. Cunningham, The physics of radiology, 4 th ed. http://www.utoledo.edu/med/depts/radther
Chapter 6 The Basic Interactions between Photons and Charged Particles with Matter Radiation Dosimetry I Text: H.E Johns and J.R. Cunningham, The physics of radiology, 4 th ed. http://www.utoledo.edu/med/depts/radther
X-ray diffraction geometry
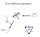 X-ray diffraction geometry Setting controls sample orientation in the diffraction plane. most important for single-crystal diffraction For any poly- (or nano-) crystalline specimen, we usually set: 1 X-ray
X-ray diffraction geometry Setting controls sample orientation in the diffraction plane. most important for single-crystal diffraction For any poly- (or nano-) crystalline specimen, we usually set: 1 X-ray
1 Crystal Structures. of three-dimensional crystals. Here we use two-dimensional examples to illustrate the concepts.
 3 1 Crystal Structures A crystal is a periodic array of atoms. Many elements and quite a few compounds are crystalline at low enough temperatures, and many of the solid materials in our everyday life (like
3 1 Crystal Structures A crystal is a periodic array of atoms. Many elements and quite a few compounds are crystalline at low enough temperatures, and many of the solid materials in our everyday life (like
Lecture 3 - Compton Scattering
 Lecture 3 - Compton Scattering E. Daw March 0, 01 1 Review of Lecture Last time we recalled that in special relativity, as in pre-relativistic dynamics, the total energy in an interaction or collision
Lecture 3 - Compton Scattering E. Daw March 0, 01 1 Review of Lecture Last time we recalled that in special relativity, as in pre-relativistic dynamics, the total energy in an interaction or collision
X-RAY SPECTRA. Theory:
 12 Oct 18 X-ray.1 X-RAY SPECTRA In this experiment, a number of measurements involving x-rays will be made. The spectrum of x-rays emitted from a molybdenum target will be measured, and the experimental
12 Oct 18 X-ray.1 X-RAY SPECTRA In this experiment, a number of measurements involving x-rays will be made. The spectrum of x-rays emitted from a molybdenum target will be measured, and the experimental
Structural characterization. Part 1
 Structural characterization Part 1 Experimental methods X-ray diffraction Electron diffraction Neutron diffraction Light diffraction EXAFS-Extended X- ray absorption fine structure XANES-X-ray absorption
Structural characterization Part 1 Experimental methods X-ray diffraction Electron diffraction Neutron diffraction Light diffraction EXAFS-Extended X- ray absorption fine structure XANES-X-ray absorption
Overview of scattering, diffraction & imaging in the TEM
 Overview of scattering, diffraction & imaging in the TEM Eric A. Stach Purdue University Scattering Electrons, photons, neutrons Radiation Elastic Mean Free Path (Å)( Absorption Length (Å)( Minimum Probe
Overview of scattering, diffraction & imaging in the TEM Eric A. Stach Purdue University Scattering Electrons, photons, neutrons Radiation Elastic Mean Free Path (Å)( Absorption Length (Å)( Minimum Probe
Photons in the universe. Indian Institute of Technology Ropar
 Photons in the universe Photons in the universe Element production on the sun Spectral lines of hydrogen absorption spectrum absorption hydrogen gas Hydrogen emission spectrum Element production on the
Photons in the universe Photons in the universe Element production on the sun Spectral lines of hydrogen absorption spectrum absorption hydrogen gas Hydrogen emission spectrum Element production on the
Crystallography Reading: Warren, Chapters 2.1, 2.2, 2.6, 8 Surface symmetry: Can be a clue to underlying structure. Examples:
 Crystallography Reading: Warren, Chapters 2.1, 2.2, 2.6, 8 Surface symmetry: Can be a clue to underlying structure. Examples: Snow (SnowCrystals.com) Bismuth (Bao, Kavanagh, APL 98 66103 (2005) Hexagonal,
Crystallography Reading: Warren, Chapters 2.1, 2.2, 2.6, 8 Surface symmetry: Can be a clue to underlying structure. Examples: Snow (SnowCrystals.com) Bismuth (Bao, Kavanagh, APL 98 66103 (2005) Hexagonal,
X-ray Diffraction. Diffraction. X-ray Generation. X-ray Generation. X-ray Generation. X-ray Spectrum from Tube
 X-ray Diffraction Mineral identification Mode analysis Structure Studies X-ray Generation X-ray tube (sealed) Pure metal target (Cu) Electrons remover inner-shell electrons from target. Other electrons
X-ray Diffraction Mineral identification Mode analysis Structure Studies X-ray Generation X-ray tube (sealed) Pure metal target (Cu) Electrons remover inner-shell electrons from target. Other electrons
 Synchrotron Power Cosmic rays are astrophysical particles (electrons, protons, and heavier nuclei) with extremely high energies. Cosmic-ray electrons in the galactic magnetic field emit the synchrotron
Synchrotron Power Cosmic rays are astrophysical particles (electrons, protons, and heavier nuclei) with extremely high energies. Cosmic-ray electrons in the galactic magnetic field emit the synchrotron
The interaction of radiation with matter
 Basic Detection Techniques 2009-2010 http://www.astro.rug.nl/~peletier/detectiontechniques.html Detection of energetic particles and gamma rays The interaction of radiation with matter Peter Dendooven
Basic Detection Techniques 2009-2010 http://www.astro.rug.nl/~peletier/detectiontechniques.html Detection of energetic particles and gamma rays The interaction of radiation with matter Peter Dendooven
Drickamer type. Disk containing the specimen. Pressure cell. Press
 ε-fe Drickamer type Press Pressure cell Disk containing the specimen Low Temperature Cryostat Diamond Anvil Cell (DAC) Ruby manometry Re gasket for collimation Small size of specimen space High-density
ε-fe Drickamer type Press Pressure cell Disk containing the specimen Low Temperature Cryostat Diamond Anvil Cell (DAC) Ruby manometry Re gasket for collimation Small size of specimen space High-density
Quantum and Atomic Physics - Multiple Choice
 PSI AP Physics 2 Name 1. The Cathode Ray Tube experiment is associated with: (A) J. J. Thomson (B) J. S. Townsend (C) M. Plank (D) A. H. Compton 2. The electron charge was measured the first time in: (A)
PSI AP Physics 2 Name 1. The Cathode Ray Tube experiment is associated with: (A) J. J. Thomson (B) J. S. Townsend (C) M. Plank (D) A. H. Compton 2. The electron charge was measured the first time in: (A)
Scattering Lecture. February 24, 2014
 Scattering Lecture February 24, 2014 Structure Determination by Scattering Waves of radiation scattered by different objects interfere to give rise to an observable pattern! The wavelength needs to close
Scattering Lecture February 24, 2014 Structure Determination by Scattering Waves of radiation scattered by different objects interfere to give rise to an observable pattern! The wavelength needs to close
1. Nuclear Size. A typical atom radius is a few!10 "10 m (Angstroms). The nuclear radius is a few!10 "15 m (Fermi).
 1. Nuclear Size We have known since Rutherford s! " scattering work at Manchester in 1907, that almost all the mass of the atom is contained in a very small volume with high electric charge. Nucleus with
1. Nuclear Size We have known since Rutherford s! " scattering work at Manchester in 1907, that almost all the mass of the atom is contained in a very small volume with high electric charge. Nucleus with
