Replenishment of volatile-rich mafic magma into a degassed chamber drives mixing and eruption of Tungurahua volcano
|
|
|
- Adam Blake
- 5 years ago
- Views:
Transcription
1 Bull Volcanol (2014) 76:872 DOI /s RESEARCH ARTICLE Replenishment of volatile-rich mafic magma into a degassed chamber drives mixing and eruption of Tungurahua volcano Madison L. Myers & Dennis J. Geist & Michael C. Rowe & Karen S. Harpp & Paul J. Wallace & Josef Dufek Received: 19 March 2014 /Accepted: 10 September 2014 # Springer-Verlag Berlin Heidelberg 2014 Abstract In July and August of 2006 and May of 2010, Tungurahua volcano, Ecuador, produced pyroclastic flowforming eruptions, representing increased explosivity compared to the Strombolian events that characterized its behavior since its renewal in Volatiles (H 2 O, CO 2, S, Cl) and major elements were analyzed in 35 melt inclusions hosted in olivine and pyroxene phenocrysts in tephra from both events to reconstruct the pre-eruptive magmatic conditions and mechanisms that led to these more explosive episodes. Melt inclusion composition paired with host phenocryst zonation indicate mixing of two distinct magmas: a volatile-rich ( 4.0 wt% H 2 Oand 1,800 ppm S) basaltic andesite containing olivine phenocrysts and a degassed ( 1.0 wt% H 2 Oand ppm S) andesite with plagioclase and pyroxene phenocrysts that contain andesitic to dacitic melt inclusions. We attribute the lower volatile concentrations in the evolved melt inclusions to degassing that occurred during residence in Editorial responsibility: J.E. Gardner M. L. Myers (*): P. J. Wallace Department of Geological Sciences, University of Oregon, Eugene, OR 97403, USA mmyers3@uoregon.edu D. J. Geist: K. S. Harpp Department of Geological Sciences, University of Idaho, 3022, Moscow, ID 83843, USA M. C. Rowe School of Environment, University of Auckland, Auckland 1142, New Zealand K. S. Harpp Department of Geology, Colgate University, Hamilton, NY 13346, USA J. Dufek School of Earth and Atmospheric Sciences, Georgia Institute of Technology, Atlanta, GA 30332, USA a shallow reservoir, where fractional crystallization led to the production of dacitic melt. Our melt inclusion data confirm the hypothesis made on the basis of phenocryst zoning profiles (J Volcanol Geotherm Res 199:69 84, 2011) that the intrusion of a volatile-rich basaltic andesite into a more evolved chamber and subsequent mixing led to explosive eruption in Melt inclusions from the 2006 and 2010 eruptive products have comparable volatile and major element compositions. High H 2 O concentrations in melt inclusions from 2010 olivine indicate little diffusive loss from the melt inclusions following mixing with the degassed andesitic reservoir, which requires that the 2010 eruption be the result of a new recharge event and not remobilization of the 2006 hybrid. Keywords Tungurahua. Melt inclusions. Recharge. Magma mixing Introduction Understanding the processes that control the explosivity of a volcanic system, especially those that control the change from a more effusive to explosive eruption, is crucial for hazards monitoring. It is well known that the dynamics and eruptive strength of all volcanic eruptions are strongly controlled by the concentrations of water (H 2 O), and to a lesser extent carbon dioxide (CO 2 ), within the magma (Wilson et al. 1980; Sparks et al. 1997; Davidson and Kamenetsky 2007). Thus, volatile concentrations are important for understanding the increased activity associated with more explosive episodes. Melt inclusions provide a way of measuring the preeruptive volatile concentrations within deeply stored magma, as after entrapment, melt inclusions are largely isolated from the host melt (e.g., Anderson 1975; Roedder 1984; Wallace 2005; Métrich and Wallace 2008; Blundy et al. 2010, and references therein). Typically, melt inclusions preserve
2 872, Page 2 of 17 Bull Volcanol (2014) 76:872 volatile concentrations that are higher than those of the eventually erupted magma (Wallace 2005; Métrich and Wallace 2008). Melt inclusions have also been found to record the progressive degassing of magma over the course of an eruption, which in turn can govern the explosivity (Anderson et al. 1989; Roggensack et al. 1997; Oppenheimer et al. 2003; Sparks 2003; Blundy and Cashman 2005). Furthermore, comparisons of crystal chemistry and the compositions of glassy melt inclusions can provide information on the presence of different magma batches, the timing of degassing and mixing, and other magmatic evolutionary processes that occur before eruption (Danyushevsky et al. 2000; Blundy and Cashman 2005; Nichols and Wysoczanski 2007; Johnson et al. 2011). This paper expands on the petrologic data presented by Samaniego et al. (2011) for rocks recently erupted from Tungurahua volcano by interpreting compositional data from melt inclusions in juvenile scoria bombs from the 2006 and 2010 pyroclastic density current deposits (Hall et al. 2013). Samaniego et al. (2011) used petrological evidence to show that the 2006 eruptions were the result of mafic recharge into a shallow, more evolved chamber, which drove higher energy eruptions. In the present study, melt inclusions hosted in olivine, orthopyroxene, and clinopyroxene were analyzed for H 2 O, CO 2, sulfur (S), and chlorine (Cl), along with major elements, with the purpose of reconstructing the processes that occurred in Tungurahua s magma chamber prior to these more explosive eruptions and thereby testing the recharge hypothesis. Our data support and expand upon the Samaniego et al. (2011) interpretation by quantifying the volatile concentrations associated with the recharged mafic magma and shallowly stored andesitic magma erupted in We suggest that this model can also be applied to the 2010 event and adds to growing evidence from melt inclusion studies for the importance of recharge and mixing of magmas in the evolution of andesitic volcanoes and the role of recharge as an eruption trigger (e.g., Carrasco-Núñez and Rose 1995; Murphy et al. 2000; Edmonds et al. 2001; Roberge et al. 2009). Regional setting and eruptive history Tungurahua (elevation 5,023 m) is located within the eastern Cordillera of the Ecuadorian Andes (1.467 S, W), 120 km SE of Quito (Fig. 1). In 1999, Tungurahua renewed volcanic activity after 80 years of quiescence. Eruptions during the first 7 years were dominated by Strombolian activity, with frequent ash and tephra emissions (Arellano et al. 2008). In 2006, Tungurahua experienced an increase in eruptive strength, resulting in several pyroclastic-flow-forming eruptions (Samaniego et al. 2011). These more energetic eruptions also occurred in 2008, 2010, 2011, 2012, and Tungurahua remains active as of August 2014 and continues to be a concern because of its steep relief ( 3,000 m), posing a threat to the population of Baños, a town of 18,000 inhabitants located on the lower northern flanks of the volcano (Arellano et al. 2008). This threat is magnified by the structure of Tungurahua s crater, which causes pyroclastic flows to descend the north and west flanks of the edifice (Kelfoun et al. 2009). Tungurahua volcano has been monitored since 1988 by the Instituto Geofisico de la Escuela Politécnica Nacional using seismological, geochemical, thermal, geodetic, acoustic, and other observational techniques (Fee et al. 2010), and a comprehensive hazard map was published by Hall et al. (2002). Historic eruptions and 1999 renewal Over historic times, Tungurahua has experienced an eruptive cycle almost every century, each lasting about a decade. The current cycle has persisted beyond this predicted interval, making understanding the causes of its more explosive phases and continued activity crucial for ongoing hazards monitoring. Historically, activity tends to be dominated by Strombolian and Vulcanian eruptions, although Plinian eruptions are also documented (Hall et al. 1999). The Strombolian and Vulcanian events are characterized by regional tephra fallout, pyroclastic and debris flows, and blocky lava flows (Samaniego et al. 2011), but pyroclastic products dominate each cycle (Hall et al. 1999; Arellano et al. 2008). Seismic and infrasonic stations were installed on the flanks of the volcano after activity recommenced in 1999 (Arellano et al. 2008). Arellano et al. (2008) found that passive degassing prior to 2006 accounted for 90 % of the observed SO 2 emissions at Tungurahua, most of which occurs during the frequent effusive eruptions. During 1999 and 2005, low eruption rates suggest a moderate magma ascent rate from the reservoir and through the conduit system (Samaniego et al. 2011). Pairing gas compositions and seismicity indicate that volcanic activity during these opening eruptive phases originated from a conduit system with a depth range between 1 and 5 km below the summit (Molina et al. 2005; Arellano et al. 2008). The 2006 and 2010 eruptions In early April 2006, long-period seismic activity was detected 5 to 15 km below the summit, followed by an increase in SO 2 discharge (Arellano et al. 2008; Carn et al. 2008; Fee et al. 2010; Samaniego et al. 2011). From May 10 16, 2006, several explosive eruptions were recorded, with ash columns reaching 19 km above the volcano. Cataclysmic pyroclastic-flow-forming eruptions occurred on July 14 and August of 2006, with flows reaching the base of the volcano (Samaniego et al. 2011; Hall et al. 2013). Following the emplacement of pyroclastic flows, lavas erupted (Hall et al. 2013). The cumulative eruptive volume for the July 14 event totals m 3 of pyroclastic and fallout
3 Bull Volcanol (2014) 76:872 Page 3 of 17, 872 Fig. 1 Aerial image of Tungurahua volcano (Google Earth); stars indicate sample locations. The town of Baños, Ecuador, is located along the northern flanks of Tungurahua, about 8 km from the main vent. The dark areas radiating from the summit are pyroclastic density deposits, mostly from the 2006 eruption. Location of Tungurahua volcano (bottom right inset), where triangles depict some of Ecuador s most well-known volcanoes. Dashed lines represent the Western (WC) and Eastern Cordilleras (EC), with Tungurahua falling along the EC material, about 12 % the volume of the August eruption ( m 3, Troncoso et al. 2006; Kelfoun et al. 2009; Eychenne et al. 2012;Halletal.2013). Between 2006 and 2010, activity returned to the lowerenergy Strombolian eruptions, similar to those observed from 1999 to 2006, although one major eruption occurred in 2008, producing m 3 of material and 17.5 cm of uplift on the upper western flank (Biggs et al. 2010). On May 26 and 28, 2010, another strong eruption sequence occurred, producing 1 3 km long pyroclastic flows on the N, NW, W, and SW flanks of Tungurahua. Although the 2010 eruption produced pyroclastic flows, it was not nearly as voluminous as the 2006 climactic events. Petrology Most of Tungurahua s historic eruptive cycles have begun with dacitic compositions (64 66 wt% SiO 2 ) and ended with the eruption of andesite (56 59 wt% SiO 2 ), corresponding to a change from more explosive to effusive eruptive behavior (Hall et al. 1999). Samaniego et al. (2011) published a comprehensive petrologic study of the eruptive material produced between 1999 and 2006, focusing on the July and August 2006 eruptions. All of the rocks analyzed, including the lava, pyroclastic flow deposits, and tephra, have andesitic compositions (58 59 wt% SiO 2 ) and contain plagioclase (5 15 vol.%), clinopyroxene (2 4 vol.%), orthopyroxene (2 4 vol.%), and magnetite ± trace olivine as phenocrysts. This is interpreted as indicating the existence of a homogeneous andesitic reservoir over the entire eruptive cycle (Samaniego et al. 2011). Samaniego et al. (2011) noted the presence of resorption textures in plagioclase and pyroxene phenocrysts from the climactic 2006 eruptions, along with μm thick anorthite (An) and Mg-rich rims. Pairing this information with deep (8 10 km) seismicity and increased gas emissions, Samaniego et al. (2011) conclude that the explosive eruptions in 2006 were caused by the intrusion of a mafic magma into an andesitic reservoir, with subsequent mixing and pressurization
4 872, Page 4 of 17 Bull Volcanol (2014) 76:872 of the chamber. Inputs of mafic magma are commonly observed as eruptive triggers in intermediate systems (Carrasco- Núñez and Rose 1995; Murphy et al. 2000; Edmonds et al. 2001; Roberge et al and references therein), making recharge a plausible hypothesis for the cause of these suddenly more explosive eruptions in No chemical or petrographic differences were observed in juvenile samples from the July 14 and August 16 17, 2006, eruptive products (Samaniego et al. 2011); however, the August event produced a tephra layer that contained a trace amount of dacitic pumice (<1 2 vol.%). Two-pyroxene, pyroxene-liquid, and plagioclase-liquid thermobarometers applied to juvenile andesite indicate crystallization temperatures ranging between 950 and 1,015 C (Samaniego et al. 2011). The phenocryst assemblage (and an assumption of equilibrium) was used to estimate a pressure of crystallization between 1,000 and 2,500 bars, on the basis of published experimental studies on hydrous andesitic magmas, which were saturated with the observed phenocrysts in this pressure range. This places the magma storage region between 7.5 and 9.5 km below the summit, coincident with the hypocenters of deep seismic activity observed prior to the 2006 eruptions (Arellano et al. 2008; Samaniego et al. 2011). Methods Juvenile scoria bombs were collected from the 2006 and May 2010 pyroclastic flow deposits during July The use of rapidly quenched bombs with glassy rinds reduces the likelihood of post-eruptive modification of melt inclusions. Wholerock composition of the three bombs, two from the 2006 deposits and one from the 2010 deposit, were analyzed by X-ray fluorescence at the Geoanalytical Lab at Washington State University, using the methods of Johnson et al. (1999). Samples were made into thin sections and inspected visually for melt inclusions in phenocrysts, then hand-crushed and picked for mineral separates. Glassy melt inclusions (MI) were found in olivine, clinopyroxene, orthopyroxene, and plagioclase phenocrysts in both the 2006 and 2010 bombs. Olivine and pyroxene grains contain glassy MIs large enough for analysis (>50 μm), making them preferred for this study due to analytical constraints. Plagioclase-hosted inclusions from these eruptions tend to contain daughter crystals and are typically smaller ( 20 μm) compared to the olivine- and pyroxene-hosted MIs. Nearly all of the analyzed MIs are vapor and shrinkage bubble free, suggesting that volatile loss from entrapment concentrations is minimal (Severs et al. 2007; Gaetani et al. 2012). Individual pyroxene and olivine grains were doubly polished to a 1 μm finish for analysis by Fourier transform infrared spectroscopy (FTIR) at the University of Oregon. Infrared spectra were collected between 6,000 and 1,000 cm 1, where the main peaks of interest for this study were the total OH peak at 3,550 cm 1 and the carbonate doublet at 1,435 and 1,515 cm 1. The concentration of CO 2 was calculated using an average height of the two peaks in the carbonate doublet (Fine and Stolper 1985). At more evolved compositions, CO 2 is stored as molecular CO 2 (2,350 cm 1 ), however this peak was absent from all spectra. Replicate spectra were acquired for each MI as well as a spectrum for the host mineral (see below). Thickness was measured using both a digital micrometer (±2 μm) and the reflectance interference fringe method described by Wysoczanski and Tani (2006). One difficulty we encountered was that some MIs could not be doubly exposed, either because of fracturing of the mineral host or to preserve two MIs within the same crystal. To correct for interference by the host mineral, a correction ratio was applied by subtracting the host mineral spectrum from the MI spectrum, using the method described by Nichols and Wysoczanski (2007). The reproducibility associated with this correction was found to be excellent, with the standard deviation before and after double exposure of 0.08 wt% (Nichols and Wysoczanski 2007). Although this method was created to deal with olivine interferences, the same principles were applied to correct for pyroxene interferences, using multiple wavelength overlaps to constrain variability. Absorbances were converted to H 2 O concentrations using the Beer-Lambert law, where c i =M i A/ρ d ε. Inthis,c i is the concentration of the absorbing species (in wt%), M i is the molecular weight of the species (g mol 1 ), A is the absorbance height of the relevant vibration band, ρ is the sample density (g L 1 ), d is the thickness of the wafer analyzed (cm), and ε is the molar absorption coefficient (L mol 1 cm 1 ). Total H 2 O concentration was calculated using the total OH peak at 3,550 cm 1.Weusedanε value of 70±6.9 L mol 1 cm 1 for the pyroxene-hosted andesitic to dacitic MIs, taken from King et al. (2002), whereas the more mafic andesitic MIs in olivine require an ε value of 63±3 L mol 1 cm 1 (Dixon et al. 1988). An ε value of 350 L mol 1 cm 1 was used for the CO 2 doublet, calculated using the MI composition (Dixon and Pan 1995). Peak heights were calculated using a straight-line background correction (Dixon et al. 1995). Glass density was calculated from the major element compositions as described by Luhr (2001). Combined uncertainties in density, absorption coefficient, and thickness cause uncertainty to be approximately 10 % (Dixon et al. 1988), where most of this error is due to the thickness measurement. Based on these uncertainties, average 1 standard deviation uncertainties for H 2 O are ±0.25 wt% and for CO 2 ±20 ppm. After FTIR analysis, the crystal wafers were mounted and analyzed for major elements (Mg, Fe, Si, Mn, Al, Ca, Na, K, P, Ti, Ni, Cr) using the JEOL JXA-8500 F electron microprobe at Washington State University. Analysis was conducted on MIs, major mineral phases, including core to rim transects,
5 Bull Volcanol (2014) 76:872 Page 5 of 17, 872 and the matrix glass from several locations within each sample. For host crystal analysis, beam current varied between 30 and 50 na, using a beam diameter of 7 μm. Beam conditions were set with an accelerating voltage of 15 kv, a beam current of 30 na, and a beam diameter of 5 7 μm for all glass analyses. To minimize the effects of Na migration in the glasses, Na was among one of the first elements analyzed and a zero-time intercept correction was applied. Matrix glass and MIs were also analyzed for S and Cl. All concentrations were above detection limits, although the matrix glass S concentrations approach these values. Reproducibility and accuracy of melt inclusion major element, S, and Cl abundances is based on repeated analysis of VG-2 standard glass (Table 2). Error analysis (reported as % relative standard deviation) indicates that melt inclusion compositions are generally reproducible to better than 2 %, with higher RSD for S (3.4 %), Cl (7.8 %), Na 2 O(6%),P 2 O 5 (12 %), and MnO (21 %). Although none of the plagioclase-hosted MIs were prepared for analysis by FTIR because of their small size, glassy MIs in plagioclase in thin section were also analyzed by EMPA. It should be noted however that plagioclase-hosted MI were mostly contained within larger sieve textured phenocrysts and thus represent only one phase of plagioclase crystallization. Results Whole-rock and phenocryst compositions Whole-rock major element compositions of all three scoria samples are within analytical uncertainty, with SiO 2 =58.40± 0.05 wt% (Table 1). These compositions fall within the range of the andesites erupted during Strombolian events from Tungurahua between 1999 and 2005 (58 59 wt% SiO 2 )and are close to the range of andesites from previous historic eruptions ( wt% SiO 2, Samaniego et al. 2011). Plagioclase occurs in three textural populations in samples from 2006 to 2010: euhedral phenocrysts, strongly sieved phenocrysts, and microlites. The phenocryst textures described by Samaniego et al. (2011) are also present in our 2006 and 2010 samples, including resorption embayments to differing degrees in all phenocrysts, and strong, stepwise reverse zonation within μm of the rim in plagioclase (average of An 64±3 core and An 72±4 rim) and clinopyroxene phenocrysts (average of Mg#=71±3 core and Mg#=77±4 rim, where Mg#=100 MgO/(MgO+FeO T ). These disequilibrium textures are independent of phenocryst size. One noteworthy difference in the 2010 samples is their finer grain size, due to the smaller population of the mm size fraction. Clinopyroxene phenocrysts are mostly euhedral, whereas orthopyroxene tends to be embayed. A few 2010 orthopyroxene crystals have slight normal zoning ( 1 Mg# Table 1 Whole-rock major elemental (wt%) analyses from 2006 to 2010 eruptive products measured in this study Sample name TU10-02 PJ TU10-06 PJ TU PJ Sample type Scoriae/bomb Scoriae/bomb Scoriae/bomb Eruption year SiO TiO Al2O FeO* MnO MgO CaO Na2O K2O P2O Total Ni Cr Sc V Ba Rb Sr Zr Y Nb Ga Cu Zn Pb La Ce Th Nd U unit), but otherwise orthopyroxene is unzoned (Fig. 2). Olivine is normally zoned in both the 2006 and 2010 samples, but to different extents olivine phenocrysts have cores of Fo 80±2 that sharply transition within the outer μmto Fo 75±2. The 2010 olivine cores have core compositions of Fo 79±0.5 dropping to Fo 77±2 within 20 μm of the rim. Melt inclusion compositions The major element compositions of the 52 analyzed MIs (26 each from 2006 to 2010) range from basaltic andesite to dacite (Table 2). Melt inclusion compositions are normalized on an anhydrous basis to allow for direct comparison between inclusions. Measured analytical totals (major element oxides + S, Cl, H 2 O, and CO 2 ), range from 96 to 100 wt% (98.1±
6 872, Page 6 of 17 Bull Volcanol (2014) 76:872 Mg# Core Olivine OPX CPX 1.1 wt% on average). Calibration of major element oxide analysis by EMPA on anhydrous standards likely contributes significantly to low analyzed totals. Major element compositions are found to relate strongly to the host phase, where clinopyroxene contains the most evolved MIs, olivine contains the most mafic MIs, and inclusions hosted in plagioclase and orthopyroxene fall in-between (Fig. 3). For example, MIs in pyroxene from the 2006 eruption range to higher silica concentrations (60 67 wt%) than those hosted within plagioclase (61 63 wt%) or olivine (54 61 wt%) and are also more evolved than the bulk rock (58 wt%). The 2010 eruptive products produce similar ranges, but extend to slightly more evolved compositions (Fig. 3). The matrix glass composition in all samples is homogeneous relative to the variation in the MIs, with SiO 2 concentrations ranging from 60 to 62 wt%. Most of the MIs have compositions within the range of wholerock data from historical eruptions (Hall et al. 1999; Samaniego et al. 2011), with the exception of TiO 2 and FeO (Fig. 3): both concentrations become increasingly offset (TiO wt% and FeO 3.0 wt%) from the historical WR trend with increasing silica concentration. H 2 O, CO 2,S,Cl Normal Zoning Reverse Zoning Mg# Rim Fig. 2 The Mg# (where Mg#=100 MgO/(MgO+FeO T ) in mol.%) of rim compositions plotted against the Mg# of the core for individual crystals illustrates zonal patterns in phenocrysts, where a 1:1 ratio represents unzoned crystals. Symbol shapes represent different minerals, whereas shading represents either the 2006 or 2010 eruption. Most clinopyroxenes are reversely zoned, whereas olivine is either unzoned or weakly normally zoned. Orthopyroxene falls mostly along the 1:1 line. No significant differences exist between 2006 and 2010 samples Of the 35 MI measured for H 2 OandCO 2 concentrations, 16 are from 2006 and 19 from 2010 samples (Table 2). Water concentrations of 2006 pyroxene-hosted MIs range from 0.4 to 2.4 wt%, a wider range than the tight clustering of wt% for MIs in the 2010 pyroxenes, with an average of 1.0 wt% for both years. The larger range in 2006 is due to orthopyroxene-hosted MIs containing greater H 2 O concentrations than those hosted in clinopyroxene, whereas in 2010 these two H 2 O ranges are indistinguishable. Olivine-hosted MIs have H 2 O concentrations that extend to 3.4 wt% for 2006 samples and 4.1 wt% for 2010, with both years containing a significant spread that extends down to 1 wt%h 2 O(Fig.4). Only two of the MIs have CO 2 concentrations above detection limits ( 50 ppm), both from the same 2006 sample. One is an olivine-hosted MI with 200 ppm CO 2 and the other is an orthopyroxene-hosted MI with 60 ppm CO 2 that also contains the highest H 2 O concentration found in any pyroxene-hosted MI. The absence of CO 2 in all but two MIs coupled with the fact that the melt inclusions do not contain vapor bubbles suggests that degassing of CO 2 occurred from ascending magmas at greater depths, before the melt inclusions were trapped (Luhr 2001; Métrich and Wallace 2008). Inclusions from both the 2006 and 2010 samples fall within two distinct populations on the basis of S concentrations (Fig. 4). One population contains 1,000 1,800 ppm S and is solely found in the more mafic olivine-hosted MIs (olivine host Mg# 78 82). The second group contains ppm S and is hosted within more evolved olivine- (Mg# 73 77), pyroxene-, and plagioclase-hosted inclusions. No sulfur contents fall between 500 and 1,000 ppm (Fig. 4). The concentration of Cl increases with differentiation in all MIs. In both years, the matrix glasses contain the lowest concentrations of Cl, as well as S, falling slightly below the overall trend of the MIs (Figs. 4 and 5). This suggests late-stage degassing of Cl and S from the system, most likely during eruption (Métrich and Wallace 2008). Discussion The majority of olivine- and pyroxene-hosted MIs are in MgO-FeO equilibrium with their host (K d =(MgO/FeO) liq / (MgO/FeO) xl,wherek d =0.26±0.03 for clinopyroxene (Putirka 2008) and 0.26±0.06 for orthopyroxene (von Seckendorff and O Neill 1993). For olivine-hosted MIs, K d depends on melt composition (Table 2), where K d =0.36±.02 for more evolved olivine-hosted inclusions (Fo 77) and 0.30 ±.02 for the mafic population (Fo>78, Ford et al. 1983). Olivine-hosted MIs that are out of equilibrium with their host were corrected by either adding or subtracting host compositions to the original melt composition (Danyushevsky et al. 2000; Table 2); no inclusion required more than 5 % modification. Most orthopyroxene-hosted MIs are also within the range of published values for K d. K d corrections were not applied to clinopyroxene- and plagioclase-hosted inclusions, as these calculations are complicated by the complexity of the host composition, meaning that some of the scatter observed within these MIs could be attributed to uncorrected postentrapment modification.
7 Bull Volcanol (2014) 76:872 Page 7 of 17, 872 Table 2 EMPA anhydrous normalized major element concentrations (wt%) and measured S and Cl (ppm), with H 2 O(wt%)andCO 2 (ppm) by FTIR, separated by year, host, and mounting format Host Mount SiO 2 TiO 2 Al 2 O 3 FeO T MnO MgO CaO Na 2 O K 2 O P 2 O 5 Analytical total H 2 O CO 2 S Cl Mg# Host OC a 2006 sample names Tu10-02 #2 Ol GM BDL 1, Tu10-02 #5 Ol GM , Olivine06 #4 Ol GM , Olivine06 #9 Ol GM , Olivine06 #10, MI1 Ol GM , Olivine06 #10, MI2 Ol GM , Olivine06 #3 Ol GM , Tu10-06 #1 Ol GM 3.42 BDL 1, NA Tu10-02 #4 Ol GM BDL Olivine06 #1 Ol GM Olivine06 #2, MI1 Ol GM Olivine06 #2, MI2 Ol GM Tu10-02 #102 Ol GM 0.62 BDL NA Tu10-02 #105a Ol GM 1.73 BDL NA Tu10-02 #106 Ol GM 1.03 BDL NA Tu10-02 #106 OPX GM BDL NA OPX Box 28 #1 OPX TS , NA OPX Box 1 #1 OPX TS , NA Tu10-02 #107 OPX GM NA Tu10-06 #102 MI 1 OPX GM 1.72 BDL NA Tu10-06 #102 MI 2 OPX GM 1.60 BDL NA Tu10-02 #1 CPX GM BDL 193 1, NA Tu10-06 #1, MI1 CPX GM BDL 191 1, NA Tu10-06 #4 CPX GM BDL 164 1, NA Tu10-02 #2 CPX GM BDL 282 1, NA CPX Box 28 #2 CPX TS , NA CPX Box 87 #1 CPX TS NA CPX Box 40 #1 CPX TS , NA Tu10-06 #1, MI2 CPX GM 0.53 BDL NA Plag Box 68 #1 Plag TS NA Plag Box 90 #1 Plag TS ,175 NA Plag Box 106 #1 Plag TS ,011 NA Plag Box 5 #1 Plag TS NA Plag Box 1 #2 Plag TS ,107 NA
8 872, Page 8 of 17 Bull Volcanol (2014) 76:872 Table 2 (continued) Host Mount SiO 2 TiO 2 Al 2 O 3 FeO T MnO MgO CaO Na 2 O K 2 O P 2 O 5 Analytical total H 2 O CO 2 S Cl Mg# Host OC a 2006 MG #2 MG TS NA 2006 MG #3 MG TS NA 2006 MG #1 MG TS NA Tu10-02 MG1 MG TS NA Tu10-02 MG2 MG TS NA Tu10-02 MG3 MG TS NA 2010 sample names Tu10-10L #108 Ol GM BDL 1, Tu10-10L #105, MI1 Ol GM BDL 1, Tu10-10L #105, MI2 Ol GM BDL 1, Tu10-10 #2 Ol GM BDL 488 1, Olivine06 #5 Ol GM Olivine06 #6, 2 Ol GM Olivine06 #7 Ol GM , Olivine06 #8 Ol GM , Tu10-10L #107 Ol GM 1.18 BDL NA Tu10-10L #110, MI1 OPX GM BDL 186 1, NA Tu10-10L #113, MI1 OPX GM , NA Tu10-10L #113, MI2 OPX GM BDL 425 1, NA Tu10-10L #114, MI1 OPX GM BDL 265 1, NA Tu10-10L #114, MI2 OPX GM BDL 326 1, NA Tu10-10L #1 OPX GM BDL 217 1, NA Tu10-10L #110, MI2 OPX GM 1.21 BDL NA Tu10-10L #111 OPX GM 0.86 BDL NA Pyroxene B 18 CPX TS , NA Pyroxene B 18#2 CPX TS , NA Pyroxene B 18 #3 CPX TS NA Pyroxene B 89 #1 CPX TS , NA Pyroxene B 89 #2 CPX TS , NA Tu10-10L #103, MI1 CPX GM BDL 298 1, NA Tu10-10L #103, MI2 CPX GM BDL 220 1, NA Tu10-10L #101, MI1 CPX GM 1.19 BDL NA Tu10-10L #102, MI1 CPX GM 0.84 BDL NA Tu10-10L #102, MI2 CPX GM 1.03 BDL NA Tu10-10L #104, MI1 CPX GM 1.21 BDL NA
9 Bull Volcanol (2014) 76:872 Page 9 of 17, 872 Table 2 (continued) Host Mount SiO 2 TiO 2 Al 2 O 3 FeO T MnO MgO CaO Na 2 O K 2 O P 2 O 5 Analytical total H 2 O CO 2 S Cl Mg# Host OC a Tu10-10L #109, MI1 CPX GM 1.14 BDL NA Plagioclase B138 #2 Plag TS NA Plagioclase B140 #1 Plag TS NA Plagioclase B23 #1 Plag TS NA Plagioclase B44 #1 Plag TS ,028 NA Plagioclase B138 #1 Plag TS NA 2010 matrix glass #2 MG TS NA 2010 matrix glass MG TS NA 2010 matrix glass #3 MG TS NA Avg. VG-2 (N=5) , NA VG-2 %RSD NA Accepted (De Hoog et al. 2001) , NA not measured, Ol olivine, OPX orthopyroxene, CPX clinopyroxene, Plag plagioclase, MG matrix glass, BDL below detection limits, NA not applicable a Olivine corrections (OC) show how much olivine was added or subtracted from MIs to achieve compositional equilibrium Melt inclusion populations Major element compositions of all MIs can be separated into two distinct groups on the basis of zoning characteristics of the host phenocryst and S concentrations. The more mafic inclusions have silica concentrations ranging between 54 and 59 wt% and contain the highest H 2 O( 1 4 wt%) and S concentrations (1,000 1,800 ppm). These MIs are preserved solely in olivine phenocrysts, which display normal zoning (Figs. 2 and 5). More evolved MIs have silica concentrations ranging between 60 and 67 wt% contain lower H 2 O (average 1 wt%) and S concentrations (<500 ppm) and are found within pyroxene, plagioclase, and low-fo olivine, where plagioclase and clinopyroxene are reversely zoned (Fig. 3). We attribute the range in major elemental, H 2 O, and S concentrations to crystallization occurring in two separate magma bodies, corresponding to two crystallizing assemblages. These are herein referred to as the olivine-bearing magma ('Ol magma'; basaltic andesite to low-silica andesite melts) and the plagioclase-pyroxene-bearing magma ('Pl- Pyx magma'; andesite to dacite melts). The spatial positioning, chemical evolution, and interaction of these two magma bodies are discussed in detail in the forthcoming sections. Because all populations occur within both the 2006 and 2010 samples, both eruptions must have involved the same two compositionally distinct magma types. Locations of the magma reservoirs Estimates of minimum entrapment pressure were calculated using the H 2 O solubility model of Papale et al. (2006) by assuming that melts were H 2 O-saturated at the time of entrapment. The highest H 2 O concentrations associated with the Pl- Pyx magma is 2.4 wt%, which yields minimum pressures of 500 bars. The highest H 2 O concentration in the Ol magma (4.1 wt%) indicates saturation at 1,100 bars. Using a crustal density of 2,650 kg/m 3 (Hall et al. 1999), these vapor saturation pressures place the Pl-Pyx magma at 1.9 km, whereas the minimum depth where Ol magma began crystallizing is 4.2 km. The majority of MIs from both magmas have lower H 2 O concentrations, however, meaning either entrapment took place after subsequent degassing at lower pressures, or the MIs underwent partial H 2 O diffusive loss post-entrapment. For instance, it is possible that the three inclusions hosted in olivine with low H 2 O but high S concentrations could be evidence for the later process (Fig. 4), which would likely occur over time scales of hours to days at the magmatic temperatures expected for this system post-mixing (Lloyd et al. 2013; Bucholz et al. 2013). These pressure estimates are inconsistent with those proposed by Samaniego et al. (2011) based on experimental phase equilibria, which suggested crystal growth of the Pl-Pyx magma at 2,000 bars, or km below the summit. This estimate is poorly
10 872, Page 10 of 17 Bull Volcanol (2014) 76:872 Fig. 3 Harker diagrams for all melt inclusions (MIs) and whole rock from samples used in this study (WR, +), together with WR compositions from historical eruptions (historical, ; shown in red) and previously measured WR from the 2006 eruption (WR, ; shown in orange) (Samaniego et al. 2011). All MELTS modeling applies a starting water concentration of 4.0 wt%, and an incremental pressure drop from 1,500 to 500 bars, representing the ascending Ol magmas to the level of the Pl-Pyx magma. Liquidus temperature hovered 1,080 C, and fractionation proceeded until the desired compositional evolution was achieved, typically 920 C. This fits with the thermometry presented by Samaniego et al. (2011). Two separate scenarios are shown on each plot, and a third is shown on the TiO 2 plot. All lines simulate fractional crystallization of the most primitive MI composition but with different degrees of H 2 O removal during the MELTS run. The solid black line shows the results of removing H 2 O from 4.0 to 0.5 wt%, assuming degassing initiates when SiO 2 reaches 58 wt%. The solid green line shows two stages of water removal, one from 4.0 to 2.0 wt% H 2 OatSiO 2 =57 wt%, then a second step from 2.0 to 0.5 wt% H 2 OatSiO 2 =60 wt%. The third MELTS scenario shown as the black dashed line on the TiO 2 plot represents continuous fractionation of a mafic melt starting at 4.0 wt% with no forced water loss. This closed-system model best reproduces the historical TiO 2 WR tend. The black dotted line on the FeO plot represents a mixing line between 2006 end-member compositions, one from a mafic andesite MI and another from a pyroxene-hosted dacitic MI composition. Thirty to 40 % mixing of 2006 and 2010 end-members can reproduce the matrix glass composition in both years. All error is less than symbol size
11 Bull Volcanol (2014) 76:872 Page 11 of 17, 872 Fig. 4 Sulfur and Cl versus H 2 O concentration, as well as Cl versus S for all MIs, separated by host phenocryst (shape)andyear(shading). Analytical uncertainty is shown in the top right corner and is calculated for S and Cl using the standard deviation error of the glass standard used throughout the analyses and the highest concentration measured (Table 2). The black line on the S versus H 2 Oplot(top) separates the high and low SiO 2 MI populations, where the low SiO 2 population correlates with the highest H 2 O and S concentrations hosted solely in olivine phenocrysts. Exsolution of H 2 O and S generally coincides (Fig. 6), meaning that some of the lower water concentrations in the high S/H 2 O population could be the result of H 2 O species diffusion. Chlorine versus S (bottom) clearly separates out the more primitive olivine-hosted inclusions, representing the parental, less evolved recharged basaltic-andesite magma, from the more evolved and degassed melt inclusions representing the resident, andesitic magma
12 872, Page 12 of 17 Bull Volcanol (2014) 76:872 Fig. 5 Sulfur, Cl, and H 2 O concentrations plotted against SiO 2. Sulfur concentrations fall in two distinct populations, thought to represent the degassing of S from the system during recharge of the parental andesitic magma. All matrix glass analyses falls below the trend produced by melt inclusions for Cl and S concentrations, indicating that late-stage degassing occurred during eruption. Error in SiO 2 is less than the symbol size S (ppm) Cl (ppm) H 2 O (wt.%) Olivine OPX CPX MG SiO 2 (wt.%) constrained, however, owing to compositional differences between the experiments and the natural melts (e.g., the lack of amphibole in Tungurahua andesites). Another possibility is that the melt was undersaturated with water at the depth of entrapment meaning pressure is underestimated; however, the homogenous low volatile concentrations in the Pl-Pyx magma along with the continuous passive degassing signal supports a shallow, volatile saturated system (Arellano et al. 2008). Seismicity and increased SO 2 discharge measured before major eruptions in 2006 and 2008 point to a deeper recharge source than our estimate (8 10 km, Arellano et al. 2008; Carn et al. 2008; Fee et al. 2010; Samaniego et al. 2011). Our interpretation is that increased SO 2 emissions and deeper
13 Bull Volcanol (2014) 76:872 Page 13 of 17, 872 Fig. 6 Volatile concentrations (H 2 O, Cl, S) in melt inclusions are plotted against K 2 O, which for these compositions can be used to discriminate between degassing and fractional crystallization. Generally, a positive trend indicates crystallization controls the volatile compositions, whereas a vertical drop indicates degassing. A strong degassing signal is observed in S and partiallyinh 2 O for the most mafic MIs, hosted solely in olivine. Fractional crystallization dominates the evolved compositions as the majority of volatiles have previously degassed S (ppm) Olivine Pyx Plag MG 2000 Cl (ppm) H 2 O (wt.%) K 2 O (wt.%) seismic activity (8 10 km) prior to a major eruption indicate the movement of the Ol magma to shallower levels, where olivine crystallization preserved MIs trapped at depths <4.2 km. Few, if any, crystals have been transported from the deeper levels, if this is the case. Evolution of the two magma populations To address the evolution of MIs within the andesitic reservoir, we applied MELTS (Ghiorso and Sack 1995) modeling.to start, a primitive olivine-hosted MI with 4 wt% water was
14 872, Page 14 of 17 Bull Volcanol (2014) 76:872 fractionated along the NNO buffer, using incremental pressure steps from 1.5 to 0.5 kbar, which are approximately the H 2 O saturation pressures of the Ol magma and Pl-Pyx magma, respectively. This model successfully simulates fractionation producing the compositional evolution of the MIs in the Pl- Pyx melt, with two exceptions being FeO and TiO 2.Although the MELTS models do replicate the FeO and TiO 2 concentrations of the historical whole rocks, the MIs fall along a separate trend (Fig. 3). In order to reproduce the FeO and TiO 2 MI trends, an openly degassing system is required, whereby water is manually removed in MELTS to simulate volatile exsolution during depressurization of the ascending basaltic magma. The removal of water from the system suppresses Fe-Ti oxide crystallization, allowing for the FeO and TiO 2 MI trends to be reproduced (Fig. 3). Two separate scenarios are modeled, each representing different degrees of H 2 O removal during the MELTS run, placed at compositions that overlap between the two magmatic bodies. The solid black line on Fig. 3 shows the results of lowering H 2 Ofrom 4.0 to 0.5 wt% when SiO 2 reaches 58 wt%. The solid green line shows two stages of water removal, one from 4.0 to 2.0 wt% H 2 OatSiO 2 =57 wt%, then a second step from 2.0 to 0.5 wt% H 2 OatSiO 2 =60wt%.Thesetwoscenarios recreate the compositional trends produced by MIs and whole-rock compositions for most major elements. This modeling suggests that the shallow evolution of the mafic magma is influenced by the depth at which degassing and crystallization begin in the system. MI compositions from Volcán Llaima, Chile also trend towards higher TiO 2 concentrations, similarly believed to be driven by suppression of Fe- Ti oxide stability during shallow magma evolution (Bouvet de Maisonneuve et al. 2012). According to our modeling, the intermediate composition melt inclusions may have been trapped as the Ol magma was cooling and crystallizing to produce dacitic melt. Alternatively, they may be hybrids produced by back-mixing between the Ol magma and the more evolved Pl-Pyx magma, which in turn is a daughter melt produced by fractional crystallization. Concentrations of S, Cl, and H 2 O are shown against an incompatible element, K 2 O, to evaluate the effects of crystallization and degassing within the magmatic system. Concentrations of S, and to a lesser extent H 2 O, strongly support degassing driving the evolution of the melts, especially the mafic andesite (Fig. 6). The compositional gap in S between 500 and 1,000 ppm in the two populations of olivine-hosted MIs is similar to that observed at Dotsero Volcano, Colorado, which is explained by crystallization both before and after S degassing had occurred (Rowe et al. 2011). The low S contents of the MI hosted in lower Fo olivines suggest trapping at shallower depths, possibly after mixing between the Ol and Pl-Pyx magmas. Alternatively, the low-h 2 O and S olivine-hosted MIs may have been trapped from an evolved complement of the ascending Ol magma, which is supported by their less-evolved major element compositions compared to most Pl-Pyx melts and the normal zoning in these olivine crystals (Figs. 2 and 3). Thus decompression-induced crystallization can explain much of the compositional evolution of the Ol magma, but the low S and H 2 O concentrations of all Pl-Pyx magma MIs imply that the Pl-Pyx magma was already largely degassed (Fig. 6). The shallowly stored Pl-Pyx magma may have experienced coupled fractionation and degassing, where much of the compositional evolution is driven through shallow crystallization. Reverse zoning in olivine phenocrysts and normal zoning in pyroxene and plagioclase from the Pl-Pyx magma provides strong evidence for late-stage mixing between the two endmembers after isolated compositional evolution of each magma body (Fig. 2). Furthermore, the constant andesitic wholerock compositions represented by our data (Table 1) and historical datasets (Samaniego et al. 2011) indicatethat mixing is a frequently repeated process (c.f. Wada 1995; Coombs et al. 2000). Mass balance calculations suggest a % contribution of basaltic andesite to the andesitic reservoir recreates the hybrid matrix glass compositions for both 2006 and 2010 (Fig. 3). The shift in seismic activity, increased SO 2 emissions associated with these larger eruptions and the presence of only one, thin, late-stage overgrowth rim, however, suggests that the crystals analyzed in this study did not recently experience another large mixing event (Arellano et al. 2008; Carnet al. 2008; Samaniego et al. 2011). We therefore propose a two-reservoir model for the Tungurahua magmatic plumbing system. The deeper reservoir (>4.2 km depth) contains mafic basaltic andesite melt with phenocrysts of olivine and is the parent composition to a shallower, more evolved and largely degassed reservoir. The melt composition in the upper body ranges to dacite; however, it is possible that this is a mushy reservoir whose bulk composition is andesitic as well, but has developed sections of dacitic melt (Samaniego et al. 2011). The evolution of the whole system involves isolated fractional crystallization of the two magmatic bodies and frequent mixing between them, which explains the monotonous whole-rock compositions, as well as the wide range in MI compositions. The degree to which the diversity in MIs measured in the shallow, degassed Pl-Pyx magma represents compositions derived from continuous mixing versus crystallization is not discernable, as any hybrids would have been produced by mixing of two magmas along the same liquid line of descent, but both processes are likely contributing to the diversification. It is important to note that the dacitic melt inclusions have a different composition than dacitic rocks erupted historically, which are most notable in their higher concentrations
15 Bull Volcanol (2014) 76:872 Page 15 of 17, 872 of FeO and TiO 2 (Fig. 3). We attribute this difference to the level at which degassing accompanying crystallization occurs within the system. Replenishment driving the 2006 and 2010 eruptions The 2006 eruption was almost certainly driven by mafic recharge from a deeper reservoir, as indicated by the thin reversely zoned rims on plagioclase and pyroxenes (Samaniego et al. 2011) and range in melt inclusion compositions. If this hybrid magma had been preserved until 2010, olivine-hosted MI would have lost most of its water via H 2 O species diffusion to equilibrate to the low H 2 O concentrations of the Pl-Pyx magma on a timescale of days to weeks (Bucholz et al. 2013). The prevalence of H 2 O-rich MIs in 2010 indicates that the 2006 and 2010 eruptions must have been driven by two separate mixing episodes involving similar end-member magmas. The 2010 phenocrysts do not preserve evidence for two recharge events, suggesting that the 2010 eruption sampled andesitic magma that was not affected by the 2006 mixing event (Fig. 7). Phase 3: Eruption due to replenished volatiles 1 km 3 km Phase 2: Magma mixing, volatile exsolution and pressurization Magma degassing Plag & Pyx Crystallization Andesitic Reservoir Olivine Crystallization 5 km Phase 1: Mafic Recharge from lower reservoir 7 km Samaniego et al. (2011) Magma Placement 7-9 km Mafic Magma Reservoir Fig. 7 Simplified schematic of the plumbing system underneath Tungurahua volcano, extracted using melt inclusions compositions from the 2006 and 2010 explosive eruptions. Placement of the Ol magma and Pl-Pyx magma are positioned at depth assuming the melts are water saturated (Papale et al. 2006) and using the highest water concentration in each of the two magma types. Hypocenters of volcano tectonic earthquakes (circles), determined by a 3-D P-wave velocity model, between August 1999 and May 2003 are superimposed, clustering along a vertical structure at depths of 1 5 km beneath the summit crater (Molina et al. 2005). The second shallow reservoir is required by the observation of the absence of a second set of reversely zoned rims on 2010 phenocrysts. Constant matrix glass compositions suggest thorough and efficient mixing between the Ol magma recharge and the degassed more evolved Pl-Pyx magma. The calculated viscosity of the Pl-Pyx magma is Pa s at 1,000 C, with a density between 2,300 and 2,500 kg/m 3, whereas the viscosity of the Ol magma is Pa s for temperatures between 1,000 and 1,100 C, with a density between 2,500 and 2,700 kg/m 3 (Giordano et al. 2008). The similar viscosities and densities of the two magmas perhaps aided in the efficiency of the mixing between the Ol magma and Pl-Pyx magma. Temperature estimates are based on thermobarometry (Samaniego et al. 2011) and MELTS liquidus calculations of MI major elemental compositions (Ghiorso and Sack 1995). Evolution of the shallow andesitic reservoir is driven by fractional crystallization of plagioclase and pyroxene and continuous mixing, whereas the Ol magma is a less-evolved parental melt that intrudes from a deeper reservoir, where decompression driven crystallization begins at a minimum depth of 4.2 km. The depth of the mafic, water-rich reservoir is based on the estimate from Samaniego et al. (2011) from phase equilibria as well as the detection of deep seismic events prior to more explosive episodes (Arellano et al. 2008, Samaniego et al. 2011). The replenishment of volatiles from the Ol magma is thought to pressurize the shallow, degassed Pl-Pyx magma, and drive a more explosive eruption
Volatile solubility models and their application to magma storage and transport in the mantle and he crust. Julie Roberge ESIA-Ticoman, IPN Mexico
 Volatile solubility models and their application to magma storage and transport in the mantle and he crust Julie Roberge ESIA-Ticoman, IPN Mexico Melt Inclusions What are they? How to use them volatiles
Volatile solubility models and their application to magma storage and transport in the mantle and he crust Julie Roberge ESIA-Ticoman, IPN Mexico Melt Inclusions What are they? How to use them volatiles
Petrogenetic Constraints at Mount Rainier Volcano, Washington
 Petrogenetic Constraints at Mount Rainier Volcano, Washington S. C. Kuehn and P. R. Hooper, Department of Geology, Washington State University, Pullman, WA A. E. Eggers and C. Kerrick, Department of Geology,
Petrogenetic Constraints at Mount Rainier Volcano, Washington S. C. Kuehn and P. R. Hooper, Department of Geology, Washington State University, Pullman, WA A. E. Eggers and C. Kerrick, Department of Geology,
Supplementary material
 1 GSA DATA REPOSITORY 2011128 Melnik et al. Supplementary material 1. CSD acquisition: The groundmass plagioclase CSD (Fig. 1a and DR1d) was generated from element maps of 1024 by 800 points over an area
1 GSA DATA REPOSITORY 2011128 Melnik et al. Supplementary material 1. CSD acquisition: The groundmass plagioclase CSD (Fig. 1a and DR1d) was generated from element maps of 1024 by 800 points over an area
DIFFERENTIATION OF MAGMAS BY FRACTIONAL CRYSTALLIZATION THE M&M MAGMA CHAMBER
 Geol 2312 Igneous and Metamorphic Petrology Spring 2009 Name DIFFERENTIATION OF MAGMAS BY FRACTIONAL CRYSTALLIZATION THE M&M MAGMA CHAMBER Objective: This exercise is intended to improve understanding
Geol 2312 Igneous and Metamorphic Petrology Spring 2009 Name DIFFERENTIATION OF MAGMAS BY FRACTIONAL CRYSTALLIZATION THE M&M MAGMA CHAMBER Objective: This exercise is intended to improve understanding
Supplemental Information. I. Alternative version of Figure 1. II. Sample Preparation
 GSA DATA REPOSITORY 2012195 Watkins et al. Supplemental Information I. Alternative version of Figure 1 Figure DR1. CO 2 versus H 2 O for Mono Craters pyroclasts. Circles represent spot analyses on obsidian
GSA DATA REPOSITORY 2012195 Watkins et al. Supplemental Information I. Alternative version of Figure 1 Figure DR1. CO 2 versus H 2 O for Mono Craters pyroclasts. Circles represent spot analyses on obsidian
PETROGENESIS OF A SERIES OF MAFIC SHEETS WITHIN THE VINALHAVEN PLUTON, VINALHAVEN ISLAND, MAINE
 PETROGENESIS OF A SERIES OF MAFIC SHEETS WITHIN THE VINALHAVEN PLUTON, VINALHAVEN ISLAND, MAINE DANIEL HAWKINS Western Kentucky University Research Advisor: Andrew Wulff INTRODUCTION Round Point, in the
PETROGENESIS OF A SERIES OF MAFIC SHEETS WITHIN THE VINALHAVEN PLUTON, VINALHAVEN ISLAND, MAINE DANIEL HAWKINS Western Kentucky University Research Advisor: Andrew Wulff INTRODUCTION Round Point, in the
APPENDIX A SAMPLING AND ANALYTICAL METHODS
 APPENDIX A SAMPLING AND ANALYTICAL METHODS Sampling of Klyuchevskoy Tephra and Lava Lavas available for sampling at lower altitudes on Klyuchevskoy s slopes erupted primarily from flank vents and thus
APPENDIX A SAMPLING AND ANALYTICAL METHODS Sampling of Klyuchevskoy Tephra and Lava Lavas available for sampling at lower altitudes on Klyuchevskoy s slopes erupted primarily from flank vents and thus
Notes for Use of the Cpx-Plag-Ol Thermobar Workbook Last Updated:
 Notes for Use of the Cpx-Plag-Ol Thermobar Workbook Last Updated: 7-22-05 Cpx-Plag-Ol Thermobar is an Excel workbook that can be used to calculate crystallization pressures and temperatures for clinopyroxene-
Notes for Use of the Cpx-Plag-Ol Thermobar Workbook Last Updated: 7-22-05 Cpx-Plag-Ol Thermobar is an Excel workbook that can be used to calculate crystallization pressures and temperatures for clinopyroxene-
Sample collection was restricted to pumice from plinian fallout, from thin distal ignimbrite, and from
 DATA REPOSITORY APPENDIX 1. ANALYTICAL TECHNIQUES Sample collection was restricted to pumice from plinian fallout, from thin distal ignimbrite, and from proximal lithic breccias deposits (Fig. DR1 and
DATA REPOSITORY APPENDIX 1. ANALYTICAL TECHNIQUES Sample collection was restricted to pumice from plinian fallout, from thin distal ignimbrite, and from proximal lithic breccias deposits (Fig. DR1 and
Overview of the KAHT system. Ian E.M. Smith, School of Environment, University of Auckland
 Overview of the KAHT system Ian E.M. Smith, School of Environment, University of Auckland Tonga-Kermadec-New Zealand Arc Developed on the Pacific - Australian convergent margin Mainly intraoceanic except
Overview of the KAHT system Ian E.M. Smith, School of Environment, University of Auckland Tonga-Kermadec-New Zealand Arc Developed on the Pacific - Australian convergent margin Mainly intraoceanic except
Week 7 Submarine Pyroclastic Activity (there was no lecture for week 6)
 Week 7 Submarine Pyroclastic Activity (there was no lecture for week 6) Note: some of these slides were provided by Dave Clague, MBARI Two topics: fluidal clasts and bubble wall fragments internal water
Week 7 Submarine Pyroclastic Activity (there was no lecture for week 6) Note: some of these slides were provided by Dave Clague, MBARI Two topics: fluidal clasts and bubble wall fragments internal water
Chapter 9: Trace Elements
 Chapter 9: Trace Elements Note magnitude of major element changes Figure 8.2. Harker variation diagram for 310 analyzed volcanic rocks from Crater Lake (Mt. Mazama), Oregon Cascades. Data compiled by Rick
Chapter 9: Trace Elements Note magnitude of major element changes Figure 8.2. Harker variation diagram for 310 analyzed volcanic rocks from Crater Lake (Mt. Mazama), Oregon Cascades. Data compiled by Rick
Igneous and Metamorphic Rock Forming Minerals. Department of Geology Mr. Victor Tibane SGM 210_2013
 Igneous and Metamorphic Rock Forming Minerals Department of Geology Mr. Victor Tibane 1 SGM 210_2013 Grotzinger Jordan Understanding Earth Sixth Edition Chapter 4: IGNEOUS ROCKS Solids from Melts 2011
Igneous and Metamorphic Rock Forming Minerals Department of Geology Mr. Victor Tibane 1 SGM 210_2013 Grotzinger Jordan Understanding Earth Sixth Edition Chapter 4: IGNEOUS ROCKS Solids from Melts 2011
Chapter 9: Trace Elements
 Lecture 13 Introduction to Trace Elements Wednesday, March 9, 2005 Chapter 9: Trace Elements Note magnitude of major element changes Figure 8-2. Harker variation diagram for 310 analyzed volcanic rocks
Lecture 13 Introduction to Trace Elements Wednesday, March 9, 2005 Chapter 9: Trace Elements Note magnitude of major element changes Figure 8-2. Harker variation diagram for 310 analyzed volcanic rocks
Mineral/feature Modal% Size, morphology, distinguishing optical properties
 Sample#: FIL 10-1 Rock Name: Olivine bearing, vesiculated 2-Px basaltic andesite Hand-specimen description: Highly porphyritic and vesiculated (1-5mm) medium-grained dark grey groundmass with abundant
Sample#: FIL 10-1 Rock Name: Olivine bearing, vesiculated 2-Px basaltic andesite Hand-specimen description: Highly porphyritic and vesiculated (1-5mm) medium-grained dark grey groundmass with abundant
Partial melting of mantle peridotite
 Partial melting of mantle peridotite 1100 1200 1300 1400 1500 (TºC) Depth (km) 50 100 150 Plag lherzolite (ol-opx-cpx-pl) Spinel lherzolite (Ol-opx-cpx-sp) Garnet lherzolite (Ol-opx-cpx-gar) Graphite Diamond
Partial melting of mantle peridotite 1100 1200 1300 1400 1500 (TºC) Depth (km) 50 100 150 Plag lherzolite (ol-opx-cpx-pl) Spinel lherzolite (Ol-opx-cpx-sp) Garnet lherzolite (Ol-opx-cpx-gar) Graphite Diamond
LAB 9: ULTRAMAFIC ROCKS, CUMULATES AND MELT SOURCES
 Geology 316 (Petrology) (03/26/2012) Name LAB 9: ULTRAMAFIC ROCKS, CUMULATES AND MELT SOURCES INTRODUCTION Ultramafic rocks are igneous rocks containing less than 10% felsic minerals (quartz + feldspars
Geology 316 (Petrology) (03/26/2012) Name LAB 9: ULTRAMAFIC ROCKS, CUMULATES AND MELT SOURCES INTRODUCTION Ultramafic rocks are igneous rocks containing less than 10% felsic minerals (quartz + feldspars
INTERPRETATION OF OBSERVED TEXTURES
 Chapter 4 INTERPRETATION OF OBSERVED TEXTURES 4.1 General Considerations Processes able to modify physical and chemical parameters of the volcanic system are multiple and, at times, combined (e.g., convection,
Chapter 4 INTERPRETATION OF OBSERVED TEXTURES 4.1 General Considerations Processes able to modify physical and chemical parameters of the volcanic system are multiple and, at times, combined (e.g., convection,
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 115, B11202, doi: /2010jb007433, 2010
 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 115,, doi:10.1029/2010jb007433, 2010 Magma plumbing system of the 2000 eruption of Miyakejima volcano, Japan, deduced from volatile and major component contents of
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 115,, doi:10.1029/2010jb007433, 2010 Magma plumbing system of the 2000 eruption of Miyakejima volcano, Japan, deduced from volatile and major component contents of
Essentials of Geology, 11e
 Essentials of Geology, 11e Igneous Rocks and Intrusive Activity Chapter 3 Instructor Jennifer Barson Spokane Falls Community College Geology 101 Stanley Hatfield Southwestern Illinois College Characteristics
Essentials of Geology, 11e Igneous Rocks and Intrusive Activity Chapter 3 Instructor Jennifer Barson Spokane Falls Community College Geology 101 Stanley Hatfield Southwestern Illinois College Characteristics
Lecture 36. Igneous geochemistry
 Lecture 36 Igneous geochemistry Reading - White Chapter 7 Today 1. Overview 2. solid-melt distribution coefficients Igneous geochemistry The chemistry of igneous systems provides clues to a number of important
Lecture 36 Igneous geochemistry Reading - White Chapter 7 Today 1. Overview 2. solid-melt distribution coefficients Igneous geochemistry The chemistry of igneous systems provides clues to a number of important
Magmatic Sulfur and Chlorine Abundances at Stromboli, Italy and their Role in the Formation of Vesicle-hosted Metal Alloys
 Brigham Young University BYU ScholarsArchive All Theses and Dissertations 2008-08-07 Magmatic Sulfur and Chlorine Abundances at Stromboli, Italy and their Role in the Formation of Vesicle-hosted Metal
Brigham Young University BYU ScholarsArchive All Theses and Dissertations 2008-08-07 Magmatic Sulfur and Chlorine Abundances at Stromboli, Italy and their Role in the Formation of Vesicle-hosted Metal
J. Mangas and F.J. Perez-Torrado. Departamento de Física. Universidad de Las Palmas de Gran Canaria Las Palmas de Gran Canaria.
 Magmatic processes in the oceanic lithosphere: characterization of the ultramafic and mafic materials from the Holocene volcanic centers of Bandama and La Caldera de Pinos de Gáldar (Gran Canaria, Canary
Magmatic processes in the oceanic lithosphere: characterization of the ultramafic and mafic materials from the Holocene volcanic centers of Bandama and La Caldera de Pinos de Gáldar (Gran Canaria, Canary
REVISION 1:Ascent rates of rhyolitic magma at the onset of three caldera-forming eruptions
 1 2 REVISION 1:Ascent rates of rhyolitic magma at the onset of three caldera-forming eruptions 3 4 5 6 Madison L. Myers 1,*, Paul J. Wallace 1, Colin J.N. Wilson 2, James M. Watkins 1, and Yang Liu 3 7
1 2 REVISION 1:Ascent rates of rhyolitic magma at the onset of three caldera-forming eruptions 3 4 5 6 Madison L. Myers 1,*, Paul J. Wallace 1, Colin J.N. Wilson 2, James M. Watkins 1, and Yang Liu 3 7
12 Chemistry (Mg,Fe) 2 SiO 4 Olivine is forms what is called an isomorphous solid solution series that ranges between two end members: Forsterite Mg
 11 Olivine Structure Olivine is a common green or brown rock forming minerals which consists of a solid-solution series between Forsterite (Fo) and Fayalite (Fa). It is an orthorhombic orthosilicate with
11 Olivine Structure Olivine is a common green or brown rock forming minerals which consists of a solid-solution series between Forsterite (Fo) and Fayalite (Fa). It is an orthorhombic orthosilicate with
Worked Example of Batch Melting: Rb and Sr
 Worked Example of Batch Melting: Rb and Sr Basalt with the mode: Table 9.2. Conversion from mode to weight percent Mineral Mode Density Wt prop Wt% ol 15 3.6 54 0.18 cpx 33 3.4 112.2 0.37 plag 51 2.7 137.7
Worked Example of Batch Melting: Rb and Sr Basalt with the mode: Table 9.2. Conversion from mode to weight percent Mineral Mode Density Wt prop Wt% ol 15 3.6 54 0.18 cpx 33 3.4 112.2 0.37 plag 51 2.7 137.7
Visualizing Earth Science. Chapter Overview. Volcanoes and Eruption Types. By Z. Merali and B. F. Skinner. Chapter 9 Volcanism and Other
 Visualizing Earth Science By Z. Merali and B. F. Skinner Chapter 9 Volcanism and Other Igneous Processes Volcanoes types and effects of eruption Chapter Overview Melting and cooling of rocks Geological
Visualizing Earth Science By Z. Merali and B. F. Skinner Chapter 9 Volcanism and Other Igneous Processes Volcanoes types and effects of eruption Chapter Overview Melting and cooling of rocks Geological
SR Allen, RS Fiske, Y Tamura Sumisu methods
 SR Allen, RS Fiske, Y Tamura Sumisu methods FTIR water contents from melt inclusions Quartz-hosted melt inclusions were analyzed for H 2 O and CO 2 by Fourier Transform Infrared Spectroscopy (FTIR) at
SR Allen, RS Fiske, Y Tamura Sumisu methods FTIR water contents from melt inclusions Quartz-hosted melt inclusions were analyzed for H 2 O and CO 2 by Fourier Transform Infrared Spectroscopy (FTIR) at
Minor- and Trace Element Zoning in Plagioclase From Kizimen Volcano, Kamchatka: Insights on the Magma Chamber Processes
 Minor- and Trace Element Zoning in Plagioclase From Kizimen Volcano, Kamchatka: Insights on the Magma Chamber Processes Tatiana Churikova 1,2, Gerhard Wörner 2, John Eichelberger 3, and Boris Ivanov 1
Minor- and Trace Element Zoning in Plagioclase From Kizimen Volcano, Kamchatka: Insights on the Magma Chamber Processes Tatiana Churikova 1,2, Gerhard Wörner 2, John Eichelberger 3, and Boris Ivanov 1
Petrology of Volcanic Rocks from Bequia and St. Vincent*
 Petrology of Volcanic Rocks from Bequia and St. Vincent* Michal Camejo 1, Richard Robertson 1, Elena Melekhova 2, Anna Hicks 3, and Thomas Christopher 1,4 Search and Discovery Article #51171 (15)** Posted
Petrology of Volcanic Rocks from Bequia and St. Vincent* Michal Camejo 1, Richard Robertson 1, Elena Melekhova 2, Anna Hicks 3, and Thomas Christopher 1,4 Search and Discovery Article #51171 (15)** Posted
GLY 155 Introduction to Physical Geology, W. Altermann. Grotzinger Jordan. Understanding Earth. Sixth Edition
 Grotzinger Jordan Understanding Earth Sixth Edition Chapter 4: IGNEOUS ROCKS Solids from Melts 2011 by W. H. Freeman and Company Chapter 4: Igneous Rocks: Solids from Melts 1 About Igneous Rocks Igneous
Grotzinger Jordan Understanding Earth Sixth Edition Chapter 4: IGNEOUS ROCKS Solids from Melts 2011 by W. H. Freeman and Company Chapter 4: Igneous Rocks: Solids from Melts 1 About Igneous Rocks Igneous
VOLCANIC STRATIGRAPHY AND PETROLOGY OF THE NORTHERN SNAEFELLSNES RIFT, SOUTHERN LAXÁRDALSFJÖLL, ICELAND
 VOLCANIC STRATIGRAPHY AND PETROLOGY OF THE NORTHERN SNAEFELLSNES RIFT, SOUTHERN LAXÁRDALSFJÖLL, ICELAND LEBN SCHUYLER Whitman College Sponsor: John Winter INTRODUCTION Iceland is exposed above sea level
VOLCANIC STRATIGRAPHY AND PETROLOGY OF THE NORTHERN SNAEFELLSNES RIFT, SOUTHERN LAXÁRDALSFJÖLL, ICELAND LEBN SCHUYLER Whitman College Sponsor: John Winter INTRODUCTION Iceland is exposed above sea level
Breeding et al., Data Repository Material Figure DR1. Athens. Study Area
 Breeding, Ague, and Brocker 1 Figure DR1 21 o 24 Greece o A 38 o Athens Tinos 37 o Syros Attic-Cycladic Blueschist Belt Syros Kampos B Study Area Ermoupoli N Vari Unit Cycladic HP-LT Unit Marble horizons
Breeding, Ague, and Brocker 1 Figure DR1 21 o 24 Greece o A 38 o Athens Tinos 37 o Syros Attic-Cycladic Blueschist Belt Syros Kampos B Study Area Ermoupoli N Vari Unit Cycladic HP-LT Unit Marble horizons
Engineering Geology ECIV 2204
 Engineering Geology ECIV 2204 Instructor : Dr. Jehad Hamad 2017-2016 Chapter (3) Igneous Rocks Chapter 3: Rocks: Materials of the Solid Earth Igneous Rocks Chapter 3: Rocks: Materials of the Solid Earth
Engineering Geology ECIV 2204 Instructor : Dr. Jehad Hamad 2017-2016 Chapter (3) Igneous Rocks Chapter 3: Rocks: Materials of the Solid Earth Igneous Rocks Chapter 3: Rocks: Materials of the Solid Earth
Controls on long-term low explosivity at andesitic arc volcanoes: Insights from Mount Hood, Oregon
 Controls on long-term low explosivity at andesitic arc volcanoes: Insights from Mount Hood, Oregon Alison M. Koleszar a, Adam J.R. Kent b, Paul J. Wallace c, William E. Scott d a Corresponding author:
Controls on long-term low explosivity at andesitic arc volcanoes: Insights from Mount Hood, Oregon Alison M. Koleszar a, Adam J.R. Kent b, Paul J. Wallace c, William E. Scott d a Corresponding author:
Chapter IV MINERAL CHEMISTRY
 Chapter IV MINERAL CHEMISTRY Chapter-IV MINERAL CHEMISTRY 4.1 INTRODUCTION In this chapter, chemical analyses of different minerals present in various rocks of Mashhad granitoid plutons have been presented.
Chapter IV MINERAL CHEMISTRY Chapter-IV MINERAL CHEMISTRY 4.1 INTRODUCTION In this chapter, chemical analyses of different minerals present in various rocks of Mashhad granitoid plutons have been presented.
Imagine the first rock and the cycles that it has been through.
 A rock is a naturally formed, consolidated material usually composed of grains of one or more minerals The rock cycle shows how one type of rocky material gets transformed into another The Rock Cycle Representation
A rock is a naturally formed, consolidated material usually composed of grains of one or more minerals The rock cycle shows how one type of rocky material gets transformed into another The Rock Cycle Representation
EPS 50 Lab 2: Igneous Rocks Grotzinger and Jordan, Chapter 4
 Name: EPS 50 Lab 2: Igneous Rocks Grotzinger and Jordan, Chapter 4 Introduction In the previous lab, we learned about mineral characteristics, properties and identities as well as the three basic rock
Name: EPS 50 Lab 2: Igneous Rocks Grotzinger and Jordan, Chapter 4 Introduction In the previous lab, we learned about mineral characteristics, properties and identities as well as the three basic rock
Constitution of Magmas. Magmas. Gas Law. Composition. Atomic Structure of Magma. Structural Model. PV = nrt H 2 O + O -2 = 2(OH) -
 Constitution of Magmas Magmas Best, Ch. 8 Hot molten rock T = 700-1200 degrees C Composed of ions or complexes Phase Homogeneous Separable part of the system With an interface Composition Most components
Constitution of Magmas Magmas Best, Ch. 8 Hot molten rock T = 700-1200 degrees C Composed of ions or complexes Phase Homogeneous Separable part of the system With an interface Composition Most components
Alternative Mechanisms for Volcanic Activity in Hotspot-Ridge Systems: The Northern Galapagos Province
 ABSTRACT for the Plume IV Penrose Conference Alternative Mechanisms for Volcanic Activity in Hotspot-Ridge Systems: The Northern Galapagos Province Karen S. Harpp, Colgate University, Department of Geology,
ABSTRACT for the Plume IV Penrose Conference Alternative Mechanisms for Volcanic Activity in Hotspot-Ridge Systems: The Northern Galapagos Province Karen S. Harpp, Colgate University, Department of Geology,
- low FeO*/MgO is controlled by the reaction relation oliv + liq! opx.
 Mantle melting trend to high-sio 2 - low FeO*/MgO is controlled by the reaction relation oliv + liq! opx. 4 3 2 1 Ol+Opx +Cpx+Sp +liq 79-35g 82-66 87S35 85-44 85-41c -ol +opx Mantle melting/reaction 0
Mantle melting trend to high-sio 2 - low FeO*/MgO is controlled by the reaction relation oliv + liq! opx. 4 3 2 1 Ol+Opx +Cpx+Sp +liq 79-35g 82-66 87S35 85-44 85-41c -ol +opx Mantle melting/reaction 0
Mineral chemistry of submarine lavas from Hilo Ridge, Hawaii: implications for magmatic processes within Hawaiian rift zones
 Contrib Mineral Petrol (1999) 135: 355±372 Ó Springer-Verlag 1999 Huai-Jen Yang á Frederick A. Frey David A. Clague á Michael O. Garcia Mineral chemistry of submarine lavas from Hilo Ridge, Hawaii: implications
Contrib Mineral Petrol (1999) 135: 355±372 Ó Springer-Verlag 1999 Huai-Jen Yang á Frederick A. Frey David A. Clague á Michael O. Garcia Mineral chemistry of submarine lavas from Hilo Ridge, Hawaii: implications
Differentiation and magma mixing on Kilauea s east rift zone: a further look at the eruptions of 1955 and Part II.
 Bull Volcanol (16) 57: 602 60 Q Springer-Verlag 16 ORIGINAL AER T. L. Wright 7 R. T. Helz Differentiation and magma mixing on Kilauea s east rift zone: a further look at the eruptions of 155 and 160. art
Bull Volcanol (16) 57: 602 60 Q Springer-Verlag 16 ORIGINAL AER T. L. Wright 7 R. T. Helz Differentiation and magma mixing on Kilauea s east rift zone: a further look at the eruptions of 155 and 160. art
ERTH 456 / GEOL 556 Volcanology. Lecture 06: Conduits
 1 / 28 ERTH 456 / GEOL 556 Volcanology Lecture 06: Conduits Ronni Grapenthin rg@nmt.edu MSEC 356, x5924 hours: TR 3-4PM or appt. September 12, 2016 2 / 28 How does magma get from source to surface? What
1 / 28 ERTH 456 / GEOL 556 Volcanology Lecture 06: Conduits Ronni Grapenthin rg@nmt.edu MSEC 356, x5924 hours: TR 3-4PM or appt. September 12, 2016 2 / 28 How does magma get from source to surface? What
Chapter 4 8/27/2013. Igneous Rocks. and Intrusive Igneous Activity. Introduction. The Properties and Behavior of Magma and Lava
 Introduction Chapter 4 Igneous rocks form by the cooling of magma (or lava). Large parts of the continents and all the oceanic crust are composed of. and Intrusive Igneous Activity The Properties and Behavior
Introduction Chapter 4 Igneous rocks form by the cooling of magma (or lava). Large parts of the continents and all the oceanic crust are composed of. and Intrusive Igneous Activity The Properties and Behavior
Petrologic and experimental evidence for the movement and heating of the pre-eruptive Minoan rhyodacite (Santorini, Greece)
 Contrib Mineral Petrol (1999) 135: 315±331 Ó Springer-Verlag 1999 Elizabeth Cottrell á James E. Gardner Malcolm J. Rutherford Petrologic and experimental evidence for the movement and heating of the pre-eruptive
Contrib Mineral Petrol (1999) 135: 315±331 Ó Springer-Verlag 1999 Elizabeth Cottrell á James E. Gardner Malcolm J. Rutherford Petrologic and experimental evidence for the movement and heating of the pre-eruptive
Igneous Rocks. Magma molten rock material consisting of liquid rock and crystals. A variety exists, but here are the end members:
 Igneous Rocks Magma molten rock material consisting of liquid rock and crystals. A variety exists, but here are the end members: Types of Magma Basaltic, Basic or Mafic very hot (900-1200 C) very fluid
Igneous Rocks Magma molten rock material consisting of liquid rock and crystals. A variety exists, but here are the end members: Types of Magma Basaltic, Basic or Mafic very hot (900-1200 C) very fluid
MACRORYTHMIC GABBRO TO GRANITE CYCLES OF CLAM COVE VINALHAVEN INTRUSION, MAINE
 MACRORYTHMIC GABBRO TO GRANITE CYCLES OF CLAM COVE VINALHAVEN INTRUSION, MAINE NICK CUBA Amherst College Sponsor: Peter Crowley INTRODUCTION The rocks of the layered gabbro-diorite unit of the Silurian
MACRORYTHMIC GABBRO TO GRANITE CYCLES OF CLAM COVE VINALHAVEN INTRUSION, MAINE NICK CUBA Amherst College Sponsor: Peter Crowley INTRODUCTION The rocks of the layered gabbro-diorite unit of the Silurian
GEOL 2312 Igneous and Metamorphic Petrology Spring 2016 Score / 58. Midterm 1 Chapters 1-10
 GEOL 2312 Igneous and Metamorphic Petrology Name KEY Spring 2016 Score / 58 Midterm 1 Chapters 1-10 1) Name two things that petrologists want to know about magmas (1 pt) Formation, source, composition,
GEOL 2312 Igneous and Metamorphic Petrology Name KEY Spring 2016 Score / 58 Midterm 1 Chapters 1-10 1) Name two things that petrologists want to know about magmas (1 pt) Formation, source, composition,
Textures of Igneous Rocks
 Page 1 of 6 EENS 212 Prof. Stephen A. Nelson Petrology Tulane University This document last updated on 12-Feb-2004 Introduction to Igneous Rocks An igneous rock is any crystalline or glassy rock that forms
Page 1 of 6 EENS 212 Prof. Stephen A. Nelson Petrology Tulane University This document last updated on 12-Feb-2004 Introduction to Igneous Rocks An igneous rock is any crystalline or glassy rock that forms
Lecture 25 Subduction Related Magmatism
 Lecture 25 Subduction Related Magmatism Monday, May 2 nd 2005 Subduction Related Magmatism Activity along arcuate volcanic chains along subduction zones Distinctly different from the mainly basaltic provinces
Lecture 25 Subduction Related Magmatism Monday, May 2 nd 2005 Subduction Related Magmatism Activity along arcuate volcanic chains along subduction zones Distinctly different from the mainly basaltic provinces
Electronic Appendix A: Supplementary material to accompany the manuscript, Fe 3+ / Fe in Mariana Arc basalts and primary fo 2.
 Electronic Appendix A: Supplementary material to accompany the manuscript, Fe 3+ / Fe in Mariana Arc basalts and primary fo 2. Screening for olivine interference in Fe-µ-XANES spectra When collecting Fe-µ-XANES
Electronic Appendix A: Supplementary material to accompany the manuscript, Fe 3+ / Fe in Mariana Arc basalts and primary fo 2. Screening for olivine interference in Fe-µ-XANES spectra When collecting Fe-µ-XANES
Igneous Rock Classification, Processes and Identification Physical Geology GEOL 100
 Igneous Rock Classification, Processes and Identification Physical Geology GEOL 100 Ray Rector - Instructor Major Concepts 1) Igneous rocks form directly from the crystallization of a magma or lava 2)
Igneous Rock Classification, Processes and Identification Physical Geology GEOL 100 Ray Rector - Instructor Major Concepts 1) Igneous rocks form directly from the crystallization of a magma or lava 2)
Rhyodacite magma storage conditions prior to the 3430 ybp caldera-forming eruption of Aniakchak volcano, Alaska
 Contrib Mineral Petrol (2006) 152:523 540 DOI 10.1007/s00410-006-0121-4 ORIGINAL PAPER Rhyodacite magma storage conditions prior to the 3430 ybp caldera-forming eruption of Aniakchak volcano, Alaska Jessica
Contrib Mineral Petrol (2006) 152:523 540 DOI 10.1007/s00410-006-0121-4 ORIGINAL PAPER Rhyodacite magma storage conditions prior to the 3430 ybp caldera-forming eruption of Aniakchak volcano, Alaska Jessica
GSA DATA REPOSITORY
 GSA DATA REPOSITORY 2013019 Supplemental information for The Solidus of Alkaline Carbonatite in the Deep Mantle Konstantin D. Litasov, Anton Shatskiy, Eiji Ohtani, and Gregory M. Yaxley EXPERIMENTAL METHODS
GSA DATA REPOSITORY 2013019 Supplemental information for The Solidus of Alkaline Carbonatite in the Deep Mantle Konstantin D. Litasov, Anton Shatskiy, Eiji Ohtani, and Gregory M. Yaxley EXPERIMENTAL METHODS
MIZUHO AMMA-MIYASAKA 1 * AND MITSUHIRO NAKAGAWA 2
 JOURNAL OF PETROLOGY VOLUME 44 NUMBER 12 PAGES 2113±2138 2003 DOI: 10.1093/petrology/egg072 Evolution of Deeper Basaltic and Shallower Andesitic Magmas during the AD 1469---1983 Eruptions of Miyake-Jima
JOURNAL OF PETROLOGY VOLUME 44 NUMBER 12 PAGES 2113±2138 2003 DOI: 10.1093/petrology/egg072 Evolution of Deeper Basaltic and Shallower Andesitic Magmas during the AD 1469---1983 Eruptions of Miyake-Jima
GEOL 2312 Igneous and Metamorphic Petrology Spring 2009 Sc ore / 40
 GEOL 2312 Igneous and Metamorphic Petrology Name Spring 2009 Sc ore / 40 QUIZ 3 1) Name two geologic features that provide physical evidence for the mineralogy of the earth s mantle (2 pts) Ophiolites,
GEOL 2312 Igneous and Metamorphic Petrology Name Spring 2009 Sc ore / 40 QUIZ 3 1) Name two geologic features that provide physical evidence for the mineralogy of the earth s mantle (2 pts) Ophiolites,
The Nature of Igneous Rocks
 The Nature of Igneous Rocks Form from Magma Hot, partially molten mixture of solid liquid and gas Mineral crystals form in the magma making a crystal slush Gases - H 2 O, CO 2, etc. - are dissolved in
The Nature of Igneous Rocks Form from Magma Hot, partially molten mixture of solid liquid and gas Mineral crystals form in the magma making a crystal slush Gases - H 2 O, CO 2, etc. - are dissolved in
GEOLOGY. Subject : GEOLOGY (For under graduate student.) Paper No. : Paper 02 Introduction to Geology 02
 GEOLOGY Subject : GEOLOGY (For under graduate student.) Paper No. : Paper 02 Introduction to Geology 02 Topic No. & Title : 37 Magma Bowen Series (Part 01) Academic Script What is Igneous Petrology? Igneous
GEOLOGY Subject : GEOLOGY (For under graduate student.) Paper No. : Paper 02 Introduction to Geology 02 Topic No. & Title : 37 Magma Bowen Series (Part 01) Academic Script What is Igneous Petrology? Igneous
Magmatic Processes at Subduction Zones
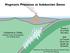 Magmatic Processes at Subduction Zones Katherine A. Kelley Graduate School of Oceanography Univ. of Rhode Island Thanks to Terry Plank Erik Hauri GVP: Liz Cottrell Simon Carn Jennifer Jay Ed Venzke Subduction
Magmatic Processes at Subduction Zones Katherine A. Kelley Graduate School of Oceanography Univ. of Rhode Island Thanks to Terry Plank Erik Hauri GVP: Liz Cottrell Simon Carn Jennifer Jay Ed Venzke Subduction
ANDESITE CONFERENCE GUIDEBOOK
 STATE OF OREGON DEPARTMENT OF GEOLOGY AND MINERAL 1069 STATE OFFICE BUILDING PORTLAND. OREGON 972Q1 INDUSTRIES ANDESITE CONFERENCE GUIDEBOOK Editor, Hollis M. Dole Cartographer, C. J. Newhouse Bulletin
STATE OF OREGON DEPARTMENT OF GEOLOGY AND MINERAL 1069 STATE OFFICE BUILDING PORTLAND. OREGON 972Q1 INDUSTRIES ANDESITE CONFERENCE GUIDEBOOK Editor, Hollis M. Dole Cartographer, C. J. Newhouse Bulletin
A Rock is a solid aggregate of minerals.
 Quartz A Rock is a solid aggregate of minerals. Orthoclase Feldspar Plagioclase Feldspar Biotite Four different minerals are obvious in this piece of Granite. The average automobile contains: Minerals
Quartz A Rock is a solid aggregate of minerals. Orthoclase Feldspar Plagioclase Feldspar Biotite Four different minerals are obvious in this piece of Granite. The average automobile contains: Minerals
Introduction. Volcano a vent where molten rock comes out of Earth
 Introduction Volcano a vent where molten rock comes out of Earth Example: Kilauea Volcano, Hawaii Hot (~1,200 o C) lava pools around the volcanic vent. Hot, syrupy lava runs downhill as a lava flow. The
Introduction Volcano a vent where molten rock comes out of Earth Example: Kilauea Volcano, Hawaii Hot (~1,200 o C) lava pools around the volcanic vent. Hot, syrupy lava runs downhill as a lava flow. The
Effect of tectonic setting on chemistry of mantle-derived melts
 Effect of tectonic setting on chemistry of mantle-derived melts Lherzolite Basalt Factors controlling magma composition Composition of the source Partial melting process Fractional crystallization Crustal
Effect of tectonic setting on chemistry of mantle-derived melts Lherzolite Basalt Factors controlling magma composition Composition of the source Partial melting process Fractional crystallization Crustal
V. B. NAUMOV 1, V. A. KOVALENKER 2 and V. L. RUSINOV 2
 CHEMICAL COMPOSITION, TRACE ELEMENTS, AND VOLATILE COMPONENTS OF MELTS: EVIDENCE FROM INCLUSIONS IN THE MINERALS OF NEOVOLCANITES FROM THE CENTRAL AND EASTERN SLOVAKIA V. B. NAUMOV 1, V. A. KOVALENKER
CHEMICAL COMPOSITION, TRACE ELEMENTS, AND VOLATILE COMPONENTS OF MELTS: EVIDENCE FROM INCLUSIONS IN THE MINERALS OF NEOVOLCANITES FROM THE CENTRAL AND EASTERN SLOVAKIA V. B. NAUMOV 1, V. A. KOVALENKER
Q. WANG, Q-K. XIA, S. Y. O REILLY, W. L. GRIFFIN, E. E. BEYER AND H. K. BRUECKNER
 Pressure- and stress-induced fabric transition in olivine from peridotites in the Western Gneiss Region (Norway): implications for mantle seismic anisotropy Q. WANG, Q-K. XIA, S. Y. O REILLY, W. L. GRIFFIN,
Pressure- and stress-induced fabric transition in olivine from peridotites in the Western Gneiss Region (Norway): implications for mantle seismic anisotropy Q. WANG, Q-K. XIA, S. Y. O REILLY, W. L. GRIFFIN,
BONINITIC MELT INCLUSIONS IN CHROME SPINEL FROM THE OGASAWARA ARCHIPELAGO
 GSA DATA REPOSITORY 2015057 BONINITIC MELT INCLUSIONS IN CHROME SPINEL FROM THE OGASAWARA ARCHIPELAGO DATA REPOSITORY for Thermal and chemical evolution of the subarc mantle revealed by spinel-hosted melt
GSA DATA REPOSITORY 2015057 BONINITIC MELT INCLUSIONS IN CHROME SPINEL FROM THE OGASAWARA ARCHIPELAGO DATA REPOSITORY for Thermal and chemical evolution of the subarc mantle revealed by spinel-hosted melt
Ascent rates of rhyolitic magma at the onset of three caldera-forming eruptions
 American Mineralogist, Volume 103, pages 952 965, 2018 Ascent rates of rhyolitic magma at the onset of three caldera-forming eruptions Madison L. Myers 1, *, Paul J. Wallace 1, Colin J.N. Wilson 2, James
American Mineralogist, Volume 103, pages 952 965, 2018 Ascent rates of rhyolitic magma at the onset of three caldera-forming eruptions Madison L. Myers 1, *, Paul J. Wallace 1, Colin J.N. Wilson 2, James
Petrology and Geochronology of Iran Tepe volcano, Eastern Rhodopes, Bulgaria: Age relationship with the Ada Tepe gold deposit. (preliminary data)
 Petrology and Geochronology of Iran Tepe volcano, Eastern Rhodopes, Bulgaria: Age relationship with the Ada Tepe gold deposit. (preliminary data) Peter Kibarov, Peter Marchev, Maria Ovtcharova, Raya Raycheva,
Petrology and Geochronology of Iran Tepe volcano, Eastern Rhodopes, Bulgaria: Age relationship with the Ada Tepe gold deposit. (preliminary data) Peter Kibarov, Peter Marchev, Maria Ovtcharova, Raya Raycheva,
Lecture 6 - Igneous Rocks and Volcanoes
 Lecture 6 - Igneous Rocks and Volcanoes Learning objectives Understand and be able to predict where and why magma will be forming at different tectonic settings Understand the factors controlling magma
Lecture 6 - Igneous Rocks and Volcanoes Learning objectives Understand and be able to predict where and why magma will be forming at different tectonic settings Understand the factors controlling magma
Supplementary Information
 Supplementary Information Supplementary Tables Major element compositions of host and daughter crystals: Table 1 reports composition for host-olivine. No daughter olivine has been found. The number for
Supplementary Information Supplementary Tables Major element compositions of host and daughter crystals: Table 1 reports composition for host-olivine. No daughter olivine has been found. The number for
Engineering Geology ECIV 2204
 Engineering Geology ECIV 2204 2017-2016 Chapter (4) Volcanoes Chapter 4: Volcanoes and Other Igneous Activity cataclysmic relating to or denoting a violent natural even Eventually the entire
Engineering Geology ECIV 2204 2017-2016 Chapter (4) Volcanoes Chapter 4: Volcanoes and Other Igneous Activity cataclysmic relating to or denoting a violent natural even Eventually the entire
Earth Science 232 Petrography
 Earth Science 232 Petrography Course notes by Shaun Frape and Alec Blyth Winter 2002 1 Petrology - Introduction Some Definitions Petra Greek for rock Logos Greek for disclosure or explanation Petrology
Earth Science 232 Petrography Course notes by Shaun Frape and Alec Blyth Winter 2002 1 Petrology - Introduction Some Definitions Petra Greek for rock Logos Greek for disclosure or explanation Petrology
Olivine-hosted melt inclusions in Hawaiian picrites: equilibration, melting, and plume source characteristics
 Chemical Geology 183 (2002) 143 168 www.elsevier.com/locate/chemgeo Olivine-hosted melt inclusions in Hawaiian picrites: equilibration, melting, and plume source characteristics Marc D. Norman a, *, Michael
Chemical Geology 183 (2002) 143 168 www.elsevier.com/locate/chemgeo Olivine-hosted melt inclusions in Hawaiian picrites: equilibration, melting, and plume source characteristics Marc D. Norman a, *, Michael
How 2 nd half labs will work
 How 2 nd half labs will work Continue to use your mineral identification skills Learn to describe, classify, interpret rock hand samples: Igneous sedimentary metamorphic volcanic plutonic (1 week) (1 wk)
How 2 nd half labs will work Continue to use your mineral identification skills Learn to describe, classify, interpret rock hand samples: Igneous sedimentary metamorphic volcanic plutonic (1 week) (1 wk)
Spot Name U-Pb ages (Ma) Plagioclase ages (Ma) Biotite age (Ma) Whole rock age (Ma)
 Table 1. Average U-Pb ages from this study in comparison with previous ages from Sherrod and Tosdal (1991, and references therein). Previous study ages are reported as ranges including uncertainty (i.e.
Table 1. Average U-Pb ages from this study in comparison with previous ages from Sherrod and Tosdal (1991, and references therein). Previous study ages are reported as ranges including uncertainty (i.e.
GY 111: Physical Geology
 UNIVERSITY OF SOUTH ALABAMA GY 111: Physical Geology Lecture 9: Extrusive Igneous Rocks Instructor: Dr. Douglas W. Haywick Last Time 1) The chemical composition of the crust 2) Crystallization of molten
UNIVERSITY OF SOUTH ALABAMA GY 111: Physical Geology Lecture 9: Extrusive Igneous Rocks Instructor: Dr. Douglas W. Haywick Last Time 1) The chemical composition of the crust 2) Crystallization of molten
Pyroxenes (Mg, Fe 2+ ) 2 Si 2 O 6 (monoclinic) and. MgSiO 3 FeSiO 3 (orthorhombic) Structure (Figure 2 of handout)
 Pyroxenes (Mg, Fe 2+ ) 2 Si 2 O 6 (monoclinic) and 20 MgSiO 3 FeSiO 3 (orthorhombic) Structure (Figure 2 of handout) Chain silicate eg Diopside Mg and Fe ions link SiO 3 chains The chain runs up and down
Pyroxenes (Mg, Fe 2+ ) 2 Si 2 O 6 (monoclinic) and 20 MgSiO 3 FeSiO 3 (orthorhombic) Structure (Figure 2 of handout) Chain silicate eg Diopside Mg and Fe ions link SiO 3 chains The chain runs up and down
Igneous Processes I: Igneous Rock Formation, Compositions, and Textures
 Igneous Processes I: Igneous Rock Formation, Compositions, and Textures Crustal Abundances of Rock Types Igneous Rocks Form by the cooling and hardening (crystallization/glassification) of magma. There
Igneous Processes I: Igneous Rock Formation, Compositions, and Textures Crustal Abundances of Rock Types Igneous Rocks Form by the cooling and hardening (crystallization/glassification) of magma. There
Straddling the tholeiitic/calc-alkaline transition: the effects of modest amounts of water on magmatic differentiation at Newberry Volcano, Oregon
 Straddling the tholeiitic/calc-alkaline transition: the effects of modest amounts of water on magmatic differentiation at Newberry Volcano, Oregon The MIT Faculty has made this article openly available.
Straddling the tholeiitic/calc-alkaline transition: the effects of modest amounts of water on magmatic differentiation at Newberry Volcano, Oregon The MIT Faculty has made this article openly available.
EMMR25 Mineralogy: Ol + opx + chlorite + cpx + amphibole + serpentine + opaque
 GSA Data Repository 2017365 Marshall et al., 2017, The role of serpentinite derived fluids in metasomatism of the Colorado Plateau (USA) lithospheric mantle: Geology, https://doi.org/10.1130/g39444.1 Appendix
GSA Data Repository 2017365 Marshall et al., 2017, The role of serpentinite derived fluids in metasomatism of the Colorado Plateau (USA) lithospheric mantle: Geology, https://doi.org/10.1130/g39444.1 Appendix
Chapter 4 Rocks & Igneous Rocks
 Chapter 4 Rocks & Igneous Rocks Rock Definition A naturally occurring consolidated mixture of one or more minerals e.g, marble, granite, sandstone, limestone Rock Definition Must naturally occur in nature,
Chapter 4 Rocks & Igneous Rocks Rock Definition A naturally occurring consolidated mixture of one or more minerals e.g, marble, granite, sandstone, limestone Rock Definition Must naturally occur in nature,
Lecture 24 Hawaii. Hawaii
 Lecture 24 Hawaii Friday, April 22 nd 2005 Hawaii The Hawaiian Islands, in the middle of the Pacific Ocean, are volcanic islands at the end of a long chain of submerged volcanoes. These volcanoes get progressively
Lecture 24 Hawaii Friday, April 22 nd 2005 Hawaii The Hawaiian Islands, in the middle of the Pacific Ocean, are volcanic islands at the end of a long chain of submerged volcanoes. These volcanoes get progressively
Igneous Rock. Magma Chamber Large pool of magma in the lithosphere
 Igneous Rock Magma Molten rock under the surface Temperature = 600 o 1400 o C Magma Chamber Large pool of magma in the lithosphere Magma chamber - most all magma consists of silicon and oxygen (silicate)
Igneous Rock Magma Molten rock under the surface Temperature = 600 o 1400 o C Magma Chamber Large pool of magma in the lithosphere Magma chamber - most all magma consists of silicon and oxygen (silicate)
SUPPLEMENTARY INFORMATION
 GSA Data Repository 080 Schorn et al., 08, Thermal buffering in the orogenic crust: Geology, https://doi.org/0.30/g4046.. SUPPLEMENTARY INFORMATION 3 PHASE DIAGRAM MODELING 4 5 6 7 8 9 0 3 4 Phase diagrams
GSA Data Repository 080 Schorn et al., 08, Thermal buffering in the orogenic crust: Geology, https://doi.org/0.30/g4046.. SUPPLEMENTARY INFORMATION 3 PHASE DIAGRAM MODELING 4 5 6 7 8 9 0 3 4 Phase diagrams
Student Name: College: Grade:
 Student Name: College: Grade: Physical Geology Laboratory IGNEOUS MINERALS AND ROCKS IDENTIFICATION - INTRODUCTION & PURPOSE: In this lab you will learn to identify igneous rocks in hand samples from their
Student Name: College: Grade: Physical Geology Laboratory IGNEOUS MINERALS AND ROCKS IDENTIFICATION - INTRODUCTION & PURPOSE: In this lab you will learn to identify igneous rocks in hand samples from their
GSA DATA REPOSITORY
 GSA DATA REPOSITORY 2012161 Allan et al. SUPPLEMENTARY INFORMATION Summary of Magma Types Table DR1 summarizes some of the key petrologic, geochemical and physical characteristics of the three magma types
GSA DATA REPOSITORY 2012161 Allan et al. SUPPLEMENTARY INFORMATION Summary of Magma Types Table DR1 summarizes some of the key petrologic, geochemical and physical characteristics of the three magma types
WORKING WITH ELECTRON MICROPROBE DATA FROM A HIGH PRESSURE EXPERIMENT CALCULATING MINERAL FORMULAS, UNIT CELL CONTENT, AND GEOTHERMOMETRY
 WORKING WITH ELECTRON MICROPROBE DATA FROM A HIGH PRESSURE EXPERIMENT CALCULATING MINERAL FORMULAS, UNIT CELL CONTENT, AND GEOTHERMOMETRY Brandon E. Schwab Department of Geology Humboldt State University
WORKING WITH ELECTRON MICROPROBE DATA FROM A HIGH PRESSURE EXPERIMENT CALCULATING MINERAL FORMULAS, UNIT CELL CONTENT, AND GEOTHERMOMETRY Brandon E. Schwab Department of Geology Humboldt State University
High-T heating stage: application for igneous petrogenesis and mantle processes - melt inclusions as key tools -
 High-T heating stage: application for igneous petrogenesis and mantle processes - melt inclusions as key tools - SZABÓ, Csaba Lithosphere Fluid Research Lab (LRG), Department of Petrology and Geochemistry,
High-T heating stage: application for igneous petrogenesis and mantle processes - melt inclusions as key tools - SZABÓ, Csaba Lithosphere Fluid Research Lab (LRG), Department of Petrology and Geochemistry,
YUKI SUZUKI 1 * AND SETSUYA NAKADA 2
 JOURNAL OF PETROLOGY VOLUME 48 NUMBER 8 PAGES1543^1567 2007 doi:10.1093/petrology/egm029 Remobilization of Highly Crystalline Felsic Magma by Injection of Mafic Magma: Constraints from the Middle Sixth
JOURNAL OF PETROLOGY VOLUME 48 NUMBER 8 PAGES1543^1567 2007 doi:10.1093/petrology/egm029 Remobilization of Highly Crystalline Felsic Magma by Injection of Mafic Magma: Constraints from the Middle Sixth
Differentiation of Magmas By Fractional Crystallization
 Wirth Magmatic Differentiation Using M&M s 1 HANDOUT Differentiation of Magmas By Fractional Crystallization Objective The objective of this exercise is to gain first-hand knowledge of the process of magmatic
Wirth Magmatic Differentiation Using M&M s 1 HANDOUT Differentiation of Magmas By Fractional Crystallization Objective The objective of this exercise is to gain first-hand knowledge of the process of magmatic
Igneous and Metamorphic Rock Forming Minerals. Department of Geology Mr. Victor Tibane SGM 210_2013
 Igneous and Metamorphic Rock Forming Minerals Department of Geology Mr. Victor Tibane 1 SGM 210_2013 Intrusive and Effusive Rocks Effusive rocks: rapid cooling small crystalls or glas Lava & ash Magmatic
Igneous and Metamorphic Rock Forming Minerals Department of Geology Mr. Victor Tibane 1 SGM 210_2013 Intrusive and Effusive Rocks Effusive rocks: rapid cooling small crystalls or glas Lava & ash Magmatic
Chapter 4 Up from the Inferno: Magma and Igneous Rocks
 Chapter 4 Up from the Inferno: Magma and Igneous Rocks Up from the Inferno: Magma and Igneous Rocks Updated by: Rick Oches, Professor of Geology & Environmental Sciences Bentley University Waltham, Massachusetts
Chapter 4 Up from the Inferno: Magma and Igneous Rocks Up from the Inferno: Magma and Igneous Rocks Updated by: Rick Oches, Professor of Geology & Environmental Sciences Bentley University Waltham, Massachusetts
From Punchbowl to Panum: Long Valley Volcanism and the Mono-Inyo Crater Chain
 From Punchbowl to Panum: Leslie Schaffer E105 2002 Final Paper Long Valley Volcanism and the Mono-Inyo Crater Chain Figure 1. After a sequence of earthquakes during the late 1970 s to the early 1980 s
From Punchbowl to Panum: Leslie Schaffer E105 2002 Final Paper Long Valley Volcanism and the Mono-Inyo Crater Chain Figure 1. After a sequence of earthquakes during the late 1970 s to the early 1980 s
The role of bubble formation in volcanic eruption
 The role of bubble formation in volcanic eruption Eyal Goldmann Division of Natural Sciences, El Camino College, Torrance, CA, 90506 Prepared for Geology 1 at El Camino College Fall 2009 1 1. Introduction
The role of bubble formation in volcanic eruption Eyal Goldmann Division of Natural Sciences, El Camino College, Torrance, CA, 90506 Prepared for Geology 1 at El Camino College Fall 2009 1 1. Introduction
Trace Elements. Today s lecture
 Trace Elements 300 Ni 200 ppm 100 0 300 Zr 200 100 0 40 50 60 70 80 SiO 2 wt. % Updates: M&M due date: Tuesday Today s lecture Topics: Trace element compositions Trace element behavior Partitioning Spider(
Trace Elements 300 Ni 200 ppm 100 0 300 Zr 200 100 0 40 50 60 70 80 SiO 2 wt. % Updates: M&M due date: Tuesday Today s lecture Topics: Trace element compositions Trace element behavior Partitioning Spider(
Case History: Mt. St. Helens
 Case History: Mt. St. Helens EAS 458 Volcanology Introduction 1980 eruption of Mt. St. Helens was particularly interesting and violent eruption with an unusual lateral blast. In the 1970 s, the USGS (Crandell(
Case History: Mt. St. Helens EAS 458 Volcanology Introduction 1980 eruption of Mt. St. Helens was particularly interesting and violent eruption with an unusual lateral blast. In the 1970 s, the USGS (Crandell(
XM1/331 XM1/331 BLFX-3 XM1/331
 a b AkC AkC strontian fluoro-apatite clinopyroxene phlogopite K-richterite XM1/331 clinopyroxene XM1/331 Fe-Ti ox c d clinopyroxene kric AkC ilmenite Sr-barite AkC XM1/331 BLFX-3 Supplementary Figure 1.
a b AkC AkC strontian fluoro-apatite clinopyroxene phlogopite K-richterite XM1/331 clinopyroxene XM1/331 Fe-Ti ox c d clinopyroxene kric AkC ilmenite Sr-barite AkC XM1/331 BLFX-3 Supplementary Figure 1.
UV-V-NIR Reflectance Spectroscopy
 UV-V-NIR Reflectance Spectroscopy Methods and Results A. Nathues Naturally-occurring inorganic substances with a definite and predictable chemical composition and physical properties Major groups: Silicates
UV-V-NIR Reflectance Spectroscopy Methods and Results A. Nathues Naturally-occurring inorganic substances with a definite and predictable chemical composition and physical properties Major groups: Silicates
CO 2 quantification in magmatic systems
 CO 2 quantification in magmatic systems Innovative coupling of X-ray micro-tomography, in-situ microanalysis and thermodynamic modelling By Laura Créon Créon et al. (2018) Problematics 7 years work to
CO 2 quantification in magmatic systems Innovative coupling of X-ray micro-tomography, in-situ microanalysis and thermodynamic modelling By Laura Créon Créon et al. (2018) Problematics 7 years work to
