Development of huperzine A and B for treatment of Alzheimer s disease
|
|
|
- Amber Pitts
- 6 years ago
- Views:
Transcription
1 Pure Appl. Chem., Vol. 79, No. 4, pp , doi: /pac IUPAC Development of huperzine A and B for treatment of Alzheimer s disease Donglu Bai Shanghai Institute of Materia Medica, Chinese Academy of Sciences, 555 Zuchongzhi Road, Shanghai, , China Abstract: Recent studies have proved that huperzine A (HupA) possesses different pharmacological actions other than the inhibition of hydrolysis of ACh. These noncholinergic roles, for instance, the antagonist effect on NMDA receptor, the protection of neuronal cells against β-amyloid, free radicals, and hypoxia-ischemia-induced injury, could be important too in Alzheimer s disease (AD) treatment. The therapeutic effects of HupA are probably based on a multitarget mechanism. By targeting dual active sites of AChE, a series of bis- and bifunctional HupB compounds with various lengths of tether were designed, synthesized, and tested for the inhibition and selectivity of AChE. The most potent bis-hupb compound exhibited increase by three orders of magnitude in AChE inhibition and two orders of magnitude in selectivity for AChE than its parent HupB. Keywords: acetylcholinesterase inhibitors; butyrocholinesterase inhibitors; huperzine A; huperzine B; Alzheimer s disease; dimers. INTRODUCTION Millions of years of plant evolution have led to the development of many secondary metabolites with various and unique chemical structures that scientists have not even thought of. So far, less than 20 % of all plant species have been evaluated chemically or biologically. Therefore, medicinal plants remain an important source in the search for new leads, new chemical entities of drugs, and new mechanisms of drug action as well [1,2]. On earth, there are at least species of higher plants. They contain a much greater diversity of bioactive compounds than any chemical library. Many more useful drugs will be found in the plant kingdom if the research projects are conducted in a systematic and logical manner [3]. Alzheimer s disease (AD) is the most common form of dementia and one of the major diseases affecting elderly people. The cholinergic enhancement strategy has now been one of the major efforts to pharmacologically palliate the cognitive symptoms. To date, the most effective approach has been the use of acetylcholinesterase (AChE) inhibitors, such as tacrine, donepezil, and rivastigmine. In China, huperzine A (HupA) is the first-choice drug in the treatment of AD. *Pure Appl. Chem. 79, (2007). An issue of reviews and research papers based on lectures presented at the 25 th International Symposium on Chemistry of Natural Products (ISCNP-25) and 5 th International Conference on Biodiversity (ICOB-5), held jointly in Kyoto, Japan, July 2006, on the theme of natural products. 469
2 470 D. BAI HUPERZINE A The Lycopodium alkaloid HupA (1) was isolated from a Chinese folk medicine, Huperzia serrata (Thunb.) Trev., by Liu and his coworker in 1986 [4 6]. This club moss has been used in China for the treatment of contusion, strain, swelling, and schizophrenia. The total alkaloids from this plant could alleviate the symptom of myasthenia gravis. Occurrence of HupA ( )-HupA occurs in several plant families such as Huperziaceae, Lycopodiaceae, and Selaginella. From 1995 to 2001, an investigation of the natural resources of the Huperziaceae family in China was carried out to catalog the occurrence, general distribution, and abundance of various species [7 9]. In the Huperziaceae family, there are two genera, huperzia and phlegmariurus. The yields of HupA in dried herb of Huperzia serrata range from to % depending on the collecting seasons and growing regions. In this genus, herteriana, ovatifolia, and serrata species have higher yields of HupA than others. In Phlegmariurus genus, all of the 11 species investigated have much higher contents of HupA than Huperzia plants. The highest contents of HupA were found in carinatus and mingcheensis species [7]. In H. serrata whole plant, the yield of HupA varied by 33 % throughout the year. Samples collected in October had the highest yield (97.6 µg/g), whereas the lowest yield (64.0 µg/g) was observed in late winter through spring. The yield of HupA also varied dramatically by tissue. The highest yield was found in leaves (111.8 µg/g), whereas the roots and sporangia had much lower levels (24.9 and 37.7 µg/g, respectively). Most recently, Ma et al. have succeeded in propagating Phlegmariurus squarrosus. The cultured plants may provide a new source of HupA [7]. Pharmacological and clinical studies of HupA The anticholinesterase activities of HupA were evaluated in vivo, and significant inhibitions of AChE were observed in the cortex, hippocampus, and striatum in rats. There clearly is a dose-dependent inhibition of AChE in the brain region by HupA. In contrast to donepezil and tacrine, HupA has a higher oral bioavailability [10,11]. Studies using microdialysis technique in rats showed that HupA elevated dose-dependently the level of ACh in cortex. (Fig. 1, results shown as mean ± SEM. *P < 0.05, **P < 0.01 vs. baseline.) The time course of cortical AChE inhibition by HupA is positively correlated with the increase of ACh. (Fig. 1, #P < 0.05, ##P < 0.01 vs. saline control.) According to the doses of inhibitors, HupA was 8- and 2-fold more potent than donepezil and rivastigmine, respectively, in increasing cortical ACh levels with a longer-lasting effect [12]. The improving effect of HupA in memory deficits is more potent on working memory than on reference memory compared with donepezil and tacrine. This effect may be beneficial in AD treatment, because the cognitive deficits in AD patients are severe for the memory of recent events [13 15].
3 Development of huperzine A and B 471 Fig. 1 Comparative effects of HupA, donepezil, and rivastigmine on the cortex ACh levels and AChE activity in rats after ip injection. To study the efficacy of HupA on memory and learning performance of students, HupA has been tested in 34 pairs of middle school students complaining of memory inadequacy by using double-blind and matched-pair method. The results of this study exhibited that HupA markedly improved the memory function of adolescent students [11,17]. Recent pharmacological studies have proved that HupA possesses different bioactivities other than the inhibition of hydrolysis of ACh. These noncholinergic effects including the antagonistic effect on NMDA receptor, the protection of neuronal cells against cytotoxicity and apoptosis induced by β-amyloid, free radicals, and hypoxia-ischemia could also be important in AD treatment [20]. Interestingly, the neuroprotective effect of HupA is not correlated with its AChE inhibition, suggesting that the therapeutic effect of HupA may be exerted via a multitarget mechanism [11,18]. Synthetic studies of HupA Because of the unique bioactivity and low yields in plants, several groups have devoted intensive efforts to the chemical synthesis of HupA. It possesses the rigid bicyclo [3.3.1]nonene skeleton and fused pyridone ring. The retrosynthetic analysis of this molecule is shown in Scheme 1. Scheme 1 The β-keto ester functionalities in key intermediate 3 would provide not only an activating group for the construction of three-carbon bridged compound 2 by tandem Michael-aldol reaction of 3 and methacrolein, but also the latent groups for the formation of both ethylidene substitution by Wittig reaction and amino group via Curtius rearrangement of the acid from ester 3. Endocyclic double bond may be formed by dehydration of the aldol product. The pyridone ring could be protected as a stable
4 472 D. BAI methoxy pyridine. Based on the synthetic strategy in Scheme 1, the total synthesis of racemic and natural ( )-HupA have been accomplished by several groups [18 20]. The first synthesis of HupA was reported by Ji s group [21]. However, the overall yield of HupA by this approach, especially the yield of dehydration of aldol adduct, is unsatisfactory. In preparation of the key intermediate 3, a four-step approach from readily available dimethyl 4-oxopimelate was developed. The enamine 4 was directly condensed with propynamide to give pyridone 5 with two desirable carbomethoxy side chains. After O-methylation, Dieckmann condensation of diester 6 gave 3 in 39 % overall yield (Scheme 2) [22 24]. Scheme 2 In order to avoid the low-yielding step of elimination of the aldol product, the palladium-catalyzed bicycloannulation of β-keto ester 3 was reported by Kozikowski s group [25] using tetramethylguanidine as base and 2-methylene-1,3-propanediol diacetate as bis-electrophile in the presence of tetrakis (triphenylphosphine)-palladium(0). The resulting methylene-bridged compound 7 was in 92 % yield. The double-bond migration was pursued with triflic acid, affording endocyclic olefine 9 in 90 % yield. Since the double-bond migration could be performed in the last step, an isomer 8 was obtained. The overall yield of HupA was 40 % from β-keto ester 3 (Scheme 3) [25]. Scheme 3
5 Development of huperzine A and B 473 Based on the same stratagy, the stereoselective synthesis of HupA was tested by using different chiral ligands instead of triphenylphosphine. Terashima s group reported that the bridged compound 7 obtained by stereoselective bicycloannulation was in 64 % ee using ferrocenylphosphine (R,S)-10 as the chiral ligand [26]. It is expected that fine-tuning of the size of the N-substituents of ligand 10 with an appropriate ω-hydroxy chain would induce a favorable effect on the enantioselectivity of the reaction. Therefore, a number of chiral bis(diphenylphosphino)ferrocenyl ligands 11 and 12 were designed, synthesized, and tested for the stereoselective allylic bicycloannulation of β-keto ester 3 with 2-methylene-1, 3-propanediol diacetate. It seems that for the better stereoselectivity of the reaction induced by the chiral ligands 11 and 12, the R 1 alkyl group should be bigger than propyl, and R 2 should be an ω-hydroxypentyl group. To our delight, a 90 % ee of the bridged compound 7 was obtained by using (R,S)-ferrocenylphosphine 12a and 12b as chiral ligands. With the most efficient chiral ligands 12a and 12b in hand, the chiral nonracemic product 7 was obtained in the desired configuration for the synthesis of natural ( )-HupA (Scheme 3). Consequently, (+)-HupA could also be obtained by (S,R)-12a or 12b as the chiral ligand for the reaction [27 29]. Analogs and derivatives of HupA Many analogs and derivatives of HupA were prepared and tested for their inhibitory activities of AChE. Unfortunately, neither the structurally simplified analogs nor the derivatives from the natural HupA exhibited the anti-ache potency as HupA itself except 10-methyl HupA and a few of the Schiff bases of ( )-HupA [19,20,30].
6 474 D. BAI ZT-1 is a Schiff base of HupA and 5-chloro-2-hydroxy-3-methoxybenzaldehyde. As a prodrug, it is hydrolyzed nonenzymatically into the active compound HupA. In vitro, the AChE inhibitory activities of ZT-1 and HupA are in the same range. In vivo, a marked dose-dependent inhibition of AChE in brain by ZT-1 was observed in rats, and it was similar to HupA. A study in monkeys showed that ZT-1 reversed the memory deficits induced by scopolamine in young adult and aged monkeys. Phase I clinical studies demonstrated it was safe and well tolerated. The oral bioavailability of ZT-1 was better than HupA. Phase II clinical trials for efficacy assessment in mild and moderate AD patients is now underway in Europe [31]. HUPERZINE B Huperzine B (HupB, 13), the minor alkaloid in the plant H. serrata, is less potent and selective in the inhibition of AChE than HupA. However, it exhibited a higher therapeutic index. In behavioral studies, HupB improved memory retention and memory retrieval in adult and aged mice, and reversed the disruption of memory retention induced by scopolamine, sodium nitrite, electroconvulsive shock, and cycloheximide in mice. Recent studies also indicated that HupB had neuroprotective effect by attenuating hydrogen peroxide-induced injury [32 34]. The first synthesis of (±)-HupB was accomplished by Bai s group [35]. The synthetic approach to HupB is straightforward and especially efficient in regard to the construction of the tetracyclic ring skeleton via a tandem Michael addition and intramolecular Mannich cyclization. This approach can also serve in the preparation of analogs of HupB. Bis- and bifunctional HupB compounds Based on the homodimer and heterodimer strategy in drug design, Pang et al. [36,37] reported that bis(7)-tacrine, the heptylene-linked tacrine dimer, possessed both optimal AChE inhibitory potency and selectivity than tacrine itself. Recent studies suggest that AChE inhibitors may simultaneously alleviate cognitive deficits and behave as disease-modifying agents by inhibiting the β-amyloid aggregation through binding to both central catalytic and peripheral active sites of AChE [38]. It is supposed that a linking chain bearing nitrogen atoms in dimers may favorably interact via cation-π-electron hydrogen bonds with some of the aromatic residues in the active gorge of AChE. The structure activity relationship (SAR) studies reveal that all O-substituted derivatives of HupA are inactive in AChE inhibition. The X-ray analysis of TcAChE-( )-HupB complex has also demonstrated that pyridone moiety in HupB is responsible for the key interaction with the catalytic site of the enzyme via hydrogen bonding [39]. Therefore, a series of bis-hupb and bifunctional HupB compounds, which may be able to bind both catalytic and peripheral binding sites of AChE, were thus designed and synthesized [40]. Bis-HupB compounds are composed of two HupB moieties linked with each other on the amine nitrogen by carbon nitrogen chains. In bifunctional HupB compounds 20, one HupB moiety of bis-hupb compounds is replaced by an aryl group.
7 Development of huperzine A and B 475 For the preparation of bis-hupb compounds, HupB was first acylated with chloroacetyl chloride or acryloyl chloride to afford chloroacetyl HupB 21 (96 % yield) and acryloyl HupB 22 (95 % yield), respectively. Reaction of piperazine, homopiperazine, cyclopropyl amine, propylamine, or α,ω-n,n'-dimethylalkanediamines with chloroacetyl HupB 21 proceeded smoothly in acetonitrile in the presence of potassium carbonate and potassium iodide, affording bis-hupb 14a, 14b, 16a, 16b, and 18a e, respectively. Michael addition of acryloyl HupB 22 and the corresponding amines mentioned above was conducted in acetonitrile, catalyzed by silica gel, furnishing bis-hupb 14c, 14d, and 18f-I, respectively (Scheme 4). Reduction of 14, 16, and 18 with LAH gave the reduced bis-hupb 15, 17, and 19, respectively [40,41].
8 476 D. BAI Scheme 4 In the preparation of bifunctional HupB compounds, α,ω-n,n'-dimethylalkanediamines were first alkylated with arylmethyl chloride, giving N,N'-dimethyl-N-arylmethylalkanediamines. Reaction of N,N'-dimethyl-N-arylmethylalkanediamines with chloroacetyl HupB 21 or acryloyl HupB 22 furnished bifunctional HupB compounds 20 (Scheme 4) [41]. All the bis- and bifunctional HupB compounds were tested for their ChE inhibition and selectivity for AChE. In bis-hupb compounds, the most potent one is bis(18)-hupb (18h), and in bifunctional HupB compounds, the most potent is bifunctional(15)-hupb (20f) (Table 1). Table 1 ChE inhibition and selectivity of bis- and bifunctional HupB compounds. a Compounds Atom numbers AChE BuChE Selectivity of tether (IC 50, nm) b (IC 50, µm) c for AChE d HupA 72.4 ± ± HupB ± ± h ± ± f ± ± a Assay performed by the modified Ellman method at ph = 7.4. Results are the mean ± SD. b Assay performed using rat cortex homogenate in aqueous solution. c Assay performed using rat serum. d Selectivity for AChE is defined as IC 50 (BuChE)/IC 50 (AChE). In comparison with HupB, most of the bis-hupb and bifunctional HupB compounds are much more potent in the inhibition of AChE. The most potent ones, 18h and 20f, exhibited increase by three orders of magnitude in AChE inhibition and by two orders of magnitude in selectivity for AChE vs. BuChE than its parent HupB. The length of the tether plays a key role in the inhibition and the selectivity of AChE. The optimal length of the tethers is in a range of atoms. On an equimolar basis, 18h is 3-fold more potent in AChE inhibition than donepezil in mice. No significant difference was observed in AChE inhibition of 18h between oral and intraperitoneal admin-
9 Development of huperzine A and B 477 istrations. Mice water-maze studies showed that 18h remarkably improved scopolamine- and ischemiainduced spatial performance deficits. In addition, 18h also attenuated β-amyloid and oxygen-glucose deprivation-induced cytotoxicity and oxidative stress as well as staurosporine-induced apoptosis [42]. Docking studies of bis (18)-HupB (18h) In order to explore the possible binding conformation and interaction mode of the bis-hupb compounds with AChE, a molecular modeling study was performed, employing the docking program DOCK4.0 based on the structure of the complex of TcAChE with HupB (PDB entry 1GPN). Since bis(18)-hupb (18h) afforded maximum inhibitory potency, it was selected for docking simulations. Both sp 3 N atoms in 18h were treated as protonated. The central catalytic pocket, peripheral pocket, and the binding gorge were taken as the targets in the docking simulation. In the search for multiple anchors, the TcAChE structure was kept fixed and the bis(18)-hupb (18h) flexible while the maximum-orientations and configurations-per-cycle parameters were set to 600 and 150, respectively [40]. The docking results demonstrated that one HupB moiety in 18h interacted with TcAChE in the central catalytic pocket near the residue Trp84, and another HupB moiety interacted in the peripheral pocket near the residue Trp279 (Fig. 2). The binding position and mode of the central catalytic HupB moiety is similar to HupB in the crystal structure of TcAChE-HupB complex (1GPN), having two hydrogen bonds with Tyr130 and hydrophobic interaction with Trp84, Gly118, Gly119, Phe290, Phe330, Phe331, and His440. The other HupB moiety in the peripheral pocket interacted with Asn280 through a hydrogen bond, and with Trp279 and Leu282 via hydrophobic interaction. The long tether could fold with a proper conformation in the gorge that might favorably interact with Tyr70, Asp72, Tyr121, Trp279, Ile287, Phe330, and Tyr334 through hydrophobic interaction (Fig. 2). The simultaneous interactions of 18h in the central pocket, gorge passage, and peripheral pocket of TcAChE suggest the reason for the high inhibitory potency of AChE. Fig. 2 Interaction between bis(18)-hupb (18h) and TcAChE.
10 478 D. BAI CONCLUSIONS HupA has better penetration through blood brain barrier, higher oral bioavailability, and longer duration of AChE inhibition in comparison with other well-known AChE inhibitors. Clinical trials have demonstrated that HupA produces significant improvements in memory deficits in aged and AD patients. The HupA AChE complex has a longer life than other prophylactic sequestering agents, so HupA has now been proposed as a pretreatment drug for organophosphonates nerve agents. In addition, HupA possesses different pharmacological actions other than the inhibition of hydrolysis of ACh. These noncholinergic roles for instance, the antagonist effect on NMDA receptor, the protection of neuronal cells against β-amyloid, free radicals, and hypoxia-ischemia-induced injury could be important too in AD treatment. The therapeutic effects of HupA are probably based on a multitarget mechanism. Studies on the interactions between AChE and bis-hupb or bifunctional HupB compounds at the atomic level by molecular docking methods and X-ray crystallographic analysis are valuable for designing new ChE inhibitors with improved therapeutic profiles for AD treatment. ACKNOWLEDGMENTS The contributions of the collaborators, Profs. Jiasen Liu, Dayuan Zhu, Xican Tang, Xuchang He, Hualiang Jiang, J. L. Sussman, Ruyun Ji, and S.S. Xu, and postgraduate students whose names are mentioned in the references are deeply appreciated. We gratefully acknowledge the financial supports from National Natural Science Foundation of China (Grants , , , , , , , and ), the State Key Program of Basic Research of China (Grant 2002CB512802), and the 863 Hi-Tech Program of China (Grants 2002AA233061, 2001AA235051, and 2001AA235041). REFERENCES 1. M. S. Butler. J. Nat. Prod. 67, 2141 (2004). 2. G. A. Cordell, M. D. Colvard. J. Ethnopharmacol. 100, 14 (2005). 3. I. Raskin, D. M. Ribnicky, S. Komarnytsky, N. Ilic, A. Poulev, N. Borisjuk, A. Brinker, D. A. Moreno, C. Ripoll, N. Yakoby, J. M. O Neal, T. Cornwell, I. Postor, B. Fridlender. Trends Biotechnol. 20, 522 (2002). 4. J. S. Liu, C. M. Yu, Y. Z. Zhou, Y. Y. Han, F. W. Wu, B. F. Qi, Y. L. Zhu. Acta Chim. Sin. 44, 1035 (1986). 5. J. S. Liu, Y. L. Zhu, C. M. Yu, Y. Z. Zhou, Y. Y. Han, F. W. Wu, B. F. Qi. Can. J. Chem. 64, 837 (1986). 6. C. M. Yu, X. C. Tang, J. S. Liu. U.S. Patent 5,177,082, Jan. 5, X. Q. Ma, C. H. Tan, D. Y. Zhu, D. R. Gang. J. Agric. Food Chem. 53, 1393 (2005). 8. X. Q. Ma, C. H. Tan, D. Y. Zhu, D. R. Gang. J. Ethnopharmacol. 104, 54 (2006). 9. X. Q. Ma, D. R. Gang. Nat. Prod. Rep. 21, 752 (2004). 10. D. H. Cheng, X. C. Tang. Pharmacol., Biochem. Behav. 60, 377 (1998). 11. H. Wang, X. C. Tang. Acta Pharmacol. Sin. 19, 27 (1998). 12. Y. Q. Liang, X. C. Tang. Neurosci. Lett. 361, 56 (2004). 13. Z. Q. Xiong, D. H. Cheng, X. C. Tang. Acta Pharmacol. Sin. 19, 128 (1998). 14. L. Y. Ou, X. C. Tang, J. X. Cai. Eur. J. Pharmacol. 433, 151 (2001). 15. T. Wang, X. C. Tang. Eur. J. Pharmacol. 349, 137 (1998). 16. R. Wang, H. Yan, X. C. Tang. Acta Pharmacol. Sin. 27, 1 (2006) and refs. cited therein. 17. Q. Q. Sun, S. S. Xu, J. L. Pan, H. M. Guo, W. Q. Cao. Acta Pharmacol. Sin. 20, 601 (1999).
11 Development of huperzine A and B D. L. Bai, Z. F. Wang, S. Feng, X. C. Tang. In Advances in Pharmaceutical Chemistry, D. L. Bai, K. X. Chen (Eds.), pp , Chemical Industry Press, Beijing (2005). 19. D. L. Bai, X. C. Tang, X. C. He. Curr. Med. Chem. 7, 355 (2000) and refs. cited therein. 20. H. L. Jiang, X. M. Luo, D. L. Bai. Curr. Med. Chem. 10, 2231 (2003). 21. L. G. Qian, R. Y. Ji. Tetrahedron Lett. 30, 2089 (1989). 22. W. P. Chen, F. Q. Yang. Chin. J. Med. Chem. 2, 34 (1992). 23. S. Feng, X. C. He, G. L. Yu, Y. Xia, D. L. Bai. Org. Prep. Proc. Int. 36, 129 (2004). 24. L. G. Qian, K. J. Gu, R. Y. Ji. Chin. J. Med. Chem. 2, 1 (1992). 25. G. Campiani, L. Q. Sun, A. P. Kozikowski, P. Aagaard, M. McKinney. J. Org. Chem. 58, 7660 (1993). 26. S. Kaneko, T. Yoshino, T. Katoh, S. Terashima. Tetrahedron Lett. 54, 5471 (1998). 27. X. C. He, B. Wang, D. L. Bai. Tetrahedron Lett. 39, 411 (1998). 28. X. C. He, B. Wang, G. L. Yu, D. L. Bai. Tetrahedron: Asymmetry 12, 3213 (2001). 29. D. L. Bai, et al. To be published. 30. A. P. Kozikowski, W. Tuckmantel. Acc. Chem. Res. 32, 641 (1999). 31. D. Y. Zhu, C. H. Tan, Y. M. Li. In Medicinal Chemistry of Bioactive Natural Products, X. T. Liang, W. S. Fang (Eds.), pp , Wiley-Interscience, Hoboken, NJ (2006). 32. J. Liu, H. Y. Zhang, L. M. Wang, X. C. Tang. Acta Pharmacol. Sin. 20, 1415 (1999). 33. H. Y. Zhang, X. C. Tang. Neurosci. Lett. 292, 41 (2000). 34. Z. F. Wang, J. Zhou, X. C. Tang. Acta Pharmacol. Sin. 23, 1193 (2002). 35. B. G. Wu, D. L. Bai. J. Org. Chem. 62, 5978 (1997). 36. Y. P. Pang, P. Quiram, T. Jelacic, F. Hong, S. Brimijoin. J. Biol. Chem. 271, (1996). 37. P. R. Carlier, Y. F. Han, E. S. H. Chow, C. P. L. Li, H. Wang, T. X. Lieu, H. S. Wong, Y. P. Pang. Bioorg. Med. Chem. 7, 351 (1999). 38. P. Munoz-Ruiz, L. Rubio, E. Garcia-Palomero, I. Dorronsoro, M. Monte-Millan, R. Valenzuela, P. Usan, C. Austria, M. Bartolini, V. Andrisano, A. Bidon-Chanal, M. Orozco, F. Javier Luque, M. Medina, A. Martinez. J. Med. Chem. 48, 7223 (2005) and refs. cited therein. 39. H. Dvir, H. L. Jiang, D. M. Wong, M. Harel, M. Chetrit, X. C. He, G. Y. Jin, G. L. Yu, X. C. Tang, I. Silman, D. L. Bai, J. L. Sussman. Biochemistry 41, (2002). 40. S. Feng, Z. F. Wang, X. C. He, S. X. Zheng, Y. Xia, H. L. Jiang, X. C. Tang, D. L. Bai. J. Med. Chem. 48, 655 (2005). 41. S. Feng, Y. Xia, D. M. Han, C. Y. Zhang, X. C. He, X. C. Tang, D. L. Bai. Bioorg. Med. Chem. Lett. 15, 523 (2005). 42. X. C. Tang, et al. To be published.
A Highly Efficient Organocatalyst for Direct Aldol Reactions of Ketones and Aldehydes
 A ighly Efficient rganocatalyst for Direct Aldol Reactions of Ketones and Aldehydes Zhuo Tang, Zhi-ua Yang, Xiao-ua Chen, Lin-Feng Cun, Ai-Qiao Mi, Yao-Zhong Jiang, and Liu-Zhu Gong Contribution from the
A ighly Efficient rganocatalyst for Direct Aldol Reactions of Ketones and Aldehydes Zhuo Tang, Zhi-ua Yang, Xiao-ua Chen, Lin-Feng Cun, Ai-Qiao Mi, Yao-Zhong Jiang, and Liu-Zhu Gong Contribution from the
I. Introduction. Peng Zhao. Liu lab
 Asymmetric Total Synthesis of Mycoleptodiscin A Shupeng Zhou, Hao Chen, Yijie Luo, Wenhao Zhang and Ang Li Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai
Asymmetric Total Synthesis of Mycoleptodiscin A Shupeng Zhou, Hao Chen, Yijie Luo, Wenhao Zhang and Ang Li Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai
Bioengineering & Bioinformatics Summer Institute, Dept. Computational Biology, University of Pittsburgh, PGH, PA
 Pharmacophore Model Development for the Identification of Novel Acetylcholinesterase Inhibitors Edwin Kamau Dept Chem & Biochem Kennesa State Uni ersit Kennesa GA 30144 Dept. Chem. & Biochem. Kennesaw
Pharmacophore Model Development for the Identification of Novel Acetylcholinesterase Inhibitors Edwin Kamau Dept Chem & Biochem Kennesa State Uni ersit Kennesa GA 30144 Dept. Chem. & Biochem. Kennesaw
Receptor Based Drug Design (1)
 Induced Fit Model For more than 100 years, the behaviour of enzymes had been explained by the "lock-and-key" mechanism developed by pioneering German chemist Emil Fischer. Fischer thought that the chemicals
Induced Fit Model For more than 100 years, the behaviour of enzymes had been explained by the "lock-and-key" mechanism developed by pioneering German chemist Emil Fischer. Fischer thought that the chemicals
Recent advances in transition metal-catalyzed or -mediated cyclization of 2,3-allenoic acids: New methodologies for the synthesis of butenolides*
 Pure Appl. Chem., Vol. 76, No. 3, pp. 651 656, 2004. 2004 IUPAC Recent advances in transition metal-catalyzed or -mediated cyclization of 2,3-allenoic acids: New methodologies for the synthesis of butenolides*
Pure Appl. Chem., Vol. 76, No. 3, pp. 651 656, 2004. 2004 IUPAC Recent advances in transition metal-catalyzed or -mediated cyclization of 2,3-allenoic acids: New methodologies for the synthesis of butenolides*
Efficient enantioselective hydrogenation of quinolines catalyzed by conjugated microporous polymers with embedded chiral BINAP ligand
 Chinese Journal of Catalysis 36 (2015) 1170 1174 available at www.sciencedirect.com journal homepage: www.elsevier.com/locate/chnjc Communication Efficient enantioselective hydrogenation of quinolines
Chinese Journal of Catalysis 36 (2015) 1170 1174 available at www.sciencedirect.com journal homepage: www.elsevier.com/locate/chnjc Communication Efficient enantioselective hydrogenation of quinolines
B X A X. In this case the star denotes a chiral center.
 Lecture 13 Chirality III October 29, 2013 We can also access chiral molecules through the use of something called chiral auxiliaries, which basically is a chiral attachment that you add to your molecule
Lecture 13 Chirality III October 29, 2013 We can also access chiral molecules through the use of something called chiral auxiliaries, which basically is a chiral attachment that you add to your molecule
Chimica Farmaceutica (Insegnamento Integrato di Chimica e Biotecnologie Farmaceutiche) Drug design (2)
 (Insegnamento Integrato di Chimica e Biotecnologie Farmaceutiche) Drug design (2) Molecular Variations in Homologous Series: Vinylogues and Benzologues HOMOLOGOUS SERIES. Definition and classification
(Insegnamento Integrato di Chimica e Biotecnologie Farmaceutiche) Drug design (2) Molecular Variations in Homologous Series: Vinylogues and Benzologues HOMOLOGOUS SERIES. Definition and classification
Chapter 19. Synthesis and Reactions of b-dicarbonyl Compounds: More Chemistry of Enolate Anions. ß-dicarbonyl compounds. Why are ß-dicarbonyls useful?
 Chapter 19 Synthesis and Reactions of b-dicarbonyl Compounds: More Chemistry of Enolate Anions ß-dicarbonyl compounds Two carbonyl groups separated by a carbon Three common types ß-diketone ß-ketoester
Chapter 19 Synthesis and Reactions of b-dicarbonyl Compounds: More Chemistry of Enolate Anions ß-dicarbonyl compounds Two carbonyl groups separated by a carbon Three common types ß-diketone ß-ketoester
Copper-catalyzed cleavage of benzyl ethers with diacetoxyiodobenzene and p-toluenesulfonamide
 General Papers ARKIVC 2008 (xii) 103-108 Copper-catalyzed cleavage of benzyl ethers with diacetoxyiodobenzene and p-toluenesulfonamide Ling He a,b, Qin Wang a, Guo-Chuan Zhou b, Lei Guo b, and Xiao-Qi
General Papers ARKIVC 2008 (xii) 103-108 Copper-catalyzed cleavage of benzyl ethers with diacetoxyiodobenzene and p-toluenesulfonamide Ling He a,b, Qin Wang a, Guo-Chuan Zhou b, Lei Guo b, and Xiao-Qi
Quantum Mechanical Models of P450 Metabolism to Guide Optimization of Metabolic Stability
 Quantum Mechanical Models of P450 Metabolism to Guide Optimization of Metabolic Stability Optibrium Webinar 2015, June 17 2015 Jonathan Tyzack, Matthew Segall, Peter Hunt Optibrium, StarDrop, Auto-Modeller
Quantum Mechanical Models of P450 Metabolism to Guide Optimization of Metabolic Stability Optibrium Webinar 2015, June 17 2015 Jonathan Tyzack, Matthew Segall, Peter Hunt Optibrium, StarDrop, Auto-Modeller
Bifunctional Asymmetric Catalysts: Design and Applications. Junqi Li CHEM Sep 2010
 Bifunctional Asymmetric Catalysts: Design and Applications Junqi Li CHEM 535 27 Sep 2010 Enzyme Catalysis vs Small-Molecule Catalysis Bronsted acid Lewis acid Lewis acid Bronsted base Activation of both
Bifunctional Asymmetric Catalysts: Design and Applications Junqi Li CHEM 535 27 Sep 2010 Enzyme Catalysis vs Small-Molecule Catalysis Bronsted acid Lewis acid Lewis acid Bronsted base Activation of both
ing equilibrium i Dynamics? simulations on AchE and Implications for Edwin Kamau Protein Science (2008). 17: /29/08
 Induced-fit d or Pre-existin ing equilibrium i Dynamics? Lessons from Protein Crystallography yand MD simulations on AchE and Implications for Structure-based Drug De esign Xu Y. et al. Protein Science
Induced-fit d or Pre-existin ing equilibrium i Dynamics? Lessons from Protein Crystallography yand MD simulations on AchE and Implications for Structure-based Drug De esign Xu Y. et al. Protein Science
The synthesis of molecules containing quaternary stereogenic centers via the intramolecular asymmetric Heck reaction. Eric Gillis 4/19/07
 The synthesis of molecules containing quaternary stereogenic centers via the intramolecular asymmetric Heck reaction Eric Gillis 4/19/07 Quaternary stereocenters in complex small molecules Retrosynthetic
The synthesis of molecules containing quaternary stereogenic centers via the intramolecular asymmetric Heck reaction Eric Gillis 4/19/07 Quaternary stereocenters in complex small molecules Retrosynthetic
Lycopodine & Huperzine A Story of Lycopodium Alkaloids
 Lycopodine & Huperzine A Story of Lycopodium Alkaloids Reporter: Supervisor: Zhe Niu Prof. Yang Prof. Chen Prof. Tang 2014/12/3 Content Background Synthetic Studies of Lycopodine An AChEI Huperzine A Summury
Lycopodine & Huperzine A Story of Lycopodium Alkaloids Reporter: Supervisor: Zhe Niu Prof. Yang Prof. Chen Prof. Tang 2014/12/3 Content Background Synthetic Studies of Lycopodine An AChEI Huperzine A Summury
Page 1 of 9. Sessional Examination (November 2017) Max Marks: 20 Date: Time: One Hour. Model Answers
 Page 1 of 9 Sessional Examination (November 2017) Class: B. Pharm-II yr (III sem) Subject: Pharma Org. Chem-II Max Marks: 20 Date: 14.11.2017 Time: One Hour Model Answers Q. 1. Solve the following (ANY
Page 1 of 9 Sessional Examination (November 2017) Class: B. Pharm-II yr (III sem) Subject: Pharma Org. Chem-II Max Marks: 20 Date: 14.11.2017 Time: One Hour Model Answers Q. 1. Solve the following (ANY
Literature Report 2. Divergent Asymmetric Total Synthesis of Mulinane Diterpenoids. Date :
 Literature Report 2 Divergent Asymmetric Total Synthesis of Mulinane Diterpenoids Reporter : Zhou-Hao Zhu Checker : Xiao-Yong Zhai Date : 2018-04-23 Liu, Y.-T.; Li, L.-P.; Xie, J.-H.*; Zhou, Q.-L. Angew.
Literature Report 2 Divergent Asymmetric Total Synthesis of Mulinane Diterpenoids Reporter : Zhou-Hao Zhu Checker : Xiao-Yong Zhai Date : 2018-04-23 Liu, Y.-T.; Li, L.-P.; Xie, J.-H.*; Zhou, Q.-L. Angew.
Research in our group encompasses the following 4 areas in Synthetic Organic Chemistry.
 Research in our group encompasses the following 4 areas in Synthetic Organic Chemistry. 1] Development of Asymmetric Catalysis 2] Total Synthesis of Natural products 3] Development of Novel Protecting-Group-Free
Research in our group encompasses the following 4 areas in Synthetic Organic Chemistry. 1] Development of Asymmetric Catalysis 2] Total Synthesis of Natural products 3] Development of Novel Protecting-Group-Free
Literature Report. Catalytic Enantioselective Synthesis of Isoindolinones through a Biomimetic Approach. : Zhong Yan : Ji Zhou :
 Literature Report Catalytic Enantioselective Synthesis of Isoindolinones through a Biomimetic Approach Reporter Checker Date : Zhong Yan : Ji Zhou : 2017-12-22 Min, C.; Lin, Y.; Seidel, D. Angew. Chem.
Literature Report Catalytic Enantioselective Synthesis of Isoindolinones through a Biomimetic Approach Reporter Checker Date : Zhong Yan : Ji Zhou : 2017-12-22 Min, C.; Lin, Y.; Seidel, D. Angew. Chem.
Keywords: anti-coagulants, factor Xa, QSAR, Thrombosis. Introduction
 PostDoc Journal Vol. 2, No. 3, March 2014 Journal of Postdoctoral Research www.postdocjournal.com QSAR Study of Thiophene-Anthranilamides Based Factor Xa Direct Inhibitors Preetpal S. Sidhu Department
PostDoc Journal Vol. 2, No. 3, March 2014 Journal of Postdoctoral Research www.postdocjournal.com QSAR Study of Thiophene-Anthranilamides Based Factor Xa Direct Inhibitors Preetpal S. Sidhu Department
Chapter 20 Carboxylic Acid Derivatives Nucleophilic Acyl Substitution
 Chapter 20 Carboxylic Acid Derivatives Nucleophilic Acyl Substitution Nomenclature: In carboxylic acid chlorides, anhydrides, esters and amides, the parent is the carboxylic acid. In each case be sure
Chapter 20 Carboxylic Acid Derivatives Nucleophilic Acyl Substitution Nomenclature: In carboxylic acid chlorides, anhydrides, esters and amides, the parent is the carboxylic acid. In each case be sure
The Legends of the Star Tetrodotoxin
 The Legends of the Star Tetrodotoxin Advisors: Prof. YANG Prof. CHEN Prof. TANG Reporter: Haixin YU History Puffer Fish Delicious food in Japan fugu Poisonous For Japanese and Egyptians Millennia For Europeans
The Legends of the Star Tetrodotoxin Advisors: Prof. YANG Prof. CHEN Prof. TANG Reporter: Haixin YU History Puffer Fish Delicious food in Japan fugu Poisonous For Japanese and Egyptians Millennia For Europeans
H. Dvir,, H. L. Jiang,, D. M. Wong,, M. Harel, M. Chetrit, X. C. He, G. Y. Jin, G. L. Yu, X. C. Tang, I. Silman, D. L. Bai,*, and J. L.
 10810 Biochemistry 2002, 41, 10810-10818 X-ray Structures of Torpedo californica Acetylcholinesterase Complexed with (+)-Huperzine A and (-)-Huperzine B: Structural Evidence for an Active Site Rearrangement,
10810 Biochemistry 2002, 41, 10810-10818 X-ray Structures of Torpedo californica Acetylcholinesterase Complexed with (+)-Huperzine A and (-)-Huperzine B: Structural Evidence for an Active Site Rearrangement,
New sesquiterpenoids from the rhizomes of Acorus tatarinowii
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 214 New sesquiterpenoids from the rhizomes of Acorus tatarinowii Xiao-Lin Feng, a Yang Yu, *a Hao
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 214 New sesquiterpenoids from the rhizomes of Acorus tatarinowii Xiao-Lin Feng, a Yang Yu, *a Hao
Reactions at α-position
 Reactions at α-position In preceding chapters on carbonyl chemistry, a common reaction mechanism observed was a nucleophile reacting at the electrophilic carbonyl carbon site NUC NUC Another reaction that
Reactions at α-position In preceding chapters on carbonyl chemistry, a common reaction mechanism observed was a nucleophile reacting at the electrophilic carbonyl carbon site NUC NUC Another reaction that
Chemistry of η 5 -fullerene metal complexes*
 Pure Appl. Chem., Vol. 73, No. 2, pp. 355 359, 2001. 2001 IUPAC Chemistry of η 5 -fullerene metal complexes* Eiichi Nakamura and Masaya Sawamura Department of Chemistry, The University of Tokyo, Tokyo
Pure Appl. Chem., Vol. 73, No. 2, pp. 355 359, 2001. 2001 IUPAC Chemistry of η 5 -fullerene metal complexes* Eiichi Nakamura and Masaya Sawamura Department of Chemistry, The University of Tokyo, Tokyo
Galbulimima Alkaloids (-)-GB 13 and (+)-GB 16
 Galbulimima Alkaloids (-)-GB 13 and (+)-GB 16 Zi, W.; Yu, S.; Ma, D. Angew. Chem. Int. Ed. 2010, 49, 5887 5890 Current Literature Jie Xu 08.28.10 Jie Xu @ Wipf Group Page 1 of 18 12/5/2010 Isolation Galbulimima
Galbulimima Alkaloids (-)-GB 13 and (+)-GB 16 Zi, W.; Yu, S.; Ma, D. Angew. Chem. Int. Ed. 2010, 49, 5887 5890 Current Literature Jie Xu 08.28.10 Jie Xu @ Wipf Group Page 1 of 18 12/5/2010 Isolation Galbulimima
DOCKING TUTORIAL. A. The docking Workflow
 2 nd Strasbourg Summer School on Chemoinformatics VVF Obernai, France, 20-24 June 2010 E. Kellenberger DOCKING TUTORIAL A. The docking Workflow 1. Ligand preparation It consists in the standardization
2 nd Strasbourg Summer School on Chemoinformatics VVF Obernai, France, 20-24 June 2010 E. Kellenberger DOCKING TUTORIAL A. The docking Workflow 1. Ligand preparation It consists in the standardization
OXFORD H i g h e r E d u c a t i o n Oxford University Press, All rights reserved.
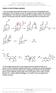 Patrick, An Introduction to dicinal hemistry 4e Answers to end-of-chapter questions 1) The mechanism below shows the release of one molecule of formaldehyde from methenamine. The mechanism can then be
Patrick, An Introduction to dicinal hemistry 4e Answers to end-of-chapter questions 1) The mechanism below shows the release of one molecule of formaldehyde from methenamine. The mechanism can then be
How to make pyridines: the Hantzsch pyridine synthesis
 ow to make pyridines: the antzsch pyridine synthesis 1191 Zinc in acetic acid (Chapter 24) reduces the oxime to the amine and we can start the synthesis by doing the conjugate addition and then reducing
ow to make pyridines: the antzsch pyridine synthesis 1191 Zinc in acetic acid (Chapter 24) reduces the oxime to the amine and we can start the synthesis by doing the conjugate addition and then reducing
Enols and Enolates. A type of reaction with carbonyl compounds is an α-substitution (an electrophile adds to the α carbon of a carbonyl)
 Enols and Enolates A type of reaction with carbonyl compounds is an α-substitution (an electrophile adds to the α carbon of a carbonyl) E+ E In the preceding chapters, we primarily studied nucleophiles
Enols and Enolates A type of reaction with carbonyl compounds is an α-substitution (an electrophile adds to the α carbon of a carbonyl) E+ E In the preceding chapters, we primarily studied nucleophiles
Exam 1 (Monday, July 6, 2015)
 Chem 231 Summer 2015 Assigned Homework Problems Last updated: Friday, July 24, 2015 Problems Assigned from Essential Organic Chemistry, 2 nd Edition, Paula Yurkanis Bruice, Prentice Hall, New York, NY,
Chem 231 Summer 2015 Assigned Homework Problems Last updated: Friday, July 24, 2015 Problems Assigned from Essential Organic Chemistry, 2 nd Edition, Paula Yurkanis Bruice, Prentice Hall, New York, NY,
Ligand-receptor interactions
 University of Silesia, Katowice, Poland 11 22 March 2013 Ligand-receptor interactions Dr. Pavel Polishchuk A.V. Bogatsky Physico-Chemical Institute of National Academy of Sciences of Ukraine Odessa, Ukraine
University of Silesia, Katowice, Poland 11 22 March 2013 Ligand-receptor interactions Dr. Pavel Polishchuk A.V. Bogatsky Physico-Chemical Institute of National Academy of Sciences of Ukraine Odessa, Ukraine
CATALYSIS MULTICATALYST SYSTEM IN ASYMMETRIC. Wiley. Department of Chemistry
 MULTICATALYST SYSTEM IN ASYMMETRIC CATALYSIS JIAN ZHOU Shanghai Key Laboratory of Green Chemistry and Chemical Processes Department of Chemistry East China Normal University Shanghai, China Wiley Preface
MULTICATALYST SYSTEM IN ASYMMETRIC CATALYSIS JIAN ZHOU Shanghai Key Laboratory of Green Chemistry and Chemical Processes Department of Chemistry East China Normal University Shanghai, China Wiley Preface
Homogeneous Catalysis - B. List
 omogeneous Catalysis - B. List 2.2.2 Research Area "rganocatalytic Asymmetric α-alkylation of Aldehydes" (B. List) Involved:. Vignola, A. Majeed Seayad bjective: α-alkylations of carbonyl compounds are
omogeneous Catalysis - B. List 2.2.2 Research Area "rganocatalytic Asymmetric α-alkylation of Aldehydes" (B. List) Involved:. Vignola, A. Majeed Seayad bjective: α-alkylations of carbonyl compounds are
Organic Chemistry, Third Edition. Chapter 24 Carbonyl condensations
 rganic Chemistry, Third Edition Chapter 24 Carbonyl condensations 1 Review: enolates LDA, -78 C TF kinetic RX R = Me, 1 alkyl R Na TF, RT RX R = Me, 1 alkyl thermodynamic R enolates = nucleophiles React
rganic Chemistry, Third Edition Chapter 24 Carbonyl condensations 1 Review: enolates LDA, -78 C TF kinetic RX R = Me, 1 alkyl R Na TF, RT RX R = Me, 1 alkyl thermodynamic R enolates = nucleophiles React
pharmaceutical industry- drug discovery
 ombinatorial hemistry: molecular diversity "Synthesis and pplications of Small Molecule Libraries." Thompson, L..; llman, J.. hem. ev.,, -00. "esign, Synthesis, and valuation of Small-Molecule Libraries.
ombinatorial hemistry: molecular diversity "Synthesis and pplications of Small Molecule Libraries." Thompson, L..; llman, J.. hem. ev.,, -00. "esign, Synthesis, and valuation of Small-Molecule Libraries.
Suggested solutions for Chapter 28
 s for Chapter 28 28 PBLEM 1 ow would you make these four compounds? Give your disconnections, explain why you chose them and then give reagents for the. 2 2 Me S Exercises in basic one- group C X disconnections.
s for Chapter 28 28 PBLEM 1 ow would you make these four compounds? Give your disconnections, explain why you chose them and then give reagents for the. 2 2 Me S Exercises in basic one- group C X disconnections.
Angela Carrillo Alocén
 Literature Presentation Angela Carrillo Alocén 9/15/10 Functional and Structural Analysis of a Key Region of the Cell Wall Inhibitor Moenomycin Fuse, S, Tsukamoto, H; Yuan, Y; Wang, T-S; Zhang, Y; Bolla,
Literature Presentation Angela Carrillo Alocén 9/15/10 Functional and Structural Analysis of a Key Region of the Cell Wall Inhibitor Moenomycin Fuse, S, Tsukamoto, H; Yuan, Y; Wang, T-S; Zhang, Y; Bolla,
Ping-Chiang Lyu. Institute of Bioinformatics and Structural Biology, Department of Life Science, National Tsing Hua University.
 Pharmacophore-based Drug design Ping-Chiang Lyu Institute of Bioinformatics and Structural Biology, Department of Life Science, National Tsing Hua University 96/08/07 Outline Part I: Analysis The analytical
Pharmacophore-based Drug design Ping-Chiang Lyu Institute of Bioinformatics and Structural Biology, Department of Life Science, National Tsing Hua University 96/08/07 Outline Part I: Analysis The analytical
Rising Novel Organic Synthesis
 Literature report Rising Novel Organic Synthesis Methods Based on the Cleavage of N-N and N-O Bonds Reporter: Zhang-Pei Chen Checker : Mu-Wang Chen Date: 04/03/2014 Kürti, L. et al. Kürti, L. et al. Science
Literature report Rising Novel Organic Synthesis Methods Based on the Cleavage of N-N and N-O Bonds Reporter: Zhang-Pei Chen Checker : Mu-Wang Chen Date: 04/03/2014 Kürti, L. et al. Kürti, L. et al. Science
Chapter 10: Carboxylic Acids and Their Derivatives
 Chapter 10: Carboxylic Acids and Their Derivatives The back of the white willow tree (Salix alba) is a source of salicylic acid which is used to make aspirin (acetylsalicylic acid) The functional group
Chapter 10: Carboxylic Acids and Their Derivatives The back of the white willow tree (Salix alba) is a source of salicylic acid which is used to make aspirin (acetylsalicylic acid) The functional group
Supporting Information. A turn-on fluorescent probe for detection of Cu 2+ in living cells based on signaling mechanism of N=N isomerization
 Electronic Supplementary Material (ESI) for New Journal of Chemistry. This journal is The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2016 Supporting Information A turn-on
Electronic Supplementary Material (ESI) for New Journal of Chemistry. This journal is The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2016 Supporting Information A turn-on
Saika Siddiqui, Bruce Gustafson, and Ramachandra, S. Hosmane*
 Third International Electronic Conference on Synthetic Organic Chemistry (ECSOC-3), www.mdpi.org/ecsoc-3.htm, September 1-30, 1999 [A0029] A New Synthetic Approach toward Ring-Expanded ("Fat") Purine Nucleobases:
Third International Electronic Conference on Synthetic Organic Chemistry (ECSOC-3), www.mdpi.org/ecsoc-3.htm, September 1-30, 1999 [A0029] A New Synthetic Approach toward Ring-Expanded ("Fat") Purine Nucleobases:
Fluorine in Peptide and Protein Engineering
 Fluorine in Peptide and Protein Engineering Rita Fernandes Porto, February 11 th 2016 Supervisor: Prof. Dr. Beate Koksch 1 Fluorine a unique element for molecule design The most abundant halogen in earth
Fluorine in Peptide and Protein Engineering Rita Fernandes Porto, February 11 th 2016 Supervisor: Prof. Dr. Beate Koksch 1 Fluorine a unique element for molecule design The most abundant halogen in earth
Aldehydes and Ketones : Aldol Reactions
 Aldehydes and Ketones : Aldol Reactions The Acidity of the a Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons a to carbonyls are unusually acidic The resulting anion is stabilized by
Aldehydes and Ketones : Aldol Reactions The Acidity of the a Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons a to carbonyls are unusually acidic The resulting anion is stabilized by
Suggested solutions for Chapter 40
 s for Chapter 40 40 PBLEM 1 Suggest mechanisms for these reactions, explaining the role of palladium in the first step. Ac Et Et BS () 4 2 1. 2. K 2 C 3 evision of enol ethers and bromination, the Wittig
s for Chapter 40 40 PBLEM 1 Suggest mechanisms for these reactions, explaining the role of palladium in the first step. Ac Et Et BS () 4 2 1. 2. K 2 C 3 evision of enol ethers and bromination, the Wittig
Chapter 16 Aldehydes and Ketones I. Nucleophilic Addition to the Carbonyl Group
 Chapter 16 Aldehydes and Ketones I. Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
Chapter 16 Aldehydes and Ketones I. Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
Catellani Reaction (Pd-Catalyzed Sequential Reaction) Todd Luo
 Catellani Reaction (Pd-Catalyzed Sequential Reaction) Todd Luo 2014.1.6 1 Content Introduction Progress of Catellani Reaction o-alkylation and Applications o-arylation and Applications Conclusion and Outlook
Catellani Reaction (Pd-Catalyzed Sequential Reaction) Todd Luo 2014.1.6 1 Content Introduction Progress of Catellani Reaction o-alkylation and Applications o-arylation and Applications Conclusion and Outlook
Supporting Information for. Silver-catalyzed intramolecular hydroamination of alkynes in
 Supporting Information for Silver-catalyzed intramolecular hydroamination of alkynes in aqueous media: efficient and regioselective synthesis for fused benzimidazoles Xu Zhang, a, b Yu Zhou, b Hengshuai
Supporting Information for Silver-catalyzed intramolecular hydroamination of alkynes in aqueous media: efficient and regioselective synthesis for fused benzimidazoles Xu Zhang, a, b Yu Zhou, b Hengshuai
CHM 320 Laboratory Projects Spring, 2009
 M 320 Laboratory Projects Spring, 2009 I. Enantioselective Reduction of Benzofuran-2-yl Methyl Ketone using Enzymes from arrots. Typically, the reduction of an unsymmetrical, achiral ketone with a hydride
M 320 Laboratory Projects Spring, 2009 I. Enantioselective Reduction of Benzofuran-2-yl Methyl Ketone using Enzymes from arrots. Typically, the reduction of an unsymmetrical, achiral ketone with a hydride
Synthesis and Utility of α-aminoboranes and α-hydroxyboranes. Morken Group Literature Meeting Thomas C. Caya May 17, 2012
 Synthesis and Utility of α-aminoboranes and α-hydroxyboranes Morken Group Literature Meeting Thomas C. Caya May 17, 2012 2 α-amino and β-amino Boronates Few methods to make these compounds. Great potential
Synthesis and Utility of α-aminoboranes and α-hydroxyboranes Morken Group Literature Meeting Thomas C. Caya May 17, 2012 2 α-amino and β-amino Boronates Few methods to make these compounds. Great potential
1. WORK PROGRESS AND ACHIEVEMENTS DURING THE PERIOD. A summary of progress towards objectives and details for each task;
 1. WORK PROGRESS AND ACHIEVEMENTS DURING THE PERIOD A summary of progress towards objectives and details for each task; In Nature, a vast array of complex biomolecules, including peptides, oligonucleotides,
1. WORK PROGRESS AND ACHIEVEMENTS DURING THE PERIOD A summary of progress towards objectives and details for each task; In Nature, a vast array of complex biomolecules, including peptides, oligonucleotides,
Fullerene peroxides in cage-opening reactions*
 Pure Appl. Chem., Vol. 78, No. 4, pp. 841 845, 2006. doi:10.1351/pac200678040841 2006 IUPAC Fullerene peroxides in cage-opening reactions* Liangbing Gan Key Laboratory of Bioorganic Chemistry and Molecular
Pure Appl. Chem., Vol. 78, No. 4, pp. 841 845, 2006. doi:10.1351/pac200678040841 2006 IUPAC Fullerene peroxides in cage-opening reactions* Liangbing Gan Key Laboratory of Bioorganic Chemistry and Molecular
A Novel Approach of Using NBS as an Effective and Convenient Oxidizing Agent for Various Compounds a Survey
 Journal of Chemistry and Chemical Sciences, Vol.8(1), 59-65, January 2018 (An International Research Journal), www.chemistry-journal.org ISSN 2229-760X (Print) ISSN 2319-7625 (Online) A Novel Approach
Journal of Chemistry and Chemical Sciences, Vol.8(1), 59-65, January 2018 (An International Research Journal), www.chemistry-journal.org ISSN 2229-760X (Print) ISSN 2319-7625 (Online) A Novel Approach
N-Heterocyclic Carbene Catalysis via Azolium Dienolates: An Efficient Strategy for Enantioselective Remote Functionalizations
 Angew. Chem. Int. Ed. 2017, 10.1002. 1 N-Heterocyclic Carbene Catalysis via Azolium Dienolates: An Efficient Strategy for Enantioselective Remote Functionalizations Reporter: En Li Supervisor: Prof. Yong
Angew. Chem. Int. Ed. 2017, 10.1002. 1 N-Heterocyclic Carbene Catalysis via Azolium Dienolates: An Efficient Strategy for Enantioselective Remote Functionalizations Reporter: En Li Supervisor: Prof. Yong
Structural biology and drug design: An overview
 Structural biology and drug design: An overview livier Taboureau Assitant professor Chemoinformatics group-cbs-dtu otab@cbs.dtu.dk Drug discovery Drug and drug design A drug is a key molecule involved
Structural biology and drug design: An overview livier Taboureau Assitant professor Chemoinformatics group-cbs-dtu otab@cbs.dtu.dk Drug discovery Drug and drug design A drug is a key molecule involved
Chapter 22: Amines. Organic derivatives of ammonia, NH 3. Nitrogen atom have a lone pair of electrons, making the amine both basic and nucleophilic
 hapter 22: Amines. rganic derivatives of ammonia, 3. itrogen atom have a lone pair of electrons, making the amine both basic and nucleophilic 22.1: Amines omenclature. (please read) sp 3 Amines are classified
hapter 22: Amines. rganic derivatives of ammonia, 3. itrogen atom have a lone pair of electrons, making the amine both basic and nucleophilic 22.1: Amines omenclature. (please read) sp 3 Amines are classified
Chem 263 Nov 19, Cl 2
 Chem 263 Nov 19, 2013 eactions of Enolates: X X alogenation X C 2 Alkylation C Aldol eaction X C Acylation Example: halogenation LDA 2 Chloroacetone is used in tear gas. chloroacetone In this reaction,
Chem 263 Nov 19, 2013 eactions of Enolates: X X alogenation X C 2 Alkylation C Aldol eaction X C Acylation Example: halogenation LDA 2 Chloroacetone is used in tear gas. chloroacetone In this reaction,
Chapter 16 Aldehydes and Ketones I Nucleophilic Addition to the Carbonyl Group
 Chapter 16 Aldehydes and Ketones I Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
Chapter 16 Aldehydes and Ketones I Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
Halogen Bond Applications in Organic Synthesis. Literature Seminar 2018/7/14 M1 Katsuya Maruyama
 Halogen Bond Applications in Organic Synthesis Literature Seminar 2018/7/14 M1 Katsuya Maruyama 1 Contents 1. Introduction 2. Property of Halogen Bond 3. Application to Organic Synthesis 2 1. Introduction
Halogen Bond Applications in Organic Synthesis Literature Seminar 2018/7/14 M1 Katsuya Maruyama 1 Contents 1. Introduction 2. Property of Halogen Bond 3. Application to Organic Synthesis 2 1. Introduction
Ring constructions by the use of fluorine substituent as activator and controller*
 Pure Appl. Chem., Vol. 72, No. 9, pp. 1685 1689, 2000. 2000 IUPAC Ring constructions by the use of fluorine substituent as activator and controller* Junji Ichikawa Department of Chemistry, Graduate School
Pure Appl. Chem., Vol. 72, No. 9, pp. 1685 1689, 2000. 2000 IUPAC Ring constructions by the use of fluorine substituent as activator and controller* Junji Ichikawa Department of Chemistry, Graduate School
sp 3 C-H insertion by α-oxo Gold Carbene B4 Kei Ito
 1 sp 3 C-H insertion by α-oxo Gold Carbene B4 Kei Ito 2016. 1. 30 1. Introduction 2 About Carbene 3 Brief history of carbene (~2000) Carbene Neutral compounds featuring a divalent carbon atom with only
1 sp 3 C-H insertion by α-oxo Gold Carbene B4 Kei Ito 2016. 1. 30 1. Introduction 2 About Carbene 3 Brief history of carbene (~2000) Carbene Neutral compounds featuring a divalent carbon atom with only
KOT 222 Organic Chemistry II
 KOT 222 Organic Chemistry II Course Objectives: 1) To introduce the chemistry of alcohols and ethers. 2) To study the chemistry of functional groups. 3) To learn the chemistry of aromatic compounds and
KOT 222 Organic Chemistry II Course Objectives: 1) To introduce the chemistry of alcohols and ethers. 2) To study the chemistry of functional groups. 3) To learn the chemistry of aromatic compounds and
2015 AP Biology Unit 2 PRETEST- Introduction to the Cell and Biochemistry
 Name: Class: _ Date: _ 2015 AP Biology Unit 2 PRETEST- Introduction to the Cell and Biochemistry Multiple Choice Identify the choice that best completes the statement or answers the question. 1) In what
Name: Class: _ Date: _ 2015 AP Biology Unit 2 PRETEST- Introduction to the Cell and Biochemistry Multiple Choice Identify the choice that best completes the statement or answers the question. 1) In what
Spacer conformation in biologically active molecules*
 Pure Appl. Chem., Vol. 76, No. 5, pp. 959 964, 2004. 2004 IUPAC Spacer conformation in biologically active molecules* J. Karolak-Wojciechowska and A. Fruziński Institute of General and Ecological Chemistry,
Pure Appl. Chem., Vol. 76, No. 5, pp. 959 964, 2004. 2004 IUPAC Spacer conformation in biologically active molecules* J. Karolak-Wojciechowska and A. Fruziński Institute of General and Ecological Chemistry,
Chapter 7 Alkenes and Alkynes I: Properties and Synthesis Elimination Reactions of Alkyl Halides"
 Chapter 7 Alkenes and Alkynes I: Properties and Synthesis Elimination Reactions of Alkyl Halides The (E)-(Z) System for Designating Alkene Diastereomers The Cahn-Ingold-Prelog convention is used to assign
Chapter 7 Alkenes and Alkynes I: Properties and Synthesis Elimination Reactions of Alkyl Halides The (E)-(Z) System for Designating Alkene Diastereomers The Cahn-Ingold-Prelog convention is used to assign
Organic Chemistry of Drug Degradation
 Organic Chemistry of Drug Degradation Li New Jersey Email: minli88@yahoo.com Publishing Contents Chapter 1 Introduction 1 1.1 Drug Impurities, and the Importance of Understanding Drug Chemistry 1 Characteristics
Organic Chemistry of Drug Degradation Li New Jersey Email: minli88@yahoo.com Publishing Contents Chapter 1 Introduction 1 1.1 Drug Impurities, and the Importance of Understanding Drug Chemistry 1 Characteristics
1/4/2011. Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds
 Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds The Acidity of the Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons to carbonyls are unusually acidic
Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds The Acidity of the Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons to carbonyls are unusually acidic
Stereodivergent Catalysis. Aragorn Laverny SED Group Meeting July
 Stereodivergent Catalysis Aragorn Laverny SED Group Meeting July 31 2018 1 Stereodivergent Catalysis In the context of asymmetric synthesis, a stereodivergent process is one that allows access to any given
Stereodivergent Catalysis Aragorn Laverny SED Group Meeting July 31 2018 1 Stereodivergent Catalysis In the context of asymmetric synthesis, a stereodivergent process is one that allows access to any given
The application of ligands with planar chirality in asymmetric synthesis*
 Pure Appl. Chem., Vol. 71, No. 8, pp. 1401±1405, 1999. Printed in Great Britain. q 1999 IUPAC The application of ligands with planar chirality in asymmetric synthesis* Li-Xin Dai,² Xue-Long Hou, Wei-Ping
Pure Appl. Chem., Vol. 71, No. 8, pp. 1401±1405, 1999. Printed in Great Britain. q 1999 IUPAC The application of ligands with planar chirality in asymmetric synthesis* Li-Xin Dai,² Xue-Long Hou, Wei-Ping
Identifying Interaction Hot Spots with SuperStar
 Identifying Interaction Hot Spots with SuperStar Version 1.0 November 2017 Table of Contents Identifying Interaction Hot Spots with SuperStar... 2 Case Study... 3 Introduction... 3 Generate SuperStar Maps
Identifying Interaction Hot Spots with SuperStar Version 1.0 November 2017 Table of Contents Identifying Interaction Hot Spots with SuperStar... 2 Case Study... 3 Introduction... 3 Generate SuperStar Maps
Amines Reading Study Problems Key Concepts and Skills Lecture Topics: Amines: structure and nomenclature
 Amines Reading: Wade chapter 19, sections 19-1-19-19 Study Problems: 19-37, 19-39, 19-40, 19-41, 19-44, 19-46, 19-47, 19-48, 19-51, 19-54 Key Concepts and Skills: Explain how the basicity of amines varies
Amines Reading: Wade chapter 19, sections 19-1-19-19 Study Problems: 19-37, 19-39, 19-40, 19-41, 19-44, 19-46, 19-47, 19-48, 19-51, 19-54 Key Concepts and Skills: Explain how the basicity of amines varies
Self-stable Electrophilic Reagents for Trifluoromethylthiolation. Reporter: Linrui Zhang Supervisor: Prof. Yong Huang Date:
 Self-stable Electrophilic Reagents for Trifluoromethylthiolation Reporter: Linrui Zhang Supervisor: Prof. Yong Huang Date: 2017-12-25 Content Introduction Trifluoromethanesulfenates: Preparation and reactivity
Self-stable Electrophilic Reagents for Trifluoromethylthiolation Reporter: Linrui Zhang Supervisor: Prof. Yong Huang Date: 2017-12-25 Content Introduction Trifluoromethanesulfenates: Preparation and reactivity
Early Stages of Drug Discovery in the Pharmaceutical Industry
 Early Stages of Drug Discovery in the Pharmaceutical Industry Daniel Seeliger / Jan Kriegl, Discovery Research, Boehringer Ingelheim September 29, 2016 Historical Drug Discovery From Accidential Discovery
Early Stages of Drug Discovery in the Pharmaceutical Industry Daniel Seeliger / Jan Kriegl, Discovery Research, Boehringer Ingelheim September 29, 2016 Historical Drug Discovery From Accidential Discovery
Amines. Amines are organic compounds containing a nitrogen functionality. primary secondary tertiary quaternary
 Amines Amines are organic compounds containing a nitrogen functionality Depending upon the number of alkyl, or aryl, groups attached to nitrogen determines its classification, or order 2 primary secondary
Amines Amines are organic compounds containing a nitrogen functionality Depending upon the number of alkyl, or aryl, groups attached to nitrogen determines its classification, or order 2 primary secondary
CHEM Chapter 23. Carbonyl Condensation Reactions (quiz) W25
 CHEM 2425. Chapter 23. Carbonyl Condensation Reactions (quiz) W25 Student: 1. Which of the following statements about Aldol reactions with either aldehydes or ketones is true? Equilibrium favors the starting
CHEM 2425. Chapter 23. Carbonyl Condensation Reactions (quiz) W25 Student: 1. Which of the following statements about Aldol reactions with either aldehydes or ketones is true? Equilibrium favors the starting
Keynotes in Organic Chemistry
 Keynotes in Organic Chemistry Second Edition ANDREW F. PARSONS Department of Chemistry, University of York, UK Wiley Contents Preface xi 1 Structure and bonding 1 1.1 Ionic versus covalent bonds 1 1.2
Keynotes in Organic Chemistry Second Edition ANDREW F. PARSONS Department of Chemistry, University of York, UK Wiley Contents Preface xi 1 Structure and bonding 1 1.1 Ionic versus covalent bonds 1 1.2
C H activation of aliphatic amines without unnecessary mask M2 Takaya Togo
 C H activation of aliphatic amines without unnecessary mask 2017.11.25 M2 Takaya Togo 1 Outline 1.Introduction 2.Free amines as DG Discovery of new activation mode Mechanistic studies Application of the
C H activation of aliphatic amines without unnecessary mask 2017.11.25 M2 Takaya Togo 1 Outline 1.Introduction 2.Free amines as DG Discovery of new activation mode Mechanistic studies Application of the
Conformational Analysis
 Conformational Analysis C01 3 C C 3 is the most stable by 0.9 kcal/mole C02 K eq = K 1-1 * K 2 = 0.45-1 * 0.048 = 0.11 C04 The intermediate in the reaction of 2 has an unfavorable syn-pentane interaction,
Conformational Analysis C01 3 C C 3 is the most stable by 0.9 kcal/mole C02 K eq = K 1-1 * K 2 = 0.45-1 * 0.048 = 0.11 C04 The intermediate in the reaction of 2 has an unfavorable syn-pentane interaction,
An Advanced Anode Material for Sodium Ion. Batteries
 Layered-Structure SbPO 4 /Reduced Graphene Oxide: An Advanced Anode Material for Sodium Ion Batteries Jun Pan, Shulin Chen, # Qiang Fu, Yuanwei Sun, # Yuchen Zhang, Na Lin, Peng Gao,* # Jian Yang,* and
Layered-Structure SbPO 4 /Reduced Graphene Oxide: An Advanced Anode Material for Sodium Ion Batteries Jun Pan, Shulin Chen, # Qiang Fu, Yuanwei Sun, # Yuchen Zhang, Na Lin, Peng Gao,* # Jian Yang,* and
Suggested solutions for Chapter 29
 s for Chapter 29 29 PRBLEM 1 or each of the following reactions (a) state what kind of substitution is suggested and (b) suggest what product might be formed if monosubstitution occured. Br 2 3 2 S 4 S
s for Chapter 29 29 PRBLEM 1 or each of the following reactions (a) state what kind of substitution is suggested and (b) suggest what product might be formed if monosubstitution occured. Br 2 3 2 S 4 S
NMR Studies of a Series of Shikimic Acid Derivatives
 Journal of the Chinese Chemical Society, 2007, 54, 1313-1320 1313 NMR Studies of a Series of Shikimic Acid Derivatives Ping Zhang ( ), Jian Huang ( ) and Fen-Er Chen* ( ) Department of Chemistry, Fudan
Journal of the Chinese Chemical Society, 2007, 54, 1313-1320 1313 NMR Studies of a Series of Shikimic Acid Derivatives Ping Zhang ( ), Jian Huang ( ) and Fen-Er Chen* ( ) Department of Chemistry, Fudan
CHAPTER 19: CARBONYL COMPOUNDS III
 CHAPTER 19: CARBONYL COMPOUNDS III A hydrogen bonded to a carbon adjacent to a carbonyl carbon is sufficiently acidic to be removed by a strong base. The carbon adjacent to a carbonyl carbon is called
CHAPTER 19: CARBONYL COMPOUNDS III A hydrogen bonded to a carbon adjacent to a carbonyl carbon is sufficiently acidic to be removed by a strong base. The carbon adjacent to a carbonyl carbon is called
Aldehydes and Ketones 2. Based on Organic Chemistry, J. G. Smith 3rde.
 Aldehydes and Ketones 2 Based on Organic Chemistry, J. G. Smith 3rde. The Wittig Reaction Wittig reaction, named for German chemist Georg Wittig, who was awarded the Nobel Prize in Chemistry in 1979 for
Aldehydes and Ketones 2 Based on Organic Chemistry, J. G. Smith 3rde. The Wittig Reaction Wittig reaction, named for German chemist Georg Wittig, who was awarded the Nobel Prize in Chemistry in 1979 for
Suggested solutions for Chapter 41
 s for Chapter 41 41 PBLEM 1 Explain how this synthesis of amino acids, starting with natural proline, works. Explain the stereoselectivity of each step after the first. C 2 C 2 3 CF 3 C 2 2 Pd 2 C 2 +
s for Chapter 41 41 PBLEM 1 Explain how this synthesis of amino acids, starting with natural proline, works. Explain the stereoselectivity of each step after the first. C 2 C 2 3 CF 3 C 2 2 Pd 2 C 2 +
COURSE UNIT DESCRIPTION. Dept. Organic Chemistry, Vilnius University. Type of the course unit
 Course unit title Organic Chemistry II Lecturer(s) Rimantas Vaitkus COURSE UNIT DESCRIPTION Department Dept. Organic Chemistry, Vilnius University Cycle First Type of the course unit Mode of delivery Period
Course unit title Organic Chemistry II Lecturer(s) Rimantas Vaitkus COURSE UNIT DESCRIPTION Department Dept. Organic Chemistry, Vilnius University Cycle First Type of the course unit Mode of delivery Period
Chapter 19: Amines. Introduction
 Chapter 19: Amines Chap 19 HW: (be able to name amines); 37, 39, 41, 42, 44, 46, 47, 48, 53-55, 57, 58 Introduction Organic derivatives of ammonia. Many are biologically active. Chap 19: Amines Slide 19-2
Chapter 19: Amines Chap 19 HW: (be able to name amines); 37, 39, 41, 42, 44, 46, 47, 48, 53-55, 57, 58 Introduction Organic derivatives of ammonia. Many are biologically active. Chap 19: Amines Slide 19-2
Introduction to Chemoinformatics and Drug Discovery
 Introduction to Chemoinformatics and Drug Discovery Irene Kouskoumvekaki Associate Professor February 15 th, 2013 The Chemical Space There are atoms and space. Everything else is opinion. Democritus (ca.
Introduction to Chemoinformatics and Drug Discovery Irene Kouskoumvekaki Associate Professor February 15 th, 2013 The Chemical Space There are atoms and space. Everything else is opinion. Democritus (ca.
Introduction to medicinal chemisry
 2017 1 st edition Introduction to medicinal chemisry Med Chem I Mohammed Nooraldeen (PhD) كيمياء دوائية )1 ) محمد نورالدين محمود Introduction to medicinal chemistry Medicinal Chemistry Biology Medicine
2017 1 st edition Introduction to medicinal chemisry Med Chem I Mohammed Nooraldeen (PhD) كيمياء دوائية )1 ) محمد نورالدين محمود Introduction to medicinal chemistry Medicinal Chemistry Biology Medicine
Published on Web 12/13/2002
 Published on Web 12/13/2002 Acetylcholinesterase Complexed with Bivalent Ligands Related to Huperzine A: Experimental Evidence for Species-Dependent Protein-Ligand Complementarity Dawn M. Wong,, Harry
Published on Web 12/13/2002 Acetylcholinesterase Complexed with Bivalent Ligands Related to Huperzine A: Experimental Evidence for Species-Dependent Protein-Ligand Complementarity Dawn M. Wong,, Harry
EMPIRICAL VS. RATIONAL METHODS OF DISCOVERING NEW DRUGS
 EMPIRICAL VS. RATIONAL METHODS OF DISCOVERING NEW DRUGS PETER GUND Pharmacopeia Inc., CN 5350 Princeton, NJ 08543, USA pgund@pharmacop.com Empirical and theoretical approaches to drug discovery have often
EMPIRICAL VS. RATIONAL METHODS OF DISCOVERING NEW DRUGS PETER GUND Pharmacopeia Inc., CN 5350 Princeton, NJ 08543, USA pgund@pharmacop.com Empirical and theoretical approaches to drug discovery have often
DAMIETTA UNIVERSITY CHEM-103: BASIC ORGANIC CHEMISTRY LECTURE
 DAMIETTA UNIVERSITY CHEM-103: BASIC ORGANIC CHEMISTRY LECTURE 6 Dr Ali El-Agamey 1 Oxidation States Easy for inorganic salts: CrO 4 2- reduced to Cr 2 O 3. KMnO 4 reduced to MnO 2. Oxidation: Gain of O,
DAMIETTA UNIVERSITY CHEM-103: BASIC ORGANIC CHEMISTRY LECTURE 6 Dr Ali El-Agamey 1 Oxidation States Easy for inorganic salts: CrO 4 2- reduced to Cr 2 O 3. KMnO 4 reduced to MnO 2. Oxidation: Gain of O,
Molecules, Medicine and Life
 Molecules, Medicine and Life Ravi P. Singh Organic Chemistry Division NCL PUNE Exciting Science 24 th March 2013 Life and its basic elements What is life? What are we made of? What is our surrounding made
Molecules, Medicine and Life Ravi P. Singh Organic Chemistry Division NCL PUNE Exciting Science 24 th March 2013 Life and its basic elements What is life? What are we made of? What is our surrounding made
Institute of Physical and Theoretical Chemistry Wroclaw University of Technology Wyb. Wyspiańskiego Wrocław, Poland
 COMPUTATIONAL METHODS IN SCIENCE AND TECHNOLOGY 3, 55-62 (1997) THE STRUCTURE, PROTON AFFINITY, ELECTROSTATIC PROPERTIES AND ITS RELATION TO BIOLOGICAL ACTIVITY OF COCAINE AND ITS DERIVATIVES. S. Roszak
COMPUTATIONAL METHODS IN SCIENCE AND TECHNOLOGY 3, 55-62 (1997) THE STRUCTURE, PROTON AFFINITY, ELECTROSTATIC PROPERTIES AND ITS RELATION TO BIOLOGICAL ACTIVITY OF COCAINE AND ITS DERIVATIVES. S. Roszak
A Simple Introduction of the Mizoroki-Heck Reaction
 A Simple Introduction of the Mizoroki-Heck Reaction Reporter: Supervisor: Zhe Niu Prof. Yang Prof. Chen Prof. Tang 2016/2/3 Content Introduction Intermolecular Mizoroki-Heck Reaction Intramolecular Mizoroki-Heck
A Simple Introduction of the Mizoroki-Heck Reaction Reporter: Supervisor: Zhe Niu Prof. Yang Prof. Chen Prof. Tang 2016/2/3 Content Introduction Intermolecular Mizoroki-Heck Reaction Intramolecular Mizoroki-Heck
N-Arylhexahydropyrimidines. Electron impact mass spectrometry
 -rylhexahydropyrimidines. Electron impact mass spectrometry M. Beatriz García, Isabel. Perillo, and Liliana R. Orelli* Departamento de Química Orgánica, Facultad de Farmacia y Bioquímica. Junín 956, (1113)
-rylhexahydropyrimidines. Electron impact mass spectrometry M. Beatriz García, Isabel. Perillo, and Liliana R. Orelli* Departamento de Química Orgánica, Facultad de Farmacia y Bioquímica. Junín 956, (1113)
Tautomerism and Keto Enol Equilibrium
 Tautomerism and Keto Enol Equilibrium Enols & enolates are important nucleophiles in organic & biochemistry. Keto-Enol Equilibrium: Tautomerisation can be catalyzed by either acids or bases. Relative stability
Tautomerism and Keto Enol Equilibrium Enols & enolates are important nucleophiles in organic & biochemistry. Keto-Enol Equilibrium: Tautomerisation can be catalyzed by either acids or bases. Relative stability
Carbonyl Compounds. Introduction
 Carbonyl Compounds Introduction 1 Introduction Two broad classes of compounds contain the carbonyl group: [1] Compounds that have only carbon and hydrogen atoms bonded to the carbonyl [2] Compounds that
Carbonyl Compounds Introduction 1 Introduction Two broad classes of compounds contain the carbonyl group: [1] Compounds that have only carbon and hydrogen atoms bonded to the carbonyl [2] Compounds that
CHEM 251 (4 credits): Description
 CHEM 251 (4 credits): Intermediate Reactions of Nucleophiles and Electrophiles (Reactivity 2) Description: An understanding of chemical reactivity, initiated in Reactivity 1, is further developed based
CHEM 251 (4 credits): Intermediate Reactions of Nucleophiles and Electrophiles (Reactivity 2) Description: An understanding of chemical reactivity, initiated in Reactivity 1, is further developed based
