Calculation of Ground-State Energy for Linear Hell;* Through Fifth Order (United Atom Treatment)
|
|
|
- Griselda Elliott
- 5 years ago
- Views:
Transcription
1 An-Najah Univ. J. Res., Vol. 12, (1998) Calculation of Ground-State Energy for Linear Hell;* Through Fifth Order (United Atom Treatment) Hell2++a zitla (.0. 12: sAWI jail Mohammed Abu-Jafar Abstract Department of Physics, An-Najah N University, Nablus, Palestine. Received: (24/2/1998), Accepted: (27/10/1998) In this paper, the nonrelativistic energies of the linear Hell" molecular ion have been computed using the multiperturbation theory for the ground state through fifth order. The results are very encouraging compared to the variational calculations. Nc_:>;,11 c:11.i11,11, gill Date 4.i.4.AdstLil jail L,A.. :;t3ln.11 Zara 1,,a31 * Lbic- " ''.-1? I. Introduction A multiperturbation method has been proposed for the calculation of the energies of molecular systems through fourth and fifth order in the energy [1,2]. The techniques involved have been developed to the point that accurate energies can be obtained for small molecular systems [3].
2 30 Calculation of Ground-State Energy The united atom treatment [4] of molecules is used for examining the behavior of the potential energy function for small values of internuclear distances. In the united-atom perturbation treatment of molecules [5,6] the unperturbed system is taken as the united atom, which corresponds to the molecule in question. Chisholm and Lodge [7,8,9] have carried out the calculations for two-electron diatomic systems (13 2, Hell+, He++ ) through second-order in the energy. Galvan, Abu-Jafar and Sanders [3] have studied the two-electron polyatomic systems (Hell2++, H3+) through third-order in the energy. The purpose of this paper is to extend the application of perturbation theory to fifth order in the energy and to the treatment of hetronuclear linear triatomic molecule HeI1 2". II. Wave Functions The zero-order wave function for this system is a properly symmetrized product of one - electron (hydrogenic) orbitals. The first - order correction to the wave function is given by AP = TIA (71,12) ± ZB6 m1 (RB, )1 (F2 ) S )tp (RB,72 ) zc (RC, rl )ls (r 2 )-4-is(F1),pi (Ro2) J (1) where RB and It, are the internuclear distances between the charge Z A and the two perturbing charges, ZB and Z e, respectively. The angle subtended by these two perturbing charges is denoted by 0 The charge ZA is chosen as the largest of the nuclear charges in the linear molecule Hell 2++ on which the electrons are centered. ti-f ia and tr are the first - order corrections in the atomic and molecular wave functions, respectively.
3 .11ohamrned Abu-Jafar 31 The multicenter integrals appear in the energy expansion coefficients. In order to simplify the evaluation of these integrals, the single-center basis sets have been used for all wave functions. energy. Hence The first - order correction in the atomic wave function is given by 4 ( N A, 41 1 kr' r2 /' i1ci q'i (2) = where the coefficients are chosen to minimize the computed total ty1a = p n m iz-11 2 Ci ri r2 pjcose u ie (3) where P12 is the operator which interchanges the subscripts 1 and 2, and 8 12 is the angle between Fl and F. The nonlinear parameter If / is optimized by the variational - perturbation method. For the atomic wave function, all terms with.e 16 and n + m + 2P. 20 have been included with a total of 501 terms. The first- order correction in the molecular wave function is given by tl' 1 C im 0 (R. ' m n i E c. T p COSei )e =1 i (4) where Cm are coefficients optimized variationally. The nonlinear parameter /3 1 is also optimized variationally. For the molecular wave function, all terms with 16 and n 20 have been used with a total of 221 terms. The second - order correction to the wave function is given by T2=q4(i'1, F2)+z 2B67(RB4Ols(F2)+ 1,5 0"1),F7(RB, F2)]+z1,F7(R,, F,)47.2)
4 32 Calculation of Ground-State Energy nun' 1S(FI ) T7' (RC F2 ZB ZC VB'Rc,8-7.])-14P2)± 1s(Fi)til (RB. Rc' e,p2)1 Am Am Z ,1? B' 1- )+ 2 Z C 11 C' l' 2 ) (5) r 1 and calculations [10]. A can be obtained from variational-perturbation sit and uf are known in a closed form [11,12]. The uf wave / 11 functions are obtained by a multiperturbation variant of the variationalperturbation method, yielding upper bounds to the corresponding g22 in the process. V ;9 and all utilize ``"" the same basis set for the trial wave 2 / 11 functions p (COS012 (1 + P12)LCfmnrin r2in p )exp[ k + F2 )]. mm Similarly, and vi both utilize the basis nt fri n p i COS 0, )exp Ai; ) III. Method The multiperturbation theory has been thoroughly discussed in scientific articles [1,2,3]. For the Hal,' molecular ion, we have two electrons and three nuclei of charges ZA, ZB and Z.. The total Hamiltonian of the Hell," system in charge -scaled atomic units can be written as H = Ho +, (6)
5 Mohammed Abu-Jafar 33 where Ho is the unperturbed Hamiltonian and the perturbation H I is given by A 11A ± ± 'twill Ho can be written in scaled a.u. as 2 z) 1 1 Ho 2 WI ± V2/ ria r2a The perturbation H 1 is the electron - electron interaction : 1 r12 (9) 1 and AA ZA is the inverse of the nuclear charge. The perturbation FL is the electron - nuclear interaction: ( 1 1 \ rib r213 (10) and Zs ZA is the ratio of the nuclear charges. The perturbation H i is given by H i 1 1 ric r 2C,/ and Ain' Zc ZA
6 34 Calculation of Ground-State Energy The expressions for the multiperturbation energy - expansion coefficients through fifth order are derived by Abu-Jafar et al. [2 ] The first - order energy coefficient for the ground state of the molecule is A m m ( CI Et + ZBEI krb1 ± ZC TC)* (12) The first- order correction to the ground state energy of a two - electron atom is s: = X. On the other hand, the first-order correction to the ground state energy of a one - electron diatomic molecule with internuclear distance R is given by 1 r 1-2R E = - R e (13) With the use of wave function y1, one can calculate the second - and third - order energy coefficients. The second - order energy is given by 62=+2ic,7(R)+2ii..1 (Pc) +ZI3e(RE) +Zci:4(.1d +2Z,3Z_ (R6r.Pc,O, (14) A m where [10], E 2 is known exactly [11], and &Aim has been derived by Chisholm and Lodge [9]. Note that the dependence of the ITI' energy on the angle 9 first appears in this order through the term e ll [3]. The third-order energy can also be obtained from the first-order wave function iff and is given by r 1 A 3 m Am Am 3'63+ 2 ZBE3(R9) +2 i 3#1 (Rc) + ZBE216 ) +ZcE 21 A'W (&,)+ ZBE12 i 2 Am ',, 2 mm 2 mm 4- ZC g12 (RC) + '' ZB ZC E, 1 B, RC' ZB ZC' El2 ' (RB, RC ' 9) 2 (R) Amur' ± ZBZCe111 B,Rc, ) (15) An-Najoh Univ. J Res., Vol. 12, (1998).
7 Mohammed Abu-Jafar 35 Several of the above coefficients are either known exactly or are obtained from variational calculations. The remaining coefficients can be computed from the first - order wave functions. With the use of wave function u/ fifth - order energy coefficients. / 2 The fourth - order energy is given by, one can calculate the fourth - and Zd-2z4BdU+2i.(R)1--zZ(16)-rzcAJ-kz2,947W-Ficaspi 2 (RC) ZB3 141RB) 1Am3 (RC) + 2ZB3 ZC 3rni" (RB' RC 7 ) +2Z2B Zc2 E2m2"' (Rs, Rc, + 2Z8Z:, 61"12: (RB'R-,9)+ZBZC 2 Amni + Z13 ZC E112 (RB RC The fifth - order energy is given by.mm (RB RC Z2BZC 461Am21 14 (RB 'RC (16) m(rb) +2 5 m (0 + 2 Am(O + 2 Ani (Rc) 3,...4m(RB) 5 Z B 6.5 ZC 6.5 ZB 32 ZC 32-1-ZB Am' (Rc) 4 Am +ZC 4 23 B 814 (RR) ZC.\14 Z (RC) m (3B) (RC) + ZC 4An: 3 2 mni + ZB ZC 6.32 (RB ' Rc ' 9) ± 2Zs2 Zc3 271 (Rs' Re' )i Amni 3 Amni +2 ZBZC 614 (1?B' RC'e) + ZBZC %'RC'0 + ZBZC 6'131 6,RA ±zez...,,e1473n1 (Rs' Rc '0 + Z B2 Zc 2/71 "1 (Rs, RC' ± Z2c EAm 2i2n1 (Rs, Rc 2 2 Amm ZBZCE122 (R s Rc 9) 2z4B Zc g4mim (Rs, Re (17) With the exception of the singly - subscripted coefficients, most of the above coefficients in eqs. (16 & 17) have been calculated here directly from the appropriate components of the second - order wave function. The total energy through fifth - order for this molecular ion, in ordinary atomic units, is then given by
8 36 Calculation of Ground-State Energy 2-m E z + Z 4 e ZB ZAZC ZBZC m Rs. Rc R (18) Results for the third-, fourth-, and fifth-order perturbation sums of the energy for Hell," are presented in Table 1 for 0 = 180, where they are also compared to the available variational calculations. IV. Results and Discussion There is no experimental evidence to show that the system Heil,: exists as a stable molecular ion, although HeH 2+ may be stable [13]. Zetik and Poshusta [14,15] have done the variational calculations over a wide range of internuclear distances for the Heir: molecule. They have found that there is no internuclear equilibrium distance for this molecule. Table I and Fig. I present the total energy through fifth-order correction for Hell. The total energies have been calculated for only the symmetric linear configuration of this molecule. When compared to the results of Zetik and Poshusta [14,15], we find that 8 3,8 4 and g s all lie below variational calculations by roughly 0.05 a.0 or so. One can only note the rapid convergence of the perturbation series produced here with a Z A of 2; so that the contribution of 8 4 & 8 5 to the total energy is likely to be relatively small. The improvement in convergence is true for heteronuclear molecules, especially for those with a single heavy ion. We can see from Table I & Fig. 1 that this molecule does not have a minimum energy, which means that it does not appear as a bound system. Hence this molecule is unstable. The multiperturbation coefficients with two "molecular" perturbations as in HOC+ are small for large R. The significant contribution to 6., 6 4 and 8 5 at large R comes from 8, A A, 6%, and 85 A, An-?rajah Univ. J. Res., Vol. 12, (1998).
9 An-Najah Univ. J Res., Vol. 12, (1998). Alohammed Abu-Jafar 37 respectively. Therefore, we can conclude from this study that the ground state of linear HeH;# is not bound. Finally, a computer program has been written in Fortran -77 in quadruple precision to compute the total energy E(R) for a given internuclear distance R, and this has been coupled to an optimizing routine to find the minimum of E(R). Table 1. Total energies (in a.u) for the linear, symmetric Hel-1; + molecule (0 = 180 '), truncated energy sums ( 6 3, 6-4, 6. 5 ) are compared to the variational results. R (a.u.) E5 c(variationai)a , ''Zetil( and Poshusta [14,15]
10 38 Calculation of Ground-State Energy Figure: 1. Energy for linear, symmetric HeF ; (0)E3; ( n) 64; (6 ) 6 5; (+) variational results of Zetik and Pushusta [14,15] / Linear HeF1 2 -H- Molecule L LLI rr rt rr-r-rrr-r-rrr, r 11,77777,177,11-177,Trrl TUT, i Inter- nuclear Distance in (a.u.) Acknowledgments The author would like to thank both the International Center for Theoretical Physics, Trieste, and the Center for Theoretical and Applied Physics, Yarmouk University, Irbid - Jordan, for their kind hospitality. References [1] Sanders, F., (1973). Multiperturbation Theory of Electron Correlation in Atoms. Phys. Rev. A, 7(6), [2] Abu-Jafar, M. Musameh, S. and Abdelraziq, I. (1996). A Fifth-order Multiperturbation Derivation of the Energy Coefficients of Polyatomic Molecules. An-Najah University J. for Research (Natural Science), 4(10), Galvan, D., Abu-Jafar, M. and Sanders, F., (1995). Multiperturbation approach to potential energy surfaces for polyatomic molecules. J. Chem. Phys., 102 (12) 1-12.
11 Mohammed Abu-Jafar 39 [4] Levine, I., (1964). United Atom Treatment of H2+.J. Chem. Phys., 41 (7), [5] Bingel, W., (1959A). United Atom Treatment of the Behavior of Potential Energy Curves of Diatomic Molecules for Small R. Chem. Phys., 30 (5), [6] Bingel, W., (1959B). United Atom Treatment of the Behavior of Potential Energy Surfaces of Polyatomic Molecules at Small Internuclear Distances. J. Chem. Phys., 30 (5), [7] Chisholm, C., (1971). A double expansion method for calculating molecular properties: Double perturbation theory for molecular species. Molecular Physics, 21 (5), [8] Chisholm,C. and Lodge, K. (1971A). A double expansion method for calculating molecular properties: Ground State energies of one-and two- electron diatomic molecular systems. Molecular Physics, 21 (5) [9] Chisholm,C. and Lodge, K. (1971B) A double expansion method for calculating molecular properties: Screening in one-and two-electron diatomic molecules. Molecular Physics, 22 (4), [10] Scherr, C. and Knight, R., (1963). Two-Electron Atoms: A Sixth- Order perturbation Study of the 1'S Ground State. Reviews of Modern Physics, 35 (3), [1 1] Dalgarno, A. and Lynn, N., (1957). An Exact Calculation of Second Order Long Range Forces. Proc. Phys. Soc. London, 70, [12] Mahootchi, A. (1975). Dissertation, S. I. U. at Carbondale. [13] Poshusta, R., Haugen, J. and Zetik, D., (1969). Ab Initio Predictions for Very Small Ions. J. Chem. Phys., 51 (8), [14] Zetik, D., (1968). Dissertation. The University of Texas at Austin. [15] Zetik, D. and Poshusta, R., (1970). Ab lnitio Potential Surfaces of HeH. J. Chem. Phys., 52,
A L A BA M A L A W R E V IE W
 A L A BA M A L A W R E V IE W Volume 52 Fall 2000 Number 1 B E F O R E D I S A B I L I T Y C I V I L R I G HT S : C I V I L W A R P E N S I O N S A N D TH E P O L I T I C S O F D I S A B I L I T Y I N
A L A BA M A L A W R E V IE W Volume 52 Fall 2000 Number 1 B E F O R E D I S A B I L I T Y C I V I L R I G HT S : C I V I L W A R P E N S I O N S A N D TH E P O L I T I C S O F D I S A B I L I T Y I N
By Soroku TOH. (Read Dec. 16, 1939 by M. KOTANI).
 Quantum-Mechanical Treatment of Helium-Hydride Molecule Ion HeH+. By Soroku TOH. (Read Dec. 16, 1939 by M. KOTANI). 1. Helium-hydride molecule ion HeH+, whose existence and process of formation was investigated
Quantum-Mechanical Treatment of Helium-Hydride Molecule Ion HeH+. By Soroku TOH. (Read Dec. 16, 1939 by M. KOTANI). 1. Helium-hydride molecule ion HeH+, whose existence and process of formation was investigated
E nuc rep = Z AZ B r AB m =0.499 hartree =13.60 ev. E nuc rep (H 2 )= m/a m =0.714 hartree =19.43 ev.
 Chemistr 31 Phsical Chemistr Homework Assignment # 7 1. Sketch qualitative contour maps of the following molecular orbitals for a diatomic molecule AB. Identif each as a bonding or an anti-bonding molecular
Chemistr 31 Phsical Chemistr Homework Assignment # 7 1. Sketch qualitative contour maps of the following molecular orbitals for a diatomic molecule AB. Identif each as a bonding or an anti-bonding molecular
The Behaviour of the First-Order Density Matrix at the Coulomb Singularities of the Schrödinger Equation
 The Behaviour of the First-Order Density Matrix at the Coulomb Singularities of the Schrödinger Equation B y WERNER A. BINGEL * Quantum Theory Project, Departments of Chemistry Physics, University of Florida,
The Behaviour of the First-Order Density Matrix at the Coulomb Singularities of the Schrödinger Equation B y WERNER A. BINGEL * Quantum Theory Project, Departments of Chemistry Physics, University of Florida,
c. What is the average rate of change of f on the interval [, ]? Answer: d. What is a local minimum value of f? Answer: 5 e. On what interval(s) is f
![c. What is the average rate of change of f on the interval [, ]? Answer: d. What is a local minimum value of f? Answer: 5 e. On what interval(s) is f c. What is the average rate of change of f on the interval [, ]? Answer: d. What is a local minimum value of f? Answer: 5 e. On what interval(s) is f](/thumbs/87/95703678.jpg) Essential Skills Chapter f ( x + h) f ( x ). Simplifying the difference quotient Section. h f ( x + h) f ( x ) Example: For f ( x) = 4x 4 x, find and simplify completely. h Answer: 4 8x 4 h. Finding the
Essential Skills Chapter f ( x + h) f ( x ). Simplifying the difference quotient Section. h f ( x + h) f ( x ) Example: For f ( x) = 4x 4 x, find and simplify completely. h Answer: 4 8x 4 h. Finding the
`G 12 */" T A5&2/, ]&>b ; A%/=W, 62 S 35&.1?& S + ( A; 2 ]/0 ; 5 ; L) ( >>S.
![`G 12 */ T A5&2/, ]&>b ; A%/=W, 62 S 35&.1?& S + ( A; 2 ]/0 ; 5 ; L) ( >>S. `G 12 */ T A5&2/, ]&>b ; A%/=W, 62 S 35&.1?& S + ( A; 2 ]/0 ; 5 ; L) ( >>S.](/thumbs/76/73242945.jpg) 01(( +,-. ()*) $%&' "#! : : % $& - "#$ :, (!" -&. #0 12 + 34 2567 () *+ '!" #$%& ; 2 "1? + @)&2 A5&2 () 25& 89:2 *2 72, B97I J$K
01(( +,-. ()*) $%&' "#! : : % $& - "#$ :, (!" -&. #0 12 + 34 2567 () *+ '!" #$%& ; 2 "1? + @)&2 A5&2 () 25& 89:2 *2 72, B97I J$K
176 5 t h Fl oo r. 337 P o ly me r Ma te ri al s
 A g la di ou s F. L. 462 E l ec tr on ic D ev el op me nt A i ng er A.W.S. 371 C. A. M. A l ex an de r 236 A d mi ni st ra ti on R. H. (M rs ) A n dr ew s P. V. 326 O p ti ca l Tr an sm is si on A p ps
A g la di ou s F. L. 462 E l ec tr on ic D ev el op me nt A i ng er A.W.S. 371 C. A. M. A l ex an de r 236 A d mi ni st ra ti on R. H. (M rs ) A n dr ew s P. V. 326 O p ti ca l Tr an sm is si on A p ps
P a g e 5 1 of R e p o r t P B 4 / 0 9
 P a g e 5 1 of R e p o r t P B 4 / 0 9 J A R T a l s o c o n c l u d e d t h a t a l t h o u g h t h e i n t e n t o f N e l s o n s r e h a b i l i t a t i o n p l a n i s t o e n h a n c e c o n n e
P a g e 5 1 of R e p o r t P B 4 / 0 9 J A R T a l s o c o n c l u d e d t h a t a l t h o u g h t h e i n t e n t o f N e l s o n s r e h a b i l i t a t i o n p l a n i s t o e n h a n c e c o n n e
بسم الله الرحمن الرحیم. Curriculum Vitae
 بسم الله الرحمن الرحیم Curriculum Vitae I. Name: Mohammed Salameh Salem Abu-Jafar II. Rank: Associate Professor of Physics (2001 Present). Theoretical Physics (Analytical & Computational Condensed Matter
بسم الله الرحمن الرحیم Curriculum Vitae I. Name: Mohammed Salameh Salem Abu-Jafar II. Rank: Associate Professor of Physics (2001 Present). Theoretical Physics (Analytical & Computational Condensed Matter
Higher-order C n dispersion coefficients for hydrogen
 Higher-order C n dispersion coefficients for hydrogen J Mitroy* and M W J Bromley Faculty of Technology, Charles Darwin University, Darwin NT 0909, Australia Received 2 November 2004; published 11 March
Higher-order C n dispersion coefficients for hydrogen J Mitroy* and M W J Bromley Faculty of Technology, Charles Darwin University, Darwin NT 0909, Australia Received 2 November 2004; published 11 March
Electron States of Diatomic Molecules
 IISER Pune March 2018 Hamiltonian for a Diatomic Molecule The hamiltonian for a diatomic molecule can be considered to be made up of three terms Ĥ = ˆT N + ˆT el + ˆV where ˆT N is the kinetic energy operator
IISER Pune March 2018 Hamiltonian for a Diatomic Molecule The hamiltonian for a diatomic molecule can be considered to be made up of three terms Ĥ = ˆT N + ˆT el + ˆV where ˆT N is the kinetic energy operator
( R) MOLECULES: VALENCE BOND DESCRIPTION VALENCE BOND APPROXIMATION METHOD. Example: H 2. at some fixed R, repeat for many different values
 5.6 004 Lecture #9 page MOLECULES: VLENCE OND DESCRIPTION VLENCE OND PPROXIMTION METHOD Example: H H = + + r r r r r R Determine E ( R) at some fixed R, repeat for many different values Electronic Schrodinger
5.6 004 Lecture #9 page MOLECULES: VLENCE OND DESCRIPTION VLENCE OND PPROXIMTION METHOD Example: H H = + + r r r r r R Determine E ( R) at some fixed R, repeat for many different values Electronic Schrodinger
Physics 622. T.R. Lemberger. Jan. 2003
 Physics 622. T.R. Lemberger. Jan. 2003 Connections between thermodynamic quantities: enthalpy, entropy, energy, and microscopic quantities: kinetic and bonding energies. Useful formulas for one mole of
Physics 622. T.R. Lemberger. Jan. 2003 Connections between thermodynamic quantities: enthalpy, entropy, energy, and microscopic quantities: kinetic and bonding energies. Useful formulas for one mole of
(1/2) M α 2 α, ˆTe = i. 1 r i r j, ˆV NN = α>β
 Chemistry 26 Spectroscopy Week # The Born-Oppenheimer Approximation, H + 2. Born-Oppenheimer approximation As for atoms, all information about a molecule is contained in the wave function Ψ, which is the
Chemistry 26 Spectroscopy Week # The Born-Oppenheimer Approximation, H + 2. Born-Oppenheimer approximation As for atoms, all information about a molecule is contained in the wave function Ψ, which is the
Topic 3: Periodicity OBJECTIVES FOR TODAY: Fall in love with the Periodic Table, Interpret trends in atomic radii, ionic radii, ionization energies &
 Topic 3: Periodicity OBJECTIVES FOR TODAY: Fall in love with the Periodic Table, Interpret trends in atomic radii, ionic radii, ionization energies & electronegativity The Periodic Table What is the periodic
Topic 3: Periodicity OBJECTIVES FOR TODAY: Fall in love with the Periodic Table, Interpret trends in atomic radii, ionic radii, ionization energies & electronegativity The Periodic Table What is the periodic
Quantum mechanics can be used to calculate any property of a molecule. The energy E of a wavefunction Ψ evaluated for the Hamiltonian H is,
 Chapter : Molecules Quantum mechanics can be used to calculate any property of a molecule The energy E of a wavefunction Ψ evaluated for the Hamiltonian H is, E = Ψ H Ψ Ψ Ψ 1) At first this seems like
Chapter : Molecules Quantum mechanics can be used to calculate any property of a molecule The energy E of a wavefunction Ψ evaluated for the Hamiltonian H is, E = Ψ H Ψ Ψ Ψ 1) At first this seems like
0 + E (1) and the first correction to the ground state energy is given by
 1 Problem set 9 Handout: 1/24 Due date: 1/31 Problem 1 Prove that the energy to first order for the lowest-energy state of a perturbed system is an upper bound for the exact energy of the lowest-energy
1 Problem set 9 Handout: 1/24 Due date: 1/31 Problem 1 Prove that the energy to first order for the lowest-energy state of a perturbed system is an upper bound for the exact energy of the lowest-energy
2m 2 Ze2. , where δ. ) 2 l,n is the quantum defect (of order one but larger
 PHYS 402, Atomic and Molecular Physics Spring 2017, final exam, solutions 1. Hydrogenic atom energies: Consider a hydrogenic atom or ion with nuclear charge Z and the usual quantum states φ nlm. (a) (2
PHYS 402, Atomic and Molecular Physics Spring 2017, final exam, solutions 1. Hydrogenic atom energies: Consider a hydrogenic atom or ion with nuclear charge Z and the usual quantum states φ nlm. (a) (2
Forbidden Electric Dipole Transitions in the Hydrogen Molecular Ion First Estimates
 Bulg. J. Phys. 44 (2017) 76 83 Forbidden Electric Dipole Transitions in the Hydrogen Molecular Ion First Estimates P. Danev Institute for Nuclear Research and Nuclear Energy, Bulgarian Academy of Sciences,
Bulg. J. Phys. 44 (2017) 76 83 Forbidden Electric Dipole Transitions in the Hydrogen Molecular Ion First Estimates P. Danev Institute for Nuclear Research and Nuclear Energy, Bulgarian Academy of Sciences,
Computational Methods. Chem 561
 Computational Methods Chem 561 Lecture Outline 1. Ab initio methods a) HF SCF b) Post-HF methods 2. Density Functional Theory 3. Semiempirical methods 4. Molecular Mechanics Computational Chemistry " Computational
Computational Methods Chem 561 Lecture Outline 1. Ab initio methods a) HF SCF b) Post-HF methods 2. Density Functional Theory 3. Semiempirical methods 4. Molecular Mechanics Computational Chemistry " Computational
Stability conditions for hydrogen-antihydrogen-like quasimolecules
 Purdue University Purdue e-pubs Birck and NCN Publications Birck Nanotechnology Center May 8 Stability conditions for hydrogen-antihydrogen-like quasimolecules Alejandro Ferrón Universidad Nacional de
Purdue University Purdue e-pubs Birck and NCN Publications Birck Nanotechnology Center May 8 Stability conditions for hydrogen-antihydrogen-like quasimolecules Alejandro Ferrón Universidad Nacional de
Lecture 10. Born-Oppenheimer approximation LCAO-MO application to H + The potential energy surface MOs for diatomic molecules. NC State University
 Chemistry 431 Lecture 10 Diatomic molecules Born-Oppenheimer approximation LCAO-MO application to H + 2 The potential energy surface MOs for diatomic molecules NC State University Born-Oppenheimer approximation
Chemistry 431 Lecture 10 Diatomic molecules Born-Oppenheimer approximation LCAO-MO application to H + 2 The potential energy surface MOs for diatomic molecules NC State University Born-Oppenheimer approximation
Chemistry 334 Part 2: Computational Quantum Chemistry
 Chemistry 334 Part 2: Computational Quantum Chemistry 1. Definition Louis Scudiero, Ben Shepler and Kirk Peterson Washington State University January 2006 Computational chemistry is an area of theoretical
Chemistry 334 Part 2: Computational Quantum Chemistry 1. Definition Louis Scudiero, Ben Shepler and Kirk Peterson Washington State University January 2006 Computational chemistry is an area of theoretical
Be H. Delocalized Bonding. Localized Bonding. σ 2. σ 1. Two (sp-1s) Be-H σ bonds. The two σ bonding MO s in BeH 2. MO diagram for BeH 2
 The Delocalized Approach to Bonding: The localized models for bonding we have examined (Lewis and VBT) assume that all electrons are restricted to specific bonds between atoms or in lone pairs. In contrast,
The Delocalized Approach to Bonding: The localized models for bonding we have examined (Lewis and VBT) assume that all electrons are restricted to specific bonds between atoms or in lone pairs. In contrast,
properties Michele Catti Dipartimento di Scienza dei Materiali Università di Milano Bicocca, Italy
 Elastic and piezoelectric tensorial properties Michele Catti Dipartimento di Scienza dei Materiali Università di Milano Bicocca, Italy (catti@mater.unimib.it) 1 Tensorial physical properties of crystals
Elastic and piezoelectric tensorial properties Michele Catti Dipartimento di Scienza dei Materiali Università di Milano Bicocca, Italy (catti@mater.unimib.it) 1 Tensorial physical properties of crystals
Quadrupole - Quadrupole and Dipole - Octupole Dispersion Forces Between Axially Symmetrie Molecules *
 B A N D a ZEITSCHRIFT FUR NATURFORSCHUNG HEFT Quadrupole - Quadrupole Dipole - Octupole Dispersion Forces Between Axially Symmetrie Molecules * ALWYN. VAN DER MERWE Department of Physics, University of
B A N D a ZEITSCHRIFT FUR NATURFORSCHUNG HEFT Quadrupole - Quadrupole Dipole - Octupole Dispersion Forces Between Axially Symmetrie Molecules * ALWYN. VAN DER MERWE Department of Physics, University of
ON MIXING AND PARTIAL MIXING
 ON MIXING AND PARTIAL MIXING BY N. A. XRIEDMAN AND D. S. ORNSTEIN 1. Introduction Let (X, a, m) denote the unit interwl with Lebesgue mesure, nd let r be n invertible ergodie mesure preserving transformation
ON MIXING AND PARTIAL MIXING BY N. A. XRIEDMAN AND D. S. ORNSTEIN 1. Introduction Let (X, a, m) denote the unit interwl with Lebesgue mesure, nd let r be n invertible ergodie mesure preserving transformation
QUANTUM MECHANICS AND MOLECULAR STRUCTURE
 6 QUANTUM MECHANICS AND MOLECULAR STRUCTURE 6.1 Quantum Picture of the Chemical Bond 6.2 Exact Molecular Orbital for the Simplest Molecule: H + 2 6.3 Molecular Orbital Theory and the Linear Combination
6 QUANTUM MECHANICS AND MOLECULAR STRUCTURE 6.1 Quantum Picture of the Chemical Bond 6.2 Exact Molecular Orbital for the Simplest Molecule: H + 2 6.3 Molecular Orbital Theory and the Linear Combination
Conical Intersections. Spiridoula Matsika
 Conical Intersections Spiridoula Matsika The Born-Oppenheimer approximation Energy TS Nuclear coordinate R ν The study of chemical systems is based on the separation of nuclear and electronic motion The
Conical Intersections Spiridoula Matsika The Born-Oppenheimer approximation Energy TS Nuclear coordinate R ν The study of chemical systems is based on the separation of nuclear and electronic motion The
EFFECTIVE CROSS SECTIONS FOR THE EXCHANGE EXCITATION OF ATOMS AND IONS BY ELECTRON IMPACT
 SOVET PHYSCS JETP VOLUME 25, NUMBER 1 JULY, 1967 EFFECTVE CROSS SECTONS FOR THE EXCHANGE EXCTATON OF ATOMS AND ONS BY ELECTRON MPACT. L. BEGMAN and L. A. VANSHTEN P. N. Lebedev Physics nstitute, Academy
SOVET PHYSCS JETP VOLUME 25, NUMBER 1 JULY, 1967 EFFECTVE CROSS SECTONS FOR THE EXCHANGE EXCTATON OF ATOMS AND ONS BY ELECTRON MPACT. L. BEGMAN and L. A. VANSHTEN P. N. Lebedev Physics nstitute, Academy
(6) 10 [vol ) Y. Nagai : On the Behaviour of P1oc. Jap. Acad., vol. 26 (1950). 2i In this paper, "capacity" means
 (6) 10 [vol. 26. 26. On the Behaviour o f the Boundary o f Riemann Surfaces, II. By Yasutaka NAGA!. Mathematical Institute, Naniwa Daigaku. (Comm. by K. KUNVGI, M.J.A., June 12, 1950.) In Part I of the
(6) 10 [vol. 26. 26. On the Behaviour o f the Boundary o f Riemann Surfaces, II. By Yasutaka NAGA!. Mathematical Institute, Naniwa Daigaku. (Comm. by K. KUNVGI, M.J.A., June 12, 1950.) In Part I of the
Molecular Orbital Theory This means that the coefficients in the MO will not be the same!
 Diatomic molecules: Heteronuclear molecules In heteronuclear diatomic molecules, the relative contribution of atomic orbitals to each MO is not equal. Some MO s will have more contribution from AO s on
Diatomic molecules: Heteronuclear molecules In heteronuclear diatomic molecules, the relative contribution of atomic orbitals to each MO is not equal. Some MO s will have more contribution from AO s on
Convergence of the multipole expansions of the polarization and dispersion interactions for atoms under confinement
 Convergence of the multipole expansions of the polarization and dispersion interactions for atoms under confinement Yong-Hui Zhang 1,2, Li-Yan Tang 2, Xian-Zhou Zhang 1, Jun Jiang 3 and J. Mitroy 3 1 Department
Convergence of the multipole expansions of the polarization and dispersion interactions for atoms under confinement Yong-Hui Zhang 1,2, Li-Yan Tang 2, Xian-Zhou Zhang 1, Jun Jiang 3 and J. Mitroy 3 1 Department
Evaluation of Coulomb and exchange integrals for higher excited states of helium atom by using spherical harmonics series
 J Math Chem () 5:86 DOI.7/s9--9997-6 ORIGINAL PAPER Evaluation of Coulomb and exchange integrals for higher excited states of helium atom by using spherical harmonics series Artit Hutem Sutee Boonchui
J Math Chem () 5:86 DOI.7/s9--9997-6 ORIGINAL PAPER Evaluation of Coulomb and exchange integrals for higher excited states of helium atom by using spherical harmonics series Artit Hutem Sutee Boonchui
FORMULAE FOR G-FUNCTION
 Revista de la Union Matematica Argentina Volumen 24, Numero 4, 1969. SOME FORMULAE FOR G-FUNCTION by B.L. Sharma 1. INTRODUCTION. In a recent paper {S} the author has defined the generalized function of
Revista de la Union Matematica Argentina Volumen 24, Numero 4, 1969. SOME FORMULAE FOR G-FUNCTION by B.L. Sharma 1. INTRODUCTION. In a recent paper {S} the author has defined the generalized function of
Electron Correlation - Methods beyond Hartree-Fock
 Electron Correlation - Methods beyond Hartree-Fock how to approach chemical accuracy Alexander A. Auer Max-Planck-Institute for Chemical Energy Conversion, Mülheim September 4, 2014 MMER Summerschool 2014
Electron Correlation - Methods beyond Hartree-Fock how to approach chemical accuracy Alexander A. Auer Max-Planck-Institute for Chemical Energy Conversion, Mülheim September 4, 2014 MMER Summerschool 2014
Tentative content material to be covered for Exam 2 (Wednesday, November 2, 2005)
 Tentative content material to be covered for Exam 2 (Wednesday, November 2, 2005) Chapter 16 Quantum Mechanics and the Hydrogen Atom 16.1 Waves and Light 16.2 Paradoxes in Classical Physics 16.3 Planck,
Tentative content material to be covered for Exam 2 (Wednesday, November 2, 2005) Chapter 16 Quantum Mechanics and the Hydrogen Atom 16.1 Waves and Light 16.2 Paradoxes in Classical Physics 16.3 Planck,
Geometric Predicates P r og r a m s need t o t es t r ela t ive p os it ions of p oint s b a s ed on t heir coor d ina t es. S im p le exa m p les ( i
 Automatic Generation of SS tag ed Geometric PP red icates Aleksandar Nanevski, G u y B lello c h and R o b ert H arp er PSCICO project h ttp: / / w w w. cs. cm u. ed u / ~ ps ci co Geometric Predicates
Automatic Generation of SS tag ed Geometric PP red icates Aleksandar Nanevski, G u y B lello c h and R o b ert H arp er PSCICO project h ttp: / / w w w. cs. cm u. ed u / ~ ps ci co Geometric Predicates
Resume. Sharif M. Sh. Musameh Physics department An- Najah University Nablus, West Bank, Palestine E mail:
 Resume Sharif M. Sh. Musameh Physics department An- Najah University Nablus, West Bank, Palestine E mail: musameh@najah.edu Education 1984 Ph.D. in Physics and minor in Mathematics. North Carolina State
Resume Sharif M. Sh. Musameh Physics department An- Najah University Nablus, West Bank, Palestine E mail: musameh@najah.edu Education 1984 Ph.D. in Physics and minor in Mathematics. North Carolina State
Comparative Analysis of Techniques for Solving the Hydraulics of Pressurized Irrigation Pipe Networks. Numan Mizyed
 An-Najah Univ. J. Res., Vol. 11, (1997) 1-21 Comparative Analysis of Techniques for Solving the Hydraulics of Pressurized Irrigation Pipe Networks Numan Mizyed Plant Production Department, Faculty of Agriculture,
An-Najah Univ. J. Res., Vol. 11, (1997) 1-21 Comparative Analysis of Techniques for Solving the Hydraulics of Pressurized Irrigation Pipe Networks Numan Mizyed Plant Production Department, Faculty of Agriculture,
T h e C S E T I P r o j e c t
 T h e P r o j e c t T H E P R O J E C T T A B L E O F C O N T E N T S A r t i c l e P a g e C o m p r e h e n s i v e A s s es s m e n t o f t h e U F O / E T I P h e n o m e n o n M a y 1 9 9 1 1 E T
T h e P r o j e c t T H E P R O J E C T T A B L E O F C O N T E N T S A r t i c l e P a g e C o m p r e h e n s i v e A s s es s m e n t o f t h e U F O / E T I P h e n o m e n o n M a y 1 9 9 1 1 E T
F l a s h-b a s e d S S D s i n E n t e r p r i s e F l a s h-b a s e d S S D s ( S o-s ltiad t e D r i v e s ) a r e b e c o m i n g a n a t t r a c
 L i f e t i m e M a n a g e m e n t o f F l a-b s ah s e d S S D s U s i n g R e c o v e r-a y w a r e D y n a m i c T h r o t t l i n g S u n g j i n L e, e T a e j i n K i m, K y u n g h o, Kainmd J
L i f e t i m e M a n a g e m e n t o f F l a-b s ah s e d S S D s U s i n g R e c o v e r-a y w a r e D y n a m i c T h r o t t l i n g S u n g j i n L e, e T a e j i n K i m, K y u n g h o, Kainmd J
Lowest vibrational states of 4 He 3 He + : Non-Born-Oppenheimer calculations
 Lowest vibrational states of He 3 He + : Non-Born-Oppenheimer calculations Monika Stanke,, * Dariusz Kȩdziera, Sergiy Bubin, Marcin Molski, 3 and Ludwik Adamowicz, Department of Chemistry, University of
Lowest vibrational states of He 3 He + : Non-Born-Oppenheimer calculations Monika Stanke,, * Dariusz Kȩdziera, Sergiy Bubin, Marcin Molski, 3 and Ludwik Adamowicz, Department of Chemistry, University of
I M P O R T A N T S A F E T Y I N S T R U C T I O N S W h e n u s i n g t h i s e l e c t r o n i c d e v i c e, b a s i c p r e c a u t i o n s s h o
 I M P O R T A N T S A F E T Y I N S T R U C T I O N S W h e n u s i n g t h i s e l e c t r o n i c d e v i c e, b a s i c p r e c a u t i o n s s h o u l d a l w a y s b e t a k e n, i n c l u d f o l
I M P O R T A N T S A F E T Y I N S T R U C T I O N S W h e n u s i n g t h i s e l e c t r o n i c d e v i c e, b a s i c p r e c a u t i o n s s h o u l d a l w a y s b e t a k e n, i n c l u d f o l
eigenvalues eigenfunctions
 Born-Oppenheimer Approximation Atoms and molecules consist of heavy nuclei and light electrons. Consider (for simplicity) a diatomic molecule (e.g. HCl). Clamp/freeze the nuclei in space, a distance r
Born-Oppenheimer Approximation Atoms and molecules consist of heavy nuclei and light electrons. Consider (for simplicity) a diatomic molecule (e.g. HCl). Clamp/freeze the nuclei in space, a distance r
Chapter 9. Molecular Geometry and Bonding Theories
 Chapter 9. Molecular Geometry and Bonding Theories 9.1 Molecular Shapes Lewis structures give atomic connectivity: they tell us which atoms are physically connected to which atoms. The shape of a molecule
Chapter 9. Molecular Geometry and Bonding Theories 9.1 Molecular Shapes Lewis structures give atomic connectivity: they tell us which atoms are physically connected to which atoms. The shape of a molecule
The Bond Number Relationship for the O-H... O Systems
 CROATICA CHEMICA ACTA CCACAA 61 (4) 815-819 (1988) CCA-1828 YU ISSN 0011-1643 UDC 541.571.9 Original Scientific Paper The Bond Number Relationship for the O-H... O Systems Slawomir J. Grabowski Institute
CROATICA CHEMICA ACTA CCACAA 61 (4) 815-819 (1988) CCA-1828 YU ISSN 0011-1643 UDC 541.571.9 Original Scientific Paper The Bond Number Relationship for the O-H... O Systems Slawomir J. Grabowski Institute
I N A C O M P L E X W O R L D
 IS L A M I C E C O N O M I C S I N A C O M P L E X W O R L D E x p l o r a t i o n s i n A g-b eanste d S i m u l a t i o n S a m i A l-s u w a i l e m 1 4 2 9 H 2 0 0 8 I s l a m i c D e v e l o p m e
IS L A M I C E C O N O M I C S I N A C O M P L E X W O R L D E x p l o r a t i o n s i n A g-b eanste d S i m u l a t i o n S a m i A l-s u w a i l e m 1 4 2 9 H 2 0 0 8 I s l a m i c D e v e l o p m e
Quantum Theory of Many-Particle Systems, Phys. 540
 Quantum Theory of Many-Particle Systems, Phys. 540 Questions about organization Second quantization Questions about last class? Comments? Similar strategy N-particles Consider Two-body operators in Fock
Quantum Theory of Many-Particle Systems, Phys. 540 Questions about organization Second quantization Questions about last class? Comments? Similar strategy N-particles Consider Two-body operators in Fock
Speed of light c = m/s. x n e a x d x = 1. 2 n+1 a n π a. He Li Ne Na Ar K Ni 58.
 Physical Chemistry II Test Name: KEY CHEM 464 Spring 18 Chapters 7-11 Average = 1. / 16 6 questions worth a total of 16 points Planck's constant h = 6.63 1-34 J s Speed of light c = 3. 1 8 m/s ħ = h π
Physical Chemistry II Test Name: KEY CHEM 464 Spring 18 Chapters 7-11 Average = 1. / 16 6 questions worth a total of 16 points Planck's constant h = 6.63 1-34 J s Speed of light c = 3. 1 8 m/s ħ = h π
Vlaamse Overheid Departement Mobiliteit en Openbare Werken
 Vlaamse Overheid Departement Mobiliteit en Openbare Werken Waterbouwkundig Laboratorium Langdurige metingen Deurganckdok: Opvolging en analyse aanslibbing Bestek 16EB/05/04 Colofon Ph o to c o ve r s h
Vlaamse Overheid Departement Mobiliteit en Openbare Werken Waterbouwkundig Laboratorium Langdurige metingen Deurganckdok: Opvolging en analyse aanslibbing Bestek 16EB/05/04 Colofon Ph o to c o ve r s h
Software Process Models there are many process model s in th e li t e ra t u re, s om e a r e prescriptions and some are descriptions you need to mode
 Unit 2 : Software Process O b j ec t i ve This unit introduces software systems engineering through a discussion of software processes and their principal characteristics. In order to achieve the desireable
Unit 2 : Software Process O b j ec t i ve This unit introduces software systems engineering through a discussion of software processes and their principal characteristics. In order to achieve the desireable
STEEL PIPE NIPPLE BLACK AND GALVANIZED
 Price Sheet Effective August 09, 2018 Supersedes CWN-218 A Member of The Phoenix Forge Group CapProducts LTD. Phone: 519-482-5000 Fax: 519-482-7728 Toll Free: 800-265-5586 www.capproducts.com www.capitolcamco.com
Price Sheet Effective August 09, 2018 Supersedes CWN-218 A Member of The Phoenix Forge Group CapProducts LTD. Phone: 519-482-5000 Fax: 519-482-7728 Toll Free: 800-265-5586 www.capproducts.com www.capitolcamco.com
CHEM3023: Spins, Atoms and Molecules
 CHEM3023: Spins, Atoms and Molecules Lecture 3 The Born-Oppenheimer approximation C.-K. Skylaris Learning outcomes Separate molecular Hamiltonians to electronic and nuclear parts according to the Born-Oppenheimer
CHEM3023: Spins, Atoms and Molecules Lecture 3 The Born-Oppenheimer approximation C.-K. Skylaris Learning outcomes Separate molecular Hamiltonians to electronic and nuclear parts according to the Born-Oppenheimer
On the Uniqueness of Molecular Orbitals and limitations of the MO-model.
 On the Uniqueness of Molecular Orbitals and limitations of the MO-model. The purpose of these notes is to make clear that molecular orbitals are a particular way to represent many-electron wave functions.
On the Uniqueness of Molecular Orbitals and limitations of the MO-model. The purpose of these notes is to make clear that molecular orbitals are a particular way to represent many-electron wave functions.
Chapter VI: Beta decay
 Chapter VI: Beta decay 1 Summary 1. General principles 2. Energy release in decay 3. Fermi theory of decay 4. Selections rules 5. Electron capture decay 6. Other decays 2 General principles (1) The decay
Chapter VI: Beta decay 1 Summary 1. General principles 2. Energy release in decay 3. Fermi theory of decay 4. Selections rules 5. Electron capture decay 6. Other decays 2 General principles (1) The decay
CHARGE EXCHANGE IN SLOW COLLISIONS OF IONS WITH HYDROGEN ISOTOPES. ADIABATIC APPROACH Inga Yu. Tolstikhina
 CHARGE EXCHANGE IN SLOW COLLISIONS OF IONS WITH HYDROGEN ISOTOPES. ADIABATIC APPROACH Inga Yu. Tolstikhina P.N.Lebedev Physical Institute, Russian Academy of Sciences Moscow, Russia Theoretical approaches
CHARGE EXCHANGE IN SLOW COLLISIONS OF IONS WITH HYDROGEN ISOTOPES. ADIABATIC APPROACH Inga Yu. Tolstikhina P.N.Lebedev Physical Institute, Russian Academy of Sciences Moscow, Russia Theoretical approaches
~,. :'lr. H ~ j. l' ", ...,~l. 0 '" ~ bl '!; 1'1. :<! f'~.., I,," r: t,... r':l G. t r,. 1'1 [<, ."" f'" 1n. t.1 ~- n I'>' 1:1 , I. <1 ~'..
 ,, 'l t (.) :;,/.I I n ri' ' r l ' rt ( n :' (I : d! n t, :?rj I),.. fl.),. f!..,,., til, ID f-i... j I. 't' r' t II!:t () (l r El,, (fl lj J4 ([) f., () :. -,,.,.I :i l:'!, :I J.A.. t,.. p, - ' I I I
,, 'l t (.) :;,/.I I n ri' ' r l ' rt ( n :' (I : d! n t, :?rj I),.. fl.),. f!..,,., til, ID f-i... j I. 't' r' t II!:t () (l r El,, (fl lj J4 ([) f., () :. -,,.,.I :i l:'!, :I J.A.. t,.. p, - ' I I I
F(t) equilibrium under H 0
 Physics 17b: Statistical Mechanics Linear Response Theory Useful references are Callen and Greene [1], and Chandler [], chapter 16. Task To calculate the change in a measurement B t) due to the application
Physics 17b: Statistical Mechanics Linear Response Theory Useful references are Callen and Greene [1], and Chandler [], chapter 16. Task To calculate the change in a measurement B t) due to the application
CHM Physical Chemistry II Chapter 12 - Supplementary Material. 1. Einstein A and B coefficients
 CHM 3411 - Physical Chemistry II Chapter 12 - Supplementary Material 1. Einstein A and B coefficients Consider two singly degenerate states in an atom, molecule, or ion, with wavefunctions 1 (for the lower
CHM 3411 - Physical Chemistry II Chapter 12 - Supplementary Material 1. Einstein A and B coefficients Consider two singly degenerate states in an atom, molecule, or ion, with wavefunctions 1 (for the lower
Stark effect of a rigid rotor
 J. Phys. B: At. Mol. Phys. 17 (1984) 3535-3544. Printed in Great Britain Stark effect of a rigid rotor M Cohen, Tova Feldmann and S Kais Department of Physical Chemistry, The Hebrew University, Jerusalem
J. Phys. B: At. Mol. Phys. 17 (1984) 3535-3544. Printed in Great Britain Stark effect of a rigid rotor M Cohen, Tova Feldmann and S Kais Department of Physical Chemistry, The Hebrew University, Jerusalem
Solutions and Ions. Pure Substances
 Class #4 Solutions and Ions CHEM 107 L.S. Brown Texas A&M University Pure Substances Pure substance: described completely by a single chemical formula Fixed composition 1 Mixtures Combination of 2 or more
Class #4 Solutions and Ions CHEM 107 L.S. Brown Texas A&M University Pure Substances Pure substance: described completely by a single chemical formula Fixed composition 1 Mixtures Combination of 2 or more
-0 and K any positive real number. Similarly we define,
 AN INEQUALITY OF SCHÜR AND AN INEQUALITY OF NEWTON K. V. MENON Let El H) denote the elementary complete symmetric functions of the rth order in ai, a2,, am respectively. If (1.1) et = e\ jl \ i / (m +
AN INEQUALITY OF SCHÜR AND AN INEQUALITY OF NEWTON K. V. MENON Let El H) denote the elementary complete symmetric functions of the rth order in ai, a2,, am respectively. If (1.1) et = e\ jl \ i / (m +
JOSEPH A. BENTLEY. Contact Information. Division of Biological and Physical Sciences P.O. Box 3262 DSU Delta State University Cleveland, MS 38733
 JOSEPH A. BENTLEY Contact Information Division of Biological and Physical Sciences P.O. Box 3262 DSU Cleveland, MS 38733 Phone: (662) 846 4482 Lab: (662) 846 4490 FAX: (662) 846 4798 E-mail: jbentley@deltastate.edu
JOSEPH A. BENTLEY Contact Information Division of Biological and Physical Sciences P.O. Box 3262 DSU Cleveland, MS 38733 Phone: (662) 846 4482 Lab: (662) 846 4490 FAX: (662) 846 4798 E-mail: jbentley@deltastate.edu
The Projector Augmented Wave method
 The Projector Augmented Wave method Advantages of PAW. The theory. Approximations. Convergence. 1 The PAW method is... What is PAW? A technique for doing DFT calculations efficiently and accurately. An
The Projector Augmented Wave method Advantages of PAW. The theory. Approximations. Convergence. 1 The PAW method is... What is PAW? A technique for doing DFT calculations efficiently and accurately. An
Charge renormalization at the large-d limit for diatomic molecules
 Charge renormalization at the large-d limit for diatomic molecules R. Bleil, A. Faliks, M. Miletic, and S. Kais Department of Chemistry, Purdue University, West Lafayette, Indiana 47907 Received 2 March
Charge renormalization at the large-d limit for diatomic molecules R. Bleil, A. Faliks, M. Miletic, and S. Kais Department of Chemistry, Purdue University, West Lafayette, Indiana 47907 Received 2 March
On the accurate evaluation of overlap integrals over Slater type orbitals using analytical and recurrence relations I.I. Guseinov. B.A.
 On the accurate evaluation of overlap integrals over Slater type orbitals using analytical and recurrence relations I.I. Guseinov Department of Physics, Faculty of Arts and Sciences, Onseiz Mart University,
On the accurate evaluation of overlap integrals over Slater type orbitals using analytical and recurrence relations I.I. Guseinov Department of Physics, Faculty of Arts and Sciences, Onseiz Mart University,
IMPROVEMENT OF AN APPROXIMATE SET OF LATENT ROOTS AND MODAL COLUMNS OF A MATRIX BY METHODS AKIN TO THOSE OF CLASSICAL PERTURBATION THEORY
 IMPROVEMENT OF AN APPROXIMATE SET OF LATENT ROOTS AND MODAL COLUMNS OF A MATRIX BY METHODS AKIN TO THOSE OF CLASSICAL PERTURBATION THEORY By H. A. JAHN {University of Birmingham) [Received 7 October 947]
IMPROVEMENT OF AN APPROXIMATE SET OF LATENT ROOTS AND MODAL COLUMNS OF A MATRIX BY METHODS AKIN TO THOSE OF CLASSICAL PERTURBATION THEORY By H. A. JAHN {University of Birmingham) [Received 7 October 947]
l ; Y l,l-1, Y l,-1+1; etc., plus a single non-degenerate function Y l,0, in axial symmetry.
 Chapter 6 Along "Reaction Paths", Orbitals Can be Connected One-to-One According to Their Symmetries and Energies. This is the Origin of the Woodward-offmann Rules I. Reduction in Symmetry As fragments
Chapter 6 Along "Reaction Paths", Orbitals Can be Connected One-to-One According to Their Symmetries and Energies. This is the Origin of the Woodward-offmann Rules I. Reduction in Symmetry As fragments
Quantum Theoretical Study on Single, Double and
 128 Quantum Theoretical Study on Single, Double and Triple Bonding between Nitrogen-Nitrogen Atoms Sigeru NAGAHARA Synopsis We intend to study the characteristics of single, double and triple bonds between
128 Quantum Theoretical Study on Single, Double and Triple Bonding between Nitrogen-Nitrogen Atoms Sigeru NAGAHARA Synopsis We intend to study the characteristics of single, double and triple bonds between
Adiabatic Coupling Constant g of the Binary Liquid Mixture Methanol Cyclohexane. Abstract
 Adiabatic Coupling Constant g of the Binary Liquid Mixture Methanol Cyclohexane Saja Omar and IssamAbdelraziq * Physics Dept. AnNajah National University, Nablus, Palestine Corresponding Author: IssamAbdelraziq
Adiabatic Coupling Constant g of the Binary Liquid Mixture Methanol Cyclohexane Saja Omar and IssamAbdelraziq * Physics Dept. AnNajah National University, Nablus, Palestine Corresponding Author: IssamAbdelraziq
The Relativistic Jahn-Teller Effect
 The Relativistic Jahn-Teller Effect Wolfgang Domcke Technical University of Munich Leonid V. Poluyanov Russian Academy of Sciences, Moscow 1 The Jahn-Teller Theorem: A configuration of a polyatomic molecule
The Relativistic Jahn-Teller Effect Wolfgang Domcke Technical University of Munich Leonid V. Poluyanov Russian Academy of Sciences, Moscow 1 The Jahn-Teller Theorem: A configuration of a polyatomic molecule
CP 52 Page In & Zone Sensitivity
 db SSM S R.0K R0.00K To Sheet ZA ON OFF C 0. R.K R 0.0K DUCK To Sheet Page Input Shield R 00 S PP Phantom Power 0/V L T C 0PF C L 0PF T EURO POS R0 0 R 0 R.0K 00/0V R Mic/Line.K R.K R.0K R 00 R. R0A KRD
db SSM S R.0K R0.00K To Sheet ZA ON OFF C 0. R.K R 0.0K DUCK To Sheet Page Input Shield R 00 S PP Phantom Power 0/V L T C 0PF C L 0PF T EURO POS R0 0 R 0 R.0K 00/0V R Mic/Line.K R.K R.0K R 00 R. R0A KRD
Table of C on t en t s Global Campus 21 in N umbe r s R e g ional Capac it y D e v e lopme nt in E-L e ar ning Structure a n d C o m p o n en ts R ea
 G Blended L ea r ni ng P r o g r a m R eg i o na l C a p a c i t y D ev elo p m ent i n E -L ea r ni ng H R K C r o s s o r d e r u c a t i o n a n d v e l o p m e n t C o p e r a t i o n 3 0 6 0 7 0 5
G Blended L ea r ni ng P r o g r a m R eg i o na l C a p a c i t y D ev elo p m ent i n E -L ea r ni ng H R K C r o s s o r d e r u c a t i o n a n d v e l o p m e n t C o p e r a t i o n 3 0 6 0 7 0 5
z E z *" I»! HI UJ LU Q t i G < Q UJ > UJ >- C/J o> o C/) X X UJ 5 UJ 0) te : < C/) < 2 H CD O O) </> UJ Ü QC < 4* P? K ll I I <% "fei 'Q f
 I % 4*? ll I - ü z /) I J (5 /) 2 - / J z Q. J X X J 5 G Q J s J J /J z *" J - LL L Q t-i ' '," ; i-'i S": t : i ) Q "fi 'Q f I»! t i TIS NT IS BST QALITY AVAILABL. T Y FRNIS T TI NTAIN A SIGNIFIANT NBR
I % 4*? ll I - ü z /) I J (5 /) 2 - / J z Q. J X X J 5 G Q J s J J /J z *" J - LL L Q t-i ' '," ; i-'i S": t : i ) Q "fi 'Q f I»! t i TIS NT IS BST QALITY AVAILABL. T Y FRNIS T TI NTAIN A SIGNIFIANT NBR
MANY ELECTRON ATOMS Chapter 15
 MANY ELECTRON ATOMS Chapter 15 Electron-Electron Repulsions (15.5-15.9) The hydrogen atom Schrödinger equation is exactly solvable yielding the wavefunctions and orbitals of chemistry. Howev er, the Schrödinger
MANY ELECTRON ATOMS Chapter 15 Electron-Electron Repulsions (15.5-15.9) The hydrogen atom Schrödinger equation is exactly solvable yielding the wavefunctions and orbitals of chemistry. Howev er, the Schrödinger
CATAVASII LA NAȘTEREA DOMNULUI DUMNEZEU ȘI MÂNTUITORULUI NOSTRU, IISUS HRISTOS. CÂNTAREA I-A. Ήχος Πα. to os se e e na aș te e e slă ă ă vi i i i i
 CATAVASII LA NAȘTEREA DOMNULUI DUMNEZEU ȘI MÂNTUITORULUI NOSTRU, IISUS HRISTOS. CÂNTAREA I-A Ήχος α H ris to os s n ș t slă ă ă vi i i i i ți'l Hris to o os di in c ru u uri, în tâm pi i n ți i'l Hris
CATAVASII LA NAȘTEREA DOMNULUI DUMNEZEU ȘI MÂNTUITORULUI NOSTRU, IISUS HRISTOS. CÂNTAREA I-A Ήχος α H ris to os s n ș t slă ă ă vi i i i i ți'l Hris to o os di in c ru u uri, în tâm pi i n ți i'l Hris
11/29/2014. Problems with Valence Bond Theory. VB theory predicts many properties better than Lewis Theory
 Problems with Valence Bond Theory VB theory predicts many properties better than Lewis Theory bonding schemes, bond strengths, bond lengths, bond rigidity however, there are still many properties of molecules
Problems with Valence Bond Theory VB theory predicts many properties better than Lewis Theory bonding schemes, bond strengths, bond lengths, bond rigidity however, there are still many properties of molecules
Structure of diatomic molecules
 Structure of diatomic molecules January 8, 00 1 Nature of molecules; energies of molecular motions Molecules are of course atoms that are held together by shared valence electrons. That is, most of each
Structure of diatomic molecules January 8, 00 1 Nature of molecules; energies of molecular motions Molecules are of course atoms that are held together by shared valence electrons. That is, most of each
Section 5.8. (i) ( 3 + i)(14 2i) = ( 3)(14 2i) + i(14 2i) = {( 3)14 ( 3)(2i)} + i(14) i(2i) = ( i) + (14i + 2) = i.
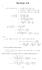 1. Section 5.8 (i) ( 3 + i)(14 i) ( 3)(14 i) + i(14 i) {( 3)14 ( 3)(i)} + i(14) i(i) ( 4 + 6i) + (14i + ) 40 + 0i. (ii) + 3i 1 4i ( + 3i)(1 + 4i) (1 4i)(1 + 4i) (( + 3i) + ( + 3i)(4i) 1 + 4 10 + 11i 10
1. Section 5.8 (i) ( 3 + i)(14 i) ( 3)(14 i) + i(14 i) {( 3)14 ( 3)(i)} + i(14) i(i) ( 4 + 6i) + (14i + ) 40 + 0i. (ii) + 3i 1 4i ( + 3i)(1 + 4i) (1 4i)(1 + 4i) (( + 3i) + ( + 3i)(4i) 1 + 4 10 + 11i 10
P a g e 3 6 of R e p o r t P B 4 / 0 9
 P a g e 3 6 of R e p o r t P B 4 / 0 9 p r o t e c t h um a n h e a l t h a n d p r o p e r t y fr om t h e d a n g e rs i n h e r e n t i n m i n i n g o p e r a t i o n s s u c h a s a q u a r r y. J
P a g e 3 6 of R e p o r t P B 4 / 0 9 p r o t e c t h um a n h e a l t h a n d p r o p e r t y fr om t h e d a n g e rs i n h e r e n t i n m i n i n g o p e r a t i o n s s u c h a s a q u a r r y. J
Exhibit 2-9/30/15 Invoice Filing Page 1841 of Page 3660 Docket No
 xhibit 2-9/3/15 Invie Filing Pge 1841 f Pge 366 Dket. 44498 F u v 7? u ' 1 L ffi s xs L. s 91 S'.e q ; t w W yn S. s t = p '1 F? 5! 4 ` p V -', {} f6 3 j v > ; gl. li -. " F LL tfi = g us J 3 y 4 @" V)
xhibit 2-9/3/15 Invie Filing Pge 1841 f Pge 366 Dket. 44498 F u v 7? u ' 1 L ffi s xs L. s 91 S'.e q ; t w W yn S. s t = p '1 F? 5! 4 ` p V -', {} f6 3 j v > ; gl. li -. " F LL tfi = g us J 3 y 4 @" V)
This is the author s version of a work that was submitted for publication prior to peer-review. This is known as the pre-print.
 This is the author s version of a work that was submitted for publication prior to peer-review. This is known as the pre-print. Citation for author s submitted version Zhang, Yong-Hui, Tang, Li-Yan, Zhang,
This is the author s version of a work that was submitted for publication prior to peer-review. This is known as the pre-print. Citation for author s submitted version Zhang, Yong-Hui, Tang, Li-Yan, Zhang,
Diatomic Molecules. 7th May Hydrogen Molecule: Born-Oppenheimer Approximation
 Diatomic Molecules 7th May 2009 1 Hydrogen Molecule: Born-Oppenheimer Approximation In this discussion, we consider the formulation of the Schrodinger equation for diatomic molecules; this can be extended
Diatomic Molecules 7th May 2009 1 Hydrogen Molecule: Born-Oppenheimer Approximation In this discussion, we consider the formulation of the Schrodinger equation for diatomic molecules; this can be extended
INSTRUCTIONS: 7. Relax and do well.
 EM 1314 Name Exam III TA Name John III. Gelder November 16, 1992 Lab Section INSTRUTIONS: 1. This examination consists of a total of 7 different pages. The last page includes a periodic table and some
EM 1314 Name Exam III TA Name John III. Gelder November 16, 1992 Lab Section INSTRUTIONS: 1. This examination consists of a total of 7 different pages. The last page includes a periodic table and some
In this lecture, we will go through the hyperfine structure of atoms. The coupling of nuclear and electronic total angular momentum is explained.
 Lecture : Hyperfine Structure of Spectral Lines: Page- In this lecture, we will go through the hyperfine structure of atoms. Various origins of the hyperfine structure are discussed The coupling of nuclear
Lecture : Hyperfine Structure of Spectral Lines: Page- In this lecture, we will go through the hyperfine structure of atoms. Various origins of the hyperfine structure are discussed The coupling of nuclear
Higher Orders Instability of a Hollow Jet Endowed with Surface Tension
 Mechanics and Mechanical Engineering Vol. 2, No. (2008) 69 78 c Technical University of Lodz Higher Orders Instability of a Hollow Jet Endowed with Surface Tension Ahmed E. Radwan Mathematics Department,
Mechanics and Mechanical Engineering Vol. 2, No. (2008) 69 78 c Technical University of Lodz Higher Orders Instability of a Hollow Jet Endowed with Surface Tension Ahmed E. Radwan Mathematics Department,
Approximation Methods in QM
 Chapter 3 Approximation Methods in QM Contents 3.1 Time independent PT (nondegenerate)............... 5 3. Degenerate perturbation theory (PT)................. 59 3.3 Time dependent PT and Fermi s golden
Chapter 3 Approximation Methods in QM Contents 3.1 Time independent PT (nondegenerate)............... 5 3. Degenerate perturbation theory (PT)................. 59 3.3 Time dependent PT and Fermi s golden
!IhIIiIiIII IIIBIIIKI AD-A N PAGE ELECTE AFOSR.TF :3 8. om1 No 0704o0ea
 AD-A267 0820N PAGE Form Approved om1 No 0704o0ea Publi ~lqe I hour per response. including the time for reviewing Ir$uaclon. searching esisting data sources. gath t ecollection of Information. Send comments
AD-A267 0820N PAGE Form Approved om1 No 0704o0ea Publi ~lqe I hour per response. including the time for reviewing Ir$uaclon. searching esisting data sources. gath t ecollection of Information. Send comments
PHYSICAL CHEMISTRY I. Chemical Bonds
 PHYSICAL CHEMISTRY I Chemical Bonds Review The QM description of bonds is quite good Capable of correctly calculating bond energies and reaction enthalpies However it is quite complicated and sometime
PHYSICAL CHEMISTRY I Chemical Bonds Review The QM description of bonds is quite good Capable of correctly calculating bond energies and reaction enthalpies However it is quite complicated and sometime
Physics 828 Problem Set 7 Due Wednesday 02/24/2010
 Physics 88 Problem Set 7 Due Wednesday /4/ 7)a)Consider the proton to be a uniformly charged sphere of radius f m Determine the correction to the s ground state energy 4 points) This is a standard problem
Physics 88 Problem Set 7 Due Wednesday /4/ 7)a)Consider the proton to be a uniformly charged sphere of radius f m Determine the correction to the s ground state energy 4 points) This is a standard problem
NO-Sl~l 966 RN INTERPRETiTION OF THE 02 AMGR ELECTRON SPECTRUNl(U) 1/1
 NOSl~l 966 RN INTERPRETiTION OF THE 02 AMGR ELETRON SPETRUNl(U) 1/1 MRSHINGTON UNIV MRSHINOTON D DEPT OF HEMISTRY UNSSIIEDH SANDE ET AL. OT 95 TR2S NSSSI4BSK9852 FO74 N 1 tgeorge UASIIE G? N L L. Q.. 1111111
NOSl~l 966 RN INTERPRETiTION OF THE 02 AMGR ELETRON SPETRUNl(U) 1/1 MRSHINGTON UNIV MRSHINOTON D DEPT OF HEMISTRY UNSSIIEDH SANDE ET AL. OT 95 TR2S NSSSI4BSK9852 FO74 N 1 tgeorge UASIIE G? N L L. Q.. 1111111
MO Calculation for a Diatomic Molecule. /4 0 ) i=1 j>i (1/r ij )
 MO Calculation for a Diatomic Molecule Introduction The properties of any molecular system can in principle be found by looking at the solutions to the corresponding time independent Schrodinger equation
MO Calculation for a Diatomic Molecule Introduction The properties of any molecular system can in principle be found by looking at the solutions to the corresponding time independent Schrodinger equation
(tnaiaun uaejna) o il?smitfl?^ni7wwuiinuvitgviisyiititvi2a-a a imaviitjivi5a^ qw^ww^i fiaa!i-j?s'u'uil?g'ijimqwuwiijami.wti. a nmj 1,965,333.
 0 fltu77jjiimviu«7mi^ gi^"ijhm?'ijjw?flfi^ V m 1 /14 il?mitfl?^i7wwuiinuvitgviiyiititvi2- imviitvi^ qw^ww^i fi!i-j?'u'uil?g'iqwuwiijmi.wti twwrlf^ imii2^
0 fltu77jjiimviu«7mi^ gi^"ijhm?'ijjw?flfi^ V m 1 /14 il?mitfl?^i7wwuiinuvitgviiyiititvi2- imviitvi^ qw^ww^i fi!i-j?'u'uil?g'iqwuwiijmi.wti twwrlf^ imii2^
Correlated exponential functions in high precision calculations for diatomic molecules. Abstract
 Version 3.1 Correlated exponential functions in high precision calculations for diatomic molecules Krzysztof Pachucki Faculty of Physics University of Warsaw Hoża 69 00-681 Warsaw Poland Abstract Various
Version 3.1 Correlated exponential functions in high precision calculations for diatomic molecules Krzysztof Pachucki Faculty of Physics University of Warsaw Hoża 69 00-681 Warsaw Poland Abstract Various
I n t e r n a t i o n a l E l e c t r o n i c J o u r n a l o f E l e m e n t a r y E.7 d u, c ai ts is ou n e, 1 V3 1o-2 l6, I n t h i s a r t
 I n t e r n a t i o n a l E l e c t r o n i c J o ue rlne am l e not fa r y E d u c a t i o n, 2 0 1 4, 1 37-2 ( 16 ). H o w R e a d i n g V o l u m e A f f e c t s b o t h R e a d i n g F l u e n c y
I n t e r n a t i o n a l E l e c t r o n i c J o ue rlne am l e not fa r y E d u c a t i o n, 2 0 1 4, 1 37-2 ( 16 ). H o w R e a d i n g V o l u m e A f f e c t s b o t h R e a d i n g F l u e n c y
B ooks Expans ion on S ciencedirect: 2007:
 B ooks Expans ion on S ciencedirect: 2007: 1 INFORUM, 22-24 May, Prague Piotr Golkiewicz Account Manager Elsevier B.V. Email: p.golkiewicz@elsevier.com Mobile: +48 695 30 60 17 2 Pres entation Overview
B ooks Expans ion on S ciencedirect: 2007: 1 INFORUM, 22-24 May, Prague Piotr Golkiewicz Account Manager Elsevier B.V. Email: p.golkiewicz@elsevier.com Mobile: +48 695 30 60 17 2 Pres entation Overview
AP Human Geography Pre-Test. A. World Cities. B. Multiple Nuclei Model. D, Least Cost Theory. E. Cultural Landscape. F, Concentric Zone Model
 Paul T. Gray, Jr. Russellville High Schl - Russellville, AR paul.gray@rsdmail.k12.ar.us AP Human Gegraphy Pre-Test MATCHING- Therists in Human Gegraphy 1. Carl Sauer A. Wrld Cities 2. Jhann Heinrich vn
Paul T. Gray, Jr. Russellville High Schl - Russellville, AR paul.gray@rsdmail.k12.ar.us AP Human Gegraphy Pre-Test MATCHING- Therists in Human Gegraphy 1. Carl Sauer A. Wrld Cities 2. Jhann Heinrich vn
No. 2 lectronic state and potential energy function for UH where ρ = r r e, r being the interatomic distance and r e its equilibrium value. How
 Vol 12 No 2, February 2003 cfl 2003 Chin. Phys. Soc. 1009-1963/2003/12(02)/0154-05 Chinese Physics and IOP Publishing Ltd lectronic state and potential energy function for UH 2+* Wang Hong-Yan( Ψ) a)y,
Vol 12 No 2, February 2003 cfl 2003 Chin. Phys. Soc. 1009-1963/2003/12(02)/0154-05 Chinese Physics and IOP Publishing Ltd lectronic state and potential energy function for UH 2+* Wang Hong-Yan( Ψ) a)y,
Page Input. Shield EURO 3-STA GND. 470pF Z1B. (+20 db) 10K % C38 LINK LEVEL. To Sheet 3 GND. 120Hz. Page Level R11 15K. 7kHz GND .
 Ducker Depth 0 0 db R K R0 K SSM S Ducker Depth 00 To Sheet ZA C 0. R.K R 0K DUCK To Sheet Page Input S PT Phantom Power J EURO STA R 00 L T L T C 0/ C 0pF R0 0 R 0 C 00/0 SA Mic/Line PT C 00/0 R K R.K
Ducker Depth 0 0 db R K R0 K SSM S Ducker Depth 00 To Sheet ZA C 0. R.K R 0K DUCK To Sheet Page Input S PT Phantom Power J EURO STA R 00 L T L T C 0/ C 0pF R0 0 R 0 C 00/0 SA Mic/Line PT C 00/0 R K R.K
