DEVELOPMENT OF A FLOW-THROUGH CELL FOR ACCURATE MEASUREMENTS OF LOW ELECTROLYTIC CONDUCTIVITY
|
|
|
- Trevor Miller
- 5 years ago
- Views:
Transcription
1 XIX IMEKO World Congress Fundamental and Applied Metrology September 6 11, 2009, Lisbon, Portugal DEVELOPMENT OF A FLOW-THROUGH CELL FOR ACCURATE MEASUREMENTS OF LOW ELECTROLYTIC CONDUCTIVITY C. Boveri 1, F. Durbiano 2, D. Serazio 3 1 Politecnico di Torino, Torino, Italy, c.boveri@inrim.it 2 Istituto Nazionale di Ricerca Metrologica, Torino, Italy, f.durbiano@inrim.it 3 Istituto Nazionale di Ricerca Metrologica, Torino, Italy, d.serazio@inrim.it Abstract The present paper focuses on the development of a flow-through cell and a closed circuit that permit to carry out measurements of low electrolytic conductivity of aqueous solutions under flowing condition. The traceability path has been set as follows: samples with conductivity between 200 μs cm -1 and 50 μs cm -1 have been employed for the calibration of the geometric constant of the new cell, by comparison with the primary cell of Istituto Nazionale di Ricerca Metrologica (I.N.Ri.M.). Then the flow-through cell has been used to measure values with decreasing conductivities down to 1 μs cm -1. The measurement system capabilities have been evaluated to be limited by contamination effects. Keywords: pure water, low conductivity measurement, flow-through cell 1. INTRODUCTION Pure water is extensively used in many critical applications, which include chip fabrication for semiconductors (like microelectronics rinse waters), intravenous solutions for pharmaceuticals, and high-pressure boilers for power generation [1]. Pure water has a very low, but not quite zero, electrical conductivity. Deviation from this value is a measure of trace ionic impurities. The electrolytic conductivity method is applicable for detecting trace amounts of ionic contaminants in water [2]. Accurate and reliable (hence, traceable to International System of units) measurement results are needed. Certified reference materials or calibrated sensors should be used for this purpose. Consequently, National Metrology Institutes (NMIs) dealt with the establishment of traceability for the measurement of electrolytic conductivity [3]. At the Istituto Nazionale di Ricerca Metrologica (I.N.Ri.M.) a primary measurement system for electrolytic conductivity in the range between 50 μs cm -1 e 2 S m -1 has been developed. Numerous International Comparisons have been organised by the Comité Consultatif pour la Quantité de Matière (CCQM) in order to check the accuracies claimed by the NMIs [4-6]. Recently several NMIs (I.N.Ri.M. among them), to answer the needs of the industrial sectors described above, engaged in the extension of traceability of electrolytic conductivity towards pure water value (about 1 μs cm -1 ). Within this effort, a new International Comparison CCQM- P83 on low conductivity values, 5 μs cm -1, has been organised and it is expected in In a measurement system intended for accurate measurements below 10 μs cm -1, flow-through or in-line conductivity cells should be used, in order to prevent contamination from atmosphere and by wetted surfaces. Atmospheric carbon dioxide (CO 2 ) can reach an equilibrium concentration in water of about 1 mg l -1, and because of formation of carbonic acid can add approximately 1 μs cm -1 to the original sample conductivity [2]. In this work the new flow-through cell, and the closed circuit measurement system developed at I.N.Ri.M., are described. Moreover, the measurement traceability and first measurements of low conductivity are reported. 2. MEASUREMENT SYSTEM In order to measure the conductivity of purified water and water for injection, having a conductivity below 1 μs cm -1, a flow-through cell has been designed at I.N.Ri.M. and constructed by Disa Raffaele e F.lli glass blower. The cell is shown in Fig. 1: it is made of a Pyrex glass chamber holding two parallel and faced electrodes, positioned at a distance of 10 mm. The electrodes (thick 0.5 mm and with the diameter of 20 mm) are of smooth and pure platinum to guarantee chemical inertness and minimise impurity permeability [7]. They are connected to electrical coaxial cables with platinum wires, coated with a sheath of a boron glass with a thermal expansion coefficient matched to platinum. The platinum wires have been platinum spotwelded to the electrodes to avoid contamination of the sample. Moreover, the electrodes are removable to allow eventually a deep cleaning. The cell chamber has a capacity of about 180 ml in order to permit a good thermal capacity. To fill and to empty the chamber an input tube has been placed between the removable electrodes, and a SVL cap avoids the solution contamination. The cell includes two side pipelines, with Teflon taps, for the circulation of the sample flux. The sample inlet cross section is smaller than the outlet to guarantee the cell integrity caused by pressure fluctuations.
2 In the conjunction with the cell chamber the pipe inlet has a turn, to create a sample turbulent motion and to keep stirred the solution. A linear flux between electrodes has to be anyhow avoided because it would be confused with ion migration. All the electrodes, the pipelines, and the tube input are vertical extended for eventually introducing the cell in a liquid thermostatic bath. an air bath, see Fig. 2; the cell is put inside a beaker full of water for increasing the thermal capacity, hence the lowterm temperature stability. The significant heat dissipation of the peristaltic pump does not permit to place the entire closed circuit inside the air bath; the portion of the circuit with the pump has been placed outside, in a thermostated (23 ± 1 C ) laboratory. A picture of the instruments and the circuit outside the bath is shown in Fig. 3. Cell impedance is measured in the 20 Hz 2 MHz range with a QuadTech 7600b LCR meter. Bridge accuracy specifications is 0.07 % when measuring a conductivity of 200 μs cm -1, and 0.1 % for a conductivity of 50 μs cm -1. The specified accuracy has been considered insufficient, and has been improved fivefold with calibrations (before and after every measurement run) using Tinsley standard ac resistors having resistance values bracketing the resistance range of the sample. Impedance spectra are acquired and processed by purpose LabWindows/CVI and Matlab codes. Fig. 1. I.N.Ri.M. flow-through cell The sample flows in a closed circuit glass pipeline to prevent the CO 2 contamination, and the flux is created by a Watson-Marlow Bredel Sci-Q 323 peristaltic pump that operates with a speed of 90 rpm. Most of the pipeline circuit is constituted of Pyrex glass. For the cell connections and tube section within the pump, after some tests done with Teflon and Fluorel tubes, Tygon pipeline has been chosen, which shows up to now the best characteristics as lowest permeability and contamination. Fig. 2. Measurement system inside the air thermostatic bath At 25 C the conductivity temperature coefficient of pure water is about 5.4 % C -1 [1, 7]. Cell temperature is measured by a PT-100 thermoresistance (glued to outside wall) and a multimeter (Agilent Tech. mod. 3458), with an uncertainty of 0.01 C. Temperature control is performed by Fig. 3. Measurement system outside the air thermostatic bath Samples having different nominal conductivity values (200, 70, 50, 20, and 1 μs cm -1 ) have been investigated. The first four samples were solutions of KCl in ultra pure water, the last one has been prepared by leaving ultra pure water, poured into a capacious bottle, at rest without top for two days in order to reach the equilibrium with the laboratory CO 2. In a first stage, the geometric constant of the flowthrough cell has been calibrated by comparison with the I.N.Ri.M. primary cell. 200 μs cm -1, 70 μs cm -1, and 50 μs cm -1 solutions have been used for the calibration, because the primary cell does not allow measurements with reasonable accuracy in the lower conductivity range. The cell constant has been estimated by the mean of the values obtained for each sample, as suggested in [8]. In the second stage of the experiment, the calibrated flow-through cell has been employed for measurements of 20 and 1 μs cm -1 samples. The conductivity, k, of each sample is given by (1): k = R C cell [ 1 α ( T T )] T Re f where C cell is the geometric constant of the flow-through cell, defined by its calibration with the I.N.Ri.M. primary (1)
3 cell, α T is the temperature coefficient, T is the temperature, and T Ref is the reference temperature (25 C). Many impedance sweeps (20 Hz - 2 MHz) in the RLC bridge frequency range have been performed, and the ones showing the better temperature stability have been selected. In order to reject parasitic contributions, the solution resistance and reactance versus frequency have been plotted, and the resistance at which the minimum point of reactance is verified has been extracted. The minimum of the reactance values corresponds to the plateau range of the resistance values (bulk resistance). This value of resistance is utilized for the determination of the electrolytic conductivity value of solution. So that the polarisation effects at low frequency range and capacitive effects at high frequency range are minimised. The mean value of the minimum point resistances has been evaluated, and has been taken as representative of resistance, R, of the solution. Then R, has been corrected to T Ref and the conductivity value has been estimated by (1). For 1 μs cm -1 sample, in addition with measurements with the RLC bridge, further measurements have been conducted with a calibrated lock-in amplifier (EG&G 7265) to achieve an extended frequency range ( Hz). The need of data in such low range is important for a better understanding of the resistance behaviour versus frequency, strongly influenced by interface contributions. largest value among ones obtained by the measurements of the three solutions has been chosen as the uncertainty contribution associated to C cell, that is of m -1. In order to have the possibility to use the C cell value for the measurements with the flow system, with the closed circuit, a measurement comparison between the static system and the flow one considering the same solution has been carried out. This comparison has been undertaken with the 70 μs m -1 and the 50 μs cm -1 samples. The obtained differences were respectively of 0.01 % and of 0.02 %, which have been considered negligible with respect to the uncertainty associated to the cell constant. The flow system used 7 hours to reach the temperature stability, so each measurement kept at least 10 hours. The maximum temperature variation considered acceptable at 25 C was of 0.02 C. The solution with nominal conductivity of 20 μs cm -1 has been measured, and an example of plot with the resistance and reactance as a function of frequency is shown in Fig RESULTS The first measurement stage, the calibration of the flowthrough cell, has been carried out by repeating the measurements at least three times for each conductivity value. The calibration by comparison of the flow-through cell has been undertaken considering a static system, without the closed circuit, in order to be in the same conditions of the primary system and because the measured solutions (samples with conductivities of μs cm -1, μs cm -1, and μs cm -1 ) showed enough stability time. Table 1 resumes the results of each solution and their associated uncertainties. Table 1. Cell constants and the associated standard uncertainties obtained with the three solutions. Electrolytic conductivity (μs cm -1 ) Cell constant (m -1 ) Expanded uncertainty (m -1 ) In the third column of Table 1 the expanded uncertainties include the contributions associated to the temperature (the temperature stability was kept during the measurements within 0.01 C), to the solutions measured with the primary system, to the measurement repeatability, and to the RLC bridge. The C cell estimation has been obtained by the mean of geometric constants measured with the three solutions, and has been resulted of m -1. The Fig. 4. Example of selected resistance ( ) and reactance ( ) sweeps carried out with the RLC bridge for the 20 μs cm -1 solution. In Fig. 4, the minimum point of reactance (modulus of the value) was at 1000 Hz. During the same measurement, after the solution has reached the temperature stability, the resistance has slightly continued to decrease due to the contamination of atmosphere CO 2 and of pipeline. Taking into account the time spent to carry out a set of three measurements, the 20 μs cm -1 sample has changed of 0.01 % per hour. Table 2. Uncertainty budget for the solution with nominal conductivity of 20 μs cm -1 at 25 C. Quantity Estimate Standard Uncertainty Assumed distribution C cell normal R normal T rectangular α Τ rectangular
4 The conductivity value resulted to be μs cm -1, considering the model equation (1). The uncertainty contributions are summarized in Table 2. In the treatment of the uncertainty the relevant international documents [9] and [10] have been followed. The standard uncertainties have been combined by applying the uncertainty propagation law, where the input quantities are considered uncorrelated. The standard uncertainty resulted to be S m -1 and the expanded uncertainty S m (1.2 %). The main contribution (34.08 Ω) associated to R includes the uncertainties due to the RLC bridge calibration, the measurement repeatability, and the solution contamination due to CO 2 and pipeline. The contribution amount due to the RLC bridge is known, that is evaluated 5.49 Ω. Most of the term is due to the repetition shortage and to the contamination which have not been distinguished. The main problem of the measurements carried out with the 1 μs cm -1 sample was referred to the solution stability due to the pipeline and to the CO 2 contamination. After the solution has reached the temperature stability, the resistance value (measured with the RLC bridge) has continued to decrease during the time of the same measurement of 0.07 % per hour. Since the solution wasn t in contact with the air, that change was mostly due to the contamination of Tygon pipeline. A set of seven repetitions with the 1 μs cm -1 sample have been carried out distributed on ten days. Considering also the solution storage in a capacious bottle, with air in it, the resistance has decreased of about 1 % per hour. The uncertainty contributions are summarized in Table 3. Table 3. Uncertainty budget for the solution with nominal conductivity of 1 μs cm -1. Quantity Estimate Standard Uncertainty Assumed distribution C cell normal R normal T rectangular α Τ rectangular The obtained conductivity value was 0.82 μs cm -1, the contribution associated to the RLC bridge was of 211 Ω, and the expanded uncertainty S m -1. Another significant problem has been caused from the detection of the frequency value at which the solution resistance could be considered as representative of the bulk (no polarisation effects are included). The reactance minimum point was at 25 Hz, very close to the minimum value of the RLC bridge full scale. Consequently, another set of six measurements have been undertaken with the lockin in the range between 0.1 Hz and 50 Hz in order to define the resistance range where the parasitic effects are minimised. Before each sample measurement at the chosen frequencies, the lock-in autophase was started with a resistor with a similar resistance. The current measurements (imaginary and real terms) have been carried out and transformed respectively in reactance and resistance values. Each frequency sweep has been carried out selecting two ranges: between 0.1 Hz and 1 Hz the Time Constant (TC) was 10 s and the time between two values (delta t) was 50 s; in the frequency range 1-50 Hz, TC was 5 s and Δt 25 s. The main instrument settings were: Input Limit 3 μa (correspondent to a gain of 0 db); Sensitivity 1 μa; Voltage 0.1 V (the saturation occurs if more than 0.1 V was set); and Signal Channel Coupling DC (for the reactance measurement). A resistance and reactance measurement comparison of 1 μs cm -1 sample with the RLC bridge and with the lock-in as a function of frequency is shown in Fig. 5. It shows a good compatibility of the measurements carried out with the two instruments. The resistance seemed to be invariant to frequency down to 10 Hz. At lower frequencies interface effects occur. Fig. 5. Comparison between resistance and reactance sweeps undertaken with RLC bridge and lock-in with the 1 μs cm -1 sample (for RLC bridge: resistance ( ) and reactance ( ); for lock-in: resistance ( ) and reactance ( )). In this case the obtained conductivity value was of 0.77 μs cm -1,and the expanded uncertainty of S m -1. The uncertainty contribution due to the lock-in was 1116 Ω, whereas the one due to the repeatability and contamination was 8632 Ω. 4. CONCLUSIONS A flow-through cell and a closed circuit that permit to carry out low electrolytic conductivity measurements of aqueous solutions have been developed. After the calibration of the geometric constant of the new cell have been performed by solutions with conductivity from 200 μs cm -1 to 50 μs cm -1, it has been employed to measure solutions with conductivity of 20 μs cm -1 and 1 μs cm -1. Since the solutions with very low conductivity show frequencies at which the resistance could be considered as representative of the solution close to the minimum value of the RLC bridge full scale (25 Hz), a lockin amplifier has been employed. A good agreement has been obtained between the RLC bridge measurements and the lock-in ones both for the resistance and the reactance and the frequency, although the lock-in is not an accurate instrument for this kind of measurements, the low frequencies, and the applied voltage was low and the measured current high.
5 Moreover, some indications about uncertainty contributions of 20 μs cm -1 and 1 μs cm -1 samples have been reported. The future activities regards the improvement of the measurement system in order to increase the time stability of the solution. For example, a study on soft plastic tubes with the most suitable technical specifications will be carried out. Moreover, a solution storing system which operates under argon gas will be developed. Another improvement will consist in the test and study of a more specific and accurate instrumentation for the resistance and reactance at low frequencies. In addiction, the development of a measurement system directly connected to the outlet of a ultra pure water production system will be carried out in order to have the possibility to perform measurements of electrolytic conductivity around 0.05 μs cm -1. Concerning this value, the main aim will be to achieve a measurement uncertainty lower than 2 % as prescribed by the U.S. Pharmacopeia and enforced by the Food and Drug Administration for the manufacture and use of ultra pure water and water for injection. REFERENCES [1] T. S. Light, S. Licht, A. C. Bevilacqua, K. Morash, The fundamental conductivity and resistivity of water, Electrochemical and solid-state letters, Vol. 8, n. 1, pp. E16-E19, [2] Electrical conductivity and resistivity of a flowing high purity water sample, American Society for testing and materials ASTM Designation: , [3] F. Brinkmann, N. E. Dam, E. Deák, F. Durbiano, E. Ferrara, J. Fükö, et al., Primary methods for the measurement of electrolytic conductivity, Accredit. Qual. Assur., Vol. 8, n. 7, pp , July [4] F. Durbiano, L. Callegaro, P.P. Capra, and V. D Elia, Key Comparison CCQM-K36: Electrolytic Conductivity at 0.5 and S/m, IEN Measurement Report, IEN Technical Report n. 694, October [5] F. Durbiano, L. Callegaro, P. P. Capra, and G. Marullo Reedtz, Pilot Comparison CCQM-P47 on Electrolytic Conductivity of dilute solutions, IEN Measurement Report, IEN Technical Report n. 666, October [6] F. Durbiano, E. Ferrara, G. Marullo Reedtz, and P. P. Capra, Pilot Study CCQM-P22 on Electrolytic Conductivity, IEN Measurement Report, IEN Technical Report n. 629, May [7] Standard Test Methods for Electrical Conductivity and Resistivity of Water, American Society for testing and materials ASTM Designation: D , [8] Calibration method for conductivity cells, International Recommandation OIML 68, Edition 1985 (E). [9] European Co-operation for Accreditation, EA 4/02, Expression of the Uncertainty of Measurement in Calibration. Dec [10] ISO Guide to the Expression of Uncertainty in Measurement (1995).
ELECTROLYTIC CONDUCTIVITY AS A QUALITY INDICATOR FOR BIOETHANOL
 XX IMEKO World Congress Metrology for Green Growth September 9 14, 2012, Busan, Republic of Korea ELECTROLYTIC CONDUCTIVITY AS A QUALITY INDICATOR FOR BIOETHANOL S. Seitz 1, P. Spitzer 1, F. Durbiano 2,
XX IMEKO World Congress Metrology for Green Growth September 9 14, 2012, Busan, Republic of Korea ELECTROLYTIC CONDUCTIVITY AS A QUALITY INDICATOR FOR BIOETHANOL S. Seitz 1, P. Spitzer 1, F. Durbiano 2,
White Paper. Perform Conductivity Measurements In Compliance with USP <645>
 Perform Conductivity Measurements In Compliance with USP Water is the most widely used substance, raw material, or ingredient in the production, processing and formulation of compendial articles.
Perform Conductivity Measurements In Compliance with USP Water is the most widely used substance, raw material, or ingredient in the production, processing and formulation of compendial articles.
IMPLEMENTATION OF ETHANOL HEAT PIPE AT CETIAT
 IMPLEMENTATION OF ETHANOL HEAT PIPE AT CETIAT JO Favreau 1, E Georgin 1, B Savanier 1, A. Merlone 2 1 CETIAT, 96100 Villeurbanne, France 2 INRIM, Torino, Italy Abstract. CETIAT is a calibration laboratory
IMPLEMENTATION OF ETHANOL HEAT PIPE AT CETIAT JO Favreau 1, E Georgin 1, B Savanier 1, A. Merlone 2 1 CETIAT, 96100 Villeurbanne, France 2 INRIM, Torino, Italy Abstract. CETIAT is a calibration laboratory
Conductivity Theory and Practice
 Conductivity Theory and Practice - 1 - - when you need to be sure... Preface The importance of conductivity Conductivity measurement is an extremely widespread and useful method, especially for quality
Conductivity Theory and Practice - 1 - - when you need to be sure... Preface The importance of conductivity Conductivity measurement is an extremely widespread and useful method, especially for quality
Automated volume measurement for weihts using acoustic volumeter
 IMEKO 20th TC3, 3rd TC16 and 1st TC22 International Conference Cultivating metrological knowledge 27th to 30th November, 2007. Merida, Mexico. Automated volume measurement for weihts using acoustic volumeter
IMEKO 20th TC3, 3rd TC16 and 1st TC22 International Conference Cultivating metrological knowledge 27th to 30th November, 2007. Merida, Mexico. Automated volume measurement for weihts using acoustic volumeter
Stage 4 G-03: CONDUCTIVITY OF SOLUTIONS CP: USP. May 2015 INTRODUCTION
 002-1601PDG.pdf 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 Stage 4 G-03: CONDUCTIVITY OF SOLUTIONS CP: USP May 2015 INTRODUCTION This chapter provides information on how to apply electrical
002-1601PDG.pdf 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 Stage 4 G-03: CONDUCTIVITY OF SOLUTIONS CP: USP May 2015 INTRODUCTION This chapter provides information on how to apply electrical
ph Electrode basics ph Electrodes Model 19 B /1 =1 m 24 B /1 =1 m
 s ph electrodes are constructed from a special composition which senses the hydrogen ion concentration. This is typically composed of alkali metal ions. The alkali metal ions of the and the hydrogen ions
s ph electrodes are constructed from a special composition which senses the hydrogen ion concentration. This is typically composed of alkali metal ions. The alkali metal ions of the and the hydrogen ions
EURAMET Project 858: Hydrostatic weighing exchange of experiences. Project outline
 EURAMET Project 858: Hydrostatic weighing exchange of experiences Project outline A bilateral comparison has been carried out between the national measurement institutes INRIM, Italy and IPQ, Portugal,
EURAMET Project 858: Hydrostatic weighing exchange of experiences Project outline A bilateral comparison has been carried out between the national measurement institutes INRIM, Italy and IPQ, Portugal,
VI. EIS STUDIES LEAD NANOPOWDER
 VI. EIS STUDIES LEAD NANOPOWDER 74 26. EIS Studies of Pb nanospheres Impedance (valid for both DC and AC), a complex resistance occurs when current flows through a circuit (composed of various resistors,
VI. EIS STUDIES LEAD NANOPOWDER 74 26. EIS Studies of Pb nanospheres Impedance (valid for both DC and AC), a complex resistance occurs when current flows through a circuit (composed of various resistors,
Il nuovo Sistema Internazionale (SI) Le unità elettromagnetiche
 Il nuovo Sistema Internazionale (SI) Le unità elettromagnetiche Luca Callegaro Istituto Nazionale di Ricerca Metrologica Torino, Italy 4 Maggio 2017 Luca Callegaro (INRIM) Nuovo SI 4 Maggio 2017 1 / 27
Il nuovo Sistema Internazionale (SI) Le unità elettromagnetiche Luca Callegaro Istituto Nazionale di Ricerca Metrologica Torino, Italy 4 Maggio 2017 Luca Callegaro (INRIM) Nuovo SI 4 Maggio 2017 1 / 27
NIST CERTIFICATION OF ITS-90 FIXED-POINT CELLS FROM K TO K: METHODS AND UNCERTAINTIES
 NIST CERTIFICATION OF ITS-90 FIXED-POINT CELLS FROM 83.8058 K TO 1234.93 K: METHODS AND UNCERTAINTIES Gregory F. Strouse National Institute of Standards and Technology, Gaithersburg, Maryland, USA ABSTRACT
NIST CERTIFICATION OF ITS-90 FIXED-POINT CELLS FROM 83.8058 K TO 1234.93 K: METHODS AND UNCERTAINTIES Gregory F. Strouse National Institute of Standards and Technology, Gaithersburg, Maryland, USA ABSTRACT
Comparison Euramet 898
 Euramet 898 Electrolytic at pure water level final report 2010-02-05 Euramet Electrochemical Analysis WG Comparison Euramet 898 Electrolytic at pure water level Petra Spitzer 1, Hans Jensen 2, Bertil Magnusson
Euramet 898 Electrolytic at pure water level final report 2010-02-05 Euramet Electrochemical Analysis WG Comparison Euramet 898 Electrolytic at pure water level Petra Spitzer 1, Hans Jensen 2, Bertil Magnusson
A calibration facility for automatic weather stations
 METEOROLOGICAL APPLICATIONS Meteorol. Appl. 22: 842 846 (2015) Published online 16 November 2015 in Wiley Online Library (wileyonlinelibrary.com) DOI: 10.1002/met.1514 A calibration facility for automatic
METEOROLOGICAL APPLICATIONS Meteorol. Appl. 22: 842 846 (2015) Published online 16 November 2015 in Wiley Online Library (wileyonlinelibrary.com) DOI: 10.1002/met.1514 A calibration facility for automatic
Development of a Heat-Pipe-Based Hot Plate for Surface-Temperature Measurements
 Int J Thermophys (2009) 30:257 264 DOI 10.1007/s10765-008-0495-9 Development of a Heat-Pipe-Based Hot Plate for Surface-Temperature Measurements L. Rosso N. Koneva V. Fernicola Published online: 12 August
Int J Thermophys (2009) 30:257 264 DOI 10.1007/s10765-008-0495-9 Development of a Heat-Pipe-Based Hot Plate for Surface-Temperature Measurements L. Rosso N. Koneva V. Fernicola Published online: 12 August
APPENDIX 1 DESCRIPTION OF HOT WIRE ANEMOMETER
 146 APPENDIX 1 DESCRIPTION OF HOT WIRE ANEMOMETER Basic Principles of CTA Anemometer The hot-wire anemometer was introduced in its original form in the first half of the 0 th century. A major breakthrough
146 APPENDIX 1 DESCRIPTION OF HOT WIRE ANEMOMETER Basic Principles of CTA Anemometer The hot-wire anemometer was introduced in its original form in the first half of the 0 th century. A major breakthrough
Establishing traceability and estimating measurement uncertainty in physical, chemical and biological measurements
 Establishing traceability and estimating measurement uncertainty in physical, chemical and biological measurements Experimental Design 8.2.2013 Doc. Martti Heinonen etunimi.sukunimi@mikes.fi Outline 1.
Establishing traceability and estimating measurement uncertainty in physical, chemical and biological measurements Experimental Design 8.2.2013 Doc. Martti Heinonen etunimi.sukunimi@mikes.fi Outline 1.
TECHNICAL PAPERS. Ulrich Breuel, Barbara Werner, Petra Spitzer and Hans D. Jensen
 Experiences with Novel Secondary Conductivity Sensors within the German Calibration Service (DKD) Ulrich Breuel, Barbara Werner, Petra Spitzer and Hans D. Jensen Abstract: International efforts concentrate
Experiences with Novel Secondary Conductivity Sensors within the German Calibration Service (DKD) Ulrich Breuel, Barbara Werner, Petra Spitzer and Hans D. Jensen Abstract: International efforts concentrate
CHAPTER 5 CONVECTIVE HEAT TRANSFER COEFFICIENT
 62 CHAPTER 5 CONVECTIVE HEAT TRANSFER COEFFICIENT 5.1 INTRODUCTION The primary objective of this work is to investigate the convective heat transfer characteristics of silver/water nanofluid. In order
62 CHAPTER 5 CONVECTIVE HEAT TRANSFER COEFFICIENT 5.1 INTRODUCTION The primary objective of this work is to investigate the convective heat transfer characteristics of silver/water nanofluid. In order
XVIII IMEKO WORLD CONGRESS Metrology for a Sustainable Development September, 17 22, 2006, Rio de Janeiro, Brazil
 XVIII IMEKO WORLD CONGRESS Metrology for a Sustainable Development September, 17 22, 2006, Rio de Janeiro, Brazil A COMPARISON OF THE INITIAL UNCERTAINTY BUDGET AND AN ESTIMATE OF THE POSTERIOR UNCERTAINTIES,
XVIII IMEKO WORLD CONGRESS Metrology for a Sustainable Development September, 17 22, 2006, Rio de Janeiro, Brazil A COMPARISON OF THE INITIAL UNCERTAINTY BUDGET AND AN ESTIMATE OF THE POSTERIOR UNCERTAINTIES,
Analysis of a national low dc resistance inter-laboratories comparison
 16 th International Congress of Metrology, 04008 (2013) DOI: 10.1051/ metrology/201304008 C Owned by the authors, published by EDP Sciences, 2013 Analysis of a national low dc resistance inter-laboratories
16 th International Congress of Metrology, 04008 (2013) DOI: 10.1051/ metrology/201304008 C Owned by the authors, published by EDP Sciences, 2013 Analysis of a national low dc resistance inter-laboratories
Flow Measurement. SITRANS F M System information SITRANS F M electromagnetic flowmeters. 4/22 Siemens FI
 Function All are based on Faraday s law of induction: U M = B v d k U M = Measured voltage induced in the medium perpendicular to the magnetic field and the flow direction. The voltage is tapped at two
Function All are based on Faraday s law of induction: U M = B v d k U M = Measured voltage induced in the medium perpendicular to the magnetic field and the flow direction. The voltage is tapped at two
ORP Probe. Mini ORP. Reads. +/-2000mV. Range SMA. Connector. 95% in 1s. Response time 100 PSI. Max pressure. 60m (197 ft) Max depth 1 80 C
 Mini V 1.3 Revised 1/10/19 ORP Probe Reads Range Connector Response time Max pressure Max depth Temperature range C Cable length Internal temperature sensor Time before recalibration Life expectancy Maintenance
Mini V 1.3 Revised 1/10/19 ORP Probe Reads Range Connector Response time Max pressure Max depth Temperature range C Cable length Internal temperature sensor Time before recalibration Life expectancy Maintenance
Module 2. Measurement Systems. Version 2 EE IIT, Kharagpur 1
 Module 2 Measurement Systems Version 2 EE IIT, Kharagpur 1 Lesson 8 Measurement of Level, Humidity and ph Version 2 EE IIT, Kharagpur 2 Instructional Objectives At the end of this lesson, the student will
Module 2 Measurement Systems Version 2 EE IIT, Kharagpur 1 Lesson 8 Measurement of Level, Humidity and ph Version 2 EE IIT, Kharagpur 2 Instructional Objectives At the end of this lesson, the student will
I. Yang, C. H. Song, Y.-G. Kim & K. S. Gam
 Cryostat for Fixed-Point Calibration of Capsule-Type SPRTs I. Yang, C. H. Song, Y.-G. Kim & K. S. Gam International Journal of Thermophysics Journal of Thermophysical Properties and Thermophysics and Its
Cryostat for Fixed-Point Calibration of Capsule-Type SPRTs I. Yang, C. H. Song, Y.-G. Kim & K. S. Gam International Journal of Thermophysics Journal of Thermophysical Properties and Thermophysics and Its
Guest Forum. ph Measurement using the Harned Cell. The Screening Committee Lecture for the First Dr. Masao Horiba s Award
 Guest Forum The Screening Committee Lecture for the First Dr. Masao Horiba s Award ph Measurement using the Harned Cell -ph Values Acceptable for the International Society- Susumu Nakamura National Institute
Guest Forum The Screening Committee Lecture for the First Dr. Masao Horiba s Award ph Measurement using the Harned Cell -ph Values Acceptable for the International Society- Susumu Nakamura National Institute
ORP Probe ORP. Reads. +/-2000mV. Range. 95% in 1s. Response time 100 PSI. Max pressure. 60m (197 ft) Max depth 1 80 C. Temperature range C.
 V 2.7 Revised 11/8/17 ORP Probe Reads Range Response time Max pressure Max depth Temperature range C Cable length Internal temperature sensor Time before recalibration Life expectancy Maintenance ORP +/-2000mV
V 2.7 Revised 11/8/17 ORP Probe Reads Range Response time Max pressure Max depth Temperature range C Cable length Internal temperature sensor Time before recalibration Life expectancy Maintenance ORP +/-2000mV
Applied Fluid Mechanics
 Applied Fluid Mechanics 1. The Nature of Fluid and the Study of Fluid Mechanics 2. Viscosity of Fluid 3. Pressure Measurement 4. Forces Due to Static Fluid 5. Buoyancy and Stability 6. Flow of Fluid and
Applied Fluid Mechanics 1. The Nature of Fluid and the Study of Fluid Mechanics 2. Viscosity of Fluid 3. Pressure Measurement 4. Forces Due to Static Fluid 5. Buoyancy and Stability 6. Flow of Fluid and
Final Report August 2010
 Bilateral Comparison of 100 pf Capacitance Standards (ongoing BIPM key comparison BIPM.EM-K14.b) between the CMI, Czech Republic and the BIPM, January-July 2009 J. Streit** and N. Fletcher* *Bureau International
Bilateral Comparison of 100 pf Capacitance Standards (ongoing BIPM key comparison BIPM.EM-K14.b) between the CMI, Czech Republic and the BIPM, January-July 2009 J. Streit** and N. Fletcher* *Bureau International
Applied Fluid Mechanics
 Applied Fluid Mechanics 1. The Nature of Fluid and the Study of Fluid Mechanics 2. Viscosity of Fluid 3. Pressure Measurement 4. Forces Due to Static Fluid 5. Buoyancy and Stability 6. Flow of Fluid and
Applied Fluid Mechanics 1. The Nature of Fluid and the Study of Fluid Mechanics 2. Viscosity of Fluid 3. Pressure Measurement 4. Forces Due to Static Fluid 5. Buoyancy and Stability 6. Flow of Fluid and
ISEmax CAM40/CAS40. Technical Information
 Technical Information Online measurement of nutrient parameters Ion-selective electrode system for the continuous measurement of ammonium and nitrate Application The ion-selective electrode system works
Technical Information Online measurement of nutrient parameters Ion-selective electrode system for the continuous measurement of ammonium and nitrate Application The ion-selective electrode system works
IMPROVING SOIL MEASUREMENTS FOR BOUNDARY LAYER RESEARCH
 IMPROVING SOIL MEASUREMENTS FOR BOUNDARY LAYER RESEARCH Hannelore Bloemink, Jan Bijma Royal Netherlands Meteorological Institute (KNMI) Instrumentation Division P.O. Box 201, 3730 AE De Bilt, The Netherlands
IMPROVING SOIL MEASUREMENTS FOR BOUNDARY LAYER RESEARCH Hannelore Bloemink, Jan Bijma Royal Netherlands Meteorological Institute (KNMI) Instrumentation Division P.O. Box 201, 3730 AE De Bilt, The Netherlands
Process Control Instrumentation Technology Curtis D. Johnson Eighth Edition
 Process Control Instrumentation Technology Curtis D. Johnson Eighth Edition Pearson Education Limited Edinburgh Gate Harlow Essex CM20 2JE England and Associated Companies throughout the world Visit us
Process Control Instrumentation Technology Curtis D. Johnson Eighth Edition Pearson Education Limited Edinburgh Gate Harlow Essex CM20 2JE England and Associated Companies throughout the world Visit us
1 Phone , fax , 2 Phone , fax ,
 1 10 k high Precision transportable Setup to calibrate Multifunction Electrical instruments P.P. Capra 1 and F. Galliana 2 National Institute of Metrological Research, (INRIM) str. Cacce, 91 10135 (TURIN
1 10 k high Precision transportable Setup to calibrate Multifunction Electrical instruments P.P. Capra 1 and F. Galliana 2 National Institute of Metrological Research, (INRIM) str. Cacce, 91 10135 (TURIN
ph Probe Spear Tip Silver / silver chloride Reads Range / Resolution 95% in 1s Response time 100 PSI Max pressure 60m (197 ft) Max depth
 V 2.4 Revised 3/1/18 Spear Tip ph Probe Silver / silver chloride Reads ph Range 0 14 Resolution Response time Max pressure Max depth Temperature range C Cable length Internal temperature sensor Time before
V 2.4 Revised 3/1/18 Spear Tip ph Probe Silver / silver chloride Reads ph Range 0 14 Resolution Response time Max pressure Max depth Temperature range C Cable length Internal temperature sensor Time before
INSTRUCTION MANUAL. Type: ORP500 C, ORP500 CD, ORP222 CD, ORP223 CD EN Introduction Helpful Operating Tips. 2
 ORP/Reference Electrodes INSTRUCTION MANUAL Type: ORP500 C, ORP500 CD, ORP222 CD, ORP223 CD EN 02-05 Table of Contents 1. Introduction. 2 2. Helpful Operating Tips. 2 3. Calibration Procedure 3 3.1. Laboratory
ORP/Reference Electrodes INSTRUCTION MANUAL Type: ORP500 C, ORP500 CD, ORP222 CD, ORP223 CD EN 02-05 Table of Contents 1. Introduction. 2 2. Helpful Operating Tips. 2 3. Calibration Procedure 3 3.1. Laboratory
INTERNATIONAL COMPARISON OF PLATINUM RESISTANCE THERMOMETERS BETWEEN CHILE AND ECUADOR
 INTERNATIONAL COMPARISON OF PLATINUM RESISTANCE THERMOMETERS BETWEEN CHILE AND ECUADOR M. Araya 1, D. Almeida 2 1 National Laboratory of Temperature of Chile, LCPNT, Santiago, Chile 2 Ecuadorian Standardization
INTERNATIONAL COMPARISON OF PLATINUM RESISTANCE THERMOMETERS BETWEEN CHILE AND ECUADOR M. Araya 1, D. Almeida 2 1 National Laboratory of Temperature of Chile, LCPNT, Santiago, Chile 2 Ecuadorian Standardization
TEMPERATURE AND HUMIDITY DEPENDENCE ON STABILITY OF TORQUE MEASURING DEVICES Tassanai Sanponpute and Nittaya Arksonnarong
 IMEKO 22 nd TC3, 15 th TC5 and 3 rd TC22 International Conferences 3 to 5 February, 2014, Cape Town, Republic of South Africa TEMPERATURE AND HUMIDITY DEPENDENCE ON STABILITY OF TORQUE MEASURING DEVICES
IMEKO 22 nd TC3, 15 th TC5 and 3 rd TC22 International Conferences 3 to 5 February, 2014, Cape Town, Republic of South Africa TEMPERATURE AND HUMIDITY DEPENDENCE ON STABILITY OF TORQUE MEASURING DEVICES
Measurement Uncertainty in the DTA Temperature Calibration
 International Journal of Pure and Applied Physics ISSN 0973-1776 Volume 6, Number 4 (2010), pp. 429 437 Research India Publications http://www.ripublication.com/ijpap.htm Measurement Uncertainty in the
International Journal of Pure and Applied Physics ISSN 0973-1776 Volume 6, Number 4 (2010), pp. 429 437 Research India Publications http://www.ripublication.com/ijpap.htm Measurement Uncertainty in the
PTB S 16.5 MN HYDRAULIC AMPLIFICATION MACHINE AFTER MODERNIZATION
 IMEKO 22 nd TC3, 15 th TC5 and 3 rd TC22 International Conferences 3 to 5 February, 2014, Cape Town, Republic of South Africa PTB S 16.5 MN HYDRAULIC AMPLIFICATION MACHINE AFTER MODERNIZATION R. Kumme
IMEKO 22 nd TC3, 15 th TC5 and 3 rd TC22 International Conferences 3 to 5 February, 2014, Cape Town, Republic of South Africa PTB S 16.5 MN HYDRAULIC AMPLIFICATION MACHINE AFTER MODERNIZATION R. Kumme
ph Probe Silver / silver chloride Reads Range / Resolution 95% in 1s Response time 100 PSI Max pressure 60m (197 ft) Max depth 1 99 C
 V 2.8 Revised 1/16/18 ph Probe Silver / silver chloride Reads ph Range 0 14 Resolution Response time Max pressure Max depth Temperature range C Cable length Internal temperature sensor Time before recalibration
V 2.8 Revised 1/16/18 ph Probe Silver / silver chloride Reads ph Range 0 14 Resolution Response time Max pressure Max depth Temperature range C Cable length Internal temperature sensor Time before recalibration
Measurement of the 30 Si mole fraction in the new Avogadro silicon material by Neutron Activation and high resolution spectrometry
 Measurement of the 30 Si mole fraction in the new Avogadro silicon material by Neutron Activation and high resolution spectrometry Marco Di Luzio 1,2, Attila Stopic 3, Giancarlo D Agostino 1, John W Bennett
Measurement of the 30 Si mole fraction in the new Avogadro silicon material by Neutron Activation and high resolution spectrometry Marco Di Luzio 1,2, Attila Stopic 3, Giancarlo D Agostino 1, John W Bennett
WATER ANALYSIS The ph Value. The Redox Potential. ALMEMO ph and Redox Measurement. The Electrical Conductivity
 www.ahlborn.com WATER ANALYSIS The ph Value The ph value is a logarithmic measure for the concentration of the H ions in a hydrous solution and indicates, by a numerical value, whether the solution has
www.ahlborn.com WATER ANALYSIS The ph Value The ph value is a logarithmic measure for the concentration of the H ions in a hydrous solution and indicates, by a numerical value, whether the solution has
BUREAU INTERNATIONAL DES POIDS ET MESURES
 BUREAU INTERNATIONAL DES POIDS ET MESURES Bilateral comparison of 1 Ω and 10 kω standards (ongoing BIPM key comparisons BIPM.EM-K13.a and 13.b) between the SMD (Belgium) and the BIPM October 2017 Final
BUREAU INTERNATIONAL DES POIDS ET MESURES Bilateral comparison of 1 Ω and 10 kω standards (ongoing BIPM key comparisons BIPM.EM-K13.a and 13.b) between the SMD (Belgium) and the BIPM October 2017 Final
EA Guidelines on the Calibration of Temperature Indicators and Simulators by Electrical Simulation and Measurement
 Publication Reference EA-10/11 EA Guidelines on the Calibration of Temperature Indicators and Simulators by Electrical PURPOSE This document has been produced by EA as a means of giving advice for calibrating
Publication Reference EA-10/11 EA Guidelines on the Calibration of Temperature Indicators and Simulators by Electrical PURPOSE This document has been produced by EA as a means of giving advice for calibrating
Conductivity Meter For Liquids LCM-8716 Manual
 Conductivity Meter For Liquids LCM-8716 Manual ALFF ENGINEERING Gomweg 7, CH-8915 Hausen am Albis, Switzerland Phone: +41 1 77 66 77 6 fax: +41 1 77 66 77 7 e-mail: info@alff-engineering.ch Web: www.alff-engineering.ch
Conductivity Meter For Liquids LCM-8716 Manual ALFF ENGINEERING Gomweg 7, CH-8915 Hausen am Albis, Switzerland Phone: +41 1 77 66 77 6 fax: +41 1 77 66 77 7 e-mail: info@alff-engineering.ch Web: www.alff-engineering.ch
Direct Measurement ISE Method Method to 4.00 mg/l NO 3 N TISAB Solution
 , drinking water, 8359 DOC316.53.01239 Direct Measurement ISE Method Method 8359 0.04 to 4.00 mg/l NO 3 N TISAB Solution Scope and Application: Drinking water Test preparation How to use instrument-specific
, drinking water, 8359 DOC316.53.01239 Direct Measurement ISE Method Method 8359 0.04 to 4.00 mg/l NO 3 N TISAB Solution Scope and Application: Drinking water Test preparation How to use instrument-specific
He II Heat transfer through a Corrugated Tube - Test Report
 He II Heat transfer through a Corrugated Tube - Test Report Authors: Ch. Darve, Y. Huang, T. Nicol, T. Peterson Keywords: LHC inner triplet, heat exchanger, He II heat transfer, Kapitza resistance. Abstract
He II Heat transfer through a Corrugated Tube - Test Report Authors: Ch. Darve, Y. Huang, T. Nicol, T. Peterson Keywords: LHC inner triplet, heat exchanger, He II heat transfer, Kapitza resistance. Abstract
METHOD 9210 POTENTIOMETRIC DETERMINATION OF NITRATE IN AQUEOUS SAMPLES WITH ION-SELECTIVE ELECTRODE
 METHOD 9210 POTENTIOMETRIC DETERMINATION OF NITRATE IN AQUEOUS SAMPLES WITH ION-SELECTIVE ELECTRODE 1.0 SCOPE AND APPLICATION 1.1 This method can be used for measuring total solubilized nitrate in drinking
METHOD 9210 POTENTIOMETRIC DETERMINATION OF NITRATE IN AQUEOUS SAMPLES WITH ION-SELECTIVE ELECTRODE 1.0 SCOPE AND APPLICATION 1.1 This method can be used for measuring total solubilized nitrate in drinking
RESULTS OF ICARUS 9 EXPERIMENTS RUN AT IMRA EUROPE
 Roulette, T., J. Roulette, and S. Pons. Results of ICARUS 9 Experiments Run at IMRA Europe. in Sixth International Conference on Cold Fusion, Progress in New Hydrogen Energy. 1996. Lake Toya, Hokkaido,
Roulette, T., J. Roulette, and S. Pons. Results of ICARUS 9 Experiments Run at IMRA Europe. in Sixth International Conference on Cold Fusion, Progress in New Hydrogen Energy. 1996. Lake Toya, Hokkaido,
ENERGY DISTRIBUTION ANALYSIS IN A LOW HEAD FRANCIS TURBINE DURING THERMODYNAMIC EFFICIENCY MEASUREMENTS
 ENERGY DISTRIBUTION ANALYSIS IN A LOW HEAD FRANCIS TURBINE DURING THERMODYNAMIC EFFICIENCY MEASUREMENTS Gianalberto Grego, Fabio F. Muciaccia W.E.S.T. Srl, Milan, Italy west-hydro@libero.it ABSTRACT The
ENERGY DISTRIBUTION ANALYSIS IN A LOW HEAD FRANCIS TURBINE DURING THERMODYNAMIC EFFICIENCY MEASUREMENTS Gianalberto Grego, Fabio F. Muciaccia W.E.S.T. Srl, Milan, Italy west-hydro@libero.it ABSTRACT The
MODEL FOR UNCERTAINTY ESTIMATION IN COMPARISON CALIBRATION OF THERMOCOUPLES
 XVII IMEKO World Congress Metrology in the 3rd Millennium June 7, 003, Dubrovnik, Croatia MODEL OR UNCERTAINTY ESTIMATION IN COMPARISON CALIBRATION O THERMOCOUPLES Georges Bonnier, Eliane Renaot, DavorZvizdic,
XVII IMEKO World Congress Metrology in the 3rd Millennium June 7, 003, Dubrovnik, Croatia MODEL OR UNCERTAINTY ESTIMATION IN COMPARISON CALIBRATION O THERMOCOUPLES Georges Bonnier, Eliane Renaot, DavorZvizdic,
THERMOHYDRAULIC BEHAVIOUR OF HEII IN STRATIFIED CO-CURRENT TWO-PHASE FLOW AT HIGH VAPOR VELOCITIES
 EUROPEAN ORGANIZATION FOR NUCLEAR RESEARCH European Laboratory for Particle Physics Large Hadron Collider Project LHC Project Report 67 THERMOHYDRAULIC BEHAVIOUR OF HEII IN STRATIFIED CO-CURRENT TWO-PHASE
EUROPEAN ORGANIZATION FOR NUCLEAR RESEARCH European Laboratory for Particle Physics Large Hadron Collider Project LHC Project Report 67 THERMOHYDRAULIC BEHAVIOUR OF HEII IN STRATIFIED CO-CURRENT TWO-PHASE
Fig. Electrochemical Cell/ Potentiometric Titration
 Fig. Electrochemical Cell/ Potentiometric Titration The accurate, precise and effective potentiometric measurements can be made with the help of the following two types of electrodes namely : REFERENCE
Fig. Electrochemical Cell/ Potentiometric Titration The accurate, precise and effective potentiometric measurements can be made with the help of the following two types of electrodes namely : REFERENCE
ANALYSIS OF INDUCTIVE CURRENT PULSE DYNAMICS IN WATER ELECTROLYSES CELL
 ANALYSIS OF INDUCTIVE CURRENT PULSE DYNAMICS IN WATER ELECTROLYSES CELL Martins Vanags, Janis Kleperis, Gunars Bajars, Andrejs Lusis Institute of Solid State Physics of University of Latvia, Riga, LV-10050,
ANALYSIS OF INDUCTIVE CURRENT PULSE DYNAMICS IN WATER ELECTROLYSES CELL Martins Vanags, Janis Kleperis, Gunars Bajars, Andrejs Lusis Institute of Solid State Physics of University of Latvia, Riga, LV-10050,
CHAPTER 4 THERMAL CONDUCTIVITY AND VISCOSITY MEASUREMENTS
 50 CHAPTER 4 THERMAL CONDUCTIVITY AND VISCOSITY MEASUREMENTS 4.1 INTRODUCTION In the development of any energy-efficient heat transfer fluids for enhanced heat transfer performance, in practical applications,
50 CHAPTER 4 THERMAL CONDUCTIVITY AND VISCOSITY MEASUREMENTS 4.1 INTRODUCTION In the development of any energy-efficient heat transfer fluids for enhanced heat transfer performance, in practical applications,
Supplementary Information. Carolyn Richmonds, Megan Witzke, Brandon Bartling, Seung Whan Lee, Jesse Wainright,
 Supplementary Information Electron transfer reactions at the plasma-liquid interface Carolyn Richmonds, Megan Witzke, Brandon Bartling, Seung Whan Lee, Jesse Wainright, Chung-Chiun Liu, and R. Mohan Sankaran*,
Supplementary Information Electron transfer reactions at the plasma-liquid interface Carolyn Richmonds, Megan Witzke, Brandon Bartling, Seung Whan Lee, Jesse Wainright, Chung-Chiun Liu, and R. Mohan Sankaran*,
The design and operational theory of ph electrodes is a very complex subject, explored only briefly here. What is important to understand is that thes
 ph measurement A very important measurement in many liquid chemical processes (industrial, pharmaceutical, manufacturing, food production, etc.) is that of ph: the measurement of hydrogen ion concentration
ph measurement A very important measurement in many liquid chemical processes (industrial, pharmaceutical, manufacturing, food production, etc.) is that of ph: the measurement of hydrogen ion concentration
Capacitive Sensors. Contents
 Contents Capacitive sensors detect the change in capacitance caused by the approach of an object. Their advantage lies in the ability to detect virtually any material, from metals to oils. 4.2 Definitions,
Contents Capacitive sensors detect the change in capacitance caused by the approach of an object. Their advantage lies in the ability to detect virtually any material, from metals to oils. 4.2 Definitions,
PHYSICS 122 Lab EXPERIMENT NO. 6 AC CIRCUITS
 PHYSICS 122 Lab EXPERIMENT NO. 6 AC CIRCUITS The first purpose of this laboratory is to observe voltages as a function of time in an RC circuit and compare it to its expected time behavior. In the second
PHYSICS 122 Lab EXPERIMENT NO. 6 AC CIRCUITS The first purpose of this laboratory is to observe voltages as a function of time in an RC circuit and compare it to its expected time behavior. In the second
ECE-343 Test 1: Feb 10, :00-8:00pm, Closed Book. Name : SOLUTION
 ECE-343 Test : Feb 0, 00 6:00-8:00pm, Closed Book Name : SOLUTION C Depl = C J0 + V R /V o ) m C Diff = τ F g m ω T = g m C µ + C π ω T = g m I / D C GD + C or V OV GS b = τ i τ i = R i C i ω H b Z = Z
ECE-343 Test : Feb 0, 00 6:00-8:00pm, Closed Book Name : SOLUTION C Depl = C J0 + V R /V o ) m C Diff = τ F g m ω T = g m C µ + C π ω T = g m I / D C GD + C or V OV GS b = τ i τ i = R i C i ω H b Z = Z
Advances in Resistivity Instrumentation for UPW Systems of the Future
 Advances in Resistivity Instrumentation for UPW Systems of the Future Anthony C. Bevilacqua, Ph.D. Thornton, Inc. 1432 Main St., Waltham, Massachusetts 02451 Abstract For decades, resistivity instrumentation
Advances in Resistivity Instrumentation for UPW Systems of the Future Anthony C. Bevilacqua, Ph.D. Thornton, Inc. 1432 Main St., Waltham, Massachusetts 02451 Abstract For decades, resistivity instrumentation
Journal of Chemical and Pharmaceutical Research, 2014, 6(8): Research Article
 Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research, 2014, 6(8):118-124 Research Article ISSN : 0975-7384 CODEN(USA) : JCPRC5 Effects of current density on capacitive deionization
Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research, 2014, 6(8):118-124 Research Article ISSN : 0975-7384 CODEN(USA) : JCPRC5 Effects of current density on capacitive deionization
CONDUCTOMETRIC TITRATIONS. Reading: 1. Skoog, Holler and Nieman: Chapter 22 A. INTRODUCTION. A.1 Classification of Electroanalytical Methods
 Reading: 1. Skoog, Holler and Nieman: Chapter 22 A. INTRODUCTION A.1 Classification of Electroanalytical Methods Electroanalytical methods are methods of analysis which rely on electrical properties of
Reading: 1. Skoog, Holler and Nieman: Chapter 22 A. INTRODUCTION A.1 Classification of Electroanalytical Methods Electroanalytical methods are methods of analysis which rely on electrical properties of
General Medicine 2016/17
 ÚSTAV LÉKAŘSKÉ BIOCHEMIE A LABORATORNÍ DIAGNOSTIKY 1. LF UK Buffers, buffer capacity. Oxidoreduction, electrode processes Practical lesson on medical biochemistry General Medicine Martin Vejražka, Tomáš
ÚSTAV LÉKAŘSKÉ BIOCHEMIE A LABORATORNÍ DIAGNOSTIKY 1. LF UK Buffers, buffer capacity. Oxidoreduction, electrode processes Practical lesson on medical biochemistry General Medicine Martin Vejražka, Tomáš
MAGNETIC FIELDS & UNIFORM PLANE WAVES
 MAGNETIC FIELDS & UNIFORM PLANE WAVES Name Section Multiple Choice 1. (8 Pts) 2. (8 Pts) 3. (8 Pts) 4. (8 Pts) 5. (8 Pts) Notes: 1. In the multiple choice questions, each question may have more than one
MAGNETIC FIELDS & UNIFORM PLANE WAVES Name Section Multiple Choice 1. (8 Pts) 2. (8 Pts) 3. (8 Pts) 4. (8 Pts) 5. (8 Pts) Notes: 1. In the multiple choice questions, each question may have more than one
Evaluation of Capacitance in Motor Circuit Analysis Findings. Howard W Penrose, Ph.D., CMRP President, SUCCESS by DESIGN
 Evaluation of Capacitance in Motor Circuit Analysis Findings Howard W Penrose, Ph.D., CMRP President, SUCCESS by DESIGN Introduction The question related to the ability of low voltage testing to detect
Evaluation of Capacitance in Motor Circuit Analysis Findings Howard W Penrose, Ph.D., CMRP President, SUCCESS by DESIGN Introduction The question related to the ability of low voltage testing to detect
Metallized Polypropylene Film Capacitor Related Document: IEC
 MKP 184 Metallized Polypropylene Film Capacitor Related Document: IEC 6084-16 MAIN APPLICATIONS: High voltage, high current and high pulse operations, deflection circuits in TV sets (S-correction and fly-back
MKP 184 Metallized Polypropylene Film Capacitor Related Document: IEC 6084-16 MAIN APPLICATIONS: High voltage, high current and high pulse operations, deflection circuits in TV sets (S-correction and fly-back
Measurements with Ion Selective Electrodes: Determination of Fluoride in Toothpaste
 Experiment ISE: Measurements with Ion Selective Electrodes: Determination of Fluoride in Toothpaste 67 You have been hired by the government to check the fluoride concentration labelling on some major
Experiment ISE: Measurements with Ion Selective Electrodes: Determination of Fluoride in Toothpaste 67 You have been hired by the government to check the fluoride concentration labelling on some major
792A AC/DC Transfer Standard
 92A AC/DC Transfer Standard Technical Data Support for your most demanding ac measurement requirements ppm total uncertainty Traceable to national standards range: 2 mv to 00 V Frequency range: Hz to 1
92A AC/DC Transfer Standard Technical Data Support for your most demanding ac measurement requirements ppm total uncertainty Traceable to national standards range: 2 mv to 00 V Frequency range: Hz to 1
The Basic Capacitor. Dielectric. Conductors
 Chapter 9 The Basic Capacitor Capacitors are one of the fundamental passive components. In its most basic form, it is composed of two conductive plates separated by an insulating dielectric. The ability
Chapter 9 The Basic Capacitor Capacitors are one of the fundamental passive components. In its most basic form, it is composed of two conductive plates separated by an insulating dielectric. The ability
Comparison of measurement uncertainty budgets for calibration of sound calibrators: Euromet project 576
 NPL REPORT CMAM 73 Comparison of measurement uncertainty budgets for calibration of sound calibrators: Euromet project 576 Peter Hanes October 2001 The National Physical Laboratory is operated on behalf
NPL REPORT CMAM 73 Comparison of measurement uncertainty budgets for calibration of sound calibrators: Euromet project 576 Peter Hanes October 2001 The National Physical Laboratory is operated on behalf
Good practice guide containing experimental results and recommendations for the selection, preparation and calibration of the temperature sensors
 Good practice guide containing experimental results and recommendations for the selection, preparation and calibration of the temperature sensors 1. Scope... 2 2. Introduction... 2 3. Selection of thermocouples
Good practice guide containing experimental results and recommendations for the selection, preparation and calibration of the temperature sensors 1. Scope... 2 2. Introduction... 2 3. Selection of thermocouples
Operating Instructions for Ammonium ISE Specifications
 Operating Instructions for Ammonium ISE Specifications Range: The Ammonium Electrode responds to uncomplexed ion activity over the range 1 x 10-1M to 1 x 10-6M. Linear detection limit is about 10-5M Interference's:
Operating Instructions for Ammonium ISE Specifications Range: The Ammonium Electrode responds to uncomplexed ion activity over the range 1 x 10-1M to 1 x 10-6M. Linear detection limit is about 10-5M Interference's:
What s Your (real or imaginary) LCR IQ?
 Chroma Systems Solutions, Inc. What s Your (real or imaginary) LCR IQ? 11021, 11025 LCR Meter Keywords:. Impedance, Inductance, Capacitance, Resistance, Admittance, Conductance, Dissipation Factor, 4-Terminal
Chroma Systems Solutions, Inc. What s Your (real or imaginary) LCR IQ? 11021, 11025 LCR Meter Keywords:. Impedance, Inductance, Capacitance, Resistance, Admittance, Conductance, Dissipation Factor, 4-Terminal
sensors ISSN
 Sensors 2009, 9, 118-130; doi:10.3390/s90100118 Article OPEN ACCESS sensors ISSN 1424-8220 www.mdpi.com/journal/sensors Effect of Structural Design of Silver/Silver Chloride Electrodes on Stability and
Sensors 2009, 9, 118-130; doi:10.3390/s90100118 Article OPEN ACCESS sensors ISSN 1424-8220 www.mdpi.com/journal/sensors Effect of Structural Design of Silver/Silver Chloride Electrodes on Stability and
BC546 / 547 / 548. Small Signal Transistors (NPN) Vishay Semiconductors
 Small Signal Transistors (NPN) Features NPN Silicon Epitaxial Planar Transistors These transistors are subdivided into three groups A, B, and C according to their current gain. The type BC546 is available
Small Signal Transistors (NPN) Features NPN Silicon Epitaxial Planar Transistors These transistors are subdivided into three groups A, B, and C according to their current gain. The type BC546 is available
Technical Explanation for Axial Fans
 CSM_Axial_TG_E Introduction What Is an Axial? Axial fans are used for stable cooling in many different applications and locations. If the temperature of a device increases, the lives of its internal parts
CSM_Axial_TG_E Introduction What Is an Axial? Axial fans are used for stable cooling in many different applications and locations. If the temperature of a device increases, the lives of its internal parts
VSMOW Triple Point of Water Cells: Borosilicate versus Fused- Quartz
 VSMOW Triple Point of Water Cells: Borosilicate versus Fused- Quartz M. Zhao 1,3 and G. F. Strouse 2 1 Fluke Corporation, Hart Scientific Division, American Fork, Utah 84003, U.S.A. 2 National Institute
VSMOW Triple Point of Water Cells: Borosilicate versus Fused- Quartz M. Zhao 1,3 and G. F. Strouse 2 1 Fluke Corporation, Hart Scientific Division, American Fork, Utah 84003, U.S.A. 2 National Institute
Standard Methods for the Examination of Water and Wastewater
 2510 CONDUCTIVITY*#(1) 2510 A. Introduction Conductivity, k, is a measure of the ability of an aqueous solution to carry an electric current. This ability depends on the presence of ions; on their total
2510 CONDUCTIVITY*#(1) 2510 A. Introduction Conductivity, k, is a measure of the ability of an aqueous solution to carry an electric current. This ability depends on the presence of ions; on their total
STUDY OF THE BEST MEASUREMENT CAPABILITY IN ROCKWELL SCALE AT THE BRAZILIAN NMI INMETRO S PRIMARY HARDNESS STANDARD MACHINE
 XIX IMEKO World Congress Fundamental and Applied Metrology September 6 11, 2009, Lisbon, Portugal STUDY OF THE BEST MEASUREMENT CAPABILITY IN ROCKWELL SCALE AT THE BRAZILIAN NMI INMETRO S PRIMARY HARDNESS
XIX IMEKO World Congress Fundamental and Applied Metrology September 6 11, 2009, Lisbon, Portugal STUDY OF THE BEST MEASUREMENT CAPABILITY IN ROCKWELL SCALE AT THE BRAZILIAN NMI INMETRO S PRIMARY HARDNESS
Unique Behaviour of Nonsolvents for Polysulphides in Lithium-Sulphur Batteries.
 Electronic Supplementary Material (ESI) for Energy & Environmental Science. This journal is The Royal Society of Chemistry 214 Supplementary Information Unique Behaviour of Nonsolvents for Polysulphides
Electronic Supplementary Material (ESI) for Energy & Environmental Science. This journal is The Royal Society of Chemistry 214 Supplementary Information Unique Behaviour of Nonsolvents for Polysulphides
Semi-automatic titrators PH-Burette. A great ph-meter with integrated burette. Incredible price! Infinite possibilities
 Semi-automatic titrators PH-Burette. A great ph-meter with integrated burette. Incredible price! Infinite possibilities instruments 5 YEARS G U A R A N T E E Quality at a reasonable price The optimum combination
Semi-automatic titrators PH-Burette. A great ph-meter with integrated burette. Incredible price! Infinite possibilities instruments 5 YEARS G U A R A N T E E Quality at a reasonable price The optimum combination
Manual ph Probe & Oxidation Reduction Potential Probe Models 6528A and 6528B
 Manual ph Probe & Oxidation Reduction Potential Probe Models 6528A and 6528B Revision History File name / Revision Date Authors Previous version BX 2004 RS/ JH Unidata Manual - 6528 ph and ORP Sensors
Manual ph Probe & Oxidation Reduction Potential Probe Models 6528A and 6528B Revision History File name / Revision Date Authors Previous version BX 2004 RS/ JH Unidata Manual - 6528 ph and ORP Sensors
POTENTIOMETRIC TITRATIONS & SOLUBILITY EQUILIBRIA. Background
 POTENTIOMETRIC TITRATIONS & SOLUBILITY EQUILIBRIA Background In this experiment, students will familiarize themselves with potentiometric titration, practice using the first derivative to find the equivalence
POTENTIOMETRIC TITRATIONS & SOLUBILITY EQUILIBRIA Background In this experiment, students will familiarize themselves with potentiometric titration, practice using the first derivative to find the equivalence
CERTIFICATE OF ACCREDITATION
 CERTIFICATE OF ACCREDITATION ANSI-ASQ National Accreditation Board 500 Montgomery Street, Suite 625, Alexandria, VA 22314, 877-344-3044 This is to certify that Compania Nacional de Metrologia S.A.S - Conamet
CERTIFICATE OF ACCREDITATION ANSI-ASQ National Accreditation Board 500 Montgomery Street, Suite 625, Alexandria, VA 22314, 877-344-3044 This is to certify that Compania Nacional de Metrologia S.A.S - Conamet
Reproduction of Fleischmann and Pons experiments
 onchampt, G., L. Bonnetain, and P. Hieter. Reproduction of Fleischmann and Pons Experiments. in Sixth International Conference on Cold Fusion, Progress in New Hydrogen Energy. 1996. Lake Toya, Hokkaido,
onchampt, G., L. Bonnetain, and P. Hieter. Reproduction of Fleischmann and Pons Experiments. in Sixth International Conference on Cold Fusion, Progress in New Hydrogen Energy. 1996. Lake Toya, Hokkaido,
Final Concentration 0 excess 0.1 M 0.1 M
 PURPOSE: 1. To estimate the Acid-Ionization Constant (Ka) for acetic acid by conductivity testing comparisons. 2. To become familiar with the ph meter and ph measurements. 3. To determine the Acid-Ionization
PURPOSE: 1. To estimate the Acid-Ionization Constant (Ka) for acetic acid by conductivity testing comparisons. 2. To become familiar with the ph meter and ph measurements. 3. To determine the Acid-Ionization
THERMOCOUPLE CHARACTERISTICS TRAINER
 THERMOCOUPLE CHARACTERISTICS TRAINER (Model No : ) User Manual Version 2.0 Technical Clarification /Suggestion : / Technical Support Division, Vi Microsystems Pvt. Ltd., Plot No :75,Electronics Estate,
THERMOCOUPLE CHARACTERISTICS TRAINER (Model No : ) User Manual Version 2.0 Technical Clarification /Suggestion : / Technical Support Division, Vi Microsystems Pvt. Ltd., Plot No :75,Electronics Estate,
Electronic Supplementary Material (ESI) for Chemical Communications This journal is The Royal Society of Chemistry 2011
 Supplementary Information for Selective adsorption toward toxic metal ions results in selective response: electrochemical studies on polypyrrole/reduced graphene oxide nanocomposite Experimental Section
Supplementary Information for Selective adsorption toward toxic metal ions results in selective response: electrochemical studies on polypyrrole/reduced graphene oxide nanocomposite Experimental Section
Chapter Four. Experimental
 Chapter Four 4.1 Materials N,N-Diethyl monoethanolamine (purity 98%) used in all experiments was purchased from Spectrochem Pvt. Ltd., Mumbai. N-Ethyl monoethanolamine, N-(- aminoethyl)ethanolamine, diethanolamine,
Chapter Four 4.1 Materials N,N-Diethyl monoethanolamine (purity 98%) used in all experiments was purchased from Spectrochem Pvt. Ltd., Mumbai. N-Ethyl monoethanolamine, N-(- aminoethyl)ethanolamine, diethanolamine,
2.1. Accuracy, n- how close the indication of the thermometer is to the true value.
 AASHTO Accreditation Policy and Guidance on Thermometer Selection and Records Revised: January 25, 2018 Revision #: 0 1. Objective 1.1. The purpose of this document is to clearly define acceptance criteria
AASHTO Accreditation Policy and Guidance on Thermometer Selection and Records Revised: January 25, 2018 Revision #: 0 1. Objective 1.1. The purpose of this document is to clearly define acceptance criteria
ASSOCIATE DEGREE IN ENGINEERING RESIT EXAMINATIONS SEMESTER 1. "Electrical Eng Science"
 ASSOCIATE DEGREE IN ENGINEERING RESIT EXAMINATIONS SEMESTER 1 COURSE NAME: "Electrical Eng Science" CODE: GROUP: "[ADET 2]" DATE: December 2010 TIME: DURATION: 9:00 am "Two hours" INSTRUCTIONS: 1. This
ASSOCIATE DEGREE IN ENGINEERING RESIT EXAMINATIONS SEMESTER 1 COURSE NAME: "Electrical Eng Science" CODE: GROUP: "[ADET 2]" DATE: December 2010 TIME: DURATION: 9:00 am "Two hours" INSTRUCTIONS: 1. This
Alternating Current Circuits. Home Work Solutions
 Chapter 21 Alternating Current Circuits. Home Work s 21.1 Problem 21.11 What is the time constant of the circuit in Figure (21.19). 10 Ω 10 Ω 5.0 Ω 2.0µF 2.0µF 2.0µF 3.0µF Figure 21.19: Given: The circuit
Chapter 21 Alternating Current Circuits. Home Work s 21.1 Problem 21.11 What is the time constant of the circuit in Figure (21.19). 10 Ω 10 Ω 5.0 Ω 2.0µF 2.0µF 2.0µF 3.0µF Figure 21.19: Given: The circuit
I m. R s. Digital. R x. OhmmetersxSeries Shunt Digital. R m
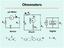 µa Meter I I s I m s E Digital x I Voltmeter x x E µa Meter m Is OhmmetersxSeries Shunt Digital EIx= = ()E sm x mxvi= x Shunt Ohmmeter Shunt s x E µa Meter I m I m V m E ) ( v I E ) ( E v E v E I When
µa Meter I I s I m s E Digital x I Voltmeter x x E µa Meter m Is OhmmetersxSeries Shunt Digital EIx= = ()E sm x mxvi= x Shunt Ohmmeter Shunt s x E µa Meter I m I m V m E ) ( v I E ) ( E v E v E I When
The ph Value The ph value is a logarithmic measure for the concentration of the H ions in a hydrous solution and indicates, by a numerical value, whet
 Content ph value, redox potential, conductivity 15.02 The Electrical Conductivity 15.03 Solute oxygen in liquids 15.03 ph one-bar measuring chains FY 96 PHEK, FY 96 PHER, FY 96 PHEN 15.04 ph insertion
Content ph value, redox potential, conductivity 15.02 The Electrical Conductivity 15.03 Solute oxygen in liquids 15.03 ph one-bar measuring chains FY 96 PHEK, FY 96 PHER, FY 96 PHEN 15.04 ph insertion
Electrical Characterization of 3D Through-Silicon-Vias
 Electrical Characterization of 3D Through-Silicon-Vias F. Liu, X. u, K. A. Jenkins, E. A. Cartier, Y. Liu, P. Song, and S. J. Koester IBM T. J. Watson Research Center Yorktown Heights, NY 1598, USA Phone:
Electrical Characterization of 3D Through-Silicon-Vias F. Liu, X. u, K. A. Jenkins, E. A. Cartier, Y. Liu, P. Song, and S. J. Koester IBM T. J. Watson Research Center Yorktown Heights, NY 1598, USA Phone:
EXPERIMENT 6. Properties of Buffers INTRODUCTION
 EXPERIMENT 6 Properties of Buffers INTRODUCTION A chemical buffer is any substance in a solution that tends to stabilize the hydronium ion concentration by neutralizing any added acid or base. Buffers
EXPERIMENT 6 Properties of Buffers INTRODUCTION A chemical buffer is any substance in a solution that tends to stabilize the hydronium ion concentration by neutralizing any added acid or base. Buffers
Light, Electricity and Liquids. By Vitaliy Zamsha and Vladimir Shevtsov
 Light, Electricity and Liquids updated By Vitaliy Zamsha and Vladimir Shevtsov Abstract The authors of this article revealed their long-awaited work, which represents some effects in liquids under the
Light, Electricity and Liquids updated By Vitaliy Zamsha and Vladimir Shevtsov Abstract The authors of this article revealed their long-awaited work, which represents some effects in liquids under the
Alternating Current Circuits
 Alternating Current Circuits AC Circuit An AC circuit consists of a combination of circuit elements and an AC generator or source. The output of an AC generator is sinusoidal and varies with time according
Alternating Current Circuits AC Circuit An AC circuit consists of a combination of circuit elements and an AC generator or source. The output of an AC generator is sinusoidal and varies with time according
EXPERIMENTAL SETUP AND PROCEDURE
 CHAPTER 3 EXPERIMENTAL SETUP AND PROCEDURE 3.1 Determination of vapour-liquid equilibria Isobaric Vapour-Liquid Equilibria date have been obtained, using a Smith and Bonner [39] type still which is a modified
CHAPTER 3 EXPERIMENTAL SETUP AND PROCEDURE 3.1 Determination of vapour-liquid equilibria Isobaric Vapour-Liquid Equilibria date have been obtained, using a Smith and Bonner [39] type still which is a modified
