In response to your prompt, we have performed the rubber band experiment in a team and
|
|
|
- Hugh Hudson
- 6 years ago
- Views:
Transcription
1 Memo To: Ms. Liticia Salter, Instructor From: Mahmudul Alam, Mohammad Sirajudeen Date: April 17, 2007 Subject: Transmittal Memo In response to your prompt, we have performed the rubber band experiment in a team and our main objective was to study and observe the change of entropy of the rubber band both in the stretched and relaxed state. Our report analyses the change of entropy both from the perspective of molecular orderliness and the energy degradation. The report also provides an explanation for why the rubber band contracts when it is exposed to heat. Our area of satisfaction was the outcome of the experiment, because it completely agreed with the theoretical explanation. As for the hair dryer, we borrowed it from a barber working in a men s saloon. Initially, we had a difficulty in convincing the barber that we are going to use it for an experiment, but finally we managed it. Please review and respond to our report at your earliest convenience. 1
2 Abstract The report presents the change of entropy in a rubber band both from the perspective of molecular orderliness and energy degradation. Rubber is an elastic hydrocarbon polymer, which unlike other substances contracts when exposed to heat because of its unique atomic structure. However, the outcome of the experiment, which mainly comprises two simple tests, demonstrated that like the other materials, the change of entropy in a rubber band also completely agrees with the theoretical explanation of entropy. Introduction The report discusses an experiment which was performed to observe and study the change of entropy in a rubber band both in relaxed and stretched state. The objective of this experiment was to determine whether the outcome holds the theory of entropy, and to provide with an explanation why the rubber band contracts if it is heated. This report contains the procedure and result of the experiment, including an analysis and a discussion of the result. Theory Entropy in a closed thermodynamic system is defined as a quantitative measure of the amount of thermal energy which is unavailable to do any work. In other words it is the measure of thermal energy which is given out or absorbed during a reaction. Entropy is actually the indicator of unavailable form of energy. For instance, let us assume a system, which is comprised of a hot body and a cold body. If somehow the hot body and the cold body come in contact, then according to the second law of thermodynamics, heat will automatically flow from the hot body to the cold body, and this thermal energy could be transformed into mechanical energy or work by a heat engine. Once the system reaches the equilibrium state, the energy of the system, according to the first law of thermodynamics and the law of energy conservation, will remain same, but then the energy cannot be used to do work any more. Although the energy is never destroyed, it tends to degrade from the useful form to the useless one, and entropy is the measurement of this degradation. Page 2
3 Entropy is also defined by the amount of disorder or randomness of a system. In the previous example, the system before reaching its equilibrium point was ordered, because the faster and more active molecules of hot body were easily separable from the less active molecules of cold body. This orderliness is destroyed at the equilibrium stage because at that point, molecules of hot body cannot be separated easily from that of cold body. With the increase in disorder, the amount of available form of energy decreases, and therefore, the messier the system, the higher the entropy. If a system absorbs or releases an amount of change in entropy is Δ Q heat energy in T temperature, then the ΔQ ΔS = T Δ Q or, Sfinal S initial = (1) T Apparatus For this experiment, we used the following apparatus: 4-5 rubber bands (in unstressed state, the rubber bands are 7 mm width and 28 cm long) a hair dryer a meter stick ( 1 m long) a hammer (weight approximately 1.25 kg) The rubber bands were provided by the instructor, while one of the team members had the hammer in his house. As for the hair dryer and the meter stick, we borrowed it from a local men s saloon and the Texas A & M University at Qatar Library (TAMUQ-L) respectively. Procedure We performed this experiment in an air-conditioned room, where the rubber bands were kept in for several hours. The experiment consists of two different types of tests, and we carried out both of the tests with more than one rubber band to come up with as precise result as possible. Page 3
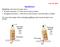
4 Procedure of Test-1: The procedure of the Test-1 is quite straightforward, since it involves stretching and shrinking only. First, one of the team members held an unstressed rubber band in his forehead to feel the temperature of the rubber band, and it was felt little bit cooler than the room temperature. Placing his thumbs at each ends of the rubber band, he then quickly stretched the rubber band to its maximum, and immediately pressed it against his forehead to determine the temperature change of the rubber band. As the next step, he then suddenly released the rubber band, and again checked the temperature when the rubber band returned to its normal length. He repeated the test several times until he felt confident about the result. To make sure that the obtained results were correct, the other team member repeated the above mentioned procedure with another rubber band. Procedure of Test 2: The basic set-up for Test2- was also quite easy and pretty clear cut. First, we hung a rubber band vertically from a hook embedded in a concrete wall, and suspended a weight from the other end of it so that the rubber band remained in a linear, upright position, parallel to the surface of the wall. As for the weight, we used a 1.25 kg hammer, because we found that it does not surpass the elasticity limit of the rubber band. Next, we plugged in the hair dryer, and while it was warming up, we measured the length of the stretched rubber band under room temperature with the meter stick. Finally, we heated the rubber band by exposing it to the hot, warm air of the hair dryer, and recorded the final length of the rubber band. While heating, the hair dryer was kept 15 cm apart from the dangling rubber band. The figure on the following page introduces a schematic diagram of the basic set up of the Test 2. Page 4
5 Figure 1: The Schematic Diagram of the Test 2 apparatus arrangement Experimental Data The following table shows the results of Test-1: The State of the Rubber Band The Temperature felt Neither Stretched nor Contracted A little bit cooler than the room temperature After quickly Stretched Warm After Contracted Cool Table 1: The result of Test 1 Page 5
6 The results of Test-2 have been tabulated in the following table: Rubber Length before Length After Change in the Band Heating (cm) Heating (cm) Length (cm) Rubber Band Rubber Band Rubber Band Table 2: The data recorded from the Test 2 Analysis The result of this small experiment very closely supported every aspect of the scientific theory of entropy. Theoretically, the entropy was supposed to increase during the relaxation of the rubber band, and from the experiment we also found that the entropy increased during the relaxation process. The vice-versa was also true, and the discussion section further analyses and interprets this result in a narrative and elaborative way. Discussion of the Result: First, for the sake of explanation, let us assume that the temperature before and after the stretch of the rubber is T1 and T2. After stretching the rubber, we felt that it became warmer, which definitely says that in this process the rubber released a specific amount of heat Page 6
7 energy. Assuming that this specific amount of energy is Q joules, the change in entropy is ΔQ ΔQ, and this is a negative amount because here T2 > T1. The negative quantity is a T T 1 2 proof that the entropy decreases when the rubber band is stretched. After contraction, we found that the rubber band feels cool, and this means that it sucked an amount of heat energy from the heat detector-our forehead, and from the surrounding atmosphere as well. If T1 and T2 is the temperature of the rubber band before and after the contraction, Q is the amount of heat that is sucked up by the band, then the change of entropy in this case is ΔQ ΔQ T T 1 2, which is positive because T1>T2. The positive quantity is the indication that the entropy increases when the rubber band is released from its stretched position. The whole phenomena of expanding or contracting of the rubber band is a reversible process, but the phenomena include a spontaneous (contraction) and a non-spontaneous (expansion) process. The Second Law of Thermodynamics says that for any spontaneous process, the overall change in the entropy must be greater than or equal zero, which means the change, should be positive. Our rubber band experiment exactly demonstrated the similar result. During the relaxation, which is spontaneous process, the heat was absorbed, and the entropy increased. On the other hand, during the expansion, which is a non-spontaneous process, the heat energy was released, and the entropy increased. This change in entropy in the rubber band can also be explained even if entropy is taken as the measurement of the orderliness of something. In this situation, entropy will be the determinant of the orderliness of the molecules present in the rubber band. The loosely fitted chains or strings of molecules of the rubber band try to line up when the rubber band is stretched. Because of this being lined up, the disorder decreases, and the entropy on the other hand increases. Similarly, the opposite things occur when the rubber band is released fro its stretched position, and the entropy goes up. Unlike other substances, the rubber contracts when it is heated, and it can be explained from its atomic structure too. The rubber consists of chains or strings of very large, threadlike Page 7
8 molecules, these molecules move very dynamically in their respective chain when the rubber is heated. Because of the motion of the molecules at the middle of the chain, the ends of the chains are drawn closer and closer, which eventually forces the stretched rubber band to contract. The similarity between the Test-1 and Test-2 is that in both cases the rubber band contracts because of absorbing heat. In test one, the rubber band absorbs heat from the surrounding, and from the heat-detector, our lips, whereas in the second test it absorbs heat from the hot air of the hair dryer. However, in the second test, the rubber band contracts more because it absorbs more heat from the hot air. Conclusion: In the conclusion, we can say that although the contraction of the rubber band due to heat seemed unusual considering other materials expand because of heating, the atomic structure of the rubber showed us that this actually happens to comply with the theory of entropy. Appendices: The modulus of elasticity plays a great role in elasticity limit of any material, and therefore the modulus of elasticity was an important factor in determining the weight of the hammer. The following is a brief discussion about the elasticity limit, and it is directly copied and pasted from the following website: < Modulus of Elasticity: Rate of change of strain here is presented as a function of stress. The slope of the straight line is a portion of a stress-strain diagram. Tangent modulus of elasticity is the slope of the stress-strain diagram at any point. Secant modulus of elasticity is stress divided by strain at any given value of stress or strain. It also is called stress-strain ratio. Page 8
9 Figure 2: Modulus of Elasticity Graph Moduli of elasticity in tension and compression are approximately equal and are known as Young's modulus. Reference Seeley, M.S., Postgraduate physics student, Cambridge, UK. Why do rubber bands contract when heated instead of expanding? Website accessed and information retrieved on April 16, 2007.< Unknown Author. Entropy, Definition and much more from Answers.com. Website accessed and information retrieved on April 16, < Unknown Author. Entropy, Wikipedia, the free Encyclopedia. Website accessed and information retrieved on April 16, 2007.< Unknown Author.Modulus of Elesticity. Website accessed and information retrieved on April 16, 2007.< Unknown Author.Dead Load. Website accessed and information retrieved on April 16, 2007.< Unknown Author.Entropy. Website accessed and information retrieved on April 16, < Page 9
10 Unknown Author.Order and Disorder. Website accessed and information retrieved on April 16, 2007.< Unknown Author.Rubber band. Website accessed and information retrieved on April 16, 2007.< Page 10
Physics 3 Summer 1989 Lab 7 - Elasticity
 Physics 3 Summer 1989 Lab 7 - Elasticity Theory All materials deform to some extent when subjected to a stress (a force per unit area). Elastic materials have internal forces which restore the size and
Physics 3 Summer 1989 Lab 7 - Elasticity Theory All materials deform to some extent when subjected to a stress (a force per unit area). Elastic materials have internal forces which restore the size and
Thermal physics revision questions
 Thermal physics revision questions ONE SECTION OF QUESTIONS TO BE COMPLETED AND MARKED EVERY WEEK AFTER HALF TERM. Section 1: Energy 1. Define the law of conservation of energy. Energy is neither created
Thermal physics revision questions ONE SECTION OF QUESTIONS TO BE COMPLETED AND MARKED EVERY WEEK AFTER HALF TERM. Section 1: Energy 1. Define the law of conservation of energy. Energy is neither created
3 Types of Heat Transfer
 3 Types of Heat Transfer The movement of heat from a warmer object to a cooler object. Heat Transfer- 1. Conduction Heat transfer by direct contact of molecules. In other words, when one molecule runs
3 Types of Heat Transfer The movement of heat from a warmer object to a cooler object. Heat Transfer- 1. Conduction Heat transfer by direct contact of molecules. In other words, when one molecule runs
Preview of Period 7: Applications of the Laws of Thermodynamics
 Preview of Period 7: Applications of the Laws of Thermodynamics 7.1 Conservation of Energy and the 1 st Law of Thermodynamics ow does conservation of energy relate to molecular motion? What is the 1 st
Preview of Period 7: Applications of the Laws of Thermodynamics 7.1 Conservation of Energy and the 1 st Law of Thermodynamics ow does conservation of energy relate to molecular motion? What is the 1 st
Conceptual Physics Fundamentals
 Conceptual Physics Fundamentals Chapter 8: TEMPERATURE, HEAT, AND THERMODYNAMICS This lecture will help you understand: Temperature Absolute Zero Internal Energy Heat Quantity of Heat The Laws of Thermodynamics
Conceptual Physics Fundamentals Chapter 8: TEMPERATURE, HEAT, AND THERMODYNAMICS This lecture will help you understand: Temperature Absolute Zero Internal Energy Heat Quantity of Heat The Laws of Thermodynamics
Thermodynamics 1 Lecture Note 2
 Thermodynamics 1 Lecture Note 2 March 20, 2015 Kwang Kim Yonsei University kbkim@yonsei.ac.kr 39 8 7 34 53 Y O N Se I 88.91 16.00 14.01 78.96 126.9 Physical Chemistry Chemistry is the study of Matter and
Thermodynamics 1 Lecture Note 2 March 20, 2015 Kwang Kim Yonsei University kbkim@yonsei.ac.kr 39 8 7 34 53 Y O N Se I 88.91 16.00 14.01 78.96 126.9 Physical Chemistry Chemistry is the study of Matter and
10.2 PROCESSES 10.3 THE SECOND LAW OF THERMO/ENTROPY Student Notes
 10.2 PROCESSES 10.3 THE SECOND LAW OF THERMO/ENTROPY Student Notes I. THE FIRST LAW OF THERMODYNAMICS A. SYSTEMS AND SURROUNDING B. PV DIAGRAMS AND WORK DONE V -1 Source: Physics for the IB Diploma Study
10.2 PROCESSES 10.3 THE SECOND LAW OF THERMO/ENTROPY Student Notes I. THE FIRST LAW OF THERMODYNAMICS A. SYSTEMS AND SURROUNDING B. PV DIAGRAMS AND WORK DONE V -1 Source: Physics for the IB Diploma Study
Thermodynamics. We can summarize the four laws of thermodynamics as follows:
 Thermodynamics Objective: To investigate the zeroth and first laws of thermodynamics. To calculate properties such as specific heat. To investigate the ideal gas law. To become familiar with basic P-V
Thermodynamics Objective: To investigate the zeroth and first laws of thermodynamics. To calculate properties such as specific heat. To investigate the ideal gas law. To become familiar with basic P-V
THERMODYNAMICS. Thermodynamics is the study of energy relationships that involve heat, mechanical work, and other aspects of energy and heat transfer.
 THERMODYNAMICS Thermodynamics is the study of energy relationships that involve heat, mechanical work, and other aspects of energy and heat transfer. Central Heating Objectives: After finishing this unit,
THERMODYNAMICS Thermodynamics is the study of energy relationships that involve heat, mechanical work, and other aspects of energy and heat transfer. Central Heating Objectives: After finishing this unit,
CPO Science Foundations of Physics. Unit 8, Chapter 27
 CPO Science Foundations of Physics Unit 8, Chapter 27 Unit 8: Matter and Energy Chapter 27 The Physical Properties of Matter 27.1 Properties of Solids 27.2 Properties of Liquids and Fluids 27.3 Properties
CPO Science Foundations of Physics Unit 8, Chapter 27 Unit 8: Matter and Energy Chapter 27 The Physical Properties of Matter 27.1 Properties of Solids 27.2 Properties of Liquids and Fluids 27.3 Properties
Physics 1501 Lecture 37
 Physics 1501: Lecture 37 Todays Agenda Announcements Homework #12 (Dec. 9): 2 lowest dropped Midterm 2 in class Wednesday Friday: review session bring your questions Todays topics Chap.18: Heat and Work»
Physics 1501: Lecture 37 Todays Agenda Announcements Homework #12 (Dec. 9): 2 lowest dropped Midterm 2 in class Wednesday Friday: review session bring your questions Todays topics Chap.18: Heat and Work»
Lecture PowerPoints. Chapter 13 Physics: Principles with Applications, 7 th edition Giancoli
 Lecture PowerPoints Chapter 13 Physics: Principles with Applications, 7 th edition Giancoli This work is protected by United States copyright laws and is provided solely for the use of instructors in teaching
Lecture PowerPoints Chapter 13 Physics: Principles with Applications, 7 th edition Giancoli This work is protected by United States copyright laws and is provided solely for the use of instructors in teaching
Entropy and the Second Law of Thermodynamics
 Entropy and the Second Law of Thermodynamics Reading Problems 7-1 7-3 7-88, 7-131, 7-135 7-6 7-10 8-24, 8-44, 8-46, 8-60, 8-73, 8-99, 8-128, 8-132, 8-1 8-10, 8-13 8-135, 8-148, 8-152, 8-166, 8-168, 8-189
Entropy and the Second Law of Thermodynamics Reading Problems 7-1 7-3 7-88, 7-131, 7-135 7-6 7-10 8-24, 8-44, 8-46, 8-60, 8-73, 8-99, 8-128, 8-132, 8-1 8-10, 8-13 8-135, 8-148, 8-152, 8-166, 8-168, 8-189
22 Which of the following correctly defines the terms stress, strain and Young modulus? stress strain Young modulus
 PhysicsndMathsTutor.com Which of the following correctly defines the terms stress, strain and Young modulus? 97/1/M/J/ stress strain Young modulus () x (area) (extension) x (original length) (stress) /
PhysicsndMathsTutor.com Which of the following correctly defines the terms stress, strain and Young modulus? 97/1/M/J/ stress strain Young modulus () x (area) (extension) x (original length) (stress) /
Figure Two. Then the two vector equations of equilibrium are equivalent to three scalar equations:
 2004- v 10/16 2. The resultant external torque (the vector sum of all external torques) acting on the body must be zero about any origin. These conditions can be written as equations: F = 0 = 0 where the
2004- v 10/16 2. The resultant external torque (the vector sum of all external torques) acting on the body must be zero about any origin. These conditions can be written as equations: F = 0 = 0 where the
Chapter 11 Heat Engines and The Second Law of Thermodynamics
 Chapter 11 Heat Engines and The Second Law of Thermodynamics Heat Engines Heat engines use a temperature difference involving a high temperature (T H ) and a low temperature (T C ) to do mechanical work.
Chapter 11 Heat Engines and The Second Law of Thermodynamics Heat Engines Heat engines use a temperature difference involving a high temperature (T H ) and a low temperature (T C ) to do mechanical work.
Class 22 - Second Law of Thermodynamics and Entropy
 Class 22 - Second Law of Thermodynamics and Entropy The second law of thermodynamics The first law relates heat energy, work and the internal thermal energy of a system, and is essentially a statement
Class 22 - Second Law of Thermodynamics and Entropy The second law of thermodynamics The first law relates heat energy, work and the internal thermal energy of a system, and is essentially a statement
Module 3 - Thermodynamics. Thermodynamics. Measuring Temperatures. Temperature and Thermal Equilibrium
 Thermodynamics From the Greek thermos meaning heat and dynamis meaning power is a branch of physics that studies the effects of changes in temperature, pressure, and volume on physical systems at the macroscopic
Thermodynamics From the Greek thermos meaning heat and dynamis meaning power is a branch of physics that studies the effects of changes in temperature, pressure, and volume on physical systems at the macroscopic
Weight and contact forces: Young's modulus, Hooke's law and material properties
 Weight and contact forces: Young's modulus, Hooke's law and material properties Many objects deform according to Hooke's law; many materials behave elastically and have a Young's modulus. In this section,
Weight and contact forces: Young's modulus, Hooke's law and material properties Many objects deform according to Hooke's law; many materials behave elastically and have a Young's modulus. In this section,
AP Physics Free Response Practice Oscillations
 AP Physics Free Response Practice Oscillations 1975B7. A pendulum consists of a small object of mass m fastened to the end of an inextensible cord of length L. Initially, the pendulum is drawn aside through
AP Physics Free Response Practice Oscillations 1975B7. A pendulum consists of a small object of mass m fastened to the end of an inextensible cord of length L. Initially, the pendulum is drawn aside through
Conceptual Physics Fundamentals
 Conceptual Physics Fundamentals Chapter 8: TEMPERATURE, HEAT, AND THERMODYNAMICS This lecture will help you understand: Temperature Absolute Zero Internal Energy Heat Quantity of Heat The Laws of Thermodynamics
Conceptual Physics Fundamentals Chapter 8: TEMPERATURE, HEAT, AND THERMODYNAMICS This lecture will help you understand: Temperature Absolute Zero Internal Energy Heat Quantity of Heat The Laws of Thermodynamics
Lecture Outline Chapter 17. Physics, 4 th Edition James S. Walker. Copyright 2010 Pearson Education, Inc.
 Lecture Outline Chapter 17 Physics, 4 th Edition James S. Walker Chapter 17 Phases and Phase Changes Ideal Gases Kinetic Theory Units of Chapter 17 Solids and Elastic Deformation Phase Equilibrium and
Lecture Outline Chapter 17 Physics, 4 th Edition James S. Walker Chapter 17 Phases and Phase Changes Ideal Gases Kinetic Theory Units of Chapter 17 Solids and Elastic Deformation Phase Equilibrium and
Activity Title: It s Either Very Hot or Very Cold Up There!
 Grades 3-5 Teacher Pages Activity Title: It s Either Very Hot or Very Cold Up There! Activity Objective(s): In this activity, and the follow-up activity next week, teams will design and conduct experiments
Grades 3-5 Teacher Pages Activity Title: It s Either Very Hot or Very Cold Up There! Activity Objective(s): In this activity, and the follow-up activity next week, teams will design and conduct experiments
Archimedes Principle
 Archimedes Principle applies in air the more air an object displaces, the greater the buoyant force on it if an object displaces its weight, it hovers at a constant altitude if an object displaces less
Archimedes Principle applies in air the more air an object displaces, the greater the buoyant force on it if an object displaces its weight, it hovers at a constant altitude if an object displaces less
Name Class Date. c. 273 K
 Exercises 24.1 Absolute Zero (page 469) 1. Is the following sentence true or false? There is no limit to how cold an object can get. 2. Define absolute zero. 3. Circle the letter of each statement about
Exercises 24.1 Absolute Zero (page 469) 1. Is the following sentence true or false? There is no limit to how cold an object can get. 2. Define absolute zero. 3. Circle the letter of each statement about
Core Chemistry UNIT 1: Matter & Energy Section 1: The Law of Conservation of Mass Section 2: States of Matter & Intro to Thermodynamics
 Core Chemistry UNIT 1: Matter & Energy Section 1: The Law of Conservation of Mass Section 2: States of Matter & Intro to Thermodynamics UNIT 1 Synapsis In our first unit we will explore matter & energy
Core Chemistry UNIT 1: Matter & Energy Section 1: The Law of Conservation of Mass Section 2: States of Matter & Intro to Thermodynamics UNIT 1 Synapsis In our first unit we will explore matter & energy
Demonstrate understanding of aspects of heat
 Demonstrate understanding of aspects of heat Heat Transfer Temperature - temperature is a measure of the average kinetic energy of the particles making up an object (measured in C or K) 0 K = -273 o C
Demonstrate understanding of aspects of heat Heat Transfer Temperature - temperature is a measure of the average kinetic energy of the particles making up an object (measured in C or K) 0 K = -273 o C
Module 3 - Thermodynamics. Thermodynamics. Measuring Temperatures. Temperature and Thermal Equilibrium
 Thermodynamics From the Greek thermos meaning heat and dynamis meaning power is a branch of physics that studies the effects of changes in temperature, pressure, and volume on physical systems at the macroscopic
Thermodynamics From the Greek thermos meaning heat and dynamis meaning power is a branch of physics that studies the effects of changes in temperature, pressure, and volume on physical systems at the macroscopic
Agenda. Chapter 10, Problem 26. All matter is made of atoms. Atomic Structure 4/8/14. What is the structure of matter? Atomic Terminology
 Agenda Today: HW Quiz, Thermal physics (i.e., heat) Thursday: Finish thermal physics, atomic structure (lots of review from chemistry!) Chapter 10, Problem 26 A boy reaches out of a window and tosses a
Agenda Today: HW Quiz, Thermal physics (i.e., heat) Thursday: Finish thermal physics, atomic structure (lots of review from chemistry!) Chapter 10, Problem 26 A boy reaches out of a window and tosses a
The Kinetic Theory of Matter. Temperature. Temperature. Temperature. Temperature. Chapter 6 HEAT
 The Kinetic Theory of Matter Hewitt/Lyons/Suchocki/Yeh Conceptual Integrated Science Chapter 6 HEAT Kinetic Theory of Matter: Matter is made up of tiny particles (atoms or molecules) that are always in
The Kinetic Theory of Matter Hewitt/Lyons/Suchocki/Yeh Conceptual Integrated Science Chapter 6 HEAT Kinetic Theory of Matter: Matter is made up of tiny particles (atoms or molecules) that are always in
ME 207 Material Science I
 ME 207 Material Science I Chapter 3 Properties in Tension and Compression Dr. İbrahim H. Yılmaz http://web.adanabtu.edu.tr/iyilmaz Automotive Engineering Adana Science and Technology University Introduction
ME 207 Material Science I Chapter 3 Properties in Tension and Compression Dr. İbrahim H. Yılmaz http://web.adanabtu.edu.tr/iyilmaz Automotive Engineering Adana Science and Technology University Introduction
Atoms and molecules are in motion and have energy
 Atoms and molecules are in motion and have energy By now you know that substances are made of atoms and molecules. These atoms and molecules are always in motion and have attractions to each other. When
Atoms and molecules are in motion and have energy By now you know that substances are made of atoms and molecules. These atoms and molecules are always in motion and have attractions to each other. When
Lecture Outline Chapter 18. Physics, 4 th Edition James S. Walker. Copyright 2010 Pearson Education, Inc.
 Lecture Outline Chapter 18 Physics, 4 th Edition James S. Walker Chapter 18 The Laws of Thermodynamics Units of Chapter 18 The Zeroth Law of Thermodynamics The First Law of Thermodynamics Thermal Processes
Lecture Outline Chapter 18 Physics, 4 th Edition James S. Walker Chapter 18 The Laws of Thermodynamics Units of Chapter 18 The Zeroth Law of Thermodynamics The First Law of Thermodynamics Thermal Processes
Temperature, Heat, and Expansion
 Thermodynamics (Based on Chapters 21-24) Temperature, Heat, and Expansion (Ch 21) Warmth is the kinetic energy of atoms and molecules. Temperature (21.1) The measure of how hot and cold things are is temperature.
Thermodynamics (Based on Chapters 21-24) Temperature, Heat, and Expansion (Ch 21) Warmth is the kinetic energy of atoms and molecules. Temperature (21.1) The measure of how hot and cold things are is temperature.
Chapter 7 Notes. Matter is made of tiny particles in constant motion
 Chapter 7 Notes Section 7.1 Matter is made of tiny particles in constant motion Atomic Theory Greek philosophers (430 BC ) Democritus and Leucippus proposed that matter is made of tiny particles called
Chapter 7 Notes Section 7.1 Matter is made of tiny particles in constant motion Atomic Theory Greek philosophers (430 BC ) Democritus and Leucippus proposed that matter is made of tiny particles called
Chapter 21: Temperature, Heat and Expansion
 Chapter 21: Temperature, Heat and Expansion All matter solid, liquid and gas is made of atoms or molecules, which are continually jiggling. As this jiggling is a movement, all these particles must have
Chapter 21: Temperature, Heat and Expansion All matter solid, liquid and gas is made of atoms or molecules, which are continually jiggling. As this jiggling is a movement, all these particles must have
So far in talking about thermodynamics, we ve mostly limited ourselves to
 251 Lecture 33 So far in talking about thermodynamics, we ve mostly limited ourselves to discussions of thermochemistry, a quantification of the heat absorbed or given off as the result of a chemical reaction.
251 Lecture 33 So far in talking about thermodynamics, we ve mostly limited ourselves to discussions of thermochemistry, a quantification of the heat absorbed or given off as the result of a chemical reaction.
Post-Show HOT AND COLD. Gases. Liquids. Solids. After the Show. Traveling Science Shows
 Traveling Science Shows Post-Show HOT AND COLD After the Show We recently presented a Hot and Cold show at your school, and thought you and your students might like to continue investigating this topic.
Traveling Science Shows Post-Show HOT AND COLD After the Show We recently presented a Hot and Cold show at your school, and thought you and your students might like to continue investigating this topic.
Hooke's Law: Stress and Strain Revisited *
 OpenStax-CNX module: m42240 1 Hooke's Law: Stress and Strain Revisited * OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 Abstract Explain
OpenStax-CNX module: m42240 1 Hooke's Law: Stress and Strain Revisited * OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 Abstract Explain
Chapter 19 Chemical Thermodynamics
 Chapter 19 Chemical Thermodynamics Spontaneous Processes Entropy and the Second Law of Thermodynamics The Molecular Interpretation of Entropy Entropy Changes in Chemical Reactions Gibbs Free Energy Free
Chapter 19 Chemical Thermodynamics Spontaneous Processes Entropy and the Second Law of Thermodynamics The Molecular Interpretation of Entropy Entropy Changes in Chemical Reactions Gibbs Free Energy Free
NORTHERN ILLINOIS UNIVERSITY PHYSICS DEPARTMENT. Physics 211 E&M and Quantum Physics Spring Lab #2: Electrostatics. qq k r
 NORTHRN ILLINOIS UNIVRSITY PHYSICS DPARTMNT Physics 11 &M and Quantum Physics Spring 018 Lab #: lectrostatics Lab Writeup Due: Mon/Wed/Thu/Fri, Jan. 9/31/Jan. 1/, 018 Background You ve learned a lot about
NORTHRN ILLINOIS UNIVRSITY PHYSICS DPARTMNT Physics 11 &M and Quantum Physics Spring 018 Lab #: lectrostatics Lab Writeup Due: Mon/Wed/Thu/Fri, Jan. 9/31/Jan. 1/, 018 Background You ve learned a lot about
Chapter 12 Thermal Energy
 Chapter 12 Thermal Energy Chapter 12 In this chapter you will: Learn how temperature relates to the potential and kinetic energies of atoms and molecules. Distinguish heat from work. Calculate heat transfer
Chapter 12 Thermal Energy Chapter 12 In this chapter you will: Learn how temperature relates to the potential and kinetic energies of atoms and molecules. Distinguish heat from work. Calculate heat transfer
Entropy and Enthalpy: Changes in Rubber Bands
 Section 8 Entropy and Enthalpy: Changes in Rubber Bands What Do You See? Learning Outcomes In this section you will Determine if a change results in an increase or decrease in entropy. Determine if a change
Section 8 Entropy and Enthalpy: Changes in Rubber Bands What Do You See? Learning Outcomes In this section you will Determine if a change results in an increase or decrease in entropy. Determine if a change
2,000-gram mass of water compared to a 1,000-gram mass.
 11.2 Heat To change the temperature, you usually need to add or subtract energy. For example, when it s cold outside, you turn up the heat in your house or apartment and the temperature goes up. You know
11.2 Heat To change the temperature, you usually need to add or subtract energy. For example, when it s cold outside, you turn up the heat in your house or apartment and the temperature goes up. You know
Thermodynamic system is classified into the following three systems. (ii) Closed System It exchanges only energy (not matter) with surroundings.
 1 P a g e The branch of physics which deals with the study of transformation of heat energy into other forms of energy and vice-versa. A thermodynamical system is said to be in thermal equilibrium when
1 P a g e The branch of physics which deals with the study of transformation of heat energy into other forms of energy and vice-versa. A thermodynamical system is said to be in thermal equilibrium when
Energy. Potential Kinetic
 Energy the ability to do work or cause change typically expressed in units of joules (J) can be transferred from one object to another two general types: Potential Kinetic Potential Energy (PE) stored
Energy the ability to do work or cause change typically expressed in units of joules (J) can be transferred from one object to another two general types: Potential Kinetic Potential Energy (PE) stored
Electricity and Energy 1 Content Statements
 Keep this in good condition, it will help you pass your final exams. The school will only issue one paper copy per pupil. An e-copy will be placed on the school s web-site. Electricity and Energy 1 Content
Keep this in good condition, it will help you pass your final exams. The school will only issue one paper copy per pupil. An e-copy will be placed on the school s web-site. Electricity and Energy 1 Content
Name Date Block LESSON CLUSTER 6: Expansion and Contraction
 LESSON CLUSTER 6: Expansion and Contraction Did you know that when you say that something is hot or cold, you are actually saying something about the molecules of that substance? Words like hot and cold
LESSON CLUSTER 6: Expansion and Contraction Did you know that when you say that something is hot or cold, you are actually saying something about the molecules of that substance? Words like hot and cold
11/29/2017 IRREVERSIBLE PROCESSES. UNIT 2 Thermodynamics: Laws of thermodynamics, ideal gases, and kinetic theory
 11/9/017 AP PHYSICS UNIT Thermodynamics: Laws of thermodynamics, ideal gases, and kinetic theory CHAPTER 13 SECOND LAW OF THERMODYNAMICS IRREVERSIBLE PROCESSES The U G of the water-earth system at the
11/9/017 AP PHYSICS UNIT Thermodynamics: Laws of thermodynamics, ideal gases, and kinetic theory CHAPTER 13 SECOND LAW OF THERMODYNAMICS IRREVERSIBLE PROCESSES The U G of the water-earth system at the
What is thermodynamics? and what can it do for us?
 What is thermodynamics? and what can it do for us? The overall goal of thermodynamics is to describe what happens to a system (anything of interest) when we change the variables that characterized the
What is thermodynamics? and what can it do for us? The overall goal of thermodynamics is to describe what happens to a system (anything of interest) when we change the variables that characterized the
CHM Solids, Liquids, and Phase Changes (r15) Charles Taylor 1/9
 CHM 111 - Solids, Liquids, and Phase Changes (r15) - 2015 Charles Taylor 1/9 Introduction In CHM 110, we used kinetic theory to explain the behavior of gases. Now, we will discuss solids and liquids. While
CHM 111 - Solids, Liquids, and Phase Changes (r15) - 2015 Charles Taylor 1/9 Introduction In CHM 110, we used kinetic theory to explain the behavior of gases. Now, we will discuss solids and liquids. While
7. The coffee cup allows for pv work because it allows for a change in volume.
 1. A black body radiator is a theoretically perfect body that absorbs all energy incident upon it (or produced within it) and then emits 100% of this energy as electromagnetic radiation. 2. First, it is
1. A black body radiator is a theoretically perfect body that absorbs all energy incident upon it (or produced within it) and then emits 100% of this energy as electromagnetic radiation. 2. First, it is
Equilibrium & Elasticity
 PHYS 101 Previous Exam Problems CHAPTER 12 Equilibrium & Elasticity Static equilibrium Elasticity 1. A uniform steel bar of length 3.0 m and weight 20 N rests on two supports (A and B) at its ends. A block
PHYS 101 Previous Exam Problems CHAPTER 12 Equilibrium & Elasticity Static equilibrium Elasticity 1. A uniform steel bar of length 3.0 m and weight 20 N rests on two supports (A and B) at its ends. A block
(Refer Slide Time: 00:58)
 Nature and Properties of Materials Professor Bishak Bhattacharya Department of Mechanical Engineering Indian Institute of Technology Kanpur Lecture 18 Effect and Glass Transition Temperature In the last
Nature and Properties of Materials Professor Bishak Bhattacharya Department of Mechanical Engineering Indian Institute of Technology Kanpur Lecture 18 Effect and Glass Transition Temperature In the last
Thermodynamic Systems, States, and Processes
 Thermodynamics Thermodynamic Systems, States, and Processes A thermodynamic system is described by an equation of state, such as the ideal gas law. The location of the state can be plotted on a p V diagram,
Thermodynamics Thermodynamic Systems, States, and Processes A thermodynamic system is described by an equation of state, such as the ideal gas law. The location of the state can be plotted on a p V diagram,
Chemical Kinetics Review Sheet
 Chemical Kinetics Review Sheet Main concepts - Chemical reactions can happen when two atoms or molecules collide with enough energy. - The greater the number of collisions the more likely a reaction can
Chemical Kinetics Review Sheet Main concepts - Chemical reactions can happen when two atoms or molecules collide with enough energy. - The greater the number of collisions the more likely a reaction can
Outline of the Course
 Outline of the Course 1) Review and Definitions 2) Molecules and their Energies 3) 1 st Law of Thermodynamics 4) 2 nd Law of Thermodynamics 5) Gibbs Free Energy 6) Phase Diagrams and REAL Phenomena 7)
Outline of the Course 1) Review and Definitions 2) Molecules and their Energies 3) 1 st Law of Thermodynamics 4) 2 nd Law of Thermodynamics 5) Gibbs Free Energy 6) Phase Diagrams and REAL Phenomena 7)
9 MECHANICAL PROPERTIES OF SOLIDS
 9 MECHANICAL PROPERTIES OF SOLIDS Deforming force Deforming force is the force which changes the shape or size of a body. Restoring force Restoring force is the internal force developed inside the body
9 MECHANICAL PROPERTIES OF SOLIDS Deforming force Deforming force is the force which changes the shape or size of a body. Restoring force Restoring force is the internal force developed inside the body
Chapter 12 Thermodynamics
 Chapter 12 Thermodynamics 12.1 Thermodynamic Systems, States, and Processes System: definite quantity of matter with real or imaginary boundaries If heat transfer is impossible, the system is thermally
Chapter 12 Thermodynamics 12.1 Thermodynamic Systems, States, and Processes System: definite quantity of matter with real or imaginary boundaries If heat transfer is impossible, the system is thermally
Chapter 14 Temperature and Heat
 Nicholas J. Giordano www.cengage.com/physics/giordano Chapter 14 Temperature and Heat Thermodynamics Starting a different area of physics called thermodynamics Thermodynamics focuses on energy rather than
Nicholas J. Giordano www.cengage.com/physics/giordano Chapter 14 Temperature and Heat Thermodynamics Starting a different area of physics called thermodynamics Thermodynamics focuses on energy rather than
Survey of Thermodynamic Processes and First and Second Laws
 Survey of Thermodynamic Processes and First and Second Laws Please select only one of the five choices, (a)-(e) for each of the 33 questions. All temperatures T are absolute temperatures. All experiments
Survey of Thermodynamic Processes and First and Second Laws Please select only one of the five choices, (a)-(e) for each of the 33 questions. All temperatures T are absolute temperatures. All experiments
International Journal of Basic & Applied Sciences IJBAS-IJENS Vol:14 No:04 9
 International Journal of Basic & Applied Sciences IJBAS-IJENS Vol:14 No:04 9 Investigation on a Practical Model to Explain the Temperature Change of a Deflating Balloon Yongkyun Lee*, Yongnam Kwon Korean
International Journal of Basic & Applied Sciences IJBAS-IJENS Vol:14 No:04 9 Investigation on a Practical Model to Explain the Temperature Change of a Deflating Balloon Yongkyun Lee*, Yongnam Kwon Korean
Infrared Experiments of Thermal Energy and Heat Transfer
 Infrared Experiments of Thermal Energy and Heat Transfer You will explore thermal energy, thermal equilibrium, heat transfer, and latent heat in a series of hands-on activities augmented by the thermal
Infrared Experiments of Thermal Energy and Heat Transfer You will explore thermal energy, thermal equilibrium, heat transfer, and latent heat in a series of hands-on activities augmented by the thermal
Joy of Science Discovering the matters and the laws of the universe
 November 12, 2012 Joy of Science Discovering the matters and the laws of the universe Key Words Universe, Energy, Quantum mechanics, Chemical reaction, Structure of matter (Earth, Evolution of life, Ecosystem,
November 12, 2012 Joy of Science Discovering the matters and the laws of the universe Key Words Universe, Energy, Quantum mechanics, Chemical reaction, Structure of matter (Earth, Evolution of life, Ecosystem,
Chapter 13 ELASTIC PROPERTIES OF MATERIALS
 Physics Including Human Applications 280 Chapter 13 ELASTIC PROPERTIES OF MATERIALS GOALS When you have mastered the contents of this chapter, you will be able to achieve the following goals: Definitions
Physics Including Human Applications 280 Chapter 13 ELASTIC PROPERTIES OF MATERIALS GOALS When you have mastered the contents of this chapter, you will be able to achieve the following goals: Definitions
Physics. Chapter 7 Energy
 Physics Chapter 7 Energy Work How long does a force act? Last week, we meant time as in impulse (Ft) This week, we will take how long to mean distance Force x distance (Fd) is what we call WORK W = Fd
Physics Chapter 7 Energy Work How long does a force act? Last week, we meant time as in impulse (Ft) This week, we will take how long to mean distance Force x distance (Fd) is what we call WORK W = Fd
Random arrang ement (disorder) Ordered arrangement (order)
 2 CHAPTER 3 In any spontaneous energy conversion, the entropy of the system increases. Regardless of the nature of energy conversion, the entropy of the universe tends toward a maximum. (more probable)
2 CHAPTER 3 In any spontaneous energy conversion, the entropy of the system increases. Regardless of the nature of energy conversion, the entropy of the universe tends toward a maximum. (more probable)
=.55 = = 5.05
 MAT1193 4c Definition of derivative With a better understanding of limits we return to idea of the instantaneous velocity or instantaneous rate of change. Remember that in the example of calculating the
MAT1193 4c Definition of derivative With a better understanding of limits we return to idea of the instantaneous velocity or instantaneous rate of change. Remember that in the example of calculating the
CHAPTER 15 ADIABATIC DEMAGNETIZATION
 1 HAER 15 ADIAAI DEAGNEIZAION 151 Introduction One way to cool a gas is as follows First compress it isothermally his means compress it in a vessel that isn t insulated, and wait for the gas to lose any
1 HAER 15 ADIAAI DEAGNEIZAION 151 Introduction One way to cool a gas is as follows First compress it isothermally his means compress it in a vessel that isn t insulated, and wait for the gas to lose any
Temperature, Thermal Energy and Heat
 Temperature You use the words hot and cold to describe temperature. Something is hot when its temperature is high. When you heat water on a stove, its temperature increases. How are temperature and heat
Temperature You use the words hot and cold to describe temperature. Something is hot when its temperature is high. When you heat water on a stove, its temperature increases. How are temperature and heat
COURSE 3.20: THERMODYNAMICS OF MATERIALS. FINAL EXAM, Dec 18, 2000
 NAME: COURSE 3.20: THERMODYNAMICS OF MATERIALS FINAL EXAM, Dec 18, 2000 PROBLEM 1 (10 POINTS) PROBLEM 2 (15 POINTS) PROBLEM 3 (12 POINTS) PROBLEM 4 (12 POINTS) PROBLEM 5 (12 POINTS) PROBLEM 6 (15 POINTS)
NAME: COURSE 3.20: THERMODYNAMICS OF MATERIALS FINAL EXAM, Dec 18, 2000 PROBLEM 1 (10 POINTS) PROBLEM 2 (15 POINTS) PROBLEM 3 (12 POINTS) PROBLEM 4 (12 POINTS) PROBLEM 5 (12 POINTS) PROBLEM 6 (15 POINTS)
Chapter 2, Lesson 5: Changing State Melting
 Chapter 2, Lesson 5: Changing State Melting Key Concepts Melting is a process that causes a substance to change from a solid to a liquid. Melting occurs when the molecules of a solid speed up enough that
Chapter 2, Lesson 5: Changing State Melting Key Concepts Melting is a process that causes a substance to change from a solid to a liquid. Melting occurs when the molecules of a solid speed up enough that
Crystalline The Solid State notes.notebook. April 15, crystalline : regular/repeating arrangement (pattern) of particles
 http://www.google.com/imgres? imgurl=http://bouman.chem.georgetown.edu/s02/lect30/e03.jpg&imgrefurl=http://bouman.che m.georgetown.edu/s02/lect30/lect30.htm&usg= TlOCX3 Zy179Iiw4QP5X nuklxi=&h=305 &w=675&sz=190&hl=en&start=27&itbs=1&tbnid=zz3jzrsdfp0ogm:&tbnh=62&tbnw=138
http://www.google.com/imgres? imgurl=http://bouman.chem.georgetown.edu/s02/lect30/e03.jpg&imgrefurl=http://bouman.che m.georgetown.edu/s02/lect30/lect30.htm&usg= TlOCX3 Zy179Iiw4QP5X nuklxi=&h=305 &w=675&sz=190&hl=en&start=27&itbs=1&tbnid=zz3jzrsdfp0ogm:&tbnh=62&tbnw=138
8th Grade. Thermal Energy Study Guide.
 1 8th Grade Thermal Energy Study Guide 2015 10 09 www.njctl.org 2 Thermal Energy Study Guide www.njctl.org 3 Part 1 Define the following terms and/or concepts 4 1 Temperature 5 2 Kinetic Energy 6 3 Thermal
1 8th Grade Thermal Energy Study Guide 2015 10 09 www.njctl.org 2 Thermal Energy Study Guide www.njctl.org 3 Part 1 Define the following terms and/or concepts 4 1 Temperature 5 2 Kinetic Energy 6 3 Thermal
Slide 1 / 67. Slide 2 / 67. 8th Grade. Thermal Energy Study Guide Slide 3 / 67. Thermal Energy. Study Guide.
 Slide 1 / 67 Slide 2 / 67 8th Grade Thermal Energy Study Guide 2015-10-09 www.njctl.org Slide 3 / 67 Thermal Energy Study Guide www.njctl.org Slide 4 / 67 Part 1 Define the following terms and/or concepts
Slide 1 / 67 Slide 2 / 67 8th Grade Thermal Energy Study Guide 2015-10-09 www.njctl.org Slide 3 / 67 Thermal Energy Study Guide www.njctl.org Slide 4 / 67 Part 1 Define the following terms and/or concepts
Electrostatics II. Introduction
 Electrostatics II Objective: To learn how excess charge is created and transferred. To measure the electrostatic force between two objects as a function of their electrical charges and their separation
Electrostatics II Objective: To learn how excess charge is created and transferred. To measure the electrostatic force between two objects as a function of their electrical charges and their separation
Temperature Energy and Heat
 CHAPTER 3 Temperature Energy and Heat 3.1 Temperature What is temperature? Why is temperature important in chemistry? How is energy related to temperature? 2 3.1 Temperature Milk fat particles are being
CHAPTER 3 Temperature Energy and Heat 3.1 Temperature What is temperature? Why is temperature important in chemistry? How is energy related to temperature? 2 3.1 Temperature Milk fat particles are being
What Is Air Temperature?
 2.2 Read What Is Air Temperature? In Learning Set 1, you used a thermometer to measure air temperature. But what exactly was the thermometer measuring? What is different about cold air and warm air that
2.2 Read What Is Air Temperature? In Learning Set 1, you used a thermometer to measure air temperature. But what exactly was the thermometer measuring? What is different about cold air and warm air that
Chapter 11. Energy in Thermal Processes
 Chapter 11 Energy in Thermal Processes Energy Transfer When two objects of different temperatures are placed in thermal contact, the temperature of the warmer decreases and the temperature of the cooler
Chapter 11 Energy in Thermal Processes Energy Transfer When two objects of different temperatures are placed in thermal contact, the temperature of the warmer decreases and the temperature of the cooler
This Week. 6/2/2015 Physics 214 Summer
 This Week Heat and Temperature Water and Ice Our world would be different if water didn t expand Engines We can t use all the energy! Why is a diesel engine more efficient? Geysers: You have to be faithful
This Week Heat and Temperature Water and Ice Our world would be different if water didn t expand Engines We can t use all the energy! Why is a diesel engine more efficient? Geysers: You have to be faithful
INTERACTIONS AND ENERGY
 CHAPTER 1 Scientists Ideas INTERACTIONS AND ENERGY In this Chapter you first learned a way of representing the motion of an object using speed-time graphs. Then you developed some ideas involving interactions
CHAPTER 1 Scientists Ideas INTERACTIONS AND ENERGY In this Chapter you first learned a way of representing the motion of an object using speed-time graphs. Then you developed some ideas involving interactions
PHYSICS. Chapter 9 Lecture FOR SCIENTISTS AND ENGINEERS A STRATEGIC APPROACH 4/E RANDALL D. KNIGHT Pearson Education, Inc.
 PHYSICS FOR SCIENTISTS AND ENGINEERS A STRATEGIC APPROACH 4/E Chapter 9 Lecture RANDALL D. KNIGHT Chapter 9 Work and Kinetic Energy IN THIS CHAPTER, you will begin your study of how energy is transferred
PHYSICS FOR SCIENTISTS AND ENGINEERS A STRATEGIC APPROACH 4/E Chapter 9 Lecture RANDALL D. KNIGHT Chapter 9 Work and Kinetic Energy IN THIS CHAPTER, you will begin your study of how energy is transferred
Chapter 20 Second Law of Thermodynamics. Copyright 2009 Pearson Education, Inc.
 Chapter 20 Second Law of Thermodynamics It is easy to produce thermal energy using work, but how does one produce work using thermal energy? This is a heat engine; mechanical energy can be obtained from
Chapter 20 Second Law of Thermodynamics It is easy to produce thermal energy using work, but how does one produce work using thermal energy? This is a heat engine; mechanical energy can be obtained from
Chapter 12. The Laws of Thermodynamics. First Law of Thermodynamics
 Chapter 12 The Laws of Thermodynamics First Law of Thermodynamics The First Law of Thermodynamics tells us that the internal energy of a system can be increased by Adding energy to the system Doing work
Chapter 12 The Laws of Thermodynamics First Law of Thermodynamics The First Law of Thermodynamics tells us that the internal energy of a system can be increased by Adding energy to the system Doing work
Spring_#7. Thermodynamics. Youngsuk Nam.
 Spring_#7 Thermodynamics Youngsuk Nam ysnam1@khu.ac.kr You can t connect the dots looking forward; you can only connect them looking backwards. So you have to trust that the dots will somehow connect in
Spring_#7 Thermodynamics Youngsuk Nam ysnam1@khu.ac.kr You can t connect the dots looking forward; you can only connect them looking backwards. So you have to trust that the dots will somehow connect in
Thermal Physics. Topics to be covered. Slide 2 / 105. Slide 1 / 105. Slide 3 / 105. Slide 4 / 105. Slide 5 / 105. Slide 6 / 105.
 Slide 1 / 105 Slide 2 / 105 Topics to be covered Thermal Physics Temperature and Thermal quilibrium Gas Laws Internal nergy Heat Work Laws of Thermodynamics Heat ngines Slide 3 / 105 Thermodynamics System
Slide 1 / 105 Slide 2 / 105 Topics to be covered Thermal Physics Temperature and Thermal quilibrium Gas Laws Internal nergy Heat Work Laws of Thermodynamics Heat ngines Slide 3 / 105 Thermodynamics System
Keep the Heat Test School Name. Team Number
 Keep the Heat Test 1-28-2012 School Name Team Number Circle the all of the correct answer to the below questions. One or more of the answers can be correct, if more than on one answer is correct, circle
Keep the Heat Test 1-28-2012 School Name Team Number Circle the all of the correct answer to the below questions. One or more of the answers can be correct, if more than on one answer is correct, circle
Unit #5- Chapter #6. Types of chemical reactions. Energy: its forms 10/15/2013. Thermodynamics
 Unit #5- Chapter #6 Thermodynamics Types of chemical reactions PRODUCT-FAVORED: when the reaction converts reactants to products completely-it may take a small amount of activation energy but releases
Unit #5- Chapter #6 Thermodynamics Types of chemical reactions PRODUCT-FAVORED: when the reaction converts reactants to products completely-it may take a small amount of activation energy but releases
Module 5: Rise and Fall of the Clockwork Universe. You should be able to demonstrate and show your understanding of:
 OCR B Physics H557 Module 5: Rise and Fall of the Clockwork Universe You should be able to demonstrate and show your understanding of: 5.2: Matter Particle model: A gas consists of many very small, rapidly
OCR B Physics H557 Module 5: Rise and Fall of the Clockwork Universe You should be able to demonstrate and show your understanding of: 5.2: Matter Particle model: A gas consists of many very small, rapidly
Unit 3: States of Matter, Heat and Gas Laws
 Unit 3 - Stevens 1 Unit 3: States of Matter, Heat and Gas Laws Vocabulary: Solid Term Definition Example Liquid Gas No definite shape, but definite volume; Particles close together, but can move around
Unit 3 - Stevens 1 Unit 3: States of Matter, Heat and Gas Laws Vocabulary: Solid Term Definition Example Liquid Gas No definite shape, but definite volume; Particles close together, but can move around
Gravitational Potential Energy
 Name: Directions: Read and answer the following questions. You can then go on to my web page and check your answers. At the conclusion, go to schoology.com and complete the PE assignment. Gravitational
Name: Directions: Read and answer the following questions. You can then go on to my web page and check your answers. At the conclusion, go to schoology.com and complete the PE assignment. Gravitational
PROGRAM OF PHYSICS. Lecturer: Dr. DO Xuan Hoi Room A
 PROGRAM OF PHYSICS Lecturer: Dr. DO Xuan Hoi Room A1. 503 E-mail : dxhoi@hcmiu.edu.vn PHYSICS 2 (FLUID MECHANICS AND THERMAL PHYSICS) 02 credits (30 periods) Chapter 1 Fluid Mechanics Chapter 2 Heat, Temperature
PROGRAM OF PHYSICS Lecturer: Dr. DO Xuan Hoi Room A1. 503 E-mail : dxhoi@hcmiu.edu.vn PHYSICS 2 (FLUID MECHANICS AND THERMAL PHYSICS) 02 credits (30 periods) Chapter 1 Fluid Mechanics Chapter 2 Heat, Temperature
Chapter 12. The Laws of Thermodynamics
 Chapter 12 The Laws of Thermodynamics First Law of Thermodynamics The First Law of Thermodynamics tells us that the internal energy of a system can be increased by Adding energy to the system Doing work
Chapter 12 The Laws of Thermodynamics First Law of Thermodynamics The First Law of Thermodynamics tells us that the internal energy of a system can be increased by Adding energy to the system Doing work
Equilibrium. the linear momentum,, of the center of mass is constant
 Equilibrium is the state of an object where: Equilibrium the linear momentum,, of the center of mass is constant Feb. 19, 2018 the angular momentum,, about the its center of mass, or any other point, is
Equilibrium is the state of an object where: Equilibrium the linear momentum,, of the center of mass is constant Feb. 19, 2018 the angular momentum,, about the its center of mass, or any other point, is
 Academic Year 2016-2017 First Term Science Revision sheets PHYSICS ( Answer key ) Name: Grade: 10 Date: Section: (A) Science Practice : Q1: Choose the letter of the choice that best answer the questions:
Academic Year 2016-2017 First Term Science Revision sheets PHYSICS ( Answer key ) Name: Grade: 10 Date: Section: (A) Science Practice : Q1: Choose the letter of the choice that best answer the questions:
Energy, Heat and Temperature. Introduction
 Energy, Heat and Temperature Introduction 3 basic types of energy: Potential (possibility of doing work because of composition or position) Kinetic (moving objects doing work) Radiant (energy transferred
Energy, Heat and Temperature Introduction 3 basic types of energy: Potential (possibility of doing work because of composition or position) Kinetic (moving objects doing work) Radiant (energy transferred
MECHANICAL PROPERTIES OF MATERIALS
 1 MECHANICAL PROPERTIES OF MATERIALS Pressure in Solids: Pressure in Liquids: Pressure = force area (P = F A ) 1 Pressure = height density gravity (P = hρg) 2 Deriving Pressure in a Liquid Recall that:
1 MECHANICAL PROPERTIES OF MATERIALS Pressure in Solids: Pressure in Liquids: Pressure = force area (P = F A ) 1 Pressure = height density gravity (P = hρg) 2 Deriving Pressure in a Liquid Recall that:
Simple Harmonic Motion Investigating a Mass Oscillating on a Spring
 17 Investigating a Mass Oscillating on a Spring A spring that is hanging vertically from a support with no mass at the end of the spring has a length L (called its rest length). When a mass is added to
17 Investigating a Mass Oscillating on a Spring A spring that is hanging vertically from a support with no mass at the end of the spring has a length L (called its rest length). When a mass is added to
Chapter 4 - Second Law of Thermodynamics
 Chapter 4 - The motive power of heat is independent of the agents employed to realize it. -Nicolas Léonard Sadi Carnot David J. Starling Penn State Hazleton Fall 2013 An irreversible process is a process
Chapter 4 - The motive power of heat is independent of the agents employed to realize it. -Nicolas Léonard Sadi Carnot David J. Starling Penn State Hazleton Fall 2013 An irreversible process is a process
Per 7 Solutions: Entropy and the Laws of Thermodynamics
 // Per 7 Solutions: Entropy and the Laws of Thermodynamics 7. Entropy ) Entropy Your instructor will discuss entropy. a) What is entropy? Entropy is a measure of the disorder of a system. disorder, the
// Per 7 Solutions: Entropy and the Laws of Thermodynamics 7. Entropy ) Entropy Your instructor will discuss entropy. a) What is entropy? Entropy is a measure of the disorder of a system. disorder, the
