METHODOLOGIES FOR IDENTIFYING SUBSETS OF THE POPULATION WHERE TWO TREATMENTS DIFFER. Gregory A. Yothers
|
|
|
- Frederica Wright
- 6 years ago
- Views:
Transcription
1 METHODOLOGIES FOR IDENTIFYING SUBSETS OF THE POPULATION WHERE TWO TREATMENTS DIFFER by Gregory A. Yothers B.S. Mathematics, The Pennsylvania State University, 1985 M.A. Statistics, The University of Pittsburgh, 1998 Submitted to the Graduate Faculty of Arts and Sciences in partial fulfillment of the requirements for the degree of Doctor of Philosophy University of Pittsburgh 2003
2 UNIVERSITY OF PITTSBURGH FACULTY OF ARTS AND SCIENCES This dissertation was presented by Gregory A. Yothers It was defended on October 27, 2003 and approved by Leon J. Gleser John L. Bryant H. Samuel Wieand Allan R. Sampson Dissertation Director ii
3 Copyright by Gregory Allen Yothers 2003 iii
4 METHODOLOGIES FOR IDENTIFYING SUBSETS OF THE POPULATION WHERE TWO TREATMENTS DIFFER Gregory A. Yothers, PhD University of Pittsburgh, 2003 Three methodologies for the problem of identifying subsets of the population where two treatments differ are proposed here. Our first methodology is applicable when the response is possibly right censored time-toevent data and we wish to compare two treatments using the stratified log-rank test on the overall sample as well as separately within the levels of a categorical co-factor used to stratify the overall test. We present a method of calculating the exact familywise type I error rate for this group of tests given the type I error for each of the component tests. We present a sensitivity analysis of power for the design of a clinical trial based on varying assumptions about the interaction between treatment and the co-factor and various allocations of the type I error to the hypothesis tests. In our second methodology, we consider comparing two treatments using a given hypothesis test on the full sample and on all possible subsets, and we separately consider restricting the subsets considered to be those defined based on half-intervals of a covariate. Rather than treating this as a family of hypothesis tests, we instead choose the minimum p-value from the group of hypothesis tests as our test statistic. Simulation is employed to find an approximate critical value for our novel test statistic. We present a method for simulating a critical p-value which allows for hypothesis testing on all subsets of the sample while controlling type I error. Our third methodology applies when we wish to compare the mean response between two treatments, but the mean response varies depending on the value of a continuous covariate in some nonlinear fashion. Our solution is to fit separate segmented linear models to each treatment to approximate the nonlinear relationship. We develop asymptotic simultaneous confidence bands for the difference of the expected value functions conditional on the covariate from two treatment groups. The technique applies to the case where the changepoints of the segmented linear models have to be estimated or the case of known changepoints. The method is used to find intervals of the covariate where the treatments differ significantly. iv
5 PREFACE This work would not have been possible with out the unending love and patience of my immediate family. My wife Robbin has had to bear more than her share of providing financially for our family and handling of routine household matters and she has not received the personal attention from me that she deserves over these last few years. My children Mitchell and Emily have not had as much of my time and attention as they deserve. I want to thank you all for your patience and perseverance; I will try to make it up to you. I want to thank my extended family; my grandmother Eva, Mom and Dad Yothers, Mom and Dad Houston, Glenn and Lisa and family, Aaron and Jen, Terri and Paul and family, and Jessica for your support over these last years and for humoring me when I was absent from family functions. I gratefully acknowledge my current employer The National Surgical Adjuvant Breast and Bowel Project (NSABP) as well as my former employer Respironics Incorporated for providing me the flexibility in my employment to pursue an advanced degree, paying the bulk of my tuition, and providing much of the computing resources necessary to accomplish this work. My committee members Doctors John Bryant, Sam Wieand, and Leon Gleser have been invaluable to me through the process of completing my education and research. I thank them for their thoughtful mentoring and guidance, their outstanding instruction, and their insightful comments for improving this research. Last but not least, I want to thank my advisor Doctor Allan Sampson for his tireless efforts in prodding me along to finally complete this research, his inspiring instruction, his editing of my work and coaching of my writing skills, and the wealth of knowledge he brought to bear in helping me tackle these topics. I will always be grateful. v
6 TABLE OF CONTENTS 1 Introduction General problem statement Standard solutions Controlling the Familywise Error for Multiple Log-Rank Tests Inference Guided Data Exploration Asymptotic Simultaneous Confidence Band for the Difference of Segmented Linear Models Comparison between Simultaneous Confidence Band for the Difference of Segmented Linear Models and Inference Guided Data Exploration by ANCOVA on Half Intervals of a Covariate Conclusion Controlling the Familywise Error Rate for Multiple Log-Rank Tests Problem Statement Background Approach to the problem Allocation of Alpha Relationship between subgroup-level test statistics and overall statistic for 2 subgroups Definition of familywise power and FWE Extension to many subgroups Recursive definition of power function Controlling the FWE Motivating Example: NSABP Protocol B Choosing Type I Error Rates or How Best to Allocate Alpha Relationship between FWE, overall α, and subgroup-level α Loss of power with no interaction between treatment and subgroup vi
7 2.6.3 Power with interaction between treatment and subgroup Power with varying sample sizes between subgroups Power with no overall effect but varying interaction between treatment and subgroup Power when a prior probability distribution is placed on the difference in effect size between subgroups Common Approaches to Controlling FWE Conclusion Inference Guided Data Exploration Problem Statement Background Approach to the problem Inference Guided Data Exploration Restricted by Minimum Subset Sample Size Two sided Z-test assuming known variance Two sided t-test Two sided t-test, upper bound Inference Guided Data Exploration Restricted by Half-Intervals of a Covariate Two sided t-test Two sided Wilcoxon rank sum test ANCOVA t-test Application Discussion Asymptotic Simultaneous Confidence Band for the Difference of Segmented Linear Models Introduction and Background Segmented Linear Model with Unknown Change Points for Two Treatments Construction of Simultaneous Confidence Band Asymptotic Normality Requirement The Estimated Covariance Matrix of the Parameter Estimates vii
8 4.3.3 Partial Derivatives Equation for Simultaneous Confidence Band Segmented Linear Models with Known Changepoints Estimation and Practical Approach Application Simulation of Coverage Probabilities Discussion Comparison to Naïve segmented Working-Hotelling band: Unknown change points Comparison to Naïve segmented Working-Hotelling band: Known change points Difficulties Presented by Multiple Intervals of Significant Difference Comparison between Simultaneous Confidence Band for the Difference of Segmented Linear Models and Inference Guided Data Exploration by ANCOVA on Half-Intervals of a Covariate Introduction Brief explanation of Simultaneous Confidence Band for the Difference of Segmented Linear Models Brief explanation of Inference Guided Data Exploration by ANCOVA on Half-Intervals of a Covariate Stopping Criteria 1, Half-Intervals Minimum p-value Stopping Criteria 2, Half-Intervals First Significant p-value Discussion of similarities and differences Simple models to illuminate the relative strengths of the methods Measures of Performance Estimation of the True Threshold Coverage by the True Region were the Groups Differ Simulations Simulation Model 1: Segmented lines with different changepoints, otherwise parallel Simulation Model 2: Treatment segmented, control single line Simulation Model 3: Step function for treatment, control single line viii
9 5.3.4 Minimum sample size and critical p-value used for half-intervals method Simulation results Discussion Coverage by the True Region where the Groups Differ Estimation of the True Threshold Relationship between bias, coverage, and MSE Areas of application for the methods (Exploratory vs. Regulatory) Conclusion Controlling the Familywise Error for Multiple Log-Rank Tests Inference Guided Data Exploration Asymptotic Simultaneous Confidence Band for the Difference of Segmented Linear Models Comparison between Simultaneous Confidence Band for the Difference of Segmented Linear Models and Inference Guided Data Exploration by ANCOVA on Half-Intervals of a Covariate Areas for future research Acknowledgements Appendix A Recursive Definition of the Power Function for Multiple Log-rank Tests Appendix B Strong Control of FWE for Multiple Log-rank Tests Appendix C Equivalence of Protection Schemes for Multiple Log-rank Tests Appendix D Asymptotic Normality of our Parameterization of the Segmented Linear Model Appendix E Derivation of the Plug-in Estimator of the Information Matrix Bibliography ix
10 LIST OF TABLES Table 1: Possible alpha allocation for 2 subgroups and FWE = Table 2: Range of FWE for protected and unprotected schemes Table 3: Numeric example of subsets wtih Z statistic Table 4: Minimum subset size, two sample Z-test, alpha = 0.05, N = 10,000 iterations per simulation Table 5: Minimum subset size, two-sample t-test, alpha = 0.05, N = 10,000 iterations per simulation Table 6: Minimum subset size, two-sample t-test, upper bound, alpha = 0.05, N = 10,000 iterations Table 7: Selected Z-scores from Table 4, Table 5, and Table Table 8: Half-intervals of covariate, two-sample t-test, alpha = 0.05, N = 10,000 iterations Table 9: Half-intervals of covariate, Wilcoxon test, alpha = 0.05, N = 10,000 iterations Table 10: Half- intervals of a covariate, ANCOVA t-test, alpha = 0.05, N = 10,000 iterations Table 11: P values of t-test comparing all patient no older than tabulated age Table 12: Empirical Non-Coverage Probabilities with Covariates Uniform on (0, 10). 80 Table 13: Empirical Non-Coverage Probabilities with Covariates Fixed and Equally Spaced Table 14: Data for method of half-intervals example Table 15: Performance of the methods under various simulation models and standard deviations x
11 LIST OF FIGURES Figure 1: NSABP Protocol B-29 Schema Figure 2: Possible alpha allocation for 2 subgroups, FWE = 0.05, and α 1 = α Figure 3: Familywise power and power of overall stratified test, RR 1 = RR 2 = Figure 4: Familywise power and power of overall stratified test, RR 1 = 0.59 and RR 2 = Figure 5: Familywise power for various pairs of reduction in event rates Figure 6: Familywise power for various pairs of reduction in event rates, unequal allocation to subgroups (1:3) Figure 7: Familywise Power for various pairs of reduction in event rates, no overall treatment effect Figure 8: Expected familywise power for various allocations of events to the control arms of the 2 subgroups Figure 9: FWE for protected and unprotected schemes Figure 10: 2-segment linear model fit to AC group Figure 11: 2-segment linear model fit to AC Tax group Figure 12: Difference in mean response of the 2 models with 95% simultaneous confidence band Figure 13: 2-Segment Linear Fit to Treatment Group 1 Data Figure 14: 2-Segment Linear Fit to Treatment Group 2 Data Figure 15: Simultaneous Confidence Interval for the Difference (Group 2 minus Group 1) Figure 16: Comparison between Naïve Working Hotelling and Our Simultaneous Confidence Bands Figure 17: Comparison between Naïve Working Hotelling and Our Simultaneous Confidence Bands Figure 18: Method of half-intervals, critical p-value = , minimum sample size = 2, panels Figure 19: E(Y X) under simulation models 1, 2, and Figure 20: Example for SCB, identified region is covered by true region xi
12 Figure 21: Mean response vs. covariate under simulation model Figure 22: Mean response vs. covariate under simulation model Figure 23: Mean response vs. covariate under simulation model Figure 24: Coverage by the true interval under simulation model Figure 25: MSE of threshold estimator under simulation model Figure 26: Coverage by the true interval under simulation model Figure 27: MSE of threshold estimator under simulation model Figure 28: Coverage by the true interval under simulation model Figure 29: MSE of threshold estimator under simulation model xii
13 1 Introduction 1.1 General problem statement Usual techniques for comparing two treatments assume that the differential response in patients receiving treatment versus control, which we refer to as the treatment effect, is constant over the population under study. In practice, this assumption is at best only approximately correct, and often there are important fluctuations in the effect of treatment due to known factors or latent factors unknown to the researchers. If we compare two treatments under the assumption that the treatment effect is constant over the population under study when in fact the effect of treatment varies due to factors, known or unknown, we are in effect testing whether the average treatment effect is different from zero. When such a comparison leads to a significant result, the results are generalized to the entire population and, as a result, if there are subsets of the population where the effect of treatment is negligible or detrimental we may be treating some patients unnecessarily or actually causing harm. Conversely, when such a comparison leads to a conclusion of accepting the null hypothesis of no treatment effect, we may deprive certain subsets of the population from access to a beneficial treatment. The primary aim of this research is to leverage known factors which may be related to the size of the treatment effect in hopes of identifying subsets of the population where treatment is or is not beneficial. A portion of this research deals with examining all subsets of the population. A finding of significant difference between the treatments when all subsets are considered may imply there are factors unknown to the researcher which are related to the size of the treatment effect. 1.2 Standard solutions It is common practice to attempt to model factors which are related (or possibly related) to the size of the response in order to reduce the variance of the parameter estimates related to the treatment comparison and provide for more powerful inference. Typically, some attempt is made to test for interaction between treatment and the other factors in the model. These interaction tests can identify situations where the treatment effect is not 1
14 constant over the population under study. However, studies are rarely planned to have adequate power for such tests (Pocock et al. (2002)). When a factor is identified which interacts with treatment, the approach to analysis must be modified to address this. For categorical factors, this generally means that separate treatment comparisons are performed within each level of the factor and the issue of multiple comparisons arises. For continuous factors, the issues can be more complex and only a few standard approaches have been proposed. For example, the parallel slopes analysis of covariance model is often employed when the response is linear with respect to a covariate. Standard practice is to test the parallel slopes assumption prior to proceeding with the analysis. Violation of the parallel slopes assumption implies a treatment by covariate interaction. A solution for the case where the slopes are not parallel was proposed by Potthoff (1964) who constructs a simultaneous confidence band for the difference in mean response between the treatments with respect to the covariate. The treatments are found to be significantly different on regions of the covariate where zero difference is not contained in the simultaneous confidence band. 1.3 Controlling the Familywise Error for Multiple Log-Rank Tests Our first methodology is described in Chapter 2 and concerns comparing two treatments when the response is possibly censored time-to-event data. More specifically, the problem is the design of an analysis plan for such data when there is some concern that the effect of treatment will be different on several levels of a cofactor. Our solution is to perform stratified log-rank tests within each level of the cofactor along with an overall log-rank test stratified over the levels of the cofactor as well as the other stratification factors. All of the tests are performed at reduced levels of type I error so that the type I error for the family of hypothesis tests is controlled to be level. Generalized methods of dealing with the multiple comparison problem such as the Bonferroni procedure could be applied to this problem, but the conservative nature of such an approach is unappealing. Other more specialized multiple comparison procedures such as Tukey s HSD, Fisher s LSD, Dunnet s MCP, Holm s, or Sidak s are applicable to the setting of ANOVA where one is interested in comparing multiple 2
15 treatments (Miller (1981) and Hsu (1996)). Since our aim is to test the same hypothesis for the overall sample as well as within each stratum, these methods do not apply. Our major contribution is a method of calculating the exact familywise error rate (FWE) for this family of hypothesis tests given the type I error of each component test. Our method can be used to find a combination of type I error rates for the individual hypothesis tests which controls the familywise error rate at level. We present a sensitivity analysis based on the design of National Surgical Adjuvant Breast and Bowel Project (NSABP) Protocol B-29 (NSABP (2000)) to show how various allocations of the type I error to the constituent tests of hypothesis affect power under various alternatives. We find this approach to be efficient and effective when the sign of the treatment effect is the same for each subgroup and there may or may not be small to moderate differences in the magnitude of the effect between subgroups. We show that ad hoc methods for dealing with the inflation of the type I error such as using a preliminary interaction test to decide whether to perform the overall test or the individual subgroup tests are largely ineffective. While such protection schemes do a better job of controlling the type I error than the unprotected approach of performing all tests at level, the approach of using a preliminary interaction test still results in roughly a doubling of the FWE. 1.4 Inference Guided Data Exploration In Chapter 3, we consider comparing two treatments using a given hypothesis test on the full sample and on every subset where each treatment has at least n min observations. Rather than treating this as a family of hypothesis tests, we instead choose the minimum p-value from the set of hypothesis tests as our test statistic. Simulation is employed to find an approximate critical value for our novel test statistic. Much as the Scheffé procedure provides a critical value for the test statistic which allows for comparing all contrasts in the analysis of variance, we derive a critical p-value which, while controlling the type I error, allows for hypothesis testing on every subset of the original sample where each treatment has at least n min observations. This technique may prove useful in providing a rule of thumb for judging significant differences when an investigator has 3
16 gone fishing in his data, i.e., performed a large number of statistical tests of hypothesis on subsets of the sample. There are problems similar to the all subsets problem in the field of spatial statistics. Worsley (1992) addressed the problem of finding regions of the brain which were active while the subjects performed some task. The volume of the brain is divided into hyperrectangles called voxels. Brain activation was measured via Positron Emission Tomography (PET) for a number of subjects and a t-statistic is computed for the change in activation at each voxel. A typical experiment may include 10 5 voxels, so the problem of determining which of the voxels experienced a significant change is non-trivial. He uses the concept of a Gaussian field to develop critical values for the t-statistics such that, under the null hypothesis of no change in activation, the probability that the t-statistic from at least one of the voxels will exceed the critical value is alpha. The difficulties with implementing an approach such as this on the current problem would involve finding an analogue for the distance between non overlapping subsets and dealing with the problem of overlapping subsets. In our work, we consider two common tests of hypothesis, the two sample Z-test and two sample t-test, and we provide tables of the critical p-value for various sample sizes for the treatment and control groups and various values of the minimum sample size in each treatment group. The exact simulation solution is found to be computationally intractable for the two sample t-test for moderate to large sample sizes. This is not the case for the Z-test. A special property of the Z-test is exploited to find the critical p-value for this test even for large sample sizes. More specifically, if we consider all pairs of sub-samples of size n i from treatment and n j from control, then the most extreme Z-score for this combination of sub-sample sizes comes from the test comparing the largest n i order statistics from treatment with the smallest n j order statistics from control or from the test comparing the smallest n i order statistics from treatment with the largest n j order statistics from control. We refer to these two combinations as tests comparing maximal order statistics to minimal order statistics. The approach of only considering subsets where maximal order statistics are compared to minimal order statistics can also be applied to the setting of the t-test, and, while it provides an estimate of the critical p-value for the 4
17 t-test which is biased upwards, we show that this approximation is superior to using the critical p-value from the Z-test as the approximation. We also consider a restriction on the subsets to be considered where the subsets are defined based on half-intervals of a covariate. For example, subsets are formed by including all observations except the k largest/smallest order statistics of the covariate from the full sample with the treatments pooled so that, for example, k = 0 corresponds to the full sample comparison. Treatment comparisons are made for the subsets corresponding to k = 0, 1, 2, until one of the treatments has less than n min observations in the subset. This restriction on the subsets considered leads to much less extreme critical p-values as opposed to considering all subsets. In a related problem, Koziol and Wu (1996) consider the problem of determining a threshold value for baseline hemoglobin below which patients are less likely to require blood transfusion when treated with r-huepo, a recombinant form of human erythropoietin. Their method is applicable to the case where the response is binary and the probability of success is monotonically related to the value of the covariate. For the approach based on half-intervals of a covariate, we provide tables of the critical p-value for three common tests of hypothesis, the two sample t-test, the normal approximation to the Wilcoxon rank sum test, and the analysis of covariance model (ANCOVA). We find that the critical p-value depends on the joint distribution of the response and the covariate for the t and Wilcoxon tests, but for the ANCOVA model, where the dependence between the response and covariate is properly modeled, the critical p-value does not depend on the joint distribution. In order to illustrate the method based on half-intervals of a covariate, we use tumor response data from NSABP Protocol B-27 (Mamounas (1997)). We identify a halfinterval of the covariate (age) where the reduction in tumor size is greater in women receiving Taxotere than in women of the same age not receiving Taxotere. 5
18 1.5 Asymptotic Simultaneous Confidence Band for the Difference of Segmented Linear Models Our final methodology is presented in Chapter 4. Here we are concerned with the comparison of two treatments when the expected value of the response for each treatment differentially depends on the value of a covariate. In particular, we are interested in situations where a linear model is inadequate to describe this relationship. We propose the use of segmented linear models as a flexible approach to describing such functional relationships. The approach that we take is related to that of Potthoff (1964), in that we construct an asymptotic simultaneous confidence band about the difference in mean response of two segmented linear models. In order to accomplish this, we extend results of Cox and Ma (1995) who give a general approach for constructing an asymptotic simultaneous confidence band for a single nonlinear regression function. Simulations are performed to ascertain the true coverage of our simultaneous confidence bands for the difference of two segmented linear regressions. When the data are sufficient to identify the underlying model, we find that the coverage of our asymptotic simultaneous confidence band is slightly conservative for large sample sizes, i.e., the proportion of simulations where the simultaneous confidence band does not cover the true difference in mean response is less than. When the covariates are drawn at random, so that there is positive probability of observing no data on one of the segments of the segmented linear model, the method still performs well for moderate to large sample sizes, however the small sample coverage is poor. The method is applied to breast cancer tumor size data from NSABP Protocol B-27 (Mamounas 1997) where we find an interval of the covariate (age) where the treatments are significantly different. 1.6 Comparison between Simultaneous Confidence Band for the Difference of Segmented Linear Models and Inference Guided Data Exploration by ANCOVA on Half Intervals of a Covariate Chapter 5 compares the methods developed in Chapter 4 and Chapter 3. Each of these methods describes a region of the covariate where the treatments differ when a significant difference is found. We consider several simulation models where the treatments have the same mean response for covariate values up to a threshold value and different mean 6
19 responses for covariate values greater than the threshold. The methods are compared based on two performance measures, the first being the probability that the identified region (with respect to the covariate) where the method claims the treatments differ is covered by the true region where the treatments differ. The second performance measure is the mean squared error (MSE) of the threshold estimate relative to the true threshold, where we define the threshold estimate to be the smallest covariate value in the identified region of significant difference. In light of the results from these performance measures we discuss how one might choose between the methods based on the aims of the application. Our simultaneous confidence band method performed best for coverage of the identified region of significant difference by the true region where the treatments differ. As a result, we recommend this method when the aim of the research is regulatory. The method of inference guided data exploration by ANCOVA on half-intervals performed best for the MSE of the estimate of the threshold value of the covariate defining the region where the treatments differ. Thus, we recommend this method when the aim of the research is exploratory. 1.7 Conclusion In Chapter 6 we conclude with a brief review of Chapters 2 through 5, reflections on our major contributions and conclusions, and directions for future research. 7
20 2 Controlling the Familywise Error Rate for Multiple Log-Rank Tests 2.1 Problem Statement Our concern lies with the problem of designing a clinical trial for possibly right censored time-to-event data where we wish to compare two treatments with an overall stratified test along with individual treatment comparisons within subgroups of the data where the grouping factor(s) is one or more of the stratification factors. We wish to perform such a family of hypothesis tests while controlling the familywise type I error. This approach is appealing when the effect of treatment may not be the same in each of the subgroups (i.e., treatment by subgroup interaction). Multiple tests of hypotheses will inflate the type I error for a given clinical trial unless steps are taken to control for multiplicity. Common methods of controlling for multiple comparisons lead to a loss of power for the individual hypotheses so that subgroup analyses are often avoided. However, subgroup analyses often serve a legitimate scientific purpose when performed in a responsible manner. The stratified log-rank test is often used as the primary analysis for time-to-event data. Log-rank or stratified log-rank tests can be used to determine the significance of the treatment effect separately within each subgroup. General methods for dealing with multiple comparisons, such as the Bonferroni procedure, are conservative and can lead to greatly reduced power. Rather than using such conservative tests, we propose for our goal the calculation of the exact familywise error (FWE) for the overall stratified logrank test along with separate log-rank or stratified log-rank tests within each subgroup. This will allow for greater flexibility in the design of clinical trials and reduce the loss of power for the individual hypotheses. 2.2 Background Generalized methods of dealing with the multiple comparison problem such as the Bonferroni procedure could be applied to this problem. The Bonferroni procedure is known to be conservative due to the nature of the underlying probability inequality. Our method exploits the fact that the overall stratified log rank statistic is a weighted sum of 8
21 the subgroup level statistics and the dependence among the relevant test statistics can be used to obtain an exact procedure which will be more powerful for each of the component hypotheses than would the Bonferroni procedure. Klein and Moeschberger (1997) give formulas for the stratified and ordinary log-rank test statistics. Both of the statistics have an asymptotic normal distribution. Other more specialized multiple comparison procedures such as Tukey s HSD, Fisher s LSD, Dunnet s MCP, Holm s, or Sidak s are applicable to the setting of ANOVA where one is interested in comparing multiple treatments (Miller (1981) and Hsu (1996)). Since our aim is to test the same hypothesis for the overall sample as well as within each subgroup, these methods do not apply. 2.3 Approach to the problem Our concern is the design and analysis of clinical trials to compare two treatments where, in addition to the overall test, we desire to test the primary hypothesis on several subgroups which partition the sample. We propose a method whereby a pre-specified familywise alpha is allocated among the overall test and the constituent subgroup tests. We find the method to be efficient in terms of familywise power when the treatment effect in each subgroup is in the same direction and the magnitude of the range of treatment effects between subgroups is not too great. The procedure can be used to make the design of a clinical trial robust against the presence of a treatment by subgroup interaction when a significant interaction is not anticipated. In Section 2.4, we derive expressions for the exact FWE and familywise power and adapt them to numerical solution. In Section 2.5, we present NSABP Protocol B-29 (NSABP (2000)) as a motivating example. Section 2.6 is a sensitivity analysis, loosely based on NSABP Protocol B-29, which illustrates how variation in design assumptions affects familywise power. We consider common approaches to dealing with a potential treatment by subgroup interaction in Section 2.7 and illustrate the deficiencies of such approaches. We close the chapter in Section 2.8 with a discussion of our conclusions. 9
22 2.4 Allocation of Alpha In this section, we propose a multiple testing approach where one performs an overall test for treatment effect along with tests within each subgroup. All tests are carried out at reduced levels of significance so that the familywise level of significance is maintained at a specified rate. All tests are based on the stratified log-rank statistic, although the ordinary log-rank statistic may be used for a subgroup test when the subgroup consists of a single stratum Relationship between subgroup-level test statistics and overall statistic for 2 subgroups In this subsection, we develop notation and describe the relationship between the subgroup level and overall log-rank test statistics for the case of k = 2 subgroups. For example, if we have one grouping factor with 2 levels and an additional stratification factor, then the two subgroup level tests will use log-rank statistics stratified for the additional stratification factor and the overall test is stratified both for the grouping factor as well as the additional stratification factor. Let L 1 and L 2 be the stratified log-rank statistics from the 1 st and 2 nd subgroup tests comparing treatment to control, respectively, and let V 1 and V 2 be the respective variances. When a subgroup consists of a single stratum, the ordinary log-rank statistic is used. Formulas for the stratified and ordinary log-rank test statistic and its variance are given in Klein and Moeschberger (1997, pp , 206). Let L 0 = L 1 + L 2 represent the overall stratified log-rank statistic comparing treatment to control aggregated over the subgroups and V 0 denote its variance. In the usual case, where failure and censoring times are independent between patients and censoring is non-informative, L 1 and L 2 are independent and V 0 = V 1 + V 2. Now, the standardized log-rank statistics are represented by Z = L V, i = 0,1,2. Since L 0 = L 1 + L 2 and V 0 = V 1 + V 2, it follows that: ( ) i i i Z = Z a + Z 1 a, where a = V V + V, 0 < a < 1. (2.4.1) The ratio a is approximately the proportion of events which occur in the first subgroup. 10
23 The standardized log rank statistics have an asymptotic standard normal distribution under the null hypothesis that the hazard rates, as a function of time, are equal up to the longest time on study (Klein and Moeschberger (1997)). Let α 0, α 1, and α 2 represent the nominal levels of significance of the two-sided tests based on Z 0, Z 1, and Z 2 and let c 0, c 1, and c 2 be the corresponding critical values (i.e., c = Φ (1 α / 2) where is the CDF of the standard normal distribution) When the assumption of proportional hazards holds, it is convenient to describe the null and alternative hypotheses in terms of the Relative Risk (RR) or hazard ratio. Often the term reduction in event rate is used, this quantity is equal to one minus the RR. Let RR i, i {0, 1, 2}, represent the relative risk in the overall sample, 1 st subgroup, and 2 nd subgroup respectively, so that RR i = 1, i {0, 1, 2}, denotes the null hypothesis and RR 1 = RR 1 Alt and RR 2 = RR 2 Alt is a specific alternative hypothesis. The overall relative risk is a function of the relative risks in the subgroups, so that, in specifying a specific alternative, it suffices to specify the alternative for the subgroups only. When the alternative hypothesis is true, the standardized test statistic Zi = Li Vi is asymptotically distributed i i Alt as N[ln( RR ) V, 1] (Schoenfeld (1981)). The expected value of the standardized logrank statistic when the proportional hazards assumption does not hold is given by Lakatos (1988). Let i be the expected value of Z i under the alternative hypothesis. Then W i = Z i i is distributed as standard normal under the alternative hypothesis. When the assumption of Alt proportional hazards holds, θ = ln ( RR ) difference between treatments, i = 0. V i i i. Under the null hypothesis of no Definition of familywise power and FWE We define familywise power to be the probability of detecting at least one significant difference for a member of our family of hypotheses when a specific alternative hypothesis holds. When the null hypothesis is true ( i = 0 in each subgroup), we refer to the familywise power as the FWE. The power against a specific alternate hypothesis can 11
24 be written as: ( α α α θ θ ) = φ ( θ ) φ ( θ ) Power 0, 1, 2, a, 1, 2 1 z1 1 z2 2 dz1dz2, (2.4.2) where φ denotes the standard normal density, and the integral is taken over the acceptance region defined by ( ) { z1, z2 : z1 c1, z2 c2, z0 c0} < < <. The integration region can be re-expressed as: c z 1 a c z 1 a ;(2.4.3) a a ( z, z ) : c < z < c, max c, < z < min c, so that it follows: ( α α α a θ θ ) Power,,,,, = ( { } ) { ( ) } θ (2.4.4) ( ) ( ) c2 1 φ z2 θ2 Φ max c1,min c1, c0 z2 1 a a θ c 1 2 ( ) Φ max c1, min c1, c0 z2 1 a a 1 dz2, where Φ is the CDF of the standard normal distribution Extension to many subgroups These results generalize to k subgroups as follows: k k k Z = Z a, where a = V V, 0 < a < 1, a = 1 0. (2.4.5) i i i i j i j i= 1 j= 1 j= 1 The ratio a i is approximately equal to the proportion of events which occur in the i th subgroup. The familywise power converges to: ( α α α a a θ θ ) Power,,,,,,,,, 0 1 k 1 k 1 k = ck c2 k k 1 φ ( zi θi ) Φ max c1,min c1, c0 zi ai a1 θ1 (2.4.6) c i 2 i 2 k c = = 2 k Φ max c1,min c1, c 0 zi ai a1 θ1 dz2 dzk. i= 2 12
25 2.4.4 Recursive definition of power function The multiple integral in equation (2.4.6) can be difficult to evaluate numerically when the number of subgroups goes beyond about 3 or 4. Fortunately there is a recursive representation of the power function that facilitates computation when there are many subgroups. Given α0, α1,, αk, a1,, ak, θ1,, θk, define: ρ r r ( z) Pr Z a z ( Z c ). (2.4.7) r i i j j i= 1 j= 1 Then, and, ( ) ( ) ( ) max, min (, ) ρ1 z = Φ c1 c1 z a1 θ1 Φ c1 θ1, (2.4.8) cr+ 1 ( ) = ( ) ( ) ρ φ θ ρ (2.4.9) r+ 1 z u r 1 r z u ar 1 du. c + + r+ 1 For k subgroups, ( α α α a a θ θ ) = ρ ( c ) ρ ( c ) Power 0, 1,,, 1,,, 1, k k, k 1 k 0 k 0. (2.4.10) See Appendix A for derivation and proof. An S-Plus function implementing the recursive method of calculating power is available from the author Controlling the FWE Given a 1,, a k, where a i is the proportion of expected events in subgroup i, controlling the familywise error to be level is a problem of choosing 0,, k such that the power (2.4.10) is equal to when all null hypotheses are true ( 1 = = k = 0). The solutions form a subspace of dimension k, so that there is no unique solution. Trial and error can be used to find a solution reasonably quickly, and the process may be expedited by constraining the nominal type I errors for the within subgroup tests to all be equal. This approach provides strong control of the FWE in the sense that for any arrangement of 13
26 true and false null hypotheses, the FWE will be no more than. See Appendix B for a discussion of strong control. 2.5 Motivating Example: NSABP Protocol B-29 The primary aim of NSABP Protocol B-29 (NSABP (2000)) was to determine whether the addition of Octreotide to Tamoxifen alone or to Tamoxifen in combination with chemotherapy (AC) prolongs disease-free survival in women with axillary node-negative, estrogen-receptor (ER) positive primary invasive breast cancer. The protocol schema is shown in Figure 1. An unusual feature of this clinical trial is that the patient has to decide along with her physician whether or not to use chemotherapy. The trial was designed this way because, at the time this trial was initiated, the standard of care for these patients was in a state of flux. Previously, the standard of care among node negative ER positive breast cancer patients had been Tamoxifen alone. The release of the results of NSABP Protocol B-20 (Fisher et al. (1997)) at about the same time as the initiation of Protocol B-29 caused a shift in the standard of care to chemotherapy. The trial is designed to have 80% power to detect a 25% decrease in event rate (i.e., RR = 0.75) using a 0.05 level two-sided overall stratified log-rank test. The trial will accrue 3000 patients over 5 years, and the final analysis is scheduled to follow the 400 th event, which is estimated to require an additional 3 years of follow-up. Physicians involved in the design of the trial thought the effect of Octreotide would be unlikely to materially interact with chemotherapy status. However, in planning the trial it was felt to be important to provide for individual tests for the effect of Octreotide in the presence of chemotherapy as well as in its absence. It was considered unacceptable to treat these subgroup analyses as post-hoc, or exploratory, so it was necessary to design an analysis plan that controlled the familywise error rate. Among the many factors considered in making this decision, a primary consideration is that, if the trial shows positive results, the pharmaceutical company providing the test drug would likely use the trial as a basis for a new treatment indication from regulatory bodies. 14
27 NSABP B-29 Schema T1 or T2 or T3; pn0; M0 ER-Positive Decision to use Chemotherapy* No Chemotherapy Chemotherapy Stratification Age Pathologic Tumor Size Stratification Age Pathologic Tumor Size Tamoxifen Tamoxifen + Octreotide AC + Tamoxifen AC + Tamoxifen + Octreotide Group 1 Group 2 Group 3 Group 4 * The decision to use AC chemotherapy must be made prior to randomization. Figure 1: NSABP Protocol B-29 Schema The stratification factors are chemotherapy (yes, no), age (<50, 50+), and pathologic tumor size (0-2, 2.1-4, and 4.1+ cm). The subsets of interest are those defined by the grouping factor chemotherapy (yes, no) and the within subset tests for a treatment effect due to Octreotide are stratified for age and pathologic tumor size. 2.6 Choosing Type I Error Rates or How Best to Allocate Alpha Relationship between FWE, overall α, and subgroup-level α The question arises as to how the type I error should be divided between the overall and the subgroup-specific tests, or rather, how much alpha should be spent on the subgroupspecific tests. For k = 2 subgroups, Table 1 and Figure 2 show a variety of combinations of the nominal size of the overall test (α 0 ) and the nominal size of the within subgroup tests (α 1 & α 2 ). For simplicity, we only consider the case where α 1 = α 2. The possibilities form a continuum between (α 0, α 1 = α 2 ) = (0.05, 0) (no subgroup specific tests) to (0, ) (no overall test). Given α exper, α 0, and the constraint α 1 = α 2, the common value of α 1 & α 2 is a function of a (the proportion of events in the first subgroup), however, as may be seen in Figure 2, the effect of varying a is weak. 15
28 Table 1: Possible alpha allocation for 2 subgroups and FWE = 0.05 a = 0.50 a = 0.25 a = 0.10 α 0 α 1 = α 2 α 0 α 1 = α 2 α 0 α 1 = α Figure 2: Possible alpha allocation for 2 subgroups, FWE = 0.05, and α 1 = α Loss of power with no interaction between treatment and subgroup Here we consider the penalty in terms of power when some of our alpha is allocated to subgroup specific tests when the treatment effect is the same in all subgroups. Figure 3 shows the case where there is an overall 25% reduction in event rate, no interaction between treatment and subgroup (RR 1 = RR 2 = 0.75), and the expected number of events 16
29 is equal in each of the k = 2 subgroups (a = 0.5). The overall stratified log-rank test at the 0.05 level has power The alpha allocation of 0 = 0.04 and 1 = 2 = yields a power of 0.79 for the overall test and a familywise power of 0.80! Thus, spending 20% of total alpha (setting 0 = 0.04) on subgroup tests leads to a very small loss of power even in the case of no interaction. Setting 0 less than about 0.03, on the other hand, leads to a rather substantial loss in power. We should point out that the small loss in power for the case of no treatment by subgroup interaction is not he only sacrifice when electing to spend some of our alpha on subgroup tests. When all of our alpha is allocated to the overall test, then rejecting the null hypothesis results in a conclusion generalizable to the entire population represented by the full sample. When some of our alpha is spent on subgroup tests, the trial may have a successful outcome (one of the null hypotheses is rejected), but the result may only be generalizable to the population represented by a subset. Thus the scope of application of a successful result may be reduced. Figure 3: Familywise power and power of overall stratified test, RR 1 = RR 2 =
30 2.6.3 Power with interaction between treatment and subgroup Next we consider how power is affected in the presence of treatment-subgroup interaction when some of our alpha is allocated to subgroup specific tests. Figure 4 shows the case of a 41% reduction in event rate in one subgroup and a 9% reduction in the other subgroup. We again assume that there are 400 total events and that the expected number of events on the control arm of each subgroup is equal. When the subgroups are pooled, we have an overall 25% reduction in event rate. The overall stratified log-rank test at the α 0 = 0.05 level has power Reducing α 0 to 0.04 reduces the power of the overall test to 0.804, but increases the familywise power to 0.906! Spending any more than 20% of alpha on subgroup tests does not materially increase the familywise power even in the presence of this very substantial interaction. Figure 4: Familywise power and power of overall stratified test, RR 1 = 0.59 and RR 2 = 0.81 In the previous subsection, we pointed out that allocating some of our alpha to subset tests has the potential of limiting the scope of application for a successful outcome when there is no interaction between treatment and subgroup. The gain in familywise power illustrated by Figure 4 is due to the increased probability of detecting the large treatment 18
31 difference in the 1 st subgroup. Even if the scope of application is limited, it may be better to get something (a finding restricted to a subgroup) than nothing (a negative trial). Now we examine how varying the magnitude of interaction affects the familywise power. Figure 5 shows a variety of pairs of reduction in event rates in the two subgroups such that when the subgroups are pooled we have a 25% reduction in event rate. We again assume that there are 400 total events and that the expected number of events on the control arm of each subgroup is equal. The overall stratified log-rank test at the α 0 = 0.05 level has power in the range Reducing α 0 to 0.04 dramatically increases the familywise power in the presence of interaction. Spending any more than 20% of alpha on subgroup tests does not materially increase the familywise power even in the presence of substantial interaction. Note that for small interaction (the pair (35, 15)), the power is maximized near 0 = 0.04.!!!! Figure 5: Familywise power for various pairs of reduction in event rates 19
32 2.6.4 Power with varying sample sizes between subgroups Until now we have only considered what happens when the numbers of events (patients) in the two subgroups are roughly equal. We next consider the case where most of the patients are assigned to the subgroup with the smaller treatment effect. Figure 6 shows a variety of pairs of reduction in event rates for comparison with Figure 5. We again assume that there are 400 total events, but now the numbers of events on the control arms of the two subgroups are not equal. The expected number of events on the control arm of subgroup 2 is three times the expected number of events on the control arm of subgroup 1. When the subgroups are pooled, the reduction in event rate varies from 25% for the pair (25, 25) to 12.5% for the pair (50, 0). We see that the familywise power suffers when most of the events (patients) are in the subgroup with a small reduction in event rate. Note that a choice of size of overall test equal to 0.04 would give near maximum power given any of the states of nature explored in Figure 6.!!!! Figure 6: Familywise power for various pairs of reduction in event rates, unequal allocation to subgroups (1:3) 20
Chapter 1 Statistical Inference
 Chapter 1 Statistical Inference causal inference To infer causality, you need a randomized experiment (or a huge observational study and lots of outside information). inference to populations Generalizations
Chapter 1 Statistical Inference causal inference To infer causality, you need a randomized experiment (or a huge observational study and lots of outside information). inference to populations Generalizations
The Design of a Survival Study
 The Design of a Survival Study The design of survival studies are usually based on the logrank test, and sometimes assumes the exponential distribution. As in standard designs, the power depends on The
The Design of a Survival Study The design of survival studies are usually based on the logrank test, and sometimes assumes the exponential distribution. As in standard designs, the power depends on The
Group Sequential Tests for Delayed Responses. Christopher Jennison. Lisa Hampson. Workshop on Special Topics on Sequential Methodology
 Group Sequential Tests for Delayed Responses Christopher Jennison Department of Mathematical Sciences, University of Bath, UK http://people.bath.ac.uk/mascj Lisa Hampson Department of Mathematics and Statistics,
Group Sequential Tests for Delayed Responses Christopher Jennison Department of Mathematical Sciences, University of Bath, UK http://people.bath.ac.uk/mascj Lisa Hampson Department of Mathematics and Statistics,
CHL 5225H Advanced Statistical Methods for Clinical Trials: Multiplicity
 CHL 5225H Advanced Statistical Methods for Clinical Trials: Multiplicity Prof. Kevin E. Thorpe Dept. of Public Health Sciences University of Toronto Objectives 1. Be able to distinguish among the various
CHL 5225H Advanced Statistical Methods for Clinical Trials: Multiplicity Prof. Kevin E. Thorpe Dept. of Public Health Sciences University of Toronto Objectives 1. Be able to distinguish among the various
Pubh 8482: Sequential Analysis
 Pubh 8482: Sequential Analysis Joseph S. Koopmeiners Division of Biostatistics University of Minnesota Week 10 Class Summary Last time... We began our discussion of adaptive clinical trials Specifically,
Pubh 8482: Sequential Analysis Joseph S. Koopmeiners Division of Biostatistics University of Minnesota Week 10 Class Summary Last time... We began our discussion of adaptive clinical trials Specifically,
Improving Efficiency of Inferences in Randomized Clinical Trials Using Auxiliary Covariates
 Improving Efficiency of Inferences in Randomized Clinical Trials Using Auxiliary Covariates Anastasios (Butch) Tsiatis Department of Statistics North Carolina State University http://www.stat.ncsu.edu/
Improving Efficiency of Inferences in Randomized Clinical Trials Using Auxiliary Covariates Anastasios (Butch) Tsiatis Department of Statistics North Carolina State University http://www.stat.ncsu.edu/
Sample size re-estimation in clinical trials. Dealing with those unknowns. Chris Jennison. University of Kyoto, January 2018
 Sample Size Re-estimation in Clinical Trials: Dealing with those unknowns Christopher Jennison Department of Mathematical Sciences, University of Bath, UK http://people.bath.ac.uk/mascj University of Kyoto,
Sample Size Re-estimation in Clinical Trials: Dealing with those unknowns Christopher Jennison Department of Mathematical Sciences, University of Bath, UK http://people.bath.ac.uk/mascj University of Kyoto,
Sample Size Determination
 Sample Size Determination 018 The number of subjects in a clinical study should always be large enough to provide a reliable answer to the question(s addressed. The sample size is usually determined by
Sample Size Determination 018 The number of subjects in a clinical study should always be large enough to provide a reliable answer to the question(s addressed. The sample size is usually determined by
DETAILED CONTENTS PART I INTRODUCTION AND DESCRIPTIVE STATISTICS. 1. Introduction to Statistics
 DETAILED CONTENTS About the Author Preface to the Instructor To the Student How to Use SPSS With This Book PART I INTRODUCTION AND DESCRIPTIVE STATISTICS 1. Introduction to Statistics 1.1 Descriptive and
DETAILED CONTENTS About the Author Preface to the Instructor To the Student How to Use SPSS With This Book PART I INTRODUCTION AND DESCRIPTIVE STATISTICS 1. Introduction to Statistics 1.1 Descriptive and
Group Sequential Designs: Theory, Computation and Optimisation
 Group Sequential Designs: Theory, Computation and Optimisation Christopher Jennison Department of Mathematical Sciences, University of Bath, UK http://people.bath.ac.uk/mascj 8th International Conference
Group Sequential Designs: Theory, Computation and Optimisation Christopher Jennison Department of Mathematical Sciences, University of Bath, UK http://people.bath.ac.uk/mascj 8th International Conference
Comparing Group Means When Nonresponse Rates Differ
 UNF Digital Commons UNF Theses and Dissertations Student Scholarship 2015 Comparing Group Means When Nonresponse Rates Differ Gabriela M. Stegmann University of North Florida Suggested Citation Stegmann,
UNF Digital Commons UNF Theses and Dissertations Student Scholarship 2015 Comparing Group Means When Nonresponse Rates Differ Gabriela M. Stegmann University of North Florida Suggested Citation Stegmann,
DESIGNING EXPERIMENTS AND ANALYZING DATA A Model Comparison Perspective
 DESIGNING EXPERIMENTS AND ANALYZING DATA A Model Comparison Perspective Second Edition Scott E. Maxwell Uniuersity of Notre Dame Harold D. Delaney Uniuersity of New Mexico J,t{,.?; LAWRENCE ERLBAUM ASSOCIATES,
DESIGNING EXPERIMENTS AND ANALYZING DATA A Model Comparison Perspective Second Edition Scott E. Maxwell Uniuersity of Notre Dame Harold D. Delaney Uniuersity of New Mexico J,t{,.?; LAWRENCE ERLBAUM ASSOCIATES,
Chapter Seven: Multi-Sample Methods 1/52
 Chapter Seven: Multi-Sample Methods 1/52 7.1 Introduction 2/52 Introduction The independent samples t test and the independent samples Z test for a difference between proportions are designed to analyze
Chapter Seven: Multi-Sample Methods 1/52 7.1 Introduction 2/52 Introduction The independent samples t test and the independent samples Z test for a difference between proportions are designed to analyze
Multiple Comparison Procedures Cohen Chapter 13. For EDUC/PSY 6600
 Multiple Comparison Procedures Cohen Chapter 13 For EDUC/PSY 6600 1 We have to go to the deductions and the inferences, said Lestrade, winking at me. I find it hard enough to tackle facts, Holmes, without
Multiple Comparison Procedures Cohen Chapter 13 For EDUC/PSY 6600 1 We have to go to the deductions and the inferences, said Lestrade, winking at me. I find it hard enough to tackle facts, Holmes, without
Statistics and Probability Letters. Using randomization tests to preserve type I error with response adaptive and covariate adaptive randomization
 Statistics and Probability Letters ( ) Contents lists available at ScienceDirect Statistics and Probability Letters journal homepage: wwwelseviercom/locate/stapro Using randomization tests to preserve
Statistics and Probability Letters ( ) Contents lists available at ScienceDirect Statistics and Probability Letters journal homepage: wwwelseviercom/locate/stapro Using randomization tests to preserve
Lecture Outline. Biost 518 Applied Biostatistics II. Choice of Model for Analysis. Choice of Model. Choice of Model. Lecture 10: Multiple Regression:
 Biost 518 Applied Biostatistics II Scott S. Emerson, M.D., Ph.D. Professor of Biostatistics University of Washington Lecture utline Choice of Model Alternative Models Effect of data driven selection of
Biost 518 Applied Biostatistics II Scott S. Emerson, M.D., Ph.D. Professor of Biostatistics University of Washington Lecture utline Choice of Model Alternative Models Effect of data driven selection of
Adaptive Designs: Why, How and When?
 Adaptive Designs: Why, How and When? Christopher Jennison Department of Mathematical Sciences, University of Bath, UK http://people.bath.ac.uk/mascj ISBS Conference Shanghai, July 2008 1 Adaptive designs:
Adaptive Designs: Why, How and When? Christopher Jennison Department of Mathematical Sciences, University of Bath, UK http://people.bath.ac.uk/mascj ISBS Conference Shanghai, July 2008 1 Adaptive designs:
Chapter Six: Two Independent Samples Methods 1/51
 Chapter Six: Two Independent Samples Methods 1/51 6.3 Methods Related To Differences Between Proportions 2/51 Test For A Difference Between Proportions:Introduction Suppose a sampling distribution were
Chapter Six: Two Independent Samples Methods 1/51 6.3 Methods Related To Differences Between Proportions 2/51 Test For A Difference Between Proportions:Introduction Suppose a sampling distribution were
Minimax-Regret Sample Design in Anticipation of Missing Data, With Application to Panel Data. Jeff Dominitz RAND. and
 Minimax-Regret Sample Design in Anticipation of Missing Data, With Application to Panel Data Jeff Dominitz RAND and Charles F. Manski Department of Economics and Institute for Policy Research, Northwestern
Minimax-Regret Sample Design in Anticipation of Missing Data, With Application to Panel Data Jeff Dominitz RAND and Charles F. Manski Department of Economics and Institute for Policy Research, Northwestern
Adaptive designs beyond p-value combination methods. Ekkehard Glimm, Novartis Pharma EAST user group meeting Basel, 31 May 2013
 Adaptive designs beyond p-value combination methods Ekkehard Glimm, Novartis Pharma EAST user group meeting Basel, 31 May 2013 Outline Introduction Combination-p-value method and conditional error function
Adaptive designs beyond p-value combination methods Ekkehard Glimm, Novartis Pharma EAST user group meeting Basel, 31 May 2013 Outline Introduction Combination-p-value method and conditional error function
A Generalized Global Rank Test for Multiple, Possibly Censored, Outcomes
 A Generalized Global Rank Test for Multiple, Possibly Censored, Outcomes Ritesh Ramchandani Harvard School of Public Health August 5, 2014 Ritesh Ramchandani (HSPH) Global Rank Test for Multiple Outcomes
A Generalized Global Rank Test for Multiple, Possibly Censored, Outcomes Ritesh Ramchandani Harvard School of Public Health August 5, 2014 Ritesh Ramchandani (HSPH) Global Rank Test for Multiple Outcomes
1 The problem of survival analysis
 1 The problem of survival analysis Survival analysis concerns analyzing the time to the occurrence of an event. For instance, we have a dataset in which the times are 1, 5, 9, 20, and 22. Perhaps those
1 The problem of survival analysis Survival analysis concerns analyzing the time to the occurrence of an event. For instance, we have a dataset in which the times are 1, 5, 9, 20, and 22. Perhaps those
Chapter 13 Section D. F versus Q: Different Approaches to Controlling Type I Errors with Multiple Comparisons
 Explaining Psychological Statistics (2 nd Ed.) by Barry H. Cohen Chapter 13 Section D F versus Q: Different Approaches to Controlling Type I Errors with Multiple Comparisons In section B of this chapter,
Explaining Psychological Statistics (2 nd Ed.) by Barry H. Cohen Chapter 13 Section D F versus Q: Different Approaches to Controlling Type I Errors with Multiple Comparisons In section B of this chapter,
Step-down FDR Procedures for Large Numbers of Hypotheses
 Step-down FDR Procedures for Large Numbers of Hypotheses Paul N. Somerville University of Central Florida Abstract. Somerville (2004b) developed FDR step-down procedures which were particularly appropriate
Step-down FDR Procedures for Large Numbers of Hypotheses Paul N. Somerville University of Central Florida Abstract. Somerville (2004b) developed FDR step-down procedures which were particularly appropriate
Wooldridge, Introductory Econometrics, 4th ed. Appendix C: Fundamentals of mathematical statistics
 Wooldridge, Introductory Econometrics, 4th ed. Appendix C: Fundamentals of mathematical statistics A short review of the principles of mathematical statistics (or, what you should have learned in EC 151).
Wooldridge, Introductory Econometrics, 4th ed. Appendix C: Fundamentals of mathematical statistics A short review of the principles of mathematical statistics (or, what you should have learned in EC 151).
Probability and Probability Distributions. Dr. Mohammed Alahmed
 Probability and Probability Distributions 1 Probability and Probability Distributions Usually we want to do more with data than just describing them! We might want to test certain specific inferences about
Probability and Probability Distributions 1 Probability and Probability Distributions Usually we want to do more with data than just describing them! We might want to test certain specific inferences about
Hypothesis T e T sting w ith with O ne O One-Way - ANOV ANO A V Statistics Arlo Clark Foos -
 Hypothesis Testing with One-Way ANOVA Statistics Arlo Clark-Foos Conceptual Refresher 1. Standardized z distribution of scores and of means can be represented as percentile rankings. 2. t distribution
Hypothesis Testing with One-Way ANOVA Statistics Arlo Clark-Foos Conceptual Refresher 1. Standardized z distribution of scores and of means can be represented as percentile rankings. 2. t distribution
Power and Sample Size Calculations with the Additive Hazards Model
 Journal of Data Science 10(2012), 143-155 Power and Sample Size Calculations with the Additive Hazards Model Ling Chen, Chengjie Xiong, J. Philip Miller and Feng Gao Washington University School of Medicine
Journal of Data Science 10(2012), 143-155 Power and Sample Size Calculations with the Additive Hazards Model Ling Chen, Chengjie Xiong, J. Philip Miller and Feng Gao Washington University School of Medicine
Monitoring clinical trial outcomes with delayed response: incorporating pipeline data in group sequential designs. Christopher Jennison
 Monitoring clinical trial outcomes with delayed response: incorporating pipeline data in group sequential designs Christopher Jennison Department of Mathematical Sciences, University of Bath http://people.bath.ac.uk/mascj
Monitoring clinical trial outcomes with delayed response: incorporating pipeline data in group sequential designs Christopher Jennison Department of Mathematical Sciences, University of Bath http://people.bath.ac.uk/mascj
a Sample By:Dr.Hoseyn Falahzadeh 1
 In the name of God Determining ee the esize eof a Sample By:Dr.Hoseyn Falahzadeh 1 Sample Accuracy Sample accuracy: refers to how close a random sample s statistic is to the true population s value it
In the name of God Determining ee the esize eof a Sample By:Dr.Hoseyn Falahzadeh 1 Sample Accuracy Sample accuracy: refers to how close a random sample s statistic is to the true population s value it
Biost 518 Applied Biostatistics II. Purpose of Statistics. First Stage of Scientific Investigation. Further Stages of Scientific Investigation
 Biost 58 Applied Biostatistics II Scott S. Emerson, M.D., Ph.D. Professor of Biostatistics University of Washington Lecture 5: Review Purpose of Statistics Statistics is about science (Science in the broadest
Biost 58 Applied Biostatistics II Scott S. Emerson, M.D., Ph.D. Professor of Biostatistics University of Washington Lecture 5: Review Purpose of Statistics Statistics is about science (Science in the broadest
Multiple Comparisons
 Multiple Comparisons Error Rates, A Priori Tests, and Post-Hoc Tests Multiple Comparisons: A Rationale Multiple comparison tests function to tease apart differences between the groups within our IV when
Multiple Comparisons Error Rates, A Priori Tests, and Post-Hoc Tests Multiple Comparisons: A Rationale Multiple comparison tests function to tease apart differences between the groups within our IV when
Contrasts and Multiple Comparisons Supplement for Pages
 Contrasts and Multiple Comparisons Supplement for Pages 302-323 Brian Habing University of South Carolina Last Updated: July 20, 2001 The F-test from the ANOVA table allows us to test the null hypothesis
Contrasts and Multiple Comparisons Supplement for Pages 302-323 Brian Habing University of South Carolina Last Updated: July 20, 2001 The F-test from the ANOVA table allows us to test the null hypothesis
Contents. Acknowledgments. xix
 Table of Preface Acknowledgments page xv xix 1 Introduction 1 The Role of the Computer in Data Analysis 1 Statistics: Descriptive and Inferential 2 Variables and Constants 3 The Measurement of Variables
Table of Preface Acknowledgments page xv xix 1 Introduction 1 The Role of the Computer in Data Analysis 1 Statistics: Descriptive and Inferential 2 Variables and Constants 3 The Measurement of Variables
Keppel, G. & Wickens, T. D. Design and Analysis Chapter 12: Detailed Analyses of Main Effects and Simple Effects
 Keppel, G. & Wickens, T. D. Design and Analysis Chapter 1: Detailed Analyses of Main Effects and Simple Effects If the interaction is significant, then less attention is paid to the two main effects, and
Keppel, G. & Wickens, T. D. Design and Analysis Chapter 1: Detailed Analyses of Main Effects and Simple Effects If the interaction is significant, then less attention is paid to the two main effects, and
Statistics in medicine
 Statistics in medicine Lecture 4: and multivariable regression Fatma Shebl, MD, MS, MPH, PhD Assistant Professor Chronic Disease Epidemiology Department Yale School of Public Health Fatma.shebl@yale.edu
Statistics in medicine Lecture 4: and multivariable regression Fatma Shebl, MD, MS, MPH, PhD Assistant Professor Chronic Disease Epidemiology Department Yale School of Public Health Fatma.shebl@yale.edu
Chapter 6. Logistic Regression. 6.1 A linear model for the log odds
 Chapter 6 Logistic Regression In logistic regression, there is a categorical response variables, often coded 1=Yes and 0=No. Many important phenomena fit this framework. The patient survives the operation,
Chapter 6 Logistic Regression In logistic regression, there is a categorical response variables, often coded 1=Yes and 0=No. Many important phenomena fit this framework. The patient survives the operation,
Examining the accuracy of the normal approximation to the poisson random variable
 Eastern Michigan University DigitalCommons@EMU Master's Theses and Doctoral Dissertations Master's Theses, and Doctoral Dissertations, and Graduate Capstone Projects 2009 Examining the accuracy of the
Eastern Michigan University DigitalCommons@EMU Master's Theses and Doctoral Dissertations Master's Theses, and Doctoral Dissertations, and Graduate Capstone Projects 2009 Examining the accuracy of the
Causal Hazard Ratio Estimation By Instrumental Variables or Principal Stratification. Todd MacKenzie, PhD
 Causal Hazard Ratio Estimation By Instrumental Variables or Principal Stratification Todd MacKenzie, PhD Collaborators A. James O Malley Tor Tosteson Therese Stukel 2 Overview 1. Instrumental variable
Causal Hazard Ratio Estimation By Instrumental Variables or Principal Stratification Todd MacKenzie, PhD Collaborators A. James O Malley Tor Tosteson Therese Stukel 2 Overview 1. Instrumental variable
Multiple Testing. Gary W. Oehlert. January 28, School of Statistics University of Minnesota
 Multiple Testing Gary W. Oehlert School of Statistics University of Minnesota January 28, 2016 Background Suppose that you had a 20-sided die. Nineteen of the sides are labeled 0 and one of the sides is
Multiple Testing Gary W. Oehlert School of Statistics University of Minnesota January 28, 2016 Background Suppose that you had a 20-sided die. Nineteen of the sides are labeled 0 and one of the sides is
13: Additional ANOVA Topics. Post hoc Comparisons
 13: Additional ANOVA Topics Post hoc Comparisons ANOVA Assumptions Assessing Group Variances When Distributional Assumptions are Severely Violated Post hoc Comparisons In the prior chapter we used ANOVA
13: Additional ANOVA Topics Post hoc Comparisons ANOVA Assumptions Assessing Group Variances When Distributional Assumptions are Severely Violated Post hoc Comparisons In the prior chapter we used ANOVA
Semiparametric Regression
 Semiparametric Regression Patrick Breheny October 22 Patrick Breheny Survival Data Analysis (BIOS 7210) 1/23 Introduction Over the past few weeks, we ve introduced a variety of regression models under
Semiparametric Regression Patrick Breheny October 22 Patrick Breheny Survival Data Analysis (BIOS 7210) 1/23 Introduction Over the past few weeks, we ve introduced a variety of regression models under
Chapter Three. Hypothesis Testing
 3.1 Introduction The final phase of analyzing data is to make a decision concerning a set of choices or options. Should I invest in stocks or bonds? Should a new product be marketed? Are my products being
3.1 Introduction The final phase of analyzing data is to make a decision concerning a set of choices or options. Should I invest in stocks or bonds? Should a new product be marketed? Are my products being
Comparing Adaptive Designs and the. Classical Group Sequential Approach. to Clinical Trial Design
 Comparing Adaptive Designs and the Classical Group Sequential Approach to Clinical Trial Design Christopher Jennison Department of Mathematical Sciences, University of Bath, UK http://people.bath.ac.uk/mascj
Comparing Adaptive Designs and the Classical Group Sequential Approach to Clinical Trial Design Christopher Jennison Department of Mathematical Sciences, University of Bath, UK http://people.bath.ac.uk/mascj
Analysing data: regression and correlation S6 and S7
 Basic medical statistics for clinical and experimental research Analysing data: regression and correlation S6 and S7 K. Jozwiak k.jozwiak@nki.nl 2 / 49 Correlation So far we have looked at the association
Basic medical statistics for clinical and experimental research Analysing data: regression and correlation S6 and S7 K. Jozwiak k.jozwiak@nki.nl 2 / 49 Correlation So far we have looked at the association
Introduction to the Analysis of Variance (ANOVA) Computing One-Way Independent Measures (Between Subjects) ANOVAs
 Introduction to the Analysis of Variance (ANOVA) Computing One-Way Independent Measures (Between Subjects) ANOVAs The Analysis of Variance (ANOVA) The analysis of variance (ANOVA) is a statistical technique
Introduction to the Analysis of Variance (ANOVA) Computing One-Way Independent Measures (Between Subjects) ANOVAs The Analysis of Variance (ANOVA) The analysis of variance (ANOVA) is a statistical technique
9 Correlation and Regression
 9 Correlation and Regression SW, Chapter 12. Suppose we select n = 10 persons from the population of college seniors who plan to take the MCAT exam. Each takes the test, is coached, and then retakes the
9 Correlation and Regression SW, Chapter 12. Suppose we select n = 10 persons from the population of college seniors who plan to take the MCAT exam. Each takes the test, is coached, and then retakes the
Sampling and Sample Size. Shawn Cole Harvard Business School
 Sampling and Sample Size Shawn Cole Harvard Business School Calculating Sample Size Effect Size Power Significance Level Variance ICC EffectSize 2 ( ) 1 σ = t( 1 κ ) + tα * * 1+ ρ( m 1) P N ( 1 P) Proportion
Sampling and Sample Size Shawn Cole Harvard Business School Calculating Sample Size Effect Size Power Significance Level Variance ICC EffectSize 2 ( ) 1 σ = t( 1 κ ) + tα * * 1+ ρ( m 1) P N ( 1 P) Proportion
3 Joint Distributions 71
 2.2.3 The Normal Distribution 54 2.2.4 The Beta Density 58 2.3 Functions of a Random Variable 58 2.4 Concluding Remarks 64 2.5 Problems 64 3 Joint Distributions 71 3.1 Introduction 71 3.2 Discrete Random
2.2.3 The Normal Distribution 54 2.2.4 The Beta Density 58 2.3 Functions of a Random Variable 58 2.4 Concluding Remarks 64 2.5 Problems 64 3 Joint Distributions 71 3.1 Introduction 71 3.2 Discrete Random
Analysis of Variance and Co-variance. By Manza Ramesh
 Analysis of Variance and Co-variance By Manza Ramesh Contents Analysis of Variance (ANOVA) What is ANOVA? The Basic Principle of ANOVA ANOVA Technique Setting up Analysis of Variance Table Short-cut Method
Analysis of Variance and Co-variance By Manza Ramesh Contents Analysis of Variance (ANOVA) What is ANOVA? The Basic Principle of ANOVA ANOVA Technique Setting up Analysis of Variance Table Short-cut Method
Statistical Methods for Alzheimer s Disease Studies
 Statistical Methods for Alzheimer s Disease Studies Rebecca A. Betensky, Ph.D. Department of Biostatistics, Harvard T.H. Chan School of Public Health July 19, 2016 1/37 OUTLINE 1 Statistical collaborations
Statistical Methods for Alzheimer s Disease Studies Rebecca A. Betensky, Ph.D. Department of Biostatistics, Harvard T.H. Chan School of Public Health July 19, 2016 1/37 OUTLINE 1 Statistical collaborations
BIAS OF MAXIMUM-LIKELIHOOD ESTIMATES IN LOGISTIC AND COX REGRESSION MODELS: A COMPARATIVE SIMULATION STUDY
 BIAS OF MAXIMUM-LIKELIHOOD ESTIMATES IN LOGISTIC AND COX REGRESSION MODELS: A COMPARATIVE SIMULATION STUDY Ingo Langner 1, Ralf Bender 2, Rebecca Lenz-Tönjes 1, Helmut Küchenhoff 2, Maria Blettner 2 1
BIAS OF MAXIMUM-LIKELIHOOD ESTIMATES IN LOGISTIC AND COX REGRESSION MODELS: A COMPARATIVE SIMULATION STUDY Ingo Langner 1, Ralf Bender 2, Rebecca Lenz-Tönjes 1, Helmut Küchenhoff 2, Maria Blettner 2 1
Person-Time Data. Incidence. Cumulative Incidence: Example. Cumulative Incidence. Person-Time Data. Person-Time Data
 Person-Time Data CF Jeff Lin, MD., PhD. Incidence 1. Cumulative incidence (incidence proportion) 2. Incidence density (incidence rate) December 14, 2005 c Jeff Lin, MD., PhD. c Jeff Lin, MD., PhD. Person-Time
Person-Time Data CF Jeff Lin, MD., PhD. Incidence 1. Cumulative incidence (incidence proportion) 2. Incidence density (incidence rate) December 14, 2005 c Jeff Lin, MD., PhD. c Jeff Lin, MD., PhD. Person-Time
Uniformly Most Powerful Bayesian Tests and Standards for Statistical Evidence
 Uniformly Most Powerful Bayesian Tests and Standards for Statistical Evidence Valen E. Johnson Texas A&M University February 27, 2014 Valen E. Johnson Texas A&M University Uniformly most powerful Bayes
Uniformly Most Powerful Bayesian Tests and Standards for Statistical Evidence Valen E. Johnson Texas A&M University February 27, 2014 Valen E. Johnson Texas A&M University Uniformly most powerful Bayes
Two-stage Adaptive Randomization for Delayed Response in Clinical Trials
 Two-stage Adaptive Randomization for Delayed Response in Clinical Trials Guosheng Yin Department of Statistics and Actuarial Science The University of Hong Kong Joint work with J. Xu PSI and RSS Journal
Two-stage Adaptive Randomization for Delayed Response in Clinical Trials Guosheng Yin Department of Statistics and Actuarial Science The University of Hong Kong Joint work with J. Xu PSI and RSS Journal
On Procedures Controlling the FDR for Testing Hierarchically Ordered Hypotheses
 On Procedures Controlling the FDR for Testing Hierarchically Ordered Hypotheses Gavin Lynch Catchpoint Systems, Inc., 228 Park Ave S 28080 New York, NY 10003, U.S.A. Wenge Guo Department of Mathematical
On Procedures Controlling the FDR for Testing Hierarchically Ordered Hypotheses Gavin Lynch Catchpoint Systems, Inc., 228 Park Ave S 28080 New York, NY 10003, U.S.A. Wenge Guo Department of Mathematical
Fall 2017 STAT 532 Homework Peter Hoff. 1. Let P be a probability measure on a collection of sets A.
 1. Let P be a probability measure on a collection of sets A. (a) For each n N, let H n be a set in A such that H n H n+1. Show that P (H n ) monotonically converges to P ( k=1 H k) as n. (b) For each n
1. Let P be a probability measure on a collection of sets A. (a) For each n N, let H n be a set in A such that H n H n+1. Show that P (H n ) monotonically converges to P ( k=1 H k) as n. (b) For each n
Type I error rate control in adaptive designs for confirmatory clinical trials with treatment selection at interim
 Type I error rate control in adaptive designs for confirmatory clinical trials with treatment selection at interim Frank Bretz Statistical Methodology, Novartis Joint work with Martin Posch (Medical University
Type I error rate control in adaptive designs for confirmatory clinical trials with treatment selection at interim Frank Bretz Statistical Methodology, Novartis Joint work with Martin Posch (Medical University
The SEQDESIGN Procedure
 SAS/STAT 9.2 User s Guide, Second Edition The SEQDESIGN Procedure (Book Excerpt) This document is an individual chapter from the SAS/STAT 9.2 User s Guide, Second Edition. The correct bibliographic citation
SAS/STAT 9.2 User s Guide, Second Edition The SEQDESIGN Procedure (Book Excerpt) This document is an individual chapter from the SAS/STAT 9.2 User s Guide, Second Edition. The correct bibliographic citation
Estimating the Mean Response of Treatment Duration Regimes in an Observational Study. Anastasios A. Tsiatis.
 Estimating the Mean Response of Treatment Duration Regimes in an Observational Study Anastasios A. Tsiatis http://www.stat.ncsu.edu/ tsiatis/ Introduction to Dynamic Treatment Regimes 1 Outline Description
Estimating the Mean Response of Treatment Duration Regimes in an Observational Study Anastasios A. Tsiatis http://www.stat.ncsu.edu/ tsiatis/ Introduction to Dynamic Treatment Regimes 1 Outline Description
An Introduction to Mplus and Path Analysis
 An Introduction to Mplus and Path Analysis PSYC 943: Fundamentals of Multivariate Modeling Lecture 10: October 30, 2013 PSYC 943: Lecture 10 Today s Lecture Path analysis starting with multivariate regression
An Introduction to Mplus and Path Analysis PSYC 943: Fundamentals of Multivariate Modeling Lecture 10: October 30, 2013 PSYC 943: Lecture 10 Today s Lecture Path analysis starting with multivariate regression
Construction and statistical analysis of adaptive group sequential designs for randomized clinical trials
 Construction and statistical analysis of adaptive group sequential designs for randomized clinical trials Antoine Chambaz (MAP5, Université Paris Descartes) joint work with Mark van der Laan Atelier INSERM
Construction and statistical analysis of adaptive group sequential designs for randomized clinical trials Antoine Chambaz (MAP5, Université Paris Descartes) joint work with Mark van der Laan Atelier INSERM
Accounting for Baseline Observations in Randomized Clinical Trials
 Accounting for Baseline Observations in Randomized Clinical Trials Scott S Emerson, MD, PhD Department of Biostatistics, University of Washington, Seattle, WA 9895, USA October 6, 0 Abstract In clinical
Accounting for Baseline Observations in Randomized Clinical Trials Scott S Emerson, MD, PhD Department of Biostatistics, University of Washington, Seattle, WA 9895, USA October 6, 0 Abstract In clinical
PubH 7470: STATISTICS FOR TRANSLATIONAL & CLINICAL RESEARCH
 PubH 7470: STATISTICS FOR TRANSLATIONAL & CLINICAL RESEARCH The First Step: SAMPLE SIZE DETERMINATION THE ULTIMATE GOAL The most important, ultimate step of any of clinical research is to do draw inferences;
PubH 7470: STATISTICS FOR TRANSLATIONAL & CLINICAL RESEARCH The First Step: SAMPLE SIZE DETERMINATION THE ULTIMATE GOAL The most important, ultimate step of any of clinical research is to do draw inferences;
401 Review. 6. Power analysis for one/two-sample hypothesis tests and for correlation analysis.
 401 Review Major topics of the course 1. Univariate analysis 2. Bivariate analysis 3. Simple linear regression 4. Linear algebra 5. Multiple regression analysis Major analysis methods 1. Graphical analysis
401 Review Major topics of the course 1. Univariate analysis 2. Bivariate analysis 3. Simple linear regression 4. Linear algebra 5. Multiple regression analysis Major analysis methods 1. Graphical analysis
Building a Prognostic Biomarker
 Building a Prognostic Biomarker Noah Simon and Richard Simon July 2016 1 / 44 Prognostic Biomarker for a Continuous Measure On each of n patients measure y i - single continuous outcome (eg. blood pressure,
Building a Prognostic Biomarker Noah Simon and Richard Simon July 2016 1 / 44 Prognostic Biomarker for a Continuous Measure On each of n patients measure y i - single continuous outcome (eg. blood pressure,
Testing a secondary endpoint after a group sequential test. Chris Jennison. 9th Annual Adaptive Designs in Clinical Trials
 Testing a secondary endpoint after a group sequential test Christopher Jennison Department of Mathematical Sciences, University of Bath, UK http://people.bath.ac.uk/mascj 9th Annual Adaptive Designs in
Testing a secondary endpoint after a group sequential test Christopher Jennison Department of Mathematical Sciences, University of Bath, UK http://people.bath.ac.uk/mascj 9th Annual Adaptive Designs in
Experimental Design and Data Analysis for Biologists
 Experimental Design and Data Analysis for Biologists Gerry P. Quinn Monash University Michael J. Keough University of Melbourne CAMBRIDGE UNIVERSITY PRESS Contents Preface page xv I I Introduction 1 1.1
Experimental Design and Data Analysis for Biologists Gerry P. Quinn Monash University Michael J. Keough University of Melbourne CAMBRIDGE UNIVERSITY PRESS Contents Preface page xv I I Introduction 1 1.1
Formal Statement of Simple Linear Regression Model
 Formal Statement of Simple Linear Regression Model Y i = β 0 + β 1 X i + ɛ i Y i value of the response variable in the i th trial β 0 and β 1 are parameters X i is a known constant, the value of the predictor
Formal Statement of Simple Linear Regression Model Y i = β 0 + β 1 X i + ɛ i Y i value of the response variable in the i th trial β 0 and β 1 are parameters X i is a known constant, the value of the predictor
Survival Analysis for Case-Cohort Studies
 Survival Analysis for ase-ohort Studies Petr Klášterecký Dept. of Probability and Mathematical Statistics, Faculty of Mathematics and Physics, harles University, Prague, zech Republic e-mail: petr.klasterecky@matfyz.cz
Survival Analysis for ase-ohort Studies Petr Klášterecký Dept. of Probability and Mathematical Statistics, Faculty of Mathematics and Physics, harles University, Prague, zech Republic e-mail: petr.klasterecky@matfyz.cz
Open Problems in Mixed Models
 xxiii Determining how to deal with a not positive definite covariance matrix of random effects, D during maximum likelihood estimation algorithms. Several strategies are discussed in Section 2.15. For
xxiii Determining how to deal with a not positive definite covariance matrix of random effects, D during maximum likelihood estimation algorithms. Several strategies are discussed in Section 2.15. For
Introduction to Crossover Trials
 Introduction to Crossover Trials Stat 6500 Tutorial Project Isaac Blackhurst A crossover trial is a type of randomized control trial. It has advantages over other designed experiments because, under certain
Introduction to Crossover Trials Stat 6500 Tutorial Project Isaac Blackhurst A crossover trial is a type of randomized control trial. It has advantages over other designed experiments because, under certain
A Monte Carlo Simulation of the Robust Rank- Order Test Under Various Population Symmetry Conditions
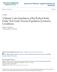 Journal of Modern Applied Statistical Methods Volume 12 Issue 1 Article 7 5-1-2013 A Monte Carlo Simulation of the Robust Rank- Order Test Under Various Population Symmetry Conditions William T. Mickelson
Journal of Modern Applied Statistical Methods Volume 12 Issue 1 Article 7 5-1-2013 A Monte Carlo Simulation of the Robust Rank- Order Test Under Various Population Symmetry Conditions William T. Mickelson
Transition Passage to Descriptive Statistics 28
 viii Preface xiv chapter 1 Introduction 1 Disciplines That Use Quantitative Data 5 What Do You Mean, Statistics? 6 Statistics: A Dynamic Discipline 8 Some Terminology 9 Problems and Answers 12 Scales of
viii Preface xiv chapter 1 Introduction 1 Disciplines That Use Quantitative Data 5 What Do You Mean, Statistics? 6 Statistics: A Dynamic Discipline 8 Some Terminology 9 Problems and Answers 12 Scales of
Multiple Testing of One-Sided Hypotheses: Combining Bonferroni and the Bootstrap
 University of Zurich Department of Economics Working Paper Series ISSN 1664-7041 (print) ISSN 1664-705X (online) Working Paper No. 254 Multiple Testing of One-Sided Hypotheses: Combining Bonferroni and
University of Zurich Department of Economics Working Paper Series ISSN 1664-7041 (print) ISSN 1664-705X (online) Working Paper No. 254 Multiple Testing of One-Sided Hypotheses: Combining Bonferroni and
Effect of investigator bias on the significance level of the Wilcoxon rank-sum test
 Biostatistics 000, 1, 1,pp. 107 111 Printed in Great Britain Effect of investigator bias on the significance level of the Wilcoxon rank-sum test PAUL DELUCCA Biometrician, Merck & Co., Inc., 1 Walnut Grove
Biostatistics 000, 1, 1,pp. 107 111 Printed in Great Britain Effect of investigator bias on the significance level of the Wilcoxon rank-sum test PAUL DELUCCA Biometrician, Merck & Co., Inc., 1 Walnut Grove
Tackling Statistical Uncertainty in Method Validation
 Tackling Statistical Uncertainty in Method Validation Steven Walfish President, Statistical Outsourcing Services steven@statisticaloutsourcingservices.com 301-325 325-31293129 About the Speaker Mr. Steven
Tackling Statistical Uncertainty in Method Validation Steven Walfish President, Statistical Outsourcing Services steven@statisticaloutsourcingservices.com 301-325 325-31293129 About the Speaker Mr. Steven
SHOPPING FOR EFFICIENT CONFIDENCE INTERVALS IN STRUCTURAL EQUATION MODELS. Donna Mohr and Yong Xu. University of North Florida
 SHOPPING FOR EFFICIENT CONFIDENCE INTERVALS IN STRUCTURAL EQUATION MODELS Donna Mohr and Yong Xu University of North Florida Authors Note Parts of this work were incorporated in Yong Xu s Masters Thesis
SHOPPING FOR EFFICIENT CONFIDENCE INTERVALS IN STRUCTURAL EQUATION MODELS Donna Mohr and Yong Xu University of North Florida Authors Note Parts of this work were incorporated in Yong Xu s Masters Thesis
β j = coefficient of x j in the model; β = ( β1, β2,
 Regression Modeling of Survival Time Data Why regression models? Groups similar except for the treatment under study use the nonparametric methods discussed earlier. Groups differ in variables (covariates)
Regression Modeling of Survival Time Data Why regression models? Groups similar except for the treatment under study use the nonparametric methods discussed earlier. Groups differ in variables (covariates)
Bios 6649: Clinical Trials - Statistical Design and Monitoring
 Bios 6649: Clinical Trials - Statistical Design and Monitoring Spring Semester 2015 John M. Kittelson Department of Biostatistics & Informatics Colorado School of Public Health University of Colorado Denver
Bios 6649: Clinical Trials - Statistical Design and Monitoring Spring Semester 2015 John M. Kittelson Department of Biostatistics & Informatics Colorado School of Public Health University of Colorado Denver
STAT 5200 Handout #7a Contrasts & Post hoc Means Comparisons (Ch. 4-5)
 STAT 5200 Handout #7a Contrasts & Post hoc Means Comparisons Ch. 4-5) Recall CRD means and effects models: Y ij = µ i + ϵ ij = µ + α i + ϵ ij i = 1,..., g ; j = 1,..., n ; ϵ ij s iid N0, σ 2 ) If we reject
STAT 5200 Handout #7a Contrasts & Post hoc Means Comparisons Ch. 4-5) Recall CRD means and effects models: Y ij = µ i + ϵ ij = µ + α i + ϵ ij i = 1,..., g ; j = 1,..., n ; ϵ ij s iid N0, σ 2 ) If we reject
Pairwise rank based likelihood for estimating the relationship between two homogeneous populations and their mixture proportion
 Pairwise rank based likelihood for estimating the relationship between two homogeneous populations and their mixture proportion Glenn Heller and Jing Qin Department of Epidemiology and Biostatistics Memorial
Pairwise rank based likelihood for estimating the relationship between two homogeneous populations and their mixture proportion Glenn Heller and Jing Qin Department of Epidemiology and Biostatistics Memorial
Structure learning in human causal induction
 Structure learning in human causal induction Joshua B. Tenenbaum & Thomas L. Griffiths Department of Psychology Stanford University, Stanford, CA 94305 jbt,gruffydd @psych.stanford.edu Abstract We use
Structure learning in human causal induction Joshua B. Tenenbaum & Thomas L. Griffiths Department of Psychology Stanford University, Stanford, CA 94305 jbt,gruffydd @psych.stanford.edu Abstract We use
BIOS 312: Precision of Statistical Inference
 and Power/Sample Size and Standard Errors BIOS 312: of Statistical Inference Chris Slaughter Department of Biostatistics, Vanderbilt University School of Medicine January 3, 2013 Outline Overview and Power/Sample
and Power/Sample Size and Standard Errors BIOS 312: of Statistical Inference Chris Slaughter Department of Biostatistics, Vanderbilt University School of Medicine January 3, 2013 Outline Overview and Power/Sample
Hypothesis Testing. Part I. James J. Heckman University of Chicago. Econ 312 This draft, April 20, 2006
 Hypothesis Testing Part I James J. Heckman University of Chicago Econ 312 This draft, April 20, 2006 1 1 A Brief Review of Hypothesis Testing and Its Uses values and pure significance tests (R.A. Fisher)
Hypothesis Testing Part I James J. Heckman University of Chicago Econ 312 This draft, April 20, 2006 1 1 A Brief Review of Hypothesis Testing and Its Uses values and pure significance tests (R.A. Fisher)
Guideline on adjustment for baseline covariates in clinical trials
 26 February 2015 EMA/CHMP/295050/2013 Committee for Medicinal Products for Human Use (CHMP) Guideline on adjustment for baseline covariates in clinical trials Draft Agreed by Biostatistics Working Party
26 February 2015 EMA/CHMP/295050/2013 Committee for Medicinal Products for Human Use (CHMP) Guideline on adjustment for baseline covariates in clinical trials Draft Agreed by Biostatistics Working Party
y response variable x 1, x 2,, x k -- a set of explanatory variables
 11. Multiple Regression and Correlation y response variable x 1, x 2,, x k -- a set of explanatory variables In this chapter, all variables are assumed to be quantitative. Chapters 12-14 show how to incorporate
11. Multiple Regression and Correlation y response variable x 1, x 2,, x k -- a set of explanatory variables In this chapter, all variables are assumed to be quantitative. Chapters 12-14 show how to incorporate
Introduction to Regression Analysis. Dr. Devlina Chatterjee 11 th August, 2017
 Introduction to Regression Analysis Dr. Devlina Chatterjee 11 th August, 2017 What is regression analysis? Regression analysis is a statistical technique for studying linear relationships. One dependent
Introduction to Regression Analysis Dr. Devlina Chatterjee 11 th August, 2017 What is regression analysis? Regression analysis is a statistical technique for studying linear relationships. One dependent
A Bayesian Nonparametric Approach to Causal Inference for Semi-competing risks
 A Bayesian Nonparametric Approach to Causal Inference for Semi-competing risks Y. Xu, D. Scharfstein, P. Mueller, M. Daniels Johns Hopkins, Johns Hopkins, UT-Austin, UF JSM 2018, Vancouver 1 What are semi-competing
A Bayesian Nonparametric Approach to Causal Inference for Semi-competing risks Y. Xu, D. Scharfstein, P. Mueller, M. Daniels Johns Hopkins, Johns Hopkins, UT-Austin, UF JSM 2018, Vancouver 1 What are semi-competing
Group comparison test for independent samples
 Group comparison test for independent samples The purpose of the Analysis of Variance (ANOVA) is to test for significant differences between means. Supposing that: samples come from normal populations
Group comparison test for independent samples The purpose of the Analysis of Variance (ANOVA) is to test for significant differences between means. Supposing that: samples come from normal populations
e author and the promoter give permission to consult this master dissertation and to copy it or parts of it for personal use. Each other use falls
 e author and the promoter give permission to consult this master dissertation and to copy it or parts of it for personal use. Each other use falls under the restrictions of the copyright, in particular
e author and the promoter give permission to consult this master dissertation and to copy it or parts of it for personal use. Each other use falls under the restrictions of the copyright, in particular
Statistical Applications in Genetics and Molecular Biology
 Statistical Applications in Genetics and Molecular Biology Volume 5, Issue 1 2006 Article 28 A Two-Step Multiple Comparison Procedure for a Large Number of Tests and Multiple Treatments Hongmei Jiang Rebecca
Statistical Applications in Genetics and Molecular Biology Volume 5, Issue 1 2006 Article 28 A Two-Step Multiple Comparison Procedure for a Large Number of Tests and Multiple Treatments Hongmei Jiang Rebecca
Hypothesis testing: Steps
 Review for Exam 2 Hypothesis testing: Steps Exam 2 Review 1. Determine appropriate test and hypotheses 2. Use distribution table to find critical statistic value(s) representing rejection region 3. Compute
Review for Exam 2 Hypothesis testing: Steps Exam 2 Review 1. Determine appropriate test and hypotheses 2. Use distribution table to find critical statistic value(s) representing rejection region 3. Compute
The Components of a Statistical Hypothesis Testing Problem
 Statistical Inference: Recall from chapter 5 that statistical inference is the use of a subset of a population (the sample) to draw conclusions about the entire population. In chapter 5 we studied one
Statistical Inference: Recall from chapter 5 that statistical inference is the use of a subset of a population (the sample) to draw conclusions about the entire population. In chapter 5 we studied one
Part III. Hypothesis Testing. III.1. Log-rank Test for Right-censored Failure Time Data
 1 Part III. Hypothesis Testing III.1. Log-rank Test for Right-censored Failure Time Data Consider a survival study consisting of n independent subjects from p different populations with survival functions
1 Part III. Hypothesis Testing III.1. Log-rank Test for Right-censored Failure Time Data Consider a survival study consisting of n independent subjects from p different populations with survival functions
2 Hand-out 2. Dr. M. P. M. M. M c Loughlin Revised 2018
 Math 403 - P. & S. III - Dr. McLoughlin - 1 2018 2 Hand-out 2 Dr. M. P. M. M. M c Loughlin Revised 2018 3. Fundamentals 3.1. Preliminaries. Suppose we can produce a random sample of weights of 10 year-olds
Math 403 - P. & S. III - Dr. McLoughlin - 1 2018 2 Hand-out 2 Dr. M. P. M. M. M c Loughlin Revised 2018 3. Fundamentals 3.1. Preliminaries. Suppose we can produce a random sample of weights of 10 year-olds
MORE ON SIMPLE REGRESSION: OVERVIEW
 FI=NOT0106 NOTICE. Unless otherwise indicated, all materials on this page and linked pages at the blue.temple.edu address and at the astro.temple.edu address are the sole property of Ralph B. Taylor and
FI=NOT0106 NOTICE. Unless otherwise indicated, all materials on this page and linked pages at the blue.temple.edu address and at the astro.temple.edu address are the sole property of Ralph B. Taylor and
Chap The McGraw-Hill Companies, Inc. All rights reserved.
 11 pter11 Chap Analysis of Variance Overview of ANOVA Multiple Comparisons Tests for Homogeneity of Variances Two-Factor ANOVA Without Replication General Linear Model Experimental Design: An Overview
11 pter11 Chap Analysis of Variance Overview of ANOVA Multiple Comparisons Tests for Homogeneity of Variances Two-Factor ANOVA Without Replication General Linear Model Experimental Design: An Overview
Bounds on Causal Effects in Three-Arm Trials with Non-compliance. Jing Cheng Dylan Small
 Bounds on Causal Effects in Three-Arm Trials with Non-compliance Jing Cheng Dylan Small Department of Biostatistics and Department of Statistics University of Pennsylvania June 20, 2005 A Three-Arm Randomized
Bounds on Causal Effects in Three-Arm Trials with Non-compliance Jing Cheng Dylan Small Department of Biostatistics and Department of Statistics University of Pennsylvania June 20, 2005 A Three-Arm Randomized
