Poisson-Boltzmann cell model for heterogeneously charged colloids
|
|
|
- Daniella Lloyd
- 6 years ago
- Views:
Transcription
1 PHYSICAL REVIEW E 80, Poisson-Boltzmann cell model for heterogeneously charged colloids Eelco Eggen and René van Roij Institute for Theoretical Physics, Utrecht University, Leuvenlaan 4, 3584 CE Utrecht, The Netherlands Received 7 July 009; published 7 October 009 We introduce the Poisson-Boltzmann cell model for spherical colloidal particles with a heterogeneous surface charge distribution. This model is obtained by generalizing existing cell models for mixtures of homogeneously charged colloidal spheres. Our model has similar features as Onsager s second-virial theory for liquid crystals, but it predicts no orientational ordering if there is no positional ordering. This implies that all phases of heterogeneously charged colloids that are liquidlike with respect to translational degrees of freedom are also isotropic with respect to particle orientation. DOI: /PhysRevE PACS numbers: 8.70.y, pv, M, 8.35.Rs I. INTRODUCTION Already a long time ago, Marcus 1, Ohtsuki et al., and Alexander et al. 3 realized that the cell model approach of Wigner and Seitz 4 to calculate the properties of electrons in solids can also be applied to colloidal matter. In this case, there are no quantum effects and, instead of a wave function, one calculates the ion distributions around charged colloidal particles dispersed in a liquid medium. In the simplest case, the colloidal dispersion consists of one colloidal species immersed in a 1:1 electrolyte solution. The colloidal particles are homogeneously charged and have a spherical shape. Additionally, the simplification of taking a spherical Wigner-Seitz cell instead of space filling is justified in fluid phases with no broken translational symmetry. A number of extensions has been made to this basic cell model. There is the eccentric Poisson-Boltzmann PB cell model,5 and the heterogeneous or polydisperse cell model 6,7 to describe mixtures. Additionally, the cell model has been extended by applying cylindrical Wigner- Seitz cells for the description of disk-shaped and rodlike particles 8,9. In the present paper, we make an extension toward particles with a heterogeneous surface charge distribution such as patterned colloids 10 or Janus particles 11. Janus particles are characterized by two distinct regions of surface area. Each of these faces has a different chemical composition, which can create spontaneous particle aggregation 1. Our aim is toward a description of selforganization of these particles from single-particle properties. The cell model can be a powerful tool, giving a simple description of these complex systems. From this description, a number of thermodynamic quantities can be derived such as free energy and osmotic pressure. We investigate how surface charge heterogeneity influences the distribution of particle orientations in the case of a homogeneous positional distribution. This case can be considered as a simplified description of a fluid of these particles but also as an oversimplified description of a solid. Since the particle interactions are implemented through the boundary conditions of the Wigner-Seitz cell, one can choose to neglect the correlations between the orientational and positional ordering. The basis of our model is the generalization of the Poisson-Boltzmann cell model by Biesheuvel et al. 6 considering mixtures, together with the insight of Onsager 13 that a distribution of orientations can be considered in the same way as mixtures. In principle, this model can be used to predict the phase behavior of a large class of colloidal and nanoparticles because an anisotropic as well as spherical particle shape can be treated. However, here we restrict ourselves to a system of spherical colloidal particles. II. CELL MODEL FOR MIXTURES We start by giving the prerequisites for the ordinary spherically symmetric cell model see Fig. 1. In this simple case, we consider a system of N identical spherically symmetric colloidal particles in a volume V. These particles have radius a and a surface charge Ze, where e is the elementary charge. The system is presumed to be in osmotic contact with a reservoir of monovalent cations and anions at total concentration s, with charge e. Both the particle and the surrounding medium are considered to be dielectric media, where in and out are the permittivities of the particle and the solvent, respectively. Within Poisson-Boltzmann theory, one relates the density profiles r of the ions to the electrostatic potential r in a fixed configuration of the colloidal particles. This complicated nonlinear N-body problem can be simplified consida R in out FIG. 1. Illustration of the Poisson-Boltzmann cell model. A colloidal particle of radius a is surrounded by ions. It is situated in the center of a spherical cell of radius R /009/804/ The American Physical Society
2 EELCO EGGEN AND RENÉ VAN ROIJ erably by regarding a single colloidal particle in the center of a Wigner-Seitz cell surrounded by the ions. This cell is assumed to have a spherical shape of radius R instead of space filling. The volume of the cell is fixed by the available volume per particle, 4 3 R3 = V N. 1 We treat the ions in a mean-field description, such that we obtain the PB equation = r 0 for 0 r a sinh r for a r R, where 1 = out / s e is the Debye screening length, and =e is the dimensionless electrostatic potential, where =1/k B T. This second-order nonlinear partial differential equation describes the electrostatic effects of the ions in the cell volume surrounding the colloidal particle. The boundary conditions are determined by the charge on the particle and the cell electroneutrality. The first boundary condition is given by r r a = r r a, 3 in r r r a = e Z 4a out r r r a, 4 which fixes the electric field at the particle surface. In Eq. 4, Z is the number of elementary charges on the particle surface. Because of the homogeneous surface charge distribution, there is no electric field inside the particle. This reduces boundary condition 4 to r r r a a = Zl B a, 5 where l B =e /4 out is the Bjerrum length. The second boundary condition fixes the electric field at the cell boundary, according to Gauss law R =0. 6 The PB equation together with the boundary conditions 5 and 6 form a closed set and describe the basic Poisson- Boltzmann cell model as studied, for example, in Refs. 3,14,15. Now we discuss the case of a mixture of equally sized homogeneously charged colloidal species with surface charge Z i e. Again, the particles have radius a and are positioned in the center of a spherical Wigner-Seitz cell of radius R. The PB equation is solved separately for each colloidal species i, and the notion of electroneutrality is applied to the system as a whole 6. Each solution i r is determined by the surface charge density on the corresponding colloidal species i a = Z il B a. 7 Given that the cells of each pair of species are considered to be neighboring, it is natural to impose a common boundary PHYSICAL REVIEW E 80, value R for the potential on every cell surface. This value is by definition equal to the average value of the electrostatic potential at the cell boundary for different species. Hence, we have i R = x j j R R i, 8 j where x i =N i /N is the molar fraction of species i, such that x i =1. 9 i The boundary value R is fixed by setting the average value of the charge contained in the cells for different species to zero. According to Gauss law, this is achieved by imposing x i i R =0. 10 i The PB equation applied to each species i together with the boundary conditions 7, 8, and 10 again form a closed set. In principle, one could allow for a different cell radius R i for each colloidal species. In this case, a factor R i must be included in the summation of Eq. 10. One subsequently imposes a set of physically motivated conditions on these radii. These conditions must comply with the fact that the average cell volume of the system is given by 4 x i R 3 3 i = V i N. 11 In the case of mixtures, where the charge of all species has the same sign, one can fix each R i by imposing the condition that the electric field must vanish at the cell boundary of each species 7. This condition is equivalent with imposing electroneutrality on the cell of each species separately. There is no guarantee, however, that this is possible for a general mixture of charged spheres. For reasons of simplicity, we will not use such an extension. Hence, in this paper, the cell radius is given by a single value R. III. EXTENSION TOWARD HETEROGENEOUS CHARGE DISTRIBUTIONS We now consider a system of spherical colloidal particles that are not homogeneously charged such as Janus particles 11. Again, the system consists of N identical spherical particles of radius a in a volume V. We apply the same Poisson- Boltzmann theory, such that the electrostatic potential obeys the PB equation. As before, we fix the position of each particle at the center of a spherical Wigner-Seitz cell of radius R, and the PB equation must be solved for each species. However, in this case we replace the species index i by an orientation ˆ, and each solution is denoted by ˆ ;r. In the spirit of Onsager, we can view such an orientational distribution as a mixture, where the different particle species have a distinct charge distribution. In this paper, we focus on charge distributions that are cylindrically symmetric with respect to the particle orientation ˆ. This charge distribution gives us the boundary con
3 POISSON-BOLTZMANN CELL MODEL FOR PHYSICAL REVIEW E 80, dˆ fˆ =1. 16 ˆω dition on the particle surface at each position anˆ, in ˆ ;anˆ = out ˆ ;anˆ, 1 nˆ in ˆ ;anˆ =4l B ˆ ;nˆ nˆ out ˆ ;anˆ, 13 where ˆ ;nˆ is the surface charge density in units of e that belongs to a particle with an orientation ˆ, and = in / out is the relative dielectric constant of the particle with respect to the surrounding solvent. The labels in and out denote the solutions inside the colloidal particle and outside the particle, respectively. Next, we must supply a generalized version of the boundary conditions on the cell surface given in Eqs. 8 and 10. The following approach is illustrated by Fig., which shows the directionality that must be included in the appropriate boundary conditions. First, we generalize the concept of a fixed cell surface potential, as given by R in Eq. 8. Because of the lack of spherical symmetry in this case, the cell surface potential becomes a function of the position Rnˆ on the cell surface. Similar to the cell model for mixtures, the value of this potential is defined as the average value of the electrostatic potential at the cell boundary for different species. However, the average value is taken at the position of opposite orientation ˆ ;Rnˆ = Rnˆ R nˆ ˆ, 14 and instead of a summation over species weighted by the molar fractions x i, we have an integral over particle orientations ˆ weighted by the orientational distribution function ODF fˆ r dˆ fˆ ˆ ;r. 15 This distribution is normalized such that FIG.. Color online Illustration of the Poisson-Boltzmann cell model for heterogeneous charge distributions. For each direction nˆ perpendicular to the cell surface, we determine the appropriate boundary conditions. ˆn The boundary condition in Eq. 14 ensures that the potential of two touching cells at positions Rnˆ and Rnˆ, respectively belonging to arbitrary species is continuous. Consequently, the definition of the cell surface potential R nˆ is such that it is always an even function R nˆ = R nˆ. 17 Finally, we have to impose a boundary condition that fixes this cell surface potential. However, if we only impose global electroneutrality on the system, we obtain a boundary condition that is too general for a solution that is not spherically symmetric. It would ensure that the average value over all species of the charge contained in each Wigner-Seitz cell vanishes. By applying Gauss law, we see that this condition is satisfied by setting the average value of the electric field integrated over the surface to zero dnˆ nˆ Rnˆ =0. 18 Interestingly, this fixes only the isotropic contribution to the cell surface potential. Therefore, we impose an additional condition that is based on the concept of continuity of the electric field flux from one cell to another. The difference between the outward flux at the cell boundary and the average inward flux of neighboring cells is represented by F R ˆ ;nˆ nˆ ˆ ;Rnˆ nˆ Rnˆ. 19 This quantity is averaged over all particle orientations and set to zero, in order to insure global electric field flux conservation F R nˆ =0, 0 which is equivalent to imposing nˆ Rnˆ = nˆ Rnˆ. 1 This condition does fix the cell surface potential, and it defines an average boundary value of the radial derivative such that it is an odd function of nˆ. IV. SPECIAL LIMITING CASES: PERFECTLY ISOTROPIC AND PERFECTLY ALIGNED In this section, we apply specific choices for the ODF. In turn, these choices yield a specific form for the boundary conditions 14 and 1. The resulting models are less intricate than the full model we presented in the previous section. Also, these models yield boundary conditions that one would expect from a naive description of such systems. Let us take a look at the model that our boundary conditions yield when we implement specific ODFs. First, we consider a perfectly isotropic orientational distribution f iso ˆ = 1 4. Since in such a system there is no preferential direction, we argue that all solutions for different particle orientations
4 EELCO EGGEN AND RENÉ VAN ROIJ are equivalent. Consequently, the cell surface potential R nˆ is independent of the position on the cell surface iso ˆ ;Rnˆ = R. 3 This result is in accordance with the notion that in the isotropic case the average over all particle orientations Eq. 15 is equal to the average over all orientations nˆ of the position on the cell surface and that this average no longer depends on either orientation. Therefore, the boundary condition 1 is equivalent to the condition that each cell is electroneutral nˆ iso Rnˆ = dnˆ nˆ iso ˆ ;Rnˆ =0. 4 Alternatively, we can choose a perfectly aligned orientational distribution f ˆ = ˆ ẑ. 5 Clearly, in this case there is only one solution to be determined ẑ;r r, and the boundary conditions 14 and 1 become Rnˆ = Rnˆ, 6 7 nˆ Rnˆ = nˆ Rnˆ. 8 Evidently, the choice of a perfectly aligned orientational distribution leads to periodic boundary conditions, where each position Rnˆ on the cell surface is identified with position Rnˆ. V. APPLICATION TO LINEARIZED POISSON- BOLTZMANN THEORY To solve the full nonlinear problem is possible numerically, but it turns out to be very involved 16. Therefore, we restrict ourselves to the linearized version of Poisson- Boltzmann theory. In this case, the nonlinear right-hand side of the PB equation is linearized around a certain value. We denote it by 0, such that the linearized Poisson-Boltzmann LPB equation is given by out ˆ ;r = cosh 0 out ˆ ;r 0 sinh 0. 9 In some cases, the value for 0 is chosen to be zero. This choice is meaningful if the concentration of colloids, as well as the total surface charge density, is low. Alternatively, its value can be set to the isotropically averaged value of the potential at the cell boundary. This choice is particularly useful when one has the boundary values of the potential and the electric field from numerical calculations of the nonlinear PB equation 16. These can be used to fit renormalized charge distributions on the particle surface using the expression in Eq. 33. Lastly, one can apply the Donnan potential as the value around which to perform the linearization. This value requires no other input than the colloid concentration, its particle radius, its total surface charge, and the reservoir salt concentration 14. In this paper, we leave 0 unspecified. Inside the colloidal particle still satisfies the Laplace equation. It is natural in this case to expand both the inner and the outer solutions in spherical harmonics. This leads to two sets of coefficients, which have to be matched at the particle surface. Inside the particle in ˆ ;r = =0 m= whereas in the electrolyte out ˆ ;r = 0 tanh 0 A,m ˆ r Y,m,, =0 m= B,m ˆ i r 30 C,m ˆ k ry,m,, 31 where = cosh 0, and i and k are the modified spherical Bessel functions of order of the first and second kinds, respectively. The boundary condition on the particle surface is given by Eqs. 1 and 13, where we decompose the charge distribution as 1 ˆ ;nˆ = =0 4 P ˆ nˆ. 3 Next, we impose the boundary conditions at the cell surface, which are given in Eqs. 14 and 1. Together, this yields the general solution for the dimensionless electrostatic potential in the cell interior out ˆ ;r = 0 tanh 0 where PHYSICAL REVIEW E 80, l B 1 1 =0 ; a, R k ri R i rk RP ˆ rˆ l B 1 =0 even 1 ; a, R R ; a, R ; a, r dˆ fˆ P ˆ rˆ, ; a, R k a a k ai R i a a i ak R, ; a, R ; a, R. 35 R The details of the derivation of the expression in Eq. 33 can be found in the Appendix. Note that the first sum over all does not depend on the ODF, whereas it does depend on the particle orientation ˆ. This contribution to the potential is purely due to the particle at the center of the cell, and it
5 POISSON-BOLTZMANN CELL MODEL FOR vanishes at the cell boundary. Conversely, the second sum over even does not depend on the particle orientation, whereas it does depend on the ODF. This means that it describes the effect of all the surrounding particles. Moreover, it vanishes in the limit of infinite dilution R. The thermodynamic potential for the ion distribution in a single cell is given by 14 cell ˆ = sout dr out ˆ ;rsinh out ˆ ;r cosh out ˆ ;r a dnˆ ˆ ;nˆ out ˆ ;anˆ, 36 where the label out at the integral symbol denotes integration over the cell interior i.e., the domain of out, and s is the reservoir salt concentration such that =8l B s.we cannot evaluate this expression analytically. Therefore, we linearize it around 0 to find where cell ˆ 0 iso int, R3 a 3 s 0 sinh 0 cosh 0, iso s 0 cosh 0 sinh 0 out dr out ˆ ;r 0, int a dnˆ ˆ ;nˆ out ˆ ;anˆ It turns out that int depends on the particle orientation and the ODF, whereas the other two terms depend on neither. The expression we obtain can be derived through another route, by expanding the original nonlinear functional of the ion profiles ˆ ;r up to second order with respect to a density,0 = s exp 0. Minimizing this functional with respect to the ion profiles yields the LPB equation 9, and the accompanying expressions for the ion profiles ˆ ;r,0 1 0 out ˆ ;r. Substitution of this expression into the functional yields Eq. 37. VI. ONSAGER-LIKE SECOND-ORDER DENSITY- FUNCTIONAL THEORY We now approximate the total free energy per colloidal particle of the system by averaging over all particle orientations. Also, we add an entropic contribution, which is analogous to mixing entropy Ff N dˆ fˆ ln4fˆ dˆ fˆ cell ˆ. 41 We neglect the translational entropic contributions of the colloidal particles because we are only interested in the effects of charge anisotropy and orientational distribution. Using the identity dnˆ ˆ ;nˆ P ˆ nˆ = P ˆ ˆ, 4 which can be easily derived from Eq. 3 using the addition theorem, we derive the following expression for the free energy: where and Ff N F 0 N dˆ fˆ ln4fˆ 1 dˆ fˆ dˆ fˆ Kˆ,ˆ, 43 F 0 N 0 0 tanh 0 Kˆ,ˆ PHYSICAL REVIEW E 80, a R3 a 3 s sinh 0 a 0 0 tanh 0 a l B 1 1 =0 ; a, R k ai R i ak R, l B 5R =0 even 1 P ˆ ˆ 44 ; a, R ; a, R. 45 Note that the second term of F 0 /N vanishes if one chooses the Donnan potential for the value of 0. Also, the entire contribution from F 0 /N vanishes upon taking the functional derivative with respect to fˆ. The structure of the free-energy functional 43 is remarkably similar to that of Onsager s second-virial theory for hard rods 13. In the case of spherocylinders, the kernel Kˆ,ˆ stems from hard-core interactions, and it is equal to the product of the rod density and the orientation-dependent excluded volume of one rod in the vicinity of another, Kˆ,ˆ = N V 4 3 D3 L D L D sin, 46 where L is the rod length, D is the rod diameter. The angle 0, between the two rod orientations is defined by cos ˆ ˆ. Onsager s second-virial theory predicts the existence of an isotropicnematic transition caused by the competition between orientational and translational entropy. In the low-density isotropic phase, the mixing term dˆ fˆ ln4fˆ is minimized by an isotropic orienta
6 EELCO EGGEN AND RENÉ VAN ROIJ PHYSICAL REVIEW E 80, K(γ) R =3a R =3.1a R =4a σ(θ)/σ0 4 purely quadrupolar distribution, which reflects in the fact that the kernel has the same orientational dependence. In both cases, the kernel has a minimum at =/. Consequently, we expect no isotropicnematic transition. In the next section, we argue that this conclusion holds for any choice of parameters. VII. BIFURCATION THEORY The ODF that minimizes the free energy 43 obeys the Euler-Lagrange equation (a) K(γ) (b) π 0 0 π R =3a R =3.1a R =4a tional distribution, whereas the contribution due to the average excluded volume 1 dˆ fˆ dˆ fˆ Kˆ,ˆ is minimized in the high-density nematic phase. This transition only occurs if the length-to-diameter ratio L/D is large, such that the kernel Kˆ,ˆ is sufficiently anisotropic, with a maximum at =/. In the limit LD, the description by Onsager is quantitative. In the present case, the kernel stems from anisotropic electrostatic interactions, and we will investigate if these can give rise to such a symmetry-breaking transition. In both cases, the kernel is rotationally invariant, i.e., it only depends on the mutual relative orientation of the unit vectors ˆ and ˆ through the dot product ˆ ˆ. Figure 3 shows the values of the kernel Kˆ,ˆ for two distinct surface charge distributions ˆ,nˆ. The angle between the axis of symmetry and the position vector on the surface is defined by cos ˆ nˆ. The top graph shows a highly peaked distribution around =0 and = in the inset. However, the kernel is much less anisotropic in this case. The lower graph has a γ 0. 0 π 0 π FIG. 3. The kernel Kˆ,ˆ for different values of the cell radius R, and two distinct charge distributions. We fixed the values a=1, =1, and l B =0.01. The inset in each graph shows the corresponding charge distribution as a function of the angle between the axis of symmetry and the position vector on the surface. The charge distribution in the top graph is scaled with 0 =10 3, whereas the scaling in the lower graph is given by =10 3. σ(θ)/σ γ 0. 0 θ θ ln4fˆ = dˆ fˆ Kˆ,ˆ, 47 where we introduced the Lagrange multiplier to ensure the normalization of f given by Eq. 16. The Euler-Lagrange equation can be rewritten in a form that always satisfies this normalization exp dˆ fˆ Kˆ,ˆ fˆ =. 48 dˆ exp dˆ fˆ Kˆ,ˆ One easily checks that f iso ˆ =1/4 is a solution of Eq. 48, describing the perfectly isotropic phase. Due to its nonlinear character, one can expect additional anisotropic solutions to this equation. Finding explicit expressions for these solutions, however, is difficult; although good insight can be obtained from a bifurcation analysis. The goal of this analysis is to determine if and for what parameters an instability can be found in the reference solution with respect to a perturbation. We choose the isotropic ODF as a reference and expand around this solution by writing fˆ =1/4fˆ, with f a small deviation. Following the same scheme as Kayser and Raveché 17 applied to Onsager s model of hard rods, which was extended by Mulder 18, we find the bifurcation equation fˆ = dˆ Kˆ,ˆ fˆ Kfˆ. 49 This is an eigenvalue equation, for which a nontrivial solution exists if the integral operator K has eigenvalues 1. The parameter value for which this occurs is called the bifurcation point, where an anisotropic solution branches off from the isotropic reference solution. The solution to the bifurcation equation 49 can be given in terms of eigenfunctions of K. On the basis of rotational-symmetry arguments, we find that these eigenfunctions are the Legendre polynomials of the dot product of the orientation ˆ with respect to an arbitrary direction, dˆ Kˆ,ˆ P ˆ ẑ = P ˆ ẑ, 50 where the eigenvalues follow from Eq. 45,
7 POISSON-BOLTZMANN CELL MODEL FOR = l B 4 5a R for even ; a, R ; a, R 0 for odd. 51 The bifurcation point is determined by = 1. However, all eigenvalues are positive. Therefore, the bifurcation equation 49 has no solution, and there is no bifurcation point. The understanding of the origin of this property of the coefficients lies in the fact that both ; a, R and ; a, R approach their minimum in the limit R a. Moreover, these limits are non-negative since lim ; a, R = 1 R a a, 5 lim ; a, R = R a 3a The breaking of orientational symmetry if it exists cannot be captured using bifurcation theory in this version of the Poisson-Boltzmann cell model. It is due to the local stability of the isotropic solution that holds for all values of the parameters. However, strictly speaking, this does not exclude another globally stable solution. In principle, the Euler-Lagrange equation 48 can be solved numerically for example, by an iteration procedure for any given charge distribution. Alternatively, it can be rewritten as a nonlinear system of equations for the expansion coefficients f of the ODF, fˆ = 1 4 f P ˆ ẑ. 54 Using the eigenvalues to rewrite the Euler-Lagrange equation, we obtain f P ˆ ẑ f = dˆ P ˆ ẑexp dˆ exp f P ˆ ẑ. 55 All solutions satisfy f 0 =1, and f =0 for odd, independent of the choice of charge distribution. Additionally, if we impose the simple form PHYSICAL REVIEW E 80, Kˆ,ˆ = 0 P ˆ ˆ, 56 then the right-hand side of Eq. 55 can be calculated analytically for any given value of. The resulting expression for = is a function of the product f, which we denote by gf. It has the property that gt0 for t0, gt 0 for t0, and g0=0. Hence, if 0, the only solution of the equation f =gf is given by f =0. Consequently, the right-hand side of Eq. 55 is identically zero for all 0. In other words, for the simple kernel 56, we can rigorously show that the isotropic reference solution is the only possible solution. On the basis of physical arguments, we expect but cannot proof rigorously that anisotropic solutions do not exist either for kernels with higher-order contributions with positive amplitudes. The results in this section strongly suggest that there must be positional order before there can be orientational ordering in suspensions of Janus or other patchy particles. In other words, we do not expect liquid-crystal phases, and the transition from an isotropic state to a fully ordered crystal phase if it exists may well be intermitted by a plasticcrystal phase. Our simple cell model, however, does not take into account the positional correlations of the plastic-crystal phase, due to the mean-field nature of the applied boundary conditions at the cell surface. VIII. CONCLUSION AND OUTLOOK We developed a simple cell model in the context of Poisson-Boltzmann theory for heterogeneously charged colloidal spheres. The boundary conditions on the colloid surface as well as on the Wigner-Seitz cell surface depend on the charge heterogeneity and the orientational distribution of the colloidal particles. Within a linear approximation to Poisson-Boltzmann theory, these boundary conditions give rise to a free-energy functional of the orientational distribution function fˆ that is very similar to the one used in Onsager s second-order virial approximation in the description of the isotropicnematic transition of hard rods 13. The present description, however, does not give rise to orientational ordering. Since our model treats the position of the colloids in a mean-field description and since we do expect some degree of orientational ordering at sufficiently high particle density this result suggests that electrostaticsinduced orientational ordering requires the existence of positional ordering. Therefore, we predict no orientational ordering in fluids of these particles, i.e., no liquid-crystal phases. The present theory, however, is based on a number of assumptions that must be addressed, and some caution is advised in the interpretation of this result. First of all, we do not expect that the choice of linearized Poisson-Boltzmann theory has a significant effect on our conclusions, although it is known that nonlinear screening affects the long-range orientation dependence of the electrostatic potential around heterogeneously charged colloidal particles 16. The reason for this is that our conclusions arise from the boundary conditions on the cell surface. In the case of charge renormalization, this linearization is applied to fit an effective charge, which describes the asymptotic behavior correctly. Therefore, all conclusions in this paper should still hold, when the charge distribution is replaced by an effective renormalized one. Furthermore, in the case of a high surface charge of dipolar character, the conclusion that there is no orientational alignment can be rather counterintuitive. Experimental evidence of this alignment always coincides with the formation of chains 19,0, with a polarity pointing in the direction along the chain, due to the dipole moment of the constituents. However, such chainlike structures have a strong orientationposition correlation that is not captured by the cell model as formulated here. Our prediction of the absence of liquid-crystal phases in these systems is consistent with
8 EELCO EGGEN AND RENÉ VAN ROIJ the types of phases found in simulations of dipolar particles such as isotropic-fluid, string-fluid, and gel phases at low density, and face-centered-cubic, hexagonal-close-packed, and body-centered-tetragonal solid phases at high density 1. Indeed, no liquid-crystal phases were found. Finally, we showed it to be impossible to find solutions that branch off from the isotropic reference solution, using bifurcation theory, because this reference solution fails to become unstable. Hypothetically, stable anisotropic solutions that do not connect to the metastable reference solution might still exist. However, our model belongs to the class of models investigated by Mulder 18 for which this type of solution has not been found. Our explicit analysis of the simple case in Sec. VII concerning a charge distribution consisting of a monopole and quadrupole charge only supports this conclusion. A potentially serious shortcoming of our model is the mean-field treatment of the colloidal particles. The present model does not include any positional or orientational correlations. The nature of these correlations can be related in a simple way to systems of oppositely charged colloidal particles,3. The number of bonds between oppositely charged particles in these systems depends on the colloid density. Also, for the dense liquid phase coexisting with a dilute vapor phase provided the Debye screening length is large enough the pair distribution function shows that a colloidal particle is surrounded by different layers of colloidal species with alternating signs of charge. The first surrounding layer has an opposite charge with respect to the particle in the origin; the next layer is like charged and so on. These systems also display multiple crystal structures, which have different coordination number. The same notion can be applied to particles of different orientations to include orientational pair correlations in the cell model. One could consider to expand the class of Poisson- Boltzmann cell models by incorporating a description of these correlations. More specifically, one can choose a different approach to the way that the surface potential R is determined. In the present models, this potential is independent of the colloidal species or orientation to which the cell belongs. Also, each colloidal species or orientation has an equivalent weight equal to its molar fraction or value of the ODF in the average of the potential and electric field flux at the cell boundary. This property is due to the meanfield description, which is used, through the assumption that the surrounding of a particle at the cell boundary is independent from the species it belongs to, or equivalently, its orientation. However, if this restriction is lifted, one may include the fact that the surroundings do depend on this property through the pair distribution function. Additionally, a jellium approximation can be applied in the same way it is applied to monodisperse systems of homogeneously charged colloidal spheres and rods 4,5. In this description, there is no need for a certain cell shape and volume. Moreover, the jellium model has a natural way to include particle pair correlations 6,7. Finally, there is an opportunity to apply the Poisson-Boltzmann cell model to nonspherical cells 8. The boundary conditions can be imposed in the same way as in this paper. However, this complicates the expression of the appropriate boundary conditions since a nonspherical shape will couple different spherical harmonic modes. The shape of these cells must be controlled by additional constraints such as the minimization of free energy. Also, the choice of shapes must be motivated by physical arguments. We leave these options for future studies. APPENDIX: DERIVATION OF THE ELECTROSTATIC POTENTIAL Inside the colloidal particle ˆ ;r satisfies the Laplace equation, whereas in the electrolyte it satisfies the LPB equation. Therefore, we have to match two general solutions, using the boundary condition on the particle surface. We do this by expanding both solutions in spherical harmonics. These expressions are given in Eqs. 30 and 31. We apply the boundary condition on the particle surface given by Eqs. 1 and 13. To this end, we expand the surface charge distribution in spherical harmonics, using Eq. 3 and the addition theorem, ˆ ;nˆ = PHYSICAL REVIEW E 80, =0 m= Y,m ˆ Y,m nˆ. A1 The arguments nˆ and ˆ of the spherical harmonic function should be interpreted as the pair of spherical angles of this orientation with respect to the reference frame. Consequently, from the boundary conditions 1 and 13, weobtain the following condition on the coefficients of out : B,m ˆ i a a a i C,m ˆ k a a a k = 4l B 1 Y,m ˆ. A Next, we apply the boundary conditions at the cell surface given in Eqs. 14 and 1. This yields a linear system of equations, which can be solved analytically. However, we can choose to split the solution into two contributions. The first contribution then satisfies the boundary conditions on the particle surface given by Eq. A as well as the condition that the potential vanishes at the cell boundary. This is already the relevant boundary condition for all odd contributions to ˆ ;r, whereas a second contribution must be added later to the even contributions in order to satisfy the full set of boundary conditions. The coefficients that belong to the first contribution will be denoted by B,m ˆ and C,m ˆ. First, we impose the vanishing potential at the cell boundary by B,m ˆ i R C,m ˆ k R =0. Together with Eq. A, this yields B,m ˆ = 4l B 1 Y,m ˆ k R, ; a, R A3 A4 C,m ˆ = 4l B 1 Y,m ˆ i R, A5 ; a, R where ; a, R is defined in Eq. 34. The orientational dependence of this first contribution is such that it and
9 POISSON-BOLTZMANN CELL MODEL FOR therefore all odd contributions only depends on the angle between ˆ and rˆ, odd ˆ ;r = l B 1 =1 odd 1 P ˆ rˆ k ri R i rk R. ; a, R A6 As previously mentioned, a second contribution must be added to the coefficients of the even contributions. With this contribution included, the solution ˆ ;r satisfies the full set of boundary conditions on the cell surface given in Eqs. 14 and 1. We denote the coefficients of this secondary contribution by B,m and C,m. Also, we show that these do not depend on the particle orientation because the two distinct boundary conditions that govern them do not. First, the boundary condition A is already satisfied by the coefficients B,m ˆ and C,m ˆ. Therefore, B,mi a a a i C,mk a a a k =0. A7 Second, the boundary condition 14 imposes a value on the coefficients that only depends on the value of the potential at the cell surface, which is necessarily independent of the orientation ˆ. Hence, B,m i R C,mk R =,m, where,m is defined by R nˆ = 0 tanh 0 =0 even m=,m Y,m nˆ. Together, these conditions yield B,m = k a a a k,m ; a, R, A8 A9 A10 C,m =i a a a i,m ; a, R. A11 Finally, the boundary condition 1 imposes a vanishing value of the weighted average of the even contributions to the electric field flux at the cell boundary. This condition can be expressed in terms of a relation between the coefficients B,m, C,m, B,m, and C,m. By substituting the values given in Eqs. A4 and A5, we arrive at B,m i R C,mk R = dˆ fˆ B,m ˆ i R C,m ˆ k R 4l B 1 = ; a, R R dˆ fˆ Y,m ˆ where we used for even, A1 k Ri R i Rk R = 1 R, A13 which can be derived from standard identities for the modified spherical Bessel functions. The conditions in Eqs. A7 and A1 are sufficient to derive similar expressions for B,m and C,m. However, the construction we use to derive the solution to the even contributions enables us to show that the cell surface potential R nˆ has the same symmetry properties as the ODF in addition to the fact that it is composed purely of even contributions, 4l B 1,m = ; a, R R dˆ fˆ Y,m ˆ, A14 where ; a, R is defined in Eq. 35. With this, we readily obtain the even contributions even ˆ ;r = l B 1 =0 even 1 ; a, Rk ri R i rk RP ˆ rˆ PHYSICAL REVIEW E 80, ; a, r ; a, R R dˆ fˆ P ˆ rˆ, A15 and we obtain the general solution for the dimensionless electrostatic potential in the cell interior given in Eq R. A. Marcus, J. Chem. Phys. 3, T. Ohtsuki, S. Mitaku, and K. Okano, Jpn. J. Appl. Phys. 17, S. Alexander, P. M. Chaikin, P. Grant, G. J. Morales, P. Pincus, and D. Hone, J. Chem. Phys. 80, E. Wigner and F. Seitz, Phys. Rev. 43, H. H. von Grünberg and L. Belloni, Phys. Rev. E 6, P. M. Biesheuvel, S. Lindhoud, R. de Vries, and M. A. Cohen Stuart, Langmuir, A. Torres, G. Téllez, and R. van Roij, J. Chem. Phys. 18,
10 EELCO EGGEN AND RENÉ VAN ROIJ 8 J.-P. Hansen and E. Trizac, Physica A 35, L. Bocquet, E. Trizac, and M. Aubouy, J. Chem. Phys. 117, S. C. Glotzer and M. J. Solomon, Nature Mater. 6, A. Walther and A. H. E. Müller, Soft Matter 4, L. Hong, A. Cacciuto, E. Luijten, and S. Granick, Nano Lett. 6, L. Onsager, Ann. N.Y. Acad. Sci. 51, H. H. von Grünberg, R. van Roij, and G. Klein, Europhys. Lett. 55, E. Trizac, L. Bocquet, M. Aubouy, and H.-H. von Grünberg, Langmuir 19, N. Boon, E. Carvajal Gallardo, S. Zhang, E. Eggen, M. Dijkstra, and R. van Roij, e-print arxiv: R. F. Kayser, Jr., and H. J. Raveché, Phys. Rev. A 17, B. M. Mulder, Phys. Rev. A 39, Z. Tang, N. A. Kotov, and M. Giersig, Science 97, PHYSICAL REVIEW E 80, S. C. Glotzer, M. J. Solomon, and N. A. Kotov, AIChE J. 50, A. Goyal, C. K. Hall, and O. D. Velev, Phys. Rev. E 77, J. B. Caballero, A. M. Puertas, A. Fernández-Barbero, and F. J. de las Nieves, J. Chem. Phys. 11, M. E. Leunissen, C. G. Christova, A.-P. Hynninen, C. P. Royall, A. I. Campbell, A. Imhof, M. Dijkstra, R. van Roij, and A. van Blaaderen, Nature London 437, E. Trizac and Y. Levin, Phys. Rev. E 69, S. Pianegonda, E. Trizac, and Y. Levin, J. Chem. Phys. 16, R. Castañeda-Priego, L. F. Rojas-Ochoa, V. Lobaskin, and J. C. Mixteco-Sánchez, Phys. Rev. E 74, T. E. Colla, Y. Levin, and E. Trizac, J. Chem. Phys. 131, H. Graf, H. Löwen, and M. Schmidt, Prog. Colloid Polym. Sci. 104,
Effective charge saturation in colloidal suspensions
 JOURNAL OF CHEMICAL PHYSICS VOLUME 117, NUMBER 17 1 NOVEMBER 2002 Effective charge saturation in colloidal suspensions Lydéric Bocquet Laboratoire de Physique de l E.N.S. de Lyon, UMR CNRS 5672, 46 Allée
JOURNAL OF CHEMICAL PHYSICS VOLUME 117, NUMBER 17 1 NOVEMBER 2002 Effective charge saturation in colloidal suspensions Lydéric Bocquet Laboratoire de Physique de l E.N.S. de Lyon, UMR CNRS 5672, 46 Allée
V. Electrostatics Lecture 24: Diffuse Charge in Electrolytes
 V. Electrostatics Lecture 24: Diffuse Charge in Electrolytes MIT Student 1. Poisson-Nernst-Planck Equations The Nernst-Planck Equation is a conservation of mass equation that describes the influence of
V. Electrostatics Lecture 24: Diffuse Charge in Electrolytes MIT Student 1. Poisson-Nernst-Planck Equations The Nernst-Planck Equation is a conservation of mass equation that describes the influence of
Attraction or repulsion between charged colloids? A connection with Debye Hückel theory
 J. Phys.: Condens. Matter 12 (2000) A263 A267. Printed in the UK PII: S0953-8984(00)07724-9 Attraction or repulsion between charged colloids? A connection with Debye Hückel theory René van Roij H H Wills
J. Phys.: Condens. Matter 12 (2000) A263 A267. Printed in the UK PII: S0953-8984(00)07724-9 Attraction or repulsion between charged colloids? A connection with Debye Hückel theory René van Roij H H Wills
1 Introduction. Green s function notes 2018
 Green s function notes 8 Introduction Back in the "formal" notes, we derived the potential in terms of the Green s function. Dirichlet problem: Equation (7) in "formal" notes is Φ () Z ( ) ( ) 3 Z Φ (
Green s function notes 8 Introduction Back in the "formal" notes, we derived the potential in terms of the Green s function. Dirichlet problem: Equation (7) in "formal" notes is Φ () Z ( ) ( ) 3 Z Φ (
arxiv:cond-mat/ v1 [cond-mat.soft] 9 Aug 1997
![arxiv:cond-mat/ v1 [cond-mat.soft] 9 Aug 1997 arxiv:cond-mat/ v1 [cond-mat.soft] 9 Aug 1997](/thumbs/89/99391124.jpg) Depletion forces between two spheres in a rod solution. K. Yaman, C. Jeppesen, C. M. Marques arxiv:cond-mat/9708069v1 [cond-mat.soft] 9 Aug 1997 Department of Physics, U.C.S.B., CA 93106 9530, U.S.A. Materials
Depletion forces between two spheres in a rod solution. K. Yaman, C. Jeppesen, C. M. Marques arxiv:cond-mat/9708069v1 [cond-mat.soft] 9 Aug 1997 Department of Physics, U.C.S.B., CA 93106 9530, U.S.A. Materials
1. Poisson-Boltzmann 1.1. Poisson equation. We consider the Laplacian. which is given in spherical coordinates by (2)
 1. Poisson-Boltzmann 1.1. Poisson equation. We consider the Laplacian operator (1) 2 = 2 x + 2 2 y + 2 2 z 2 which is given in spherical coordinates by (2) 2 = 1 ( r 2 ) + 1 r 2 r r r 2 sin θ θ and in
1. Poisson-Boltzmann 1.1. Poisson equation. We consider the Laplacian operator (1) 2 = 2 x + 2 2 y + 2 2 z 2 which is given in spherical coordinates by (2) 2 = 1 ( r 2 ) + 1 r 2 r r r 2 sin θ θ and in
Physics 127b: Statistical Mechanics. Landau Theory of Second Order Phase Transitions. Order Parameter
 Physics 127b: Statistical Mechanics Landau Theory of Second Order Phase Transitions Order Parameter Second order phase transitions occur when a new state of reduced symmetry develops continuously from
Physics 127b: Statistical Mechanics Landau Theory of Second Order Phase Transitions Order Parameter Second order phase transitions occur when a new state of reduced symmetry develops continuously from
Boundary. DIFFERENTIAL EQUATIONS with Fourier Series and. Value Problems APPLIED PARTIAL. Fifth Edition. Richard Haberman PEARSON
 APPLIED PARTIAL DIFFERENTIAL EQUATIONS with Fourier Series and Boundary Value Problems Fifth Edition Richard Haberman Southern Methodist University PEARSON Boston Columbus Indianapolis New York San Francisco
APPLIED PARTIAL DIFFERENTIAL EQUATIONS with Fourier Series and Boundary Value Problems Fifth Edition Richard Haberman Southern Methodist University PEARSON Boston Columbus Indianapolis New York San Francisco
On the Chemical Free Energy of the Electrical Double Layer
 1114 Langmuir 23, 19, 1114-112 On the Chemical Free Energy of the Electrical Double Layer Marian Manciu and Eli Ruckenstein* Department of Chemical Engineering, State University of New York at Buffalo,
1114 Langmuir 23, 19, 1114-112 On the Chemical Free Energy of the Electrical Double Layer Marian Manciu and Eli Ruckenstein* Department of Chemical Engineering, State University of New York at Buffalo,
arxiv: v1 [cond-mat.soft] 3 Feb 2011
![arxiv: v1 [cond-mat.soft] 3 Feb 2011 arxiv: v1 [cond-mat.soft] 3 Feb 2011](/thumbs/87/96173053.jpg) Jamming of hard rods I: From Onsager to Edwards Maximilien Danisch 1,2, Adrian Baule 2, and Hernan A. Makse 2 1 Physics Department, Ecole Normale Supérieure de Cachan, 61 Avenue du President Wilson, 94235
Jamming of hard rods I: From Onsager to Edwards Maximilien Danisch 1,2, Adrian Baule 2, and Hernan A. Makse 2 1 Physics Department, Ecole Normale Supérieure de Cachan, 61 Avenue du President Wilson, 94235
Contents. 1 Basic Equations 1. Acknowledgment. 1.1 The Maxwell Equations Constitutive Relations 11
 Preface Foreword Acknowledgment xvi xviii xix 1 Basic Equations 1 1.1 The Maxwell Equations 1 1.1.1 Boundary Conditions at Interfaces 4 1.1.2 Energy Conservation and Poynting s Theorem 9 1.2 Constitutive
Preface Foreword Acknowledgment xvi xviii xix 1 Basic Equations 1 1.1 The Maxwell Equations 1 1.1.1 Boundary Conditions at Interfaces 4 1.1.2 Energy Conservation and Poynting s Theorem 9 1.2 Constitutive
Melting line of charged colloids from primitive model simulations
 THE JOURNAL OF CHEMICAL PHYSICS 123, 244902 2005 Melting line of charged colloids from primitive model simulations Antti-Pekka Hynninen a and Marjolein Dijkstra Soft Condensed Matter Group, Debye Institute,
THE JOURNAL OF CHEMICAL PHYSICS 123, 244902 2005 Melting line of charged colloids from primitive model simulations Antti-Pekka Hynninen a and Marjolein Dijkstra Soft Condensed Matter Group, Debye Institute,
Effective theory of quadratic degeneracies
 Effective theory of quadratic degeneracies Y. D. Chong,* Xiao-Gang Wen, and Marin Soljačić Department of Physics, Massachusetts Institute of Technology, Cambridge, Massachusetts 02139, USA Received 28
Effective theory of quadratic degeneracies Y. D. Chong,* Xiao-Gang Wen, and Marin Soljačić Department of Physics, Massachusetts Institute of Technology, Cambridge, Massachusetts 02139, USA Received 28
Here ρ(φ) is a probability density of rod orientations (ρ 0 with total integral one). We assume that the interaction potential U(φ) is given by,
 COMM. MATH. SCI. Vol. 3, No. 1, pp. 21 26 c 25 International Press A NOTE ON THE ONSAGER MODEL OF NEMATIC PHASE TRANSITIONS I. FATKULLIN AND V. SLASTIKOV Abstract. We study Onsager s free energy functional
COMM. MATH. SCI. Vol. 3, No. 1, pp. 21 26 c 25 International Press A NOTE ON THE ONSAGER MODEL OF NEMATIC PHASE TRANSITIONS I. FATKULLIN AND V. SLASTIKOV Abstract. We study Onsager s free energy functional
1. (3) Write Gauss Law in differential form. Explain the physical meaning.
 Electrodynamics I Midterm Exam - Part A - Closed Book KSU 204/0/23 Name Electro Dynamic Instructions: Use SI units. Where appropriate, define all variables or symbols you use, in words. Try to tell about
Electrodynamics I Midterm Exam - Part A - Closed Book KSU 204/0/23 Name Electro Dynamic Instructions: Use SI units. Where appropriate, define all variables or symbols you use, in words. Try to tell about
Phase diagram for stimulus-responsive materials containing dipolar colloidal particles
 Phase diagram for stimulus-responsive materials containing dipolar colloidal particles Amit Goyal, Carol K. Hall,* and Orlin D. Velev Department of Chemical and Bimolecular Engineering, North Carolina
Phase diagram for stimulus-responsive materials containing dipolar colloidal particles Amit Goyal, Carol K. Hall,* and Orlin D. Velev Department of Chemical and Bimolecular Engineering, North Carolina
APPLIED PARTIM DIFFERENTIAL EQUATIONS with Fourier Series and Boundary Value Problems
 APPLIED PARTIM DIFFERENTIAL EQUATIONS with Fourier Series and Boundary Value Problems Fourth Edition Richard Haberman Department of Mathematics Southern Methodist University PEARSON Prentice Hall PEARSON
APPLIED PARTIM DIFFERENTIAL EQUATIONS with Fourier Series and Boundary Value Problems Fourth Edition Richard Haberman Department of Mathematics Southern Methodist University PEARSON Prentice Hall PEARSON
Ideality in Granular Mixtures: Random Packing of Non-Spherical Particles
 Ideality in Granular Mixtures: Random Packing of Non-Spherical Particles Andriy Kyrylyuk Van t Hoff Lab for Physical and Colloid Chemistry, Utrecht University, The Netherlands Outline Motivation. Spheres:
Ideality in Granular Mixtures: Random Packing of Non-Spherical Particles Andriy Kyrylyuk Van t Hoff Lab for Physical and Colloid Chemistry, Utrecht University, The Netherlands Outline Motivation. Spheres:
E&M. 1 Capacitors. January 2009
 E&M January 2009 1 Capacitors Consider a spherical capacitor which has the space between its plates filled with a dielectric of permittivity ɛ. The inner sphere has radius r 1 and the outer sphere has
E&M January 2009 1 Capacitors Consider a spherical capacitor which has the space between its plates filled with a dielectric of permittivity ɛ. The inner sphere has radius r 1 and the outer sphere has
Peculiarities of first-order phase transitions in the presence of an electric field
 Peculiarities of first-order phase transitions in the presence of an electric field Yu. Dolinsky* and T. Elperin The Pearlstone Center for Aeronautical Engineering Studies Department of Mechanical Engineering
Peculiarities of first-order phase transitions in the presence of an electric field Yu. Dolinsky* and T. Elperin The Pearlstone Center for Aeronautical Engineering Studies Department of Mechanical Engineering
Nematic order of model goethite nanorods in a magnetic field
 Nematic order of model goethite nanorods in a magnetic field H. H. Wensink and G. J. Vroege* Van t Hoff Laboratory for Physical and Colloid Chemistry, Debye Institute, Utrecht University, Padualaan 8,
Nematic order of model goethite nanorods in a magnetic field H. H. Wensink and G. J. Vroege* Van t Hoff Laboratory for Physical and Colloid Chemistry, Debye Institute, Utrecht University, Padualaan 8,
Shear Flow of a Nematic Liquid Crystal near a Charged Surface
 Physics of the Solid State, Vol. 45, No. 6, 00, pp. 9 96. Translated from Fizika Tverdogo Tela, Vol. 45, No. 6, 00, pp. 5 40. Original Russian Text Copyright 00 by Zakharov, Vakulenko. POLYMERS AND LIQUID
Physics of the Solid State, Vol. 45, No. 6, 00, pp. 9 96. Translated from Fizika Tverdogo Tela, Vol. 45, No. 6, 00, pp. 5 40. Original Russian Text Copyright 00 by Zakharov, Vakulenko. POLYMERS AND LIQUID
5 Topological defects and textures in ordered media
 5 Topological defects and textures in ordered media In this chapter we consider how to classify topological defects and textures in ordered media. We give here only a very short account of the method following
5 Topological defects and textures in ordered media In this chapter we consider how to classify topological defects and textures in ordered media. We give here only a very short account of the method following
d 1 µ 2 Θ = 0. (4.1) consider first the case of m = 0 where there is no azimuthal dependence on the angle φ.
 4 Legendre Functions In order to investigate the solutions of Legendre s differential equation d ( µ ) dθ ] ] + l(l + ) m dµ dµ µ Θ = 0. (4.) consider first the case of m = 0 where there is no azimuthal
4 Legendre Functions In order to investigate the solutions of Legendre s differential equation d ( µ ) dθ ] ] + l(l + ) m dµ dµ µ Θ = 0. (4.) consider first the case of m = 0 where there is no azimuthal
1. (3) Write Gauss Law in differential form. Explain the physical meaning.
 Electrodynamics I Midterm Exam - Part A - Closed Book KSU 204/0/23 Name Instructions: Use SI units. Where appropriate, define all variables or symbols you use, in words. Try to tell about the physics involved,
Electrodynamics I Midterm Exam - Part A - Closed Book KSU 204/0/23 Name Instructions: Use SI units. Where appropriate, define all variables or symbols you use, in words. Try to tell about the physics involved,
free space (vacuum) permittivity [ F/m]
![free space (vacuum) permittivity [ F/m] free space (vacuum) permittivity [ F/m]](/thumbs/79/79624275.jpg) Electrostatic Fields Electrostatic fields are static (time-invariant) electric fields produced by static (stationary) charge distributions. The mathematical definition of the electrostatic field is derived
Electrostatic Fields Electrostatic fields are static (time-invariant) electric fields produced by static (stationary) charge distributions. The mathematical definition of the electrostatic field is derived
Where do ions solvate?
 PRAMANA c Indian Academy of Sciences Vol. 64, No. 6 journal of June 25 physics pp. 957 961 YAN LEVIN Instituto de Física, Universidade Federal do Rio Grande do Sul, Caixa Postal 1551, CEP 9151-97, Porto
PRAMANA c Indian Academy of Sciences Vol. 64, No. 6 journal of June 25 physics pp. 957 961 YAN LEVIN Instituto de Física, Universidade Federal do Rio Grande do Sul, Caixa Postal 1551, CEP 9151-97, Porto
Theory of domain patterns in systems with long-range interactions of Coulomb type
 PHYSICAL REVIEW E 66, 6618 22 Theory of domain patterns in systems with long-range interactions of Coulomb type C. B. Muratov* Department of Mathematical Sciences, New Jersey Institute of Technology, Newark,
PHYSICAL REVIEW E 66, 6618 22 Theory of domain patterns in systems with long-range interactions of Coulomb type C. B. Muratov* Department of Mathematical Sciences, New Jersey Institute of Technology, Newark,
ON ALGORITHMS FOR BROWNIAN DYNAMICS COMPUTER SIMULATIONS
 COMPUTATIONAL METHODS IN SCIENCE AND TECHNOLOGY 4,35-42 (1998) ON ALGORITHMS FOR BROWNIAN DYNAMICS COMPUTER SIMULATIONS ARKADIUSZ C. BRAŃKA Institute of Molecular Physics, Polish Academy of Sciences, Smoluchowskiego
COMPUTATIONAL METHODS IN SCIENCE AND TECHNOLOGY 4,35-42 (1998) ON ALGORITHMS FOR BROWNIAN DYNAMICS COMPUTER SIMULATIONS ARKADIUSZ C. BRAŃKA Institute of Molecular Physics, Polish Academy of Sciences, Smoluchowskiego
PARTIAL DIFFERENTIAL EQUATIONS and BOUNDARY VALUE PROBLEMS
 PARTIAL DIFFERENTIAL EQUATIONS and BOUNDARY VALUE PROBLEMS NAKHLE H. ASMAR University of Missouri PRENTICE HALL, Upper Saddle River, New Jersey 07458 Contents Preface vii A Preview of Applications and
PARTIAL DIFFERENTIAL EQUATIONS and BOUNDARY VALUE PROBLEMS NAKHLE H. ASMAR University of Missouri PRENTICE HALL, Upper Saddle River, New Jersey 07458 Contents Preface vii A Preview of Applications and
Gas-liquid phase separation in oppositely charged colloids: stability and interfacial tension
 7 Gas-liquid phase separation in oppositely charged colloids: stability and interfacial tension We study the phase behaviour and the interfacial tension of the screened Coulomb (Yukawa) restricted primitive
7 Gas-liquid phase separation in oppositely charged colloids: stability and interfacial tension We study the phase behaviour and the interfacial tension of the screened Coulomb (Yukawa) restricted primitive
Math 342 Partial Differential Equations «Viktor Grigoryan
 Math 342 Partial ifferential Equations «Viktor Grigoryan 3 Green s first identity Having studied Laplace s equation in regions with simple geometry, we now start developing some tools, which will lead
Math 342 Partial ifferential Equations «Viktor Grigoryan 3 Green s first identity Having studied Laplace s equation in regions with simple geometry, we now start developing some tools, which will lead
DRAFT. Analytical Model for the Effective Thermal Conductivity Matrix of Anisotropic Composites
 DRAFT Proceedings of IMECE 25 ASME 21 International Mechanical Engineering Congress & Exposition November 12-18 21, Vancouver, British Columbia Canada IMECE21-466 Analytical Model for the Effective Thermal
DRAFT Proceedings of IMECE 25 ASME 21 International Mechanical Engineering Congress & Exposition November 12-18 21, Vancouver, British Columbia Canada IMECE21-466 Analytical Model for the Effective Thermal
Theoretische Physik 2: Elektrodynamik (Prof. A-S. Smith) Home assignment 11
 WiSe 22..23 Prof. Dr. A-S. Smith Dipl.-Phys. Matthias Saba am Lehrstuhl für Theoretische Physik I Department für Physik Friedrich-Alexander-Universität Erlangen-Nürnberg Problem. Theoretische Physik 2:
WiSe 22..23 Prof. Dr. A-S. Smith Dipl.-Phys. Matthias Saba am Lehrstuhl für Theoretische Physik I Department für Physik Friedrich-Alexander-Universität Erlangen-Nürnberg Problem. Theoretische Physik 2:
*blood and bones contain colloids. *milk is a good example of a colloidal dispersion.
 Chap. 3. Colloids 3.1. Introduction - Simple definition of a colloid: a macroscopically heterogeneous system where one component has dimensions in between molecules and macroscopic particles like sand
Chap. 3. Colloids 3.1. Introduction - Simple definition of a colloid: a macroscopically heterogeneous system where one component has dimensions in between molecules and macroscopic particles like sand
Dielectrics - III. Lecture 22: Electromagnetic Theory. Professor D. K. Ghosh, Physics Department, I.I.T., Bombay
 Dielectrics - III Lecture 22: Electromagnetic Theory Professor D. K. Ghosh, Physics Department, I.I.T., Bombay We continue with our discussion of dielectric medium. Example : Dielectric Sphere in a uniform
Dielectrics - III Lecture 22: Electromagnetic Theory Professor D. K. Ghosh, Physics Department, I.I.T., Bombay We continue with our discussion of dielectric medium. Example : Dielectric Sphere in a uniform
3. General properties of phase transitions and the Landau theory
 3. General properties of phase transitions and the Landau theory In this Section we review the general properties and the terminology used to characterise phase transitions, which you will have already
3. General properties of phase transitions and the Landau theory In this Section we review the general properties and the terminology used to characterise phase transitions, which you will have already
Attraction between two similar particles in an electrolyte: effects of Stern layer absorption
 Attraction between two similar particles in an electrolyte: effects of Stern layer absorption F.Plouraboué, H-C. Chang, June 7, 28 Abstract When Debye length is comparable or larger than the distance between
Attraction between two similar particles in an electrolyte: effects of Stern layer absorption F.Plouraboué, H-C. Chang, June 7, 28 Abstract When Debye length is comparable or larger than the distance between
Gas-liquid phase separation in oppositely charged colloids: Stability and interfacial tension
 THE JOURNAL OF CHEMICAL PHYSICS 125, 094502 2006 Gas-liquid phase separation in oppositely charged colloids: Stability and interfacial tension Andrea Fortini, a Antti-Pekka Hynninen, b and Marjolein Dijkstra
THE JOURNAL OF CHEMICAL PHYSICS 125, 094502 2006 Gas-liquid phase separation in oppositely charged colloids: Stability and interfacial tension Andrea Fortini, a Antti-Pekka Hynninen, b and Marjolein Dijkstra
Spin Interactions. Giuseppe Pileio 24/10/2006
 Spin Interactions Giuseppe Pileio 24/10/2006 Magnetic moment µ = " I ˆ µ = " h I(I +1) " = g# h Spin interactions overview Zeeman Interaction Zeeman interaction Interaction with the static magnetic field
Spin Interactions Giuseppe Pileio 24/10/2006 Magnetic moment µ = " I ˆ µ = " h I(I +1) " = g# h Spin interactions overview Zeeman Interaction Zeeman interaction Interaction with the static magnetic field
Equilibrium orientation of an ellipsoidal particle inside a dielectric medium with a finite electric conductivity in the external electric field
 PHYSICAL REVIEW E 71, 056611 005 Equilibrium orientation of an ellipsoidal particle inside a dielectric medium with a finite electric conductivity in the external electric field Yu. Dolinsky* and T. Elperin
PHYSICAL REVIEW E 71, 056611 005 Equilibrium orientation of an ellipsoidal particle inside a dielectric medium with a finite electric conductivity in the external electric field Yu. Dolinsky* and T. Elperin
EQUATION LANGEVIN. Physics, Chemistry and Electrical Engineering. World Scientific. With Applications to Stochastic Problems in. William T.
 SHANGHAI HONG WorlrfScientific Series krtonttimfjorary Chemical Physics-Vol. 27 THE LANGEVIN EQUATION With Applications to Stochastic Problems in Physics, Chemistry and Electrical Engineering Third Edition
SHANGHAI HONG WorlrfScientific Series krtonttimfjorary Chemical Physics-Vol. 27 THE LANGEVIN EQUATION With Applications to Stochastic Problems in Physics, Chemistry and Electrical Engineering Third Edition
Phase transitions of quadrupolar fluids
 Phase transitions of quadrupolar fluids Seamus F. O Shea Department of Chemistry, University of Lethbridge, Lethbridge, Alberta, Canada, T1K 3M4 Girija S. Dubey Brookhaven National Laboratory, Upton, New
Phase transitions of quadrupolar fluids Seamus F. O Shea Department of Chemistry, University of Lethbridge, Lethbridge, Alberta, Canada, T1K 3M4 Girija S. Dubey Brookhaven National Laboratory, Upton, New
Problem Set #4: 4.1,4.7,4.9 (Due Monday, March 25th)
 Chapter 4 Multipoles, Dielectrics Problem Set #4: 4.,4.7,4.9 (Due Monday, March 5th 4. Multipole expansion Consider a localized distribution of charges described by ρ(x contained entirely in a sphere of
Chapter 4 Multipoles, Dielectrics Problem Set #4: 4.,4.7,4.9 (Due Monday, March 5th 4. Multipole expansion Consider a localized distribution of charges described by ρ(x contained entirely in a sphere of
4 Power Series Solutions: Frobenius Method
 4 Power Series Solutions: Frobenius Method Now the ODE adventure takes us to series solutions for ODEs, a technique A & W, that is often viable, valuable and informative. These can be readily applied Sec.
4 Power Series Solutions: Frobenius Method Now the ODE adventure takes us to series solutions for ODEs, a technique A & W, that is often viable, valuable and informative. These can be readily applied Sec.
Optical bistability in metal/dielectric composite with interfacial layer
 Physica B 301 (2001) 190}195 Optical bistability in metal/dielectric composite with interfacial layer T. Pan*, J.P. Huang, Z.Y. Li CCAST, World Laboratory, P.O. Box 8730, Beijing 100080, People's Republic
Physica B 301 (2001) 190}195 Optical bistability in metal/dielectric composite with interfacial layer T. Pan*, J.P. Huang, Z.Y. Li CCAST, World Laboratory, P.O. Box 8730, Beijing 100080, People's Republic
Notes: Most of the material presented in this chapter is taken from Jackson, Chap. 2, 3, and 4, and Di Bartolo, Chap. 2. 2π nx i a. ( ) = G n.
 Chapter. Electrostatic II Notes: Most of the material presented in this chapter is taken from Jackson, Chap.,, and 4, and Di Bartolo, Chap... Mathematical Considerations.. The Fourier series and the Fourier
Chapter. Electrostatic II Notes: Most of the material presented in this chapter is taken from Jackson, Chap.,, and 4, and Di Bartolo, Chap... Mathematical Considerations.. The Fourier series and the Fourier
1. Electricity and Magnetism (Fall 1995, Part 1) A metal sphere has a radius R and a charge Q.
 1. Electricity and Magnetism (Fall 1995, Part 1) A metal sphere has a radius R and a charge Q. (a) Compute the electric part of the Maxwell stress tensor T ij (r) = 1 {E i E j 12 } 4π E2 δ ij both inside
1. Electricity and Magnetism (Fall 1995, Part 1) A metal sphere has a radius R and a charge Q. (a) Compute the electric part of the Maxwell stress tensor T ij (r) = 1 {E i E j 12 } 4π E2 δ ij both inside
CH 23. Gauss Law. A. Gauss law relates the electric fields at points on a (closed) Gaussian surface to the net charge enclosed by that surface.
 CH 23 Gauss Law [SHIVOK SP212] January 4, 2016 I. Introduction to Gauss Law A. Gauss law relates the electric fields at points on a (closed) Gaussian surface to the net charge enclosed by that surface.
CH 23 Gauss Law [SHIVOK SP212] January 4, 2016 I. Introduction to Gauss Law A. Gauss law relates the electric fields at points on a (closed) Gaussian surface to the net charge enclosed by that surface.
VII. Porous Media Lecture 32: Percolation
 VII. Porous Media Lecture 32: Percolation April 25 th, 2011 Notes by John Casey (and MZB) References: S. Torquato, Random Heterogeneous Materials (Springer 2002) D. Stauffer, Introduction to Percolation
VII. Porous Media Lecture 32: Percolation April 25 th, 2011 Notes by John Casey (and MZB) References: S. Torquato, Random Heterogeneous Materials (Springer 2002) D. Stauffer, Introduction to Percolation
Liquid crystal phase transitions in dispersions of rod-like colloidal particles
 J. Phys.: Condens. Matter 8 (1996) 9451 9456. Printed in the UK Liquid crystal phase transitions in dispersions of rod-like colloidal particles M P B van Bruggen, F M van der Kooij and HNWLekkerkerker
J. Phys.: Condens. Matter 8 (1996) 9451 9456. Printed in the UK Liquid crystal phase transitions in dispersions of rod-like colloidal particles M P B van Bruggen, F M van der Kooij and HNWLekkerkerker
arxiv: v1 [cond-mat.soft] 4 Mar 2018
![arxiv: v1 [cond-mat.soft] 4 Mar 2018 arxiv: v1 [cond-mat.soft] 4 Mar 2018](/thumbs/95/126298848.jpg) arxiv:183.1361v1 [cond-mat.soft] 4 Mar 218 Anomalous system-size dependence of electrolytic cells with an electrified oil-water interface Marise Westbroek, a Niels Boon, a and René van Roij a Manipulation
arxiv:183.1361v1 [cond-mat.soft] 4 Mar 218 Anomalous system-size dependence of electrolytic cells with an electrified oil-water interface Marise Westbroek, a Niels Boon, a and René van Roij a Manipulation
 Title Super- and subcritical hydration of Thermodynamics of hydration Author(s) Matubayasi, N; Nakahara, M Citation JOURNAL OF CHEMICAL PHYSICS (2000), 8109 Issue Date 2000-05-08 URL http://hdl.handle.net/2433/50350
Title Super- and subcritical hydration of Thermodynamics of hydration Author(s) Matubayasi, N; Nakahara, M Citation JOURNAL OF CHEMICAL PHYSICS (2000), 8109 Issue Date 2000-05-08 URL http://hdl.handle.net/2433/50350
510 Subject Index. Hamiltonian 33, 86, 88, 89 Hamilton operator 34, 164, 166
 Subject Index Ab-initio calculation 24, 122, 161. 165 Acentric factor 279, 338 Activity absolute 258, 295 coefficient 7 definition 7 Atom 23 Atomic units 93 Avogadro number 5, 92 Axilrod-Teller-forces
Subject Index Ab-initio calculation 24, 122, 161. 165 Acentric factor 279, 338 Activity absolute 258, 295 coefficient 7 definition 7 Atom 23 Atomic units 93 Avogadro number 5, 92 Axilrod-Teller-forces
Anisotropic lattice models of electrolytes
 JOURNAL OF CHEMICAL PHYSICS VOLUME 7, NUMBER 9 5 NOVEMBER 2002 Anisotropic lattice models of electrolytes Vladimir Kobelev and Anatoly B. Kolomeisky a) Department of Chemistry, Rice University, Houston,
JOURNAL OF CHEMICAL PHYSICS VOLUME 7, NUMBER 9 5 NOVEMBER 2002 Anisotropic lattice models of electrolytes Vladimir Kobelev and Anatoly B. Kolomeisky a) Department of Chemistry, Rice University, Houston,
Partial Dynamical Symmetry in Deformed Nuclei. Abstract
 Partial Dynamical Symmetry in Deformed Nuclei Amiram Leviatan Racah Institute of Physics, The Hebrew University, Jerusalem 91904, Israel arxiv:nucl-th/9606049v1 23 Jun 1996 Abstract We discuss the notion
Partial Dynamical Symmetry in Deformed Nuclei Amiram Leviatan Racah Institute of Physics, The Hebrew University, Jerusalem 91904, Israel arxiv:nucl-th/9606049v1 23 Jun 1996 Abstract We discuss the notion
Receiver. Johana Brokešová Charles University in Prague
 Propagation of seismic waves - theoretical background Receiver Johana Brokešová Charles University in Prague Seismic waves = waves in elastic continuum a model of the medium through which the waves propagate
Propagation of seismic waves - theoretical background Receiver Johana Brokešová Charles University in Prague Seismic waves = waves in elastic continuum a model of the medium through which the waves propagate
Lennard-Jones as a model for argon and test of extended renormalization group calculations
 JOURNAL OF CHEMICAL PHYSICS VOLUME 111, NUMBER 2 22 NOVEMBER 1999 Lennard-Jones as a model for argon and test of extended renormalization group calculations John A. White Department of Physics, American
JOURNAL OF CHEMICAL PHYSICS VOLUME 111, NUMBER 2 22 NOVEMBER 1999 Lennard-Jones as a model for argon and test of extended renormalization group calculations John A. White Department of Physics, American
3/22/2016. Chapter 27 Gauss s Law. Chapter 27 Preview. Chapter 27 Preview. Chapter Goal: To understand and apply Gauss s law. Slide 27-2.
 Chapter 27 Gauss s Law Chapter Goal: To understand and apply Gauss s law. Slide 27-2 Chapter 27 Preview Slide 27-3 Chapter 27 Preview Slide 27-4 1 Chapter 27 Preview Slide 27-5 Chapter 27 Preview Slide
Chapter 27 Gauss s Law Chapter Goal: To understand and apply Gauss s law. Slide 27-2 Chapter 27 Preview Slide 27-3 Chapter 27 Preview Slide 27-4 1 Chapter 27 Preview Slide 27-5 Chapter 27 Preview Slide
Colloidal Gas-Liquid-Solid Phase Diagram in Low Ionic Strength Solutions
 (S17 S) 8th Japan-China-Korea Workshop on Microgravity Sciences for Asian Microgravity Pre-Symposium Colloidal Gas-Liquid-Solid Phase Diagram in Low Ionic Strength Solutions Masamichi ISHIKAWA 1 and Ryota
(S17 S) 8th Japan-China-Korea Workshop on Microgravity Sciences for Asian Microgravity Pre-Symposium Colloidal Gas-Liquid-Solid Phase Diagram in Low Ionic Strength Solutions Masamichi ISHIKAWA 1 and Ryota
Separation of Variables in Linear PDE: One-Dimensional Problems
 Separation of Variables in Linear PDE: One-Dimensional Problems Now we apply the theory of Hilbert spaces to linear differential equations with partial derivatives (PDE). We start with a particular example,
Separation of Variables in Linear PDE: One-Dimensional Problems Now we apply the theory of Hilbert spaces to linear differential equations with partial derivatives (PDE). We start with a particular example,
Spherical cloaking with homogeneous isotropic multilayered structures
 Spherical cloaking with homogeneous isotropic multilayered structures The MIT Faculty has made this article openly available. Please share how this access benefits you. Your story matters. Citation As
Spherical cloaking with homogeneous isotropic multilayered structures The MIT Faculty has made this article openly available. Please share how this access benefits you. Your story matters. Citation As
Clusters and Percolation
 Chapter 6 Clusters and Percolation c 2012 by W. Klein, Harvey Gould, and Jan Tobochnik 5 November 2012 6.1 Introduction In this chapter we continue our investigation of nucleation near the spinodal. We
Chapter 6 Clusters and Percolation c 2012 by W. Klein, Harvey Gould, and Jan Tobochnik 5 November 2012 6.1 Introduction In this chapter we continue our investigation of nucleation near the spinodal. We
Downloaded from
 Question 1.1: What is the force between two small charged spheres having charges of 2 10 7 C and 3 10 7 C placed 30 cm apart in air? Repulsive force of magnitude 6 10 3 N Charge on the first sphere, q
Question 1.1: What is the force between two small charged spheres having charges of 2 10 7 C and 3 10 7 C placed 30 cm apart in air? Repulsive force of magnitude 6 10 3 N Charge on the first sphere, q
we1 = j+dq + = &/ET, (2)
 EUROPHYSICS LETTERS Europhys. Lett., 24 (S), pp. 693-698 (1993) 10 December 1993 On Debye-Huckel s Theory. I. M. MLADENOV Central Laboratory of Biophysics, Bulgarian Academy of Sciences Acad. G. Bonchev
EUROPHYSICS LETTERS Europhys. Lett., 24 (S), pp. 693-698 (1993) 10 December 1993 On Debye-Huckel s Theory. I. M. MLADENOV Central Laboratory of Biophysics, Bulgarian Academy of Sciences Acad. G. Bonchev
II. Equilibrium Thermodynamics Lecture 7: Statistical Thermodynamics
 II. Equilibrium Thermodynamics Lecture 7: Statistical Thermodynamics Notes by ChangHoon Lim (and MZB) Open circuit voltage of galvanic cell is To understand compositional effects on, we need to consider
II. Equilibrium Thermodynamics Lecture 7: Statistical Thermodynamics Notes by ChangHoon Lim (and MZB) Open circuit voltage of galvanic cell is To understand compositional effects on, we need to consider
Chapter 2. Dielectric Theories
 Chapter Dielectric Theories . Dielectric Theories 1.1. Introduction Measurements of dielectric properties of materials is very important because it provide vital information regarding the material characteristics,
Chapter Dielectric Theories . Dielectric Theories 1.1. Introduction Measurements of dielectric properties of materials is very important because it provide vital information regarding the material characteristics,
 Title Theory of solutions in the energy r of the molecular flexibility Author(s) Matubayasi, N; Nakahara, M Citation JOURNAL OF CHEMICAL PHYSICS (2003), 9702 Issue Date 2003-11-08 URL http://hdl.handle.net/2433/50354
Title Theory of solutions in the energy r of the molecular flexibility Author(s) Matubayasi, N; Nakahara, M Citation JOURNAL OF CHEMICAL PHYSICS (2003), 9702 Issue Date 2003-11-08 URL http://hdl.handle.net/2433/50354
HEAT CONDUCTION USING GREEN S FUNCTIONS
 HEAT CONDUCTION USING GREEN S FUNCTIONS Preface to the first edition Preface to the second edition Author Biographies Nomenclature TABLE OF CONTENTS FOR SECOND EDITION December 2009 Page viii x xii xiii
HEAT CONDUCTION USING GREEN S FUNCTIONS Preface to the first edition Preface to the second edition Author Biographies Nomenclature TABLE OF CONTENTS FOR SECOND EDITION December 2009 Page viii x xii xiii
Chapter 21 Chapter 23 Gauss Law. Copyright 2014 John Wiley & Sons, Inc. All rights reserved.
 Chapter 21 Chapter 23 Gauss Law Copyright 23-1 What is Physics? Gauss law relates the electric fields at points on a (closed) Gaussian surface to the net charge enclosed by that surface. Gauss law considers
Chapter 21 Chapter 23 Gauss Law Copyright 23-1 What is Physics? Gauss law relates the electric fields at points on a (closed) Gaussian surface to the net charge enclosed by that surface. Gauss law considers
Optical properties of spherical and anisotropic gold shell colloids
 8 Optical properties of spherical and anisotropic gold shell colloids Core/shell colloids consisting of a metal shell and a dielectric core are known for their special optical properties. The surface plasmon
8 Optical properties of spherical and anisotropic gold shell colloids Core/shell colloids consisting of a metal shell and a dielectric core are known for their special optical properties. The surface plasmon
l=0 The expansion coefficients can be determined, for example, by finding the potential on the z-axis and expanding that result in z.
 Electrodynamics I Exam - Part A - Closed Book KSU 15/11/6 Name Electrodynamic Score = 14 / 14 points Instructions: Use SI units. Where appropriate, define all variables or symbols you use, in words. Try
Electrodynamics I Exam - Part A - Closed Book KSU 15/11/6 Name Electrodynamic Score = 14 / 14 points Instructions: Use SI units. Where appropriate, define all variables or symbols you use, in words. Try
Can the imaginary part of permeability be negative?
 Can the imaginary part of permeability be negative? Vadim A. Markel* Departments of Radiology and Bioengineering, University of Pennsylvania, Philadelphia, Pennsylvania 19104, USA Received 25 February
Can the imaginary part of permeability be negative? Vadim A. Markel* Departments of Radiology and Bioengineering, University of Pennsylvania, Philadelphia, Pennsylvania 19104, USA Received 25 February
Electric Flux. If we know the electric field on a Gaussian surface, we can find the net charge enclosed by the surface.
 Chapter 23 Gauss' Law Instead of considering the electric fields of charge elements in a given charge distribution, Gauss' law considers a hypothetical closed surface enclosing the charge distribution.
Chapter 23 Gauss' Law Instead of considering the electric fields of charge elements in a given charge distribution, Gauss' law considers a hypothetical closed surface enclosing the charge distribution.
Lecture 6. NONELECTROLYTE SOLUTONS
 Lecture 6. NONELECTROLYTE SOLUTONS NONELECTROLYTE SOLUTIONS SOLUTIONS single phase homogeneous mixture of two or more components NONELECTROLYTES do not contain ionic species. CONCENTRATION UNITS percent
Lecture 6. NONELECTROLYTE SOLUTONS NONELECTROLYTE SOLUTIONS SOLUTIONS single phase homogeneous mixture of two or more components NONELECTROLYTES do not contain ionic species. CONCENTRATION UNITS percent
Symmetry Properties of Superconductors
 Australian Journal of Basic and Applied Sciences, 6(3): 81-86, 2012 ISSN 1991-8178 Symmetry Properties of Superconductors Ekpekpo, A. Department of Physics, Delta State University, Abraka, Nigeria. Abstract:
Australian Journal of Basic and Applied Sciences, 6(3): 81-86, 2012 ISSN 1991-8178 Symmetry Properties of Superconductors Ekpekpo, A. Department of Physics, Delta State University, Abraka, Nigeria. Abstract:
Internal Structures of Electron, Neutron, Proton and Nuclei particle masses are not arbitrary
 Internal Structures of Electron, Neutron, Proton and Nuclei particle masses are not arbitrary Jose P Koshy josepkoshy@gmail.com Abstract: The mass of neutron is slightly greater than1838 electrons. So
Internal Structures of Electron, Neutron, Proton and Nuclei particle masses are not arbitrary Jose P Koshy josepkoshy@gmail.com Abstract: The mass of neutron is slightly greater than1838 electrons. So
1 Solutions in cylindrical coordinates: Bessel functions
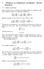 1 Solutions in cylindrical coordinates: Bessel functions 1.1 Bessel functions Bessel functions arise as solutions of potential problems in cylindrical coordinates. Laplace s equation in cylindrical coordinates
1 Solutions in cylindrical coordinates: Bessel functions 1.1 Bessel functions Bessel functions arise as solutions of potential problems in cylindrical coordinates. Laplace s equation in cylindrical coordinates
Introductions to ExpIntegralEi
 Introductions to ExpIntegralEi Introduction to the exponential integrals General The exponential-type integrals have a long history. After the early developments of differential calculus, mathematicians
Introductions to ExpIntegralEi Introduction to the exponential integrals General The exponential-type integrals have a long history. After the early developments of differential calculus, mathematicians
V. Electrostatics. MIT Student
 V. Electrostatics Lecture 26: Compact Part of the Double Layer MIT Student 1 Double-layer Capacitance 1.1 Stern Layer As was discussed in the previous lecture, the Gouy-Chapman model predicts unphysically
V. Electrostatics Lecture 26: Compact Part of the Double Layer MIT Student 1 Double-layer Capacitance 1.1 Stern Layer As was discussed in the previous lecture, the Gouy-Chapman model predicts unphysically
collisions inelastic, energy is dissipated and not conserved
 LECTURE 1 - Introduction to Granular Materials Jamming, Random Close Packing, The Isostatic State Granular materials particles only interact when they touch hard cores: rigid, incompressible, particles
LECTURE 1 - Introduction to Granular Materials Jamming, Random Close Packing, The Isostatic State Granular materials particles only interact when they touch hard cores: rigid, incompressible, particles
Electrolytes. Chapter Basics = = 131 2[ ]. (c) From both of the above = = 120 8[
![Electrolytes. Chapter Basics = = 131 2[ ]. (c) From both of the above = = 120 8[ Electrolytes. Chapter Basics = = 131 2[ ]. (c) From both of the above = = 120 8[](/thumbs/83/88791608.jpg) Chapter 1 Electrolytes 1.1 Basics Here we consider species that dissociate into positively and negatively charged species in solution. 1. Consider: 1 H (g) + 1 Cl (g) + ()+ () = { } = (+ )+ ( ) = 167[
Chapter 1 Electrolytes 1.1 Basics Here we consider species that dissociate into positively and negatively charged species in solution. 1. Consider: 1 H (g) + 1 Cl (g) + ()+ () = { } = (+ )+ ( ) = 167[
1 Fundamentals. 1.1 Overview. 1.2 Units: Physics 704 Spring 2018
 Physics 704 Spring 2018 1 Fundamentals 1.1 Overview The objective of this course is: to determine and fields in various physical systems and the forces and/or torques resulting from them. The domain of
Physics 704 Spring 2018 1 Fundamentals 1.1 Overview The objective of this course is: to determine and fields in various physical systems and the forces and/or torques resulting from them. The domain of
List of Comprehensive Exams Topics
 List of Comprehensive Exams Topics Mechanics 1. Basic Mechanics Newton s laws and conservation laws, the virial theorem 2. The Lagrangian and Hamiltonian Formalism The Lagrange formalism and the principle
List of Comprehensive Exams Topics Mechanics 1. Basic Mechanics Newton s laws and conservation laws, the virial theorem 2. The Lagrangian and Hamiltonian Formalism The Lagrange formalism and the principle
arxiv:cond-mat/ v3 [cond-mat.soft] 12 Sep 2001
![arxiv:cond-mat/ v3 [cond-mat.soft] 12 Sep 2001 arxiv:cond-mat/ v3 [cond-mat.soft] 12 Sep 2001](/thumbs/81/83614310.jpg) EUROPHYSICS LETTERS 15 August 2000 Europhys. Lett., 51 (4), pp. 461 468 (2000) arxiv:cond-mat/0006501v3 [cond-mat.soft] 12 Sep 2001 Ground state of two unlike charged colloids: An analogy with ionic bonding
EUROPHYSICS LETTERS 15 August 2000 Europhys. Lett., 51 (4), pp. 461 468 (2000) arxiv:cond-mat/0006501v3 [cond-mat.soft] 12 Sep 2001 Ground state of two unlike charged colloids: An analogy with ionic bonding
CHAPTER 3 POTENTIALS 10/13/2016. Outlines. 1. Laplace s equation. 2. The Method of Images. 3. Separation of Variables. 4. Multipole Expansion
 CHAPTER 3 POTENTIALS Lee Chow Department of Physics University of Central Florida Orlando, FL 32816 Outlines 1. Laplace s equation 2. The Method of Images 3. Separation of Variables 4. Multipole Expansion
CHAPTER 3 POTENTIALS Lee Chow Department of Physics University of Central Florida Orlando, FL 32816 Outlines 1. Laplace s equation 2. The Method of Images 3. Separation of Variables 4. Multipole Expansion
PHYSICS. Chapter 24 Lecture FOR SCIENTISTS AND ENGINEERS A STRATEGIC APPROACH 4/E RANDALL D. KNIGHT
 PHYSICS FOR SCIENTISTS AND ENGINEERS A STRATEGIC APPROACH 4/E Chapter 24 Lecture RANDALL D. KNIGHT Chapter 24 Gauss s Law IN THIS CHAPTER, you will learn about and apply Gauss s law. Slide 24-2 Chapter
PHYSICS FOR SCIENTISTS AND ENGINEERS A STRATEGIC APPROACH 4/E Chapter 24 Lecture RANDALL D. KNIGHT Chapter 24 Gauss s Law IN THIS CHAPTER, you will learn about and apply Gauss s law. Slide 24-2 Chapter
Electric fields in matter
 Electric fields in matter November 2, 25 Suppose we apply a constant electric field to a block of material. Then the charges that make up the matter are no longer in equilibrium: the electrons tend to
Electric fields in matter November 2, 25 Suppose we apply a constant electric field to a block of material. Then the charges that make up the matter are no longer in equilibrium: the electrons tend to
The 3 dimensional Schrödinger Equation
 Chapter 6 The 3 dimensional Schrödinger Equation 6.1 Angular Momentum To study how angular momentum is represented in quantum mechanics we start by reviewing the classical vector of orbital angular momentum
Chapter 6 The 3 dimensional Schrödinger Equation 6.1 Angular Momentum To study how angular momentum is represented in quantum mechanics we start by reviewing the classical vector of orbital angular momentum
Scaling relations for parallel disc Capacitance with Dielectric media
 Scaling relations for parallel disc Capacitance with Dielectric media C. V. Krishnamurthy, Gokul Raj R Measurement and Modeling Lab Department of Physics, Indian Institute of Technology Madras Chennai-600036,
Scaling relations for parallel disc Capacitance with Dielectric media C. V. Krishnamurthy, Gokul Raj R Measurement and Modeling Lab Department of Physics, Indian Institute of Technology Madras Chennai-600036,
Where, ε 0 = Permittivity of free space and = Nm 2 C 2 Therefore, force
 Exercises Question.: What is the force between two small charged spheres having charges of 2 0 7 C and 3 0 7 C placed 30 cm apart in air? Answer.: Repulsive force of magnitude 6 0 3 N Charge on the first
Exercises Question.: What is the force between two small charged spheres having charges of 2 0 7 C and 3 0 7 C placed 30 cm apart in air? Answer.: Repulsive force of magnitude 6 0 3 N Charge on the first
Chapter 4. Electrostatic Fields in Matter
 Chapter 4. Electrostatic Fields in Matter 4.1. Polarization 4.2. The Field of a Polarized Object 4.3. The Electric Displacement 4.4. Linear Dielectrics 4.5. Energy in dielectric systems 4.6. Forces on
Chapter 4. Electrostatic Fields in Matter 4.1. Polarization 4.2. The Field of a Polarized Object 4.3. The Electric Displacement 4.4. Linear Dielectrics 4.5. Energy in dielectric systems 4.6. Forces on
Chapter 7. Pickering Stabilisation ABSTRACT
 Chapter 7 Pickering Stabilisation ABSTRACT In this chapter we investigate the interfacial properties of Pickering emulsions. Based upon findings that indicate these emulsions to be thermodynamically stable,
Chapter 7 Pickering Stabilisation ABSTRACT In this chapter we investigate the interfacial properties of Pickering emulsions. Based upon findings that indicate these emulsions to be thermodynamically stable,
THE MODIFIED YOUNG S EQUATION FOR THE CONTACT ANGLE OF A SMALL SESSILE DROP FROM AN INTERFACE DISPLACEMENT MODEL
 International Journal of Modern Physics B, Vol. 13, No. 7 (1999) 355 359 c World Scientific Publishing Company THE MODIFIED YOUNG S EQUATION FOR THE CONTACT ANGLE OF A SMALL SESSILE DROP FROM AN INTERFACE
International Journal of Modern Physics B, Vol. 13, No. 7 (1999) 355 359 c World Scientific Publishing Company THE MODIFIED YOUNG S EQUATION FOR THE CONTACT ANGLE OF A SMALL SESSILE DROP FROM AN INTERFACE
J10M.1 - Rod on a Rail (M93M.2)
 Part I - Mechanics J10M.1 - Rod on a Rail (M93M.2) J10M.1 - Rod on a Rail (M93M.2) s α l θ g z x A uniform rod of length l and mass m moves in the x-z plane. One end of the rod is suspended from a straight
Part I - Mechanics J10M.1 - Rod on a Rail (M93M.2) J10M.1 - Rod on a Rail (M93M.2) s α l θ g z x A uniform rod of length l and mass m moves in the x-z plane. One end of the rod is suspended from a straight
Physics PhD Qualifying Examination Part I Wednesday, January 21, 2015
 Physics PhD Qualifying Examination Part I Wednesday, January 21, 2015 Name: (please print) Identification Number: STUDENT: Designate the problem numbers that you are handing in for grading in the appropriate
Physics PhD Qualifying Examination Part I Wednesday, January 21, 2015 Name: (please print) Identification Number: STUDENT: Designate the problem numbers that you are handing in for grading in the appropriate
Phase diagram of hard-core repulsive Yukawa particles with a density-dependent truncation: asimplemodelforcharged colloids
 INSIUE OF PHYSICSPUBLISHING JOURNAL OFPHYSICS: CONDENSED MAER J. Phys.: Condens. Matter 15 (2003) S3557 S3567 PII: S0953-8984(03)66493-3 Phase diagram of hard-core repulsive Yukawa particles with a density-dependent
INSIUE OF PHYSICSPUBLISHING JOURNAL OFPHYSICS: CONDENSED MAER J. Phys.: Condens. Matter 15 (2003) S3557 S3567 PII: S0953-8984(03)66493-3 Phase diagram of hard-core repulsive Yukawa particles with a density-dependent
Effect of fibre shape on transverse thermal conductivity of unidirectional composites
 Sādhanā Vol. 4, Part 2, April 25, pp. 53 53. c Indian Academy of Sciences Effect of fibre shape on transverse thermal conductivity of unidirectional composites B RAGHAVA RAO,, V RAMACHANDRA RAJU 2 and
Sādhanā Vol. 4, Part 2, April 25, pp. 53 53. c Indian Academy of Sciences Effect of fibre shape on transverse thermal conductivity of unidirectional composites B RAGHAVA RAO,, V RAMACHANDRA RAJU 2 and
Renormalization Group for the Two-Dimensional Ising Model
 Chapter 8 Renormalization Group for the Two-Dimensional Ising Model The two-dimensional (2D) Ising model is arguably the most important in statistical physics. This special status is due to Lars Onsager
Chapter 8 Renormalization Group for the Two-Dimensional Ising Model The two-dimensional (2D) Ising model is arguably the most important in statistical physics. This special status is due to Lars Onsager
Molecular Driving Forces
 Molecular Driving Forces Statistical Thermodynamics in Chemistry and Biology SUBGfittingen 7 At 216 513 073 / / Ken A. Dill Sarina Bromberg With the assistance of Dirk Stigter on the Electrostatics chapters
Molecular Driving Forces Statistical Thermodynamics in Chemistry and Biology SUBGfittingen 7 At 216 513 073 / / Ken A. Dill Sarina Bromberg With the assistance of Dirk Stigter on the Electrostatics chapters
