Large scale genomic prediction using singular value decomposition of the genotype matrix
|
|
|
- Brianne Paul
- 6 years ago
- Views:
Transcription
1 Genetics Selection Evolution RESEARCH ARTICLE Open Access Large scale genomic prediction using singular value decomposition of the genotype matrix Jørgen Ødegård *, Ulf Indahl 2, Ismo Strandén 3 and Theo H. E. Meuwissen 2 Abstract Background: For marker effect models and genomic animal models, computational requirements increase with the number of loci and the number of genotyped individuals, respectively. In the latter case, the inverse genomic relationship matrix GRM) is typically needed, which is computationally demanding to compute for large datasets. Thus, there is a great need for dimensionality-reduction methods that can analyze massive genomic data. For this purpose, we developed reduced-dimension singular value decomposition SVD) based models for genomic prediction. Methods: Fast SVD is performed by analyzing different chromosomes/genome segments in parallel and/or by restricting SVD to a limited core of genotyped individuals, producing chromosome- or segment-specific principal components PC). Given a limited effective population size, nearly all the genetic variation can be effectively captured by a limited number of PC. Genomic prediction can then be performed either by PC ridge regression PCRR) or by genomic animal models using an inverse GRM computed from the chosen PC PCIG). In the latter case, computation of the inverse GRM will be feasible for any number of genotyped individuals and can be readily produced row- or element-wise. Results: Using simulated data, we show that PCRR and PCIG models, using chromosome-wise SVD of a core sample of individuals, are appropriate for genomic prediction in a larger population, and results in virtually identical predicted breeding values as the original full-dimension genomic model r =.000). Compared with other algorithms e.g. algorithm for proven and young animals, APY), the chromosome-wise SVD-based) PCRR and PCIG models were more robust to size of the core sample, giving nearly identical results even down to 500 core individuals. The method was also successfully tested on a large multi-breed dataset. Conclusions: SVD can be used for dimensionality reduction of large genomic datasets. After SVD, genomic prediction using dense genomic data and many genotyped individuals can be done in a computationally efficient manner. Using this method, the resulting genomic estimated breeding values were virtually identical to those computed from a full-dimension genomic model. Background In recent years, genomic prediction ] has revolutionized animal and plant breeding methods. With decreasing genotyping costs, the number of genotyped individuals has increased exponentially over years, with up to full sequence of genomic information available for prediction. Genomic prediction can be performed *Correspondence: jorgen.odegard@aquagen.no AquaGen AS, P.O. Box 240, 7462 Trondheim, Norway Full list of author information is available at the end of the article using two families of genomic models: marker effects models MEM) e.g. SNP-best linear unbiased prediction BLUP), BayesA, BayesB, BayesC, etc.), and animal models that use a genomic relationship matrix GRM). The latter can be further divided into genomic models that include genotyped animals only genomic BLUP, i.e. GBLUP) and single-step GBLUP ssgblup) models 2, 3] that combine genotyped and ungenotyped animals. The advantage of genomic animal models is that they fit nicely within the traditional linear models framework, The Authors) 208. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original authors) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver publicdomain/zero/.0/) applies to the data made available in this article, unless otherwise stated.
2 Page 2 of 2 and can essentially be adapted to any kind of linear or generalized linear animal model single-trait, multi-trait, random regression, etc.). However, with the increasing number of genotyped individuals and increasing density of genotypes, the computational requirements of genomic prediction models increase accordingly. Hence, MEM analysis of full sequence data, e.g. using Bayesian variable selection models, will be very demanding in terms of computing time. For ssgblup 2, 3], the inverse of the GRM is computed prior to analysis, which may be practically impossible when the number of genotyped animals becomes very large e.g. > 00,000). To address the latter, Misztal et al. 4] proposed the algorithm for proven and young animals APY), which uses a core sample of individuals to compute an approximate inverse of the GRM for all animals. However, in some cases, the total GRM does not have full rank, and thus no inverse. Therefore, Fernando et al. 5] suggested exact methods to obtain ssgblup solutions. One of the options that they proposed was to model animal genetic effects as linear combinations of independent factors. In the following section, we propose a related strategy that applies singular value decomposition SVD) to perform large-scale genomic evaluation, both for MEM and animal genomic models. Thus, our study aims at: ) using SVD and principal component PC) ridge regression PCRR) for genomic prediction as an alternative to MEM, using up to full sequence genomic data, and 2) applying SVD techniques for computation of exact inverses of PC-based GRM, using dimensionality reduction. Methods Marker effect models Assume that dense single nucleotide polymorphism SNP) genotypes for k loci are available for N animals. Omitting fixed effects for simplicity, the simplest MEM called SNP-BLUP) can be specified as ]: y = Xb + e, where y is a vector of phenotypes, X is an N k centered) matrix of genotype dosage for all SNPs and all animals, b N 0, Iσm) 2 is a vector of SNP allele substitution effects, σm 2 is the variance of SNP effects, e N 0, Iσe 2 ) is a vector of random residuals, and σe 2 is the residual variance. The SNP-BLUP equations 6] are: X X + λi ]ˆb = X y, 2) where λ = σ e 2 is the ratio of residual variance to SNP σm 2 effects variance. Here, we assume that the SNP effects variance is: σm 2 = σg 2 2, where σ 2 k i= p i p i ) g is the total ) additive genetic variance and p i is the allele frequency at locus i. The dimension of the equation system is equal to the number of loci k). Hence, if k is large e.g. full sequence), solving this system of equations may be difficult. Gblup A GBLUP animal) model, equivalent to the above SNP- BLUP model i.e. assuming all animals have data) is 7]: y = g + e, 3) ) where e is as defined above and g N 0, Gσg 2 is a vector of additive genetic effects. Now, the equation system becomes 7]: I + λ g G ] ĝ = y, 4) where λ = σ e 2, i.e. the ratio of residual to total additive genetic variance. The GRM G is a function of σg 2 the observed genotypes, e.g. based on VanRaden s Method 8], G = ρ XX, where ρ = 2p p), with p being a vector of SNP allele frequencies in the population. In populations of limited effective size N e ), the genomic relationships are a result of the segregation of a limited number of haplotype segments, and thus, G may not be positive definite, implying that its inverse does not exist. In such cases, G is still positive semidefinite, i.e., for any non-zero vector z of N real numbers: z Gz = ρ z XX z = ρ u u 0 u = X z ). We defined an approximated GRM: G = G + Iθ) = ρ XX + Iρθ ), where θ is a small number e.g. 0 3 ). The matrix G is positive definite, and thus invertible, as: z Gz = ρ u u + θ z z > 0. Adding θ to the GRM diagonal elements has a negligible effect on the solutions and may be viewed as fitting a tiny) fraction of the residual as a part of the additive genetic effects, and thus is essentially equivalent to the original GBLUP model. Although G exists, computing it by direct brute-force inversion will be increasingly challenging, and eventually impossible, as the number of genotyped individuals increases e.g. for N > 00,000). Another option is to specify the equation system as 9]: G + λg I ] ĝ = Gy, 5) which do not require an invertible G. However, for typical single-step evaluations, the inverse of the GRM is still needed 2, 3]. The dimension of the GRM is equal to number of genotyped animals N, which may be smaller than number of loci k at least for dense genomic data). In the opposite case, when the number of genotyped animals exceeds the number of loci, G does not exist, but the
3 Page 3 of 2 exact inverse of G can be calculated by the Woodbury formula 0]: G = ρ XX + Iρθ ) ) ) X = ρ Iρ θ Iρ θ X I k + X Iρ θ X Iρ θ = I X X X + I k ρθ ) ) X, θ where I and I k are identity matrices of rank N animals), and k number of loci), respectively. This implies that the k k) matrix X X + I k ρθ ) has to be inverted rather than the N N) GRM. Still, for large k, computing a direct inverse may be computationally difficult. We will show later how dimensionality reduction of genomic data can be used for the efficient computation of the inverse of large GRM. Principal component ridge regression PCRR) Related animals typically share large segments of DNA, and for dense genomic data, substantial linkage disequilibrium LD) is expected between closely linked loci. Hence, the genomic variation, even with up to full sequence data, is likely largely explained by a smaller number of underlying components, i.e., using principal component analysis, majority of the genomic variation can be described by a limited number of principal components PC). For a genotype matrix X of size N k), assuming that N < k more markers than individuals), an economy-sized i.e. only keeping PC with eigenvalues > 0) SVD e.g. ] would be: X = USV, where U is N N), V is N k), and S is a diagonal matrix of dimension N, with singular values on the diagonal square root of eigenvalues). Furthermore, U U = UU = I, and V V = I, while VV = I for N < k ). The SVD rectangular matrices) and eigenvalue decomposition symmetric matrices) have previously been used in genomic models 2, 3]. The SNP-BLUP model can be re-parametrized into a PCRR model 3] by defining s = V b PC regression coefficients). The model can be specified as: y = Ts + e, where e is as defined above, the score matrix T = US = XV, and s N 0, Iσ 2 m). There is an exact relationship between solutions to Henderson s mixed model equations HMME) that correspond to the PCRR and MEM models, given as ˆb = Vŝ 4]. As ŝ = V ˆb, this implies that ˆb = VV ˆb, even when VV = I for example when the number of loci exceeds the number of animals), a proof of which is in the Appendix. We illustrate this with the following small numerical example. 6) 7) 8) Consider centered genotypes of four individuals with five loci as: X = 0, 0 0 which has more loci than animals. In addition, the genotypes of the four animals are not linearly independent, yielding a genotype matrix of rank 3. Assume that the four individuals have the following phenotypes: y = effect solutions: ˆb =. Then, using λ = gives the following SNP As X has rank 3, it can be decomposed as X = USV, keeping the first three components. The SVD matrices of X are: U = S = diag , and.7557 Hence, VV ˆb = ˆb, although VV = I. The SNP-BLUP equations can then be re-arranged into an equivalent PCRR equation system see Appendix): ] S 2 + λi ŝ = T y. 9), V = Then, VV = , and VV ˆb =
4 Page 4 of 2 Note that T T = SU US = S 2. Predictions of individual genetic effects can then be obtained as: ĝ = Tŝ. In this system of equations, there are at most) N independent effects to be estimated, rather than k effects number of loci), and both S 2 and I are diagonal matrices. Hence, the entire left-hand side of the BLUP equation system is diagonal, with diagonal elements S 2 ii + λ). This equation system is extremely easy to solve, even for very large y and many genotypes and animals. The main challenge thus lies in performing SVD of matrix X. Performing large scale SVD analyses on genomic data Although both population size and the number of loci can be substantial, the effective number of loci is limited by N e, which may be rather small in farmed animal populations. According to Meuwissen et al. 5], the effective number of loci in a population is: M e = 0) 2N el log2n e ), where L is the genome length in Morgans. For example, for a population of N e = 200 and L = 20, M e = 335, i.e. about 67 effective loci per Morgan. This can be explained by genomic data coming from larger haplotype blocks with restricted recombination, and a reduced number of PC can thus explain all or nearly all genetic variation, even for very large populations, when N e is limited the smallest PC may actually capture genotyping errors or extremely rare alleles). Still, computing a low-rank approximation of X through SVD of the entire genotype dataset can be computationally very demanding for large N and k. One possibility is to perform SVD on a subset of the individuals, which will be referred to as the core sample, equivalent to the core sample of the APY algorithm 4], and use the results for reduced-rank approximation of the entire genomic dataset. The core sample should be representative of the population and sufficiently large such that all or nearly all genetic variation is captured, but at the same time be restricted to a computationally manageable size. More specifically, a reduced matrix, e.g. n rows individuals) of the genotype matrix X are extracted, resulting in the matrix: X n = U n S n V n. ) For a population with limited M e, it is expected that a representative and moderately sized core sample would span nearly all genetic variation in the population. Hence, for increasing n, the most important eigenvectors of X n X n will approach the most important eigenvectors of the entire X X, i.e. the first few columns in V n likely approach the first few columns in V. Hence, V n can be used to approximate the scores for the non-core animals. In the case where SVD is performed on the entire dataset, the score matrix is: T = XV = USV V = US ). For a reduced-dimension model, the score matrix is: T q = XV q, where V q includes the first q eigenvectors of V. As now SVD is performed on a smaller core sample, the reduced-dimension score matrix can be estimated by replacing V q with V nq i.e. the first q eigenvectors of V n ): C = XV nq. The model can now be written as: y = Cs + e, and the PCRR equation system becomes: C C + λi ] ŝ = C y. Now, ŝ = V nq ˆb i.e. V nq has replaced V q from the entire population). Note that C C is not a diagonal matrix. The dimension of this equation system genomic effects) is the number of chosen components based on the core sample), q n). Hence, given that an SVD can be performed on the n k genomic dataset of the core sample, a direct solution to the maximum) n n PCRR equation system would be straightforward. Alternatively, dimensionality reduction and SVD of the entire genomic data set can be performed in three steps: ) SVD on genomic data of the core sub-sample; 2) dimensionality reduction of the entire genomic data X set using Eq. 2), resulting in the reduced-dimension matrix C; and 3) SVD of C without further dimensionality reduction), resulting in a score matrix ˆT of the entire genomic data set X. Hence: C = U C S C V C = ˆTV C. Now: X CV nq = ˆTV C V nq = ˆT ˆV. The model is now: y = ˆTt + e. 2) 3) 4) 5) 6) Here, ˆt = V Cŝ = V C V nq ˆb = ˆV ˆb. Note that ˆT has the same dimension as C, but ˆT ˆT = S 2 C diagonal) and ˆV ˆV = I. The PCRR equation system is thus: S 2 C + λi ]ˆt = ˆT y, 7) for which the coefficient matrix is diagonal, making the equation system easy to solve. A small numerical example illustrates the method. Consider the genotypes of five individuals the four given in the earlier example and an additional animal): X = 0. This centered genotype
5 Page 5 of 2 matrix still has rank 3 and, thus, there is room for dimension reduction. The genotype of the last individual is identical to the first individual, and thus we consider the first four individuals as core sample and use this in SVD keeping the first three components): X n = Matrix C can be used directly in PCRR. Assume that the five individuals have the following phenotypes assuming no fixed effects): y = and λ =. 0.7 Then, solving the equation system: C C + λi ] ŝ = C y, yields ŝ = and ĝ = Cŝ = Note that C C is not a diagonal matrix. Alternatively, a second-stage SVD can be performed, giving C = U C S C V C = ˆTV C. Now: ˆT = U C S C = Here, ˆT ˆT = S 2 C 0 0 = U n S n V n C = XV n3 = diagonal) and solving the equation system S 2 C + λi]ˆt = ˆT y yields ˆt = , and ĝ = ˆTˆt = 0.222, i.e. exactly the same animal solutions as above. Performing SVD in parallel on genome segments The SVD can be performed independently in parallel) on different genome segments in this case, chromosomes). This implies that different but not necessarily fully independent) sets of PC are chosen for each segment. For the core sample, the economy-sized SVD of chromosome i is thus: X in = U in S in V in, 8) T in = U in S in. 9) As above, the approximated score matrix ˆT of X can be computed in three steps: ) perform chromosome-wise SVD on a core sample of genomic data for each chromosome same core individuals for all chromosomes); 2) compute chromosome-specific reduced rank C i = X i V inq for all individuals core and non-core) and concatenate these into C = ] C C 2... C c ; and 3) perform SVD of C = U C S C V C and compute the reduced dimension score matrix ˆT = U C S C without further rank reduction). The entire genotype matrix across all chromosomes can then be approximated as: V nq 0 0 V X C = ˆTV nq 0 0 C = ˆT ˆV. 0 0 V cnq 0 0 V cnq 20) The model and equation system are then as described above Eqs. 6 and 7). As above, matrix C can also be used directly in PCRR, although the mixed model coefficient matrix may be dense but of reduced dimensionality). For each chromosome, the effective number of segregating loci is much smaller than for the whole genome, implying that fewer PC < n) will be needed per chromosome than for the whole genome. The total number of chosen PC at most n c, where c is the number of chromosomes) is q i, where q i is the number of chosen PC for chromosome i. Still, since SVD of the core sample genomic data is performed chromosome-wise, the final number of chosen PC may potentially exceed the number of animals in the core subpopulation. This implies that genetic variation of the core and non-core subpopulations is assumed to be explained by a limited number of common components i.e. haplotype blocks), and that the number of components that segregate in the core may be larger than the number of core individuals. In contrast, the APY algorithm assumes that all genetic variation is explained by the additive genetic effects of the core individuals, rather than by the haplotype blocks that segregate among those individuals. Principal component based algorithm for inverting the GRM PCIG) Single-step genomic analyses are widely used in the analysis of real data. As mentioned earlier, the original) singlestep equation system requires the inverse of the GRM
6 Page 6 of 2 G ) to be computed prior to analysis. If inversion is done by brute force, large-scale analyses that potentially include millions of genotyped animals will be virtually impossible to perform. However, in the following section we describe how the GRM for such data can be effectively approximated through SVD techniques and how the exact inverse of an approximated GRM can be obtained. If SVD of C can be performed as described above: X ˆT ˆV. A PC-based GRM then is: G = ρ XX ρ ˆT ˆV ˆV ˆT = ρ ˆT ˆT, where ρ = 2p p), with p being a vector of SNP allele frequencies in the population. An actual inverse of G may not exist, as XX may not have full rank even with very dense SNP data), while a reducedrank ˆT ˆT rank < N) is never invertible. As above, this problem can be circumvented by replacing G with G = ρ ˆT ˆT + Iθ = ρ ˆT ˆT + Iρθ 2) ), where θ is a small value e.g. 0 3 ) to ensure that G is positive definite and thus can be inverted. Using the Woodbury formula 0], the exact inverse of G is: ) G = ρ ˆT ˆT + Iρθ ) ) = ρ Iρ θ Iρ θ ˆT I p + ˆT Iρ θ ˆT ˆT Iρ θ = ) I ˆT S 2 C pρθ) θ + I ˆT, 22) where I p is an identity matrix of dimension q i number of chosen PC summed over all chromosomes). The only matrix that needs to be inverted explicitly is S 2 C + I pρθ ), which is diagonal. Hence, given that S 2 C and ˆT are available, computing G is not very demanding. Furthermore, the inverse relationships can be computed row by row as: G i = θ ) ) I i ˆT i S 2 C + I pρθ ˆT. The above inverse of GRM requires an SVD of the C matrix as described in the stepwise procedures above). However, since CC = ˆTV c V c ˆT = ˆT ˆT, the above inverse of the GRM can also be computed as: G = ρ CC + Iρθ ) ) ) C = ρ Iρ θ Iρ θ C I p + C Iρ θ C Iρ θ = θ I C C C + I p ρθ ) C ). 23) Thus, the only explicit inverse needed here is C C + I p ρθ ), which is of full rank and has dimension q i. For example, q i 0,000 components may be sufficient to describe essentially all genetic variation, even for a large genotyped population if it has limited N e. Under these assumptions, an inverse of GRM can be computed for any number of genotyped individuals. QR based algorithm for inverting GRM QRIG) Fernando et al. 5] suggested a QR decomposition of the X matrix, which is generally faster than SVD. A QR decomposition of matrix X n, of dimension k n, with n < k, is: X n = Q nr n i.e. X n = R n Q n, 24) where Q n is a k n matrix with orthogonal columns i.e. Q n Q n = I, Q n Q n =I), while R n is a n n upper triangular matrix. Furthermore, R n = X nq n. The genomic relationship matrix for the core sample is: G n = ρ X nx n = ρ R n Q n Q nr n = ρ R n R n. 25) As in the APY algorithm, this method assumes that nearly) all genetic variation is captured by the additive genetic effects of individuals in the core sample. For the entire dataset X sorted such that the core sample comes first), this implies that: X = R Q R ] ] n 0 Q n 0 ˆR 26) n 0 = ˆR 0 0 Q n, R where ˆR ] = n ˆR n, ˆR n = X nq n, and X n is the genotype matrix of all non-core individuals. The GRM can thus be approximated as: G ρ ˆR Q n Q n ˆR = ρ ˆR ˆR, 27) where ˆR is a n N matrix, which is considerably smaller than the original X N k). This approach is equivalent to strategy IV of Fernando et al. 5] except that core animals are assumed to explain nearly all genomic variation rather than all genomic variation exactly). Here, genotypes of all animals are expressed as linear functions of genotypes of a reduced set of animals rows in the genotype matrix). In their case, this result was used to compute a reduced set of components. Here, instead we use ˆR to compute a QR-based inverse of GRM QRIG) as: ) G = ρ ˆR ˆR + Iρθ 28) = ) ) I ˆR ˆR ˆR + I p ρθ ˆR. θ Thus, the only part that needs to be inverted explicitly ) is the n n matrix ˆR ˆR + I p ρθ. The QR factorization
7 Page 7 of 2 can, as for SVD, be parallelized through chromosomewise factorizations. Then, an overall QR is performed on the combined R matrix: R n R 2n... = Q n R n, 29) R in where R in is an R matrix that is obtained from QR factorization of the genomic data on chromosome i and R n is a genome-wide matrix. The QRIG algorithm is well suited for reduced-rank approximations down to the size of the core sample. However, reducing rank below the size of the core sample would not be optimal e.g. in chromosome-wise analysis), as this implies a reduction in the size of the core sample. For such situations, the PCIG approach is more appropriate because this method uses all available information in the core sample to estimate a reduced number of contributing components. Weighted genomic relationship matrix As in MEM, different loci can be given different relative weights in the genomic animal model by weighting SNPs differently in the calculation of the GRM, as: G = ρ XDX = ρ USV DVSU = ρ TFT, 30) where D is a diagonal matrix of locus weights proportional to the variance of the effect of each locus) and ρ = 2p D p), with p being a vector of allele frequencies in the population, and F = V DV. In the simplest case, i.e. using VanRaden Method 2 6], elements of D are: D i = 2p i p i ). The GRM can then be approximated as: G ρ CV nq DV nqc = ρ CF nc, 3) where F n = V nq DV nq, i.e. a symmetric matrix with dimension equal to the number of chosen components. The exact inverse of the approximated genomic relationship matrix is: G = ρ CF n C + Iρθ ) = ρ Iρ θ Iρ θ C = θ F n ) ) C I C C C + Fn ρθ. ) ) C + C Iρ θ C Iρ θ 32) Using this method, a weighted genomic relationship matrix can be used even for single-step animal models. Simulation study A simulation study was performed to verify the reducedrank approximation of genomic data. The simulated species had 20 chromosomes of Morgan each. Simulation of sequence data followed the approach of Meuwissen and Goddard 7], except that their scaling argument was not applied here, in order not to scale down the computations. The historical effective population size was 000, which also reflects its actual size, since simulation of new generations followed Wright s idealized population structure. To create LD and mutation-drift equilibria, the historical population was simulated for 0,000 generations. The per meiosis and per base pair mutation rate was 0 8 and mutations followed the infinite sites model 8]. After the initial 0,000 generations, N e was reduced to 00 over 0 generations to mimic a livestock population. In the last generation, 0,000 animals were generated and their genotypes and phenotypes were used in genetic analysis. The total number of segregating loci in generation 0,000 was 53,836, of which about half 279,504) were still segregating in the last generation generation 0,00). Per chromosome, 200 SNPs with a minor allele frequency higher than 0.0 were randomly sampled as causative SNPs, i.e causative SNPs 2p in total. Genotypes were standardized to 2pj p j, j) 2p j 2pj p j) and 2 2p j 2pj p j) for the genotypes 0 0, 0 and, respectively, where p j is the frequency of the allele, and collected in the genotype matrix X. True genetic values of the animals were obtained as: TBV = αxb, 33) where b is a 53,836 ) vector, including 4000 quantitative trait loci QTL) SNPs that were declared as QTL) effects, which were sampled from a normal distribution, and effects of non-causative SNPs set to 0. All QTL effects were scaled by α such that total additive genetic variance in generation 0,00 was σg 2 =.0. Residual environmental effects were sampled from N 0, Iσe 2 ), with σe 2 set such that heritability was 0.25, 0.50 or No fixed effects were simulated. The resulting dataset was analyzed with several statistical models: ) Ordinary GBLUP y = µ + Zg + e, ) g N 0, Gσg 2. 2) Reduced-rank PCRR chromosome-wise SVD) y = µ + ˆTs + e, ) s N 0, Iσm 2, g = ˆTs.
8 Page 8 of 2 3) GBLUP using reduced-dimension approximations of GRM a. Chromosome-wise SVD PCIG-C) b. Genome-wide SVD PCIG-G) c. QR-based genome-wide) d. APY genome-wide) y = µ + Zg + e, ) g N 0, Gσg 2, where G is an approximation of G, using the PCIG-G, PCIG-C, QRIG, or APY algorithms. The chromosome-wise SVD PCRR or PCIG-C) was performed independently for each chromosome based on a core sample of 500, 000 or 2000 individuals. For each chromosome, the number of components was set such that > 99% of the chromosome-specific genomic variation in the core) was explained by the chosen PC. These PC were then used to compute ˆT. For the PCIG-G, an economy-sized SVD was performed across all chromosomes for the core sample 500 to 2000 individuals) and, thus, the final number of components was equal to the core sample size. The QR-based algorithm was based on all genotypes of the core sample, while the APY algorithm was based on genomic relationships of core sample individuals. All models and algorithms were compared based on their accuracy of predicting the true breeding values of validation animals that had masked phenotypes. Validation animals were randomly sampled among non-core animals with a probability of 0%). Data preparation and statistical analyses were performed using Julia software scripts All solutions were obtained by solving the mixed model equations directly. Real data analysis The PCIG-C and APY algorithms were also used in a single-step multi-trait genomic evaluation of a real dataset, which was comprised of data from the Irish beef cattle carcass evaluation and included 8.33 million animals with records on nine traits. The model used was identical excluding genetic groups) to the standard Irish beef cattle evaluation model 9]. There were 3.35 million animals in the pedigree, of which 63,277 were genotyped. Genotyping was done by using the Illumina Bovine SNP50 Bead Chip Illumina, San Diego, USA), of which 54,620 SNPs on 29 autosomes were included in the analysis after quality edits). The population was heterogeneous and included genotypes of animals from 4 breeds. Hence, the dataset was challenging in the sense that a large core sample was needed to capture genetic variation in all breeds. For PCIG-C, the number of components per chromosome was set such that it explained a given percentage from 90 to 95%) of the chromosomespecific genomic variation and core sample sizes were 30,000 to 50,000. The resulting estimated breeding values EBV) using the PCIG-C and APY inverse GRM were compared with the original EBV based on direct inversion of G + Iθ). The analysis was conducted using an iterative solver in the MIX99 software using the preconditioned conjugate gradient method and iteration on data. A value of θ = 0 3 was added to the diagonal elements of the GRM to ensure that the matrix was positive definite. Two simple Julia scripts are attached, demonstrating ) how to use SVD methods to compute reduceddimension approximations of a larger genomic data using a core sample Additional file ), and 2) how to combine reduced-dimension genomic data from multiple chromosomes in computation of an inverse approximated genomic relationship matrix Additional file 2). Results Simulation study When based on the same PC, the PCIG and PCRR models are equivalent, except that for PCIG a small number is added to the diagonal elements of the GRM prior to inversion. Thus, for the simulation study, the results of PCIG and PCRR were nearly identical and only results for PCIG are shown. The correlations of the EBV from each model with the EBV of the full-dimensional GBLUP are in Fig.. In general, across core sample sizes 500 to 2000) and heritabilities 0.25 to 0.90), the PCIG-C model resulted in very similar EBV as GBLUP, with EBV correlations ranging from to.000. The results were less favorable for models PCIG-G, APY and QRIG EBV correlations ranging from to 0.984). Differences of the PCIG-C from the other models were largest for the lowest core sample sizes 500) and highest heritability 0.90). With respect to accuracy of selection correlation between EBV and true breeding value), GBLUP and PCIG-C had very similar and generally higher accuracies than the other models Fig. 2), 0.82, 0.88 and 0.95 for heritability equal to 0.25, 0.50 and 0.90, respectively. Differences in accuracy of GBLUP/PCIG-C from the other models were largest at the lowest core samples and highest heritabilities e.g. for a core sample of 500 and heritability 0.90, accuracy was 0.95 for GBLUP/PCIG-C vs. 0.8 for the other methods). At the lowest core sample size 500), genomic relationships were so crudely
9 Page 9 of 2 Correla on Correla on Correla on a b c Size of core sample PCIG-C PCIG-G APY QRIG Size of core sample PCIG-C PCIG-G APY QRIG Size of core sample PCIG-C PCIG-G APY QRIG Fig. Correlations of genomic estimated breeding values for validation animals obtained using chromosome-wise PCIG PCIG-C), genome-wise PCIG PCIG-G), QR-based inversion QRIG) and the APY GBLUP APY) with those obtained using ordinary GBLUP. Based on simulated data with heritability equal to 0.25 a), 0.50 b), and 0.90 c) described by PCIG-G, APY and QRIG that very little information was obtained by changing the heritability from 0.25 to At higher core sample sizes, the differences between GBLUP/PCIG-C and the other methods were smaller, but not negligible, even at core sample sizes up to As a result, PCIG-C was much more robust to core sample size and achieved comparable results to the full-dimension GBLUP, even at the smallest core sizes tested. Using PCIG-C, the average number of PC needed to capture at least 99% of the genomic variation per chromosome was 239, 298 and 340 for, respectively, 500, 000 and 2000 animals in the core 4770, 5959 and Accuracu of selec on Accuracy of selec on Accuracy of selec on a b c Size of core sample PCIG-C PCIG-G APY QRIG Size of core sample PCIG-C PCIG-G APY QRIG Size of core sample PCIG-C PCIG-G APY QRIG Fig. 2 Accuracies of genomic estimated breeding values correlation with true breeding values) for validation animals, using chromosomewise PCIG PCIG-C), genome-wise PCIG PCIG-G), QR-based inversion QRIG) and the APY GBLUP APY). Based on simulated data with heritability equal to 0.25 a), 0.50 b), and 0.90 c). Accuracies of the original GBLUP method were essentially identical to those from PCIG- C and are not shown 6795 components across all chromosomes). Hence, for this data structure, the genomic relationships could be effectively approximated with a limited number of chromosome-specific PC, even when estimated from core sample sizes down to 500 individuals. With respect to computing time, QR decomposition QRIG) required down to 8% less computing time than SVD PCIG models) when applied to genomic data on single chromosomes ~ 25 k loci). The relative difference in computation time between QR and SVD was largest at smaller core samples. However, at small core samples,
10 Page 0 of 2 both methods were fast, making the relative difference in computing time less important. Multi breed beef cattle data For the real data analysis of the multiple-breed beef cattle population using PCIG-C, the results were essentially identical for core sample sizes of 30,000 and 50,000, hence only results of the latter are presented. Correlations of EBV from the PCIG-C single step models with EBV from the original GBLUP direct inversion) model were high for all traits to 0.999, to 0.999, and.000 when the chosen components explained 85, 90 and 95% of chromosomal genomic variance, respectively). The corresponding numbers of chosen components were 30,208 85%), 34,655 90%), and 40,40 95%). APY based on a core sample size of 50,000 individuals resulted in almost identical ranking of animals based on EBV as the original model rank correlations ranging from to.000), while the rank of animals based on an APY of a core sample of 30,000 individuals corresponding in rank i.e. no of PC) with PCIG-C 85%) had a slightly lower correlation with the rank from the original model to 0.996). For a similar rank ~ 30,000) of the GRM number of chosen PC in PCIG and number of core animals in APY), PCIG- C needed a smaller number of iterations to converge 35 to 385 vs. 69 to 756, for PCIG-C and APY, respectively). Computing times could not be compared directly, as these may have been influenced by other jobs running simultaneously on the computer cluster. Discussion When based on the same PC, the PCRR and PCIG algorithms gave identical EBV. However, the PCIG algorithm is more flexible in that it can easily be incorporated into existing single-step genomic animal models. The results of the current study show that all reduced-dimension algorithms PCRR, PCIG-C, PCIG-G, APY and QRIG) approach the GBLUP solutions when core sample size becomes large. However, the PCRR and PCIG-C algorithms were, by far, the most robust to reductions in core sample size. For the simulated data, the EBV were virtually identical to the EBV obtained with full-dimension GBLUP for all core sample sizes even down to 500 individuals) and heritabilities, with correlations between EBV ranging from to.000. For the other methods, accuracy of selection dropped considerably at smaller core sample sizes 500 and 000), especially with high heritability. In the real data analysis of a multi-breed beef cattle population, core sample size was generally large and differences between methods were thus smaller, but still in the favor of PCIG-C compared with APY. The PCIG algorithm can be used to calculate the exact inverse of an approximated GRM, even for extremely large genomic datasets that potentially contain millions of individuals and loci, using a limited number of PC per chromosome. The SVD-based PCIG-C uses all genetic data from the core sample to identify the more important PC for each chromosome, and the GRM is based on these. The method can be heavily parallelized, since SVD is performed separately for each chromosome. The number of PC needed to describe the relationship structure of a population depends on the effective number of segregating genomic segments in the population, which for large populations of limited N e is typically much smaller than the actual population size N). After SVD, the inverse of GRM using PCIG) can be computed easily, and potentially row- or element-wise, which gives room for further parallelization. Hence, computing time can be reduced substantially. Using iteration on data, rows of the inverse of GRM can be computed directly during iteration and, thus, the entire inverse GRM does not need to be stored explicitly. In contrast, when performing brute force inversion of the entire GRM, memory requirements increase quadratically and numbers of computations increase cubically with the number of animals in the population 20]. Compared with PCIG, QRIG algorithm based on QR-decomposition was slightly faster and has potential for parallelization by chromosome). However, this model is less well suited for dimensionality reduction below core sample size e.g. per chromosome) and is more sensitive to size of the core sample. Thus, we prefer the PCIG-C over the QRIG algorithm. The PCIG algorithm proposed in this study is related to the APY algorithm 4], since both methods use genomic data in a core sample to approximate the inverse) GRM of all animals. In APY, the core sample must be sufficiently small such that the inverse of the core GRM can be computed directly, and the remaining elements of the entire inverse of GRM are computed based on the inverse relationships of the core individuals and the relationships between core and non-core individuals. Furthermore, APY assumes that the non-core part of the inverse GRM is diagonal, while PCIG makes no such assumptions. Using PCIG, the GRM is approximated by a limited number of PC and by adding a small number to the diagonal elements, while the inverse of this matrix is computed by exact methods. Hence, given that the GRM can be appropriately approximated using PC estimated from the core sample, the computed inverse of GRM from PCIG will necessarily also be appropriate, which explains why solutions from reduced-rank PCIG-C were nearly identical to those obtained from full-dimension GBLUP in this study, even at the smallest core sample sizes. The genome-wide
11 Page of 2 PCIG-G gave similar solutions as APY and genome-wide) QRIG, which can be explained by the fact that the maximum number of components in genome-wide analysis is limited by the size of the core sample, while the maximum number of components in chromosome-wise PCIG-C is larger size of core sample x number of chromosomes). For PCIG-G, APY and QRIG this is an especially limiting factor in smaller core samples, as observed with the simulated dataset, e.g. for these genome-wide methods a core size of 500 imply that the GRM is approximated by, at most, 500 components PC or animal effects) while up to 0,000 PC may be used in the PCRR/PCIG-C models. Genetic analyses based on chromosome-wise SVD of a core sample assumes that genetic variation associated with each chromosome can be explained by the chosen chromosome-specific components i.e. haplotype blocks), and that the same components are present and responsible for genetic variation in the entire population. In contrast, the APY algorithm assumes that all genetic variation in the population is explained by the additive genetic effects of individuals in the core sample, i.e. that breeding values of non-core individuals are merely functions of breeding values of the core individuals. This implies that, if accuracies of core individuals approach unity e.g. bulls with large daughter groups), accuracy of the entire genotyped population is also assumed to approach unity, even for newly born genotyped individuals, which is not likely to be true. Even if thousands of historical bulls with progeny are included in the core sample, the EBV of a genotyped calf is not expected to be perfect. In PCIG-C, a more realistic approach is taken, since the accuracy of non-core animals depends on the precision of the estimated effects of the underlying PC, rather than on the accuracy of the EBV of core animals. The number of underlying components may exceed the number of core individuals and, thus, a high accuracy of the EBV of core animals does not imply high accuracies for all underlying components. Thus, as the EBV of non-core animals are functions of these components, genotyped newborn animals are not necessarily assumed to be predicted accurately, even if the core animals are accurate. In real data, population structures may be more complex and stratified. Hence, real data analyses of complex populations may require larger core samples, e.g. as in the real multi-population dataset analyzed here. The methods used herein, only consider simple SNP- BLUP or genomic animal models, where, a priori, genetic variance is evenly distributed across the genome. However, such simplistic models likely do not use the full potential of high-density or sequence data, which may include genotypes of the causative mutations themselves. One alternative is to combine SVD techniques with methods that allow for different weighting of the SNPs in the model i.e. approximating Bayesian variable selection models). This approach is described and evaluated in a separate study 2]. Conclusions We propose SVD-based methods for genomic prediction. Although SVD may be computationally demanding, the analysis can be performed on a reduced core sample of individuals and/or in parallel on different genome segments, making fast computation possible. After SVD, large-scale genomic analysis can be performed either by PC ridge regression PCRR) or by a genomic animal model GBLUP), with the GRM and its inverse defined by the chosen PC PCIG). The principal component-based GRM is not of full rank but can be made invertible by adding a small number to the diagonal of the entire matrix, and its exact inverse can be easily obtained using the Woodbury formula. The inverse of the SVD-based GRM can be computed row- or element-wise, and the entire matrix does not need to be stored explicitly, e.g. when applying iteration on data. Based on simulated data, PCRR/PCIG models based on chromosome-wise SVD of genomic data from a limited core sample resulted in essentially identical solutions for the entire population as the full-dimension GBLUP model correlations between EBV =.000), while other methods genome-wide SVD, QRIG and APY) were less accurate, especially at smaller core sample sizes. Additional files Additional file. How to do reduced-dimension approximation of a larger genomic data set from a specific chromosome, using SVD of data on the same chromosome in a smaller core sample. Additional file 2. Combining reduced-dimension genomic data from multiple chromosomes in computation of an inverse approximated genomic relationship matrix. Authors contributions JØ performed the statistical analysis and wrote the manuscript. THEM produced the simulated data set, participated in developing the computational approach, and helped write the manuscript. UI improved computational strategies. IS performed statistical analysis of the large-scale real data set. All authors read and approved the final manuscript. Author details AquaGen AS, P.O. Box 240, 7462 Trondheim, Norway. 2 Norwegian University of Life Sciences, P.O. Box 5003, 432 Ås, Norway. 3 Natural Resources Institute Finland Luke), Humppilantie 4, Jokioinen, Finland. Acknowledgements The study was partly funded by The Research Council of Norway through Project No : From whole genome sequence to precision breeding. Competing interests The authors declare that they have no competing interests. Ethics approval and consent to participate Not applicable.
12 Page 2 of 2 Publisher s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. Appendix In principal component regression, the original SNP solutions can be obtained from the principal component solutions as 4]: ˆb = Vŝ. Define ˆβ = VV ˆb and ŝ = V ˆb. This is true, if it holds that ˆβ = ˆb, which we will prove now. The columns of V are orthogonal, V V = I, while, in many cases, VV = I e.g. when the number of loci exceeds the rank of the genotype matrix). As X = USV = TV, multiplying X with VV yields: X VV ) = TV VV = TV = X. Hence, for any vector ǫ of length equal to number of loci, XVV ǫ = Xǫ. This implies that, X ˆβ = X ˆb, i.e. the predicted genetic effect of an animal is identical, whether based on ˆβ or ˆb. The HMME excluding fixed effects) using an MEM is: X X + λi ]ˆb = X y. Using that X VV ) = X and VV ) X = X, this equation system can easily be modified into an equation system for ˆβ. First, we pre-multiply with VV ) : X X + λvv ]ˆb = X y, as ˆβ = VV ˆb and X ˆβ = X ˆb, the equation above is identical to: X X + λi ] ˆβ = X y. The resulting equation system is of full rank and identical to the original MEM equation system. Thus, there is a single solution vector, implying that: ˆβ = ˆb. The SNP-BLUP equations can be reformulated into equivalent PCRR equations as follows: X X + λi ]ˆb = X y, VT TV + λi ]ˆb = VT y. Pre-multiplying the equation above with V yields: T T + λi ] V ˆb = T y. Define: ŝ = V ˆb: T T + λi ] ŝ = T y. The PCRR and SNP-BLUP equations are thus equivalent. Received: 25 January 207 Accepted: 2 January 208 References. Meuwissen THE, Hayes BJ, Goddard ME. Prediction of total genetic value using genome-wide dense marker maps. Genetics. 200;57: Legarra A, Aguilar I, Misztal I. A relationship matrix including full pedigree and genomic information. J Dairy Sci. 2009;92: Christensen OF, Lund MS. Genomic prediction when some animals are not genotyped. Genet Sel Evol. 200;42:2. 4. Misztal I, Legarra A, Aguilar I. Using recursion to compute the inverse of the genomic relationship matrix. J Dairy Sci. 204;97: Fernando RL, Cheng H, Garrick DJ. An efficient exact method to obtain GBLUP and single-step GBLUP when the genomic relationship matrix is singular. Genet Sel Evol. 206;48: Henderson CR. Best linear unbiased estimation and prediction under a selection model. Biometrics. 975;3: VanRaden P. Genomic measures of relationship and inbreeding. Interbull Bull. 2007;37: VanRaden PM. Efficient estimation of breeding values from dense genomic data. J Dairy Sci. 2007;90: Henderson CR. Applications of linear models in animal breeding. In: Schaeffer LR, editor. Applications of linear models in animal breeding. Guelph: University of Guelph; 984. ISBN-0: , ISBN-3: Woodbury MA. Inverting modified matrices. Memorandum Report 42, Statistical Research Group, Princeton, New Jersey; Lay DC. Linear algebra and its applications. Reading: Addison-Wesley; de los Campos G, Gianola D, Rosa GJM, Weigel KA, Crossa J. Semi-parametric genomic-enabled prediction of genetic values using reproducing kernel Hilbert spaces methods. Genet Res Camb). 200;92: Tusell L, Perez-Rodriguez P, Forni S, Wu XL, Gianola D. Genome-enabled methods for predicting litter size in pigs: a comparison. Animal. 203;7: Hastie T, Tibshirani R. Efficient quadratic regularization for expression arrays. Biostatistics. 2004;5: Meuwissen T, Hayes B, Goddard M. Accelerating improvement of livestock with genomic selection. Annu Rev Anim Biosci. 203;: VanRaden PM. Efficient methods to compute genomic predictions. J Dairy Sci. 2008;9: Meuwissen T, Goddard M. Accurate prediction of genetic values for complex traits by whole-genome resequencing. Genetics. 200;85: Kimura M. The number of heterozygous nucleotide sites maintained in a finite population due to steady flux of mutations. Genetics. 969;6: Evans RD, Kearney JF, McCarthy J, Cromie A, Pabiou T. Beef performance evaluations in a multi-layered and mainly crossbred population. In: Proceedings of the 0th world congress on genetics applied to livestock production: 7 22 August 204; Vancouver; Misztal I. Inexpensive computation of the inverse of the genomic relationship matrix in populations with small effective population size. Genetics. 206;202: Meuwissen THE, Indahl UG, Ødegård J. Variable selection models for genomic selection using whole-genome sequence data and singular value decomposition. Genet Sel Evol. 207;49:94.
Limited dimensionality of genomic information and effective population size
 Limited dimensionality of genomic information and effective population size Ivan Pocrnić 1, D.A.L. Lourenco 1, Y. Masuda 1, A. Legarra 2 & I. Misztal 1 1 University of Georgia, USA 2 INRA, France WCGALP,
Limited dimensionality of genomic information and effective population size Ivan Pocrnić 1, D.A.L. Lourenco 1, Y. Masuda 1, A. Legarra 2 & I. Misztal 1 1 University of Georgia, USA 2 INRA, France WCGALP,
Extension of single-step ssgblup to many genotyped individuals. Ignacy Misztal University of Georgia
 Extension of single-step ssgblup to many genotyped individuals Ignacy Misztal University of Georgia Genomic selection and single-step H -1 =A -1 + 0 0 0 G -1-1 A 22 Aguilar et al., 2010 Christensen and
Extension of single-step ssgblup to many genotyped individuals Ignacy Misztal University of Georgia Genomic selection and single-step H -1 =A -1 + 0 0 0 G -1-1 A 22 Aguilar et al., 2010 Christensen and
Genotyping strategy and reference population
 GS cattle workshop Genotyping strategy and reference population Effect of size of reference group (Esa Mäntysaari, MTT) Effect of adding females to the reference population (Minna Koivula, MTT) Value of
GS cattle workshop Genotyping strategy and reference population Effect of size of reference group (Esa Mäntysaari, MTT) Effect of adding females to the reference population (Minna Koivula, MTT) Value of
GBLUP and G matrices 1
 GBLUP and G matrices 1 GBLUP from SNP-BLUP We have defined breeding values as sum of SNP effects:! = #$ To refer breeding values to an average value of 0, we adopt the centered coding for genotypes described
GBLUP and G matrices 1 GBLUP from SNP-BLUP We have defined breeding values as sum of SNP effects:! = #$ To refer breeding values to an average value of 0, we adopt the centered coding for genotypes described
Crosses. Computation APY Sherman-Woodbury «hybrid» model. Unknown parent groups Need to modify H to include them (Misztal et al., 2013) Metafounders
 Details in ssgblup Details in SSGBLUP Storage Inbreeding G is not invertible («blending») G might not explain all genetic variance («blending») Compatibility of G and A22 Assumption p(u 2 )=N(0,G) If there
Details in ssgblup Details in SSGBLUP Storage Inbreeding G is not invertible («blending») G might not explain all genetic variance («blending») Compatibility of G and A22 Assumption p(u 2 )=N(0,G) If there
Lecture 28: BLUP and Genomic Selection. Bruce Walsh lecture notes Synbreed course version 11 July 2013
 Lecture 28: BLUP and Genomic Selection Bruce Walsh lecture notes Synbreed course version 11 July 2013 1 BLUP Selection The idea behind BLUP selection is very straightforward: An appropriate mixed-model
Lecture 28: BLUP and Genomic Selection Bruce Walsh lecture notes Synbreed course version 11 July 2013 1 BLUP Selection The idea behind BLUP selection is very straightforward: An appropriate mixed-model
G-BLUP without inverting the genomic relationship matrix
 G-BLUP without inverting the genomic relationship matrix Per Madsen 1 and Jørgen Ødegård 2 1 Center for Quantitative Genetics and Genomics Department of Molecular Biology and Genetics, Aarhus University
G-BLUP without inverting the genomic relationship matrix Per Madsen 1 and Jørgen Ødegård 2 1 Center for Quantitative Genetics and Genomics Department of Molecular Biology and Genetics, Aarhus University
MIXED MODELS THE GENERAL MIXED MODEL
 MIXED MODELS This chapter introduces best linear unbiased prediction (BLUP), a general method for predicting random effects, while Chapter 27 is concerned with the estimation of variances by restricted
MIXED MODELS This chapter introduces best linear unbiased prediction (BLUP), a general method for predicting random effects, while Chapter 27 is concerned with the estimation of variances by restricted
arxiv: v1 [stat.me] 10 Jun 2018
![arxiv: v1 [stat.me] 10 Jun 2018 arxiv: v1 [stat.me] 10 Jun 2018](/thumbs/79/80338220.jpg) Lost in translation: On the impact of data coding on penalized regression with interactions arxiv:1806.03729v1 [stat.me] 10 Jun 2018 Johannes W R Martini 1,2 Francisco Rosales 3 Ngoc-Thuy Ha 2 Thomas Kneib
Lost in translation: On the impact of data coding on penalized regression with interactions arxiv:1806.03729v1 [stat.me] 10 Jun 2018 Johannes W R Martini 1,2 Francisco Rosales 3 Ngoc-Thuy Ha 2 Thomas Kneib
FOR animals and plants, many genomic analyses with SNP
 INVESTIGATION Inexpensive Computation of the Inverse of the Genomic Relationship Matrix in Populations with Small Effective Population Size Ignacy Misztal 1 Animal and Dairy Science, University of Georgia,
INVESTIGATION Inexpensive Computation of the Inverse of the Genomic Relationship Matrix in Populations with Small Effective Population Size Ignacy Misztal 1 Animal and Dairy Science, University of Georgia,
Lecture 8 Genomic Selection
 Lecture 8 Genomic Selection Guilherme J. M. Rosa University of Wisconsin-Madison Mixed Models in Quantitative Genetics SISG, Seattle 18 0 Setember 018 OUTLINE Marker Assisted Selection Genomic Selection
Lecture 8 Genomic Selection Guilherme J. M. Rosa University of Wisconsin-Madison Mixed Models in Quantitative Genetics SISG, Seattle 18 0 Setember 018 OUTLINE Marker Assisted Selection Genomic Selection
(Genome-wide) association analysis
 (Genome-wide) association analysis 1 Key concepts Mapping QTL by association relies on linkage disequilibrium in the population; LD can be caused by close linkage between a QTL and marker (= good) or by
(Genome-wide) association analysis 1 Key concepts Mapping QTL by association relies on linkage disequilibrium in the population; LD can be caused by close linkage between a QTL and marker (= good) or by
A simple method to separate base population and segregation effects in genomic relationship matrices
 Plieschke et al. Genetics Selection Evolution (2015) 47:53 DOI 10.1186/s12711-015-0130-8 Genetics Selection Evolution RESEARCH ARTICLE Open Access A simple method to separate base population and segregation
Plieschke et al. Genetics Selection Evolution (2015) 47:53 DOI 10.1186/s12711-015-0130-8 Genetics Selection Evolution RESEARCH ARTICLE Open Access A simple method to separate base population and segregation
Using the genomic relationship matrix to predict the accuracy of genomic selection
 J. Anim. Breed. Genet. ISSN 0931-2668 ORIGINAL ARTICLE Using the genomic relationship matrix to predict the accuracy of genomic selection M.E. Goddard 1,2, B.J. Hayes 2 & T.H.E. Meuwissen 3 1 Department
J. Anim. Breed. Genet. ISSN 0931-2668 ORIGINAL ARTICLE Using the genomic relationship matrix to predict the accuracy of genomic selection M.E. Goddard 1,2, B.J. Hayes 2 & T.H.E. Meuwissen 3 1 Department
FaST linear mixed models for genome-wide association studies
 Nature Methods FaS linear mixed models for genome-wide association studies Christoph Lippert, Jennifer Listgarten, Ying Liu, Carl M Kadie, Robert I Davidson & David Heckerman Supplementary Figure Supplementary
Nature Methods FaS linear mixed models for genome-wide association studies Christoph Lippert, Jennifer Listgarten, Ying Liu, Carl M Kadie, Robert I Davidson & David Heckerman Supplementary Figure Supplementary
Animal Model. 2. The association of alleles from the two parents is assumed to be at random.
 Animal Model 1 Introduction In animal genetics, measurements are taken on individual animals, and thus, the model of analysis should include the animal additive genetic effect. The remaining items in the
Animal Model 1 Introduction In animal genetics, measurements are taken on individual animals, and thus, the model of analysis should include the animal additive genetic effect. The remaining items in the
An indirect approach to the extensive calculation of relationship coefficients
 Genet. Sel. Evol. 34 (2002) 409 421 409 INRA, EDP Sciences, 2002 DOI: 10.1051/gse:2002015 Original article An indirect approach to the extensive calculation of relationship coefficients Jean-Jacques COLLEAU
Genet. Sel. Evol. 34 (2002) 409 421 409 INRA, EDP Sciences, 2002 DOI: 10.1051/gse:2002015 Original article An indirect approach to the extensive calculation of relationship coefficients Jean-Jacques COLLEAU
Multiple-Trait Across-Country Evaluations Using Singular (Co)Variance Matrix and Random Regression Model
 Multiple-rait Across-Country Evaluations Using Singular (Co)Variance Matrix and Random Regression Model Esa A. Mäntysaari M Agrifood Research Finland, Animal Production, SF 31600 Jokioinen 1. Introduction
Multiple-rait Across-Country Evaluations Using Singular (Co)Variance Matrix and Random Regression Model Esa A. Mäntysaari M Agrifood Research Finland, Animal Production, SF 31600 Jokioinen 1. Introduction
Repeated Records Animal Model
 Repeated Records Animal Model 1 Introduction Animals are observed more than once for some traits, such as Fleece weight of sheep in different years. Calf records of a beef cow over time. Test day records
Repeated Records Animal Model 1 Introduction Animals are observed more than once for some traits, such as Fleece weight of sheep in different years. Calf records of a beef cow over time. Test day records
Package bwgr. October 5, 2018
 Type Package Title Bayesian Whole-Genome Regression Version 1.5.6 Date 2018-10-05 Package bwgr October 5, 2018 Author Alencar Xavier, William Muir, Shizhong Xu, Katy Rainey. Maintainer Alencar Xavier
Type Package Title Bayesian Whole-Genome Regression Version 1.5.6 Date 2018-10-05 Package bwgr October 5, 2018 Author Alencar Xavier, William Muir, Shizhong Xu, Katy Rainey. Maintainer Alencar Xavier
Recent advances in statistical methods for DNA-based prediction of complex traits
 Recent advances in statistical methods for DNA-based prediction of complex traits Mintu Nath Biomathematics & Statistics Scotland, Edinburgh 1 Outline Background Population genetics Animal model Methodology
Recent advances in statistical methods for DNA-based prediction of complex traits Mintu Nath Biomathematics & Statistics Scotland, Edinburgh 1 Outline Background Population genetics Animal model Methodology
Lecture 9. QTL Mapping 2: Outbred Populations
 Lecture 9 QTL Mapping 2: Outbred Populations Bruce Walsh. Aug 2004. Royal Veterinary and Agricultural University, Denmark The major difference between QTL analysis using inbred-line crosses vs. outbred
Lecture 9 QTL Mapping 2: Outbred Populations Bruce Walsh. Aug 2004. Royal Veterinary and Agricultural University, Denmark The major difference between QTL analysis using inbred-line crosses vs. outbred
Impact of Using Reduced Rank Random Regression Test-Day Model on Genetic Evaluation
 Impact of Using Reduced Rank Random Regression Test-Day on Genetic Evaluation H. Leclerc 1, I. Nagy 2 and V. Ducrocq 2 1 Institut de l Elevage, Département Génétique, Bât 211, 78 352 Jouy-en-Josas, France
Impact of Using Reduced Rank Random Regression Test-Day on Genetic Evaluation H. Leclerc 1, I. Nagy 2 and V. Ducrocq 2 1 Institut de l Elevage, Département Génétique, Bât 211, 78 352 Jouy-en-Josas, France
PCA vignette Principal components analysis with snpstats
 PCA vignette Principal components analysis with snpstats David Clayton October 30, 2018 Principal components analysis has been widely used in population genetics in order to study population structure
PCA vignette Principal components analysis with snpstats David Clayton October 30, 2018 Principal components analysis has been widely used in population genetics in order to study population structure
Maternal Genetic Models
 Maternal Genetic Models In mammalian species of livestock such as beef cattle sheep or swine the female provides an environment for its offspring to survive and grow in terms of protection and nourishment
Maternal Genetic Models In mammalian species of livestock such as beef cattle sheep or swine the female provides an environment for its offspring to survive and grow in terms of protection and nourishment
Modification of negative eigenvalues to create positive definite matrices and approximation of standard errors of correlation estimates
 Modification of negative eigenvalues to create positive definite matrices and approximation of standard errors of correlation estimates L. R. Schaeffer Centre for Genetic Improvement of Livestock Department
Modification of negative eigenvalues to create positive definite matrices and approximation of standard errors of correlation estimates L. R. Schaeffer Centre for Genetic Improvement of Livestock Department
Multi-population genomic prediction. Genomic prediction using individual-level data and summary statistics from multiple.
 Genetics: Early Online, published on July 18, 2018 as 10.1534/genetics.118.301109 Multi-population genomic prediction 1 2 Genomic prediction using individual-level data and summary statistics from multiple
Genetics: Early Online, published on July 18, 2018 as 10.1534/genetics.118.301109 Multi-population genomic prediction 1 2 Genomic prediction using individual-level data and summary statistics from multiple
Chapter 5 Prediction of Random Variables
 Chapter 5 Prediction of Random Variables C R Henderson 1984 - Guelph We have discussed estimation of β, regarded as fixed Now we shall consider a rather different problem, prediction of random variables,
Chapter 5 Prediction of Random Variables C R Henderson 1984 - Guelph We have discussed estimation of β, regarded as fixed Now we shall consider a rather different problem, prediction of random variables,
A relationship matrix including full pedigree and genomic information
 J Dairy Sci 9 :4656 4663 doi: 103168/jds009-061 American Dairy Science Association, 009 A relationship matrix including full pedigree and genomic information A Legarra,* 1 I Aguilar, and I Misztal * INRA,
J Dairy Sci 9 :4656 4663 doi: 103168/jds009-061 American Dairy Science Association, 009 A relationship matrix including full pedigree and genomic information A Legarra,* 1 I Aguilar, and I Misztal * INRA,
Lecture WS Evolutionary Genetics Part I 1
 Quantitative genetics Quantitative genetics is the study of the inheritance of quantitative/continuous phenotypic traits, like human height and body size, grain colour in winter wheat or beak depth in
Quantitative genetics Quantitative genetics is the study of the inheritance of quantitative/continuous phenotypic traits, like human height and body size, grain colour in winter wheat or beak depth in
Causal Graphical Models in Quantitative Genetics and Genomics
 Causal Graphical Models in Quantitative Genetics and Genomics Guilherme J. M. Rosa Department of Animal Sciences Department of Biostatistics & Medical Informatics OUTLINE Introduction: Correlation and
Causal Graphical Models in Quantitative Genetics and Genomics Guilherme J. M. Rosa Department of Animal Sciences Department of Biostatistics & Medical Informatics OUTLINE Introduction: Correlation and
Prediction of genetic Values using Neural Networks
 Prediction of genetic Values using Neural Networks Paulino Perez 1 Daniel Gianola 2 Jose Crossa 1 1 CIMMyT-Mexico 2 University of Wisconsin, Madison. September, 2014 SLU,Sweden Prediction of genetic Values
Prediction of genetic Values using Neural Networks Paulino Perez 1 Daniel Gianola 2 Jose Crossa 1 1 CIMMyT-Mexico 2 University of Wisconsin, Madison. September, 2014 SLU,Sweden Prediction of genetic Values
Animal Models. Sheep are scanned at maturity by ultrasound(us) to determine the amount of fat surrounding the muscle. A model (equation) might be
 Animal Models 1 Introduction An animal model is one in which there are one or more observations per animal, and all factors affecting those observations are described including an animal additive genetic
Animal Models 1 Introduction An animal model is one in which there are one or more observations per animal, and all factors affecting those observations are described including an animal additive genetic
Bare minimum on matrix algebra. Psychology 588: Covariance structure and factor models
 Bare minimum on matrix algebra Psychology 588: Covariance structure and factor models Matrix multiplication 2 Consider three notations for linear combinations y11 y1 m x11 x 1p b11 b 1m y y x x b b n1
Bare minimum on matrix algebra Psychology 588: Covariance structure and factor models Matrix multiplication 2 Consider three notations for linear combinations y11 y1 m x11 x 1p b11 b 1m y y x x b b n1
Lecture 9 Multi-Trait Models, Binary and Count Traits
 Lecture 9 Multi-Trait Models, Binary and Count Traits Guilherme J. M. Rosa University of Wisconsin-Madison Mixed Models in Quantitative Genetics SISG, Seattle 18 0 September 018 OUTLINE Multiple-trait
Lecture 9 Multi-Trait Models, Binary and Count Traits Guilherme J. M. Rosa University of Wisconsin-Madison Mixed Models in Quantitative Genetics SISG, Seattle 18 0 September 018 OUTLINE Multiple-trait
GENOMIC SELECTION WORKSHOP: Hands on Practical Sessions (BL)
 GENOMIC SELECTION WORKSHOP: Hands on Practical Sessions (BL) Paulino Pérez 1 José Crossa 2 1 ColPos-México 2 CIMMyT-México September, 2014. SLU,Sweden GENOMIC SELECTION WORKSHOP:Hands on Practical Sessions
GENOMIC SELECTION WORKSHOP: Hands on Practical Sessions (BL) Paulino Pérez 1 José Crossa 2 1 ColPos-México 2 CIMMyT-México September, 2014. SLU,Sweden GENOMIC SELECTION WORKSHOP:Hands on Practical Sessions
Eiji Yamamoto 1,2, Hiroyoshi Iwata 3, Takanari Tanabata 4, Ritsuko Mizobuchi 1, Jun-ichi Yonemaru 1,ToshioYamamoto 1* and Masahiro Yano 5,6
 Yamamoto et al. BMC Genetics 2014, 15:50 METHODOLOGY ARTICLE Open Access Effect of advanced intercrossing on genome structure and on the power to detect linked quantitative trait loci in a multi-parent
Yamamoto et al. BMC Genetics 2014, 15:50 METHODOLOGY ARTICLE Open Access Effect of advanced intercrossing on genome structure and on the power to detect linked quantitative trait loci in a multi-parent
BLUP without (inverse) relationship matrix
 Proceedings of the World Congress on Genetics Applied to Livestock Production, 11, 5 BLUP without (inverse relationship matrix E. Groeneveld (1 and A. Neumaier ( (1 Institute of Farm Animal Genetics, Friedrich-Loeffler-Institut,
Proceedings of the World Congress on Genetics Applied to Livestock Production, 11, 5 BLUP without (inverse relationship matrix E. Groeneveld (1 and A. Neumaier ( (1 Institute of Farm Animal Genetics, Friedrich-Loeffler-Institut,
Pedigree and genomic evaluation of pigs using a terminal cross model
 66 th EAAP Annual Meeting Warsaw, Poland Pedigree and genomic evaluation of pigs using a terminal cross model Tusell, L., Gilbert, H., Riquet, J., Mercat, M.J., Legarra, A., Larzul, C. Project funded by:
66 th EAAP Annual Meeting Warsaw, Poland Pedigree and genomic evaluation of pigs using a terminal cross model Tusell, L., Gilbert, H., Riquet, J., Mercat, M.J., Legarra, A., Larzul, C. Project funded by:
Linear Regression (1/1/17)
 STA613/CBB540: Statistical methods in computational biology Linear Regression (1/1/17) Lecturer: Barbara Engelhardt Scribe: Ethan Hada 1. Linear regression 1.1. Linear regression basics. Linear regression
STA613/CBB540: Statistical methods in computational biology Linear Regression (1/1/17) Lecturer: Barbara Engelhardt Scribe: Ethan Hada 1. Linear regression 1.1. Linear regression basics. Linear regression
Bases for Genomic Prediction
 Bases for Genomic Prediction Andres Legarra Daniela A.L. Lourenco Zulma G. Vitezica 2018-07-15 1 Contents 1 Foreword by AL (it only engages him) 5 2 Main notation 6 3 A little bit of history 6 4 Quick
Bases for Genomic Prediction Andres Legarra Daniela A.L. Lourenco Zulma G. Vitezica 2018-07-15 1 Contents 1 Foreword by AL (it only engages him) 5 2 Main notation 6 3 A little bit of history 6 4 Quick
Applications of Randomized Methods for Decomposing and Simulating from Large Covariance Matrices
 Applications of Randomized Methods for Decomposing and Simulating from Large Covariance Matrices Vahid Dehdari and Clayton V. Deutsch Geostatistical modeling involves many variables and many locations.
Applications of Randomized Methods for Decomposing and Simulating from Large Covariance Matrices Vahid Dehdari and Clayton V. Deutsch Geostatistical modeling involves many variables and many locations.
Chapter 6 Linkage Disequilibrium & Gene Mapping (Recombination)
 12/5/14 Chapter 6 Linkage Disequilibrium & Gene Mapping (Recombination) Linkage Disequilibrium Genealogical Interpretation of LD Association Mapping 1 Linkage and Recombination v linkage equilibrium ²
12/5/14 Chapter 6 Linkage Disequilibrium & Gene Mapping (Recombination) Linkage Disequilibrium Genealogical Interpretation of LD Association Mapping 1 Linkage and Recombination v linkage equilibrium ²
Properties of Matrices and Operations on Matrices
 Properties of Matrices and Operations on Matrices A common data structure for statistical analysis is a rectangular array or matris. Rows represent individual observational units, or just observations,
Properties of Matrices and Operations on Matrices A common data structure for statistical analysis is a rectangular array or matris. Rows represent individual observational units, or just observations,
Heritability estimation in modern genetics and connections to some new results for quadratic forms in statistics
 Heritability estimation in modern genetics and connections to some new results for quadratic forms in statistics Lee H. Dicker Rutgers University and Amazon, NYC Based on joint work with Ruijun Ma (Rutgers),
Heritability estimation in modern genetics and connections to some new results for quadratic forms in statistics Lee H. Dicker Rutgers University and Amazon, NYC Based on joint work with Ruijun Ma (Rutgers),
Alternative implementations of Monte Carlo EM algorithms for likelihood inferences
 Genet. Sel. Evol. 33 001) 443 45 443 INRA, EDP Sciences, 001 Alternative implementations of Monte Carlo EM algorithms for likelihood inferences Louis Alberto GARCÍA-CORTÉS a, Daniel SORENSEN b, Note a
Genet. Sel. Evol. 33 001) 443 45 443 INRA, EDP Sciences, 001 Alternative implementations of Monte Carlo EM algorithms for likelihood inferences Louis Alberto GARCÍA-CORTÉS a, Daniel SORENSEN b, Note a
VARIANCE COMPONENT ESTIMATION & BEST LINEAR UNBIASED PREDICTION (BLUP)
 VARIANCE COMPONENT ESTIMATION & BEST LINEAR UNBIASED PREDICTION (BLUP) V.K. Bhatia I.A.S.R.I., Library Avenue, New Delhi- 11 0012 vkbhatia@iasri.res.in Introduction Variance components are commonly used
VARIANCE COMPONENT ESTIMATION & BEST LINEAR UNBIASED PREDICTION (BLUP) V.K. Bhatia I.A.S.R.I., Library Avenue, New Delhi- 11 0012 vkbhatia@iasri.res.in Introduction Variance components are commonly used
Chapter 12 REML and ML Estimation
 Chapter 12 REML and ML Estimation C. R. Henderson 1984 - Guelph 1 Iterative MIVQUE The restricted maximum likelihood estimator (REML) of Patterson and Thompson (1971) can be obtained by iterating on MIVQUE,
Chapter 12 REML and ML Estimation C. R. Henderson 1984 - Guelph 1 Iterative MIVQUE The restricted maximum likelihood estimator (REML) of Patterson and Thompson (1971) can be obtained by iterating on MIVQUE,
Best unbiased linear Prediction: Sire and Animal models
 Best unbiased linear Prediction: Sire and Animal models Raphael Mrode Training in quantitative genetics and genomics 3 th May to th June 26 ILRI, Nairobi Partner Logo Partner Logo BLUP The MME of provided
Best unbiased linear Prediction: Sire and Animal models Raphael Mrode Training in quantitative genetics and genomics 3 th May to th June 26 ILRI, Nairobi Partner Logo Partner Logo BLUP The MME of provided
Accounting for read depth in the analysis of genotyping-by-sequencing data
 Accounting for read depth in the analysis of genotyping-by-sequencing data Ken Dodds, John McEwan, Timothy Bilton, Rudi Brauning, Rayna Anderson, Tracey Van Stijn, Theodor Kristjánsson, Shannon Clarke
Accounting for read depth in the analysis of genotyping-by-sequencing data Ken Dodds, John McEwan, Timothy Bilton, Rudi Brauning, Rayna Anderson, Tracey Van Stijn, Theodor Kristjánsson, Shannon Clarke
Solving Large Test-Day Models by Iteration on Data and Preconditioned Conjugate Gradient
 Solving Large Test-Day Models by Iteration on Data and Preconditioned Conjugate Gradient M. LIDAUER, I. STRANDÉN, E. A. MÄNTYSAARI, J. PÖSÖ, and A. KETTUNEN Animal Production Research, Agricultural Research
Solving Large Test-Day Models by Iteration on Data and Preconditioned Conjugate Gradient M. LIDAUER, I. STRANDÉN, E. A. MÄNTYSAARI, J. PÖSÖ, and A. KETTUNEN Animal Production Research, Agricultural Research
Chapter 11 MIVQUE of Variances and Covariances
 Chapter 11 MIVQUE of Variances and Covariances C R Henderson 1984 - Guelph The methods described in Chapter 10 for estimation of variances are quadratic, translation invariant, and unbiased For the balanced
Chapter 11 MIVQUE of Variances and Covariances C R Henderson 1984 - Guelph The methods described in Chapter 10 for estimation of variances are quadratic, translation invariant, and unbiased For the balanced
MODELLING STRATEGIES TO IMPROVE GENETIC EVALUATION FOR THE NEW ZEALAND SHEEP INDUSTRY. John Holmes
 MODELLING STRATEGIES TO IMPROVE GENETIC EVALUATION FOR THE NEW ZEALAND SHEEP INDUSTRY John Holmes A thesis submitted for the degree of Doctor of Philosophy at the University of Otago, Dunedin, New Zealand
MODELLING STRATEGIES TO IMPROVE GENETIC EVALUATION FOR THE NEW ZEALAND SHEEP INDUSTRY John Holmes A thesis submitted for the degree of Doctor of Philosophy at the University of Otago, Dunedin, New Zealand
Association Testing with Quantitative Traits: Common and Rare Variants. Summer Institute in Statistical Genetics 2014 Module 10 Lecture 5
 Association Testing with Quantitative Traits: Common and Rare Variants Timothy Thornton and Katie Kerr Summer Institute in Statistical Genetics 2014 Module 10 Lecture 5 1 / 41 Introduction to Quantitative
Association Testing with Quantitative Traits: Common and Rare Variants Timothy Thornton and Katie Kerr Summer Institute in Statistical Genetics 2014 Module 10 Lecture 5 1 / 41 Introduction to Quantitative
Raphael Mrode. Training in quantitative genetics and genomics 30 May 10 June 2016 ILRI, Nairobi. Partner Logo. Partner Logo
 Basic matrix algebra Raphael Mrode Training in quantitative genetics and genomics 3 May June 26 ILRI, Nairobi Partner Logo Partner Logo Matrix definition A matrix is a rectangular array of numbers set
Basic matrix algebra Raphael Mrode Training in quantitative genetics and genomics 3 May June 26 ILRI, Nairobi Partner Logo Partner Logo Matrix definition A matrix is a rectangular array of numbers set
Singular Value Decomposition
 Chapter 6 Singular Value Decomposition In Chapter 5, we derived a number of algorithms for computing the eigenvalues and eigenvectors of matrices A R n n. Having developed this machinery, we complete our
Chapter 6 Singular Value Decomposition In Chapter 5, we derived a number of algorithms for computing the eigenvalues and eigenvectors of matrices A R n n. Having developed this machinery, we complete our
Linear Models for the Prediction of Animal Breeding Values
 Linear Models for the Prediction of Animal Breeding Values R.A. Mrode, PhD Animal Data Centre Fox Talbot House Greenways Business Park Bellinger Close Chippenham Wilts, UK CAB INTERNATIONAL Preface ix
Linear Models for the Prediction of Animal Breeding Values R.A. Mrode, PhD Animal Data Centre Fox Talbot House Greenways Business Park Bellinger Close Chippenham Wilts, UK CAB INTERNATIONAL Preface ix
Genomic best linear unbiased prediction method including imprinting effects for genomic evaluation
 Nishio Satoh Genetics Selection Evolution (2015) 47:32 DOI 10.1186/s12711-015-0091-y Genetics Selection Evolution RESEARCH Open Access Genomic best linear unbiased prediction method including imprinting
Nishio Satoh Genetics Selection Evolution (2015) 47:32 DOI 10.1186/s12711-015-0091-y Genetics Selection Evolution RESEARCH Open Access Genomic best linear unbiased prediction method including imprinting
Reduced Animal Models
 Reduced Animal Models 1 Introduction In situations where many offspring can be generated from one mating as in fish poultry or swine or where only a few animals are retained for breeding the genetic evaluation
Reduced Animal Models 1 Introduction In situations where many offspring can be generated from one mating as in fish poultry or swine or where only a few animals are retained for breeding the genetic evaluation
7. Symmetric Matrices and Quadratic Forms
 Linear Algebra 7. Symmetric Matrices and Quadratic Forms CSIE NCU 1 7. Symmetric Matrices and Quadratic Forms 7.1 Diagonalization of symmetric matrices 2 7.2 Quadratic forms.. 9 7.4 The singular value
Linear Algebra 7. Symmetric Matrices and Quadratic Forms CSIE NCU 1 7. Symmetric Matrices and Quadratic Forms 7.1 Diagonalization of symmetric matrices 2 7.2 Quadratic forms.. 9 7.4 The singular value
Should genetic groups be fitted in BLUP evaluation? Practical answer for the French AI beef sire evaluation
 Genet. Sel. Evol. 36 (2004) 325 345 325 c INRA, EDP Sciences, 2004 DOI: 10.1051/gse:2004004 Original article Should genetic groups be fitted in BLUP evaluation? Practical answer for the French AI beef
Genet. Sel. Evol. 36 (2004) 325 345 325 c INRA, EDP Sciences, 2004 DOI: 10.1051/gse:2004004 Original article Should genetic groups be fitted in BLUP evaluation? Practical answer for the French AI beef
5. Best Linear Unbiased Prediction
 5. Best Linear Unbiased Prediction Julius van der Werf Lecture 1: Best linear unbiased prediction Learning objectives On completion of Lecture 1 you should be able to: Understand the principle of mixed
5. Best Linear Unbiased Prediction Julius van der Werf Lecture 1: Best linear unbiased prediction Learning objectives On completion of Lecture 1 you should be able to: Understand the principle of mixed
Computational Aspects of Aggregation in Biological Systems
 Computational Aspects of Aggregation in Biological Systems Vladik Kreinovich and Max Shpak University of Texas at El Paso, El Paso, TX 79968, USA vladik@utep.edu, mshpak@utep.edu Summary. Many biologically
Computational Aspects of Aggregation in Biological Systems Vladik Kreinovich and Max Shpak University of Texas at El Paso, El Paso, TX 79968, USA vladik@utep.edu, mshpak@utep.edu Summary. Many biologically
Genetic evaluation for three way crossbreeding
 Genetic evaluation for three way crossbreeding Ole F. Christensen olef.christensen@mbg.au.dk Aarhus University, Center for Quantitative Genetics and Genomics EAAP 2015, Warszawa Motivation (pigs) Crossbreeding
Genetic evaluation for three way crossbreeding Ole F. Christensen olef.christensen@mbg.au.dk Aarhus University, Center for Quantitative Genetics and Genomics EAAP 2015, Warszawa Motivation (pigs) Crossbreeding
Matrices and Vectors. Definition of Matrix. An MxN matrix A is a two-dimensional array of numbers A =
 30 MATHEMATICS REVIEW G A.1.1 Matrices and Vectors Definition of Matrix. An MxN matrix A is a two-dimensional array of numbers A = a 11 a 12... a 1N a 21 a 22... a 2N...... a M1 a M2... a MN A matrix can
30 MATHEMATICS REVIEW G A.1.1 Matrices and Vectors Definition of Matrix. An MxN matrix A is a two-dimensional array of numbers A = a 11 a 12... a 1N a 21 a 22... a 2N...... a M1 a M2... a MN A matrix can
Forecast comparison of principal component regression and principal covariate regression
 Forecast comparison of principal component regression and principal covariate regression Christiaan Heij, Patrick J.F. Groenen, Dick J. van Dijk Econometric Institute, Erasmus University Rotterdam Econometric
Forecast comparison of principal component regression and principal covariate regression Christiaan Heij, Patrick J.F. Groenen, Dick J. van Dijk Econometric Institute, Erasmus University Rotterdam Econometric
GENERALIZED LINEAR MIXED MODELS: AN APPLICATION
 Libraries Conference on Applied Statistics in Agriculture 1994-6th Annual Conference Proceedings GENERALIZED LINEAR MIXED MODELS: AN APPLICATION Stephen D. Kachman Walter W. Stroup Follow this and additional
Libraries Conference on Applied Statistics in Agriculture 1994-6th Annual Conference Proceedings GENERALIZED LINEAR MIXED MODELS: AN APPLICATION Stephen D. Kachman Walter W. Stroup Follow this and additional
p(d g A,g B )p(g B ), g B
 Supplementary Note Marginal effects for two-locus models Here we derive the marginal effect size of the three models given in Figure 1 of the main text. For each model we assume the two loci (A and B)
Supplementary Note Marginal effects for two-locus models Here we derive the marginal effect size of the three models given in Figure 1 of the main text. For each model we assume the two loci (A and B)
Lecture 2: Genetic Association Testing with Quantitative Traits. Summer Institute in Statistical Genetics 2017
 Lecture 2: Genetic Association Testing with Quantitative Traits Instructors: Timothy Thornton and Michael Wu Summer Institute in Statistical Genetics 2017 1 / 29 Introduction to Quantitative Trait Mapping
Lecture 2: Genetic Association Testing with Quantitative Traits Instructors: Timothy Thornton and Michael Wu Summer Institute in Statistical Genetics 2017 1 / 29 Introduction to Quantitative Trait Mapping
EXERCISES FOR CHAPTER 7. Exercise 7.1. Derive the two scales of relation for each of the two following recurrent series:
 Statistical Genetics Agronomy 65 W. E. Nyquist March 004 EXERCISES FOR CHAPTER 7 Exercise 7.. Derive the two scales of relation for each of the two following recurrent series: u: 0, 8, 6, 48, 46,L 36 7
Statistical Genetics Agronomy 65 W. E. Nyquist March 004 EXERCISES FOR CHAPTER 7 Exercise 7.. Derive the two scales of relation for each of the two following recurrent series: u: 0, 8, 6, 48, 46,L 36 7
Genetic Parameter Estimation for Milk Yield over Multiple Parities and Various Lengths of Lactation in Danish Jerseys by Random Regression Models
 J. Dairy Sci. 85:1596 1606 American Dairy Science Association, 2002. Genetic Parameter Estimation for Milk Yield over Multiple Parities and Various Lengths of Lactation in Danish Jerseys by Random Regression
J. Dairy Sci. 85:1596 1606 American Dairy Science Association, 2002. Genetic Parameter Estimation for Milk Yield over Multiple Parities and Various Lengths of Lactation in Danish Jerseys by Random Regression
Evolution of phenotypic traits
 Quantitative genetics Evolution of phenotypic traits Very few phenotypic traits are controlled by one locus, as in our previous discussion of genetics and evolution Quantitative genetics considers characters
Quantitative genetics Evolution of phenotypic traits Very few phenotypic traits are controlled by one locus, as in our previous discussion of genetics and evolution Quantitative genetics considers characters
Distinctive aspects of non-parametric fitting
 5. Introduction to nonparametric curve fitting: Loess, kernel regression, reproducing kernel methods, neural networks Distinctive aspects of non-parametric fitting Objectives: investigate patterns free
5. Introduction to nonparametric curve fitting: Loess, kernel regression, reproducing kernel methods, neural networks Distinctive aspects of non-parametric fitting Objectives: investigate patterns free
Introduction to Machine Learning. PCA and Spectral Clustering. Introduction to Machine Learning, Slides: Eran Halperin
 1 Introduction to Machine Learning PCA and Spectral Clustering Introduction to Machine Learning, 2013-14 Slides: Eran Halperin Singular Value Decomposition (SVD) The singular value decomposition (SVD)
1 Introduction to Machine Learning PCA and Spectral Clustering Introduction to Machine Learning, 2013-14 Slides: Eran Halperin Singular Value Decomposition (SVD) The singular value decomposition (SVD)
Calculation of IBD probabilities
 Calculation of IBD probabilities David Evans and Stacey Cherny University of Oxford Wellcome Trust Centre for Human Genetics This Session IBD vs IBS Why is IBD important? Calculating IBD probabilities
Calculation of IBD probabilities David Evans and Stacey Cherny University of Oxford Wellcome Trust Centre for Human Genetics This Session IBD vs IBS Why is IBD important? Calculating IBD probabilities
BASIC NOTIONS. x + y = 1 3, 3x 5y + z = A + 3B,C + 2D, DC are not defined. A + C =
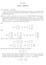 CHAPTER I BASIC NOTIONS (a) 8666 and 8833 (b) a =6,a =4 will work in the first case, but there are no possible such weightings to produce the second case, since Student and Student 3 have to end up with
CHAPTER I BASIC NOTIONS (a) 8666 and 8833 (b) a =6,a =4 will work in the first case, but there are no possible such weightings to produce the second case, since Student and Student 3 have to end up with
APPENDIX A. Background Mathematics. A.1 Linear Algebra. Vector algebra. Let x denote the n-dimensional column vector with components x 1 x 2.
 APPENDIX A Background Mathematics A. Linear Algebra A.. Vector algebra Let x denote the n-dimensional column vector with components 0 x x 2 B C @. A x n Definition 6 (scalar product). The scalar product
APPENDIX A Background Mathematics A. Linear Algebra A.. Vector algebra Let x denote the n-dimensional column vector with components 0 x x 2 B C @. A x n Definition 6 (scalar product). The scalar product
REDUCED ANIMAL MODEL FOR MARKER ASSISTED SELECTION USING BEST LINEAR UNBIASED PREDICTION. R.J.C.Cantet 1 and C.Smith
 REDUCED ANIMAL MODEL FOR MARKER ASSISTED SELECTION USING BEST LINEAR UNBIASED PREDICTION R.J.C.Cantet 1 and C.Smith Centre for Genetic Improvement of Livestock, Department of Animal and Poultry Science,
REDUCED ANIMAL MODEL FOR MARKER ASSISTED SELECTION USING BEST LINEAR UNBIASED PREDICTION R.J.C.Cantet 1 and C.Smith Centre for Genetic Improvement of Livestock, Department of Animal and Poultry Science,
Quantitative genetics theory for genomic selection and efficiency of breeding value prediction in open-pollinated populations
 Scientia Agricola http://dx.doi.org/0.590/003-906-04-0383 Quantitative genetics theory for genomic selection and efficiency of breeding value prediction in open-pollinated populations 43 José Marcelo Soriano
Scientia Agricola http://dx.doi.org/0.590/003-906-04-0383 Quantitative genetics theory for genomic selection and efficiency of breeding value prediction in open-pollinated populations 43 José Marcelo Soriano
MODEL-FREE LINKAGE AND ASSOCIATION MAPPING OF COMPLEX TRAITS USING QUANTITATIVE ENDOPHENOTYPES
 MODEL-FREE LINKAGE AND ASSOCIATION MAPPING OF COMPLEX TRAITS USING QUANTITATIVE ENDOPHENOTYPES Saurabh Ghosh Human Genetics Unit Indian Statistical Institute, Kolkata Most common diseases are caused by
MODEL-FREE LINKAGE AND ASSOCIATION MAPPING OF COMPLEX TRAITS USING QUANTITATIVE ENDOPHENOTYPES Saurabh Ghosh Human Genetics Unit Indian Statistical Institute, Kolkata Most common diseases are caused by
B553 Lecture 5: Matrix Algebra Review
 B553 Lecture 5: Matrix Algebra Review Kris Hauser January 19, 2012 We have seen in prior lectures how vectors represent points in R n and gradients of functions. Matrices represent linear transformations
B553 Lecture 5: Matrix Algebra Review Kris Hauser January 19, 2012 We have seen in prior lectures how vectors represent points in R n and gradients of functions. Matrices represent linear transformations
A reduced animal model with elimination of quantitative trait loci equations for marker-assisted selection
 Original article A reduced animal model with elimination of quantitative trait loci equations for marker-assisted selection S Saito H Iwaisaki 1 Graduate School of Science and Technology; 2 Department
Original article A reduced animal model with elimination of quantitative trait loci equations for marker-assisted selection S Saito H Iwaisaki 1 Graduate School of Science and Technology; 2 Department
MA 575 Linear Models: Cedric E. Ginestet, Boston University Regularization: Ridge Regression and Lasso Week 14, Lecture 2
 MA 575 Linear Models: Cedric E. Ginestet, Boston University Regularization: Ridge Regression and Lasso Week 14, Lecture 2 1 Ridge Regression Ridge regression and the Lasso are two forms of regularized
MA 575 Linear Models: Cedric E. Ginestet, Boston University Regularization: Ridge Regression and Lasso Week 14, Lecture 2 1 Ridge Regression Ridge regression and the Lasso are two forms of regularized
Genetic Parameters for Stillbirth in the Netherlands
 Genetic Parameters for Stillbirth in the Netherlands Arnold Harbers, Linda Segeren and Gerben de Jong CR Delta, P.O. Box 454, 68 AL Arnhem, The Netherlands Harbers.A@CR-Delta.nl 1. Introduction Stillbirth
Genetic Parameters for Stillbirth in the Netherlands Arnold Harbers, Linda Segeren and Gerben de Jong CR Delta, P.O. Box 454, 68 AL Arnhem, The Netherlands Harbers.A@CR-Delta.nl 1. Introduction Stillbirth
Computational Methods. Eigenvalues and Singular Values
 Computational Methods Eigenvalues and Singular Values Manfred Huber 2010 1 Eigenvalues and Singular Values Eigenvalues and singular values describe important aspects of transformations and of data relations
Computational Methods Eigenvalues and Singular Values Manfred Huber 2010 1 Eigenvalues and Singular Values Eigenvalues and singular values describe important aspects of transformations and of data relations
Quantitative genetics theory for genomic selection and efficiency of genotypic value prediction in open-pollinated populations
 4 Scientia Agricola http://dx.doi.org/0.590/678-99x-05-0479 Quantitative genetics theory for genomic selection and efficiency of genotypic value prediction in open-pollinated populations José Marcelo Soriano
4 Scientia Agricola http://dx.doi.org/0.590/678-99x-05-0479 Quantitative genetics theory for genomic selection and efficiency of genotypic value prediction in open-pollinated populations José Marcelo Soriano
COMP6237 Data Mining Covariance, EVD, PCA & SVD. Jonathon Hare
 COMP6237 Data Mining Covariance, EVD, PCA & SVD Jonathon Hare jsh2@ecs.soton.ac.uk Variance and Covariance Random Variables and Expected Values Mathematicians talk variance (and covariance) in terms of
COMP6237 Data Mining Covariance, EVD, PCA & SVD Jonathon Hare jsh2@ecs.soton.ac.uk Variance and Covariance Random Variables and Expected Values Mathematicians talk variance (and covariance) in terms of
1 Springer. Nan M. Laird Christoph Lange. The Fundamentals of Modern Statistical Genetics
 1 Springer Nan M. Laird Christoph Lange The Fundamentals of Modern Statistical Genetics 1 Introduction to Statistical Genetics and Background in Molecular Genetics 0 0 1 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0
1 Springer Nan M. Laird Christoph Lange The Fundamentals of Modern Statistical Genetics 1 Introduction to Statistical Genetics and Background in Molecular Genetics 0 0 1 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0
Linear Algebra - Part II
 Linear Algebra - Part II Projection, Eigendecomposition, SVD (Adapted from Sargur Srihari s slides) Brief Review from Part 1 Symmetric Matrix: A = A T Orthogonal Matrix: A T A = AA T = I and A 1 = A T
Linear Algebra - Part II Projection, Eigendecomposition, SVD (Adapted from Sargur Srihari s slides) Brief Review from Part 1 Symmetric Matrix: A = A T Orthogonal Matrix: A T A = AA T = I and A 1 = A T
Singular Value Decompsition
 Singular Value Decompsition Massoud Malek One of the most useful results from linear algebra, is a matrix decomposition known as the singular value decomposition It has many useful applications in almost
Singular Value Decompsition Massoud Malek One of the most useful results from linear algebra, is a matrix decomposition known as the singular value decomposition It has many useful applications in almost
Quantitative characters - exercises
 Quantitative characters - exercises 1. a) Calculate the genetic covariance between half sibs, expressed in the ij notation (Cockerham's notation), when up to loci are considered. b) Calculate the genetic
Quantitative characters - exercises 1. a) Calculate the genetic covariance between half sibs, expressed in the ij notation (Cockerham's notation), when up to loci are considered. b) Calculate the genetic
Simulation Study on Heterogeneous Variance Adjustment for Observations with Different Measurement Error Variance
 Simulation Study on Heterogeneous Variance Adjustment for Observations with Different Measurement Error Variance Pitkänen, T. 1, Mäntysaari, E. A. 1, Nielsen, U. S., Aamand, G. P 3., Madsen 4, P. and Lidauer,
Simulation Study on Heterogeneous Variance Adjustment for Observations with Different Measurement Error Variance Pitkänen, T. 1, Mäntysaari, E. A. 1, Nielsen, U. S., Aamand, G. P 3., Madsen 4, P. and Lidauer,
Prediction of the Confidence Interval of Quantitative Trait Loci Location
 Behavior Genetics, Vol. 34, No. 4, July 2004 ( 2004) Prediction of the Confidence Interval of Quantitative Trait Loci Location Peter M. Visscher 1,3 and Mike E. Goddard 2 Received 4 Sept. 2003 Final 28
Behavior Genetics, Vol. 34, No. 4, July 2004 ( 2004) Prediction of the Confidence Interval of Quantitative Trait Loci Location Peter M. Visscher 1,3 and Mike E. Goddard 2 Received 4 Sept. 2003 Final 28
2. LINEAR ALGEBRA. 1. Definitions. 2. Linear least squares problem. 3. QR factorization. 4. Singular value decomposition (SVD) 5.
 2. LINEAR ALGEBRA Outline 1. Definitions 2. Linear least squares problem 3. QR factorization 4. Singular value decomposition (SVD) 5. Pseudo-inverse 6. Eigenvalue decomposition (EVD) 1 Definitions Vector
2. LINEAR ALGEBRA Outline 1. Definitions 2. Linear least squares problem 3. QR factorization 4. Singular value decomposition (SVD) 5. Pseudo-inverse 6. Eigenvalue decomposition (EVD) 1 Definitions Vector
Assignment 2 (Sol.) Introduction to Machine Learning Prof. B. Ravindran
 Assignment 2 (Sol.) Introduction to Machine Learning Prof. B. Ravindran 1. Let A m n be a matrix of real numbers. The matrix AA T has an eigenvector x with eigenvalue b. Then the eigenvector y of A T A
Assignment 2 (Sol.) Introduction to Machine Learning Prof. B. Ravindran 1. Let A m n be a matrix of real numbers. The matrix AA T has an eigenvector x with eigenvalue b. Then the eigenvector y of A T A
Selection on Multiple Traits
 Selection on Multiple Traits Bruce Walsh lecture notes Uppsala EQG 2012 course version 7 Feb 2012 Detailed reading: Chapter 30 Genetic vs. Phenotypic correlations Within an individual, trait values can
Selection on Multiple Traits Bruce Walsh lecture notes Uppsala EQG 2012 course version 7 Feb 2012 Detailed reading: Chapter 30 Genetic vs. Phenotypic correlations Within an individual, trait values can
Lecture 24: Multivariate Response: Changes in G. Bruce Walsh lecture notes Synbreed course version 10 July 2013
 Lecture 24: Multivariate Response: Changes in G Bruce Walsh lecture notes Synbreed course version 10 July 2013 1 Overview Changes in G from disequilibrium (generalized Bulmer Equation) Fragility of covariances
Lecture 24: Multivariate Response: Changes in G Bruce Walsh lecture notes Synbreed course version 10 July 2013 1 Overview Changes in G from disequilibrium (generalized Bulmer Equation) Fragility of covariances
Supplementary Materials for Molecular QTL Discovery Incorporating Genomic Annotations using Bayesian False Discovery Rate Control
 Supplementary Materials for Molecular QTL Discovery Incorporating Genomic Annotations using Bayesian False Discovery Rate Control Xiaoquan Wen Department of Biostatistics, University of Michigan A Model
Supplementary Materials for Molecular QTL Discovery Incorporating Genomic Annotations using Bayesian False Discovery Rate Control Xiaoquan Wen Department of Biostatistics, University of Michigan A Model
Genomic prediction using haplotypes in New Zealand dairy cattle
 Graduate Theses and Dissertations Iowa State University Capstones, Theses and Dissertations 2016 Genomic prediction using haplotypes in New Zealand dairy cattle Melanie Kate Hayr Iowa State University
Graduate Theses and Dissertations Iowa State University Capstones, Theses and Dissertations 2016 Genomic prediction using haplotypes in New Zealand dairy cattle Melanie Kate Hayr Iowa State University
Genetic parameters for various random regression models to describe total sperm cells per ejaculate over the reproductive lifetime of boars
 Published December 8, 2014 Genetic parameters for various random regression models to describe total sperm cells per ejaculate over the reproductive lifetime of boars S. H. Oh,* M. T. See,* 1 T. E. Long,
Published December 8, 2014 Genetic parameters for various random regression models to describe total sperm cells per ejaculate over the reproductive lifetime of boars S. H. Oh,* M. T. See,* 1 T. E. Long,
