May 3, Abstract. Studies of ion-molecule reactions at low temperatures are difficult because stray electric
|
|
|
- Della Terry
- 6 years ago
- Views:
Transcription
1 New method to study ion-molecule reactions at low temperatures and application to the H H 2 H H reaction P. Allmendinger, J. Deiglmayr, O. Schullian, K. Höveler, J. A. Agner, H. Schmutz, F. Merkt arxiv: v1 [physics.chem-ph] 2 May 2017 Laboratorium für Physikalische Chemie, ETH Zürich, CH-8093 Zurich, Switzerland May 3, 2017 Abstract Studies of ion-molecule reactions at low temperatures are difficult because stray electric fields in the reaction volume affect the kinetic energy of charged reaction partners. We describe a new experimental approach to study ion-molecule reactions at low temperatures and present, as example, a measurement of the H H 2 H H reaction with the H+ 2 ion prepared in a single rovibrational state at collision energies in the range E col /k B = 5-60 K. To reach such low collision energies, we use a merged-beam approach and observe the reaction within the orbit of a Rydberg electron, which shields the ions from stray fields. The first beam is a supersonic beam of pure ground-state H 2 molecules and the second is a supersonic beam of H 2 molecules excited to Rydberg-Stark states of principal quantum number n selected in the range Initially, the two beams propagate along axes separated by an angle of 10. To merge the two beams, the Rydberg molecules in the latter beam are deflected using a surface-electrode Rydberg-Stark deflector. The collision energies of the merged beams are determined by measuring the velocity distributions of the two beams and they are adjusted by changing the temperature of the pulsed valve used to generate the ground-state H 2 beam and by adapting the electric-potential functions to the electrodes of the deflector. The collision energy is varied down to below E col /k B = 10 K, i.e., below E col 1 mev, with an energy resolution of 100 µev. We demonstrate that the Rydberg electron acts as a spectator and does not affect the cross sections, which are found to closely 1
2 follow a classical-langevin-capture model in the collision-energy range investigated. Because all neutral atoms and molecules can be excited to Rydberg states, this method of studying ion-molecule reactions is applicable to reactions involving singly-charged cations. 1 Introduction Ion-molecule reactions are very important chemical processes in low-density gaseous environments, which justifies the considerable attention they have received over the years. Many ionmolecule reactions have low reaction barriers or are barrierless and have large rate coefficients, even at very low temperatures. Consequently, they represent ideal systems with which to study chemical processes at temperatures close to 0 K, where one expects quantum-mechanical effects to become important (see, e.g., Refs. [1, 2, 3, 4, 5] and references therein). Studies of ionmolecule reactions at low temperature are also important for the development of reliable models of astrochemical reaction cycles [6, 7, 8, 9]. The desire to study ion-molecule reactions at ever lower temperatures has stimulated a continuous stream of innovation for more than a century and numerous review articles provide a good overview of the progress achieved, experimentally and theoretically, in the understanding of low-temperature ion-molecule chemistry [10, 11, 12]. Recently developed methods with which ion-molecule reactions can be studied at low temperatures include the use of uniform supersonic flows [13], of multipole ion guides and buffer-gas cooling [14, 15], and the use of ultracold ions in Coulomb crystals [16, 17, 18]. A major difficulty encountered in experimental studies of ion-molecule reactions at the lowest temperature arises from stray electric fields. A variation of the electric potential of only 1 mv imparts 1 mev kinetic energy onto a singly-charged ion and thus hinders a control of the collision energy (E col ) below this level, i.e., below E col /k B = 12 K, where k B is Boltzmann s constant. For this reason, even some of the most important ionmolecule reactions in the universe, such as the reaction H H 2 H H, have not been studied experimentally so far at temperatures below about 50 K. 2
3 We present here an experimental approach to study ion-molecule reactions at collision energies below E col = k B 10 K which relies on the combination of the following three essential aspects: (i) Rather than studying an ion-molecule reaction in free space, we study it within the orbit of a Rydberg electron of high principal quantum number (n > 20), which thus only acts as a distant spectator to the ion-molecule reaction. The advantage of this approach is that the reaction is shielded from electric stray fields by the Rydberg electron. That ion-molecule reactions can be studied in this way has been advocated and demonstrated by several authors [19, 20, 21, 22, 23]. (ii) To reach low collision energies, we merge two pulsed supersonic beams produced by pulsed valves capable of generating short pulses of about 10 µs duration. When the gas pulses propagate over large distances in a vacuum chamber, the molecules separate spatially according to their respective velocities and this enables the selection of molecules with very narrow velocity distributions. This advantage has been elegantly exploited in studies of reactions between neutral molecules at very low temperatures [24, 25, 26, 27, 28]. (iii) To merge a beam of Rydberg molecules with a beam of ground-state molecules, we use an on-chip Rydberg- Stark surface-electrode deflector [29], which also serves the purpose of selecting a very restricted phase-space volume and thereby enhances the collision-energy resolution of the experiment. This approach is in principle applicable to a broad range of ion-molecule reactions because all atoms and molecules can be excited to Rydberg states. To illustrate it, we present a measurement of the relative integral cross section of the H 2 + H 2 H H + e reaction (H 2 designates an H 2 molecule excited to a Rydberg state), which was shown to be equivalent to the reaction H H 2 H H [19]. This reaction is very important in astrochemistry and has been extensively studied experimentally [30, 31, 32, 33, 19] and theoretically [34, 35], in the low temperature regime most recently by Dashevskaya et al. [36] and Sanz-Sanz et al. [37]. In their efforts to measure the integral cross section for this reaction at low collision energies, Glenewinkel-Meyer and Gerlich have been able to obtain reliable values down to E col /k B = 60 K [38]. Their data, with which the most recent theoretical results are compared [37], still 3
4 represent the experimental reference on this reaction at low collision energies. The results we present here extend the collision-energy range from the lowest collision energies reported by Glenewinkel-Meyer and Gerlich to E col /k B = 5 K, with selected measurements below 2 K. 2 Experiment 2.1 Experimental setup and procedure A schematic view of the experimental setup is presented in Fig. 1. It consists of differentially pumped beam-source chambers and a reaction chamber with a time-of-flight mass spectrometer. The beam-source chambers are separated from the reaction chamber by a 6-cm-long region containing a Rydberg-Stark surface-electrode deflector. Two pulsed collimated supersonic beams of pure H 2 are formed in the beam-source chambers using liquid-nitrogen-cooled pulsed valves and initially propagate at an angle of 10. After passing a skimmer, the two beams enter a high-vacuum chamber and are further collimated just before the reaction zone (see Fig. 1). Just before the two beams cross, the molecules in one of these two beams are excited from the X 1 Σ + g (v = 0, J = 0) ground state to long-lived nkm Rydberg-Stark states with principal quantum number n in the range and the ion core in the X + 2 Σ + g (v + = 0, N + = 0) state using the resonant three-photon excitation sequence nkm[x + 2 Σ + g (0, 0)] I 1 Π g (0, 2) B 1 Σ + u (3, 1) X 1 Σ + g (0, 0). (1) This excitation sequence and the three lasers used for the successive excitation steps have been described in Refs. [39, 40]. In the following, we refer to the beam containing Rydberg H 2 molecules as the Rydberg H 2 beam and to the other beam as the ground-state H 2 beam, which consists of a statistical mixture of para and ortho H 2 molecules in their X 1 Σ + g (v = 0, N = 0) (25%) and (v = 0, N = 1) (75%) ground state. A surface-electrode deflector, consisting of a set of electrodes on a bent printed circuit board (PCB) (see Ref. [29] for details), is used to deflect the Rydberg molecules and to merge the two 4
5 supersonic beams. A shutter labeled C in Fig. 1 prevents undeflected Rydberg molecules from entering the reaction zone. The deflector can also be used to select molecules with a narrower velocity distribution than the initial beam and to accelerate or decelerate them. The relative central velocity of the two beams is set either by adjusting the temperature of the pulsed valve generating the ground-state H 2 beam while keeping the velocity of the Rydberg H 2 beam constant, or by adjusting the waveforms of the electric potentials applied to the electrodes of the deflector. Because a precise knowledge of the velocity distributions of the two beams is crucial for the determination of the energy dependence of the cross sections, they are measured independently. The velocity distribution of the ground-state H 2 beam is determined by photoexciting the H 2 molecules to long-lived Rydberg states at the spot labeled A in Fig. 1. The photoexcitation is carried out before the molecules enter into the reaction zone using the same excitation sequence (Eq. (1)) as used to generate the Rydberg molecules in the second beam. The long-lived Rydberg molecules then fly as neutral particles until they reach the microchannel-plate (MCP) detector labeled MCP1 in Fig. 1 and located beyond the reaction zone. The velocity distribution of the molecules excited at spot A during the 5-ns-long laser-excitation pulse is directly determined from the distribution of flight times to the detector. The full velocity distribution of the beam is reconstructed from measurements in which the delay between the valve-opening time and the laser-photoexcitation pulse is systematically varied over the entire duration of the gas pulse (see Fig. 2 and Section 2.2). The three-dimensional velocity distribution and the main geometrical parameters of the experimental configurations (e.g., the valve-opening time and the distances between the valve front plate, the excitation spot, the reaction zone and the detectors) are used as input parameters for the simulation of the trajectories of the ground-state H 2 and the Rydberg H 2 molecules in the vacuum chambers, as described in Section 3. The procedure used to determine the velocity and density distributions of the deflected Rydberg H 2 beam has been described in Ref. [29], to which we refer the reader for details (see also Section 3). 5
6 The merged beams enter a reaction zone located within a cylindrical stack made of three electrodes, with which the reaction products are extracted towards an imaging detector (MCP with phosphor screen ( MCP2 ) and CCD camera) in a direction perpendicular to the mergedbeam-propagation axis. The extraction electrodes have a diameter of 7 cm and are designed so that pulsed extraction fields can be applied in a Wiley-McLaren configuration [41] to extract the ions and detect them mass selectively. The electric-field distribution generated in the reaction zone upon application of a potential difference across the stack is determined using a commercial simulation program (SIMION 8.1 [42]). The same program is also used to simulate the trajectories of the H + 3 ions produced by the reaction and to calculate their time-of-flight distribution. An essential aspect of the experiments presented in this article is linked to the ability of generating short pulses of cold molecules in well-collimated supersonic beams. The techniques required to generate short-pulse molecular beams are well established [43, 44]. For experiments in which the velocity of a supersonic beam is tuned by varying the valve temperature, solenoid valves offer distinct advantages because they can be operated at cryogenic temperatures (see, e.g., Refs. [45, 46]). To achieve pulse lengths of about 10 µs for the experiments presented in this article, special attention was paid to the miniaturization of the design following general principles established in the development of earlier valves [43, 45, 44]. Our home-built pulsed valves consist of a 1.5-mm-diameter cylindrical plunger (Vacuumschmelze Vacoflux50) located inside a ceramic tube. The plunger is spring-preloaded with a coil spring towards a mm-thick polyimide disk sealing the front plate with a central orifice of 0.35 mm diameter. The plunger is actuated with a coil (0.4-mm-diameter copper wire, 65 windings). No specific measure is taken to guide or enhance the magnetic fields near the plunger. Instead, high pulsed currents of about 100 A are applied to the coil. To avoid eddy currents, the coil is surrounded by Al 2 O 3 ceramic spacers. With this design, valve opening times as short as 13 µs could be achieved and were used in the present work. The valve assembly is fully encapsulated in a copper body with epoxy (Stycast 6
7 2850ft). The pulsed valve is linked to a liquid-nitrogen Dewar using copper braids and the valve temperature is regulated by heating. Another essential aspect of the measurement procedure is that it is accompanied by numerical simulations of the reaction from the preparation of the molecules in the supersonic beams prior to the reaction to the detection of the product ions. The simulation procedure is outlined in Section Determination of velocity distributions and collision energies The measurement of the velocity distribution of the H 2 molecules in the ground-state beam is carried out by photoexcitation to long-lived Rydberg states at the position marked A in Fig. 1 and recording their times of flight to the MCP detector labeled MCP1 in Fig. 1. Because of the short duration of the valve opening, the length of the gas pulse at the photoexcitation spot is almost entirely determined by the velocity distribution of the molecules in the supersonic beam and by the experimental geometry. The distances between the valve orifice and the skimmer and the photoexcitation spot are 7 and 80 cm, respectively. The fastest molecules in the beam arrive at the photoexcitation spot first and are excited at the shortest delay times between the opening of the pulsed valve and the laser pulse. Consequently, their flight times from the photoexcitation spot until ionization at the detector MCP1 are shorter than the flight times of molecules photoexcited at longer delays. This behavior is illustrated by the time-of-flight distributions presented in panel (a) of Fig. 2. When operating the valve at a temperature of 17 C (left part of the panel), the H 2 molecules in the supersonic beam have velocities around 2600 m/s. The molecules excited by the lasers at the shortest (longest) delays arrive at the detector first (last) and their flight times from the excitation spot to MCP1 are 40 µs (46 µs), corresponding to a velocity of 2780 m/s (2420 m/s). Cooling the valve to 140 C reduces the speed of the supersonic expansion to about 1700 m/s, as illustrated by the time-of-flight profiles displayed on the right-hand side of Fig. 2a. These 7
8 measurements demonstrate that pulsed laser excitation enables one to select molecules with a much narrower velocity distribution than the overall beam because of the dispersion taking place as the molecules travel from the valve orifice to the photoexcitation spot. This dispersion also naturally selects the velocity of the molecules present in the reaction zone at a given time. This selection is essential to achieve a high energy resolution in the measurement of reaction cross sections. The time-of-flight traces presented in Fig. 2a reveal that the velocity distributions are not uniform, but consist, for the short pulses generated by our valve, of two components. Indeed, when pulsing high currents to achieve an intense gas flow through the orifice of the valve, bouncing of the plunger is difficult to prevent [47]. This second component is emitted by the valve at a later time. Consequently, the molecules released in this second pulse that are probed by the photoexcitation pulse have a higher velocity than the molecules originating from the main pulse. This behavior is clearly observable in the traces displayed in Fig. 2a, as highlighted by the red trace, where the asterisk designates the contribution from the second gas pulse. Fortunately, the second component is well separated in time from the first and is only minor, so that its contribution to the reaction can either be eliminated by a careful adjustment of the timing of the experiment or precisely accounted for in the analysis. The overall velocity distribution of the H 2 molecules in the supersonic beam extracted from time-of-flight measurements such as those presented in panel (a) is depicted in Fig. 2b for a valve temperature of 123 C. The velocity distributions follow in a straightforward manner from the known distance between laser-excitation spot and MCP surface. The velocity distribution is centered around 1820 m/s with a full width at half maximum of about 120 m/s. The dispersion taking place between the valve orifice and the reaction zone, however, strongly reduces the velocity distribution of the molecules contributing to the reaction. To model this dispersion and precisely characterize the velocity of the molecules reaching the reaction zone at a given time, we use the particle-trajectory-simulation program described in Section 3. The velocity distributions 8
9 obtained from measurements such as those presented in panels (a) and (b) are used as input distributions to the simulations of the ground-state H 2 beam and to extract the mean velocity of the main component of the gas pulse as a function of the temperature of the valve (see Fig. 2d). To validate these distributions, we carried out an independent measurement of the relative density of the H 2 molecules in the beam, and monitored the H + 2 ion signal generated in the region labeled B in Fig. 1 by electron-impact ionization as a function of the trigger time and the temperature of the valve. The ionization signal was monitored after extraction of the H + 2 ions toward the MCP detector labeled MCP2 in Fig. 1 and located at the end of the flight tube. The results of a typical measurement are displayed as dots in Fig. 2c, where they are compared to the results of a numerical simulation obtained with our particle-trajectory-simulation program (red trace). The simulations and their good agreement with the experimental results enable us to decompose the intensity distribution into the two components of the gas pulse, which are displayed as green and blue traces for the main component of the gas pulse and the minor component originating from the plunger rebounce, respectively. This quantification enables us to accurately predict the relative density and the velocities of the H 2 ground-state molecules present at a given time and a given position in the reaction zone and for a given valve temperature, which is crucial to determine reliable relative reaction cross sections. The combination of simulations and experiment indicates that the spatial extent of the ground-state-h 2 -molecule packets along the beam-propagation direction is about 10 cm in the reaction zone. The ground-state-h 2 - molecule packets are thus longer than the diameter (7 cm) of the ion-extraction electrodes. The velocity distribution of the Rydberg H 2 beam at the exit of the Rydberg-Stark deflector can also be precisely determined, as explained in Ref. [29]. The spatial length of the H 2 - Rydberg-molecule packet along the beam-propagation direction in the reaction zone is only a few millimeters and thus much shorter than the length of the packet of ground-state H 2 molecules because (1) photoexcitation at the entrance of the deflector selects a narrow slice of the original velocity distribution, (2) the deflector is operated in a guiding mode with a small trap volume 9
10 of 1 mm 3 and preserves the velocity component in the instantaneous propagation direction of the beam, with minimal heating to at most 300 mk [29], and (3) the reaction zone is located immediately after the deflector (see Fig. 1). The distribution of collision energies between the ground-state and the Rydberg H 2 molecules is determined by the spatial overlap of the short H 2 -Rydberg-molecule packet with the long ground-state H 2 packet in the time interval during which the reaction is monitored. As time proceeds, the H 2 Rydberg molecules interact with ground-state H 2 molecules of changing average velocity. Consequently, the average collision energy changes with time as the H 2 -Rydbergmolecule packet moves across the ground-state-h 2 -molecule packet. This effect reduces the collision-energy resolution of the measurements. Because of the large average kinetic energy of the H + 3 reaction products (about 0.17 ev, corresponding to an average velocity of about 3500 m/s in the center-of-mass frame [32]), most of the H + 3 product ions leave the reaction zone within 10 µs of their formation. The probability that an H + 3 product ion is still in the reaction zone at a given time after the reaction decreases with time and can be estimated from the kinetic-energy distribution of the reaction products (see Section 3). The collision-energy resolution thus initially deteriorates with increasing interaction time and saturates to a value of about k B 1 K when the interaction time approaches 10 µs. This effect can be accurately modelled from a known or assumed product-kinetic-energy distribution using our particle-trajectory-simulation program. The time interval during which the reaction is monitored can be restricted experimentally by applying two pulsed electric fields across the stack of extraction electrodes surrounding the reaction zone (see Fig. 1). The first pulse sweeps all ions out of the reaction zone and initializes the reaction-observation time, which lasts until the application of the second pulse. This second pulse exclusively extracts the H + 3 ions produced by reactions having taken place during the fieldfree interval between the two pulses. It also field ionizes the H 2 Rydberg molecules that have not reacted provided that their principal quantum number is high enough for field ionization. For all 10
11 experiments presented below, we chose a value of 26 V/cm for both pulses, which is insufficient to field ionize the Rydberg states in the range of n values between 20 and 40 prepared optically, but efficiently field ionizes Rydberg states with n beyond 60 produced by blackbody-radiationinduced transitions as the H 2 Rydberg molecules propagate from the entrance of the deflector to the reaction zone [48]. When adjusting the length of the interval between the two field pulses, a compromise must be made. Indeed, too short an interval reduces the number of H + 3 ions whereas too long an interval deteriorates the collision-energy resolution of the measurements for the reasons given above. With an interval of 3 µs, the collision-energy resolution is limited to k B 300 mk by the temperature of the Rydberg H 2 beam. 3 Particle-trajectory simulation of collision experiments In this section, we describe in detail the simulations of the H + 3 -product-ion yield of the H+ 2 + H 2 reaction. The simulations are based on 1. trajectories r(t) of deflected Rydberg H 2 molecules. The trajectories are obtained by classical, numerical propagation of particles with randomly sampled initial conditions as described in Ref. [29]. 2. the density distribution ρ H2 ( r, t) and the velocity distribution v H2 ( r, t) of the ground-state H 2 molecules. The distributions are obtained by analytic propagation of measured velocity and density profiles as described in Section a pseudo-first-order rate model for the reaction of isolated Rydberg H 2 molecules with the much more abundant ground-state H 2 molecules under conditions where the total reaction probability per Rydberg molecule is much less than one. 4. the differential cross sections as a function of the recoil energy and the scattering angle based on previous experimental measurements [32]. 11
12 5. the detection probability of the product ions resulting from classical trajectory simulations of the time-of-flight detection process. The simulation of the reaction and detection process is implemented numerically by the Monte-Carlo method. To illustrate the general concept of the simulations, we describe in the following the fate of a fictive Rydberg H 2 molecule created in the moving trap above the chip-based deflector. The trajectory of this particle (item 1 in the list presented above), which is determined purely by classical mechanics, is calculated by solving Newton s equation of motion in the applied inhomogeneous electric fields during deflection, and in free space once the particle is released from the trap. The accuracy of calculations of this type has been verified in numerous experimental studies [40, 29]. In the next step, the Monte-Carlo simulation of the reaction process (item 3) samples randomly the probability for a reaction to occur along the Rydberg trajectory P reaction (t, t + t) = k eff ρ H2 ( r(t), t) t, (2) where k eff is an effective reaction-rate coefficient which is assumed to be constant over the range of collision energies probed along the trajectory of the H 2 Rydberg molecule. Random sampling of equation (2) faithfully reproduces the distribution of product ions if the total reaction probability per Rydberg molecule is much less than one, i.e., if the collision-free path length is much larger than the dimensions of the interaction region. Experimentally this condition is fulfilled because there is no measurable reduction of the number of Rydberg molecules, detected after the reaction zone, when the beam of ground-state H 2 molecules is introduced. The velocity vectors of the product ions are randomly sampled from the assumed differential cross sections (item 4). The classical motion of the product ions before and during extraction in the time-of-flight stack (see Fig. 1) is simulated, again by solving Newton s equation of motion in time-dependent electric fields (item 5). The number of simulated product ions which reach the detector (MCP2 in Fig. 1) within the experimental time-of-flight window is counted. The number of counted product ions, averaged over many Rydberg-molecule trajectories, is the result of the simulation to which the experimental measurements are compared. The 12
13 simulations are expected to reproduce the experimental observations only up to factor, which depends on the number of ground-state H 2 molecules in the pulse and the detection efficiency of the MCP. This factor does not, however, depend on the collision energy, so that any energydependent variation of the ratio between the simulated and the measured number of product ions can be directly related to an energy-dependence of k eff. In the following, we describe in more detail the determination of the density and velocity distributions of the ground-state H 2 molecules from measured time-of-flight distributions (item 2), and the distribution of product-ion-velocity vectors on the basis of differential cross sections (item 4). 3.1 Velocity and density distributions of the ground-state H 2 molecules The determination of density and velocity distributions of the ground-state H 2 molecules is based on the laser excitation of H 2 molecules to a high-n-rydberg state at a well-defined spot with z = z exc (labeled A in Fig. 1) and time t exc, as described in Section 2.2. The measured time of flight t TOF of the molecules from z exc to the front of MCP1 at position z MCP1 (see Fig. 1) yields directly the velocity of the excited molecules in the beam direction (chosen as the z axis in the following). For a given temperature of the valve T valve, t TOF depends only on the difference t = t exc t valve between the valve-opening time t valve and t exc and one finds v( t) = z MCP1 z exc, (3) t TOF ( t) where we have omitted the implicit dependence on T valve. To simulate a scattering experiment performed for a fixed value of t valve, the ground-state H 2 velocity needs to be known at arbitrary positions ( r, t). We therefore propagate v( t i ), measured at discrete values t i of the excitation delay, to new positions z i (t) = z exc + v( t i ) (t t i t valve ). (4) Because the measured velocity distribution v( t) is a monotonously-decreasing function, this procedure yields uniquely defined values v(z i (t), t), which are interpolated in z to obtain v(z, t). 13
14 The radial x and y components of the velocity vectors are determined geometrically, assuming propagation along a straight line with origin at the valve orifice. The number of Rydberg molecules detected on MCP1 after laser excitation at a delay t is proportional to the number N (z exc, t) of ground-state molecules in the laser-excitation volume at z = z exc. For the simulation of the reaction (item 3), the density of ground-state molecules is required as a function of arbitrary coordinates r and times t. To this end, we first transform the distribution N (z exc, t), measured in the time domain at a fixed position, into a spatial distribution at a fixed time. This is done numerically using the procedure described above for the determination of v(z, t) by propagating N (z exc, t) to new positions z i according to Ñ(z i (t), t) = N (z exc, t i ) ( ) d t, (5) dz i where the derivative, which is evaluated numerically, ensures the conservation of the particle number N exc = Ñ(z, t)dz at all times t. The relative density of ground-state H 2 molecules along z at time t is then given by ρ(z i, t) = 1 N exc 1 (z i z valve ) 2 Ñ(z i, t). (6) The factor 1/(z z valve ) 2 accounts for the radial expansion of the ground-state H 2 pulse as it propagates away from the point-like valve orifice. We then obtain ρ(z, t), again by interpolation. The quantity ρ(z, t), which is determined separately at many different temperatures of the valve, does not depend any more on experimental parameters such as the excitation volume, the absolute density of ground-state molecules, or the probability for exciting a ground-state molecule into a long-lived Rydberg state. To account for the variation of the absolute groundstate-molecule density with T valve, we multiply ρ(z, t) by a factor which is proportional to the total number of ground-state molecules in the gas pulse as measured by electron-impact ionization (see Section 2.1) and obtain ρ T valve H 2 (z, t). In principle, the proportionality constant could be determined from the efficiency of the electron-impact-ionization process and the gain factor of the MCP detector. In this work, however, we simulate only relative product-ion yields, which 14
15 are scaled to the measured data by a single, global constant. For comparison with scattering experiments measured at a valve temperature T valve where no velocity and density profiles were measured, ρ T valve H 2 (z, t) was interpolated. The density ρ T valve H 2 (z, t) does not depend on the radial distance from the beam axis, as verified by calculations based on the direct-simulation-monte-carlo (DSMC) method [49]. However, the geometric truncation of the beam by the skimmer and additional apertures (see Fig. 1) is explicitly taken into account. 3.2 Distribution of product-ion-velocity vectors The distribution of velocity vectors of the product ions in the laboratory frame depends on the center-of-mass-velocity vector v c.m. of the colliding pair, the collision energy E col, the differential cross section dσ de r dσ dφ with respect to the recoil energy E r, and the differential cross sections dσ dθ and with respect to the center-of-mass polar (θ) and azimuthal (φ) angles. The initial relative velocity of the reactants is obtained from the calculated trajectory r(t) of a Rydberg molecule and from the velocity distribution of ground-state H 2 molecules v H2 ( r, t) as v rel,e ( r(t), t) = r(t) v H2 ( r(t), t). (7) The corresponding collision energy is E col ( r(t), t) = 1 2 µ e v rel,e ( r(t), t) 2, (8) where µ e is the reduced mass of the reactants. The final relative velocity of the reaction products, is given by the recoil energy, as 2 v rel,p ( r(t), t) = E r, (9) µ p where µ p is the reduced mass of the reaction products. Previous studies observed that, for low collision energies, about one third of the available energy E tot, i.e., the sum of E col and the exothermicity of the reaction, appears as recoil energy of the products [32]. For the simulation, 15
16 the recoil energies are randomly sampled from the normalized differential cross section dσ de r shown in Fig. 3. The form of dσ de r was modeled using the differential cross section measured by Pollard et al. [32] for E tot = 3.25 ev and scaled linearly to the value of E tot 1.7 ev in our experiments. The polar angle θ of the product-velocity vectors with respect to the initial relative velocity v rel,e ( r(t), t) is randomly sampled from the normalized differential cross section dσ dθ, which was assumed to be uniformly distributed following observations made by Pollard et al. [32]. In their studies at low collision energies (E col = 1.5 ev, Fig. 11(a) of Ref. [32]), H + 3 ions were observed to scatter preferentially into solid angles along the collision axis with only a weak forward-backward asymmetry, which is expected for a strongly exothermic reaction with no internal barrier. The azimuthal angle was also sampled randomly from a uniform distribution. The velocity vector of the H + 3 product ion in the laboratory frame, taking into account the conservation of momentum, is then given by v H + ( r(t), t) = v c.m. + 3 m H m H + m H + 3 v rel,p ( r(t), t). (10) A classical trajectory simulation for a H + 3 ion created at t init and position r(t) with velocity vector v H + ( r(t), t) in the time-of-flight mass spectrometer is then performed in SIMION to test 3 if this ion would be detected in the experiment (item 5). 4 Results 4.1 Determination of relative reaction cross sections The collision energies can be adjusted in several ways: (i) by changing the temperature of the valve releasing the ground-state H 2 beam and thus its velocity (see Fig. 2d); (ii) by varying the velocity of the Rydberg H 2 beam selected by the deflector or by accelerating the Rydberg H 2 beam during the deflection [29]; (iii) by modifying the trigger time of the valve releasing the ground-state H 2 beam and thus modifying the velocity of the ground-state H 2 molecules interacting with the Rydberg H 2 molecules in the reaction zone. In the experiments presented 16
17 in this article, the collision-energy dependence of the cross section was recorded by monitoring the H + 3 product yield for different temperatures of the valve releasing the ground-state H 2 molecules and by systematically varying the trigger time of the valve opening with respect to the laser pulse used to excite the H 2 molecules in the second beam to Rydberg states. Fig. 4 compares two ion-time-of-flight spectra recorded by extracting the ions from the reaction zone with a pulsed electric field of 26 V/cm. The measurement was carried out at a collision energy of k B 2.4 K with H 2 Rydberg states initially prepared in the n = 22, k = 12, m = 3 Rydberg-Stark state. The valves used to produce the H 2 ground-state beam and the Rydberg H 2 beam were operated at temperatures of 133 and 170 C, respectively. To record the red and black traces, which were each obtained by averaging over 50 experimental cycles, the ground-state H 2 beam was turned on and off, respectively. In the former case, H + 3 product ions are observed in addition to the H + 2 ions produced by pulsed field ionization of the H 2 Rydberg molecules. This H + 2 signal only represents the (small) fraction of the initially prepared n = 22 Rydberg molecules that underwent a blackbody-radiation-induced transition to Rydberg states with n > 60, as mentioned in Section 2.1. This signal has the same strngth in both traces shown in Fig. 4, which indicates that only very few of the initially prepared H 2 Rydberg molecules undergo a reaction. The assumption of low reaction probability per H 2 Rydberg molecule, which is used in the simulations (see Section 3), is thus justified. This measurement also demonstrates that no significant H + 3 signal is generated from the Rydberg H 2 beam alone but that both beam are necessary to observe H + 3 reaction products. In reactions with H 2 Rydberg molecules prepared in states with principal quantum number n between 22 and 37, we verified that the number of detected H + 3 ions does not depend significantly on the amplitude of the pulsed electric extraction field, which was varied between 10 and 60 V/cm. This demonstrates that the H + 3 ions do not result from field-ionization of H 3 Rydberg molecules, in agreement with the observation of Pratt et al. [19] that long-lived H 3 Rydberg molecules do not contribute significantly to the products of the collision. Such molecules would 17
18 indeed decay by autoionization before the ion-extraction pulse is applied. 4.2 Comparison of cross sections measured at different n values The H + 3 ion signal obtained from the reaction, though small, is sufficient to record excitation spectra of the Rydberg states of H 2. The spectrum displayed in Fig. 5c was obtained by monitoring the H + 3 ion signal resulting from the H 2 + H 2 reaction at a collision energy of 90 µev (E col /k B = 1 K). The intensity distribution in this spectrum was used to quantify the effects of a possible dependence of the reaction cross section on the principal quantum number of the excited H 2 Rydberg states. To this end, two other excitation spectra of the same series were recorded and are presented in Figs. 5a and b. The first of these (Fig. 5a) was obtained by monitoring the ionization of the H 2 Rydberg molecules as these reach the detector MCP1 (see Fig. 1). When recording this spectrum, the ground-state H 2 beam was turned off. The intensity distribution in this spectrum reflects both the excitation probability and the decay of the Rydberg states from the excitation spot (see Fig. 1) to the detector surface. It is very similar to that observed in Fig. 5c, with the main difference that the intensities of the transitions to lower (higher) Rydberg states appear slightly weaker (stronger) because of the decay of the Rydberg states during the flight time of the molecules to the detector. Indeed, the decay rate of Rydberg states decreases with increasing n value, which introduces a bias favoring the detection of high Rydberg states in Fig. 5a. To correct for this effect, additional spectra were recorded using a movable MCP detector (labeled D in Fig. 1), which enabled us to quantify the n dependence of the decay and to remove its effect on the intensity distributions. The ratio of the intensity of the H + 3 signal from Fig. 5c to the intensity of the H 2 signal from Fig. 5a, but corrected for the decay, is presented in Fig. 6 for n values between 24 and 35 for two representative sets of measurements carried out at collision energies E col /k B = 1 K (black dots and error bars) and 79 K (blue dots and error bars), corresponding to the lowest 18
19 and highest collision energies studied in this work. A linear regression of these data yielded a slope of 0.003±0.013, which indicates that the cross-sections for the reaction forming H + 3 do not reveal any n dependence within the precision limit of our measurements, i.e., within 1.3 %, in the investigated range of Rydberg states (n = 24-35). Over this range of n values, the classical radius of the Rydberg-electron orbit, which scales as n 2, increases by a factor of more than 2, and the Rydberg-electron density close to the H + 2 ion core, which scales as n 4 in Rydberg- Stark states, decreases by a factor of about 5. We therefore conclude that the influence of the Rydberg electron on the reaction is negligible and that the cross-sections extracted from our measurements faithfully represent the cross sections of the H H 2 H H reaction within the 1.3 % precision limit mentioned above. The excitation spectrum displayed in Fig. 5b was recorded by monitoring the field ionization signal of the H 2 Rydberg molecules in the reaction zone with a pulsed field of 26 V/cm under the same experimental conditions as used to record the spectrum depicted in Fig. 5c. Such a field only efficiently ionizes Rydberg states with n values beyond 60. The mechanism leading to the observation of the H + 2 signal observed in this spectrum is blackbody-radiation-induced transitions to n > 60 Rydberg states taking place in the 50 µs interval between photoexcitation at the entrance of the deflector and the detection in the reaction zone. The gradual increase of the intensity of the field-ionization signal with n in this spectrum is fully consistent with this mechanism and can be modeled with the procedure described in detail in Ref. [48]. The reason for showing this spectrum in Fig. 5 is that it enables us to rule out any significant contribution to the H + 3 product yield from reactions of the H+ 2 ions produced by pulsed field ionization. Would such reactions significantly contribute to the H + 3 product yield, the H+ 3 signal would reveal an increase with n, in contrast with the experimental observations. Analysis of the experimental data leads to the conclusion that no significant contribution to the H + 3 signal is produced from such reactions. 19
20 4.3 Collision-energy dependence of the relative cross sections in the range E col /k B = 5-60 K Panels (a) and (d) of Fig. 7 display false-color plots of the H + 3 product yield recorded as a function of the delay time between the opening of the valve releasing the H 2 ground-state beam and the ion-extraction pulse (horizontal axis) and of the delay time between the laser pulse used to prepare the H 2 Rydberg molecules at the entrance of the deflector and the rising edge of the electric-field pulse used to extract the ions in the reaction zone (vertical axis). Varying the opening time of the valve releasing the H 2 ground-state beam enables us to vary the kinetic energy of the ground-state H 2 molecules that react with the Rydberg H 2 molecules in the reaction zone, and so to vary the collision energy, as explained in Subsection 2.2. The experiments were carried out at a ground-state H 2 valve temperature of 140 C (panel (a), mean velocity of 1680 m/s) and 25 C (panel (d), mean velocity of 2670 m/s) and a mean velocity of the Rydberg H 2 beam of 1540 m/s, corresponding to mean collision energies E col /k B of 1 K and 79 K, respectively. The H + 3 signal intensity along the vertical axis first increases with increasing delay of the ion-extraction pulse because a progressively larger fraction of the ground-state H 2 beam has interacted with the Rydberg H 2 molecules by the time the pulse is applied. The H + 3 ion signal then returns to zero when the extraction pulse is applied after all H + 3 ions have moved out of the reaction zone. The variation of H + 3 signal intensity along the horizontal axis primarily reflects the density of ground-state H 2 molecules because the rate coefficient k eff = vσ(v) of the reaction is independent of the collision energy, at least within the validity limits of the classical-langevin-capture model. This is illustrated by the cuts through the images made at an ion-extraction time of 52 µs depicted as black traces in the upper panels of Figs. 7(c) and (f). These two panels also show simulations of the relative H + 3 product yield assuming a pure classical-langevin-capture behavior as blue, green, and red traces for the contributions of the main pulse, of the plunger-rebounce pulse and their sum, respectively. 20
21 The lower panels of Figs. 7(c) and (f) illustrate the dependence of the collision energy on the delay between the opening time of the ground-state H 2 valve (t valve ) and the laser pulse used to generate the H 2 Rydberg states (t exc ) for the main gas pulse (blue dots with error bars) and for the minor pulse caused by the plunger rebounce (green dots with error bars). The H + 3 signal at each value of t exc t valve is proportional to the rate coefficient (see Eq. (2)). Because all other parameters determining the H + 3 signal are known up to a global scaling factor from the measurements and simulations of velocity and density distributions (see Sections 2.1 and 3), the energy dependence of the rate coefficient can be reconstructed by matching the simulated H + 3 signal to the measured signal at each point of the data set. The data set involves a large number of measurements such as those depicted in Figs. 7(a) and (d), recorded for many different temperatures of the ground-state H 2 valve. Our values of the rate coefficient thus emerge from a highly redundant data set and are robust against statistical signal fluctuations collected at particular sets of experimental conditions. The extracted rate coefficients faithfully reproduce all recorded data, as illustrated with the two examples presented in Fig. 7. The known collision energy allows us to convert the measured rate coefficients into cross sections (see Fig. 10 below). The images presented in Fig. 7 are also sensitive to the assumed distribution of H + 3 production velocity vectors (see Subsection 3.2, in particular Eq. (10)) because the H + 3 product ions emitted in the directions parallel or anti-parallel to the collision vector (and thus predominantly moving in a direction parallel or anti-parallel to the merged-beams-propagation direction) have different detection probabilities as a result of purely geometrical considerations. Indeed, H + 3 ions emitted in the merged-beams propagation direction leave the reaction zone sooner than those emitted in the opposite direction because of the center-of-mass motion of the reactants. This aspect is illustrated in more detail in Fig. 8, which presents three calculations corresponding to the experimental conditions used to record the data presented in Fig. 7(a), but assuming H + 3 emission parallel to the H+ 2 + H 2 collision vector (panel (c)), H + 3 emission antiparallel to this vector (panel (a)), and assuming a uniform distribution of the differential cross 21
22 section dσ dθ (panel (b)) (see Subsection 3.2). The experimental data only closely resemble the simulations assuming a uniform dσ dθ distribution, which supports the conclusions drawn by Pollard et al. [32] that the H + 3 product ions are emitted predominantly along the collision axis with only weak (if at all present) backward-forward asymmetry. The cross section can also be determined from the two-extraction-pulse experiments described in Subsection 2.2. The outcome of experiments in which the trigger time of the first (discrimination) and second (detection) pulses were varied independently are presented in Fig. 9 where they are compared to numerical simulations. The data-less triangle on the lower right side of the figure corresponds to situations where the discrimination pulse is applied after the detection pulse, whereas the data-less triangle on the upper-left side of the figure corresponds to situations where the second pulse is applied too long after the first pulse so that either no signal is observed any more or the energy resolution is degraded. The electric potentials used to generate the discrimination and detection pulses produce very high fields at the entrance of the reaction zone, where the electrodes of the stack are only spaced by a few millimeters (see Fig. 1). The large electric fields caused in that area by the discrimination pulse effectively prevent the detection of product ions generated in the corresponding time window, which is indicated by the grey-shaded bars in panels (a) and (b) of Fig. 9. The procedure to determine the cross sections from this data set is the same as described for Fig. 7. The excellent agreement between simulation and experimental results supports the validity of the assumed differential cross sections (see Section 3). Fig. 10 presents the energy-dependent cross sections extracted from our measurements as well as cross sections reported in earlier studies in the form of a double-logarithmic plot. The black data points are the absolute integral cross sections obtained by Glenewinkel-Meyer and Gerlich [38], the dashed black line represents a pure Langevin-capture behavior, and the green line corresponds to the classical-molecular-dynamics-with-quantum-transitions (MDQT) calculations of Sanz-Sanz et al. [37], respectively. The blue and red dots represent our measurements using 22
23 n = 31 and n = 22 H 2 Rydberg states, respectively, and scaled (arbitrarily) so as to match the cross section calculated by Sanz-Sanz et al. [37] (their equation (27)) at the collision energy E col /k B = 30 K. The vertical error bars indicate the standard deviations of the cross sections and the horizontal bars represent the energy resolution of our measurements. Although our data are compatible with the data of Sanz-Sanz et al. [37] within the resolution and uncertainty limits of our measurement, our data set indicates a slightly steeper slope which is almost identical to the slope of the data of Glenewinkel-Meyer and Gerlich in the range of collision energies between 5 and 10 mev. Our two data sets obtained for different n values are consistent, which further demonstrates that the Rydberg electron does not significantly modify the cross sections. The general behavior revealed by the cross sections presented in Fig. 10 indicates that the classical-langevin-capture model can be used in good approximation when extrapolating the cross-sections of the H + 2 +H 2 H H to low collision energies, over the entire range of energies relevant for astrochemistry - down to E col /k B = 5 K, at least for the lowest rovibrational levels of H 2 and H + 2. Deviations caused by quantum-mechanical effects are only expected to occur at lower energies than those investigated here [36]. 5 Conclusions We have presented a new approach to study ion-molecule reactions at low collision energies, which relies on the equivalence between ion-molecule reactions in free-space and within the orbit of a highly excited Rydberg electron. This equivalence, which we have experimentally verified to high precision with Rydberg-Stark states of principal quantum number in the range 22-40, enables one to avoid the adverse effects of stray electric fields on the collision-energy resolution of cross-section measurements. Indeed, the Rydberg electron effectively shields the reaction from such fields without significantly affecting its outcome. To reach collision energies below E col /k B = 10 K, we have exploited a Rydberg-Stark surface- 23
24 electrode deflector to merge a beam of ground-state molecules with a beam of molecules excited to high Rydberg states. We chose the H H 2 H H reaction to illustrate the main features of this new approach because of the importance of this reaction in astrophysics and because recent calculations of integral cross sections have been reported for this reaction [37] with which our results can be compared. Our results indicate that the classical-langevin-capture model for ion-induced-dipole interactions represents an excellent approximation in the range of collision energies investigated, down to E col /k B = 5 K, as pointed out earlier [36, 37]. To extract reliable relative cross sections over a broad range of collision energies, care had to be taken to carefully measure the velocity distributions of both beams in the reaction zone and to fully simulate all aspects of the reaction, from the preparation of the molecules in the supersonic beam to the detection of the product ions. The numerical simulation of the experiment enabled us to verify the observation made earlier and at higher collision energies by Pollard et al. [32] that the product distribution is peaked in the backward and forward directions with no strong forward-backward asymmetry. It also enabled us to assess the collision-energy-resolution limit of the current experiment (k B 0.35 K), which originates from the translational temperature of the H 2 Rydberg molecule released by the deflector. Our simulations indicate that reducing the depth of the moving electric trap used to deflect the Rydberg molecules should permit an improvement of the resolution to about 100 mk. Experiments are currently underway to reach collision energies below 1 K and to study the deviations from the classical-langevin-capture model predicted by Dashevskaya et al. [36]. The method presented and illustrated in this article has the advantage of being applicable to a broad range of ion-molecule reactions. It also permits the full selection of the quantum state of the ion through excitation of Rydberg states belonging to series converging on specific rovibrational levels of the ion core. The experiments presented here were carried out with H + 2 ions in their X 2 Σ + g (v + = 0, N + = 0) ground state and with a statistical mixture of para and ortho H 2 molecules in their X 1 Σ + g (v = 0, N = 0) (25%) and (v = 0, N = 1) (75%) ground state. 24
25 Experiments with a pure beam of para H 2 molecules as well as with HD, D 2, HD + and D + 2 are envisaged as next steps in our investigations. Reactions involving H + and He +, which we can control with our surface-electrode decelerator [50, 51], could be investigated without substantial modifications of our apparatus and experimental procedure. Acknowledgments We thank Prof. J. Troe and Prof. E. Nikitin for their encouragements and fruitful discussions. Several results presented here were first presented in November 2015 at a Bunsen Discussion Meeting held in Göttingen in honor of Professor Jürgen Troe, to whom it is a great pleasure to dedicate this article. This work is supported financially by the Swiss National Science Foundation under project Nr and the NCCR QSIT. References [1] S. C. Althorpe and D. C. Clary. Ann. Rev. Phys. Chem. 2003, 54, [2] E. E. Nikitin, J. Troe, Phys. Chem. Chem. Phys. 2005, 7, [3] I. W. M. Smith, Angew. Chem. Int. Edit. 2006, 45, [4] M. T. Bell, A. D. Gingell, J. M. Oldham, T. P. Softley, S. Willitsch, Farad. Discuss. 2009, 142, [5] B. Gao, Phys. Rev. A 2011, 83, [6] E. Herbst, W. Klemperer, Astrophys. J. 1973, 185, [7] E. Herbst, Space Science Rev. 2003, 106, [8] V. Wakelam, I. W. M. Smith, E. Herbst, J. Troe, W. Geppert, H. Linnartz, K. Oeberg, E. Roueff, M. Agundez, P. Pernot, H. M. Cuppen, J. C. Loison, D. Talbi, Space Science Rev. 2010, 156,
26 [9] T. Oka, Phil. Trans. Royal Soc. Lond. A 2012, 370(1978), [10] D. Smith, Int. J. Mass Spec. Ion Proc. 1993, 129, [11] D. C. Clary, Science 1998, 279(5358), [12] D. Gerlich, M. Smith, Physica Scripta 2006, 73, C25 C31. [13] B. R. Rowe, J.-B. Marquette, C. Rebrion, J. Chem. Soc., Faraday Trans , 85, [14] D. Gerlich, Physica Scripta 1995, T59, [15] R. Wester, J. Phys. B 2009, 42, [16] M. Drewsen, I. Jensen, J. Lindballe, N. Nissen, R. Martinussen, A. Mortensen, P. Staanum, D. Voigt, Int. J. Mass Spec. 2003, 229, [17] S. Willitsch, M. T. Bell, A. D. Gingell, T. P. Softley, Phys. Chem. Chem. Phys. 2008, 10, [18] S. Willitsch, Int. Rev. Phys. Chem. 2012, 31, [19] S. T. Pratt, J. L. Dehmer, P. M. Dehmer, W. A. Chupka, J. Chem. Phys. 1994, 101, [20] P. M. Dehmer, W. A. Chupka J. Phys. Chem. 1995, 99, [21] E. Wrede, L. Schnieder, K. Seekamp-Schnieder, B. Niederjohann, K. H. Welge, Phys. Chem. Chem. Phys. 2005, 7, [22] D. Dai, C. C. Wang, G. Wu, S. A. Harich, H. Song, M. Hayes, R. T. Skodje, X. Wang, D. Gerlich, X. Yang, Phys. Rev. Lett. 2005, 95, [23] M. Matsuzawa, Phys. Rev. A 2010, 82,
27 [24] A. B. Henson, S. Gersten, Y. Shagam, J. Narevicius, E. Narevicius, Science 2012, 338(6104), [25] E. Lavert-Ofir, Y. Shagam, A. B. Henson, S. Gersten, J. K los, P. S. Żuchowski, J. Narevicius, E. Narevicius, Nature Chem. 2014, 6, [26] J. Jankunas, B. Bertsche, K. Jachymski, M. Hapka, A. Osterwalder, J. Chem. Phys. 2014, 140, [27] B. Bertsche, J. Jankunas, A. Osterwalder, CHIMIA Int. J. Chem. 2014, 68, [28] J. Jankunas, K. Jachymski, M. Hapka, A. Osterwalder, J. Chem. Phys. 2015, 142, [29] P. Allmendinger, J. Deiglmayr, J. A. Agner, H. Schmutz, F. Merkt, Phys. Rev. A 2014, 90, [30] Scott L. Anderson, F. A. Houle, D. Gerlich, Y. T. Lee, J. Chem. Phys. 1991, 75, [31] J. D. Shao, C. Y. Ng, J. Chem. Phys. 1986, 84, [32] J. E. Pollard, L. K. Johnson, D. A. Lichtin, R. B. Cohen, J. Chem. Phys. 1991, 95, [33] S. R. Mackenzie, T. P. Softley, J. Chem. Phys. 1994, 101, [34] C. W. Eaker, G. C. Schatz, J. Phys. Chem. 1985, 89, [35] M. Baer, C. Y. Ng, J. Chem. Phys. 1990, 93, [36] E. I. Dashevskaya, I. Litvin, E. E. Nikitin, J. Troe, J. Chem. Phys. 2005, 122, [37] C. Sanz-Sanz, A. Aguado, O. Roncero, F. Naumkin, J. Chem. Phys. 2015, 143, [38] T. Glenewinkel-Meyer, D. Gerlich, Israel J. Chem. 1997, 37, [39] S. D. Hogan, Ch. Seiler, F. Merkt, Phys. Rev. Lett. 2009, 103,
28 [40] Ch. Seiler, S. D. Hogan, F. Merkt, Phys. Chem. Chem. Phys. 2011, 13, [41] W. C. Wiley, I. H. McLaren, Rev. Sci. Instr. 1955, 26, [42] Scientific Instrument Services, Inc. SIMION 8.1. Ringoes, New Jersey, USA, [43] W. R. Gentry, C. F. Giese, Rev. Sci. Instr. 1978, 49, [44] U. Even, J. Jortner, D. Noy, N. Lavie, C. Cossart-Magos, J. Chem. Phys. 2000, 112, [45] O. F. Hagena, Rev. Sci. Instr , 62, [46] D. Pentlehner, R. Riechers, B. Dick, A. Slenczka, U. Even, N. Lavie, R. Brown, K. Luria, Rev. Sci. Instr. 2009, 80, [47] W. Christen, J. Chem. Phys. 2013, 139, [48] Ch. Seiler, J. A. Agner, P. Pillet, F. Merkt, J. Phys. B 2016, 49, [49] O. Schullian, J. Loreau, N. Vaeck, A. van der Avoird, B. R. Heazlewood, C. J. Rennick, T. P. Softley, Mol. Phys. 2015, 113, [50] S. D. Hogan, P. Allmendinger, H. Saßmannshausen, H. Schmutz, F. Merkt, Phys. Rev. Lett. 2012, 108, [51] P. Allmendinger, J. A. Agner, H. Schmutz, F. Merkt, Phys. Rev. A 2013, 88,
29 Figures MCP 2 Pulsed valve Skimmer Ion TOF detection Ground-state beam Beam collimator A C Rydberg molecule beam PCB B D Pulsed valve Skimmer e - -gun Beam source region Deflection Laser excitation Reaction region MCP 1 Figure 1: Schematic representation of the merged-beam apparatus used to study ion-molecule reactions at low collision energies, with the two skimmed supersonic beams initially propagating at an angle of 10, the Rydberg-Stark deflector made of a bent printed circuit board (PCB) and used to merge the beams after laser excitation, the reaction zone, and the linear time-offlight spectrometer used to detect reactants and products separately. (MCP1) and (MCP2) Microchannel-plate detectors to monitor the flight times of Rydberg H 2 molecules and the iontime-of-flight spectra, respectively. (A) Laser excitation spot used in measurements of the velocity distribution of the undeflected beam of ground-state H 2 molecules. (B) Region in which the density profile of the ground-state molecules in the undeflected beam is measured by electronimpact ionization induced by an electron gun. (C) Aperture used to prevent the undeflected Rydberg molecules from entering the reaction region. (D) Movable microchannel-plate detector used for measurements of the velocity distribution and the lifetimes of the Rydberg molecules. 29
30 30
31 ) (b) - = - * * * ( * (µ ) (d) ( / ) ( / ) - ( ) (c) ( ) (a) (µ ) ( ) Figure 2: Determination of the density and velocity distributions of H2 molecules in the groundstate beam. (a) Time-of-flight profiles of long-lived H2 Rydberg molecules prepared by photoexcitation at the position marked A in Fig. 1 at different time delays between the valve opening and the photoexcitation laser pulse and for valve temperatures of 17 C (left) and 140 C (right). The red trace highlights the measurement carried out at the center of the gas pulse and the asterisk indicates the contribution from the plunger-rebounce pulse. (b) Velocity distribution extracted for the main pulse (full line) and the plunger rebounce pulse (dashed line) from time-of-flight measurements such as those depicted in (a) but for a valve temperature of 123 C. (c) Dots: Relative H2 density in the ground-state beam measured by electron-impact ionization in the region labeled B in Fig. 1. Red line: Particle trajectory simulation of the beam density based on the velocity distribution presented in panel (b) and decomposed into contributions from the main pulse (green line) and the pulse originating from the plunger rebounce (blue line). (d) Mean velocity (dots) and full width at half maximum of the velocity distribution (vertical bars) as a function of the valve temperature. 31
32 σ / ( ) () Figure 3: Normalized differential cross section dσ/de r of the H H 2 H H reaction as a function of product recoil energy E r used in the simulations. 32
33 + ( ) + * + * Figure 4: Ion time-of-flight spectra recorded in the reaction zone with the ground-state beam turned on (red trace, shifted along vertical axis for clarity) and off (black trace). 33
34 + = = + = = * ( ) + + ( - ) Figure 5: Spectra of the H 2 Rydberg series converging on the X + 2 Σ + g (v + = 0, N + = 0, 2) ionization thresholds recorded from the I 1 Π g (v = 0, N = 0) intermediate state. (a) Signal corresponding to the ionization of the Rydberg molecules as they impinge on the MCP detector located beyond the reaction zone (labeled MCP1 in Fig. 1). (b) H + 2 signal obtained by pulsed field ionization in the reaction zone and extraction of the ions to the MCP detector located at the end of the time-of-flight tube (labeled MCP2 in Fig. 1). (c) H + 3 signal resulting from the H 2 + H 2 reaction at a collision energy of 90 µev (E col /k B = 1 K) and extracted to the MCP located at the end of the time-of-flight tube. 34
35 ( ) / = / = Figure 6: Ratio R of the H + 3 signal resulting from the H 2 +H 2 reaction to the H 2 signal detected on MCP1 (signal in Fig. 5(a)) corrected for the measured n-dependent lifetimes. The blue and black data points were obtained for valve temperatures of 140 C and 25 C, respectively, corresponding to E col /k B = 1 K and 79 K, respectively. The error bars represent one standard deviation. Dashed line: Linear function with slope of ± obtained by linear regression of the experimental data points (see text for details). 35
36 - - (a) (b) (c) = µ (d) - (e) (f) = µ ( ) ( ) ( ) ( ) ( ) - Figure 7: (a, b, d, and e) False-color images of the H + 3 signal resulting from the H 2 +H 2 reaction as a function of the H 2 -valve switch-on time t valve (horizontal axis) and of the time of the ionextraction pulse (vertical axis) with respect to the Rydberg-excitation laser-pulse trigger time t exc. Panels (a) and (d) present the experimental data recorded at the valve temperatures of (a) -140 C and (d) 25 C. The corresponding simulated images, obtained assuming a uniformly distributed differential cross section dσ dθ are shown in panels (b) and (e), respectively. (c and f) Upper panel: Cuts through the experimental and simulated images at the ion extraction time of 52 µs. The black, red, blue, and green traces correspond to the experimental data, their simulations, and the contributions to the simulations from the main pulse and the plungerrebounce pulse, respectively. Lower Panel: Dependence of the collision energy on the delay between t valve and t exc. The blue dots with error bars correspond to the main pulse, the green dots with error bars correspond to the plunger-rebounce pulse and the red line to the average of the two energies weighted by their contribution to the detected H + 3 signal. 36
37 (a) (b) (c) Figure 8: Simulations of the experimental results presented in Fig. 7(a) assuming (a) a maximal forward anisotropy of the product emission, (b) a uniformly distributed differential cross section dσ dθ (already shown in Fig.7(b)) and (c) a maximal backward anisotropy of the product emission. 37
38 (a) (b) Figure 9: Two-extraction-pulse method to reduce the reaction time. Panels (a) and (b) display, respectively, the measured and simulated H + 3 reaction yield extracted by the second extraction pulse as a function of the trigger times of the two extraction pulses. The lower-right data-less triangle corresponds to situations where the second pulse is applied before the first one. In the upper-left data-less triangle, the second pulse is applied more than 20 µs after the first pulse and does not yield additional information on the reaction. The area shaded in grey corresponds to the time interval during which all ions are eliminated by the large field generated near the entrance of the reaction zone by the pulsed potential used to generate the first field pulse. See text for additional information. 38
arxiv: v1 [physics.chem-ph] 21 Dec 2016
![arxiv: v1 [physics.chem-ph] 21 Dec 2016 arxiv: v1 [physics.chem-ph] 21 Dec 2016](/thumbs/90/104434880.jpg) Observation of enhanced rate coefficients in the H + + H H + 3 + H reaction at low collision energies Pitt Allmendinger a, Johannes Deiglmayr a, Katharina Höveler, Otto Schullian, and Frédéric Merkt b
Observation of enhanced rate coefficients in the H + + H H + 3 + H reaction at low collision energies Pitt Allmendinger a, Johannes Deiglmayr a, Katharina Höveler, Otto Schullian, and Frédéric Merkt b
arxiv: v1 [physics.atom-ph] 16 Apr 2019
![arxiv: v1 [physics.atom-ph] 16 Apr 2019 arxiv: v1 [physics.atom-ph] 16 Apr 2019](/thumbs/96/128749498.jpg) Submitted to Molecular Physics Vol. 00, No. 00, Month 200x, 1 12 ARTICLE arxiv:1904.07628v1 [physics.atom-ph] 16 Apr 2019 Fluorescence-lifetime-limited trapping of Rydberg helium atoms on a chip V. Zhelyazkova
Submitted to Molecular Physics Vol. 00, No. 00, Month 200x, 1 12 ARTICLE arxiv:1904.07628v1 [physics.atom-ph] 16 Apr 2019 Fluorescence-lifetime-limited trapping of Rydberg helium atoms on a chip V. Zhelyazkova
CHAPTER D3 TOF ION OPTICS
 Back to Basics Section D: Ion Optics CHAPTER D3 TOF ION OPTICS TABLE OF CONTENTS QuickGuide...399 Summary...401 Background...403 EquationsofMotionofIons...403 Resolution...405 Reflectron...407 Comparison
Back to Basics Section D: Ion Optics CHAPTER D3 TOF ION OPTICS TABLE OF CONTENTS QuickGuide...399 Summary...401 Background...403 EquationsofMotionofIons...403 Resolution...405 Reflectron...407 Comparison
Laser Dissociation of Protonated PAHs
 100 Chapter 5 Laser Dissociation of Protonated PAHs 5.1 Experiments The photodissociation experiments were performed with protonated PAHs using different laser sources. The calculations from Chapter 3
100 Chapter 5 Laser Dissociation of Protonated PAHs 5.1 Experiments The photodissociation experiments were performed with protonated PAHs using different laser sources. The calculations from Chapter 3
Differential Cross Section Measurements in Ion-molecule Collisions
 Differential Cross Section Measurements in Ion-molecule Collisions Song Cheng Department of Physics and Astronomy, University of Toledo, Toledo, Ohio 43606 A 14 m long beam line dedicated to study very
Differential Cross Section Measurements in Ion-molecule Collisions Song Cheng Department of Physics and Astronomy, University of Toledo, Toledo, Ohio 43606 A 14 m long beam line dedicated to study very
Appendix A Detector Calibration
 Appix A Detector Calibration The scattering pattern from single clusters analyzed in Sect. 3.5 have been obtained with a large area detector which allows for spatially resolved measurement of the scattered
Appix A Detector Calibration The scattering pattern from single clusters analyzed in Sect. 3.5 have been obtained with a large area detector which allows for spatially resolved measurement of the scattered
Time of Flight Mass Spectroscopy and Velocity Map Imaging
 Time of Flight Mass Spectroscopy and Velocity Map Imaging Geet Ghanshyam January 26, 2013 Velocity map imaging (VMI) is used to study the dynamics of various dissociative electron attachment (DEA) processes
Time of Flight Mass Spectroscopy and Velocity Map Imaging Geet Ghanshyam January 26, 2013 Velocity map imaging (VMI) is used to study the dynamics of various dissociative electron attachment (DEA) processes
Manipulating Rydberg atoms and molecules in the gas phase and near chip surfaces
 RQI Winter School Obergurgl, 12 February 2013 Manipulating Rydberg atoms and molecules in the gas phase and near chip surfaces Ch. Seiler, P. Allmendinger, S. D. Hogan, H. Saßmannshausen, J. Deiglmayr
RQI Winter School Obergurgl, 12 February 2013 Manipulating Rydberg atoms and molecules in the gas phase and near chip surfaces Ch. Seiler, P. Allmendinger, S. D. Hogan, H. Saßmannshausen, J. Deiglmayr
EXPERIMENT 2-6. e/m OF THE ELECTRON GENERAL DISCUSSION
 Columbia Physics: Lab -6 (ver. 10) 1 EXPERMENT -6 e/m OF THE ELECTRON GENERAL DSCUSSON The "discovery" of the electron by J. J. Thomson in 1897 refers to the experiment in which it was shown that "cathode
Columbia Physics: Lab -6 (ver. 10) 1 EXPERMENT -6 e/m OF THE ELECTRON GENERAL DSCUSSON The "discovery" of the electron by J. J. Thomson in 1897 refers to the experiment in which it was shown that "cathode
Mass Analyzers. Principles of the three most common types magnetic sector, quadrupole and time of flight - will be discussed herein.
 Mass Analyzers After the production of ions in ion sources, the next critical step in mass spectrometry is to separate these gas phase ions according to their mass-to-charge ratio (m/z). Ions are extracted
Mass Analyzers After the production of ions in ion sources, the next critical step in mass spectrometry is to separate these gas phase ions according to their mass-to-charge ratio (m/z). Ions are extracted
hν' Φ e - Gamma spectroscopy - Prelab questions 1. What characteristics distinguish x-rays from gamma rays? Is either more intrinsically dangerous?
 Gamma spectroscopy - Prelab questions 1. What characteristics distinguish x-rays from gamma rays? Is either more intrinsically dangerous? 2. Briefly discuss dead time in a detector. What factors are important
Gamma spectroscopy - Prelab questions 1. What characteristics distinguish x-rays from gamma rays? Is either more intrinsically dangerous? 2. Briefly discuss dead time in a detector. What factors are important
In Situ Imaging of Cold Atomic Gases
 In Situ Imaging of Cold Atomic Gases J. D. Crossno Abstract: In general, the complex atomic susceptibility, that dictates both the amplitude and phase modulation imparted by an atom on a probing monochromatic
In Situ Imaging of Cold Atomic Gases J. D. Crossno Abstract: In general, the complex atomic susceptibility, that dictates both the amplitude and phase modulation imparted by an atom on a probing monochromatic
Introduction to the Q Trap LC/MS/MS System
 www.ietltd.com Proudly serving laboratories worldwide since 1979 CALL +1.847.913.0777 for Refurbished & Certified Lab Equipment ABI Q Trap LC/MS/MS Introduction to the Q Trap LC/MS/MS System The Q Trap
www.ietltd.com Proudly serving laboratories worldwide since 1979 CALL +1.847.913.0777 for Refurbished & Certified Lab Equipment ABI Q Trap LC/MS/MS Introduction to the Q Trap LC/MS/MS System The Q Trap
Supplementary Figure 1 Level structure of a doubly charged QDM (a) PL bias map acquired under 90 nw non-resonant excitation at 860 nm.
 Supplementary Figure 1 Level structure of a doubly charged QDM (a) PL bias map acquired under 90 nw non-resonant excitation at 860 nm. Charging steps are labeled by the vertical dashed lines. Intensity
Supplementary Figure 1 Level structure of a doubly charged QDM (a) PL bias map acquired under 90 nw non-resonant excitation at 860 nm. Charging steps are labeled by the vertical dashed lines. Intensity
SUPPLEMENTARY INFORMATION
 doi:1.138/nature1878 I. Experimental setup OPA, DFG Ti:Sa Oscillator, Amplifier PD U DC U Analyzer HV Energy analyzer MCP PS CCD Polarizer UHV Figure S1: Experimental setup used in mid infrared photoemission
doi:1.138/nature1878 I. Experimental setup OPA, DFG Ti:Sa Oscillator, Amplifier PD U DC U Analyzer HV Energy analyzer MCP PS CCD Polarizer UHV Figure S1: Experimental setup used in mid infrared photoemission
Chapter 37 Early Quantum Theory and Models of the Atom. Copyright 2009 Pearson Education, Inc.
 Chapter 37 Early Quantum Theory and Models of the Atom Planck s Quantum Hypothesis; Blackbody Radiation Photon Theory of Light and the Photoelectric Effect Energy, Mass, and Momentum of a Photon Compton
Chapter 37 Early Quantum Theory and Models of the Atom Planck s Quantum Hypothesis; Blackbody Radiation Photon Theory of Light and the Photoelectric Effect Energy, Mass, and Momentum of a Photon Compton
Rydberg spectroscopy of Zeeman-decelerated beams of metastable Helium molecules
 Rydberg spectroscopy of Zeeman-decelerated beams of metastable Helium molecules Paul Jansen, Michael Motsch, Daniel Sprecher, Frédéric Merkt Laboratorium für Physikalische Chemie, ETH Zürich Motivation
Rydberg spectroscopy of Zeeman-decelerated beams of metastable Helium molecules Paul Jansen, Michael Motsch, Daniel Sprecher, Frédéric Merkt Laboratorium für Physikalische Chemie, ETH Zürich Motivation
Neutron Transport Calculations Using Monte-Carlo Methods. Sean Lourette Fairport High School Advisor: Christian Stoeckl
 Neutron Transport Calculations Using Monte-Carlo Methods Sean Lourette Fairport High School Advisor: Christian Stoeckl Laboratory for Laser Energetics University of Rochester Summer High School Research
Neutron Transport Calculations Using Monte-Carlo Methods Sean Lourette Fairport High School Advisor: Christian Stoeckl Laboratory for Laser Energetics University of Rochester Summer High School Research
CHAPTER D4 ORTHOGONAL TIME OF FLIGHT OPTICS
 Back to Basics Section D: Ion Optics CHAPTER D4 ORTHOGONAL TIME OF FLIGHT OPTICS TABLE OF CONTENTS QuickGuide...413 Summary...415 Introduction...417 The physical basis of orthogonal TOF....... 419 Pulsedmainbeamsofions...421
Back to Basics Section D: Ion Optics CHAPTER D4 ORTHOGONAL TIME OF FLIGHT OPTICS TABLE OF CONTENTS QuickGuide...413 Summary...415 Introduction...417 The physical basis of orthogonal TOF....... 419 Pulsedmainbeamsofions...421
Photoelectric Effect Experiment
 Experiment 1 Purpose The photoelectric effect is a key experiment in modern physics. In this experiment light is used to excite electrons that (given sufficient energy) can escape from a material producing
Experiment 1 Purpose The photoelectric effect is a key experiment in modern physics. In this experiment light is used to excite electrons that (given sufficient energy) can escape from a material producing
EQUIPMENT Beta spectrometer, vacuum pump, Cs-137 source, Geiger-Muller (G-M) tube, scalar
 Modern Physics Laboratory Beta Spectroscopy Experiment In this experiment, electrons emitted as a result of the radioactive beta decay of Cs-137 are measured as a function of their momentum by deflecting
Modern Physics Laboratory Beta Spectroscopy Experiment In this experiment, electrons emitted as a result of the radioactive beta decay of Cs-137 are measured as a function of their momentum by deflecting
BETA-RAY SPECTROMETER
 14 Sep 07 β-ray.1 BETA-RAY SPECTROMETER In this experiment, a 180, constant-radius magnetic spectrometer consisting of an electromagnet with a Geiger-Muller detector, will be used to detect and analyze
14 Sep 07 β-ray.1 BETA-RAY SPECTROMETER In this experiment, a 180, constant-radius magnetic spectrometer consisting of an electromagnet with a Geiger-Muller detector, will be used to detect and analyze
Interface (backside) & Extraction Lens
 Plasma Interface Interface (backside) & Extraction Lens Extraction Lens (-2000 volts) ION OPTICS Tip of the sampler cone is positioned to be in the region of maximum ionization Ions no longer under control
Plasma Interface Interface (backside) & Extraction Lens Extraction Lens (-2000 volts) ION OPTICS Tip of the sampler cone is positioned to be in the region of maximum ionization Ions no longer under control
Lab 5 - ELECTRON CHARGE-TO-MASS RATIO
 81 Name Date Partners Lab 5 - ELECTRON CHARGE-TO-MASS RATIO OBJECTIVES To understand how electric and magnetic fields impact an electron beam To experimentally determine the electron charge-to-mass ratio
81 Name Date Partners Lab 5 - ELECTRON CHARGE-TO-MASS RATIO OBJECTIVES To understand how electric and magnetic fields impact an electron beam To experimentally determine the electron charge-to-mass ratio
Fundamentals of Mass Spectrometry. Fundamentals of Mass Spectrometry. Learning Objective. Proteomics
 Mass spectrometry (MS) is the technique for protein identification and analysis by production of charged molecular species in vacuum, and their separation by magnetic and electric fields based on mass
Mass spectrometry (MS) is the technique for protein identification and analysis by production of charged molecular species in vacuum, and their separation by magnetic and electric fields based on mass
Basic structure of SEM
 Table of contents Basis structure of SEM SEM imaging modes Comparison of ordinary SEM and FESEM Electron behavior Electron matter interaction o Elastic interaction o Inelastic interaction o Interaction
Table of contents Basis structure of SEM SEM imaging modes Comparison of ordinary SEM and FESEM Electron behavior Electron matter interaction o Elastic interaction o Inelastic interaction o Interaction
Two-stage Rydberg charge exchange in a strong magnetic field
 Two-stage Rydberg charge exchange in a strong magnetic field M. L. Wall, C. S. Norton, and F. Robicheaux Department of Physics, Auburn University, Auburn, Alabama 36849-5311, USA Received 21 June 2005;
Two-stage Rydberg charge exchange in a strong magnetic field M. L. Wall, C. S. Norton, and F. Robicheaux Department of Physics, Auburn University, Auburn, Alabama 36849-5311, USA Received 21 June 2005;
Modern Physics Laboratory Beta Spectroscopy Experiment
 Modern Physics Laboratory Beta Spectroscopy Experiment Josh Diamond and John Cummings Fall 2009 Abstract In this experiment, electrons emitted as a result of the radioactive beta decay of 137 55 Cs are
Modern Physics Laboratory Beta Spectroscopy Experiment Josh Diamond and John Cummings Fall 2009 Abstract In this experiment, electrons emitted as a result of the radioactive beta decay of 137 55 Cs are
Positron-Electron Annihilation
 Positron-Electron Annihilation Carl Akerlof September 13, 008 1. Introduction This experiment attempts to explore several features of positron-electron annihilation. One of the attractive aspects of e
Positron-Electron Annihilation Carl Akerlof September 13, 008 1. Introduction This experiment attempts to explore several features of positron-electron annihilation. One of the attractive aspects of e
CEE 772: Instrumental Methods in Environmental Analysis
 Updated: 10 December 2014 Print version CEE 772: Instrumental Methods in Environmental Analysis Lecture #21 Mass Spectrometry: Mass Filters & Spectrometers (Skoog, Chapt. 20, pp.511-524) (Harris, Chapt.
Updated: 10 December 2014 Print version CEE 772: Instrumental Methods in Environmental Analysis Lecture #21 Mass Spectrometry: Mass Filters & Spectrometers (Skoog, Chapt. 20, pp.511-524) (Harris, Chapt.
Lecture 8: Mass Spectrometry
 intensity Lecture 8: Mass Spectrometry Relative abundance m/z 1 Ethylbenzene experiment CH 2 CH 3 + m/z = 106 CH 2 + m/z = 91 C 8 H 10 MW = 106 CH + m/z = 77 + 2 2 What information can we get from MS spectrum?
intensity Lecture 8: Mass Spectrometry Relative abundance m/z 1 Ethylbenzene experiment CH 2 CH 3 + m/z = 106 CH 2 + m/z = 91 C 8 H 10 MW = 106 CH + m/z = 77 + 2 2 What information can we get from MS spectrum?
Mass Spectrometry in MCAL
 Mass Spectrometry in MCAL Two systems: GC-MS, LC-MS GC seperates small, volatile, non-polar material MS is detection devise (Agilent 320-MS TQ Mass Spectrometer) Full scan monitoring SIM single ion monitoring
Mass Spectrometry in MCAL Two systems: GC-MS, LC-MS GC seperates small, volatile, non-polar material MS is detection devise (Agilent 320-MS TQ Mass Spectrometer) Full scan monitoring SIM single ion monitoring
Chemistry Instrumental Analysis Lecture 35. Chem 4631
 Chemistry 4631 Instrumental Analysis Lecture 35 Principle components: Inlet Ion source Mass analyzer Ion transducer Pumps Signal processor Mass analyzers Quadrupole Time of Flight Double Focusing Ion
Chemistry 4631 Instrumental Analysis Lecture 35 Principle components: Inlet Ion source Mass analyzer Ion transducer Pumps Signal processor Mass analyzers Quadrupole Time of Flight Double Focusing Ion
D. To the right (Total 1 mark)
 1. An electron passes the north pole of a bar magnet as shown below. What is the direction of the magnetic force on the electron? A. Into the page B. Out of the page C. To the left D. To the right 2. A
1. An electron passes the north pole of a bar magnet as shown below. What is the direction of the magnetic force on the electron? A. Into the page B. Out of the page C. To the left D. To the right 2. A
Saturation Absorption Spectroscopy of Rubidium Atom
 Saturation Absorption Spectroscopy of Rubidium Atom Jayash Panigrahi August 17, 2013 Abstract Saturated absorption spectroscopy has various application in laser cooling which have many relevant uses in
Saturation Absorption Spectroscopy of Rubidium Atom Jayash Panigrahi August 17, 2013 Abstract Saturated absorption spectroscopy has various application in laser cooling which have many relevant uses in
Lecture 8: Mass Spectrometry
 intensity Lecture 8: Mass Spectrometry Relative abundance m/z 1 Ethylbenzene CH 2 CH 3 + m/z = 106 CH 2 + m/z = 91 C 8 H 10 MW = 106 CH + m/z = 77 + 2 2 What information can be obtained from a MS spectrum?
intensity Lecture 8: Mass Spectrometry Relative abundance m/z 1 Ethylbenzene CH 2 CH 3 + m/z = 106 CH 2 + m/z = 91 C 8 H 10 MW = 106 CH + m/z = 77 + 2 2 What information can be obtained from a MS spectrum?
Precision Measurement of the Ionization Energy of the GK 1 Σ + g (v = 1, N = 1) State of Molecular Hydrogen.
 Precision Measurement of the Ionization Energy of the GK 1 Σ + g (v = 1, N = 1) State of Molecular Hydrogen. Maximilian Beyer, Daniel Sprecher and Frédéric Merkt Laboratory of Physical Chemistry, ETH Zurich,
Precision Measurement of the Ionization Energy of the GK 1 Σ + g (v = 1, N = 1) State of Molecular Hydrogen. Maximilian Beyer, Daniel Sprecher and Frédéric Merkt Laboratory of Physical Chemistry, ETH Zurich,
Today, I will present the first of two lectures on neutron interactions.
 Today, I will present the first of two lectures on neutron interactions. I first need to acknowledge that these two lectures were based on lectures presented previously in Med Phys I by Dr Howell. 1 Before
Today, I will present the first of two lectures on neutron interactions. I first need to acknowledge that these two lectures were based on lectures presented previously in Med Phys I by Dr Howell. 1 Before
Angular Correlation Experiments
 Angular Correlation Experiments John M. LoSecco April 2, 2007 Angular Correlation Experiments J. LoSecco Notre Dame du Lac Nuclear Spin In atoms one can use the Zeeman Effect to determine the spin state.
Angular Correlation Experiments John M. LoSecco April 2, 2007 Angular Correlation Experiments J. LoSecco Notre Dame du Lac Nuclear Spin In atoms one can use the Zeeman Effect to determine the spin state.
arxiv: v1 [physics.plasm-ph] 10 Nov 2014
![arxiv: v1 [physics.plasm-ph] 10 Nov 2014 arxiv: v1 [physics.plasm-ph] 10 Nov 2014](/thumbs/88/117240344.jpg) arxiv:1411.2464v1 [physics.plasm-ph] 10 Nov 2014 Effects of fast atoms and energy-dependent secondary electron emission yields in PIC/MCC simulations of capacitively coupled plasmas A. Derzsi 1, I. Korolov
arxiv:1411.2464v1 [physics.plasm-ph] 10 Nov 2014 Effects of fast atoms and energy-dependent secondary electron emission yields in PIC/MCC simulations of capacitively coupled plasmas A. Derzsi 1, I. Korolov
Project P2 - The weak charge of the proton
 Institute for Nuclear Physics, University of Mainz E-mail: beckerd@kph.uni-mainz.de K. Gerz, S. Baunack, K. S. Kumar, F. E. Maas The goal of Project P2 is to determine the electroweak mixing angle sin
Institute for Nuclear Physics, University of Mainz E-mail: beckerd@kph.uni-mainz.de K. Gerz, S. Baunack, K. S. Kumar, F. E. Maas The goal of Project P2 is to determine the electroweak mixing angle sin
(a) Write down the total Hamiltonian of this system, including the spin degree of freedom of the electron, but neglecting spin-orbit interactions.
 1. Quantum Mechanics (Spring 2007) Consider a hydrogen atom in a weak uniform magnetic field B = Bê z. (a) Write down the total Hamiltonian of this system, including the spin degree of freedom of the electron,
1. Quantum Mechanics (Spring 2007) Consider a hydrogen atom in a weak uniform magnetic field B = Bê z. (a) Write down the total Hamiltonian of this system, including the spin degree of freedom of the electron,
Citation for published version (APA): Mollema, A. K. (2008). Laser cooling, trapping and spectroscopy of calcium isotopes s.n.
 University of Groningen Laser cooling, trapping and spectroscopy of calcium isotopes Mollema, Albert Kornelis IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you
University of Groningen Laser cooling, trapping and spectroscopy of calcium isotopes Mollema, Albert Kornelis IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you
Chapter 1 The discovery of the electron 1.1 Thermionic emission of electrons
 Chapter 1 The discovery of the electron 1.1 Thermionic emission of electrons Learning objectives: What are cathode rays and how were they discovered? Why does the gas in a discharge tube emit light of
Chapter 1 The discovery of the electron 1.1 Thermionic emission of electrons Learning objectives: What are cathode rays and how were they discovered? Why does the gas in a discharge tube emit light of
Transverse momentum of ionized atoms and diatomic molecules acquired in collisions with fast highly-charged heavy ion. V. Horvat and R. L.
 Transverse momentum of ionized atoms and diatomic molecules acquired in collisions with fast highly-charged heavy ion V. Horvat and R. L. Watson The momenta of ions and electrons emerging from collisions
Transverse momentum of ionized atoms and diatomic molecules acquired in collisions with fast highly-charged heavy ion V. Horvat and R. L. Watson The momenta of ions and electrons emerging from collisions
v = E B FXA 2008 UNIT G485 Module Magnetic Fields BQv = EQ THE MASS SPECTROMETER
 UNIT G485 Module 1 5.1.2 Magnetic Fields 11 Thus, in order for the particle to suffer NO DEFLECTION and so exit the device at Y : From which : MAGNETIC FORCE UP = ELECTRIC FORCE DOWN BQv = EQ THE MASS
UNIT G485 Module 1 5.1.2 Magnetic Fields 11 Thus, in order for the particle to suffer NO DEFLECTION and so exit the device at Y : From which : MAGNETIC FORCE UP = ELECTRIC FORCE DOWN BQv = EQ THE MASS
Harris: Quantitative Chemical Analysis, Eight Edition
 Harris: Quantitative Chemical Analysis, Eight Edition CHAPTER 21: MASS SPECTROMETRY CHAPTER 21: Opener 21.0 Mass Spectrometry Mass Spectrometry provides information about 1) The elemental composition of
Harris: Quantitative Chemical Analysis, Eight Edition CHAPTER 21: MASS SPECTROMETRY CHAPTER 21: Opener 21.0 Mass Spectrometry Mass Spectrometry provides information about 1) The elemental composition of
CEE 772 Lecture #27 12/10/2014. CEE 772: Instrumental Methods in Environmental Analysis
 Updated: 10 December 2014 Print version CEE 772: Instrumental Methods in Environmental Analysis Lecture #21 Mass Spectrometry: Mass Filters & Spectrometers (Skoog, Chapt. 20, pp.511 524) (Harris, Chapt.
Updated: 10 December 2014 Print version CEE 772: Instrumental Methods in Environmental Analysis Lecture #21 Mass Spectrometry: Mass Filters & Spectrometers (Skoog, Chapt. 20, pp.511 524) (Harris, Chapt.
EEE4106Z Radiation Interactions & Detection
 EEE4106Z Radiation Interactions & Detection 2. Radiation Detection Dr. Steve Peterson 5.14 RW James Department of Physics University of Cape Town steve.peterson@uct.ac.za May 06, 2015 EEE4106Z :: Radiation
EEE4106Z Radiation Interactions & Detection 2. Radiation Detection Dr. Steve Peterson 5.14 RW James Department of Physics University of Cape Town steve.peterson@uct.ac.za May 06, 2015 EEE4106Z :: Radiation
(Total for Question = 5 marks) PhysicsAndMathsTutor.com
 1 Rutherford designed an experiment to see what happened when alpha particles were directed at a piece of gold foil. Summarise the observations and state the conclusions Rutherford reached about the structure
1 Rutherford designed an experiment to see what happened when alpha particles were directed at a piece of gold foil. Summarise the observations and state the conclusions Rutherford reached about the structure
Rb, which had been compressed to a density of 1013
 Modern Physics Study Questions for the Spring 2018 Departmental Exam December 3, 2017 1. An electron is initially at rest in a uniform electric field E in the negative y direction and a uniform magnetic
Modern Physics Study Questions for the Spring 2018 Departmental Exam December 3, 2017 1. An electron is initially at rest in a uniform electric field E in the negative y direction and a uniform magnetic
Ion Induced Beam disruption Mechanism
 Ion Induced Beam disruption Mechanism C. Vermare CEA, Polygone d Expérimentation de Moronvilliers, France H. Davis, D.C. Moir, R. Olson Los Alamos National Laboratory, NM, USA T. Hughes Mission Research
Ion Induced Beam disruption Mechanism C. Vermare CEA, Polygone d Expérimentation de Moronvilliers, France H. Davis, D.C. Moir, R. Olson Los Alamos National Laboratory, NM, USA T. Hughes Mission Research
Lab 5 - ELECTRON CHARGE-TO-MASS RATIO
 79 Name Date Partners OBJECTIVES OVERVIEW Lab 5 - ELECTRON CHARGE-TO-MASS RATIO To understand how electric and magnetic fields impact an electron beam To experimentally determine the electron charge-to-mass
79 Name Date Partners OBJECTIVES OVERVIEW Lab 5 - ELECTRON CHARGE-TO-MASS RATIO To understand how electric and magnetic fields impact an electron beam To experimentally determine the electron charge-to-mass
Møller Polarimetry on Atomic Hydrogen
 E.Chudakov June 21, 2011 Møller Polarimetry on Atomic Hydrogen 1 Møller Polarimetry on Atomic Hydrogen E.Chudakov 1 1 JLab Meeting at UVA Outline E.Chudakov June 21, 2011 Møller Polarimetry on Atomic Hydrogen
E.Chudakov June 21, 2011 Møller Polarimetry on Atomic Hydrogen 1 Møller Polarimetry on Atomic Hydrogen E.Chudakov 1 1 JLab Meeting at UVA Outline E.Chudakov June 21, 2011 Møller Polarimetry on Atomic Hydrogen
MAGNETIC DEFLECTION. OBJECTIVE: To observe the effect of a magnetic field on an electron beam. To measure the Earth s magnetic field.
 MAGNETIC DEFLECTION OBJECTIVE: To observe the effect of a magnetic field on an electron beam. To measure the Earth s magnetic field. THEORY: Moving charges exert forces on one another that are not observed
MAGNETIC DEFLECTION OBJECTIVE: To observe the effect of a magnetic field on an electron beam. To measure the Earth s magnetic field. THEORY: Moving charges exert forces on one another that are not observed
Supplementary Material for In situ frequency gating and beam splitting of vacuum- and extreme-ultraviolet pulses
 Supplementary Material for In situ frequency gating and beam splitting of vacuum- and extreme-ultraviolet pulses Rajendran Rajeev, Johannes Hellwagner, Anne Schumacher, Inga Jordan, Martin Huppert, Andres
Supplementary Material for In situ frequency gating and beam splitting of vacuum- and extreme-ultraviolet pulses Rajendran Rajeev, Johannes Hellwagner, Anne Schumacher, Inga Jordan, Martin Huppert, Andres
Atomic Spectra. d sin θ = mλ (1)
 Atomic Spectra Objectives: To measure the wavelengths of visible light emitted by atomic hydrogen and verify that the measured wavelengths obey the empirical Rydberg formula. To observe emission spectra
Atomic Spectra Objectives: To measure the wavelengths of visible light emitted by atomic hydrogen and verify that the measured wavelengths obey the empirical Rydberg formula. To observe emission spectra
Instrumental Analysis. Mass Spectrometry. Lecturer:! Somsak Sirichai
 303351 Instrumental Analysis Mass Spectrometry Lecturer:! Somsak Sirichai Mass Spectrometry What is Mass spectrometry (MS)? An analytic method that employs ionization and mass analysis of compounds in
303351 Instrumental Analysis Mass Spectrometry Lecturer:! Somsak Sirichai Mass Spectrometry What is Mass spectrometry (MS)? An analytic method that employs ionization and mass analysis of compounds in
Chapter 28. Atomic Physics
 Chapter 28 Atomic Physics Quantum Numbers and Atomic Structure The characteristic wavelengths emitted by a hot gas can be understood using quantum numbers. No two electrons can have the same set of quantum
Chapter 28 Atomic Physics Quantum Numbers and Atomic Structure The characteristic wavelengths emitted by a hot gas can be understood using quantum numbers. No two electrons can have the same set of quantum
10. Wavelength measurement using prism spectroscopy
 Spk 0. Wavelength measurement using prism spectroscopy 0. Introduction The study of emitted spectra of electromagnetic waves by excited atoms makes for one of the most important methods to investigate
Spk 0. Wavelength measurement using prism spectroscopy 0. Introduction The study of emitted spectra of electromagnetic waves by excited atoms makes for one of the most important methods to investigate
Vibrational Spectra of Chloroform, Freon-11 and Selected Isotopomers in the THz Frequency Region
 Vibrational Spectra of Chloroform, Freon-11 and Selected Isotopomers in the THz Frequency Region Christa Haase, Jinjun Liu, Frédéric Merkt, Laboratorium für physikalische Chemie, ETH Zürich current address:
Vibrational Spectra of Chloroform, Freon-11 and Selected Isotopomers in the THz Frequency Region Christa Haase, Jinjun Liu, Frédéric Merkt, Laboratorium für physikalische Chemie, ETH Zürich current address:
MS/MS .LQGVRI0606([SHULPHQWV
 0DVV6SHFWURPHWHUV Tandem Mass Spectrometry (MS/MS) :KDWLV0606" Mass spectrometers are commonly combined with separation devices such as gas chromatographs (GC) and liquid chromatographs (LC). The GC or
0DVV6SHFWURPHWHUV Tandem Mass Spectrometry (MS/MS) :KDWLV0606" Mass spectrometers are commonly combined with separation devices such as gas chromatographs (GC) and liquid chromatographs (LC). The GC or
MONTE-CARLO SIMULATIONS OF TIME- RESOLVED, OPTICAL READOUT DETECTOR for PULSED, FAST-NEUTRON TRANSMISSION SPECTROSCOPY (PFNTS)
 MONTE-CARLO SIMULATIONS OF TIME- RESOLVED, OPTICAL READOUT DETECTOR for PULSED, FAST-NEUTRON TRANSMISSION SCTROSCOPY (PFNTS) a*, David Vartsky a, I. Mardor a, M. B. Goldberg a, D. Bar a, G. Feldman a,
MONTE-CARLO SIMULATIONS OF TIME- RESOLVED, OPTICAL READOUT DETECTOR for PULSED, FAST-NEUTRON TRANSMISSION SCTROSCOPY (PFNTS) a*, David Vartsky a, I. Mardor a, M. B. Goldberg a, D. Bar a, G. Feldman a,
MAGNETIC EFFECT OF CURRENT
 MAGNETIC EFFECT OF CURRENT VERY SHORT ANSWER QUESTIONS Q.1 Who designed cyclotron? Q.2 What is the magnetic field at a point on the axis of the current element? Q.3 Can the path of integration around which
MAGNETIC EFFECT OF CURRENT VERY SHORT ANSWER QUESTIONS Q.1 Who designed cyclotron? Q.2 What is the magnetic field at a point on the axis of the current element? Q.3 Can the path of integration around which
Role of nuclear spin in photoionization: Hyperfine-resolved photoionization of Xe and multichannel quantum defect theory analysis
 Role of nuclear spin in photoionization: Hyperfine-resolved photoionization of Xe and multichannel quantum defect theory analysis H. J. Wörner, M. Grütter, E. Vliegen, and F. Merkt* Laboratorium für Physikalische
Role of nuclear spin in photoionization: Hyperfine-resolved photoionization of Xe and multichannel quantum defect theory analysis H. J. Wörner, M. Grütter, E. Vliegen, and F. Merkt* Laboratorium für Physikalische
Theory English (Official)
 Q3-1 Large Hadron Collider (10 points) Please read the general instructions in the separate envelope before you start this problem. In this task, the physics of the particle accelerator LHC (Large Hadron
Q3-1 Large Hadron Collider (10 points) Please read the general instructions in the separate envelope before you start this problem. In this task, the physics of the particle accelerator LHC (Large Hadron
Copyright 2008, University of Chicago, Department of Physics. Experiment VI. Gamma Ray Spectroscopy
 Experiment VI Gamma Ray Spectroscopy 1. GAMMA RAY INTERACTIONS WITH MATTER In order for gammas to be detected, they must lose energy in the detector. Since gammas are electromagnetic radiation, we must
Experiment VI Gamma Ray Spectroscopy 1. GAMMA RAY INTERACTIONS WITH MATTER In order for gammas to be detected, they must lose energy in the detector. Since gammas are electromagnetic radiation, we must
MASS SPECTROMETRY. Topics
 MASS SPECTROMETRY MALDI-TOF AND ESI-MS Topics Principle of Mass Spectrometry MALDI-TOF Determination of Mw of Proteins Structural Information by MS: Primary Sequence of a Protein 1 A. Principles Ionization:
MASS SPECTROMETRY MALDI-TOF AND ESI-MS Topics Principle of Mass Spectrometry MALDI-TOF Determination of Mw of Proteins Structural Information by MS: Primary Sequence of a Protein 1 A. Principles Ionization:
Experiment objectives: measure the ratio of Planck s constant to the electron charge h/e using the photoelectric effect.
 Chapter 1 Photoelectric Effect Experiment objectives: measure the ratio of Planck s constant to the electron charge h/e using the photoelectric effect. History The photoelectric effect and its understanding
Chapter 1 Photoelectric Effect Experiment objectives: measure the ratio of Planck s constant to the electron charge h/e using the photoelectric effect. History The photoelectric effect and its understanding
Lab 6 - Electron Charge-To-Mass Ratio
 Lab 6 Electron Charge-To-Mass Ratio L6-1 Name Date Partners Lab 6 - Electron Charge-To-Mass Ratio OBJECTIVES To understand how electric and magnetic fields impact an electron beam To experimentally determine
Lab 6 Electron Charge-To-Mass Ratio L6-1 Name Date Partners Lab 6 - Electron Charge-To-Mass Ratio OBJECTIVES To understand how electric and magnetic fields impact an electron beam To experimentally determine
and another with a peak frequency ω 2
 Physics Qualifying Examination Part I 7-Minute Questions September 13, 2014 1. A sealed container is divided into two volumes by a moveable piston. There are N A molecules on one side and N B molecules
Physics Qualifying Examination Part I 7-Minute Questions September 13, 2014 1. A sealed container is divided into two volumes by a moveable piston. There are N A molecules on one side and N B molecules
Wave Motion and Sound
 Wave Motion and Sound 1. A back and forth motion that repeats itself is a a. Spring b. Vibration c. Wave d. Pulse 2. The number of vibrations that occur in 1 second is called a. A Period b. Frequency c.
Wave Motion and Sound 1. A back and forth motion that repeats itself is a a. Spring b. Vibration c. Wave d. Pulse 2. The number of vibrations that occur in 1 second is called a. A Period b. Frequency c.
PhysicsAndMathsTutor.com 1
 PhysicsAndMathsTutor.com 1 1. Millikan determined the charge on individual oil droplets using an arrangement as represented in the diagram. The plate voltage necessary to hold a charged droplet stationary
PhysicsAndMathsTutor.com 1 1. Millikan determined the charge on individual oil droplets using an arrangement as represented in the diagram. The plate voltage necessary to hold a charged droplet stationary
Secondary Ion Mass Spectroscopy (SIMS)
 Secondary Ion Mass Spectroscopy (SIMS) Analyzing Inorganic Solids * = under special conditions ** = semiconductors only + = limited number of elements or groups Analyzing Organic Solids * = under special
Secondary Ion Mass Spectroscopy (SIMS) Analyzing Inorganic Solids * = under special conditions ** = semiconductors only + = limited number of elements or groups Analyzing Organic Solids * = under special
Model Answer (Paper code: AR-7112) M. Sc. (Physics) IV Semester Paper I: Laser Physics and Spectroscopy
 Model Answer (Paper code: AR-7112) M. Sc. (Physics) IV Semester Paper I: Laser Physics and Spectroscopy Section I Q1. Answer (i) (b) (ii) (d) (iii) (c) (iv) (c) (v) (a) (vi) (b) (vii) (b) (viii) (a) (ix)
Model Answer (Paper code: AR-7112) M. Sc. (Physics) IV Semester Paper I: Laser Physics and Spectroscopy Section I Q1. Answer (i) (b) (ii) (d) (iii) (c) (iv) (c) (v) (a) (vi) (b) (vii) (b) (viii) (a) (ix)
Lab 6 - ELECTRON CHARGE-TO-MASS RATIO
 101 Name Date Partners OBJECTIVES OVERVIEW Lab 6 - ELECTRON CHARGE-TO-MASS RATIO To understand how electric and magnetic fields impact an electron beam To experimentally determine the electron charge-to-mass
101 Name Date Partners OBJECTIVES OVERVIEW Lab 6 - ELECTRON CHARGE-TO-MASS RATIO To understand how electric and magnetic fields impact an electron beam To experimentally determine the electron charge-to-mass
Miniature electrostatic electron energy analyzers and S-shaped deflector
 REVIEW OF SCIENTIFIC INSTRUMENTS 79, 023104 2008 Miniature electrostatic electron energy analyzers and S-shaped deflector J. F. Williams, 1,2 X. Chen, 3 and P. Wilkie 1,2 1 Centre for Atomic, Molecular
REVIEW OF SCIENTIFIC INSTRUMENTS 79, 023104 2008 Miniature electrostatic electron energy analyzers and S-shaped deflector J. F. Williams, 1,2 X. Chen, 3 and P. Wilkie 1,2 1 Centre for Atomic, Molecular
Photoelectron Spectroscopy using High Order Harmonic Generation
 Photoelectron Spectroscopy using High Order Harmonic Generation Alana Ogata Yamanouchi Lab, University of Tokyo ABSTRACT The analysis of photochemical processes has been previously limited by the short
Photoelectron Spectroscopy using High Order Harmonic Generation Alana Ogata Yamanouchi Lab, University of Tokyo ABSTRACT The analysis of photochemical processes has been previously limited by the short
An ion follows a circular path in a uniform magnetic field. Which single change decreases the radius of the path?
 T5-1 [237 marks] 1. A circuit is formed by connecting a resistor between the terminals of a battery of electromotive force (emf) 6 V. The battery has internal resistance. Which statement is correct when
T5-1 [237 marks] 1. A circuit is formed by connecting a resistor between the terminals of a battery of electromotive force (emf) 6 V. The battery has internal resistance. Which statement is correct when
Ratio of Charge to Mass (e/m) for the Electron
 Objective: In this experiment you will determine the ratio of charge to mass (e/m) of the electron, by measuring the deflecting of electrons as they move through a magnetic field. Apparatus: e/m apparatus
Objective: In this experiment you will determine the ratio of charge to mass (e/m) of the electron, by measuring the deflecting of electrons as they move through a magnetic field. Apparatus: e/m apparatus
Chap. 3. Elementary Quantum Physics
 Chap. 3. Elementary Quantum Physics 3.1 Photons - Light: e.m "waves" - interference, diffraction, refraction, reflection with y E y Velocity = c Direction of Propagation z B z Fig. 3.1: The classical view
Chap. 3. Elementary Quantum Physics 3.1 Photons - Light: e.m "waves" - interference, diffraction, refraction, reflection with y E y Velocity = c Direction of Propagation z B z Fig. 3.1: The classical view
MANIPAL INSTITUTE OF TECHNOLOGY
 SCHEME OF EVAUATION MANIPA INSTITUTE OF TECHNOOGY MANIPA UNIVERSITY, MANIPA SECOND SEMESTER B.Tech. END-SEMESTER EXAMINATION - MAY SUBJECT: ENGINEERING PHYSICS (PHY/) Time: 3 Hrs. Max. Marks: 5 Note: Answer
SCHEME OF EVAUATION MANIPA INSTITUTE OF TECHNOOGY MANIPA UNIVERSITY, MANIPA SECOND SEMESTER B.Tech. END-SEMESTER EXAMINATION - MAY SUBJECT: ENGINEERING PHYSICS (PHY/) Time: 3 Hrs. Max. Marks: 5 Note: Answer
Harmonic Generation for Photoionization Experiments Christian J. Kornelis Physics REU Kansas State University
 Harmonic Generation for Photoionization Experiments Christian J. Kornelis Physics REU Kansas State University The Basic Setup for the KLS Photoionization Experiment V. Kumarappan Femtosecond Pump-Probe
Harmonic Generation for Photoionization Experiments Christian J. Kornelis Physics REU Kansas State University The Basic Setup for the KLS Photoionization Experiment V. Kumarappan Femtosecond Pump-Probe
ELECTRON IMPACT IONIZATION OF HELIUM [(e,2e) & (e,3e)] INVESTIGATED WITH COLD TARGET RECOIL-ION MOMENTUM SPECTROSCOPY
![ELECTRON IMPACT IONIZATION OF HELIUM [(e,2e) & (e,3e)] INVESTIGATED WITH COLD TARGET RECOIL-ION MOMENTUM SPECTROSCOPY ELECTRON IMPACT IONIZATION OF HELIUM [(e,2e) & (e,3e)] INVESTIGATED WITH COLD TARGET RECOIL-ION MOMENTUM SPECTROSCOPY](/thumbs/71/65949100.jpg) ELECTRON IMPACT IONIZATION OF HELIUM [(e,2e) & (e,3e)] INVESTIGATED WITH COLD TARGET RECOIL-ION MOMENTUM SPECTROSCOPY E. Erturk, 1 L. Spielberger, 1 M. Achler, 1 L. Schmidt, 1 R. Dorner, 1 Th. Weber, 1
ELECTRON IMPACT IONIZATION OF HELIUM [(e,2e) & (e,3e)] INVESTIGATED WITH COLD TARGET RECOIL-ION MOMENTUM SPECTROSCOPY E. Erturk, 1 L. Spielberger, 1 M. Achler, 1 L. Schmidt, 1 R. Dorner, 1 Th. Weber, 1
Praktikum zur. Materialanalytik
 Praktikum zur Materialanalytik Energy Dispersive X-ray Spectroscopy B513 Stand: 19.10.2016 Contents 1 Introduction... 2 2. Fundamental Physics and Notation... 3 2.1. Alignments of the microscope... 3 2.2.
Praktikum zur Materialanalytik Energy Dispersive X-ray Spectroscopy B513 Stand: 19.10.2016 Contents 1 Introduction... 2 2. Fundamental Physics and Notation... 3 2.1. Alignments of the microscope... 3 2.2.
Quantitative Assessment of Scattering Contributions in MeV-Industrial X-ray Computed Tomography
 11th European Conference on Non-Destructive Testing (ECNDT 2014), October 6-10, 2014, Prague, Czech Republic More Info at Open Access Database www.ndt.net/?id=16530 Quantitative Assessment of Scattering
11th European Conference on Non-Destructive Testing (ECNDT 2014), October 6-10, 2014, Prague, Czech Republic More Info at Open Access Database www.ndt.net/?id=16530 Quantitative Assessment of Scattering
Semiconductor Physics and Devices
 Introduction to Quantum Mechanics In order to understand the current-voltage characteristics, we need some knowledge of electron behavior in semiconductor when the electron is subjected to various potential
Introduction to Quantum Mechanics In order to understand the current-voltage characteristics, we need some knowledge of electron behavior in semiconductor when the electron is subjected to various potential
Experiment 3 1. The Michelson Interferometer and the He- Ne Laser Physics 2150 Experiment No. 3 University of Colorado
 Experiment 3 1 Introduction The Michelson Interferometer and the He- Ne Laser Physics 2150 Experiment No. 3 University of Colorado The Michelson interferometer is one example of an optical interferometer.
Experiment 3 1 Introduction The Michelson Interferometer and the He- Ne Laser Physics 2150 Experiment No. 3 University of Colorado The Michelson interferometer is one example of an optical interferometer.
Absolute Integral and Differential Cross Sections for the Reactive Scattering of H - + D 2 and D - + H 2
 J. Phys. Chem. A 1997, 101, 6441-6447 6441 Absolute Integral and Differential Cross Sections for the Reactive Scattering of H - + D 2 and D - + H 2 E. Haufler, S. Schlemmer, and D. Gerlich* Institut für
J. Phys. Chem. A 1997, 101, 6441-6447 6441 Absolute Integral and Differential Cross Sections for the Reactive Scattering of H - + D 2 and D - + H 2 E. Haufler, S. Schlemmer, and D. Gerlich* Institut für
Stability and loss in an ion-trap resonator
 PHYSICAL REVIEW A, VOLUME 65, 042703 Stability and loss in an ion-trap resonator H. B. Pedersen, D. Strasser, O. Heber, M. L. Rappaport, and D. Zajfman* Department of Particle Physics, Weizmann Institute
PHYSICAL REVIEW A, VOLUME 65, 042703 Stability and loss in an ion-trap resonator H. B. Pedersen, D. Strasser, O. Heber, M. L. Rappaport, and D. Zajfman* Department of Particle Physics, Weizmann Institute
(Refer Slide Time 00:09) (Refer Slide Time 00:13)
 (Refer Slide Time 00:09) Mass Spectrometry Based Proteomics Professor Sanjeeva Srivastava Department of Biosciences and Bioengineering Indian Institute of Technology, Bombay Mod 02 Lecture Number 09 (Refer
(Refer Slide Time 00:09) Mass Spectrometry Based Proteomics Professor Sanjeeva Srivastava Department of Biosciences and Bioengineering Indian Institute of Technology, Bombay Mod 02 Lecture Number 09 (Refer
ICPMS Doherty Lecture 1
 ICPMS Doherty Lecture 1 Mass Spectrometry This material provides some background on how to measure isotope abundances by means of mass spectrometry. Mass spectrometers create and separate ionized atoms
ICPMS Doherty Lecture 1 Mass Spectrometry This material provides some background on how to measure isotope abundances by means of mass spectrometry. Mass spectrometers create and separate ionized atoms
February 18, In the parallel RLC circuit shown, R = Ω, L = mh and C = µf. The source has V 0. = 20.0 V and f = Hz.
 Physics Qualifying Examination Part I 7- Minute Questions February 18, 2012 1. In the parallel RLC circuit shown, R = 800.0 Ω, L = 160.0 mh and C = 0.0600 µf. The source has V 0 = 20.0 V and f = 2400.0
Physics Qualifying Examination Part I 7- Minute Questions February 18, 2012 1. In the parallel RLC circuit shown, R = 800.0 Ω, L = 160.0 mh and C = 0.0600 µf. The source has V 0 = 20.0 V and f = 2400.0
Experimental Methods of Particle Physics
 Experimental Methods of Particle Physics (PHY461) Fall 015 Olaf Steinkamp 36-J- olafs@physik.uzh.ch 044 63 55763 Overview 1) Introduction / motivation measurement of particle momenta: magnetic field early
Experimental Methods of Particle Physics (PHY461) Fall 015 Olaf Steinkamp 36-J- olafs@physik.uzh.ch 044 63 55763 Overview 1) Introduction / motivation measurement of particle momenta: magnetic field early
object objective lens eyepiece lens
 Advancing Physics G495 June 2015 SET #1 ANSWERS Field and Particle Pictures Seeing with electrons The compound optical microscope Q1. Before attempting this question it may be helpful to review ray diagram
Advancing Physics G495 June 2015 SET #1 ANSWERS Field and Particle Pictures Seeing with electrons The compound optical microscope Q1. Before attempting this question it may be helpful to review ray diagram
THEORETICAL PROBLEM 2 DOPPLER LASER COOLING AND OPTICAL MOLASSES
 THEORETICAL PROBLEM 2 DOPPLER LASER COOLING AND OPTICAL MOLASSES The purpose of this problem is to develop a simple theory to understand the so-called laser cooling and optical molasses phenomena. This
THEORETICAL PROBLEM 2 DOPPLER LASER COOLING AND OPTICAL MOLASSES The purpose of this problem is to develop a simple theory to understand the so-called laser cooling and optical molasses phenomena. This
Studies of Charge Separation Characteristics for Higher Density Plasma. in a Direct Energy Converter Using Slanted Cusp Magnetic Field
 J. Plasma Fusion Res. SERES, Vol. 9 (1) Studies of Charge Separation Characteristics for Higher Density Plasma in a Direct Energy Converter Using Slanted Cusp Magnetic Field Akio TANGUCH, Norifumi SOTAN,
J. Plasma Fusion Res. SERES, Vol. 9 (1) Studies of Charge Separation Characteristics for Higher Density Plasma in a Direct Energy Converter Using Slanted Cusp Magnetic Field Akio TANGUCH, Norifumi SOTAN,
RANGE OF ALPHAS. Advanced Laboratory, Physics 407 University of Wisconsin Madison, Wisconsin 53706
 (revised 10/20/10) RANGE OF ALPHAS Advanced Laboratory, Physics 407 University of Wisconsin Madison, Wisconsin 53706 Abstract A silicon solid state detector is used to measure the energy of alphas which
(revised 10/20/10) RANGE OF ALPHAS Advanced Laboratory, Physics 407 University of Wisconsin Madison, Wisconsin 53706 Abstract A silicon solid state detector is used to measure the energy of alphas which
Doppler-Free Spectroscopy of Hyperfine Zeeman Effects in Rubidium
 Doppler-Free Spectroscopy of Hyperfine Zeeman Effects in Rubidium Samuel Bader and Leo Zhou MIT Department of Physics (Dated: May 19, 2013) The hyperfine Zeeman effect is observed via Doppler-free spectroscopy
Doppler-Free Spectroscopy of Hyperfine Zeeman Effects in Rubidium Samuel Bader and Leo Zhou MIT Department of Physics (Dated: May 19, 2013) The hyperfine Zeeman effect is observed via Doppler-free spectroscopy
Cluster fusion in a high magnetic field
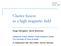 Santa Fe July 28, 2009 Cluster fusion in a high magnetic field Roger Bengtson, Boris Breizman Institute for Fusion Studies, Fusion Research Center The University of Texas at Austin In collaboration with:
Santa Fe July 28, 2009 Cluster fusion in a high magnetic field Roger Bengtson, Boris Breizman Institute for Fusion Studies, Fusion Research Center The University of Texas at Austin In collaboration with:
