MoLE Gas Laws Activities
|
|
|
- Cameron Mitchell
- 6 years ago
- Views:
Transcription
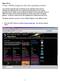
1 MoLE Gas Laws Activities To begin this assignment you must be able to log on to the Internet using Internet Explorer (Microsoft) 4.5 or higher. If you do not have the current version of the browser, go to and follow the instructions on the page. You will need Internet Explorer for your particular operating system. If you have any difficulties contact your instructor. Once the browser is running, type the following address into the location-input line near the top of the Internet Explorer window: This will load the Gas Simulation. Once you have the simulation is running your screen will look like what is shown in left hand section of Figure I. below. Control Bar Region Gas Sample Region Graphing Region Figure I. There are three important regions that require some discussion. The Gas Sample Region has the most activity. It is a container with a plunger. To explore the behavior of the gas sample you can change the variables located in the Control Bar Region. The Control Bar Region shows five scrollbars: pressure (in units of atmospheres), volume (in units of liters), mol of gas (one for He gas and the other for Ne gas), and temperature (in units of Kelvins). To the left of each scrollbar is a radio button. When selected, that particular variable (called the dependent variable)
2 is calculated based on the value of the other four variables. In the default mode the pressure scrollbar's radio button is selected so the pressure of the gas sample is calculated. As a simple exploration try moving each of the scrollbars and observe the effect on the gas sample. These effects will be addressed in more detail in this experiment. There are three buttons immediately below the Control Bar Region. The Pause button will suspend the motion in the gas sample, the Reset button returns the program to the default conditions, and the Enable Tracking button turns a red tracking line on and off. Below the Pause Button is a fourth button different from the previous three in that it is a dropdown menu. Clicking and holding the mouse button will reveal three choices: Velocities (default); Relations (graphing); and Help. Figure II. The default mode is Velocities. The Velocity Distribution Region shows a plot of the velocity distribution in the gas sample (see Figure II.). The y-axis of this plot represents the number of particles. The x-axis represents the range of velocities starting at zero. The bars in this plot represent the velocities of the particles in the Gas Sample Region. As the velocities of the particles change, the plot is redrawn. The smooth curved line in the plot represents the ideal distribution of the velocities for the gas sample. The vertical line represents the average (rootmean-square average) velocity of the sample. Observe the behavior of this region while changing each of the variables in the Control Bar Region. In the Gas Sample Region, one of the particles is labeled with a red dot. This same particle is identified in red in the velocity distribution plot. If you click on the enable tracking button, you can follow the path of the particle. The length of the tail represents a fixed unit of time, and consequently can be used as a measure of the velocity of the particle. If you pause the motion of the particles, you can click on different particles to get a measure of their velocity. A second choice from the drop down menu is the Relations view. This choice reveals an x,y graph with a dropdown menu on each axis. Selecting the dropdown menu on either axis provides a list of the variables shown in the Control Bar Region. The two buttons, Enable and Multiple are used when plotting pairs of variables. If you select pressure for the y-axis and volume for the x-axis, and the click on Enable, these same variables are activated in the Control Bar Region. By scrolling the volume slide bar in the Control Bar Region you will trace out a plot that will look similar to Figure III.
3 Figure III. The Multiple button allows two or more plots to be superimposed. Select Pressure and Volume as the variable to be plotted on your graph. Then click on the Multiple button and adjust the temperature to 400 K. Click on the Enable button and scroll the Volume slide bar. Click on the Disable button and change the temperature to 200 K. Click on the Enable button and scroll the Volume slide bar. Repeat this process for the minimum temperature. You will see a graph that looks similar to Figure IV. Figure IV. Got the hang of it? If you have any questions check with another student in the class, your instructor.
4 Gas Pressure and Volume Relationships Exp. Computer Simulation -A Name Lab Section Problem Statement: How are the pressure and volume of a gas sample related? I. Data Collection: A. Open the Gas Law Simulation program and observe and describe, in the space below, the activity in the Gas Sample window. Consider using the some or all of the following terms in your description: particles, atoms, molecules, collisions, velocity, energy, force. B. One of the objects in the window is colored differently than the others. Enable the tracking function and trace the path of a particle from one side of the screen to the other in the space below. Explain any changes in velocity or direction that you observe.
5 C. Record the values for pressure, volume and temperature on the digital read-outs of the Control Bar window. D. Observe the action in the Velocity Distribution window. Relate what you see with the behavior of the objects in the Gas Sample window. Click the Pause button and sketch and label the graph in the space below. E. Using the controls in the Control Bar window, fix Pressure as a dependent variable by clicking on its radio button. Change the volume of the container using the Volume slider bar and observe what happens to the pressure of the system. Also observe what happens in the Velocity Distribution window. Explain how the activity in the Gas Sample window accounts for your observations.
6 F. Collect five additional observations of volume/pressure relationships and record all of your data in the following table. Data Table Pressure Volume _ II. Data Analysis: What patterns are shown in these data? It might be helpful to graph the data. Try to come up with an algebraic equation that expresses the pattern you found.
7 III. Interpretation and Conclusions: A. How are the pressure and volume of a gas sample related? B. Relate what you observed concerning the motion of objects in this activity with your observations in experiment E-1A of your Inquiries into Chemistry laboratory manual. C. Using your data, predict the pressure of a gas sample at a volume of 100L. Show how you made your prediction. Please take a moment to fill out a brief survey of your opinions of this activity at:
8 Gas Pressure and Temperature Relationships Exp. Computer Simulation -B Name Lab Section Problem Statement: How are the pressure and temperature of a gas sample related? I. Data Collection: A. Record the values for pressure, volume and temperature on the digital read-outs of the Controls window. B. Observe the action in the Velocity Distribution window. Relate what you see with the behavior of the objects in the Gas Sample window. Click the Pause button and sketch and label the graph in the space below. B. Using the controls in the Control Bar window, change the temperature in the container and observe what happens to the pressure of the system. Also observe what happens in the Velocity Distribution window. Explain how the activity in the Gas Sample window accounts for your observations.
9 C. Collect five additional observations of pressure/temperature relationships and record all of your data in the following table. Data Table Pressure Temperature _ II. Data Analysis: A. What patterns are shown in these data? It might be helpful to graph the data. Try to come up with an algebraic equation that expresses the pattern you found.
10 III. Interpretation and Conclusions: A. How are the pressure and temperature of a gas sample related? B. Relate what you observed concerning the motion of objects in this activity with your observations in experiment E-1 B of your Inquiries into Chemistry laboratory manual. C. Using your data, predict the pressure of a gas sample at a temperature of 10 Kelvins. Show how you made your prediction.
11 Gas Systems Computer Simulation System 1 Investigate the relationship between the volume and temperature of a gas sample at constant pressure and amount. System 2 Investigate how the number of gas particles effects the pressure of a gas sample. System 3 Compare and interpret pressure vs. volume relationships of gas samples at different temperatures. (You can plot multiple graphs in the plotting window by clicking on the mutiple button.) System 4 Investigate the average speed (distance per unit time) of particles in a gas as a function of pressure, volume, amount, or temperature. (You can expose the path traveled by a particle during a time interval by activating the "enable tracking" button in the Control Bar window, and then clicking on a particle.) System 5 Investigate the average number of collisions between particles or with the container walls in a gas sample as a function of pressure, volume, amount, or temperature. (You can expose the path traveled by a particle during a time interval by activating the "enable tracking" button in the Control Bar window, and then clicking on a particle.) System 6 Investigate any of the above systems using different kinds of particles or combinations of particles. System 7 Investigate any other gas system or investigate a modification of any of the above systems.
12 Research Statements Use evidence from the MoLE simulation to prove or disprove the following assertions. 1. The pressure exerted by a combination of gases is equal to the sum of the pressure exerted by the individual gases. 2. The speed of a gas particle increases with increasing pressure when the volume is decreased. 3. The pressure exerted by a gas depends on its molar mass. 4. The speed of a gas particle depends on its molar mass. 5. The kinetic energy of a gas particle depends on its molar mass. 6. The collision of a gas particle with the walls of its container does not effect its speed. 7. The collision of a gas particle with another gas particle does not effect its speed. 8. The average speed of two like gas particles before a collision is equal to the average speed after the collision. 9. The average speed of two unlike gas particles before a collision is equal to the average speed after the collision.
MoLE Gas Laws Activities *
 MoLE Gas Laws Activities * To begin this assignment you must be able to log on to the Internet using Internet Explorer (Microsoft) 4.5 or higher. If you do not have the current version of the browser,
MoLE Gas Laws Activities * To begin this assignment you must be able to log on to the Internet using Internet Explorer (Microsoft) 4.5 or higher. If you do not have the current version of the browser,
Gas Pressure and Temperature Relationships *
 Gas Pressure and Temperature Relationships * MoLE Activities To begin this assignment you must be able to log on to the Internet (the software requires OSX for mac users). Type the following address into
Gas Pressure and Temperature Relationships * MoLE Activities To begin this assignment you must be able to log on to the Internet (the software requires OSX for mac users). Type the following address into
Introduction to MoLEs Activities Computer Simulations *
 Introduction to MoLEs Activities Computer Simulations * Molecular Level Experiments (MoLEs) are chemistry laboratory experiments based on computer simulations. There are two types of simulation programs
Introduction to MoLEs Activities Computer Simulations * Molecular Level Experiments (MoLEs) are chemistry laboratory experiments based on computer simulations. There are two types of simulation programs
Mass and Particle Relationships
 Mass and Particle Relationships Name Lab Section Log on to the Internet. Type the following address into the location-input line of your browser: http://cheminfo.chem.ou.edu/~mra/ccli2004/srgm1.htm This
Mass and Particle Relationships Name Lab Section Log on to the Internet. Type the following address into the location-input line of your browser: http://cheminfo.chem.ou.edu/~mra/ccli2004/srgm1.htm This
Acid/Base Interactions
 Acid/Base Interactions Name Lab Section Log on to the Internet. Type the following address into the location-input line of your browser: http://cheminfo.chem.ou.edu/~mra/ccli2004/acids+bm.htm This will
Acid/Base Interactions Name Lab Section Log on to the Internet. Type the following address into the location-input line of your browser: http://cheminfo.chem.ou.edu/~mra/ccli2004/acids+bm.htm This will
Shifting Reactions A
 Shifting Reactions A Name Lab Section Log on to the Internet. Type the following address into the location-input line of your browser: http://introchem.chem.okstate.edu/dcicla/ergbm.htm This will load
Shifting Reactions A Name Lab Section Log on to the Internet. Type the following address into the location-input line of your browser: http://introchem.chem.okstate.edu/dcicla/ergbm.htm This will load
Shifting Reactions B
 Shifting Reactions B Name Lab Section Log on to the Internet. Type the following address into the location-input line of your browser: http://introchem.chem.okstate.edu/dcicla/ergbn.htm This will load
Shifting Reactions B Name Lab Section Log on to the Internet. Type the following address into the location-input line of your browser: http://introchem.chem.okstate.edu/dcicla/ergbn.htm This will load
Introduction to Gases Guided Inquiry
 Introduction to Gases Guided Inquiry Part 1 - The Kinetic Molecular Theory Adapted from a POGIL authored by Linda Padwa and David Hanson, Stony Brook University Why? The kinetic-molecular theory is a model
Introduction to Gases Guided Inquiry Part 1 - The Kinetic Molecular Theory Adapted from a POGIL authored by Linda Padwa and David Hanson, Stony Brook University Why? The kinetic-molecular theory is a model
Speeds of a Chemical Reaction
 Speeds of a Chemical Reaction Name Lab Section Log on to the Internet. Type the following address into the location-input line of your browser: http://cheminfo.chem.ou.edu/~mra/ccli2004/k2rm.htm This will
Speeds of a Chemical Reaction Name Lab Section Log on to the Internet. Type the following address into the location-input line of your browser: http://cheminfo.chem.ou.edu/~mra/ccli2004/k2rm.htm This will
Acid/Base Classifications
 Acid/Base Classifications Name Lab Section Log on to the Internet. Type the following address into the location-input line of your browser: http://cheminfo.chem.ou.edu/~mra/ccli2004/acidsm.htm This will
Acid/Base Classifications Name Lab Section Log on to the Internet. Type the following address into the location-input line of your browser: http://cheminfo.chem.ou.edu/~mra/ccli2004/acidsm.htm This will
The purpose of this lab is to investigate phases of matter, temperature, and heat energy.
 9460218_CH07_p081-090.qxd 1/20/10 9:46 PM Page 81 7 TEMPERATURE AND HEAT PURPOSE The purpose of this lab is to investigate phases of matter, temperature, and heat energy. SIMULATIONS States of Matter Figure
9460218_CH07_p081-090.qxd 1/20/10 9:46 PM Page 81 7 TEMPERATURE AND HEAT PURPOSE The purpose of this lab is to investigate phases of matter, temperature, and heat energy. SIMULATIONS States of Matter Figure
Catalysts. Lab Section. Log on to the Internet. Type the following address into the location-input line of your browser:
 Catalysts Name Lab Section Log on to the Internet. Type the following address into the location-input line of your browser: http://cheminfo.chem.ou.edu/~mra/ccli2004/kcatbgm.htm This will load a Particulate
Catalysts Name Lab Section Log on to the Internet. Type the following address into the location-input line of your browser: http://cheminfo.chem.ou.edu/~mra/ccli2004/kcatbgm.htm This will load a Particulate
After obtaining access to the Jmol program you should see an image on your computer screen that looks similar to the following figure. Figure 1.
 A C T I V I T I E S Molecular Modeling Introduction to Molecular Modeling In the following laboratory activities you will examine three-dimensional models of molecules using the computer-based molecular
A C T I V I T I E S Molecular Modeling Introduction to Molecular Modeling In the following laboratory activities you will examine three-dimensional models of molecules using the computer-based molecular
Gases. Section 13.1 The Gas Laws Section 13.2 The Ideal Gas Law Section 13.3 Gas Stoichiometry
 Gases Section 13.1 The Gas Laws Section 13.2 The Ideal Gas Law Section 13.3 Gas Stoichiometry Click a hyperlink or folder tab to view the corresponding slides. Exit Section 13.1 The Gas Laws State the
Gases Section 13.1 The Gas Laws Section 13.2 The Ideal Gas Law Section 13.3 Gas Stoichiometry Click a hyperlink or folder tab to view the corresponding slides. Exit Section 13.1 The Gas Laws State the
Center of Mass. Evaluation copy
 Center of Mass Experiment 19 INTRODUCTION In the most of the previous experiments you have examined the motion of a single object as it underwent a variety of motions. You learned that an object subject
Center of Mass Experiment 19 INTRODUCTION In the most of the previous experiments you have examined the motion of a single object as it underwent a variety of motions. You learned that an object subject
Collisions and conservation laws
 (ta initials) first name (print) last name (print) brock id (ab17cd) (lab date) Experiment 4 Collisions and conservation laws Prelab preparation Print a copy of this experiment to bring to your scheduled
(ta initials) first name (print) last name (print) brock id (ab17cd) (lab date) Experiment 4 Collisions and conservation laws Prelab preparation Print a copy of this experiment to bring to your scheduled
Simulation: Gas Laws FOR THE TEACHER
 Simulation: Gas Laws FOR THE TEACHER Summary In this simulation, students will investigate three of the fundamental gas laws, including Boyle s Law, Charles Law and Gay-Lussac s Law. Students will have
Simulation: Gas Laws FOR THE TEACHER Summary In this simulation, students will investigate three of the fundamental gas laws, including Boyle s Law, Charles Law and Gay-Lussac s Law. Students will have
Project: Equilibrium Simulation
 UNIT 02: BCLN CHEMISTRY 12 - Rev. July, 2015 Project: Simulation Potential Credits: /20 Name: Goal: In this project you will explore the concept of reversible reactions and calculating K eq using some
UNIT 02: BCLN CHEMISTRY 12 - Rev. July, 2015 Project: Simulation Potential Credits: /20 Name: Goal: In this project you will explore the concept of reversible reactions and calculating K eq using some
Simulating Future Climate Change Using A Global Climate Model
 Simulating Future Climate Change Using A Global Climate Model Introduction: (EzGCM: Web-based Version) The objective of this abridged EzGCM exercise is for you to become familiar with the steps involved
Simulating Future Climate Change Using A Global Climate Model Introduction: (EzGCM: Web-based Version) The objective of this abridged EzGCM exercise is for you to become familiar with the steps involved
Energy Analysis of a Mass Oscillating on a Spring Masses and Springs Simulation
 Energy Analysis of a Mass Oscillating on a Spring Masses and Springs Simulation Using FIREFOX only, go to http://www.colorado.edu/physics/phet (or Google phet ) Click on Simulations, then Masses and Springs
Energy Analysis of a Mass Oscillating on a Spring Masses and Springs Simulation Using FIREFOX only, go to http://www.colorado.edu/physics/phet (or Google phet ) Click on Simulations, then Masses and Springs
Activity 8b - Electric Field Exploration
 Name Date Activity 8b - Electric Field Exploration Pd Go to the following website: http://phet.colorado.edu Find the heading Run our Simulations and click On Line. Under the Simulations heading, select
Name Date Activity 8b - Electric Field Exploration Pd Go to the following website: http://phet.colorado.edu Find the heading Run our Simulations and click On Line. Under the Simulations heading, select
Motion II. Goals and Introduction
 Motion II Goals and Introduction As you have probably already seen in lecture or homework, and if you ve performed the experiment Motion I, it is important to develop a strong understanding of how to model
Motion II Goals and Introduction As you have probably already seen in lecture or homework, and if you ve performed the experiment Motion I, it is important to develop a strong understanding of how to model
Student Exploration: Temperature and Particle Motion
 Name: Date: Student Exploration: Temperature and Particle Motion Vocabulary: absolute zero, Kelvin scale, kinetic energy, molecule, temperature, Prior Knowledge Questions (Do these BEFORE using the Gizmo.)
Name: Date: Student Exploration: Temperature and Particle Motion Vocabulary: absolute zero, Kelvin scale, kinetic energy, molecule, temperature, Prior Knowledge Questions (Do these BEFORE using the Gizmo.)
Student Exploration: Energy of a Pendulum
 Name: Date: Student Exploration: Energy of a Pendulum Vocabulary: conservation of energy, gravitational potential energy, kinetic energy, pendulum, potential energy, velocity Prior Knowledge Questions
Name: Date: Student Exploration: Energy of a Pendulum Vocabulary: conservation of energy, gravitational potential energy, kinetic energy, pendulum, potential energy, velocity Prior Knowledge Questions
Cart on a Ramp. Evaluation Copy. Figure 1. Vernier Dynamics Track. Motion Detector Bracket
 Cart on a Ramp Computer 3 This experiment uses an incline and a low-friction cart. If you give the cart a gentle push up the incline, the cart will roll upward, slow and stop, and then roll back down,
Cart on a Ramp Computer 3 This experiment uses an incline and a low-friction cart. If you give the cart a gentle push up the incline, the cart will roll upward, slow and stop, and then roll back down,
Momentum ~ Lab Name:
 Momentum ~ Lab Name: Instructions: Using a pencil, answer the following questions. The lab is marked, based on effort, completeness, neatness, and accuracy. Do your best! Part 1: Explosion In this section,
Momentum ~ Lab Name: Instructions: Using a pencil, answer the following questions. The lab is marked, based on effort, completeness, neatness, and accuracy. Do your best! Part 1: Explosion In this section,
Student Exploration: Bohr Model of Hydrogen
 Name: Date: Student Exploration: Bohr Model of Hydrogen Vocabulary: absorption spectrum, Bohr model, electron volt, emission spectrum, energy level, ionization energy, laser, orbital, photon [Note to teachers
Name: Date: Student Exploration: Bohr Model of Hydrogen Vocabulary: absorption spectrum, Bohr model, electron volt, emission spectrum, energy level, ionization energy, laser, orbital, photon [Note to teachers
CC Algebra 2H Transforming the parent function
 CC Algebra H Transforming the Name: March. Open up the geometer s sketchpad document on Mr. March s website (It s under CC Algebra Unit Algebra Review). Make sure ou maimize both windows once ou open the
CC Algebra H Transforming the Name: March. Open up the geometer s sketchpad document on Mr. March s website (It s under CC Algebra Unit Algebra Review). Make sure ou maimize both windows once ou open the
Lab: Electric Fields Hockey *
 Name: Lab: Electric Fields Hockey * Objective: To investigate experimentally the concept of the electric field and to map (to represent graphically) some electric field lines for particular configurations
Name: Lab: Electric Fields Hockey * Objective: To investigate experimentally the concept of the electric field and to map (to represent graphically) some electric field lines for particular configurations
Burning a Hydrocarbon II
 Burning a Hydrocarbon II Name Lab Section Problem Statement: How are the masses of products limited by the amounts of reactants? I. Data Collection: A. Go to http://cheminfo.chem.ou.edu/~mra/home.html
Burning a Hydrocarbon II Name Lab Section Problem Statement: How are the masses of products limited by the amounts of reactants? I. Data Collection: A. Go to http://cheminfo.chem.ou.edu/~mra/home.html
Date: Summer Stem Section:
 Page 1 of 7 Name: Date: Summer Stem Section: Summer assignment: Build a Molecule Computer Simulation Learning Goals: 1. Students can describe the difference between a molecule name and chemical formula.
Page 1 of 7 Name: Date: Summer Stem Section: Summer assignment: Build a Molecule Computer Simulation Learning Goals: 1. Students can describe the difference between a molecule name and chemical formula.
Harmonic Motion. Mass on a Spring. Physics 231: General Physics I Lab 6 Mar. 11, Goals:
 Physics 231: General Physics I Lab 6 Mar. 11, 2004 Names: Harmonic Motion Goals: 1. To learn about the basic characteristics of periodic motion period, frequency, and amplitude 2. To study what affects
Physics 231: General Physics I Lab 6 Mar. 11, 2004 Names: Harmonic Motion Goals: 1. To learn about the basic characteristics of periodic motion period, frequency, and amplitude 2. To study what affects
Lab 12 Pressure-Temperature Relationship in Gases
 Lab 12 Pressure-Temperature Relationship in Gases INTRODUCTION /PURPOSE/PLE LAB QUESTION Gases are made up of molecules that are in constant motion and exert pressure when they collide with the walls of
Lab 12 Pressure-Temperature Relationship in Gases INTRODUCTION /PURPOSE/PLE LAB QUESTION Gases are made up of molecules that are in constant motion and exert pressure when they collide with the walls of
CHEMISTRY Matter and Change. Chapter 13: Gases
 CHEMISTRY Matter and Change Chapter 13: Gases CHAPTER 13 Table Of Contents Section 13.1 Section 13.2 Section 13.3 The Gas Laws The Ideal Gas Law Gas Stoichiometry Click a hyperlink to view the corresponding
CHEMISTRY Matter and Change Chapter 13: Gases CHAPTER 13 Table Of Contents Section 13.1 Section 13.2 Section 13.3 The Gas Laws The Ideal Gas Law Gas Stoichiometry Click a hyperlink to view the corresponding
Lab: Newton s Second Law
 Ph4_ConstMass2ndLawLab Page 1 of 9 Lab: Newton s Second Law Constant Mass Equipment Needed Qty Equipment Needed Qty 1 Mass and Hanger Set (ME-8967) 1 Motion Sensor (CI-6742) 1 String (SE-8050) 1 m Balance
Ph4_ConstMass2ndLawLab Page 1 of 9 Lab: Newton s Second Law Constant Mass Equipment Needed Qty Equipment Needed Qty 1 Mass and Hanger Set (ME-8967) 1 Motion Sensor (CI-6742) 1 String (SE-8050) 1 m Balance
Chem 1 Kinetics. Objectives. Concepts
 Chem 1 Kinetics Objectives 1. Learn some basic ideas in chemical kinetics. 2. Understand how the computer visualizations can be used to benefit the learning process. 3. Understand how the computer models
Chem 1 Kinetics Objectives 1. Learn some basic ideas in chemical kinetics. 2. Understand how the computer visualizations can be used to benefit the learning process. 3. Understand how the computer models
Gases! n Properties! n Kinetic Molecular Theory! n Variables! n The Atmosphere! n Gas Laws!
 Gases n Properties n Kinetic Molecular Theory n Variables n The Atmosphere n Gas Laws Properties of a Gas n No definite shape or volume n Gases expand to fill any container n Thus they take the shape of
Gases n Properties n Kinetic Molecular Theory n Variables n The Atmosphere n Gas Laws Properties of a Gas n No definite shape or volume n Gases expand to fill any container n Thus they take the shape of
Ideal Gas Law Experiment
 rev 05/2018 Ideal Gas Law Experiment Equipment List Qty Item Part number 1 Ideal Gas Law Apparatus TD-8596A 1 Pressure Sensor Absolute CI-6532A 1 Analog Adaptor Introduction The purpose of this lab is
rev 05/2018 Ideal Gas Law Experiment Equipment List Qty Item Part number 1 Ideal Gas Law Apparatus TD-8596A 1 Pressure Sensor Absolute CI-6532A 1 Analog Adaptor Introduction The purpose of this lab is
CHARTING THE HEAVENS USING A VIRTUAL PLANETARIUM
 Name Partner(s) Section Date CHARTING THE HEAVENS USING A VIRTUAL PLANETARIUM You have had the opportunity to look at two different tools to display the night sky, the celestial sphere and the star chart.
Name Partner(s) Section Date CHARTING THE HEAVENS USING A VIRTUAL PLANETARIUM You have had the opportunity to look at two different tools to display the night sky, the celestial sphere and the star chart.
Charles Law: V 1 = V 2 T 1 T 2
 Name: Gas Laws Background In this investigation you will examine three gas laws including Boyle s Law, Charles Law and Gay-Lussac s Law. You will explore how manipulating the variables of volume (L), pressure
Name: Gas Laws Background In this investigation you will examine three gas laws including Boyle s Law, Charles Law and Gay-Lussac s Law. You will explore how manipulating the variables of volume (L), pressure
Remember that C is a constant and ë and n are variables. This equation now fits the template of a straight line:
 CONVERTING NON-LINEAR GRAPHS INTO LINEAR GRAPHS Linear graphs have several important attributes. First, it is easy to recognize a graph that is linear. It is much more difficult to identify if a curved
CONVERTING NON-LINEAR GRAPHS INTO LINEAR GRAPHS Linear graphs have several important attributes. First, it is easy to recognize a graph that is linear. It is much more difficult to identify if a curved
Worksheet for Exploration 6.1: An Operational Definition of Work
 Worksheet for Exploration 6.1: An Operational Definition of Work This Exploration allows you to discover how work causes changes in kinetic energy. Restart. Drag "handy" to the front and/or the back of
Worksheet for Exploration 6.1: An Operational Definition of Work This Exploration allows you to discover how work causes changes in kinetic energy. Restart. Drag "handy" to the front and/or the back of
First Order Rate Laws and the Kinetics Mechanism Simulation Program
 First Order Rate Laws and the Kinetics Mechanism Simulation Program The Kinetics Mechanism Simulation program (kine.html) is useful for building models in a wide variety of areas, including population
First Order Rate Laws and the Kinetics Mechanism Simulation Program The Kinetics Mechanism Simulation program (kine.html) is useful for building models in a wide variety of areas, including population
Physics 1050 Experiment 6. Moment of Inertia
 Physics 1050 Moment of Inertia Prelab uestions These questions need to be completed before entering the lab. Please show all workings. Prelab 1 Sketch a graph of torque vs angular acceleration. Normal
Physics 1050 Moment of Inertia Prelab uestions These questions need to be completed before entering the lab. Please show all workings. Prelab 1 Sketch a graph of torque vs angular acceleration. Normal
TEACHER NOTES SCIENCE NSPIRED
 Science Objectives Students will explore the force two charges exert on each other. Students will observe how the force depends upon the magnitude of the two charges and the distance separating them. Students
Science Objectives Students will explore the force two charges exert on each other. Students will observe how the force depends upon the magnitude of the two charges and the distance separating them. Students
Electric Fields and Equipotentials
 OBJECTIVE Electric Fields and Equipotentials To study and describe the two-dimensional electric field. To map the location of the equipotential surfaces around charged electrodes. To study the relationship
OBJECTIVE Electric Fields and Equipotentials To study and describe the two-dimensional electric field. To map the location of the equipotential surfaces around charged electrodes. To study the relationship
LAB 2 - ONE DIMENSIONAL MOTION
 Name Date Partners L02-1 LAB 2 - ONE DIMENSIONAL MOTION OBJECTIVES Slow and steady wins the race. Aesop s fable: The Hare and the Tortoise To learn how to use a motion detector and gain more familiarity
Name Date Partners L02-1 LAB 2 - ONE DIMENSIONAL MOTION OBJECTIVES Slow and steady wins the race. Aesop s fable: The Hare and the Tortoise To learn how to use a motion detector and gain more familiarity
Newton s Third Law and Conservation of Momentum 1 Fall 2017
 Introduction Newton s Third Law and Conservation of omentum 1 Fall 217 The purpose of this experiment is to study the forces between objects that interact with each other, especially in collisions, and
Introduction Newton s Third Law and Conservation of omentum 1 Fall 217 The purpose of this experiment is to study the forces between objects that interact with each other, especially in collisions, and
for MiLAB Desktop Experiments in Physics imagine explore learn
 Experiments in Physics for MiLAB Desktop imagine explore learn www.einsteinworld.com 4 5 6 7 This book contains 48 student experiments in Physics. For your convenience we have added an index in which
Experiments in Physics for MiLAB Desktop imagine explore learn www.einsteinworld.com 4 5 6 7 This book contains 48 student experiments in Physics. For your convenience we have added an index in which
1. What is the value of the quantity PV for one mole of an ideal gas at 25.0 C and one atm?
 Real Gases Thought Question: How does the volume of one mole of methane gas (CH4) at 300 Torr and 298 K compare to the volume of one mole of an ideal gas at 300 Torr and 298 K? a) the volume of methane
Real Gases Thought Question: How does the volume of one mole of methane gas (CH4) at 300 Torr and 298 K compare to the volume of one mole of an ideal gas at 300 Torr and 298 K? a) the volume of methane
General Chemistry Lab Molecular Modeling
 PURPOSE The objectives of this experiment are PROCEDURE General Chemistry Lab Molecular Modeling To learn how to use molecular modeling software, a commonly used tool in chemical research and industry.
PURPOSE The objectives of this experiment are PROCEDURE General Chemistry Lab Molecular Modeling To learn how to use molecular modeling software, a commonly used tool in chemical research and industry.
Laws versus Theories
 Announcements Text HW due tomorrow (Friday) Online HW #3 (Type 1) due Monday, September 17 by 7:00 p.m. Online HW #3 (Type 2) due Wednesday, September 19 by 7:00 p.m. Lab write-up for Gases Lab due Wednesday,
Announcements Text HW due tomorrow (Friday) Online HW #3 (Type 1) due Monday, September 17 by 7:00 p.m. Online HW #3 (Type 2) due Wednesday, September 19 by 7:00 p.m. Lab write-up for Gases Lab due Wednesday,
3 Charged Particle Motion in a Magnetic Field
 3 Charged Particle Motion in a Magnetic Field When you have completed the Particle Annihilation section and read all the text (especially section 2.2), click the Next button in the Particle Annihilation
3 Charged Particle Motion in a Magnetic Field When you have completed the Particle Annihilation section and read all the text (especially section 2.2), click the Next button in the Particle Annihilation
The CSC Interface to Sky in Google Earth
 The CSC Interface to Sky in Google Earth CSC Threads The CSC Interface to Sky in Google Earth 1 Table of Contents The CSC Interface to Sky in Google Earth - CSC Introduction How to access CSC data with
The CSC Interface to Sky in Google Earth CSC Threads The CSC Interface to Sky in Google Earth 1 Table of Contents The CSC Interface to Sky in Google Earth - CSC Introduction How to access CSC data with
Structures in the Magnetosphere 2. Hover the curser over a point on the image. The coordinates and value at that point should appear.
 Investigating the Magnetosphere Introduction In this investigation, you will explore the properties of the regions of the magnetosphere using simulation results from the BATS-R-US model from the magnetosphere
Investigating the Magnetosphere Introduction In this investigation, you will explore the properties of the regions of the magnetosphere using simulation results from the BATS-R-US model from the magnetosphere
MASSACHUSETTS INSTITUTE OF TECHNOLOGY Physics Department. Experiment 03: Work and Energy
 MASSACHUSETTS INSTITUTE OF TECHNOLOGY Physics Department Physics 8.01 Fall Term 2010 Experiment 03: Work and Energy Purpose of the Experiment: In this experiment you allow a cart to roll down an inclined
MASSACHUSETTS INSTITUTE OF TECHNOLOGY Physics Department Physics 8.01 Fall Term 2010 Experiment 03: Work and Energy Purpose of the Experiment: In this experiment you allow a cart to roll down an inclined
PHYSICS LAB Experiment 7 Fall 2004 CONSERVATION OF MOMENTUM & COLLISIONS
 PHYSICS 83 - LAB Experiment 7 Fall 004 CONSERVATION OF MOMENTUM & COLLISIONS In this experiment we will study how the total vector momentum of an isolated system is conserved (remains constant) in collisions.
PHYSICS 83 - LAB Experiment 7 Fall 004 CONSERVATION OF MOMENTUM & COLLISIONS In this experiment we will study how the total vector momentum of an isolated system is conserved (remains constant) in collisions.
Lab 12 - Conservation of Momentum And Energy in Collisions
 Lab 12 - Conservation of Momentum And Energy in Collisions Name Partner s Name I. Introduction/Theory Momentum is conserved during collisions. The momentum of an object is the product of its mass and its
Lab 12 - Conservation of Momentum And Energy in Collisions Name Partner s Name I. Introduction/Theory Momentum is conserved during collisions. The momentum of an object is the product of its mass and its
1 D Collisions and Impulse
 SP211 Lab: Six 1-D Collisions and Impulse PHYSICS LAB 6 SP211 1 D Collisions and Impulse I. Introduction A. Linear momentum is an important physical quantity associated with motion. In fact, Newton actually
SP211 Lab: Six 1-D Collisions and Impulse PHYSICS LAB 6 SP211 1 D Collisions and Impulse I. Introduction A. Linear momentum is an important physical quantity associated with motion. In fact, Newton actually
Key Performance Task
 COURSE UNIT PERIOD PAGE SPH3U Energy Conservation of Mechanical Energy 1 of 2 Overall Expectation D2. investigate energy transformations and the law of conservation of energy, and solve related problems
COURSE UNIT PERIOD PAGE SPH3U Energy Conservation of Mechanical Energy 1 of 2 Overall Expectation D2. investigate energy transformations and the law of conservation of energy, and solve related problems
Math 261 Sampling Distributions Lab Spring 2009
 Math 261 Sampling Distributions Lab Spring 2009 Name: Purpose After completing this lab, you should be able to distinguish between the distribution of the population, distribution of the sample, and the
Math 261 Sampling Distributions Lab Spring 2009 Name: Purpose After completing this lab, you should be able to distinguish between the distribution of the population, distribution of the sample, and the
Learning to Use Scigress Wagner, Eugene P. (revised May 15, 2018)
 Learning to Use Scigress Wagner, Eugene P. (revised May 15, 2018) Abstract Students are introduced to basic features of Scigress by building molecules and performing calculations on them using semi-empirical
Learning to Use Scigress Wagner, Eugene P. (revised May 15, 2018) Abstract Students are introduced to basic features of Scigress by building molecules and performing calculations on them using semi-empirical
Exploring Potential Energy, Kinetic energy and Conservation of Energy: Part 1:
 WARM UP 3-4 mins: exploring energy with Phet Skate Park. Directions: 1) QUIETLY get a computer, and, with your partner,-search (Google) for: Phet Skate Park: Phet Skate Park 2) Click on the first link
WARM UP 3-4 mins: exploring energy with Phet Skate Park. Directions: 1) QUIETLY get a computer, and, with your partner,-search (Google) for: Phet Skate Park: Phet Skate Park 2) Click on the first link
Applet Exercise 1: Introduction to the Virtual Lab and Wave Fundamentals
 Applet Exercise 1: Introduction to the Virtual Lab and Wave Fundamentals Objectives: 1. Become familiar with the Virtual Lab display and controls.. Find out how sound loudness or intensity depends on distance
Applet Exercise 1: Introduction to the Virtual Lab and Wave Fundamentals Objectives: 1. Become familiar with the Virtual Lab display and controls.. Find out how sound loudness or intensity depends on distance
Forces and Newton s Second Law
 Forces and Newton s Second Law Goals and Introduction Newton s laws of motion describe several possible effects of forces acting upon objects. In particular, Newton s second law of motion says that when
Forces and Newton s Second Law Goals and Introduction Newton s laws of motion describe several possible effects of forces acting upon objects. In particular, Newton s second law of motion says that when
Introduction to the College of DuPage NEXLAB Website
 Introduction to the College of DuPage NEXLAB Website The purpose of this lab is to familiarize yourself with our website so that you will have an easier time following along in class and will be able to
Introduction to the College of DuPage NEXLAB Website The purpose of this lab is to familiarize yourself with our website so that you will have an easier time following along in class and will be able to
You may use g = 10 m/s 2, sin 60 = 0.87, and cos 60 = 0.50.
 1. A child pulls a 15kg sled containing a 5kg dog along a straight path on a horizontal surface. He exerts a force of a 55N on the sled at an angle of 20º above the horizontal. The coefficient of friction
1. A child pulls a 15kg sled containing a 5kg dog along a straight path on a horizontal surface. He exerts a force of a 55N on the sled at an angle of 20º above the horizontal. The coefficient of friction
Using Tables and Graphing Calculators in Math 11
 Using Tables and Graphing Calculators in Math 11 Graphing calculators are not required for Math 11, but they are likely to be helpful, primarily because they allow you to avoid the use of tables in some
Using Tables and Graphing Calculators in Math 11 Graphing calculators are not required for Math 11, but they are likely to be helpful, primarily because they allow you to avoid the use of tables in some
Name Student ID # Instructor Lab Period Date Due. Lab 1 Polynomials
 Name Student ID # Instructor Lab Period Date Due Lab 1 Polynomials Objectives 1. To become familiar with the definition of a polynomial. 2. To examine some of the properties of the graphs of polynomials.
Name Student ID # Instructor Lab Period Date Due Lab 1 Polynomials Objectives 1. To become familiar with the definition of a polynomial. 2. To examine some of the properties of the graphs of polynomials.
Investigating Food Dyes in Sports Beverages. Sample
 Investigating Food Dyes in Sports Beverages Investigation 1 There are many different brands of beverages that fall under the general category of sports drinks. Most of these beverages contain an FD&C food
Investigating Food Dyes in Sports Beverages Investigation 1 There are many different brands of beverages that fall under the general category of sports drinks. Most of these beverages contain an FD&C food
REPLACE DAMAGED OR MISSING TEXTBOOK BARCODE LABEL
 Destiny Textbook Manager allows users to create and print replacement barcode labels for textbooks. In this tutorial you will learn how to: Replace damaged textbook barcode label(s) Replace missing textbook
Destiny Textbook Manager allows users to create and print replacement barcode labels for textbooks. In this tutorial you will learn how to: Replace damaged textbook barcode label(s) Replace missing textbook
Name Student ID # Instructor Lab Period Date Due. Lab 5 Continuity
 Name Student ID # Instructor Lab Period Date Due Lab 5 Continuity Objectives 1. To visually represent the concept of continuity. 2. To develop an informal intuition for continuity. Continuity A fuction
Name Student ID # Instructor Lab Period Date Due Lab 5 Continuity Objectives 1. To visually represent the concept of continuity. 2. To develop an informal intuition for continuity. Continuity A fuction
Safety: BE SURE TO KEEP YOUR SMART CART UPSIDE-DOWN WHEN YOU RE NOT ACTIVELY USING IT TO RECORD DATA.
 Why do people always ignore Objective: 1. Determine how an object s mass affects the friction it experiences. 2. Compare the coefficient of static friction to the coefficient of kinetic friction for each
Why do people always ignore Objective: 1. Determine how an object s mass affects the friction it experiences. 2. Compare the coefficient of static friction to the coefficient of kinetic friction for each
Preparations and Starting the program
 Preparations and Starting the program https://oldwww.abo.fi/fakultet/ookforskning 1) Create a working directory on your computer for your Chemkin work, and 2) download kinetic mechanism files AAUmech.inp
Preparations and Starting the program https://oldwww.abo.fi/fakultet/ookforskning 1) Create a working directory on your computer for your Chemkin work, and 2) download kinetic mechanism files AAUmech.inp
5-Sep-15 PHYS101-2 GRAPHING
 GRAPHING Objectives 1- To plot and analyze a graph manually and using Microsoft Excel. 2- To find constants from a nonlinear relation. Exercise 1 - Using Excel to plot a graph Suppose you have measured
GRAPHING Objectives 1- To plot and analyze a graph manually and using Microsoft Excel. 2- To find constants from a nonlinear relation. Exercise 1 - Using Excel to plot a graph Suppose you have measured
Student Exploration: Free-Fall Laboratory
 Name: Date: Student Exploration: Free-Fall Laboratory Vocabulary: acceleration, air resistance, free fall, instantaneous velocity, terminal velocity, velocity, vacuum Prior Knowledge Questions (Do these
Name: Date: Student Exploration: Free-Fall Laboratory Vocabulary: acceleration, air resistance, free fall, instantaneous velocity, terminal velocity, velocity, vacuum Prior Knowledge Questions (Do these
Activity P20: Conservation of Mechanical Energy (Force Sensor, Photogate)
 Name Class Date Activity P20: Conservation of Mechanical Energy (Force Sensor, Photogate) Concept DataStudio ScienceWorkshop (Mac) ScienceWorkshop (Win) Energy P20 Mechanical Energy.DS P23 Cons. Mechanical
Name Class Date Activity P20: Conservation of Mechanical Energy (Force Sensor, Photogate) Concept DataStudio ScienceWorkshop (Mac) ScienceWorkshop (Win) Energy P20 Mechanical Energy.DS P23 Cons. Mechanical
PHY 221 Lab 3 Vectors and Motion in 1 and 2 Dimensions
 PHY 221 Lab 3 Vectors and Motion in 1 and 2 Dimensions Print Your Name Print Your Partners' Names Instructions Before lab, read the Introduction, and answer the Pre-Lab Questions on the last page of this
PHY 221 Lab 3 Vectors and Motion in 1 and 2 Dimensions Print Your Name Print Your Partners' Names Instructions Before lab, read the Introduction, and answer the Pre-Lab Questions on the last page of this
Empirical Gas Laws (Parts 1 and 2) Pressure-volume and pressure-temperature relationships in gases
 Empirical Gas Laws (Parts 1 and 2) Pressure-volume and pressure-temperature relationships in gases Some of the earliest experiments in chemistry and physics involved the study of gases. The invention of
Empirical Gas Laws (Parts 1 and 2) Pressure-volume and pressure-temperature relationships in gases Some of the earliest experiments in chemistry and physics involved the study of gases. The invention of
One Dimensional Collisions 1 Fall 2018
 One Dimensional Collisions 1 Fall 2018 Name: Partners: Introduction The purpose of this experiment is to perform experiments to learn about momentum, impulse and collisions in one dimension. Write all
One Dimensional Collisions 1 Fall 2018 Name: Partners: Introduction The purpose of this experiment is to perform experiments to learn about momentum, impulse and collisions in one dimension. Write all
The Mass of Jupiter Student Guide
 The Mass of Jupiter Student Guide Introduction: In this lab, you will use astronomical observations of Jupiter and its satellites to measure the mass of Jupiter. We will use the program Stellarium to simulate
The Mass of Jupiter Student Guide Introduction: In this lab, you will use astronomical observations of Jupiter and its satellites to measure the mass of Jupiter. We will use the program Stellarium to simulate
SOLID 1. Make sure your state of matter is set on solid. Write your observations below:
 Chemistry Ms. Ye Name Date Block Properties of Matter: Particle Movement Part 1: Follow the instructions below to complete the activity. Click on the link to open the simulation for this activity: http://phet.colorado.edu/sims/states-of-matter/states-of-matterbasics_en.jnlp***note:
Chemistry Ms. Ye Name Date Block Properties of Matter: Particle Movement Part 1: Follow the instructions below to complete the activity. Click on the link to open the simulation for this activity: http://phet.colorado.edu/sims/states-of-matter/states-of-matterbasics_en.jnlp***note:
BD1. Reciprocal space and Fourier transforms. IB Mineral Sciences Module B: Reciprocal Space, Symmetry and Crystallography
 Reciprocal space and Fourier transforms The aim of this practical is to give you a chance to develop your intuition about the relationship between real space and reciprocal space. There are two halves
Reciprocal space and Fourier transforms The aim of this practical is to give you a chance to develop your intuition about the relationship between real space and reciprocal space. There are two halves
3D Molecule Viewer of MOGADOC (JavaScript)
 3D Molecule Viewer of MOGADOC (JavaScript) Movement of the Molecule Rotation of the molecule: Use left mouse button to drag. Translation of the molecule: Use right mouse button to drag. Resize the molecule:
3D Molecule Viewer of MOGADOC (JavaScript) Movement of the Molecule Rotation of the molecule: Use left mouse button to drag. Translation of the molecule: Use right mouse button to drag. Resize the molecule:
Part 1: Play Purpose: Play! See how everything works before we try to find any relationships.
 PhET Gas Law Simulation Name: http://phet.colorado.edu/new/simulations/sims.php?sim=gas_properties If the direct link does not work, use Google, and use the search terms Phet Gas properties. You can download
PhET Gas Law Simulation Name: http://phet.colorado.edu/new/simulations/sims.php?sim=gas_properties If the direct link does not work, use Google, and use the search terms Phet Gas properties. You can download
Applications of Newton's Laws
 Applications of Newton's Laws Purpose: To apply Newton's Laws by applying forces to objects and observing their motion; directly measuring these forces that are applied. Apparatus: Pasco track, Pasco cart,
Applications of Newton's Laws Purpose: To apply Newton's Laws by applying forces to objects and observing their motion; directly measuring these forces that are applied. Apparatus: Pasco track, Pasco cart,
Connect the Vernier spectrometer to your lap top computer and power the spectrometer if necessary. Start LoggerPro on your computer.
 Connect the Vernier spectrometer to your lap top computer and power the spectrometer if necessary. Start LoggerPro on your computer. The screen shown in Fig. 1 may be displayed. If status line displays
Connect the Vernier spectrometer to your lap top computer and power the spectrometer if necessary. Start LoggerPro on your computer. The screen shown in Fig. 1 may be displayed. If status line displays
Dynamics Track Momentum, Energy, and Collisions
 Dynamics Track Momentum, Energy, and Collisions Student Handout Collisions between objects create some interesting questions about which conservation laws apply. In this lab you will be comparing elastic
Dynamics Track Momentum, Energy, and Collisions Student Handout Collisions between objects create some interesting questions about which conservation laws apply. In this lab you will be comparing elastic
Lab 3 Momentum Change and Impulse
 Lab 3 Momentum Change and Impulse Objectives: < To measure the change in momentum of a cart in a collision and the impulse acting on it during the collision and to compare these values as a test of the
Lab 3 Momentum Change and Impulse Objectives: < To measure the change in momentum of a cart in a collision and the impulse acting on it during the collision and to compare these values as a test of the
EXPERIMENT 7: ANGULAR KINEMATICS AND TORQUE (V_3)
 TA name Lab section Date TA Initials (on completion) Name UW Student ID # Lab Partner(s) EXPERIMENT 7: ANGULAR KINEMATICS AND TORQUE (V_3) 121 Textbook Reference: Knight, Chapter 13.1-3, 6. SYNOPSIS In
TA name Lab section Date TA Initials (on completion) Name UW Student ID # Lab Partner(s) EXPERIMENT 7: ANGULAR KINEMATICS AND TORQUE (V_3) 121 Textbook Reference: Knight, Chapter 13.1-3, 6. SYNOPSIS In
PHY221 Lab 2 - Experiencing Acceleration: Motion with constant acceleration; Logger Pro fits to displacement-time graphs
 Page 1 PHY221 Lab 2 - Experiencing Acceleration: Motion with constant acceleration; Logger Pro fits to displacement-time graphs Print Your Name Print Your Partners' Names You will return this handout to
Page 1 PHY221 Lab 2 - Experiencing Acceleration: Motion with constant acceleration; Logger Pro fits to displacement-time graphs Print Your Name Print Your Partners' Names You will return this handout to
How Do I Create a Hubble Diagram to show the expanding universe?
 How Do I Create a Hubble Diagram to show the expanding universe? An extremely important topic in astronomy is the expansion of the universe. Although the expanding universe is nearly always discussed in
How Do I Create a Hubble Diagram to show the expanding universe? An extremely important topic in astronomy is the expansion of the universe. Although the expanding universe is nearly always discussed in
Activity P08: Newton's Second Law - Constant Force (Force Sensor, Motion Sensor)
 Activity P08: Newton's Second Law - Constant Force (Force Sensor, Motion Sensor) Concept DataStudio ScienceWorkshop (Mac) ScienceWorkshop (Win) Newton s Laws P08 Constant Force.DS P11 Constant Force P11_CONF.SWS
Activity P08: Newton's Second Law - Constant Force (Force Sensor, Motion Sensor) Concept DataStudio ScienceWorkshop (Mac) ScienceWorkshop (Win) Newton s Laws P08 Constant Force.DS P11 Constant Force P11_CONF.SWS
The Bohr Atom. PHYS 1301 F98 Prof. T.E. Coan Last edit: 6 Aug 98. Introduction
 1 The Bohr Atom PHYS 1301 F98 Prof. T.E. Coan Last edit: 6 Aug 98 Introduction In this week's computer simulation, we will examine the behavior of a simplified model of the hydrogen atom. This model, the
1 The Bohr Atom PHYS 1301 F98 Prof. T.E. Coan Last edit: 6 Aug 98 Introduction In this week's computer simulation, we will examine the behavior of a simplified model of the hydrogen atom. This model, the
Stoichiometric Reactor Simulation Robert P. Hesketh and Concetta LaMarca Chemical Engineering, Rowan University (Revised 4/8/09)
 Stoichiometric Reactor Simulation Robert P. Hesketh and Concetta LaMarca Chemical Engineering, Rowan University (Revised 4/8/09) In this session you will learn how to create a stoichiometric reactor model
Stoichiometric Reactor Simulation Robert P. Hesketh and Concetta LaMarca Chemical Engineering, Rowan University (Revised 4/8/09) In this session you will learn how to create a stoichiometric reactor model
Module 2A Turning Multivariable Models into Interactive Animated Simulations
 Module 2A Turning Multivariable Models into Interactive Animated Simulations Using tools available in Excel, we will turn a multivariable model into an interactive animated simulation. Projectile motion,
Module 2A Turning Multivariable Models into Interactive Animated Simulations Using tools available in Excel, we will turn a multivariable model into an interactive animated simulation. Projectile motion,
Lab 4: Gauss Gun Conservation of Energy
 Lab 4: Gauss Gun Conservation of Energy Before coming to Lab Read the lab handout Complete the pre-lab assignment and hand in at the beginning of your lab section. The pre-lab is written into this weeks
Lab 4: Gauss Gun Conservation of Energy Before coming to Lab Read the lab handout Complete the pre-lab assignment and hand in at the beginning of your lab section. The pre-lab is written into this weeks
The Kinetic-Molecular Theory of Gases
 The Kinetic-Molecular Theory of Gases kinetic-molecular theory of gases Originated with Ludwig Boltzman and James Clerk Maxwell in the 19th century Explains gas behavior on the basis of the motion of individual
The Kinetic-Molecular Theory of Gases kinetic-molecular theory of gases Originated with Ludwig Boltzman and James Clerk Maxwell in the 19th century Explains gas behavior on the basis of the motion of individual
Physics Labs with Computers, Vol. 1 P14: Simple Harmonic Motion - Mass on a Spring A
 Activity P14: Simple Harmonic Motion - Mass on a Spring (Force Sensor, Motion Sensor) Concept DataStudio ScienceWorkshop (Mac) ScienceWorkshop (Win) Harmonic motion P14 SHM.DS P19 SHM Mass on a Spring
Activity P14: Simple Harmonic Motion - Mass on a Spring (Force Sensor, Motion Sensor) Concept DataStudio ScienceWorkshop (Mac) ScienceWorkshop (Win) Harmonic motion P14 SHM.DS P19 SHM Mass on a Spring
Graphing Motion (Part 1 Distance)
 Unit Graphing Motion (Part 1 Distance) Directions: Work in your group (2 or 3 people) on the following activity. Choose ONE group member to be the subject (the person who walks to/from motion detector).
Unit Graphing Motion (Part 1 Distance) Directions: Work in your group (2 or 3 people) on the following activity. Choose ONE group member to be the subject (the person who walks to/from motion detector).
