Macroion solutions in the cell model studied by field theory and Monte Carlo simulations
|
|
|
- Richard Baker
- 6 years ago
- Views:
Transcription
1 Macroion solutions in the cell model studied by field theory and Monte Carlo simulations Leo Lue 1, a) and Per Linse 1) Department of Chemical and Process Engineering, University of Strathclyde James Weir Building, 75 Montrose Street, Glasgow G1 1XJ, United Kingdom ) Physical Chemistry, Department of Chemistry Lund University, P.O. Box 14, S-1 00 Lund, Sweden (Dated: 9 October 014) Aqueous solutions of charged spherical macroions with variable dielectric permittivity and their associated counterions are examined within the cell model using a field theory and Monte Carlo simulations. The field theory is based on separation of fields into short- and long-wavelength terms, which are subjected to different statistical-mechanical approximations. The simulations were performed by using a new, accurate, and fast algorithm for numerical evaluation of the electrostatic polarization interaction. The field theory provides counterion distributions outside a macroion in good agreement with the simulation results over the full range from weak to strong electrostatic coupling. A low-dielectric macroion leads to a displacement of the counterions away from the macroion. a) Electronic mail: leo.lue@strath.ac.uk 1
2 I. INTRODUCTION Solutions of charged colloidal particles occur in nearly all biological systems and in many industrially important formulations. The nature of the electrical double layer composed of colloidal surface charges and its neutralizing mobile counterions has a large influence on structural and thermodynamic properties of these systems 1. The Poisson-Boltzmann (PB) theory has been successful in describing many properties of the electric double layer and is responsible for shaping much of our current understanding of the behavior of solutions containing charged colloids. This theory works best when the electrostatic interactions in the system are relatively weak, i.e., low colloidal surface charge density, monovalent counterions, high dielectric constant, and/or high temperature. The breakdown of the PB theory at strong electrostatic interactions is primarily due to its neglect of correlations between the counterions, which becomes increasingly important at such conditions. Many theories have been developed to account for correlation effects, such as modified PB approaches 4, liquid state theories such as the hypernetted-chain and mean-spherical approximations 5 7, loop expansions 8,9, variational approximations 10, to field theoretic formulations, and densityfunctional theories 4,11,1. These different approaches improve upon the PB theory to varying degrees; however, they all tend to breakdown when the electrostatic interactions become even stronger. When the electrostatic interactions are extremely strong, a strong-coupling expansion method has been developed 13,14 that accurately describes the electrostatic double layer. Even though the PB theory is known to fail for highly electrostatically coupled systems, it has been found that it can still be used to quantitatively fit properties of these systems provided a renormalized charge is used (see Ref. 15 and references given therein). Such a use is sometimes physically justified by the strong attraction of counterions to the surface, which often are considered to be bound to the surface. Several theoretical approaches have been developed to relate the renormalized charge to the underlying stociometric charge of the colloids. The renormalized jellium model 16 19, is such a relatively recent theoretical approach. In the investigations referred to above, the dielectric constant of the colloids is the same as the surrounding solvent. However, in most aqueous colloidal solutions, the colloids have a dielectric constant lower than that of the surrounding solvent. This gives rise to an additional repelling force acting on the counterions arising from the surface charges induced at the dielectric discontinuity. Often this repulsion is viewed as arising from image charges (or image charge densities) located
3 on the other side of the dielectric discontinuity as viewed from the counterions 0. Simulation of systems with dielectric discontinuities are computationally intensive, due to the many-body nature of the charge polarization at these discontinuities. Much of the early simulation work for systems with dielectric heterogeneities was limited to planar geometries where the imagecharge description becomes simple. However, in spherical geometry the image-charge casting leads to a varying linear image charge density, and the calculations become computationally much more involved. Consequently, there have been relatively few such studies 1,. In a very recent work, dos Santos et al. 3 proposed a much faster algorithm that is accurate for low-dielectric spheres in a high-dielectric medium. Also recently, extenstions to other more complex geometries, such as pairs of interacting dielectric spheres 4 and parallel cylinders 5 have been made. Different theoretical approaches have been developed to analyze systems with dielectric heterogeneities. First, due to the neglect of ion-ion correlations, in spherical geometry there are no additional image-charge forces in the PB theory. For the planar geometry, early approaches 6,7 added the image-charge interaction directly to the PB theory as an additional external potential acting on the ions and this approach has been extended to weakly curved interfaces 8. Later, these imagecharge interaction were included more rigorously in integral equation approaches, such as the hypernetted chain approximation 9 3. Field-theoretic methods based on the loop expansion and variational approximation 36 have also been applied to planar geometry. A variational fieldtheoretical approach has been used to study image-charge effects in spherical 10 and cylindrical 37 geometries and a loop expansion approach has been used to examine two interacting dielectric spheres 38. The approaches mentioned so far are only accurate for weak electrostatic coupling. A strong-coupling theory have been developed for strong electrostatic coupling. It has been expanded to include systems with dielectric heterogeneities, and this has been applied to systems in planar geometries 39, for systems of parallel cylinders 5,40, and spheres 41. In this work, we examine the properties of salt-free solutions of spherical macroions, including the effects of a low dielectric interior, within the context of the cell model. Recently, a simple approximate field theory has been developed 4,43 that works well from the weak- through to the strong-coupling regimes, even for systems with dielectric heterogeneities. It relies on dividing the electrostatic interactions in the system into short and long-wavelength contributions and treating each within separate approximation schemes. While this theory can be used in arbitrary geometries, it has so far only been applied to planar systems, where it works fairly well. Here, we asses the predictions of this field-theory approach 4,43 for the spherical cell model by comparing to es- 3
4 sentially exact Monte Caro simulation data. The latter were obtained by using a new and more quickly converging expansion for the electrostatic energy of a system with a spherical dielectric interface. Our algorithm shares many features with the one by dos Santos et al. 3, but our derivation is mathematically simpler and is formally exact for all values of the dielectric constant. The cell model is described in the next section, and in Section III details of the systems that we study are given. Then in Section IV, we give a brief overview of the splitting theory and its application to the spherical cell model. Details of the simulation methods are provided in Section V. Section VI provides our numerical findings. The main conclusions of our work are summarized in Section VII. II. BACKGROUND A. Electrostatic interaction energy Consider a system composed of a fixed charge density distribution and mobile point charges. The total charge density Q at r is given by Q(r) = Σ(r) + k q k δ d (r r k ), (1) where Σ(r) denotes the fixed charge density distribution and where q k is the charge and r k the position of a mobile point charge k, with δ d being the d-dimensional Dirac delta function, here d = 3. If the system is embedded in a spatially varying dielectric medium described by ε(r), then the electrostatic interaction energy U of the system is given by U = 1 where G 0 is the Green s function of Poisson s equation drdr Q(r)G 0 (r, r )Q(r ) 1 q kg free (r k, r k ), () k 1 4π ε(r) G 0(r, r ) = δ(r r ), (3) 4
5 and G free is the Green s function for a system in a medium with a uniform dielectric constant ε, which is equal to G free (r, r ) = 1 ε r r. (4) The Green s function of the system G 0 can be considered as a sum of (i) a contribution G free from a uniform system and (ii) a contribution G 0, which is the change due to heterogeneities of the dielectric medium according to G 0 (r, r ) = G free (r, r ) + G 0 (r, r ) (5) The electrostatic interaction energy U of the system can be decomopsed according to U = U ΣΣ + U qσ + U qq + U pol (6) where U ΣΣ is the interaction energy of the fixed charge distribution, U Σq the Coulomb interaction between the fixed charge distribution and mobile point charges, U qq the direct Coulomb interaction between the mobile point charges, and U pol the polarization contribution to the interaction between the mobile point charges. These different terms are related to the various Green s functions as U ΣΣ = 1 dr dr Σ(r)G 0 (r, r )Σ(r ) (7) U qσ = u qσ (r k ) = q k drg 0 (r k, r)σ(r) (8) k k U qq = u qq (r j, r k ) = q j q k G free (r j, r k ) j<k j<k (9) U pol = 1 u pol (r j, r k ) = 1 q j q k G 0 (r j, r k ) (10) j,k which also defines the quantities u qσ (r k ), u qq (r j, r k ), and u pol (r j, r k ). j,k B. The primitive model and the spherical cell model The primitive model of asymmetric electrolytes constitutes a firm basis for a statisticalmechanical description of solutions of charged macromolecules. Within this model, ionic species 5
6 are represented by charged hard spheres differing in charge and size, whereas the solvent enters the model only through its relative dielectric constant. In this study, solutions contain only charged spherical macroions and their counterions, forming an electrically neutral system, are considered; no simple salt is added. This implies that we can make the further simplification that counterions are point-like, i.e., their hard-sphere radii are zero. In the spherical cell model, the solution of the asymmetric electrolyte is divided into subvolumes, each containing one macroion and a corresponding amount of counterions and solvent. This model is most frequently used to examine the distribution of ions near a macroion and to obtain approximate thermodynamic results. The symmetry of the cell should follow closely the symmetry of the macroion. The obvious choice is a concentric spherical cell placed around the macroion. The influence of the remaining solution is taken into account in a mean-field manner. The cell model is most useful when the macroions repel each other and becomes less appropriate when they possess attractive interactions. Usually, the dielectric constant of the macroion is taken to be equal to that of the solvent. However, here we will consider the case where the interior of the macroion possesses a dielectric constant lower than that of the solvent, which is a more realistic description for colloids in aqueous solutions. Given the primitive and spherical cell models, the interaction energy U of the system can be expressed as U = k u qσ (r k ) + j<k u qq (r j, r k ) + k u ext (r k ) + 1 u pol (r j, r k ). (11) j,k The Coulomb and excluded-volume interaction between the macroion and counterion k, u qσ (r k ), is given by u qσ (r k ) =, for r k < R M Qq k (1), for r k R M εr k with Q denoting the macroion charge, q k the ionic charge, R M the macroion radius, ε the relative dielectric constant of the solvent, and r k the radial position of the counterion. The Coulomb interaction between counterions j and k, u qq (r j, r k ), is given by u qq (r j, r k ) = 6 q jq k ε r j r k. (13)
7 The confining external potential energy u ext acting on counterion k becomes 0, for r k R cell u ext (r k ) = (14), for r k > R cell where R cell denotes the radius of the spherical cell. In the case that the macroion has the same dielectric constant as the surrounding solution (homogeneous dielectric solution), the polarization energy u pol is zero. However, if the macroion has a dielectric constant ε ε (heterogeneous dielectric solution), a polarization surface charge density will appear at the dielectric discontinuity and u pol becomes nonzero. For simplicity, let the dielectric discontinuity be spherical with radius R d. Then, the potential energy involving the polarization surface charge density and the counterions can be cast as an ionic self-term u pol (r k, r k ) and an ion-ion term u pol (r j, r k ) with u pol (r j, r k ) = q jq k ε l l=1 m= l [ 4π l + 1 ε ε ε(1 + 1/l) + ε ] ( R l+1 d r l+1 j r l+1 k ) Y lm (θ j, φ j )Y lm(θ k, φ k ) (15) applicable to both j = k and j k, where r = (r, θ, φ), Y lm denotes the spherical harmonics, and the complex conjugate. In the following, the dielectric discontinuity will be placed at the hard-sphere surface of the macroion, i.e., R d = R M. This maximizes the effect of the polarization surface charge density. The interaction energy associated with the surface polarization can be re-expressed in several ways with different convergence properties and computational efficiency. III. SYSTEMS In this work, we consider four aqueous solutions containing macroions and their counterions, which were studied in Ref. 44. As our primitive model systems are composed only of macroions and point counterions, the system is fully specified by three reduced parameters. Here, we choose (i) the charge ratio Q/q, (ii) the electrostatic coupling parameter Ξ defined by Ξ = Q l B R M (16) 7
8 which is one parameter (of many choices) that characterizes the strength of the electrostatic coupling in the system, and (iii) the macroion volume fraction φ M given by φ M = 4πR3 M 3 ρ M (17) where ρ M is the macroion number density. In Eq. (16), l B = (q/e 0 ) λ B is a modified Bjerrum length, where λ B is the Bjerrum length λ B = e 0 εk B T (18) with e 0 the fundamental unit of charge, k B being the Boltzmann constant, and T the absolute temperature. Physically, the modified Bjerrum length is the distance between two counterions at which their electrostatic interaction energy becomes equal to the thermal energy k B T. Table I provides the values of Q/q, Ξ, and φ M of the four systems. The electrostatic coupling parameter Ξ ranges from weak (Ξ 1) to strong (Ξ 1) coupling in steps of approximately one order of magnitude from System IV to Systems I and II to System III. The macroion volume fraction φ M = 0.01 is used throughout, which implies a cell to macroion radius ratio R cell /R M = Table I also provides typical values of Q, q, and R M for colloids in aqueous solution at ambient temperature consistent with the specifications of Systems I IV. Thus, Systems I, II, and III could represent solutions of charged surfactant micelles with (i) radius R M = 0 Å, where the micelle is weakly charged in System I and highly charged in Systems II and III and (ii) monovalent counterions in Systems I and II and divalent ones in System III. Finally, System IV could represent a solution of silica particles with radius R M = 160 Å with monovalent counterions. Note, the colloid charge in System IV is the same as in Systems II and III. IV. THE SPLITTING THEORY A. General This section provides a brief outline of an approximate field theory, which we refer to as the splitting theory, applied to a system composed of a fixed charge distribution Σ and mobile point charges of magnitude q. For further details, see Ref. 43. The basic idea behind the theory is to convert the partition function of the system to a functional integral over two interaction fields: 8
9 TABLE I. Specification of the four systems considered in terms of charge ratio Q/q, electrostatic coupling parameter Ξ, and macroion volume fraction φ M as well as corresponding macroion charge Q, counterion charge q, and macroion radius R M for typical aqueous colloidal solutions at ambient temperature. a System Q/q Ξ φ M Q q R M b σ/l B e 0 e 0 Å I II III IV a T = 300 K and ε = b σ is a parameter of the splitting theory, determined by Eq. (5). The reported values are for the homogeneous systems. one varying over small wavelengths and the other varying over large wavelengths. The functional integration for each of these fields is evaluated using different approaches, viz. a virial expansion for the short-wavelength field and a mean-field approximation for the long-wavelength field. Thus, the variations in the electric potential due to the varying counterion configurations are at shortwavelengths taken into account but on long-wavelengths a mean-field description prevails. The separation between short and long wavelength is achieved by dividing the Green s function G 0 of Poisson s equation according to G 0 = G s + G l (19) where G l = PG 0 and G s = (1 P)G 0 with P being an operator which splits the long-wavelength variations of the interaction field from the short-wavelength variations. The choice of the operator is somewhat arbitrary, and, in this work, we select P = [1 σ + σ 4 4 ] 1. The interaction energy U can be rewritten in terms of these two Green s functions as U = 1 drdr Q(r)G l (r, r )Q(r ) + q G s (r j, r k ) j,k + ] [u(r k ) q G s(r k, r k ) + 1 drdr Σ(r)G s (r, r )Σ(r ) k (0) 9
10 where u is a one-body interaction energy, which is felt by each counterion due to the presence of the fixed charge and dielectric heterogeneities in the system, and is given by u(r) = q dr G s (r, r )Σ(r ) + q G 0(r, r) q PG free(r, r) + u ext (r). (1) The first term is the short-wavelength interaction of the point charge with the fixed charges, while the second term is the interaction of the point charges with the image charges generated by the dielectric heterogeneities. The third term is the energy of the long-wavelength correlation hole. By performing the Hubbard-Stratonovich transformation 45,46 on both the long-wavelength and short-wavelength electrostatic interactions, the grand partition function of the system can be written as a functional integral over a field ψ s which fluctuates on short lengthscales and a field ψ l which fluctuates on long lengthscales. The integration over ψ s is approximated using a first order cumulant expansion, and the integration over ψ l is evaluated using the mean-field approximation. With these approximations, the Helmholtz free energy F becomes 4,43 βf [ρ, Σ] [ ] dr ρ(r) ln ρ(r)λ d 1 + dr ρ(r)βu(r) 1 drdr i β ψ l (r)g 1 l (r, r )i ψ l (r ) + dr[qρ(r) + Σ(r)]i ψ l (r ) + β drdr Σ(r)G s (r, r )Σ(r ) () where ρ(r) is the number density of the mobile ions, Λ is their thermal wavelength, and ψ l (r) is the mean value of the long-wavelength field. The first two terms represent the free energy of the counterions interacting with the one-body energy. The third term is the free energy of the long-wavelength electrostatic field, while the fourth term is the coupling energy between the counterions and the long-wavelength electrostatic field. The final term is the short-range electrostatic interaction energy of the fixed charge distribution in the system. The mean value of the long-wavelength field ψ l is given by δf δi ψ l (r) = 0 (3) which leads to 1 ε(r) φ(r) = Σ(r) + qρ(r) (4) 4π 10
11 where i ψ l = Pβφ. In an exact description, the properties of the system are independent of the splitting parameter σ. However, as the functional integrals entering Eq. () are approximate ones, the values of predicted properties of the system do depend on σ. In order to minimize this dependence, the value of the splitting parameter σ is determined through F σ = 0 (5) Consequently, to first order in σ, the predicted properties are independent of the splitting parameter. We can now express any equilibrium property of the system from the free energy functional given in Eq. (). For example, the dimensionless chemical potential γ of the counterions is given by γ(r) = δ βf [ρ, Σ] δρ(r) = ln ρ(r)λ d + βu(r) + qi ψ l (r), (6) and the internal energy of the system by U = βf [ρ, Σ] β = dr ρ(r)u(r) + 1 dr[qρ(r) + Σ(r)]i ψ l (r) + 1 drdr Σ(r)G s (r, r )Σ(r ). (7) B. Application to the spherical cell model In this section, we apply the splitting theory described in the Section IV A to the spherical cell model described in Section II B. Thus, the system consists of a central spherical macroion of radius R M with a dielectric constant ε and its counterions, which are immersed in a medium of dielectric constant ε. For a fixed and uniform charge distribution on a spherical shell of radius R M, the shortwavelength contribution to the self energy of the macroion is 1 drdr Σ(r)G s (r, r )Σ(r ) = Q 3εσ 11 ( σ R M ) [ ( )] 1 e 3R/σ RM cos. (8) σ
12 The interior of the spherical macroion has a dielectric constant of ε. The resulting shift of the Green s function, owing to ε ε, becomes G 0 (r, r) = 1 εr M l=1 ( ) ( ) l+ 1 η RM, (9) 1 + 1/l + η r where η ε /ε. Note, in the equation following Eq. (46) in Ref. 10 the factor should be omitted. The one-body interaction energy of the counterions is given by u(r) = qq σ 3εσ rr M + q εr l=1 [ ( e 3 r R M r RM σ cos σ ( 1 η RM 1 + 1/l + η r where the external potential energy u ext is given in Eq. (14). ) ( )] e 3 r+r M r + RM σ cos σ ) l+1 q 3εσ + u ext(r). For the spherical cell model, the free energy given in Eq. () reduces to (30) βf [ρ, Σ] Rcell R M [ ] Rcell 4πr dr ρ(r) ln ρ(r)λ d 1 + Rcell ε 4πr dr i 8π ψ l(r)φ (r) + R M + β drdr Σ(r)G s (r, r )Σ(r ). R M Rcell 4πr dr ρ(r)βu(r) R M 4πr dr qρ(r)i ψ l (r) + Qi ψ l (R M ) (31) Minimizing the free energy with respect to the long-wavelength field ψ l leads to Poisson s equation in spherical coordinates [cf. Eq. (4)] ε[r φ (r)] = 4πr qρ(r) (3) with the boundary conditions φ (R M ) = Q εr M and φ (R cell ) = 0. The long-wavelength field is obtained from the electric potential through the relation ψ l = Pβφ. The counterion density ρ(r) is related to i ψ l through [see Eq. (6)] ρ(r) = Λ d e γ βu(r) qi ψ l (r) (33) 1
13 The value of the chemical potential γ is determined by ensuring that the total number of counterions in the cell neutralizes the macroion charge. Within the cell model, the pressure of the system can be obtained from the free energy by the relation βp = (βf ) V = 1 (βf ). (34) 4πRcell R cell Using the expression for the free energy given in Eq. (31), we find that the pressure predicted by the splitting theory is βp = ρ(r cell ). (35) Therefore, we see that the splitting theory, like the PB theory, obeys the contact value theorem, which is exact 47. V. SIMULATION DETAILS A. General The Monte Carlo (MC) simulations of the four systems as defined in Section III were performed in the canonical ensemble, i.e., at constant number of particles, volume, and temperature. Each simulation involved 10 7 MC trial moves per counterion. The value of trial displacements were selected differently for the four systems. Counterion distribution functions were determined by using a histogram width of 0.01R M (464 bins). The uncertainties are smaller than the size of the symbols used to present the simulation data in Section VI. However, in the logarithmic plots the first points appear significantly shifted upwards owing to the very rapid density variation at r R M. As that does not affect our conclusions, corrections of those shifts have not been made. Statistical uncertainties were calculated using block averaging by subdividing each simulation into twenty equally sized blocks. The integrated MC/molecular dynamic/brownian dynamics simulation package MOLSIM for molecular systems was employed
14 B. Evaluation of the polarization energy We have employed a novel, efficient, and accurate scheme to evaluate the polarization energy U pol = 1 with u pol (r j, r k ) given by Eq. (15). u pol (r j, r k ) (36) j,k First, the polarization energy U pol can be expressed in several, mathematically equivalent, but numerically different ways. Employing the simplifying notation η ε ε and we obtain: t jk R d r j R d r k. (A) After introducing the multipole moment Q lm defined by Q lm k q k r l+1 k Y lm (θ k, φ k ), (37) we have U pol = 1 ε l l=1 m= l [ 4π l η 1 + 1/l + η ] R l+1 d Q lm Q lm. (38) (B) After denoting the angle between the vectors connecting the positions r j and r k by γ jk and applying the addition theorem for spherical harmonics, we get u pol (r j, r k ) = q jq k εr d [ l=1 where P l is the l th Legendre polynomial η 1 + 1/l + η ] t l+1 jk P l(cos γ jk ) (39)
15 (C) After introducing the exact expansion 1 η 1 + 1/l + η = 1 η 1 + η 1 η 1 (1 + η) l + 1 η(1 η) 1 (1 + η) (1 + 1/l + η)(1 + 1/l) ( ) (40) 1 l and using the generating function of the Legendre polynomial, Eq. (39) can be re-written in a more quickly convergent sum according to u pol (r j, r k ) = u (I) pol (r j, r k ) + u (II) pol (r j, r k ) + u (III) pol (r j, r k ) (41) with u (I) pol (r j, r k ) = q jq k 1 η εr d 1 + η t jk(1 t jk cos γ jk + t jk) 1/ (4) 1 η u (II) pol (r j, r k ) = q jq k εr d u (III) pol (r j, r k ) = q jq k εr d (1 + η) ln 1 cos γ jk [(1 t jk cos γ jk + t jk )1/ + t jk cos γ jk ] { 1 η [ (1 + η) η 1 1 t jk l 1 + 1/l + η For self-terms, j = k, we have cos γ kk = 1, and Eqs. (4) (44) result to u (I) pol (r k, r k ) = q jq k 1 η εr d 1 + η u (II) pol (r k, r k ) = q jq k 1 η εr d u (III) pol (r k, r k ) = q jq k εr d t kk l=1 ] t l+1 jk 1 + 1/l P l(cos γ jk ) } (43) (44) 1 t kk (45) (1 + η) ln(1 t kk) (46) { 1 η [ ] } (1 + η) η 1 1 t l+1 kk t kk (47) l 1 + 1/l + η 1 + 1/l l=1 Second, the number of mathematical operations involved in scheme (A) scales as O(N(l max) ), (B) as O(N l max), and (C) as O(N l max ) with N being the number of counterions. This shows that, for systems with few ions, scheme (B) is preferred over (A); the opposite holds for a large number of ions. However, the re-expression of the sum over l in scheme (C) makes l max l max for the same accuracy, where l max denotes the the largest l used in Eqs. (38) or (39) and l max the largest l used in Eq. (44). From the data given in Table II, we found convergence already for l max = 0, and hence the scaling of the polarization interaction also becomes O(N ), as for the 15
16 Coulombic ion-ion interaction [cf. Eqs. (11) and (13)]. The polarization energy of the heterogeneous dielectric solution was evaluated using scheme (C), i.e., Eqs. (36) and (41) (44). Table II shows that the error of truncating Eq. (44) at l max = 0 is comparable with the statistical uncertainty of the simulations. Our experience is that including the polarization energy using this scheme lengthens the MC simulations roughly by a factor of two. The expressions given in scheme (C) are formally exact. In special case where η = 0, the infinite series in Eq. (44) does not need to be evaluated, and the polarization energy reduces to the closed form expression previously obtained in Ref. 3, which is exact. The approximate expression for the polarization energy given in Eqs. (5) (9) in Ref. 3 is related to that in scheme (C), with the exception of: (i) an extra factor of 1 + η in Eq. (43), and (ii) the neglect of the term given by Eq. (44). TABLE II. Reduced electrostatic interaction energy U/[(N + 1)k B T ] a of the four systems examined by the splitting theory and Monte Carlo simulations using different l max. Method Dielec. b c l max System I II III IV Theory Homo Hetero MC Homo (8) (7) 30.60(1) () Hetero (6) 1.856(6) 7.010(1) 0.799() Hetero (5) 1.854(5) 7.010(1) () Hetero (6) 1.858(6) 7.009(1) () a Statistical uncertainty (one standard deviation) in parenthesis. b Homo: ε = and Hetero: ε = 1. c Upper limit of the sum in Eq. (44). VI. RESULTS Properties of the four colloidal systems obtained by applying the splitting theory and by Monte Carlo simulations will be presented together with some complementary results from the PB equation. Results of homogeneous dielectric systems will be given first and followed by results of heterogeneous dielectric systems. 16
17 System I System II System III System IV ρ(r) / ρ avg r / R M - 1 FIG. 1. Normalized counterion number density ρ(r)/ρ avg as a function of the scaled radial distance r/r M from the center of the macroion for System I (red), System II (black), System III (green), and System IV (blue) as obtained from the Poisson-Boltzmann theory (data from Ref. 44) (dotted curves), the splitting theory (dashed curves), and Monte Carlo simulations (solid curves). The results of Systems I and IV of the PB equation are identical and all data for these two systems essentially collapse on a single curve. A. Homogeneous dielectric solution We will here examine the case where the dielectric constant of the macroions ε is the same as that of the surrounding solvent ε, i.e., ε = ε. Figure 1 shows the normalized counterion number density as a function of the radial position for the four systems described in Section III and investigated using the PB theory 44 (dotted curves), the splitting theory described in Section IV (dashed curves), and Monte Carlo simulations described in Section V (solid curves). The corresponding values of the splitting parameter σ are given in Table I for these homogeneous dielectric systems. Table II provides the electrostatic interaction energy of the four systems. Generally, there is an accumulation of the counterions near the macroion with the maximal density appearing at contact r = R M (Fig. 1), arising from the attractive electrostatic macroioncounterion interaction. In Systems I and IV, the counterion distributions deviate only moderately 17
18 from a uniform distribution, which originates from the fact that the electrostatic coupling parameter Ξ is comparably small. The distribution of the counterions becomes much more nonuniform in Systems II and III, where Ξ = 5 and 40, respectively. The predictions of the PB theory agree well with the MC simulation data for Systems I and IV, possessing low values of Ξ. At larger values of Ξ, the PB approximation underestimates the accumulation of the small counterions near the macroion, leading to a density that is too large further away. Noticeably, within the spherical cell model, the osmotic pressure is given by βp = ρ(r cell ), so the PB approximation overestimates the osmotic pressure at large Ξ. The length σ, entering in the splitting operator P of the splitting theory, can be interpreted as the size of a correlation hole around each counterion. The optimal σ as a function of the electrostatic coupling parameter Ξ is given in Fig. for three different colloidal sizes R M. In the strong-coupling limit (Ξ 1), the dependence of the free energy of the system on the splitting parameter is dominated by the one-body energy of the counterions [second term in Eq. ()] and the short-range electrostatic interaction energy of the macroion charge [last term in Eq. ()]. The balance between these two terms leads 4,43 to the scaling relation σ Ξ 1/ with a prefactor basically independent of R M /l B (Fig. ). The splitting parameter diverges at Ξ = q lb /(R M ), which is the value of the coupling parameter which corresponds to a macroion with Q = q. In the limit σ, G l = 0 and G s = G 0, and thus the splitting theory reduces to an exact description of a macroion with a single counterion ion, where the density profile becomes ρ(r) e βqq/(εr). In contrast, the PB equation does not reduce properly to this limit, as it does not account for the discreteness of the counterions. For this particular geometry, the PB theory does not become exact in the weak coupling limit, due to the finite and limited number of counterions in the system. In the weak coupling limit, the PB approximation becomes a good approximation only when the number of counterions remains substantial, i.e., Q/q 1. Figure 3 displays the counterion number density profile at different macroion charge Q, keeping the macroion radius R M, macroion volume fraction φ, and the counterion charge q fixed. This is equivalent to increasing the coupling parameter Ξ at constant R cell /R M and R M /l B. In panel (a), Q = 0, 40, and 100 at R M /l B = , and in panel (b), Q = 80, 10 3, 10 4, and 10 5 at R M /l B = As the charge of the macroion is increased, the counterions become increasingly attracted to the macroion surface. In the strong-coupling regime (Ξ 1), we see that there are two distinct regions in the counterion distribution: a tightly bound inner layer, and 18
19 σ / l B R M / l B = R M / l B = R M / l B = Ξ FIG.. Reduced splitting parameter σ/l B as a function of the electrostatic coupling parameter Ξ for η = 1 (solid curves) and η = 1/ (dashed curves) at R cell /R M = 4.64 with R M /l B = (black curves), R M /l B = (red curves), and R M /l B =.4909 (green curves). The solid and dashed curves are mostly superimposed. a diffuse outer layer. The width of the inner layer is of the order of σ. At the same value of the coupling constant, the counterions are more closely associated to macroions of larger size (cf. Figs. 3b and 3a). This is due to the smaller repulsions between the counterions bound to the larger macroion because they are, on average, further apart. B. Heterogeneous dielectric solution Now, we assess the ability of the splitting theory to describe counterion distributions when the macroion possesses a dielectric constant ε = 1, while keeping the dielectric constant of the solvent at ε = The radial distribution of the counterions around the macroion for each of the four systems examined are given in Fig. 4, as predicted by the splitting theory (lines) and by Monte Carlo simulation (symbols), presented using a linear (left column) and logarithmic (right column) abscissa. The corresponding data for ε = are also included. Generally, while the maxima of the counterion distributions appear at r = R M for a homoge- 19
20 ρ(r) / ρ avg (a) (b) Ξ = 10.1 Ξ = Ξ = r / R M - 1 Ξ = Ξ = Ξ = Ξ = r / R M - 1 FIG. 3. Normalized counterion number density ρ(r)/ρ avg as a function of the scaled radial distance r/r M 1 from the center of the macroion for the radii ratio R cell /R M = 4.64 and macroion radius (a) R M /l B = and (b) R M /l B =.4909 for homogeneous (ε = ε) dielectric solutions. In (a), ( Q/q, Ξ) = (10, 10.1) (red curve), (40, ) corresponding to System III (green curve), and (100, 101.) (blue curve); and in (b), ( Q/q, Ξ) = (80, ) corresponding to System IV (black curve), (10 3, ) (red curve), (10 4, 9.885) (green curve), and (10 5, 98.85) (blue curve). The locations where r R M = 3σ are also indicated (vertical dotted lines). neous dielectric permeability, for ε < ε (i) the counterion density is zero at r = R M and (ii) the maxima appears r > R M. Observation (i) originates from the fact that the additional repulsive potential U pol operating between the counterions and the induced polarization charges diverges at r = R d = R M [see Eqs. (41) (44)], and finding (ii) results from the combination of U pol with the attractive Coulomb macroion counterion interaction. These principal features agree with those found and discussed in previous publications on the same topic 1 3. In more detail, the predictions of the splitting theory agree to a very high degree with the simulation data for Systems I and IV. Only at distances shorter than 0.01R M, detectable differences are found (see right column of Fig. 4). In System II, noticeable differences are seen up to r 1.5R M 0
21 ρ(r) / ρ avg ρ(r) / ρ avg (a) (c) (e) (g) r / R M 10-3 (b) (d) (f) (h) r/r M - 1 FIG. 4. Normalized counterion number density ρ(r)/ρ avg as a function of the scaled radial distance r/r M from the center of the macroion for System I (a,b), II (c,d), III (e,f), and IV (g,h) with ε = ε (black) and ε = 1 (red) as obtained from the splitting theory (curves) and Monte Carlo simulations (symbols). The abscissa is on a linear scale in the left column and on a logarithmic scale in the right column. Results of the splitting theory and Monte Carlo simulations are nearly indistinguishable in the left column. (Figs. 4c and d). In System III, which has the strongest electrostatic coupling, the prediction of the splitting theory is only qualitatively correct (Fig. 4e and f). It accurately predicts the location of the peak of the counterion distribution; however, it underestimates the counterion densities at short distances, and overestimates the density at large distances. Similar systems have been examined recently in Ref. 49, where they develop a modified Poisson-Boltzmann equation to account for the presence of the dielectric interface. This works 1
22 well in the far-field region but not near the interface. When this modified PB equation is coupled together with a theory for the strongly-correlated region near the interface, it accurately describes the counterion density profiles simulated in the work. Interestingly, the splitting theory reduces to their modified PB equation in the limit that σ 0. This suggests that using a spatially varying splitting parameter could improve the theory developed in this work, particularly the predictions far from the macroion. Figure 5 displays the radial counterion density profile with macroion charges as for the systems given in Fig. 3. We see that the location of the density maxima (on a relative scale) moves closer to the macroion surface (i) as the macroion charge increases and (ii) as the macroion size increases. The former is due to the increased counterion macroion Coulomb attraction, and the latter to the decreased polarization repulsion. In the strong-coupling regime, there are also here two distinct layers of counterions. Finally, the variation of the value of the splitting parameter σ with the strength of the electrostatic coupling Ξ was shown in Fig.. The values of σ for the systems with a dielectric heterogeneity becomes slightly larger, but still close to those for homogeneous dielectric system. The differences in σ increase (i) as the Bjerrum length becomes larger than the macroion radius and (ii) as the image charge interactions becomes stronger. VII. CONCLUSIONS We have studied models of charged spherical colloids in salt-free solution, including cases where the dielectric constant of the macroion interior is lower than that of the surrounding solution. A new method for evaluating the polarization energy arising from the dielectric heterogeneity has been developed and employed in Monte Carlo simulations of these systems. This method has been found to converge extremely rapidly and offers great computational advantages over the previous methods (with the exception of the just published work by dos Santos et al. 3, which shares features with the method presented here), when the number of counterions in the system is sufficiently low. We have also assessed the accuracy of a recently developed field theory. This splitting theory predicts counterion density profiles that compare well those from Monte Carlo simulations. That holds even in the presence of a dielectric interface; however, the theory overestimates the pressure and potential energy of the system. At high electrostatic couplings, the theory predicts counterion density profiles that qualitatively divide into a two distinct regions: one strongly bound inner layer
23 Ξ = 10.1 Ξ = Ξ = 101. (a) (b) ρ(r) / ρ avg r / R M - 1 Ξ = Ξ = Ξ = Ξ = r / R M - 1 FIG. 5. As in Fig. 3 but for a heterogeneous (ε = 1 ε) dielectric solution. and one diffuse outer layer. The thickness of the bound layer scales with the splitting parameter σ. ACKNOWLEDGMENTS Financial support by the Swedish Research Council (VR) through the Linnaeus grant Organizing Molecular Matter (OMM) center of excellence ( ) and through individual grants to PL ( ) are gratefully acknowledged. REFERENCES 1 D. F. Evans and H. Wennerström, The Colloidal Domain: Where Physics, Chemistry, Biology, and Technology Meet, nd ed. (Wiley-VCH, New York, 1999). C. W. Outhwaite and L. B. Bhuiyan, J. Chem. Soc., Faraday Trans. 79, 707 (1983). 3
24 3 C. Outhwaite and L. Bhuiyan, Mol. Phys. 74, 367 (1991). 4 L. B. Bhuiyan and C. W. Outhwaite, Condens. Matter Phys. 8, 87 (005). 5 M. Fushiki, Chem. Phys. Lett. 154, 77 (1989). 6 E. Gonzalez-Tovar and M. Lozada-Cassou, J. Phys. Chem. 93, 3761 (1989). 7 G. I. Guerrero-García, E. González-Tovar, M. Lozada-Cassou, and F. de J. Guevara-Rodríguez, J. Chem. Phys. 13, (005). 8 R. D. Coalson and A. Duncan, J. Chem. Phys. 97, 5653 (199). 9 R. R. Netz and H. Orland, Eur. Phys. J. E 1, 03 (000). 10 R. A. Curtis and L. Lue, J. Chem. Phys. 13, (005). 11 Y.-X. Yu, J. Wu, and G.-H. Gao, J. Chem. Phys. 10, 73 (004). 1 T. Goel and C. N. Patra, J. Chem. Phys. 17, (007). 13 B. I. Shklovskii, Phys. Rev. E 60, 580 (1999). 14 A. G. Moreira and R. R. Netz, Phys. Rev. Lett. 87, (001). 15 J. Dobnikar, R. Castañeda-Priego, H. H. von Grünberg, and E. Trizac, New J. Phys. 8, 77 (006). 16 E. Trizac and Y. Levin, Phys. Rev. E 69, (004). 17 S. Pianegonda, E. Trizac, and Y. Levin, J. Chem. Phys. 16, (007). 18 T. E. Colla, Y. Levin, and E. Trizac, J. Chem. Phys. 131, (009). 19 T. E. Colla and Y. Levin, J. Chem. Phys. 133 (010), / M. M. Hatlo and L. Lue, Soft Matter 4, 158 (008). 1 P. Linse, J. Phys. Chem. 90, 681 (1986). R. Messina, J. Chem. Phys. 117, 1106 (00). 3 A. P. dos Santos, A. Bakhshandeh, and Y. Levin, J. Chem. Phys. 135, (011). 4 J. Reščič and P. Linse, J. Chem. Phys. 19, (008). 5 M. Kanduc, A. Naji, and R. Podgornik, J. Chem. Phys. 13, 4703 (010). 6 C. Wagner, Physik. Z. 5, 474 (194). 7 L. Onsager and N. N. T. Samaras, J. Chem. Phys., 58 (1934). 8 J. Groenewold, J. Chem. Phys. 107, 9668 (1997). 9 R. Kjellander and S. Marcelja, J. Chem. Phys. 8, 1 (1985). 30 R. Kjellander and S. Marcelja, Chem. Phys. Lett. 14, 485 (1987). 31 P. Attard, D. J. Mitchell, and B. W. Ninham, J. Chem. Phys. 88, 4987 (1988). 3 P. Attard, D. J. Mitchell, and B. W. Ninham, J. Chem. Phys. 89, 4358 (1988). 4
25 33 R. Netz, Eur. Phys. J. E 5, 189 (001). 34 R. Netz, Eur. Phys. J. E 5, 557 (001). 35 D. S. Dean and R. R. Horgan, Phys. Rev. E 69, (004). 36 M. M. Hatlo, R. A. Curtis, and L. Lue, J. Chem. Phys. 18, (008). 37 S. Buyukdagli, M. Manghi, and J. Palmeri, J. Chem. Phys. 134, (011). 38 N. Ben-Tal and R. D. Coalson, J. Chem. Phys. 101, 5148 (1994). 39 Y. S. Jho, M. Kanduc, A. Naji, R. Podgornik, M. W. Kim, and P. A. Pincus, Phys. Rev. Lett. 101 (008), /PhysRevLett A. Arnold and C. Holm, Eur. Phys. J. E 7, 1 (008). 41 A. Naji and R. R. Netz, Europhys. J. E 13, 43 (004), /epje/e x. 4 M. M. Hatlo and L. Lue, Soft Matter 5, 15 (009). 43 M. M. Hatlo and L. Lue, EPL (Europhysics Letters) 89, 500 (010). 44 P. Linse, in Advanced Computer Simulation Approaches for Soft Matter Sciences II, Advances in Polymer Science, Vol. 185, edited by C. Holm and K. Kremer (Springer Berlin / Heidelberg, 005) pp R. L. Stratonovich, Dokl. Akad. Nauk SSSR 115, 1097 (1957). 46 J. Hubbard, Phys. Rev. Lett. 3, 77 (1959). 47 H. Wennerström, B. Jönsson, and P. Linse, J. Chem. Phys. 76, 4665 (198). 48 P. Linse, MOLSIM, Version 5.0, Lund University, Sweden (011). 49 A. Bakhshandeh, A. P. dos Santos, and Y. Levin, Phys. Rev. Lett. 107, (011). 5
A FIELD THEORETIC APPROACH TO THE ELECTRIC INTERFACIAL LAYER. MIXTURE OF TRIVALENT ROD-LIKE AND MONOVALENT POINT-LIKE IONS BETWEEN CHARGED WALLS.
 Modern Physics Letters B c World Scientific Publishing Company A FIELD THEORETIC APPROACH TO THE ELECTRIC INTERFACIAL LAYER. MIXTURE OF TRIVALENT ROD-LIKE AND MONOVALENT POINT-LIKE IONS BETWEEN CHARGED
Modern Physics Letters B c World Scientific Publishing Company A FIELD THEORETIC APPROACH TO THE ELECTRIC INTERFACIAL LAYER. MIXTURE OF TRIVALENT ROD-LIKE AND MONOVALENT POINT-LIKE IONS BETWEEN CHARGED
Electrostatics of membrane adhesion
 Electrostatics of membrane adhesion S. Marcelja Department of Applied Mathematics, Research School of Physical Sciences and Engineering, The Australian National University, Canberra ACT 6, Australia ABSTRACT
Electrostatics of membrane adhesion S. Marcelja Department of Applied Mathematics, Research School of Physical Sciences and Engineering, The Australian National University, Canberra ACT 6, Australia ABSTRACT
Generalizations for the Potential of Mean Force between Two Isolated Colloidal Particles from Monte Carlo Simulations
 Journal of Colloid and Interface Science 252, 326 330 (2002) doi:10.1006/jcis.2002.8497 Generalizations for the Potential of Mean Force between Two Isolated Colloidal Particles from Monte Carlo Simulations
Journal of Colloid and Interface Science 252, 326 330 (2002) doi:10.1006/jcis.2002.8497 Generalizations for the Potential of Mean Force between Two Isolated Colloidal Particles from Monte Carlo Simulations
Density-functional theory for the structures and thermodynamic properties of highly asymmetric electrolyte and neutral component mixtures
 PHYSICAL REVIEW E 70, 031109 (2004) Density-functional theory for the structures and thermodynamic properties of highly asymmetric electrolyte and neutral component mixtures Zhidong Li and Jianzhong Wu*
PHYSICAL REVIEW E 70, 031109 (2004) Density-functional theory for the structures and thermodynamic properties of highly asymmetric electrolyte and neutral component mixtures Zhidong Li and Jianzhong Wu*
Gas-liquid phase separation in oppositely charged colloids: stability and interfacial tension
 7 Gas-liquid phase separation in oppositely charged colloids: stability and interfacial tension We study the phase behaviour and the interfacial tension of the screened Coulomb (Yukawa) restricted primitive
7 Gas-liquid phase separation in oppositely charged colloids: stability and interfacial tension We study the phase behaviour and the interfacial tension of the screened Coulomb (Yukawa) restricted primitive
Binary Hard-Sphere Mixtures Within Spherical Pores
 Journal of the Korean Physical Society, Vol. 35, No. 4, October 1999, pp. 350 354 Binary Hard-Sphere Mixtures Within Spherical Pores Soon-Chul Kim Department of Physics, Andong National University, Andong
Journal of the Korean Physical Society, Vol. 35, No. 4, October 1999, pp. 350 354 Binary Hard-Sphere Mixtures Within Spherical Pores Soon-Chul Kim Department of Physics, Andong National University, Andong
arxiv: v1 [physics.bio-ph] 27 Nov 2013
![arxiv: v1 [physics.bio-ph] 27 Nov 2013 arxiv: v1 [physics.bio-ph] 27 Nov 2013](/thumbs/77/75209850.jpg) 1 arxiv:1311.7138v1 [physics.bio-ph] 27 Nov 2013 On the ion-mediated interaction between protein and DNA M. Barbi CNRS LPTMC UMR 7600, Université Pierre et Marie Curie-Paris 6 4 place Jussieu, 75252 Paris
1 arxiv:1311.7138v1 [physics.bio-ph] 27 Nov 2013 On the ion-mediated interaction between protein and DNA M. Barbi CNRS LPTMC UMR 7600, Université Pierre et Marie Curie-Paris 6 4 place Jussieu, 75252 Paris
Test-charge theory for the electric double layer
 PHYSICAL REVIEW E 70, 0602 (2004) Test-charge theory for the electric double layer Yoram Burak* and David Andelman School of Physics and Astronomy, Raymond and Beverly Sackler Faculty of Exact Sciences,
PHYSICAL REVIEW E 70, 0602 (2004) Test-charge theory for the electric double layer Yoram Burak* and David Andelman School of Physics and Astronomy, Raymond and Beverly Sackler Faculty of Exact Sciences,
arxiv:cond-mat/ v3 [cond-mat.soft] 12 Sep 2001
![arxiv:cond-mat/ v3 [cond-mat.soft] 12 Sep 2001 arxiv:cond-mat/ v3 [cond-mat.soft] 12 Sep 2001](/thumbs/81/83614310.jpg) EUROPHYSICS LETTERS 15 August 2000 Europhys. Lett., 51 (4), pp. 461 468 (2000) arxiv:cond-mat/0006501v3 [cond-mat.soft] 12 Sep 2001 Ground state of two unlike charged colloids: An analogy with ionic bonding
EUROPHYSICS LETTERS 15 August 2000 Europhys. Lett., 51 (4), pp. 461 468 (2000) arxiv:cond-mat/0006501v3 [cond-mat.soft] 12 Sep 2001 Ground state of two unlike charged colloids: An analogy with ionic bonding
Electrostatic Double Layer Force: Part III
 NPTEL Chemical Engineering Interfacial Engineering Module 3: Lecture 4 Electrostatic Double Layer Force: Part III Dr. Pallab Ghosh Associate Professor Department of Chemical Engineering IIT Guwahati, Guwahati
NPTEL Chemical Engineering Interfacial Engineering Module 3: Lecture 4 Electrostatic Double Layer Force: Part III Dr. Pallab Ghosh Associate Professor Department of Chemical Engineering IIT Guwahati, Guwahati
Interactions between Charged Surfaces Mediated by Stiff, Multivalent Zwitterionic Polymers
 Page 1 of 1 Soft Matter PAPER www.rsc.org/softmatter Soft Matter Interactions between Charged Surfaces Mediated by Stiff, Multivalent Zwitterionic Polymers Klemen Bohinc a, Jurij Reščič b and Leo Lue c
Page 1 of 1 Soft Matter PAPER www.rsc.org/softmatter Soft Matter Interactions between Charged Surfaces Mediated by Stiff, Multivalent Zwitterionic Polymers Klemen Bohinc a, Jurij Reščič b and Leo Lue c
Interactions between charged surfaces mediated by molecules with spatially distributed charges*
 Pure Appl. Chem., Vol. 85, No. 1, pp. 15 26, 2013. http://dx.doi.org/10.1351/pac-con-12-04-08 2012 IUPAC, Publication date (Web): 13 December 2012 Interactions between charged surfaces mediated by molecules
Pure Appl. Chem., Vol. 85, No. 1, pp. 15 26, 2013. http://dx.doi.org/10.1351/pac-con-12-04-08 2012 IUPAC, Publication date (Web): 13 December 2012 Interactions between charged surfaces mediated by molecules
arxiv: v1 [cond-mat.stat-mech] 1 Oct 2009
![arxiv: v1 [cond-mat.stat-mech] 1 Oct 2009 arxiv: v1 [cond-mat.stat-mech] 1 Oct 2009](/thumbs/74/70932354.jpg) Shell Structure of Confined Charges at Strong Coupling J. Wrighton, J. Dufty, M. Bonitz, 2 and H. Kählert 2 Department of Physics, University of Florida, Gainesville, FL 326 arxiv:9.76v [cond-mat.stat-mech]
Shell Structure of Confined Charges at Strong Coupling J. Wrighton, J. Dufty, M. Bonitz, 2 and H. Kählert 2 Department of Physics, University of Florida, Gainesville, FL 326 arxiv:9.76v [cond-mat.stat-mech]
Electrostatic correlations and fluctuations for ion binding to a finite length polyelectrolyte
 THE JOURNAL OF CHEMICAL PHYSICS 122, 044903 2005 Electrostatic correlations and fluctuations for ion binding to a finite length polyelectrolyte Zhi-Jie Tan and Shi-Jie Chen a) Department of Physics and
THE JOURNAL OF CHEMICAL PHYSICS 122, 044903 2005 Electrostatic correlations and fluctuations for ion binding to a finite length polyelectrolyte Zhi-Jie Tan and Shi-Jie Chen a) Department of Physics and
Effect of Polyelectrolyte Adsorption on Intercolloidal Forces
 5042 J. Phys. Chem. B 1999, 103, 5042-5057 Effect of Polyelectrolyte Adsorption on Intercolloidal Forces Itamar Borukhov, David Andelman,*, and Henri Orland School of Physics and Astronomy, Raymond and
5042 J. Phys. Chem. B 1999, 103, 5042-5057 Effect of Polyelectrolyte Adsorption on Intercolloidal Forces Itamar Borukhov, David Andelman,*, and Henri Orland School of Physics and Astronomy, Raymond and
Local molecular field theory for effective attractions between like charged objects in systems with strong Coulomb interactions
 Local molecular field theory for effective attractions between like charged objects in systems with strong Coulomb interactions Yng-Gwei Chen and John D. Weeks Departments of Physics and Chemistry and
Local molecular field theory for effective attractions between like charged objects in systems with strong Coulomb interactions Yng-Gwei Chen and John D. Weeks Departments of Physics and Chemistry and
arxiv: v1 [cond-mat.soft] 21 Sep 2016
![arxiv: v1 [cond-mat.soft] 21 Sep 2016 arxiv: v1 [cond-mat.soft] 21 Sep 2016](/thumbs/91/106037610.jpg) Mean-field beyond mean-field: the single particle view for moderately to strongly coupled charged fluids Ladislav Šamaj Institute of Physics, Slovak Academy of Sciences, Bratislava, Slovakia Alexandre
Mean-field beyond mean-field: the single particle view for moderately to strongly coupled charged fluids Ladislav Šamaj Institute of Physics, Slovak Academy of Sciences, Bratislava, Slovakia Alexandre
Polymer Solution Thermodynamics:
 Polymer Solution Thermodynamics: 3. Dilute Solutions with Volume Interactions Brownian particle Polymer coil Self-Avoiding Walk Models While the Gaussian coil model is useful for describing polymer solutions
Polymer Solution Thermodynamics: 3. Dilute Solutions with Volume Interactions Brownian particle Polymer coil Self-Avoiding Walk Models While the Gaussian coil model is useful for describing polymer solutions
Where do ions solvate?
 PRAMANA c Indian Academy of Sciences Vol. 64, No. 6 journal of June 25 physics pp. 957 961 YAN LEVIN Instituto de Física, Universidade Federal do Rio Grande do Sul, Caixa Postal 1551, CEP 9151-97, Porto
PRAMANA c Indian Academy of Sciences Vol. 64, No. 6 journal of June 25 physics pp. 957 961 YAN LEVIN Instituto de Física, Universidade Federal do Rio Grande do Sul, Caixa Postal 1551, CEP 9151-97, Porto
Chapter 4. Electrostatic Fields in Matter
 Chapter 4. Electrostatic Fields in Matter 4.1. Polarization 4.2. The Field of a Polarized Object 4.3. The Electric Displacement 4.4. Linear Dielectrics 4.5. Energy in dielectric systems 4.6. Forces on
Chapter 4. Electrostatic Fields in Matter 4.1. Polarization 4.2. The Field of a Polarized Object 4.3. The Electric Displacement 4.4. Linear Dielectrics 4.5. Energy in dielectric systems 4.6. Forces on
Theoretische Physik 2: Elektrodynamik (Prof. A-S. Smith) Home assignment 11
 WiSe 22..23 Prof. Dr. A-S. Smith Dipl.-Phys. Matthias Saba am Lehrstuhl für Theoretische Physik I Department für Physik Friedrich-Alexander-Universität Erlangen-Nürnberg Problem. Theoretische Physik 2:
WiSe 22..23 Prof. Dr. A-S. Smith Dipl.-Phys. Matthias Saba am Lehrstuhl für Theoretische Physik I Department für Physik Friedrich-Alexander-Universität Erlangen-Nürnberg Problem. Theoretische Physik 2:
1. (3) Write Gauss Law in differential form. Explain the physical meaning.
 Electrodynamics I Midterm Exam - Part A - Closed Book KSU 204/0/23 Name Instructions: Use SI units. Where appropriate, define all variables or symbols you use, in words. Try to tell about the physics involved,
Electrodynamics I Midterm Exam - Part A - Closed Book KSU 204/0/23 Name Instructions: Use SI units. Where appropriate, define all variables or symbols you use, in words. Try to tell about the physics involved,
Incorporation of excluded volume correlations into Poisson-Boltzmann theory. Abstract
 PREPRINT Incorporation of excluded volume correlations into Poisson-Boltzmann theory Dmytro Antypov, 1, Marcia C. Barbosa, 2, 3, 1, and Christian Holm 1 Max-Planck-Institut für Polymerforschung, Ackermannweg
PREPRINT Incorporation of excluded volume correlations into Poisson-Boltzmann theory Dmytro Antypov, 1, Marcia C. Barbosa, 2, 3, 1, and Christian Holm 1 Max-Planck-Institut für Polymerforschung, Ackermannweg
Multimedia : Boundary Lubrication Podcast, Briscoe, et al. Nature , ( )
 3.05 Nanomechanics of Materials and Biomaterials Thursday 04/05/07 Prof. C. Ortiz, MITDMSE I LECTURE 14: TE ELECTRICAL DOUBLE LAYER (EDL) Outline : REVIEW LECTURE #11 : INTRODUCTION TO TE ELECTRICAL DOUBLE
3.05 Nanomechanics of Materials and Biomaterials Thursday 04/05/07 Prof. C. Ortiz, MITDMSE I LECTURE 14: TE ELECTRICAL DOUBLE LAYER (EDL) Outline : REVIEW LECTURE #11 : INTRODUCTION TO TE ELECTRICAL DOUBLE
lim = F F = F x x + F y y + F z
 Physics 361 Summary of Results from Lecture Physics 361 Derivatives of Scalar and Vector Fields The gradient of a scalar field f( r) is given by g = f. coordinates f g = ê x x + ê f y y + ê f z z Expressed
Physics 361 Summary of Results from Lecture Physics 361 Derivatives of Scalar and Vector Fields The gradient of a scalar field f( r) is given by g = f. coordinates f g = ê x x + ê f y y + ê f z z Expressed
Hydrogels in charged solvents
 Hydrogels in charged solvents Peter Košovan, Christian Holm, Tobias Richter 27. May 2013 Institute for Computational Physics Pfaffenwaldring 27 D-70569 Stuttgart Germany Tobias Richter (ICP, Stuttgart)
Hydrogels in charged solvents Peter Košovan, Christian Holm, Tobias Richter 27. May 2013 Institute for Computational Physics Pfaffenwaldring 27 D-70569 Stuttgart Germany Tobias Richter (ICP, Stuttgart)
Monopole polarization of C 60 fullerene shell
 Monopole polarization of C 6 fullerene shell M. Ya. Amusia 1, and A. S. Baltenkov 3 1 Racah Institute of Physics, the Hebrew University, Jerusalem, 9194 Israel Ioffe Physical-Technical Institute, St. Petersburg,
Monopole polarization of C 6 fullerene shell M. Ya. Amusia 1, and A. S. Baltenkov 3 1 Racah Institute of Physics, the Hebrew University, Jerusalem, 9194 Israel Ioffe Physical-Technical Institute, St. Petersburg,
Density Functional Theory for Planar Electric Double Layers: Closing the Gap between Simple and Polyelectrolytes
 J. Phys. Chem. B 2006, 110, 7473-7484 7473 Density Functional Theory for Planar Electric Double Layers: Closing the Gap between Simple and Polyelectrolytes Zhidong Li and Jianzhong Wu* Department of Chemical
J. Phys. Chem. B 2006, 110, 7473-7484 7473 Density Functional Theory for Planar Electric Double Layers: Closing the Gap between Simple and Polyelectrolytes Zhidong Li and Jianzhong Wu* Department of Chemical
Proteins in solution: charge-tuning, cluster formation, liquid-liquid phase separation, and crystallization
 HERCULES Specialized Course: Non-atomic resolution scattering in biology and soft matter Grenoble, September 14-19, 2014 Proteins in solution: charge-tuning, cluster formation, liquid-liquid phase separation,
HERCULES Specialized Course: Non-atomic resolution scattering in biology and soft matter Grenoble, September 14-19, 2014 Proteins in solution: charge-tuning, cluster formation, liquid-liquid phase separation,
Physics 127b: Statistical Mechanics. Lecture 2: Dense Gas and the Liquid State. Mayer Cluster Expansion
 Physics 27b: Statistical Mechanics Lecture 2: Dense Gas and the Liquid State Mayer Cluster Expansion This is a method to calculate the higher order terms in the virial expansion. It introduces some general
Physics 27b: Statistical Mechanics Lecture 2: Dense Gas and the Liquid State Mayer Cluster Expansion This is a method to calculate the higher order terms in the virial expansion. It introduces some general
Charge fluctuations and counterion condensation
 PHYSICAL REVIEW E, VOLUME 65, 0550 Charge fluctuations and counterion condensation A. W. C. Lau, D. B. Lukatsky, P. Pincus, 3 and S. A. Safran Department of Physics and Astronomy, University of Pennsylvania,
PHYSICAL REVIEW E, VOLUME 65, 0550 Charge fluctuations and counterion condensation A. W. C. Lau, D. B. Lukatsky, P. Pincus, 3 and S. A. Safran Department of Physics and Astronomy, University of Pennsylvania,
2 Structure. 2.1 Coulomb interactions
 2 Structure 2.1 Coulomb interactions While the information needed for reproduction of living systems is chiefly maintained in the sequence of macromolecules, any practical use of this information must
2 Structure 2.1 Coulomb interactions While the information needed for reproduction of living systems is chiefly maintained in the sequence of macromolecules, any practical use of this information must
Incorporation of excluded-volume correlations into Poisson-Boltzmann theory
 Incorporation of excluded-volume correlations into Poisson-Boltzmann theory Dmytro Antypov, 1, * Marcia C. Barbosa, 2, and Christian Holm 3,1, 1 Max-Planck-Institut für Polymerforschung, Ackermannweg 10,
Incorporation of excluded-volume correlations into Poisson-Boltzmann theory Dmytro Antypov, 1, * Marcia C. Barbosa, 2, and Christian Holm 3,1, 1 Max-Planck-Institut für Polymerforschung, Ackermannweg 10,
Conductors and Insulators
 Conductors and Insulators Lecture 11: Electromagnetic Theory Professor D. K. Ghosh, Physics Department, I.I.T., Bombay Self Energy of a Charge Distribution : In Lecture 1 we briefly discussed what we called
Conductors and Insulators Lecture 11: Electromagnetic Theory Professor D. K. Ghosh, Physics Department, I.I.T., Bombay Self Energy of a Charge Distribution : In Lecture 1 we briefly discussed what we called
Electrolyte Solutions
 Chapter 8 Electrolyte Solutions In the last few chapters of this book, we will deal with several specific types of chemical systems. The first one is solutions and equilibria involving electrolytes, which
Chapter 8 Electrolyte Solutions In the last few chapters of this book, we will deal with several specific types of chemical systems. The first one is solutions and equilibria involving electrolytes, which
Critical point of electrolyte mixtures
 Critical point of electrolyte mixtures THE JOURNAL OF CHEMICAL PHYSICS 123, 084903 2005 Antti-Pekka Hynninen and Marjolein Dijkstra Soft Condensed Matter Group, Debye Institute, Utrecht University, Princetonplein
Critical point of electrolyte mixtures THE JOURNAL OF CHEMICAL PHYSICS 123, 084903 2005 Antti-Pekka Hynninen and Marjolein Dijkstra Soft Condensed Matter Group, Debye Institute, Utrecht University, Princetonplein
3 Chapter. Gauss s Law
 3 Chapter Gauss s Law 3.1 Electric Flux... 3-2 3.2 Gauss s Law (see also Gauss s Law Simulation in Section 3.10)... 3-4 Example 3.1: Infinitely Long Rod of Uniform Charge Density... 3-9 Example 3.2: Infinite
3 Chapter Gauss s Law 3.1 Electric Flux... 3-2 3.2 Gauss s Law (see also Gauss s Law Simulation in Section 3.10)... 3-4 Example 3.1: Infinitely Long Rod of Uniform Charge Density... 3-9 Example 3.2: Infinite
1. Thomas-Fermi method
 1. Thomas-Fermi method We consider a system of N electrons in a stationary state, that would obey the stationary Schrödinger equation: h i m + 1 v(r i,r j ) Ψ(r 1,...,r N ) = E i Ψ(r 1,...,r N ). (1.1)
1. Thomas-Fermi method We consider a system of N electrons in a stationary state, that would obey the stationary Schrödinger equation: h i m + 1 v(r i,r j ) Ψ(r 1,...,r N ) = E i Ψ(r 1,...,r N ). (1.1)
1. Poisson-Boltzmann 1.1. Poisson equation. We consider the Laplacian. which is given in spherical coordinates by (2)
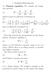 1. Poisson-Boltzmann 1.1. Poisson equation. We consider the Laplacian operator (1) 2 = 2 x + 2 2 y + 2 2 z 2 which is given in spherical coordinates by (2) 2 = 1 ( r 2 ) + 1 r 2 r r r 2 sin θ θ and in
1. Poisson-Boltzmann 1.1. Poisson equation. We consider the Laplacian operator (1) 2 = 2 x + 2 2 y + 2 2 z 2 which is given in spherical coordinates by (2) 2 = 1 ( r 2 ) + 1 r 2 r r r 2 sin θ θ and in
Molecular Modeling -- Lecture 15 Surfaces and electrostatics
 Molecular Modeling -- Lecture 15 Surfaces and electrostatics Molecular surfaces The Hydrophobic Effect Electrostatics Poisson-Boltzmann Equation Electrostatic maps Electrostatic surfaces in MOE 15.1 The
Molecular Modeling -- Lecture 15 Surfaces and electrostatics Molecular surfaces The Hydrophobic Effect Electrostatics Poisson-Boltzmann Equation Electrostatic maps Electrostatic surfaces in MOE 15.1 The
we1 = j+dq + = &/ET, (2)
 EUROPHYSICS LETTERS Europhys. Lett., 24 (S), pp. 693-698 (1993) 10 December 1993 On Debye-Huckel s Theory. I. M. MLADENOV Central Laboratory of Biophysics, Bulgarian Academy of Sciences Acad. G. Bonchev
EUROPHYSICS LETTERS Europhys. Lett., 24 (S), pp. 693-698 (1993) 10 December 1993 On Debye-Huckel s Theory. I. M. MLADENOV Central Laboratory of Biophysics, Bulgarian Academy of Sciences Acad. G. Bonchev
Chapter 21 Chapter 23 Gauss Law. Copyright 2014 John Wiley & Sons, Inc. All rights reserved.
 Chapter 21 Chapter 23 Gauss Law Copyright 23-1 What is Physics? Gauss law relates the electric fields at points on a (closed) Gaussian surface to the net charge enclosed by that surface. Gauss law considers
Chapter 21 Chapter 23 Gauss Law Copyright 23-1 What is Physics? Gauss law relates the electric fields at points on a (closed) Gaussian surface to the net charge enclosed by that surface. Gauss law considers
On the Chemical Free Energy of the Electrical Double Layer
 1114 Langmuir 23, 19, 1114-112 On the Chemical Free Energy of the Electrical Double Layer Marian Manciu and Eli Ruckenstein* Department of Chemical Engineering, State University of New York at Buffalo,
1114 Langmuir 23, 19, 1114-112 On the Chemical Free Energy of the Electrical Double Layer Marian Manciu and Eli Ruckenstein* Department of Chemical Engineering, State University of New York at Buffalo,
Gases and the Virial Expansion
 Gases and the irial Expansion February 7, 3 First task is to examine what ensemble theory tells us about simple systems via the thermodynamic connection Calculate thermodynamic quantities: average energy,
Gases and the irial Expansion February 7, 3 First task is to examine what ensemble theory tells us about simple systems via the thermodynamic connection Calculate thermodynamic quantities: average energy,
A cylinder in a magnetic field (Jackson)
 Problem 1. A cylinder in a magnetic field (Jackson) A very long hollow cylinder of inner radius a and outer radius b of permeability µ is placed in an initially uniform magnetic field B o at right angles
Problem 1. A cylinder in a magnetic field (Jackson) A very long hollow cylinder of inner radius a and outer radius b of permeability µ is placed in an initially uniform magnetic field B o at right angles
l=0 The expansion coefficients can be determined, for example, by finding the potential on the z-axis and expanding that result in z.
 Electrodynamics I Exam - Part A - Closed Book KSU 15/11/6 Name Electrodynamic Score = 14 / 14 points Instructions: Use SI units. Where appropriate, define all variables or symbols you use, in words. Try
Electrodynamics I Exam - Part A - Closed Book KSU 15/11/6 Name Electrodynamic Score = 14 / 14 points Instructions: Use SI units. Where appropriate, define all variables or symbols you use, in words. Try
Attraction between two similar particles in an electrolyte: effects of Stern layer absorption
 Attraction between two similar particles in an electrolyte: effects of Stern layer absorption F.Plouraboué, H-C. Chang, June 7, 28 Abstract When Debye length is comparable or larger than the distance between
Attraction between two similar particles in an electrolyte: effects of Stern layer absorption F.Plouraboué, H-C. Chang, June 7, 28 Abstract When Debye length is comparable or larger than the distance between
A half submerged metal sphere (UIC comprehensive
 Problem 1. exam) A half submerged metal sphere (UIC comprehensive A very light neutral hollow metal spherical shell of mass m and radius a is slightly submerged by a distance b a below the surface of a
Problem 1. exam) A half submerged metal sphere (UIC comprehensive A very light neutral hollow metal spherical shell of mass m and radius a is slightly submerged by a distance b a below the surface of a
8.333: Statistical Mechanics I Problem Set # 5 Due: 11/22/13 Interacting particles & Quantum ensembles
 8.333: Statistical Mechanics I Problem Set # 5 Due: 11/22/13 Interacting particles & Quantum ensembles 1. Surfactant condensation: N surfactant molecules are added to the surface of water over an area
8.333: Statistical Mechanics I Problem Set # 5 Due: 11/22/13 Interacting particles & Quantum ensembles 1. Surfactant condensation: N surfactant molecules are added to the surface of water over an area
arxiv: v1 [cond-mat.soft] 29 Aug 2017
![arxiv: v1 [cond-mat.soft] 29 Aug 2017 arxiv: v1 [cond-mat.soft] 29 Aug 2017](/thumbs/74/70978477.jpg) Ionic Size Effects on the Poisson-Boltzmann Theory Thiago Colla, 1,a) Lucas Nunes Lopes, 2 and Alexandre P. dos Santos 2,3,b) 1) Instituto de Física, Universidade Federal de Ouro Preto, CEP 35400-000,
Ionic Size Effects on the Poisson-Boltzmann Theory Thiago Colla, 1,a) Lucas Nunes Lopes, 2 and Alexandre P. dos Santos 2,3,b) 1) Instituto de Física, Universidade Federal de Ouro Preto, CEP 35400-000,
Computer simulation methods (2) Dr. Vania Calandrini
 Computer simulation methods (2) Dr. Vania Calandrini in the previous lecture: time average versus ensemble average MC versus MD simulations equipartition theorem (=> computing T) virial theorem (=> computing
Computer simulation methods (2) Dr. Vania Calandrini in the previous lecture: time average versus ensemble average MC versus MD simulations equipartition theorem (=> computing T) virial theorem (=> computing
Lecture 13: Electromagnetic Theory Professor D. K. Ghosh, Physics Department, I.I.T., Bombay. Poisson s and Laplace s Equations
 Poisson s and Laplace s Equations Lecture 13: Electromagnetic Theory Professor D. K. Ghosh, Physics Department, I.I.T., Bombay We will spend some time in looking at the mathematical foundations of electrostatics.
Poisson s and Laplace s Equations Lecture 13: Electromagnetic Theory Professor D. K. Ghosh, Physics Department, I.I.T., Bombay We will spend some time in looking at the mathematical foundations of electrostatics.
The Behavior of 2:1 and 3:1 Electrolytes at Polarizable Interfaces
 The Behavior of 2:1 and 3:1 Electrolytes at Polarizable Interfaces Tímea Nagy 1, Mónika Valiskó 1, Douglas Henderson 2, and Dezső Boda 1,2 1 Department of Physical Chemistry, University of Pannonia, P.O.
The Behavior of 2:1 and 3:1 Electrolytes at Polarizable Interfaces Tímea Nagy 1, Mónika Valiskó 1, Douglas Henderson 2, and Dezső Boda 1,2 1 Department of Physical Chemistry, University of Pannonia, P.O.
A New Uniform Phase Bridge Functional: Test and Its Application to Non-uniform Phase Fluid
 Commun. Theor. Phys. (Beijing, China) 39 (2003) pp. 231 237 c International Academic Publishers Vol. 39, No. 2, February 15, 2003 A New Uniform Phase Bridge Functional: Test and Its Application to Non-uniform
Commun. Theor. Phys. (Beijing, China) 39 (2003) pp. 231 237 c International Academic Publishers Vol. 39, No. 2, February 15, 2003 A New Uniform Phase Bridge Functional: Test and Its Application to Non-uniform
Bound states of two particles confined to parallel two-dimensional layers and interacting via dipole-dipole or dipole-charge laws
 PHYSICAL REVIEW B VOLUME 55, NUMBER 8 15 FEBRUARY 1997-II Bound states of two particles confined to parallel two-dimensional layers and interacting via dipole-dipole or dipole-charge laws V. I. Yudson
PHYSICAL REVIEW B VOLUME 55, NUMBER 8 15 FEBRUARY 1997-II Bound states of two particles confined to parallel two-dimensional layers and interacting via dipole-dipole or dipole-charge laws V. I. Yudson
Using Fundamental Measure Theory to Treat the Correlation Function of the Inhomogeneous Hard-Sphere Fluid
 Using Fundamental Measure Theory to Treat the Correlation Function of the Inhomogeneous Hard-Sphere Fluid Jeff B. Schulte, Patrick A. Kreitzberg, Chris V. Haglund, and David Roundy Department of Physics,
Using Fundamental Measure Theory to Treat the Correlation Function of the Inhomogeneous Hard-Sphere Fluid Jeff B. Schulte, Patrick A. Kreitzberg, Chris V. Haglund, and David Roundy Department of Physics,
Colloid Chemistry. La chimica moderna e la sua comunicazione Silvia Gross.
 Colloid Chemistry La chimica moderna e la sua comunicazione Silvia Gross Istituto Dipartimento di Scienze di e Scienze Tecnologie Chimiche Molecolari ISTM-CNR, Università Università degli Studi degli Studi
Colloid Chemistry La chimica moderna e la sua comunicazione Silvia Gross Istituto Dipartimento di Scienze di e Scienze Tecnologie Chimiche Molecolari ISTM-CNR, Università Università degli Studi degli Studi
Modified Maxwell-Garnett equation for the effective transport coefficients in inhomogeneous media
 J. Phys. A: Math. Gen. 3 (998) 7227 7234. Printed in the UK PII: S0305-4470(98)93976-2 Modified Maxwell-Garnett equation for the effective transport coefficients in inhomogeneous media Juris Robert Kalnin
J. Phys. A: Math. Gen. 3 (998) 7227 7234. Printed in the UK PII: S0305-4470(98)93976-2 Modified Maxwell-Garnett equation for the effective transport coefficients in inhomogeneous media Juris Robert Kalnin
Chapter 1 The Electric Force
 Chapter 1 The Electric Force 1. Properties of the Electric Charges 1- There are two kinds of the electric charges in the nature, which are positive and negative charges. - The charges of opposite sign
Chapter 1 The Electric Force 1. Properties of the Electric Charges 1- There are two kinds of the electric charges in the nature, which are positive and negative charges. - The charges of opposite sign
How to define the direction of A??
 Chapter Gauss Law.1 Electric Flu. Gauss Law. A charged Isolated Conductor.4 Applying Gauss Law: Cylindrical Symmetry.5 Applying Gauss Law: Planar Symmetry.6 Applying Gauss Law: Spherical Symmetry You will
Chapter Gauss Law.1 Electric Flu. Gauss Law. A charged Isolated Conductor.4 Applying Gauss Law: Cylindrical Symmetry.5 Applying Gauss Law: Planar Symmetry.6 Applying Gauss Law: Spherical Symmetry You will
A Fast Algorithm for Treating Dielectric Discontinuities in Charged Spherical Colloids
 Interdiscip Sci Comput Life Sci (212) 4: 19 26 DOI: 1.17/s12539-12-113-1 A Fast Algorithm for Treating Dielectric Discontinuities in Charged Spherical Colloids Zhenli XU (Department of Mathematics, and
Interdiscip Sci Comput Life Sci (212) 4: 19 26 DOI: 1.17/s12539-12-113-1 A Fast Algorithm for Treating Dielectric Discontinuities in Charged Spherical Colloids Zhenli XU (Department of Mathematics, and
Soft Matter - Theoretical and Industrial Challenges Celebrating the Pioneering Work of Sir Sam Edwards
 Soft Matter - Theoretical and Industrial Challenges Celebrating the Pioneering Work of Sir Sam Edwards One Hundred Years of Electrified Interfaces: The Poisson-Boltzmann theory and some recent developments
Soft Matter - Theoretical and Industrial Challenges Celebrating the Pioneering Work of Sir Sam Edwards One Hundred Years of Electrified Interfaces: The Poisson-Boltzmann theory and some recent developments
Monte Carlo simulation for the potential of mean force between ionic colloids in solutions of asymmetric salts
 JOURNAL OF CHEMICAL PHYSICS VOLUME 111, NUMBER 15 15 OCTOBER 1999 Monte Carlo simulation for the potential of mean force between ionic colloids in solutions of asymmetric salts J. Z. Wu, D. Bratko, H.
JOURNAL OF CHEMICAL PHYSICS VOLUME 111, NUMBER 15 15 OCTOBER 1999 Monte Carlo simulation for the potential of mean force between ionic colloids in solutions of asymmetric salts J. Z. Wu, D. Bratko, H.
Physics 127c: Statistical Mechanics. Weakly Interacting Fermi Gas. The Electron Gas
 Physics 7c: Statistical Mechanics Wealy Interacting Fermi Gas Unlie the Boson case, there is usually no ualitative change in behavior going from the noninteracting to the wealy interacting Fermi gas for
Physics 7c: Statistical Mechanics Wealy Interacting Fermi Gas Unlie the Boson case, there is usually no ualitative change in behavior going from the noninteracting to the wealy interacting Fermi gas for
Critical parameters of unrestricted primitive model electrolytes with charge asymmetries up to 10:1
 JOURNAL OF CHEMICAL PHYSICS VOLUME 119, NUMBER 16 22 OCTOBER 2003 Critical parameters of unrestricted primitive model electrolytes with charge asymmetries up to 10:1 Daniel W. Cheong and Athanassios Z.
JOURNAL OF CHEMICAL PHYSICS VOLUME 119, NUMBER 16 22 OCTOBER 2003 Critical parameters of unrestricted primitive model electrolytes with charge asymmetries up to 10:1 Daniel W. Cheong and Athanassios Z.
Unusual Entropy of Adsorbed Methane on Zeolite Templated Carbon. Supporting Information. Part 2: Statistical Mechanical Model
 Unusual Entropy of Adsorbed Methane on Zeolite Templated Carbon Supporting Information Part 2: Statistical Mechanical Model Nicholas P. Stadie*, Maxwell Murialdo, Channing C. Ahn, and Brent Fultz W. M.
Unusual Entropy of Adsorbed Methane on Zeolite Templated Carbon Supporting Information Part 2: Statistical Mechanical Model Nicholas P. Stadie*, Maxwell Murialdo, Channing C. Ahn, and Brent Fultz W. M.
Electrodynamics I Midterm - Part A - Closed Book KSU 2005/10/17 Electro Dynamic
 Electrodynamics I Midterm - Part A - Closed Book KSU 5//7 Name Electro Dynamic. () Write Gauss Law in differential form. E( r) =ρ( r)/ɛ, or D = ρ, E= electricfield,ρ=volume charge density, ɛ =permittivity
Electrodynamics I Midterm - Part A - Closed Book KSU 5//7 Name Electro Dynamic. () Write Gauss Law in differential form. E( r) =ρ( r)/ɛ, or D = ρ, E= electricfield,ρ=volume charge density, ɛ =permittivity
Circumventing the pathological behavior of path-integral Monte Carlo for systems with Coulomb potentials
 Circumventing the pathological behavior of path-integral Monte Carlo for systems with Coulomb potentials M. H. Müser and B. J. Berne Department of Chemistry, Columbia University, New York, New York 10027
Circumventing the pathological behavior of path-integral Monte Carlo for systems with Coulomb potentials M. H. Müser and B. J. Berne Department of Chemistry, Columbia University, New York, New York 10027
Supplementary Information for: Controlling Cellular Uptake of Nanoparticles with ph-sensitive Polymers
 Supplementary Information for: Controlling Cellular Uptake of Nanoparticles with ph-sensitive Polymers Hong-ming Ding 1 & Yu-qiang Ma 1,2, 1 National Laboratory of Solid State Microstructures and Department
Supplementary Information for: Controlling Cellular Uptake of Nanoparticles with ph-sensitive Polymers Hong-ming Ding 1 & Yu-qiang Ma 1,2, 1 National Laboratory of Solid State Microstructures and Department
 Title Super- and subcritical hydration of Thermodynamics of hydration Author(s) Matubayasi, N; Nakahara, M Citation JOURNAL OF CHEMICAL PHYSICS (2000), 8109 Issue Date 2000-05-08 URL http://hdl.handle.net/2433/50350
Title Super- and subcritical hydration of Thermodynamics of hydration Author(s) Matubayasi, N; Nakahara, M Citation JOURNAL OF CHEMICAL PHYSICS (2000), 8109 Issue Date 2000-05-08 URL http://hdl.handle.net/2433/50350
1. (3) Write Gauss Law in differential form. Explain the physical meaning.
 Electrodynamics I Midterm Exam - Part A - Closed Book KSU 204/0/23 Name Electro Dynamic Instructions: Use SI units. Where appropriate, define all variables or symbols you use, in words. Try to tell about
Electrodynamics I Midterm Exam - Part A - Closed Book KSU 204/0/23 Name Electro Dynamic Instructions: Use SI units. Where appropriate, define all variables or symbols you use, in words. Try to tell about
3/22/2016. Chapter 27 Gauss s Law. Chapter 27 Preview. Chapter 27 Preview. Chapter Goal: To understand and apply Gauss s law. Slide 27-2.
 Chapter 27 Gauss s Law Chapter Goal: To understand and apply Gauss s law. Slide 27-2 Chapter 27 Preview Slide 27-3 Chapter 27 Preview Slide 27-4 1 Chapter 27 Preview Slide 27-5 Chapter 27 Preview Slide
Chapter 27 Gauss s Law Chapter Goal: To understand and apply Gauss s law. Slide 27-2 Chapter 27 Preview Slide 27-3 Chapter 27 Preview Slide 27-4 1 Chapter 27 Preview Slide 27-5 Chapter 27 Preview Slide
Molecular Driving Forces
 Molecular Driving Forces Statistical Thermodynamics in Chemistry and Biology SUBGfittingen 7 At 216 513 073 / / Ken A. Dill Sarina Bromberg With the assistance of Dirk Stigter on the Electrostatics chapters
Molecular Driving Forces Statistical Thermodynamics in Chemistry and Biology SUBGfittingen 7 At 216 513 073 / / Ken A. Dill Sarina Bromberg With the assistance of Dirk Stigter on the Electrostatics chapters
Experimental Soft Matter (M. Durand, G. Foffi)
 Master 2 PCS/PTSC 2016-2017 10/01/2017 Experimental Soft Matter (M. Durand, G. Foffi) Nota Bene Exam duration : 3H ecture notes are not allowed. Electronic devices (including cell phones) are prohibited,
Master 2 PCS/PTSC 2016-2017 10/01/2017 Experimental Soft Matter (M. Durand, G. Foffi) Nota Bene Exam duration : 3H ecture notes are not allowed. Electronic devices (including cell phones) are prohibited,
Perspective: Coulomb fluids-weak coupling, strong coupling, in between and beyond
 Perspective: Coulomb fluids-weak coupling, strong coupling, in between and beyond Naji, Ali; Kanduc, Matej; Forsman, Jan; Podgornik, Rudolf Published in: Journal of Chemical Physics DOI: 10.1063/1.4824681
Perspective: Coulomb fluids-weak coupling, strong coupling, in between and beyond Naji, Ali; Kanduc, Matej; Forsman, Jan; Podgornik, Rudolf Published in: Journal of Chemical Physics DOI: 10.1063/1.4824681
PHYSICS. Chapter 24 Lecture FOR SCIENTISTS AND ENGINEERS A STRATEGIC APPROACH 4/E RANDALL D. KNIGHT
 PHYSICS FOR SCIENTISTS AND ENGINEERS A STRATEGIC APPROACH 4/E Chapter 24 Lecture RANDALL D. KNIGHT Chapter 24 Gauss s Law IN THIS CHAPTER, you will learn about and apply Gauss s law. Slide 24-2 Chapter
PHYSICS FOR SCIENTISTS AND ENGINEERS A STRATEGIC APPROACH 4/E Chapter 24 Lecture RANDALL D. KNIGHT Chapter 24 Gauss s Law IN THIS CHAPTER, you will learn about and apply Gauss s law. Slide 24-2 Chapter
1. Electricity and Magnetism (Fall 1995, Part 1) A metal sphere has a radius R and a charge Q.
 1. Electricity and Magnetism (Fall 1995, Part 1) A metal sphere has a radius R and a charge Q. (a) Compute the electric part of the Maxwell stress tensor T ij (r) = 1 {E i E j 12 } 4π E2 δ ij both inside
1. Electricity and Magnetism (Fall 1995, Part 1) A metal sphere has a radius R and a charge Q. (a) Compute the electric part of the Maxwell stress tensor T ij (r) = 1 {E i E j 12 } 4π E2 δ ij both inside
Surface Tension of Electrolyte Solutions: A Self-consistent Theory
 Surface Tension of Electrolyte Solutions: A Self-consistent Theory Tomer Markovich, David Andelman Raymond and Beverly Sackler School of Physics and Astronomy Tel Aviv University, Ramat Aviv, Tel Aviv
Surface Tension of Electrolyte Solutions: A Self-consistent Theory Tomer Markovich, David Andelman Raymond and Beverly Sackler School of Physics and Astronomy Tel Aviv University, Ramat Aviv, Tel Aviv
Surprising Challenges
 December 12, 2013 12:41 PSP Book - 9in x 6in 01-ch01 Chapter 1 Surprising Challenges V. Adrian Parsegian Department of Physics, University of Massachusetts, Amherst 01003, USA parsegian@physics.umass.edu
December 12, 2013 12:41 PSP Book - 9in x 6in 01-ch01 Chapter 1 Surprising Challenges V. Adrian Parsegian Department of Physics, University of Massachusetts, Amherst 01003, USA parsegian@physics.umass.edu
Phase Equilibria and Molecular Solutions Jan G. Korvink and Evgenii Rudnyi IMTEK Albert Ludwig University Freiburg, Germany
 Phase Equilibria and Molecular Solutions Jan G. Korvink and Evgenii Rudnyi IMTEK Albert Ludwig University Freiburg, Germany Preliminaries Learning Goals Phase Equilibria Phase diagrams and classical thermodynamics
Phase Equilibria and Molecular Solutions Jan G. Korvink and Evgenii Rudnyi IMTEK Albert Ludwig University Freiburg, Germany Preliminaries Learning Goals Phase Equilibria Phase diagrams and classical thermodynamics
Physical Applications: Convexity and Legendre transforms
 Physical Applications: Convexity and Legendre transforms A.C. Maggs CNRS+ESPCI, Paris June 2016 Physical applications Landau theories for dielectric response Asymmetric electrolytes with finite volume
Physical Applications: Convexity and Legendre transforms A.C. Maggs CNRS+ESPCI, Paris June 2016 Physical applications Landau theories for dielectric response Asymmetric electrolytes with finite volume
Attraction or repulsion between charged colloids? A connection with Debye Hückel theory
 J. Phys.: Condens. Matter 12 (2000) A263 A267. Printed in the UK PII: S0953-8984(00)07724-9 Attraction or repulsion between charged colloids? A connection with Debye Hückel theory René van Roij H H Wills
J. Phys.: Condens. Matter 12 (2000) A263 A267. Printed in the UK PII: S0953-8984(00)07724-9 Attraction or repulsion between charged colloids? A connection with Debye Hückel theory René van Roij H H Wills
arxiv:cond-mat/ v1 [cond-mat.soft] 9 Aug 1997
![arxiv:cond-mat/ v1 [cond-mat.soft] 9 Aug 1997 arxiv:cond-mat/ v1 [cond-mat.soft] 9 Aug 1997](/thumbs/89/99391124.jpg) Depletion forces between two spheres in a rod solution. K. Yaman, C. Jeppesen, C. M. Marques arxiv:cond-mat/9708069v1 [cond-mat.soft] 9 Aug 1997 Department of Physics, U.C.S.B., CA 93106 9530, U.S.A. Materials
Depletion forces between two spheres in a rod solution. K. Yaman, C. Jeppesen, C. M. Marques arxiv:cond-mat/9708069v1 [cond-mat.soft] 9 Aug 1997 Department of Physics, U.C.S.B., CA 93106 9530, U.S.A. Materials
Dielectrics. Lecture 20: Electromagnetic Theory. Professor D. K. Ghosh, Physics Department, I.I.T., Bombay
 What are dielectrics? Dielectrics Lecture 20: Electromagnetic Theory Professor D. K. Ghosh, Physics Department, I.I.T., Bombay So far we have been discussing electrostatics in either vacuum or in a conductor.
What are dielectrics? Dielectrics Lecture 20: Electromagnetic Theory Professor D. K. Ghosh, Physics Department, I.I.T., Bombay So far we have been discussing electrostatics in either vacuum or in a conductor.
CHAPTER 3 POTENTIALS 10/13/2016. Outlines. 1. Laplace s equation. 2. The Method of Images. 3. Separation of Variables. 4. Multipole Expansion
 CHAPTER 3 POTENTIALS Lee Chow Department of Physics University of Central Florida Orlando, FL 32816 Outlines 1. Laplace s equation 2. The Method of Images 3. Separation of Variables 4. Multipole Expansion
CHAPTER 3 POTENTIALS Lee Chow Department of Physics University of Central Florida Orlando, FL 32816 Outlines 1. Laplace s equation 2. The Method of Images 3. Separation of Variables 4. Multipole Expansion
Fall 2004 Physics 3 Tu-Th Section
 Fall 2004 Physics 3 Tu-Th Section Claudio Campagnari Lecture 9: 21 Oct. 2004 Web page: http://hep.ucsb.edu/people/claudio/ph3-04/ 1 Last time: Gauss's Law To formulate Gauss's law, introduced a few new
Fall 2004 Physics 3 Tu-Th Section Claudio Campagnari Lecture 9: 21 Oct. 2004 Web page: http://hep.ucsb.edu/people/claudio/ph3-04/ 1 Last time: Gauss's Law To formulate Gauss's law, introduced a few new
Supporting Information for Conical Nanopores. for Efficient Ion Pumping and Desalination
 Supporting Information for Conical Nanopores for Efficient Ion Pumping and Desalination Yu Zhang, and George C. Schatz,, Center for Bio-inspired Energy Science, Northwestern University, Chicago, Illinois
Supporting Information for Conical Nanopores for Efficient Ion Pumping and Desalination Yu Zhang, and George C. Schatz,, Center for Bio-inspired Energy Science, Northwestern University, Chicago, Illinois
Computer simulation methods (1) Dr. Vania Calandrini
 Computer simulation methods (1) Dr. Vania Calandrini Why computational methods To understand and predict the properties of complex systems (many degrees of freedom): liquids, solids, adsorption of molecules
Computer simulation methods (1) Dr. Vania Calandrini Why computational methods To understand and predict the properties of complex systems (many degrees of freedom): liquids, solids, adsorption of molecules
Lennard-Jones as a model for argon and test of extended renormalization group calculations
 JOURNAL OF CHEMICAL PHYSICS VOLUME 111, NUMBER 2 22 NOVEMBER 1999 Lennard-Jones as a model for argon and test of extended renormalization group calculations John A. White Department of Physics, American
JOURNAL OF CHEMICAL PHYSICS VOLUME 111, NUMBER 2 22 NOVEMBER 1999 Lennard-Jones as a model for argon and test of extended renormalization group calculations John A. White Department of Physics, American
Review. Spring Semester /21/14. Physics for Scientists & Engineers 2 1
 Review Spring Semester 2014 Physics for Scientists & Engineers 2 1 Notes! Homework set 13 extended to Tuesday, 4/22! Remember to fill out SIRS form: https://sirsonline.msu.edu Physics for Scientists &
Review Spring Semester 2014 Physics for Scientists & Engineers 2 1 Notes! Homework set 13 extended to Tuesday, 4/22! Remember to fill out SIRS form: https://sirsonline.msu.edu Physics for Scientists &
EFFECTS OF ADDED ELECTROLYTES ON THE STRUCTURE OF CHARGED POLYMERIC MICELLES
 Soft Materials, 3(2&3): 89 120, (2006) Copyright # Taylor & Francis Group, LLC ISSN 1539-445X print/1539-4468 online DOI: 10.1080/15394450600801228 EFFECTS OF DDED ELECTROLYTES ON THE STRUCTURE OF CHRGED
Soft Materials, 3(2&3): 89 120, (2006) Copyright # Taylor & Francis Group, LLC ISSN 1539-445X print/1539-4468 online DOI: 10.1080/15394450600801228 EFFECTS OF DDED ELECTROLYTES ON THE STRUCTURE OF CHRGED
V. Electrostatics Lecture 24: Diffuse Charge in Electrolytes
 V. Electrostatics Lecture 24: Diffuse Charge in Electrolytes MIT Student 1. Poisson-Nernst-Planck Equations The Nernst-Planck Equation is a conservation of mass equation that describes the influence of
V. Electrostatics Lecture 24: Diffuse Charge in Electrolytes MIT Student 1. Poisson-Nernst-Planck Equations The Nernst-Planck Equation is a conservation of mass equation that describes the influence of
Supplementary Information for A Magnetic Wormhole
 Supplementary Information for A Magnetic Wormhole Jordi Prat-Camps, Carles Navau, and Alvaro Sanchez Departament de Física, Universitat Autònoma de Barcelona, 08193 Bellaterra, Barcelona, Catalonia, Spain
Supplementary Information for A Magnetic Wormhole Jordi Prat-Camps, Carles Navau, and Alvaro Sanchez Departament de Física, Universitat Autònoma de Barcelona, 08193 Bellaterra, Barcelona, Catalonia, Spain
V. Electrostatics. MIT Student
 V. Electrostatics Lecture 26: Compact Part of the Double Layer MIT Student 1 Double-layer Capacitance 1.1 Stern Layer As was discussed in the previous lecture, the Gouy-Chapman model predicts unphysically
V. Electrostatics Lecture 26: Compact Part of the Double Layer MIT Student 1 Double-layer Capacitance 1.1 Stern Layer As was discussed in the previous lecture, the Gouy-Chapman model predicts unphysically
1 Electromagnetic concepts useful for radar applications
 Electromagnetic concepts useful for radar applications The scattering of electromagnetic waves by precipitation particles and their propagation through precipitation media are of fundamental importance
Electromagnetic concepts useful for radar applications The scattering of electromagnetic waves by precipitation particles and their propagation through precipitation media are of fundamental importance
v(r i r j ) = h(r i )+ 1 N
 Chapter 1 Hartree-Fock Theory 1.1 Formalism For N electrons in an external potential V ext (r), the many-electron Hamiltonian can be written as follows: N H = [ p i i=1 m +V ext(r i )]+ 1 N N v(r i r j
Chapter 1 Hartree-Fock Theory 1.1 Formalism For N electrons in an external potential V ext (r), the many-electron Hamiltonian can be written as follows: N H = [ p i i=1 m +V ext(r i )]+ 1 N N v(r i r j
Resonances and dipole moments in dielectric, magnetic, and magnetodielectric cylinders an overview
 Appl Phys A (2011) 103: 789 793 DOI 10.1007/s00339-010-6219-6 Resonances and dipole moments in dielectric, magnetic, and magnetodielectric cylinders an overview A. Dirksen S. Arslanagic O. Breinbjerg Received:
Appl Phys A (2011) 103: 789 793 DOI 10.1007/s00339-010-6219-6 Resonances and dipole moments in dielectric, magnetic, and magnetodielectric cylinders an overview A. Dirksen S. Arslanagic O. Breinbjerg Received:
Center for Theoretical Physics, Department of Applied Physics, Twente University, P.O. Box 217, 7500 AE Enschede, The Netherlands
 Physica A 193 (1993) 413-420 North-Holland Distribution of ions around a charged sphere P. Strating and F.W. Wiegel Center for Theoretical Physics, Department of Applied Physics, Twente University, P.O.
Physica A 193 (1993) 413-420 North-Holland Distribution of ions around a charged sphere P. Strating and F.W. Wiegel Center for Theoretical Physics, Department of Applied Physics, Twente University, P.O.
Research Statement. Shenggao Zhou. November 3, 2014
 Shenggao Zhou November 3, My research focuses on: () Scientific computing and numerical analysis (numerical PDEs, numerical optimization, computational fluid dynamics, and level-set method for interface
Shenggao Zhou November 3, My research focuses on: () Scientific computing and numerical analysis (numerical PDEs, numerical optimization, computational fluid dynamics, and level-set method for interface
Stochastic Poisson-Boltzmann Equation for Charged Fluids
 Stochastic Poisson-Boltzmann Equation for Charged Fluids Li Wan Department of Physics, Wenzhou University, Wenzhou 325035, P. R. China Ning-Hua Tong Department of Physics, Renmin University of China, 100872
Stochastic Poisson-Boltzmann Equation for Charged Fluids Li Wan Department of Physics, Wenzhou University, Wenzhou 325035, P. R. China Ning-Hua Tong Department of Physics, Renmin University of China, 100872
