,t] ph =r oph. + (r oph
|
|
|
- Magnus Sharp
- 5 years ago
- Views:
Transcription
1 104 Chapter 6. The Solid Mass Photon and Atomic Spectra 1. The Solid Mass Photon In chapter 1 it was proved from the lst postulate of special relativity that the speed of light is vector ally additive and consequently that Maxwell s Equations are false. In this chapter the first steps will be taken to provide the equations and dynamics to replace Maxwell s Equations and classical electrodynamics with equations and dynamics based on the solid mass atom and the solid mass photon. Photons are hypothesized to be solid mass particles produced as collision products of two atoms (Discussed below), or as explosion products of an exploding atom (Chapter 3 section 10 and chapter 10, section 5). As concerns photons produced by collision of atoms. Colliding solid mass atoms compress the atomic mass surface at the contact point resulting in percussive and shear waves that fan out between isopycnals from the contact point and re- converge at a point directly opposite the contact point releasing a solid mass particle here after called a photon. The physical details of this process are discussed in chapter 6, section 4. Experimentally, atoms at very different temperatures can produce a given optical color, e.g. red. The surface of the sun at the accepted temperature of 6,000 o K, a tungsten filament at (800)( o K) and an excited Geissler Tube at 350o K all produce red light. This means that if the photons that produce red light are in thermal equilibrium with their parent atoms i.e. T ph,r =T At where,r,r = KT ph,r and =, then red light photons emitted at different T, have unequal kinetic energies. By hypothesis, all red light photons have the same momentum. See chapter 6, section 9. The internal dynamics of an isolated photon are governed by equations of the form 3.15: 6.1 where h[r,t] ph =r+ (r,t) ph, U ph = ph = (r,t) ph, where (r,0) ph =0, h[r oph,t] ph =r oph + (r oph,t) ph. h ph and r are measured from a coordinate frame at rest w.r.t. the center of mass of the photon to any point within or on the surface of the photon. r oph, represents the space averaged surface of the photon at t=0. Due to vibrational energy loss or gain by collision with atoms or with other photons, C 1,ph is a function of t during collision and a constant between collisions. Ignoring field forces acting on the photon, the total energy of the photon TE ph is: TE ph = +m ph C 1,ph. A given mass sample for large enough T, T>0 o K, is constantly emitting photons from the sample surface and is thus constantly losing energy to the external world. Assuming that the sample is not undergoing chemical or nuclear reactions, in order
2 105 to keep the sample at constant temperature T, a constant supply of energy must be supplied to the sample: e S = e ex. Where e S is the total photon and conductive thermal energy lost per second by the sample and provided to the external world at the sample surface and e ex is the total energy per second provided by the external world to the sample at the sample surface. e ex is provided by direct conductive thermal contact between the given mass and the external world and by photons emitted by the external world. If the mass sample at temperature T>0 o K is in empty outer space with no incoming photon energy, then the sample will spontaneously emit solid mass photons thereby losing energy continuously and the sample s temperature will approach T >0 o K as t >. THERE IS NO GROUND STATE FOR THE SOLID MASS ATOM FOR T>0 o K. Using 3.8, the field strength (r) for the solid mass photon is written below. 6.2 p 2, r r ph p= 2, r r ph where r is the distance from the center of the photon to any point within the photon Consider an isolated photon in vacuum for which. 0 with 0 r r ph where r ph is the average photon radius. From 3.28 for a photon with average photon radius r ph :
3 106 and using 6.2,. In order to compute, it is assumed that see chapter 3, section 9. = 2, p= 2 =, 3 < p 2 has been computed for p=0 and is listed in table Photon Binding Energy From chapter 6, section 2 and the development of 3.29, the binding energy of the photon is given by: 6.3 BE ph = m ph C 1 = Table 6.1 n 1 n 2 (ev) m ph (gm) (3.2) (3.2) (3.2) (3.2) (3.2) (3.2) (3.6) (3.6) (3.2) (3.2)10-34
4 107 Table 6.1 lists m ph as a function of speed 10 n 1 and kinetic energy n 2 (ev). Solving n 2 (ev)= ( ) for mph yields: m ph =(3.2)10-2(6+n 1) n2 Table 6.2 lists r ph as a function of speed 10 n 1, kinetic energy n 2 (ev) and binding energy be ph (ev). Evaluating 6.3 for r ph yields: r ph = (6.4)10 2(9-2n 1) ( ). Table 6.2 n 1 n 2 (ev) be ph (ev) r ph (cm) (6.4) (6.4) (6.4) (6.4) (6.4) (6.4) (7.7) (1.5) (6.4) (6.4)10-28 Table 6.3 lists as a function of speed 10 n 1, kinetic energy n 2 (ev), binding energy be ph (ev) and p=0. Using from chapter 6, section 2, becomes: = 10 n 1( ), for 2<p 0 and =, for 3 < p 2
5 108 Table 6.3 n 1 n 2 (ev) be ph (ev) p (7.1)10 7 (2.2)10 8 (7.1)10 8 (2.2)10 9 (7.1)10 9 (2.2)10 10 (2.1)10 10 (6.7)10 10 (7.1)10 10 (2.2) Newton's 2 nd Law Rewritten Newton's Laws were originally written for mass points. Mass points do not exist and consequently Newton's Laws must be rewritten for liquids, solids and gases made up of continuous mass atoms each of which is separated by empty space. In general each atom in a liquid or a solid is coupled to two or more adjacent atoms while each atom in a gas maybe coupled to 0,1,2,,6 atoms, the exact number depending on the coupled elements involved. Let (,t) represent the position of any point within or on the surface of a continuous mass atom at time t as measured from an inertial frame S. represents any point within or on the surface of the atom at t=0. (,t)=[ + (,t)], (,0)=, (,0)=, (,t)= (,t),. The position of the center of mass of an atom of mass m At is given by: 6.4 Where V=V(t) is the volume of the atom as a function of time and S=S(t) is the bounding surface of V(t)., and are also measured from inertial frame S. Let ( ) represent the pressure due to contact forces created by charge neutral
6 109 atoms and subatomic particles bouncing off of the atomic surface S(t) maintaining conservation of energy and momentum. Newton's second law becomes: 6.5(i) + = = Or using alternative notation: 6.5(ii) (t) Tc + (t) Tf = is the vector sum total of all external contact forces acting on S(t) where Tc Tc. is the vector sum total of all externally caused field forces acting on the material Tf in volume V(t) where Tf and where ( ) is the force per unit volume due to externally caused field forces. Classically given (t) Tc + (t) Tf, and (,0) and (,0) for a mass point, one can solve 6.5(ii) for (,t), (,t),. However 6.5(ii) is, strictly speaking, not correct. Consider; At time t o atom #1 is struck by another atom in the radial direction. The collision increases the internal energy of atom #1 by m At C 1 =m At C 1,f m At C 1,i >0 where m At C 1,i <0 represents the internal energy of the atom before collision and m At C 1,f <0 represents the internal energy of atom #1 after collision. A deformation force (t) D produced by (t) Tc will cause a change in the internal energy m At C 1 where (t) D = and is the deformation pressure acting on the surface of the atom. (t) D goes into changing the density structure ( ), the mean radius and the internal and surface wave energy. In order that 6.5(ii) hold rigorously, it is necessary that m At C 1 << KE T where KE T is the translational kinetic energy of the atom. In the general case for which m At C 1 cannot be ignored: 6.5(iii) (t) Tc (t) D + (t) Tf =
7 Newton s Laws Rewritten for Two Solid Mass Atoms Colliding Elastically FIGURE A Central to the Newtonian concept of force, acceleration and velocity is the concept of the coordinate frame from which they are measured, i.e. the inertial frame. Consider two atoms on a center on center collision course, Fig A. At t=0 and before the two atoms collide, the center of mass of the 2 atoms is at the origin so that m 1 (0)= m 2 (0). If experimentally, m 1 (t)= m 2 (t), and assuming a mutual field force created by m 1 and m 2 is the only force acting on m 1 and m 2, then 21 =m 1 1 (t)= m 2 2 (t)= 12 : Newton s 3rd Law holds. Now suppose that one is in a frame such that Newton s 3 rd Law holds. Consequently, m 1 1 (t)= m 2 2 (t), m 1 1 (t)+m 2 2 (t)=m 1 1 (0)+m 2 2 (0)=const. and m 1 (t) m 1 (0) m 1 1 (0)t = m 2 (t)+m 2 (0)+m 2 2 (0)t consequently, m 1 (t)= m 2 (t) iff. m 1 (0)= m 2 (0) and m 1 1 (0)= m 2 2 (0). Let m il represent the mass of the inertial frame S. In order that S remain a non-accelerating inertial frame, it is required that << (R 1 ) = (R 2 ) where is the force on S due to m 1, plus the force on S due to m 2, and (R 1 ) is the force on m 1 due to m 2 and (R 2 ) is the force on m 2 due to m 1. Atom #1 has mass m 1 and initial radius r 1,i and atom #2 has mass m 2 and initial radius r 2,i with r 1,i r 2,i. The collision lasts t c sec. where t c =t f,c -t i,c. At t i,c, the center to center distance S i is S i =r 1,i +r 2,i =R 1,i +R 2,i and at t f,c the center to center distance is S f =r 1,f +r 2,f =R 1,f +R 2,f where due to deformation, S f may not be equal to S i. The force acting on atom #1 due to atom #2 during the collision is (S) 21 and the force acting on atom #2 due to atom #1 during the collision is (S) 12 where S(t i,c )=S i and S(t f,c )=S f : t i,c t t f,c. (r 1,S) 21 is the force on atom #1 due to atom #2 as if atom #1 were a point mass centered on the center of mass of atom #1 and (r 2,S) 12 is the force on atom #2 due to atom #1 as if atom #2 were a point mass centered on the center of mass of atom #2. (r 1,S) 21 is the sum of 2 forces, (r 1,S) 21 =[ (S) 21 +[ (r 1 ) 21
8 111 where [ (r 1 ) 21 is the contact force on #1 due to #2 and [ (S) 21 is the chemical bond force on #1 due to #2. Similarly (r 2,S) 12 =[ (r 2 ) 12 +[ (S) 12. Using figure B, S=R 1 +R 2 =r 1 +r 2, 21 = =, 12 = =. FIGURE B Using Figure A, the conservation of energy equations become: 6.5(iv) [ KE(S) 1 =[ = [ V 1, and [ KE(S) 2 =[ = [ V 2. Assume you are in a frame such that BEFORE and AFTER the collision Newton s 3 rd Law holds, [ (S) 21 = [ (S) 12 and therefore, m 1 (t)= m 2 (t)+m 1 (0)+m 2 (0)+{m 1 1 (0)+m 2 2 (0)}t and [ (S) 21 d = [ (S) 12 d =[ (S) 12 { d [ 1 (0)+ 2 (0)]dt} and [ (S) 21 d =[ (S) 12 d iff., m 1 = m 2 and [ 1 (0)+ 2 (0)]=0 and it is not at all clear that in general, =[. Given that [ (S) 21 = [ (S) 12 and 21 =, 12 = and m 1 m 2 it will be proved that: 6.5(iv a ) =[ iff. [ (S) 21 is an inverse square function. If m 1 =m 2 and [ 1 (0)+ 2 (0)]=0 then 6.5(iv a ) holds for any integrable functions such that [ (S) 21 = [ (S) 12. With S=R 1 +R 2 =r 1 +r 2 and 21 =, 12 =, and d ={ d + [ 1 (0)+ 2 (0)]dt} by direct substitution:
9 112 [ = [ + [m 1 1 (0)+m 2 2 (0)][ and [ = [ + [m 1 1 (0)+m 2 2 (0)][ Expand the integral in a power series: =. If =[ then term for term, a n ( ) n (S n )= a n ( ) n (S n ) and simplifying yields, (m 2 ) n+1 =(m 1 ) n+1 with solutions n=-1 and/or m 2 =m 1. It has been proved that given [ (S) 21 = [ (S) 12 and 21 =, 12 = and m 1 m 2, that =[ iff. [ (S) 21 is an inverse square function. If m 1 =m 2 and [ 1 (0)+ 2 (0)]=0 then =[ holds for any integrable functions for which [ (S) 21 = [ (S) 12. Equating results: 6.5(v) [ KE(S) 1 =[ = [ V 1 =[ KE(S) 2 =[ = [ V 2 iff. [ (S) 21 = [ (S) 12 and [ (S) 21 and [ (S) 12 are inverse square functions, From which it immediately follows that the change in total energy=0 6.5(vi) [ KE(S) 1 +[ KE(S) 2 +[ V 1 +[ V 2 =0 [ KE(S) 1 =[ KE(S) 2 = [ V 1 = [ V 2 Although the above analysis is for 2 atoms attracted by a field force before and after collision, the analysis holds for any 2 macroscopic objects attracted by a field force.
10 113 Note that although [ =[ for any integrable function [ (S) 21 subject to [ (S) 21 = [ (S) 12 and 21 = 12 : [ [ KE(S) 1 as [ KE(S) 1 =[ = [ + [m 1 1 (0)+m 2 2 (0)][ [ A similar argument holds to show that [ KE(S) 2,cb. If [ (S) 21 = [ (S) 12 but [ (S) 21 and [ (S) 12 are not inverse square functions, then 6.5(iv) still holds but [ [ and therefore [ (S) 21 and [ (S) 12 do not represent physical, experimental reality. Turning now to the atoms at collision; from appendix 10A, the total internal energy of the atoms is: [T 1 +[V 1 = <0 for 3<p 1 0 [T 2 +[V 2 = <0 for 3<p 2 0 Ignoring the cb force for the moment, and using Figure C below, define x 1 =r 1,i x 2 =r 2,i r. All measurements are made from inertial frame S. r and FIGURE C The conservation of energy equations for the 2 atoms in collision c during time t, t i,c t t f,c are:
11 (vii) KE(r 1 ) 1,c + T 1,c =[ = V 1,c and KE(r 2 ) 2,c + T 2,c =[ = V 2,c Using 3.4, 3.8 and 3.19 derive the pressure P 1 (x 1 ) at x 1 in the interior of isolated m 1, yielding: P 1 (x 1 )= [1 ]= [1 ( ) ] ( ) Similarly, in the interior of isolated m 2, P 2 (x 2 )= ( ). Using Figure D, L= cos, R= sin, x=x 1 ( L)=x 1 (1 cos ), =cos -1 (1- ). FIGURE D With o cos -1 (1- ), the collision force [f(x 1 ) 21 acting on m 1 due to collision with m 2 during time t i,c t t i,f, is: [f(x 1 ) 21 = =2 =. Integrating [f(x 1 ) 21 yields: [f(x 1 ) 21 = [ ( -1)sin 2 -cos + cos (sin 2 +2)] = ( ) 2 (1- ) B 1 ( ) 2 where B 1. In vector form, [ (x 1 ) 21 = ( ) 2. Similarly, [f(x 2 ) 12 = ( ) 2 =B 2 where B 2. In vector form, [ (x 2 ) 12 = ( ) 2. The force is created at the collision surface, Fig. B along the line of collision LC, Figure D. Assuming Newton s 3 rd Law [ (x 1 ) 21 = [ (x 2 ) 12 find:
12 (vii a ) = [ ] ( ) 2 for non-identical atoms. Using fig. B, [ (x 1 ) 21 =m 1 = [ (x 2 ) 12 = m as 2 measured from inertial frame S. If [ (x 1 ) 21 =m 1 then from symmetry considerations [ (x 2 ) 12 =m 2 and therefore [ (x 1 ) 21 =m 1 = [ (x 2 ) 12 = m 2 but m 1 = [ ] ( ) 2 m 2 m 2, consequently [ (x 1 ) 21 m 1 and from symmetry considerations, [ (x 2 ) 12 m 2 With [ (x 1 ) 21 = B 1 = B 2 and [ (x 2 ) 12 =B 2 =B 1 find: [ =[ =[ = B 1 similarly, [ =[ =[ = B 2. Setting B 1 = B 2 find x 1 =( ) ( ) ( ) x 2 which is inconsistent with 6.5(vii a ). Therefore if [ (x 1 ) 21 = [ (x 2 ) 12 then [ [. This solution is non-physical as it means there is no connection between the kinetic energy and potential energy of m 1 and the kinetic energy and potential energy of m 2. If however, [ =[ then, 6.5(vii a ) is false and [ (x 1 ) 21 [ (x 2 ) 12 i.e. Newton s 3 rd law is false and with R 1 =R(t) 1, R 2 =R(t) 2 : Considering and to be made of two linearly independent functions and where TKE TKE IKE stands for Translational Kinetic Energy and IKE stands for Internal Kinetic Energy, find: KE(t) 1,c =[ = KE(t) 2,c =[ and T(t) 1,c =[ = V(t) 1,c = T(t) 2,c =[ = V(t) 2,c 6.5(viiia) KE(t) 1,c = KE(t) 2,c 6.5(viiib) T(t) 1,c = V(t) 1,c = T(t) 2,c = V(t) 2,c The total change in energy=0. If photons are created, then the energy to create the photon must be taken into consideration.
13 116 If one assumes that the atoms are perfect spheres during collision, then due to symmetry considerations, the internal momentum of each atom sums to zero. The conservation of momentum equations = m 1 (t) 1 = = m 2 (t) 2 yield: m 1 (t) 1 + m 2 (t) 2 =m 1 (t i,c ) 1 + m 2 (t i,c ) 2 and differentiating yields [ (t) 21 = [ (t) 12 which is false. Consequently the conservation of momentum equations become: 6.5(ix) m 1 (t) 1 + (t) 1 +m 2 (t) 2 + (t) 2 =m 1 (t i,c ) 1 + (t) 1,i + m 2 (t i,c ) 2 + (t) 2,i =const. Where the s represent the internal momentum due to collision caused atom asymmetry and where (t) 1 =, (t) 2 = and [T 1 +[V 1 = <0, [T 2 +[V 2 = <0. Rewrite 6.5(viiia) and 6.5(ix) in linear form. 6.5(xa) m 1 (t) m 2 (t)= m 1 (t i,c ) m 2 (t i,c )=K 1 =const., t i,c t t f,c m 1 (t) 1 (t) m 2 (t)+ 2 (t)=m 1 (t i,c ) 1 (t i,c ) m 2 (t i,c )+ 2 (t i,c )=K 2 =const. It is left as a problem to solve 6.5(xa) in terms of the functions 1 (t) and 2 (t) and the constants, m 1, m 2, K 1, K 2. With 1 (t i,c )=0 and 2 (t i,c )=0, K 2 becomes: K 2 =m 1 (t i,c ) m 2 (t i,c ) Returning to 6.5(viiib). T(t) 1,c = (t)= m 1, T(t) 2,c = (t)= m 1 V(t) 1,c = ( ) ( ), V(t) 2,c = ( ) and writing out 6.5(viiib) yields: 6.5(xb) (t)= ( )= (t)= And in particular, 6.5(xc) 2 (t)= [ ] = [ ] And 1 (t)= ( ) 2 (t) And = =K 3 =const. 6. Creation of a Solid Mass Photon-Energetics of a Tungsten Filament Light Bulb Photons are hypothesized to be solid mass particles produced as collision products of two atoms. Two colliding solid mass atoms, fig. 6.2, compress the atomic mass surface at the contact point resulting in the creation of a Percussive Wave followed by a Shear Wave.
14 Percussive Wave (PW). The collision generated PW with percussive wave speed V p fans out along the surface of the atom from the contact point at A and re converges at point B directly opposite the contact point A, as labeled in figure It is helpful to remember that the atoms in a transition metal solid strike one another with frequency /sec, the molecules in a liquid /sec, and the molecules in a gas at S.T.P /sec. Let E pw represent the total percussive wave energy generated on the surface of atom #1 by collision with atom #2. It is hypothesized that there exists an atomic species dependent threshold energy E ThP such that: a. If E pw E ThP the PW will compress the photon mass m ph at point B as in Fig and 3. b. If E pw <E ThP the PW will not compress the photon mass m ph at point B and E pw will travel back and reconverge at point A. 2. Shear wave (SW). The SW fans out along the surface of the atom starting from the contact point at A with shear wave speed V s and re converges at point B directly opposite the contact point as labeled in figure Typically for macroscopic material solids, 2 ( ) 2 3 and it is hypothesized that for the solid mass atom, 2 ( ) 2 3 also holds. Let E sw represent the total shear wave energy generated on the surface of atom #1 by collision with atom #2. It is hypothesized that there exists an atomic species dependent threshold energy E ThS such that: a. If E sw > E ThS the SW will compress and accelerate the photon mass m ph at point A expelling a solid mass photon with translational kinetic energy m ph v 2, momentum m ph and binding energy. See Fig.6.2-4,5 and 6. b. If E sw E ThS the SW will not compress and accelerate the photon mass m ph at point A. To recapitulate: In order to produce a photon with translational kinetic energy m ph v 2, momentum m ph and binding energy, a collision between atom#1 and atom #2 is required such that atom#1 (The photon emitting atom) is caused to produce shear and percussive wave energies m ph v 2 + =E pw +E sw =E ThP +E ThS, where it is assumed that there is a 100% conversion of E pw +E sw to m ph v 2 + and where E ThP +E ThS = m 1 C 1 =the change in internal energy of atom #1 due to
15 118 collision with atom #2. The case m 1 C 1 E ThP +E ThS is discussed below. As regards the temperature dependence of E ThP +E ThS, see #11 below Note that in order to expel the photon with momentum m ph v, figure 6.2-4, requires that the shear wave exert a net vector force across the ends of the proto-photon in the + direction. In figure 6.2, the open square represents a right circular cylinder which is the uncompressed mass of the proto- photon with mass m ph and the black circle represents the spherical compressed mass of the photon. FIGURE 6.2 Atom #2 Atom #1 5A Average Force Between Two Colliding Atoms The following analysis proceeds on the assumption that this is the physically correct origin of photons. Consider the magnitude of the average force = that two colliding atoms exert on one another during the time c that they are in direct contact with one another. See figure is measured from inertial frame S. Assuming the incident and final velocity of atom #1 is i = U i and f =U f then, is: = =. Assuming the special case U i =U f =, (No photon creation), becomes: 6.6 = In order to derive the speed of sound formula for the transition metals 4.14, it was assumed in chapter 4, section 3 that and consequently: >>. Evaluating R o for tungsten using Table 4.1 yields: R ow (800 o K)=(8.1)10-11 cm and evaluating the inequalities yields: <<(1.1)10-14 sec. and >>(4.3)10-4 dy.
16 119 The average time t r that it takes two colliding atoms to come to rest is t r = c and <<(5.5)10-15 sec. and using c =10-16 sec yields: =(4.7)10-2 dy. During collision, the distance r o is shortened by >0 to (r o ). Figure 6.3. What is the value of assuming the de-acceleration is constant? FIGURE 6.3 ( ) W = U i = c = [(0.58)10 8 ] =(1.9)10-13 cm. Note that ( )= ( )= m(u i ) 2 as required. With p=0 (Constant density), the radius of the proto photon is: =. Using 3.4; The surface density of W with p=0 is 35 and assuming the density of a proto photon is also 35gm/cm 3 and using mph = gm (Derived below): =[ ] =(1.4)10-12 cm= (1.4)10-4 <<r At. Now assume the volume of the proto photon V cyp is the right circular cylinder in figure 6.2 where V cyp = (r cyp ) 2 L cyp =2 (r cyp ) 3. Assuming V cyp = V php where V php is the volume of the spherical proto photon (Chapter 6, section 1), V php = = (1.4) = (1.1)10-35 cm 3 yields: r cyp =(1.2)10-12 cm., A cyp = (1.2) =(4.5)10-24 cm 2, L cyp =(3.0)10-12 cm, V cyp = (1.1)10-35 cm 3. Consider atom #1 after it has been struck by atom #2 and is accelerating in the Let h(h o,t) ph =[h o + (h o,t)] ph represent the position of the center of mass of the proto photon with (h o,0)=0. Figure 6.4a shows the position of the proto photon inside the atom as measured from inertial frame S at rest w.r.t. the center of mass of direction.
17 120 two colliding atoms #1 and #2 only one of which is shown. At t=0, (0) ph =0, (0 + ) ph >0 where (t) ph is the speed of the proto photon at time t. FIGURE 6.4a F =f([h o + + (h o +,t)] ph ) +f([h o + (h o,t)] ph ) represents the sum of the forces acting across the yz faces of the proto photon, (See figure 6.4 and 6.2-4),where f([h o + + (h o +,t)] ph ) 0 and f([h o + (h o,t)] ph ) 0. From 6.5 F =m ph ph. Expand h ph in a power series in t with (0) ph =0, (0) ph =0, (0) ph =0. This yields, h ph =h o +a 2 t 2 +a 4 t , a 2 >0 and approximate h ph by h ph =h o +a 2 t 2 with ph =2a 2 t+4a 4 t3, ph =2a 2 +12a 4 t2 and ph =24a 4 t. Define t f by h(t f ) ph =2r o h o +a 2 and c f 2a 2 and ph 2a 2. Solve for a 2 and t f yielding a 2, t f, F=m ph ph m ph. Table 6.4 lists t f and F as functions of the initial position h o of the center of mass of a proto photon for the special case r o =(1.3) 10-8 cm, (The tungsten atom), at T=(800)( o K). The energy equivalence of T=(800)( o K) is 0.1ev. A-priori the value of E ThP +E ThS is not known. It is assumed that after N collisions with Tungsten atoms (N 10 6 ) the emitted photons are in thermal equilibrium with their parent atoms. Assuming the photon speed at it leaves the atom is c f =(3.0)10 10, the photon mass is m ph =(3.6)10-34 gm with binding energy BE ph 0.1ev. The total energy to produce a photon in thermal equilibrium at T=(800)( o K) is KE ph + BE ph 0.2ev. In general c f (3.0)10 10, and c pheq =(3.0)10 10, is achieved by multiple collisions of the newly created photon with Tungsten atoms and resultant increase in KE ph and decrease in r ph due to compression with resultant increase in BE ph.
18 121 TABLE 6.4 t f (sec) F(dyn) 1 (8.5)10-19 (1.2) (4.3)10-19 (4.8) (8.5)10-20 (1.2) (8.5)10-21 (1.2)10-3 For an analysis of negligible terms missing in the computation of F(dyn), see sec.13, appendix 6A. 5B Over Pressure Necessary to Compress and Accelerate a Photon In the above, the force F necessary to accelerate the photon mass from c o =0 to c f from its point of origin within the tungsten atom to the surface of the atom was derived. In what follows an approximate value to the over pressure P necessary to compress the photon from its original volume to its final volume is derived. For ease of computation, two simplifying assumptions are made. 1. The right cylindrical proto photon of fig. 6.4 is replaced by a sphere, see fig. 6.4b, where the initial volume of the sphere,, is equal to the initial volume of the cylinder; = = (1.1)10-35 cm 3. r php is the radius of the spherical proto photon. 2. While in the atom and being compressed from the spherical proto photon into the photon, the spherical proto photon is in time dependent energy equilibrium with itself + ( )=C(t) 2 <0. See fig. 6.4b. is measured from frame S at rest w.r.t. the center of mass of the spherical proto photon where is the position of mass element m within the proto photon where (,t)=[ + (,t)], (,0)=, 0 r r pph. Also, = ( ) where m= ( ) V. Let P(t) represent the pressure acting in a direction normal to the spherical surface of the proto photon. For a transition metal from 4.6, PV=, however for the proto photon there is no empty space in the proto photon analogous to R o (T) for the transition metals. It is hypothesized that for the proto photon for constants >0 and K 1 >0: PV =K 1 and PdV=K 1 V dv. The work done in compressing a spherical proto photon into a spherical photon is W= = V ( -1) = [(V f ) ( -1) (V i ) ( -1) ] with W >0. Compiling results:
19 122 FIGURE 6.4b 6.7a PV =K 1 >0, W=, 1, >1 6.7b W=P i V i ln Let P i =P(0) W, P f =P(t f ) W, and from above, V i =V php (0)=(1.1)10-35 cm 3. Given T=(800)( o K) with energy equivalence 0.1ev, and assuming that after attaining thermal equilibrium the photon has kinetic energy KE ph =0.1ev with BE ph =0.1ev and using table 6.2 the radius of the photon is r ph (t f )=(7.7)10-25 cm with V f =V ph (t f )=(1.9)10-72 cm 3. Using 4.5, R o (800)=(8.1)10-11 cm and using 4.7, P(0) W =(2.0)1012. Evaluating K 1 for tungsten at t=0: K 1W =P(0) W [V php (0)] =(2.0)10 12 [(1.1)10-35 ] ergcm 3( -1). From 6.7a W= =(2.2)10-23 erg Computed values of W in ev as a function of are listed in table 6.5. TABLE 6.5 W(ev) K 1W (erg) (cm 3( -1) ) 1 (1.2)10-9 (2.2) (6.6)10-7 (7.0) (1.6)10-3 (2.2) (3.4) (1.8) (7.2) (1.8)10 4 (2.3)10-37
20 123 A graph of W as a function of is given in graph 6.1. During the creation of a photon inside an atom on the surface of an incandescent tungsten filament, the force and energy necessary to compress a proto photon into a photon come from the initial percussive and shear wave energy E pw i +E generated by swi collision with adjacent atoms in the tungsten filament. The change in wave energy (E pw +E sw ) necessary to produce a photon is (E pw +E sw )=E pw f +E (E swf pw i +E )<0. Assume the following: swi a. At t=0, two identical tungsten atoms with equal translational KE and equal and opposite momenta collide, and all of the KE of atom #2, see fig. 6.2, goes into producing E pw i +E in atom #1. swi b. All of the initial wave energy E pw i +E, goes into producing a photon so that swi E pw f +E swf =0. Log( W(ev)) GRAPH 6.1- Log Plot T=(800)( o K), KE ph =0.1ev, BE ph =0.1ev, c ph =(3.0)10 10, Then at the instant o at which atom #1 emits a photon, V( ) W =V( ) php =0 and using conservation of energy and momentum yields: m W =( )( m ph )<< m ph.
21 124 At t=0, the two atoms collide and the translational kinetic energy of atom #2, KE(0) W = m W, goes into creating E pw i +E swi so that m W =E pw i +E swi where: E pw i +E swi = m W + m ph m ph C 1,ph m ph W m ph m ph C 1,ph m ph W. Expanding the m ph C 1,ph term: m ph C 1,ph = H( ) H where r phf is the radius of the emitted photon and r php is the radius of the proto photon. Expanding the m ph W term: m ph W = m ph [ (r o ) W (h o ) W ]= [( )((p+3) ( ) p+2 ) 1] where h o is the position of the proto photon at t=0, r o is the radius of the tungsten atom and 3<p 0 where the density (r) of the tungsten atom is (r)= with 0 r r o. Compiling results: 6.8 m W =E pw i +E swi m ph + + [( )((p+3) ( ) p+2 ) 1] Let F(t) T represent the total absolute value of the compressive force acting on the (assumed) spherical tungsten proto photon where F(t) T =P(t) W A php (t). A php (t) is the total area of the proto photon sphere at time t. 6.9 F(t) T =P(t) W A php (t) In what follows, an order of magnitude approximation of F(t) T will be computed. Given that the temperature of an incandescent tungsten filament is T=(800)( o K) with energy equivalence 0.1ev, assume the following: a. After emission by the tungsten atom at t= f and after compression and collision with ~ 10 6 tungsten atoms, the emitted photons have average translational kinetic energy KE ph =0.1ev, average binding energy BE ph = 0.1ev and speed c ph eq =(3)1010. Using m ph (c ph eq ) 2 =0.1ev, yields m ph =(3.6)10-34 gm b. Using BE ph = =0.1ev yields photon final radius r( f ) ph =(7.7)10-25 cm, cross sectional area A( f ) ph = (r( f ) ph ) 2 =(1.8)10-48 cm 2 and volume V( f ) ph = (r( f ) ph ) 3 = (1.9)10-72 cm 3.
22 125 c. Assuming the density of the tungsten atom is constant (p=0), the proto photon at the instant of creation t=0, has the same density as the density of the tungsten atom = 35 =. Solving for yields, =(1.4)10-12 cm, cross sectional area A(0) php = ( ) 2 =(6.1)10-24 cm 2 and volume V(0) php = ( ) 3 = (1.1)10-35 cm 3. Compute P(t) W and F(t) T using PV =K 1W and 6.9. With h o =2r o r(0) ph (See figure 6.4), P(0) W is the average surface pressure on tungsten at t=0. Evaluate P(0) W using 4.7 at (800)( o K) with (Using 4.5), R o (800)=(8.1) This yields: P(0) W ={ }= (2.0) With W=0.2ev and using table 6.5, = P(0) W [V ph (0)] =(2.0)10 12 [(1.1)10-35 ] = (1.8)10-32 erg(cm) 3( -1) and consequently P( f ) W =[(1.8)10-32 ][(1.9)10-72 ] =(4.2)10 58 Tabulated results are listed in table 6.6. t TABLE 6.6 P(t) W ( ) A ph (t)cm 2 F(t) T (dy) V ph (t)cm 3 0 (2)10 12 (6)10-24 (1)10-11 (1)10-35 t f (4)10 58 (2)10-48 (8)10 10 (2) C Light Emission from a Tungsten Filament Consider how many photons per sec, /sec, are emitted by a tungsten filament in a 100W light bulb. Given the dimensions of the filament, Diameter=(2.5)10-2 cm, Length=(3.6)10 2 cm, Surface Area= DL=28cm 2. The cross sectional area of a tungsten atom is (2r At ) 2 = (6.25)10-16 cm 2. The number of atoms on the surface of the filament, # At, is therefore, # At =(28)/(6.25)10-16 =(4.5)10 16 tungsten atoms. Let a o P in be the electric power converted to photon kinetic energy per second by the filament where P in =100W=10 9 erg/sec=(6.2)10 20 ev/sec, and 0<a o <1 as measured from inertial frame S at rest w.r.t. the tungsten filament. Given the temperature of the tungsten filament is 800( o K) with energy equivalence 0.1ev, the number of photons emitted by the filament per sec is therefore: /fil.sec=a o P in /KE ph =
23 126 a o (6.2)10 20 /(0.10)=(6.2)10 21 a o. With a measured efficiency for a tungsten 100W light bulb of a o = energy out /energy in =(2.3)10-2, a 100W bulb emits 2.3W as photons. The /fil.sec=(6.2)10 21 (2.3)10-2 =(1.4)10 20 and the /atomsec=[(1.4)10 20 ]/(4.5)10 16 =(3.1)10 3 or 1 every (3.2)10-4 sec. From chapter 4, section 3: Let represent the average time interval measured from the instant that atom #1 is in contact with atom #2 until the instant that atom #1 is again in contact with atom #2: 2 [2 m] 1/2 /[KT] 1/2. From 4.5 and chapter 4, reference 1: = =(2.5)10-8 [ ]10-2 = (8.3)10-11 cm. and =(17)10-11 [2 (3.1)10-22 ] 1/2 /[(1.4)10-14 (8)] 1/2 =(2.2)10-14 sec. One tungsten atom on the surface of the filament, collides with five of its neighbors at the rate of, =8/ = (3.6)10 14 col.sec -1. Therefore, 1 photon for every tungsten atom is created for every (3.6)10 14 (3.2)10-4 =(1.2)10 11 collisions, so that in terms of the number of collisions necessary to create one photon, the creation of one photon is a very rare process. Let atom #1 be the atom that releases a photon on collision with atom #2. At T=800 o K, how many of those (1.2)10 11 collisions does atom #1 have energy sufficient to create one photon, where E Cr is the energy necessary to create one photon with TrKe= m ph where c f is not necessarily equal to (3) The change in binding energy from proto photon to photon is BE php = and the energy to create a photon is: 6.10 E Cr = + as measured from inertial frame S. See figure 6.4. E Cr goes into compressing the photon and accelerating the photon. Let N represent the number of collisions between atom #1 and its five neighbor atoms and let N E be the number of those collisions whose result is that atom #1 has kinetic energy E as measured from S' at rest with respect to the tungsten filament. N E is given by:
24 N E = Let E Cr be the solution of 6.11 with N E =1, N=(1.2)10 11 and KT=.067ev at T=800 o K This yields E Cr =1.8 ev=(2.9)10-12 erg. Solving for m ph and BE ph using KE ph =0.1ev and assuming c f =(3)1010 yields: m ph =(3.6)10-34 gm=(2.1) amu and BE ph = 1.7ev. Solving for r ph using BE ph = yields: r ph =(4.8)10-26 cm. For simplicity, consider the collision of two isolated atoms. Let atom #2 and atom #1 be on the x-axis with #2 to the left of #1. At t=0 atom #2 collides with atom #1 at the origin and at t=t ph, atom #1 ejects a photon down the positive x-axis as in fig. 6.2 and at time t s the two atoms separate. The origin is the center of mass of the two atoms. Let F(s,t) W1 represent the sum total of percussive, shear and atomic field forces acting on tungsten atom #1 at time t and point s, on and within atom #1, and let F(s,t) W2 represent the sum total of percussive, shear and atomic field forces acting on tungsten atom #2 at time t and point s, on and within atom #2. Consider 0<t pr,i <t pr,f <t sh,i <t sh,f t ph < t s where t pr,i is the time at which the percussive wave starts to compress the protophoton and t pr,f is the time at which the percussive wave finishes compressing the protophoton. t sh,i is the time at which the shear wave starts to compress the protophoton and t sh,f is the time at which the shear wave finishes compressing the protophoton. A photon is ejected by atom #1 at t ph and the 2 atoms separate at t s. With 0 t t s, conservation of momentum yields: = + +{ + }=
25 128 = V W2 is the volume of atom #2 and V W1 is the volume of atom #1. The approximation used in the 3 rd line of 6.13 and 6.14 assumes that the speed of the photon at t=t ph is much larger that the escape speed from the tungsten atom, c(t ph ) ph >>V es. Note that the sum of the momenta at t=0 is in the direction and consequently the sum of the momenta for t>0 is also zero and therefore the and components of each of the two integrals in 6.13 is zero. Conservation of energy yields: 6.14 KE(0) 2 +m 2 C(0) 2 +KE(0) 1 +m 1 C(0) 1 =KE(t) 2 +m 2 C(t) 2 +E(t) 2,W +KE(t) 1 +m 1 C(t) 1 +E(t) 1,W = KE( ) 2 +m 2 C( ) 2 +E( ) 2,W +KE( ) 1 +m 1 C( ) 1 +E( ) 1,W = KE(t ph ) 2 +m 2 C(t ph ) 2 +E(t ph ) 2,W +KE(t ph ) 1 +m 1 C(t ph ) 1 + (t ph ) ph + KE(t s ) 2 +m 2 C(t s ) 2 +KE(t s ) 1 +m 1 C(t s ) 1 + (t s ) ph + Where KE(t) n is the translational kinetic energy, C(t) n is the total internal energy of the n th atom and E(t) n,w is the internal and surface wave energy of the n th atom. The C's are defined by It has been tacitly assumed that KE(0) 1 < BE ph < KE(0) 2 so that atom #2 does not create a photon and atom #1 does create a photon. Using 6.13 with m 2 =m 1 =m At and assuming that all of the translational KE of atom #2 goes into creation of a photon with speed c(t s ) ph : 6.15a V(0) 2 = V(0) 1 V(t s ) 2 +V(t s ) 1 +( )c(t s ) ph and V(t s ) 2 =( ) V(0) 1, V(t s ) 1 =( ) V(0) 1, so that:
26 b V(0) 2 = V(0) 1 +( )c(t s ) ph, V(0) 2 >V(0) 1 And with KE(0) 2 =E Cr 6.16 m At (V(0) [c(t s ) ph ] 2 + The central hypothesis as regards photon production: Each element and isotope at temperature T has the potential to produce photons with unique mass m ph, unique radius r ph and unique c(t s ) ph : If atom #2 has: (i) KE 2,i <, No photon is created by atom #1. (ii) KE 2,i =, 1 photon is created by atom #1 but remains in the atom. (iii) <KE 2,I < + [V es ] 2, If c(t s ) ph < V es, 1 photon is created by atom #1 but is captured by the atom. (iv) KE 2,I = + [V es ] 2, If c(t s ) ph =V es, 1 photon is created by atom #1 and escapes from the atom with c ph =0 at infinity in vacuum. (v) KE 2,i = + [c(t s ) ph ] 2 > + [V es ] 2, 1 photon is created by atom #1 and escapes from the atom with c ph > 0 at infinity in vacuum. (vi) KE 2,i > + [c(t s ) ph ] 2, does not occur being superceded by case (v) If correct, the above hypothesis predicts that atoms at temperature T omit mono energetic photons, however after reflection from ~10 6 atoms, the photons will have a Maxwellian distribution of energies. As regards (i): The collision between atom #2 and #1 is elastic and the initial kinetic energies are exchanged: m At V(0) = m At V(t At ) and m At V(0) = m At V(t At ). The wave pressure P W created at in atom #1, figure 6.2, 1 and 2, is P( ) W < B( ) where B( ) is the bulk modulus of atom #1 at the location of the protophoton. The pressure is too small to compress the proto photon into a photon. As regards (ii) through (v): The collision between atom #2 and #1 is not elastic. The wave pressure P W created at in atom #1, is P( ) W =B( ). The following are true by hypothesis.
27 All photons in a given spectroscopic line have the same momentum m ph c ph but not necessarily the same energy. 2. Photons in two different lines have different m ph c ph but may or may not have the same. 3. All photons in the optical of the same m ph c ph have the same color as determined by a normal human eye but not necessarily the same kinetic energy. 4. All photons in the optical of the same color have the same m ph c ph but not necessarily the same kinetic energy. 5. All photons of the same kinetic energy may have different colors. 6. It follows that two separate lines are of different colors and two separate colors are of different lines. 7. All photons in a given spectroscopic line have the same momentum m ph c ph and will not be separable upon further diffraction using a spectroscopic grating. See figure 6.8. Considering a tungsten light bulb. Tungsten has 5 stable isotopes and each isotope may have a distinct photon kinetic energy spectrum, however (By hypothesis), all atoms of the same tungsten isotope in a tungsten light bulb emit monoenergetic photons with the same momentum. After ~10 6 collisions, this results in a spectroscopic ally determined continuous energy spectrum with mean kinetic energy = KT. As above, solving for m ph assuming that =c o =(3.0)10 10, at 800 o K yields, m ph =(3.6)10-34 gm. By direct computation using 6.11, 75% of these photons will have speeds between 0.45c o and 1.3c o. It is predicted that the speed of red light v R from isotopic-ally pure tungsten is not equal to the speed of violet light v V from isotopic ally pure tungsten. The tungsten filament radiates (1.4)10 20 in the optical. The power lost by the filament in the production of (1.4)10 20 is: P=(1.4)10 20 E Cr =(1.4)10 20 { + }. With E Cr =1.8ev, see 6.12, P=(1.4)10 20 (1.8)=(2.5)10 20 ev/fil.sec=40w/fil. which means that 60W/fil is transformed into heat +infrared photons. The heat is conducted away from the filament by atomic collisions between the tungsten atoms of the filament and the metal light bulb socket and between the tungsten atoms of the filament and the gas surrounding the filament. 7. Creation of Line Spectrum from Solid Mass Photons The failure of classical electrodynamicists to derive the energy density spectrum of the electromagnetic energy emitted by a black body, a kaolin clay block, spawned the creation of quantum mechanics. By considering the atom to be an energy
28 131 quantized harmonic oscillator, Max Planck in December 1900 derived the energy density spectrum of the electromagnetic energy emitted by a kaolin clay block. In 1910 Niels Bohr derived the wavelengths of the discrete electromagnetic spectrum emitted by excited hydrogen, the Balmer Series, from the Bohr-Rutherford model of the atom. And in 1926, Erwin Schr dinger derived the Balmer Series from Schr dinger s Equation. Continuing the development of line and continuous spectrum radiation begun in chapter 6, section 5: A given atom has an internal energy IE=IKE+IPE, as measured from inertial frame S at rest w.r.t. the center of mass of the atom emitting the photon, where IKE is the internal kinetic energy and IPE is the internal potential energy. For the case of an isolated atom with radial symmetry and using 3.15 and 3.28, =0, r o =const. and 6.17 where the subscript o on U, and C refers to the =0 state. For this case, the internal energy IE of the atom is called the internal residual energy IE re as measured from inertial frame S. IE re =IKE re +V o =m At C o where IKE re is the internal residual kinetic energy, IKE re = and V o is the total potential energy,. r o is tabulated in table 4.1. For the 0 case assuming radial symmetry, 3.15 becomes: = =C 1. h o is the instantaneous radius, and r 1 is the mean atomic radius. For this case, IE=IKE+V=m At C 1 where the internal kinetic energy IKE is IKE= and V=. h=r+ (r,t), (r,0)=0, 0 r r 1, and h o h(r 1,t)=r 1 + (r 1,t), (r 1,t) 0, where r 1 = and = 0. The difference in energy IE between the two states is given by: 6.18 IE=IE IE re =m At (C 1 C o )=m At + As examined in chapter 7, charge, ionization and electric current effects are
29 132 hypothesized to be due to IE 0. If IE =0 then there are no charge, ionization or electric current effects. The origin of the photons emitted by a solid, liquid or gas is analyzed according to the energy source responsible for the energy E Cr = + (6.10), necessary to create a photon. The variables that determine the physical properties of the photon are its mass, velocity, and radius and these determine its m ph c f, m ph and creation energy E Cr = +. As used here, the terms Charged atom and Excited Atom mean an atom in radial oscillation i.e. IE 0. See Line Spectra Line spectra from metal electrode excited gas in glass tubes have been observed for over 125 years. It is generally assumed that the reported spectra from a glass tube filled with atom X is: 1. Not due to photons emitted by doped atoms Y in the glass of the glass tube. 2. Not due to excitation of the atoms of the glass tube by direct contact with sputtered metal electrode atoms and the subsequent emission of photons by the atoms of the glass. 3. Not due to secondary excitation of the atoms of the gas or the glass by externally applied photon or high frequency sound sources. In what follows, we will examine the validity of #1 above. The Balmer Series The characteristics of the spectra emitted by hydrogen excited by an electric source coupled to the gas through a conducting electrode, depends on the pressure of the gas, the applied voltage, the geometry of the electrode and the geometry of the confining tube. If for example a modern glass tube filled with hydrogen is emitting a continuous spectrum at 20Atms, by decreasing the pressure, the continuous spectrum will resolve into four discrete bands in the visible. On further reduction of pressure, the bands will resolve into four discrete lines in the visible (Red, green, blue, violet). The Ultraviolet portion of the series (A series of lines with decreasing separation distance in the ultraviolet) has as its source, White Dwarf Stars. This is the Balmer Series. As regards the four discrete lines in the visible. Let S be an inertial frame at rest w.r.t the metal electrodes. Balmer Series photons typically are produced by hydrogen atoms at a temperature of T 350 o K with mean KE=0.045 ev.
30 133 The red light photons emitted by a tungsten 100W light bulb have KE ph,w =0.1 ev with momentum m ph,w c o =(1.1)10-23 and m ph,w =(3.6)10-34 gm. The momentum of the red light photons emitted by tungsten is by hypothesis equal to the momentum of the Balmer Series red light photons: p ph,w = p ph,h and IF the photons comprising the red line have kinetic energy KE ph,h =0.045 ev. their speed c ph,h is c ph,h =(1.3)10 10 with m ph,h =(8.5)10-34 gm. It is generally believed that the red light discharge of the tube is due to excitation of the hydrogen gas by electrons emitted from the cathode. This cannot be the case as a continuous luminous discharge producing the Balmer Series can occur in curvilinear tubes where the emitting gas is: a. separated from both anode and cathode by several layers of glass. b. not on a line of sight between the two electrodes. c. is uniform throughout the length of the glass. i.e. there is no cathode glow. It is hypothesized that the electrodes charge the hydrogen gas by direct contact with the negative electrode and the charge is spread throughout the gas by direct contact between the atoms of the gas. Physically increased charge means increased amplitude of the surface oscillation of the atoms of the gas. When the charge energy on one of the hydrogen atoms in the molecule is E Cr, the hydrogen molecule emits 1 red line photon with momentum p ph,h =(1.1) A-priori neither the speed nor the mass of a red line photon are known and consequently the kinetic energy KE ph,h of a red line photon at this point is not known. As the red glow is uniform throughout the tube, the hydrogen molecule does not pick up E Cr on one contact with the negative electrode. The green, blue and violet line photons of the Balmer Series are by hypothesis created by the adsorption of red line photons by metal atoms doped in the glass (Li, Be, Ca) and the subsequent expulsion of green, blue, and violet photons respectively by a process of optical pumping as discussed in chapter 6, section 7. This can be experimentally determined by: 1 Determining chemically whether or not there are doped atoms in the glass used to create the Balmer Series. 2. Construct a Geissler Tube from chemically pure SiO 2 and if the hypothesis is correct, only the red line will be created. By hypothesis, 1 or more red line photons striking Li atoms produce the green line, 1 or more red line photons striking Be atoms produce the blue line and 1 or more red line photons sriking Ca atoms produce the violet line. The physical dimensions of the cylindrical glass tube as used in an instructional physics laboratory are r GT = cm, L GT =10cm, V=10cm 3. The H 2 pressure in the tube is 10-2 Atm=10 4
31 134 and the number of H 2 molecules N H2 is N H2 =(2.4) computed for T=300 o K. The radius of H, r H is: r H =(0.51) 10-8 cm and the radius of the hydrogen molecule r H2 is r H2 =(1.0) 10-8 cm. The mean free path of a hydrogen molecule is, = = =(3.4) 10-3 cm. The temperature of the H 2 gas when emitting in the optical is 350 o K and the average speed of the H 2 molecule is = =(1.9) The average time t H2 between collisions of H 2 with H 2 is t H2 = =(1.8) 10-8 sec. with collision frequency f= = The radiated power in the optical of a 100W tungsten light bulb is 2.2W, and let the radiated power of the red line emitted outside the luminescent glass tube be represented by P GT. The creation of the Balmer Series red line photons is modeled as follows. Starting with all hydrogen molecules with no excitation energy, at t=0 hydrogen molecule #1 collides with the negative electrode and picks up energy e from the negative electrode. t H2 =(1.8) 10-8 sec. later, e is transferred by direct collision from Hydrogen molecule #1 to Hydrogen molecule #2. The collision process continues until all molecules in the tube have excitation energy e, then 2 e, then 3 e until finally all molecules in the tube have N e=e cr at which time the tube discharges, and all molecules simultaneously emit one red photon with momentum p ph,h =(1.1) With P GT as above, let t ep represent the time interval between the discharge of the tube and the next discharge of the tube: t ep = = ( p ph,h c ph,h )=(1.3)10-5 ( )sec. with a repetition rate f= =(7.7)10 4 ( ). The validity of the expression for t ep can be determined by directly measuring t ep, c ph,h and P GT : e.g. if P GT =10 6 and c ph,h =10 8 then t ep =(1.3)10-3 sec and f=(7.7)10 2. The power in to the tube is represented by P in. Given the cross section of the electrodes is 1cm 2, the number of molecules hitting the electrode per second is
32 n =(1.1)10 22, and each collision takes away e=(0.91)10-22 P in erg. Where P in =5W=(5)10 7 : e=(4.6)10-15 erg. The tube is idealized at time t, to consist of N molecules each at the center of a cube of volume =(4.2)10-18 cm 3, with center to center distance x=(1.61)10-6 cm and cross sectional area ( x) 2 =(2.59)10-12 cm 2. ( x) 2 on each electrode receives (1.1)10 22 ( x) 2 =(2.8)10 10 with (3.6)10-11.and there is a hit in ( x) 2 at t i =(3.6)10-11 i, with i=0,1,2, There are N 1 = =(6.25)10 6 molecules in a line between the two electrodes. With average speed in a given x direction = = (4.8)10 4, if a molecule leaves the electrode at time t i with oscillation energy e, e is approximated to arrive at L o x(i+ ), at arrival time t a i by t ai =t i +(L o x(i+ ))( ) where t i is the time at which a molecule with oscillation energy e leaves the electrode. All N 1 molecules have energy e at time t a N 1 =t N1 +(L o x(n 1 + ))( ) t N1 =(2.25)10-4 sec and N T t N1 = t ep for some N T where =(5.9)10-2 ( ) and with c ph,h and P GT as above, N T =6. The energy to create 1 photon E cr is given by E cr =KE ph + BE ph 2KE ph =p ph,h c ph,h and E cr is also given by E cr =( ) t ep =( )( p ph,h c ph,h ) p ph,h c ph,h with consequent 2. With P in and P GT as above, =50. Note that with P in =(5)10 7 : E cr =(2.75)10-14 erg and as the energy equivalence of T=350 o K is (7.2)10-14 erg, E cr < the equilibrium energy of the gas inside the tube. This is not necessarily impossible; See sec.7. If it should turn out that E cr =(7.2)10-14 erg, with P GT and c ph,h as above, then P in =(1.3)10 8 =13W. As above, the photons comprising the Balmer Series red line have momentum (By hypothesis) equal to that of red light photons emitted by a tungsten 100W light bulb: p ph,h =m ph,h c ph,h =(1.1)10-23 and as above with c ph,h =(1.3)10 10 : m ph,h equals m ph,h =(8.5)10-34, KE ph,h =0.045ev and if BE ph,h =KE ph,h then BE ph,h = = (7.2)10-14 erg, and r ph,h becomes: r ph,h =(1.0)10-23 cm
Chapter 9. Rutherford Scattering, Radioactive Decay, Energetic Atomic Collisions
 260 Chapter 9. Rutherford Scattering, Radioactive Decay, Energetic Atomic Collisions 1. Rutherford Scattering We reexamine Rutherford scattering, (Reference 9.1) with in the context of neutral solid mass
260 Chapter 9. Rutherford Scattering, Radioactive Decay, Energetic Atomic Collisions 1. Rutherford Scattering We reexamine Rutherford scattering, (Reference 9.1) with in the context of neutral solid mass
Chapter 37 Early Quantum Theory and Models of the Atom. Copyright 2009 Pearson Education, Inc.
 Chapter 37 Early Quantum Theory and Models of the Atom Planck s Quantum Hypothesis; Blackbody Radiation Photon Theory of Light and the Photoelectric Effect Energy, Mass, and Momentum of a Photon Compton
Chapter 37 Early Quantum Theory and Models of the Atom Planck s Quantum Hypothesis; Blackbody Radiation Photon Theory of Light and the Photoelectric Effect Energy, Mass, and Momentum of a Photon Compton
CHAPTER 3 The Experimental Basis of Quantum Theory
 CHAPTER 3 The Experimental Basis of Quantum Theory 3.1 3.2 3.3 3.4 3.5 3.6 3.7 3.8 3.9 Discovery of the X Ray and the Electron Determination of Electron Charge Line Spectra Quantization As far as I can
CHAPTER 3 The Experimental Basis of Quantum Theory 3.1 3.2 3.3 3.4 3.5 3.6 3.7 3.8 3.9 Discovery of the X Ray and the Electron Determination of Electron Charge Line Spectra Quantization As far as I can
is the minimum stopping potential for which the current between the plates reduces to zero.
 Module 1 :Quantum Mechanics Chapter 2 : Introduction to Quantum ideas Introduction to Quantum ideas We will now consider some experiments and their implications, which introduce us to quantum ideas. The
Module 1 :Quantum Mechanics Chapter 2 : Introduction to Quantum ideas Introduction to Quantum ideas We will now consider some experiments and their implications, which introduce us to quantum ideas. The
Earlier we learned that hot, opaque objects produce continuous spectra of radiation of different wavelengths.
 Section7: The Bohr Atom Earlier we learned that hot, opaque objects produce continuous spectra of radiation of different wavelengths. Continuous Spectrum Everyone has seen the spectrum produced when white
Section7: The Bohr Atom Earlier we learned that hot, opaque objects produce continuous spectra of radiation of different wavelengths. Continuous Spectrum Everyone has seen the spectrum produced when white
Stellar Astrophysics: The Interaction of Light and Matter
 Stellar Astrophysics: The Interaction of Light and Matter The Photoelectric Effect Methods of electron emission Thermionic emission: Application of heat allows electrons to gain enough energy to escape
Stellar Astrophysics: The Interaction of Light and Matter The Photoelectric Effect Methods of electron emission Thermionic emission: Application of heat allows electrons to gain enough energy to escape
PSI AP Physics How was it determined that cathode rays possessed a negative charge?
 PSI AP Physics 2 Name Chapter Questions 1. How was it determined that cathode rays possessed a negative charge? 2. J. J. Thomson found that cathode rays were really particles, which were subsequently named
PSI AP Physics 2 Name Chapter Questions 1. How was it determined that cathode rays possessed a negative charge? 2. J. J. Thomson found that cathode rays were really particles, which were subsequently named
Quantum Mechanics. Particle in a box All were partial answers, leading Schrödinger to wave mechanics
 Chemistry 4521 Time is flying by: only 15 lectures left!! Six quantum mechanics Four Spectroscopy Third Hour exam Three statistical mechanics Review Final Exam, Wednesday, May 4, 7:30 10 PM Quantum Mechanics
Chemistry 4521 Time is flying by: only 15 lectures left!! Six quantum mechanics Four Spectroscopy Third Hour exam Three statistical mechanics Review Final Exam, Wednesday, May 4, 7:30 10 PM Quantum Mechanics
Nicholas J. Giordano. Chapter 29. Atomic Theory. Marilyn Akins, PhD Broome Community College
 Nicholas J. Giordano www.cengage.com/physics/giordano Chapter 29 Atomic Theory Marilyn Akins, PhD Broome Community College Atomic Theory Matter is composed of atoms Atoms are assembled from electrons,
Nicholas J. Giordano www.cengage.com/physics/giordano Chapter 29 Atomic Theory Marilyn Akins, PhD Broome Community College Atomic Theory Matter is composed of atoms Atoms are assembled from electrons,
Paper 2. Section B : Atomic World
 Paper 2 Section B : Atomic World Q.2 Multiple-choice questions A B C D 2.1 25.19 15.78 9.18 49.68 2.2 25.79 20.39 41.97 11.72 2.3 18.35 9.76 48.84 22.65 2.4 9.27 18.87 27.90 43.50 2.5 63.47 4.28 10.99
Paper 2 Section B : Atomic World Q.2 Multiple-choice questions A B C D 2.1 25.19 15.78 9.18 49.68 2.2 25.79 20.39 41.97 11.72 2.3 18.35 9.76 48.84 22.65 2.4 9.27 18.87 27.90 43.50 2.5 63.47 4.28 10.99
CHAPTER 27 Quantum Physics
 CHAPTER 27 Quantum Physics Units Discovery and Properties of the Electron Planck s Quantum Hypothesis; Blackbody Radiation Photon Theory of Light and the Photoelectric Effect Energy, Mass, and Momentum
CHAPTER 27 Quantum Physics Units Discovery and Properties of the Electron Planck s Quantum Hypothesis; Blackbody Radiation Photon Theory of Light and the Photoelectric Effect Energy, Mass, and Momentum
Quantum Physics and Atomic Models Chapter Questions. 1. How was it determined that cathode rays possessed a negative charge?
 Quantum Physics and Atomic Models Chapter Questions 1. How was it determined that cathode rays possessed a negative charge? 2. J. J. Thomson found that cathode rays were really particles, which were subsequently
Quantum Physics and Atomic Models Chapter Questions 1. How was it determined that cathode rays possessed a negative charge? 2. J. J. Thomson found that cathode rays were really particles, which were subsequently
1 The Cathode Rays experiment is associated. with: Millikan A B. Thomson. Townsend. Plank Compton
 1 The Cathode Rays experiment is associated with: A B C D E Millikan Thomson Townsend Plank Compton 1 2 The electron charge was measured the first time in: A B C D E Cathode ray experiment Photoelectric
1 The Cathode Rays experiment is associated with: A B C D E Millikan Thomson Townsend Plank Compton 1 2 The electron charge was measured the first time in: A B C D E Cathode ray experiment Photoelectric
The... of a particle is defined as its change in position in some time interval.
 Distance is the. of a path followed by a particle. Distance is a quantity. The... of a particle is defined as its change in position in some time interval. Displacement is a.. quantity. The... of a particle
Distance is the. of a path followed by a particle. Distance is a quantity. The... of a particle is defined as its change in position in some time interval. Displacement is a.. quantity. The... of a particle
Particle Detectors and Quantum Physics (2) Stefan Westerhoff Columbia University NYSPT Summer Institute 2002
 Particle Detectors and Quantum Physics (2) Stefan Westerhoff Columbia University NYSPT Summer Institute 2002 More Quantum Physics We know now how to detect light (or photons) One possibility to detect
Particle Detectors and Quantum Physics (2) Stefan Westerhoff Columbia University NYSPT Summer Institute 2002 More Quantum Physics We know now how to detect light (or photons) One possibility to detect
Chapter 6. Quantum Theory and the Electronic Structure of Atoms Part 1
 Chapter 6 Quantum Theory and the Electronic Structure of Atoms Part 1 The nature of light Quantum theory Topics Bohr s theory of the hydrogen atom Wave properties of matter Quantum mechanics Quantum numbers
Chapter 6 Quantum Theory and the Electronic Structure of Atoms Part 1 The nature of light Quantum theory Topics Bohr s theory of the hydrogen atom Wave properties of matter Quantum mechanics Quantum numbers
Chapter 39. Particles Behaving as Waves
 Chapter 39 Particles Behaving as Waves 39.1 Electron Waves Light has a dual nature. Light exhibits both wave and particle characteristics. Louis de Broglie postulated in 1924 that if nature is symmetric,
Chapter 39 Particles Behaving as Waves 39.1 Electron Waves Light has a dual nature. Light exhibits both wave and particle characteristics. Louis de Broglie postulated in 1924 that if nature is symmetric,
I. Multiple Choice Questions (Type-I)
 I. Multiple Choice Questions (Type-I) 1. Which of the following conclusions could not be derived from Rutherford s α -particle scattering experiement? (i) Most of the space in the atom is empty. (ii) The
I. Multiple Choice Questions (Type-I) 1. Which of the following conclusions could not be derived from Rutherford s α -particle scattering experiement? (i) Most of the space in the atom is empty. (ii) The
Chapter 28. Atomic Physics
 Chapter 28 Atomic Physics Quantum Numbers and Atomic Structure The characteristic wavelengths emitted by a hot gas can be understood using quantum numbers. No two electrons can have the same set of quantum
Chapter 28 Atomic Physics Quantum Numbers and Atomic Structure The characteristic wavelengths emitted by a hot gas can be understood using quantum numbers. No two electrons can have the same set of quantum
THE EDUCARE (SIROHI CLASSES) TEST SERIES 2018
 THE EDUCARE (SIROHI CLASSES) TEST SERIES 2018 XII PHYSICS TEST MODERN PHYSICS NAME-... DATE-.. MM- 25 TIME-1 HR 1) Write one equation representing nuclear fusion reaction. (1) 2) Arrange radioactive radiations
THE EDUCARE (SIROHI CLASSES) TEST SERIES 2018 XII PHYSICS TEST MODERN PHYSICS NAME-... DATE-.. MM- 25 TIME-1 HR 1) Write one equation representing nuclear fusion reaction. (1) 2) Arrange radioactive radiations
Quantum and Atomic Physics - Multiple Choice
 PSI AP Physics 2 Name 1. The Cathode Ray Tube experiment is associated with: (A) J. J. Thomson (B) J. S. Townsend (C) M. Plank (D) A. H. Compton 2. The electron charge was measured the first time in: (A)
PSI AP Physics 2 Name 1. The Cathode Ray Tube experiment is associated with: (A) J. J. Thomson (B) J. S. Townsend (C) M. Plank (D) A. H. Compton 2. The electron charge was measured the first time in: (A)
1. What is the minimum energy required to excite a mercury atom initially in the ground state? ev ev ev
 Page 1 of 10 modern bank Name 25-MAY-05 1. What is the minimum energy required to excite a mercury atom initially in the ground state? 1. 4.64 ev 3. 10.20 ev 2. 5.74 ev 4. 10.38 ev 2. The diagram represents
Page 1 of 10 modern bank Name 25-MAY-05 1. What is the minimum energy required to excite a mercury atom initially in the ground state? 1. 4.64 ev 3. 10.20 ev 2. 5.74 ev 4. 10.38 ev 2. The diagram represents
Early Quantum Theory and Models of the Atom
 Early Quantum Theory and Models of the Atom Electron Discharge tube (circa 1900 s) There is something ( cathode rays ) which is emitted by the cathode and causes glowing Unlike light, these rays are deflected
Early Quantum Theory and Models of the Atom Electron Discharge tube (circa 1900 s) There is something ( cathode rays ) which is emitted by the cathode and causes glowing Unlike light, these rays are deflected
1) Introduction 2) Photo electric effect 3) Dual nature of matter 4) Bohr s atom model 5) LASERS
 1) Introduction 2) Photo electric effect 3) Dual nature of matter 4) Bohr s atom model 5) LASERS 1. Introduction Types of electron emission, Dunnington s method, different types of spectra, Fraunhoffer
1) Introduction 2) Photo electric effect 3) Dual nature of matter 4) Bohr s atom model 5) LASERS 1. Introduction Types of electron emission, Dunnington s method, different types of spectra, Fraunhoffer
Modern Physics for Scientists and Engineers International Edition, 4th Edition
 Modern Physics for Scientists and Engineers International Edition, 4th Edition http://optics.hanyang.ac.kr/~shsong Review: 1. THE BIRTH OF MODERN PHYSICS 2. SPECIAL THEORY OF RELATIVITY 3. THE EXPERIMENTAL
Modern Physics for Scientists and Engineers International Edition, 4th Edition http://optics.hanyang.ac.kr/~shsong Review: 1. THE BIRTH OF MODERN PHYSICS 2. SPECIAL THEORY OF RELATIVITY 3. THE EXPERIMENTAL
Chapter 5 Light and Matter
 Chapter 5 Light and Matter Stars and galaxies are too far for us to send a spacecraft or to visit (in our lifetimes). All we can receive from them is light But there is much we can learn (composition,
Chapter 5 Light and Matter Stars and galaxies are too far for us to send a spacecraft or to visit (in our lifetimes). All we can receive from them is light But there is much we can learn (composition,
QUANTUM MECHANICS AND MOLECULAR SPECTROSCOPY
 QUANTUM MECHANICS AND MOLECULAR SPECTROSCOPY CHEM 330 B. O. Owaga Classical physics Classical physics is based on three assumptions i. Predicts precise trajectory for particles with precisely specified
QUANTUM MECHANICS AND MOLECULAR SPECTROSCOPY CHEM 330 B. O. Owaga Classical physics Classical physics is based on three assumptions i. Predicts precise trajectory for particles with precisely specified
AP Physics B Summer Assignment
 BERGEN COUNTY TECHNICAL SCHOOL AP Physics B Summer Assignment 2011 Solve all problems on separate paper. This will be due the first week of school. If you need any help you can e-mail Mr. Zavorotniy at
BERGEN COUNTY TECHNICAL SCHOOL AP Physics B Summer Assignment 2011 Solve all problems on separate paper. This will be due the first week of school. If you need any help you can e-mail Mr. Zavorotniy at
ATOMIC SPECTRA. Objective:
 1 ATOMIC SPECTRA Objective: To measure the wavelengths of visible light emitted by atomic hydrogen and verify the measured wavelengths against those predicted by quantum theory. To identify an unknown
1 ATOMIC SPECTRA Objective: To measure the wavelengths of visible light emitted by atomic hydrogen and verify the measured wavelengths against those predicted by quantum theory. To identify an unknown
Physics 1C Lecture 29A. Finish off Ch. 28 Start Ch. 29
 Physics 1C Lecture 29A Finish off Ch. 28 Start Ch. 29 Particle in a Box Let s consider a particle confined to a one-dimensional region in space. Following the quantum mechanics approach, we need to find
Physics 1C Lecture 29A Finish off Ch. 28 Start Ch. 29 Particle in a Box Let s consider a particle confined to a one-dimensional region in space. Following the quantum mechanics approach, we need to find
CHAPTER 3 The Experimental Basis of Quantum
 CHAPTER 3 The Experimental Basis of Quantum 3.1 Discovery of the X Ray and the Electron 3.2 Determination of Electron Charge 3.3 Line Spectra 3.4 Quantization 3.5 Blackbody Radiation 3.6 Photoelectric
CHAPTER 3 The Experimental Basis of Quantum 3.1 Discovery of the X Ray and the Electron 3.2 Determination of Electron Charge 3.3 Line Spectra 3.4 Quantization 3.5 Blackbody Radiation 3.6 Photoelectric
X-Rays from Atoms. These are called K α X-rays See table 29.1 for the energy of K α X-rays produced by some elements. Section 29.3
 X-Rays from Atoms The highest photon energy available in a hydrogen atom is in the ultraviolet part of the electromagnetic spectrum Other atoms can emit much more energetic photons larger Z, more electric
X-Rays from Atoms The highest photon energy available in a hydrogen atom is in the ultraviolet part of the electromagnetic spectrum Other atoms can emit much more energetic photons larger Z, more electric
Preview. Atomic Physics Section 1. Section 1 Quantization of Energy. Section 2 Models of the Atom. Section 3 Quantum Mechanics
 Atomic Physics Section 1 Preview Section 1 Quantization of Energy Section 2 Models of the Atom Section 3 Quantum Mechanics Atomic Physics Section 1 TEKS The student is expected to: 8A describe the photoelectric
Atomic Physics Section 1 Preview Section 1 Quantization of Energy Section 2 Models of the Atom Section 3 Quantum Mechanics Atomic Physics Section 1 TEKS The student is expected to: 8A describe the photoelectric
The Photoelectric Effect
 Stellar Astrophysics: The Interaction of Light and Matter The Photoelectric Effect Methods of electron emission Thermionic emission: Application of heat allows electrons to gain enough energy to escape
Stellar Astrophysics: The Interaction of Light and Matter The Photoelectric Effect Methods of electron emission Thermionic emission: Application of heat allows electrons to gain enough energy to escape
Observation of Atomic Spectra
 Observation of Atomic Spectra Introduction In this experiment you will observe and measure the wavelengths of different colors of light emitted by atoms. You will first observe light emitted from excited
Observation of Atomic Spectra Introduction In this experiment you will observe and measure the wavelengths of different colors of light emitted by atoms. You will first observe light emitted from excited
Ch 7 Quantum Theory of the Atom (light and atomic structure)
 Ch 7 Quantum Theory of the Atom (light and atomic structure) Electromagnetic Radiation - Electromagnetic radiation consists of oscillations in electric and magnetic fields. The oscillations can be described
Ch 7 Quantum Theory of the Atom (light and atomic structure) Electromagnetic Radiation - Electromagnetic radiation consists of oscillations in electric and magnetic fields. The oscillations can be described
CHAPTER 3 Prelude to Quantum Theory. Observation of X Rays. Thomson s Cathode-Ray Experiment. Röntgen s X-Ray Tube
 CHAPTER Prelude to Quantum Theory.1 Discovery of the X Ray and the Electron. Determination of Electron Charge. Line Spectra.4 Quantization.5 Blackbody Radiation.6 Photoelectric Effect.7 X-Ray Production.8
CHAPTER Prelude to Quantum Theory.1 Discovery of the X Ray and the Electron. Determination of Electron Charge. Line Spectra.4 Quantization.5 Blackbody Radiation.6 Photoelectric Effect.7 X-Ray Production.8
General Physics (PHY 2140)
 General Physics (PHY 140) Lecture 33 Modern Physics Atomic Physics Atomic spectra Bohr s theory of hydrogen http://www.physics.wayne.edu/~apetrov/phy140/ Chapter 8 1 Lightning Review Last lecture: 1. Atomic
General Physics (PHY 140) Lecture 33 Modern Physics Atomic Physics Atomic spectra Bohr s theory of hydrogen http://www.physics.wayne.edu/~apetrov/phy140/ Chapter 8 1 Lightning Review Last lecture: 1. Atomic
Course Name: AP Physics. Team Names: Jon Collins. Velocity Acceleration Displacement
 Course Name: AP Physics Team Names: Jon Collins 1 st 9 weeks Objectives Vocabulary 1. NEWTONIAN MECHANICS and lab skills: Kinematics (including vectors, vector algebra, components of vectors, coordinate
Course Name: AP Physics Team Names: Jon Collins 1 st 9 weeks Objectives Vocabulary 1. NEWTONIAN MECHANICS and lab skills: Kinematics (including vectors, vector algebra, components of vectors, coordinate
Chapter Six: X-Rays. 6.1 Discovery of X-rays
 Chapter Six: X-Rays 6.1 Discovery of X-rays In late 1895, a German physicist, W. C. Roentgen was working with a cathode ray tube in his laboratory. He was working with tubes similar to our fluorescent
Chapter Six: X-Rays 6.1 Discovery of X-rays In late 1895, a German physicist, W. C. Roentgen was working with a cathode ray tube in his laboratory. He was working with tubes similar to our fluorescent
Chapter 1. From Classical to Quantum Mechanics
 Chapter 1. From Classical to Quantum Mechanics Classical Mechanics (Newton): It describes the motion of a classical particle (discrete object). dp F ma, p = m = dt dx m dt F: force (N) a: acceleration
Chapter 1. From Classical to Quantum Mechanics Classical Mechanics (Newton): It describes the motion of a classical particle (discrete object). dp F ma, p = m = dt dx m dt F: force (N) a: acceleration
Explain how Planck resolved the ultraviolet catastrophe in blackbody radiation. Calculate energy of quanta using Planck s equation.
 Objectives Explain how Planck resolved the ultraviolet catastrophe in blackbody radiation. Calculate energy of quanta using Planck s equation. Solve problems involving maximum kinetic energy, work function,
Objectives Explain how Planck resolved the ultraviolet catastrophe in blackbody radiation. Calculate energy of quanta using Planck s equation. Solve problems involving maximum kinetic energy, work function,
Planck s Quantum Hypothesis Blackbody Radiation
 Planck s Quantum Hypothesis Blackbody Radiation The spectrum of blackbody radiation has been measured(next slide); it is found that the frequency of peak intensity increases linearly with temperature.
Planck s Quantum Hypothesis Blackbody Radiation The spectrum of blackbody radiation has been measured(next slide); it is found that the frequency of peak intensity increases linearly with temperature.
E n = n h ν. The oscillators must absorb or emit energy in discrete multiples of the fundamental quantum of energy given by.
 Planck s s Radiation Law Planck made two modifications to the classical theory The oscillators (of electromagnetic origin) can only have certain discrete energies determined by E n = n h ν with n is an
Planck s s Radiation Law Planck made two modifications to the classical theory The oscillators (of electromagnetic origin) can only have certain discrete energies determined by E n = n h ν with n is an
Exam 2 Development of Quantum Mechanics
 PHYS40 (Spring 00) Riq Parra Exam # (Friday, April 1 th, 00) Exam Development of Quantum Mechanics Do NOT write your name on this exam. Write your class ID number on the top right hand corner of each problem
PHYS40 (Spring 00) Riq Parra Exam # (Friday, April 1 th, 00) Exam Development of Quantum Mechanics Do NOT write your name on this exam. Write your class ID number on the top right hand corner of each problem
Physics 1C Lecture 29B
 Physics 1C Lecture 29B Emission Spectra! The easiest gas to analyze is hydrogen gas.! Four prominent visible lines were observed, as well as several ultraviolet lines.! In 1885, Johann Balmer, found a
Physics 1C Lecture 29B Emission Spectra! The easiest gas to analyze is hydrogen gas.! Four prominent visible lines were observed, as well as several ultraviolet lines.! In 1885, Johann Balmer, found a
Chapter 9: Quantization of Light
 Chapter 9: Quantization of Light Max Planck started the revolution of quantum theory by challenging the classical physics and the classical wave theory of light. He proposed the concept of quantization
Chapter 9: Quantization of Light Max Planck started the revolution of quantum theory by challenging the classical physics and the classical wave theory of light. He proposed the concept of quantization
Lecture PowerPoints. Chapter 27 Physics: Principles with Applications, 7th edition Giancoli
 Lecture PowerPoints Chapter 27 Physics: Principles with Applications, 7th edition Giancoli This work is protected by United States copyright laws and is provided solely for the use of instructors in teaching
Lecture PowerPoints Chapter 27 Physics: Principles with Applications, 7th edition Giancoli This work is protected by United States copyright laws and is provided solely for the use of instructors in teaching
Properties of Electromagnetic Radiation Chapter 5. What is light? What is a wave? Radiation carries information
 Concepts: Properties of Electromagnetic Radiation Chapter 5 Electromagnetic waves Types of spectra Temperature Blackbody radiation Dual nature of radiation Atomic structure Interaction of light and matter
Concepts: Properties of Electromagnetic Radiation Chapter 5 Electromagnetic waves Types of spectra Temperature Blackbody radiation Dual nature of radiation Atomic structure Interaction of light and matter
UNIT : QUANTUM THEORY AND THE ATOM
 Name St.No. Date(YY/MM/DD) / / Section UNIT 102-10: QUANTUM THEORY AND THE ATOM OBJECTIVES Atomic Spectra for Hydrogen, Mercury and Neon. 1. To observe various atomic spectra with a diffraction grating
Name St.No. Date(YY/MM/DD) / / Section UNIT 102-10: QUANTUM THEORY AND THE ATOM OBJECTIVES Atomic Spectra for Hydrogen, Mercury and Neon. 1. To observe various atomic spectra with a diffraction grating
Einstein. Quantum Physics at a glance. Planck s Hypothesis (blackbody radiation) (ultraviolet catastrophe) Quantized Energy
 Quantum Physics at a glance Quantum Physics deals with the study of light and particles at atomic and smaller levels. Planck s Hypothesis (blackbody radiation) (ultraviolet catastrophe) Quantized Energy
Quantum Physics at a glance Quantum Physics deals with the study of light and particles at atomic and smaller levels. Planck s Hypothesis (blackbody radiation) (ultraviolet catastrophe) Quantized Energy
Chapter 27 Early Quantum Theory and Models of the Atom Discovery and Properties of the electron
 Chapter 27 Early Quantum Theory and Models of the Atom 27-1 Discovery and Properties of the electron Measure charge to mass ratio e/m (J. J. Thomson, 1897) When apply magnetic field only, the rays are
Chapter 27 Early Quantum Theory and Models of the Atom 27-1 Discovery and Properties of the electron Measure charge to mass ratio e/m (J. J. Thomson, 1897) When apply magnetic field only, the rays are
Chapter 38 and Chapter 39
 Chapter 38 and Chapter 39 State of 19th and very early 20th century physics: Light: 1. E&M Maxwell s equations > waves; J. J. Thompson s double slit experiment with light 2. Does light need a medium? >
Chapter 38 and Chapter 39 State of 19th and very early 20th century physics: Light: 1. E&M Maxwell s equations > waves; J. J. Thompson s double slit experiment with light 2. Does light need a medium? >
Chapter 7. The Quantum- Mechanical Model of the Atom. Chapter 7 Lecture Lecture Presentation. Sherril Soman Grand Valley State University
 Chapter 7 Lecture Lecture Presentation Chapter 7 The Quantum- Mechanical Model of the Atom Sherril Soman Grand Valley State University The Beginnings of Quantum Mechanics Until the beginning of the twentieth
Chapter 7 Lecture Lecture Presentation Chapter 7 The Quantum- Mechanical Model of the Atom Sherril Soman Grand Valley State University The Beginnings of Quantum Mechanics Until the beginning of the twentieth
THE NATURE OF THE ATOM. alpha particle source
 chapter THE NATURE OF THE ATOM www.tutor-homework.com (for tutoring, homework help, or help with online classes) Section 30.1 Rutherford Scattering and the Nuclear Atom 1. Which model of atomic structure
chapter THE NATURE OF THE ATOM www.tutor-homework.com (for tutoring, homework help, or help with online classes) Section 30.1 Rutherford Scattering and the Nuclear Atom 1. Which model of atomic structure
Radiation - Electromagnetic Waves (EMR): wave consisting of oscillating electric and magnetic fields that move at the speed of light through space.
 Radiation - Electromagnetic Waves (EMR): wave consisting of oscillating electric and magnetic fields that move at the speed of light through space. Photon: a quantum of light or electromagnetic wave. Quantum:
Radiation - Electromagnetic Waves (EMR): wave consisting of oscillating electric and magnetic fields that move at the speed of light through space. Photon: a quantum of light or electromagnetic wave. Quantum:
The Franck-Hertz Experiment David Ward The College of Charleston Phys 370/Experimental Physics Spring 1997
 The Franck-Hertz Experiment David Ward The College of Charleston Phys 370/Experimental Physics Spring 1997 Abstract One of the most important experiments supporting quantum theory is the Franck- Hertz
The Franck-Hertz Experiment David Ward The College of Charleston Phys 370/Experimental Physics Spring 1997 Abstract One of the most important experiments supporting quantum theory is the Franck- Hertz
Photoelectric Effect
 Photoelectric Effect The ejection of electrons from a surface by the action of light striking that surface is called the photoelectric effect. In this experiment, as you investigate the photoelectric effect,
Photoelectric Effect The ejection of electrons from a surface by the action of light striking that surface is called the photoelectric effect. In this experiment, as you investigate the photoelectric effect,
Electronic structure of atoms
 Chapter 1 Electronic structure of atoms light photons spectra Heisenberg s uncertainty principle atomic orbitals electron configurations the periodic table 1.1 The wave nature of light Much of our understanding
Chapter 1 Electronic structure of atoms light photons spectra Heisenberg s uncertainty principle atomic orbitals electron configurations the periodic table 1.1 The wave nature of light Much of our understanding
Chapter 28. Atomic Physics
 Chapter 28 Atomic Physics Sir Joseph John Thomson J. J. Thomson 1856-1940 Discovered the electron Did extensive work with cathode ray deflections 1906 Nobel Prize for discovery of electron Early Models
Chapter 28 Atomic Physics Sir Joseph John Thomson J. J. Thomson 1856-1940 Discovered the electron Did extensive work with cathode ray deflections 1906 Nobel Prize for discovery of electron Early Models
Because light behaves like a wave, we can describe it in one of two ways by its wavelength or by its frequency.
 Light We can use different terms to describe light: Color Wavelength Frequency Light is composed of electromagnetic waves that travel through some medium. The properties of the medium determine how light
Light We can use different terms to describe light: Color Wavelength Frequency Light is composed of electromagnetic waves that travel through some medium. The properties of the medium determine how light
EXPERIMENT 5. The Franck-Hertz Experiment (Critical Potentials) Introduction
 EXPERIMENT 5 The Franck-Hertz Experiment (Critical Potentials) Introduction In the early part of the twentieth century the structure of the atom was studied in depth. In the process of developing and refining
EXPERIMENT 5 The Franck-Hertz Experiment (Critical Potentials) Introduction In the early part of the twentieth century the structure of the atom was studied in depth. In the process of developing and refining
LECTURE # 17 Modern Optics Matter Waves
 PHYS 270-SPRING 2011 LECTURE # 17 Modern Optics Matter Waves April 5, 2011 1 Spectroscopy: Unlocking the Structure of Atoms There are two types of spectra, continuous spectra and discrete spectra: Hot,
PHYS 270-SPRING 2011 LECTURE # 17 Modern Optics Matter Waves April 5, 2011 1 Spectroscopy: Unlocking the Structure of Atoms There are two types of spectra, continuous spectra and discrete spectra: Hot,
NYS STANDARD/KEY IDEA/PERFORMANCE INDICATOR 5.1 a-e. 5.1a Measured quantities can be classified as either vector or scalar.
 INDICATOR 5.1 a-e September Unit 1 Units and Scientific Notation SI System of Units Unit Conversion Scientific Notation Significant Figures Graphical Analysis Unit Kinematics Scalar vs. vector Displacement/dis
INDICATOR 5.1 a-e September Unit 1 Units and Scientific Notation SI System of Units Unit Conversion Scientific Notation Significant Figures Graphical Analysis Unit Kinematics Scalar vs. vector Displacement/dis
1P22/1P92 Exam Review Problems 2013 Friday, January 14, :03 AM. Chapter 20
 Exam Review Problems 2011 Page 1 1P22/1P92 Exam Review Problems 2013 Friday, January 14, 2011 10:03 AM Chapter 20 True or false? 1 It's impossible to place a charge on an insulator, because no current
Exam Review Problems 2011 Page 1 1P22/1P92 Exam Review Problems 2013 Friday, January 14, 2011 10:03 AM Chapter 20 True or false? 1 It's impossible to place a charge on an insulator, because no current
LECTURE # 19 Dennis Papadopoulos End of Classical Physics Quantization Bohr Atom Chapters 38 39
 PHYS 270-SPRING 2011 LECTURE # 19 Dennis Papadopoulos End of Classical Physics Quantization Bohr Atom Chapters 38 39 April 14, 2011 1 HOW TO MEASURE SPECTRA Spectroscopy: Unlocking the Structure of Atoms
PHYS 270-SPRING 2011 LECTURE # 19 Dennis Papadopoulos End of Classical Physics Quantization Bohr Atom Chapters 38 39 April 14, 2011 1 HOW TO MEASURE SPECTRA Spectroscopy: Unlocking the Structure of Atoms
A fluorescent tube is filled with mercury vapour at low pressure. After mercury atoms have been excited they emit photons.
 Q1.(a) A fluorescent tube is filled with mercury vapour at low pressure. After mercury atoms have been excited they emit photons. In which part of the electromagnetic spectrum are these photons? What is
Q1.(a) A fluorescent tube is filled with mercury vapour at low pressure. After mercury atoms have been excited they emit photons. In which part of the electromagnetic spectrum are these photons? What is
Semiconductor Physics and Devices
 Introduction to Quantum Mechanics In order to understand the current-voltage characteristics, we need some knowledge of electron behavior in semiconductor when the electron is subjected to various potential
Introduction to Quantum Mechanics In order to understand the current-voltage characteristics, we need some knowledge of electron behavior in semiconductor when the electron is subjected to various potential
Absorber Alpha emission Alpha particle Atom. Atomic line spectra Atomic mass unit Atomic number Atomic structure. Background radiation
 Material that prevent radioactive emission from passing through it Release of alpha particle from unstable nucleus(a 2+ helium ion or a helium nucleus) The nucleus of a helium atom (two protons and two
Material that prevent radioactive emission from passing through it Release of alpha particle from unstable nucleus(a 2+ helium ion or a helium nucleus) The nucleus of a helium atom (two protons and two
2008 Brooks/Cole 2. Frequency (Hz)
 Electromagnetic Radiation and Matter Oscillating electric and magnetic fields. Magnetic field Electric field Chapter 7: Electron Configurations and the Periodic Table Traveling wave moves through space
Electromagnetic Radiation and Matter Oscillating electric and magnetic fields. Magnetic field Electric field Chapter 7: Electron Configurations and the Periodic Table Traveling wave moves through space
ASTRO Fall 2012 LAB #7: The Electromagnetic Spectrum
 ASTRO 1050 - Fall 2012 LAB #7: The Electromagnetic Spectrum ABSTRACT Astronomers rely on light to convey almost all of the information we have on distant astronomical objects. In addition to measuring
ASTRO 1050 - Fall 2012 LAB #7: The Electromagnetic Spectrum ABSTRACT Astronomers rely on light to convey almost all of the information we have on distant astronomical objects. In addition to measuring
We also find the development of famous Schrodinger equation to describe the quantization of energy levels of atoms.
 Lecture 4 TITLE: Quantization of radiation and matter: Wave-Particle duality Objectives In this lecture, we will discuss the development of quantization of matter and light. We will understand the need
Lecture 4 TITLE: Quantization of radiation and matter: Wave-Particle duality Objectives In this lecture, we will discuss the development of quantization of matter and light. We will understand the need
The energy of the emitted light (photons) is given by the difference in energy between the initial and final states of hydrogen atom.
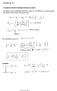 Lecture 20-21 Page 1 Lectures 20-21 Transitions between hydrogen stationary states The energy of the emitted light (photons) is given by the difference in energy between the initial and final states of
Lecture 20-21 Page 1 Lectures 20-21 Transitions between hydrogen stationary states The energy of the emitted light (photons) is given by the difference in energy between the initial and final states of
Name Final Exam December 7, 2015
 Name Final Exam December 7, 015 This test consists of five parts. Please note that in parts II through V, you can skip one question of those offered. Part I: Multiple Choice (mixed new and review questions)
Name Final Exam December 7, 015 This test consists of five parts. Please note that in parts II through V, you can skip one question of those offered. Part I: Multiple Choice (mixed new and review questions)
Prof. Jeff Kenney Class 5 June 1, 2018
 www.astro.yale.edu/astro120 Prof. Jeff Kenney Class 5 June 1, 2018 to understand how we know stuff about the universe we need to understand: 1. the spectral analysis of light 2. how light interacts with
www.astro.yale.edu/astro120 Prof. Jeff Kenney Class 5 June 1, 2018 to understand how we know stuff about the universe we need to understand: 1. the spectral analysis of light 2. how light interacts with
Models of the Atom. Spencer Clelland & Katelyn Mason
 Models of the Atom Spencer Clelland & Katelyn Mason First Things First Electrons were accepted to be part of the atom structure by scientists in the1900 s. The first model of the atom was visualized as
Models of the Atom Spencer Clelland & Katelyn Mason First Things First Electrons were accepted to be part of the atom structure by scientists in the1900 s. The first model of the atom was visualized as
Chapter 27. Quantum Physics
 Chapter 27 Quantum Physics Need for Quantum Physics Problems remained from classical mechanics that relativity didn t explain Blackbody Radiation The electromagnetic radiation emitted by a heated object
Chapter 27 Quantum Physics Need for Quantum Physics Problems remained from classical mechanics that relativity didn t explain Blackbody Radiation The electromagnetic radiation emitted by a heated object
AP PHYSICS 1 Learning Objectives Arranged Topically
 AP PHYSICS 1 Learning Objectives Arranged Topically with o Big Ideas o Enduring Understandings o Essential Knowledges o Learning Objectives o Science Practices o Correlation to Knight Textbook Chapters
AP PHYSICS 1 Learning Objectives Arranged Topically with o Big Ideas o Enduring Understandings o Essential Knowledges o Learning Objectives o Science Practices o Correlation to Knight Textbook Chapters
We start with a reminder of a few basic concepts in probability. Let x be a discrete random variable with some probability function p(x).
 1 Probability We start with a reminder of a few basic concepts in probability. 1.1 discrete random variables Let x be a discrete random variable with some probability function p(x). 1. The Expectation
1 Probability We start with a reminder of a few basic concepts in probability. 1.1 discrete random variables Let x be a discrete random variable with some probability function p(x). 1. The Expectation
Particle nature of light & Quantization
 Particle nature of light & Quantization A quantity is quantized if its possible values are limited to a discrete set. An example from classical physics is the allowed frequencies of standing waves on a
Particle nature of light & Quantization A quantity is quantized if its possible values are limited to a discrete set. An example from classical physics is the allowed frequencies of standing waves on a
PHYS General Physics II Lab The Balmer Series for Hydrogen Source. c = speed of light = 3 x 10 8 m/s
 PHYS 1040 - General Physics II Lab The Balmer Series for Hydrogen Source Purpose: The purpose of this experiment is to analyze the emission of light from a hydrogen source and measure and the wavelengths
PHYS 1040 - General Physics II Lab The Balmer Series for Hydrogen Source Purpose: The purpose of this experiment is to analyze the emission of light from a hydrogen source and measure and the wavelengths
The Franck-Hertz Experiment Physics 2150 Experiment No. 9 University of Colorado
 Experiment 9 1 Introduction The Franck-Hertz Experiment Physics 2150 Experiment No. 9 University of Colorado During the late nineteenth century, a great deal of evidence accumulated indicating that radiation
Experiment 9 1 Introduction The Franck-Hertz Experiment Physics 2150 Experiment No. 9 University of Colorado During the late nineteenth century, a great deal of evidence accumulated indicating that radiation
PRACTICE QUESTION PAPER WITH SOLUTION CLASS XI PHYSICS
 PRACTICE QUESTION PAPER WITH SOLUTION CLASS XI PHYSICS. A given quantity has both magnitude and direction. Is it necessarily a vector? Justify your answer.. What is the rotational analogue of the force?.
PRACTICE QUESTION PAPER WITH SOLUTION CLASS XI PHYSICS. A given quantity has both magnitude and direction. Is it necessarily a vector? Justify your answer.. What is the rotational analogue of the force?.
PHYS 4 CONCEPT PACKET Complete
 PHYS 4 CONCEPT PACKET Complete Written by Jeremy Robinson, Head Instructor Find Out More +Private Instruction +Review Sessions WWW.GRADEPEAK.COM Need Help? Online Private Instruction Anytime, Anywhere
PHYS 4 CONCEPT PACKET Complete Written by Jeremy Robinson, Head Instructor Find Out More +Private Instruction +Review Sessions WWW.GRADEPEAK.COM Need Help? Online Private Instruction Anytime, Anywhere
General Physics (PHY 2140)
 General Physics (PHY 2140) Lecture 27 Modern Physics Quantum Physics Blackbody radiation Plank s hypothesis http://www.physics.wayne.edu/~apetrov/phy2140/ Chapter 27 1 Quantum Physics 2 Introduction: Need
General Physics (PHY 2140) Lecture 27 Modern Physics Quantum Physics Blackbody radiation Plank s hypothesis http://www.physics.wayne.edu/~apetrov/phy2140/ Chapter 27 1 Quantum Physics 2 Introduction: Need
Photoelectric Effect Experiment
 Experiment 1 Purpose The photoelectric effect is a key experiment in modern physics. In this experiment light is used to excite electrons that (given sufficient energy) can escape from a material producing
Experiment 1 Purpose The photoelectric effect is a key experiment in modern physics. In this experiment light is used to excite electrons that (given sufficient energy) can escape from a material producing
The Emission Spectra of Light
 The Emission Spectra of Light Objectives: Theory: 1.... measured the wavelength limits of the color bands in the visible spectrum, 2.... measured the wavelengths of the emission lines of the hydrogen Balmer
The Emission Spectra of Light Objectives: Theory: 1.... measured the wavelength limits of the color bands in the visible spectrum, 2.... measured the wavelengths of the emission lines of the hydrogen Balmer
Physics 9e/Cutnell. correlated to the. College Board AP Physics 2 Course Objectives
 correlated to the College Board AP Physics 2 Course Objectives Big Idea 1: Objects and systems have properties such as mass and charge. Systems may have internal structure. Enduring Understanding 1.A:
correlated to the College Board AP Physics 2 Course Objectives Big Idea 1: Objects and systems have properties such as mass and charge. Systems may have internal structure. Enduring Understanding 1.A:
Classical and Planck picture. Planck s constant. Question. Quantum explanation for the Wein Effect.
 6.1 Quantum Physics. Particle Nature of Light Particle nature of Light Blackbody Radiation Photoelectric Effect Properties of photons Ionizing radiation Radiation damage x-rays Compton effect X-ray diffraction
6.1 Quantum Physics. Particle Nature of Light Particle nature of Light Blackbody Radiation Photoelectric Effect Properties of photons Ionizing radiation Radiation damage x-rays Compton effect X-ray diffraction
Chapter 37 Early Quantum Theory and Models of the Atom
 Chapter 37 Early Quantum Theory and Models of the Atom Units of Chapter 37 37-7 Wave Nature of Matter 37-8 Electron Microscopes 37-9 Early Models of the Atom 37-10 Atomic Spectra: Key to the Structure
Chapter 37 Early Quantum Theory and Models of the Atom Units of Chapter 37 37-7 Wave Nature of Matter 37-8 Electron Microscopes 37-9 Early Models of the Atom 37-10 Atomic Spectra: Key to the Structure
Chapter 5 Electrons In Atoms
 Chapter 5 Electrons In Atoms 5.1 Revising the Atomic Model 5.2 Electron Arrangement in Atoms 5.3 Atomic Emission Spectra and the Quantum Mechanical Model 1 Copyright Pearson Education, Inc., or its affiliates.
Chapter 5 Electrons In Atoms 5.1 Revising the Atomic Model 5.2 Electron Arrangement in Atoms 5.3 Atomic Emission Spectra and the Quantum Mechanical Model 1 Copyright Pearson Education, Inc., or its affiliates.
PHYS 3313 Section 001 Lecture #14
 PHYS 3313 Section 001 Lecture #14 Monday, March 6, 2017 The Classic Atomic Model Bohr Radius Bohr s Hydrogen Model and Its Limitations Characteristic X-ray Spectra 1 Announcements Midterm Exam In class
PHYS 3313 Section 001 Lecture #14 Monday, March 6, 2017 The Classic Atomic Model Bohr Radius Bohr s Hydrogen Model and Its Limitations Characteristic X-ray Spectra 1 Announcements Midterm Exam In class
Downloaded from
 Chapter 13 (Kinetic Theory) Q1. A cubic vessel (with face horizontal + vertical) contains an ideal gas at NTP. The vessel is being carried by a rocket which is moving at a speed of500 ms in vertical direction.
Chapter 13 (Kinetic Theory) Q1. A cubic vessel (with face horizontal + vertical) contains an ideal gas at NTP. The vessel is being carried by a rocket which is moving at a speed of500 ms in vertical direction.
Atom Physics. Chapter 30. DR JJ UiTM-Cutnell & Johnson 7th ed. 1. Model of an atom-the recent model. Nuclear radius r m
 Chapter 30 Atom Physics DR JJ UiTM-Cutnell & Johnson 7th ed. 1 30.1 Rutherford Scattering and the Nuclear Atom Model of an atom-the recent model Nuclear radius r 10-15 m Electron s position radius r 10-10
Chapter 30 Atom Physics DR JJ UiTM-Cutnell & Johnson 7th ed. 1 30.1 Rutherford Scattering and the Nuclear Atom Model of an atom-the recent model Nuclear radius r 10-15 m Electron s position radius r 10-10
UNIVERSITY OF SASKATCHEWAN Department of Physics and Engineering Physics
 UNIVERSITY OF SASKATCHEWAN Department of Physics and Engineering Physics Physics 115.3 Physics and the Universe FINAL EXAMINATION December 9, 011 NAME: (Last) Please Print (Given) Time: 3 hours STUDENT
UNIVERSITY OF SASKATCHEWAN Department of Physics and Engineering Physics Physics 115.3 Physics and the Universe FINAL EXAMINATION December 9, 011 NAME: (Last) Please Print (Given) Time: 3 hours STUDENT
ATOMIC STRUCTURE, ELECTRONS, AND PERIODICITY
 ATOMIC STRUCTURE, ELECTRONS, AND PERIODICITY All matter is made of atoms. There are a limited number of types of atoms; these are the elements. (EU 1.A) Development of Atomic Theory Atoms are so small
ATOMIC STRUCTURE, ELECTRONS, AND PERIODICITY All matter is made of atoms. There are a limited number of types of atoms; these are the elements. (EU 1.A) Development of Atomic Theory Atoms are so small
Discussion Review Test #2. Units 12-19: (1) (2) (3) (4) (5) (6)
 Discussion Review Test #2 Units 12-19: (1) (2) (3) (4) (5) (6) (7) (8) (9) Galileo used his observations of the changing phases of Venus to demonstrate that a. the sun moves around the Earth b. the universe
Discussion Review Test #2 Units 12-19: (1) (2) (3) (4) (5) (6) (7) (8) (9) Galileo used his observations of the changing phases of Venus to demonstrate that a. the sun moves around the Earth b. the universe
Chapter 8: Electrons in Atoms Electromagnetic Radiation
 Chapter 8: Electrons in Atoms Electromagnetic Radiation Electromagnetic (EM) radiation is a form of energy transmission modeled as waves moving through space. (see below left) Electromagnetic Radiation
Chapter 8: Electrons in Atoms Electromagnetic Radiation Electromagnetic (EM) radiation is a form of energy transmission modeled as waves moving through space. (see below left) Electromagnetic Radiation
Sharif University of Technology Physics Department. Modern Physics Spring 2016 Prof. Akhavan
 Sharif University of Technology Physics Department Modern Physics Spring 2016 Prof. Akhavan Problems Set #5. Due on: 03 th of April / 15 th of Farvardin. 1 Blackbody Radiation. (Required text book is Modern
Sharif University of Technology Physics Department Modern Physics Spring 2016 Prof. Akhavan Problems Set #5. Due on: 03 th of April / 15 th of Farvardin. 1 Blackbody Radiation. (Required text book is Modern
Physics Homework Set I Su2015
 1) The particles which enter into chemical reactions are the atom's: 1) _ A) protons. B) positrons. C) mesons. D) electrons. E) neutrons. 2) Which of the following type of electromagnetic radiation has
1) The particles which enter into chemical reactions are the atom's: 1) _ A) protons. B) positrons. C) mesons. D) electrons. E) neutrons. 2) Which of the following type of electromagnetic radiation has
