-1/2 +1/2 -1/2 +1/2. σ - σ + g. g +1/2 -1/2
|
|
|
- Madison Jackson
- 5 years ago
- Views:
Transcription
1 LECTURE 9 Aside: Maxwell Velocity Distribution Before we talk about the laser cooling of atoms, I just want to mention that if you have a gas of atoms in equilibrium, and you treat them as classical particles, then they will have Maxwell{Boltzmann statistics ( e,e ). In particular, their energies are kinetic energies (E = 2 mv2 ), so that their velocity distribution is given by P (v) =Ce,mv2 =2 () where = =k B T and C is a constant that can be determined from the normalization condition. Notice that the velocity distribution is a Gaussian centered at v = (the gas isn't going anywhere in particular) and that a range of velocities are represented. Laser Cooling (References: Nobel lectures of Chu, Cohen{Tannoudji, and Phillips, Rev. Mod. Phys. 7, 685{742 (998).) Lasers are reminiscent of the ray guns that one sees in science ction movies. As we mentioned in the last lecture, a laser with less power than a typical light bulb can burn a hole through a metal plate. However, in recent years, lasers have been used, not to heat, but to cool atomic gases to the lowest temperatures that man has been able to achieve: afewnanokelvin above absolute zero. Iwant to describe some the tricks that have been devised to accomplish this cooling. These developments are the basis of the 997 Nobel prize in physics which was awarded to Steven Chu, Claude N. Cohen{Tannoudji, and William Phillips. We mentioned in lecture 4 that light can apply radiation pressure to matter. This gives comets their tails. Microscopically when an atom absorbs a photon of energy h, it will receive a momentum impulse h=c along the direction of the incoming photon ~p in (p in = h=c). In order to absorb a photon of frequency, the atom must have an allowed transition between 2 energy levels where E = h. If the atom emits a photon with momentum ~p out, the atom will recoil in the opposite direction. Thus the atom experiences a net momentum change ~p atom = ~p in, ~p out due to this incoherent scattering process. Since the scattered photon has no preferred direction, the net eect is due to the absorbed photons, resulting in a scattering force ~F scatt = N~p in, where N is the number of photons scattered per second. Typical scattering rates for atoms excited by a laser tuned to a strong resonance line are on the order of 7 to 8 /sec. As an example, the velocity ofa sodium atom changes by 3 cm/sec per absorbed photon. The scattering force can be 5 times the gravitational acceleration on earth, feeble compared to electromagnetic forces on charged particles, but stronger than any other long{range force that aects neutral particles. Doppler Cooling Recall how the doppler shift works. If you move along a beam of light toward its source with velocity v, the light is blue shifted. This is because the distance a wave travels in one period T of oscillation is ct while the distance you move in one period is vt. You can think of vt as the amount by which awavelength is squeezed. The doppler
2 shifted wavelength is = ct, vt =(c, v)t = c, v Notice that this is shorter (bluer) than the original wavelength. frequency is higher and is given by = c = c c, v (2) The doppler shifted If you move away from the source of light, then the light is red shifted. Just change v!,v in the above equations to see that the wavelength gets longer and the frequency drops. The red shift of the spectral lines from stars and galaxies is how we know that the universe is expanding. Now let's apply this to laser cooling. Take 2identical laser beams counterpropagating in opposite directions. Let's put some atoms in the beams' path and tune the lasers below the atomic resonance. If the atoms are standing still, they won't absorb any photons because the frequency is less than what is needed for electrons to make a transition to a higher energy level. But a moving atom doppler shifts the beam opposing its motion closer to resonance and shifts the beam co{propagating with the motion away from resonance. So chances are that it will absorb more photons from the beam opposing its motion than from the beam in the direction of its motion. Thus the atom will experience a net force opposing its motion. By slowing the atoms down, we are cooling them. In the limit where atoms are moving slowly enough so that the dierence in the absorption due to the Doppler eect is linearly proportional to the velocity, this force will result in viscous damping ~F =,~v. We can upgrade to 6 laser beams pointed at the origin, with each pair of counterpropagating beams along one of the coordinate axes x, y, and z. There is also a heating eect due to the random kicks an atom receives by randomly scattering photons from counterpropagating beams that surround the atoms. Scattering consists of absorbing a photon and then emitting another photon in some random direction and recoiling as a result. Balancing the eects of heating and cooling, one gets an estimate of how cold the atoms can get. For alkali atoms the temperature is of order K. Not only does the laser light cool the atoms, it also tends to conne them. An atom receiving random kicks in a viscous medium is analogous to the Brownian motion of a dust particle immersed in water. In the laser case, the viscous medium is a sea of photons which have been dubbed \optical molasses." The rst experiments showing that optical molasses works was done by Steve Chu and his collaborators. They achieved a temperature of 85 K. Sisyphus Cooling K may sound pretty cold, but laser cooling can get even colder thanks to the Sisyphus eect. To understand this eect, let's rst consider the following case of optical pumping. It uses resonant excitation of atoms by circularly polarized light for transferring to the atoms part of the angular momentum carried by the light beam. It is based on the fact that dierent Zeeman sublevels in the atomic ground state have in general (3) 2
3 dierent absorption rates for incoming polarized light. (Notation: +,polarized light has positive helicity and the photons carry + angular momentum. If you face the oncoming wave, the polarization vector rotates counterclockwise and the wave is left{ circularly polarized. Similarly,,polarized light has negative helicity, the photons carry, angular momentum, and it is right{circularly polarized. polarized light is linearly polarized light which is a linear combination of + and, and carries no net angular momentum.) e e -/2 +/2 σ - σ + g -/2 g +/2 For example, for a J g = =2 $ J e = =2 transition, only atoms in the sublevel M g =,=2 can absorb +,polarized light. They are excited into the sublevel M e =+=2 of J e ==2 from which they can fall back into the sublevel M g =+=2 by spontaneous emission of a linearly polarized photon. They then remain trapped in this state because no further +,transition can take place. It is possible in this way to obtain high degrees of spin orientation in atomic ground states. Because of this coupling between the M g =,=2 sublevel and the excited state via + photons, the energy of the M g =,=2 sublevel is shifted. Similarly M g = +=2 atoms can absorb,,polarized light and be excited into the M e =,=2 sublevel from which they can fall back into the sublevel M g =,=2 by spontaneous emission of a linearly polarized photon. Thus the M g = +=2 sublevel has its energy shifted. σ - σ + g -/2 g +/2 Now consider a laser conguration consisting of 2 counterpropagating plane waves along the z axis, with orthogonal linear polarizations and with the same frequency and the same intensity. Because the phase shift between the 2 waves increases linearly with z, the polarization of the total eld changes from + to, and vice versa every =4. In between it is elliptical or linear. Consider now the simple case where the atomic ground state has an angular momentum J g = =2. The two Zeeman sublevels M g = =2 undergo dierent energy level shifts (called \light shifts") depending on the laser polarization, so that the Zeeman degeneracy in zero magnetic eld is removed. This gives the energy diagram showing spatial modulations of the Zeeman splitting between the two sublevels with a period =2. When the atom absorbs a photon followed by spontaneous emission of a photon, optical pumping transfers between the two sublevels occurs with the direction depending on the polarization: M g =,=2! M g =+=2for + polarization, M g =+=2! M g = 3
4 ,=2 for, polarization. Here also, the spatial modulation of the laser polarization results in a spatial modulation of the optical pumping rates with a period of =2. σ + σ - σ + beam polarization beam polarization λ/4 λ/4 z g -/2 g +/2 Spatial modulation of the light polarization results in correlation between the spatial modulation of light shifts and optical pumping rates. By properly tuning the frequency of the laser light, optical pumping always transfers atoms from the higher Zeeman sublevel to the lower one. One always loses energy. Suppose now that the atom is moving to the right, starting from the bottom of an energy valley, for example in the state M g =+=2 at a place where the polarization is +. As the atom climbs the potential energy hill, its kinetic energy is converted into potential energy. At the top of the hill it has the maximum probability to be optically pumped into the lower sublevel, i.e., the bottom of the energy valley. And the process starts all over again. This is like the story of Sisyphus in Greek mythology, who was condemned by the gods to roll the stone up the hill, only to have it roll back down before he reached the top. He would then have to start all over again. Dissipation occurs because the spontaneously emitted photon has an energy higher than the absorbed photon. (This is an example of an anti{stokes Raman process.) So the atom is always losing energy and hence is being cooled. Using Sisyphus cooling, temperature of a few K can be achieved. Cooling is further aided by the fact that one can arrange it so that when an atom has velocity v ', it no longer absorbs light, and so won't be heated by photon absorption. This is called subrecoil laser cooling. (In case you're wondering why an atom at the top of the hill (in the M g =+=2, say) doesn't make a direct transition into the valley (to the M g =,=2, say) and avoid the trouble of making an intermediate stop in the excited state, it's because the density of 4
5 nal states is too small. Recall Fermi's Golden Rule from lecture 6: R f i = 2 h j <fjhji >j2 N(E) (4) where N(E) is the density of nal states with energy E. Recall from lecture that the density of photon states N(!)! 2. The energy dierence between M g = +=2 and M g =,=2 is very small, so the density of nal photon states is very tiny as well. This makes the transition rate very small.) Raman Eect In our discussion of the Sisyphus eect, we saw an example where the atom went from one state to another via an excited state. The atom absorbed a photon of frequency, made a transition from state to an excited state, then spontaneously emitted another photon of frequency and made a transition down to state 2. The frequency dierence between the absorbed and emitted photons is, = (E, E 2 )=h. The scenario we have just described is called the Raman eect. Raman scattering refers to the process where the incident and emitted photons have a frequency dierence that corresponds to a characteristic frequency of the atom, molecule, or solid. In some cases the frequency dierence corresponds to a phonon (vibrational quantum) or, for molecules, to a characteristic rotational frequency. Bose{Einstein Condensation and Superuidity (Reference: Robert B. Leighton, Principles of Modern Physics, McGraw{Hill (959).) Interesting things happen at very low temperatures and Bose{Einstein condensation is one of them. Recall that there is no statistical limit to the number bosons that can occupy a single state. In a Bose condensed state, an appreciable fraction of the particles is in the lowest energy level at temperatures below T C. These particles are in the same state and can be described by the same wavefunction. In other words a macroscopic number of particles are in one coherent state. (We saw this in the case of the photons in a laser beam.) If we write = j je i, then this state is described by a given phase. The oldest known physical manifestation of Bose condensation is superuid 4 He. A 4 He atom has total angular momentum zero and is therefore a boson. At T C = 2:8 K liquid helium becomes superuid. The transition temperature is called the,point because the shape of the specic heat curve at T C is shaped like. One cools liquid helium by pumping on it to get rid of the hot atoms (evaporative cooling). It boils a little. Then at the transition it boils vigorously and suddenly stops. Eisberg and Resnick has a picture of this on page 43. The reason for this behavior is that the thermal conductivity increases by a factor of about 6 at the transition, so that the superuid is no longer able to sustain a temperature gradient. To make a bubble, heat has to locally vaporize the uid and make itmuch hotter than the surrounding uid. This is no longer possible in the superuid state. Perhaps the hallmark of a superuid is that it has no viscosity. As a result the superuid can ow through tiny capillary tubes that normal liquid can't get through. Superuid 4 He is often described byatwo{uid model, i.e., it is thought of as consisting of 5
6 2 uids, one of which is normal and the other is superuid. It's the superuid component which is able to ow through the capillary tube. So if you use this method to measure the coecient of viscosity, you nd that it suddenly drops to zero at the,point. One can see the eect of both components by putting a torsional oscillator consisting of a stack thin, light, closely spaced mica disks immersed in the liquid. If the liquid has a high viscosity, the liquid between the disks is dragged along and contributes signicantly to the moment of inertia of the disks. If the viscosity is small, the moment of inertia is more nearly equal to that of the disks alone. Using this method, no discontinuity is found in the coecient of viscosity at the,point. Another weird thing that superuid helium does is escape from a beaker by crawling up the sides, owing down the outside, and dripping o the bottom. The helium atoms are attracted by thevan der Waals forces of the walls of the container, and they are able to ow up the walls because of the lack of viscosity. The rate of ow can be 3 cm per second or more. The superuid helium can surmount quite a high wall, on the order of several meters in height. (Brief aside to explain the van der Waals force: As an electron moves in a molecule, there exists at any instant of time a separation of positive and negative charge in the molecule. The latter has, therefore, an electric dipole moment p which varies in time. If another molecule exists nearby, it will have a dipole moment induced by the rst molecule. These two dipoles are attracted to each other. This is the van der Waals force.) We can show mathematically that there is a macrosopic population of the lowest energy state in the following way. Consider a gas of noninteracting bosons. Let the energy levels be measured from the lowest energy level, i.e., let the zero point energy be the zero of energy. Then the chemical potential must be negative, otherwise the Bose{Einstein distribution would be negative for some of the levels. Recall from lecture 3 that the Bose{Einstein distribution gives the average number of particles in state s: <n s >= e (Es,), is adjusted so that the total number of particles is N: (5) X s <n s > (6) Let me give a sneak preview: If we assume a continuous distribution of states, starts out negative and gets bigger as the temperature decreases. equals its upper limit of zero at some temperature T C,belowwhichwe can no longer satisfy (6) because the right hand side can't deliver enough particles. This leads us to treat the lowest energy level separately and we nd that we can satisfy (6) by keeping the extra particles we need in the lowest state. Now let's do the math. In order to turn the sum over s in (6) into an integral, let's assume a continuous density of states. In lecture we found that if k{space is isotropic, 6
7 i.e., the same in every direction, then the number of states in a spherical shell lying between radii k and k + dk is k dk = V (2) 3 (4k2 dk) = V 2 2 k2 dk (7) Now if the energy of the bosons is purely kinetic energy and continuous, then and or Also (8) implies that Plugging () and () into (7) yields E = h2 k 2 2m de = h2 kdk m (8) (9) kdk = mde h 2 () k = p 2mE h () E de = V m 3 2! =2 E =2 de (2) 2 3 h So we can rewrite (6) as Z e (E,), EdE = V m 3 2 h 3 2! =2 Z E =2 e (E,), de Now recall that for a geometric series So X p= e,p(e,) = e (E,), =, e,(e,) e (E,) = e (E,) Plugging this into (3) leads to V m 3 2 h 3 2! =2 Z, e,(e,) (3) E =2 e,(e,) 7 X p= X p= e,p(e,) (4) e,p(e,) de (5)
8 Let x = E. Then V m 3 2 h 3 2 Let s = p + and y = sx. Then V m 3 2 h 3 2 The denition of a gamma function is! =2 (k B T ) 3=2 X p=! =2 (k B T ) 3=2 X,(n) = Z Z e (p+) x =2 e,(p+)x dx (6) s= e Z s y =2 e,y dy (7) s 3=2 y n, e,y dy n> (8) So Z y =2 e,y dy =,( 3 2 ) (9) Thus V! m 3 =2,( 3 2 h )(k BT ) 3=2 X s=! e s s 3=2 Now on the right hand side, as the temperature T decreases, must increase to keep the product constant and equal to N. T can be made as small as we wish, but, which we said must be negative, cannot be greater than zero. But the product must be a constant. So (2) is only valid above a certain critical temperature T C. Below this temperature, our treatment breaks down. Where did we go wrong? The aw lies in the fact that we assumed that the states are continuously distributed. However, since we are interested in very low temperatures which involves the occupation of the lowest lying energy levels, we may expect that the actual discrete nature of the level distribution might play an essential role in the lowest temperature range. So let us treat the lowest level E = separately. We will assume that it is not degenerate with any other levels and we will assume that the remaining levels are continuously distributed from E =to E = as described by (2). So in our summation (6) we will treat the lowest level separately: (2) = e,, + V m 3 2 h 3 2! =2 Z E =2 e (E,), de e,, + T T C 3=2 f()n (2) (22) where the second quantity on the right is just the right hand side of (2), written in terms of the critical temperature T C. f( =)=because T C is dened such that the right hand sideof(2)equalsn with T = T C and =. 8
9 We see that it is now possible to satisfy this new equation with negative values of for all temperatures, since the rst term becomes innite as!. The inclusion of the lowest energy level as a separate term in our treatment has thus removed the previous diculty of not being able to account for all of the particles at temperatures below T C. If we now inquire into what this equation means physically, we see that, at temperatures below T C, the chemical potential will take on such values that those particles which are not included in the continuous distribution will be found in the lowest level. That is, a kind of condensation occurs; it is such that an appreciable fraction of the particles is in the lowest energy level at temperatures below T C. If we write (22) as T 3=2 n + f()n (23) TC and realize that f( ) at low temperatures, then we nd the population n of the lowest level to be, approximately, n = N " T 3=2 #, TC (24) At T = T C, n = while at T =, n = N. Using n = (e,, ),, we nd that at low temperatures =,k B T ln + (25) n Notice that is negative. As T!,! : (T! )!, + ln( N +)! as N!and T! (26) Considering superuid helium as a 2 component uid with normal and superuid components is consistent with having some of the particles in the lowest energy level and the rest in higher energy levels. There is no microscopic theory of superuid helium, though computer simulations by Ceperley have been quite successful in reproducing its properties. One of the complications is that the helium atoms are so closely packed that they are strongly interacting; they're in a liquid state. It would be closer to the ideal case to have a system of bosons which are weakly interacting. (Reference: H.{J. Miesner and W. Ketterle, \Bose{Einstein Condensation in Dilute Atomic Gases," Solid State Communications 7, 629 (998) and references therein.) This has recently been achieved in the case of alkali atoms such as rubidium, sodium, and lithium. Using a combination of optical and magnetic traps together with laser cooling and evaporative cooling, several research groups have achieved Bose condensation in dilute weakly interacting vapors of alkali atoms. In these systems the thermal debroglie wavelength exceeds the mean distance between atoms. Nanokelvin temperatures and densities of 5 cm,3 have been achieved. (Compare this to a mole of liquid which has a typical density of 23 cm,3.) At nanokelvin temperatures the thermal debroglie 9
10 wavelength exceeds m which is about times the average spacing between atoms. In these experiments they have actually been able to directly observe the macroscopic population of the zero momentum eigenstate. In addition the coherence resulting from being in macroscopic wavefunctions has been demonstrated by observing the interference between two independent condensates. Two spatially separated condensates were released from the magnetic trap and allowed to overlap during ballistic expansion of the gases. Interference patterns were observed that are analogous to the pattern produced in a double{slit experiment in optics.
The amazing story of Laser Cooling and Trapping
 The amazing story of Laser Cooling and Trapping following Bill Phillips Nobel Lecture http://www.nobelprize.org/nobel_prizes/physics/ laureates/1997/phillips-lecture.pdf Laser cooling of atomic beams 1
The amazing story of Laser Cooling and Trapping following Bill Phillips Nobel Lecture http://www.nobelprize.org/nobel_prizes/physics/ laureates/1997/phillips-lecture.pdf Laser cooling of atomic beams 1
THEORETICAL PROBLEM 2 DOPPLER LASER COOLING AND OPTICAL MOLASSES
 THEORETICAL PROBLEM 2 DOPPLER LASER COOLING AND OPTICAL MOLASSES The purpose of this problem is to develop a simple theory to understand the so-called laser cooling and optical molasses phenomena. This
THEORETICAL PROBLEM 2 DOPPLER LASER COOLING AND OPTICAL MOLASSES The purpose of this problem is to develop a simple theory to understand the so-called laser cooling and optical molasses phenomena. This
Precision Interferometry with a Bose-Einstein Condensate. Cass Sackett. Research Talk 17 October 2008
 Precision Interferometry with a Bose-Einstein Condensate Cass Sackett Research Talk 17 October 2008 Outline Atom interferometry Bose condensates Our interferometer One application What is atom interferometry?
Precision Interferometry with a Bose-Einstein Condensate Cass Sackett Research Talk 17 October 2008 Outline Atom interferometry Bose condensates Our interferometer One application What is atom interferometry?
Bose-Einstein Condensate: A New state of matter
 Bose-Einstein Condensate: A New state of matter KISHORE T. KAPALE June 24, 2003 BOSE-EINSTEIN CONDENSATE: A NEW STATE OF MATTER 1 Outline Introductory Concepts Bosons and Fermions Classical and Quantum
Bose-Einstein Condensate: A New state of matter KISHORE T. KAPALE June 24, 2003 BOSE-EINSTEIN CONDENSATE: A NEW STATE OF MATTER 1 Outline Introductory Concepts Bosons and Fermions Classical and Quantum
Physics Nov Cooling by Expansion
 Physics 301 19-Nov-2004 25-1 Cooling by Expansion Now we re going to change the subject and consider the techniques used to get really cold temperatures. Of course, the best way to learn about these techniques
Physics 301 19-Nov-2004 25-1 Cooling by Expansion Now we re going to change the subject and consider the techniques used to get really cold temperatures. Of course, the best way to learn about these techniques
Laser Cooling and Trapping of Atoms
 Chapter 2 Laser Cooling and Trapping of Atoms Since its conception in 1975 [71, 72] laser cooling has revolutionized the field of atomic physics research, an achievement that has been recognized by the
Chapter 2 Laser Cooling and Trapping of Atoms Since its conception in 1975 [71, 72] laser cooling has revolutionized the field of atomic physics research, an achievement that has been recognized by the
Physics Nov Bose-Einstein Gases
 Physics 3 3-Nov-24 8- Bose-Einstein Gases An amazing thing happens if we consider a gas of non-interacting bosons. For sufficiently low temperatures, essentially all the particles are in the same state
Physics 3 3-Nov-24 8- Bose-Einstein Gases An amazing thing happens if we consider a gas of non-interacting bosons. For sufficiently low temperatures, essentially all the particles are in the same state
Laser cooling and trapping
 Laser cooling and trapping William D. Phillips wdp@umd.edu Physics 623 14 April 2016 Why Cool and Trap Atoms? Original motivation and most practical current application: ATOMIC CLOCKS Current scientific
Laser cooling and trapping William D. Phillips wdp@umd.edu Physics 623 14 April 2016 Why Cool and Trap Atoms? Original motivation and most practical current application: ATOMIC CLOCKS Current scientific
CHAPTER 9 Statistical Physics
 CHAPTER 9 Statistical Physics 9.1 Historical Overview 9.2 Maxwell Velocity Distribution 9.3 Equipartition Theorem 9.4 Maxwell Speed Distribution 9.5 Classical and Quantum Statistics 9.6 Fermi-Dirac Statistics
CHAPTER 9 Statistical Physics 9.1 Historical Overview 9.2 Maxwell Velocity Distribution 9.3 Equipartition Theorem 9.4 Maxwell Speed Distribution 9.5 Classical and Quantum Statistics 9.6 Fermi-Dirac Statistics
Chapter 7: Quantum Statistics
 Part II: Applications - Bose-Einstein Condensation SDSMT, Physics 204 Fall Introduction Historic Remarks 2 Bose-Einstein Condensation Bose-Einstein Condensation The Condensation Temperature 3 The observation
Part II: Applications - Bose-Einstein Condensation SDSMT, Physics 204 Fall Introduction Historic Remarks 2 Bose-Einstein Condensation Bose-Einstein Condensation The Condensation Temperature 3 The observation
Chapter 7: Quantum Statistics
 Part II: Applications - Bose-Einstein Condensation SDSMT, Physics 203 Fall Introduction Historic Remarks 2 Bose-Einstein Condensation Bose-Einstein Condensation The Condensation Temperature 3 The observation
Part II: Applications - Bose-Einstein Condensation SDSMT, Physics 203 Fall Introduction Historic Remarks 2 Bose-Einstein Condensation Bose-Einstein Condensation The Condensation Temperature 3 The observation
Rb, which had been compressed to a density of 1013
 Modern Physics Study Questions for the Spring 2018 Departmental Exam December 3, 2017 1. An electron is initially at rest in a uniform electric field E in the negative y direction and a uniform magnetic
Modern Physics Study Questions for the Spring 2018 Departmental Exam December 3, 2017 1. An electron is initially at rest in a uniform electric field E in the negative y direction and a uniform magnetic
Chapter 37 Early Quantum Theory and Models of the Atom. Copyright 2009 Pearson Education, Inc.
 Chapter 37 Early Quantum Theory and Models of the Atom Planck s Quantum Hypothesis; Blackbody Radiation Photon Theory of Light and the Photoelectric Effect Energy, Mass, and Momentum of a Photon Compton
Chapter 37 Early Quantum Theory and Models of the Atom Planck s Quantum Hypothesis; Blackbody Radiation Photon Theory of Light and the Photoelectric Effect Energy, Mass, and Momentum of a Photon Compton
ESSENTIAL QUANTUM PHYSICS PETER LANDSHOFF. University of Cambridge ALLEN METHERELL. University of Central Florida GARETH REES. University of Cambridge
 ESSENTIAL QUANTUM PHYSICS PETER LANDSHOFF University of Cambridge ALLEN METHERELL University of Central Florida GARETH REES University of Cambridge CAMBRIDGE UNIVERSITY PRESS Constants of quantum physics
ESSENTIAL QUANTUM PHYSICS PETER LANDSHOFF University of Cambridge ALLEN METHERELL University of Central Florida GARETH REES University of Cambridge CAMBRIDGE UNIVERSITY PRESS Constants of quantum physics
The phases of matter familiar for us from everyday life are: solid, liquid, gas and plasma (e.f. flames of fire). There are, however, many other
 1 The phases of matter familiar for us from everyday life are: solid, liquid, gas and plasma (e.f. flames of fire). There are, however, many other phases of matter that have been experimentally observed,
1 The phases of matter familiar for us from everyday life are: solid, liquid, gas and plasma (e.f. flames of fire). There are, however, many other phases of matter that have been experimentally observed,
PHYS 3313 Section 001 Lecture # 24
 PHYS 3313 Section 001 Lecture # 24 Wednesday, April 29, Dr. Alden Stradling Equipartition Theorem Quantum Distributions Fermi-Dirac and Bose-Einstein Statistics Liquid Helium Laser PHYS 3313-001, Spring
PHYS 3313 Section 001 Lecture # 24 Wednesday, April 29, Dr. Alden Stradling Equipartition Theorem Quantum Distributions Fermi-Dirac and Bose-Einstein Statistics Liquid Helium Laser PHYS 3313-001, Spring
X-Rays from Atoms. These are called K α X-rays See table 29.1 for the energy of K α X-rays produced by some elements. Section 29.3
 X-Rays from Atoms The highest photon energy available in a hydrogen atom is in the ultraviolet part of the electromagnetic spectrum Other atoms can emit much more energetic photons larger Z, more electric
X-Rays from Atoms The highest photon energy available in a hydrogen atom is in the ultraviolet part of the electromagnetic spectrum Other atoms can emit much more energetic photons larger Z, more electric
CHAPTER 9 Statistical Physics
 CHAPTER 9 Statistical Physics 9.1 9.2 9.3 9.4 9.5 9.6 9.7 Historical Overview Maxwell Velocity Distribution Equipartition Theorem Maxwell Speed Distribution Classical and Quantum Statistics Fermi-Dirac
CHAPTER 9 Statistical Physics 9.1 9.2 9.3 9.4 9.5 9.6 9.7 Historical Overview Maxwell Velocity Distribution Equipartition Theorem Maxwell Speed Distribution Classical and Quantum Statistics Fermi-Dirac
Quantum Physics & From Ideas to Implementation. Underlying concepts in the syllabus
 Quantum Physics & From Ideas to Implementation Underlying concepts in the syllabus 1 1 What is Quantum Physics? Wave-particle duality Tells us that energy comes in packets, particles are wave-like. Systems
Quantum Physics & From Ideas to Implementation Underlying concepts in the syllabus 1 1 What is Quantum Physics? Wave-particle duality Tells us that energy comes in packets, particles are wave-like. Systems
Select/Special Topics in Atomic Physics Prof. P. C. Deshmukh Department of Physics Indian Institute of Technology, Madras
 Select/Special Topics in Atomic Physics Prof. P. C. Deshmukh Department of Physics Indian Institute of Technology, Madras Lecture - 39 Zeeman Effect Fine Structure, Hyperfine Structure Elemental, Rudimentary
Select/Special Topics in Atomic Physics Prof. P. C. Deshmukh Department of Physics Indian Institute of Technology, Madras Lecture - 39 Zeeman Effect Fine Structure, Hyperfine Structure Elemental, Rudimentary
CHAPTER 27 Quantum Physics
 CHAPTER 27 Quantum Physics Units Discovery and Properties of the Electron Planck s Quantum Hypothesis; Blackbody Radiation Photon Theory of Light and the Photoelectric Effect Energy, Mass, and Momentum
CHAPTER 27 Quantum Physics Units Discovery and Properties of the Electron Planck s Quantum Hypothesis; Blackbody Radiation Photon Theory of Light and the Photoelectric Effect Energy, Mass, and Momentum
Planck s Quantum Hypothesis Blackbody Radiation
 Planck s Quantum Hypothesis Blackbody Radiation The spectrum of blackbody radiation has been measured(next slide); it is found that the frequency of peak intensity increases linearly with temperature.
Planck s Quantum Hypothesis Blackbody Radiation The spectrum of blackbody radiation has been measured(next slide); it is found that the frequency of peak intensity increases linearly with temperature.
Many-Body Problems and Quantum Field Theory
 Philippe A. Martin Francois Rothen Many-Body Problems and Quantum Field Theory An Introduction Translated by Steven Goldfarb, Andrew Jordan and Samuel Leach Second Edition With 102 Figures, 7 Tables and
Philippe A. Martin Francois Rothen Many-Body Problems and Quantum Field Theory An Introduction Translated by Steven Goldfarb, Andrew Jordan and Samuel Leach Second Edition With 102 Figures, 7 Tables and
Chapter 28. Atomic Physics
 Chapter 28 Atomic Physics Quantum Numbers and Atomic Structure The characteristic wavelengths emitted by a hot gas can be understood using quantum numbers. No two electrons can have the same set of quantum
Chapter 28 Atomic Physics Quantum Numbers and Atomic Structure The characteristic wavelengths emitted by a hot gas can be understood using quantum numbers. No two electrons can have the same set of quantum
Atoms and Spectroscopy
 Atoms and Spectroscopy Lecture 3 1 ONE SMALL STEP FOR MAN ONE GIANT LEAP FOR MANKIND 2 FROM ATOMS TO STARS AND GALAXIES HOW DO WE KNOW? Observations The Scientific Method Hypothesis Verifications LAW 3
Atoms and Spectroscopy Lecture 3 1 ONE SMALL STEP FOR MAN ONE GIANT LEAP FOR MANKIND 2 FROM ATOMS TO STARS AND GALAXIES HOW DO WE KNOW? Observations The Scientific Method Hypothesis Verifications LAW 3
Chapter 5 Light and Matter
 Chapter 5 Light and Matter Stars and galaxies are too far for us to send a spacecraft or to visit (in our lifetimes). All we can receive from them is light But there is much we can learn (composition,
Chapter 5 Light and Matter Stars and galaxies are too far for us to send a spacecraft or to visit (in our lifetimes). All we can receive from them is light But there is much we can learn (composition,
Vegard B. Sørdal. Thermodynamics of 4He-3He mixture and application in dilution refrigeration
 Vegard B. Sørdal Thermodynamics of 4He-3He mixture and application in dilution refrigeration 1. Introduction 2. Crogenic methods Contents of the presentation 3. Properties of Helium 4. Superfluid Helium
Vegard B. Sørdal Thermodynamics of 4He-3He mixture and application in dilution refrigeration 1. Introduction 2. Crogenic methods Contents of the presentation 3. Properties of Helium 4. Superfluid Helium
Ultra-cold gases. Alessio Recati. CNR INFM BEC Center/ Dip. Fisica, Univ. di Trento (I) & Dep. Physik, TUM (D) TRENTO
 Ultra-cold gases Alessio Recati CNR INFM BEC Center/ Dip. Fisica, Univ. di Trento (I) & Dep. Physik, TUM (D) TRENTO Lectures L. 1) Introduction to ultracold gases Bosonic atoms: - From weak to strong interacting
Ultra-cold gases Alessio Recati CNR INFM BEC Center/ Dip. Fisica, Univ. di Trento (I) & Dep. Physik, TUM (D) TRENTO Lectures L. 1) Introduction to ultracold gases Bosonic atoms: - From weak to strong interacting
OpenStax-CNX module: m The Bohr Model. OpenStax College. Abstract
 OpenStax-CNX module: m51039 1 The Bohr Model OpenStax College This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 4.0 By the end of this section, you will
OpenStax-CNX module: m51039 1 The Bohr Model OpenStax College This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 4.0 By the end of this section, you will
Lecture 4: Absorption and emission lines
 Lecture 4: Absorption and emission lines Senior Astrophysics 2018-03-13 Senior Astrophysics () Lecture 4: Absorption and emission lines 2018-03-13 1 / 35 Outline 1 Absorption and emission line spectra
Lecture 4: Absorption and emission lines Senior Astrophysics 2018-03-13 Senior Astrophysics () Lecture 4: Absorption and emission lines 2018-03-13 1 / 35 Outline 1 Absorption and emission line spectra
Properties of Electromagnetic Radiation Chapter 5. What is light? What is a wave? Radiation carries information
 Concepts: Properties of Electromagnetic Radiation Chapter 5 Electromagnetic waves Types of spectra Temperature Blackbody radiation Dual nature of radiation Atomic structure Interaction of light and matter
Concepts: Properties of Electromagnetic Radiation Chapter 5 Electromagnetic waves Types of spectra Temperature Blackbody radiation Dual nature of radiation Atomic structure Interaction of light and matter
Molecular spectroscopy
 Molecular spectroscopy Origin of spectral lines = absorption, emission and scattering of a photon when the energy of a molecule changes: rad( ) M M * rad( ' ) ' v' 0 0 absorption( ) emission ( ) scattering
Molecular spectroscopy Origin of spectral lines = absorption, emission and scattering of a photon when the energy of a molecule changes: rad( ) M M * rad( ' ) ' v' 0 0 absorption( ) emission ( ) scattering
Quantum Mechanica. Peter van der Straten Universiteit Utrecht. Peter van der Straten (Atom Optics) Quantum Mechanica January 15, / 22
 Quantum Mechanica Peter van der Straten Universiteit Utrecht Peter van der Straten (Atom Optics) Quantum Mechanica January 15, 2013 1 / 22 Matrix methode Peter van der Straten (Atom Optics) Quantum Mechanica
Quantum Mechanica Peter van der Straten Universiteit Utrecht Peter van der Straten (Atom Optics) Quantum Mechanica January 15, 2013 1 / 22 Matrix methode Peter van der Straten (Atom Optics) Quantum Mechanica
Ultracold atoms and molecules
 Advanced Experimental Techniques Ultracold atoms and molecules Steven Knoop s.knoop@vu.nl VU, June 014 1 Ultracold atoms laser cooling evaporative cooling BEC Bose-Einstein condensation atom trap: magnetic
Advanced Experimental Techniques Ultracold atoms and molecules Steven Knoop s.knoop@vu.nl VU, June 014 1 Ultracold atoms laser cooling evaporative cooling BEC Bose-Einstein condensation atom trap: magnetic
PHYS 219 General Physics: Electricity, Light and Modern Physics
 PHYS 219 General Physics: Electricity, Light and Modern Physics Final exam is scheduled on Thursday May 2 @ 8 10 AM In Physics 112 It will cover five Chapters 25, 27, 28, 29, and 30. Review lecture notes,
PHYS 219 General Physics: Electricity, Light and Modern Physics Final exam is scheduled on Thursday May 2 @ 8 10 AM In Physics 112 It will cover five Chapters 25, 27, 28, 29, and 30. Review lecture notes,
Building Blocks for Quantum Computing Part IV. Design and Construction of the Trapped Ion Quantum Computer (TIQC)
 Building Blocks for Quantum Computing Part IV Design and Construction of the Trapped Ion Quantum Computer (TIQC) CSC801 Seminar on Quantum Computing Spring 2018 1 Goal Is To Understand The Principles And
Building Blocks for Quantum Computing Part IV Design and Construction of the Trapped Ion Quantum Computer (TIQC) CSC801 Seminar on Quantum Computing Spring 2018 1 Goal Is To Understand The Principles And
From laser cooling to BEC First experiments of superfluid hydrodynamics
 From laser cooling to BEC First experiments of superfluid hydrodynamics Alice Sinatra Quantum Fluids course - Complement 1 2013-2014 Plan 1 COOLING AND TRAPPING 2 CONDENSATION 3 NON-LINEAR PHYSICS AND
From laser cooling to BEC First experiments of superfluid hydrodynamics Alice Sinatra Quantum Fluids course - Complement 1 2013-2014 Plan 1 COOLING AND TRAPPING 2 CONDENSATION 3 NON-LINEAR PHYSICS AND
UNIT 7 ATOMIC AND NUCLEAR PHYSICS
 1 UNIT 7 ATOMIC AND NUCLEAR PHYSICS PHYS:1200 LECTURE 33 ATOMIC AND NUCLEAR PHYSICS (1) The physics that we have presented thus far in this course is classified as Classical Physics. Classical physics
1 UNIT 7 ATOMIC AND NUCLEAR PHYSICS PHYS:1200 LECTURE 33 ATOMIC AND NUCLEAR PHYSICS (1) The physics that we have presented thus far in this course is classified as Classical Physics. Classical physics
Chapter 16 Lecture. The Cosmic Perspective Seventh Edition. Star Birth Pearson Education, Inc.
 Chapter 16 Lecture The Cosmic Perspective Seventh Edition Star Birth 2014 Pearson Education, Inc. Star Birth The dust and gas between the star in our galaxy is referred to as the Interstellar medium (ISM).
Chapter 16 Lecture The Cosmic Perspective Seventh Edition Star Birth 2014 Pearson Education, Inc. Star Birth The dust and gas between the star in our galaxy is referred to as the Interstellar medium (ISM).
Dept. of Physics, MIT Manipal 1
 Chapter 1: Optics 1. In the phenomenon of interference, there is A Annihilation of light energy B Addition of energy C Redistribution energy D Creation of energy 2. Interference fringes are obtained using
Chapter 1: Optics 1. In the phenomenon of interference, there is A Annihilation of light energy B Addition of energy C Redistribution energy D Creation of energy 2. Interference fringes are obtained using
In Situ Imaging of Cold Atomic Gases
 In Situ Imaging of Cold Atomic Gases J. D. Crossno Abstract: In general, the complex atomic susceptibility, that dictates both the amplitude and phase modulation imparted by an atom on a probing monochromatic
In Situ Imaging of Cold Atomic Gases J. D. Crossno Abstract: In general, the complex atomic susceptibility, that dictates both the amplitude and phase modulation imparted by an atom on a probing monochromatic
Atoms, Molecules and Solids. From Last Time Superposition of quantum states Philosophy of quantum mechanics Interpretation of the wave function:
 Essay outline and Ref to main article due next Wed. HW 9: M Chap 5: Exercise 4 M Chap 7: Question A M Chap 8: Question A From Last Time Superposition of quantum states Philosophy of quantum mechanics Interpretation
Essay outline and Ref to main article due next Wed. HW 9: M Chap 5: Exercise 4 M Chap 7: Question A M Chap 8: Question A From Last Time Superposition of quantum states Philosophy of quantum mechanics Interpretation
Quantum optics of many-body systems
 Quantum optics of many-body systems Igor Mekhov Université Paris-Saclay (SPEC CEA) University of Oxford, St. Petersburg State University Lecture 2 Previous lecture 1 Classical optics light waves material
Quantum optics of many-body systems Igor Mekhov Université Paris-Saclay (SPEC CEA) University of Oxford, St. Petersburg State University Lecture 2 Previous lecture 1 Classical optics light waves material
Photons: Explained and Derived by Energy Wave Equations
 Photons: Explained and Derived by Energy Wave Equations Jeff Yee jeffsyee@gmail.com May 2, 2018 Summary Photons are curious packets of energy that exhibit particle-like behavior but they are also known
Photons: Explained and Derived by Energy Wave Equations Jeff Yee jeffsyee@gmail.com May 2, 2018 Summary Photons are curious packets of energy that exhibit particle-like behavior but they are also known
Example of a Plane Wave LECTURE 22
 Example of a Plane Wave http://www.acs.psu.edu/drussell/demos/evanescentwaves/plane-x.gif LECTURE 22 EM wave Intensity I, pressure P, energy density u av from chapter 30 Light: wave or particle? 1 Electromagnetic
Example of a Plane Wave http://www.acs.psu.edu/drussell/demos/evanescentwaves/plane-x.gif LECTURE 22 EM wave Intensity I, pressure P, energy density u av from chapter 30 Light: wave or particle? 1 Electromagnetic
Chapter 16: Star Birth
 Chapter 16 Lecture Chapter 16: Star Birth Star Birth 16.1 Stellar Nurseries Our goals for learning: Where do stars form? Why do stars form? Where do stars form? Star-Forming Clouds Stars form in dark clouds
Chapter 16 Lecture Chapter 16: Star Birth Star Birth 16.1 Stellar Nurseries Our goals for learning: Where do stars form? Why do stars form? Where do stars form? Star-Forming Clouds Stars form in dark clouds
Astronomy. Chapter 15 Stellar Remnants: White Dwarfs, Neutron Stars, and Black Holes
 Astronomy Chapter 15 Stellar Remnants: White Dwarfs, Neutron Stars, and Black Holes are hot, compact stars whose mass is comparable to the Sun's and size to the Earth's. A. White dwarfs B. Neutron stars
Astronomy Chapter 15 Stellar Remnants: White Dwarfs, Neutron Stars, and Black Holes are hot, compact stars whose mass is comparable to the Sun's and size to the Earth's. A. White dwarfs B. Neutron stars
Revolution in Physics. What is the second quantum revolution? Think different from Particle-Wave Duality
 PHYS 34 Modern Physics Ultracold Atoms and Trappe Ions Today and Mar.3 Contents: a) Revolution in physics nd Quantum revolution b) Quantum simulation, measurement, and information c) Atomic ensemble and
PHYS 34 Modern Physics Ultracold Atoms and Trappe Ions Today and Mar.3 Contents: a) Revolution in physics nd Quantum revolution b) Quantum simulation, measurement, and information c) Atomic ensemble and
Quantum Mechanics. Particle in a box All were partial answers, leading Schrödinger to wave mechanics
 Chemistry 4521 Time is flying by: only 15 lectures left!! Six quantum mechanics Four Spectroscopy Third Hour exam Three statistical mechanics Review Final Exam, Wednesday, May 4, 7:30 10 PM Quantum Mechanics
Chemistry 4521 Time is flying by: only 15 lectures left!! Six quantum mechanics Four Spectroscopy Third Hour exam Three statistical mechanics Review Final Exam, Wednesday, May 4, 7:30 10 PM Quantum Mechanics
Chapter 3. Electromagnetic Theory, Photons. and Light. Lecture 7
 Lecture 7 Chapter 3 Electromagnetic Theory, Photons. and Light Sources of light Emission of light by atoms The electromagnetic spectrum see supplementary material posted on the course website Electric
Lecture 7 Chapter 3 Electromagnetic Theory, Photons. and Light Sources of light Emission of light by atoms The electromagnetic spectrum see supplementary material posted on the course website Electric
van Quantum tot Molecuul
 10 HC10: Molecular and vibrational spectroscopy van Quantum tot Molecuul Dr Juan Rojo VU Amsterdam and Nikhef Theory Group http://www.juanrojo.com/ j.rojo@vu.nl Molecular and Vibrational Spectroscopy Based
10 HC10: Molecular and vibrational spectroscopy van Quantum tot Molecuul Dr Juan Rojo VU Amsterdam and Nikhef Theory Group http://www.juanrojo.com/ j.rojo@vu.nl Molecular and Vibrational Spectroscopy Based
Semiconductor Physics and Devices
 Introduction to Quantum Mechanics In order to understand the current-voltage characteristics, we need some knowledge of electron behavior in semiconductor when the electron is subjected to various potential
Introduction to Quantum Mechanics In order to understand the current-voltage characteristics, we need some knowledge of electron behavior in semiconductor when the electron is subjected to various potential
Fundamentals and New Frontiers of Bose Einstein Condensation
 Experimental realization of Bose Einstein condensation (BEC) of dilute atomic gases [Anderson, et al. (1995); Davis, et al. (1995); Bradley, et al. (1995, 1997)] has ignited a virtual explosion of research.
Experimental realization of Bose Einstein condensation (BEC) of dilute atomic gases [Anderson, et al. (1995); Davis, et al. (1995); Bradley, et al. (1995, 1997)] has ignited a virtual explosion of research.
Physics PhD Qualifying Examination Part I Wednesday, August 26, 2015
 Physics PhD Qualifying Examination Part I Wednesday, August 26, 2015 Name: (please print) Identification Number: STUDENT: Designate the problem numbers that you are handing in for grading in the appropriate
Physics PhD Qualifying Examination Part I Wednesday, August 26, 2015 Name: (please print) Identification Number: STUDENT: Designate the problem numbers that you are handing in for grading in the appropriate
Chapter 16 Lecture. The Cosmic Perspective Seventh Edition. Star Birth Pearson Education, Inc.
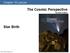 Chapter 16 Lecture The Cosmic Perspective Seventh Edition Star Birth Star Birth 16.1 Stellar Nurseries Our goals for learning: Where do stars form? Why do stars form? Where do stars form? Star-Forming
Chapter 16 Lecture The Cosmic Perspective Seventh Edition Star Birth Star Birth 16.1 Stellar Nurseries Our goals for learning: Where do stars form? Why do stars form? Where do stars form? Star-Forming
Chapter 39. Particles Behaving as Waves
 Chapter 39 Particles Behaving as Waves 39.1 Electron Waves Light has a dual nature. Light exhibits both wave and particle characteristics. Louis de Broglie postulated in 1924 that if nature is symmetric,
Chapter 39 Particles Behaving as Waves 39.1 Electron Waves Light has a dual nature. Light exhibits both wave and particle characteristics. Louis de Broglie postulated in 1924 that if nature is symmetric,
Star-Forming Clouds. Stars form in dark clouds of dusty gas in interstellar space. The gas between the stars is called the interstellar medium.
 Star Birth Chapter 16 Lecture 16.1 Stellar Nurseries The Cosmic Perspective Our goals for learning: Where do stars form? Why do stars form? Seventh Edition Star Birth Where do stars form? Star-Forming
Star Birth Chapter 16 Lecture 16.1 Stellar Nurseries The Cosmic Perspective Our goals for learning: Where do stars form? Why do stars form? Seventh Edition Star Birth Where do stars form? Star-Forming
Chapters 31 Atomic Physics
 Chapters 31 Atomic Physics 1 Overview of Chapter 31 Early Models of the Atom The Spectrum of Atomic Hydrogen Bohr s Model of the Hydrogen Atom de Broglie Waves and the Bohr Model The Quantum Mechanical
Chapters 31 Atomic Physics 1 Overview of Chapter 31 Early Models of the Atom The Spectrum of Atomic Hydrogen Bohr s Model of the Hydrogen Atom de Broglie Waves and the Bohr Model The Quantum Mechanical
Atomic Physics (Phys 551) Final Exam Solutions
 Atomic Physics (Phys 551) Final Exam Solutions Problem 1. For a Rydberg atom in n = 50, l = 49 state estimate within an order of magnitude the numerical value of a) Decay lifetime A = 1 τ = 4αω3 3c D (1)
Atomic Physics (Phys 551) Final Exam Solutions Problem 1. For a Rydberg atom in n = 50, l = 49 state estimate within an order of magnitude the numerical value of a) Decay lifetime A = 1 τ = 4αω3 3c D (1)
Introduction to cold atoms and Bose-Einstein condensation (II)
 Introduction to cold atoms and Bose-Einstein condensation (II) Wolfgang Ketterle Massachusetts Institute of Technology MIT-Harvard Center for Ultracold Atoms 7/7/04 Boulder Summer School * 1925 History
Introduction to cold atoms and Bose-Einstein condensation (II) Wolfgang Ketterle Massachusetts Institute of Technology MIT-Harvard Center for Ultracold Atoms 7/7/04 Boulder Summer School * 1925 History
8 Wavefunctions - Schrödinger s Equation
 8 Wavefunctions - Schrödinger s Equation So far we have considered only free particles - i.e. particles whose energy consists entirely of its kinetic energy. In general, however, a particle moves under
8 Wavefunctions - Schrödinger s Equation So far we have considered only free particles - i.e. particles whose energy consists entirely of its kinetic energy. In general, however, a particle moves under
Chapter 4 Arrangement of Electrons in Atoms. 4.1 The Development of a New Atomic Model
 Chapter 4 Arrangement of Electrons in Atoms 4.1 The Development of a New Atomic Model Properties of Light Electromagnetic Radiation: EM radiation are forms of energy which move through space as waves There
Chapter 4 Arrangement of Electrons in Atoms 4.1 The Development of a New Atomic Model Properties of Light Electromagnetic Radiation: EM radiation are forms of energy which move through space as waves There
LASER. Light Amplification by Stimulated Emission of Radiation
 LASER Light Amplification by Stimulated Emission of Radiation Laser Fundamentals The light emitted from a laser is monochromatic, that is, it is of one color/wavelength. In contrast, ordinary white light
LASER Light Amplification by Stimulated Emission of Radiation Laser Fundamentals The light emitted from a laser is monochromatic, that is, it is of one color/wavelength. In contrast, ordinary white light
Modern Physics Departmental Exam Last updated November 2013
 Modern Physics Departmental Exam Last updated November 213 87 1. Recently, 2 rubidium atoms ( 37 Rb ), which had been compressed to a density of 113 atoms/cm 3, were observed to undergo a Bose-Einstein
Modern Physics Departmental Exam Last updated November 213 87 1. Recently, 2 rubidium atoms ( 37 Rb ), which had been compressed to a density of 113 atoms/cm 3, were observed to undergo a Bose-Einstein
Chapter 31 Atomic Physics
 100 92 86 100 92 84 100 92 84 98 92 83 97 92 82 96 91 80 96 91 76 95 91 74 95 90 68 95 89 67 95 89 66 94 87 93 86 No. of Students in Range Exam 3 Score Distribution 25 22 20 15 10 10 5 3 2 0 0 0 0 0 0
100 92 86 100 92 84 100 92 84 98 92 83 97 92 82 96 91 80 96 91 76 95 91 74 95 90 68 95 89 67 95 89 66 94 87 93 86 No. of Students in Range Exam 3 Score Distribution 25 22 20 15 10 10 5 3 2 0 0 0 0 0 0
FI 3103 Quantum Physics
 FI 3103 Quantum Physics Alexander A. Iskandar Physics of Magnetism and Photonics Research Group Institut Teknologi Bandung General Information Lecture schedule 17 18 9136 51 5 91 Tutorial Teaching Assistant
FI 3103 Quantum Physics Alexander A. Iskandar Physics of Magnetism and Photonics Research Group Institut Teknologi Bandung General Information Lecture schedule 17 18 9136 51 5 91 Tutorial Teaching Assistant
PHYSICS 250 May 4, Final Exam - Solutions
 Name: PHYSICS 250 May 4, 999 Final Exam - Solutions Instructions: Work all problems. You may use a calculator and two pages of notes you may have prepared. There are problems of varying length and difficulty.
Name: PHYSICS 250 May 4, 999 Final Exam - Solutions Instructions: Work all problems. You may use a calculator and two pages of notes you may have prepared. There are problems of varying length and difficulty.
Particle nature of light & Quantization
 Particle nature of light & Quantization A quantity is quantized if its possible values are limited to a discrete set. An example from classical physics is the allowed frequencies of standing waves on a
Particle nature of light & Quantization A quantity is quantized if its possible values are limited to a discrete set. An example from classical physics is the allowed frequencies of standing waves on a
Requirements for scaleable QIP
 p. 1/25 Requirements for scaleable QIP These requirements were presented in a very influential paper by David Divincenzo, and are widely used to determine if a particular physical system could potentially
p. 1/25 Requirements for scaleable QIP These requirements were presented in a very influential paper by David Divincenzo, and are widely used to determine if a particular physical system could potentially
STATES OF MATTER NOTES..
 STATES OF MATTER NOTES.. While you are reading, answer the following which will help you with the States of Matter Project. What is matter (definition): What are the states of matter and what are the characteristics/properties
STATES OF MATTER NOTES.. While you are reading, answer the following which will help you with the States of Matter Project. What is matter (definition): What are the states of matter and what are the characteristics/properties
Part I. Principles and techniques
 Part I Principles and techniques 1 General principles and characteristics of optical magnetometers D. F. Jackson Kimball, E. B. Alexandrov, and D. Budker 1.1 Introduction Optical magnetometry encompasses
Part I Principles and techniques 1 General principles and characteristics of optical magnetometers D. F. Jackson Kimball, E. B. Alexandrov, and D. Budker 1.1 Introduction Optical magnetometry encompasses
Supersolids. Bose-Einstein Condensation in Quantum Solids Does it really exist?? W. J. Mullin
 Supersolids Bose-Einstein Condensation in Quantum Solids Does it really exist?? W. J. Mullin This is a lively controversy in condensed matter physics. Experiment says yes. Theory says no, or at best maybe.
Supersolids Bose-Einstein Condensation in Quantum Solids Does it really exist?? W. J. Mullin This is a lively controversy in condensed matter physics. Experiment says yes. Theory says no, or at best maybe.
Optical Properties of Lattice Vibrations
 Optical Properties of Lattice Vibrations For a collection of classical charged Simple Harmonic Oscillators, the dielectric function is given by: Where N i is the number of oscillators with frequency ω
Optical Properties of Lattice Vibrations For a collection of classical charged Simple Harmonic Oscillators, the dielectric function is given by: Where N i is the number of oscillators with frequency ω
PHYS 393 Low Temperature Physics Set 2: Liquid Helium-4
 PHYS 393 Low Temperature Physics Set 2: Liquid Helium-4 Christos Touramanis Oliver Lodge Lab, Office 319 c.touramanis@liverpool.ac.uk He 4 atom Two protons, two neutrons in nucleus: I=0 Two electrons in
PHYS 393 Low Temperature Physics Set 2: Liquid Helium-4 Christos Touramanis Oliver Lodge Lab, Office 319 c.touramanis@liverpool.ac.uk He 4 atom Two protons, two neutrons in nucleus: I=0 Two electrons in
14. Structure of Nuclei
 14. Structure of Nuclei Particle and Nuclear Physics Dr. Tina Potter Dr. Tina Potter 14. Structure of Nuclei 1 In this section... Magic Numbers The Nuclear Shell Model Excited States Dr. Tina Potter 14.
14. Structure of Nuclei Particle and Nuclear Physics Dr. Tina Potter Dr. Tina Potter 14. Structure of Nuclei 1 In this section... Magic Numbers The Nuclear Shell Model Excited States Dr. Tina Potter 14.
QM1 - Tutorial 1 The Bohr Atom and Mathematical Introduction
 QM - Tutorial The Bohr Atom and Mathematical Introduction 26 October 207 Contents Bohr Atom. Energy of a Photon - The Photo Electric Eect.................................2 The Discrete Energy Spectrum
QM - Tutorial The Bohr Atom and Mathematical Introduction 26 October 207 Contents Bohr Atom. Energy of a Photon - The Photo Electric Eect.................................2 The Discrete Energy Spectrum
Electronic structure of atoms
 Chapter 1 Electronic structure of atoms light photons spectra Heisenberg s uncertainty principle atomic orbitals electron configurations the periodic table 1.1 The wave nature of light Much of our understanding
Chapter 1 Electronic structure of atoms light photons spectra Heisenberg s uncertainty principle atomic orbitals electron configurations the periodic table 1.1 The wave nature of light Much of our understanding
Introduction to Sources: Radiative Processes and Population Inversion in Atoms, Molecules, and Semiconductors Atoms and Molecules
 OPTI 500 DEF, Spring 2012, Lecture 2 Introduction to Sources: Radiative Processes and Population Inversion in Atoms, Molecules, and Semiconductors Atoms and Molecules Energy Levels Every atom or molecule
OPTI 500 DEF, Spring 2012, Lecture 2 Introduction to Sources: Radiative Processes and Population Inversion in Atoms, Molecules, and Semiconductors Atoms and Molecules Energy Levels Every atom or molecule
The Nature of Light I: Electromagnetic Waves Spectra Kirchoff s Laws Temperature Blackbody radiation
 The Nature of Light I: Electromagnetic Waves Spectra Kirchoff s Laws Temperature Blackbody radiation Electromagnetic Radiation (How we get most of our information about the cosmos) Examples of electromagnetic
The Nature of Light I: Electromagnetic Waves Spectra Kirchoff s Laws Temperature Blackbody radiation Electromagnetic Radiation (How we get most of our information about the cosmos) Examples of electromagnetic
Chemistry 101 Chapter 11 Modern Atomic Theory
 Chemistry 101 Chapter 11 Modern Atomic Theory Electromagnetic radiation: energy can be transmitted from one place to another by lightmore properly called electromagnetic radiation. Many kinds of electromagnetic
Chemistry 101 Chapter 11 Modern Atomic Theory Electromagnetic radiation: energy can be transmitted from one place to another by lightmore properly called electromagnetic radiation. Many kinds of electromagnetic
New theory of light and ether
 New theory of light and ether Author: Udrea Sergiu, Montreal, Canada http://www.lightethertheory.com/ mail to: serudr@yahoo.com, serudr@gmail.com Abstract Light as a wave needs a medium of propagation.
New theory of light and ether Author: Udrea Sergiu, Montreal, Canada http://www.lightethertheory.com/ mail to: serudr@yahoo.com, serudr@gmail.com Abstract Light as a wave needs a medium of propagation.
Quantum Mechanics. Exam 3. Photon(or electron) interference? Photoelectric effect summary. Using Quantum Mechanics. Wavelengths of massive objects
 Exam 3 Hour Exam 3: Wednesday, November 29th In-class, Quantum Physics and Nuclear Physics Twenty multiple-choice questions Will cover:chapters 13, 14, 15 and 16 Lecture material You should bring 1 page
Exam 3 Hour Exam 3: Wednesday, November 29th In-class, Quantum Physics and Nuclear Physics Twenty multiple-choice questions Will cover:chapters 13, 14, 15 and 16 Lecture material You should bring 1 page
Nuclear Physics. (PHY-231) Dr C. M. Cormack. Nuclear Physics This Lecture
 Nuclear Physics (PHY-31) Dr C. M. Cormack 11 Nuclear Physics This Lecture This Lecture We will discuss an important effect in nuclear spectroscopy The Mössbauer Effect and its applications in technology
Nuclear Physics (PHY-31) Dr C. M. Cormack 11 Nuclear Physics This Lecture This Lecture We will discuss an important effect in nuclear spectroscopy The Mössbauer Effect and its applications in technology
Saturation Absorption Spectroscopy of Rubidium Atom
 Saturation Absorption Spectroscopy of Rubidium Atom Jayash Panigrahi August 17, 2013 Abstract Saturated absorption spectroscopy has various application in laser cooling which have many relevant uses in
Saturation Absorption Spectroscopy of Rubidium Atom Jayash Panigrahi August 17, 2013 Abstract Saturated absorption spectroscopy has various application in laser cooling which have many relevant uses in
Electric Potential Energy: Potential Difference
 OpenStax-CNX module: m42324 1 Electric Potential Energy: Potential Difference OpenStax College This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 Abstract
OpenStax-CNX module: m42324 1 Electric Potential Energy: Potential Difference OpenStax College This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 Abstract
Waves & Oscillations
 Physics 42200 Waves & Oscillations Lecture 32 Electromagnetic Waves Spring 2016 Semester Matthew Jones Electromagnetism Geometric optics overlooks the wave nature of light. Light inconsistent with longitudinal
Physics 42200 Waves & Oscillations Lecture 32 Electromagnetic Waves Spring 2016 Semester Matthew Jones Electromagnetism Geometric optics overlooks the wave nature of light. Light inconsistent with longitudinal
Preview. Atomic Physics Section 1. Section 1 Quantization of Energy. Section 2 Models of the Atom. Section 3 Quantum Mechanics
 Atomic Physics Section 1 Preview Section 1 Quantization of Energy Section 2 Models of the Atom Section 3 Quantum Mechanics Atomic Physics Section 1 TEKS The student is expected to: 8A describe the photoelectric
Atomic Physics Section 1 Preview Section 1 Quantization of Energy Section 2 Models of the Atom Section 3 Quantum Mechanics Atomic Physics Section 1 TEKS The student is expected to: 8A describe the photoelectric
Optical Lattices. Chapter Polarization
 Chapter Optical Lattices Abstract In this chapter we give details of the atomic physics that underlies the Bose- Hubbard model used to describe ultracold atoms in optical lattices. We show how the AC-Stark
Chapter Optical Lattices Abstract In this chapter we give details of the atomic physics that underlies the Bose- Hubbard model used to describe ultracold atoms in optical lattices. We show how the AC-Stark
1. Why photons? 2. Photons in a vacuum
 1 Photons 1. Why photons? Ask class: most of our information about the universe comes from photons. What are the reasons for this? Let s compare them with other possible messengers, specifically massive
1 Photons 1. Why photons? Ask class: most of our information about the universe comes from photons. What are the reasons for this? Let s compare them with other possible messengers, specifically massive
Lecture 0. NC State University
 Chemistry 736 Lecture 0 Overview NC State University Overview of Spectroscopy Electronic states and energies Transitions between states Absorption and emission Electronic spectroscopy Instrumentation Concepts
Chemistry 736 Lecture 0 Overview NC State University Overview of Spectroscopy Electronic states and energies Transitions between states Absorption and emission Electronic spectroscopy Instrumentation Concepts
Superfluidity and Superconductivity
 Superfluidity and Superconductivity These are related phenomena of flow without resistance, but in very different systems Superfluidity: flow of helium IV atoms in a liquid Superconductivity: flow of electron
Superfluidity and Superconductivity These are related phenomena of flow without resistance, but in very different systems Superfluidity: flow of helium IV atoms in a liquid Superconductivity: flow of electron
Lecture 21: Lasers, Schrödinger s Cat, Atoms, Molecules, Solids, etc. Review and Examples. Lecture 21, p 1
 Lecture 21: Lasers, Schrödinger s Cat, Atoms, Molecules, Solids, etc. Review and Examples Lecture 21, p 1 Act 1 The Pauli exclusion principle applies to all fermions in all situations (not just to electrons
Lecture 21: Lasers, Schrödinger s Cat, Atoms, Molecules, Solids, etc. Review and Examples Lecture 21, p 1 Act 1 The Pauli exclusion principle applies to all fermions in all situations (not just to electrons
Introduction to Atomic Physics and Quantum Optics
 Physics 404 and Physics 690-03 Introduction to Atomic Physics and Quantum Optics [images courtesy of Thywissen group, U of T] Prof. Seth Aubin Office: room 255, Small Hall, tel: 1-3545 Lab: room 069, Small
Physics 404 and Physics 690-03 Introduction to Atomic Physics and Quantum Optics [images courtesy of Thywissen group, U of T] Prof. Seth Aubin Office: room 255, Small Hall, tel: 1-3545 Lab: room 069, Small
1. As a macroscopic analogy, think of an idealized pool table on which there is no friction. Let s consider a few scenarios.
 1 Worksheet AM1: Coupling Interactions In complex atoms, the electrons interact with each other. Naturally, the interactions affect the energy. Also, due to these interactions, the individual electrons
1 Worksheet AM1: Coupling Interactions In complex atoms, the electrons interact with each other. Naturally, the interactions affect the energy. Also, due to these interactions, the individual electrons
Revision Guide. Chapter 7 Quantum Behaviour
 Revision Guide Chapter 7 Quantum Behaviour Contents CONTENTS... 2 REVISION CHECKLIST... 3 REVISION NOTES... 4 QUANTUM BEHAVIOUR... 4 Random arrival of photons... 4 Photoelectric effect... 5 PHASE AN PHASORS...
Revision Guide Chapter 7 Quantum Behaviour Contents CONTENTS... 2 REVISION CHECKLIST... 3 REVISION NOTES... 4 QUANTUM BEHAVIOUR... 4 Random arrival of photons... 4 Photoelectric effect... 5 PHASE AN PHASORS...
When I hear of Schrödinger s cat, I reach for my gun. --Stephen W. Hawking. Lecture 21, p 1
 When I hear of Schrödinger s cat, I reach for my gun. --Stephen W. Hawking Lecture 21, p 1 Lecture 21: Lasers, Schrödinger s Cat, Atoms, Molecules, Solids, etc. Review and Examples Lecture 21, p 2 Act
When I hear of Schrödinger s cat, I reach for my gun. --Stephen W. Hawking Lecture 21, p 1 Lecture 21: Lasers, Schrödinger s Cat, Atoms, Molecules, Solids, etc. Review and Examples Lecture 21, p 2 Act
Chapter 27 Lecture Notes
 Chapter 27 Lecture Notes Physics 2424 - Strauss Formulas: λ P T = 2.80 10-3 m K E = nhf = nhc/λ fλ = c hf = K max + W 0 λ = h/p λ - λ = (h/mc)(1 - cosθ) 1/λ = R(1/n 2 f - 1/n 2 i ) Lyman Series n f = 1,
Chapter 27 Lecture Notes Physics 2424 - Strauss Formulas: λ P T = 2.80 10-3 m K E = nhf = nhc/λ fλ = c hf = K max + W 0 λ = h/p λ - λ = (h/mc)(1 - cosθ) 1/λ = R(1/n 2 f - 1/n 2 i ) Lyman Series n f = 1,
70 YEAR QUEST ENDS IN SUCCESS BOSE-EINSTEIN CONDENSATION 2001 NOBEL PRIZE IN PHYSICS
 70 YEAR QUEST ENDS IN SUCCESS BOSE-EINSTEIN CONDENSATION 2001 NOBEL PRIZE IN PHYSICS 8.044, LECTURE 33, SPRING 2004 BOSE-EINSTEIN CONDENSATION IS A QUANUM MECHANICAL EFFECT Image removed due to copyright
70 YEAR QUEST ENDS IN SUCCESS BOSE-EINSTEIN CONDENSATION 2001 NOBEL PRIZE IN PHYSICS 8.044, LECTURE 33, SPRING 2004 BOSE-EINSTEIN CONDENSATION IS A QUANUM MECHANICAL EFFECT Image removed due to copyright
Nuclear Decays. Alpha Decay
 Nuclear Decays The first evidence of radioactivity was a photographic plate, wrapped in black paper and placed under a piece of uranium salt by Henri Becquerel on February 26, 1896. Like many events in
Nuclear Decays The first evidence of radioactivity was a photographic plate, wrapped in black paper and placed under a piece of uranium salt by Henri Becquerel on February 26, 1896. Like many events in
 Math Questions for the 2011 PhD Qualifier Exam 1. Evaluate the following definite integral 3" 4 where! ( x) is the Dirac! - function. # " 4 [ ( )] dx x 2! cos x 2. Consider the differential equation dx
Math Questions for the 2011 PhD Qualifier Exam 1. Evaluate the following definite integral 3" 4 where! ( x) is the Dirac! - function. # " 4 [ ( )] dx x 2! cos x 2. Consider the differential equation dx
