Error analysis in Barker s method: extension to ternary systems
|
|
|
- Vernon Lyons
- 5 years ago
- Views:
Transcription
1 Fluid hase quilibria rror analysis in Barer s method: extension to ternary systems I.M.A. Fonseca, L.Q. Lobo ) Departamento de ngenharia Quımica, UniÕersidade de Coimbra, 3000 Coimbra, ortugal Received 1 December 1994; accepted 7 November 1998 Abstract An extension of Barer s method to ternary systems is briefly outlined. xpressions for the standard deviations of the excess molar Gibbs free energy and the equilibrium pressure as functions of composition are obtained. These expressions are applied to accurate experimental data for the cryogenic Ž liquid. mixture CH 3F q NOq Xe recently measured in our laboratory. q 1999 lsevier Science B.V. All rights reserved. Keywords: VL low-pressure; Ternary systems; rrors; Barer s method 1. Introduction In the experimental determination of vapour liquid equilibrium Ž VL. using isothermal static cells the measured properties are the equilibrium pressure exp of the mixture and the amount of substance ni of each component introduced into the cell. Reduction of these data to obtain x y phase diagrams is usually made by using Barer s method wx 1. This method has been widely used for binary and ternary systems but in most cases the statistical analysis of the errors associated with it has not been reported. Goral wx described a procedure to carry out such analysis for binary systems, and wx Kolasinsa et al. 3 applied it assuming the Wilson model for the excess molar Gibbs free energy G. In this paper we have extended this ind of study to ternary mixtures, and applied it to VL data for the system CH FŽ 1. qn OqXe Ž 3. and its constituent binaries, at K wx 3 4, recently obtained in our laboratory by a modified version of the accurate low-temperature technique introduced and developed by Davies et al. wx 5 and Fonseca and Lobo wx 6. Since the equilibrium pressure is a nonlinear function of the adjustable parameters in the assumed G model expression, Barer s method embodies a nonlinear regression technique. Therefore, the ) Corresponding author. Tel.: q ; fax: q r99r$ - see front matter q 1999 lsevier Science B.V. All rights reserved. II: S
2 06 ( ) I.M.A. Fonseca, L.Q. LoborFluid hase quilibria uncertainties associated with such parameters are to be obtained from the variance covariance matrix wx 7. Using the propagation law of errors the standard deviations surfaces for and G as functions of composition for ternary systems can be calculated. In this paper we report on such calculations.. Barer s method: outline of application to ternary mixtures In essence Barer s method optimizes the parameters in the assumed expression for the liquid composition dependence of G through an iterative procedure which minimizes the sum of the squares of the pressure residuals R, R s y. Ž 1. exp The calculated equilibrium pressure is obtained from Ý i i i i i s x g ) b, where xi is the mole fraction of component i in the liquid mixture, gi is its activity coefficient, and b is a factor which Ž mainly. i taes into account the vapour-phase non-ideality, usually through a two-term virial equation of state, ½½ ÝÝ ) b sexp Ž V )yb. y y y y d yd :RT. Ž 3. i i ii i i ji j j In these expressions y represents the mole fraction of i in the vapour, ) and V ) i i i are the vapour pressure and the liquid molar volume of pure component i, respectively, Bii is the second virial coefficient of i, and dijsbijybiiyb jj. Initial estimates for the values of the mole fractions in the vapour yi are found by assuming Raoult s law, and in the next iterations by taing y s xy ) r b. Ž 4. i i i i i xpressions for the activity coefficients are derived from the rigorous thermodynamic relationship L L ln gis n G r n i T,,n, 5 j where n L sý n L and the superscript L i i refers to the liquid mixture. For ternary systems we have Ž chosen to represent the reduced quantity g sg rrt. in the form Ý ij 13 all binaries g s g qg, Ž 6. where the binary terms g, and the ternary one g the mole fractions in the liquid, and ij 13 ½ 5 are assumed to be Redlich Kister functions of ij i j ij ijž i j. ijž i j. g sx x A qb x yx qc x yx, 7 g sx x x c yc x yc x
3 ( ) I.M.A. Fonseca, L.Q. LoborFluid hase quilibria In these expressions A, B, C, c, c and c are the adjustable parameters mentioned above. From ij ij ij 0 1 qs. 5 and 6 the activity coefficient of component 1 becomes ln g1sx Ž A1qB1qC1. x1ž 1yx1. q Ž A1yB1qC1. xž 1y x1. q4c1 x1 xž 3 x1y. qx3 Ž A13qB13qC13. x1ž 1yx1. q Ž A13yB13qC13. x13 = Ž 1y x1. q4c13 x1 x3ž 3 x1y. qx x3 1C3 x x3yž A3yB3qC3. x3 yž A3qB3qC3. x qx x3 c0ž 1y x1. yc1 x1y Ž 1y3 x1.ž c1 x1qc x.. Similar expressions for ln g and ln g are obtained from q. Ž 9. 3 by circular change of subscripts. The activity coefficients are functions of the liquid composition and of the parameters present in the G model. Values for the parameters A ij, Bij and Cij are arrived at from binary data alone while those for the parameters c 0, c1 and c are found by regression of the experimental data for the ternary mixtures, i.e., by solving the matrix equation where L d c s T, Ž 10. ž / ž /ž / ž /ž c c c c c / Ý Ý Ý ž /ž / ž / ž /ž / ž c /ž c / ž c /ž c / ž c / 0 1 ÝR pž c 0 / 1 ÝR pž c 1 / ÝR pž c / L s Ý Ý Ý, c c c c c Ý Ý Ý dc 0 d c s dc, and T s. Ž 11. dc All the summations in these matrices are taen over all the M ternary experimental points. The elements of matrix wlx are obtained directly from q. Ž.. The elements d c Ž s 0,1,. represent increments in the c parameters, being obtained from q. Ž 10. by inversion: y1 d c s L T. Ž 1. Ž 9.
4 08 ( ) I.M.A. Fonseca, L.Q. LoborFluid hase quilibria Table 1 Values of the parameters in qs. Ž.Ž. 6 8 with their respective standard deviations wx 4 A s0.148" B sy0.086" C s0.0645" A s1.7470" B sy0.1467" C s0.1957" A3s1.16" B3s0.0715" C3s0.0506"0.01 c0sy0.6711" c1sy0.4088" csy0.5585" Ž The iterations start by assuming c sc sc s0 i.e., g s , and the process is then recycled with new values for the element matrices wlx y1 and wtx by taing corrected values of c given by cscž last iteration. qdc, Ž 13. until a situation is reached for which the d c become smaller than a pre-defined value Ž e.g., y6 dc -10 in this wor.. 3. rror analysis The calculation of matrix wlx y1 wd x, immediately yields wx 7 the so called variance covariance matrix D s L s, Ž 14. y1 where M Ý 1 s s R rž Mym., Ž 15. represents the variance of the equilibrium pressure, and m is the number of adjustable parameters in q. Ž 8.. The diagonal elements D in the symmetrical matrix wdx ii are the variances of the parameters. Both Dii and the covariances Dij Ž i.e., the non-diagonal elements. are affected by the experimental uncertainty incorporated in s as shown by q. Ž 14. p. Three independent variance covariance matrices for the binary systems are obtained exactly in the same way. They are also independent from that for the ternary system. Indeed each set of binary data Ž and the ternary one. concerns a different mixture embodying different experimental uncertainties and originating different values of s Ž 15.. p Table Values of the covariances of the parameters in qs. Ž. 6 Ž. 8 covž A, B. s1.58=10 covž A, C. sy6.953=10 covž B, C. sy3.605=10 covž A, B. s3.7=10 covž A, C. sy.449=10 covž B, C. sy1.559=10 covž A, B. sy.46=10 covž A, C. sy5.519=10 covž B, C. sy8.334=10 covž c, c. sy7.401=10 covž c, c. s8.109=10 covž c, c. sy4.179=10 y5 y5 y5 1 1 y6 1 1 y6 1 1 y y y y4 3 3 y3 3 3 y3 3 3 y
5 ( ) I.M.A. Fonseca, L.Q. LoborFluid hase quilibria The uncertainty associated with any other variable which is itself a function of the optimized parameters mentioned above, namely the G function, is readily obtained as follows. Let f be a function of parameters a, b, and c, fsfž a,b,c., Ž 16. whose uncertainties may assume any values between ys and qs Ž sa, b, c.. Then, through the propagation law of errors, the variance s is given as wx 7 f s sf s qf s qf s f a a b b c c q FF a bcovž a,b. q FFcov a c Ž a,c. q FFcov b c Ž b,c. Ž 17. where the usual convention has been used to indicate the partial derivatives, e.g., F sž Fra. a b,c. xpressions similar to q. Ž 17. omitting the covariance terms have been used. However, these terms can often mae a significant contribution to the estimated value of s f, and therefore should not be neglected wx 8. Applying q. 17 to g defined by q. 6, an expression for the standard deviation surface of G as a function of the composition of the liquid mixture is obtained: ½ ½ 4 G 1 A 1 1 B 1 1 C s s RT x x s q x yx s q x yx s q x yx cov A, B 3 Ž 1. Ž 1 1. Ž 1. Ž 1 1. q x y x cov A,C q x y x cov B,C q... similar terms for the binaries Ž 1,3. and Ž, qž x x x. = s qs x 1 3 c0 c1 1 1r qsc xycov c 0,c1 x1ycov c 0,c xqcov c 1,c x1x. 18 The total number of parameters whose uncertainties contribute to s G for each binary mixture, and three for the ternary term. 55 is, therefore, twelve: three Fig. 1. erspective view of the standard deviation surface s calculated from q. Ž 18. G, with parameters listed in Tables 1 and. s is represented as a function of the mole fractions of the three components. G
6 10 ( ) I.M.A. Fonseca, L.Q. LoborFluid hase quilibria In a similar way the standard deviation surface of the equilibrium pressure s liquid composition is obtained by applying q. Ž 17. to as defined in q. Ž.: ½ ž / A1 ž / B1 ž / C1 ž /ž / 1 1 A1 B1 C1 A1 B1 s s s q s q s q covž A, B. ž /ž / ž /ž / q covž A 1,C1. q covž B 1,C1. A C B C q... similar terms for the binaries Ž 1,3. and Ž, as a function of the ž / c c c Ž ž / 1 ž / c c c ž c /ž c / ž c / r c c1 c q s q s q s q cov c,c q = ž / ž /ž / covž c,c. q covž c,c.. Ž Results and conclusions The values of the parameters in q. Ž 6. obtained by Barer s method for CH FŽ 1. qn 0qXe Ž 3. 3, at K, using experimental data recently obtained in our laboratory wx 4 are summarized with their respective standard deviations in Table 1. The calculated covariances are listed in Table. The standard deviations surfaces s G and s are represented as functions of the liquid mole fractions in Figs. 1 and, respectively. Both perspective views in these figures clearly show that the standard deviations in the parameters for the binary mixtures affect the calculated values of s G and s for Fig.. erspective view of the standard deviation surface s calculated from q. Ž 19., with parameters listed in Tables 1 and. s is represented as a function of the mole fractions of the three components.
7 ( ) I.M.A. Fonseca, L.Q. LoborFluid hase quilibria the ternary system. The higher values of these deviations near the N O qxež 3. binary region are a consequence of the comparatively much higher values of the parameters standard deviations for this binary mixture. The value of s for the equimolar ternary mixture is about 6 J mol y1 G, half of which arises from s G 13. References wx 1 J.A. Barer, Aust. J. Chem. 6 Ž wx M. Goral, Z. hys. Chemie, Leipzig 58 Ž wx 3 G. Kolasinsa,. Oracz, Z. hys. Chemie, Leipzig 60 Ž wx 4 I.M.A. Fonseca, L.Q. Lobo, Fluid hase quilibria 113 Ž wx 5 R.H. Davies, A.G. Duncan, G. Saville, L.A.K. Staveley, Trans. Faraday Soc. 63 Ž wx 6 I.M.A. Fonseca, L.Q. Lobo, Fluid hase quilibria 47 Ž wx 7 G... Box, W.. Hunter, J.S. Hunter, Statistics for xperimenters, Wiley, New Yor, wx 8 W.. Wentworth, J. Chem. duc. 4 Ž
Two Posts to Fill On School Board
 Y Y 9 86 4 4 qz 86 x : ( ) z 7 854 Y x 4 z z x x 4 87 88 Y 5 x q x 8 Y 8 x x : 6 ; : 5 x ; 4 ( z ; ( ) ) x ; z 94 ; x 3 3 3 5 94 ; ; ; ; 3 x : 5 89 q ; ; x ; x ; ; x : ; ; ; ; ; ; 87 47% : () : / : 83
Y Y 9 86 4 4 qz 86 x : ( ) z 7 854 Y x 4 z z x x 4 87 88 Y 5 x q x 8 Y 8 x x : 6 ; : 5 x ; 4 ( z ; ( ) ) x ; z 94 ; x 3 3 3 5 94 ; ; ; ; 3 x : 5 89 q ; ; x ; x ; ; x : ; ; ; ; ; ; 87 47% : () : / : 83
A DARK GREY P O N T, with a Switch Tail, and a small Star on the Forehead. Any
 Y Y Y X X «/ YY Y Y ««Y x ) & \ & & } # Y \#$& / Y Y X» \\ / X X X x & Y Y X «q «z \x» = q Y # % \ & [ & Z \ & { + % ) / / «q zy» / & / / / & x x X / % % ) Y x X Y $ Z % Y Y x x } / % «] «] # z» & Y X»
Y Y Y X X «/ YY Y Y ««Y x ) & \ & & } # Y \#$& / Y Y X» \\ / X X X x & Y Y X «q «z \x» = q Y # % \ & [ & Z \ & { + % ) / / «q zy» / & / / / & x x X / % % ) Y x X Y $ Z % Y Y x x } / % «] «] # z» & Y X»
r sat,l T sr sat,l T rf rh Ž 4.
 Fluid Phase Equilibria 150 151 1998 215 223 Extended corresponding states for pure polar and non-polar fluids: an improved method for component shape factor prediction Isabel M. Marrucho a, James F. Ely
Fluid Phase Equilibria 150 151 1998 215 223 Extended corresponding states for pure polar and non-polar fluids: an improved method for component shape factor prediction Isabel M. Marrucho a, James F. Ely
Vapor liquid equilibria for the binary system 2,2 dimethylbutane + 1,1 dimethylpropyl methyl ether (TAME) at , , and 338.
 Fluid Phase Equilibria 221 (2004) 1 6 Vapor liquid equilibria for the binary system 2,2 dimethylbutane + 1,1 dimethylpropyl methyl ether (TAME) at 298.15, 318.15, and 338.15 K Armando del Río a, Baudilio
Fluid Phase Equilibria 221 (2004) 1 6 Vapor liquid equilibria for the binary system 2,2 dimethylbutane + 1,1 dimethylpropyl methyl ether (TAME) at 298.15, 318.15, and 338.15 K Armando del Río a, Baudilio
MODELING OF PHASE EQUILIBRIA FOR BINARY AND TERNARY MIXTURES OF CARBON DIOXIDE, HYDROGEN AND METHANOL
 MODELING OF PHASE EQUILIBRIA FOR BINARY AND TERNARY MIXTURES OF CARBON DIOXIDE, HYDROGEN AND METHANOL Neil R. Foster *, Keivan Bezanehtak, Fariba Dehghani School of Chemical Engineering and Industrial
MODELING OF PHASE EQUILIBRIA FOR BINARY AND TERNARY MIXTURES OF CARBON DIOXIDE, HYDROGEN AND METHANOL Neil R. Foster *, Keivan Bezanehtak, Fariba Dehghani School of Chemical Engineering and Industrial
Terminal attractor optical associative memory with adaptive control parameter
 1 June 1998 Ž. Optics Communications 151 1998 353 365 Full length article Terminal attractor optical associative memory with adaptive control parameter Xin Lin a,), Junji Ohtsubo b, Masahiko Mori a a Electrotechnical
1 June 1998 Ž. Optics Communications 151 1998 353 365 Full length article Terminal attractor optical associative memory with adaptive control parameter Xin Lin a,), Junji Ohtsubo b, Masahiko Mori a a Electrotechnical
Vapour-Liquid Equilibrium for the Binary System Dimethylformamide Ethylene Glycol*
 Vapour-Liquid Equilibrium for the Binary System Dimethylformamide Ethylene Glycol* E. GRACZOVÁ** and + J. SUROVÝ Department of Chemical and Biochemical Engineering, Faculty of Chemical and Food Technology,
Vapour-Liquid Equilibrium for the Binary System Dimethylformamide Ethylene Glycol* E. GRACZOVÁ** and + J. SUROVÝ Department of Chemical and Biochemical Engineering, Faculty of Chemical and Food Technology,
Quadratic mixing rules for equations of state. Origins and relationships to the virial expansion
 67 Fluid Phase Equilibria Journal Volume 91, Pages 67-76. 1993 Quadratic mixing rules for equations of state. Origins and relationships to the virial expansion Kenneth R. Hall * and Gustavo A. Iglesias-Silva
67 Fluid Phase Equilibria Journal Volume 91, Pages 67-76. 1993 Quadratic mixing rules for equations of state. Origins and relationships to the virial expansion Kenneth R. Hall * and Gustavo A. Iglesias-Silva
Department of Chemical Engineering, Slovak Technical Bratislava. Received 8 October 1974
 Calculation of the activity coefficients and vapour composition from equations of the Tao method modified for inconstant integration step Ax. П. Ternary systems J. DOJČANSKÝ and J. SUROVÝ Department of
Calculation of the activity coefficients and vapour composition from equations of the Tao method modified for inconstant integration step Ax. П. Ternary systems J. DOJČANSKÝ and J. SUROVÝ Department of
Comparison of different mixing rules for prediction of density and residual internal energy of binary and ternary Lennard Jones mixtures
 Fluid Phase Equilibria 178 (2001) 87 95 Comparison of different mixing rules for prediction of density and residual internal energy of binary and ternary Lennard Jones mixtures Jian Chen a,, Jian-Guo Mi
Fluid Phase Equilibria 178 (2001) 87 95 Comparison of different mixing rules for prediction of density and residual internal energy of binary and ternary Lennard Jones mixtures Jian Chen a,, Jian-Guo Mi
Densities and Viscosities of the Ternary Mixtures Water + Butyl Acetate + Methanol and Water + Ethyl Propionate + Methanol at 303.
 926 J. Chem. Eng. Data 2000, 45, 926-931 Densities and Viscosities of the Ternary Mixtures Water + Butyl Acetate + Methanol and Water + Ethyl Propionate + Methanol at 303.15 K Zoran P. Visak, Abel G. M.
926 J. Chem. Eng. Data 2000, 45, 926-931 Densities and Viscosities of the Ternary Mixtures Water + Butyl Acetate + Methanol and Water + Ethyl Propionate + Methanol at 303.15 K Zoran P. Visak, Abel G. M.
A. H. Hall, 33, 35 &37, Lendoi
 7 X x > - z Z - ----»»x - % x x» [> Q - ) < % - - 7»- -Q 9 Q # 5 - z -> Q x > z»- ~» - x " < z Q q»» > X»? Q ~ - - % % < - < - - 7 - x -X - -- 6 97 9
7 X x > - z Z - ----»»x - % x x» [> Q - ) < % - - 7»- -Q 9 Q # 5 - z -> Q x > z»- ~» - x " < z Q q»» > X»? Q ~ - - % % < - < - - 7 - x -X - -- 6 97 9
Phase equilibria properties of binary and ternary systems containing isopropyl ether + isobutanol + benzene at K.
 Phase equilibria properties of binary and ternary systems containing isopropyl ether + isobutanol + benzene at 313.15 K. R.M. Villamañán 1, M.C. Martín 2, C.R. Chamorro 2, M.A. Villamañán 2, J.J. Segovia
Phase equilibria properties of binary and ternary systems containing isopropyl ether + isobutanol + benzene at 313.15 K. R.M. Villamañán 1, M.C. Martín 2, C.R. Chamorro 2, M.A. Villamañán 2, J.J. Segovia
Linear Least Squares Fitting
 Linear Least Squares Fitting Bhas Bapat IISER Pune Nov 2014 Bhas Bapat (IISER Pune) Linear Least Squares Fitting Nov 2014 1 / 16 What is Least Squares Fit? A procedure for finding the best-fitting curve
Linear Least Squares Fitting Bhas Bapat IISER Pune Nov 2014 Bhas Bapat (IISER Pune) Linear Least Squares Fitting Nov 2014 1 / 16 What is Least Squares Fit? A procedure for finding the best-fitting curve
Liquid Mixtures of Xenon with Fluorinated Species: Xenon + Sulfur Hexafluoride
 5284 J. Phys. Chem. B 2007, 111, 5284-5289 Liquid Mixtures of Xenon with Fluorinated Species: Xenon + Sulfur Hexafluoride Lino M. B. Dias,, Eduardo J. M. Filipe,*, Clare McCabe, Telma Cordeiro, and Jorge
5284 J. Phys. Chem. B 2007, 111, 5284-5289 Liquid Mixtures of Xenon with Fluorinated Species: Xenon + Sulfur Hexafluoride Lino M. B. Dias,, Eduardo J. M. Filipe,*, Clare McCabe, Telma Cordeiro, and Jorge
LOWHLL #WEEKLY JOURNAL.
 # F 7 F --) 2 9 Q - Q - - F - x $ 2 F? F \ F q - x q - - - - )< - -? - F - - Q z 2 Q - x -- - - - 3 - % 3 3 - - ) F x - \ - - - - - q - q - - - - -z- < F 7-7- - Q F 2 F - F \x -? - - - - - z - x z F -
# F 7 F --) 2 9 Q - Q - - F - x $ 2 F? F \ F q - x q - - - - )< - -? - F - - Q z 2 Q - x -- - - - 3 - % 3 3 - - ) F x - \ - - - - - q - q - - - - -z- < F 7-7- - Q F 2 F - F \x -? - - - - - z - x z F -
A new segmental local composition model for calculation of thermodynamic properties of binary polymer solutions
 Scientia Iranica C (204) 2(6), 2087{2097 Sharif University of Technology Scientia Iranica Transactions C: Chemistry and Chemical ngineering www.scientiairanica.com A new segmental local composition model
Scientia Iranica C (204) 2(6), 2087{2097 Sharif University of Technology Scientia Iranica Transactions C: Chemistry and Chemical ngineering www.scientiairanica.com A new segmental local composition model
LOWELL WEEKLY JOURNAL
 Y -» $ 5 Y 7 Y Y -Y- Q x Q» 75»»/ q } # ]»\ - - $ { Q» / X x»»- 3 q $ 9 ) Y q - 5 5 3 3 3 7 Q q - - Q _»»/Q Y - 9 - - - )- [ X 7» -» - )»? / /? Q Y»» # X Q» - -?» Q ) Q \ Q - - - 3? 7» -? #»»» 7 - / Q
Y -» $ 5 Y 7 Y Y -Y- Q x Q» 75»»/ q } # ]»\ - - $ { Q» / X x»»- 3 q $ 9 ) Y q - 5 5 3 3 3 7 Q q - - Q _»»/Q Y - 9 - - - )- [ X 7» -» - )»? / /? Q Y»» # X Q» - -?» Q ) Q \ Q - - - 3? 7» -? #»»» 7 - / Q
GG303 Lecture 6 8/27/09 1 SCALARS, VECTORS, AND TENSORS
 GG303 Lecture 6 8/27/09 1 SCALARS, VECTORS, AND TENSORS I Main Topics A Why deal with tensors? B Order of scalars, vectors, and tensors C Linear transformation of scalars and vectors (and tensors) II Why
GG303 Lecture 6 8/27/09 1 SCALARS, VECTORS, AND TENSORS I Main Topics A Why deal with tensors? B Order of scalars, vectors, and tensors C Linear transformation of scalars and vectors (and tensors) II Why
MECH 5312 Solid Mechanics II. Dr. Calvin M. Stewart Department of Mechanical Engineering The University of Texas at El Paso
 MECH 5312 Solid Mechanics II Dr. Calvin M. Stewart Department of Mechanical Engineering The University of Texas at El Paso Table of Contents Preliminary Math Concept of Stress Stress Components Equilibrium
MECH 5312 Solid Mechanics II Dr. Calvin M. Stewart Department of Mechanical Engineering The University of Texas at El Paso Table of Contents Preliminary Math Concept of Stress Stress Components Equilibrium
Chemistry 5350 Advanced Physical Chemistry Fall Semester 2013
 Chemistry 5350 Advanced Physical Chemistry Fall Semester 2013 Mierm Examination: Thermodynamics and Kinetics Name: October 10, 2013 Constants and Conversion Factors Gas Constants: 8.314 J mol 1 K 1 ; 8.314
Chemistry 5350 Advanced Physical Chemistry Fall Semester 2013 Mierm Examination: Thermodynamics and Kinetics Name: October 10, 2013 Constants and Conversion Factors Gas Constants: 8.314 J mol 1 K 1 ; 8.314
Calculating thermodynamic properties from perturbation theory I. An analytic representation of square-well potential hard-sphere perturbation theory
 Ž. Fluid Phase Equilibria 154 1999 1 1 Calculating thermodynamic properties from perturbation theory I. An analytic representation of square-well potential hard-sphere perturbation theory Bing-Jian Zhang
Ž. Fluid Phase Equilibria 154 1999 1 1 Calculating thermodynamic properties from perturbation theory I. An analytic representation of square-well potential hard-sphere perturbation theory Bing-Jian Zhang
Homework 1/Solutions. Graded Exercises
 MTH 310-3 Abstract Algebra I and Number Theory S18 Homework 1/Solutions Graded Exercises Exercise 1. Below are parts of the addition table and parts of the multiplication table of a ring. Complete both
MTH 310-3 Abstract Algebra I and Number Theory S18 Homework 1/Solutions Graded Exercises Exercise 1. Below are parts of the addition table and parts of the multiplication table of a ring. Complete both
Accuracy of vapour ^ liquid critical points computed from cubic equations of state
 High Temperatures ^ High Pressures 2000 volume 32 pages 449 ^ 459 15 ECTP Proceedings pages 433 ^ 443 DOI:10.1068/htwu303 Accuracy of vapour ^ liquid critical points computed from cubic equations of state
High Temperatures ^ High Pressures 2000 volume 32 pages 449 ^ 459 15 ECTP Proceedings pages 433 ^ 443 DOI:10.1068/htwu303 Accuracy of vapour ^ liquid critical points computed from cubic equations of state
AAE COMBUSTION AND THERMOCHEMISTRY
 5. COMBUSTIO AD THERMOCHEMISTRY Ch5 1 Overview Definition & mathematical determination of chemical equilibrium, Definition/determination of adiabatic flame temperature, Prediction of composition and temperature
5. COMBUSTIO AD THERMOCHEMISTRY Ch5 1 Overview Definition & mathematical determination of chemical equilibrium, Definition/determination of adiabatic flame temperature, Prediction of composition and temperature
On intramolecular and intermolecular hydrogen bonding
 Ž. Fluid Phase Equilibria 156 1999 51 56 On intramolecular and intermolecular hydrogen bonding Doukeni Missopolinou, Costas Panayiotou ) Department of Chemical Engineering, UniÕersity of Thessaloniki,
Ž. Fluid Phase Equilibria 156 1999 51 56 On intramolecular and intermolecular hydrogen bonding Doukeni Missopolinou, Costas Panayiotou ) Department of Chemical Engineering, UniÕersity of Thessaloniki,
Closed-Form Solution Of Absolute Orientation Using Unit Quaternions
 Closed-Form Solution Of Absolute Orientation Using Unit Berthold K. P. Horn Department of Computer and Information Sciences November 11, 2004 Outline 1 Introduction 2 3 The Problem Given: two sets of corresponding
Closed-Form Solution Of Absolute Orientation Using Unit Berthold K. P. Horn Department of Computer and Information Sciences November 11, 2004 Outline 1 Introduction 2 3 The Problem Given: two sets of corresponding
P A L A C E P IE R, S T. L E O N A R D S. R a n n o w, q u a r r y. W WALTER CR O TC H, Esq., Local Chairman. E. CO O PER EVANS, Esq.,.
 ? ( # [ ( 8? [ > 3 Q [ ««> » 9 Q { «33 Q> 8 \ \ 3 3 3> Q»«9 Q ««« 3 8 3 8 X \ [ 3 ( ( Z ( Z 3( 9 9 > < < > >? 8 98 ««3 ( 98 < # # Q 3 98? 98 > > 3 8 9 9 ««««> 3 «>
? ( # [ ( 8? [ > 3 Q [ ««> » 9 Q { «33 Q> 8 \ \ 3 3 3> Q»«9 Q ««« 3 8 3 8 X \ [ 3 ( ( Z ( Z 3( 9 9 > < < > >? 8 98 ««3 ( 98 < # # Q 3 98? 98 > > 3 8 9 9 ««««> 3 «>
The Product Operator Formalism
 2 The Product Operator Formalism 1. INTRODUCTION In this section we will see that the density matrix at equilibrium can be expressed in terms of the spin angular momentum component I z of each nucleus.
2 The Product Operator Formalism 1. INTRODUCTION In this section we will see that the density matrix at equilibrium can be expressed in terms of the spin angular momentum component I z of each nucleus.
P(N,V,T) = NRT V. = P(N,V,T) dv
 CHEM-443, Fall 2016, Section 010 Student Name Quiz 1 09/09/2016 Directions: Please answer each question to the best of your ability. Make sure your response is legible, precise, includes relevant dimensional
CHEM-443, Fall 2016, Section 010 Student Name Quiz 1 09/09/2016 Directions: Please answer each question to the best of your ability. Make sure your response is legible, precise, includes relevant dimensional
Fluid Phase Equilibria (2001)
 Fluid Phase Equilibria 187 188 (2001) 299 310 Vapor liquid equilibria for the binary systems decane + 1,1-dimethylethyl methyl ether (MTBE) and decane + 1,1-dimethylpropyl methyl ether (TAME) at 308.15,
Fluid Phase Equilibria 187 188 (2001) 299 310 Vapor liquid equilibria for the binary systems decane + 1,1-dimethylethyl methyl ether (MTBE) and decane + 1,1-dimethylpropyl methyl ether (TAME) at 308.15,
Accurate thermodynamic-property models for CO 2 -rich mixtures
 Available online at www.sciencedirect.com Energy Procedia 37 (2013 ) 2914 2922 GHGT-11 Accurate thermodynamic-property models for CO 2 -rich mixtures Roland Span*, Johannes Gernert, Andreas Jäger Thermodynamics,
Available online at www.sciencedirect.com Energy Procedia 37 (2013 ) 2914 2922 GHGT-11 Accurate thermodynamic-property models for CO 2 -rich mixtures Roland Span*, Johannes Gernert, Andreas Jäger Thermodynamics,
Experimental Investigation of Excess molar enthalpies of binary mixtures formed by ethyl acetate (with Cyclohexane or 1-Butanol or 1- Hexene)
 Experimental Investigation of Excess molar enthalpies of binary mixtures formed by ethyl acetate (with Cyclohexane or 1-Butanol or 1- Hexene) Mahendra V. Guddad 1*, K. L. Shivabasappa 2, Bhausaheb L. Pangarkar
Experimental Investigation of Excess molar enthalpies of binary mixtures formed by ethyl acetate (with Cyclohexane or 1-Butanol or 1- Hexene) Mahendra V. Guddad 1*, K. L. Shivabasappa 2, Bhausaheb L. Pangarkar
Asymptotic Multivariate Kriging Using Estimated Parameters with Bayesian Prediction Methods for Non-linear Predictands
 Asymptotic Multivariate Kriging Using Estimated Parameters with Bayesian Prediction Methods for Non-linear Predictands Elizabeth C. Mannshardt-Shamseldin Advisor: Richard L. Smith Duke University Department
Asymptotic Multivariate Kriging Using Estimated Parameters with Bayesian Prediction Methods for Non-linear Predictands Elizabeth C. Mannshardt-Shamseldin Advisor: Richard L. Smith Duke University Department
Surface and interfacial tensions of the systems water + n-butyl acetate + methanol and water + n-pentyl acetate + methanol at 303.
 Fluid Phase Equilibria 08 (003) 1 1 Surface and interfacial tensions of the systems water + n-butyl acetate + methanol and water + n-pentyl acetate + methanol at 303.15 K B.M.S. Santos, A.G.M. Ferreira,
Fluid Phase Equilibria 08 (003) 1 1 Surface and interfacial tensions of the systems water + n-butyl acetate + methanol and water + n-pentyl acetate + methanol at 303.15 K B.M.S. Santos, A.G.M. Ferreira,
Vapor-liquid equilibrium
 Vapor-liquid equilibrium From Wikipedia, the free encyclopedia Vapor-liquid equilibrium, abbreviated as VLE by some, is a condition where a liquid and its vapor (gas phase) are in equilibrium with each
Vapor-liquid equilibrium From Wikipedia, the free encyclopedia Vapor-liquid equilibrium, abbreviated as VLE by some, is a condition where a liquid and its vapor (gas phase) are in equilibrium with each
G T = G f (product) G f (reactant) = kj/mol Ka (550 O C) = exp(8840/(8.314*( )) = 3.369
 (7.) An equimolar mixture of H and CO can be obtained by the reaction of steam with coal. Compute the equilibrium compositions at 550 C based on an equimolar feed of H, CO, and HO. The reaction is H O
(7.) An equimolar mixture of H and CO can be obtained by the reaction of steam with coal. Compute the equilibrium compositions at 550 C based on an equimolar feed of H, CO, and HO. The reaction is H O
MECH 5312 Solid Mechanics II. Dr. Calvin M. Stewart Department of Mechanical Engineering The University of Texas at El Paso
 MECH 5312 Solid Mechanics II Dr. Calvin M. Stewart Department of Mechanical Engineering The University of Texas at El Paso Table of Contents Thermodynamics Derivation Hooke s Law: Anisotropic Elasticity
MECH 5312 Solid Mechanics II Dr. Calvin M. Stewart Department of Mechanical Engineering The University of Texas at El Paso Table of Contents Thermodynamics Derivation Hooke s Law: Anisotropic Elasticity
Kirkwood-Buff Integrals for Aqueous Urea Solutions Based upon the Quantum Chemical Electrostatic Potential and Interaction Energies
 Supporting Information for Kirkwood-Buff Integrals for Aqueous Urea Solutions Based upon the Quantum Chemical Electrostatic Potential and Interaction Energies Shuntaro Chiba, 1* Tadaomi Furuta, 2 and Seishi
Supporting Information for Kirkwood-Buff Integrals for Aqueous Urea Solutions Based upon the Quantum Chemical Electrostatic Potential and Interaction Energies Shuntaro Chiba, 1* Tadaomi Furuta, 2 and Seishi
Fluid data consistency testing incorporation into the ThermoDataEngine
 Fluid data consistency testing incorporation into the ThermoEngine Vladimir Diky 2, John O'Connell 1 *, Jens Abildskov 3, Kenneth Kroenlein 2, Michael Frenkel 2 1 Professor Emeritus, University of Virginia,
Fluid data consistency testing incorporation into the ThermoEngine Vladimir Diky 2, John O'Connell 1 *, Jens Abildskov 3, Kenneth Kroenlein 2, Michael Frenkel 2 1 Professor Emeritus, University of Virginia,
Feasibility Study of Heterogeneous Batch Extractive Distillation
 European Symposium on Computer Arded Aided Process Engineering 15 L. Puigjaner and A. Espuña (Editors) 2005 Elsevier Science B.V. All rights reserved. Feasibility Study of Heterogeneous Batch Extractive
European Symposium on Computer Arded Aided Process Engineering 15 L. Puigjaner and A. Espuña (Editors) 2005 Elsevier Science B.V. All rights reserved. Feasibility Study of Heterogeneous Batch Extractive
We can see from the gas phase form of the equilibrium constant that pressure of species depend on pressure. For the general gas phase reaction,
 Pressure dependence Equilibrium constant We can see from the gas phase form of the equilibrium constant that the equilibrium concentrations of species depend on pressure. This dependence is inside the
Pressure dependence Equilibrium constant We can see from the gas phase form of the equilibrium constant that the equilibrium concentrations of species depend on pressure. This dependence is inside the
LOWELL JOURNAL. MUST APOLOGIZE. such communication with the shore as Is m i Boimhle, noewwary and proper for the comfort
 - 7 7 Z 8 q ) V x - X > q - < Y Y X V - z - - - - V - V - q \ - q q < -- V - - - x - - V q > x - x q - x q - x - - - 7 -» - - - - 6 q x - > - - x - - - x- - - q q - V - x - - ( Y q Y7 - >»> - x Y - ] [
- 7 7 Z 8 q ) V x - X > q - < Y Y X V - z - - - - V - V - q \ - q q < -- V - - - x - - V q > x - x q - x q - x - - - 7 -» - - - - 6 q x - > - - x - - - x- - - q q - V - x - - ( Y q Y7 - >»> - x Y - ] [
Measurement and Calculation of Physico-Chemical Properties of Binary Mixtures Containing Xylene and 1- Alkanol
 Chemical Methodologies 2(2018) 308-314 Chemical Methodologies Journal homepage: http://chemmethod.com Original Research article Measurement and Calculation of Physico-Chemical Properties of Binary Mixtures
Chemical Methodologies 2(2018) 308-314 Chemical Methodologies Journal homepage: http://chemmethod.com Original Research article Measurement and Calculation of Physico-Chemical Properties of Binary Mixtures
Volumetric Study of the Binary Mixtures Containing a Branched Hexane and an Isomeric Chlorobutane
 78 Journal of Applied Solution Chemistry and Modeling, 205, 4, 78-84 Volumetric Study of the Binary Mixtures Containing a Branched Hexane and an Isomeric Chlorobutane Hernando Guerrero, Félix M. Royo and
78 Journal of Applied Solution Chemistry and Modeling, 205, 4, 78-84 Volumetric Study of the Binary Mixtures Containing a Branched Hexane and an Isomeric Chlorobutane Hernando Guerrero, Félix M. Royo and
Phase Separation Degree of Freedom Analysis. Binary Vapor-Liquid Systems. Azeotropic Systems. - Gibbs phase rule F C P 2 -General analysis
 Lecture 5. Single Equilibrium Stages (1) Phase Separation [Ch. 4] Degree of Freedom Analysis - Gibbs phase rule F CP2 -General analysis Binary Vapor-Liquid Systems - Examples of binary system - Phase equilibrium
Lecture 5. Single Equilibrium Stages (1) Phase Separation [Ch. 4] Degree of Freedom Analysis - Gibbs phase rule F CP2 -General analysis Binary Vapor-Liquid Systems - Examples of binary system - Phase equilibrium
Outline of the Course
 Outline of the Course 1) Review and Definitions 2) Molecules and their Energies 3) 1 st Law of Thermodynamics Conservation of Energy. 4) 2 nd Law of Thermodynamics Ever-Increasing Entropy. 5) Gibbs Free
Outline of the Course 1) Review and Definitions 2) Molecules and their Energies 3) 1 st Law of Thermodynamics Conservation of Energy. 4) 2 nd Law of Thermodynamics Ever-Increasing Entropy. 5) Gibbs Free
MECH 6661 lecture 9/1 Dr. M. Medraj Mech. Eng. Dept. - Concordia University
 Thermodynamic Models Multicomponent Systems Outline Thermodynamic Models Regular Solution Models Sublattice Model Associated Solutions Cluster Variation Model Quasichemical Model Cluster Expansion Model
Thermodynamic Models Multicomponent Systems Outline Thermodynamic Models Regular Solution Models Sublattice Model Associated Solutions Cluster Variation Model Quasichemical Model Cluster Expansion Model
IlE. Simulation of a Distillation Column on an IBM PC, Accounting for Real Behaviour of Electrolyte Solutions * c I
 ~~ Chem. Eng. Technol. 14 (1991) 295-300 295 Simulation of a Distillation Column on an IBM PC, Accounting for Real Behaviour of Electrolyte Solutions * Andre Bosen and Manfred Roth** This contribution
~~ Chem. Eng. Technol. 14 (1991) 295-300 295 Simulation of a Distillation Column on an IBM PC, Accounting for Real Behaviour of Electrolyte Solutions * Andre Bosen and Manfred Roth** This contribution
Linear Algebra Review
 Linear Algebra Review Yang Feng http://www.stat.columbia.edu/~yangfeng Yang Feng (Columbia University) Linear Algebra Review 1 / 45 Definition of Matrix Rectangular array of elements arranged in rows and
Linear Algebra Review Yang Feng http://www.stat.columbia.edu/~yangfeng Yang Feng (Columbia University) Linear Algebra Review 1 / 45 Definition of Matrix Rectangular array of elements arranged in rows and
Inverse of a Square Matrix. For an N N square matrix A, the inverse of A, 1
 Inverse of a Square Matrix For an N N square matrix A, the inverse of A, 1 A, exists if and only if A is of full rank, i.e., if and only if no column of A is a linear combination 1 of the others. A is
Inverse of a Square Matrix For an N N square matrix A, the inverse of A, 1 A, exists if and only if A is of full rank, i.e., if and only if no column of A is a linear combination 1 of the others. A is
Note: items marked with * you should be able to perform on a closed book exam. Chapter 10 Learning Objective Checklist
 Note: items marked with * you should be able to perform on a closed book exam. Chapter 10 Learning Objective Checklist Sections 10.1-10.13 find pure component properties on a binary P-x-y or T-x-y diagram.*
Note: items marked with * you should be able to perform on a closed book exam. Chapter 10 Learning Objective Checklist Sections 10.1-10.13 find pure component properties on a binary P-x-y or T-x-y diagram.*
Thermodynamics of Reactive Systems The Equilibrium Constant
 Lecture 27 Thermodynamics of Reactive Systems The Equilibrium Constant A. K. M. B. Rashid rofessor, Department of MME BUET, Dhaka Today s Topics The Equilibrium Constant Free Energy and Equilibrium Constant
Lecture 27 Thermodynamics of Reactive Systems The Equilibrium Constant A. K. M. B. Rashid rofessor, Department of MME BUET, Dhaka Today s Topics The Equilibrium Constant Free Energy and Equilibrium Constant
LOWELL WEEKLY JOURNAL
 Y G y G Y 87 y Y 8 Y - $ X ; ; y y q 8 y $8 $ $ $ G 8 q < 8 6 4 y 8 7 4 8 8 < < y 6 $ q - - y G y G - Y y y 8 y y y Y Y 7-7- G - y y y ) y - y y y y - - y - y 87 7-7- G G < G y G y y 6 X y G y y y 87 G
Y G y G Y 87 y Y 8 Y - $ X ; ; y y q 8 y $8 $ $ $ G 8 q < 8 6 4 y 8 7 4 8 8 < < y 6 $ q - - y G y G - Y y y 8 y y y Y Y 7-7- G - y y y ) y - y y y y - - y - y 87 7-7- G G < G y G y y 6 X y G y y y 87 G
Prediction of phase equilibria in waterralcoholralkane systems
 Fluid Phase Equilibria 158 160 1999 151 163 Prediction of phase equilibria in waterralcoholralkane systems Epaminondas C. Voutsas ), Iakovos V. Yakoumis, Dimitrios P. Tassios Laboratory of Thermodynamics
Fluid Phase Equilibria 158 160 1999 151 163 Prediction of phase equilibria in waterralcoholralkane systems Epaminondas C. Voutsas ), Iakovos V. Yakoumis, Dimitrios P. Tassios Laboratory of Thermodynamics
Name: Discussion Section:
 CBE 141: Chemical Engineering Thermodynamics, Spring 2018, UC Berkeley Midterm 2 March 22, 2018 Time: 80 minutes, closed-book and closed-notes, one-sided 8 ½ x 11 equation sheet allowed Please show all
CBE 141: Chemical Engineering Thermodynamics, Spring 2018, UC Berkeley Midterm 2 March 22, 2018 Time: 80 minutes, closed-book and closed-notes, one-sided 8 ½ x 11 equation sheet allowed Please show all
Reduced rank regression in cointegrated models
 Journal of Econometrics 06 (2002) 203 26 www.elsevier.com/locate/econbase Reduced rank regression in cointegrated models.w. Anderson Department of Statistics, Stanford University, Stanford, CA 94305-4065,
Journal of Econometrics 06 (2002) 203 26 www.elsevier.com/locate/econbase Reduced rank regression in cointegrated models.w. Anderson Department of Statistics, Stanford University, Stanford, CA 94305-4065,
A Probability Review
 A Probability Review Outline: A probability review Shorthand notation: RV stands for random variable EE 527, Detection and Estimation Theory, # 0b 1 A Probability Review Reading: Go over handouts 2 5 in
A Probability Review Outline: A probability review Shorthand notation: RV stands for random variable EE 527, Detection and Estimation Theory, # 0b 1 A Probability Review Reading: Go over handouts 2 5 in
Determination of the nonsolvent polymer interaction parameter χ 13 in the casting solutions
 Journal of Membrane Science 174 (2000) 87 96 Determination of the nonsolvent polymer interaction parameter χ 13 in the casting solutions Zhansheng Li, Chengzhang Jiang Dalian Institute of Chemical Physics,
Journal of Membrane Science 174 (2000) 87 96 Determination of the nonsolvent polymer interaction parameter χ 13 in the casting solutions Zhansheng Li, Chengzhang Jiang Dalian Institute of Chemical Physics,
THERMODYNAMIC EXCESS PROPERTIES IN BINARY MIXTURES OF MOLECULES OF DIFFERENT SIZES AND SHAPES. Introduction
 THERMODYNAMIC EXCESS PROPERTIES IN BINARY MIXTURES OF MOLECULES OF DIFFERENT SIZES AND SHAPES Dana Dragoescu, A. Barhala and Rodica Vilcu Isothermal vapour-liquid equilibrium (VLE) data for binary mixtures
THERMODYNAMIC EXCESS PROPERTIES IN BINARY MIXTURES OF MOLECULES OF DIFFERENT SIZES AND SHAPES Dana Dragoescu, A. Barhala and Rodica Vilcu Isothermal vapour-liquid equilibrium (VLE) data for binary mixtures
Chemical Thermodynamics. Chapter 18
 Chemical Thermodynamics Chapter 18 Thermodynamics Spontaneous Processes Entropy and Second Law of Thermodynamics Entropy Changes Gibbs Free Energy Free Energy and Temperature Free Energy and Equilibrium
Chemical Thermodynamics Chapter 18 Thermodynamics Spontaneous Processes Entropy and Second Law of Thermodynamics Entropy Changes Gibbs Free Energy Free Energy and Temperature Free Energy and Equilibrium
Density, Excess Molar Volumes of Water-Ethanol Binary Mixtures at Various Temperatures
 2017 IJSRST Volume 3 Issue 10 Print ISSN: 2395-6011 Online ISSN: 2395-602X National Conference on Research and Developments in Synthetic Organic Chemistry Density, Excess Molar Volumes of Water-Ethanol
2017 IJSRST Volume 3 Issue 10 Print ISSN: 2395-6011 Online ISSN: 2395-602X National Conference on Research and Developments in Synthetic Organic Chemistry Density, Excess Molar Volumes of Water-Ethanol
Department of mathematics MA201 Mathematics III
 Department of mathematics MA201 Mathematics III Academic Year 2015-2016 Model Solutions: Quiz-II (Set - B) 1. Obtain the bilinear transformation which maps the points z 0, 1, onto the points w i, 1, i
Department of mathematics MA201 Mathematics III Academic Year 2015-2016 Model Solutions: Quiz-II (Set - B) 1. Obtain the bilinear transformation which maps the points z 0, 1, onto the points w i, 1, i
2x (x 2 + y 2 + 1) 2 2y. (x 2 + y 2 + 1) 4. 4xy. (1, 1)(x 1) + (1, 1)(y + 1) (1, 1)(x 1)(y + 1) 81 x y y + 7.
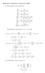 Homework 8 Solutions, November 007. (1 We calculate some derivatives: f x = f y = x (x + y + 1 y (x + y + 1 x = (x + y + 1 4x (x + y + 1 4 y = (x + y + 1 4y (x + y + 1 4 x y = 4xy (x + y + 1 4 Substituting
Homework 8 Solutions, November 007. (1 We calculate some derivatives: f x = f y = x (x + y + 1 y (x + y + 1 x = (x + y + 1 4x (x + y + 1 4 y = (x + y + 1 4y (x + y + 1 4 x y = 4xy (x + y + 1 4 Substituting
Speeds of sound and isothermal compressibility of ternary liquid systems: Application of Flory s statistical theory and hard sphere models
 PRAMANA c Indian Academy of Sciences Vol. 70, No. 4 journal of April 2008 physics pp. 731 738 Speeds of sound and isothermal compressibility of ternary liquid systems: Application of Flory s statistical
PRAMANA c Indian Academy of Sciences Vol. 70, No. 4 journal of April 2008 physics pp. 731 738 Speeds of sound and isothermal compressibility of ternary liquid systems: Application of Flory s statistical
Weighted Least Squares
 Weighted Least Squares The standard linear model assumes that Var(ε i ) = σ 2 for i = 1,..., n. As we have seen, however, there are instances where Var(Y X = x i ) = Var(ε i ) = σ2 w i. Here w 1,..., w
Weighted Least Squares The standard linear model assumes that Var(ε i ) = σ 2 for i = 1,..., n. As we have seen, however, there are instances where Var(Y X = x i ) = Var(ε i ) = σ2 w i. Here w 1,..., w
THERMODYNAMIC CONSISTENCY TESTS FOR PHASE EQUILIBRIUM IN LIQUID SOLUTE+SUPERCRITICAL SOLVENT MIXTURES
 THERMODYNAMIC CONSISTENCY TESTS FOR PHASE EQUILIBRIUM IN LIQUID SOLUTE+SUPERCRITICAL SOLVENT MIXTURES José O. Valderrama 1, and Víctor H. Alvarez 1 Fac. de Ingeniería, Depto. Ing. Mecánica, Univ. de la
THERMODYNAMIC CONSISTENCY TESTS FOR PHASE EQUILIBRIUM IN LIQUID SOLUTE+SUPERCRITICAL SOLVENT MIXTURES José O. Valderrama 1, and Víctor H. Alvarez 1 Fac. de Ingeniería, Depto. Ing. Mecánica, Univ. de la
Solubilities of some new refrigerants in water
 Fluid Phase Equilibria 173 (2000) 97 107 Solubilities of some new refrigerants in water Alexandre A.F. Miguel, Abel G.M. Ferreira, Isabel M.A. Fonseca Department of Chemical Engineering, University of
Fluid Phase Equilibria 173 (2000) 97 107 Solubilities of some new refrigerants in water Alexandre A.F. Miguel, Abel G.M. Ferreira, Isabel M.A. Fonseca Department of Chemical Engineering, University of
Review Article. VLE Properties from ISM Equation of State: Application to Pure and Mixture
 Review Article PHYSICAL CHEMISTRY RESEARCH Published by the Iranian Chemical Society www.physchemres.org info@physchemres.org Phys. Chem. Res., Vol., No. 1, 16-, March 015. DOI: 10.06/pcr.015.798 VLE Properties
Review Article PHYSICAL CHEMISTRY RESEARCH Published by the Iranian Chemical Society www.physchemres.org info@physchemres.org Phys. Chem. Res., Vol., No. 1, 16-, March 015. DOI: 10.06/pcr.015.798 VLE Properties
Elasticité de surface. P. Muller and A. Saul Surf. Sci Rep. 54, 157 (2004).
 Elasticité de surface P. Muller and A. Saul Surf. Sci Rep. 54, 157 (2004). The concept I Physical origin Definition Applications Surface stress and crystallographic parameter of small crystals Surface
Elasticité de surface P. Muller and A. Saul Surf. Sci Rep. 54, 157 (2004). The concept I Physical origin Definition Applications Surface stress and crystallographic parameter of small crystals Surface
Chapter 5 Matrix Approach to Simple Linear Regression
 STAT 525 SPRING 2018 Chapter 5 Matrix Approach to Simple Linear Regression Professor Min Zhang Matrix Collection of elements arranged in rows and columns Elements will be numbers or symbols For example:
STAT 525 SPRING 2018 Chapter 5 Matrix Approach to Simple Linear Regression Professor Min Zhang Matrix Collection of elements arranged in rows and columns Elements will be numbers or symbols For example:
Modified Raoult's Law and Excess Gibbs Free Energy
 ACTIVITY MODELS 1 Modified Raoult's Law and Excess Gibbs Free Energy Equilibrium criteria: f V i = L f i For vapor phase: f V i = y i i P For liquid phase, we may use an activity coefficient ( i ), giving:
ACTIVITY MODELS 1 Modified Raoult's Law and Excess Gibbs Free Energy Equilibrium criteria: f V i = L f i For vapor phase: f V i = y i i P For liquid phase, we may use an activity coefficient ( i ), giving:
Thermodynamics (Classical) for Biological Systems Prof. G. K. Suraishkumar Department of Biotechnology Indian Institute of Technology Madras
 Thermodynamics (Classical) for Biological Systems Prof. G. K. Suraishkumar Department of Biotechnology Indian Institute of Technology Madras Module No. # 04 Thermodynamics of Solutions Lecture No. # 25
Thermodynamics (Classical) for Biological Systems Prof. G. K. Suraishkumar Department of Biotechnology Indian Institute of Technology Madras Module No. # 04 Thermodynamics of Solutions Lecture No. # 25
1 EM algorithm: updating the mixing proportions {π k } ik are the posterior probabilities at the qth iteration of EM.
 Université du Sud Toulon - Var Master Informatique Probabilistic Learning and Data Analysis TD: Model-based clustering by Faicel CHAMROUKHI Solution The aim of this practical wor is to show how the Classification
Université du Sud Toulon - Var Master Informatique Probabilistic Learning and Data Analysis TD: Model-based clustering by Faicel CHAMROUKHI Solution The aim of this practical wor is to show how the Classification
LB 220 Homework 4 Solutions
 LB 220 Homework 4 Solutions Section 11.4, # 40: This problem was solved in class on Feb. 03. Section 11.4, # 42: This problem was also solved in class on Feb. 03. Section 11.4, # 43: Also solved in class
LB 220 Homework 4 Solutions Section 11.4, # 40: This problem was solved in class on Feb. 03. Section 11.4, # 42: This problem was also solved in class on Feb. 03. Section 11.4, # 43: Also solved in class
Topic 7 - Matrix Approach to Simple Linear Regression. Outline. Matrix. Matrix. Review of Matrices. Regression model in matrix form
 Topic 7 - Matrix Approach to Simple Linear Regression Review of Matrices Outline Regression model in matrix form - Fall 03 Calculations using matrices Topic 7 Matrix Collection of elements arranged in
Topic 7 - Matrix Approach to Simple Linear Regression Review of Matrices Outline Regression model in matrix form - Fall 03 Calculations using matrices Topic 7 Matrix Collection of elements arranged in
Physical Chemistry Chapter 4 The Properties of Mixtures
 Physical Chemistry Chapter 4 The Properties of Mixtures by Izirwan Bin Izhab FKKSA izirwan@ump.edu.my Chapter Description Aims Determine the fugacity and fugacity coefficients for pure species using generic
Physical Chemistry Chapter 4 The Properties of Mixtures by Izirwan Bin Izhab FKKSA izirwan@ump.edu.my Chapter Description Aims Determine the fugacity and fugacity coefficients for pure species using generic
Non-linear least-squares and chemical kinetics. An improved method to analyse monomer-excimer decay data
 Journal of Mathematical Chemistry 21 (1997) 131 139 131 Non-linear least-squares and chemical kinetics. An improved method to analyse monomer-excimer decay data J.P.S. Farinha a, J.M.G. Martinho a and
Journal of Mathematical Chemistry 21 (1997) 131 139 131 Non-linear least-squares and chemical kinetics. An improved method to analyse monomer-excimer decay data J.P.S. Farinha a, J.M.G. Martinho a and
Chapter 2: Basic Governing Equations
 -1 Reynolds Transport Theorem (RTT) - Continuity Equation -3 The Linear Momentum Equation -4 The First Law of Thermodynamics -5 General Equation in Conservative Form -6 General Equation in Non-Conservative
-1 Reynolds Transport Theorem (RTT) - Continuity Equation -3 The Linear Momentum Equation -4 The First Law of Thermodynamics -5 General Equation in Conservative Form -6 General Equation in Non-Conservative
' Liberty and Umou Ono and Inseparablo "
 3 5? #< q 8 2 / / ) 9 ) 2 ) > < _ / ] > ) 2 ) ) 5 > x > [ < > < ) > _ ] ]? <
3 5? #< q 8 2 / / ) 9 ) 2 ) > < _ / ] > ) 2 ) ) 5 > x > [ < > < ) > _ ] ]? <
Calculation of Excess Adiabatic Compressibility from Ternary Liquid Experimental Data
 Calculation of Excess Adiabatic Compressibility from Ternary Liquid Experimental Data Vladimír Hanta 1, Lenka Morávková 2 1 Department of Computing and Control Engineering, Faculty of Chemical Engineering,
Calculation of Excess Adiabatic Compressibility from Ternary Liquid Experimental Data Vladimír Hanta 1, Lenka Morávková 2 1 Department of Computing and Control Engineering, Faculty of Chemical Engineering,
Liquids and Solutions
 Liquids and Solutions Introduction This course examines the properties of liquids and solutions at both the thermodynamic and the molecular level. The main topics are: Liquids, Ideal and Regular Solutions,
Liquids and Solutions Introduction This course examines the properties of liquids and solutions at both the thermodynamic and the molecular level. The main topics are: Liquids, Ideal and Regular Solutions,
Crrelation of Isoprene-Maleic Anhydride Reaction Rate Constants in
 123 Received December 26, 2005 Accepted for Publication March 31, 2006 2006 Soc. Mater. Eng. Resour. Japan(C) Crrelation of Isoprene-Maleic Anhydride Reaction Rate Constants in Mixed Solvents Haruo KATo,
123 Received December 26, 2005 Accepted for Publication March 31, 2006 2006 Soc. Mater. Eng. Resour. Japan(C) Crrelation of Isoprene-Maleic Anhydride Reaction Rate Constants in Mixed Solvents Haruo KATo,
Phase equilibrium studies of impure CO 2 systems to underpin developments of CCS technologies
 Phase equilibrium studies of impure CO 2 systems to underpin developments of CCS technologies Jie Ke, Martyn Poliakoff and Michael W. George School of Chemistry The University of Nottingham 1 September,
Phase equilibrium studies of impure CO 2 systems to underpin developments of CCS technologies Jie Ke, Martyn Poliakoff and Michael W. George School of Chemistry The University of Nottingham 1 September,
A note on a difference type estimator for population mean under two phase sampling design
 Khan and Al Hossain SpringerPlus 0165:73 DOI 10.1186/s40064-016-368-1 RESEARCH Open Access A note on a dference type estimator for population mean under two phase sampling design Mursala Khan 1* and Abdullah
Khan and Al Hossain SpringerPlus 0165:73 DOI 10.1186/s40064-016-368-1 RESEARCH Open Access A note on a dference type estimator for population mean under two phase sampling design Mursala Khan 1* and Abdullah
Lecture 13: Simple Linear Regression in Matrix Format. 1 Expectations and Variances with Vectors and Matrices
 Lecture 3: Simple Linear Regression in Matrix Format To move beyond simple regression we need to use matrix algebra We ll start by re-expressing simple linear regression in matrix form Linear algebra is
Lecture 3: Simple Linear Regression in Matrix Format To move beyond simple regression we need to use matrix algebra We ll start by re-expressing simple linear regression in matrix form Linear algebra is
DETERMINATION AND PREDICTION OF THE ISOBARIC VAPOR- LIQUID-LIQUID EQUILIBRIUM DATA
 DETERMINATION AND PREDICTION OF THE ISOBARIC VAPOR- LIQUID-LIQUID EQUILIBRIUM DATA Koichi Iwakabe and Hitoshi Kosuge Department of Chemical Engineering, Tokyo Institute of Technology 12-1, Ookayama-2,
DETERMINATION AND PREDICTION OF THE ISOBARIC VAPOR- LIQUID-LIQUID EQUILIBRIUM DATA Koichi Iwakabe and Hitoshi Kosuge Department of Chemical Engineering, Tokyo Institute of Technology 12-1, Ookayama-2,
Mathematics 222a Quiz 2 CODE 111 November 21, 2002
 Student s Name [print] Student Number Mathematics 222a Instructions: Print your name and student number at the top of this question sheet. Print your name and your instructor s name on the answer sheet.
Student s Name [print] Student Number Mathematics 222a Instructions: Print your name and student number at the top of this question sheet. Print your name and your instructor s name on the answer sheet.
We introduce methods that are useful in:
 Instructor: Shengyu Zhang Content Derived Distributions Covariance and Correlation Conditional Expectation and Variance Revisited Transforms Sum of a Random Number of Independent Random Variables more
Instructor: Shengyu Zhang Content Derived Distributions Covariance and Correlation Conditional Expectation and Variance Revisited Transforms Sum of a Random Number of Independent Random Variables more
The Phase Field Method
 The Phase Field Method To simulate microstructural evolutions in materials Nele Moelans Group meeting 8 June 2004 Outline Introduction Phase Field equations Phase Field simulations Grain growth Diffusion
The Phase Field Method To simulate microstructural evolutions in materials Nele Moelans Group meeting 8 June 2004 Outline Introduction Phase Field equations Phase Field simulations Grain growth Diffusion
n i,j+1/2 q i,j * qi+1,j * S i+1/2,j
 Helsinki University of Technology CFD-group/ The Laboratory of Applied Thermodynamics MEMO No CFD/TERMO-5-97 DATE: December 9,997 TITLE A comparison of complete vs. simplied viscous terms in boundary layer
Helsinki University of Technology CFD-group/ The Laboratory of Applied Thermodynamics MEMO No CFD/TERMO-5-97 DATE: December 9,997 TITLE A comparison of complete vs. simplied viscous terms in boundary layer
LOWELL WEEKLY JOURNAL. ^Jberxy and (Jmott Oao M d Ccmsparftble. %m >ai ruv GEEAT INDUSTRIES
 ? (») /»» 9 F ( ) / ) /»F»»»»»# F??»»» Q ( ( »»» < 3»» /» > > } > Q ( Q > Z F 5
? (») /»» 9 F ( ) / ) /»F»»»»»# F??»»» Q ( ( »»» < 3»» /» > > } > Q ( Q > Z F 5
MANY BILLS OF CONCERN TO PUBLIC
 - 6 8 9-6 8 9 6 9 XXX 4 > -? - 8 9 x 4 z ) - -! x - x - - X - - - - - x 00 - - - - - x z - - - x x - x - - - - - ) x - - - - - - 0 > - 000-90 - - 4 0 x 00 - -? z 8 & x - - 8? > 9 - - - - 64 49 9 x - -
- 6 8 9-6 8 9 6 9 XXX 4 > -? - 8 9 x 4 z ) - -! x - x - - X - - - - - x 00 - - - - - x z - - - x x - x - - - - - ) x - - - - - - 0 > - 000-90 - - 4 0 x 00 - -? z 8 & x - - 8? > 9 - - - - 64 49 9 x - -
i;\-'i frz q > R>? >tr E*+ [S I z> N g> F 'x sa :r> >,9 T F >= = = I Y E H H>tr iir- g-i I * s I!,i --' - = a trx - H tnz rqx o >.F g< s Ire tr () -s
 5 C /? >9 T > ; '. ; J ' ' J. \ ;\' \.> ). L; c\ u ( (J ) \ 1 ) : C ) (... >\ > 9 e!) T C). '1!\ /_ \ '\ ' > 9 C > 9.' \( T Z > 9 > 5 P + 9 9 ) :> : + (. \ z : ) z cf C : u 9 ( :!z! Z c (! $ f 1 :.1 f.
5 C /? >9 T > ; '. ; J ' ' J. \ ;\' \.> ). L; c\ u ( (J ) \ 1 ) : C ) (... >\ > 9 e!) T C). '1!\ /_ \ '\ ' > 9 C > 9.' \( T Z > 9 > 5 P + 9 9 ) :> : + (. \ z : ) z cf C : u 9 ( :!z! Z c (! $ f 1 :.1 f.
Design of Ideal Batch Reactors operated under Isothermal Conditions (It is important to have this note set with you during all lecture classes.
 CP33 Set # July-October 3 Design of Ideal Batch Reactors operated under Isothermal Conditions It is important to have this note set with you during all lecture classes. A batch reactor is such that a batch
CP33 Set # July-October 3 Design of Ideal Batch Reactors operated under Isothermal Conditions It is important to have this note set with you during all lecture classes. A batch reactor is such that a batch
Economics 620, Lecture 5: exp
 1 Economics 620, Lecture 5: The K-Variable Linear Model II Third assumption (Normality): y; q(x; 2 I N ) 1 ) p(y) = (2 2 ) exp (N=2) 1 2 2(y X)0 (y X) where N is the sample size. The log likelihood function
1 Economics 620, Lecture 5: The K-Variable Linear Model II Third assumption (Normality): y; q(x; 2 I N ) 1 ) p(y) = (2 2 ) exp (N=2) 1 2 2(y X)0 (y X) where N is the sample size. The log likelihood function
Analysis of Carleman Representation of Analytical Recursions
 JOURNAL OF MATHEMATICAL ANALYSIS AND APPLICATIONS 224, 8190 1998 ARTICLE NO. AY985986 Analysis of Carleman Representation of Analytical Recursions G. Berolaio Department of Physics, Bar-Ilan Uniersity,
JOURNAL OF MATHEMATICAL ANALYSIS AND APPLICATIONS 224, 8190 1998 ARTICLE NO. AY985986 Analysis of Carleman Representation of Analytical Recursions G. Berolaio Department of Physics, Bar-Ilan Uniersity,
pv m = RT + Bp The simplest equation for calculating fugacity, given an equation of state, is Z=1 + B RT p
 Chem 42/523 Chemical hermodynamics Homework Assignment # 5 1. *Assume O 2 gas obeys the virial equation pv m = R + Bp with B = 12.5 cm 3 mol 1 at 298.15 K. Calculate the fugacity of oxygen at p = 1. MPa
Chem 42/523 Chemical hermodynamics Homework Assignment # 5 1. *Assume O 2 gas obeys the virial equation pv m = R + Bp with B = 12.5 cm 3 mol 1 at 298.15 K. Calculate the fugacity of oxygen at p = 1. MPa
OLI Tips #52 Alloy Manager
 The Right Chemistry OLI Tips #52 Alloy Manager Calculation of the activity of individual components in alloys. The development of this activity model was performed at the Oa Ridge National Laboratory.
The Right Chemistry OLI Tips #52 Alloy Manager Calculation of the activity of individual components in alloys. The development of this activity model was performed at the Oa Ridge National Laboratory.

 «4 [< «
«4 [< «