Finite element and finite volume-element simulation of pseudo-ecgs and cardiac alternans
|
|
|
- Berniece Potter
- 5 years ago
- Views:
Transcription
1 Research Article Received 3 July 2013 Published online 10 April 2014 in Wiley Online Library (wileyonlinelibrary.com) DOI: /mma.3127 MOS subject classification: 35K57; 65M60 Finite element and finite volume-element simulation of pseudo-ecgs and cardiac alternans Marie Dupraz a, Simonetta Filippi b, Alessio Gizzi b, Alfio Quarteroni a,c and Ricardo Ruiz-Baier d * Communicated by S. Wise In this paper, we are interested in the spatio-temporal dynamics of the transmembrane potential in paced isotropic and anisotropic cardiac tissues. In particular, we observe a specific precursor of cardiac arrhythmias that is the presence of alternans in the action potential duration. The underlying mathematical model consists of a reaction diffusion system describing the propagation of the electric potential and the nonlinear interaction with ionic gating variables. Either conforming piecewise continuous finite elements or a finite volume-element scheme are employed for the spatial discretization of all fields, whereas operator splitting strategies of first and second order are used for the time integration. We also describe an efficient mechanism to compute pseudo-ecg signals, and we analyze restitution curves and alternans patterns for physiological and pathological cardiac rhythms. Copyright 2014 John Wiley & Sons, Ltd. Keywords: finite element discretization; finite volume-element method; reaction diffusion system; operator splitting; cardiac alternans; pseudo-ecg; spatio-temporal dynamics 1. Introduction Cardiac arrhythmias represent one of the major causes of morbidity and mortality in industrialized countries. They are associated with important risk factors and comorbidities as hypertension, diabetes, and other aging pathologies, in particular with strokes. Arrhythmias represent, in fact, a pathology included in the priority investigation lines of the European research program HORION International strategies have been oriented toward the development of new and more efficient methods and devices of prevention, as well as medical care in order to face the growing costs connected with an increasing life expectancy. The study of such a complex phenomenon requires, therefore, a Systems Biology multidisciplinary and integrative approach, where clinical and experimental investigations are integrated by in silico analysis. It is well known that heart tissue is a complex biological excitable media that supports rotating activation waves [1, 2] which induce regular and irregular rhythms leading to life threatening arrhythmias. Spiral (two dimensional) and scroll (three dimensional) waves have been experimentally observed and theoretically predicted during tachycardia and out-of phase stimuli. They are recognized to be responsible for fibrillation scenarios [3, 4] both in normal and altered thermal conditions [5, 6]. Based on these considerations, the mathematical modeling of cardiac electrical activity has received a great deal of interest in the last few decades [7]. On the lines of the pioneering work of Hodgkin Huxley, their experimental methodology and theoretical formalism [8], a variety of mathematical models have been introduced to capture some important features of this complex phenomenon, typically regarding the heart as an excitable medium, therefore studying the underlying mechanisms within the framework of reaction diffusion systems (see, e.g., [9]). The quantity and quality of such studies has been also boosted by the increasing capacity of modern computers and numerical techniques (see, e.g., [10]). Virtually, all major types of numerical methods have been successfully employed to simulate 1046 a Modeling and Scientific Computing, MATHICSE, École Polytechnique Fédérale de Lausanne,CH-1015 Lausanne, Switzerland b Nonlinear Physics and Mathematical Modeling Lab, University Campus Bio-Medico of Rome, I Rome, Italy c MOX - Politecnico di Milano, piazza Leonardo da Vinci 32, Milano, Italy d Institute of Earth Sciences, FGSE, University of Lausanne, CH-1015 Lausanne, Switzerland * Correspondence to: Ricardo Ruiz-Baier, Institute of Earth Sciences, FGSE, University of Lausanne, CH-1015 Lausanne, Switzerland. ricardo.ruizbaier@unil.ch
2 M. DUPRA ET AL. the so-called monodomain or bidomain equations in 3D geometries, including finite differences [11, 12], finite volume formulations [13, 14], finite elements (FEs) [15 19], and spectral discretizations [20]. In this work, we focus on the monodomain mathematical formalism to study emerging spatio-temporal dynamics and alternans behavior in two-dimensional and three-dimensional domains. The underlying system of equations consists of a parabolic PDE describing the propagation of membrane depolarization, nonlinearly coupled with a set of ODEs governing the dynamics of gating variables, and ionic concentrations. The membrane kinetics are modeled adopting the four-variable minimal phenomenological model for cardiac action potential propagation from [21]. Despite the current level of ionic models complexity (state-of-the-art models may include more than sixty variables, see, e.g., [22]), the minimal model allows us to reproduce key experimental-based cardiac action potential restitution properties and to investigate pro-arrhythmic states with comparable accuracy to more sophisticated ionic models. In the present work, we further introduce dedicate numerical solvers based on piecewise linear FEs and operator splitting strategies for the space-time discretization. Differently from other computational work in cardiac electrophysiology, these schemes, under some particular conditions, are able to provide accurate and efficient numerical approximations. In addition, we introduce a finite volumeelement (FVE) method, where one of the main goals is to improve the mass conservation properties of the overall discretization. FVE methods are nonstandard hybrid discretizations that combine some features of classical FE methods and finite volume schemes, such as smooth derivability of error estimates in natural norms and local conservativity of fluxes. We recall that the main idea is to write a finite volume method on an adjoint mesh and to use a piecewise constant interpolation operator to recast the formulation as a Petrov Galerkin scheme. Different flavors of these methods have been typically applied in the simulation of flow processes [23 28], and up to our best knowledge, the present paper is the first one addressing FVE discretizations specifically tailored for cardiac-related problems. We point out that a somewhat related method has also been applied to cardiac electrophysiology in [29]; however, our method is more closely linked to the formulations introduced in [30 33] for reaction diffusion systems. Another goal of this paper is to quantitatively assess the accuracy of the proposed numerical schemes in terms of correct reproduction of several electrophysiological scenarios of interest in cardiac modeling. The phenomenon of alternating variations in the amplitude of, for example, membrane action potential waves over successive cardiac cycles is commonly known as cardiac alternans (see, e.g., [34 36]). Our extended numerical study in two and three dimensions allows us to characterize the behavior of these alternans in terms of reconstructed ECG signals (called pseudo-ecg [60]), conduction velocity (CV), and action potential duration (APD) restitution curves [37], in the framework of the so-called forward problem in electrocardiology. Such a complex topic exhibits several unknowns both from the experimental and theoretical points of view. Moreover, different numerical results are still debated in terms of which mathematical models are able to correctly reproduce the spatio-temporal onset and transition of alternans patterns toward ventricular fibrillation. In this work, we show the ability of the proposed numerical schemes to accurately render space and time alternans patterns and discuss and analyze in detail the tissue-dependent properties (i.e., myocardial fiber rotational anisotropy [38]) of the studied phenomena. The remainder of this paper is organized as follows. Section 2 outlines the mathematical model of cardiac electrophysiology including the monodomain equations, the choice of ionic model, and presents the main elements of ECG modeling and observation of alternans. We describe in Section 3 the FE and FVE discretizations along with the operator splitting employed in our numerical framework. A set of illustrative examples is collected in Section 4, and we close with some remarks and discussion in Section Governing equations, pseudo-ecg modeling, and alternans dynamics 2.1. The monodomain model Let R 3 denote a domain with smooth and consider a finite time interval.0, T/ for a fixed T > 0. The so-called monodomain equation (also known as cable equation) results from the Kirchhoff s current law, and it addresses single cell excitation, that is, depolarization and repolarization dynamics, describing the propagation of the transmembrane potential through the tissue via a diffusive Fourier formalism [39]. It consists in the following reaction diffusion system where the unknown quantities are the transmembrane potential v D v.x, t/ andavectorfieldofs ionic variables (named gating variables) i D i.x, t/: C m t v r rv/ C 1 I ion.v, i/ D I app in.0, T, d t i m.v, i/ D 0 in.0, T. (2.1) Here, C m is the membrane capacitance per unit area, is the surface-to-volume ratio of the myocardium, I ion is the sum of the ionic currents, I app is an extracellular current density stimulus, and D f I C. s f / f 0 f 0 is a transversely isotropic conductivity tensor representing different myocardial propagation velocities f, s in the parallel and perpendicular myofiber directions, f 0, s 0, respectively (see, e.g., [17]). As usual, the t,d t, r,andr represent the partial and total derivatives with respect to time t, spatial gradient and divergence operators, respectively. The cardiac muscle consists of densely packed cells organized in such a way that the mean electric conductivity is much higher in the direction of the fibers f 0. In Cartesian coordinates, we have 0 f cos 2.z/ C s sin 2 1.z/. f s / sin.z/ cos.z/ 0 f s / sin.z/ cos.z/ f sin 2.z/ C s cos 2.z/ 0 A, 0 0 s where.z/ denotes the angle between the fiber and the y axis in each plane (Figure 1). 1047
3 M. DUPRA ET AL. Figure 1. Sketch of the 3D slab of cardiac tissue employed in the simulations. The fibers vary transmurally from 60ı at z D 1 to 60ı on z D 0. Such conditions are representative of the left ventricle wall. System (2.1) is complemented with zero-flux boundary conditions for the transmembrane potential Œ.x/rv n D 0 representing that the tissue is isolated, and suitable initial potential and ionic distributions v.x, 0/ D v0, i.x, 0/ D i0 are identified. The specification of the reaction terms Iion and m determines, respectively, the ionic currents across the cell membrane and concentration of ionic quantities, which will be considered in the model. Depending on the target application, cell models with different levels of physiological detail can be incorporated, ranging from phenomenological (which reproduce the shape and structure of the action potential) to physiologically accurate descriptions of the microscopic subcellular dynamics (see a mathematical analysis of a class of reaction diffusion systems governing membrane dynamics in, e.g., [40]). Here, we focus on the phenomenological minimal model for human species, introduced in [21]. This four-field model is capable to accurately recover several physiological and pathological conditions. These include, for instance, resting potential and excitation thresholds, shape-duration of the action potential and restitution dynamics, CV, and wave break. The kinetics are specified as Iion.v, i/ D i1 H.v 1 /.v 1 /.vv v/= fi C.v v0 /.1 H.v 2 // = 0 C H.v 2 /= 3,0 H.v 2 /i2 i3 = si, H.v 1 //.i1,inf i1 /= 1 H.v 1 /i1 = 1C B C C C m.v, i/ D H.v 2 //.i2,inf i2 / = 2 H.v 2 /it2 = 2 A,..1 C tanh.k3.v v3 /// =2 i3 / = 3 where H is the Heaviside function while switches and infinite values are defined as 1 H v 1 v 1,1 C H v 1 1,2, D 2,1 C 2,2 2,1 1 C tanh k2 v v2 =2, 1 D 2 3,0 D 3,0,1 C. 3,0,2 3,0,1 /.1 C tanh.k3,0.v v3,0 /// =2, 3 D..1 H.v 2 // 3,1 C H.v 2 / 3,2, 0 D..1 H.v 0 // 0,1 C H.v 0 / 0,2, 1, v < 1, 0, u 1 D.1 H.v 0 //.1 v= 2,1 / C H.v 0 /i2,1. i1,inf D i2,inf All parameter values are specified in Table I Pseudo-ECG representation 1048 Electrocardiogram is the most used device to record the external potential at the torso surface. These data are employed to assess the properties of the electrical activity in the heart. To recover this signal, one could solve a full heart torso coupled problem (see, e.g., [41, 42]), or alternatively, obtain an approximation (which is, of course, less accurate) called pseudo-ecg. Basically, the latter consists in averaging the potential propagation through the torso recorded at a given electrode e. Let xe denote the position of e at the torso N Assuming that the extra-myocardial matrix and the torso possess an isotropic conductivity D I, for a given surface (that is, xe ). > 0, and assuming that impressed density current is rv, we can write the following approximation for the shape of the ECG at xe v.x, t/ dx. (2.2) ECG.xe / :D kx xe k Copyright 2014 John Wiley & Sons, Ltd. Math. Meth. Appl. Sci. 2015,
4 M. DUPRA ET AL. Table I. Typical values for model and discretization parameters. Parameters Ionic cell model parameters 0 D 0.005, 1 D 0.3, 2 D 0.13, 1 D 0.1, 3,0,1 D 91, 3,0,2 D 0.8, 3,1 D , 3,2 D 4, 0,1 D 410, 0,2 D 7, i2,1 D 0.5, v v D 1.61, 1,1 D 80, 1,2 D C 1 D , 2,1 D 70, 2,2 D 8, C 2 D 280, k2 D 200, v 2 D 0.016, fi D 0.078, k 3,0 D 2.1, v 3,0 D 0.6, k 3 D , v 3 D , si D , 2,1 D 0.01 Monodomain model parameters C m D 1F=cm 2, D 1400 cm, f D cm 2 =ms, s D cm 2 =ms Discretization and pacing parameters D.0, 1/.0, 1/.0, / cm 3, hd0.005 cm, t D0.01 ms, `D5, ıt D5 ms, D2ms Values The derivation of (2.2) relies on Ohm s law, Green s formula, and the relations between a potential, currents, and a point source [43]. Such an approximation therefore becomes useful to get further information concerning the electrical activity of the heart, specially when in vivo action potential tissue measurements are not feasible Dynamics of cardiac alternans Alternans of APD at the cellular and tissue levels is usually associated with alternans of the T-wave in the ECG signal [44 46], that is, there exists an alternating variation in the amplitude or shape of the T-wave. With such an expression, it is possible to identify a condition that rises during fast pacing of the tissue, resulting in a regular and stable alternations of the action potential between higher longer and smaller shorter waves, in time. Although the mechanisms for alternating signals have been theoretically explored in zero-dimensional settings (see, e.g., [34 36]), only a few experimental and theoretical evidences have been discussed in higher spatial dimensions [47 52]. Both in-phase (concordant) and out-of-phase (discordant) patterns have been recently shown to arise because of several different and cumulative effects [53, 54], encompassing: (i) concordant phases can develop from discordant alternans as the pacing period is decreased; (ii) multiple stationary nodal lines may exist, as alternans exhibit full three-dimensional dynamics; (iii) the complex spatio-temporal alternans patterns are very sensitive to both the stimulation site and simulation history. These evidences indicate the hidden predisposition of such a behavior to chaotic regimes and how little is our understanding of the phenomenon. The most adopted alternans analysis is based on the study of the so-called restitution curve of the APD (Section 4). According to onedimensional map theory, Nolasco and Dahlen [37] defined a criterion for alternans onset based on the slope of such a curve. In this work, we make use of such a criterion, but we further analyze the resulting spatio-temporal dynamics by adopting the following definition of alternans at a generic location in the Cartesian space: j APD.x, y, z/n j > APD.x, y, z/ n D APD.x, y, z/ nc1 APD.x, y, z/ n! j APD.x, y, z/ n j Alternans Nodal line (2.3) In particular, we compute the difference between two consecutive APDs, at pacing n and n C 1, thus comparing the absolute difference with a reference threshold D 2 ms, we distinguish between non alternating (Nodal line) and alternating (Alternans) regions. Such a procedure allows us to determine the temporal distribution of action potential alternans across the domain. In fact, by plotting the actual difference APD.x, y, z/ n in the mapped field, we can visualize in-phase and out-of-phase regions (Figure 7). 3. Discretization 3.1. Finite element setting The spatial domain is discretized into tetrahedral elements K of diameter h K providing a conforming partition T h of meshsize h :D max h K consisting of N h vertexes. By P 1.K/, we denote the space of affine functions defined in K. The classical finite dimensional space of continuous piecewise linear functions is (see, e.g., [55]) V h D v h 2 C 0. N/ : v h j K 2 P 1.K/, 8K 2 T h, and it is spanned by a set of Lagrangian basis functions f i g Nh id1. The spatial semidiscrete Galerkin formulation for (2.1) consists in finding v h 2 V h, i h 2 ŒV h s such that for each t 2.0, T/ C m t v h.t/w h dx C rv h.t/ rw h dx C 1 d t i h.t/ j h dx I ion.v h.t/, i h.t//w h dx D I app w h dx 8w h 2 V h, m.v h.t/, i h.t// j h dx D 0 8j h 2 ŒV h s. 1049
5 M. DUPRA ET AL. Let us denote mass and stiffness matrices respectively by M, A, with components m kl :D X K2T K h k l dx, a kl :D X r k r l dx, K2T K h and define the vectors V D.v/ j, J D.i/ j, I ion D.I ion / j, I app D.I app / j, j D 1, :::, N h, where the subindices denote that the quantity corresponds to the node j of T h. Then the semidiscrete FE approximation of the monodomain reaction diffusion model reads C m 1 M@ t V AV C 1 MI ion D MI app, d t J D m.v, J/ Finite volume-element formulation Let T h be a dual partition of into finite volumes (here polygons) K j centered on each node x j, j D 1, :::, N h of the primal mesh T h. Let b K denote the barycenter of a generic element K 2 T h. Dual elements are constructed by joining the barycenter b K of each primal element K sharing the vertex x j, with the midpoints of the faces intersecting x j. A finite dimensional space associated to piecewise constant functions defined on this dual mesh is defined as follows: Vh nv o :D h 2 L 2./ : v h j K j 2 P 0 Kj, 8Kj 2 Th, and a connection between V h and V h? is defined by the piecewise constant interpolator h : V h! Vh (cf., e.g., [32]) specifically defined by XN h. h v h /.x/ D v h.x j / j.x/ for x 2, where j stands for the characteristic function on the control volume K j? 2 Th. Its vectorial counterpart is denoted by h : ŒV h s! V s h, s 1. Let wh 2 V h,j h 2 ŒV h s. Multiplication of the first and second equations in (2.1) by the test functions h w h 2 V h?, hj h 2 V s h (respectively), integration by parts over each control volume K j 2 Th and summation yields a semidiscrete finite volume method written in the form 1 X K j 2T h.c t v h.t/ C.I ion.v h.t/, i h.t//, h w h / X Kj 2Th X K j 2T h K j 2T h. rv h.t/ n/ h w h ds D X. d t i h.t/ m.v h.t/, i h.t//, h j h / D 0, K j 2T h Iapp, h w h, for all w h 2 V h, j h 2 ŒV h s, and associated to the initial data v h.0/ D h v.0/, where h : H 1./! V h is a projection defined by the diffusion operator as follows: XN h w h.x j / r.v h v/ n D 0, 8w h 2 V Using, for example, [31,32], it is possible to reformulate this problem as a Petrov Galerkin scheme: For t > 0, find.v h.t/, i h.t// 2 ŒV h sc1 such that C m t v h.t/w h dx C rv h.t/ rw h dx C 1 d t i h.t/ h j h dx I ion.v h.t/, i h.t// h w h dx D I app h w h dx 8 h w h 2 Vh, m.v h.t/, i h.t// h j h dx D 0 8 h j h 2 Vh (3.1) s Time integration and operator splitting The time interval.0, T/ is discretized into N t subintervals of length t D T=N t. We will denote with a superscript n N t the quantities associated to a time instant t n D n t. At each time step, the ionic current I ion at each x 2 is approximated by the so-called ionic current interpolation (see, e.g., [18]), where the nodal values of the electric current are interpolated linearly onto the quadrature point, that is, XN h I ioni D I ion.v j, i j / i.x/,
6 M. DUPRA ET AL. where v i, i i again denote the value of the potential and ionic concentrations at the node i of T h. By doing so, the assembly of the RHS is very efficient. A further gain in efficiency is obtained with the FVE formulation (3.1), because in that case, we have a ionic current interpolation of the form XN h I ioni Q D I ion.v j, i j / i.x/. (3.2) For sake of both performance and accuracy in recovering the wave front, we split the monodomain equations solving first the pure reaction part of the cell model consisting on a set of nodal ODEs, followed by the (time-dependent) diffusive step (see, e.g., [41]). The way that the time derivatives are approximated in each step of this splitting has an important effect on the accuracy of the final solution. For the reaction, part we employ an explicit Euler scheme for the time integration, whereas the diffusion part of the splitting is discretized in time with a second order backward differentiation formula (BDF2, see, e.g., [56]). These considerations will be explored in Section 4. For V n, J n known, we compute V nc1, J nc1 in three steps, using the intermediate variables V nc?, V nc??, I nc?? ion,from C m V nc? D C m V n ti n ion, Cm 1 3M C 2A V nc?? D C m 1 M 4V nc? V n, t t C m V nc1 D C m V nc?? ti nc?? ion. Here, the vector I ion and the FE mass matrix M are replaced by Q I ion (3.2) and the FVE mass matrix QM whenever a FVE scheme is employed. In addition, we incorporate mass lumping to the algorithm (the mass matrix is substituted by a diagonal approximation). In some scenarios, this technique could induce numerical artifacts (see the discussion in [57]); however, for the cases studied herein, we found that it does not affect the accuracy of the numerical approximation, also preserved by the FVE discretization. Energy-based stability estimates for coupled and splitting-based FE techniques on the bidomain equations can be found in, for example, [58, 59] Pseudo-ECG computation The computation of the ECG signal associated to each electrode (2.2) requires the approximation of the function f h D v h and its integral [60]. Writing formally the following weak form of this relation in allows us to employ a low order FE approximation v h of v (otherwise, v h should be at least in Vh 2 D w h 2 C 0 N : w h j K 2 P 2.K/, 8K 2 T h,wherep2.k/ is the space of quadratic polynomials defined on K): rv h rw h dx D f h w h dx, 8w h 2 V h. (3.3) Starting from (3.3), if we now assume that f h 2 V h,thenv h D P N h v j j and f h D P N h f j j, which entails that computing f h yields the system XN h XN h f j j k dx D v j r j r k dx, k D 1, :::, N h, or in matrix form: Find f D.f 1, :::, f Nh / such that Mf D OAV, (3.4) where OA has elements Oa kl :D P K2T h RK r k r l dx. Then, (2.2) is approximated by the quantity XN h f j ECG h.x e / D, r j where r j :Dkx j x e k is the distance from any mesh point to the electrode (located evidently outside ). 4. Numerical results The computational domain consists of a slab of cm. The geometry and a series of meshes were generated using the open source mesh manipulator GMSH [61]. All simulations presented in this section have been implemented in the framework of the LGPL parallel FE library LifeV ( and run on one to eight nodes of the cluster Bellatrix at the EPF Lausanne (each with two Sandy Bridge processors running at 2.2 GHz, with eaight cores each, 32 GB of RAM, Infiniband QDR 2 : 1 connectivity, and GPFS filesystem). All model parameters, unless otherwise specified, are taken as in Table I. The accuracy of the FE and FVE approximations has been assessed by solving the isotropic monodomain equations with the simpler Rogers McCulloch kinetics [19], where initial and boundary conditions are imposed such that the exact solution is given by hp i 1. v.x, y, z, t/ D 1 C exp 1=2.x t/ An experimental convergence of O.h/ is observed for the transmembrane potential in the H 1 -norm for both discretizations (Figure 2 (right), see also [13]). Further code validation has been performed by focusing initially on the accuracy of the CV under the assumption of tissue isotropy, that is, we put f D s D 0.001, cm 2 =ms. In principle, the meshsize should be taken small enough to reproduce the correct CV, also for conductivity values given specifically as in Section 2. We have solved the monodomain equations for a sequence of refined meshes, and as observed in Figure 2 (left), when h decreases, the CV goes asymptotically to the physiological value that agrees with [21], and in particular, a meshsize smaller than h D 0.01 cm is needed. We 1051
7 M. DUPRA ET AL. also see that a first order splitting yields too large CV, and moreover, convergence is not attained. On the other hand, we are able to capture the correct value of CV employing a second order splitting method (Figure 2, right). Next, we consider the anisotropic case. Fibers and sheetlets directions.f 0, s 0 / have been generated analytically as depicted in Figure 1 and were assigned to each vertex of the corresponding mesh. An initial stimulus is applied at one corner of the domain, and we observe the influence of the pacing location on the shape and speed of propagation of the transmembrane potential. Steep depolarization wavefronts are typically observed, which require high spatial resolutions. Figure 3 shows the differences between the wave propagation in two different scenarios. We observe a more significant diffusion of v along the fibers direction. A local CV of 66.3 cm=s has been obtained in the fibers direction and of 31.4 cm=s in the cross-fibers direction. The ratio between these values is in accordance with the theoretical one p s = f given in [62]. We now turn to the analysis of restitution curves, obtained by plotting the APD versus the duration of the previous diastolic interval (DI). These data are generated via a pacing down protocol (see, e.g., [53]), where starting from a first cycle length (CL), defining a reduction factor ıt and a number of ` stimulations (typically depending on the complexity of the ionic model at hand), we pace ` times at a fixed CL and then reduce the pacing interval by ıt. This procedure is applied until a fixed minimal CL is reached, where or capture is no longer possible or fibrillation arises. For each pacing frequency, we record the last two APDs and DIs (if no alternans appear, only the last values are needed). These values are computed at fixed locations, far enough from the pacing site. In this case, we employ ` D 5and ıt D 5 ms. The stimuli are applied at a corner of the domain generating spherical waves propagating across the tissue. For the isotropic case, the data of interest are recorded at several points, far from the stimulus location and in the direction of the propagation (i.e., along the diagonal of the xy plane). For the anisotropic case, the restitution curves and recording locations are depicted in Figure 4. We observe that irrespective of the location, the shape of the restitution curves is conserved, whereas the stable APD (represented by the corresponding visible threshold) varies across the domain. Pseudo-ECG signals have been obtained from solving (3.4). A block-gmres solver has been used combined with right ML preconditioner with a tolerance of 10 10, a maximum of 200 iterations, and presmoothing with symmetric Gauss Seidel (see, e.g., [63]). An Amesos-KLU solver is employed for the coarse level, with an aggregation threshold of 1% [64]. Figure 5 shows pseudo-ecgs corresponding to fixed pacing CL simulations without alternans, performed on a 1D cable, a 2D surface, and the 3D slab. In all cases, a satisfactory Figure 2. Conduction velocity versus meshsize on a cable computed with the minimal model (left), comparison of conduction velocities computed with FE and first or second order splittings of the minimal model in a 3D slab (middle), and spatial convergence of the FVE and FE approximations of the transmembrane potential at t D 1 ms, with respect to an exact solution employing Rogers McCulloch kinetics (right) Figure 3. FVE approximation of the transmembrane potential at t D 12 ms. Here, we focus on the effect of anisotropy and pacing location (arrow) on the propagation of the action potential at t D 12 ms, pacing from (0,0,0) (left), and pacing from (0,1,0) (right) for the minimal model.
8 M. DUPRA ET AL. Figure 4. Restitution curves for the minimal model (left) along with recording locations within a 2D slice of anisotropic tissue (right). Figure 5. Pseudo-ECG profiles for the minimal model computed on a cable (left), a square surface (middle) and a 3D slab (right) using a FE (top, solid blue) and a FVE space discretization (bottom, dashed red). Figure 6. Action potential and related pseudo-ecg signal for the minimal model under isotropic conditions (left) along with local restitution data for an effective cycle length of 420 ms (right). 1053
9 M. DUPRA ET AL. Figure 7. FE approximate spatial distribution of APD exhibiting discordant alternans at t D 7000 ms and t D 5000 ms (left-top and left-bottom, respectively) and action potential recordings at different locations obtained by pacing at (0,0,0) (right-top) and (0,1,0) (right-bottom) qualitative agreement with respect to the expected structure of the signal is observed. In particular, the QRS complex and T-wave are reproduced according to physiological regimes [41]. The differences between FE and FVE formulations are barely noticeable in this case. Finally, we describe the dynamics of cardiac alternans as defined in (2.3). It is well known that high pacing frequencies may generate APD alternans (see, e.g., [53] and references therein). We here employ a so-called quiescent pacing protocol, where from a resting initial condition, we immediately start pacing at the desired frequency. We observe that a pacing period of 210 ms already induces alternation of the APD. Only one of two action potentials is kept, and therefore, the effective cycle length is of 420 ms. Under the assumption of tissue isotropy, we observe an homogeneous spatial distribution for the generated alternans, which are spatially concordant (Figure 6, left-top). The presence of alternans is also noticed from Figure 6 (right), where the restitution curve related to this specific CL exhibits a splitting (long short repetition). As predicted, we observe a correlation between APD alternans and T-wave alternans (Figure 6, left), and an alternation in the amplitude of the action potential is correlated with alternation of the QRS complex amplitude in the pseudo-ecg counterpart. We now take into account the tissue anisotropy and pace from two different locations (0,0,0) and (0,1,0). As in the previous case, only one out of two action potentials is kept. The resulting spatial distribution of transient alternans is no longer homogeneous and spatially discordant alternans are observed. In addition, the fibers direction has an important effect in the alternans distribution. From Figure 7, we see that both the shape of the action potential and the alternans pattern depend on the pacing site too. At the recording locations A, B, we observe out-of-phase alternans (discordant alternans), whereas at C, we observe a nodal line, though the pacing location is surrounded by an in-phase alternans pattern. We also study the presence of spiral waves in the tissue in both isotropic and anisotropic cases using the proposed FVE method. Because the obtained wavelength is smaller in this case, we employ the Rogers McCulloch model. As expected, both cases present perturbed pseudo-ecg signals. In the isotropic case, because the spiral wave does not stabilize, the initial form of the signal can be easily identified; however, the QRS complex shows some abnormalities, see Figure 8. In the anisotropic case, the spiral wave reaches a periodic state, and after stabilization, the general form of the signal is completely destroyed. The fact that spiral waves are associated with tachycardia and fibrillation suggests that the pseudo-ecg reproduces correctly the underlying rhythm disorders.
10 M. DUPRA ET AL. Figure 8. Snapshots of the FVE approximation of the transmembrane potential at several time instants and pseudo-ecg signal reconstructed with the Rogers McCulloch model on isotropic (top) and anisotropic tissue conditions (bottom). 5. Concluding remarks Alternans patterns, action potential repolarization, and spiral waves breakup are often associated with serious pro-arrhythmic states in cardiac tissue. Therefore, understanding the nonlinear spatio-temporal mechanisms underlying these phenomena is of crucial importance for the medical practice. In this paper, we have focused in particular on the development of cardiac alternans exploring a phenomenological mathematical model fine tuned on cardiac electrophysiological data both on 1D, 2D, and 3D idealized domains. A careful validation of the code was performed reproducing correct CV and APD shapes and wavelengths (Figure 2). In the threedimensional case, fibers rotational anisotropy throughout the thickness of the domain was also considered in the model (Figure 3), highlighting that the influence of the pacing location is affected by the fibers architecture in the tissue. In particular, two totally different action potential waves are generated with a net measurable reduction of the propagation velocity when a pacing site orthogonal to the fibers direction is employed. A specific analysis of the APD restitution curves (Figure 4) allows us to emphasize that the onset of the APD-DI heterogeneities are also due to the presence of fibers in the tissue. Although the shape of the restitution curves is similar at different locations in the domain, the starting point with a slope greater than 1 arises for different value of APD. Such an observation is also in line with the numerical quantification of spatial discordant alternans observed in our three-dimensional simulations (Figure 7). The same observation is further 1055
11 M. DUPRA ET AL. reinforced through the analysis of the pseudo-ecg signals. In fact, simulations performed on isotropic (1D, 2D, and 3D) domains and at low pacing frequencies do not show any alternans in the ECG profiles (Figure 5), whereas the same quantities analyzed at higher pacing frequency clearly discriminate an alternating regime (splitting) both in concordant (Figure 6) and discordant (Figure 7) cases by varying the T-wave duration and the QRS amplitude. Finally, we analyzed the profiles of the pseudo-ecg signal in the case of a stable arrhythmia, that is, a single spiral in the tissue (Figure 8). By comparing isotropic and anisotropic three-dimensional slabs, we correctly observe as the last case results in much more realistic ECG signals, very close to a prefibrillating scenario typical of diseased heart tissues. In addition, from a numerical perspective, our results suggest that, at least for the minimal model, a second order splitting combined with mass lumping is an accurate enough choice to recover the correct conduction velocities. However, complex intramural patterns are also present in the behavior of the potential and its corresponding ECG reconstructions. These aspects deserve further investigation. Current extensions of this work include the study of excitation contraction phenomena and the assessment of mechanical alternans in cardiomyocytes and in the whole muscle using an active strain framework (see, e.g., [65 67]). A further extension toward thermal and mechanical couplings is also under investigation: in particular, the specific aim is to account for more realistic scenarios in order to validate recent low energy defibrillating techniques [68 71]. Our mathematical framework is in accordance with other recent works addressing the problem of spiral pinning and unpinning in biological excitable and deformable tissues, with particular reference to the heart tissue [68, 72, 73]. Our work represents an extension to these studies, which were typically treating isotropic, two-dimensional domains with over-simplified phenomenological models. The approach here proposed, moreover, allows extensions and applications for related problems in biological excitable media, that is, intestine [74 76] and brain [77, 78] as well as other biochemical systems [79]. The robustness of the pseudo-ecg calculation in such a complex phenomenon is quite appealing and promising and will be further explored in combination with advanced numerical techniques of signal analysis. The introduction of innovative and predictive synthetic indicators (see, e.g., [80]) for the clinical practice will be also carefully considered in a forthcoming contribution. We also plan to carry out the convergence analysis of FVE formulations for monodomain and bidomain equations following [31]. Further improvements of this work, more oriented to numerical methods, may include the assessment of the influence of space adaptivity in cardiac electrophysiology [4] and electromechanics, and the development of efficient and scalable preconditioners that would allow an increased performance [17, 81]. Acknowledgements The authors acknowledge the financial support by the European Research Council through the Advanced Grant Mathcard, Mathematical Modelling and Simulation of the Cardiovascular System, Project and the International Center for Relativistic Astrophysics Network, ICRANet. Fruitful discussions with Simone Rossi (EPF Lausanne) are also gratefully acknowledged References 1. Winfree AT. When Time Breaks Down: The Three-Dimensional Dynamics of Electrochemical Waves and Cardiac Arrhythmias.Princeton University Press: Princeton, Bini D, Cherubini C, Filippi S, Gizzi A, Ricci PE. On spiral waves arising in natural systems. Communications in Computational Physics 2010; 8: Cherry EM, Fenton FH. Visualization of spiral and scroll waves in simulated and experimental cardiac tissue. New Journal of Physics 2008; 10: Deuflhard P, Erdmann B, Roitzsch R, Lines GR. Adaptive finite element simulation of ventricular fibrillation dynamics. Physical Review E: Statistical, Nonlinear, and Soft Matter Physics 2012; 85: Luengviriya C, Luengviriya J, Sutthiopad M, Müller SC. Influence of temperature on a spiral wave in excitable chemical media. IPCBEE 2012; 38: Fenton FH, Gizzi A, Cherubini C, Pomella N, Filippi S. Role of temperature on nonlinear cardiac dynamics. Physical Review E: Statistical, Nonlinear, and Soft Matter Physics 2013; : Panfilov AV, Holden AV (eds). Computational Biology of the Heart eds. Wiley: Chichester, Hodgkin AL, Huxley AF. A quantitative description of membrane current and its application to conduction and excitation in nerve. Journal of Physiology 1952; 117: Cherubini C, Filippi S. Lagrangian field theory of reaction-diffusion. Physical Review E: Statistical, Nonlinear, and Soft Matter Physics 2009; 80: Bordas R, Carpentieri B, Fotia G, Maggio F, Nobes R, Pitt-Francis J, Southern J. Simulation of cardiac electrophysiology on next-generation high-performance computers.philosophical Transactions of the Royal Society of London A 2009; 367: Heidenreich EA, Rodriguez JF, Gaspar FJ, Doblaré M. Fourth-order compact schemes with adaptive time step for monodomain reaction-diffusion equations. Journal of Computational Physics 2008; 216: Skouibine K, Trayanova N, Moore P. A numerically efficient model for simulation of defibrillation in an active bidomain sheet of myocardium. Mathematical Biosciences 2000; 166: Bendahmane M, Bürger R, Ruiz-Baier R. A finite volume scheme for cardiac propagation in media with isotropic conductivities. Mathematics and Computers in Simulation 2010; 80: Harrild DM, Henriquez CS. A finite volume model of cardiac propagation. Annals of Biomedical Engineering 1997; 25: Aliev RR, Panfilov AV. A simple two-variable model of cardiac excitation. Chaos Solitons & Fractals 1996; 7: Bourgault Y, Ethier M, LeBlanc VG. Simulation of electrophysiological waves with an unstructured finite element method. ESAIM: Mathematical Modelling and Numerical Analysis 2003; 37: Colli Franzone P, Pavarino LF. A parallel solver for reaction-diffusion systems in computational electro-cardiology. Mathematical Models and Methods in Applied Sciences 2004; 14:
12 M. DUPRA ET AL. 18. Pathmanathan P, Murams GR, Southern J, Whiteley JP. The significant effect if the choice of ionic current integration method in cardiac electro-physiological simulations. International Journal of Numerical Methods in Biomedical Engineering 2011; 27: Rogers JM, McCulloch AD. A collocation-galerkin finite element model of cardiac action potential propagation. IEEE Transactions on Biomedical Engineering 1994; 41: Arthurs CJ, Bishop MJ, Kay D. Efficient simulation of cardiac electrical propagation using high order finite elements. Journal of Computational Physics 2012; 231: Bueno-Orovio A, Cherry EM, Fenton FH. Minimal model for human ventricular action potential in tissue. Journal of Theoretical Biology 2008; 253: Iyer V, Mazhari R, Winslow RL. A computational model of the human left-ventricular epicardial myocyte. Biophysical Journal 2004; 87: Bürger R, Ruiz-Baier R. Torres H. A stabilized finite volume element formulation for sedimentation-consolidation processes. SIAM Journal of Scientific Computing 2012; 34:B265 B Cai. On the finite volume element method. Numerische Mathematik 1991; 58: Ewing RE, Lazarov RD, Lin Y. Finite volume element approximations of nonlocal reactive flows in porous media. Numerical Methods for Partial Differential Equations 2000; 16: Girault V. A combined finite element and marker and cell method for solving Navier-Stokes equations. Numerische Mathematik 1976; 26: Quarteroni A, Ruiz-Baier R. Analysis of a finite volume element method for the Stokes problem. Numerische Mathematik 2011; 118: Ruiz-Baier R, Torres H. Numerical solution of a multidimensional sedimentation problem using finite volume-element methods. Applied Numerical Mathematics 2014, DOI /j.apnum Penland RC, Harrild DM, Henriquez CS. Modeling impulse propagation and extracellular potential distributions in anisotropic cardiac tissue using a finite volume element discretization 2002; 4: Bürger R, Ruiz-Baier R, Tian C. Stability analysis and finite volume element discretization for delay-driven spatial patterns in a predator-prey model. Journal of Mathematical Analysis and Applications. submitted Lazarov R, Tomov S. A posteriori error estimates for finite volume element approximations of convection-diffusion-reaction equations. Computers & Geosciences 2002; 6: Lin, Ruiz-Baier R, Tian C. Finite volume element approximation of an inhomogeneous Brusselator model with cross-diffusion. Journal of Computational Physics 2014; 256: Phongthanapanich S, Dechaumphai P. Finite volume element method for analysis of unsteady reaction diffusion problems. Acta Mechanica Sinica 2009; 25: Sun J, Amellal F, Glass L, Billette J. Alternans and period-doubling bifurcations in atrioventricular nodal conduction. Journal of Theoretical Biology 1995; 173: Courtemanche M, Glass L, Keener JP. Instabilities of a propagating pulse in a ring of excitable media. Physical Review Letters 1993; 70: Ito H, Glass L. Theory of reentrant excitation in a ring of cardiac tissue. Physica D 1992; 56: Nolasco JB, Dahlen RW. A graphic method for the study of alternation of cardiac action potentials. Journal of Applied Physiology 1968; 25: Kocica MJ, Corno AF, Carreras-Costa F, Ballester-Rodes M, Moghbel MC, Cueva CN, Lackovic V, Kanjuh VI, Torrent-Guasp F. The helical ventricular myocardial band: global, three-dimensional, functional architecture of the ventricular myocardium. European Journal of Cardio-Thoracic Surgery 2006; 1:S Pullan AJ, Buist ML, Cheng LK. Mathematically Modeling the Electrical Activity of the Heart: From Cell to Body Surface and Back. World Scientific Publishing Company: Singapore, Veneroni M. Reaction-diffusion systems for the microscopic cellular model of the cardiac electric field. Mathematicsl Methods in the Applied Sciences 2006; 29: Sundnes J, Lines GT, Tveito A. An operator splitting method for solving the bidomain equations coupled to a volume conductor model for the torso. Mathematical Biosciences 2005; 194: Boulakia M, Cazeau S, Fernández MA, Gerbeau JF, emzemi N. Mathematical modeling of electrocardiograms: a numerical study. Annals of Biomedical Engineering 2010; 38: Aslanidi OV, Clayton RH, Lambert JL, Holden AV. Dynamical and cellular electrophysiological mechanisms of ECG changes during ischaemia. Journal of Theoretical Biology 2005; 237: Rosenbaum DS, Jackson LE, Smith JM, Garan H, Ruskin JN, Cohen RJ. Electrical alternans and vulnerability to ventricular arrhythmias 1994; 330: Pastore JM, Girouard SD, Laurita KR, Akar FG, Rosenbaum DS. Mechanism linking T-wave alternans to the genesis of cardiac fibrillation. Circulation 1999; 99: Watanabe MA, Fenton FH, Evans SJ, Hastings HM, Karma A. Mechanisms for discordant alternans. Journal of Cardiovascular Electrophysiology 2001; 12: Qu, Garfinkel A, Chen PS, Weiss JN. Mechanisms of discordant alternans and induction of reentry in simulated cardiac tissue. Circulation 2000; 102: Walker ML, Wan X, Kirsch GE, Rosenbaum DS. Hysteresis effect implicates calcium cycling as a mechanism of repolarization alternans. Circulation 2003; 108: Walker ML,Rosenbaum DS.Cellular alternans as mechanism of cardiac arrhythmogenesis.heart Rhythm 2005; 2: de Diego C, Pai RK, Dave AS, Lynch A, Thu M, Chen F, Xie L H, Weiss JN, Valderrábano M. Spatially discordant alternans in cardiomyocyte monolayers. American Journal of Physiology-Heart and Circulatory Physiology 2008; 294:H1417 H iv O, Morales E, Song Y, Peng X, Odening KE, Buxton AE, Karma A, Koren G, Choi BR. Origin of complex behaviour of spatially discordant alternans in a transgenic rabbit model of type 2 long QT syndrome. Journal of Physiology 2009; 587: Weinberg S, Malhotra N, Tung L. Vulnerable windows define susceptibility to alternans and spatial discordance. American Journal of Physiology-Heart and Circulatory Physiology 2010; 298:H1727 H Gizzi A, Cherry EM, Gilmour RF, Luther S, Filippi S, Fenton FH. Effects of pacing site and stimulation history on alternans dynamics and the development of complex spatiotemporal patterns in cardiac tissue. Frontiers in Physiology 2013; 19(4): Visweswaran R, McIntyre SD, Ramkrishnan K, hao X, Tolkacheva EG. Spatiotemporal evolution and prediction of [Ca(2+)]i and APD alternans in isolated rabbit hearts. Journal of Cardiovascular Electrophysiology 2013; 24:
13 M. DUPRA ET AL. 55. Quarteroni A. Numerical Models for Differential Problems, MS& A series, vol. 2. Springer-Verlag: Milan, QuarteroniA, SaccoR, SaleriF. Numerical Mathematics, Series: Texts in Applied Mathematics 2nd ed, Vol. 37. Springer-Verlag: Milan, Pathmanathan P, Bernabeu MO, Niederer SA, Gavaghan DJ, Kay D. Computational modelling of cardiac electrophysiology: explanation of the variability of results from different numerical solvers. International Journal of Numerical Methods in Biomedical Engineering 2012; 28: Ethier M, Bourgault Y. Semi-implicit time-discretization schemes for the bidomain model. SIAM Journal on Numerical Analysis 2008; 46: Fernández MA, emzemi N. Decoupled time-marching schemes in computational cardiac electrophysiology and ECG numerical simulation. Mathematical Biosciences 2010; 226: Clayton RH, Holden AV. Computational framework for simulating the mechanisms and ECG of re-entrant ventricular fibrillation. Physiological Measurement 2002; 23: Geuzaine C, Remacle JF. Gmsh: a three-dimensional finite element mesh generator with built-in pre- and post-processing facilities. International Journal for Numerical Methods in Engineering 2009; 79: Fenton FH, Karma A. Vortex dynamics in three-dimensional continuous myocardium with fiber rotation: filament instability and fibrillations. Chaos 1998; 8: Sala M. An object-oriented framework for the development of scalable parallel multilevel preconditioners. ACM Transactions on Mathematical Software 2006; 32(3): Sala M, Stanley K, Heroux M. Amesos: a set of general interfaces to sparse direct solver libraries. Proceedings of PARA 06 Conference, Umea, Sweden, 2006, Ruiz-Baier R, Gizzi A, Rossi S, Cherubini C, Laadhari A, Filippi S, Quarteroni A. Mathematical modeling of active contraction in isolated cardiomyocytes. Mathematical Medicine and Biology 2013, DOI /imammb/dqt Laadhari A, Ruiz-Baier R, Quarteroni A. Fully Eulerian finite element approximation of a fluid-structure interaction problem in cardiac cells. International Journal for Numerical Methods in Engineering 2013; 96: Nobile F, Quarteroni A, Ruiz-Baier R. An active strain electromechanical model for cardiac tissue. International Journal of Numerical Methods in Biomedical Engineering 2012; 28: Cherubini C, Filippi S, Gizzi A. Electroelastic unpinning of rotating vortices in biological excitable media. Physical Review E: Statistical, Nonlinear, and Soft Matter Physics 2012; 85: Cherubini C, Filippi S, Nardinocchi P, Teresi L. An electromechanical model of cardiac tissue: constitutive issues and electrophysiological effects. Progress in Biophysics and Molecular biology 2008; 97: Luther S, Fenton FH, Kornreich BG, Squires A, Bittihn P, Hornung D, abel M, Flanders J, Gladuli A, Campoy L, Cherry EM, Luther G, Hasenfuss G, Krinsky VI, Pumir A, Gilmour RF Jr, Bodenschatz E. Low-energy control of electrical turbulence in the heart. Nature 2011; 475: Pumir A, Sinha S, Sridhar S, Argentina M, Hörning M, Filippi S, Cherubini C, Luther S, Krinsky V. Wave-train-induced termination of weakly anchored vortices in excitable media. Physical Review E: Statistical, Nonlinear, and Soft Matter Physics 2010; 81: Hörning M. Termination of pinned vortices by high-frequency wave trains in heartlike excitable media with anisotropic fiber orientation. Physical Review E 2012; 86: Yuan G, hang H, Xu A, Wang G. Attractive and repulsive contributions of localized excitability inhomogeneities and elimination of spiral waves in excitable media. Physical Review E 2012; 88: Gizzi A, Cherubini C, Migliori S, Alloni R, Portuesi R, Filippi S. On the electrical intestine turbulence induced by temperature changes. Physical Biology 2010; 7: O Grady G, Du P, Paskaranandavadivel N, Angeli TR, Lammers WJ, Asirvatham SJ, Windsor JA, Farrugia G, Pullan AJ, Cheng LK. Rapid high-amplitude circumferential slow wave propagation during normal gastric pacemaking and dysrhythmias. Neurogastroenterology and Motility 2012; 24: e Lammers WJ.Arrhythmias in the gut.neurogastroenterology and Motility 2013; 25: Kochs E. Electrophysiological monitoring and mild hypothermia. Journal of Neurosurgical Anesthesiology 1995; 7: aremba J.Hyperthermia in ischemic stroke.medical Science Monitor 2004; 10:RA Jiménez A, Steinbock O. Stationary vortex loops induced by filament interaction and local pinning in a chemical reaction-diffusion system. Physical Review Letters 2012; 109: Gizzi A, Bernaschi M, Bini D, Cherubini C, Filippi S, Melchionna S, Succi S. Three-band decomposition analysis of wall shear stress in pulsatile flows. Physical Review E 2011; 83: Plank G, Liebmann M, Weber dos Santos R, Vigmond EJ, Haase G. Algebraic multigrid preconditioner for the cardiac bidomain model. IEEE Transactions on Biomedical Engineering 2007; 54:
Boundary-induced reentry in homogeneous excitable tissue
 Boundary-induced reentry in homogeneous excitable tissue Fernando Siso-Nadal, 1 Niels F. Otani, 2 Robert F. Gilmour, Jr., 2 and Jeffrey J. Fox 1 1 Gene Network Sciences, Ithaca, New York 1485, USA 2 Department
Boundary-induced reentry in homogeneous excitable tissue Fernando Siso-Nadal, 1 Niels F. Otani, 2 Robert F. Gilmour, Jr., 2 and Jeffrey J. Fox 1 1 Gene Network Sciences, Ithaca, New York 1485, USA 2 Department
Introduction to Physiology V - Coupling and Propagation
 Introduction to Physiology V - Coupling and Propagation J. P. Keener Mathematics Department Coupling and Propagation p./33 Spatially Extended Excitable Media Neurons and axons Coupling and Propagation
Introduction to Physiology V - Coupling and Propagation J. P. Keener Mathematics Department Coupling and Propagation p./33 Spatially Extended Excitable Media Neurons and axons Coupling and Propagation
A note on discontinuous rate functions for the gate variables in mathematical models of cardiac cells
 Procedia Computer Science (2) (22) 6 945 95 Procedia Computer Science www.elsevier.com/locate/procedia International Conference on Computational Science ICCS 2 A note on discontinuous rate functions for
Procedia Computer Science (2) (22) 6 945 95 Procedia Computer Science www.elsevier.com/locate/procedia International Conference on Computational Science ICCS 2 A note on discontinuous rate functions for
Lecture Notes 8C120 Inleiding Meten en Modelleren. Cellular electrophysiology: modeling and simulation. Nico Kuijpers
 Lecture Notes 8C2 Inleiding Meten en Modelleren Cellular electrophysiology: modeling and simulation Nico Kuijpers nico.kuijpers@bf.unimaas.nl February 9, 2 2 8C2 Inleiding Meten en Modelleren Extracellular
Lecture Notes 8C2 Inleiding Meten en Modelleren Cellular electrophysiology: modeling and simulation Nico Kuijpers nico.kuijpers@bf.unimaas.nl February 9, 2 2 8C2 Inleiding Meten en Modelleren Extracellular
Numerical simulations of cardiac arrhythmias and defibrillation
 Numerical simulations of cardiac arrhythmias and defibrillation M. Hanslien, J. Sundnes and G.T. Lines Simula Research Laboratory and Department of Informatics, University of Oslo Abstract This paper is
Numerical simulations of cardiac arrhythmias and defibrillation M. Hanslien, J. Sundnes and G.T. Lines Simula Research Laboratory and Department of Informatics, University of Oslo Abstract This paper is
arxiv: v1 [q-bio.to] 8 May 2017
![arxiv: v1 [q-bio.to] 8 May 2017 arxiv: v1 [q-bio.to] 8 May 2017](/thumbs/96/127247949.jpg) Thermo-Electric Nonlinear Diffusion arxiv:175.392v1 [q-bio.to] 8 May 217 Nonlinear diffusion & thermo-electric coupling in a two-variable model of cardiac action potential A. Gizzi, 1 A. Loppini, 1 R.
Thermo-Electric Nonlinear Diffusion arxiv:175.392v1 [q-bio.to] 8 May 217 Nonlinear diffusion & thermo-electric coupling in a two-variable model of cardiac action potential A. Gizzi, 1 A. Loppini, 1 R.
Rate-dependent propagation of cardiac action potentials in a one-dimensional fiber
 PHYICAL REVIEW E 70, 061906 (2004) Rate-dependent propagation of cardiac action potentials in a one-dimensional fiber John W. Cain, 1, * Elena G. Tolkacheva, 2 David G. chaeffer, 1,4 and Daniel J. Gauthier
PHYICAL REVIEW E 70, 061906 (2004) Rate-dependent propagation of cardiac action potentials in a one-dimensional fiber John W. Cain, 1, * Elena G. Tolkacheva, 2 David G. chaeffer, 1,4 and Daniel J. Gauthier
2011 NSF-CMACS Workshop on Atrial Fibrillation (5 th day )
 2011 NSF-CMACS Workshop on Atrial Fibrillation (5 th day ) Flavio H. Fenton Department of Biomedical Sciences College of Veterinary Medicine, Cornell University, NY and Max Planck Institute for Dynamics
2011 NSF-CMACS Workshop on Atrial Fibrillation (5 th day ) Flavio H. Fenton Department of Biomedical Sciences College of Veterinary Medicine, Cornell University, NY and Max Planck Institute for Dynamics
Parameters for Minimal Model of Cardiac Cell from Two Different Methods: Voltage-Clamp and MSE Method
 Parameters for Minimal Model of Cardiac Cell from Two Different Methods: oltage-clamp and MSE Method Soheila Esmaeili 1, * and Bahareh beheshti 1 Department of Biomedical engineering, ran University of
Parameters for Minimal Model of Cardiac Cell from Two Different Methods: oltage-clamp and MSE Method Soheila Esmaeili 1, * and Bahareh beheshti 1 Department of Biomedical engineering, ran University of
Scroll Waves in Anisotropic Excitable Media with Application to the Heart. Sima Setayeshgar Department of Physics Indiana University
 Scroll Waves in Anisotropic Excitable Media with Application to the Heart Sima Setayeshgar Department of Physics Indiana University KITP Cardiac Dynamics Mini-Program 1 Stripes, Spots and Scrolls KITP
Scroll Waves in Anisotropic Excitable Media with Application to the Heart Sima Setayeshgar Department of Physics Indiana University KITP Cardiac Dynamics Mini-Program 1 Stripes, Spots and Scrolls KITP
Multiple Mechanisms of Spiral Wave Breakup in a Model of Cardiac Electrical Activity
 Multiple Mechanisms of Spiral Wave Breakup in a Model of Cardiac Electrical Activity Flavio H. Fenton and Elizabeth M. Cherry Center for Arrhythmia Research at Hofstra University and The Heart Institute,
Multiple Mechanisms of Spiral Wave Breakup in a Model of Cardiac Electrical Activity Flavio H. Fenton and Elizabeth M. Cherry Center for Arrhythmia Research at Hofstra University and The Heart Institute,
Scroll Waves in Anisotropic Excitable Media with Application to the Heart. Sima Setayeshgar Department of Physics Indiana University
 Scroll Waves in Anisotropic Excitable Media with Application to the Heart Sima Setayeshgar Department of Physics Indiana University KITP Cardiac Dynamics Mini-Program 1 Stripes, Spots and Scrolls KITP
Scroll Waves in Anisotropic Excitable Media with Application to the Heart Sima Setayeshgar Department of Physics Indiana University KITP Cardiac Dynamics Mini-Program 1 Stripes, Spots and Scrolls KITP
Recovery dynamics of the Fenton-Karma membrane model and their implications for modeling pharmacologically-induced atrial fibrillation
 Recovery dynamics of the Fenton-Karma membrane model and their implications for modeling pharmacologically-induced atrial fibrillation Matt Gonzales Department of Bioengineering University of California,
Recovery dynamics of the Fenton-Karma membrane model and their implications for modeling pharmacologically-induced atrial fibrillation Matt Gonzales Department of Bioengineering University of California,
The Physics of the Heart. Sima Setayeshgar
 The Physics of the Heart Sima Setayeshgar Department of Physics Indiana University Indiana Unversity Physics REU Seminar, July 27 2005 1 Stripes, Spots and Scrolls Indiana Unversity Physics REU Seminar,
The Physics of the Heart Sima Setayeshgar Department of Physics Indiana University Indiana Unversity Physics REU Seminar, July 27 2005 1 Stripes, Spots and Scrolls Indiana Unversity Physics REU Seminar,
The role of short term memory and conduction velocity restitution in alternans formation
 The role of short term memory and conduction velocity restitution in alternans formation Ning Wei a, Yoichiro Mori a, Elena G. Tolkacheva b, a School of Mathematics, University of Minnesota, Minneapolis,
The role of short term memory and conduction velocity restitution in alternans formation Ning Wei a, Yoichiro Mori a, Elena G. Tolkacheva b, a School of Mathematics, University of Minnesota, Minneapolis,
arxiv: v2 [physics.bio-ph] 14 Aug 2008
![arxiv: v2 [physics.bio-ph] 14 Aug 2008 arxiv: v2 [physics.bio-ph] 14 Aug 2008](/thumbs/95/122824011.jpg) Action potential restitution and hysteresis in a reaction-diffusion system with pacing rate dependent excitation threshold J. M. Starobin arxiv:0805.4444v2 [physics.bio-ph] 14 Aug 2008 University of North
Action potential restitution and hysteresis in a reaction-diffusion system with pacing rate dependent excitation threshold J. M. Starobin arxiv:0805.4444v2 [physics.bio-ph] 14 Aug 2008 University of North
6.3.4 Action potential
 I ion C m C m dφ dt Figure 6.8: Electrical circuit model of the cell membrane. Normally, cells are net negative inside the cell which results in a non-zero resting membrane potential. The membrane potential
I ion C m C m dφ dt Figure 6.8: Electrical circuit model of the cell membrane. Normally, cells are net negative inside the cell which results in a non-zero resting membrane potential. The membrane potential
Learning Cycle Linear Hybrid Automata for Excitable Cells
 Learning Cycle Linear Hybrid Automata for Excitable Cells Sayan Mitra Joint work with Radu Grosu, Pei Ye, Emilia Entcheva, I V Ramakrishnan, and Scott Smolka HSCC 2007 Pisa, Italy Excitable Cells Outline
Learning Cycle Linear Hybrid Automata for Excitable Cells Sayan Mitra Joint work with Radu Grosu, Pei Ye, Emilia Entcheva, I V Ramakrishnan, and Scott Smolka HSCC 2007 Pisa, Italy Excitable Cells Outline
Condition for alternans and its control in a two-dimensional mapping model of paced cardiac dynamics
 Condition for alternans and its control in a two-dimensional mapping model of paced cardiac dynamics Elena G. Tolkacheva, 1,4 Mónica M. Romeo, 2,4 Marie Guerraty, 2 and Daniel J. Gauthier 1,3,4 1 Department
Condition for alternans and its control in a two-dimensional mapping model of paced cardiac dynamics Elena G. Tolkacheva, 1,4 Mónica M. Romeo, 2,4 Marie Guerraty, 2 and Daniel J. Gauthier 1,3,4 1 Department
3-dimensional simulation of long QT syndrome: early afterdepolarizations and reentry Ray Huffaker, Boris Kogan
 3-dimensional simulation of long QT syndrome: early afterdepolarizations and reentry Ray Huffaker, Boris Kogan Abstract Early afterdepolarizations (EADs), thought to be highly arrhythmogenic in the case
3-dimensional simulation of long QT syndrome: early afterdepolarizations and reentry Ray Huffaker, Boris Kogan Abstract Early afterdepolarizations (EADs), thought to be highly arrhythmogenic in the case
Classifying Mechanisms of Spiral Wave Breakup Underlying Cardiac Fibrillation Using Quantitative Metrics
 Rochester Institute of Technology RIT Scholar Works Theses Thesis/Dissertation Collections 8--04 Classifying Mechanisms of Spiral Wave Breakup Underlying Cardiac Fibrillation Using Quantitative Metrics
Rochester Institute of Technology RIT Scholar Works Theses Thesis/Dissertation Collections 8--04 Classifying Mechanisms of Spiral Wave Breakup Underlying Cardiac Fibrillation Using Quantitative Metrics
Application to Experiments
 Chapter 12 Application to Experiments In the past three decades there have been a large number of papers reporting the experimental observation of chaos. In this chapter I describe two rather different
Chapter 12 Application to Experiments In the past three decades there have been a large number of papers reporting the experimental observation of chaos. In this chapter I describe two rather different
Mathematical Biology. Criterion for stable reentry in a ring of cardiac tissue. John W. Cain
 J. Math. Biol. DOI 10.1007/s00285-007-0100-z Mathematical Biology Criterion for stable reentry in a ring of cardiac tissue John W. Cain Received: 11 September 2006 / Revised: 29 March 2007 Springer-Verlag
J. Math. Biol. DOI 10.1007/s00285-007-0100-z Mathematical Biology Criterion for stable reentry in a ring of cardiac tissue John W. Cain Received: 11 September 2006 / Revised: 29 March 2007 Springer-Verlag
Local discontinuous Galerkin methods for elliptic problems
 COMMUNICATIONS IN NUMERICAL METHODS IN ENGINEERING Commun. Numer. Meth. Engng 2002; 18:69 75 [Version: 2000/03/22 v1.0] Local discontinuous Galerkin methods for elliptic problems P. Castillo 1 B. Cockburn
COMMUNICATIONS IN NUMERICAL METHODS IN ENGINEERING Commun. Numer. Meth. Engng 2002; 18:69 75 [Version: 2000/03/22 v1.0] Local discontinuous Galerkin methods for elliptic problems P. Castillo 1 B. Cockburn
Coupled dynamics of voltage and calcium in paced cardiac cells
 Coupled dynamics of voltage and calcium in paced cardiac cells Yohannes Shiferaw, 1,2 Daisuke Sato, 1 and Alain Karma 1 1 Department of Physics and Center for Interdisciplinary Research on Complex Systems,
Coupled dynamics of voltage and calcium in paced cardiac cells Yohannes Shiferaw, 1,2 Daisuke Sato, 1 and Alain Karma 1 1 Department of Physics and Center for Interdisciplinary Research on Complex Systems,
Origins of Spiral Wave Meander and Breakup in a Two-Dimensional Cardiac Tissue Model
 Annals of Biomedical Engineering, Vol. 28, pp. 755 771, 2000 Printed in the USA. All rights reserved. 0090-6964/2000/28 7 /755/17/$15.00 Copyright 2000 Biomedical Engineering Society Origins of Spiral
Annals of Biomedical Engineering, Vol. 28, pp. 755 771, 2000 Printed in the USA. All rights reserved. 0090-6964/2000/28 7 /755/17/$15.00 Copyright 2000 Biomedical Engineering Society Origins of Spiral
The Physics of the Heart. Sima Setayeshgar
 The Physics of the Heart Sima Setayeshgar Department of Physics Indiana University Indiana Unversity Physics REU Seminar: August 1, 2007 1 Stripes, Spots and Scrolls Indiana Unversity Physics REU Seminar:
The Physics of the Heart Sima Setayeshgar Department of Physics Indiana University Indiana Unversity Physics REU Seminar: August 1, 2007 1 Stripes, Spots and Scrolls Indiana Unversity Physics REU Seminar:
Analysis of a Modified Feedback Control Technique for Suppressing Electrical Alternans in Cardiac Tissue
 Analysis of a Modified Feedback Control Technique for Suppressing Electrical Alternans in Cardiac Tissue Srinivasan V. Narayanan 1 and John W. Cain 2 1 Dept. of Biomedical Engineering, Virginia Commonwealth
Analysis of a Modified Feedback Control Technique for Suppressing Electrical Alternans in Cardiac Tissue Srinivasan V. Narayanan 1 and John W. Cain 2 1 Dept. of Biomedical Engineering, Virginia Commonwealth
Mathematical Models. Chapter Modelling the Body as a Volume Conductor
 Chapter 2 Mathematical Models 2.1 Modelling the Body as a Volume Conductor As described in the previous chapter, the human body consists of billions of cells, which may be connected by various coupling
Chapter 2 Mathematical Models 2.1 Modelling the Body as a Volume Conductor As described in the previous chapter, the human body consists of billions of cells, which may be connected by various coupling
Periodic Behavior in Cardiac Tissue: Dynamics of Spatially Discordant Calcium Alternans
 University of Colorado, Boulder CU Scholar Applied Mathematics Graduate Theses & Dissertations Applied Mathematics Spring 1-1-213 Periodic Behavior in Cardiac Tissue: Dynamics of Spatially Discordant Calcium
University of Colorado, Boulder CU Scholar Applied Mathematics Graduate Theses & Dissertations Applied Mathematics Spring 1-1-213 Periodic Behavior in Cardiac Tissue: Dynamics of Spatially Discordant Calcium
Divergence Formulation of Source Term
 Preprint accepted for publication in Journal of Computational Physics, 2012 http://dx.doi.org/10.1016/j.jcp.2012.05.032 Divergence Formulation of Source Term Hiroaki Nishikawa National Institute of Aerospace,
Preprint accepted for publication in Journal of Computational Physics, 2012 http://dx.doi.org/10.1016/j.jcp.2012.05.032 Divergence Formulation of Source Term Hiroaki Nishikawa National Institute of Aerospace,
Biomechanics. Soft Tissue Biomechanics
 Biomechanics cross-bridges 3-D myocardium ventricles circulation Image Research Machines plc R* off k n k b Ca 2+ 0 R off Ca 2+ * k on R* on g f Ca 2+ R0 on Ca 2+ g Ca 2+ A* 1 A0 1 Ca 2+ Myofilament kinetic
Biomechanics cross-bridges 3-D myocardium ventricles circulation Image Research Machines plc R* off k n k b Ca 2+ 0 R off Ca 2+ * k on R* on g f Ca 2+ R0 on Ca 2+ g Ca 2+ A* 1 A0 1 Ca 2+ Myofilament kinetic
Modelling the effect of gap junctions on tissue-level cardiac electrophysiology
 Modelling the effect of gap junctions on tissue-level cardiac electrophysiology Doug Bruce Pras Pathmanathan Jonathan P. Whiteley douglas.bruce@oriel.ox.ac.uk pras.pathmanathan@cs.ox.ac.uk jonathan.whiteley@cs.ox.ac.uk
Modelling the effect of gap junctions on tissue-level cardiac electrophysiology Doug Bruce Pras Pathmanathan Jonathan P. Whiteley douglas.bruce@oriel.ox.ac.uk pras.pathmanathan@cs.ox.ac.uk jonathan.whiteley@cs.ox.ac.uk
Asymptotic approximation of an ionic model for cardiac restitution
 Asymptotic approximation of an ionic model for cardiac restitution David G. Schaeffer,3, Wenjun Ying 2, and Xiaopeng Zhao 2,3 Department of Mathematics and 2 Biomedical Engineering and 3 Center for Nonlinear
Asymptotic approximation of an ionic model for cardiac restitution David G. Schaeffer,3, Wenjun Ying 2, and Xiaopeng Zhao 2,3 Department of Mathematics and 2 Biomedical Engineering and 3 Center for Nonlinear
Wave propagation in an excitable medium with a negatively sloped restitution curve
 CHAOS VOLUME 12, NUMBER 3 SEPTEMBER 2002 Wave propagation in an excitable medium with a negatively sloped restitution curve A. V. Panfilov a) Department of Theoretical Biology, Utrecht University, Padualaan
CHAOS VOLUME 12, NUMBER 3 SEPTEMBER 2002 Wave propagation in an excitable medium with a negatively sloped restitution curve A. V. Panfilov a) Department of Theoretical Biology, Utrecht University, Padualaan
Electrophysiological Modeling of Membranes and Cells
 Bioeng 6460 Electrophysiology and Bioelectricity Electrophysiological Modeling of Membranes and Cells Frank B. Sachse fs@cvrti.utah.edu Overview Recapitulation Electrical Modeling of Membranes Cardiac
Bioeng 6460 Electrophysiology and Bioelectricity Electrophysiological Modeling of Membranes and Cells Frank B. Sachse fs@cvrti.utah.edu Overview Recapitulation Electrical Modeling of Membranes Cardiac
Basic mechanisms of arrhythmogenesis and antiarrhythmia
 EHRA EDUCATIONAL REVIEW AND PREPARATORY COURSE ON INVASIVE CARDIAC ELECTROPHYSIOLOGY EUROPEAN HEART HOUSE, February 2011 Basic mechanisms of arrhythmogenesis and antiarrhythmia Antonio Zaza Università
EHRA EDUCATIONAL REVIEW AND PREPARATORY COURSE ON INVASIVE CARDIAC ELECTROPHYSIOLOGY EUROPEAN HEART HOUSE, February 2011 Basic mechanisms of arrhythmogenesis and antiarrhythmia Antonio Zaza Università
2013 NSF-CMACS Workshop on Atrial Fibrillation
 2013 NSF-CMACS Workshop on A Atrial Fibrillation Flavio H. Fenton School of Physics Georgia Institute of Technology, Atlanta, GA and Max Planck Institute for Dynamics and Self-organization, Goettingen,
2013 NSF-CMACS Workshop on A Atrial Fibrillation Flavio H. Fenton School of Physics Georgia Institute of Technology, Atlanta, GA and Max Planck Institute for Dynamics and Self-organization, Goettingen,
TAKING MATH TO THE HEART: Pulse Propagation in a Discrete Ring
 TAKING MATH TO THE HEART: Pulse Propagation in a Discrete Ring HASSAN SEDAGHAT Department of Mathematics, Virginia Commonwealth University, Richmond, VA 23284-2014, USA The Discrete Ring or Loop A (discrete)
TAKING MATH TO THE HEART: Pulse Propagation in a Discrete Ring HASSAN SEDAGHAT Department of Mathematics, Virginia Commonwealth University, Richmond, VA 23284-2014, USA The Discrete Ring or Loop A (discrete)
Key words. cable equation, Neumann boundary condition, voltage conservation, finite difference, phase field, cardiac model, monodomain
 Abstract. We present a finite difference method for modeling the propagation of electrical waves in cardiac tissue using the cable equation with homogeneous Neumann boundary conditions. This method is
Abstract. We present a finite difference method for modeling the propagation of electrical waves in cardiac tissue using the cable equation with homogeneous Neumann boundary conditions. This method is
Reproducing Cardiac Restitution Properties Using the Fenton Karma Membrane Model
 Annals of Biomedical Engineering, Vol. 33, No. 7, July 2005 ( 2005) pp. 907 911 DOI: 10.1007/s10439-005-3948-3 Reproducing Cardiac Restitution Properties Using the Fenton Karma Membrane Model ROBERT A.
Annals of Biomedical Engineering, Vol. 33, No. 7, July 2005 ( 2005) pp. 907 911 DOI: 10.1007/s10439-005-3948-3 Reproducing Cardiac Restitution Properties Using the Fenton Karma Membrane Model ROBERT A.
The behaviour of high Reynolds flows in a driven cavity
 The behaviour of high Reynolds flows in a driven cavity Charles-Henri BRUNEAU and Mazen SAAD Mathématiques Appliquées de Bordeaux, Université Bordeaux 1 CNRS UMR 5466, INRIA team MC 351 cours de la Libération,
The behaviour of high Reynolds flows in a driven cavity Charles-Henri BRUNEAU and Mazen SAAD Mathématiques Appliquées de Bordeaux, Université Bordeaux 1 CNRS UMR 5466, INRIA team MC 351 cours de la Libération,
MODELLING OF RECIPROCAL TRANSDUCER SYSTEM ACCOUNTING FOR NONLINEAR CONSTITUTIVE RELATIONS
 MODELLING OF RECIPROCAL TRANSDUCER SYSTEM ACCOUNTING FOR NONLINEAR CONSTITUTIVE RELATIONS L. X. Wang 1 M. Willatzen 1 R. V. N. Melnik 1,2 Abstract The dynamics of reciprocal transducer systems is modelled
MODELLING OF RECIPROCAL TRANSDUCER SYSTEM ACCOUNTING FOR NONLINEAR CONSTITUTIVE RELATIONS L. X. Wang 1 M. Willatzen 1 R. V. N. Melnik 1,2 Abstract The dynamics of reciprocal transducer systems is modelled
Reentry in heterogeneous cardiac tissue described by the Luo-Rudy ventricular action potential model
 Am J Physiol Heart Circ Physiol 284: H542 H548, 2003. First published October 10, 2002; 10.1152/ajpheart.00608.2002. Reentry in heterogeneous cardiac tissue described by the Luo-Rudy ventricular action
Am J Physiol Heart Circ Physiol 284: H542 H548, 2003. First published October 10, 2002; 10.1152/ajpheart.00608.2002. Reentry in heterogeneous cardiac tissue described by the Luo-Rudy ventricular action
Bifurcation analysis of a normal form for excitable media: Are stable dynamical alternans on a ring possible?
 CHAOS 18, 013129 2008 Bifurcation analysis of a normal form for excitable media: Are stable dynamical alternans on a ring possible? Georg A. Gottwald a School of Mathematics and Statistics and Centre for
CHAOS 18, 013129 2008 Bifurcation analysis of a normal form for excitable media: Are stable dynamical alternans on a ring possible? Georg A. Gottwald a School of Mathematics and Statistics and Centre for
Adaptive Time Space Discretization for Combustion Problems
 Konrad-Zuse-Zentrum fu r Informationstechnik Berlin Takustraße 7 D-14195 Berlin-Dahlem Germany JENS LANG 1,BODO ERDMANN,RAINER ROITZSCH Adaptive Time Space Discretization for Combustion Problems 1 Talk
Konrad-Zuse-Zentrum fu r Informationstechnik Berlin Takustraße 7 D-14195 Berlin-Dahlem Germany JENS LANG 1,BODO ERDMANN,RAINER ROITZSCH Adaptive Time Space Discretization for Combustion Problems 1 Talk
Simulating Cardiac Electromechanics using Abaqus UEL
 1 Simulating Cardiac Electromechanics using Abaqus UEL Introduction From a finite elements point of view, modeling the complex beating of heart tissues involves solving strongly coupled electromechanical
1 Simulating Cardiac Electromechanics using Abaqus UEL Introduction From a finite elements point of view, modeling the complex beating of heart tissues involves solving strongly coupled electromechanical
Introduction to electrophysiology. Dr. Tóth András
 Introduction to electrophysiology Dr. Tóth András Topics Transmembran transport Donnan equilibrium Resting potential Ion channels Local and action potentials Intra- and extracellular propagation of the
Introduction to electrophysiology Dr. Tóth András Topics Transmembran transport Donnan equilibrium Resting potential Ion channels Local and action potentials Intra- and extracellular propagation of the
1. Introduction. The Stokes problem seeks unknown functions u and p satisfying
 A DISCRETE DIVERGENCE FREE WEAK GALERKIN FINITE ELEMENT METHOD FOR THE STOKES EQUATIONS LIN MU, JUNPING WANG, AND XIU YE Abstract. A discrete divergence free weak Galerkin finite element method is developed
A DISCRETE DIVERGENCE FREE WEAK GALERKIN FINITE ELEMENT METHOD FOR THE STOKES EQUATIONS LIN MU, JUNPING WANG, AND XIU YE Abstract. A discrete divergence free weak Galerkin finite element method is developed
APPM 2360 Project 3 Mathematical Investigation of Cardiac Dynamics
 APPM 2360 Project 3 Mathematical Investigation of Cardiac Dynamics Due: Thursday, December 6, 2018 by 4:59 p.m. Submit as a PDF to Assignments on Canvas 1 Introduction Cardiac Arrhythmia, or irregular
APPM 2360 Project 3 Mathematical Investigation of Cardiac Dynamics Due: Thursday, December 6, 2018 by 4:59 p.m. Submit as a PDF to Assignments on Canvas 1 Introduction Cardiac Arrhythmia, or irregular
Segmented medical images based simulations of Cardiac electrical activity and electrocardiogram: a model comparison
 Segmented medical images based simulations of Cardiac electrical activity and electrocardiogram: a model comparison Charles Pierre, Olivier Rousseau, Yves Bourgault To cite this version: Charles Pierre,
Segmented medical images based simulations of Cardiac electrical activity and electrocardiogram: a model comparison Charles Pierre, Olivier Rousseau, Yves Bourgault To cite this version: Charles Pierre,
Reentry produced by small-scale heterogeneities in a discrete model of cardiac tissue
 Journal of Physics: Conference Series PAPER OPEN ACCESS Reentry produced by small-scale heterogeneities in a discrete model of cardiac tissue To cite this article: Sergio Alonso and Markus Bär 216 J. Phys.:
Journal of Physics: Conference Series PAPER OPEN ACCESS Reentry produced by small-scale heterogeneities in a discrete model of cardiac tissue To cite this article: Sergio Alonso and Markus Bär 216 J. Phys.:
White Rose Research Online URL for this paper: Version: Accepted Version
 This is a repository copy of A coupled 3D-1D numerical monodomain solver for cardiac electrical activation in the myocardium with detailed Purkinje network. White Rose Research Online URL for this paper:
This is a repository copy of A coupled 3D-1D numerical monodomain solver for cardiac electrical activation in the myocardium with detailed Purkinje network. White Rose Research Online URL for this paper:
The Department of Electrical Engineering. nkrol Mentors: Dr. Mohammad Imtiaz, Dr. Jing Wang & Dr. In Soo Ahn
 Bradley University The Department of Electrical Engineering nkrol Mentors: Dr. Mohammad Imtiaz, Dr. Jing Wang & Dr. In Soo Ahn AN ELECTRICAL ENGINEERING PERSPECTIVE OF THE HUMAN HEART This research project
Bradley University The Department of Electrical Engineering nkrol Mentors: Dr. Mohammad Imtiaz, Dr. Jing Wang & Dr. In Soo Ahn AN ELECTRICAL ENGINEERING PERSPECTIVE OF THE HUMAN HEART This research project
Introduction to Heat and Mass Transfer. Week 8
 Introduction to Heat and Mass Transfer Week 8 Next Topic Transient Conduction» Analytical Method Plane Wall Radial Systems Semi-infinite Solid Multidimensional Effects Analytical Method Lumped system analysis
Introduction to Heat and Mass Transfer Week 8 Next Topic Transient Conduction» Analytical Method Plane Wall Radial Systems Semi-infinite Solid Multidimensional Effects Analytical Method Lumped system analysis
ROLE OF BIDOMAIN MODEL OF CARDIAC TISSUE IN THE DYNAMICS OF PHASE SINGULARITIES
 ROLE OF BIDOMAIN MODEL OF CARDIAC TISSUE IN THE DYNAMICS OF PHASE SINGULARITIES Jianfeng Lv Submitted to the faculty of the University Graduate School in partial fulfillment of the requirement for the
ROLE OF BIDOMAIN MODEL OF CARDIAC TISSUE IN THE DYNAMICS OF PHASE SINGULARITIES Jianfeng Lv Submitted to the faculty of the University Graduate School in partial fulfillment of the requirement for the
Lecture 9 Approximations of Laplace s Equation, Finite Element Method. Mathématiques appliquées (MATH0504-1) B. Dewals, C.
 Lecture 9 Approximations of Laplace s Equation, Finite Element Method Mathématiques appliquées (MATH54-1) B. Dewals, C. Geuzaine V1.2 23/11/218 1 Learning objectives of this lecture Apply the finite difference
Lecture 9 Approximations of Laplace s Equation, Finite Element Method Mathématiques appliquées (MATH54-1) B. Dewals, C. Geuzaine V1.2 23/11/218 1 Learning objectives of this lecture Apply the finite difference
New nonlocal approaches for modelling the electrophysiology of the human heart
 New nonlocal approaches for modelling the electrophysiology of the human heart Newport, June, 2013 Kevin Burrage, (Oxford and QUT) kevin.burrage@cs.ox.ac.uk Overview Modelling spatial heterogeneity in
New nonlocal approaches for modelling the electrophysiology of the human heart Newport, June, 2013 Kevin Burrage, (Oxford and QUT) kevin.burrage@cs.ox.ac.uk Overview Modelling spatial heterogeneity in
An Approach for Applying Data Assimilation Techniques for Studying Cardiac Arrhythmias
 Rochester Institute of Technology RIT Scholar Works Theses Thesis/Dissertation Collections 5-3-214 An Approach for Applying Data Assimilation Techniques for Studying Cardiac Arrhythmias Stephen T. Scorse
Rochester Institute of Technology RIT Scholar Works Theses Thesis/Dissertation Collections 5-3-214 An Approach for Applying Data Assimilation Techniques for Studying Cardiac Arrhythmias Stephen T. Scorse
March 9, :18 Int J. Bifurcation and Chaos/INSTRUCTION FILE Morfu2v2 EFFECT OF NOISE AND STRUCTURAL INHOMOGENEITIES IN REACTION DIFFUSION MEDIA
 March 9, 2007 10:18 Int J. Bifurcation and Chaos/INSTRUCTION FILE Int J. Bifurcation and Chaos Submission Style EFFECT OF NOISE AND STRUCTURAL INHOMOGENEITIES IN REACTION DIFFUSION MEDIA S. Morfu Laboratoire
March 9, 2007 10:18 Int J. Bifurcation and Chaos/INSTRUCTION FILE Int J. Bifurcation and Chaos Submission Style EFFECT OF NOISE AND STRUCTURAL INHOMOGENEITIES IN REACTION DIFFUSION MEDIA S. Morfu Laboratoire
AProofoftheStabilityoftheSpectral Difference Method For All Orders of Accuracy
 AProofoftheStabilityoftheSpectral Difference Method For All Orders of Accuracy Antony Jameson 1 1 Thomas V. Jones Professor of Engineering Department of Aeronautics and Astronautics Stanford University
AProofoftheStabilityoftheSpectral Difference Method For All Orders of Accuracy Antony Jameson 1 1 Thomas V. Jones Professor of Engineering Department of Aeronautics and Astronautics Stanford University
PALADINS: Scalable Time-Adaptive Algebraic Splitting and Preconditioners for the Navier-Stokes Equations
 2013 SIAM Conference On Computational Science and Engineering Boston, 27 th February 2013 PALADINS: Scalable Time-Adaptive Algebraic Splitting and Preconditioners for the Navier-Stokes Equations U. Villa,
2013 SIAM Conference On Computational Science and Engineering Boston, 27 th February 2013 PALADINS: Scalable Time-Adaptive Algebraic Splitting and Preconditioners for the Navier-Stokes Equations U. Villa,
Higher order optimization and adaptive numerical solution for optimal control of monodomain equations in cardiac electrophysiology
 Higher order optimization and adaptive numerical solution for optimal control of monodomain equations in cardiac electrophysiology Chamakuri Nagaiah and Karl Kunisch Institute of Mathematics and Scientific
Higher order optimization and adaptive numerical solution for optimal control of monodomain equations in cardiac electrophysiology Chamakuri Nagaiah and Karl Kunisch Institute of Mathematics and Scientific
The Mathematical Modelling of Inhomogeneities in Ventricular Tissue
 The Mathematical Modelling of Inhomogeneities in entricular Tissue T.K. SHAJAHAN 1, SITABHRA SINHA 2, and RAHUL PANDIT 1, * 1 Centre for Condensed Matter Theory, Department of Physics, Indian Institute
The Mathematical Modelling of Inhomogeneities in entricular Tissue T.K. SHAJAHAN 1, SITABHRA SINHA 2, and RAHUL PANDIT 1, * 1 Centre for Condensed Matter Theory, Department of Physics, Indian Institute
ADAPTIVE SOLUTIONS OF NONLINEAR PARABOLIC EQUATIONS WITH APPLICATION TO HYPERTHERMIA TREATMENTS
 ADAPTIVE SOLUTIONS OF NONLINEAR PARABOLIC EQUATIONS WITH APPLICATION TO HYPERTHERMIA TREATMENTS Bodo Erdmann, Jens Lang, Martin Seebaß Konrad-Zuse-Zentrum für Informationstechnik Berlin Takustraße 7 14195
ADAPTIVE SOLUTIONS OF NONLINEAR PARABOLIC EQUATIONS WITH APPLICATION TO HYPERTHERMIA TREATMENTS Bodo Erdmann, Jens Lang, Martin Seebaß Konrad-Zuse-Zentrum für Informationstechnik Berlin Takustraße 7 14195
Abstract. 1. Introduction
 Journal of Computational Mathematics Vol.28, No.2, 2010, 273 288. http://www.global-sci.org/jcm doi:10.4208/jcm.2009.10-m2870 UNIFORM SUPERCONVERGENCE OF GALERKIN METHODS FOR SINGULARLY PERTURBED PROBLEMS
Journal of Computational Mathematics Vol.28, No.2, 2010, 273 288. http://www.global-sci.org/jcm doi:10.4208/jcm.2009.10-m2870 UNIFORM SUPERCONVERGENCE OF GALERKIN METHODS FOR SINGULARLY PERTURBED PROBLEMS
Introduction to Heat and Mass Transfer. Week 9
 Introduction to Heat and Mass Transfer Week 9 補充! Multidimensional Effects Transient problems with heat transfer in two or three dimensions can be considered using the solutions obtained for one dimensional
Introduction to Heat and Mass Transfer Week 9 補充! Multidimensional Effects Transient problems with heat transfer in two or three dimensions can be considered using the solutions obtained for one dimensional
The Restitution Portrait: A New Method for Investigating Rate-Dependent Restitution
 698 The Restitution Portrait: A New Method for Investigating Rate-Dependent Restitution SOMA S. KALB, M.S.E., HANA M. DOBROVOLNY, M.S., ELENA G. TOLKACHEVA, PH.D., SALIM F. IDRISS, M.D., PH.D.,, WANDA
698 The Restitution Portrait: A New Method for Investigating Rate-Dependent Restitution SOMA S. KALB, M.S.E., HANA M. DOBROVOLNY, M.S., ELENA G. TOLKACHEVA, PH.D., SALIM F. IDRISS, M.D., PH.D.,, WANDA
MagnetoHemoDynamics in MRI devices
 MagnetoHemoDynamics in MRI devices Conference on Mathematics of Medical Imaging June -4, Jean-Frédéric Gerbeau INRIA Paris-Rocquencourt & Laboratoire J-L. Lions France Joint work with A. Drochon (UTC),
MagnetoHemoDynamics in MRI devices Conference on Mathematics of Medical Imaging June -4, Jean-Frédéric Gerbeau INRIA Paris-Rocquencourt & Laboratoire J-L. Lions France Joint work with A. Drochon (UTC),
Principles of Nuclear Magnetic Resonance Microscopy
 Principles of Nuclear Magnetic Resonance Microscopy Paul T. Callaghan Department of Physics and Biophysics Massey University New Zealand CLARENDON PRESS OXFORD CONTENTS 1 PRINCIPLES OF IMAGING 1 1.1 Introduction
Principles of Nuclear Magnetic Resonance Microscopy Paul T. Callaghan Department of Physics and Biophysics Massey University New Zealand CLARENDON PRESS OXFORD CONTENTS 1 PRINCIPLES OF IMAGING 1 1.1 Introduction
Simulation of Cardiac Action Potentials Background Information
 Simulation of Cardiac Action Potentials Background Information Rob MacLeod and Quan Ni February 7, 2 Introduction The goal of assignments related to this document is to experiment with a numerical simulation
Simulation of Cardiac Action Potentials Background Information Rob MacLeod and Quan Ni February 7, 2 Introduction The goal of assignments related to this document is to experiment with a numerical simulation
Anisotropic meshes for PDEs: a posteriori error analysis and mesh adaptivity
 ICAM 2013, Heraklion, 17 September 2013 Anisotropic meshes for PDEs: a posteriori error analysis and mesh adaptivity Simona Perotto MOX-Modeling and Scientific Computing Department of Mathematics, Politecnico
ICAM 2013, Heraklion, 17 September 2013 Anisotropic meshes for PDEs: a posteriori error analysis and mesh adaptivity Simona Perotto MOX-Modeling and Scientific Computing Department of Mathematics, Politecnico
Real-time computer simulations of excitable media: Java as a scientific language and as a wrapper for C and Fortran programs
 Real-time computer simulations of excitable media: Java as a scientific language and as a wrapper for C and Fortran programs Flavio H. Fenton a,b, *, Elizabeth M. Cherry c, Harold M. Hastings b, Steven
Real-time computer simulations of excitable media: Java as a scientific language and as a wrapper for C and Fortran programs Flavio H. Fenton a,b, *, Elizabeth M. Cherry c, Harold M. Hastings b, Steven
Computer Science Department Technical Report. University of California. Los Angeles, CA
 Computer Science Department Technical Report University of California Los Angeles, CA 995-1596 COMPUTER SIMULATION OF THE JAFRI-WINSLOW ACTION POTENTIAL MODEL Sergey Y. Chernyavskiy November 1998 Boris
Computer Science Department Technical Report University of California Los Angeles, CA 995-1596 COMPUTER SIMULATION OF THE JAFRI-WINSLOW ACTION POTENTIAL MODEL Sergey Y. Chernyavskiy November 1998 Boris
Isogeometric Analysis of the electrophysiology in the human heart: numerical simulation of the bidomain equations on the atria
 MOX-Report No. 22/218 Isogeometric Analysis of the electrophysiology in the human heart: numerical simulation of the bidomain equations on the atria Pegolotti, L.; Dede', L.; Quarteroni, A. MOX, Dipartimento
MOX-Report No. 22/218 Isogeometric Analysis of the electrophysiology in the human heart: numerical simulation of the bidomain equations on the atria Pegolotti, L.; Dede', L.; Quarteroni, A. MOX, Dipartimento
3D constitutive modeling of the passive heart wall
 3D constitutive modeling of the passive heart wall Thomas S.E. Eriksson Institute of Biomechanics - Graz University of Technology Department of Biophysics - Medical University of Graz SFB status seminar,
3D constitutive modeling of the passive heart wall Thomas S.E. Eriksson Institute of Biomechanics - Graz University of Technology Department of Biophysics - Medical University of Graz SFB status seminar,
Passive and Active Deformation Processes of 3D Fibre-Reinforced Caricatures of Cardiovascular Tissues
 Excerpt from the Proceedings of the COMSOL Conference 2009 Milan Passive and Active Deformation Processes of 3D Fibre-Reinforced Caricatures of Cardiovascular Tissues Antonio DiCarlo 1, Paola Nardinocchi
Excerpt from the Proceedings of the COMSOL Conference 2009 Milan Passive and Active Deformation Processes of 3D Fibre-Reinforced Caricatures of Cardiovascular Tissues Antonio DiCarlo 1, Paola Nardinocchi
Conduction velocity restitution in models of electrical excitation in the heart
 Conduction velocity restitution in models of electrical excitation in the heart Who? From? When? School of Mathematics and Statistics University of Glasgow December 7, 2011 / Liverpool References and credits
Conduction velocity restitution in models of electrical excitation in the heart Who? From? When? School of Mathematics and Statistics University of Glasgow December 7, 2011 / Liverpool References and credits
Controlling Chaos in the Heart: Some Mathematics Behind Terminating Cardiac Arrhythmias
 Controlling Chaos in the Heart: Some Mathematics Behind Terminating Cardiac Arrhythmias John W. Cain September 24, 2013 Abstract Precisely coordinated rhythmic contraction of heart muscle tissue is essential
Controlling Chaos in the Heart: Some Mathematics Behind Terminating Cardiac Arrhythmias John W. Cain September 24, 2013 Abstract Precisely coordinated rhythmic contraction of heart muscle tissue is essential
Stability and Convergence of a Finite Volume Method for a Reaction-Diffusion system of Equations in Electro-Cardiology
 Stability and Convergence of a Finite Volume Method for a Reaction-Diffusion system of Equations in Electro-Cardiology Yves Coudière Charles Pierre Laboratoire Jean Leray, Nantes University and CNRS -
Stability and Convergence of a Finite Volume Method for a Reaction-Diffusion system of Equations in Electro-Cardiology Yves Coudière Charles Pierre Laboratoire Jean Leray, Nantes University and CNRS -
One-Dimensional Numerical Solution of the Maxwell-Minkowski Equations
 Tamkang Journal of Science and Engineering, Vol. 12, No. 2, pp. 161168 (2009) 161 One-Dimensional Numerical Solution of the Maxwell-Minkowski Equations Mingtsu Ho 1 and Yao-Han Chen 2 1 Department of Electronic
Tamkang Journal of Science and Engineering, Vol. 12, No. 2, pp. 161168 (2009) 161 One-Dimensional Numerical Solution of the Maxwell-Minkowski Equations Mingtsu Ho 1 and Yao-Han Chen 2 1 Department of Electronic
Lectures on. Constitutive Modelling of Arteries. Ray Ogden
 Lectures on Constitutive Modelling of Arteries Ray Ogden University of Aberdeen Xi an Jiaotong University April 2011 Overview of the Ingredients of Continuum Mechanics needed in Soft Tissue Biomechanics
Lectures on Constitutive Modelling of Arteries Ray Ogden University of Aberdeen Xi an Jiaotong University April 2011 Overview of the Ingredients of Continuum Mechanics needed in Soft Tissue Biomechanics
Discontinuous Galerkin methods for nonlinear elasticity
 Discontinuous Galerkin methods for nonlinear elasticity Preprint submitted to lsevier Science 8 January 2008 The goal of this paper is to introduce Discontinuous Galerkin (DG) methods for nonlinear elasticity
Discontinuous Galerkin methods for nonlinear elasticity Preprint submitted to lsevier Science 8 January 2008 The goal of this paper is to introduce Discontinuous Galerkin (DG) methods for nonlinear elasticity
Cardiac Electrophysiology on the Moving Heart
 Cardiac Electrophysiology on the Moving Heart (... or a short story on shape analysis) Felipe Arrate FOCUS PROGRAM ON GEOMETRY, MECHANICS AND DYNAMICS The Legacy of Jerry Marsden The Fields Institute July
Cardiac Electrophysiology on the Moving Heart (... or a short story on shape analysis) Felipe Arrate FOCUS PROGRAM ON GEOMETRY, MECHANICS AND DYNAMICS The Legacy of Jerry Marsden The Fields Institute July
A High-Order Discontinuous Galerkin Method for the Unsteady Incompressible Navier-Stokes Equations
 A High-Order Discontinuous Galerkin Method for the Unsteady Incompressible Navier-Stokes Equations Khosro Shahbazi 1, Paul F. Fischer 2 and C. Ross Ethier 1 1 University of Toronto and 2 Argonne National
A High-Order Discontinuous Galerkin Method for the Unsteady Incompressible Navier-Stokes Equations Khosro Shahbazi 1, Paul F. Fischer 2 and C. Ross Ethier 1 1 University of Toronto and 2 Argonne National
Drift-Diffusion Simulation of the Ephaptic Effect in the Triad Synapse of the Retina
 Drift-Diffusion Simulation of the Ephaptic Effect in the Triad Synapse of the Retina Jeremiah Jones PhD Thesis Defense, Applied Mathematics SCHOOL OF MATHEMATICAL AND STATISTICAL SCIENCES April 5, 2013
Drift-Diffusion Simulation of the Ephaptic Effect in the Triad Synapse of the Retina Jeremiah Jones PhD Thesis Defense, Applied Mathematics SCHOOL OF MATHEMATICAL AND STATISTICAL SCIENCES April 5, 2013
Suppression of Spiral Waves and Spatiotemporal Chaos Under Local Self-adaptive Coupling Interactions
 Commun. Theor. Phys. (Beijing, China) 45 (6) pp. 121 126 c International Academic Publishers Vol. 45, No. 1, January 15, 6 Suppression of Spiral Waves and Spatiotemporal Chaos Under Local Self-adaptive
Commun. Theor. Phys. (Beijing, China) 45 (6) pp. 121 126 c International Academic Publishers Vol. 45, No. 1, January 15, 6 Suppression of Spiral Waves and Spatiotemporal Chaos Under Local Self-adaptive
Multigrid Methods for Elliptic Obstacle Problems on 2D Bisection Grids
 Multigrid Methods for Elliptic Obstacle Problems on 2D Bisection Grids Long Chen 1, Ricardo H. Nochetto 2, and Chen-Song Zhang 3 1 Department of Mathematics, University of California at Irvine. chenlong@math.uci.edu
Multigrid Methods for Elliptic Obstacle Problems on 2D Bisection Grids Long Chen 1, Ricardo H. Nochetto 2, and Chen-Song Zhang 3 1 Department of Mathematics, University of California at Irvine. chenlong@math.uci.edu
Helsinki University of Technology Publications on Engineering Physics Teknillisen korkeakoulun teknillisen fysiikan julkaisuja
 Helsinki University of Technology Publications on Engineering Physics Teknillisen korkeakoulun teknillisen fysiikan julkaisuja Espoo 2004 TKK-F-A829 A HYBRID MODEL OF CARDIAC ELECTRICAL CONDUCTION Kim
Helsinki University of Technology Publications on Engineering Physics Teknillisen korkeakoulun teknillisen fysiikan julkaisuja Espoo 2004 TKK-F-A829 A HYBRID MODEL OF CARDIAC ELECTRICAL CONDUCTION Kim
Real-time computer simulations of excitable media:
 BioSystems 64 (2002) 73 96 www.elsevier.com/locate/biosystems Real-time computer simulations of excitable media: JAVA as a scientific language and as a wrapper for C and FORTRAN programs Flavio H. Fenton
BioSystems 64 (2002) 73 96 www.elsevier.com/locate/biosystems Real-time computer simulations of excitable media: JAVA as a scientific language and as a wrapper for C and FORTRAN programs Flavio H. Fenton
Fast Solvers for Unsteady Incompressible Flow
 ICFD25 Workshop p. 1/39 Fast Solvers for Unsteady Incompressible Flow David Silvester University of Manchester http://www.maths.manchester.ac.uk/~djs/ ICFD25 Workshop p. 2/39 Outline Semi-Discrete Navier-Stokes
ICFD25 Workshop p. 1/39 Fast Solvers for Unsteady Incompressible Flow David Silvester University of Manchester http://www.maths.manchester.ac.uk/~djs/ ICFD25 Workshop p. 2/39 Outline Semi-Discrete Navier-Stokes
INTERGRID OPERATORS FOR THE CELL CENTERED FINITE DIFFERENCE MULTIGRID ALGORITHM ON RECTANGULAR GRIDS. 1. Introduction
 Trends in Mathematics Information Center for Mathematical Sciences Volume 9 Number 2 December 2006 Pages 0 INTERGRID OPERATORS FOR THE CELL CENTERED FINITE DIFFERENCE MULTIGRID ALGORITHM ON RECTANGULAR
Trends in Mathematics Information Center for Mathematical Sciences Volume 9 Number 2 December 2006 Pages 0 INTERGRID OPERATORS FOR THE CELL CENTERED FINITE DIFFERENCE MULTIGRID ALGORITHM ON RECTANGULAR
A Symmetric and Low-Frequency Stable Potential Formulation for the Finite-Element Simulation of Electromagnetic Fields
 A Symmetric and Low-Frequency Stable Potential Formulation for the Finite-Element Simulation of Electromagnetic Fields Martin Jochum, Ortwin Farle, and Romanus Dyczij-Edlinger Abstract A low-frequency
A Symmetric and Low-Frequency Stable Potential Formulation for the Finite-Element Simulation of Electromagnetic Fields Martin Jochum, Ortwin Farle, and Romanus Dyczij-Edlinger Abstract A low-frequency
Locating Order-Disorder Phase Transition in a Cardiac System
 Locating Order-Disorder Phase Transition in a Cardiac System Hiroshi Ashikaga *a and Ameneh Asgari-Targhi b a Cardiac Arrhythmia Service, Johns Hopkins University School of Medicine, 6 N Wolfe Street,
Locating Order-Disorder Phase Transition in a Cardiac System Hiroshi Ashikaga *a and Ameneh Asgari-Targhi b a Cardiac Arrhythmia Service, Johns Hopkins University School of Medicine, 6 N Wolfe Street,
Towards the numerical simulation of electrocardiograms
 owards the numerical simulation of electrocardiograms Muriel Boulakia 2, Miguel A. Fernández 1 Jean-Frédéric Gerbeau 1, and Nejib Zemzemi 1 1 REO team, INRIA Rocquencourt, France WWW home page: http://www-rocq.inria.fr/reo
owards the numerical simulation of electrocardiograms Muriel Boulakia 2, Miguel A. Fernández 1 Jean-Frédéric Gerbeau 1, and Nejib Zemzemi 1 1 REO team, INRIA Rocquencourt, France WWW home page: http://www-rocq.inria.fr/reo
Multigrid Methods and their application in CFD
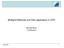 Multigrid Methods and their application in CFD Michael Wurst TU München 16.06.2009 1 Multigrid Methods Definition Multigrid (MG) methods in numerical analysis are a group of algorithms for solving differential
Multigrid Methods and their application in CFD Michael Wurst TU München 16.06.2009 1 Multigrid Methods Definition Multigrid (MG) methods in numerical analysis are a group of algorithms for solving differential
SPATIOTEMPORAL DYNAMICS OF PACING IN MODELS OF ANATOMICAL REENTRY
 SPATIOTEMPORAL DYNAMICS OF PACING IN MODELS OF ANATOMICAL REENTRY Sitabhra Sinha and David J. Christini Division of Cardiology, Weill Medical College of Cornell University, New York, NY, 10021, USA Centre
SPATIOTEMPORAL DYNAMICS OF PACING IN MODELS OF ANATOMICAL REENTRY Sitabhra Sinha and David J. Christini Division of Cardiology, Weill Medical College of Cornell University, New York, NY, 10021, USA Centre
High Order Accurate Runge Kutta Nodal Discontinuous Galerkin Method for Numerical Solution of Linear Convection Equation
 High Order Accurate Runge Kutta Nodal Discontinuous Galerkin Method for Numerical Solution of Linear Convection Equation Faheem Ahmed, Fareed Ahmed, Yongheng Guo, Yong Yang Abstract This paper deals with
High Order Accurate Runge Kutta Nodal Discontinuous Galerkin Method for Numerical Solution of Linear Convection Equation Faheem Ahmed, Fareed Ahmed, Yongheng Guo, Yong Yang Abstract This paper deals with
Implicit Solution of Viscous Aerodynamic Flows using the Discontinuous Galerkin Method
 Implicit Solution of Viscous Aerodynamic Flows using the Discontinuous Galerkin Method Per-Olof Persson and Jaime Peraire Massachusetts Institute of Technology 7th World Congress on Computational Mechanics
Implicit Solution of Viscous Aerodynamic Flows using the Discontinuous Galerkin Method Per-Olof Persson and Jaime Peraire Massachusetts Institute of Technology 7th World Congress on Computational Mechanics
Models and Measurements of the Anisotropic Cardiac Bidomain
 Models and Measurements of the Anisotropic Cardiac Bidomain John P. Wikswo Instituto de Matemática Pura e Aplicada Rio De Janeiro, Brazil 6 May 2002 Living State Physics Group, Department of Physics &
Models and Measurements of the Anisotropic Cardiac Bidomain John P. Wikswo Instituto de Matemática Pura e Aplicada Rio De Janeiro, Brazil 6 May 2002 Living State Physics Group, Department of Physics &
