Chm 363. Spring 2017, Exercise Set 1 Quantum Mechanics Atoms, Ionization Energies Multielectronic Atoms Symmetry. Mr. Linck
|
|
|
- Duane Johns
- 5 years ago
- Views:
Transcription
1 Chm 363 Spring 2017, Exercise Set 1 Quantum Mechanics Atoms, Ionization Energies Multielectronic Atoms Symmetry Mr. Linck Version 3.0 January 23, Schrödinger s Equation Schrödinger s equation for a one dimensional system without a potential energy says that a constant times the second derivative of an acceptable wave function must yield a constant (denoted k below) times that wave function back again, or: 2 2 ψ 2m x 2 = kψ Show that under these conditions of potential that ψ = cos(kx) is a suitable solution. 1.2 Solutions to Schrödinger s Equation Show that the functions ψ = sin(kx) and ψ = e kx are also solutions. 1.3 Boundary Conditions Acceptable wave functions must satisfy boundary conditions. For the problem at hand, a parve on a pole with no potential energy, the wave function must disappear at x = 0 and at x = L, where L is the length of the pole. Show that neither ψ = cos(kx) nor ψ = e kx satisfy this condition at x = Boundary Conditions Show that the boundary condition at x = L requires the value of k in ψ = sin(kx) to be nπ L. 1.5 The Wave Function for a POP Make a rough plot of the wave function versus x for a parve on a pole. HINT: Nothing magic here; the wave function is given by ψ = sin(nπx/l) which is a simple function to plot no matter what value of n you choose. 1.6 Hydrogen Atom Quantum Numbers What are the conventional symbols for the three quantum numbers in a hydrogen atom? What property or properties does each approximately measure?
2 Energy Levels in the Hydrogen Atom Make an energy level diagram (horizontal lines, one for each orbital, spread along a vertical energy level axis) for the hydrogen atom energy levels as a function of n. Put numerical values in for the energies and label each level with the quantum numbers. Go up to the n = 3 level. 1.8 Spectral Lines in Emission in the Hydrogen Atom Calculate the wavelength (in nm) for the transition from 3p to 2s in the H atom. Why is this an emission? 1.9 Spectral Lines and Relative Energies Excited Hg emits a photon in the blue region of the spectrum, at nm. Calculate the energy of this photon. Find the energy of a mole of these and compare your answer to the energy needed to break a typical C-H bond (usually given in kj/mole) Spectral Lines and Relative Energies An excited atom emits a photon in the ultraviolet region of the spectrum. Does this have more or less energy than that emitted from Hg (in the last problem)? 1.11 Radial Wave Functions If the radial wave function for a 3s electron is: ( ) ( 1 3/2 ( ) ( ) ) r r 2 ψ = /3 e r 3a 0 3a 0 3a 0 3a 0 find the values of r when the function is nodal. HINT: This is a simple math problem; set ψ to zero and solve for r a Plotting Hydrogen Atom Wave Functions Why do we have such a convoluted way of plotting hydrogen atom wave functions? Why are they more difficult that POP wave functions? 1.13 Radial Wave Functions Make a rough plot of the radial wave function for a hydrogen atom with quantum numbers of 3s, 3p, 4s, and 4f Probability at a Given Distance Make a rough plot of the probability of finding a 2s and 2p electron at a given distance from the nucleus versus the distance. Put your plots on the same scale Probability at a Given Distance Make a rough plot of the probability of finding a 2s and 3s electron at a given distance from the nucleus versus the distance. Put your plots on the same scale Probability at a Given Distance Make a rough plot of the probability of finding a 1s and 2p electron at a given distance from the nucleus versus the distance. Put your plots on the same scale.
3 Probability at a Given Distance Make a rough plot of the probability of finding a 2s, 4p, and 4f elecctron at a given distance from the nuclues versus the distance. Put your plots on the same scale Angular Momentum Functions The allowed values of the quantum number l depend on the value of the quantum number n. How many l values are allowed for n = 4? 1.19 Angular Momentum Functions How many m l (also called, l z ) values are allowed for l = 2? for l = 3? in general, for l = l? 1.20 Angular Momentum of Hydrogen Atoms Make a vector diagram for the situation where l = 2. HINT: Remember that the length of an angular momentum vector is related to the quantum number l through the relationship: length = l(l + 1) (1) 1.21 Angular Momentum Code In normal discussion about wave functions, say 1s, there is that strange letter. It stands for the l values. What is the code for various l values from l = 0 to 4? 1.22 Angular Momentum of Hydrogen Atoms Make a vector diagram for the situation l = 1, m l = 0. HINT: m l and l z are equivalent expressions of the quantum number Angular Momentum of Hydrogen Atoms Make a vector diagram for the situation l = 2, m l = Vector Addition Make a diagram where you add two vectors, both of length 1, such that the final vector is two units long Vector Addition Make a diagram where you add two vectors, both of length 1, such that the final vector is one unit long Vector Addition Make a diagram where you add two vectors, both of length 1, such that the final vector is the null vector Vector Addition If I asked you give the result of adding two vectors, what would you say? 1.28 Quantum Angular Momentum Vectors It is worth noting this result: Make a table of the ratio of the length of a quantum angular momentum vector to the value of l, i.e., a table of What trend do you observe? l(l+1) l, for l from 1 to 5 in integer steps.
4 Figure 1: The Relationship between Cartesian and Spherical Coordinates 1.29 Quantum Vector Addition From the data in the last problem, what must happen if you add two lower l value vectors to make a larger one with maximum total angular momentum, labeled L = l 1 + l 2? HINT: I am looking for a general answer, not some specific one Quantum Angular Momentum Truth If you have a quantum mechanical angular momentum vector of length 20, what is it s l value? 1.31 Quantum Angular Momentum Truth If you have a quantum mechanical angular momentum vector with l = 3, what is its length? 1.32 Quantum Angular Momentum Truth If you have a quantum mechanical angular momentum vector of length 20, what are the legal projections of this vector on the z axis? That is, what are the allowed l z (or m l ) values? 1.33 Cartesian and Spherical Coordinates Consult Figure 1 and convert the given Cartesian coordinates [x, y, z] into spherical coordinates [r, θ, φ]. You should not need a calculator or computer. [1, 0, 0]; [0, 1, 0]; [0, 0, 1]; [1, 1, 0] Cartesian and Spherical Coordinates For each of the following convert the spherical coordinates [r, θ, φ] into Cartesian coordinates [x, y, z]. You should not need a calculator or computer. [1, π/2, π/2]; [1, π, 0]; [1, π/2, 3π/2] Cartesian and Spherical Coordinates Find the expressions that relate x, y, and z to r, θ, and φ; three equations. See Figure 1
5 Figure 2: The 2p x wave function with z = 0, arbitrary units Figure 3: The 2p x wave function with z = 3, arbitrary units, but same as for Figure Angular Momentum Functions and Spatial Functions If the angular part of the natural (actually the functions that are eigenfunctions of the angular momentum operator) 2p, m = 1 and m = -1, wave functions for a hydrogen atom are: ψ m=1 = 3/(4π)Sin[θ]e iφ ψ m= 1 = 3/(4π)Sin[θ]e iφ show that adding these produces a function we might call p x. HINT: You need to look up the value of the sum e ix + e ix, and you will need to refer to Figure Hydrogen Wave Function as x and y Change In Figures 2 and 3 are given the hydrogen atom wave function for quantum numbers 2p x with z held constant. Be sure you understand these pictures Angular Wave Functions Make a sketch of the angular wave function for a 2s electron; a 3s electron Angular Wave Functions Make a sketch of the angular wave function for a 2p x electron; a 3p z electron.
6 Figure 4: The 3d xy wave function with z = 0, arbitrary units Angular Wave Functions Make a sketch of the angular wave function for all five possible 3d electrons Angular Wave Functions Sketch the angular dependence of all three p orbitals from the same view point. Label your axes Angular Wave Functions Sketch the angular dependence of all five d orbitals from the same view point. Make your z axis up and down on the paper Angular Wave Functions Show the angular function d xy behaves as does the function xy, that is, the function x times y. Figure 4 is a sketch of that function with z held frozen at zero Angular Wave Functions Use the intuition gained in the last problem to describe the angular dependence of f xyz Angular Wave Functions Read the article by Becker, J. Chem. Educ., 1964, 41, 358 and be able to describe the angular properties of the seven f orbitals Angular Wave Functions What is the sign of Becker s function A (labeled so in his Table 1) at x=1, y=0, z=0? at (0, 1, 0)? at (-1, 0, 0)? 1.47 Angular Wave Functions Becker s function C, also known as f z(5z 2 3r 2 ), has several nodal cones at various values of θ. Find them from the functional form given in Table 1. HINT: A computer with Mathematica might be useful Angular Wave Functions Compare what one might call f z 3 from the last problem with d z 2.
7 Figure 5: The 3p x wave function with z = 0, arbitrary units Angular Wave Functions From the form f x(5z 2 r 2 ), evaluate the function when x = Angular Wave Functions Show the function from the last problem changes sign when x is changed to -x Angular Wave Functions What happens to the sign of the function of problem 49 when z goes to -z? 1.52 Angular Wave Functions With information from the last three problems, put in signs in the lobs of the function given by Becker in his figure Even and Odd Functions Look at the angular functions s, p, d, and f. For each go from a point labeled x, y, z to a point -x, -y, -z. Under that transformation, does the function change sign or not? Do you see a pattern? Those functions that remain of the same sign are called g and those that change are called u. HINT: This is a symmetry result that we will soon get to more formally Angular Wave Functions In Figure 5 is a plot (arbitrary units) of a 3p x wave function. Account for the striking difference from Figure 2. HINT: How many features are different? 1.55 Ionization Energy What is the factor that causes the ionization energy of N to be greater than B? 1.56 Gauss s Theorem A critical theorem from classical electrostatics to know and appreciate is Gauss s theorem. This theorem pertains to the charge evenly distributed on a sphere; let s say for our purposes, on a hollow metallic sphere. The theorem states: if you are outside the charged sphere, the charge appears to you as if it is located at the center of the sphere. Give a rationalization of why Gauss s theorem is correct; afterall, some of the charge is close to you, the observer.
8 Figure 6: Janet s Periodic Table 1.57 Ionization Energy Using your knowledge of the ground state electronic configuration of Li, suggest whether a 2s or a 2p orbital is more stable in a Li atom. Can you deduce a reason for this behavior from the probability at a given distance plots of a 2s electron and a 2p electron? If you have trouble making sense of this, picture yourself riding on a 2s electron looking toward the nucleus and apply Gauss s theorem. What charge do you see as you sweep out all probable positions? (HINT: The value will not be a constant.) Do the same as you ride a 2p electron. Could the name penetration be given to the difference? Why? What is penetrated? 1.58 Ionization Energy What is the factor that causes the ionization energy of Be to be greater than B? 1.59 Ionization Energy What is the factor that causes the ionization energy of N to be greater than P? 1.60 Ionization Energy What is the factor that causes the ionization energy of P to be greater than S? 1.61 Ionization Energy What are the four factors influencing ionization energy? 1.62 Ionization Energy Which of the four factors are important in the difference in IE between Li and Cs? 1.63 Ionization Energy Which of the four factors are important in the difference in IE between Li and Be? 1.64 Ionization Energy Which of the four factors are important in the difference in IE between H[5s 1 ] and Rb? 1.65 Ionization Energy Order each of the following sets in terms of increasing I.E. C O Ne Li Na Mg C Si P F Cl Br 1.66 Periodic Table In Figure 6 is a version of a periodic table proposed by Janet. What is it good at showing?
9 Periodic Table What factor does the Janet periodic table give up representing clearly? 1.68 Ionization Energy Predict the largest IE of each of the following pairs and explain your reasoning: Li Na C N Cr Zn I Te Mg Al Al Ge Cl Br 1.69 Atomic Radii How would you expect atomic radii to vary from left to right in the periodic table? Look up some values to verify your supposition Atomic Radii How would you expect atomic radii to vary from top to bottom in the periodic table? Look up some values to verify your supposition Ionization Energy How would you expect the IE of the mononegative ions to vary from left to right across the first row in the periodic table? 1.72 Ionization Energy A rule for IE has been suggested. If you take the sum of the IE of an element in column I with the element in column VII of the same row, you will get the same answer as doing so with col. II + col VI, or col III + col V, or twice col IV. Test this with first row elements Ionization Energy How do you rationalize the rule in the last problem? electron/electron repulsion and penetration. HINT: Use your knowledge of 1.74 Ionization Energy Define species X as having a 2s 2 configuration and species Y as having a 2s 2 2p 1 configuration. The relative ionization energies for some of these isoelectronic comparisons are given in Table 1. Explain the data Ionization Energy The first five ionization energies of the N atom are 14.53, 29.59, 47.43, 77.4, and ev. Verbalize how you would make sense of these values Ionization Energy Predict the first six ionization energies of the S atom given the second is 23.4 ev Ionization Energy The first IE of B is 800 kj/mole. Predict (roughly) the second, third, and fourth IE of B.
10 X Y IE X IE Y Be B 1.12 B + C C 2+ N N 3+ O O 4+ F Table 1: Relative Ionization Energies for Some Isoelectronic Species 1.78 The 4s/3d Issue Problems involving this issue rely upon a paper by L. G. Vanquickenborne, K. Pierloot, and D. Devoghel (Inorg. Chem., 1989, 28, ). The 4s orbital is less stable than the 3d in a hydrogen atom. This continues in multielectronic atoms until carbon, where a switch occurs and 3d becomes less stable. Suggest a reason why He through B has the hydrogen arrangement, but C and greater (at least for a number of elements see below) have the opposite arrangement The 4s/3d Issue Suggest a reason that after carbon, 4s is more stable than 3d The 4s/3d Issue What are the valence electrons in Ge? Given that result, which orbital is more stable in Ge, 3d or 4s? Why? 1.81 The 4s/3d Issue Given the facts from the last two problems, there must be a second cross-over of the energies of 3d and 4s. in which 3d again becomes more stable. Calculations suggest this occurs just past Ca, at Sc. The issue becomes then a question of why the configuration of the transition metals is largely 4s 2 3d n and not 3d n+2. The other feature that must be considered, according to Vanquickenborne, et. al., is electron-electron repulsion. What would you expect for the electron-electron repulsion between two 4s electrons compared to that between two 3d electrons? 1.82 The 4s/3d Issue Detailed calculations indicate that the electron-electron repulsion difference is sufficiently large that the configuration 4s 2 3d n is generally the most stable configuration. For Sc, for instance, the approximate energy of the 3d level is -24 ev and that of the 4s level is -19 ev. The 3d/3d, 3d/4s, and 4s/4s electron-electron repulsions are about 13 ev, 6 ev, and 7 ev, respectively. Make a rough calculation of the energy of the three possible configurations of Sc, those with 1, 2, and 3 electrons in the d orbitals. HINT: Pay attention to the number and kind of electrons that are repelling and count the number of pairs correctly.
11 The 4s/3d Issue The Vanquickenborne analysis supports the configuration of the transition metal ions. That analysis shows that the 3d orbital is stabilized by charge much more than 4s is. Can you give a reason? 1.84 Ionization Energy and Chemical Properties How big a change in ionization energy is there between Na and Ar? How big between Sc and Zn? What do you think this does to the relative chemical behavior of these two sets of elements? 1.85 Ionization Energy The ionization energy of Li(1s 2 2s 1 ) is 5.39 ev; that of Li(1s 2 2p 1 ) is 3.54 ev. How much energy does it take to excite the first material to the second? What wavelength of light is necessary to carry out this conversion? Compare the two values with the IE s of H(2s 1 ) and H(2p 1 ) and give a rationalization that makes sense within our scheme of factors to consider in determining IE Constancy of IE of Transition Metals Generally, it is a 4s electron that is removed in the first IE of the first row transition metal atoms. This statement is commonly given, but what it really means needs careful thought. One cannot really say which electron is removed; all one can do is give the configuration of the system before and after that removal. The statement, therefore, really means that if the configuration of the atom is 3d n 4s 2, the positive transition metal ion is 3d n 4s 1 or 3d n+1 rather than 3d n 1 4s 2. If we stick to our simple statement above, why does the IE of transition metal atoms change so slightly as the nuclear charge goes up? 1.87 Ionization Energy Look up the values of the ionization energies of boron through Ne and plot them. Draw straight lines through appropriate data Ionization Energy If the distance between the extrapolated line drawn through the IE of B, C, and N and the line drawn through the IE of O, F, and Ne is about 430 kj/mole, what would you expect for the separation between the corresponding lines drawn through Al, Si, and P and S, Cl, Ar? 1.89 Russell-Saunders Coupling Draw three short lines horizonally across, as in. Label each for the m l value that a p electron can have. Now for a p 3 configuration, put in electrons such that the sum of the m l (or l z ) values is maximal Russell-Saunders Coupling Again draw three short lines horizonally across. Label each for the m l value that a p electron can have. Now for a p 3 configuration, put in electrons such that the sum of the s z values is maximal Russell-Saunders Coupling How many different arrangement of electrons can you have for a p 3 configuration?
12 Russell-Saunders Coupling Make a chart in which each of the arrangements that you predicted in the last problem is classified according to its value of M L (the sum of the m l ) and its M S (the sum of the s z ) Russell-Saunders Coupling Find the Russell-Saunders states for a p 3 configuration. HINT: You must use quantum logic to do so, but that is easy Russell-Saunders Coupling Find the Hund s first rule ground state for a p 3 configuration Russell-Saunders Coupling Show that the contribution to the R-S state from a full shell is nothing Russell-Saunders Coupling Find the R-S states for a d 2 configuration Russell-Saunders Coupling Find the Hund s first and second rule ground state for a d 2 configuration Russell-Saunders Coupling Find the Hund s first and second rule ground state for a d 4 configuration. HINT: This is only asking for the ground state. To find them all is a chore. To find the ground state is easy if you apply the rules as you start the table rather than after you finish analyzing with it Russell-Saunders Coupling Find the ground R-S state for a d 2 s 1 configuration Spectral Lines within Russell-Saunders States At low resolution, there are six transitions in the emission spectrum of C + that occur within the 2s2p 2 configuration. Four of these are forbidden transitions (and hence are weak); the other two are allowed. The selection rules for allowed transitions between Russell-Saunders states are that L = ±1 and that S = 0. What Russell-Saunders states are involved? Which transitions are allowed and which forbidden? Spin-Orbit Coupling Give the spin-orbit states for a 1 D state. HINT: duh? Spin-Orbit Coupling Give the spin-orbit states for a 4 G state Hund s Third Rule Which is the most stable spin-orbit state, 3 P 2 or 3 P 0? HINT: I know what I am saying Hund s Third Rule Which is the most stable spin-orbit state for an O atom, 3 P 2 or 3 P 0? Russell-Saunders Coupling What is the degeneracy of the 4 I state of an f 3 configuration?
13 Russell-Saunders Coupling Is the 4 I state the R-S ground state of an f 3 configuration? Spin-Orbit Coupling What is the spin-orbit coupled ground state of the 4 I state of an f 3 configuration? Degeneracy of States What is the degeneracy of the spin-orbit coupled ground state of the 4 I state of an f 3 configuration Atomic Spectra The so-called Fraunhofer lines occur in the solar spectrum. What are they and what causes them? Atomic Spectra A Fraunhofer line in the solar spectrum at nm is caused be neutral iron atoms. What transition (between what states of what configuration) is responsible for this line? Explain in detail how this process occurs. HINT: You might look at Atomic Spectra A line in the ultraviolet region of the spectrum of excited nitrogen atoms occurs at nm. It is presumably due to a transtion from the 4 P 5/2 (2p 2 3s 1 ) to the ground state of the atom. Two other transitions are close by. Will they be of higher or lower energy? Give your reasoning Spin-Orbit Coupling The energies of the three spin orbital coupled states of the ground R-S state of a C atom in the p 2 configuration are (in cm 1 ): 3 P 0, 0; 3 P 1, 16.4; 3 P 2, Show that if you take into account degeneracy that these states are centered around an average value of about 29.6 cm 1. Draw an approximately accurate energy level diagram for this situation with the three spin-orbit states branching off from the average value. An example is shown in Figure Review of IE The first IE of C, N, and O are 11.25, 14.53, and ev. Explain the trend Review of IE The first IE of Si, P, and S are 8.15, 10.48, and ev. Why are these values all lower than those of the last problem? IE and Spin-Orbit Coupling The first IE of Sn, Sb, and Te are 7.34, 8.64, and 9.01 ev. Explain this trend. HINT: Try making diagrams such as you made in problem 112 for both the ground state of the atom and the ground state of the ion IE and Spin-Orbit Coupling The first IE of Pb, Bi, and Po are 7.42, 7.28, and 8.43 ev. What do these data do to your analysis of the last several problems? HINT: See the last problem.
14 Figure 7: Example Energy Level Diagram for Problem VOIE The same kind of averaging over states that we used with spin-orbit coupling in problem 112 can be used with the states caused by electron-electron repulsion. What is a VOIE? Average of States Energy The energies of states of a carbon atom in the p 2 configuration are: 3 P = 0; 1 D = cm 1, and 1 S = cm 1. What is the average energy of the s 2 p 2 configuration of C? VOIE What can you deduce about the splitting of the states of a plus one carbon ion in the p 1 configuration? What is the average energy of the s 2 p 1 configuration of C +? Find the VOIE of a C 2p electron Russell-Saunders Coupling Use Charlotte Moore s tables, shown in Figures 8 and 9 to find the VOIE of the p orbital electrons in N. HINT: The normal ionization energy of N atom is cm VOIE interpretation Why does the gap between the p orbital VOIE and the s orbital VOIE increase from B to Ne? And now on to SYMMETRY
15 Figure 8: Part of the Table for Energy Levels in N Atom, Charlotte E. Moore, NSRDS-NBS 3, 80p. (1975). Figure 9: Part of the Table for Energy Levels in N + Ion, Charlotte E. Moore, NSRDS-NBS 3, 80p. (1975).
16 Figure 10: Figure for problem 123 Figure 11: Figure for problem Symmetry Operation A symmetry operation leaves, for a closed-eyed observer, an object [blank]. Fill in the blank two words Symmetry Operations What symmetry operation carries out the process shown in Figure 10? Hereafter this object will be called M. HINT: The top side of the balls are colored orange and the bottom side blue for ease of discerning the operations; they are also numbered for this purpose. Of course, neither the color nor the numbers really exist from the point of view of the symmetry Symmetry Operations What symmetry operation carries out the operation shown in Figure 11? Symmetry Operations What single symmetry operation accomplishes the operation shown in Figure 12? Symmetry Operations Since the operation in Figure 12 does what the two in Figures 10 and 11 do, the operation in Figure 12 must be the product of the two in the other figures. Write this succinctly Symmetry Operations Find the product of the operation C 3 σ up/dn for object M, where C 3 is a clockwise rotation of the object by 2π/3 and σ up/dn is reflection in a plane perpendicular to the plane of the object and up and down as we view it.
17 Figure 12: Figure for problem Symmetry Operations Find the inverse of each of the three symmetry operations (hereafter, SO) from problems Symmetry Operations Proper symmetry operations are those that you can actually do on the object; generally rotations. Find the proper symmetry operations in H 2 O NH 3 PtCl 2 4, a square planar complex CH Symmetry Operations Give a justification for the statement: The product of any two proper S.O. is also proper Symmetry Operations Improper symmetry operations are SO that you cannot really do on a physical object. They include reflections in planes internal to the object. What does it require to rigorously define the location of a reflection plane? Symmetry Operations Improper symmetry operations also include the center of inversion, called i, in which anything located at (x, y, z) is moved to (-x, -y, -z). Does object M have an i? Symmetry Operations Improper symmetry operations have a third kind of operation, a rotation-reflection. This S n operation is a rotation by 360/n degrees followed by a reflection in a plane perpendicular to that axis of rotation. Does object M have an S n? Symmetry Operations Find the improper symmetry operations in the compounds given in problem 129.
18 Symmetry Operations Build a multiplication table for the SO of object M Symmetry Operations For the group of object M, find some SO that do not commute Symmetry Operations For the group of object M, find some SO that do commute Group Theory List the four properties needed for a group Group Theory In a group multiplication table each element of the group occurs once and only once in any given row or column. Prove this has to be true. HINT: Assume for arbitrary elements F, G, H, and R that: FG = R HG = R Then multiply from the right with the inverse of G and show that the equations above can only be true if F = H Subgroups Is there a sub-group in the group of M? What SO are in it? Group Theory The order of a group is simply the number of operations in it. What is the order of the group of M? Of the sub-group? Class Theory If X and U are both in the group of object M, evaluate X 1 U X for all operations X in the group of object M when U = C 3. The set of operations you get as a result are said to be in the same class Class Theory What elements of the SO of the group of M are in the same class as C 3? Class Theory Inspect your answer from the last problem and see if you can believe the following: SO are in the same class if they are operations of the same kind about the same kind of axis Class Theory Find the class structure for the groups in problem Symmetry Operations What are the SO in 1, see Figure 13? Symmetry Operations What are the SO in 2?
19 Figure 13: Figure for problems Symmetry Operations What are the SO in 3? Symmetry Operations What are the SO in 4? Symmetry Operations What are the SO in 5? Symmetry Operations In molecules 3, 4, and 5, we usually allow for rapid rotation about bonds (such as the C-O in 3 and 4, and the B-O in 5). What are the SO present if we do so? HINT: This rotation has the effect of replacing the stereochemically positioned hydrogen atoms with a smeared, or averaged, position so that the whole OH group becomes symmetrical One Dimensional Representations Use your data from the group of object M. Show that each of the following one dimensional matrices (called X and Y for now), associated with the corresponding SO indicated, faithfully reproduce the group multiplication properties. E C 3z C 3z C 2(1) C 2(2) C 2(3) σ h S 3 S 3 σ v(1) σ v(2) σ v(3) X Y
20 Two Dimensional Representations Use the following data of two dimensional matrices (called Z and Q for now) associated with SO of the sub-group of object M and show that matrix multiplication faithfully reproduces the group multiplication table. Z Q E C 3z C 3z C 2(1) C 2(2) C 2(3) Block Diagonal Representations Notice that the matrices in the last problem are all block diagonal, that is, of the form a b c 0 0 d e f They were formed by combining X with itself (in Z) and separately with Y (in Q). Show that when multiplying the matrices of Q together that there is never a situation where the number in the 2,2 position influences that in the 1,1 position. The rule: Block diagonal matrices can be reduced to a set of smaller matrices. Block diagonalization will be critical to our progress Multiplying Matrices Here are two three by three matrices a 0 0 A = 0 b c Find the product AQ. 0 d e Q = Matrices in Block Diagonal Form Examine your answer to the last problem. Show that it has the same block diagonal form as each of the two starting matrices Building Representations Imagine object M, let the z axis be perpendicular to the triangle and through its center. Put a p z orbital at the center of the triangle. Now ask what happens to the orbital when each symmetry operation of the subgroup of M (given in problem 153) is applied to that orbital. Find a number that does the same thing to the p orbital. If you associate that number with the corresponding symmetry operation you have formed a representation. The rule: Functions generate representations.
21 Figure 14: Figure for problems Building Representations Where (in general terms) did the matrices in problem 153 come from? Symmetry Operations Find the symmetry operations on object N in Figure 14 assuming that the object is not turned so that the back side of the rectangle shows (you could, for instance, assume the backside was really blue) Building Representations Use the function x (or the vector x shown in Figure 14) and your symmetry operations from the last problem to generate a representation Building Representations Use the function y (or the vector y shown in Figure 14) and your symmetry operations from problem 159 to generate a representation Symmetry Operations Find the symmetry operations on object N in Figure 14 assuming that the object is identical on the top and bottom Building a Representation Use object N from the last problem and consider the two vectors shown in Figure 14. Also let the vector z be perpendicular to the plane of the rectangle and go through the center. Build the matrix that represents the operation C 2z generated by these three vectors, all at once. HINTS: (1) What the operation does is convert q into r is the language to use; for instance, in the first entry below the operation converts x to -x. (2) It will be a three dimensional representation generated as follows. Build a row vector of the three vectors, and then determine what happens to them under a symmetry operation, say C 2z ( ) ( ) C 2z x y z = x y z Now you want to find the matrix that does the same: ( ) a b c ( ) x y z d e f = x y z g h i
22 Figure 15: Figure for problems 165 and 170 Figure 16: Figure for problems 167 and Building a Representation Finish the representation begun in the last problem for all the rest of the SO of object N Building a Representation Find the proper SO of the object O. Build the representation generated by the three vectors x, y, and z. HINT: In contrast to the previous problems, this one will have off-diagonal terms in the matrix Building a Representation from Ligand Orbitals Use object M. Take a sphere on each corner of the triangle. Find the representation generated by those three spheres. Notice how we slowly, and sneakily, move toward real chemistry: a sphere is beginning to look like a hydrogen atom 1s orbital! HINT: You might label them a to c just so you can tell things apart. Also, remember they are spheres, their top is the same as their bottom Building a Representation Use object P. I have tried to draw a p orbital at each corner with the orange color representing the positive angular phase and the blue the negative. Find the representation generated by these four functions. HINTS: For later reference, it would be useful to name the functions. I did this by calling the upper left function a and proceeded clockwise using b through d. There are eight operations in the full group of this object.
23 Remark: Problems 168 to 177 deal with the reduction of a matrix representation. This involves manipulations that change an arbitrary representation into block diagonal form. These problems involve some amount of (not difficult) matrix manipulation (matrix algebra) and are not absolutely needed for using group theory once we develop character theory. If you decide to skip these problems, you should carry away at least a rough understanding of the following: To reduce a representation into block diagonal form requires that the basis set of the representation be changed Reduction of a Representation Here is a very simple example. It involves the symmetry group C 2v, a group we will see often. The operations in this group are E, C 2z, σ xz, and σ yz. Here is what happens to two functions under these operations: ( ) ( ) E a b = a b ( ) ( ) C 2z a b = b a ( ) ( ) σ xz a b = b a ( ) ( ) σ yz a b = a b Build the matrices that correspond to each of these operations. HINT: Your should put the numbers e, r, t, and y into the matrix associated with C 2z such that the following equation is valid: ( ) a b e r ( ) = b a t y Reduction of a Representation Now take the linear combinations of the functions a and b of the last problem: f = a + b g = a b Since you know what happens to each of a and b under the C 2v operations, you can figure out what happens to f and g. Do so and build the appropriate matrices. Notice that they are in block diagonal form. Conclusion: The change in basis set from a and b to f and g has changed the representation into block diagonal form; or, as the professional would say, has reduced the representation Reduction of a Representation Here is another example, although to keep the tediium to a minimum, we only consider two of the symmetry operations. Consider object O. Pretend the orange circles are H 1s orbitals and label them from the upper right starting with a clockwise to d. Construct the matrix that does what the C 4 (clockwise rotation by π/2) does to those four functions. Also construct the matrix that does what the C 2,x=y rotation does to the four functions.
24 Reduction of a Representation Now take the linear combinations of the four hydrogen atom functions from the last problem: f = a + b + c + d g = a b + c d h = a c i = b d Since you know what happens to each of a through d under the two operations of the last problem, you can figure out what happens to f through i. Do so. Notice that they are in block diagonal form with f by itself, g by itself, and h and i mixed. Conclusion: The change in basis set from a, b, c and d to f, g, h and i has reduced the representation to block diagonal form Abstract Reduction of a Representation Now we work through the formal process of reduction. through d. Arrange them as a row vector, ( ) v = a b c d Consider a set of functions, a We now let some symmetry operation, O, operate on v, Ov. We let the matrix that represents this operation, the kind of matrix you have been building in recent problems, be represented by the symbol Γ v O in the basis of v (which therefore generates the superscript); thus Ov = v Γ v O. Now we construct a matrix T that takes the v into the new functions, the linear combinations that will block diagonalize the representation; in symbols, v T = x, where x is a row vector composed of the new functions, f through i : ( ) x = f g h i We have by analogy with the above, Ox = x Γ x O. Put into this last expression the equivalence of x given in terms of T and v Abstract Reduction of a Representation Your expression from the last problem reads: O v T = v T Γ x O We can now multiply both sides of this expression by T 1, the inverse of T. What do you get? Abstract Reduction of a Representation Your result from the last problem is O v = v T Γ x O T 1 However, we already know that O v = v Γ v O. You can equate the two expression you have for O v. What result do you get?
25 Abstract Reduction of a Representation From the last problem you have, after removing the common v from both sides: Γ x O = T 1 Γ v O T Write the corresponding expression for Γ x O in terms of Γv O Reduction of a Representation The answers that you should have gotten for the proper symmetry operations in problem 166 are given below in the order E, C 3, C 3, C 2a, C 2b, and C 2c (where your order may be somewhat different if you numbered your functions in a different way. These functions label the hydrogen atoms as a at the top, b on the lower right, and c on the lower left and the matrices listed have them in the order a through c. Figure 17: Matrices for proper rotations of ligand s orbitals in a triangle. The matrices above are the Γ v O for all v in the proper symmetry operations of a triangle. I claim the T matrix is Find the matrices in Γ x O. HINT: If you are doing this by hand multiplication of the matrices, just do a couple, say C 3 and C 2b ; if you are using Mathematica, it is a breeze to do them all. n Reduction of a Representation In this problem we work through the reduction of a representation. If you don t like doing this, don t worry. We will generate an easier way to do it. However, the principle behind the method is important. (I would use Mathematica to do this if I were you, but multiplication by hand is easy enough if you don t know the program.) Consider the representation generated in problem 167. I claim that the following linear combinations of functions should be considered as ψ 1 through ψ 4 : 1 2 ψ 1 = a + b + c + d ψ 2 = a + b c d ψ 3 = a b + c d ψ 4 = a b c + d Construct the T matrix and reduce the representation that you got in problem 167
26 Reduced Representations There are some representations that are irreducible. Clearly this is true of the one dimensional ones. It is also true of certain higher order representations. Those representations that are not reducible are called irreducible representations. How many irreducible representations did you get in problem 177? Irreducible Representations The number of irreducible representations in any group of symmetry operations is equal to the number of classes in that set of symmetry operations. How many irreducible representations are in the group of problem 143? Matrix Property The trace of a matrix is the sum of the upper-left to lower-right diagonal elements, that is, i a ii. What is the trace of the matrices given below? / Characters of a Representation When matrices are members of a representation, their traces are called their characters. What are the characters for the matrices in Figure 17? Characters of a Representation Use your answer from the last problem to show that the characters for members of the same class are the same Generating Characters of a Representation Functions generate representations and characters come from the representation. Can we get directly from functions to characters? Of course! The rule is to compute the number of functions that go into themselves under the given symmetry operation. That is the character of the representation. In most cases this is very easy; only when functions get mixed partially into each other (such as the functions x and y in an object with triangular symmetry) does this given any difficulty. Use this method to find the character of the representation generated by four hydrogen s functions located at the corners of object N (see Figure 14) Generating Characters of a Representation Find the characters for the representation generated by hydrogen 1s functions at the corners of a square, Figure 15. HINT: Take advantage of our rule about characters being the same for elements in the same class. HINT: This object has 16 symmetry operations in 10 classes Irreducible Representations How many irreducible representations are in the group of the last problem?
27 Generating Characters of a Representation Find the characters for the representation generated by a carbon atom (with a 2s wave function and three 2p functions) located at the center of a square, Figure 15. HINT: Take advantage of our rule about characters being the same for elements in the same class. HINT: Can a 2s function ever get changed into a 2p function? Generating Characters of a Representation Find the characters generated by a set of three p orbitals, each on a corner of a triangle, and each pointing with its positive lobe pointing at the center of the triangle Generating Characters of a Representation and the Beauty of Symmetry Find the characters generated by a set of three p orbitals, each on a corner of a triangle, two of which have their positive lobe pointing at the center of the triangle, whereas the third has its negative lobe doing so. NOTE: Same answer as last problem. Symmetry doesn t care Generating Characters of a Representation Find the characters generated by a set of three p orbitals, each on a corner of a triangle, and each perpendicular to the line from the center of the triangle to the corner, and in the plane of the triangle Generating Characters of a Representation Find the characters generated by a set of three p orbitals, each on a corner of a triangle, and each perpendicular to the line from the center of the triangle to the corner, and perpendicular to the plane of the triangle Characters for Irreducible Representations The characters of irreducible representations are given in what are called character tables. Here is the character table for the group of symmetry operations of object M, a group called C 3v : C 3v E 2C 3 3σ v A z, z 2 A R z E (x,y) (R x,r y ) (x 2 - y 2, xy) (xz, yz) What are the dimensions of the three irreducible representations? Review: To Reduce a Representation What do you do to reduce a representation?
28 Using Character Theory to Reduce a Representation It is easy to reduce a representation using character theory. You don t even have to know what the new basis set is. All you have to do is to use a formula that is relatively easily (but not here) derived. To find the number of times that an irreducible representation is in a reducible one, n a, you use the three-handed rule : n a = 1 h g c χ(γ c red ) χ(γc a) (2) c where the sum is over all classes, h is the number of symmetry operations in the group, g c is the number of symmetry operations in the class, χ(γ c red ) is the characer of the reducible representation for the class, and χ(γ c a) is the characer of the irreducible representation a for the class. For the C 3v group, use the equation to find the number of time that the A 1 irreducible representation is in a reducible representation with characters of (E) 5, (C 3 ) 2, (σ v ) Using Character Theory to Reduce a Representation For the C 3v group, use equation 2 to find the number of time that the A 2 irreducible representation is in a reducible representation with characters of (E) 5, (C 3 ) 2, (σ v ) Using Character Theory to Reduce a Representation For the D 4d group, use equation 2 to find the number of time that the A 1 irreducible representation is in a reducible representation with characters of (E) 4, (2S 8 ) 0, (2C 4 ) 0, (2S 3 8 ) 0, (C 2) 4, (4C 2 ) 0, (4σ d ) 2. D 4d E 2S 8 2C 4 2S 3 8 C 2 4C 2 4σ d A z 2 A R z B B z E (x,y) E (xy, x 2 -y 2 ) E (Rx, R y ) (xz, yz) Using Character Theory to Reduce a Representation For the D 4d group, use equation 2 to find the number of time that the B 1 irreducible representation is in a reducible representation with characters of (E) 4, (2S 8 ) 0, (2C 4 ) 0, (2S 3 8 ) 0, (C 2) 4, (4C 2 ) 0, (4σ d ) Using Character Theory to Reduce a Representation For the D 4d group, use equation 2 to find the number of time that the B 2 irreducible representation is in a reducible representation with characters of (E) 4, (2S 8 ) 0, (2C 4 ) 0, (2S 3 8 ) 0, (C 2) 4, (4C 2 ) 0, (4σ d ) 2.
29 Using Character Theory to Reduce a Representation For the D 4d group, use equation 2 to find the number of time that the E 2 irreducible representation is in a reducible representation with characters of (E) 4, (2S 8 ) 0, (2C 4 ) 0, (2S 3 8 ) 0, (C 2) 4, (4C 2 ) 0, (4σ d ) Using the Three Hand Rule Here is an interesting application of equation 2. Think logically and answer this question: How many times is the A 1 irreducible representation (in C 3v symmetry, or any other symmetry) in the A 2 irreducible representation? Using the Three Hand Rule The implication of the last problem is that is we apply equation 2 to two different irreducible representations, we must come up with zero as an answer. Try it for the C 3v group Using the Three Hand Rule Here is another interesting application of equation 2. Think logically and answer this question: How many times is the A 1 irreducible representation (in C 3v symmetry, or any other symmetry) in the A 1 irreducible representation? Using the Three Hand Rule The implication of the last problem is that is we apply equation 2 to the same different irreducible representation, we must come up with one as an answer. Try it for the C 3v group. REMARK (if you are initerested in such things): You can think of the list of characters of irreducible representations as components of orthogonal vectors in a multidimensional space Reduction of a Representation For the D 4d group, determine the result of applying equation 2 to the set of characters: (E) 4, (2S 8 ) 0, (2C 4 ) 4, (2S 3 8 ) 0, (C 2) 4, (4C 2 0, (4σ d ) Reduction of a Representation What is the meaning of the result of the last problem? Reduction of a Representation Here are the characters for a reducible representation in C 6v symmetry. Find the irreducible representations in this reducible one: HINT: The order of elements is the same as in the character table. C 6v E 2C 6 2C 3 C 2 3σ v 3σ d A z, z 2 A R z B B E (x, y) (R x, R y ) (xz, yz) E (x 2 -y 2, xy)
30 Generation and Reduction of a Representation Imagine benzene and assume that it has C 6v symmetry (which is actually just a subgroup of the D 6h symmetry that benzene has). Find and reduce the representation generated by the six 2p z orbitals on the carbon atoms; these orbitals are perpendicular to the plane of the benzene ring. HINT: If in the reduction of a representation you do not get an integer for the number of times that the a th irreducible representation is in the reducible one, you made a mistake in the characters of the reducible representation Group Identification Imagine C 5 H 5 in the form of a regular pentagon. To what point group does this molecular belong? Generation of a Representation Find the characters of the representation generated by the C 2p z orbitals, those perpendicular to the plane of the molecule Reduction of a Representation Reduce the representation found in the last problem. D 5h E 2C 5 2C 2 5 5C 2 σ h 2S 5 2S 3 5 5σ v A z 2 A R z E 1 2 2cos(2π/5) 2cos(4π/ cos(2π/5) 2cos(4π/5 0 (x, y) E 2 2 2cos(4π/5) 2cos(2π/ cos(4π/5) 2cos(2π/5 0 (x 2 -y 2, xy) A A z E 1 2 2cos(2π/5) 2cos(4π/ cos(2π/5) -2cos(4π/5 0 (R x, R y ) (xz, yz) E 2 2 2cos(4π/5) 2cos(2π/ cos(4π/5) -2cos(2π/ Finding and Reducing a Representation For NH 3, find the representation generated by the 1s orbitals on the H atoms. Reduce it Finding and Reducing a Representation Find the representation generated by the 2s function on the N in NH Finding and Reducing a Representation Find and reduce the representation generated by the three 2p functions on the N of NH The Right Hand Side of a Character Table Does your answer to the last problem suggest anything to you about the right hand side of a character table?
Chm 331 Fall 2015, Exercise Set 4 NMR Review Problems
 Chm 331 Fall 015, Exercise Set 4 NMR Review Problems Mr. Linck Version.0. Compiled December 1, 015 at 11:04:44 4.1 Diagonal Matrix Elements for the nmr H 0 Find the diagonal matrix elements for H 0 (the
Chm 331 Fall 015, Exercise Set 4 NMR Review Problems Mr. Linck Version.0. Compiled December 1, 015 at 11:04:44 4.1 Diagonal Matrix Elements for the nmr H 0 Find the diagonal matrix elements for H 0 (the
Chapter 10: Modern Atomic Theory and the Periodic Table. How does atomic structure relate to the periodic table? 10.1 Electromagnetic Radiation
 Chapter 10: Modern Atomic Theory and the Periodic Table How does atomic structure relate to the periodic table? 10.1 Electromagnetic Radiation Electromagnetic (EM) radiation is a form of energy that exhibits
Chapter 10: Modern Atomic Theory and the Periodic Table How does atomic structure relate to the periodic table? 10.1 Electromagnetic Radiation Electromagnetic (EM) radiation is a form of energy that exhibits
PAPER No. 7: Inorganic Chemistry - II (Metal-Ligand Bonding, Electronic Spectra and Magnetic Properties of Transition Metal Complexes
 Subject Chemistry Paper No and Title Module No and Title Module Tag 7, Inorganic chemistry II (Metal-Ligand Bonding, Electronic Spectra and Magnetic Properties of Transition Metal Complexes) 10, Electronic
Subject Chemistry Paper No and Title Module No and Title Module Tag 7, Inorganic chemistry II (Metal-Ligand Bonding, Electronic Spectra and Magnetic Properties of Transition Metal Complexes) 10, Electronic
Atoms with More than One Electron
 Fun with the Periodic Table Activity 6 Atoms with More than One Electron GOALS In this activity you will: View the spectra of various materials. Graphically analyze patterns in the amounts of energy required
Fun with the Periodic Table Activity 6 Atoms with More than One Electron GOALS In this activity you will: View the spectra of various materials. Graphically analyze patterns in the amounts of energy required
Chm 363. Spring 2017, Exercise Set 2 Main Group Molecular Orbital Diagrams Distortion of Structures Vibrational Analysis. Mr.
 Chm 363 Spring 2017, Exercise Set 2 Main Group Molecular Orbital Diagrams Distortion of Structures Vibrational Analysis Mr. Linck Version 2.0 January 31, 2017 2.1 Building an MO Diagram The first step
Chm 363 Spring 2017, Exercise Set 2 Main Group Molecular Orbital Diagrams Distortion of Structures Vibrational Analysis Mr. Linck Version 2.0 January 31, 2017 2.1 Building an MO Diagram The first step
Because light behaves like a wave, we can describe it in one of two ways by its wavelength or by its frequency.
 Light We can use different terms to describe light: Color Wavelength Frequency Light is composed of electromagnetic waves that travel through some medium. The properties of the medium determine how light
Light We can use different terms to describe light: Color Wavelength Frequency Light is composed of electromagnetic waves that travel through some medium. The properties of the medium determine how light
Chem 673, Problem Set 5 Due Thursday, November 29, 2007
 Chem 673, Problem Set 5 Due Thursday, November 29, 2007 (1) Trigonal prismatic coordination is fairly common in solid-state inorganic chemistry. In most cases the geometry of the trigonal prism is such
Chem 673, Problem Set 5 Due Thursday, November 29, 2007 (1) Trigonal prismatic coordination is fairly common in solid-state inorganic chemistry. In most cases the geometry of the trigonal prism is such
Chm 363. Spring 2017, Exercise Set 3 Transition Metal Bonding and Spectra. Mr. Linck. Version 1.5 March 9, 2017
 Chm 363 Spring 2017, Exercise Set 3 Transition Metal Bonding and Spectra Mr. Linck Version 1.5 March 9, 2017 3.1 Transition Metal Bonding in Octahedral Compounds How do the metal 3d, 4s, and 4p orbitals
Chm 363 Spring 2017, Exercise Set 3 Transition Metal Bonding and Spectra Mr. Linck Version 1.5 March 9, 2017 3.1 Transition Metal Bonding in Octahedral Compounds How do the metal 3d, 4s, and 4p orbitals
Light. Light (con t.) 2/28/11. Examples
 Light We can use different terms to describe light: Color Wavelength Frequency Light is composed of electromagnetic waves that travel through some medium. The properties of the medium determine how light
Light We can use different terms to describe light: Color Wavelength Frequency Light is composed of electromagnetic waves that travel through some medium. The properties of the medium determine how light
Modern Atomic Theory. (a.k.a. the electron chapter!) Chemistry 1: Chapters 5, 6, and 7 Chemistry 1 Honors: Chapter 11
 Modern Atomic Theory (a.k.a. the electron chapter!) 1 Chemistry 1: Chapters 5, 6, and 7 Chemistry 1 Honors: Chapter 11 ELECTROMAGNETIC RADIATION 2 Electromagnetic radiation. 3 4 Electromagnetic Radiation
Modern Atomic Theory (a.k.a. the electron chapter!) 1 Chemistry 1: Chapters 5, 6, and 7 Chemistry 1 Honors: Chapter 11 ELECTROMAGNETIC RADIATION 2 Electromagnetic radiation. 3 4 Electromagnetic Radiation
Group Members: Your Name In Class Exercise #6. Photon A. Energy B
 Group Members: Your Name In Class Exercise #6 Shell Structure of Atoms Part II Photoelectron Spectroscopy Photoelectron spectroscopy is closely related to the photoelectric effect. When high energy photons
Group Members: Your Name In Class Exercise #6 Shell Structure of Atoms Part II Photoelectron Spectroscopy Photoelectron spectroscopy is closely related to the photoelectric effect. When high energy photons
8. Which of the following could be an isotope of chlorine? (A) 37 Cl 17 (B) 17 Cl 17 (C) 37 Cl 17 (D) 17 Cl 37.5 (E) 17 Cl 37
 Electronic Structure Worksheet 1 Given the following list of atomic and ionic species, find the appropriate match for questions 1-4. (A) Fe 2+ (B) Cl (C) K + (D) Cs (E) Hg + 1. Has the electron configuration:
Electronic Structure Worksheet 1 Given the following list of atomic and ionic species, find the appropriate match for questions 1-4. (A) Fe 2+ (B) Cl (C) K + (D) Cs (E) Hg + 1. Has the electron configuration:
A DOT STRUCTURE FOR A LARGER MOLECULE ETHANOL! Count valence electrons
 212 A DOT STRUCTURE FOR A LARGER MOLECULE Count valence electrons Pick central atom and draw skeletal structure - central atom is usually the one that needs to gain the most electrons! - skeletal structure
212 A DOT STRUCTURE FOR A LARGER MOLECULE Count valence electrons Pick central atom and draw skeletal structure - central atom is usually the one that needs to gain the most electrons! - skeletal structure
The Electronic Theory of Chemistry
 JF Chemistry CH1101 The Electronic Theory of Chemistry Dr. Baker bakerrj@tcd.ie Module Aims: To provide an introduction to the fundamental concepts of theoretical and practical chemistry, including concepts
JF Chemistry CH1101 The Electronic Theory of Chemistry Dr. Baker bakerrj@tcd.ie Module Aims: To provide an introduction to the fundamental concepts of theoretical and practical chemistry, including concepts
1 Electrons and Chemical Bonding
 CHAPTER 13 1 Electrons and Chemical Bonding SECTION Chemical Bonding BEFORE YOU READ After you read this section, you should be able to answer these questions: What is chemical bonding? What are valence
CHAPTER 13 1 Electrons and Chemical Bonding SECTION Chemical Bonding BEFORE YOU READ After you read this section, you should be able to answer these questions: What is chemical bonding? What are valence
LECTURE 4: HOW TO GENERATE ELECTRONIC CONFIGURATIONS FOR ATOMS AND IONS
 LECTURE 4: HOW TO GENERATE ELECTRONIC CONFIGURATIONS FOR ATOMS AND IONS We are about to do something incredibly useful create the electronic configurations for every kind of neutral or charged atom we
LECTURE 4: HOW TO GENERATE ELECTRONIC CONFIGURATIONS FOR ATOMS AND IONS We are about to do something incredibly useful create the electronic configurations for every kind of neutral or charged atom we
The Electronic Structure of Atoms
 The Electronic Structure of Atoms Classical Hydrogen-like atoms: Atomic Scale: 10-10 m or 1 Å + - Proton mass : Electron mass 1836 : 1 Problems with classical interpretation: - Should not be stable (electron
The Electronic Structure of Atoms Classical Hydrogen-like atoms: Atomic Scale: 10-10 m or 1 Å + - Proton mass : Electron mass 1836 : 1 Problems with classical interpretation: - Should not be stable (electron
C H E M 1 CHEM 101-GENERAL CHEMISTRY CHAPTER 6 THE PERIODIC TABLE & ATOMIC STRUCTURE INSTR : FİLİZ ALSHANABLEH
 C H E M 1 CHEM 101-GENERAL CHEMISTRY CHAPTER 6 THE PERIODIC TABLE & ATOMIC STRUCTURE 0 1 INSTR : FİLİZ ALSHANABLEH CHAPTER 6 THE PERIODIC TABLE & ATOMIC STRUCTURE The Electromagnetic Spectrum The Wave
C H E M 1 CHEM 101-GENERAL CHEMISTRY CHAPTER 6 THE PERIODIC TABLE & ATOMIC STRUCTURE 0 1 INSTR : FİLİZ ALSHANABLEH CHAPTER 6 THE PERIODIC TABLE & ATOMIC STRUCTURE The Electromagnetic Spectrum The Wave
Unit 7. Atomic Structure
 Unit 7. Atomic Structure Upon successful completion of this unit, the students should be able to: 7.1 List the eight regions of the electromagnetic spectrum in the designated order and perform calculations
Unit 7. Atomic Structure Upon successful completion of this unit, the students should be able to: 7.1 List the eight regions of the electromagnetic spectrum in the designated order and perform calculations
3.1 Hydrogen Spectrum
 3.1 Hydrogen Spectrum Light is electromagnetic radiation that can be produced at different energy levels. High energy light has a short wavelength (λ) and a high frequency (ƒ, ν) (gamma rays, x-rays, ultraviolet).
3.1 Hydrogen Spectrum Light is electromagnetic radiation that can be produced at different energy levels. High energy light has a short wavelength (λ) and a high frequency (ƒ, ν) (gamma rays, x-rays, ultraviolet).
Atomic Structure, Periodic Table, and Other Effects: Chapter 8 of Rex and T. Modern Physics
 Atomic Structure, Periodic Table, and Other Effects: Chapter 8 of Rex and T Modern Physics 11/16 and 11/19/2018 1 Introduction In Chapter 7, we studied the hydrogen atom. What about other elements, e.g.,
Atomic Structure, Periodic Table, and Other Effects: Chapter 8 of Rex and T Modern Physics 11/16 and 11/19/2018 1 Introduction In Chapter 7, we studied the hydrogen atom. What about other elements, e.g.,
2008 Brooks/Cole 2. Frequency (Hz)
 Electromagnetic Radiation and Matter Oscillating electric and magnetic fields. Magnetic field Electric field Chapter 7: Electron Configurations and the Periodic Table Traveling wave moves through space
Electromagnetic Radiation and Matter Oscillating electric and magnetic fields. Magnetic field Electric field Chapter 7: Electron Configurations and the Periodic Table Traveling wave moves through space
General Chemistry 1 CHM201 Unit 4 Practice Test. 4. What is the orbital designation for an electron with the quantum numbers n 4, 3?
 General Chemistry 1 CHM201 Unit 4 Practice Test 1. An orbital s orientation in space is determined by a. the quantum number. d. the n quantum number. b. the m l quantum number. e. both the and m l quantum
General Chemistry 1 CHM201 Unit 4 Practice Test 1. An orbital s orientation in space is determined by a. the quantum number. d. the n quantum number. b. the m l quantum number. e. both the and m l quantum
Chapter 8. Periodic Properties of the Element
 Chapter 8 Periodic Properties of the Element Mendeleev (1834 1907) Ordered elements by atomic mass Saw a repeating pattern of properties Periodic law when the elements are arranged in order of increasing
Chapter 8 Periodic Properties of the Element Mendeleev (1834 1907) Ordered elements by atomic mass Saw a repeating pattern of properties Periodic law when the elements are arranged in order of increasing
5 Symmetries and point group in a nut shell
 30 Phys520.nb 5 Symmetries and point group in a nut shell 5.1. Basic ideas: 5.1.1. Symmetry operations Symmetry: A system remains invariant under certain operation. These operations are called symmetry
30 Phys520.nb 5 Symmetries and point group in a nut shell 5.1. Basic ideas: 5.1.1. Symmetry operations Symmetry: A system remains invariant under certain operation. These operations are called symmetry
- Light has properties of WAVES such as DIFFRACTION (it bends around small obstructions).
 170 LIGHT wavelength Diffraction frequency = wavelengths / time = - Light has properties of WAVES such as DIFFRACTION (it bends around small obstructions). - Einstein noted that viewing light as a particle
170 LIGHT wavelength Diffraction frequency = wavelengths / time = - Light has properties of WAVES such as DIFFRACTION (it bends around small obstructions). - Einstein noted that viewing light as a particle
Ch 8 Electron Configurations and Periodicity (Periodic table)
 Ch 8 Electron Configurations and Periodicity (Periodic table) - An e 1 configuration is an atom s particular distribution of e 1 among the available subshells and orbitals. For example, the ground state
Ch 8 Electron Configurations and Periodicity (Periodic table) - An e 1 configuration is an atom s particular distribution of e 1 among the available subshells and orbitals. For example, the ground state
Multielectron Atoms.
 Multielectron Atoms. Chem 639. Spectroscopy. Spring 00 S.Smirnov Atomic Units Mass m e 1 9.109 10 31 kg Charge e 1.60 10 19 C Angular momentum 1 1.055 10 34 J s Permittivity 4 0 1 1.113 10 10 C J 1 m 1
Multielectron Atoms. Chem 639. Spectroscopy. Spring 00 S.Smirnov Atomic Units Mass m e 1 9.109 10 31 kg Charge e 1.60 10 19 C Angular momentum 1 1.055 10 34 J s Permittivity 4 0 1 1.113 10 10 C J 1 m 1
Chem 673, Problem Set 5 Due Tuesday, December 2, 2008
 Chem 673, Problem Set 5 Due Tuesday, December 2, 2008 (1) (a) Trigonal bipyramidal (tbp) coordination is fairly common. Calculate the group overlaps of the appropriate SALCs for a tbp with the 5 d-orbitals
Chem 673, Problem Set 5 Due Tuesday, December 2, 2008 (1) (a) Trigonal bipyramidal (tbp) coordination is fairly common. Calculate the group overlaps of the appropriate SALCs for a tbp with the 5 d-orbitals
Quantum Theory & Electronic Structure of Atoms. It s Unreal!! Check your intuition at the door.
 Quantum Theory & Electronic Structure of Atoms It s Unreal!! Check your intuition at the door. 1 Quantum Theory of the Atom Description of the atom and subatomic particles. We will focus on the electronic
Quantum Theory & Electronic Structure of Atoms It s Unreal!! Check your intuition at the door. 1 Quantum Theory of the Atom Description of the atom and subatomic particles. We will focus on the electronic
CHEM 130 Exp. 8: Molecular Models
 CHEM 130 Exp. 8: Molecular Models In this lab, we will learn and practice predicting molecular structures from molecular formulas. The Periodic Table of the Elements IA 1 H IIA IIIA IVA VA VIA VIIA 3 5
CHEM 130 Exp. 8: Molecular Models In this lab, we will learn and practice predicting molecular structures from molecular formulas. The Periodic Table of the Elements IA 1 H IIA IIIA IVA VA VIA VIIA 3 5
- Why are phase labels required? Because phase changes either absorb or release energy. ... what does this mean?
 157 SINCE the enthalpy change does NOT depend on path, this means that we can use standard values for enthalpy to predict the heat change in reactions that we have not tested in a calorimeter. THERMOCHEMICAL
157 SINCE the enthalpy change does NOT depend on path, this means that we can use standard values for enthalpy to predict the heat change in reactions that we have not tested in a calorimeter. THERMOCHEMICAL
Test Bank for General Chemistry Atoms First 2nd Edition by John E. McMurry and Robert C. Fay
 Test Bank for General Chemistry Atoms First 2nd Edition by John E. McMurry and Robert C. Fay Link download full: https://digitalcontentmarket.org/download/test-bank-for-general-chemistry-atoms-f irst-2nd-edition-by-mcmurry-and-fay/
Test Bank for General Chemistry Atoms First 2nd Edition by John E. McMurry and Robert C. Fay Link download full: https://digitalcontentmarket.org/download/test-bank-for-general-chemistry-atoms-f irst-2nd-edition-by-mcmurry-and-fay/
Professor K. Section 8 Electron Configuration Periodic Table
 Professor K Section 8 Electron Configuration Periodic Table Schrödinger Cannot be solved for multielectron atoms We must assume the orbitals are all hydrogen-like Differences In the H atom, all subshells
Professor K Section 8 Electron Configuration Periodic Table Schrödinger Cannot be solved for multielectron atoms We must assume the orbitals are all hydrogen-like Differences In the H atom, all subshells
-"l" also contributes ENERGY. Higher values for "l" mean the electron has higher energy.
 170 - Giving the four parameters will uniquely identify an electron around an atom. No two electrons in the same atom can share all four. These parameters are called QUANTUM NUMBERS. PRINCIPAL QUANTUM
170 - Giving the four parameters will uniquely identify an electron around an atom. No two electrons in the same atom can share all four. These parameters are called QUANTUM NUMBERS. PRINCIPAL QUANTUM
Notes: Electrons and Periodic Table (text Ch. 4 & 5)
 Name Per. Notes: Electrons and Periodic Table (text Ch. 4 & 5) NOTE: This set of class notes is not complete. We will be filling in information in class. If you are absent, it is your responsibility to
Name Per. Notes: Electrons and Periodic Table (text Ch. 4 & 5) NOTE: This set of class notes is not complete. We will be filling in information in class. If you are absent, it is your responsibility to
Periodicity and the Electronic Structure of Atoms 國防醫學院生化學科王明芳老師
 Periodicity and the Electronic Structure of Atoms 國防醫學院生化學科王明芳老師 2018-10-2 1 2 Light and the Electromagnetic Spectrum Electromagnetic energy ( light ) is characterized by wavelength, frequency, and amplitude.
Periodicity and the Electronic Structure of Atoms 國防醫學院生化學科王明芳老師 2018-10-2 1 2 Light and the Electromagnetic Spectrum Electromagnetic energy ( light ) is characterized by wavelength, frequency, and amplitude.
= proton (positive charge) = electron (negative charge) = neutron (no charge) A Z. ,, and are notations that represent isotopes of carbon.
 ChemQuest 8 Name: Date: Hour: Information: Structure of the Atom Note the following symbols: (they are not to scale) = proton (positive charge) = electron (negative charge) = neutron (no charge) The following
ChemQuest 8 Name: Date: Hour: Information: Structure of the Atom Note the following symbols: (they are not to scale) = proton (positive charge) = electron (negative charge) = neutron (no charge) The following
Lecture Presentation. Chapter 8. Periodic Properties of the Element. Sherril Soman Grand Valley State University Pearson Education, Inc.
 Lecture Presentation Chapter 8 Periodic Properties of the Element Sherril Soman Grand Valley State University Nerve Transmission Movement of ions across cell membranes is the basis for the transmission
Lecture Presentation Chapter 8 Periodic Properties of the Element Sherril Soman Grand Valley State University Nerve Transmission Movement of ions across cell membranes is the basis for the transmission
The Hydrogen Atom. Dr. Sabry El-Taher 1. e 4. U U r
 The Hydrogen Atom Atom is a 3D object, and the electron motion is three-dimensional. We ll start with the simplest case - The hydrogen atom. An electron and a proton (nucleus) are bound by the central-symmetric
The Hydrogen Atom Atom is a 3D object, and the electron motion is three-dimensional. We ll start with the simplest case - The hydrogen atom. An electron and a proton (nucleus) are bound by the central-symmetric
Quantum Theory and Electron Configurations
 Chapter 5 Chapter 5 Quantum Theory and Electron Configurations It s all about color In terms of atomic models, so far: Dalton (1803) = Tiny, solid particle Thomson (1897) = Plum Pudding model Electrons
Chapter 5 Chapter 5 Quantum Theory and Electron Configurations It s all about color In terms of atomic models, so far: Dalton (1803) = Tiny, solid particle Thomson (1897) = Plum Pudding model Electrons
1. Following Dalton s Atomic Theory, 2. In 1869 Russian chemist published a method. of organizing the elements. Mendeleev showed that
 20 CHEMISTRY 11 D. Organizing the Elements The Periodic Table 1. Following Dalton s Atomic Theory, By 1817, chemists had discovered 52 elements and by 1863 that number had risen to 62. 2. In 1869 Russian
20 CHEMISTRY 11 D. Organizing the Elements The Periodic Table 1. Following Dalton s Atomic Theory, By 1817, chemists had discovered 52 elements and by 1863 that number had risen to 62. 2. In 1869 Russian
Goals for Today. Clarify some Rydberg Concepts Absorption vs. emission
 Note: Due to recent changes the exam 2 material for these slides ends at Ionization Energy Exceptions. You can omit Lewis Structures through General Formal Charge Rules. CH301 Unit 2 QUANTUM NUMBERS AND
Note: Due to recent changes the exam 2 material for these slides ends at Ionization Energy Exceptions. You can omit Lewis Structures through General Formal Charge Rules. CH301 Unit 2 QUANTUM NUMBERS AND
Chapter 7. Characteristics of Atoms. 7.1 Electromagnetic Radiation. Chapter 7 1. The Quantum Mechanical Atom. Atoms: How do we study atoms?
 Chapter 7 The Quantum Mechanical Atom 1 Characteristics of Atoms Atoms: possess mass contain positive nuclei contain electrons occupy volume have various properties attract one another combine to form
Chapter 7 The Quantum Mechanical Atom 1 Characteristics of Atoms Atoms: possess mass contain positive nuclei contain electrons occupy volume have various properties attract one another combine to form
Structure and Bonding of Organic Molecules
 Chem 220 Notes Page 1 Structure and Bonding of Organic Molecules I. Types of Chemical Bonds A. Why do atoms forms bonds? Atoms want to have the same number of electrons as the nearest noble gas atom (noble
Chem 220 Notes Page 1 Structure and Bonding of Organic Molecules I. Types of Chemical Bonds A. Why do atoms forms bonds? Atoms want to have the same number of electrons as the nearest noble gas atom (noble
-"l" also contributes ENERGY. Higher values for "l" mean the electron has higher energy.
 175 - Giving the four parameters will uniquely identify an electron around an atom. No two electrons in the same atom can share all four. These parameters are called QUANTUM NUMBERS. PRINCIPAL QUANTUM
175 - Giving the four parameters will uniquely identify an electron around an atom. No two electrons in the same atom can share all four. These parameters are called QUANTUM NUMBERS. PRINCIPAL QUANTUM
s or Hz J atom J mol or -274 kj mol CHAPTER 4. Practice Exercises ΔE atom = ΔE mol =
 CHAPTER 4 Practice Exercises 4.1 10 1 2.1410 s or Hz 4.3 ΔE atom = ΔE mol = 4.5610 J atom 19 1 2.7410 J mol or -274 kj mol 5 1-1 4.5 excitation energy = 471 kj mol 1 + 275 kj mol 1 = 746 kj mol 1 Hg 4.7
CHAPTER 4 Practice Exercises 4.1 10 1 2.1410 s or Hz 4.3 ΔE atom = ΔE mol = 4.5610 J atom 19 1 2.7410 J mol or -274 kj mol 5 1-1 4.5 excitation energy = 471 kj mol 1 + 275 kj mol 1 = 746 kj mol 1 Hg 4.7
Chapter 8: Periodic Properties of the Elements
 C h e m i s t r y 1 A : C h a p t e r 8 P a g e 1 Chapter 8: Periodic Properties of the Elements Homework: Read Chapter 8. Work out sample/practice exercises Check for the MasteringChemistry.com assignment
C h e m i s t r y 1 A : C h a p t e r 8 P a g e 1 Chapter 8: Periodic Properties of the Elements Homework: Read Chapter 8. Work out sample/practice exercises Check for the MasteringChemistry.com assignment
1. As a macroscopic analogy, think of an idealized pool table on which there is no friction. Let s consider a few scenarios.
 1 Worksheet AM1: Coupling Interactions In complex atoms, the electrons interact with each other. Naturally, the interactions affect the energy. Also, due to these interactions, the individual electrons
1 Worksheet AM1: Coupling Interactions In complex atoms, the electrons interact with each other. Naturally, the interactions affect the energy. Also, due to these interactions, the individual electrons
! Chemical!Bond!! Lewis!Diagram!(HI!#13)! o Ionic!and!covalent!bond!(M!+!NM!or!NM!+!NM)!(Complete!transfer!of!e S!or!sharing!of!e S )!
 !! Unit*2.*Atomic*Theory*! Molar!mass!calculation!using!the!abundance!of!isotopes!of!an!element!!!! Electron!configuration!(both!full!notation!and!core!notation)!(HI!#12)! o Neutral!atom,!anion,!cation!(ensure!you!know!the!rules!associated!with!ions)!
!! Unit*2.*Atomic*Theory*! Molar!mass!calculation!using!the!abundance!of!isotopes!of!an!element!!!! Electron!configuration!(both!full!notation!and!core!notation)!(HI!#12)! o Neutral!atom,!anion,!cation!(ensure!you!know!the!rules!associated!with!ions)!
CHEM 1311A. E. Kent Barefield. Course web page.
 CHEM 1311A E. Kent Barefield Course web page http://web.chemistry.gatech.edu/~barefield/1311/chem1311a.html Two requests: cell phones to silent/off no lap tops in operation during class Bring your transmitter
CHEM 1311A E. Kent Barefield Course web page http://web.chemistry.gatech.edu/~barefield/1311/chem1311a.html Two requests: cell phones to silent/off no lap tops in operation during class Bring your transmitter
Accelerated Chemistry Study Guide The Periodic Table, Chapter 5
 Accelerated Chemistry Study Guide The Periodic Table, Chapter 5 Terms, definitions, and people Dobereiner Newlands Mendeleev Moseley Periodic table Periodic Law group family period Page 1 of 38 alkali
Accelerated Chemistry Study Guide The Periodic Table, Chapter 5 Terms, definitions, and people Dobereiner Newlands Mendeleev Moseley Periodic table Periodic Law group family period Page 1 of 38 alkali
Chapter 11 Modern Atomic Theory
 167 Chapter 11 Modern Atomic Theory Review Skills 11.1 The Mysterious Electron Standing Waves and Guitar Strings Electrons as Standing Waves Waveforms for Hydrogen Atoms Particle Interpretation of the
167 Chapter 11 Modern Atomic Theory Review Skills 11.1 The Mysterious Electron Standing Waves and Guitar Strings Electrons as Standing Waves Waveforms for Hydrogen Atoms Particle Interpretation of the
Midterm Exam I. CHEM 181: Introduction to Chemical Principles September 24, 2015 Key
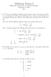 Midterm Exam I CHEM 8: Introduction to Chemical Principles September 24, 205 Key. A Li 2+ ion in an unknown, highly excited electronic state, first emits a photon at a wavelength of 4.36 µm, and following
Midterm Exam I CHEM 8: Introduction to Chemical Principles September 24, 205 Key. A Li 2+ ion in an unknown, highly excited electronic state, first emits a photon at a wavelength of 4.36 µm, and following
Be H. Delocalized Bonding. Localized Bonding. σ 2. σ 1. Two (sp-1s) Be-H σ bonds. The two σ bonding MO s in BeH 2. MO diagram for BeH 2
 The Delocalized Approach to Bonding: The localized models for bonding we have examined (Lewis and VBT) assume that all electrons are restricted to specific bonds between atoms or in lone pairs. In contrast,
The Delocalized Approach to Bonding: The localized models for bonding we have examined (Lewis and VBT) assume that all electrons are restricted to specific bonds between atoms or in lone pairs. In contrast,
Chem 6 Practice Exam 2
 These problems are from past Chem 6 exams. Each exam contained a page of universal constant values and common equations; yours will, too, along with a Periodic Table! The answers follow after all the questions.
These problems are from past Chem 6 exams. Each exam contained a page of universal constant values and common equations; yours will, too, along with a Periodic Table! The answers follow after all the questions.
Molecular Geometry and Polarity 1
 Experiment Molecular Geometry and Polarity 1 Objectives At the end of this activity you should be able to: o Write Lewis structures for molecules. o Classify bonds as nonpolar covalent, polar covalent,
Experiment Molecular Geometry and Polarity 1 Objectives At the end of this activity you should be able to: o Write Lewis structures for molecules. o Classify bonds as nonpolar covalent, polar covalent,
PART 2 Electronic Structure and the Periodic Table. Reference: Chapter 7 8 in textbook
 PART 2 Electronic Structure and the Periodic Table Reference: Chapter 7 8 in textbook 1 Experiment to Discover Atom Structure -particle: He 2+ mass number = 4 Nucleus and Electron Model 2 Atomic Structure
PART 2 Electronic Structure and the Periodic Table Reference: Chapter 7 8 in textbook 1 Experiment to Discover Atom Structure -particle: He 2+ mass number = 4 Nucleus and Electron Model 2 Atomic Structure
What is a Bond? Chapter 8. Ionic Bonding. Coulomb's Law. What about covalent compounds?
 Chapter 8 What is a Bond? A force that holds atoms together. Why? We will look at it in terms of energy. Bond energy- the energy required to break a bond. Why are compounds formed? Because it gives the
Chapter 8 What is a Bond? A force that holds atoms together. Why? We will look at it in terms of energy. Bond energy- the energy required to break a bond. Why are compounds formed? Because it gives the
C 2 '' σ v ' C 2 ' "side on" "in-plane" 2S determine how the MO transforms under each symmetry operation, Figure 3.
 Lecture Model nswers to Problems Self-study Problems / Exam Preparation determine the point group of o use VESPR (from st year) to determine that is planar, then use the flow chart. is the molecule linear?
Lecture Model nswers to Problems Self-study Problems / Exam Preparation determine the point group of o use VESPR (from st year) to determine that is planar, then use the flow chart. is the molecule linear?
Electromagnetic Radiation. Chapter 12: Phenomena. Chapter 12: Quantum Mechanics and Atomic Theory. Quantum Theory. Electromagnetic Radiation
 Chapter 12: Phenomena Phenomena: Different wavelengths of electromagnetic radiation were directed onto two different metal sample (see picture). Scientists then recorded if any particles were ejected and
Chapter 12: Phenomena Phenomena: Different wavelengths of electromagnetic radiation were directed onto two different metal sample (see picture). Scientists then recorded if any particles were ejected and
Chem 6, 10 Section Spring Exam 2 Solutions
 Exam 2 Solutions 1. (4 + 6 + 5 points) Dartmouth s FM radio station, WDCR, broadcasts by emitting from its antenna photons of frequency 99.3 MHz (99.3 10 6 Hz). (a) What is the energy of a single WDCR
Exam 2 Solutions 1. (4 + 6 + 5 points) Dartmouth s FM radio station, WDCR, broadcasts by emitting from its antenna photons of frequency 99.3 MHz (99.3 10 6 Hz). (a) What is the energy of a single WDCR
5.5. Representations. Phys520.nb Definition N is called the dimensions of the representations The trivial presentation
 Phys50.nb 37 The rhombohedral and hexagonal lattice systems are not fully compatible with point group symmetries. Knowing the point group doesn t uniquely determine the lattice systems. Sometimes we can
Phys50.nb 37 The rhombohedral and hexagonal lattice systems are not fully compatible with point group symmetries. Knowing the point group doesn t uniquely determine the lattice systems. Sometimes we can
Periodic Table Workbook
 Key Ideas: The placement or location of elements on the Periodic Table gives an indication of physical and chemical properties of that element. The elements on the Periodic Table are arranged in order
Key Ideas: The placement or location of elements on the Periodic Table gives an indication of physical and chemical properties of that element. The elements on the Periodic Table are arranged in order
SPARKS CH301. Why are there no blue fireworks? LIGHT, ELECTRONS & QUANTUM MODEL. UNIT 2 Day 2. LM15, 16 & 17 due W 8:45AM
 SPARKS CH301 Why are there no blue fireworks? LIGHT, ELECTRONS & QUANTUM MODEL UNIT 2 Day 2 LM15, 16 & 17 due W 8:45AM QUIZ: CLICKER QUESTION Which of these types of light has the highest energy photons?
SPARKS CH301 Why are there no blue fireworks? LIGHT, ELECTRONS & QUANTUM MODEL UNIT 2 Day 2 LM15, 16 & 17 due W 8:45AM QUIZ: CLICKER QUESTION Which of these types of light has the highest energy photons?
Nucleus. Electron Cloud
 Atomic Structure I. Picture of an Atom Nucleus Electron Cloud II. Subatomic particles Particle Symbol Charge Relative Mass (amu) protons p + +1 1.0073 neutrons n 0 1.0087 electrons e - -1 0.00054858 Compare
Atomic Structure I. Picture of an Atom Nucleus Electron Cloud II. Subatomic particles Particle Symbol Charge Relative Mass (amu) protons p + +1 1.0073 neutrons n 0 1.0087 electrons e - -1 0.00054858 Compare
Quantum Theory of Angular Momentum and Atomic Structure
 Quantum Theory of Angular Momentum and Atomic Structure VBS/MRC Angular Momentum 0 Motivation...the questions Whence the periodic table? Concepts in Materials Science I VBS/MRC Angular Momentum 1 Motivation...the
Quantum Theory of Angular Momentum and Atomic Structure VBS/MRC Angular Momentum 0 Motivation...the questions Whence the periodic table? Concepts in Materials Science I VBS/MRC Angular Momentum 1 Motivation...the
0. Introduction 1 0. INTRODUCTION
 0. Introduction 1 0. INTRODUCTION In a very rough sketch we explain what algebraic geometry is about and what it can be used for. We stress the many correlations with other fields of research, such as
0. Introduction 1 0. INTRODUCTION In a very rough sketch we explain what algebraic geometry is about and what it can be used for. We stress the many correlations with other fields of research, such as
Chem Symmetry and Introduction to Group Theory. Symmetry is all around us and is a fundamental property of nature.
 Chem 59-65 Symmetry and Introduction to Group Theory Symmetry is all around us and is a fundamental property of nature. Chem 59-65 Symmetry and Introduction to Group Theory The term symmetry is derived
Chem 59-65 Symmetry and Introduction to Group Theory Symmetry is all around us and is a fundamental property of nature. Chem 59-65 Symmetry and Introduction to Group Theory The term symmetry is derived
Unit 1, Lesson 01: Summary of Atomic Structure so far
 Unit 1, Lesson 01: Summary of Atomic Structure so far Atoms are made of sub-atomic particles: Protons: found in the nucleus, charge of 1+, mass of 1 amu (u) Neutrons: found in nucleus, no charge, mass
Unit 1, Lesson 01: Summary of Atomic Structure so far Atoms are made of sub-atomic particles: Protons: found in the nucleus, charge of 1+, mass of 1 amu (u) Neutrons: found in nucleus, no charge, mass
The early periodic table based on atomic weight. (Section 5.1) Lets review: What is a hydrogen atom? 1 electron * nucleus H 1 proton
 PERIODICITY AND ATOMIC STRUCTURE CHAPTER 5 How can we relate the structure of the atom to the way that it behaves chemically? The process of understanding began with a realization that many of the properties
PERIODICITY AND ATOMIC STRUCTURE CHAPTER 5 How can we relate the structure of the atom to the way that it behaves chemically? The process of understanding began with a realization that many of the properties
- Some properties of elements can be related to their positions on the periodic table.
 180 PERIODIC TRENDS - Some properties of elements can be related to their positions on the periodic table. ATOMIC RADIUS - The distance between the nucleus of the atoms and the outermost shell of the electron
180 PERIODIC TRENDS - Some properties of elements can be related to their positions on the periodic table. ATOMIC RADIUS - The distance between the nucleus of the atoms and the outermost shell of the electron
All chemical bonding is based on the following relationships of electrostatics: 2. Each period on the periodic table
 UNIT VIII ATOMS AND THE PERIODIC TABLE 25 E. Chemical Bonding 1. An ELECTROSTATIC FORCE is All chemical bonding is based on the following relationships of electrostatics: The greater the distance between
UNIT VIII ATOMS AND THE PERIODIC TABLE 25 E. Chemical Bonding 1. An ELECTROSTATIC FORCE is All chemical bonding is based on the following relationships of electrostatics: The greater the distance between
Chemical Bonding and Molecular Models
 25 Chemical Bonding and Molecular Models A chemical bond is a force that holds groups of two or more atoms together and makes them function as a unit. Bonding involves only the valence (outer shell) electrons
25 Chemical Bonding and Molecular Models A chemical bond is a force that holds groups of two or more atoms together and makes them function as a unit. Bonding involves only the valence (outer shell) electrons
Bohr Model of Atom: electrons move around nucleus in orbits similar to how planets orbit the sun energy levels for electrons are quantized
 Chemistry I: Quantum Mechanics Notes Bohr Model of Atom: electrons move around nucleus in orbits similar to how planets orbit the sun energy levels for electrons are quantized Major developments that put
Chemistry I: Quantum Mechanics Notes Bohr Model of Atom: electrons move around nucleus in orbits similar to how planets orbit the sun energy levels for electrons are quantized Major developments that put
Quantum Electron Model Chapter 5 Mr. Hines
 Quantum Electron Model Chapter 5 Mr. Hines Part A - INTRODUCTION TO THE QUANTUM ELECTRON MODEL 1 Recall basic knowledge from chapter 4 energy levels, valence electrons, periods, and groups 2 Describe atoms
Quantum Electron Model Chapter 5 Mr. Hines Part A - INTRODUCTION TO THE QUANTUM ELECTRON MODEL 1 Recall basic knowledge from chapter 4 energy levels, valence electrons, periods, and groups 2 Describe atoms
Angular Momentum Quantization: Physical Manifestations and Chemical Consequences
 Angular Momentum Quantization: Physical Manifestations and Chemical Consequences Michael Fowler, University of Virginia 7/7/07 The Stern-Gerlach Experiment We ve established that for the hydrogen atom,
Angular Momentum Quantization: Physical Manifestations and Chemical Consequences Michael Fowler, University of Virginia 7/7/07 The Stern-Gerlach Experiment We ve established that for the hydrogen atom,
Chemistry 543--Final Exam--Keiderling May 5, pm SES
 Chemistry 543--Final Exam--Keiderling May 5,1992 -- 1-5pm -- 174 SES Please answer all questions in the answer book provided. Make sure your name is clearly indicated and that the answers are clearly numbered,
Chemistry 543--Final Exam--Keiderling May 5,1992 -- 1-5pm -- 174 SES Please answer all questions in the answer book provided. Make sure your name is clearly indicated and that the answers are clearly numbered,
Copyright 2010 Sponholtz Productions, LLC Page 1
 Introduction to the Atom Key Terms: abbreviated electron configuration - combines the inert, noble core electrons with the remaining, outermost electrons, which are commonly called valence electrons. angular
Introduction to the Atom Key Terms: abbreviated electron configuration - combines the inert, noble core electrons with the remaining, outermost electrons, which are commonly called valence electrons. angular
The Hydrogen Atom According to Bohr
 The Hydrogen Atom According to Bohr The atom We ve already talked about how tiny systems behave in strange ways. Now let s s talk about how a more complicated system behaves. The atom! Physics 9 4 Early
The Hydrogen Atom According to Bohr The atom We ve already talked about how tiny systems behave in strange ways. Now let s s talk about how a more complicated system behaves. The atom! Physics 9 4 Early
Name: Unit 3 Guide-Electrons In Atoms
 Name: Unit 3 Guide-Electrons In Atoms Importance of Electrons Draw a complete Bohr model of the atom. Write an element s electron configuration. Know how the symbols used in ECs relate to electron properties
Name: Unit 3 Guide-Electrons In Atoms Importance of Electrons Draw a complete Bohr model of the atom. Write an element s electron configuration. Know how the symbols used in ECs relate to electron properties
 CHAPTER 6 CHEMICAL BONDING TEXT BOOK EXERCISE Q.1. Select the correct statement. i. An ionic compound A + B - is most likely to be formed when ii. iii. a. the ionization energy of A is high and electron
CHAPTER 6 CHEMICAL BONDING TEXT BOOK EXERCISE Q.1. Select the correct statement. i. An ionic compound A + B - is most likely to be formed when ii. iii. a. the ionization energy of A is high and electron
Chapter 7. Atomic Structure
 Chapter 7 Atomic Structure Light Made up of electromagnetic radiation. Waves of electric and magnetic fields at right angles to each other. Parts of a wave Wavelength Frequency = number of cycles in one
Chapter 7 Atomic Structure Light Made up of electromagnetic radiation. Waves of electric and magnetic fields at right angles to each other. Parts of a wave Wavelength Frequency = number of cycles in one
Chem 105 Final Exam. Here is the summary of the total 225 points plus 10 bonus points. Carefully read the questions. Good luck!
 May 3 rd, 2012 Name: CLID: Score: Chem 105 Final Exam There are 50 multiple choices that are worth 3 points each. There are 4 problems and 1 bonus problem. Try to answer the questions, which you know first,
May 3 rd, 2012 Name: CLID: Score: Chem 105 Final Exam There are 50 multiple choices that are worth 3 points each. There are 4 problems and 1 bonus problem. Try to answer the questions, which you know first,
Notes: Unit 6 Electron Configuration and the Periodic Table
 Name KEY Block Notes: Unit 6 Electron Configuration and the Periodic Table In the 1790's Antoine Lavoisier compiled a list of the known elements at that time. There were only 23 elements. By the 1870's
Name KEY Block Notes: Unit 6 Electron Configuration and the Periodic Table In the 1790's Antoine Lavoisier compiled a list of the known elements at that time. There were only 23 elements. By the 1870's
Unit 3: Electron configuration and periodicity
 Unit 3: Electron configuration and periodicity Group 1 BOHR MODELS Group 18 H Group 2 Group 13 Group 14 Group 15 Group 16 Group 17 He Li Be B C N O F Ne Na Mg Al Si P S Cl Ar K Ca His theory couldn t
Unit 3: Electron configuration and periodicity Group 1 BOHR MODELS Group 18 H Group 2 Group 13 Group 14 Group 15 Group 16 Group 17 He Li Be B C N O F Ne Na Mg Al Si P S Cl Ar K Ca His theory couldn t
I. Multiple Choice Questions (Type-I)
 I. Multiple Choice Questions (Type-I) 1. Which of the following conclusions could not be derived from Rutherford s α -particle scattering experiement? (i) Most of the space in the atom is empty. (ii) The
I. Multiple Choice Questions (Type-I) 1. Which of the following conclusions could not be derived from Rutherford s α -particle scattering experiement? (i) Most of the space in the atom is empty. (ii) The
Chem What is the difference between an orbit (Bohr model) and an orbital (quantum mechanical model)?
 Reading: sections 6.5-6.6 As you read this material, ask yourself the following questions: What are wave functions and orbitals, how do orbitals differ from orbits? What can we learn about an electron
Reading: sections 6.5-6.6 As you read this material, ask yourself the following questions: What are wave functions and orbitals, how do orbitals differ from orbits? What can we learn about an electron
Chapter 9 Ionic and Covalent Bonding
 Chem 1045 Prof George W.J. Kenney, Jr General Chemistry by Ebbing and Gammon, 8th Edition Last Update: 06-April-2009 Chapter 9 Ionic and Covalent Bonding These Notes are to SUPPLIMENT the Text, They do
Chem 1045 Prof George W.J. Kenney, Jr General Chemistry by Ebbing and Gammon, 8th Edition Last Update: 06-April-2009 Chapter 9 Ionic and Covalent Bonding These Notes are to SUPPLIMENT the Text, They do
Electron Configuration and Chemical Periodicity
 Electron Configuration and Chemical Periodicity The Periodic Table Periodic law (Mendeleev, Meyer, 1870) periodic reoccurrence of similar physical and chemical properties of the elements arranged by increasing
Electron Configuration and Chemical Periodicity The Periodic Table Periodic law (Mendeleev, Meyer, 1870) periodic reoccurrence of similar physical and chemical properties of the elements arranged by increasing
CHEM Course web page. Outline for first exam period
 CHEM 3 Course web page http://web.chemistry.gatech.edu/~barefield/3/chem3a.html Outline for first exam period Atomic structure and periodic properties Structures and bonding models for covalent compounds
CHEM 3 Course web page http://web.chemistry.gatech.edu/~barefield/3/chem3a.html Outline for first exam period Atomic structure and periodic properties Structures and bonding models for covalent compounds
Atomic Theory. Quantum Mechanics
 Atomic Theory Quantum Mechanics Quantum Mechanics The ol solar system model of the atom does have some practical uses It tells us that protons and neutrons are in the nucleus, and electrons are in orbitals
Atomic Theory Quantum Mechanics Quantum Mechanics The ol solar system model of the atom does have some practical uses It tells us that protons and neutrons are in the nucleus, and electrons are in orbitals
Unit 1 Part 2 Atomic Structure and The Periodic Table Introduction to the Periodic Table UNIT 1 ATOMIC STRUCTURE AND THE PERIODIC TABLE
 UNIT 1 ATOMIC STRUCTURE AND THE PERIODIC TABLE PART 2 INTRODUCTION TO THE PERIODIC TABLE Contents 1. The Structure of the Periodic Table 2. Trends in the Periodic Table Key words: group, period, block,
UNIT 1 ATOMIC STRUCTURE AND THE PERIODIC TABLE PART 2 INTRODUCTION TO THE PERIODIC TABLE Contents 1. The Structure of the Periodic Table 2. Trends in the Periodic Table Key words: group, period, block,
QUANTUM MECHANICS AND ATOMIC STRUCTURE
 5 CHAPTER QUANTUM MECHANICS AND ATOMIC STRUCTURE 5.1 The Hydrogen Atom 5.2 Shell Model for Many-Electron Atoms 5.3 Aufbau Principle and Electron Configurations 5.4 Shells and the Periodic Table: Photoelectron
5 CHAPTER QUANTUM MECHANICS AND ATOMIC STRUCTURE 5.1 The Hydrogen Atom 5.2 Shell Model for Many-Electron Atoms 5.3 Aufbau Principle and Electron Configurations 5.4 Shells and the Periodic Table: Photoelectron
Gateway 125, 126, 130 Fall 2006 HW 3 p1. Figure 1 was designed to represent an atom. Critique the model by answering the following questions:
 Gateway 125, 126, 130 Fall 2006 HW 3 p1 Figure 1 was designed to represent an atom. Critique the model by answering the following questions: Figure 1: Representation of an Atom red grey blue 1) What are
Gateway 125, 126, 130 Fall 2006 HW 3 p1 Figure 1 was designed to represent an atom. Critique the model by answering the following questions: Figure 1: Representation of an Atom red grey blue 1) What are
2. For the following two compounds between oxygen and hydrogen: 3. Tell what discoveries were made by each of the following scientists:
 EXTRA HOMEWORK 1A 1. When Dalton proposed that matter was composed of atoms, why was his Atomic Theory accepted? 2. For the following two compounds between oxygen and hydrogen: Mass of O Mass of H Compound
EXTRA HOMEWORK 1A 1. When Dalton proposed that matter was composed of atoms, why was his Atomic Theory accepted? 2. For the following two compounds between oxygen and hydrogen: Mass of O Mass of H Compound
When I lecture we will add more info, so leave spaces in your notes
 Title and Highlight Topic: EQ: Date Reflect Question: Reflect on the material by asking a question (its not suppose to be answered from notes) NOTES: Write out the notes from my website. Use different
Title and Highlight Topic: EQ: Date Reflect Question: Reflect on the material by asking a question (its not suppose to be answered from notes) NOTES: Write out the notes from my website. Use different
Finite Mathematics : A Business Approach
 Finite Mathematics : A Business Approach Dr. Brian Travers and Prof. James Lampes Second Edition Cover Art by Stephanie Oxenford Additional Editing by John Gambino Contents What You Should Already Know
Finite Mathematics : A Business Approach Dr. Brian Travers and Prof. James Lampes Second Edition Cover Art by Stephanie Oxenford Additional Editing by John Gambino Contents What You Should Already Know
How many grams of sodium metal is required to completely react with 2545 grams of chlorine gas?
 EXAMPLE PROBLEM: How many grams of sodium metal is required to completely react with 2545 grams of chlorine gas? 1 - Convert 2545 grams of chlorine to moles chlorine using formula weight 2 - Convert moles
EXAMPLE PROBLEM: How many grams of sodium metal is required to completely react with 2545 grams of chlorine gas? 1 - Convert 2545 grams of chlorine to moles chlorine using formula weight 2 - Convert moles
Chem 110 General Principles of Chemistry
 Chem 110 General Principles of Chemistry Chapter 5 lectronic Structure of Atoms Chapter 5 deals with the electronic structure of atoms. It is because of the different numbers of electrons in atoms and
Chem 110 General Principles of Chemistry Chapter 5 lectronic Structure of Atoms Chapter 5 deals with the electronic structure of atoms. It is because of the different numbers of electrons in atoms and
