THE COMPOSITION OF THE CHASSIGNY METEORITE
|
|
|
- Alberta Washington
- 5 years ago
- Views:
Transcription
1 THE COMPOSITION OF THE CHASSIGNY METEORITE Brian Mason, J.A. Nelen Smithsonian Institution Wadlington, DC P. Muir, S.R. Taylor Australian National University Canberra, A.C.T. 2600, Australia Spark source mass spectrometric analysis of the Chassigny meteorite has given the following data (in ppm): Rb 0.4, Sr 7.2, Y 0.64, Zr 1.5, Nb 0.32, Ba 7.1, La 0.39, Ce 1.12, Pr 0.13, Nd 0.54, Sm 0.11, Eu 0.038, Gd 0.11, Tb 0.02,Dy 0.12, Ho 0.03, Er 0.09, Yb 0.10, Pb 1.0, Th 0.057, U These data, in conjunction with major element composition and mineralogical and textural features, indicate that this meteorite is an olivinerich cumulate, possibly genetically related to the nakhlites. INTRODUCTION The Chassigny meteorite fell on October 3, 1815, near the village of Chassigny on the plateau of Langres in the province of Haute-Marne, France. One stone (or possibly more) was recovered. It had broken into numerous fragments on impact; the total weight collected was about 4 kg, the largest fragment being about 1 kg. The largest piece now known is one of 246 g in the Museum d'histoire Naturelle, Paris; small pieces are widely distributed in collections. Chassigny is a unique meteorite, an olivine achondrite showing no close relationship to any other achondrite. Although its major element composition has been established by several analyses, there are few published data on minor and trace elements. To fill this gap, and possibly to shed some light on the relationship of Chassigny to other meteorites, we obtained material from the specimen in the Smithsonian Institution (USNM 624). MAJOR ELEMENT COMPOSITION Chassigny was first analysed by Vauquelin in This analysis, while incomplete by modern standards, was adequate to establish that Chassigny consisted largely of iron-rich olivine. Five analyses from the literature are given in Table I, along with the composition calculated from a modal analysis and the composition of the individual minerals. The model analysis was published by Prinz et al. (1974) in volume percent (olivine 91.6, pyroxene 5.0, feldspar 1.7, chromite 1.4, melt inclusions 0.3), and was converted to weight percent by using the appropriate densities for the individual minerals. The pyroxene has been arbitrarily divided equally between clinopyroxene and orthopyroxene, and the 0.3% melt inclusions were omitted from the calculation. The mineral compositions are given in Table 2. Meteoritics, Vol. 11, No.1, March 31,
2 Table 1 Chemical analyses of the Chassigny meteorite Si Ti Alz CrZ FeO MoO MgO CaO NazO K P 2 0 S Sum Q m* * 1OOMg(Mg+Mn+Fe) 1. Damour, 1862; the total includes 3.77% insoluble, which Damour identified as chromite and pyroxene. 2. Dyakonova and Kharitonova, 1960; they report no FeS, and 0.06% Ni, 3. Jerernine et at., 1962; they report 0.60% FeS, 0.24% H 20-, and no Ni. 4. Jerome, McCarthy et al., 1974; the analysis was given as elements and has been converted to oxides to facilitate comparison with the other analyses. 6. Calculated from the modal analysis (Prinz et al., 1974) and mineral compositions (Table 2). Table 2 Mineral compositions in the Chassigny meteorite, by microprobe analysis. Olivine Clinopyroxene Orthopyroxene Plagioclase Chromite Si0 2 Ti0 2 Ah03 CrZ03 FeO MnO MgO CaO NazO KzO Sum < < < 0.D <
3 All five analyses show good agreement for the major components Si0 2, FeO, and MgO, and the figures are consistent with those derived from the mode. Damour's analysis is essentially that of the olivine, since he analysed the portion soluble in RN0 3, and reported the remainder as insoluble, commenting that it consisted of chromite and pyroxene. His value for Kp is clearly erroneous, possibly introduced from his reagents, and the presence of Cr203 in the acid-soluble material is puzzling, since the olivine contains only traces of this component. The values for some of the minor components are fairly consistent for all analyses, but for a few there are notable discrepancies. These discrepancies are not likely to be due to sampling difficulties, since Chassigny is a relatively fine-grained meteorite and even a small sample should be adequate. The most notable discrepancies are in AIP3' Here we see the inadequacy of the older wet-chemical determination of small amounts of this component, analyses 2 and 3, against more recent determinations, analyses 4 and 5. Analyses 4 and 5 give lower Ah03 values than those determined from the mode, but the difference is small and may be due to a slight overestimate of plagioclase in the mode. The alkali figures determined from the mode are consistent with the lowest figures for these components in the chemical analyses. Analysis 5 is notably discrepant in CaO content from the other determinations and is certainly erroneous for this component. The small amount of P20S is consistent with the presence of accessory chlorapatite reported by Prinz et al. (1974). Jeremine et al. (1962) reported 0.60% FeS, whereas Dyakonova and Kharitonova (1960) reported the absence of FeS. The truth lies somewhere in between; examination of a polished thin section shows small amounts of sulfides, considerably less than 0.6%. Prinz et al. (1974) record troilite, pyrite, and pentlandite as minor minerals. The 0.06% Ni reported in analysis 2 is confirmed by Jerome (1970), who found 475 ppm Ni (by emission spectrometry); this nickel evidently accounts for the pentlandite, but whether some of this element is also present in olivine is not known. TRACE ELEMENTS Trace elements were determined by spark source mass spectrography, using the technique described by Taylor (1965, 1971). This technique uses the rare earth element Lu as an internal standard, so its abundance cannot be directly determined. The rare earth Tm suffers a serious interference from a multiple carbon molecule, so its abundance is also not determined. Abundances of both Tm and Lu can be estimated from the rare earth pattern. Accuracy and precision of the method are dependent on the total number of exposures used to calculate the abundance of each element, as well as other factors. For this study four photoplates were exposed, each with about 15 exposures, and determinations for many elemen15 were based on more than one isotope. This resulted in the measured abundance of each element being based on 8 to 30 determinations. The precision obtained for all elements was 23
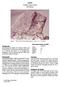
4 about ± 5%. Results obtained by this technique on lunar samples agree well with results obtained on the same samples by other methods (Taylor et al., 1973). This and other comparisons indicate that the accuracy of the method is about ± 10%. Quantitative abundance data were obtained for the following elements (in ppm): Rb 004, Sr 7.2, Y 0.64, Zr 1.5, Nb 0.32, Ba 7.1, La 0.39, Ce 1.12, Pr 0.13, Nd 0.54, Sm 0.11, Eu 0.038, Gd 0.11, Tb 0.02, Dy 0.12, Ho 0.03, Er 0.09, Yb 0.10, Pb 1.0, U 0.021, Th Additional trace element data are given by Jerome (1970); he reported Ba 5, Sc 8, V 50 (by emission spectrometry), and Co 140.6, Sc 5.58 (by INAA). DISCUSSION As mentioned previously, Chassigny is a unique meteorite, showing no close relationship to any other achondrite. Mineralogically it shows a qualitative resemblance to the chondrites, with dominant olivine and minor pyroxene, plagioclase, and chromite. The olivine composition (Fa32) is similar to that of the most iron-rich olivine in common chondrites; clinopyroxene is lower in CaO and orthopyroxene higher in CaO than most pyroxene in chondrites (Mason, 1968), but are comparable to pyroxene compositions in the Shaw chondrite (Fredriksson and Mason, 1967); plagioclase is sodiumrich, similar to that in chondrites and unlike the calcium-rich plagioclase typical of most achondrites. For Chassigny the major mineralogical differences from chondrites are the absence and near-absence of nickel-iron and troilite. Texturally Chassigny has always been classed as an achondrite. However, Jeremine et al. (1962) recognized what they described as "chondres naissants," small spherical aggregates consisting mainly of pyroxene. In a thin section of Chassigny in the Smithsonian collection we have recognized structures similar to those described by Jerernine et al. but are unde cided on their interpretation. Whether they are truly incipient chondrules, or chondrules which have been almost erased by recrystallization, or some unrelated structures is difficult to determine from the limited evidence. The texture is that of an aggregate of euhedral to subhedral olivine crystals (size range 0.5 to 1.8 mm) with minor pyroxene and a little interstitial plagioclase; minute chromite grains are included in the olivine, and larger chromite grains are interstitial to the olivine and pyroxene. The texture strongly suggests a cumulate origin. The compositional relationship between Chassigny and the average chondrite is illustrated in Fig. 1. This shows that the major elements Mg, Fe, and Si are similar in abundance, with Mg showing minor enrichment in Chassigny. The only other elements showing relative enrichment in Chassigny are Cr, Mn, and Ba, and La, Ce, and Pr, illustrated in Fig. 2. The enrichment in Cr is explained by the relatively increased chromite content of Chassigny; the Mn enrichment is probably due to the high olivine content, but the source of the Ba enrichment is not immediately apparent. Relative to chondrites, 24
5 10 Fig. 1 I'I'.sa " ".Sr Sc lizr C {p K " ()3 1()4 loll AVERAGE CHONDRITE Plot (logarithmic scale) of elemental abundances (in ppm) in the Chassigny meteorite versus average chondritic abundances. The upper broken line represents 5 times, the lower broken line 0.2 times chondritic abundances. " F. Mg Si.Co AI.Na Mn.Ti 20 s 15 G >-.0 iii G0.7 Q: 0.5 f- %!2 0.4 OIl 0.3 La c. Pr Nd (Pm) Sm Eu Gd Tb Dy Ha Er Tm Vb Fig. 2 REE abundances, normalized to average chondritic abundances, in the Chassigny meteorite. 25
6 Chassigny is notably depleted in AI, Na, K (and Rb), P, Co, Ni, Zr, and to a lesser extent in Ca and Ti; 8 is also strongly depleted. This suggests that if Chassigny was derived from material of chondritic composition, this derivation included the elimination of most of the metal, sulfide, feldspar, and phosphate components. Binz et al. (1975) have pointed out that Chassigny shows compositional similarities to the ureilites; this is certainly true for most ofthe major components, although the FeOMgO ratio for the urelites is consistently lower than this ratio for Chassigny. The urelites are quite different from Chassigny in texture, and Chassigny lacks the carbonaceous matter characteristic of the ureilites. The rare earth distribution, Fig. 2, is an intriguing one. La and Ce are enhanced at a level of 1.3 times chondrites, and the relative concentration falls off rapidly to Sm at 0.5 times chondrites and then remains relatively uniform at this level; there may be a very slight positive Eu anomaly. The distribution pattern resembles that of terrestrial lherzolite and dunite xenoliths in basalts, as exemplified by those described by Frey and Green (1974); these are enriched in La and Ce at 2 to five times chondrites, with rapidly diminishing enrichment to Sm and Eu, and rather uniform concentration of heavier REE at 0.2 to 0.6 times chondrites. This distribution pattern is not controlled by the REE abundances in the dominant mineral, olivine; olivine, both in meteorites (Masuda, 1968) and in terrestrial dunites (Philpotts et al., 1972), has extremely low REE abundances, for most elements at less than 0.1 times chondrites. The distribution pattern in Chassigny must therefore represent a composite of the minor and trace constituents - pyroxene, plagioclase, apatite, and the 0.3% melt inclusions described by Prinz et al. (1974). Of particular significance is the absence of any marked Eu anomaly. If Chassigny were a cumulate from a melt of chondritic composition, with the elimination of most ofthe feldspar, then this meteorite should show a strong negative Eu anomaly, since meteoritic plagioclase is characterized by a strong positive Eu anomaly. The absence of this anomaly indicates that the REE distribution pattern in Chassigny cannot be explained by the simple subtraction of plagioclase (and phosphate) from material of chondritic composition. A reviewer has pointed out that the REE distribution in Chassigny is similar to that in the nakhlites (Schmitt and Smith, 1963), but at much lower concentrations, about 0.2 times those in the nakhlites. This suggests the possibility of a relationship between Chassigny and the nakhlites. The nakhlites have a much higher CaO content and FeOMgO ratio than Chassigny (mineralogically expressed by their consisting largely of diopsidic pyroxene with a minor amount of iron-rich olivine). If Chassigny is genetically related to the nakhlites, it probably represents an early cumulate from a common parent liquid. 26
7 REFERENCES Binz, CM., M. Ikramuddin and M.E. Lipschutz, Contents of eleven trace elements in ureilite achondrites. Geochim. Cosmochim. Acta 39, Damour, A., Note sur la pierre meteoritique de Chassigny. Compt. Rend. Acad. Sci Paris 55, Dyakonova, M.1. and V.Y. Kharitonova, Results of the chemical analyses of some stony and iron meteorites in the collection of the Academy ofscience ofthe U.S.S.R. Meteoritika 18, Fredriksson, K. and B. Mason, The Shaw meteorite. Geochim. Cosmochim.Acta 31, Frey, F.A. and D.H. Green, The mineralogy, geochemistry and origin of lherzolite inclusions in Victorian basanites. Geochim: Cosmochim: Acta 38, Jeremine, E., J. Orcel and A. Sandrea, Etude mineralogique et structurale de la meteorite de Chassigny. Bull. Soc. franc. Mineral. CristaL 85, Jerome, D.Y., Composition and origin of some achondritic meteorites. Dissertation, University oforegon, 113 pp, Mason, B., Pyroxenes in meteorites. Lithos 1, Masuda, A., Lanthanide concentrations in the olivine phase of the Brenham pallasite. Earth Planet. Sci. Lett. 5, McCarthy, T.S., A.J. Erlank, J.P. Willisand L.B. Ahrens, New chemical analyses of six achondrites and one chondrite. Meteoritics 9, Philpotts, J.A., C.C. Schnetzler and H.B. Thomas, Petrogenetic implications of some new geochemical data on eclogitic and ultrabasic inclusions. Geochim. Cosmochim. Acta 36, Prinz, M., P.H. Hlava and K. Keil, The Chassigny meteorite: a relatively iron-rich cumulate dunite.metevritics 9, Taylor, S.R., Geochemical analysis by spark source mass spectrography. Geochim. Cosmochim. Acta 29, Taylor, S.R., Geochemical application of spark source mass spectrography 11. Photoplate data processing. Geochim. Cosmochim. Acta 35, Taylor, S.R., M.P. Gorton, P. Muir, W.B. Nance, R. Rudowski and N. Ware, Composition of the Descartes region, lunar highlands. Geochim. Cosmochim. Acta 37, Manuscript received
IV. Governador Valadares clinopyroxenite, 158 grams find
 IV. Governador Valadares clinopyroxenite, 158 grams find Figure IV-1. Photograph of Governador Valadares (158 grams) from Dr. Fernanda Ferrucci via Dr. Giuseppe Cavarretta. Photo taken by L. Spinozzi.
IV. Governador Valadares clinopyroxenite, 158 grams find Figure IV-1. Photograph of Governador Valadares (158 grams) from Dr. Fernanda Ferrucci via Dr. Giuseppe Cavarretta. Photo taken by L. Spinozzi.
Worked Example of Batch Melting: Rb and Sr
 Worked Example of Batch Melting: Rb and Sr Basalt with the mode: Table 9.2. Conversion from mode to weight percent Mineral Mode Density Wt prop Wt% ol 15 3.6 54 0.18 cpx 33 3.4 112.2 0.37 plag 51 2.7 137.7
Worked Example of Batch Melting: Rb and Sr Basalt with the mode: Table 9.2. Conversion from mode to weight percent Mineral Mode Density Wt prop Wt% ol 15 3.6 54 0.18 cpx 33 3.4 112.2 0.37 plag 51 2.7 137.7
Chapter 9: Trace Elements
 Lecture 13 Introduction to Trace Elements Wednesday, March 9, 2005 Chapter 9: Trace Elements Note magnitude of major element changes Figure 8-2. Harker variation diagram for 310 analyzed volcanic rocks
Lecture 13 Introduction to Trace Elements Wednesday, March 9, 2005 Chapter 9: Trace Elements Note magnitude of major element changes Figure 8-2. Harker variation diagram for 310 analyzed volcanic rocks
Supplementary Figure 1 Map of the study area Sample locations and main physiographic features of the study area. Contour interval is 200m (a) and 40m
 Supplementary Figure 1 Map of the study area Sample locations and main physiographic features of the study area. Contour interval is 200m (a) and 40m (b). Dashed lines represent the two successive ridge
Supplementary Figure 1 Map of the study area Sample locations and main physiographic features of the study area. Contour interval is 200m (a) and 40m (b). Dashed lines represent the two successive ridge
EMMR25 Mineralogy: Ol + opx + chlorite + cpx + amphibole + serpentine + opaque
 GSA Data Repository 2017365 Marshall et al., 2017, The role of serpentinite derived fluids in metasomatism of the Colorado Plateau (USA) lithospheric mantle: Geology, https://doi.org/10.1130/g39444.1 Appendix
GSA Data Repository 2017365 Marshall et al., 2017, The role of serpentinite derived fluids in metasomatism of the Colorado Plateau (USA) lithospheric mantle: Geology, https://doi.org/10.1130/g39444.1 Appendix
Spot Name U-Pb ages (Ma) Plagioclase ages (Ma) Biotite age (Ma) Whole rock age (Ma)
 Table 1. Average U-Pb ages from this study in comparison with previous ages from Sherrod and Tosdal (1991, and references therein). Previous study ages are reported as ranges including uncertainty (i.e.
Table 1. Average U-Pb ages from this study in comparison with previous ages from Sherrod and Tosdal (1991, and references therein). Previous study ages are reported as ranges including uncertainty (i.e.
Meteorites free samples from the solar system
 Meteorites free samples from the solar system It is easier to believe that Yankee professors would lie, than that stones would fall from heaven [Thomas Jefferson, 3rd president of the USA] 2.1 Collection
Meteorites free samples from the solar system It is easier to believe that Yankee professors would lie, than that stones would fall from heaven [Thomas Jefferson, 3rd president of the USA] 2.1 Collection
TRACE ELEMENT ANALYSIS OF DIAMOND BY LAM ICPMS: STANDARDISATION, RESULTS AND DIRECTIONS
 TRACE ELEMENT ANALYSIS OF DIAMOND BY LAM ICPMS: STANDARDISATION, RESULTS AND DIRECTIONS W.L. Griffin 1, 3, Sonal Rege 1, Rondi M. Davies 1, 2, Simon Jackson 1, Suzanne Y. O Reilly 1 1.ARC National Key
TRACE ELEMENT ANALYSIS OF DIAMOND BY LAM ICPMS: STANDARDISATION, RESULTS AND DIRECTIONS W.L. Griffin 1, 3, Sonal Rege 1, Rondi M. Davies 1, 2, Simon Jackson 1, Suzanne Y. O Reilly 1 1.ARC National Key
Trace Elements. Today s lecture
 Trace Elements 300 Ni 200 ppm 100 0 300 Zr 200 100 0 40 50 60 70 80 SiO 2 wt. % Updates: M&M due date: Tuesday Today s lecture Topics: Trace element compositions Trace element behavior Partitioning Spider(
Trace Elements 300 Ni 200 ppm 100 0 300 Zr 200 100 0 40 50 60 70 80 SiO 2 wt. % Updates: M&M due date: Tuesday Today s lecture Topics: Trace element compositions Trace element behavior Partitioning Spider(
XM1/331 XM1/331 BLFX-3 XM1/331
 a b AkC AkC strontian fluoro-apatite clinopyroxene phlogopite K-richterite XM1/331 clinopyroxene XM1/331 Fe-Ti ox c d clinopyroxene kric AkC ilmenite Sr-barite AkC XM1/331 BLFX-3 Supplementary Figure 1.
a b AkC AkC strontian fluoro-apatite clinopyroxene phlogopite K-richterite XM1/331 clinopyroxene XM1/331 Fe-Ti ox c d clinopyroxene kric AkC ilmenite Sr-barite AkC XM1/331 BLFX-3 Supplementary Figure 1.
TABLE DR2. Lu-Hf ISOTOPIC DATA FOR WHOLE ROCK SAMPLES AND ZIRCONS [Lu] [Hf]
![TABLE DR2. Lu-Hf ISOTOPIC DATA FOR WHOLE ROCK SAMPLES AND ZIRCONS [Lu] [Hf] TABLE DR2. Lu-Hf ISOTOPIC DATA FOR WHOLE ROCK SAMPLES AND ZIRCONS [Lu] [Hf]](/thumbs/72/67409273.jpg) TABLE DR1. LOWER CRUSTAL GRANULITE XENOLITH DERIVATION AND MINERALOGY Sample Kimberlite Type Mineralogy KX1-1 Lace s gt + qz + sa + rt (sil, ky, gr, su, cor, zr, mz) KX1-2 Lace s gt + sa + qz + rt (sil,
TABLE DR1. LOWER CRUSTAL GRANULITE XENOLITH DERIVATION AND MINERALOGY Sample Kimberlite Type Mineralogy KX1-1 Lace s gt + qz + sa + rt (sil, ky, gr, su, cor, zr, mz) KX1-2 Lace s gt + sa + qz + rt (sil,
Petrogenesis of angrites
 Pergamon doi:10.1016/s0016-7037(03)00310-7 Geochimica et Cosmochimica Acta, Vol. 67, No. 24, pp. 4775 4789, 2003 Copyright 2003 Elsevier Ltd Printed in the USA. All rights reserved 0016-7037/03 $30.00.00
Pergamon doi:10.1016/s0016-7037(03)00310-7 Geochimica et Cosmochimica Acta, Vol. 67, No. 24, pp. 4775 4789, 2003 Copyright 2003 Elsevier Ltd Printed in the USA. All rights reserved 0016-7037/03 $30.00.00
Summary of test results for Daya Bay rock samples. by Patrick Dobson Celia Tiemi Onishi Seiji Nakagawa
 Summary of test results for Daya Bay rock samples by Patrick Dobson Celia Tiemi Onishi Seiji Nakagawa October 2004 Summary A series of analytical tests were conducted on a suite of granitic rock samples
Summary of test results for Daya Bay rock samples by Patrick Dobson Celia Tiemi Onishi Seiji Nakagawa October 2004 Summary A series of analytical tests were conducted on a suite of granitic rock samples
Chapter 9: Trace Elements
 Chapter 9: Trace Elements Note magnitude of major element changes Figure 8.2. Harker variation diagram for 310 analyzed volcanic rocks from Crater Lake (Mt. Mazama), Oregon Cascades. Data compiled by Rick
Chapter 9: Trace Elements Note magnitude of major element changes Figure 8.2. Harker variation diagram for 310 analyzed volcanic rocks from Crater Lake (Mt. Mazama), Oregon Cascades. Data compiled by Rick
Earth Materials I Crystal Structures
 Earth Materials I Crystal Structures Isotopes same atomic number, different numbers of neutrons, different atomic mass. Ta ble 1-1. Su mmar y of quantu m num bers Name Symbol Values Principal n 1, 2,
Earth Materials I Crystal Structures Isotopes same atomic number, different numbers of neutrons, different atomic mass. Ta ble 1-1. Su mmar y of quantu m num bers Name Symbol Values Principal n 1, 2,
Composition of the Earth and its reservoirs: Geochemical observables
 Composition of the Earth and its reservoirs: Geochemical observables Cin-Ty A. Lee Rice University MYRES-I 2004 The Earth is dynamic and heterogeneous Atmosphere Midocean Ridge Plume Ocean Crust Oceanic
Composition of the Earth and its reservoirs: Geochemical observables Cin-Ty A. Lee Rice University MYRES-I 2004 The Earth is dynamic and heterogeneous Atmosphere Midocean Ridge Plume Ocean Crust Oceanic
The mantle metasomatism: diversity and impact What the mantle xenoliths tell us?
 The mantle metasomatism: diversity and impact What the mantle xenoliths tell us? Mantle metasomatism Physical and chemical processes that are implemented during the flow of magmas and / or fluids within
The mantle metasomatism: diversity and impact What the mantle xenoliths tell us? Mantle metasomatism Physical and chemical processes that are implemented during the flow of magmas and / or fluids within
Fe,Mg,Mn-bearing phosphates in the GRA meteorite: Occurrences and mineral chemistry
 American Mineralogist, Volume 84, pages 1354 1359, 1999 Fe,Mg,Mn-bearing phosphates in the GRA 95209 meteorite: Occurrences and mineral chemistry CHRISTINE FLOSS* McDonnell Center for the Space Sciences
American Mineralogist, Volume 84, pages 1354 1359, 1999 Fe,Mg,Mn-bearing phosphates in the GRA 95209 meteorite: Occurrences and mineral chemistry CHRISTINE FLOSS* McDonnell Center for the Space Sciences
The mineralogy of the Yaringie Hill meteorite A new H5 chondrite from South Australia
 Meteoritics & Planetary Science 44, Nr 11, 1687 1693 (2009) Abstract available online at http://meteoritics.org The mineralogy of the Yaringie Hill meteorite A new H5 chondrite from South Australia Ralf
Meteoritics & Planetary Science 44, Nr 11, 1687 1693 (2009) Abstract available online at http://meteoritics.org The mineralogy of the Yaringie Hill meteorite A new H5 chondrite from South Australia Ralf
Geochemical analysis unveils frictional melting process in a
 GSA Data Repository 219116 1 2 3 Geochemical analysis unveils frictional melting process in a subduction zone fault Tsuyoshi Ishikawa and Kohtaro Ujiie 4 Supplemental Material 6 7 8 9 METHOD TABLES (Tables
GSA Data Repository 219116 1 2 3 Geochemical analysis unveils frictional melting process in a subduction zone fault Tsuyoshi Ishikawa and Kohtaro Ujiie 4 Supplemental Material 6 7 8 9 METHOD TABLES (Tables
Breeding et al., Data Repository Material Figure DR1. Athens. Study Area
 Breeding, Ague, and Brocker 1 Figure DR1 21 o 24 Greece o A 38 o Athens Tinos 37 o Syros Attic-Cycladic Blueschist Belt Syros Kampos B Study Area Ermoupoli N Vari Unit Cycladic HP-LT Unit Marble horizons
Breeding, Ague, and Brocker 1 Figure DR1 21 o 24 Greece o A 38 o Athens Tinos 37 o Syros Attic-Cycladic Blueschist Belt Syros Kampos B Study Area Ermoupoli N Vari Unit Cycladic HP-LT Unit Marble horizons
Element Cube Project (x2)
 Element Cube Project (x2) Background: As a class, we will construct a three dimensional periodic table by each student selecting two elements in which you will need to create an element cube. Helpful Links
Element Cube Project (x2) Background: As a class, we will construct a three dimensional periodic table by each student selecting two elements in which you will need to create an element cube. Helpful Links
THE MONTE MAGGIORE PERIDOTITE (CORSICA)
 MONTE MAGGIORE CAPO CORSO CORSICA Giovanni B. Piccardo THE MONTE MAGGIORE PERIDOTITE (CORSICA) FIELD RELATIONSHIPS MORB Gabbro Spinel (ex-garnet) pyroxenites L ESCURSIONE A MONTE MAGGIORE The Monte Maggiore
MONTE MAGGIORE CAPO CORSO CORSICA Giovanni B. Piccardo THE MONTE MAGGIORE PERIDOTITE (CORSICA) FIELD RELATIONSHIPS MORB Gabbro Spinel (ex-garnet) pyroxenites L ESCURSIONE A MONTE MAGGIORE The Monte Maggiore
Effect of tectonic setting on chemistry of mantle-derived melts
 Effect of tectonic setting on chemistry of mantle-derived melts Lherzolite Basalt Factors controlling magma composition Composition of the source Partial melting process Fractional crystallization Crustal
Effect of tectonic setting on chemistry of mantle-derived melts Lherzolite Basalt Factors controlling magma composition Composition of the source Partial melting process Fractional crystallization Crustal
M} iiicnumlsum. and Erakot. Descriptions of Two Meteorites: Karoonda BY BRIAN MASON1 AND H. B. WIIK2
 M} iiicnumlsum PUBLISHED BY THE AMERICAN MUSEUM OF NATURAL HISTORY CENTRAL PARK WEST AT 79TH STREET, NEW YORK 24, N.Y. NUMBER 2 II 5 DECEMBERI4, I962 Descriptions of Two Meteorites: Karoonda and Erakot
M} iiicnumlsum PUBLISHED BY THE AMERICAN MUSEUM OF NATURAL HISTORY CENTRAL PARK WEST AT 79TH STREET, NEW YORK 24, N.Y. NUMBER 2 II 5 DECEMBERI4, I962 Descriptions of Two Meteorites: Karoonda and Erakot
BONINITIC MELT INCLUSIONS IN CHROME SPINEL FROM THE OGASAWARA ARCHIPELAGO
 GSA DATA REPOSITORY 2015057 BONINITIC MELT INCLUSIONS IN CHROME SPINEL FROM THE OGASAWARA ARCHIPELAGO DATA REPOSITORY for Thermal and chemical evolution of the subarc mantle revealed by spinel-hosted melt
GSA DATA REPOSITORY 2015057 BONINITIC MELT INCLUSIONS IN CHROME SPINEL FROM THE OGASAWARA ARCHIPELAGO DATA REPOSITORY for Thermal and chemical evolution of the subarc mantle revealed by spinel-hosted melt
Minor element zoning and trace element geochemistry of pallasites
 Meteoritics & Planetary Science 38, Nr 8, 1217 1241 (2003) Abstract available online at http://meteoritics.org Minor element zoning and trace element geochemistry of pallasites Weibiao HSU1, 2* 1 Purple
Meteoritics & Planetary Science 38, Nr 8, 1217 1241 (2003) Abstract available online at http://meteoritics.org Minor element zoning and trace element geochemistry of pallasites Weibiao HSU1, 2* 1 Purple
GSA Data Repository
 GSA Data Repository 2015244 1. Method of Statistical Analysis Appendix DR1 One has to be careful and use only samples with complete Sm-Eu-Gd concentration data to study Eu/Eu* in the crust. This is because
GSA Data Repository 2015244 1. Method of Statistical Analysis Appendix DR1 One has to be careful and use only samples with complete Sm-Eu-Gd concentration data to study Eu/Eu* in the crust. This is because
Figure of rare earth elemental abundances removed due to copyright restrictions.
 Figure of rare earth elemental abundances removed due to copyright restrictions. See figure 3.1 on page 26 of Tolstikhin, Igor and Jan Kramers. The Evolution of Matter: From the Big Bang to the Present
Figure of rare earth elemental abundances removed due to copyright restrictions. See figure 3.1 on page 26 of Tolstikhin, Igor and Jan Kramers. The Evolution of Matter: From the Big Bang to the Present
APPENDIX TABLES. Table A2. XRF analytical results for samples from drill hole AP5 (Areachap)
 APPENDIX TABLES Table A2. XRF analytical results for samples from drill hole AP5 (Areachap) Sample No. AP5/19 AP5/20 AP5/21 AP5/22 AP5/23 AP5/24 AP5/25AP5/26AP5/27AP5/28AP5/29AP5/30AP5/31AP5/32 AP5/33
APPENDIX TABLES Table A2. XRF analytical results for samples from drill hole AP5 (Areachap) Sample No. AP5/19 AP5/20 AP5/21 AP5/22 AP5/23 AP5/24 AP5/25AP5/26AP5/27AP5/28AP5/29AP5/30AP5/31AP5/32 AP5/33
23. A New Type of Antarctic Achondrites and their to S Asteroids and Chondrites
 No. 8] Proc. Japan Acad., 68, Ser. B (1992) 115 23. A New Type of Antarctic Achondrites and their to S Asteroids and Chondrites Relationship By Hiroshi TAKEDA,*) Jun SAITO,*)'**) and Takahiro HIROI***)
No. 8] Proc. Japan Acad., 68, Ser. B (1992) 115 23. A New Type of Antarctic Achondrites and their to S Asteroids and Chondrites Relationship By Hiroshi TAKEDA,*) Jun SAITO,*)'**) and Takahiro HIROI***)
The Periodic Table. Periodic Properties. Can you explain this graph? Valence Electrons. Valence Electrons. Paramagnetism
 Periodic Properties Atomic & Ionic Radius Energy Electron Affinity We want to understand the variations in these properties in terms of electron configurations. The Periodic Table Elements in a column
Periodic Properties Atomic & Ionic Radius Energy Electron Affinity We want to understand the variations in these properties in terms of electron configurations. The Periodic Table Elements in a column
Petrogenesis of lunar meteorite Northwest Africa 2977: Constraints from in situ microprobe results
 Meteoritics & Planetary Science 45, Nr 12, 1929 1947 (2011) doi: 10.1111/j.1945-5100.2010.01131.x Petrogenesis of lunar meteorite Northwest Africa 2977: Constraints from in situ microprobe results Ai-Cheng
Meteoritics & Planetary Science 45, Nr 12, 1929 1947 (2011) doi: 10.1111/j.1945-5100.2010.01131.x Petrogenesis of lunar meteorite Northwest Africa 2977: Constraints from in situ microprobe results Ai-Cheng
Nucleus. Electron Cloud
 Atomic Structure I. Picture of an Atom Nucleus Electron Cloud II. Subatomic particles Particle Symbol Charge Relative Mass (amu) protons p + +1 1.0073 neutrons n 0 1.0087 electrons e - -1 0.00054858 Compare
Atomic Structure I. Picture of an Atom Nucleus Electron Cloud II. Subatomic particles Particle Symbol Charge Relative Mass (amu) protons p + +1 1.0073 neutrons n 0 1.0087 electrons e - -1 0.00054858 Compare
DRAFT Cataclastic Dunite grams
 72415-72418 Cataclastic Dunite 58.74 grams DRAFT Figure 1: Boulder 3 at Station 2, with large dunite clast (72415-72418). The bright spot is the location of samples. A sample of the boulder matrix (72435)
72415-72418 Cataclastic Dunite 58.74 grams DRAFT Figure 1: Boulder 3 at Station 2, with large dunite clast (72415-72418). The bright spot is the location of samples. A sample of the boulder matrix (72435)
Differentiation & Thermal Evolution
 Differentiation & Thermal Evolution 1 1 Meteorite Classification: Iron Meteorites 2 Meteorite Classification: Iron Meteorites 2 Meteorite Classification Basic Types of Meteorites: - Stony (93% of falls)
Differentiation & Thermal Evolution 1 1 Meteorite Classification: Iron Meteorites 2 Meteorite Classification: Iron Meteorites 2 Meteorite Classification Basic Types of Meteorites: - Stony (93% of falls)
8. Relax and do well.
 CHEM 1515 Exam II John II. Gelder October 14, 1993 Name TA's Name Lab Section INSTRUCTIONS: 1. This examination consists of a total of 8 different pages. The last two pages include a periodic table, a
CHEM 1515 Exam II John II. Gelder October 14, 1993 Name TA's Name Lab Section INSTRUCTIONS: 1. This examination consists of a total of 8 different pages. The last two pages include a periodic table, a
Solutions and Ions. Pure Substances
 Class #4 Solutions and Ions CHEM 107 L.S. Brown Texas A&M University Pure Substances Pure substance: described completely by a single chemical formula Fixed composition 1 Mixtures Combination of 2 or more
Class #4 Solutions and Ions CHEM 107 L.S. Brown Texas A&M University Pure Substances Pure substance: described completely by a single chemical formula Fixed composition 1 Mixtures Combination of 2 or more
72335 Impact melt Breccia grams
 Impact melt Breccia 108.9 grams Figure 1: Location of on boulder #2 on landslide off of South Massif. Boulder is ~ 2-3 meters high. AS17-137-20918. Transcript Hey, that s a different rock, Gene (station
Impact melt Breccia 108.9 grams Figure 1: Location of on boulder #2 on landslide off of South Massif. Boulder is ~ 2-3 meters high. AS17-137-20918. Transcript Hey, that s a different rock, Gene (station
Chem Exam 1. September 26, Dr. Susan E. Bates. Name 9:00 OR 10:00
 Chem 1711 Exam 1 September 26, 2013 Dr. Susan E. Bates Name 9:00 OR 10:00 N A = 6.022 x 10 23 mol 1 I A II A III B IV B V B VI B VII B VIII I B II B III A IV A V A VI A VII A inert gases 1 H 1.008 3 Li
Chem 1711 Exam 1 September 26, 2013 Dr. Susan E. Bates Name 9:00 OR 10:00 N A = 6.022 x 10 23 mol 1 I A II A III B IV B V B VI B VII B VIII I B II B III A IV A V A VI A VII A inert gases 1 H 1.008 3 Li
Supporting Information
 Supporting Information Bindi et al. 10.1073/pnas.1111115109 Fig. S1. Electron microprobe X-ray elemental maps for the grain reported in Fig. 1B. Experimental details are given in Experimental Methods.
Supporting Information Bindi et al. 10.1073/pnas.1111115109 Fig. S1. Electron microprobe X-ray elemental maps for the grain reported in Fig. 1B. Experimental details are given in Experimental Methods.
Chem GENERAL CHEMISTRY I MIDTERM EXAMINATION
 Concordia University CHEM 205 Fall 2009, B LAST NAME: FIRST NAME: STUDENT ID: Chem 205 - GENERAL CHEMISTRY I MIDTERM EXAMINATION PLEASE READ THIS BOX WHILE WAITING TO START INSTRUCTIONS: Calculators are
Concordia University CHEM 205 Fall 2009, B LAST NAME: FIRST NAME: STUDENT ID: Chem 205 - GENERAL CHEMISTRY I MIDTERM EXAMINATION PLEASE READ THIS BOX WHILE WAITING TO START INSTRUCTIONS: Calculators are
GEOL 2312 Igneous and Metamorphic Petrology Spring 2016 Score / 58. Midterm 1 Chapters 1-10
 GEOL 2312 Igneous and Metamorphic Petrology Name KEY Spring 2016 Score / 58 Midterm 1 Chapters 1-10 1) Name two things that petrologists want to know about magmas (1 pt) Formation, source, composition,
GEOL 2312 Igneous and Metamorphic Petrology Name KEY Spring 2016 Score / 58 Midterm 1 Chapters 1-10 1) Name two things that petrologists want to know about magmas (1 pt) Formation, source, composition,
Abstract. 1. Introduction
 Abstract 1. Introduction 2. Geological position and host volcanics 3. Summary of primary mineralogy of Tirich and other peridotites of Dzhilinda River Figure 1. Composition of clinopyroxenes from the Dzhilinda
Abstract 1. Introduction 2. Geological position and host volcanics 3. Summary of primary mineralogy of Tirich and other peridotites of Dzhilinda River Figure 1. Composition of clinopyroxenes from the Dzhilinda
Lecture 38. Igneous geochemistry. Read White Chapter 7 if you haven t already
 Lecture 38 Igneous geochemistry Read White Chapter 7 if you haven t already Today. Magma mixing/afc 2. Spot light on using the Rare Earth Elements (REE) to constrain mantle sources and conditions of petrogenesis
Lecture 38 Igneous geochemistry Read White Chapter 7 if you haven t already Today. Magma mixing/afc 2. Spot light on using the Rare Earth Elements (REE) to constrain mantle sources and conditions of petrogenesis
The exam must be written in ink. No calculators of any sort allowed. You have 2 hours to complete the exam. Periodic table 7 0
 Email: The exam must be written in ink. No calculators of any sort allowed. You have 2 hours to complete the exam. CEM 610B Exam 3 Spring 2002 Instructor: Dr. Brian Pagenkopf Page Points 2 6 3 7 4 9 5
Email: The exam must be written in ink. No calculators of any sort allowed. You have 2 hours to complete the exam. CEM 610B Exam 3 Spring 2002 Instructor: Dr. Brian Pagenkopf Page Points 2 6 3 7 4 9 5
8. Relax and do well.
 CHEM 1215 Exam III John III. Gelder November 11, 1998 Name TA's Name Lab Section INSTRUCTIONS: 1. This examination consists of a total of 7 different pages. The last page includes a periodic table and
CHEM 1215 Exam III John III. Gelder November 11, 1998 Name TA's Name Lab Section INSTRUCTIONS: 1. This examination consists of a total of 7 different pages. The last page includes a periodic table and
Geogenic versus Anthropogenic Metals and Metalloids
 Geogenic versus Anthropogenic Metals and Metalloids Geochemical methods for evaluating whether metals and metalloids are from geogenic versus anthropogenic sources 1 Definitions Geogenic from natural geological
Geogenic versus Anthropogenic Metals and Metalloids Geochemical methods for evaluating whether metals and metalloids are from geogenic versus anthropogenic sources 1 Definitions Geogenic from natural geological
Dating. AST111 Lecture 8a. Isotopic composition Radioactive dating
 Dating Martian Lafayette Asteroid with patterns caused by the passaged through the atmosphere. Line on the fusion crust were caused by beads of molten rock. AST111 Lecture 8a Isotopic composition Radioactive
Dating Martian Lafayette Asteroid with patterns caused by the passaged through the atmosphere. Line on the fusion crust were caused by beads of molten rock. AST111 Lecture 8a Isotopic composition Radioactive
Written by Alex Ruzicka and Melinda Hutson Department of Geology, Portland State University
 1 of 10 posted September 30, 2005 Portales Valley: Not Just Another Ordinary Chondrite --- A melted meteorite gives a snapshot of the heat and shock that wracked an asteroid during the first stages of
1 of 10 posted September 30, 2005 Portales Valley: Not Just Another Ordinary Chondrite --- A melted meteorite gives a snapshot of the heat and shock that wracked an asteroid during the first stages of
Lecture 36. Igneous geochemistry
 Lecture 36 Igneous geochemistry Reading - White Chapter 7 Today 1. Overview 2. solid-melt distribution coefficients Igneous geochemistry The chemistry of igneous systems provides clues to a number of important
Lecture 36 Igneous geochemistry Reading - White Chapter 7 Today 1. Overview 2. solid-melt distribution coefficients Igneous geochemistry The chemistry of igneous systems provides clues to a number of important
7. Relax and do well.
 CHEM 1215 Exam II John II. Gelder October 7, 1998 Name TA's Name Lab Section INSTRUCTIONS: 1. This examination consists of a total of 5 different pages. The last page includes a periodic table and a solubility
CHEM 1215 Exam II John II. Gelder October 7, 1998 Name TA's Name Lab Section INSTRUCTIONS: 1. This examination consists of a total of 5 different pages. The last page includes a periodic table and a solubility
AMHERST COLLEGE Department of Geology Geology 41: Environmental and Solid Earth Geophysics
 AMHERST COLLEGE Department of Geology Geology 41: Environmental and Solid Earth Geophysics Lab 1: Meteorites EQUIPMENT: notebook and pen only In this lab, we will examine thin sections and hand samples
AMHERST COLLEGE Department of Geology Geology 41: Environmental and Solid Earth Geophysics Lab 1: Meteorites EQUIPMENT: notebook and pen only In this lab, we will examine thin sections and hand samples
8. Relax and do well.
 CHEM 1314 3;30 pm Theory Exam III John III. Gelder November 13, 2002 Name TA's Name Lab Section INSTRUCTIONS: 1. This examination consists of a total of 8 different pages. The last page include a periodic
CHEM 1314 3;30 pm Theory Exam III John III. Gelder November 13, 2002 Name TA's Name Lab Section INSTRUCTIONS: 1. This examination consists of a total of 8 different pages. The last page include a periodic
Mineralogy and petrology of two ordinary chondrites and their correlation with other meteorites
 MINERALOGIA, 40, No. 1 4: 107 116 (2009) DOI: 10.2478/v10002-009-0009-9 www.mineralogia.pl MINERALOGICAL SOCIETY OF POLAND POLSKIE TOWARZYSTWO MINERALOGICZNE Short note Mineralogy and petrology of two
MINERALOGIA, 40, No. 1 4: 107 116 (2009) DOI: 10.2478/v10002-009-0009-9 www.mineralogia.pl MINERALOGICAL SOCIETY OF POLAND POLSKIE TOWARZYSTWO MINERALOGICZNE Short note Mineralogy and petrology of two
INSTRUCTIONS: CHEM Exam I. September 13, 1994 Lab Section
 CHEM 1314.05 Exam I John I. Gelder September 13, 1994 Name TA's Name Lab Section Please sign your name below to give permission to post, by the last 4 digits of your student I.D. number, your course scores
CHEM 1314.05 Exam I John I. Gelder September 13, 1994 Name TA's Name Lab Section Please sign your name below to give permission to post, by the last 4 digits of your student I.D. number, your course scores
Atoms and the Periodic Table
 Atoms and the Periodic Table Parts of the Atom Proton Found in the nucleus Number of protons defines the element Charge +1, mass 1 Parts of the Atom Neutron Found in the nucleus Stabilizes the nucleus
Atoms and the Periodic Table Parts of the Atom Proton Found in the nucleus Number of protons defines the element Charge +1, mass 1 Parts of the Atom Neutron Found in the nucleus Stabilizes the nucleus
grams g g g g Regolith Breccia
 15306 134.2 grams 15315 35.6 g 15324 32.3 g 15325 57.8 g 15330 57.8 g Regolith Breccia Figure 1a,b: Photo of dust-covered 15306. Sample is 7 cm across. S71-43064 and 067. Introduction 15306 is a regolith
15306 134.2 grams 15315 35.6 g 15324 32.3 g 15325 57.8 g 15330 57.8 g Regolith Breccia Figure 1a,b: Photo of dust-covered 15306. Sample is 7 cm across. S71-43064 and 067. Introduction 15306 is a regolith
12052 Pigeonite Basalt 1866 grams
 12052 Pigeonite Basalt 1866 grams Revised Figure 1: Photo of broken side of 12052 showing vugs. Sample is 6.5 cm high. NASA # S70-44633. Summary of Age Data for 12052 Ar/Ar Rb/Sr Nyquist 1977 (recalculated)
12052 Pigeonite Basalt 1866 grams Revised Figure 1: Photo of broken side of 12052 showing vugs. Sample is 6.5 cm high. NASA # S70-44633. Summary of Age Data for 12052 Ar/Ar Rb/Sr Nyquist 1977 (recalculated)
Late-stage apatite: a potential HREEenriched. minerals in carbonatites
 Late-stage apatite: a potential HREEenriched co-product of LREE minerals in carbonatites Sam Broom-Fendley Camborne School of Mines, University of Exeter, UK Frances Wall Gus Gunn Aoife Brady Will Dawes
Late-stage apatite: a potential HREEenriched co-product of LREE minerals in carbonatites Sam Broom-Fendley Camborne School of Mines, University of Exeter, UK Frances Wall Gus Gunn Aoife Brady Will Dawes
8. Relax and do well.
 EM 1515.001-006 Exam II John II. Gelder March 5, 2002 Name TA's Name Section INSTRUTIONS: 1. This examination consists of a total of 8 different pages. The last three pages include a periodic table, a
EM 1515.001-006 Exam II John II. Gelder March 5, 2002 Name TA's Name Section INSTRUTIONS: 1. This examination consists of a total of 8 different pages. The last three pages include a periodic table, a
Chemistry 431 Practice Final Exam Fall Hours
 Chemistry 431 Practice Final Exam Fall 2018 3 Hours R =8.3144 J mol 1 K 1 R=.0821 L atm mol 1 K 1 R=.08314 L bar mol 1 K 1 k=1.381 10 23 J molecule 1 K 1 h=6.626 10 34 Js N A = 6.022 10 23 molecules mol
Chemistry 431 Practice Final Exam Fall 2018 3 Hours R =8.3144 J mol 1 K 1 R=.0821 L atm mol 1 K 1 R=.08314 L bar mol 1 K 1 k=1.381 10 23 J molecule 1 K 1 h=6.626 10 34 Js N A = 6.022 10 23 molecules mol
610B Final Exam Cover Page
 1 st Letter of Last Name NAME: 610B Final Exam Cover Page No notes or calculators of any sort allowed. You have 3 hours to complete the exam. CHEM 610B, 50995 Final Exam Fall 2003 Instructor: Dr. Brian
1 st Letter of Last Name NAME: 610B Final Exam Cover Page No notes or calculators of any sort allowed. You have 3 hours to complete the exam. CHEM 610B, 50995 Final Exam Fall 2003 Instructor: Dr. Brian
HANDOUT SET GENERAL CHEMISTRY I
 HANDOUT SET GENERAL CHEMISTRY I Periodic Table of the Elements 1 2 3 4 5 6 7 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 IA VIIIA 1 2 H He 1.00794 IIA IIIA IVA VA VIA VIIA 4.00262 3 Li 6.941 11 Na 22.9898
HANDOUT SET GENERAL CHEMISTRY I Periodic Table of the Elements 1 2 3 4 5 6 7 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 IA VIIIA 1 2 H He 1.00794 IIA IIIA IVA VA VIA VIIA 4.00262 3 Li 6.941 11 Na 22.9898
GSA DATA REPOSITORY
 GSA DATA REPOSITORY 2013011 Chen et al. ANALITICAL METHODS Microprobe analysis Microprobe analyses of minerals were done on a JEOL Superprobe JXA 8100 at the Key Laboratory of Orogenic Belts and Crustal
GSA DATA REPOSITORY 2013011 Chen et al. ANALITICAL METHODS Microprobe analysis Microprobe analyses of minerals were done on a JEOL Superprobe JXA 8100 at the Key Laboratory of Orogenic Belts and Crustal
DR Item Table DR1. Sample no Rock-type Oxide, wt% Trace Elements, ppm REE, ppm
 DR Item 2017318 for Sarıfakıoğlu, E., Dilek, Y., and Sevin, M., 2017, New synthesis of the Izmir-Ankara-Erzincan suture zone and the Ankara mélange in northern Anatolia based on new geochemical and geochronological
DR Item 2017318 for Sarıfakıoğlu, E., Dilek, Y., and Sevin, M., 2017, New synthesis of the Izmir-Ankara-Erzincan suture zone and the Ankara mélange in northern Anatolia based on new geochemical and geochronological
Magmatic Ore Deposits:
 Magmatic Ore Deposits: A number of processes that occur during cooling and crystallization of magmatic bodies can lead to the separation and concentration of minerals. 1- Pegmatites 2- Layered intrusions
Magmatic Ore Deposits: A number of processes that occur during cooling and crystallization of magmatic bodies can lead to the separation and concentration of minerals. 1- Pegmatites 2- Layered intrusions
The Periodic Table of the Elements
 The Periodic Table of the Elements All matter is composed of elements. All of the elements are composed of atoms. An atom is the smallest part of an element which still retains the properties of that element.
The Periodic Table of the Elements All matter is composed of elements. All of the elements are composed of atoms. An atom is the smallest part of an element which still retains the properties of that element.
HANDOUT SET GENERAL CHEMISTRY II
 HANDOUT SET GENERAL CHEMISTRY II Periodic Table of the Elements 1 2 3 4 5 6 7 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 IA VIIIA 1 2 H He 1.00794 IIA IIIA IVA VA VIA VIIA 4.00262 3 Li 6.941 11 Na 22.9898
HANDOUT SET GENERAL CHEMISTRY II Periodic Table of the Elements 1 2 3 4 5 6 7 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 IA VIIIA 1 2 H He 1.00794 IIA IIIA IVA VA VIA VIIA 4.00262 3 Li 6.941 11 Na 22.9898
Radiometric Dating (tap anywhere)
 Radiometric Dating (tap anywhere) Protons Neutrons Electrons Elements on the periodic table are STABLE Elements can have radioactive versions of itself called ISOTOPES!! Page 1 in your ESRT has your list!
Radiometric Dating (tap anywhere) Protons Neutrons Electrons Elements on the periodic table are STABLE Elements can have radioactive versions of itself called ISOTOPES!! Page 1 in your ESRT has your list!
What monitoring techniques are appropriate and effective for detecting CO2 migration in groundwater: isotope-based monitoring Philippe Négrel
 What monitoring techniques are appropriate and effective for detecting CO2 migration in groundwater: isotope-based monitoring Philippe Négrel Acting in complicity with Pauline Humez. Results from Pauline
What monitoring techniques are appropriate and effective for detecting CO2 migration in groundwater: isotope-based monitoring Philippe Négrel Acting in complicity with Pauline Humez. Results from Pauline
grams grams Poikilitic Impact Melt Breccia
 64567 13.8 grams 64569-14.3 grams Poikilitic Impact Melt Breccia Figure 1: Photo of 64567. Scale in mm. S72-55386 Mineralogical Mode by Simonds et al. (1973) 64567 64569 Plagioclase 69% 57 Pyroxene 10
64567 13.8 grams 64569-14.3 grams Poikilitic Impact Melt Breccia Figure 1: Photo of 64567. Scale in mm. S72-55386 Mineralogical Mode by Simonds et al. (1973) 64567 64569 Plagioclase 69% 57 Pyroxene 10
INSTRUCTIONS: Exam III. November 10, 1999 Lab Section
 CHEM 1215 Exam III John III. Gelder November 10, 1999 Name TA's Name Lab Section INSTRUCTIONS: 1. This examination consists of a total of 7 different pages. The last page includes a periodic table and
CHEM 1215 Exam III John III. Gelder November 10, 1999 Name TA's Name Lab Section INSTRUCTIONS: 1. This examination consists of a total of 7 different pages. The last page includes a periodic table and
Determination of rare earth and refractory trace element abundances in early solar system objects by ion microprobe
 Determination of rare earth and refractory trace element abundances in early solar system objects by ion microprobe S Sahijpal 1, K K Marhas 2 and J N Goswami 2 1 Department of Physics, Panjab University,
Determination of rare earth and refractory trace element abundances in early solar system objects by ion microprobe S Sahijpal 1, K K Marhas 2 and J N Goswami 2 1 Department of Physics, Panjab University,
Petrogenetic Constraints at Mount Rainier Volcano, Washington
 Petrogenetic Constraints at Mount Rainier Volcano, Washington S. C. Kuehn and P. R. Hooper, Department of Geology, Washington State University, Pullman, WA A. E. Eggers and C. Kerrick, Department of Geology,
Petrogenetic Constraints at Mount Rainier Volcano, Washington S. C. Kuehn and P. R. Hooper, Department of Geology, Washington State University, Pullman, WA A. E. Eggers and C. Kerrick, Department of Geology,
K. 27 Co. 28 Ni. 29 Cu Rb. 46 Pd. 45 Rh. 47 Ag Cs Ir. 78 Pt.
 1 IA 1 H Hydrogen 1.01 Atomic number Element symbol Element name Atomic mass VIIIA 1 H 1.01 IIA IIIA IVA VA VIA VIIA 2 He 4.00 Metalloids 3 Li 6.94 4 Be 9.01 5 B 10.81 6 C 12.01 7 N 14.01 8 O 16.00 9 F
1 IA 1 H Hydrogen 1.01 Atomic number Element symbol Element name Atomic mass VIIIA 1 H 1.01 IIA IIIA IVA VA VIA VIIA 2 He 4.00 Metalloids 3 Li 6.94 4 Be 9.01 5 B 10.81 6 C 12.01 7 N 14.01 8 O 16.00 9 F
(C) Pavel Sedach and Prep101 1
 (C) Pavel Sedach and Prep101 1 (C) Pavel Sedach and Prep101 1 (C) Pavel Sedach and Prep101 2 (C) Pavel Sedach and Prep101 2 (C) Pavel Sedach and Prep101 3 (C) Pavel Sedach and Prep101 3 (C) Pavel Sedach
(C) Pavel Sedach and Prep101 1 (C) Pavel Sedach and Prep101 1 (C) Pavel Sedach and Prep101 2 (C) Pavel Sedach and Prep101 2 (C) Pavel Sedach and Prep101 3 (C) Pavel Sedach and Prep101 3 (C) Pavel Sedach
CHEM 10113, Quiz 5 October 26, 2011
 CHEM 10113, Quiz 5 October 26, 2011 Name (please print) All equations must be balanced and show phases for full credit. Significant figures count, show charges as appropriate, and please box your answers!
CHEM 10113, Quiz 5 October 26, 2011 Name (please print) All equations must be balanced and show phases for full credit. Significant figures count, show charges as appropriate, and please box your answers!
Chapter 12 The Atom & Periodic Table- part 2
 Chapter 12 The Atom & Periodic Table- part 2 Electrons found outside the nucleus; negatively charged Protons found in the nucleus; positive charge equal in magnitude to the electron s negative charge Neutrons
Chapter 12 The Atom & Periodic Table- part 2 Electrons found outside the nucleus; negatively charged Protons found in the nucleus; positive charge equal in magnitude to the electron s negative charge Neutrons
65055 Basaltic Impact Melt grams
 65055 Basaltic Impact Melt 500.8 grams Figure 1: Photo of 65055 showing large zap pit. Cube is 1 cm. S72-43869. Introduction According to the Apollo 16 Catalog by Ryder and Norman, 65055 is an aluminous,
65055 Basaltic Impact Melt 500.8 grams Figure 1: Photo of 65055 showing large zap pit. Cube is 1 cm. S72-43869. Introduction According to the Apollo 16 Catalog by Ryder and Norman, 65055 is an aluminous,
DRAFT Coarse-fines 569 grams
 10085 Coarse-fines 569 grams DRAFT Figure 1: Selected coarse fines from 10085. Scale is in mm. Photo from Wood et al. 1969. Introduction!0085 and 10084 were created during the Apollo 11 preliminary examination
10085 Coarse-fines 569 grams DRAFT Figure 1: Selected coarse fines from 10085. Scale is in mm. Photo from Wood et al. 1969. Introduction!0085 and 10084 were created during the Apollo 11 preliminary examination
7) Applications of Nuclear Radiation in Science and Technique (1) Analytical applications (Radiometric titration)
 7) Applications of Nuclear Radiation in Science and Technique (1) (Radiometric titration) The radioactive material is indicator Precipitation reactions Complex formation reactions Principle of a precipitation
7) Applications of Nuclear Radiation in Science and Technique (1) (Radiometric titration) The radioactive material is indicator Precipitation reactions Complex formation reactions Principle of a precipitation
CHEM Come to the PASS workshop with your mock exam complete. During the workshop you can work with other students to review your work.
 It is most beneficial to you to write this mock midterm UNDER EXAM CONDITIONS. This means: Complete the midterm in 1.5 hours. Work on your own. Keep your notes and textbook closed. Attempt every question.
It is most beneficial to you to write this mock midterm UNDER EXAM CONDITIONS. This means: Complete the midterm in 1.5 hours. Work on your own. Keep your notes and textbook closed. Attempt every question.
M10/4/CHEMI/SPM/ENG/TZ1/XX+ CHEMISTRY. Wednesday 12 May 2010 (afternoon) 45 minutes INSTRUCTIONS TO CANDIDATES
 M10/4/EMI/SPM/ENG/TZ1/XX+ 22106110 EMISTRY standard level Paper 1 Wednesday 12 May 2010 (afternoon) 45 minutes INSTRUTIONS TO ANDIDATES Do not open this examination paper until instructed to do so. Answer
M10/4/EMI/SPM/ENG/TZ1/XX+ 22106110 EMISTRY standard level Paper 1 Wednesday 12 May 2010 (afternoon) 45 minutes INSTRUTIONS TO ANDIDATES Do not open this examination paper until instructed to do so. Answer
Lecture 31. Planetary Accretion the raw materials and the final compositions
 Lecture 31 Planetary Accretion the raw materials and the final compositions Reading this week: White Ch 11 (sections 11.1-11.4) Today 1. Boundary conditions for Planetary Accretion Growth and Differentiation
Lecture 31 Planetary Accretion the raw materials and the final compositions Reading this week: White Ch 11 (sections 11.1-11.4) Today 1. Boundary conditions for Planetary Accretion Growth and Differentiation
APPENDIX 7. QUALITY CONTROL AND QUALITY ASSURANCE (QA/QC) REPORT Virginia T. McLemore
 APPENDIX 7. QUALITY CONTROL AND QUALITY ASSURANCE (QA/QC) REPORT Virginia T. McLemore INTRODUCTION The samples and field data (including field observations and measurements) are the basic component of
APPENDIX 7. QUALITY CONTROL AND QUALITY ASSURANCE (QA/QC) REPORT Virginia T. McLemore INTRODUCTION The samples and field data (including field observations and measurements) are the basic component of
[YOUR NAME] [GROUP PARTNER NAMES IF APPLICABLE] [DATE] [COURSE ID] for [CLIENT NAME] [CLIENT CONTACT INFORMATION]
![[YOUR NAME] [GROUP PARTNER NAMES IF APPLICABLE] [DATE] [COURSE ID] for [CLIENT NAME] [CLIENT CONTACT INFORMATION] [YOUR NAME] [GROUP PARTNER NAMES IF APPLICABLE] [DATE] [COURSE ID] for [CLIENT NAME] [CLIENT CONTACT INFORMATION]](/thumbs/94/119688806.jpg) Department of Earth & Atmospheric Sciences Minerals Lab; Attn: Dr. Kackstaetter Campus Box 22, P.O.Box 173362 Denver, CO 80217-3362 [PROJECT TITLE] [Your title should summarize the purpose of the paper
Department of Earth & Atmospheric Sciences Minerals Lab; Attn: Dr. Kackstaetter Campus Box 22, P.O.Box 173362 Denver, CO 80217-3362 [PROJECT TITLE] [Your title should summarize the purpose of the paper
M10/4/CHEMI/SPM/ENG/TZ2/XX+ CHEMISTRY. Wednesday 12 May 2010 (afternoon) 45 minutes INSTRUCTIONS TO CANDIDATES
 M10/4/CHEMI/SPM/ENG/TZ/XX+ 106116 CHEMISTRY standard level Paper 1 Wednesday 1 May 010 (afternoon) 45 minutes INSTRUCTIONS TO CANDIDATES Do not open this examination paper until instructed to do so. Answer
M10/4/CHEMI/SPM/ENG/TZ/XX+ 106116 CHEMISTRY standard level Paper 1 Wednesday 1 May 010 (afternoon) 45 minutes INSTRUCTIONS TO CANDIDATES Do not open this examination paper until instructed to do so. Answer
8. Relax and do well.
 CHEM 1014 Exam I John I. Gelder September 16, 1999 Name TA's Name Lab Section Please sign your name below to give permission to post your course scores on homework, laboratories and exams. If you do not
CHEM 1014 Exam I John I. Gelder September 16, 1999 Name TA's Name Lab Section Please sign your name below to give permission to post your course scores on homework, laboratories and exams. If you do not
GSA DATA REPOSITORY
 GSA DATA REPOSITORY 2012161 Allan et al. SUPPLEMENTARY INFORMATION Summary of Magma Types Table DR1 summarizes some of the key petrologic, geochemical and physical characteristics of the three magma types
GSA DATA REPOSITORY 2012161 Allan et al. SUPPLEMENTARY INFORMATION Summary of Magma Types Table DR1 summarizes some of the key petrologic, geochemical and physical characteristics of the three magma types
8. Relax and do well.
 CHEM 1314.03 Exam I John I. Gelder September 25, 1997 Name TA's Name Lab Section Please sign your name below to give permission to post, by the last 4 digits of your student I.D. number, your course scores
CHEM 1314.03 Exam I John I. Gelder September 25, 1997 Name TA's Name Lab Section Please sign your name below to give permission to post, by the last 4 digits of your student I.D. number, your course scores
REGOLITH GEOCHEMISTRY OF THE NORTH KIMBERLEY, WESTERN AUSTRALIA: A STRONG PROXY FOR BEDROCK
 REGOLITH GEOCHEMISTRY OF THE NORTH KIMBERLEY, WESTERN AUSTRALIA: A STRONG PROXY FOR BEDROCK Paul A. Morris 1 1 Geological Survey of Western Australia, 100 Plain Street, East Perth 6004, Western Australia;
REGOLITH GEOCHEMISTRY OF THE NORTH KIMBERLEY, WESTERN AUSTRALIA: A STRONG PROXY FOR BEDROCK Paul A. Morris 1 1 Geological Survey of Western Australia, 100 Plain Street, East Perth 6004, Western Australia;
Guide to the Extended Step-Pyramid Periodic Table
 Guide to the Extended Step-Pyramid Periodic Table William B. Jensen Department of Chemistry University of Cincinnati Cincinnati, OH 452201-0172 The extended step-pyramid table recognizes that elements
Guide to the Extended Step-Pyramid Periodic Table William B. Jensen Department of Chemistry University of Cincinnati Cincinnati, OH 452201-0172 The extended step-pyramid table recognizes that elements
The Credo and Fenbark meteorites, new finds of common chondrites from north-west of Kalgoorlie, Western Australia
 MINERALOGICAL MAGAZINE, JUNE 1969, VOL, 37, NO. 286 The Credo and Fenbark meteorites, new finds of common chondrites from north-west of Kalgoorlie, Western Australia G. J. H. McCALL Reader in Geology,
MINERALOGICAL MAGAZINE, JUNE 1969, VOL, 37, NO. 286 The Credo and Fenbark meteorites, new finds of common chondrites from north-west of Kalgoorlie, Western Australia G. J. H. McCALL Reader in Geology,
7. Relax and do well.
 CHEM 1215 Exam II John II. Gelder October 7, 1998 Name TA's Name Lab Section INSTRUCTIONS: 1. This examination consists of a total of 5 different pages. The last page includes a periodic table and a solubility
CHEM 1215 Exam II John II. Gelder October 7, 1998 Name TA's Name Lab Section INSTRUCTIONS: 1. This examination consists of a total of 5 different pages. The last page includes a periodic table and a solubility
Table 1: Summary of petrographic characteristics for porphyritic samples from Campbell and Chatham islands, New Zealand phenocrysts sample location
 Table 1: Summary of petrographic characteristics for porphyritic samples from Campbell and Chatham islands, New Zealand phenocrysts sample location deposit type rock type age/unit >5%
Table 1: Summary of petrographic characteristics for porphyritic samples from Campbell and Chatham islands, New Zealand phenocrysts sample location deposit type rock type age/unit >5%
CHEM 171 EXAMINATION 1. October 9, Dr. Kimberly M. Broekemeier. NAME: Key
 CHEM 171 EXAMINATION 1 October 9, 008 Dr. Kimberly M. Broekemeier NAME: Key I A II A III B IV B V B VI B VII B VIII I B II B III A IV A V A VI A VII A inert gase s 1 H 1.008 Li.941 11 Na.98 19 K 9.10 7
CHEM 171 EXAMINATION 1 October 9, 008 Dr. Kimberly M. Broekemeier NAME: Key I A II A III B IV B V B VI B VII B VIII I B II B III A IV A V A VI A VII A inert gase s 1 H 1.008 Li.941 11 Na.98 19 K 9.10 7
SCIENCE 1206 UNIT 2 CHEMISTRY. September 2017 November 2017
 SCIENCE 1206 UNIT 2 CHEMISTRY September 2017 November 2017 UNIT OUTLINE 1. Review of Grade 9 Terms & the Periodic Table Bohr diagrams Evidence for chemical reactions Chemical Tests 2. Naming & writing
SCIENCE 1206 UNIT 2 CHEMISTRY September 2017 November 2017 UNIT OUTLINE 1. Review of Grade 9 Terms & the Periodic Table Bohr diagrams Evidence for chemical reactions Chemical Tests 2. Naming & writing
Crystal structure control of the dissolution of rare earth elements in water-mineral interactions
 Geochemical Journal, Vol. 40, pp. 437 to 446, 2006 Crystal structure control of the dissolution of rare earth elements in water-mineral interactions SHIN-NOSUKE SHIBATA,* TSUYOSHI TANAKA and KOSHI YAMAMOTO
Geochemical Journal, Vol. 40, pp. 437 to 446, 2006 Crystal structure control of the dissolution of rare earth elements in water-mineral interactions SHIN-NOSUKE SHIBATA,* TSUYOSHI TANAKA and KOSHI YAMAMOTO
LAACHER SEE REVISITED: HIGH SPATIAL RESOLUTION ZIRCON DATING IMPLIES RAPID FORMATION OF A ZONED MAGMA CHAMBER -
 LAACHER SEE REVISITED: HIGH SPATIAL RESOLUTION ZIRCON DATING IMPLIES RAPID FORMATION OF A ZONED MAGMA CHAMBER - DATA REPOSITORY ANALYTICAL PROCEDURES Ion microprobe U-Th measurements Th-U dating was performed
LAACHER SEE REVISITED: HIGH SPATIAL RESOLUTION ZIRCON DATING IMPLIES RAPID FORMATION OF A ZONED MAGMA CHAMBER - DATA REPOSITORY ANALYTICAL PROCEDURES Ion microprobe U-Th measurements Th-U dating was performed
