Chapter 7. Studies on GAP Based Propellant Formulations, Cure Kinetics and Rheology
|
|
|
- Ambrose Hoover
- 5 years ago
- Views:
Transcription
1 Chapter 7 Studies on GAP Based Propellant Formulations, Cure Kinetics and Rheology 7.1 Introduction The search for more energetic and eco-friendly propellants has lead to studies on large number of compounds like GAP, POLYGLYN, BAMO, AMMO, ADN, HNF and CL The important considerations in the development of new formulations include higher performance parameters like density specific impulse, minimum signature, eco-friendliness and low friction and impact sensitivity. The cost involved and reliability are also important. The operational solid motors widely use HTPB/AP/Al based propellant, which has reached a saturation level in terms of the above mentioned requirements. The high content of HCl in the exhaust of AP based propellant is considered as a major concern. 4-7 New generation propellant formulations with energetic binders like GAP and oxidiser systems like ADN, HNF and CL-20 show lot of promise in this respect. 8 GAP has been reported to be compatible with high energy oxidisers. 9 GAP based propellant system and its characteristics have been dealt with in literature Ballistic properties of GAP based energetic composites involving NC/NG, HMX and AP has been presented by 13, 14 Kubota et.al. A comparison of GAP based propellant with that of HTPB for gun propellant application has been presented by Schedlbauer. 15 GAP/AN propellant system have been studied for chemical stability, combustion behaviour and sensitivity by Menke et.al. 16 GAP crosslinked with TDI and IPDI has been evaluated as integrated ram rocket propellants by Sahu et.al. 17 Studies on the pyrolysis of GAP/RDX/BTTN propellant formulation have been presented by Ross et.al. 18 Investigation of the GAP based propellant for gas generator application has been presented by Helmy
2 In this study, evaluation of theoretical performance parameters like specific impulse and density impulse of various GAP based propellant formulations and propellant characteristics like burn rate, mechanical properties and rheological behaviour were carried out. Rheological evaluation of the binder and propellant slurry are important steps for assessing the processability of the propellant system. 20 The rheological behaviour of the polymeric binder and that of uncured propellant slurry is to be understood to optimise the process parameters like mixing time, mixing temperature, rate of casting or slurry feed rate and pot life of propellant. Realisation of defect free propellant grain depends on thorough understanding of the nature and effect of rheological behaviour of propellant binder and slurry. For the binder, the viscosity depends largely on the molecular weight, temperature and rate of shear. The relationship between molecular weight and viscosity was given by Bueche, 21 which was later modified by a number of interpretations. The effect of temperature on viscosity was first enunciated by Andrade. 22 Study of rheological characteristics of the system could help in the selection of the processing technique for a particular propellant system. 23, 24 Rheology of highly solid loaded system is influenced by a number of factors like particle size, size distribution and shape of solid additives, rate of shear, binder viscosity, rate of cure reaction and process temperature. A large number of studies were presented by many authors on the effect of various parameters on rheology of suspensions with low solid loading Study on the kinetics of polymer network formation could throw light on the rheological characteristics. 29 Reproducibility of ballistic and mechanical properties of propellant requires uniform distribution of solid additives in the matrix. This is possible only if the propellant slurry has controlled rheological characteristics and also good processability. Rheological characterisation could help to understand the flow pattern of the propellant slurry, measurement techniques required, effect of compositional 210
3 variables, effect of process variables and requirement of processing techniques. Propellant flow characteristics change as the cure reaction proceeds. Though a large volume of studies on the synthesis, characterisation and energetic formulations based on GAP are available in the literature, data on rheological evaluation of GAP and GAP based formulations are scarce. Hence, there is scope to study the rheological characteristics of GAP. In this study, first the effect of plasticiser content on the viscosity of GAP was evaluated. Different types of plasticisers were evaluated for this purpose. The viscosity build up of GAP resin due to curing reaction with different diisocyanates was evaluated at different temperatures. GAP based propellant system was studied to understand the effect of different variables on the rheological behaviour of the system. A selected formulation of GAP-HTPB blend based propellant was also evaluated for this purpose. Chapter 7 comprises of two parts viz Part I and Part II. Part I Studies on GAP Based Propellant Formulations 7.2 Theoretical performance evaluation of GAP based propellant formulations Theoretical computations were carried out with the help of computer codes to evaluate various combinations of energetic ingredients to arrive at an optimum result. NASA-SP is one of the most widely used programme code for the purpose. The inputs required for the evaluation of performance parameters by NASA-SP-273 include concentration of oxidiser, binder, metallic fuel, molecular formulae of the constituents and heat of formation. For the purpose of comparison of various propellant formulations, the evaluation was done for standard conditions of rocket operation at 70 ksc pressure with isentropic expansion to 1 atmospheric pressure and vacuum conditions. The 211
4 expansion of the combustion products are assumed to be at equilibrium and a nozzle expansion ratio of 10 was employed for the study. The theoretical computations were carried out with different formulations. Figure 7.1 shows the results of evaluation of various combinations of high energy oxidisers with GAP. Figure 7.1 Effect of the solid loading on specific impulse of GAP based propellant formulations with 18% Al. Comparison of data shows that, HTPB based compositions with AP and Al (18%) could contribute to a peak specific impulse (Isp) value of 265 seconds with 86% solid loading. Further increase in solid loading was found to have no influence on Isp. GAP with ADN and Al with solid loading of 82% was found to provide a peak Isp of 275 seconds which indicate a good performance characteristic. The near plateau region observed in the Isp profile in the range of 76 to 86% solid loading indicate an important advantage in terms of flexibility to tailor the propellant formulation to meet processing requirement of propellant. GAP with HNF and Al was found to provide highest Isp value of 280 seconds at 84% solid loading. However, incompatibility of HNF with diisocyanate curatives and particle shape of 212
5 HNF are difficult problems for propellant formulations. The relative low value of Isp (260 seconds) seen for GAP with CL-20 and Al at 86% solid loading could be due to the comparatively low oxygen balance of (11%) of CL-20. The comparison shows that GAP with advanced oxidiser systems could provide significant improvement in performance parameters compared to conventional propellants. For the purpose of comparison with conventional propellant formulations, the specific impulse of GAP-AP propellant was also determined for both aluminised and non aluminised formulations. Figure 7.2 shows the results of theoretical computations. The data show that the specific impulse of aluminised propellant is higher than that of non aluminised propellant for both sea level and vacuum conditions. Figure 7.2 Comparison of Isp of GAP-AP propellant with and without Al 213
6 The higher Isp of aluminised propellant results from the higher energy out put from the combustion of aluminium. It is also noted that for all the formulations, the peak Isp is observed in the range of 75 to 80% solid loading. The peak vacuum Isp value of 291 s was observed for aluminised GAP-AP formulation Vacuum specific impulse of GAP based propellant formulations The vacuum specific impulse of GAP based propellant formulations with advanced oxidisers was also estimated for comparison. Figure 7.3 shows the vacuum Isp of GAP based propellant with advanced oxidiser systems in comparison with HTPB based propellant. The vacuum Isp values are also found to be significantly higher than that of conventional propellant formulations based on HTPB and AP. Figure 7.3 Comparison of vacuum Isp of GAP and HTPB based propellants Density impulse of GAP based propellant formulations Density specific impulse is the product of density and specific impulse of propellant. Figure 7.4 shows comparison of density specific impulse of different 214
7 propellant formulations with that of HTPB-AP based systems with different solid loadings. Figure 7.4 Comparison of density Isp of GAP and HTPB based formulations Density specific impulse is an important parameter for comparison of different propellants in terms of their volume limited performance capability. From the data it is seen that GAP with CL-20 shows maximum density specific impulse of 525 s g/cc at 90% solid loading. The higher density Isp is the result of higher density and energetics of CL-20 compared to HNF or ADN. However, such a high solid loaded formulation is difficult to work with. The GAP and advanced oxidiser based propellant compositions with peak performance characteristics are shown in table 7.1. With Al content of 14%, CL-20/Al/GAP based propellant could provide a density Isp of 545 s g/cc. However, a total solid loading of 90% could pose difficulty in propellant processing. 215
8 Table 7.1 Peak performance characteristics of propellants based on GAP with ADN, HNF and CL-20 Propellant ADN/Al/GAP HNF/Al/GAP CL-20/Al/GAP Composition 64/18/18 60/18/16 76/14/10 Peak Isp (s) Peak Vac.Isp (s) Peak density Isp (s g/cc) Density (g/cc) Flame temp (K) Molecular weight of products Evaluation of propellant formulations Composite propellant formulations with GAP as binder were prepared for the study. AP was used as the oxidiser in all the propellant formulations. The propellant formulations were selected based on the optimum performance characteristics arrived at from the theoretical performance evaluation and also based on the gumstock property evaluations done as explained in chapter 5. NCO/OH ratio of unity and crosslinker content of 5% with respect to binder content was selected for all the trials. The propellant formulations were prepared in such a way as to process defect free specimens for evaluation of mechanical and ballistic properties. In order to study the effect of particle size of AP on the properties of the propellant, propellant mix with combination of coarse and fine AP and with fine AP alone were prepared. A solid loading of 75% was utilised for the study. Propellants with optimum aluminium content of 18% and with low aluminium content of 2% were also prepared for the study. In order to modify the slurry flow characteristics, DOA was used as the plasticiser. DOA content of 15% with respect to binder content was used in the propellant formulations. 216
9 7.3.1 Propellant experimentations Materials GAP resin with molecular weight 2000 (by VPO) and hydroxyl value of 45 mg KOH/g was used for the propellant processing. The crosslinking agent used was a 2:1 mix of TMP and butane diol, mixed and dried for extended periods under vacuum to remove moisture to the extent of 0.1%. TDI with purity higher than 99% as available from commercial sources was used as the curing agent. AP with purity higher than 99% produced in VSSC was used. Two grades of AP namely coarse and fine grades with average particle size of 300 µ and 40 µ respectively were used for the study. Aluminium powder (of average particle size µ) with purity higher than 99%, as available from commercial sources was used. Copper chromite available from commercial source was used. Curing catalyst used for the study was prepared by mixing DBTDL in toluene with a volumetric ratio of 1: Equipments Propellant processing was carried out using a small scale horizontal sigma blade mixer. A photograph of the sigma mixer used is shown in figure7.5. The mixer is equipped with hydraulic arrangement for tilting the mixer bowl for loading and unloading and all safety measures to carry out safe processing of propellant. 217
10 Figure 7.5 Horizontal sigma mixer used for propellant mixing The propellant specimens were prepared by casting the propellant slurry using vacuum casting set up. The vacuum casting set up consists of a hopper and casting chamber which can be evacuated to a vacuum level of 5-10 mm Hg. A photograph of the vacuum casting set up is shown in figure7.6. Figure7.6 Vacuum casting set up used for propellant processing 218
11 The propellant slurry was fed into the hopper of the vacuum casting set up. The propellant is slowly fed through the valve into an evacuated container. After the casting was completed, the propellant was kept in an air oven and cured at 60 0 C for 15 days. Mechanical properties of the propellant samples were determined using Instron testing machine. The dumbbell specimens were prepared as mentioned in section 2.4 in chapter 2. The burn rate measurements were done for the propellant samples sliced from the cured block. The measurements were done using the acoustic method as mentioned in section 2.9 at different pressures, namely 20, 40 and 60 ksc Propellant processing GAP based propellant slurry was prepared with TDI as curing agents for the study. AP with coarse to fine ratio of 2:1 was selected for the study. The estimation of stochiometric quantities of resin and curing agent for preparing the propellant formulations were done as shown below. (i) Hydroxyl number + Acid number of binder (ii) Hydroxyl number of crosslinking agent (iii) Purity of diisocyanate (TDI) (iv) Purity of TDI, NCO/OH equivalent ratio (v) Quantity of resin (vi) = x = y = C = R = D gm (vii) Quantity of crosslinker = E gm (1:2 by weight ratio of 1,4-butanediol and 1,1,1- trimethylol propane) 219
12 (7.3) x) TDI required for D gm of resin = D x A x R 561 C 87 = F (7.4) 8.16 (7.5) xii) Catalyst added ( DBTDL in toluene solution = H gm (0.326% by weight of binder). Weight percentage of the ingredients for a typical propellant formulation prepared is shown in table 7.2. Table 7.2 Typical propellant formulation prepared for the study Propellant ingredient Materials Percentage by weight (%) Binder composition NCO/OH ratio =1 GAP DOA Crosslinker TDI Catalyst Oxidiser AP (coarse) AP fine Metal fuel Aluminium
13 For processing the slurry, first GAP resin was mixed with DOA and charged into the mixer. This was followed with addition of crosslinking agent. Aluminium powder was added next and mixed. The oxidiser was added in small lots and mixed thoroughly. In one formulation, AP fine alone was used. Mixing of AP was followed by addition and mixing of curing agent. Finally DBTDL in toluene solution was added and mixed. The mixing schedule followed for the propellant slurry was as shown in table 7.3. A process temperature of 50 0 C was maintained during mixing. Table 7.3 Mixing schedule followed for preparation of propellant Propellant ingredient Mixing time (min) Premix (GAP+DOA+Crosslinker) 5 Aluminium powder 5 ½ AP coarse 5 ½ AP coarse 5 ½ AP fine 5 ½ AP fine 5 Mixing 30 TDI 20 Catalyst 20 Total 100 The mix was evacuated and then fed into a vacuum casting setup. The propellant was vacuum cast at room temperature and cured at 60 0 C for 15 days. Samples were machined out from the cured propellant for evaluation of mechanical and ballistic properties. The effect of AP content in the propellant on the properties was evaluated by preparing formulations with different grades of AP. In the formulations prepared with fine grade AP, low aluminium content of 2% was used. Propellant formulation with copper chromite as burn rate modifier and formulations 221
14 with variable GAP resin content were also prepared and evaluated. Samples were cut from the cured propellant and evaluated for mechanical and burn rate properties Results and discussion The mechanical properties determined for propellant samples prepared with different formulations are shown in table 7.4. Table 7.4 Mechanical properties of different GAP based propellant formulations Properties Tensile strength(ksc) Elongation (%) Modulus (ksc) Shore A hardness Solid loading -75% AP content - 57% AP coarse to fine ratio - 2:1 Al content - 18% Propellant formulation Solid loading -70% AP content - 68% AP fine only Al content - 2% Solid loading -70% AP content - 67% AP fine only Al content - 2% C C content -1% The burning rate of the propellant samples were evaluated at different pressures as mentioned in section 2.9 in chapter 2. From the data generated, the burn rate law was derived for specific propellant formulations. For GAP propellant with 18% aluminium content, the burn rate was found to increase with increasing pressure 13, 17 as expected for composite propellants. Table 7.5 shows the burn rate data generated for aluminised GAP propellant at three different pressures. 222
15 Table 7.5 Burn rate data generated for aluminised GAP propellant Propellant formulation Burn rate at different pressures (mm s -1 ) 30 ksc 40 ksc 60 ksc GAP -17 % Solid loading -75% AP 57 % coarse to fine ratio 2:1 Al - 18% The burn rate law was determined from a logarithmic plot of burn rate versus pressure. The burn rate law for the aluminised propellant derived from the plot (figure 7.7) can be represented as shown in equation 7.6 Where r is the burn rate in mm s -1 and P is the pressure in ksc. Figure 7.7 Logarithmic plot of burn rate vs pressure 223
16 The effect of GAP content on the burn rate of low aluminised GAP-AP propellant was evaluated. The GAP resin content was varied from 18.3 to 26.3% by weight in the experiments. AP content was varied from 62 to 70% with particle size of 40 µ, Aluminum content of 2% and copper chromite content of 1% were used for the study. The burn rate was determined at 70 ksc pressure. Table 7.6 shows the variation of burn rate with GAP resin content in the propellant. The study showed that high burn rate of 29.4 mm s -1 at 70 ksc pressure could be achieved for GAP propellant with a solid loading of 67%. Table 7.6 Effect of GAP resin content on burn rate of propellant GAP content AP content Solid Burn rate ( %) (%) loading (%) at 70 ksc (mm s -1 ) Studies on the ignitability of GAP based propellant formulation Ignitability of GAP based propellant formulations have been studied and reported for advanced applications such as microthruster propellants In this study, different GAP based propellant formulations were evaluated for ignitability using low power nichrome wire based ignition systems. 7.5 Propellant system for microthrusters The propellant system that can be used for this application should meet specific requirements. The important factors are 224
17 i. The propellant should be energetic enough to meet the mission needs. ii. Propellant should be processable and it should form defect free grain when filled and cured inside the cavity. iii. It should have low ignition temperature. iv. The flame temperature should not be too high. v. It should be stable in the space environments for long time of storage. vi. Above all, it should be safe and should function reliably. Different types of propellant charges including pyrotechnic materials like sodium azide, lead styphanate and composite propellant based on GAP and ammonium perchlorate have been reported in the literature for micro thrusters. Since the GAP based composite propellant was found to meet almost all the requirements satisfactorily, it was considered as the primary choice Experimental GAP polymer contains energetic azide group in the molecule, which increase the gaseous content of combustion products during thermal decomposition. GAP can also perform as a monopropellant without any oxidiser. Also, the flame temperature of GAP based propellant is comparatively lower than many other composite solid propellants. In the trials carried out, GAP based propellant system was used. Many propellant trials were carried out for GAP based formulations with different oxidiser combinations. The AP propellant was made by mixing GAP with other ingredients and curing it after filling it in the microthruster cavities. For curing, the resin was mixed with crosslinker and curing agent composition. The oxidisers used for the trials included AP and Potassium Nitrate. In order to minimise the chlorine compounds and other corrosive elements in the combustion products, the oxidiser content was minimised. 225
18 Materials The sources of GAP, crosslinker, TDI, AP, copper chromite and catalyst used were same as mentioned in section Fine grade of AP of average particle size 40 µ was used for the study. Potassium nitrate with average particle size 40 µ as available from commercial source was also used as oxidizer Sample preparation For preparing the propellant, GAP resin was first vacuum dried. Resin was then mixed with 5% crosslinker. The oxidiser was dispersed thoroughly by hand mixing. A small quantity (0.05%) of copper chromite was also mixed. TDI was added as curing agent. After curator addition, 2 mg of catalyst was added and mixed thoroughly. The mix was then degassed and then filled into the microthruster cavities using a nitrogen jet. Filling was done repeatedly to ensure that the cavities are fully filled with propellant. The assembly was then placed inside a hot air oven at 60 0 C for 48 hrs for curing. The micro thruster array was prepared by drilling 2 mm size cavities at 2 mm apart on a hylam substrate of size 50 x 50 cm 2. A nichrome wire based ignition system was developed. The nichrome wire was soldered on to Cu strips, which are adhesively bonded on both sides of the microthruster array. The nichrome wire was placed across the thruster cavity. Figure 7.8 shows the thruster arrays along with electrical contact for ignition system. 226
19 Figure 7.8 Micro thruster assembly prepared with 2 mm size thrusters Testing A novel ignition system was developed to ignite the propellant by using a nichrome wire segment as a heat source on the surface of the cured propellant. While propellant filling, it was made sure that the nichrome wire and propellant are in contact. The nichrome wire bridge was tested using current supplied from a 6 Volt battery. In the ignition circuit, electrical power supply was established by connecting the Cu strips to 6 Volt battery through contact switches. Each thruster was provided with independent ignition system. The electrical contact was designed to supply the necessary electrical power independently to each thruster Results and discussion The independent ignition and sustained burning of the individual thruster was observed visually. The details of the propellant formulation tested and observations made are presented in table 7.7. After the trials, further tests were carried out with an optimised AP content of 5%. In all the trials carried out with AP, ignition and the 227
20 sustained burning of the propellant could be seen without affecting the adjacent thrusters. Table 7.7 Test results of the GAP based propellant formulations Propellant formulation Observation during test Post test observation Cured GAP without AP and Cu Cr 2 O 4 Bridge wire fired. Propellant not ignited GAP with 2% KN and 0.05% Cu Cr 2 O 7 Igniter fired. No firing of propellant GAP with 0.05% Cu Cr 2 O 4 GAP with 2% KN and 0.05% Cu Cr 2 O 4 GAP with 2% AP and 0.05% Cu Cr 2 O 4 GAP with 5% AP and 0.05% Cu Cr 2 O 4 GAP with 10% AP and 0.05% Cu Cr 2 O 4 Igniter fired. No firing of propellant Igniter fired. No firing of propellant Ignition of propellant and sustained combustion seen. Burning of propellant seen. Jet seen clearly. Smoke was seen in large proportion. Ignition of propellant and sustained combustion seen. Smoke seen. Propellant is intact after test. Igniter bridge wire broken. Propellant is not burned. Propellant is not burned. Propellant is not burned. Propellant is consumed and charring of the cavity observed. Propellant is consumed and charring of the cavity seen. Propellant is consumed and charring of the cavity noted. 7.6 Conclusion Theoretical performance evaluation of GAP with different oxidiser systems was carried out. Evaluation of the effect of solid loading on the performance showed that formulation of GAP with HNF and aluminium with a solid loading of 78% 228
21 could provide a peak specific impulse of 280 s. For aluminised GAP-HNF propellant a peak vacuum Isp of 305 s was observed at a solid loading of 78%. The vacuum Isp of GAP based propellant with ADN or HNF was found to be much higher that that of GAP-AP propellant. Highest density impulse of the order of 545 s g/cc was observed for GAP CL-20 based propellant. The study showed that GAP with advanced oxidisers can significantly improve the performance capability of the propellant. As part of the study, GAP based propellant was processed and evaluated by conventional means. The mechanical properties were evaluated for different GAP based propellant formulations. The test results showed good mechanical properties for the cured propellant. Burn rate evaluation showed that, high burn rate of the order of 29.4 mm s -1 could be achieved for aluminized GAP-AP propellant with 70% solid loading. Studies carried out for ignitability of GAP showed that GAP with 5 weight% fine grade AP can be satisfactorily used for advanced propulsion application such as microthruster systems. Part II Studies on Rheology and Cure Kinetics of GAP and GAP based Propellant 7.7 Basic concepts of rheological evaluation The fundamental concepts of study of rheology include definition of flow field under consideration, rheological model which describes the system under study and experimental techniques for determination of rheological properties. A rheological model establishes the relationship between shear stress, shear rate and 229
22 shear strain. For Newtonian fluids, the shear stress-shear rate relationship is given by the equation 7.7. Where τ is shear stress, ηis the viscosity and du/dy is the rate of shear. In this expression, the viscosity is a constant. Majority of the non-newtonian fluids which include polymeric fluids fall under the category of pseudo plastic materials. The shear stress versus shear rate relationship in such cases is given by the equation 7.8. Where τ is the shear stress, K is consistency index in N s 2 M -2 and ν is the dimension less pseudo plasticity index. The fluid viscosity is given by the equation 7.9. Where η is the viscosity with units Pa.s. These expressions describe non-newtonian fluids which follow the Power law. Proper selection of values of K and ν helps to predict the flow pattern of Power law fluids. Detailed theoretical treatment of applications of flow theory to polymer processing has been presented by number of authors Viscosity data of the polymer compounds is an important input for the rheological characterisation. A variety of viscometers are used for determination of viscosity of fluids. 37 The different types include rotational viscometers, capillary 230
23 viscometer, orifice viscometer, rising bubble viscometer, pantographs and plasticorders. Torque rheometers are used to test thermoplastic and thermosetting elastomers. Composite propellant slurry is a colloidal suspension of crystalline solids in a polymeric binder. The shear stress versus shear rate curves of the slurry show a hysterisis pattern. Studies have shown that, flow pattern of propellant slurry inside the motor case during casting process could influence the orientation of the oxidiser particles due to the shearing action generated, finally leading to a non uniform burn front propagation. 38 Relationship between flow along mandrel and walls, velocity gradient and pattern generated inside motor case due to free fall of propellant paste have been reported in literature. 39 Propellant slurry with 86% solid loading has shown to follow Herschel Bulkley 40, 41 type rheological equation. The equation is given by Where τ 0 is the yield stress and ν is the pseudo plasticity index. The hysterisis shown by the shear stress versus shear rate curve of the slurry is due to the thixotropic nature of the propellant slurry. The area under the hysterisis loop represents the energy loss in destroying the structure of the system and is called the thixotropic index. As the curing reaction progresses, the yield stress, consistence index and thixotropic index increases. The yield stress denotes the shear stress required to overcome the resistance offered by the slurry to flow. 42 Once the yield stress is overcome, the reduction in apparent viscosity of the slurry results as the particles and polymer molecules orient in the direction of applied stress. This leads to the pseudo plastic behaviour of the slurry. The thixotropicity or time dependant 231
24 behaviour of the propellant slurry results from the hindrance offered to the process of orientation of filler particles at higher filler loading due to inter particle interaction. Propellant processability is strongly influenced by temperature. Temperature increase accelerates the cure reaction, viscosity, yield stress and thixotropic index. Initially, the fluidity is increased by temperature leading to a decrease in the viscosity. With increase in rate of curing reaction, the fluidity decreases due to polymer network formation resulting in the increase in viscosity, yield stress and thixotropy. It is desirable for propellant slurry to have minimum yield stress, minimum viscosity and minimum thixotropicity for good processability. The processing temperature for the propellant slurry is optimised with these factors in consideration. From the time required for rheological parameters to increase from an initial value to a particular limit after curing agent addition, it is possible to have an idea of the kinetics of curing reaction. 43, 44 One method is to consider that the rate of reaction is proportional to the reciprocal of time required to double the viscosity or yield stress. From this consideration, the activation energy of the rate process can be deduced using the modified Arrhenius relationship as shown below. Using this expression a plot of ln (1/t) vs (1/T) can be made and from the slope of the straight line, the activation energy can be determined. Where t is the time to double the viscosity or yield stress, A is the preexponential factor, E is the activation energy, T is the absolute temperature and R is the universal gas constant. 7.8 Effect of plasticiser content on the rheological behaviour of GAP The use of plasticiser in polymer systems has been dealt with in detail by Sears et.al. 45 Usual solid propellant formulations contain around 3 to 5% of 232
25 plasticiser to enhance the processability. 46 A detailed account of compatibility studies carried out for GAP in terms of mechanical properties with ester and hydrocarbon type plasticiser systems are provided in chapter 5. Studies were also carried out with energetic plasticiser systems. It was found that, ester type plasticisers like dioctyladipate (DOA) and dioctylphthalate (DOP) are compatible with GAP. The effect of DOA, DOP and different energetic azido plasticiser systems like 1,6-hexanediol bis (azidoacetate) (HDBAA), 2-ethyl-1,3-hexanediol bis (azidoacetate) (EHDBAA) and diethylene glycol bis(azidoacetate) (DEGBAA) were evaluated for this purpose Experimental The effect of concentration of plasticiser on the viscosity of GAP resin was evaluated by preparing mixes of GAP with varying concentration of DOA, DOP and the three azido plasticisers. The plasticiser concentrations used for the study were 0, 10, 20, 30, 40 and 50 parts per 100 parts of GAP. The viscosity of the mix of each of the formulations was evaluated at 30 0 C. The shear rate employed for the measurement was varied over a wide range Materials The source of GAP used for the study was as mentioned in section DOA and DOP, as available from commercial sources were used for the study. The azido plasticisers, HDBAA, EHDBAA and DEGBAA were synthesised in VSSC Instrumental All the measurements were carried out using Brookfield viscometer as mentioned in section 2.6. For resin-plasticiser combination, disc spindle no. 4 was used. The spindle rpm was varied in the range from 0.5 to 100 rpm for varying the 233
26 shear rate. The shear stress and shear rate were estimated from the machine parameters, geometrical parameters of the spindle, dial reading and viscosity Results and discussion An expected trend of reduction in viscosity was noted for GAP with increase in plasticiser content. The reduction in the viscosity of GAP with ester type plasticiser content is shown in figure 7.9 and reduction in viscosity with the three azido plasticisers is shown in figure A constant shear rate of 100 s -1 was followed for the experiments. Figure 7.9 Effect of DOA and DOP content on viscosity of GAP resin 234
27 Figure 7.10 Effect of azido plasticisers on viscosity of GAP resin It was observed that the rate of reduction in viscosity decreases as the plasticiser concentration exceeds 25% in the case of all the plasticisers. This could be due to limited miscibility arising out of change over to secondary plasticiser system. 45 The viscosity evaluation showed that, the azido plasticisers are in general far superior in modifying the flow characteristics of GAP. The observation of better compatibility of azido plasticisers could be explained from the fact that the presence of polar azido groups in both the systems lead to better chemical and thermodynamic feasibility of mixing. From the figure 7.10, it can be noted that for a 50% concentration of azido plasticiser HDBAA, the viscosity of GAP was reduced to nearly 6.3% of initial value. From the rheological measurements, the shear rate versus shear stress relationship of GAP and GAP plasticised with DOA were evaluated. The shear stress and shear rate was estimated from the instrument parameters and viscosity values measured. Figure 7.11 shows a plot of shear rate versus shear stress relationship for GAP and GAP with DOA content of 10 and 20%. 235
28 Figure 7.11 Shear stress vs shear rate relationship for GAP and GAP with DOA The shear stress versus shear rate relationship shows a good linear relationship indicating the neat and plasticised resin follow behaviour close to Newtonian. 7.9 Studies on curing of GAP by viscometry and IR spectroscopy Viscosity build up data of the polymer in the pre-gel stage could be used as a valuable input to study the kinetics of the process and the temperature effect. 47 The chemical nature of the curatives, temperature and presence of crosslinker and catalyst have strong influence on the viscosity build up of the network. In this study, the curing reaction of GAP-TDI system was evaluated by viscometry. The effect of temperature on the viscosity build up and kinetic parameters of the process were evaluated. The cure reaction of GAP was also followed by IR spectroscopy for comparison. Three different curing agents viz, TDI, IPDI and MDCI were employed. The influence of crosslinker and catalyst on the viscosity build up of GAP due to cure reaction was also studied. The concentrations of crosslinker and catalyst were 236
29 selected based on the gum-stock property evaluation carried out as mentioned in chapter 5. The study was done for GAP-TDI and GAP-IPDI systems Experimental Materials The sources of GAP, TDI, IPDI, MDCI, crosslinker and catalyst used for the study were same as mentioned in section Instrumental Viscosity during the cure reaction was measured using Brookfield viscometer model RVDV II +. The curing mixture was degassed before charging into the sample cell. The sample cell used was a small sample adapter of 10 ml capacity and the spindle used was S-21. The sample adapter was placed in a constant temperature hot water circulation bath. Viscosity of the curing polymer was measured at various intervals. The IR spectroscopy was done using Nicolet 510 P model FTIR spectroscope. The intensity of the peaks in the spectra was derived using a built in software available with the FTIR spectroscope. The samples were kept inside a thermostat heated by an IR lamp for obtaining isothermal conditions Testing For kinetic study, GAP resin was mixed with the curing agents and then evacuated for 10 minutes before filling into the viscometer cup. The viscosity measurement was done at four different temperatures viz 30, 40, 50 and 60 0 C. The viscosity data was recorded at regular intervals of time for 5 to 6 hours. The IR spectra of the samples were recorded by using a drop of the sample from the mix prepared as mentioned earlier. The samples were smeared between the 237
30 NaCl cells as a thin film for the measurement. The IR spectra were recorded at 30, 40, 50 and 60 0 C at regular intervals of time for 5 to 6 hours for the study. For viscosity build up study of GAP, crosslinker and catalyst combination, the samples were made by mixing GAP first with cross linking agent followed by curing agent. After that, the catalyst was added, mixed and evacuated for 10 minutes. The mix was then filled into the viscometer cup for viscosity measurement. The measurement was done at 30, 45 and 60 0 C Results and discussion The rate of viscosity build up of GAP was found to be comparatively lower than other binder systems like HTPB. 29 The reason for the low rate observed could be assigned to the secondary nature of hydroxyl groups of GAP. Figure 7.12 shows the viscosity build up of GAP with TDI as curative at 30, 40, 50 and 60 0 C. It was noted that when IPDI and MDCI were used as curing agents, the viscosity build up was very low even at elevated temperature of 60 0 C. Figure 7.12 Viscosity build up of GAP with TDI as curing agent at different temperatures 238
31 The higher reactivity of TDI compared to IPDI and MDCI could be assigned to the prominent electron withdrawing effect associated with the aromatic system. 48 From the data, it was observed that at higher temperature, the viscosity of the mix was lower due to the increase in mobility of the polymer molecule. The rate of viscosity build up was found to steadily increase with temperature. It has been reported that viscosity build up of polyurethane system follow exponential relationship with time at constant temperature as the viscosity build up follows first order kinetics. 49 The exponential relationship is shown in equation Where, η t is the viscosity at time t after curing agent addition, η 0 is initial viscosity and k v is the rate constant for viscosity build up. The rate constant can be determined by linearising the exponential relationship as shown in equation From the slope of plot of ln (η t ) versus t, the rate constant k is determined. Figure 7.13 shows the linearised viscosity time relationship with a straight line fit. Figure 7.13 Plots of ln (viscosity) vs time at various temperatures for GAP-TDI system 239
32 The plots show clearly a two stage pattern. The first stage is found to be faster than the second. The stage separation may be due to the difference in the reactivities of the two diisocyanate groups (ortho and para) of TDI. NCO group in the ortho position is less reactive than the one in the para position due to steric hindrance at the ortho position caused by the 1-methyl group. 50 Both o- and p-nco groups are activated by each other through mesomeric electron withdrawing effect. The depletion of p-nco groups in the initial phase of cure reaction may further cause deactivation of the o-nco groups in addition to the steric hindrance. However, the difference in the reactivities narrows down with increase in temperature. This was confirmed by the observation that the ratio of the rate constants for the first and second stage (k 1 /k 2 ) decreases with temperature. It was also observed that there is no stage separation at 60 0 C. The rate constants determined for the two stages at each temperature and the ratio of the rate constants are shown in table 7.8. Table 7.8 Rate constants for viscosity build up of GAP TDI system Rate constant (minute -1 ) Temp. ( 0 C) I st II nd stage k stage (k 1 ) 1 /k 2 (k 2 ) E E E E E E E From the viscosity build up data, activation energy and activation enthalpy of the process were also determined using the Arrhenius (7.14) and Eyring equations (7.15) respectively. 240
33 Where k is the rate constant; E is activation energy; T is temperature in Kelvin scale and R is gas constant, A is the pre-exponential factor or Arrhenius frequency factor. In the Eyring equation, H * is activation enthalpy, k N is Boltzman constant, h is Planks constant and S * is activation entropy. Arrhenius and Eyring plots are depicted in figures 7.14 and 7.15 respectively. The corresponding activation parameters are listed in table 7.9. Figure 7.14 Arrhenius plots for viscosity build up of GAP-TDI system 241
34 Figure 7.15 Eyring plots for viscosity build up of GAP TDI system Using the Arrhenius relationship, a plot of ln k against 1/T is made and from the slope of straight line plot, activation energy is determined. From the Eyring relationship, slopes of the straight line plots between ln (k/t) and 1/T, H * is obtained and the intercepts give activation entropy. Table 7.9 Activation energy and activation entropy determined from Arrhenius and Eyring equations for viscosity build up of GAP-TDI system Reaction stage Activation energy (kj mol -1 ) Activation entropy (J mol -1 K -1 ) Stage Stage Viscometric studies on GAP curing in the presence of crosslinker and catalyst were carried out. Figures 7.16 and 7.17 show the viscosity build up profile for GAP- TDI and GAP-IPDI systems respectively. 242
35 Figure 7.16 Viscosity build up profile for GAP-TDI system with crosslinker and catalyst Figure 7.17 Viscosity build up profile for GAP-IPDI system with crosslinker and catalyst Viscosity build up of GAP with TDI and IPDI as curatives in presence of catalyst was evaluated and compared. The rate of viscosity build up of GAP with IPDI was lower than that with TDI when the crosslinking was done in the presence of catalyst. The data show that in the case of GAP-TDI at higher temperature, the reaction rate increases rapidly after 100 minutes of curative addition, which leads to 243
36 higher rate of viscosity build up. In the case of GAP-IPDI system, the reaction rate and viscosity follows an identical rate of build up even at higher temperature due to the low reactivity of aliphatic diisocyanate. Using the modified Arrhenius relationship (equation 7.11) for time to double the viscosity of the curing polymer, the activation energy for viscosity build up was determined for GAP-TDI and GAP IPDI systems. Figure 7.18 shows the kinetic plot for GAP-TDI and GAP-IPD systems with crosslinker and catalyst. The activation energy for viscosity build up noted for GAP-TDI system was kj mol -1 and that for GAP-IPDI system was kj mol -1. It was observed that the presence of catalyst helps to reduce activation energy for the crosslinking considerably. Figure 7.18 Kinetic plot for viscosity build up of GAP-TDI and GAP-IPDI systems with crosslinker and catalyst Study of the cure kinetics of GAP by IR spectroscopy was carried out. The path of the reaction can be easily followed by recording FTIR spectra of the curing mixture at various time intervals. For instance, the IR spectra of GAP-TDI mixture immediately after mixing and after 3 hours are shown in Figures It can be 244
37 observed that there is a sharp reduction in the absorbance of NCO peak (2273cm -1 ) after 3 hours of reaction. Figure 7.19 FTIR spectra of the GAP-TDI sample (a) immediately after mixing the ingredients and (b) after 3 hours Reduction in the intensity of the peak at 2273 cm -1 corresponds to the consumption of NCO groups while increase in the intensity of the peak at 1726 cm -1 indicates the formation of urethane groups due to reaction between hydroxyl and diisocyanate groups. The absorption bands at 2100 cm -1 due to stretching of azide group and CH stretching at 2930 cm -1 remain almost unaffected throughout the course of reaction. For this reason, the ratio between the absorbance of NCO (2273 cm -1 ) and that of CH stretching (2930 cm -1 ) is taken as a measure of concentration of diisocyanate groups for the purpose of evaluating kinetic parameters. It has been established that the reaction between hydroxyl and diisocyanate groups follow 2 nd 245
38 order kinetics. 51 The kinetic expression when the ratio between the equivalents of NCO and OH groups is unity is as given below: where, [C NCO ] 0 and [C NCO ] t are concentrations of NCO groups at the start of the reaction and at any given time t, and k is 2 nd order rate constant. When the absorbance is considered for concentration term, the kinetic equation may take the form as and Thus, plotting 1/[A] t against t yields straight lines and slopes of which are the 2 nd order rate constants for the reaction between GAP and the diisocyanate curative. Figures 7.20 to 7.22 depict the 2 nd order plots for GAP-TDI, GAP-IPDI and GAP- MDCI respectively. 246
39 Figure 7.20 Second order kinetic plots for GAP-TDI system Figure 7.21 Second order kinetic plots for GAP-IPDI system 247
40 Figure 7.22 Second order kinetic plots for GAP-MDCI system The second order rate constants were obtained for various diisocyanates namely TDI, IPDI and MDCI each at different temperatures viz: 30, 40, 50 and 60 C. For all the cases, linear plots with good correlation coefficients were obtained, indicating that the reactions between GAP and diisocyanate curatives follow a second order kinetics as reported for other similar systems. 48, 51, 52 The slopes of the straight line plots are the rate constants for the reactions. The second order rate constants thus obtained for the three diisocyanates are listed in table
41 Table 7.10 Kinetic data for GAP crosslinking from IR spectroscopic study Isocyanate TDI Stage I Stage II Second order rate constant at different temperatures (k) (mol -1 minute -1 ) 30 0 C 40 0 C 50 0 C 60 0 C 3.49 x x x x x 10-2 IPDI Stage I Stage II 2.20 x x x x x 10-3 MDCI 2.85 x x x x 10-3 k TDI /k IPDI k TDI /k MDCI k IPDI /k MDCI It can be seen that at any given temperature, the rate constants for the three diisocyanate compounds can be arranged in the order TDI > IPDI > MDCI. This is very much in accordance with reported trend obtained with conventional chemical kinetic approaches. A comparison of second order plot for reaction between GAP and the three isocyantaes at 30 0 C is shown in figure
42 Figure 7.23 Second order kinetic plots for the reaction between GAP and various diisocyanate compounds at 30 0 C Due to electron withdrawing mesomeric effect, which is very important for aromatic isocyanates, 29 TDI is more reactive than the aliphatic isocyanates used in the study. Between the two cyclo-aliphatic isocyanates used in the present study, IPDI is expected to be more reactive than MDCI as one of the two isocyanate groups in IPDI is primary in nature and the other is secondary. Both the isocyanate groups in MDCI are secondary and can be expected to be far more sluggish in its reaction with hydroxyl groups. A deviation from the general behaviour was observed with TDI and IPDI at 30 0 C. Both exhibit a two stage reaction pathway. With TDI, the first stage was faster than the second, while with IPDI the second stage was faster than the first; which is a typical example for autocatalysis. MDCI did not exhibit any stage separation. It can be further noted that the ratio of the rate constants of higher reactive to a lower reactive isocyanate steadily reduces with temperature, indicating that rise in temperature narrows down difference between the reactivities. Highest fall in the ratio occurs between TDI and MDCI. 250
43 As mentioned earlier, the activation energy and activation entropy for the reaction between GAP and the three isocyanates were also determined using the data generated by IR spectroscopic studies by means of Arrhenius equation (7.14) and Eyring equation (7.15) respectively. Arrhenius and Eyring plots are depicted in figures 7.24 and 7.25 respectively. The corresponding activation parameters are listed in table The values obtained for Activation energy (E) and activation enthalpy ( H * ) are in conformity with the trend in the reactivities of the diisocyanates with GAP. Figure 7.24 Arrhenius plots for GAP with TDI, IPDI and MDCI 251
44 Figure 7.25 Eyring plots for GAP with TDI, IPDI and MDCI Table 7.11 Activation parameters for the reaction between GAP and various isocyanates Diisocyanate Activation Energy (kj mol -1 ) Activation enthalpy (kj mol -1 ) Activation entropy (J mol -1 K -1 ) TDI IPDI MDCI It is always desirable to draw a relationship between kinetic parameters and viscosity of the curing mixture. Such a correlation would help to predict the viscosity of the curing mixture at a given time during the pre-gel phase. Of the many attempts made, the parameters 1/(1-p) and ln η t give rise to linear correlations with fairly good correlation coefficients, where p is the extent of reaction between NCO and OH 252
A Glossary of Terms Used in the Adhesives, Coatings and Elastomers (ACE) Sector
 A Glossary of Terms Used in the Adhesives, Coatings and Elastomers (ACE) Sector Abrasion resistance The ability of the coating membrane to resist mechanical action such as foot traffic and particles, which
A Glossary of Terms Used in the Adhesives, Coatings and Elastomers (ACE) Sector Abrasion resistance The ability of the coating membrane to resist mechanical action such as foot traffic and particles, which
CH5716 Processing of Materials
 CH5716 Processing of Materials Ceramic Thick Film Processing Lecture MC5 Slurry Characterisation Specific Surface Area Powder size & specific surface area (area per unit wt) closely related As particle
CH5716 Processing of Materials Ceramic Thick Film Processing Lecture MC5 Slurry Characterisation Specific Surface Area Powder size & specific surface area (area per unit wt) closely related As particle
Rocket Propulsion. Combustion chamber Throat Nozzle
 Rocket Propulsion In the section about the rocket equation we explored some of the issues surrounding the performance of a whole rocket. What we didn t explore was the heart of the rocket, the motor. In
Rocket Propulsion In the section about the rocket equation we explored some of the issues surrounding the performance of a whole rocket. What we didn t explore was the heart of the rocket, the motor. In
Influence of concentration, type and particle size of fillers on the dynamic mechanical behaviour of elastomeric HTPB binder
 Influence of concentration, type and particle size of fillers on the dynamic mechanical behaviour of elastomeric HTPB binder Dr. Manfred A. Bohn*, Mauricio FerrapontoffLemos**, *, Günter Mussbach***, *
Influence of concentration, type and particle size of fillers on the dynamic mechanical behaviour of elastomeric HTPB binder Dr. Manfred A. Bohn*, Mauricio FerrapontoffLemos**, *, Günter Mussbach***, *
STUDIES ON ISOTHERMAL URETHANE POLY MERlZATlON
 POLY M.-PLAST. TECHNOL. ENG., 30(2&3), 227-238 (1 991) STUDIES ON ISOTHERMAL URETHANE POLY MERlZATlON REJI JOHN, E. T. THACHIL, P. MVINDRAN, N. R. NEELAKANTAN, AND N. SUBRAMAMAN Department of Chemical
POLY M.-PLAST. TECHNOL. ENG., 30(2&3), 227-238 (1 991) STUDIES ON ISOTHERMAL URETHANE POLY MERlZATlON REJI JOHN, E. T. THACHIL, P. MVINDRAN, N. R. NEELAKANTAN, AND N. SUBRAMAMAN Department of Chemical
Preparation and Characterization of Eco-Friendly Hydrogen Peroxide Based Gel Oxidizer
 Proceedings of 7 th Asian-Pacific Conference on Aerospace Technology and Science May 23-26, 2013, Taiwan Preparation and Characterization of Eco-Friendly Hydrogen Peroxide Based Gel Oxidizer B.V.S.Jyoti
Proceedings of 7 th Asian-Pacific Conference on Aerospace Technology and Science May 23-26, 2013, Taiwan Preparation and Characterization of Eco-Friendly Hydrogen Peroxide Based Gel Oxidizer B.V.S.Jyoti
Optical & Spectroscopic Insight into Rheology. SR Kim
 Optical & Spectroscopic Insight into Rheology SR Kim 14.11.2014 Contents Rheology and Microscopy Rheology and Simultaneous FT-IR Analysis 2 3 RHEOLOGY AND MICROSCOPY What does Rheology Do? Put a defined
Optical & Spectroscopic Insight into Rheology SR Kim 14.11.2014 Contents Rheology and Microscopy Rheology and Simultaneous FT-IR Analysis 2 3 RHEOLOGY AND MICROSCOPY What does Rheology Do? Put a defined
Catalytic bead sensors are used primarily to detect
 Chapter 3 Catalytic Combustible Gas Sensors Catalytic bead sensors are used primarily to detect combustible gases. They have been in use for more than 50 years. Initially, these sensors were used for monitoring
Chapter 3 Catalytic Combustible Gas Sensors Catalytic bead sensors are used primarily to detect combustible gases. They have been in use for more than 50 years. Initially, these sensors were used for monitoring
Studies of rheological properties of suspension of heterogeneous rocket propellant based on HTPB rubber
 Studies of rheological properties of suspension of heterogeneous rocket propellant based on HTPB rubber Bogdan FLORCZAK Institute of Industrial Organic Chemistry, Warsaw, Poland; Ewelina BEDNARCZYK, Andrzej
Studies of rheological properties of suspension of heterogeneous rocket propellant based on HTPB rubber Bogdan FLORCZAK Institute of Industrial Organic Chemistry, Warsaw, Poland; Ewelina BEDNARCZYK, Andrzej
Malleable, Mechanically Strong, and Adaptive Elastomers. Enabled by Interfacial Exchangeable Bonds
 Malleable, Mechanically Strong, and Adaptive Elastomers Enabled by Interfacial Exchangeable Bonds Zhenghai Tang, Yingjun Liu, Baochun Guo,*, and Liqun Zhang*, Department of Polymer Materials and Engineering,
Malleable, Mechanically Strong, and Adaptive Elastomers Enabled by Interfacial Exchangeable Bonds Zhenghai Tang, Yingjun Liu, Baochun Guo,*, and Liqun Zhang*, Department of Polymer Materials and Engineering,
Propanone can be formed when glucose comes into contact with bacteria in the absence of air. Deduce the role of the bacteria in this reaction.
 Q1.(a) Propanone can be formed when glucose comes into contact with bacteria in the absence of air. Balance the following equation for this reaction of glucose to form propanone, carbon dioxide and water....c
Q1.(a) Propanone can be formed when glucose comes into contact with bacteria in the absence of air. Balance the following equation for this reaction of glucose to form propanone, carbon dioxide and water....c
5 Energy from chemicals
 5 Energy from chemicals Content 5.1 Enthalpy 5.2 Hydrogen fuel cell Learning Outcomes Candidates should be able to: (a) (b) (c) (d) (e) describe the meaning of enthalpy change in terms of exothermic (H
5 Energy from chemicals Content 5.1 Enthalpy 5.2 Hydrogen fuel cell Learning Outcomes Candidates should be able to: (a) (b) (c) (d) (e) describe the meaning of enthalpy change in terms of exothermic (H
IMPROVEMENT IN MECHANICAL PROPERTIES OF MODIFIED GRAPHENE/EPOXY NANOCOMPOSITES
 18 TH INTERNATIONAL CONFERENCE ON COMPOSITE MATERIALS IMPROVEMENT IN MECHANICAL PROPERTIES OF MODIFIED 1 Introduction Since first successfully separated from graphite by micromechanical cleavage [1], graphene
18 TH INTERNATIONAL CONFERENCE ON COMPOSITE MATERIALS IMPROVEMENT IN MECHANICAL PROPERTIES OF MODIFIED 1 Introduction Since first successfully separated from graphite by micromechanical cleavage [1], graphene
Rheological and mechanical properties of epoxy composites modified with montmorillonite nanoparticles
 Plasticheskie Massy, No. 3, 2011, pp. 56 60 Rheological and mechanical properties of epoxy composites modified with montmorillonite nanoparticles S.O. Il in, 1 I.Yu. Gorbunova, 2 E.P. Plotnikova, 1 and
Plasticheskie Massy, No. 3, 2011, pp. 56 60 Rheological and mechanical properties of epoxy composites modified with montmorillonite nanoparticles S.O. Il in, 1 I.Yu. Gorbunova, 2 E.P. Plotnikova, 1 and
Chapter 1. Composite Solid Propellant Binders Current Status and Advances
 Chapter 1 Composite Solid Propellant Binders Current Status and Advances 1.1 Introduction All modern developments in the area of solid propulsion systems are mostly based on composite propellants. Storable
Chapter 1 Composite Solid Propellant Binders Current Status and Advances 1.1 Introduction All modern developments in the area of solid propulsion systems are mostly based on composite propellants. Storable
IFE Level 3 Diploma in Fire Science and Fire Safety (VRQ)
 Unit 1: Fire Engineering Science Unit Reference Number: A/505/6005 Introduction This unit focuses on fire engineering science and fire behaviour. The content of the unit has been designed to reflect the
Unit 1: Fire Engineering Science Unit Reference Number: A/505/6005 Introduction This unit focuses on fire engineering science and fire behaviour. The content of the unit has been designed to reflect the
Studies on Furan Polymer Concrete
 Studies on Furan Polymer Concrete Rajesh Katiyar 1, Shobhit Shukla 2 1Associate Professor, Department of Chemical engineering, H.B.T.U., Kanpur-208002, India 2Research Scholar, Department of Chemical engineering
Studies on Furan Polymer Concrete Rajesh Katiyar 1, Shobhit Shukla 2 1Associate Professor, Department of Chemical engineering, H.B.T.U., Kanpur-208002, India 2Research Scholar, Department of Chemical engineering
Jet Propulsion. Lecture Ujjwal K Saha, Ph. D. Department of Mechanical Engineering Indian Institute of Technology Guwahati
 Lecture - 27 Prepared under QIP-CD Cell Project Jet Propulsion Ujjwal K Saha, Ph. D. Department of Mechanical Engineering Indian Institute of Technology Guwahati 1 Propellant Properties High specific impulse
Lecture - 27 Prepared under QIP-CD Cell Project Jet Propulsion Ujjwal K Saha, Ph. D. Department of Mechanical Engineering Indian Institute of Technology Guwahati 1 Propellant Properties High specific impulse
UNIT ONE BOOKLET 6. Thermodynamic
 DUNCANRIG SECONDARY ADVANCED HIGHER CHEMISTRY UNIT ONE BOOKLET 6 Thermodynamic Can we predict if a reaction will occur? What determines whether a reaction will be feasible or not? This is a question that
DUNCANRIG SECONDARY ADVANCED HIGHER CHEMISTRY UNIT ONE BOOKLET 6 Thermodynamic Can we predict if a reaction will occur? What determines whether a reaction will be feasible or not? This is a question that
The application of nano aluminum powder on solid propellant
 The application of nano aluminum powder on solid propellant Metal incendiary agent is one of the important components of modern solid propellant, which can improve the explosion heat and density of propellant.
The application of nano aluminum powder on solid propellant Metal incendiary agent is one of the important components of modern solid propellant, which can improve the explosion heat and density of propellant.
Rocket Propulsion Prof. K. Ramamurthi Department of Mechanical Engineering Indian Institute of Technology, Madras
 Rocket Propulsion Prof. K. Ramamurthi Department of Mechanical Engineering Indian Institute of Technology, Madras Lecture 23 Burn Rate of Solid Propellants and Equilibrium Pressure in Solid Propellant
Rocket Propulsion Prof. K. Ramamurthi Department of Mechanical Engineering Indian Institute of Technology, Madras Lecture 23 Burn Rate of Solid Propellants and Equilibrium Pressure in Solid Propellant
Determination of the Internal Ballistic Properties of Solid Heterogeneous Rocket Propellants
 Determination of the Internal Ballistic Properties of Solid Heterogeneous... 589 Central European Journal of Energetic Materials, 2014, 11(4), 589-601 ISSN 2353-1843 Determination of the Internal Ballistic
Determination of the Internal Ballistic Properties of Solid Heterogeneous... 589 Central European Journal of Energetic Materials, 2014, 11(4), 589-601 ISSN 2353-1843 Determination of the Internal Ballistic
11.1 Survey of Spacecraft Propulsion Systems
 11.1 Survey of Spacecraft Propulsion Systems 11.1 Survey of Spacecraft Propulsion Systems In the progressing Space Age, spacecrafts such as satellites and space probes are the key to space exploration,
11.1 Survey of Spacecraft Propulsion Systems 11.1 Survey of Spacecraft Propulsion Systems In the progressing Space Age, spacecrafts such as satellites and space probes are the key to space exploration,
Applied Thermodynamics - II
 Gas Turbines Sudheer Siddapureddy sudheer@iitp.ac.in Department of Mechanical Engineering Jet Propulsion - Classification 1. A heated and compressed atmospheric air, mixed with products of combustion,
Gas Turbines Sudheer Siddapureddy sudheer@iitp.ac.in Department of Mechanical Engineering Jet Propulsion - Classification 1. A heated and compressed atmospheric air, mixed with products of combustion,
CHAPTER-5 BISMALEIMIDE-ALLYL NOVOLAC OLIGOMERS: SYNTHESIS AND CURE KINETICS
 CHAPTER-5 BISMALEIMIDE-ALLYL VLAC LIGMERS: SYTHESIS AD CURE KIETICS This chapter deals with Bismaleimide resin systems, with a novolac back bone, containing both maleimide and allyl functionalities incorporated
CHAPTER-5 BISMALEIMIDE-ALLYL VLAC LIGMERS: SYTHESIS AD CURE KIETICS This chapter deals with Bismaleimide resin systems, with a novolac back bone, containing both maleimide and allyl functionalities incorporated
Mr.N.Srikar M.Pharm.,(Ph.D) KRISHNA TEJA PHARMACY COLLEGE
 . Mr.N.Srikar M.Pharm.,(Ph.D) KRISHNA TEJA PHARMACY COLLEGE CONTENTS Introduction Definition Importance Newton's laws Types of flow Viscosity Measurements of viscosity Pharmaceutical applications 2 INTRODUCTION
. Mr.N.Srikar M.Pharm.,(Ph.D) KRISHNA TEJA PHARMACY COLLEGE CONTENTS Introduction Definition Importance Newton's laws Types of flow Viscosity Measurements of viscosity Pharmaceutical applications 2 INTRODUCTION
(03) WMP/Jun10/CHEM4
 Thermodynamics 3 Section A Answer all questions in the spaces provided. 1 A reaction mechanism is a series of steps by which an overall reaction may proceed. The reactions occurring in these steps may
Thermodynamics 3 Section A Answer all questions in the spaces provided. 1 A reaction mechanism is a series of steps by which an overall reaction may proceed. The reactions occurring in these steps may
Viscoelastic Characterization of Different Solid Rocket Propellants Using the Maxwell Spring-Dashpot Model
 Viscoelastic Characterization of Different Solid Rocket Propellants... 189 Central European Journal of Energetic Materials, 2012, 9(3), 189-199 ISSN 1733-7178 Viscoelastic Characterization of Different
Viscoelastic Characterization of Different Solid Rocket Propellants... 189 Central European Journal of Energetic Materials, 2012, 9(3), 189-199 ISSN 1733-7178 Viscoelastic Characterization of Different
Chemistry Assessment Unit AS 2
 Centre Number 71 Candidate Number ADVANCED SUBSIDIARY (AS) General Certificate of Education January 2011 Chemistry Assessment Unit AS 2 assessing Module 2: Organic, Physical and Inorganic Chemistry [AC121]
Centre Number 71 Candidate Number ADVANCED SUBSIDIARY (AS) General Certificate of Education January 2011 Chemistry Assessment Unit AS 2 assessing Module 2: Organic, Physical and Inorganic Chemistry [AC121]
CHEM5. General Certificate of Education Advanced Level Examination June Unit 5 Energetics, Redox and Inorganic Chemistry
 Centre Number Surname Candidate Number For Examiner s Use Other Names Candidate Signature Examiner s Initials General Certificate of Education Advanced Level Examination June 2010 Question 1 2 Mark Chemistry
Centre Number Surname Candidate Number For Examiner s Use Other Names Candidate Signature Examiner s Initials General Certificate of Education Advanced Level Examination June 2010 Question 1 2 Mark Chemistry
ISSN ; e-issn Copyright 2017 Institute of Industrial Organic Chemistry, Poland
 Central European Journal of Energetic Materials ISSN 1733-7178; e-issn 2353-1843 Cent. Eur. J. Energ. Mater. 2017, 14(4): 917-932; DOI: 10.22211/cejem/76704 Experimental Approaches to Develop a High Thrust
Central European Journal of Energetic Materials ISSN 1733-7178; e-issn 2353-1843 Cent. Eur. J. Energ. Mater. 2017, 14(4): 917-932; DOI: 10.22211/cejem/76704 Experimental Approaches to Develop a High Thrust
3.2.1 Energetics. Enthalpy Change. 263 minutes. 259 marks. Page 1 of 41
 ..1 Energetics Enthalpy Change 6 minutes 59 marks Page 1 of 41 Q1. (a) Define the term standard molar enthalpy of formation, ΔH f. (b) State Hess s law. (c) Propanone, CO, burns in oxygen as shown by the
..1 Energetics Enthalpy Change 6 minutes 59 marks Page 1 of 41 Q1. (a) Define the term standard molar enthalpy of formation, ΔH f. (b) State Hess s law. (c) Propanone, CO, burns in oxygen as shown by the
Cherry Hill Tuition A Level Chemistry OCR (A) Paper 9 THIS IS A NEW SPECIFICATION
 THIS IS A NEW SPECIFICATION ADVANCED SUBSIDIARY GCE CHEMISTRY A Chains, Energy and Resources F322 * OCE / 1 9 2 3 4* Candidates answer on the Question Paper OCR Supplied Materials: Data Sheet for Chemistry
THIS IS A NEW SPECIFICATION ADVANCED SUBSIDIARY GCE CHEMISTRY A Chains, Energy and Resources F322 * OCE / 1 9 2 3 4* Candidates answer on the Question Paper OCR Supplied Materials: Data Sheet for Chemistry
Multi-mode revisited
 Multi-mode revisited Testing the application of shift factors S.J.M Hellenbrand 515217 MT 7.29 Coaches: Ir. L.C.A. van Breemen Dr. Ir. L.E. Govaert 2-7- 7 Contents Contents 1 Introduction 2 I Polymers
Multi-mode revisited Testing the application of shift factors S.J.M Hellenbrand 515217 MT 7.29 Coaches: Ir. L.C.A. van Breemen Dr. Ir. L.E. Govaert 2-7- 7 Contents Contents 1 Introduction 2 I Polymers
Binding Interaction Between Dantocol and RDX. Chris Williams. Supervised by Dr Stephen Clarke Co-Supervised Dr Simon Mathew & Dr Ian Lochert (DSTO)
 Binding Interaction Between Dantocol and RDX Chris Williams Supervised by Dr Stephen Clarke Co-Supervised Dr Simon Mathew & Dr Ian Lochert (DST) Introduction Components of polymer bonded explosives (PBX)
Binding Interaction Between Dantocol and RDX Chris Williams Supervised by Dr Stephen Clarke Co-Supervised Dr Simon Mathew & Dr Ian Lochert (DST) Introduction Components of polymer bonded explosives (PBX)
Reaction Kinetics. Reaction kinetics is the study of the rates of reactions and the factors which affect the rates. Hebden Unit 1 (page 1 34)
 Hebden Unit 1 (page 1 34) Reaction kinetics is the study of the rates of reactions and the factors which affect the rates. 2 1 What are kinetic studies good for? 3 How to speed up: 1. Paint drying 2. Setting
Hebden Unit 1 (page 1 34) Reaction kinetics is the study of the rates of reactions and the factors which affect the rates. 2 1 What are kinetic studies good for? 3 How to speed up: 1. Paint drying 2. Setting
IFE Level 3 Diploma in Fire Science and Fire Safety
 IFE Level 3 Diploma in Fire Science and Fire Safety Unit 1: Fire Engineering Science Unit Reference Number: A/505/6005 Introduction This unit focuses on fire engineering science and fire behaviour. The
IFE Level 3 Diploma in Fire Science and Fire Safety Unit 1: Fire Engineering Science Unit Reference Number: A/505/6005 Introduction This unit focuses on fire engineering science and fire behaviour. The
Coimisiún na Scrúduithe Stáit State Examinations Commission
 2009. M 37 Coimisiún na Scrúduithe Stáit State Examinations Commission LEAVING CERTIFICATE EXAMINATION 2009 PHYSICS AND CHEMISTRY ORDINARY LEVEL MONDAY, 15 JUNE MORNING 9:30 TO 12:30 Six questions to be
2009. M 37 Coimisiún na Scrúduithe Stáit State Examinations Commission LEAVING CERTIFICATE EXAMINATION 2009 PHYSICS AND CHEMISTRY ORDINARY LEVEL MONDAY, 15 JUNE MORNING 9:30 TO 12:30 Six questions to be
Aqueous Colloidal Processing and green sheet properties of. Lead Zirconate Titanate (PZT) ceramics made by Tape. Casting.
 Aqueous Colloidal Processing and green sheet properties of Lead Zirconate Titanate (PZT) ceramics made by Tape Casting. A. Navarro, J.R.Alcock and R.W.Whatmore Nanotechnology Dept, SIMS, Cranfield University,
Aqueous Colloidal Processing and green sheet properties of Lead Zirconate Titanate (PZT) ceramics made by Tape Casting. A. Navarro, J.R.Alcock and R.W.Whatmore Nanotechnology Dept, SIMS, Cranfield University,
Supporting Information
 Supporting Information Thermoset Shape-Memory Polyurethane with Intrinsic Plasticity Enabled by Transcarbamoylation Ning Zheng, Zizheng Fang, Weike Zou, Qian Zhao,* and Tao Xie* anie_201602847_sm_miscellaneous_information.pdf
Supporting Information Thermoset Shape-Memory Polyurethane with Intrinsic Plasticity Enabled by Transcarbamoylation Ning Zheng, Zizheng Fang, Weike Zou, Qian Zhao,* and Tao Xie* anie_201602847_sm_miscellaneous_information.pdf
Rocket Propulsion Overview
 Rocket Propulsion Overview Seitzman Rocket Overview-1 Rocket Definition Rocket Device that provides thrust to a vehicle by accelerating some matter (the propellant) and exhausting it from the rocket Most
Rocket Propulsion Overview Seitzman Rocket Overview-1 Rocket Definition Rocket Device that provides thrust to a vehicle by accelerating some matter (the propellant) and exhausting it from the rocket Most
Introduction to the Combustion of Energetic Materials
 Introduction to the Combustion of Energetic Materials Steve Son There is not a law under which any part of this universe is governed which does not come into play, and is not touched upon, in [the phenomena
Introduction to the Combustion of Energetic Materials Steve Son There is not a law under which any part of this universe is governed which does not come into play, and is not touched upon, in [the phenomena
Pharmaceutics I. Unit 6 Rheology of suspensions
 Pharmaceutics I اينالديصيدلينيات 1 Unit 6 Rheology of suspensions 1 Rheology, the science of the flow or deformation of matter (liquid or soft solid) under the effect of an applied force. It addresses
Pharmaceutics I اينالديصيدلينيات 1 Unit 6 Rheology of suspensions 1 Rheology, the science of the flow or deformation of matter (liquid or soft solid) under the effect of an applied force. It addresses
YUsend-1 Solid Propellant Microthruster Design, Fabrication and Testing
 YUsend-1 Solid Propellant Microthruster Design, Fabrication and Testing 24 th AIAA/USU Conference on Small Satellites Authors: Kartheephan Sathiyanathan, Regina Lee, Hugh Chesser (York University) Charles
YUsend-1 Solid Propellant Microthruster Design, Fabrication and Testing 24 th AIAA/USU Conference on Small Satellites Authors: Kartheephan Sathiyanathan, Regina Lee, Hugh Chesser (York University) Charles
Drilling Fluid Thixotropy & Relevance
 ANNUAL TRANSACTIONS OF THE NORDIC RHEOLOGY SOCIETY, VOL. 13, 2005 Drilling Fluid Thixotropy & Relevance Richard Jachnik1, 1Baker Hughes INTEQ, Stoneywood Park North, Dyce, Aberdeen, Scotland, UK ABSTRACT
ANNUAL TRANSACTIONS OF THE NORDIC RHEOLOGY SOCIETY, VOL. 13, 2005 Drilling Fluid Thixotropy & Relevance Richard Jachnik1, 1Baker Hughes INTEQ, Stoneywood Park North, Dyce, Aberdeen, Scotland, UK ABSTRACT
Rocket Dynamics. Forces on the Rocket
 Rocket Dynamics Forces on the Rockets - Drag Rocket Stability Rocket Equation Specific Impulse Rocket otors F Thrust Forces on the Rocket Equation of otion: Need to minimize total mass to maximize acceleration
Rocket Dynamics Forces on the Rockets - Drag Rocket Stability Rocket Equation Specific Impulse Rocket otors F Thrust Forces on the Rocket Equation of otion: Need to minimize total mass to maximize acceleration
Quiz 1 Introduction to Polymers
 090109 Quiz 1 Introduction to Polymers In class we discussed the definition of a polymer first by comparing polymers with metals and ceramics and then by noting certain properties of polymers that distinguish
090109 Quiz 1 Introduction to Polymers In class we discussed the definition of a polymer first by comparing polymers with metals and ceramics and then by noting certain properties of polymers that distinguish
AS Paper 1 and 2 Kc and Equilibria
 AS Paper 1 and 2 Kc and Equilibria Q1.When one mole of ammonia is heated to a given temperature, 50 per cent of the compound dissociates and the following equilibrium is established. NH 3(g) ½ N 2 (g)
AS Paper 1 and 2 Kc and Equilibria Q1.When one mole of ammonia is heated to a given temperature, 50 per cent of the compound dissociates and the following equilibrium is established. NH 3(g) ½ N 2 (g)
On the Rheological Parameters Governing Oilwell Cement Slurry Stability
 ANNUAL TRANSACTIONS OF THE NORDIC RHEOLOGY SOCIETY, VOL. 12, 2004 On the Rheological Parameters Governing Oilwell Cement Slurry Stability Roni Gandelman, Cristiane Miranda, Kleber Teixeira, André L. Martins
ANNUAL TRANSACTIONS OF THE NORDIC RHEOLOGY SOCIETY, VOL. 12, 2004 On the Rheological Parameters Governing Oilwell Cement Slurry Stability Roni Gandelman, Cristiane Miranda, Kleber Teixeira, André L. Martins
3.2.2 Kinetics. Effect of temperature. 145 minutes. 145 marks. Page 1 of 22
 3.. Kinetics Effect of temperature 145 minutes 145 marks Page 1 of Q1. (a) State what is meant by the term activation energy of a reaction. (b) (c) State in general terms how a catalyst increases the rate
3.. Kinetics Effect of temperature 145 minutes 145 marks Page 1 of Q1. (a) State what is meant by the term activation energy of a reaction. (b) (c) State in general terms how a catalyst increases the rate
Flexible Packaging Adhesives The Basics. Larry Jopko Rohm and Haas Company. Abstract
 Flexible Packaging Adhesives The Basics Larry Jopko Rohm and Haas Company Abstract Flexible packaging adhesives are predominately based on urethane and acrylic chemistry. Backbone options create unique
Flexible Packaging Adhesives The Basics Larry Jopko Rohm and Haas Company Abstract Flexible packaging adhesives are predominately based on urethane and acrylic chemistry. Backbone options create unique
Contents. Preface XIII. 1 General Introduction 1 References 6
 VII Contents Preface XIII 1 General Introduction 1 References 6 2 Interparticle Interactions and Their Combination 7 2.1 Hard-Sphere Interaction 7 2.2 Soft or Electrostatic Interaction 7 2.3 Steric Interaction
VII Contents Preface XIII 1 General Introduction 1 References 6 2 Interparticle Interactions and Their Combination 7 2.1 Hard-Sphere Interaction 7 2.2 Soft or Electrostatic Interaction 7 2.3 Steric Interaction
Rheological Characterization of Medical Thermoplastic Polyurethanes
 Rheological Characterization of Medical Thermoplastic Polyurethanes Ian Pierson, Emily Chen, Ajay D Padsalgikar Abbott, Rogers, MN Abstract The use of thermoplastic polyurethanes (TPUs) in the medical
Rheological Characterization of Medical Thermoplastic Polyurethanes Ian Pierson, Emily Chen, Ajay D Padsalgikar Abbott, Rogers, MN Abstract The use of thermoplastic polyurethanes (TPUs) in the medical
Take home Exam due Wednesday, Aug 26. In class Exam will be the that morning class multiple choice questions.
 Announcements Take home Exam due Wednesday, Aug 26. In class Exam will be the that morning class. 15-20 multiple choice questions. Updated projects Aug 28: answer what lab chemistry needs to get done to
Announcements Take home Exam due Wednesday, Aug 26. In class Exam will be the that morning class. 15-20 multiple choice questions. Updated projects Aug 28: answer what lab chemistry needs to get done to
Periodic table with the elements associated with commercial polymers in color.
 Polymers 1. What are polymers 2. Polymerization 3. Structure features of polymers 4. Thermoplastic polymers and thermosetting polymers 5. Additives 6. Polymer crystals 7. Mechanical properties of polymers
Polymers 1. What are polymers 2. Polymerization 3. Structure features of polymers 4. Thermoplastic polymers and thermosetting polymers 5. Additives 6. Polymer crystals 7. Mechanical properties of polymers
Electronic Supplementary Information for New Journal of Chemistry
 Electronic Supplementary Material (ESI) for New Journal of Chemistry. This journal is The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2015 Electronic Supplementary Information
Electronic Supplementary Material (ESI) for New Journal of Chemistry. This journal is The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2015 Electronic Supplementary Information
Study on Military Polyurethane Adhesive
 Research Paper Study on Military Polyurethane Adhesive Changming Zhang 1 *, Zhanggen Huang 1, Jun Ping Li 2 and Xiao Juan Lu 2 1 State Key Lab. of Coal Conversion, Institute of Coal Chemistry, Chinese
Research Paper Study on Military Polyurethane Adhesive Changming Zhang 1 *, Zhanggen Huang 1, Jun Ping Li 2 and Xiao Juan Lu 2 1 State Key Lab. of Coal Conversion, Institute of Coal Chemistry, Chinese
Polymer Reaction Engineering
 Polymer Reaction Engineering Polymerization Techniques Bulk Solution Suspension Emulsion Interfacial Polymerization Solid-State Gas-Phase Plasma Polymerization in Supercritical Fluids Bulk Polymerization
Polymer Reaction Engineering Polymerization Techniques Bulk Solution Suspension Emulsion Interfacial Polymerization Solid-State Gas-Phase Plasma Polymerization in Supercritical Fluids Bulk Polymerization
ST STITHIANS COLLEGE DEPARTMENT OF PHYSICAL SCIENCE PRELIMINARY EXAMINATIONS 2012 GRADE 12 PHYSICAL SCIENCE: PAPER 2
 Page 1 of 15 ST STITHIANS COLLEGE DEPARTMENT OF PHYSICAL SCIENCE PRELIMINARY EXAMINATIONS 2012 GRADE 12 PHYSICAL SCIENCE: PAPER 2 Examiners Moderators: Miss S Nargaroo (Girls College), Mr SB Lambson Mr
Page 1 of 15 ST STITHIANS COLLEGE DEPARTMENT OF PHYSICAL SCIENCE PRELIMINARY EXAMINATIONS 2012 GRADE 12 PHYSICAL SCIENCE: PAPER 2 Examiners Moderators: Miss S Nargaroo (Girls College), Mr SB Lambson Mr
Mechanical properties of polymers: an overview. Suryasarathi Bose Dept. of Materials Engineering, IISc, Bangalore
 Mechanical properties of polymers: an overview Suryasarathi Bose Dept. of Materials Engineering, IISc, Bangalore UGC-NRCM Summer School on Mechanical Property Characterization- June 2012 Overview of polymer
Mechanical properties of polymers: an overview Suryasarathi Bose Dept. of Materials Engineering, IISc, Bangalore UGC-NRCM Summer School on Mechanical Property Characterization- June 2012 Overview of polymer
Biopolymers as sustainable fillers for elastomers
 Biopolymers as sustainable fillers for elastomers Jane Clarke Loughborough University What are biopolymers and why are they sustainable? Biopolymers are polymers synthesised by plants (and animals) Carbohydrates
Biopolymers as sustainable fillers for elastomers Jane Clarke Loughborough University What are biopolymers and why are they sustainable? Biopolymers are polymers synthesised by plants (and animals) Carbohydrates
Contents. Preface... xvii
 Contents Preface... xvii CHAPTER 1 Idealized Flow Machines...1 1.1 Conservation Equations... 1 1.1.1 Conservation of mass... 2 1.1.2 Conservation of momentum... 3 1.1.3 Conservation of energy... 3 1.2
Contents Preface... xvii CHAPTER 1 Idealized Flow Machines...1 1.1 Conservation Equations... 1 1.1.1 Conservation of mass... 2 1.1.2 Conservation of momentum... 3 1.1.3 Conservation of energy... 3 1.2
2/18/2013. Spontaneity, Entropy & Free Energy Chapter 16. The Dependence of Free Energy on Pressure Sample Exercises
 Spontaneity, Entropy & Free Energy Chapter 16 16.7 The Dependence of Free Energy on Pressure Why is free energy dependent on pressure? Isn t H, enthalpy independent of pressure at constant pressure? No
Spontaneity, Entropy & Free Energy Chapter 16 16.7 The Dependence of Free Energy on Pressure Why is free energy dependent on pressure? Isn t H, enthalpy independent of pressure at constant pressure? No
1.11 Redox Equilibria
 1.11 Redox Equilibria Electrochemical cells Electron flow A cell has two half cells. The two half cells have to be connected with a salt bridge. Simple half cells will consist of a metal (acts an electrode)
1.11 Redox Equilibria Electrochemical cells Electron flow A cell has two half cells. The two half cells have to be connected with a salt bridge. Simple half cells will consist of a metal (acts an electrode)
race to the Second Edition Preface to the First Edition 'kno\t'ledgements Contents
 Contents race to the Second Edition Preface to the First Edition 'kno\t'ledgements Chemistry and Basic Intermediates. Introduction. Basic Chemistry Basic Structure of a Polyurethane Elastomer. Synthesis
Contents race to the Second Edition Preface to the First Edition 'kno\t'ledgements Chemistry and Basic Intermediates. Introduction. Basic Chemistry Basic Structure of a Polyurethane Elastomer. Synthesis
INFLUENCE OF ISOMER RATIO OF TOLUENE DI ISOCYANATE ON SOLID PROPELLANT BEHAVIOR
 INFLUENCE OF ISOMER RATIO OF TOLUENE DI ISOCYANATE ON SOLID PROPELLANT BEHAVIOR CH Devi Vara Prasad 1, Srinivas Babu N 2, Lakshmana Rao V 3, Ranganathan V 4 1 Scientist, Solid Propellant Plant, Satish
INFLUENCE OF ISOMER RATIO OF TOLUENE DI ISOCYANATE ON SOLID PROPELLANT BEHAVIOR CH Devi Vara Prasad 1, Srinivas Babu N 2, Lakshmana Rao V 3, Ranganathan V 4 1 Scientist, Solid Propellant Plant, Satish
Modeling and Simulation of Fabrication, Transport, and Combustion of. Flow Environments
 Modeling and Simulation of Fabrication, Transport, and Combustion of Nano-Engineered Energetic Materials (NEEM) in Flow Environments Vigor Yang The Pennsylvania State University Presented at Nano-Engineered
Modeling and Simulation of Fabrication, Transport, and Combustion of Nano-Engineered Energetic Materials (NEEM) in Flow Environments Vigor Yang The Pennsylvania State University Presented at Nano-Engineered
CHEM J-11 June /01(a)
 CHEM1001 2014-J-11 June 2014 22/01(a) Combustion of 15.0 g of coal provided sufficient heat to increase the temperature of 7.5 kg of water from 286 K to 298 K. Calculate the amount of heat (in kj) absorbed
CHEM1001 2014-J-11 June 2014 22/01(a) Combustion of 15.0 g of coal provided sufficient heat to increase the temperature of 7.5 kg of water from 286 K to 298 K. Calculate the amount of heat (in kj) absorbed
TECHNICAL UPDATE. Ricon Resins Peroxide Curing Data and Use as a Reactive Plasticizer in Polyphenylene Ether Based CCL and PWB
 TARGET MARKETS/ APPLICATIONS Copper clad laminate (CCL) and printed wiring [Circuit] boards (PWB) Structural composites Radomes Aerospace applications ADDITIONAL INFO SDS /TDS: Ricon 100, 154, 157, 184,
TARGET MARKETS/ APPLICATIONS Copper clad laminate (CCL) and printed wiring [Circuit] boards (PWB) Structural composites Radomes Aerospace applications ADDITIONAL INFO SDS /TDS: Ricon 100, 154, 157, 184,
CHEMISTRY HIGHER LEVEL
 *P15* PRE-LEAVING CERTIFICATE EXAMINATION, 2009 CHEMISTRY HIGHER LEVEL TIME: 3 HOURS 400 MARKS Answer eight questions in all These must include at least two questions from Section A All questions carry
*P15* PRE-LEAVING CERTIFICATE EXAMINATION, 2009 CHEMISTRY HIGHER LEVEL TIME: 3 HOURS 400 MARKS Answer eight questions in all These must include at least two questions from Section A All questions carry
Energy Changes in Chemical Reactions
 Unit 3 Energetics Unit 3-1 Section 3.1 Energy Changes in Chemical Reactions ( 1 ) Conservation of energy An object which is capable of doing work is said to possess energy. There are many forms of energy:
Unit 3 Energetics Unit 3-1 Section 3.1 Energy Changes in Chemical Reactions ( 1 ) Conservation of energy An object which is capable of doing work is said to possess energy. There are many forms of energy:
The Influence of Molecular Weight on Explosive Hazard. Energetic Polyphosphazenes a new category of binders for energetic formulations
 The Influence of Molecular Weight on Explosive Hazard Kason Bala and Peter Golding Energetic Polyphosphazenes a new category of binders for energetic formulations Peter Golding and Stephen J Trussell Bomb
The Influence of Molecular Weight on Explosive Hazard Kason Bala and Peter Golding Energetic Polyphosphazenes a new category of binders for energetic formulations Peter Golding and Stephen J Trussell Bomb
CHAPTER 4 THERMAL HAZARD ASSESSMENT OF FIREWORKS MIXTURE USING ACCELERATING RATE CALORIMETER (ARC)
 68 CHAPTER 4 THERMAL HAZARD ASSESSMENT OF FIREWORKS MIXTURE USING ACCELERATING RATE CALORIMETER (ARC) 4.1 INTRODUCTION Accelerating Rate Calorimeter (ARC) is one of the versatile experimental tools available
68 CHAPTER 4 THERMAL HAZARD ASSESSMENT OF FIREWORKS MIXTURE USING ACCELERATING RATE CALORIMETER (ARC) 4.1 INTRODUCTION Accelerating Rate Calorimeter (ARC) is one of the versatile experimental tools available
SUPPORTING INFORMATION
 SUPPORTING INFORMATION Rapid, puncture-initiated healing via oxygen-mediated polymerization Scott R. Zavada, Nicholas R. McHardy, Keith L. Gordon, and Timothy F. Scott* Experimental Section Materials:
SUPPORTING INFORMATION Rapid, puncture-initiated healing via oxygen-mediated polymerization Scott R. Zavada, Nicholas R. McHardy, Keith L. Gordon, and Timothy F. Scott* Experimental Section Materials:
Pharmaceutics I صيدالنيات 1. Unit 6
 Pharmaceutics I صيدالنيات 1 Unit 6 1 Rheology of suspensions Rheology, the study of flow, addresses the viscosity characteristics of powders, fluids, and semisolids. Materials are divided into two general
Pharmaceutics I صيدالنيات 1 Unit 6 1 Rheology of suspensions Rheology, the study of flow, addresses the viscosity characteristics of powders, fluids, and semisolids. Materials are divided into two general
Q1. (a) State what is meant by the term activation energy of a reaction. (1)
 Q1. (a) State what is meant by the term activation energy of a reaction. (c) State in general terms how a catalyst increases the rate of a chemical reaction. The curve below shows the Maxwell Boltzmann
Q1. (a) State what is meant by the term activation energy of a reaction. (c) State in general terms how a catalyst increases the rate of a chemical reaction. The curve below shows the Maxwell Boltzmann
(02) WMP/Jun10/CHEM2
 Energetics 2 Section A Answer all the questions in the spaces provided. 1 An equation for the equilibrium reaction between hydrogen, iodine and hydrogen iodide is shown below. H 2 (g) + I 2 (g) 2HI(g)
Energetics 2 Section A Answer all the questions in the spaces provided. 1 An equation for the equilibrium reaction between hydrogen, iodine and hydrogen iodide is shown below. H 2 (g) + I 2 (g) 2HI(g)
Based on the kinetic molecular theory of gases, which one of the following statements is INCORRECT?
 1 Based on the kinetic molecular theory of gases, which one of the following statements is INCORRECT? A) The collisions between gas molecules are perfectly elastic. B) At absolute zero, the average kinetic
1 Based on the kinetic molecular theory of gases, which one of the following statements is INCORRECT? A) The collisions between gas molecules are perfectly elastic. B) At absolute zero, the average kinetic
Characterization of Polymerization of Isocyanate Resin and Phenolic Resins of Different Molecular weights. Part I: morphology and structure analysis
 SWST 2015 International Convention Characterization of Polymerization of Isocyanate Resin and Phenolic Resins of Different Molecular weights. Part I: morphology and structure analysis Xiaomei Liu Department
SWST 2015 International Convention Characterization of Polymerization of Isocyanate Resin and Phenolic Resins of Different Molecular weights. Part I: morphology and structure analysis Xiaomei Liu Department
PhysicsAndMathsTutor.com. Advanced Subsidiary Unit 1: The Core Principles of Chemistry
 Write your name here Surname Other names Pearson Edexcel International Advanced Level Centre Number Chemistry Advanced Subsidiary Unit 1: The Core Principles of Chemistry Candidate Number Friday 27 May
Write your name here Surname Other names Pearson Edexcel International Advanced Level Centre Number Chemistry Advanced Subsidiary Unit 1: The Core Principles of Chemistry Candidate Number Friday 27 May
NITRILE RUBBER (NBR) NANOCOMPOSITES BASED ON DIFFERENT FILLER GEOMETRIES (Nanocalcium carbonate, Carbon nanotube and Nanoclay)
 CHAPTER 5 NITRILE RUBBER (NBR) NANOCOMPOSITES BASED ON DIFFERENT FILLER GEOMETRIES (Nanocalcium carbonate, Carbon nanotube and Nanoclay) 5.1 Introduction Nanocalcium carbonate (NCC) is a particulate nanofiller
CHAPTER 5 NITRILE RUBBER (NBR) NANOCOMPOSITES BASED ON DIFFERENT FILLER GEOMETRIES (Nanocalcium carbonate, Carbon nanotube and Nanoclay) 5.1 Introduction Nanocalcium carbonate (NCC) is a particulate nanofiller
Properties of Compounds
 Chapter 6. Properties of Compounds Comparing properties of elements and compounds Compounds are formed when elements combine together in fixed proportions. The compound formed will often have properties
Chapter 6. Properties of Compounds Comparing properties of elements and compounds Compounds are formed when elements combine together in fixed proportions. The compound formed will often have properties
N10/4/CHEMI/SP2/ENG/TZ0/XX CHEMISTRY STANDARD LEVEL PAPER 2. Thursday 11 November 2010 (afternoon) Candidate session number.
 N10/4/CHEMI/SP2/ENG/TZ0/XX 88106105 CHEMISTRY STANDARD LEVEL PAPER 2 Thursday 11 November 2010 (afternoon) 1 hour 15 minutes 0 0 Candidate session number INSTRUCTIONS TO CANDIDATES Write your session number
N10/4/CHEMI/SP2/ENG/TZ0/XX 88106105 CHEMISTRY STANDARD LEVEL PAPER 2 Thursday 11 November 2010 (afternoon) 1 hour 15 minutes 0 0 Candidate session number INSTRUCTIONS TO CANDIDATES Write your session number
Compatibility Investigations on Novel Energetic Formulations of Nitrogen Rich Azole [Bistetrazolylamine(BTA)]
![Compatibility Investigations on Novel Energetic Formulations of Nitrogen Rich Azole [Bistetrazolylamine(BTA)] Compatibility Investigations on Novel Energetic Formulations of Nitrogen Rich Azole [Bistetrazolylamine(BTA)]](/thumbs/93/113068484.jpg) NDIA 216 Insensitive Munitions & Energetic Materials Technology Symposium Compatibility Investigations on Novel Energetic Formulations of Nitrogen Rich Azole [Bistetrazolylamine(BTA)] Sasidharan Nimesh*,
NDIA 216 Insensitive Munitions & Energetic Materials Technology Symposium Compatibility Investigations on Novel Energetic Formulations of Nitrogen Rich Azole [Bistetrazolylamine(BTA)] Sasidharan Nimesh*,
Rheological Properties of ABS at Low Shear Rates: Effects of Phase Heterogeneity
 Malaysian Polymer Journal, Vol 4, No, p9-36, 9 Available online at wwwfkkksautmmy/mpj Rheological Properties of ABS at Low Shear Rates: Effects of Phase Heterogeneity Asif Ali Qaiser *, Yasir Qayyum and
Malaysian Polymer Journal, Vol 4, No, p9-36, 9 Available online at wwwfkkksautmmy/mpj Rheological Properties of ABS at Low Shear Rates: Effects of Phase Heterogeneity Asif Ali Qaiser *, Yasir Qayyum and
F G C G H H C B B A A DD E E
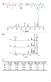 a D E F G H D E I F G H F F I DD E E G H H b c [1] 0 (M) [2a] 0 (M) [1] (M) [2a] (M) [3a] (M) K (M -1 ) a) 0.160 0.139 0.049 0.028 0.111 81 b) 0.147 0.180 0.025 0.058 0.122 84 c) 0.157 0.270 0.013 0.126
a D E F G H D E I F G H F F I DD E E G H H b c [1] 0 (M) [2a] 0 (M) [1] (M) [2a] (M) [3a] (M) K (M -1 ) a) 0.160 0.139 0.049 0.028 0.111 81 b) 0.147 0.180 0.025 0.058 0.122 84 c) 0.157 0.270 0.013 0.126
Tolonate X FLO 100. Bio-based & low viscosity aliphatic isocyanate polymer
 Tolonate X FLO 100 Bio-based & low viscosity aliphatic isocyanate polymer Discover the of Tolonate TM X FLO 100 Tolonate X FLO 100 is a new, partially bio-based, solvent-free and low viscosity aliphatic
Tolonate X FLO 100 Bio-based & low viscosity aliphatic isocyanate polymer Discover the of Tolonate TM X FLO 100 Tolonate X FLO 100 is a new, partially bio-based, solvent-free and low viscosity aliphatic
Bonus Final Exam 3. 1 Calculate the heat of reaction,δh 0 rxn, for the following reaction as written at 298 K: g 2H 2 CH 4. g CF 4.
 Bonus Final Exam 3 1 Calculate the heat of reaction,δh rxn, for the following reaction as written at 298 K: CH 4 2F 2 CF 4 2H 2 substance CH 4 CF 4 ΔH f kj/mol 75 68 (A) ΔH rxn 23 kj (B) ΔH rxn 914 kj
Bonus Final Exam 3 1 Calculate the heat of reaction,δh rxn, for the following reaction as written at 298 K: CH 4 2F 2 CF 4 2H 2 substance CH 4 CF 4 ΔH f kj/mol 75 68 (A) ΔH rxn 23 kj (B) ΔH rxn 914 kj
Effect of Polyacramide as Drilling Fluid in Kuttanadu Soil
 Effect of Polyacramide as Drilling Fluid in Kuttanadu Soil JayaV, Syam N. Professor, Department of Civil Engineering, GECBH, Thiruvannathapuram, Kerala, India Junior Engineer (Civil), Dam Safety Wing,
Effect of Polyacramide as Drilling Fluid in Kuttanadu Soil JayaV, Syam N. Professor, Department of Civil Engineering, GECBH, Thiruvannathapuram, Kerala, India Junior Engineer (Civil), Dam Safety Wing,
MECHANICAL PROPERTIES
 MECHANICAL PROPERTIES Rheology S.C. BAYNE, 1 J.Y. Thompson 2 1 University of Michigan School of Dentistry, Ann Arbor, MI 48109-1078 sbayne@umich.edu 2 Nova Southeastern College of Dental Medicine, Ft.
MECHANICAL PROPERTIES Rheology S.C. BAYNE, 1 J.Y. Thompson 2 1 University of Michigan School of Dentistry, Ann Arbor, MI 48109-1078 sbayne@umich.edu 2 Nova Southeastern College of Dental Medicine, Ft.
Rotational viscometers
 42 Non-Newtonian Flow in the Process Industries Rotational viscometers Due to their relative importance as tools for the rheological characterisation of non-newtonian fluid behaviour, we concentrate on
42 Non-Newtonian Flow in the Process Industries Rotational viscometers Due to their relative importance as tools for the rheological characterisation of non-newtonian fluid behaviour, we concentrate on
U N I T T E S T P R A C T I C E
 South Pasadena AP Chemistry Name 6 Thermodynamics Period Date U N I T T E S T P R A C T I C E Part 1 Multiple Choice You should allocate 25 minutes to finish this portion of the test. No calculator should
South Pasadena AP Chemistry Name 6 Thermodynamics Period Date U N I T T E S T P R A C T I C E Part 1 Multiple Choice You should allocate 25 minutes to finish this portion of the test. No calculator should
Properties of polypropylene modified with elastomers
 Plasticheskie Massy, No. 5, 2005, pp. 31 34 Properties of polypropylene modified with elastomers G. M. Danilova-Volkovskaya Rostov State Academy of Agricultural Engineering Selected from International
Plasticheskie Massy, No. 5, 2005, pp. 31 34 Properties of polypropylene modified with elastomers G. M. Danilova-Volkovskaya Rostov State Academy of Agricultural Engineering Selected from International
Conclusion and Future Work
 Chapter 7 7. Chapter 7 and Future Work Chapter 7 Abstract This chapter gives the details of correlations of the spectroscopic investigation results with those available from other studies and also summarizes
Chapter 7 7. Chapter 7 and Future Work Chapter 7 Abstract This chapter gives the details of correlations of the spectroscopic investigation results with those available from other studies and also summarizes
FOX7/GAP ROCKET PROPELLANTS FOR A SHOULDER LAUNCHED PROJECTILE
 27TH INTERNATIONAL SYMPOSIUM ON BALLISTICS FREIBURG, GERMANY, APRIL 22 2, 13 FOX7/GAP ROCKET PROPELLANTS FOR A SHOULDER LAUNCHED PROJECTILE Hendrik Lips 1 and Klaus Menke 2 1 Dynamit Nobel Defence, 57299
27TH INTERNATIONAL SYMPOSIUM ON BALLISTICS FREIBURG, GERMANY, APRIL 22 2, 13 FOX7/GAP ROCKET PROPELLANTS FOR A SHOULDER LAUNCHED PROJECTILE Hendrik Lips 1 and Klaus Menke 2 1 Dynamit Nobel Defence, 57299
AP Chem Chapter 14 Study Questions
 Class: Date: AP Chem Chapter 14 Study Questions 1. A burning splint will burn more vigorously in pure oxygen than in air because a. oxygen is a reactant in combustion and concentration of oxygen is higher
Class: Date: AP Chem Chapter 14 Study Questions 1. A burning splint will burn more vigorously in pure oxygen than in air because a. oxygen is a reactant in combustion and concentration of oxygen is higher
Review for Exam #1. Review of Mathematics. Weighted Mean
 Review for Exam #1 Review of Mathematics Weighted Mean A certain property of material 1 is P 1 and that of material is P If x 1 amount (weight or volume) of material 1 is mixed with x amount of material,
Review for Exam #1 Review of Mathematics Weighted Mean A certain property of material 1 is P 1 and that of material is P If x 1 amount (weight or volume) of material 1 is mixed with x amount of material,
Chapter 3 Rheological Studies of Polymer Gel Systems
 Rheological Studies of Polymer Gel Systems CHAPTER 3 RHEOLOGICAL STUDIES OF POLYMER GEL SYSTEMS 3.1. INTRODUCTION The rheological behavior of polymer gel solution is the fundamental parameter for its application
Rheological Studies of Polymer Gel Systems CHAPTER 3 RHEOLOGICAL STUDIES OF POLYMER GEL SYSTEMS 3.1. INTRODUCTION The rheological behavior of polymer gel solution is the fundamental parameter for its application
A. 2.5 B. 5.0 C. 10. D. 20 (Total 1 mark) 2. Consider the following reactions. N 2 (g) + O 2 (g) 2NO(g) 2NO 2 (g) 2NO(g) + O 2 (g)
 1. When 100 cm 3 of 1.0 mol dm 3 HCl is mixed with 100 cm 3 of 1.0 mol dm 3 NaOH, the temperature of the resulting solution increases by 5.0 C. What will be the temperature change, in C, when 50 cm 3 of
1. When 100 cm 3 of 1.0 mol dm 3 HCl is mixed with 100 cm 3 of 1.0 mol dm 3 NaOH, the temperature of the resulting solution increases by 5.0 C. What will be the temperature change, in C, when 50 cm 3 of
Determination and Assessment of the Rheological Properties of Pastes for Screen Printing Ceramics
 ANNUAL TRANSACTIONS OF THE NORDIC RHEOLOGY SOCIETY, VOL. 17, 2009 Determination and Assessment of the Rheological Properties of Pastes for Screen Printing Ceramics John W. Phair 1 and Andreas F-J. Kaiser
ANNUAL TRANSACTIONS OF THE NORDIC RHEOLOGY SOCIETY, VOL. 17, 2009 Determination and Assessment of the Rheological Properties of Pastes for Screen Printing Ceramics John W. Phair 1 and Andreas F-J. Kaiser
