Q1. Intensity of M+1 peak x Intensity of M peak 2.57% x 46.60% Number of carbon atoms = Number of carbon atoms =
|
|
|
- Bernadette Clark
- 5 years ago
- Views:
Transcription
1 The mass spectrum of an unknown compound has a molecular ion peak with a relative intensity of 46.60% and an M+1 peak of 2.57%. How many carbon atoms are in the compound? (Fill in an integer number) Number of carbon atoms = Number of carbon atoms = Q1 Intensity of M+1 peak x Intensity of M peak 2.57% x 46.60% Correct Answer = 5
2 Order of Coverage (Exam 1) Homework Assignment Due Date 1 B IR Functional s (wdeadline) Tuesday, August 23 2 B Mass Spec - Molecular Ion (wdeadline) Wednesday, August 24 3 B Mass Spec - Isotope Effects (wdeadline) Thursday, August 25 4 B Number of Peaks 1H NMR Spectra (wdeadline) Friday, August 26 5 B Number of Peaks 13C NMR (wdeadline) Saturday, August 27 6 B Theoretical NMR Chemical Shift (wdeadline) Sunday, August 28 7 B Theoretical NMR Integration (wdeadline) Monday, August 29 8 B Theor. NMR Spin-Spin Splitting (wdeadline) Tuesday, August 30 9 B NMR Spectroscopy Problems (wdeadline) Wednesday, August B C NMR Structure ID (wdeadline) Thursday, September 1 11 B A Nomenclature Alkyl Halides (wdeadline) Friday, September 2 12 B B Alkyl Halide Nomenclature (wdeadline) Saturday, September 3 13 B A Halogenation of Alkanes (wdeadline) Sunday, September 4 14 B B Halogenation of Alkanes (wdeadline) Monday, September 5
3 Order of Coverage (Exam 1) Homework Assignment Due Date 15 B A Oxidation and Anti-oxidants (wdeadline) Tuesday, September 6 16 B Aromaticity (wdeadline) Wednesday, September 7 17 B B Arene Nomenclature (wdeadline) Thursday, September 8 18 B A Halogenation of Arenes (wdeadline) Friday, September 9 19 B B Halogenation of Arenes (wdeadline) Friday, September 9 20 B A Arene Rxns Inorganic Acids (wdeadline) Saturday, September B B Arene Rxns Inorganic Acids (wdeadline) Saturday, September B A Friedel-Crafts (wdeadline) Sunday, September B B Friedel-Crafts (wdeadline) Sunday, September B Arene Mechanistic Issues (wdeadline) Wednesday, September B B Arene Mechanisms (wdeadline) Wednesday, September B A Nucleophilic Aromatic Subs (wdeadline) Thursday, September B B Nucleophilic Aromatic Subs (wdeadline) Friday, September 14 Exam 1 September 18, 19, 20
4 Exam 1 Time: Tuesday, September 20: 7:00 9:00PM Wednesday, September 21: 7:00 9:00PM OR Thursday, September 22: 7:00 10:00PM Location Soc/Anthro Testing Center Chapters will be covered in this order: Chapter 11, 14, 15, 19, 13 Practice Exams are Posted B A Practice Exam 1A B B Practice Exam 1B Deadline for alternate arrangements is Monday, 9/19/2016 at 4:30 PM (i.e., close of business) An oral make-up exam will be required for making up the exam for all students not taking the exam on the above dates or having already made prior arrangements
5 Chemically Equivalent Hydrogens Equivalent hydrogens are H-atoms that are completely interchangeable as to their role in the molecule. One way is to determine whether H s are equivalent is to replace each H with a different group and see if you get a different compound.
6 Chemically Equivalent Hydrogens One way is to determine whether H s are equivalent is to replace each H with a different group and see if you get a different compound. All H s in ethane are chemically equivalent! Replacement of any H with an F results in the same compound!
7 Chemically Equivalent Hydrogens Propane has two (2) groups of chemically equivalent hydrogens. Substitution of any one of the blue H s results in 1-fluoropropane, while substitution of either of the red H s results in 2-fluoropropane
8 How many different groups of chemically equivalent hydrogen atoms are in the following compound? Give a number. A B B A Answer = Q2
9 How many different groups of chemically equivalent hydrogen atoms are in the following compound? Give a number. B B Answer = 3 A C A Q3
10 How many different groups of chemically equivalent hydrogen atoms are in the following compound? Give a number. A Answer = 4 A B C D Q4
11 How many different groups of chemically equivalent hydrogen atoms are in the following compound? Give a number. Answer = Q5
12 How many different groups of chemically equivalent hydrogen atoms are in the following compound? Give a number. E D C Answer = 5 D C B A Q6
13 How many different groups of chemically equivalent hydrogen atoms are in the following compound? Give a number. D, cis to the C H E, trans to the C H D, cis to the C H C B A Answer = 5, due to cis, transissues E, trans to the C H Q7
14 How many different groups of chemically equivalent carbon atoms are in the following compound? Give F E a number. D Answer = 6 E D C B A Q8
15 How many different groups of chemically equivalent carbon atoms are in the following compound? Give D D C a number. B A Answer = Q9
16 Background Material ch/virttxtjml/spectrpy/nmr/nmr1.htm (Last accessed August 23, 2016) OR Google NMR Tutorial
17 What can I learn from 1 H NMR? Each group of chemically equivalent hydrogens gives a signal! Three pieces of information from each signal Chemical Shift Deshielding from Nearest Neighbors Electronegativity (e.g., O, N, C=O, Ar) Pi system effects Integration Number of H s on that carbon atom Spin-spin splitting Number of H s on next carbon atom
18 A Modern Spectrometer Room 110 Clark Hall Cost: $600,000
19 Schematic view of an NMR Spectrometer
20 Introduction to Chemical Shift H nucleus (among others) has a magnetic moment, due to spinning of the charged nucleus. In a magnetic field, there are two possible energetic states where the nuclei are stable. The energy range for changing from one allowed state is in the radio frequency range and is measured in Nuclear Magnetic Resonance.
21 Chemical Shift Depends Primarily on the Electron Cloud Surrounding the Nucleus An electronegative group will remove electrons from the cloud and deshield the nucleus More electron density results in a more shielded nucleus
22 1 H Chemical Shifts Deshielded Shielded
23 Penn s View of 1 H Chemical Shifts Deshielded Strong Mild Electron Electron Withdrawing Withdrawing s s Shielded
24 Integration Area under the peak is proportional to the number of hydrogen atoms
25 Chemical Shift and Integration Examples 4 6
26 Reusch s View of 1 H Chemical Shifts
27 Chemical Shift
28 Approximate Values of Chemical Shifts for 1 H NMR Type of Proton Approximate Chemical Shift ( ) Type of Proton Approximate Chemical Shift ( ) -CH Ar-H CH I-C-H C=C-CH Br-C-H Cl-C-H Ar-CH RNH 2 Variable R-O-H Variable R-O-CH ArOH Variable R 2 C=CHR RCO 2 H Variable
29 Chemical Shift and Integration Examples 2 6
30 Chemical Shift and Integration Examples 3 3
31 Chemical Shift and Integration Examples
32 Chemical Shift and Integration Examples
33 Chemical Shift and Integration Examples
34 Spin-Spin Splitting
35 Splitting Patterns
36 Splitting Patterns
37 Spin-Spin Splitting of C4H8O2
38
39 Worked Example C 7 H 8 O R OH
40 Worked Example (C 8 H 9 BrO)
41 Worked Example (C 4 H 8 O 2 ) Integration: 1 1 6
42 Worked Example (C 5 H 12 O 2 )
43 Worked Example (C 5 H 6 O)
Give the major product(s) of the following reaction.
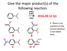 Give the major product(s) of the following reaction. HNO3 H2SO4 2016-09-12 Q1 A B E. There is no reaction or the correct product is not listed here. C D Give the major product(s) of the following reaction.
Give the major product(s) of the following reaction. HNO3 H2SO4 2016-09-12 Q1 A B E. There is no reaction or the correct product is not listed here. C D Give the major product(s) of the following reaction.
A B C D E F G Q1. heat
 Give the final product of the following reaction. Give your answer as a text answer. If more than one species is correct, put your answers in alphabetical order. HNO3 H2SO4 A B C D E F G 2016-09-09 Q1
Give the final product of the following reaction. Give your answer as a text answer. If more than one species is correct, put your answers in alphabetical order. HNO3 H2SO4 A B C D E F G 2016-09-09 Q1
Experiment 2 - NMR Spectroscopy
 Experiment 2 - NMR Spectroscopy OBJECTIVE to understand the important role of nuclear magnetic resonance spectroscopy in the study of the structures of organic compounds to develop an understanding of
Experiment 2 - NMR Spectroscopy OBJECTIVE to understand the important role of nuclear magnetic resonance spectroscopy in the study of the structures of organic compounds to develop an understanding of
Give the major organic product(s) of the following reaction.
 Give the major organic product(s) of the following reaction. 1) NaNO2, HCl 0 o C 2) CuCN 2016-09-23 Q1 A D B C E F There is no reaction or the correct product is not listed here. 5 of 7 Give the major
Give the major organic product(s) of the following reaction. 1) NaNO2, HCl 0 o C 2) CuCN 2016-09-23 Q1 A D B C E F There is no reaction or the correct product is not listed here. 5 of 7 Give the major
NMRis the most valuable spectroscopic technique for organic chemists because it maps the carbon-hydrogen framework of a molecule.
 Chapter 13: Nuclear magnetic resonance spectroscopy NMRis the most valuable spectroscopic technique for organic chemists because it maps the carbon-hydrogen framework of a molecule. 13.2 The nature of
Chapter 13: Nuclear magnetic resonance spectroscopy NMRis the most valuable spectroscopic technique for organic chemists because it maps the carbon-hydrogen framework of a molecule. 13.2 The nature of
ORGANIC - EGE 5E CH NUCLEAR MAGNETIC RESONANCE SPECTROSCOPY
 !! www.clutchprep.com CONCEPT: PURPOSE OF ANALYTICAL TECHNIQUES Classical Methods (Wet Chemistry): Chemists needed to run dozens of chemical reactions to determine the type of molecules in a compound.
!! www.clutchprep.com CONCEPT: PURPOSE OF ANALYTICAL TECHNIQUES Classical Methods (Wet Chemistry): Chemists needed to run dozens of chemical reactions to determine the type of molecules in a compound.
Give a common name for the following compound.
 Give a common name for the following compound. A. Benzyl Phenoate B. Phenyl Phenoate C. Benzyl Benzoate D. Phenyl Benzoate E. Phenyl Phenylacetate F. Phenyl Phenylethanoate G. Phenyl Benzylacetate 2016-10-07
Give a common name for the following compound. A. Benzyl Phenoate B. Phenyl Phenoate C. Benzyl Benzoate D. Phenyl Benzoate E. Phenyl Phenylacetate F. Phenyl Phenylethanoate G. Phenyl Benzylacetate 2016-10-07
NUCLEAR MAGNETIC RESONANCE AND INTRODUCTION TO MASS SPECTROMETRY
 NUCLEAR MAGNETIC RESONANCE AND INTRODUCTION TO MASS SPECTROMETRY A STUDENT SHOULD BE ABLE TO: 1. Identify and explain the processes involved in proton ( 1 H) and carbon-13 ( 13 C) nuclear magnetic resonance
NUCLEAR MAGNETIC RESONANCE AND INTRODUCTION TO MASS SPECTROMETRY A STUDENT SHOULD BE ABLE TO: 1. Identify and explain the processes involved in proton ( 1 H) and carbon-13 ( 13 C) nuclear magnetic resonance
A B C D E F Q1
 Give the major organic product(s) of the following reaction. Give your answer as a text answer, with the correct answers being listed in alphabetical order. (Example: xxxx a b) 1) Li 2) 2016-11-11 Q1 3)
Give the major organic product(s) of the following reaction. Give your answer as a text answer, with the correct answers being listed in alphabetical order. (Example: xxxx a b) 1) Li 2) 2016-11-11 Q1 3)
A B C D Q1
 Think about this reaction in terms of mechanism. All of the intermediates of the reaction are provided. Give the intermediates in order of their appearance along the reaction coordinate. (Example: xxxx
Think about this reaction in terms of mechanism. All of the intermediates of the reaction are provided. Give the intermediates in order of their appearance along the reaction coordinate. (Example: xxxx
NMR = Nuclear Magnetic Resonance
 NMR = Nuclear Magnetic Resonance NMR spectroscopy is the most powerful technique available to organic chemists for determining molecular structures. Looks at nuclei with odd mass numbers or odd number
NMR = Nuclear Magnetic Resonance NMR spectroscopy is the most powerful technique available to organic chemists for determining molecular structures. Looks at nuclei with odd mass numbers or odd number
NMR spectra of some simple molecules. Effect of spinning: averaging field inhomogeneity (nmr1.pdf pg 2)
 NMR spectra of some simple molecules Effect of spinning: averaging field inhomogeneity (nmr1.pdf pg 2) N S H 0 H o Because the protons have a magnetic field associated with them, the field changes as across
NMR spectra of some simple molecules Effect of spinning: averaging field inhomogeneity (nmr1.pdf pg 2) N S H 0 H o Because the protons have a magnetic field associated with them, the field changes as across
Give the major organic product(s) of the following reaction.
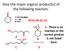 Give the major organic product(s) of the following reaction. 1) CH3CH2MgBr 2) H3O 2016-09-26 Q1 + E. There is no reaction or the correct product A B is not listed here. C D Explanation CH3CH2MgBr 1) CH3CH2MgBr
Give the major organic product(s) of the following reaction. 1) CH3CH2MgBr 2) H3O 2016-09-26 Q1 + E. There is no reaction or the correct product A B is not listed here. C D Explanation CH3CH2MgBr 1) CH3CH2MgBr
CH Exam #4 (Take Home) Date Due: 11/25,26/2013
 CH2710 - Exam #4 (Take Home) Date Due: 11/25,26/2013 Section I - Multiple Choice - Choose the BEST answer from the choices given and place the letter of you choice in the space provided. 1. Energy absorbed
CH2710 - Exam #4 (Take Home) Date Due: 11/25,26/2013 Section I - Multiple Choice - Choose the BEST answer from the choices given and place the letter of you choice in the space provided. 1. Energy absorbed
Nuclear Magnetic Resonance H-NMR Part 1 Introduction to NMR, Instrumentation, Sample Prep, Chemical Shift. Dr. Sapna Gupta
 Nuclear Magnetic Resonance H-NMR Part 1 Introduction to NMR, Instrumentation, Sample Prep, Chemical Shift Dr. Sapna Gupta Introduction NMR is the most powerful tool available for organic structure determination.
Nuclear Magnetic Resonance H-NMR Part 1 Introduction to NMR, Instrumentation, Sample Prep, Chemical Shift Dr. Sapna Gupta Introduction NMR is the most powerful tool available for organic structure determination.
H3O+ heat I Q1
 Give the major organic product(s) of the following reaction. Give your answer as a text answer, with the correct answers being listed in alphabetical order. (Example: xxxx a b) H3O + heat A B C D E F G
Give the major organic product(s) of the following reaction. Give your answer as a text answer, with the correct answers being listed in alphabetical order. (Example: xxxx a b) H3O + heat A B C D E F G
CHEM311 FALL 2005 Practice Exam #3
 CHEM311 FALL 2005 Practice Exam #3 Instructions: This is a multiple choice / short answer practice exam. For the multiple-choice questions, there may be more than one correct answer. If so, then circle
CHEM311 FALL 2005 Practice Exam #3 Instructions: This is a multiple choice / short answer practice exam. For the multiple-choice questions, there may be more than one correct answer. If so, then circle
Chapter 15 Lecture Outline
 Organic Chemistry, First Edition Janice Gorzynski Smith University of Hawaii Chapter 5 Lecture Outline Introduction to NMR Two common types of NMR spectroscopy are used to characterize organic structure:
Organic Chemistry, First Edition Janice Gorzynski Smith University of Hawaii Chapter 5 Lecture Outline Introduction to NMR Two common types of NMR spectroscopy are used to characterize organic structure:
Chapter 14. Nuclear Magnetic Resonance Spectroscopy
 Organic Chemistry, Second Edition Janice Gorzynski Smith University of Hawai i Chapter 14 Nuclear Magnetic Resonance Spectroscopy Prepared by Rabi Ann Musah State University of New York at Albany Copyright
Organic Chemistry, Second Edition Janice Gorzynski Smith University of Hawai i Chapter 14 Nuclear Magnetic Resonance Spectroscopy Prepared by Rabi Ann Musah State University of New York at Albany Copyright
Spectroscopy. Empirical Formula: Chemical Formula: Index of Hydrogen Deficiency (IHD)
 Spectroscopy Empirical Formula: Chemical Formula: Index of Hydrogen Deficiency (IHD) A)From a structure: B)From a molecular formula, C c H h N n O o X x, Formula for saturated hydrocarbons: Subtract the
Spectroscopy Empirical Formula: Chemical Formula: Index of Hydrogen Deficiency (IHD) A)From a structure: B)From a molecular formula, C c H h N n O o X x, Formula for saturated hydrocarbons: Subtract the
Chapter 13: Molecular Spectroscopy
 Chapter 13: Molecular Spectroscopy Electromagnetic Radiation E = hν h = Planck s Constant (6.63 x 10-34 J. s) ν = frequency (s -1 ) c = νλ λ = wavelength (nm) Energy is proportional to frequency Spectrum
Chapter 13: Molecular Spectroscopy Electromagnetic Radiation E = hν h = Planck s Constant (6.63 x 10-34 J. s) ν = frequency (s -1 ) c = νλ λ = wavelength (nm) Energy is proportional to frequency Spectrum
OAT Organic Chemistry - Problem Drill 19: NMR Spectroscopy and Mass Spectrometry
 OAT Organic Chemistry - Problem Drill 19: NMR Spectroscopy and Mass Spectrometry Question No. 1 of 10 Question 1. Which statement concerning NMR spectroscopy is incorrect? Question #01 (A) Only nuclei
OAT Organic Chemistry - Problem Drill 19: NMR Spectroscopy and Mass Spectrometry Question No. 1 of 10 Question 1. Which statement concerning NMR spectroscopy is incorrect? Question #01 (A) Only nuclei
Name: Student Number: University of Manitoba - Department of Chemistry CHEM Introductory Organic Chemistry II - Term Test 1
 Name: Student Number: University of Manitoba - Department of Chemistry CHEM 2220 - Introductory Organic Chemistry II - Term Test 1 Thursday, February 11, 2016; 7-9 PM This is a 2-hour test, marked out
Name: Student Number: University of Manitoba - Department of Chemistry CHEM 2220 - Introductory Organic Chemistry II - Term Test 1 Thursday, February 11, 2016; 7-9 PM This is a 2-hour test, marked out
4) protons experience a net magnetic field strength that is smaller than the applied magnetic field.
 1) Which of the following CANNOT be probed by an spectrometer? See sect 15.1 Chapter 15: 1 A) nucleus with odd number of protons & odd number of neutrons B) nucleus with odd number of protons &even number
1) Which of the following CANNOT be probed by an spectrometer? See sect 15.1 Chapter 15: 1 A) nucleus with odd number of protons & odd number of neutrons B) nucleus with odd number of protons &even number
ORGANIC - CLUTCH CH ANALYTICAL TECHNIQUES: IR, NMR, MASS SPECT
 !! www.clutchprep.com CONCEPT: PURPOSE OF ANALYTICAL TECHNIQUES Classical Methods (Wet Chemistry): Chemists needed to run dozens of chemical reactions to determine the type of molecules in a compound.
!! www.clutchprep.com CONCEPT: PURPOSE OF ANALYTICAL TECHNIQUES Classical Methods (Wet Chemistry): Chemists needed to run dozens of chemical reactions to determine the type of molecules in a compound.
Química Orgânica I. Nuclear Magnetic Resonance Spectroscopy (I) Ciências Farmacêuticas Bioquímica Química AFB QO I 2007/08 1 AFB QO I 2007/08 2
 Química Orgânica I Ciências Farmacêuticas Bioquímica Química AFB QO I 2007/08 1 Nuclear Magnetic Resonance Spectroscopy (I) AFB QO I 2007/08 2 1 Adaptado de: Organic Chemistry, 6th Edition; L. G. Wade,
Química Orgânica I Ciências Farmacêuticas Bioquímica Química AFB QO I 2007/08 1 Nuclear Magnetic Resonance Spectroscopy (I) AFB QO I 2007/08 2 1 Adaptado de: Organic Chemistry, 6th Edition; L. G. Wade,
ORGANIC - CLUTCH CH ANALYTICAL TECHNIQUES: IR, NMR, MASS SPECT
 !! www.clutchprep.com CONCEPT: PURPOSE OF ANALYTICAL TECHNIQUES Classical Methods (Wet Chemistry): Chemists needed to run dozens of chemical reactions to determine the type of molecules in a compound.
!! www.clutchprep.com CONCEPT: PURPOSE OF ANALYTICAL TECHNIQUES Classical Methods (Wet Chemistry): Chemists needed to run dozens of chemical reactions to determine the type of molecules in a compound.
Objective 4. Determine (characterize) the structure of a compound using IR, NMR, MS.
 Objective 4. Determine (characterize) the structure of a compound using IR, NMR, MS. Skills: Draw structure IR: match bond type to IR peak NMR: ID number of non-equivalent H s, relate peak splitting to
Objective 4. Determine (characterize) the structure of a compound using IR, NMR, MS. Skills: Draw structure IR: match bond type to IR peak NMR: ID number of non-equivalent H s, relate peak splitting to
Principles of Molecular Spectroscopy: Electromagnetic Radiation and Molecular structure. Nuclear Magnetic Resonance (NMR)
 Principles of Molecular Spectroscopy: Electromagnetic Radiation and Molecular structure Nuclear Magnetic Resonance (NMR) !E = h" Electromagnetic radiation is absorbed when the energy of photon corresponds
Principles of Molecular Spectroscopy: Electromagnetic Radiation and Molecular structure Nuclear Magnetic Resonance (NMR) !E = h" Electromagnetic radiation is absorbed when the energy of photon corresponds
Experiment 11: NUCLEAR MAGNETIC RESONANCE SPECTROSCOPY
 Experiment 11: NUCLEAR MAGNETIC RESONANCE SPECTROSCOPY Purpose: This is an exercise to introduce the use of nuclear magnetic resonance spectroscopy, in conjunction with infrared spectroscopy, to determine
Experiment 11: NUCLEAR MAGNETIC RESONANCE SPECTROSCOPY Purpose: This is an exercise to introduce the use of nuclear magnetic resonance spectroscopy, in conjunction with infrared spectroscopy, to determine
William H. Brown & Christopher S. Foote
 Requests for permission to make copies of any part of the work should be mailed to:permissions Department, Harcourt Brace & Company, 6277 Sea Harbor Drive, Orlando, Florida 32887-6777 William H. Brown
Requests for permission to make copies of any part of the work should be mailed to:permissions Department, Harcourt Brace & Company, 6277 Sea Harbor Drive, Orlando, Florida 32887-6777 William H. Brown
4) protons experience a net magnetic field strength that is smaller than the applied magnetic field.
 1) Which of the following CANNOT be probed by an NMR spectrometer? See sect 15.1 Chapter 15: 1 A) nucleus with odd number of protons & odd number of neutrons B) nucleus with odd number of protons &even
1) Which of the following CANNOT be probed by an NMR spectrometer? See sect 15.1 Chapter 15: 1 A) nucleus with odd number of protons & odd number of neutrons B) nucleus with odd number of protons &even
Unit 11 Instrumentation. Mass, Infrared and NMR Spectroscopy
 Unit 11 Instrumentation Mass, Infrared and NMR Spectroscopy Spectroscopic identification of organic compounds Qualitative analysis: presence but not quantity (i.e. PEDs) Quantitative analysis: quantity
Unit 11 Instrumentation Mass, Infrared and NMR Spectroscopy Spectroscopic identification of organic compounds Qualitative analysis: presence but not quantity (i.e. PEDs) Quantitative analysis: quantity
1. neopentyl benzene. 4 of 6
 I. 1 H NMR spectroscopy A. Theory 1. The protons and neutrons in atomic nuclei spin, as does the nucleus itself 2. The circulation of nuclear charge can generate a nuclear magnetic moment, u, along the
I. 1 H NMR spectroscopy A. Theory 1. The protons and neutrons in atomic nuclei spin, as does the nucleus itself 2. The circulation of nuclear charge can generate a nuclear magnetic moment, u, along the
CHEMISTRY 263 HOME WORK
 Lecture Topics: CHEMISTRY 263 HOME WORK Module7: Hydrogenation of Alkenes Hydrogenation - syn and anti- addition - hydrogenation of alkynes - synthesis of cis-alkenes -synthesis of trans-alkenes Text sections:
Lecture Topics: CHEMISTRY 263 HOME WORK Module7: Hydrogenation of Alkenes Hydrogenation - syn and anti- addition - hydrogenation of alkynes - synthesis of cis-alkenes -synthesis of trans-alkenes Text sections:
The rest of topic 11 INTRODUCTION TO ORGANIC SPECTROSCOPY
 The rest of topic 11 INTRODUCTION TO ORGANIC SPECTROSCOPY 1. Mass spectrometry: SPECTROSCOPIC TECHNIQUES - A technique capable of identifying the presence of various mass segments of organic molecules.
The rest of topic 11 INTRODUCTION TO ORGANIC SPECTROSCOPY 1. Mass spectrometry: SPECTROSCOPIC TECHNIQUES - A technique capable of identifying the presence of various mass segments of organic molecules.
EASTERN ARIZONA COLLEGE General Organic Chemistry I
 EASTERN ARIZONA COLLEGE General Organic Chemistry I Course Design 2015-2016 Course Information Division Science Course Number CHM 235 (SUN# CHM 2235) Title General Organic Chemistry I Credits 4 Developed
EASTERN ARIZONA COLLEGE General Organic Chemistry I Course Design 2015-2016 Course Information Division Science Course Number CHM 235 (SUN# CHM 2235) Title General Organic Chemistry I Credits 4 Developed
Structure Determination: Nuclear Magnetic Resonance Spectroscopy
 Structure Determination: Nuclear Magnetic Resonance Spectroscopy Why This Chapter? NMR is the most valuable spectroscopic technique used for structure determination More advanced NMR techniques are used
Structure Determination: Nuclear Magnetic Resonance Spectroscopy Why This Chapter? NMR is the most valuable spectroscopic technique used for structure determination More advanced NMR techniques are used
CHEM311 FALL 2005 Practice Exam #3
 EM311 FALL 2005 Practice Exam #3 Instructions: This is a multiple choice / short answer practice exam. For the multiple-choice questions, there may be more than one correct answer. If so, then circle as
EM311 FALL 2005 Practice Exam #3 Instructions: This is a multiple choice / short answer practice exam. For the multiple-choice questions, there may be more than one correct answer. If so, then circle as
- 1/2. = kb o = hνν + 1/2. B o increasing magnetic field strength. degenerate at B o = 0
 NMR EXPERIMENT When magnetically active nuclei are placed into an external magnetic field, the magnetic fields align themselves with the external field into two orientations. During the experiment, electromagnetic
NMR EXPERIMENT When magnetically active nuclei are placed into an external magnetic field, the magnetic fields align themselves with the external field into two orientations. During the experiment, electromagnetic
CHEM Chapter 13. Nuclear Magnetic Spectroscopy (Homework) W
 CHEM 2423. Chapter 13. Nuclear Magnetic Spectroscopy (Homework) W Short Answer 1. For a nucleus to exhibit the nuclear magnetic resonance phenomenon, it must be magnetic. Magnetic nuclei include: a. all
CHEM 2423. Chapter 13. Nuclear Magnetic Spectroscopy (Homework) W Short Answer 1. For a nucleus to exhibit the nuclear magnetic resonance phenomenon, it must be magnetic. Magnetic nuclei include: a. all
Chapter 13 Nuclear Magnetic Resonance Spectroscopy
 Organic Chemistry, 6 th Edition L. G. Wade, Jr. Chapter 13 Nuclear Magnetic Resonance Spectroscopy Jo Blackburn Richland College, Dallas, TX Dallas County Community College District 2006, Prentice Hall
Organic Chemistry, 6 th Edition L. G. Wade, Jr. Chapter 13 Nuclear Magnetic Resonance Spectroscopy Jo Blackburn Richland College, Dallas, TX Dallas County Community College District 2006, Prentice Hall
Proton NMR. Four Questions
 Proton NMR Four Questions How many signals? Equivalence Where on spectrum? Chemical Shift How big? Integration Shape? Splitting (coupling) 1 Proton NMR Shifts Basic Correlation Chart How many 1 H signals?
Proton NMR Four Questions How many signals? Equivalence Where on spectrum? Chemical Shift How big? Integration Shape? Splitting (coupling) 1 Proton NMR Shifts Basic Correlation Chart How many 1 H signals?
Module 13: Chemical Shift and Its Measurement
 Subject Chemistry Paper No and Title Module No and Title Module Tag Paper 12: Organic Spectroscopy CHE_P12_M13_e-Text TABLE OF CONTENTS 1. Learning Outcomes 2. Introduction 3. Shielding and deshielding
Subject Chemistry Paper No and Title Module No and Title Module Tag Paper 12: Organic Spectroscopy CHE_P12_M13_e-Text TABLE OF CONTENTS 1. Learning Outcomes 2. Introduction 3. Shielding and deshielding
16.1 Introduction to NMR Spectroscopy. Spectroscopy. Spectroscopy. Spectroscopy. Spectroscopy. Spectroscopy 4/11/2013
 What is spectroscopy? NUCLEAR MAGNETIC RESONANCE (NMR) spectroscopy may be the most powerful method of gaining structural information about organic compounds. NMR involves an interaction between electromagnetic
What is spectroscopy? NUCLEAR MAGNETIC RESONANCE (NMR) spectroscopy may be the most powerful method of gaining structural information about organic compounds. NMR involves an interaction between electromagnetic
Module9. Nuclear Magnetic Resonance Spectroscopy Nuclear Magnetic Resonance (NMR) spectroscopy - Chemical shift - Integration of signal area
 1 CHEMISTRY 263 HOME WORK Lecture Topics: Module7. Hydrogenation of Alkenes The Function of the Catalyst - Syn and anti- addition Hydrogenation of Alkynes - Syn- addition of hydrogen: Synthesis of cis-alkenes
1 CHEMISTRY 263 HOME WORK Lecture Topics: Module7. Hydrogenation of Alkenes The Function of the Catalyst - Syn and anti- addition Hydrogenation of Alkynes - Syn- addition of hydrogen: Synthesis of cis-alkenes
Instrumental Chemical Analysis
 L15 Page1 Instrumental Chemical Analysis Nuclear Magnetic Resonance Dr. Ahmad Najjar Philadelphia University Faculty of Pharmacy Department of Pharmaceutical Sciences 1 st semester, 2017/2018 Nuclear Magnetic
L15 Page1 Instrumental Chemical Analysis Nuclear Magnetic Resonance Dr. Ahmad Najjar Philadelphia University Faculty of Pharmacy Department of Pharmaceutical Sciences 1 st semester, 2017/2018 Nuclear Magnetic
Electrophiles are attracted to the π bond Addition sees a π bond replaced with a σ bond There are many different types of addition reactions:
 Nucleophiles and Electrophiles Nucleophiles are the atoms that donates the electron pairs and is added to the molecules (In the example above this is the CN) Electrophiles are the atoms that seek electron
Nucleophiles and Electrophiles Nucleophiles are the atoms that donates the electron pairs and is added to the molecules (In the example above this is the CN) Electrophiles are the atoms that seek electron
NMR Spectroscopy. Chapter 19
 NMR Spectroscopy Chapter 19 Nuclear Magnetic Resonance spectroscopy is a powerful analytical technique used to characterize organic molecules by identifying carbon-hydrogen frameworks within molecules.
NMR Spectroscopy Chapter 19 Nuclear Magnetic Resonance spectroscopy is a powerful analytical technique used to characterize organic molecules by identifying carbon-hydrogen frameworks within molecules.
C NMR Spectroscopy
 13.14 13 C NMR Spectroscopy 1 H and 13 C NMR compared: both give us information about the number of chemically nonequivalent nuclei (nonequivalent hydrogens or nonequivalent carbons) both give us information
13.14 13 C NMR Spectroscopy 1 H and 13 C NMR compared: both give us information about the number of chemically nonequivalent nuclei (nonequivalent hydrogens or nonequivalent carbons) both give us information
3.15 Nuclear Magnetic Resonance Spectroscopy, NMR
 3.15 Nuclear Magnetic Resonance Spectroscopy, NMR What is Nuclear Magnetic Resonance - NMR Developed by chemists and physicists together it works by the interaction of magnetic properties of certain nuclei
3.15 Nuclear Magnetic Resonance Spectroscopy, NMR What is Nuclear Magnetic Resonance - NMR Developed by chemists and physicists together it works by the interaction of magnetic properties of certain nuclei
January 30, 2018 Chemistry 328N
 Lecture 4 Some More nmr January 30, 2018 Tricks for solving unknowns Review. Empirical formula is lowest common denominator ratio of atomic composition From Homework: unknown has an empirical formula of
Lecture 4 Some More nmr January 30, 2018 Tricks for solving unknowns Review. Empirical formula is lowest common denominator ratio of atomic composition From Homework: unknown has an empirical formula of
16.1 Introduction to NMR. Spectroscopy
 16.1 Introduction to NMR What is spectroscopy? Spectroscopy NUCLEAR MAGNETIC RESNANCE (NMR) spectroscopy may be the most powerful method of gaining structural information about organic compounds. NMR involves
16.1 Introduction to NMR What is spectroscopy? Spectroscopy NUCLEAR MAGNETIC RESNANCE (NMR) spectroscopy may be the most powerful method of gaining structural information about organic compounds. NMR involves
4) protons experience a net magnetic field strength that is smaller than the applied magnetic field.
 1) Which of the following CANNOT be probed by an spectrometer? See sect 16.1 Chapter 16: 1 A) nucleus with odd number of protons & odd number of neutrons B) nucleus with odd number of protons &even number
1) Which of the following CANNOT be probed by an spectrometer? See sect 16.1 Chapter 16: 1 A) nucleus with odd number of protons & odd number of neutrons B) nucleus with odd number of protons &even number
ORGANIC - BROWN 8E CH NUCLEAR MAGNETIC RESONANCE.
 !! www.clutchprep.com CONCEPT: 1 H NUCLEAR MAGNETIC RESONANCE- GENERAL FEATURES 1 H (Proton) NMR is a powerful instrumental method that identifies protons in slightly different electronic environments
!! www.clutchprep.com CONCEPT: 1 H NUCLEAR MAGNETIC RESONANCE- GENERAL FEATURES 1 H (Proton) NMR is a powerful instrumental method that identifies protons in slightly different electronic environments
UCF - ORGANIC CHEMISTRY 2 - PROF. GERASIMOVA UCF PROF. GERASIMOVA EXAM REVIEW 1.
 UCF PROF. GERASIMOVA EXAM REVIEW 1 www.clutchprep.com 1 PRACTICE: Determine the index of hydrogen deficiency (degrees of unsaturation) for the following molecule. Antipsychotic - Haloperidol = C 21 H 23
UCF PROF. GERASIMOVA EXAM REVIEW 1 www.clutchprep.com 1 PRACTICE: Determine the index of hydrogen deficiency (degrees of unsaturation) for the following molecule. Antipsychotic - Haloperidol = C 21 H 23
Spectroscopy in Organic Chemistry. Types of Spectroscopy in Organic
 Spectroscopy in Organic Chemistry Spectroscopy Spectrum dealing with light, or more specifically, radiation Scope to see Organic Spectroscopy therefore deals with examining how organic molecules interact
Spectroscopy in Organic Chemistry Spectroscopy Spectrum dealing with light, or more specifically, radiation Scope to see Organic Spectroscopy therefore deals with examining how organic molecules interact
Name: Student Number: University of Manitoba - Department of Chemistry CHEM Introductory Organic Chemistry II - Term Test 1
 Name: Student Number: University of Manitoba - Department of Chemistry CHEM 2220 - Introductory Organic Chemistry II - Term Test 1 Thursday, February 12, 2015; 7-9 PM This is a 2-hour test, marked out
Name: Student Number: University of Manitoba - Department of Chemistry CHEM 2220 - Introductory Organic Chemistry II - Term Test 1 Thursday, February 12, 2015; 7-9 PM This is a 2-hour test, marked out
4. NMR spectra. Interpreting NMR spectra. Low-resolution NMR spectra. There are two kinds: Low-resolution NMR spectra. High-resolution NMR spectra
 1 Interpreting NMR spectra There are two kinds: Low-resolution NMR spectra High-resolution NMR spectra In both cases the horizontal scale is labelled in terms of chemical shift, δ, and increases from right
1 Interpreting NMR spectra There are two kinds: Low-resolution NMR spectra High-resolution NMR spectra In both cases the horizontal scale is labelled in terms of chemical shift, δ, and increases from right
Chapter 13: Nuclear Magnetic Resonance (NMR) Spectroscopy direct observation of the H s and C s of a molecules
 hapter 13: Nuclear Magnetic Resonance (NMR) Spectroscopy direct observation of the s and s of a molecules Nuclei are positively charged and spin on an axis; they create a tiny magnetic field + + Not all
hapter 13: Nuclear Magnetic Resonance (NMR) Spectroscopy direct observation of the s and s of a molecules Nuclei are positively charged and spin on an axis; they create a tiny magnetic field + + Not all
Chapter 4: Aromatic Compounds. Bitter almonds are the source of the aromatic compound benzaldehyde
 Chapter 4: Aromatic Compounds Bitter almonds are the source of the aromatic compound benzaldehyde Sources of Benzene Benzene, C 6 H 6, is the parent hydrocarbon of the especially stable compounds known
Chapter 4: Aromatic Compounds Bitter almonds are the source of the aromatic compound benzaldehyde Sources of Benzene Benzene, C 6 H 6, is the parent hydrocarbon of the especially stable compounds known
Nuclear Magnetic Resonance (NMR) Spectroscopy Introduction:
 Nuclear Magnetic Resonance (NMR) Spectroscopy Introduction: Nuclear magnetic resonance spectroscopy (NMR) is the most powerful tool available for organic structure determination. Like IR spectroscopy,
Nuclear Magnetic Resonance (NMR) Spectroscopy Introduction: Nuclear magnetic resonance spectroscopy (NMR) is the most powerful tool available for organic structure determination. Like IR spectroscopy,
1. Predict the structure of the molecules given by the following spectral data: a Mass spectrum:m + = 116
 Additional Problems for practice.. Predict the structure of the molecules given by the following spectral data: a Mass spectrum:m + = IR: weak absorption at 9 cm - medium absorption at cm - NMR 7 3 3 C
Additional Problems for practice.. Predict the structure of the molecules given by the following spectral data: a Mass spectrum:m + = IR: weak absorption at 9 cm - medium absorption at cm - NMR 7 3 3 C
PAPER No. 12: ORGANIC SPECTROSCOPY. Module 19: NMR Spectroscopy of N, P and F-atoms
 Subject Chemistry Paper No and Title Module No and Title Module Tag Paper 12: Organic Spectroscopy CHE_P12_M19_e-Text TABLE OF CONTENTS 1. Learning Outcomes 2. 15 N NMR spectroscopy 3. 19 F NMR spectroscopy
Subject Chemistry Paper No and Title Module No and Title Module Tag Paper 12: Organic Spectroscopy CHE_P12_M19_e-Text TABLE OF CONTENTS 1. Learning Outcomes 2. 15 N NMR spectroscopy 3. 19 F NMR spectroscopy
Lecture 14 Organic Chemistry 1
 CHEM 232 Organic Chemistry I at Chicago Lecture 14 Organic Chemistry 1 Professor Duncan Wardrop February 25, 2010 1 CHEM 232 Organic Chemistry I at Chicago Mass Spectrometry Sections: 13.24-13.25 2 Spectroscopy
CHEM 232 Organic Chemistry I at Chicago Lecture 14 Organic Chemistry 1 Professor Duncan Wardrop February 25, 2010 1 CHEM 232 Organic Chemistry I at Chicago Mass Spectrometry Sections: 13.24-13.25 2 Spectroscopy
Chapter 14 Spectroscopy
 hapter 14 Spectroscopy There are four major analytical techniques used for identifying the structure of organic molecules 1. Nuclear Magnetic Resonance or NMR is the single most important technique for
hapter 14 Spectroscopy There are four major analytical techniques used for identifying the structure of organic molecules 1. Nuclear Magnetic Resonance or NMR is the single most important technique for
NMR Nuclear Magnetic Resonance Spectroscopy p. 83. a hydrogen nucleus (a proton) has a charge, spread over the surface
 NMR Nuclear Magnetic Resonance Spectroscopy p. 83 a hydrogen nucleus (a proton) has a charge, spread over the surface a spinning charge produces a magnetic moment (a vector = direction + magnitude) along
NMR Nuclear Magnetic Resonance Spectroscopy p. 83 a hydrogen nucleus (a proton) has a charge, spread over the surface a spinning charge produces a magnetic moment (a vector = direction + magnitude) along
Learning Guide for Chapter 11 - Alkenes I
 Learning Guide for Chapter 11 - Alkenes I I. Introduction to alkenes - p 1 bond structure, classifying alkenes, reactivity, physical properties, occurrences and uses, spectroscopy, stabilty II. Unsaturation
Learning Guide for Chapter 11 - Alkenes I I. Introduction to alkenes - p 1 bond structure, classifying alkenes, reactivity, physical properties, occurrences and uses, spectroscopy, stabilty II. Unsaturation
Using NMR and IR Spectroscopy to Determine Structures Dr. Carl Hoeger, UCSD
 Using NMR and IR Spectroscopy to Determine Structures Dr. Carl Hoeger, UCSD The following guidelines should be helpful in assigning a structure from NMR (both PMR and CMR) and IR data. At the end of this
Using NMR and IR Spectroscopy to Determine Structures Dr. Carl Hoeger, UCSD The following guidelines should be helpful in assigning a structure from NMR (both PMR and CMR) and IR data. At the end of this
Chapter 13 Structure t Determination: Nuclear Magnetic Resonance Spectroscopy
 John E. McMurry www.cengage.com/chemistry/mcmurry Chapter 13 Structure t Determination: ti Nuclear Magnetic Resonance Spectroscopy Revisions by Dr. Daniel Holmes MSU Paul D. Adams University of Arkansas
John E. McMurry www.cengage.com/chemistry/mcmurry Chapter 13 Structure t Determination: ti Nuclear Magnetic Resonance Spectroscopy Revisions by Dr. Daniel Holmes MSU Paul D. Adams University of Arkansas
HOWEVER please produce a poster no smaller than 11 x 17 inches which represents the contents of your presentation for submission on June 8th:
 Notice to CH 242 Students This represents the new material that will be ~50% of the Cumulative final on Tuesday June 8th. The other review material will be similar to previous exams/practice exams homework
Notice to CH 242 Students This represents the new material that will be ~50% of the Cumulative final on Tuesday June 8th. The other review material will be similar to previous exams/practice exams homework
Required Materials For complete material(s) information, refer to
 Butler Community College Science, Technology, Engineering, and Math Division Robert Carlson Revised Fall 2017 Implemented Spring 2018 COURSE OUTLINE Organic Chemistry 1 Course Description CH 240. Organic
Butler Community College Science, Technology, Engineering, and Math Division Robert Carlson Revised Fall 2017 Implemented Spring 2018 COURSE OUTLINE Organic Chemistry 1 Course Description CH 240. Organic
Lecture 2 nmr Spectroscopy
 Lecture 2 nmr Spectroscopy Pages 427 430 and Chapter 13 Molecular Spectroscopy Molecular spectroscopy: the study of the frequencies of electromagnetic radiation that are absorbed or emitted by substances
Lecture 2 nmr Spectroscopy Pages 427 430 and Chapter 13 Molecular Spectroscopy Molecular spectroscopy: the study of the frequencies of electromagnetic radiation that are absorbed or emitted by substances
Nuclear Magnetic Resonance Spectroscopy: Tools for Structure Determination
 Nuclear Magnetic Resonance Spectroscopy: Tools for Structure Determination Chung-Ming Sun Department of Applied Chemistry National Chiao Tung University Hualien 300, Taiwan Introduction NMR (Nuclear Magnetic
Nuclear Magnetic Resonance Spectroscopy: Tools for Structure Determination Chung-Ming Sun Department of Applied Chemistry National Chiao Tung University Hualien 300, Taiwan Introduction NMR (Nuclear Magnetic
Paper 12: Organic Spectroscopy
 Subject Chemistry Paper No and Title Module No and Title Module Tag Paper 12: Organic Spectroscopy 31: Combined problem on UV, IR, 1 H NMR, 13 C NMR and Mass - Part III CHE_P12_M31 TABLE OF CONTENTS 1.
Subject Chemistry Paper No and Title Module No and Title Module Tag Paper 12: Organic Spectroscopy 31: Combined problem on UV, IR, 1 H NMR, 13 C NMR and Mass - Part III CHE_P12_M31 TABLE OF CONTENTS 1.
Basic Concepts of NMR: Identification of the Isomers of C 4 O 2. by 1 H NMR Spectroscopy
 Basic Concepts of NM: Identification of the Isomers of C H 8 O by H NM Spectroscopy Objectives NM spectroscopy is a powerful tool in determining the structure of compounds. Not only is it able to give
Basic Concepts of NM: Identification of the Isomers of C H 8 O by H NM Spectroscopy Objectives NM spectroscopy is a powerful tool in determining the structure of compounds. Not only is it able to give
Course Syllabus. Department: Science & Technology. Date: April I. Course Prefix and Number: CHM 212. Course Name: Organic Chemistry II
 Department: Science & Technology Date: April 2012 I. Course Prefix and Number: CHM 212 Course Name: Organic Chemistry II Course Syllabus Credit Hours and Contact Hours: 5 credit hours and 7 (3:3:1) contact
Department: Science & Technology Date: April 2012 I. Course Prefix and Number: CHM 212 Course Name: Organic Chemistry II Course Syllabus Credit Hours and Contact Hours: 5 credit hours and 7 (3:3:1) contact
NUCLEAR MAGNETIC RESONANCE SPECTROSCOPY
 NMR Spectroscopy 1 NULEAR MAGNETI RESONANE SPETROSOPY Involves interaction of materials with the low-energy radiowave region of the electromagnetic spectrum Origin of Spectra Theory All nuclei possess
NMR Spectroscopy 1 NULEAR MAGNETI RESONANE SPETROSOPY Involves interaction of materials with the low-energy radiowave region of the electromagnetic spectrum Origin of Spectra Theory All nuclei possess
Chapter 13 Spectroscopy
 hapter 13 Spectroscopy Infrared spectroscopy Ultraviolet-Visible spectroscopy Nuclear magnetic resonance spectroscopy Mass Spectrometry 13.1 Principles of Molecular Spectroscopy: Electromagnetic Radiation
hapter 13 Spectroscopy Infrared spectroscopy Ultraviolet-Visible spectroscopy Nuclear magnetic resonance spectroscopy Mass Spectrometry 13.1 Principles of Molecular Spectroscopy: Electromagnetic Radiation
ORGANIC - BRUICE 8E CH MASS SPECT AND INFRARED SPECTROSCOPY
 !! www.clutchprep.com CONCEPT: PURPOSE OF ANALYTICAL TECHNIQUES Classical Methods (Wet Chemistry): Chemists needed to run dozens of chemical reactions to determine the type of molecules in a compound.
!! www.clutchprep.com CONCEPT: PURPOSE OF ANALYTICAL TECHNIQUES Classical Methods (Wet Chemistry): Chemists needed to run dozens of chemical reactions to determine the type of molecules in a compound.
4) protons experience a net magnetic field strength that is smaller than the applied magnetic field.
 1) Which of the following CANNOT be probed by an spectrometer? See sect 16.1 Chapter 16: 1 A) nucleus with odd number of protons & odd number of neutrons B) nucleus with odd number of protons &even number
1) Which of the following CANNOT be probed by an spectrometer? See sect 16.1 Chapter 16: 1 A) nucleus with odd number of protons & odd number of neutrons B) nucleus with odd number of protons &even number
CHEMISTRY Organic Chemistry Laboratory II Spring 2019 Lab #5: NMR Spectroscopy
 Team Members: Unknown # CHEMISTRY 244 - Organic Chemistry Laboratory II Spring 2019 Lab #5: NMR Spectroscopy Purpose: You will learn how to predict the NMR data for organic molecules, organize this data
Team Members: Unknown # CHEMISTRY 244 - Organic Chemistry Laboratory II Spring 2019 Lab #5: NMR Spectroscopy Purpose: You will learn how to predict the NMR data for organic molecules, organize this data
Indirect Coupling. aka: J-coupling, indirect spin-spin coupling, indirect dipole-dipole coupling, mutual coupling, scalar coupling (liquids only)
 Indirect Coupling aka: J-coupling, indirect spin-spin coupling, indirect dipole-dipole coupling, mutual coupling, scalar coupling (liquids only) First, two comments about direct coupling Nuclear spins
Indirect Coupling aka: J-coupling, indirect spin-spin coupling, indirect dipole-dipole coupling, mutual coupling, scalar coupling (liquids only) First, two comments about direct coupling Nuclear spins
Chem 2320 Exam 1. January 30, (Please print)
 Chem 2320 Exam 1 January 30, 2006 Name: (first) (last) (Please print) Last 4 digits of I.D. I. Multiple Choice ( /20) Score /60 II /15 III /25 Total score /100 I. Multiple choice questions. (3 points each).
Chem 2320 Exam 1 January 30, 2006 Name: (first) (last) (Please print) Last 4 digits of I.D. I. Multiple Choice ( /20) Score /60 II /15 III /25 Total score /100 I. Multiple choice questions. (3 points each).
A-LEVEL A-LEVEL CHEMISTRY CHEMISTRY NOTES
 A-LEVEL A-LEVEL CHEMISTRY CHEMISTRY NOTES snaprevise.co.uk I have designed and compiled these beautiful notes to provide a detailed but concise summary of this module. I have spent a lot of time perfecting
A-LEVEL A-LEVEL CHEMISTRY CHEMISTRY NOTES snaprevise.co.uk I have designed and compiled these beautiful notes to provide a detailed but concise summary of this module. I have spent a lot of time perfecting
Chapter 24. Amines. Based on McMurry s Organic Chemistry, 7 th edition
 Chapter 24. Amines Based on McMurry s Organic Chemistry, 7 th edition Amines Organic Nitrogen Compounds Organic derivatives of ammonia, NH 3, Nitrogen atom with a lone pair of electrons, making amines
Chapter 24. Amines Based on McMurry s Organic Chemistry, 7 th edition Amines Organic Nitrogen Compounds Organic derivatives of ammonia, NH 3, Nitrogen atom with a lone pair of electrons, making amines
Chemistry 3720 Old Exams. Practice Exams & Keys
 Chemistry 3720 ld Exams Practice Exams & Keys 2015-17 Spring 2017 Page File 3 Spring 2017 Exam 1 10 Spring 2017 Exam 1 Key 16 Spring 2017 Exam 2 23 Spring 2017 Exam 2 Key 29 Spring 2017 Exam 3 36 Spring
Chemistry 3720 ld Exams Practice Exams & Keys 2015-17 Spring 2017 Page File 3 Spring 2017 Exam 1 10 Spring 2017 Exam 1 Key 16 Spring 2017 Exam 2 23 Spring 2017 Exam 2 Key 29 Spring 2017 Exam 3 36 Spring
C h a p t e r S i x t e e n: Nuclear Magnetic Resonance Spectroscopy. An 1 H NMR FID of ethanol
 0.2 0.4 0.6 0.8 1.0 1.2 1.4 1.6 1.8 2.0 2.2 2.4 2.6 2.8 3.0 3.2 3.4 3.6 C h a p t e r S i x t e e n: Nuclear Magnetic Resonance Spectroscopy An 1 NMR FID of ethanol Note: Problems with italicized numbers
0.2 0.4 0.6 0.8 1.0 1.2 1.4 1.6 1.8 2.0 2.2 2.4 2.6 2.8 3.0 3.2 3.4 3.6 C h a p t e r S i x t e e n: Nuclear Magnetic Resonance Spectroscopy An 1 NMR FID of ethanol Note: Problems with italicized numbers
The Use of NMR Spectroscopy
 Spektroskopi Molekul Organik (SMO): Nuclear Magnetic Resonance (NMR) Spectroscopy All is adopted from McMurry s Organic Chemistry The Use of NMR Spectroscopy Used to determine relative location of atoms
Spektroskopi Molekul Organik (SMO): Nuclear Magnetic Resonance (NMR) Spectroscopy All is adopted from McMurry s Organic Chemistry The Use of NMR Spectroscopy Used to determine relative location of atoms
Department of Chemistry SUNY/Oneonta. Chem Organic Chemistry I
 Department of Chemistry SUNY/Oneonta Chem 221 - Organic Chemistry I Examination #4 - ANSWERS - December 11, 2000 Answer to question #32 corrected 12/13/00, 8:30pm. INSTRUCTIONS This examination is in multiple
Department of Chemistry SUNY/Oneonta Chem 221 - Organic Chemistry I Examination #4 - ANSWERS - December 11, 2000 Answer to question #32 corrected 12/13/00, 8:30pm. INSTRUCTIONS This examination is in multiple
(2) Read each statement carefully and pick the one that is incorrect in its information.
 Organic Chemistry - Problem Drill 17: IR and Mass Spectra No. 1 of 10 1. Which statement about infrared spectroscopy is incorrect? (A) IR spectroscopy is a method of structure determination based on the
Organic Chemistry - Problem Drill 17: IR and Mass Spectra No. 1 of 10 1. Which statement about infrared spectroscopy is incorrect? (A) IR spectroscopy is a method of structure determination based on the
12.1 The Nature of Organic molecules
 12.1 The Nature of Organic molecules Organic chemistry: : The chemistry of carbon compounds. Carbon is tetravalent; it always form four bonds. Prentice Hall 2003 Chapter One 2 Organic molecules have covalent
12.1 The Nature of Organic molecules Organic chemistry: : The chemistry of carbon compounds. Carbon is tetravalent; it always form four bonds. Prentice Hall 2003 Chapter One 2 Organic molecules have covalent
Qualitative Analysis of Unknown Compounds
 Qualitative Analysis of Unknown Compounds 1. Infrared Spectroscopy Identification of functional groups in the unknown All functional groups are fair game (but no anhydride or acid halides, no alkenes or
Qualitative Analysis of Unknown Compounds 1. Infrared Spectroscopy Identification of functional groups in the unknown All functional groups are fair game (but no anhydride or acid halides, no alkenes or
Chapter 16 Nuclear Magnetic Resonance Spectroscopy
 hapter 16 Nuclear Magnetic Resonance Spectroscopy The Spinning Proton A spinning proton generates a magnetic field, resembling that of a small bar magnet. An odd number of protons in the nucleus creates
hapter 16 Nuclear Magnetic Resonance Spectroscopy The Spinning Proton A spinning proton generates a magnetic field, resembling that of a small bar magnet. An odd number of protons in the nucleus creates
Tuesday, January 13, NMR Spectroscopy
 NMR Spectroscopy NMR Phenomenon Nuclear Magnetic Resonance µ A spinning charged particle generates a magnetic field. A nucleus with a spin angular momentum will generate a magnetic moment (μ). If these
NMR Spectroscopy NMR Phenomenon Nuclear Magnetic Resonance µ A spinning charged particle generates a magnetic field. A nucleus with a spin angular momentum will generate a magnetic moment (μ). If these
CHEM 322 Laboratory Methods in Organic Chemistry. Introduction to NMR Spectroscopy
 EM 322 Laboratory Methods in Organic hemistry Introduction to NMR Spectroscopy What structural information does NMR spectroscopy provide? 1) hemical shift (δ) data reveals the molecular (functional group)
EM 322 Laboratory Methods in Organic hemistry Introduction to NMR Spectroscopy What structural information does NMR spectroscopy provide? 1) hemical shift (δ) data reveals the molecular (functional group)
Lecture 02 Nuclear Magnetic Resonance Spectroscopy Principle and Application in Structure Elucidation
 Application of Spectroscopic Methods in Molecular Structure Determination Prof. S. Sankararaman Department of Chemistry Indian Institution of Technology Madras Lecture 02 Nuclear Magnetic Resonance Spectroscopy
Application of Spectroscopic Methods in Molecular Structure Determination Prof. S. Sankararaman Department of Chemistry Indian Institution of Technology Madras Lecture 02 Nuclear Magnetic Resonance Spectroscopy
Chem 325 NMR Intro. The Electromagnetic Spectrum. Physical properties, chemical properties, formulas Shedding real light on molecular structure:
 Physical properties, chemical properties, formulas Shedding real light on molecular structure: Wavelength Frequency ν Wavelength λ Frequency ν Velocity c = 2.998 10 8 m s -1 The Electromagnetic Spectrum
Physical properties, chemical properties, formulas Shedding real light on molecular structure: Wavelength Frequency ν Wavelength λ Frequency ν Velocity c = 2.998 10 8 m s -1 The Electromagnetic Spectrum
Chemistry 1110 Exam 4 Study Guide
 Chapter 10 Chemistry 1110 Exam 4 Study Guide 10.1 Know that unstable nuclei can undergo radioactive decay. Identify alpha particles, beta particles, and/or gamma rays based on physical properties such
Chapter 10 Chemistry 1110 Exam 4 Study Guide 10.1 Know that unstable nuclei can undergo radioactive decay. Identify alpha particles, beta particles, and/or gamma rays based on physical properties such
Lecture 5 Still More nmr
 Lecture 5 Still More nmr three spins February 5, 2019 Supplemental Problem Chlorocyclobutane Origins of Signal Splitting B 0 H b Magnetic field of H b subtracts from the applied field; H b signal appears
Lecture 5 Still More nmr three spins February 5, 2019 Supplemental Problem Chlorocyclobutane Origins of Signal Splitting B 0 H b Magnetic field of H b subtracts from the applied field; H b signal appears
