on-line analyzer systems for safety and control of chlorine production plants by Chris Du Bois by Thibaut Bettini Buenos Aires Argentina Nov.
|
|
|
- Marlene Short
- 5 years ago
- Views:
Transcription
1 on-line analyzer systems for safety and control of chlorine production plants by Chris Du Bois Exc. VP Systems Integration Div. AppliTek by Thibaut Bettini Project Engineer AppliTek Buenos Aires Argentina Nov. 2016
2 anode cathode MEMBRANE Re-Generation Chemicals ION EXCHANGER A ION EXCHANGER B Re-Generation ION EXCHANGER C BRINE DE- CHLORINATOR 200 AppliTek NV/SA, Vacuum EZ-Brine on-line calcium (Ca 2+ ) and magnesium (Mg 2+ ) in Ultra-Purified brine caustic soda (NaOH) Further Chlorine Processing Water sodium carbonate (Na 2 CO 3 ) hydroxides (NaOH) Return Brine or Depleted Brine Filtration Aid DEPLETED BRINE TANK HYDROGEN PURIFICATIO N Salt Raw Saturated Brine SETTLING Pre-Purified Brine FILTRATION Chemicals WET chlorine (Cl 2 ) hydrogen (H 2 ) WHY brine QUALITY Sludge Solids Precipitated AVOID membrane damage CaCO 3 ( ) range: ppb Mg(OH) 2 ( ) 5 EZ-Brine calcium (Ca 2+ ) & magnesium (Mg 2+ ) in Ultra-Purified Brine hydrogen chloride (HCl) 4 ACIDIFICATION 6 BRINE STORAGE 320 g/l NaCl Ultra-Purified Brine Depleted Brine Saturate d Brine Cl 2 H 2 + _ ELECTROLYZER 33% 30% NaOH Water NaOH caustic soda (NaOH) Where in Process?
3 4 5 6 EZ-Brine on-line calcium (Ca 2+ ) and magnesium (Mg 2+ ) in Ultra-Purified brine AppliTek delivers an analyzer system guaranteeing analysis results other analyzer suppliers selling only an analyzer Field Proven Best Available Technology Analysis Result = Analyzer + Application + Preconditioning WHY on-line EZ-Brine?
4 4 5 6 EZ-Brine on-line calcium (Ca 2+ ) and magnesium (Mg 2+ ) in Ultra-Purified brine AppliTek delivers an analyzer system guaranteeing analysis results HVAC temperature control sample + analyzer Sample Cooler / Heater Field Proven Best Available Technology Why on-line EZ-Brine?
5 4 5 6 EZ-Brine on-line calcium (Ca 2+ ) and magnesium (Mg 2+ ) in Ultra-Purified brine control of elephant control of mosquito Challenge! amalgam chlorine plant membrane chlorine plant
6 4 5 6 EZ-Brine on-line calcium (Ca 2+ ) and magnesium (Mg 2+ ) in Ultra-Purified brine typical EZ-Brine range: 0 20 ppb Ca ++ & Mg ++ (detection limit < 1 ppb) 1 ppb = 1 part per billion 1 ppb equals finding approx. one coin in the Andes mountains +20 ppb of Ca ++ & Mg ++ in brine can already damage your membrane chlorine production 7,000,000,000 mm 7,000,000 meter 7,000 km 7 mm Andes mountains length equals to 1 ppb
7 4 5 6 EZ-Brine on-line calcium (Ca 2+ ) and magnesium (Mg 2+ ) in Ultra-Purified brine on-line Ca 2+ & Mg 2+ control has a Direct Financial impact. membrane damage by Ca 2+ & Mg 2+ is nonreturnable high costs! Electrical Power Cost Increase Membrane Replacement Cost (typical life-time > 5 years) Shut down Cost (production loss) Labor Cost Why EZ-Brine?
8 4 5 6 EZ-Brine on-line calcium (Ca 2+ ) and magnesium (Mg 2+ ) in Ultra-Purified brine Analysis Colorimetric method injection of brine sample in EZ-Brine analyzer addition of Sodium hydroxide (NaOH) buffer Alkaline addition of color reagent (HNB) red complex Initial absorbance value is measured at ( ) 610 nm addition of EDTA RED complex is destroyed blue Final Absorbance value is measured at ( ) 610 nm calculation of result: Lambert Beer s Law ABS = Є. b. C Є = molar absorptivity (l. cm-1. mol-1) b = path length (cm) C = concentration (mol/liter) measuring range: ppb Ca ++ & Mg ++ detection limit: better than 0.6 ppb analysis frequency: 1 analysis / minutes How EZ-Brine?
9 4 5 6 EZ-Brine on-line calcium (Ca 2+ ) and magnesium (Mg 2+ ) in Ultra-Purified brine Unique Colorimetric Design: special highly sensitive photometer driven by a unique algorithm LED Detector How EZ-Brine?
10 4 5 6 EZ-Brine on-line calcium (Ca 2+ ) and magnesium (Mg 2+ ) in Ultra-Purified brine Purified BRINE Time Stream ABS0 (mau) ABS1 (mau) ABS2 (mau) ABS21 (mau) Result (ppb Ca ++ & Mg ++) room temperature ( C) Results EZ-Brine 07:05: :00: :56: :51: :46: Average (ppb Ca ++ & Mg ++ ) Stdev (ppb Ca ++ & Mg ++ ) Purified BRINE spiked with 20ppb of Calcium (Ca ++ ) Time Stream ABS0 (mau) ABS1 (mau) ABS2 (mau) ABS21 (mau) Result (ppb Ca ++ & Mg ++ ) room temperature ( C) 06:41: :36: :31: :27: :22: Average (ppb Ca ++ & Mg ++ ) Stdev (ppb Ca ++ & Mg ++ ) Accuracy (ppb Ca ++ & Mg ++ )
11 4 5 6 EZ-Brine on-line calcium (Ca 2+ ) and magnesium (Mg 2+ ) in Ultra-Purified brine Field Example TX USA AnaShell single compartment protective shelter 2 x multi stream EZ-Brine on-line analyzer systems
12 4 5 6 EZ-Brine on-line calcium (Ca 2+ ) and magnesium (Mg 2+ ) in Ultra-Purified brine Field Example TN USA AnaShell single compartment protective shelter 2 x multi stream EZ-Brine on-line analyzer systems
13 4 5 6 EZ-Brine on-line calcium (Ca 2+ ) and magnesium (Mg 2+ ) in Ultra-Purified brine Field Example Frankfurt - Germany AnaShell protective shelter including double stream EZ-Brine on-line calcium (Ca 2+ ) and magnesium (Mg 2+ ) in Ultra-purified brine
14 4 5 6 Field Example EZ-Brine on-line calcium (Ca 2+ ) and magnesium (Mg 2+ ) in Ultra-Purified brine LA - USA AnaShell protective cabinet 2 x EZ-BRINE on-line analyzer system for Ca ++ & Mg ++ in ultra-purified brine
15 4 5 6 Field Example EZ-Brine on-line calcium (Ca 2+ ) and magnesium (Mg 2+ ) in Ultra-Purified brine Samutprakarn - Thailand AnaShell single-compartment protective shelter 1 x EZ-Brine on-line Analyzer system for Ca++ & Mg++ in ultra-purified brine
16 anode cathode MEMBRANE Re-Generation Chemicals ION EXCHANGER A ION EXCHANGER B Re-Generation ION EXCHANGER C BRINE DE- CHLORINATOR 200 AppliTek NV/SA, Vacuum caustic soda (NaOH) Further Chlorine Processing Water sodium carbonate (Na 2 CO 3 ) hydroxides (NaOH) Return Brine or Depleted Brine Filtration Aid DEPLETED BRINE TANK HYDROGEN PURIFICATION Salt Raw Saturated Brine SETTLING Pre-Purified Brine FILTRATION Chemicals WET chlorine (Cl 2 ) hydrogen (H 2 ) Sludge Precipitated CaCO 3 ( ) Mg(OH) 2 ( ) Solids Depleted Brine Cl 2 H 2 + _ EARLY WARNING hydrogen detection PROTECT your Chlorine Plant against hydrogen Explosion FAST hydrogen (H 2 ) in WET chlorine (Cl 2 ) by multi wavelength INFRARED < 30 seconds response time hydrogen chloride (HCl) ACIDIFICATION BRINE STORAGE 320 g/l NaCl Ultra-Purified Brine Saturated Brine ELECTROLYZER 33% 30% NaOH Water NaOH caustic soda (NaOH) FAST oxygen (O 2 ) in WET chlorine (Cl 2 ) by Alternating Pressure Paramagnetic analyzer Where in Process?
17 Vacuum references a study on hydrogen-chlorine low explosibility limits, performed by the laboratory of the safety engineering department of BASF in Ludwigshafen, Germany. The conclusion of the study: maximum allowable value of 3 ± 0.2 % H 2 in Cl 2 at 50 C Lesson learned after Explosion in drying section of a membrane electrolysis unit: the absolute necessity to have a fast detection of hydrogen concentration increase at the outlet of the cells plus an additional monitoring system of the possible membrane damages (voltage / current, quality of products ) Why on-line H 2 in hot-wet Chlorine?
18 Vacuum n 1 Cardinal Rule of safe production of chlorine by WCC Why on-line H 2 in hot-wet Chlorine?
19 Misconceptions! hydrogen is a light gas Why on-line H 2 in hot-wet Chlorine? No Risk hydrogen is not an hydrocarbon No Risk May 6, 1937 Lakehurst, New Jersey USA Hindenburg disaster Plant Safety Critical Equipment
20 High H 2 present High Energy present Defined as: General Purpose Zone ( Non-explosion Proof ) Low voltage High Current = High Energy High Electrical Current NO electrical Ex Proof Technology possible chlorine Electrolysis Process on-line Hydrogen Analyzer system Field Proven Technology Fast Response (T90 = 15 seconds) Good results during start-up Cell Room HAZOP approved Fast on-line analysis of hydrogen (H 2 ) in hot-wet chlorine (Cl 2 ) gas to keep the gas matrix within the flammability limits.
21 hydrogen is not waiting to ignite until you are ready with analysis!!! Every second counts!!! Response time of < 35 seconds T 90 Do not install an analyzer systems that needs minutes to show results T 90 = time needed to detect 90% of Full Scale range when you switch from 0 to 100% at sample point
22 by Gas-Chromatograph (GC) 1 by Thermal Conductivity (TC) 2 by Multi-Wavelength INFRARED (MW-IR) 3 Thermal Conductivity UV light + Multi-Wavelength INFRARED UV light + H 2 + Cl 2 2 HCl H 2 + Cl 2 2 HCl differential measurement Delta TC before - and after oxidation by UV UV reactor H 2 and Cl 2 forms HCl by UV HCl is measured by MW-IR UV reactor % HCl stoechiometrically equal to H 2 Discontinuous measurement Long analysis time (several min.!) Can only be used for Quality Control Cannot be used for Process Control Cannot be used for SAFETY Complex Technology High Maintenance Low Up-time Not Selective measurement for H 2 Calibration Matrix - dependable Does not react first 6 12 min. after Plant Start-up Cannot be used for SAFETY during Plant Start-up Selective measurement for H 2 Calibration Matrix - independable Fast Response (T90 < 20 seconds) Shows Good Plant Start-up Best Available Technology Field Proven High Up-time
23 4 20 ma 200 AppliTek NV/SA, Thermostatically controlled electrically traced thick wall PFA insulated sample bundle % vol HCl stoechiometrically equals to H 2 Process Valve by Customer DIP-tube Sample Take Off Point ( STOP ) # 1 Main Header Salt Washing Water Removal Salt Filtration Pressure Control Flow Control Sample Aspiration Flame Arrestors UV reactor H 2 + Cl 2 2HCl HCl analysis ( corresponds to H 2 ) Multi-wavelength IR analyzer Calibration Preconditioning HMI Human Interface How on-line H 2 in hot-wet Chlorine? Drain FIELD Drain Pump AnaShell corrosion resistant protective cabinet
24 Height = 2 m (6 ft 56) 200 AppliTek NV/SA, Vortex Cooler typical ± 10 m (32 ft) Thermostatically controlled electrically traced thick wall PFA insulated sample bundle Multi-wavelength IR analyzer Logic Control double compartment corrosion resistant insulated protected cabinet IP56 / NEMA 4X Heat Shrink Sample Bundle Entry Salt Washing Water Removal Salt Filtration UV reaction (H 2 + Cl 2 2HCl) Pressure Control Flow Control Sample Aspiration Flame Arrestor Protection Process Valve by Customer DIP-tube STOP Main Chlorine Header Electrical Compartment 1 height = 2 m (6 ft 56) Width = 1 m (3 ft 2) Depth = 0 m 5 (1 ft 64) Analytical Compartment 2 height = 2 m (6 ft 56) Width = 2 m (6 ft 56) Depth = 0 m 5 (1 ft 64) Width = 3 m (9 ft 4) How on-line H 2 in hot-wet Chlorine? Condensate Drain Pump UV - Reactor H 2 + Cl 2 2HCl Water Condensation
25 Start-up Membrane type Chlorine Plant by Gas-Chromatograph (GC) 0.4% H 2 in Cl 2 5 min. 30 s 1 by Multi-Wavelength InfraRed (MW-IR) 3 Air Dilution Multi-Wavelength InfraRed (MW-IR) Hydrogen Start-up Peak ± 5 min. Load (ka) by Thermal Conductivity (TC) 0% H 2 in Cl 2 2 Field Results
26 Field Example Switzerland AnaShell double compartment protective cabinet 1 x single stream on-line Analyzer system for hydrogen in hot-wet chlorine
27 Field Example LA USA AnaShell triple compartment protective cabinet 1 x multi stream on-line Analyzer system for hydrogen in hot-wet chlorine
28 Field Example Brazil AnaShell triple compartment protective cabinet 1 x multi stream on-line Analyzer system for hydrogen in hot-wet chlorine
29 Example Argentina AnaShell double compartment protective cabinet 1 x multi stream on-line Analyzer system for hydrogen in hot-wet chlorine
30 Field Example Belgium AnaShell triple compartment protective cabinet 1 x multi stream on-line Analyzer system for hydrogen in hot-wet chlorine 1 x single stream on-line Analyzer system for oxygen in hot-wet chlorine
31 Field Example Banten - Indonesia AnaShell multi compartment protective cabinet 1 x on-line Analyzer system for hydrogen in hot-wet chlorine
32 Field Example LA, USA AnaShell multi compartment protective cabinet 3 x on-line Analyzer system for hydrogen in hot-wet chlorine ( Multi Wavelength IR )
33 Field Example LA, USA AnaShell multi compartment protective cabinet 3 x on-line Analyzer system for hydrogen in hot-wet chlorine ( Multi Wavelength IR )
34 Field Example Japan AnaShell double compartment protective cabinet 1 x on-line Analyzer system for hydrogen in hot wet chlorine ( Multi Wavelength IR )
35 Field Example Finland 2 x AnaShell double compartment protective cabinet 2 x on-line Analyzer system for hydrogen in hot wet chlorine ( Multi Wavelength IR )
36 Field Example The Netherlands 2 pc AnaShell multi-compartment protective cabinets 1 x on-line Analyzer system for hydrogen in hot wet chlorine ( by multi-wavelength IR ) 1 x on-line Analyzer system for oxygen in hot wet chlorine ( by alternating pressure paramagnetic analyzer )
37 References repeat orders
38 Works Good Looks Good 24 / 7 Worldwide Support Use High Quality - State of the Art BAT Single Source Responsibility International After Sales Service Our goal is to make your plant more safe make your plant more profitable save your plant money Our Mission repeat orders
Hydrogen & Chlorine monitoring for the Chlor-Alkali Industry
 technical datasheet Application Note - AN9041 Hydrogen & Chlorine monitoring for the Chlor-Alkali Industry Measurement Technology Limited (MTL) part of Eaton's Crouse-Hinds business, design and manufacture
technical datasheet Application Note - AN9041 Hydrogen & Chlorine monitoring for the Chlor-Alkali Industry Measurement Technology Limited (MTL) part of Eaton's Crouse-Hinds business, design and manufacture
Electrolysis System CHLORINSITU V (PLUS)
 Output of 100 3.500 g/h of ultra-pure active chlorine (hypochlorous acid) Electrolysis systems of the type CHLORINSITU V generate ultrapure active chlorine using the unique vacuum method with the membrane
Output of 100 3.500 g/h of ultra-pure active chlorine (hypochlorous acid) Electrolysis systems of the type CHLORINSITU V generate ultrapure active chlorine using the unique vacuum method with the membrane
Monitoring Nitrogen Trichloride in Chlorine Manufacture. solvent. O NaOH + 1 /2H 2
 Application Summary Analytes: (nitrogen trichloride) Detector: OMA-300 Process Analyzer Process Stream: headspace gas above saturated CCl 4 solvent Typical Measurement Range: 0-20,000 ppm Introduction
Application Summary Analytes: (nitrogen trichloride) Detector: OMA-300 Process Analyzer Process Stream: headspace gas above saturated CCl 4 solvent Typical Measurement Range: 0-20,000 ppm Introduction
Reuse of Produced Water for Electrolytic Oxidant Production: Challenges and Solutions
 Reuse of Produced Water for Electrolytic Oxidant Production: Challenges and Solutions Produced Water Produced water is extracted from wells at a rate far greater than hydrocarbon extraction ~8 bblproduced
Reuse of Produced Water for Electrolytic Oxidant Production: Challenges and Solutions Produced Water Produced water is extracted from wells at a rate far greater than hydrocarbon extraction ~8 bblproduced
Environmental Series - Trace contaminant analysis in brine using a Thermo Scientific icap 6000 Series Duo ICP
 Application Note: 40986 Environmental Series - Trace contaminant analysis in brine using a Thermo Scientific icap 6000 Series Duo ICP Karen Harper, Applications Group Leader, Thermo Scientific, Cambridge,
Application Note: 40986 Environmental Series - Trace contaminant analysis in brine using a Thermo Scientific icap 6000 Series Duo ICP Karen Harper, Applications Group Leader, Thermo Scientific, Cambridge,
ADI 2040 Process Analyzer
 ADI 2040 Process Analyzer Multi-purpose wet chemical analysis Multi-purpose Wet Chemical Analysis ADI 2040 The Metrohm Applikon ADI 2040 Process Analyzer is a multipurpose wet chemical analyzer, that has
ADI 2040 Process Analyzer Multi-purpose wet chemical analysis Multi-purpose Wet Chemical Analysis ADI 2040 The Metrohm Applikon ADI 2040 Process Analyzer is a multipurpose wet chemical analyzer, that has
Chlorine-alkali electrolysis
 Chlorine-alkali electrolysis Inline analytical technology for: brine purification electrolysis chlorine gas drying NaOH concentration hydrochloric acid production Increasing q Robust, acc With high LiquiSonic
Chlorine-alkali electrolysis Inline analytical technology for: brine purification electrolysis chlorine gas drying NaOH concentration hydrochloric acid production Increasing q Robust, acc With high LiquiSonic
3. Chemical industry. Because of their modular design, the instruments in the TOC-L series can be equipped for any possible measurement
 3. Chemical industry The most commonly used compound in the chemical industry is water not only as a solvent in processing, but also as an energy carrier in the cooling or heating cycle. As vast amounts
3. Chemical industry The most commonly used compound in the chemical industry is water not only as a solvent in processing, but also as an energy carrier in the cooling or heating cycle. As vast amounts
Nitrogen, ammonia, colorimetry, salicylate-hypochlorite, automated-segmented flow
 1. Application Nitrogen, ammonia, colorimetry, salicylate-hypochlorite, automated-segmented flow Parameters and Codes: Nitrogen, ammonia, dissolved, I-2522-90 (mg/l as N): 00608 Nitrogen, ammonia, total-in-bottom-material,
1. Application Nitrogen, ammonia, colorimetry, salicylate-hypochlorite, automated-segmented flow Parameters and Codes: Nitrogen, ammonia, dissolved, I-2522-90 (mg/l as N): 00608 Nitrogen, ammonia, total-in-bottom-material,
ESP FLOWSHEET SIMULATION APPLICATION BRIEF Chlor-Alkali Simulation
 ESP FLOWSHEET SIMULATION APPLICATION BRIEF Chlor-Alkali Simulation Revised April 10, 2012 1 The anode in an electrochemical cell is the strongest oxidizer known, as it is fully capable of taking an electron
ESP FLOWSHEET SIMULATION APPLICATION BRIEF Chlor-Alkali Simulation Revised April 10, 2012 1 The anode in an electrochemical cell is the strongest oxidizer known, as it is fully capable of taking an electron
Anion and Cation analysis with Professional IC - automatic dilution and sample preparation with SPM
 IC Application Work AW CH6-1048-012011 Anion and Cation analysis with Professional IC - automatic dilution and sample preparation with SPM Branch: Chemical industry; Water, wastewater, environmental protection,
IC Application Work AW CH6-1048-012011 Anion and Cation analysis with Professional IC - automatic dilution and sample preparation with SPM Branch: Chemical industry; Water, wastewater, environmental protection,
Electrolyzed Water as a Novel Sanitizer in the Food Industry: Current Trends and Future Perspectives
 Electrolyzed Water as a Novel Sanitizer in the Food Industry: Current Trends and Future Perspectives PHILIP NEL VP TECHNICAL AND R&D RADICAL WATERS CITREX CHILE A NEW ECO-SANITISER Electrochemically Activated
Electrolyzed Water as a Novel Sanitizer in the Food Industry: Current Trends and Future Perspectives PHILIP NEL VP TECHNICAL AND R&D RADICAL WATERS CITREX CHILE A NEW ECO-SANITISER Electrochemically Activated
SGL Group`s outstanding expertise HCl synthesis units with steam generation
 SGL Group`s outstanding expertise HCl synthesis units with steam generation CLOROSUR BUENOS AIRES, ARGENTINA NOV 2016 DC0 public - (GUADARRAMAJO) HCl syntheses with steam generation at a glance Criteria
SGL Group`s outstanding expertise HCl synthesis units with steam generation CLOROSUR BUENOS AIRES, ARGENTINA NOV 2016 DC0 public - (GUADARRAMAJO) HCl syntheses with steam generation at a glance Criteria
Module 20: Corrosion Control and Sequestering Answer Key (edited April 2017)
 Module 20: Corrosion Control and Sequestering Answer Key (edited April 2017) Bureau of Safe Drinking Water, Department of Environmental Protection 1 UNIT 1 EXERCISE Exercise for Unit 1 Background and Properties
Module 20: Corrosion Control and Sequestering Answer Key (edited April 2017) Bureau of Safe Drinking Water, Department of Environmental Protection 1 UNIT 1 EXERCISE Exercise for Unit 1 Background and Properties
CHLORINE PROCESS ECONOMICS PROGRAM. Report No. 61A. Supplement A. by YEN CHEN YEN. May A private report by the STANFORD RESEARCH INSTITUTE
 Report No. 61A CHLORINE Supplement A by YEN CHEN YEN May 1074 A private report by the PROCESS ECONOMICS PROGRAM STANFORD RESEARCH INSTITUTE I I MENLO PARK, CALIFORNIA CONTENTS 1 2 3 INTRODUCTION... 1 SUMMARY...
Report No. 61A CHLORINE Supplement A by YEN CHEN YEN May 1074 A private report by the PROCESS ECONOMICS PROGRAM STANFORD RESEARCH INSTITUTE I I MENLO PARK, CALIFORNIA CONTENTS 1 2 3 INTRODUCTION... 1 SUMMARY...
PRACTICAL 3 ph AND BUFFERS
 PRACTICAL 3 ph AND BUFFERS ph and Buffers Structure 3.1 Introduction 3.2 ph and Buffers: Basic Concept 3.2.1 ph 3.2.2 Buffers and Buffer Solutions 3.3 Methods for Determining ph Experiment 1: Measurement
PRACTICAL 3 ph AND BUFFERS ph and Buffers Structure 3.1 Introduction 3.2 ph and Buffers: Basic Concept 3.2.1 ph 3.2.2 Buffers and Buffer Solutions 3.3 Methods for Determining ph Experiment 1: Measurement
Stoichiometry ( ) ( )
 Stoichiometry Outline 1. Molar Calculations 2. Limiting Reactants 3. Empirical and Molecular Formula Calculations Review 1. Molar Calculations ( ) ( ) ( ) 6.02 x 10 23 particles (atoms or molecules) /
Stoichiometry Outline 1. Molar Calculations 2. Limiting Reactants 3. Empirical and Molecular Formula Calculations Review 1. Molar Calculations ( ) ( ) ( ) 6.02 x 10 23 particles (atoms or molecules) /
CCS140 and CCS141. Technical Information. Sensors for free chlorine Amperometric, membrane-covered sensors for installation in the CCA250 assembly
 Technical Information CCS140 and CCS141 Sensors for free chlorine Amperometric, membrane-covered sensors for installation in the CCA250 assembly Application Oxidising agents such as chlorine or anorganic
Technical Information CCS140 and CCS141 Sensors for free chlorine Amperometric, membrane-covered sensors for installation in the CCA250 assembly Application Oxidising agents such as chlorine or anorganic
What is a chemical property of matter?
 What is a chemical property of matter? A. Chemical properties of matter involve a chemical change. 1. Observations of matter that involve a chemical change cause new matter to be formed. a. Chemical changes
What is a chemical property of matter? A. Chemical properties of matter involve a chemical change. 1. Observations of matter that involve a chemical change cause new matter to be formed. a. Chemical changes
Modified Industrial Chlor Alkali Process for Reduction in Power Consumption
 Modified Industrial Chlor Alkali Process for Reduction in Power Consumption Abhishek Sinha, Anurag Ranjak Department of Chemical Engineering, Indian Institute of Technology, Delhi, India Abstract: Chlor-alkali
Modified Industrial Chlor Alkali Process for Reduction in Power Consumption Abhishek Sinha, Anurag Ranjak Department of Chemical Engineering, Indian Institute of Technology, Delhi, India Abstract: Chlor-alkali
IGCSE (9-1) Edexcel - Chemistry
 IGCSE (9-1) Edexcel - Chemistry Principles of Chemistry Chemical Formulae, Equations and Calculations NOTES 1.25: Write word equations and balanced chemical equations (including state symbols): For reactions
IGCSE (9-1) Edexcel - Chemistry Principles of Chemistry Chemical Formulae, Equations and Calculations NOTES 1.25: Write word equations and balanced chemical equations (including state symbols): For reactions
KENYA NATIONAL EXAMINATION COUNCIL REVISION MOCK EXAMS 2016 TOP NATIONAL SCHOOLS KAPSABET BOYS CHEMISTRY PAPER 2 TIME: 2 HOURS
 KENYA NATIONAL EXAMINATION COUNCIL REVISION MOCK EXAMS 2016 TOP NATIONAL SCHOOLS KAPSABET BOYS CHEMISTRY PAPER 2 TIME: 2 HOURS SCHOOLS NET KENYA Osiligi House, Opposite KCB, Ground Floor Off Magadi Road,
KENYA NATIONAL EXAMINATION COUNCIL REVISION MOCK EXAMS 2016 TOP NATIONAL SCHOOLS KAPSABET BOYS CHEMISTRY PAPER 2 TIME: 2 HOURS SCHOOLS NET KENYA Osiligi House, Opposite KCB, Ground Floor Off Magadi Road,
5. SEPARATION OF MIXTURES, PURIFICATION OF SOLIDS Objectives
 Name: Date:.. 5. SEPARATION OF MIXTURES, PURIFICATION OF SOLIDS Objectives Introduction to basic chemical laboratory operations: grinding, dissolving, decanting, centrifuging, filtration, crystallization.
Name: Date:.. 5. SEPARATION OF MIXTURES, PURIFICATION OF SOLIDS Objectives Introduction to basic chemical laboratory operations: grinding, dissolving, decanting, centrifuging, filtration, crystallization.
Nitrate plus Nitrite and Nitrite in Seawater by Segmented Flow Analysis (SFA)
 Methodology Nitrate plus Nitrite and Nitrite in Seawater by Segmented Flow Analysis (SFA) (Cartridge Part #A002603) 1.0 Scope and Application 1.1 This method is used to determine the concentration of nitrate
Methodology Nitrate plus Nitrite and Nitrite in Seawater by Segmented Flow Analysis (SFA) (Cartridge Part #A002603) 1.0 Scope and Application 1.1 This method is used to determine the concentration of nitrate
Chapter 18. Electrochemistry
 Chapter 18 Electrochemistry Section 17.1 Spontaneous Processes and Entropy Section 17.1 http://www.bozemanscience.com/ap-chemistry/ Spontaneous Processes and Entropy Section 17.1 Spontaneous Processes
Chapter 18 Electrochemistry Section 17.1 Spontaneous Processes and Entropy Section 17.1 http://www.bozemanscience.com/ap-chemistry/ Spontaneous Processes and Entropy Section 17.1 Spontaneous Processes
Draw one line from each solution to the ph value of the solution. Solution ph value of the solution
 1 The ph scale is a measure of the acidity or alkalinity of a solution. (a) Draw one line from each solution to the ph value of the solution. Solution ph value of the solution 5 Acid 7 9 Neutral 11 13
1 The ph scale is a measure of the acidity or alkalinity of a solution. (a) Draw one line from each solution to the ph value of the solution. Solution ph value of the solution 5 Acid 7 9 Neutral 11 13
Frequently asked question about our new Platinum Water Ionizer
 Frequently asked question about our new Platinum Water Ionizer How many electrodes does the Platinum Water Ionizer have? Answer: 2, One positive and One negative How many electrolysis plates does the Platinum
Frequently asked question about our new Platinum Water Ionizer How many electrodes does the Platinum Water Ionizer have? Answer: 2, One positive and One negative How many electrolysis plates does the Platinum
What Do You Think? Investigate GOALS. Part A: Precipitation of Calcium
 H 2 Woes Activity 6 Water Softening GOALS In this activity you will: Investigate the equilibria behind water softening. Determine which reduces water hardness more, precipitation reactions or ion-exchange
H 2 Woes Activity 6 Water Softening GOALS In this activity you will: Investigate the equilibria behind water softening. Determine which reduces water hardness more, precipitation reactions or ion-exchange
Name... Class... Date...
 The electrolysis of aqueous solutions of ionic compounds Specification references C4.3.1 The process of electrolysis C4.3.4 Electrolysis of aqueous solutions C4.3.5 Representation of reactions at s as
The electrolysis of aqueous solutions of ionic compounds Specification references C4.3.1 The process of electrolysis C4.3.4 Electrolysis of aqueous solutions C4.3.5 Representation of reactions at s as
CHAPTER ELECTROCHEMISTRY
 149 CHAPTER ELECTROCHEMISTRY 1. On electrolysis of dilute sulphuric acid using platinum electrodes, the product obtained at the anode will be hydrogen oxygen hydrogen sulphide sulphur dioxide 2. Which
149 CHAPTER ELECTROCHEMISTRY 1. On electrolysis of dilute sulphuric acid using platinum electrodes, the product obtained at the anode will be hydrogen oxygen hydrogen sulphide sulphur dioxide 2. Which
SO 4... [2], to an excess of dilute sulfuric acid. A student adds a sample of solid potassium carbonate, K [3]
![SO 4... [2], to an excess of dilute sulfuric acid. A student adds a sample of solid potassium carbonate, K [3] SO 4... [2], to an excess of dilute sulfuric acid. A student adds a sample of solid potassium carbonate, K [3]](/thumbs/83/87669036.jpg) 1 Chemicals called acids have been known throughout history The word acid comes from the Latin acidus meaning sour Dilute sulfuric acid, H 2 SO 4, is a common laboratory acid (a) State the formulae of
1 Chemicals called acids have been known throughout history The word acid comes from the Latin acidus meaning sour Dilute sulfuric acid, H 2 SO 4, is a common laboratory acid (a) State the formulae of
Exercise 2-2. Titration of a Strong Acid EXERCISE OBJECTIVES
 Exercise 2-2 Titration of a Strong Acid EXERCISE OBJECTIVES To describe the effect of a ph variation on a chemical indicator; To titrate water containing a strong base solution with a strong acid solution;
Exercise 2-2 Titration of a Strong Acid EXERCISE OBJECTIVES To describe the effect of a ph variation on a chemical indicator; To titrate water containing a strong base solution with a strong acid solution;
Mole Concept 5.319% = = g sample =
 Mole - a counting system Avogadro s number = 6.0 10 3 Mole Concept Chemical calculation involving mass: Empirical formula: The simplest formula that shows the relative numbers of the different kinds of
Mole - a counting system Avogadro s number = 6.0 10 3 Mole Concept Chemical calculation involving mass: Empirical formula: The simplest formula that shows the relative numbers of the different kinds of
Classifying Chemical Reactions: Lab Directions
 Classifying Chemical Reactions: Lab Directions Please Return Background: The power of chemical reactions to transform our lives is visible all around us in our homes, in our cars, even in our bodies. Chemists
Classifying Chemical Reactions: Lab Directions Please Return Background: The power of chemical reactions to transform our lives is visible all around us in our homes, in our cars, even in our bodies. Chemists
Student Book links Specification links Links to prior learning Suggested teaching order
 Teaching plan 5.1.2 Molecular formulae Student Book links Specification links Links to prior learning Suggested teaching order 5.1.2 5.03 5.04 5.05 Core practical 1 Learning objectives Empirical formulae
Teaching plan 5.1.2 Molecular formulae Student Book links Specification links Links to prior learning Suggested teaching order 5.1.2 5.03 5.04 5.05 Core practical 1 Learning objectives Empirical formulae
Single Method On-line Analyzers
 Single Method On-line Analyzers ADI 2016 Titrolyzer ADI 2018 Ion-Analyzer ADI 2019 Process Colorimeter ADI 2016 / 2018 / 2019 Automatic and dependable On-line Ion Analysis The single method Process Analyzers
Single Method On-line Analyzers ADI 2016 Titrolyzer ADI 2018 Ion-Analyzer ADI 2019 Process Colorimeter ADI 2016 / 2018 / 2019 Automatic and dependable On-line Ion Analysis The single method Process Analyzers
Application Your benefits
 Technical Information CCS140 and CCS141 Sensors for free chlorine Amperometric, membrane-covered sensors for installation in the CCA250 flow assembly Application Oxidizing agents such as chlorine or anorganic
Technical Information CCS140 and CCS141 Sensors for free chlorine Amperometric, membrane-covered sensors for installation in the CCA250 flow assembly Application Oxidizing agents such as chlorine or anorganic
Classifying Chemical Reactions
 1 Classifying Chemical Reactions Analyzing and Predicting Products Introduction The power of chemical reactions to transform our lives is visible all around us-in our cars, even in our bodies. Chemists
1 Classifying Chemical Reactions Analyzing and Predicting Products Introduction The power of chemical reactions to transform our lives is visible all around us-in our cars, even in our bodies. Chemists
Single Method On-line Analyzers. ADI 2016 Titrolyzer ADI 2018 Ion-Analyzer ADI 2019 Process Colorimeter
 Single Method On-line Analyzers ADI 2016 Titrolyzer ADI 2018 Ion-Analyzer ADI 2019 Process Colorimeter Automatic and dependable on-line ion analysis 02 The single method process analyzers from Metrohm
Single Method On-line Analyzers ADI 2016 Titrolyzer ADI 2018 Ion-Analyzer ADI 2019 Process Colorimeter Automatic and dependable on-line ion analysis 02 The single method process analyzers from Metrohm
5 Energy from chemicals
 5 Energy from chemicals Content 5.1 Enthalpy 5.2 Hydrogen fuel cell Learning Outcomes Candidates should be able to: (a) (b) (c) (d) (e) describe the meaning of enthalpy change in terms of exothermic (H
5 Energy from chemicals Content 5.1 Enthalpy 5.2 Hydrogen fuel cell Learning Outcomes Candidates should be able to: (a) (b) (c) (d) (e) describe the meaning of enthalpy change in terms of exothermic (H
Mercury Stack Monitor SM-4
 Member of the envea Group Mercury Stack Monitor SM-4 Automatic continuous emission monitor (CEM) for mercury Continuous operation Sample dilution directly at stack - works with virtually any sample matrix
Member of the envea Group Mercury Stack Monitor SM-4 Automatic continuous emission monitor (CEM) for mercury Continuous operation Sample dilution directly at stack - works with virtually any sample matrix
BUT FIRST LET S REVIEW IONS AND BONDING. What is the Lewis dot diagram for Magnesium? 2+ 2-
 ELECTROLYTES BUT FIRST LET S REVIEW IONS AND BONDING What is the Lewis dot diagram for Magnesium? Mg 2 2- S What is the Lewis dot diagram for Sulfur? How would these 2 elements complete the octet rule?
ELECTROLYTES BUT FIRST LET S REVIEW IONS AND BONDING What is the Lewis dot diagram for Magnesium? Mg 2 2- S What is the Lewis dot diagram for Sulfur? How would these 2 elements complete the octet rule?
Determination of some components in mineral water
 Determination of some components in mineral water There are numerous mineral water springs in Slovakia. The effect of mineral water on human health depends on the composition of salts (ions) dissolved
Determination of some components in mineral water There are numerous mineral water springs in Slovakia. The effect of mineral water on human health depends on the composition of salts (ions) dissolved
Unit - 3 ELECTROCHEMISTRY VSA QUESTIONS (1 - MARK QUESTIONS) 3. Mention the purpose of salt-bridge placed between two half-cells of a galvanic cell?
 Unit - 3 ELECTROCHEMISTRY 1. What is a galvanic cell? VSA QUESTIONS (1 - MARK QUESTIONS) 2. Give the cell representation for Daniell Cell. 3. Mention the purpose of salt-bridge placed between two half-cells
Unit - 3 ELECTROCHEMISTRY 1. What is a galvanic cell? VSA QUESTIONS (1 - MARK QUESTIONS) 2. Give the cell representation for Daniell Cell. 3. Mention the purpose of salt-bridge placed between two half-cells
3. Which of the following would create a chemical change when it is added to a glass of warm milk?
 Test Name: Science 8 Assessment 6 ReviewScan Test Code: 9668632 Date: Your Name: Teacher: Class Name: 1. Which step in these instructions for making scrambled eggs results in a chemical change? First,
Test Name: Science 8 Assessment 6 ReviewScan Test Code: 9668632 Date: Your Name: Teacher: Class Name: 1. Which step in these instructions for making scrambled eggs results in a chemical change? First,
Exercise 4-3. Titration of Weak Acids EXERCISE OBJECTIVE DISCUSSION OUTLINE. The 5% rule DISCUSSION
 Exercise 4-3 Titration of Weak Acids EXERCISE OBJECTIVE Titrate both a weak acid solution and a weak polyprotic acid solution with a strong base solution. Plot a graph using the titration data, analyze
Exercise 4-3 Titration of Weak Acids EXERCISE OBJECTIVE Titrate both a weak acid solution and a weak polyprotic acid solution with a strong base solution. Plot a graph using the titration data, analyze
Atomic Absorption Spectroscopy and Atomic Emission Spectroscopy
 Atomic Absorption Spectroscopy and Atomic Emission Spectroscopy A. Evaluation of Analytical Parameters in Atomic Absorption Spectroscopy Objective The single feature that contributes most to making atomic
Atomic Absorption Spectroscopy and Atomic Emission Spectroscopy A. Evaluation of Analytical Parameters in Atomic Absorption Spectroscopy Objective The single feature that contributes most to making atomic
Gaseous CEMS technology
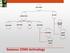 GAS CEMS EXTRACTIVE IN-SITU COLD DRY HOT WET DRY DILUTION PROBE/ POINT IN-STACK CROSS STACK OUT OF STACK Gaseous CEMS technology Probe (at stack) Heated filter Heated sample line Condenser Pump Analyzers
GAS CEMS EXTRACTIVE IN-SITU COLD DRY HOT WET DRY DILUTION PROBE/ POINT IN-STACK CROSS STACK OUT OF STACK Gaseous CEMS technology Probe (at stack) Heated filter Heated sample line Condenser Pump Analyzers
High-Pressure Electrolytic Carbonate Eluent Generation Devices and Their Applications in Ion Chromatography Systems
 High-Pressure Electrolytic Carbonate Eluent Generation Devices and Their Applications in Ion Chromatography Systems Yan Liu, Zhongqing Lu, and Chris Pohl; Thermo Fisher Scientific, Sunnyvale, CA USA Overview
High-Pressure Electrolytic Carbonate Eluent Generation Devices and Their Applications in Ion Chromatography Systems Yan Liu, Zhongqing Lu, and Chris Pohl; Thermo Fisher Scientific, Sunnyvale, CA USA Overview
In the exam you will be asked to tackle questions such as the one below.
 Get started AO3 2 Preparing salts This unit will help you to plan, describe and understand an experiment to prepare a salt. In the exam you will be asked to tackle questions such as the one below. Exam-style
Get started AO3 2 Preparing salts This unit will help you to plan, describe and understand an experiment to prepare a salt. In the exam you will be asked to tackle questions such as the one below. Exam-style
2B Air, Oxygen, Carbon Dioxide and Water
 Air, Oxygen, Carbon Dioxide and Water Air, oxygen and carbon dioxide are important chemicals in our everyday lives. Knowledge of their properties helps us to develop an understanding of the role they play.
Air, Oxygen, Carbon Dioxide and Water Air, oxygen and carbon dioxide are important chemicals in our everyday lives. Knowledge of their properties helps us to develop an understanding of the role they play.
Lab: Cabbages in Chemistry 3pts ec printing in COLOR / 2pts B&W
 Lab: Cabbages in Chemistry 3pts ec printing in COLOR / 2pts B&W Telltale Colors 1. In the mixing tray, place 5 drops of the chemical in 13 compartments 2. DIP test the ph paper & record the ph measurement
Lab: Cabbages in Chemistry 3pts ec printing in COLOR / 2pts B&W Telltale Colors 1. In the mixing tray, place 5 drops of the chemical in 13 compartments 2. DIP test the ph paper & record the ph measurement
Soil Cation Analysis Using High-Performance Capillary Zone Electrophoresis Last Modified: October 20, 2006
 Soil Cation Analysis Using High-Performance Capillary Zone Electrophoresis Last Modified: October 20, 2006 Introduction: Capillary electrophoresis (CE) is a relatively new, but rapidly growing separation
Soil Cation Analysis Using High-Performance Capillary Zone Electrophoresis Last Modified: October 20, 2006 Introduction: Capillary electrophoresis (CE) is a relatively new, but rapidly growing separation
LlkJ-/ rpdf Pollution Prevention - Source Reduction with Electrodialytic Processes
 w- LlkJ-/ rpdf Pollution Prevention - Source Reduction with Electrodialytic Processes by Daniel J. Vaughan This paper is focused on how not to make waste or how to prevent pollution at the source. I know
w- LlkJ-/ rpdf Pollution Prevention - Source Reduction with Electrodialytic Processes by Daniel J. Vaughan This paper is focused on how not to make waste or how to prevent pollution at the source. I know
VOCABULARY Define. 1. stoichiometry. 2. composition stoichiometry. 3. reaction stoichiometry. 4. unknown. 5. mole ratio
 CHAPTER 9 HOMEWORK 9-1 (pp. 275 279) Define. 1. stoichiometry 2. composition stoichiometry 3. reaction stoichiometry 4. unknown 5. mole ratio SKILL BUILDER On a separate sheet of paper, write five possible
CHAPTER 9 HOMEWORK 9-1 (pp. 275 279) Define. 1. stoichiometry 2. composition stoichiometry 3. reaction stoichiometry 4. unknown 5. mole ratio SKILL BUILDER On a separate sheet of paper, write five possible
CIE Chemistry A-Level Practicals for Papers 3 and 5
 CIE Chemistry A-Level Practicals for Papers 3 and 5 Ion Identification Group 2 Ions Identification Example -3 1. Place 10 drops of 0.1 mol dm barium chloride in a clean test tube. Must be clean to ensure
CIE Chemistry A-Level Practicals for Papers 3 and 5 Ion Identification Group 2 Ions Identification Example -3 1. Place 10 drops of 0.1 mol dm barium chloride in a clean test tube. Must be clean to ensure
Buffer Preparation. Learning Objectives:
 Proteomics Buffer Preparation Buffer Preparation Maintaining the optimum ph during the biological sample processing is to maintain the proper functional and structural aspects of the sample. It is important
Proteomics Buffer Preparation Buffer Preparation Maintaining the optimum ph during the biological sample processing is to maintain the proper functional and structural aspects of the sample. It is important
EXPERIMENT 6 Empirical Formula of a Compound
 EXPERIMENT 6 Empirical Formula of a Compound INTRODUCTION Chemical formulas indicate the composition of compounds. A formula that gives only the simplest ratio of the relative number of atoms in a compound
EXPERIMENT 6 Empirical Formula of a Compound INTRODUCTION Chemical formulas indicate the composition of compounds. A formula that gives only the simplest ratio of the relative number of atoms in a compound
Chemistry 1011 TOPIC TEXT REFERENCE. Electrochemistry. Masterton and Hurley Chapter 18. Chemistry 1011 Slot 5 1
 Chemistry 1011 TOPIC Electrochemistry TEXT REFERENCE Masterton and Hurley Chapter 18 Chemistry 1011 Slot 5 1 18.5 Electrolytic Cells YOU ARE EXPECTED TO BE ABLE TO: Construct a labelled diagram to show
Chemistry 1011 TOPIC Electrochemistry TEXT REFERENCE Masterton and Hurley Chapter 18 Chemistry 1011 Slot 5 1 18.5 Electrolytic Cells YOU ARE EXPECTED TO BE ABLE TO: Construct a labelled diagram to show
Every living and nonliving things is made up of matter. MATTER: anything that has mass & takes up space. What does all matter have in common?
 the basics Every living and nonliving things is made up of matter MATTER: anything that has mass & takes up space What does all matter have in common? Smallest unit of matter ALL matter is made of particles
the basics Every living and nonliving things is made up of matter MATTER: anything that has mass & takes up space What does all matter have in common? Smallest unit of matter ALL matter is made of particles
A-LEVEL TRANSITION COURSE SUMMER 2018 PART 2: USING CHEMICAL EQUATIONS
 A-LEVEL TRANSITION COURSE SUMMER 2018 PART 2: USING CHEMICAL EQUATIONS MASS AQUEOUS VOLUME ` MOLAR MASS GASEOUS VOLUME MOLES CONCENTRATION REVISION FROM LESSON 1 How many moles? 1) Jahin weighs a sample
A-LEVEL TRANSITION COURSE SUMMER 2018 PART 2: USING CHEMICAL EQUATIONS MASS AQUEOUS VOLUME ` MOLAR MASS GASEOUS VOLUME MOLES CONCENTRATION REVISION FROM LESSON 1 How many moles? 1) Jahin weighs a sample
EXPERIMENT: LIMITING REAGENT. NOTE: Students should have moles of reactants in DATASHEET converted into masses in grams prior to the lab period.
 Revised 12/2015 EXPERIMENT: LIMITING REAGENT Chem 1104 Lab NOTE: Students should have moles of reactants in DATASHEET converted into masses in grams prior to the lab period. INTRODUCTION Limiting reactant
Revised 12/2015 EXPERIMENT: LIMITING REAGENT Chem 1104 Lab NOTE: Students should have moles of reactants in DATASHEET converted into masses in grams prior to the lab period. INTRODUCTION Limiting reactant
ELECTROLYTES & NEUTRALIZATION
 ELECTROLYTES & NEUTRALIZATION BUT FIRST LET S REVIEW IONS AND BONDING What is the Lewis dot diagram for Magnesium? Mg 2 2- S What is the Lewis dot diagram for Sulfur? How would these 2 elements bond? What
ELECTROLYTES & NEUTRALIZATION BUT FIRST LET S REVIEW IONS AND BONDING What is the Lewis dot diagram for Magnesium? Mg 2 2- S What is the Lewis dot diagram for Sulfur? How would these 2 elements bond? What
Unit 4: Chemical Changes (Higher Content)
 Metals react with oxygen to produce metal oxides. E.g. Copper + Oxygen > Copper Oxide The reactions are oxidation reactions because the metals gain oxygen. Reactivity of Metals Metal Extraction Metals
Metals react with oxygen to produce metal oxides. E.g. Copper + Oxygen > Copper Oxide The reactions are oxidation reactions because the metals gain oxygen. Reactivity of Metals Metal Extraction Metals
Electrochemistry. Review oxidation reactions and how to assign oxidation numbers (Ch 4 Chemical Reactions).
 Electrochemistry Oxidation-Reduction: Review oxidation reactions and how to assign oxidation numbers (Ch 4 Chemical Reactions). Half Reactions Method for Balancing Redox Equations: Acidic solutions: 1.
Electrochemistry Oxidation-Reduction: Review oxidation reactions and how to assign oxidation numbers (Ch 4 Chemical Reactions). Half Reactions Method for Balancing Redox Equations: Acidic solutions: 1.
DR/4000 PROCEDURE SELENIUM. 4. Measure 100 ml of sample into a second 500-mL erlenmeyer flask (label the flask sample ).
 Method 8194 DR/4000 PROCEDURE Diaminobenzidine Method* (0 to 1.000 mg/l) Scope and Application: For water and wastewater; distillation is required for determining total selenium. See the Distillation procedure
Method 8194 DR/4000 PROCEDURE Diaminobenzidine Method* (0 to 1.000 mg/l) Scope and Application: For water and wastewater; distillation is required for determining total selenium. See the Distillation procedure
TRANS-NZOIA COUNTY KCSE REVISION MOCK EXAMS 2015
 TRANS-NZOIA COUNTY KCSE REVISION MOCK EXAMS 2015 TIME: 2 HOURS233/2 CHEMISTRY PAPER 2 (THEORY) SCHOOLS NET KENYA Osiligi House, Opposite KCB, Ground Floor Off Magadi Road, Ongata Rongai Tel: 0711 88 22
TRANS-NZOIA COUNTY KCSE REVISION MOCK EXAMS 2015 TIME: 2 HOURS233/2 CHEMISTRY PAPER 2 (THEORY) SCHOOLS NET KENYA Osiligi House, Opposite KCB, Ground Floor Off Magadi Road, Ongata Rongai Tel: 0711 88 22
Chemical properties of acids and bases
 Student s Name: Date: Chemical properties of acids and bases Background Acids and bases are two classes of compounds that have wide application in industrial and household chemistry. Acids are substances
Student s Name: Date: Chemical properties of acids and bases Background Acids and bases are two classes of compounds that have wide application in industrial and household chemistry. Acids are substances
In 1807 Davy did an electrolysis experiment to produce potassium. Davy first tried to electrolyse a solid potassium salt to produce potassium
 Q1. This question is about potassium. (a) Humphrey Davy was a professor of chemistry. In 1807 Davy did an electrolysis experiment to produce potassium. Davy first tried to electrolyse a solid potassium
Q1. This question is about potassium. (a) Humphrey Davy was a professor of chemistry. In 1807 Davy did an electrolysis experiment to produce potassium. Davy first tried to electrolyse a solid potassium
for free revision past papers visit:
 NAME ADM NO:. STUNDENT S SIGNATURE DATE.. SCHOOL 233/2 FORM THREE CHEMISTRY THEORY Paper 2 END YEAR 2017 EXAMS. Time: 2 Hrs FORM THREE CHEMISTRY 233/2 INSTRUCTIONS TO CANDIDATES Write your Name and Index
NAME ADM NO:. STUNDENT S SIGNATURE DATE.. SCHOOL 233/2 FORM THREE CHEMISTRY THEORY Paper 2 END YEAR 2017 EXAMS. Time: 2 Hrs FORM THREE CHEMISTRY 233/2 INSTRUCTIONS TO CANDIDATES Write your Name and Index
Experiment 16. Desalination of Sea Water E16-1
 Experiment 16 Desalination of Sea Water E16-1 E16-2 The Task The goal of this experiment is to investigate the nature and some properties of sea water. Skills At the end of the laboratory session you should
Experiment 16 Desalination of Sea Water E16-1 E16-2 The Task The goal of this experiment is to investigate the nature and some properties of sea water. Skills At the end of the laboratory session you should
Determination of Trace Cations in Power Plant Waters Containing Morpholine
 Application Note 8 Determination of Trace Cations in Power Plant Waters Containing Morpholine INTRODUCTION Morpholine and ammonium are used as additives in power plant waters. Morpholine acts as a corrosion
Application Note 8 Determination of Trace Cations in Power Plant Waters Containing Morpholine INTRODUCTION Morpholine and ammonium are used as additives in power plant waters. Morpholine acts as a corrosion
Characteristics & Types of Chemical Reactions
 Characteristics & Types of Chemical Reactions What is a chemical reaction? Another name for a chemical change Bonds break and atoms of one or more substances are rearranged and new bonds form New substance(s)
Characteristics & Types of Chemical Reactions What is a chemical reaction? Another name for a chemical change Bonds break and atoms of one or more substances are rearranged and new bonds form New substance(s)
Chapter 1 Chemical Reactions & Equations
 CBSE Class 10th NCERT Science Chapter 1 Chemical Reactions & Equations Intext Questions On Page 6 Question 1: Why should a magnesium ribbon be cleaned before burning in air? Magnesium is an extremely reactive
CBSE Class 10th NCERT Science Chapter 1 Chemical Reactions & Equations Intext Questions On Page 6 Question 1: Why should a magnesium ribbon be cleaned before burning in air? Magnesium is an extremely reactive
(for tutoring, homework help, or help with online classes)
 www.tutor-homework.com (for tutoring, homework help, or help with online classes) 1. chem10b 18.2-30 What is the final stage in municipal water treatment? A. aeration B. settling C. removal of added fluoride
www.tutor-homework.com (for tutoring, homework help, or help with online classes) 1. chem10b 18.2-30 What is the final stage in municipal water treatment? A. aeration B. settling C. removal of added fluoride
INSTRUCTIONS TO CANDIDATES
 NAME. INDEX No.. ADM. NO. CANDIDATE S SIGNATURE 233/2 Date: 30 th July 2013 CHEMISTRY Time: 10.45 12.45 p.m. Paper 2 (Theory) Time: 2 Hours JULY/AUGUST 2013 2013 RABAI DISTRICT MOCK EXAMINATION Kenya Certificate
NAME. INDEX No.. ADM. NO. CANDIDATE S SIGNATURE 233/2 Date: 30 th July 2013 CHEMISTRY Time: 10.45 12.45 p.m. Paper 2 (Theory) Time: 2 Hours JULY/AUGUST 2013 2013 RABAI DISTRICT MOCK EXAMINATION Kenya Certificate
Management of Potentially Toxic Substances
 The Science of Chemical Safety Essential Toxicology - 5 Management of Potentially Toxic Substances John Duffus & Howard Worth IUPAC Educators Resource Material IUPAC Management of Toxic Substances Management
The Science of Chemical Safety Essential Toxicology - 5 Management of Potentially Toxic Substances John Duffus & Howard Worth IUPAC Educators Resource Material IUPAC Management of Toxic Substances Management
Santa Monica College Chemistry 11
 Types of Reactions Objectives The objectives of this laboratory are as follows: To perform several types of simple chemical reactions, To become familiar with some common observable signs of chemical reactions,
Types of Reactions Objectives The objectives of this laboratory are as follows: To perform several types of simple chemical reactions, To become familiar with some common observable signs of chemical reactions,
Sodium Chloride - Analytical Standard
 Sodium Chloride - Analytical Standard Determination of Total Mercury Former numbering: ECSS/CN 312-1982 & ESPA/CN-E-106-1994 1. SCOPE AND FIELD OF APPLICATION The present EuSalt Analytical Standard describes
Sodium Chloride - Analytical Standard Determination of Total Mercury Former numbering: ECSS/CN 312-1982 & ESPA/CN-E-106-1994 1. SCOPE AND FIELD OF APPLICATION The present EuSalt Analytical Standard describes
Electrochemistry Worksheets
 Electrochemistry Worksheets Donald Calbreath, Ph.D. Say Thanks to the Authors Click http://www.ck12.org/saythanks (No sign in required) To access a customizable version of this book, as well as other interactive
Electrochemistry Worksheets Donald Calbreath, Ph.D. Say Thanks to the Authors Click http://www.ck12.org/saythanks (No sign in required) To access a customizable version of this book, as well as other interactive
CHEMISTRY HIGHER LEVEL
 *P15* Pre-Leaving Certificate Examination, 2012 Triailscrúdú na hardteistiméireachta, 2012 CHEMISTRY HIGHER LEVEL TIME: 3 HOURS 400 MARKS Answer eight questions in all These must include at least two questions
*P15* Pre-Leaving Certificate Examination, 2012 Triailscrúdú na hardteistiméireachta, 2012 CHEMISTRY HIGHER LEVEL TIME: 3 HOURS 400 MARKS Answer eight questions in all These must include at least two questions
Describe in full the colour change at the end-point of this titration. ... (1)
 Q1. (a) A solution of barium hydroxide is often used for the titration of organic acids. A suitable indicator for the titration is thymol blue. Thymol blue is yellow in acid and blue in alkali. In a titration
Q1. (a) A solution of barium hydroxide is often used for the titration of organic acids. A suitable indicator for the titration is thymol blue. Thymol blue is yellow in acid and blue in alkali. In a titration
New On-Line High-Pressure Electrolytic Eluent Generators for Ion Chromatography
 New On-Line High-Pressure Electrolytic Eluent Generators for Ion Chromatography Yan Liu, Zhongqing Lu, and Chris Pohl, Thermo Fisher Scientific, Sunnyvale, CA USA Overview Purpose: In this work, new high-pressure
New On-Line High-Pressure Electrolytic Eluent Generators for Ion Chromatography Yan Liu, Zhongqing Lu, and Chris Pohl, Thermo Fisher Scientific, Sunnyvale, CA USA Overview Purpose: In this work, new high-pressure
MAHESH TUTORIALS I.C.S.E.
 MAHESH TUTORIALS I.C.S.E. GRADE - X (2017-2018) Exam No. : MT/ICSE/SEMI PRELIM - II - SET -A 020 Periodic Table, Chemical bonding, Acid, Bases and Salts, Practical Work, Mole Concept, Electrolysis Chemistry
MAHESH TUTORIALS I.C.S.E. GRADE - X (2017-2018) Exam No. : MT/ICSE/SEMI PRELIM - II - SET -A 020 Periodic Table, Chemical bonding, Acid, Bases and Salts, Practical Work, Mole Concept, Electrolysis Chemistry
Titration of a strong acid with a strong base with Cobra4
 Titration of a strong acid with a strong base with Cobra4 TEC Related topics Strong and weak acids and bases, ph value, titration curves, equivalence point, potentiometry. Principle Hydrochloric acid is
Titration of a strong acid with a strong base with Cobra4 TEC Related topics Strong and weak acids and bases, ph value, titration curves, equivalence point, potentiometry. Principle Hydrochloric acid is
ADI 201Y Single Method On-line Analyzers
 Applikon Analytical / ADI201Y Single Method On-line Analyzers ADI 201Y Single Method On-line Analyzers ADI 2016 Titrolyzer ADI 2018 Ion-Analyzer ADI 2019 Process Colorimeter ADI 2016 / 2018 / 2019 Automatic
Applikon Analytical / ADI201Y Single Method On-line Analyzers ADI 201Y Single Method On-line Analyzers ADI 2016 Titrolyzer ADI 2018 Ion-Analyzer ADI 2019 Process Colorimeter ADI 2016 / 2018 / 2019 Automatic
Chemical Reactions: Introduction to Reaction Types
 Chemical Reactions: Introduction to Reaction Types **Lab Notebook** Record observations for all of the chemical reactions carried out during the lab in your lab book. These observations should include:
Chemical Reactions: Introduction to Reaction Types **Lab Notebook** Record observations for all of the chemical reactions carried out during the lab in your lab book. These observations should include:
Physical Change - alters the form or appearance of a substance but does not change it into a new, different substance
 Chemical Reactions Physical Change - alters the form or appearance of a substance but does not change it into a new, different substance Chemical Change (aka chemical reaction) - forms one or more new
Chemical Reactions Physical Change - alters the form or appearance of a substance but does not change it into a new, different substance Chemical Change (aka chemical reaction) - forms one or more new
Downloaded from
 Subject: Chemistry Class: XI Chapter: The s-block Elements Top concepts. The s-block elements of the periodic table are those in which the last electron enters the outermost s-orbital. Elements of group
Subject: Chemistry Class: XI Chapter: The s-block Elements Top concepts. The s-block elements of the periodic table are those in which the last electron enters the outermost s-orbital. Elements of group
Acids and Bases 2 Science Notes JC-Learn. JC-Learn. Science Notes Acids and Bases 2. 1 P a g e
 JC-Learn Science Notes Acids and Bases 2 1 P a g e Acids and Bases 2 The two most common laboratory acids are hydrochloric acid (HCl) and sulfuric acid (H2SO4). The two most common laboratory bases are
JC-Learn Science Notes Acids and Bases 2 1 P a g e Acids and Bases 2 The two most common laboratory acids are hydrochloric acid (HCl) and sulfuric acid (H2SO4). The two most common laboratory bases are
BASU. Healthcare. Knowledge brings the greatest benefit
 BASU Healthcare Knowledge brings the greatest benefit Knowledge brings the greatest benefit BASU is a privately owned company in Austria. We design and manufacture high quality products using simple reagents.
BASU Healthcare Knowledge brings the greatest benefit Knowledge brings the greatest benefit BASU is a privately owned company in Austria. We design and manufacture high quality products using simple reagents.
CHLORINE RECOVERY FROM HYDROGEN CHLORIDE
 CHLORINE RECOVERY FROM HYDROGEN CHLORIDE The Project A plant is to be designed for the production of 10,000 metric tons per year of chlorine by the catalytic oxidation of HCl gas. Materials Available 1.
CHLORINE RECOVERY FROM HYDROGEN CHLORIDE The Project A plant is to be designed for the production of 10,000 metric tons per year of chlorine by the catalytic oxidation of HCl gas. Materials Available 1.
Wet FGD Chemistry and Performance Factors
 Wet FGD Chemistry and Performance Factors Gordon Maller URS Corporation Presented at: 2008 Power Gen Conference December 1, 2008 Presentation Outline FGD Chemistry Overview Effect of Key Process Variables
Wet FGD Chemistry and Performance Factors Gordon Maller URS Corporation Presented at: 2008 Power Gen Conference December 1, 2008 Presentation Outline FGD Chemistry Overview Effect of Key Process Variables
Supporting Information for. Sodium Hydroxide Production from Seawater Desalination Brine: Process Design and Energy efficiency
 Supporting Information for Sodium Hydroxide Production from Seawater Desalination Brine: Process Design and Energy efficiency Fengmin Du a, David E. M. Warsinger a, Tamanna I. Urmi a, Gregory P. Thiel
Supporting Information for Sodium Hydroxide Production from Seawater Desalination Brine: Process Design and Energy efficiency Fengmin Du a, David E. M. Warsinger a, Tamanna I. Urmi a, Gregory P. Thiel
CHEMISTRY 13 Electrochemistry Supplementary Problems
 1. When the redox equation CHEMISTRY 13 Electrochemistry Supplementary Problems MnO 4 (aq) + H + (aq) + H 3 AsO 3 (aq) Mn 2+ (aq) + H 3 AsO 4 (aq) + H 2 O(l) is properly balanced, the coefficients will
1. When the redox equation CHEMISTRY 13 Electrochemistry Supplementary Problems MnO 4 (aq) + H + (aq) + H 3 AsO 3 (aq) Mn 2+ (aq) + H 3 AsO 4 (aq) + H 2 O(l) is properly balanced, the coefficients will
The electrolysis of sodium chloride solution produces useful substances. covalent ionic non-metallic
 1 The electrolysis of sodium chloride solution produces useful substances. (a) (i) Choose a word from the box to complete the sentence. covalent ionic non-metallic Electrolysis takes place when electricity
1 The electrolysis of sodium chloride solution produces useful substances. (a) (i) Choose a word from the box to complete the sentence. covalent ionic non-metallic Electrolysis takes place when electricity
Drinking water is allowed to contain up to 1.3 parts per million of copper (by mass) and be considered safe. What does parts per million (ppm) mean?
 E One in a Million Drinking water is allowed to contain up to 1. parts per million of copper (by mass) and be considered safe. What does parts per million (ppm) mean? Living things and the environment
E One in a Million Drinking water is allowed to contain up to 1. parts per million of copper (by mass) and be considered safe. What does parts per million (ppm) mean? Living things and the environment
Exercise 2-4. Titration of a Buffer Solution EXERCISE OBJECTIVES
 Exercise 2-4 Titration of a Buffer Solution EXERCISE OBJECTIVES To define the terms buffer solution and buffer capacity; To titrate a buffer solution with a weak acid solution; To plot a graph using the
Exercise 2-4 Titration of a Buffer Solution EXERCISE OBJECTIVES To define the terms buffer solution and buffer capacity; To titrate a buffer solution with a weak acid solution; To plot a graph using the
