Dr M. Mehrdad University of Guilan, Department of Chemistry, Rasht, Iran
|
|
|
- Cecily Hodges
- 5 years ago
- Views:
Transcription
1 Dr M. Mehrdad University of Guilan, Department of Chemistry, Rasht, Iran
2 MO theory considers the electrons in molecules to occupy MOs that are formed by linear combinations (addition and subtraction) of all the atomic orbitals on all the atoms in the structure. In MOT, electrons are not confined to an individual atom plus the bonding region with another atom. Instead, electrons are contained in MOs that are highly delocalized spread across the entire molecule. MOT is based on the Schrödinger equation. Ψ = EΨ : amiltonian operator Ψ: wavefunction describing an orbital E: the energy of an electron in a particular orbital obtain Ψ and this equation Ψ = Σc i φ i (linear combinations of all the atomic orbitals) c i = coefficient φ i = atomic orbital To construct group MO s, one needs to understand how to combine AO s properly. 2 This procedure is called qualitative molecular orbital theory(qmot)
3 Rules of QMOT 1. Consider valence orbitals only (e.g., for Carbon, 2s, 2p x, 2p y and 2p z ) 2. Form completely delocalized MO s as linear combinations of s and p AO s. Remember, combination of n AO s gives n MO s 3. MO s must be either symmetric or antisymmetric with respect to the symmetry operations of the molecule. 4. Compose MO s for structures of higher symmetry and then produce MOs for related, but less symmetric systems by systematic distortion of the MOs for higher symmetry. For example, for the C 2 system, start with linear C (D h ) then bend the system (C 2v ). 5. Molecules with similar molecular structures, e.g., C 3 and N 3, have qualitatively similar MO s, the major difference being the number of valence electrons that occupy the common MO system. 6. The total energy of the system is the sum of the MO energies of the individual valence electrons. ( occupied MO s) 7. If the two highest energy MO s of a given symmetry derive primarily from different kinds of AO s (e.g., s and p), then mix the two MO s to form hybrid orbitals. For example, for the A 2 system (p.3), mix C and E orbitals to form hybrid C and E. 3
4 8. When two orbitals interact, the lower energy orbital is stabilized and the higher energy orbital is destabilized. The out-of-phase or antibonding interaction between the two starting orbitals always raises the energy more than the corresponding in-phase or bonding interaction lowers the energy.. (energy of stabilization, e stab, is always smaller that energy of destabilization, e destab. Thus, 4electron-2center interaction is always repulsive. ) 4
5 9. When two orbitals interact, the lower energy orbital mixes into itself the higher energy one in a bonding way, whereas the higher energy orbital mixes into itself the lower energy one in an antibonding way. (If orbitals of different energy interact (b), the one of lower energy, B, will contribute more in binding orbital; the one of higher energy, A, will contribute more in antibonding orbital.) b) 5
6 10. The smaller the initial energy gap between the two interacting orbitals, the stronger the mixing interaction. 11. The larger the overlap between interacting orbitals, the larger the interaction. (σ-bonds are stronger than π-bonds.) 12. The more electronegative elements have lower energy AO s. 13. A change in the geometry of a molecule will produce a large change in the energy of a particular MO if the geometry change results in changes of AO overlap that are large. 14. The AO coefficients are large in high energy MO s with many nodes or complicated nodal surfaces. 15. Energies of orbitals of the same symmetry classification cannot cross each other. Instead such orbitals mix and diverge. 6
7 example 7
8 8
9 Symmetry Elements E: Identity operation عنصر یکسانی C n : Proper rotation محور چرخشی 3 C C 3 C 2 3 C C 3 C 6 C 2 9
10 Symmetry Elements i: Inversion عمل وارونگی Cl Cl Cl W Cl Cl Cl i Cl Cl Cl W Cl Cl Cl C 4 s h : orizontal Mirror Plane صفحه آیینه ای افقی s h C 2 s v : Vertical Mirror Plane صفحه آیینه ای عمودی Br Br s v Br Br 10
11 Symmetry Elements S n : Improper rotation: combination C n and s h عمل چرخشی انعکاسی S 2 is equivalent to inversion (i) S C s h S 2 Me center of symmetry Me Me Me Me C 2 Me Me s h Me Me Me Me Me 11
12 Groups with no proper rotation axis C 1 : Only E (i.e. no symmetry elements) C s : E and s C i : E and i S n : E, S n (S 1 = C s ; S 2 = C i ) Groups with one proper rotation axis C n : E, C n only Symmetry Groups C nv : E, C n, and n s v (linear unsymmetrical molecules are C v ) C nh : E, C n, and s h Dihedral Groups: Groups with n C 2 axes to C n D n : E, C n, and n C 2 axes to C n D nh : E, C n, n C 2 axes, and s h (linear symmetrical molecules are D h ) D nd : E, C n, n C 2 axes, and n s v Cubic Groups: Groups with more than one C n (n 3) T d : symmetry of a regular tetrahedron: 4 C 3 O h : symmetry of a regular octagon: 6 C 4 I h : symmetry of a regular icosahedron: 12 C 5 12
13 Cl F C C 1 Br Br Br C s Br F Cl F Cl C i Br Br S 4 Br Br C 3 N O C S F C 3 C 3v C v F C 2h C 2 D 2 D 6h O C O D h D 3d O C O O C C C W C C C O O T d O O h I h 13
14 Symmetry Decision Tree C nh Yes No No s h? s v? C n C v or D h More than one C n (n 3) Cubic T, O, I No S 2n colinear w/ C n? Yes C nv Yes Yes Linear? No Yes Find principal axes C n is the principal axis? No nc 2 to C n? n vertical mirror planes No S 2n None Yes Yes C s, C i or C 1 s h? No s v? Yes D nd Yes No D nh D n Physical Chemistry, Joseph. Noggle, 2nd ed., Scott Foresman & Co, Glenview, IL, 1996, pg
15 To illustrate the procedures of qualitative MO theory, we will build the MO s of planar C 3. We choose planar C 3 because it is more symmetrical. We will be using: - three 1s orbitals, - one C 2s orbital, - and three C 2p orbitals. 15
16 1. First, mix the carbon 2s orbital with the three AO s in-phase to produce orbital A which is symmetric with respect to the C 3 -axis of symmetry of the molecule. [Focus on the low-lying, bonding MO s, the orbitals of which are mixed in-phase (bonding).] Using out-of-phase mixing, one gets the high energy, antibonding orbital E. 16
17 2. Next, use the carbon p-orbitals. The p z AO cannot mix with any of the orbitals, since they all lie on the nodal plane of this orbital. We thus have an MO that is simply an atomic p-orbital, D. The p x and p y AO s can mix with the 1s orbitals of the atoms to give favorable interaction patterns, as seen in MO s B and C. B and C are degenerate of the same energy. 17
18 Orbitals A, B, C and D are the group orbitals for planar C 3. 18
19 MOs for Planar A 3 19
20 20
21 A diagram that follows orbital energies as a function of angular distortions is termed a Walsh diagram. Pyramidalization lowers the energy of orbital A slightly (slight - interaction); it raises the energies of B and C more because of the loss of overlap between the p orbitals and the hydrogen orbitals. 21
22 The biggest impact, however, is on orbital D. This orbital is non-bonding when planar, but becomes increasingly bonding upon pyramidalization. Orbitals A-C are strongly C- bonding, whether planar or pyramidal. In a VB model, we would want three C- bonds, each with two electrons, for a total of six C- bonding electrons. The two models agree. With QMOT, we still have three C- bonds, described by three occupied MOs that are strongly C- bonding. 22
23 Consistent with Rule 7, 7. If the two highest energy MO s of a given symmetry derive primarily from different kinds of AO s then mix the two MO s to form hybrid orbitals. MO s D and E, having the same symmetry, but one based on a carbon p orbital and the other using a 2s orbital, leads to mixing of these two orbitals to form D and E. D now looks more like a lone pair orbital, and it resembles an sp i hybrid at carbon. 23
24 24
25 Group orbitals a collection of partially delocalized orbitals that is consistently associated with a functional group or similar collection of atoms in a molecule. A, B, C, D (or D ) are the Group orbitals of the methyl group, and we can use these orbitals to model the bonding in any molecules that contain the methyl group. 1. Low-lying C- bonding orbitals derived from carbon 2s orbitals and of s-symmetry are termed s(c 3 ) orbitals. 2. The C- bonding orbitals that are derived from carbon 2p orbitals, of -symmetry, are termed (C3) orbitals. They are a degenerate pair. 3. The other orbital of s-symmetry, that derived from the carbon p z AO, points away from the s and is termed the s(out) orbital. orbitals/html/page11.htm 25
26 26
Chapter 9. Molecular Geometry and Bonding Theories
 Chapter 9. Molecular Geometry and Bonding Theories PART I Molecular Shapes Lewis structures give atomic connectivity: they tell us which atoms are physically connected to which atoms. The shape of a molecule
Chapter 9. Molecular Geometry and Bonding Theories PART I Molecular Shapes Lewis structures give atomic connectivity: they tell us which atoms are physically connected to which atoms. The shape of a molecule
Shapes of Molecules. Lewis structures are useful but don t allow prediction of the shape of a molecule.
 Shapes of Molecules Lewis structures are useful but don t allow prediction of the shape of a molecule. H O H H O H Can use a simple theory based on electron repulsion to predict structure (for non-transition
Shapes of Molecules Lewis structures are useful but don t allow prediction of the shape of a molecule. H O H H O H Can use a simple theory based on electron repulsion to predict structure (for non-transition
Chapter 9. Molecular Geometry and Bonding Theories
 Chapter 9. Molecular Geometry and Bonding Theories 9.1 Molecular Shapes Lewis structures give atomic connectivity: they tell us which atoms are physically connected to which atoms. The shape of a molecule
Chapter 9. Molecular Geometry and Bonding Theories 9.1 Molecular Shapes Lewis structures give atomic connectivity: they tell us which atoms are physically connected to which atoms. The shape of a molecule
Hybridization and Molecular Orbital (MO) Theory
 ybridization and Molecular Orbital (MO) Theory Chapter 10 istorical Models G.N.Lewis and I. Langmuir (~1920) laid out foundations Ionic species were formed by electron transfer Covalent molecules arise
ybridization and Molecular Orbital (MO) Theory Chapter 10 istorical Models G.N.Lewis and I. Langmuir (~1920) laid out foundations Ionic species were formed by electron transfer Covalent molecules arise
1s atomic orbital 2s atomic orbital 2s atomic orbital (with node) 2px orbital 2py orbital 2pz orbital
 Atomic Orbitals 1s atomic orbital 2s atomic orbital 2s atomic orbital (with node) 2px orbital 2py orbital 2pz orbital Valence Bond Theory and ybridized Atomic Orbitals Bonding in 2 1s 1s Atomic Orbital
Atomic Orbitals 1s atomic orbital 2s atomic orbital 2s atomic orbital (with node) 2px orbital 2py orbital 2pz orbital Valence Bond Theory and ybridized Atomic Orbitals Bonding in 2 1s 1s Atomic Orbital
Chapter 9 Molecular Geometry and Bonding Theories
 Lecture Presentation Chapter 9 Geometry James F. Kirby Quinnipiac University Hamden, CT Shapes Lewis Structures show bonding and lone pairs, but do not denote shape. However, we use Lewis Structures to
Lecture Presentation Chapter 9 Geometry James F. Kirby Quinnipiac University Hamden, CT Shapes Lewis Structures show bonding and lone pairs, but do not denote shape. However, we use Lewis Structures to
CHAPTER TEN MOLECULAR GEOMETRY MOLECULAR GEOMETRY V S E P R CHEMICAL BONDING II: MOLECULAR GEOMETRY AND HYBRIDIZATION OF ATOMIC ORBITALS
 CHAPTER TEN CHEMICAL BONDING II: AND HYBRIDIZATION O ATOMIC ORBITALS V S E P R VSEPR Theory In VSEPR theory, multiple bonds behave like a single electron pair Valence shell electron pair repulsion (VSEPR)
CHAPTER TEN CHEMICAL BONDING II: AND HYBRIDIZATION O ATOMIC ORBITALS V S E P R VSEPR Theory In VSEPR theory, multiple bonds behave like a single electron pair Valence shell electron pair repulsion (VSEPR)
QUANTUM MECHANICS AND MOLECULAR STRUCTURE
 6 QUANTUM MECHANICS AND MOLECULAR STRUCTURE 6.1 Quantum Picture of the Chemical Bond 6.2 Exact Molecular Orbital for the Simplest Molecule: H + 2 6.3 Molecular Orbital Theory and the Linear Combination
6 QUANTUM MECHANICS AND MOLECULAR STRUCTURE 6.1 Quantum Picture of the Chemical Bond 6.2 Exact Molecular Orbital for the Simplest Molecule: H + 2 6.3 Molecular Orbital Theory and the Linear Combination
The symmetry properties & relative energies of atomic orbitals determine how they react to form molecular orbitals. These molecular orbitals are then
 1 The symmetry properties & relative energies of atomic orbitals determine how they react to form molecular orbitals. These molecular orbitals are then filled with the available electrons according to
1 The symmetry properties & relative energies of atomic orbitals determine how they react to form molecular orbitals. These molecular orbitals are then filled with the available electrons according to
Constructing a MO of NH 3. Nitrogen AO symmetries are
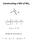 Constructing a MO of NH 3 Nitrogen AO symmetries are To develop a MO scheme for NH 3 assume that only the 2s and2p orbitals of nitrogen interact with the hydrogen 1s orbitals (i.e., the nitrogen 1s orbital
Constructing a MO of NH 3 Nitrogen AO symmetries are To develop a MO scheme for NH 3 assume that only the 2s and2p orbitals of nitrogen interact with the hydrogen 1s orbitals (i.e., the nitrogen 1s orbital
We can keep track of the mixing of the 2s and 2p orbitals in beryllium as follows:
 We can keep track of the mixing of the 2s and 2p orbitals in beryllium as follows: The beryllium sp orbitals overlap with hydrogen Is orbitals (the hydrogen's electrons are shown in the above orbital diagram
We can keep track of the mixing of the 2s and 2p orbitals in beryllium as follows: The beryllium sp orbitals overlap with hydrogen Is orbitals (the hydrogen's electrons are shown in the above orbital diagram
Chapter 10: Chemical Bonding II. Bonding Theories
 Chapter 10: Chemical Bonding II Dr. Chris Kozak Memorial University of Newfoundland, Canada Bonding Theories Previously, we saw how the shapes of molecules can be predicted from the orientation of electron
Chapter 10: Chemical Bonding II Dr. Chris Kozak Memorial University of Newfoundland, Canada Bonding Theories Previously, we saw how the shapes of molecules can be predicted from the orientation of electron
Molecular Orbital Theory. Molecular Orbital Theory: Electrons are located in the molecule, not held in discrete regions between two bonded atoms
 Molecular Orbital Theory Valence Bond Theory: Electrons are located in discrete pairs between specific atoms Molecular Orbital Theory: Electrons are located in the molecule, not held in discrete regions
Molecular Orbital Theory Valence Bond Theory: Electrons are located in discrete pairs between specific atoms Molecular Orbital Theory: Electrons are located in the molecule, not held in discrete regions
Be H. Delocalized Bonding. Localized Bonding. σ 2. σ 1. Two (sp-1s) Be-H σ bonds. The two σ bonding MO s in BeH 2. MO diagram for BeH 2
 The Delocalized Approach to Bonding: The localized models for bonding we have examined (Lewis and VBT) assume that all electrons are restricted to specific bonds between atoms or in lone pairs. In contrast,
The Delocalized Approach to Bonding: The localized models for bonding we have examined (Lewis and VBT) assume that all electrons are restricted to specific bonds between atoms or in lone pairs. In contrast,
Chapter 9. Chemical Bonding II: Molecular Geometry and Bonding Theories
 Chapter 9 Chemical Bonding II: Molecular Geometry and Bonding Theories Topics Molecular Geometry Molecular Geometry and Polarity Valence Bond Theory Hybridization of Atomic Orbitals Hybridization in Molecules
Chapter 9 Chemical Bonding II: Molecular Geometry and Bonding Theories Topics Molecular Geometry Molecular Geometry and Polarity Valence Bond Theory Hybridization of Atomic Orbitals Hybridization in Molecules
Wave Equations of Polyatomic Molecules
 Wave Equations of Polyatomic Molecules! Approximate wave functions are sought by combining atomic wave functions for the bonded atoms.! Several different approaches have been taken to constructing trial
Wave Equations of Polyatomic Molecules! Approximate wave functions are sought by combining atomic wave functions for the bonded atoms.! Several different approaches have been taken to constructing trial
Chapter 4 Symmetry and Chemical Bonding
 Chapter 4 Symmetry and Chemical Bonding 4.1 Orbital Symmetries and Overlap 4.2 Valence Bond Theory and Hybrid Orbitals 4.3 Localized and Delocalized Molecular Orbitals 4.4 MX n Molecules with Pi-Bonding
Chapter 4 Symmetry and Chemical Bonding 4.1 Orbital Symmetries and Overlap 4.2 Valence Bond Theory and Hybrid Orbitals 4.3 Localized and Delocalized Molecular Orbitals 4.4 MX n Molecules with Pi-Bonding
Chemistry Lecture Notes
 Molecular orbital theory Valence bond theory gave us a qualitative picture of chemical bonding. Useful for predicting shapes of molecules, bond strengths, etc. It fails to describe some bonding situations
Molecular orbital theory Valence bond theory gave us a qualitative picture of chemical bonding. Useful for predicting shapes of molecules, bond strengths, etc. It fails to describe some bonding situations
Chapter 10 Chemical Bonding II: Molecular Shapes, Valence Bond Theory, and Molecular Orbital Theory
 10.1 Artificial Sweeteners: Fooled by Molecular Shape 425 10.2 VSEPR Theory: The Five Basic Shapes 426 10.3 VSEPR Theory: The Effect of Lone Pairs 430 10.4 VSEPR Theory: Predicting Molecular Geometries
10.1 Artificial Sweeteners: Fooled by Molecular Shape 425 10.2 VSEPR Theory: The Five Basic Shapes 426 10.3 VSEPR Theory: The Effect of Lone Pairs 430 10.4 VSEPR Theory: Predicting Molecular Geometries
Chemistry: The Central Science. Chapter 9: Molecular Geometry and Bonding Theory
 Chemistry: The Central Science Chapter 9: Molecular Geometry and Bonding Theory The shape and size of a molecule of a particular substance, together with the strength and polarity of its bonds, largely
Chemistry: The Central Science Chapter 9: Molecular Geometry and Bonding Theory The shape and size of a molecule of a particular substance, together with the strength and polarity of its bonds, largely
Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals Chapter 10
 Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals Chapter 10 Linear Trigonal 180 o planar 120 o Tetrahedral 109.5 o Trigonal Bipyramidal 120 and 90 o Octahedral 90 o linear Linear
Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals Chapter 10 Linear Trigonal 180 o planar 120 o Tetrahedral 109.5 o Trigonal Bipyramidal 120 and 90 o Octahedral 90 o linear Linear
Chapter 10 Theories of Covalent Bonding
 Chapter 10 Theories of Covalent Bonding 1 Atomic Orbitals Molecules Bonding and 2 Molecular Structure Questions How are molecules held together? Why is O 2 paramagnetic? And how is this property connected
Chapter 10 Theories of Covalent Bonding 1 Atomic Orbitals Molecules Bonding and 2 Molecular Structure Questions How are molecules held together? Why is O 2 paramagnetic? And how is this property connected
Coordination Chemistry: Bonding Theories. Molecular Orbital Theory. Chapter 20
 Coordination Chemistry: Bonding Theories Molecular Orbital Theory Chapter 20 Review of the Previous Lecture 1. Discussed magnetism in coordination chemistry and the different classification of compounds
Coordination Chemistry: Bonding Theories Molecular Orbital Theory Chapter 20 Review of the Previous Lecture 1. Discussed magnetism in coordination chemistry and the different classification of compounds
Chapter 9: Molecular Geometries and Bonding Theories Learning Outcomes: Predict the three-dimensional shapes of molecules using the VSEPR model.
 Chapter 9: Molecular Geometries and Bonding Theories Learning Outcomes: Predict the three-dimensional shapes of molecules using the VSEPR model. Determine whether a molecule is polar or nonpolar based
Chapter 9: Molecular Geometries and Bonding Theories Learning Outcomes: Predict the three-dimensional shapes of molecules using the VSEPR model. Determine whether a molecule is polar or nonpolar based
Be H. Delocalized Bonding. Localized Bonding. σ 2. σ 1. Two (sp-1s) Be-H σ bonds. The two σ bonding MO s in BeH 2. MO diagram for BeH 2
 The Delocalized Approach to Bonding: The localized models for bonding we have examined (Lewis and VBT) assume that all electrons are restricted to specific bonds between atoms or in lone pairs. In contrast,
The Delocalized Approach to Bonding: The localized models for bonding we have examined (Lewis and VBT) assume that all electrons are restricted to specific bonds between atoms or in lone pairs. In contrast,
Chapter 10 Chemical Bonding II
 Chapter 10 Chemical Bonding II Valence Bond Theory Valence Bond Theory: A quantum mechanical model which shows how electron pairs are shared in a covalent bond. Bond forms between two atoms when the following
Chapter 10 Chemical Bonding II Valence Bond Theory Valence Bond Theory: A quantum mechanical model which shows how electron pairs are shared in a covalent bond. Bond forms between two atoms when the following
Chapter 10. Structure Determines Properties! Molecular Geometry. Chemical Bonding II
 Chapter 10 Chemical Bonding II Structure Determines Properties! Properties of molecular substances depend on the structure of the molecule The structure includes many factors, including: the skeletal arrangement
Chapter 10 Chemical Bonding II Structure Determines Properties! Properties of molecular substances depend on the structure of the molecule The structure includes many factors, including: the skeletal arrangement
Molecular shape is only discussed when there are three or more atoms connected (diatomic shape is obvious).
 Chapter 10 Molecular Geometry (Ch9 Jespersen, Ch10 Chang) The arrangement of the atoms of a molecule in space is the molecular geometry. This is what gives the molecules their shape. Molecular shape is
Chapter 10 Molecular Geometry (Ch9 Jespersen, Ch10 Chang) The arrangement of the atoms of a molecule in space is the molecular geometry. This is what gives the molecules their shape. Molecular shape is
Chapter 9 Molecular Geometry and Bonding Theories
 Chapter 9 Molecular Geometry and Bonding Theories 9.1 Molecular Shapes Lewis structures give atomic connectivity (which atoms are physically connected). By noting the number of bonding and nonbonding electron
Chapter 9 Molecular Geometry and Bonding Theories 9.1 Molecular Shapes Lewis structures give atomic connectivity (which atoms are physically connected). By noting the number of bonding and nonbonding electron
Chapter 9. Molecular Geometries and Bonding Theories. Lecture Presentation. John D. Bookstaver St. Charles Community College Cottleville, MO
 Lecture Presentation Chapter 9 Theories John D. Bookstaver St. Charles Community College Cottleville, MO Shapes The shape of a molecule plays an important role in its reactivity. By noting the number of
Lecture Presentation Chapter 9 Theories John D. Bookstaver St. Charles Community College Cottleville, MO Shapes The shape of a molecule plays an important role in its reactivity. By noting the number of
Chapter 9 Molecular Geometry Valence Bond and Molecular Orbital Theory
 Chapter 9 Molecular Geometry Valence Bond and Molecular Orbital Theory Chapter Objectives: Learn the basics of Valence Bond Theory and Molecular Orbital Theory and how they are used to model covalent bonding.
Chapter 9 Molecular Geometry Valence Bond and Molecular Orbital Theory Chapter Objectives: Learn the basics of Valence Bond Theory and Molecular Orbital Theory and how they are used to model covalent bonding.
Molecular Structure and Orbitals
 CHEM 1411 General Chemistry Chemistry: An Atoms First Approach by Zumdahl 2 5 Molecular Structure and Orbitals Chapter Objectives: Learn the basics of Valence Bond Theory and Molecular Orbital Theory and
CHEM 1411 General Chemistry Chemistry: An Atoms First Approach by Zumdahl 2 5 Molecular Structure and Orbitals Chapter Objectives: Learn the basics of Valence Bond Theory and Molecular Orbital Theory and
Chemistry 3211 Coordination Chemistry Part 3 Ligand Field and Molecular Orbital Theory
 Chemistry 3211 Coordination Chemistry Part 3 Ligand Field and Molecular Orbital Theory Electronic Structure of Six and Four-Coordinate Complexes Using Crystal Field Theory, we can generate energy level
Chemistry 3211 Coordination Chemistry Part 3 Ligand Field and Molecular Orbital Theory Electronic Structure of Six and Four-Coordinate Complexes Using Crystal Field Theory, we can generate energy level
EXAM II Material. Part I Chemical Bonding I Lewis Theory Chapter 9 pages A. Drawing electron dot structures HOW TO:
 CHEMISTRY 112 LECTURE EXAM II Material Part I Chemical Bonding I Lewis Theory Chapter 9 pages 376-386 A. Drawing electron dot structures HOW TO: 1. Write e- dot structure for the individual atoms. 2. a)
CHEMISTRY 112 LECTURE EXAM II Material Part I Chemical Bonding I Lewis Theory Chapter 9 pages 376-386 A. Drawing electron dot structures HOW TO: 1. Write e- dot structure for the individual atoms. 2. a)
Symmetry and Molecular Orbitals (I)
 Symmetry and Molecular Orbitals (I) Simple Bonding Model http://chiuserv.ac.nctu.edu.tw/~htchiu/chemistry/fall-2005/chemical-bonds.htm Lewis Structures Octet Rule Resonance Formal Charge Oxidation Number
Symmetry and Molecular Orbitals (I) Simple Bonding Model http://chiuserv.ac.nctu.edu.tw/~htchiu/chemistry/fall-2005/chemical-bonds.htm Lewis Structures Octet Rule Resonance Formal Charge Oxidation Number
Chapter 5. Molecular Orbitals
 Chapter 5. Molecular Orbitals MO from s, p, d, orbitals: - Fig.5.1, 5.2, 5.3 Homonuclear diatomic molecules: - Fig. 5.7 - Para- vs. Diamagnetic Heteronuclear diatomic molecules: - Fig. 5.14 - ex. CO Hybrid
Chapter 5. Molecular Orbitals MO from s, p, d, orbitals: - Fig.5.1, 5.2, 5.3 Homonuclear diatomic molecules: - Fig. 5.7 - Para- vs. Diamagnetic Heteronuclear diatomic molecules: - Fig. 5.14 - ex. CO Hybrid
Chapter 9. Covalent Bonding: Orbitals
 Chapter 9. Covalent onding: Orbitals Models to explain the structures and/or energies of the covalent molecules Localized Electron (LE) onding Model Lewis Structure Valence Shell Electron Pair Repulsion
Chapter 9. Covalent onding: Orbitals Models to explain the structures and/or energies of the covalent molecules Localized Electron (LE) onding Model Lewis Structure Valence Shell Electron Pair Repulsion
Chapter 10: Chemical Bonding II: Molecular Shapes; VSEPR, Valence Bond and Molecular Orbital Theories
 C h e m i s t r y 1 A : C h a p t e r 1 0 P a g e 1 Chapter 10: Chemical Bonding II: Molecular Shapes; VSEPR, Valence Bond and Molecular Orbital Theories Homework: Read Chapter 10: Work out sample/practice
C h e m i s t r y 1 A : C h a p t e r 1 0 P a g e 1 Chapter 10: Chemical Bonding II: Molecular Shapes; VSEPR, Valence Bond and Molecular Orbital Theories Homework: Read Chapter 10: Work out sample/practice
structure prediction, chemical bonding
 1 structure prediction, chemical bonding 2 Lewis structures Atoms listed in order of increasing EN, no connectivity implied CSPF (PNF 2 ) 4 3 after the Lewis Structure determine the steric number number
1 structure prediction, chemical bonding 2 Lewis structures Atoms listed in order of increasing EN, no connectivity implied CSPF (PNF 2 ) 4 3 after the Lewis Structure determine the steric number number
Molecular Geometry and Bonding Theories. Chapter 9
 Molecular Geometry and Bonding Theories Chapter 9 Molecular Shapes CCl 4 Lewis structures give atomic connectivity; The shape of a molecule is determined by its bond angles VSEPR Model Valence Shell Electron
Molecular Geometry and Bonding Theories Chapter 9 Molecular Shapes CCl 4 Lewis structures give atomic connectivity; The shape of a molecule is determined by its bond angles VSEPR Model Valence Shell Electron
Valence Bond Theory Considers the interaction of separate atoms brought together as they form a molecule. Lewis structures Resonance considerations
 CHEM 511 chapter 2 page 1 of 11 Chapter 2 Molecular Structure and Bonding Read the section on Lewis dot structures, we will not cover this in class. If you have problems, seek out a general chemistry text.
CHEM 511 chapter 2 page 1 of 11 Chapter 2 Molecular Structure and Bonding Read the section on Lewis dot structures, we will not cover this in class. If you have problems, seek out a general chemistry text.
Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals
 Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals Chapter 10 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 1 Valence shell electron
Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals Chapter 10 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 1 Valence shell electron
RDCH 702 Lecture 4: Orbitals and energetics
 RDCH 702 Lecture 4: Orbitals and energetics Molecular symmetry Bonding and structure Molecular orbital theory Crystal field theory Ligand field theory Provide fundamental understanding of chemistry dictating
RDCH 702 Lecture 4: Orbitals and energetics Molecular symmetry Bonding and structure Molecular orbital theory Crystal field theory Ligand field theory Provide fundamental understanding of chemistry dictating
Chapter 9. Molecular Geometry and Bonding Theories
 Chapter 9 Molecular Geometry and Bonding Theories MOLECULAR SHAPES 2 Molecular Shapes Lewis Structures show bonding and lone pairs do not denote shape Use Lewis Structures to determine shapes Molecular
Chapter 9 Molecular Geometry and Bonding Theories MOLECULAR SHAPES 2 Molecular Shapes Lewis Structures show bonding and lone pairs do not denote shape Use Lewis Structures to determine shapes Molecular
Chapter 4 Symmetry and Chemical Bonding
 Chapter 4 Symmetry and Chemical Bonding 4.1 Orbital Symmetries and Overlap 4.2 Valence Bond Theory and Hybrid Orbitals 4.3 Localized and Delocalized Molecular Orbitals 4.4 MX n Molecules with Pi-Bonding
Chapter 4 Symmetry and Chemical Bonding 4.1 Orbital Symmetries and Overlap 4.2 Valence Bond Theory and Hybrid Orbitals 4.3 Localized and Delocalized Molecular Orbitals 4.4 MX n Molecules with Pi-Bonding
A Rigorous Introduction to Molecular Orbital Theory and its Applications in Chemistry. Zachary Chin, Alex Li, Alex Liu
 A Rigorous Introduction to Molecular Orbital Theory and its Applications in Chemistry Zachary Chin, Alex Li, Alex Liu Quantum Mechanics Atomic Orbitals and Early Bonding Theory Quantum Numbers n: principal
A Rigorous Introduction to Molecular Orbital Theory and its Applications in Chemistry Zachary Chin, Alex Li, Alex Liu Quantum Mechanics Atomic Orbitals and Early Bonding Theory Quantum Numbers n: principal
Chapter 9. Molecular Geometries and Bonding Theories. Lecture Presentation. John D. Bookstaver St. Charles Community College Cottleville, MO
 Lecture Presentation Chapter 9 Theories John D. Bookstaver St. Charles Community College Cottleville, MO Shapes The shape of a molecule plays an important role in its reactivity. By noting the number of
Lecture Presentation Chapter 9 Theories John D. Bookstaver St. Charles Community College Cottleville, MO Shapes The shape of a molecule plays an important role in its reactivity. By noting the number of
Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals
 Chemical Bonding II: and ybridization of Atomic rbitals Chapter 10 Valence shell electron pair repulsion (VSEPR) model: Predict the geometry of the molecule from the electrostatic repulsions between the
Chemical Bonding II: and ybridization of Atomic rbitals Chapter 10 Valence shell electron pair repulsion (VSEPR) model: Predict the geometry of the molecule from the electrostatic repulsions between the
Chemistry 2000 Lecture 8: Valence bond theory
 Chemistry 000 Lecture 8: Valence bond theory Marc R. Roussel January 9, 08 Marc R. Roussel Valence bond theory January 9, 08 / 5 MO theory: a recap A molecular orbital is a one-electron wavefunction which,
Chemistry 000 Lecture 8: Valence bond theory Marc R. Roussel January 9, 08 Marc R. Roussel Valence bond theory January 9, 08 / 5 MO theory: a recap A molecular orbital is a one-electron wavefunction which,
Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals
 Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals 1 Chemical Bonding II Molecular Geometry (10.1) Dipole Moments (10.2) Valence Bond Theory (10.3) Hybridization of Atomic Orbitals
Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals 1 Chemical Bonding II Molecular Geometry (10.1) Dipole Moments (10.2) Valence Bond Theory (10.3) Hybridization of Atomic Orbitals
Molecular Orbital Theory This means that the coefficients in the MO will not be the same!
 Diatomic molecules: Heteronuclear molecules In heteronuclear diatomic molecules, the relative contribution of atomic orbitals to each MO is not equal. Some MO s will have more contribution from AO s on
Diatomic molecules: Heteronuclear molecules In heteronuclear diatomic molecules, the relative contribution of atomic orbitals to each MO is not equal. Some MO s will have more contribution from AO s on
Using Symmetry to Generate Molecular Orbital Diagrams
 Using Symmetry to Generate Molecular Orbital Diagrams review a few MO concepts generate MO for XH 2, H 2 O, SF 6 Formation of a bond occurs when electron density collects between the two bonded nuclei
Using Symmetry to Generate Molecular Orbital Diagrams review a few MO concepts generate MO for XH 2, H 2 O, SF 6 Formation of a bond occurs when electron density collects between the two bonded nuclei
CHEMISTRY - ZUMDAHL 2E CH.4 - MOLECULAR STRUCTURE AND ORBITALS.
 !! www.clutchprep.com CONCEPT: ELECTRONIC GEOMETRY When drawing a compound you have to take into account two different systems of geometrical shape. The simpler system known as electronic geometry or shape
!! www.clutchprep.com CONCEPT: ELECTRONIC GEOMETRY When drawing a compound you have to take into account two different systems of geometrical shape. The simpler system known as electronic geometry or shape
NH 3 H 2 O N 2. Why do they make chemical bonds? Molecular Orbitals
 N 2 NH 3 H 2 O Why do they make chemical bonds? 5 Molecular Orbitals Why do they make chemical bonds? Stabilization Bond energy Types of Chemical Bonds Metallic Bond Ionic Bond Covalent Bond Covalent Bond
N 2 NH 3 H 2 O Why do they make chemical bonds? 5 Molecular Orbitals Why do they make chemical bonds? Stabilization Bond energy Types of Chemical Bonds Metallic Bond Ionic Bond Covalent Bond Covalent Bond
Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals Chapter 1
 Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals Chapter 1 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. How to get the book of
Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals Chapter 1 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. How to get the book of
AP Chemistry - Problem Drill 15: Lewis Structures and VSEPR Theory
 AP Chemistry - Problem Drill 15: Lewis Structures and VSEPR Theory No. 1 of 10 1. Which shape would have sp 3 hybridization? (A) Linear (B) Bent (C) Tetrahedron (D) Trigonal planar (E) Octahedron C. Correct.
AP Chemistry - Problem Drill 15: Lewis Structures and VSEPR Theory No. 1 of 10 1. Which shape would have sp 3 hybridization? (A) Linear (B) Bent (C) Tetrahedron (D) Trigonal planar (E) Octahedron C. Correct.
: Bond Order = 1.5 CHAPTER 5. Practice Questions
 CAPTER 5 Practice Questions 5.1 5.3 S 5.5 Ethane is symmetrical, so does not have a dipole moment. owever, ethanol has a polar group at one end and so has a dipole moment. 5.7 xygen has the valence electron
CAPTER 5 Practice Questions 5.1 5.3 S 5.5 Ethane is symmetrical, so does not have a dipole moment. owever, ethanol has a polar group at one end and so has a dipole moment. 5.7 xygen has the valence electron
MO theory is better for spectroscopy (Exited State Properties; Ionization)
 CHEM 2060 Lecture 25: MO Theory L25-1 Molecular Orbital Theory (MO theory) VB theory treats bonds as electron pairs. o There is a real emphasis on this point (over-emphasis actually). VB theory is very
CHEM 2060 Lecture 25: MO Theory L25-1 Molecular Orbital Theory (MO theory) VB theory treats bonds as electron pairs. o There is a real emphasis on this point (over-emphasis actually). VB theory is very
CHEMISTRY - MCMURRY 7E CH.7 - COVALENT BONDING AND ELECTRON DOT STRUCTURES
 !! www.clutchprep.com CONCEPT: ELECTRONIC GEOMETRY When drawing a compound you have to take into account two different systems of geometrical shape. The simpler system known as electronic geometry or shape
!! www.clutchprep.com CONCEPT: ELECTRONIC GEOMETRY When drawing a compound you have to take into account two different systems of geometrical shape. The simpler system known as electronic geometry or shape
General Chemistry I (2012) Lecture by B. H. Hong
 3.8 The Limitations of Lewis's Theory 3.9 Molecular Orbitals The valence-bond (VB) and molecular orbital (MO) theories are both procedures for constructing approximate wavefunctions of electrons. The MO
3.8 The Limitations of Lewis's Theory 3.9 Molecular Orbitals The valence-bond (VB) and molecular orbital (MO) theories are both procedures for constructing approximate wavefunctions of electrons. The MO
Chemistry 1B, Fall 2012 Lectures 15-16
 Chemistry 1B Fall 2012 Quantum Mechanics of the Covalent Bond for chapter 14 animations and links see: http://switkes.chemistry.ucsc.edu/teaching/chem1b/www_other_links/ch14_links.htm 1 LISTEN UP!!! WE
Chemistry 1B Fall 2012 Quantum Mechanics of the Covalent Bond for chapter 14 animations and links see: http://switkes.chemistry.ucsc.edu/teaching/chem1b/www_other_links/ch14_links.htm 1 LISTEN UP!!! WE
Chapter 4. Molecular Structure and Orbitals
 Chapter 4 Molecular Structure and Orbitals Chapter 4 Table of Contents (4.1) (4.2) (4.3) (4.4) (4.5) (4.6) (4.7) Molecular structure: The VSEPR model Bond polarity and dipole moments Hybridization and
Chapter 4 Molecular Structure and Orbitals Chapter 4 Table of Contents (4.1) (4.2) (4.3) (4.4) (4.5) (4.6) (4.7) Molecular structure: The VSEPR model Bond polarity and dipole moments Hybridization and
Chapter 9. Covalent Bonding: Orbitals
 Chapter 9 Covalent Bonding: Orbitals Chapter 9 Table of Contents 9.1 Hybridization and the Localized Electron Model 9.2 The Molecular Orbital Model 9.3 Bonding in Homonuclear Diatomic Molecules 9.4 Bonding
Chapter 9 Covalent Bonding: Orbitals Chapter 9 Table of Contents 9.1 Hybridization and the Localized Electron Model 9.2 The Molecular Orbital Model 9.3 Bonding in Homonuclear Diatomic Molecules 9.4 Bonding
Chemistry 1B, Fall 2013 Lectures 15-16
 Chemistry 1, Fall 2013 Lectures 1516 Chemistry 1 Fall 2013 Lectures 1516 Quantum Mechanics of the Covalent ond LISTEN UP!!! WE WILL E COVERING SECOND PRT OF CHPTER 14 (pp 676688) FIRST You will go CRZY
Chemistry 1, Fall 2013 Lectures 1516 Chemistry 1 Fall 2013 Lectures 1516 Quantum Mechanics of the Covalent ond LISTEN UP!!! WE WILL E COVERING SECOND PRT OF CHPTER 14 (pp 676688) FIRST You will go CRZY
Carbon Compounds. Chemical Bonding Part 1b
 Carbon Compounds Chemical Bonding Part 1b Board Notes Introduction to VSEPR Organic Formulas Various Representations " dimethyl ether C 2 H 6 O " propyl alcohol C 3 H 8 O 3D representations " Wedges and
Carbon Compounds Chemical Bonding Part 1b Board Notes Introduction to VSEPR Organic Formulas Various Representations " dimethyl ether C 2 H 6 O " propyl alcohol C 3 H 8 O 3D representations " Wedges and
Review questions CHAPTER 5. Practice exercises 5.1 F F 5.3
 CHAPTER 5 Practice exercises 5.1 S 5.3 5.5 Ethane is symmetrical, so does not have a dipole moment. However, ethanol has a polar H group at one end and so has a dipole moment. 5.7 xygen has the valence
CHAPTER 5 Practice exercises 5.1 S 5.3 5.5 Ethane is symmetrical, so does not have a dipole moment. However, ethanol has a polar H group at one end and so has a dipole moment. 5.7 xygen has the valence
Structure and Bonding of Organic Molecules
 Chem 220 Notes Page 1 Structure and Bonding of Organic Molecules I. Types of Chemical Bonds A. Why do atoms forms bonds? Atoms want to have the same number of electrons as the nearest noble gas atom (noble
Chem 220 Notes Page 1 Structure and Bonding of Organic Molecules I. Types of Chemical Bonds A. Why do atoms forms bonds? Atoms want to have the same number of electrons as the nearest noble gas atom (noble
Topic 2. Structure and Bonding Models of Covalent Compounds of p-block Elements
 Topic 2 2-1 Structure and Bonding Models of Covalent Compounds of p-block Elements Bonding 2-2 Many different approaches to describe bonding: Ionic Bonding: Elements with large electronegativity differences;
Topic 2 2-1 Structure and Bonding Models of Covalent Compounds of p-block Elements Bonding 2-2 Many different approaches to describe bonding: Ionic Bonding: Elements with large electronegativity differences;
Bonding and Physical Properties The Molecular Orbital Theory
 Bonding and Physical Properties The Molecular Orbital Theory Ø Developed by F. Hund and R. S. Mulliken in 1932 Ø Diagram of molecular energy levels Ø Magnetic and spectral properties Paramagnetic vs. Diamagnetic
Bonding and Physical Properties The Molecular Orbital Theory Ø Developed by F. Hund and R. S. Mulliken in 1932 Ø Diagram of molecular energy levels Ø Magnetic and spectral properties Paramagnetic vs. Diamagnetic
Orbitals and energetics
 Orbitals and energetics Bonding and structure Molecular orbital theory Crystal field theory Ligand field theory Provide fundamental understanding of chemistry dictating radionuclide complexes Structure
Orbitals and energetics Bonding and structure Molecular orbital theory Crystal field theory Ligand field theory Provide fundamental understanding of chemistry dictating radionuclide complexes Structure
Molecular Orbital Theory
 Molecular Orbital Theory Paramagnetic properties of O 2 pranjoto utomo Covalent Bonding Theory Valence Bond Theory useful for deriving shapes/polarity simple but inaccurate/deficient Molecular Orbital
Molecular Orbital Theory Paramagnetic properties of O 2 pranjoto utomo Covalent Bonding Theory Valence Bond Theory useful for deriving shapes/polarity simple but inaccurate/deficient Molecular Orbital
Molecular Orbital Theory (MOT)
 Molecular Orbital Theory (MOT) In this section, There are another approach to the bonding in metal complexes: the use of molecular orbital theory (MOT). In contrast to crystal field theory, the molecular
Molecular Orbital Theory (MOT) In this section, There are another approach to the bonding in metal complexes: the use of molecular orbital theory (MOT). In contrast to crystal field theory, the molecular
Unit 6: Molecular Geometry
 Unit 6: Molecular Geometry Molecular Geometry [6-5] the polarity of each bond, along with the geometry of the molecule determines Molecular Polarity. To predict the geometries of more complicated molecules,
Unit 6: Molecular Geometry Molecular Geometry [6-5] the polarity of each bond, along with the geometry of the molecule determines Molecular Polarity. To predict the geometries of more complicated molecules,
MOLECULAR ORBITAL AND VALENCE BOND THEORY EXPLAINED (HOPEFULLY)
 MOLEULAR ORBITAL AND VALENE BOND TEORY EXPLAINED (OPEFULLY) Quantum Mechanics is a very difficult topic, with a great deal of detail that is extremely complex, yet interesting. owever, in this Organic
MOLEULAR ORBITAL AND VALENE BOND TEORY EXPLAINED (OPEFULLY) Quantum Mechanics is a very difficult topic, with a great deal of detail that is extremely complex, yet interesting. owever, in this Organic
What Do Molecules Look Like?
 What Do Molecules Look Like? The Lewis Dot Structure approach provides some insight into molecular structure in terms of bonding, but what about 3D geometry? Recall that we have two types of electron pairs:
What Do Molecules Look Like? The Lewis Dot Structure approach provides some insight into molecular structure in terms of bonding, but what about 3D geometry? Recall that we have two types of electron pairs:
CHEMISTRY 112 LECTURE EXAM II Material
 CHEMISTRY 112 LECTURE EXAM II Material Part I Chemical Bonding I Lewis Theory Chapter 9 pages 376-386 A. Drawing electron dot structures HOW TO: 1. Write e- dot structure for the individual atoms. 2. a)
CHEMISTRY 112 LECTURE EXAM II Material Part I Chemical Bonding I Lewis Theory Chapter 9 pages 376-386 A. Drawing electron dot structures HOW TO: 1. Write e- dot structure for the individual atoms. 2. a)
Molecular Geometry. Dr. Williamson s Molecular Geometry Notes. VSEPR: Definition of Terms. Dr. V.M. Williamson Texas A & M University Student Version
 Molecular Geometry Dr. V.M. Williamson Texas A & M University Student Version Valence Shell Electron Pair Repulsion- VSEPR 1. Valence e- to some extent 2. Electron pairs move as far away as possible to
Molecular Geometry Dr. V.M. Williamson Texas A & M University Student Version Valence Shell Electron Pair Repulsion- VSEPR 1. Valence e- to some extent 2. Electron pairs move as far away as possible to
Chapter 10. VSEPR Model: Geometries
 Chapter 10 Molecular Geometry VSEPR Model: Geometries Valence Shell Electron Pair Repulsion Theory Electron pairs repel and get as far apart as possible Example: Water Four electron pairs Farthest apart
Chapter 10 Molecular Geometry VSEPR Model: Geometries Valence Shell Electron Pair Repulsion Theory Electron pairs repel and get as far apart as possible Example: Water Four electron pairs Farthest apart
Molecular Geometry. Dr. Williamson s Molecular Geometry Notes. VSEPR: Definition of Terms. VSEPR: Electronic Geometries VSEPR
 Molecular Geometry Dr. V.M. Williamson Texas A & M University Student Version Valence Shell Electron Pair Repulsion- VSEPR 1. Valence e- to some extent 2. Electron pairs move as far away as possible to
Molecular Geometry Dr. V.M. Williamson Texas A & M University Student Version Valence Shell Electron Pair Repulsion- VSEPR 1. Valence e- to some extent 2. Electron pairs move as far away as possible to
We can model covalent bonding in molecules in essentially two ways:
 CHEM 2060 Lecture 22: VB Theory L22-1 PART FIVE: The Covalent Bond We can model covalent bonding in molecules in essentially two ways: 1. Localized Bonds (retains electron pair concept of Lewis Structures)
CHEM 2060 Lecture 22: VB Theory L22-1 PART FIVE: The Covalent Bond We can model covalent bonding in molecules in essentially two ways: 1. Localized Bonds (retains electron pair concept of Lewis Structures)
Chapter 9. and Bonding Theories
 Chemistry, The Central Science, 11th edition Theodore L. Brown, H. Eugene LeMay, Jr., and Bruce E. Bursten Chapter 9 Theories John D. Bookstaver St. Charles Community College Cottleville, MO Shapes The
Chemistry, The Central Science, 11th edition Theodore L. Brown, H. Eugene LeMay, Jr., and Bruce E. Bursten Chapter 9 Theories John D. Bookstaver St. Charles Community College Cottleville, MO Shapes The
GHW#3 Louisiana Tech University, Chemistry 281. POGIL exercise on Chapter 2. Covalent Bonding: VSEPR, VB and MO Theories. How and Why?
 GHW#3 Louisiana Tech University, Chemistry 281. POGIL exercise on Chapter 2. Covalent Bonding: VSEPR, VB and MO Theories. How and Why? How is Valence Shell Electron Pair Repulsion Theory developed from
GHW#3 Louisiana Tech University, Chemistry 281. POGIL exercise on Chapter 2. Covalent Bonding: VSEPR, VB and MO Theories. How and Why? How is Valence Shell Electron Pair Repulsion Theory developed from
Group 1 Group 2 Group 3 Group 4 Group 5 Group 6 Group 7 Group 8. Na Mg Al Si P S Cl Ar
 CHM 111 Chapters 7 and 8 Worksheet and Study Guide Purpose: This is a guide for your as you work through the chapter. The major topics are provided so that you can write notes on each topic and work the
CHM 111 Chapters 7 and 8 Worksheet and Study Guide Purpose: This is a guide for your as you work through the chapter. The major topics are provided so that you can write notes on each topic and work the
Page III-8-1 / Chapter Eight Lecture Notes MAR. Two s orbitals overlap. One s & one p. overlap. Two p orbitals. overlap MAR
 Bonding and Molecular Structure: Orbital ybridization and Molecular Orbitals Chapter 8 Page III-8-1 / Chapter Eight Lecture Notes Advanced Theories of Chemical Bonding Chemistry 222 Professor Michael Russell
Bonding and Molecular Structure: Orbital ybridization and Molecular Orbitals Chapter 8 Page III-8-1 / Chapter Eight Lecture Notes Advanced Theories of Chemical Bonding Chemistry 222 Professor Michael Russell
Chemical Bonding. Lewis Theory-VSEPR Valence Bond Theory Molecular Orbital Theory
 Chemical Bonding Lewis Theory-VSEPR Valence Bond Theory Molecular Orbital Theory Problems with Valence Bond Theory VB theory predicts properties better than Lewis theory bonding schemes, bond strengths,
Chemical Bonding Lewis Theory-VSEPR Valence Bond Theory Molecular Orbital Theory Problems with Valence Bond Theory VB theory predicts properties better than Lewis theory bonding schemes, bond strengths,
Molecular Orbitals. Chapter 9. Sigma bonding orbitals. Sigma bonding orbitals. Pi bonding orbitals. Sigma and pi bonds
 Molecular Orbitals Chapter 9 Orbitals and Covalent Bond The overlap of atomic orbitals from separate atoms makes molecular orbitals Each molecular orbital has room for two electrons Two types of MO Sigma
Molecular Orbitals Chapter 9 Orbitals and Covalent Bond The overlap of atomic orbitals from separate atoms makes molecular orbitals Each molecular orbital has room for two electrons Two types of MO Sigma
Valence Bond Theory. Localized Electron Model. Hybridize the Orbitals! Overlap and Bonding. Atomic Orbitals are. mmmkay. Overlap and Bonding
 Valence Bond Theory Atomic Orbitals are bad mmmkay Overlap and Bonding Lewis taught us to think of covalent bonds forming through the sharing of electrons by adjacent atoms. In such an approach this can
Valence Bond Theory Atomic Orbitals are bad mmmkay Overlap and Bonding Lewis taught us to think of covalent bonds forming through the sharing of electrons by adjacent atoms. In such an approach this can
Molecular Geometry and Bonding Theories. Molecular Shapes. Molecular Shapes. Chapter 9 Part 2 November 16 th, 2004
 Molecular Geometry and Bonding Theories Chapter 9 Part 2 November 16 th, 2004 8 Molecular Shapes When considering the geometry about the central atom, we consider all electrons (lone pairs and bonding
Molecular Geometry and Bonding Theories Chapter 9 Part 2 November 16 th, 2004 8 Molecular Shapes When considering the geometry about the central atom, we consider all electrons (lone pairs and bonding
Molecular Orbital Theory. WX AP Chemistry Chapter 9 Adapted from: Luis Bonilla Abel Perez University of Texas at El Paso
 Molecular Orbital Theory WX AP Chemistry Chapter 9 Adapted from: Luis Bonilla Abel Perez University of Texas at El Paso Molecular Orbital Theory The goal of molecular orbital theory is to describe molecules
Molecular Orbital Theory WX AP Chemistry Chapter 9 Adapted from: Luis Bonilla Abel Perez University of Texas at El Paso Molecular Orbital Theory The goal of molecular orbital theory is to describe molecules
General Physical Chemistry II
 General Physical Chemistry II Lecture 10 Aleksey Kocherzhenko October 7, 2014" Last time " promotion" Promotion and hybridization" [He] 2s 2 2p x 1 2p y 1 2p z0 " 2 unpaired electrons" [He] 2s 1 2p x 1
General Physical Chemistry II Lecture 10 Aleksey Kocherzhenko October 7, 2014" Last time " promotion" Promotion and hybridization" [He] 2s 2 2p x 1 2p y 1 2p z0 " 2 unpaired electrons" [He] 2s 1 2p x 1
Chapter 9. and Bonding Theories. Molecular Shapes. What Determines the Shape of a Molecule? 3/8/2013
 Chemistry, The Central Science, 10th edition Theodore L. Brown, H. Eugene LeMay, Jr., and Bruce E. Bursten Chapter 9 Theories John D. Bookstaver St. Charles Community College St. Peters, MO 2006, Prentice-Hall,
Chemistry, The Central Science, 10th edition Theodore L. Brown, H. Eugene LeMay, Jr., and Bruce E. Bursten Chapter 9 Theories John D. Bookstaver St. Charles Community College St. Peters, MO 2006, Prentice-Hall,
Chapter Molecules are 3D. Shapes and Bonds. Chapter 9 1. Chemical Bonding and Molecular Structure
 Chapter 9 Chemical Bonding and Molecular Structure 1 Shape 9.1 Molecules are 3D Angle Linear 180 Planar triangular (trigonal planar) 120 Tetrahedral 109.5 2 Shapes and Bonds Imagine a molecule where the
Chapter 9 Chemical Bonding and Molecular Structure 1 Shape 9.1 Molecules are 3D Angle Linear 180 Planar triangular (trigonal planar) 120 Tetrahedral 109.5 2 Shapes and Bonds Imagine a molecule where the
CHEM 110 Exam 2 - Practice Test 1 - Solutions
 CHEM 110 Exam 2 - Practice Test 1 - Solutions 1D 1 has a triple bond. 2 has a double bond. 3 and 4 have single bonds. The stronger the bond, the shorter the length. 2A A 1:1 ratio means there must be the
CHEM 110 Exam 2 - Practice Test 1 - Solutions 1D 1 has a triple bond. 2 has a double bond. 3 and 4 have single bonds. The stronger the bond, the shorter the length. 2A A 1:1 ratio means there must be the
Molecular shape is determined by the number of bonds that form around individual atoms.
 Chapter 9 CH 180 Major Concepts: Molecular shape is determined by the number of bonds that form around individual atoms. Sublevels (s, p, d, & f) of separate atoms may overlap and result in hybrid orbitals
Chapter 9 CH 180 Major Concepts: Molecular shape is determined by the number of bonds that form around individual atoms. Sublevels (s, p, d, & f) of separate atoms may overlap and result in hybrid orbitals
Chapter 13 Conjugated Unsaturated Systems
 Conjugated Unsaturated Systems 13.1 Introduction Allyl radical C 2 C C 2 C C C Allyl cation C 2 C C 2 C C C 1,3-Butadiene C 2 C C C 2 C C C C Molecules with delocalized π bonds are called conjugated unsaturated
Conjugated Unsaturated Systems 13.1 Introduction Allyl radical C 2 C C 2 C C C Allyl cation C 2 C C 2 C C C 1,3-Butadiene C 2 C C C 2 C C C C Molecules with delocalized π bonds are called conjugated unsaturated
Ch. 9- Molecular Geometry and Bonding Theories
 Ch. 9- Molecular Geometry and Bonding Theories 9.0 Introduction A. Lewis structures do not show one of the most important aspects of molecules- their overall shapes B. The shape and size of molecules-
Ch. 9- Molecular Geometry and Bonding Theories 9.0 Introduction A. Lewis structures do not show one of the most important aspects of molecules- their overall shapes B. The shape and size of molecules-
Rethinking Hybridization
 Rethinking Hybridization For more than 60 years, one of the most used concepts to come out of the valence bond model developed by Pauling was that of hybrid orbitals. The ideas of hybridization seemed
Rethinking Hybridization For more than 60 years, one of the most used concepts to come out of the valence bond model developed by Pauling was that of hybrid orbitals. The ideas of hybridization seemed
PG-TRB MO & VB THEORY. POLYTECHNIC-TRB MATERIALS MATHS/COMPUTER SCIENCE/IT/ECE/EEE MECH/CIVIL/CHEMISTRY/PHYSICS ENGLISH /AVAILABLE.
 COACHING CENTRE-PG-TRB- CHEMISTRY-MO & VB THEORY -STUDY MATERIAL- CONTACT: 8072230063 PG-TRB CHEMISTRY MO & VB THEORY POLYTECHNIC-TRB MATERIALS MATHS/COMPUTER SCIENCE/IT/ECE/EEE MECH/CIVIL/CHEMISTRY/PHYSICS
COACHING CENTRE-PG-TRB- CHEMISTRY-MO & VB THEORY -STUDY MATERIAL- CONTACT: 8072230063 PG-TRB CHEMISTRY MO & VB THEORY POLYTECHNIC-TRB MATERIALS MATHS/COMPUTER SCIENCE/IT/ECE/EEE MECH/CIVIL/CHEMISTRY/PHYSICS
Andrew Rosen *Note: If you can rotate a molecule to have one isomer equal to another, they are both the same
 *Note: If you can rotate a molecule to have one isomer equal to another, they are both the same *Note: For hybridization, if an SP 2 is made, there is one unhybridized p orbital (because p usually has
*Note: If you can rotate a molecule to have one isomer equal to another, they are both the same *Note: For hybridization, if an SP 2 is made, there is one unhybridized p orbital (because p usually has
At the end of this lesson, students should be able to :
 At the end of this lesson, students should be able to : (a) Explain Valence Shell Electron Pair Repulsion theory (VSEPR) (b) Draw the basic molecular shapes: linear, planar, tetrahedral, and octahedral.
At the end of this lesson, students should be able to : (a) Explain Valence Shell Electron Pair Repulsion theory (VSEPR) (b) Draw the basic molecular shapes: linear, planar, tetrahedral, and octahedral.
