Strategies for Organic Synthesis
|
|
|
- Melanie Owens
- 5 years ago
- Views:
Transcription
1 Strategies for rganic Synthesis ne of the things that makes chemistry unique among the sciences is synthesis. Chemists make things. New pharmaceuticals, food additives, materials, agricultural chemicals, coatings, adhesives and all sorts of other useful new molecules are prepared from simpler more readily available starting materials. There are two aspects to organic synthesis, first the development of a synthetic strategy or plan of action and second the actual implementation of that plan in a chemical laboratory. This exercise addresses the first aspect of the problem, the development of strategies for synthesis. The second aspect, the actual synthesis, requires real skill and training, something that you can start to acquire by taking an organic laboratory course. At Stony ook we offer two such courses, CE 327, a course that meets the minimum requirements for biology majors and premeds, and CE 383, a course that meets the requirements for chemistry majors and others interested in a research career. Students who master such skills readily find jobs in the chemistry, pharmaceutical and other industries that employ chemists. This figure shows a distillation apparatus that you would use in the organic chemistry laboratory. For example you might use it to purify a new ester that you synthesized from a carboxylic acid and an alcohol. For more information go to the organic chemistry laboratory web site at: The three questions: When planning an organic synthesis there are usually different questions that one must ask. 1. ow can I build the desired carbon skeleton? 2. ow do I introduce the necessary functional groups? 3. ow do I control the regio- and stereochemistry of my reactions? In the real world one needs to know a vast amount of organic chemistry to plan a successful and efficient synthesis of a complex molecule. There are thousands of known reactions; regio and stereochemistry are very difficult to control. owever, in this exercise we have simplified things by limiting our universe of reactions to the ones shown on the last page. All the molecules we discuss can be synthesized using this limited set of reactions. owever, with this limited set of reactions we can still write out syntheses of an infinite set of possible molecules. And we must still consider each of the three questions stated above.
2 ow do you plan a synthesis? The simple answer is to work backwards, one step at a time, implementing known reactions. ften there will be more than one possible reaction sequence. A little thought devoted to each of the three questions above will lead you to the proper reaction sequence more quickly. ow about an example? k. ow would you synthesize the molecule shown at the right starting with organic compounds containing three carbons or less? ow do I start? Count the carbon atoms and identify your functional groups. You need to know the functional groups so you know which reactions in your reaction library can be used to yield the given molecule. It is useful to count the carbon atoms so you will know the minimum number of carbon-carbon bond forming reactions you will eventually need in your synthesis. In this case recognize that the compound is an ester and that it is made from two fragments, a 2 carbon piece and a 5 carbon piece. Since we have to make the 5 carbon piece from starting materials of 3 carbons or less it would be nice if we could eventually figure out a way to make a C-C bond in the middle of the fragment by combining two smaller molecules. But that step is not yet obvious so let start with the ester synthesis. ester 2 carbons 5 carbons It would be nice if we could figure out how to make a C-C bond some where about here The ester synthesis is easy. You need an alcohol and an acid chloride. The acid chloride has 2 carbon atoms only, so it is a legal starting material, but the alcohol has 5 carbon atoms. This molecule must be synthesized. It becomes our new target molecule. So how do you synthesize an alcohol? For the ester you only had one reaction to choose from, but for the alcohol your reaction library has several different reactions that yield alcohols. This gives you multiple alternatives for this step. You could use an acid catalyzed addition of water to an alkene. ydroboration is not appropriate, in this case, but it would be a good choice if we needed a primary alcohol. You could use a substitution reaction starting with an alkyl bromide, but those reactions also work best if you want a primary alcohol. You could reduce a ketone with B 4. The alcohol could be the product of an acetylide reaction with an aldehyde followed by hydrogenation. ow do you choose? Let us not choose yet, we will write out the best possibilities.
3 We have identified four possible routes to our alcohol and have labeled them. Let us consider each possibility A in turn. A and B both involve the acid catalyzed addition of water to a double bond. B is obviously better than A, because path A would also give 3-pentyl alcohol, something B we do not want. This is a example of controlling the regiochemistry of the synthesis B 4 2 Path C, the reduction of a ketone would work, but how would we make the ketone? We would make it from the oxidation of the alcohol so we would have a circular path, not very useful. PCC C + D Path D requires more insight, but it gives us everything we need, the alcohol functionality and the needed C-C bond forming reaction. Thus of the four possible paths either B or D would work. Path D is finished because it starts with molecules with three carbon atoms or less, but path B needs to be completed. Path D is an example of a some what hidden solution. Your last step to a molecule may involve the hydrogenation of a double or triple bond. ow would we make 1-pentene, the alkene we need for path B? We could make it by eliminating water from an alcohol, but that would lead to a circular path since we would need to make the alcohol from the alkene. Instead we can make the alkene by adding hydrogen to an alkyne. This naturally leads us to the necessary C-C bond forming reaction using an acetylide substitution reaction. 2 So we have two paths for the final total synthesis. Let us write them both down. Both are correct answers to the original question. Which path is better? That is a decision you would make in the actual laboratory. It would depend upon such things as actual reaction yields and availability of the starting materials. n an exam you would need to give only one of these two paths
4 Synthesis Problems. Use the reactions given on the CE 142 rganic eactions Chart 1. For each of the following molecules give two synthetic routes starting with molecules containing four carbons or less. a. b. C 3 2. Show how you could synthesize the following alkenes starting with molecules containing four carbons or less. More than one step is required. a. b. 3. Show how you could synthesize the following alcohols starting with molecules containing four carbons or less. More than one step is required. a. b. c. 4. Give syntheses for the following molecules starting with molecules containing four carbons or less. int: Make an alcohol first and then do an oxidation. a. b. c. 5. Give syntheses for each of the following molecules starting with molecules containing four carbons or less. Multiple steps will be required. a. b. c. 6. Give a synthesis of the following molecule using starting materials containing three carbons or less. Note: This problem is not really hard, but it is long and requires about nine steps. C 3
5 The rganic eactions of CE / 2 1. B / - + / S 2 2 /N / 2 N 2 N Carboxylic acid derivatives B 4 eductions - - Alcohols and ethers Cr 3 PCC 2 + PCC xidations of alcohols [2+4] cycloaddition N 2 2 Li Mg Li + Li Mg Li or Mg + 2 Li or Mg rganonmetallics eactions involving or 2 Acetylide Chemistry This chart will be given on Exam 3 and the Final
6 CE 142 rganic eactions Problem Set 1. Give a synthesis of the following molecules. Some will require more than one step. You can start with any molecule of your choice. C 3 2. Give a synthesis for the following alcohol. Your organic starting materials can contain no more than four carbon atoms. Note: there are several ways to do this. Try to find more than one. 3. Show how you could use a organometallic compound to synthesize the following alcohols. You can use any starting reagent you would like as long as you use one organometallic compound. C 3 4. ow could you increase the length of an alcohol by one carbon? int: Use Grignard chemsitry.?
7 5. ow can you convert a trans-double bond to a cis-double bond?? 6. You have never seen the reaction below, but it is similar to the acid catalyzed addition of water to an alkene. Write a curved arrow mechanism You can synthesize an ether by the reaction of an alkyl bromide () with and alkoxide ( - ). Gina tried two different ways of making the ether shown below. K 3 C + C 3 C 3 C 3 3 C + K C 3 C 3 3 C C 3 C 3 C 3 C 3 C 4 8 The first method worked very well, but the second method did not give the ether. Instead it gave a different molecule of formula C 4 8. Explain what happened and explain why it did not happen in the case of the first method. 8. In workshop you demonstrated that the [2 + 4] cycloaddition reaction was symmetry allowed. + What two molecules would react via a [2 + 4] cycloaddition to give the molecule shown below?
When planning an organic synthesis there are usually different questions that one must ask.
 CE 132 Work Shop Exercise Strategies for rganic Synthesis ne of the things that makes chemistry unique among the sciences is synthesis. Chemists make things. New pharmaceuticals, food additives, materials,
CE 132 Work Shop Exercise Strategies for rganic Synthesis ne of the things that makes chemistry unique among the sciences is synthesis. Chemists make things. New pharmaceuticals, food additives, materials,
Suggested solutions for Chapter 28
 s for Chapter 28 28 PBLEM 1 ow would you make these four compounds? Give your disconnections, explain why you chose them and then give reagents for the. 2 2 Me S Exercises in basic one- group C X disconnections.
s for Chapter 28 28 PBLEM 1 ow would you make these four compounds? Give your disconnections, explain why you chose them and then give reagents for the. 2 2 Me S Exercises in basic one- group C X disconnections.
c. Oxidizing agent shown here oxidizes 2º alcohols to ketones and 1º alcohols to carboxylic acids. 3º alcohols DO NOT REACT.
 Exam 1 (Ch 17 and Review of CEM 331) Answer Key: 1. ne-step Questions: You need to know reagents for reagent arrows and to be able to draw products. I know a lot of them seem to look alike its your job
Exam 1 (Ch 17 and Review of CEM 331) Answer Key: 1. ne-step Questions: You need to know reagents for reagent arrows and to be able to draw products. I know a lot of them seem to look alike its your job
Retrosynthetic Analysis & Synthesis Problems
 Chem 232 D. J. Wardrop wardropd@uic.edu Retrosynthetic Analysis & Synthesis Problems As we draw towards the end of the semester, we will be paying more attention to questions regarding synthesis, which
Chem 232 D. J. Wardrop wardropd@uic.edu Retrosynthetic Analysis & Synthesis Problems As we draw towards the end of the semester, we will be paying more attention to questions regarding synthesis, which
Alcohol Synthesis. Dr. Sapna Gupta
 Alcohol Synthesis Dr. Sapna Gupta Synthesis of Alcohols Alcohols can be synthesized from several functional groups. Nucleophilic substitution of O - on alkyl halide ydration of alkenes water in acid solution
Alcohol Synthesis Dr. Sapna Gupta Synthesis of Alcohols Alcohols can be synthesized from several functional groups. Nucleophilic substitution of O - on alkyl halide ydration of alkenes water in acid solution
REALLY, REALLY STRONG BASES. DO NOT FORGET THIS!!!!!
 CHEM 345 Problem Set 4 Key Grignard (RMgX) Problem Set You will be using Grignard reagents throughout this course to make carbon-carbon bonds. To use them effectively, it will require some knowledge from
CHEM 345 Problem Set 4 Key Grignard (RMgX) Problem Set You will be using Grignard reagents throughout this course to make carbon-carbon bonds. To use them effectively, it will require some knowledge from
Loudon Chapter 14 Review: Reactions of Alkynes Jacquie Richardson, CU Boulder Last updated 1/16/2018
 An alkyne is any molecule with a triple bond between two carbon atoms. This triple bond consists of one σ bond and two π bonds: the σ bond exists on a straight line between carbon atoms, while one π bond
An alkyne is any molecule with a triple bond between two carbon atoms. This triple bond consists of one σ bond and two π bonds: the σ bond exists on a straight line between carbon atoms, while one π bond
Loudon Chapter 10 Review: Alcohols & Thiols Jacquie Richardson, CU Boulder Last updated 4/26/2016
 Alcohols (R) and thiols (RS) have many reactions in common with alkyl halides, but they don t do everything exactly the same. The main difference between this and alkyl halide chemistry is that unlike
Alcohols (R) and thiols (RS) have many reactions in common with alkyl halides, but they don t do everything exactly the same. The main difference between this and alkyl halide chemistry is that unlike
Identifying Functional Groups. Why is this necessary? Alkanes. Why is this so important? What is a functional group? 2/1/16
 Identifying Functional Groups The Key to Survival Why is this so important? ver and over again, you will be asked to do reactions, the details to which you will receive in lecture and via your textbook.
Identifying Functional Groups The Key to Survival Why is this so important? ver and over again, you will be asked to do reactions, the details to which you will receive in lecture and via your textbook.
Week 11 Problem Set (Solutions) 4/24, 4/25, 4/26
 Week 11 Problem Set (Solutions) 4/24, 4/25, 4/26 Concepts Covered Alkynes Oxidation Alcohols Reactions/Reagents Deprotonation of Alcohols/Alkynes Jones Oxidation (Cr 2 O 7 ) Dess-Martin Periodinane (DMP)
Week 11 Problem Set (Solutions) 4/24, 4/25, 4/26 Concepts Covered Alkynes Oxidation Alcohols Reactions/Reagents Deprotonation of Alcohols/Alkynes Jones Oxidation (Cr 2 O 7 ) Dess-Martin Periodinane (DMP)
Lecture Notes Chem 51C S. King. Chapter 20 Introduction to Carbonyl Chemistry; Organometallic Reagents; Oxidation & Reduction
 Lecture Notes Chem 51C S. King Chapter 20 Introduction to Carbonyl Chemistry; rganometallic Reagents; xidation & Reduction I. The Reactivity of Carbonyl Compounds The carbonyl group is an extremely important
Lecture Notes Chem 51C S. King Chapter 20 Introduction to Carbonyl Chemistry; rganometallic Reagents; xidation & Reduction I. The Reactivity of Carbonyl Compounds The carbonyl group is an extremely important
Homework Problem Set 9 Iverson CH320M/328M Due Friday, November 30
 omework Problem Set 9 Iverson C0M/8M Due Friday, November 0 NAME (Print): SIGNATURE: Chemistry 0M/8M Dr. ent Iverson 9th omework November 9, 08 Please print the first three letters of your last name in
omework Problem Set 9 Iverson C0M/8M Due Friday, November 0 NAME (Print): SIGNATURE: Chemistry 0M/8M Dr. ent Iverson 9th omework November 9, 08 Please print the first three letters of your last name in
CHM 292 Final Exam Answer Key
 CHM 292 Final Exam Answer Key 1. Predict the product(s) of the following reactions (5 points each; 35 points total). May 7, 2013 Acid catalyzed elimination to form the most highly substituted alkene possible
CHM 292 Final Exam Answer Key 1. Predict the product(s) of the following reactions (5 points each; 35 points total). May 7, 2013 Acid catalyzed elimination to form the most highly substituted alkene possible
Chapter 12. Alcohols from Carbonyl Compounds Oxidation-Reduction & Organometallic Compounds. Structure
 Chapter 12 Alcohols from Carbonyl Compounds xidation-eduction & rganometallic Compounds Created by Professor William Tam & Dr. Phillis Chang Structure ~ 120 o ~ 120 o C ~ 120 o Carbonyl carbon: sp 2 hybridized
Chapter 12 Alcohols from Carbonyl Compounds xidation-eduction & rganometallic Compounds Created by Professor William Tam & Dr. Phillis Chang Structure ~ 120 o ~ 120 o C ~ 120 o Carbonyl carbon: sp 2 hybridized
Loudon Chapter 19 Review: Aldehydes and Ketones CHEM 3331, Jacquie Richardson, Fall Page 1
 Loudon Chapter 19 eview: Aldehydes and Ketones CEM 3331, Jacquie ichardson, Fall 2010 - Page 1 Beginning with this chapter, we re looking at a very important functional group: the carbonyl. We ve seen
Loudon Chapter 19 eview: Aldehydes and Ketones CEM 3331, Jacquie ichardson, Fall 2010 - Page 1 Beginning with this chapter, we re looking at a very important functional group: the carbonyl. We ve seen
ORGANIC - CLUTCH CH ALCOHOLS AND CARBONYL COMPOUNDS.
 !! www.clutchprep.com CONCEPT: INTRO TO REDOX Oxidation reactions involve an increase in the content of a molecule Reduction reactions involve an increase in the content of a molecule EXAMPLE: Label the
!! www.clutchprep.com CONCEPT: INTRO TO REDOX Oxidation reactions involve an increase in the content of a molecule Reduction reactions involve an increase in the content of a molecule EXAMPLE: Label the
ALCOHOLS: Properties & Preparation
 ALLS: Properties & Preparation General formula: -, where is alkyl or substitued alkyl. Ar-: phenol - different properties. Nomenclature 1. ommon names: Name of alkyl group, followed by word alcohol. 2.
ALLS: Properties & Preparation General formula: -, where is alkyl or substitued alkyl. Ar-: phenol - different properties. Nomenclature 1. ommon names: Name of alkyl group, followed by word alcohol. 2.
Learning Guide for Chapter 15 - Alcohols (II)
 Learning Guide for Chapter 15 - Alcohols (II) I. Introduction to alcohol reactivity II. Reactions of alcohols with acids III. Reactions of alcohols with electrophiles alogenated phosphorus and sulfur compounds
Learning Guide for Chapter 15 - Alcohols (II) I. Introduction to alcohol reactivity II. Reactions of alcohols with acids III. Reactions of alcohols with electrophiles alogenated phosphorus and sulfur compounds
(Neither an oxidation or reduction: Addition or loss of H +, H 2 O, HX).
 eactions of Alcohols Alcohols are versatile organic compounds since they undergo a wide variety of transformations the majority of which are either oxidation or reduction type reactions. xidation is a
eactions of Alcohols Alcohols are versatile organic compounds since they undergo a wide variety of transformations the majority of which are either oxidation or reduction type reactions. xidation is a
Oxidation of alcohols Chromic Acid KMnO4 PCC Swern 1 ROH RCOOH RCOOH RCHO RCHO 2 ROH Ketone Ketone Ketone Ketone 3 ROH NR NR NR NR
 Chapter 11 xidation in rganic terms xidation Reduction Neither Addition of, X2; loss of 2 Addition of 2; loss of or X2 Anytime you oxidize one carbon but reduce another (ex. Adding and to adjacent carbons)
Chapter 11 xidation in rganic terms xidation Reduction Neither Addition of, X2; loss of 2 Addition of 2; loss of or X2 Anytime you oxidize one carbon but reduce another (ex. Adding and to adjacent carbons)
8-3 This exercise is worked out on page 293 as "Working with Concepts".
 Copyright 2009 James K Whitesell 8-1 This exercise combines your knowledge of basic nomenclature rules and concepts of stereochemistry introduced in Chapters 4 and 5. 8-2 Keep in mind that faculty at UCSD
Copyright 2009 James K Whitesell 8-1 This exercise combines your knowledge of basic nomenclature rules and concepts of stereochemistry introduced in Chapters 4 and 5. 8-2 Keep in mind that faculty at UCSD
CHEMISTRY Topic #4: Electrophilic Addition Reactions of Alkenes and Alkynes Fall 2018 Dr. Susan Findlay
 EMISTRY 2600 Topic #4: Electrophilic Addition Reactions of Alkenes and Alkynes Fall 2018 Dr. Susan Findlay rganic Reactions (EM 2500 Review) Most reactions in organic chemistry fall into one (or more)
EMISTRY 2600 Topic #4: Electrophilic Addition Reactions of Alkenes and Alkynes Fall 2018 Dr. Susan Findlay rganic Reactions (EM 2500 Review) Most reactions in organic chemistry fall into one (or more)
Learning Guide for Chapter 17 - Dienes
 Learning Guide for Chapter 17 - Dienes I. Isolated, conjugated, and cumulated dienes II. Reactions involving allylic cations or radicals III. Diels-Alder Reactions IV. Aromaticity I. Isolated, Conjugated,
Learning Guide for Chapter 17 - Dienes I. Isolated, conjugated, and cumulated dienes II. Reactions involving allylic cations or radicals III. Diels-Alder Reactions IV. Aromaticity I. Isolated, Conjugated,
What is in Common for the Following Reactions, and How Do They Work?
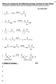 What is in Common for the Following Reactions, and ow Do They Work? You should eventually be able to draw the mechanism for these (and other) reactions 13 Key Intermediate 1 Br-Br Na Br 2 C 3 -I Me NaMe
What is in Common for the Following Reactions, and ow Do They Work? You should eventually be able to draw the mechanism for these (and other) reactions 13 Key Intermediate 1 Br-Br Na Br 2 C 3 -I Me NaMe
CHEM3331: Fundamentals of Organic Chemistry I Prof. Ognjen Š. Miljanić December 11, 2012
 HEM3331: Fundamentals of rganic hemistry I Final Exam Prof. gnjen Š. Miljanić December 11, 2012 Name: Last First Student ID Number: ead all directions very carefully, think about your answer, and then
HEM3331: Fundamentals of rganic hemistry I Final Exam Prof. gnjen Š. Miljanić December 11, 2012 Name: Last First Student ID Number: ead all directions very carefully, think about your answer, and then
HONORS ORGANIC CHEM. HAHS MRS. RICHARDS
 NRS RGANIC CEM. AS MRS. RICARDS RGANIC CEMISTRY: FINAL EXAM REVIEW List of Topics: While the exam will specifically focus on material from Quarter 2, an understanding of several important concepts from
NRS RGANIC CEM. AS MRS. RICARDS RGANIC CEMISTRY: FINAL EXAM REVIEW List of Topics: While the exam will specifically focus on material from Quarter 2, an understanding of several important concepts from
Physical Properties. Alcohols can be: CH CH 2 OH CH 2 CH 3 C OH CH 3. Secondary alcohol. Primary alcohol. Tertiary alcohol
 Chapter 10: Structure and Synthesis of Alcohols 100 Physical Properties Alcohols can be: CH 3 CH 3 CH CH 2 OH * Primary alcohol CH 3 OH CH * CH 2 CH 3 Secondary alcohol CH 3 CH 3 * C OH CH 3 Tertiary alcohol
Chapter 10: Structure and Synthesis of Alcohols 100 Physical Properties Alcohols can be: CH 3 CH 3 CH CH 2 OH * Primary alcohol CH 3 OH CH * CH 2 CH 3 Secondary alcohol CH 3 CH 3 * C OH CH 3 Tertiary alcohol
CHEM 303 Organic Chemistry II Exam II 11-April-2006 Answers
 CEM 303 rganic Chemistry II Exam II 11-April-2006 Answers 1) Compound D, C 8 14, is converted by C 2 =PPh 3 into E, C 9 16. Treatment of D with LiAl 4 yields two isomeric products, F and G, both of formula
CEM 303 rganic Chemistry II Exam II 11-April-2006 Answers 1) Compound D, C 8 14, is converted by C 2 =PPh 3 into E, C 9 16. Treatment of D with LiAl 4 yields two isomeric products, F and G, both of formula
Chapter 9 Aldehydes and Ketones Excluded Sections:
 Chapter 9 Aldehydes and Ketones Excluded Sections: 9.14-9.19 Aldehydes and ketones are found in many fragrant odors of many fruits, fine perfumes, hormones etc. some examples are listed below. Aldehydes
Chapter 9 Aldehydes and Ketones Excluded Sections: 9.14-9.19 Aldehydes and ketones are found in many fragrant odors of many fruits, fine perfumes, hormones etc. some examples are listed below. Aldehydes
2.222 Practice Problems 2003
 2.222 Practice Problems 2003 Set #1 1. Provide the missing starting compound(s), reagent/solvent, or product to correctly complete each of the following. Most people in the class have not done this type
2.222 Practice Problems 2003 Set #1 1. Provide the missing starting compound(s), reagent/solvent, or product to correctly complete each of the following. Most people in the class have not done this type
2.1. The Main Types of Organic Reactions. Addition, Substitution, and Elimination Reactions
 The Main Types of rganic Reactions Figure 2.1(A) shows raw fruit. The crisp, sharp-tasting fruit becomes soft and sweet when it is cooked. Figure 2.1(B) shows a chemist accelerating the tranformation of
The Main Types of rganic Reactions Figure 2.1(A) shows raw fruit. The crisp, sharp-tasting fruit becomes soft and sweet when it is cooked. Figure 2.1(B) shows a chemist accelerating the tranformation of
When H and OH add to the alkyne, an enol is formed, which rearranges to form a carbonyl (C=O) group:
 Next Up: Addition of, : The next two reactions are the Markovnikov and non-markovnikov additions of and to an alkyne But you will not see alcohols form in this reaction! When and add to the alkyne, an
Next Up: Addition of, : The next two reactions are the Markovnikov and non-markovnikov additions of and to an alkyne But you will not see alcohols form in this reaction! When and add to the alkyne, an
(1) Recall the different types of intermolecular interactions. (2) Look at the structure and determine the correct answer.
 MCAT rganic Chemistry - Problem Drill 11: Carboxylic Acids Question No. 1 of 10 Question 1. What kinds of interactions are holding together the carboxylic acid dimer shown? Question #01 3 C C 3 (A) Van
MCAT rganic Chemistry - Problem Drill 11: Carboxylic Acids Question No. 1 of 10 Question 1. What kinds of interactions are holding together the carboxylic acid dimer shown? Question #01 3 C C 3 (A) Van
Chem 263 Notes March 2, 2006
 Chem 263 Notes March 2, 2006 Average for the midterm is 102.5 / 150 (approx. 68%). Preparation of Aldehydes and Ketones There are several methods to prepare aldehydes and ketones. We will only deal with
Chem 263 Notes March 2, 2006 Average for the midterm is 102.5 / 150 (approx. 68%). Preparation of Aldehydes and Ketones There are several methods to prepare aldehydes and ketones. We will only deal with
Chapter 20: Aldehydes and Ketones
 Chapter 20: Aldehydes and Ketones [Chapter 20 Sections: 20.1-20.7, 20.9-10.10, 20.13] 1. Nomenclature of Aldehydes and Ketones ' ketone aldehyde f both aldehydes and ketones, the parent chain is the longest
Chapter 20: Aldehydes and Ketones [Chapter 20 Sections: 20.1-20.7, 20.9-10.10, 20.13] 1. Nomenclature of Aldehydes and Ketones ' ketone aldehyde f both aldehydes and ketones, the parent chain is the longest
ALCOHOLS AND PHENOLS; ETHERS AND EPOXIDES; THIOLS AND SULFIDES
 ALCOHOLS AND PHENOLS; ETHERS AND EPOXIDES; THIOLS AND SULFIDES A STUDENT SHOULD BE ABLE TO: 1. Give the IUPAC name when given the structure, and draw the structure given the name of open-chain and monocyclic
ALCOHOLS AND PHENOLS; ETHERS AND EPOXIDES; THIOLS AND SULFIDES A STUDENT SHOULD BE ABLE TO: 1. Give the IUPAC name when given the structure, and draw the structure given the name of open-chain and monocyclic
CHE 142 Study Guide for Final Exam Spring 2002
 CE 142 Study Guide f Final Exam Spring 2002 Biological Molecules. 1. Draw a three dimensional structure of the dipeptide: Ala-Gly. 2. The amino acid lysine is has pka values of 2.16, 9.18, and 10.79. What
CE 142 Study Guide f Final Exam Spring 2002 Biological Molecules. 1. Draw a three dimensional structure of the dipeptide: Ala-Gly. 2. The amino acid lysine is has pka values of 2.16, 9.18, and 10.79. What
Tips for taking exams in 852
 Comprehensive Tactical Methods in rganic Synthesis W. D. Wulff 1) Know the relative reactivity of carbonyl compounds Tips for taking exams in 852 Cl > > ' > > ' N2 eg: 'Mg Et ' 1equiv. 1equiv. ' ' Et 50%
Comprehensive Tactical Methods in rganic Synthesis W. D. Wulff 1) Know the relative reactivity of carbonyl compounds Tips for taking exams in 852 Cl > > ' > > ' N2 eg: 'Mg Et ' 1equiv. 1equiv. ' ' Et 50%
First 2-Hour Exam. By printing your name below, you pledge that
 Chem 3331 Fall 2004 Exam # 1 1 Name First 2-our Exam By printing your name below, you pledge that "n my honor, as a University of Colorado at Boulder student, I have neither given nor received unauthorized
Chem 3331 Fall 2004 Exam # 1 1 Name First 2-our Exam By printing your name below, you pledge that "n my honor, as a University of Colorado at Boulder student, I have neither given nor received unauthorized
Alkynes. Alkynes-hydrocarbons with a carbon-carbon triple bond.
 Alkynes Alkynes-hydrocarbons with a carbon-carbon triple bond. The carbon-carbon triple bond results from the interaction of two sp hybridized carbon atoms. 180 degree angle. Linear. The carbon-carbon
Alkynes Alkynes-hydrocarbons with a carbon-carbon triple bond. The carbon-carbon triple bond results from the interaction of two sp hybridized carbon atoms. 180 degree angle. Linear. The carbon-carbon
Nuggets of Knowledge for Chapter 17 Dienes and Aromaticity Chem 2320
 Nuggets of Knowledge for Chapter 17 Dienes and Aromaticity Chem 2320 I. Isolated, cumulated, and conjugated dienes A diene is any compound with two or C=C's is a diene. Compounds containing more than two
Nuggets of Knowledge for Chapter 17 Dienes and Aromaticity Chem 2320 I. Isolated, cumulated, and conjugated dienes A diene is any compound with two or C=C's is a diene. Compounds containing more than two
b.p.=100 C b.p.=65 C b.p.=-25 C µ=1.69 D µ=2.0 D µ=1.3 D
 Alcohols I eading: Wade chapter 10, sections 10-1- 10-12 Study Problems: 10-35, 10-37, 10-38, 10-39, 10-40, 10-42, 10-43 Key Concepts and Skills: Show how to convert alkenes, alkyl halides, and and carbonyl
Alcohols I eading: Wade chapter 10, sections 10-1- 10-12 Study Problems: 10-35, 10-37, 10-38, 10-39, 10-40, 10-42, 10-43 Key Concepts and Skills: Show how to convert alkenes, alkyl halides, and and carbonyl
Please note: We routinely xerox a number of exams following initial grading to guard against receiving altered answers during the regrading process.
 NAME (Print): SIGNATURE: Chemistry 320N Dr. Brent Iverson 1st Midterm Feb. 18, 2016 Please print the first three letters of your LAST name in the three boxes Please Note: This test may be a bit long, but
NAME (Print): SIGNATURE: Chemistry 320N Dr. Brent Iverson 1st Midterm Feb. 18, 2016 Please print the first three letters of your LAST name in the three boxes Please Note: This test may be a bit long, but
Chapter 10. Reactions of Alcohols, Amines, Ethers, and Epoxides
 Chapter 10. Reactions of Alcohols, Amines, Ethers, and Epoxides Learning objectives: 1. Provide both IUPAC and common (when applicable) names for alcohols and ethers. 2. Describe the physical properties
Chapter 10. Reactions of Alcohols, Amines, Ethers, and Epoxides Learning objectives: 1. Provide both IUPAC and common (when applicable) names for alcohols and ethers. 2. Describe the physical properties
Chem 263 Nov 7, elimination reaction. There are many reagents that can be used for this reaction. Only three are given in this course:
 hem 263 Nov 7, 2013 Preparation of Ketones and Aldehydes from Alcohols xidation of Alcohols [] must have at least 1 E elimination reaction [] = oxidation; removal of electrons [] = reduction; addition
hem 263 Nov 7, 2013 Preparation of Ketones and Aldehydes from Alcohols xidation of Alcohols [] must have at least 1 E elimination reaction [] = oxidation; removal of electrons [] = reduction; addition
CHEMISTRY Topic #8: Oxidation and Reduction Reactions Fall 2018 Dr. Susan Findlay
 CEMISTRY 2600 Topic #8: xidation and Reduction Reactions Fall 2018 Dr. Susan Findlay xidation States of Carbon Carbon can have any oxidation state from -4 (C 4 ) to +4 (C 2 ). As a general rule, increasing
CEMISTRY 2600 Topic #8: xidation and Reduction Reactions Fall 2018 Dr. Susan Findlay xidation States of Carbon Carbon can have any oxidation state from -4 (C 4 ) to +4 (C 2 ). As a general rule, increasing
Chapter 20: Aldehydes and Ketones
 hapter 20: Aldehydes and Ketones [hapter 20 Sections: 20.1-20.7, 20.9-10.10, 20.13] 1. Nomenclature of Aldehydes and Ketones ketone ' aldehyde 2. eview of the Synthesis of Aldehydes and Ketones Br Br f
hapter 20: Aldehydes and Ketones [hapter 20 Sections: 20.1-20.7, 20.9-10.10, 20.13] 1. Nomenclature of Aldehydes and Ketones ketone ' aldehyde 2. eview of the Synthesis of Aldehydes and Ketones Br Br f
Learning Guide for Chapter 13 - Alkynes
 Learning Guide for Chapter 13 - Alkynes I. Introduction to Alkynes - p 1 II. Natural ccurrence and Uses of Alkynes - p 5 III. Physical Properties of Alkynes - p 7 IV. Spectroscopy of Alkynes - p 7 V. Nomenclature
Learning Guide for Chapter 13 - Alkynes I. Introduction to Alkynes - p 1 II. Natural ccurrence and Uses of Alkynes - p 5 III. Physical Properties of Alkynes - p 7 IV. Spectroscopy of Alkynes - p 7 V. Nomenclature
Exam 1 (Monday, July 6, 2015)
 Chem 231 Summer 2015 Assigned Homework Problems Last updated: Friday, July 24, 2015 Problems Assigned from Essential Organic Chemistry, 2 nd Edition, Paula Yurkanis Bruice, Prentice Hall, New York, NY,
Chem 231 Summer 2015 Assigned Homework Problems Last updated: Friday, July 24, 2015 Problems Assigned from Essential Organic Chemistry, 2 nd Edition, Paula Yurkanis Bruice, Prentice Hall, New York, NY,
Background Information
 ackground nformation ntroduction to Condensation eactions Condensation reactions occur between the α-carbon of one carbonyl-containing functional group and the carbonyl carbon of a second carbonyl-containing
ackground nformation ntroduction to Condensation eactions Condensation reactions occur between the α-carbon of one carbonyl-containing functional group and the carbonyl carbon of a second carbonyl-containing
ORGANIC CHEMISTRY. Wiley STUDY GUIDE AND SOLUTIONS MANUAL TO ACCOMPANY ROBERT G. JOHNSON JON ANTILLA ELEVENTH EDITION. University of South Florida
 STUDY GUIDE AND SOLUTIONS MANUAL TO ACCOMPANY ORGANIC CHEMISTRY ELEVENTH EDITION T. W. GRAHAM SOLOMONS University of South Florida CRAIG B. FRYHLE Pacific Lutheran University SCOTT A. SNYDER Columbia University
STUDY GUIDE AND SOLUTIONS MANUAL TO ACCOMPANY ORGANIC CHEMISTRY ELEVENTH EDITION T. W. GRAHAM SOLOMONS University of South Florida CRAIG B. FRYHLE Pacific Lutheran University SCOTT A. SNYDER Columbia University
Chapter 8 Reactions of Alkenes
 Chapter 8 Reactions of Alkenes Electrophilic Additions o Regio vs stereoselectivity Regio where do the pieces add? Markovnikov s rule hydrogen will go to the side of the double bond with most hydrogens.
Chapter 8 Reactions of Alkenes Electrophilic Additions o Regio vs stereoselectivity Regio where do the pieces add? Markovnikov s rule hydrogen will go to the side of the double bond with most hydrogens.
Loudon Chapter 20 & 21 Review: Carboxylic Acids & Derivatives CHEM 3331, Jacquie Richardson, Fall Page 1
 Loudon Chapter 20 & 21 eview: Carboxylic Acids & Derivatives CEM 3331, Jacquie ichardson, Fall 2010 - Page 1 These two chapters cover compounds which are all at the three bonds to more electronegative
Loudon Chapter 20 & 21 eview: Carboxylic Acids & Derivatives CEM 3331, Jacquie ichardson, Fall 2010 - Page 1 These two chapters cover compounds which are all at the three bonds to more electronegative
Note: You must have your answers written in pen if you want a regrade!!!!
 NAME (Print): SIGNATURE: Chemistry 310N Dr. Brent Iverson 2nd Midterm March 27, 2008 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long,
NAME (Print): SIGNATURE: Chemistry 310N Dr. Brent Iverson 2nd Midterm March 27, 2008 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long,
Reducing Agents. Linda M. Sweeting 1998
 Reducing Agents Linda M. Sweeting 1998 Reduction is defined in chemistry as loss of oxygen, gain of hydrogen or gain of electrons; the gain of electrons enables you to calculate an oxidation state. Hydride
Reducing Agents Linda M. Sweeting 1998 Reduction is defined in chemistry as loss of oxygen, gain of hydrogen or gain of electrons; the gain of electrons enables you to calculate an oxidation state. Hydride
Organic Chemistry Semester II 1st unit specialty reactions (mechanisms not emphasized)
 rganic Chemistry Semester II 1st unit specialty reactions (mechanisms not emphasized) 1. X addition to an alkyne powerful method to add halogens (Cl,, I) to an alkyne to create geminal dihalides (pages
rganic Chemistry Semester II 1st unit specialty reactions (mechanisms not emphasized) 1. X addition to an alkyne powerful method to add halogens (Cl,, I) to an alkyne to create geminal dihalides (pages
CHEM 203. Final Exam December 18, This a closed-notes, closed-book exam. You may use your set of molecular models
 CEM 203 Final Exam December 18, 2013 Your name: This a closed-notes, closed-book exam You may use your set of molecular models This test consists of 10 pages Time: 2h 30 min 1. / 20 2. / 20 3. / 30 4.
CEM 203 Final Exam December 18, 2013 Your name: This a closed-notes, closed-book exam You may use your set of molecular models This test consists of 10 pages Time: 2h 30 min 1. / 20 2. / 20 3. / 30 4.
acetaldehyde (ethanal)
 hem 263 Nov 2, 2010 Preparation of Ketones and Aldehydes from Alkenes zonolysis 1. 3 2. Zn acetone 1. 3 2. Zn acetone acetaldehyde (ethanal) Mechanism: 3 3 3 + - oncerted reaction 3 3 3 + ozonide (explosive)
hem 263 Nov 2, 2010 Preparation of Ketones and Aldehydes from Alkenes zonolysis 1. 3 2. Zn acetone 1. 3 2. Zn acetone acetaldehyde (ethanal) Mechanism: 3 3 3 + - oncerted reaction 3 3 3 + ozonide (explosive)
Organic Chemistry Unit Review Package
 Name: Worksheet 7.viii Organic Chemistry Unit Review Package Generalized Organic Chemistry Naming Procedure Grouped into three general phases. 1. Identification phase (finding all important/correct information)
Name: Worksheet 7.viii Organic Chemistry Unit Review Package Generalized Organic Chemistry Naming Procedure Grouped into three general phases. 1. Identification phase (finding all important/correct information)
Chapter Name the following compounds: (a) 2,5-dimethyl-3-hexyne (b) 3,3-dimethyl-1-butyne (c) 2,4-octadiene-6-yne (d) 3,3-dimethyl-4-octyne (e)
 hapter 8 8.1 Name the following compounds: 3 3 3 3 2,5-dimethyl-3-hexyne 3 3 3 3,3-dimethyl-1-butyne (c) 3 3 2,4-octadiene-6-yne (d) 3 3 2 2 2 3 3 3,3-dimethyl-4-octyne (e) 3 3 3 2 3 3 2,5,5-trimethyl-3-heptyne
hapter 8 8.1 Name the following compounds: 3 3 3 3 2,5-dimethyl-3-hexyne 3 3 3 3,3-dimethyl-1-butyne (c) 3 3 2,4-octadiene-6-yne (d) 3 3 2 2 2 3 3 3,3-dimethyl-4-octyne (e) 3 3 3 2 3 3 2,5,5-trimethyl-3-heptyne
Chapter 8: Chemistry of Alkynes (C n H 2n-2 )
 hapter 8: hemistry of Alkynes ( n 2n-2 ) Bonding & hybridization Both are sp-hybridized Bond angles = 180 o 1 σ + 2 π bonds Linear around lassification R R R' σ bond energy: 88 kcal/mol π bond energy:
hapter 8: hemistry of Alkynes ( n 2n-2 ) Bonding & hybridization Both are sp-hybridized Bond angles = 180 o 1 σ + 2 π bonds Linear around lassification R R R' σ bond energy: 88 kcal/mol π bond energy:
OCH 3. 3-butyn-2-ol 3-hydroxy-1-butyne
 Alkynes, Part I eading: Wade chapter 9, sections 9-1- 9-8 Study Problems: 9-29, 9-32, 9-36 Key oncepts and Skills: Explain why alkynes are more acidic than alkanes or alkenes; show how to generate nucleophilic
Alkynes, Part I eading: Wade chapter 9, sections 9-1- 9-8 Study Problems: 9-29, 9-32, 9-36 Key oncepts and Skills: Explain why alkynes are more acidic than alkanes or alkenes; show how to generate nucleophilic
enol unstable; can't be isolated
 Name 1 ke alkenes, alkynes are stabilized by alkyl groups, so internal alkynes (i.e. disubstituted alkynes) are generally more stable than terminal alkynes (i.e. monosubstituted alkynes). With this in
Name 1 ke alkenes, alkynes are stabilized by alkyl groups, so internal alkynes (i.e. disubstituted alkynes) are generally more stable than terminal alkynes (i.e. monosubstituted alkynes). With this in
CHEM2077 HONORS ORGANIC CHEMISTRY SYLLABUS
 CHEM2077 HONORS ORGANIC CHEMISTRY SYLLABUS 1. STRUCTURE AND BONDING a] Atomic structure and bonding b] Hybridization and MO Theory c] Drawing chemical structures 2. POLAR COVALENT BONDS: ACIDS AND BASES
CHEM2077 HONORS ORGANIC CHEMISTRY SYLLABUS 1. STRUCTURE AND BONDING a] Atomic structure and bonding b] Hybridization and MO Theory c] Drawing chemical structures 2. POLAR COVALENT BONDS: ACIDS AND BASES
Alcohols. Have seen many reactions to synthesize alcohols: In this chapter we will study reactions of the alcohols
 Alcohols ave seen many reactions to synthesize alcohols: In this chapter we will study reactions of the alcohols Oxidation Need to understand the nomenclature of organic reduction/oxidation In general
Alcohols ave seen many reactions to synthesize alcohols: In this chapter we will study reactions of the alcohols Oxidation Need to understand the nomenclature of organic reduction/oxidation In general
Spring Term 2012 Dr. Williams (309 Zurn, ex 2386)
 Chemistry 242 Organic Chemistry II Spring Term 2012 Dr. Williams (309 Zurn, ex 2386) Web Page: http://math.mercyhurst.edu/~jwilliams/ jwilliams@mercyhurst.edu (or just visit Department web site and look
Chemistry 242 Organic Chemistry II Spring Term 2012 Dr. Williams (309 Zurn, ex 2386) Web Page: http://math.mercyhurst.edu/~jwilliams/ jwilliams@mercyhurst.edu (or just visit Department web site and look
Detailed Course Content
 Detailed Course Content Chapter 1: Carbon Compounds and Chemical Bonds The Structural Theory of Organic Chemistry 4 Chemical Bonds: The Octet Rule 6 Lewis Structures 8 Formal Charge 11 Resonance 14 Quantum
Detailed Course Content Chapter 1: Carbon Compounds and Chemical Bonds The Structural Theory of Organic Chemistry 4 Chemical Bonds: The Octet Rule 6 Lewis Structures 8 Formal Charge 11 Resonance 14 Quantum
This reactivity makes alkenes an important class of organic compounds because they can be used to synthesize a wide variety of other compounds.
 This reactivity makes alkenes an important class of organic compounds because they can be used to synthesize a wide variety of other compounds. Mechanism for the addition of a hydrogen halide What happens
This reactivity makes alkenes an important class of organic compounds because they can be used to synthesize a wide variety of other compounds. Mechanism for the addition of a hydrogen halide What happens
CARBONYL COMPOUNDS: OXIDATION-REDUCTION REACTION
 CARBONYL COMPOUNDS: OXIDATION-REDUCTION REACTION Introduction Several functional groups contain the carbonyl group Carbonyl groups can be converted into alcohols by various reactions Structure of the Carbonyl
CARBONYL COMPOUNDS: OXIDATION-REDUCTION REACTION Introduction Several functional groups contain the carbonyl group Carbonyl groups can be converted into alcohols by various reactions Structure of the Carbonyl
Exam I 19 April 2004 Name:
 Chem 317 S04 Page 1 of 7 Kantorowski Exam I 19 April 2004 Name: This exam contains 6 pages of questions confirm this once you begin The last page is a spectral data table that can be removed You will have
Chem 317 S04 Page 1 of 7 Kantorowski Exam I 19 April 2004 Name: This exam contains 6 pages of questions confirm this once you begin The last page is a spectral data table that can be removed You will have
Organic Chemistry 1 CHM 2210 Exam 4 (December 10, 2001)
 Exam 4 (December 10, 2001) Name (print): Signature: Student ID Number: There are 12 multiple choice problems (4 points each) on this exam. Record the answers to the multiple choice questions on THIS PAGE.
Exam 4 (December 10, 2001) Name (print): Signature: Student ID Number: There are 12 multiple choice problems (4 points each) on this exam. Record the answers to the multiple choice questions on THIS PAGE.
Synthetic possibilities Chem 315 Beauchamp 1
 Synthetic possibilities hem Beauchamp Propose reasonable syntheses f the following target molecules (TM-#). You can use the given starting materials and any typical ganic reagents studied in our course
Synthetic possibilities hem Beauchamp Propose reasonable syntheses f the following target molecules (TM-#). You can use the given starting materials and any typical ganic reagents studied in our course
Chapter 17: Carbonyl Compounds II
 Chapter 17: Carbonyl Compounds II Learning bjectives: 1. ecognize and assign names to aldehydes and ketones. 2. Write the mechanism for nucleophilic addition and nucleophilic addition-elimination reactions
Chapter 17: Carbonyl Compounds II Learning bjectives: 1. ecognize and assign names to aldehydes and ketones. 2. Write the mechanism for nucleophilic addition and nucleophilic addition-elimination reactions
Score: Homework Problem Set 6 Iverson CH320N Due Friday, March 10. NAME (Print): Chemistry 320N Dr. Brent Iverson 6th Homework March 3, 2017
 omework Problem Set 6 Iverson 320 Due Friday, March 10 AME (Print): SIGATURE: hemistry 320 Dr. Brent Iverson 6th omework March 3, 2017 Please print the first three letters of your last name in the three
omework Problem Set 6 Iverson 320 Due Friday, March 10 AME (Print): SIGATURE: hemistry 320 Dr. Brent Iverson 6th omework March 3, 2017 Please print the first three letters of your last name in the three
REACTION AND SYNTHESIS REVIEW
 REACTION AND SYNTHESIS REVIEW A STUDENT SHOULD BE ABLE TO PREDICT PRODUCTS, IDENTIFY REACTANTS, GIVE REACTION CONDITIONS, PROPOSE SYNTHESES, AND PROPOSE MECHANISMS (AS LISTED BELOW). REVIEW THE MECHANISM
REACTION AND SYNTHESIS REVIEW A STUDENT SHOULD BE ABLE TO PREDICT PRODUCTS, IDENTIFY REACTANTS, GIVE REACTION CONDITIONS, PROPOSE SYNTHESES, AND PROPOSE MECHANISMS (AS LISTED BELOW). REVIEW THE MECHANISM
DAMIETTA UNIVERSITY. Energy Diagram of One-Step Exothermic Reaction
 DAMIETTA UNIVERSITY CHEM-103: BASIC ORGANIC CHEMISTRY LECTURE 5 Dr Ali El-Agamey 1 Energy Diagram of One-Step Exothermic Reaction The vertical axis in this graph represents the potential energy. The transition
DAMIETTA UNIVERSITY CHEM-103: BASIC ORGANIC CHEMISTRY LECTURE 5 Dr Ali El-Agamey 1 Energy Diagram of One-Step Exothermic Reaction The vertical axis in this graph represents the potential energy. The transition
Aldehydes and Ketones
 Aldehydes and Ketones Preparation of Aldehydes xidation of Primary Alcohols --- 2 P 1o alcohol ydroboration of a Terminal Alkyne, followed by Tautomerization --- 1. B 3, TF 2. 2 2, K 2 terminal alkyne
Aldehydes and Ketones Preparation of Aldehydes xidation of Primary Alcohols --- 2 P 1o alcohol ydroboration of a Terminal Alkyne, followed by Tautomerization --- 1. B 3, TF 2. 2 2, K 2 terminal alkyne
CHAPTER 9. ALKYNES: AN INTRODUCTION TO ORGANIC SYNTHESIS
 CAPTER 9. ALKYNES: AN INTRDUCTIN T RGANIC SYNTESIS Alkyne Nomenclature. Like alkenes, number so the alkyne gets the lowest number. Name the following two molecules: 3 C C CC 2 C 2 C(C 3 ) 2 Cl If, however,
CAPTER 9. ALKYNES: AN INTRDUCTIN T RGANIC SYNTESIS Alkyne Nomenclature. Like alkenes, number so the alkyne gets the lowest number. Name the following two molecules: 3 C C CC 2 C 2 C(C 3 ) 2 Cl If, however,
The Organic Acids. Carboxylic Acids * *
 arboxylic Acids The rganic Acids Some Notation: Acids and their conjugate bases pka ~ 15 - weak acid arboxylic Acid pka ~ 4 moderate acid - arboxylate Anion pka ~ -7 very strong acid l - l arboxylic acids
arboxylic Acids The rganic Acids Some Notation: Acids and their conjugate bases pka ~ 15 - weak acid arboxylic Acid pka ~ 4 moderate acid - arboxylate Anion pka ~ -7 very strong acid l - l arboxylic acids
Chemistry 2030 Introduction to Organic Chemistry Fall Semester 2017 Dr. Rainer Glaser
 Chemistry 2030 Introduction to Organic Chemistry Fall Semester 2017 Dr. Rainer Glaser Examination #3 Nucleophilic Substitutions & Eliminations, Alcohols, and Ethers Thursday, November 2, 2017, 8:25-9:15
Chemistry 2030 Introduction to Organic Chemistry Fall Semester 2017 Dr. Rainer Glaser Examination #3 Nucleophilic Substitutions & Eliminations, Alcohols, and Ethers Thursday, November 2, 2017, 8:25-9:15
Learning Guide for Chapter 14 - Alcohols (I)
 Learning Guide for Chapter 14 - Alcohols (I) I. Introduction to Alcohols and Thiols II. Acid/base Behavior of Alcohols, Phenols, and Thiols III. Nomenclature of Alcohols IV. Synthesis of Alcohols Previous
Learning Guide for Chapter 14 - Alcohols (I) I. Introduction to Alcohols and Thiols II. Acid/base Behavior of Alcohols, Phenols, and Thiols III. Nomenclature of Alcohols IV. Synthesis of Alcohols Previous
Module9. Nuclear Magnetic Resonance Spectroscopy Nuclear Magnetic Resonance (NMR) spectroscopy - Chemical shift - Integration of signal area
 1 CHEMISTRY 263 HOME WORK Lecture Topics: Module7. Hydrogenation of Alkenes The Function of the Catalyst - Syn and anti- addition Hydrogenation of Alkynes - Syn- addition of hydrogen: Synthesis of cis-alkenes
1 CHEMISTRY 263 HOME WORK Lecture Topics: Module7. Hydrogenation of Alkenes The Function of the Catalyst - Syn and anti- addition Hydrogenation of Alkynes - Syn- addition of hydrogen: Synthesis of cis-alkenes
TOPIC 3 - ALDEHYDES AND KETONES (Chapters 12 & 16)
 TPIC 3 - ALDEYDES AND KETNES (Chapters 12 & 16) Lecture 15 Web12 12.1 Introduction 16.1 Introduction 16.2 Nomenclature of Aldehydes and Ketones 16.3 ysical Properties 12.2 xidation Reduction Reactions
TPIC 3 - ALDEYDES AND KETNES (Chapters 12 & 16) Lecture 15 Web12 12.1 Introduction 16.1 Introduction 16.2 Nomenclature of Aldehydes and Ketones 16.3 ysical Properties 12.2 xidation Reduction Reactions
Chapter 18: Carbonyl Compounds II
 Chapter 18: Carbonyl Compounds II Learning bjectives: 1. ecognize and assign names to aldehydes and ketones. 2. Write the mechanism for nucleophilic addition and nucleophilic addition-elimination reactions
Chapter 18: Carbonyl Compounds II Learning bjectives: 1. ecognize and assign names to aldehydes and ketones. 2. Write the mechanism for nucleophilic addition and nucleophilic addition-elimination reactions
Name. Department of Chemistry SUNY/Oneonta. Chem Organic Chemistry I. Examination #3 - November 8, 2004 ANSWERS
 Name Department of hemistry SUNY/neonta hem 221 - rganic hemistry I Examination #3 - November 8, 2004 ANSWERS INSTRUTINS --- This examination has two parts. The first part is in multiple choice format;
Name Department of hemistry SUNY/neonta hem 221 - rganic hemistry I Examination #3 - November 8, 2004 ANSWERS INSTRUTINS --- This examination has two parts. The first part is in multiple choice format;
Bond length (pm) Bond strength (KJ/mol)
 hapter 10: Alkyl alides = F, l,, I Alkyl halide Aryl halide Vinyl halide 10.1 Naming alkyl halides- ead 10.2 Structure of alkyl halides Table 10.1 alomethane 3 -F 3 -l 3-3 -I Bond length (pm) 139 178 193
hapter 10: Alkyl alides = F, l,, I Alkyl halide Aryl halide Vinyl halide 10.1 Naming alkyl halides- ead 10.2 Structure of alkyl halides Table 10.1 alomethane 3 -F 3 -l 3-3 -I Bond length (pm) 139 178 193
Chemistry 3720 Old Exams. Practice Exams & Keys
 Chemistry 3720 ld Exams Practice Exams & Keys 2015-17 Spring 2017 Page File 3 Spring 2017 Exam 1 10 Spring 2017 Exam 1 Key 16 Spring 2017 Exam 2 23 Spring 2017 Exam 2 Key 29 Spring 2017 Exam 3 36 Spring
Chemistry 3720 ld Exams Practice Exams & Keys 2015-17 Spring 2017 Page File 3 Spring 2017 Exam 1 10 Spring 2017 Exam 1 Key 16 Spring 2017 Exam 2 23 Spring 2017 Exam 2 Key 29 Spring 2017 Exam 3 36 Spring
CHEMISTRY 341. Final Exam Tuesday, December 16, Problem 1 15 pts Problem 9 8 pts. Problem 2 5 pts Problem pts
 CEMISTRY 341 Final Exam Tuesday, December 16, 1997 Name NAID Problem 1 15 pts Problem 9 8 pts Problem 2 5 pts Problem 10 21 pts Problem 3 26 pts Problem 11 15 pts Problem 4 10 pts Problem 12 6 pts Problem
CEMISTRY 341 Final Exam Tuesday, December 16, 1997 Name NAID Problem 1 15 pts Problem 9 8 pts Problem 2 5 pts Problem 10 21 pts Problem 3 26 pts Problem 11 15 pts Problem 4 10 pts Problem 12 6 pts Problem
Chapter 7. Alkenes: Reactions and Synthesis
 Chapter 7. Alkenes: Reactions and Synthesis 1 Synthesis of Alkenes: Elimination Reactions 1. Dehydrohalogenation of alkyl halides. loss of requires CH 2 CH 2 Cl Zaitsev s Rule: CH 2 C 2. Dehydration of
Chapter 7. Alkenes: Reactions and Synthesis 1 Synthesis of Alkenes: Elimination Reactions 1. Dehydrohalogenation of alkyl halides. loss of requires CH 2 CH 2 Cl Zaitsev s Rule: CH 2 C 2. Dehydration of
1. (6 points) Provide IUPAC accepted names for the following compounds. 2. (6 points) Provide a structure for the following compounds.
 Chem52 omework Set 1 This homework set is similar to a Chem 51 final exam. Please provide answers in the spaces provided or, preferably, on attached sheets. Point values are listed only to give you the
Chem52 omework Set 1 This homework set is similar to a Chem 51 final exam. Please provide answers in the spaces provided or, preferably, on attached sheets. Point values are listed only to give you the
2.222 Introductory Organic Chemistry II Midterm Exam
 NAME: STUDENT NUMBE: Page 1 of 7 University of Manitoba Department of hemistry 2.222 Introductory rganic hemistry II Midterm Exam Wednesday February 20, 2002 Put all answers in the spaces provided. If
NAME: STUDENT NUMBE: Page 1 of 7 University of Manitoba Department of hemistry 2.222 Introductory rganic hemistry II Midterm Exam Wednesday February 20, 2002 Put all answers in the spaces provided. If
12. Aldehydes & Ketones (text )
 2009, Department of Chemistry, The University of Western ntario 12.1 12. Aldehydes & Ketones (text 13.1 13.11) A. Structure and Nomenclature The carbonyl group is present in aldehydes and ketones and is
2009, Department of Chemistry, The University of Western ntario 12.1 12. Aldehydes & Ketones (text 13.1 13.11) A. Structure and Nomenclature The carbonyl group is present in aldehydes and ketones and is
ORGANIC - CLUTCH CH ALDEHYDES AND KETONES: NUCLEOPHILIC ADDITION
 !! www.clutchprep.com CONCEPT: ALDEHYDE NOMENCLATURE Replace the suffix of the alkane -e with the suffix On the parent chain, the carbonyl is always terminal, and receive a location As substituents, they
!! www.clutchprep.com CONCEPT: ALDEHYDE NOMENCLATURE Replace the suffix of the alkane -e with the suffix On the parent chain, the carbonyl is always terminal, and receive a location As substituents, they
Synthesis and Structure of Alcohols Alcohols can be considered organic analogues of water.
 Synthesis and Structure of Alcohols Alcohols can be considered organic analogues of water. Alcohols are usually classified as primary, secondary and tertiary. Alcohols with the hydroxyl bound directly
Synthesis and Structure of Alcohols Alcohols can be considered organic analogues of water. Alcohols are usually classified as primary, secondary and tertiary. Alcohols with the hydroxyl bound directly
Chem 263 March 28, 2006
 Chem 263 March 28, 2006 Properties of Carboxylic Acids Since carboxylic acids are structurally related to both ketones and aldehydes, we would expect to see some similar structural properties. The carbonyl
Chem 263 March 28, 2006 Properties of Carboxylic Acids Since carboxylic acids are structurally related to both ketones and aldehydes, we would expect to see some similar structural properties. The carbonyl
Carbon-heteroatom single bonds basic C N C X. X= F, Cl, Br, I Alkyl Halide C O. epoxide Chapter 14 H. alcohols acidic H C S C. thiols.
 hapter 13: Alcohols and Phenols 13.1 Structure and Properties of Alcohols Alkanes arbon - arbon Multiple Bonds arbon-heteroatom single bonds basic Alkenes X X= F, l,, I Alkyl alide amines hapter 23 nitro
hapter 13: Alcohols and Phenols 13.1 Structure and Properties of Alcohols Alkanes arbon - arbon Multiple Bonds arbon-heteroatom single bonds basic Alkenes X X= F, l,, I Alkyl alide amines hapter 23 nitro
Spectroscopy. 184 Inspirational chemistry. Index sheets. Chemical aerobics
 184 Inspirational chemistry Spectroscopy Index 7.5 5 sheets Three activities that could be used to teach spectroscopy are presented within this resource. A good introduction to spectroscopy is to do the
184 Inspirational chemistry Spectroscopy Index 7.5 5 sheets Three activities that could be used to teach spectroscopy are presented within this resource. A good introduction to spectroscopy is to do the
MOLECULAR REPRESENTATIONS AND INFRARED SPECTROSCOPY
 MOLEULAR REPRESENTATIONS AND INFRARED SPETROSOPY A STUDENT SOULD BE ABLE TO: 1. Given a Lewis (dash or dot), condensed, bond-line, or wedge formula of a compound draw the other representations. 2. Give
MOLEULAR REPRESENTATIONS AND INFRARED SPETROSOPY A STUDENT SOULD BE ABLE TO: 1. Given a Lewis (dash or dot), condensed, bond-line, or wedge formula of a compound draw the other representations. 2. Give
Chemistry Exam 2. The Periodic Table
 Name: Last First MI Chemistry 234-002 Exam 2 Spring 2017 Dr. J. sbourn Instructions: The first 18 questions of this exam should be answered on the provided Scantron. You must use a pencil for filling in
Name: Last First MI Chemistry 234-002 Exam 2 Spring 2017 Dr. J. sbourn Instructions: The first 18 questions of this exam should be answered on the provided Scantron. You must use a pencil for filling in
KOT 222 Organic Chemistry II
 KOT 222 Organic Chemistry II Course Objectives: 1) To introduce the chemistry of alcohols and ethers. 2) To study the chemistry of functional groups. 3) To learn the chemistry of aromatic compounds and
KOT 222 Organic Chemistry II Course Objectives: 1) To introduce the chemistry of alcohols and ethers. 2) To study the chemistry of functional groups. 3) To learn the chemistry of aromatic compounds and
