The NASA Ames PAH IR Spectroscopic Database: Computational Version 3.00 with Updated Content and the Introduction of Multiple Scaling Factors
|
|
|
- Ariel Hood
- 5 years ago
- Views:
Transcription
1 2018. The American Astronomical Society. All rights reserved. The NASA Ames PAH IR Spectroscopic Database: Computational Version 3.00 with Updated Content and the Introduction of Multiple Scaling Factors Charles W. Bauschlicher, Jr. 1, A. Ricca 2,3, C. Boersma 3, and L. J. Allamandola 3 1 NASA Ames Research Center, MS 2303, Moffett Field, CA , USA; Charles.W.Bauschlicher@nasa.gov 2 Carl Sagan Center, SETI Institute, 189 Bernardo Avenue, Suite 200, Mountain View, CA 94043, USA 3 NASA Ames Research Center, MS 2456, Moffett Field, CA , USA Received 2017 September 25; revised 2017 December 5; accepted 2017 December 5; published 2018 February 5 Abstract Version 3.00 of the library of computed spectra in the NASA Ames PAH IR Spectroscopic Database (PAHdb) is described. Version 3.00 introduces the use of multiple scale factors, instead of the single scaling factor used previously, to align the theoretical harmonic frequencies with the experimental fundamentals. The use of multiple scale factors permits the use of a variety of basis sets; this allows new PAH species to be included in the database, such as those containing oxygen, and yields an improved treatment of strained species and those containing nitrogen. In addition, the computed spectra of 2439 new PAH species have been added. The impact of these changes on the analysis of an astronomical spectrum through databasefitting is considered and compared with a fit using Version 2.00 of the library of computed spectra. Finally, astronomical constraints are defined for the PAH spectral libraries in PAHdb. Key words: astrochemistry astronomical databases: miscellaneous ISM: lines and bands methods: numerical molecular data techniques: spectroscopic 1. Introduction In 2010, the inaugural version ( 2010) of the NASA Ames PAH IR Spectroscopic Database 4 (PAHdb), containing a large collection of theoretical and experimental infrared (IR) spectra of polycyclic aromatic hydrocarbons (PAHs), was made publicly available. The library of computed PAH spectra featured 583 density functional theory (DFT) spectra. In 2013, PAHdb underwent a major update in the form of a version 2.00 release (Boersma et al. 2014). This version extended the library of computed PAH spectra to a total of 700 theoretical spectra, which featured larger PAHs, and included advanced tools for the analysis of the IR spectra, such as, for example, the nonnegative least squares fitting of imported astronomical spectra. This paper reports on the version 3.00 update to the library of computed PAH spectra that adds 2439 spectra and revises the treatment of scale factors. The vibrational frequencies are computed using the harmonic approximation. To account for limitations in the level of theory and for anharmonic corrections, the computed frequencies are scaled to improve the agreement with the experimental fundamentals. Since the C H stretching anharmonicities are known to be larger than those of the other modes, Bauschlicher & Langhoff (1997) initially used two scale factors to fit the naphthalene results to the band positions from two matrixisolation experiments (Szczepanski & Vala 1993; Hudgins & Sandford 1998). Using the B3LYP (Stephens et al. 1994) hybrid (Becke 1993) functional in conjunction with a 431G basis set (Frisch et al. 1984), it was found that the two scale factors were essentially identical, and that the agreement with the experimental fundamentals was very good. However, Bauschlicher & Ricca (2010) noted that for some PAHs, the B3LYP functional could fail due the presence of lowlying excited states. This failure was easy to detect due to unreasonably large IR intensities. In some cases, using a 4 generalized gradient approximation (GGA), i.e., a nonhybrid functional, such as BP86 (Perdew 1986; Becke 1988), could circumvent the failure. As with B3LYP, the two scale factors were essentially identical and only one factor was used. However, when both functionals worked, the scaled B3LYP frequencies were in better agreement with experiment than the scaled BP86 results. Thus, the computational part of the database included results obtained using the B3LYP/431G approach when available or the BP86/431G level of theory when the B3LYP/431G results were unavailable. Using the B3LYP or BP86 functional in conjunction with the 431G basis set allowed the study of numerous PAHs. However, this level of theory was not without its limitations. The basis set is too small for systems containing oxygen atoms. In addition, a comparison of the computed IR data for strained systems like C 60,C 70, and benzyne, as well as a series of acridine molecules (Mattioda et al. 2017) with experiment showed somewhat larger errors for strained or nitrogencontaining systems than found for planar PAH molecules containing only carbon and hydrogen atoms. Clearly, these systems required a higher level of theory. Bauschlicher & Ricca (2010) showed that switching to a different hybrid functional did not improve the results over B3LYP, nor did changing the GGA used improve the results compared with the BP86. This showed that the choice of functional was not the root of the problem and therefore larger basis sets were needed to improve the description of these systems. However, using basis sets larger than 431G with only two scale factors actually made the agreement with experiment worse (Bauschlicher & Langhoff 1997; Bauschlicher & Ricca 2010). A clue to the problem with larger basis sets was provided by a comparison of the B3LYP/431G and the BP86/431G results, where there was a systematic difference in the μm region, with B3LYP better describing the very strong band near 11 μm. This suggests that the C H stretch, the 6 9 μm, and 9 15 μm regions should be scaled separately. Switching from two to three scale factors improved the accuracy of the BP86/431G 1
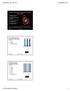
2 Figure 1. Pie charts showing the distribution of charge (left) and composition (right) for all species in the computational part of the database, version PAH spectra, bringing it into better agreement with experiment and with the B3LYP/431G results. Using three scaling factors also improved the agreement with experiment as the basis set quality was increased. Thus, using three scale factors allowed the inclusion of results obtained using larger basis sets into the database. Note that even three scale factors can be insufficient. As noted previously (Bauschlicher & Ricca 2013), the CC triple bond requires yet a different scale factor. In Section 2 the newly added species to the computational library are discussed. Section 3 features a discussion on scale factors. Section 4 considers the impact of the changes on the analysis of an astronomical spectrum through databasefitting and introduces the concept of the astronomical PAH subset to the database. This paper closes with a summary and conclusions in Section Added Species and Composition PAHs containing five and sevenmembered rings (Bauschlicher 2015), including C 60 and C 70, have been added with molecular UIDs ranging from 717 to 737. Circumcircumcircumanthracene (C 190 H 34 ), circumcircumcircumtetracene (C 210 H 36 ), and those PAHs containing bay regions described in Peeters et al. (2017) have been added with molecular UIDs ranging from 738 to 774. The other oval PAH molecules mentioned in Peeters et al. (2017) were included in version PAHs with side groups have been assigned molecular UIDs ranging from 775 to 812 (Bauschlicher 2016). The naphthobenzopyrene anion from the study of Mattioda et al. (2014) that was computed using a 631G * basis set was added with molecular UID 813 and hexacene, heptacene, and octacene have molecular UIDs 814 to 822; the IR spectra for the other species described in Mattioda et al. (2014) were computed using the B3LYP/431G approach and were already in the database. PAHs with molecular UIDs from 823 to 828 were some unpublished C 54 H 18 species; one had an extra hydrogen added, while the others had one or more fivemembered rings. Perylene (Langhoff 1996) was added with molecular UID 829. The species from our recent B3LYP/631G * study (Mattioda et al. 2017) of some neutral acridine species and their PAH parents have been added (molecular UIDs 830 to 832) or replaced species that were computed using the 431G basis set. Species with molecular UIDs that range from 833 to 836 are the most stable species from our study (Bauschlicher & Ricca 2014) of the loss of C 2 H 2. Species with molecular UIDs from 837 to 2357 are the neutral C n H y species of Mackie et al. (2015), while their neutral dehydrogenated C n species have molecular UIDs ranging from 2358 to Figure 1 shows the distribution of charge and composition for all of the PAHs included in the computational part of version 3.00 of the database. Clearly, most of the species are neutrals and have a formula of the form C x H y, i.e., they are pure PAHs. A much more detailed breakdown of the molecules included in version 2.00 and 3.00 of the database is given in Table 1. From this table it is clear that most of the 2439 added species are neutral PAHs containing less than 50 carbon atoms. This is because Mackie et al. (2015) studied all of the neutral C x H y and C x PAHs with eight or fewer fused rings. However, there is a near doubling of the species with more than 100 carbons. Few of the new PAH species contain nitrogen and none of the new PAH species contain oxygen, magnesium, or iron. The breakdown of the molecules included in the theoretical part of the database by number of carbon atoms is shown in Figure 2. Most species of the database involve molecules with between 21 and 50 carbon atoms and most of the species are pure PAHs. In fact, the database should now include all neutral PAHs with 8 or fewer fused rings. Version 2.00 had about equal numbers of neutrals and cations, with a reasonable population of multiply charged cations and anions of the larger species. For version 3.00, this charge balance is no longer true, as most of the added species are neutrals. Figures 2(c) and (d) are analogous to Figures 2(a) and (b), but exclude the dehydrogenated C x species. The insights that can be deduced from Figures 2(a) and (b) also apply to Figures 2(c) and (d). Figures 2(e) and (f) show the composition for the C x species in the database. They are essentially all neutral and contain between 21 and 50 carbons. 3. Scale Factors As described in the introduction, the decision was made to switch to three scale factors with ranges as follows: (1) C H stretching bands (greater than 2500 cm 1 ; less than 4 μm), (2) bands between 2500 and cm 1 (i.e., between 4 and 9 μm), and (3) bands between and 0 cm 1 (i.e., greater than 9 μm). Note that in our original study, we determined the scale factors by minimizing å i n = 1 () si * we () i n(), i where n is the number of IR bands for which there are experimental data, s(i) is the scale factor, which is a function of the harmonic frequency, w e, that is obtained from DFT, and ν is the experimental fundamental value. In this work we switch to i n 1 si we i n i 6 å = (()* () ()) since we are willing to increase the small errors somewhat to reduce the maximum error. We scaled our harmonic DFT frequencies to better reproduce the experimental fundamentals, therefore we needed one or more experimental spectra to determine the scale factors. Ideally, we would have used the spectra of many large PAH molecules. Since the experimental library of the database contains 75 PAHs, with the largest being C 50 H 22, this might have seemed straightforward. However, a comparison of the computed and experimental spectra for the large PAHs showed that assignments were not easy to make. The experimental IR spectra commonly have weak bands in regions where theoretical spectra have no allowed bands; these are presumably overtones or combination bands that are not included in the harmonic approximation. Even after discarding these extra experimental bands, lining up the computed and experimental bands was not easy. In some cases the band positions suggested one assignment, while the band intensities suggest another. For example, consider two nearby theory bands A(theory) and B(theory) and two nearby experimental bands A(exp) and B(exp), where band positions might have 2
3 Table 1 Breakdown of the Molecules Included in Version 3.00 of the Computational Database by Charge, Composition, and Size Charge Number of Carbon Atoms Total All Molecules all 76(76) 211(195) 839(74) 1663(104) 140(104) 114(98) 96(49) 3139(700) neutral 39(39) 115(98) 795(37) 1590(35) 43(27) 37(31) 35(19) 2654(286) anion 3(3) 10(11) 8(4) 22(20) 16(13) 21(20) 27(14) 107(85) cation 34(34) 86(86) 29(26) 45(43) 65(48) 51(42) 34(16) 344(295) / 0(0) 0(0) 7(7) 6(6) 16(16) 5(5) 0(0) 34(34) PAHs Without Substitutions all 26(26) 121(107) 814(50) 1648(89) 108(72) 100(84) 96(49) 2913(477) neutral 12(12) 70(55) 784(27) 1589(34) 41(25) 37(31) 35(19) 2568(203) anion 3(3) 8(9) 8(4) 22(20) 16(13) 21(20) 27(14) 105(83) cation 11(11) 43(43) 18(15) 31(29) 41(24) 37(28) 34(16) 215(166) / 0(0) 0(0) 4(4) 6(6) 10(10) 5(5) 0(0) 25(25) PAHs with Nitrogen all 10(10) 48(46) 18(17) 9(9) 18(18) 8(8) 0(0) 111(108) neutral 5(5) 24(22) 11(10) 0(0) 0(0) 0(0) 0(0) 40(37) anion 0(0) 2(2) 0(0) 0(0) 0(0) 0(0) 0(0) 2(2) cation 5(5) 22(22) 7(7) 9(9) 12(12) 8(8) 0(0) 63(63) / 0(0) 0(0) 0(0) 0(0) 6(6) 0(0) 0(0) 6(6) PAHs with Oxygen all 40(40) 42(42) 0(0) 0(0) 2(2) 0(0) 0(0) 84(84) neutral 22(22) 21(21) 0(0) 0(0) 0(0) 0(0) 0(0) 43(43) anion 0(0) 0(0) 0(0) 0(0) 0(0) 0(0) 0(0) 0(0) cation 18(18) 21(21) 0(0) 0(0) 2(2) 0(0) 0(0) 41(41) / 0(0) 0(0) 0(0) 0(0) 0(0) 0(0) 0(0) 0(0) PAHs with Magnesium or Iron all 0(0) 0(0) 7(7) 6(6) 12(12) 6(6) 0(0) 31(31) neutral 0(0) 0(0) 0(0) 1(1) 2(2) 0(0) 0(0) 3(3) anion 0(0) 0(0) 0(0) 0(0) 0(0) 0(0) 0(0) 0(0) cation 0(0) 0(0) 4(4) 5(5) 10(10) 6(6) 0(0) 25(25) / 0(0) 0(0) 3(3) 0(0) 0(0) 0(0) 0(0) 3(3) PAHs without Hydrogen all 0(0) 10(0) 295(2) 495(0) 10(4) 3(3) 0(0) 813(9) neutral 0(0) 10(0) 294(1) 495(0) 4(2) 1(1) 0(0) 804(4) anion 0(0) 0(0) 0(0) 0(0) 2(0) 0(0) 0(0) 2(0) cation 0(0) 0(0) 1(1) 0(0) 4(2) 1(1) 0(0) 6(4) / 0(0) 0(0) 0(0) 0(0) 0(0) 1(1) 0(0) 1(1) Note. The number of species in each class in version 2.00 is given in parentheses. suggested assigning A(theory) with A(exp) and B(theory) with B(exp), but with A(theory) and B(exp) having larger intensities than B(theory) and A(exp). Thus, on the basis of the intensities, one would have reversed the assignment. Uncertainties in the band assignments for large PAHs have led us to take a different approach, namely we have used naphthalene, for which the assignments of the bands are definitive and there are several experimental bands to determine the scale factors. We have then used these scale factors to compute the spectra of larger PAH molecules. The good agreement of the (scaled) computed and experimental spectra for larger species has been used to validate this approach. In version 3.00 we used the gasphase experimental spectrum of naphthalene instead of the average of two matrixisolation spectra (Szczepanski & Vala 1993; Hudgins & Sandford 1998). The scale factors were obtained by fitting to the 17 allowed IR bands determined by Pirali et al. (2009; this includes the 16 bands in Table 1 of their paper and the band at 1601 cm 1 shown in their Figure 2) the 4 A g and 2 B 2g bands summarized by Behlen et al. (1981) and the one A u and one B 1g bands summarized by Cané et al. (1998). Figure 3 compares all of the available laboratorydetermined matrixisolated band positions in PAHdb with those determined using either a single or multiple scale factors. The figure shows that there is only a very slight (0.02%) increase in spread when moving to multiple scale factors based on gasphase experiments. The average and maximum errors in the band position for the B3LYP functional, as a function of the basis set, are given in Table 2. The results are given for the Poplestyle (Frisch et al. 1984) basis sets in the top part of the table and for the 3
4 Figure 2. Breakdown of the species in the computational part of the database, version 3.00, by number of carbon atoms: (a) by composition, (b) by charge, (c) by composition excluding the C x species, (d) by charge excluding the C x species, (e) by composition for the C x species, and (f) by charge for the C x species. correlationconsistent valence polarized (ccpv) basis sets of Dunning (1989) in the bottom of the table. Note that the B3LYP/431G errors are different from those reported previously, since we are now comparing with a different, much larger, set of experimental values. As the basis sets improve, for both the Poplestyle and correlationconsistent style sets, the average and maximum errors tend to decrease. Note that the ccpvqz basis set actually has a slightly larger error than the ccpvtz basis set and the 6311G(2d, 2p) basis set has somewhat larger errors than the 6311G ** basis set; that is, all of the largest basis sets have about the same errors. We also note that the same experimental bands have the largest error for all basis sets and computing the anharmonic frequencies actually increases the maximum error. The problem bands in naphthalene are the a u band at 195, the b 2u band at , and the a g band at cm 1. None of these bands appear in the older matrixisolation experiments. It would be interesting to understand the origin of the difference between theory and experiment for these three bands. The gasphase frequencies are in good agreement with the average of matrixisolation results, so the change in the experimental data used for the scale factor fits makes only small changes in the scale factors. For example, the single scale factor for the B3LYP/ 431G changed from to While this switch might slightly degrade the agreement between theory and matrixisolation experiments, it will hopefully improve the agreement Figure 3. Comparison of the distributions (red line) of the ratio of PAH band positions established from matrixisolation experiments over the DFTcalculated band positions using either a single scale factor (top) or multiple scale factors (bottom). Shown is a fitted Gaussian (blue dotted line) and indicated are the mean of the fitted Gaussian (μ) and the width of the fitted Gaussian (σ). Table 2 Scale Factors and the Average and Maximum Errors (in cm 1 ) as a Function of Basis Sets for the B3LYP Functional Basis Set Scale Factors Avg. Error Max Error G G G * G ** G ** G(2d, 2p) ccpvdz ccpvtz ccpvqz Note. Scale factor 1 is for 0 to cm 1, 2 is for to 2500 cm 1, and 3 is for greater than 2500 cm 1. with observations, which correspond to gasphase molecules; see Section 4. As noted in the introduction, the B3LYP/631G * harmonic frequencies with three scale factors were in better agreement with experiment than were the B3LYP/431G results for PAHs containing heteroatoms, such as acridine species. Also note that even larger basis sets can be used and reduce the errors even further. With current programs and computers it is possible to use the ccpvtz set on a system as large as C 150 H 30. Thus, using the three scale factors allows an improved treatment for 4
5 smaller species where a larger basis set can be used and puts the BP86/431G approach on more equal footing with the B3LYP/431G approach. The naphthalene results suggest that most of our scaled bands should be within 5 10 cm 1 of the experimental band positions and a few could have an error as large as 15 cm 1. This level of accuracy is sufficient to gain insight into astronomical observations. 4. Astronomical Considerations In version 2.00 of the PAHdb (Boersma et al. 2014), the capability to directly fit an astronomical spectrum was added. Since then, the databasefitting approach has proven to be a valuable addition to the astronomer s toolkit(e.g., Andrews et al. 2015; Zhang & Kwok 2015; Boersma et al. 2016). Toconsider the astronomical impact of the updates to the library of computed spectra, the databasefitting approach is applied to the same SpitzerIRS (Houck et al. 2004; Werneretal. 2004) spectrum from Boersma et al. (2014) to decompose the PAH emission into contributing PAH subclasses, i.e., charge and size. This spectrum, reproduced from Boersma et al. (2013; position II), probes emission from the diffuse region near the northwest photodissociation region (PDR) of NGC Before fitting, any molecular hydrogen lines were removed, the spectrum was corrected for extinction and a warm dust continuum was subtracted. The reader is directed to Boersma et al. (2013) for details. In Boersma et al. (2014), to illustrate the fitting approach that was then made publicly available via PAHdb, no constraints were placed on the PAHs that should be considered for inclusion or exclusion in the fit. This was done to highlight the general inconsistencies that arise with our current understanding of the PAH model, specifically those that arise when comparing with our first fit results that were presented in Boersma et al. (2013). That exercise emphasized the need to properly constrain the PAHs to be considered for inclusion in a fit by taking into account the physical conditions in the astronomical environment. Here, full advantage is taken of that knowledge and only PAHs in the computational part of the database meeting the following astronomical constraints are considered for a fit: 1. contain more than 20 carbon atoms; 2. are either a pure PAH or PANH; 3. are not superhydrogenated; 4. have no aliphatic side groups; 5. are not fully dehydrogenated, excluding C 60 and C 70, which are included in the fit. The size limit (1) is imposed as PAHs with less than 20 carbon atoms are quickly destroyed due to their instability with respect to photodissociation in the emission region (Allamandola et al. 1989; Puget & Léger 1989). Only pure PAHs those containing only carbon and hydrogen and PANHs those containing carbon, hydrogen, and nitrogen are considered (2) as other heteroatom inclusion is deemed less significant 5 (Hudgins et al. 2005). Superhydrogenated and PAHs containing 5 Note that while oxygen substitution for carbon atoms within the hexagonal carbon skeleton is considered highly unlikely due to stability arguments, it has been shown that H addition, as well as replacement of peripheral H atoms with OH and = O (forming aromatic alcohols and ketones), readily occurs in PAH/ water ice experiments simulating photoprocessing in dense clouds (Bernstein et al. 1999; Cook et al. 2015; de Barros et al. 2017). These species will be released from the ices at the edge of the cloud and will be part of the PAH family feedstock into the PDR. Perusal of Table 1 shows these species are currently underrepresented in the library of computed spectra. aliphatic side groups (3) are not included at this stage because at best they add only minor substructure to the 5 15 μm regionof the spectrum. This will change with the availability of JWST spectra, which will include the μm region where the CHstretch aromatic and aliphatic signatures are clearcut (Geballe et al. 1989; Joblinetal. 1996). The possible contribution of emission from fully dehydrogenated PAHs (5) to the astronomical PAH spectrum is intriguing; however, it is highly unlikely that a significant fraction of the PAHs will be fully dehydrogenated in the emission region considered here (Mackie et al. 2015). However, fullerenes (C 60 and C 70 ) have not been excluded, as there is clear observational evidence for their presence in harsh radiation environments (e.g., (Cami et al. 2010; Sellgren et al. 2010; Boersma et al. 2012; Berné et al. 2013). Imposing these constraints results in a pool of 283 and 1877 spectra to be considered when using the library of computed PAH spectra in version 2.00 and 3.00, respectively, of the PAHdb. A PAH emission model must be applied to transform the integrated PAH absorption cross sections available in the database into PAH emission spectra. Here, each PAH is excited by a 7 ev photon, the computed band positions are redshifted 15 cm 1 to simulate anharmonic effects; each transition is convolved with a Gaussian emission profile with a FWHM of 15 cm 1 ; and the model takes the full temperature cascade into account (see 2010; Boersma et al. 2013). The AmesPAHdbIDLSuite is utilized to perform the database fit. This suite is a collection of IDL 6 object classes (a set of programs utilizing IDL s objectoriented programming capabilities) and can be obtained from the PAHdb website 7 or from the GitHub development repository. 8 Figure 4 presents the results of the fit, directly comparing the results obtained using versions 2.00 (left), 3.00 (middle), and 3.00 (right). Version 3.00 contains the same collection of PAHs as in version 3.00, but instead of using multiple scale factors, it uses a single scale factor (0.958 for B3LYP and for BP86 as in version 2.00, and for the fullerenes as in version 3.00). This allows one to separate the effects of adding more species from those produced by introducing multiple scale factors. The top row of the figure shows that the fits are good and that they are very comparable to each other. The second row of the figure shows the breakdown of the spectrum in terms of size. The contribution from large PAHs (ncarbon > 50) and small PAHs (ncarbon 50) is about in all three panels. The bottom row of Figure 4 shows the breakdown of the spectrum by charge. Again, the differences between the left, middle, and right panels are generally small. Figure 5 presents an overview of the chemical structures involved in each of the fits, ordered according to their contribution to the fit in terms of flux. The figure shows that a variety of PAH structures is needed, including those with nitrogen and with defects, as well as fullerenes. The number of PAHs required for the fit is about the same when using either version 2.00 (29), 3.00 (27), or 3.00 (27) of the computed library. We compute the error in the fits as the area of the absolute value of the residual over the area of the astronomical spectra. This integration is done in cm 1 space since it is linear in 6 IDL.aspx
6 Figure 4. The 5 15 μm SpitzerIRS spectrum of position II from Boersma et al. (2013) of the reflection nebula NGC 7023 decomposed using the spectra from version 2.00 (left column), 3.00 (middle column), and 3.00 (right column) using of the computed libraries of the NASA Ames PAH IR Spectroscopic Database. Top row: observed spectrum ( ), fit (red). Middle row: size breakdown of the spectrum into contributions from small (NC 50; red) and large (NC > 50; green) PAHs. The observed spectrum and total fit are shown as symbols and a black line, respectively. Bottom row: charge breakdown of the spectrum into contributions from PAH anions (red), neutrals (green), and cations (blue). The observed spectrum and the total fit are shown as symbols and a black line, respectively. See Section 4 for details. energy. The errors are given in Figure 4 and they show an increase when moving from the computational database at version 2.00 to version 3.00, but a reduction when using version It should be noted that the contribution to the fit of the molecules treated using the BP86 approach is reduced when using version 3.00 compared to using version Part of the motivation for introducing three scale factors was to prevent the BP86 results from being used to incorrectly fit the red wing of the 11.2 μm band. However, it appears that new molecules in version 3.00 have replaced the BP86 contribution to the spectra. Not surprisingly the BP86 contribution to the fit using version 3.00 is also very small. About half of the difference in the fit between version 3.00 and 3.00 comes from the PANH molecules. When three scale factors are used, the very small reduction in the scale factor in the 6 9 μm range reduces the contribution to the fit from the PANHs. In the long run, the computational spectra for the nitrogencontaining molecules should be replaced with those obtained using larger basis sets. The slightly larger error obtained when using version 3.00 compared to using 3.00 is not because the 3.00 version is necessarily better, but because version 3.00 has reduced the importance of species included in the 3.00 fit due to errors in the band position associated with a single scale factor. That is, the difference between versions 3.00 and 3.00 indicates that the following areas need improvement: (i) important classes of molecules are underrepresented and need to be included, (ii) a more thorough computational treatment that includes anharmonicity, and (iii) a superior treatment of the emission process that includes anharmonicity is needed. These limitations force the inclusion of incorrect species in the fit due to small errors in the older scale factor. Table 3 crossreferences the PAHs in each fit and presents the chemical formula of each PAH and its contribution in terms of flux (f) and number density (a). In all three cases, about 35% of the emission is produced by the same two PAHs. Of the remaining PAHs, most contributing at 5%, there are eight (8) unique to version 2.00 and one (1) to version Between versions 2.00 and 3.00 there are eleven (11) new PAHs required for the fit. While the quality of the overall fit is generally good, it has difficulties matching some of the emission. Notable are the blue side of the 6.2 μm PAH feature and the PAH emission 6
7 The Astrophysical Journal Supplement Series, 234:32 (10pp), 2018 February Figure 5. Overview of the PAH structures contributing to the decomposition of the 5 15 μm SpitzerIRS spectrum of position II from Boersma et al. (2013) of the reflection nebula NGC 7023 decomposed using the spectra from version 2.00 (top), 3.00 (middle), and 3.00 (bottom) using the computed libraries of the NASA Ames PAH IR Spectroscopic Database. The structures are presented in order of their contribution to the fit in terms of flux. Table 3 shows, for each structure, the chemical formula, its contribution to the total number of PAHs (a) and its contribution to the total flux (f) in percent. See Section 4 for details. originating between 10 and 15 μm. In addition, the contribution from PAH anions to the spectrum seems unreasonably large (see the discussion in (Boersma et al. 2012). These difficulties are mostly due to the limited variation within the appropriate class of PAH species in PAHdb. It has been suggested that the blue side of the 6.2 μm band can be attributed to PAHs containing nitrogen, PANHs (e.g., Peeters et al. 2002; Hudgins et al. 2005, class A). Version 3.00 of the computed library holds 55 PANHs meeting the astronomical criteria listed above. Of these, most form a series where the loci of the nitrogen atom is permuted, not different large molecular structures containing nitrogen. 7
8 8 Table 3 Overview of the PAHs Contributing to the Decomposition of the 5 15 μm SpitzerIRS Spectrum of Position II from Boersma et al. (2013) of the Reflection Nebula NGC 7023 Decomposed Using the Spectra from Version 2.00 (left), 3.00 (Middle), and 3.00 (Right) of the Computed Libraries of the NASA Ames PAH IR Spectroscopic Database Version 2.00 Version 3.00 Version 3.00 Ref. Formula f [%] a [%] Ref. Formula f [%] a [%] Ref. Formula f [%] a [%] 2.1 = 3.1 = = 3.1 = = 2.1 = = 3.2 = 3.2 C52H18N = 3.2 = 2.2 C52H18N = 2.2 = 3.2 C52H18N = 3.3 = = 3.5 = = 2.5 = 3.4 C42H = 3.10 = 3.4 C 31 H 14 N = 3.3 = 2.5 C42H = 2.4 = 3.10 C31H14 N = 3.4 = 3.3 C42H = 3.9 C52H = 2.3 = = 3.11 = 3.6 C 48 H = 3.17 = = 2.6 = 3.11 C 48 H = 3.6 = = 3.13 = 2.8 C32H = 2.10 = 3.8 C23H12N = 3.7 = 3.13 C32H = 3.7 = 2.10 C23H12N = 2.9 = 3.9 C36H = 3.9 = 3.8 C36H = 3.8 = 2.9 C36H = 3.5 C52H = 3.8 = 3.7 C23H12N = 3.4 = 2.4 C31H14 N = 2.13 = 3.12 C90H = 3.24 = 3.15 C96H = 3.6 = 2.6 C 48 H = 2.12 = 3.13 C52H18N = 3.13 = 3.11 C52H18N = 3.10 = 2.13 C90H = 3.27 C146H = 3.12 = 3.10 C90H = 3.11 = 2.12 C52H18N = 2.8 = 3.7 C32H = 3.14 C42H = 2.14 C42H = 3.17 C34H C 98 H = 3.22 C34H = 2.11 = 3.24 C96H C36H = 3.16 C = 3.16 C = 3.19 = 3.19 C32H = 3.14 C34H = 2.7 = C150H = 3.26 C26H = 2.26 C170H = 3.27 C32H = 3.19 = 2.17 C32H = 2.17 = 3.19 C32H = 3.23 C24H C128H = 2.29 C32H C23H12N = 3.21 C34H = 3.21 C34H C102H C 28 H = 3.15 C34H C24H = 2.20 C24H = 2.24 C112H = 3.23 C112H = 3.15 = 2.11 C96H C80H C24H C40H = 2.27 C22H = 3.18 C170H C30H = 3.18 C26H = 3.25 C22H = 3.12 C146H = 2.19 C32H C82H = 3.20 C32H Note. The PAHs are presented in order of their contribution to the fit in terms of flux. For each PAH we provide the chemical formula, its contribution to the total number of PAHs (a), and its contribution to the total flux ( f ) as a percent. Figure 5 presents the chemical structure for each PAH. See Section 4 for details. The Astrophysical Journal Supplement Series, 234:32 (10pp), 2018 February
9 The emission between 10 and 15 μm is attributed to the CH outofplane (CH oop ) vibrational motion of peripheral hydrogen atoms. Each of the distinct bands in the μm region is associated with a particular hydrogen adjacency class the number of neighboring hydrogen atoms protruding a single aromatic ring, namely, the 11.2, 12.0, 12.7, and 13.5 μm PAH bands are associated with the solo, duo, trio, and quartet adjacency classes, respectively (e.g., Hony et al. 2001). Currently, the computed library is limited in the number of large PAHs with varying adjacency classes. Most of the larger PAHs (ncarbon > 50) have condensed structures and are part of a series where one characteristic is permuted, e.g., the location of a sevenmembered ring defect (Bauschlicher 2015). Hence the need for the acenes in the fit to add trio and quartet CH outofplane bending bands. Although the presence of PAH anions in space has been suggested (e.g., Bregman & Temi 2005; 2009), the uncannily large contribution here is attributed to improper treatment of anharmonicity. One of the effects of anharmonicity is that it introduces a red wing into the emission profile. Since the PAH band positions of PAH anions systematically fall to the red of their neutral and cationic counterparts, they tend to fill in the red wings instead. The way forward to overcome these difficulties is straightforward and includes (1) the computation of the spectra of a large variety of PANHs and adding them to PAHdb; (2) the computation of a large variety of spectra from large PAHs covering all hydrogen adjacency classes and adding them to PAHdb; and (3) extending PAHdb to include the computed PAH spectra that treat the effects of anharmonicity and/or enhance the employed PAH emission model to better treat anharmonicity. 5. Summary and Conclusions The computational part of the database has been modified to support multiple scale factors. While the B3LYP/431G results are slightly changed, the BP86/431G results are improved by the use of three scale factors, namely the systematic difference between the B3LYP and BP86 for the band at 11.2 μm has been reduced. More importantly, the use of multiple scale factors reduces the error for the larger basis set to be similar to or smaller than that found for the 431G basis set. This allows the addition of spectra computed using a larger basis set to the PAHdb. This yields improved results for species containing oxygen and nitrogen and for strained systems. We can now deal with strained triple bonds like those found in benzyne. The database now contains C 60 and C 70 neutral and ions computed using the 631G * basis set and includes a special scale factor for strained systems in this basis set. In addition to changing the handling of scale factors, 2439 species have been added. Most of these are the neutral species from Mackie et al. (2015). However, the computational part of database version 3.00 has almost twice as many molecules with more than 100 carbons than contained in version This includes species with bay regions, which has been attributed to the band at 8.2 μm (Peeters et al. 2017). A subset of PAH spectra from the updated version 3.00 library of computed spectra meeting astronomically relevant criteria were used in combination with a PAH emission model to fit and analyze an astronomical spectrum originating in the northwest PDR of the reflection nebula NGC These results were compared to those obtained from a fit using version 2.00 of the library and using the updated library with a single scale factor instead (version 3.00 ). Overall, the results are on a par with each other, especially when considering emission originating from the different PAH subclasses, i.e., charge and size. The noticeable reduction in the error when assuming a single scale factor (version 3.00 ) over multiple scale factors (version 3.00) is interpreted as the latter emphasizing the currently limited variation within each of the PAH subclasses (i.e., charge, size, structure, composition) in the PAHdb spectral library, as the use of multiple scale factors is considered appropriate from a molecular spectroscopic standpoint. Some of the remaining significant limitations of the computational spectroscopic library of PAHdb in matching astronomical PAH emission spectra can be overcome by (1) the computation and addition of compact and irregular large PANH spectra in each charge state; (2) the computation and addition of spectra from large PAHs covering all hydrogen adjacency classes and charge states; and (3) the computation and addition of PAH spectra for large PAHS that treat anharmonicity and/or employ a PAH emission model that better treats anharmonicity. Lastly, those who make use of the data and tools of the NASA Ames PAH IR Spectroscopic Database are kindly asked to refer to this paper, Boersma et al. (2014) and A. L. Mattioda et al. (2017, in preparation). A.R. acknowledges support from NASA s APRA program (NNX17AE71G). C.B. is grateful for an appointment at NASA Ames Research Center through the San Jose State University Research Foundation (NNX14AG80A and NNX17AJ88A) and acknowledges support from NASA s ADAP program (NNH14ZDA001N). L.J.A. gratefully acknowledges support from NASA s ADAP program (NNH16ZDA001N) and is thankful for an appointment at the NASA Ames Research Center through the Bay Area Environmental Research Institute (80NSSC17M0014). Facility: Spitzer (IRS). Software: MPFIT (Markwardt 2009), IDL Astronomy User s Library (Landsman 1993), AmesPAHdbIDLSuite (Boersma et al. 2014). ORCID ids A. Ricca https: /orcid.org/ C. Boersma https: /orcid.org/ x References Allamandola, L. J., Tielens, G. G. M., & Barker, J. R. 1989, ApJS, 71, 733 Andrews, H., Boersma, C., Werner, M. W., et al. 2015, ApJ, 807, 99 Bauschlicher, C. W. 2015, CP, 448, 43 Bauschlicher, C. W. 2016, CPL, 665, 100 Bauschlicher, C. W., Boersma, C., Ricca, A., et al. 2010, ApJS, 189, 341 Bauschlicher, C. W., & Langhoff, S. R. 1997, AcSpA, 53, 1225 Bauschlicher, C. W., Peeters, E., & Allamandola, L. J. 2009, ApJ, 697, 311 Bauschlicher, C. W., & Ricca, A. 2010, MolPh, 108, 2647 Bauschlicher, C. W., Jr., & Ricca, A. 2013, ApJ, 776, 102 Bauschlicher, C. W., Jr., & Ricca, A. 2014, Theor. Chem. Acc., 133, 1479 Becke, A. D. 1988, PhRvA, 38, 3098 Becke, A. D. 1993, JChPh, 98, 5648 Behlen, F. M., McDonald, D. B., Sethuraman, V., & Rice, S. A. 1981, JChPh, 75, 5685 Berné, O., Mulas, G., & Joblin, C. 2013, A&A, 550, L4 Bernstein, M. P., Sandford, S. A., Allamandola, L. J., et al. 1999, Sci, 283, 1135 Boersma, C., Bauschlicher, C. W., Ricca, A., et al. 2014, ApJS, 211, 8 Boersma, C., Bregman, J., & Allamandola, L. J. 2016, ApJ, 832, 51 Boersma, C., Bregman, J. D., & Allamandola, L. J. 2013, ApJ, 769, 117 9
10 Boersma, C., Rubin, R. H., & Allamandola, L. J. 2012, ApJ, 753, 168 Bregman, J., & Temi, P. 2005, ApJ, 621, 831 Cami, J., BernardSalas, J., Peeters, E., & Malek, S. E. 2010, Sci, 329, 1180 Cané, E., Palmieri, P., Tarroni, R., Trombetti, A., & Handy, N. 1998, JChPh, 106, 9004 Cook, A. M., Ricca, A., Mattioda, A. L., et al. 2015, ApJ, 799, 14 de Barros, A. L. F., Mattioda, A. L., Ricca, A., CruzDiaz, G. A., & Allamandola, L. J. 2017, ApJ, 848, 112 Dunning, T. H., Jr. 1989, JChPh, 90, 1007 Frisch, M. J., Pople, J. A., & Binkley, J. S. 1984, JChPh, 80, 3265 Geballe, T. R., Tielens, A. G. G. M., Allamandola, L. J., Moorhouse, A., & Brand, P. W. J. L. 1989, ApJ, 341, 278 Hony, S., Van Kerckhoven, C., Peeters, E., et al. 2001, A&A, 370, 1030 Houck, J. R., Roellig, T. L., van Cleve, J., et al. 2004, ApJS, 154, 18 Hudgins, D. L., & Sandford, S. A. 1998, JPCA, 102, 329 Hudgins, D. M., Bauschlicher, C. W., & Allamandola, L. J. 2005, ApJ, 632, 316 Joblin, C., Tielens, A. G. G. M., Allamandola, L. J., & Geballe, T. R. 1996, ApJ, 458, 610 Landsman, W. B. 1993, in ASP Conf. Ser. 52, Astronomical Data Analysis Software and Systems II, ed. R. J. Hanisch, R. J. V. Brissenden, & J. Barnes (San Francisco, CA: ASP), 246 Langhoff, S. R. 1996, JPhCh, 100, 2819 Mackie, C. J., Peeters, E., Bauschlicher, C. W., Jr., & Cami, J. 2015, ApJ, 799, 131 Markwardt, C. B. 2009, in ASP Conf. Ser. 411, Astronomical Data Analysis Software and Systems XVIII, ed. D. A. Bohlender, D. Durand, & P. Dowler (San Francisco, CA: ASP), 251 Mattioda, A. L., Bauschlicher, C. W., Bregman, J. D., et al. 2014, AcSpA, 130, 639 Mattioda, A. L., Bauschlicher, C. W., Ricca, A., et al. 2017, AcSpA, 181, 286 Peeters, E., Bauschlicher, C. W., Jr., Allamandola, L. J., et al. 2017, ApJ, 836, 198 Peeters, E., Hony, S., Van Kerckhoven, C., et al. 2002, A&A, 390, 1089 Perdew, J. P. 1986, PhRvB, 33, 8822 Pirali, O., Vervloet, M., Mulas, G., Malloci, G., & Joblin, C. 2009, PCCP, 11, 3443 Puget, J. L., & Léger, A. 1989, ARA&A, 27, 161 Sellgren, K., Werner, M. W., Ingalls, J. G., et al. 2010, ApJL, 722, L54 Stephens, P. J., Devlin, F. J., Chabalowski, C. F., & Frisch, M. J. 1994, JPhCh, 98, Szczepanski, J., & Vala, M. 1993, ApJ, 414, 646 Werner, M. W., Roellig, T. L., Low, F. J., et al. 2004, ApJS, 154, 1 Zhang, Y., & Kwok, S. 2015, ApJ, 798, 37 10
ANALYZING ASTRONOMICAL OBSERVATIONS WITH THE NASA AMES PAH DATABASE
 PAHs and the Universe C. Joblin and A.G.G.M. Tielens (eds) EAS Publications Series, 46 (2011) 117-122 www.eas.org ANALYZING ASTRONOMICAL OBSERVATIONS WITH THE NASA AMES PAH DATABASE J. Cami 1, 2 Abstract.
PAHs and the Universe C. Joblin and A.G.G.M. Tielens (eds) EAS Publications Series, 46 (2011) 117-122 www.eas.org ANALYZING ASTRONOMICAL OBSERVATIONS WITH THE NASA AMES PAH DATABASE J. Cami 1, 2 Abstract.
arxiv: v1 [astro-ph] 7 Feb 2008
![arxiv: v1 [astro-ph] 7 Feb 2008 arxiv: v1 [astro-ph] 7 Feb 2008](/thumbs/83/88512079.jpg) The infrared spectra of very large, compact, highly symmetric, polycyclic aromatic hydrocarbons (PAHs) Charles W. Bauschlicher, Jr. 1, Els Peeters 2,3,4, Louis J. Allamandola 5 arxiv:0802.1071v1 [astro-ph]
The infrared spectra of very large, compact, highly symmetric, polycyclic aromatic hydrocarbons (PAHs) Charles W. Bauschlicher, Jr. 1, Els Peeters 2,3,4, Louis J. Allamandola 5 arxiv:0802.1071v1 [astro-ph]
Astrochimistry Spring 2013 Lecture 4: Interstellar PAHs NGC HST
 Astrochimistry Spring 2013 Lecture 4: Interstellar PAHs NGC 7023 - HST Julien Montillaud 8th February 2013 Outline I. From Unidentified to Aromatic Infrared Bands (7 p.) I.1 Historical background I.2 Observational
Astrochimistry Spring 2013 Lecture 4: Interstellar PAHs NGC 7023 - HST Julien Montillaud 8th February 2013 Outline I. From Unidentified to Aromatic Infrared Bands (7 p.) I.1 Historical background I.2 Observational
arxiv: v1 [astro-ph.ga] 29 Jun 2011
![arxiv: v1 [astro-ph.ga] 29 Jun 2011 arxiv: v1 [astro-ph.ga] 29 Jun 2011](/thumbs/73/68434711.jpg) Astronomy & Astrophysics manuscript no. final c ESO 2 June 3, 2 arxiv:6.5899v [astro-ph.ga] 29 Jun 2 Coupled Blind Signal Separation and Spectroscopic Database Fitting of the Mid Infrared PAH Features
Astronomy & Astrophysics manuscript no. final c ESO 2 June 3, 2 arxiv:6.5899v [astro-ph.ga] 29 Jun 2 Coupled Blind Signal Separation and Spectroscopic Database Fitting of the Mid Infrared PAH Features
arxiv: v1 [astro-ph.ga] 5 Dec 2018
![arxiv: v1 [astro-ph.ga] 5 Dec 2018 arxiv: v1 [astro-ph.ga] 5 Dec 2018](/thumbs/91/107040438.jpg) Draft version December 7, 2018 Typeset using LATEX twocolumn style in AASTeX62 Examining the class B-to-A shift of the 7.7 µm PAH band with the NASA Ames PAH IR Spectroscopic Database Matthew J. Shannon
Draft version December 7, 2018 Typeset using LATEX twocolumn style in AASTeX62 Examining the class B-to-A shift of the 7.7 µm PAH band with the NASA Ames PAH IR Spectroscopic Database Matthew J. Shannon
Class 3. The PAH Spectrum, what does it tell us??
 Class 3 The PAH Spectrum, what does it tell us?? PAH Vibrations! CH str! CC str! CC str /CH ip! CH oop! 3! 4! 5! 6! 7! 8! 9! 10! 15! Wavelength (µm)! NASA Ames! Astrochemisty Lab! Vibration - S. Langhoff!
Class 3 The PAH Spectrum, what does it tell us?? PAH Vibrations! CH str! CC str! CC str /CH ip! CH oop! 3! 4! 5! 6! 7! 8! 9! 10! 15! Wavelength (µm)! NASA Ames! Astrochemisty Lab! Vibration - S. Langhoff!
Chemical Enrichment of the ISM by Stellar Ejecta
 Chemical Enrichment of the ISM by Stellar Ejecta Sun Kwok The University of Hong Kong IAU GA Beijing, Special Session 12, August 31, 2012 Molecular synthesis in the late stages of stellar evolution It
Chemical Enrichment of the ISM by Stellar Ejecta Sun Kwok The University of Hong Kong IAU GA Beijing, Special Session 12, August 31, 2012 Molecular synthesis in the late stages of stellar evolution It
THE MID-INFRARED LABORATORY SPECTRA OF NAPHTHALENE (C 10 H 8 ) IN SOLID H 2 O
 The Astrophysical Journal, 607:346 360, 2004 May 20 # 2004. The American Astronomical Society. All rights reserved. Printed in U.S.A. THE MID-INFRARED LABORATORY SPECTRA OF NAPHTHALENE (C 10 H 8 ) IN SOLID
The Astrophysical Journal, 607:346 360, 2004 May 20 # 2004. The American Astronomical Society. All rights reserved. Printed in U.S.A. THE MID-INFRARED LABORATORY SPECTRA OF NAPHTHALENE (C 10 H 8 ) IN SOLID
University of Groningen. Infrared emission features Boersma, Christiaan
 University of Groningen Infrared emission features Boersma, Christiaan IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the
University of Groningen Infrared emission features Boersma, Christiaan IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the
A. G. G. M. TIELENS SRON, Kapteyn Astronomical Institute, P.O. Box 800, 9700 AV Groningen, The Netherlands
 THE ASTROPHYSICAL JOURNAL, 560:261È271, 2001 October 10 ( 2001. The American Astronomical Society. All rights reserved. Printed in U.S.A. THEORETICAL MODELING OF INFRARED EMISSION FROM NEUTRAL AND CHARGED
THE ASTROPHYSICAL JOURNAL, 560:261È271, 2001 October 10 ( 2001. The American Astronomical Society. All rights reserved. Printed in U.S.A. THEORETICAL MODELING OF INFRARED EMISSION FROM NEUTRAL AND CHARGED
Exploring ISM dust with IRSIS. Emmanuel DARTOIS IAS-CNRS
 Exploring ISM dust with IRSIS Emmanuel DARTOIS IAS-CNRS IRSIS meeting 05-12-2007 Overview Intestellar ice mantles Hydrocarbons in the galaxy and outside Polycyclic Aromatic Hydrocarbons (PAHs) Interstellar
Exploring ISM dust with IRSIS Emmanuel DARTOIS IAS-CNRS IRSIS meeting 05-12-2007 Overview Intestellar ice mantles Hydrocarbons in the galaxy and outside Polycyclic Aromatic Hydrocarbons (PAHs) Interstellar
Aromatic Features in M101 HII Regions and Starburst Galaxies
 **FULL TITLE** ASP Conference Series, Vol. **VOLUME**, **YEAR OF PUBLICATION** **NAMES OF EDITORS** Aromatic Features in M101 HII Regions and Starburst Galaxies Karl D. Gordon, Charles Engelbracht, J.D.T.
**FULL TITLE** ASP Conference Series, Vol. **VOLUME**, **YEAR OF PUBLICATION** **NAMES OF EDITORS** Aromatic Features in M101 HII Regions and Starburst Galaxies Karl D. Gordon, Charles Engelbracht, J.D.T.
Astrochemistry (2) Interstellar extinction. Measurement of the reddening
 Measurement of the reddening The reddening of stellar colours casts light on the properties of interstellar dust Astrochemistry (2) Planets and Astrobiology (2016-2017) G. Vladilo The reddening is measured
Measurement of the reddening The reddening of stellar colours casts light on the properties of interstellar dust Astrochemistry (2) Planets and Astrobiology (2016-2017) G. Vladilo The reddening is measured
arxiv: v1 [astro-ph.sr] 15 May 2015
![arxiv: v1 [astro-ph.sr] 15 May 2015 arxiv: v1 [astro-ph.sr] 15 May 2015](/thumbs/87/96798259.jpg) ON THE ORIGIN OF THE 11.3 MICRON UNIDENTIFIED INFRARED EMISSION FEATURE arxiv:1505.03971v1 [astro-ph.sr] 15 May 2015 SeyedAbdolreza Sadjadi, Yong Zhang ( 張泳 ), and Sun Kwok ( 郭新 ) Space Astronomy Laboratory,
ON THE ORIGIN OF THE 11.3 MICRON UNIDENTIFIED INFRARED EMISSION FEATURE arxiv:1505.03971v1 [astro-ph.sr] 15 May 2015 SeyedAbdolreza Sadjadi, Yong Zhang ( 張泳 ), and Sun Kwok ( 郭新 ) Space Astronomy Laboratory,
The NASA Ames PAH IR Spectroscopic Database
 Chapter 5 The NASA Ames PAH IR Spectroscopic Database C. Boersma, L.J. Allamandola, C.W. Bauschlicher Jr., A. Ricca, J. Cami, A.L. Mattioda, A.G.G.M. Tielens Abstract Some thirty-five years after the initial
Chapter 5 The NASA Ames PAH IR Spectroscopic Database C. Boersma, L.J. Allamandola, C.W. Bauschlicher Jr., A. Ricca, J. Cami, A.L. Mattioda, A.G.G.M. Tielens Abstract Some thirty-five years after the initial
A SMALL FULLERENE (C 24 ) MAY BE THE CARRIER OF THE 11.2 MICRON UNIDENTIFIED INFRARED BAND
 accepted for publication in The Astrophysical Journal A SMALL FULLERENE (C 24 ) MAY BE THE CARRIER OF THE 11.2 MICRON UNIDENTIFIED INFRARED BAND L. S. Bernstein 1, R. M. Shroll 1, D. K. Lynch 2, & F. O.
accepted for publication in The Astrophysical Journal A SMALL FULLERENE (C 24 ) MAY BE THE CARRIER OF THE 11.2 MICRON UNIDENTIFIED INFRARED BAND L. S. Bernstein 1, R. M. Shroll 1, D. K. Lynch 2, & F. O.
AKARI Near-Infrared Spectroscopy Towards Young Stellar Objects
 AKARI Near-Infrared Spectroscopy Towards Young Stellar Objects Aleksandra Ardaseva 1,TakashiOnaka 2 1 University of St Andrews, United Kingdom 2 Department of Astronomy, Graduate School of Science, University
AKARI Near-Infrared Spectroscopy Towards Young Stellar Objects Aleksandra Ardaseva 1,TakashiOnaka 2 1 University of St Andrews, United Kingdom 2 Department of Astronomy, Graduate School of Science, University
LABORATORY INFRARED SPECTROSCOPY OF GASEOUS NEGATIVELY CHARGED POLYAROMATIC HYDROCARBONS
 The Astrophysical Journal, 787:17 (11pp), 214 June 1 C 214. The American Astronomical Society. All rights reserved. Printed in the U.S.A. doi:1.188/4-637x/787/2/17 LABORATORY INFRARED SPECTROSCOPY OF GASEOUS
The Astrophysical Journal, 787:17 (11pp), 214 June 1 C 214. The American Astronomical Society. All rights reserved. Printed in the U.S.A. doi:1.188/4-637x/787/2/17 LABORATORY INFRARED SPECTROSCOPY OF GASEOUS
LABORATORY INFRARED SPECTRA OF POLYCYCLIC AROMATIC NITROGEN HETEROCYCLES: QUINOLINE AND PHENANTHRIDINE IN SOLID ARGON AND H 2 O
 The Astrophysical Journal, 626:909 918, 2005 June 20 # 2005. The American Astronomical Society. All rights reserved. Printed in U.S.A. LABORATORY INFRARED SPECTRA OF POLYCYCLIC AROMATIC NITROGEN HETEROCYCLES:
The Astrophysical Journal, 626:909 918, 2005 June 20 # 2005. The American Astronomical Society. All rights reserved. Printed in U.S.A. LABORATORY INFRARED SPECTRA OF POLYCYCLIC AROMATIC NITROGEN HETEROCYCLES:
Christine Joblin Institut de Recherche en Astrophysique et Planétologie Université de Toulouse [UPS] CNRS
![Christine Joblin Institut de Recherche en Astrophysique et Planétologie Université de Toulouse [UPS] CNRS Christine Joblin Institut de Recherche en Astrophysique et Planétologie Université de Toulouse [UPS] CNRS](/thumbs/95/123499834.jpg) Christine Joblin Institut de Recherche en Astrophysique et Planétologie Université de Toulouse [UPS] CNRS Negative ions and molecules in astrophysics Gothenburg and Onsala, Sweden 22-24/08/2011 Outline
Christine Joblin Institut de Recherche en Astrophysique et Planétologie Université de Toulouse [UPS] CNRS Negative ions and molecules in astrophysics Gothenburg and Onsala, Sweden 22-24/08/2011 Outline
University of Groningen. Infrared emission features Boersma, Christiaan
 University of Groningen Infrared emission features Boersma, Christiaan IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the
University of Groningen Infrared emission features Boersma, Christiaan IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the
8: Composition and Physical state of Interstellar Dust
 8: Composition and Physical state of Interstellar Dust James Graham UC, Berkeley 1 Reading Tielens, Interstellar Medium, Ch. 5 Mathis, J. S. 1990, AARA, 28, 37 Draine, B. T., 2003, AARA, 41, 241 2 Nature
8: Composition and Physical state of Interstellar Dust James Graham UC, Berkeley 1 Reading Tielens, Interstellar Medium, Ch. 5 Mathis, J. S. 1990, AARA, 28, 37 Draine, B. T., 2003, AARA, 41, 241 2 Nature
PDF hosted at the Radboud Repository of the Radboud University Nijmegen
 PDF hosted at the Radboud Repository of the Radboud University Nijmegen The following full text is a publisher's version. For additional information about this publication click this link. http://hdl.handle.net/2066/169460
PDF hosted at the Radboud Repository of the Radboud University Nijmegen The following full text is a publisher's version. For additional information about this publication click this link. http://hdl.handle.net/2066/169460
Interstellar Dust and Extinction
 University of Oxford, Astrophysics November 12, 2007 Outline Extinction Spectral Features Emission Scattering Polarization Grain Models & Evolution Conclusions What and Why? Dust covers a range of compound
University of Oxford, Astrophysics November 12, 2007 Outline Extinction Spectral Features Emission Scattering Polarization Grain Models & Evolution Conclusions What and Why? Dust covers a range of compound
Department of Chemistry, University of California, Berkeley, CA ; Received 2002 May 28; accepted 2002 July 22
 The Astrophysical Journal Supplement Series, 143:455 467, 2002 December # 2002. The American Astronomical Society. All rights reserved. Printed in U.S.A. SINGLE-PHOTON INFRARED EMISSION SPECTROSCOPY OF
The Astrophysical Journal Supplement Series, 143:455 467, 2002 December # 2002. The American Astronomical Society. All rights reserved. Printed in U.S.A. SINGLE-PHOTON INFRARED EMISSION SPECTROSCOPY OF
Status of the Diffuse Interstellar Band Problem
 Status of the Diffuse Interstellar Band Problem Ben McCall Department of Chemistry and Department of Astronomy University of Illinois at Urbana-Champaign APO DIB Collaboration: Tom Fishman (Chicago), Scott
Status of the Diffuse Interstellar Band Problem Ben McCall Department of Chemistry and Department of Astronomy University of Illinois at Urbana-Champaign APO DIB Collaboration: Tom Fishman (Chicago), Scott
Physics and Chemistry of the Interstellar Medium
 Physics and Chemistry of the Interstellar Medium Sun Kwok The University of Hong Kong UNIVERSITY SCIENCE BOOKS Sausalito, California * Preface xi The Interstellar Medium.1.1 States of Matter in the ISM
Physics and Chemistry of the Interstellar Medium Sun Kwok The University of Hong Kong UNIVERSITY SCIENCE BOOKS Sausalito, California * Preface xi The Interstellar Medium.1.1 States of Matter in the ISM
LABORATORY SPECTROSCOPY OF PROTONATED PAH MOLECULES RELEVANT FOR INTERSTELLAR CHEMISTRY
 PAHs and the Universe C. Joblin and A.G.G.M. Tielens (eds) EAS Publications Series, 46 (2011) 103-108 www.eas.org LABORATORY SPECTROSCOPY OF PROTONATED PAH MOLECULES RELEVANT FOR INTERSTELLAR CHEMISTRY
PAHs and the Universe C. Joblin and A.G.G.M. Tielens (eds) EAS Publications Series, 46 (2011) 103-108 www.eas.org LABORATORY SPECTROSCOPY OF PROTONATED PAH MOLECULES RELEVANT FOR INTERSTELLAR CHEMISTRY
Beyond the Visible -- Exploring the Infrared Universe
 Beyond the Visible -- Exploring the Infrared Universe Prof. T. Jarrett (UCT) Infrared Window Telescopes ISM -- Galaxies Infrared Window Near-infrared: 1 to 5 µm Mid-infrared: 5 to 50 µm
Beyond the Visible -- Exploring the Infrared Universe Prof. T. Jarrett (UCT) Infrared Window Telescopes ISM -- Galaxies Infrared Window Near-infrared: 1 to 5 µm Mid-infrared: 5 to 50 µm
The Interstellar Medium in Galaxies: SOFIA Science
 The Interstellar Medium in Galaxies: SOFIA Science Margaret Meixner (STScI) Xander Tielens (NASA/Ames/Leiden Univ.), Jesse Dotson (NASA/ARC), Bruce Draine (Princeton), Mark Wolfire (U. Maryland), Jackie
The Interstellar Medium in Galaxies: SOFIA Science Margaret Meixner (STScI) Xander Tielens (NASA/Ames/Leiden Univ.), Jesse Dotson (NASA/ARC), Bruce Draine (Princeton), Mark Wolfire (U. Maryland), Jackie
Laser Dissociation of Protonated PAHs
 100 Chapter 5 Laser Dissociation of Protonated PAHs 5.1 Experiments The photodissociation experiments were performed with protonated PAHs using different laser sources. The calculations from Chapter 3
100 Chapter 5 Laser Dissociation of Protonated PAHs 5.1 Experiments The photodissociation experiments were performed with protonated PAHs using different laser sources. The calculations from Chapter 3
arxiv: v1 [astro-ph.ga] 28 Jan 2016
![arxiv: v1 [astro-ph.ga] 28 Jan 2016 arxiv: v1 [astro-ph.ga] 28 Jan 2016](/thumbs/94/119192526.jpg) Submitted, 19-11-2015; Accepted 22-01-2016. Preprint typeset using L A TEX style emulateapj v. 5/2/11 POLYCYCLIC AROMATIC HYDROCARBON EMISSION IN SPITZER/IRS MAPS I: CATALOG AND SIMPLE DIAGNOSTICS D. J.
Submitted, 19-11-2015; Accepted 22-01-2016. Preprint typeset using L A TEX style emulateapj v. 5/2/11 POLYCYCLIC AROMATIC HYDROCARBON EMISSION IN SPITZER/IRS MAPS I: CATALOG AND SIMPLE DIAGNOSTICS D. J.
Aromatic emission from the ionised mane of the Horsehead nebula ABSTRACT
 A&A 471, 205 212 (2007) DOI: 10.1051/0004-6361:20066172 c ESO 2007 Astronomy & Astrophysics Aromatic emission from the ionised mane of the Horsehead nebula M. Compiègne 1,A.Abergel 1, L. Verstraete 1,
A&A 471, 205 212 (2007) DOI: 10.1051/0004-6361:20066172 c ESO 2007 Astronomy & Astrophysics Aromatic emission from the ionised mane of the Horsehead nebula M. Compiègne 1,A.Abergel 1, L. Verstraete 1,
Polycyclic Aromatic Hydrocarbon from the Magellanic Clouds
 Polycyclic Aromatic Hydrocarbon from the Magellanic Clouds Hony and Spitzer/SAGE-SPEC team Galliano, Seok, Neelamkodan, Kemper, Madden, Indebetouw, Gordon, Sandstrom 11 Aug 2017 S. Hony (Heidelberg University)
Polycyclic Aromatic Hydrocarbon from the Magellanic Clouds Hony and Spitzer/SAGE-SPEC team Galliano, Seok, Neelamkodan, Kemper, Madden, Indebetouw, Gordon, Sandstrom 11 Aug 2017 S. Hony (Heidelberg University)
Astronomy. Astrophysics. Evolution of polycyclic aromatic hydrocarbons in photodissociation regions. Hydrogenation and charge states
 A&A 552, A5 (23) DOI:.5/4-3/222757 c ESO 23 Astronomy & Astrophysics Evolution of polycyclic aromatic hydrocarbons in photodissociation regions Hydrogenation and charge states J. Montillaud,2,3, C. Joblin,2,
A&A 552, A5 (23) DOI:.5/4-3/222757 c ESO 23 Astronomy & Astrophysics Evolution of polycyclic aromatic hydrocarbons in photodissociation regions Hydrogenation and charge states J. Montillaud,2,3, C. Joblin,2,
POLYCYCLIC AROMATIC HYDROCARBON CONTRIBUTION TO THE INFRARED OUTPUT ENERGY OF THE UNIVERSE AT Z 2
 The Astrophysical Journal Supplement Series, 154:112 117, 2004 September # 2004. The American Astronomical Society. All rights reserved. Printed in U.S.A. POLYCYCLIC AROMATIC HYDROCARBON CONTRIBUTION TO
The Astrophysical Journal Supplement Series, 154:112 117, 2004 September # 2004. The American Astronomical Society. All rights reserved. Printed in U.S.A. POLYCYCLIC AROMATIC HYDROCARBON CONTRIBUTION TO
Excited States Calculations for Protonated PAHs
 52 Chapter 3 Excited States Calculations for Protonated PAHs 3.1 Introduction Protonated PAHs are closed shell ions. Their electronic structure should therefore be similar to that of neutral PAHs, but
52 Chapter 3 Excited States Calculations for Protonated PAHs 3.1 Introduction Protonated PAHs are closed shell ions. Their electronic structure should therefore be similar to that of neutral PAHs, but
INVESTIGATION OF THE ULTRAVIOLET, VISIBLE, AND NEAR-INFRARED ABSORPTION SPECTRA OF HYDROGENATED POLYCYCLIC AROMATIC HYDROCARBONS AND THEIR CATIONS
 The Astrophysical Journal, 628:555 566, 2005 July 20 # 2005. The American Astronomical Society. All rights reserved. Printed in U.S.A. INVESTIGATION OF THE ULTRAVIOLET, VISIBLE, AND NEAR-INFRARED ABSORPTION
The Astrophysical Journal, 628:555 566, 2005 July 20 # 2005. The American Astronomical Society. All rights reserved. Printed in U.S.A. INVESTIGATION OF THE ULTRAVIOLET, VISIBLE, AND NEAR-INFRARED ABSORPTION
E. Rauls. Department of Theoretical Physics, Faculty of Natural Sciences, University of Paderborn, D Paderborn, Germany;
 The Astrophysical Journal, 679:531Y536, 2008 May 20 # 2008. The American Astronomical Society. All rights reserved. Printed in U.S.A. A CATALYZED ROUTES TO MOLECULAR HYDROGEN FORMATION AND HYDROGEN ADDITION
The Astrophysical Journal, 679:531Y536, 2008 May 20 # 2008. The American Astronomical Society. All rights reserved. Printed in U.S.A. A CATALYZED ROUTES TO MOLECULAR HYDROGEN FORMATION AND HYDROGEN ADDITION
Fullerenes and PAHs: from laboratory to the detection in interstellar space
 Highlights of Spanish Astrophysics VII, Proceedings of the X Scientific Meeting of the Spanish Astronomical Society held on July 9-13, 2012, in Valencia, Spain. J. C. Guirado, L.M. Lara, V. Quilis, and
Highlights of Spanish Astrophysics VII, Proceedings of the X Scientific Meeting of the Spanish Astronomical Society held on July 9-13, 2012, in Valencia, Spain. J. C. Guirado, L.M. Lara, V. Quilis, and
(2) Read each statement carefully and pick the one that is incorrect in its information.
 Organic Chemistry - Problem Drill 17: IR and Mass Spectra No. 1 of 10 1. Which statement about infrared spectroscopy is incorrect? (A) IR spectroscopy is a method of structure determination based on the
Organic Chemistry - Problem Drill 17: IR and Mass Spectra No. 1 of 10 1. Which statement about infrared spectroscopy is incorrect? (A) IR spectroscopy is a method of structure determination based on the
Lecture 5. Interstellar Dust: Chemical & Thermal Properties
 Lecture 5. Interstellar Dust: Chemical & Thermal Properties!. Spectral Features 2. Grain populations and Models 3. Thermal Properties 4. Small Grains and Large Molecules -------------------------------------------------
Lecture 5. Interstellar Dust: Chemical & Thermal Properties!. Spectral Features 2. Grain populations and Models 3. Thermal Properties 4. Small Grains and Large Molecules -------------------------------------------------
Unscrambling the Egg. Yvonne Pendleton NASA Ames Research Center. JWST Workshop Nov. 14, 2017
 Unscrambling the Egg Yvonne Pendleton NASA Ames Research Center JWST Workshop Nov. 14, 2017 From interstellar dust to new stars and planets Comparisons between material forming new planetary systems and
Unscrambling the Egg Yvonne Pendleton NASA Ames Research Center JWST Workshop Nov. 14, 2017 From interstellar dust to new stars and planets Comparisons between material forming new planetary systems and
Lecture 18 - Photon Dominated Regions
 Lecture 18 - Photon Dominated Regions 1. What is a PDR? 2. Physical and Chemical Concepts 3. Molecules in Diffuse Clouds 4. Galactic and Extragalactic PDRs References Tielens, Ch. 9 Hollenbach & Tielens,
Lecture 18 - Photon Dominated Regions 1. What is a PDR? 2. Physical and Chemical Concepts 3. Molecules in Diffuse Clouds 4. Galactic and Extragalactic PDRs References Tielens, Ch. 9 Hollenbach & Tielens,
ROTATIONAL SPECTRA OF SMALL PAHs: ACENAPHTHENE, ACENAPHTHYLENE, AZULENE, AND FLUORENE
 The Astrophysical Journal, 662:1309 Y 1314, 27 June 20 # 27. The American Astronomical Society. All rights reserved. Printed in U.S.A. A ROTATIONAL SPECTRA OF SMALL PAHs: ACENAPHTHENE, ACENAPHTHYLENE,
The Astrophysical Journal, 662:1309 Y 1314, 27 June 20 # 27. The American Astronomical Society. All rights reserved. Printed in U.S.A. A ROTATIONAL SPECTRA OF SMALL PAHs: ACENAPHTHENE, ACENAPHTHYLENE,
Astronomy 106, Fall September 2015
 Today in Astronomy 106: molecules to molecular clouds to stars Aromatic (benzene-ring) molecules in space Formation of molecules, on dust-grain surfaces and in the gas phase Interstellar molecular clouds
Today in Astronomy 106: molecules to molecular clouds to stars Aromatic (benzene-ring) molecules in space Formation of molecules, on dust-grain surfaces and in the gas phase Interstellar molecular clouds
ELECTRONIC SPECTROSCOPY OF FUV-IRRADIATED DIAMONDOIDS: A COMBINED EXPERIMENTAL AND THEORETICAL STUDY
 published in The Astrophysical Journal 729 (2011) 91, Received 2010 November 25; accepted 2011 January 5 Preprint typeset using L A TEX style emulateapj v. 11/10/09 ELECTRONIC SPECTROSCOPY OF FUV-IRRADIATED
published in The Astrophysical Journal 729 (2011) 91, Received 2010 November 25; accepted 2011 January 5 Preprint typeset using L A TEX style emulateapj v. 11/10/09 ELECTRONIC SPECTROSCOPY OF FUV-IRRADIATED
Radiant energy is proportional to its frequency (cycles/s = Hz) as a wave (Amplitude is its height) Different types are classified by frequency or
 CHEM 241 UNIT 5: PART B INFRA-RED RED SPECTROSCOPY 1 Spectroscopy of the Electromagnetic Spectrum Radiant energy is proportional to its frequency (cycles/s = Hz) as a wave (Amplitude is its height) Different
CHEM 241 UNIT 5: PART B INFRA-RED RED SPECTROSCOPY 1 Spectroscopy of the Electromagnetic Spectrum Radiant energy is proportional to its frequency (cycles/s = Hz) as a wave (Amplitude is its height) Different
The Cornell Atlas of Spitzer Spectra (CASSIS) and recent advances in the extraction of complex sources
 The Cornell Atlas of Spitzer Spectra (CASSIS) and recent advances in the extraction of complex sources V. Lebouteiller (CEA & Cornell University) D.J. Barry, G.C. Sloan, H.W.W. Spoon, D.W. Weedman, J.R.
The Cornell Atlas of Spitzer Spectra (CASSIS) and recent advances in the extraction of complex sources V. Lebouteiller (CEA & Cornell University) D.J. Barry, G.C. Sloan, H.W.W. Spoon, D.W. Weedman, J.R.
Experiment 11: NUCLEAR MAGNETIC RESONANCE SPECTROSCOPY
 Experiment 11: NUCLEAR MAGNETIC RESONANCE SPECTROSCOPY Purpose: This is an exercise to introduce the use of nuclear magnetic resonance spectroscopy, in conjunction with infrared spectroscopy, to determine
Experiment 11: NUCLEAR MAGNETIC RESONANCE SPECTROSCOPY Purpose: This is an exercise to introduce the use of nuclear magnetic resonance spectroscopy, in conjunction with infrared spectroscopy, to determine
kev e - and H + ECR source Shock wave Molecular ices 3 C 2 H 2 C 6 H 6 2 C 3 H 3 Dust impact Europa
 B Sivaraman kev e - and H + Shock wave Molecular ices ECR source 3 C 2 H 2 C 6 H 6 Europa Dust impact 2 C 3 H 3 C 6 H 6 Temperature, K 273 K 70 / 80 90 K 50 60 K < 20 K New molecules (ISM) C 60 - C 70
B Sivaraman kev e - and H + Shock wave Molecular ices ECR source 3 C 2 H 2 C 6 H 6 Europa Dust impact 2 C 3 H 3 C 6 H 6 Temperature, K 273 K 70 / 80 90 K 50 60 K < 20 K New molecules (ISM) C 60 - C 70
arxiv: v1 [astro-ph.ga] 21 Nov 2018
![arxiv: v1 [astro-ph.ga] 21 Nov 2018 arxiv: v1 [astro-ph.ga] 21 Nov 2018](/thumbs/90/103887410.jpg) Article The Astrochemistry Implications of Quantum Chemical Normal Modes Vibrational Analysis SeyedAbdolreza Sadjadi ID & Quentin Andrew Parker ID Laboratory for Space Research and the Department of Physics,
Article The Astrochemistry Implications of Quantum Chemical Normal Modes Vibrational Analysis SeyedAbdolreza Sadjadi ID & Quentin Andrew Parker ID Laboratory for Space Research and the Department of Physics,
Lecture 14 Organic Chemistry 1
 CHEM 232 Organic Chemistry I at Chicago Lecture 14 Organic Chemistry 1 Professor Duncan Wardrop February 25, 2010 1 CHEM 232 Organic Chemistry I at Chicago Mass Spectrometry Sections: 13.24-13.25 2 Spectroscopy
CHEM 232 Organic Chemistry I at Chicago Lecture 14 Organic Chemistry 1 Professor Duncan Wardrop February 25, 2010 1 CHEM 232 Organic Chemistry I at Chicago Mass Spectrometry Sections: 13.24-13.25 2 Spectroscopy
Theoretical Electronic and Rovibrational Studies for Anions of Interest to the DIBs
 Georgia Southern University From the SelectedWorks of Ryan C. Fortenberry 2013 Theoretical Electronic and Rovibrational Studies for Anions of Interest to the DIBs Ryan C. Fortenberry, Georgia Southern
Georgia Southern University From the SelectedWorks of Ryan C. Fortenberry 2013 Theoretical Electronic and Rovibrational Studies for Anions of Interest to the DIBs Ryan C. Fortenberry, Georgia Southern
The ionisation state of PAHs in interstellar environments
 Astron. Astrophys. 323, 534 540 (1997) ASTRONOMY AND ASTROPHYSICS The ionisation state of PAHs in interstellar environments E. Dartois and L. d Hendecourt IAS-CNRS, Université Paris XI, Batiment 121, F-91405
Astron. Astrophys. 323, 534 540 (1997) ASTRONOMY AND ASTROPHYSICS The ionisation state of PAHs in interstellar environments E. Dartois and L. d Hendecourt IAS-CNRS, Université Paris XI, Batiment 121, F-91405
Comparison between 30 micron sources in different galaxies
 Journal of Physics: Conference Series PAPER OPEN ACCESS Comparison between 30 micron sources in different galaxies To cite this article: Marcin Gadkowski et al 2016 J. Phys.: Conf. Ser. 728 062007 View
Journal of Physics: Conference Series PAPER OPEN ACCESS Comparison between 30 micron sources in different galaxies To cite this article: Marcin Gadkowski et al 2016 J. Phys.: Conf. Ser. 728 062007 View
On the aliphatic versus aromatic content of the carriers of the unidentified infrared emission features
 Advance Access publication 2016 July 20 doi:10.1093/mnras/stw1740 On the aliphatic versus aromatic content of the carriers of the unidentified infrared emission features X. J. Yang, 1,2 R. Glaser, 3 Aigen
Advance Access publication 2016 July 20 doi:10.1093/mnras/stw1740 On the aliphatic versus aromatic content of the carriers of the unidentified infrared emission features X. J. Yang, 1,2 R. Glaser, 3 Aigen
Relaxation of energized PAHs
 Relaxation of energized PAHs 2018 1 st year PhD student Supervisor: Dr. Christine Joblin May 16, 2018 Mid-Infrared Spectra Emission features 3.3, 6.2, 7.7, 8.6, 11.2, 12.7 µm Vibrational modes of PAHs
Relaxation of energized PAHs 2018 1 st year PhD student Supervisor: Dr. Christine Joblin May 16, 2018 Mid-Infrared Spectra Emission features 3.3, 6.2, 7.7, 8.6, 11.2, 12.7 µm Vibrational modes of PAHs
Structure and Bonding of Organic Molecules
 Chem 220 Notes Page 1 Structure and Bonding of Organic Molecules I. Types of Chemical Bonds A. Why do atoms forms bonds? Atoms want to have the same number of electrons as the nearest noble gas atom (noble
Chem 220 Notes Page 1 Structure and Bonding of Organic Molecules I. Types of Chemical Bonds A. Why do atoms forms bonds? Atoms want to have the same number of electrons as the nearest noble gas atom (noble
Table 8.2 Detailed Table of Characteristic Infrared Absorption Frequencies
 Table 8.2 Detailed Table of Characteristic Infrared Absorption Frequencies The hydrogen stretch region (3600 2500 cm 1 ). Absorption in this region is associated with the stretching vibration of hydrogen
Table 8.2 Detailed Table of Characteristic Infrared Absorption Frequencies The hydrogen stretch region (3600 2500 cm 1 ). Absorption in this region is associated with the stretching vibration of hydrogen
Structure Determination. How to determine what compound that you have? One way to determine compound is to get an elemental analysis
 Structure Determination How to determine what compound that you have? ne way to determine compound is to get an elemental analysis -basically burn the compound to determine %C, %H, %, etc. from these percentages
Structure Determination How to determine what compound that you have? ne way to determine compound is to get an elemental analysis -basically burn the compound to determine %C, %H, %, etc. from these percentages
arxiv: v1 [astro-ph.ga] 30 Jul 2012
![arxiv: v1 [astro-ph.ga] 30 Jul 2012 arxiv: v1 [astro-ph.ga] 30 Jul 2012](/thumbs/92/108174026.jpg) Mon. ot. R. Astron. Soc. 000, 1 10 (2012) Printed 31 July 2018 (M LATX style file v2.2) Spatial distribution and interpretation of the 3.3 µm PAH emission band of the Red Rectangle arxiv:1207.6990v1 [astro-ph.ga]
Mon. ot. R. Astron. Soc. 000, 1 10 (2012) Printed 31 July 2018 (M LATX style file v2.2) Spatial distribution and interpretation of the 3.3 µm PAH emission band of the Red Rectangle arxiv:1207.6990v1 [astro-ph.ga]
THE UNUSUAL HYDROCARBON EMISSION FROM THE EARLY CARBON STAR HD : THE CONNECTION BETWEEN AROMATICS AND ALIPHATICS
 The Astrophysical Journal, 664:1144Y1153, 2007 August 1 # 2007. The American Astronomical Society. All rights reserved. Printed in U.S.A. A THE UNUSUAL HYDROCARBON EMISSION FROM THE EARLY CARBON STAR HD
The Astrophysical Journal, 664:1144Y1153, 2007 August 1 # 2007. The American Astronomical Society. All rights reserved. Printed in U.S.A. A THE UNUSUAL HYDROCARBON EMISSION FROM THE EARLY CARBON STAR HD
Simulating chemistry on interstellar dust grains in the laboratory
 From Stars to Life, April 3-6 2013, Gainesville FL Simulating chemistry on interstellar dust grains in the laboratory Nicolas Polfer University of Florida Department of Chemistry http://www.cosmotography.com/images/cosmic_nurseries.html
From Stars to Life, April 3-6 2013, Gainesville FL Simulating chemistry on interstellar dust grains in the laboratory Nicolas Polfer University of Florida Department of Chemistry http://www.cosmotography.com/images/cosmic_nurseries.html
Physics 224 The Interstellar Medium
 Physics 224 The Interstellar Medium Lecture #11: Dust Composition, Photoelectric Heating, Neutral Gas Outline Part I: Dust Heating & Cooling continued Part III: Dust Emission & Photoelectric Heating Part
Physics 224 The Interstellar Medium Lecture #11: Dust Composition, Photoelectric Heating, Neutral Gas Outline Part I: Dust Heating & Cooling continued Part III: Dust Emission & Photoelectric Heating Part
Interstellar Medium and Star Birth
 Interstellar Medium and Star Birth Interstellar dust Lagoon nebula: dust + gas Interstellar Dust Extinction and scattering responsible for localized patches of darkness (dark clouds), as well as widespread
Interstellar Medium and Star Birth Interstellar dust Lagoon nebula: dust + gas Interstellar Dust Extinction and scattering responsible for localized patches of darkness (dark clouds), as well as widespread
CH 3. mirror plane. CH c d
 CAPTER 20 Practice Exercises 20.1 The index of hydrogen deficiency is two. The structural possibilities include two double bonds, a double do 20.3 (a) As this is an alkane, it contains only C and and has
CAPTER 20 Practice Exercises 20.1 The index of hydrogen deficiency is two. The structural possibilities include two double bonds, a double do 20.3 (a) As this is an alkane, it contains only C and and has
Physical Chemistry Lab II CHEM 4644 Spring 2011 Final Exam 5 questions at 3 points each equals 15 total points possible.
 Physical Chemistry Lab II Name: KEY CHEM 4644 Spring 2011 Final Exam 5 questions at 3 points each equals 15 total points possible. Constants: c = 3.00 10 8 m/s h = 6.63 10-34 J s 1 Hartree = 4.36 10-18
Physical Chemistry Lab II Name: KEY CHEM 4644 Spring 2011 Final Exam 5 questions at 3 points each equals 15 total points possible. Constants: c = 3.00 10 8 m/s h = 6.63 10-34 J s 1 Hartree = 4.36 10-18
Interstellar Chemistry
 4 Interstellar Chemistry Pascale Ehrenfreund Leiden Observatory, Leiden, The Netherlands The space between the stars, called the interstellar medium (ISM), is composed primarily of H and He gases incorporating
4 Interstellar Chemistry Pascale Ehrenfreund Leiden Observatory, Leiden, The Netherlands The space between the stars, called the interstellar medium (ISM), is composed primarily of H and He gases incorporating
Mapping the Spatial Distribution of H 2 in Nearby Galaxies with the Spitzer Infrared Spectrograph
 The Evolving ISM in the Milky Way & Nearby Galaxies Mapping the Spatial Distribution of H 2 in Nearby Galaxies with the Spitzer Infrared Spectrograph Gregory Brunner 1, Reginald Dufour 1, Kartik Sheth
The Evolving ISM in the Milky Way & Nearby Galaxies Mapping the Spatial Distribution of H 2 in Nearby Galaxies with the Spitzer Infrared Spectrograph Gregory Brunner 1, Reginald Dufour 1, Kartik Sheth
B. J. McCall, J. Thorburn, L. M. Hobbs, T. Oka, and D. G. York
 The Astrophysical Journal, 559:L49 L53, 200 September 20 200. The American Astronomical Society. All rights reserved. Printed in U.S.A. REJECTION OF THE C DIFFUSE INTERSTELLAR BAND HYPOTHESIS B. J. McCall,
The Astrophysical Journal, 559:L49 L53, 200 September 20 200. The American Astronomical Society. All rights reserved. Printed in U.S.A. REJECTION OF THE C DIFFUSE INTERSTELLAR BAND HYPOTHESIS B. J. McCall,
Lecture 11. IR Theory. Next Class: Lecture Problem 4 due Thin-Layer Chromatography
 Lecture 11 IR Theory Next Class: Lecture Problem 4 due Thin-Layer Chromatography This Week In Lab: Ch 6: Procedures 2 & 3 Procedure 4 (outside of lab) Next Week in Lab: Ch 7: PreLab Due Quiz 4 Ch 5 Final
Lecture 11 IR Theory Next Class: Lecture Problem 4 due Thin-Layer Chromatography This Week In Lab: Ch 6: Procedures 2 & 3 Procedure 4 (outside of lab) Next Week in Lab: Ch 7: PreLab Due Quiz 4 Ch 5 Final
Protonated Polycyclic Aromatic Hydrocarbons and the Interstellar Medium
 1 Chapter 1 Protonated Polycyclic Aromatic Hydrocarbons and the Interstellar Medium 1.1 Interstellar Molecules Although hydrogen is the most abundant element in the universe, it is other elements that
1 Chapter 1 Protonated Polycyclic Aromatic Hydrocarbons and the Interstellar Medium 1.1 Interstellar Molecules Although hydrogen is the most abundant element in the universe, it is other elements that
The Interstellar Medium
 The Interstellar Medium Fall 2014 Lecturer: Dr. Paul van der Werf Oortgebouw 565, ext 5883 pvdwerf@strw.leidenuniv.nl Assistant: Kirstin Doney Huygenslaboratorium 528 doney@strw.leidenuniv.nl Class Schedule
The Interstellar Medium Fall 2014 Lecturer: Dr. Paul van der Werf Oortgebouw 565, ext 5883 pvdwerf@strw.leidenuniv.nl Assistant: Kirstin Doney Huygenslaboratorium 528 doney@strw.leidenuniv.nl Class Schedule
Diffuse Interstellar Medium
 Diffuse Interstellar Medium Basics, velocity widths H I 21-cm radiation (emission) Interstellar absorption lines Radiative transfer Resolved Lines, column densities Unresolved lines, curve of growth Abundances,
Diffuse Interstellar Medium Basics, velocity widths H I 21-cm radiation (emission) Interstellar absorption lines Radiative transfer Resolved Lines, column densities Unresolved lines, curve of growth Abundances,
REVIEW: VALENCE ELECTRONS CHEMICAL BONDS: LEWIS SYMBOLS: CHEMICAL BONDING. What are valence electrons?
 REVIEW: VALENCE ELECTRONS 13 CHEMICAL BONDING What are valence electrons? Which groups on the periodic table readily give up electrons? What group readily accepts electrons? CHEMICAL BONDS: What are chemical
REVIEW: VALENCE ELECTRONS 13 CHEMICAL BONDING What are valence electrons? Which groups on the periodic table readily give up electrons? What group readily accepts electrons? CHEMICAL BONDS: What are chemical
Electronic and optical properties of PAH families: a (time-dependent) DFT study
 Electronic and optical properties of PAH families: a (time-dependent) DFT study Giancarlo Cappellini1 Giuliano Malloci 1 1 Alessandro Mattoni1 Giacomo Mulas2 CNR-IOM 2 SLACS UniCA Cagliari Cagliari SIF-L'Aquila
Electronic and optical properties of PAH families: a (time-dependent) DFT study Giancarlo Cappellini1 Giuliano Malloci 1 1 Alessandro Mattoni1 Giacomo Mulas2 CNR-IOM 2 SLACS UniCA Cagliari Cagliari SIF-L'Aquila
Organic Compound Identification Using Infrared Spectroscopy. Description
 Return to paper Organic Compound Identification Using Infrared Spectroscopy Dr. Walt Volland, Bellevue Community College All rights reserved 1999, Bellevue, Washington Description This exercise is intended
Return to paper Organic Compound Identification Using Infrared Spectroscopy Dr. Walt Volland, Bellevue Community College All rights reserved 1999, Bellevue, Washington Description This exercise is intended
MID-INFRARED SPECTRA OF POLYCYCLIC AROMATIC HYDROCARBON EMISSION IN HERBIG Ae/Be STARS
 The Astrophysical Journal, 632:956 963, 2005 October 20 # 2005. The American Astronomical Society. All rights reserved. Printed in U.S.A. MID-INFRARED SPECTRA OF POLYCYCLIC AROMATIC HYDROCARBON EMISSION
The Astrophysical Journal, 632:956 963, 2005 October 20 # 2005. The American Astronomical Society. All rights reserved. Printed in U.S.A. MID-INFRARED SPECTRA OF POLYCYCLIC AROMATIC HYDROCARBON EMISSION
Some HI is in reasonably well defined clouds. Motions inside the cloud, and motion of the cloud will broaden and shift the observed lines!
 Some HI is in reasonably well defined clouds. Motions inside the cloud, and motion of the cloud will broaden and shift the observed lines Idealized 21cm spectra Example observed 21cm spectra HI densities
Some HI is in reasonably well defined clouds. Motions inside the cloud, and motion of the cloud will broaden and shift the observed lines Idealized 21cm spectra Example observed 21cm spectra HI densities
The 22 micron emission feature in supernova remnants and massive star-forming regions
 The Evolving ISM in the Milky Way & Nearby Galaxies The 22 micron emission feature in supernova remnants and massive star-forming regions Takashi Onaka 1, Thomas. L. Roellig 2, Yoko Okada 3, and Kin-Wing
The Evolving ISM in the Milky Way & Nearby Galaxies The 22 micron emission feature in supernova remnants and massive star-forming regions Takashi Onaka 1, Thomas. L. Roellig 2, Yoko Okada 3, and Kin-Wing
More information can be found in Chapter 12 in your textbook for CHEM 3750/ 3770 and on pages in your laboratory manual.
 CHEM 3780 rganic Chemistry II Infrared Spectroscopy and Mass Spectrometry Review More information can be found in Chapter 12 in your textbook for CHEM 3750/ 3770 and on pages 13-28 in your laboratory manual.
CHEM 3780 rganic Chemistry II Infrared Spectroscopy and Mass Spectrometry Review More information can be found in Chapter 12 in your textbook for CHEM 3750/ 3770 and on pages 13-28 in your laboratory manual.
1. INTRODUCTION. Received 2003 January 28; accepted 2003 March 31
 The Astrophysical Journal, 592:947 952, 2003 August 1 # 2003. The American Astronomical Society. All rights reserved. Printed in U.S.A. THE ROLE OF POLYCYCLIC AROMATIC HYDROCARBONS IN ULTRAVIOLET EXTINCTION.
The Astrophysical Journal, 592:947 952, 2003 August 1 # 2003. The American Astronomical Society. All rights reserved. Printed in U.S.A. THE ROLE OF POLYCYCLIC AROMATIC HYDROCARBONS IN ULTRAVIOLET EXTINCTION.
Astrochemistry the summary
 Astrochemistry the summary Astro 736 Nienke van der Marel April 27th 2017 Astrochemistry When the first interstellar molecules were discovered, chemists were very surprised. Why? Conditions in space are
Astrochemistry the summary Astro 736 Nienke van der Marel April 27th 2017 Astrochemistry When the first interstellar molecules were discovered, chemists were very surprised. Why? Conditions in space are
Ram Pressure Stripping in NGC 4330
 The Evolving ISM in the Milky Way & Nearby Galaxies Ram Pressure Stripping in NGC 4330 Anne Abramson 1 & Jeffrey D. P. Kenney 1 1 Astronomy Department, Yale University, P.O. Box 208101 New Haven, CT 06520-8101
The Evolving ISM in the Milky Way & Nearby Galaxies Ram Pressure Stripping in NGC 4330 Anne Abramson 1 & Jeffrey D. P. Kenney 1 1 Astronomy Department, Yale University, P.O. Box 208101 New Haven, CT 06520-8101
arxiv: v2 [astro-ph] 31 Oct 2008
![arxiv: v2 [astro-ph] 31 Oct 2008 arxiv: v2 [astro-ph] 31 Oct 2008](/thumbs/93/112964597.jpg) Submitted to The Astrophysical Journal Letters Is the Universe More Transparent to Gamma Rays than Previously Thought? Floyd W. Stecker arxiv:0807.4880v2 [astro-ph] 31 Oct 2008 Astrophysics Science Division,
Submitted to The Astrophysical Journal Letters Is the Universe More Transparent to Gamma Rays than Previously Thought? Floyd W. Stecker arxiv:0807.4880v2 [astro-ph] 31 Oct 2008 Astrophysics Science Division,
NIR Silicate features and Statistics from IRAS data
 NIR Silicate features and Statistics from IRAS data Ranjan Gupta Inter University Center for Astronomy and Astrophysics Pune-411007, India NIR Silicate features and Statistics from IRAS data p.1/46 Abstract
NIR Silicate features and Statistics from IRAS data Ranjan Gupta Inter University Center for Astronomy and Astrophysics Pune-411007, India NIR Silicate features and Statistics from IRAS data p.1/46 Abstract
Verification of COS/FUV Bright Object Aperture (BOA) Operations at Lifetime Position 3
 Instrument Science Report COS 2015-05(v1) Verification of COS/FUV Bright Object Aperture (BOA) Operations at Lifetime Position 3 Andrew Fox 1, John Debes 1, & Julia Roman-Duval 1 1 Space Telescope Science
Instrument Science Report COS 2015-05(v1) Verification of COS/FUV Bright Object Aperture (BOA) Operations at Lifetime Position 3 Andrew Fox 1, John Debes 1, & Julia Roman-Duval 1 1 Space Telescope Science
Probing Bonding Using Infrared Spectroscopy Chem
 Probing Bonding Using Infrared Spectroscopy Chem 112-2011 INTRODUCTION First, watch the short video on how to record an infrared spectrum using an infrared spectrometer, linked at: http://employees.oneonta.edu/viningwj/chem112/labs/ir_video/ir_video_controller.swf
Probing Bonding Using Infrared Spectroscopy Chem 112-2011 INTRODUCTION First, watch the short video on how to record an infrared spectrum using an infrared spectrometer, linked at: http://employees.oneonta.edu/viningwj/chem112/labs/ir_video/ir_video_controller.swf
Kinetics of nitric oxide desorption from carbonaceous surfaces
 Fuel Processing Technology 77 78 (2002) 453 458 www.elsevier.com/locate/fuproc Kinetics of nitric oxide desorption from carbonaceous surfaces Alejandro Montoya a, Fanor Mondragón a, Thanh N. Truong b,
Fuel Processing Technology 77 78 (2002) 453 458 www.elsevier.com/locate/fuproc Kinetics of nitric oxide desorption from carbonaceous surfaces Alejandro Montoya a, Fanor Mondragón a, Thanh N. Truong b,
Dust in the Diffuse Universe
 Dust in the Diffuse Universe Obscuring Effects Chemical Effects Thermal Effects Dynamical Effects Diagnostic Power Evidence for Grains: Chemical Effects Catalyzes molecular hydrogen formation. Depletion
Dust in the Diffuse Universe Obscuring Effects Chemical Effects Thermal Effects Dynamical Effects Diagnostic Power Evidence for Grains: Chemical Effects Catalyzes molecular hydrogen formation. Depletion
Noordwijk, The Netherlands
 Noordwijk, The Netherlands 1 Contents Program 2 PAHs and JWST 7 PAH emission features in space 14 PAH Spectroscopy and the Search for Signatures 27 Formation & Processing of PAHs 39 Relationships between
Noordwijk, The Netherlands 1 Contents Program 2 PAHs and JWST 7 PAH emission features in space 14 PAH Spectroscopy and the Search for Signatures 27 Formation & Processing of PAHs 39 Relationships between
VIBRATION-ROTATION SPECTRUM OF CO
 Rice University Physics 332 VIBRATION-ROTATION SPECTRUM OF CO I. INTRODUCTION...2 II. THEORETICAL CONSIDERATIONS...3 III. MEASUREMENTS...8 IV. ANALYSIS...9 April 2011 I. Introduction Optical spectroscopy
Rice University Physics 332 VIBRATION-ROTATION SPECTRUM OF CO I. INTRODUCTION...2 II. THEORETICAL CONSIDERATIONS...3 III. MEASUREMENTS...8 IV. ANALYSIS...9 April 2011 I. Introduction Optical spectroscopy
The 158 Micron [C II] Line: A Measure of Global Star Formation Activity in Galaxies Stacey et al. (1991) ApJ, 373, 423
![The 158 Micron [C II] Line: A Measure of Global Star Formation Activity in Galaxies Stacey et al. (1991) ApJ, 373, 423 The 158 Micron [C II] Line: A Measure of Global Star Formation Activity in Galaxies Stacey et al. (1991) ApJ, 373, 423](/thumbs/81/83212129.jpg) The 158 Micron [C II] Line: A Measure of Global Star Formation Activity in Galaxies Stacey et al. (1991) ApJ, 373, 423 Presented by Shannon Guiles Astronomy 671 April 24, 2006 Image:[C II] map of the galaxy
The 158 Micron [C II] Line: A Measure of Global Star Formation Activity in Galaxies Stacey et al. (1991) ApJ, 373, 423 Presented by Shannon Guiles Astronomy 671 April 24, 2006 Image:[C II] map of the galaxy
Infra-red Spectroscopy
 Molecular vibrations are associated with the absorption of energy (infrared activity) by the molecule as sets of atoms (molecular moieties) vibrate about the mean center of their chemical bonds. Infra-red
Molecular vibrations are associated with the absorption of energy (infrared activity) by the molecule as sets of atoms (molecular moieties) vibrate about the mean center of their chemical bonds. Infra-red
The Diffuse Interstellar Bands and Carbon Chains
 The Diffuse Interstellar Bands and Carbon Chains Ben McCall Miller Institute for Basic Research in Science Department of Chemistry and Department of Astronomy University of California at Berkeley U. Chicago:
The Diffuse Interstellar Bands and Carbon Chains Ben McCall Miller Institute for Basic Research in Science Department of Chemistry and Department of Astronomy University of California at Berkeley U. Chicago:
7. Dust Grains & Interstellar Extinction. James R. Graham University of California, Berkeley
 7. Dust Grains & Interstellar Extinction James R. Graham University of California, Berkeley Visual Extinction Presence of interstellar gas or nebulae has a long history Existence of absorbing interstellar
7. Dust Grains & Interstellar Extinction James R. Graham University of California, Berkeley Visual Extinction Presence of interstellar gas or nebulae has a long history Existence of absorbing interstellar
2. Infrared spectroscopy
 2. Infrared spectroscopy 2-1Theoretical principles An important tool of the organic chemist is Infrared Spectroscopy, or IR. IR spectra are acquired on a special instrument, called an IR spectrometer.
2. Infrared spectroscopy 2-1Theoretical principles An important tool of the organic chemist is Infrared Spectroscopy, or IR. IR spectra are acquired on a special instrument, called an IR spectrometer.
CHEM 103: Chemistry in Context
 CHEM 103: Chemistry in Context Unit 4.2 Atmospheric Chemistry: the chemistry of global climate change Reading: Chapter 3 Unit 4.2: Chemistry Behind Global Climate Change Solar energy balance Earth s surface
CHEM 103: Chemistry in Context Unit 4.2 Atmospheric Chemistry: the chemistry of global climate change Reading: Chapter 3 Unit 4.2: Chemistry Behind Global Climate Change Solar energy balance Earth s surface
Studies of diffuse UV radiation
 Bull. Astr. Soc. India (2007) 35, 295 300 Studies of diffuse UV radiation N. V. Sujatha and Jayant Murthy Indian Institute of Astrophysics, Bangalore 560 034, India Abstract. The upcoming TAUVEX mission
Bull. Astr. Soc. India (2007) 35, 295 300 Studies of diffuse UV radiation N. V. Sujatha and Jayant Murthy Indian Institute of Astrophysics, Bangalore 560 034, India Abstract. The upcoming TAUVEX mission
